#8279
Incidence of Ceramic-on-Ceramic Bearing Fractures in Total Hip Arthroplasty: A 26-Year Single Tertiary Referral Center Study
HONG SEOK Kim - Seoul National University Hospital - Seoul, South Korea
Young-Seung Ko - Seoul National University Hospital - Seoul, Korea (Republic of)
*Jeong Joon Yoo - Seoul National University College of Medicine - SEOUL, South Korea
*Email: jjyos@snu.ac.kr
Introduction:
Ceramic-on-ceramic (CoC) bearings have been widely used in total hip arthroplasty (THA) due to their excellent wear properties. However, ceramic component fractures remain a concern. This study aimed to report the incidence of modern ceramic component fracture and identify factors that might influence this risk.
Methods:
We conducted a retrospective review of 4,719 hips that underwent THA with modern CoC bearings at a single institution between 1997 and 2023. We determined the incidence and the associated risk factors of CoC bearing fracture.
Results:
Out of the 4,719 THA procedures, there were 24 revisions (0.51%) for CoC bearing fracture. Specifically, revisions for fracture were to zero out of 2,254 (0.0%) Biolox Delta heads, 17 of 2,465 (0.690%) Biolox Forte heads, 2 of 2,254 (0.089%) Biolox Delta liners and 5 of 2,465 (0.203%) Biolox Forte liners. All ceramic head fractures occurred in 28mm-size short neck head.
Conclusion:
We report the largest single-center study of CoC bearing fractures to date, without using registry data. Although the risk of revision for fracture of CoC bearings is low, previous research has not accurately estimated this risk. Our data was comparable with recent evidence suggesting that the latest generation of ceramic components has significantly decreased the incidence of head fracture, but not liner fracture.
#8513
TiN, TiNbN, ZrN Coated Joint Replacements Past, Present, and Future of Ceramic Coatings
Antonio Santana - IHI Ionbond AG - Dulliken, Switzerland
*Susann Schmidt - IHI Ionbond AG - Dulliken, Switzerland
*Email: susann.schmidt@ionbond.com
Introduction:
Successful knee replacement procedures are estimated to last currently 15 – 20 years in the human body before a revision is needed [1]. The reasons for revision surgeries are various and studies lack to show implications on the choice of material that is implanted. Coating orthopedic implants with high performance ceramics such as TiN, TiNbN monolayer or ZrN multilayer coatings were shown to reduce UHMWPE wear and metal ion release to the body [2,3]. Thus, improving the implant surface characteristics utilizing monolayer ceramic coatings such as TiN, TiNbN generally increase implant lifetimes and ceramic coatings can mitigate the risk of revision surgeries at any stage of implantation [4]. In this paper we bring further insights how the coating technology, chemistry and architecture can vary the results in wear and ion release as well as mechanical properties suggesting how to make improvements to improve wear and implant longevity.
Methods:
For the study mirror polished (Ra < 0.05 µm) orthopaedic implants and flat test samples are coated with generation II physical vapor deposition (PVD) using steered cathodic arc in form of monolayer and multilayer and chemical vapor deposition (CVD). These already marketed generation II coatings are compared with coatings prepared by pulsed magnetron sputtering (PMS) - a generation III coating technology. The coating processes are performed to corresponding standard operating procedures. All surfaces are polished to a roughness of Ra < 0.05 µm prior to testing.
The coating performance is evaluated by simulator tests i.a.w. ASTM F732, while the coating biocompatibility is confirmed according to ISO 10993. Fundamental and application relevant coating properties such as coating roughness, composition and substrate adhesion are evaluated for TiN and TiNbN chemistries on implant components and test samples in agreement with their corresponding ISO standard.
Results:
The coating performance and related properties are compared, set into context with the application technique and related to basic material properties. The coatings prepared by PVD cathodic arc and CVD show dense coating structures, yet elevated roughness values. Here, the TiN shows preferred defect densities over TiNbN because of inherent Nb material properties. Further, CVD coatings show perfect coverage on complex substrates such as hip cups and heads, figure 1. Both coating chemistries TiN and TiNbN show smooth, virtually defect free surfaces as prepared by pulsed magnetron sputtering. Other coating properties are regarded similar. Coating and counter body wear is affected by substrate defect densities and initial roughness values, which is corroborated by simulator test results.
Conclusion:
Both ceramic coatings - TiN and TiNbN - are viable options to improve un-coated implant characteristics. The inherent increased roughness of PVD cathodic arc and CVD coatings require special attention in post-coat processing. This is especially true for TiNbN. Initially higher coating roughness values may lead to additional pathways for ion release from the implant substrates. In contrary, the coating application by pulsed magnetron sputtering leads to virtually defect free surfaces which lead to improve ion release from the substrate and wear characteristics.
Figures
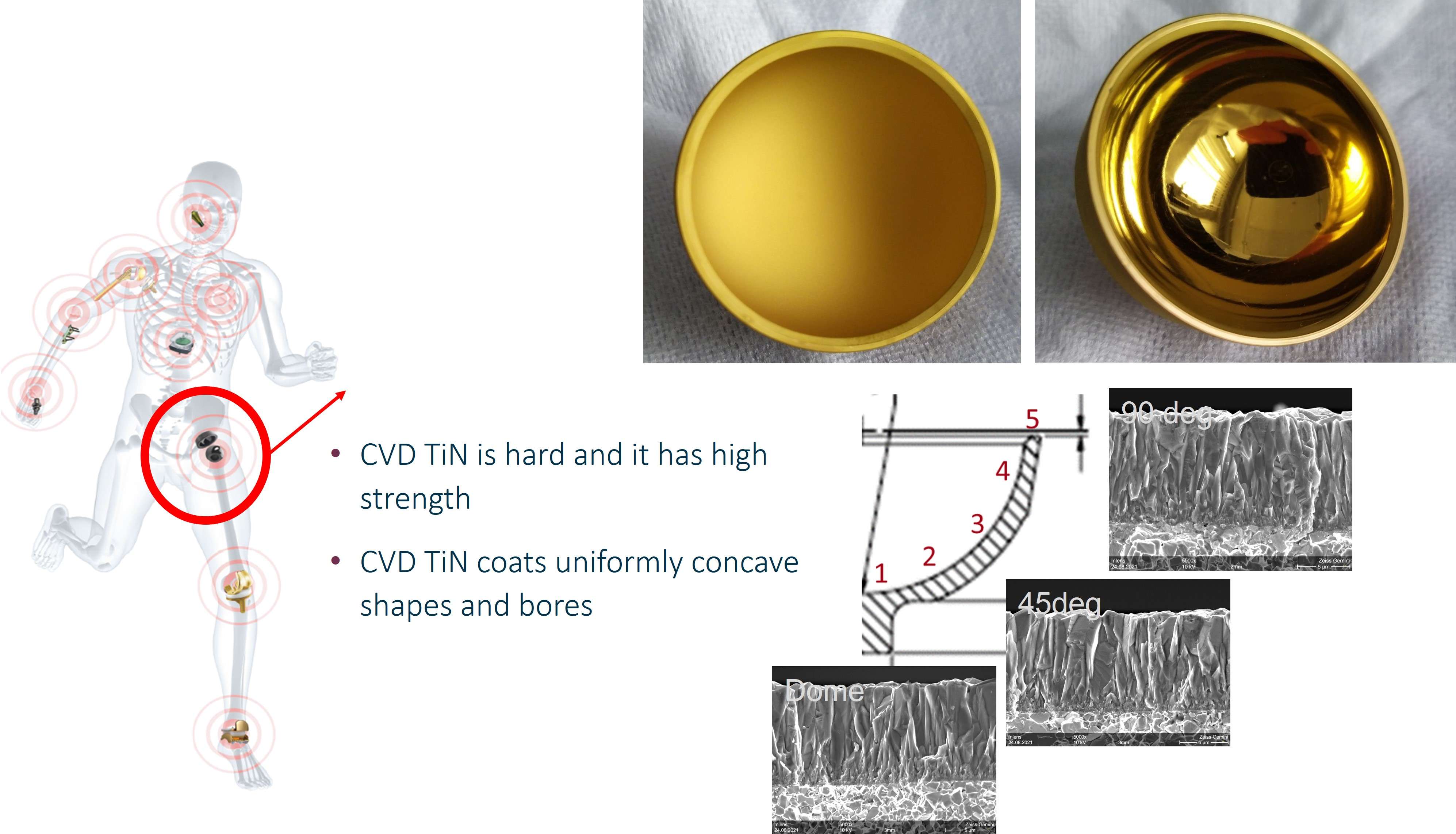
Figure 1#8205
Magnetic Resonance Safety Evaluation of a Novel AMC Ceramic TKA and Comparison of MR Image Artifacts to a CoCr TKA of Analogous Design
*Alessandro Alan Porporati - CeramTec GmbH - Plochingen, Germany
Yvonne Moedinger - CeramTec GmbH - Plochingen, Germany
Eric D. Anttila - MED Institute Inc. - West Lafayette, USA
Grant M. Baker - MED Institute Inc. - West Lafayette, USA
David C. Gross - MED Institute Inc. - West Lafayette, USA
*Email: a.porporati@ceramtec.de
Keywords: ceramic, alumina matrix composite, zirconia-toughened alumina, knee prosthesis, total knee arthroplasty, magnetic resonance imaging, image artifact
Introduction: Scanning metal knee implants in magnetic resonance imaging (MRI) systems creates image artifacts that complicate imaging-based diagnosis of the peri-implant region after total knee arthroplasty (TKA). MRI safety hazards could effectively be minimized by using metal-free knee prostheses that offer the potential for higher quality diagnostic images.
Methods: A novel ceramic TKA device without metallic components (i.e., metal-free) composed of BIOLOX®delta (CeramTec GmbH, Plochingen, Germany), a zirconia-toughened alumina matrix composite (AMC), was tested in an MR environment. Safety hazards were assessed resulting from the device placed into the MR environment at 3T (Tesla). American Society for Testing and Materials (ASTM) standard test methods were used for evaluating the magnetically induced displacement force, magnetically induced torque, and radiofrequency (RF)-induced heating. MR image artifacts of the AMC ceramic knee were evaluated according to ASTM standards and compared to a cobalt-chromium (CoCr) knee implant. Additionally, an Evan’s Magnetic Susceptibility Balance was used to assess the volumetric magnetic susceptibility of AMC, CoCr and titanium (Ti) metal alloys, which describes the interaction of the materials with the applied magnetic field.
Results: Magnetically induced displacement force and magnetically induced torque results indicate that the AMC ceramic knee does not pose a significant risk in a clinical MRI environment. Moreover, minimal RF-induced heating (below 1°C) of the device was observed after 15 minutes of scan time. Minimal image artifacts were induced by the AMC ceramic knee during MRI (7 mm) in comparison to the CoCr knee (88 mm). The AMC ceramic material showed extremely low magnetic susceptibility (2 ppm), compared to CoCr (820 - 2885 ppm) and Ti (157 - 190 ppm) alloys, which underlines that it is a nonmetallic and nonmagnetic material well suited for the manufacturing of MR Safe orthopedic implants.
Conclusion: The herein investigated AMC ceramic knee, which is currently under development and is not cleared or approved by the FDA for distribution in the United States, is a novel metal-free knee implant that could provide a valuable alternative to commercially available metal TKA devices in MRI applications. The BIOLOX®delta ceramic knee is composed of nonconductive, nonmetallic, and nonmagnetic materials. There are no known hazards resulting from exposure of this implant to a magnetic resonance environment, suggesting that the AMC ceramic knee can be regarded as MR Safe. The AMC ceramic knee can be scanned with superior imaging results in 1.5T and 3T MRI systems, which is an advantage compared to metal alternatives on the market.
Figures
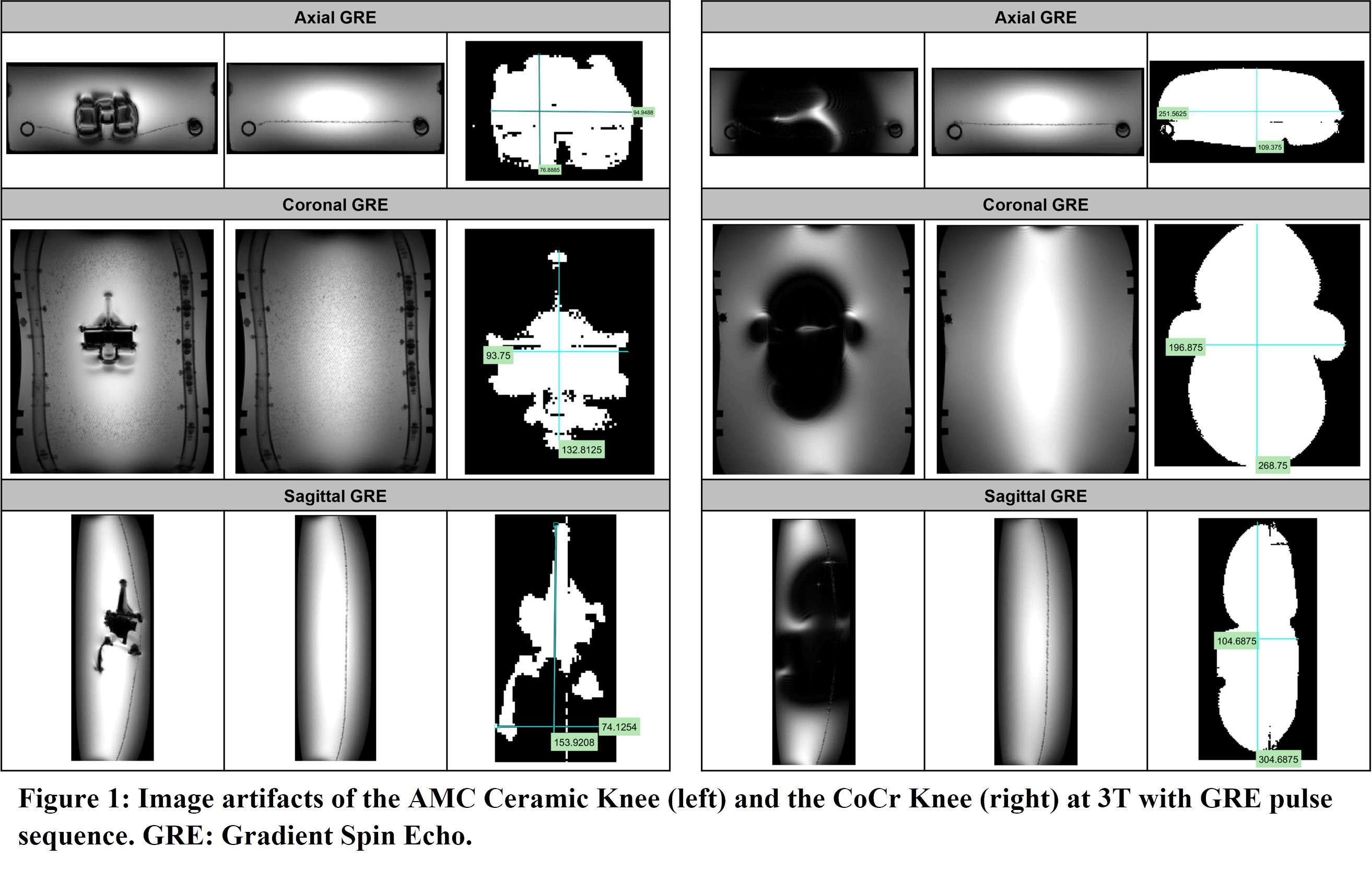
Figure 1#8568
Pyrocarbon Interposition Shoulder Arthroplasty: Analysis of Tissue Remodeling in Human and Sheep Model
*Ghassene Ouenzerfi - Wright Medical - Montbonnot saint Martin, France
Michel Hassler - Wright Medical - Montbonnot, France
Ana-Maria Sfarghiu - Laboratoire de Mécanique des Contacts et des Structures - Villeurbanne, France
Nina Attik - Univ Lyon, UCBL Lyon 1, UMR CNRS 5615, Laboratoire des Multimatériaux et Interfaces, - lyon, France
Remy Gauthier - Univ Lyon, CNRS, INSA Lyon, UCBL, MATEIS, - Villeurbanne, France
Helene Follet - INSERM UMR 1033, Université de Lyon - Lyon, France
Jean Paul Roux - INSERM UMR 1033, Université de Lyon - Lyon, France
Etienne Massardier - LAMCOS, Université de Lyon - Villeurbanne, France
Imbert De Gaudemaris - LAMCOS, Université de Lyon, - villeurbanne, France
*Email: ghassene.ouenzerfi@wright.com
Introduction:
In vitro data demonstrate the potential benefits of the Pyrocarbon (PyC) as a bearing material against cartilage or bone. PyC Free Interposition Arthroplasty (PFIA) has been used with positive outcomes for over 10 years for hand and wrist joint replacements [1,2,3]. Recently this concept was introduced on shoulder, to deal with bone stock preservation and treatment of young and middle-aged patients challenges. The aim of this study is to assess the adaptation and regeneration of bone tissue when rubbing against PyC by analyzing both retrieval from human and animal tissues.
Materials and methods:
1) Human retrieval analysis
Six patients implanted through PFIA and who underwent revision surgery (France) were considered. The time between initial implantation and revision surgery, and the reason for revision as well as the level of adherence of the neo-formed tissue are given in Fig 1.
2) Animal Retrieval analysis
7 sheeps underwent shoulder surgery using PFIA : 4 with CrCo implants, and 3 with PyC ones. After being bred for 3 years, they were sacrificed and neo-formed tissues on humeral side were analyzed using histological and micro-CT scan technics.
Results:
1) Human retrieval analysis
The neo-formed tissue poorly bonded to bone (--) appeared as a fibrocellular tissue, with low presence of collagen II and aggrecan markers. Those loosely (-) and slightly (+) bonded to bone presented some characteristics markers of cartilage, such as the presence of glycosaminoglycans. Finally, firmly bonded neo-formed tissues (++) presented cartilage-like characteristics, with a strong presence of collagen II and aggrecans. The interlock with bone can be seen in Figure 2.a. It is worth noticing that the neo-tissue never adheres to the PyC surface, allowing for a gliding motion of the implant on the humeral bone cavity.
Load transmission from the implant to the humeral bone differs depending on the binding degree of this neo-tissue on bone.
2) Animal retrieval analysis
Densification of the subchondral bone in contact with the implants was found for both materials. This phenomenon was more present with Cr-Co implants. Trabeculae are thicker in CrCo, but with a lower connectivity. Bone-implant interface is more dense than normal trabecular bone, it can be then considered as “corticalisation” (Fig 3). Resorption areas can be observed, especially with CrCo implants. The histological analysis (Fig 2.b) showed the presence of neo-cartilage tissue. The quality of tissue was more regular with PyC implant.
Conclusion:
Both animal and human retrieval analysis showed the presence of neocartilage tissues after rubbing PyC against bone. PyC implants seem to be well tolerated and transmitted strain in a better way than CrCo.
The design and the material seem to both play a crucial role on the way how bone is mechanically loaded which impact the remodeling and the healing of the tissue.
REFERENCES:
[1] Hannoun et al. European Cell and Materials. 2019. [2] Garret et all. JSES. 2017. [3] Bellmère et al. Hand Surg Rehab. 2018
Figures
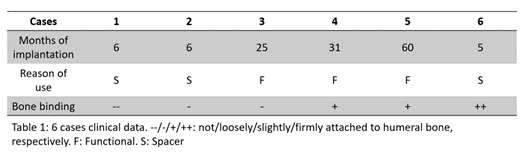
Figure 1
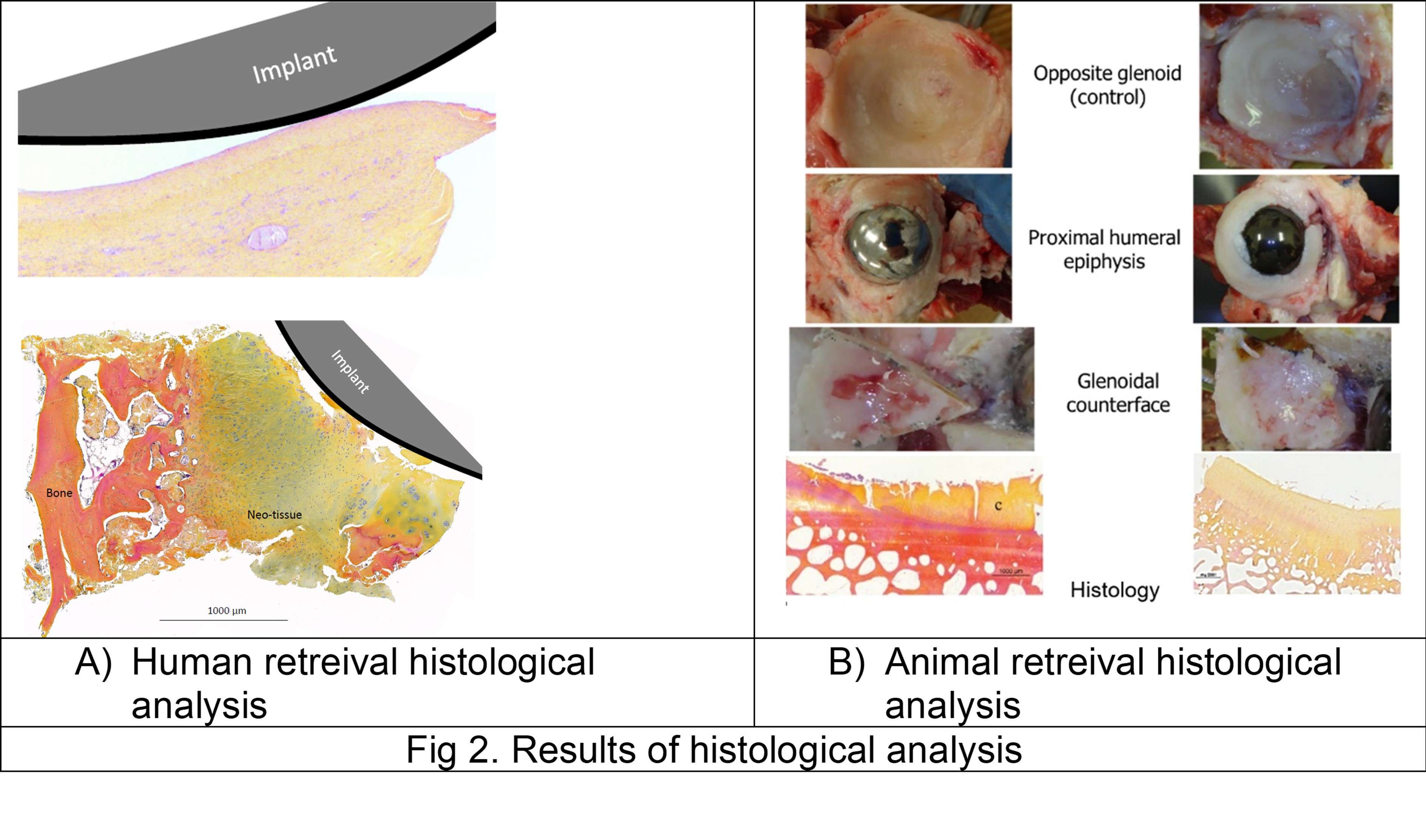
Figure 2
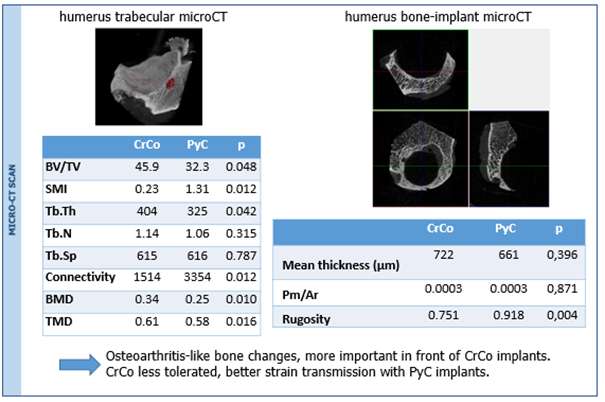
Figure 3#8748
Analgesic Doped UHMWPE for Therapeutic Implant Materials in Total Joint Arthroplasty
Nicoletta Inverardi - Massachusetts General Hospital - Boston, United States of America
Sashank Lekkala - Massachusetts General Hospital - Boston, United States of America
Keith Wannomae - Massachusetts General Hospital - Boston, USA
Brad Micheli - Massachusetts General Hospital - Boston, USA
Hany Bedair - Massachusetts General Hospital - Boston, USA
Orhun Muratoglu - Massachusetts General Hospital - Boston, USA
*Ebru Oral - Massachusetts General Hospital - Boston, USA
*Email: eoral@partners.org
Introduction
Pain management after total joint arthroplasty is often addressed by systemic delivery of opioids. Local delivery systems of non-opioid analgesic drugs have been investigated by blending UHMWPE with analgesic drugs1. The drug dosing is limited by the decrease in mechanical properties that is shown in these phase-separated materials after blending and molding. In this work, we studied an alternative process to supplement UHMWPE with the analgesic drugs lidocaine and bupivacaine, which is carried out after molding through a diffusion process. The aim is to obtain a therapeutic UHMWPE-based implant material with improved mechanical properties.
Methods
Diffusion doping was carried out by soaking UHMWPE samples inside the melted drug (the free-base form of lidocaine or bupivacaine) at 120 °C for 4 hours. The UHMWPE sample geometries were either prismatic strip (~3mm thick) or cylindrical pin (9mm diameter) depending on the following testing. Samples were weighed before and after doping, and the diffusion profile along the thickness was measured by FTIR. The drug index was calculated as the ratio of the areas under the analgesic characteristic peak (i.e., 1165 cm-1 and 960 cm-1 for lidocaine and bupivacaine, respectively) and the polyethylene skeletal absorbance at 1895 cm-1. Samples for tensile testing were die-cut and tensile tests were run at a crosshead speed of 10 mm/min (ASTM D638-10, n=4). The drug elution was investigating by eluting machined samples (3×5×20 mm3, n=6) in de-ionized water and calculating their concentration by UV spectroscopy.
Results
Diffusion doping was successfully performed leading to around 6-9 mg/cm2 doped drug to surface area. The diffusion profile of the drug along the thickness of the sample is shown in Figure 1. The tensile properties in terms of ultimate tensile strength (UTS) and elongation at break (EAB) did not significantly decrease compared to virgin UHMWPE. The drug release rate was extrapolated to the surface of a knee implant (100 cm2). The results showed that the day 1 dose was from 16 to 91 mg/day, for bupivacaine and lidocaine respectively. This amount is in the same order of magnitude of the minimum effective dose achieved after peri-articular injection2.
Conclusion
Diffusion doping of free-base analgesic drugs into UHMWPE might be a viable strategy to obtain implant materials for local delivery of pain medication without negatively affecting their mechanical properties.
References
- Grindy et al., Acta Biomaterialia 93 (2019) 63–73
- Karlsen et al., PLoS One 12 (2017) e0173107.
Acknowledgements
This work was supported in part by the Office of the Assistant Secretary of Defense for Health Affairs (Peer Reviewed Medical Research Program, Award No. W81XWH-17-1-0614). Opinions, interpretations, conclusions, and recommendations are those of the author and are not necessarily endorsed by the Department of Defense.
Keywords: UHMWPE, drug delivery, pain management, diffusion doping
Figures
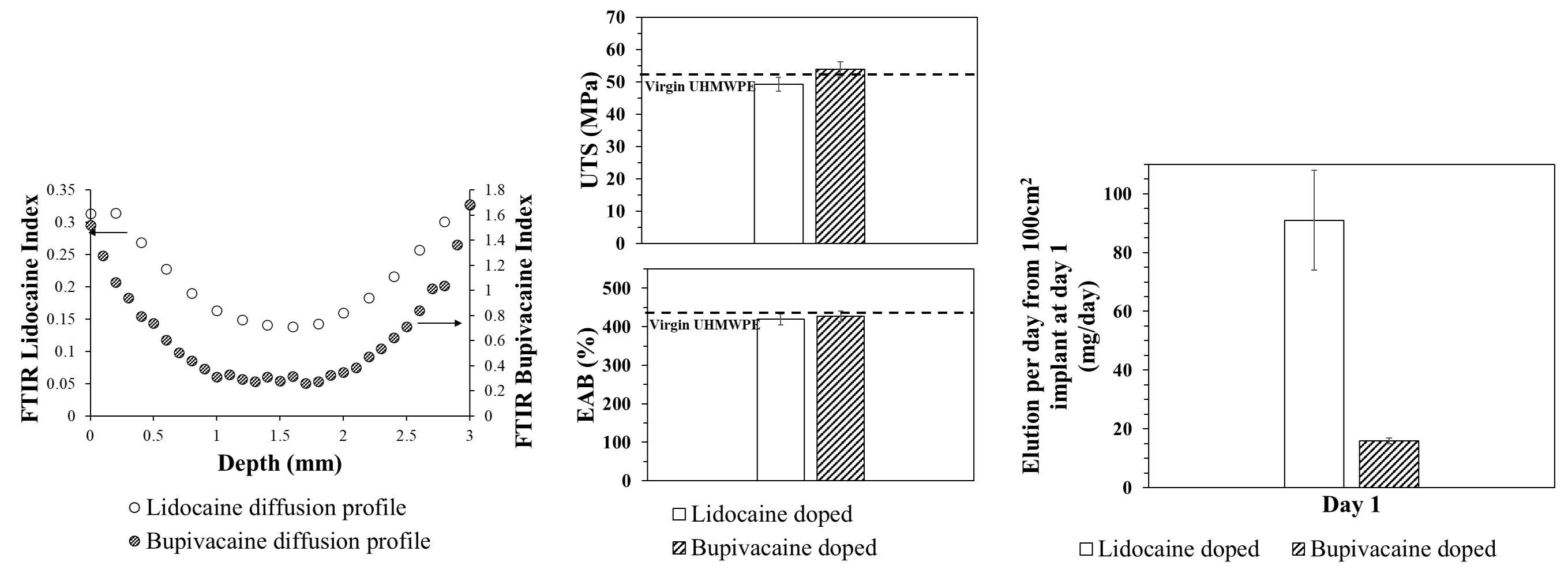
Figure 1#8318
Rifampin Loaded Antibiotic Cement Maintains Structural Integrity While Inhibiting Bacterial Growth
*Jacob Laperche - Frank H. Netter School of Medicine Quinnipiac University - Middletown, United States of America
Caitlin Barrett - University Orthopedics - Providence, USA
Dioscaris Garcia - Brown University - Providence, USA
Valentin Antoci - University Orthopedics - Providence, USA
Drew Clippert - Brown University/ University Orthopedics - Providence, United States of America
Abigail Boduch - Brown University Department of Orthopaedics - Providence, USA
Jillian Glasser - University Orthopedics Inc. - Providence, USA
*Email: jacoblaperche@gmail.com
Introduction: Implant related infection continues to be a pressing issue in orthopedic surgery. Due to the scope of this issue, significant research has been done on the prevention and treatment of PJI, with a focus on antimicrobial biomaterial design and biofilm eradication. This study investigates mechanical and antibacterial properties of polymethyl methacrylate (PMMA) with various concentrations of added rifampin.
Methods: Mechanical test samples were created by adding 0 to 200mg of Rifadin IV into a bag of SmartSet HV Bone Cement. Mechanical strength was tested, with a 70 MPa cutoff. Separate 100mg rifampin and control samples were incubated with P. aeruginosa for 6, 12, or 24 hours. Samples were stained using Dylight 594-conjugated anti-LPS antibodies and imaged with confocal microscopy. Student’s t-tests were performed to measure differences in bacterial coverage. Kirby Bauer assays on P. aeruginosa were also performed. Zones of inhibition were measured at 24 and 48hrs.
Results: There was no statistical difference between the mean compressive strength for PMMA control cylinders and those doped with 30, 50, and 100mg of rifampin. Higher doses of rifampin weakened the cement below the cut-off. The 100mg rifampin concentration also had significantly less bacterial presence at 12 and 24 hours (p=0.045 and p=0.0191). No zones of inhibition were seen on the Kirby Bauer assays for the samples with added rifampin.
Conclusion: Rifampin is universally accepted as an adjuvant to other antibiotics in the fight against resistant organisms. This is the first study focusing on clinically relevant doses of rifampin and the associated elution efficacy of the antibiotic. The ability of the rifampin-loaded PMMA to maintain mechanical integrity while demonstrating significant antimicrobial activity shows promise for future studies of the usage of this antibiotic in bone cement for prevention and treatment of PJI.
#8358
Osseointegration of Antimicrobial Iodine Surface-Treated Implants in an Ovine Model
*Devendra Gorhe - Zimmer Biomet - Warsaw, USA
Lucia Pontiroli - Zimmer Biomet - Swindon, United Kingdom
Imran Khan - Zimmer Biomet Inc - Swindon, United Kingdom
*Email: devendra.gorhe@zimmerbiomet.com
INTRODUCTION: Antimicrobial coatings and treatments based on silver, copper, bismuth, gallium, and antibiotics are described in the literature for the management of Periprosthetic Joint Infection (PJI) [1, 2]. Iodine is a well-known antiseptic with a long history of clinical use [3]. Its oxidative properties make it effective against bacteria [4], but its effect on bone remodeling when applied to an implant surface is not known. This study evaluated the effect of an Iodine surface treatment applied to Ti6Al4V orthopedic devices. Bone attachment was assessed at various time points during a GLP (Good Laboratory Practice) sheep study as per ISO 10993-6 [5].
METHODS: This study involved 18 sheep with three implantation time points (4, 12, and 26 weeks) and Ti6Al4V alloy implants with three surface iodine concentrations - High (160-200 µg/cm2), Medium (80-120 µg/cm2) and Low (25-50 µg/cm2). The Ti6Al4V implants were 8 mm diameter cylindrical parts coated with a Porous Plasma Spray (PPS) Ti6Al4V coating. Iodine was incorporated electrochemically as described previously by Tsuchiya et al. [4]. Histology, histomorphometric analysis, and mechanical push-out testing from cancellous bone were conducted. The clinical pathology assessment was carried out through hematology, serum chemistry and thyroid chemistry (total thyroxine (TT4), total triiodothyronine (TT3) and free thyroxine (FT4)) assays.
RESULTS: All Iodine-treated groups had higher bone-in-contact (BIC, %) than Controls at 4 weeks, in both cortical and cancellous bone, with no differences observed at 12 and 26 weeks (Figure 1). This observation was corroborated by the findings of mechanical push-out tests: comparable mean push-out forces were observed between groups (i.e., the three treatment Iodine doses and Controls) within each time point, or between time points (4, 12, and 26 weeks) for each groups (Figure 2). The Histological analysis and histomorphometry of bone tissue exhibited no local toxicity or fibrous tissue formation (Figure 3), irrespective of iodine dose. There were no clinical pathology or gross necropsy findings to suggest health or organ impairment, inflammation or infection related to the Test or Control articles for any of the 18 animals. The serum iodine concentration or thyroid markers remained steady in all study groups, and no changes were detected in urine iodine concentration in the selected groups. There was no evidence of a systemic response to the test articles based on histopathology of non-target tissues and clinical pathology findings. There was one early mortality, but this was attributed to post-operative patella luxation complications with both stifles and was unrelated to the test articles.
Conclusion: The study confirmed that there was no local toxicity and no delayed bone attachment to the test articles due to presence of iodine, as evidenced by the higher %BIC for all iodine doses as compared to the Controls at 4 weeks, and no difference with Controls at later time points. Comparable push-out forces were observed between Control and Iodine-treated implants. No abnormal trends were found for serum thyroid hormone and urine iodine levels.
Figures
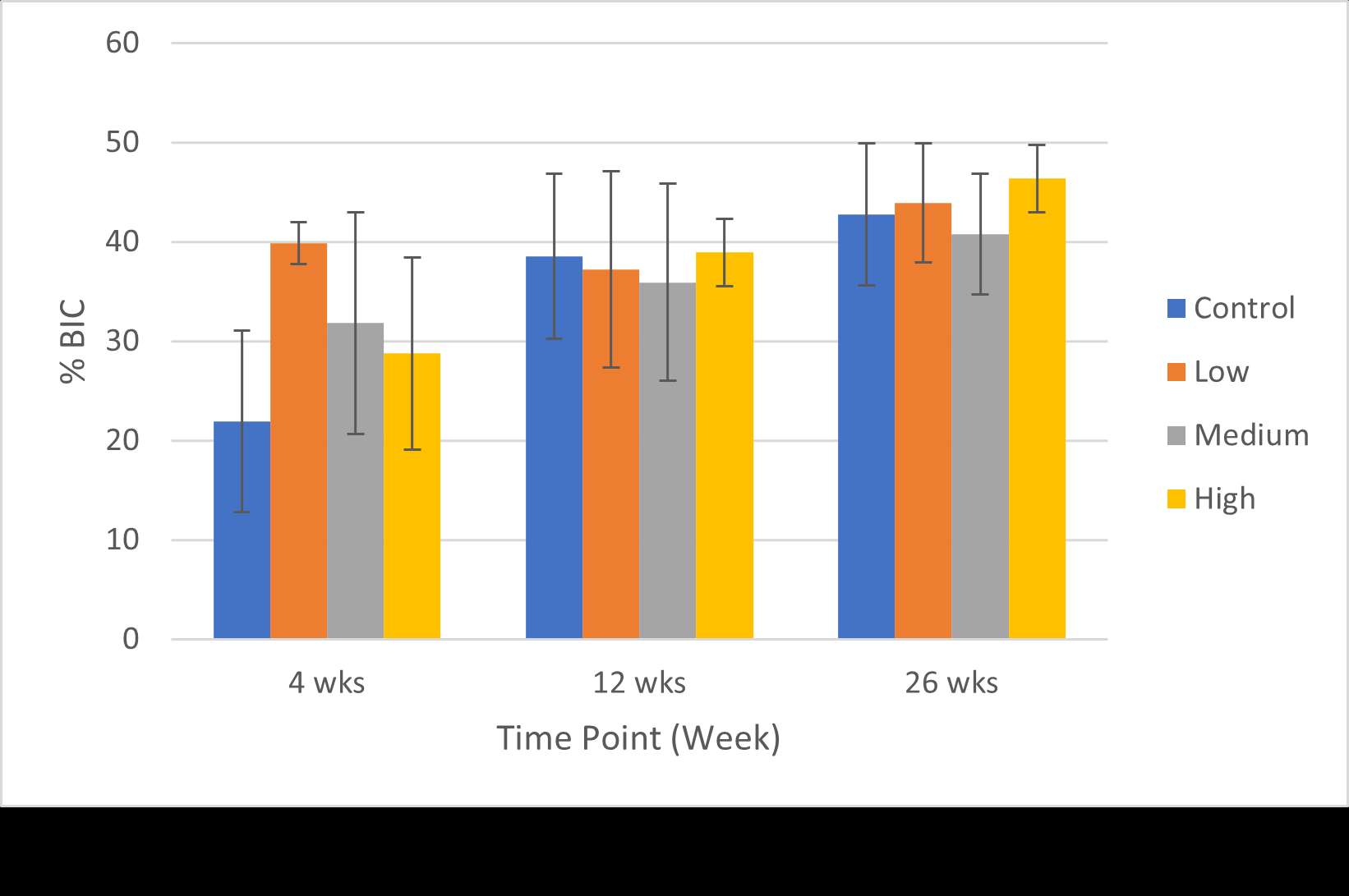
Figure 1
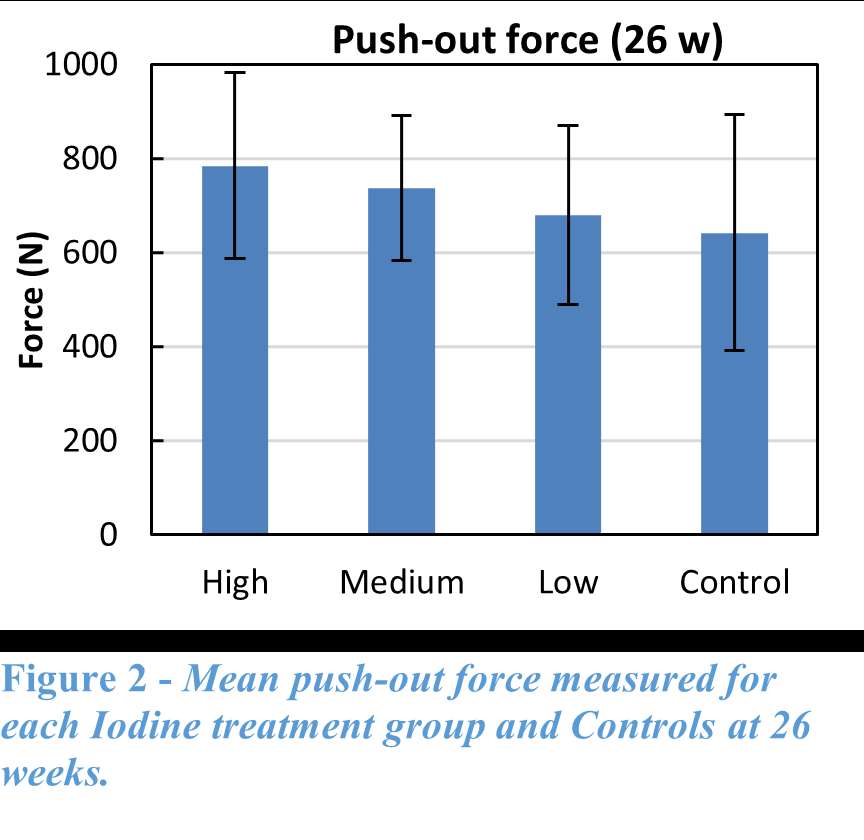
Figure 2
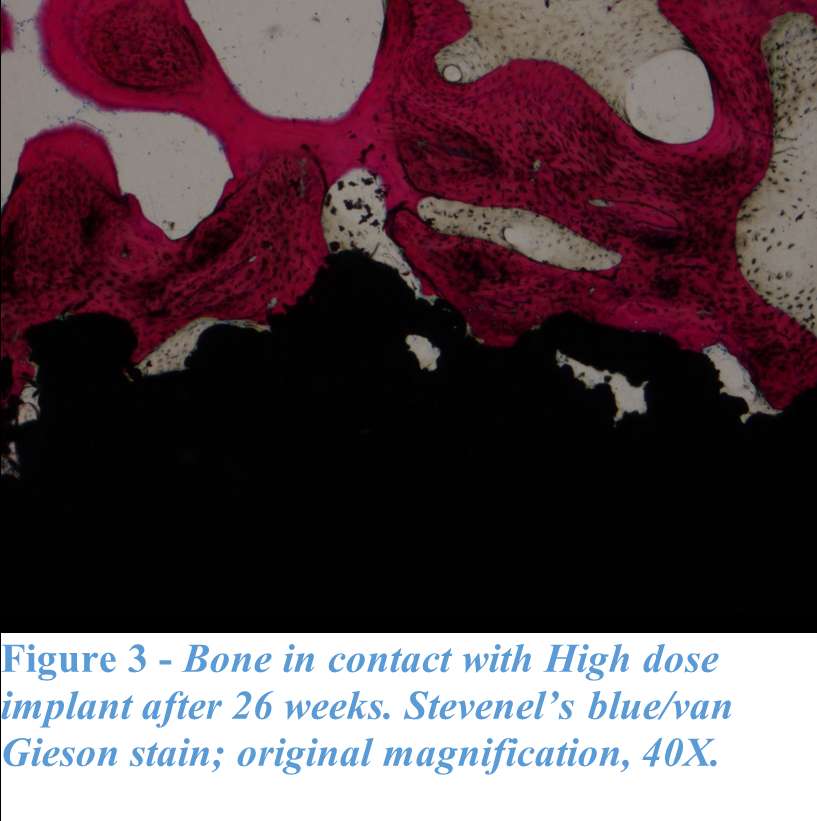
Figure 3#8797
The Immune Response Against Staphylococcus Aureus in a Rat Subcutaneous Model
Yingfang Fan - Massachusetts General Hospital - Boston, USA
Madeline McCanne - Massachusetts General Hospital - Boston, USA
Jean Yuh - Massachusetts General Hospital - Boston, USA
Sashank Lekkala - Massachusetts General Hospital - Boston, United States of America
Amita Sekar - Massachusetts General Hospital - Boston, USA
*Ebru Oral - Massachusetts General Hospital - Boston, USA
*Email: eoral@partners.org
Introduction: Our goal is to design drug-delivery devices for antibiotics based on the risk of infection to decrease the incidence of periprosthetic infection. Our study focuses on evaluating the differences in the immune response to a laboratory strain (ATCC 12600) susceptible to local antibiotics and a multi-drug resistant clinical strain (L1101) of Staphylococcus aureus in vitro and in vivo.
Methods: Under the approved MGH IACUC protocol 2021N000127, we implanted stainless steel plates (10x3x1 mm) subcutaneously on the dorsum of 102 Sprague Dawley rats(n=6/animal). The rats were divided into groups receiving different bacterial inoculate: 105 CFU ATCC 12600 (gentamicin-sensitive MSSA, n=21), 108 CFU ATCC 12600 (n=30), 105 CFU L1101 (gentamicin-resistant MRSA, n=21), 108 CFU L1101 (n=30). Additionally, a non-infected control group (n=15) was included. All groups were sacrificed on postoperative days (POD) 1, 3, 7, or 21. We tested plasma α-2-macroglobulin (a2M) concentration via ELISA (Abcam ab157730), tissue TNF-α, macrophage colony-stimulating factor (MCSF-1), T-cell markers, VEGFα, matrix metalloproteinase-1 (MMP1), MMP3, and MMP13 gene expression via RT-PCR, tissue TNF-α, IL-6 protein level via immunofluorescent staining, and tissue capsule thickness via H&E. Statistical significance was evaluated using one-way ANOVA followed by the Tukey test.
Results: Post-surgery systemic inflammation persisted until day 7, with higher inoculum inducing more inflammation (Figure 1). TNFα gene expression, a marker of local inflammation, increased early in animals receiving L1101 and decreased in those receiving the laboratory strain (Figure 2). Notably, the expression levels of MCSF-1 and T-cell markers (CD4, CD5, CD6, and CD8) were significantly upregulated in response to infection, and their profiles varied between the two strains, suggesting distinct rates of T-cell apoptosis. Histological analysis revealed increased vascularity, thicker capsules, and the presence of neutrophils surrounding the capsule until POD 7 in all groups (Figure 3). The 12600 group showed lower MMP-1 expression than the L1101 group on POD 7, while the 12600 LI group exhibited the highest MMP3 and MMP13 expression on POD 3. Moreover, the HI L1101 group had lower MCSF-1 and VEGFα levels compared to all three groups on POD 3. These findings highlight distinct gene expression patterns associated with infection, indicating altered tissue remodeling and angiogenesis. Moreover, all infected groups exhibited downregulation of the VEGFα-CXCR pathway and displayed impaired wound healing on POD 1. L1101 infection demonstrated a slow onset and more aggressiveness compared to 12600 at the early time point based on the expression of inflammatory cytokines and T-cell markers.
Conclusion: Both the lab and clinical S. aureus strains elicited inflammatory responses similar to the acute phase of a foreign body response, inducing innate immune cell infiltration and T-cell apoptosis. However, infection progression patterns varied, with high-dose 12600 showing a slow onset and decline, while high-dose L1101 infection had a rapid onset and subsequent decline. Infected implants underwent fibrotic encapsulation and vascularization, resembling the chronic phase of the foreign body response. These findings highlight differences in immune responses to various bacterial strains, informing our understanding of infection mechanisms and aiding the development of targeted interventions to improve patient outcomes.
Figures
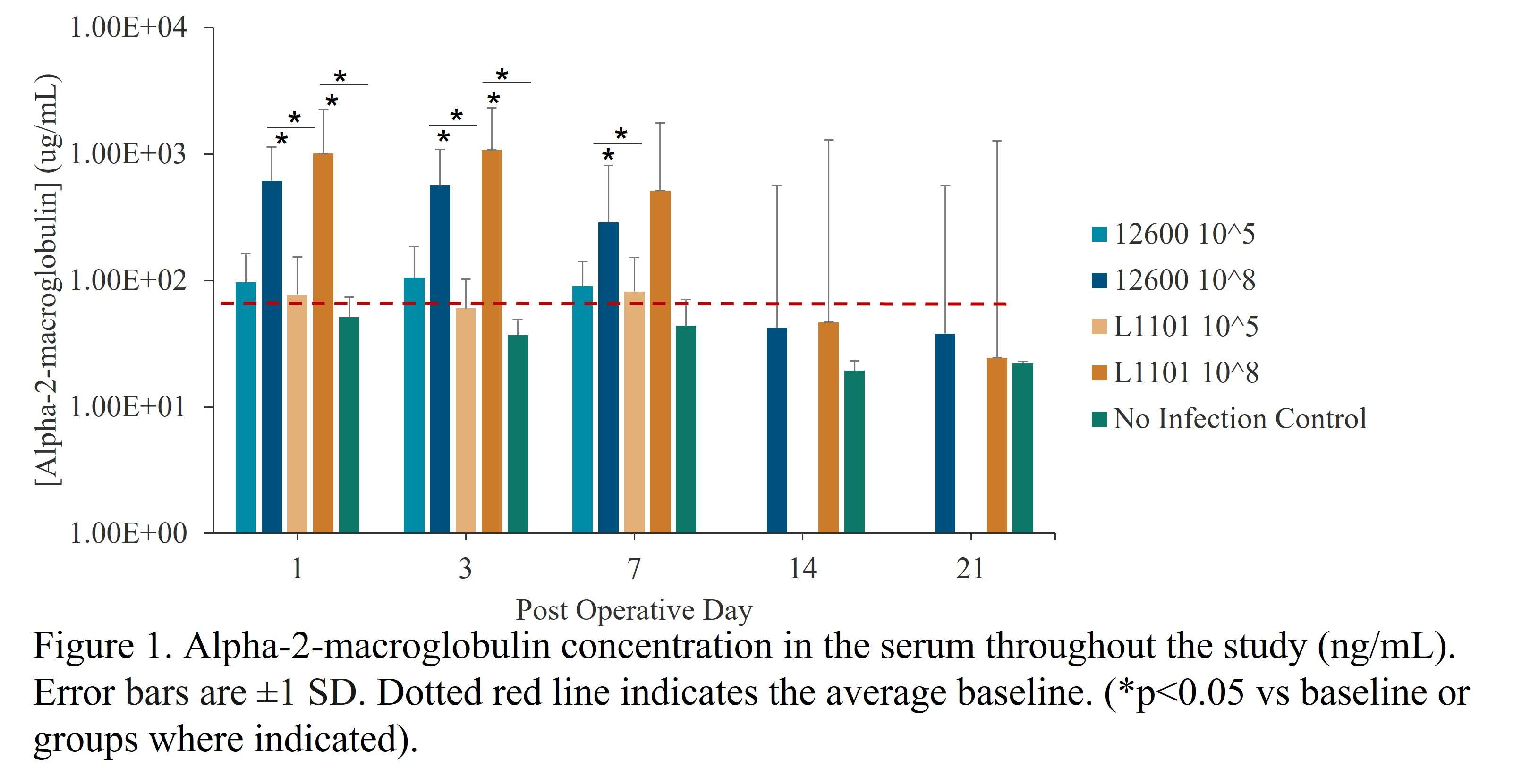
Figure 1
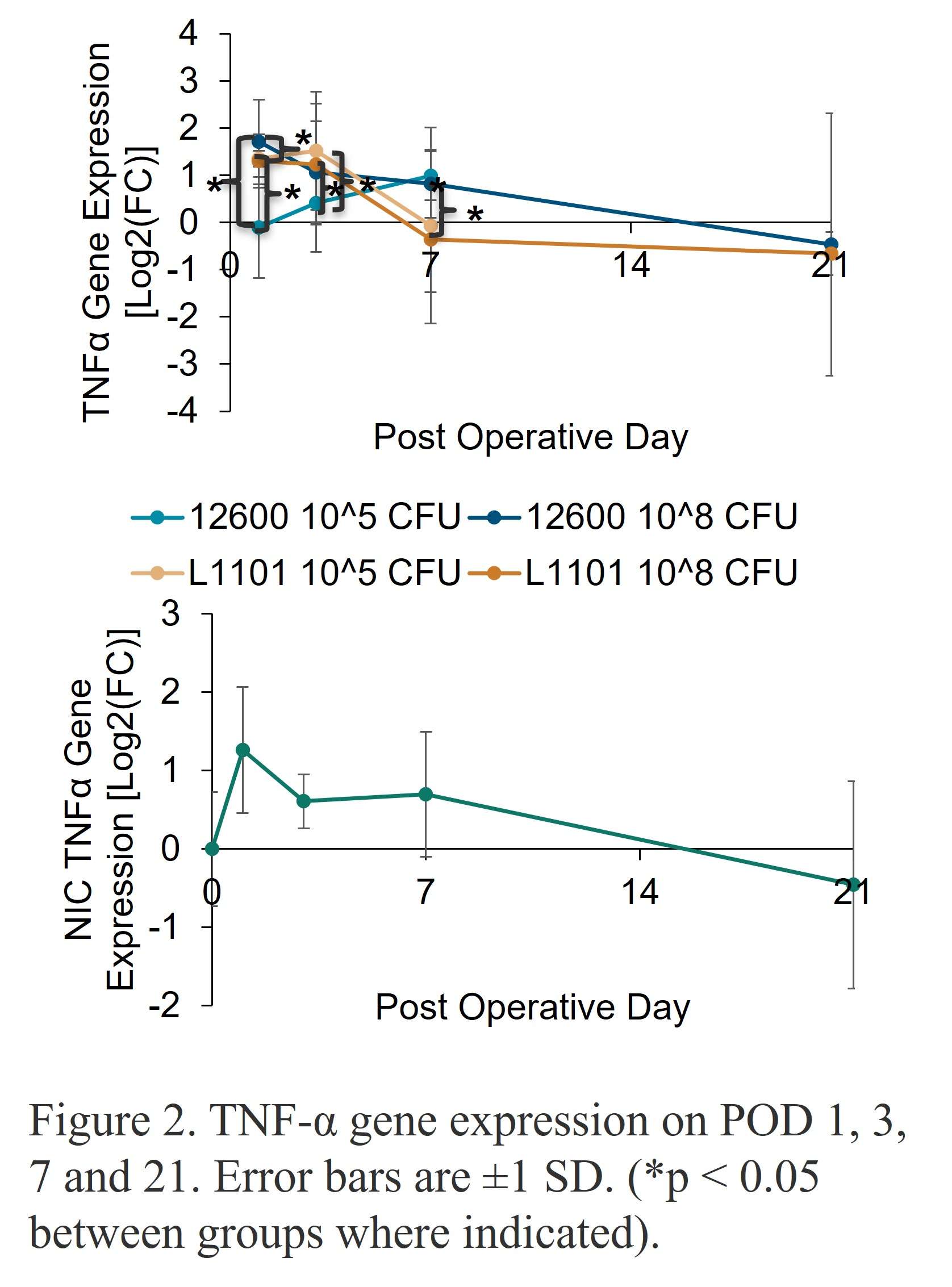
Figure 2
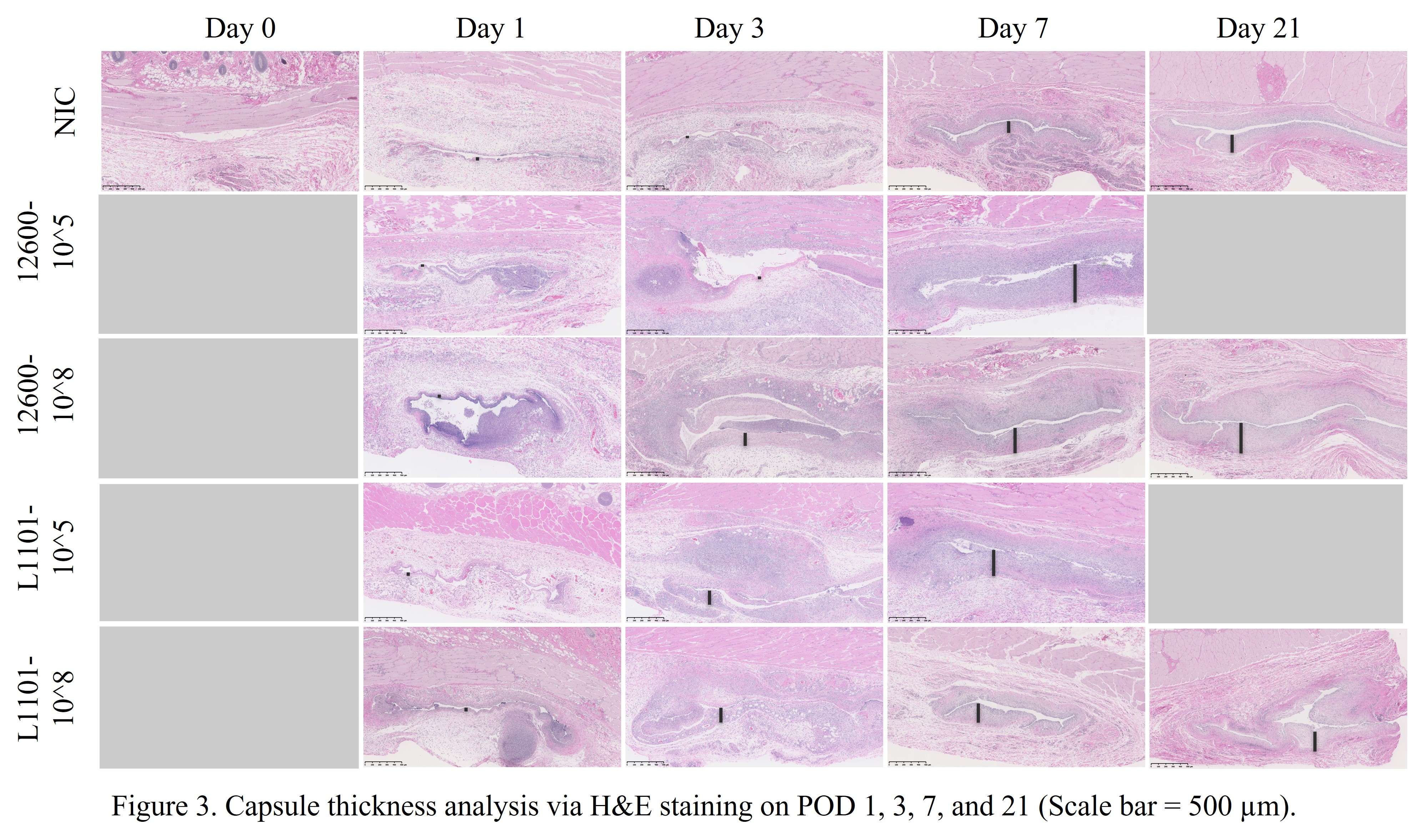
Figure 3#8235
In Silico Clinical Trials: Our Learnings
*Philippe Favre - Zimmer Biomet - Winterthur, Switzerland
Jeffrey Bischoff - Zimmer, Inc. - Warsaw, USA
Christine Mueri - Zimmer Biomet - Winterthur, Switzerland
Adam Henderson - Zimmer Biomet - Winterthur, Switzerland
Lukas Connolly - Zimmer Biomet - Winterthur, Switzerland
Hadi SeyedHosseini - Zimmer Biomet - Winterthur, Switzerland
Ghislain Maquer - Zimmer Biomet - Winterthur, Switzerland
*Email: philippe.favre@zimmerbiomet.com
Introduction
Clinical data requirements have recently risen because of the new European Medical Device Regulation (MDR). Concurrently, collecting clinical data is difficult for rare demographics or indications, patients are lost to follow-up, and separate clinical trials are often required for design variants. There is a pressing need to develop innovative strategies for addressing contemporary regulatory requirements in a more effective manner.
In silico clinical trials (ISCT) can address many challenges in enriching clinical trials with computer simulations. Possible barriers in setting up an ISCT for a regulatory submission were previously identified [1]. Here we review how previously identified barriers were addressed during the process of developing and validating an ISCT pipeline and refresh our outlook on ISCT for industrial applications.
Overcoming barriers
The four previously identified barriers for ISCT implementation [1] were addressed as follows:
- Defining the relevant patient harms to address with ISCT - Lacking external guidance on determining patient harms to include in an ISCT, a novel framework was developed that independently considers the risk associated with the harm, the impact of treatment on the harm likelihood of occurrence, and technical feasibility of evaluating the harm via ISCT. This framework heavily relies on existing clinical data, and maximizes clinical impact of the ISCT.
- Creating a versatile software pipeline: A fully automated ISCT pipeline has been developed with acceptable (<90min) throughput time per model and 95% solving success rate. This demonstrates the feasibility of running large scale population models in a reasonable timeframe on standard hardware.
- Ensuring model credibility: Lacking guidance for establishing model credibility for an ISCT, a risk-based approach for planning and executing clinical validation activities was developed, following closely the ASME V&V40 philosophy [2]. This approach introduces separate credibility activities and assessments for clinical validation, by applying the technical framework to replicate known clinical results prior to executing the intended ISCT.
- Limiting regulatory uncertainty – Advances were regularly communicated with both internal and external regulatory authorities, and with our peers within dedicated conferences and organization. These engagements help ensure the technical validity of the ISCT approach, and identify potential concerns from academic, industrial, and regulatory stakeholders that can be mitigated with continuing advances in guidance or technical implementation.
Outlook
Gaps in contemporary guidance on ISCT application have resulted in development of initial frameworks to identify appropriate harms, and to use clinical data to demonstrate validity. Further, a robust, automated pipeline has been developed to support population-level analyses. Nevertheless, substantial technical work is required to apply these frameworks to new ISCT applications, including adapting the surgical procedure and representation of in vivo use conditions. Nevertheless, the benefits of ISCT to address challenges in clinical data acquisition support continued investment by the community in this regulatory and clinical strategy.
[1] Favre, P. et al. In Silico Clinical Trials in the Orthopedic Device Industry: From Fantasy to Reality?. Ann Biomed Eng 49, 3213–3226 (2021).
[2] ASME-V&V40. Assessing Credibility of Computational Modeling through Verification and V
#8430
Evaluating the Varus-Valgus and Internal-External Constraint Level of a Mid-Level Constraint Total Knee System Using Finite Element Analysis
*Yupin Shi - Hospital for Special Surgery - New York, United States of America
Joseph Lipman - Hospital for Special Surgery - New York, USA
Fernando Quevedo Gonzalez - Hospital for Special Surgery - New York, USA
Peter Sculco - Hospital for Special Surgery - New York, USA
*Email: shiy@hss.edu
Introduction: Modern total knee systems can mix and match femoral components and bearings of different sizes. However, no study has shown whether the VV and IE constraint angles remain the same among different combinations. The objectives of this study were to computationally evaluate the VV and IE constraint angles of femoral components paired with bearings in different compatible combinations using finite element analysis (FEA).
Methods: We chose the Zimmer Biomet Persona Posterior Stabilized (PS) femoral component, which is compatible with the Constrained Posterior Stabilized (CPS) bearings in various sizes. Available bearings in 6-9/CD and 6-9/GH and extreme femoral components compatible with these bearings, including 6-Narrow, 9-Narrow, and 9-Standard were laser scanned using HandyScan (Creaform, Laval, Qc) to generate their computer-aided design models by reverse engineering in DesignX (3D Systems). The geometries were imported into the FE software Abaqus (Dassault Systems, Providence, RI) and assembled at a 30° flexion. The models were meshed with 1mm linear tetrahedral elements. The femoral articular surfaces were extracted and modeled as rigid; the bearings were modeled as Ultra-High Molecular Weight Polyethylene (E=1016 MPa, υ=0.46). For VV constraint, the femoral component could move in medial-lateral and superior-inferior directions but was fixed about the flexion-extension and internal-external axis, and in anterior-posterior directions; the bearing was locked in all degrees of freedom. For IE constraint, the femoral component could move in superior-inferior directions and about the varus-valgus axis but was fixed in all other degrees of freedom; the bearing could move in anterior-posterior and medial-lateral directions but was fixed about the flexion-extension and varus-valgus axis, and in superior-inferior directions. The friction coefficient was 0.02. For VV constraint, 50N and 3000N axial compression loads were applied while the femoral component was rotated in the coronal plane. For IE constraint, 300N and 3000N axial compression loads were applied while the bearing was rotated in the axial plane. 50N is the approximate axial load applied during the intraoperative assessment of knee stability. 300N and 3000N are the maximum axial loads during walking and stair climbing. Moment vs rotation angle curves and the constraint angle (rotation angles to contact) were reported.
Results: The VV constraint angle at 50N were ±1.3?, ±1.3? and ±0.8? for 6-Narrow, 9-Narrow and 9-Standard with 6-9/CD, and were ±1.5?, ±1.5? and ±1.1? for 6-Narrow, 9-Narrow and 9-Standard with 6-9/GH. The VV constraint angle at 3000N were ±1.4?, ±0.9? and ±0.8? for 6-Narrow, 9-Narrow and 9-Standard with 6-9/CD, and were ±1.6?, ±2.1? and ±1.6? for 6-Narrow, 9-Narrow and 9-Standard with 6-9/GH. The IE constraint angle at 300N were ±3.0?, ±2.7? and ±1.8? for 6-Narrow, 9-Narrow and 9-Standard with 6-9/CD, and were ±3.4?, ±3.1? and ±2.4? for 6-Narrow, 9-Narrow and 9-Standard with 6-9/GH. The IE constraint angle at 3000N were ±3.3?, ±2.8? and ±2.0? for 6-Narrow, 9-Narrow and 9-Standard with 6-9/CD, and were ±3.7?, ±3.3? and ±2.6? for 6-Narrow, 9-Narrow and 9-Standard with 6-9/GH.
Conclusion: The VV and IE constraint angle is larger for larger bearings and narrower femoral components; however, the differences were small.
Figures
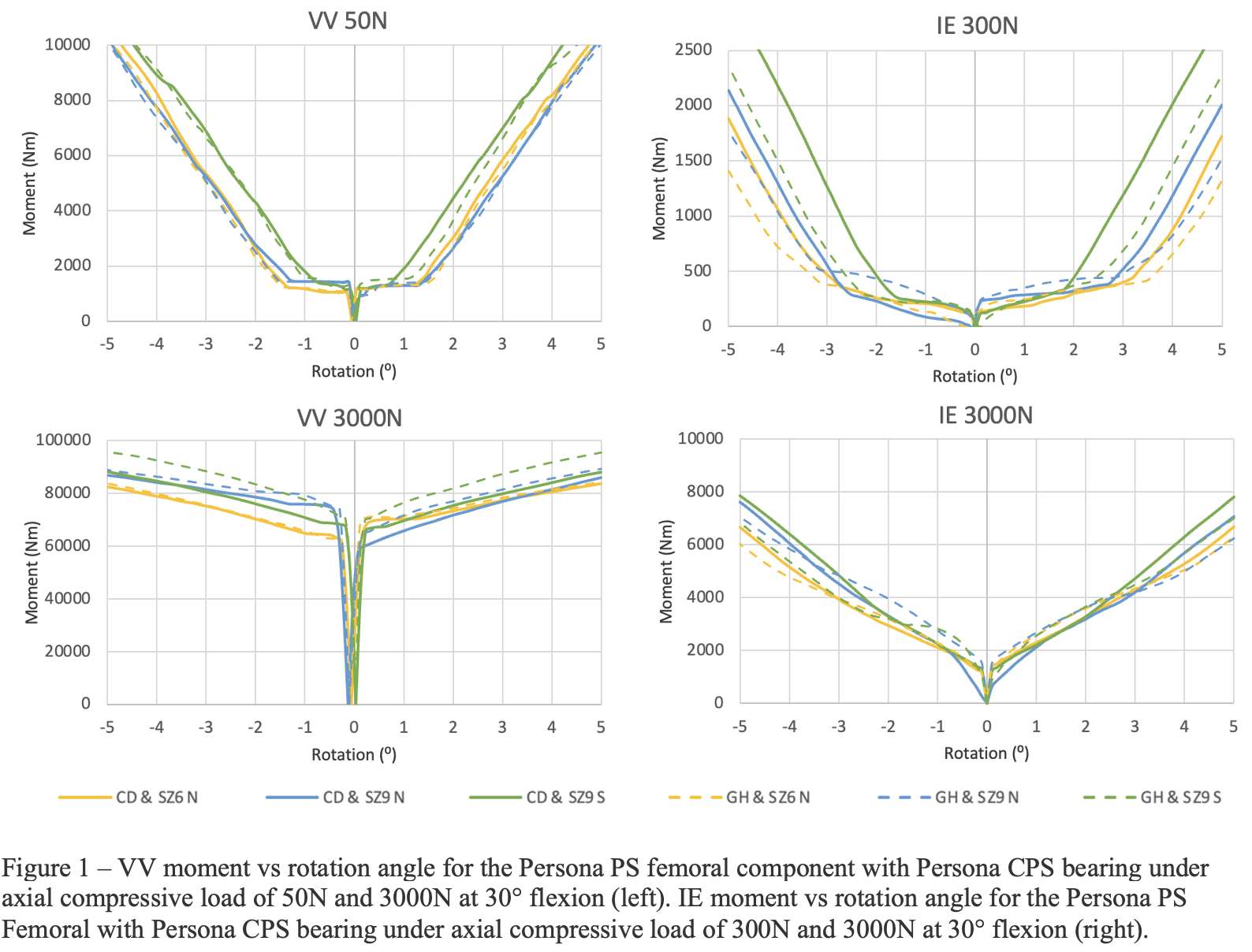
Figure 1
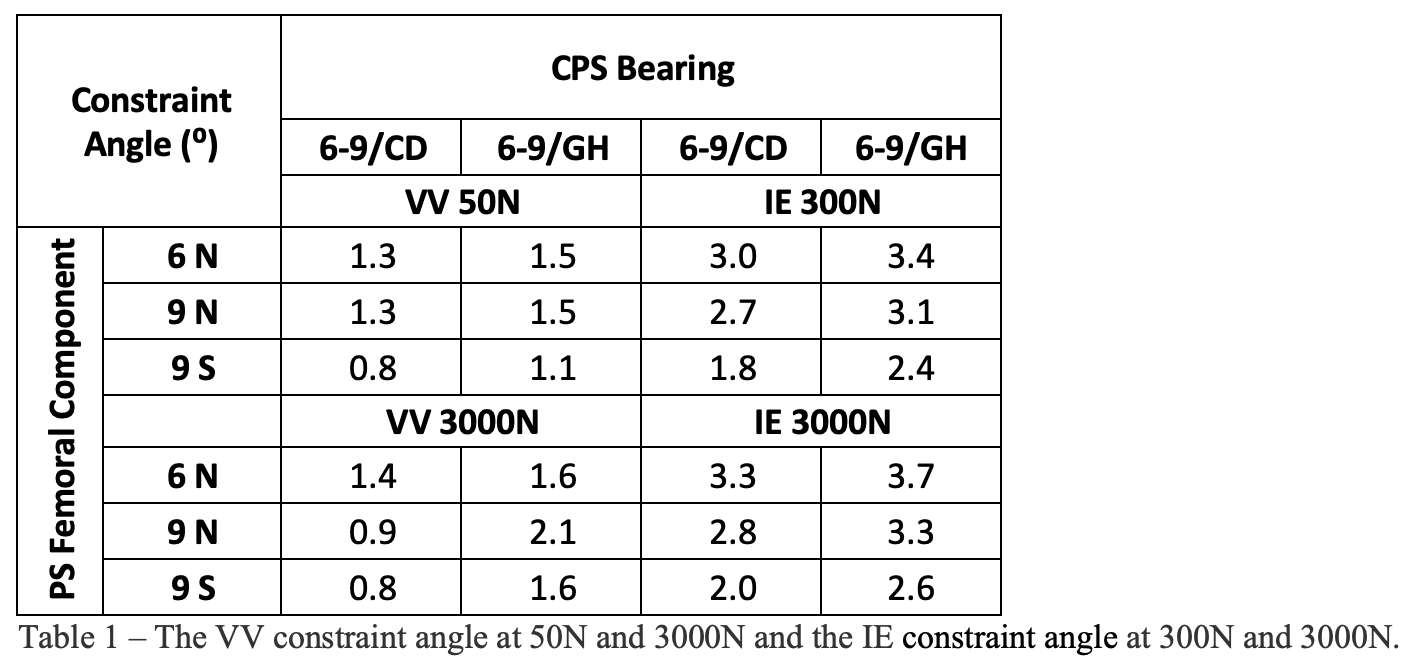
Figure 2#8236
Evaluation of an Anatomically Shaped Lateral Meniscus Prosthesis Using Finite Element Analysis
*Thom Bitter - Raboud University Nijmegen Medical Centre - Nijmegen, Netherlands
Branco van Minnen - RadboudUMC - Nijmegen, Netherlands
Albert van der Veen - Atro Medical B.V. - Nijmegen, Netherlands
Tony van Tienen - Atro Medical BV - Nijmegen, Netherlands
Dennis Janssen - Radboud University Nijmegen Medical Centre - Nijmegen, Netherlands
*Email: Thom.Bitter@radboudumc.nl
Introduction
To relieve pain and improve knee function, an anatomical medial meniscus prosthesis has been developed [1]. Due to a growing need for a solution in the lateral compartment, there is interest in the development of a lateral meniscus prosthesis. In the pre-clinical stage, evaluation using the finite element method (FE) can provide valuable insights into the function and structural integrity of the prosthesis. In this study an anatomical lateral meniscus prosthesis was therefore evaluated using FE simulations to investigate the strength and load sharing capabilities of the implant.
Methods
An FE model was created of a knee in which the bones were rigid, and the cartilage, native meniscus and prosthesis materials were modeled using hyperelastic material models. The prosthesis consists of Bionate® II 80A polycarbonate urethane (PCU) body, with Bionate® 75D PCU horns (DSM Biomedical, Berkeley, CA, USA). The material properties of these materials were determined using uniaxial tensile tests in a water bath at 37°C. The ACL, PCL, LCL, and MCL were simplified using pretensioned springs. The meniscus 75D was fixated to the tibial plateau using tapes through the horns, which in the FE model was mimicked using linear pretensioned springs to allow for small movements and rotations of the horns. After initiating contact between the different structures, a 1.000 N axial load was applied to the femur. When the full load was reached, a flexion rotation was applied to the femur. The outcome measures were stresses, strains and contact pressures of both the prosthesis and the cartilage.
Results
Under the 1.000 N axial load the prosthesis showed strains of up to 43% (Figure 2). The contact pressures were distributed over the prosthesis and cartilage, and there was load sharing between the medial and lateral side. The cartilage contact pressures had a maximum value of 11 MPa. After 25 degrees of flexion the strains and contact pressures increased to 57 % and 16 MPa respectively.
Discussion
The strains in the prosthesis during the flexion movement reached relatively high values. However, uniaxial tensile tests with the prosthesis material showed the material is capable of withstanding strains of over 100% without damage or plastic deformation. The 57% is well below this level, which is promising for the survival of the prosthesis. Load sharing between prosthesis and cartilage and also the medial and lateral compartment was present, which suggests the implant can reduce the contact pressures on the cartilage, and thereby relieve pain and possibly reduce the progression of osteoarthritis.
Further simulations and experiments are required for validation of the simulations and for a full evaluation of the prosthesis.
References:
[1] van Minnen BS, van der Veen AJ, van de Groes SAW, Verdonschot NJJ, van Tienen TG (2022) An anatomically shaped medial meniscus prosthesis is able to partially restore the contact mechanics of the meniscectomized knee joint. J Exp Orthop 9:91
Figures

Figure 1
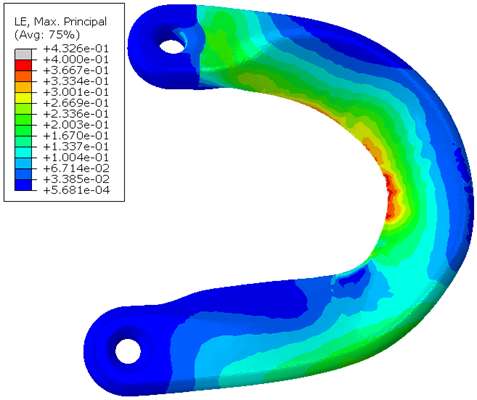
Figure 2
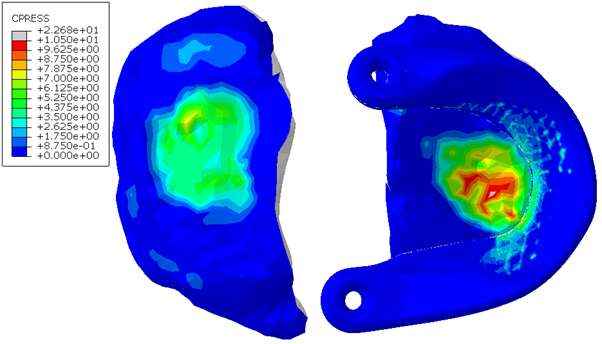
Figure 3#8130
Femoral and Tibial Trabecular Bone Shows Up to 82% Stress Relaxation Which Could Affect the Simulated Initial Stability of Cementless Implants
*Thomas Gersie - RadboudUMC - Nijmegen, Netherlands
Thom Bitter - Raboud University Nijmegen Medical Centre - Nijmegen, Netherlands
David Wolfson - DePuy International Ltd - Leeds, United Kingdom
Robert Freeman - DePuy International Ltd. - Leeds, United Kingdom
Nico Verdonschot - Radboudumc - Nijmegen, Netherlands
Dennis Janssen - Radboud University Nijmegen Medical Centre - Nijmegen, Netherlands
*Email: thomas.gersie@radboudumc.nl
Introduction
Computational models of orthopedic interventions, such as total joint reconstructions, depend on bone biomechanics. Accurately determining bone material properties is therefore crucial for the reliability of these models. While trabecular bone is often modelled as a linear elastic material, the mechanical response of trabecular bone is, in fact, time-dependent. Although this viscoelastic behavior is often ignored for simplicity, it could have a significant effect on biomechanics, especially in analyzing the primary stability of press-fit implants.
In a prior study, the optimal testing time for a stress relaxation experiment and the stress relaxation response of bovine trabecular bone up to 24 hours was quantified. In this study, the stress relaxation response of the human tibial and femoral trabecular bone is quantified and explained how this behavior could be incorporated into computational models to simulate realistic press-fit conditions of cementless implants.
Methods
31 Femoral and 33 tibial trabecular bone cylinders were harvested from 6 donor cadavers (5 female, age range: 53 – 90). The cylinders were compressed for 30 minutes on four consecutive days with an increasing static strain from 0.2 to 0.8%, with 0.2% increments. After each experiment the sample was allowed to recover for 24 hours. A water basin filled with physiological saline at 37°C was used to keep the specimens hydrated during the mechanical testing. All experimental data was extrapolated to 24 hours and then a Modified superposition model was fit.
Results
Stress relaxation was similar for the human tibia and femur, while it was significantly higher for all strain levels than in bovine bone, which was investigated in our previous study (Figure 1). After 24 hours, stress relaxation ranging from 39% to 82% was observed (Figure 2). A mean stress relaxation of 54% was observed. No correlation was found between the stress relaxation rate and the applied strain. Therefore, the viscoelastic behavior of the trabecular bone can be described using three levels of stress relaxation (min, mean, max) observed in this dataset, which is visualized in Figure 2.
Conclusion
The significant level of stress relaxation in human trabecular bone impacts the primary fixation of press-fit implants and the magnitude of micromotions that occur at the interface between the implant and bone. Previous research has shown that the mechanical properties of bone can significantly affect micromotions calculated in computational models as well. Therefore, including viscoelastic bone material response in these models may lead to more realistic predictions and simulations of primary fixation for press-fit implants. Unfortunately, no material model could be developed to simulate the stress relaxation in relation to the initial strain level and the BMD. However, the minimal, mean and maximal levels of stress relaxation observed in this study could be included in simulations of total joint reconstructions. This inclusion could provide insights into the potential effect of bone viscoelasticity on the initial stability of cementless implants.
Acknowledgements
This collaboration project is co-funded by the PPP allowance made available by Health~Holland, Top Sector Life Sciences & Health, to stimulate public-private partnerships, and DePuy Synthes (Leeds, UK).
Figures
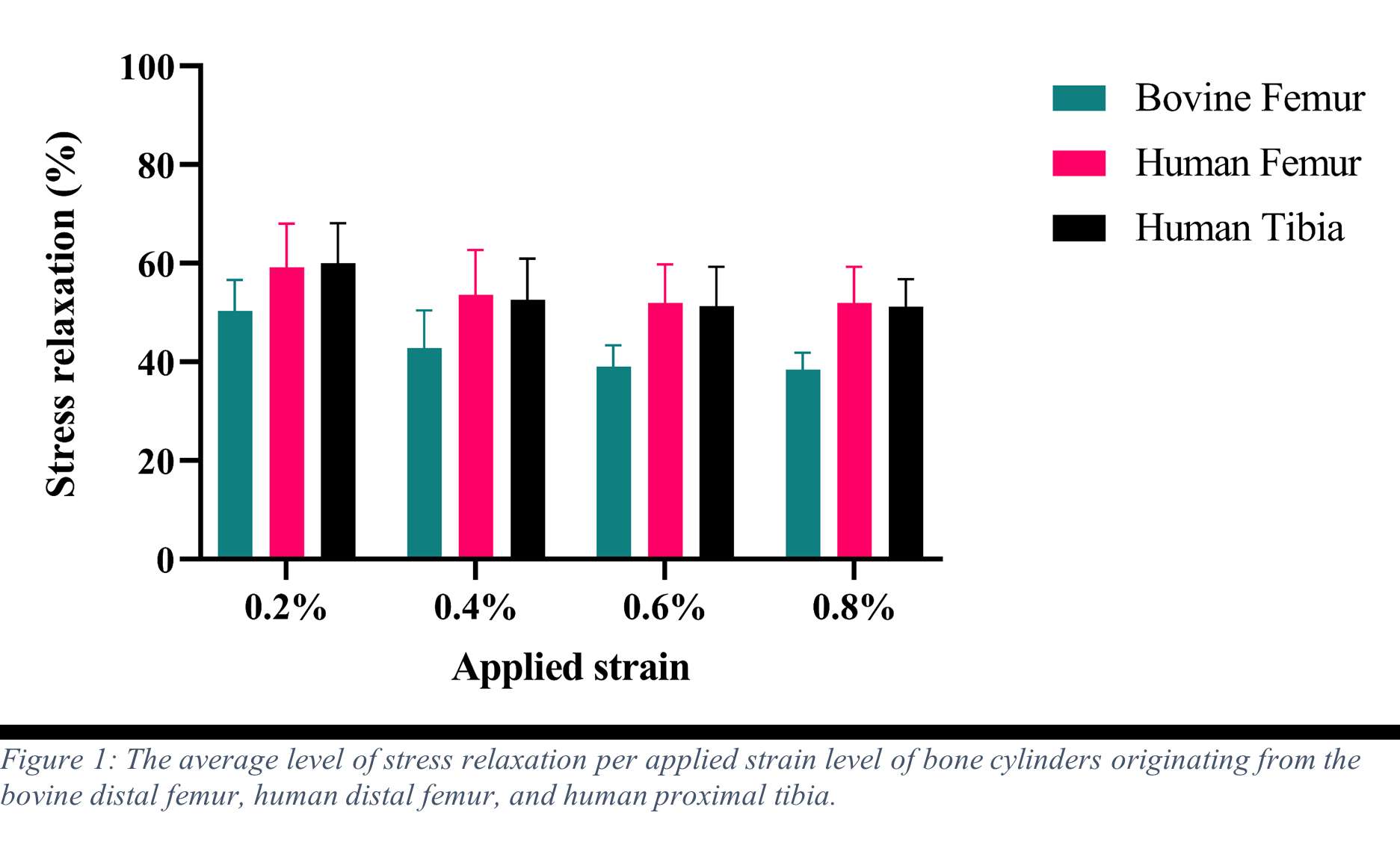
Figure 1
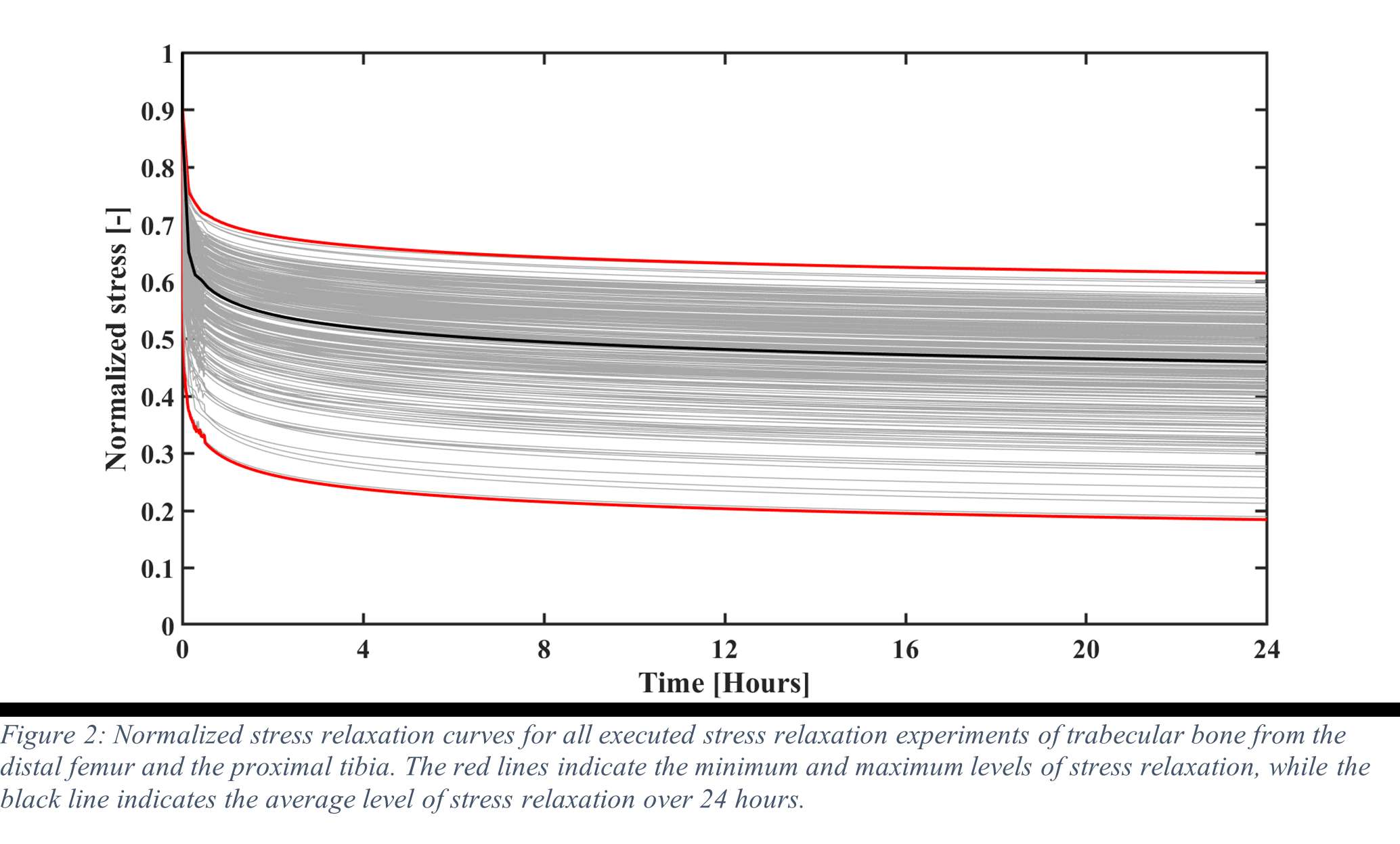
Figure 2#8785
Effect of Foot Position on Total Knee Arthroplasty in the Golfing Patient: A Video Fluoroscopic and Advanced Modelling Study
*Nils Horn
Renate List - ETH Zurich - Zurich, Switzerland
Pascal Schuetz - ETH Zurich - Zurich, Switzerland
William Taylor - Swiss Federal Institute of Technology Zurich - Zürich, Switzerland
Stefan Preiss - Schulthess Klinik - Zurich, Switzerland
Hamed Hosseini - ETH - Zurich, Switzerland
*Email: horn.nils@gmail.com
Introduction:
The health benefits of golf are well established and it remains a popular sport in the elderly population. The return to play after Total Knee Arthroplasty (TKA) is recommended. However some patients do complain about pain after play. The longevity of the implant in a rotational sport can be a concern. The effect of foot position on implant loads are still unknown.
Methods:
The 3D kinematics of five experienced golfers with a fixed-bearing TKA were studied with standard motion capture, force plates and video fluoroscopy. Each subject performed 5 golf swings with the lead foot in 0 degree, self-selected and 30 degree externally rotated pose. A previously validated OpenSim model was scaled to each subjects anthropometry based on the skin-marker locations. Translations, rotations and knee contact forces of the tibiofemoral and patellofemoral joint were estimated throughout the complete cycle of each golf swing.
Results:
The agreement between the model predictions and the fluoroscopic kinematics confirmed the modeling framework. The results indicated the peak tibiofemoral contact forces ranging from 1.9 to 3.9 body weight (BW) with no significant differences between 0 und 30 degree for the medial compartment but significant differences for the lateral compartment during the end range motions of the golf swing. The peak patellofemoral contact force for 0 degree (2.1 BW) was larger than that of 30 degree (1.7 BW).
Conclusion:
The clear variation of forces across the medial and lateral tibiofemoral compartment and the patellofemoral joint indicates that loading of a total knee replacement of the leading leg in a golfing patient can be modulated by adjusting the stance and technique. Therefore subjects can consider biomechanical optimisation of their golfing approach to potentially reduce the risk of injury and improve the longevity of their implant. Further research continues to optimize the sports specific demands of the growing patient population with joint replacements.
#8521
Stability of Glenoid Baseplate in Reverse Shoulder Arthroplasty: Use of a Finite Element Model to Predict Micromotion Measured Using an in Vitro Motion Capture Approach
*Thomas Ferro - Limacorporate - Villanova di San Daniele del Friuli, Italy
Andrea Fattori - Lima Corporate - Villanova Di San Daniele del Friuli, Italy
Michele Pressacco - LimaCorporate Spa - Villanova di San Daniele del Friuli, Italy
*Email: thomas.ferro@limacorporate.com
Introduction. Since one of the most common complications in Reverse Shoulder Arthroplasty (RSA) is glenoid baseplate loosening, it is fundamental to promote appropriate osseous ingrowth between the baseplate and the bone to achieve primary stability and long-term fixation. Several studies recommend 150µm maximum micromotion threshold at bone/implant interface to promote bone integration. Glenoid baseplate stability tests aimed to assess implant performances are standardized according to ASTM F2028 norm and require measuring in vitro micromotion of the component under simulated physiologic loads. The method poses challenges in the stability assessment because of difficulties in measuring micromotions at bone/implant interface in hidden areas or in identifying the highest relative motion areas; additionally, it limits the possibility to test several different configurations when changing implant design parameters (e.g. baseplate sizes, screw number, etc.) or to assess worst case scenarios. Finite Element Analysis (FEA) represents a viable method to easily assess micromotion and stability through simulated test setups; however, due to the complexity of the physical tests, the correspondent Finite Element (FE) model requires proper parameters setting to be able to provide reliable results. The aim of this study is to compare a FE model results with laboratory results while testing a glenoid component platform according to ASTM F2028, with micromotion recorded by means of motion capture technology.
Methods. A glenoid component platform that includes modular and monolithic baseplate and that allows multiple configurations in terms of baseplate geometries, peg length and screws placement was analyzed through FEA to study all the allowed combinations to find the worst-case micromotion configuration while applying the pre-cycling setup described by ASTM F2028 for RSA (Fig.1). In vitro tests were physically performed on two identified worst-case configurations obtained in the previous FEA: six samples for each configuration were implanted following the surgical technique and were tested according to the norm. Test output was the pre-cycling relative displacement optically measured with a motion capture system (GOM, GmbH, Braunschweig, Germany) on two couples of visible points (E1/B1, E2/B2) to be directly compared with the results obtained in the same position through the FE models (Fig.1). FEA and laboratory results were than expressed in terms of percentage of the highest micromotion obtained during the tests for the comparison.
Results. FEA results are in good agreement with the laboratory results and allowed to predict trends (Fig.2, Fig.3) for all the relative displacement components measured (shear, axial and transversal) and for the total value as well. From a quantitative point of view, the deviation between test results and FEA was between 9÷15%, with always an underestimated total micromotion by the FEA.
Discussion and Conclusions. Even if it is very challenging to predict micromotion because of the little order of magnitude of the measured values, of the errors introduced during the construct preparation and of the test setup preparation, appropriate FEA is able to predict trends and can be used to identify worst case configurations leading to a minimization of physical tests and allowing the estimation of the micromotions even in not visible areas.
Figures
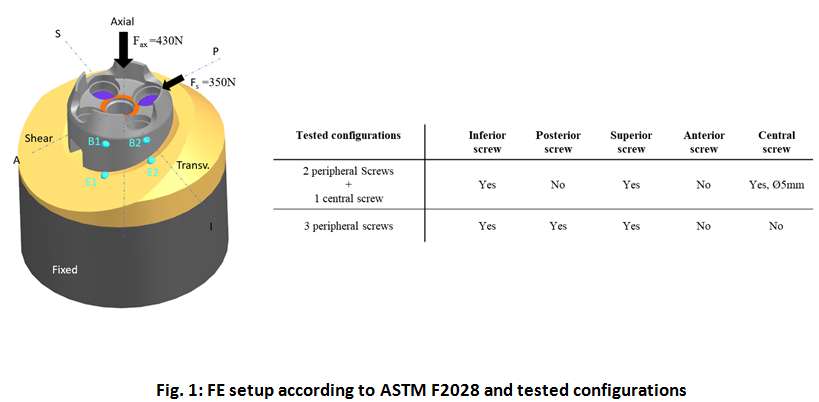
Figure 1
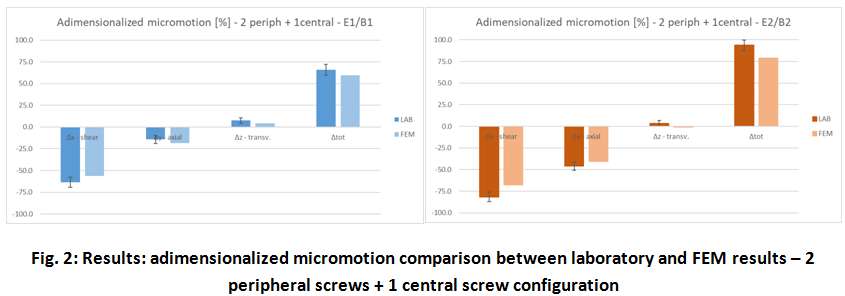
Figure 2
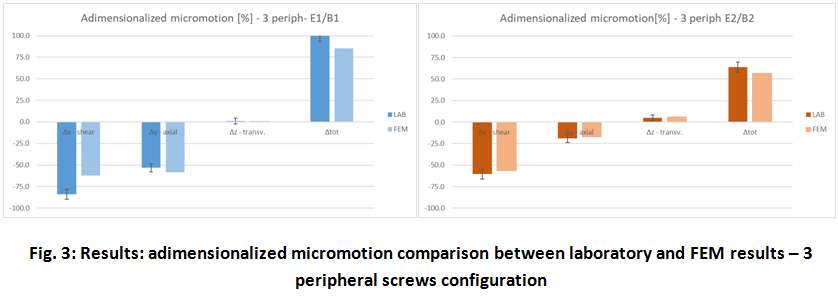
Figure 3#8218
Comparative Study of a Novel Cannulated Screw and Locking Neutralization Plate Construct Versus Tension Band Wiring for Patella Fractures: Experimental and Finite Element Analysis
*Sunjung Kim - University of Illinois Chicago - Chicago, United States of America
Rohan Wangikar - Unniversity of Illinois Chicago - Chicago, USA
*Email: skim6487@uic.edu
Introduction
Patellar fractures can lead to significant pain and disability, and selecting an optimal fixation system for these fractures remains a subject of debate. This study compares the mechanical performance of a novel cannulated screw and locking neutralization plate construct with tension band wiring (TBW) for patellar fractures using experimental testing and finite element analysis (FEA).
Methods
A total of 22 human cadaveric patellas were acquired and transversely fractured. Half of the specimens were repaired using the novel cannulated screw and locking neutralization plate construct, while the other half was fixed using TBW—biomechanical testing apparatus allowed for accurate simulation of flexion and extension motions. Optotrak markers were placed on both sides of the fracture for precise spatial measurements. Cyclic testing consisting of 500 flexion and extension cycles evaluated the stability and durability of the fixation constructs. Fracture gap size was measured by analyzing the difference in distance between the Optotrak markers before and after cycling. Load-to-failure testing was conducted, applying forces directly to the proximal and distal tendons. Finite element analysis (FEA) simulations were also performed to analyze the mechanical behavior of the fixation constructs. FEA models of the fractured patellas with the respective fixation constructs were created. The FEA simulations allowed for assessing stress distribution and deformation patterns within the fixation systems under varying loading conditions. The results from the FEA analysis were used to validate and complement the experimental testing findings.
Results
At the endpoint of 500 cycles, the patellas fixed with the novel cannulated screw and locking neutralization plate construct exhibited an average fracture site gap of 0.09mm (SD=0.12mm), significantly smaller than the average gap of 0.77mm (SD=0.54mm) observed in the TBW group. The load to failure of the novel construct was, on average, 1359N (SD=393.30), significantly higher than the load to failure of 780.1N (SD=233) observed for the TBW group (Fig. 1). Finite element analysis (FEA) simulations supported the experimental findings, showing that the plate fixation system could withstand higher loads than the tension band fixation system. In the plate fixation system, the maximum stress of 1317.1 MPa was located at the patella’s base. In contrast, for the tension band fixation system, the maximum pressure of 347.15 MPa was located at the apex of the lateral side (Fig. 2).
Conclusion
The comparative study demonstrated that the novel cannulated screw and locking neutralization plate construct outperformed tension band wiring regarding fracture site gap and load to failure. The smaller fracture site gap observed with the novel construct suggests improved stability and potential for enhanced healing. Additionally, the higher load to failure of the novel construct indicates its ability to withstand greater forces experienced by the patella. These findings contribute to developing more effective treatment options for patellar fractures, ultimately leading to improved patient outcomes.
Figures
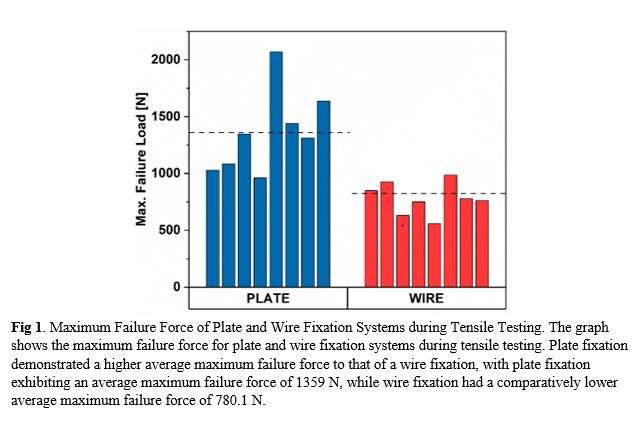
Figure 1
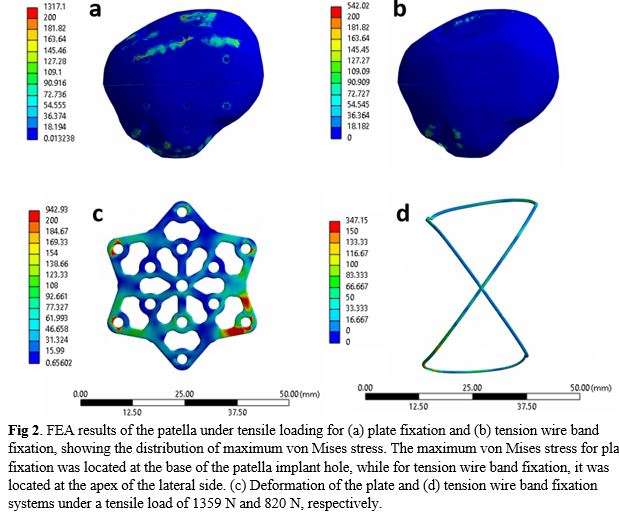
Figure 2#8256
Credibility Assessment of Computational Models for Modular Junction Dissociation
*Mehul Dharia - Zimmer Biomet, Inc. - Warsaw, USA
Maged Awadalla - Zimmer Biomet - Warsaw, USA
Saandeep Mani - Zimmer Biomet - Warsaw, USA
Kimberly Mimnaugh - Zimmer Biomet, Inc - Warsaw, USA
Philippe Favre - Zimmer Biomet - Winterthur, Switzerland
Jeffrey Bischoff - Zimmer, Inc. - Warsaw, USA
*Email: mehul.dharia@zimmerbiomet.com
INTRODUCTION: Understanding and evaluating the strength of modular junctions in orthopaedics is important for implant success. Computational modeling can be used to evaluate novel modular junction designs, provided sufficient credibility of the model can be demonstrated. In this study, a finite element analysis (FEA) model was created for evaluating axial and torsional pull-off strength of a modular junction including head, ring adapter, and lock adapter. Head-ring is a conical taper junction and ring-lock is a conical-spherical taper junction. The dissociation strength predictions were compared to bench-top experiments and model credibility was assessed as per the ASME VV40-2018 framework.
METHODS: For the two experiments (axial pull-off and torsional torque-out), the head-ring was first assembled followed by the ring-lock, both under 2000N static load. The axial pull-off force (axial strength) and torsional torque-out moment (torsional strength) to dissociate lock or ring connections were measured as well as the mode of dissociation (ring-from-head or lock-from-ring), and angle at torque-out. FEA simulated both experimental tests (Fig.1) and coupled and uncoupled uncertainty quantification based on several model form and model input sensitivity analyses (geometrical tolerances, ring tilt during assembly, friction coefficient effects, etc.) were performed. Predicted values for the three outputs, as well as the dissociation mode, were compared to experimental measurements.
RESULTS: Experiments measured axial pull-off (n=9), torsional torque-out (n=5), and the angle at torque-out dissociation as shown in Fig. 2. While dissociation occurred at the ring-lock interface in both tests, the axial test also found dissociation at head-ring interface in select samples without significantly impacting pull-off strength. The predicted pull-off/torque-out and angle at torque-out after accounting for uncertainties significantly overlapped with the benchtop measurements distribution (Fig.2), demonstrating agreement between the models and the benchtop tests. The predicted dissociation mode, and its non-influence on peak pull-off/torque-out strength, also matched with that observed in the experimental measurements.
DISCUSSION: While the above mentioned qualitative and quantitative comparisons of multiple outputs between models and its comparators (tests) demonstrated a high level of agreement, it only represents one component (validation assessment) of the overall model credibility process. Several other aspects were carefully planned and examined. Verification model: A validated FEA software was used, detailed mesh convergence and model peer reviews were performed. Validation model: A wide variety of uncoupled and coupled sensitivity analyses were performed and propagated to final predictions. Validation comparator: Statistically significant number of samples and multiple conditions were tested. The overall credibility of the models simulating axial pull-off and torsional torque-out was determined as high. Applicability: The credibility established here should be justified for using this modeling approach in evaluating future designs. To the authors’ knowledge, this is the first study to demonstrate high credibility for a modular junction dissociation model per ASME VV40-2018 framework.
CONCLUSIONS: A highly credible FEA modeling approach as per ASME VV40-2018 can virtually evaluate dissociation risk of complex modular systems, increasing trust for evaluating the strength of novel modular systems.
Figures
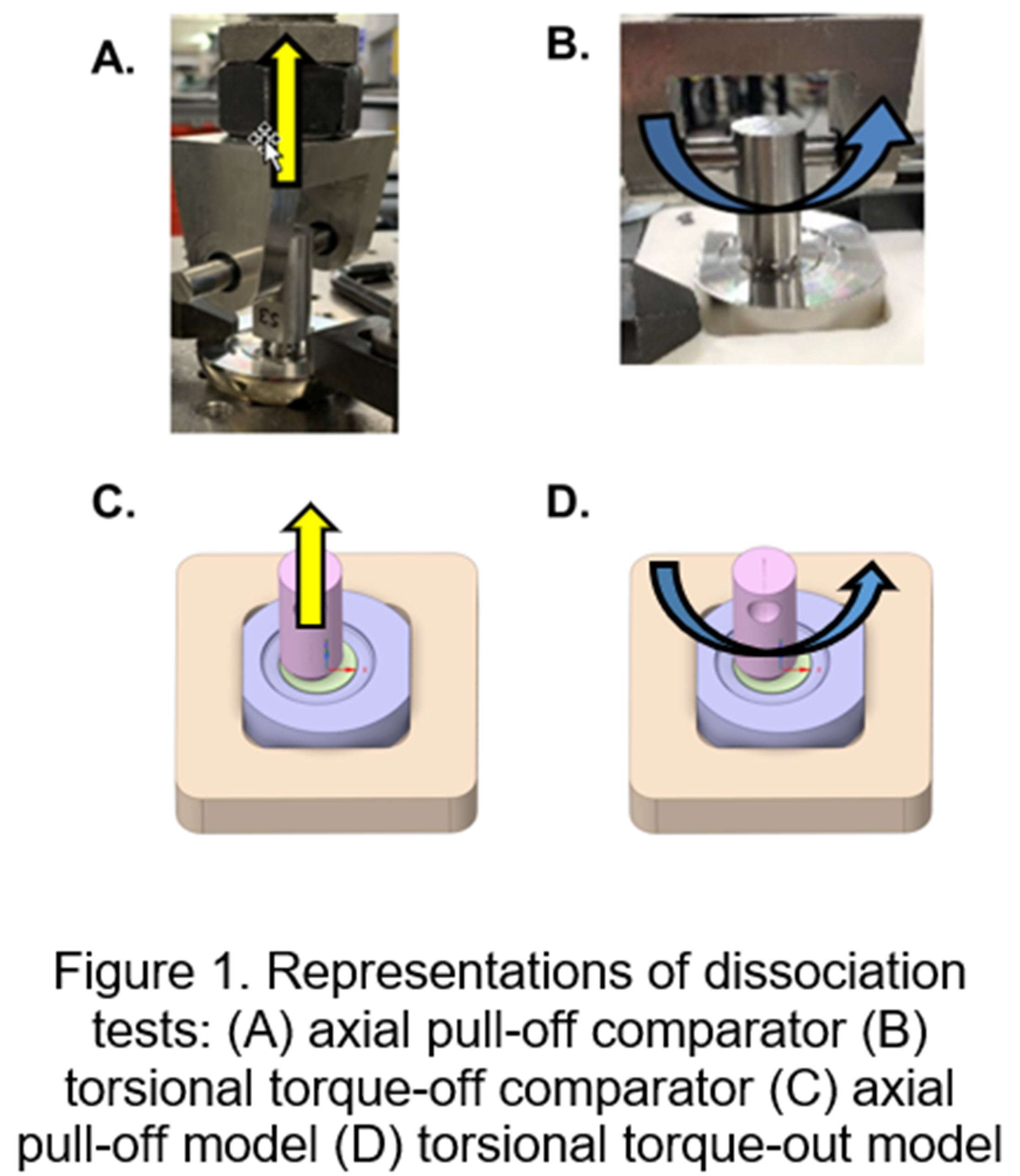
Figure 1
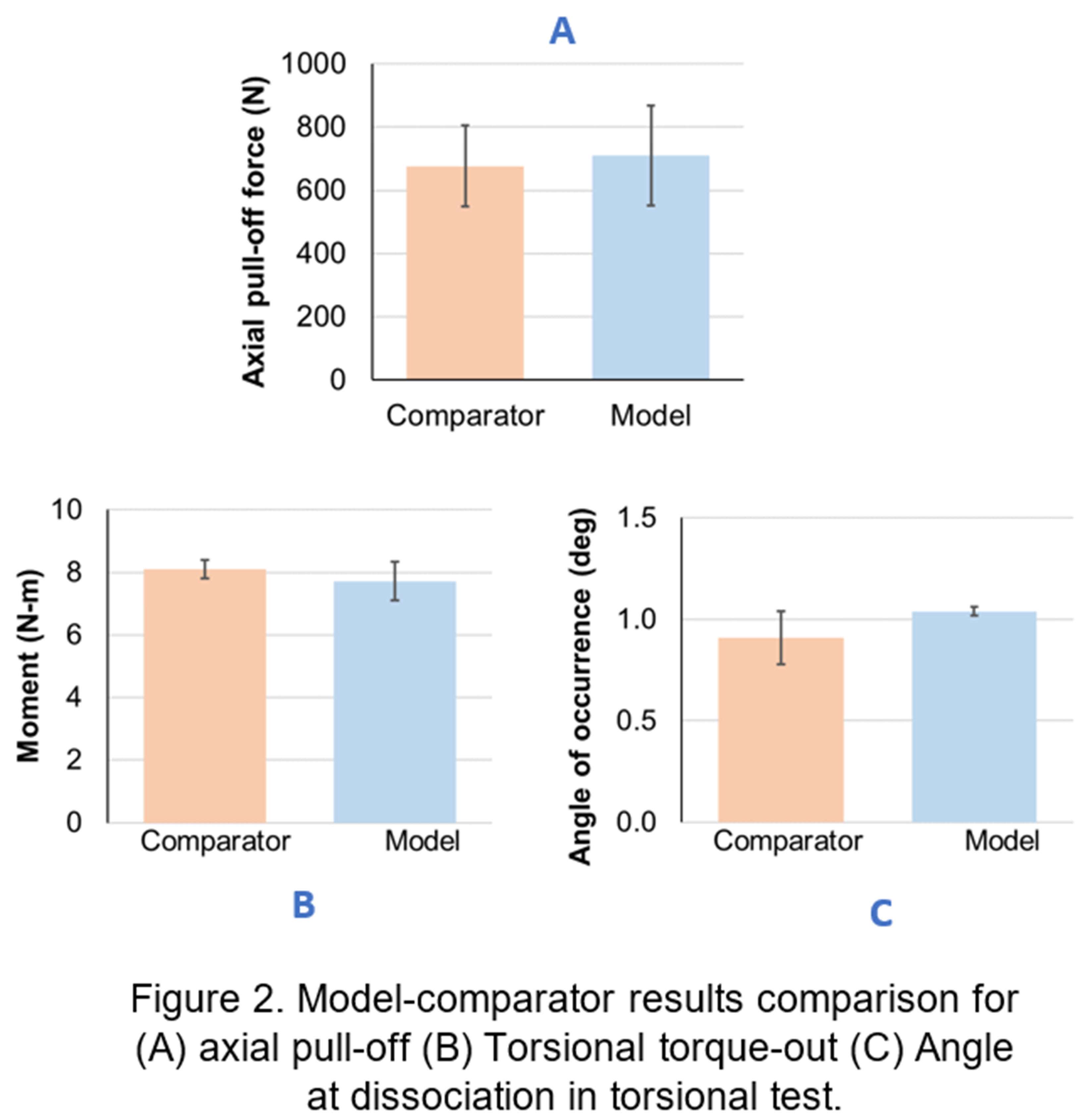
Figure 2#8435
Locking Strength of Variable Angle Adapter: Is It Comparable to Morse Taper?
*Maged Awadalla - Zimmer Biomet - Warsaw, USA
Mehul Dharia - Zimmer Biomet, Inc. - Warsaw, USA
Saandeep Mani - Zimmer Biomet - Warsaw, USA
Kimberly Mimnaugh - Zimmer Biomet, Inc - Warsaw, USA
Philippe Favre - Zimmer Biomet - Winterthur, Switzerland
Jeffrey Bischoff - Zimmer, Inc. - Warsaw, USA
*Email: maged.awadalla@zimmerbiomet.com
INTRODUCTION: Most of current total joint replacements offer a fixed head-stem connection using a morse taper (fixed angle adapter). However, a variable angle prosthesis allows for a range of inclination and version to recreate the anatomy, helping restore joint flexibility and natural kinematics. The head-adapter connection consists of a conical taper junction and adapter-stem connection is a conical-spherical taper junction (Figure 1). The aim of this study was to evaluate the locking strength of the variable angle adapter compared to a traditional fixed angle adapter using experimental methods. Computational analysis was applied to further investigate contact behavior. This study hypothesized that a variable angle adapter can achieve equivalent locking strength to that of a fixed angle adapter.
METHODS: Nine variable and six fixed angle adapters were assembled using a 2000N load using MTS uniaxial testing frame. Following assembly, the head was fixed, and the variable and fixed angle adapter was pulled until dissociation occurred, and maximum pull-off force was recorded. A finite element analysis (FEA) was used to reproduce the experimental test set-up. All contacts in the FEA simulation were frictional. Using a converged mesh, the FEA simulation started with assembly of the modular connections (2000N), followed by relaxation step, and then pull off. The maximum force along the taper axis during the pull-off step was recorded and compared to the experimental outcomes. Coupled and uncoupled uncertainty quantification based on several model form and model input sensitivity analyses (geometrical tolerances, ring tilt during assembly, friction coefficient effects, etc.) were performed on the variable angle adapter. After model validation, the influence of least material condition (LMC) and maximum material condition (MMC) on dissociation force were investigated.
RESULTS: Experimentally, the mean pull-off force for the variable angle adapter was 33% greater than fixed angle adapter. During experimental analysis, two dissociation modes were observed for the variable angle adapter (head-adapter, and adapter-stem), compared to one dissociation mode for the fixed angle adapter (head-adapter). The variability in dissociation mode did not statistically influence pull-off force. FEA predictions matched the experimental outcomes showing that the variable angle adapter pull-off force was 36% greater than the fixed angle adapter (Figure 2A). Although, variability in testing fixture could in-part play a role, the FEA model showed that, under the same test setup, the increase in the variable angle adapter pull-off force may be due to an 86% decrease in contact area at the conical-spherical taper junction compared to fixed angle adapter, leading to 83% increase in adapter-stem contact pressure after full assembly (Figure 2B) and 85% increase in frictional stress during pull-off. Furthermore, the FEA model showed that adapter-stem contact orientation and pressure, LMC-MMC condition, and shear force are in-part the cause for the variation in dissociation mode found in variable angle adapter.
CONCLUSION: Variable angle adapters offer the possibility to achieve the connection strength of traditional morse tapers, while better accommodating surgical and/or patient variability.
Figures
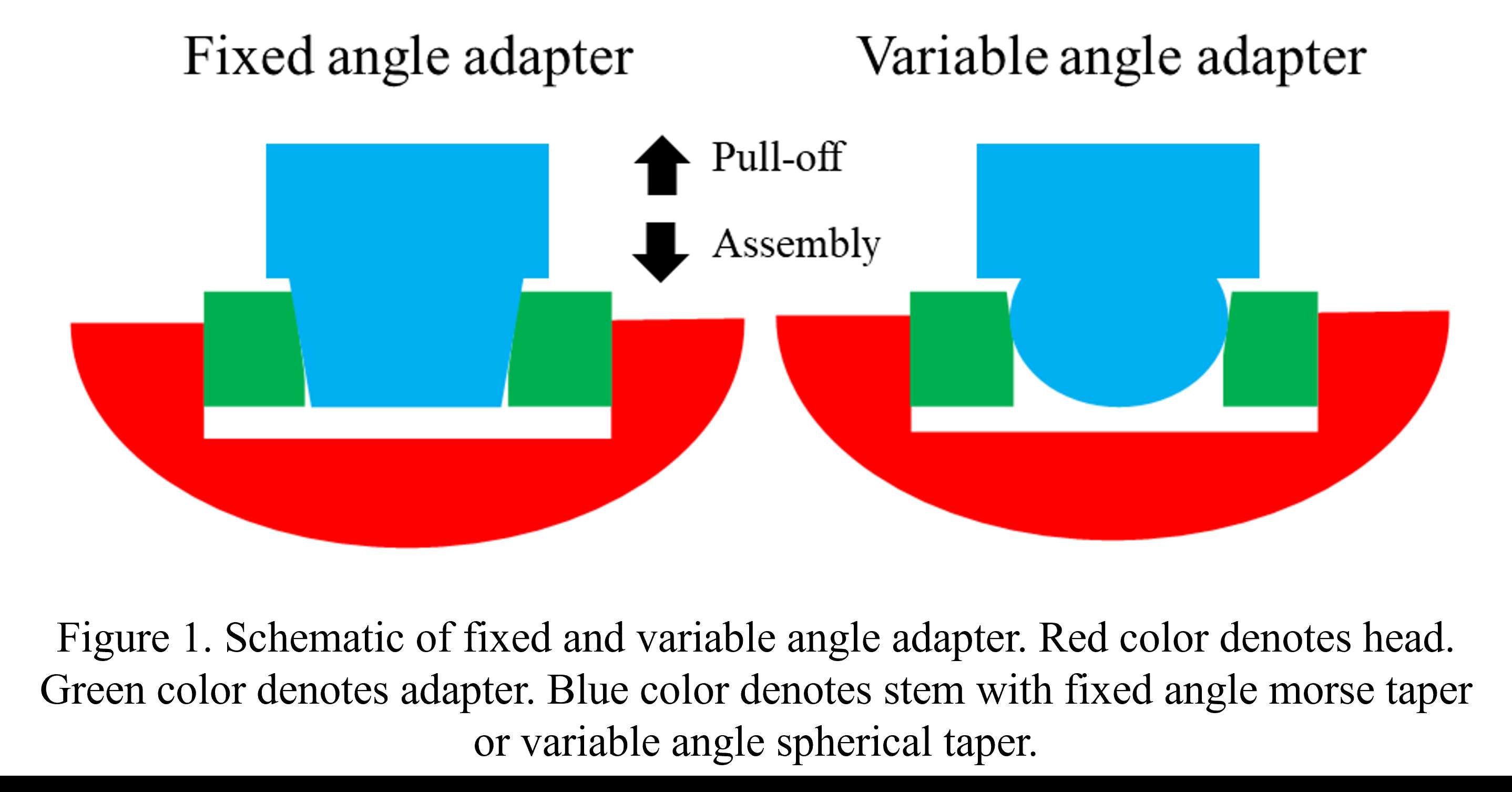
Figure 1
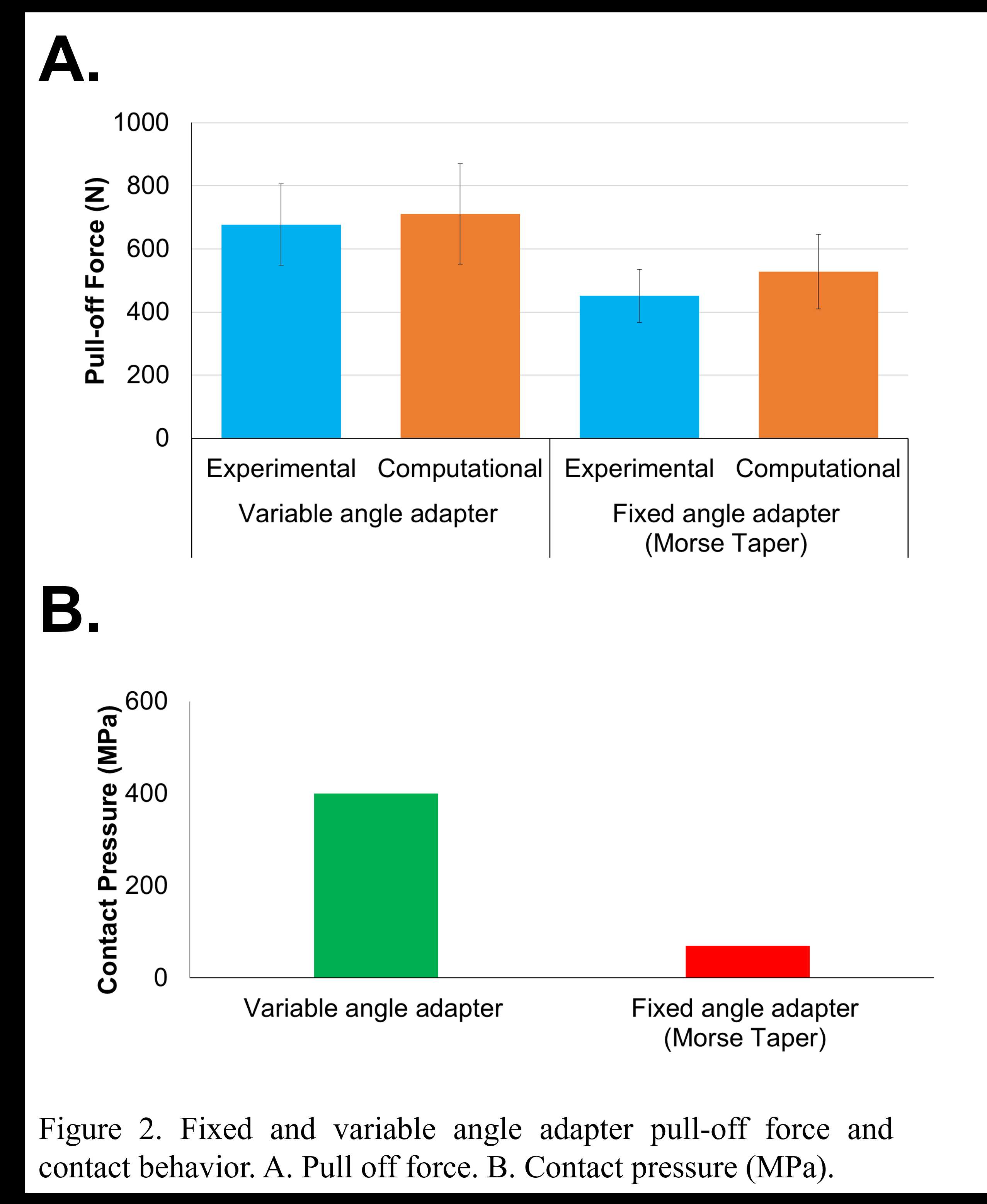
Figure 2#8693
Effect of Mobile and Fixed-Bearing Knee Arthroplasty Design on Kinematics in Golf
*Nils Horn
Renate List - ETH Zurich - Zurich, Switzerland
Pascal Schuetz - ETH Zurich - Zurich, Switzerland
Stefan Preiss - Schulthess Klinik - Zurich, Switzerland
Tomas Drobny
*Email: horn.nils@gmail.com
Introduction. The health benefits of Golf and physical activity are well established. The return to play after TKA is recommended. In a more active and well informed golf playing patient population the demand to get back to play a rotational sport is often discussed. The effect of implant design (mobile-bearing (MB) versus fixed-bearing (FB)) on the kinematics throughout a full golf swing are still unknown. This is the first study that provides recommendations regarding the choice of TKA implant and foot position for golf playing patients.
Methods. The 3D kinematics of a MB (n=5) and a FB (n=6) TKA in the leading leg were quantified and compared during the golf swing with three different foot positions by videofluoroscopy in a single centered observational study. The 3D tibiofemoral kinematics were analyzed by single plane videofluoroscopy and subsequent 2D/ 3D registration. The rotational capabilities of the MB and FB TKA were additionally assessed with a passive rotation device.
Results. The data showed a significant difference between the two TKA designs in terms of rotational capabilities and movement pattern. The MB TKA design allowed a higher maximal internal tibial rotation at address and end of follow through. In the FB TKA design, a more restricted movement pattern was observed. With a more externally rotated lead foot, a larger ROM resulted from a greater external tibial rotation in the backswing and distributed the ROM more evenly throughout the swing. During the passive tests, the MB TKA design allowed a wider rotational motion compared to the FB design.
Conclusions. The present study shows the more anatomical and larger tibiofemoral rotation of the MB design compared to the FB and gives great insight into the kinematics of the knee joint during a golf swing for patients with TKA. An external rotated lead foot is therefore recommended to provide the most rotational capabilities to the avid golfer.
#8469
Knee Pivot Motion During Weight-Bearing Activity in a Large Cohort of Healthy Asymptomatic Individuals
Alix Cagnin - Emovi Inc - Montreal, Canada
*Rémi Courteille - École de Technologies Supérieure - Montreal, Canada
Nicola Hagemeister - Ecole de technologie superieure - Montreal, Canada
Laurence CHEZE - Université Claude Bernard Lyon 1 - Lyon, France
Alex Fuentes - Emovi - Montreal, Canada
Pascal-Andre Vendittoli - Hopital Maisonneuve Rosemont-Universite de Montreal - montreal, Canada
*Email: remi.courteille.1@ens.etsmtl.ca
Introduction: Understanding the normal function of the knee and its complex mechanisms is crucial to achieve better outcomes in total knee arthroplasty. Although surgeons have access to multiple static parameters of interest to guide their decision-making, more information on the knee’ dynamic behavior is needed. While the knee pivot motion is a parameter of interest in implant designs, there is little consensus on its behavior, especially during weight-bearing activities. This study aimed at presenting a method to characterize the knee pivot motion and the position of the center of rotation (COR) during gait in healthy asymptomatic individuals.
Methods: Seventy-seven (77) healthy individuals (142 knees) participated. Three-dimensional knee kinematics were captured non-invasively in clinic using the KneeKG™ system (Emovi, Canada) during treadmill walking (Fig.1). Knee pivot motion was assessed by projecting transepicondylar axis (TEA) in the transverse plane (i.e., tibial plateau) throughout the gait cycle (GC). The tibial plateau was normalized from -1 (lateral condyle) to +1 (medial condyle) with 0 being the center of the knee and was divided in four zones: the lateral (from -1.4 to -0.20), central (-0.20 to +0.20), medial (+0.20 to +1.4), and extra-articular zones (<-1.4 or >+1.4). The COR location, corresponding to the intersection of two consecutive TEA projections, was determined at each GC percentage and used to characterize the pivot motion pattern. Pattern I corresponded to an antero-posterior translation of the TEA (i.e., no significant rotation) when the COR was located in the extra-articular zone. Patterns II to IV corresponded to a rotation respectively around a lateral COR (II), a medial COR (III) or a central COR (IV, Fig.2). The predominant pivot pattern was determined independently in five sub-phases of the GC: loading (from 0 to 15% of the GC), mid-stance (15-30%), end-stance (30-45%), push-off (45-60%), and swing (60-100%).
Results: There was 52.8% of women and the mean age was 32.8 years (95%CI: 31.2;34.5). Pattern IV (i.e., central pivot) was the most frequent pattern in each sub-phase except during push-off (i.e., lateral pivot; Table 1). Interestingly, the proportion of individuals who presented a central pivot (i.e., predominantly a rotation around a point located within 20% of the center of the tibia) decreased progressively during stance (from 69.0% at loading to 22.5% during push-off). The highest proportion of both lateral and medial pivot patterns occurred during push-off (32.4% and 27.5% respectively) when the knee flexed typically between 8° and 20°.
Conclusion: This study presented a non-invasive method using the KneeKG™ system to quantify knee pivot motion during weight-bearing activity in clinical setting in contrast to fluoroscopic studies. Results suggest that knee pivot motion pattern during gait is variable in healthy individuals, and that it may vary throughout the GC, supporting the relevance of assessing it within sub-phases. While additional studies are needed to refine these preliminary findings, it is a promising avenue to better understand this dynamic parameter of interest. Future work should assess pivot motion in pathologic knees and post-arthroplasty to move towards more personalized surgeries to restore knee function and achieve better outcomes.
Figures
Figure 1
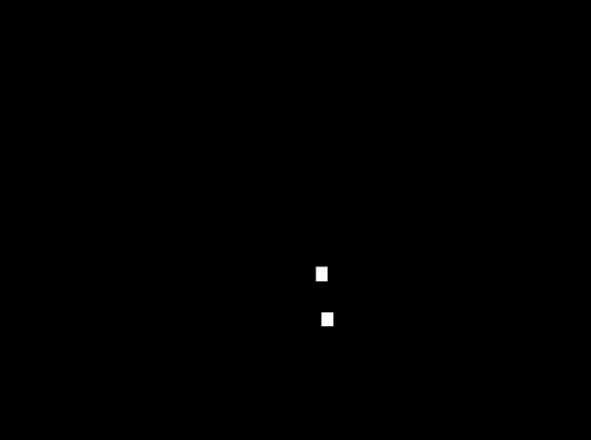
Figure 2
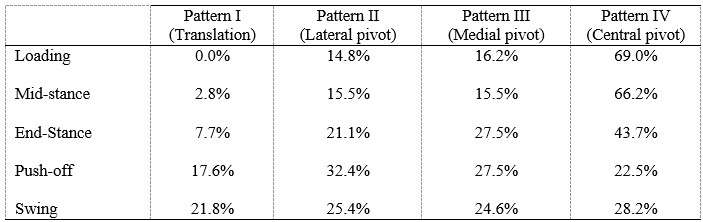
Figure 3#8473
Knee Pivot Motion Pattern During Weight-Bearing Activity May Be Associated With Patient Outcomes After Total Knee Arthroplasty
Alix Cagnin - Emovi Inc - Montreal, Canada
Rémi Courteille - École de Technologies Supérieure - Montreal, Canada
*Alex Fuentes - Emovi - Montreal, Canada
Pierre Ranger - Hopital Jean-Talon - Montreal, Canada
Laurence CHEZE - Université Claude Bernard Lyon 1 - Lyon, France
Nicola Hagemeister - Ecole de technologie superieure - Montreal, Canada
*Email: afuentes@emovi.ca
Introduction: Up to 20% of patients remain unsatisfied after total knee arthroplasty (TKA) and report functional limitations. While this is partly explained by the knee kinematics not being fully restored post-surgery, few studies specifically explored the associations between patient-reported outcome measures (PROMs) and knee pivot motion patterns. Although it is accepted that the knee’ center of rotation (COR) position changes as the knee flexes, this pivot motion behaviour and its relationships to clinical outcomes are not well understood in TKA patients, especially during weight-bearing activity. This study explored if the pivot motion pattern during stance post-TKA is associated with PROMs.
Methods: A retrospective study was conducted on 37 patients (43 knees) who underwent TKA. All knees had patella resurfacing and benefitted from a similar implant (Genesis-II/Legion, posterior-stabilizing, Smith&Nephew, N-Z). Three-dimensional (3D) knee kinematics were captured in clinic using the KneeKGTM system (Emovi, Canada) between 12- and 36-month post-surgery (Fig.1). Knee pivot motion was assessed by projecting the transepicondylar axis (TEA) in the transverse plane (i.e., tibial plateau) throughout stance during treadmill walking. The knee COR was determined by the intersection of two consecutive TEA projections at each percentage of the stance phase and its location was used to classify pivot motion in four patterns (Fig.2). Pattern I corresponded to an antero-posterior translation of the TEA. Patterns II to IV corresponded to a rotation respectively around a lateral COR (II), a medial COR (III) or a central COR (IV). The predominant pivot pattern was determined independently in four sub-phases of stance: loading (from 0 to 25%), mid-stance (25-50%), end-stance (50-75%), and push-off (75-100%). Knees who presented the same pivot motion pattern in all four sub-phases were classified as “single pivot” and the others as “multi-pivot”. Patients completed a Knee Injury and Osteoarthritis Outcome Score (KOOS) assessing the knee condition on symptoms, pain, function during activity of daily living (ADL), function during sport/recreative activities (SPORT), and quality of life (QOL) using scores from 0 (extreme symptoms) to 100 (no symptoms). KOOS scores were compared between groups using Student T-tests.
Results: There was 51.4% of women, the mean age was 66.7 years (95%CI: 63.8;69.5). All KOOS scores, except for symptoms (p=0.21), showed statistically significant differences between groups (all p≤0.05, Table 1). Patients with “single pivot” pattern, corresponding to knees maintaining the same pattern throughout stance, reported significantly and clinically poorer scores related to pain, function during ADL and SPORT, and QOL (ranging from 13 to 27-point difference). Interestingly, all “single pivot” knees exhibited a central pivot motion (i.e., a COR within 33% of the center of the tibia).
Conclusion: Results suggest that knee pivot motion pattern during stance is associated with PROMs post-TKA. Patients exhibiting a “single pivot” pattern reported more pain and less function and QOL. While additional studies are needed to confirm these findings and explore the differences in outcomes post-TKA between different patterns (i.e., lateral/medial pivot motion), this supports the relevance of assessing this dynamic parameter of interest post- but also pre-surgery to help better understand residual mechanical dysfunctions.
Figures
Figure 1
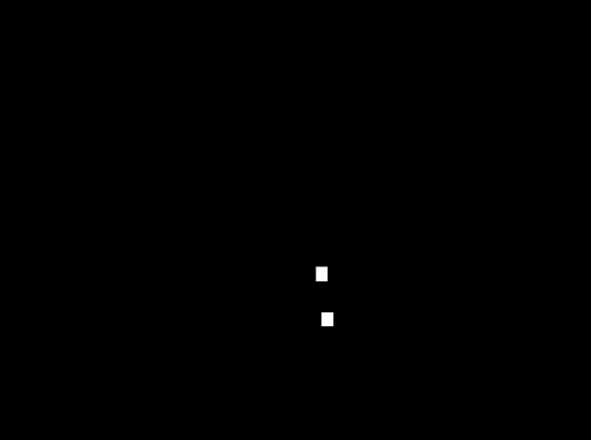
Figure 2
Figure 3#8632
Kinematic Comparison of Cruciate Retaining Total Knee Arthroplasty With Physiological Articular Surface
*Teruya Ishibashi - Osaka University - Suita, Japan
*Email: t.bashi001@gmail.com
Introduction
It remains to be controversial whether or not to retain posterior cruciate ligament (PCL) in total knee arthroplasty (TKA). In healthy knee, articular surface geometry influences its kinematics in balance with ligaments. We hypothesize that a cruciate retaining (CR) TKA with a physiological surface geometry would exhibit normal kinematics with a functioning posterior cruciate ligament (PCL).
Materials and methods
We analyzed 20 knees (20 Patients, 1 male and 19 female) who underwent successful TKA and agreed to participate in the current investigation under institutional review board approval. 10 knees implanted with CR TKA (JOURNEY ΙΙ, Smith & Nephew) as Group J and 10 knees were implanted CR TKA (FINE Total Knee System, Teijin Nakashima Medical) as Group F. Mean age at the time of surgery were 73.8 ± 7.8 and 71.6 ± 5.7 years in Group J and F, respectively. Each patient was asked to perform squatting. To estimate the spatial position and orientation of the components, a 2D/3D registration technique was used. The technique uses computer-assisted design models to reproduce the spatial position of the femoral and tibial components from single view fluoroscopic images. We evaluated the knee flexion angle, femoral external rotation (ER) angle and anteroposterior (AP) translation of the nearest point from femoral component and tibial axial plane for both medial and lateral sides (MAP and LAP). 3D bone models were reconstructed using pre- and post-operative CT. As previous report, the distance between femoral and tibial insertion of PCL was calculated as the length of PCL. Repeated ANOVA with post hoc was used to compare the flexion angle, AP translation, ER angle between group J and F. Values of p < 0.05 were considered statistically significant.
Results
The range of motion were -4.3 ± 8.3°/108.3 ± 16.5° in Group J and -5.6 ± 7.3°/111.5 ± 12.0° in Group F. The femoral condyle exhibited gradually externally rotated in both group F, and the mean amount of ER angle were 12.3 ± 3.5° in Group J and 14.8 ± 3.5° in Group F (Figure 1). The MAP exhibited posterior translation from 0° to 30° in both groups, more anteriorly translated from 40° in Group J compared to Group F (Figure 2). The LAP exhibited posterior translation from 0° to 120° in both groups (Figure 3). The PCL length exhibited similar elongation pattern in both groups (Figure 4).
Discussion
Two types of CR TKA with a physiological surface geometry exhibited similar rotational behavior, but their AP positions were quite different. The cause was not likely to result from the PCL length.
Figures
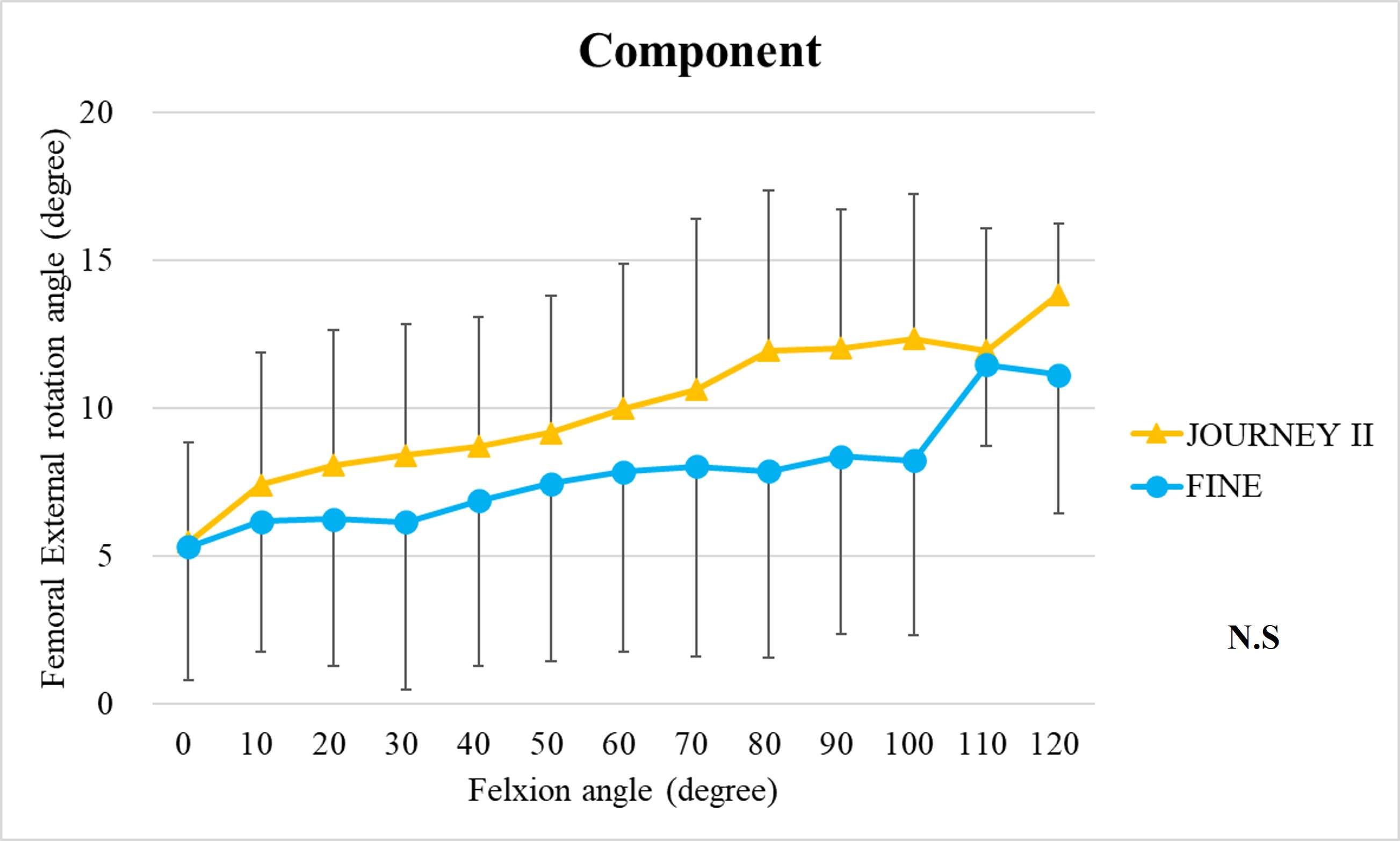
Figure 1
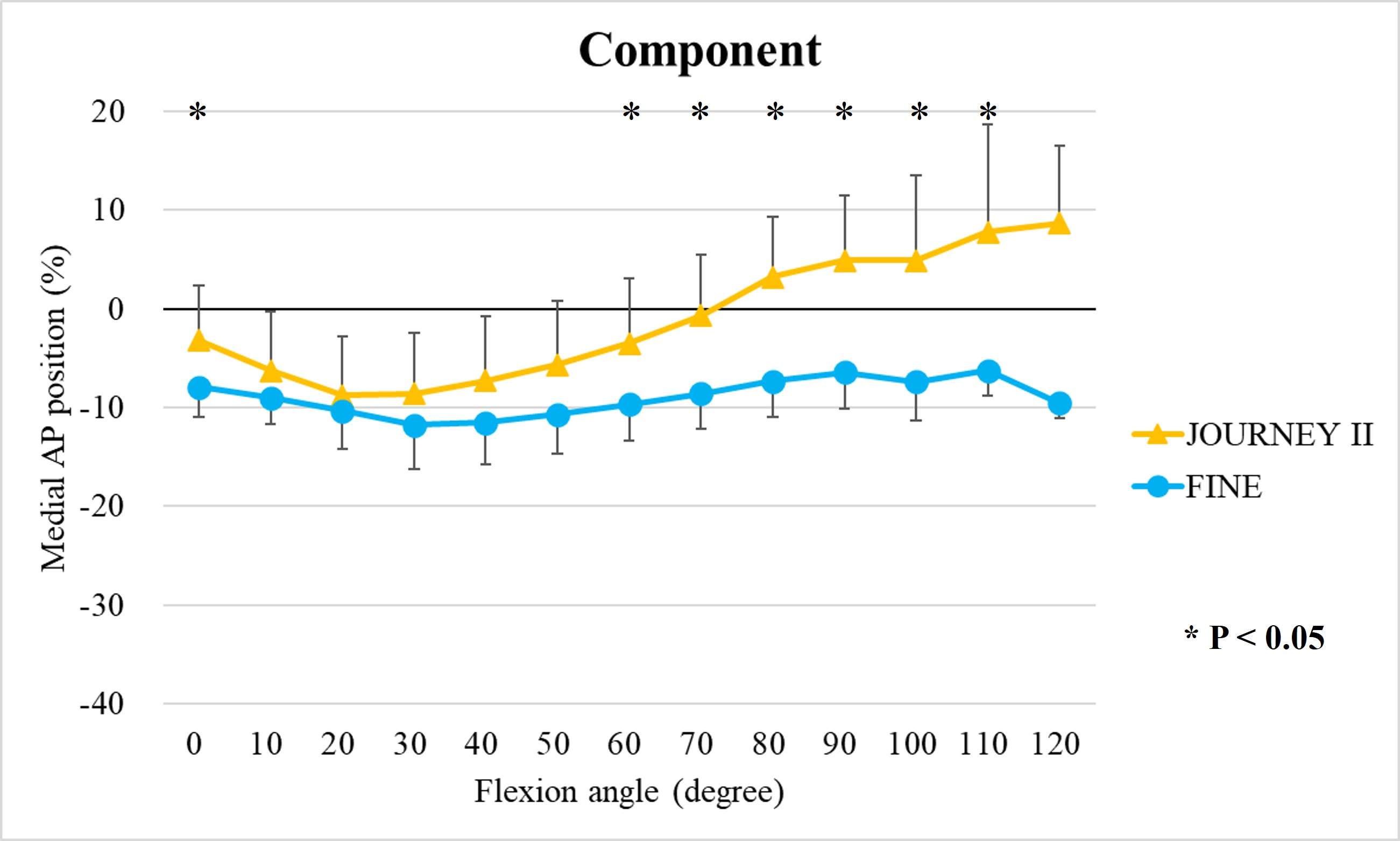
Figure 2
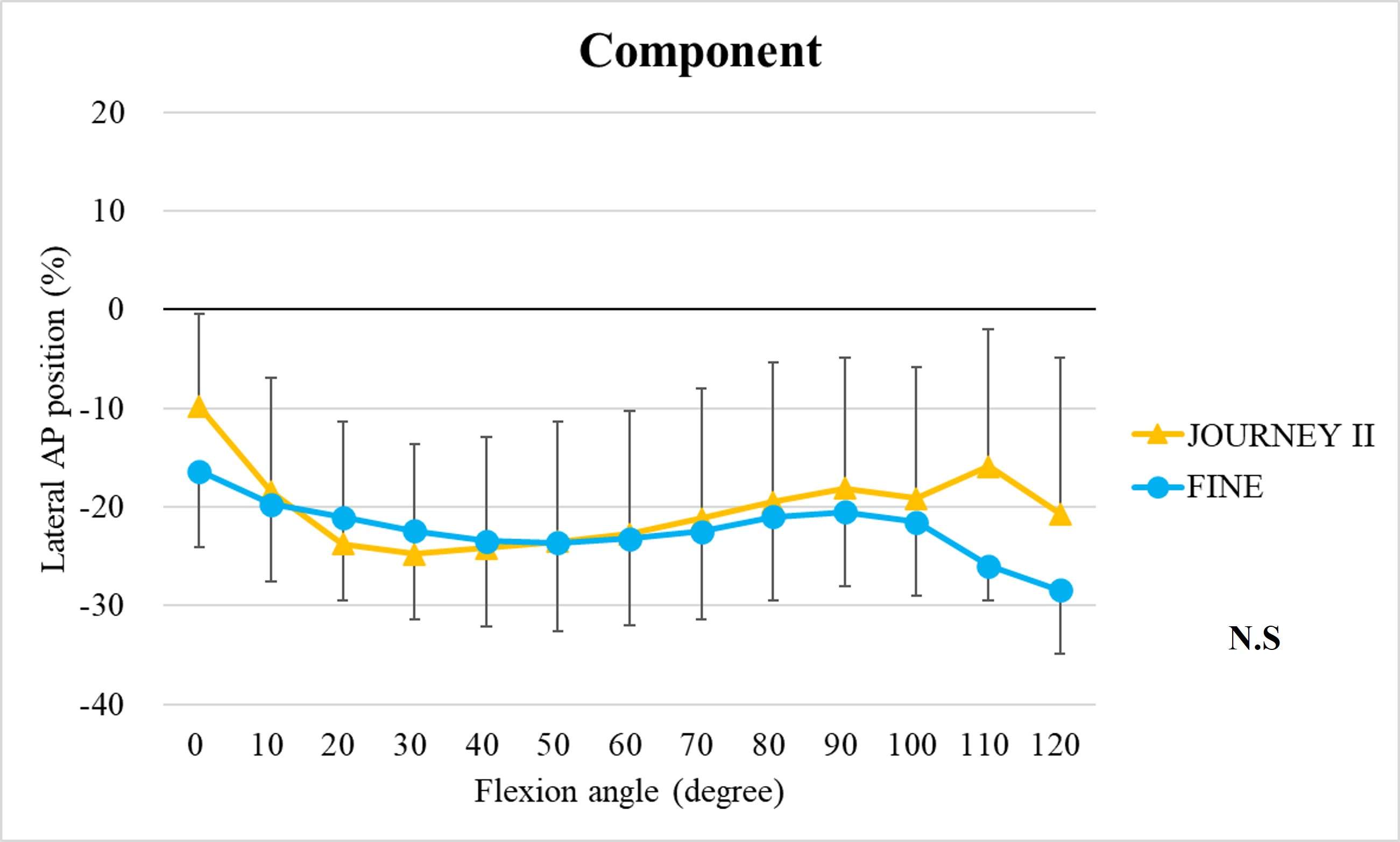
Figure 3
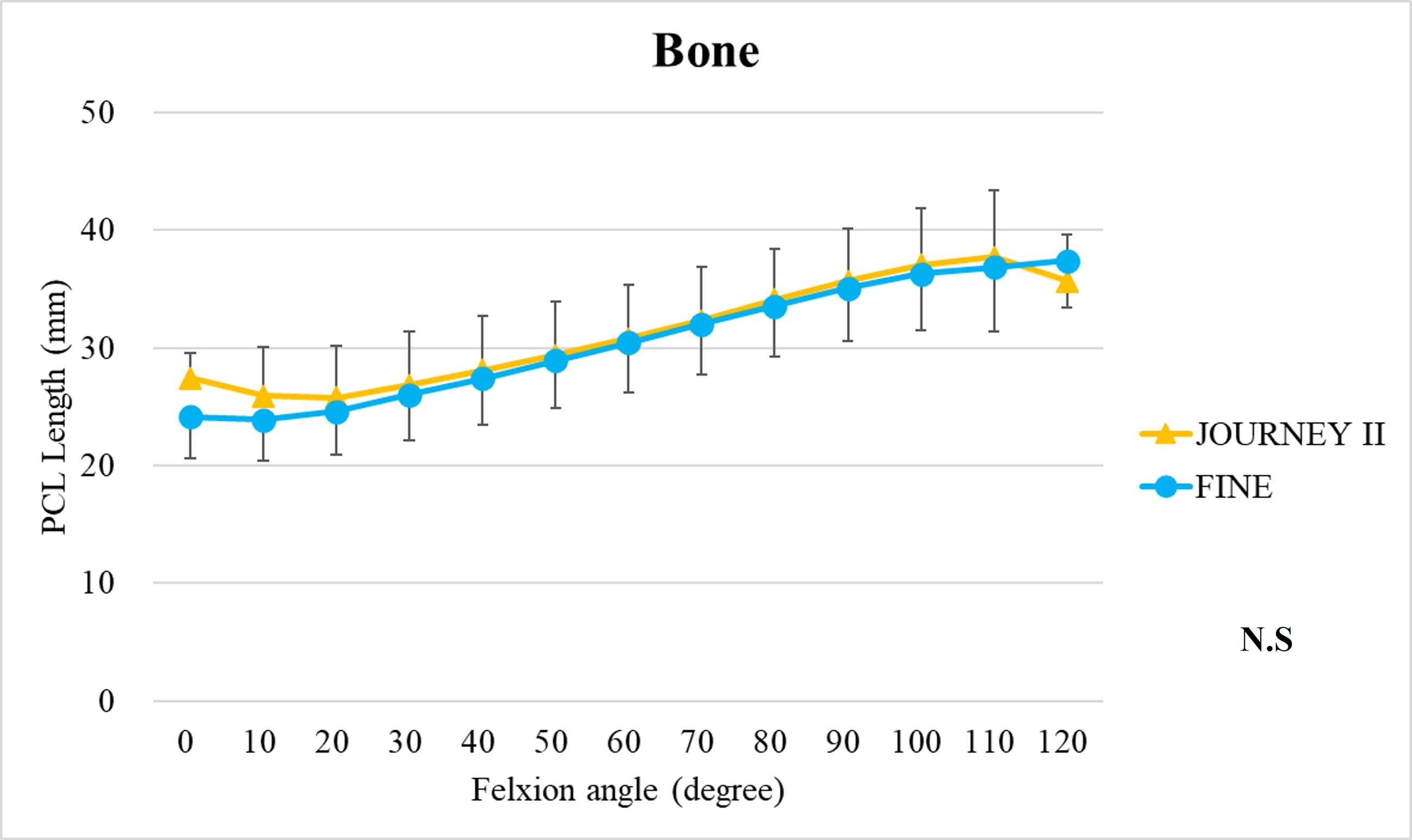
Figure 4#8555
Knee Joint Kinematic, Kinetic, and Musculoskeletal Associations With Medial Knee Osteophyte Size in Patients With Severe Knee Osteoarthritis
*Annemarie Laudanski - Dalhousie University - Halifax, Canada
Nadim Ammoury - Dalhousie University - Halifax, Canada
Glen Richardson - Dalhousie University & Capital Health - Halifax, Canada
Michael Dunbar - Dalhousie University - Halifax, Canada
Cheryl Hubley-Kozey - Dalhousie University - Halifax, Canada
Janie Wilson - Dalhousie University - Ancaster, Canada
*Email: annemarie.laudanski@dal.ca
Introduction
Knee osteoarthritis (OA) is a debilitating disease, manifesting through pain, mobility limitations, and joint deformities, which, in end-stage, can only be treated with total knee arthroplasty (TKA). Yet the reality of post-TKA outcomes reflect a variety of perceived pain- and function-based improvements with dissatisfaction reported by nearly 1 in 5 [1]. While radiographic features, including joint-space narrowing and osteophyte (OST) severity, represent distinct pathological processes in OA advancement [2,3], potential associations between biomechanical changes and OST size in a population with severe OA remain unexplored. Therefore, the objective of this study was to investigate the effects of OST severity, independently from JSN, on knee joint kinematics, kinetics, and muscular co-contraction during gait to elucidate potential considerations for the phenotyping of patients with severe OA.
Methods
This investigation constitutes a secondary analysis of 27 participants with severe knee OA. Anterior-posterior and lateral radiographs were obtained from each participant and two raters provided medial OST scores and JSN (0-3) using the OARSI standardized atlas [4]. Each participant performed gait analyses one-week pre- and one-year post-TKA, consisting of self-paced overground walking during which synchronous kinematic (NDI), kinetic (AMTI), and electromyographic (Bortec Inc.) data were collected. Biomechanical data were processed using procedures previously described [5,6] to obtain knee adduction angles, peak external knee adduction moments (pKAMs), and co-contraction indices (CCIs) for the vastus medialis-medial hamstring (VMMH) and vastus medialis-medial gastrocnemius (VMMG). Two-factor ANOVAs served to examine OST score and session (pre- and post-TKA) main and interaction effects for frontal plane angles during stance, pKAM, and VMMH and VMMG CCIs at pKAM and during early stance (0-25% gait cycle).
Results
Participants were divided into three groups based on OST scores (Table 1). Statistical analyses revealed no differences in frontal plane kinematics; however, in the frontal plane kinetics, significant decreases in pKAM post-TKA (p=0.007) and a trend towards lower pKAM as OST severity increased (p=0.065) were observed (Figure 1), supporting previous findings that OST development may be associated with altered joint loading [4]. While not statistically significant, trends revealed decreasing co-contraction at pKAM across all groups post-TKA, the greatest among those with the highest OST scores in both muscle groups (Figure 2). The highest OST group also presented with significantly higher VMMH co-contraction in early stance (p=0.015) suggesting larger osteophytes may result in increased co-contraction about the knee potentially increasing joint stability during early stance while the knee is most extended.
Conclusions
Differences in osteophyte scores within individuals with severe OA should further be investigated given their unique relation to joint loading and musculoskeletal activity. This information may prove valuable in person-specific surgical planning for improved TKA outcomes and patient satisfaction.
References
[1] Arokoski et al., Scand. J. Med. Sci Sport. 10:186-198, 2000.[2] Nobel et al., Clin Orthop Relat Res. 35-42, 2006
[2] Kraus et al., Plos One 5(3): 1-11, 2010
[3] Ishii et al., J Orthop Res. 38:639-644, 2020
[4] Altman & Gold, Osteoarthr Cartil. 15(Sup.1):A1-A56, 2007
[5] Landry et al., J Biomech. 40:1754-1761, 2007
[6] Hubley-Kozey et al., Clin Biomech. 24:407-414, 2009
Figures
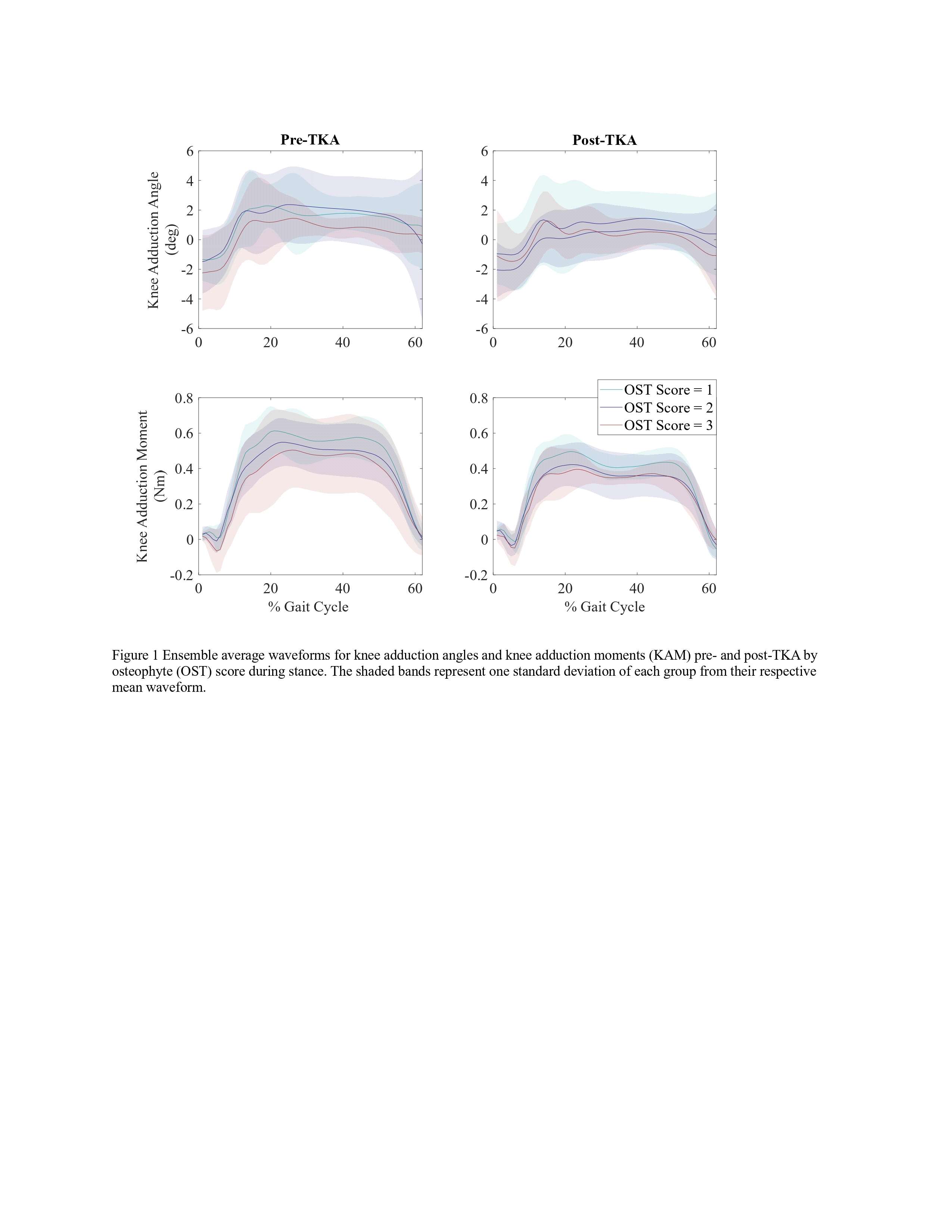
Figure 1
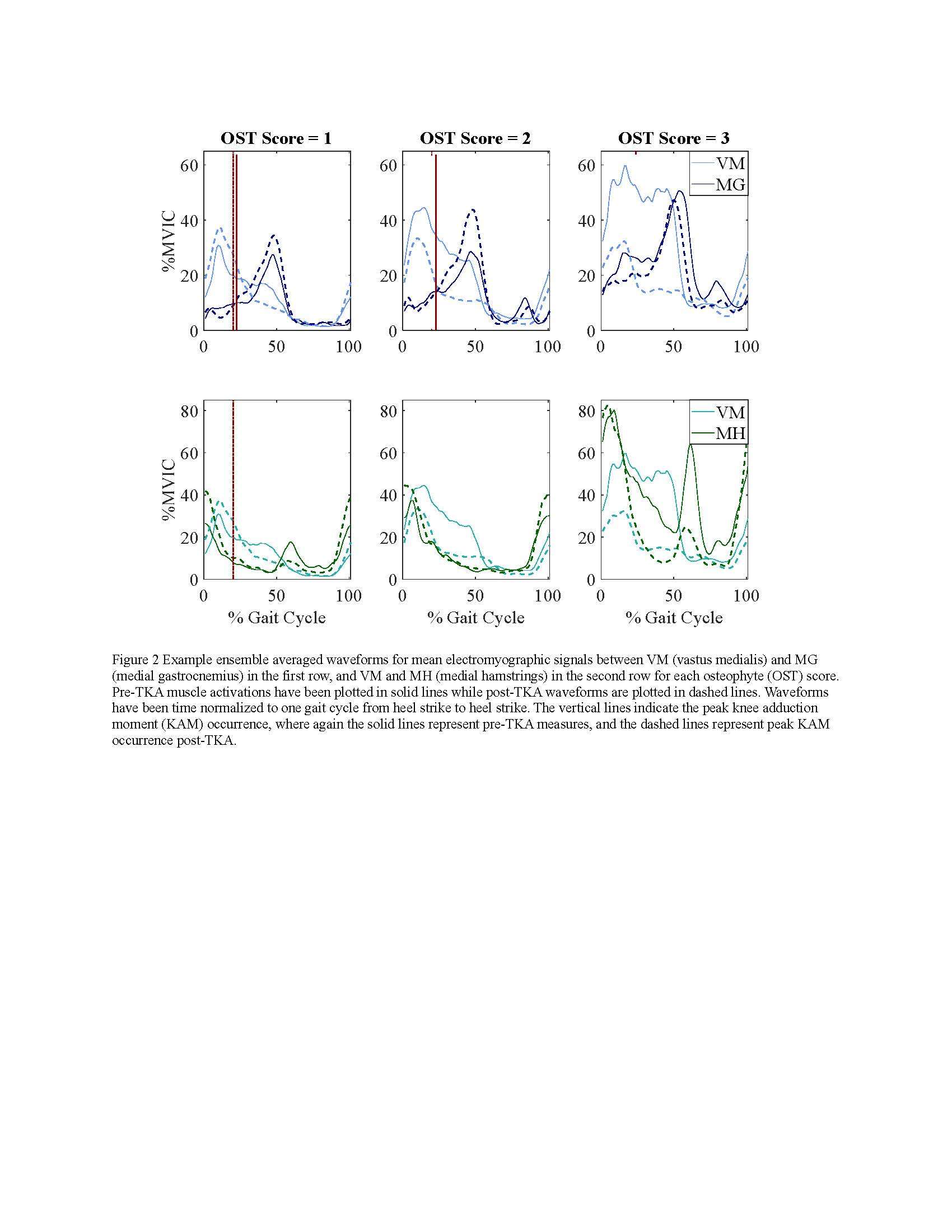
Figure 2
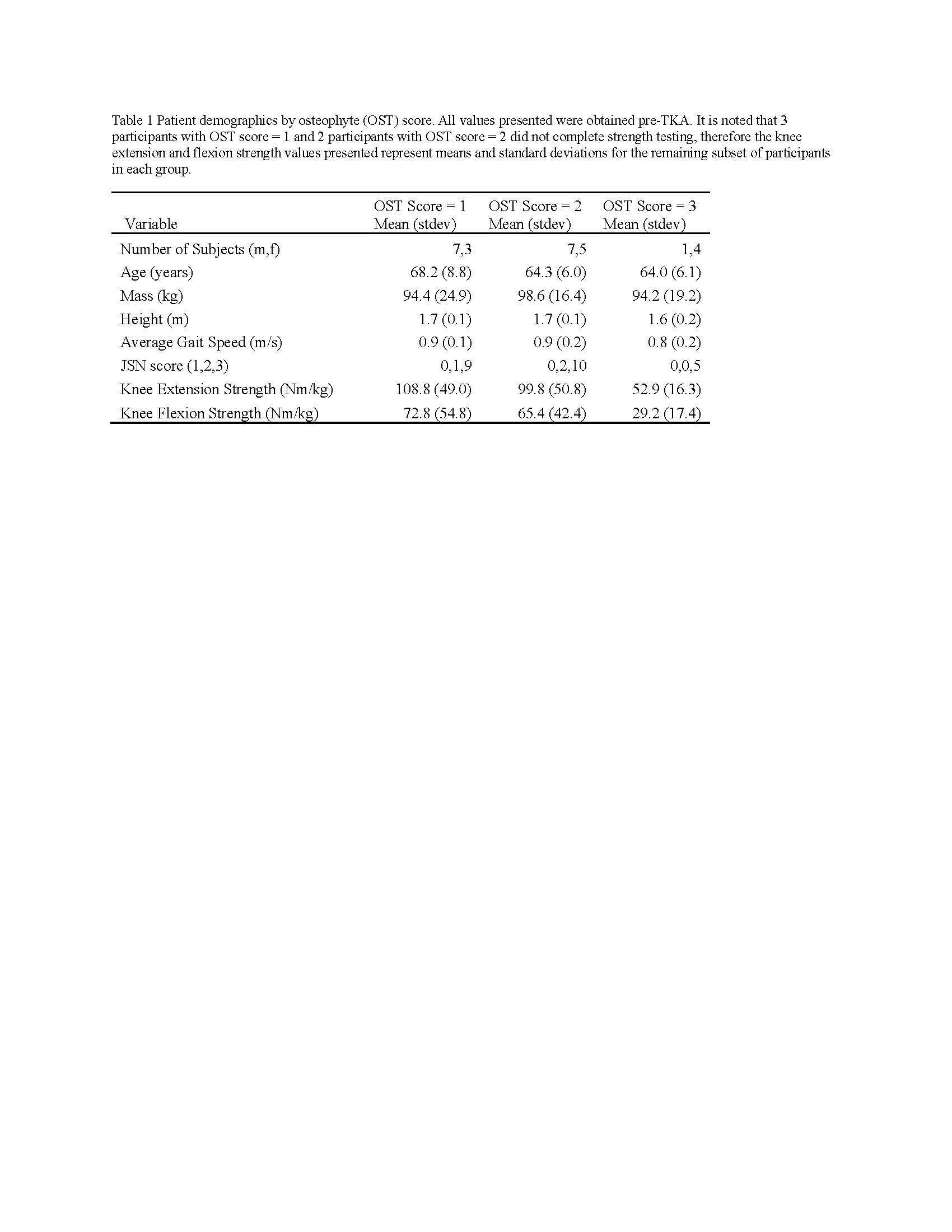
Figure 3#8574
An Automated Approach for 3D-to-2D Registration of Total Knee Arthroplasty Fluoroscopy Using a Single Deep Learning Neural Network and Hybrid Optimization
*Viet-Dung Nguyen - The University of Tennessee at Knoxville - Knoxville, United States of America
Michael LaCour - University of Tennessee - Knoxville, USA
Richard Komistek - The University of Tennessee - Knoxville, USA
*Email: vnguye28@vols.utk.edu
Introduction: Machine Learning (ML) is advancing technology that can help reduce workload and improve overall efficiency by automating tasks that are typically done manually. One such example is 3D-to-2D image registration, which has been the “gold standard” for in vivo orthopedics kinematics analyses for 30 years, helping determine postoperative total knee arthroplasty (TKA) kinematic outcomes, as seen in Figure 1. Unfortunately, these techniques can be time-consuming and labor-intensive, especially when conducting larger studies on hundreds of participants. Thus, the orthopaedic applications of machine learning are potentially extremely impactful. This work proposes an approach to using a deep neural network and hybrid optimization to register 3D TKA components onto their corresponding fluoroscopic images.
Methods: Utilizing a previous dataset of more than 30,000 fluoroscopic images, a novel methodology is proposed for automatic 3D-to-2D TKA registration. The algorithm starts with all models oriented in an initial zero position and can also create 3D CAD implant models for each subject. A predicted pose is then computed using a single deep learning neural network. This neural network consists of one input layer representing the fluoroscopic image and three output layers for class (femur or tibia), 2D box, and transformations. Since the training data is not uniformly distributed, including various implant types and different fluoroscopic units, this causes the accuracy of machine learning to be low, as shown in Table 1. Nonetheless, the machine learning outputs are sufficient to be initial poses that are then refined using a hybrid optimization technique. The hybrid optimization consists of two phases, using edge-based optimization first to converge quickly to an initial solution and then optional intensity-based optimization to achieve improved accuracy.
Results: A sample outcome of the proposed automated registration methodology is shown in Figure 2. The top-left and bottom-left images demonstrate the findings of the deep learning stage. While the machine learning results exhibit commendable speed in real-time, their accuracy level is not yet sufficient. In terms of the ground truth comparison (assumed to be finalized manual registrations), the deep learning model records an accuracy of 5 mm for in-plane errors, 10.35 mm for out-of-plane errors, and 5.71 degrees for rotation errors. As observable qualitatively from the images on the left-hand side in Figure 2, the registration precision of the deep learning technique is proximate to the silhouettes of the fluoroscopic images, although it does not yet satisfactorily for kinematics analyses. Upon utilizing hybrid optimization techniques, the accuracy is greatly improved, demonstrating 0.23 mm for in-plane errors, 0.98 mm for out-of-plane errors, and 0.90 degrees for rotation errors, as presented in Table 1.
Conclusion: In this study, a novel machine learning framework is proposed for registering three-dimensional computer-aided design (CAD) implant models automatically and accurately onto two-dimensional X-ray or fluoroscopic images. This novel methodology can potentially revolutionize 3D-to-2D registration by automating the process, allowing for fast implementation in a variety of clinical and research settings. The computational results show competitive accuracy, and this approach also can be extended to other joints such as hips and ankles.
Figures
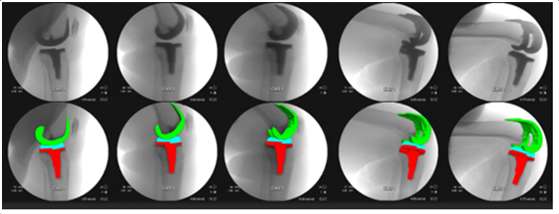
Figure 1
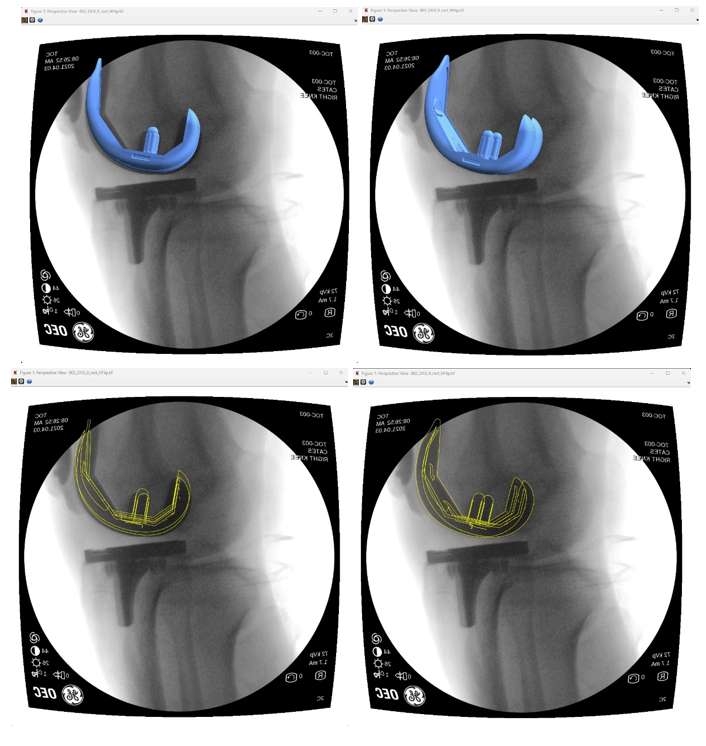
Figure 2
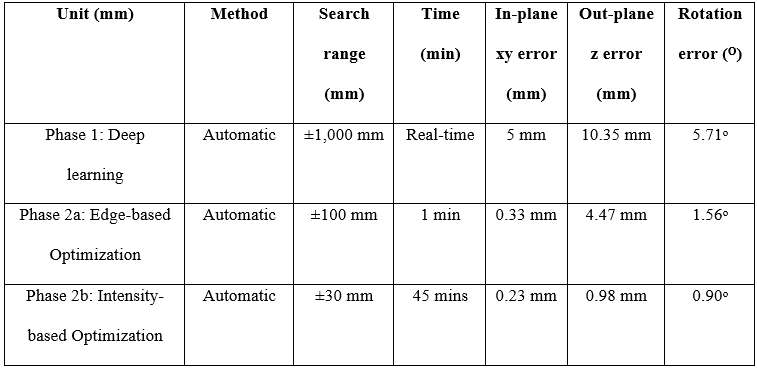
Figure 3#8613
In Vivo Knee Kinematics: Does Anterior Stabilization in a TKA Make a Difference?
*Lauren Smith - University of Tennessee, Knoxville - Knoxville, USA
Richard Komistek - The University of Tennessee - Knoxville, USA
Michael LaCour - University of Tennessee - Knoxville, USA
*Email: lsmit186@vols.utk.edu
Introduction:
The bi-cruciate stabilized (BCS) TKA features a dual cam-post mechanism to substitute for the ACL and the PCL, while the posterior stabilized (PS) TKA provides a singular cam-post mechanism to replicate the functionality of the PCL alone. Will adequate substitution of both cruciate ligaments result in more normal-like kinematics than seen in traditional PS TKAs? This research study assesses the importance of replicating the function of the ACL in BCS TKAs compared to its PS counterpart and the normal knee.
Methods:
The in vivo 3D kinematics were determined for 54 subjects having a PS TKA (20 Type # 1, 14 Type #2), 20 subjects having a BCS TKA and 10 having a normal knee. Kinematics were obtained using validated fluoroscopic and 3D-to-2D registration techniques. All subjects were asked to perform a weight-bearing deep knee bend (DKB) maneuver intended to flex the knee from full extension to maximum flexion. The kinematic parameters assessed for each subject were lateral and medial anterior/posterior (LAP, MAP) condylar position and axial orientation (Figure 1).
Results:
At full extension, the BCS subjects demonstrated a more anterior position of both condyles compared to the PS TKA. The BCS TKA was statistically more anterior than both PS TKA #1 (p< 0.0001) and the PS TKA #2 (p=0.0328). In early flexion, the BCS TKAs experienced greater rollback and more normal-like kinematics. Once the knees reached mid-flexion, all TKA exhibited similar kinematic patterns. Therefore, both the normal knee and BCS TKA experienced statistically more PFR of the lateral condyle during this flexion range than PS TKA #1 (p<0.0001) and PS TKA #2 (p<0.0001) subjects. From full extension to maximum knee flexion, the BCS TKA subjects demonstrated greater posterior femoral rollback and axial rotation. Detailed kinematics can be found in Table 1, while a visual representation of these motion patterns can be seen in Figure 3.
Discussion:
Since the BCS TKA design has two cam/post mechanisms to mirror the functionality of not only the PCL but also the ACL, it can be discerned from this data that it replicates more normal-like kinematics in comparison to the analyzed PS TKAs. It is assumed that since the anterior cam/post interaction with the BCS TKA, they demonstrated a more anteriorized position at full extension, much like the normal knee, which can likely be attributed to the design accounting for the functionality of the ACL. Similarly, in early flexion, the BCS TKA displayed much higher amounts of posterior femoral rollback of both medial and lateral condyles, as one would expect to see in the normal knee. Once in deeper flexion (90°+) and the PCL becomes dominant, all designs begin to translate posteriorly, as they all share posterior cam/post designs to mimic the PCL. Absence of the ACL seems to affect the performance of these implants during the crucial part of early flexion.
Figures
Figure 1
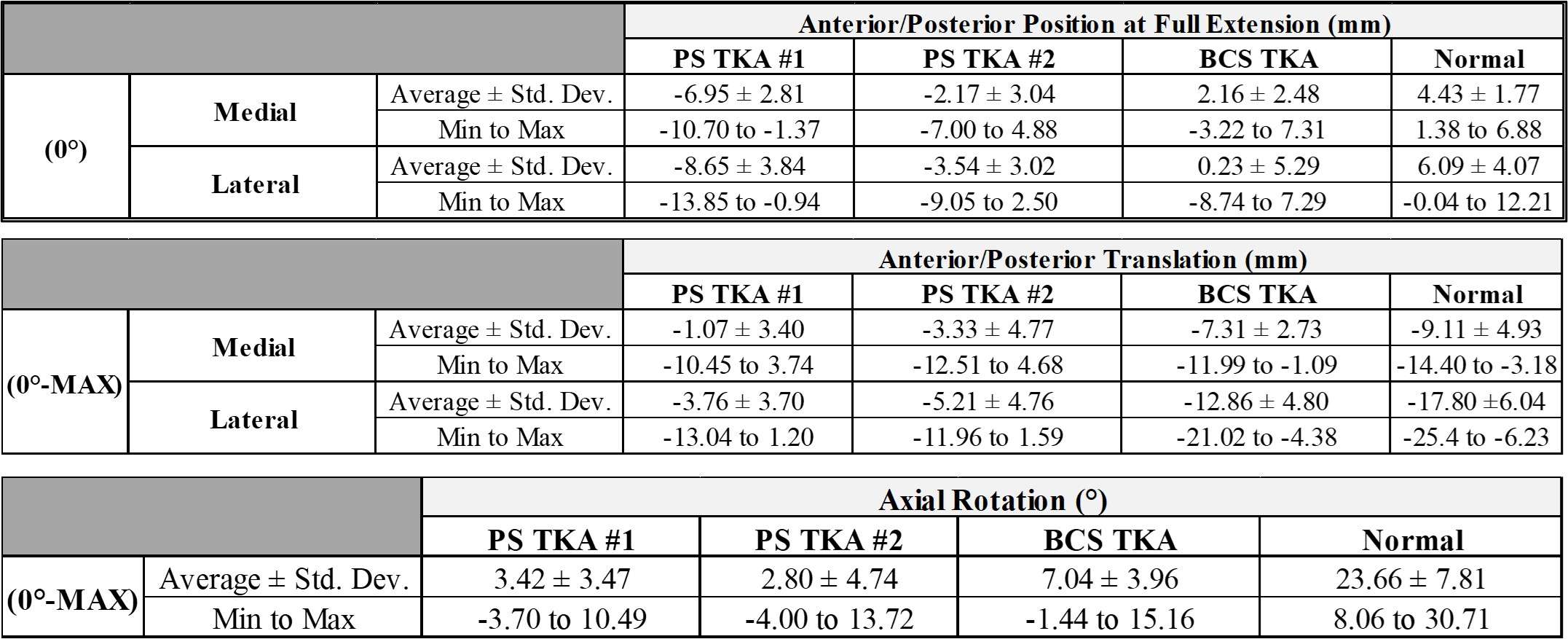
Figure 2
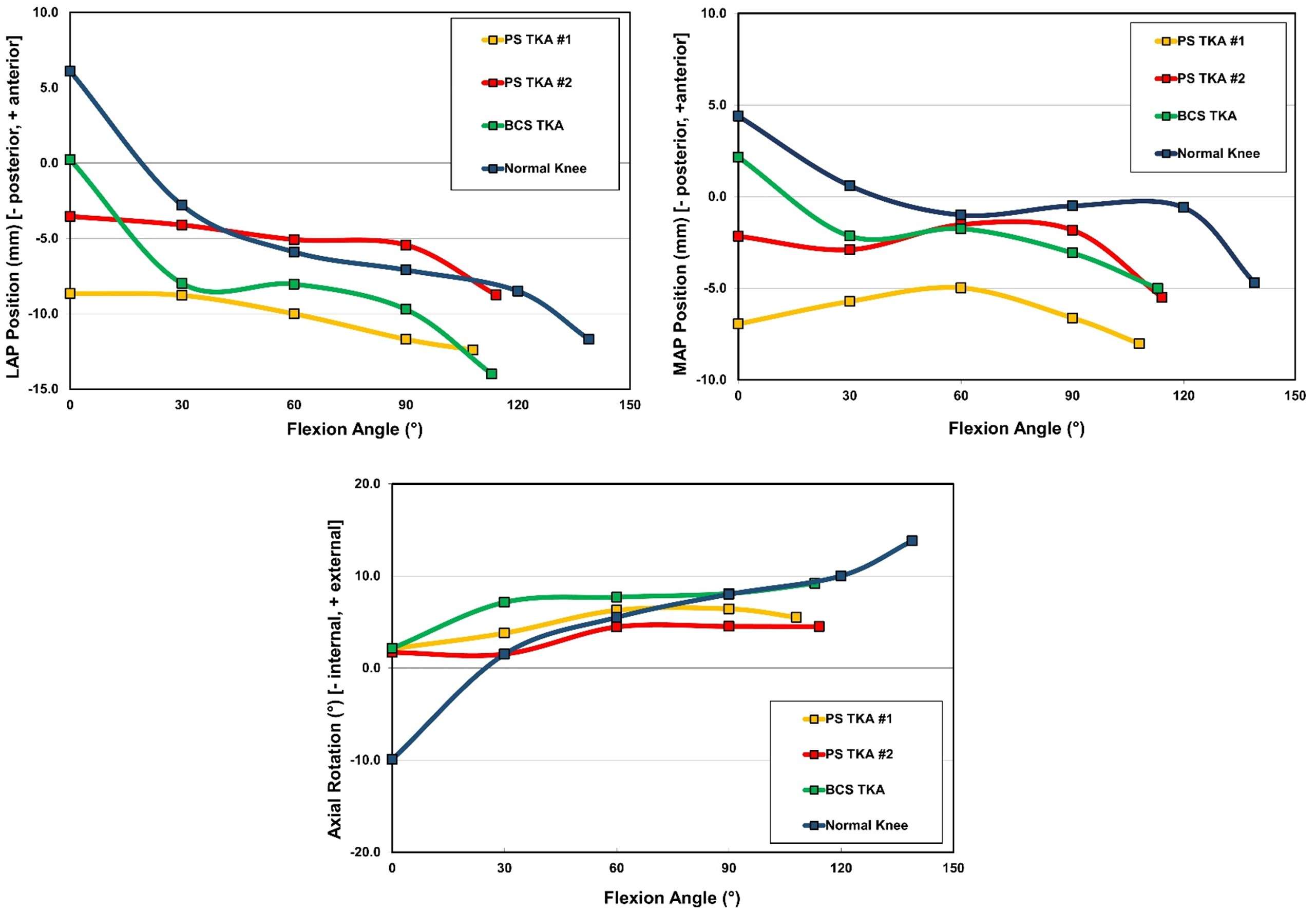
Figure 3#8307
Serial Release of the PCL Fibers Does Not Guarantee a Uniform Reduction in Femoral Rollback in Cruciate-Retaining TKA: A Computational Study
Cynthia Kahlenberg - Hospital for Special Surgery - New York City, USA
Brian Chalmers - Hospital for Special Surgery - New York CIty, USA
William Long - NYU Langone Health - New York, USA
Timothy Wright - Hospital for Special Surgery - New York, USA
Geoffrey H Westrich - Hospital for Special Surgery - New York, USA
David J. Mayman - Hospital for Special Surgery - New York, USA
Carl Imhauser - USA
Peter K. Sculco - Hospital for Special Surgery - NYC, USA
*Reza Pourmodheji - Hospital for Special Surgery - New York, United States of America
*Email: pourmodhejir@hss.edu
Introduction: A major goal of cruciate-retaining (CR) total knee arthroplasty (TKA) is to achieve femoral rollback by retaining the posterior cruciate ligament (PCL), thus providing a large range of knee flexion [1]. In knees with excessively tight flexion gaps, partial or total release of the PCL is often used intraoperatively [2]. However, the effect of serial release of selected PCL fibers on femoral rollback in CR-TKA is not well understood. Therefore, we employed a computational framework to quantify how serially releasing the PCL fibers affects posterior translation of the medial and lateral femoral condyles through a range of knee flexion from 0 to 90°.
Methods: Computational models derived from six independent cadaveric left knees (male; age: 60.9±13.6 years) were virtually implanted with CR femoral components and inserts (Persona, Zimmer-Biomet, Warsaw, IN). The computational modeling framework utilized a multibody dynamics framework including 33 line-elements representing the PCL, collateral, and capsular ligaments and a rigid body contact formulation to describe the articular interactions. The PCL was composed of seven elements, identified by a numbering convention from the most posterior and medial femoral insertion as fiber 1 to the most anterior and lateral femoral insertion as fiber 7 (Fig. 1). The knee was flexed from 0 to 90° under 500 N of compression to represent a test of passive flexion. The flexion test was run with all PCL fibers intact (PCL retained) and after serially releasing each fiber starting with fiber 7 to fiber 1. Femoral medial and lateral rollback was quantified as the distance between the contact points at 0° and 90° of flexion in the anterior-posterior (AP) direction (anterior was positive). A Kolmogorov-Smirnov test (p<0.05) revealed that our data were not normally distributed; therefore, we reported medians and quartiles. To compare femoral rollback between the different PCL conditions, we used a nonparametric Wilcoxon signed-rank test (α = 0.05).
Results: Releasing all of the PCL fibers reduced femoral rollback for both the medial and later condyles (Fig. 2). Sequentially resecting the fibers showed the largest affect when fibers 4-7 were sectioned with a median reduction in femoral rollback of 0.97 [0.4 1.4] mm (p=0.06) and 0.86 [0.4 1.1] mm (p=0.03) in the medial and lateral compartments, respectively (Fig. 3).
Conclusion: Our model revealed that serially releasing the PCL fibers reduced the femoral rollback nonuniformly. Interestingly, our findings were subject-specific: no single specific PCL fiber across our cohort of six specimens was responsible for controlling femoral rollback, though release of fibers 4 and 5 had the largest impact. Our results suggest that release of the PCL fibers should be done with caution, as it likely impacts femoral rollback. Strategic release of PCL fibers for optimal kinematics requires further patient-specific studies incorporating the effect of specific implants and other soft tissue and bone resection.
Acknowledgment: The Clark and Kirby Foundations. Implants donated by Zimmer Biomet, Inc.
References: [1] Scott et al., J Arthroplasty, 2008. [2] Williams et al. Clin. Orthop. Relat. Res, 1996. [3] Arima et al Clin. Orthop. Relat. Res. 1998.
Figures
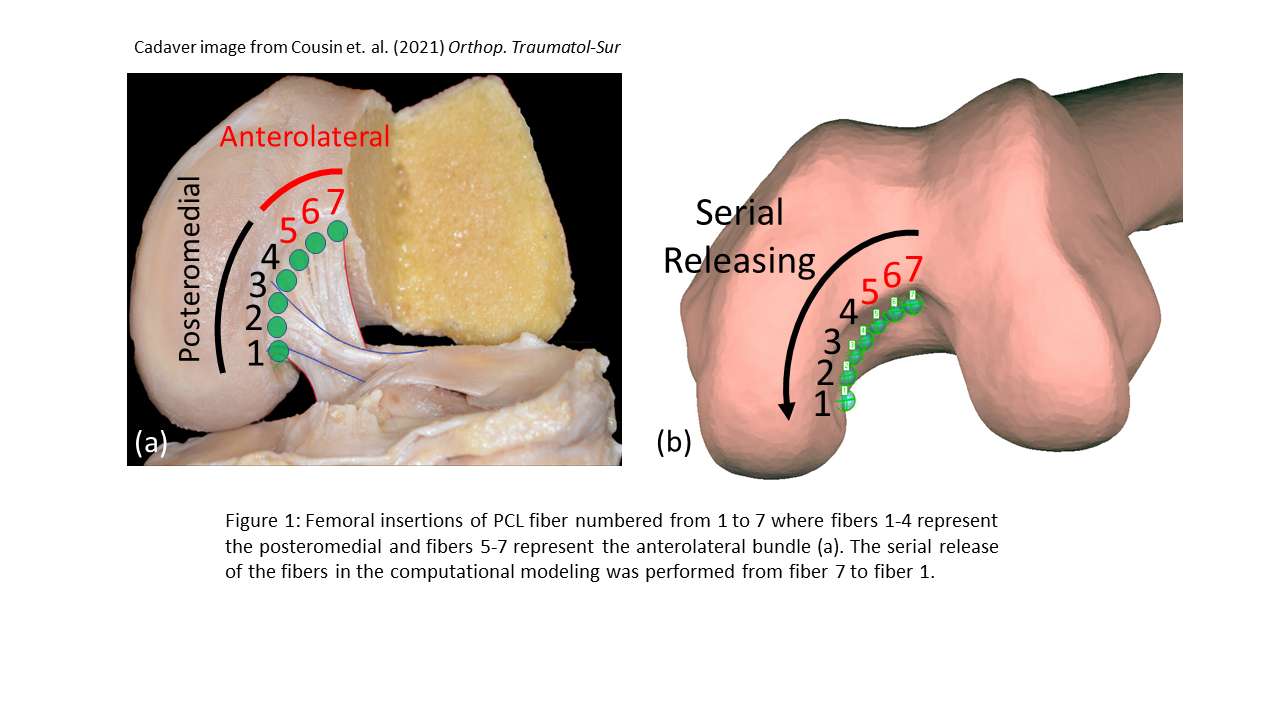
Figure 1
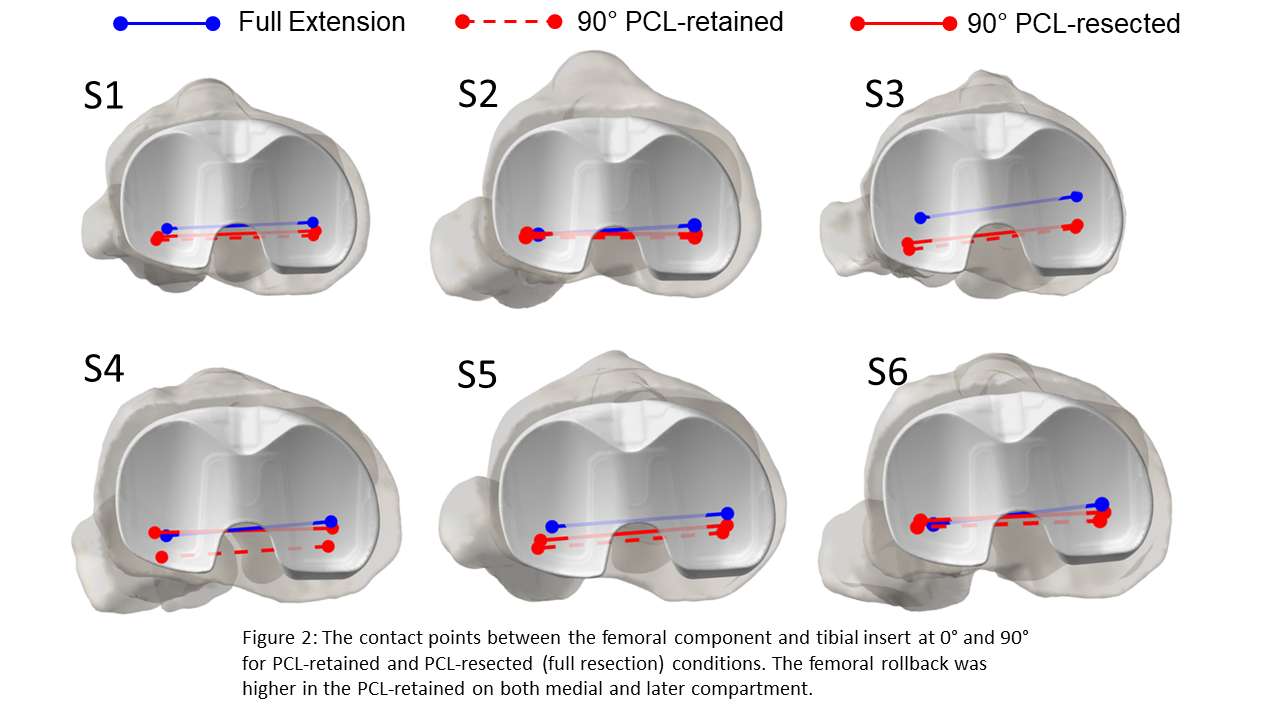
Figure 2
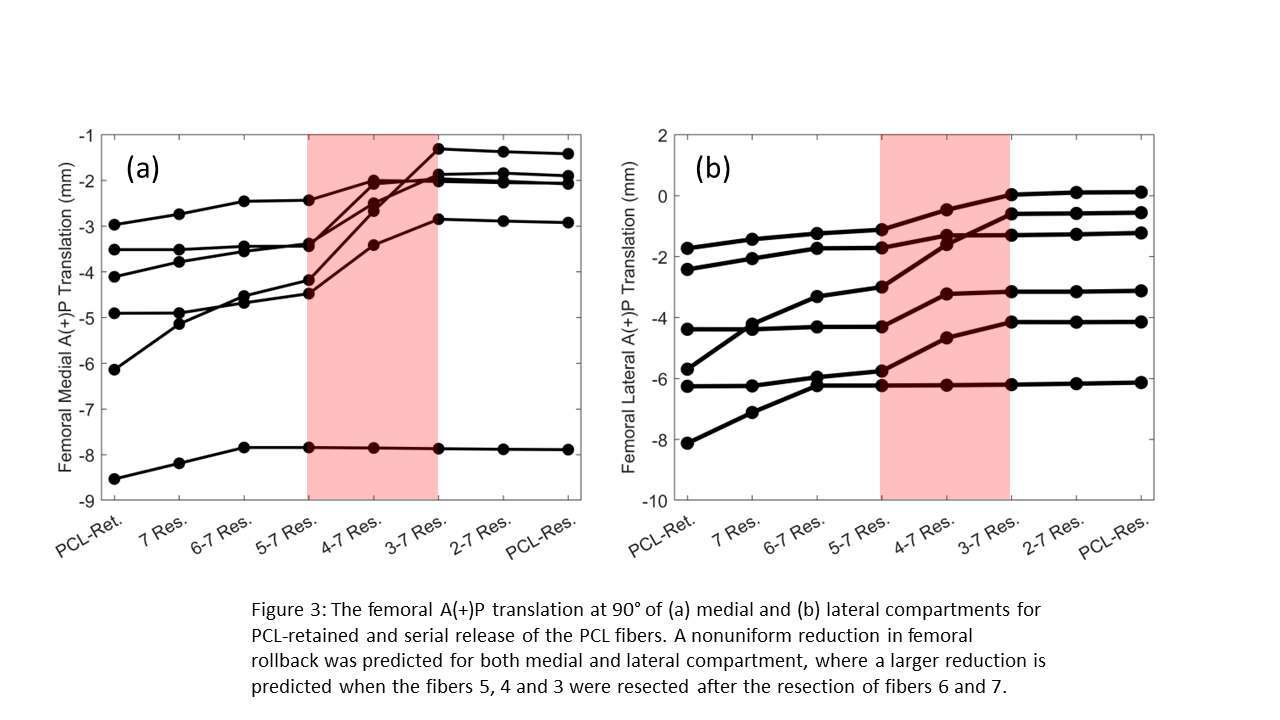
Figure 3#8213
Relating Functional Kinematics to Implant Design in Total Knee Arthroplasty Based on the Location of Standardised Joint Segment Frames: An Application of REFRAME
Michael Utz - Aesculap AG - Tuttlingen, Germany
Ariana Ortigas Vasquez - Aesculap AG - Tuttlingen, Germany
*Adrian Sauer - Aesculap AG - Tuttlingen, Germany
Allan Maas - Aesculap - Tuttlingen, Germany
William Taylor - Swiss Federal Institute of Technology Zurich - Zürich, Switzerland
Thomas M. Grupp - Aesculap AG - Tuttlingen, Germany
Matthias Woiczinski - University Hospital of Munich (LMU) - Munich, Germany
*Email: adrian.sauer@aesculap.de
Introduction
Recent attempts to improve patient satisfaction after total knee arthroplasty (TKA) include the identification of kinematic phenotypes. The goal behind clustering patients based on knee kinematic patterns during activities of daily living into a handful of “phenotypes” is to associate each group with a particular surgical strategy (implant design, alignment, etc.) that best addresses their specific needs. Previous work has focused on tackling this challenge by attempting to find statistically significant clusters of kinematics signals (i.e. time series data) leveraging methods such as k-means clustering, principal component analysis, and statistical parametric mapping [1,2]. However, recent studies have demonstrated the large degree of variability in kinematic signals that is associated with even very minor inconsistencies in local segment frame orientation and position [3]. As a result, the clinical significance of patient groups identified based on these datasets with high-dimensionality and variability has so far been tenuous, especially without proper standardisation.
In this study, we instead explore the feasibility of classifying patients based on the location of their femoral joint centres (relative to the anatomical centre, i.e. midpoint between condyles) after reference frame standardisation with REFRAME [3], simplifying the defining variables from up to six time series to a single 3D vector.
Methods
The native tibiofemoral kinematics of eight cadaver knees (3 female, 3 right) were assessed in a knee rig [4] during deep squat based on optical measurements. Data collection was repeated after implantation of a cruciate retaining (CR) femoral component and a CR tibial inlay. Next, two other implant configurations were tested: CR femur + medial stabilised (MS) inlay, and CR femur + lateral stabilised (LS) inlay. Finally, the posterior cruciate ligament was sacrificed, posterior stabilising (PS) components were implanted, and kinematics were recorded again.
Kinematic signals were then standardised using REFRAME [3], solving for the optimal position of the femoral origin based on a minimisation of the variance of femoral (3D) translations relative to the tibia. The optimised position of the femoral origin therefore represented the functional rotation centre. Paired t-tests statistically characterised the differences between mean origins in each direction.
Results
Implementation of REFRAME to standardise local tibiofemoral segment frames led to relocation of the femoral origins (relative to originally defined anatomical origins). Mean optimised positions were consistent with expectations of where functional rotation centres would be located for different implant designs (medial for MS, lateral for LS, and posterior and distal for PS). Mean origin positions for both PS and CR were more lateral than for LS. These visually discernible patters (Figure 1) were supported by statistically significant differences along the x- and y-axes for MS, LS and PS, and along the z-axis for PS (Table 1).
Conclusion
REFRAME implementation demonstrated potential to enable the correct identification of TKA implant design based on tibiofemoral kinematic signals during a deep squat. By focusing on femoral origin position after frame standardisation, patient classification is simplified from attempting to cluster highly variable time series signals to merely assessing the 3D location of the functional centre relative to the anatomical.
Figures
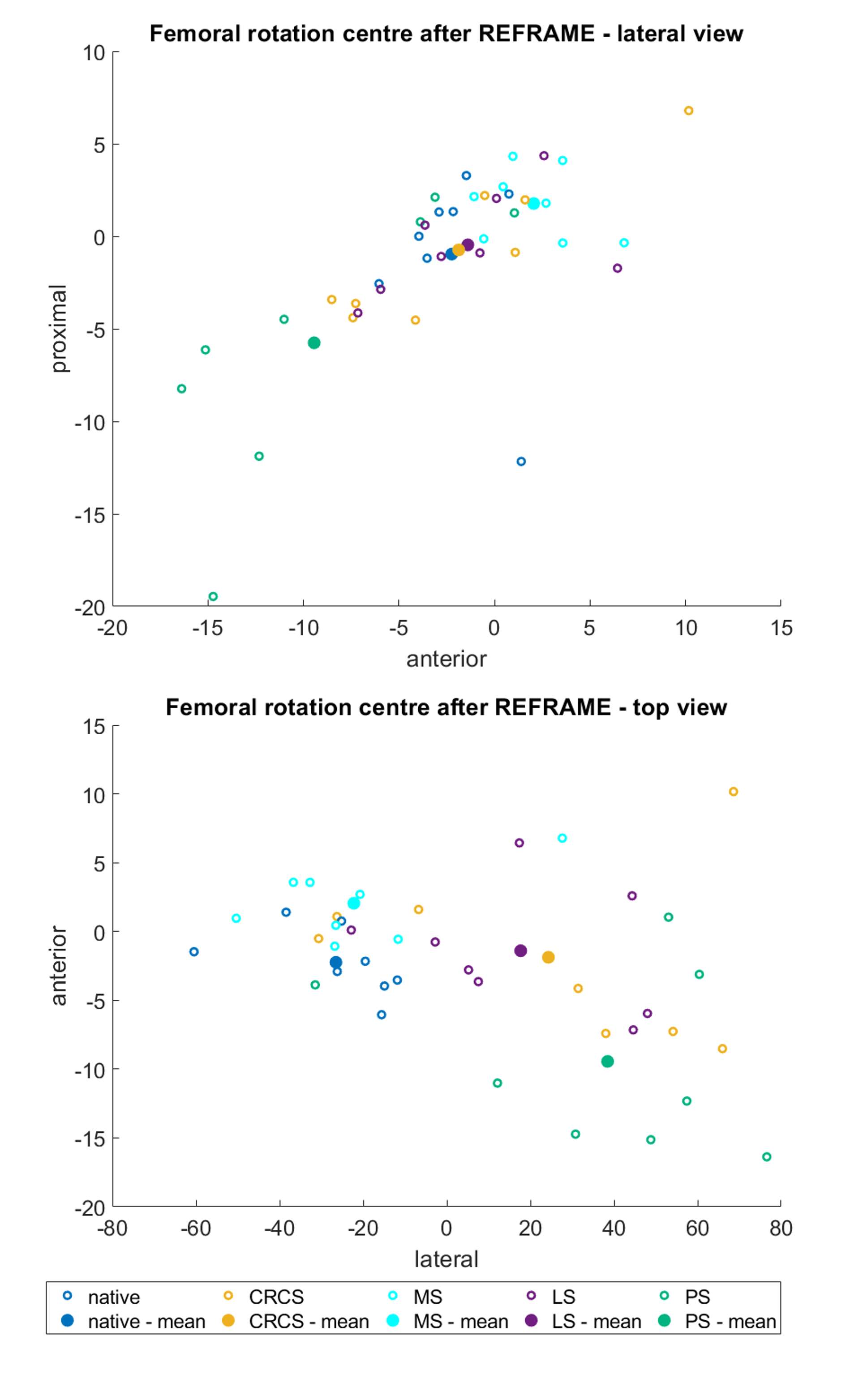
Figure 1
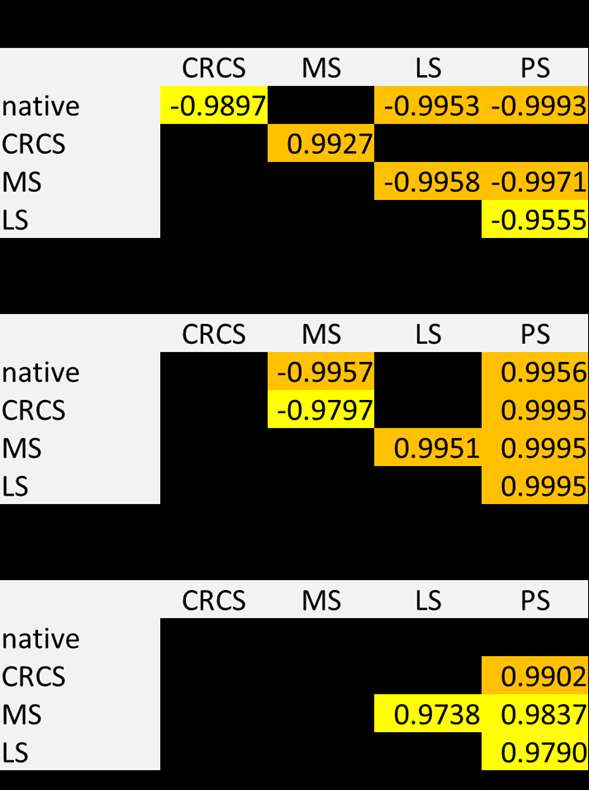
Figure 2#8605
Kinematic Outcomes of a Bi-Cruciate Stabilized (BCS) TKA Implanted by Multiple Surgeons: Assessing Surgeon Variability
*Lauren Smith - University of Tennessee, Knoxville - Knoxville, USA
Richard Komistek - The University of Tennessee - Knoxville, USA
Michael LaCour - University of Tennessee - Knoxville, USA
steven haas - Hospital for Special Surgery - New York, United States of America
Harold Cates - Tennessee Orthopaedic Clinics - Knoxville, USA
Jan Victor - Ghent University - Gent, Belgium
Manuel Vieira da Silva - Hospital de Braga - Braga, Portugal
*Email: lsmit186@vols.utk.edu
Introduction:
Unlike most TKA designs, the bi-cruciate stabilized (BCS) TKA features a dual cam-post mechanism to substitute for the functionality of both the ACL and the PCL. The ability to replicate functionality of both cruciate ligaments is thought to lead to more normal-like kinematics. However, while different implant systems have been shown to alter TKA kinematics, the question of how other factors such as surgical technique and surgeon variability affect post-operative mechanics remains a topic of discussion. This research study strives to control TKA type and design variability and compare the kinematic results of a single BCS TKA system implanted by 4 different surgeons.
Methods:
In vivo 3D kinematics were determined for 80 subjects having a BCS TKA implanted by four different surgeons (20, 20, 20 and 20, respectively) and 10 having a normal knee. The study was IRB-approved, and all knees were deemed well-functioning by their respective surgeon. Kinematics were obtained using validated fluoroscopic and 3D-to-2D registration techniques. All subjects were asked to perform a weight-bearing deep knee bend (DKB) maneuver intended to flex the knee from full extension to maximum flexion. The kinematic parameters assessed for each subject were lateral and medial anterior/posterior (LAP, MAP) condylar position and axial orientation (Figure 1).
Results:
All four BCS TKAs saw varying ROM. However, Haas demonstrated a significantly higher flexion than all other surgeons, averaging 132o of weight-bearing flexion (p<0.0001). In both early flexion (0-30) and late flexion (90-Max), both condyles of all 4 BCS TKAs translated posteriorly, as one would expect in the normal knee. When reviewing the lateral condyle motion, the Haas cohort exhibited a significantly higher amount of rollback in late flexion than the other surgeons (p=0.0001,p=0.0007,p=0.0006). Conversely, during mid-flexion, all surgeons except Haas demonstrated small amounts of paradoxical anterior sliding. In general, all cohorts also saw positive, external femorotibial axial rotation from full extension to maximum flexion. Detailed kinematics can be found in Table 1, while a visual representation of these motion patterns can be seen in Figure 3.
Discussion:
Since the BCS TKA design has two cam/post mechanisms to mirror the functionality of both the PCL and the ACL, it can be discerned from this data that post-operative kinematics are fairly similar to the normal knee, however, lesser in magnitude. There was variability between the cohorts, indicating that surgeon variability appears to have a large effect on the data. Ultimately, this means that even among a TKA design with more ligament-substituting constraint, there is still variability with respect to ROM and condylar motion patterns. It could be assumed that this variability is due to both the patient and surgical implantation and possibly the rehabilitation process. In this analysis of 4 different surgeonsthere was a general trend of the condyles positioned anteriorly at full extension, indicating that the anterior cam is functioning to replicate the ACL, and posterior rollback does occur during late flexion, indicating that the posterior cam is function to replicate the PCL.
Figures
Figure 1
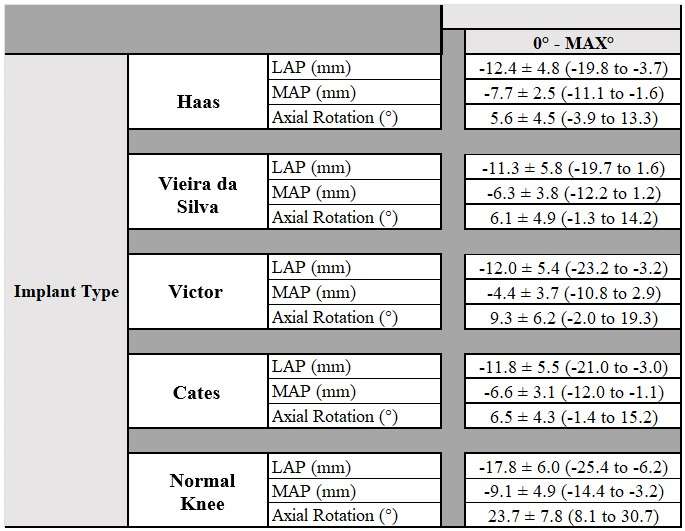
Figure 2
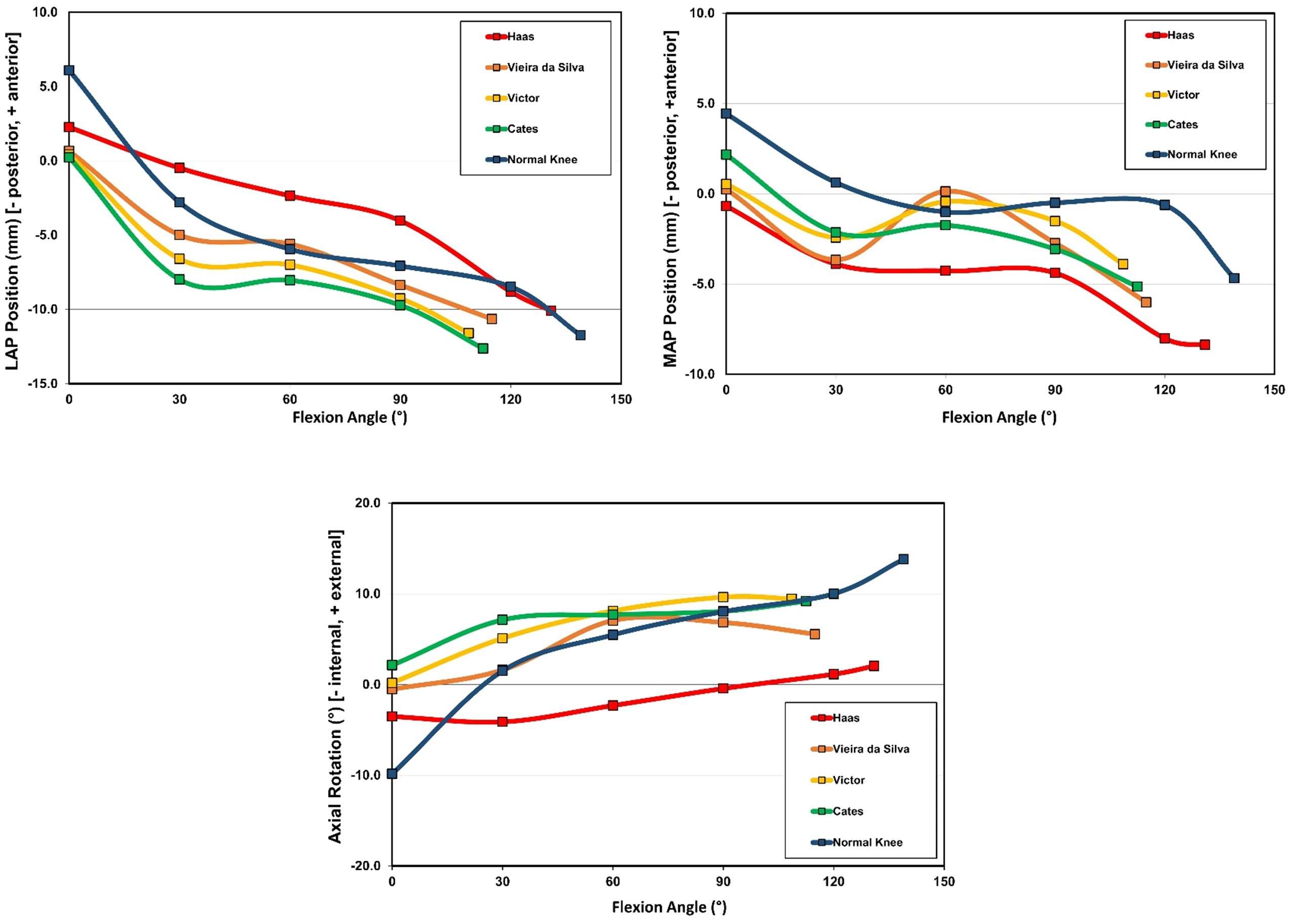
Figure 3#8786
Bearing Science (Non-)Friction: Effects of Stress and Speed on Friction Coefficients
Alexander Eischeid - University of Nebraska Medical Center - USA
Joel Weisenburger - UNMC - Omaha, USA
*Hani Haider - UNMC - Omaha, USA
*Email: hhaider@unmc.edu
INTRODUCTION:
With wear remaining a cause for revision and implant failure, preclinical wear testing of implants must mimic the in-patient environment, especially lubrication, to ensure accuracy in simulating and assessing bearing wear patterns. This study tested the effect of compressive stress and linear sliding speed on the lubrication of implant material articulation.
METHODS:
A friction measuring machine was custom-built, in a pin-on-disk configuration, allowing a very large dynamic range of stresses to be imposed and friction to be generated and measured very accurately. Minimizing frictional errors within the machine was possible utilizing air-bearing technology. A disk of highly polished CoCr (Ra: 0.04±0.01 µm) was rotated under a 6 mm diameter ultra-high molecular weight polyethylene (UHMWPE) pin machined from GUR-1020 bar stock (25-40 kGy gamma irradiated, non-crosslinked). Bovine serum was placed on the disk for lubrication (see details below). Pin assembly movement, permitted by a frictionless air-bearing, was counteracted by a torque cell connected with a variable-length lever arm. The friction coefficient was determined from frictional forces paired with axial load measurement logged in real time and averaged. An experimental test matrix varied stresses (0.6MPa, 5MPa, 7.5MPa, and 10MPa) and speeds (10mm/s, 25mm/s, 50mm/s, and 80mm/s) of articulation in which friction was measured. The lubrication was ISO standard bovine calf serum (GE Healthcare, Logan, UT) diluted with ethylenediaminetetraacetic acid (7.45g/L, Fischer Scientific, Hampton, NH) to protein concentrations of 20g/L and 30g/L. Three human synovial fluid samples obtained from total knee arthroplasty patients (IRB approved) were tested for friction coefficient comparison. All tests were performed at 22°C.
RESULTS:
In all serum trials, increasing speed only nominally decreased friction coefficient (Fig. 1). On average, increasing speed 8-fold (10mm/s to 80mm/s) decreased friction coefficient by only 0.016 ± 0.004 which, while statistically significant (p=2.6E-6), likely has little clinical significance. Serum friction coefficients exhibited greater effects of stress dependence with friction decreasing by an average 0.07±0.005 (p=8.3E-5) as stress increased from 0.6MPa to 10MPa.
Synovial fluid displayed a similar but smaller magnitude decrease in friction coefficient due to increasing speed (Fig. 2), averaging a difference of 0.009±0.007 (p=0.0018) from 10mm/s to 80mm/s. Increasing stress with synovial fluid produced the opposite trend to serum, increasing friction coefficient by 0.016±0.003 (p=0.0008).
CONCLUSION:
Articulation speed seems unimportant in the wide range used which were representative of in vitro testing. Interestingly, increasing compressive stress on the articulating surface produced opposite effects when comparing serum to synovial fluid in terms of the friction coefficient (Fig. 3). At low stress (0.6MPa) the synovial fluid showed less than half the friction of serum. The corollary to that, importantly, was that at higher stresses reached in hips (~5MPa and even higher in knees) synovial fluid would have no less friction than serum. Further investigation into the properties of human synovial fluid (maybe its higher viscosity) may explain the reason.
Preclinical testing with serum as the lubricant is at least safe in high loading/stress simulations and may be too harsh (and safer still) in low stress tests compared to in vivo.
Figures
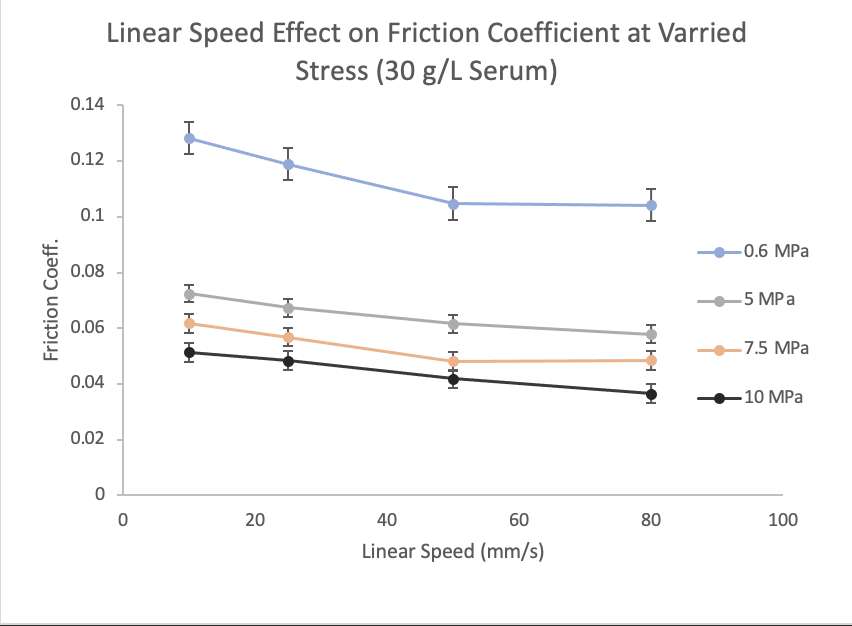
Figure 1
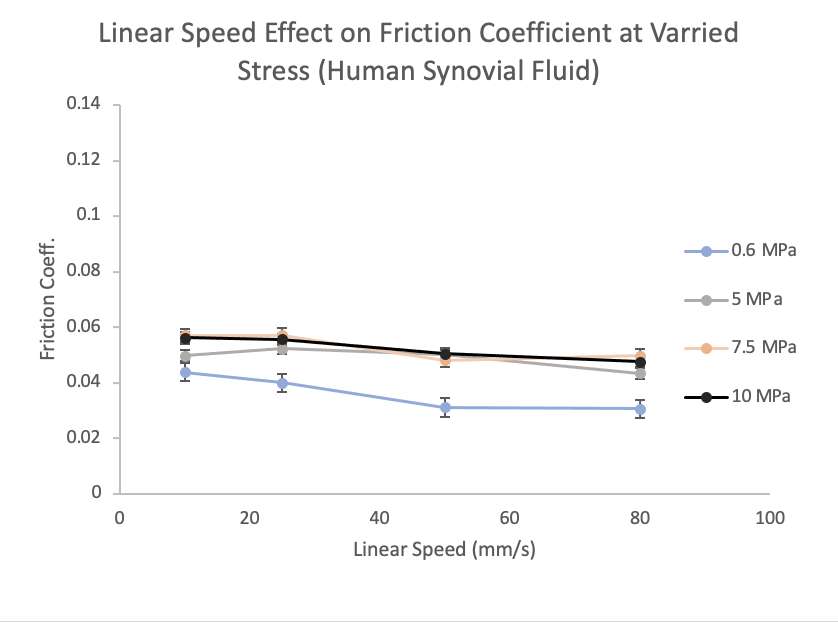
Figure 2
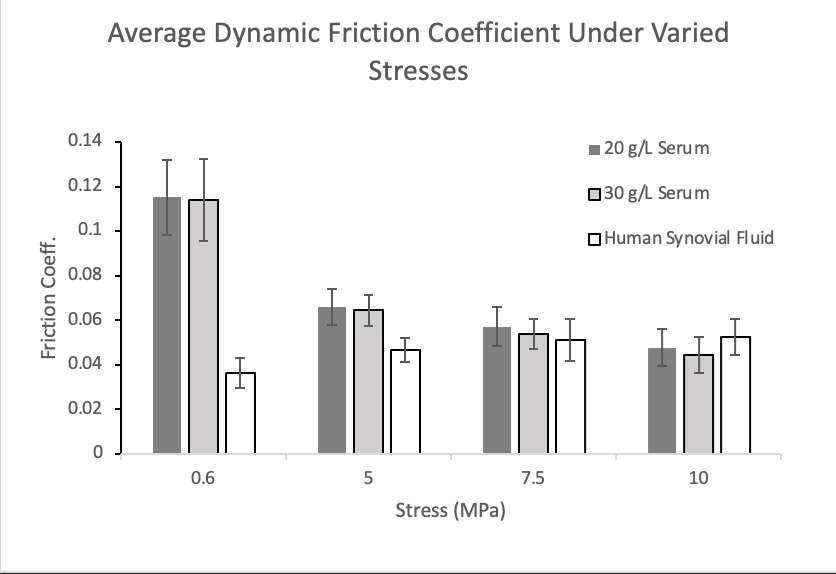
Figure 3#8570
Metal Damage Mechanisms of Total Ankle Replacements
*Shona Haston - Newcastle University - Newcastle upon Tyne, United Kingdom
David Langton - ExplantLab - Newcastle upon Tyne, United Kingdom
David Townshend - Northumbria NHS Trust - North Shields, United Kingdom
Rohan Bhalekar - ExplantLab - Newcastle upon Tyne, United Kingdom
Thomas Joyce - Newcastle University - Newcastle upon Tyne, United Kingdom
*Email: S.Haston@newcastle.ac.uk
Introduction:
Total ankle replacement (TAR) has higher revision rates than other lower limb total joint replacements. Published explant analysis studies are limited and those to date have primarily focused on damage mode analysis of the polyethylene components. This explant analysis study aimed to characterise the damage mechanisms present on the metal tibial and talar components of various contemporary failed TARs in order test the hypothesis that appreciable metal debris is produced from these devices.
Methods:
The articulating and non-articulating surfaces of a cohort of 27 explanted TARs, comprised of 3 mobile bearing and 5 fixed bearing designs, were analysed visually using light microscopy as well as macroscopically. The talar components of all the explanted devices included were manufactured from cobalt-chromium alloy, and the tibial components were manufactured from either cobalt-chromium alloy (83%) or titanium alloy (17%).
Tibial and talar component articulating surfaces, along with the polyethylene inserts bearing and backside surfaces, were analysed for common damage modes. For metal components on which pitting was identified, non-contacting profilometry was performed on pitted and unpitted areas to confirm material loss. Scanning electronic microscopy with energy dispersive X-ray spectroscopy was performed to determine the composition of embedded debris identified in polyethylene inserts. The non-articulating surfaces of the metal components were analysed for changes to the porous coatings in terms of loss of coating and changes in reflectivity.
Results:
Pitting and scratching were the most commonly observed damage features. Microscopic analysis revealed pitting on the articulating surfaces of 95% of talar components and 52% of tibial components (Figure 1). Non-contacting 3D profilometry confirmed material loss from these pits, with significantly higher measured maximum valley depth values for pitted areas compared to unpitted areas (p<0.05).
Pitting was identified on more tibial components manufactured from cobalt-chromium alloy than titanium alloy (63% versus 0%), though abrasive changes indicative of micromotion at the fixed bearing interface was identified on one (20%) of the titanium alloy tibial component inferior surfaces.
Sliding plane scratching (i.e., linear scratches following the direction of the antero-posterior movement of the ankle) was visible macroscopically on the bearing surfaces of 78% of talar components. This scratching indicates the presence of hard third body particles at this articulation in vivo.
Embedded debris, determined to be cobalt-chromium and titanium via scanning electronic microscopy with energy dispersive X-ray spectroscopy, was identified on 19% of polyethylene inserts. Both bearing and backside surfaces of polyethylene inserts exhibited this embedded metallic debris.
The non-articulating surfaces of the metal components showed coating loss and/or changes in reflectivity in 80% of cases.
Conclusion:
This explant study identified common damage to the metal components of various explanted TAR in the form of pitting and scratching, particularly in cobalt-chromium alloy components. The findings indicate the presence of third body debris at the bearing, most likely accounted for by coating particles from the non-articulating surfaces of the metallic components. These findings demonstrate the phenomenon of metal debris release from contemporary TARs; a potentially under-recognised issue which should be considered in future study of failed TAR.
Figures
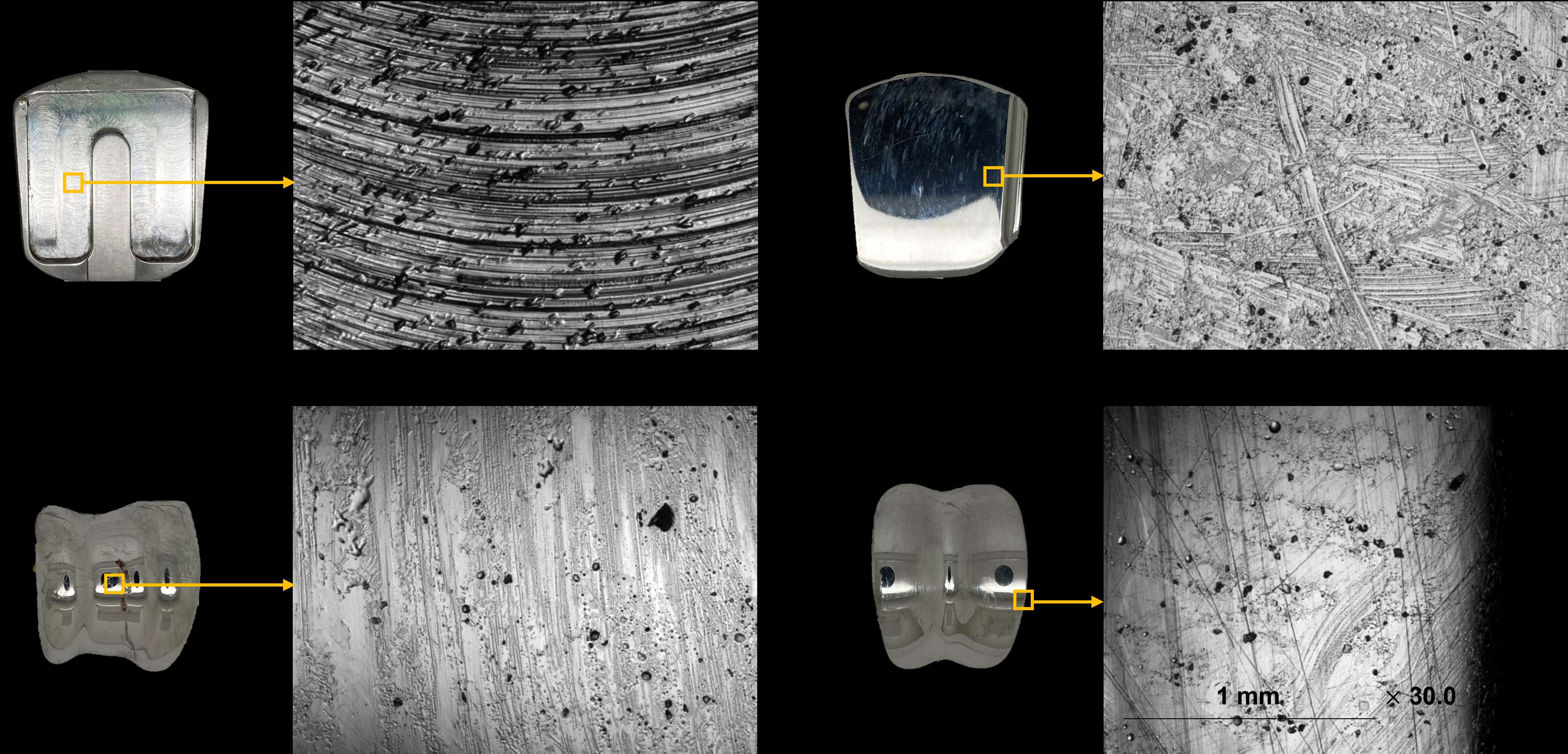
Figure 1#8518
Differences in Wear Modes, Debris Type, and Tissue Response Between Anatomic and Reverse Shoulder Arthroplasties
*Deborah Hall - Rush University Medical Center - Chicago, USA
Stanley Liu - Rush University Medical Center - Chicago, USA
Niraj Lawande - Rush University Medical Center - Chicago, USA
Gregory Nicholson - Rush University Medical Center - Chicago, USA
Grant Garrigues - Rush University Medical Center - Chicago, USA
Robin Pourzal - Rush University Med Ctr - Chicago, USA
*Email: deborah_hall@rush.edu
Introduction: Little attention has been given to potential long-term complications due to tissue reactions to implant debris, and potential loosening of the implant due to particle-induced osteolysis in total shoulder arthroplasties (TSA). Some recent studies have shown that implant wear with subsequent immune cell response does occur in TSAs. Here we examine a series of retrieved anatomic (aTSAs) and reverse (rTSAs) designs to determine extent of implant damage and to characterize the nature of the corresponding periprosthetic tissue responses and how these relate to implant loosening.
Methods: TSA components and periprosthetic tissues were retrieved from 63 patients (35 aTSA, 28 rTSA). None of the implants in this study were removed for infection. Damage to the implants was characterized using light microscopy. Head/stem taper junction damage was graded 1-4 as minimal, mild, moderate or marked. Metal bearing surfaces was graded 1-3 (mild, moderate, marked). Damage on polyethylene (PE) was given a score based on the summation of scoring for extent of wear scar, presence of polishing, 3-body wear, rim damage, delamination, and grooves and scratches for a maximum score of 14. H&E stained sections of periprosthetic soft tissues were evaluated for the extent and type of cellular response. A semi-quantitative system was used to score (1=rare to 4=marked) the overall number of particle-laden macrophages, foreign body giant cells, lymphocytes, plasma cells, eosinophils, and neutrophils. Implant damage and histopathological patterns were compared between the two TSA groups using the Mann-Whitney and Spearman tests.
Results: PE bearing surface scores were greater in the aTSA group (8.6±2.4, 4.8±1.7, p≤0.000). The PE bearing surfaces of aTSAs were dominated by 3-body wear and plastic deformation, whereas the rTSA PE components exhibited mainly polishing and scratching. Cases with severe rim damage occurred mainly due to delamination and plastic deformation in aTSA, and in rTSA due to notching (8 of 28 cases) (Figure 1). Metal surface damage occurred in a few cases in both groups. Only one aTSA case exhibited marked taper corrosion. Metal particles were seen in 77% of aTSAs and 93% of rTSAs. More bone cement and suture debris was seen in the aTSA group compared to the rTSA (p≤0.004). In both groups the primary nature of the inflammatory response was a moderate to marked macrophage response to wear particles (86% of cases). The particle-laden macrophages tended to occur in broad sheets and contained metal, PE, bone cement and suture debris. There was no difference in cellular response between aTSA and rTSA.
Conclusion: The principal difference in the design, fixation and biomechanical loading of the two TSA types resulted in different wear mechanisms and modes, and subsequently, different types and sizes of implant debris. Both groups exhibited a strong macrophage response to a combination of these different types of debris—PE, metal, bone cement and suture. Especially PE particles may differ in size between groups due to different acting wear mechanisms that depend on the design of the implant, but also the occurrence of rim damage, which may also affect the extent of macrophage response.
#8654
The Effects of Third Body Debris on an Alternative Implant Material in Total Knee Arthroplasty
Isadora Guarino - Dartmouth College - Hanover, United States of America
Thomas Cisneros - University of New Mexico - New Mexico, USA
Afton Limberg - Dartmouth College - Hanover, United States of America
Rebecca Thomson - Dartmouth College - Hanover, United States of America
*Douglas Van Citters - Dartmouth College - Hanover, USA
*Email: dvancitters@Dartmouth.edu
Introduction: Cobalt-chrome (CoCr) alloy has been the material of choice for TKA femurs, but concerns about patient hypersensitivity have led to the introduction of cobalt-alternatives to address mechanical and biocompatibility properties. Historically, titanium-based femoral components have not performed as well as cobalt components due to third body damage (Nasser CORR 1990; McKellop ORS 1980). The objective of this study is to compare damage and wear of bearing couples in a pin on disk test with the incorporation of third body debris representing worst case scenarios of liberated bone cement and porous coating beads.
Methods: UHMWPE pins (never irradiated GUR 1050) were articulated in a square pattern against (1) Wrought CoCr (CoCr), (2) N+ Ion-Implanted Ti6Al4V (N+ Ti), and (3) Ion Beam Enhanced Deposition TiN Ti (IBED Ti). Polyethylene cylinders were surfaced with a microtome and pre-soaked before being tested at a contact stress of 14 MPa for 1M cycles in dilute bovine serum. Bone cement chips (5 mm) and 1.5 mg of CP titanium beads (0.25 mm) were inserted in the articulation before starting to simulate worst-case in vivo conditions. Wear rate was determined by measuring polymer mass loss every 250k cycles. Metal puck damage was analyzed using white light interferometry.
Results: At 1M cycles the CoCr puck exhibited the least damage, as indicated by the peak-to-valley height of the material surface, under both bone cement and titanium debris conditions (2.27µm cement, 2.41µm CP-Ti). In contrast, IBED Ti demonstrated increased damage with bone cement, measuring 8.35µm, followed by N+ Ti with 6.48µm. N+ Ti exhibited the highest overall damage with CP-Ti debris, measuring 24.31µm, followed by IBED Ti with 4.78µm. Fig. 2 shows pins articulated against the N+ Ti experienced the greatest material loss under both conditions. Steady state wear rates for pins articulating against IBED Ti and CoCr were statistically similar under both debris conditions. Mass loss may be attributed to a high kurtosis value associated with puck damage, as the overall surface roughness values remained below 1 µm for all articulating conditions.
Conclusions: The CoCr coupon exhibited the lowest damage, as indicated by PV and Sa, under bone cement and titanium debris conditions. While this finding suggests that CoCr implants may possess superior damage resistance compared to titanium-based materials it should be cautioned that the material in the wrought condition may be harder than the typical cast condition found in femurs. In vivo damage is to be expected, and under bone cement debris conditions the N+ Ti and IBED Ti both showed damage consistent with retrieved CoCr femurs. When simulated liberated CP-Ti beads are introduced, the IBED Ti exhibited minimal additional damage while the N+ Ti exhibited extensive damage. Polyethylene wear under all conditions was low for CoCr and IBED Ti to the point of being statistically indistinguishable. In all cases the N+ Ti showed significantly higher wear rates. In this test, the IBED Ti surface appears robust in the face of expected third-body debris and does not result in significantly increased wear of the polymer pin.
Figures
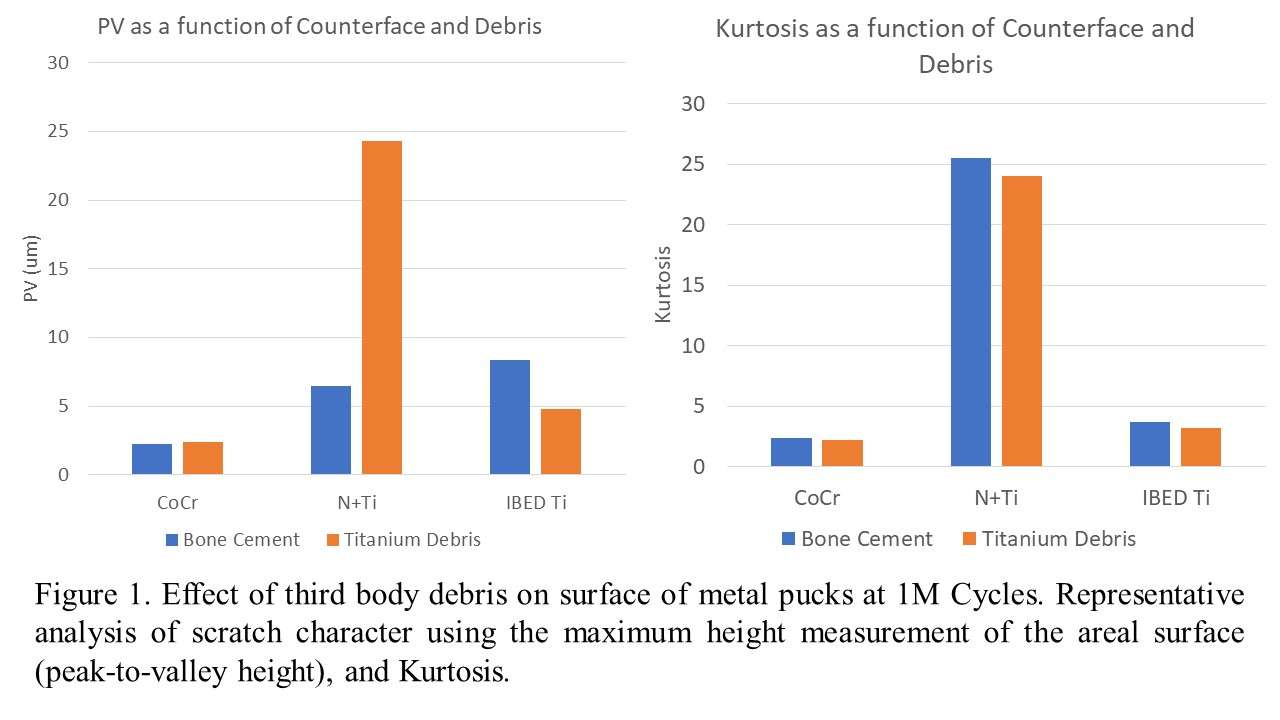
Figure 1
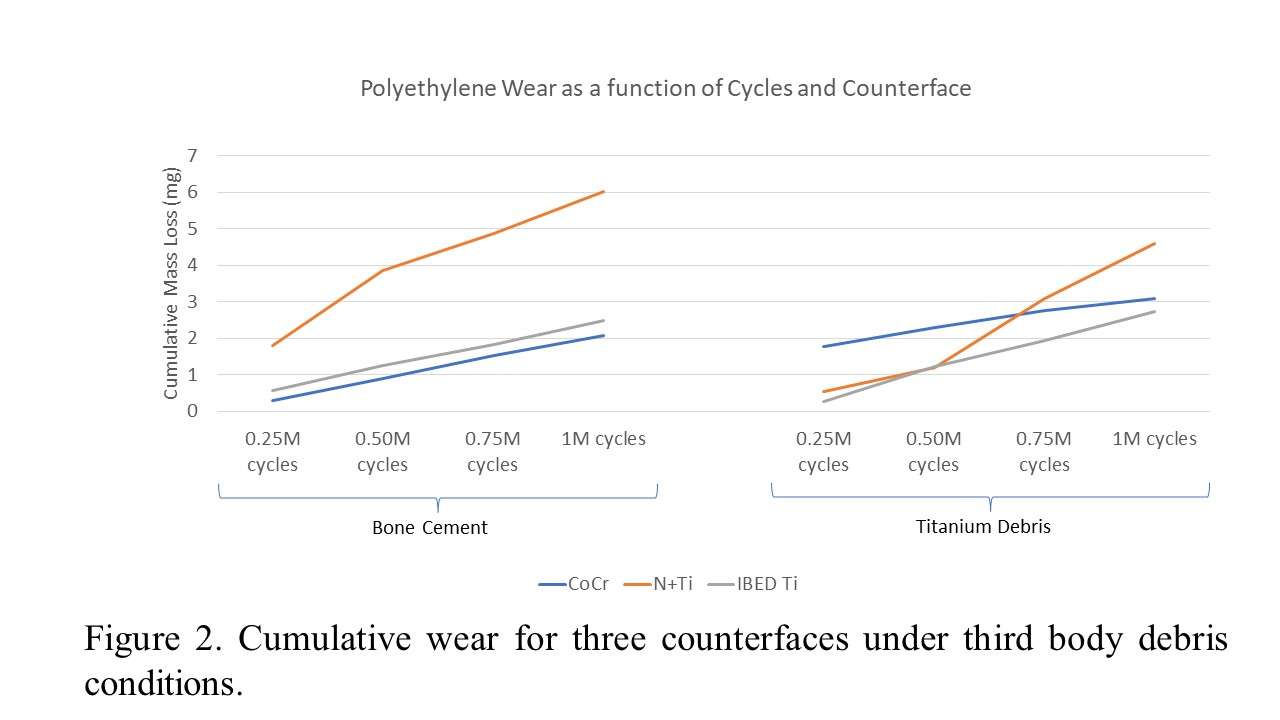
Figure 2#8231
In Vitro Wear Behavior of Knee Implants at Different Load Levels: The Impact of the Test Fluid
*Stefan Schroeder - Heidelberg University Hospital - Heidelberg, Germany
Maximilian Uhler - Laboratory of Biomechnics and Implant Research - Heidelberg, Germany
Mareike Schonhoff - Laboratory of Biomechanics and Implant Research - Heidelberg, Germany
Timo Nees - Heidelberg University Hospital - Heidelberg, Germany
Tanja Wonerow - Helmut-Schmidt-University - Hamburg, Germany
Jens Nuppnau - Helmut-Schmidt-University - Hamburg, Germany
Frank Mantwill - Helmut-Schmidt-University - Hamburg, Germany
Philippe Kretzer - Laboratory of Biomechanics and Implant Research, University of Heidelberg - Heidelberg, Germany
*Email: Stefan.Schroeder@med.uni-heidelberg.de
In vitro knee wear simulation is a suitable method to investigate the wear behavior of different knee implants. Implant materials as well as implant designs can be optimized using this approach without the necessity of any patients’ involvement. According to the ISO standard, calf serum with a protein content of 20 g/l is used for knee wear simulations acting as synovial fluid replacement. However, in contrast to natural knee joints, in which the synovial fluid is constantly renewed, the test fluid of in vitro knee wear simulations stays typically in the test chamber for 0.5x106 cycles. Previously, it was demonstrated that the rheological properties of the calf serum changes during different test durations, which might be due to denaturation and degradation of proteins. These findings raise the question, if the rheological properties of the test fluid will vary, when different axial loads are applied with the same kinematics and if this may have an impact on the wear rate.
A displacement-controlled AMTI knee simulator was used for the comparison of three different load levels. For the medium load curve a maximum axial load of 2600 N (100%) was applied according to the ISO standard. For simulating a low and a high load level, the load curves were compressed and expanded to maximum loads of 1300 N (50%) and 3900 N (150%) respectively. The flexion-extension, anterior-posterior translation and internal-external rotation were equal for the three conditions. The total test duration of each group was 2.5x106 cycles and gravimetric wear measurements of the polyethylene inserts were performed every 0.5x106 cycles. Rheological measurements of the test fluids after a test interval of 0.5x106 cycles were performed for the three groups and from raw serum at zero cycles as reference using Physica MCR 702 rheometer to investigate dynamic viscosity.
The lowest gravimetric wear rate was found for the lowest load level (50%). Interestingly, the medium (100%) and high (150%) load level showed similar wear rates, with a slightly lower wear rate for the high load level (see Figure 1). When comparing the rheological results, the load level 100% showed a slightly lower dynamic viscosity than the 50% load level. The 150% load level led to the highest dynamic viscosity (see Figure 2). Only the reference test fluid showed a clear shear thinning behavior.
The results show that a load level of 100% led to a higher wear rate and a slightly lower dynamic viscosity than a load level of 50%. Surprisingly, the load level of 150% led to a slightly lower wear rate than the 100% load level but showed the highest dynamic viscosity. The high shear forces at the 150% load level might lead to an accelerated degradation and denaturation of the proteins resulting in a favorable tribological behavior. Future studies need to prove, if the protein-containing test fluid can replicate the in vivo situation or need to be replaced with an alternative synthetic test fluid.
Figures
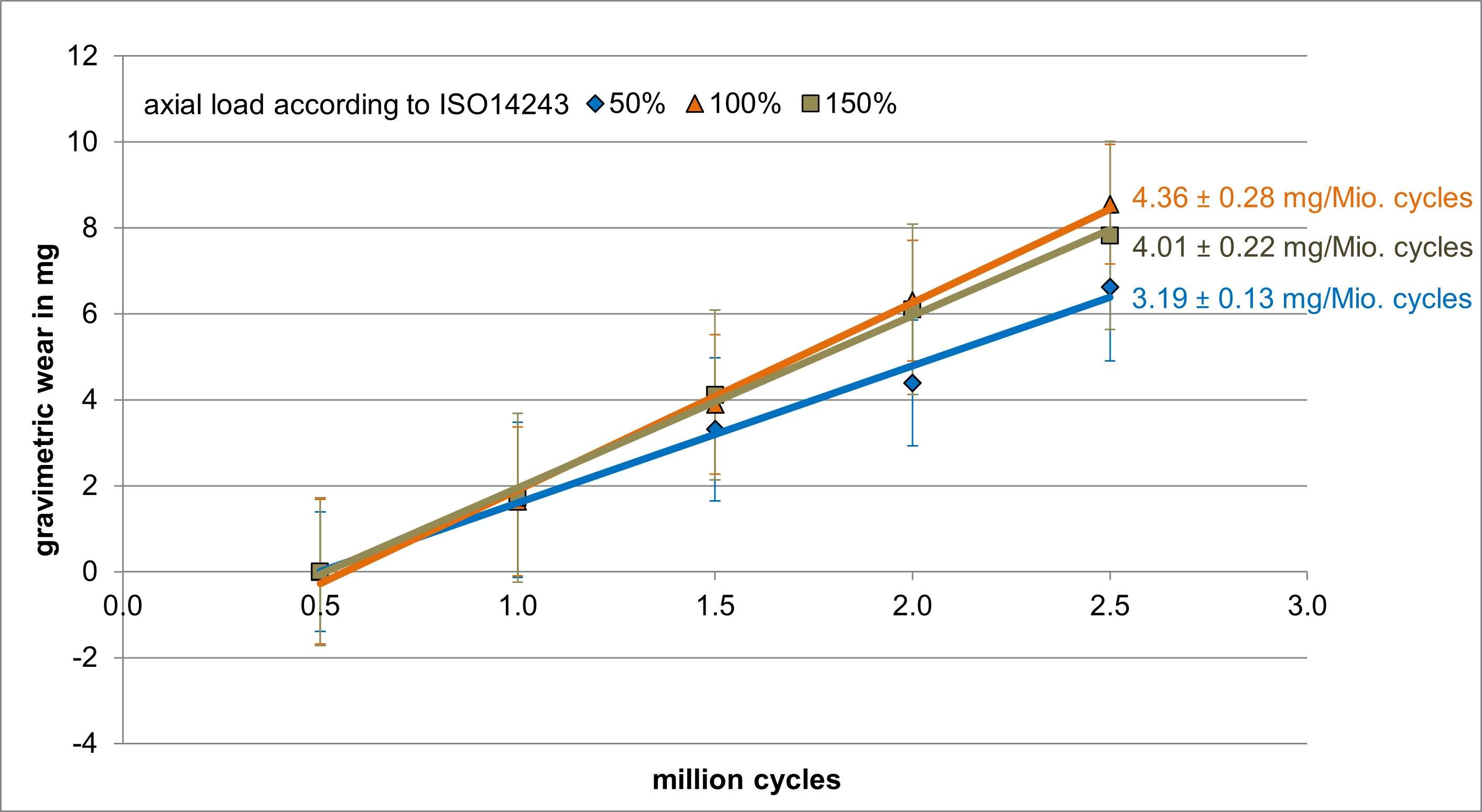
Figure 1
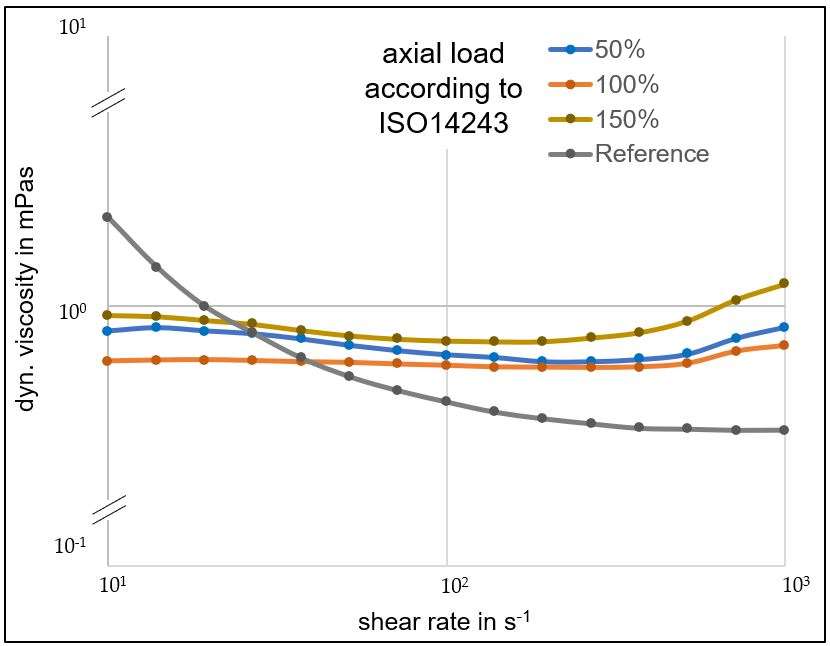
Figure 2#8622
Fretting/Corrosion of Titanium Alloy Dual Mobility Insert Taper With and Without IBED TiN Coating.
*Chris Weaber - TJO Inc. - Salt Lake City, USA
Dermott McHugh - Total Joint Orthopedics - Salt Lake City, USA
Eric Ouellette - Exponent - Philadelphia, USA
Tom Collier - Total Joint Orthopedics - Salt Lake City, USA
*Email: cweaber@tjoinc.com
Introduction: Cobalt chrome alloy dual mobility inserts, and titanium alloy acetabular shell taper interfaces may be susceptible to fretting/corrosion (1). The possible clinical effects of fretting corrosion have spurred interest in novel materials and designs that may further mitigate this risk. A novel ion beam enhanced deposition (IBED) TiN coating process has been developed for titanium alloy dual mobility insert components. This study aims to compare the taper interface fretting/corrosion performance of a titanium alloy dual mobility insert and titanium alloy acetabular shell, both with and without IBED TiN coating on the taper interface of the insert.
Methods: Three dual mobility inserts with IBED TiN coating on the taper interface, and three inserts without IBED TiN coating on the taper interface were tested in a short-term incremental cycle fretting corrosion test. Prior to assembly with an acetabular shell and testing, all taper surfaces were documented using a Keyence VHX-6000 digital optical microscope at 5X and 30X magnification. Acetabular shells were potted into a test fixture, and the inserts were assembled into the shells per ASTM F2009-20 with 0.05mm/s displacement rate to a peak load of 2kN, using a spherical indenter sized to the ID of the insert. Assembled constructs were submerged in PBS, held at 37 °C and the tapers (working electrode) were held potentio-statically at -50mV vs. Ag/AgCl. Transient current following voltage application was allowed to equilibrate for one hour before cyclic loading. Cyclic loading was applied (Sinusoidal, 1Hz, R=0.1, 600 cycles per increment) with increasing maximum load increments of 100N from 500N-1000N, and increments of 200N from 1200N-5000N of maximum load (total of 15,600 cycles). Fretting current was recorded continuously throughout the fretting corrosion test. After testing, tapers were disassembled, rinsed with de-ionized water and photo documented.
Fretting Corrosion testing was performed by Exponent (Philadelphia, PA)
Results: As shown in Figure 2, uncoated tapers showed, in general, slightly lower currents than coated tapers at the 3kN and 5kN load. Figure 1 also shows that uncoated tapers generally had minimal fretting currents compared to the TiN coated tapers. Two of the three coated samples showed increasing fretting currents with increasing loads. Conversely none of the uncoated samples showed an increasing trend. Additionally, a detectable instantaneous fretting current response was evident from coated constructs, whereas uncoated samples did not show cycle to cycle current peaks. Localized regions or bands of surface abrasion were noted, consistent with asperity-asperity contact. There was no visual sign of corrosion damage by way of pitting, mechanically assisted corrosion, or other mechanisms.
Conclusion: The short-term nature of this test is not expected to reveal significant damage to tapers; however, the results suggest less fretting current of the uncoated dual mobility inserts and titanium alloy acetabular shell taper interfaces.
These data suggest that an as-machined finish on the dual mobility titanium alloy inserts and titanium alloy acetabular shell taper interface may prove to be a minimally corrosive combination over the long term in an in vitro test environment. Long-term testing is needed to confirm this hypothesis.
Figures
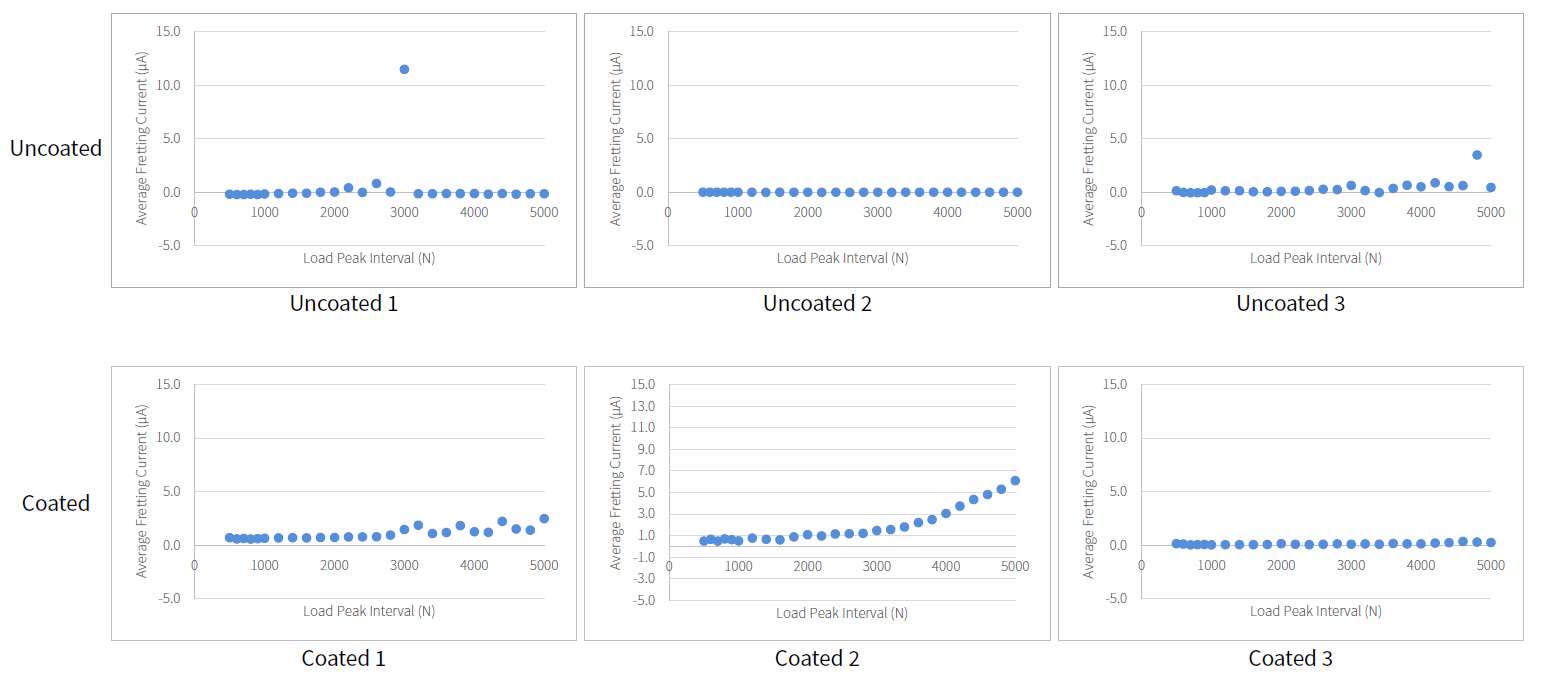
Figure 1
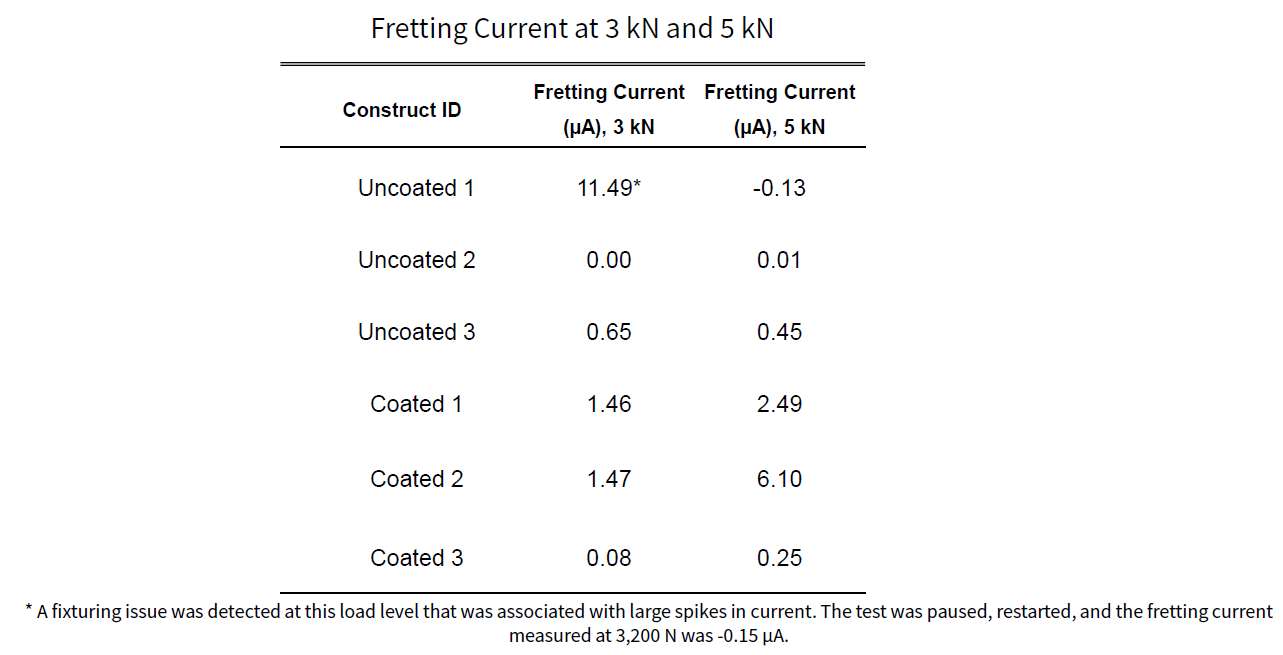
Figure 2#8400
Understanding the Tribology of an All-Polymer Total Knee Replacement
Raelene Cowie - University of Leeds - Leeds, United Kingdom
Adam Briscoe - Invibio Ltd - Lytham St. Annes, United Kingdom
*Louise M. Jennings - University of Leeds - Leeds, United Kingdom
*Email: l.m.jennings@leeds.ac.uk
Introduction
Over the last 10 years investigations into the suitability of PEEK-OPTIMA™ polymer (unfilled PEEK) as the femoral component material of total knee replacements in terms of its wear performance have been carried out. Initial investigations in a simple geometric pin-on-plate configuration were used to systematically investigate different parameters relating to the tribology of the bearing couple before moving to whole joint simulation of the tibiofemoral and patellofemoral joints, including investigation of adverse conditions such as third body wear.
Methods
Pin-on-plate simulation was used to understand the optimum environmental conditions (lubricant protein concentration and temperature) under which to investigate the device, and to investigate the influence of cross-shear and contact pressure on wear. Full joint simulation of the tibiofemoral and patellofemoral joints was carried out in a 6 station Prosim Electromechanical knee simulator under conditions of optimal joint alignment in a clean lubricant. To start to understand the influence of more adverse conditions, third body wear studies using porcine cortical bone and PMMA cement were performed. All total joint wear simulation was carried out under displacement controlled conditions [1,2]. The wear of the all-polymer bearing couple was compared to conventional materials cobalt chrome-on-UHMWPE. In the joint simulation studies, the cobalt chrome implants were of similar initial surface topography and geometry and the investigations of the all-polymer and metal-on-polyethylene implants were carried out side-by-side. Wear was determined gravimetrically. Data are expressed as mean ± 95% confidence limits, statistical analysis was carried out to compare PEEK-OPTIMA™ to cobalt chrome using ANOVA with significance taken at p<0.05.
Results
Pin-on-plate wear simulation in 25% bovine serum (16 g/l) carried out under room temperature conditions minimised test artefacts that were otherwise associated with no protein in the lubricant, high protein conditions and elevated temperature (~35°C). These parameters were used throughout. With increasing contact pressure, there was a decrease in wear factor, consistent with studies of UHMWPE-on-cobalt chrome [3] (Figure 1). Under uniaxial motion, wear was very low, introducing rotation increased the wear factor, a similar trend to cobalt chrome but with lower values [3] (Figure 2). There was no significant difference in the wear rate of UHMWPE against PEEK-OPTIMA™ or cobalt chrome in either the tibiofemoral or patellofemoral joints when tested in clean lubricant or lubricant contaminated with third body particles of bone or PMMA cement (Figure 3). However, when investigated with PMMA cement, extensive scratching was visible on both femoral component materials, particularly PEEK-OPTIMA™ and lubricant discolouration occurred in the cobalt chrome-on-UHMWPE bearing couple.
Conclusion
The pin-on-plate simulation has shown similar wear trends of UHMWPE against PEEK-OPTIMA™ and cobalt chrome under different contact pressure and cross-shear conditions. In the whole joint wear simulation studies carried out to date, the wear of UHMWPE against PEEK-OPTIMA™ has been similar to (p>0.05) UHMWPE against cobalt chrome in both the tibiofemoral and patellofemoral joints, including under third body conditions in the tibiofemoral joint.
1.McEwen, H.M.J., et al.2005.J Biomechanics 38(2):357-365.
2.Maiti, R., et al.2014.JOEIM 228(2):175-181.
3. Abdelgaied, A., et al.2018 JMBBM 78:282-291.
Figures
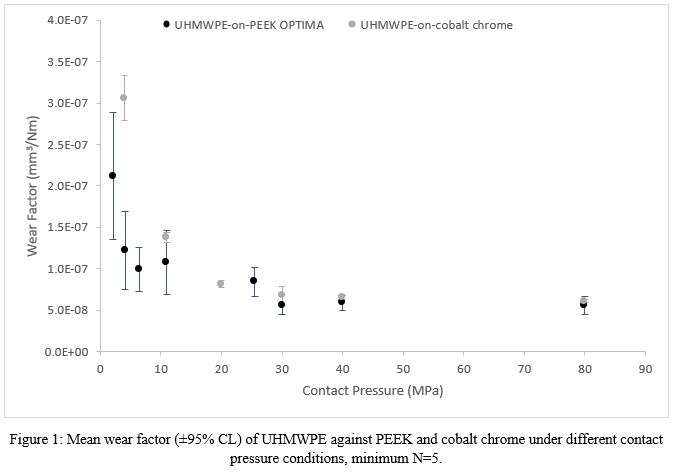
Figure 1
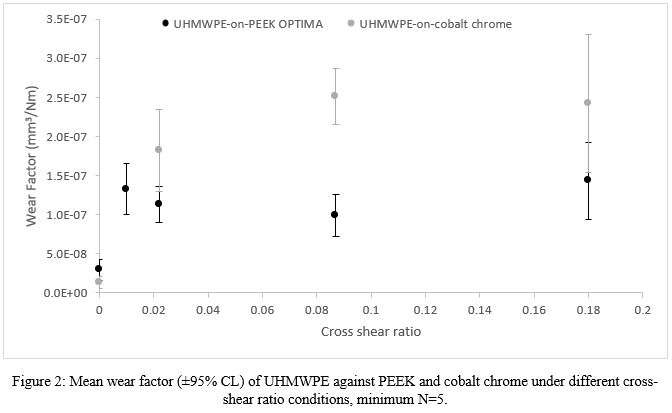
Figure 2
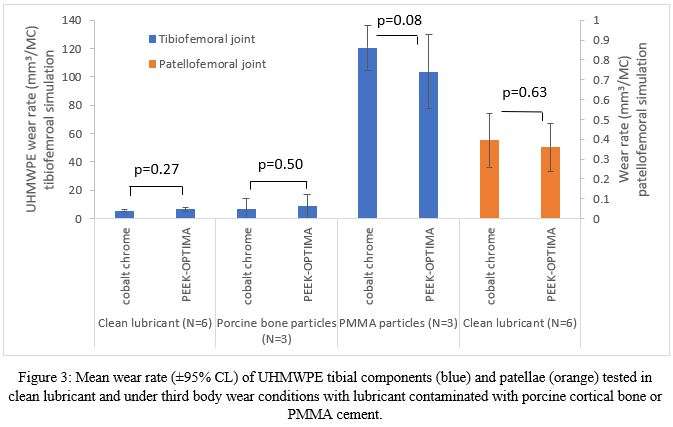
Figure 3#8783
Bearing Science (Non-)Friction: How Bovine Serum and Its Concentrations Compare to Friction With Human Synovial Fluid
Alexander Eischeid - University of Nebraska Medical Center - USA
Joel Weisenburger - UNMC - Omaha, USA
*Hani Haider - UNMC - Omaha, USA
*Email: hhaider@unmc.edu
INTRODUCTION:
Arthroplasty implant bearing wear results from friction, so accurately characterizing friction of bearing couples is important. Given the likely correlation between forces of friction on the wear rates of implant bearing couples, understanding of the factors that affect friction will help guide further innovation. In this study, we present comprehensive testing of the effects on friction coefficient of varied orientations and serum protein concentrations, and compare them to human synovial fluid .
METHODS:
A friction measuring machine was custom-built, in a pin-on-disk configuration, allowing a very large dynamic range of stresses to be imposed. Generated friction was measured very accurately, minimizing frictional errors within the machine by utilizing air-bearing technology. A disk of highly polished CoCr (Ra: 0.04 ± 0.01 µm) was rotated under a 6 mm diameter ultra-high molecular weight polyethylene (UHMWPE) pin machined from GUR-1020 bar stock (25-40 kGy gamma irradiated, non-crosslinked). Bovine serum samples were placed on the disk for lubrication (see details below). Pin assembly movement, permitted by a frictionless air bearing, was counteracted by a torque cell-connected variable lever arm. Friction coefficient was determined from frictional forces paired with axial load measurements logged in real time and averaged. With constant linear pin sliding speed (25 mm/s) and stress (10 MPa), each pin was placed in 0°, 90°, and 180° relative axial rotations (orientations) to capture any systematic variations in UHMWPE molecular chain orientations. Diluted bovine calf serum was used as lubricant (GE Healthcare, Logan, UT) with ethylenediaminetetraacetic acid (EDTA) (7.45 g/L, Fischer Scientific, Hampton, NH). The serum was diluted with deionized water to various set protein concentrations (10 g/L, 20 g/L, 30 g/L, 40 g/L, and 54 g/L (undiluted), and a control of pure deionized water all tested at 22°C (room temperature). In comparison, 9 human synovial fluid samples from 8 patients receiving total knee arthroplasty (IRB approved) were tested at the same compressive stress, temperature, and articulation speed as above.
RESULTS:
A decrease in dynamic friction coefficient resulted (Fig. 1) as serum concentration increased (0.0578±0.0060 at 0 g/L to 0.0370±0.00293 at 54 g/L). UHMWPE orientation was determined to have no significant effect on friction coefficient for three tested pins (p = 0.60, 0.84, 0.96). Human synovial fluid testing (Fig. 2) showed an average friction coefficient of 0.0487±0.00488 (ranging 0.0427-0.0581), close to serum with protein concentration between 30 and 40 g/L. Thus, synovial fluid produces less friction compared to current ISO implant wear testing standard concentrations of 20 g/L (0.0516 ± 0.00411) for knee tests and 30 g/L (0.0493 ± 0.00277) for hip tests.
CONCLUSION:
Current ISO testing standard concentrations for hip and knee testing are realistic if not relatively harsh, leaning towards patients with poorly lubricating synovial fluid, thus supporting their continued use in testing protocols.
Figures
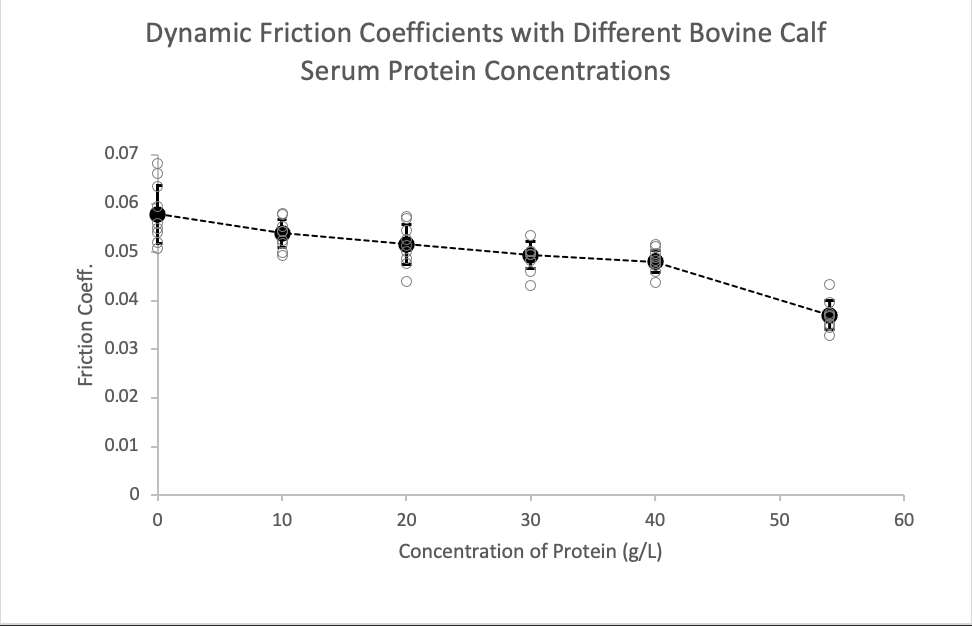
Figure 1
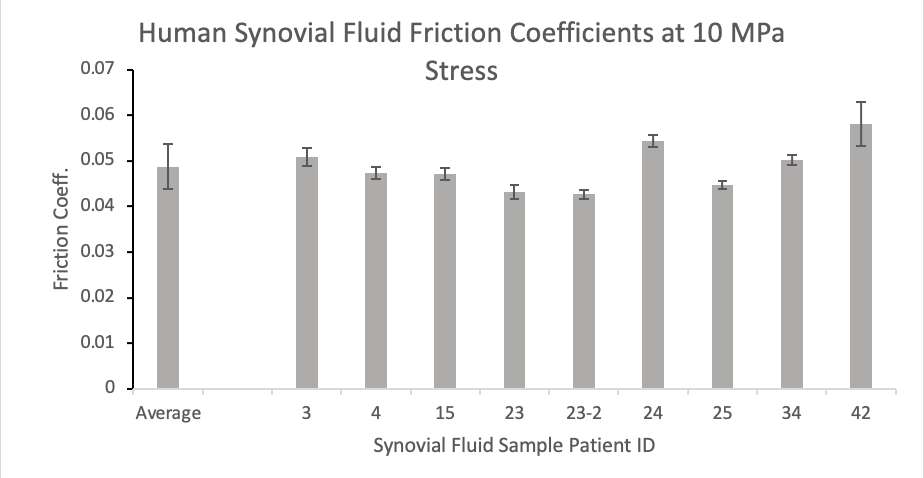
Figure 2#8794
Performance of an ADLC-on-Titanium Femur Under Abrasive Preclinical Testing Conditions
*Ryan Siskey - Exponent - Philadelphia, USA
Alex Avendano - Exponent - Philadelphia, USA
Lisa Lallo - Exponent - Philadelphia, PA, USA
Ruth Heckler - DJO Surgical - Austin, USA
Kapil Raghuraman - Enovis - Ausitn, USA
John Vinciguerra - DJO Surgical - Austin, USA
*Email: RSISKEY@EXPONENT.com
Introduction
Advancements in modern ADLC coatings make them an attractive solution for providing wear resistance in orthopaedic total joint applications. A prior study found ADLC remains intact and relatively unchanged through 5 MC of clean wear testing. Abrasive conditions resulted in increased UHMWPE wear rates that reduced when the abrasive condition was removed. The ADLC femoral components were intentionally damaged during the abrasive phase to challenge the bearing couple, but this prior work suffered from limited characterization of the femoral component and was primarily focused on the wear of the UHMWPE components. The aim of this study was to evaluate the tribological performance of the ADLC femoral component under abrasive pre-clinical conditions. Additionally, new methodologies for characterization of damage and damage progression under wear scenarios are explored.
Materials and Methods
A total knee replacement system consisting of a crosslinked and vitamin E stabilized UHMWPE tibial bearing and, previously tested and intentionally damaged, ADLC-coated titanium femoral was evaluated. The methods prescribed in ISO 14243-1 were used to guide the input parameters for the test. All components were cleaned and dried, photodocumented, and weighed for mass loss throughout the test. Samples were also characterized using SEM, interferometry, and microCT to document the damage modes and effects of abrasive damage. Surface mapping of the articulating region was undertaken using a Keyence VR5200 surface profilometer before and after testing. The resulting surface maps were compared from the beginning and end of the test to assess the penetration and estimated volumetric material loss of the femoral components.
Results
Consistent with prior testing, the UHMWPE tibial inserts demonstrated evidence of microabrasive damage modes typical of UHMWPE-on-hard bearings. The existing damage to the ADLC coating demonstrated no evidence of coating breakdown or progression of the previously created abrasive damage. The gravimetric analysis, photodocumentation, SEM, and surface penetration measurements confirmed the intentional damage of the femoral component showed minimal to no additional titanium substrate exposure in the region of contact. However, the femoral component mass loss measurements do not have sufficient resolution to assess component material loss and are at best a relative estimate of changes over the course of the test (Table 1). Surface penetration mapping was validated to be able to measure surface changes down to 2 µm of thickness change. Initial surface changes due to abrasive damage were found to be on the order of 10 µm, consistent with coating removal, and results after 2.0MC will be presented.
Discussion
Overall, the results suggest the ADLC remains intact through 2.0MC of abrasive wear testing and demonstrate wear mechanisms and wear rates consistent with now common vitamin-E inclusive cross-linked UHWMPE-on-hard bearing couples. While intentional abrasive damage increased the wear rate of the tibial inserts, it did not result in runaway wear and the resulting wear rates were comparable to conventional TKA under clean conditions. Most notably, a method to assess the femoral component with sufficient resolution to assess penetration and estimate volumetric loss was developed and validated.
Figures
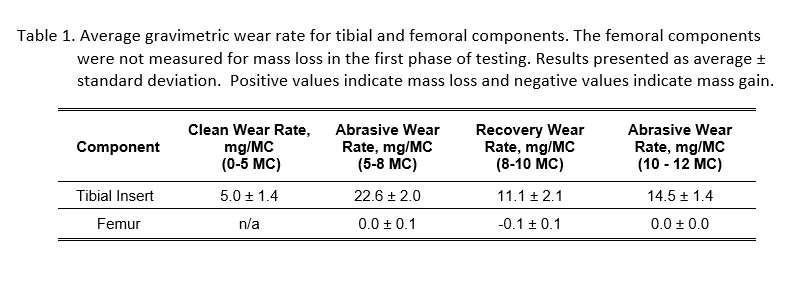
Figure 1#8228
In Vivo Hip Separation and Edge Loading for Novel Neutral and Lipped Liner Types
*Michael LaCour - University of Tennessee - Knoxville, USA
Garett Dessinger - The University of Tennessee - Knoxville, USA
Jarrod Nachtrab - University of Thennessee - Knoxville, USA
Thang Nguyen - University of Tennessee - Knoxville, USA
Manh Ta - Stryker Corporation - Fort Lauderdale, USA
Richard Komistek - The University of Tennessee - Knoxville, USA
*Email: mlacour@utk.edu
INTRODUCTION
Hip instability has long been an area of research for total hip arthroplasty (THA), as dislocation is one of the leading causes of failure and revision surgery. Other aspects of hip instability, such as hip separation, micromotion, edge loading, and impulse loads, can lead to decreased contact areas, increased contact stresses, impulse loading conditions, and ultimately increased wear rates. To reduce dislocation rates, lipped liners have been designed with an elevated portion of the rim intended to maintain greater contact area within the liner. The objective of this study is to investigate the effectiveness of lipped liners using mathematical modeling to compare the hip separation, edge loading, and overall stability mechanics of a lipped versus neutral liner.
METHODS
This study uses a previously validated mathematical model of the hip joint. Ten subject-specific bones were input into the model, shown in Figure 1. The model was used to evaluate stance phase of gait for each subject. For all simulations, the stem was placed in the algorithm-determined “ideal” position, with the goal of placing the implanted femoral head as close to the preoperative anatomical femoral head as possible. This initial position of the cup/liner was established as the “0 mm medialization offset” position. The cup/liner was then shifted by 4 mm, 8 mm, and 10 mm into the acetabulum in both the supero-medial direction (blue arrow) and the pure medial direction (orange arrow), shown in Figure 2, to simulate intraoperative acetabular offset changes. All analyses were conducted for both a novel EMPHASYS THA lipped liner and neutral liner (DePuy Synthes, Warsaw, IN).
RESULTS
For cup shifting in the super-medial direction, the two liner systems showed negligible differences at 0 mm and 4 mm offset. However, the lipped liner showed a reduction in hip separation of 0.3 mm ± 0.2 mm at 8 mm offset and 0.5 mm ± 0.2 mm at 10 mm offset. Both of these differences were statistically significant (p = 0.02 and p < 0.0001, respectively), indicating that the lipped liner yielded reductions in hip separation and micromotion compared to the neutral liner.
More significant than the separation reduction, the lipped liner showed an overall reduction in edge loading compared to the neutral liner (Figure 3). In these cases, the contact area was reduced by 66 mm² ± 24 mm² at 4 mm offset (p < 0.0001), 109 mm² ± 54 mm² at 8 mm (p = 0.0002), and 82 mm² ± 55 mm² at 10 mm offset (p = 0.001). These changes correspond to improvements in contact area as high as a 33%.
DISCUSSION
For all subjects in this study who experienced hip separation due to component medialization, the lipped liner performed as well or better than the neutral liner, leading to statistical and clinical benefit. The findings in our study correlate well with registries that have documented that lipped liner dislocation or failure rates are less than neutral lines, even though lipped liners are often used in more difficult cases.
Figures
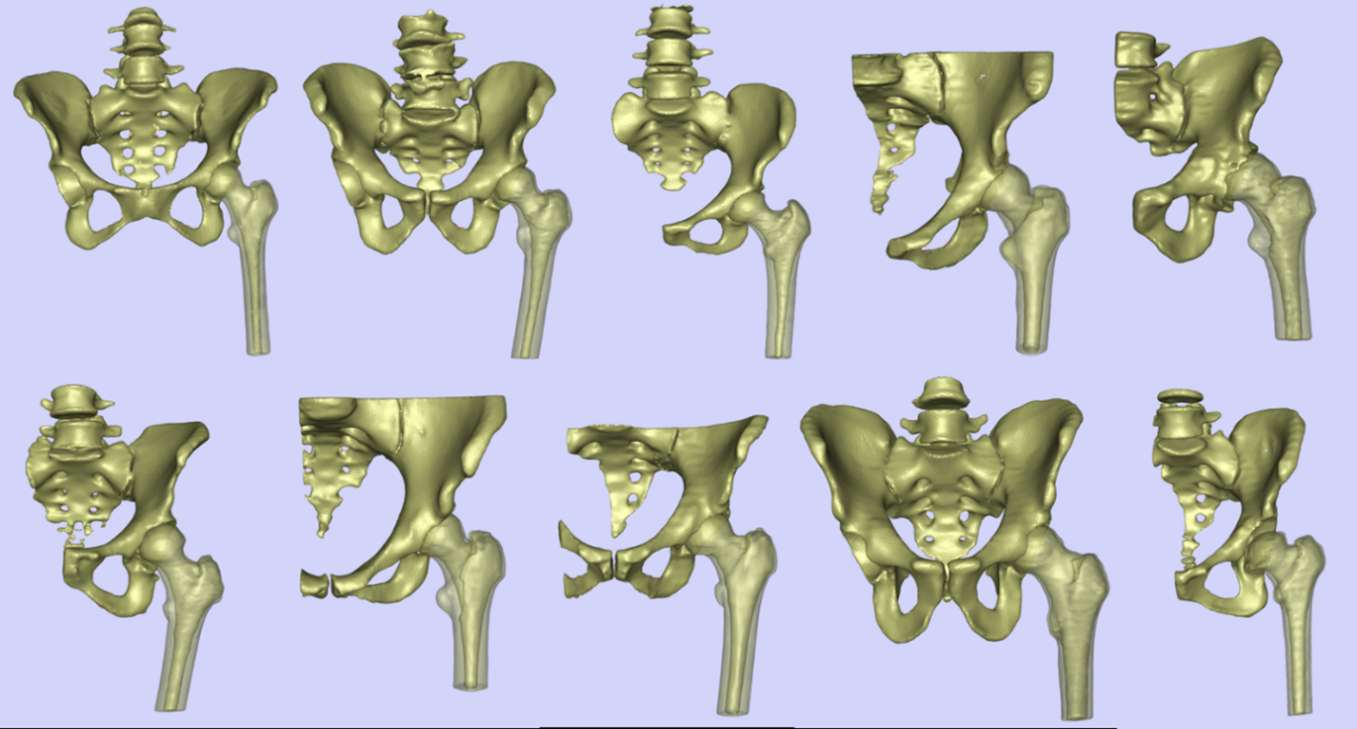
Figure 1
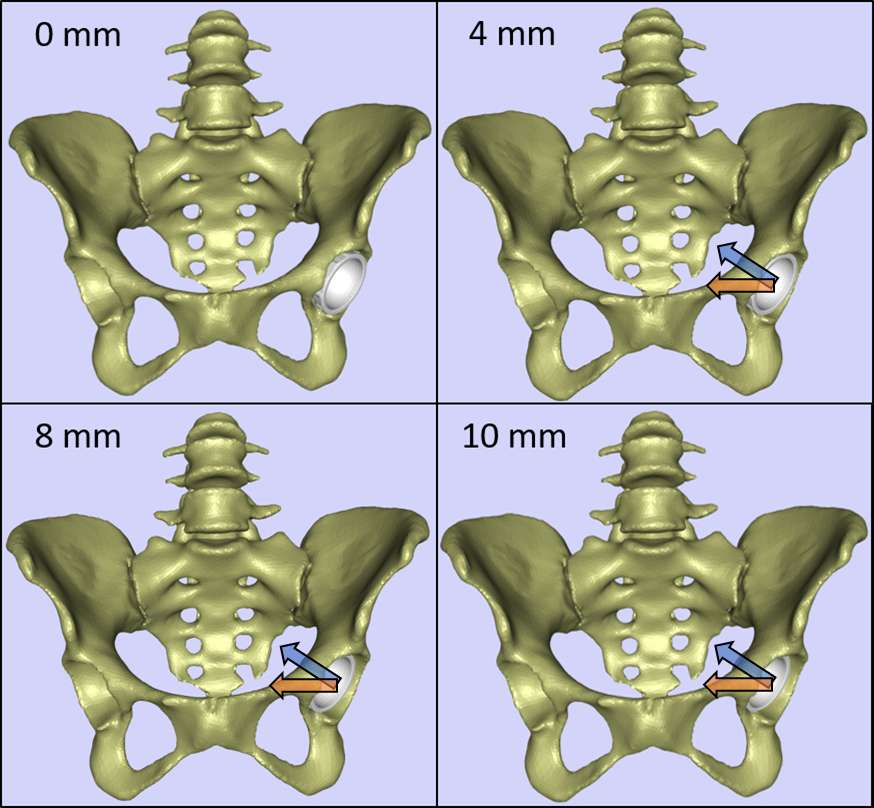
Figure 2
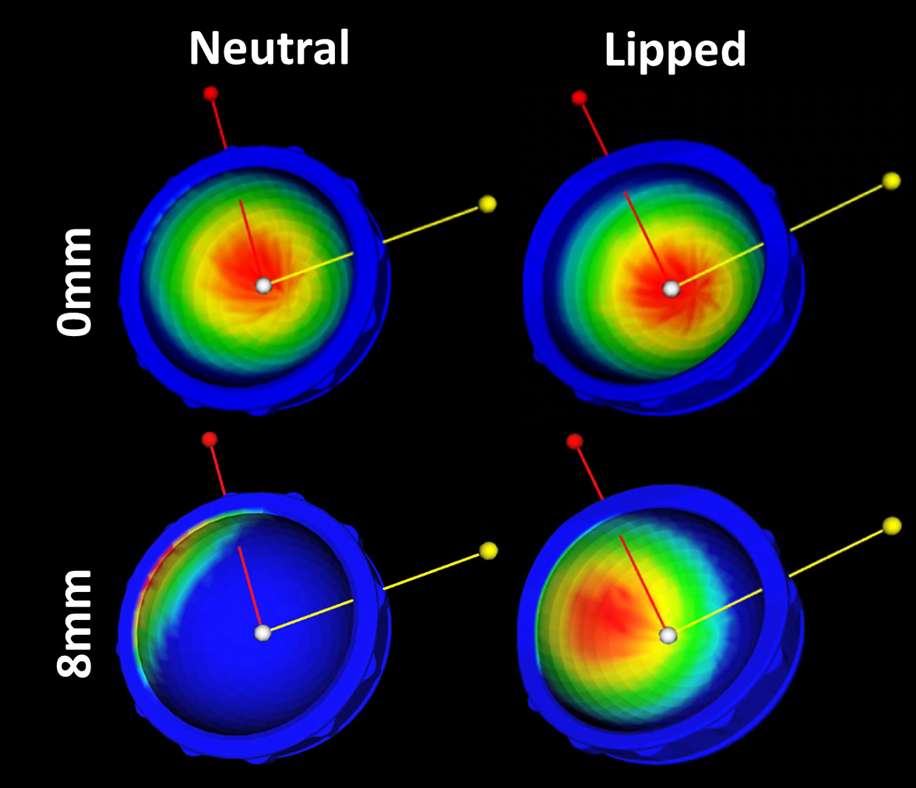
Figure 3#8510
Stress Shielding in Total Shoulder Arthroplasty: An Automatic Analysis of 3D Volumetric Filling Ratio on a Novel 3D-Printed Humeral Short Stem With an Open Design
*Andrea Fattori - Lima Corporate - Villanova Di San Daniele del Friuli, Italy
Axel Pavan - Limacorporate Spa - Cividale del Friuli, Italy
Cristiano Pizzamiglio - Limacorporate S.p.A. - Villanova Di San Daniele Del Friuli, Italy
Michele Pressacco - LimaCorporate Spa - Villanova di San Daniele del Friuli, Italy
*Email: andrea.fattori@limacorporate.com
Introduction. Total Shoulder Arthroplasty is an effective treatment for several degenerative conditions of the glenohumeral joint. Recently, short humeral stem designs have been introduced to preserve bone and simplify future revisions by leveraging the metaphyseal fixation while preserving the diaphysis; however, recent studies raised the concern about stress shielding (SS) observed with some of these devices. SS involves radiographic bone adaptation mainly resulting in proximal bone resorption, with risk of stem loosening or subsidence. Several authors showed that a higher risk of SS is associated with higher filling ratios (FR) of the stem in the humerus; therefore, an implant design that can guarantee primary stability while reducing the FR is key to prevent SS. The objective of this study is to assess the volumetric filling ratio (VFR) of a newly developed 3D-printed humeral short stem (PRIMA Stem, LimaCorporate) with an open design and a narrow distal tip aimed at reducing the FR, calculated with an automated procedure in several implant retroversion angles (RA).
Methods. Bone boundaries of a multi-ethnic sample of fifty-four humeri were segmented from CT-scanned images of both cadaveric specimens and living donors. Landmarks were automatically detected using the algorithm provided by the Scalismo library, while the open source-based FIGURA system was used to automatically perform virtual surgeries following the surgical technique (135° neck-shaft angle cut), and to evaluate the VFRs. Four values of RA were considered: anatomical, zero, twenty and thirty degrees. The VFRs were defined as the ratio between metaphyseal [diaphyseal] bone volume removed during surgery and the inner metaphyseal [diaphyseal] volume. The metaphyseal region extended from the resection level down to 40% of stem’s length, while the diaphyseal one included the remaining part of the stem [Fig.1]. Subjects with metaphyseal VFR lower than 0.38 were considered at low risk of SS (Celik 2020) and for each RA a one-tailed one-sample t-test was performed to assess whether the VFR is lower than that threshold. To the best of our knowledge, no threshold for the diaphyseal VFR is available, thus only a descriptive analysis was performed. For each VFR, a one-way repeated measures ANOVA, followed by post-hoc tests with Bonferroni correction, was carried out to understand whether the RA may affect the VFR.
Results. For all RAs, the metaphyseal VFR was approximately normally distributed, and its mean was statistically and practically lower than the SS threshold (p<0.001, δ>1.0), [Fig.2]. Specifically, the anatomical retroversion had the highest VFR (μ=0.35±0.03; δ=1.17). Regarding the diaphyseal VFR, the anatomical retroversion had again the highest sample mean (μ=0.17±0.03). As the RA decreases, the VFR decreased as well for both the metaphyseal (p<0.001, η2P=0.61) and diaphyseal (p<0.001, η2P=0.30) regions.
Conclusions. The results suggest that a stem with an open design and narrow distal tip may present lower risk of SS thanks to a reduced VFR; additionally, lower RAs may positively affect the VFR. While the methodology can help in assessing the risk of SS in different configurations, clinical data will be needed to confirm a lower incidence of SS with this novel stem.
Figures
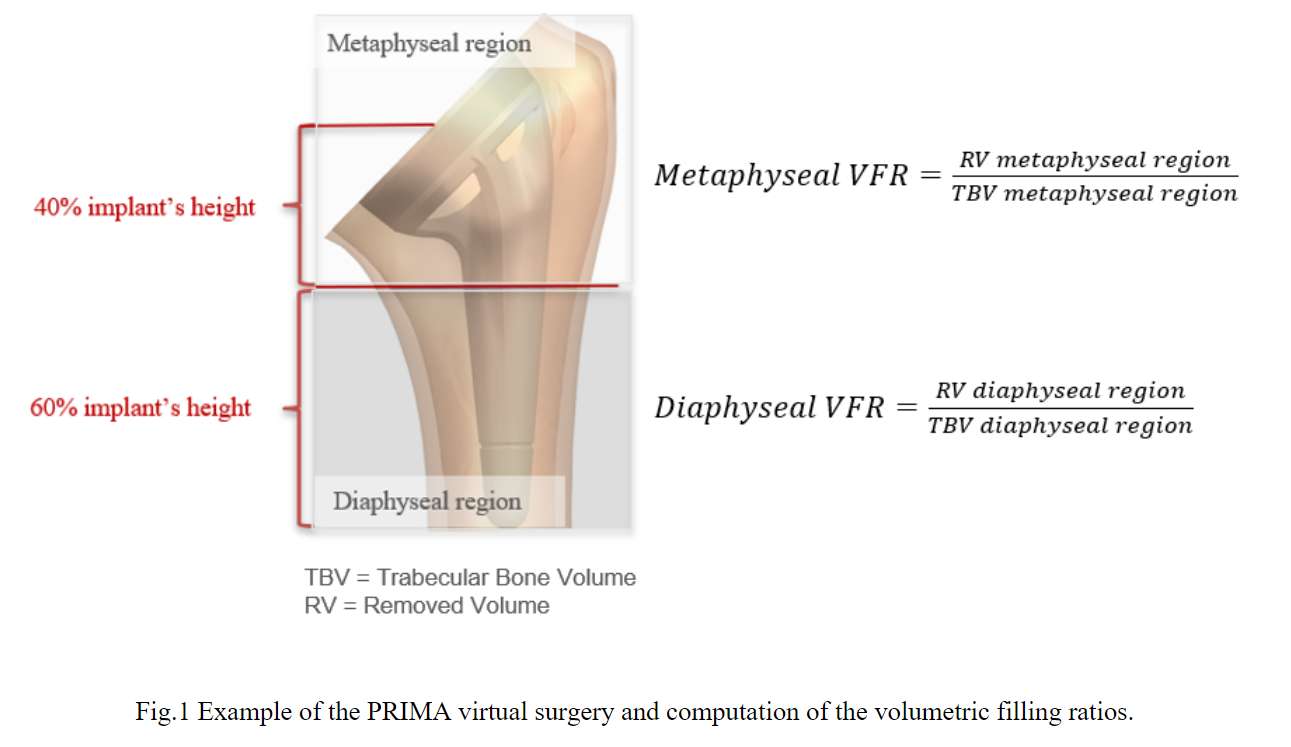
Figure 1
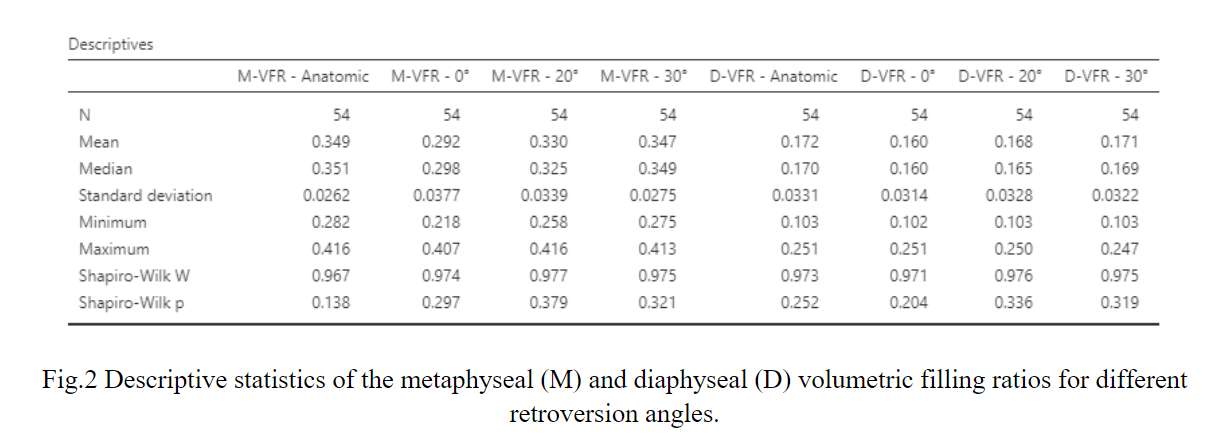
Figure 2#8422
The Effects of Glenoid Baseplate Peripheral Screw Number on Micromotion in Reverse Total Shoulder Arthroplasty
*Elise Martin - University at Buffalo - Buffalo, USA
Thomas Duquin - Univesity at Buffalo - Buffalo, USA
Mark Ehrensberger - University of Buffalo - USA
*Email: elisemar@buffalo.edu
Introduction: Glenoid baseplate loosening continues to be one of the leading causes of failure in reverse total shoulder arthroplasty. Oftentimes the glenoid baseplate is attached to the glenoid using a series of screws and therefore we wanted to examine how differing numbers of peripheral screws could impact stability of the glenoid baseplate. There are several potential advantages to maintaining the already limited bone stock available in the glenoid by reducing the screw number and therefore the goal of this study is to examine how many screws can be removed whilst maintaining adequate fixation.
Methods: Three constructs, each consisting of 10 samples, were implanted with a central screw (3.5x30mm) and differing numbers of locking peripheral screws: 4-screw, 2-screw (superior and inferior screw only), and 0 screw. The superior and inferior screws were 30mm in length and the anterior and posterior screws were 15mm in length. The samples were prepared by implanting in 15 pcf poly rigid polyurethane foam and embedded within polymethylmethacrylate (PMMA) to allow for a snug fit within the testing apparatus. An initial and final displacement test was conducted which applied a static shear (350N ± 15N) and normal (430N ± 15N) load while simultaneously measuring displacement along the axis of the two applied forces using two linear variable differential transducers. In-between the two displacement tests, a cyclic load pattern was applied by swinging the samples through an arc of abduction (+30 degrees to -15 degrees) at ¼ Hz for 10,000 cycles while maintain a normal load of 750N.
Results: The displacement measured in both the shear and compressive directions indicated an increase in displacement on average for the 0-screw condition compared to the 2-, and 4-screw configurations although not by a statistically significant amount. Additionally, there was a significant increase between the initial and final conditions for both the 4-screw and 0-screw constructs when examining both shear (4-screw: 67.88+/-32.14micron vs. 99.50+/-32.15micron; p=0.021, 0-screw: 95.55+/-57.01micron vs. 211.06+/-140.07micron; p=0.016) and compressive (4-screw: 107.17+/-36.27micron vs. 179.64+/-101.75micron; p=0.028, 0-screw: 135.85+/-120.61micron vs. 284.916+/-233.33micron; p=0.045) displacements. This trend does not follow for the 2-screw case for either shear (97.22+/-46.3micron vs. 113.25+/-79.92micron; p=0.295) or compression (113.00+/-81.74micron vs. 108.01+/-79.64micron; p=0.446) displacement. The average displacement values for both shear and compressive displacement are shown in figure 1. An additional feature of note is the excessively large error bars on the 0-screw case indicating a large amount of discrepancy case to case due to the instability of this construct.
Conclusion: A reverse total shoulder glenoid baseplate is stable with either four peripheral screws or just superior and inferior peripheral screws but becomes unstable and unreliable when all 4 peripheral screws are removed.
Figures
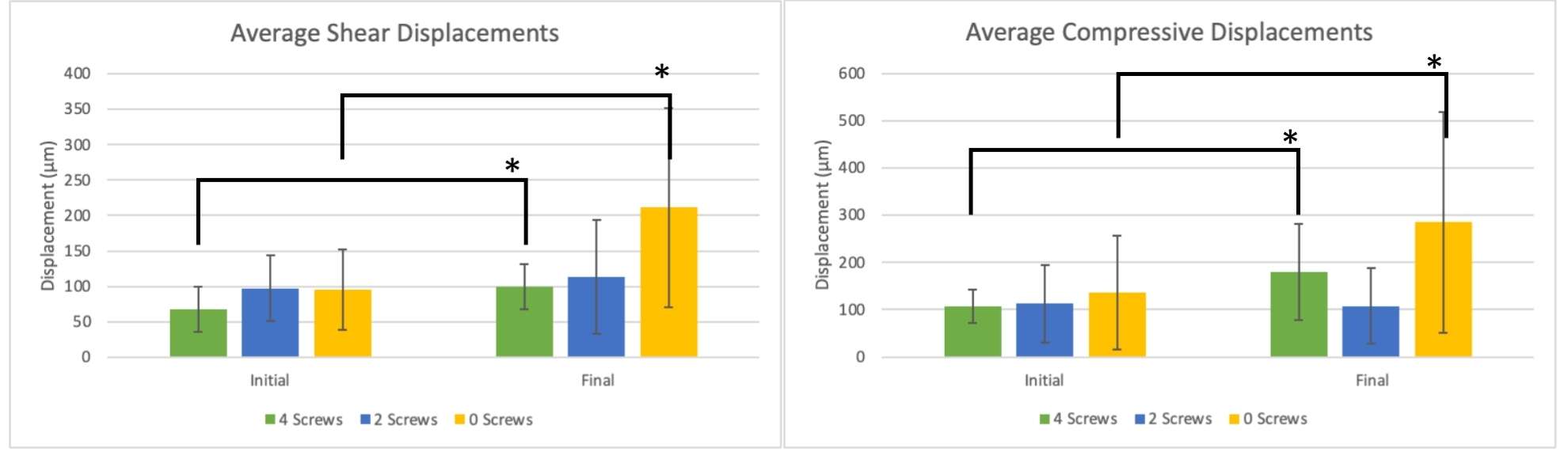
Figure 1#8798
Inferior Glenoid Component Inclination Can Reduce Shear Forces in Total Shoulder Arthroplasty
Jonathan Glenday - Hospital for Special Surgery - New York, USA
David Dines - Hospital for Special Surgery - New York, USA
Joshua S Dines - Hospital for Special Surgery - New York, USA
Samuel Taylor - Hospital for Special Surgery - New York, USA
Lawrence Gulotta - Hospital for Special Surgery - New York, USA
*Andreas Kontaxis - Hospital For Special Surgery - New York, USA
*Email: kontaxisa@hss.edu
Introduction: Total Shoulder Arthroplasty (TSA) is commonly used to alleviate pain and restore function in osteoarthritic (OA) shoulders. Despite the wide use of TSA, there are still concerns about glenoid fixation, stability and wear that may be a result of excessive glenohumeral shear forces. The common TSA surgical guidelines recommend that glenoid component placement should restore neutral version, but little is known how the inclination can affect muscle and joint contact forces. This study uses an established computerized shoulder biomechanical model to investigate how glenoid component inclination affects the biomechanics of TSA.
Methods: The Newcastle Shoulder Model (NSM) was used to calculate glenohumeral joint forces after TSA. The model consists of 6 rigid bones (thorax, clavicle, scapula, humerus, radius, and ulna), it has 31 muscles, and it simulates functional shoulder motions including clavicle and scapula kinematics. Five CTs from pre-op OA patients were utilized to customize the NSM and create 5 OA models. A shoulder orthopedic surgeon performed a virtual TSA to the models using a Biomet Comprehensive® Total Shoulder system with glenoid inclinations of 0°, 10°and 20° (3 configurations). To investigate how the integrity of the rotator cuff (RC) muscle may affect the results, two different RC setups were also tested; i) healthy RC and ii) attenuated superior cuff (supraspinatus cross sectional area reduced by 70%). Glenoid joint contact forces (analyzed in compressive and shear) and muscle forces were computed for abduction and scapula plane elevation motions.
Results: Glenoid inclination had a significant effect on glenohumeral joint contact forces (Fig. 1). Shear forces were decreased with 10° and 20° inferior inclination, compared to 0°, in the models with the healthy RC (p<0.001). The reduction of shear forces was even larger for the models with the attenuated superior RC muscles, where the 20° glenoid inclination showed the largest reduction of shear forces (44.2% compared to 0°, p<0.001). In general, the attenuated RC models showed higher shear forces compared to the healthy RC models, except from when the glenoid was placed with 20° of inclination (132N±84N and 132±84N for healthy and attenuated RC respectively, p=0.534)
Discussion/Conclusions: Overall, inferior glenoid component inclination in TSA can reduce glenoid shear forces, which often result in polyethylene wear or loosening. In this study, inferior glenoid inclination resisted the upward deltoid pulling and reduced the shear forces. However, 20° of inclination showed to benefit mostly the models with the attenuated supraspinatus muscle where the increased deltoid force resulted in increased glenoid shear force. Overall, the data suggest that inferior glenoid component inclination may be beneficial for TSA, and more aggressive inclination should be considered for patients with attenuated superior cuff muscles. However, this investigation did not take into consideration glenoid bone quality and how it may be affected by the inferior reaming. Future studies should also focus on finite element analysis to understand more on how the glenoid inclination can affect glenoid fixation in TSA.
Figures
Figure 1#8438
Humeral Neck Bone Mineral Density Is Systematically Affected by the Resection Height
*Ingmar Fleps - Warsaw, United States of America
Mirella Lopez Picazo - 3D-Shaper Medical - Barcelona, Spain
Ghislain Maquer - Zimmer Biomet - Winterthur, Switzerland
Christine Mueri - Zimmer Biomet - Winterthur, Switzerland
Taku Hatta - Joint Surgery Sports Clinic Ishinomaki - Ishinomaki, Japan
Ludovic Humbert - 3D-Shaper Medical - Barcelona, Spain
Jeffrey Bischoff - Zimmer, Inc. - Warsaw, USA
Philippe Favre - Zimmer Biomet - Winterthur, Switzerland
*Email: ingmar.fleps@zimmerbiomet.com
Introduction: Bone quality is a key parameter when deciding for stemless total shoulder arthroplasty. As there is often a discrepancy between the preoperative surgical plan and the reality of the OR [1]–[3], it is important to determine how surgical parameters influence the bone quality at the site of implantation. The objective of this study is to assess changes in bone quality related to resection plane inclination angle, retroversion angle, and height.
Methods: Proximal humeri (N=42) were segmented from 33 CT scans, and a baseline virtual resection was defined through the anatomical humeral neck. A retroversion of 25° was assumed for all humeri due to the lack of the distal condyles in the scan field of view. Surgical variation was then simulated by varying the resection plane inclination angle (α), retroversion angle (β), and height (x) (Figure 1) [1]–[3]. A total of 100 resections (design points) were simulated following a Latin hypercube sampling within the variable distributions. For each humerus, the largest cylindrical volume of interest (VOI) with a height-to-radius ratio of 1.35 (based on typical stemless implant dimensions) was determined that could fit within the metaphyseal, trabecular bone for all simulated resections. The mean BMD was calculated from the calibrated CT scans (B-MAS200, Kyoto Kagaku, Kyoto, Japan) in the VOI for each design point. The sensitivity of the CT-based BMD on the surgical variability was analyzed using a multivariate linear regression model that included α, β, x, and the BMD of the baseline resection plane (x=0, α=0, β=0) as independent variables.
Results: The mean deviation of the BMD in the VOI from the baseline BMD across all design points was 24.3 mg/cm3. Baseline BMD, resection height (x) and deviation in the retroversion angle were significantly associated with the measured BMD within the VOIs. The inclination angle did not significantly contribute to the model. Baseline BMD alone explained 67% of the variability of the BMD within the various VOIs (Table 1, Model 1), adding the resection height increased the explained variability to 95% (Model 2). Adding the retroversion angle deviation to the model (Model 3) marginally improved the explained variability by the model. The standard deviation of the error associated with Model 2 was below 9.6 mg/cm3.
Conclusion: The variability in the humeral head resection resulted in considerable differences in the measured BMD compared to BMD measured for the baseline resection of the anatomical neck. A large part of these differences could be attributed to a systematic effect related to the resection height, whereas variability in the resection retroversion and inclination angle had negligible impact.
Acknowledgements: This study was funded by grant number 1521 of Eurostars 3- call 2.
References:
[1] T. Suter et al., Arch. Orthop. Trauma Surg., vol. 142, no. 11, pp. 3141–3147, Nov. 2022
[2] C. D. Joyce et al., J. Shoulder Elb. Surg., vol. 31, no. 8, pp. 1674–1681, Aug. 2022
[3] J. T. Rojas et al., J. Shoulder Elb. Surg., vol. 31, no. 9, pp. 1929–1937, Sep. 2022
#8441
Sensitivity Analysis of the Volume of Interest When Measuring Bone Mineral Density for Stemless Shoulder Arthroplasty
*Ingmar Fleps - Warsaw, United States of America
Mirella Lopez Picazo - 3D-Shaper Medical - Barcelona, Spain
Ghislain Maquer - Zimmer Biomet - Winterthur, Switzerland
Christine Mueri - Zimmer Biomet - Winterthur, Switzerland
Taku Hatta - Joint Surgery Sports Clinic Ishinomaki - Ishinomaki, Japan
Ludovic Humbert - 3D-Shaper Medical - Barcelona, Spain
Jeffrey Bischoff - Zimmer, Inc. - Warsaw, USA
Philippe Favre - Zimmer Biomet - Winterthur, Switzerland
*Email: ingmar.fleps@zimmerbiomet.com
Introduction: A bone-sparing stemless implant can provide a surgical alternative to stemmed total shoulder arthroplasty (TSA), provided that bone quality in the metaphysis is sufficient to ensure its stability. Sensitivity analyses [1] [2] evaluated regional bone density variations in the proximal humerus, but focused on volumes not necessarily relevant for stemless TSA. In this study, we investigated the influence of the selected volume on the measured BMD, focusing on regions initiating from the resection plane.
Methods: Shoulders (N=42) were segmented from 33 CT scans and a virtual resection of the anatomical humeral neck was performed. Patients were scanned along with including a calibration phantom (B-MAS200, Kyoto Kagaku, Kyoto, Japan). Two subsequent BMD analyses were conducted on the trabecular compartment. Method A involved fitting the maximum cylinder with a height-to-radius ratio of 1.35 (based on typical stemless implant dimensions) and extruded along the neck axis starting at the resection plane to each humerus. Cylindrical volumes were then generated from this baseline following a full factorial design with three radii (100%, 75%, 50% from baseline) and five heights (100%, 80%, 60%, 40%, 20% from baseline) (Figure 1). Method B consisted of a series of “sections” with increasing thicknesses (3, 6, 9, 12, and 15 mm) all initiating from the resection surface. Analysis of Variance (ANOVA) for repeated measures was used to test the influence of measurement volume for each method separately.
Results: Using Method A, the cylinder radius was positively associated with BMD while cylinder height was negatively associated with BMD (Figure 2). Thus, the cylinder with the largest diameter and smallest height (100% radius, 20% height) featured the highest mean BMD. BMD differences between cylinder heights and radii were all significant except between 100% and 80% height. The BMD extracted with Method B was higher than for Method A. The section thickness for Method B significantly correlated with BMD and significant differences were found between all five thicknesses in terms of BMD.
Conclusion: Differences in BMD between cylinder sizes indicate that BMD values vary with implant size. Bone density values extracted with Method B where higher because they included the dense trabecular bone close to the cortex. Utilizing this denser bone as support would likely be beneficial for stemless implants. Therefore, a shallow and wide implant would be supported by better quality bone. However, mechanically there is likely a trade-off between supporting the moments geometrically with a longer implant and supporting the implant with denser bone.
Acknowledgements: This study was funded by grant number 1521 of Eurostars 3- call 2.
References:
[1] H. Alidousti et al., J. Shoulder Elb. Surg., vol. 26, no. 9, pp. 1653–1661, Sep. 2017
[2] J. M. Reeves et al., JSES Int., vol. 5, no. 3, pp. 525–531, May 2021
Figures
Figure 1
Figure 2#8281
Glenoid Components Peg's Perforation and Primary Stability in Reverse Shoulder Arthroplasty: A Morphological-Based Optimization of a Glenoid Baseplate's Peg Design
*Nicola Del Negro - LimaCorporate Spa - Villanova di San Daniele del Friuli, Italy
Andrea Fattori - Lima Corporate - Villanova Di San Daniele del Friuli, Italy
Cristiano Pizzamiglio - Limacorporate S.p.A. - Villanova Di San Daniele Del Friuli, Italy
Michele Pressacco - LimaCorporate Spa - Villanova di San Daniele del Friuli, Italy
*Email: Nicola.DelNegro@limacorporate.com
Introduction: In Reverse Shoulder Arthroplasty, it is important to properly choose the glenoid components to ensure adequate bone preservation and primary implant stability. Among all the existing solutions, the ones most used could be a baseplate with a central peg enhanced with an optional central compressive screw or a baseplate with a central long post without screw; the baseplates could in turn be non-wedged or wedged designs. The aim of this study was to define a peg’s length threshold for a given glenoid component design (wedged and non-wedged) beyond which the external cortex of the scapula would be perforated and not allow the use of a central compressive screw.
Methods: The periosteal and endosteal bone cortex boundaries of a sample of thirty-eight scapulae were segmented from Computed Tomography images of cadaveric specimens. Scapular landmarks were automatically detected using the non-rigid registration algorithm provided by the Scalismo library and the anatomic measurements were computed using the open source-based FIGURA system. Anatomic scapular and glenoid reference systems were automatically defined on each anatomy. In a first analysis, a simulacrum of a non-wedged Ø25mm baseplate with a R35mm curved backside and a Ø11mm peg with conical tip was placed with 0° of version and 0° of tilt on the glenoids, flush with the inferior rim of the scapula. The minimum glenoid coverage was set to 80%. For each anatomy, the implanted simulacrum was modified by extending the peg’s tip until perforation of scapular cortex (Fig.1). In a second analysis, the assessment was repeated by including a 15° wedged baseplate simulacrum to be used in glenoids with natural tilt or version exceeding typical normal values (Fig.2), to optimize the amount of bone removal.
Results: The perforation-tolerant peg lengths distributions were analyzed (Fig.3). When implanting non-wedged baseplates only, the mean peg length without perforation was 15.6mm and the interquartile range was 9.32mm (9.81 to 19.1mm). A peg shorter than 14mm allowed no perforation in 2/3 of the analyzed scapulae. When using the 15° wedged baseplates option in abnormal scapulae, the mean peg length without perforation increased to just 16.2mm and the interquartile range was 8.38mm (10.7 to 19.1mm); however, this led to an average 72% of bone removal sparing compared to non-augmented ones.
Conclusions: In a glenoid platform with a 25mm baseplate and defined backside curvature, according to this morphological study, the use of a Ø11mm peg shorter than 14mm allowed to achieve no glenoid perforation in 2/3 of the cases; for these cases, a central compressive screw might then be used to enhance compression and primary stability within the glenoid vault. The use of wedged baseplate did not affect much the perforation-tolerant peg length; however, it significantly reduced the amount of bone removed. With a peg length greater than the third quartile of the distribution (19.1mm), the likelihood of scapular perforation increases, and a central screw might not be always used to enhance compression; in these cases, a longer peg perforating the cortex without central screw might be desirable to enhance stability.
Figures
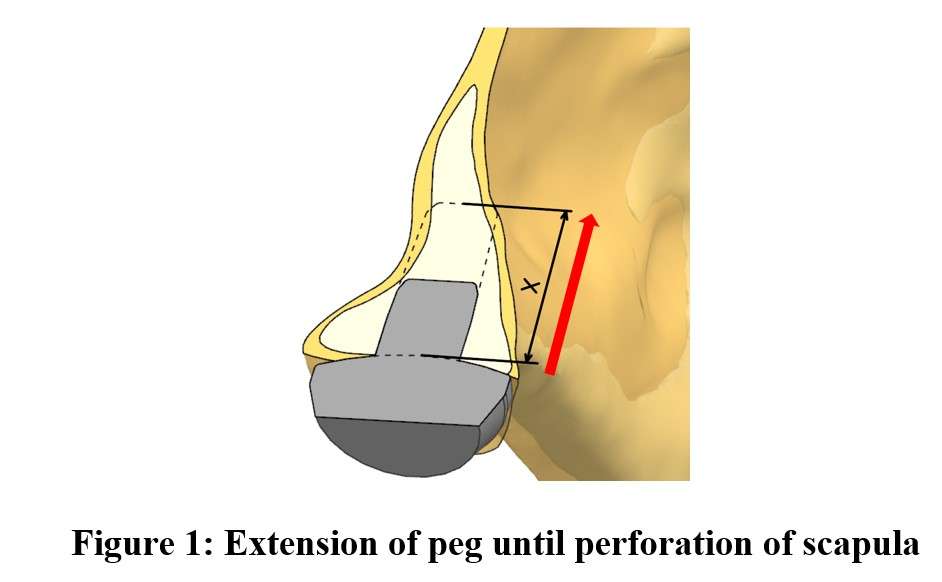
Figure 1
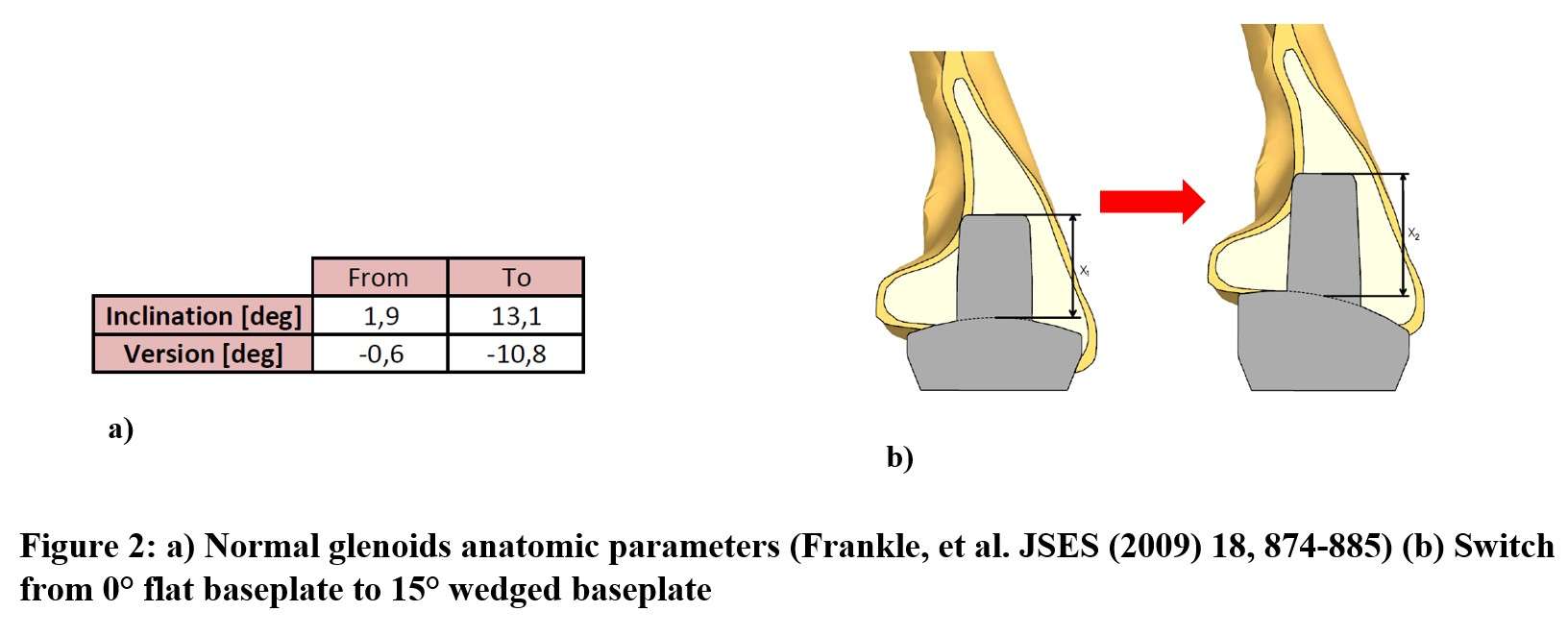
Figure 2
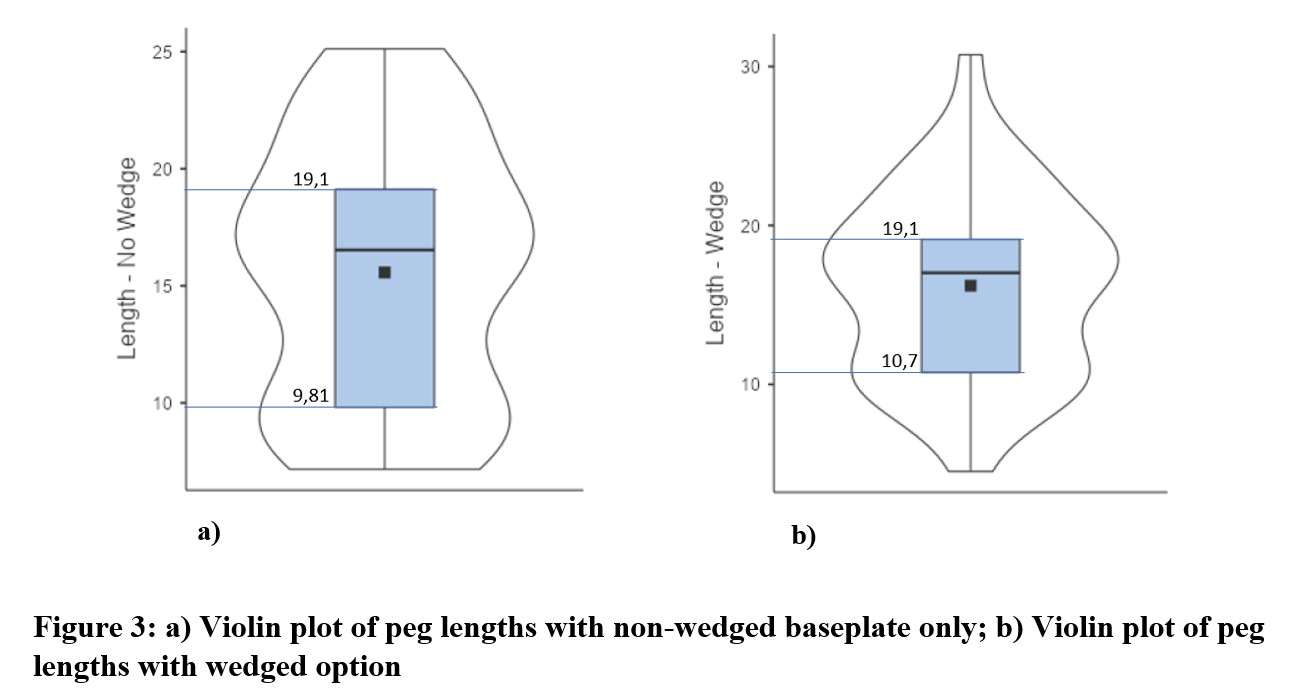
Figure 3#8479
Evaluating the Modular Connection Performance of Anatomic Shoulder Replacement System
*Ravikumar Varadarajan - Zimmer Biomet - Warsaw, USA
Jeffrey Bischoff - Zimmer, Inc. - Warsaw, USA
*Email: ravikumar.varadarajan@zimmerbiomet.com
Introduction:
Modern shoulder arthroplasty systems are comprised of various implant components designed to provide the modularity and adaptability necessary to facilitate individual anatomical adjustment and restoration of the glenohumeral joint. Clinical complications due to repetitive relative surface micromotion between the mating components at the taper junctions is known to cause fretting corrosion and release of metal debris in hip arthroplasty [1]. While clinical complications are not reported for shoulder arthroplasty due to taper corrosion, few retrieval studies have characterized corrosion and fretting damage in shoulders [2,3]. The objective of this study was to develop a test method to investigate the modular connection performance of an anatomic shoulder replacement system.
Methods:
Anatomic implant components from the IdentityTM Humeral System (Zimmer Biomet, Warsaw, IN) were utilized in this study. The modular implants comprise of a Humeral Stem, Stem Adapter, Head Adapter, and the Humeral Head (Figure 1). Test components were cleaned and weighed before assembly. Accelerated corrosion fatigue testing was performed using closed-loop servo hydraulic load frames that simulated physiological loading conditions (Figure 2). The test rig was designed to apply cyclic loads under a test environment that accelerate fretting corrosion at the modular junctions. Five test specimens (test group) were subjected to a maximum load of 870 N orientated at 40 and 20 degrees in the coronal and sagittal plane, respectively [4,5]. For elevations greater than 80 degrees, 52 motions/hour have been reported for anatomic shoulder arthroplasty patients [6], resulting in ~ 5 million cycles over a 20-year period. Therefore, testing was conducted for 5 million cycles at 3 Hz in ringers solution maintained at 50?C and 3.5 pH. After fatigue testing, the implant components were disassembled, cleaned, and weighed to determine mass loss at the modular junctions. Three test specimens (control group) were cleaned, weighed, assembled, and placed in the same environment but not subjected to cyclic loading as was the test group.
Results:
The results showed that there was no significant fretting or corrosion observed at any of the modular junctions tested. Visual inspection revealed no signs of damage or wear on any component surfaces, and no evidence of material loss due to corrosion was observed. Additionally, there were no instances of mechanical failure or loosening observed during testing. Mass loss of the test group was significantly less than a baseline 6 degree taper femoral system [7] introduced to the market more than 40 years ago, Figure 3.
Conclusion:
An accelerated test method was developed to investigate the modular connection performance of an anatomic shoulder replacement system. The findings from this study indicate that the IdentityTM Shoulder System is resistant to fretting fatigue under accelerated corrosion conditions.
References:
1. Mistry et al., Journal of Orthopedics and Traumatology.,17(1),p1-6,2016
2. Hornung et al., Journal of Shoulder Elbow Surgery,31(11),P2381-2391,2022
3. Eckert et al., Biomedical Research International, PMC4940522,2016
4. Orthoload.com
5. Fryar et. al. Mean body weight, height, waist circumference, and body mass index among adults: United States National Health Statistics Reports. 2018
6. Langohr et al., Journal of Shoulder Elbow Surgery,27(2),p325-332,2018
7. https://www.zimmerbiomet.com/content/dam/zimmer-web/documents/en-IN/pdf/medical-professionals/hip/CLS-Brevius-with-Kinectiv-Technology-White-Paper-06.02248.012-10-2011.pdf
Figures

Figure 1
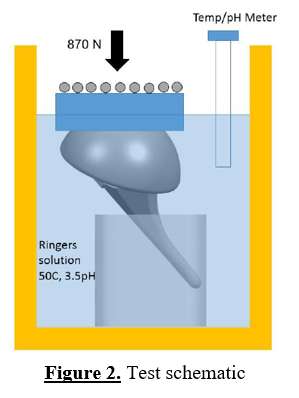
Figure 2
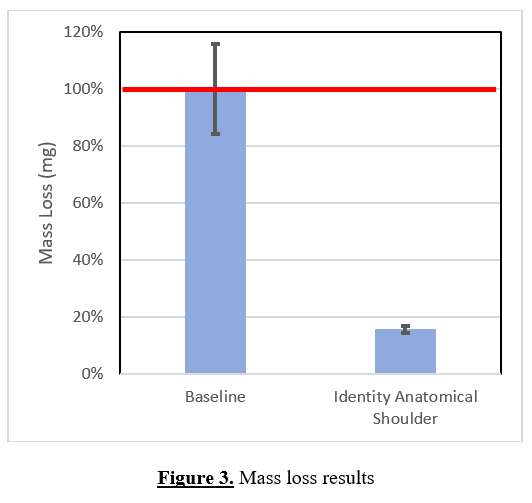
Figure 3#8392
Comparative Analysis of Loosening of Two Humeral Stems Using an in Silico Clinical Trial
*Ghislain Maquer - Zimmer Biomet - Winterthur, Switzerland
Christine Mueri - Zimmer Biomet - Winterthur, Switzerland
Adam Henderson - Zimmer Biomet - Winterthur, Switzerland
Lukas Connolly - Zimmer Biomet - Winterthur, Switzerland
Jeffrey Bischoff - Zimmer, Inc. - Warsaw, USA
Philippe Favre - Zimmer Biomet - Winterthur, Switzerland
*Email: ghislain.maquer@zimmerbiomet.com
Introduction: Manufacturers rely on pre-clinical benchtop tests to establish product safety and performance of new products, until clinical data becomes available. In silico clinical trials (ISCT) have the potential to provide ancillary evidence with population-based finite element (FE) models1. Stem loosening is one of the main complications in anatomical Total Shoulder Arthroplasty and represents a major harm to be included in an ISCT application for a shoulder stem. The goal of this study was to evaluate the relative risk of stem loosening between two contemporary shoulder arthroplasty systems, using an ISCT approach.
Methods: High stem-bone interface micromotion may inhibit bone in-growth and may result in loosening2. A modeling framework was recently validated for stem micromotion3 based on the ASME V&V40 standard. Using this validated approach, a series of CT-based FE models were virtually implanted with two different short stems: a recently introduced device (Identity™ Shoulder System) and a clinically successful system (Comprehensive® Total Shoulder System). Seven humeri were taken from an internal 3D bone database (Fig. 1A-B). Virtual surgery was performed following their respective surgical techniques (Fig. 1C) and stem size and positioning were optimized to fit the largest possible size while minimizing cortical impingement. The implanted bones were imported to a FE software for meshing (Fig. 1D) and contact definitions. Boundary conditions including joint reaction and muscle loads representative of 80° of glenohumeral abduction4 were applied (Fig. 1F). Bone material properties were assigned to each bone element based on the greyscale values of the CT image (Fig. 1E). Finally, micromotion at the bone-implant interface was extracted in the porous coated region (Fig. 1F).
Results: Interface micromotion was significantly lower for Identity than for Comprehensive (Figure 2), which seems to be attributable to a more tapered, volume-filling proximal stem profile that provides more resistance to axial stem motion in the humeral metaphysis (Figure 3).
Conclusion: This study illustrates the potential of ISCT to augment clinical data, by providing insights to the differential outcome of two stems within the same patient cohort, an analysis that would be impossible in a traditional clinical trial. This further allows identifying design and anatomical specificities that play a role in stem interface micromotion. Future work will consist in further modeling the variability found in the clinical application, by consideration of surgical, bone quality and loading variability, and including a larger cohort of bone models. ISCT studies have a great potential to increase confidence in new device performance before market release.
References:
1. Favre, P. et al. In Silico Clinical Trials in the Orthopedic Device Industry: From Fantasy to Reality? Ann. Biomed. Eng. (2021) doi:10.1007/s10439-021-02787-y.
2. Pilliar, R. M., Lee, J. M. & Maniatopoulos, C. Observations on the effect of movement on bone ingrowth into porous-surfaced implants. Clin. Orthop. Relat. Res. 208, 108–113 (1986).
3. Maquer, G. et al. First Steps Towards Enriching Clinical Trials For Orthopaedic Implants With Simulated Data. in EFORT CONGRESS LISBON 2022.
4. Favre, P. et al. An integrated model of active glenohumeral stability. J. Biomech. 45, 2248–2255 (2012).
Figures
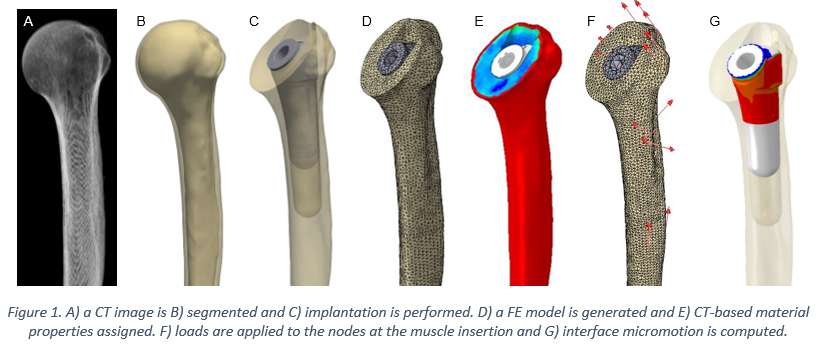
Figure 1
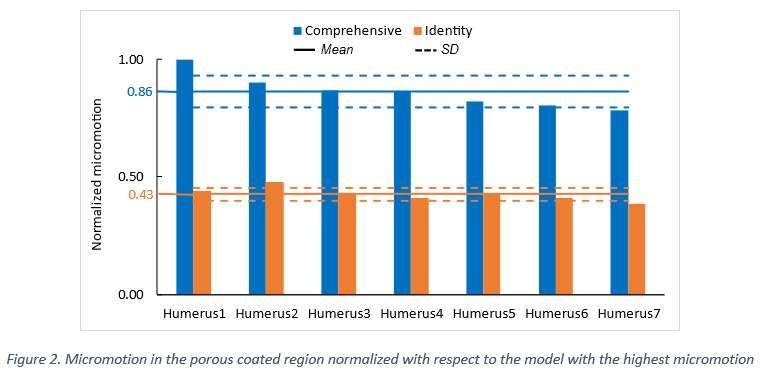
Figure 2
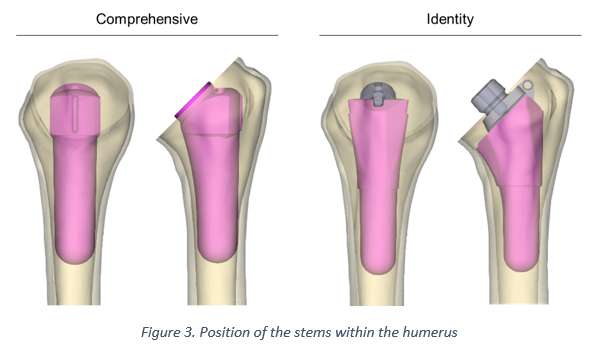
Figure 3#8412
Comparisons Between in-Situ Corrosion Rates of Modular Hip Replacement Tapers Under ASTM F1875, ISO 14242, and Physiological Profiles
*Samuel Perry - University of Leeds - Leeds, GB
Gregory Pryce - Instite of Biogical and Medical Engineering - University of Leeds - Leeds, United Kingdom
Michael Bryant - University of Leeds - Leeds, United Kingdom
Richard Hall - University of Leeds - Leeds, United Kingdom
Gregory deBoer - Institute of Thermofluids, University of Leeds - Leeds, United Kingdom
Andrew Robert Beadling - University of Leeds - Leeds, United Kingdom
*Email: mn17s2p@leeds.ac.uk
INTRODUCTION: Over 89,000 total hip arthroplasties (THAs) were carried out in the United Kingdom in 2021, with the majority using a modular taper interface in-between femoral head and femoral neck components [1]. It is known that forces from daily activities leads to micro-motion between taper interface components [2]. The resulting corrosion is a significant cause of adverse reactions for THA, possibly causing up to 4.7% of revisions [2]. However, current standards use simplified loading patterns unrepresentative of physiological motion and loading, with ASTM F1875 using only a sinusoidal uniaxial methodology. This study aimed to develop an in-situ methodology to measure and compare taper corrosion rates between standard pre-clinical testing profiles and physiological profiles.
MATERIALS & METHOD: A ProSim electro-mechanical single-station universal joint simulator (Simulation Solutions, UK) was used to replicate ASTM F1875-98 and ISO 14242-1:2014 test profiles, as well as physiological profiles of patients walking, fast walking, and lunging. Physiological profiles were derived from motion capture and multi-body musculoskeletal modelling [3]. Five 28mm diameter CoCr femoral heads were loaded through 2KN onto rough, proximally matched high nitrogen stainless steel taper samples, before being placed in a testing fixture angled according to ISO 7206-4. Samples were submerged in PBS according to ASTM F1875-98, and connected via a short silicone tube to an Ag/AgCl combined counter and reference electrode. Open circuit potential was measured for 30 minutes, before samples were held at a 100mV potentiostatic polarisation for 45 minutes without load. Loading profiles were subsequently tested in the aforementioned order for 1000 cycles each, with profiles separated by unloaded phases 25 minutes long. Recorded currents were assessed using a custom MATLAB script (MathsWorks, USA), which separated loading profiles and calculated average and peak currents. Charge transfer was also calculated, as it is proportional to corrosion rate.
RESULTS: For both peak and average current values, as well as charge transfer, average values were highest for the fast walk profile, followed by ISO 14242, walking, and lunge. ASTM F1875 obtained the lowest averages in all measurements, with corrosion rates and current rates less than 35% of those obtained from ISO 14242, and less than 20% of those obtained from fast walking. Statistically significant differences were found between all profiles and ASTM F1875 (P<0.01), except for lunge. Differences in corrosion rates between fast walking and ISO 14242 were also not statistically significant (P=0.0883).
CONCLUSIONS: Uniaxial testing, as described in ASTM F1875, provides low and simple torques at the taper junction, not representative of those experienced in-vivo. ISO 14242, walking, and fast walking profiles all experienced significantly higher rates of taper corrosion than ASTM F1875, while having lower peak axial forces. This data suggests that the addition of kinematics significantly alters the corrosion response, and that even ISO 14242 may not provide representative corrosion responses for high intensity activity. Further investigation of corrosion responses under physiologically relevant profiles is encouraged.
REFERENCES: [1] National Joint Registry., 2023; [2] Mueller et al., J Mech Behav Biomed Mater 2021; [3] Lunn et al., J. Arthroplasty 2020;
Figures
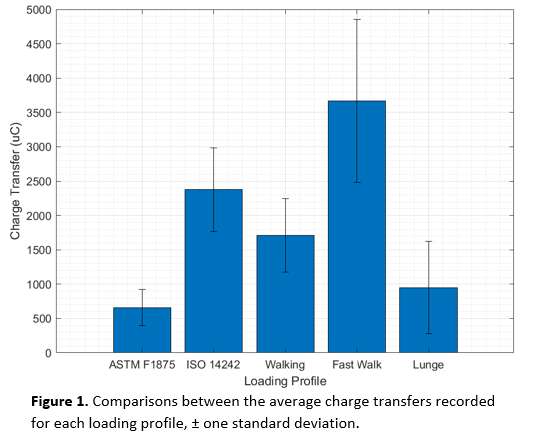
Figure 1
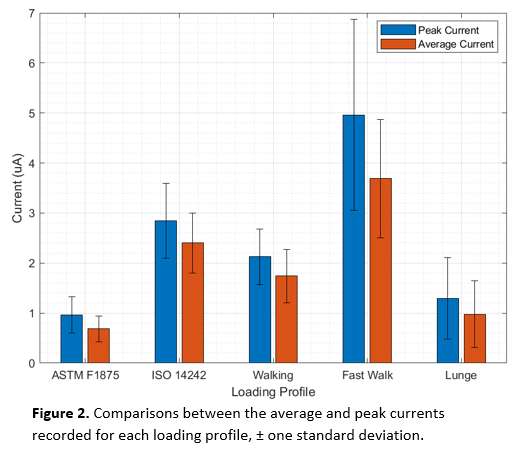
Figure 2#8683
Benchmarking Mechanical Performance of 3D-Printed Silicon Nitride-PEEK Spinal Fusion Devices
*Ryan Bock - SINTX Technologies - Salt Lake City, USA
Cemile Basgul - Drexel University - Philadelphia, USA
Paul DeSantis - Drexel University - Philadelphia, United States of America
Tabitha Derr - Drexel University - Philadelphia, USA
Hannah Spece - Drexel University - Philadelphia, USA
Douglas Hoxworth - SINTX Technologies - Salt Lake City, USA
Aliza Rabinowitz - Drexel University - Philadelphia, United States of America
Noreen Hickok
Steven M. Kurtz - Drexel - Philadelphia, USA
*Email: RBock@sintx.com
INTRODUCTION: Polyetheretherketone (PEEK) possesses many amenable properties for use in spinal fusion devices, such as moduli similar to bone, adequate strength, ease of processing, lack of a magnetic moment, and ease of sterilization [1]. However, its relatively low surface energy and radiotransparency can present challenges for prevention of implant-associated infection, osseointegration, and post-operative assessment of implant position and fusion. Radiopaque markers are used to help assess position, and more recently bioactive agents incorporated as part of composite blends have been employed to enhance osseointegration and mitigate infection risk. Silicon nitride (SN), already used in spinal fusion as a monolithic ceramic device, has previously demonstrated antibacterial and osteogenic properties [2]. A novel 3D printed SN-PEEK composite spinal fusion device is under development to provide the familiar fit and feel of PEEK supplemented by SN’s bioactivity. This work describes the results of standardized device mechanical tests conducted on sample implants printed from the new composite material as part of this development effort.
METHODS: Sample cervical spinal fusion devices in three configurations (solid, porous, and porous+window – Fig. 1) were 3D printed by fused filament fabrication (FFF) (R1, 3DS-Kumovis) from PEEK and composite 15 vol% SN-PEEK filaments. This concentration of SN was selected to be approach but remain lower than the percolation threshold to avoid deleterious effects on the composite’s processability and mechanical properties. Devices were tested in accordance with ASTM F2077 [3] in static compression, compression-shear, and torsion. Test data were benchmarked against published mechanical test data for spine devices currently cleared in the U.S. market [4].
RESULTS: In compression and compression-shear, devices printed from PEEK and SN-PEEK exceed the 75th percentile of performance versus device data shared in Peck [4]. In compression, inclusion of porosity increased stiffness while inclusion of a window mildly reduced stiffness. No effect was observed as a function of material. In shear, similar geometric effects were observed, and composite samples were ~40-50% stiffer than neat PEEK samples. In torsion, the solid design was the stiffest, and similar increases for the composite relative to the monolith were observed. Observed yield moments (~5-10 Nm, see Fig. 2) and ultimate moments (~5.5-11 Nm, see Fig. 3) for all devices were well above the 5% threshold (~2.5 Nm) established in Peck [4] with solid devices being more robust than porous, and composite devices being more robust than monoliths.
CONCLUSION: These experiments confirm that implants printed by FFF from SN-PEEK composite filament possess the required static mechanical properties to withstand physiologic loads in the spinal fusion device application. Along with favorable bacterial assay, biocompatibility, and osteoblast interaction data presented elsewhere, these data justify continued development of 3D-printed SN-PEEK composite spinal fusion devices. Ongoing and future work includes dynamic testing in accordance ASTM F2077, further biocompatibility testing, and large animal model in vivo studies to gather further data needed to support a device filing.
REFERENCES: [1] SM Kurtz (Ed) (2019), PEEK Biomaterials Handbook, Elsevier [2] TJ Webster, Acta biomater. (2012) [3] ASTM F2077 (2022).[4] JH Peck, J. Biomech. (2017).
#8174
Porcine Femurs as Surrogate for in-Vitro Testing of Uncemented Hip Revision Stems
*Julius Boettcher - TUHH Hamburg University of Technology - Hamburg, Germany
Kay Sellenschloh - TUHH Hamburg University of Technology - Hamburg, Germany
Gerd Huber - TUHH Hamburg University of Technology - Hamburg, Germany
Benjamin Ondruschka - Medical Center Hamburg-Eppendorf (UKE) - Hamburg, Germany
Michael Morlock - TUHH Hamburg University of Technology - Hamburg, Germany
*Email: julius.boettcher@tuhh.de
INTRODUCTION
Implant loosening is a major reason for revision in uncemented revision total joint arthroplasty [1]. Manufacturers aim to strengthen the stem-to-bone connection to improve primary stability. Tapered stems with longitudinal splines embedded in the diaphyseal cortex provide sufficient stability for bone ingrowth [2]. Porcine bone is commonly used as a surrogate for human femurs in vitro without validation [3]. The aim of this study was to investigate the suitability of porcine femoral bone as a surrogate for preclinical testing of the primary stability of revision stems in humans.
MATERIALS AND METHODS
Five pairs of human femurs (4 male, 1 female, 69.2 ± 11 years) and 14 incoherent porcine femurs were used in this study. Major proximal bone defects were simulated in the human femurs by performing an extended trochanteric osteotomy. Human and porcine femurs were implanted with a splined modular revision hip stem (Reclaim™ DePuy Synthes, nporcine = 7, nhuman = 5) while a prototype design with wider, shorter secondary splines interspaced between primary splines that are equivalent to those found on Reclaim stems was implanted in the contralateral human femurs and a similar number of porcine bones to analyse whether the influence of design differences is similar for both bone materials (Figure.1). Implantation was performed with a drop tower. Energy levels were increased (2 J, 3 J, 4 J, 5 J) until the templated implant position was reached or incremental seating was less than 0.5 mm (DIC, Aramis 3D, MV 100, GOM, Germany). Torque to failure was applied under a static preload (porcine: 100 N, human: 500 N) at 0.5 °/s (MiniBionix II, MTS, MN). Shear forces were prevented by a double cardan joint configuration. Spline indentation into bone was evaluated by digital microscopy (VHX-7100, Keyence, Japan) for the porcine bone. For the human femurs, contact analysis within the femoral canal was performed by superimposing CT-images and laser scans.
RESULTS
The prototype design showed significantly higher torsional resistance in the human femurs (p = 0.039, Figure.2), but not in the porcine model (p = 0.71). Both designs had less than 0.3 mm cortical imprint depth in porcine bone, showing no difference in contact. In human femurs, cortical contact of the stem was significantly higher for the prototype design (p = 0.046, Figure.3A). Average cortical imprint depth was not significantly different between the stem designs but the depth range was smaller for the prototype design (p = 0.502, Figure.3B).
DISCUSSION & CONCLUSION
Higher cortical bone density in porcine femurs may explain different stem design results compared to human bone. The longer thin splines didn't imprint enough, preventing the secondary splines of the prototype design from contacting the bone. In human femurs, wider and shorter splines contact cortical bone, preventing further indentation and migration. The resulting increased contact area may improve torsional resistance. Using porcine bone as a surrogate to compare stem designs wasn't suitable, highlighting the need to validate surrogate models before use.
The support of DePuy Synthes in providing the implants and surgical instruments is gratefully acknowledged.
REFERENCES
[1] AAOS, AJRR, 2022
[2] Hancock et al., HIP INT, 2019
[3] Pearce et al., EUR CELLS MATER, 2007
Figures
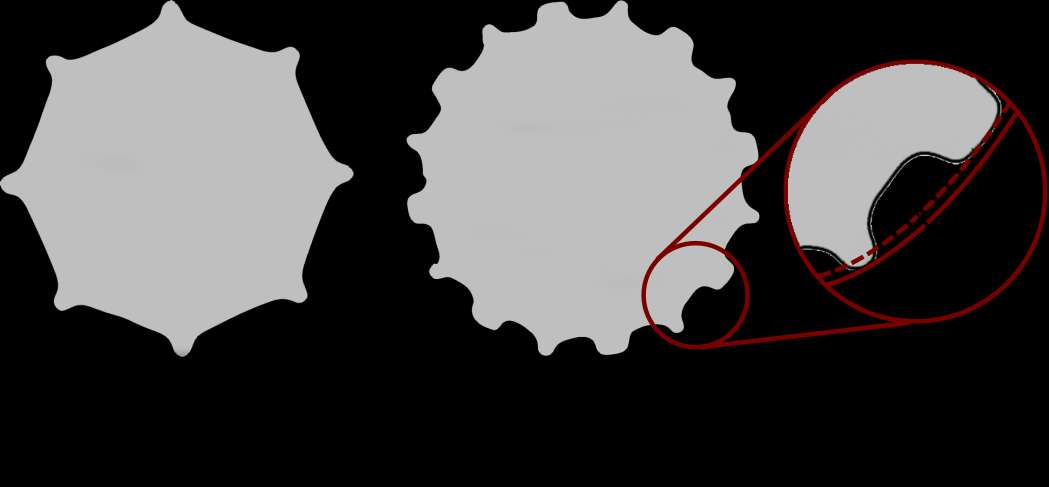
Figure 1
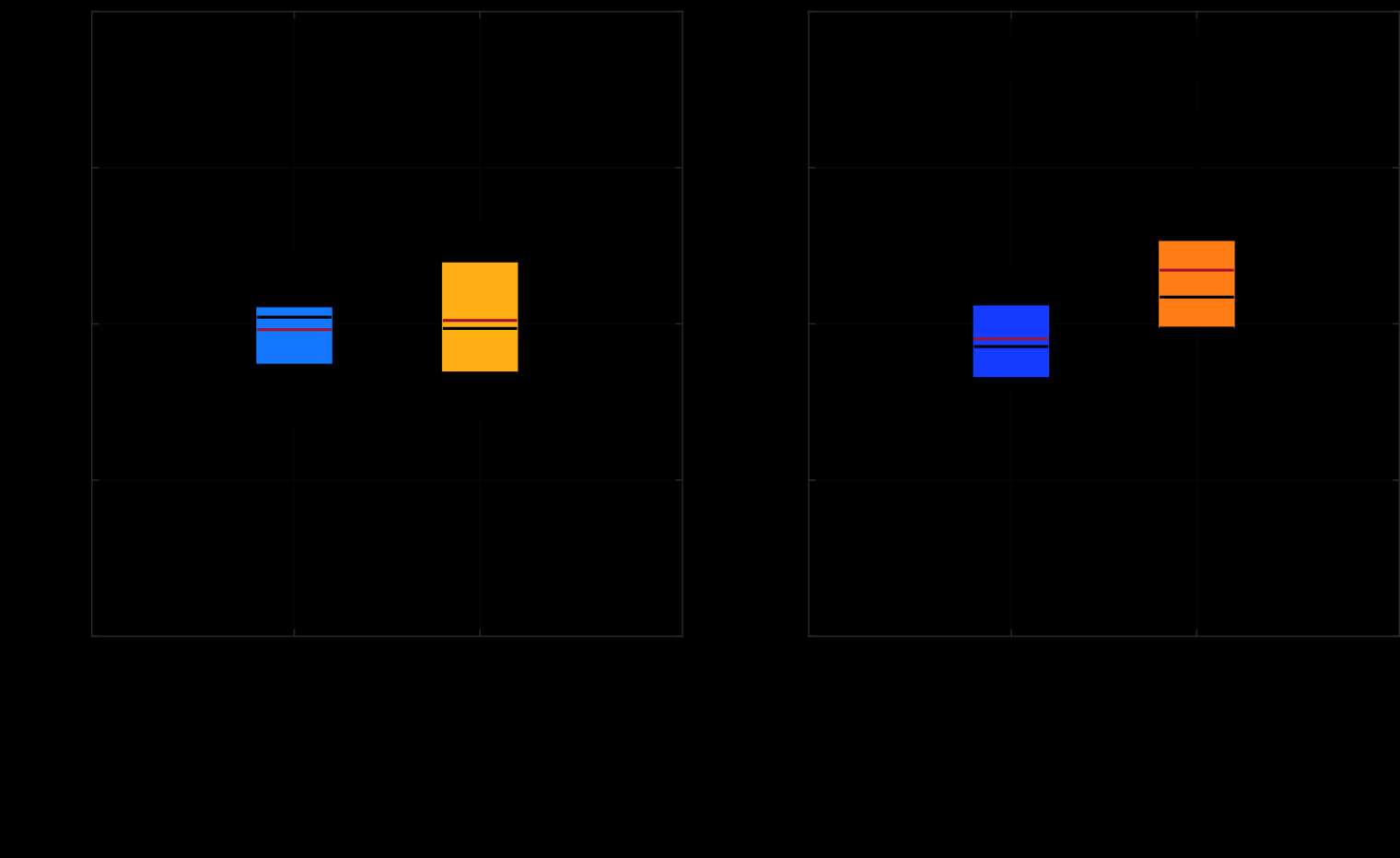
Figure 2
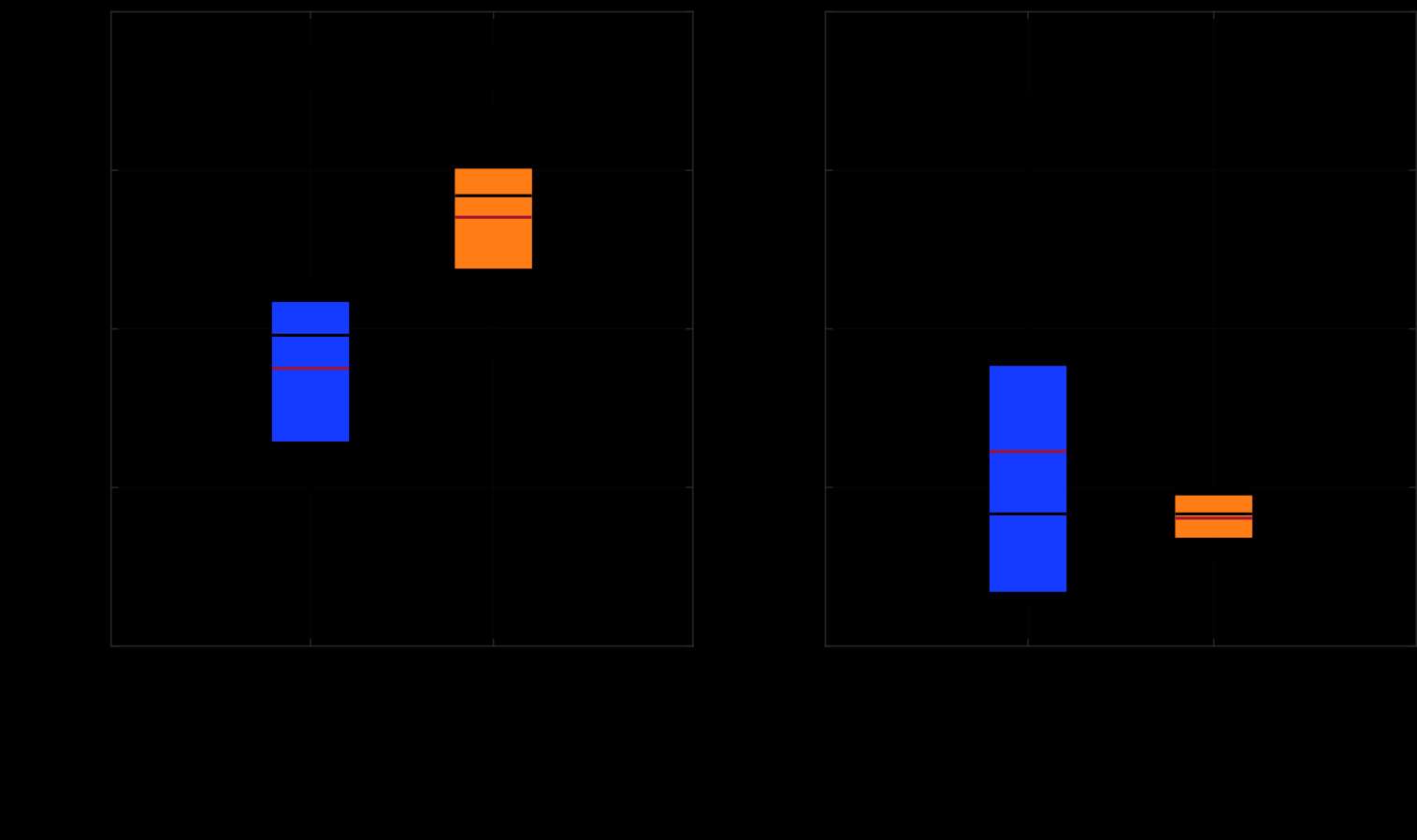
Figure 3#8627
Utilizing Phase-Stepping to Expand DIC Capabilities: Femoral Components and Tibial Trays
Gregory Pepe - Stryker - Ridgewood, NJ, United States of America
*Akeem Downer - Stryker Orthopedics - Mahwah, United States of America
Ananthkrishnan Gopalakrishnan - Stryker Orthopaedics - Mahwah, USA
Paul Andreasen - Stryker Orthopaedics - Mahwah, USA
*Email: akeem.downer@stryker.com
Introduction: Phase-stepping is a strategy that can be employed on cyclic tests that captures an image at one moment of a cycle, then another image at a desired phase offset on the next possible cycle, repeating to a desired number of images. This allows a camera setup to capture more representative images for a given cycle than its maximum framerate would normally allow. This study used digital image correlation (DIC), a non-contact optical metrology method, to measure major strain experienced under different standardized tests for tibial trays and femoral components. A machine vision strobe was used to provide adequate brightness for low exposure time image capture, minimizing motion blur.
Methods: A black and white speckled pattern was applied to a tibial tray and femoral components to be analyzed by the ARAMIS 5M camera system (GOM GmbH) using DIC, with images captured using a phase-stepping algorithm in ARAMIS Professional 2019 software. Lighting was provided by an MVS-5000 machine vision strobe (Excelitas Technologies Corp., MA).
Two types of tests were run in this study. The first follows ASTM F1800-19E1 [1], a standard for cyclic fatigue testing of metal tibial trays. A CoCr tibial tray was used, with a cyclic load from 90N to 900N applied to its unfixed side. The second follows ASTM F3210-22 [2], a standard for cyclic fatigue testing of metallic total knee femoral components. Two total knee CR femoral components of the smallest size were used, one each of CoCr and Ti alloy. The maximum load applied to the samples was based on a performance requirement corresponding to peak loads from chair rise activity [3]. Both tests were run at both 10Hz and 20Hz, using a floating bearing load applicator to allow for transverse motion as advised in the standards. Five phase-stepped loading cycles were captured and analyzed for peak major strain in each test. The camera system setup and the software’s strain analysis is provided in Figure 1.
Results: At 10Hz and 20Hz, the peak major strain occurred at the tibial tray’s PCL notch and the femoral components’ articular distal medial condyle surface (Figure 1, red areas). No significant difference (p>0.05) in peak major strain was found between the two loading frequencies in any test (Figure 2). A significant difference was found between femoral component materials regardless of frequency (10Hz: p=0.000; 20Hz: p=0.000), expectedly due to Ti alloy having approximately half the elastic modulus of CoCr.
Conclusion: Both the tibial tray and femoral component tests showed no significant peak major strain differences between 10Hz and 20Hz loading frequencies. This verifies the frequency range allowed in ASTM F3210-22 and may imply that ASTM F1800-19E1 can be performed at least up to 20Hz with a floating bearing load applicator with no appreciable difference on the measured strains, which may lead to faster overall implant evaluation test times. These results were able to be obtained by relatively low-framerate cameras using the phase-stepping technique (Figure 3), which may be used in other similarly framerate-limited optical measurement systems to enhance their capabilities.
Figures
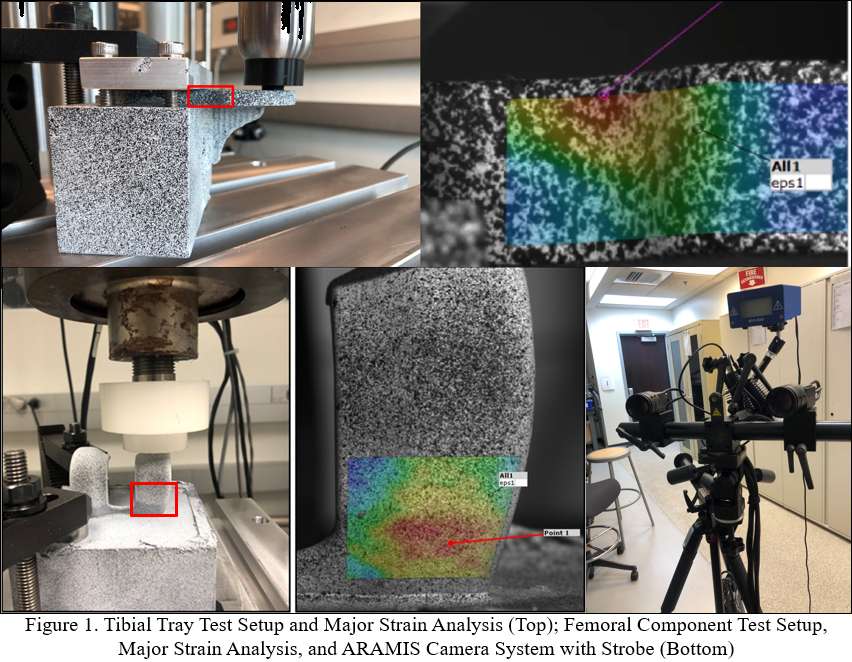
Figure 1
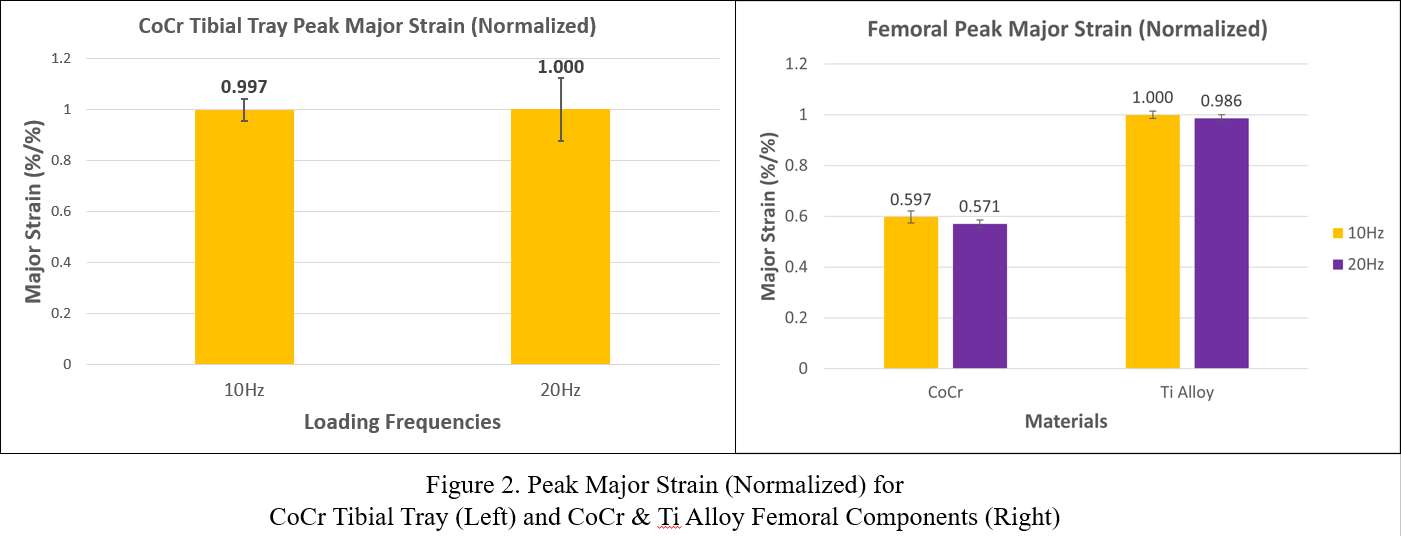
Figure 2
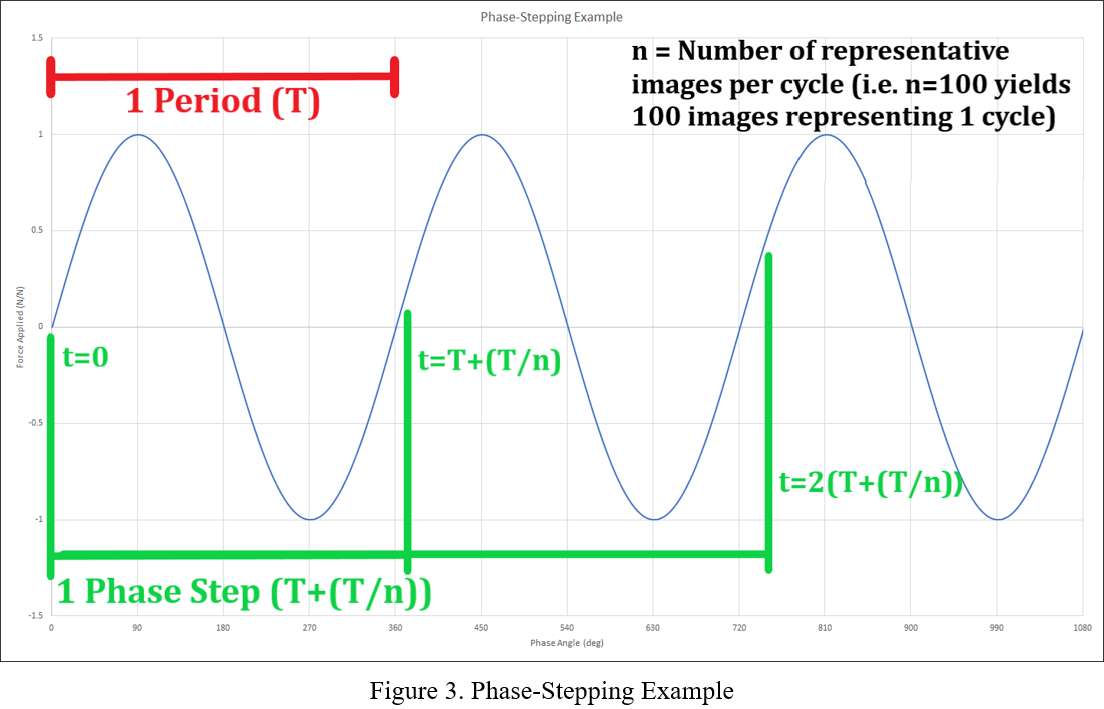
Figure 3#8516
How Does Design and Tray Size Affect Cementless Tibia Stability?
*John Kyle Mueller - Zimmer Biomet - Warsaw, USA
Charles Lawrie - Baptist Health South Florida - Coral Gables, USA
Charlie Parduhn - Zimmer Biomet - Warsaw, USA
Jeffrey Bischoff - Zimmer, Inc. - Warsaw, USA
Cory Trischler - Zimmer Biomet - Warsaw, USA
Marc Bandi - Zimmer GmbH - Winterthur, Switzerland
Eik Siggelkow - Zimmer GmbH - Winterthur, Switzerland
*Email: johnkyle.mueller@zimmerbiomet.com
Introduction:
Initial biomechanical stability of cementless total knee arthroplasty components is critical to allow for bony ingrowth and long-term fixation. Factors including keel design, peripheral spike shape and location, press fit and others affect initial implant fixation. However, the impact of tray shape is largely unknown. In this study. We compare the difference in stability between an anatomic and asymmetric tibia tray designs on stability in an invitro model.
Methods: This study evaluates the initial stability of two cementless tibia designs implanted in validated biomechanical testing model. Components were implanted into a polyurethane foam that replicates the stiffness of cortical and cancellous bone and were tested for stability in simulated level walking (2500 cycles) and stair descent (130 cycles). (Table 1) A clinically successful keeled asymmetric cementless tibia plate with round peripheral pegs (Asymmetric) [1] (n=5) and a new commercially available keeled additively manufactured anatomically shaped tibia plate with square pegs (Anatomic) (n=5) were loaded using a 6 Degree of Freedom (DOF) robotic system instrumented with a load cell. Design specific cruciate retaining loads and kinematics for level walking and stair descent were determined using a computational model of the knee [2]. The micromotion of each construct was measured using digital image correlation (DIC) and data post processed using custom scripts (Matlab R2013b, Mathworks, Nattick, MA). To quantify the impact of tray size on stability, a smaller sized Anatomic tray (Small Anatomic) (n=5) was implanted in a foam tibia model appropriate for the tray size. A scaled virtual biomechanic knee (VBK) model was created for the smaller size tibia and body weight was scaled accordingly.
Results: The maximum 3D micromotion of the Anatomic tray was less than the clinically successful Asymmetric tray. During walking the 3D micromotion was 205 +/- 54 µm (lateral) and 84 +/- 5 µm (posterior lateral) for the Asymmetric and Anatomic tibia plates, respectively (p<0.001) (Figure 1). For stair descent the 3D micromotion was 151 +/- 65 µm (anterior lateral) and 92 +/- 17 µm (lateral) for the Asymmetric and Anatomic plates, respectively. The maximum 3D micromotion of the Small Anatomic tray was less than the maximum Asymmetric tray (p=0.001) (Figure 2). Small tray micromotion was 1.3 +/- 0.2 times (p=0.115) and 1.0 +/- 0.1 times (p=0.305) the maximum 3D micromotion of the Anatomic tray for level walking and stair descent, respectively, although these increases were not statistically different.
Conclusion: This study showed an Anatomic tray experienced decreased micromotion compared to a clinically successful asymmetric design. The difference in micromotion from the small to mid-sized tray was not statistically significant.
References:
[1] Hofmann et al. Ten-to 14-year clinical followup of the cementless Natural Knee system. Clin Orthopaedics Relat Res 2001;388:85-94.
[2] Siggelkow et al. Valdation of specimen specific robotically calibrated knee virtual models using robot experiments. Proceedings CMBBE 2012
Figures
Figure 1
Figure 2
Figure 3#8454
Pre-Clinical Edge Loading Testing of Hip Replacements: Do We Need to Incorporate Cup Version?
*Lee Etchels - University of Leeds - Leeds, United Kingdom
Nicholas Cooper - University of Leeds - Leeds, United Kingdom
Ruth Wilcox - University of Leeds - Leeds, United Kingdom
Alison Jones - University of Leeds - Leeds, United Kingdom
*Email: l.w.etchels@leeds.ac.uk
Introduction
Current pre-clinical ISO standardised testing of total hip replacements aims to evaluate the resilience of new implant designs to long term damage mechanisms resulting from frequent rim loading. The current standard (ISO 14242:4) positions the cup at a fixed 65° of inclination and 0° of version, and rim loading is generated during swing phase when the joint contact force is lower. Previous work [1] using the Leeds Osteoarthritis Hip Cohort established that during gait the range of version angles seen during swing phase ranged from ~0° to 40°, with a consistent increase in version from toe off to heel strike (mean +6°, 29/30 subjects).
Methods
As the purpose of the standard is to test under relatively severe conditions, cup orientation cases were generated to provide a sensitivity study representing the maximum version, and the maximum change in version during swing phase, across our available patient measurements. Three variations from the standard ISO case (#1) were tested: 40° of static version (#2); dynamic version 10-0-10° (heel-strike – toe-off – heel strike, linear ramp between) (#3); dynamic version 40-30-40° (#4). A previously developed dynamic explicit deformable finite element [2] approach, validated for kinematics [2], was used with a 36mm metal-on-polyethylene liner geometry and an elastic-plastic material model.
Results
Cases with high version angles caused the greatest movement of the contact while on the rim (Figure 1, plots 2 and 4) and highest peak contact forces on the rim (Table 1). Cases with a dynamic version angle during swing phase (Figure 1, plot 3 and 4) generated the highest peak contact pressures on the rim and greater overall plastic strain within the liner.
Conclusions
Orienting the cup with a version angle > 0°, both statically and dynamically, altered both the kinematics and the resulting contact conditions. Version angles caused the contact to slide around the rim during swing phase which could increase the contact pressures and plastic deformation. These findings suggest that consideration of more representative orientations may be important in the development of new hip replacement designs with altered liner geometry or material combinations. Although experimental validation will be required, the computational modelling supports the need to develop the necessary experimental methodologies and investigate whether the current ISO 14242:4 test standard should be augmented via the addition of cup version.
References
1. Etchels, L. et al., (2022). Assessment of cup orientation during gait: implications for preclinical hip replacement testing. 33rd Congress of the International Society for Technology in Arthroplasty. Maui, US.
2. Etchels, L. et al., (2023). Dynamic finite element analysis of hip replacement edge loading: Balancing precision and run time in a challenging model. Journal of the Mechanical Behavior of Biomedical Materials, 105865. https://doi.org/10.1016/J.JMBBM.2023.105865
Acknowledgements
The authors would like to thank the Leeds Biomedical Research Centre for providing patient data and processing support, and the EPSRC (grant number EP/W003139/1) for funding this project.
Figures
Figure 1
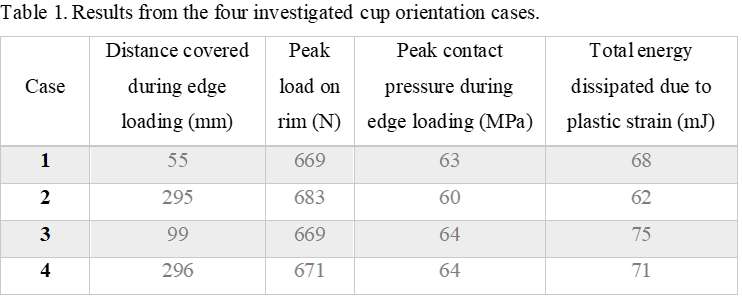
Figure 2#8447
Evaluation of the Interlocking Mechanism Between Insert And Tibial Component of Fixed Bearing Total Knee Replacements
*Kim Haeussler - CeramTec GmbH - Plochingen, Germany
Anne Gebert de Uhlenbrock - CeramTec GmbH - Lauf, Germany
Markus Flohr - CeramTec GmbH - Plochingen, Germany
Alessandro Alan Porporati - CeramTec GmbH - Plochingen, Germany
*Email: k.haeussler@ceramtec.de
Introduction
Interlocking mechanisms between tibial components and inserts of fixed bearing total knee replacements are intended to prevent dissociation of the tibial component and to limit backside wear by reducing interface micromotion. In the course of the development of a novel ceramic total knee replacement adequate testing methods including the definition of acceptance criteria for the evaluation of different interlocking mechanisms were needed. Posterior stabilized systems usually have a post-cam mechanism and thus have been defined in this study as worst-case concerning the interlocking mechanism, which commonly consist of form-fit and/or press-fit mechanisms. Dissociation of the insert from the tibial component is a rare but serious complication, which typically occurs in the early postoperative period, ranging from days to 2 years after surgery. Two clinical disassociation principles are generally described as follows:
- anterior lift-off of the insert in flexion resulting in an anterior dislocation and
- posterior lift-off in extension, with a posterior dislocation of the insert
Methods
Quasi-static test setups were developed for the two most relevant loading directions (anterior-posterior, posterior-anterior) (Figure 1). All tested inserts were axially assembled and afterwards, the resisting moments were determined for different designs of the locking mechanism, sizes of the components and geometric tolerances. Additionally, the influence of soaking the insert was evaluated. As another influencing factor, cyclic preconditioning using test methods derived from ASTM F2722 and ASTM F2723 was applied to the specimens to evaluate the fatigue behavior of the interlocking mechanism (Figure 1). For each clinical dissociation principle acceptance criteria were developed based on in vivo relevant load cases.
Results
A comprehensive testing methodology for the evaluation of the interlocking mechanism between insert und tibial component of fixed bearing total knee replacements could be developed. Statistically significant differences between different designs and sizes (Figure 2), geometric tolerances (Figure 3) and soaking procedures (Figure 3) have been determined. Additionally, distinct effects of cyclic pre-conditioning could be observed (Figure 3). Although, differences were found between designs, all tested specimens fulfilled the pre-defined acceptance criteria.
Conclusion
Testing and understanding of the interlocking mechanisms are crucial for the development of fixed bearing total knee replacements. Clear influencing factors of the behavior of the interlocking mechanism could be detected. Especially, cyclic pre-conditioning, design and size had large effects on the resisting moments. During the development of a specific interlocking mechanism, it is therefore recommended to consider various size dependent influencing factors in terms of realistic material loading and interface mechanics to examine their effects on the entire product portfolio. The study of the behavior of the interlocking mechanisms using sophisticated methods depending on the specific design should therefore be used to guarantee a safe knee arthroplasty system.
Figures
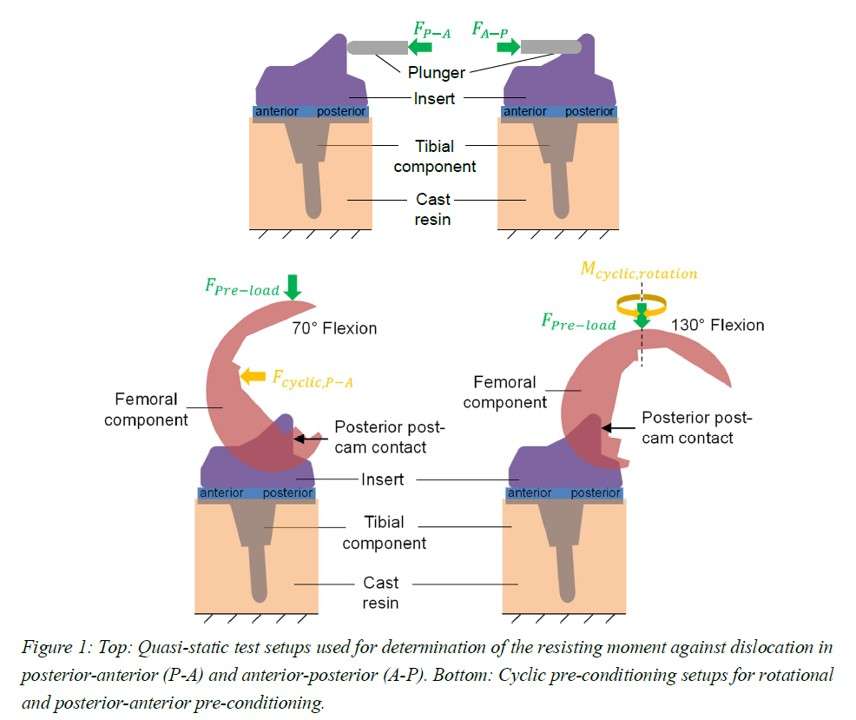
Figure 1
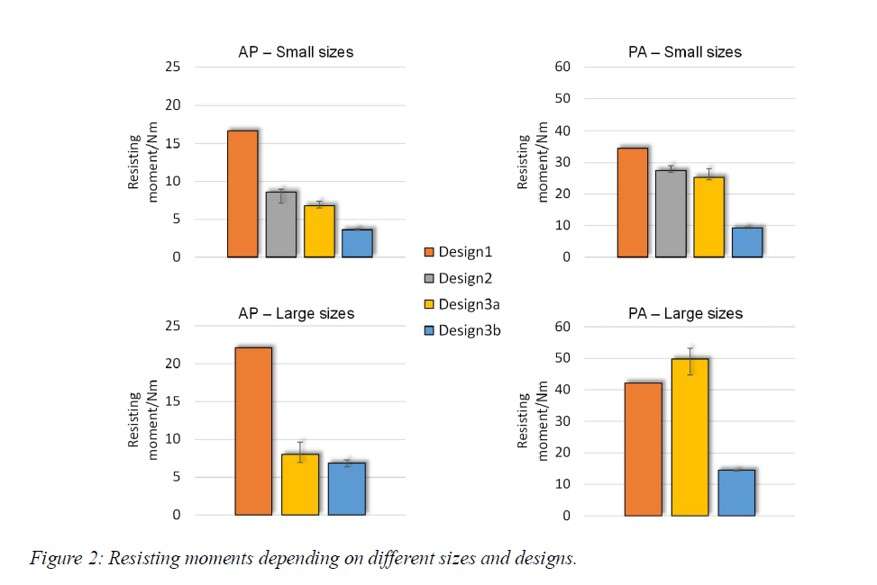
Figure 2
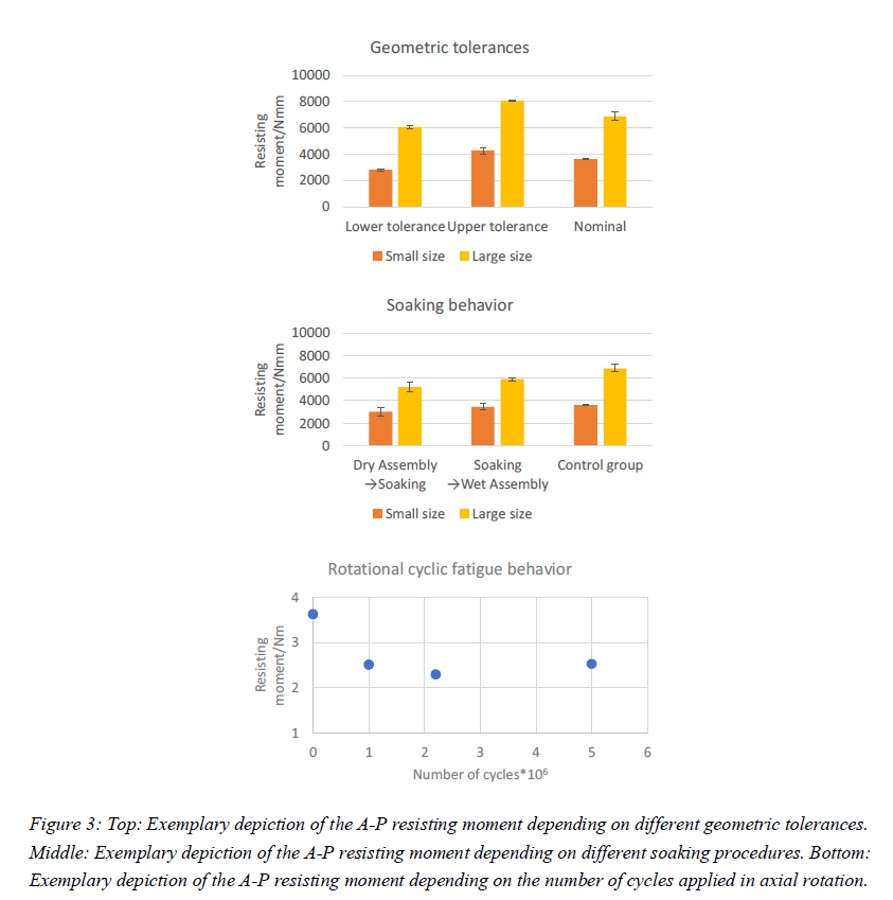
Figure 3#8520
Hip Stem Micromotion Monitoring Using a Hybrid RF-Optical Measurement System
*Souptick Chanda - Indian Institute of Technology Guwahati - Guwahati, India
Saurav Kumar Roy - IIEST Shibpur - Howrah, India
Rik Chattopadhyay - IIEST Shibpur - Howrah, India
Debasis Mitra - IIEST Shibpur - Howrah, India
*Email: csouptick@iitg.ac.in
Introduction: The incidences of THA has increased globally over the years. Post-operative stability of hip stems has a lot of bearing on the success of THA. Conventional imaging techniques like X-rays cannot be used for continuous monitoring of stem micromovements. Besides, they are known to have radiation hazards, and hence cannot be prescribed indiscriminately. We are, therefore, proposing a technique based on microwave sensor which is integrated with an optical system for continuous monitoring of stem micromotion as well as the healing stages after a hip surgery.
Methods: Microwave based techniques have recently been used as diagnostic tool to detect and assess the bone fractures [1]. In this simulation work, a wearable antenna is used as a sensor which is placed over the human thigh and the reflection coefficient plot has been used for tracking the movements of the hip stem. At first, a human thigh model consisting of skin, fat, and muscle layer is created in CST microwave studio and then a generic CAD model of a cementless hip stem is inserted virtually into the resected femur. The simulations are done for the antenna placed over the human thigh model and the scattering parameters are plotted. There is a change in resonance frequency with the micromotion of the metal implant since the dielectric properties of the thigh region change due to the conducting nature of the metal implant. The schematic of the proposed technique is shown in Fig. 1.
Results: The change in signal frequency is small and thus is undetectable by conventional RF measurement techniques. An optical system combined with RF antenna measures this small change in signal frequency. First, a carrier signal is generated using an optical source and the RF signal will amplitude modulate this signal using MZM. From the MZM, the modulated signal has two sidebands carrying the frequency information of the signal. One of these sidebands is sent to the optical wavemeter. This wavemeter measures the optical wavelength with a resolution of femtometer [2]. This wavemeter setup is done using uncorrelated intensity distribution pattern as studied in [3].
Conclusion: There are very few techniques which can give resolution of 1 MHz for micromotion detection. However, none of these are portable and low-cost solutions. The present scheme can help develop a system which is portable and low cost for precise measurement of signal wavelength. Finally, the dielectric profile can be monitored with this scheme which can be clinically understood by a doctor. Once validated, the entire scheme can further be extended in future for fracture detection of long bones.
References:
[1] S. N. Mahmood et al. "Recent Advances in Wearable Antenna Technologies: a Review"
[2] G. D. Bruce et al. "Femtometer-resolved simultaneous measurement of multiple laser wavelengths in a speckle wavemeter"
[3] B. Redding et al. “Compact spectrometer based on a disordered photonic chip”
Acknowledgement: This work acknowledges SERB, DST, Government of India.
Figures
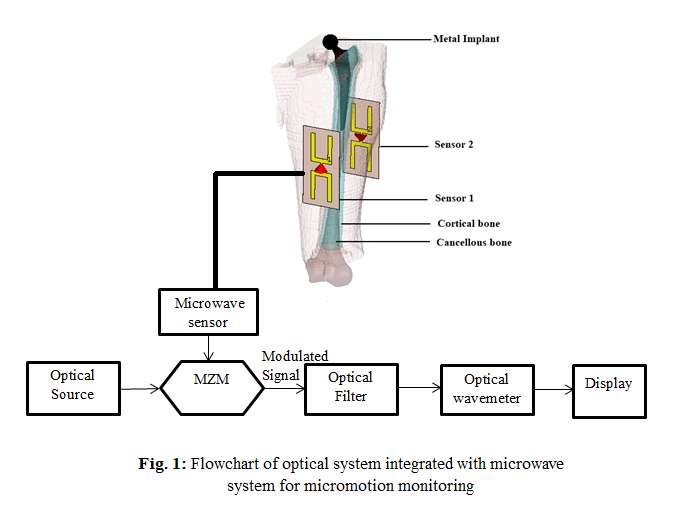
Figure 1#8425
Characterization of Acoustic Emissions Resulting From Friction of Different Bone Tissue Against Implant Material: A New Approach for Implant Loosening Detection
*Magnus Reulbach - Hannover Medical School - Hannover, Germany
Longwei Cong - Leibniz Universität Hannover - Hannover, Germany
Tim Kobelt - Leibniz University Hannover - Hannover, Germany
Stefan Zimmermann - Leibniz University Hannover - Hannover, Germany
Bernd-Arno Behrens - Leibniz University Hannover - Garbsen, Germany
Eike Jakubowitz - Hanover medical Schhol - Hannover, Germany
*Email: reulbach.magnus@mh-hannover.de
Introduction:
During the loosening process of hip arthroplasties tolerable relative movements of 30-100 µm between stem and bone will be exceeded and induce a typical loosening cascade leading to a gradual fixation loss. This loosening is often recognized only late by pain and, thus, a significant bone loss appears in most cases. This in turn is associated with higher complexity of revision surgeries. The introduction of acoustic emission (AE) analysis seems to be a promising approach to detect this early relative stem movements, since there is no reliable diagnostic method up to date. In experimental in-vitro investigations, isolated relative movements between bone and implant substrates will be induced in order to characterize AE signals caused due to implant loosening. The inclusion of influencing parameters such as implant material, surface roughness, pressure at bone-implant interface and different bone tissues lead to a wide variety of test combinations. The possibility of substituting human with animal bone for in-vitro testing would allow simplified sample preparation and a remarkable cost reduction. The aim of this preliminary study was to investigate whether AE-signals differ between bone tissue of different species.
Material and methods:
According to DIN-ISO-5832-3, Ti6Al4V wrought alloy was used as the implant substrate. The implant surface roughness according to uncemented hip implants was generated using corundum blasting (Ra 4.11±0.33 µm) according to Jakubowitz et al. (2017). Bone substrates were taken from bovine and porcine cortical bone. To characterize AE signals a test rig was developed to simulate relative motions between bone and implant metal substrates in a standardized manner (Figure 1). A bone-implant contact pressure of 5 MPa was applied. AE signals of 125 relative movements for each species were captured using a piezoelectric sound sensor (VS45-H, Vallen Systeme GmbH, frequency range: 20-450 kHz) placed directly on implant substrates. Data was processed with the AMSY-6 system and the software Vallen AE Suite Vers. R2020.1124.2 (Vallen Systeme GmbH). The algorithms for AE hit detection, denoising and characterization were coded in Python (Python Software Foundation, version 3.9). The machine-learning algorithm Random Forest Gini Feature Importance (RFFI) was used to evaluate the dissimilarities of time and frequency features of AE-hits (Muir et al. 2021) between different bone-material groups.
Results:
During single relative movement events, multiple AE-signal hits were detected (Figure 2). The peak frequency turned out to be the highest importance feature with RFFI (Figure 3). The graphical representation of the peak frequency distribution of all detected hits (n=3427) shows a clear accumulation around 110 kHz for bovine bone substrates, which is not apparent for porcine samples (Diagram on the right in Figure 3).
Conclusion/Outlook:
The study's results provide a first important milestone for THR loosening detection by AE-analysis. It became evident that there are differences in AE-signal characteristics resulting from bone tissue of different species. Further in vitro experiments are currently carried out to verify whether the differences described in the present study also show up when comparing with human bone tissue.
Acknowledgements:
Funded by the Deutsche Forschungsgemeinschaft (DFG, German Research Foundation) – SFB/TRR-298-SIIRI – Project-ID 426335750
Figures
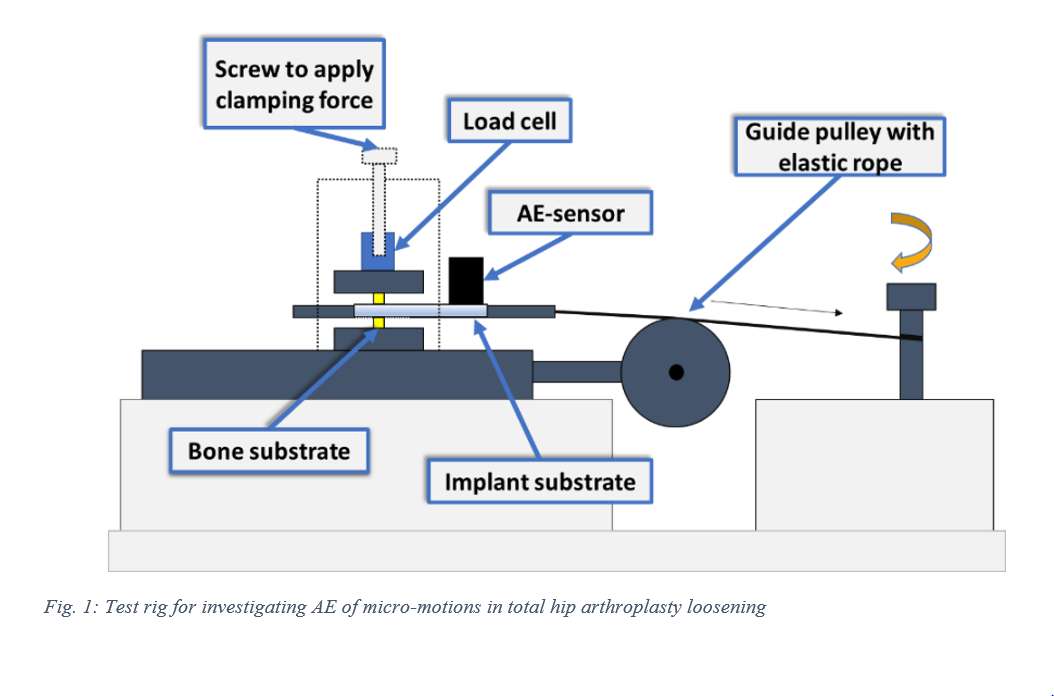
Figure 1
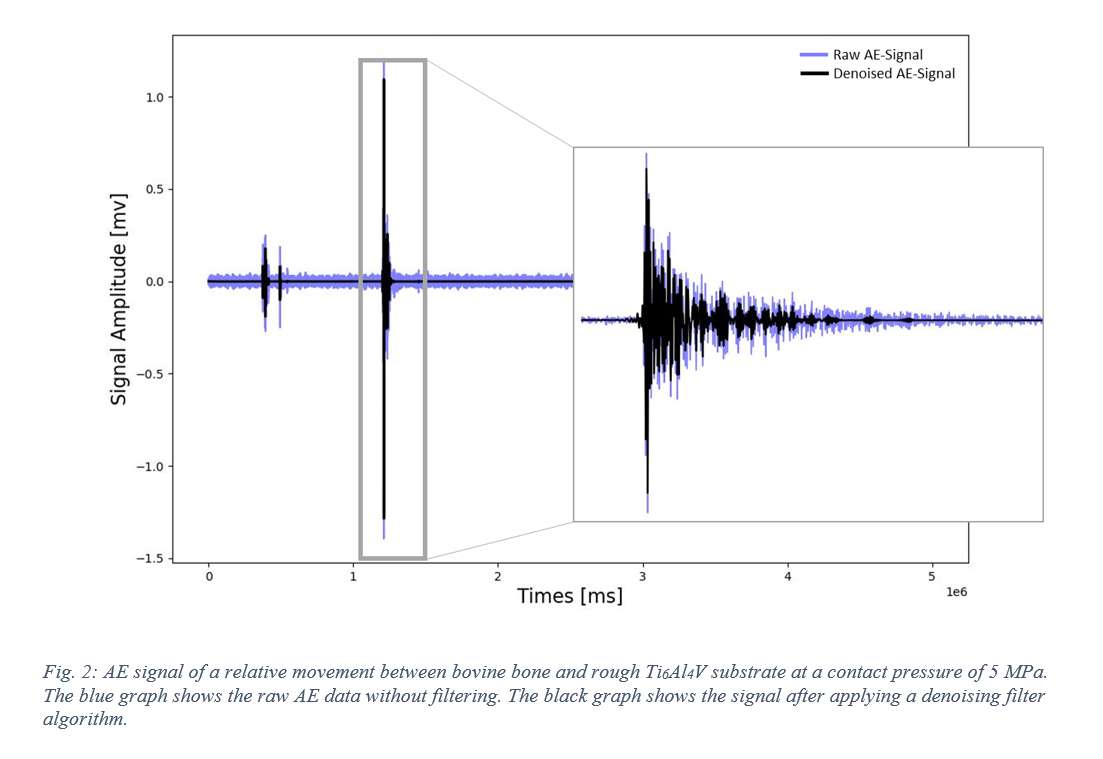
Figure 2
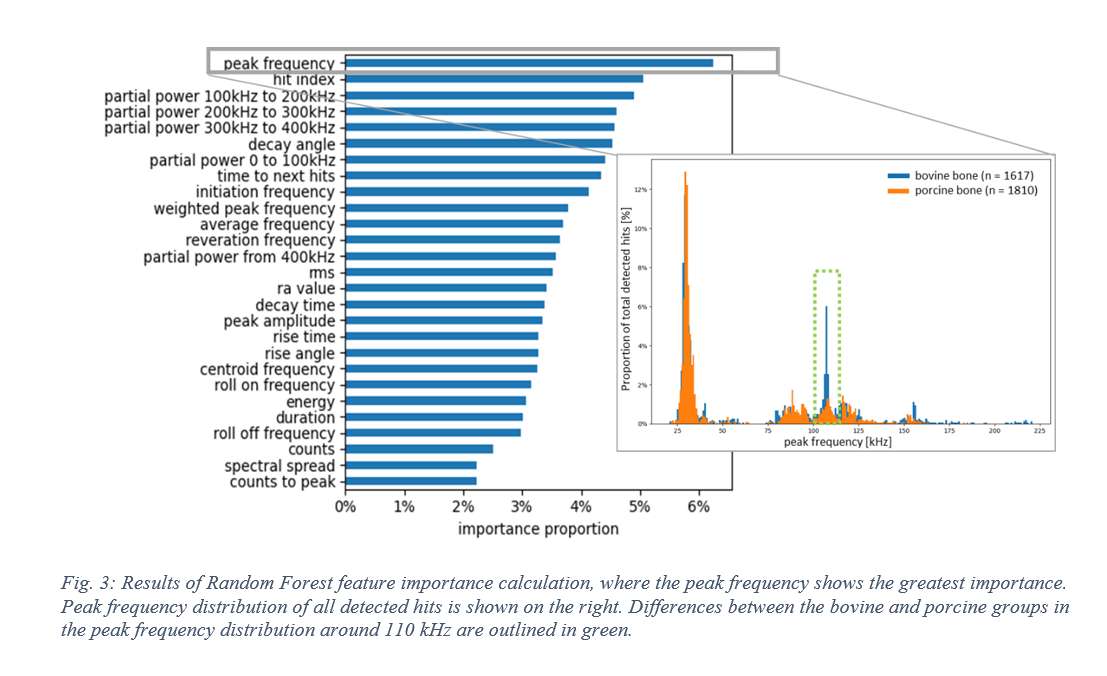
Figure 3#8263
Frictional Sensitivity Assessment During a Passive Knee Drop Test in a Surrogate Total Knee Model
*Kevin Abbruzzese - Stryker - Mahwah, USA
Andre Freligh - Stryker Orthopaedics - MAHWAH, United States of America
Scott Logan - Stryker Orthopaedics - Mahwah, USA
Sally LiArno - Stryker Orthopaedics - Bergenfield, USA
*Email: kevin.abbruzzese@stryker.com
Introduction:
Osteoarthritis is a degenerative joint disease that is commonly associated with knee stiffness. This can affect biomechanical properties at the knee which can be attributed to frictional changes between joint surfaces. The pendulum knee drop (PKD) test is a widely used method for assessing knee stiffness by measuring passive knee oscillations over time. A surrogate total knee arthroplasty bone model with representative soft tissue components was used to assess the frictional response of different material components. The PKD test was performed on the knee model to determine the impact of frictional changes on knee stiffness. Range of motion (ROM) was assessed and stiffness and damping factors were estimated to determine knee stiffness.
Methods:
To assess knee stiffness changes between materials with different frictional properties, the PKD test was performed on a mechanical bone total knee model with representative soft tissue, Figure 1. An inertial measurement unit (IMU) sensor was placed on the tibia to record ROM. Three trials were performed for each of the three different implant materials (TKA, Nylon, and Connex), with the order of materials randomized for each trial. The model femur was fixed to a table and the femur and tibia implant materials for each group were applied to the model. The knee was dropped from full extension at the calibrated reference position and allowed to oscillate until rest. The IMU sensor was used to measure knee ROM and the log decrement ratio and damping coefficients were calculated for each condition.
Results:
A total of nine trials were conducted, with three trials for each material. An Anderson darling test was performed to assess data for normality. The data was non-normally distributed and a Kruskal-Wallis test was used to assess significance between material groups. Significant differences were detected between all material groups (p<0.05). The TKA and Connex group were significantly stiffer than the nylon group. The Connex group resulted in the greatest stiffness as compared to all other trials, Figure 2. An increase in coefficient of friction between materials led to significantly stiffer knees as observed with the log decrement coefficient. The same trends were observed for the damping coefficient.
Conclusion:
This study aimed to investigate knee stiffness by varying implant materials in a knee arthroplasty model using the pendulum knee drop (PKD) test. Frictional properties were assessed by comparing three groups representative of clinically relevant populations like osteoarthritis, knee arthroplasty and hyaline cartilage. The results demonstrated a significant increase in knee stiffness in the Connex group, which had higher frictional effects. Conversely, the nylon group, which represents highly lubricious materials, resulted in decreased stiffness. The PKD test was sensitive enough to detect simulated physiological changes in knee stiffness based on frictional characteristics. These findings have important clinical implications, as they may aid in identifying or characterizing the severity of osteoarthritic conditions or frictional changes at the joint surface. This study provides valuable insights into the relationship between knee stiffness based on material properties and demonstrates the sensitivity of this test to detect changes in representative physiological conditions.
Figures
Figure 1#8163
Should Lymphocyte Transformation Testing Be Done in Patients With Multiple Allergies?
*Gerhard Maale - Medical Center Plano - Plano, USA
*Email: gerhardmaale53@gmail.com
INTRODUCTION: Metal-ion allergies are observed in 82.3% of patients experiencing multidirectional instability (MDI) and arthrofibrosis. This is an early cause of failure in 1.5% of primary total knee arthroplasties (PTKA). The question is whether or not patients with a multiplicity of allergies should be tested for metal-ion allergies as well prior to PTKA. Third party insurance or CMS does not cover the expense for this study.
METHODS: All patients exhibiting a hypersensitivity to metal ion by Lymphocyte Transformation Test (LTT) were analyzed for other allergies. This cohort consisted of 200 patients with known metal allergies. They were analyzed for other known allergies based on hospital charts. These allergies were broken down to metals and other allergies.
RESULTS: Out of the 200 patients diagnosed with a metal allergy, 164 (82%) had at least 1 associated other allergy. In this group, the average age was 66 with a 2:1 female to male ratio indicating the allergy is gender specific. The 164 patients had an average of 4.2 allergies per patient. Of the 200 patients with nickel hypersensitivity, 120 (60%) had at least 1 allergy to an additional metal.
CONCLUSION: In this group of patients, there was a high correlation evident between the presence of nickel allergies and the number of associated other allergies, including to other metals (82%). When other multiple allergies are seen, because of this correlation LTT testing preoperatively would be recommended as this group of patients are of a higher risk to metal-ion allergies. When patients have metal allergies, they require hypoallergenic devices that are different than routine PTKAs.
#8160
Multi-Directional Instability and Arthrofibrosis Associated With Metal Ion Allergies Mimicking Infections
*Gerhard Maale - Medical Center Plano - Plano, USA
*Email: gerhardmaale53@gmail.com
Introduction: Patients can experience multiple problems following a primary Total Knee Arthroplasty (TKA). These problems include infection, component loosening, multi-directional instability (MDI), and arthrofibrosis. MDI following a primary TKA is a clinical syndrome characterized by global ligament laxity, pain while getting up from a seated position, audible clunking of the implant in varus-valgus stressing, instability in gait, and a warm knee effusion. These patients present with restricted range of motion (arthrofibrosis). Is MDI associated with arthrofibrosis commonly seen with metal-ion allergies in failed TKA patients?
Methods: In this cohort, patients presenting with clinical MDI and restricted range of motion following a primary TKA were subject to a Metal-Lymphocyte Transformation Test (LTT). These studies were done prior to TKA revision. Specific metal-ion allergies were reviewed retrospectively. All patients had pre-op imagery including WBC, CT, and Tri-phase bone scans. Biopsies and cultures were performed at the time of revision.
Results: Of the 277 patients experiencing MDI with arthrofibrosis, 200 patients (72.2%) tested positive for nickel hypersensitivity and 228 (82.3%) were allergic to some metal in the implant . Of the 200 patients with nickel allergy, 67 had multiple metal-ion allergies. When comparing metal-ion allergies in the population of total knees (1.5%) to the patients with MDI in arthrofibrosis and also gender-specificity, the p-value is < 0.001 using Fischer’s exact study.
Conclusion: MDI associated with arthrofibrosis accounts for 1.5% of failures in primary TKAs. It is associated with metal ion allergies in 82.3% of the cases. Nickel is the most common metal allergy (72.2% of the MDI’s). It is gender-specific 2:1 female to male. MDI with arthrofibrosis is the second most common cause of failure in primary TKAs, only second to infection.
#8408
The Rate of Decline in Renal Function After Metal-on-Metal THA Is Improved by Revision Surgery for the Treatment of Adverse Reactions to Metal Debris.
*Masahiko Haneda - Kobe University Graduate School of Medicine - Kobe, Japan
Naoko Shima - Hyogo Rehabilitation Center - Kobe, Japan
*Email: konnvitz2@yahoo.co.jp
Introduction: Metal-on-metal total hip arthroplasty (MoM-THA) requires attention to the postoperative decline in renal function. The purpose of this study are 1) to investigate the change of renal function after MoM-THA, 2) to investigate the relationship between adverse reactions to metal debris (ARMD) and renal function, and 3) to investigate the renal function after revision surgery.
Methods: Of 369 MoM-THA cases using Conserve plus cup (Wright Medical) performed at our hospital from November 2007 to March 2011, 249 cases (28 males and 221 females) were included in this study in the MoM-THA group. Cases with bilateral MoM-THA, with a postoperative follow-up of less than 1 year, in which dialysis was introduced, or cases in which revision was performed at other hospitals were excluded. Fifty patients who underwent non-MoM-THA (ceramic on ceramic or ceramic on polyethylene) with minimum 5-year follow-up at our hospital from June 2002 to February 2017 were included in the non-MoM-THA group. Age, estimated glomerular filtration rate (eGFR, mL/min/1.73m2) as renal function at primary THA, revision surgery, and last follow-up, and serum cobalt (Co) and chromium (Cr) ion concentrations at revision surgery were investigated. Statistical analysis was performed using Spearman's rank correlation coefficient, Mann-Whitney U test, and Wilcoxon signed-rank test.
Result: There were no significant differences in age (67.1 years in the MoM-THA group, 63.7 years in the non-MoM-THA group, p=0.075) and eGFR at primary THA (81.4 in the MoM-THA group, 85.0 in the non-MoM-THA group, p=0.224). The mean follow-up period was 9.9 years in the MoM-THA group and 8.2 years in the non-MoM-THA group (p=0.001). Annual eGFR decline was significantly faster in the MoM-THA group compared to the non-MoM-THA group (-2.0 vs -1.2, p=0,003, Fig.1). One hundred forty-eight of 249 cases in the MoM-THA group developed ARMD, and the AMRD- group was older than the ARMD+ group (74.5 vs 62.1 years, p<0.001) and lower preoperative eGFR (77.8 vs 83.8, p=0.005), but no difference in the annual eGFR decline (-2.0 vs -1.9, p=0.603) was observed. Of the 148 patients in the ARMD+ group, 80 underwent revision surgery, of which 53 patients could be followed up for at least 1 year after surgery. The mean follow-up period between primary THA and revision surgery was 6.6 years and the mean follow-up period after revision surgery was 5.7 years, with no significant difference (p=0.176). The annual eGFR decline slowed with revision surgery (-2.1 from primary THA to revision surgery, -0.9 from revision surgery to last follow-up, p=0.006, Fig.2). No correlation was found between eGFR and Co or Cr ion concentrations at the time of revision surgery in 55 patients for whom serum Co and Cr ion concentrations could be measured (Co: r=-0.075, p=0.5869; Cr: r=0.188, p=0.170, Fig.3).
Conclusion: The annual eGFR decline in the MoM-THA group decreased about twice as fast as that in the non-MoM-THA group, but the annual eGFR decline slowed with revision surgery. No significant relationships were observed between the presence of ARMD and the annual eGFR decline, and between serum Co or Cr ion concentrations and eGFR.
Figures
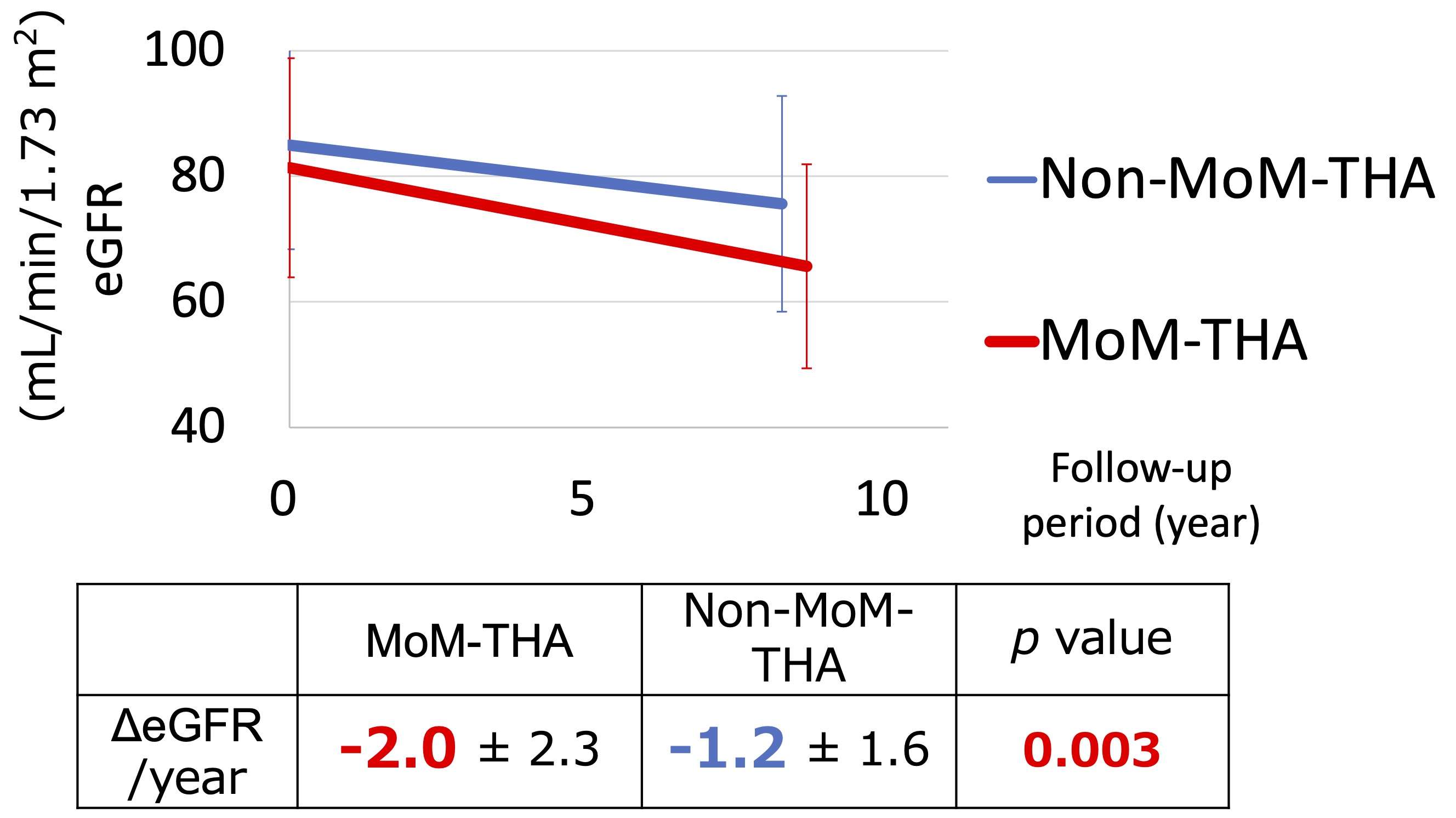
Figure 1
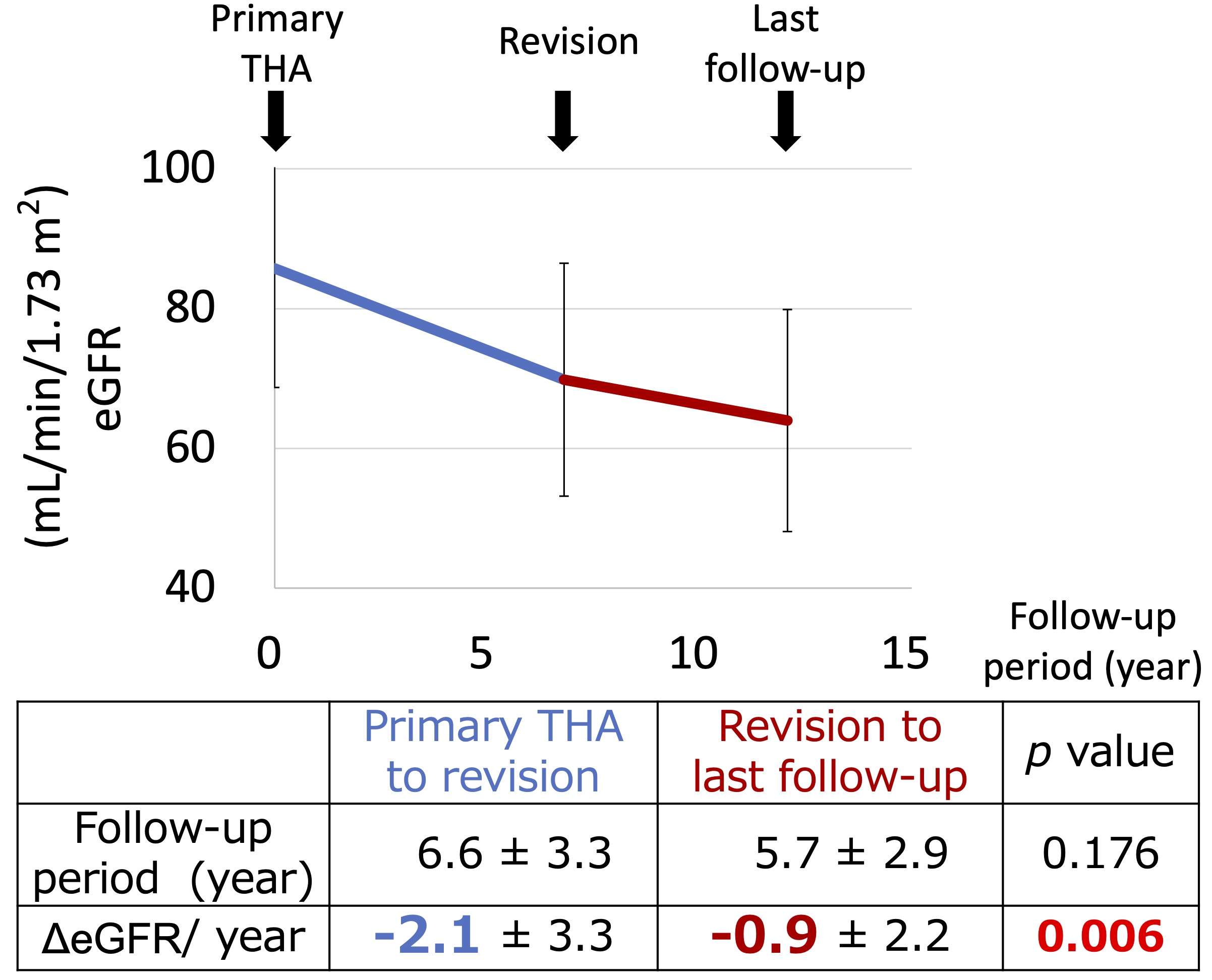
Figure 2
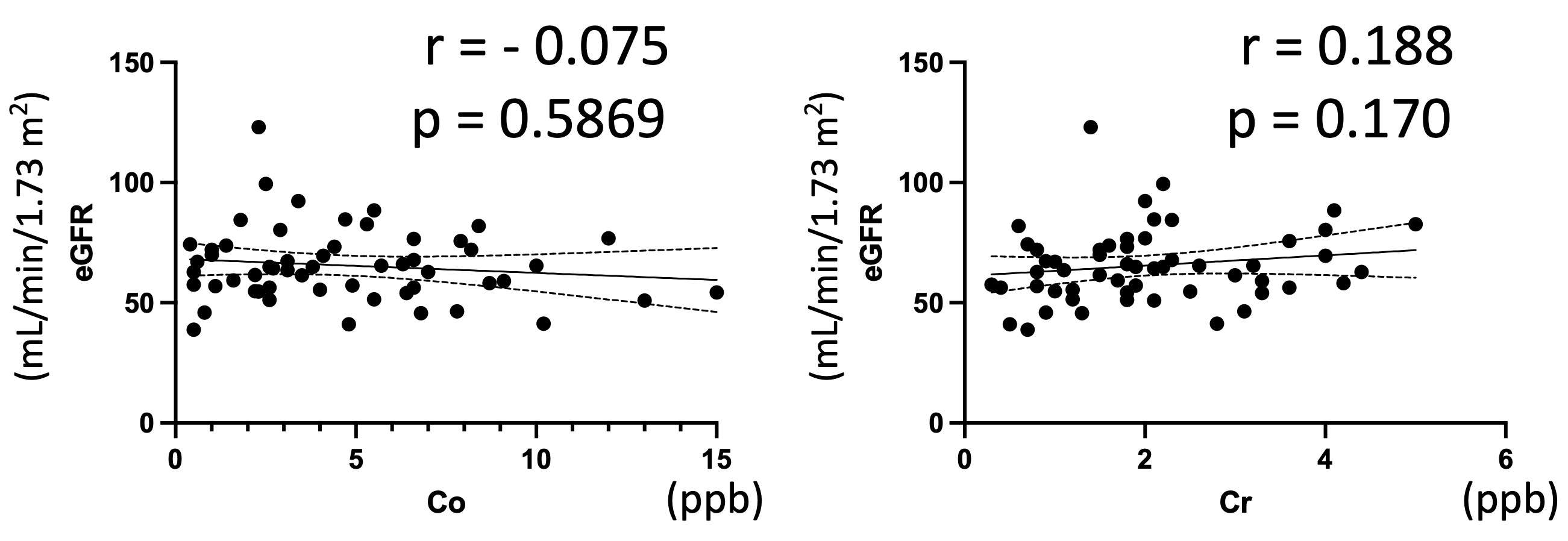
Figure 3#8409
The Incidence of ARMD and Revision Rate After Metal-on-Metal THA With a Large-Diameter Femoral Head
*Naoko Shima - Hyogo Rehabilitation Center - Kobe, Japan
Masahiko Haneda - Kobe University Graduate School of Medicine - Kobe, Japan
*Email: naoko.shima.4947@gmail.com
Introduction: Metal-on-metal total hip arthroplasty (MoM-THA) with a large-diameter femoral head has advantages of joint stability and less wear. However, adverse reactions to metal debris (ARMD) after MoM-THA is concerned. This study aimed to investigate the incidence of ARMD and revision rate after MoM-THA, and to report our algorithm for revision.
Materials and Methods: From 2007 to 2011, 337 patients (373 hips) underwent MoM-THA with one type of cup, large-diameter femoral head, and stem with modular-neck at our institution. There were 36 males (39 hips) and 301 females (334 hips) with a mean age of 64 years. Bone lesions including osteolysis on plain radiographs, pseudotumor on computed tomography (CT) or magnetic resonance imaging (MRI) were diagnosed as ARMD.
Results: ARMD was diagnosed in 194 of 373 hips (52.0%), with a mean time to onset of 5.3 years. Revision was performed in 102 hips (27.3%), of which 99 hips (26.5%) were due to ARMD. Three hips were replaced due to periprosthetic femoral shaft fracture, cup dislocation, or infection. The mean age at revision was 68.4 years, and the time from primary THA to revision for ARMD treatment was 7.0 years. All revisions were performed using a posterior approach. Periarticular pseudotumor was resected as much as possible while carefully preserving neurovascular structures in accordance with the following principles: (1) modular-neck is replaced in case tranionosis is observed; (2) stem is preserved in case no implant loosening and no malalignment; (3) a cementless component is used in case the bone on the acetabular side is in good condition; (4) either ceramic-on-polyethylene or ceramic-on-ceramic bearing surfaces are selected in case adequate stability was achieved with trial components, often with 32 to 36 mm ceramic heads; (5) the gluteus maximus muscle transfer is considered in case with considerable instability due to soft tissue imbalance. Our strategy for revision surgerry after MoM-THA is shown in Figure 1. Cup, head, and modular-neck were replaced in 73 of the 99 hips that underwent revision. All implants were replaced in 26 hips, of which 8 stems were replaced with cementless, and 18 with cemented stems. There were no cases in which only head and modular-neck were replaced. Cementless cups were used in 90 hips. Of these, 85 hips used ceramic-on-polyethylene bearing surfaces, 1 hip for dual mobility, 1 hip for large MoM bearing, and 3 hips for ceramic-on-ceramic. Cemented cups with ceramic-on-polyethylene bearing surfaces were used in the remaining 9 hips, of which support rings were used in 6 hips. Bone grafts were performed in 35 hips with marked osteolysis. After 99 revisions, complications occurred in 7 patients including 2 of infection, 1 of femoral shaft fracture, 1 of aseptic loosening, and 3 of dislocation.
Re-revision was required in 5 hips, because of 2 infections, 1 aseptic loosening around a cup, 1 femoral shaft fracture, and 1 dislocation.
Conclusion: Our study revealed that MoM-THA with a large-diameter femoral head has a high risk of ARMD, and revision using our algorithm results in fewer complications and re-revision.
Figures
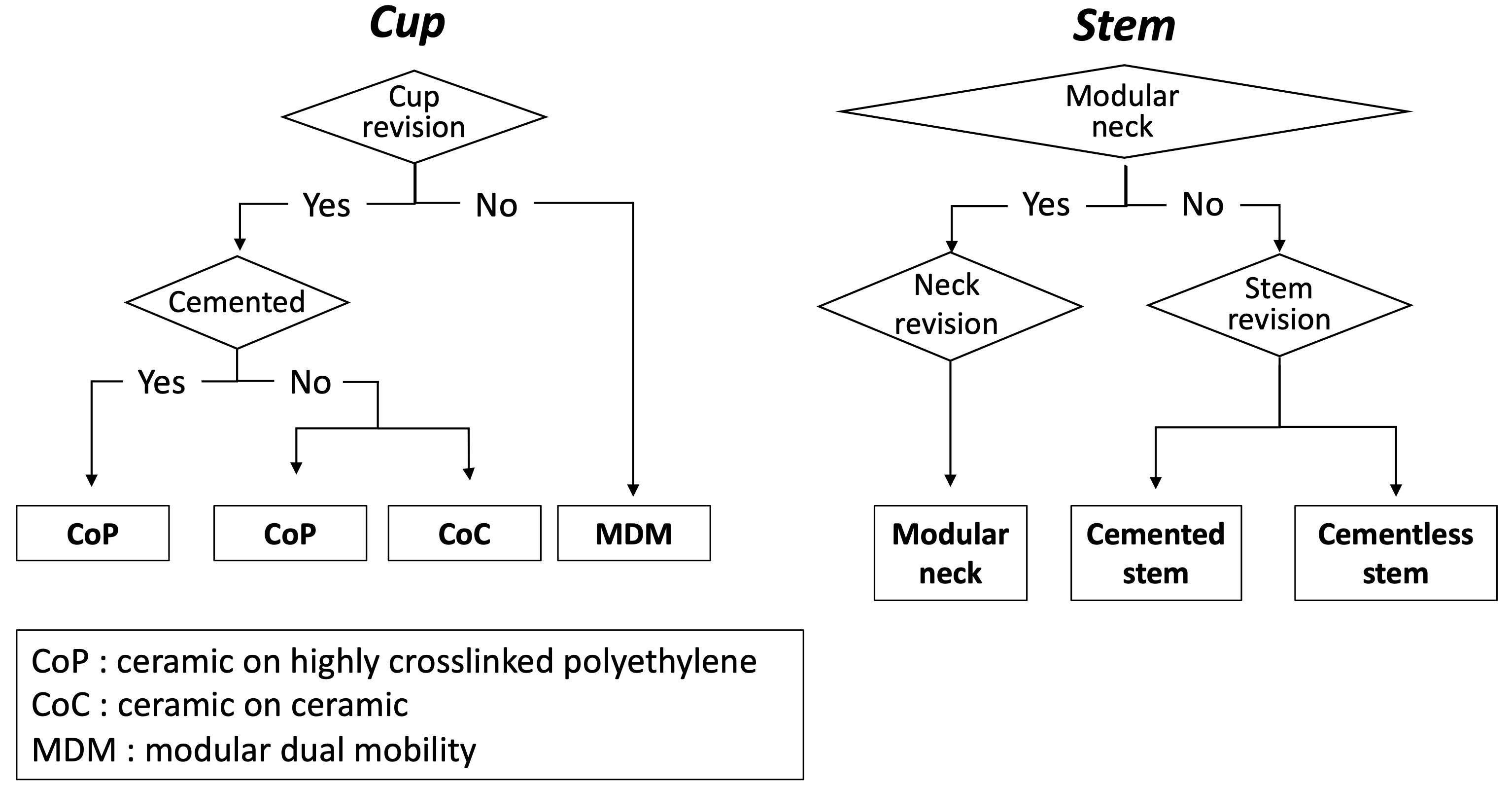
Figure 1#8471
In Vitro Effects of Lymphocytes and Macrophages on CoCrMo and TiAlV Alloys
*Lisa Phan - University of Tennessee Health Science Center - Memphis, USA
William Mihalko - University of Tennessee - Memphis, USA
Madison Brown - University of Tennessee Health Science Center - Memphis, USA
Richard Smith - Univerisity of Tennessee Health Science Center - Memphis, USA
Brian Morrow - University of Tennessee Health Science Center - Memphis, USA
*Email: qyd488@uthsc.edu
Introduction
This study explores the effects of inflammatory cell-induced corrosion (ICIC) on the surface chemistry of orthopaedic implant alloys. ICIC refers to the direct attack and corrosion of implant surfaces by inflammatory cells. This study aims to observe the effects of cellular corrosion when macrophages are co-cultured with lymphocytes. We hypothesized that the oxide levels of disks would be higher in activated cell groups compared to non-activated cell groups, and in macrophage and lymphocyte groups compared to macrophage-only groups.
Methods
ASTM F1537 CoCrMo and ASTM F136 TiAlV disks were prepared. The macrophages were either non-activated or activated using a growth medium that included IFNγ and LPS, and either cultured alone on the disks or with T helper lymphocytes. Once the cells were cultured on the disks for 30 days the disks surface was examined with a SEM and the oxide levels on the surface were analyzed with EDS.
Results
SEM images showed features with widths similar to those of inflammatory cells and were identified as valleys. These features may have been caused by ICIC and did not originate from the sanding process since they crossed in a different direction. The Disks + Non-Activated Medium groups for both alloys had significantly higher oxide levels compared to all other groups (p< 0.001). Furthermore, the Activated MO + Lymphocytes + CoCrMo disks group had significantly higher oxide levels than the Nonactivated MO + CoCrMo disks (p = 0.002) and Activated MO + CoCrMo groups (p < 0.001).
Conclusion
The findings of this study may be useful in developing strategies to prevent ICIC and related adverse reactions to metal debris associated with TJA surgeries. This study highlights the importance of understanding the role of inflammatory response in affecting the surface chemistry of orthopaedic implants and the need for further research in this area.
References
- Natu, S., Sidaginamale, R. P., Gandhi, J., Langton, D. J. & Nargol, A. V. F. Adverse reactions to metal debris: histopathological features of periprosthetic soft tissue reactions seen in association with failed metal on metal hip arthroplasties. J Clin Pathol 65, 409–418 (2012).
- Langton, D. J. et al. Adverse reaction to metal debris following hip resurfacing: the influence of component type, orientation and volumetric wear. J Bone Joint Surg Br 93, 164–171 (2011).
- Kwon, Y. M. et al. ‘Asymptomatic’ pseudotumors after metal-on-metal hip resurfacing arthroplasty: prevalence and metal ion study. J Arthroplasty 26, 511–518 (2011).
- Cook, R. B. et al. Pseudotumour formation due to tribocorrosion at the taper interface of large diameter metal on polymer modular total hip replacements. J Arthroplasty 28, 1430–1436 (2013).
- Gilbert, J. L. et al. Direct in vivo inflammatory cell-induced corrosion of CoCrMo alloy orthopedic implant surfaces. J Biomed Mater Res Part A 103, 211–223 (2014).
- Miller, K. C., Holloway, M. B., Morrow, B. R., Smith, R. A. & Mihalko, W. M. In-Vitro Cell-Induced Corrosion by Macrophages on Cobalt-Chromium-Molybdenum Alloy. J Arthroplasty 37, S355–S363 (2022).
Figures
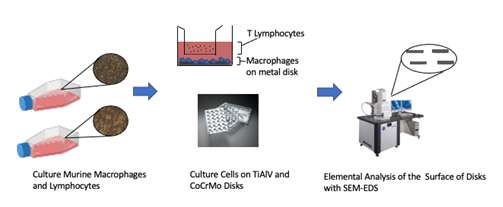
Figure 1

Figure 2
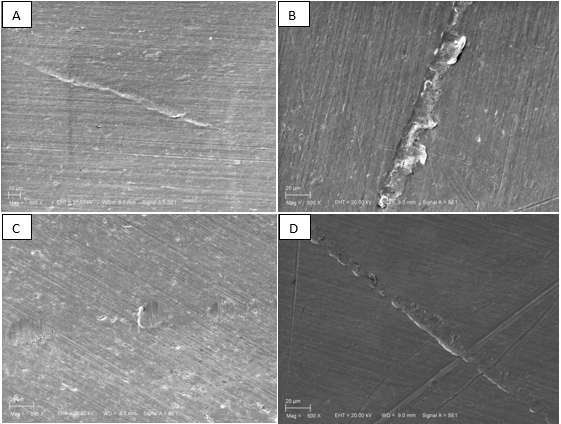
Figure 3#8317
Failure of a Femoral Revision Knee Component With Chemical Corrosion and Elevated Metal Ions: A Case Report
*Aarti Shenoy - Hospital for Special Surgery - New York, USA
Christopher Holland - Duke University Medical Center - Durham, USA
Paul F Lachiewicz - Chapel Hill, USA
*Email: shenoya@hss.edu
Introduction: Modular taper junction failure in a total knee arthroplasty (TKA) implant is a rare complication. Corrosion at these interfaces remains under-reported in the literature. We present here a case of late, atraumatic failure of a modern, revision modular TKA femoral component with elevated preoperative serum cobalt and chromium ion levels.
Case Presentation: The patient, a 146 kg (BMI 40.4), 61-year-old man, underwent a two-stage reimplantation with cemented modular stemmed femoral component, tibial component, and posterior-stabilized (PS) polyethylene liner (Sigma; DePuy Synthes Johnson & Johnson, Warsaw, IN, USA), 13 years previously, after a failed primary TKA complicated by periprosthetic joint infection (PJI). Patient experienced no issues for the subsequent 11 years and was ambulatory with no support and no history of trauma. Patient presented with a slight limp, trace effusion of the right knee, full extension, no extensor lag, and flexion at 65 degrees. Laboratory results are as follows: hematocrit 41.6, hemoglobin 13 gm/dl, erythrocyte sedimentation rate 40, C-reactive protein 1.22, with negative infectious work up. Anterior-posterior radiograph showed osteolysis in the anterior femur and proximal medial tibia, and a radiolucent line between the proximal end of the stem extension and the condylar femoral component (Fig. 1). Serum metal ion levels showed cobalt 7.3 ppb and chromium 4.6 ppb (Quest Diagnostics, Chantilly, VA, USA). Metal synovitis was observed intraoperatively. The condylar component (CC) appeared to be loosened slightly while the tibial and patella components were well-fixed. A revision femoral component and stem were cemented into the distal femur and a “PS plus” polyethylene liner was placed. Pathologic examination of the synovium was performed and retrieved components were visually examined under light microscopy, with specific surface damage features captured using digital optical microscopy. Pathologic examination of the synovium, under light microscopy, showed a diffuse histiocytic infiltrate, with fine cytoplasmic black granules, and focal lymphocytic cuffing of small vessels, indicative of aseptic lymphocyte dominant vasculitis-associate lesion[1]. At four months postoperatively, the serum cobalt had decreased to 1.4 ppb, and serum chromium to 1.1ppb, and to 0.9 ppb, and 1.1 ppb respectively at six months.
Retrieved femoral component was disassembled into the condylar component (CC), stem, and bolt using minimal torque. The taper junction formed a flat-on-flat contact between the bolt and the CC, which exhibited extensive corrosion damage. The bolt showed corrosion damage over most of the surface, with etching type material loss in some regions (Fig. 2). The condylar component taper region exhibited a roughened appearance all around the taper surface (Fig. 3, marked).
Discussion: This unique case of failure at the condylar and stem extension junction of a modern, modular revision femoral component 11 years after implantation occurred with a non-constrained PS liner in place, which to our knowledge, has not been reported. The corrosion damage at the bolt taper surface appears to be typical of intergranular corrosion as described in previous publications [2], [3].Material loss observed on all taper surfaces likely contributed to the metal ions detected in serum, and in the stained periprosthetic tissue samples.
Figures
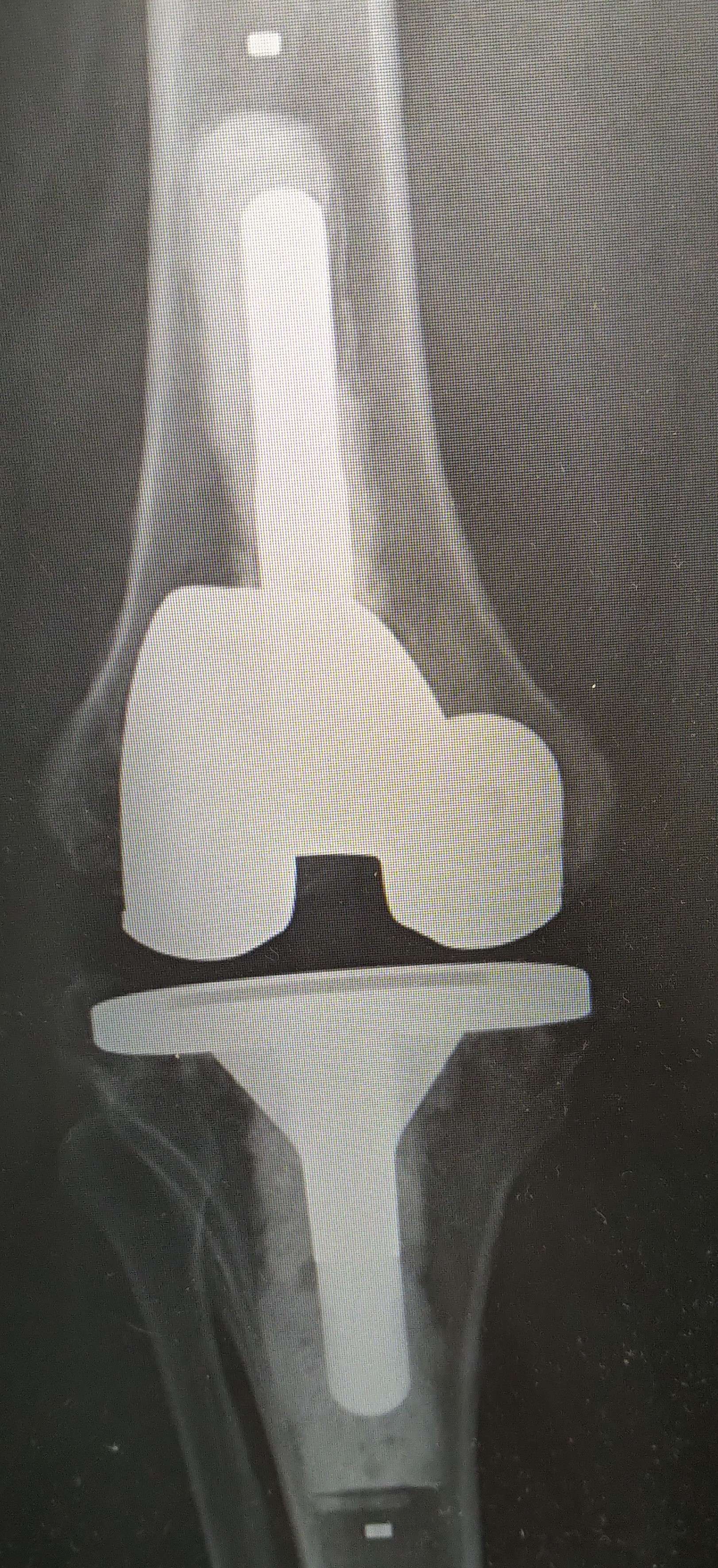
Figure 1

Figure 2

Figure 3#8630
Electrochemical Properties of Synovial Fluid and Their Correlation to Patient Reported Outcomes
*Madison Brown - University of Tennessee Health Science Center - Memphis, USA
Lisa Phan - University of Tennessee Health Science Center - Memphis, USA
Jordan Justice - University of Tennessee Health Science Center - Memphis, USA
Chandler Sears - University of Tennessee Health Science Center - Memphis, USA
Marcus Ford - Campbell Clinic Orthopaedics - Germantown, USA
John Crockarell - Campbell Clinic Orthopaedics - Germantown, USA
James Harkess - Campbell Clinic - Collierville, USA
James Guyton - Campbell Clinic Orthopaedics - Germantown, USA
Marc Mihalko - Campbell Clinic Orthopaedics - Germantown, USA
William Mihalko - University of Tennessee - Memphis, USA
*Email: mbrow275@uthsc.edu
Introduction: Total knee arthroplasty (TKA) is now one of the most performed elective surgeries with estimates predicting 1.26 million annual TKAs by 2030. However, the satisfaction rate may be as low as 80% and tens of thousands of patients are subjected to revision surgery. TKA systems consist of a Ti-6Al-4V baseplate and CoCrMo femoral component. Patient biology may initiate corrosion at the device interface in vivo, facilitating auto-catalytic behavior where initial corrosion promotes further corrosion. In this study, we evaluate the corrosion potential of synovial fluid (SF) obtained immediately prior to TKA. We hypothesize that the electrochemical corrosion rate will correlate with patient satisfaction.
Methods: Human synovial fluid samples were collected in the operating room at the time of TKA. A three-electrode low volume electrochemical cell was used with an ASTM F1537 CoCrMo or ASTM F136 TiAlV working electrode, platinum counter electrode, and Ag/AgCl reference electrode. Three electrochemical tests were performed on each alloy to obtain the electrochemical properties: open circuit potential (OCP); electrochemical impedance spectroscopy (EIS); and linear polarization (LP). The patient’s stiffness, pain, and daily function were self-reported using a Knee injury and Osteoarthritis Outcome Score, Joint Replacement (KOOSJR) survey after at least 3 months post-op. Corrosion rates were calculated and compared to find statistical outliers. A statistical outlier was classified as being two standard deviations above the mean. Outliers for corrosion rates that were identified had their KOOSJR score compared to others for significance as determined by a non-paired t test.
Results: The mean corrosion rates without outliers for TiAlV and CoCrMo are 0.008 ± 0.006 (n = 66) and 0.035 ± 0.09 (n = 86) mm/yr respectively. There are two outliers (0.028 and 0.036 mm/yr) in the TiAlV group and six for CoCrMo (0.183, 0.302, 0.314, 0.326, 0.332, 0.511 mm/yr). Three of the six patients with high CoCrMo corrosion rates, determined as outliers (0.183, 0.302, and 0.332 mm/yr) have corresponding KOOSJR scores available during this time in the study. Currently, there is only one TiAlV outlier (0.036 mm/yr). No relationship was found between corrosion rates and KOOSJR scores.
Conclusion: A subset of patients have higher electrochemical corrosion rates. Investigating the electrochemistry between SF and implant metal can provide insight into patient pain and TKA failure. Our study is ongoing, and we plan to enroll more patients and follow them up to a year after surgery to investigate the relationship between electrochemical properties and patient outcomes.
#8480
Comparison of Tribo-Corrosion Behaviours of Selective Laser Melted CoCrMo and Wrought CoCrMo
Edona Hyla - University of Leeds - Leeds, GB
*Qingyue Shi - Imperial College London - LONDON, GB
Andrew Robert Beadling - University of Leeds - Leeds, United Kingdom
Gregory deBoer - Institute of Thermofluids, University of Leeds - Leeds, United Kingdom
Richard Hall - University of Leeds - Leeds, United Kingdom
Connor Myant
Michael Bryant - University of Leeds - Leeds, United Kingdom
*Email: esperanzalovegood@gmail.com
Conventional CoCrMo alloys are commonly used in orthopaedics due to their favourable biocompatibility and mechanical properties. However, their use is still debated due to concerns about toxicity, especially after the recent Metal-on-Metal and Taper-Trunnion failures. The advent of selective laser melting (SLM) additive manufacturing (AM) of CoCrMo alloys has opened up the possibility of patient-specific and more complex implants. Nevertheless, questions have been raised regarding their wear-corrosion performance and their equivalence to conventional CoCrMo devices. Therefore, this study aims to compare the tribo-corrosion behaviours of AM- and wrought CoCrMo (W-CoCrMo) alloys to evaluate the performance of patient-specific prostheses.
AM-CoCrMo was fabricated with AconityMIDI SLM machine, using gas-atomized Co−28Cr−6Mo alloy powder. All specimens were polished to a mirror finish with a Ra<0.05, cleaned in an ultrasonic bath containing acetone for 10min and rinsed with deionized water before testing. One specimen from each group was electro-etched with 10% oxalic acid at 5V for 15s for microstructural examination. To simulate microscale abrasion processes commonly seen in vivo a micro-tribometer was used. A diamond tip (Rtip=12.5µm) was used to slide with a normal force of Fn=200mN for 1.5mm at 1.5Hz for 2700 cycles. To evaluate the corrosion, a 3-electrode electrochemical cell was integrated. Current transients were recorded during sliding motion, while immersed in Phosphate Buffered Saline, under potentiostatic polarization conditions (+100mV vs Ag/AgCl). Vertical scanning interferometry (VSI) and scanning electron microscopy (SEM) were used for post-analyses.
Following 500 cycles, the coeffictiont of friction of both AM- and W-CoCrMo stabilized with no significant difference. (Fig.1a, Tab.1). The low CoF values are attributed to high contact stresses, which align with previous research.[2] Fig. 1b and Table 1 showed a lower charge transfer for AM-CoCrMo compared to W-CoCrMo. This can be attributed to the finer carbide structure and regular distribution in AM-CoCrMo alloys, as reported in previous studies albeit under static conditions.[3] The AM-CoCrMo exhibited honeycomb-shaped cellular grains and melt pool boundaries (Fig.2c), whereas the W-CoCrMo showed larger equiaxed grains (Fig.2d). Subsequent to the tribocorrosion test, SEM images show plastic deformation as the main wear mechanism for both samples, but twinning is more dominant in W-CoCrMo (Fig.2e and 2f). Larger grain sizes in W-CoCrMo may cause single-crystal or coordinated multi-crystalline deformation due to the diamond tip engaging only with a few equiaxed grains.[4] It has been reported that the cellular boundaries in AM-CoCrMo potentially exhibit a higher wear resistance than the interior of the cell.[5] The presence of higher plastic deformation therefore can be explained due to the smaller grain boundary area present in W-CoCrMo.
This study suggests that AM-CoCrMo has a potential advantage over conventional W-CoCrMo alloys in terms of tribocorrosion resistance, which is a critical factor in orthopaedic applications. This advantage is likely attributed to the unique microstructure of the AM-CoCrMo specimens, which can be customized and optimized to meet specific needs.
Acknowledgements: Funding from European Union's Horizon-2020 programme, Marie Sk?odowska-Curie grant No.956004.
[1]Kim et al., J Alloys Compd, 2020
[2]Martinez-Nogues et al., Tribol Int, 2016
[3]Dong et al., Corros Sci, 2020
[4]Mace and Gilbert, Wear, 2022
[5]Liu et al., “Tribol Int, 2022
Figures
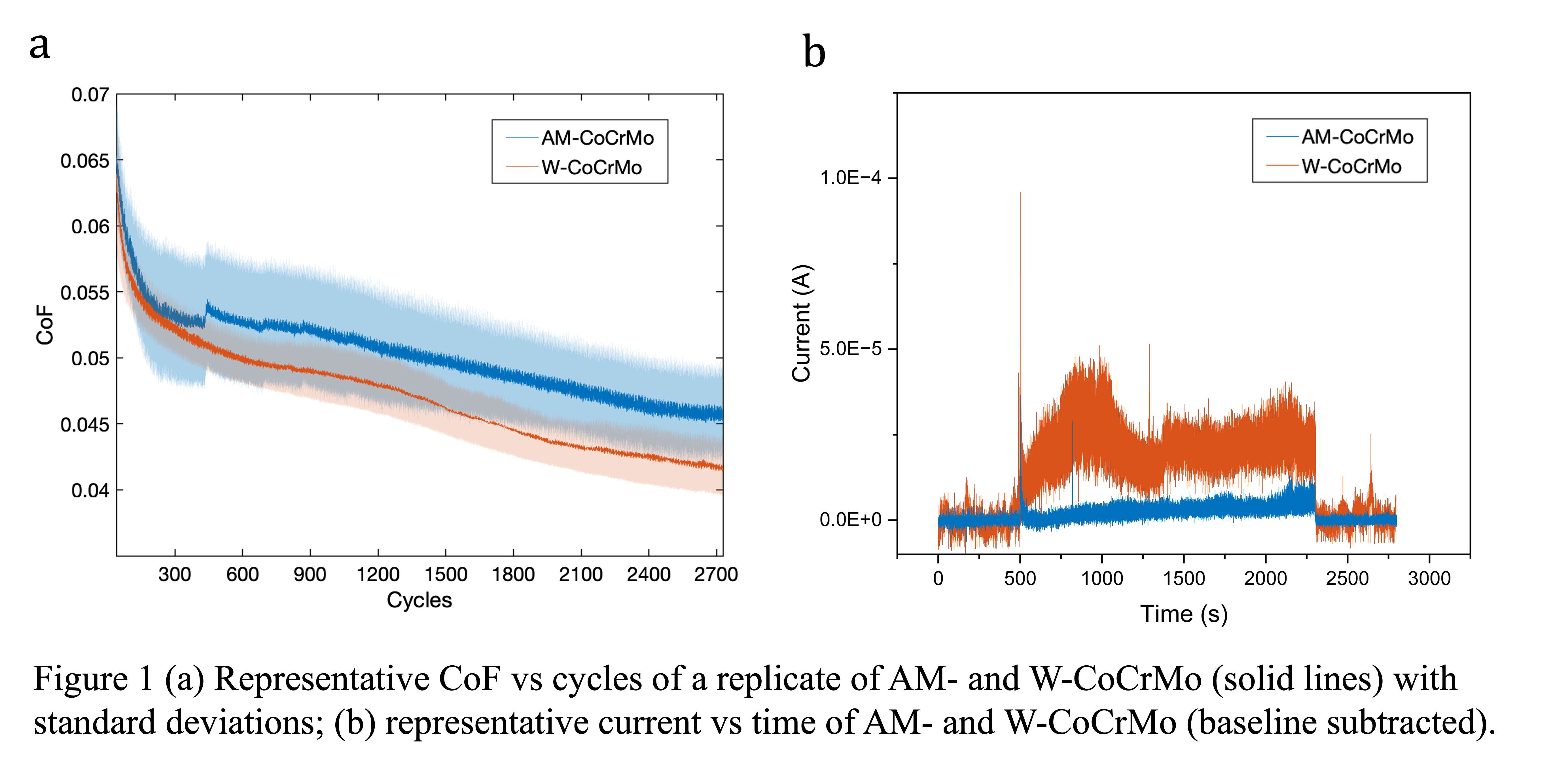
Figure 1
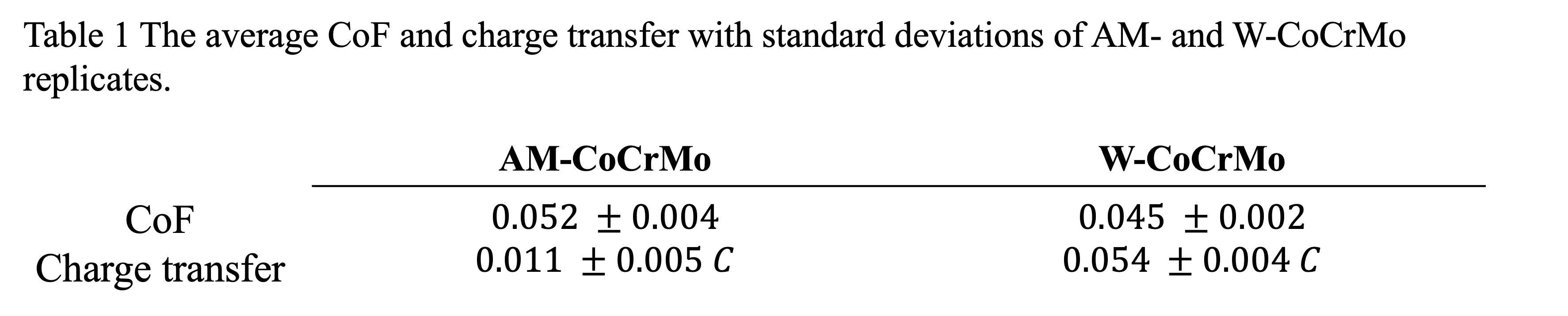
Figure 2
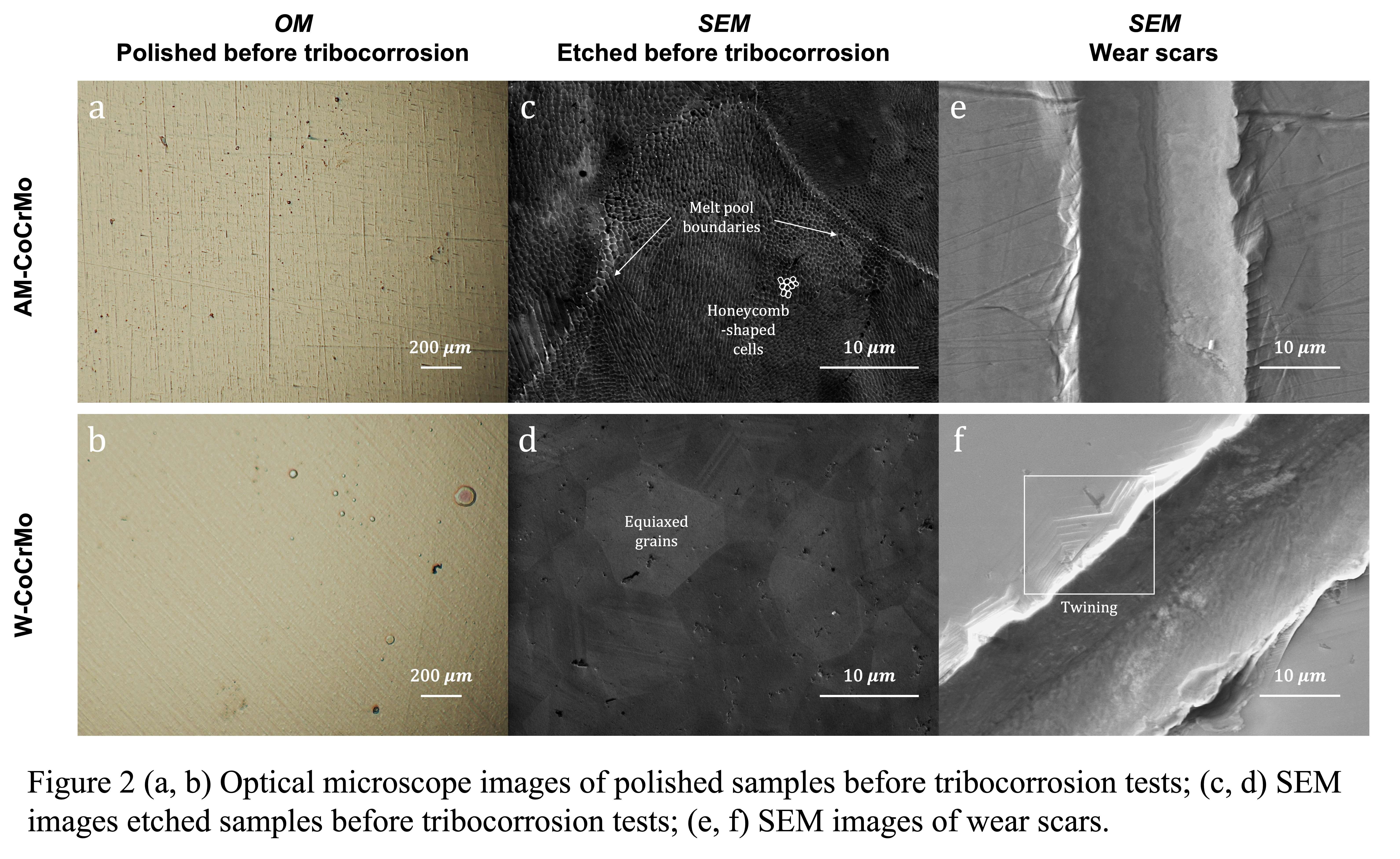
Figure 3#8531
Real-Time AFM-Based Nanoscale Tribocorrosion Analysis - Tribogram: A New Analysis Approach
Hwaran Lee - Clemson University
*Jeremy Gilbert - Clemson University - Charleston, USA
*Email: jlgilbe@clemson.edu
Introduction
Tribocorrosion occurs due to micromotion between metallic implants, the most clinically relevant being modular taper junctions of hip implants. Although atomic level deformation from micromotion could cause microscale tribocorrosion, leading to adverse metal ions and particle release, nanoscale single asperity wear mechanisms using a sub-micron radius asperity have not been investigated to date. This study used a diamond atomic force microscopy (AFM) probe tip to mimic the cyclic micromotion within modular taper junctions by producing multiple scratches along a single line. This project aimed to develop a new approach to measuring topographical changes (quantitative and qualitative results) in real-time on metallic oxide films during surface fretting. We describe this approach as a “tribogram”. We hypothesized that the quantitatively converted results from visualized AFM images could bring new insights for understanding nanoscale wear mechanisms.
Methods
CoCrMo (ASTM F1537, low carbon) alloy was polished on 240, 320, 400, and 600 grit emery paper under flowing water. Each sample was polished with 1 mm alumina powder in deionized water and washed under flowing deionized water and 70% ethanol. A diamond AFM probe (Radius~500 nm, k=350 N/m, NM-TC, Bruker) generated multiple scratches (128-line, 8 μm ´ 8 μm, 0.5 Hz, 90°) in one single line on the CoCrMo alloy surface using disabled slow scan under contact mode (Fig. 2b). The deflection sensitivity was 122.71 nm/V, and the applied voltage was 4 V. We analyzed height changes using AFM height sensor results divided at 0, 32, 64, 96, and 128 lines in Nanoscope software (version 9.4).
Results
The AFM tribogram (Fig. 1) visualized topographical changes via color variations across numbers of scratches (scratch #). The 3-dimensional image (x = length, y = scratch #, z = depth) presented the progression of the metal surface over wear cycles. Fig. 2a, which consists of individual scratch #’s, quantitatively demonstrates the evolution of topography and how much the diamond tip penetrated via height analysis of 5 lines. The surface wear depth increased with scratch #. The greatest height change (20 nm) was observed at the beginning of scratching from lines 0 to 32. Another feature was that four sliding distance positions (dashed lines) were worn out more than others (Fig. 3). The distances between the deep grooves were 2-4 μm, similar to the grain boundary size of CoCrMo alloy (3-4 μm). Therefore, there might be a relationship between grain boundary and preferential deformation. The deformation could occur more easily at grain boundaries during fretting corrosion, leading to a higher occurrence of intergranular corrosion.
Conclusion
This study developed a new method to identify tribological effects on oxide films of CoCrMo alloy surfaces in real-time and introduced the concept of tribogram – a direct measure of topography during wearing. The AFM height images and converted quantitative data may lead to a better understanding of how many scratches will significantly damage metal alloys, depth changes, and topographical details. In conclusion, this new tribology analysis using AFM could improve nano- and microscale wear mechanisms in modular taper junctions of hip replacements.
Figures
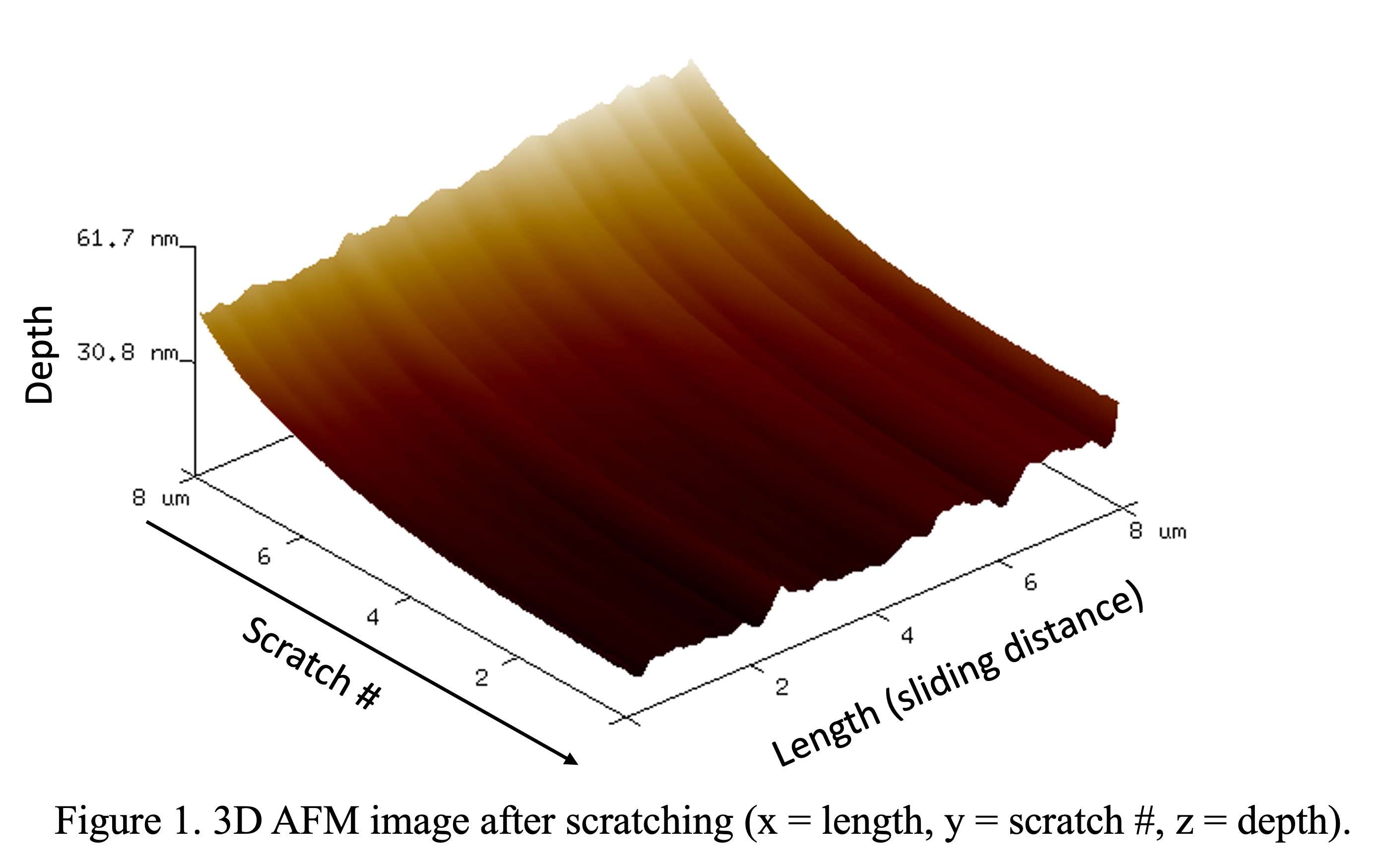
Figure 1
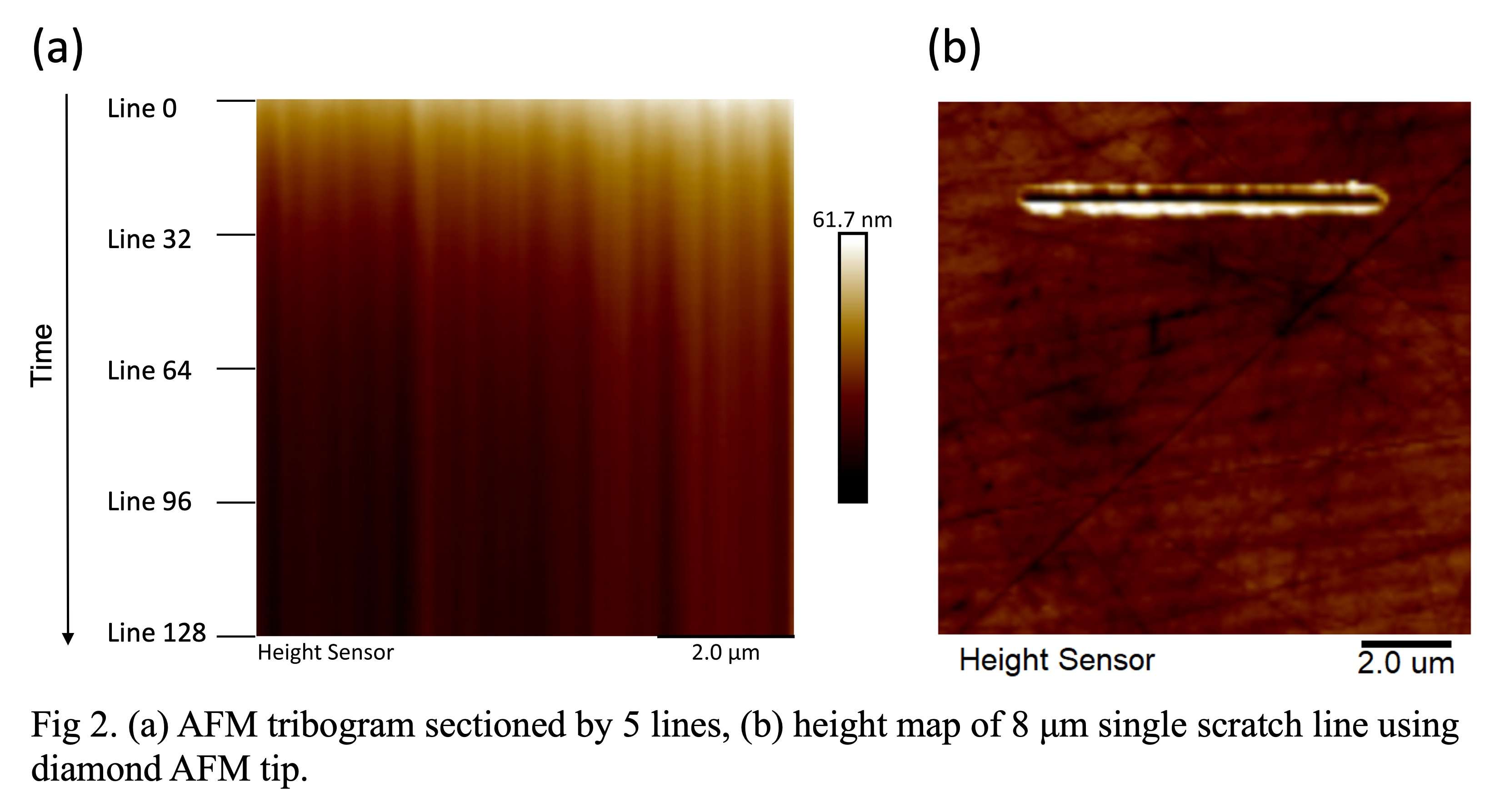
Figure 2
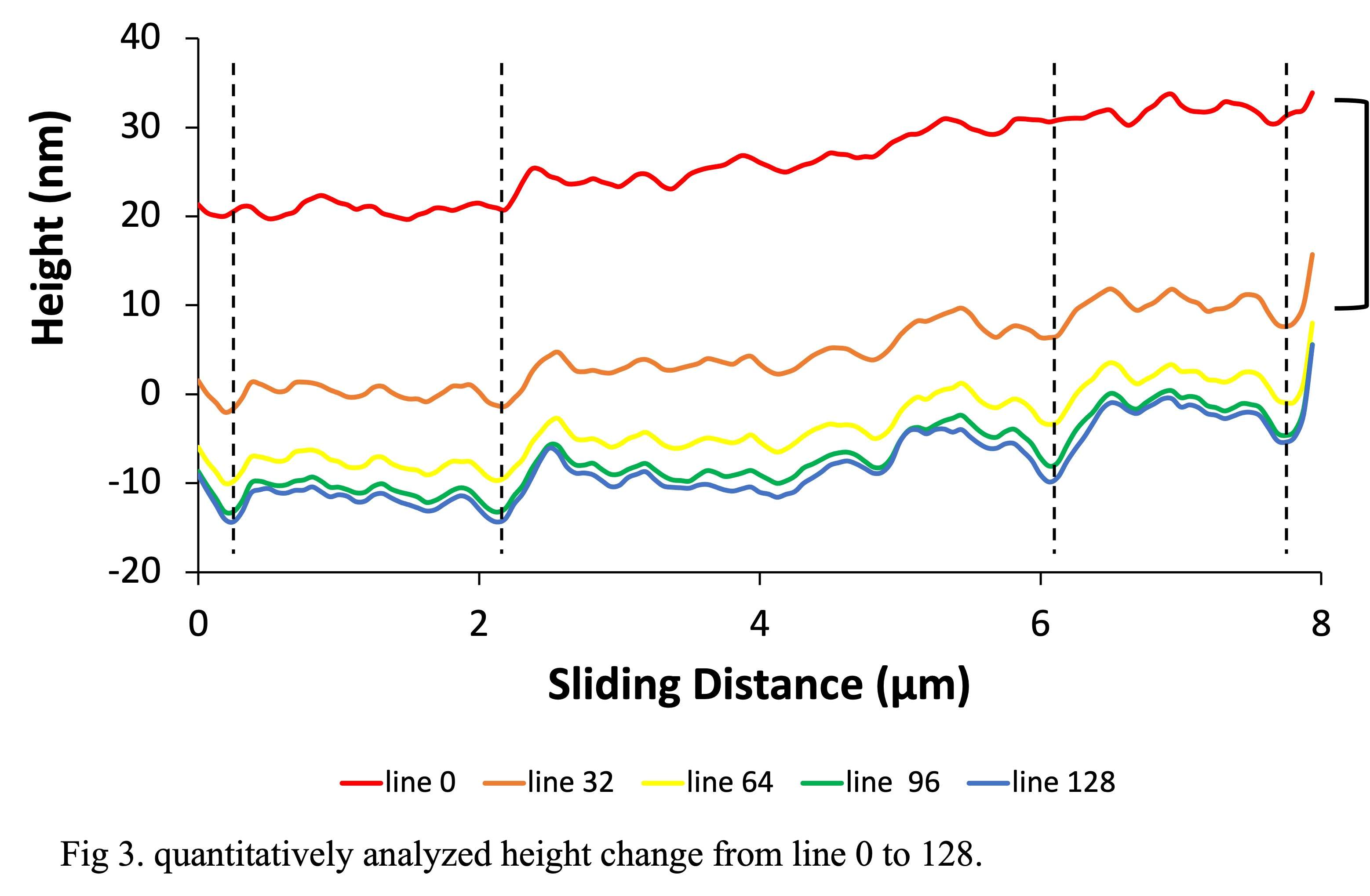
Figure 3#8169
Metal Allergy in Knee Arthroplasty: Current Evidence and Future Trends in Better Detection
*Ram Venkatesh - Leeds Teaching Hospitals - Leeds, GB
Hemant Pandit - Leeds Institute of Rheumatic and Musculoskeletal Medicine - Leeds, United Kingdom
*Email: kneejointsurgery@gmail.com
Introduction: Management of patients with history of metal allergy can be challenging when they present with
symptomatic knee arthritis needing a total knee arthroplasty (TKA). This scoping review summarises current
literature and provides an evidence-based logical approach for managing such patients.
Methods: Studies evaluating TKA patients with metal hypersensitivity/allergy were identified by searching
Cochrane Central Register of Controlled Trials, Ovid MEDLINE, and Embase, from their inception to April
2023. All studies reporting on diagnosing or managing metal hypersensitivity in TKA were included. Data were
extracted and summarized based on study design, patient population, interventions listed including
complications encountered and reported outcomes.
Results: We included 47 heterogeneous studies (two randomized controlled trials, eight comparative studies, 23
case series, and 14 case reports). The evidence indicates that true metal hypersensitivity is difficult to diagnose
with certainty with current armamentarium of screening tests preoperatively. A significant proportion of these
patients can have subclinical / overt psychological distress and this can negatively impact their clinical
outcomes. Hypoallergenic implants are viable alternatives for patients with self-reported/confirmed metal
hypersensitivity if declared preoperatively; however, no long-term outcomes are available for them and
informed consent is paramount. For patients presenting with painful TKA, metal hypersensitivity is a diagnosis
of exclusion where patch skin testing, lymphocyte transformation test, and synovial biopsies are useful adjuncts
before revision surgery is undertaken to hypoallergenic implants with shared decision-making and informed
consent. In these cases, a multidisciplinary, team-based approach with input from immunologists, radiologists,
and infectious disease physicians, orthopaedic surgeons and mechanical engineers are vital in appropriate
diagnosis and treatment options. Outcomes of revision surgery with hypoallergenic implants can improve
symptom in ~60% of cases.
Conclusion: Current assessment tools and existing evidence regarding true metal hypersensitivity in patients
presenting with history of metal allergy and needing a TKA is inadequate. There is currently no consensus on
diagnostic criteria for immune failure, an allergic reaction, to a TKA. Patient perceptions, surgeon outlook,
informed consent with multi-disciplinary approach and judicious use of diagnostic tests will help in optimising
post-operative outcomes. Further research is needed to better diagnose and manage patients presenting with
suspected metal allergy.
#8165
Retrieval Analysis of Universal II and Remotion Wrists
*Göksu Kandemir - Newcastle University - Newcastle Upon Tyne, United Kingdom
Thomas Joyce - Newcastle University - Newcastle upon Tyne, United Kingdom
*Email: goksu.kandemir@newcastle.ac.uk
Keywords: explant analysis; wrist implants; total wrist arthroplasty; metal-on-polymer
Introduction
Total wrist arthroplasty (TWA) lacks the success seen with hip and knee joint replacement. Many different designs of TWA have been offered. Two of the most common designs of metal-on-polymer TWA are the Universal II and the ReMotion. For the first time a comparative explant analysis of the two designs was undertaken.
Methods
Two different types of artificial wrist implants, Universal II and Remotion wrists, that utilised elliptical metal-on-polymer articulations were analysed. The radial and carpal plates of the Remotion wrists were cobalt chromium (CoCr), and the inserts were ultrahigh molecular weight polyethylene (UHMWPE). The radial plates of the Universal II wrists were CoCr, their carpal plates were titanium, and their inserts were UHMWPE. There were 2 Remotion wrists (medium and small-sized) and 8 Universal II wrists (large (1), medium (2), small (2), and extra small-sized (3)) available for analysis. Using a Zygo NewView 5000 non-contacting profilometer, surface topographies of the components were investigated at the nanoscale. At the macroscale, the explanted components were visually analysed for damage and damage scores were assigned to each component. The articulating surfaces and the backside of the polymeric inserts were analysed based on seven different damage modes: surface deformation, pitting, embedded debris, scratching, burnishing, abrasion, and delamination. The metallic radial plates and carpal plates were assessed based on five different damage modes; grooving, indentation, pitting, abrasion and deformation.
Results
All metallic surfaces of the Remotion wrists became rougher, the articulating surfaces of the inserts became smoother, and the backside of the inserts became rougher in vivo. All radial plates of the Universal II wrists became rougher, 4 of the carpal plates became smoother, the articulating surfaces of all of the inserts became smoother and the backside of 7 inserts became rougher in vivo.
The metallic surfaces were scratched, and inserts were burnished, suggesting abrasive and adhesive wear to be the dominant wear mechanisms in vivo. The damage observed on the components of Universal II wrists was significantly more than the damage observed on the Remotion wrists. Therefore, the damage scores assigned were higher for Universal II components. Unlike Remotion inserts, Universal II inserts showed delamination. The articulating surface of the inserts of the Remotion wrist had an average damage score of 17.49, which was 64.14 for the articulating surface of the Universal II inserts. The average damage scores of the back surfaces of the inserts were 3.14 for the Remotions and 11.52 for the Universal IIs. The Remotion radial plates had an average damage score of 3.80, whereas the Universal II radial plates had an average damage score of 9.50. The Remotion carpal plates had an average damage score of 7.26, whereas the Universal II carpal plates had an average damage score of 10.65.
Conclusion
Components of the Remotion wrists did not show major damage, whereas the components of the Universal II wrists showed substantial damage. The polyethylene inserts showed the severest damage.
#8424
Third Body Wear in-Vivo: An Analysis of 18 Retrieved Femoral THA Components
Ilya Borukhov - Stryker Orthopedics - Mahwah, USA
*Sally LiArno - Stryker Orthopaedics - Bergenfield, USA
*Email: Sally.LiArno@stryker.com
INTRODUCTION
Roughening of the metallic femoral surface in-vivo after total hip arthroplasty (THA) has been reported previously and can occur due to recurrent dislocations or third-body abrasive damage[1,2]. Sources of third-body abrasive particles can vary greatly and can include bone, bone cement, metallic, or ceramic debris. It is important to understand the damage created by third-body wear mechanisms in-vivo in order to stress THA materials in-vitro. Furthermore, soon-to-be published revisions of ISO 21535 will require wear testing under aggressive third-body conditions although no standard for it exists currently. To facilitate development of clinically relevant third-body wear testing, we retrieved metallic components of THA that indicated potential third-body wear mechanisms and assessed the surface damage.
METHODS
A total of 18 CoCr femoral THA components from a single manufacturer were obtained after filtering the retrieval database using keywords that would indicate third-body wear. Reason for revision and implantation time was summarized. A single investigator identified damaged regions of the femoral components for surface texture assessment. Surface texture parameters were measured using a white light interferometer (WLI) (NewView 6300, Zygo, Middlefield, CT). A minimum of 4 images, 0.702-x-0.526mm, were captured in the identified regions for each femoral component. Form was removed using a 4th-order polynomial. Surface roughness (Sa,nm) and average peak-to-valley height (Rz,µm) were reported. The same procedure was performed on new femoral heads (n=5) of the same material for comparison. The data for all components were grouped, box plots for each surface parameter generated, and median, 5th and 95th-percentile were calculated for each surface parameter.
RESULTS
Of the 18 CoCr femoral components retrieved, 3 were dual mobility and 15 were fixed bearing. The most frequent bearing size was 36mm (n=13) with a single component each of size 28, 32, 38, 46, and 48mm. The reasons for revision were implant fracture (n=5), loosening (n=4), dislocation (n=2), pain (n=4), infection (n=1), metallosis (n=1) and product recall (n=1). Implantation time was on average 4.92±3.98 years (range:0.02-11.00 years, n=15). A total of 229 surface scans were acquired on retrieved components and 20 on new components. Median Sa for new components was 4.3nm (3.5-5.3nm) and was 35.5nm (4.7-223.6nm) for retrieved components (Figure 1). Rz increased from 0.9µm (0.1-2.1µm) for new components to 2.6µm (0.6-4.7µm) for retrieved components. Figure 2 shows some representative WLI images from various components.
DISCUSSION
The results in this study compare well to that of Ito et al. who found a mean roughness of 120±110nm (range:10-450nm) in 94 retrieved femoral heads [1]. We found that the distribution of roughness within and across retrieved components was highly nonnormal. This is likely due to the variability and randomness in the causes of third-body wear, depending on particle source, severity, and activity. Across all surfaces analyzed, we found femoral components were roughened 8X (based on median) to 42X (based on 95th percentile). In order to advance in-vitro simulation of next generation bearing materials and designs, emphasis should be placed on test methods to evaluate implants under aggressive conditions and replicate clinically relevant damage.
REFERENCES
[1]Ito.,J.Arthroplasty,25(2):302-308,2010. [2]Mai.,Am.J.Orthop,39(10):495-500,2010.
Figures
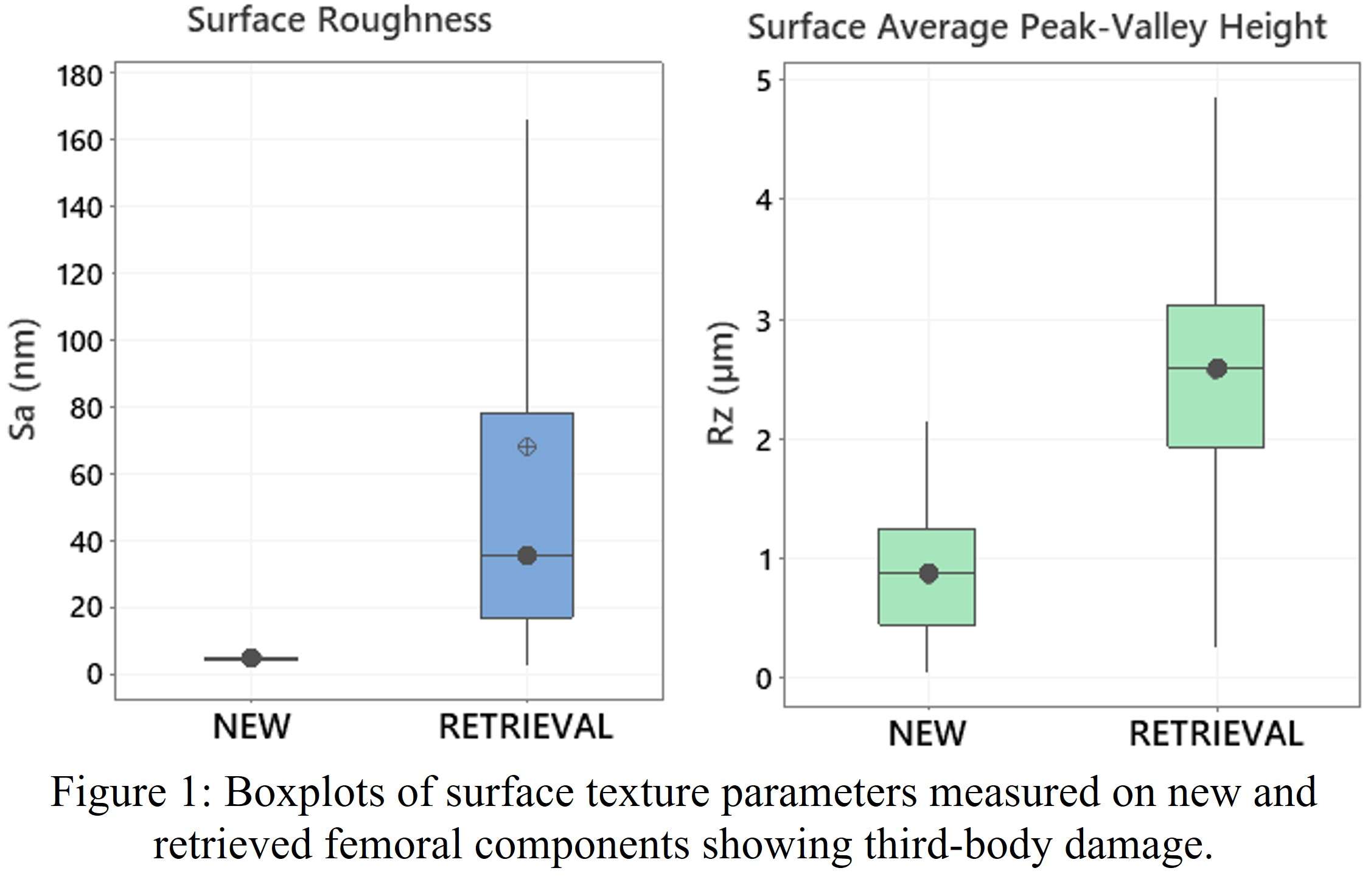
Figure 1
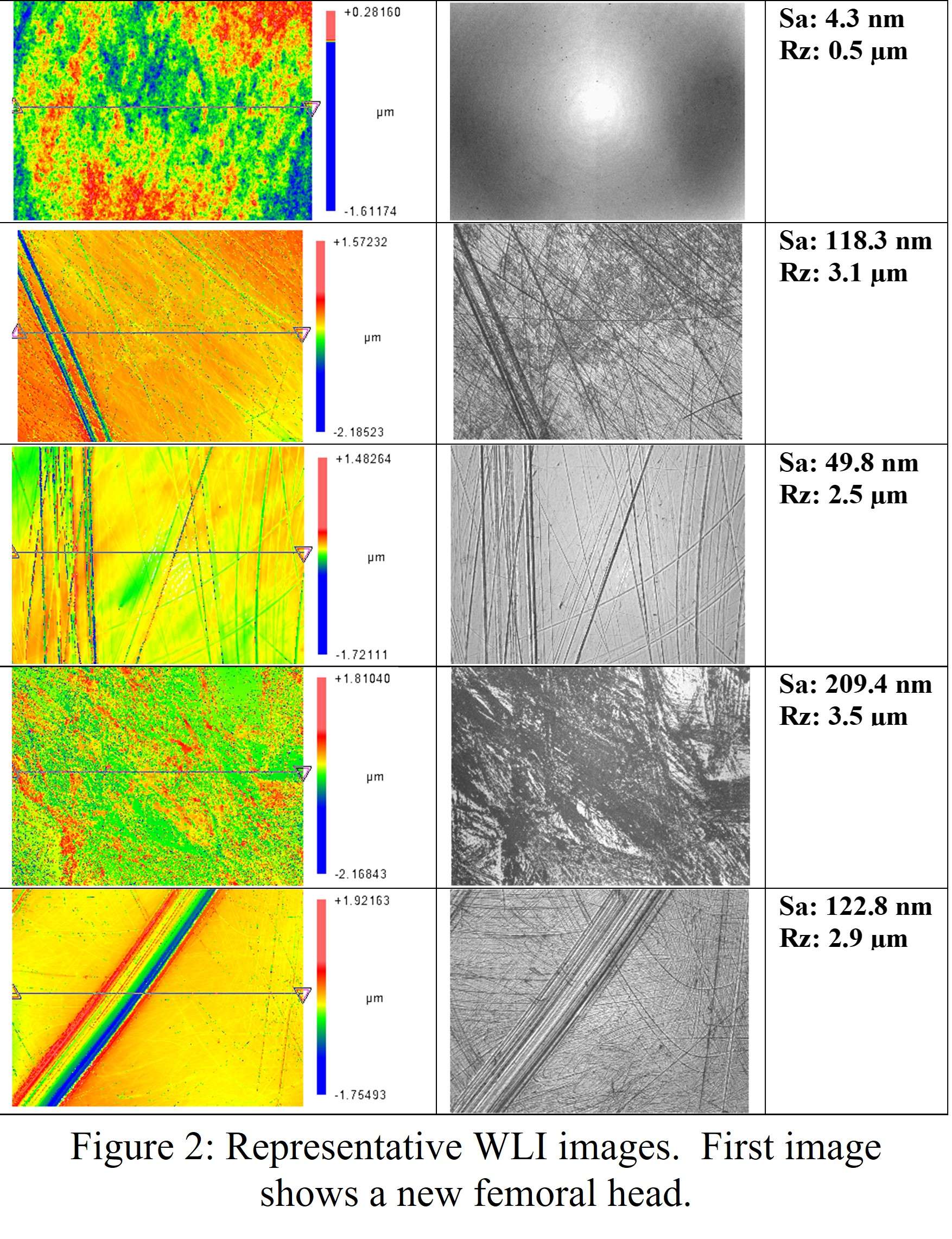
Figure 2#8315
Corrosion in Modular Dual Mobility Acetabular Components: A Retrieval Analysis Study
*Aarti Shenoy - Hospital for Special Surgery - New York, USA
Ashley Pekmezian - Hospital for Special Surgery - New York, USA
Timothy Wright - Hospital for Special Surgery - New York, USA
Douglas E Padgett - Hospital for Special Surgery - New York, USA
*Email: shenoya@hss.edu
INTRODUCTION: The use of modular dual mobility (MDM) acetabular components is rising in THA as a mitigating strategy for dislocation and instability related failure. However, mechanically-assisted crevice corrosion (MACC) concerns at the shell-liner interface remain. We performed a retrieval analysis on liners from two commercially available designs in clinical use in the U.S. to investigate corrosion damage types and severity.
METHODS: G7® Acetabular Dual Mobility System (Zimmer Biomet, “G-7”) and MDM® X3® Dual Mobility System (Stryker, “Stryker”) liners retrieved between 2017 and 2022 were selected from an IRB-approved institutional retrieval system and matched by patient demographics (patient age, sex, BMI and length of implantation), resulting in 10 pairs. Reasons for revision of Stryker liners were instability in 5 (45%), infection in 3 (27%), suspected corrosion in 1 (9%), pelvic discontinuity in 1 (9%), and for G7 were instability in 6 (60%), aseptic loosening in 3 (30%) and infection in 1 (10%). Fretting and corrosion type damage was visually scored by two independent observers (with scores of 1 to 4 indicating none, mild, moderate, severe damage, respectively) based on a modified Goldberg scale. Each device was divided into two primary regions: the taper region and the dome region, each further divided into four quadrants. Individual scores across each region were tallied, and an average score was assigned to each device. Average fretting and corrosion scores were compared separately and as a total between the two groups and between the dome and taper region within each group using student’s t-tests with p<0.05 considered as statistically significant. Four samples from each group, with unique surface damage features observed during visual scoring, were selected for scanning electron microscopy (SEM, Carl Zeiss FE-SEM 300) analysis.
RESULTS: Corrosion damage scores were low overall but significantly higher in G7 (p=0.0055) and specifically in the dome region (p=0.00003) compared to Stryker. Overall average damage scores for both groups did not differ (average scores: Stryker=25.3, G7=28.7; p=0.554). Both liners exhibited comparable fretting damage scores in the dome and taper regions (Stryker, dome=4.8, Stryker, taper=8.3, G7, dome=4.6, G7, taper=7.5). Damage features in each design differed: Stryker demonstrated parallel fretting scars on the tops of the machining lines within the taper region (Fig. 1), while G7 demonstrated more discoloration and pitting type damage on the dome and taper regions (Fig. 2). G7 also showed signs of material loss (the darker spots marked in Fig. 2).
DISCUSSION: Among our cohort, G7 liners exhibited more non-mechanical modes of corrosion. SEM analysis indicates G7 surfaces appeared rougher and might worsen crevice formation as compared to the Stryker which retained more of an as-machined finish. Retrieved G7 liners had discolored regions mostly on the dome that under SEM appeared to be interspersed with pits and material loss. Surface finish and taper geometry differences between Stryker and G7 liners could influence taper mechanics leading to unique corrosion mechanisms that should be studied further with more samples and samples obtained after longer times in service.
Figures
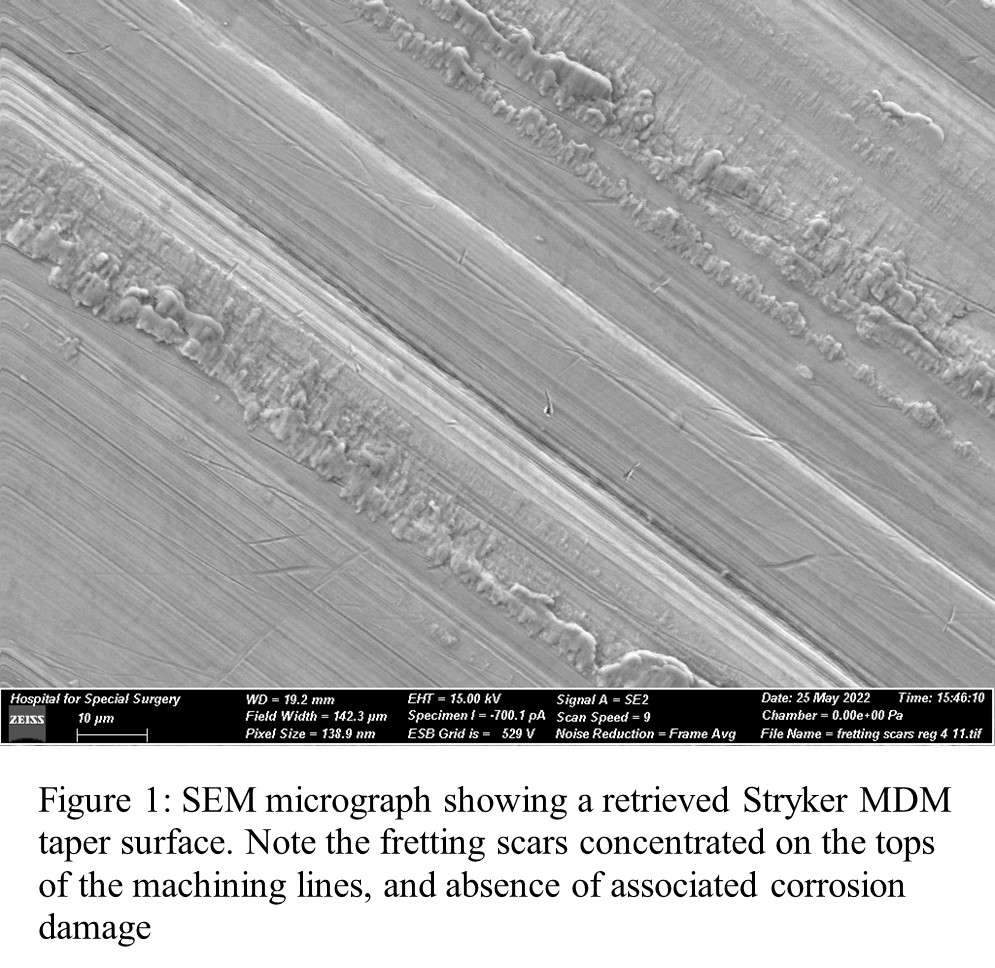
Figure 1
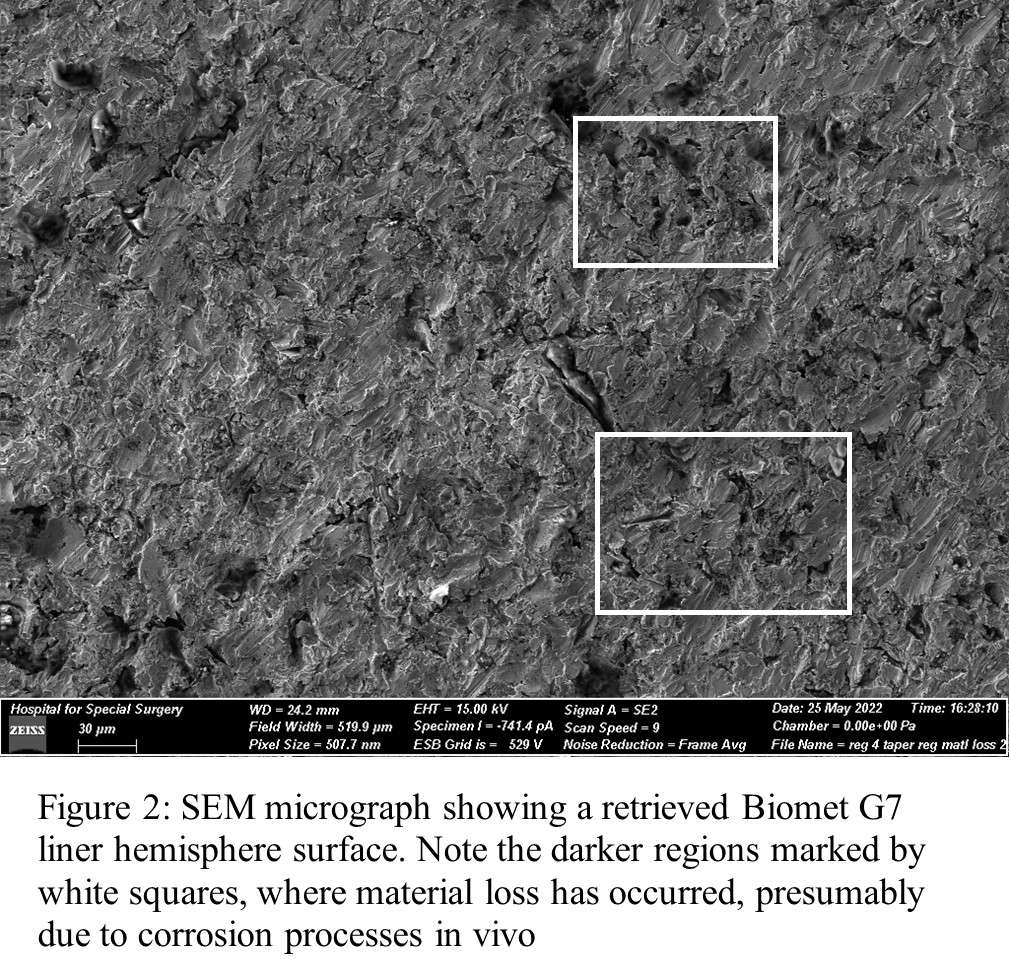
Figure 2#8670
Arthroplasty Reasons for Retrieval Follow Innovation Over Time and Can Guide Us About the Future
*Rebecca Thomson - Dartmouth College - Hanover, United States of America
Kori Jevsevar - Dartmouth College - Hanover, USA
Afton Limberg - Dartmouth College - Hanover, United States of America
Zachary Currier - Dartmouth College - Norwich, United States of America
Alexander Orem - Dartmouth Health - Lebanon, United States of America
Douglas Van Citters - Dartmouth College - Hanover, USA
*Email: rebecca.j.thomson.th@dartmouth.edu
Introduction:
Over the last fifty years, clinicians and engineers have been responsive to the reasons for revision of total joint arthroplasty components. Failures highlighted in the literature have led to material, design, and surgical improvements. To measure success, trends in innovation should reflect trends in why implants are revised. The objective of the current study is to identify the reasons for revision in a large retrieval laboratory to illuminate whether significant technological changes are reflected in an evolution in the reason for revision.
Methods:
An analysis of reasons for retrieval (RFR) of an IRB-approved database was performed with a tested predictor variable of the decade of index surgery. The database search began with 1995 as this was the period when a new digital record was developed that allowed categorization and sorting by institution, reason for revision, and device type (n=11,631 devices). Decade cut-offs were selected to reflect the prevailing technology of that era. Data is categorized by decade of device implantation and then by reason for retrieval. Primary reasons for retrieval were noted by surgeons at the time of removal. The implants were further separated into knees and hips during that period to elucidate trends.
Results:
Selection bias is minimized by incorporating a retrieval strategy that prioritizes 100% capture and analysis across multiple institutions, resulting in a retrieval database that reflects trends in hospital utilization. The highest reasons for retrieval (overall) indicate loosening and infection across eras of implantation and joint type. When divided into decades, changes in the reasons for retrieval represent more of the innovation and design changes made to the implants. For instance, revision due to polyethylene problems is maximized in knees and hips in the 1980’s and 1990’s before gamma-air processing was identified as a predictor of early failure. While this reason for revision is less prominent in devices manufactured after the advent of crosslinking (early 2000’s) hips see an increase in metal-related failures in the era of MoM, while knees move away from material-related reasons for revision and distribute amongst pain, infection, and loosening. Notably, for devices implanted more recently (2010’s, 2020’s) non-device-related reasons are expected to predominate as short duration failures are many fewer and are typically related to early infection or loosening.
Conclusion:
Material changes and design approaches show both patient benefits and drawbacks in the continuous retrieval record. Further studies should be done to understand in vivo duration, all types of arthroplasties, and potential correlation to patient demographics. Overall, reasons for retrievals in the joint registry trend toward reflecting the innovations and design changes in the industry.
Figures
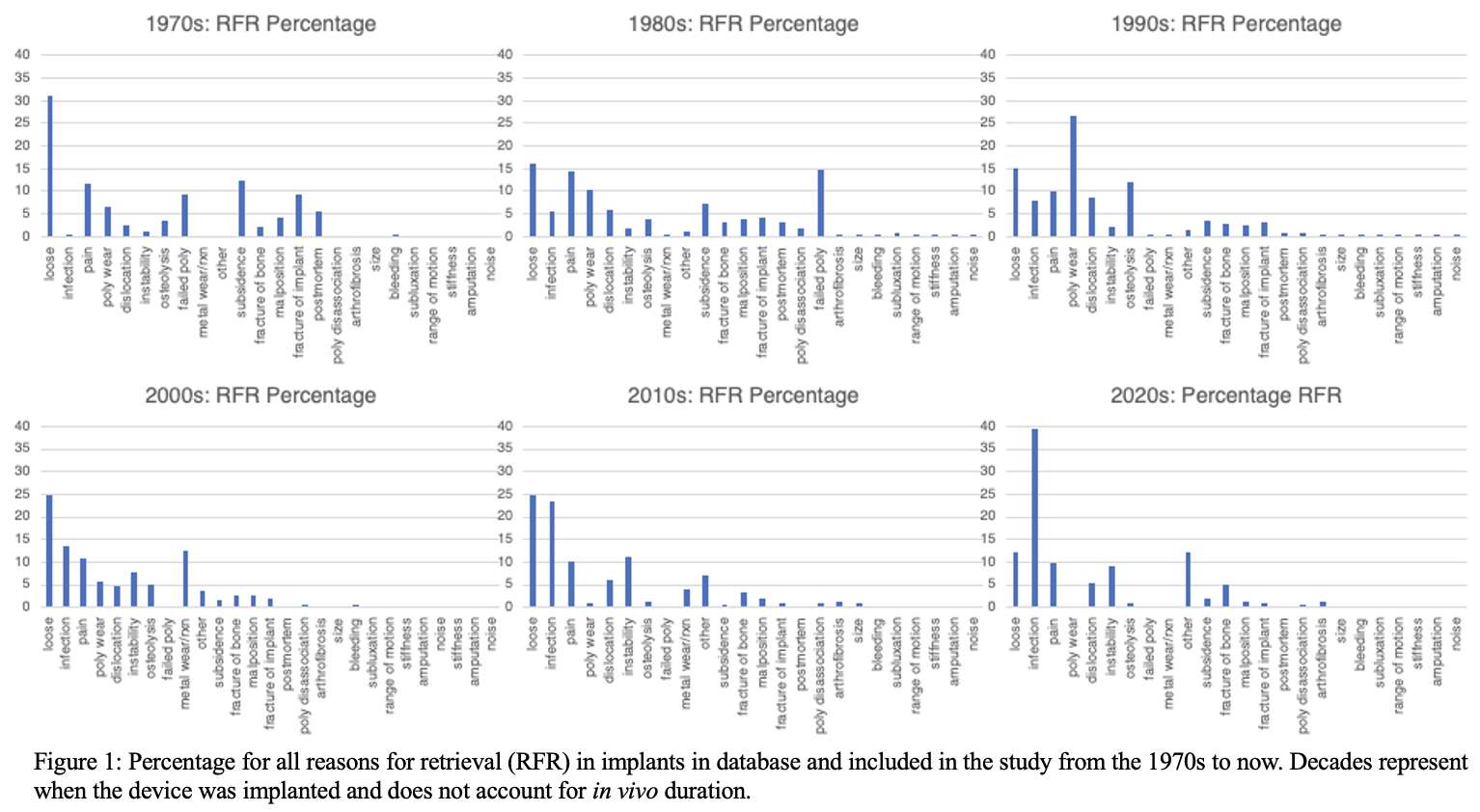
Figure 1
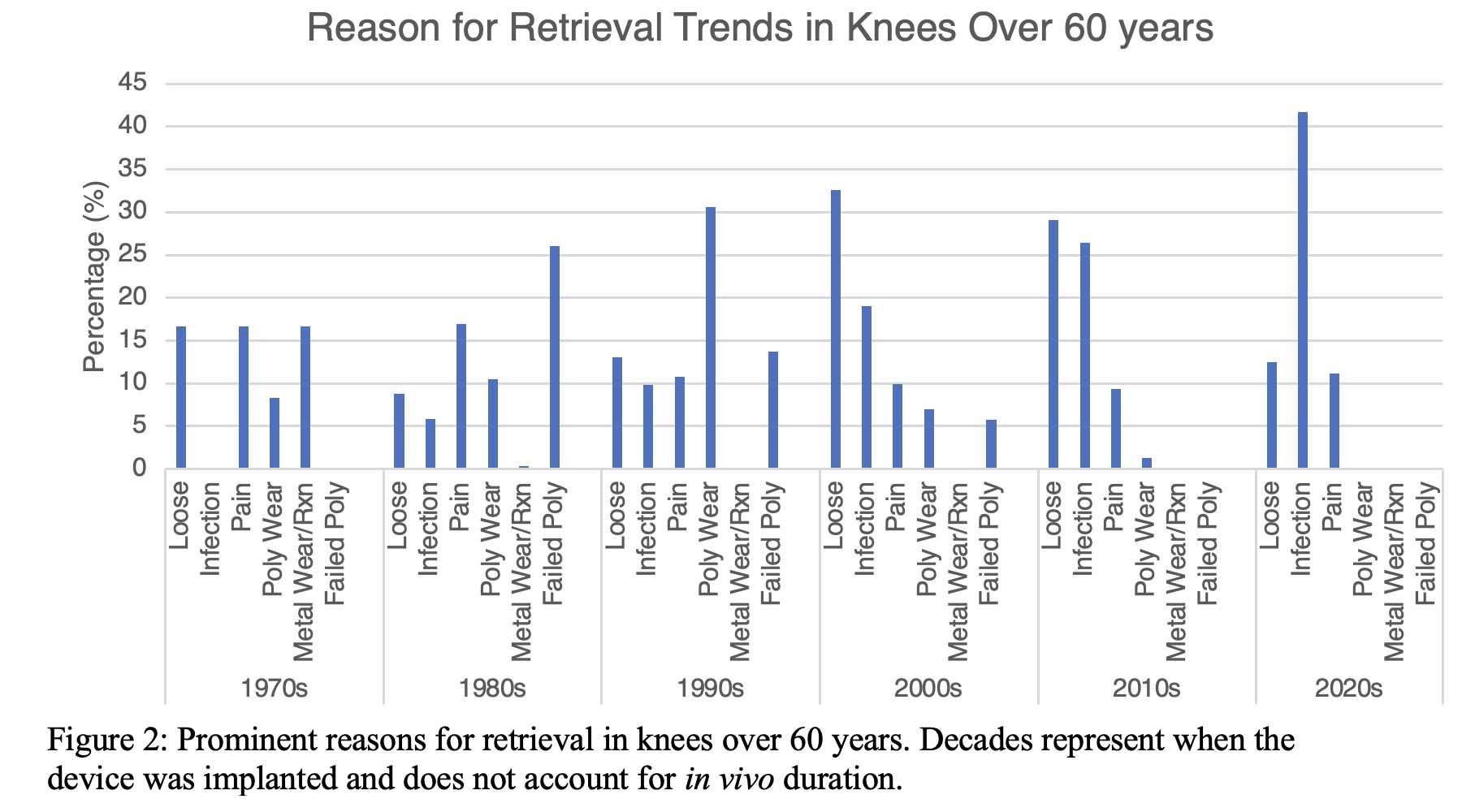
Figure 2
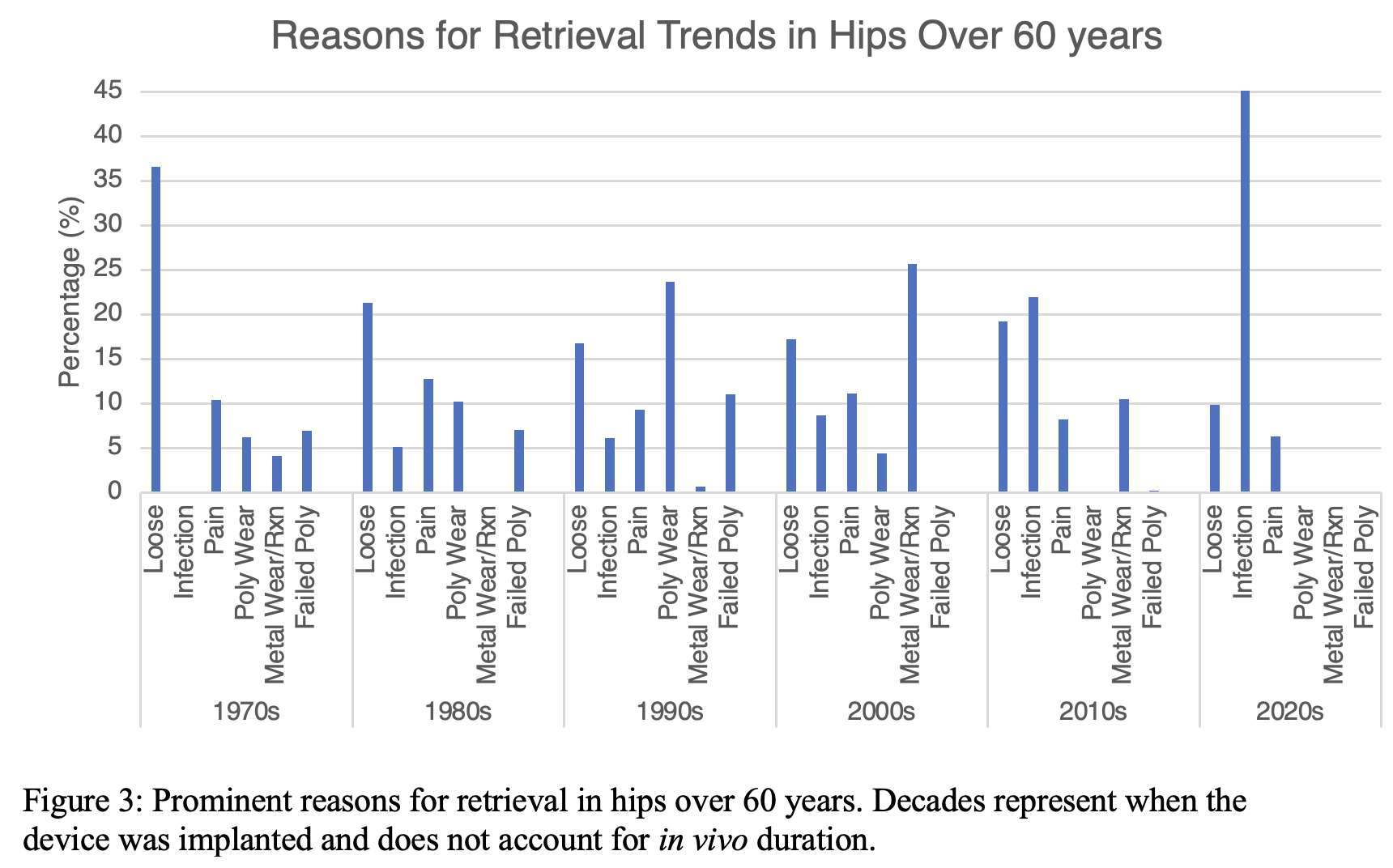
Figure 3#8679
Reasons for Retrieval in Community Hospitals Compared to an Academic Medical Center
*Rebecca Thomson - Dartmouth College - Hanover, United States of America
Kori Jevsevar - Dartmouth College - Hanover, USA
Peder Solberg - Dartmouth College - Hanover, United States of America
Zachary Currier - Dartmouth College - Norwich, United States of America
Alexander Orem - Dartmouth Health - Lebanon, United States of America
Douglas Van Citters - Dartmouth College - Hanover, USA
*Email: rebecca.j.thomson.th@dartmouth.edu
Introduction:
High-volume versus low-volume care has been a conversation between providers and is an area of interest for establishing high value outcomes. Previous research has suggested that higher volume academic hospitals tend to provide lower cost procedures with similar or improved outcomes compared with community settings1. Retrieval laboratories have the potential to experience selection bias when receiving devices based on numerous factors, including the hospital where the index procedure was performed, and the mechanism by which implants are collected, amongst many others. The objective of this study is to identify differences in the reason for revision at a large academic hospital as experienced by a retrieval laboratory when devices are categorized by index implantation at the academic hospital or in a community setting.
Methods:
An analysis of 11,631 implants retrieved from a single academic medical center since 1995 was performed to understand where arthroplasty devices were initially implanted and their subsequent reasons for retrievals. Nearly 100% of revision surgeries at this facility transfer the arthroplasty devices to a retrieval laboratory, reducing selection bias compared to surgeon judgement of damage. All devices that did not have a known implanting surgeon or location were excluded (n=103 devices). Community hospitals were characterized using the AHA definition, including all public, nonfederal, short-term general and special hospitals2.
Results:
Overall, there were no major differences between reasons for retrieval (RFR) for devices originating between community hospitals and the academic medical center. Figures 1 and 2 represent the RFR for knees and hips when implanted at the academic center compared to community hospitals. Hips are more commonly taken out for dislocation and metal reaction compared to knees. The two largest differences, as seen in Figure 3 include infection and loosening, with the subsequent differences in percentage of RFR was below 5%. Typically, infection cases, once referred to a larger hospital, will repetitively return to that hospital to be treated; this is seen in Figure 3 with an increase in infection RFR in the medical center. However, based on the reasons for retrieval seen in this study, there is no significant difference between community hospitals and the academic medical center.
Conclusion:
A robust retrieval strategy can reduce selection bias for devices originally implanted at community hospitals versus a large academic medical center. In this particular region, it does not appear that reasons for revision as experienced by the retrieval laboratory are meaningfully related to the location of the index surgery.
Citations:
1. Siddiqi A, Alamanda VK, Barrington JW, et al. Effects of Hospital and Surgeon Volume on Patient Outcomes After Total Joint Arthroplasty: Reported From the American Joint Replacement Registry. J Am Acad Orthop Surg. 2022;30(11):E811-E821. doi:10.5435/JAAOS-D-21-00946
2. Fast facts on U.S. hospitals, 2022: AHA. American Hospital Association. Accessed May 15, 2023. https://www.aha.org/statistics/fast-facts-us-hospitals.
Figures
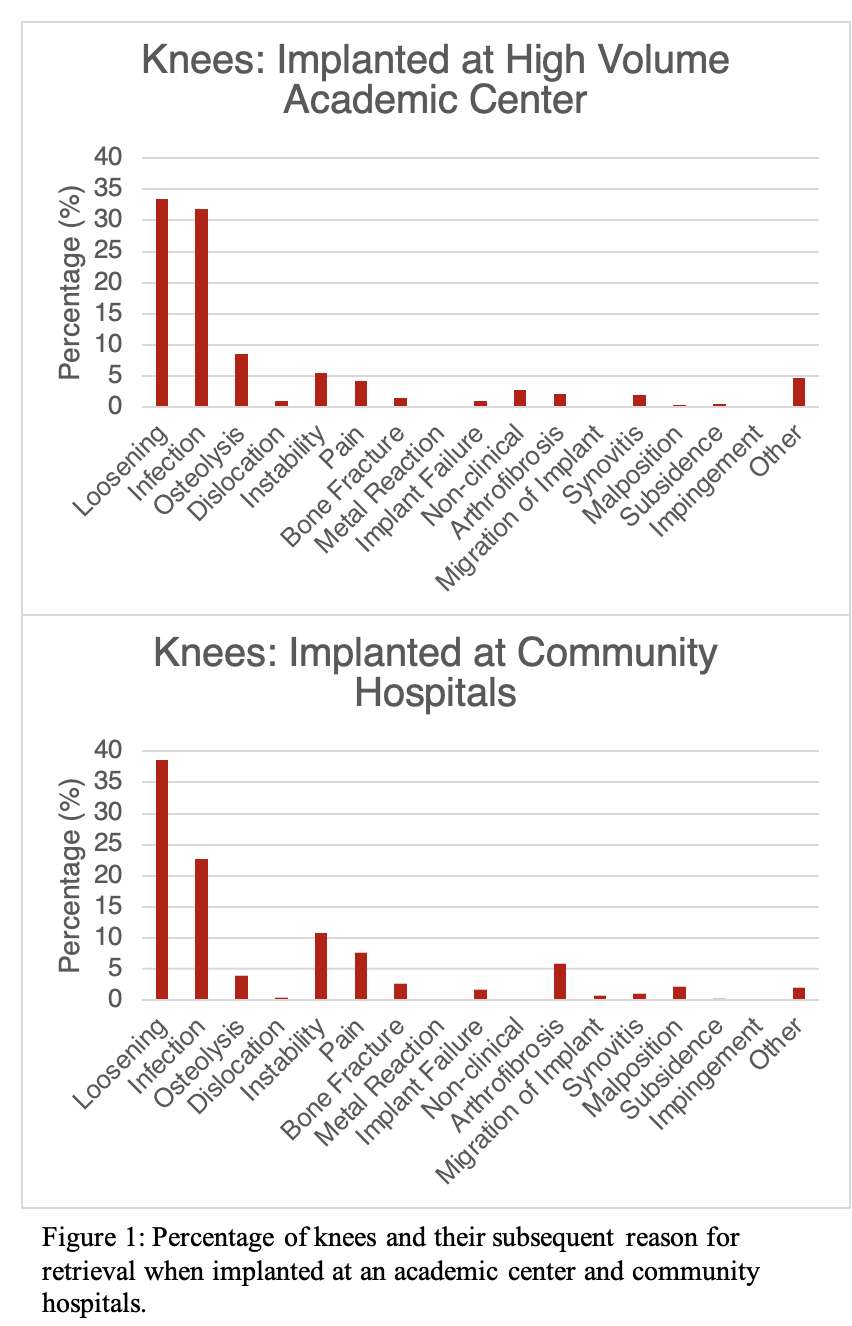
Figure 1
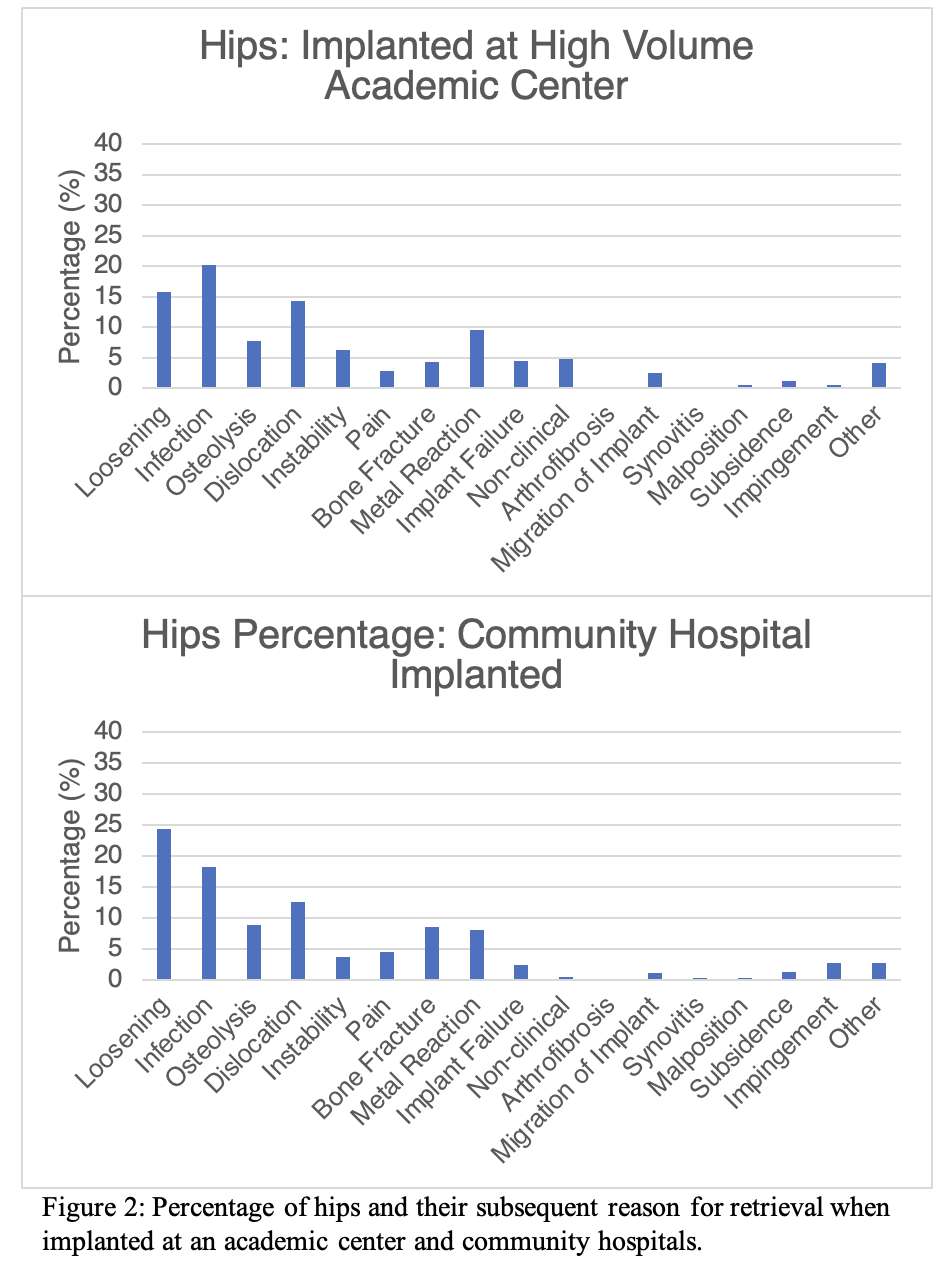
Figure 2
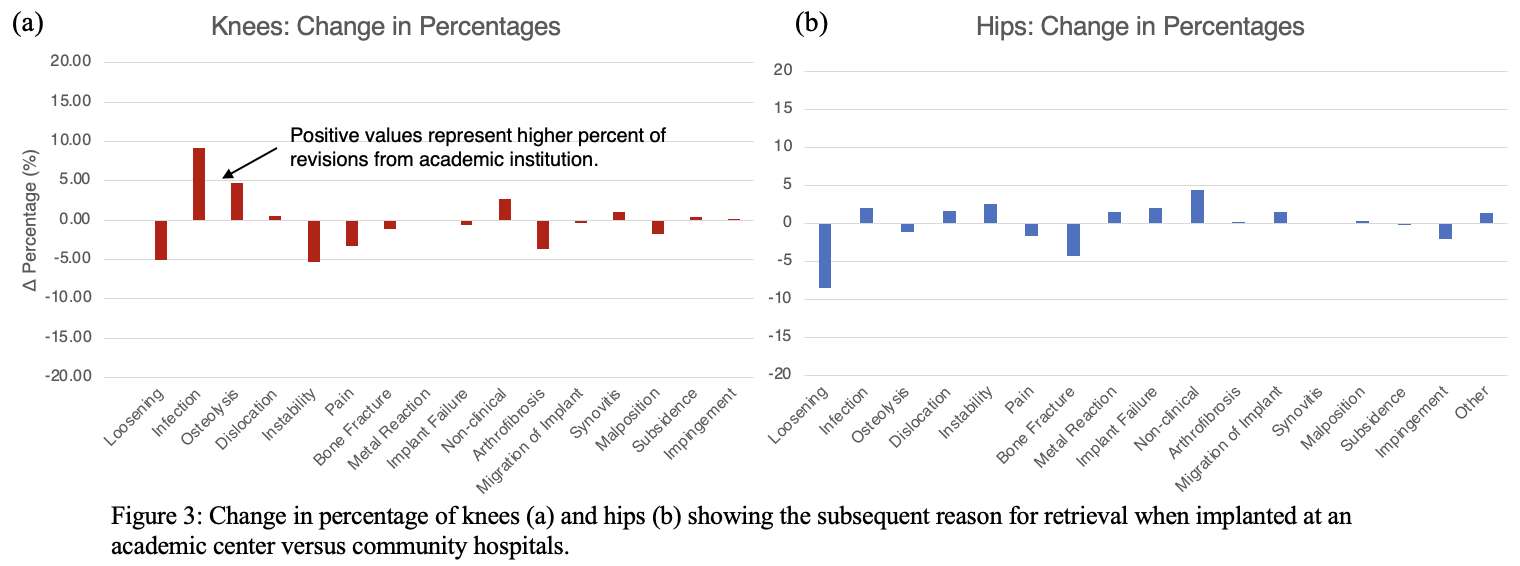
Figure 3#8752
First Report of Clinical Information and Characteristics of Hip Arthroplasty Explants Collected at a Brazilian Retrieval Analysis Center
Renir Reis Damasceno Neto - LEBm / UFSC - Florianopolis, Brazil
*Carlos Rodrigo De Mello Roesler - Universidade Federal de Santa Catarina - Florianópolis, Brazil
Matheus Henrique Linhares da Silva - UFSC - Florianopolis, Brazil
Joao Antonio Matheus Guimaraes - INTO - Rio de Janeiro, Brazil
Lourenco Pinto Peixoto - INTO - Rio de Janeiro, Brazil
Marina Dias Rosa - UFSC - Florianopolis, Brazil
Patricia Ortega Cubillos - UFSC - Florianopolis, Brazil
*Email: r.roesler@ufsc.br
INTRODUCTION
Revisions due to implant failure represent small number considering all revision reported on registers. In this way, the identification of implant-related problems is slow and may result in a large number of implantations with a poor implant design prior its detection. Explant analysis systems are prone to complement National Joint Registers findings by screening all revisions attributed to implant failures. This paper reports the characteristics of the first 188 hip arthroplasty explants collected on the partner hospital.
METHODS
For all collected implants, we had patient consent through an Informed Consent and Clarification Form. The materials were collected at a reference partner hospital, sanitized according to ISO 12891-1 standards and stored individually (Figure 1). Demographic and clinical information is collected through a pre-operative form filled out by the partner hospital, with information from patient records. Explant characteristics are obtained during the postoperative analysis stage, performed by the center researchers, based on implant traceability codes and manufacturer catalog data. The collected information refers to explants received between November 2020 and December 2022.
RESULTS AND DISCUSSION
In 2020, the center received 20 out of the 188 explants in the period under analysis, followed by 67 in 2021, and finally 101 in 2022. Excluding the short period of 2020, this represents a 50.7% growth.
Regarding the available demographic data: 77 patients were female (41.0%) and 108 were male (57.4%). Data on the sex of 3 patients were not available. Regarding the reasons for revision, there is a predominance of aseptic loosening (37.8%), which is usually related to long-term wear, followed by infection (21.3%), usually a short-term complication (up to one year after surgery). Insert or acetabular component wear also has a high number of cases (12.2%). The average implantation time was 12.6 years, with 52 cases revised within 5 years.
The characteristics of hip arthroplasty implants collected shows the dominance of two femoral stem designs: Force Closed (23.4%) and Tapered Round (21.3%), followed by the Fully Coated model (12.7%). Regarding the fixation of the femoral stems, 60.4% of explants show uncemented fixation, while cemented ones account for 39.6%. As for the fixation of acetabular components, 55.3% are uncemented, while cemented ones account for 28.2% (Figure 2).
CONCLUSION
The data collected at the center provides an initial overview of hip arthroplasty revision surgeries on the partner hospital, with a predominantly male patient population (57.4%), the main indication being aseptic loosening (37.7%), and an average implantation time of 12.6 years. The relationship of the clinical and demographic information with implant characteristics creates a background for research and development of new implant technologies (Figure 3).
Figures

Figure 1
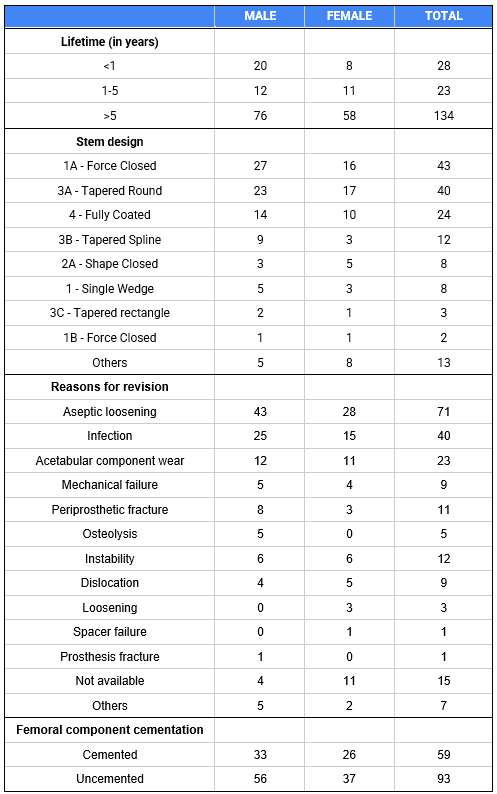
Figure 2
Figure 3#8536
Results of a Zirconium Ceramicized Femoral Component in Total Knee Arthroplasty: A Retrieval Study
*Afton Limberg - Dartmouth College - Hanover, United States of America
Zachary Currier - Dartmouth College - Norwich, United States of America
Kori Jevsevar - Dartmouth College - Hanover, USA
Matthew Abdel - Mayo Clinic - Rochester, USA
Alexander Orem - Dartmouth Health - Lebanon, United States of America
Douglas Van Citters - Dartmouth College - Hanover, USA
*Email: afton.limberg@gmail.com
Introduction: Advanced materials for total knee arthroplasty (TKA) including crosslinked bearings and cobalt-free components show promise for reducing revision rate and improving outcomes. Longer duration studies attributing mechanical success to material choices are challenging to design owing to the complexity of in vivo radiological wear analysis. Recent large-scale retrieval studies with cobalt femurs have illuminated wear rates in knees and identified key factors contributing to wear reduction, including sex, age, crosslinked polyethylene, polished trays, and implant conformity. The objective of the current study is to test whether an alternative metal in the femur changes these factors.
Methods: An IRB approved retrieval database was searched to identify 107 zirconium ceramicized femoral TKA components over a period from 2004-2023; 67 had a bearing surface of polyethylene (PE) and 40 had a bearing surface of cross-linked polyethylene (XLPE). All had a polished tibial tray. The mean duration of implantation was 58 months (2-178 months) for the PE group and 33 months (5-70 months) for the XLPE group. The most common reasons for retrieval were instability in the PE group and infection in the XLPE group. The patients were 61% females for the PE group and 58% females for the XLPE group, with a mean age of 64 years and 62 years, respectively. Mean BMI was 31.9 kg/m2 for the PE group and 32.8 kg/m2 for the XLPE group. Dimensional change (creep plus wear) was measured from the thickness of the polyethylene insert using short duration (<16 months) and never implanted devices as a reference. Oxidation (maximum ketone oxidation index) was measured via FTIR using a cross-section of the medial portion of the polyethylene at <3 months post-explant. Statistical analyses were conducted using groups matched for in vivo duration and a p-value<0.05 was considered significant.
Results: Dimensional change rate and in vivo oxidation rate were statistically indistinguishable between the two bearing types (Figures 1 & 2). Mean dimensional change rate for the PE group was 0.018±0.031 mm/year and 0.024±0.034 mm/year for the XLPE group. These values were not statistically different (p=0.39). Mean oxidation rates were also not statistically different, with the mean rate for the PE group being 0.048±0.049 a.u./year and the mean value for the XLPE group being 0.056±0.045 a.u./year (p=0.461). While attempts were made to match patient populations in the two groups, small differences still exist. Previous work showed that younger age and male sex lead to higher dimensional change rates, suggesting that patient factors combined with the polished tibial tray may account for the lack of statistical difference between these two groups.
Conclusion: Overall, no significant differences were identified for dimensional change rate or oxidation rate for tibial inserts against a zirconium ceramicized femoral component. The dimensional change results were consistent with the literature1, and similarly the oxidation rates were comparable to what has previously been reported for TKAs2, 3. Future studies should compare these values to those from cobalt-chromium based implants.
References:1. Currier et al. BJJ 2021; 2. Currier et al. JBJS 2023; 3. Currier et al. JOA 2015
Figures
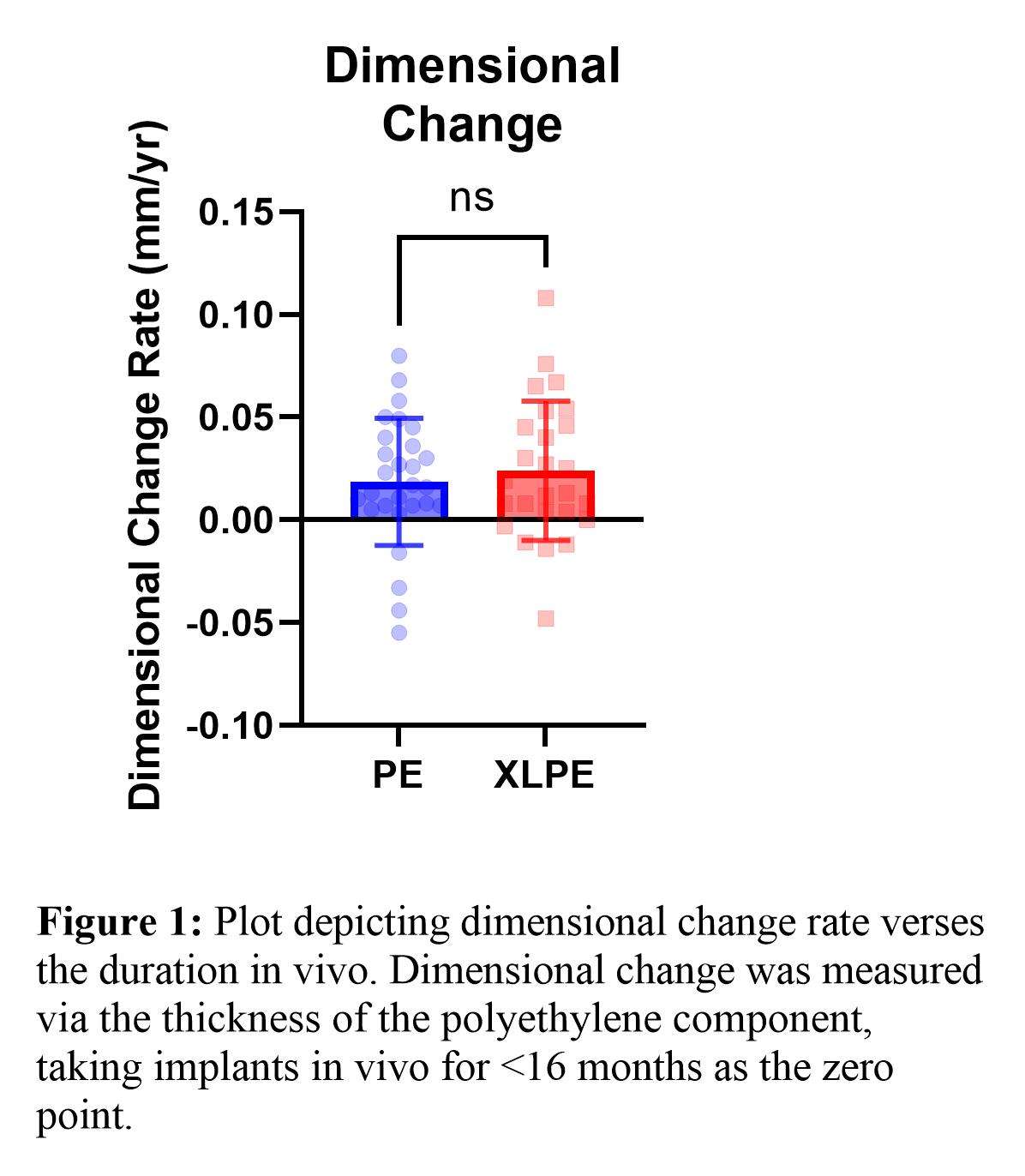
Figure 1
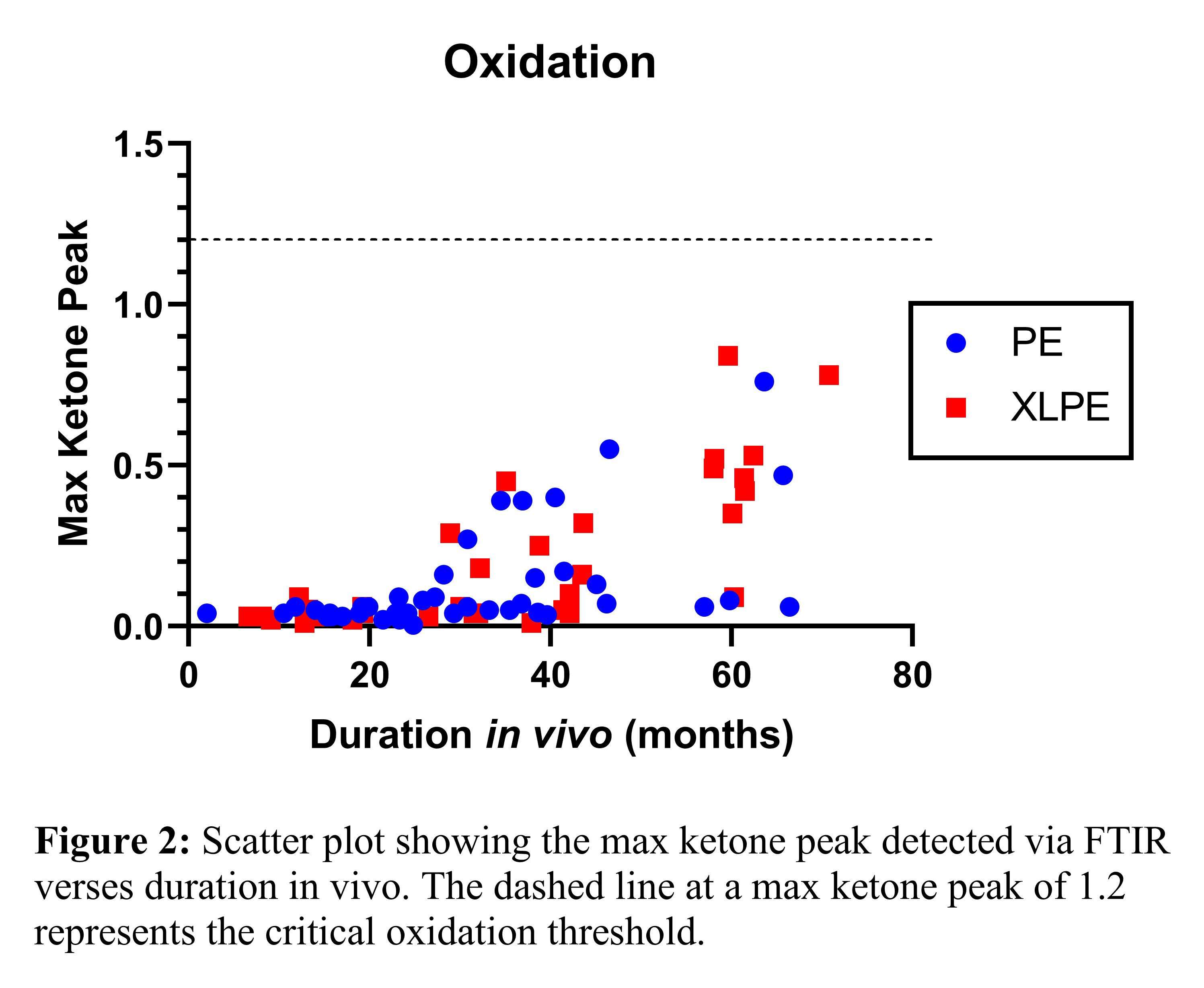
Figure 2#8725
Damage Assessment of Primary Tibial Inserts Retrieved Up to 5 Years
*Antonio Fernandes - Universidade Federal de Santa Catarina - Florianópolis, Brazil
Alan De Paula Mozella - Instituto Nacional de Traumatologia e Ortopedia Jamil Haddad São Cristóvão - Rio de Janeiro, Brazil
Enrico Fava - Federal University of Santa Catarina - Florianópolis, Brazil
Gean Vitor Salmoria - Universidade Federal de Santa Catarina - Florianópolis, Brazil
Carlos Rodrigo De Mello Roesler - Universidade Federal de Santa Catarina - Florianópolis, Brazil
Patricia Ortega Cubillos - UFSC - Florianopolis, Brazil
*Email: antonio.lebm@gmail.com
Introduction
Retrieval analysis offer a way to document implant or material-related problems leading to revision, supplying inputs for material and design improvements. Although the ultra-high molecular weight polyethylene (UHMWPE) resistance to wear, damage of tibial inserts is a recognized unwanted behavior. This paper aims to evaluate fourteen explants of posterior stabilized tibial inserts (PS) of primary total knee arthroplasty (TKAp), collected by a Brazilian retrieval analysis center in order to compare different damage modes and severity with clinical, demographic and design data.
Methods
Fourteen TKAp tibial inserts were randomly selected, all from cemented sets, PS type, with a fixed base. In addition to service time and revision indication, demographic characteristics related to each patient were collected, such as: age, gender, laterality and body mass index (BMI).
Each selected tibial insert was analyzed at 10x magnification using a method adapted from the one proposed by Hood, where 4 groups with 4 regions each were considered: medial condyle (M), regions 1 to 4; lateral condyle (L), regions 5 to 8; post (P), regions 9 to 12 (Figure 1). In addition to the 7 damage modes proposed by Hood (pitting, delamination, particle adhesion, scratching, surface deformation, burnishing and abrasion), the total area affected by any damage mode was considered.
Finally, the results of the macroscopic damage analysis were used to compare: groups M, L and P; and the regions within each group.
Results
Seven different manufacturers were identified among the fourteen explants. 78.6% were retrieved of female patients while 63.4% affected the right knee. All patients were over 63 years old at the time of retrieval. The most frequent indications for revision were aseptic loosening (5), infection (3) and periprosthetic fracture (3). The average BMI was 33.0.
The frequencies and severity had a positive correlation. The most frequent damages mode was pitting, delamination and scratching. In comparison with M and L groups, the P group had more delamination. Between the condylar groups, the medial regions had highest frequencies of pitting, delamination, and surface deformation (Figure 2).
In group M, the regions with the highest frequencies and severity of damage were 2 and 3; in group L, they were regions 6 and 7; in group P, region 12 was the most affected (Figure 3).
Only 8.4% of all severities indicated more than 50% area affected by damage per region. In contrast, around 70% of the severities indicated up to 10%.
Conclusion
The damage data among the three groups indicates that up to 5 years post operatory, the condyles are more affected than post, majorly in the anterior regions or each condyle. The posterior region of the post was the most affected, possibly because of the post-cam mechanism. Overall the damage modes and severities were low, and along with the main indications for revision, it can be concluded that the damage behavior of the inserts were similar and no relationship with the clinical data was observed.
Figures
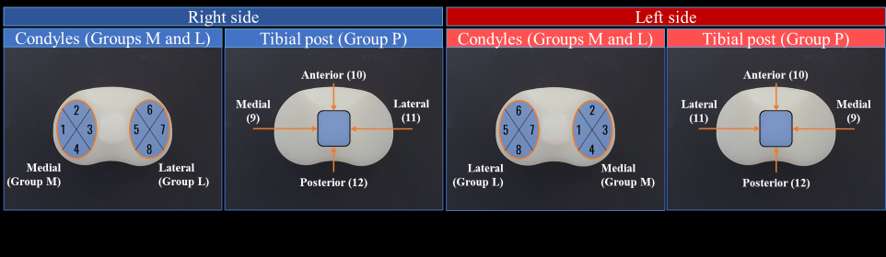
Figure 1
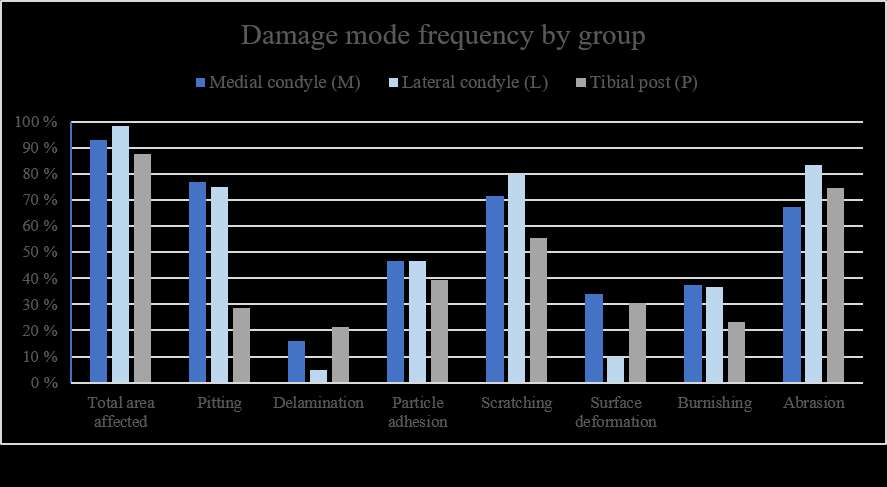
Figure 2
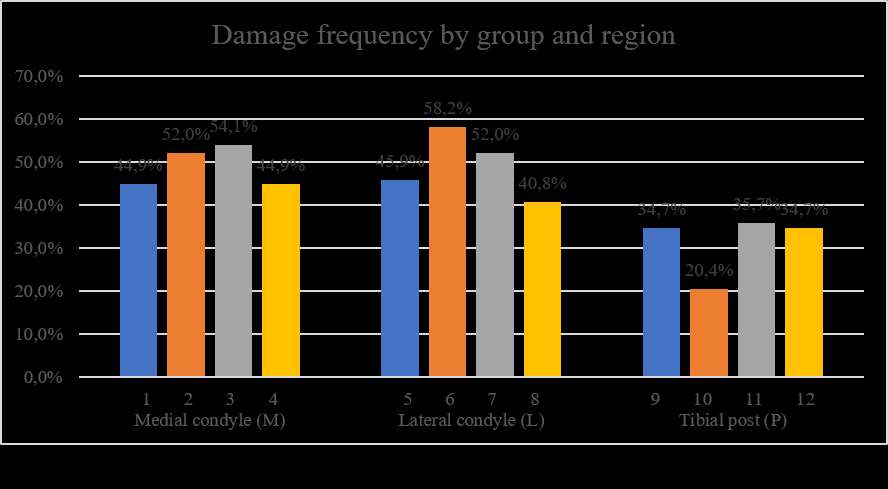
Figure 3#8270
Retrieval Analysis of Titanium Nitride-Coated Orthopaedic Implants
*cemile basgul - Drexel University - Philadelphia, United States of America
Daniel MacDonald - Drexel University - Philadelphia, USA
Greg Klein - Hartzband Center for Hip and Knee Replacement - Hackensack, USA
Nicolas Piuzzi - Cleveland Clinic - Cleveland, USA
Steven M. Kurtz - Drexel - Philadelphia, USA
*Email: cb997@drexel.edu
Introduction
Ceramic coatings such as titanium nitride (TiN) have gained popularity as a surface modification technique for implants due to their excellent biocompatibility, wear resistance, and corrosion resistance. However, concerns exist regarding issues such as 3rd body wear, increased wear of ultra-high-molecular-weight polyethylene (UHMWPE) and cohesive failure of the coating. This study aims to evaluate the in vivo performance of TiN-coated knee and hip implants, specifically examining fixation interfaces, articulating surfaces, and wear-related damage.
Methods
A total of 8 arthroplasty retrievals (3 knee and 5 hip) were collected during revision surgery from multiple institutions. The TiN-coated implants were from 3 different manufacturers (Endotech, Link, and Depuy) and included various designs (B-P, endo-model and pinnacle). Implantation years ranged from 1993 to 2016. The cohort had an average implantation time of 4.25 years (range: 1.75 to 5.25) and 17.5 years (range: 0.25 to 26) for knee and hip implants respectively. Average patient BMIs were 37 ± 2.98 and 28.5 ± 4.09 kg/m2 for knee and hip implants, respectively. The revised components included knee implants (3 femoral implants, 2 tibial trays, and 1 tibial insert) and hip implants (4 liners, 3 shells, 5 femoral heads, and 1 femoral stem). The reasons for revision were PE Wear (2/8), loosening (2/8), pain (2/8), infection (1/8), and instability (1/8). In the hip analysis, the surfaces of femoral heads, the taper surface of the femoral stem, and the rim and articulating surfaces of polyethylene liners were evaluated using a semiquantitative scoring method [1]. The knee analysis involved dividing the condyles and tibial trays into four zones and scoring accordingly [2]. Surface roughness was assessed using weight light interferometry (WLI) (Zygo NewView 9000) on specified regions, and scanning electron microscopy (SEM) and energy dispersive spectroscopy (EDS) were used for surface morphology, chemistry, and particle characterization.
Results
For hips, there was mild (2.4/4) corrosion for femoral head tapers, high scratching (2.6/3) was observed for femoral heads. For polyethylene liners the main mechanism was burnishing (2.4/3), followed by embedded debris (1/3) and scratching (0.8/3). The failure of coating was visually detected in 4 cases (3 femoral heads and 1 tibial tray (Fig. 1)). For knee implants low burnishing and scratching were observed (3.3/12) for both mechanisms. The knee surfaces proved to be hydrophilic according to the wettability. The roughness measured (Sa) for the knee retrievals was 90.7 nm with 90% CI [79,101.4]. The depth of the scratches observed on the tibial trays varied between 0.3 and 1.3 µm.
Conclusion
This study aimed to assess the in vivo damage of TiN coated knee and hip implants. The findings indicated that the primary damage mechanisms observed for TiN coatings were scratching, corrosion, and burnishing. Long-term femoral heads (25 years) showed cohesive failure of the coating, whereas short-term knee retrievals (less than 4 years) exhibited predominantly intact coatings, with only minor chipping and localized substrate exposure.
#8439
In Vivo Wear and Oxidation Analysis of Retrieved Temporal Mandibular Joint Replacement Prostheses of One Design
Catherine Yuh - Rush University Medical Center - Chicago, USA
*Deborah Hall - Rush University Medical Center - Chicago, USA
Louis G. Mercuri - Rush University Medical Center - Chicago, USA
Kevin E. Cordero - Stryker Cranial Maxillofacial - Kalamazoo, USA
Robin Pourzal - Rush University Med Ctr - Chicago, USA
*Email: deborah_hall@rush.edu
Introduction: Total temporomandibular joint (TMJ) replacement (TMJR) is the salvage treatment for end-stage TMJ pathology. Unlike total hip (THR) and knee replacements (TKR), limited knowledge exists on bearing surface wear and its impact on implant longevity. This study assessed ultra-high molecular weight polyethylene (UHMWPE) in vivo wear and oxidation in retrieved TMJR fossae.
Methods: We examined 24 surgically-retrieved TMJR fossae (average duration: 57.4 months, range 0.2-240). Surface damage features observed under stereomicroscopy were recorded. Laser scanning confocal microscopy was used to measure linear wear penetration of wear scars. Fossae were then sectioned (200 µm thick) for measurement of UHMWPE oxidation indices (OIs) using FTIR spectroscopy according to ASTM F2102-06.
Results: Distinct wear scars were observed in 17 of 24 of the fossa polyethylene (PE) bearing surfaces. The wear scars were mostly round to oval in shape (range: 1-6 mm Ø) (Fig. 1A). Three PE bearings did not exhibit any damage to the articulating surface, and two had uniform surface wear without a distinct wear scar. Additionally, 3 bearings had notable wear outside the articulating surface at the rim and side of the bearing. The primary wear feature associated with most PE wear scars was polishing in all cases. Another feature that occurred in several cases was protuberances which were either remains of the original surface topography or resembled the so-called striated patterns as previously reported for tibial liner wear observations from TKRs (Fig. 1B, C). Most bearings also had fine scratches located within the wear scar (Fig. 1D). The average maximum linear wear was 87.35 µm ± 110.17 µm. Wear and duration were linearly correlated (R2 =0.47, p=0.001, Figure 2), with an estimated wear rate of 0.82 µm/month (Fig. 2). For UHMWPE oxidation, most fossae exhibited consistent OIs through the thickness. Samples with distinct wear scars tended to have higher OIs within the wear region at the subsurface (Figure 3), with the OI increasing with longer duration (R2=0.27, p=0.018).
Conclusion: Mild wear and minimal oxidation were observed in surgically-retrieved TMJR fossae. One case without a distinct wear scar but polishing of the entire bearing surface could be explained by loose fossa screws that likely prevented a proper bedding-in of the condyle. Overall, any deviations from normal wear behavior were explained by dislocation or loose components. The observations of striated patterns are interesting because this is also common in tibial liners. Potential explanations for this pattern include nonconforming surface conditions and lack of pure sliding. Patterns of oxidation found in fossa wear scars were consistent with observations reported in total knee arthroplasty literature that found elevated OI within wear scars, which was attributed to load and infiltration of various reactive oxygen species. It needs to be noted that shelf-oxidation could not be considered because the shelf time before implantation and after removal was not known. However, it appears that its effect was rather minimal. As TMJR is increasing as a treatment for TMJ pathology, fundamental knowledge of wear mechanisms associated with implant design/material is essential to guarantee implant longevity.
Figures
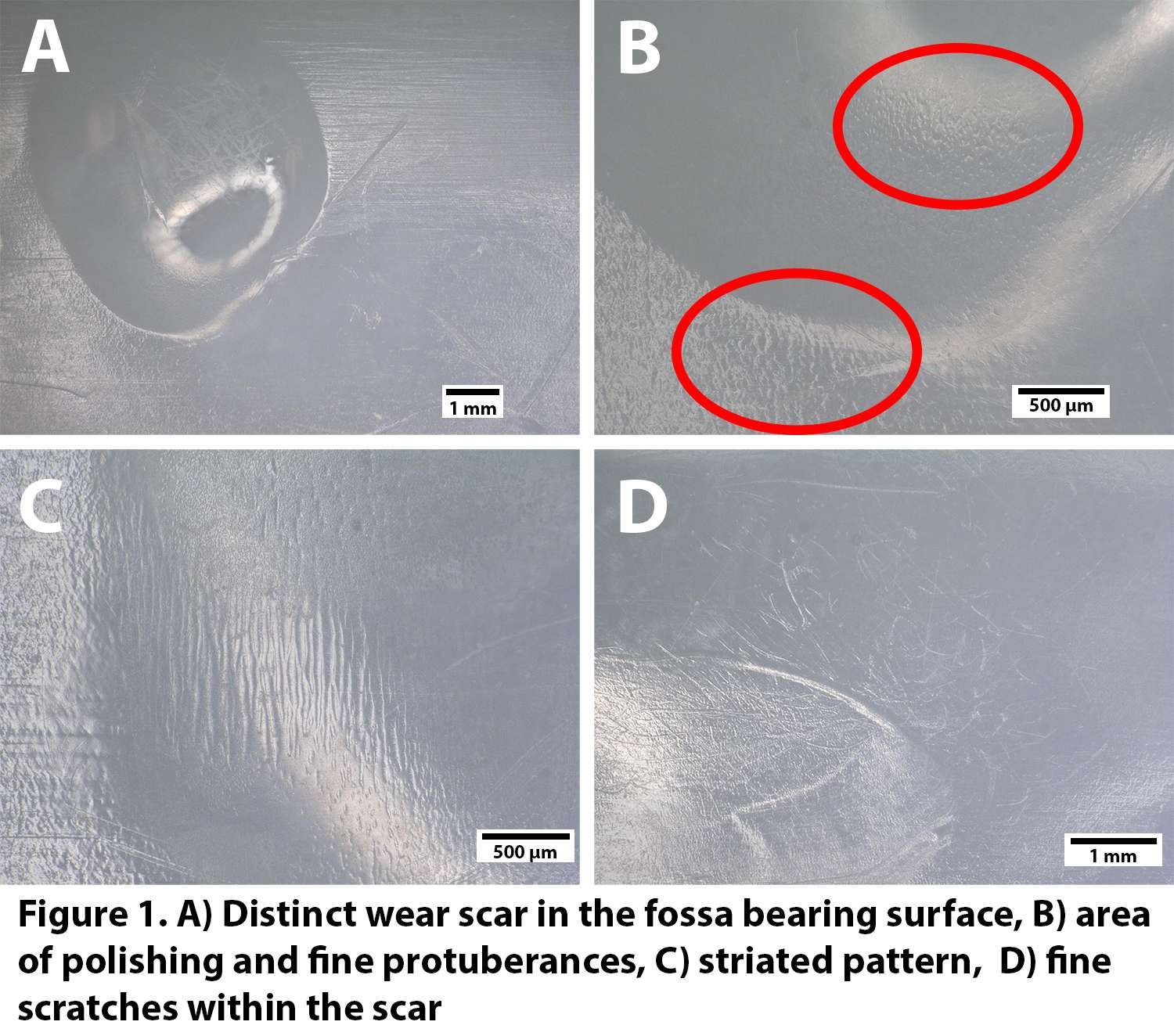
Figure 1
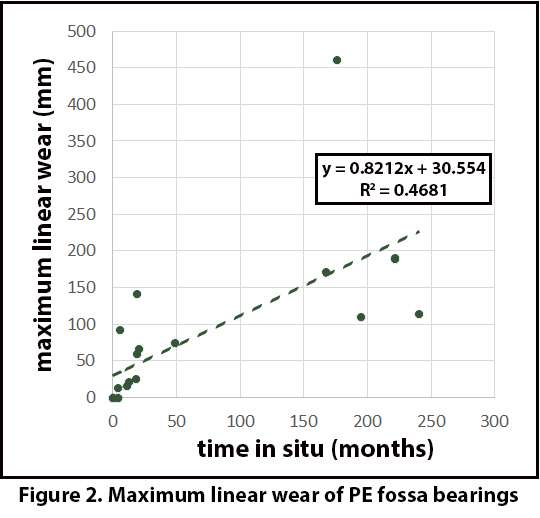
Figure 2
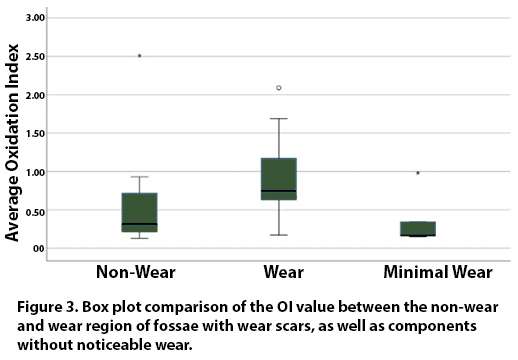
Figure 3#8334
Cup Design in Total Hip Arthroplasty With Regard to Acetabular Bone Viscoelasticity
Miriam Ruhr - TUHH Hamburg University of Technology - Hamburg, Germany
*Gerd Huber - TUHH Hamburg University of Technology - Hamburg, Germany
Michael Morlock - TUHH Hamburg University of Technology - Hamburg, Germany
*Email: g.huber@tuhh.de
Introduction
Hip arthroplasty requires high primary stability with limited cup deformation to prevent insufficient seating or excessive deformation of the liner after assembly. The primary stability of press-fit cups mainly depends on the radial interface forces at the cup rim, which is dependent on the viscoelastic properties of the human acetabular bone [1,2]. Changes in cup design promise the chance to enable high primary stability with low implantation force while taking viscoelasticity into account. The purpose of this study was to investigate the primary stability and cup deformation of acetabular press-fit cups for systematic design variations, considering the viscoelasticity of the acetabular bone.
Methods
Cyclic compression tests on human acetabular bone specimens (Ø 16 mm × 5 mm; n = 13) were performed to calibrate a numerical viscoelastic material model using Prony Series and a Neo-Hooke model (MCalibration, Veryst Engineering, Needham, MA). Experimental measurements of the time-dependent cup deformation after implantation of cups (wall-thickness 3.8 mm) in excised human acetabula [3], were additionally used to create a finite element (FE) model of cup implantation and subsequent levering out (Abaqus 2019, Dassault Systèmes Simulia Corp., Johnston, RI).
This FE model was then used to simulate the influence of i) homogeneous and ii) gradual changes of wall-thickness and iii) psoas cut-out variations (Figure 1) on the time-dependent deformation, the required implantation force and the lever-out force that represents primary stability.
Results
Acetabular bone stiffness was affected by specimens’ bone mineral density (p = 0.032), whereas the viscoelastic properties were not affected by it (p > 0.139). Experimentally a force relaxation of approximately 30% was observed within the first 300 s. In vitro, cup strain relaxation after the last impact increased with higher strain and was lower for the thin design (11.2 %, p = 0.021, R2 = 0.17) compared to the thick design (26.1 %, p < 0.001, R2 = 0.32; Figure 2).
The FE model exhibited that higher wall-thickness of the cups resulted in lower cup deformation and higher lever-out force, but came along with higher implantation forces (Figure 2 A). Alike thinner cups and a larger cut-out depth reduced implantation forces and exhibited a trend towards lower lever-out force. However, the ratio between lever-out and implantation force is particular favourable for thin wall-thickness and cut-outs.
Conclusion
High implantation forces bear the risk of bone damage or insufficient cup seating if the force cannot sufficiently be applied during implantation. Due to their smaller force relaxation after implantation, thin cups and cups with a psoas cut-out may achieve similar stability with reduced implantation forces.
References
[1] Dold et al., Proc Inst Mech Eng H P I MECH ENG H 2016
[2] Berahmani et al., J Mech Behav Biomed Mater 2015
[3] Ruhr et al., J Orthop Res 2021
Acknowledgements
Financial support by DePuy Synthes is kindly acknowledged.
Figures
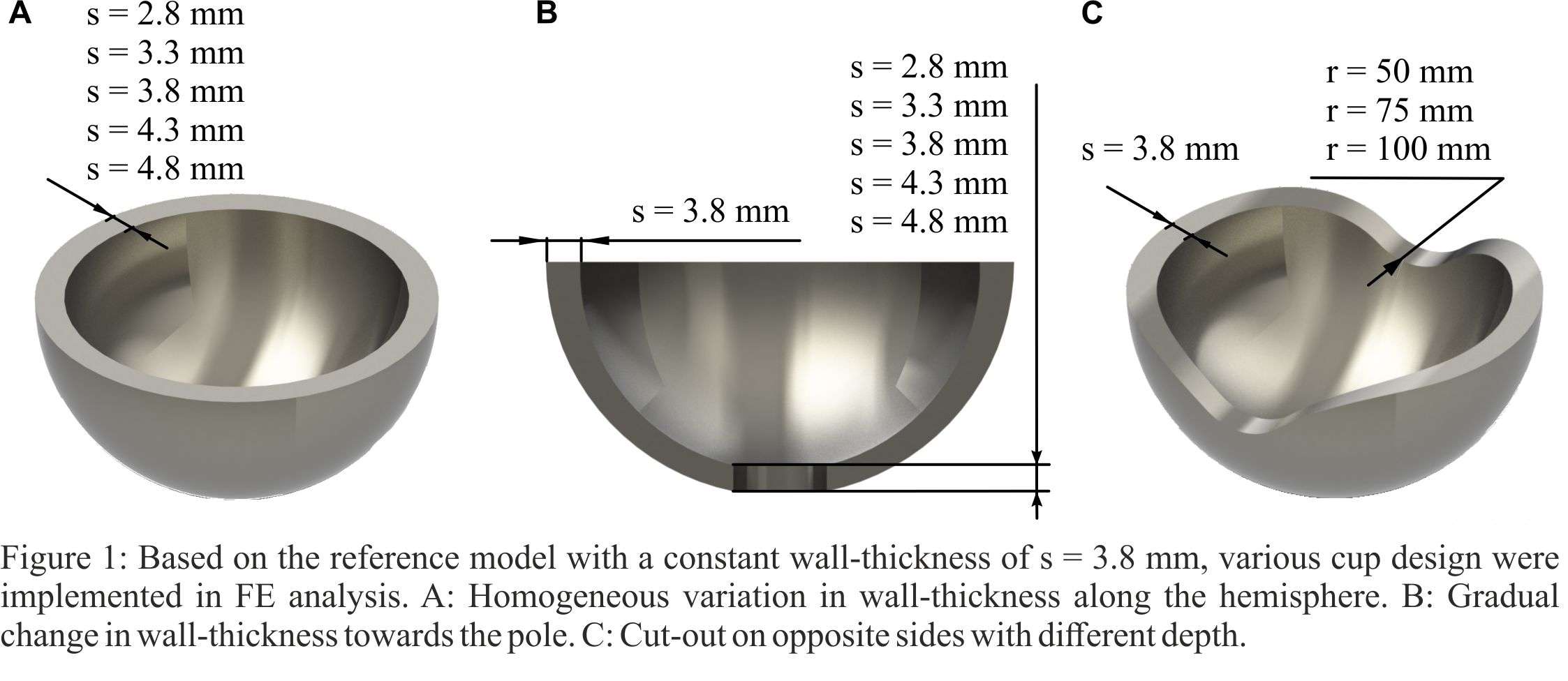
Figure 1
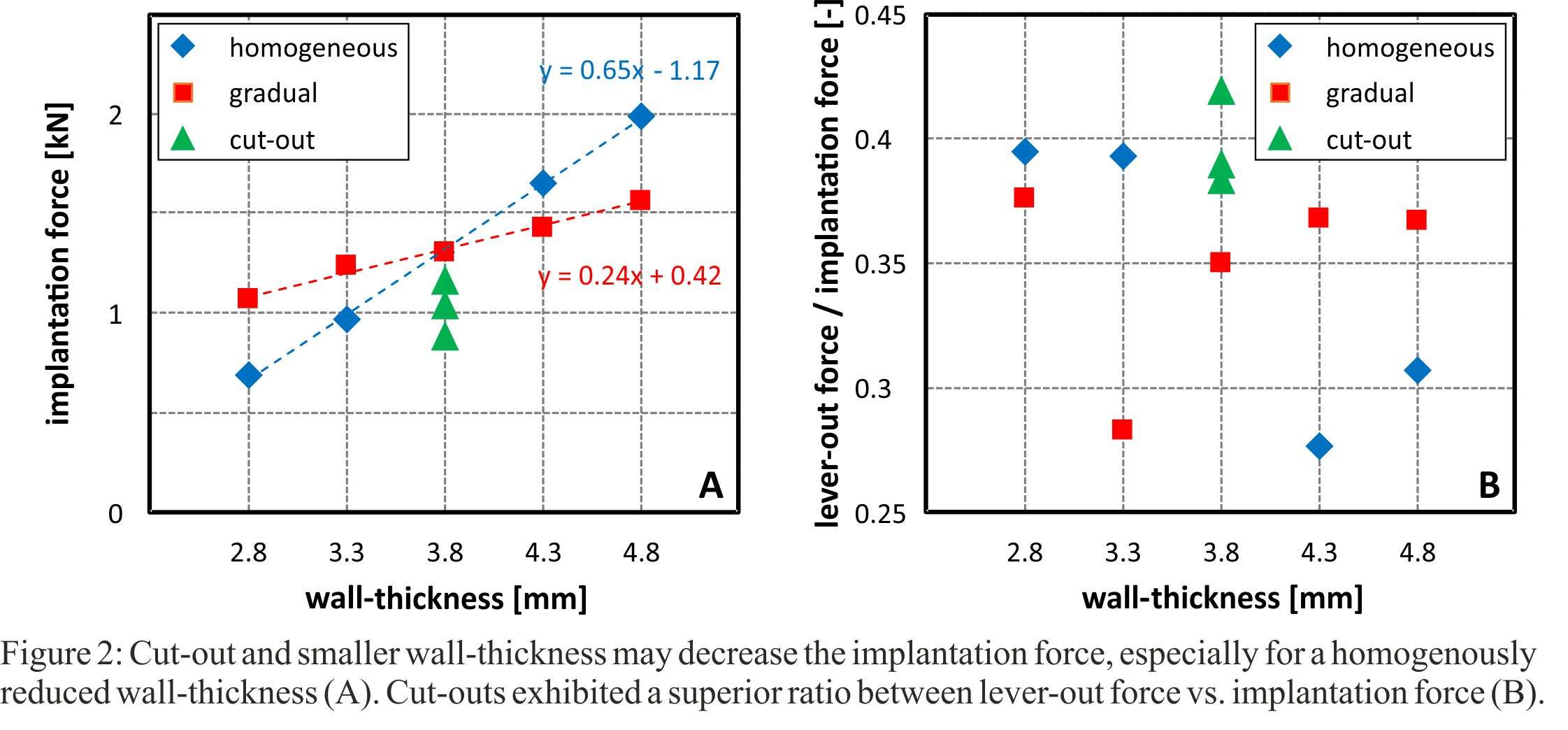
Figure 2#8192
Radiographic Fit and Fill Analysis of a Metaphyseal Filling Collared Stem Designed Using a Large CT Database
Joshua Rainey - University of Utah - Salt Lake City, United States of America
Ahmad Faizan - Stryker - Mahwah, USA
Joshua Peterson - Stryker Orthopaedics - Mahwan, USA
Manoshi Bhowmik-Stoker - Stryker Orthopaedics - Mahway, USA
Bryan D. Springer - OrthoCarolina Hip and Knee Center - Charlotte, USA
*Jeremy Gililland - University of Utah - Salt Lake City, USA
*Email: jeremy.gililland@hsc.utah.edu
Introduction: Numerous cementless hip stems are available that attempt to optimize fit and stability in total hip arthroplasty. Special attention is now paid to femoral morphology given its influence on fit and biological fixation of cementless stems [1, 2]. Prior studies have investigated the coronal dimension in the proximal femur given its key role in metaphyseal-fitting stem design [3]. Morphometric stem designs also promote enhanced fit and reduce calcar fractures [4]. However, further variations in femoral morphology have not been studied extensively. In this study, we performed additional morphometric analysis of proximal femurs to determine how the sagittal dimension may influence hip stem design. This data was then utilized to design a new collared, metaphyseal-filling triple taper stem. Radiographic fit and fill analysis was then performed to examine the fit and fill characteristics of this stem in both the coronal and sagittal planes, in addition to collar overhang.
Methods: CT scans of 1321 adult femurs from a database (SOMA, Stryker, Mahwah, NJ) were utilized to analyze femoral morphology. Figure 1 depicts constructed reference points on a template femur for measurement purposes. Transverse planes perpendicular to a sagittal plane through the femoral axis were created. Two planes proximal and eleven planes distal to the lesser trochanter were created at 10 mm intervals to measure the sagittal width of the femur at each plane. Trends observed in this data then led to the development of a collared hip stem (Insignia®, Stryker, Mahwah, NJ), which was then implanted into 59 hips (55 patients) using a direct anterior approach. Six-week post-operative radiographs were analyzed per previously described coronal fit and fill measurement methods and a novel method of measuring sagittal fit and fill [5].
Results: Sagittal width measurements along the length of a typical femur are shown in Figure 2, which represents the 126 femurs that correspond to size 4 of Accolade II (Stryker, Mahwah, NJ). Further analyses were performed for other femoral sizes to accommodate varying stem sizes. These sagittal width measurements were then averaged over the length of the femur at 10 mm intervals from the lesser trochanter and used in the development of this novel hip stem. Table 1 summarizes the results of radiographic analyses on all patients. The coronal proximal and distal fill were 85.02 ± 8.06% and 75.21 ± 9.71%, respectively. The sagittal proximal and distal fill were 86.51 ±8.77% and 59.17 ± 8.66%, respectively. Mean calcar collar coverage was 80.64 ± 19.6%. All patients had fully seated collars, and 89.8% had no evidence of collar overhang with only six cases having a collar length greater than the calcar length with a mean collar overhang of 0.7 ± 0.4 mm.
Conclusion: The sagittal and coronal dimensions of the proximal femur were used to develop a novel, collared, metaphyseal-filling triple taper stem. Radiographic analysis demonstrated excellent proximal radiographic fit and fill in both the sagittal and coronal dimensions and confirms the design intent of this novel stem. Further investigation will include obtaining long-term clinical outcomes to assess implant longevity.
Figures
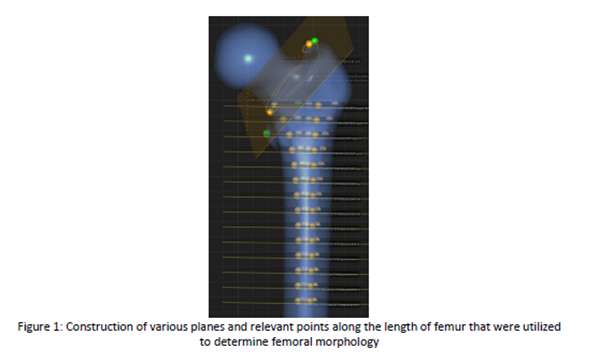
Figure 1
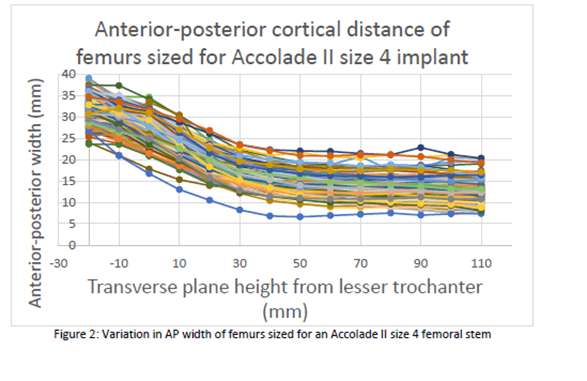
Figure 2
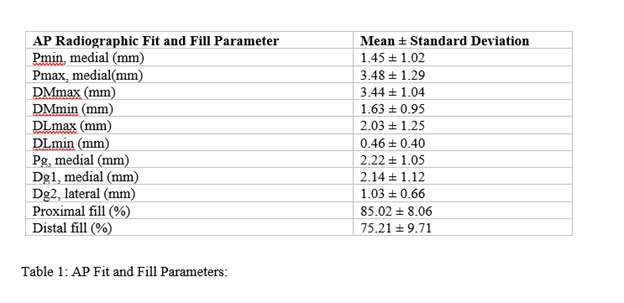
Figure 3#8164
A Novel Evaluation Method for Primary Stability of Artificial Acetabular Shells Considering Vertical Load During Level Walking
*Kazuhiro Yoshida - Kitasato University - Sagamihara, Japan
Kensuke Fukushima - Kitasato University - Sagamihara, Japan
Rina Sakai - Kitasato University - Sagamihara, Japan
Katsufumi Uchiyama - Kitasato Univ. - Sagamihara-city, JAPAN
Naonobu Takahira - kitasato university, School of Allied Health Sciences - sagamihara, Japan
Masanobu Ujihira - Kitasato university - sagamihara, Japan
*Email: yossie@kitasato-u.ac.jp
Introduction
Uncemented acetabular shell primary stability is essential for optimal clinical outcomes. Push-out testing, rotation testing, and lever-out testing are major evaluation methods of primary stability between the shell and bone. However, these test methods do not consider shell loads during daily activity and shell installation angle. Firstly, we propose a novel primary stability evaluation method for the acetabular shell that considers load during level walking and installation angles, such as acetabular inclination and anteversion. Secondly, primary stability evaluations are compared between the novel primary stability test and the conventional lever-out test.
Materials and methods
The traditional lever-out test and the novel primary stability test were performed with the uncemented type acetabular shell (Continuum Acetabular Shell, Ref# 00-8757-050-01, Zimmer Biomet G.K.). This shell has a 50 mm outer diameter and a highly porous tantalum 3D surface. An acetabular bone model was prepared using a cellular rigid polyurethane foam block with 15 pcf density (Sawbones, Pacific Research Laboratories Inc.) as a synthetic bone substrate. Press fit condition was 1 mm under-reaming.
Schematic diagrams of the lever-out test and the novel primary stability test were shown in Fig. 1 and 2. In the novel primary test, the jig for fixturing the acetabular shell was designed such that the shell center and shaft center of rotation coincided with an acetabular inclination of 40° and anteversion of 20°. The vertical load, corresponding to walking load, was set to the wear test standard for artificial hip joints of 3 kN, according to ISO 14242-1. The vertical load was applied by an air cylinder controlled by a pressure-type electro-pneumatic proportional valve (VEP3121-1, SMC Corporation), and vertical load value monitored by the load cell (LMR-S-5KNSA2, Kyowa Electronic instruments Co., Ltd.). Torque was applied to the shell an angular displacement of 5 °/min in each test.
Resulsts and Discussion
A comparison of the peak torque between the lever-out test and the novel primary stability test is shown in Fig. 3. The primary stability by the novel primary stability test was 5.4 times greater than that by the lever-out test, a significant difference. Failure mode of the novel primary stability test was that the acetabular shell rotated while maintaining the center of rotation in the reamed recess, and similar to the clinical failure. However, failure mode of the lever-out test was that the upper end of the shell acted as a fulcrum, with the lower end pulling out of the acetabular bone model.
Conclusion
The novel primary stability test method developed in this research can apply physiological walking loads and extension motions to the acetabular shell. This testing method better reflects primary stability in vivo than compared to the traditional lever-out test.
Acknowledgements
This work was supported by JSPS KAKENHI [Grant Number JP21K12755].
Figures
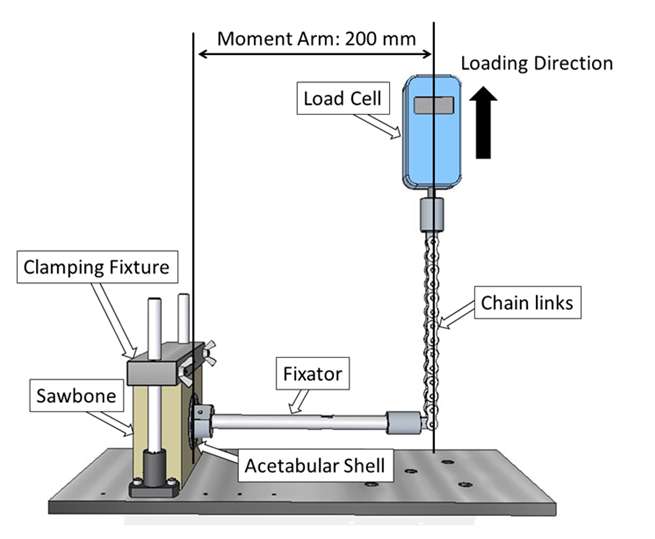
Figure 1
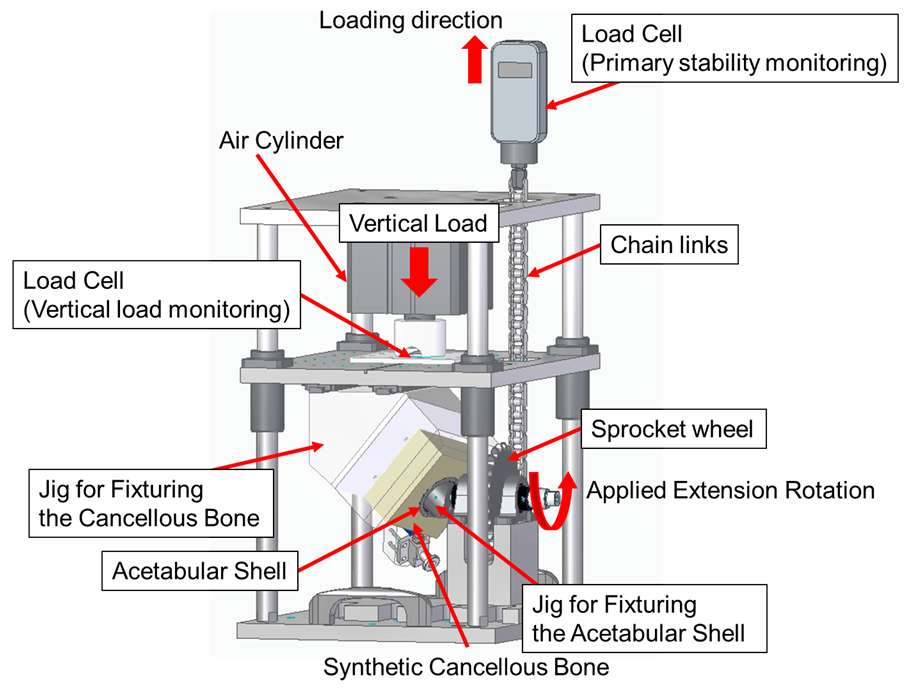
Figure 2
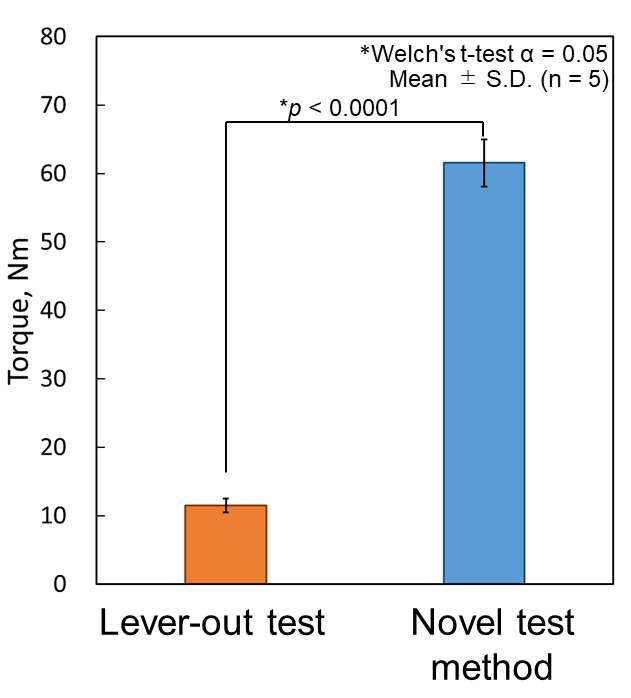
Figure 3#8223
Determination of Mechanics and Component Positioning and in Vivo Mechanics for a Novel Compaction Broach THA Stem and a Proximal Press Fit Blade THA Using a Validated Mathematical Model
*Michael LaCour - University of Tennessee - Knoxville, USA
Thang Nguyen - University of Tennessee - Knoxville, USA
Jarrod Nachtrab - University of Thennessee - Knoxville, USA
Michel Bonnin - Centre Orthopedique Santy - Lyon, France
Richard Komistek - The University of Tennessee - Knoxville, USA
*Email: mlacour@utk.edu
INTRODUCTION
Femoral stem design affects many factors of total hip arthroplasty (THA), including surgical outcomes, technique, fixation, positioning, and more. This study uses mathematical modeling to compare a recently designed compaction broaching stem (EMPHASYSTM, DePuy Synthes) to a well-known blade-style proximal press fit stem (Tri-Lock® BPS, DePuy Synthes). The objective of this study is to evaluate the implanted femoral head location compared to the anatomical head, the volumetric bone reaming required, and the postoperative mechanics (joint stability, contact area, contact stress) for each stem after implantation.
METHODS
A previously validated hip forward solution model (FSM) has been developed for implant evaluations. The model allows for virtual analyses of surgical techniques, placements, designs, soft tissue modifications, and resulting biomechanics. The model initially aligns the stem axis with the proximal canal axis. As the model “inserts” the stem into the canal, a separate model between the cortical wall and stem surface adjusts component positioning, thereby naturally adjusting stem version to avoid contact with cortical bone, until the desired position is reached.
The FSM was used to determine contact mechanics for ten subjects who previously underwent CT scan bone segmentation and fluoroscopic gait evaluation (Figure 1). Each model subject was implanted with both EMPHASYS stem + EMPHASYS Cup and the Tri-Lock stem + Pinnacle Cup. Parameters of interest include the distance between the implanted non-implanted femoral heads, volumetric cortical removal, and postoperative mechanics from gait, including contact area and contact stress.
RESULTS
EMPHASYS consistently placed the implanted femoral head closer to the preoperative head compared to Tri-Lock, indicating improved potential for component accuracy. The average distance between head centers was 3.1 ± 1.7 mm for EMPHASYS and 6.1 ± 2.8 mm for Tri-Lock (p = 0.001) (Figure 2).
The EMPHASYS stem generally required less cortical bone removal than the Tri-Lock stem to achieve these positions. Seven out of 10 participants experienced less bone removal using EMPHASYS, while 1/10 participants experienced less bone removal using Tri-Lock. EMPHASYS required an average of 15 ± 47 mm3 of cortical bone removal, while Tri-Lock required an average of 39 ± 53 mm3 (p = 0.162).
Lastly, EMPHASYS displayed less edge loading and an increase in contact area. Specifically, EMPHASYS experienced an average contact area of 889 ± 98 mm2, while Tri-Lock experienced 397 ± 66 mm2 (p < 0.0001). Accordingly, EMPHASYS also experienced only 3.1 ± 0.7 MPa of contact stress, on average, compared to 8.0 ± 2.6 MPa for Tri-Lock (p < 0.0001) (Figure 3).
DISCUSSION
While THA is already considered successful, this study shows that there is still room for improvement, both from a design perspective as well as a surgical alignment perspective. Additional sizing options and component design improvements may lead to improvement in clinical outcomes, but it is not the only avenue. Accurate component alignment, combined with optimized designs, can improve postoperative outcomes and stability of total hip replacements, as the novel EMPHASYS design generated closer potential placements, reduced cortical bone removal, and reduced contact stresses compared to the Tri-Lock BPS stem.
Figures
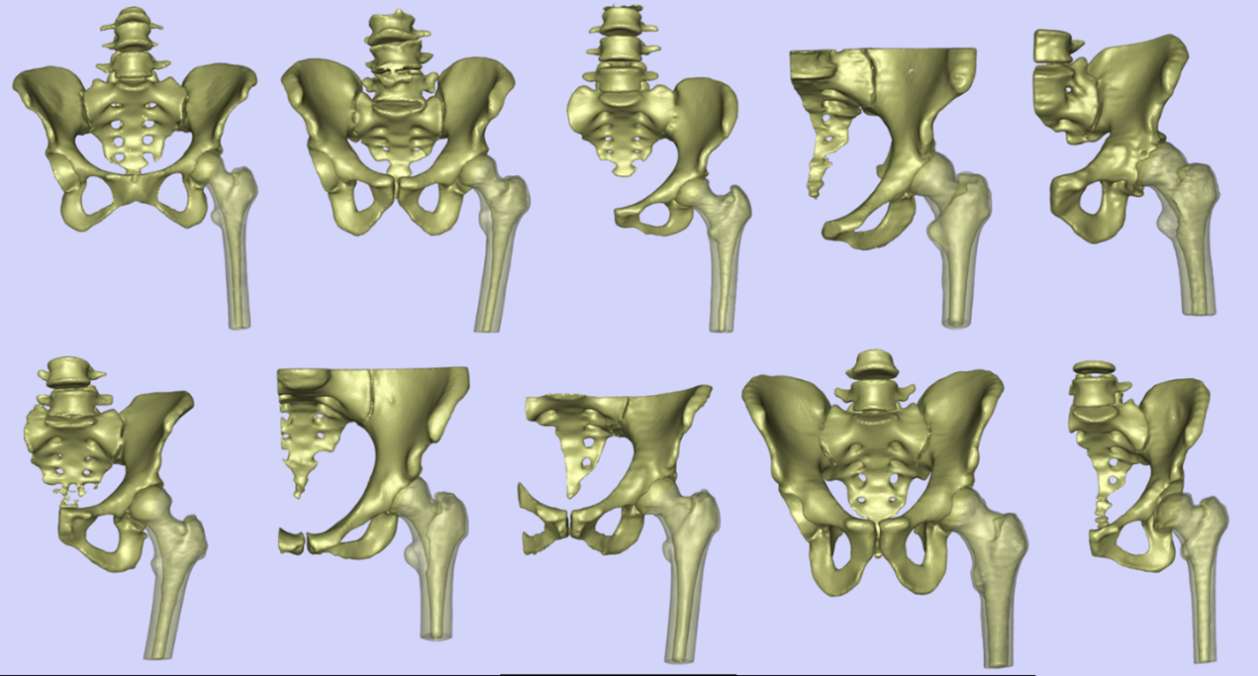
Figure 1
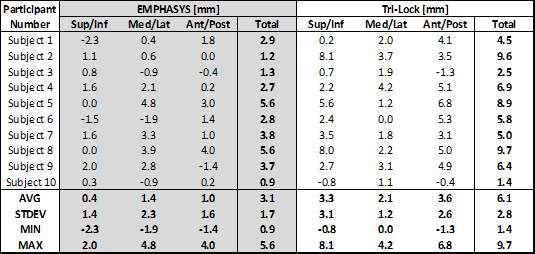
Figure 2
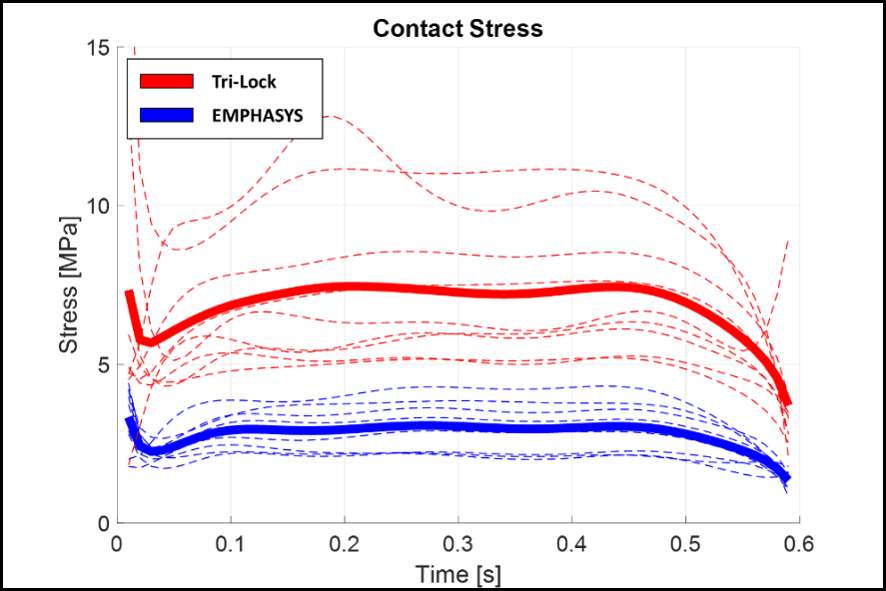
Figure 3#8175
Is Vibratory Implant Insertion a Promising Technique for Press-Fit Acetabular Cups?
*Yasaman Niki - Technische Universität Hamburg (TUHH) - hamburg, Germany
Michael Morlock - TUHH Hamburg University of Technology - Hamburg, Germany
Gerd Huber - TUHH Hamburg University of Technology - Hamburg, Germany
Kambiz Behzadi - Behzadi Medical Device - Pleasanton, USA
*Email: y.niki@tuhh.de
Introduction:
Periprosthetic fractures or loosening are major reasons for uncemented total hip arthroplasty (THA) revision [1, 2]. Press-fit implants are impacted into undersized bone cavities. Insufficient impaction forces result in insufficient implantation depth, insufficient fixation and loosening. Excessive impaction forces can generate high hoop stresses around the implant, increasing the risk of periprosthetic fractures [4], especially in poor-quality bone [5]. An increased impaction frequency was shown to reduce the impaction forces [6], which might allow achieving the desired implant position and primary stability with forces below the magnitude for critical bone damage.
This study compares vibratory insertion to consecutive single blows for acetabular cup implantation in a surrogate polyurethane (PU) model simulating different bone densities.
Method:
Acetabular cups (Pinnacle, 52 mm diameter, Gription Coating, DePuy Synthes, Leeds, UK) were implanted into 1 mm undersized cavities in PU foams (SYNBONE Inc., Davos, Switzerland) with densities of 15 and 30 pounds per cubic foot (PCF) using two devices with different impaction frequencies: a powered impaction device (1 Hz, KINCISETM, DePuy Synthes, Warsaw, IN, USA) and a vibratory implant insertion device (60 Hz, Behzadi Medical Device, Pleasanton, CA, USA). Two different offset loads were applied during impaction, accounting for intra-surgeon differences in applied forces. Impaction forces were measured during implantation (Fig. 1). After implantation, the cup position and the resulting polar gap were determined by aligning pre- and post-implantation 3D laser scans. The lever-out moment of the cup was used as a measure for primary stability (Z010, Zwick Roell, Ulm, BW, Germany).
Results:
Cup implantation reached the appropriate depth with either device in 15 PCF foams (polar gap < 2 mm, p = 0.105; Fig. 2). Seating in 30 PCF foam could not be achieved, resulting in polar gaps > 2 mm, which is clinically not recommended [7] (mostly for vibratory insertion; Fig. 2). In 15 PCF foam, the offset load did not influence the impaction force magnitude or the lever-out moment (p = 0.482, p = 0.778), allowing to pool the offset load groups. The impaction forces (p < 0.001) and the lever-out moments (p = 0.001) were significantly higher for the powered impaction device (Fig. 3).
Conclusion:
A 1 mm press fit in 30 PCF foam did not allow cup seating with either device. This highlights the importance of accounting for bone density when selecting the press-fit magnitude. PU-foam surrogate models are not well suited for testing dynamic implantation techniques utilizing the viscoelasticity of bone. Despite the model limitations, this study has shown that vibratory insertion can potentially reduce fracture risk due to decreased impaction forces. Whether the achieved primary stability is sufficient has to be shown using viscoelastic models, i.e. real bone.
References:
[1] Apostu et al., App. Sci. 2022 [4] Karachalios et at., HIP Int. 2020
[2] Kieboon et al., Arch. Orthop. Trauma Surg. 2022 [5] Ruhr et al., Bone Jt. J 2023
[3] Berry et al., J. Arth. 2002 [6] Nakasone et al., J. Arth. 2012
Figures
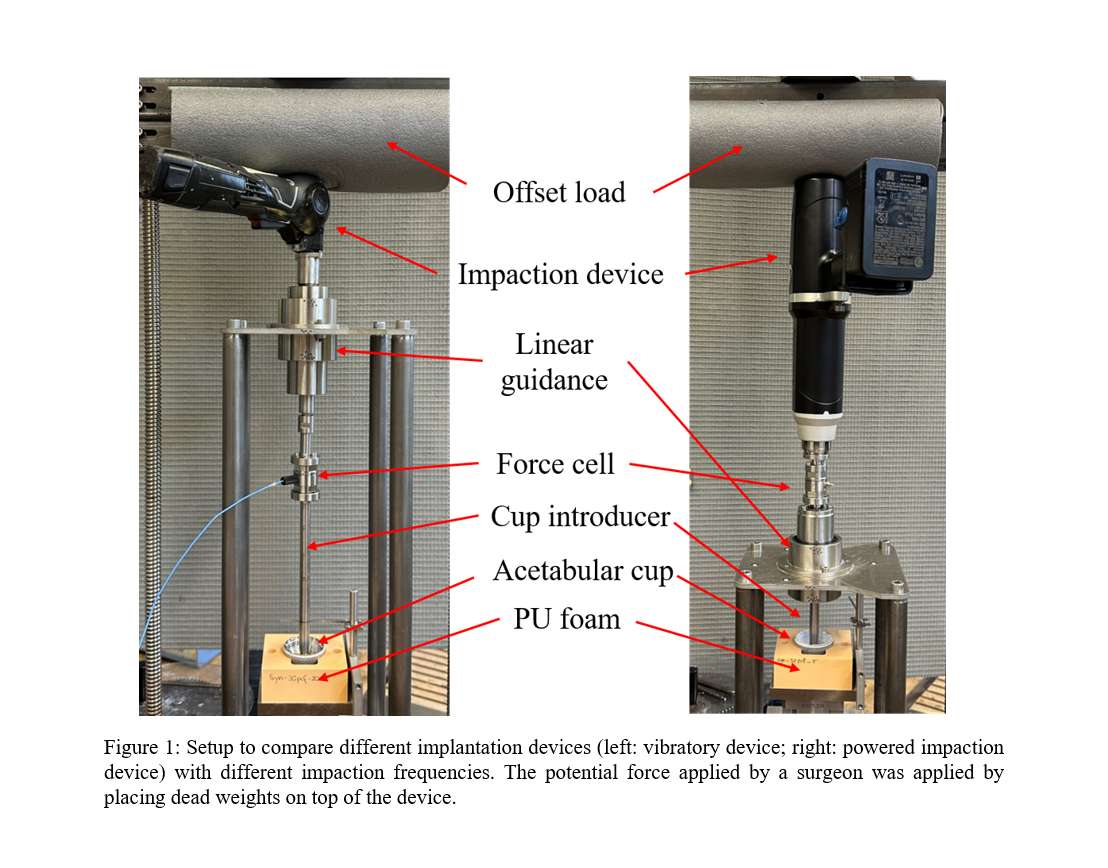
Figure 1
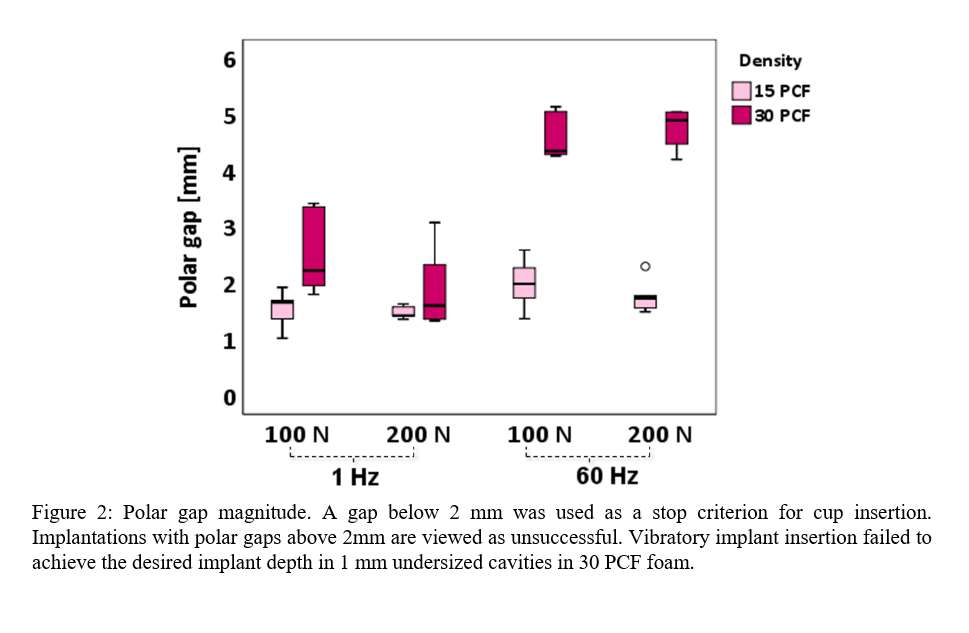
Figure 2
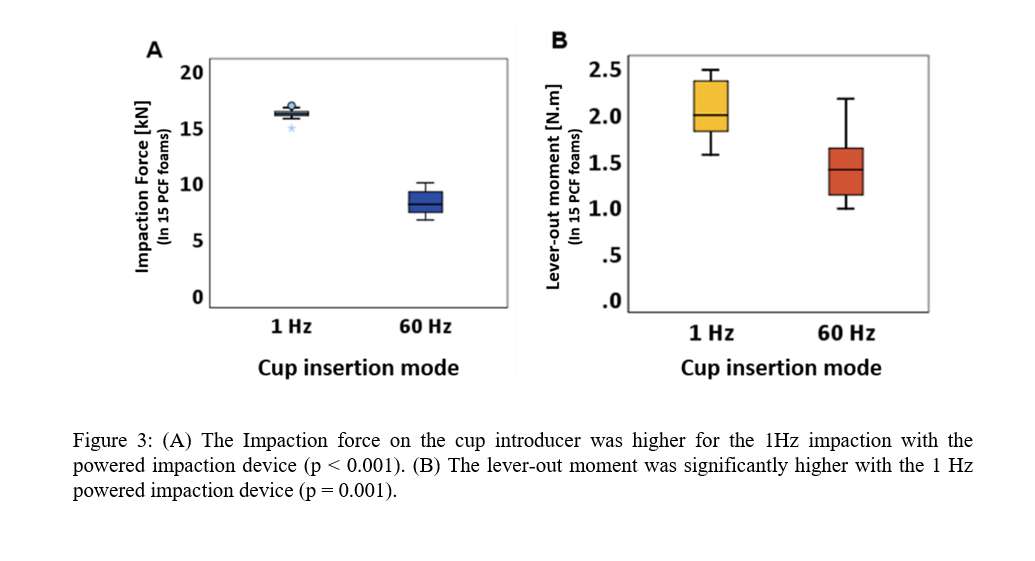
Figure 3#8393
Non-Carbon, Ordinary Traction Table Do Not Obstruct Fluoroscopic Assistance for Total Hip Arthroplasty via Direct Anterior Approach
*Seiya Ishii - Juntedndo University School of Medicine - Tokyo, Japan
Osamu Mutou - Yokohama tsurugamine hospital - Kanagawa, Japan
Yasuhiro Homma - Juntendo University - Tokyo, Japan
Tomonori Baba - Juntendo University - Tokyo, Japan
Muneaki Ishijima - Juntendo University - Tokyo, Japan
Koju Hayashi - Juntendo university - Tokyo, Japan
*Email: seiya.isii@gmail.com
Introduction: Total hip arthroplasty (THA) using a carbon-based traction table allows intraoperative seamless use of fluoroscopy, which enhances the accuracy of component placement and reduces the risk of intraoperative fracture. However, the expensive cost of carbon traction tables limited their use to hospitals performing a high volume of THA patients. The aim of this study is to evaluate the effectiveness and safety of THA via direct anterior approach (DAA) using an ordinary non-carbon traction table, which is frequently used for osteosynthesis of proximal femoral fracture.
Methods: A consecutive 82 patients who received THA at our hospital have been retrospectively analyzed. A limited number of THA have been performed using a mobile traction table specifically designed for DAA. However, due to the high costs associated with renting this specialized table for each use, we stopped using this traction table and started to use an ordinary non-carbon traction table to perform DAA-THA. Exclusion criteria are the use of the traction table specifically designed for DAA, 37 patients using a non-carbon traction table via DAA with fluoroscopic assistance and as a control, 39 THAs via posterior approach (PA) were finally included in this analysis. The radiographic outcome was analyzed using a postoperative anterior-posterior radiograph. Radiographic and clinical evaluations were conducted to assess the outcomes, including implant positioning, and complications.
Results: There were no significant differences in the basic characteristics of the patients. The DAA group had a significantly shorter operative time (DAA; 77.3 ± 24.1 vs PA; 89.4 ± 20.9, p = 0.025). There were no significant differences in blood loss, acetabular component inclination, or anteversion angle between the DAA and PA groups. Intraoperative femoral and acetabular fractures occurred in one case each in the PA group. There were no cases of infection, dislocation, or implant loosening. The DAA group had a higher ratio of acetabular components within the safe zone compared to the PA group (DAA; 97.3% vs PA; 79.5%, p = 0.029).
Conclusion: The use of a non-carbon traction table for DAA-THA with fluoroscopic assistance offers potential benefits in terms of improved accuracy of implant positioning and lower complication rates. This approach can make DAA-THA more accessible to hospitals that do not have expensive carbon traction tables. The aging population and increasing demand for THA make it crucial to explore cost-effective alternatives without compromising patient outcomes. While some reports suggest that DAA-THA without traction tables can yield comparable outcomes, most of these studies are from high-volume centers with highly skilled surgeons. The use of a traction table may facilitate the learning curve and reduce complications, such as femoral periprosthetic fractures and component loosening. Further research with larger cohorts is necessary to validate the safety and the potential risks such as pudendal nerve injury associated with the use of non-carbon traction tables for DAA-THA.
#8667
Decrease in Broaching Time Using Automated Broaching in DATHA
*Vibhu Banala - Montefiore Medical Center
Eli Kamara - Lenox Hill Hospital - New York, USA
Zeynep Seref-Ferlengez - Montefiore Medical Center - New York, USA
Yoav Zvi - Montefiore Medical Center - Bronx, USA
Shoran Tamura - Albert Einstein College of Medicine - Bronx, USA
*Email: banalavibhu@gmail.com
Introduction:
Manual broaching (MB) of the femur during a direct anterior total hip arthroplasty (DATHA) can be difficult due to exposure and positioning of the patient. Automatic broaching (AB) is a new technology that may assist in DATHA, but complications and learning curve have not previously been reported. The purpose of this study is to analyze a single fellowship trained arthroplasty surgeon’s transition from MB to AB by reporting complications associated with AB broaching and the changes in broaching time.
Methods:
A retrospective chart review was performed on 124 patients who underwent DATHA. 19 and 105 patients underwent manual and automatic broaching, respectively. The 105 AB cases were categorized into three chronological groups (n=35/group), assigned groups as MB, ABI/ABII/ABIII, respectively. Broaching times were collected, reported as average (±Standard Deviation) and times are compared among groups by Kruskal-Wallis tests. Cases where broaching time was not available were excluded (n=8) from the broaching time analysis.
Results:
Average broaching times were31 (±9) for the MB group and 19 (±5), 21 (±7), and 19 (±6) minutes for AB groups I, II, and III, respectfully (p<0.01). The overall AB group (n=105) had an average broaching time of 20 (±6). This was 11 minutes shorter that the overall MB broaching time. The calcar fracture rate in the MB group was 0/19 versus 2/105 in the AB group. The calcar fractures occurred earlier in the learning curve (Groups AB1 & ABII).
Conclusion:
Automated broaching showed a consistent decrease in broaching times when compared to manual broaching. There was no significant change in broaching time found in our cohort, however two cases of calcar fracture were present in the first two automated broaching groups, hinting at an increased complication rate during this transition.
#8525
Assessing Anatomical Version Angles in THA: Is There a Difference With Regard to Compaction Broach vs Blade Stems?
Andrew Jacobs - DePuy Synthes - Warsaw, USA
Thang Nguyen - University of Tennessee - Knoxville, USA
Jarrod Nachtrab - University of Thennessee - Knoxville, USA
Richard Komistek - The University of Tennessee - Knoxville, USA
*Michael LaCour - University of Tennessee - Knoxville, USA
*Email: mlacour@utk.edu
Introduction: Femoral version in Total Hip Arthroplasty (THA) can affect the stability and functionality of the implant. Unfortunately, the stem version angle isn’t routinely maintained, as the femoral head is removed without referencing the anatomical angles. Therefore, the femoral stem is aligned within the canal without restoring anatomical neck version, which could lead to significant changes with respect to the anatomical neck version angle (Figure 1). The objective of this study is to utilize two different techniques for positioning the stem during surgery to assess the effects of neck version using an implant positioning algorithm within a previously validated forward solution hip model.
Methods: A forward solution implant algorithm (Figure 2) was used to assess stem position by either (1) the “canal fit” module, where the stem is fit in the path of least resistance in the canal (Figure 1-B) or (2) the “anatomical fit” module, where the implant femoral head center is aligned based on the anatomical femoral head center to minimize the distance between the two centers (Figure 1-A). Mismatches between the component version angle and preoperative bone version angle, measured in the transverse plane, are denoted as 0º (neutral) when the femoral stem neck aligns with the anatomical femoral neck. The study was conducted on ten virtual subjects, implanted using both methodologies, incorporating three stem types: EMPHASYS, Corail, and Tri-Lock BPS (DePuy Synthes). The results assessed included the stem version angle, stem head center distances, and the cortical bone removal volume.
Results: In general, the canal fit position requires less cortical bone removal than anatomical fit, but the anatomical fit maintained a closer implanted femoral head center with respect to the anatomical femoral head center. During the canal fit assessment, the EMPHASYS stem experienced 3.0 mm distance from anatomical head center, compared to Tri-Lock having 6.1 mm. During the anatomical fit assessment, the EMPHASYS stem experienced 0.13 cm3 of bone removal while the Tri-Lock stem experienced 0.46 cm3 (Figure 3). The anatomical fit method maintains stem version better for all three stem types compared to the canal fit method. Interestingly, while using the canal fit method, the EMPHASYS and Corail stems only demonstrated 2º and 3º of version mismatch on average respectively, while the Tri-Lock stem induced 7º. Overall, the EMPHASYS stem produced the closest head center and version angle with the least bone removal required.
Conclusion: In this study, the compaction broach stems (EMPHASYS and Corail) experienced more optimal results than the blade-style stem (Tri-Lock). Interestingly, although it was thought that the blade system stem is more bone preserving, in this study both the EMPHASYS and Corail stems required less bone removal than the Tri-Lock stem. Although anatomical stem alignment method in this study produces a more anatomical fit, this alignment may not be achievable intraoperatively without cortical bone reaming, and it has been determined that improper stem version could lead to in-toeing and out-toeing of the foot. Therefore, it could be assumed that Corail and EMPHASYS stems may minimize change in foot position after surgery.
Figures
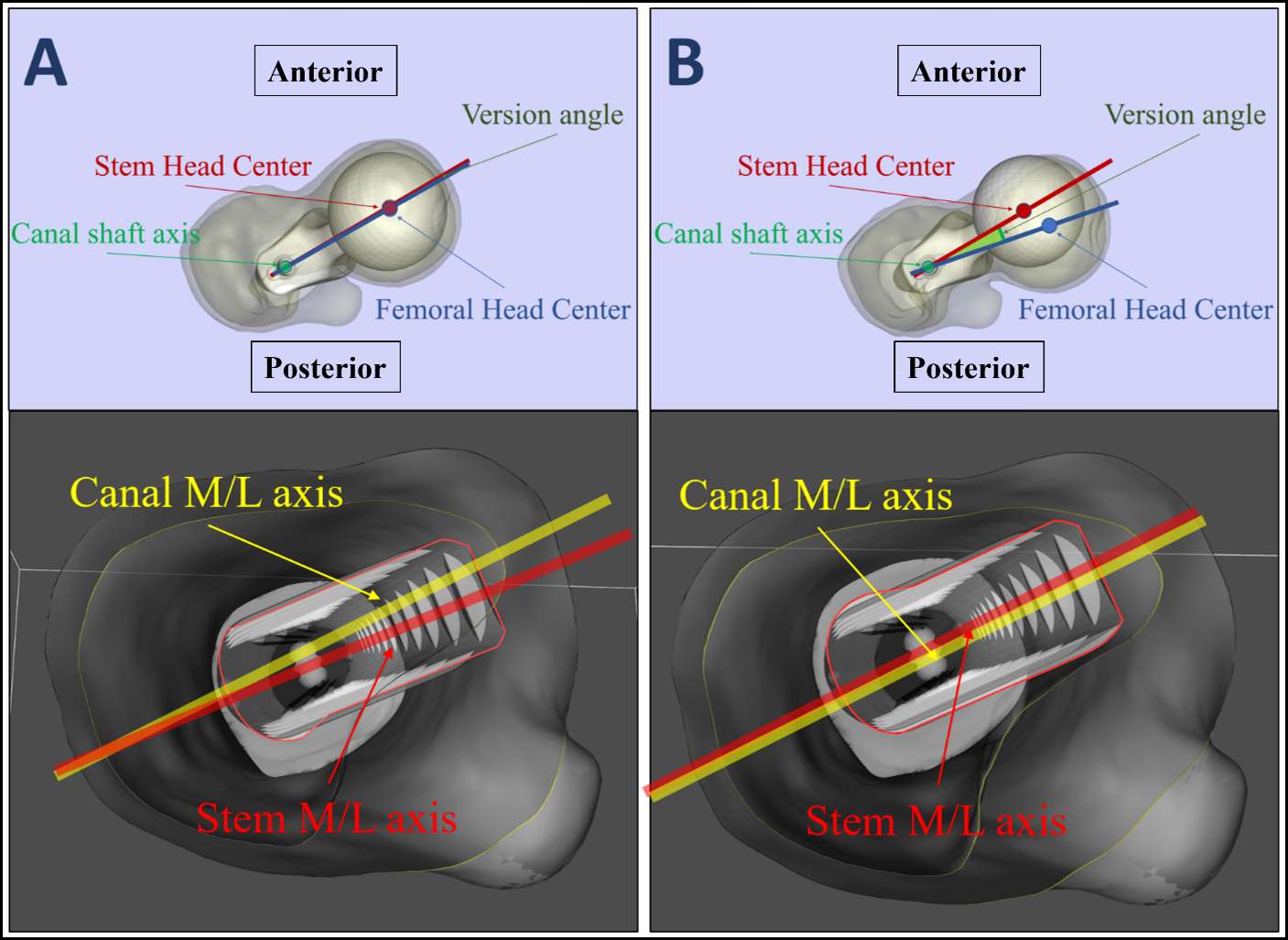
Figure 1
Figure 2
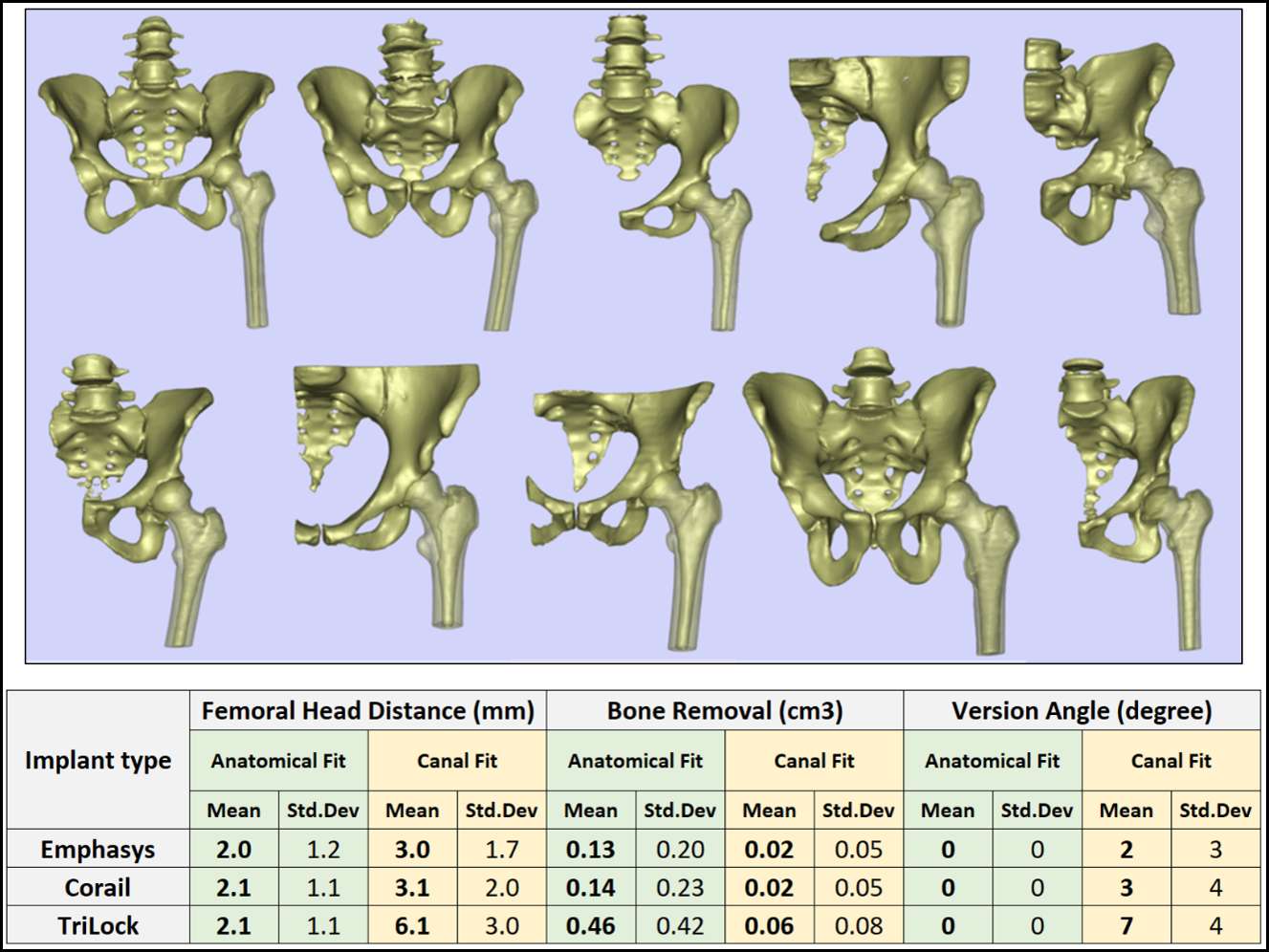
Figure 3#8099
Determining the Optimum Dimensions of 3D-Printed Cutting Jigs for Use in Orthopaedic Surgery: A Biomechanical Study
*Dean Owyang - Imperial College London - London, United Kingdom
Yiu Hin Kwan - Tan Tock Seng Hospital - Singapore, Singapore
Wei Loong Sean Ho - Tan Tock Seng Hospital - Singapore, Singapore
Gui Jie Michael Yam - Tan Tock Seng Hospital - Singapore, Singapore
*Email: DO815@ic.ac.uk
Keywords: Additive Manufacturing, 3D-Printing, Patient-Specific Instruments (PSIs).
INTRODUCTION
3D printing technology is rapidly gaining recognition in the field of Orthopaedic Surgery. Customized surgical cutting guides can be manufactured with the aid of 3D printing. These Patient-Specific Instruments (PSIs) have shown to increase accuracy, reduce operative time and lead to an overall improvement in alignment. However, as 3D printing is still a relatively new development in Orthopaedic Surgery, there is a scarcity of existing literature and information regarding the optimal design and dimensions of an ideal jig – one which is compact, durable, and able to make consistently accurate cuts intraoperatively.
Therefore, our study aims to determine the optimum design and dimensions of surgical jigs that can produce accurate cuts and are surgically practical. This can help serve as a guide for the future creation of surgical PSIs.
MATERIALS AND METHODS
A lab study was designed to mimic actual intraoperative surgical cuts. PSIs were 3D printed in medical grade, in variable thicknesses and widths of the PSI sawblade cutting slots. The PSIs were mounted to wooden blocks and surgical cuts were made using an oscillating sawblade mimicking intraoperative surgical cuts. A senior Orthopaedic surgeon and a junior medical doctor performed 36 cuts each. The cuts on the wooden blocks were analyzed, and the degree of deviation from the orientation plane for each cut was measured.
RESULT/DISCUSSION
A total of 72 cuts were performed. A two-way ANOVA was then run on the 72 measured data points to examine the effect of the depth and width of the jig on the error in degrees. There was a significant interaction between the effects of the depth and width of the jig on the error in degrees. Simple main effects analysis showed that at a depth of 5mm and 100% width, it was significantly more accurate than a width of 105% (p<0.05), 110% (p<0.001), and 120% (p<0.001).
At a depth of 10mm, the 100% width was only significantly more accurate than the 120% width (p<0.05), but no difference with the rest. Finally, when the depth was 15mm, the width did not make a difference in accuracy.
Hence, we found that the most accurate parameters for jig construction in this series were a jig width of 100% and a depth of 5mm.
CONCLUSION
Accuracy in Orthopaedic Surgery directly affects surgical outcomes. 3D-printed Orthopaedic surgical PSIs are a viable alternative to conventional methods as they have been shown to increase accuracy, improve alignment and reduce operative time. Hence, it is imperative to identify optimum design parameters of PSIs that allow consistently accurate cuts.
Our study is the first to explore and propose tangible parameters that can be used for jig construction in the future to obtain reproducible accurate cuts. We hope that the results will guide improved 3D PSI design in the future, leading to overall improved surgical outcomes.
Figures

Figure 1
Figure 2
Figure 3#8466
Using Technology to Measure and Minimize Torques in Hip Arthroplasty
*Rima Nasser - Imperial College - London, GB
Justin Cobb - Imperial College - London, United Kingdom
Jonathan Jeffers - Imperial College London - London, United Kingdom
Kabelan Karunaseelan - Imperial College London - DUNSTABLE, United Kingdom
Takuro Ueno - Kanazawa University - Kanazawa, Japan
*Email: r.nasser@imperial.ac.uk
Introduction: The direct anterior approach (DAA) to hip arthroplasty is claimed to be minimally invasive and reported to have clinical advantages over other approaches. In hip resurfacing, the DAA has the potential added advantage of preserving the femoral head blood supply. It may however create more injury to the soft tissues due to excessive leverage. Adequate visualization of the acetabulum and proximal femur requires high torques if tissue release is inadequate. Our aim was to study the contribution of different segments of the hip capsule to the amount of torque required for satisfactory exposure during anterior approach resurfacing arthroplasty.
Methods: The hip joints of 9 cadavers were approached using the DAA after a custom fixture consisting of a 6-axis force/torque sensor and motion sensor was fastened to the femoral shaft. The torques generated to visualize the acetabulum and elevate the femur were measured during the sequential release of each of the following capsular structures [Fig 1]: Iliofemoral ligament, pubofemoral ligament, zona orbicularis (transverse cut), inferior ischiofemoral ligament, superior-posterior capsule, circumferential capsulotomy, the conjoined tendon, and the piriformis. The position of the limb in space at the time of the measurement was recorded as was the anterior translation of the femur under a force of 70 N.
Results: After the usual initial dissection and a proximal iliofemoral capsulotomy, the segment of ischiofemoral ligament from 7-8 o’clock contributed to a mean 25% of overall external rotational restraint (1.5 Nm) and was, therefore, the largest restrictor of exposure of the acetabulum. Another part of the ischiofemoral ligament (10-12 o’clock) was the largest restrictor of exposure of the proximal femur, contributing to 25 % (1 Nm) of overall extension restraint. Once a circumferential capsulotomy had been performed, releasing the external rotators had a minimal contribution to the torque generated during joint exposure (≤5%). [Fig 2] A mean femoral elevation of 7 mm in the anterior direction was achieved with a 70 N elevation force. The largest mean increase in elevation was observed after the release of the ischiofemoral ligament (10-12 o’clock), accounting for 30% of the total elevation. [Fig 3]
Conclusion: In our cadaveric study, following a proximal circumferential capsular release, the forces needed to deliver adequate visualization of the hip joint through a DAA reduced well below any threshold of muscle damage. Adequate exposure of both the femoral head and acetabulum for hip resurfacing can be delivered with minimal torque, without a traction table or any special attachments, and without external rotator release.
#8402
Increase in Femoral Offset Leads to Changes in Muscle Lengths Throughout Range of Motion
James Germano - Orlin and Cohen Orthopedic Group - New York, USA
David Liu - Gold Coast Centre for Bone and Joint Surgery - Tugun, Australia
Duncan Bakke - Formus Labs - Auckland, New Zealand
*Marco Schneider - Formus Labs - Auckland, New Zealand
Thor F Besier - University of Auckland - Auckland, New Zealand
*Email: marco@formuslabs.com
Introduction
Femoral offset is a key consideration to restore native hip biomechanics when performing total hip arthroplasty. Increases in offset lead to larger moment arms for the hip abductors, in theory helping with recovery post-operatively [1] and reducing the risk of bone-on-bone impingement by increasing the clearance between the trochanteric edges and the pelvis [2]. However, increasing femoral offset can also lengthen the adductor muscles, leading to potential overstretching and altered muscle dynamics (Figure 1). This study investigated the influence of femoral offset on adductor muscle lengths throughout a sit-to-stand motion.
Methods
A template musculoskeletal model of the lower limb (OpenSim, Stanford, USA) was customised to enable simulations of femoral offset. For the purpose of this study, only adductor muscles were analysed, including the adductor magnus, longus, and brevis, and the pectineus, as well as the hamstrings. Femoral offset was incrementally adjusted from +0 to +20mm (lateral). A sit-to-stand motion was simulated for each offset value, and the changes in muscle length during the motion were calculated.
Results
Increasing femoral offset from +0 to +20mm influenced the proximal adductor muscles more than the distal muscles. For example, a +20mm offset increased the length of the proximal adductor magnus by 13% (~16 mm) (Figure 2), compared to distal regions of the muscle, which changed maximal length by <1mm (Figure 3). This finding highlights the potential for non-uniform strains throughout the muscle following changes in femoral offset. In the shorter adductor muscles, including the adductors brevis, adductor longus, and the pectineus, we found up to 16 mm length change. For the pectineus, this represents an increase of 18.6% at the point of maximum trunk flexion. In longer muscles, such as the hamstrings, the change in length was only ~1% throughout the motion.
Conclusion
This is one of the first steps in understanding the complex interaction between bone and muscle and the dynamic change in their relationship from sit to stand. The findings of this study illustrate the varied influence of femoral offset on muscle-tendon tissue, which could have important consequences on clinical outcomes, such as muscular pain, stiffness, and dislocation risk. Shorter muscles that are aligned to the medial-lateral axis experienced the greatest changes in length, compared to longer superior-inferior muscles, which was expected. Patient geometry will likely play a role on the relative changes in muscle tension relative to femoral offset, as well as patient-specific kinematics. As such, three-dimensional dynamic analyses with patient-specific anatomy and dynamics are necessary to quantify the complex interactions between implant size, position, and functional outcomes, and to enable planning for optimal implant positioning in total hip arthroplasty.
References
- McGrory BJ et al. (1995). J Bone Joint Surg Br. 77, 865-9.
- Ito K, et al. (2001). J Bone Joint Surg Br. 83, 171-6.
Figures
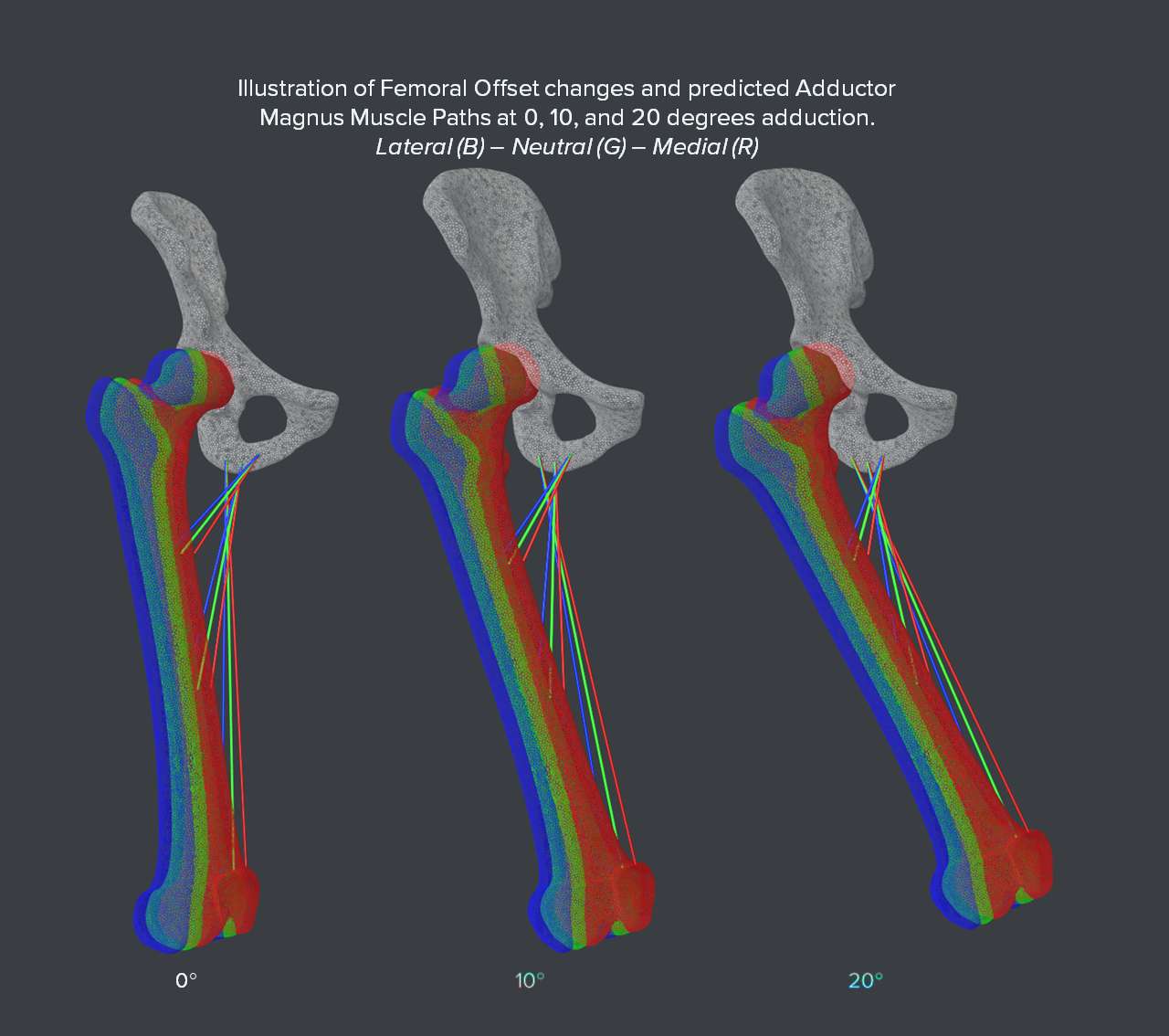
Figure 1
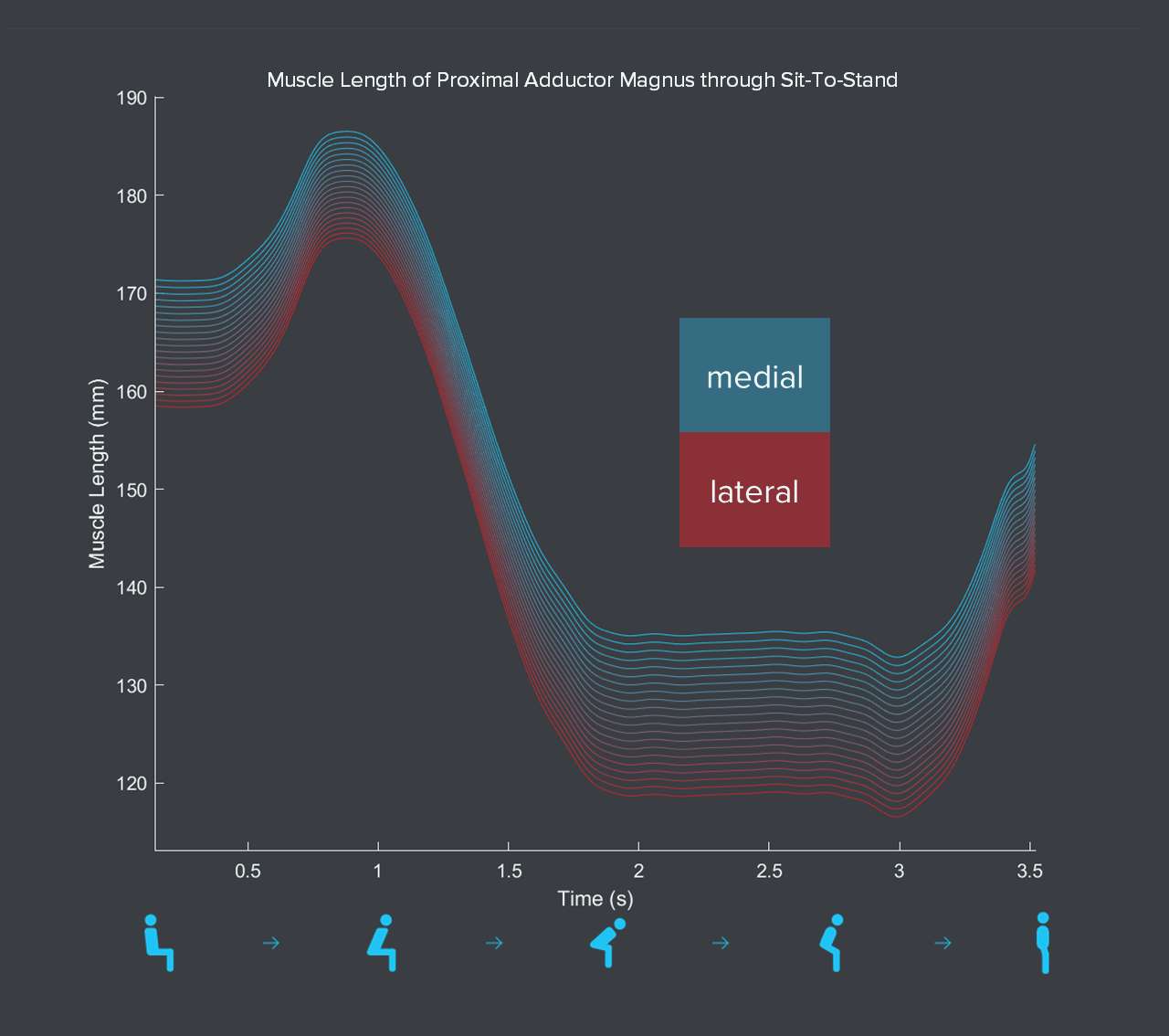
Figure 2
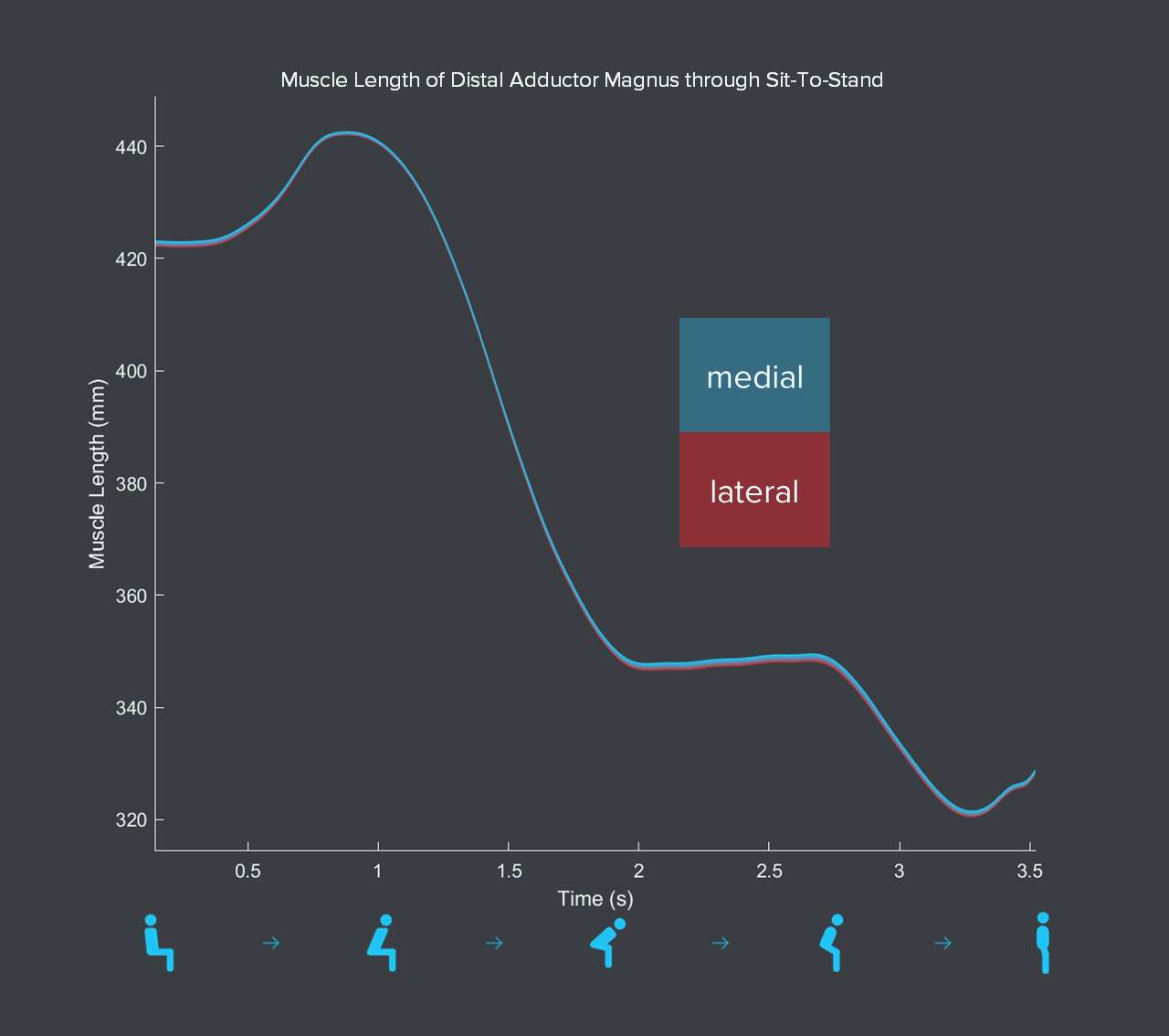
Figure 3#8156
Measuring Intra-Operative Femoral Version With Fluoroscopy in Direct Anterior Total Hip Arthroplasty
*Jhase Sniderman - Toronto, Canada
Michael Kain - Boston University - Boston, USA
*Email: jhasesniderman@gmail.com
Introduction: Instability following total hip arthroplasty (THA) remains one of the most common reasons for revision surgery. While there are multiple factors that can contribute to instability, the position of the components is of critical importance. Theoretical safe zones and combined version targets have been established for component positioning. The recent adoption of technology and a further understanding of the hip-spine relationship has led to renewed interest in optimizing the position of the acetabular component. However, significant gaps exist in measuring femoral version and understanding its contribution to hip stability.
Objective: This study aims to demonstrate a reliable way to measure femoral version intraoperatively through the direct anterior approach.
Methods: Patients were prospectively enrolled to undergo an intraoperative assessment of their native femoral version and femoral component during direct anterior THA between August 2022 and March 2023. All patients had a preoperative computer tomography (CT) scanogram of their lower extremities. A workflow was established that would allow for the fluoroscopic assessment of femoral version as part of routine THA. Femoral version was measured prior to neck osteotomy, with the broach in situ and at the time of definitive stem insertion. CT scanograms were then reviewed postoperatively and the femoral version was measured. Statistical analysis was performed comparing preoperative to intraoperative measurements with the use of t-tests.
Results: 15 patients underwent direct anterior THA with the use of this specific fluoroscopic protocol. The mean native femoral anteversion on CT scanogram was 15.1 degrees (range 3.5-37). Mean native femoral version measured intraoperatively was 14.2 degrees. Mean intraoperative broach and definitive stem version were 14.9 and 16.5 degrees. There was no significant difference between native femoral version when measured by CT scan or intraoperative fluoroscopy (p=0.9). There was similarly no significant difference between native femoral version and final stem version in this cohort of patients (p=0.65).
Conclusion: This is the first study to accurately measure femoral version intraoperatively with the use of fluoroscopy during direct anterior THA. There was little change in overall femoral version with the use of a metaphyseal filling stem. Surgeons thus wishing to achieve a combined version target can modify the position of their acetabular component based on the relative inflexible position of the femoral component. This technique can be incorporated into any surgical workflow when utilizing fluoroscopy during THA.
#8565
Dynamics of Intraoperative Pelvic Alignment During Total Hip Arthroplasty in the Supine Position
Tristan Jones - Naviswiss Inc - Tampa, United States of America
Eric M Slotkin - Reading Hospital, Orthopaedic Associates of Reading, - West Reading, Pennsylvania, USA
*Stefan Kreuzer - INOV8 Orthopedics - Houston, USA
Alejandro Gonzalez Della Valle - Hospital for Special Surgery - New York, USA
*Email: skreuzer@inov8hc.com
INTRODUCTION
Recently, the direct anterior approach (DAA) for total hip arthroplasty (THA) has become increasingly popular. One of the major advantages proposed is the innate stability and reproducibility of the pelvis in the supine position on the operating table. This proposed benefit has the potential to reduce the risks associated with component malorientation, which have been well published. The purpose of this study was to examine the dynamics in pelvic position in the sagittal plane at different time points (initial registration, cup insertion and trial reduction) during the DAA procedure.
METHOD
A total of 43 patients undergoing THA via a DAA were included. All patients received a pre-operative CT scan and a miniaturized navigation device (Naviswiss, AG) was used to execute the intra-operative image-based registration. Further, all patients were positioned on the operating table with the adjunctive use of a leg positioning device. The alignment of the pelvis was tracked and recorded at the start of the registration process, again at the point of acetabular cup insertion, and finally at trial reduction. The pelvic positions were noted and compared with respect to the sagittal plane and were recorded within the case log files of the platform.
RESULT
At the initial registration time point, the position of the pelvis was anteriorly rotated by a mean of 5.1° (range -9.6° to 15.7°, SD 5.4°). At cup insertion, the pelvis had continued to rotate anteriorly by an additional mean of 1.0° (range -0.9° to 5.4°, SD 1.3°). At trial reduction, the pelvis then rotated posteriorly by a mean of -0.6° (range -3.0° to 1.7°, SD 0.9°).
CONCLUSION
The initial supine sagittal position of the pelvis in a DAA demonstrates a high degree of patient variability. However, the magnitude of change of the pelvic position throughout the THA procedure is low, with minimal variability shown. Predicted changes in the orientation of the cup by more than 5° version were seen in no cases between any 2 of the times points assessed.
#8113
Pelvic Kinematics During Operative Motions in Direct Anterior Total Hip Arthroplasty on an Orthopedic Table
*Kathryn Colone - University of Denver - Denver, USA
Yashar Behnam - University of Denver - Denver, USA
Daniele Marras - University of Denver - Denver, USA
Casey Myers - University of Denver - Denver, USA
Chadd Clary - University of Denver - Denver, USA
Jacqueline Lamb - MIzuho OSI - Union City, USA
*Email: Kathryn.Colone@du.edu
Introduction: Direct anterior approach total hip arthroplasty (DA-THA) with patients positioned supine has become an increasingly popular surgical technique. Acetabular cup inclination and anteversion play important roles in clinical outcomes by affecting hip range of motion, joint stability, and dislocation risk. Pelvic positioning throughout DA-THA affects cup positioning, where a 1° increase in pelvic flexion causes 0.5° to 1° change in cup anteversion [1,2]. Previous studies using a traction table during DA-THA have evaluated radiographic imaging preoperatively and after component placement to investigate pelvic tilt but have not captured pelvic movement throughout the procedure [3]. The objective of this study was to understand pelvis kinematics during DA-THA facilitated by a Hana table (Mizuho OSI).
Methods: Two pelvis-to-toe specimens (four hips) were tested in this study (66, 68 yrs; 24.8, 31.82 kg/m2 BMI). Specimens were placed supine on a Hana table with the pelvis unconstrained and feet secured to the leg spars (Fig. 1). Optical markers were rigidly attached to the pelvis, femurs, and tibias to record bone kinematics using an NDI Optotrak Certus. Pelvis kinematics were evaluated during four motions associated with DA-THA on the intact hip: 1) 90° external rotation of the operative leg, 2) hyperextension and adduction, 3) return to neutral extension and abduction, 4) 90° internal rotation of the operative leg with traction (Fig. 20). After testing, fiducial markers were placed in the pelvis and femurs, probed within the motion capture system, denuded, and optically scanned to register the bony anatomy to the optical markers. Anatomic coordinate systems were established for the pelvis and used to describe pelvis kinematics during the experiment. Kinematics were described relative to the original pelvis orientation in the supine position.
Results: External rotation of the boot was associated with minimal pelvic movement (pelvic flexion (PF):1.5 ±0.7°, lateral tilt (LT): -5.2±1.1°, pelvic rotation (PR): 2.5±1.1°). During hyperextension/adduction of the hip, substantial PF and LT were observed (9.8±1.9° and -20.6±1.1°, respectively) with minimal PR. All three angles decreased after the leg was returned to neutral extension/abduction. The highest PF occurred during relocation, whereas large LT angles were observed during hyperextension/adduction and traction during relocation (Fig. 3).
Conclusion: While two-dimensional radiographic imaging has been analyzed to compare PF before and after hip implantation, little is known about the progression of PF during specific motions of DA-THA using a traction table to facilitate implant placement [4]. This study provides a quantitative analysis of pelvic motions using three-dimensional modalities. Hyperextension/adduction and traction were associated with substantial pelvic motion. 10° of PF could cause up to 10° of difference in the intended cup version, suggesting a necessity to understand intraoperative pelvic motions to optimize component placement and patient outcomes. While the lack of torso likely led to an overestimation of pelvic movement, this study provides a framework for subsequent testing to investigate kinematics in specimens with a torso more reflective of clinical conditions.
References: 1. Parilla J Arthroplasty (2019). 2. Yang Orthop Surg (2019). 3. Roettges J Arthroplasty (2018). 4. Aichmair BMC Musculoskelet Disord (2020).
Figures
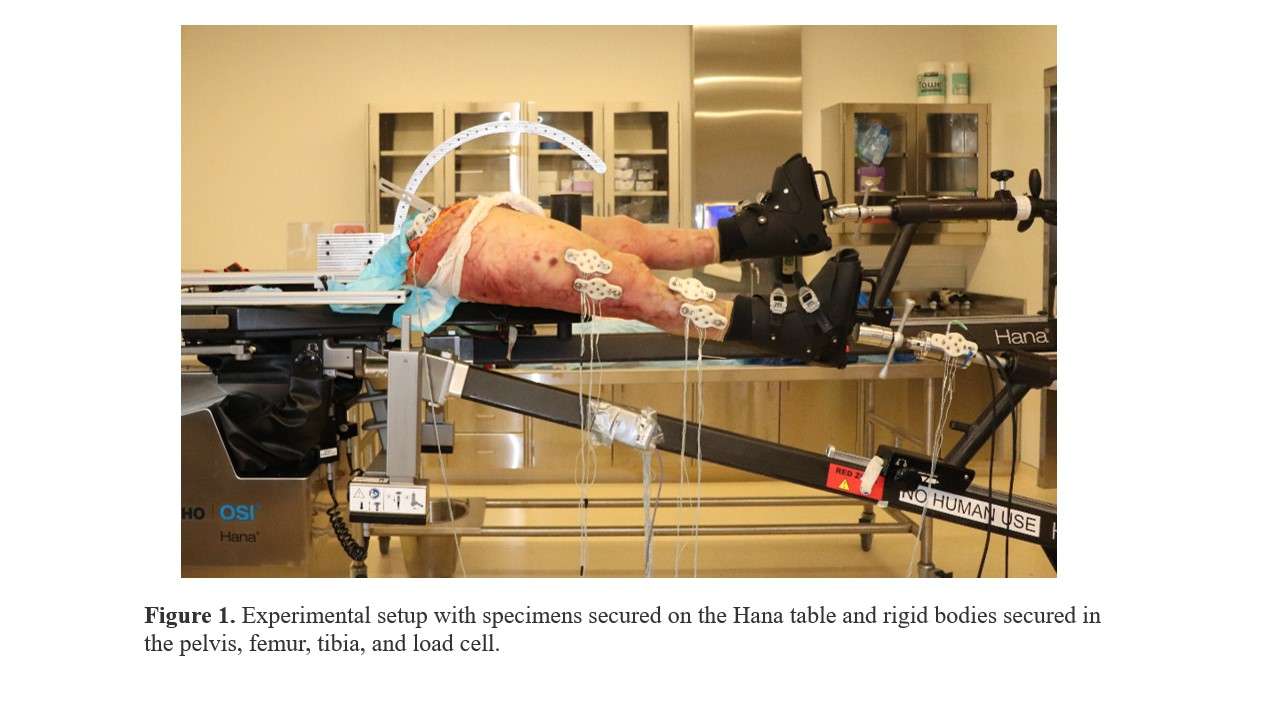
Figure 1
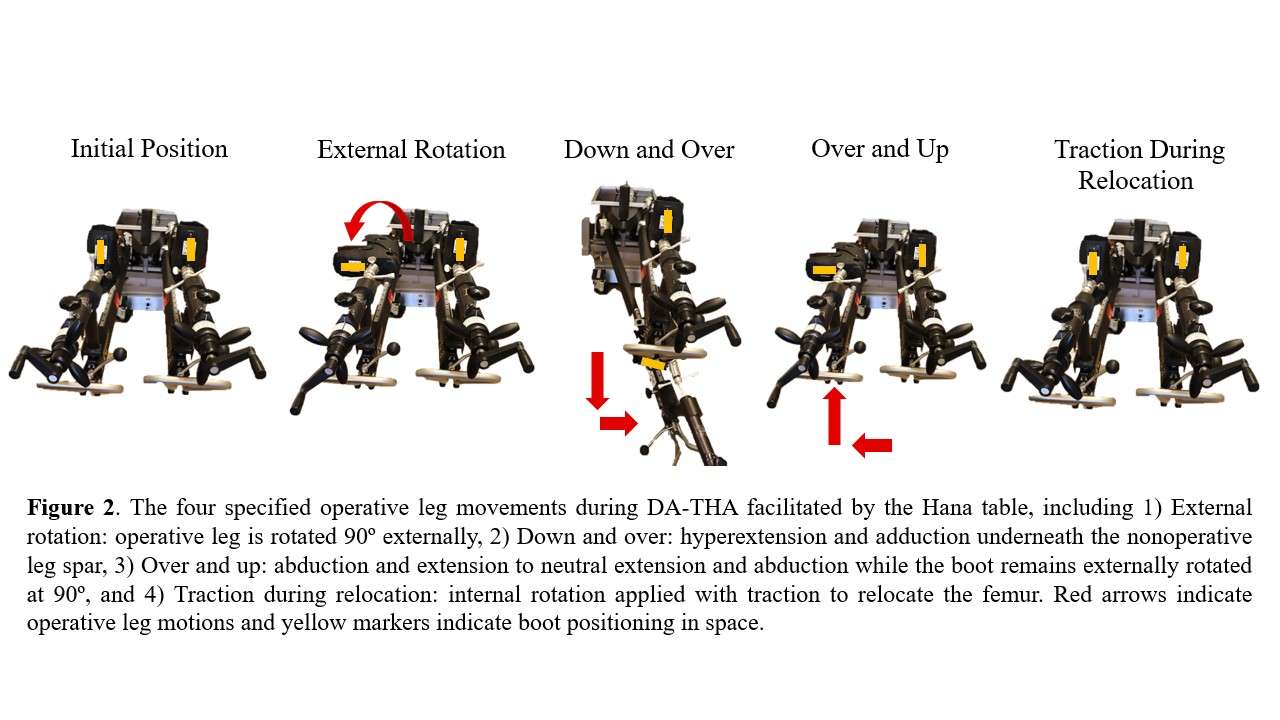
Figure 2
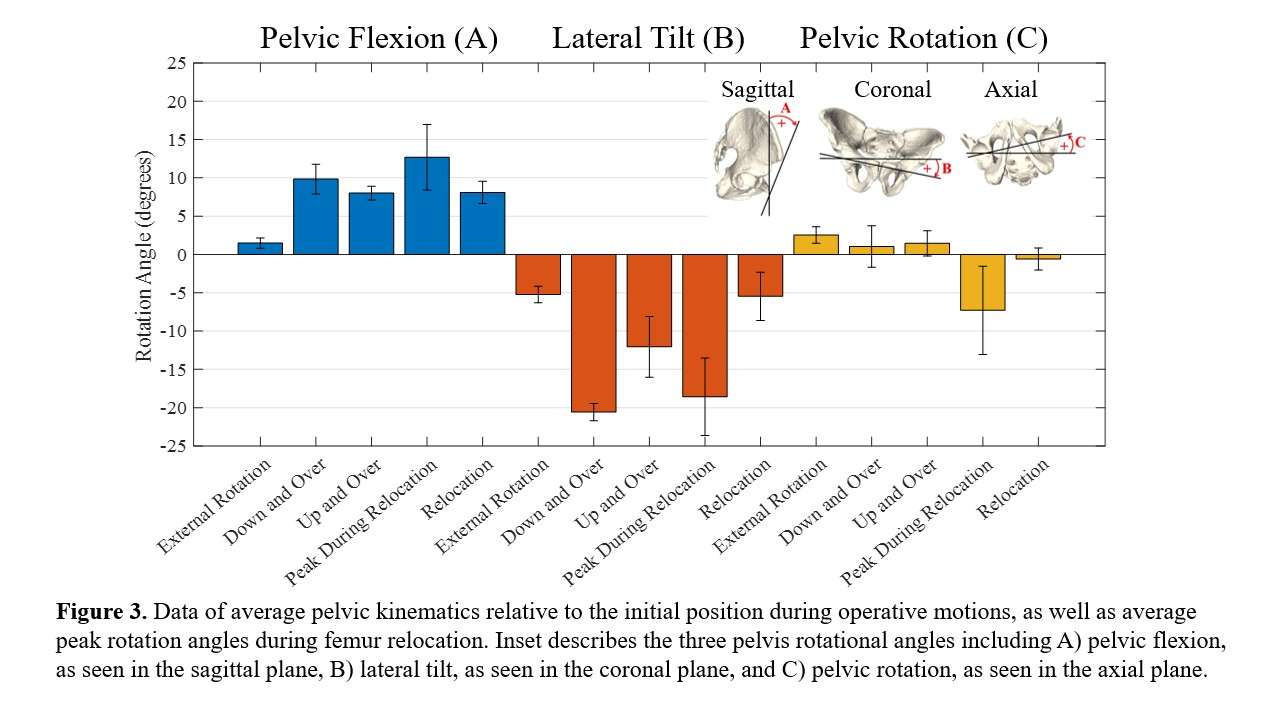
Figure 3#8431
Innominate Bone Rearrangement Following Large Acetabular Reconstruction
*Angelika Ramesh - London, GB
Johann Henckel - Royal National Orthopaedic Hospital - London, United Kingdom
Alister Hart - Royal National Orthopaedic Hospital - London, United Kingdom
Sara De Angelis - University College London - London, GB
Anna Di Laura - Royal National Orthopaedic Hospital and Dept. MechEng at UCL - London, United Kingdom
Harry Hothi - London Implant Retrieval Centre - Stanmore, United Kingdom
*Email: zcemram@ucl.ac.uk
Introduction
In the surgical management of massive acetabular defects, it is reported that early implant migration can occur without eventual implant loosening and failure. Post-operative assessment of the joint reconstruction is complex due to the challenge in establishing the movement of the implant relative to the innominate bone and how the innominate bone rearranges itself around the implant.
We aimed to measure and quantify changes in the morphology of the innominate bone.
Methods
This observational retrospective study used the immediate (timepoint 1) and 1-year post-operative (timepoint 2) CT scans of 22 patients with Paprosky type IIIB defects, treated with 3D printed custom-made acetabular implants.
3-Dimensional reconstructions of the patients’ bony pelvis were produced from the CT images using specialised rendering software solutions, for the relative comparison of the two imaging timepoints (immediate post-operative, 1-year post-operative). In cases where the structure of the innominate bone had changed too much to allow registration agreement between both scans (i.e. root mean square error RMSE) we then performed implant-to-implant registration using the implants as a fiducial marker (Fig. 1). From this second exercise we were able to quantify the anatomical changes of the innominate bone together with those instances where the implants moved relative to the bone. Further software analysis of the images of the whole pelvises allowed us to understand the relative movement of three components of the innominate bone (ilium, ischium and pubis).
Results
The mean RMSE of the innominate bone co-registration was 0.98 (range = 0.50 - 1.50).
The mean centroid distance between the position of the innominate bone relative to one another was found to be 2.7 mm (range = 0.6 – 6.0 mm).
We found that the shape of the innominate bone changed in 7 cases (32%) . The pelvic component that showed greatest change was the pubis, followed by the ischium.
All cases that presented with pre-operative pelvic discontinuity (4 out of 22, 18%) showed relative bony rearrangement.
Conclusion
When performing CT implant monitoring in patients with large acetabular defects, changes of the innominate bone can occur. We were able to quantify these changes by studying the bone morphology at two separate timepoints. Surgeons and engineers should be aware when monitoring these patients.
Figures
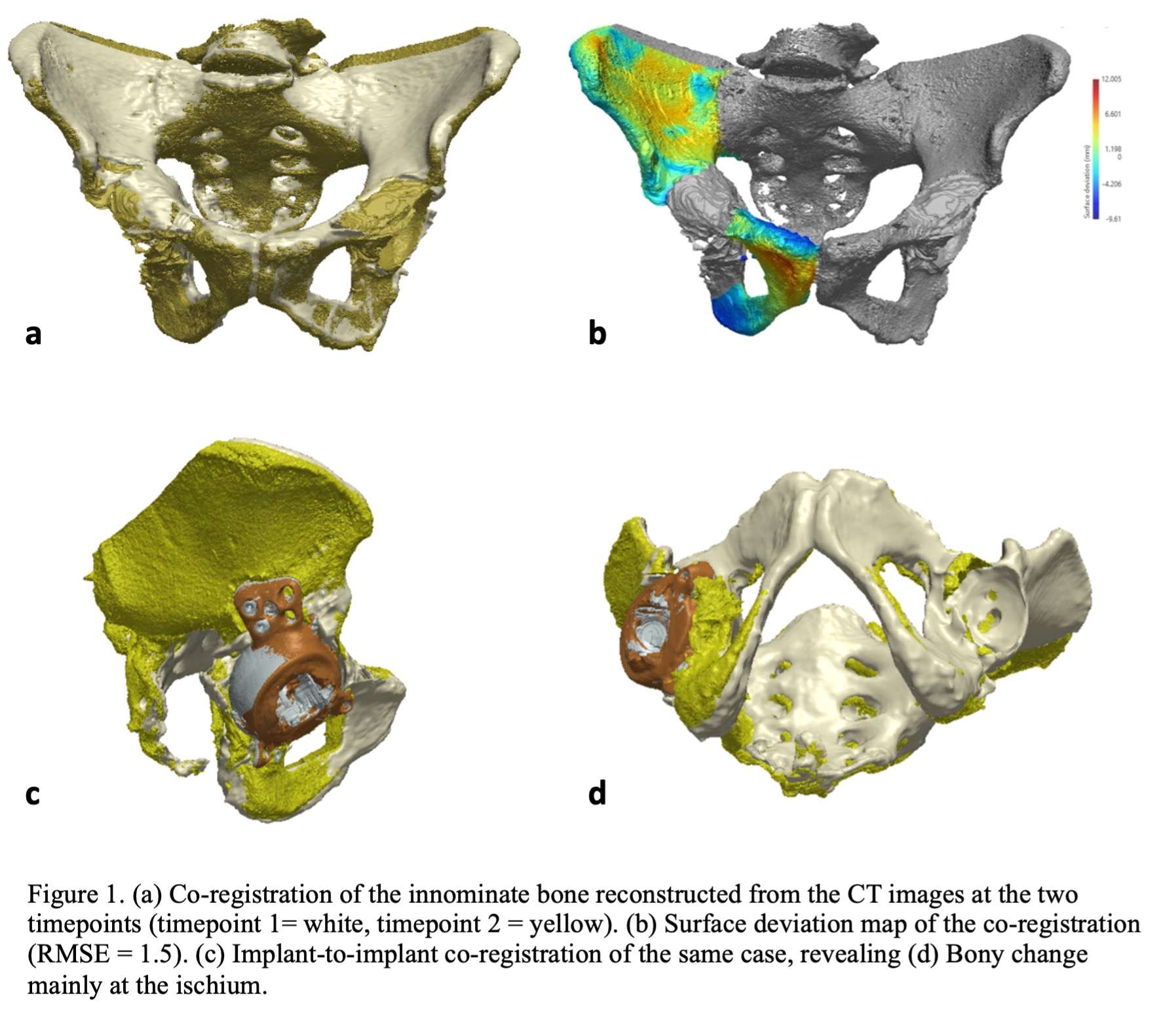
Figure 1#8278
Total Hip Arthroplasty With Extra-Small Femoral Stem in Hypoplastic Femurs: A Case-Series Study
HONG SEOK Kim - Seoul National University Hospital - Seoul, South Korea
Young-Seung Ko - Seoul National University Hospital - Seoul, Korea (Republic of)
*Jeong Joon Yoo - Seoul National University College of Medicine - SEOUL, South Korea
*Email: jjyos@snu.ac.kr
Background:
Total hip arthroplasty (THA) in patients with hypoplastic femurs presents a significant challenge to orthopedic surgeons due to the limited space available for implant placement. Therefore, the use of extra-small femoral stems has been proposed as a solution to this problem, but there is limited data on its outcomes. We aimed to evaluate the clinical and radiological outcomes of THA in patients with hypoplastic femurs using the CM stem (Corentec, Cheonan, Korea), an extra-small femoral stem.
Methods:
We included 6 hips of 4 patients. The mean age of patients is 40.5 years (range, 19-60 years). The mean height was 135.1cm (range, 113.6-150.0 cm) with a mean body mass index of 25.7 (range, 21.3-31.1 kg/m2). The diagnoses for THA were sequelae of septic arthritis in childhood, pseudoachondroplasia, spondyloepiphyseal dysplasia, and juvenile rheumatoid arthritis. The clinical outcome was assessed using the modified Harris Hip Score (mHHS), while the radiological outcome was evaluated using radiographs. The mean follow-up was 2.2 years (range, 20-27 months).
Results:
The mean mHHS improved from 48.4 preoperatively to 88.8 at the final follow-up. Radiologically, all stems showed good osteointegration with no cases of stem loosening, migration, or implant fracture at the latest follow-up.
Conclusion:
The use of extra-small femoral stems in THA for hypoplastic femurs can provide good clinical and radiological outcomes with minimal complications. We suggest that this femoral stem could be a viable option for patients with hypoplastic femurs. Further studies with larger sample sizes and longer follow-up periods are warranted.
Figures
Figure 1#8500
Evaluation of Three Hip Implants Across the Three Dorr Canal Types
*Jarrod Nachtrab - University of Thennessee - Knoxville, USA
Thang Nguyen - University of Tennessee - Knoxville, USA
Michael LaCour - University of Tennessee - Knoxville, USA
Richard Komistek - The University of Tennessee - Knoxville, USA
*Email: jnachtra@vols.utk.edu
INTRODUCTION:
Total hip arthroplasty (THA) has been considered one of the most successful orthopaedic procedures in the modern era, but in the past most THAs have struggled to maintain good fits across all Dorr Types. As preoperative planning technology continues to improve, the accuracy of intraoperative positioning of THA components will continue to be improved along with it. The objective of this study is to analyze and compare the femoral stem component fit between three different implants for all Dorr canal types.
METHODS:
A total of 58 proximal femurs from segmentation of CT scans (20 Dorr Type A, 20 Dorr Type B, and 18 Dorr Type C) were evaluated in this study. A previously validated pre-operative planning tool was used to evaluate the proximal portion of each subject-specific femoral bone model and automatically suggest a stem placement. The three stem systems used in this study, Tri-Lock, Corail, and Emphasys (a new hip system designed with the stem and cup together) are shown in Figure 1. The distance from the implanted head center (IHC) to the anatomical head center (AHC) was found (Figure 2). The volume of bone removal needed for each stem was calculated by finding the volume of the stem that overlapped with the cortical bone (Figure 3). Contact percentage was calculated through finding the area on the stem that was in contact with the cortical wall as a percentage of the total contact area of the stem.
RESULTS:
Dorr Type A:
The Emphasys average IHC to AHC was 0.4mm±0.2mm (0.2mm–1.0mm), Corail average was 0.6mm±0.3mm (0.2mm–1.6mm), and Tri-Lock’s average was 0.5mm±0.2mm (0.3mm–1.0mm). Both Emphasys and Corail had 0.0cm3 of cortical bone reaming removal on average, whereas Tri-Lock had 0.3cm3 on average. Emphasys yielded a low overall contact percentage (0.1%) within the canal when compared to Tri-Lock (10.4%) and Corail (0.9%).
Dorr Type B:
The Emphasys average IHC to AHC was 0.5mm±0.2mm (0.1mm–0.9mm), Corail’s average was 0.5mm±0.2mm (0.2mm–0.9mm), and Tri-Lock’s average was 0.6mm±0.2mm (0.3mm–1.0mm). Both Emphasys and Corail had 0.0cm3 of cortical bone reaming on average, whereas Tri-Lock had 0.6cm3 on average. Emphasys yielded a low overall contact percentage (1.0%) within the canal when compared to Tri-Lock (14.4%) and Corail (1.7%).
Dorr Type C:
The Emphasys average IHC to AHC was 0.3mm±0.1mm (0.2mm–0.6mm), Corail’s average was 0.4mm±0.2mm (0.2mm–0.6mm), and Tri-Lock’s average was 0.6mm±0.2mm (0.3mm–0.8mm). Both Emphasys and Corail had 0.0cm3 of cortical bone reaming on average, whereas Tri-Lock had 0.5cm3 on average. Emphasys yielded a low overall contact percentage (0.1%) within the canal when compared to Tri-Lock (10.5%) and Corail (0.3%).
CONCLUSION:
The results demonstrate that Emphasys had a more consistent fit across all Dorr Types. Both Emphasys and Corail required less reaming of the cortical bone when compared to Tri-Lock, but the Emphasys design led to a minimized IHC to AHC distance compared to Corail. While small mismatches in stem orientation may seem clinically negligible, these misalignments can quickly compound with additional cup misalignments, leading to more undesirable postoperative mechanics.
Figures
Figure 1
Figure 2
Figure 3#8096
30-Years-Outcome Following Limb Salvage With Endoprosthetic Reconstruction in a Ewing Sarcoma of the Lower Limb Involving Multiple Revisions and Complications
Laszlo Toth - Kantonsspital Baselland - Bruderholz, Switzerland
*Andrej Nowakowski - Kantonsspital Baselland - Bruderholz, Switzerland
*Email: andrej.nowakowski@unibas.ch
Introduction: Bone sarcomas of the lower extremities are rare malignancies occurring mostly amongst adolescent and young adult age groups. In a metastasis free situation with adequate therapy, the overall survival rate can reach up to 90%. Since the end of the 20th century limb salvage has become the standard therapeutic procedure. Assessing the quality of life we may include the functionality of the extremity, the risk of further revision and everyday suitability of the limb.
Case presentation: A 9-year-old male patient was suffering from a primary Ewing sarcoma of the right distal femur. Subsequent to radical tumor resection and systemic oncologic therapy an endoprosthetic reconstruction was performed. The patient suffered from two mechanical failures of the prosthesis components, two aseptic loosenings, and three implant associated infections. In total, he underwent 40 surgical interventions with an overall hospital stay of 437 days with multiple changes of the prosthesis. Three decades after the initial diagnosis the patient is satisfied with his own leg, enjoying excellent function and a good quality of life.
Discussion: In the modern reconstructive surgery the possibility of limb salvage has greatly expanded due to advanced technical opportunities. However, according to the literature there is no significant difference between limb salvage and amputation regarding long-term overall survival, overall quality of life, the psycho-socio-economic outcome or patient satisfaction. An important advantage of limb salvage is the greater everyday functionality. In cases involving complications, the treatment can demand an enormous personal, medical, and financial investment on behalf of the patient, the medical team, and insurance company to avoid an ablative surgery. Amputation could have a negative impact on the patient's psychological well-being, particularly if they strove to retain it. Comprehensive communication about the long-term risks, advantages, and disadvantages of the different therapeutic options is necessary as part of the informed consent process.
Conclusion: Despite the great costs, personal effort and the possible complications, limb salvage could be a suitable method to achieve functionally beneficial outcomes and patient satisfaction in sarcomas of the lower extremities. However, occasionally in some cases it requires multiple - from minor up to the most complex - revisions to maintain the functionality of the extremity.
#8097
The Necessity of Interdisciplinary Approaches as Well as Thorough Diagnostic Evaluation in the Aging Population
Michael Robert Klauser - Kantonsspital Baselland - Bruderholz, Switzerland
Debora Nowakowski - Praxis Zweichirurgen - Basel, Switzerland
*Andrej Nowakowski - Kantonsspital Baselland - Bruderholz, Switzerland
*Email: andrej.nowakowski@unibas.ch
Introduction
The elderly population is more susceptible to various medical conditions including heart disease and joint problems. These patients can present with overlapping, atypical and non-specific symptoms, leading to challenges in diagnosis and treatment. We present a case of an 87-year-old male with history of heart disease and coronary angiography who suffered from groin pain. After exclusion of a potential hernia in another healthcare facility, a CT scan was done for further evaluation, after the presence of a foreign body (FB) in his thigh had been reported. The scan revealed a broken part of the angiography catheter, located at least 1.4 cm away from the femoral nerve and vessels as well as a severe case of coxarthrosis. Still convinced that the FB was the main reason for pain, the general practitioner referred the patient to surgical colleagues for foreign body removal.
Methods
After thorough clinical examination through our colleagues, coxarthrosis seemed to be the main cause of the pain, so that the patient was finally referred to our department for further evaluation. With a local anesthetic hip injection, the hip was identified as being the leading cause of pain. The patient received a total hip prosthesis and had the FB removed at our department simultaneously.
Results
During hospitalization early mobilization was emphasized on and wound healing proceeded without complications. The patient was discharged in a good general condition and completely asymptomatic. At the three-month follow up, the patient was still satisfied and pain free.
Conclusion
This case highlights the importance of a thorough evaluation and collaboration among healthcare professionals when diagnosing complex medical conditions in the older population. In this patient with groin pain and history of heart disease as well as coronary angiography, the cause of pain was not immediately apparent. This case highlights the importance of having interdisciplinary collaborations and the use of cost-effective diagnostic tools to provide effective treatment.
#8144
Enhanced Mid-Resection Workflow Technique for Severe Varus Deformity Correction Using Robotic-Arm Assisted Total Knee Arthroplasty
*Suhas Boenerjous-Abel - Bronx-Lebanon Hospital - Bronx, USA
*Email: drsuhas09@gmail.com
Introduction:
Optimal alignment and soft-tissue balance are important factors for long term survivorship and good functional outcomes after total knee arthroplasty (TKA). However, patients with severe varus deformities of the knee pose a challenge during TKA, as these are often complicated by instability and require higher constraint. Robotic technology in total knee arthroplasty has been proven to improve accuracy of component positioning, achieve alignment targets, and balance the knee objectively. However, the utility of robotics in correcting severe varus deformities of the knee has not been well established in literature. The aim of this paper was to investigate the effectiveness of existing workflows for achieving pre-balance and propose a novel surgical workflow, in the management of severe varus deformities with robotic TKA. We propose an algorithmic approach to guide surgeons in selecting the appropriate workflow.
Methods:
Among the existing Mako (Stryker, Kalamazoo, Michigan) RA-TKA workflows, the pre-resection workflow is used when the knee can be pre-balanced by adjusting the component position based on the functional alignment philosophy. On the other hand, the mid-resection workflow is reserved for complex cases where the distal femur and proximal tibial cuts are performed to balance the extension gap first, followed by flexion gap balancing. For severe varus deformities, neither workflow could achieve pre-balance, necessitating the development of a novel technique. One of the reasons for the extension gap not balancing is the presence of large osteophytes and a tight posterior capsule which are currently inaccessible. The robot's ability to execute precise bone cuts allows for a provisional postero-medial femoral bone cut thus gaining access and aids in removal of these inaccessible structures. The flexion gap is subsequently matched to the extension gap by altering the axial positioning of the femoral component.
Conclusion:
Mako RA-TKA provides real-time data on the influence of changes in bone resection, osteophyte removal on ligament balancing. This allows surgeons to carefully titrate changes in component positioning to avoid unpredictable gaps and residual instability compared to manual TKA. This novel “enhanced mid-resection workflow” technique establishes the utility of the RA-TKA in balancing severe varus deformities of the knee. We also propose an algorithm which simplifies and helps surgeons choose between the three workflows to pre-balance knees irrespective of the severity of the varus deformity.
Figures
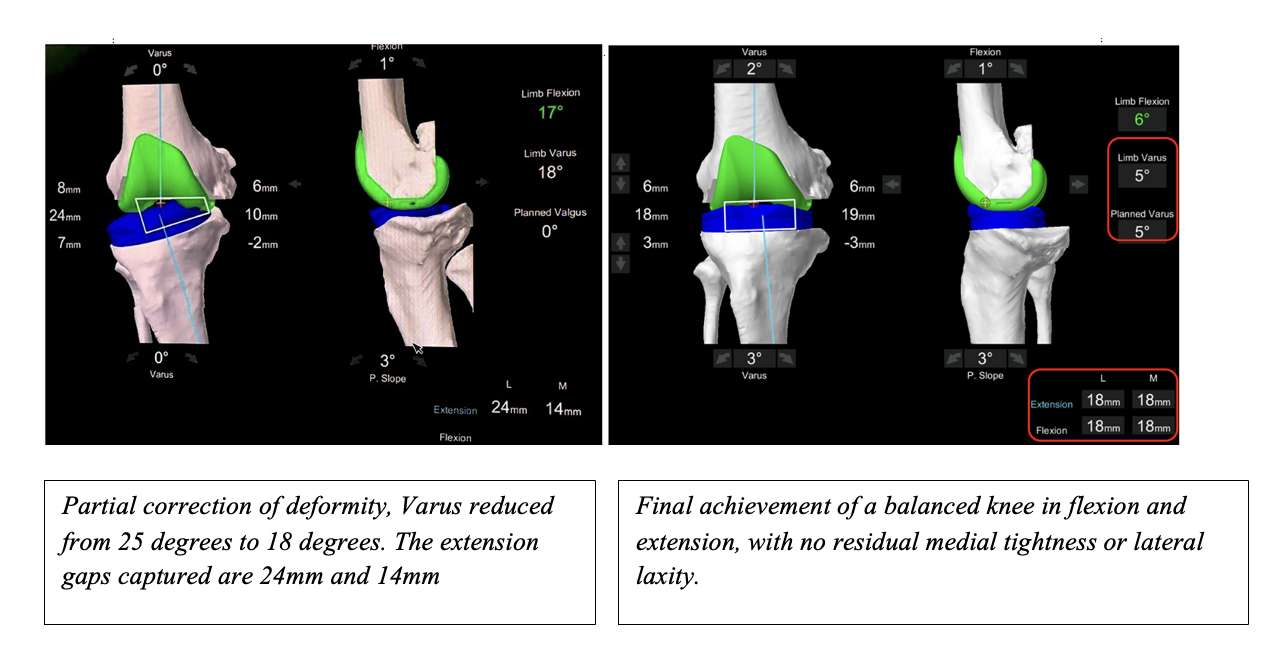
Figure 1
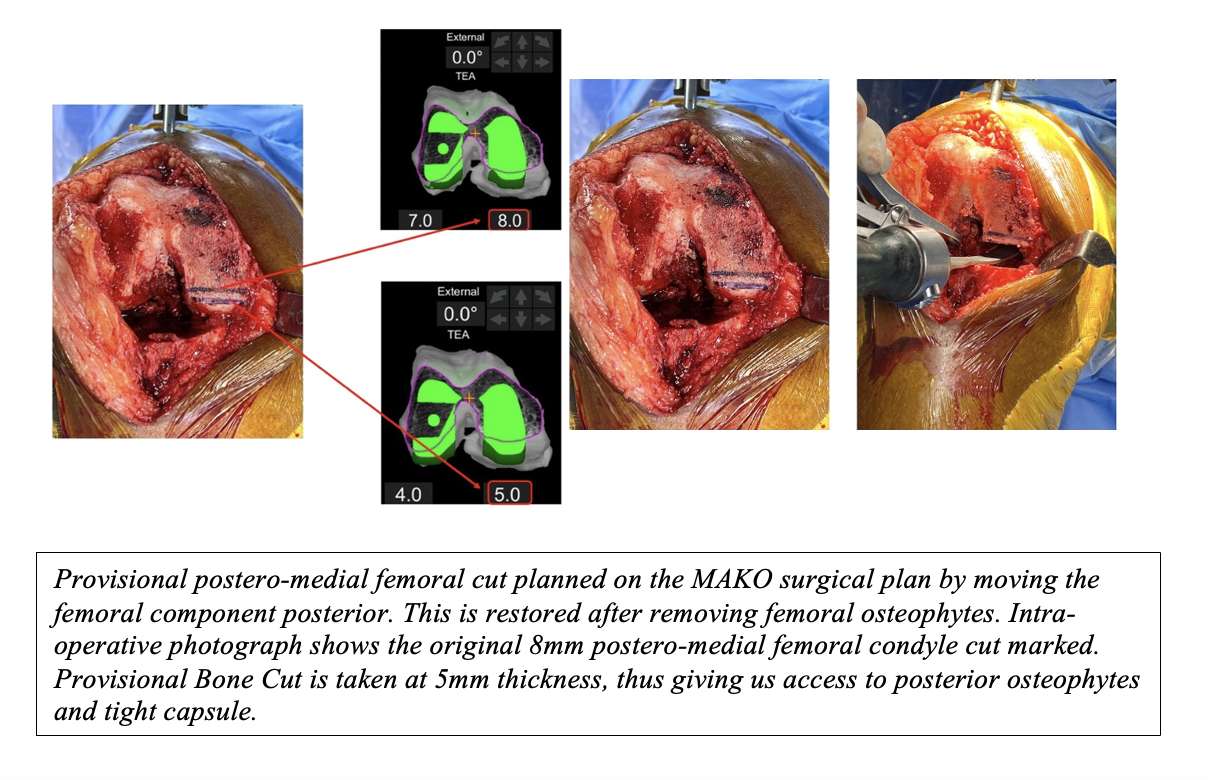
Figure 2
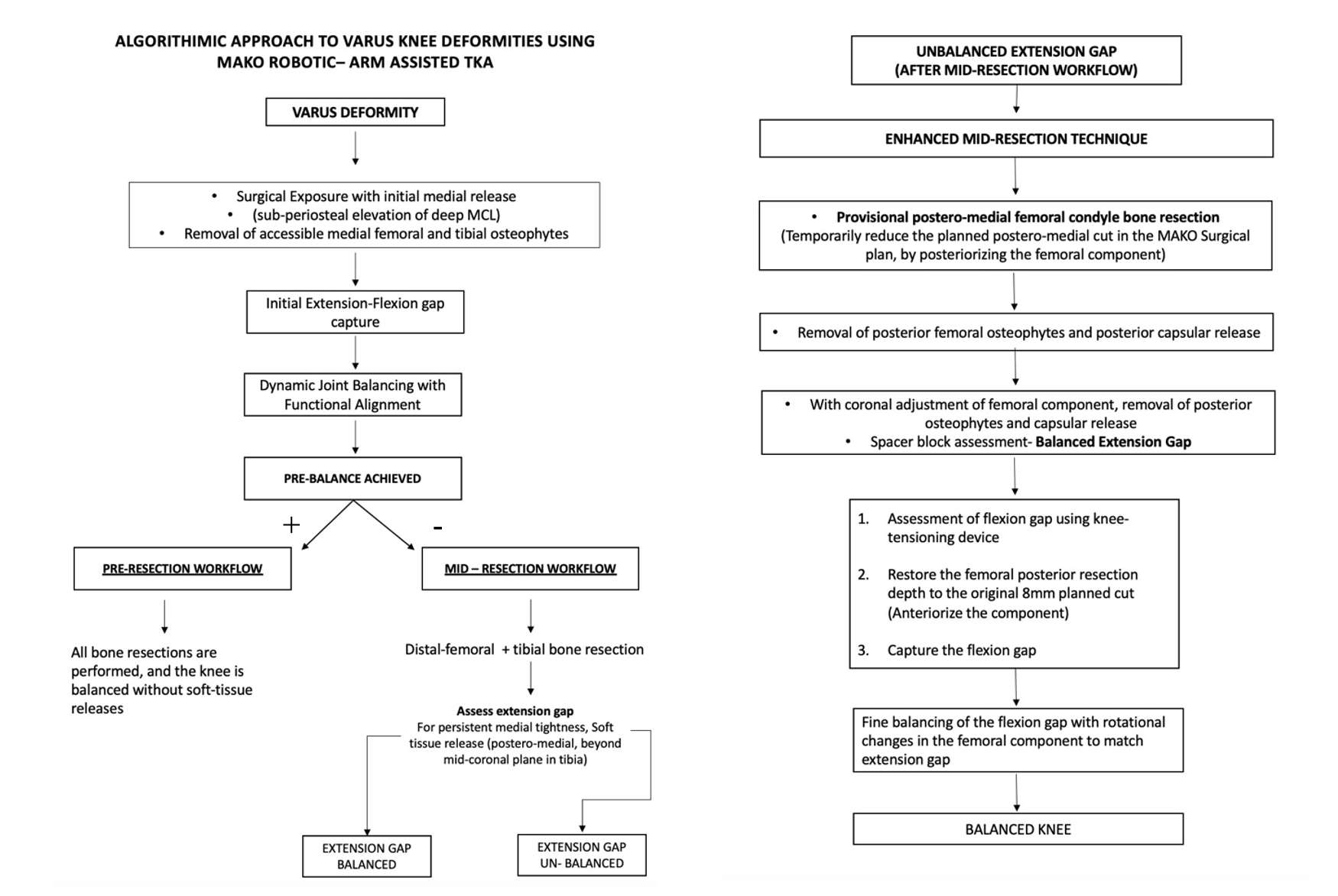
Figure 3#8159
15-Year Follow-Up of Metal-on-Metal Hip Replacements in Young Patients
*Ahmed Abohassan - East Cheshire NHS Trust - Macclesfield, GB
Syam Morapudi - Macclesfield General Hospital - Macclesfield, United Kingdom
Muhammed Umar - Macclesfield District General Hospital - Macclesfield, United Kingdom
Steven Kershaw - Macclesfield District General Hospital - Macclesfield, United Kingdom
Michael Flatman - East Cheshire NHS Trust - MacClesfield, United Kingdom
Raviprasad Kattimani - East Cheshire NHS Trust - MacClesfield, United Kingdom
*Email: drahmedhassan94@gmail.com
Introduction: Metal-on-metal (MoM) total hip replacements (THR) were introduced to avoid the problems associated with polyethylene liner wear. It is well known that they are prone to Adverse Reactions to Metal Debris and subsequent failure, and they are no longer recommended for use. It is recommended that those patients who received them are regularly reviewed to detect these complications. All patients received an uncemented Corail femoral stem and an uncemented Pinnacle femoral head. Methods: local ethical approval was received for a retrospective review of the departmental database for all patients who received a MoM THR when they were 55 years old, or younger. Electronic patient records were reviewed for clinic letters, operation notes, and serum chromium and cobalt ion levels. Patients were contacted by telephone to gain consent to send them a paper Oxford Hip Score (OHS) questionnaire. Data was analysed using SPSS. Results: 109 procedures on 90 patients with a mean follow-up of 15 years. Sixteen patients have died, and at last review three have been lost to follow-up. Twelve prostheses (11%) required revision; 1 for subsidence, 2 for infection, 6 for metallosis, 3 for femoral loosening. Mean time to revision was 7.4 years. We are awaiting analysis of OHS scores and they will be included in the final presentation. Conclusion: MoM THR are no longer recommended for use, and they should receive long-term follow-up. However, this data can reassure those patients and their healthcare professionals that the majority of MoM THR continue to perform satisfactorily
#8240
Clinical Experience Using Patient-Specific 3D CT-Based Surgical Planning and Cutting Guides in High Tibial Osteotomy
*Kelly Mills - Sint Maartenskliniek - Nijmegen, Netherlands
Petra Heesterbeek - Sint Maartenskliniek - Nijmegen, Netherlands
José Smolders - St Maartenskliniek - Nijmegen, Netherlands
*Email: k.mills@maartenskliniek.nl
Introduction:
In osteotomy around the knee deformities are corrected by altering the load-bearing axis, which helps to reduce pain and delay the progression of osteoarthritis. The success of osteotomy surgery highly depends on achieving the correct deformity correction angle. One way to improve osteotomy accuracy may be the use of patient-specific 3D CT-based operative planning and using patient-specific cutting guides (PSCGs). The present randomized study was set up to compare the accuracy and feasibility of a high tibial osteotomy (HTO) which is 3D CT-planned and executed with the use of PSCGs (PSCG group) to an HTO that is also 3D CT-planned but conventionally executed (conventional group).
Methods:
In this case series a total of 12 patients scheduled for HTO, with a varus tibial deformity, were randomly divided over both groups. All patients with rotational or severe slope deformities in need for correction in several planes were excluded. Based on a pre-operative CT-scan a 3D planning of the surgery was made for all patients. Additionally, a weightbearing long-leg standing x-ray was used to plan the surgery conventionally using the Miniaci method. An open-wedge HTO, using the 3D CT-planned correction angle as target, was carried out on all patients. PSCGs were used for those randomized to this group. The planned and achieved corrections (conventionally and 3D CT-based) were evaluated and compared using the Medial Proximal Tibial Angle (MPTA) and Posterior Proximal Tibial Angle (PPTA). Surgery time, costs and adverse events were registered in both groups.
Results:
There were no significant differences between the groups in difference between planned and achieved correction for both MPTA and PPTA (Fig 1). Noteworthy is that post-operative MPTA in the PSCG group tends to be under-corrected. Even though the aim was no slope correction, the PPTA increased in both groups after surgery. The surgery time was significantly higher for the PSCG group (Median 73 minutes (IQR 66-80) vs 51 minutes (46-60)), but with a decrease in PSCG surgery time over the subsequent cases. Additionally, the extra costs for a PSCG-surgery compared to conventional HTO in our hospital were €1013 ($1107) (preoperative CT scan, planning and printing of PSCG by external party, company-specific plate). In terms of adverse events: one PSCG patient experienced wound infection and in one PSCG patient the 3D CT-guided planning suggested a correction angle in degrees that did not match the number of mm.
Discussion and conclusion:
The use of PSCGs did not result in significantly more accurate correction. The costs and surgery time were higher with PSCGs, but surgery time decreased over time. This learning curve was also of influence in PPTA, as the surgeons learned how to properly cut through the distal cortex using PSCGs. The implementation of the process in the clinic was challenging, as it required tasks and collaborations for various hospital departments (purchasing department, radiology, OR planning) and with an external party that deviates from regular pathways and routines. All in all, it seems that PSCGs do not add value in monoplane HTOs, in centres with relatively low volumes.
Figures
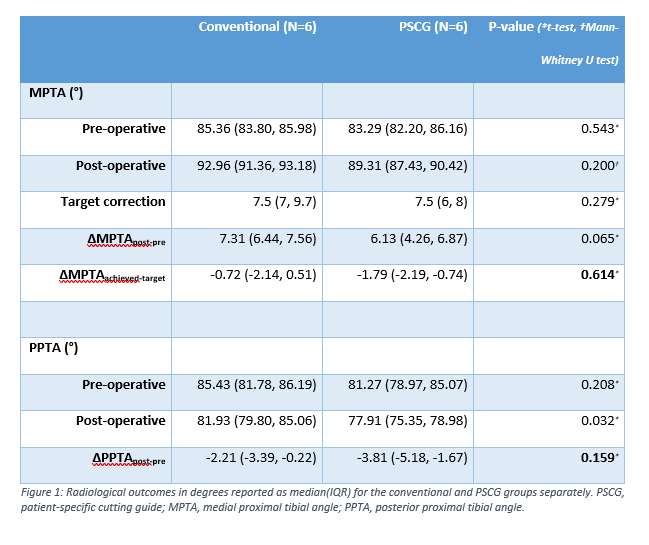
Figure 1#8313
Accuracy of Additional Bone Resection for Severe Varus Knee Deformity With Tibial Bone Defect in Robotic Total Knee Arthroplasty
*Yasushi Oshima - Nippon Medical School - Japan
Norishige Iizawa - Nippon Medical School - Tokyo, Japan
Tokifumi Majima - Nippon Medical School - Tokyo, Japan
*Email: y-oshima@nms.ac.jp
Introduction:
With advancements in artificial prostheses and surgical techniques, the clinical outcomes of the recent total knee arthroplasty (TKA) for knee osteoarthritis (KOA) have been known to be promising in terms of implant longevity and improvement in performing daily activities. However, TKA for treating severe varus knee deformity with a tibial bone defect remains difficult, because additional bone resection and metal augmentation is required. Recently, robotic technologies have been introduced in primary TKA, and their accuracy and safety have been demonstrated. Based on this, we used robotics for severe varus KOA with a bone defect, and the accuracy of the technologies was evaluated.
Methods:
This study included patients who have had primary robotic TKA with metal augmentations. Rheumatoid and inflammatory arthritis as well as valgus knee deformities were excluded. This study was approved by the Institutional Review Board.
After completing the femoral and tibial bone resections and obtaining balanced extension and flexion gaps in the Mako system (Stryker, USA), the remaining bone defect on the tibial surface was evaluated. Once the depth of the defect was greater than 5 mm and the width was greater than one-third of the medial tibial plateau, additional bone resection was performed using robotic technology, and the metal augmentation was inserted.
Results:
Overall, this study included four patients with six knees. According to the preoperative standing radiographic findings, the average lateral femorotibial angle (FTA) was 194.2° ± 3.4°. Following bone resections and soft tissue balancing, the medial half of the tibia was further horizontally resected 5 mm using robotic technology, followed by applying a tibial stem extension and a half block metal augmentation. The average FTA was improved to 177.5° ± 2.3°, with the beta and delta angles of the tibial components being 90.2° ± 0.8° and 2.5° ± 0.8°, respectively.
Conclusions:
Because positioning the additional bone resection jig at the same level as the original jig level is difficult after several surgical processes in the conventional TKA procedure, the additional bone resection becomes inaccurate. In comparison, the additional bone resection level was calculated on a computer, and the bone resection was performed precisely via robotic surgery. As the original program was not designed to apply the half block, some ingenuity is required with the 5 mm vertical resection first and then the horizontal resection of the half of the tibial surface. Thus, new software programs to resect the half of the bone surface are still expected to be developed. However, the robotic technology with metal augmentation is a promising technique for treating severe varus knee deformities.
#8375
A Novel Mesh-Free Model for Accurately Simulating Material Damage During Orthopaedic Screw Pull-Out Per the ASTM F543-17 A3 Standard
Sloan Kulper - Lifespans - Wong Chuk Hang, Hong Kong
*Janice Oentaryo - Lifespans, Ltd. - Wong Chuk Hang, Hong Kong
Rezaul Tharim - Lifespans, Ltd. - Wong Chuk Hang, Hong Kong
Erica Ueda - Lifespans, Ltd. - Wong Chuk Hang, Hong Kong
*Email: janice@lifespans.net
Mesh-free computational models of ASTM F543-17 A3 (screw pull-out) were generated using a novel simulation software system and its predictions were compared to physical experimental test results. Generic HA 3.5, HA 4.0, and HA 4.5 bone screws were fabricated (316L stainless steel, ISO Fine tolerance, n=3 each), inserted into pilot holes in the PU foam, and then pulled out while recording force vs. displacement data. Blocks of solid rigid polyurethane foam measuring 58 x 65 x 40 mm were prepared from 20 PCF foam (n=3 for each screw design), and 15 PCF foam (n=3 for HA 4.5 screw only). Models of the implant and foam blocks were constructed in the novel mesh-free computational modeling system at a resolution of 200 μm/particle and simulated pull-out tests were performed. The maximum peak pull-out loads of the HA 3.5 screws from 20 PCF foam were 692 N (average) and 706 N in the physical and simulated tests , respectively. For the HA 4.0 screws the maximum pull-out loads were 816 N (average) and 713 N from 20 PCF foam in the physical and simulated tests, respectively. For the HA 4.5 screws the maximum peak pull-out loads were 509 N (average) and 508 N from 15 PCF foam in the physical and simulated tests, respectively; maximum pull-out loads were 798 N (average) and 820 N from 20 PCF foam in the physical and simulated tests, respectively. The average CCC (concordance correlation coefficient) between simulation and experiment maximum pull-out loads was >0.90, suggesting excellent concordance, however the simulations over-predicted loads following the peak. Computational time on a 32-core cloud-computing instance was less than 12 hours for each simulated test. The novel mesh-free computational modeling system in the present study can accurately predict the maximum pull-out loads of several typical orthopedic screws in two common PU foam grades per ASTM F543-17 A3, suggesting that this system can be used to quickly predict the likelihood that a candidate design will pass without the need of a physical prototype or testing laboratory.
Figures
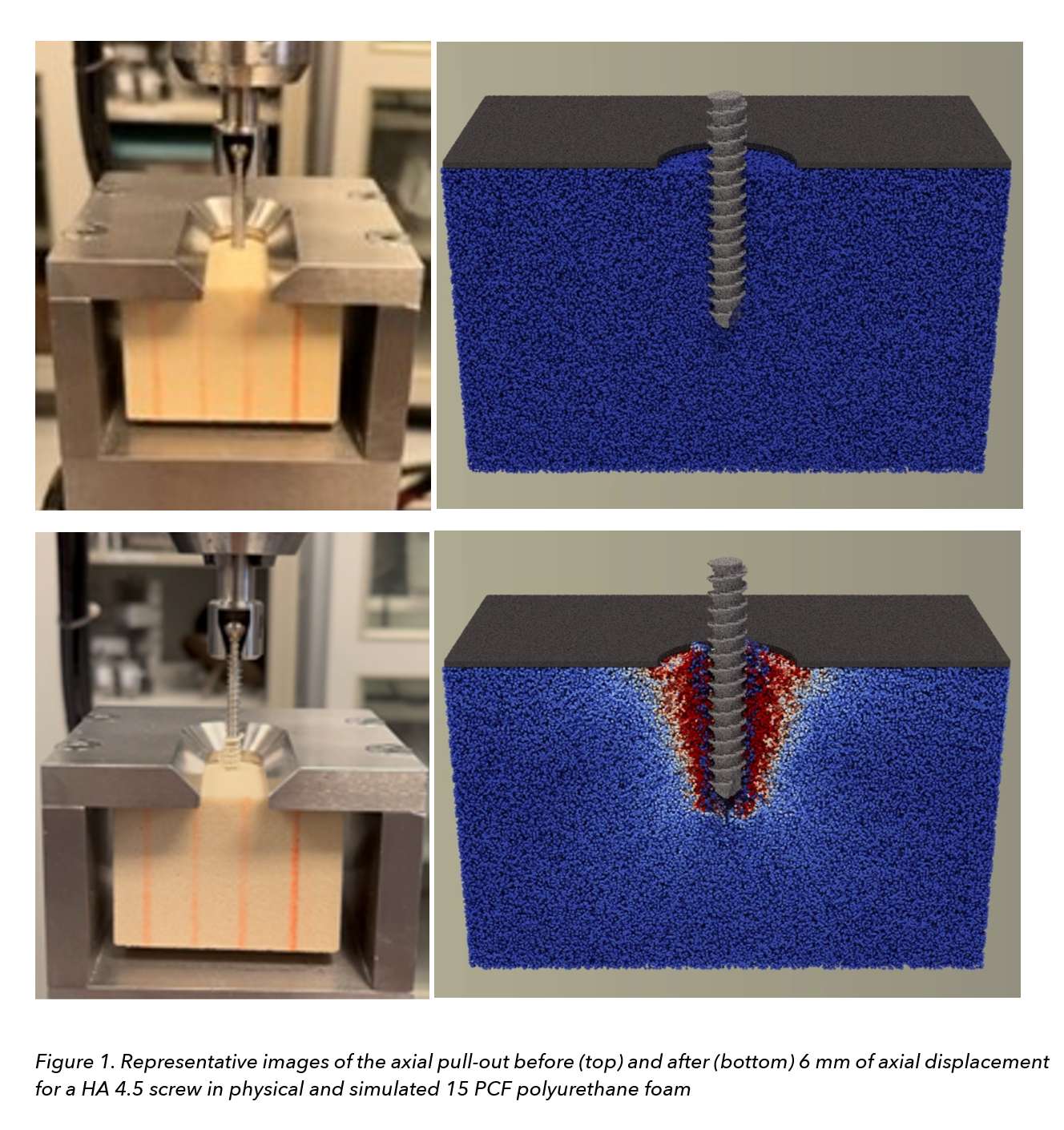
Figure 1
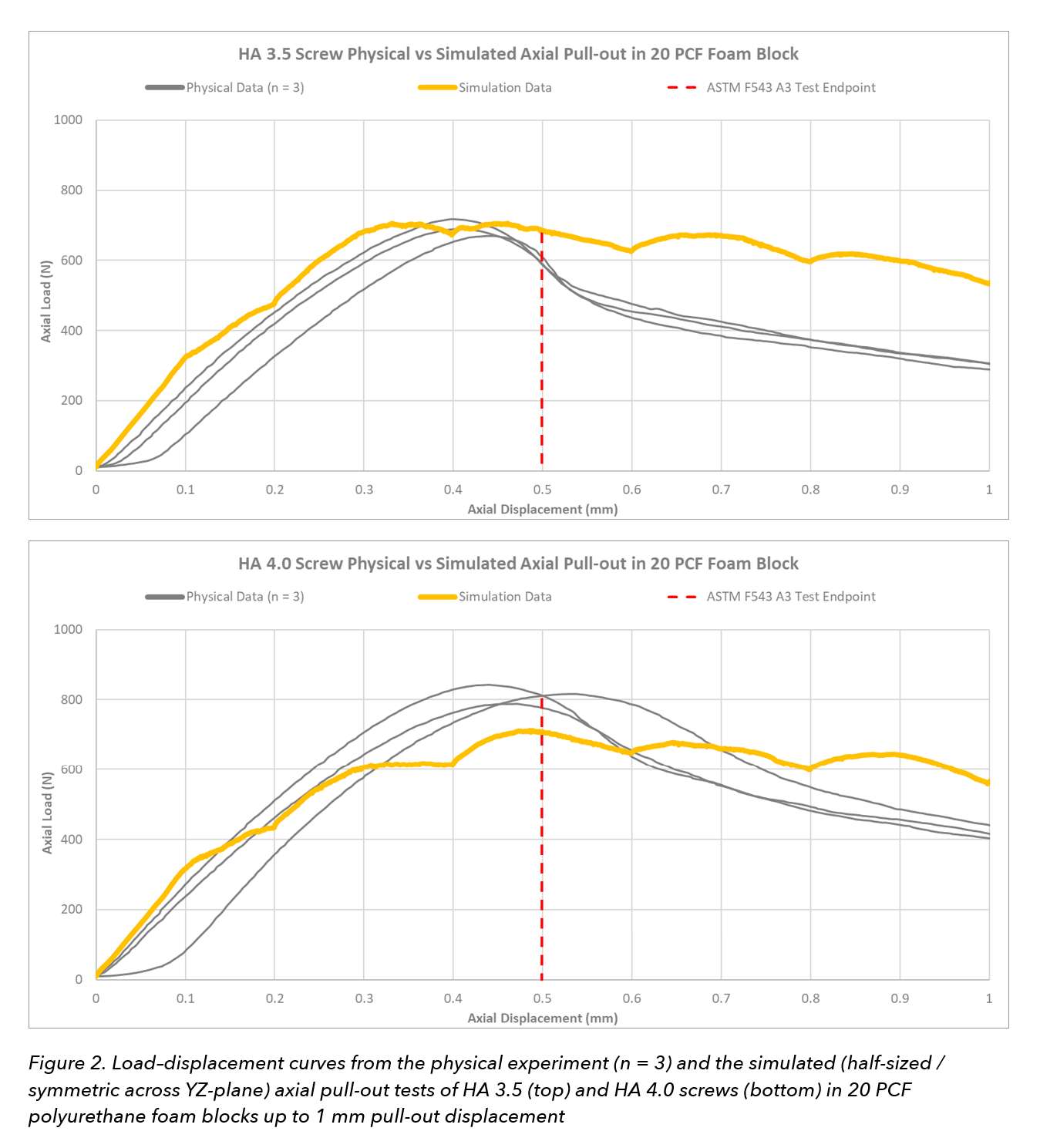
Figure 2
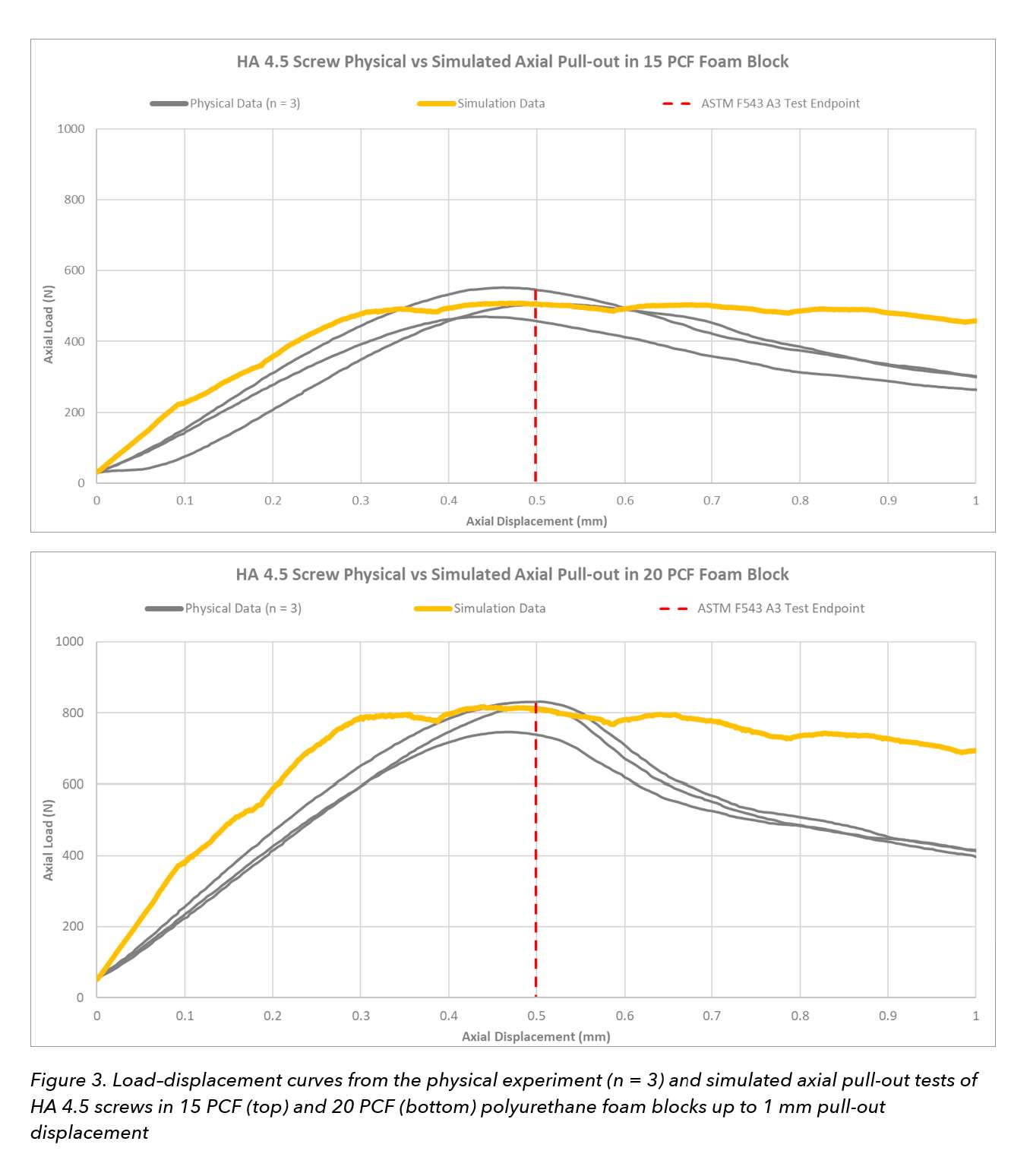
Figure 3#8405
Patient-Specific Remote Pain Monitoring: Case-Report of a Patient With an Infected Wound
*Ricardo Antunes - Stryker - Glasgow, GB
Emily Hampp - Stryker - Mahwah, USA
Paul Jacob - Oklahoma Joint Reconstruction Institute - Oklahoma City, USA
Elaine Justice - Oklahoma Joint Reconstruction Institute - Oklahoma City, USA
Matthias Verstraete - Stryker - Fort Lauderdale, USA
*Email: ricardo.antunes@stryker.com
After total knee arthroplasty (TKA), managing postoperative pain can be crucial for successful rehabilitation. Pain serves as an indicator of potential complications, including nerve injuries, fractures, infection, and dislocations. However, assessing pain in patients is subjective and relies on self-assessment reports. Antunes et al. (In Press) developed a pain score model that predicts remotely collected scores over a 90-day recovery period and quantifies deviations from the expected reference recovery. The model issues alerts for pain scores exceeding set thresholds, which may allow for early automated detection of patients requiring additional attention. In this case study, we evaluate the effectiveness of the pain score model in objectively identifying complications during recovery by presenting a TKA patient who developed a postoperative infection.
A patient (53 y/o, female, BMI 19.75, no co-morbidities) undergoing unilateral primary TKA was enrolled in a clinical study using a remote wearable monitoring device and phone application. The patient was prompted by the mobile phone application to report daily pain scores on a 11-point scale. In addition, the system allowed remote monitoring of range of motion, daily steps, activity time, prescribed exercise completion, and patient-reported outcome surveys. Patient pain scores were incrementally used as input to the model as described in Antunes et al. (In Press). This simulates the process by which new patient scores are assessed as they become available during the patients’ recovery. At each of these incremental steps, the quantile metric was calculated as a measure of the likelihood of the reported pain score based on the predicted distribution. A range of potential threshold values was compared against the calculated quantile metric to assess the detection of timing of infection.
The patient was evaluated in clinic 3-wks postoperatively and observations were deemed to be normal and improving compared to the preoperative period. After 29 days postoperatively, the patient-reported pain scores started increasing with a lower likelihood (quantile < 0.12), culminating in the highest score reported on day 34 with quantile of 0.01 (Figure 1). The patient’s abnormal recovery was identified following a review of various remotely collected parameters by the clinical staff at 34 days after surgery, resulting in a clinic visit on that day where an acute infection was identified. An irrigation and debridement procedure, with polyethylene exchange and placement of deep wound VAC was then performed at 37 days post-operatively.
Based on the quantile values, a threshold of 0.15 would generate an alert for the scores reported 3-days before the time of detection of infection (day 29 instead of day 32). This threshold would also trigger an alert on scores reported 9-14 days after surgery, representing a false-positive alert. This is deemed acceptable to safely deploy this patient-specific pain model for remote monitoring. A lower threshold of 0.05 would result on an alert triggering only on day 34, when infection was identified in practice.
The presented case suggests that model-triggered pain score alerts can be used to bring attention to clinically relevant cases, such as the occurrence an infection, for further assessment during recovery.
Figures
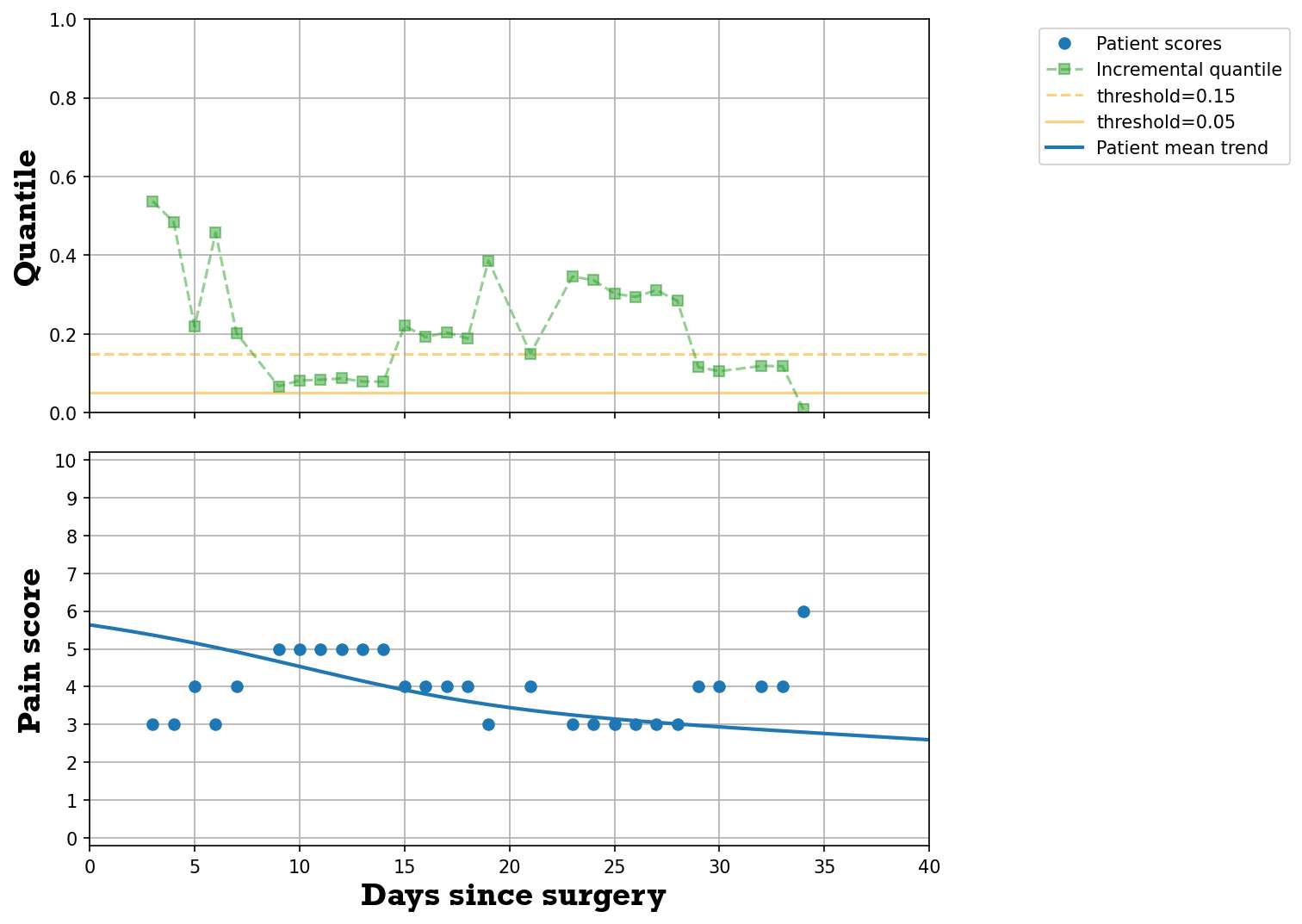
Figure 1#8407
Medialisation of the Acetabular Cup Impacts Hip Abductor Function
Atul Kamath - Cleveland Clinic - Cleveland, USA
David Liu - Gold Coast Centre for Bone and Joint Surgery - Tugun, Australia
Duncan Bakke - Formus Labs - Auckland, New Zealand
*Marco Schneider - Formus Labs - Auckland, New Zealand
Thor F Besier - University of Auckland - Auckland, New Zealand
*Email: marco@formuslabs.com
Introduction
In total hip arthroplasty (THA), medialization of the cup while preserving global offset is performed to optimize lever arms. Though this leaves the leg in approximately the same position while the hip joint rests neutral, every change in the point of rotation causes ever-larger discrepancies in the end location of the limb at the edges of the range of motion (Figure 1). This study aimed to isolate the change in medialized cup position to determine its potential effects on the function of the hip abductors musculature. As a secondary aim, we analyzed how moving the cup superiorly or inferiorly when maximally medialized altered the results.
Methods
A template musculoskeletal model of the lower limb (OpenSim, Stanford, USA) was customized to enable simulations of cup medialization. The abductor muscles, including the gluteus minimus and medius (GM), tensor fascia latae (TFL), piriformis, and sartorius, are examined herein, but representations of all muscles were included in the model. Cup medialization was increased incrementally from -5 mm (lateral) to +15 mm (medial) from the native position. A sit-to-stand motion was simulated for each cup position, and the adduction/abduction (A/A) muscle moment arms at each time point were analyzed. In addition, alteration in the superior and inferior (S/I) directions were analyzed in a similar manner (+/- 10 mm from original) at the point of furthest medialization (+15 mm) to ascertain the effects of vertical change. For all perturbations, the global offset was preserved.
Results
Generally, cup medialization (and superiorization) magnified the abduction moment arms by varying amounts across the entire motion and minimized adduction moment arms during lean-forward (with the exception of anterior GMs). The effects of S/I translation of the cup compounded the effects of medialization. For example, moment arms for the TFL changed very little during the standing phase, by up to 5 mm during the seated phase, and by over 12 mm in both directions during the lean-forward phase (Figure 2). Notably, while medialization decreases the adduction moment arm during this phase, superiorization can counter this loss in moment arm. Conversely, inferiorization can decrease the TFL moment arm by more than its maximum value, causing the muscle to act in abduction instead. The overall pattern of superiorization effect was common amongst most of the abductors, with the exceptions of the sartorius (unchanged) and the piriformis, where the effect of superiorization was inverted during lean-forward.
Conclusion
The general wisdom of medialising the hip joint during THA for the purpose of aiding abductor function is supported by moment arm analysis, but its effects throughout activities are hard to predict without simulation. Additionally, patient-specific morphology and kinematics will likely influence the relationship between cup medialization and muscle function. Standing analyses fail to capture the problems caused at the edges of the range of motion, and blind adherence to this principle could go as far as to alter the direction of abduction/adduction achieved by flexing a muscle during motion (in the case of TFL with medial and inferior placement).
Figures
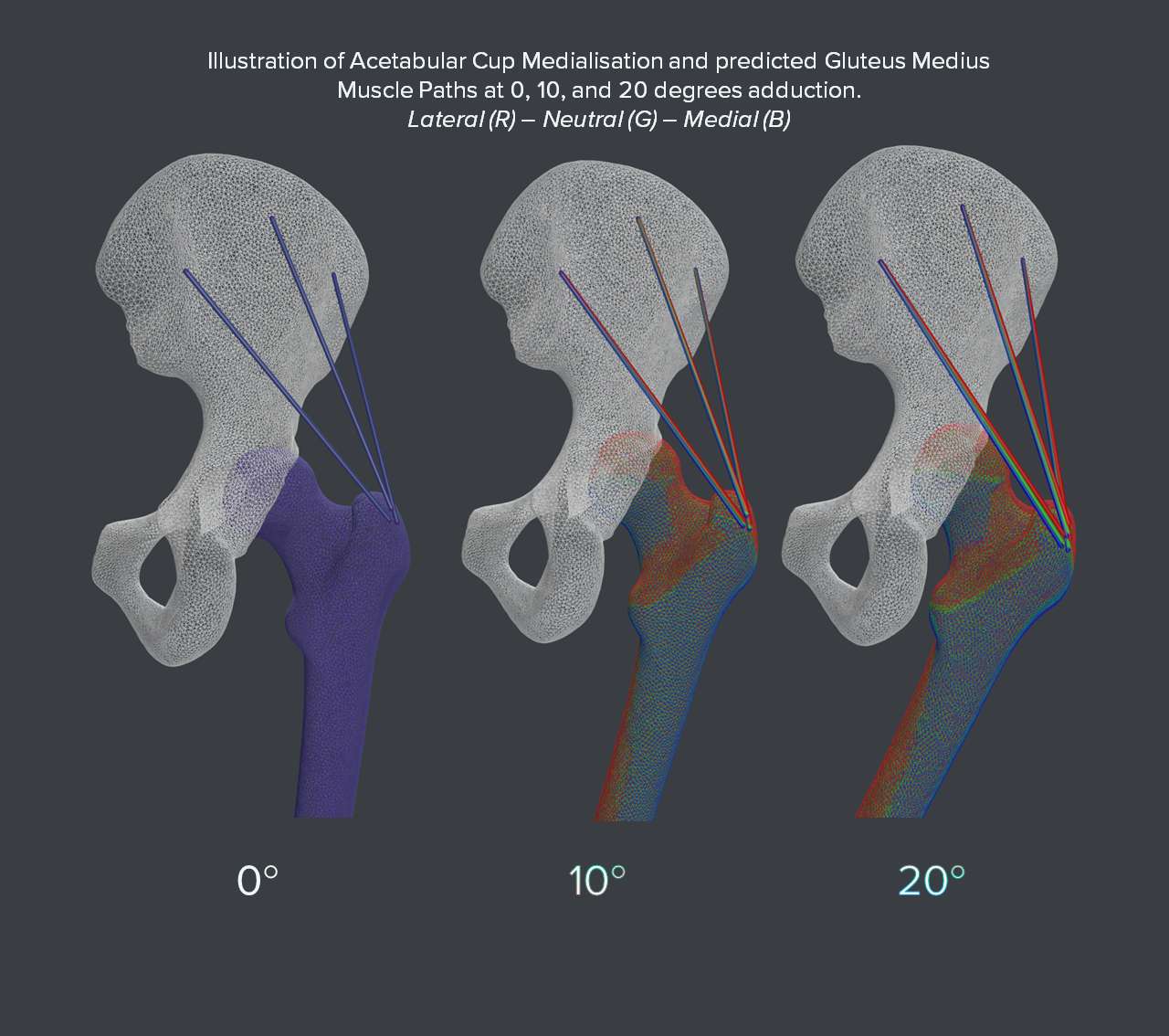
Figure 1
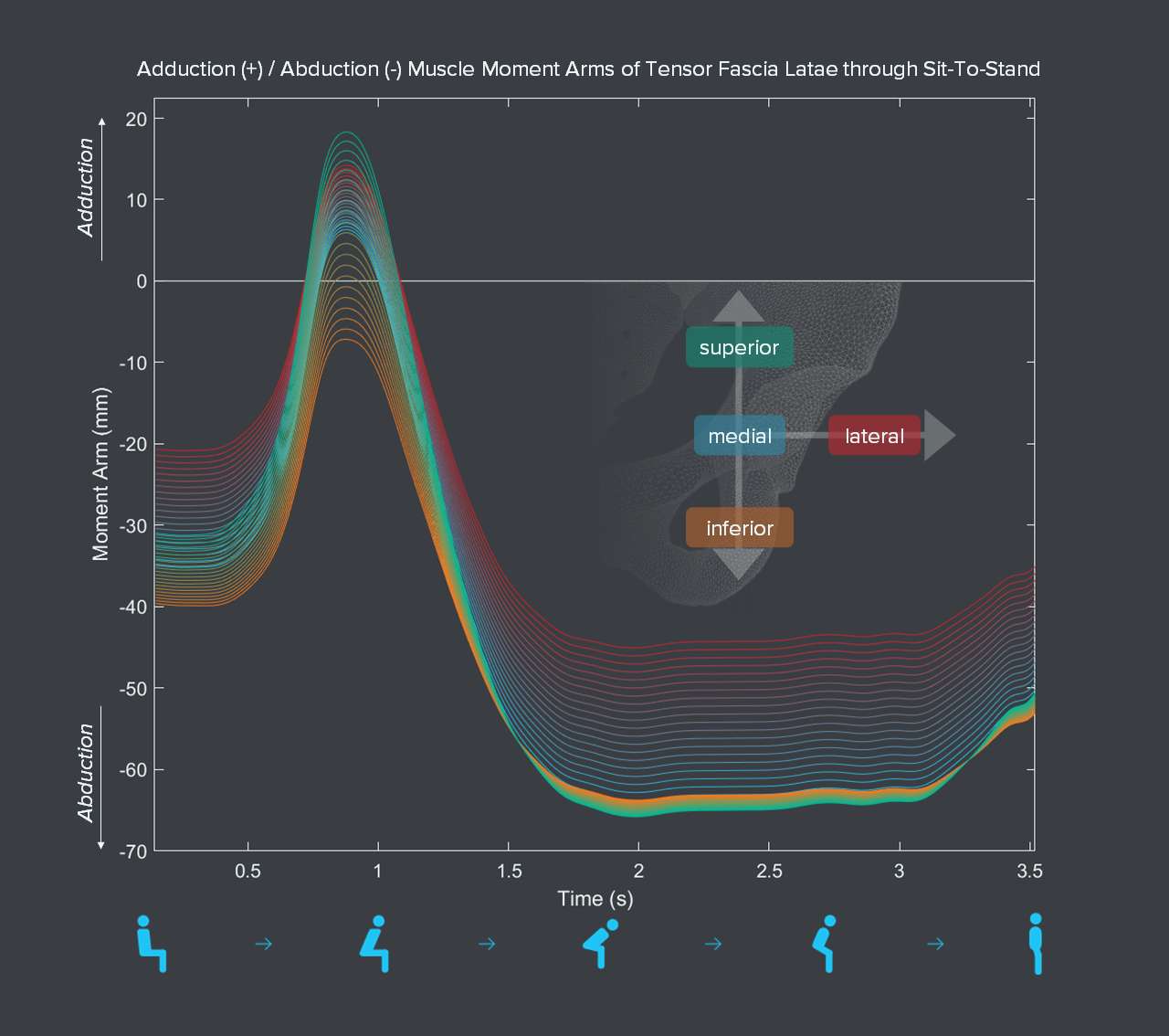
Figure 2#8450
Hip Resurfacing Indicates More Favorable Gait Parameters Post-Operatively as Compared to Total Hip Arthroplasty: A Systematic Review
*Benjamin Domb - American Hip Institute - Des Plaines, USA
Ali P. Parsa - American Hip Institute - Des Plaines, USA
Tracy George - American Hip Institute - Des Plaines, USA
Rachel E. Bruning - American Hip Institute - Des Plaines, USA
Paulo P. Padilla - American Hip Institute - Des Plaines, USA
Mark F. Schinsky - American Hip Institute - Des Plaines, USA
*Email: DrDomb@americanhipinstitute.org
In the past decade, an increasing number of younger, active patients, including athletes, sought hip resurfacing (HR) arthroplasties. There is supporting evidence that hip resurfacing might have a quicker recovery, better post-operative range of motion, and reduced risk for dislocation compared to total hip arthroplasty (THA). The purpose of the present systematic review was to study the variations in post-operative gait parameters among patients with hip osteoarthritis who have undergone hip resurfacing or THA.
According to the guidelines of the Preferred Reporting Items for Systematic Review and Meta-Analyses (PRISMA), a literature search was conducted in October 2022. Criteria for inclusion in the present study included articles that were randomized controlled trials, non-randomized comparative studies, and retrospective studies that reported post-operative gait parameters.
206 patients (224 Hips) were included in the present analysis and an average of 25 patients (range, 9-48) were included in all 8 studies. The mean age of patients reported in these 8 studies ranged from 44.4 to 67 years. Of all included studies, the patient population was, on average, comprised of 54.3% male and 45.7% female patients. Among all patients undergoing HR and THA, there were comparable numbers of males in each group (range 58.3-82.1 and 60.0-88.9 percent, respectively). Six studies indicated that hip resurfacing patients reached a greater average walking speed post-operatively as compared to THA patients, in addition to greater average stride lengths, stance times, and abilities to perform uphill walking. Three studies also showed similar outcomes in flat surface walking among hip resurfacing and total hip arthroplasty patients. Hip flexor/extensor moments and abductor moments were in favor with HR patients.
The present evidence suggests faster return to normal gait patterns in patients following hip resurfacing as compared to THA; specifically, faster return to normal walking velocity, inclined surfacing walking, and flat surface walking.
#8472
MRI Indications for Total Hip Arthroplasty in Patients Without Advanced Osteoarthritis
*Benjamin Domb - American Hip Institute - Des Plaines, USA
Zayd Chishti - American Hip Institute - Des Plaines, USA
Saiswarnesh Padmanabhan - American Hip Institute - Des Plaines, USA
Andrew J. Curley - American Hip Institute - Des Plaines, USA
Mark F. Schinsky - American Hip Institute - Des Plaines, USA
*Email: DrDomb@americanhipinstitute.org
This study aims to explore Magnetic Resonance Imaging (MRI) indications?for total hip arthroplasty (THA) in middle-aged and older adults with recalcitrant hip symptoms without radiographic evidence of advanced hip osteoarthritis.?
Patients with preoperative MRI data who underwent THA?between 2015 and 2020 and had a preoperative Tönnis grade of 0 or 1 were included. Patients were excluded if they had prior ipsilateral hip surgery, Legg-Calve-Perthes disease, or were under worker’s compensation. MRI findings were reviewed by the radiologists and senior surgeon to determine the indication for total hip arthroplasty in patients with intact joint space.
Forty-three hips were eligible for analysis. The patient cohort consisted of 31?females (72.1 %). The mean age and BMI were 53.1 years and 28.7 kg/m2, respectively. ?Five patients over the age of 35 were diagnosed with dysplasia, and due to the age of the patient, PAO was not preferred. There were two categories of patients that were either classified as having localized or generalized chondral damage based on MRI findings. Thirty hips were classified as having generalized?chondral damage, and 8 were classified as having localized chondral damage.
In patients with recalcitrant hip pain in the setting of mild osteoarthritis, generalized chondral damage on preoperative MRI was the most common indication for THA, while localized general chondral damage and dysplastic patients who were not candidates on PAO were other findings that led the surgeon to perform a THA.?
#8557
Learning Curve for Acetabular Registration in a Novel, Miniaturized, CT-Based Navigation Technology for THA
*Tristan Jones - Naviswiss Inc - Tampa, United States of America
Eric M Slotkin - Reading Hospital, Orthopaedic Associates of Reading, - West Reading, Pennsylvania, USA
Stefan Kreuzer - INOV8 Orthopedics - Houston, USA
Alejandro Gonzalez Della Valle - Hospital for Special Surgery - New York, USA
Justin Ong - HSS - New York, USA
*Email: tristan.jones@naviswiss.us
INTRODUCTION
The utilization of CT-based technologies to plan and execute a total hip arthroplasty (THA) has increased. CT based planning is frequently utilized in robotic-assisted THA. However, CT-based planning and navigation is also available in non-robotic platforms which are less costly, have a smaller footprint, and require a simpler intra-operative set up process. These technologies provide improved acetabular cup placement but require additional time to execute the necessary intra-operative registration process. This study aimed to objectify the length of time required to carry out this registration process using a miniaturized navigation system for THA.
METHOD
A total of 28 patients undergoing THA via a postero-lateral approach were included. All patients received a pre-operative CT scan and had implant positions planned using 3D planning software (Naviplan, Naviswiss). All registration times were saved and collected using the time stamps contained in the case log files, while procedure times were collated from the surgical records.
RESULT
The mean time to execute the image-based registration process was 4:09 minutes (range 2:59 – 6:10). Across the first 5 cases, the mean registration time was 4:59, with a mean case time of 85 minutes. For the next 23 cases, the mean registration time accelerated to 3:56, while the mean case time was 84 minutes. A T-test showed no significant learning effect of the learning curve on the case time (p-value 0.49) and a significant learning curve effect on the image based registration process (p-value 0.001).
CONCLUSION
This miniaturized THA navigation system displayed an efficient method for executing the image-based, intra-operative registration process. Mean registration time was less than 4 minutes following the first 5x cases, which may be considered the learning curve for the image-based workflow.
#8562
Dynamics of Intraoperative Pelvic Alignment During Total Hip Arthroplasty in the Lateral Decubitus Position
*Eric M Slotkin - Reading Hospital, Orthopaedic Associates of Reading, - West Reading, Pennsylvania, USA
Stefan Kreuzer - INOV8 Orthopedics - Houston, USA
Tristan Jones - Naviswiss Inc - Tampa, United States of America
Alejandro Gonzalez Della Valle - Hospital for Special Surgery - New York, USA
Justin Ong - HSS - New York, USA
*Email: eslotkindo@gmail.com
INTRODUCTION
A posterior-lateral approach for total hip arthroplasty (THA) has been one of the most commonly used techniques in recent history. This operative position relies on robust fixation of the patient’s pelvis on the operating table in a neutral position to avoid pelvic motion and unintended malposition of the acetabular component. The purpose of this study was to examine the dynamics in pelvic position in the coronal and transverse planes at different time points (initial registration and cup insertion) during the postero-lateral procedure.
METHOD
A total of 12 patients undergoing THA via a postero-lateral approach were included. All patients received a pre-operative CT scan and used a miniaturized navigation device (Naviswiss, AG) to execute the intra-operative image-based registration. The alignment of the pelvis was tracked and recorded at the start of the registration process and then again at the point of acetabular cup insertion. The pelvic positions were noted with respect to the coronal and transverse plane orientations and were saved and recorded within the case log files of the platform.
RESULT
From a coronal perspective, at the initial registration time point, the position of the pelvis was obliquely oriented by a mean of 1° (range -5 to 2°, SD 2). At cup insertion, this mean had become 0° (range -8 to 5°, SD 4). From a transverse perspective, at the initial registration time point, the position of the pelvis was rotated by a mean of 5° (range -6 to 15°, SD 7). At cup insertion, this mean position had become 8° (range -7 to 19°, SD 8).
CONCLUSION
Despite careful positioning of the patient in the lateral decubitus position, initial coronal and transverse alignment of the pelvis is variable and may change significantly throughout the THA procedure. There is a trend towards increasing transverse rotation (‘anterior roll’) between registration and acetabular cup insertion. These deviations from neutral have the potential to increase the likelihood of malpositioned acetabular components if the surgeon is unaware of these patient dynamics. CT-based navigation has the potential to reduce the described effects.
#8596
Is Pyrocarbon Material Inducing Articular Cartilage Regeneration ? : In Vitro Study
*Michel Hassler - Wright Medical - Montbonnot, France
Imbert De Gaudemaris - LAMCOS, Université de Lyon, - villeurbanne, France
Nina Attik - Univ Lyon, UCBL Lyon 1, UMR CNRS 5615, Laboratoire des Multimatériaux et Interfaces, - lyon, France
Remy Gauthier - Univ Lyon, CNRS, INSA Lyon, UCBL, MATEIS, - Villeurbanne, France
Ana-Maria Sfarghiu - Laboratoire de Mécanique des Contacts et des Structures - Villeurbanne, France
Amanda Lo Van - Univ Lyon, INSA Lyon, UCBL, UMR CNRS 5259 - Villeurbanne, France
*Email: michel.hassler@wright.com
In patients with osteoarthritis and an intact rotator cuff, hemi-shoulder arthroplasty (HSA) can be a viable option as it offers the advantage of keeping the native glenoid intact. However, glenoid erosion and diminution of bone stock remains a concern. Ongoing research aim to develop bone-friendly materials able to stimulate tissue repair and preserve cartilage. With this goal, a Pyrocarbon Interposition Shoulder Arthroplasty (PISA) was used for the treatment of complex shoulder pathologies and preservation of bone stock for young patients. Clinical results are encouraging (1, 2) and recent in vitro studies on animal cellular culture show that pyrocarbon (PyC) surfaces stimulates chondrocytes and bone cells proliferation (3).
Regarding this background, the aim of this study is to evaluate the effect of PyC surfaces on the potential of human cartilage regeneration. For that, we developped primary cells culture of chondrocytes extracted from human femoral head and labrum (in collaboration with Lyon Edouard Herriot Hospital) Cells were cultured during 17 days between 2 flats of PyC or 2 flats of PMMA (Polymethyl methacrylate), with a 500mµ space between the flats.
Preliminary results show that labrum cells grow faster than femoral head’s ones, and that cells grow 3 times faster on PyC than on PMMA (figure 1). However, a cellular necrosis was observed in the center of the flats. This was attributed to a poor diffusion of the culture media inside the membrane that has grown during the 17 days of static culture.
Further studies will be performed on dynamic situation using a biotriboreactor system allowing better prediction the human chondrocytes biological behavior in various physiological or pathological situations.
References : 1. J. Garret et al., « Pyrocarbon interposition shoulder arthroplasty: preliminary results from a prospective multicenter study at 2 years of follow-up », J. Shoulder Elbow Surg., vol. 26, no 7, p. 1143?1151, juill. 2017, doi: 10.1016/j.jse.2017.01.002
2. J. Garret, A. Godenèche, P. Boileau et al., Midterm results of pyrocarbon interposition shoulder arthroplasty: good outcomes after posttraumatic osteonecrosis without malunion of the tuberosities, JSES International (2022), https://doi.org/10.1016/j.jseint.2022.05.007
3. A. Hannoun et al., « Pyrocarbon versus cobalt-chromium in the context of spherical interposition implants: an in vitro study on cultured chondrocytes », Eur. Cell. Mater., vol. 37, p. 1?15, 07 2019, doi: 10.22203/eCM.v037a01
Figures
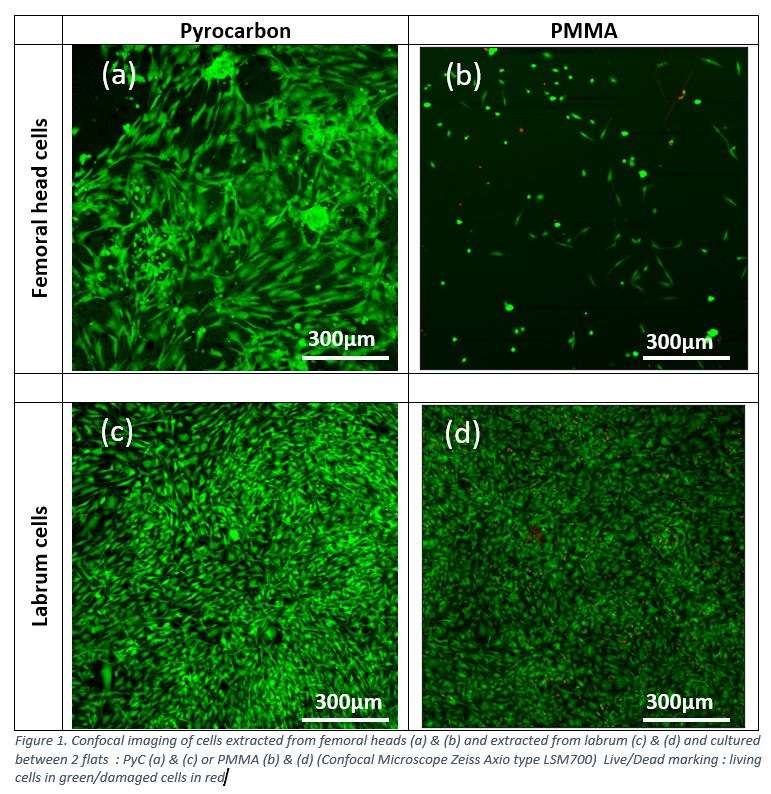
Figure 1#8602
High Accuracy of a New Augmented Reality Assisted Technique for Total Knee Arthroplasty: An in Vivo Study
*Philippe Van Overschelde - Medisch Centrum Latem - Sint-Martens-Latem, Belgium
*Email: vanoverschelde@gmail.com
Total knee arthroplasty remains the standard of care for treating end-stage osteoarthritis of the knee joint. Approximately 15 - 20 % of the patients are dissatisfied following surgery. To improve outcomes, some authors suggest a personalized alignment with narrow and specific margins as ideal target in TKA. In an attempt to achieve this goal different techniques are being introduced in the field of TKA surgery. An augmented reality solution was explored and tested as a cheaper alternative to robotic TKA.
In this study the Knee + system was used (Pixee Medical, Besancon, France). The device offers orthopaedic surgeons intraoperative assistance for implant positioning with the help of augmented reality glasses. The primary research goal is to evaluate the accuracy of an augmented reality based navigation system in the frontal plane by direct comparison of the planned angular values, the intraoperative obtained values and the angles as measured on postop full leg radiographs. The secondary research goal is to assess the feasibility of the system in terms of safety and surgical time compared to robotic TKA (Rosa, Zimmer Biomet Warsaw, USA)
This retrospective study evaluated 124 patients in the augmented reality group. All patients were followed up for at least one year. For the coronal plane the mean absolute difference between the planned versus the measured HKA was 0.51° (CI: -0.96; - 0.05). The average skin-to-skin surgical time was 76 minutes (124 patients) for the AR group and 98 minutes (100 patients) for the robotic group. No complications related to the use of the AR device were reported where 1 supracondylar fracture occurred in the robotic group.
The most important finding of the present study is that this novel technology demonstrated high accuracy in reproducing the planned angular values in a clinical setting. Augmented reality might therefore be a more cost-effective way to achieve personalized alignment in TKA.
#8642
Scoping Literature Review of Health Economic Evaluations of Implant Materials
*Ruben Mujica-Mota - University of Leeds - Leeds, GB
Carina Wang - Invibio - Leeds, GB
*Email: r.e.mujica-mota@leeds.ac.uk
Introduction
Rapid innovation in joint implants presents a challenge for health technology assessment bodies and reimbursement agencies to assess value for money, especially in demonstrating a link between implant material and patient outcomes. We conducted a scoping review to identify previous cost-effectiveness studies of implant materials in hip, knee or joint replacement as well as assessing the methodological frameworks used in this area.
Methods
A systematic search of electronic bibliographic databases was conducted for English language studies of polymers, metal or ceramic prostheses for patients undergoing hip, knee or joint replacement, published since January 2000. We extracted information from identified studies on revision rates, complications, preference-based patient-reported outcomes, quality-adjusted life years and healthcare costs. In addition, the type of economic model used and the assumptions behind the parameters chosen and any other methodological differences.
Results
After screening of titles and abstracts of 864 records and full-text screening of 35 articles, four studies met inclusion criteria for review, three were economic evaluations in total knee arthroplasty (TKA) and one additional in total hip arthroplasty (THA). The evaluated TKA technologies were: low-wear advanced articular bearings relative to standard articulation; all polyethylene tibia implants vs. metal backed implants; and premium TKA components vs. low priced standard TKA components. Two studies adopted the US provider and third party-payer perspectives, and the third study was an evaluation from the German societal perspective including out of pocket costs and productivity losses to patients. Two studies used Markov models that evaluated outcomes using quality-adjusted life years and the third study was an observational analysis of revision rates of primary TKA in joint registry data. The THA study used data from the UK and Swedish Arthroplasty Registers to compare 24 implant combinations defined in terms of bearing surface, fixation technique and femoral head size. This study found that the risk of first revision varied between an early high risk phase up to 2 years post primary operation; a middle low risk phase from 2 to 10 year post primary THR; and a late phase from 10 years onwards, of rising risks of revision due to aseptic loosening and wear. None of these studies was based on direct individual patient data on evidence of the association of radiographic outcomes and implant revision, and none reported evidence on patient reported outcomes associated with outcomes other than revisions operations.
Conclusion
There is limited evidence on economic evaluation studies of implant materials in hip and knee replacements. Key areas of uncertainty relate to how bone remodelling, stress shielding and radiolucency relate to patient reported outcomes.
#8645
Assessing the Impact of Conformity Index in Post-Stabilized Knee Prostheses on Polyethylene Tibial Post Contact Stress
José Luís Medeiros Thiesen - Federal University of Santa Catarina - Florianópolis, Brazil
*Carlos Rodrigo De Mello Roesler - Universidade Federal de Santa Catarina - Florianópolis, Brazil
Eduardo Alberto Fancello - Mechanical Design and Analysis Group (GRANTE) - Florianopolis, Brazil
Amaury Sousa Sá - Universidade Federal de Santa Catarina - Florianópolis, Brazil
Caio Morgado - Universidade Federal de Santa Catarina - Florianópolis, Brazil
*Email: r.roesler@ufsc.br
Total Knee Arthroplasty (TKA) is a surgical procedure commonly used to treat endstage osteoarthritis or rheumatoid arthritis [1]. The success of the TKA procedure and the functionality and longevity of the replaced joint depend on the kinematic performance of the TKA device [2]. Various factors can influence and predict the kinematic performance of the prosthesis, and one important design parameter is the conformity index. This index refers to the ratio between the curvature of the femoral component and the tibial insert, measured in the sagittal plane. Prostheses with high conformity exhibit a greater similarity between the radius of the femoral component and the tibial insert, resulting in a ratio approaching one. Increased conformity leads to a larger contact area between the components, reducing contact pressure [3]. Consequently, these prostheses experience less displacement and rotation under similar loading conditions compared to those with lower conformity. However, it is common for the tibial insert of highly conforming prostheses to be susceptible to pitting and delamination [4]. In posterior-stabilized (PS) knee prostheses, the so-called tibial post plays a crucial role in achieving the desired joint kinematics after TKA. The tibial post replaces the posterior cruciate ligament, stabilizing the knee and promoting femoral rollback during flexion. Several case reports have documented polyethylene tibial post breakage following TKA surgery [5]. Given the direct relationship between the prosthesis conformity index and the distribution of contact pressure on the tibial insert, we hypothesize that conformity is associated with flexural stress applied to the polyethylene tibial post in posterior-stabilized knee prostheses. This study aims to investigate three types of prostheses with varying conformity indexes, manufactured by different companies. Finite element analyses were conducted for each prosthesis to simulate their mechanical behavior under different knee flexion angles. The reaction force developed in the polyethylene post under quasi-static loading was evaluated. Our findings suggest that an increase in the conformity of posterior-stabilized knee prostheses may lead to higher contact pressure between the femoral cam and the tibial post.
References
[1] Elena Losina et al. The Dramatic Increase in Total Knee Replacement Utilization Rates in the United States Cannot Be Fully Explained by Growth in Population Size and the Obesity Epidemic:.The Journal of Bone and Joint Surgery-American Volume, 94(3):201–207, February 2012.
[2] Chadd W. Clary et al. The influence of total knee arthroplasty geometry on mid-flexion stability: An experimental and finite element study. Journal of Biomechanics, 46(7):1351–1357, April 2013.
[3] Z M Jin and T Stewart. Contact pressure prediction in total knee joint replacements Part 2: Application to the design of total knee joint replacements. 1995.
[4] Alison L. Galvin et al. Effect of conformity and contact stress on wear in fixed-bearing total knee prostheses. Journal of Biomechanics, 42(12):1898–1902, August 2009.
[5] Cynthia A. Kahlenberg et al. Clinical and Biomechanical Characteristics of Posterior-Stabilized Polyethylene Post Fractures in Total Knee Arthroplasty: A Retrieval Analysis. The Journal of Arthroplasty, page S0883540323001419, February 2023.
#8692
A Computer Vision System for Fully Automatic Tracking of Patient-Specific Native Knee Kinematics in Stereo-Radiography Sequences
*William Burton - University of Denver - Denver, United States of America
Landon Hamilton - University of Denver - Denver, United States of America
Casey Myers - University of Denver - Denver, USA
Kevin Shelburne - University of Denver - Denver, USA
Paul Rullkoetter - University of Denver - Denver, USA
*Email: will.burton@du.edu
Introduction
In vivo measurement of native knee kinematics in stereo-radiography sequences enables functional analysis of patient-specific biomechanics. Previous studies have demonstrated the accuracy of kinematic tracking [1], but acquiring such measurements is tedious. Tracking is performed manually or semi-automatically, often requiring multiple days of human effort to process a single patient activity. Reducing human effort required for obtaining joint-level kinematics may increase the scope of feasible use cases. Automated solutions could enable pre-surgical workflows or diagnostic applications. The current work therefore evaluates a computer vision system for fully automatic tracking of native knee kinematics in stereo-radiography.
Methods
53 subjects performed dynamic activities in front of a high-speed stereo-radiography (HSSR) system which captured movement of the right knee [2]. Patient-specific anatomies reconstructed from computed tomography (CT) data were used to manually track tibiofemoral (TF) and patellofemoral (PF) kinematics with interactive software. Processed trials were partitioned into training and test cohorts. The test cohort included 20 trials consisting of 4 activities performed by 5 distinct subjects. A computer vision system predicted 6-degree of freedom (6-DoF) poses of the femur, patella, and combined tibia-fibula in stereo-radiography (Fig. 1). First, radiographs were automatically annotated with segmentation maps and semantic key points using machine learning. Next, initial bone pose estimates were obtained by matching predicted key points to anatomical landmarks using numerical optimization. Finally, bone pose estimates were refined by maximizing similarities between CT-based digitally-reconstructed radiographs (DRRs) and radiographs masked by predicted segmentation maps. The computer vision system was applied to all test trials and TF and PF kinematics were computed from predicted bone poses. Predicted kinematics were evaluated against manually tracked values.
Results
The proposed system enabled fully automatic tracking in stereo-radiography (Fig. 2). Median absolute differences between predicted and manually tracked TF kinematics were 0.51, 1.21, and 1.42° for FE, VV, and IE TF rotation DoFs; and 1.03, 0.70, and 0.73 mm for ML, AP, and SI translation DoFs. Median absolute differences for PF kinematics were 2.00, 4.28, and 2.59° for FE, VV, and IE PF rotation DoFs; and 1.04, 0.76, and 1.08 mm for ML, AP, and SI translation DoFs (Fig. 3).
Discussion
Accurate measurement of native knee kinematics provides a quantitative basis for evaluating human movement. This process is primarily performed in research environments due to the technical expertise and human effort required for estimating bone poses in radiograph frames. The proposed system has demonstrated that native knee kinematics can be accurately and automatically estimated with computer vision, suggesting increased scope of use. While previous work introduced a similar system based on model-image registration with surface meshes [3], observed results suggest that CT-based DRRs may enable more accurate pose refinement. Test trials were processed in around 3 hours each by the system, which improves on manual or semi-automatic processing times whilst reducing or obviating the need for human effort.
References
[1] Bey MJ, et al. (2008) Accuracy of biplane.
[2] Hamilton LD, et al. (2017) A framework for identifying.
[3] Burton WS, et al. (2021) Automatic tracking of healthy.
Figures
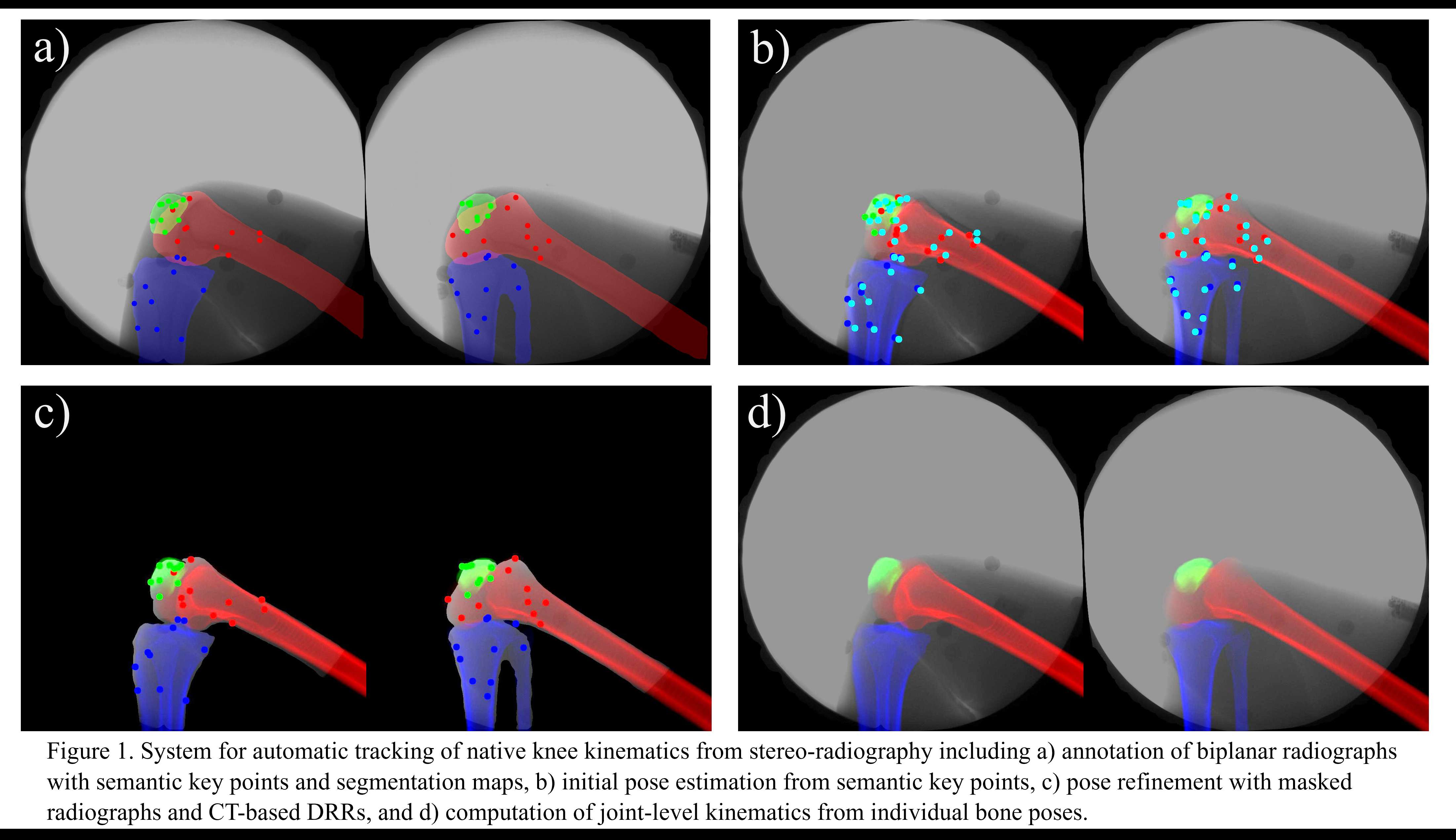
Figure 1
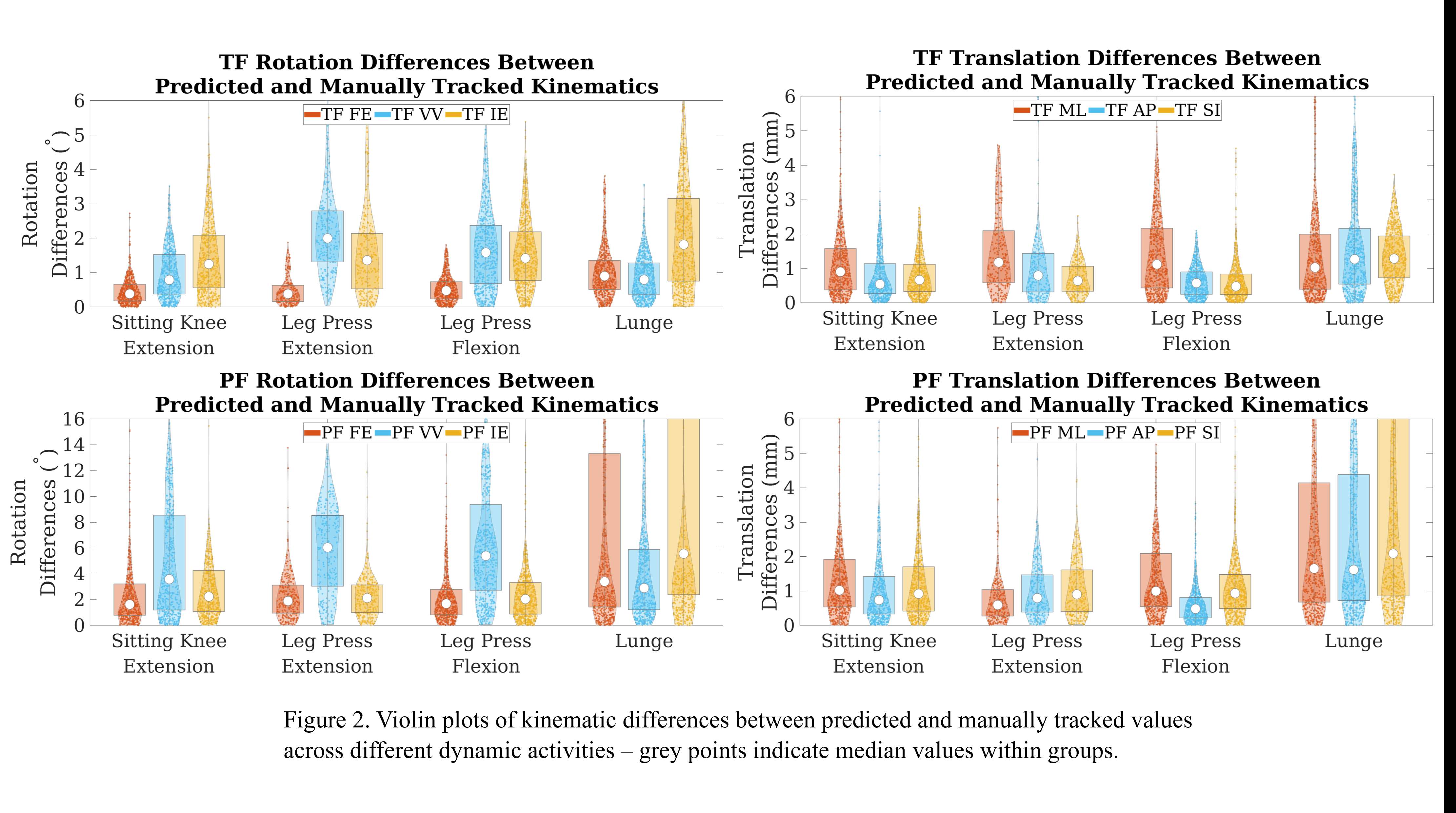
Figure 2
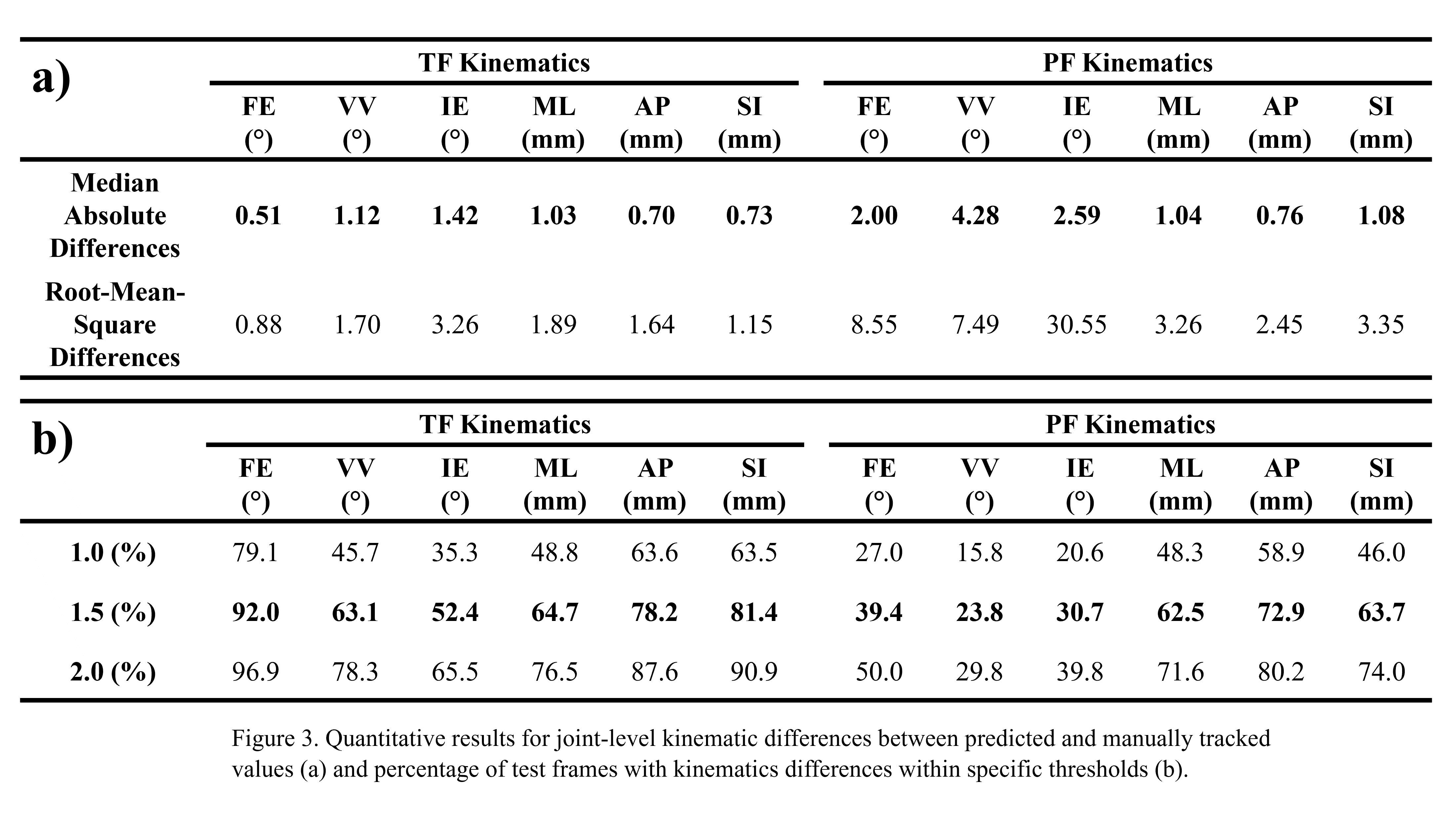
Figure 3#8731
Perioperative Outcomes in Total Knee Arthroplasty Following Preoperative Usage of a Novel Knee Bracer
*Spencer Ward - NYU Langone Orthopedic Hospital - New York, USA
Morteza Meftah - NYU Hospital for Joint Diseases - New York, USA
Ran Schwarzkopf - NYU Langone Medical Center Hospital for Joint Diseases - New York, USA
Theodore Di Pauli Von Treuheim - NYU Langone Health - New York, USA
Hayley Raymond - NYU - New York, United States of America
*Email: Spencer.Ward@nyulangone.org
Introduction
Total knee arthroplasty (TKA) is a commonly used procedure to treat refractory knee osteoarthritis (OA), although it is associated with postoperative pain, which may prevent early ambulation, recovery, and may lead increase opioid consumption in the early recovery period. This study aimed to assess the effects of preoperative use of a novel knee brace on the immediate perioperative outcomes following TKA.
Methods
This was a retrospective cohort study of eight patients who underwent primary, unilateral TKA at a single academic institution by a single surgeon who were prescribed a novel Guardian RehabilitatorTM brace (Biodynamic Technologies, New York, NY) prior to surgery. These patients were then 1:1 matched by age, sex, body mass index (BMI), and American Society of Anesthesiologists (ASA) score to a group of eight patients from the same surgeon who did not receive a brace prior to surgery. Primary outcomes were inpatient active range of motion (ROM), ROM at the first postoperative visit, pain and functional scores (Activity Measure for Post-Acute Care [AMPAC], and visual analog scale [VAS] scores), and inpatient opioid consumption converted to morphine milligram equivalents per day (MMEs/day). Independent samples t-tests were used to evaluate continuous variables and chi-square analysis was utilized for categorical variables.
Results
Patients in the brace cohort had a statistically significantly higher active range of motion at first postoperative visit compared to those who did not receive the brace (p=0.026), active range of motion did not significantly differ at the first postoperative office visit. Despite a small clinically decreased postoperative MME/day during hospitalization in patients receiving a preoperative brace (8.8 vs 12.4 MMEs/day), it did not reach statistical significance (p=0.153). There was no difference found in AMPAC scores, or VAS scores.
Conclusion
While the effect of preoperative use of a novel brace prior to TKA may improve active range of motion in the immediate postoperative period during hospitalization, it may not improve overall pain and functionality during this period of time. Further studies with larger cohorts are needed to further elucidate the benefits of trialing a preoperative brace prior to TKA.
#8733
Knee Stability and Patient Satisfaction in Cruciate-Retaining Total Knee Arthroplasty
*Shuichi Matsuda - Kyoto University - Kyoto city, Japan
Shinichi Kuriyama - kyoto university - kyoto, Japan
*Email: smat522@kuhp.kyoto-u.ac.jp
Introduction: Achieving anterior-posterior stability is crucial in posterior cruciate ligament (PCL) retaining total knee arthroplasty (CR-TKA), but questions remain on the optimal PCL tension to achieve patient satisfaction. This study assessed the relationship between postoperative PCL laxity and patient satisfaction at 2-year follow-up.
Methods: Forty-four varus osteoarthritis knees undergoing CR-TKA were included. PCL tension was adjusted by resizing the femoral component and modifying the posterior tibial slope, without PCL release.
Stress radiograph was taken to quantify postoperative PCL tension 6 months after TKA (Figure 1). PCL laxity was defined as the difference in radiographic anterior-posterior tibial translation without or with posterior tibial stress. The effect of PCL laxity on the 2-year postoperative 2011 Knee Society Score (KSS) was determined.
Results: The 2011 KSS total score improved significantly from preoperatively (87.9) to postoperatively (142.4). The mean satisfaction score was 32/40, and 96% of the patients reported “neutral satisfaction” or better after TKA.
Intraoperatively, no PCL release was performed to adjust PCL tension. The femoral component was changed to the “minus-size” in 27 knees due to excessive femoral rollback, and the tibia was recut to increase posterior slope in 6 cases with persistent excessive rollback after femoral downsizing. The final average intraoperative femoral rollback was 2.7mm less than that before arthroplasty.
Mean PCL laxity was 2.3 mm on postoperative stress radiographs, and four subgroups were defined according to PCL laxity: laxity ≤ 0 mm (5 knees); 0 mm < laxity ≤ 2 mm (19); 2 mm < laxity ≤ 4 mm (10); and laxity > 4 mm (10). Postoperative satisfaction scores were significantly highest in the subgroup with 2–4 mm laxity (Figure 2). Postoperative symptoms score, expectations score, and functional activities score were also highest in the subgroup with 2-4 mm laxity.
Conclusion: In CR-TKA without ligament release, PCL tension can be adjusted based on femoral rollback, and moderatePCL laxity (2–4 mm) achieved excellent postoperative patient satisfaction.
#8738
Is the Subluxation Percentage of the Crowe Classification a Useful Treatment Strategy for Total Hip Arthroplasty?
*Keiji Otaka - Nagoya university - Nagoya, Japan
Yasuhiko Takegami - Nagoya University - Nagoya, Japan
Yusuke Osawa - Nagoya University - Shizuoka, Japan
Hiroki Iida - Nagoya University - Nagoya, Japan
Yuto Ozawa - Nagoya University - Nagoya, Japan
Hiroto Funahashi - Nagoya university - Nagoya, Japan
Hiroaki Ido - Nagoya University - Nagoya, Japan
Takamune Asamoto - Nagoya University - Nagoya, Japan
Shinya Tanaka - Nagoya university - Nagoya, Japan
*Email: keiji_ohtaka@yahoo.co.jp
Introduction
It has been reported that a cup center-edge (cup-CE) angle greater than 0 degree is required to obtain adequate fixation of the cementless cup in a total hip arthroplasty (THA). The Crowe classification is an indicator of the degree of subluxation, and may be a useful predictor for treatment strategies for cup placement. In this study, we examined the relationship between the subluxation percentage in the Crowe classification and the cup-CE angle when a cementless cup is placed in the true acetabulum, and evaluated the cutoff value for the subluxation percentage at which a cementless cup can be placed in the true acetabulum.
Methods
We retrospectively reviewed 127 patients (144 hips) who underwent THA between April 2012 and March 2015 in our hospital. All patients were female, the mean age was 63.2 years, and the mean BMI was 23.5 kg/m². The subluxation percentage in the Crowe classification was calculated as the ratio of the distance from the interteardrop line to the head-neck junction to 1/5 of the height of the pelvis. We planned to place a cementless cup in the true acetabulum and measured the cup-CE angle. The relationship between the subluxation percentage and the cup-CE angle was evaluated and the cutoff value for the subluxation percentage that gained a cup-CE angle greater than 0 degree was determined from the receiver operating characteristic curve.
Results
According to the Crowe classification, 90 hips (62.5%) were graded as group ?, 27 (18.8%) were group ?, 18 (12.5%) were group ?, and 9 (6.3%) were group ?. For the Crowe group ?, ?, ? and ? respectively, the mean subluxation percentages were 21.9%, 58.5%, 84.4% and 143%, the mean preoperative planned cup sizes were 48.5mm, 47.8mm, 45.9mm and 42.7mm, and the mean cup-CE angles were 15.2 degrees, 3.89 degrees, 0.11 degrees and 10.7 degrees. The cutoff value for the subluxation percentage to obtain a cup-CE angle greater than 0 degree was 60% (AUC 0.863, sensitivity 0.860, specificity 0.826).
Conclusion
When the subluxation percentage in the Crowe classification is greater than 60%, cementless cup fixation to the true acetabulum is often difficult, and high-hip center reconstruction or bulk bone graft should be considered.
#8747
Does Proximity to Surgeons' Office Impact Utilization of Telemedicine Visits in Total Joint Arthroplasty?
*Vinaya Rajahraman - New York, United States of America
Elizabeth Stiles - NYU Orthopedic Hospital - New York, USA
Jonathan Katzman - NYU Langone - New York, United States of America
Muhammad Haider - NYU Orthopedic Hospital - New York, USA
Roy Davidovitch - NYU Langone Health - New York, USA
Ran Schwarzkopf - NYU Langone Medical Center Hospital for Joint Diseases - New York, USA
Joshua Rozell - NYU Langone Orthopedic Hospital - New York, USA
Casey Cardillo - NYU Orthopedic Hospital - New York, USA
*Email: v.rajahraman@gmail.com
Introduction:
Telemedicine visits have been increasing for total joint arthroplasty (TJA) patients, especially since the start of the COVID-19 pandemic. Further research is necessary to determine the characteristics of the patient population that utilizes telemedicine and the effectiveness of these visits in addressing patient concerns. This study aims to evaluate the relationship between demographics of TJA patients who have used telemedicine and their proximity to their primary clinic.
Methods:
We retrospectively reviewed all patients at our institution who had a telehealth clinic encounter between May 2018 and July 2020. Distances between patients’ home zip codes and primary clinic zip codes were calculated using the CDXGeoData (Randolph, NJ) ZIP code distance tool. Demographic characteristics of the population was collected and linear regression was utilized to control for each demographic variable.
Results:
A total of 1,055 patients had a telemedicine visit. There was no significant difference in distance between home and clinic zip codes between different groups regarding age (p=0.805), sex (p=0.092), race (p=0.422), marital status (p=0.227), and insurance type (p=0.105). No difference was seen even after adjusting for each variable separately (age, p=0.263; sex, p=0.154; race, p=0.271; marital status, p=0.405; insurance type, p=0.189). The majority of the patients live within the five boroughs of New York City (70.9%).
Conclusion:
No relationship was found between distance from patients’ home to clinic zip codes and demographics including age, sex, race, marital status, and insurance type in those that utilized telemedicine. Telemedicine may be a useful way to provide personalized care to patients for appropriate encounters regardless of distance they live from the clinic, and can potentially serve to improve patient satisfaction as a screening tool to assess the need for an in-person visit.
Figures
Figure 1
Figure 2
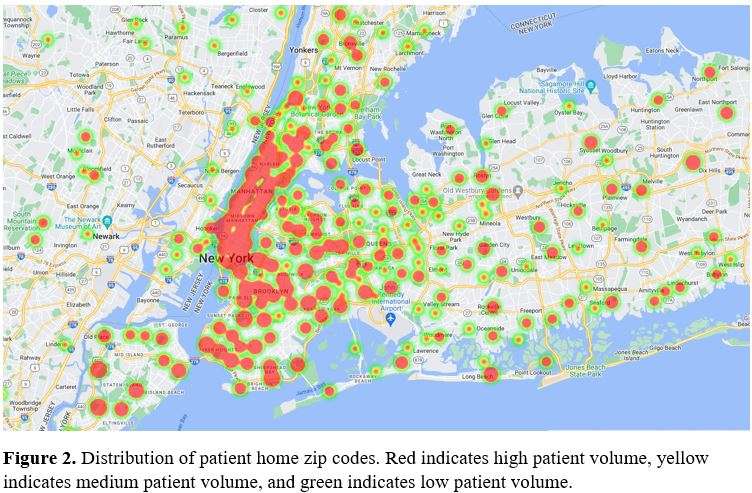
Figure 3#8784
Identifying Sources of Error in Computer Navigated Total Knee Arthroplasties Using a Metric on SE(3) and Sensitivity Analyses
*Nicole Martensson - Western University - London, Canada
Delaney Stevens - Western University - London, Canada
Kellee Stephens - Western University - London, Canada
Kenneth McIsaac - Western University - London, Canada
Brent Lanting - London Health Sciences Centre - London, Canada
*Email: nicole.martensson@outlook.com
Throughout the procedure of a computer-navigated total knee arthroplasty (TKA), there are many opportunities for sources of error to be introduced. Identifying these errors can improve surgical outcomes. There is also a lack of accessible methods in available literature for clinicians to perform research in this area using engineering analysis techniques. This project aimed to provide a greater understanding of the sources of error that can occur pre-bone cut. Possible sources of error include the bony landmark selections and the placement of the cut guide.
Using artificial bone models and a 3D point capture system concurrently with a computer-navigation system, the data points collected during the procedure were mimicked. It was found that variability of point selection varied between landmarks with some being more precise than others. Bone reference frames were then calculated using these landmark points. By painting the surface of the saw blade, the cut plane values were obtained and a cut plane reference frame was estimated. These frames were easily represented with homogeneous transformation matrices. One method of comparing transformation matrices is with a metric on SE(3), simplified in this thesis to be the Frobenius norm.
It was found that bone reference frames with the highest metric were the ones with the highest error in femur or tibia center points. It was also found that there was no clear correlation between the bone reference frame error and cut plane error, implying that other sources must be taken into account. Sensitivity analyses were performed to observe the outcome error of the bone reference frame and cut plane in regards to error in the landmark selection. The results from this support other results from the experiments: that landmark points used for the origin of the reference frames have the greatest effect on the system output.
Several methods showed that different landmark points have varying effects on the metric on SE(3). These differences can be interpolated to different sources of error. The methods in this project can also easily be applied to other computer-navigated systems for analysis.
#8791
Treatment of the Disruption of Knee Extension Mechanism After Total Knee Arthroplasty
Toshihiro Seki - Yamaguchi University Graduate School of Medicine - Ube, Japan
*Kazushige Seki - Yamaguchi University - Ube, Japan
Takashi Imagama - Yamaguchi University - Ube, Japan
Takashi Sakai - Yamaguchi University Graduate School of Medicine - Ube, Japan
*Email: tc-mezamashi@hotmail.co.jp
?Purpose? Knee extensor mechanism injuries after total knee arthroplasty (hereafter TKA) are a relatively rare complication, but are often difficult to treat. Generally, conservative treatment is considered ineffective and surgical treatment is often performed when extension lag is present or when there is a displaced fracture. In this study, we report on a retrospective study of the outcomes of 13 patients with a failed knee extension mechanism after TKA. ?Subjects and methods? 1712 patients who underwent TKA between 2011 and 2019 were included. Of these, 11 patients with knee extensor mechanism injuries and 13 joints that were observable were included. The mean age at the time of TKA was 76.7 (58-78) years, the mean time from TKA to extensor mechanism injury was 6.0 months (0 months to 4.5 years) and the mean follow-up was 2 years 4 months (1 year to 5 years 9 months). The breakdown of extension mechanism failure sites included eight patellar fractures, four patellar tendon ruptures and one quadriceps rupture. Three patellar fractures with fracture dislocation and extension lag were treated surgically and five patients were treated conservatively. Four patients with patellar tendon rupture and one with quadriceps rupture were treated surgically. The clinical and radiological evaluation included range of motion, presence or absence of extension lag, and bony fusion. ?Results?The range of motion after surgery in the four patients with patellar tendon ruptures averaged 0° in extension and 97.5° in flexion, with an extension lag of more than 10° in one case. One patient with quadriceps rupture had flexion limitation of 0° in extension and 75° in flexion, but no extension lag. In three patients with patellar fractures who underwent surgical treatment, bony fusion was achieved in one case and extension lag of more than 10° was observed in two cases. Conservative treatment resulted in bony fusion in 3 out of 5 cases, and extension lag of more than 10° in 1 case. ?Discussion? disruption of the extension mechanism is one of the most difficult and serious complications to treat after TKA. Aggressive surgical treatment is recommended for quadriceps ruptures and patellar ligament ruptures, while careful selection of treatment strategy is necessary for patellar fractures, as there have been cases of revision surgery.
#8379
Fast and Accurate Automated Segmentation of Pelvis and Femur Bones From CT Scans Is Robust to Sex, Age, and Ethnicity, and Enables 3D Preoperative Planning of THA Surgery.
*Marco Schneider - Formus Labs - Auckland, New Zealand
Lilian Lim - FormusLabs - Auckland, New Zealand
Chris Rapson - Formus Labs - Auckland, New Zealand
Alex Carleton - FormusLabs - Auckland, New Zealand
Ravik Hira - Formus Labs - Auckland, New Zealand
Thor F Besier - University of Auckland - Auckland, New Zealand
Ju Zhang - Formus Labs - Auckland, New Zealand
*Email: marco@formuslabs.com
Introduction
Accurate and rapid segmentation of the pelvis and femur cortical bone is clinically critical for 3D preoperative planning of total hip arthroplasty (THA) surgery. Image segmentation methods, such as convolutional neural networks (CNNs), can automatically segment cortical bone in CT images [1], although it is not known how the segmentation accuracy is influenced by sex, age, or ethnicity. In this study, we evaluated the accuracy of the Formus CNN in segmenting a dataset of CT images of the hip of a US population as a function of sex, age, and ethnicity.
Methods
60 CT scans of the pelvis were obtained from cadaveric adults aged 40-70 years. The scans included 26 females and 34 males of African American, Hispanic, and White/Caucasian ethnicities. The datasets were processed using the Formus CNN which automatically segmented the pelvis and the femur. Segmentation accuracy was compared between the automatically segmented geometries and the manually segmented ground truth generated in Mimics by a panel of US-board registered radiologists experienced in 3D image segmentation. Accuracy was evaluated for the external surface of the femur and hemipelvis, as well as the inner cortical surface of the femur. The metrics used to evaluate the accuracy of the segmentations were the surface-to-surface mean absolute distance (MAD), Dice score (an overlap index between 0 and 1), and Hausdorff distance (HD). Segmentation accuracy was compared between males and females, under-55 and 55-and-over age groups, and across ethnicities. Segmentation time was compared between manual and automated segmentation.
Results
Compared to manual segmentations, the automated Formus CNN segmented the hemipelvis with an average Dice score of 0.949, an average MAD of 1.15 mm, and an average HD of 3.041mm for the acetabulum. The CNN segmented the femur with an average Dice score of 0.967, an average MAD of 1.353 mm, and an HD of 2.839 for the femoral head. The inner cortical surface of the femur was segmented with a MAD of 1.017 mm and an HD of 2.801 mm. Segmentation accuracy was comparable across males and females, ethnicities, under-55 and 55-and-over groups. The Formus CNN returned a complete segmentation in 10 mins, compared to manual segmentation, which took 60 mins, on average.
Conclusion
All segmentations were much faster than manual segmentation and exceeded the clinical acceptance criteria of: Dice score equal or greater than 0.9, MAD equal or less than 2 mm, and HD equal or less than 5 mm in the femoral head and acetabulum. These results demonstrate the high accuracy and robustness of the Formus CNN in automatically obtaining patient-specific morphologies of the pelvis and femur, including the inner cortical surface, regardless of sex, age, and ethnicity for 3D preoperative planning of THA surgery. This has additional implications for patient-specific modelling workflows such as musculoskeletal modeling and finite element analysis.
References
-
Zhang J et al. (2012). MICCAI 2012 International Workshop, Nice, France
Figures
Figure 1
Figure 2#8465
Improving Care Efficiency in Adult Reconstruction Clinics: Validation of a 9-Question Survey for Diagnosing Hip Osteoarthritis
Drew Clippert - Brown University/ University Orthopedics - Providence, United States of America
Caitlin Barrett - University Orthopedics - Providence, USA
*Valentin Antoci - University Orthopedics - Providence, USA
Jacob Laperche - Frank H. Netter School of Medicine Quinnipiac University - Middletown, United States of America
*Email: valentin.antoci@gmail.com
Introduction: Within the field of adult reconstruction, there is an increasing demand for care related to joint pain as the United States population continues to age and life expectancy grows older. Despite this substantial increase in patient population, the rate of fellowship trained adult reconstruction surgeons is growing more steadily. Therefore, it is not only important for the patients but also for the surgeons to utilize clinic time efficiently. Multiple studies have shown that patient satisfaction with care is strongly correlated to in-office wait times, with one study indicating it as the strongest predictor of overall satisfaction. To improve patient quality of care and to reduce clinical burden on adult reconstruction surgeons, we devised a 9-question survey provided to patients along with traditional intake paperwork to assess the chief complaint and previous interventions as a way to increase care efficiency in the clinic and improve surgeon planning.
Methods: New patients with hip pain who had no history of prior joint replacement were provided with a 9-question choice multiple survey along with standard intake paperwork at the beginning of their visit. This survey and the questions utilized were developed in conjunction with the attending adult reconstruction surgeon whose clinic they were administered in, and this study aims at validation of these questions as predictors for care. After completion of the survey, points were awarded to each question answer as either 0, 1, or 2 depending on the response and total point scores along with individual question scores were recorded. Additionally, patient diagnosis code (hip osteoarthritis, greater trochanteric bursitis, strain, lower back pain) and surgeon decisions regarding the next steps in care were recorded. Statistical analysis of the mean difference was performed with a p-value of .05 for significance.
Results: Analysis showed that the survey was able to accurately differentiate hip osteoarthritis from a diagnosis of greater trochanteric bursitis, strain, or lower back pain based upon cumulative score. Patients who were coded as having hip osteoarthritis had an average total survey score 1.570 + .405, 2.424 + .857 and 1.258 + .456 points higher than those coded for greater trochanteric bursitis, strain, and lower back pain, respectively.
Conclusion: In this study, we found that our 9-question survey was able to accurately distinguish between patients with hip osteoarthritis and those with other conditions, which could greatly benefit adult reconstruction clinics. The survey proved to be an effective tool in assessing the patients' chief complaints and previous interventions and providing an initial osteoarthritis diagnosis with accuracy. With the rise of artificial intelligence and machine learning in the field of medicine, survey utilization may become increasingly important for optimizing patient care. These surveys provide a reliable, standardized, and efficient way to gather important information from patients, which could improve patient satisfaction and overall quality of care provided by adult reconstruction surgeons.
#8179
Radiographic Findings Associated With Mild Hip Dysplasia in 3,869 Patients Using With a Deep Learning Measurement Tool
*Seong Jun Jang - Hospital for Special Surgery - New York, United States of America
Daniel Driscoll - Hospital for Special Surgery - New York, USA
Christopher G. Anderson - Virginia Commonwealth University - Richmond, USA
Ruba Sokrab - Hospital for Special Surgery - New York, USA
Dimitrios Flevas - Hospital for Special Surgery - New York, USA
David J. Mayman - Hospital for Special Surgery - New York, USA
Jonathan Vigdorchik - Hospital for Special Surgery - New York, USA
Seth A. Jerabek - Hospital for Special Surgery - New York, USA
Peter K. Sculco - Hospital for Special Surgery - NYC, USA
*Email: seongjang22@gmail.com
Abstract
Introduction
Hip dysplasia is considered one of the leading etiologies contributing to hip degeneration and the eventual need for total hip arthroplasty (THA). In this study, we validated a deep learning (DL) algorithm to measure angles relevant to hip dysplasia and applied this algorithm to determine the prevalence of dysplasia in a large population based on incremental radiographic cutoffs.
Methods
All patients from the Osteoarthritis Initiative (OAI) with baseline AP pelvis radiographs and without previous THAs were included. A DL algorithm automated three angles associated with hip dysplasia: the modified lateral center edge angle (LCEA), Tönnis angle, and modified Sharp’s angle. The algorithm was validated against two independent readers, and all angles were measured in a cohort of 3,869 patients (61.29.2 years, 57.1% female). The percentile distributions and prevalence of dysplastic hips were analyzed using each angle.
Results
The algorithm had no significant difference (p>0.05) in measurements (paired difference:0.3-0.7) and had excellent agreement for dysplasia classification (kappa=0.78-0.88) against readers. In 140 minutes, 23,214 measurements were automated for 3,869 patients. LCEA and Sharp’s angles were higher and the Tönnis angle was lower (p<0.01) in females. The dysplastic hip prevalence varied from 2.5% to 20% utilizing the following cutoffs: 17.325.5(LCEA), 9.4-15.6(Tönnis), and 41.3-45.9(Sharps).
Conclusion
A DL algorithm was developed to measure and classify hips on the spectrum of mild hip dysplasia. The reported prevalence of dysplasia in a large patient cohort was dependent on both the measurement and threshold, with 12.4% of patients having dysplasia radiographic indices indicative of higher THA risk.
Keywords: Hip Dysplasia; Artificial Intelligence; Deep Learning; Osteoarthritis
Introduction
Hip osteoarthritis(OA) is the most common joint disease in which degenerative changes develop in the cartilage and surrounding bone, leading to pain, stiffness, loss of function, and limitations to daily activities.[1] Anatomical deformities in hip shape are considered key predisposing factors for the development of OA.[2] In particular, hip dysplasia is one of the prominent risk factors contributing to premature degenerative osteoarthritis, and it involves an abnormality in the inclination, version, and volume of the acetabulum. This often results in a greater amount of hip joint reactive forces concentrated on a smaller surface area. This focal wear ultimately leads to early degenerative changes in the hip joint and may necessitate the need for total hip arthroplasty (THA).[3]
Despite the risks associated with hip dysplasia and the potential need for THA, assessing hip dysplasia in adults remains unclear, especially in cases with mild acetabular deformity. Although rates between 3% and 5% are generally reported, the reported prevalence of hip dysplasia ranges from 1.7% to 20% in the general population based on different studies.[4-6] The wide range of prevalence may be attributed to variations in gender and ethnicity between cohorts.[6-8] More importantly, it may also be a result of variations in the method of radiographic diagnosis. Several hip dysplasia radiographic indices, including the Sharp’s angle, the Tönnis angle, and the lateral center-edge angle (LCEA), are used in literature to provide certain threshold values,[9-11] but there exists considerable uncertainty regarding the optimal cutoff for dysplasia classification. [12] Furthermore, there are even differences in how these angles are measured between studies.[13-16] These inconsistencies ultimately contribute to the discrepancy in the assessment of hip dysplasia using these indices.[3, 17]
The differences in both the radiographic index and measurement method introduce subjectivity in classifying hip dysplasia. Creating an objective measurement tool using emerging technology and applying it in a large cohort could uncover the exact variations inherent in dysplasia classification and gradation. Specifically, deep learning (DL) algorithms have already been used in several orthopaedic studies to investigate radiographic parameters, including those relevant to hip dysplasia.[18-20]. Given that the radiographic evaluation of hip dysplasia can guide management decisions, these algorithms also have important clinical implications and applications. Namely, it may provide the objective extraction of radiographic indices with the potential to aid in the early and accurate detection and evaluation of mild hip dysplasia.
The main aims of this study are to 1) develop and validate a deep learning algorithm to automate angles relevant to hip dysplasia and 2) to apply this algorithm to a large cohort of patients with or at risk of developing osteoarthritis to determine the prevalence of dysplasia radiographic findings. We hypothesize 1) that the algorithm will be able to produce measurements with surgeon-level accuracy and 2) that the prevalence of dysplasia will vary substantially depending on the application of threshold values derived from different radiological parameters.
Methods
Patient and Image Selection
All patients from the Osteoarthritis Initiative(OAI) were included in this study. The OAI is a collection of publicly available data from 4,796 patients who were prospectively enrolled in multiple institutions between 2004-2015.[21] This analysis of a public database was exempt from IRB review. From the OAI, patients with anteroposterior(AP) pelvis radiographs taken at the baseline enrollment were included. Any patient with a history of THA at the time of imaging were excluded. After inclusion and exclusion, the final cohort included 3,869 pelvic images from independent patients. The mean age was 61.29.2 (57.1% female).
Dysplasia Angles
Three angles associated with hip dysplasia were studied.[14-16, 22] They included the modified Sharps angle, the Tönnis angle, and the modified lateral center-edge angle (LCEA). As described by Agus et al., the modified Sharp's angle is the angle subtended by the line connecting the lateral sourcil edge to the ipsilateral teardrop and the line connecting the bilateral teardrops.[23] The Tönnis angle is the angle subtended by the line between the lateral and medial edge of the acetabular sourcil and the line connecting the bilateral pelvic teardrops. The modified LCEA, as described by Ogata et al., is the angle subtended by the line connecting the center of the femoral head to the lateral sourcil edge and the line approximating the vertical pelvic axis.[24](Figure 1)
Image Segmentation
Recent studies have leveraged the use of image segmentation with convolutional neural networks to measure clinically relevant orthopaedic parameters on radiographs including acetabular inclination and anteversion[25], hip joint center[26], and angles of dysplasia.[18, 19, 27] A convolutional neural network is a form of a DL model used in computer vision tasks. Image segmentation deep learning models produce annotated outputs of each pixel in an image as belonging to a specific object. We utilized image segmentation using a U-Net, a type of convolutional neural network, to automatically annotate relevant bony landmarks of interest as objects necessary for angle measurements.(See Supplemental Methods for Model Creation and Metrics)
DL Algorithm Validation of Angle Measurements
A power analysis using an alpha of 0.05 and a power of 0.80 to detect a clinically relevant difference of 2 degrees between the deep learning algorithm and reader measurements produced a sample size of 73 hips. After algorithm creation, its accuracy in measuring relevant dysplasia angles was tested on 112 independent hips not used for model creation. Two trained readers, including a fellowship-trained orthopaedic surgeon, produced the ground truth measurements for comparison. The model’s and reader’s classification for dysplastic hips based on literature-reported cutoffs[22] for the angles were compared to ensure accuracy in dysplasia detection. After validation, the model was then applied to the entire image cohort.
Statistical Analysis
Trained-reader measurements and DL measurements were compared using the inter-class correlation coefficient(ICC) and an independent t-test after testing for normality. Bland-Altman plot analyses assessed for systematic errors. Reader and algorithm agreement for classifying hips as dysplastic were assessed using Cohen’s kappa. The Pearson correlation coefficient was also determined between the three angles.
In the entire cohort, percentile distributions of hip dysplasia based on incremental radiographic cutoffs for the three angles were calculated. The angles were also compared between males and females using an independent t-test.
All statistical analyses were conducted in Python using pingouin(V0.5.3), sklearn(V1.2.2), and scipy(V1.10.1) packages.
Results
Deep Learning Algorithm Performance
The deep learning model was optimized within 100 epochs of training. The optimized model had a multi-class DSC of 0.89. When the deep learning algorithm was applied to the entire cohort of images, it measured three angles for each hip at a rate of 2.17 seconds. For the entire cohort of 3,869 images(7,738 hips), this totaled to 23,214 measurements in 140 minutes.
Deep Learning vs Surgeon Measurements
The ICC between the two trained readers was 0.81-0.91 for the three angles(Figure 2). The ICC between the mean of the readers and the deep learning algorithm was higher with all ICCs >0.88(Table 1). There was no significant difference between the readers and the deep learning-produced measurements on the sub-cohort of 112 hips(p>0.05) for all three angles. The mean paired difference was highest for the LCEA (, SD:2.6, IQR 2.3–0.9). The Bland-Altman analysis revealed no significant bias in the deep learning measurements(Figure 3).
Utilizing a cutoff of 22.5 for the modified LCEA, 10.0 for the Tönnis angle, and 40.5 for the modified Sharp’s angle, the kappa was 0.88, 0.78, and 0.81, respectively, between the algorithm and readers for classifying this sub-cohort of hips as dysplastic or nondysplastic. Classification matrices are depicted in Figure 4.
Modified LCEA
The average LCEA was 31.1(7)(Table 2). There was a significant difference between males and females with females having a higher modified LCEA. When considering the percentile cutoffs for the modified LCEA, a value of 25.5 indicated the 20th percentile (20% prevalence) whereas a value of 19.5 indicated the 5th percentile (5% prevalence)(Figure 5).
Modified Sharp’s Angle
The average Sharp’s Angle was 38.0 (4)(Table 2). There was a significant difference between males and females with females having a higher modified Sharp’s angle. A value of 41.3 indicated the 80th percentile (20% prevalence) whereas a value of 44.5 indicated the 95th percentile (5% prevalence)(Figure 6).
Tönnis Angle
The average Tönnisangle was 5.1 (5)(Table 2). Males had a higher Tönnis angle (p<0.01). A value of 9.4 indicated the 80th percentile whereas a value of 13.7 indicated the 95th percentile(Figure 7).
Angle Relationships
The Pearson correlation coefficient was -0.79 (p<0.01) between the modified LCEA and Tönnis angle, -0.67 (p<0.01) between the modified Sharp’s and modified LCEA, and 0.59 (p<0.01) between the modified Sharp’s and Tönnis angle.
Discussion
The main finding of this study was that an imaging-based DL algorithm was developed to accurately automate angles relevant to hip dysplasia on pelvis radiographs and classify hips on the spectrum of hip dysplasia. Its measurement capabilities were validated against the mean values of two trained human observers, including a fellowship-trained orthopaedic surgeon. There was no statistical difference for all measurements. Thus, the algorithm demonstrated analytical capabilities that were in accordance with landmarks and angles relevant to arthroplasty surgeons in practice. Furthermore, the application of this validated tool on a large, older patient cohort demonstrated that the reported prevalence of dysplasia is dependent on both the measurement used to define dysplasia and its specified cutoffs.
Deep learning has been implemented in several medical disciplines, including orthopaedic surgery, to investigate the propensity for automated algorithms to identify clinically relevant features on visual data including ultrasound images, radiographs, and advanced imaging.[26, 28-31] In computer vision, DL functions by applying neural networks to images to experientially learn patterns associated with image features. Information derived from imaging-based analyses may then be further used as clinical applications or to provide insight into outcomes and overall prognosis.[26, 32, 33] The current study applied DL to automate various hip joint measurements relevant to dysplasia on AP pelvis radiographs with strong accuracy and consistency. The agreement (ICCs) between the readers and the deep learning algorithm was >0.88 for all angles, and there was no statistical difference in angle measurements. Critically, the time the algorithm needed to measure three angles for each hip was only 2.17 seconds. With the integration of this tool into hospital image systems, there is value in speeding up clinical and research workflows relevant to hip dysplasia and in decreasing the burden of manual measurements on clinicians and researchers for large cohort studies. To demonstrate this application, this study further utilized this algorithm on a cohort of 3,869 images (7,738 hips), and the algorithm produced 23,214 unique measurements in 140 minutes for analysis.
The analysis of these automated measures demonstrated that the prevalence of radiographic indices indicative of hip dysplasia is dependent on the measurement and cutoff used to define dysplasia. Indeed, despite the risks associated with dysplasia and need for long-term THA, defining hip dysplasia in adults remains unstandardized, especially in less severe cases with mild acetabular deformity. The prevalence of hip dysplasia in the general population has been reported in the range from 1.7%-20% based on the type of study using various radiographic definitions.[4-6] In this study, the prevalence of dysplastic hips in this cohort varied from 2.5%-20% utilizing the following cutoffs: 17.325.5 for LCEA, 15.6 for Tönnis angle, and 45.9-41.3 for Sharps angle. These results are in close agreement with the findings of Laborie et al., which determined that cut-off values of 17-18 for the modified LCEA, and 15-16 for the Tonnis angle, and 46-47 for the modified Sharps angle resulted in a 2.5% prevalence of developmental hip dysplasia in a Norwegian population.[34] Interestingly, their study analyzed 2,011 individuals at skeletal maturity with an average age of 18.6(SD 0.6), and all measurements required the manual identification of landmarks on AP pelvis radiographs by an observer. This current study not only utilized DL to rapidly automate these measurements in a larger cohort without the need of an observer (n = 3,869), it also concurred with Laborie et al’s[34] population analyses of these angles in an older cohort with a mean age of 61.2(SD 9.2) years. This suggests that the radiographic markers of dysplasia may be consistent in populations at varying ages after skeletal maturity. Importantly, an exact threshold to determine the true prevalence of hip dysplasia is still debated.[34] Jang et al. recently analyzed the risk of long-term THA in this cohort of patients from the OAI and reported that a modified LCEA <22.4 and a Tönnis angle >10.8 conferred a higher risk of THA. Using these values as a clinically relevant threshold for classifying hip dysplasia, the prevalence of dysplasia associated with THA risk in this cohort would be 12.4% using either the LCEA or Tönnis angle. Jang et al. also reported that a modified LCEA <19.2 conferred an even higher risk of THA, which 4.5% of hips in this cohort had.[35]
It is critical to note that this study used specific definitions of radiographic measurements of hip dysplasia. The modified Sharp’s angle measured the femoral head coverage using the lateral margin of the sourcil, which was the subchondral bony condensation in the acetabular roof.[14] Similarly, the modified LCEA used the lateral margin of the sourcil as previously described by Ogata et al.[16] Many studies have utilized the LCEA described by Wilberg and the Sharp’s angle described by Sharp, which both measure to the lateral edge of the acetabular roof.[13-16] Studies comparing these methods have found that measuring to the edge of the acetabular roof may overestimate coverage and have proposed the lateral edge of the sourcil as a more reliable and clinically relevant landmark.[36, 37] Therefore, this study investigated the modified LCEA and Sharp’s angles, and the reported findings are only relevant to these specific definitions.
In this study, we demonstrated the application and value of a DL tool to provide rapid radiographic measurements of dysplasia. The clinical value of this algorithm is a potential screening tool for mild hip dysplasia in patients undergoing assessment for hip pathology. Critically, patients with hip OA complain of pain that can be a disabling symptom,[1] and radiographs are routinely obtained as part of an orthopaedic evaluation.[22] Although 3-dimensional imaging, such as computed tomography scanning (CT scan) and magnetic resonance imaging (MRI), is available, plain radiographs remain a gold standard for the initial evaluation, and a more economical screening option.[3, 38] Anatomical deformities that can be identified on plain radiographs may represent an important predisposing factor for OA development,[2, 39] and previous studies have shown that hip dysplasia, especially in borderline cases, is often overlooked radiologically and can lead to delayed diagnoses.[18] By having an automated tool quantifying deformities relevant to hip dysplasia even in subtle cases, patients that are predisposed to develop OA may be more readily identified at clinical visits or in large patient registries. This study demonstrated that after setting cutoffs of 22.5 for the modified LCEA, 10.0 for the Tönnis angle, and 40.5 for the modified Sharp’s angle for hip dysplasia, the kappa was 0.88, 0.78, and 0.81, respectively, between the algorithm and observers. Thus, there is potential in leveraging DL for dysplasia screening in the setting of hip pathology. Nonetheless, for this application to be executed properly and further developed, the algorithms must be externally validated, radiographs must be screened for good quality, measurements must be standardized, and the ongoing possibility of measurement error and misinterpretation by the DL algorithm must be kept in mind.
This study had several limitations. First, all cohort analyses of radiographic indices were conducted on automated measurements using a DL algorithm. However, these measurements were all validated in a powered sub-cohort and the final analyses were in concordance with previous studies. Furthermore, most of the OAI radiographs did not exhibit large migration of the femoral head in the acetabulum. Therefore, this algorithm is only relevant to mild dysplasia where the acetabulum and sourcil are radiographically intact. Finally, other indices of hip dysplasia including the alpha angle, the acetabular depth-width ratio, and the femoral head extrusion index were not automated nor analyzed in this cohort but are of future interest.
Conclusion
The development of a radiographic DL algorithm for hip dysplasia parameters provides automation to the evaluation and assessment of an anatomical risk factor for OA. The algorithm demonstrated strong performance and reliability in measuring angles relevant to hip dysplasia, and its application on a large cohort revealed key variations in using these angles to determine hip dysplasia prevalence. Using literature thresholds to classify hip dysplasia with a higher risk for needing eventual THA, the prevalence would be 12.4% in this cohort. Further development and validation of this tool also hold clinical value in allowing for the rapid screening of patients with mild dysplasia.
References
1. Hunter, D.J. and S. Bierma-Zeinstra, Osteoarthritis. The Lancet, 2019. 393(10182): p. 1745-1759.
2. Baker-Lepain, J.C. and N.E. Lane, Relationship between joint shape and the development of osteoarthritis. Current opinion in rheumatology, 2010. 22(5): p. 538-543.
3. Stubbs, A.J., et al., Classic Measures of Hip Dysplasia Do Not Correlate with Three-Dimensional Computer Tomographic Measures and Indices. HIP International, 2011. 21(5): p. 549-558.
4. Gosvig, K.K., et al., Prevalence of Malformations of the Hip Joint and Their Relationship to Sex, Groin Pain, and Risk of Osteoarthritis: A Population-Based Survey. The Journal of Bone and Joint Surgery-American Volume, 2010. 92(5): p. 1162-1169.
5. Jacobsen, S. and S. Sonne-Holm, Hip dysplasia: a significant risk factor for the development of hip osteoarthritis. A cross-sectional survey. Rheumatology, 2005. 44(2): p. 211-218.
6. Engesaeter, I.Ø., et al., Prevalence of radiographic findings associated with hip dysplasia in a population-based cohort of 2081 19-year-old Norweggians. Bone Joint J, 2013(2): p. 95-279.
7. Inoue, K., et al., Prevalence of hip osteoarthritis and acetabular dysplasia in French and Japanese adults, in Rheumatology. 2000. p. 745-748.
8. Laborie, L.B., et al., Radiographic measurements of hip dysplasia at skeletal maturity--new reference intervals based on 2,038 19-year-old Norwegians. Skeletal radiology, 2013. 42(7): p. 925-935.
9. Sharp, I.K., ACETABULAR DYSPLASIA. The Journal of Bone and Joint Surgery. British volume, 1961. 43-B(2): p. 268-272.
10. Tönnis, D., Congenital Dysplasia and Dislocation of the Hip in Children and Adults. 1987: p. 116–121.
11. Tannast, M., et al., What Are the Radiographic Reference Values for Acetabular Under- and Overcoverage? Clinical Orthopaedics & Related Research, 2015. 473(4): p. 1234-1246.
12. Troelsen, A., et al., Assessment of hip dysplasia and osteoarthritis: Variability of different methods. Acta Radiologica, 2010. 51(2): p. 187-193.
13. Wiberg, G., Studies on dysplastic acetabula and congenital
subluxation of the hip joint: with special reference to the complication of
osteoarthritis. Acta Chir Scand, 1939. 83: p. 1-135.
14. Agus, H., et al., How should the acetabular angle of Sharp be measured on a pelvic radiograph? Journal of Pediatric Orthopedics, 2023. 22(2): p. 228-231.
15. Hanson, J., et al., Discrepancies in measuring acetabular coverage: revisiting the anterior and lateral center edge angles. Journal of hip preservation surgery, 2015. 2(3).
16. Ogata, S., et al., Acetabular cover in congenital dislocation of the hip. The Journal of Bone and Joint Surgery. British volume, 1990. 72-B(2): p. 190-196.
17. Clohisy, J.C., et al., Radiographic Evaluation of the Hip has Limited Reliability. Clinical Orthopaedics & Related Research, 2009. 467(3): p. 666-675.
18. Jensen, J., et al., A Deep Learning Algorithm for Radiographic Measurements of the Hip in Adults-A Reliability and Agreement Study. Diagnostics (Basel, Switzerland), 2022. 12(11).
19. Park, H., et al., Diagnostic Performance of a New Convolutional Neural Network Algorithm for Detecting Developmental Dysplasia of the Hip on Anteroposterior Radiographs. Korean journal of radiology, 2021. 22(4).
20. Li, Q., et al., Auxiliary diagnosis of developmental dysplasia of the hip by automated detection of Sharp's angle on standardized anteroposterior pelvic radiographs. Medicine, 2019. 98(52): p. e18500.
21. Lester, G., The Osteoarthritis Initiative: A NIH Public-Private Partnership. HSS J, 2012. 8(1): p. 62-3.
22. Mannava, S., et al., Comprehensive Clinical Evaluation of Femoroacetabular Impingement: Part 2, Plain Radiography. Arthrosc Tech, 2017. 6(5): p. e2003-e2009.
23. Agus, H., et al., How should the acetabular angle of sharp be measured on a pelvic radiograph? Journal of Pediatric Orthopaedics, 2002. 22(2): p. 228-231.
24. Ogata, S., et al., Acetabular cover in congenital dislocation of the hip. J Bone Joint Surg Br, 1990. 72(2): p. 190-6.
25. Rouzrokh, P., et al., A Deep Learning Tool for Automated Radiographic Measurement of Acetabular Component Inclination and Version After Total Hip Arthroplasty. J Arthroplasty, 2021. 36(7): p. 2510-2517 e6.
26. Jang, S.J., et al., John Charnley Award: Deep Learning Prediction of Hip Joint Center on Standard Pelvis Radiographs. J Arthroplasty, 2022.
27. Li, Q., et al., Auxiliary diagnosis of developmental dysplasia of the hip by automated detection of Sharp's angle on standardized anteroposterior pelvic radiographs. Medicine (Baltimore), 2019. 98(52): p. e18500.
28. Karnuta, J.M., et al., Artificial Intelligence for Automated Implant Identification in Total Hip Arthroplasty: A Multicenter External Validation Study Exceeding Two Million Plain Radiographs. The Journal of Arthroplasty, 2022: p. S0883540322002728.
29. Arunachalam, H.B., et al., Viable and necrotic tumor assessment from whole slide images of osteosarcoma using machine-learning and deep-learning models. PLOS ONE, 2019. 14(4): p. e0210706.
30. Tang, H., N. Sun, and S. Shen, Improving Generalization of Deep Learning Models for Diagnostic Pathology by Increasing Variability in Training Data: Experiments on Osteosarcoma Subtypes. Journal of Pathology Informatics, 2021. 12(1): p. 30.
31. Ghasseminia, S., et al., Interobserver Variability of Hip Dysplasia Indices on Sweep Ultrasound for Novices, Experts, and Artificial Intelligence. J Pediatr Orthop, 2022. 42(4): p. e315-e323.
32. Tolpadi, A.A., et al., Deep Learning Predicts Total Knee Replacement from Magnetic Resonance Images. Scientific Reports, 2020. 10(1): p. 6371.
33. Lu, L., et al., Deep learning for the prediction of early on-treatment response in metastatic colorectal cancer from serial medical imaging. Nature Communications, 2021. 12(1): p. 6654.
34. Laborie, L.B., et al., Radiographic measurements of hip dysplasia at skeletal maturity--new reference intervals based on 2,038 19-year-old Norwegians. Skeletal Radiol, 2013. 42(7): p. 925-35.
35. Jang, S.J., et al., An Interpretable Machine Learning Model for Predicting 10-Year Total Hip Arthroplasty Risk. J Arthroplasty, 2023.
36. Omeroglu, H., et al., Measurement of center-edge angle in developmental dysplasia of the hip: a comparison of two methods in patients under 20 years of age. Skeletal radiology, 2002. 31(1).
37. Hanson, J.A., et al., Discrepancies in measuring acetabular coverage: revisiting the anterior and lateral center edge angles. J Hip Preserv Surg, 2015. 2(3): p. 280-6.
38. Philippon, M.J., et al., Joint Space Predicts THA After Hip Arthroscopy in Patients 50 Years and Older. Clinical Orthopaedics & Related Research, 2013. 471(8): p. 2492-2496.
39. Murray, R.O., The Aetiology of Primary Osteoarthritis of the Hip. The British Journal of Radiology, 1965. 38(455): p. 810-824.
Figure Legend
Figure 1: Deep learning segmentation outputs and automated angle measures.
Figure 2: Deep learning algorithm measurement against reader means.
Figure 3: Bland-Altman analysis of measurements against reader means.
Figure 4: Classification matrices for classifying hips as dysplastic or non-dysplastic based on literature-specified thresholds
Figure 5: Histogram of the modified LCEA distribution (n = 7,738 hips)
Figure 6: Histogram of the modified Sharp’s angle distribution (n = 7,738 hips)
Figure 7: Histogram of the Tönnis angle distribution (n = 7,738 hips)
Table 1: Comparison of measurements between algorithm and readers
Table 2: Percentile distribution of dysplasia angles in cohort
Figures
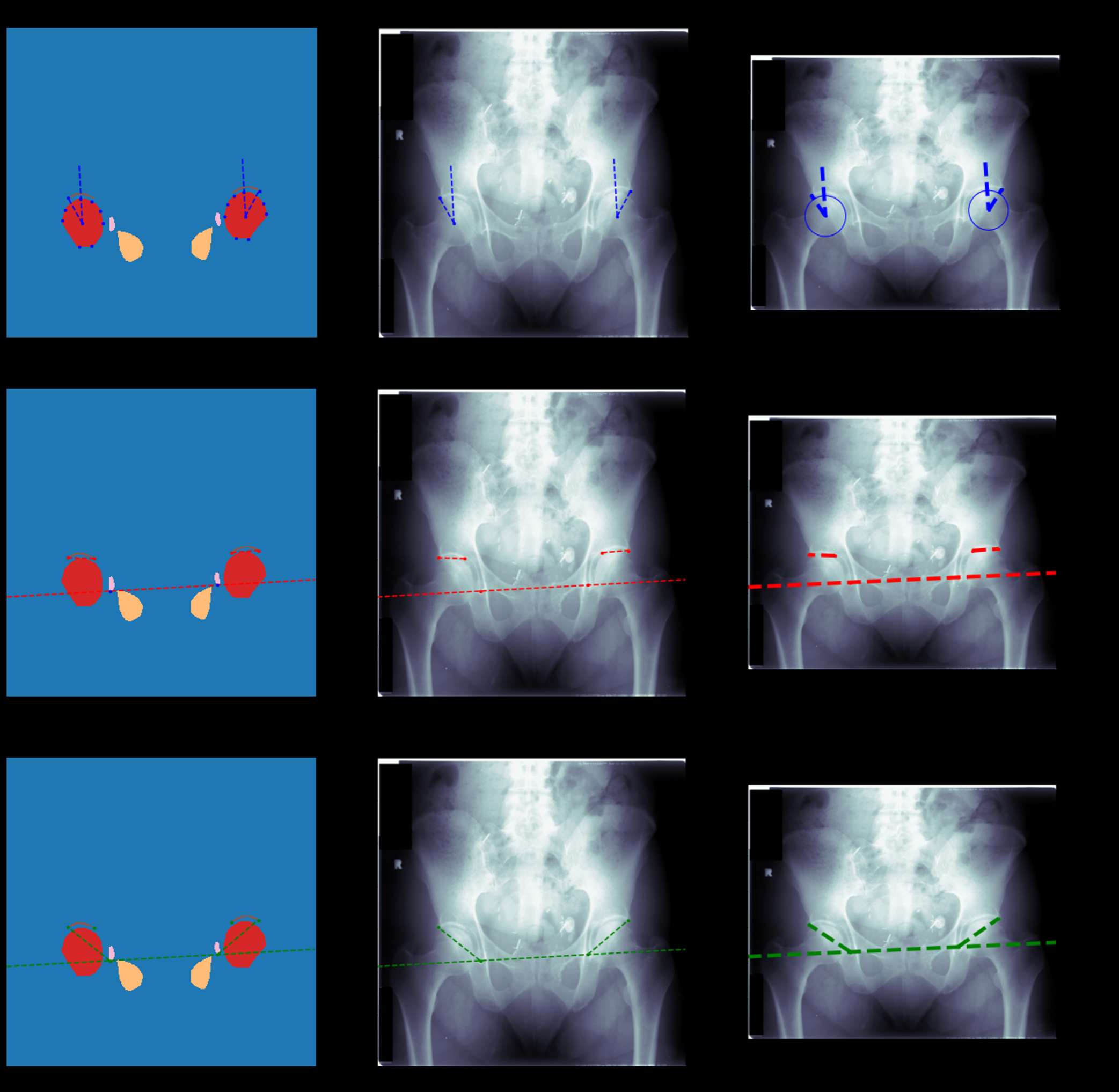
Figure 1
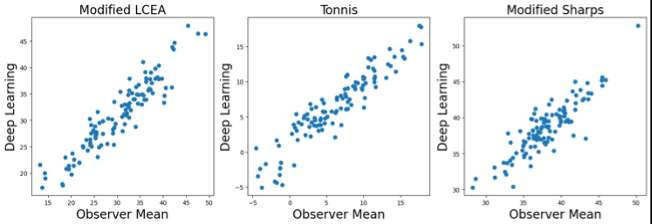
Figure 2
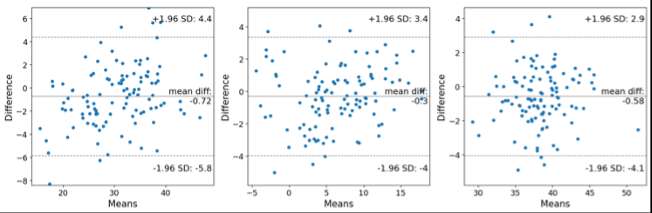
Figure 3
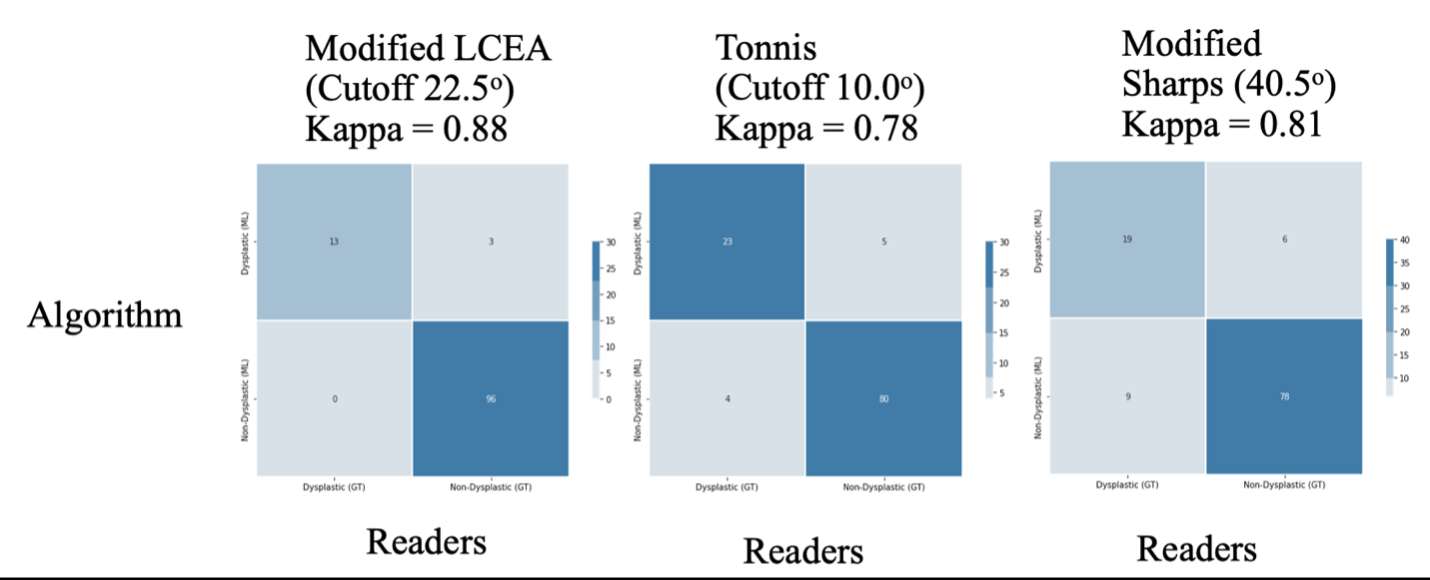
Figure 4
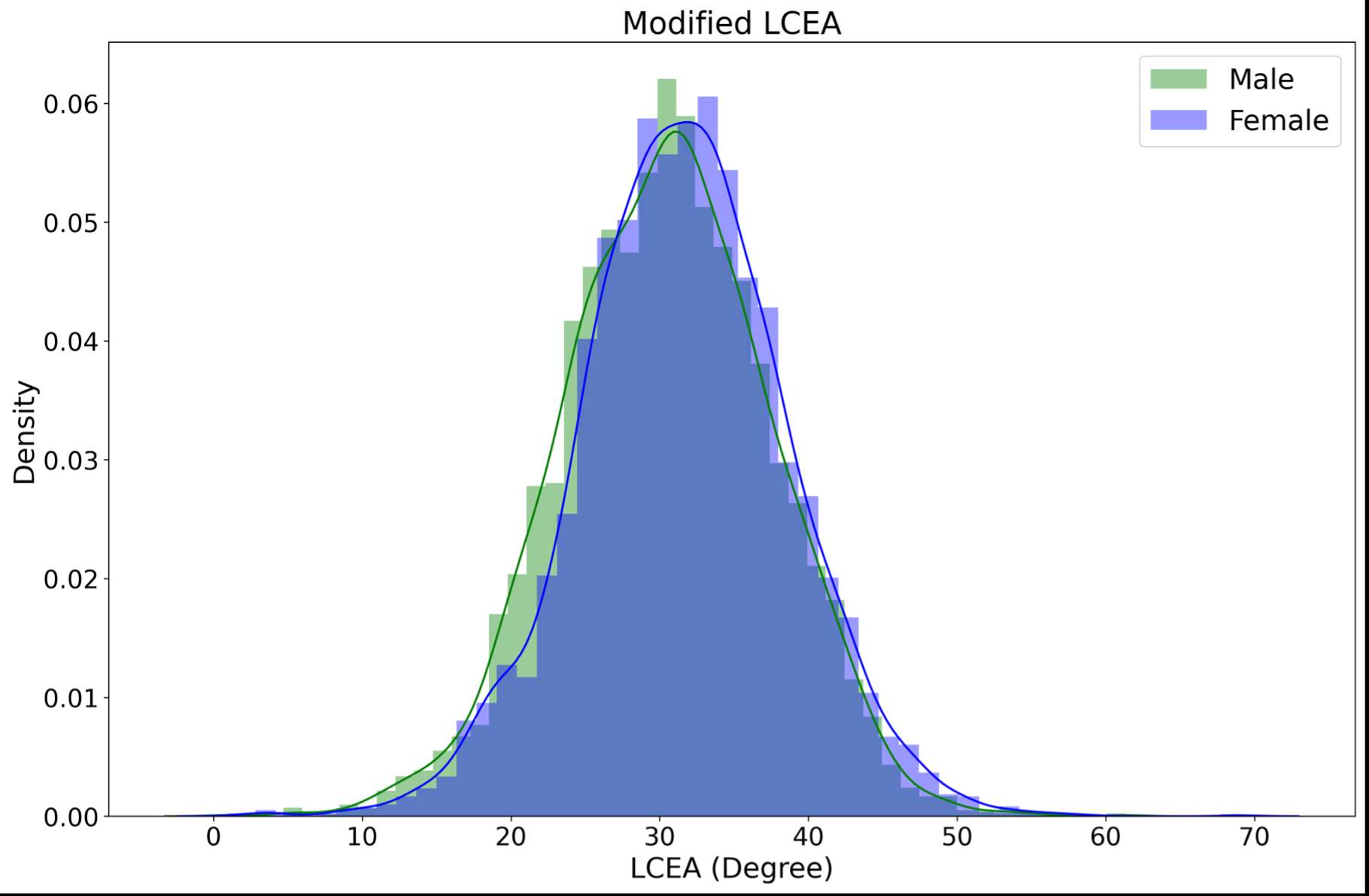
Figure 5
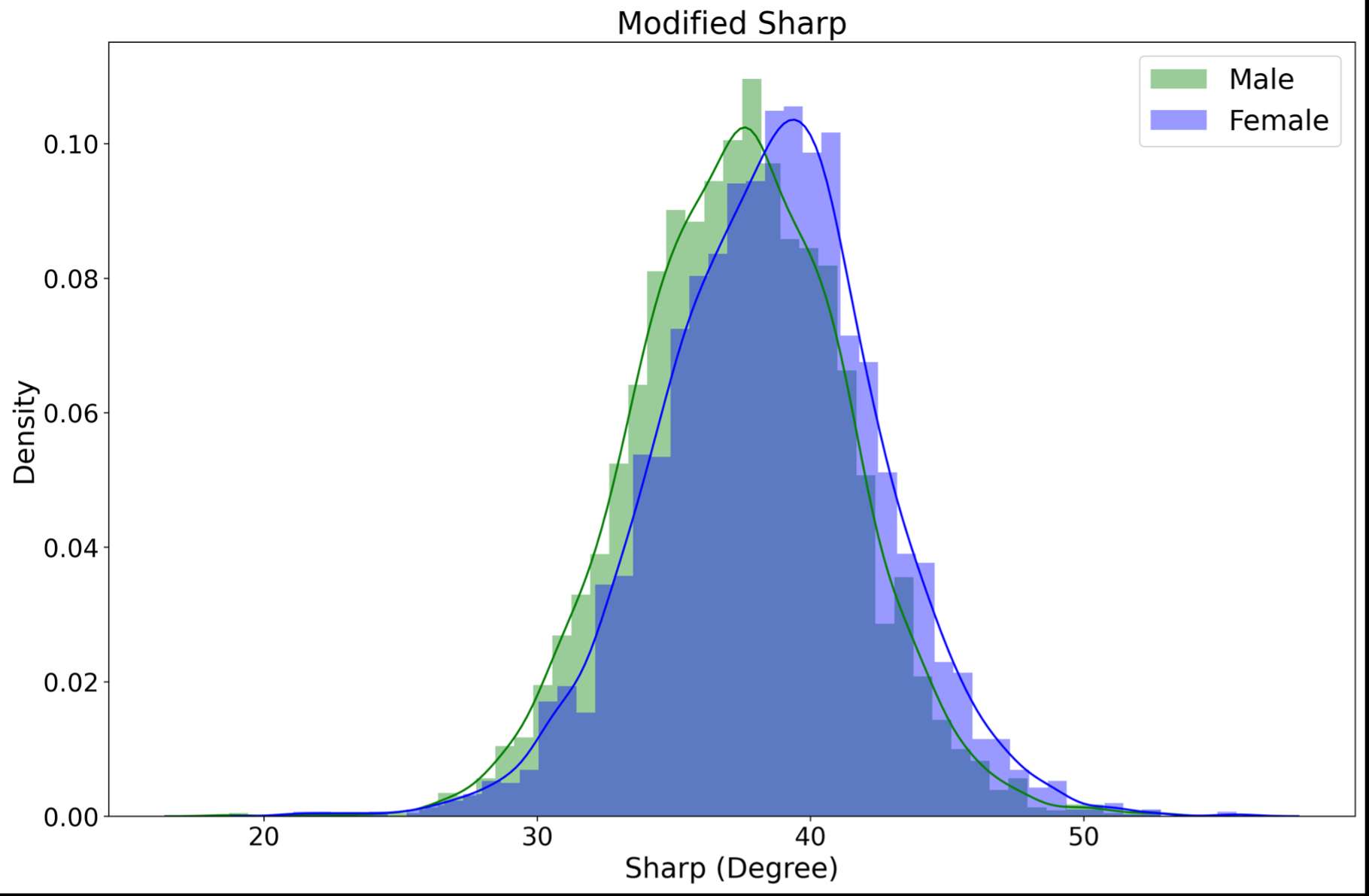
Figure 6
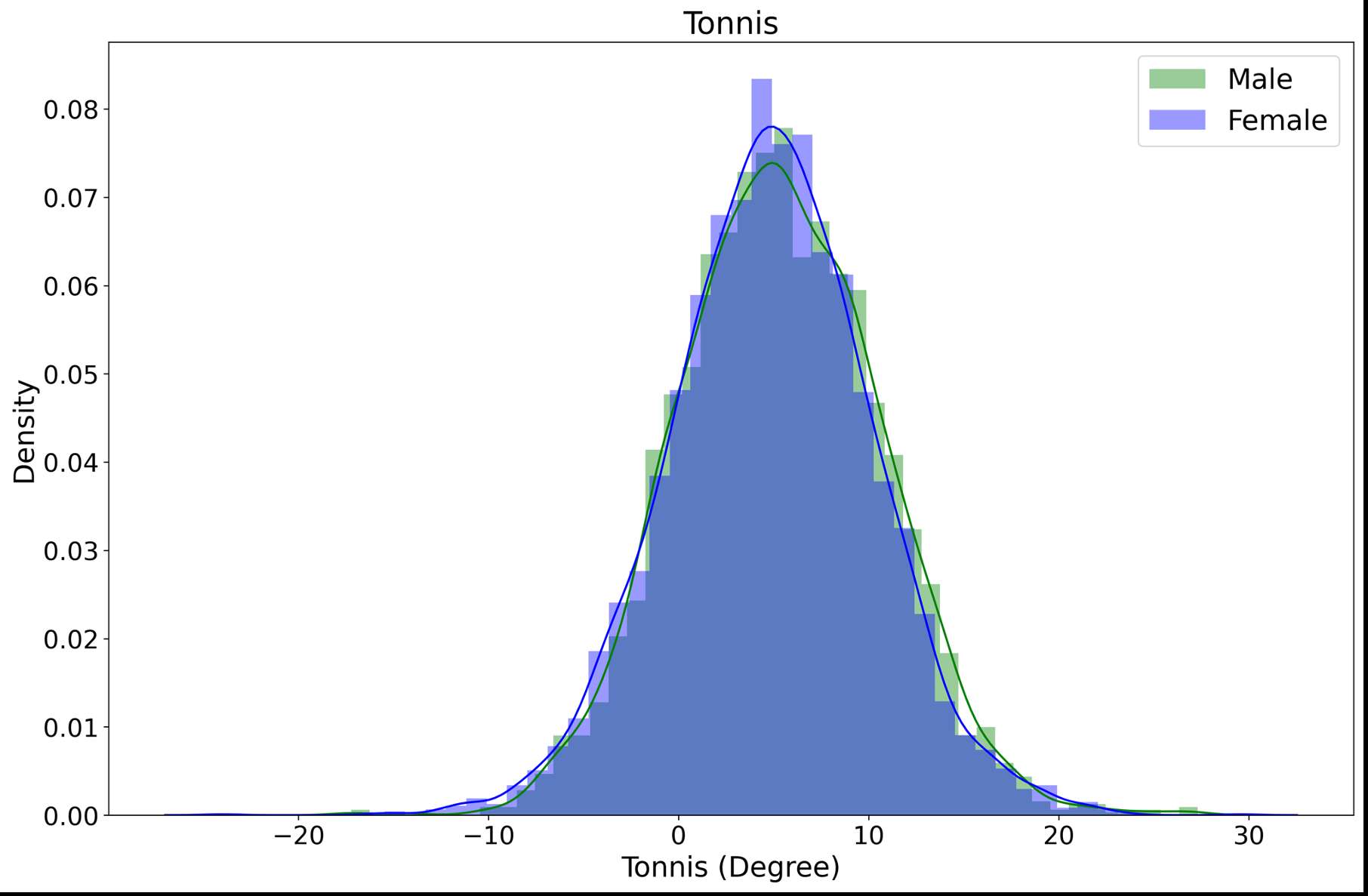
Figure 7
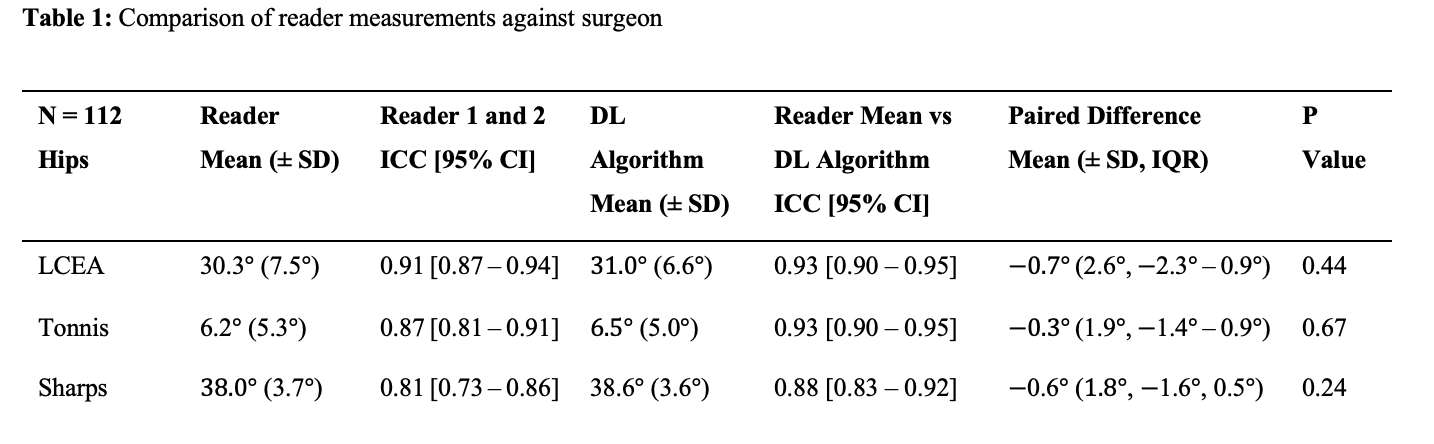
Figure 8
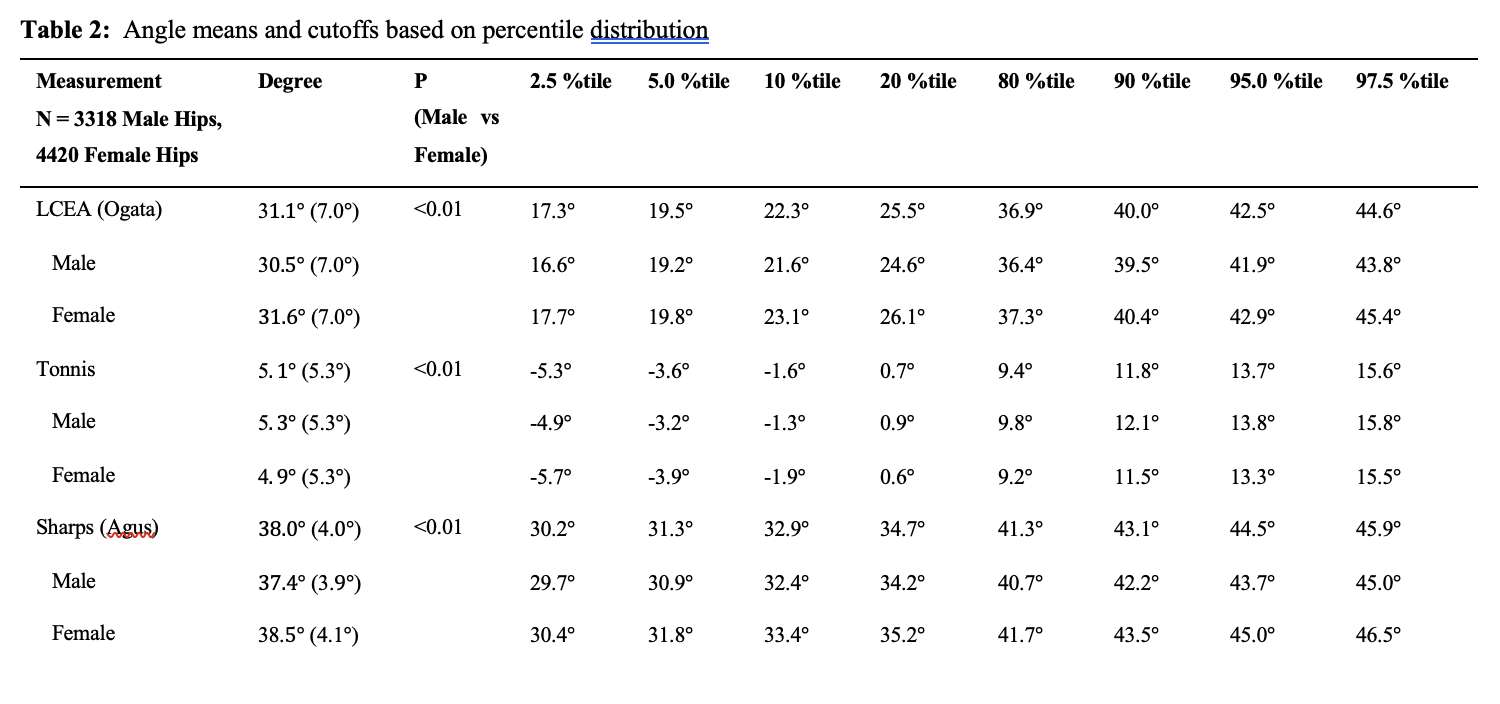
Figure 9#8357
Automated Landmark Detection and Derivation of Anatomic Measurements in Pelvic Anteroposterior Radiographs
*Gerard Smith - Corin - Sydney, Australia
Edgar Wakelin - OMNI Life Sciences - Raynham, USA
David Dewar - Hospital for Special Surgery - New York, USA
Ellie Hobba - University of Wollongong - Wollongong, Australia
Christopher Plaskos - Corin - Raynham, USA
Jim Pierrepont - Corin - Cirencester, United Kingdom
*Email: gerard.smith@coringroup.com
In total hip replacement (THA) Anteroposterior (AP) radiographs and derived measurements are routinely required to provide critical insights for optimal implant positioning and sizing, leg length restoration, and joint stability. However, manual techniques remain time-consuming and prone to variability. This study aims to evaluate the accuracy of a deep learning algorithm for automating orthopaedic parameter calculations by extracting landmarks from AP radiographs.
A U-Net deep learning segmentation algorithm was developed by leveraging an EfficientNet backbone and 297 calibrated AP radiographs, manually segmented by a skilled engineer. This process yielded segmentation masks highlighting anatomical regions of interest, which were employed to instruct the model. The masks were then used to define landmark positions on the AP radiographs and subsequent computation of orthopaedic measurements. During model development, images are divided into training (70%), validation (20%), and testing (10%) sets, resizing them to 512x512 pixels. To enhance model performance and generalizability, image augmentation techniques were incorporated in model training. Augmentations included transformative procedures (scaling, translation, and rotation) and content adjustments (Gaussian noise, brightness, and contrast). Leg length discrepancy was assessed using three reference lines relative to the lesser trochanter: 1) obturator foramen, 2) teardrop, and 3) ischia. The femoral axis was defined using proximal femoral diaphysis and distal femoral canal landmarks. The accuracy of the algorithm was evaluated in terms of intersection over union (IOU) of the segmented regions and mean absolute error (MAE) between the predicted landmarks and the ground truth. The error in offset, leg length discrepancy, and caput-collum-diaphyseal (CCD) angle was also compared.
The model produced an average IOU value of 0.85 for all segmentation masks. An example output of an AP radiograph with predicted masks is shown in Figure 1. The MAE±standard-deviation for the predicted landmarks compared to the ground truth is summarised in Table 1 and error in acetabular and femoral offset, LLD, and CCD angle is summarised in Table 2. The largest prediction errors were associated with the Ischial (2.1mm) and Proximal Diaphysis (3.1mm) landmarks. However, all remaining landmarks demonstrated a mean error under 2mm. Despite these larger errors, the CCD (1.7°), femoral offset (1.5mm), and LLD (2.2mm) measurements were not highly affected as the inaccuracy occurred along axes that do not impact the corresponding measurements - y axis for femoral landmarks and x-axis for ischial landmarks. On average, the model delivered inferences within 250 milliseconds.
The deep learning algorithm shows promise as an efficient and accurate tool for defining landmarks in AP radiographs and automating the calculation of clinically relevant parameters. Future research should focus on further validation of the algorithm in larger and more diverse patient populations.
Figures
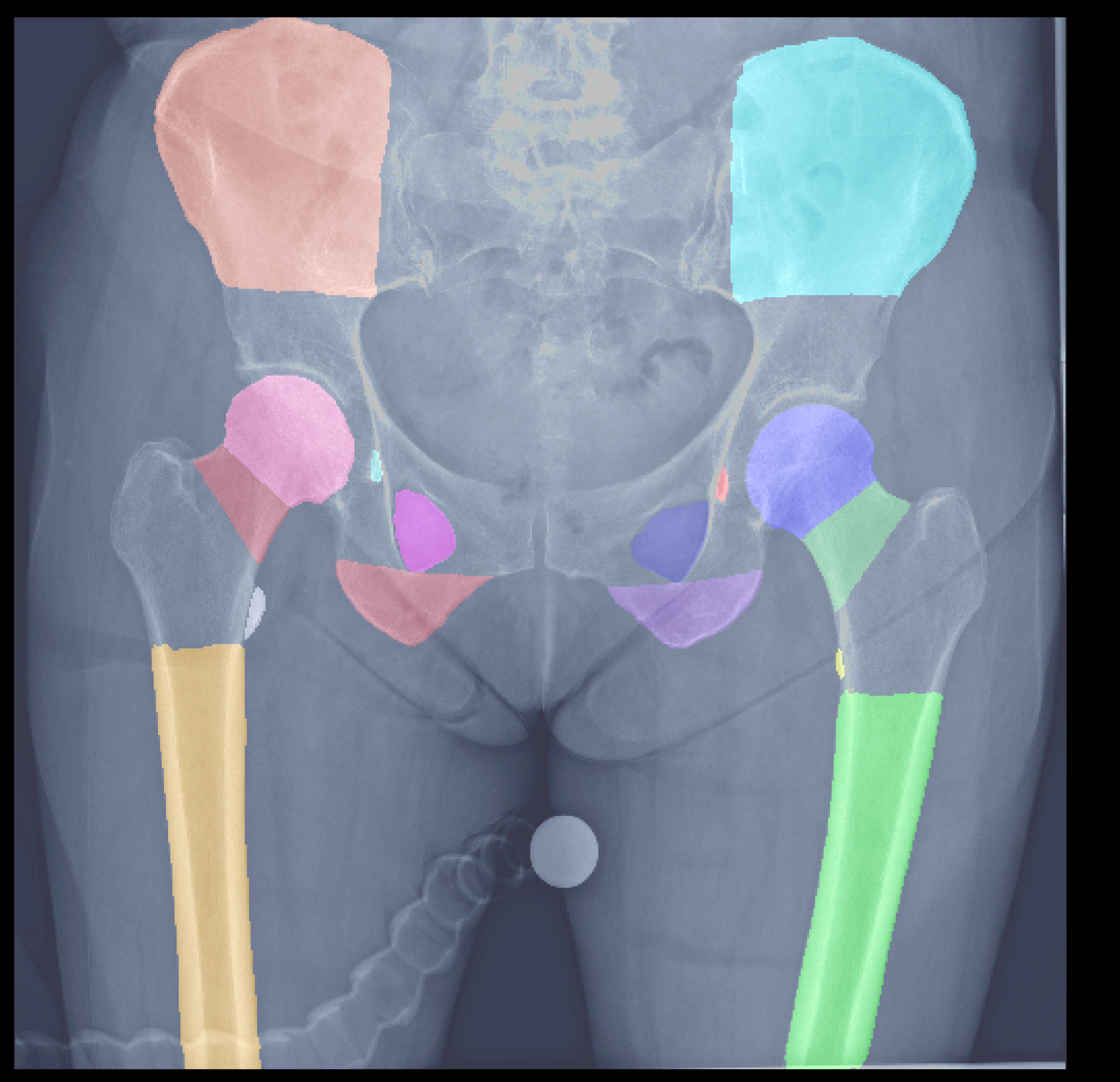
Figure 1
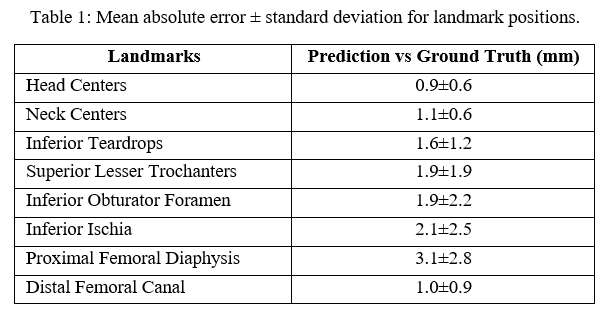
Figure 2
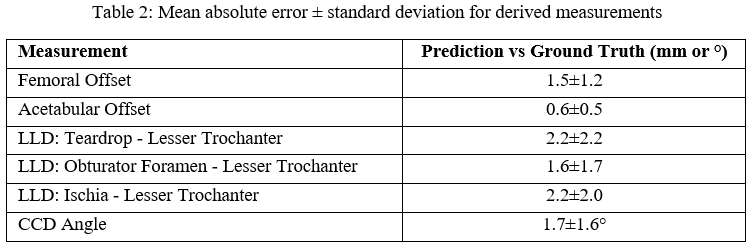
Figure 3#8780
Autonomous Extraction of Stifle Joint Kinematics From Single-Plane Fluoroscopic Images
*Sasank Desaraju - University of Florida - Gainesville, United States of America
Andrew Jensen - University of Florida - Gainesville, USA
Scott Banks - University of Florida - Gainesville, USA
Selena Tinga - Cornell University - Ithaca, USA
Stephen Jones - University of Florida - Gainesville, USA
*Email: sasank.desaraju@ufl.edu
Introduction: Many patients, nearly 20%, who undergo total knee arthroplasty are dissatisfied with their new knees [1].
Understanding pre-operative kinematics can help orthopedists plan surgical intervention that optimizes patient satisfaction, perhaps through accurately reproducing native kinematics. Additionally, comparison between pre-op and post-op kinematics can help determine better surgical techniques and implants.
Purpose/Aim of Study: Our study aims to develop and test a computational pipeline to autonomously extract tibiofemoral joint kinematics information from fluoroscopy images of canine stifles given surface mesh models of the bones.
Materials and Methods: The dataset images consists of 2,079 images of 10 canine patients walking on a treadmill before and after a tibial plateau leveling osteotomy of one leg. Gait cycles of pre-op, contralateral, and post-op trials were manually annotated to create 6-degree-of-freedom kinematics for one leg (femur and tibia) in each frame. One patient’s data has been fully withheld from training the neural network and was termed the “naive” set. The rest of the data has been split randomly into training, validation, and test sets.
The pipeline first estimates conserved keypoints on the femur and tibia using a convolutional neural network. The image keypoints are then compared to the 3D location of the keypoints on the femur and tibia bone models (Figure 1). The resulting perspective-n-point problem is solved analytically to estimate the poses of the femur and tibia. Finally, the transformation between the femur and tibia is calculated to find the joint kinematics.
Results/Findings: The perspective-n-point solver has been developed and validated using ground-truth keypoint data. Development of the deep neural network models for keypoint prediction is currently in progress. The current iteration uses one model for the femur and one model for the tibia. Both models use the Resnet-152 convolutional neural network architecture with pretrained weights from PyTorch.
Discussion: Figure 1 shows an X-ray image with ground-truth keypoints, one bone projection in blue, and an overlaid bone projection in magenta. The blue projection is ground-truth kinematics from manual annotation whereas the magenta is derived from the ground-truth keypoints. The close overlap of the two projections suggests that the pipeline’s accuracy will depend on the accuracy of the neural network predictions of the keypoint locations.
Conclusion: Novel methods are being introduced and evaluated for extracting joint kinematics from single-plane fluoroscopic images for pre-operative kinematics assessments.
References:
[1] Scott, C. E. H., Howie, C. R., MacDonald, D. & Biant, L. C. Predicting Dissatisfaction Following Total Knee Replacement: A Prospective Study of 1217 Patients. The Journal of Bone and Joint Surgery. British volume 92-B, 1253–1258 (2010).
[2] Paris D. L. Flood and Scott A. Banks, Automated Registration of 3-D Knee Implant Models to Fluoroscopic Images Using Lipschitzian Optimization, IEEE Transactions on Medical Imaging 37 (2018), no. 1, 326–335.
[3] M.R. Mahfouz, W.A. Hoff, R.D. Komistek, and D.A. Dennis, A robust method for registration of three-dimensional knee implant models to two-dimensional fluoroscopy images, IEEE Transactions on Medical Imaging 22 (2003), no. 12, 1561–1574.
Figures
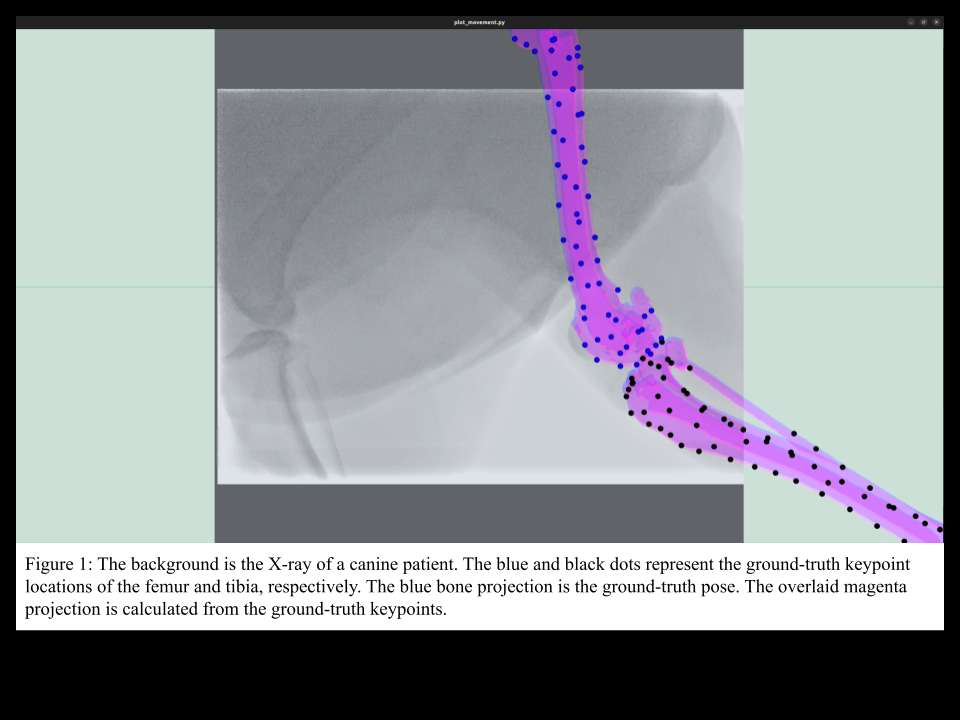
Figure 1#8191
An Artificial Intelligence Chatbot Is an Accurate and Useful Online Patient Resource Prior to Total Knee Arthroplasty
*Walter Taylor - Hospital for Special Surgery - New York, NY, United States of America
Ryan Cheng - Hospital for Special Surgery - New York, USA
Aaron Weinblatt - Hospital for Special Surgery - New York, USA
William Long - NYU Langone Health - New York, USA
*Email: taylorivw@hss.edu
Introduction: Online information is a useful resource for patients seeking advice on their orthopaedic care. However, variable information quality and biased advertisement-driven guidance on the internet have raised serious concerns among researchers investigating online health information-seeking behavior. While traditional websites provide responses to specific frequently asked questions (FAQs), sophisticated artificial intelligence (AI) tools may be able to provide the same information to patients in a more accessible, relevant, accurate manner. Chat Generative Pre-trained Transformer (ChatGPT) is a powerful AI chatbot that has been shown to effectively draw on its large reserves of information in a conversational context with a user. The purpose of this study was to assess the accuracy and reliability of ChatGPT-generated responses to FAQs regarding total knee arthroplasty (TKA).
Methods: We distributed a survey that challenged arthroplasty surgeons to identify which of two responses to FAQs on our institution’s website was human-written and which was generated by ChatGPT (Figure 1). All questions were TKA-related. The second portion of the survey investigated the potential to further leverage ChatGPT to assist with translation and accessibility as a means to better meet the needs of our diverse patient population (Table 1).
Results: Surgeons correctly identified the ChatGPT-generated responses 4 out of 10 times (range: 0-7). No consensus was reached on any of the responses to the FAQs (Table 2). Additionally, over 90% of our surgeons strongly encouraged the use of ChatGPT to more effectively accommodate the diverse patient populations that seek information from our hospital’s online resources (Table 3).
Conclusion: ChatGPT provided accurate, reliable answers to our website’s FAQs. Surgeons also agreed that ChatGPT’s ability to provide targeted, language-specific responses to FAQs would be of benefit to our diverse patient population. These findings suggest a potential use-case for ChatGPT which has yet to be explored in the literature: the tool’s use as an effective clinical communicator. Further research is warranted to examine ChatGPT’s application in this clinical context and as a potential tool to improve the accessibility of existing patient resources.
Keywords: ChatGPT; artificial intelligence; patient education; total knee arthroplasty; health equity; eHealth literacy
Figures

Figure 1
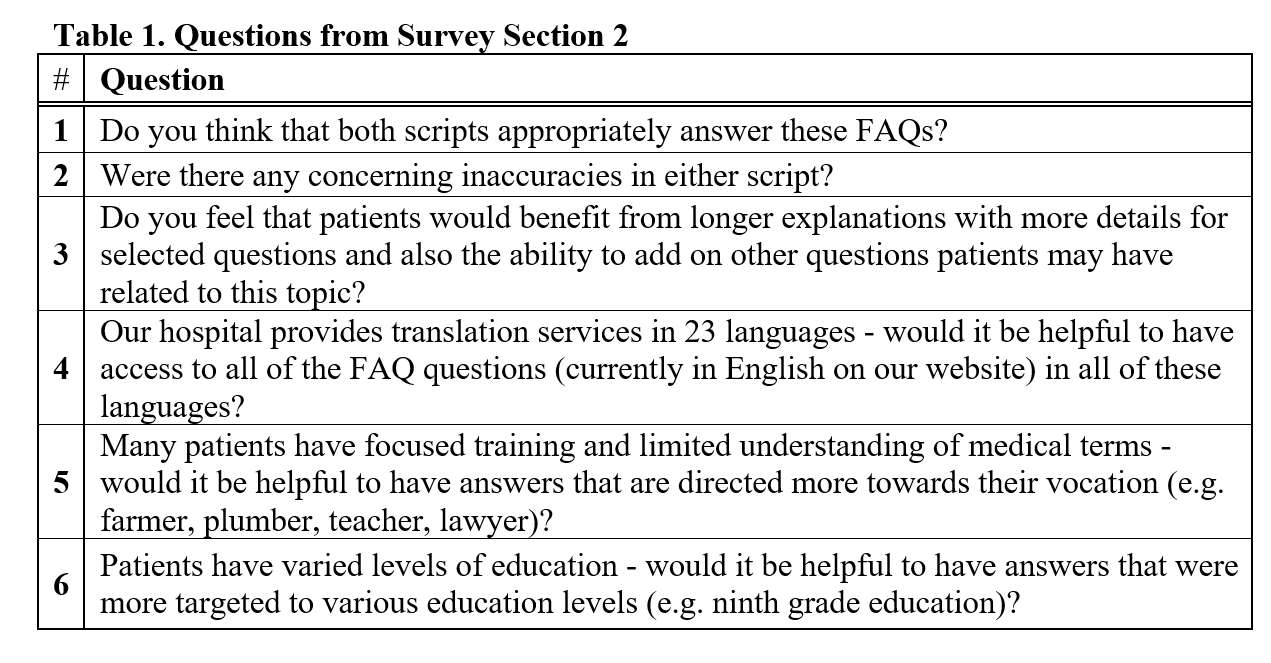
Figure 2
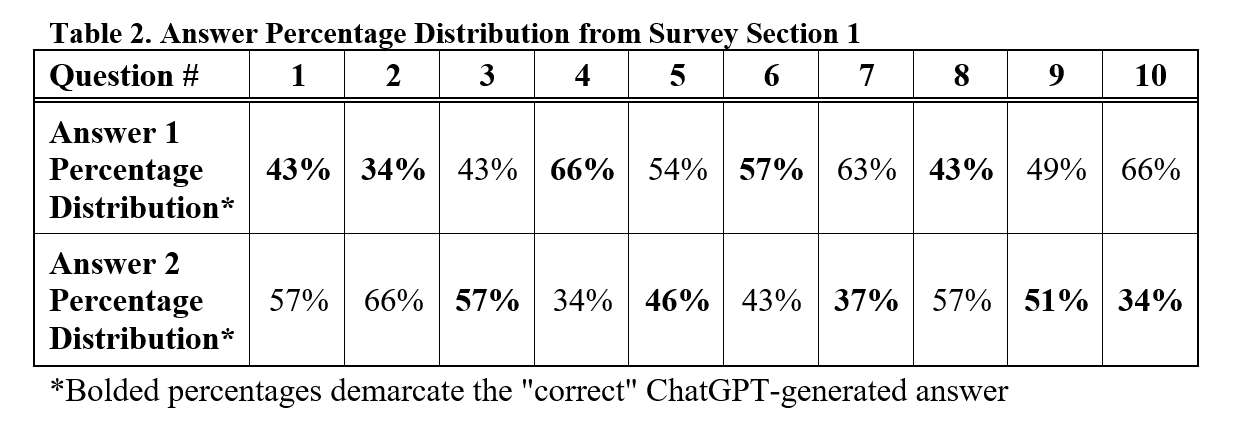
Figure 3
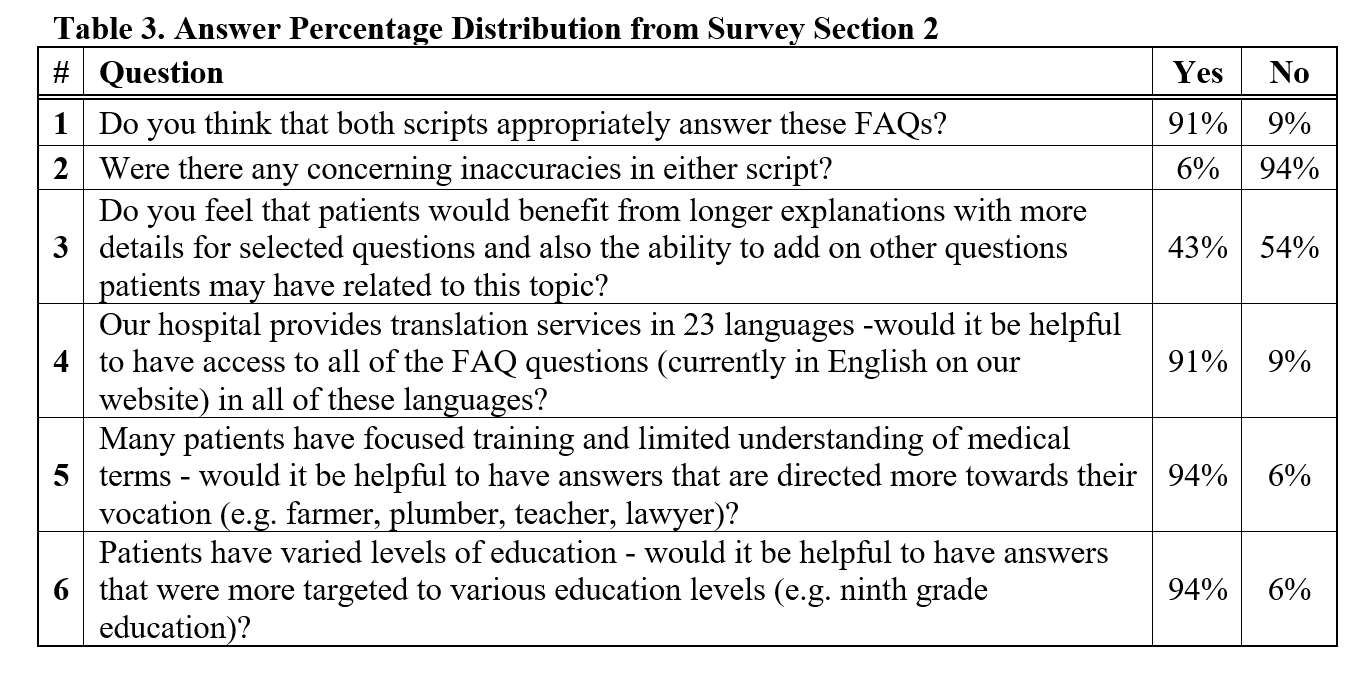
Figure 4#8220
AI Detection of Pain From Facial Expression – the New PROMS?
*Ishaan Jagota - 360 Med Care - Sydney, Australia
Joshua Twiggs - University of Sydney - Sydney, Australia
Brad Miles - 360 Knee Systems - Pymble, Australia
Justin Roe - North Sydney Orthopaedic and Sports Medicine Centre - Sydney, Australia
Frances Yu - 360 Med Care - Pymble, Australia
Qipeng Shen - 360 Med Care - Epping, Australia
*Email: ishaan.jagota@360med.care
Introduction:
Some level of adverse post-operative knee pain has been reported to occur in 10-30% of all TKAs. Despite advances in pain management, it remains a challenge to accurately measure and manage pain in these patients. Precise identification of where and how pain is occurring is crucial to identifying further intervention that might improve patient outcomes. Current pain measurement is primarily undertaken with Patient-Reported Outcome Measures (PROMs), which lack precision when considering specific functional motions that provoke pain. In this pilot study, our aim is to develop an artificial intelligence (AI) pain detection model using facial analysis to improve pain assessment.
Method:
The study involved the use of OpenCV and MediaPipe Face Mesh to create a face tracking model that can isolate the mesh of a person's face from an image and real-time video. The dataset was collected from Pain E-motion Faces Database (PEMF) of individuals who undertook pain experiments including laser and algometer-evoked pain. Two models were created using convolutional neural network (CNN) models. The first model detected the presence of pain (binary) and the second model focused on measuring the intensity of pain on a scale of 0 to 10. In addition, a series of practical use case scenarios using a standard phone camera was attempted in which the subject’s face was tracked during a series of functional motions such as sit to stand and chair-based knee flexion. A summary of the workflow is outlined in Figure 1.
Results:
The preliminary AI model for detecting the presence of pain achieved an accuracy of ~70% on the test set under an 80/20 training/testing split. During practical use case testing, the model successfully identified moments of subject-simulated pain expressions associated with specific points in the action cycle (initial weightbearing on sit to stand and straightening the knee out when chair based). The second model for detecting pain intensity provided meaningful results but requires field testing & validation with patients experiencing actual pain to compare to survey or other pain responses.
Conclusion:
This pilot study has demonstrated the potential of using facial expressions for the detection and measurement of pain through the development of an AI pain detection model. The models developed in this study were able to detect the presence of pain and measure its intensity in clinically meaningful use cases. Further clinical research is needed to validate its effectiveness and feasibility in different patient populations during clinical assessment. Following this validation, the AI pain detection model developed in this study could be integrated into routine clinical practice to provide better pain management and intervention planning for patients.
Figures
Figure 1#8464
Improving Care Efficiency in Adult Reconstruction Clinics: Validation of a 9-Question Survey for Diagnosing Knee Osteoarthritis
Drew Clippert - Brown University/ University Orthopedics - Providence, United States of America
Caitlin Barrett - University Orthopedics - Providence, USA
*Valentin Antoci - University Orthopedics - Providence, USA
Jacob Laperche - Frank H. Netter School of Medicine Quinnipiac University - Middletown, United States of America
*Email: valentin.antoci@gmail.com
Introduction: Within the field of adult reconstruction, there is an increasing demand for care related to joint pain as the United States population continues to age and life expectancy grows older. Despite this substantial increase in patient population, the rate of fellowship trained adult reconstruction surgeons is growing more steadily. Therefore, it is not only important for the patients but also for the surgeons to utilize clinic time efficiently. Multiple studies have shown that patient satisfaction with care is strongly correlated to in-office wait times, with one study indicating it as the strongest predictor of overall satisfaction. To improve patient quality of care and to reduce clinical burden on adult reconstruction surgeons, we devised a 9-question survey provided to patients along with traditional intake paperwork to assess the chief complaint and previous interventions as a way to increase care efficiency in the clinic and improve surgeon planning.
Methods: New patients with knee pain who had no history of prior joint replacement were provided with a 9-question choice multiple survey along with standard intake paperwork at the beginning of their visit. This survey and the questions utilized were developed in conjunction with the attending adult reconstruction surgeon whose clinic they were administered in, and this study aims at validation of these questions as predictors for care. After completion of the survey, points were awarded to each question answer as either 0, 1, or 2 depending on the response and total point scores along with individual question scores were recorded. Additionally, patient diagnosis code (patellofemoral, knee osteoarthritis, internal derangement) and surgeon decisions regarding the next steps in care were recorded. Statistical analysis of the mean difference was performed with a p-value of .05 for significance.
Results: Analysis showed that the survey was able to accurately differentiate knee osteoarthritis from a diagnosis of patellofemoral or internal derangement based upon cumulative score. Patients who were coded as having knee osteoarthritis had an average total survey score 2.707 + .541 and 1.592 + .522 points higher than those coded for patellofemoral or internal derangement of the knee, respectively.
Conclusion: In this study, we found that our 9-question survey was able to accurately distinguish between patients with knee osteoarthritis and those with other conditions, which could greatly benefit adult reconstruction clinics. The survey proved to be an effective tool in assessing the patients' chief complaints and previous interventions and providing an initial osteoarthritis diagnosis with accuracy. With the rise of artificial intelligence and machine learning in the field of medicine, survey utilization may become increasingly important for optimizing patient care. These surveys provide a reliable, standardized, and efficient way to gather important information from patients, which could improve patient satisfaction and overall quality of care provided by adult reconstruction surgeons.
#8351
Assessing Ability for ChatGPT to Answer Total Knee Arthroplasty-Related Questions
*Matthew Magruder - Maimonides Medical Center - Brooklyn, USA
Ariel Rodriguez - Maimonides Medical Center - Brooklyn, United States of America
Orry Erez - Maimonides Medical Center - Brooklyn, USA
Che Hang Jason Wong - Maimonides Medical Center - Brooklyn, USA
Nicolas Piuzzi - Cleveland Clinic - Cleveland, USA
Peter Sculco - Hospital for Special Surgery - New York, USA
Michael Mont - Sinai Hospital of Baltimore - Baltimore, USA
*Email: mmagruder@maimonidesmed.org
Introduction
The ability of artificial intelligence (AI) to assist providers in the field of orthopaedics has been a topic of increasing interest in recent years. Applications for AI in arthroplasty are widespread and include use in clinical decision making, in the operating room and in research. In this study, we aim to assess the quality of ChatGPT answers when asked questions related to total knee arthroplasty (TKA).
Methods
ChatGPT prompts were created by turning 15 of the AAOS Clinical Practice Guidelines into questions. All prompts were conducted in isolation of one another (as to not bias ChatGPT answers). A survey was created using Google Forms which included screenshots of each prompt and answer for the 15 questions. Surgeons were asked to grade ChatGPT answers from 1-5 based on 6 answer characteristics: 1) Relevance; 2) Accuracy; 3) Clarity; 4) Completeness; 5) Evidence-based; 6) Consistency. All prompts were prefaced with the AAOS Clinical Practice Guideline from which it was derived so surgeons could answer as accurately as possible. Three surgeons who completed Adult Joint Reconstruction fellowships completed the survey. Questions were subclassified into groups based on subject of the prompt: 1) Risk factors: effect of preoperative risk factors on postoperative outcomes; 2) Implant/Intraoperative: implant and intraoperative decision making, and 3) Pain/Functional Outcome: Interventions that affect postoperative pain and functional outcomes. Average and standard deviation for all answers, as well for each subgroup were calculated. Inter-rater reliability (IRR) was also calculated. Statistical analysis was completed using Excel (Microsoft, Washington, U.S.).
Results
All answer characteristics were graded as being above average (i.e. a score > 3). The answer characteristic with the highest grade was relevance (4.11 ± 0.95), followed by clarity (3.96 ± 0.96), evidence-based (3.91 ± 1.01), accuracy (3.76 ± 1.00), consistency (3.62 ± 1.09) and completeness (3.60 ± 1.00) (Table 1). Implant/Intraop subcategory demonstrated overall lowest grades across all answer characteristics, with no characteristic being higher than 4 out of 5. Risk factors and Implant/Intraop subcategories were similar in overall grade. IRR was calculated to be 0.24.
Discussion
Our preliminary investigation demonstrates that ChatGPT can answer questions regarding the well-established clinical guidelines in total knee arthroplasty with above-average veracity. Further work is required to determine the extent to which this natural processing AI can provide high quality responses regarding arthroplasty and how it could be used by surgeons.
Figures
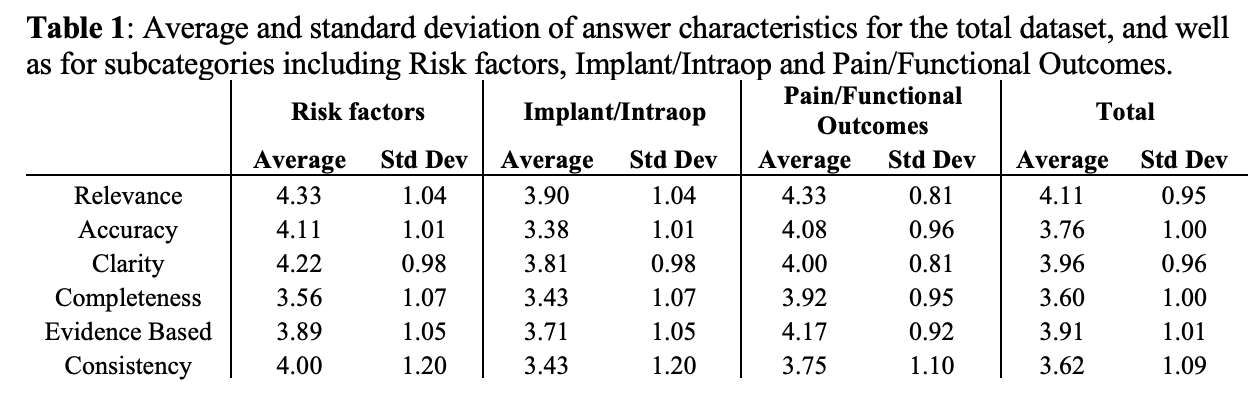
Figure 1#8696
Evaluating Pelvic Parameters Before and After the Medial Open Wedge High Tibial Osteotomy Using EOS System
*Byung Hak Oh - Konyang University Hospital - Daejeon, South Korea
*Email: sebslab@hanmail.net
INTRODUCTION: Medial Open Wedge High tibial osteotomy (MOWHTO) can lead to unintended leg lengthening effects, which may in turn affect the parameters and alignment of the pelvis and spine. Although some studies have analyzed pre- and post-operative changes in certain parameters for patients who underwent MOWHTO with plain radiographs, only few studies have employed the EOS system as a diagnostic method. The aim of this study was to examine the effect of MOWHTO on pelvic parameters by comparing the pre- and post-operative pelvic alignment of patients with knee osteoarthritis (OA) to that of healthy young individuals utilizing EOS system.
MATERIALS & METHOD: Between October 2019 to May 2021, a total of 31 patients who treated with MOWHTO for knee OA and control groups of 101 healthy young individuals without a history of trauma or pain in the knee, pelvis, or spine were enrolled. Using EOS system, changes of pelvic parameters before and after surgery were measured and compared to that of healthy young individuals including pelvic incidence (PI), sacral slope (SS), sagittal pelvic tilt (SPT), lateral pelvic tilt (LPT), pelvis axial rotation (PAR).
RESULTS: No significant differences were observed in PI(p=0.160), SS(p=0.639), LPT(p=0.498), PAR(p=0.447) between pre- and post-operative groups. However, SPT between pre- and post-operative groups (P=0.021) and pre-operative and control groups (P=0.028) changed significantly and SPT between post-operative groups and control groups (p=0.165) showed no significant differences. The mean values for SPT were 15.00±9.29 for pre-operative, 13.36±7.85 for post-operative and 11.18±6.37 for control groups.
CONCLUSIONS: MOWHTO changes no significant differences in pelvic parameters measured by EOS system except SPT. The SPT values of patients who underwent MOWHTO surgery were closer to those of healthy young individuals, indicating MOWHTO might help reducing abnormalities that can lead to spinal problems.
#8606
Phantomless Density Calibration Compared Against Mindways Model 3
*Nathaniel Pyle - DJO Surgical - Austin, USA
Carla Winsor - University of Wisconsin - Madison, USA
Bryan Kirking - Enovis - Austin, USA
Perry J Pickhardt - UW Madison - Madison, USA
*Email: nathaniel.pyle@enovis.com
Introduction
CT data can be used to estimate bone mineral density (BMD) models of human anatomy, with voxel-specific or element-specific material properties. In an era of patient-specific implants, accurate BMD metrics will be needed for custom implant design and long-term monitoring. The gold standard method is to include a calibration phantom consisting of cylinders with known densities in the imaging volume. However, many CT scans are executed without the inclusion of a calibration phantom and including the phantom can introduce imaging artifacts. This has driven a research effort to develop means of establishing quantitative bone density (QCT) from CT images without a phantom. Many phantom-less variants have been attempted and even standardized (ASTM E1935-97:2019). In this investigation, the output of two different phantomless methods is compared calibration curves from a Mindways Model 3 BMD Phantom for 8 different patients. Three of the 8 patients included had metal hardware in the scan.
The phantomless methods implemented and assessed are the “Traditional” method and the “ASTM” method. Each method relies on segmenting in-scan materials, such as air, muscle, fat and/or blood from the scan. In the Traditional method, a regression line is fit against the segmented Houndsfield (HU) values and assumed densities. In the ASTM method, the assumed densities are corrected using a scan-specific effective energy estimate that is determined using the linear attenuation of the segmented materials.
Methods
CT scans of 8 patients were segmented using Materialise Mimics. Segmentation masks of the femur, skeletal muscle, fat, air, and aortic blood were created (Figure 1). Patient-specific calibration curves were defined using the phantom, Traditional phantomless and ASTM phantomless methods using all possible material combinations of 3 or more of the segmented reference materials. Each femur was meshed with a tetrahedral mesh and element specific densities were mapped to the mesh using the BoneMat v3 algorithm. The BoneMat v3 algorithm was rewritten in Python 3 for this analysis. From each mapped femur mesh, the average density was calculated for all calibration approaches. Bland-Altman plots were generated for all calibration method/material set composite BMD results, using the Mindways Model 3 BMD as the reference.
Results and Discussion
The method-material combination with the least RMS error was the Traditional method using Air, Adipose and Blood as reference tissues (Figure 2). However, if this method is used to estimate cortical BMD, it will likely underestimate the density. The slope of the calibration curve does not match that of the Mindways Model 3 BMD. The composite BMD values match well because there are significantly more low-density cancellous elements than cortical in the femur meshes. The method-material combination with the closest slope to that of the Mindways Model 3 was the ASTM method using all 5 reference materials (Air, Adipose, Blood, Skeletal Muscle, Cortical Bone), but there was a significant bias between those result lines (Figure 3). Additionally, the three patients with metal included did not seem to affect the robustness of the phantomless methods.
Figures
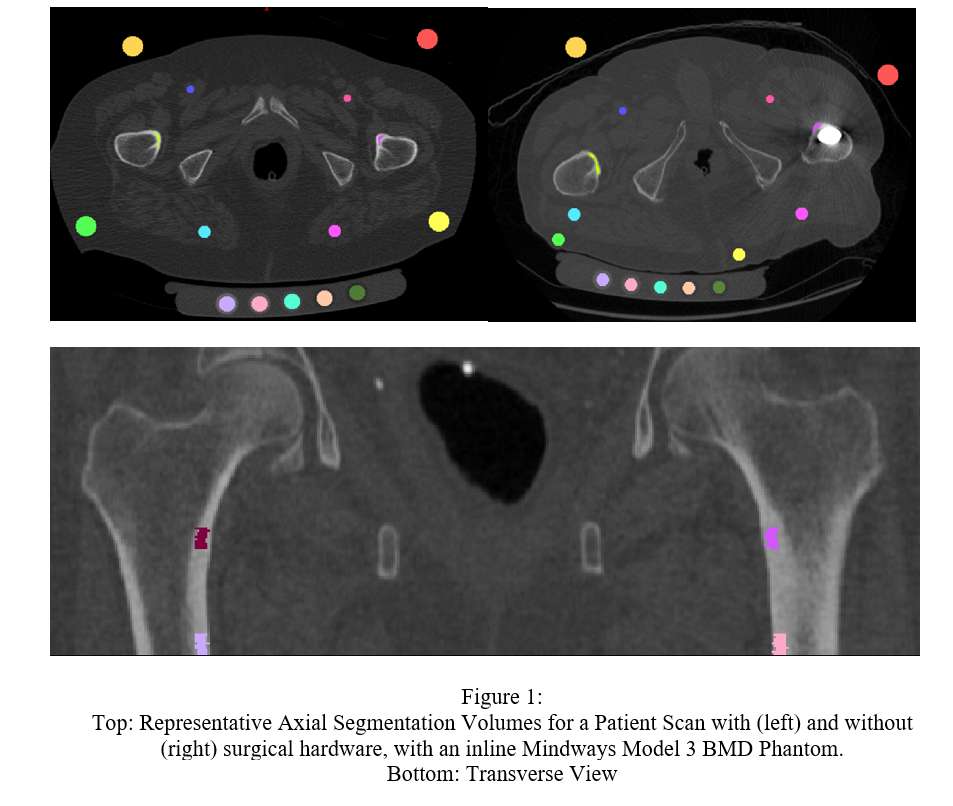
Figure 1
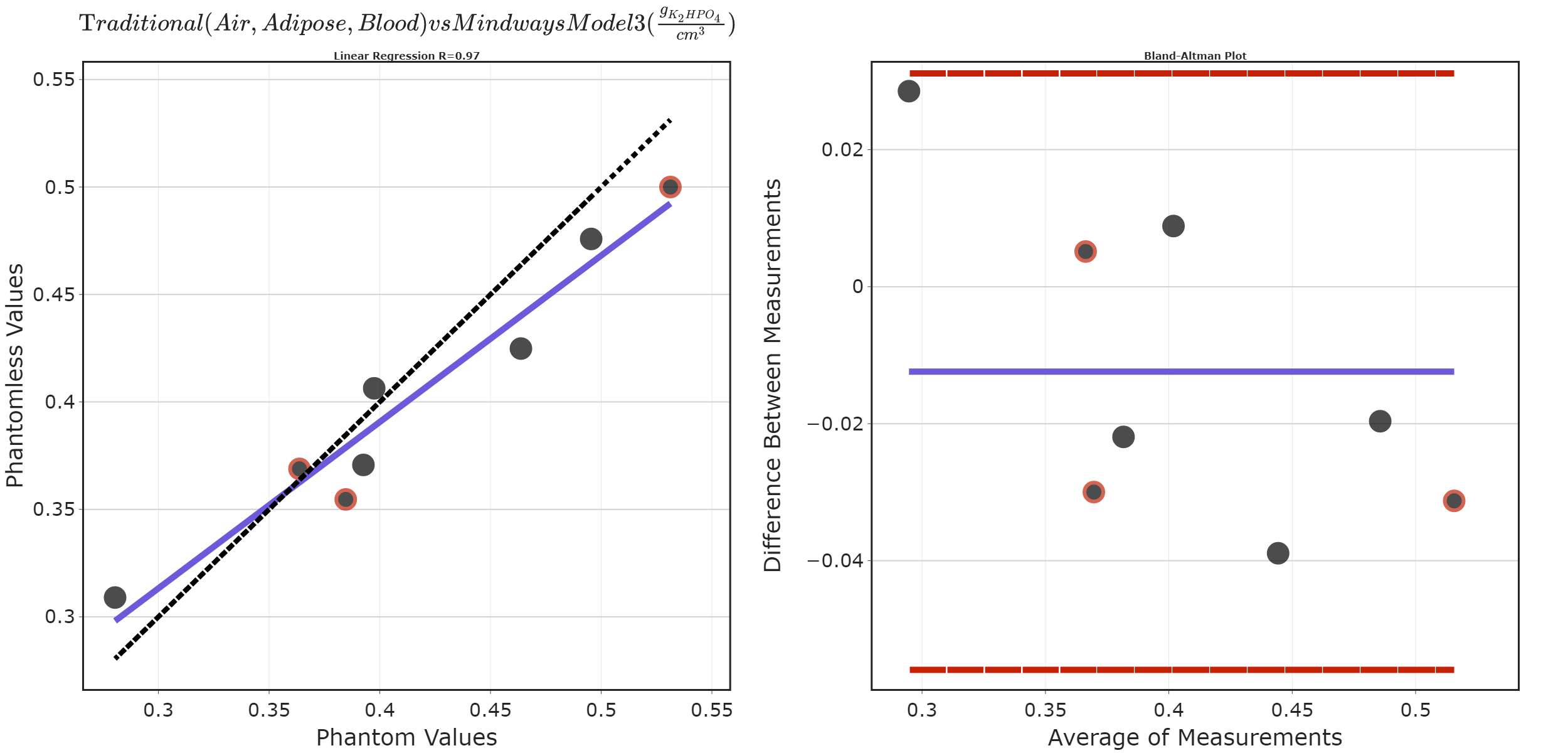
Figure 2
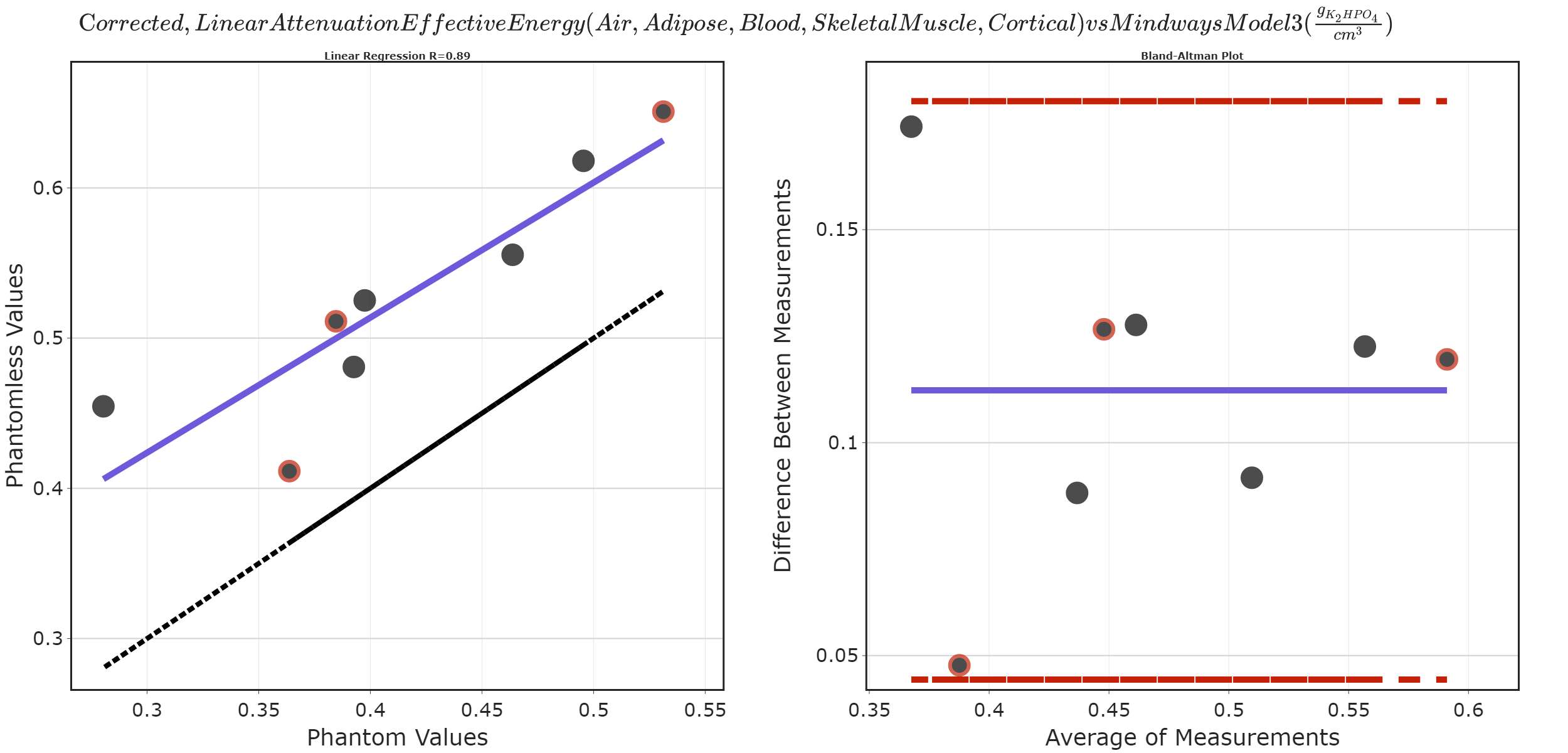
Figure 3#8291
Impact of Radiographic Projection on Assessment of Internal Femoral Geometry and Templating Stem Size
Oscar Denton - Queens University Belfast - Belfast, GB
*Alex Lennon - Queen's University Belfast - Belfast, United Kingdom
David Beverland - Musgrave Park Hospital - Belfast, United Kingdom
Janet Hill - Musgrave Park Hospital - Belfast, United Kingdom
Nicholas Dunne - Dublin City University - Dublin, Ireland
*Email: a.lennon@qub.ac.uk
Introduction
Projection error is an accepted limitation of planar radiographs when evaluating 3D structures. In the context of total hip arthroplasty (THA), the impact is notable in femoral offset (FO), neck-shaft angle (NSA) and femoral height (FH). Despite this, radiographs are used as standard templating images. When templating, the radiograph is not only used to inform modular neck combinations but also to size stems, assuming the internal metaphyseal-diaphyseal geometry is accurately represented. This work aims to elucidate the impact of variable orientation during radiograph acquisition on the appearance of the metaphyseal-diaphyseal region of the femur.
Methods
The metaphyseal-diaphyseal geometry was defined by its canal-to-calcar ratio (CCR), canal flare index (CFI), cortical index (CI), isthmus width (IsW) and isthmus location (IsL) (measured along the femoral axis from the height of the lesser trochanter); Fig. 1. Segmented femoral geometries (n = 228) were imported to MATLAB (MathWorks Inc., USA) where ray casting was used to simulate radiographs. For each femur, radiographs were simulated with -30° to 30° rotation (neutral was internally rotated by anteversion) in 10° increments, and 0° to 30° flexion in 10° increments (Fig. 2), leading to 28 radiographs at distinct orientations for each femur. From each simulated radiograph, the edge of the femoral geometry was automatically isolated, and the aforementioned features were automatically measured. Trends in these measurements could then be observed changing, across the dataset, as orientation during radiograph acquisition changed.
The size and position of each size of the stem (CORAIL, DePuy Synthes) was then optimised based on 2D contour in each simulated radiograph. The optimisation targeted the largest stem size that could sit within the diaphysis without intersection whilst also targeting an even 2mm gap between inner cortices and stem.
Results
In neutral radiographs mean values across all geometries were: CCR 0.61 (std 0.09), CFI 3.97 (std 0.70), CI 0.58 (std 0.07), IsW 11.5 mm (std 2.11 mm) and IsL 117.6 mm (std 16.76 mm). Except for CI, which varied significantly more with rotation than flexion, all variables were influenced by both rotation and flexion. CCR, CFI, CI and IsW all demonstrated turning points at approximately 15-20° external rotation (Fig. 3). Given neutral is internally rotated by anteversion, this turning point appears to occur once the applied internal rotation is counteracted.
Up to 10° internal rotation and 20° external rotation, the average stem size was unchanged compared to neutral for all flexions. At 20° internal rotation the correct stem could only be templated in the absence of flexion.
Conclusion
Alteration of combined external rotation and flexion was shown to impact the appearance of the internal metaphyseal-diaphyseal geometry in simulated femur radiographs via changes in CCR, CFI, CI, IsW, and IsL. This variation suggests decision-making dependent on intramedullary components could be negatively impacted by a combination of unquantified rotation and flexion during radiograph acquisition. Despite variation in the appearance of the diaphysis, the correct stem size tends to be assessed with malrotation up to 10° internal and 20° external rotation.
Figures
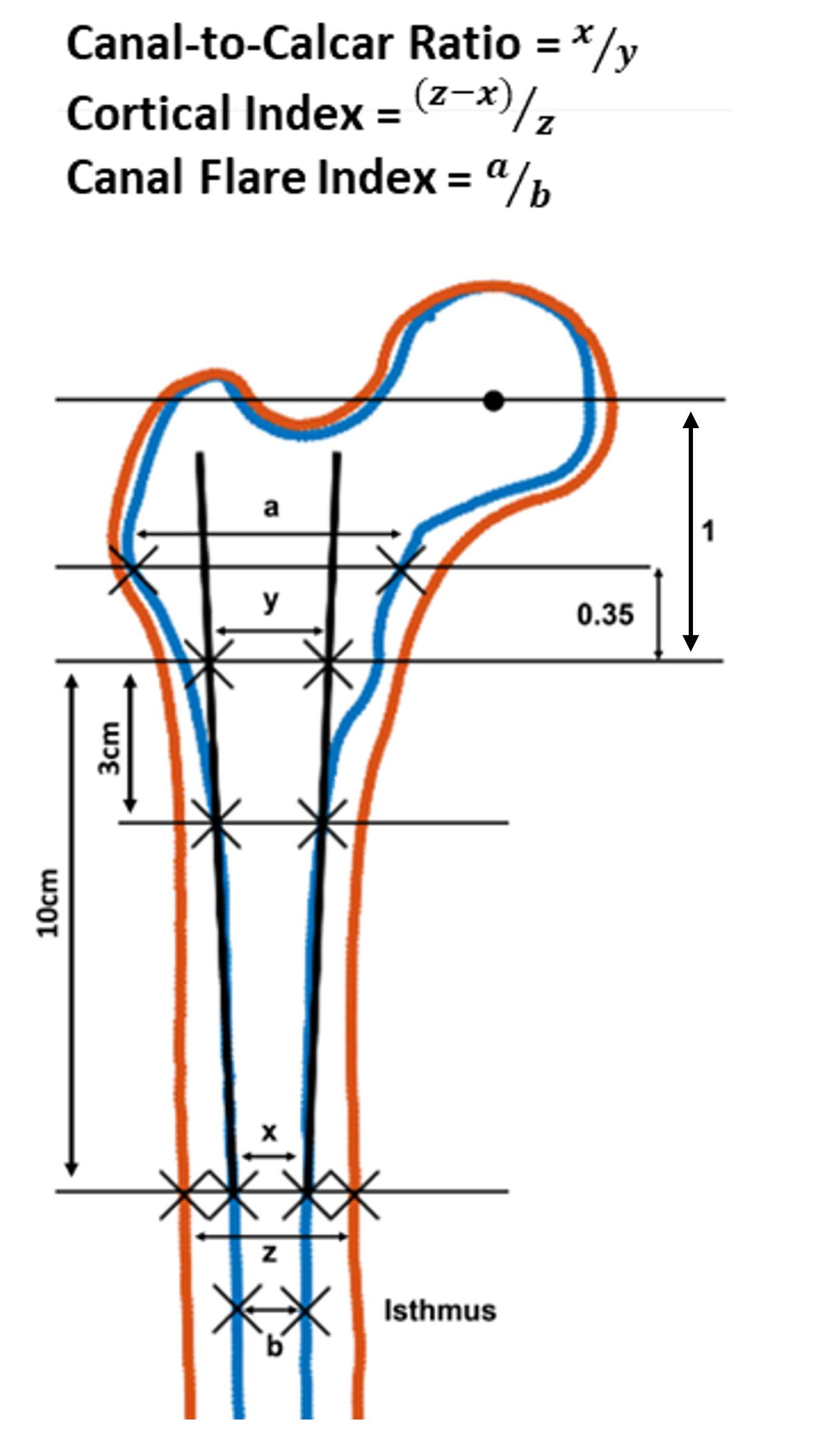
Figure 1
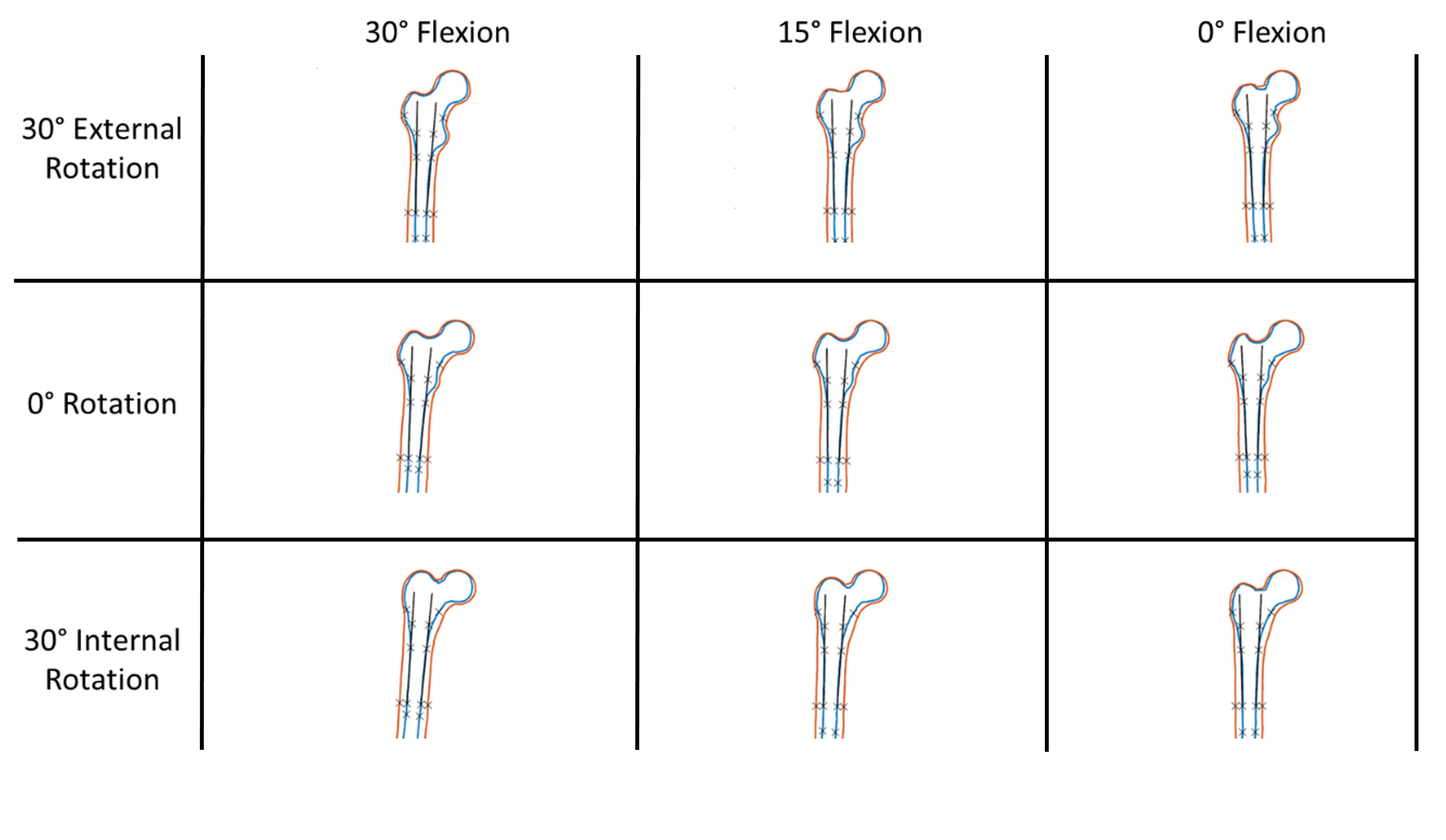
Figure 2
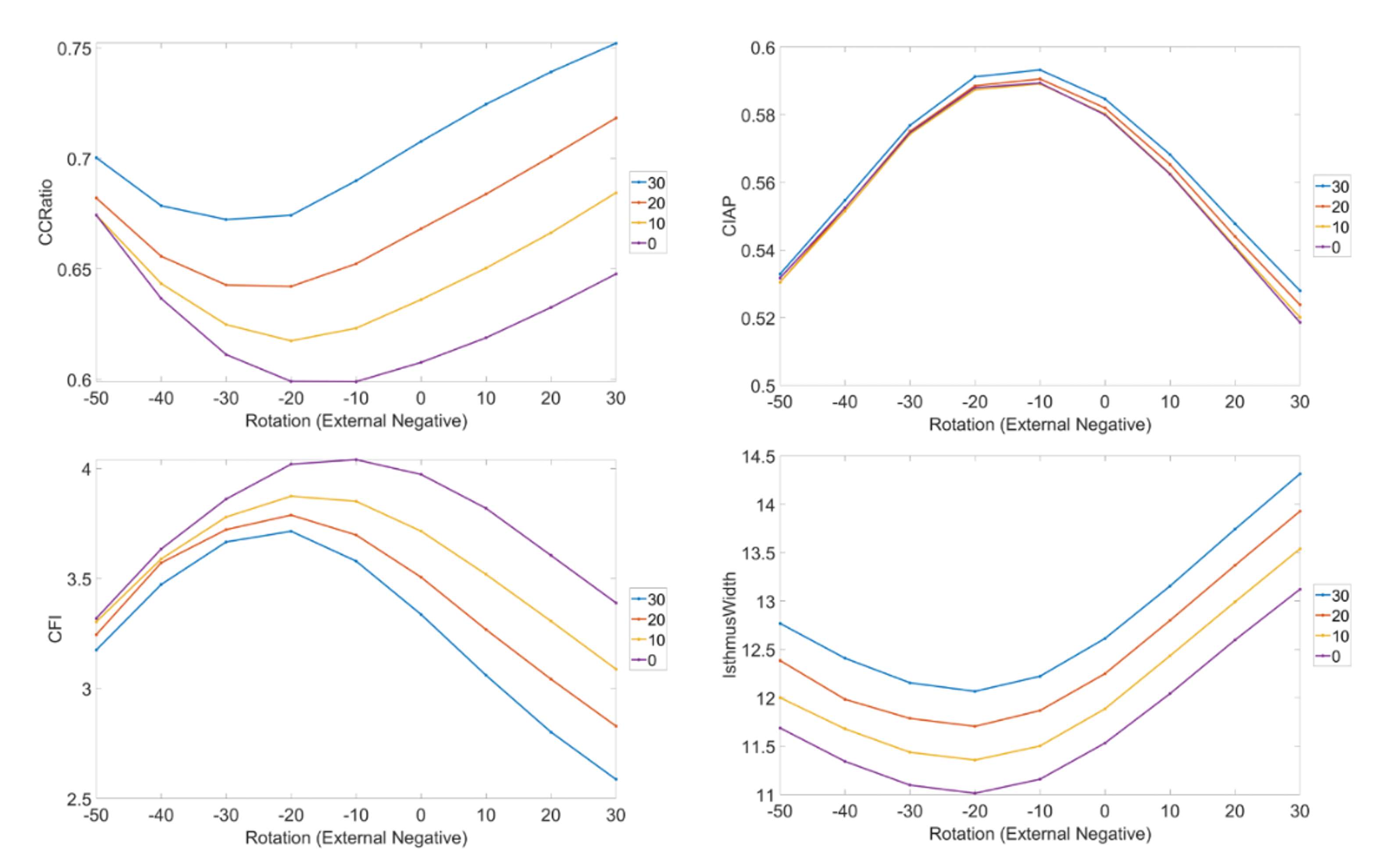
Figure 3#8677
Determining Axial Rotation of the Pelvis at the Time of an AP Radiograph: A Novel APP Based Algorithm.
*Friedrich Boettner - Hospital for Special Surgery - New York, United States of America
Ajay Premkumar - Hospital for Special Surgery - New York, USA
Lyubomir Haralambiev - Hospital for Special Surgery - New York, USA
*Email: drboettner@email.de
Introduction:
Axial rotation of the pelvis impacts inclination and anteversion measurements of the acetabular component on plain radiographs. Currently the axial rotation is not taken into account when providing acetabular component measurements on plain radiographs. The current study evaluates a novel App based algorithm to determine pelvic axial rotation at the time of an AP pelvis radiograph.
Material and. Method
An acetabular component was inserted into a radiopaque sawbone. AP and lateral radiographs were taken of the sawbone to calibrate the App. The pelvis was rotated 15 times (Minimum: 1.25 deg., Maximum 9.65 deg.) to the right and 16 times (Minimum: 1.5 deg., Maximum 11.3 deg.) to the left. The amount of axial rotation was determined using a digital caliper and an AP pelvis radiographs was taken for each position. Using a novel App based calculation algorithm the amount of axial rotation was determined from the AP Pelvis radiograph.
Results
The App determined the pelvis position at the time of the AP Pelvis radiograph with an average accuracy of 0.8 deg. compared to the digital caliper measurement. The difference to the caliper measurement ranged from 0 to 2.8 degree.
Summary
The algorithm used for this App based measurement accurately determined axial rotation of the pelvis at the time of an AP pelvic radiograph. The technology can be used for more accurate determination of acetabular component position on standard radiographs and for intraoperative C-arm imaging.
Figures

Figure 1
Figure 2#8739
Validation of a Medical Device for Varus/Valgus Stress Radiographs of the Arthritic Knee
*Thomas Hamilton - University of Oxford - Oxford, GB
Stephen Mellon - University of Oxford - Oxford, United Kingdom
David Murray - University of Oxford - Oxford, United Kingdom
*Email: thomas.hamilton@ndorms.ox.ac.uk
Introduction
Patient selection for unicompartmental knee arthroplasty (UKA) based on the pathoanatomy of disease is critical for success. Several radiographic atlas based decision aids have been validated and associated with excellent mid-term survival and function outcomes in those who meet criteria compared to those that don’t.
Stress radiographs have been demonstrated to enhance the accuracy of atlas based decision aids by around 5% with these decision aids have better accuracy at identifying patients suitable for UKA compared to other approaches including artificial intelligence/machine learning that do not utilise these views. Stress radiographs, typically performed by the radiographer or surgeon, require additional training and personal and expose the practitioner to excess radiation and as a consequence are only used by 17% of surgeons. This study describes the development and validation of a medical device for performing stress radiographs of the arthritic knee.
Methods
The device was developed using the IDEAL-D (Idea, Development, Exploration, Assessment, Long-term study - Device) framework. Figure 1. Following ethical approval a fluoroscopic validation study of 9 knees (9 patients) was undertaken where varus and valgus stress of 0 Newton, 10 Newton, 20 Newton and 30 Newton force were sequentially applied of the knee using the device and compared to the gold standard of clinician performed stress radiograph. For each force a verbal pain score was recorded. Images were measured using custom measuring software (Matlab, Massachusetts, USA) in a random order with the assessor blinded to the force applied.
Results
Varus: Bland-Altman analysis demonstrated that at 0N and 10N varus force the device underestimated medial JSW by 5.6mm (95%CI 2.9 to 8.3) and 0.6mm (95%CI 0.1 to 1.1) respectively compared to manual, clinician performed varus stress. Whereas at 20N (mean difference 0.04 mm (95%CI -0.61 to 0.70 mm) Figure 2) and 30 N (mean difference 0.09 mm (95%CI -0.41 to 0.59 mm) force good accuracy was seen between then device and gold standard of clinician performed views. The mean VAS pain score on clinician performed, varus stress was 4.0 and except for the 30N force (mean 4.9) was significantly lower with all other forces.
Valgus: Bland-Altman analysis demonstrated that at 0N valgus force the device underestimated lateral JSW by 0.8mm (95%CI 0.4 to 1.3) compared to manual, clinician performed valgus stress. Whereas at 10N (mean difference 0.01 mm (95%CI 0.7 to 0.7 mm) Figure 3), 20N (mean difference 0.2mm (95%CI 0.7 to 0.2) and 30N (mean difference 0.3mm (95%CI 0.2 to 0.7) force good accuracy was seen between then device and gold standard of clinician performed views. The mean VAS pain score on clinician performed, valgus stress was 3.7 and except for the 30N force (mean 5.4) was significantly lower with all other forces.
Conclusion
This validation study demonstrated the perfomrance of the medical device was equivelent to manual, clinician perfomred, stress radiographs. For varus stress 20N force was required and for valgus stress 10N. The use of the stress device was associated with lower pain scores than clinician performed views.
Figures

Figure 1
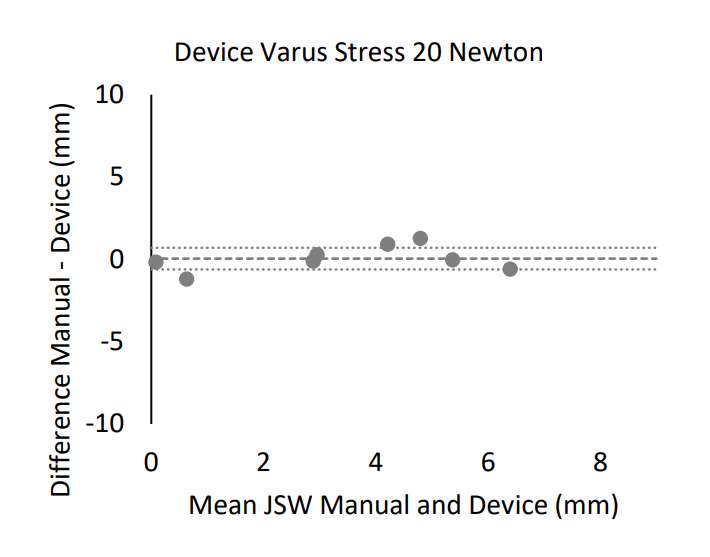
Figure 2
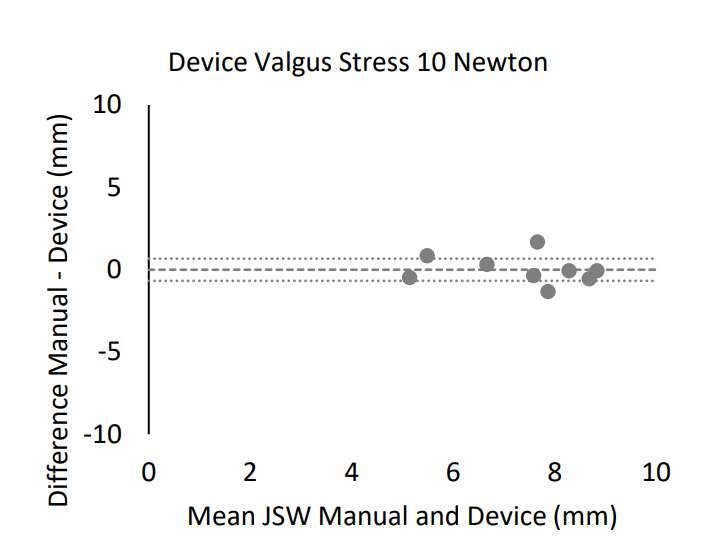
Figure 3#8663
NextAR TKA: Intraoperative, 3-Dimensional, Dynamic Ligament Assessment to Achieve Optimal Balance of the Knee
*Daniele Ascani - Medacta International SA - Chiasso, Switzerland
John Mercuri - Geisinger - Scranton, USA
Massimiliano Bernardoni - Medacta International SA - Castel San Pietro, Switzerland
*Email: ascani@medacta.ch
Introduction:
Many patients are dissatisfied with the functional result of their total knee arthroplasty (TKA). Achieving optimal, personalized soft tissue balancing is essential to a successful TKA. Surgeons are challenged with making data-driven, intra-operative decisions regarding the ligamentous structures when CT-based planning only evaluates bony anatomy. This has driven the development of intraoperative techniques to achieve optimal ligament balance. This report of twenty surgical cases demonstrates the capabilities of a new knee balancing technology, NextAR TKA. By using real time information about the ligament’s length, the software is able to predict the behavior of the ligaments before performing bony resections, thereby allowing the surgeon make highly-detailed intraoperative adjustments.
Methods:
Each knee was preoperatively planned and executed using the NextAR system. The system utilizes a single-use tracking system (small infrared camera and active tracker), augmented reality glasses, and a computer monitor. NextAR shows the real-time, dynamic length of the knee collateral ligaments by calculating the origins and the insertions of the ligaments on CT images using a previously published method. This allows the surgeon to capture the preoperative state of the ligaments and the kinematics of the knee. These data are the input for a predictive algorithm that demonstrates the post-operative behavior of the knee ligaments compared to the pre-operative state before bony resections. The surgeon can then optimize the position of the implants and observe in real-time what effect the reconstruction will have on the knee ligaments. (Figure 1)
Results:
On all twenty cases, the NextAR system demonstrated that the medial collateral ligament is fairly isometric throughout the entire range of motion, while the lateral collateral ligament (LCL) becomes more lax in mid and deep flexion. Adjustments made during the intra-operative ligament analysis allowed the surgeon to anticipate the required bony cuts and personalize the position of the implants based on the specific characteristics of the patients’ soft tissue without needing any recuts. Intraoperative and post-operative physical exam of the knees demonstrated a well-balanced knee with appropriate patellar tracking. NextAR software similarly demonstrated accurate recreation of the ligament kinematics (Figure 2). Finally, post-operative radiographs were evaluated to confirm that the implants were accurately placed relative to the intra-operative plan (Figure 3).
Conclusion:
The NextAR system successfully measured the dynamic displacement of the knee collateral lateral ligament by using an augmented reality system. The true innovation of this system allows the surgeon to balance the knee envelope by directly measuring the 3D displacement of the collateral knee ligaments. This new analysis—termed 3D gap balancing—allows the surgeon to incorporate an intraoperative analysis of the internal-external rotation of the tibia. This information is crucial to recreating the correct length and tension of the LCL in positions of flexion. 3D gap balancing highlights the importance of flexion kinematics where the contact point of the lateral femoral condyle moves posterior on the plateau as the tibia internally rotates. Using ligament data, it is possible to optimize the position of the implants and recreate patient-specific balance and kinematics through a full range of motion.
Figures
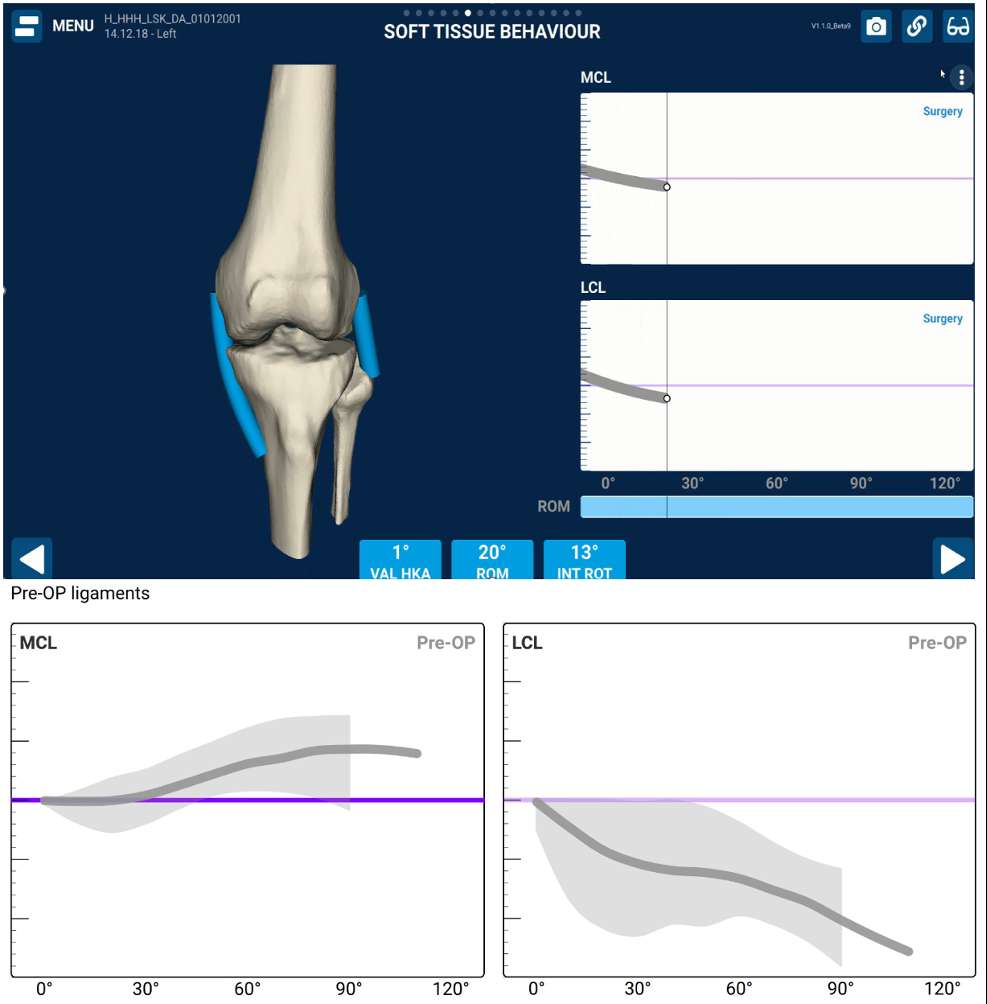
Figure 1
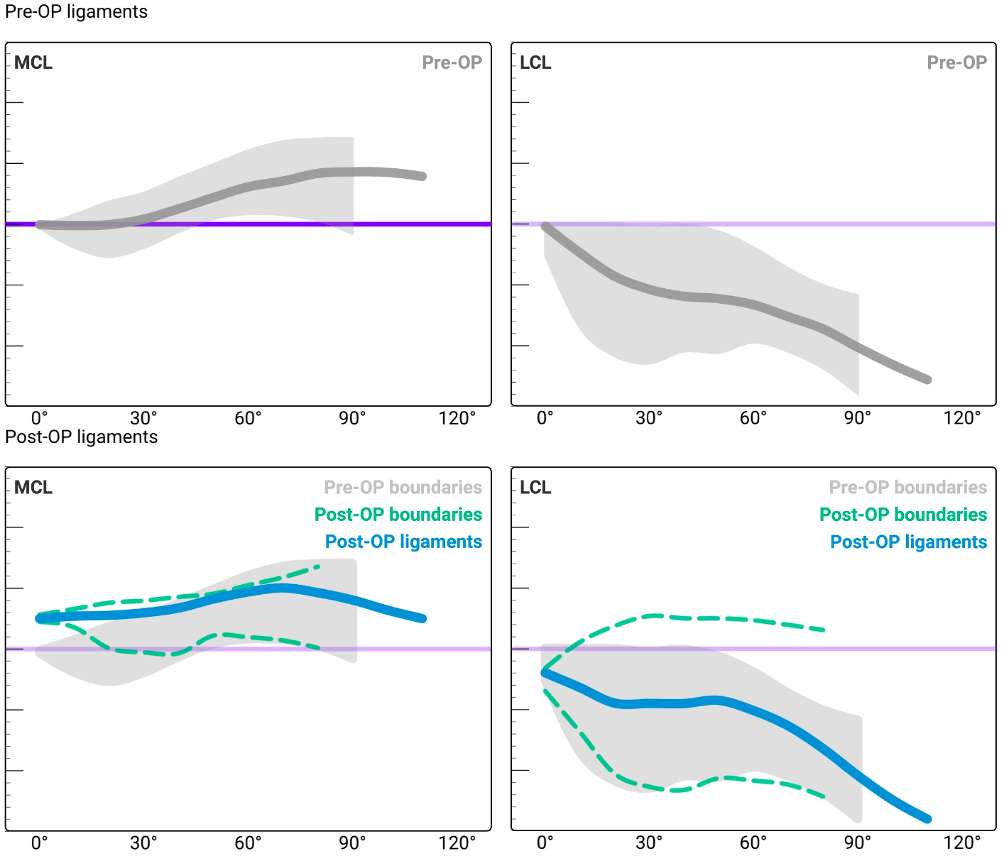
Figure 2
Figure 3#8586
Comparison of 3D Bone Models of the Knee From MDCT and CBCT Imaging
*Stuti Singh - CurveBeam AI - Seattle, United States of America
Philippe Van Overschelde - Medisch Centrum Latem - Sint-Martens-Latem, Belgium
Conrad Ivie - Tennessee Orthopaedic Clinics - Knoxville, USA
Yu Peng - CurveBeam AI - Hatfield, USA
*Email: stuti.singh@curvebeam.com
Introduction: Applications of cone-beam CT (CBCT) imaging have attracted increasing interest as hip and knee replacement technology advances. CBCT captures data using a cone-shaped X-ray beam and provides high-resolution scans. CBCT has several advantages over traditional multi-detector CT (MDCT), including higher-resolution imaging, lower radiation dose, ability to scan in a weight-bearing position, and a smaller device footprint. However, CBCT images generally demonstrate lower contrast and more noise than MDCT images. CT images are often rendered as 3D bone models, which are used for applications such as pathological assessment and surgical planning. Advanced surgical technologies like robotic assisted surgery and augmented reality require precise modelling to achieve desired results.
To validate the clinical value of CBCT-based 3D bone modelling for surgical planning, it is necessary to evaluate the performance of CBCT-based 3D models against MDCT. This study compared 3D bone models of the knee joint derived from CBCT and MDCT imaging to determine whether CBCT can be a viable alternative for 3D bone modelling.
Methods: CBCT (HiRise CT, CurveBeam AI, United States) and MDCT (GE LightSpeed VCT, GE HealthCare, Chicago, United States or Siemens SOMATOM Force CT, Siemens Healthineers, Erlangen, Germany) studies were acquired from four participants. The maximum interval between scans for each participant was 10 days.
The modelling of four bones of the knee joint (distal femur, proximal tibia, proximal fibula, and patella) was compared between CBCT and MDCT. The bones were manually segmented by an experienced medical image annotator and were reviewed independently. Three-dimensional bone models were generated from the segmentation and comparisons were made using the 3D mesh similarity measurement. The marching cubes algorithm was utilized to translate the different image resolutions of CBCT and MDCT to real-world dimensions via the pixel spacing and slice thickness and to generate 3D meshes from the binary segmentation of each bone. Then, the iterative-closest point algorithm rigidly registered each individual CBCT bone mesh to the corresponding MDCT bone mesh for the mesh distance calculation.
Results: The absolute distance between the MDCT and the CBCT-based bone models averaged below 0.35 mm across all bones of all the participants and ranged from 0.003 mm to 0.35 mm, indicating that CBCT can provide accurate 3D bone models of the knee joint. The best agreement was found for the patella (0.039 mm ± 0.03 mm), followed by the tibia (0.043 mm ± 0.02 mm), femur (0.056 mm ± 0.05 mm), and fibula (0.158 mm ± 0.11 mm). Figure 1 shows a visual comparison of the differences between MDCT and CBCT tibia bone models.
Conclusion: This study indicates that CBCT scans offer a reasonable alternative to traditional MDCT in 3D bone modelling of the knee. CBCT could improve the workflow, patient experience, and accessibility for personalized surgical planning for total joint replacement. This could in turn expand the accessibility of advanced surgical techniques such as robotics and augmented reality. Further research will be required to determine if the weight-bearing positioning of CBCT could also improve patient outcomes.
Figures
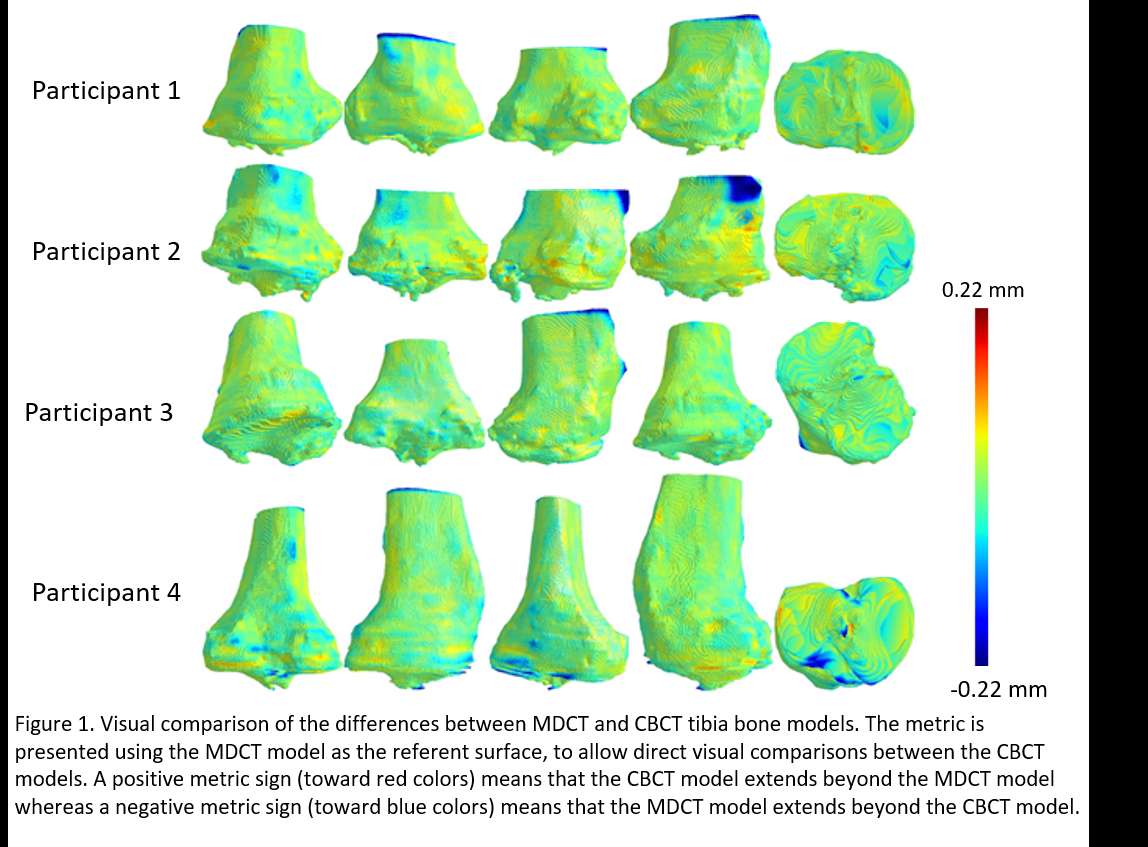
Figure 1#8526
Characterization of Proximal Tibia Bone Density in Patients Undergoing Primary Total Knee Arthroplasty
*Fernando Quevedo Gonzalez - Hospital for Special Surgery - New York, USA
Tracy Borsinger - Hospital for Special Surgery - New York, United States of America
Joseph Lipman - Hospital for Special Surgery - New York, USA
Cale Pagan - Hospital for Special Surgery - New York, United States of America
Theofilos Karasavvidis - Hospital for Special Surgery - New York, USA
Peter Sculco - Hospital for Special Surgery - New York, USA
Cynthia Kahlenberg - Hospital for Special Surgery - New York City, USA
Eytan Debbi - Cedars-Sinai Medical Center - Los Angeles, USA
Timothy Wright - Hospital for Special Surgery - New York, USA
David J. Mayman - Hospital for Special Surgery - New York, USA
Jonathan Vigdorchik - Hospital for Special Surgery - New York, USA
*Email: quevedogonzalezf@hss.edu
Introduction: Mechanical failures, including aseptic loosening and component subsidence, are common causes for revision in total knee arthroplasty (TKA) [1]. The risk of loosening or subsidence has been related to the bone mineral density (BMD) [2], which is a biomechanical marker for bone stiffness and strength [3]. However, our understanding of the differences in BMD distribution in the proximal tibia among TKA patients remains limited, hampering our ability to devise effective surgical plans to avoid mechanical failures of TKA. Our goals were to characterize the tibial BMD in patients undergoing TKA and determine whether differences exist between patients that received cemented and cementless TKA implants.
Methods: We retrospectively reviewed 25 patients (16 males, 9 females, ages: 52–87 years, BMIs: 22–41 kg/m2) that underwent CT-based robotic-assisted primary TKA with posterior-stabilized or cruciate retaining implants (Stryker, Mahwah, NJ) from September 2022 to March 2023, without metallic hardware on the affected or contralateral knee and for whom CT-scans included a K2HPO4 BMD reference phantom (Mindways Software, Austin, TX). We manually segmented the tibia from CT-scans (Mimics, Materialise, Leuven, Belgium) and identified the anatomic landmarks from the robotic surgical plan to determine the cutting surface performed during surgery. The BMD phantom allowed us to convert Hounsfield Units to mg/cm3 of K2HPO4. We computed the average BMD at the entire tibial cut surface, as well as the medial and lateral halves (Fig. 1). We also computed the average BMD at 2 mm spaced cuts from 2 mm above to 10 mm below the planned cut. We compared the BMD between patients with cemented and cementless fixation.
Results: The mean BMD was higher (p<0.001) for the medial half (range: 142.4–344.4 mg/cm3) than the lateral half (range: 75.7–281.0 mg/cm3) of the tibiae (Fig. 2). Six patients received cemented implants (5 females aged 56–84 years with BMIs 25–37 kg/m2, 1 male aged 87 years with BMI 32 kg/m2), and 19 patients received cementless implants (4 females aged 58–71 years with BMIs 22–33 kg/m2, 15 males aged 52–75 years with BMIs 26–41 kg/m2), based on intraoperative surgical choice. Cementless implants had higher mean BMD than cemented implants for the entire tibial cut (146.6–289.8 mg/cm3 vs 140.7–208.4 mg/cm3, p=0.04) and the lateral half of the cut (105.6–281.0 mg/cm3 vs 75.7–176.9 mg/cm3, p=0.03), but not for the medial half (142.4–344.4 mg/cm3 vs 164.1–269.5 mg/cm3, p=0.08). In all patients, the BMD decreased with increasing distance below the planned tibial cut (Fig. 3).
Discussion: Although such detailed BMD-based analysis was not part of the surgical decision making, our preliminary work demonstrates that surgeons chose cementless fixation for patients with higher BMD. While the BMD threshold for cementless implants remains unknown this work demonstrates differences in BMD across patients, indicating that advanced understanding of the spatial distribution of BMD could improve pre-surgical planning and long-term outcomes of TKR.
References: [1] Sharkey, et al. J Arthroplasty. 2014; [2] Petersen, et al. J Arthroplasty. 1999; [3] Morgan, et al., J Biomech. 2004
Figures
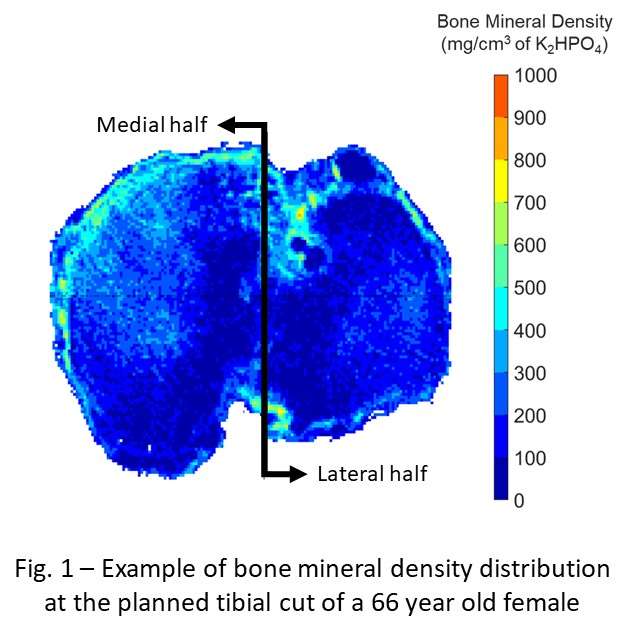
Figure 1
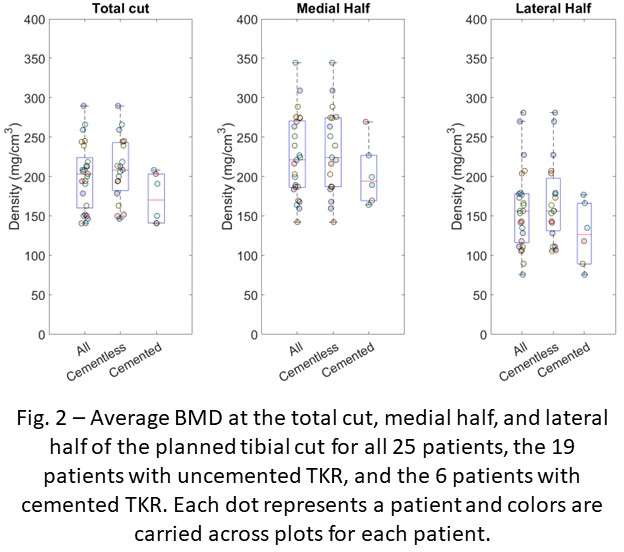
Figure 2
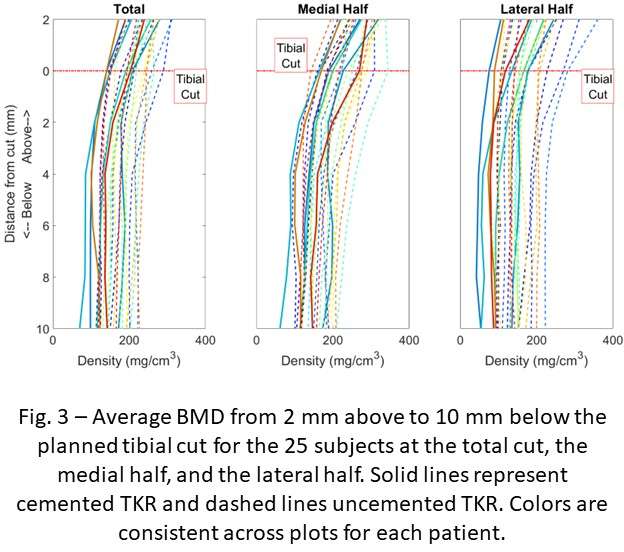
Figure 3#8561
Clinically Meaningful Changes in Cup Anteversion and Inclination Angles Are Observed on Anteroposterior X-Rays Due to Parallax Distortion.
*Emily McIntosh - Intellijoint Surgical - Kitchener, Canada
Kieran Eveleigh - Intellijoint Surgical - Kitchener, Canada
*Email: e.mcintosh@intellijointsurgical.com
Introduction: Radiographic imaging is an important part of total hip arthroplasty. Pre and post operative x-rays are consistently used to diagnose, template, and review the outcome of the surgery. Despite the widespread usage, there are well known errors associated with x-ray imaging. Parallax distortion is a phenomenon which affects the appearance of an object’s projection when it is off-center from the x-ray’s central ray. The purpose of this study was to quantify the parallax distortion via the differences in the angular measurements of the same cup orientation placed in 9 different positions on a pegboard.
Methods: Nine x-rays were taken of the same set-up with only the cup’s horizontal and vertical distance from the central ray changing. A Corin 55 mm acetabular cup (Corin, Pymble, Australia) was fixed to a wooden mount so that the angle (43° / 29°) would remain consistent throughout the protocol. The mount was then secured to the pegboard with two screws which fit into the slots, ensuring only horizontal and vertical translations of the cup. The pegboard was 23 x 23 cm, and the 9 positions were arranged in 3 columns by 3 rows. Each x-ray was combined into a single array (see Figure 1) in the same configuration where it was on the pegboard using MATLAB (Mathworks, Natick, USA). The distance between each row and column was 7.6 cm. The source to image distance (SID) was the standard 40 in/101.6 cm distance for a supine pelvis x-ray. The source-object distance (SOD) was 86 cm. The two authors measured each cup inclination and anteversion angle twice, independently, using Intellijoint VIEW (Intellijoint Surgical, Kitchener, Canada). Inter and intra-rater reliability were calculated.
Results: Inter and intra-rater reliability were excellent, with both exceeding 95%. The inclination angle range was 6° (40° - 46°) while the anteversion angle ranged by 13° (20° - 33°). The largest anteversion change from the true cup angle (9°) occurred when the cup was inferior and lateral to the central ray. The average measured cup values can be viewed in Figure 1.
Conclusion: This basic, bench-top study clearly demonstrated an important consideration for standard-of-care anteroposterior pelvis x-ray imaging. Post-operative cup positions are typically measured on standard anteroposterior x-rays where the central ray is directed at the pubis, rather than the surgical hip. Clinically meaningful differences in cup angles do arise due to parallax distortion. These differences would be magnified in larger pelvises and with an anatomically inconsistent central ray.
Keywords: Cup angles, parallax, x-ray, anteversion, inclination
Figures
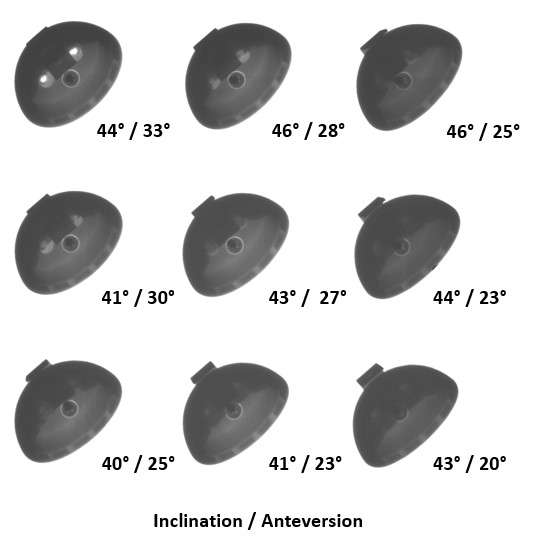
Figure 1#8694
Material Mapping Agnostic Partial Volume Artifact Correction for CT Images
Aren Beagley - University of Victoria - Victoria, Canada
*Joshua Giles - University of Victoria - Victoria, Canada
*Email: jwgiles@uvic.ca
Introduction:
Creating subject-specific finite element (FE) models with heterogenous CT-derived properties improves simulation accuracy. Heterogenous material properties are determined by converting CT Hounsfield units (HU) to density, then applying a density-modulus relationship, and mapping modulus values to the model elements. This assumes the CT accurately reflects bone properties but in regions with sharp density-gradients (like cortical-soft tissue boundaries) partial-volume artifacts (PVA’s) cause underestimates of the true values. Previous methods to correct PVA’s during FE model creation perform the correction as part of mapping materials onto the FE meshes or require an estimation of the CT point-spread function and preprocessing images via deconvolution. Therefore, we developed a PVA correction method that works on the CT scan without knowledge of the point-spread function and which is agnostic to the material mapping strategy to be used.
Methods:
Nine fresh-frozen porcine hind-limbs were acquired and the fibulae were dissected and three-point bending tests were performed following the ASABE standard. A strain rosette was placed at the center of each fibula to measure peak strain during testing. PVA correction was applied directly to the CT scans using a custom Python script to interpolate PVA voxel values from adjacent unaffected voxels (Fig. 1). FE simulations were generated and solved in Abaqus. To the authors’ knowledge, there are no published density-modulus relationships for porcine fibulae, nor any that could account for the specimens’ varying skeletal maturity. To address these challenges, computationally derived subject-specific density-modulus relationships were determined using a response surface methodology published by Eberle et al (2013). Separate relationships were determined for the uncorrected and PVA-corrected simulations. Simulation error was estimated as the relative difference in whole bone stiffness from experimental results and the relative difference in experimental principal strain magnitude compared to the principal strain magnitude computed from the surface nodes corresponding to the strain rosette location on each specimen.
Results:
Currently, simulation results have only been completed for one specimen, and this work is ongoing. The optimal subject-specific density-modulus relationship for the uncorrected and PVA-corrected CT scans were found to be 10920ρ2.5 and 11222ρ1.792 (Figs 2 & 3). Relative strain errors of 5.89% and 7.60% were found for the uncorrected and PV-corrected simulations respectively. The relative elastic modulus error was 6.46% and 9.05% respectively.
Conclusion:
Preliminary results suggest that the PVA-correction may not reduce simulation error when applied with subject-specific density-modulus relationships. Comparison of the subject-specific density-modulus relationships provide an insight as to the cause. The uncorrected density-modulus relationship assigns elastic moduli as high as 35 GPa to cortical bone and sets most trabecular bone to less than 1 GPA, given the published cortical bone density threshold of 818 mg/cc. While the PVA-corrected density-modulus relationship assigns elastic moduli in the range of 10-25 GPA and sets most trabecular bone to a range between 4-7 GPa. As such, while the error of the PVA-corrected simulations is slightly higher the derived density-modulus relationship better aligns with expected anatomical values for elastic modulus of cortical and trabecular bone, especially given the skeletal immaturity of the porcine specimens.
Figures#8453
Factors That Influence Resource Utilization Following Primary Lower Extremity Total Joint Arthroplasty
*Jimmy Daher - Ochsner Medical Center - New Orleans, USA
Bhumit Desai - Ochsner Medical Center - New Orleans, United States of America
Willard Moore III - Ochsner Medical Center - New Orleans, USA
Cruz Velasco-Gonzalez - Ochsner Medical Center - New Orleans, USA
George Chimento - Ochsner Health System - New Orleans, USA
*Email: jimmy.daher@lau.edu
Introduction:
In an effort to decrease medically unnecessary emergency department (ED) visits, we established a “Total Joint Hotline” (TJH) at our institution for patients who underwent primary total knee arthroplasty (TKA) or total hip arthroplasty (THA). The intent was to provide patients around the clock access to a member of the adult reconstruction care team in the post-operative period. The purpose of this study is to determine which patient and procedure related factors drove utilization of the TJH.
Methods:
We prospectively collected data on patients undergoing primary THA and TKA at a single institution during a 6-month period in 2021. Patients were assigned a wristband at their pre-operative clinic visit with a phone number answered directly by a member of the adult reconstruction care team. Patients were instructed to call with any issues encountered during their post-operative course and their concerns were triaged by either providing reassurance, arranging clinic follow-up, or directing them to the ED. Phone logs were recorded for demographics and comorbidity data. Statistical analysis consisted of univariable negative binomial regression with alpha set at 0.05.
Results:
372 TJA (M: 141, F: 231, THA:129, TKA: 243) were performed. 51.6% patients utilized the TJH post-operatively. There was no difference in the average number of calls for age (p=0.4512), gender (p=0.3588), BMI (p=0.7378), or smoking (p=0.7450). Patients undergoing TKA made an average of 2.1 more calls than patients undergoing THA [2.1 (1.5, 2.9); p=<0.0001]. Patients with fibromyalgia also made an average of 2.1 more calls than patients without fibromyalgia [2.1 (1.1, 3.9); p=0.026]. Patients with depression (p=0.0469) and those on psychiatric medication (p=0.0469) made 1.4 and 1.6 more calls respectively. Patients with history of anxiety and bipolar disorder have a similar trend of increased number of calls (x1.3, p=0.113 and x2.4, p=0.101, respectively) although p-value was not significant.
Conclusion:
This study shows that TKA patients with either fibromyalgia, depression or those on psychiatric medication are likely to utilize more resources in the post-operative period. This information can be used to counsel patients and prepare staff. Ideally methods of intervening and optimizing these patients pre-operatively should be developed.
#8311
Assessing the Effect Covid Protocols on the Timing of Orthopedic Operating Room Events and Efficiency
*Jacob Laperche - Frank H. Netter School of Medicine Quinnipiac University - Middletown, United States of America
Valentin Antoci - University Orthopedics - Providence, USA
Drew Clippert - Brown University/ University Orthopedics - Providence, United States of America
*Email: jacoblaperche@gmail.com
Introduction: The Covid-19 pandemic resulted in numerous changes to policies and practices within healthcare systems. These changes, when implemented, often resulted in perceived delays to typical practice including the timing of events in and around orthopedic operations. This study aimed to assess if there was a delay in operative events as a result of the covid-19 pandemic and protocols.
Methods: The timing of events for two adult reconstruction orthopedic surgeons operating at a single hospital were collected for two different time periods. The pre-covid era encompassed operations from January 1st, 2018 to June 30th, 2018. The covid-era encompassed January 1st, 2021 to June 30th, 2021. The timing of surgical events recorded included setup start, setup complete, in room time, procedure start time, procedure finish time and out of room time. The timing between each of these events was calculated and averaged per era for total hip arthroplasty (THA) and total knee arthroplasty (TKA) procedures and compared to the other era using independent sample t-tests.
Results: Calculated times were total set up time (TST = setup start to set up complete), total patient arrival time (TPAT = set up complete to in room), total anesthesia time (TAT = in room to procedure start), total procedure time (TPT = procedure start to finish), and total nursing time (TNT = procedure finish to out of room). In THA, the TAT in 2018 was significantly shorter compared to 2021, 28:53 minutes and 33:26 minutes (one sided p=0.003). No other time measures differed in THA. The TKA group TST was significantly shorter in the 2021 era at 15:10 minutes compared to 20:36 minutes in 2018 (one sided p=0.03). The TAT was 29:12 minutes in 2021 compared to 24:01 minutes in 2018 (one sided p<0.001).
Conclusions: Total anesthesia time increased during covid protocols for THA and TKA procedures compared to pre-covid protocols by around 5 minutes for both. Total set up time was shorter during covid protocols for TKA. Increased anesthesia time may contribute to perceived delays in the entire operative process. Covid protocols and precautions may have resulted in the increased time of anesthesia events.
Figures
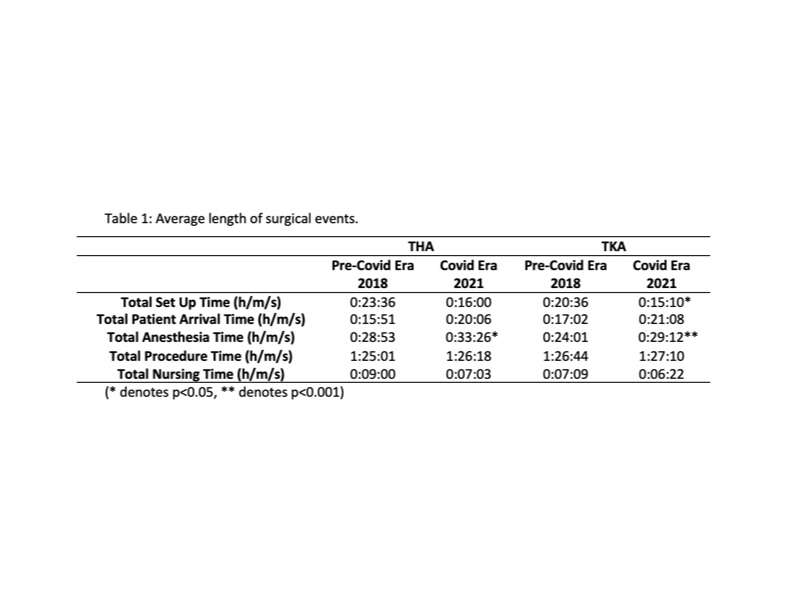
Figure 1#8314
The Removal of TKA From the CMS Inpatient Only List: What Was the Immediate Impact?
*Jacob Laperche - Frank H. Netter School of Medicine Quinnipiac University - Middletown, United States of America
Caitlin Barrett - University Orthopedics - Providence, USA
Drew Clippert - Brown University/ University Orthopedics - Providence, United States of America
Dioscaris Garcia - Brown University - Providence, USA
Valentin Antoci - University Orthopedics - Providence, USA
*Email: jacoblaperche@gmail.com
Introduction: Total knee arthroplasty (TKA) is the standard of treatment for end-stage knee osteoarthritis. With increasing TKA demand, there is a priority for optimization of TKA associated costs. On January 1, 2018, the Centers for Medicare and Medicaid (CMS) officially removed TKA from their inpatient-only list. To our knowledge there is no published study which has examined the immediate impact of Medicare's transition of TKA to the outpatient list. We hypothesized there would be no significant difference between patient reported post-operative functional outcomes or readmission, while length of stay (ALOS), and discharge to secure nursing facility (SNF) for patients undergoing TKA following the transition should be significantly lower.
Methods: We completed a retrospective review of electronic health records for patients who underwent TKA between January 1, 2017-June 30, 2017 (pre-CMS), or January 1, 2018-June 30, 2018 (post-CMS). Patients completed FORCE-TJR surveys which assessed patient reported outcomes prior to and following TKA. Hospital statistics for the two time points were determined and compared.
Results: Analysis of the pre-CMS and post-CMS transition cohorts indicated no significant difference in ADL, Pain, or PCS pre-operatively or 12-months post-operatively. Additionally, there was no difference in the median change between pre- and post-operative ADL scores (p=0.866). The median change between pre- and post-operative Pain scores approached significance with a p-value of 0.054. The pre-CMS transition group stayed significantly longer in the hospital post-operatively and were more likely to be discharged to a SNF. No difference was seen in readmission rates within 30-days of surgery (p=.253).
Conclusion: Results showed that patients had similar scores for activities of daily living, quality of life, pain, and pain catastrophizing 12-months following their TKA. Movement of TKA from the Medicare inpatient only list did not have an immediate negative impact for patient reported outcomes and 30-day readmissions at our institution in the 6-month transition period.
#8105
Out-Patient Total Hip and Knee Arthroplasty Exchange. a Feasibility Study.
*Jean-Yves Jenny - University Hospital Strasbourg - Illkirch, France
*Email: Jean-yves.jenny@chru-strasbourg.fr
Introduction
The number of total hip (THA) and knee (TKA) exchanges increases significantly with the number of primary implantations, thus increasing the hospitalization burden of health care facilities. Although outpatient THA and TKA remains a minority procedure today, it is a validated procedure that offers the same safety as conventional hospitalization. It is therefore attractive to apply the outpatient procedure to THA and TKA exchange. The aim of the present study was to analyze the feasibility of this outpatient procedure in a university department.
Methods
The study was conducted in a tertiary care facility by one surgeon experienced with THA and TKA exchange and doing routine outpatient primary THA and TKA. All THA and TKA exchanges performed during the years 2019 and 2020 were included. The outpatient THA and TKA exchange was introduced gradually, first for patients considered as having a low risk of complication, and eventually for any patients living not alone at home. All patients were followed during 12 months. Demographic data, ASA classification, indication for prosthesis exchange, length of hospitalization, occurrence of rehospitalization, complications and reoperations, and functional results were collected. Patients were divided into out-patient and in-patient groups, and the date of both groups were compared with appropriate statistical tests. The primary criterion was the rate of complications during the first post-operative year.
Results
120 cases of THA and TKA exchanges were collected. There were 49 men and 71 women, with a mean age of 73 years. Most patients were classified ASA 3. 61 revisions were performed for chronic periprosthetic infection, and 59 for aseptic reason, mainly late loosening. 24 procedures were performed as out-patient surgery (12 septic and 12 aseptic cases), and 96 as in-patient surgery (49 septic and 47 aseptic). Length of hospitalization after in-patient surgery was 4.5 days for aseptic cases and 7.2 days for septic cases. Complication rate after one year was not different in the in-patient group (12.5%) and in the out-patient group (13.1%).
Conclusion
Out-patient THA and TKA exchange may be a valuable option by selected patients. The rate of complications is not increased after out-patient procedure. This policy may allow significant cost-saving without compromising the outcome.
#8673
Can Modifiable Lifestyle Risk Factors Impact Healthcare Resource Utilization and Costs After Shoulder Arthroplasty?
Carlos Fernandez - JFK / U Miami Orthopedic Surgery Program - Lantana, USA
*Vani Sabesan - Cleveland Clinic Florida - Weston, USA
Anna Redden - FAU - boca raton, USA
Clyde Fomunung - Palm Beach Shoulder Service - Lantana, USA
Howard Routman - Atlantis Orthopedics - Atlantis, USA
*Email: sabes001@gmail.com
Hospital readmission rates are a significant measure of both quality of care and healthcare costs. Furthermore, many modifiable lifestyle risk factors can influence readmission rates for patients following surgery. The purpose of our study was to identify of key modifiable lifestyle risk factors that might pose the greatest risk for hospital readmissions and additional ER visits after Shoulder Arthroplasty (SA).
A national healthcare system with a large system wide database was queried to identify 1,721 patients who underwent SA between 2017 and 2021. Specific modifiable lifestyle risk factors such as smoking tobacco, narcotics use, BMI, and hypertension were collected. Logistic regression and odds ratio point estimate analysis was utilized to assess for associations between hospital readmission statuses and lifestyle risk factors. Hospital readmission rates were assessed within 30 or 90 days of surgery and were used as markers of healthcare costs and resource utilization. Also, return to ER within 90 days rates were included in the analysis.
The cohort consisted of 61.35% female (n = 1,056) with a mean age of 71 years and BMI of 29.4. For every whole number increase in BMI, patients were 1% more likely to be readmitted both within 30 days (1.010, 95% CI: 1.001 – 1.019, p = 0.0299) and within 90 days (1.009, 95% CI: 1.002 – 1.015, p = 0.0081). There was no significant association found between BMI and an ER visit (p = 0.65). Moreover, smokers were indicated to be 21% more likely to be readmitted within 30 days (1.210, 95% CI: 1.002 – 1.461, p = 0.0479) and 28% more likely to be readmitted within 90 days following surgery (1.279, 95% CI: 1.123 – 1.457, p = 0.0002). Similarly, smokers were 44% more likely to have an ER visit when compared to non-smokers (1.440, 95% CI: 1.190 – 1.741, p = 0.0002). Additionally, patients who used narcotics were 23% more likely to be readmitted within 90 days than those who didn’t (1.231, 95% CI: 1.015 – 1.494, p = 0.0352).
Our results report modifiable lifestyle risk factors have a significant impact on increased risk of readmission after SA up to 23%. There was an incremental effect of BMI on hospital readmissions. By mitigating these risk factors, this would likely result in a drastic decline in the cost of healthcare for patients undergoing SA.
#8766
Trends in Revenue, Cost, and Contribution Margin of Patients With a High Comorbidity Burden Undergoing Total Hip Arthroplasty From 2013 to 2021
*Itay Ashkenazi - NYU Langone Health - New-York, United States of America
Jonathan Katzman - NYU Langone - New York, United States of America
Jeremiah Thomas - NYU Langone Health - Scarsdale, United States of America
Muhammad Haider - NYU Orthopedic Hospital - New York, USA
Casey Cardillo - NYU Orthopedic Hospital - New York, USA
Roy Davidovitch - NYU Langone Health - New York, USA
Morteza Meftah - NYU Hospital for Joint Diseases - New York, USA
Ran Schwarzkopf - NYU Langone Medical Center Hospital for Joint Diseases - New York, USA
Patrick Connolly - NYU Langone Health - New York, USA
*Email: itay.ashkenazi@gmail.com
Introduction
With the increasing utilization of total hip arthroplasty (THA) in patients with a high comorbidity burden (HCB), coinciding with modifications to reimbursement models over the past decade, evaluation of the financial impact of HCB on THA over time is warranted. This study aimed to investigate trends in revenue and cost associated with THA in HCB patients.
Methods
Of the 13,439 patients who underwent primary, elective THA between 2013 and 2021 at our institution, we retrospectively analyzed patients considered to be with HCB (Charlson comorbidity index [CCI] 5 and American Society of Anesthesiology [ASA] scores of 3 or 4). Patient demographics and perioperative data were collected, as well as revenue, cost, and contribution margin (CM) of the inpatient episode. Changes over time for these financial markers as a percentage of 2013 values were analyzed. Linear regression analysis was used to determine trend significance. Of the 1,017 HCB patients screened for the study, 978 patients who had complete financial data were included in the final analyses.
Results
Between 2013 and 2021, direct cost have increased significantly (P=0.002), along with a non-significant trend in increased total cost (P=0.056). While revenue remained steady during the study period (P=0.486), the CM decreased markedly to a low of 38.0% of 2013 values, although not statistically significant (P=0.222). Rates of 90-day complications and home discharge remained steady throughout the study period.
Conclusion
Trends of increased costs for HCB patients undergoing THA were not matched by an equivalent increase in revenue, leading to dwindling CMs throughout the past decade. This trend is concerning as it may lead to decreased access to care for HCB patients. Re-evaluation of reimbursement models for THA that account for patients’ HCB may be necessary to ensure the financial viability of these procedures and preserve broad access to care.
Figures
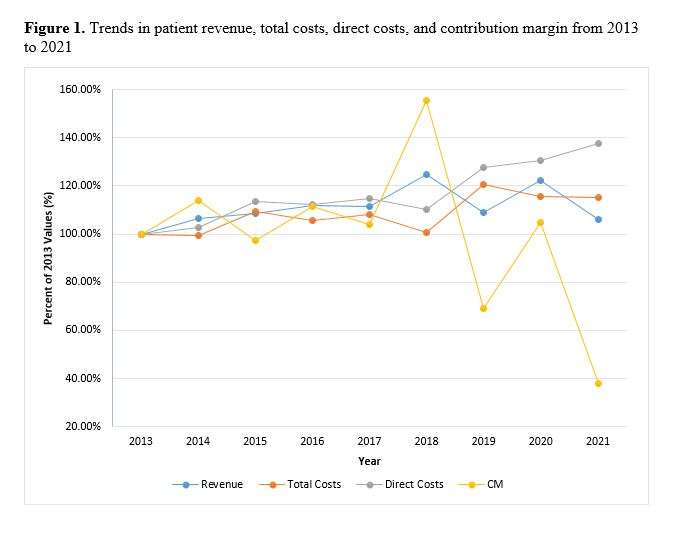
Figure 1
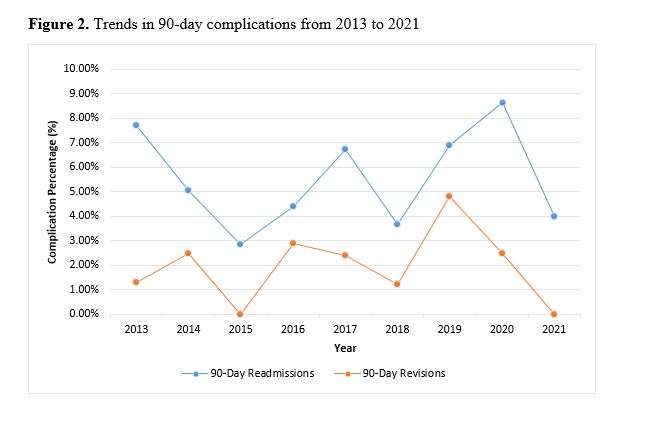
Figure 2
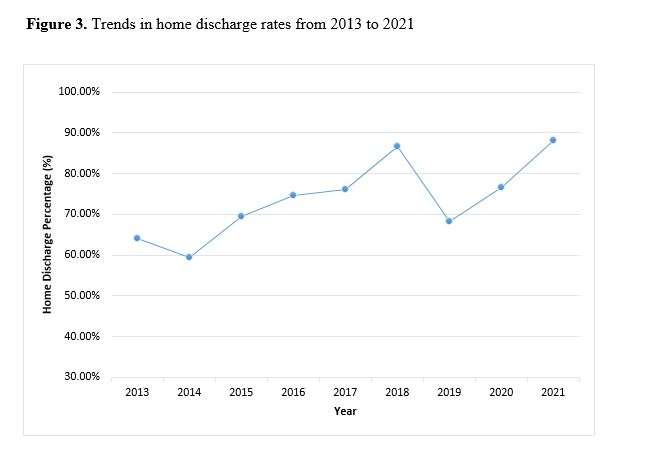
Figure 3

Figure 4
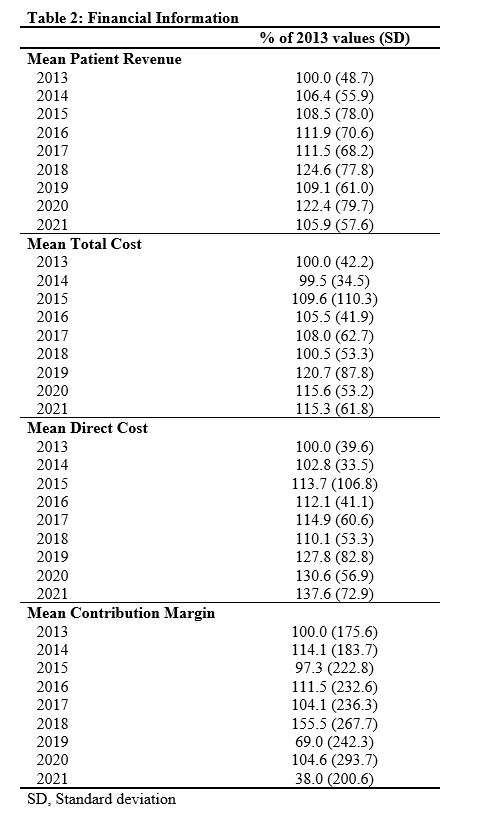
Figure 5
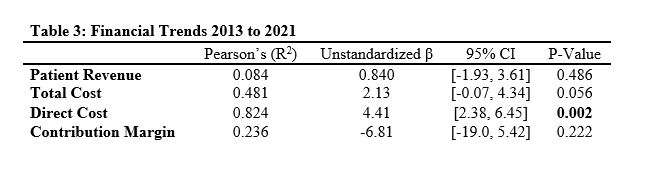
Figure 6

Figure 7
Figure 8#8148
Reduction in Rate of Implant Waste Associated With Robotic-Assisted Total Hip Arthroplasty
*Tony Shen - Hospital for Special Surgery - New York, USA
Ryan Cheng - Hospital for Special Surgery - New York, USA
Yu-Fen Chiu - Hospital for Special Surgery - New York, USA
Mark P. Figgie - Hospital for Special Surgery - New York, USA
Geoffrey H Westrich - Hospital for Special Surgery - New York, USA
*Email: shent@hss.edu
Background: Implant waste during total hip arthroplasty (THA) is costly to the United States healthcare system. Studies examining factors affecting implant waste are few and limited. The purpose of this study was to examine whether the use of enabling technologies during THA results in a smaller proportion and cost of wasted implants compared to navigation-guided and manual THA.
Methods: We retrospectively identified 104,420 implants either implanted or wasted during 18,329 primary THAs performed on 16,724 patients between January 2018 and June 2022 at our institution. THAs were separated by technology used: (1) robotic-assisted (n = 4,171), (2) imageless navigation (n = 6,887), and (3) manual (n = 7,721). The primary outcome of interest was the rate of implant waste during primary THA.
Results: Robot-assisted THA resulted in a lower proportion (1.5%) and cost ($21.97 per case) of implant waste compared to navigation-guided (2.0%, $42.60 per case) and manual (1.9%, $38.21 per case) THA. Both navigated and manual THA were more likely to waste acetabular shells (odds ratio (OR) 4.5, 3.1) and polyethylene liners (OR 2.2, 2.0) compared to robotic-assisted THA after adjusting for demographic and peri-operative factors, such as surgeon experience. Furthermore, acetabular shell waste differed significantly depending on shell size, where navigation-guided and manual THA wasted significantly more shells 56mm and larger compared to robot-assisted procedures, but not for shells 50mm and smaller. While implant waste decreased with increasing surgeon experience for procedures performed manually or with navigation, waste rates for robot-assisted THAs did not differ based on surgical experience.
Conclusion: Individual implant waste rates may vary depending on the type of technology used intraoperatively. In addition to providing more accurate component placement and restoration of leg length, the use of robotics may also, reduce the rate of acetabular component waste during THA, and ultimately, minimize overall surgical costs.
#8286
Market Resilience of Orthopaedic Hip/Knee Arthroplasty Sales During COVID-19
*Mitchell Ng - Maimonides Medical Center - Brooklyn, United States of America
Ahmed Emara - Cleveland Clinic - Cleveland, USA
Matthew Magruder - Maimonides Medical Center - Brooklyn, USA
Aaron Lam - Maimonides Medical Center - Brooklyn, USA
Afshin Razi - Maimonides Medical Center - Brooklyn, USA
Che Hang Jason Wong - Maimonides Medical Center - Brooklyn, USA
Michael Mont - Sinai Hospital of Baltimore - Baltimore, USA
Nicolas Piuzzi - Cleveland Clinic - Cleveland, USA
*Email: mitchng77@gmail.com
Introduction: The coronavirus 2019 (COVID-19) pandemic led to a significant decrease in elective surgical volume and orthopaedic device sales. The aim of this paper was to quantify this decrease and the related financial impact on the largest hip/knee arthroplasty companies by: 1) tracking individual hip/knee company valuations; 2) calculating aggregate changes in overall hip/knee arthroplasty market valuations; and 3) quantifying quarterly hip/knee revenues relative to prior years.
Materials/Methods: Financial data on the top five hip/knee arthroplasty companies by size between January 1, 2019, and October 1, 2020, was collected from a Wall Street financial database, S&P Capital IQ. Changes in valuation of these companies were compared against benchmark market indices, the S&P500 and Vanguard Healthcare ETF. U.S. hip/knee arthroplasty-specific revenue for Q1 and Q2 of 2019 and 2020 was collected from Securities Exchange Commission 10-Q forms. Quarterly revenue changes were calculated using 1-2Q19 revenues as baselines and aggregate to approximate the overall hip/knee arthroplasty market.
Results: The top five hip/knee companies lost $179.2 billion (32.7% loss) in market value from pre COVID-19 market highs to COVID-19 market lows (March 2020), while S&P500 and Vanguard Healthcare ETF decreased 36.1 and 33.2%, respectively. From market lows to October 2020, arthroplasty companies rallied 38.6% while the S&P500 and Vanguard Healthcare ETF regained 43.5 and 56.4% respectively. Notably, this occurred while aggregate 1Q/2Q20 revenue lagged 7.1/41.8% relative to 2019, with an overall decrease of $1.58B (24.8%).
Conclusion: Similar to the overall market and healthcare sector, the top five hip/knee arthroplasty companies have recovered from their COVID market lows. Our results reveal the valuations of hip/knee companies remained robust during COVID, even as revenues fell, likely due to strong investor confidence in the industry outlook and greater overall use of the healthcare system.
#8704
TKA Instrument Tray Reduction: Ambulatory Surgical Center Costs and Safety
*Robert Eberle - Maxx Orthopedics - Apex, USA
Greg Daubs, MD - Desert Orthopedic Center - Henderson, USA
Chad Hanson - Desert Orthopedic Center - Henderson, USA
*Email: eberlewriting@gmail.com
Introduction:
Chronically overstocking instrument trays for surgery is a common practice and is historically done to avoid missing instruments during a procedure, and processing a single instrument is approximately $0.51. The purpose of this study is to introduce an intra-operative instrument tray reduction strategy specific to a primary total knee arthroplasty (TKA) system, and to report on the influence of the instrument tray reduction implementation on related facility cost reductions and patient safety / outcomes through 2-years post-operatively.
Methods:
A prospective, continuous series of 34 primary TKAs in 34 patients was performed by a single surgeon. All procedures were performed in an ASC and patients were discharged the same day as surgery. To show safety across all patients in which TKA tray reduction was utilized, patient demographics, co-morbidities, and follow-up outcomes through 90 days and 1- and 2-years were recorded. In addition, common TKA-specific trays and instrument counts was assessed for processing cost comparisons.
Results:
Of the 34 patients studied, average age at surgery of 64.3 ±7.5 years (range: 35.4 – 75.5 years). The average patient BMI was 31.9 ±5.2 (range: 22.1 – 41.1). Patients presented with an average of 2.4 significant co-morbidities (range: 0 – 6). In addition, there were no adverse events related to the TKA procedure through 2-years. The reduced instrument tray processing cost was calculated at $55 and was significantly less than the other four ranging from $142 to $179.
Conclusion:
Our results have shown significant processing cost reductions for the proposed instrument tray processing costs compared to various competitive standard TKA specific instrument tray utilization (61% to 69%) without sacrificing patient safety or outcome. Further study with larger numbers is necessary. In addition, application is warranted to study the impact on operative room efficiencies including room set-up, turn-over, and the cost per square footage of instrument tray storage.
#8312
Optimizing Technology for Efficiency Across the Surgical Episode of Care
*Danielle Edwards - Hospital for Special Surgery - Manhattan, United States of America
Kelly Gritschke - Hospital for Special Surgery - New York, USA
*Email: edwardsd@hss.edu
Introduction:
As demand and costs associated with total joint arthroplasty continue to rise, it is important to focus on perioperative care to improve patient outcomes and decrease costs. Providing adequate access to care for preoperative patient education and early postoperative physical therapy (PT) is imperative. In the past our team used reports to evaluate the total time required for postoperative surgical patients to schedule either a post-acute virtual PT visit or a postoperative PT visit at one of our multiple outpatient rehabilitation facilities. To improve access to care, we created work queues for use at the preoperative physical therapy visit to collect the patient’s immediate post-acute virtual care and postoperative physical therapy location request for scheduling prior to their surgical procedure.
Methods
We leveraged technology within our electronic medical record (EMR) to create two work queues to receive information attained during the preoperative PT visit, related to the patient’s postoperative care. A work queue was created in which the preoperative PT can request post-acute virtual therapy in a workflow that worked in harmony with the case managers and social workers, which allowed all discharge planners to seamlessly order post-acute virtual therapy. An additional work queue was developed to capture the patient’s preference of their postoperative outpatient PT location and MD specified date for outpatient therapy. The information regarding the patient’s desired HSS postoperative outpatient facility was gathered during the preoperative physical therapy visit and the clinical documentation within the EMR, pulled the patient data from their visit into scheduling orders that went into the work queue.
Results
As of March 2023, the preoperative physical therapy team is seeing 100% of qualifying patients (figure 1). These new work queues allowed for multiple improvements, including better access to care (figure 2), improved staffing plans as each department was informed in advanced of the patient’s surgery and improved patient satisfaction, as patients preoperatively, were able to pick either their post-acute service or postoperative rehab location, as well as schedule their appointment at a time that was ideal. In addition, efficiency increased for the rehabilitation scheduling team, as they had decreased last minute requests and needs to juggle the schedules to fit in the surgical patient. We now can use reports and dashboards to determine the number of patients scheduled for their postoperative visits using these new work queues, in addition to looking at our access to care for rehabilitation needs for post-surgical patients (figure 3).
Conclusions
Using these new work queues allows for patient focused healthcare, as we worked to improve the patients’ journey across their surgical episode of care, as they now answer one question regarding their postoperative rehabilitation needs which then cascades into all the necessary departments and areas within the EMR, allowing for increased efficiency for the team scheduling both post-acute and outpatient rehabilitation, as these rehabilitation services are scheduled prior to the surgery.
Figures
Figure 1
Figure 2
Figure 3#8460
Investigating Knee Motion Symmetry in Total Knee Arthroplasty Patients Receiving Different Implant Designs
Nicholas Ryan - University of Ottawa - Ottawa, Canada
Eric Darmon - Université de Technologie de Compiègne - Compiègne, France
*Mario Lamontagne - University of Ottawa - Ottawa, Canada
Geoffrey F Dervin - Ottawa, Ontario, CAN
*Email: mlamon@uottawa.ca
Introduction
Osteoarthritis (OA) is a common condition that causes pain and stiffness and can adversely affect a sufferer’s movement patterns. When conservative options prove insufficient, severe knee OA is usually treated through total knee arthroplasty (TKA), which is a viable solution to ease symptoms and improve quality of life1. Although altered movement patterns often remain unresolved after surgery2-3. The choice of appropriate implant may help to restore better knee motion and loading patterns. Medial Pivot (MP) and Posterior-Stabilized (PS) are, amongst others, two types of implant designs commonly used in TKA surgery. Lower body symmetry is an important measurement to look at the improvement of movement and loading patterns after surgery. However, few studies comparing the effect of implant design on symmetry before and after TKA surgery have been performed. The purpose of this study was to investigate changes in lower body symmetry among knee OA patients after TKA surgery, and to examine whether an MP or PS implant provides greater improvement than the other.
Methods
Twenty-nine knee OA patients at Kellgren-Lawrence stage 4were recruited prior to undergoing TKA. The implant received was randomly assigned, and the participant was also blinded to the selection. Fifteen received the MP implant (MicroPort EVOLUTION®, F=7/M=8, age=63.9±5.4 years, BMI=27.6±3.3kg/m2) and fourteen received the PS implant (Zimmer NexGen®, F=6/M=8, age=65.6±7.8 years, BMI=30.1±3.7kg/m2). Twelve healthy participants (F=6/M=6, age=64.2±5.0 years, BMI=24.7±2.1kg/m2) were matched by age, height and body mass index (CTRL group). Three-dimensional motion capture analysis using a 45-marker, full-body marker set4 was performed within 1 month prior to and 12 months after surgery. Symmetry angle (SA) of knee range of motion (ROM) in the sagittal and frontal plane was calculated pre- and post-operatively as a measure of overall asymmetry (absolute values) and side-specific asymmetry (raw values5). Non-parametric tests were used to compare between- and within-group differences.
Results
For overall asymmetry in the sagittal plane, SA of the ROM for both groups was significantly more asymmetrical than the CTRL group pre-operatively, and remained so following surgery. Post-operatively, only the PS group showed a significant within-group reduction in asymmetry. The MP and PS groups were significantly different pre-operatively, but not after surgery. Pre-operatively in the frontal plane, only the PS group was significantly different to the CTRL group. No significant within-group reduction was observed for either patient group following surgery. The MP and PS groups were significantly different pre-operatively, but not after surgery.
For side-specific asymmetry, both patient groups showed significant asymmetry pre-operatively. In the sagittal plane (Figure 1), greater ROM was observed on the non-affected side, but in the frontal plane (Figure 2), greater ROM was observed on the affected side. Both groups showed significant within-group reductions in SA post-operatively, in both planes.
Conclusion
Although overall asymmetry only reached the level of the CTRL group in the frontal plane (not in the sagittal plane), TKA was successful at reducing side-specific asymmetry in knee ROM among patients suffering from knee OA in both planes. Both implant designs appeared to provide similar levels of improvement.
Figures
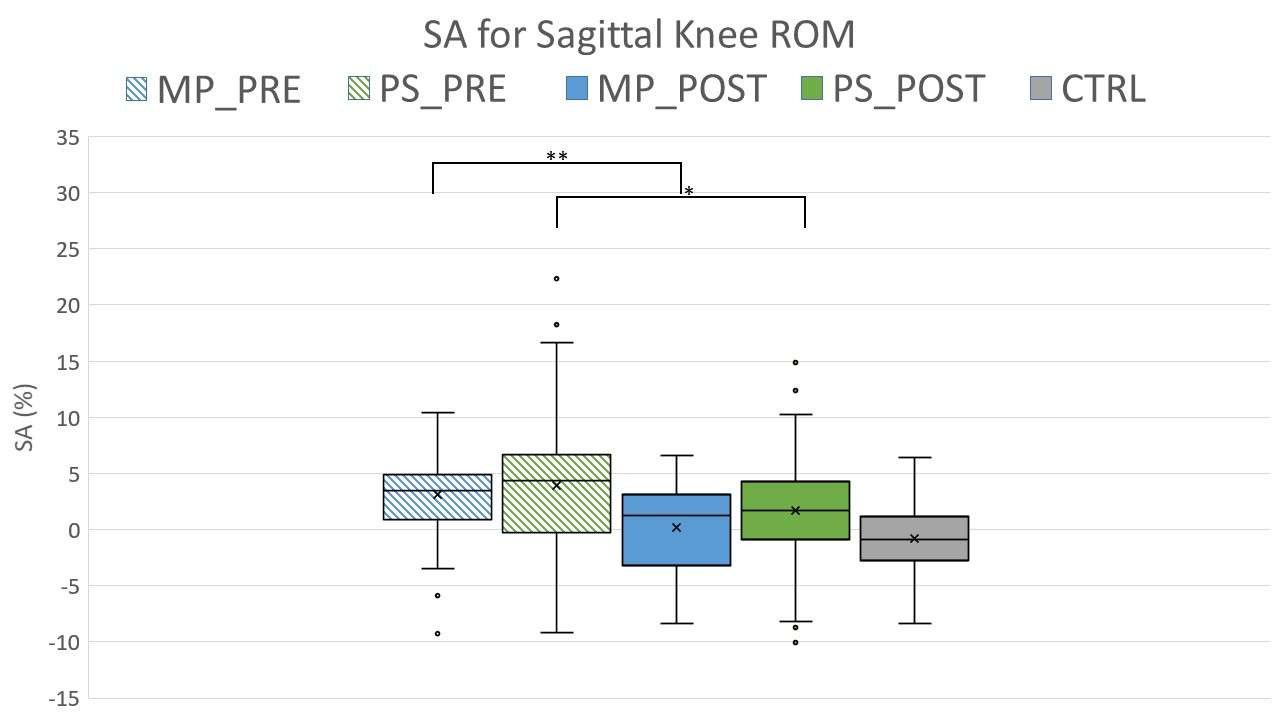
Figure 1
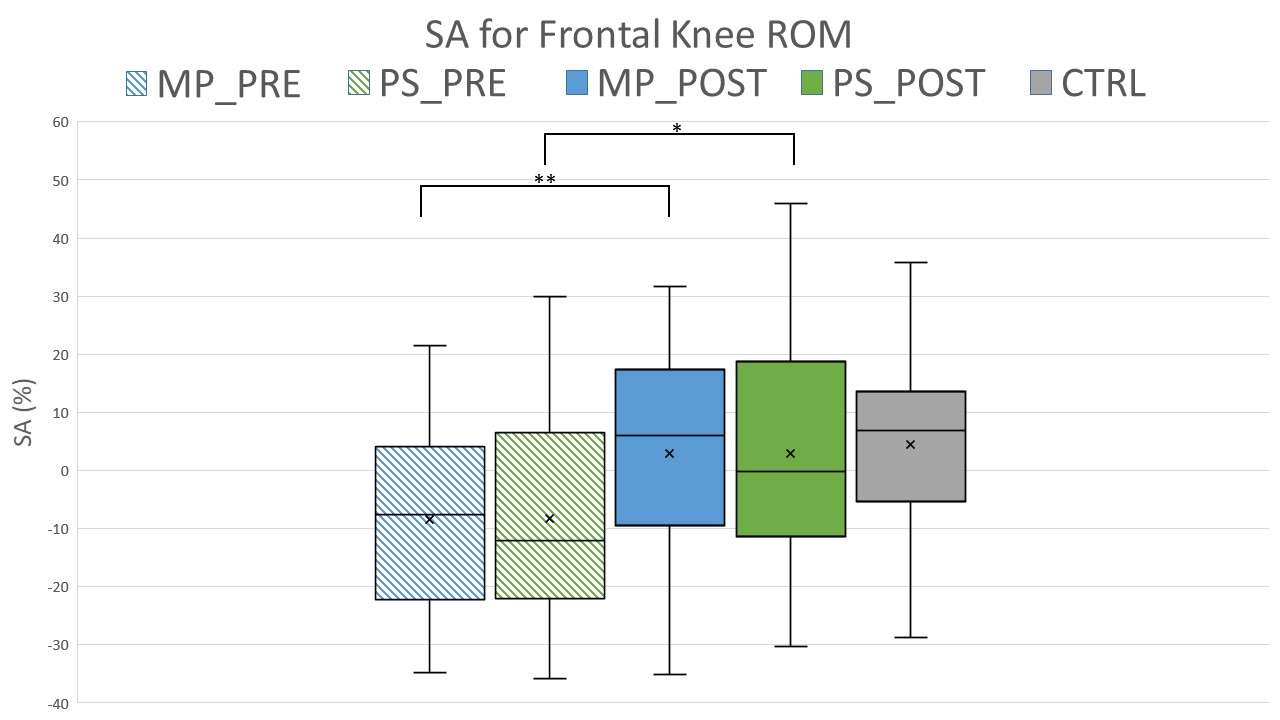
Figure 2#8243
Single Leg Stance and Timed Up and Go Tests Are Correlated With Objective Mobility Measures Following Arthroplasty
*Roberta Redfern - Zimmer Biomet - Pemberville, USA
Mike Anderson - Zimmer Biomet - Lehi, USA
Jason Cholewa - Zimmer Biomet - Warsaw, USA
Dave Van Andel - Zimmer Biomet - Grand Rapids, USA
*Email: roberta.redfern@zimmerbiomet.com
Clinical evaluation of musculoskeletal disorders, including evaluation of recovery following arthroplasty, includes a number of functional tests including the single leg stance (SLS) and timed up and go (TUG) tests. These tests require significant clinician time and patient compliance with in-person follow-up. This study aimed to determine whether step counts collected by wearable technology are associated with results of these functional tests.
Patients randomized to receive a smartwatch and care management platform were included in this analysis. Daily step counts were collected by the wearable and mobile device prior to lower limb joint arthroplasty and up to one year post-operatively. Clinical evaluations included the SLS and TUG pre-operatively and at 30- and 90-days post-operatively; the association of test times to step counts at these timepoints were evaluated by Pearson correlation test. A generalized linear model was created to estimate the effects of pre-operative steps, age, procedure, and 3-month step counts on 3-month TUG and SLS times.
385 patients were randomized to receive the wearable and mobile application and provided step count and functional test data. Average age of patients was 63.3±9.5 years; 48 (12.5%) underwent partial knee arthroplasty, 162 (42.2%) total knee arthroplasty, and 174 (45.3%) total hip arthroplasty. Considering all procedures, step counts at 1 and 3 months were moderately correlated with SLS times at the 1m and 3m assessments (r=0.31 and 0.35, both p<0.0001). Step counts at all time periods were negatively correlated with 3-month TUG results (r=-0.30 to -0.35, all p<0.001). Similar trends were observed in the TKA and THA populations, where stronger correlations were present between 3-month step counts and 3-month SLS (r=0.40, p =0.0003) in the THA population. On multivariate regression, only 3-month step counts and THA procedure were associated with 3-month TUG results. Age and pre-operative step counts were the only variables associated with SLS times at 3-months.
Step counts as collected by wearable technology were related to functional tests collected during in-person clinical assessments in this cohort. Remote monitoring of patient activity pre- and post-operatively may be a useful indicator of patient function, particularly when completion of these evaluations is not feasible.
#8197
Correlation Between Coronal Alignment and Early Postoperative Walking Ability After Bilateral TKA.
*Takaaki Nakai - Itami city hospital - Itami, Japan
Kousuke Sakata - Itami cita hospital - Itami, Japan
Yuto Nishioka - Itami City Hospital - Itami, Japan
Atsunori Ohnishi - Itami city hospital - Itami, Japan
Tsuyoshi Nakai - Itami city hospital - Itami, Japan
*Email: taknakai000m@gmail.com
Introduction: Prior studies associated coronal alignment after total knee arthroplasty (TKA) with implant survivorship and functional outcomes. But the influence of residual varus alignment on clinical outcomes after TKA is still controversial. The aim of this study was to evaluate the relationship between preoperative/postoperative coronal alignment and clinical results in patients with bilateral TKA.
Methods: Between January 2019 and December 2021, 97 patients (82 women and 15 men) who underwent bilateral TKA with Zimmer's LPS-flex mobile bearing at a single hospital were retrospectively investigated. The original diagnosis of all patients was osteoarthritis. Femorotibial angle (FTA) was measured radiologically and used to evaluate coronal alignment pre- and postoperatively.?FTA was compared in two groups of patients who were able to walk with a cane and those who walked with a walker at 2 weeks postoperatively.
Results: In the 69 patients (58 women and 11 men) who were able to walk with a cane at 2 weeks after surgery, the mean preoperative FTA was 185.0 ± 5.5° on the right and 184.6 ± 5.1°on the left; the postoperative FTA was 174.4 ± 1.4° on the right and 174.7 ± 1.6°on the left. The pre- and postoperative FTA changes were -10.6 ± 5.4° on the right and -9.9 ± 4.7 on the left. On the other hand, in 28 patients (24 women and 4 men) who were able to walk with a walker at 2 weeks postoperatively, the preoperative FTA was 185.5 ± 6.0° on the right and 187.1 ± 5.4° on the left; the postoperative FTA was 174.4 ± 1.4° on the right and 175.3 ± 1.5° on the left. The pre- and postoperative FTA changes were -10.8±5.9° on the right and -11.8±5.5° on the left. The preoperative FTA on the left was significantly related to walking ability in the early postoperative period.
Conclusion: In this study, we examined FTA and walking ability at 2 weeks postoperatively. There was a significant difference between preoperative FTA of the unilateral knee and postoperative walking ability. But there was no clear difference in postoperative FTA and clinical outcomes. The early postoperative walking ability could be influenced by various factors such as age and preoperative muscle mass. Further investigation is required to achieve appropriate alignment of components in TKA for optimal functional outcomes.
#8115
Robotic-Assisted Total Knee Arthroplasty Is Associated With Earlier Return of Symmetrical Limb Function Compared to Conventional Total Knee Arthroplasty: A Prospective Cohort Study
*Faseeh Zaidi - University of Auckland - Auckland, New Zealand
Scott Bolam - University of Auckland - Auckland, New Zealand
Michael Goplen - University of Alberta - Edmonton, Canada
Ted Yeung - University of Auckland - Auckland, New Zealand
Jacob T Munro - University of Auckland - Auckland, New Zealand
Michael M Hanlon - Auckland City Hospital - Auckland, New Zealand
Thor F Besier - University of Auckland - Auckland, New Zealand
Andrew Paul Monk - University of Auckland - Auckland, New Zealand
Craig Michael Goplen - University of Alberta - Edmonton, Canada
*Email: szai535@aucklanduni.ac.nz
Introduction: Robotic-assisted total knee arthroplasty (TKA) has demonstrated significant benefits, including improved accuracy of component positioning compared to conventional jig-based TKA. However, previous studies have often failed to associate these findings with clinically significant improvements in patient-reported outcome measures (PROMs). Wearable inertial measurement units (IMUs) offer a more nuanced and objective assessment of a patient's functional recovery after TKA. To date, there are no studies that have used wearable IMUs to evaluate early outcomes after robotic-assisted TKA when compared to conventional TKA. The aim of this study is to compare outcomes of patients undergoing robotic-assisted and conventional TKA in the early postoperative period using both traditional PROMS and novel wearable IMUs.
Methods: Patients with symptomatic end-stage knee osteoarthritis undergoing primary TKA were included in this study. Functional outcomes were assessed using ankle-worn IMUs and PROMs. IMU-based outcomes included impact load, impact asymmetry, maximum knee flexion angle and bone stimulus. PROMs included Oxford Knee Score (OKS), EuroQol-Five Dimension (EQ-5D-5L), EuroQol Visual Analogue Scale (EQ-VAS), and Forgotten Joint Score (FJS-12) were evaluated at preoperative baseline, weeks 2 to 6 postoperatively, and at 3-month postoperative follow-up.
Results: A total of 100 patients with symptomatic end-stage knee osteoarthritis undergoing primary TKA (44 in the robotic-assisted group and 56 in the conventional group) were included in this study. Robotic-assisted TKA was associated with significant improvements in impact asymmetry (82.3% vs. 22.4%; p<0.01), cumulative impact load (146.6% vs 37%; p<0.01), bone stimulus (25.1% vs 13.6%; p<0.01) and maximum knee flexion angle (37.8% vs. 24.6%; p<0.01) at postoperative week 6, compared with conventional TKA. Of note, RA-TKA demonstrated an earlier return to symmetrical limb loading compared to conventional TKA, with operative limb IMU-based function reaching 80% that of the non-operative limb by postoperative week 3. There were no differences in OKS, EQ-5D-5L, EQ-VAS, and FJS-12 at any time-point between the two groups.
Conclusion: Robotic-assisted TKA was associated with significant improvements in range of motion, impact load, bone stimulus, and earlier return of symmetrical limb function using IMUs. Traditional PROMs showed no difference in outcome at all time points when compared to conventional TKA.
#8524
Does the Knee Adduction Moment Measured From Static Radiographs Correlate With Dynamic Moments Measured During Gait?
*Fernando Quevedo Gonzalez - Hospital for Special Surgery - New York, USA
David J. Mayman - Hospital for Special Surgery - New York, USA
Theofilos Karasavvidis - Hospital for Special Surgery - New York, USA
Cale Pagan - Hospital for Special Surgery - New York, United States of America
Joseph Lipman - Hospital for Special Surgery - New York, USA
Timothy Wright - Hospital for Special Surgery - New York, USA
Peter K. Sculco - Hospital for Special Surgery - NYC, USA
Cynthia Kahlenberg - Hospital for Special Surgery - New York City, USA
Jonathan Vigdorchik - Hospital for Special Surgery - New York, USA
Eytan Debbi - Hospital for Special Surgery - NY, USA
*Email: quevedogonzalezf@hss.edu
Introduction: Most failures of total knee arthroplasties (TKA) have a mechanical origin [1]; therefore, understanding the knee joint loads is of paramount importance. The knee adduction moment (KAM) is the most important biomechanical marker for the medial-lateral load distribution between knee compartments. However, measuring the KAM requires a costly, time consuming, and highly specialized test in a dedicated motion analysis laboratory (MAL), hampering its routine clinical use. Our goal is to develop a radiographic-based static KAM measure to routinely evaluate TKA patients. As a first step in determining if the static KAM can be used as a surrogate of dynamic knee joint loading, we enrolled four patients in an IRB-approved pilot study to determine the feasibility of comparing the radiographic-based static KAM with that measured dynamically during gait in an MAL.
Methods: Four male TKA patients (age 48–76 years, BMI 22.6–30.3 kg/m2) walked barefoot preoperatively and 6 weeks postoperatively in our institution’s MAL. We simultaneously measured whole-body kinematics by tracking skin surface reflective markers with 12 cameras at 100 Hz and ground reaction forces (GRF) with four floor-embedded force plates (AMTI, Watertown, MA; Bertec, Columbus, OH) at 2,000 Hz. The dynamic KAM was calculated in the tibial frontal plane [2] by multiplying the GRF and the perpendicular distance from its line of action to the knee center, defined as the midpoint between medial and lateral epicondylar markers. The same four patients underwent standard-of-care biplanar radiographs (EOS imaging, Paris, France) at the same timepoints during bipedal and single leg stance (Fig. 1). Radiographs were synchronized with force plate measurements of each leg’s GRF (ACS Dual, AMTI, Watertown, MA). The knee skin markers, retained from the MAL, were radiopaque, allowing comparison between dynamic and static KAM. The radiographic KAM was computed in the frontal plane by multiplying the GRF and its distance to the knee center. We compared the peak dynamic KAM (wherever it occurred during the gait cycle) against the static radiographic KAM during bipedal and single leg stance.
Results: Preoperatively, the dynamic KAM range was 2.4%–4.4% of the subject’s bodyweight (BW) times height (Ht), and only two subjects exhibited the “double-hump” profile characteristic of healthy gait (Fig. 2-a). Postoperatively, the dynamic KAM was lower (1.6%–2.9% BW?Ht), and only one subject retained the double-hump profile (Fig. 2-b). For all subjects and timepoints, the radiographic KAM during single leg stance (preoperative: 0.8%–4.6% BW?Ht; postoperative: 1.8%–3.4% BW?Ht) was larger than during bipedal stance (preoperative: 0.4%–1.0% BW?Ht; postoperative: 0.4%–2.0% BW?Ht). While the sample size was small, the single leg radiographic KAM strongly corresponded with the peak dynamic KAM, especially postoperatively (Fig. 3).
Conclusion: In our initial clinical study, we observed a promising correspondence between the radiographic static KAM during single leg stance, which can be routinely obtained in TKA patients, and the peak dynamic KAM during gait. Our next steps include increasing the sample size and analyzing other timepoints in the gait cycle.
References: [1] AJRR Annual Report, 2022; [2] Shull, et al., J Biomech. 2013
Figures
Figure 1
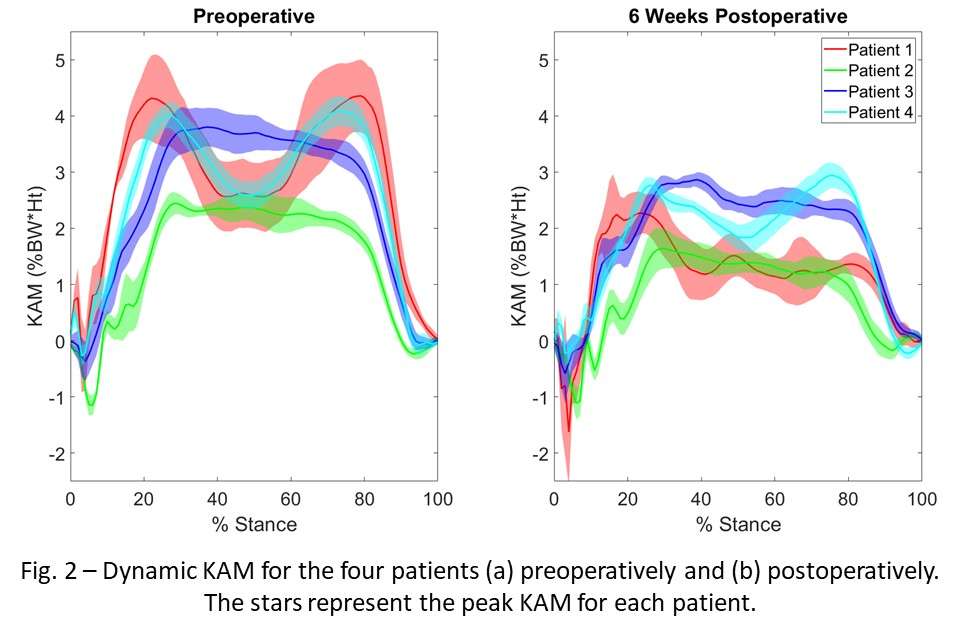
Figure 2
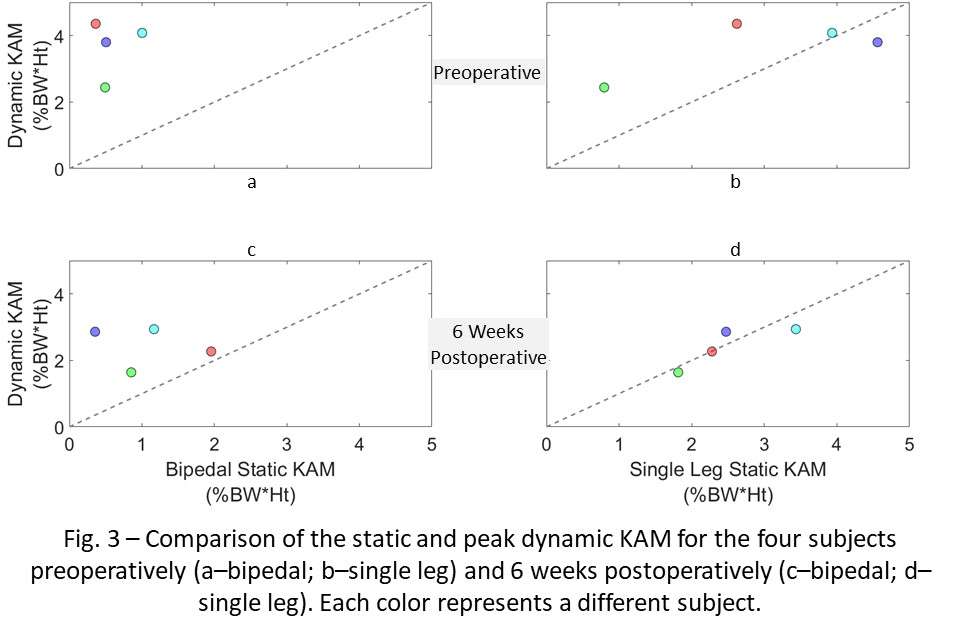
Figure 3#8549
Determination of Knee-Specific Movement Capacity From Articular Contact Geometry
*Ahmet Erdemir - Cleveland Clinic - Cleveland, United States of America
Neda Abdollahi - Cleveland Clinic - Cleveland, USA
Snehal Chokhandre - Cleveland Clinic - Cleveland, USA
*Email: erdemira@ccf.org
Introduction. Knee injuries are common and knee surgeries such as total knee arthroplasty and cruciate ligament repair are voluminous. Each knee has unique mechanics that plays a significant role in pathological degradation, risk of injury, and recovery after intervention. Utilization of movement metrics to describe the biomechanical state and function of the knee is imperative. For example, movement capacity is a measure of knee’s permissible bounds of motion dictated by joint geometrical constraints, akin to joint laxity. However, current methods for comprehensive quantification of such biomechanical markers require sophisticated experimentation or laborious modeling and simulation. The goal of this study is to develop and prototype an anatomy driven analysis strategy to comprehensively estimate knee-specific movement capacity, where movement capacity is defined by all kinematic configurations of the tibiofemoral joint afforded by articular contact surfaces.
Methods. A contact location sampling strategy generates a population of joint poses (3 translations, 3 rotations) (provisional patent application submitted). All possible contact pairs on medial and lateral articulations of tibial cartilage are matched with their femoral counterparts and for each, an optimization strategy aligns contact locations to calculate tibiofemoral joint translations and rotations (Figure 1). The generated kinematic configurations are further reduced by removal of outliers (based on z-score). Some of these poses were then excluded through a filtering process in order to distinguish the contributions of cartilage and menisci geometry articulations. The femoral contact surface’s penetration to tibial cartilages and menisci were calculated for each pose using signed-distance function. At first step, filtering was performed only based on the penetration of femoral and tibial cartilage surfaces. Second round of filtering was performed by thresholding the penetration of femoral contact surface to meniscal surfaces. For demonstration of the framework, data from oks003 specimen of Open Knee(s) were used. Geometries of femur and tibia cartilage, and menisci, which were segmented from MRI, were utilized to calculate movement capacity. Movement capacity was qualitatively evaluated against knee-specific joint mechanics for unloaded envelope of knee motion (all data points of laxity and combined loading tests < 1 N and 0.1 Nm) and for loaded envelope of knee motion (kinematics bounds at 0?, 30?, 60? and 90? flexion from laxity and combined loading data).
Results. Proposed method determines joint pose for any given joint contact pair in medial and lateral compartments (Figure 1). When performed on all possible contact pairings and filtered to exclude poses violating surface penetrations, movement capacity afforded by cartilage contact only or both by cartilage and meniscus contact (Figure 2) can be determined, as an alternative to quantify the envelope of knee joint kinematics.
Conclusion. A contact-constrained, efficient joint pose sampling strategy was developed to determine movement capacity as an indicator of a knee’s feasible motion bounds. The method relies solely on anatomy, e.g., from clinical imaging, removing the burden of joint testing and simulations. Thus, it can be incorporated in clinical workflows where movement capacity can be used for phenotyping, diagnosis, and surgical planning.
Acknowledgements. NIBIB, NIH R01EB024573 and R01EB025212.
Figures
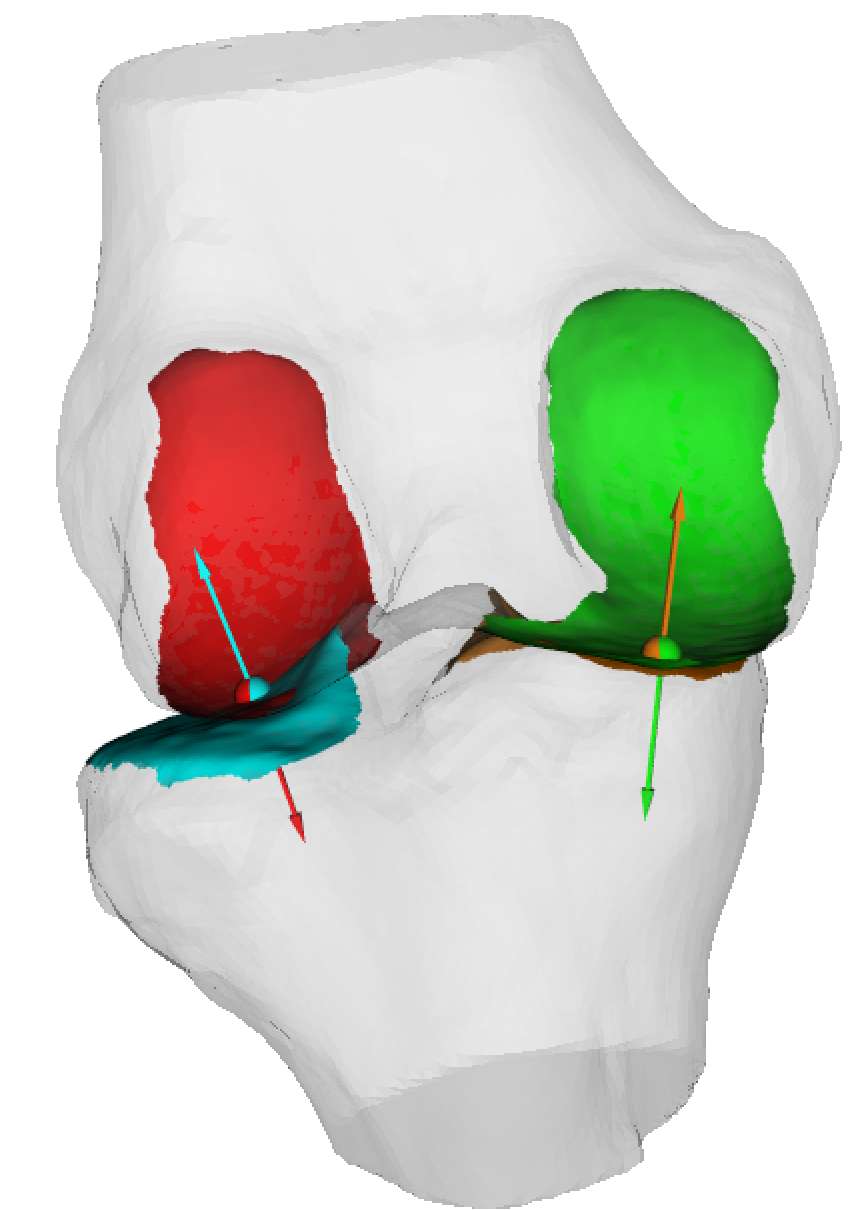
Figure 1
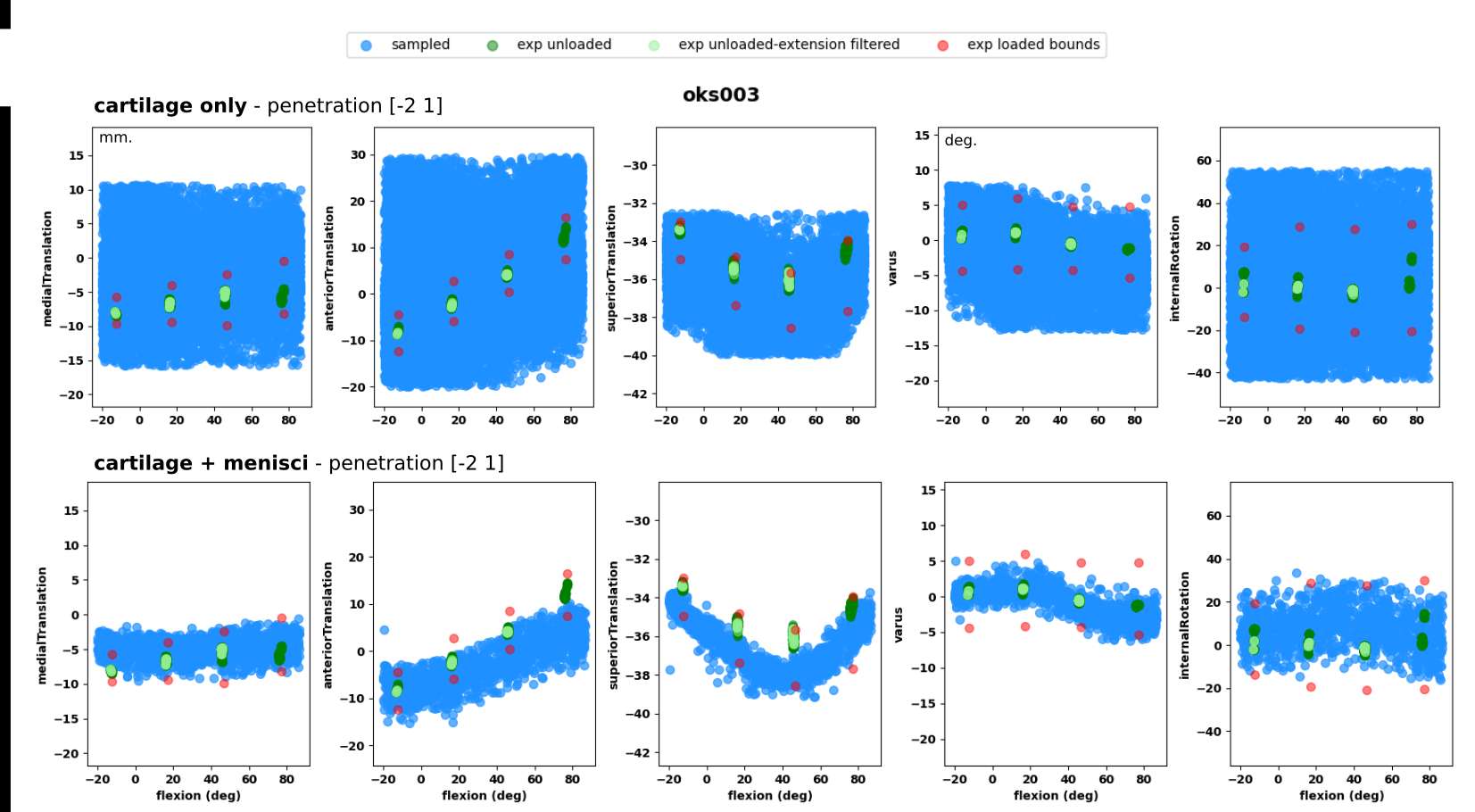
Figure 2#8376
Impact of Intra-Articular and Extra-Articular Deformity on the Femoral Trans-Epicondylar Axis
*Arun Mullaji - Breach Candy Hospital - Mumbai, India
*Email: arunmullaji@gmail.com
Introduction: The trans-epicondylar axis (TEA) is considered an important landmark based on its reported orthogonal relation to the femoral mechanical axis (FMA) in the coronal plane and its value in orienting the rotation of the femoral component in the axial plane. Various studies have addressed this issue in only normal knees, only varus or only valgus knees, usually with minimal deformity.
Aims: To assess the implications of significantly large intra-articular (varus and valgus) and extra-articular femoral deformity (bowing) on the orientation of the TEA and compare neutral, varus and valgus deformed knees. This has important consequences not only in conventional TKA surgery but also in navigated and robotic surgery in which TEA is identified and relied upon to make surgical decisions regarding bone resection and femoral component placement, as well as ligament balancing.
Methods: 210 knees undergoing TKA underwent a CT scan which included the hip, knee and ankle in a study approved by the ethics committee. TEA and FMA were identified using standard landmarks. MLDFA, MPTA, tibial mechanical axis (TMA) and computed HKA (FMA + TMA), Whitesides line (WL), posterior condylar axis (PCA) and anatomical femoral axis (AA) were defined and measured as per standard nomenclature. Knees were classed into varus (HKA<178o), neutral (178-182o), valgus (>182o).
Results: There were 102 knees with varus (mean 176o, 168-180o), 85 neutral, and 23 with valgus (mean 183o, 181-190o). FMA-AA, FMA-TEA, TEA-MLDFA were significantly different in varus, neutral and valgus knees (ANOVA p<0.001 and also on posthoc Tukey test)). TEA-WL and TEA-PCA were not different on ANOVA. HKA had a negative significant correlation with FMA-TEA (r -0.5, p<0.001) as also FMA-AA (r -0.4, p<0.001).
Discussion: These findings have important implications in alignment and balance in conventional and robotic TKA which are dependent on these landmarks. Furthermore, alterations in TEA orientation in varus and valgus knees explains the rationale for sliding epicondylar osteotomy described for severe deformities.
Figures
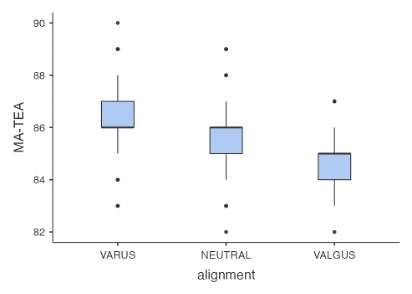
Figure 1#8195
Patient Factors Impacting the Localization of the Popliteal Artery Before TKA as Assessed by MRI
*Yoshinori Ishii - Ishii Orthpaedic & Rehabilitation Clinic - Gyoda, Japan
Hideo Noguchi - Ishii Orthopaedic & Rehabilitation Clinic - Gyoda, Japan
Junko Sato - Ishii Orthopaedic & Rehabilitation Clinic - Gyoda, Japan
Ikuko Takahashi - Ishii Orthopaedic & Rehabilitation Clinic - Gyoda, Japan
Kai Ishii - Kouseiren Takaoka Hospital - Takaoka, Japan
Yasumitsu Toribatake - Kouseiren Takaoka Hospital - Takaoka, Japan
*Email: yishii0324@gmail.com
Introduction: Intraoperative injury to the popliteal artery (PA) should be avoided during total knee arthroplasty (TKA). This study was performed to clarify the preoperative localization of the PA and the patient factors that impact its localization as a preventive measure.
Methods: Ninety-seven patients (110 knees; 18 men, 79 women) with osteoarthritis who underwent primary TKA were retrospectively reviewed (Table 1). All magnetic resonance imaging (MRI) examinations were performed with the knee in maximum extension according to the patient’s degree of flexion contracture using a 0.3-Tesla scanner (AIRIS Vento 0.3T; FUJIFILM Healthcare Co., Ltd., Tokyo, Japan). The patient was scanned while lying in the supine position. Preoperative sagittal MRI was used to measure the distance between the PA and the closest point at three levels: the femoral epicondyle (DPF), the tibial articular surface (DPAS), and the posterior tibial cortex (DPT). Previously reported patient-related factors analyzed in this study were age, sex, body weight, body height, and body mass index. Additional factors analyzed were the American Society of Anesthesiologists grade, Kellgren–Lawrence radiographic grade, Hospital for Special Surgery score, knee flexion/extension angle, range of motion, and tibiofemoral angle. The same observer performed all measurements to eliminate interobserver variation. Test–retest reliability was assessed by the same observer using the intraclass correlation coefficient in 25 participants at a 1-month interval. The intraclass correlation coefficient was calculated to be 0.988 (0.974–0.995) at DPF, 0.954 (0.891–0.98) at DPAS, and 0.962 (0.916–0.983) at DPT. All variables are expressed in millimeters as median (interquartile range).
Results: The median distance was 10.35 (7.90–12.34) mm for DPF, 6.32 (5.12–8.57) mm for DPAS, and 3.76 (2.28–5.26) mm for DPT. Body height and weight showed weak correlations with DPF (r = 0.324, p < 0.001 and r = 0.207, p = 0.03, respectively). DPF was smaller in women [9.82 (7.64–12.23) mm] than in men [11.27 (10.26–12.75) mm] (p = 0.004). A larger flexion angle and range of motion showed a weak negative correlation with DPT (r = −0.282, p = 0.003 and r = −0.236, p = 0.016, respectively). Multiple regression analysis revealed that DPF was related to body height (β = 0.341, p < 0.001) and that DPT was related to the flexion angle (β = −0.264, p = 0.005). DPAS was not related to any analyzed factors in this study.
Conclusions: Special attention should be paid to women with a small physique on the femoral side and/or patients with a large flexion angle on the tibial side as a strategy to prevent PA-related complications. The strength of this work is that to the best of our knowledge, it is the first study involving a detailed evaluation of the factors that impact the localization of the PA using a multivariate analysis. In addition, these results may provide important information not only for TKA surgery, but also for multimodal anesthesia for TKA and arthroscopic cystectomy safely and reliably in the popliteal area.
Figures
Figure 1#8125
Condylar Motion of Human Cadaveric Knees Before and After Sacrificing Both Cruciate Ligaments
*Saskia Anna Brendle - Aesculap AG / LMU Munich - Tuttlingen, Germany
Sven Krueger - Aesculap AG Research & Development - Tuttlingen, Germany
Joachim Grifka - Regensburg University Medical Center - 93077 Bad Abbach, Germany
Peter E. Mueller - Hospital of the Ludwig-Maximilians-University of Munich - Munich, Germany
Thomas M. Grupp - Aesculap AG - Tuttlingen, Germany
*Email: saskia.brendle@aesculap.de
Introduction
Even though knee prostheses have improved greatly and became one of the most reliable joint replacements, numerous studies point out that only approximately 80 % of patients are satisfied with the results of their total knee arthroplasty (TKA) [1]. It is hypothesized that recreating native knee kinematics is beneficial regarding patient satisfaction after TKA [2]. However, it is not clear whether all native knees show the same kinematic pattern and would therefore be suitable for the same TKA design. For this reason, the aim of this study was to characterize the condylar motion of native knees and to investigate changes after sacrificing the anterior cruciate ligament (ACL) and posterior cruciate ligament (PCL), respectively, in order to identify different implant requirements.
Methods
In this in vitro study, nine fresh-frozen human cadaveric knees were tested on a six-degrees-of-freedom joint-motion-simulator (Advanced Mechanical Technologies Inc., Watertown, USA). The neutral path of motion of each knee was recorded by applying continuous knee flexion and extension from 0° to 90° with 50 N compression force at different stages of resection (native, after resection of the ACL and after resection of both cruciate ligaments), with all other forces/moments maintained at 0 N/Nm. Prior to each resection stage, the knee capsule was opened using a medial parapatellar approach and closed by sutures. In order to track the relative position of femur and tibia during testing, each specimen underwent a complex 3D fitting process (ARAMIS 12M, GOM Metrology GmbH, Braunschweig, Germany) using segmented CT-scans containing landmark based femoral and tibial coordinate systems. Knowledge of the relative positions of femoral and tibial coordinate systems and their according bone geometries allowed the projection of the flexion axis and medial and lateral flexion facet centers (MFC and LFC) onto the tibial plane at different flexion angles and therefore the measurement of condylar motion throughout the arc of flexion. Anterior-posterior (AP) translation of the MFC and LFC of each specimen was calculated and normalized to the native medial AP position at 0° flexion.
Results
AP translation of the MFC and LFC and consequently condylar motion varied between the specimens and stages of resection. Specimen P08, for example, showed similar AP translation of the MFC and the LFC, resulting in a symmetrical femoral rollback in the native condition (Figure 1a). Femoral rollback decreased after sacrificing the ACL and disappeared mostly after sacrificing both cruciate ligaments (Figure 2). In contrast, specimen P12 showed almost no posterior translation of the MFC, whereas a large posterior translation was observed for the LFC (Figure 1b). Consequently, a medial pivot was present in the native condition and was maintained after sacrificing the cruciate ligaments (Figure 3).
Conclusion
It was shown that the condylar motion of native knees and the effect of ACL and PCL resection varies greatly and therefore each knee has individual requirements regarding implant design and alignment to mimic native knee kinematics. A further study will investigate which implant designs best replicate the specimens’ native knee kinematics.
References
- Bourne et al. 2010
- Angerame et al. 2019
Figures
Figure 1
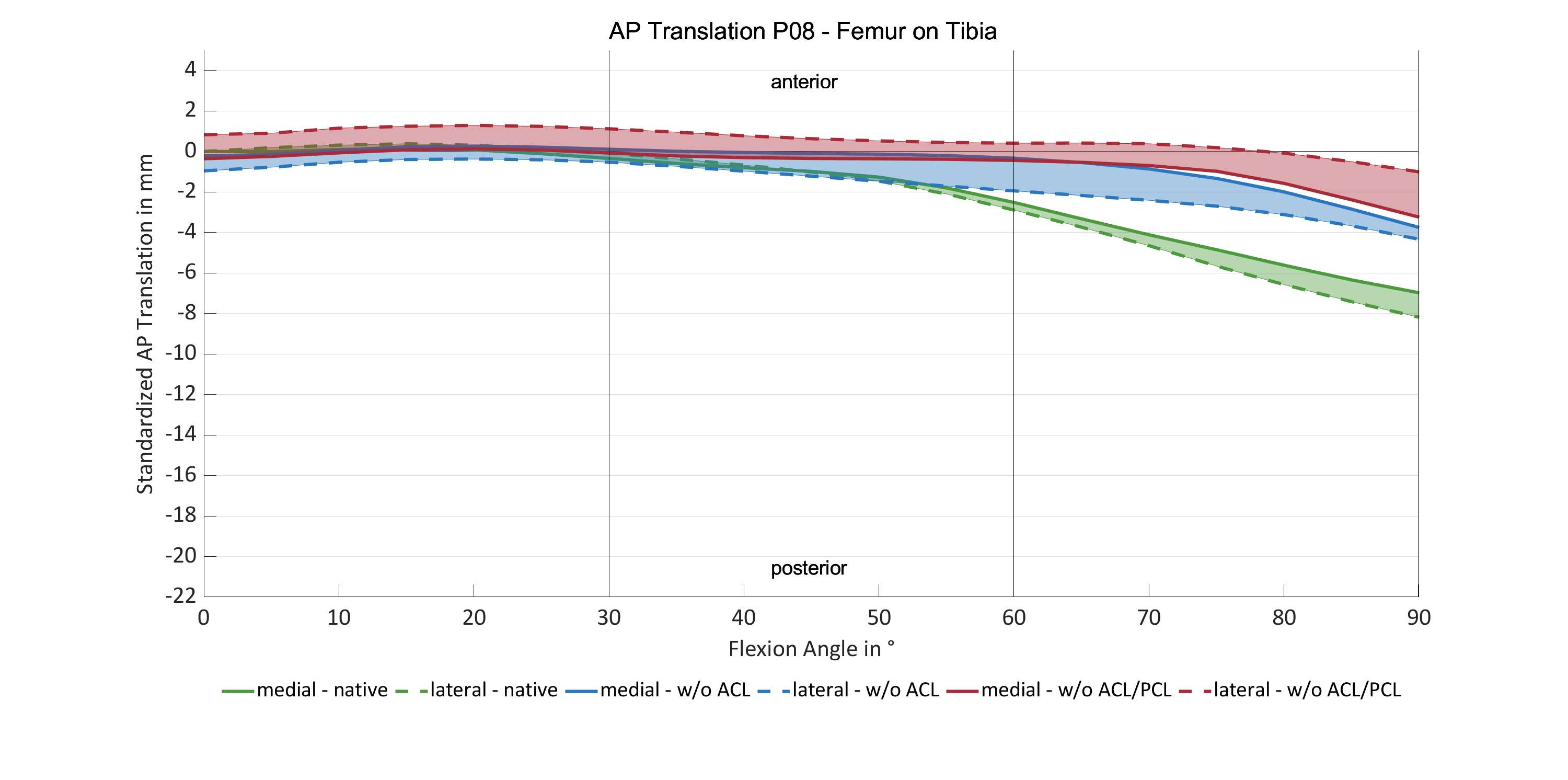
Figure 2
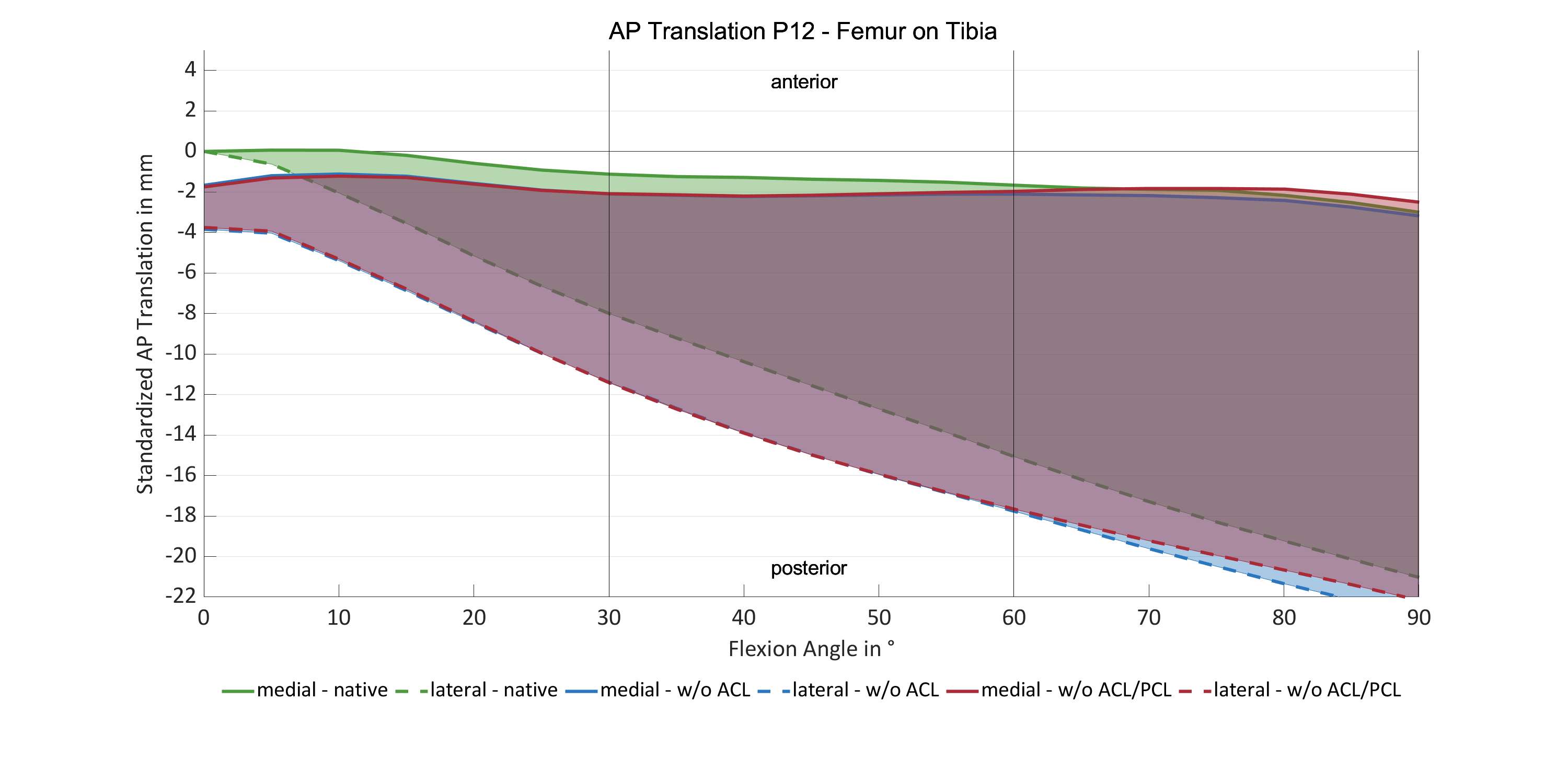
Figure 3#8556
Evaluation of the Kinematic Pattern of a Modern Posterior-Stabilized Implant Design Utilising Robotic-Arm Assistance.
*Fabio Mancino - University College London Hospitals NHS Foundation Trust - London, United Kingdom
Andreas Fontalis - University College London Hospital - London, United Kingdom
Ricci Plastow - University College London Hospitals NHS Foundation Trust - London, United Kingdom
ahmed magan - University College London Hospital - London, GB
Fares Haddad - University College London Hospital - London, United Kingdom
*Email: fabio.mancino90@gmail.com
Introduction
Physiological knee kinematics in normal knees demonstrate a medial pivot pattern and femoral rollback during flexion. Functional alignment, aims to re-establish the constitutional alignment and ligament tension, and as such the aforementioned outcomes have been extensively studied. Nevertheless, there exists a notable dearth of literature pertaining to the evaluation of kinematic patterns subsequent to TKA, mainly attributable to the lack of readily accessible tools for quantifying kinematics. The aim of this study was to evaluate the proportion of patients achieving a medial kinematic pattern following robotic-arm assisted TKA with a contemporary PS implant design, using functional alignment.
Methods
Our prospective study encompassed 60 consecutive patients undergoing primary TKA for osteoarthritis with a modern, PS implant design. All procedures were performed with robotic-arm assistance employing functional alignment, aiming to restore the patient’s pre-arthritic knee anatomy by restoring the native joint line height and obliquity. Following final implant positioning, sensor-embedded tibial trials were used to assess the kinematic pattern. Passive range of motion from maximum extension to maximum flexion was performed for each knee. Two senior, fellowship trained surgeons observed the kinematic tracking of the tibiofemoral articulation and center of load on the sensor display monitor. Furthermore, the differential load pressure was captured intraoperatively at 10°, 45° and 90° to substantiate the kinematic pattern.
Results
Our cohort included 38 females and 22 males with a mean age of 68 years with end-stage knee osteoarthritis and a coronal deformity <20°. The medial pivot kinematic pattern of the tibiofemoral articulation was found in 35 patients (58%). Load pattern and peak load pressures were compared between medial and non-medial pivot kinematic pattern. Overall, the mean medial load value was 26.3 ± 3.9 lbs at 10°, 29.2 ± 3.6 at 45°, and 32.5 ± 3.6 at 90°. Laterally, the overall mean load was 18.4 ±4.9 at 10°, 22.9 ± 4.6 at 45°, and 20.8 ± 4.6 at 90°. When evaluating the TKAs with medial pivot pattern, the mean medial load was 26.9 ± 3.9 lbs at 10°, 29.8 ± 3.9 lbs at 45°, and 32.7 ± 3.9 lbs at 90°, while the mean lateral load was 19.2 ± 5.2 lbs at 10°, 23.5 ± 4.9 lbs at 45°, and 19.1 ± 4.1 lbs at 90°. Mean differential load pressure between the compartments was <15 lbs at 10°, 45°, and 90° in both groups, corroborating mediolateral stability of the tibiofemoral articulation.
Conclusion
In our cohort, using robotic arm assistance with functional alignment we observed a medial pivot kinematic pattern in approximately 60% of the cases, with the use of a modern PS implant design. Further longer-term studies are warranted to validate these findings on a larger scale and evaluate the impact of the kinematic pattern on clinical and patient-reported outcomes.
#8250
Differences in Tibiofemoral Contact Locations Between Bicruciate-Retaining and Posterior Cruciate-Retaining TKA
*Kelly Mills - Sint Maartenskliniek - Nijmegen, Netherlands
Bart Kaptein - LUMC - Leiden, Select Country
Simon Van Laarhoven - Sint Maartenskliniek - Ubbergen, Netherlands
Petra Heesterbeek - Sint Maartenskliniek - Nijmegen, Netherlands
*Email: k.mills@maartenskliniek.nl
Introduction:
The tibiofemoral contact point (CP) location has been reported to be related to functional and clinical outcome after TKA. In this study we compared the CP of a bicruciate-retaining (BCR) TKA with a posterior cruciate retaining (CR) TKA. The difference between these two implants is the retention of anterior cruciate ligament (ACL) in the BCR-TKA. Thereby the BCR-TKA is designed to allow for the natural soft tissues to control the motion in the knee. Therefore the aim of this study was to see whether we see a more natural knee motion in the BCR-TKA compared to the CR-TKA, by judging CP locations.
Methods:
A total of 10 patients with the Journey II CR implant and 10 with the Journey II XR implant (BCR) were included from two simultaneous prospective cohort studies. At three months postoperatively weight-bearing radiostereometric (RSA) images were taken, in extension and in 90° flexion. CP locations were determined from these RSA radiographs using Model-based RSA software and compared between the implants. These CPs were determined as the minimal distance locations between femur and tibia, for the medial and lateral department separately. All CP locations are reported as a ratio from 0 to 1, with 0 being the most anterior point of the tibial inserts and 1 the most posterior.
Results:
There were no significant differences between the groups in terms of age, side, gender or BMI. The lateral BCR-TKA CP was significantly more posterior compared to the CR-TKA CP in 90° flexion (Median 0.67(IQR 0.62-0.72) vs 0.56(0.49-0.59); p=0.002). In terms of displacement of the CP location from extension to 90° flexion the BCR-TKA laterally shows more lateral compared to the CR-TKA (Median 0.25(IQR 0.21-0.29) vs 0.12(0.07-0.18; p=0.012) (Figure 1). There is also a trend towards a higher posterior displacement medially in the BCR-TKA group (Median 0.09(IQR 0.04-0.13) vs 0.01(-0.02-0.08); p=0.070). The variability in CP locations was higher for the BCR-TKA in extension.
Discussion and Conclusion:
This preliminary comparison shows that both implants are medially stable. The BCR-TKA shows more lateral physiological rollback from extension to flexion, indicating that the more flat tibial inserts of this design allows for the ligaments to control the motion as they do in the natural knee. The BCR-TKA also showed a trend towards more medial posterior displacement from extension to flexion, which could also play a role in the lateral rollback as these are coupled motions. In extension the variability in CP locations of the BCR-TKA was higher in both compartments. This could indicate imperfect balancing of the ACL in some patients. At the time of the conference we expect to share a 15 vs. 15 patients comparison of these data.
Figures
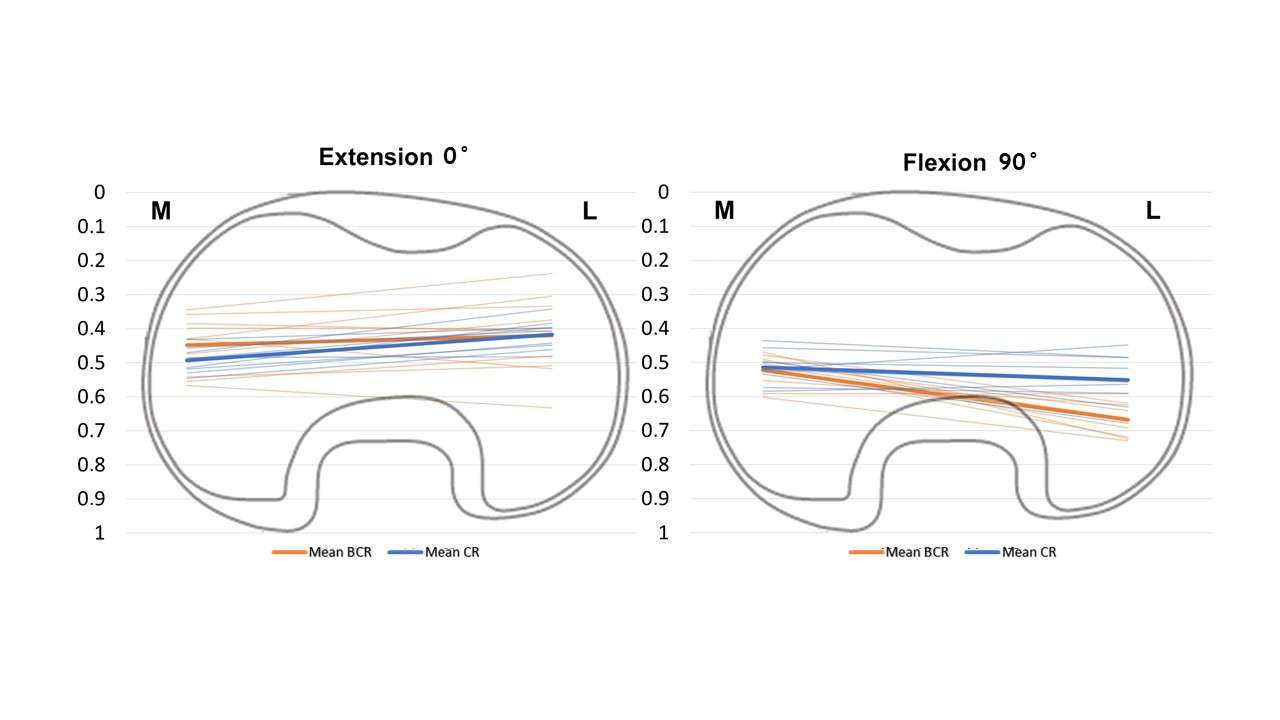
Figure 1#8713
DRR Acceleration Using Inexpensive GPUs for Model-Image Registration Based Joint Kinematic Measurements
*Satoru Ikebe - Fukuoka, Japan
Takeshi Shimoto - Fukuoka Institute of Technology - Fukuoka, Japan
Hidehiko Higaki - Kyushu Sangyo University - Fukuoka, Japan
Scott Banks - University of Florida - Gainesville, USA
*Email: ikebe.s@ufl.edu
Introduction: Model-image registration methods are commonly used in research to measure three-dimensional joint kinematics from single-plane and bi-plane x-ray images. These methods have the potential to be beneficial if used clinically, but current techniques are too slow or expensive to be clinically practical. One technical element of these methods for measuring natural bone motion is the use of digital reconstructed radiographs (DRRs). DRRs can be very expensive to compute or require expensive and fast computer hardware. The goal of this technical development was to implement a numerically efficient Siddon-Jacobs algorithm for computing DRRs on a consumer-grade graphics processing unit (GPU) to assess the computational speedup relative to traditional CPU-based calculations.
Methods: The traditional CPU method positions a CT-based voxel volume bone model in a virtual projection space, and then explicitly projects each voxel onto a region of pixels according to its radiographic density. The new GPU implementation adopts the Siddon-Jacobs algorithm, which computes an intensity for each pixel in the region of interest by performing an attenuation line summation through the voxel model positioned in the virtual projection space. This method is implemented in the Compute Unified Device Architecture (CUDA) programming language. A consumer-grade laptop computer outfit with a modest ($200 USD) graphics card was used for this experiment. The time required to render 120 DRRs of a voxelized femur model using a CPU-only traditional calculation and a parallelized implementation programmed in CUDA and run on the GPU were recorded. In addition, full model-image registration was performed for 50 x-rays of ten healthy human knees.
Results: The GPU computation of DRRs was approximately 1000 times faster than the CPU computation (62ms versus 62s, Table 1). Joint kinematics measured using the CPU and GPU methods were equivalent, with RMS differences of less than 0.5mm or 0.5deg (Table 2).
Conclusion: A clinically practical joint kinematics measurement method could benefit a wide range of patients and surgical procedures. Current model-image registration methods for measuring joint kinematics are too cumbersome or computationally expensive to be clinically practical. We demonstrate that an efficient parallel implementation of DRRs run on consumer-grade GPUs can accelerate the measurements by several orders of magnitude while maintaining measurement accuracy. This technical approach may support the introduction of a clinical joint kinematics examination in the near future.
Figures
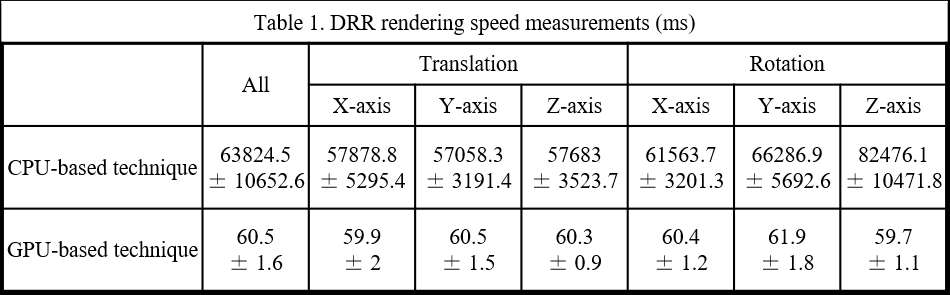
Figure 1
Figure 2#8610
Achieving High Weight-Bearing Flexion With an ACL Retaining TKA: Is Surgeon an Even Larger Difference Maker?
*Lauren Smith - University of Tennessee, Knoxville - Knoxville, USA
Richard Komistek - The University of Tennessee - Knoxville, USA
Michael LaCour - University of Tennessee - Knoxville, USA
Michael Ries - Reno Orthopedic Clinic - Reno, USA
Jan Victor - Ghent University - Gent, Belgium
Mark Freeman - Erlanger Health System - Chattanooga, USA
*Email: lsmit186@vols.utk.edu
Introduction:
Throughout the history of total knee arthroplasty (TKA), management techniques of the cruciate ligaments are a common topic of discussion. The definition of a successful TKA procedure is still up for debate; is it patient satisfaction, overall range-of-motion, normal-like kinematics, or a combination of all three? Various fluoroscopic studies have focused on determination of kinematic differences between designs, highlighting various amounts of weight-bearing flexion, but do surgeons make an even larger difference regarding overall implant success? This research study not only highlights the importance that ACL retention can have on knee kinematics but assesses the influence that the surgeon can have on post-operative kinematics while implanting a Bi-Cruciate Retaining (BCR) TKA, compared to the normal knee.
Methods:
The in vivo 3D kinematics were determined for 29 subjects having a BCR TKA implanted by three different surgeons (10, 9 and 10, respectively) and 10 having a normal knee. All knees were deemed well-functioning by their respective surgeons. Kinematics were obtained using fluoroscopy and a validated 3D-to-2D registration technique. All subjects were asked to perform a weight-bearing deep knee bend (DKB) maneuver intended to flex the knee from full extension to maximum flexion. The kinematic parameters assessed for each subject were lateral and medial anterior/posterior (LAP, MAP) condylar position and axial orientation (Figure 1).
Results:
During DKB, the Ries cohort exhibited the largest ROM (129.1°) overall (p<0.0004), with the other two groups displaying an almost identical ROM (104.7°,105.4°). The Freeman cohort demonstrated a significantly more anteriorized initial position than both remaining cohorts (p=0.0003), which we expect to see in a normal knee, allowing the condyles to experience higher amounts of rollback during knee flexion. In early-flexion (0-30) and late flexion (90-Max), all 3 cohorts exhibited progressive rollback of both femoral condyles. Mid-flexion highlights small amounts of anterior sliding for all 3 cohorts, as one can expect to see in any implanted TKA. Detailed kinematics can be found in Table 1, while a visual representation of these motion patterns can be seen in Figure 3.
Discussion:
The results of this study demonstrate that surgeon variability does have an influence on post-operative TKA kinematics. While previous BCR TKAs experience less weight-bearing flexion, subjects in this study achieved higher amounts of knee flexion. Subjects in this study with a BCS TKA experienced a more anteriorized initial position at full extension than other implanted TKAs previously analyzed. The difficulty of this procedure, as well as varying patient ligament strengths could be two big contributors to the diverse kinematic profiles yielded from this study. Nonetheless, despite some initial variability, all 3 cohorts typically experienced progressive rollback and kinematic patterns similar to the normal knee, indicating that, despite surgeon variability, retaining both the ACL and the PCL appears to yield overall kinematic patterns that are similar to the normal knee, indicating the importance of ACL functionality postoperatively.
Figures
Figure 1
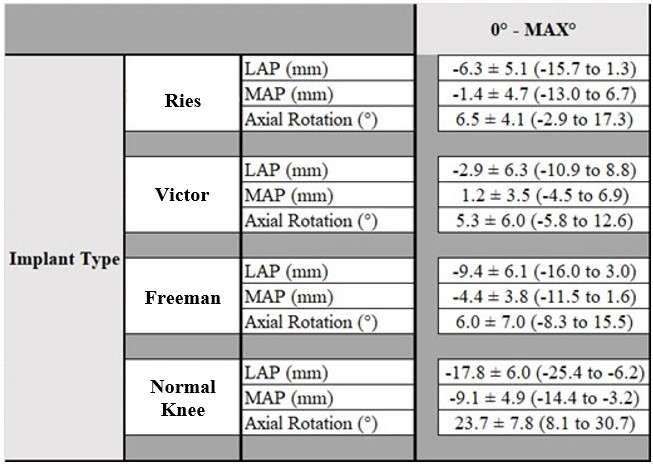
Figure 2
Figure 3#8321
Early Safety and Performance Data on the Novel PEEK-Optima Metal Free TKA System
*Frederique Vanermen - Orthopaedic Center Antwerp - Knokke, Belgium
Willem Geuskens - UZ Leuven - Leuven, Belgium
Peter Verdonk - UZ Gent - Gent, Belgium
*Email: frederique.vanermen@gmail.com
Early Safety and Performance data on the novel PEEK-Optima metal free TKA system
Frederique Vanermen, MD; Willem Geuskens, MD; Kristien Vuylsteke Msc; Peter Verdonk MD, PhD
ORTHOCA, Antwerp, Belgium
Introduction:
Total knee arthroplasty is a cost-effective procedure since it provides a substantial improvement in quality of life. Nevertheless, there is a well-known patient dissatisfaction rate of 15-20%. Prosthetic material related potential contributing factors to patient dissatisfaction are metal allergy, stress shielding, temperature sensitivity, etc. A hypoallergenic, non-cobalt-chrome containing prosthesis might be a valuable alternative to classic metal-based bearings. PEEK is chemically inert, biocompatible and has excellent mechanical properties such as stiffness, toughness and durability, and a modulus close to that of natural cortical bone. In addition, due to the absence of ferromagnetic substances, MRI and X-ray imaging is much easier. Therefore, this case series evaluated the potential of PEEK-OPTIMA as an alternative joint bearing material.
Methods:
This case series assessed the clinical, radiographic and MRI outcomes of 10 patients treated for uni-lateral, end-stage symptomatic knee osteoarthritis with a cemented PEEK-OPTIMA femoral component and an all-polyethyleen CR or PS tibial tray (Freedom Knee system, Maxx Orthopedics Inc, USA). The patella was resurfaced. Inclusion criteria consisted of nickel allergy and age of 50-75 years old. Range of motion (ROM), Knee Society Scores (KSS), Short Form 36 (SF-36), Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC), Oxford Knee Score (OKS) and standard PA and lateral radiographs as well as MRI scans were assessed pre- and postoperatively. The test used for the statistical analysis was the Mann-Whitney U test.
Results:
The mean follow-up period was 12 months with a minimum of 3 months. Both clinical and patient subjective outcomes improved significantly (p < 0.05). The mean extension and flexion range of motions improved from 3 degrees (Range 0 to 15) to 0 degrees (Range 0 to 2) and from 112 degrees (range 100 to 125) to 117 degrees (Range 85 to 130) respectively. All control radiographs showed satisfactory alignment without any signs of loosening, or failure of the components. MRI imaging was able to evaluate all soft tissues and prosthetic components: no fractures or loosening of the femoral nor tibial component were observed.
Conclusion:
This is the first report on a novel PEEK-Optima femoral bearing in Total knee arthroplasty. Early follow-up results show satisfactory safety, clinical and radiological outcomes. This novel implant has some potential advantages over metal implants since it allows improved visualization on X-ray and MR imaging and provides a true alternative for patients with a possible sensitivity to metal.
#8702
Novel Polymer Femoral Component as a Metal-Free Alternative for Tka
Asit Shah - Englewood Health - Englewood, USA
Shaan Sadhwani - Touro College of Osteopathic Medicine - New York, USA
*Robert Eberle - Maxx Orthopedics - Apex, USA
*Email: eberlewriting@gmail.com
INTRODUCTION
The use of cobalt chromium alloy (CoCr) in total knee arthroplasty (TKA) is routinely accepted as an articulating material against various polyethylene inserts. However, CoCr femoral components for TKA are of complex design, costly to manufacture, adversely influence bone stresses, obscures periprosthetic bone imaging, and releases metal ions during the service life of the implanted component. The purpose of this study is to test the feasibility of injection-molded polyetheretherketone (PEEK) as a weight-bearing articulating material for primary TKA use in humans.
METHODS
The PEEK femoral component is manufactured as an injection molded version of the cobalt chrome (CoCr) femoral component used in the system. Therefore, the bearing surface geometry of the femoral components were identical. The PEEK femoral component underwent extensive benchtop destructive and non-destructive testing to determine the feasibility of advancing further controlled study in-vivo.
RESULTS
In all instances of benchtop testing, including mechanical, fixation, wear, imaging, finite element modeling and biocompatibility, we report results that demonstrate performance that mitigates the risks of the pre-determined failure mechanisms in comparison to the incumbent CoCr component. Following extensive review of the combined test results by independent clinical and technical key opinion leaders it was determined that the residual risks of the PEEK femoral component are outweighed by the expected patient benefits.
CONCLUSION
From the testing and results within the design profile, we found that PEEK as a material for femoral components is favorable as an articulating and weight-bearing surface for primary TKA applications. The advantages of PEEK for primary TKA include significant manufacturing efficiencies over CoCr, modulus similar to bone, avoidance of imaging artifacts and no release of metal ions thus avoiding patient metal alloy sensitivity / allergy. These findings warrant further study of safety and efficacy in humans via the US-FDA Investigational Device Exemption pathway prior to commercialization and sale in the US.
#8201
Good Fit of Modern Symmetric Tibia Component Designs on Asian and Caucasian Statistical Shape Models
*Ingrid Dupraz - Aesculap AG - Tuttlingen, Germany
Philipp Hauff - Aesculap AG Research and Development - Tuttlingen, Germany
Brigitte Altermann - Aesculap - Tuttlingen, Germany
Yukihide Minoda - Osaka City University Graduate School of Medicine - Osaka, Japan
Nikolai Kornilov - Vreden National Medical Research Center of Traumatology and Orthopedi - Saint Petersburg, Russia
Thomas M. Grupp - Aesculap AG - Tuttlingen, Germany
*Email: ingrid.dupraz@aesculap.de
Introduction
The anatomical fit of tibia components remains an important factor in the daily practice of Total Knee Arthroplasty. Modern implant portfolios strive for the right balance between best accommodating the large variability of anatomies, while limiting available sizes for manageable logistics and costs. Statistical Shape Models (SSM) are a useful tool to efficiently assess the anatomical fit of TKA implants, as the anatomy of a wide part of the population is captured in a limited number of 3D models. The goal of this study was to assess the anatomical fit of 2 modern and 2 older, clinically established tibia implant designs using Asian and Caucasian SSMs.
Methods
A Caucasian and an Asian SSM were built on 120 Caucasian and 112 Asian OA anatomies. 7 bones in different sizes were derived from the Caucasian SSM and 5 sizes from the Asian SSM [1]. The AP axis was defined by the Akagi Line, which was projected on the axial plane (orthogonal to the mechanical axis). The tibia was virtually cut at 8mm under the deepest point of the medial plateau (assuming a cartilage thickness of 2mm), orthogonal to the mechanical axis. The tibia components were virtually implanted using the Materialise 3-matic® Software. The placements were performed by a senior Asian (resp. Caucasian) surgeon for the Asian SSM (resp. Caucasian SSM). Following rules were applied: 1.5 mm overhang was tolerated laterally and medially; 0.5mm overhang was tolerated anteriorly and postero-laterally; up to 6° of internal rotation was tolerated. Following implants were placed: oneKNEE® (Aesculap) and Attune® (Depuy-Synthes) as modern designs, Columbus® (Aesculap) and Sigma® (Depuy-Synthes) as older designs. To avoid any impact of the notch area on the coverage, the notch was closed on all implants. Tibia coverage was computed as the percentage of the bone surface covered by the implant. Dunnett’s test was performed to check for statistical comparisons in coverage between the oneKNEE® and the other designs. Cortical support was visually assessed by localizing the surface of the implant supported by cortical bone.
Results
The bony coverage ranged from 89% to 93% for the oneKNEE® design (resp. 87-92% for Attune®) compared to 83-86% for the Columbus® design (resp. 84-91% for Sigma®) [Fig. 1]. Especially on Asian SSMs, oneKNEE® achieved a higher coverage than older designs [Fig. 2]. Modern designs were supported by cortical bone in all areas, while older designs missed cortical support on the medial or lateral cortex in half of the Asian models [Fig. 3]. Also, the oneKNEE® design showed a very good fit with the anterior tibial contour.
Conclusion
Modern implant designs achieved a very good bony surface coverage and cortical support on all sizes of the Asian and Caucasian SSMs. The optimized shape and increased size offerings of modern tibia implants enable to cover the large variability in the anatomy of the population.
References
[1] Dupraz, I.; Bollinger, A.; Deckx, J.; Schierjott, R.A.; Utz, M.; Jacobs, M. Using Statistical Shape Models to Optimize TKA Implant Design. Appl. Sci. 2022, 12, 1020. https://doi.org/10.3390/app12031020
Figures
Figure 1
Figure 2
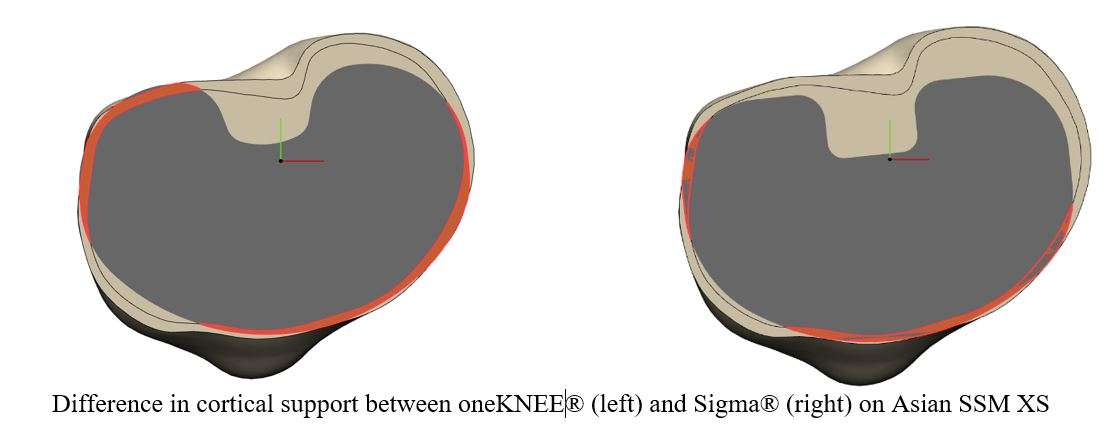
Figure 3#8219
Role of FEA and Experimental Data in Validation of Contact Pressures and Pressure Distribution in Knee Joints With Chondral Defects of Varying Sizes
*Sunjung Kim - University of Illinois Chicago - Chicago, United States of America
*Email: skim6487@uic.edu
Introduction
Chondral defects in the knee joint are a common condition that can lead to pain, swelling and tenderness, joint stiffening, and decreased mobility. Several surgical interventions are available to restore the structural integrity of the cartilage and improve joint function and range of motion, however, the optimal intervention strategy is not well defined. One of the factors that can influence the success of surgical intervention is the size and location of the cartilage defect, as well as the resulting contact pressures on the edges and surroundings of the osteochondral defect (OCD). The purpose of this study was to investigate the effect of defect size and location on contact pressures and pressure distribution in the knee joint using both compression loading of the knee in full extension and 30 degrees of flexion using both biomechanical testing and virtual testing using finite element analysis (FEA).
Methods
Mechanical testing was performed on six human cadaveric knee joints with chondral defects of varying sizes (3, 5, 7, and 10 mm) created on the medial femoral condyle (MFC) and lateral femoral condyle (LFC). Contact pressures were measured at full extension and 30 degrees of flexion using Tekscan sensors. FEA models created using CT scan images were used to validate the experimental testing results and provide further insight into stress-strains results from contact pressures under normal knee loading conditions.
Results
The study found that increasing defect size led to a significant increase in contact pressures on both the MFC and LFC, with the highest pressures observed near the 5 mm defect size. The location of the peak contact pressure points on the MFC and LFC also shifted with an increase in defect size (Fig. 1). Furthermore, FEA analysis revealed that the pressure distribution on the femoral cartilage varied significantly with defect size, with the 7 mm defect size being the most critical in terms of affecting overall contact pressure distribution (Fig. 2).
Conclusion
The findings of this study have important implications for the treatment of cartilage defects in the knee joint. The results suggest that larger cartilage defects are associated with higher contact pressures, which can lead to further cartilage damage and joint degeneration. Therefore, early intervention is essential to prevent the progression of cartilage defects and subsequent joint degeneration. Surgical interventions such as microfracture, autologous chondrocyte implantation, and osteochondral grafting may be considered to restore the structural integrity of the cartilage and improve joint function. However, the optimal intervention strategy should consider the size and location of the defect, as well as the overall contact pressure distribution on the femoral cartilage. Future studies using FEA and patient-specific CTs along with bone quality assessment be beneficial in addressing the patient’s age, daily activities for loading conditions purposes, and overall knee joint constraints. This study provides valuable insights into the behavior of chondral defects observed both in real cadaveric knees compared to in vitro models using FEA. The latter is a framework for addressing surgical intervention and treatment strategies for repairs of patients with OCD.
Figures
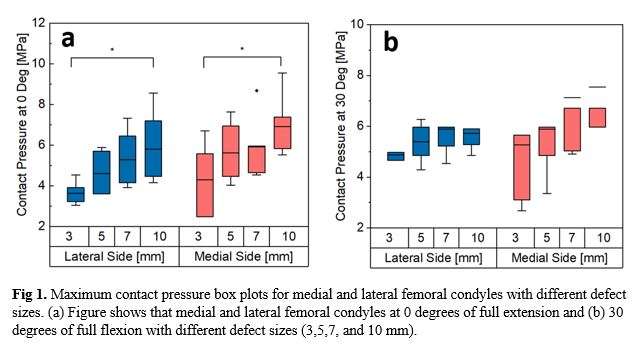
Figure 1
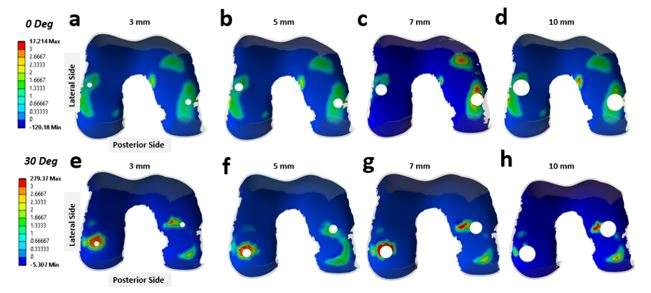
Figure 2#8196
Validation of Flexion Gap Evaluation Tool for Robot-Assisted TKA
*Ryuichi Gejo - Department of Orthopaedic Surgery, University of Toyama - Toyama-City, Japan
Makiko Nogami - Toyama University - Toyama, Japan
Hayato Mine - University of Toyama - Toyama, Japan
Takashi Tamura - University of Toyama - Toyama, Japan
Yoshiharu Kawaguchi - University of Toyama - Toyama, Japan
*Email: rgejo@med.u-toyama.ac.jp
Introduction: The TKA surgical robot (ROSA Knee) can evaluate soft tissue balance before osteotomy using various tools. One of these tools is the femoral rotational tool, which can evaluate the size and inclination of the flexion gap before osteotomy of the anterior and posterior femur. The purpose of this study was to verify the validity of the values expressed by the femoral rotational tool.
Methods: The subjects were 40 knees in which TKA was performed using the ROSA Knee, 25 knees with the Persona PS and 15 knees with the CR. According to the modified gap-balancing technique, after osteotomy of the distal femur and proximal tibia and before anteroposterior femoral osteotomy, a flexion gap was formed with a 40-lb opening force using a seesaw type tensor (FuZion, Figure 1). The femoral rotational tool of the ROSA Knee was used to display the flexion gap tilt angle (positive for lateral opening) and the medial and lateral gap values on a monitor (Figure 2) in real time. The gap tilt angle and the medial and lateral gap values were compared with the respective values shown in the tensor.
Results: The mean ± SD of the gap tilt angle was 5.0 ± 2.6°/6.2 ± 2.5° for tensor/ROSA, respectively, and there was a strong positive correlation (R = 0.89) between the two, but the mean value for ROSA was significantly (P < 0.001) larger. The medial gap was 10.4±2.0mm/9.3±2.4mm for tensor/ROSA, with a strong positive correlation (R=0.90) between the two, but significantly (P<0.001) smaller for ROSA. The lateral gap was 14.1±2.1mm/14.0±2.4mm for tensor/ROSA, and there was a strong positive correlation (R=0.89) between the two, with no significant difference.
Conclusion: There was a strong correlation between tensor and ROSA Knee for both gap tilt angle and medial and lateral gap values in the flexed position, indicating the validity of the femoral rotational tool for the modified gap-balancing technique. On the other hand, the ROSA indicated a slightly larger gap tilt angle and a smaller medial gap than the value indicated by the sensor. Possible reasons for this include the possibility that the gap measurement points of the two tools do not coincide and differences in their respective gap calculation algorithms. Therefore, care should be taken in interpreting the respective values indicated by the femoral rotational tool.
Figures
Figure 1
Figure 2#8732
Evaluating Joint Laxity in Robotic-Assisted and Conventional Total Knee Arthroplasty and Intact Knees
*Emma Donnelly - Western University - London, Canada
Brent Lanting - London Health Sciences Centre - London, Canada
Ryan Willing - Western University - London, Canada
Samira Vakili - Western University - London, Canada
Steven MacDonald - London Health Sciences Centre - London, Canada
Megan Richards - Northern Light Orthopedics - Bangor, USA
*Email: edonne@uwo.ca
The goal of total knee arthroplasty (TKA) is to relieve pain and restore mobility. Robotic-assisted TKA surgery offers the ability to optimize prosthesis positioning and thus improve surgical outcomes[1]. Whether or not these more sophisticated surgical tools result in the post-operative knee behaving more like a natural knee than after conventional approaches, however, has not been fully established. Therefore, this study aims to use a joint motion simulator to evaluate and compare joint laxity of cadaver knees following TKA using conventional and robotic surgical techniques. We hypothesize that robotic-assisted TKA will yield laxity results more similar to intact knees than conventional TKA.
Eleven pairs of knees (5 males, 6 females, age: 53-74 years) underwent TKA using a cruciate retaining, rotating platform system. From each pair, one TKA was completed using conventional methods, while the contralateral side used robot assistance (VELYS™, DePuy Synthes, IN, USA). In all cases, neutral mechanical TKA alignment strategies were used. Robotic-assisted TKA alignment was occasionally adjusted to avoid requiring additional soft tissue releases. An additional 6 knees (5 males, age: 74-91 years) comprised the intact cohort. For each knee, biomechanical testing was done using a six degree of freedom (DOF) joint motion simulator (VIVO, Advanced Mechanical Technologies Inc., MA, USA). Once mounted, neutral kinematics of the joints were measured in the anterior/posterior (AP), internal/external (IE), and varus/valgus (VV) directions while flexing and extending the knees between 15° and 90° with 30 N of compression, but otherwise unconstrained. Next, at static flexion angles of 15°, 30°, 60° and 90°, isolated forces (anterior/posterior forces of 40 N and 80 N, respectively) and torques (±4 N/m IE rotation; ±8 N/m VV) were applied. Laxity was defined as the magnitude of departure from the neutral motion path in the observed DOFs. Split-plot ANOVAs were performed for each DOF, assessing the effects of flexion angle and joint condition (intact, conventional TKA and robotic-assisted TKA). A p-value < 0.05 indicated statistical significance.
Significant differences between flexion angles were identified in all six tested directions but none within the main effect of joint condition. Though no other statistically significant differences were found, total VV laxity trended smaller in robotic-assisted TKA knees than both conventional TKA and intact knees; both of which were similar across all flexion angles(Fig.1). The total IE envelope was similar across all joint conditions at all flexion angles(Fig.2). This was also true for the total AP laxity envelope at 15° and 30°, but at higher angles it was noticeably smaller in intact knee(Fig.3).
These results suggest that knee joint laxities after TKA are similar to those of an intact knee, using either conventional methods or a robotic assist. Only in high flexion do TKA knees appear to be less effective at resisting anterior translation under direct loading, potentially an effect of the implant system used. In TKA knees, anterior translation greater than 10 mm may be indication for a more constrained implant[2]. These results may help further inform TKA planning.
[1]Song et al., Clin.Orthop., 2013;471(1):118-126
[2]Garner et al., J.Arthroplasty, 2021;36(11):3765-3772
Figures
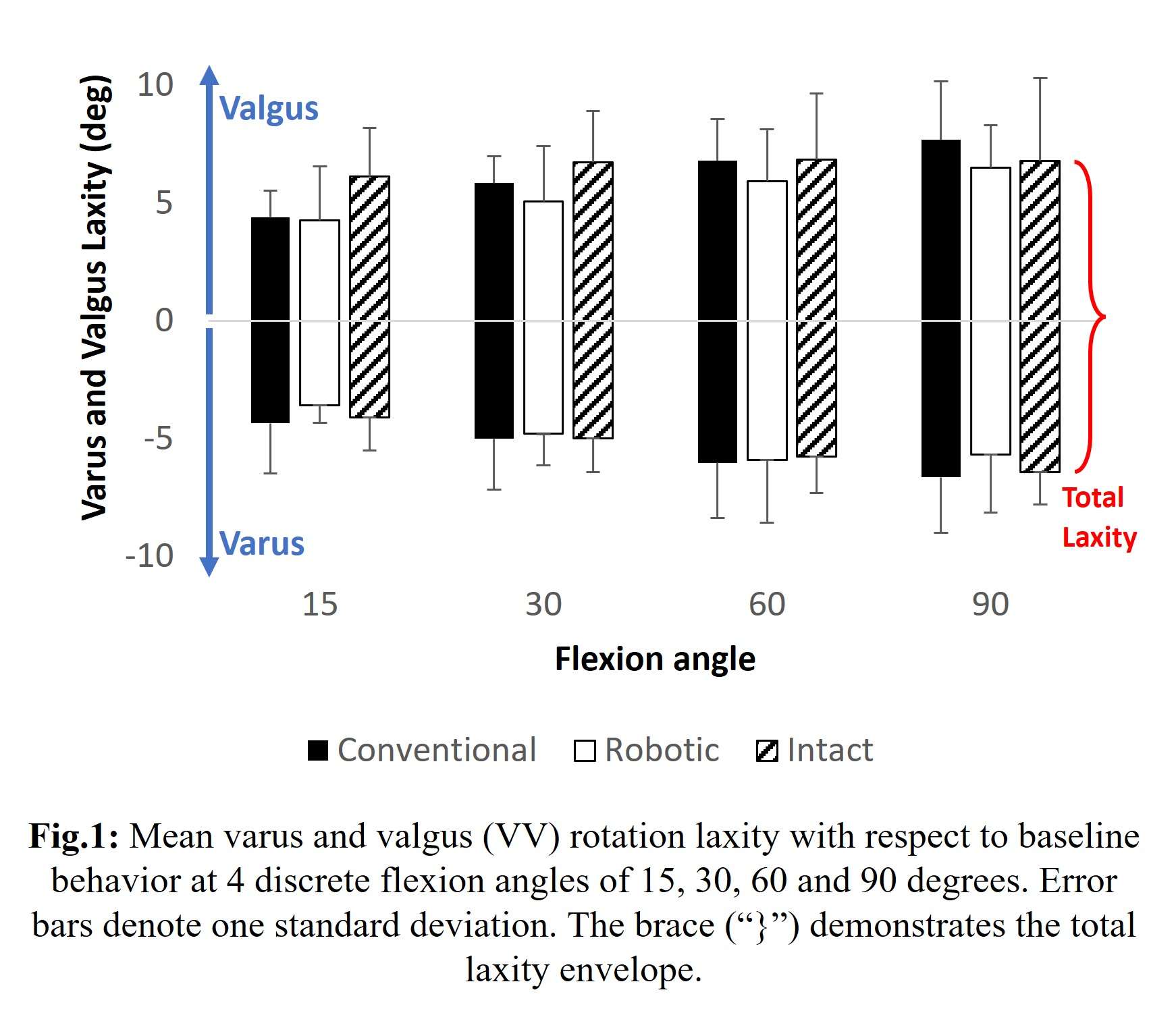
Figure 1
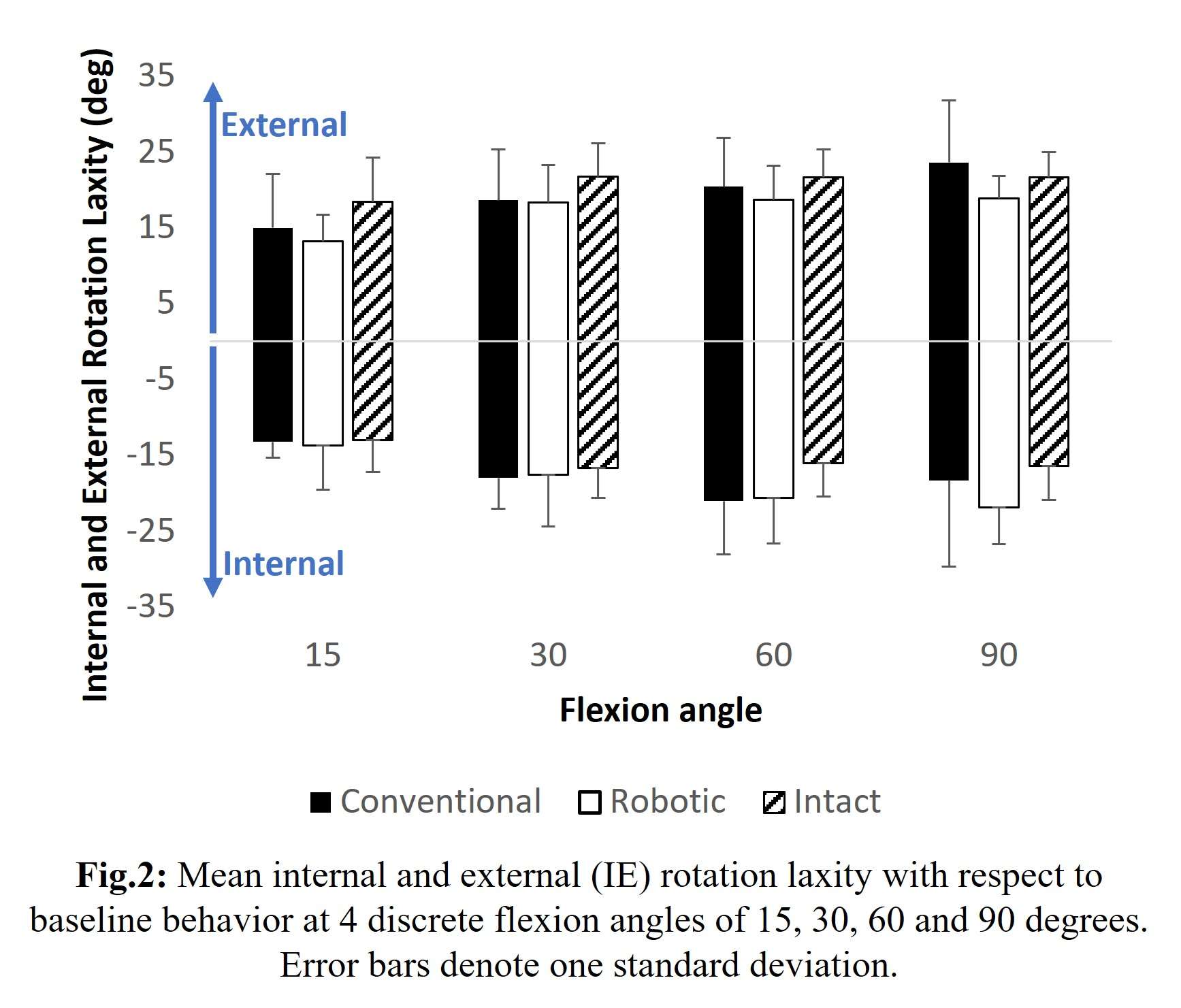
Figure 2
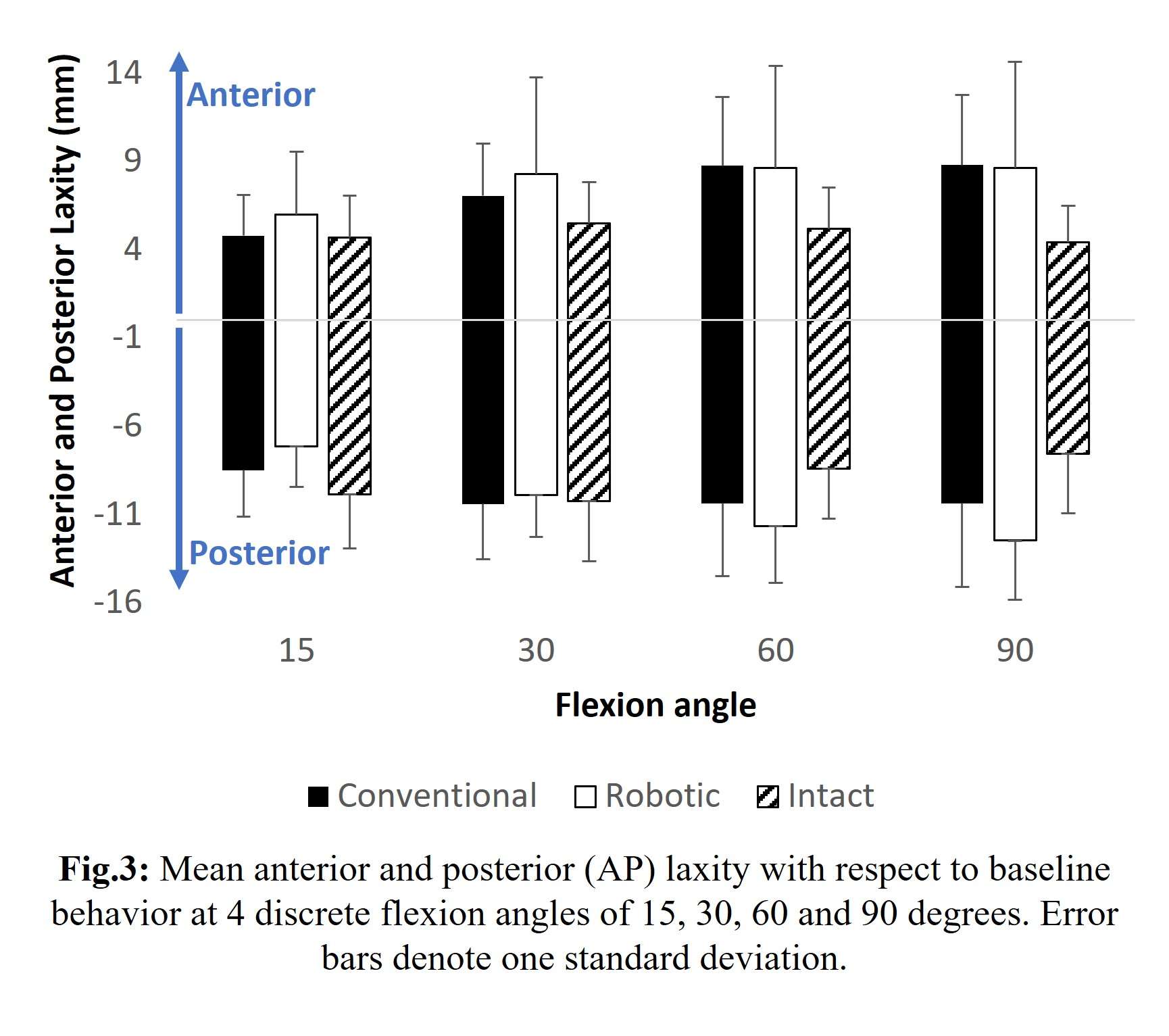
Figure 3#8589
Ligament Balance in Image-Based Robotic-Assisted TKA With Functional Positioning: An Objective Assessment Using Intraoperative Sensor Technology
*Cecile Batailler - Lyon, France
Sebastien Lustig - Hôpital de la Croix Rousse - Lyon, France
Julien Erard - Croix Rousse Hospital - Lyon, France
*Email: cecile-batailler@hotmail.fr
Introduction
Functional positioning for total knee arthroplasty (TKA) is an evolution of kinematic alignment as enabling technology has progressed. The principle is to obtain a personalized alignment using an image-based robotic-assisted system to restore knee kinematics in the three space plans while minimizing soft tissue releases. This study aimed to assess the ligament balancing of robotic-assisted TKA in extension, mid-flexion, and flexion with a functional positioning using intraoperative sensor-guided technology. We hypothesized that robotic-assisted TKA performed by functional positioning would achieve good ligament balancing all along the arc of knee flexion.
Methods
This prospective monocentric study included consecutive patients undergoing image-based robotic-assisted TKA performed with functional positioning between November 2022 and May 2023. Inclusion criteria were end-stage osteoarthritis on varus knee (HKA<183°) with no collateral ligament insufficiency. Exclusion criteria are previous femoral or tibial osteotomy or fracture and flexion contracture >10°. Thirty primary TKA were included with a mean age of 70.4 years old ±8.4, a mean body mass index of 27.9 kg/m2 ±3.8, and a mean preoperative knee alignment of 174.1° ±4.7° [162;183]. All patients had a preoperative knee CT scan which allowed an individualized 3D TKA planning according to principles of functional positioning. After robotic-assisted bone cuts, trial components were inserted, and soft-tissue balance (measured in pound-force (lbf)) was assessed using sensor-guided technology (VERASENSE; OrthoSensor, USA) at 10°, 45°, and 90° of knee flexion. A well-balanced knee was defined by mediolateral difference inferior to 15 lbf, and medial and lateral compartment loads low to 40 lbf at 10°, 45°, and 90° of knee flexion.
Results
93% of TKA (n=28) with functional positioning achieved a well-balanced knee during the first trial. 100% of TKA achieved a well-balanced knee at the end of the surgery. Two patients have needed a tibial re-cut to obtain balanced knees. On the 207 values recorded with the sensors during the surgery, there was only 3% of outliers. The mean mediolateral differences at 10°, 45°, and 90° of knee flexion during the first trial were 3.4 lbf ±9.3 ; 2.8 lbf ±5.8 ; 0.1 lbf ±8.9, respectively. The mean medial compartment load at 10°, 45°, and 90° of flexion during the first trial were 17.2 lbf ±12.8 ; 20.4 lbf ±14.7 ; 14.0 lbf ±10.4, respectively. The mean lateral compartment load at 10°, 45°, and 90° of flexion during the first trial were 20.6 lbf ±13.2 ; 17.6 lbf ±10.8 ; 14.3 lbf ±7.8, respectively. The mean postoperative knee alignment of 178.0° ±2.4° [172;182].
Conclusion
The functional positioning with image-based robotic-assisted system allowed to restore a well-balanced knee all along the arc of knee flexion after primary TKA in varus deformity. An additional cut adjustment was rarely necessary.
#8640
Robotic-Assisted Total Knee Arthroplasty Can Increase Frequency of Achieving Target Limb Alignment in Primary Total Knee Arthroplasty
*Bradley Lambert - Houston Methodist Hospital - Houston, USA
Austin Wininger - Houston Methodist Hospital - Houston, USA
Thomas Sullivan - Houston Methodist Hospital - Houston, USA
Timothy Brown - Houston Methodist Hospital - Houston, USA
Stephen Incavo - Houyston Methodist Hospital - Houston, USA
Kwan Park - Houston Methodist Hospital - Houston, USA
*Email: bslambert@houstonmethodist.org
INTRODUCTION: Robotic-assisted total knee arthroplasty (rTKA) has been shown to reduce the number of alignment outliers and to improve component positioning compared to manual TKA (mTKA). The primary purpose of this investigation was to compare the frequency of achieving target postoperative limb alignment and component positioning in rTKA compared to mTKA.
METHODS: A retrospective comparative study was performed on 250 patients undergoing primary TKA by one of two fellowship trained arthroplasty surgeons. Surgeon A performed predominantly rTKA (N=103) with the ROSA knee system (Zimmer Biomet, Warsaw, IN) and less frequently mTKA (N=44) with conventional instrumentation. Surgeon B performed only mTKA (N=103). Target limb alignment (hip-knee-ankle angle) for Surgeon A was 0° for all cases and for Surgeon B was 2° varus for varus knees and 0° for valgus knees. Radiographic measurements were determined independently by two reviewers. For all parameters, two separate analyses were made: (Analysis-1) Surgeon A (rTKA) vs. Surgeon B (mTKA); (Analysis-2) Surgeon A (rTKA) vs. Surgeon A (mTKA). For each analysis, a t-test was used to compare patient demographics and alignment measures between the two techniques. Chi-square analysis was used to compare frequency of postoperative alignment in the target zone (±2 from predefined target). An ANCOVA (co-varied on baseline measures and repeated on time: pre-op, 3mo, 6mo post-op) was used for within- and between-surgeon comparisons of PROMs (KOOS Jr.). Type-I Error was set alpha=0.05 for all analyses.
RESULTS: Analysis 1 - Surgeon A (rTKA) vs. Surgeon B (mTKA): Among valgus limbs, Surgeon A was observed to fall within the postoperative alignment target zone at a higher frequency (69.23%) compared to Surgeon B (44.12%) (P=0.026) and was, on average, closer to the center of the target zone (P=0.040). For comparison of implant component alignment among varus limbs, differences were observed between surgeons for the Alpha (P<0.001), Gamma (P=0.045), and Phi (P<0.001) angles. For comparison among valgus limbs, a difference between surgeons was observed for the Phi angle (P=0.001). Analysis 2 - Surgeon A (rTKA) vs. Surgeon A (mTKA): When analyzed across all patients combined, rTKA cases were observed to be closer to the center of the target zone compared to mTKA cases (P=0.038). For comparison of implant component alignment among varus limbs, differences between technique were observed for the Beta (P=0.040), Gamma (P<0.001), and Phi (P<0.001) angles. For valgus limbs, differences in component alignment between techniques were observed for the Gamma (P=0.040) and Phi (P=0.007) angles. For the Beta angle, rTKA cases were observed to be closer to the center of the target zone on average (P=0.046). When comparing techniques across all patients combined, differences in alignment were observed for the Gamma (P<0.001) and Phi (P<0.001) angles). No differences were detected for KOOS Jr. Score for either analysis.
CONCLUSION: Although experienced surgeons may achieve target limb alignment correction with similar frequency when comparing rTKA to mTKA for all cases, rTKA may play an important role for achieving target postoperative limb alignment in more challenging cases, such as a valgus knee.
Figures
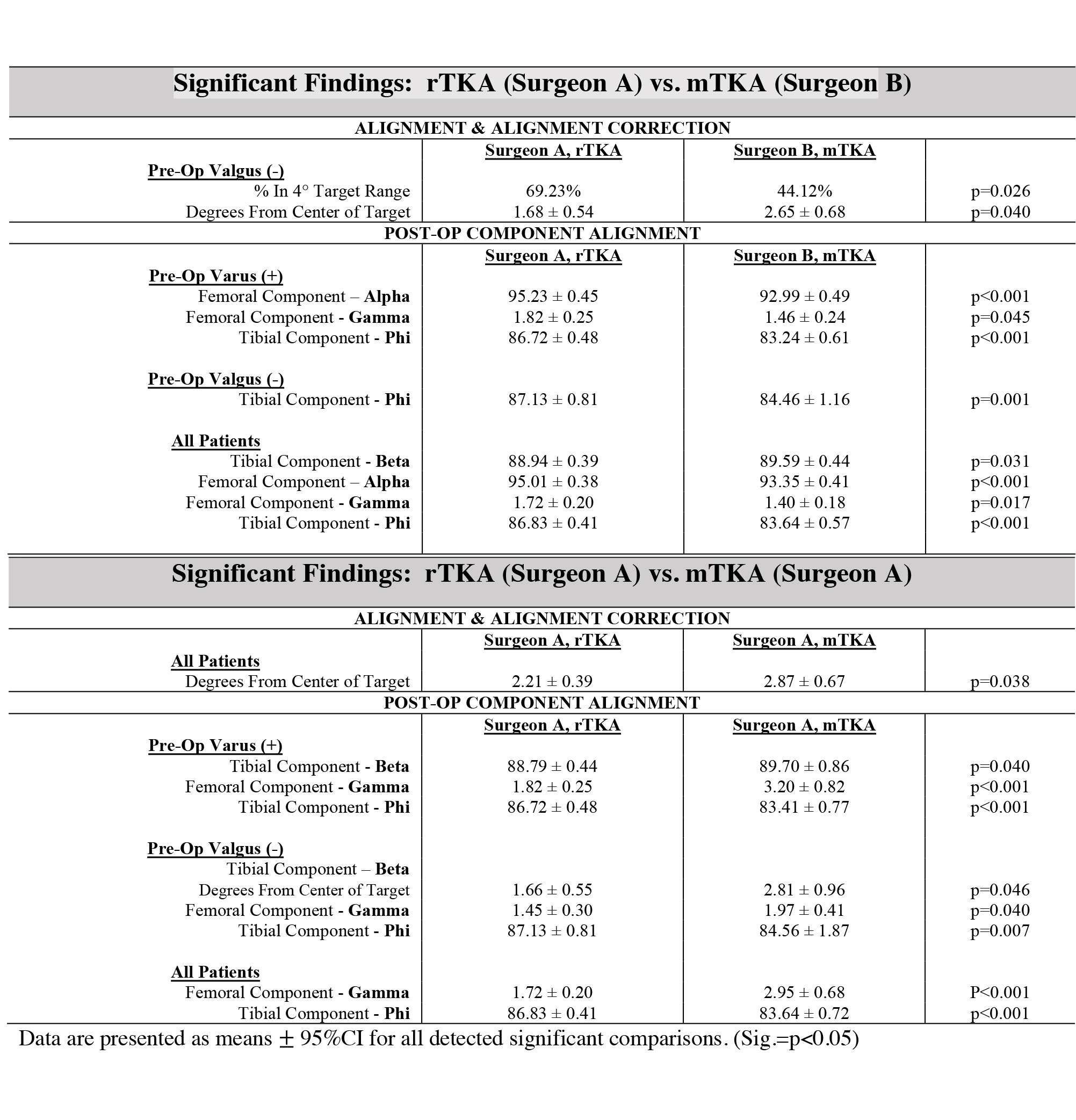
Figure 1#8264
Robotic Total Knee Arthroplasty Is Associated With Earlier Return of Postoperative Range of Motion
Travis Weiner - Columbia University Medical Center - New York, USA
Emily Ferreri - Columbia University Medical Center - New York, USA
Nana Sarpong - Columbia University Medical Center - New York, USA
Roshan Shah - Columbia University Medical Center - New York, USA
*John Cooper - Columbia University - New York, USA
*Email: jcooper02@gmail.com
Introduction
Postoperative range of motion (ROM) is an important measure for the functional outcome and overall success after total knee arthroplasty (TKA). While robotic knee systems have been shown to reduce pain and improve early function, the return of postoperative ROM specifically has not been adequately studied. The purpose of this study was to compare postoperative ROM in robotic and conventional TKA.
Methods
We retrospectively reviewed 674 primary TKAs by a single surgeon between Jan. 2018 and Feb. 2023. Patients that did not have both a 2-week follow-up (mean 17.23; range 12-28 days) and 8-week follow-up (mean 59.19; range 29-105 days) were excluded. The population was divided into two cohorts based on technique utilized: robotic vs. conventional. Conventional knees were performed with a measured resection technique with post-resection soft tissue balancing. Robotic knees were performed with one of three robotic platforms. Preoperative extension and flexion data, postoperative extension and flexion data at 2-week and 8-week follow-ups, and manipulation under anesthesia data were collected. ROM was defined as flexion minus extension. Chi-square tests were used to examine for differences between categorical variables and t-tests were used to examine for differences between continuous variables.
Results
A total of 307 robotic and 265 conventional knees were included. There were no differences in demographics, mean follow-up, or preoperative ROM between groups. The robotic group had significantly more flexion (99.20° vs 96.98°; p=0.034) and ROM (97.81° vs 95.56°; p=0.047) at the 2-week follow-up. The loss in ROM at the 2-week follow-up from preoperative ROM was significantly less for the robotic group (-11.21° vs -14.16°; p=0.031). There were no significant differences between groups in extension at either the 2-week or 8-week follow-up, in flexion at the 8-week follow-up, and ROM at the 8-week follow-up. There were also no significant differences between groups in the gain in ROM at the 8-week follow-up when compared to preoperative ROM.
Conclusion
In a review of 647 consecutive primary TKAs, the use of robotic techniques leads to an improvement in postoperative flexion, ROM, and ROM when compared to preoperative ROM at 2 weeks follow-up. These findings could partially explain the quicker recovery associated with robotic TKA when compared to conventional TKA.
#8657
Early Results for Robotic TKA in Patients in 7th Decade With High BMI and Valgus Alignment
*Shantanu Patil - SRM Medical College, SRM Institute of Science and Technology - Kattankulathur, India
Ashok Kumar P. S. - CHENNAI, India
Pichai Suryanarayan - India
Vijay C Bose - AJRI - chennai, India
Kalaivanan Kanniyan - Asian Joint Reconstruction Institute AJRI @SIMS Hospitals - Chennai, India
Siddharth Karthic - SIMS Hospital - Chennai, India
Arul Prasanna - SIMS Hospital - Chennai, India
*Email: shantanup@srmist.edu.in
- Introduction: Total Knee Arthroplasty (TKA) in patients with valgus knee deformity is a challenging task. Surgical planning needs to account for the bony remodeling along with the soft tissue contractures in order to construct a stable aligned joint. Inadequate improper surgical releases and bone cuts are predictive of instability leading to early failure. Technological advances over the past decades have improved the surgeons’ ability to mitigate complication rates by meticulous preparation and seamless execution of the plan. The use of robotics in TKA has already helped in decreasing intra- and post-operative complications as a result of the intricate groundwork prior to surgery. The current study analyses short-term outcomes of a cohort of patients undergoing TKA for Valgus deformity
- Methods: A cohort of 43 knees (38 patients, mean age 65.3 ± 7.3, range 50-77 yrs.; 32F:4M) with clinical and radiological valgus deformity at the knee joint were identified from the prospective database at a specialty arthroplasty centre in a tertiary care hospital. All patients were evaluated for surgical fitness prior to surgery. The patients underwent TKA with the use of semi-automated hand-held robotic device performed by fellowship trained surgeon with over 12 years’ experience. Initial and final alignment data, knee balancing gaps, component orientation and soft tissue releases were collected intraoperatively. Pre- and Post-operative radiographs and oxford knee scores were collected at 6 months , 1 year and 3 years post operatively
- Results: The pre-operative valgus deformity radiologically ranged from 2° to 30° (mean 12.8° ± 6.8°). The patients were older (BMI 31.7 ± 5.3, range 23.0 - 48.1); and nearly 75% suffered from at least one comorbidity such as Diabetes, Hypertension, Cardiac disease and hypothyroidism. All patients underwent a standard post-operative rehabilitation protocol and received appropriate modifications to the same. Intraoperatively, femoral and tibial component placement was monitored along with the flexion extension gaps. Average femoral component position was 0.2° valgus, 1.5° flexion and 0.3° in ER. Average tibial component position was 0.1° valgus, 3.2° slope and 0.1° in ER. Flexion and extension gaps were within 2 mm for all knees. For component position, the planned alignment was achieved in 95 % of the knees. The latest radiographs showed well seated and well-fixed components. All knees were corrected within 3° of mechanical neutral (Mean 1.3° ± 2.0°) 2 patients developed transient post-surgery foot drop which recovered within 6 months. In both the cases, a thicker insert was chosen for stability.
- Conclusion: Valgus knees present challenges during arthroplasty surgeries and can cause complications if there is inadequate planning and improper execution of the same. Use of the handheld semi-autonomous robotic devices mitigates the complications and offers the patients a safer and more predictable outcome.
1. Rajgopal A et al Long-term results of total knee arthroplasty for valgus knees: soft-tissue release technique and implant selection. J Orthop Surg (Hong Kong). 2011 Apr; 19(1):60-3. Doi: 10.1177/230949901101900114. PMID: 21519079)
#8563
The Learning Curve Associated With a New Robotic Total Knee Arthroplasty System
*John Cooper - Columbia University - New York, USA
Travis Weiner - Columbia University Medical Center - New York, USA
Alirio J deMeireles - Columbia University Medical Center - New York, USA
Xavier Ferrer - Columbia University Medical Center - New York, USA
Roshan Shah - Columbia University Medical Center - New York, USA
*Email: jcooper02@gmail.com
Introduction
Given the recent shift toward robot-assisted total knee arthroplasty (TKA) it is important to understand the impact of this new technology on surgical workflows. While the learning curve of other robotic systems has been investigated, the learning curve for the system used in this study has not been defined before. The purpose of this study was to 1) compare robot-assisted versus manual operative times of fellowship-trained arthroplasty surgeons as well as 2) investigate the learning curve associated with transitioning from manual to robot-assisted TKA.
Methods
A total of 120 robot-assisted TKAs performed by 2 fellowship-trained arthroplasty surgeons were analyzed. Each of the 60 cases per surgeon were sequentially grouped into 3 clusters of 20. Inclusion criteria were use of the surgical robot and primary TKA. Exclusion criteria included revision surgery, conversion TKA, and age <18. Mean operative time and patient demographic information were recorded for all cases. Cumulative summation analysis was performed to analyze the learning curve. Student’s t-tests were conducted to compare robot-assisted operative times to manual operative times.
Results
This was a retrospective cohort study of 120 sequential robot-assisted TKAs. For surgeon 1, there were no statistically significant differences in operative time between neither mean robot-assisted TKA nor any individual 20-case cohort groups when compared to mean manual TKA (Table 1). For surgeon 2, cases 1-20 and cases 21-40 had significantly longer operative times – 118.75 mins and 132.6 mins, respectively, compared to mean manual TKA operative time of 107.8 mins (p<0.05). Cumulative summation analysis suggests a learning curve of between 10-15 cases (Figure 1).
Conclusions
This study demonstrates a relatively swift learning curve for the implementation of robot-assisted TKA. Of the two surgeons studied, one demonstrated no significant increase in operative times across any case grouping, while the second demonstrated an increase for the first 40 cases before returning to levels comparable to that of manual TKA. This data helps to inform surgeons on their decision to implement robot-assisted TKA in their practice.
Figures
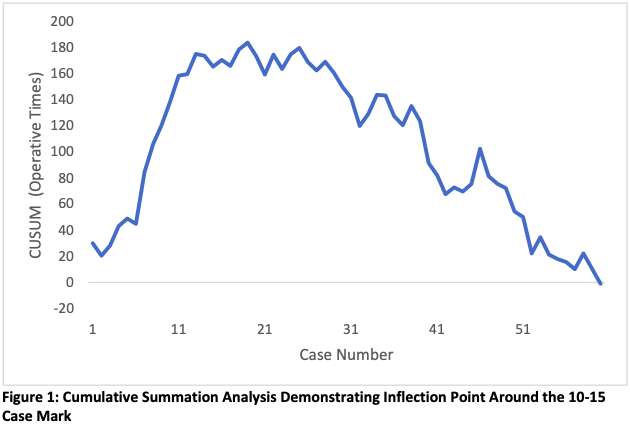
Figure 1
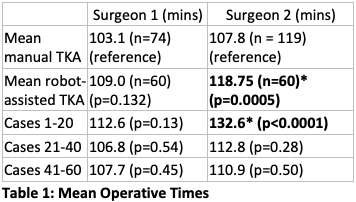
Figure 2#8767
The Utilization of Robotic Technology to Restore Knee Alignment Based on CPAK Classification in Varus Knees Following Total Knee Arthroplasty
*Weston Buehring - New York, United States of America
Vishavpreet Singh - NYU Orthopedic Hospital - New York, USA
Patrick Meere - New York University Hospital for Joint Diseases - New York, USA
Matthew Hepinstall - NYU Langone Health - New York, USA
Morteza Meftah - NYU Hospital for Joint Diseases - New York, USA
Catherine Digangi - NYU Orthopedic Hospital - New York, USA
*Email: westonbuehring2013@gmail.com
Introduction
The Coronal Plane Alignment of the Knee (CPAK) classification method is a recently implemented tool that can be utilized to describe preoperative and postoperative alignment in total knee arthroplasty (TKA) using arithmetic hip-knee-ankle (HKA) and joint line obliquity (JLO) angles. Sparse data exists regarding the effect robotics has on restoration of lower limb alignment and if clinical outcomes are influenced by restoration to a neutral position or maintaining their preoperative, varus CPAK classification. Therefore, this study sought to describe the effect Mako TKA has on preoperative varus knees in which CPAK cohorts were either restored to a neutral alignment or maintained in a varus position postoperatively.
Methods
We retrospectively reviewed 383 primary, elective Mako (Stryker) TKA cases. Within this cohort, preoperative and postoperative HKA and JLO angles were obtained using TraumaCad software. The HKA angle was calculated by subtracting the medial proximal tibial angle (MPTA) from the lateral distal femoral angle (LDFA). The JLO angle was calculated by finding the sum of the MPTA and LDFA angles. To determine preoperative varus cases, HKA angles < -2 were identified. Classification into CPAK groups were performed as follows: CPAK I (varus HKA, JLO < 177), CPAK IV (varus HKA, JLO 177 to 183), CPAK VII (varus HKA, JLO > 183). Additionally, HKA angles -2 to 2 were classified as neutral and HKA angles >2 were classified as valgus. Demographic data, clinical outcomes, and Knee injury and Osteoarthritis Outcome Score, Joint Replacement (KOOS, JR) scores were collected and compared using Chi-square tests.
Results
In total, 235 preoperative varus knees were identified, of which 196 (83.4%) were classified as CPAK I and 39 (16.6%) were classified as CPAK IV. Postoperatively, it was found that 207 cases (88.1%) switched to a different CPAK group (referred to as “new CPAK”) while 28 cases (11.9%) remained in their preoperative, varus CPAK group (referred to as “old CPAK”). Preoperative HKA angles were similar between the new CPAK cohort and the old CPAK cohort (9.52 vs. 8.25, respectively; p=0.084). However, postoperative HKA angles did statistically differ between these 2 cohorts, with the new CPAK cohort having a more neutral postoperative HKA angle relative to the old CPAK cohort (1.98 vs. 3.97; p<0.001). The new CPAK group was also more likely to be classified into CPAK I preoperatively relative to our comparison group (88.4 vs. 46.4%; p<0.001). Lastly, short-term outcomes and KOOS, JR scores did not statistically differ between the new CPAK cohort and those that retained their preoperative CPAK classification.
Conclusion
This study highlights the ability of the MAKO robot to restore native knee alignment as well as describes the comparable outcomes observed when CPAK classification groups are changed to a more neutral alignment or maintained in a postoperative varus state. In addition, this study demonstrates that keeping varus knees in an alignment similar to their preoperative state can avoid soft tissue release and does not incur worse clinical or patient-reported outcomes compared to knees more drastically altered to conform to a neutral alignment.
#8443
Can Imageless Navigation Accurately Determine CPAK in Total Knee Arthroplasty?
Adam Edelstein - Northwestern University - Chicago, USA
Linda Suleiman - Northwestern Unversity - Chicago, USA
Simon Coffey - Nepean Hospital - Sydney, Australia
Christopher Plaskos - Corin - Raynham, USA
*Alex Orsi - Corin - Raynham, USA
*Email: Alex.Orsi@coringroup.com
Coronal plane alignment of the knee (CPAK) is a well-established technique for categorizing phenotypes. CPAK is a function of joint line obliquity (JLO), and arithmetic hip-knee-ankle angle (aHKA), which are calculated from the medial proximal tibial angle (MPTA) and lateral distal femoral angle (LDFA) as measured on long-leg radiographs.
This investigation aims to understand how accurately imageless surgical navigation predicts CPAK parameters using generic cartilage wear assumptions, and if there is a wear assumption which minimizes error.
Sixty-one navigated TKAs were retrospectively reviewed. MPTA and LDFA were measured from pre-op long leg radiographs by a senior research engineer and an orthopaedic surgeon. Intraclass correlation coefficients (ICC) were assessed between observers.
MPTA and LDFA were also calculated from intraoperative surgical navigation landmark data. An optimal wear assumption was determined using a parametric analysis which performed over 27,000 unique combinations of adjustments for medial and lateral distal femoral and proximal tibial wear in increments of 0.5 mm from 0 to 3 mm, as a function of pre-op varus or valgus deformity in 1° increments from 0±3°.
JLO and aHKA were calculated from the radiographic and surgical navigation measurements.
ICCs between the two x-ray observers were rated as excellent for all CPAK parameters [Fig. 1]: MPTA = 0.96, LDFA = 0.95, JLO = 095, aHKA = 0.97, and were averaged across the observers. 73% and 97% of patients were within the same, and within one CPAK group, respectively.
The following wear assumption minimized error across all CPAK parameters, and best reproduced CPAK distribution:
≥1° Varus: 2mm medial distal femur, 1.5mm medial proximal tibia
>2?° Valgus: 0.5mm lateral distal femur, 1mm lateral proximal tibia
Differences (Mean±SD) between navigation and radiographic measurements were reported as follows: MPTA: -0.1±2.5, LDFA: -0.1±1.6, JLO: -0.1±2.9, aHKA: 0±3.1.
49% and 94% of patients were within the same, and within one CPAK group, respectively when comparing navigation and radiographic measurements, [Fig. 2].
Radiographic CPAK measurements were highly repeatable, and similar to Tarassoli et al. who reported an ICC of 0.95 for aHKA [1].
The aHKA error between radiographic and imageless navigation in the present study (0±3.1) is less than error between radiographic-aHKA and CT-aHKA (0.3±5.3), and error between radiographic-aHKA and stressed HKA (0.1±4.2) reported by Tarassoli et al. [1]. The results from this investigation suggest imageless navigation can be used to adequately predict CPAK parameters, with similar accuracy to CT based methods.
[1] Tarassoli, P, et al. Knee Surg Sports Traumatol Arthrosc 2022; 30(0): 2980
Keywords: Total knee arthroplasty, CPAK, accuracy
Figures
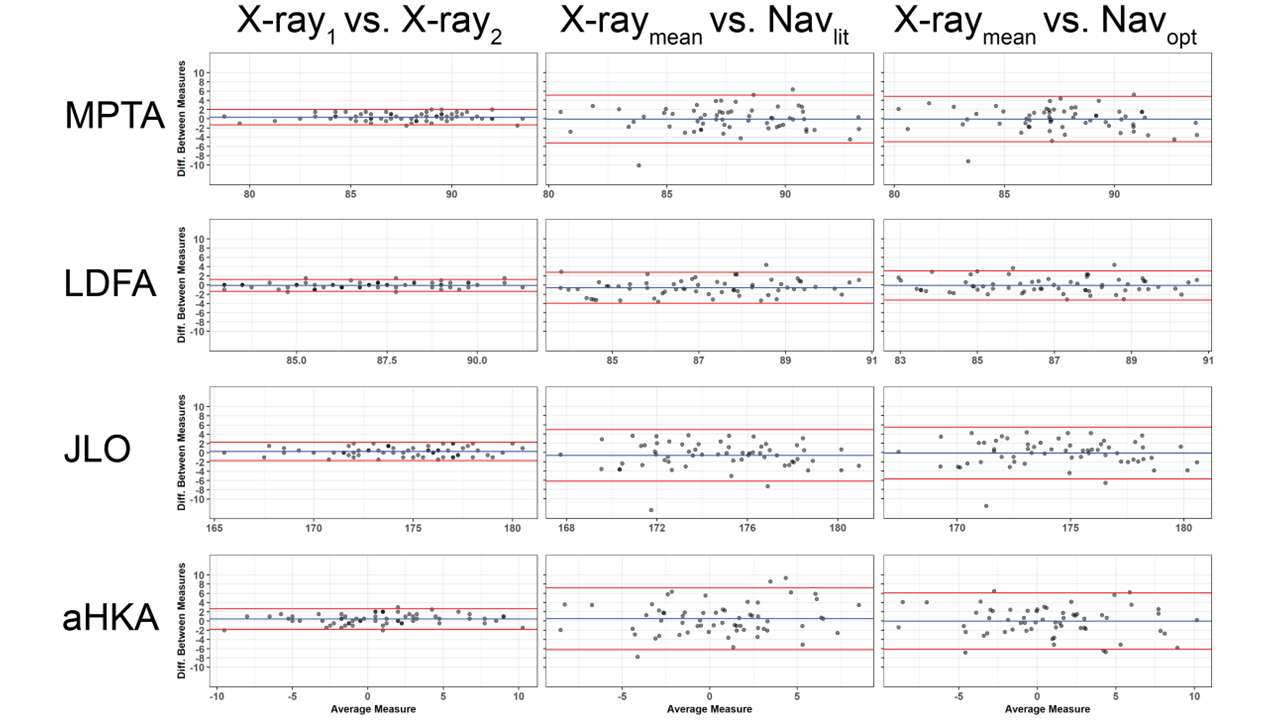
Figure 1
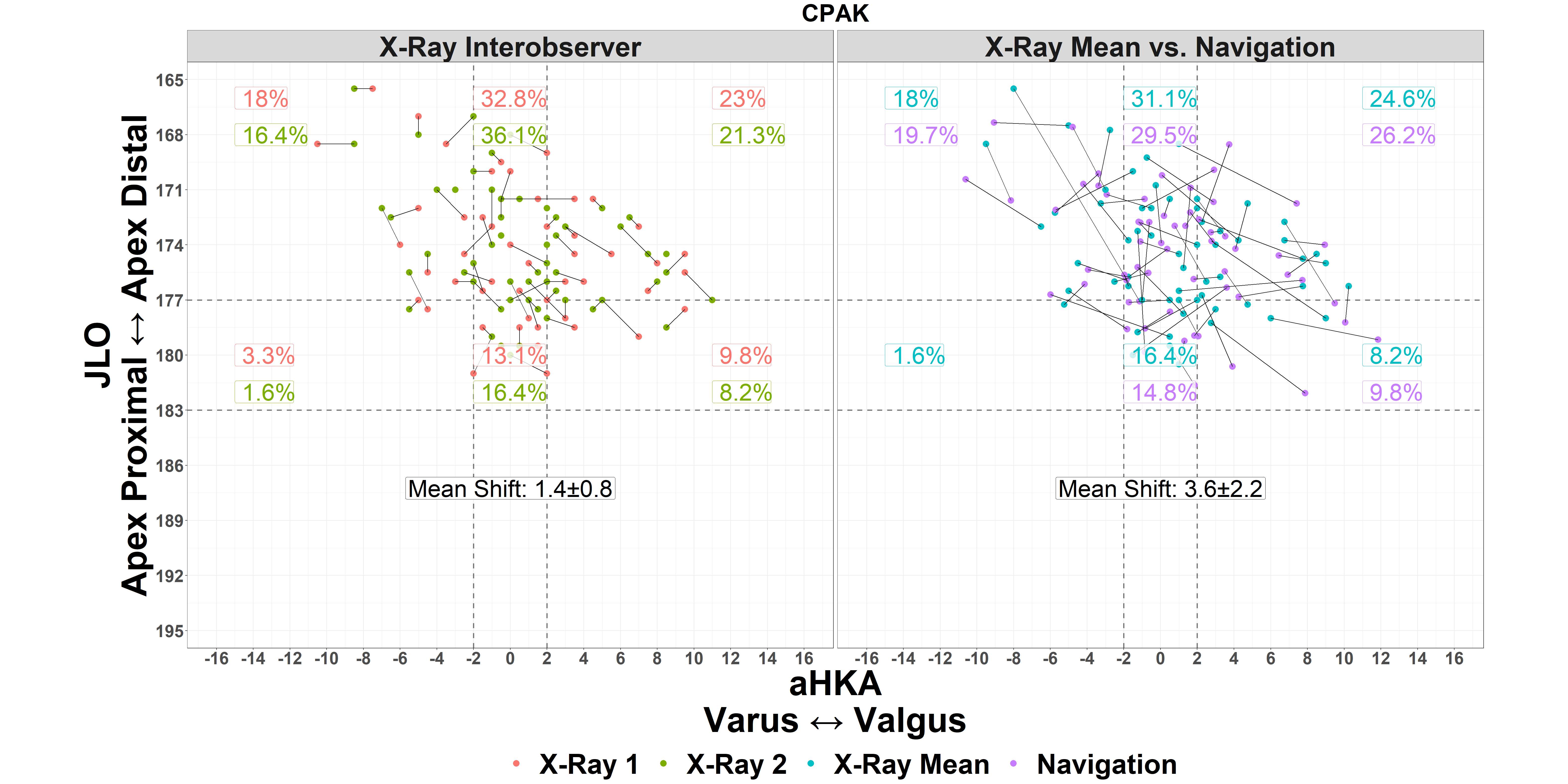
Figure 2#8760
Quantifying Balance Intra-Operatively With a Robotic Surgical Assistant in Total Knee Arthroplasty.
Mike Anderson - Zimmer Biomet - Lehi, USA
Joseph Brook - Zimmer Biomet - London, United Kingdom
Karl Surmacz - Zimmer Biomet - Swindon, United Kingdom
*Adam Henderson - Zimmer Biomet - Winterthur, Switzerland
*Email: adam.henderson@zimmerbiomet.com
Introduction
Joint space narrowing of the knee due to osteoarthritic changes has been associated with increased laxity. The ability to quantify laxity and balance intra-operatively has been limited to finite element analysis, pressure-based sensors, and manual tensioners. A contemporary robotic surgical assistant in total knee arthroplasty has been shown to improve accuracy, but we are unaware of any data demonstrating its ability to adequately quantify intra-operative balance. The purpose of this study was to assess the intra-operative data collected from the robotic system regarding soft-tissue balance at an initial and final evaluation.
Methods
We performed a descriptive analysis of anonymized data from a commercial database for patients who underwent robotic assisted primary unilateral TKA between May 2019 and April 2023, and had their data uploaded into the commercial data warehouse (n=1,093) by one of five surgeons. Surgeons were selected as these were the surgeons with the greatest number of cases who had a minimum of 100 cases uploaded. Pearson’s correlation was used to assess the relationship between flexion and extension imbalance and is represented graphically by the orientation and the ovality of the ellipse (Figure 1). Institutional review boards deemed this study exempt from full review and provided a waiver of consent and authorization for secondary analysis.
Results
The mean age of the population was 66.09 ± 8.61. The mean body mass index was 31.79 ± 5.88. The majority (52.3%) were female. The initial balance assessment intra-operatively revealed that 36.74% of patients had >2.5mm of imbalance in extension and 39.7% had >2.5 mm of imbalance in flexion. At final evaluation the imbalance >2.5 mm decreased to 3.35% in extension and 6.45% in flexion. There was a moderate to strong correlation (r=0.40 – 0.60 for each surgeon, Figure 1a) in the initial evaluation of balance in flexion and extension where levels of imbalance in extension are associated with similar values in flexion. There was a weak correlation at the final knee evaluation between balance in flexion and extension (r=-0.03 – 0.24 for each surgeon, Figure 1b). This is consistent with the surgeon’s ability to balance the compartments independently in flexion and extension.
Conclusion
This data demonstrates the ability to quantify intraoperative soft-tissue balance using a robotic surgical assistant in total knee arthroplasty. The data are consistent with increased patient variability at initial evaluation followed by surgeon guided balance at final evaluation. The lack of correlation at final balance is consistent with the ability to independently balance the knee in both flexion and extension. The data may be useful in determining the effects of various balance levels on post-operative outcomes and provide a means to assess balance without pressure sensors or finite element analysis. Further analysis is needed to assess the relationship between the pre-operative arthritic changes and laxity. Additionally, future studies should be conducted to assess the effect of balance quantified intra-operatively and post-operative outcomes.
Figures
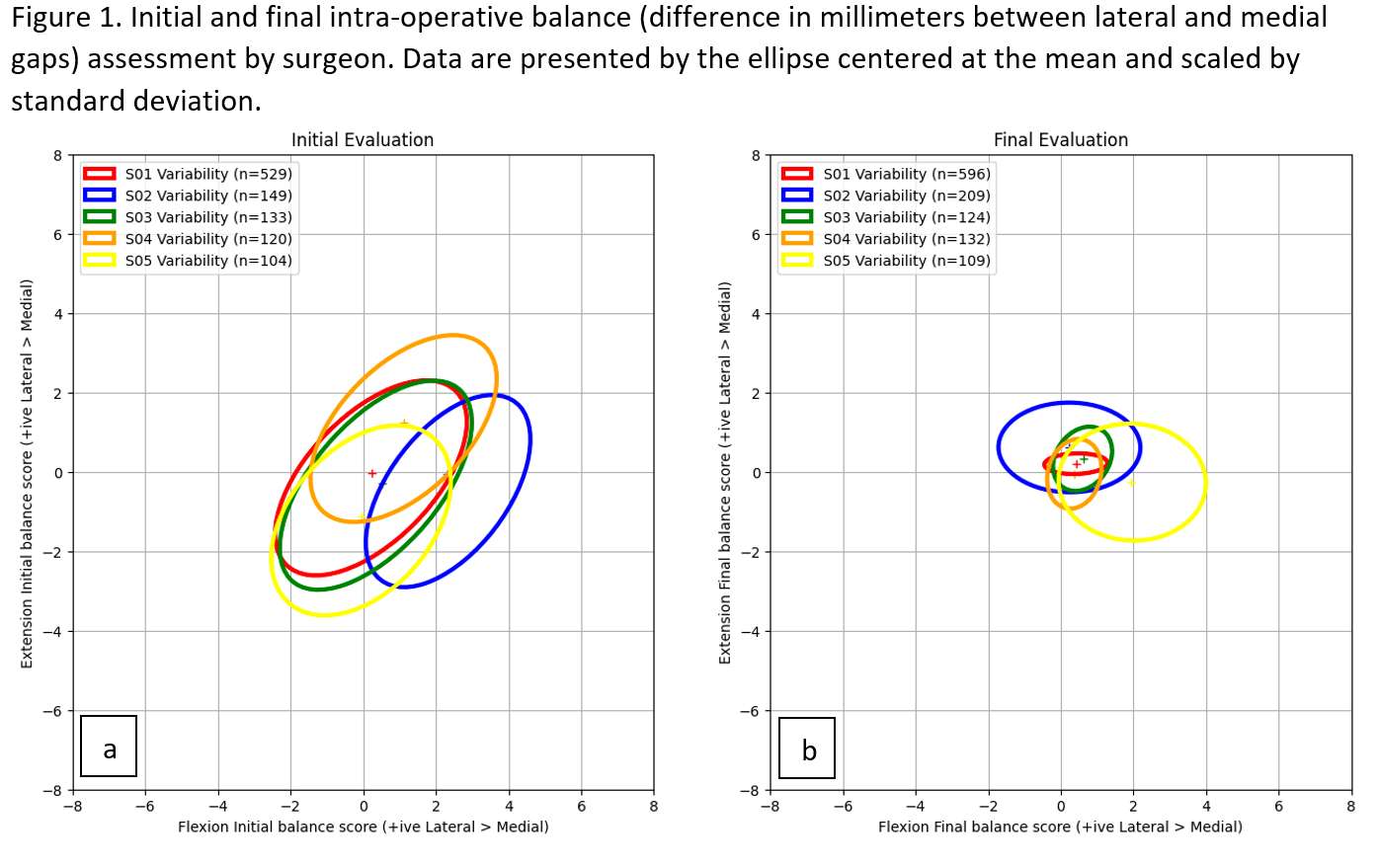
Figure 1#8578
Multi-Modality Comparison of CPAK Measurements for TKA Planning
*Stuti Singh - CurveBeam AI - Seattle, United States of America
Yu Peng - CurveBeam AI - Hatfield, USA
Philippe Van Overschelde - Medisch Centrum Latem - Sint-Martens-Latem, Belgium
*Email: stuti.singh@curvebeam.com
Total knee replacement (TKR) is a common surgical intervention for patients with chronic knee conditions. Earlier TKR was primarily based on visual assessment of the knee joint during surgery. Advancements in imaging technology allow surgeons to assess the condition of the joint and surrounding anatomy beforehand and create a surgical plan. CPAK measurements (Coronal Plane Alignment of the Knee) are a common tool used to plan the position of a knee replacement.
X-Ray can be acquired while the patient is standing, allowing for visualization of the knee joint under load. However, the images are two-dimensional which limits visualization of anatomical structures. Supine CT can be used to acquire a 3D dimensional image of the joint, but it does not accommodate a standing position. A newer modality, Weight-Bearing Cone Beam CT (WBCBCT) provides a 3D scan in a standing position. In this study, CPAK TKR surgical planning measurements were done on WBCBCT, Standing X-Ray, and supine CT scout scans of the same patients. The objective was to determine if there was a significant difference in measurement results between the three modalities.
An analysis was performed on 7 patients who were candidates for TKR. All patients were scanned at the same institution. Each patient underwent standing X-ray, supine CT scout, and WBCBCT scans prior to surgery and the three scans were acquired in a span of no more than 256 days with a mean span of 103 days. CPAK measurements were performed by a CT technician and a biomedical scientist using the RadiAnt DICOM Viewer. Each measurer conducted two measurements for each of the following angles: Hip-Knee-Ankle angle (HKA), Lateral Distal Femoral Angle (LDFA), and Medial Proximal Tibial Angle (MPTA). The inter- and intra-observers’ reproducibility errors were calculated for each measurement for each modality. For the inter-modality comparison, a two-sided paired t-test was performed.
The measured angles on WBCBCT, for the affected leg, were HKA 5.9±2.5°, LDFA 88.7±1.2°, MPTA 86.5±2.2° (n=7). The reproducibility errors on WBCBCT were HKA (0.38°), LDFA (0.82°), and MPTA (0.69°). Comparable reproducibility errors were observed for standing X-ray (HKA: 0.36°; LDFA: 0.45°; MPTA:1.38°) and supine CT scout (FTA: 0.34°; LDFA: 1.12°; MPTA:0.55°).
No statistically significant (p<0.05) difference is found, with a p-value=0.17-0.70 found across the three angle-measurements from three modalities made for each patient (n=7).
The results suggest that the Standing X-Ray, WBCBCT, and Supine CT Scout scans may provide comparable CPAK measurements and that these modalities may be interchangeable in terms of long-leg measurements for TKR planning. However, this study had several limitations including a small sample size, sometimes significant time between the scans, and CPAK measurements only accounting for the coronal plane alignment. A larger patient sample size and study of other measurements in the surgical planning process could provide more insight into if the 3D or weight-bearing properties of some modalities provide additional value in planning the position of knee implants.
#8101
The Coronal Plane Alignment of the Knee Classification Does Not Correlate With the Lower Limb Phenotype.
*Jean-Yves Jenny - University Hospital Strasbourg - Illkirch, France
Florent Baldairon - University Hospital Strasbourg - Strasbourg, France
*Email: Jean-yves.jenny@chru-strasbourg.fr
Introduction
The coronal anatomy of the lower limb is highly variable in subjects undergoing total knee arthroplasty (TKA). It is therefore important to be able to define the initial anatomy from the osteoarthritis anatomy, to define the most suitable prosthetic anatomy for the implantation of a TKA.
Hirschmann et al defined the different global, femoral and tibial phenotypes in the coronal plane. MacDessi et al defined another classification based on a combined analysis of the coronal orientation of the femur and tibia and the obliquity of the joint line (coronal plane alignment of the knee – CPAK classification). The objective of this study was to compare the phenotype classification and the CPAK classification in the same patient at the time of TKA.
Methods
This study was conducted retrospectively after approval by the institutional ethics committee (# CE-2021-32), in accordance with the procedures of the Declaration of Helsinki. All patients operated for TKA by two experienced surgeons between 2011 and 2020 were eligible. All patients were operated with the help of an image-free navigation system (OrthoPilot ®, Aesculap, Tuttlingen, FRG) allowing the precise measurement of different anatomical parameters of the operated knee before implantation [4]. Five hundred and twenty cases were randomly selected from those with a complete anatomical analysis. The following parameters were collected: the mechanical femorotibial angle (HKA), the native femoral angle measured on the lateral and distal side (LDFA), the native tibial angle measured on the distal and medial side (MDTA).
For phenotypes classification, numerical data of HKA, LDFA and MDTA angles were expressed with their mean and standard deviation. For CPAK classification, the arithmetic HKA (aHKA) was calculated as the difference between MDTA and LDFA. Joint line obliquity (JLO) was calculated as the sum of MDTA and LDFA angles. The CPAK class was then defined according to MacDessi et al.
The main criterion was the numerical value of the HKA and aHKA angles in the same subject. These values were compared by Student’s t test for paired series and then by calculation of the linear correlation coefficient. Secondary comparisons were performed according to Table 1. All statistical tests were performed at the 5% threshold.
Results
The measured HKA had a mean of 3.0° (standard deviation of 6.0°). The calculated aHKA had a mean of 1.8° (standard deviation 4.8°). There was a significant difference between the value of the two measurements in the same subject (p=0.005). There was a weak negative correlation between the values of the two measurements in the same subject. In addition, there was no relationship between HKA values and joint line obliquity values or CPAK class.
Conclusion
There was a significant difference and a weak correlation between the values of the HKA and aHKA measures in the same subject. The two analysis techniques used provide different information, and their correlation is only partial. These two techniques therefore appear to be complementary rather than exclusive. The clinical relevance of using these techniques during TKA remains unknown.
#8385
Ethnical Differences in CPAK Distribution Using 3d Segmented Bones
*Adam Henderson - Zimmer Biomet - Winterthur, Switzerland
Mike Anderson - Zimmer Biomet - Lehi, USA
Jeffrey Bischoff - Zimmer, Inc. - Warsaw, USA
*Email: adam.henderson@zimmerbiomet.com
Introduction
Coronal plane alignment of the knee (CPAK) [1] has recently been used as a means of describing knee joint morphology. The classification system uses nine distinct categories based on the arithmetic hip-knee-ankle angle (aHKA) and joint line obliquity (JLO). Previous literature has suggested country specific phenotypes using long-leg radiographs focusing on individual countries [2]. We sought to determine if 3D segmented bone models would provide an alternative method to standing full-length x-rays to quantify varying knee phenotypes across several populations.
Methods
An internal database of fully anonymized CT segmented leg bone models (n=1173 knees; 544: 629 Left: Right; 583: 590 Female: Male) was used to determine the CPAK category for each knee. Race was defined using categories from the National Institute of Health (USA) and our population consisted of both Asian and White races. For the Asian knees, ethics approval for 4 different regions was obtained while the White races were sourced from cadavers where no ethics approval was required.
A projected angle was determined for each femur and tibia independently (Figure 1). The lateral distal femoral angle (LDFA) was calculated as the projected angle of the femur distal axis and femur mechanical axis to the femur posterior tangent plane while the medial proximal tibial angle (MPTA) was calculated as the projected angle of the tibia low points and tibia mechanical axis to the tibia posterior tangent plane. The CPAK classification [1] was then calculated based on these two measurements.
Results
As shown in Figure 2 in the Asian knees (n=808) the most common CPAK categories were I and II (43 & 44% respectively), followed by III (10%). In the White knees (n=364) the same three categories were found to be the most common; however, the order and distribution were different with the most common categories being II (45%) followed by I (29%) and III (17%).
When separating the Asian knees by region (Figure 3), the most common CPAK category was I for India (54%) and Korea (48%) and category II for China (48%) and Japan (52%). The second most common category was II for India (33%) and Korea (44%) and category I for China (37%) and Japan (35%). For all Asian countries the third most common category was III ranging from 7-16%.
Discussion
This study provides data on how the distribution of CPAK categories varies by race and region when calculated from 3d segmented bones. It has been previously demonstrated that aHKA which is one component of a CPAK classification can be determined from CT [3]. However, as this study found a lower percentage of CPAK categories IV-XI, further investigation is required to determine if JLO calculated from 3d landmarks on CT bones needs to be adapted to match the values from 2d standing full length x rays.
References
[1] MacDessi et al. Bone Joint J 2021;103-B (2):329–337.
[2] Pagan et al. The Journal of Arthroplasty, 2023. In press. doi.org/10.1016/j.arth.2023.03.047.
[3] Tarassoli et al. Knee Surgery, Sports Traumatology, Arthroscopy (2022) 30:2980–2990
Figures
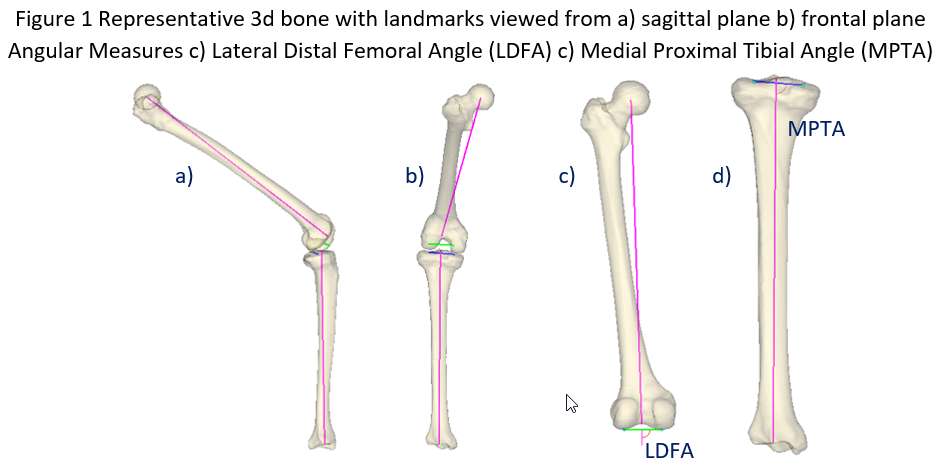
Figure 1
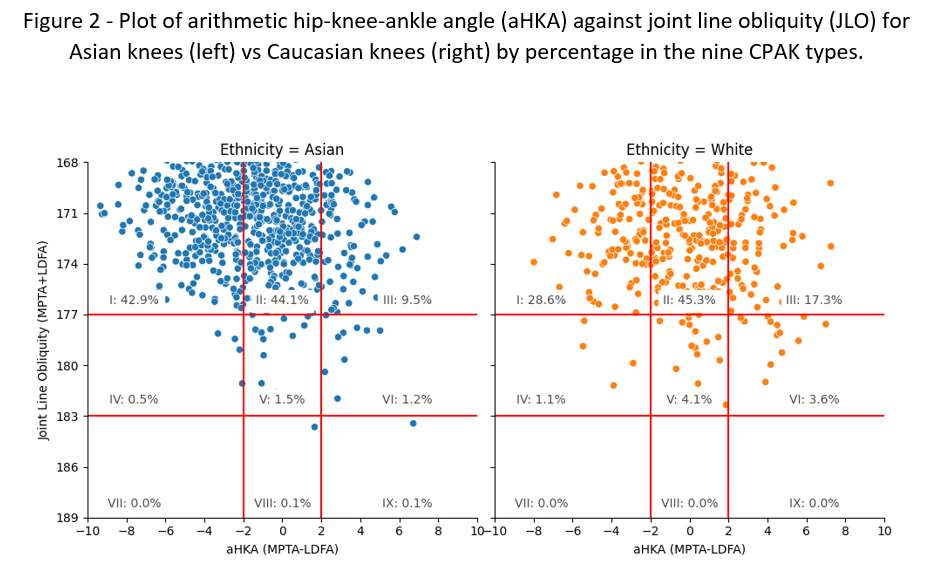
Figure 2
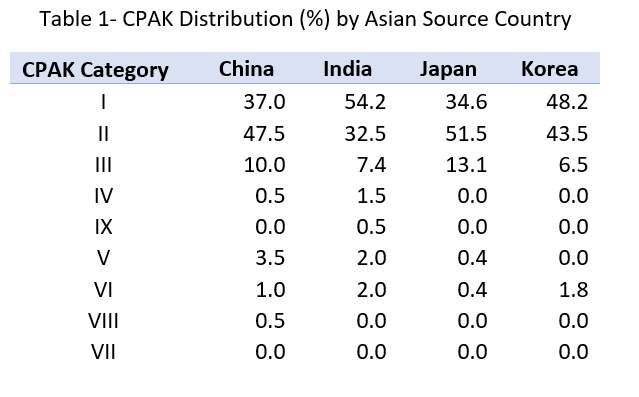
Figure 3#8265
Implant Sensitivity Assessment During a Passive Knee Drop Test in a Surrogate Total Knee Model
*Kevin Abbruzzese - Stryker - Mahwah, USA
Andre Freligh - Stryker Orthopaedics - MAHWAH, United States of America
Scott Logan - Stryker Orthopaedics - Mahwah, USA
Sally LiArno - Stryker Orthopaedics - Bergenfield, USA
Stefano Bini - University of California San Francisco - San Francisco, USA
Jared Weir
*Email: kevin.abbruzzese@stryker.com
Introduction:
Achieving optimal outcomes in total knee arthroplasty (TKA) relies on maintaining an appropriate balance of soft tissues. Surgeons often rely on subjective manual techniques to assess soft tissue balance. Such techniques vary and are difficult to quantify and replicate. To address this, a newly-developed pendulum knee drop (PKD) technique, offers a quantifiable approach to reliably estimate the soft tissues stiffness of the knee joint. It measures the amplitude and decay rate of the oscillations when the leg is passively swung. The objective of this study was to evaluate stiffness and damping in a mechanical total knee arthroplasty model by employing a passive knee drop test with an inertial measurement unit (IMU) and various tibial implants. Through the assessment of range of motion (ROM) and estimation of stiffness and damping factors, we aimed to accurately characterize knee stiffness.
Methods:
To assess knee stiffness changes with different implant insert thicknesses, the PKD test was performed on a mechanical bone total knee model with springs and nylon braided rope (Figure 1). An IMU sensor was placed on the tibia to record ROM. Three trials were performed for each of the three different insert thicknesses (9, 11, and 13 mm), with the order of insert thickness randomized for each trial. The femur of the mechanical bone model was fixed to a table and the insert was implanted in the tibial baseplate of the model. The knee was then dropped from full extension or the calibrated reference position and allowed to oscillate until rest. The IMU sensor was used to measure knee ROM and the log decrement ratio and damping coefficients were calculated for each condition. The data was non-normally distributed and a Kruskal-Wallis test was used to assess significance between insert groups.
Results:
Significant differences were detected between all insert groups (p<0.05). The 11 and 13 mm inserts resulted in significantly stiffer knee models compared to the 9 mm insert. The 13 mm insert resulted in the greatest stiffness when compared to all other trial inserts. An increase in insert trial thickness led to significantly stiffer knees as observed with the log decrement coefficient (Figure 2). The same trends were observed for the damping coefficient.
Conclusion:
This study aimed to investigate the effect of varying implant trial thicknesses on knee stiffness following total knee arthroplasty using the pendulum knee drop test with a surrogate bone model. A significant non-linear increase in knee stiffness with increasing implant trial thickness was observed. This suggests that an increase in implant thickness may lead to increases in stiffness, which appears to be exponential, where every 2 mm increase in thickness resulted in a substantial increase in stiffness. Overall, the study provides valuable insights into the relationship between implant trial thickness and knee stiffness which may have clinical implications in the selection of the appropriate implant size for patients undergoing total knee arthroplasty.
Figures
Figure 1#8284
Surgical Trends for Managing Knee Osteonecrosis: A 2010 to 2020 United States Nationwide Study
*Mitchell Ng - Maimonides Medical Center - Brooklyn, United States of America
Adam Gordon - maimonides Medical Center - Brooklyn, USA
Aaron Lam - Maimonides Medical Center - Brooklyn, USA
Matthew Magruder - Maimonides Medical Center - Brooklyn, USA
Nikhil Vasireddi - Case Western Reserve University School of Medicine - Cleveland, USA
Nicolas Piuzzi - Cleveland Clinic - Cleveland, USA
Afshin Razi - Maimonides Medical Center - Brooklyn, USA
Orry Erez - Maimonides Medical Center - Brooklyn, USA
Giles Scuderi
Michael Mont - Sinai Hospital of Baltimore - Baltimore, USA
Ariel Rodriguez - Maimonides Medical Center - Brooklyn, United States of America
*Email: mitchng77@gmail.com
INTRODUCTION: KneeOsteonecrosis (ON) is rare, with an estimated incidence of 0.01 to 0.17 per 1,000 person years. Although there are at least two separate entities under this name (true secondary osteonecrosis and spontaneous osteonecrosis), current management is directed towards delaying end-stage arthritis, with joint-preserving surgeries considered in pre-collapse and some post-collapse lesions with intact articular cartilage. Our study aimed to: 1) quantify total operative procedures with rates normalized to the United States population; 2) compare arthroplasty versus joint-preserving procedural trends; 3) determine rates of specific operative techniques and demographics in patients aged <50 versus >50 years.
METHODS: Using a nationwide database, 8,269 patients diagnosed with knee ON underwent surgical treatment from 2010 to 2020. Documented surgical procedures included total knee arthroplasty (TKA), unicompartmental knee arthroplasty (UKA), and core decompression. Primary outcomes included total procedural utilization, with sub-analysis comparing arthroplasty versus joint-preserving procedures, and age-stratified by under/over 50 years. Linear regressions were evaluated for trends in procedural volume over time.
RESULTS: From 2010 to 2014, 0.54% of all knee procedures were to treat knee ON compared to 0.71% from 2015 to 2020 (p<0.001).Overall rates of TKA (85.4%) and UKA (10.3%) far exceeded the rates of joint preserving procedures (4.3%). Comparing 2010 to 2014 versus 2015 to 2019, joint-preserving procedures proportionally increased (0.7 to 5.0%, p<0.001). Patients <50 years had significantly more joint-preserving procedures (19.5 vs. 2.7%) over the past decade. Overall, TKA was the most common procedure (7,062;85.40%), following by UKA 853;10.32%) and core decompression (354;4.28%).
CONCLUSION:
To our knowledge, this is the first study to characterize surgical trends in management of knee ON. Overall surgical volume for managing knee ON has continued to increase, outpacing population growth. Patients who have knee ON are most commonly managed with arthroplasty procedures, specifically TKA. Younger aged patients (<50 years) are more likely to undergo joint-preserving procedures, namely core decompression.
#8330
Collecting Long-Term (5 to 10-Years) Patient-Reported Outcome Measures May Be Unnecessary for Total Knee Arthroplasties
Pedro Rullan - Cleveland Clinic Foundation - Cleveland, USA
Ignacio Pasqualini - Cleveland Clinic Foundation - Cleveland, USA
Alison Klika - Cleveland Clinic - Cleveland, USA
Jianhua Shen - Stryker - Mahwah, USA
Manoshi Bhowmik-Stoker - Stryker Orthopaedics - Mahway, USA
*Emily Hampp - Stryker - Mahwah, USA
Nicolas Piuzzi - Cleveland Clinic - Cleveland, USA
*Email: emily.hampp@stryker.com
Introduction: The clinical relevance ratio (CRR) was developed to account for the loss of follow-up in clinical studies reporting patient-reported outcomes measures (PROMs). However, no study has tested its use with original outcome data for total knee arthroplasties (TKA). Therefore, this study aimed to (1) determine the proportion of patients that had a clinically significant improvement in PROMs at each follow-up visit following TKA; and (2) calculate the CRR over time for PROMs following TKA.
Methods: Fourindependent studies reporting PROMs at baseline to 10 years for 1,416 patients who underwent primary TKA in Europe or the United States were aggregated. 1,587 TKAs performed from 2005 to 2017 were included. A distribution-based minimal clinically important difference (MCID) threshold was used to determine which patients had a clinically significant improvement in PROMs. The CRR was calculated by dividing the number of cases that met the MCID threshold by the number of cases at the beginning of the study. The maximum follow-up time was ten years.
Results: The proportion of TKA patients that had a clinically significant improvement in PROMs at each follow-up visit is summarized separately for U.S. and E.U. studies. For U.S. studies, MCID attainment was higher for KSS-KS, KSS-SAT, OKS, compared to other PROMs. Similarly, for E.U. studies, MCID attainment was greatest for KOOS-Pain, KOOS-ADL, KOOS-QOL, and KSS-KS. General health PROMs, such as the EQ5D-VAS, MCS, and LEAS score had the lowest percentages of score improvements. Improvements in PROM scores were relatively similar between 1- and 5-year follow-up visits, with a few PROMs decreasing in trend between 5- and 10-years of follow-up (Figure 1). However, the CRR decreased over time for all PROMs reported in the TKA studies (Figure 2). The tipping point where the CRR began decreasing for TKA studies was at the 1-year follow-up time point.
Conclusion: The clinical relevance ratio for PROMs decreases significantly after short-term follow-up periods for TKA patients. Long-term PROM collection at 5 to 10 years and analysis may be unnecessary following TKA. Arthroplasty surgeons should focus on 1-year PROMs to assess clinically significant improvements after TKA.
Figures
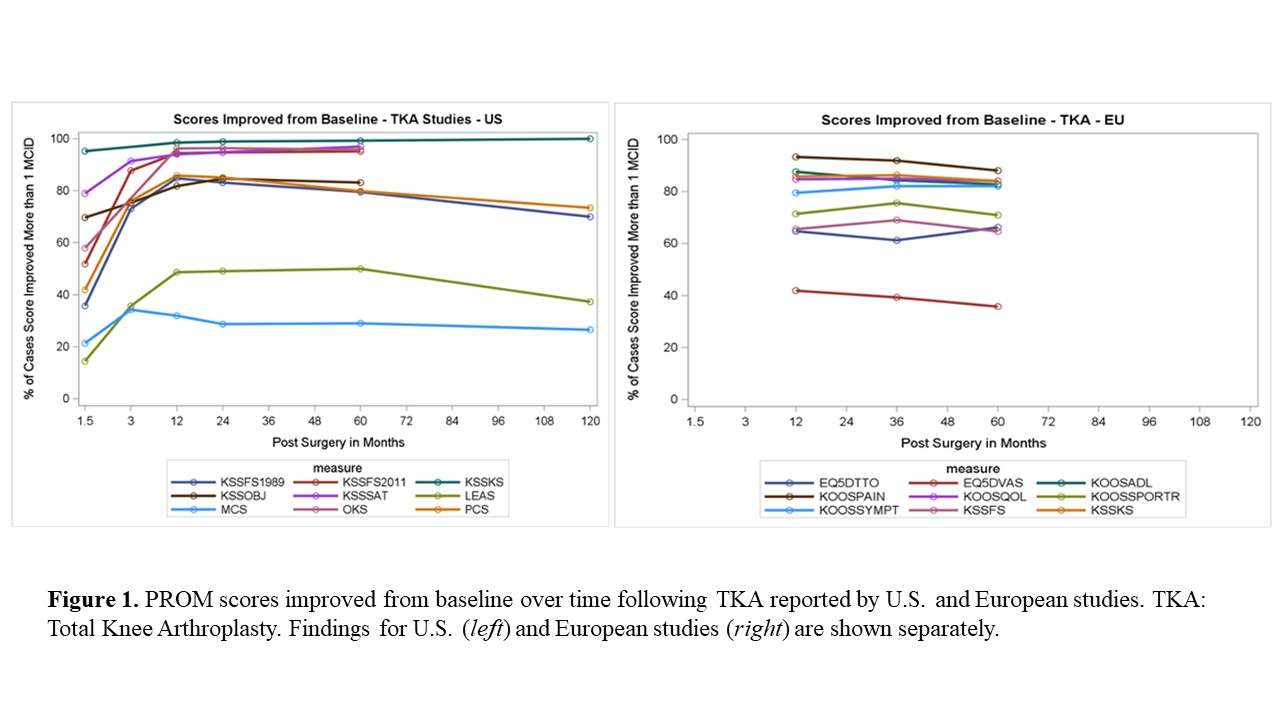
Figure 1
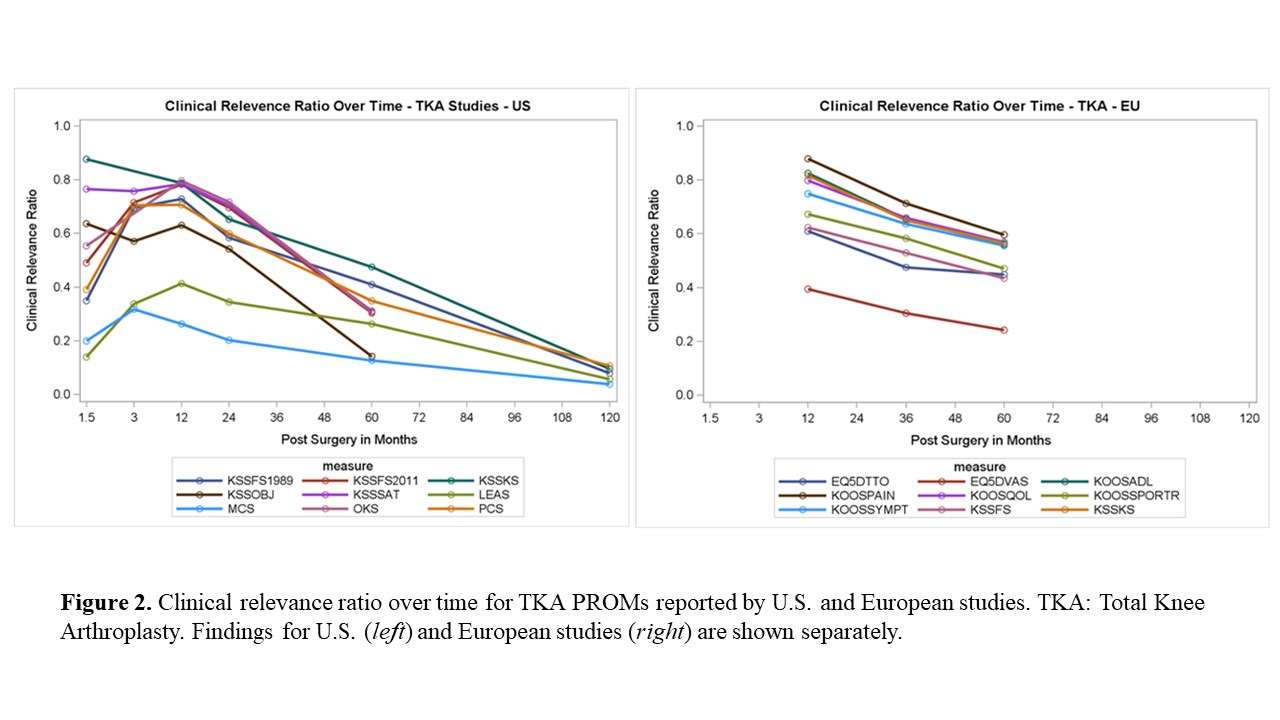
Figure 2#8173
Reconstruction of Large Tibial Bone Defects With Cement in Revision Total Knee Arthroplasty
Tae Jung Kim - SMG-SNU Boramae Medical Center/Seoul National University College of Medicine - Seoul, Korea (Republic of)
Jisu Park - SMG–SNU BORAMAE MEDICAL CENTER - Seoul, Korea (Republic of)
MinKi Kim - Seoul national university - Seoul, Korea (Republic of)
Tae Woo Kim - Seoul National University College of Medicine, SMG-SNU Boramae Medical Center - Seoul, South Korea
Moon Jong Chang - SMG-SNU Boramae Medical Center - seoul, South Korea
Chong Bum Chang - Seoul National University College of Medicine - Seoul, South Korea
*Seung-Baik Kang - Boramae Medical Center/ Seoul National University College of Medicine - Seoul, South Korea
*Email: ossbkang@gmail.com
Background : The aims of this study were to (1) evaluate 2-year radiologic outcomes after cement filling for large tibial bone defects during revision total knee arthroplasty (RTKA), and (2) determine whether cement filling for large tibial bone defects with sufficient stem length and fixation can be considerable surgical option for RTKA.
Methods : 67 patients who underwent RTKA with complete follow-up data (at least 2 years after RTKA) were categorized into group A (small ; cement filling < 5mm, N=27), group B (medium ; 5mm < cement filling < 10mm, N=20) and group C (large ; 10mm < cement filling < 60mm, N=20). The outcomes were compared by using the radiolucent line, loosening, component position (α, β, γ, and δ angles based on Knee Society roentgenographic evaluation system, adductor tubercle joint line distance), limb alignment (hip-knee-ankle angle) and tibia stem length.
Results : There were no significant difference among the three groups in radiolucent line and loosening at the last follow up (p > 0.05). There was also no significant difference in limb alignment and component position between immediate postoperative and the last follow up in all three groups (p > 0.05). Stem length (mm) of group A, B and C were 175.4 ± 38.4, 196.8 ± 36.8 and 202.5 ± 29.6, respectively, (p < 0.035).
Conclusion : Cement filling for large tibial defect with sufficient stem length and fixation can be considerable surgical option for RTKA.
Figures
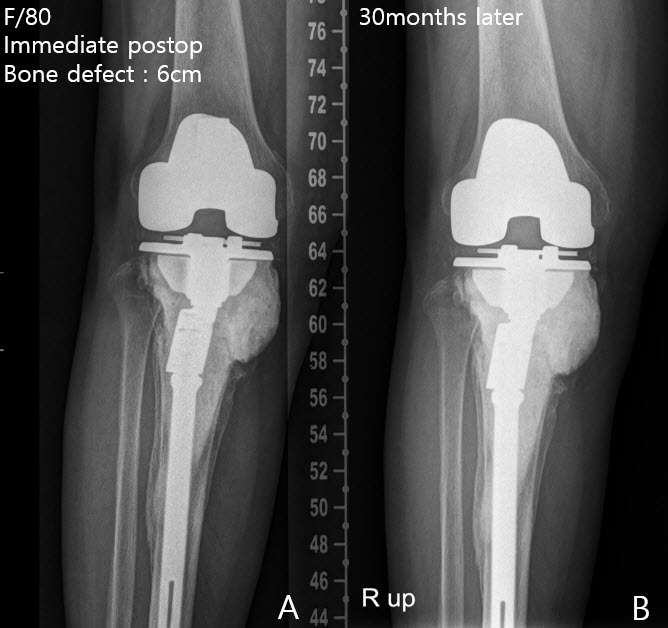
Figure 1
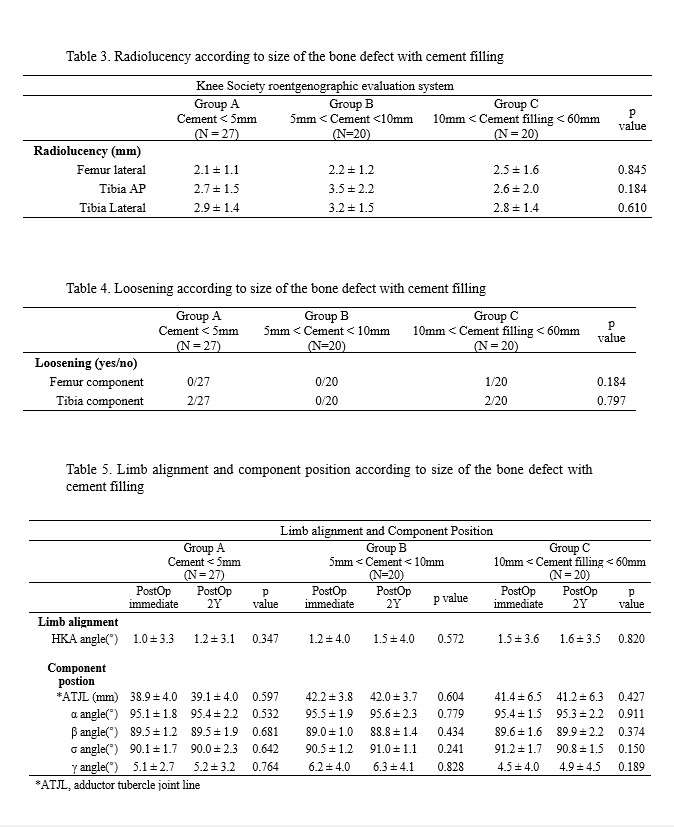
Figure 2#8618
Accuracy of Surgeon-Derived Versus CT-Based Bony Landmark Registration During Robotic-Assisted TKA: A Comparative Clinical Study
*Suhas Boenerjous-Abel - Bronx-Lebanon Hospital - Bronx, USA
*Email: drsuhas09@gmail.com
Accuracy of Surgeon-Derived versus CT-Based Bony landmark registration during Robotic-assisted TKA: A Comparative Clinical Study
ABSTRACT
Background
Robot-assisted total knee arthroplasty (RA-TKA) aims to improve implant positioning accuracy and reduce outliers in achieving limb alignment. Concerns about potential errors during landmark registration in imageless system registration have mostly been studied on cadavers or saw-bone models. This is one of the first study to compare the accuracy and reproducibility of bony landmark registration using CT-based and surgeon-derived landmarks during robotic assisted TKA.
Methods
This study was a prospective observational analysis on 90 patients undergoing RA-TKA. After completion of bone registration with an error less than 0.2mm, the surgeon was then required to register pre-defined anatomical landmarks using the bone registration probe entirely relying on the tactile and visual feedback from the operating field. A Python script was then used to analyse and compare the corresponding landmarks between the surgeon-derived points and the CT data.
Results
84 patients with a mean age of 62.6 years (SD= 8.9) were eligible for final analysis. Trans epicondylar axis (TEA) error with surgeon-derived points ranged from 7.9 degrees of internal rotation to 9.7 degrees of external rotation compared to CT based clinical TEA. Mean 3-D distance error of the medial and lateral epicondyle was 6.1mm (SD-3.1) and 5.4mm (SD-2.3) respectively. Along the coronal plane, majority of the surgeon-derived points were found to be over-cutting both the medial (N-72, 85.7%) and lateral femoral condyle (N-61, 72.6%); and also the medial (N-60, 71.4%) and lateral posterior femoral condyle (N-53, 63%). Tibial resection error ranged from 2.4mm proximally to 3.6mm distally in the medial plateau and 3.8mm proximally to 6.4mm distally along the lateral tibial plateau.
Conclusion
Surgeon-derived bony landmarks showed a wide variability when compared to 3D CT image based landmarks, which may need to be further evaluated in larger and systematic cohorts in order to reduce the chances of potential risk of component malalignment in image less RA-TKA
Keywords: accuracy; bone registration; error; robotic TKA; Imageless TKA
INTRODUCTION
One of the aims of robot-assisted total knee arthroplasty (RA-TKA) is to enhance the accuracy and precision of implant positioning and reduce outliers in achieving the planned limb alignment. Several studies have reported improved accuracy and reduced variability in implant alignment with RA-TKA, however its effect on long-term implant survival is yet to be established. Two broad categories of RA-TKA systems exist: image-based and image-less systems. They differ in how the anatomic landmarks are registered and how 3D virtual model is created. Image-based systems rely on pre-operative imaging, such as CT scan, to create a 3D virtual model of the knee, while image-less systems require surgeons to register landmarks and bone intraoperatively. While image-based systems offer the advantage of a preoperative 3D virtual plan, they also have downsides, which include the cost of imaging, the inconvenience of having to undergo imaging at a specific center with fixed protocols, and the additional burden of radiation exposure [1–3]
The accuracy of robotic or computer-assisted total knee arthroplasty (TKA) primarily depends on the reliable registration of bony landmarks.[4] Image-based systems use the CT scan to mark the anatomic landmarks and employ a process called segmentation, which creates a 3D model from CT scans. This model is used preoperatively to plan component sizes, position, bone resection depth, and alignment goals. During surgery, the patient's anatomy is matched to the 3D model by collecting mapped points, allowing the robotic system to determine relative position of the knee with regards to the cutting tools. This imageless systems in contrast lack the ability to pre-plan the procedure and solely rely on intraoperative registration of the patient's anatomy to generate a 3D virtual model. The accuracy of registration in imageless systems depends entirely on the surgeon's ability to input the data correctly. While imageless systems offer cost savings and avoid radiation exposure, they have their own drawbacks since the registration cannot be verified against a detailed 3D image.
Literature has highlighted concerns regarding potential errors during the registration process of imageless systems, as reported in various studies.[5–10] Inaccurate registration and suboptimal alignment of components have been associated with poor functional outcomes and reduced longevity in total knee replacement (TKR).[11,12] The majority of studies investigating this issue have been conducted in vitro using cadavers or saw bone models, revealing significant coronal and rotational errors. However, due to the lack of in-vivo studies, we conducted one of the first in vivo prospective studies taking advantage of the robotic software to compare CT-based landmarks with surgeon-derived landmarks in the same patient undergoing Mako (RATKA).
Our study aimed to assess and compare the accuracy and reproducibility of bony landmark registration between CT-based landmarks and surgeon-derived landmarks using the MAKO robotic software.
METHODS
This study was a prospective observational analysis of surgeon accuracy in intra-operative bony landmark registration compared to 3D CT-based landmark registration by the Image-based MAKO-robotic TKA software, in 90 consecutive patients undergoing robotic-arm assisted TKA (RATKA), at a single high-volume arthroplasty centre. The study was conducted between June 2022 to April 2023 after approval by the Institutional Ethics Committee (SIEC/2022/500).
90 patients underwent Three-dimensional Computed Tomography (3D-CT) image-based RATKA, performed using the Mako system (Stryker, Kalamazoo, MI, USA). Patients with primary OA of the knee, with varus or valgus deformity of any severity, were included in the study. Patients with previous knee surgeries, prior high-tibial or femoral osteotomies, post-traumatic arthritis, and extra-articular deformities were excluded.
CT- based landmark registration technique
After segmenting the CT scan of the patient, the MAKO Product Specialist (MPS) identifies specific anatomical points on the femur and tibia based on the three-dimensional reconstruction of the patient's lower limb. These points are as follows:
Femur:
- Medial and lateral epicondyles (Clinical TEA) - the most prominent points on the femur.
- Femur knee center - the central point of the femur near the knee.
- Femur head center - the central point of the femur's head.
Tibia:
- Medial and lateral malleolus - the bony prominences on the inner and outer sides of the ankle.
- Tibia knee center - the central point of the tibia near the knee.
- Rotational landmark - a specific point used for rotational reference.
Following this, the MPS identifies additional landmarks for resection on both the femur and tibia, which include:
- Femur:
- Distal-most point on the medial and lateral condyle.
- Most prominent point on the medial and lateral posterior condyle.
- Tibia:
- Medial and lateral tibial condyles
Surgical technique
All patients were operated with a standard medial para-patellar approach. After completion of the arthrotomy, two bi-cortical 3.2mm bone-pins were placed in the femur and tibia each for the placement of optical arrays. The surgeon then proceeds with gross and fine registration of bone based on the 3D-CT based model created by a process of segmentation, in the MAKO software. The bone registration process first involves the surgeon picking/ registering 40 points each on the distal femur and proximal tibia, based on a pre-defined distribution pattern (Figure.1a &b). This is followed by a process of verification (Figure.2). The mako software allows the procedure to continue when the registration error is less than 0.5mm. However, in this study we set a lower cut-off of 0.2mm, to minimize error.
Landmark Registration: Technique
After completion of bone registration with an error equal to less than 0.2mm, a single senior author experienced in image-less navigation systems was then required to point pre-defined anatomical landmarks using the bone registration probe. This is done using the pre-surgical planning screen of the MAKO software. The surgeon picks these landmarks entirely relying on the tactile and visual feedback he gets from the operating field without visualizing the MAKO screen. The landmarks captured include:
- Most prominent points on the Medial and Lateral epicondyles- representing the clinical transepicondylar axis (TEA)
- Medial and lateral distal femoral articular surface (single most prominent point)
- Medial and lateral posterior femoral articular surface (single most prominent point)
- Medial and lateral tibial plateau resection points (a point at the depth of the defect on the involved side, and the highest point on the un-involved side)
The landmarks derived on the 3D CT model are shown in Figure.3.
The logs for each case were extracted using a custom Python script (Python Software Foundation, Version 3.11.2) and organized in an Excel spreadsheet. Axes were plotted and angles were calculated. Planar and 3D distances between the surgeon-derived intra-operative landmark registration points and their corresponding 3D CT-derived landmarks were compared and mapped in x, y, and z coordinates. The x-axis on represents the medial-lateral axis, while the y-axis represents the anterior-posterior axis, and the z-axis represents the superior-inferior axis on both the femur and tibia.
The formulae used in the excel to calculate the Euclidean distance between CT and Captured Landmarks was = (SQRT(SUMXMY2(x1,y1,z1:x2,y2,z2))), where x1,y1,z1 are the coordinates for CT-derived landmark and x2,y2,z2 are the coordinates for their respective surgeon captured landmark.
The surgeon-derived intra-operative landmark registration points were compared with the 3D CT-derived landmarks, on the CT files post-operatively to evaluate variations in accuracy based on:
- Differences in 3D location of landmarks registered (based on variations on the cartesian coordinates system)
- Difference in CT-based and surgeon-derived Trans-epicondylar axes (TEA) and Posterior Condylar Axes (PCA) (Figure.4.)
- Over-resection and under-resection of the various femoral and tibial bone cuts.
The coordinate of the CT-derived (ideal) position of the bony landmarks (xn, yn, zn) was calculated by the equation (xn, yn, zn) = {(x1, y1, z1)+(x2, y2, z2) + …. + (x100, y100, z100)}/100. The error in identifying the surgeon-derived anatomic landmark was expressed as a deviation from this ideal point. Figures outside 2 standard deviations were excluded. By doing this, the variability of the surgeon-derived points was calculated and it was then compared to the average of the 90 CT-based landmark points.
Based on the variations in landmark registration, the software code was designed to calculate differences in bone resection (over-resection and under-resection) and axial component positioning of the femur (internal versus external rotation). RA-TKA was finally carried out using the CT-based points as final reference and routine primary TKA was carried out.
Statistical Analysis
Data normality was confirmed with the Shapiro-Wilk test. Continuous variables were presented as means with standard deviation and range. Error was represented in degrees (angles) and millimeters (distances). Comparison of mean error was analysed using the independent samples t-test. Data is presented as histograms and p-value less than 0.05 was considered significant with 95% confidence interval.
RESULTS
Summary statistics of the patient population are shown in Table 1. The study included ninety patients, with an average age of 62.6 years (SD-8.9). The majority of the patients were female, comprising 54 individuals (64.3%). These patients had an average varus deformity of 10.6 degrees (SD-2.7). After the exclusion of outliers, a total of 84 patients were eligible for final evaluation. All distances and angles are reported as the difference between the surgeon-derived landmark points when compared to the CT-based point using MAKO software. A summary of the findings is summarized in Table 2.
Trans-Epicondylar axis
On calculation of the TEA using the surgeon-derived points on the medial and lateral epicondyle, error in TEA registration showed a wide variation ranging from 7.9 degrees of internal rotation to 9.7 degrees of external rotation when compared to the true TEA (CT- derived).
Medial Epicondyle
Mean 3-D distance error of the surgeon was 6.1mm (SD-3.1) from the true centre. Error along the antero-posterior axis ranged from 9.4mm anteriorly to 13.2mm posteriorly and similarly error along the supero-inferior axis ranged from 11.4mm superiorly to 13.2mm inferiorly.
Lateral Epicondyle
Mean 3-D distance error of the surgeon 5.4mm (SD-2.3) from the true centre. Error along the antero-posterior axis ranged from 7.2mm anteriorly to 8mm posteriorly and similarly error along the supero-inferior axis ranged from 6.8mm superiorly to 9.6mm inferiorly.
Resection depths
Distal femur
Distal femur resection error on the medial femoral condyle was found to range from 3.4mm proximally to 1.7mm distally on the medial condyle with an overall mean of 0.9mm (SD-0.97) proximal; whereas on the lateral femoral condyle, it ranged from 2.9mm proximally to 2.2mm distally with an overall mean difference of 0.66mm (SD-1).
Majority of the surgeon-derived points were found to be over-cutting both the medial (N-72, 85.7%) and lateral femoral condyle (N-61, 72.6%)
Posterior femur
Posterior femoral resection error varied from 6.5mm anterior (overcutting) to 2.6mm posterior (undercutting) on the lateral condyle and ranged from 6.6mm anterior (overcutting) to 3.3mm posterior (undercutting).
Majority of the surgeon-derived points were found to be over-cutting both the medial (N-60, 71.4%) and lateral femoral condyle (N-53, 63%)
Proximal tibia
Tibial resection error which was measured along the supero-inferior axis ranged from 2.4mm proximally to 3.6mm distally in the medial plateau and ranged from 3.8mm proximally to 6.4mm distally along the lateral tibial plateau.
It was also found that a majority of the surgeon-derived points on the medial tibial plateau were under-cutting (N-49, 58.3%) whereas on the lateral tibial plateau, the surgeon had more tendency to over-cut (N-52, 61.9%).
DISCUSSION
The aim of this prospective study was to compare the variations in accuracy of bony landmark registration done by surgeons versus 3D-CT based landmark registration in patients undergoing primary robotic TKA. This is the first study to compare surgeon accuracy to gold-standard radiological landmarks in the clinical setting, using robotic software. Published evidence has proven the superiority of computer navigation and robotic technology over manual techniques in the accuracy of target alignment and component positioning.[13,14] Robotics has been proven to improve the accuracy and precision of bone resection.[15,16] However, not all robotic systems are the same. Image-less navigation or robotics still rely on surgeon-derived bony landmarks for bone registration, as opposed to image-based robotic systems in which the anatomical landmarks are marked on the 3D-CT based virtual bone models. The landmarks derived from 3D-CT scans are inherently more accurate than intra-operative surgeon-derived points in image-free systems. However, the variations in landmark registration has not been reported in robotic TKA.[17]
Our findings demonstrated a wide variation in surgeon-derived landmark registration during RATKA. One of the most important technical factors of TKA concerns the axial positioning of the femoral component. There are several intra-operative anatomical landmarks to optimize femoral component rotation, and it has been established that the use of the Trans-Epicondylar Axis (TEA) is the most reliable technique. Surgeons aim to place the component parallel to the TEA to obtain a balanced rectangular flexion gap and optimize patello-femoral kinematics during TKA. In this study, there was a wide variation in surgeon derived TEA versus the CT image-based measurements. The variation ranged from 7.9 degrees of internal rotation to 9.7 degrees of external rotation when compared to the clinical TEA (CT- derived) which was in agreement with few other studies. [5,6,18]
The variation in the TEA was mainly because of the 3-Dimensional variation in the registration of the femoral epicondyles. Variations of the medial epicondyle registration by surgeons in the sagittal plane ranged from 9.4mm anterior to 13.2mm posteriorly and 11.4mm superiorly to 13.2mm inferiorly in the coronal plane. These findings show that surgeons can have significant variations in component positioning because of the possible inaccuracy in image-free bone registration which was in agreement with the invitro study conducted by Lustig et al. [19]
E.T. Davis et al. studied errors during the registration process during imageless computer navigation for total knee arthroplasty. This was a cadaver-based study and five surgeons performed bone registration on fresh frozen cadavers, using the Stryker Knee Navigation system. They reported an error of 11.1 degrees of external rotation and 6.3 degrees of internal rotation of the TEA (Mean -3.2 degrees, SD=3.9). This paper reported a significant change in the mechanical axis of the femur due to variations in the registration of the femoral centre (which varied from 6.5mm lateral to 5.0mm medial to the actual centre). This can significantly affect component position during image-free navigation or robotics TKA.[20] Yau et al published similar findings with a maximum error of 10.8mm in the registration of the distal femoral centre which resulted in a femoral mechanical axis deviation of 1.3 degrees. [21]
Lustig et al., reported their findings about the accuracy of landmark acquisition using an in-vitro laboratory set-up. The navigation probe was used to select pre-defined points on an experimental apparatus and the error was reported. Although the error reported for distances and angles was low, this is only to prove the accuracy of the navigation system. The accuracy of the navigation hardware and approximation software is still limited by surgeon accuracy in picking landmarks in the clinical setting. [19]
Studies have shown that surgical landmarks such as the epicondyles are difficult to accurately mark without image guidance[PM1] .[7,9,22,23] Epicondyles are covered with soft-tissues and are not easy to palpate accurately in all cases. In the cadaver study by Davis et al, the reference landmark was marked after complete removal and stripping of soft-tissues.[20] Image-based robotics takes care of this problem, as the pre-operative CT scan provides accurate registration of the landmarks.[15] Cheng et al in their systematic review, reported reduced reliability of image-free navigation systems in axial positioning of the component.[24] A lot of emphasis has been placed on the coronal axis deviations after TKA, however, axial alignment is equally important.
According to Amanatullah et al, registering the medial and lateral femoral epicondyles as landmarks presents the greatest challenge, displaying significant intra-observer and inter-observer variability. The study also revealed that the safe zone for the antero-posterior axis of the epicondyles was less than 2mm. As a result, the authors concluded that this small margin of error could potentially explain why imageless navigation systems do not show improved outcomes compared to conventional total knee arthroplasty (TKA).[25] Alignment of TKA prosthesis is vital for early and late clinical outcomes.[11,12] The findings of this study demonstrate the limitations of image-free navigation or robotic systems, due to high degree of variation in bony landmark registration done by surgeons. Despite approximation software, designed to minimize the error, improper bone registration can compromise component positioning during total knee arthroplasty.
There are a few limitations to this study. Firstly, the sample size is small, which may limit the generalizability of the findings. Additionally, the analysis only included measurements taken by a single surgeon, as it would have been challenging to handle multiple streams of data simultaneously for different landmarks at the same time. Furthermore no comparative examination of post-operative alignment using both procedures was performed, which could have provided useful insights into the effectiveness of the explored methodology. Finally, the study did not evaluate clinical and functional outcomes, which are critical for determining the practical implications of the findings. Future large-scale randomized control trials are necessary to draw definitive conclusions between the two robotic-assisted TKA systems.
This study has notable strengths. Firstly, it stands out as one of the few in vivo studies to examine the disparity between landmarks chosen by surgeons and those derived from CT scans on the same patient. The analysis of mean error in each point was conducted using both 3D Euclidean distance and individual axis distance measurements, enhancing accuracy and comprehension. Gaining insight into the specific error tendencies of surgeons can be tremendously valuable for enhancing their accuracy in imageless systems by providing guidance for correction and improvement.
CONCLUSION
Surgeon-derived bony landmarks showed a wide variability when compared to 3D CT image based landmarks, which may need to be further evaluated in larger and systematic cohorts in order to reduce the chances of potential risk of component malalignment in image less RA-TKA
REFERENCES
[1] Kayani B, Konan S, Ayuob A, Onochie E, Al-Jabri T, Haddad FS. Robotic technology in total knee arthroplasty: a systematic review. EFORT Open Rev 2019;4:611–7. https://doi.org/10.1302/2058-5241.4.190022.
[2] Jacofsky DJ, Allen M. Robotics in Arthroplasty: A Comprehensive Review. J Arthroplasty 2016;31:2353–63. https://doi.org/10.1016/J.ARTH.2016.05.026.
[3] Picard F, Deep K, Jenny JY. Current state of the art in total knee arthroplasty computer navigation. Knee Surg Sports Traumatol Arthrosc 2016;24:3565–74. https://doi.org/10.1007/S00167-016-4337-1.
[4] Brin YS, Livshetz I, Antoniou J, Greenberg-Dotan S, Zukor DJ. Precise landmarking in computer assisted total knee arthroplasty is critical to final alignment. Journal of Orthopaedic Research 2010;28:1355–9. https://doi.org/10.1002/jor.21139.
[5] Yau WP, Leung A, Liu KG, Yan CH, Wong LLS, Chiu KY. Interobserver and Intra-observer Errors in Obtaining Visually Selected Anatomical Landmarks During Registration Process in Non-Image-Based Navigation-Assisted Total Knee Arthroplasty. Journal of Arthroplasty 2007;22:1150–61. https://doi.org/10.1016/j.arth.2006.10.010.
[6] Yau WP, Leung A, Liu KG, Yan CH, Wong LS, Chiu KY. Errors in the identification of the transepicondylar and anteroposterior axes of the distal femur in total knee replacement using minimally-invasive and conventional approaches: a cadaver study. J Bone Joint Surg Br 2008;90:520–6. https://doi.org/10.1302/0301-620X.90B4.19841.
[7] Jerosch J, Peuker E, Philipps B, Filler T. Interindividual reproducibility in perioperative rotational alignment of femoral components in knee prosthetic surgery using the transepicondylar axis. Knee Surg Sports Traumatol Arthrosc 2002;10:194–7. https://doi.org/10.1007/S00167-001-0271-X.
[8] Fuiko R, Kotten B, Zettl R, Ritschl P. [The accuracy of palpation from orientation points for the navigated implantation of knee prostheses]. Orthopade 2004;33:338–43. https://doi.org/10.1007/S00132-003-0570-7.
[9] Jenny JY, Boeri C. Low reproducibility of the intra-operative measurement of the transepicondylar axis during total knee replacement. Acta Orthop Scand 2004;75:74–7. https://doi.org/10.1080/00016470410001708150.
[10] Siston RA, Patel JJ, Goodman SB, Delp SL, Giori NJ. The variability of femoral rotational alignment in total knee arthroplasty. J Bone Joint Surg Am 2005;87:2276–80. https://doi.org/10.2106/JBJS.D.02945.
[11] Valkering KP, Breugem SJ, van den Bekerom MP, Tuinebreijer WE, van Geenen RCI. Effect of rotational alignment on outcome of total knee arthroplasty. Acta Orthop 2015;86:432–9. https://doi.org/10.3109/17453674.2015.1022438.
[12] Nicoll D, Rowley DI. Internal rotational error of the tibial component is a major cause of pain after total knee replacement. J Bone Joint Surg Br 2010;92:1238–44. https://doi.org/10.1302/0301-620X.92B9.23516.
[13] Shah SM. After 25 years of computer-navigated total knee arthroplasty, where do we stand today? Arthroplasty (London, England) 2021;3:41. https://doi.org/10.1186/s42836-021-00100-9.
[14] Shatrov J, Parker D. Computer and robotic – assisted total knee arthroplasty: a review of outcomes. J Exp Orthop 2020;7:1–15. https://doi.org/10.1186/S40634-020-00278-Y/TABLES/5.
[15] Miyasaka T, Kurosaka D, Saito M, Omori T, Ikeda R, Marumo K. Accuracy of Computed Tomography–Based Navigation-Assisted Total Knee Arthroplasty: Outlier Analysis. Journal of Arthroplasty 2017;32:47–52. https://doi.org/10.1016/j.arth.2016.05.069.
[16] Biant LC, Yeoh K, Walker PM, Bruce WJM, Walsh WR. The accuracy of bone resections made during computer navigated total knee replacement. Do we resect what the computer plans we resect? Knee 2008;15:238–41. https://doi.org/10.1016/j.knee.2008.01.012.
[17] Tabatabaee RM, Rasouli MR, Maltenfort MG, Fuino R, Restrepo C, Oliashirazi A. Computer-Assisted Total Knee Arthroplasty: Is There a Difference Between Image-Based and Imageless Techniques? Journal of Arthroplasty 2018;33:1076–81. https://doi.org/10.1016/j.arth.2017.11.030.
[18] Yau WP, Leung A, Chiu KY, Tang WM, Ng TP. Intraobserver errors in obtaining visually selected anatomic landmarks during registration process in nonimage-based navigation-assisted total knee arthroplasty: A cadaveric experiment. Journal of Arthroplasty 2005;20:591–601. https://doi.org/10.1016/j.arth.2005.02.011.
[19] Lustig S, Fleury C, Goy D, Neyret P, Donell ST. The accuracy of acquisition of an imageless computer-assisted system and its implication for knee arthroplasty. Knee 2011;18:15–20. https://doi.org/10.1016/j.knee.2009.12.010.
[20] Davis ET, Pagkalos J, Gallie PAM, Macgroarty K, Waddell JP, Schemitsch EH. Defining the Errors in the Registration Process During Imageless Computer Navigation in Total Knee Arthroplasty: A Cadaveric Study. Journal of Arthroplasty 2014;29:698–701. https://doi.org/10.1016/j.arth.2013.06.034.
[21] Yau WP, Leung A, Chiu KY, Tang WM, Ng TP. Intraobserver errors in obtaining visually selected anatomic landmarks during registration process in nonimage-based navigation-assisted total knee arthroplasty: A cadaveric experiment. Journal of Arthroplasty 2005;20:591–601. https://doi.org/10.1016/j.arth.2005.02.011.
[22] Lustig S, Lavoie F, Selmi TAS, Servien E, Neyret P. Relationship between the surgical epicondylar axis and the articular surface of the distal femur: an anatomic study. Knee Surg Sports Traumatol Arthrosc 2008;16:674–82. https://doi.org/10.1007/S00167-008-0551-9.
[23] van der Linden–van der Zwaag HMJ, Valstar ER, van der Molen AJ, Nelissen RGHH. Transepicondylar axis accuracy in computer assisted knee surgery: a comparison of the CT-based measured axis versus the CAS-determined axis. Comput Aided Surg 2008;13:200–6. https://doi.org/10.3109/10929080802240134.
[24] Cheng T, Zhang G, Zhang X. Imageless navigation system does not improve component rotational alignment in total knee arthroplasty. Journal of Surgical Research 2011;171:590–600. https://doi.org/10.1016/j.jss.2010.05.006.
[25] Amanatullah DF, Di Cesare PE, Meere PA, Pereira GC. Identification of the landmark registration safe zones during total knee arthroplasty using an imageless navigation system. Journal of Arthroplasty 2013;28:938–42. https://doi.org/10.1016/j.arth.2012.12.013.
LIST OF TABLES
|
Mean Age in Years (SD)
|
62.6 (8.9)
|
|
Gender Distribution
Male
Female
|
30 (35.7%)
53 (64.3%)
|
|
ASA Distribution
ASA I
ASA II
|
13 (15.5%)
71 (84.5%)
|
|
Mean Charlson Comorbidity Index (SD)
|
1.76 (0.87)
|
|
Mean BMI (SD)
|
28.3 (3.4)
|
|
Mean Pre-operative Coronal Deformity in degrees (SD)
|
10.6 (2.7)
|
|
Mean Pre-operative Sagittal Deformity in degrees (SD)
|
3.6 (4.2)
|
Table 1: Patient demographic details
|
Landmark
|
Plane of error
|
Mean
|
SD
|
Range (Min-Max)
|
|
Medial epicondyle (mm)
|
3D (Euclidean distance)
|
6.1
|
3.1
|
1.3-13.7
|
|
Lateral epicondyle (mm)
|
3D (Euclidean distance)
|
5.4
|
2.3
|
1.2-10.4
|
|
Distal Femur- Medial (mm)
|
Coronal (Supero-inferior)
|
0.9 (overcut)
|
0.97
|
1.7 (undercut) -3.4 (overcut)
|
|
Distal Femur- Lateral (mm)
|
Coronal (Supero-inferior)
|
0.65 (overcut)
|
1
|
2.2 (undercut) -2.9 (overcut)
|
|
Posterior Femur- Medial (mm)
|
Sagittal (Antero-posterior)
|
0.9 (overcut)
|
1.7
|
2.6 (undercut) -6.5 (overcut)
|
|
Posterior Femur- Lateral (mm)
|
Sagittal (Antero-posterior)
|
0.5 (overcut)
|
1.5
|
3.3 (undercut) -6.6 (overcut)
|
|
Tibia- Medial (mm)
|
Coronal (Supero-inferior)
|
0.2 (overcut)
|
0.9
|
2.4 (undercut) -3.6 (overcut)
|
|
Tibia- Lateral (mm)
|
Coronal (Supero-inferior)
|
0.6 (overcut)
|
1.5
|
3.9 (undercut) -6.3 (overcut)
|
|
TEA axis (degrees)
|
Axial
|
0.4 (internal rotation)
|
3.3
|
7.9 (internal rotation)- 9.7 (external rotation)
|
Table 2: Summary of resection and rotational error between surgeon-derived bony landmarks vs. CT- derived landmarks
LIST OF FIGURES

Figure 1: Bone registration and verification: (a) Images of 3D CT based model of the knee on the mako TKA software, with fine bony registration points. The registration pattern is part of standard mako pre-surgical planning and includes a total of 40 points each on the femur and tibia

Figure 2: Bone verification: Images of 3D CT based model of the knee on the mako TKA software, with bone verification points. The registration pattern is part of standard mako pre-surgical planning and includes a total of 6 points each on the femur and tibia

Figure 3:3-D model of the femur and tibia showing the surgeon-derived bony points on the MAKO pre-planning screen.

Figure 4: Novel software-generated post-surgical 3D model showing surgeon derived versus CT-Derived TEA and PCA

Figure 5: TEA axis variation

Figure 6: Medial and lateral distal femoral resection error

Figure 7: Medial and lateral posterior femoral resection error

Figure 8: Medial and lateral proximal tibial resection error
Figures
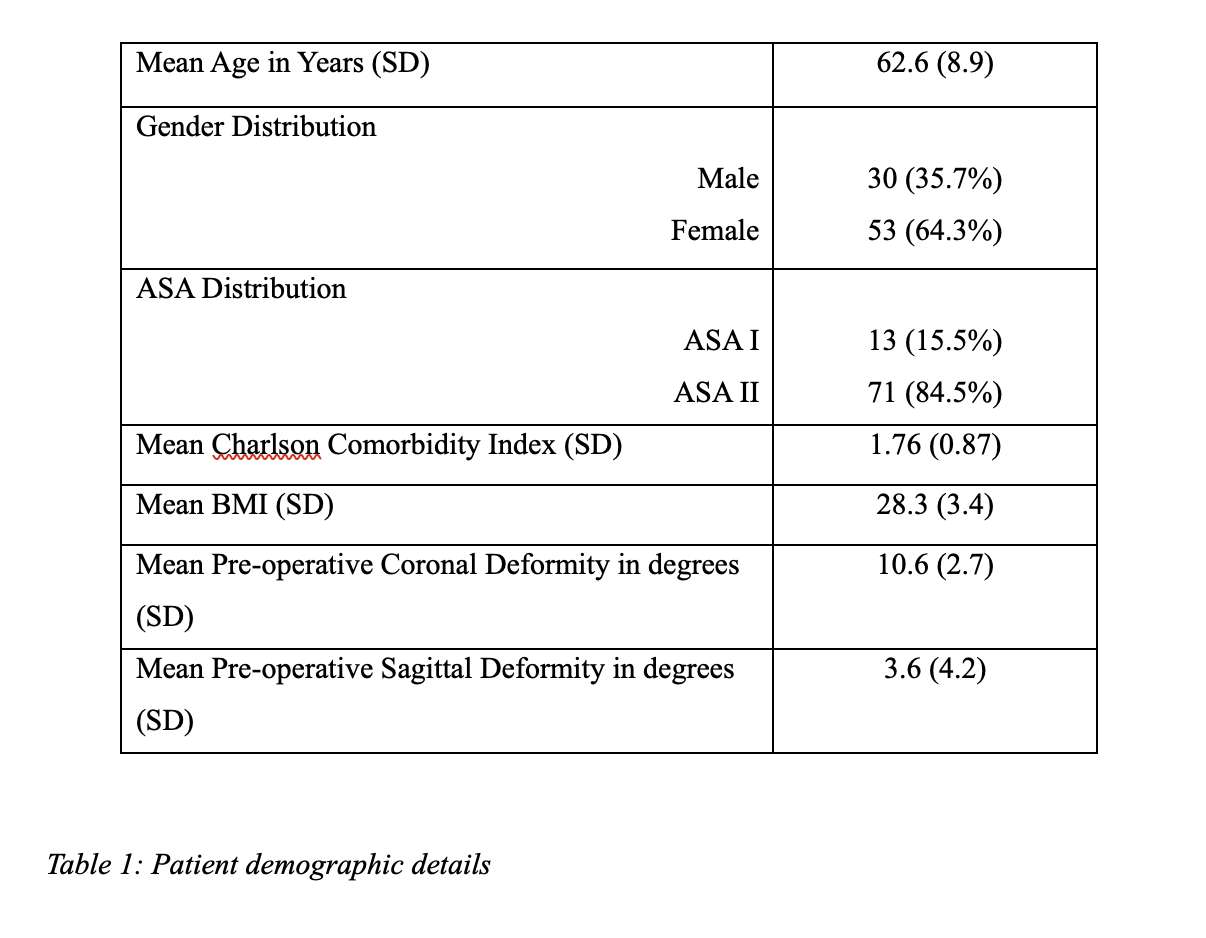
Figure 1
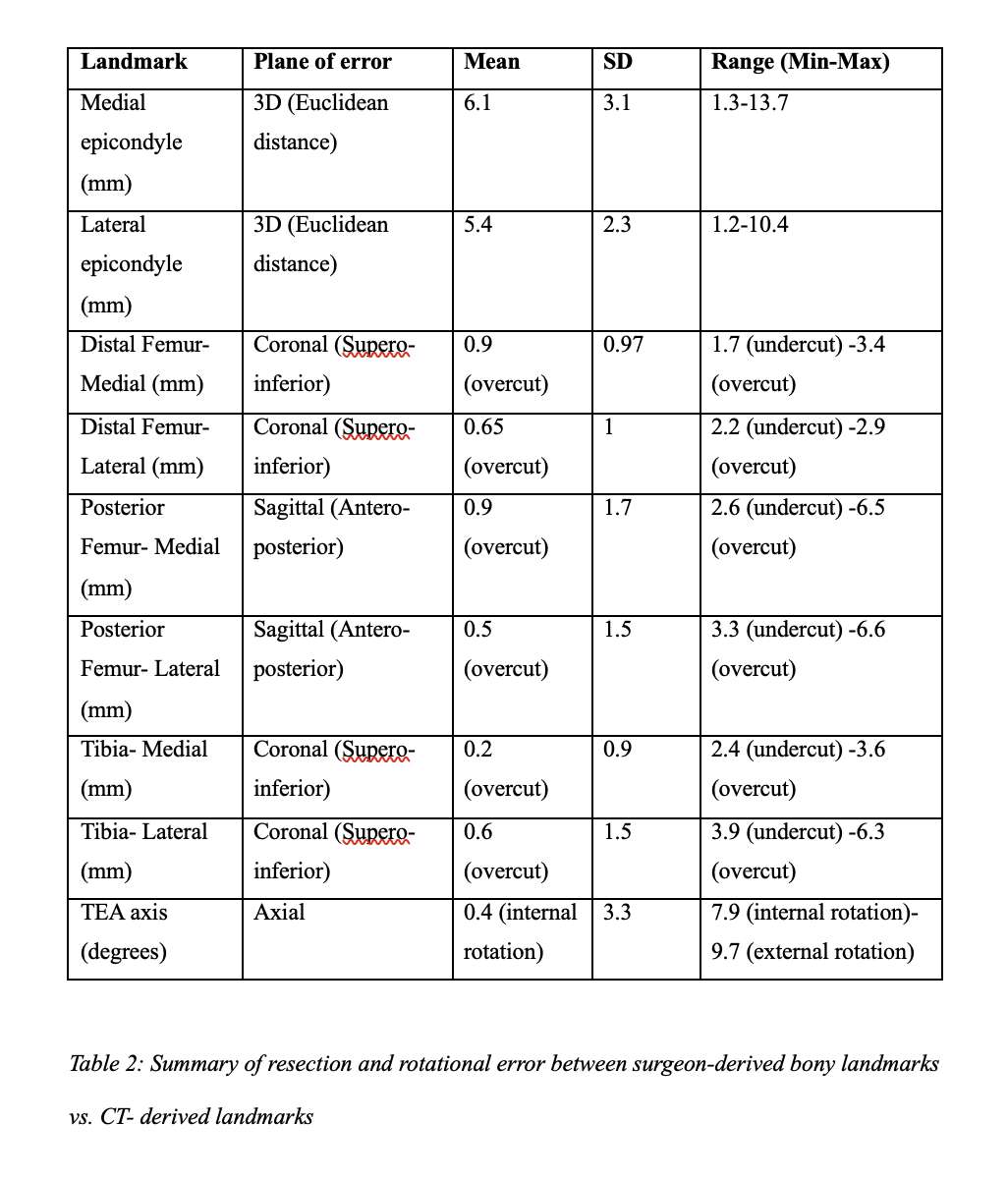
Figure 2
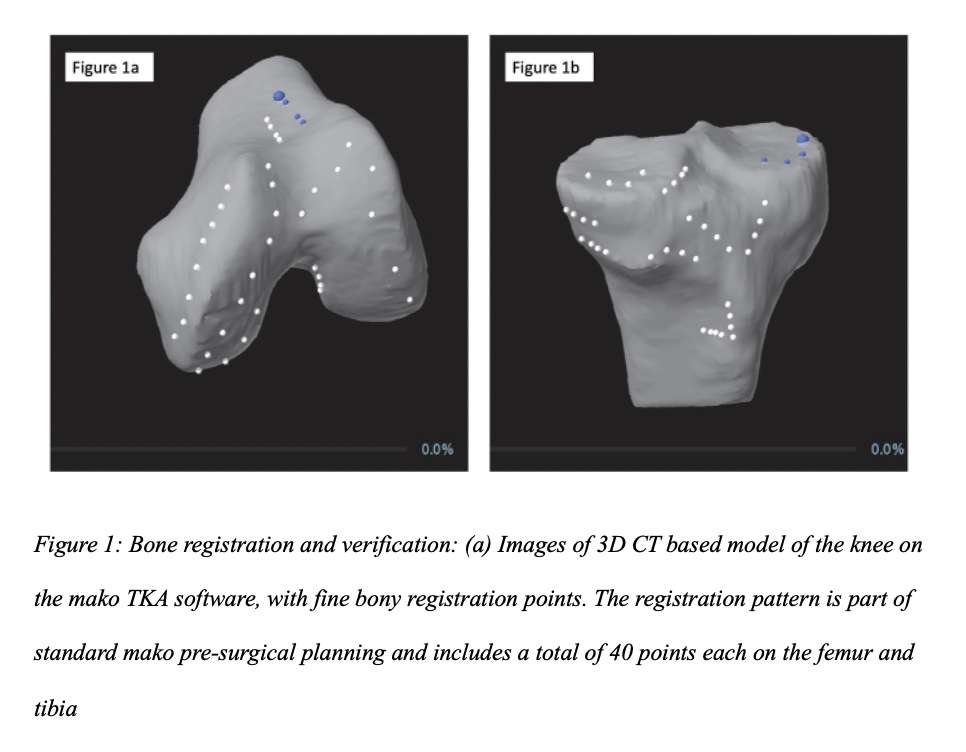
Figure 3
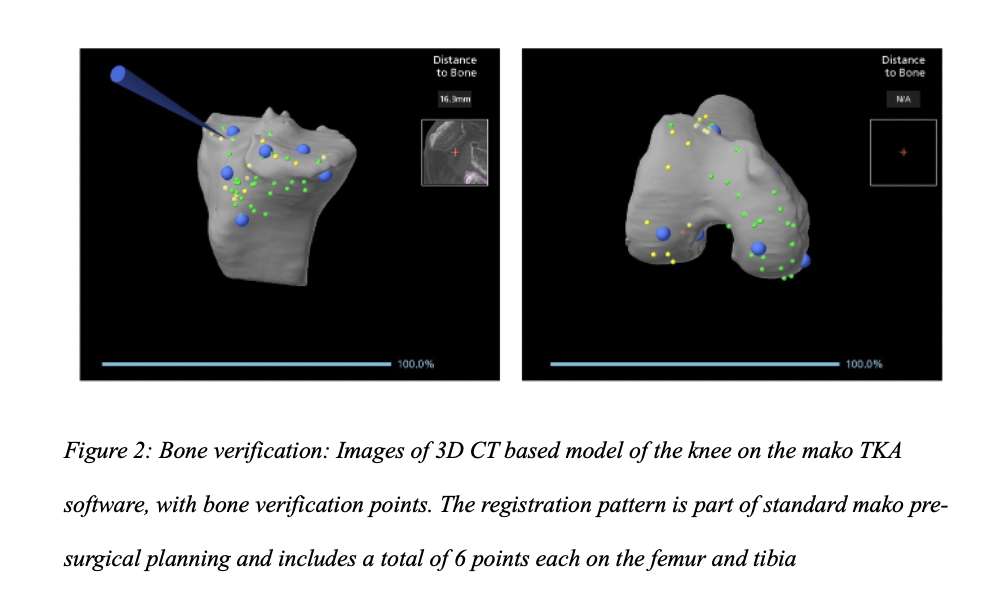
Figure 4
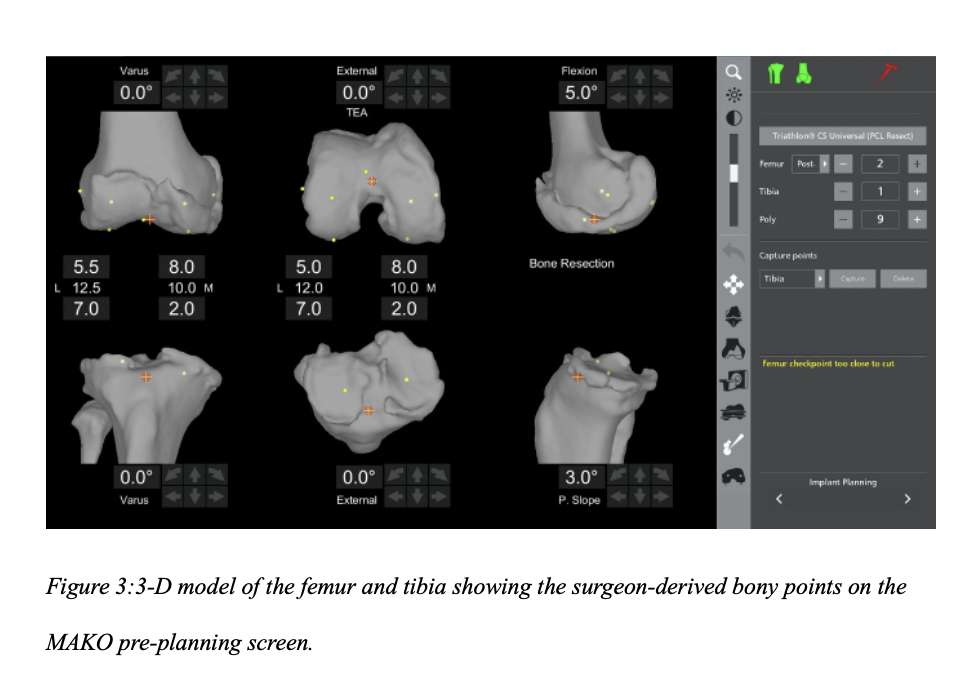
Figure 5
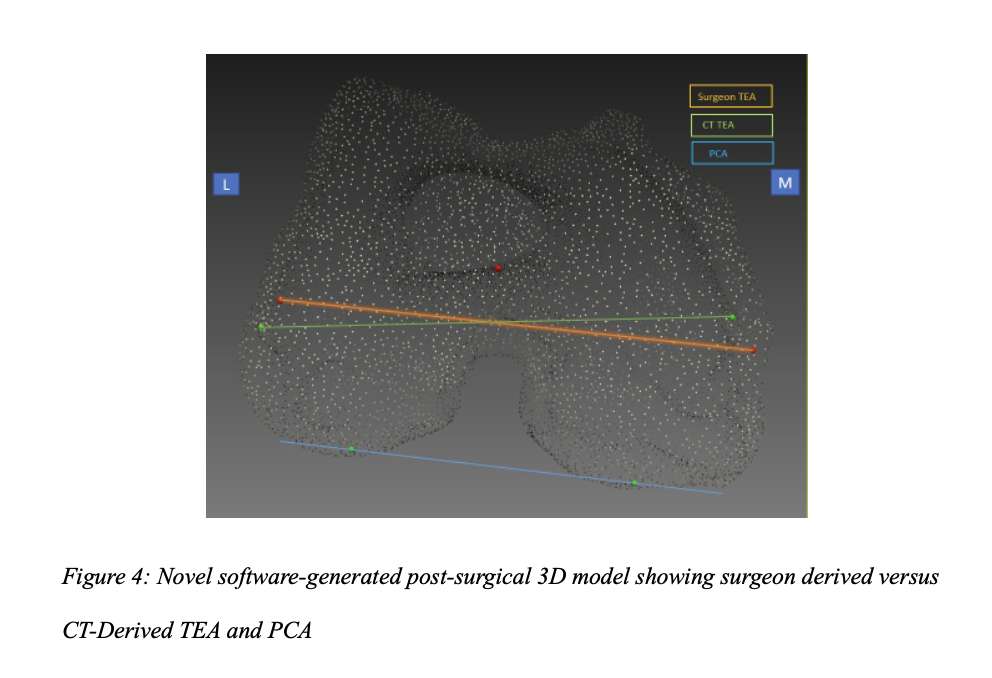
Figure 6
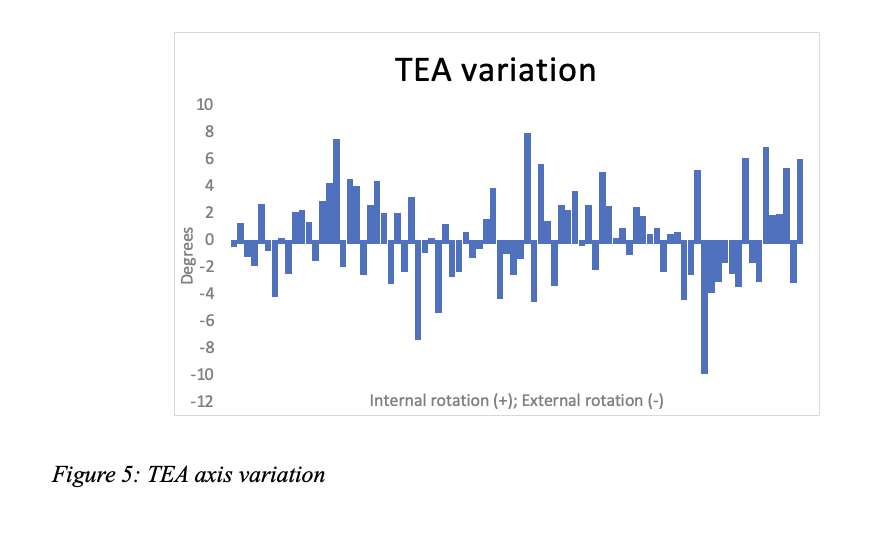
Figure 7
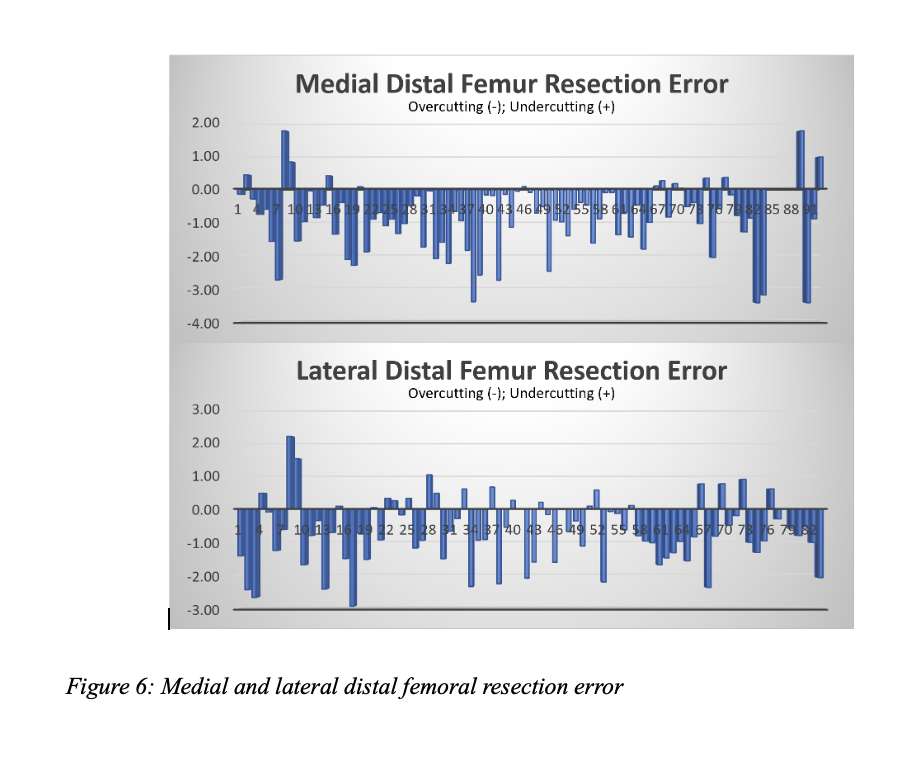
Figure 8
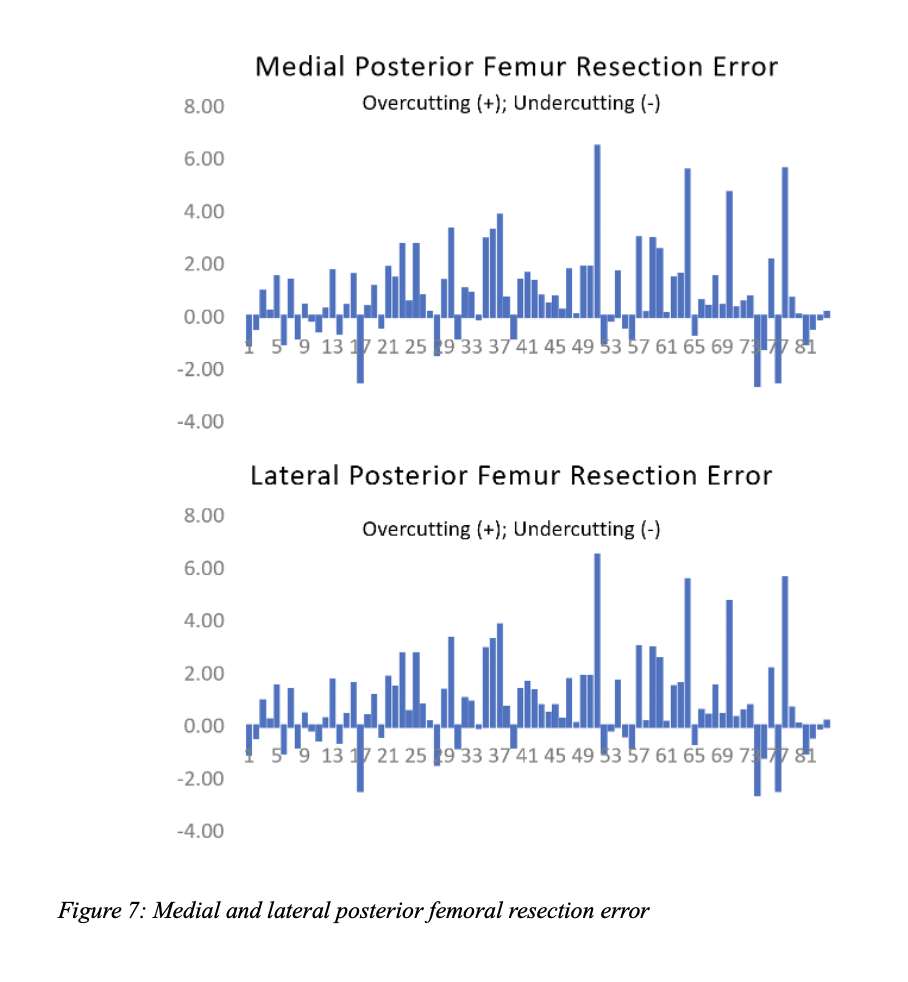
Figure 9
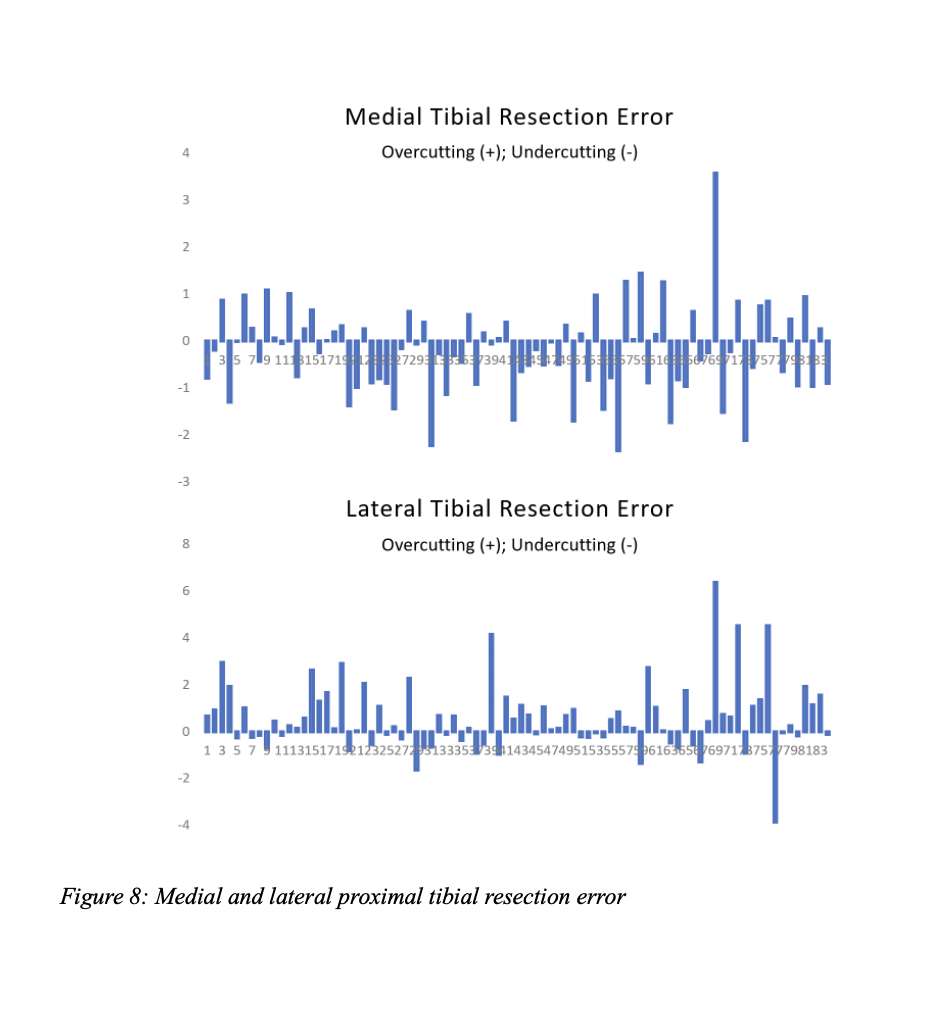
Figure 10#8189
Construct Stability of Revision Total Knee Arthroplasty With Tibial Cones: Preliminary Results of a Radiostereometric Analysis (RSA)
*Kelly Mills - Sint Maartenskliniek - Nijmegen, Netherlands
Maartje Belt - Sint Maartenskliniek - Ubbergen, Netherlands
Gijs Van Hellemondt - Ubbergen (Near Nijmegen), Belgium
Petra Heesterbeek - Sint Maartenskliniek - Nijmegen, Netherlands
*Email: k.mills@maartenskliniek.nl
Introduction
In (re-)revision total knee arthroplasty (TKA), cones can be used to ensure sufficient fixation of the prothesis in the bone in case of suboptimal metaphyseal bone stock (Fig. 1). Whether this construct results in stable and safe fixation of the implant, remains to be investigated. We aimed to investigate the stability of the fixation of the Legion revision TKA with tibial cones in the bone until 5 years postoperatively.
Methods
Twenty five patients who underwent a revision TKA with a tibial cone (Smith+Nephew, Memphis (TN), USA) were included in this prospective study. During surgery, tantalum markers were inserted in the tibial bone. It is believed that these markers are fixed in the bone and they form a 3D model of the tibia bone. The stability of the implant was assessed by measuring micromotion (total translation (TT) and total rotation (TR)) of the tibial component with respect to the 3D bone model by using model-based RSA (RSAcore, Leiden, the Netherlands). Radiographs were made post-operative (baseline), after 6 weeks, 3 months, 6 months, 1 year, 2 years, and 5 years. Clinical results were evaluated using the Oxford Knee Score (OKS), KOOS-PS, Knee Society Score (KSS), VAS pain and VAS satisfaction.
Results
Currently, all 25 patients were included and of those, 22 patients completed the 6 months follow-up, 19 the 1-year follow-up and 11 the 2-year follow-up. Median (IQR = Percentile 25 – Percentile 75) age at the time of surgery was 64 years (IQR 61-68.75 years), and median BMI was 30.4 (IQR 24.2-32.6). Median TT at 1 year was 0.43 mm (IQR 0.29-1.06) (Fig. 2), median TR was 0.63? (IQR 0.33-1.23) (Fig. 3). 5 patients showed TT>1mm, 7 patients showed TR >1?. One patient was lost to follow after 1 year due to a repeat revision TKA for loosening. At 1 year the median clinical scores were: VAS pain was 4 (IQR 2-6), VAS satisfaction was 6 (IQR 3-8), OKS 30.5 (IQR 27.3-39), KSS clinical 92 (IQR 70-95) and functional 60 (IQR 45-70), and KOOS-PS was 39.45 (35.3-43.5).
Discussion and Conclusion
So far, results on group level show a stable fixation, although there are outliers with more micromotion. One patient had a repeat revision TKA for loosening, not evidently related to migration as measured with RSA. At the time of the conference, we expect to share 22/25 1-year migration results and 16/25 2-year.
Figures
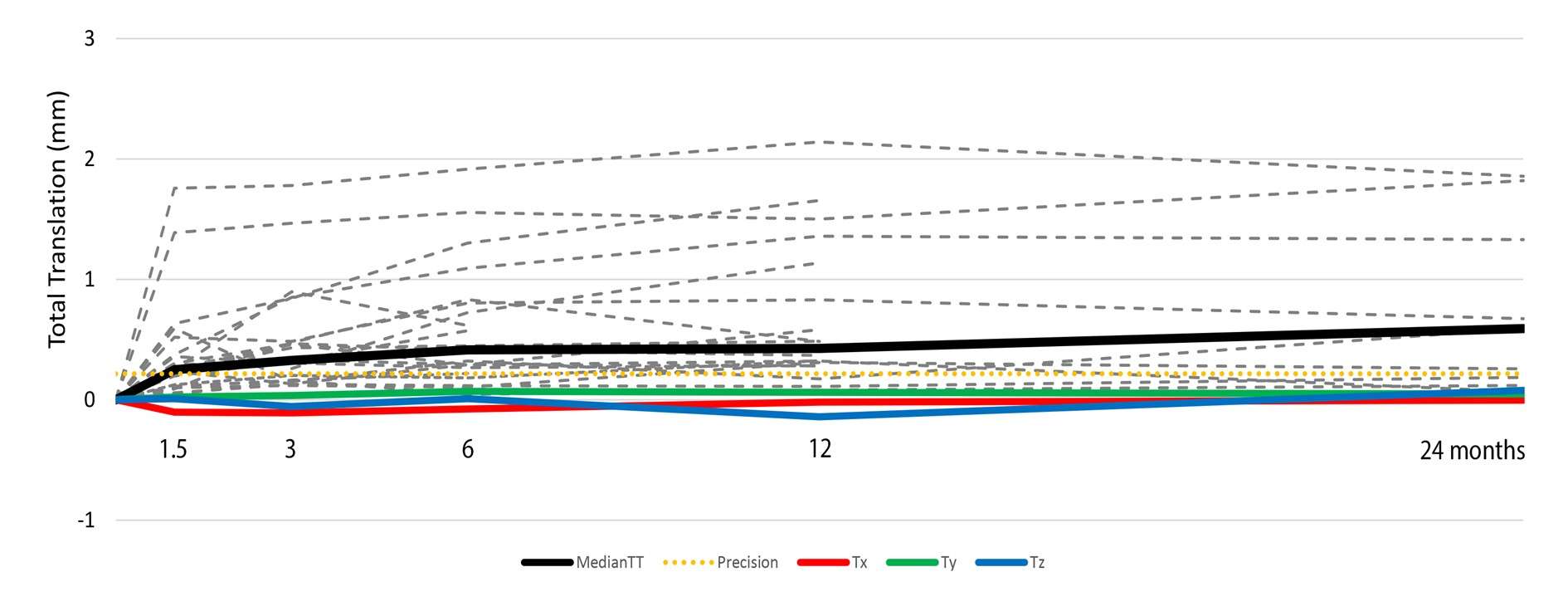
Figure 1
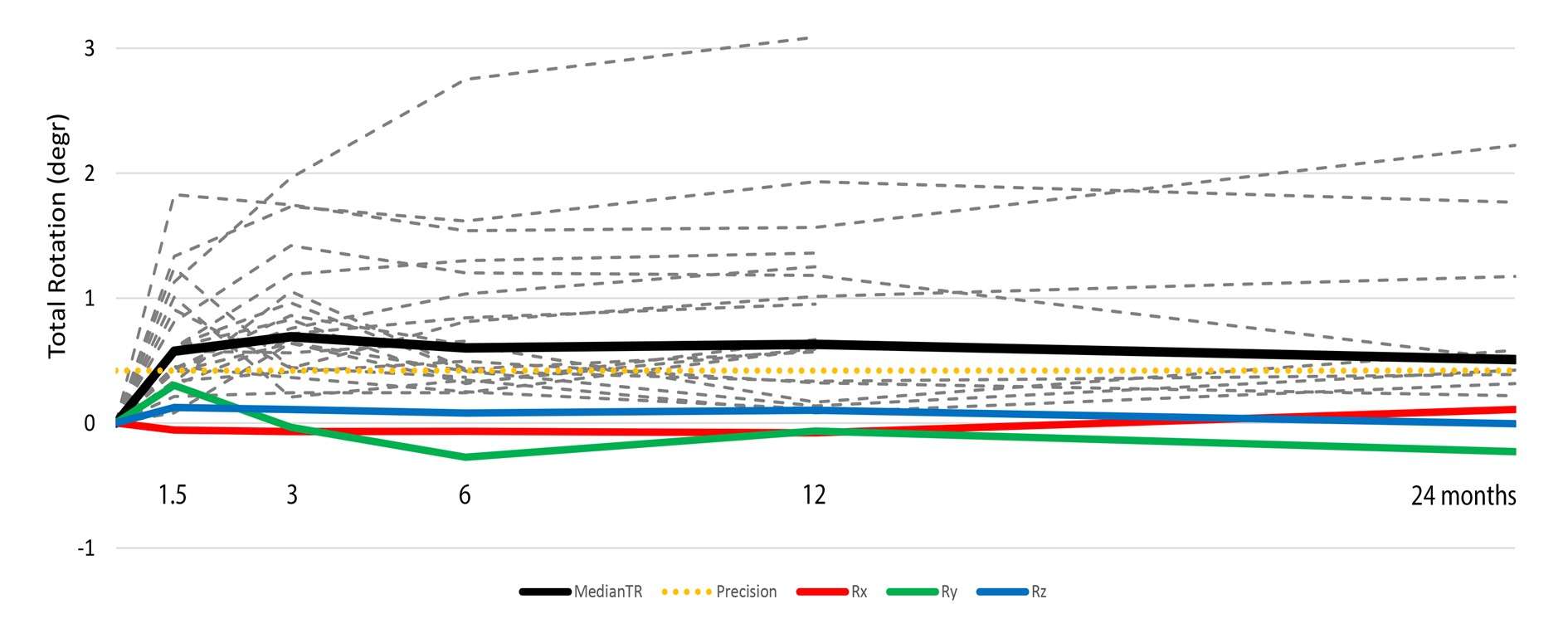
Figure 2
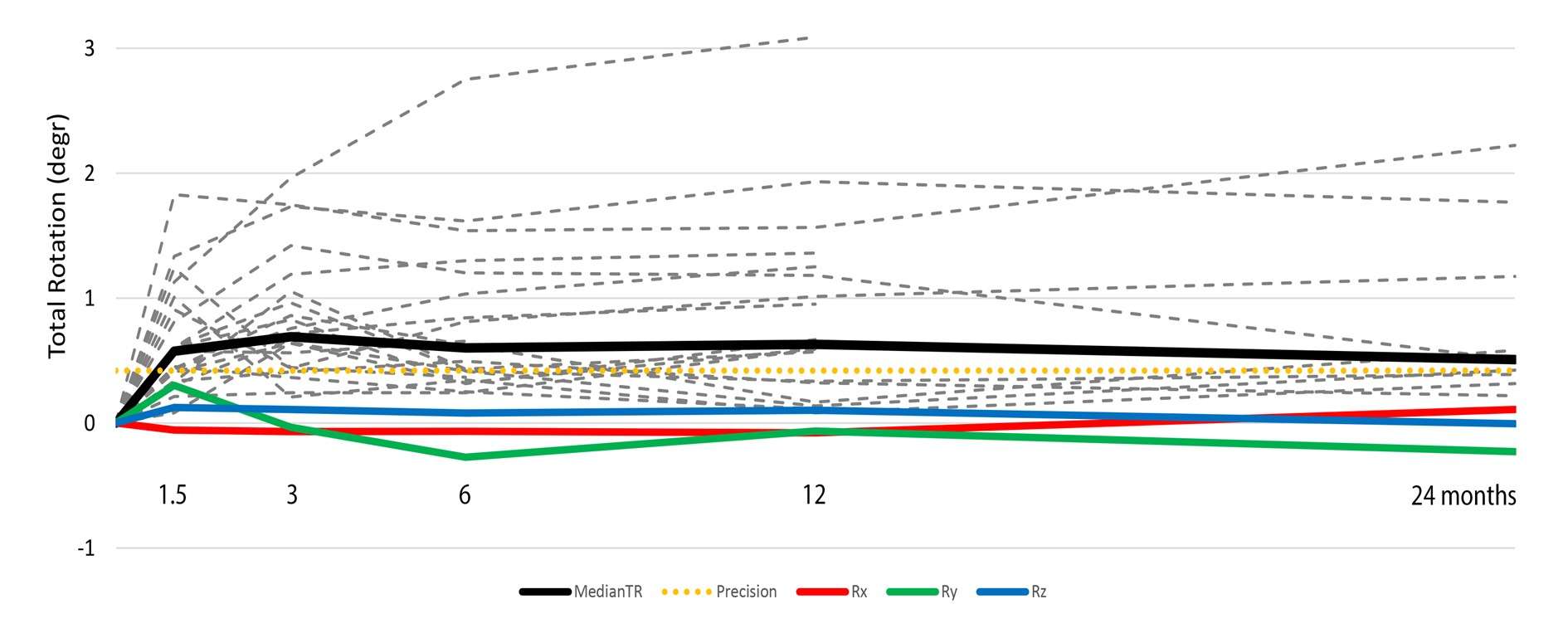
Figure 3#8287
Surgeon Delay in Geriatric Hip Fractures: Overall Time to Surgery Matters, but Not Time From Optimization to Surgery
*Mitchell Ng - Maimonides Medical Center - Brooklyn, United States of America
Matthew Magruder - Maimonides Medical Center - Brooklyn, USA
Ivan Golub - Maimonides Medical Center - Brooklyn, USA
Kevin Kang - Maimonides Medical Center - Brooklyn, USA
Lucas Voyvodic - Maimonides Medical Center - Brooklyn, USA
Ameer Tabbaa - x, United States of America
*Email: mitchng77@gmail.com
Introduction: Current U.S. and Canadian guidelines strongly recommend hip fracture surgery within 48 hours of injury to decrease relative morbidity/mortality. Multiple studies have focused on medical optimization as the key component of time to surgery. However, there is inherent bias in these analyses as patients with multiple co-morbidities and/or overall sicker medical status often take longer to optimize than healthier patients, resulting in a longer time to surgery and overall higher risk for post-operative complications. To this end, the aim of this study was to evaluate the time from medical optimization to surgery to minimize the inherent bias and determine if “real surgical delay” is associated with : 1) mortality and 2) overall complications for geriatric hip fracture patients.
Methods: A retrospective chart review of operative geriatric hip fractures treated from 2015-2018 at a single, level one trauma center was conducted, treated with percutaneous pinning, cephalomedullary nailing, hip hemiarthroplasty or total hip arthroplasty acutely. Univariate logistic regression was performed to identify potential association between time from medical optimization to surgery, and post-operative complication rates. For mortality, the Wilcoxon test was used to compare times from medical optimization to surgery for patients who were successfully discharged following surgery to those who were not.
Results: A total of 972 hip fractures were treated operatively, with a median time from medical optimization to surgery of 12.3 hours (4.9–21.7, 1st-3rd quartiles). Univariate logistic regression models did not identify an association between time from medical optimization to surgery and complication rate. As such, no optimal cutoff time could be established for complication rate. For the cohort of patients that were successfully discharged, median time from medical optimization to surgery was 12.1 hours (4.9–21.6, 1st-3rd quartiles). For the cohort of patients were not successfully discharged, median time from medical optimization to surgery was 19.4 hours (9.2–28.7, 1st-3rd quartiles). This difference, however, was not statistically significant (p = 0.16).
Conclusion: “Real surgical delay”, or time from medical optimization to surgery is not associated with increased complications or with in-patient mortality for geriatric hip fracture patients treated with arthroplasty or non-arthroplasty surgical procedures. Of note, with few exceptions, our institution adhered to the 48-hour time window from injury to hip surgery. We maintain the belief that timely surgery following medical optimization plays a crucial role in the outcomes of geriatric hip fracture patients.
#8167
Association of Serum Ferritin With Bone Turnover Markers and Bone Mineral Density in Children and Adolescents: Results From NHANES 1999-2002
*Zhi Chen - Fuzhou, China
*Email: 810316156@qq.com
Background: Recently, the effect of iron on bone metabolism is gaining increasing attention, but the results remain controversial. This study aimed to explore the relationships of serum ferritin with bone mineral density (BMD) and bone turnover markers in this specific population.
Methods: We utilized data from National Health and Nutrition Examination Survey (NHANES) 1999-2002 for analysis. Data on lumbar BMD, serum bone alkaline phosphatase (sBAP), urinary N-telopeptides (uNTx), serum ferritin and covariates were extracted and analyzed. Weighted multivariate linear regression analyses and smooth curve fittings were conducted to evaluate the association of ferritin with BMD, sBAP and uNTx. When nonlinearity was identified, threshold effect analysis was performed using two-piecewise linear regression model.
Results: A total of 1527 participants were included. The ferritin was positively linked with BMD in unadjusted model, but not in adjusted models. Subgroup analyses demonstrated similar results in boys. The ferritin was negatively linked with sBAP and uNTx in all models. In subgroup analyses, these associations remained significant in boys, but not in girls. Smooth curve fittings showed nonlinear relationships of ferritin with sBAP and uNTx, with the infection point identified at 41 ng/mL and 16 ng/mL.
Conclusions: Our study revealed that ferritin was positively related to BMD, and negatively related to sBAP and uNTx in children and adolescents. These findings improved our understanding about the effects of iron on bone metabolism, and shed new light on the assessing and improving of bone health in children and adolescents.
Keywords: ferritin, bone mineral density, bone turnover marker, NHANES
#8397
Significant Analgesic Benefits of Perioperative Duloxetine in Patients Who Have Depressive Symptoms Undergoing Total Hip Arthroplasty: A Randomized Controlled Trial
Zi-Chuan Ding - West China Hospital, Sichuan University - Chengdu, China
*Zongke Zhou - Sichuan University - Chengdu, China
Weinan Zeng - Southwest Hospital, Third Military Medical University - Chongqing, China
*Email: zongke@126.com
Introduction: Major symptoms of depression are commonly observed in patients requiring total hip arthroplasty (THA), and this is associated with increased pain scores and opioid consumption. We aimed to investigate the analgesic effect of duloxetine in these high-risk patients.
Methods: Among 263 patients scheduled for primary unilateral THA, 67 patients who scored at least 8 on the 17-item Hamilton Depression Scale (HAMD) were enrolled in this study. Patients were randomized to the duloxetine group (60 mg daily, from the day of surgery to postoperative day 6) or the placebo group. The postoperative visual analog scale (VAS) score during walking, the VAS score during hip flexion, and resting VAS score was measured. Postoperative morphine consumption, hip range of motion (ROM), Harris hip score (HHS), Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC) function, postoperative length of stay (LOS), and adverse events were recorded.
Results: The duloxetine group had significantly lower VAS scores during walking and hip flexion from postoperative day 3 to week 3 than the placebo group. With regard to the resting VAS score, duloxetine showed a better analgesic effect from postoperative day 3 to week 2 than placebo. Patients in the duloxetine group had less consumption of morphine. The duloxetine group exhibited better hip function scores, including ROM, HHS, and WOMAC function scores than the placebo group. No significant difference was observed in LOS or adverse events between groups.
Conclusion: Perioperative short-term duloxetine provides advantages in decreasing pain, reducing morphine consumption, and increasing hip function in THA patients who have depressive symptoms.
#8107
Preoperative Celecoxib Use Does Not Increase Risk of Myocardial Infarction and May Be Protective Against Thromboembolic Complications Following Total Joint Arthroplasty
*Matthew Magruder - Maimonides Medical Center - Brooklyn, USA
Vincent Yao - Sophie Davis Biomedical Education Program at the CUNY School of Medicine - New York, USA
Ariel Rodriguez - Maimonides Medical Center - Brooklyn, United States of America
Mitchell Ng - Maimonides Medical Center - Brooklyn, United States of America
Che Hang Jason Wong - Maimonides Medical Center - Brooklyn, USA
*Email: mmagruder@maimonidesmed.org
Introduction
Celecoxib is a selective cyclooxygenase-2 (COX-2) inhibitor used to treat pain in patients with primary osteoarthritis and after total joint arthroplasty (TJA). The benefits of this medication over other NSAIDs include improved pain relief without side effects related to COX-1 inhibition, like gastritis and development of peptic ulcers. However, some research has demonstrated increased risk of myocardial infarctions (MI) in patients treated with this medication. Prior research has been limited by relatively small sample sizes. Therefore, the aims of this study are to evaluate whether patients treated with celecoxib following TJA have increased risk of postoperative 1) MIs, 2) thromboembolic complications, 3) transfusions, and 4) readmissions.
Methods
Using an administrative claim database (PearlDiver), a retrospective query from January 1, 2010 to October 31, 2020 was performed. All patients who underwent primary total knee arthroplasty (TKA) and total hip arthroplasty (THA) for osteoarthritis who had an active prescription for celecoxib within 30 days prior to the procedure were included. All patients with a history of MI were excluded from the analysis. Study group patients were successfully 1:5 propensity score matched with controls based on age, gender, body-mass index (BMI) and Charlson-comorbidity index, yielding a total of 230,587 TKA patients (Celecoxib = 38,433, control = 192,154) and 129,611 THA patients (Celecoxib = 21,603, control = 108,008). Outcomes evaluated included 90-day rates of MI, deep vein thrombosis (DVT), pulmonary embolism (PE), venous thromboembolism (VTE), transfusion and readmission. Multivariate regression was used to calculate odds ratios (ORs), 95% confidence intervals (95% CIs), and p-values. Welch’s t-tests were used to test for significant differences in lengths of stay and costs of care between cohorts. A p value <0.001 was the significance threshold.
Results
Celecoxib cohort demonstrated significantly equivalent rates of post-operative MI’s following TKA (0.16% vs 0.23%; OR 0.72; p=0.018) and THA (0.25% vs 0.22%; OR 1.14; p=0.359) compared with matched controls (Table 1). In the TKA analysis, rates of DVT (3.11% vs 3.25%; OR 0.95; p<0.001) and transfusions (5.57% vs 7.09%; OR 0.76; p<0.001) were significantly lower in the celecoxib cohort. Rates of PE (2.56% vs 2.71%; OR 0.94; p=0.106), VTE (4.45% vs 4.77%; OR 0.93; p=0.008) and readmission (5.35% vs 5.39%; OR 0.99; p=0.73) were not significantly different between the groups. In the THA analysis, rates of DVT (2.57% vs 3.02%; OR 0.84; p<0.001), VTE (3.64% vs 4.30%; OR 0.83; p<0.001), transfusion (6.57% vs 10.09%; OR 0.65; p<0.001) and readmission (5.49% vs 6.06%; OR 0.89; p=0.001) were all significantly lower in the celecoxib cohort. Rates of PE (2.10% vs 2.40%; OR 0.87; p=0.007) were not statistically significant between the THA cohorts.
Conclusion
In this study, celecoxib use did not demonstrate increased risk of myocardial infarctions following TKA and THA. Further, the celecoxib cohort experienced either significantly decreased rates or no significant difference in rates of DVT, PE, VTE, transfusions and readmissions compared with matched controls. The potential thromboembolic benefits may be due in part to unknown pharmacologic mechanisms of action or improved pain control leading to increased mobility in the study cohort.
Figures
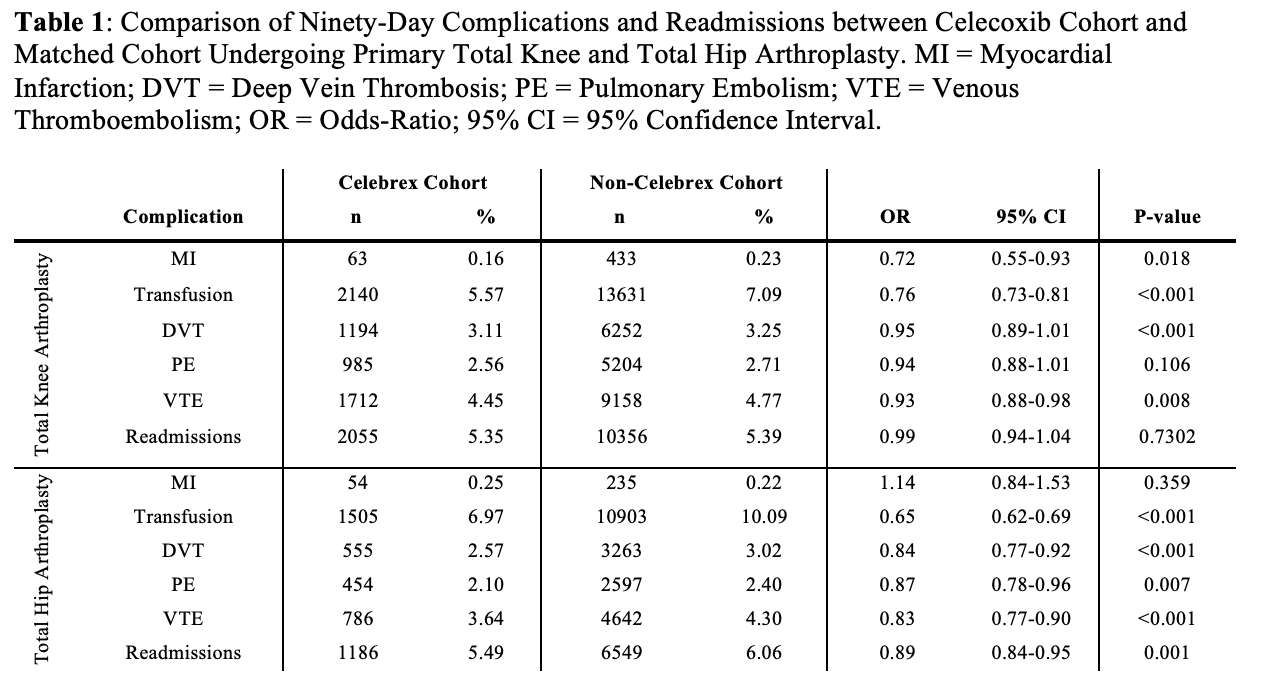
Figure 1#8168
Association Between Serum Cotinine and Muscle Mass: Results From NHANES 2011-2018
*Zhi Chen - Fuzhou, China
*Email: 810316156@qq.com
Purpose: At present, the detrimental effect of cigarette smoking on muscle mass is poorly understood. This study aimed to investigate the association between exposure to cigarette smoke, defined based on serum cotinine, and muscle mass in the US population.
Methods: We utilized National Health and Nutrition Examination Survey data between 2011 and 2018 for analysis. Data on serum cotinine, muscle mass (quantified by appendicular skeletal muscle mass index, ASMI), and covariates were extracted and analyzed. Weighted multivariate linear regression analyses and smooth curve fittings were performed to investigate the association between serum cotinine and ASMI. Subgroup analyses were stratified by race and smoking status. When nonlinearity was detected, the threshold effects were analyzed.
Results: In total, 9908 participants were included for analysis. The serum level of cotinine was negatively associated with ASMI in fully adjusted model. Furthermore, comparing participants in the highest vs. the lowest tertile of serum cotinine, we found that ASMI decreased by 0.151 Kg/m2. In subgroup analysis stratified by race, the association between serum cotinine and ASMI remained significant in all races. In addition, the association remained significant among current and former smokers, but not among those who never smoked. Smooth curve fittings showed nonlinear relationships between serum cotinine and ASMI, with the infection points identified at 344 ng/mL.
Conclusions: Our study revealed that serum cotinine is negatively related to muscle mass. This finding improves our understanding of the deleterious effects of cigarette smoking on muscle mass and highlights the importance of smoking cessation for muscle health.
#8157
Effects of Multiple IV Dexamethasone on Clinical Symptoms After TKA
Jisu Park - SMG–SNU BORAMAE MEDICAL CENTER - Seoul, Korea (Republic of)
MinKi Kim - Seoul national university - Seoul, Korea (Republic of)
Tae Jung Kim - SMG-SNU Boramae Medical Center/Seoul National University College of Medicine - Seoul, Korea (Republic of)
Tae Woo Kim - Seoul National University College of Medicine, SMG-SNU Boramae Medical Center - Seoul, South Korea
Moon Jong Chang - SMG-SNU Boramae Medical Center - seoul, South Korea
Chong Bum Chang - Seoul National University College of Medicine - Seoul, South Korea
*Seung-Baik Kang - Boramae Medical Center/ Seoul National University College of Medicine - Seoul, South Korea
*Email: ossbkang@gmail.com
Introduction
In recent times, many medical centers have been using intravenous (IV) perioperative dexamethasone, and several studies have been conducted to evaluate its effects on pain, postoperative nausea and vomiting (PONV), and postoperative discomfort through a large number of patients. Despite numerous studies conducted on dexamethasone, there has been limited research on the frequency and dose of its use.
This study is a retrospective, observational study that compares the effects of multiple low dose of perioperative intravenous (IV) dexamethasone versus no IV dexamethasone on postoperative pain, nausea and vomiting, and medication usage. The study aims to evaluate the efficacy of perioperative multiple low dose IV dexamethasone through a comparison of the two groups.
Methods
This retrospective study was conducted on 420 patients without diabetes. Patients were divided into two groups according to the use of IV dexamethasone (Dexa or No Dexa). For the Dexa, 10mg IV dexamethasone was administered twice (surgery day, postoperative day 1) and 5mg IV dexamethasone was administered once (postoperative day 2). The study measured VAS scores for pain in both groups, as well as the usage of opioid painkiller. To observe nausea and vomiting, the frequency of nausea or vomiting and the usage of antiemetics were measured.
Results
The data is divided into two groups, one group where intravenous (IV) dexamethasone was not administered (No Dexa, n = 210) and another group where it was administered (Dexa, n = 210).
The Dexa group had lower VAS scores compared to the No Dexa group on all postoperative days (POD 1 to 5), with a statistically significant difference. (Figure 1-A) The incidence of PONV was significantly lower in the Dexa group compared to the No Dexa group on surgery day and on postoperative day 1, 2. On postoperative day 3, however, the incidence of PONV was higher in the Dexa group compared to the No Dexa group. On postoperative days 4 and 5, there was no statistically significant difference in two groups. (Figure 1-B)
The cumulative opioid drug dose was significantly lower in the Dexa group compared to the No Dexa group on the day of surgery and on postoperative day 1. (Figure 1-D) The amount of antiemetics used postoperatively was significantly higher in the No Dexa group compared to the Dexa group on the day of surgery and on postoperative day 2, while it was significantly higher in the Dexa group compared to the No Dexa group on postoperative day 3. (Figure 1-C)
Conclusion
The study found that patients in the Dexa group experienced less pain compared to those in the No Dexa group up to five postoperative days, and that the incidence of PONV was significantly reduced in the Dexa group compared to the No Dexa group on surgery day and on postoperative day 1 and 2. Additionally, the use of opioid drug and the consumption of antiemetics were also significantly reduced in the Dexa group.
Figures
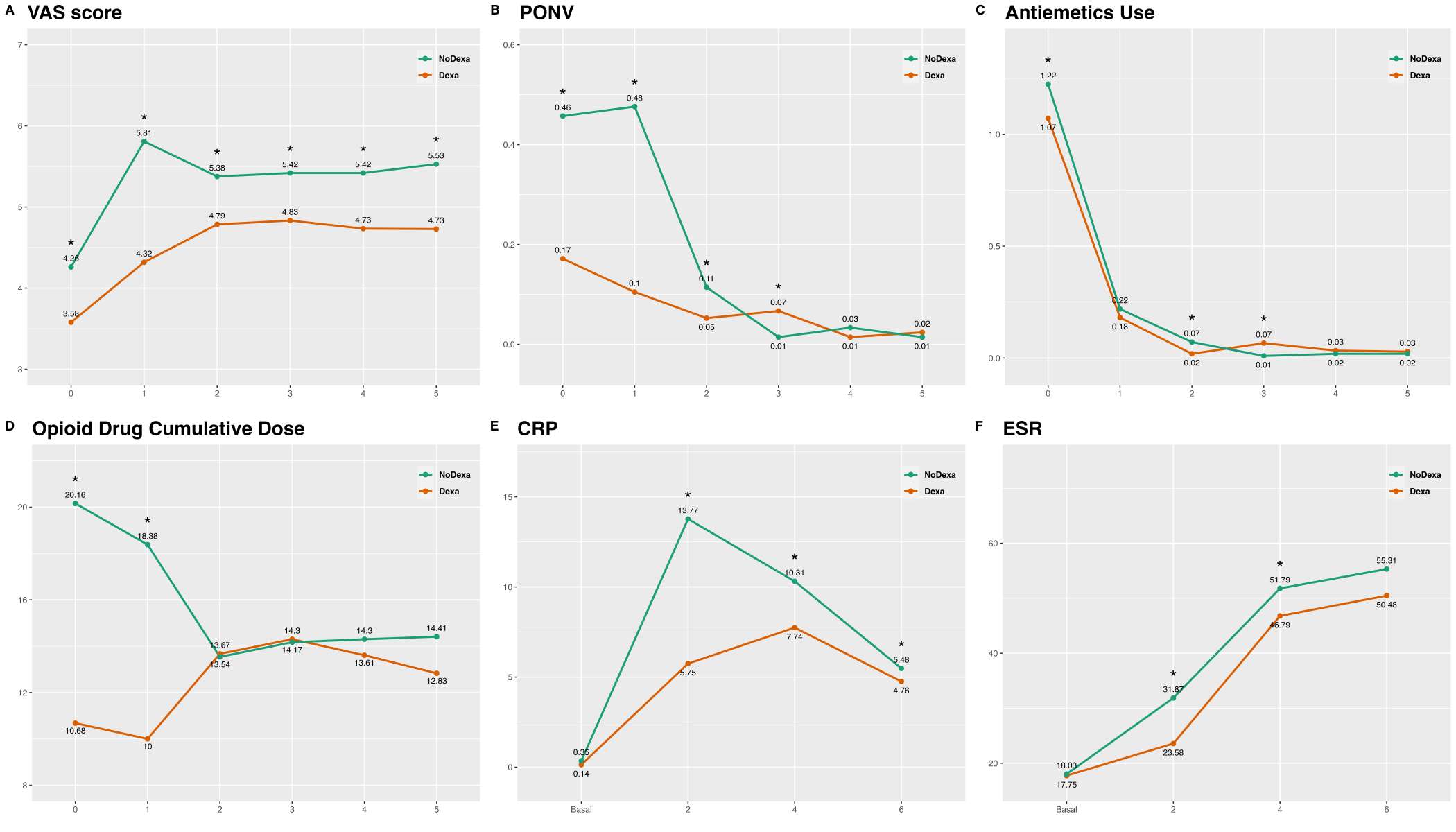
Figure 1#8592
Crohns Disease Is Associated With Higher Rates of Implant-Related Complications Following Primary Total Knee Arthroplasty
*Ariel Rodriguez - Maimonides Medical Center - Brooklyn, United States of America
Radha Pandya - Maimonides Medical Center - Brooklyn, USA
Vanathi Ganesan - Maimonides Medical Center - Brooklyn, USA
Matthew Magruder - Maimonides Medical Center - Brooklyn, USA
Che Hang Jason Wong - Maimonides Medical Center - Brooklyn, USA
Afshin Razi - Maimonides Medical Center - Brooklyn, USA
Jack Choueka - Maimonides Medical Center - Brooklyn, USA
Gabriel Lama - Maimonides Medical Center - Staten Island, United States of America
*Email: arielr418@gmail.com
Introduction: Crohn’s Disease (CD) and primary total knee arthroplasty (TKA) have risen in prevalence in Western countries. This study further investigates the impact of CD on (1) in-hospital length of stay (LOS); (2) readmission rates; (3) implant-related complications; and (4) costs of care in TKA patients.
Methods: A retrospective query from the PearlDiver database was performed from January 1st, 2005 to March 31st, 2014 for TKA patients with and without CD. CD patients were matched to controls in a 1:5 ratio by age, sex, and medical comorbidities, yielding 96,229 patients (CD=16,039; non-CD=80,190).
Results: CD patients had significantly longer LOS (3- vs. 2- days, p<0.0001). CD patients had significantly higher incidence and odds (19.80 vs. 14.91%; OR: 1.40, p<0.0001) of 90-day readmissions and of total medical and implant-related complications (6.88 vs. 4.88%; OR: 1.43, p<0.0001). CD patients incurred higher day of surgery ($18,365.98 vs. $16,192.00; p<0.0001) and 90-day costs ($21,337.46 vs. $19,101.42; p<0.0001).
Conclusion: This study demonstrates longer in-hospital LOS, higher rates of readmissions, implant-related complications, and costs of care among CD patients following primary TKA.
Keywords: Total Knee Arthroplasty; Crohn’s Disease; Peri-Prosthetic Joint Infections, Readmissions, Revisions
#8775
Smoking Cessation and Relapse Effects on Patient-Reported Outcomes Following Total Knee Arthroplasty
Christian Roberts - Campbell Clinic / University of Tennessee College of Medicine - Memphis, United States of America
*Andrew Couture - Campbell Clinic - Memphis, USA
William Mihalko - University of Tennessee - Memphis, USA
Marcus Ford - Campbell Clinic Orthopaedics - Germantown, USA
John Crockarell - Campbell Clinic Orthopaedics - Germantown, USA
Karen Derefinko - University of Tennessee Health Science Center - Memphis, USA
James Guyton - Campbell Clinic Orthopaedics - Germantown, USA
James Harkess - Campbell Clinic - Collierville, USA
*Email: acouture@campbellclinic.com
Introduction: Smoking is a known risk factor for poor outcomes following total knee arthroplasty (TKA) surgery. Despite this, many patients continue to smoke before and after surgery, leading to increased complications and worse patient-reported outcomes (PROs). The purpose of this study was to identify factors that could be modified to increase smoking abstinence and prevent relapse among these patients which will, hopefully, lead to more satisfactory outcomes. Specifically, we aimed to evaluate the prevalence of cigarette smoking relapse, quitting behavior, and identify smoking cessation predictors among patients who underwent TKA surgery.
Methods: This study involved a retrospective analysis of medical records and a survey of patients who underwent TKA surgery at a single institution. We identified a total of 200 patients, including 100 pre-operative smokers and 100 pre-operative nonsmokers. Medical records of the pre-operative nonsmokers were reviewed to obtain their KOOS JR scores and demographic data. The pre-operative smokers were contacted and asked a series of questions about their smoking behavior before and after surgery using a standardized smoking habits questionnaire. The data collected included the number of cigarettes smoked per day, the number of household members who smoke, quitting behavior, and the use of smoking cessation aids. Their KOOS JR responses were also recorded.
Results: The age range of the patients in the study was 28-86 years, with a mean age of 65.20 (SD=8.92), with nonsmokers being slightly older than smokers (mean=67.59 vs. 62.82, p<.001). Non-smokers had significantly better KOOS JR outcomes compared to smokers, with lower overall scores (mean=80.47 vs. 75.18, p=.016) and less pain on stairs (p=0.025), difficulty rising from sitting (p=0.006), and bending to the floor (p=0.002). Smokers reported a mean of 13.65 (SD=11.13) cigarettes per day. Before surgery, 63% of smokers had quit, primarily at the surgeon's request (61%). Prescription medication (59%) and nicotine replacement therapy (38%) were common cessation methods. However, smoking cessation prior to surgery did not have a significant impact on rates of returning to smoking post-operatively with 49% of patients returning to smoking (p=0.410). Of this group, 50% returned to smoking within one week.
Conclusion: The results of this study suggest that non-smokers have better outcomes following TKA than smokers, as they experience less pain and have better knee health. These findings are consistent with existing literature and serve to reaffirm that post-operative outcomes are better in patients who do not smoke. The study also highlights the high rate of smoking relapse among patients who quit smoking before surgery, with over half of them returning to smoking after surgery. While smoking relapse may not have a significant impact on pain after TKA, it can affect healing time and the overall health of patients. Therefore, smoking cessation interventions should be implemented both before and after surgery to improve outcomes and prevent smoking relapse. This study provides valuable information for clinicians and researchers working with patients undergoing TKA and may serve as the basis for future research and interventions.
Figures

Figure 1

Figure 2
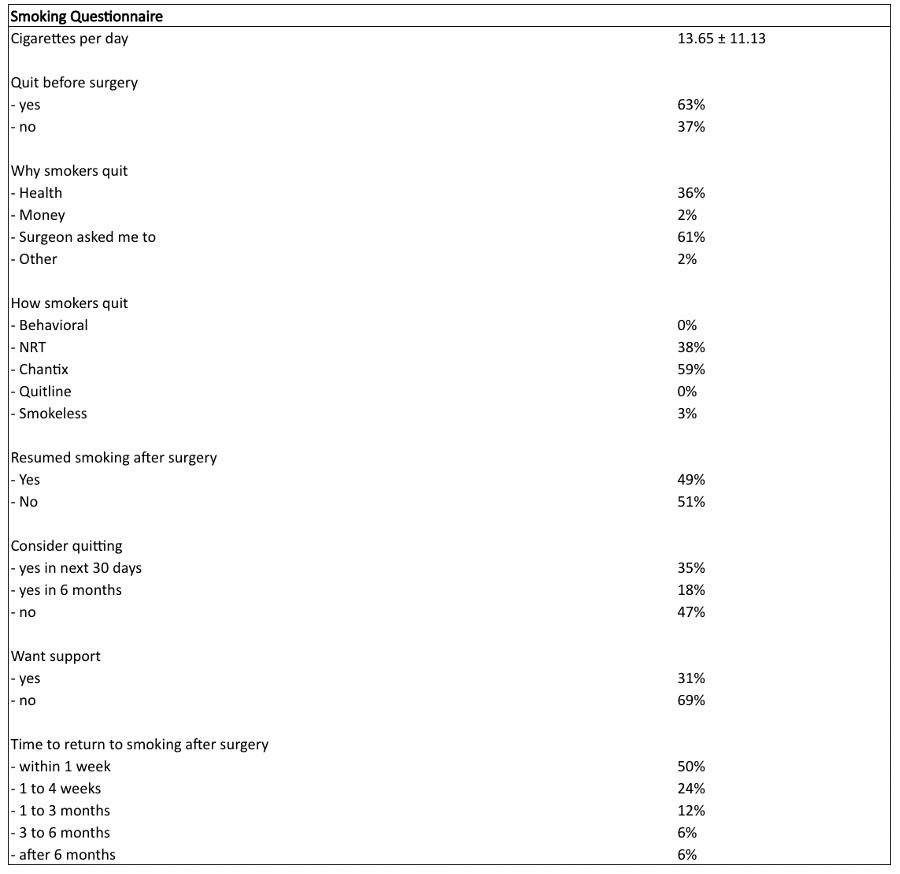
Figure 3#8781
Spinal Versus General Anesthesia for Outpatient Total Hip and Knee Arthroplasty in the Ambulatory Surgery Center: A Matched-Cohort Study
*Tyler Calkins - University of Tennesse Health Science Center - Campbell Clinic Orthopaedics - Memphis, United States of America
Evan Johnson - University of Tennessee Health Science Center - Campbell Clinic Orthopaedics - Memphis, USA
Renn Eason - University of Tennessee Health Science Center - Memphis, USA
William Mihalko - University of Tennessee - Memphis, USA
Marcus Ford - UTHSC Campbell Clinic - Memphis, United States of America
*Email: tcalkins@uthsc.edu
Introduction
Spinal anesthesia is the most popular outpatient regimen, but scenarios exist where induction is unsuccessful, unobtainable, or against patient preference. We compared the safety and efficacy of same day discharge (SDD) total hip (THA) and knee (TKA) arthroplasty utilizing spinal versus general endotracheal anesthesia in a free-standing ambulatory surgery center (ASC).
Methods
This is a retrospective matched cohort study of all THA and TKA from January 2014 to December 2020 in an ASC. General anesthesia was utilized in 105 patients (58 TKA and 47 THA). These were nearest-neighbor matched utilizing same surgeon, age, gender, body mass index and American Society of Anesthesiologists Physical Status Classification (ASA) to 58 TKA and 47 THA with spinal anesthesia, with no statistical demographic differences (p≥0.679). The primary outcome was the rate of successful SDD. Secondary outcomes included pain (0-10), nausea, medical complications in the post-acute care unit (PACU) and 90-day complications. Chi square analysis and paired t-tests were utilized.
Results
All spinal anesthetic patients underwent SDD compared to 103 (98%) general anesthetic patients (p=0.498). General anesthesia was associated with fewer minutes to discharge from PACU (227 vs 260, p=0.015), ambulation (166 vs 204, p<0.001) and urination (175 vs 202, p=0.050). General anesthesia patients had higher 1-hour (5.2 vs 1.5, p<0.001) and 2-hour (3.2 vs 1.5, p<0.001) postoperative pain, consumed more milligram morphine equivalents (13 vs 8, p<0.001) and experienced more nausea (48% versus 26%, p<0.001). With the numbers available for study, 90-day complications (8 vs 7), admissions (2 vs 3) and reoperations (5 vs 2) were similar among spinal and general anesthesia, respectively (p≥0.246).
Conclusion
General endotracheal anesthesia had an acceptable rate of SDD and 90-day complications and was associated with faster discharge from the ASC with earlier time to ambulation and urination postoperatively. These patients experienced more pain and nausea compared to those with spinal anesthesia.
#8289
Liposomal Bupivicaine Does Not Decrease Post-Operative Pain in Patients With Intracapsular Femoral Neck Fracture Treated With Arthroplasty: A Double-Blinded Randomized, Controlled Trial
*Mitchell Ng - Maimonides Medical Center - Brooklyn, United States of America
Lucas Voyvodic - Maimonides Medical Center - Brooklyn, USA
Matthew Magruder - Maimonides Medical Center - Brooklyn, USA
Ivan Golub - Maimonides Medical Center - Brooklyn, USA
Afshin Razi - Maimonides Medical Center - Brooklyn, USA
Kevin Kang - Maimonides Medical Center - Brooklyn, USA
*Email: mitchng77@gmail.com
Objectives: Liposomal bupivacaine is a long lasting local anesthetic agent developed for use in the surgical setting to help manage pain postoperatively. Recent studies have supported its efficacy following primary total joint arthroplasty, but little is known about its effectiveness in hip fracture patients. The objective of this study was to evaluate the effect of liposomal bupivacaine for patients with intracapsular hip fractures treated with hip hemiarthroplasty on: 1) post-operative pain, 2) function and 3) overall hospital course.
Methods: This was a single center, randomized prospective double-blinded study of 50 patients with isolated intracapsular femoral neck fractures from 2018 to 2022. Inclusion criteria were patients 65 years or older without dementia, treated with hip hemiarthroplasty through posterior approach. The study group consisted of 25 patients treated with intra-operative Exparel injections, while the control group consisted of 25 patients treated with standard multimodal IV/oral analgesia, and an intraoperative injection of saline. Primary outcomes were visual analogue scale (VAS) pain scores taken 4, 8, 12, 24 and 48 hours postoperatively, total morphine milligram equivalents (MME) at 12, 24 and 48 hours postoperatively, delirium at 24 and 48 hours postoperatively and time to ambulate with physical therapy. Secondary outcomes included: length of stay, discharge disposition (home vs skilled nursing facility vs inpatient rehab), and any adverse event or complication. Two-sample T-tests were conducted to identify differences, using a two-sided P-value level of 0.05 as statistically significant.
Results: There was no significant difference found in any of the outcomes measured between liposomal bupivacaine relative to the control cohort. Most notably, there were no differences in patients’ average pain scores at 4,8,12,24, or 48 hours (2.26 vs 2.7 NRS; p=0.34), total morphine equivalents used post-operatively (11.73 vs 9.98 MME; p=0.71), time to ambulation with physical therapy (1.08 v 1.44 days, p=0.07), and post-operative day of discharge (4.00 vs 3.88 days; p=0.82).
Conclusions: The results of our study suggest use of liposomal bupivacaine is not associated with significantly improved postoperative pain, function or shorter hospital course relative to saline following hip hemiarthroplasty for femoral neck fractures. Given the cost of liposomal bupivacaine over standard post-operative pain modalities, it is worth examining its use in the setting of geriatric hip fractures.
#8590
Failure of Liposomal Bupivacaine in Managing Postoperative Pain Following Shoulder Surgery
Carlos Fernandez - JFK / U Miami Orthopedic Surgery Program - Lantana, USA
*Vani Sabesan - Cleveland Clinic Florida - Weston, USA
Clyde Fomunung - Palm Beach Shoulder Service - Lantana, USA
Ajay Desai - Florida Atlantic University Charles E Schmidt College of Medicine - Boca Raton, USA
Alessia Lavin - Palm Beach Shoulder Service Atlantis Orthopaedics - Lake Worth, United States of America
Juan Lozano - FIU - Miami, USA
William Srouj - FIU - MIami, USA
Garrett Jackson - Chicago, United States of America
*Email: sabes001@gmail.com
Background: Amidst a national opioid crisis, increased focus has been placed on reducing opioid consumption. Novel multimodal pain protocols, including the use of liposomal bupivacaine (LB) have shown success in minimizing opioid usage after orthopaedic surgery. There is disputing evidence of the effectiveness of LB use in shoulder surgery and the purpose of our study was to analyze the risk factors and determinants of LB failure for postoperative pain control after orthopedic shoulder surgeries.
Methods: A retrospective review of 121 patients undergoing shoulder surgery treated by two fellowship trained shoulder surgeons at a single institution was performed from 2018-2020. All patients received a standardized multimodal pain management protocol that included an LB interscalene block. Local infiltration of LB was also administered at closure. Visual analog scales (VAS) were administered at 24, 48, 72 hours, and 7 days postoperatively. LB failure was defined as VAS score >7 any time within the 7-day postoperative period. Twenty-one patients were included in the liposomal bupivacaine failure (LBF) group and 100 in the liposomal bupivacaine success (LBS) group. Patient demographics, pain scores, and opioid consumption were compared between groups.
Results: Baseline characteristics were comparable, except the LBF cohort had a greater proportion of females (p=0.03). The LBF group had 20% of patients with a history of chronic opioid use compared to none in the LBS group (p=0.003). Logistic regression showed strong and independent associations between failure of LB and performing surgeon, chronic opioid use, gender, and history of prior surgery. Patients in the LBF group had an average of 20 Total Morphine equivalents (TME) 7-14 days following surgery compared to 0 TME (p=0.002) in the LBS group, and an average of 2.9 opioid prescriptions within the first 6 months compared to 1.7 in the LBS group (p=0.028).
Conclusions: Performing surgeon, gender, history of opioid use, and prior surgeries were all identified as strong, independent risk factors for failure of LB to control postoperative pain. In this study, performing surgeon is likely indicative of the impact of differences in patient education on postoperative pain expectations and alternate nonopioid regimens. We recommend that surgeons incorporate further preoperative patient education, and to take into account the specific risk factors when deciding to incorporate LB into their multimodal postoperative pain management plan for patients undergoing shoulder surgery
#8288
Liposomal Bupivicaine Does Not Decrease Post-Operative Pain in Patients With Intracapsular Femoral Neck Fracture Treated With Hemiarthroplasty: A Double-Blinded Randomized, Controlled Trial
*Mitchell Ng - Maimonides Medical Center - Brooklyn, United States of America
Lucas Voyvodic - Maimonides Medical Center - Brooklyn, USA
Matthew Magruder - Maimonides Medical Center - Brooklyn, USA
Afshin Razi - Maimonides Medical Center - Brooklyn, USA
Kevin Kang - Maimonides Medical Center - Brooklyn, USA
Ivan Golub - Maimonides Medical Center - Brooklyn, USA
*Email: mitchng77@gmail.com
Objectives: Liposomal bupivacaine is a long lasting local anesthetic agent developed for use in the surgical setting to help manage pain postoperatively. Recent studies have supported its efficacy following primary total joint arthroplasty, but little is known about its effectiveness in hip fracture patients. The objective of this study was to evaluate the effect of liposomal bupivacaine for patients with intracapsular hip fractures treated with hip hemiarthroplasty on: 1) post-operative pain, 2) function and 3) overall hospital course.
Methods: This was a single center, randomized prospective double-blinded study of 50 patients with isolated intracapsular femoral neck fractures from 2018 to 2022. Inclusion criteria were patients 65 years or older without dementia, treated with hip hemiarthroplasty through posterior approach. The study group consisted of 25 patients treated with intra-operative Exparel injections, while the control group consisted of 25 patients treated with standard multimodal IV/oral analgesia, and an intraoperative injection of saline. Primary outcomes were visual analogue scale (VAS) pain scores taken 4, 8, 12, 24 and 48 hours postoperatively, total morphine milligram equivalents (MME) at 12, 24 and 48 hours postoperatively, delirium at 24 and 48 hours postoperatively and time to ambulate with physical therapy. Secondary outcomes included: length of stay, discharge disposition (home vs skilled nursing facility vs inpatient rehab), and any adverse event or complication. Two-sample T-tests were conducted to identify differences, using a two-sided P-value level of 0.05 as statistically significant.
Results: There was no significant difference found in any of the outcomes measured between liposomal bupivacaine relative to the control cohort. Most notably, there were no differences in patients’ average pain scores at 4,8,12,24, or 48 hours (2.26 vs 2.7 NRS; p=0.34), total morphine equivalents used post-operatively (11.73 vs 9.98 MME; p=0.71), time to ambulation with physical therapy (1.08 v 1.44 days, p=0.07), and post-operative day of discharge (4.00 vs 3.88 days; p=0.82).
Conclusions: The results of our study suggest use of liposomal bupivacaine is not associated with significantly improved postoperative pain, function or shorter hospital course relative to saline following hip hemiarthroplasty for femoral neck fractures. Given the cost of liposomal bupivacaine over standard post-operative pain modalities, it is worth examining its use in the setting of geriatric hip fractures.
#8246
Impact of Chronic Opioid Use Prior to Arthroplasty on Mobility Recovery: A Propensity Matched Study
*Roberta Redfern - Zimmer Biomet - Pemberville, USA
Mike Anderson - Zimmer Biomet - Lehi, USA
Jason Cholewa - Zimmer Biomet - Warsaw, USA
Dave Van Andel - Zimmer Biomet - Grand Rapids, USA
*Email: roberta.redfern@zimmerbiomet.com
Introduction
Several studies have suggested that use of opioid medications prior to total joint arthroplasty may be associated with poorer post-operative outcomes. However, few studies have reported the impact on post-operative mobility and recovery of objective mobility metrics. The goal of this study was to compare objective and patient-reported mobility outcomes in patients with and without chronic pre-operative opioid use (≥90 days).
Methods
Secondary data analysis of a large multicenter, prospective observational cohort study in which patients utilized a smartphone-based care management platform with smartwatch for self-directed rehabilitation following lower limb arthroplasty. Postoperative mobility outcomes were measured by patient-reported ability to walk unassisted at 90 days post-operative, step counts as collected by smartwatches, and responses to the mobility dimension of the EQ-5D-5L. Patients with at least 180 days of follow-up available were included. Mobility metrics in a subgroup of patients propensity matched 2:1 based on age, BMI, sex, procedure, Charnley class, ambulatory status, orthopedic procedure history, and anxiety were compared.
Results
4051 patients were identified; 166 (4.1%) used opioids for at least 90 days prior to arthroplasty. Comparing the overall cohort, significantly fewer patients with long-term opioid use reported walking unassisted at 90 days post-operatively (86.8% vs 93.8%, p<0.002). Pre-operative step counts were similar between groups (5010.8 vs 5363.4, p=0.20), but were significantly lower post-operatively in opioid users through six months (4750.5 vs 5913.7, p=0.002). Fewer opioid users reported no or slight difficulties on the mobility dimension of the EQ-5D-5L pre-operatively (19.7 vs 33.0%, p<0.001), which persisted through six months (80.6% vs 92%, p<0.0001). 153 pre-operative opioid users were propensity score matched to 306 patients. The proportion who returned to walking unassisted at 90 days was similar (86.8% vs 90.3%, p=0.37). Step counts were similar pre-operatively and at 1-month post-operatively between groups but were significantly lower in opioid users at 3 and 6-months post-operative (4823.4 vs 5808.4, p=0.03). Fewer opioid users reported no or slight problems in walking about pre-operatively (19.4% vs 30.3%, p=0.007), and at all timepoints through 6 months (80.8% vs 89.6%, p=0.003).
Conclusions
Subjective and objective measures of post-operative mobility were significantly reduced in patients who utilized opioid medications for more than 90 days pre-operatively in this cohort of patients undergoing lower limb arthroplasty. These effects continued to be apparent in a subgroup of well-matched patients, suggesting that mobility recovery following arthroplasty may be associated with pre-operative opioid use.
#8343
Improved Perioperative Narcotic Usage Patterns in Patients Treated With a Fluoroscopy-Based Robotically-Assisted THA Platform When Compared to Manual Technique
Graham Buchan - Cleveland Clinic Lerner College of Medicine - Cleveland, United States of America
Zachary Bernhard - Cleveland Clinic - South Pointe Hospital - Warrensville Heights, USA
Christian Hecht - Cleveland Clinic - Cleveland, USA
*Atul Kamath - Cleveland Clinic - Cleveland, USA
*Email: kamatha@ccf.org
Introduction: The use of robotic-assisted technologies for THA has expanded in recent years due to the promise of increased precision of acetabular cup placement. However, there is a paucity of literature regarding opioid prescribing and consumption patterns surrounding robotic-assisted THA (RA-THA). We sought to compare perioperative and early postoperative opioid consumption in patients undergoing direct anterior approach (DAA)-THA with the use of a novel, fluoroscopic-assisted RA-THA system compared to opioid consumption associated with fluoroscopic-assisted, manual technique.
Methods: We performed a retrospective cohort analysis on a consecutive series of patients who received manual THA (mTHA) and fluoroscopy-based RA-THA.We compared the average amount of postoperative narcotics in morphine milligram equivalents (MME) given to each cohort within 6-weeks of surgery, including during the in-hospital and post-discharge periods. We also compared the proportion of patients that received <400 MME postoperatively. Analyses were performed on the overall cohort, as well as stratified by opioid-naïve and opioid-tolerant patients, determined by patients’ preoperative Narx score.
Results: The RA-THA cohort had significantly lower total postoperative narcotic use compared to the mTHA cohort (103.7 vs. 127.8 MME; p<0.025). This difference was similarly seen amongst opioid-tolerant patients (123.6 vs. 181.3 MME; p=0.042), but we did not detect a significant difference amongst opioid-naïve patients (93.8 vs. 114.0 MME; p=0.052) (Figure 1). No differences were seen between groups with respect to the proportion of patients who received <400 MME in the overall and stratified analyses, with >99% of RA-THA receiving <400 MME narcotics including 97.5% of opioid-tolerant patients (Table 1). The RA-THA cohort had lower total in-hospital narcotics use compared to the mTHA cohort (42.3 vs. 66.4 MME; p<0.001), consistent across opioid-naïve and opioid-tolerant patients.No differences were seen in post-discharge opioid use between groups.
Conclusions: Fluoroscopy-based RA-THA is associated with lower postoperative opioid use, including during the immediate peri-operative period, when compared to manual techniques. Importantly, these differences in improved narcotic usage with the RA-THA platform were consistent for both opioid-naive and opioid-tolerant patients. Lastly, patients who underwent RA-THA had no increased opioid consumption in the 6-week postoperative period. This may have importance in rapid recovery protocols and mitigating episode burden of care.
Keywords: Total hip arthroplasty (THA), Robotic-THA, narcotics, opioids, outcomes
Figures
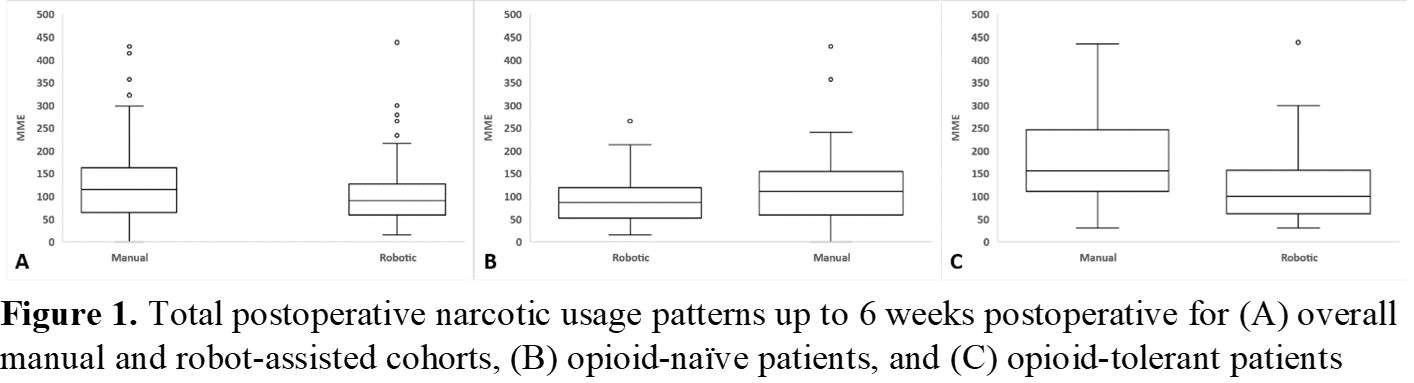
Figure 1
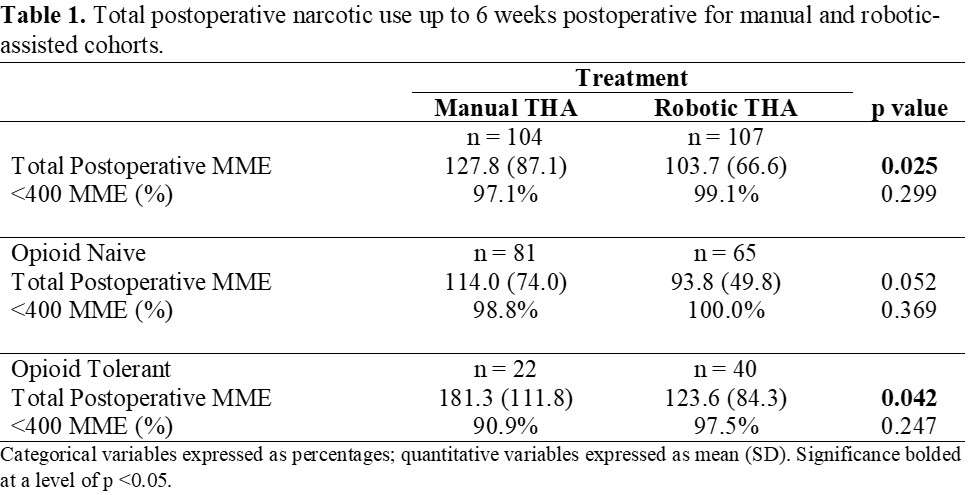
Figure 2#8768
Computer Assisted Cryotherapy (CAC) Reduce Opioids Use After Total Knee Arthroplasty (TKA): A Safe Postoperative Pain Management
veronica giuliani - sant'andrea la sapienza roma - roma, Italy
raffaella alonzo - san paolo - civitavecchia, Italy
Alessandro Carcangiu - S. Paolo Hospital, Civitavecchia (Rome) - Rome, Italy
Mattia Fabbri - san paolo - civitavecchia, Italy
Stefania De Santis - Sant'Andrea Hospital II University of Rome "La Sapienza" - rome, Italy
Ferdinando Iannotti - Sapienza - Roma, Italy
Andrea Gattelli - san paolo - civitavecchia, Italy
Giuseppe La Cava - san paolo - civitavecchia, Italy
Giorgio Bruni - san paolo - civitavecchia, Italy
*Carmelo D'Arrigo
*Email: m.battista@thetriumph.com
Introduction: Despite advances in anesthetic techniques and pain management, TKA is associated with pain and discomfort due to tissue damage, inflammatory response, local swelling, edema and stiffness. The aim of this study was to evaluate the effectiveness and safeness of CAC in pain control after primary TKA. Methods: Patients undergoing TKA between July 2020 and Jenuary 2023 were prospectively enrolled and randomly assigned in two Groups. Inclusion criteria were primary TKA replacement due to painful osteoarthritis and the ability to follow instructions and return to follow-up. Exclusion criteria were revision TKA, knee infection, extension deficit more than 15° and/or flexion less than 110°. Group I received a standardized multimodal pain control protocol using NSAID and Opioids. In Group II, patients received the same pain control protocol and underwent CAC with a standardized protocol. This protocol consisted of two application daily of 3 hours at a temperature of 10-12° and an intermittent compression up to 50 mmhg. The main outcomes were the VAS registered at 1, 3, 5 days postoperatively and opioids consumption noted during the first 5 post- operative days. Secondary outcomes were ROM, swelling, blood transfusions, infections, thrombotic events and length of hospital stay. Statistical analysis was performed by an indipendent analyst using student’s t tests and chi-square tests for categorial variables. P value was considered statistically significant at p<0.01. Results: 213 consecutive patients were eligible for enrollment. 14 patients were excluded leaving 200 patients (Group I: 100 patients; Group II: 100 patients). In both groups a significant (p<0.01) reduction in VAS Pain was registered between day 1 and day 5 postoperatively. However, when comparing both Groups, a significant difference (p<0.01) was found at 1, 3, and 5 days postoperatively with better pain control in the CAC group. When considering analgesics consumption, a significant difference (p<0.01) was found between the two groups with a lower administration of opioids, including morphine, in the CAC group. Particularly, a mean consumption of 232,65 mg and 26,53 mg of Tramadol was registered respectively in Group I and Group II. Moreover, a mean administration of 19,3 mg of morphine was registered in Group I compared to a mean consumption of 0,61 mg in Group II. Despite better results in Group II particularly in terms of NSAID use, better ROM, lower local swelling, blood transfusion and length of hospital stay, no statistically significant differences were registered. Discussion and Conclusion: Postoperative computer assisted cryotherapy (CAC) is effective in terms of pain control after primary TKA as it was associated with reduced pain and less administration of opioids during the early postoperative period. Moreover CAC seems to be a safe procedure with no adverse effect registered in our series. CAC is a safe and effective procedure following primary TKA resulting in less experienced pain and opioids use in the first postoperative days. The procedure with CAC is well tolerated improving patients satisfaction. We can speculate that this protocol can have potential favorable effect in the rehabilitation period and final outcomes.
#8244
Impact of Chronic Pre-Operative Opioid Use on Post-Operative Patient Reported Outcomes: A Propensity Matched Study
*Roberta Redfern - Zimmer Biomet - Pemberville, USA
Mike Anderson - Zimmer Biomet - Lehi, USA
Jason Cholewa - Zimmer Biomet - Warsaw, USA
Dave Van Andel - Zimmer Biomet - Grand Rapids, USA
*Email: roberta.redfern@zimmerbiomet.com
Introduction
Pre-operative opioid consumption has been reported to be associated with poorer outcomes, including lower patient-reported outcome measures (PROMs) and satisfaction following joint arthroplasty. However, few studies have matched cohorts based on patient characteristics beyond age and BMI. This study aimed to investigate the impact of chronic opioid use (≥90 days) prior to lower limb joint arthroplasty on PROMs and satisfaction in well-matched cohort of patients.
Methods
Secondary analysis of a multicenter prospective observational cohort study of patients who utilized a smartphone-based care management platform for self-directed rehabilitation following arthroplasty. Those who used opioids ≥90 days prior to arthroplasty were identified and compared to those who did not. Only those with at least 180 days of follow-up were included. Propensity score matching using the nearest neighbor method to match 2:1 was completed including age, BMI, sex, procedure, Charnley class, ambulatory status, orthopedic procedure history, and anxiety. KOOS JR and HOOS JR scores, changes in these scores, and satisfaction were compared between groups.
Results
4051 patients were investigated; 153 chronic opioid users were identified and matched to 306 patients who did not use opioids ≥90 days pre-operatively. On average patients were 61.9 years of age (62.0±10.2 vs 61.9±10.5, p=0.96) and 54.2% female (54.6% vs 53.6%, p=0.84). 7.4% of patients underwent partial knee arthroplasty (PKA), 43.4% underwent total hip arthroplasty, and 49.2% underwent total knee arthroplasty (TKA). KOOS JR scores and changes in KOOS JR scores did not vary significantly by group at any time point through six months with the numbers available, nor did satisfaction scores. Chronic opioid users undergoing THA presented with significantly lower HOOS JR preoperatively (53.2±13.3 vs 46.2±12.1, p=0.0004) and at 1-month post-operative. HOOS JR scores were similar between opioid use groups at 3 and 6-months post-operative (84.0±16.5 vs 81.9±14.1, p=0.48); change in HOOS JR scores did not vary at any time. Opioid users in the THA group reported similar satisfaction at 90 days post-operative (32.7±8.1 vs 29.4±11.0, p=0.07). In the TKA population, KOOS JR scores did not vary at any timeframe pre-operatively (48.9±13.3 vs 47.1±14.4, p=0.38) through 6-months (71.6±12.5 vs 75.8±13.8, p=0.73). Satisfaction scores at 90 days were similar in the TKA group between those with and without chronic pre-operative opioid use (27.6±9.8 vs 68.6±9.5, p=0.52).
Conclusions
In a cohort of chronic opioid users matched based on additional factors including Charnley class, ambulatory status, orthopedic procedure history, and anxiety, PROMs were similar in each procedure group at 6-months post-operatively. Patient satisfaction at 90-days did not appear to be affected by chronic pre-operative opioid use. Studies investigating the impact of pre-operative opioid use on outcomes should match for additional characteristics beyond age, BMI, and sex where possible.
#8635
Subacromial Injection: Effect on Preoperative Pain and Function in Arthroscopic Rotator Cuff Repair
*Carlos Fernandez - JFK / U Miami Orthopedic Surgery Program - Lantana, USA
Vani Sabesan - Cleveland Clinic Florida - Weston, USA
Sarah Girshfeld - Florida Atlantic University Charles E Schmidt College of Medicine - Boca Raton, USA
Clyde Fomunung - Palm Beach Shoulder Service - Lantana, USA
Gabriel Lama - Florida Atlantic University Charles E Schmidt College of Medicine - Boca Raton, USA
Ali Mohamed - FAU - Boca raton, USA
Howard Routman - Atlantis Orthopedics - Atlantis, USA
Garrett Jackson - Chicago, United States of America
*Email: carlosfernandezpeaguda@gmail.com
Background: Subacromial corticosteroid injections are widely used as conservative treatment to reduce pain and improve function in patients with rotator cuff pathology. However, there is limited clinical evidence for surgeons who use steroid injections in predicting which patients will ultimately undergo arthroscopic repair (RCR). The purpose of this study was to identify associated factors that impact patient decision to proceed with RCR following steroid injection.
Methods: A retrospective review was performed on 295 patients who underwent RCR by a single fellowship-trained orthopedic surgeon from 2017-2019. Patients were divided into two groups: 85 patients who received at least one steroid injection within one year prior to surgery were in the injection group (IG) and 203 were included in the control group (CG). Patient demographics, strength, range of motion (ROM), pain scores, and subjective shoulder value (SSV) collected from their preoperative visit were analyzed between groups.
Results: The cohort consisted of 58% males with an average age of 58.1years, BMI of 29.8, and a mean time of 2.3 months from injection to preoperative visit. There were no significant differences in demographics aside from a higher proportion of males in the IG (56%) than the NG (50.7%) (p = 0.04). Average preoperative ROM was significantly higher in IG for forward flexion (137.6º vs 127.2º, p = 0.03) and external rotation (60.8º vs 54.8º, p < 0.01). Average supraspinatus strength (4.19 vs 3.99, p = 0.01) and average infraspinatus strength (4.86 v. 4.7, p = 0.046) were also statistically higher in the injection group. There were no significant differences between groups for preoperative pain scores, satisfaction, or SSV.
Conclusions: Patients who receive a subacromial steroid injection prior to arthroscopic rotator cuff repair demonstrated improved range of motion and rotator cuff strength. Despite the better function, patients who received a subacromial injection continued to experience similar pain, satisfaction and SSV scores, likely leading to need for surgical intervention (RCR). This indicates that pain level and subjective shoulder assessments appear to be a stronger predictor than function preoperatively for patients to undergo surgical RCR.
#8492
Knee Pivot Motion After Unicomprtmental Arthroplasty May Impact Clinical Outcomes
*Rémi Courteille - École de Technologies Supérieure - Montreal, Canada
Laurence CHEZE - Université Claude Bernard Lyon 1 - Lyon, France
Cecile Batailler - Lyon North University Hospital - Lyon, France
Sebastien Lustig - Hôpital de la Croix Rousse - Lyon, France
NICOLA HAGEMEISTER - École de technologie supérieure - Montreal, Canada
Alex Fuentes - Emovi - Montreal, Canada
*Email: remi.courteille.1@ens.etsmtl.ca
Introduction: Unicompartmental knee arthroplasty (UKA) is often recommended as a treatment for advanced medial compartment knee osteoarthritis in young patients. While some studies suggest that UKA can reproduce normal knee kinematics, publications looking at the knee pivot motion during functional tasks, also known as the center of rotation (COR) behavior, are scarce in this population. The goal of this study was to assess the knee pivot motion during gait before and after UKA and evaluate if there are association with clinical outcomes.
Methods: Fifty-six (56) participants who underwent UKA were included in this study. All patients received the same implant (HLS Uni Evolution, Tornier®) at the Croix Rousse hospital (Lyon, France). Knee landmarks and three-dimensional (3D) kinematics were captured using the KneeKG® system (Emovi Inc., Canada) before and 6 months after UKA. Knee pivot motion was assessed by projecting the transepicondylar axis (TEA) positions in the transverse plane (i.e., tibial plateau) throughout the gait cycle (GC). The COR was defined by the intersection of two consecutive TEA projections. We then classified knee pivot motion in four patterns (Figure 1). Pattern I presented no rotation (i.e., antero-posterior translation of the TEA). Patterns II corresponded to a rotation around a lateral COR, pattern III, a medial COR and pattern IV a central COR (within 33% of the center of the tibia). The predominant knee pivot pattern was determined independently in five sub-phases of the GC: loading (from 0 to 15% of the GC), mid-stance (15-30%), end-stance (30-45%), push-off (45-60%), and swing (60-100%). All patients filled out the Forgotten Joint Score (FJS) questionnaire 6 months post-surgery. The higher the score on a 0-100 scale, the less the patient is aware of his operated knee (i.e., the better). Participants who presented the same knee pivot motion pattern pre- and post-UKA were classified as “non-changers” and the others as “changers”. FJS scores were compared between changers and non-changers using Student T-tests.
Results: There was 48.2% of women and the mean age was 65.9 years (95%CI: 63.78; 68.07). The distribution of changers and non-changers in each sub-phase as well as mean FJS scores and associated p-values are presented in Table 1. Interestingly, the proportion of changers increased progressively during stance (from 42.9% at loading to 64.3% at end-stance). Patients whose knee pivot motion pattern did not change during push-off reported significantly better FJS scores compared to changers (p=0.03). There were no significant differences between both groups in the other sub-phases (p>0.05).
Conclusion: Results suggest that patients who maintain similar knee pivot motion pattern during push-off after UKA may have better clinical outcomes compared to the ones whose pattern was altered after the surgery. While additional studies are needed to confirm the associations between knee pivot mechanism and outcomes in UKA, this study presents a method to assess joint pivot motion using a non-invasive medical device in clinical settings. This method is promising to better understand knee pivot motion in weight-bearing conditions prior to surgery which ultimately may help achieve better outcomes by defining more personalized surgeries.
Figures
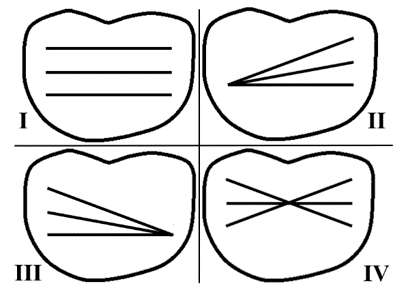
Figure 1
Figure 2#8120
Robot-Assisted Partial Knee Replacement Versus Standard Total Knee Replacement (RoboKnees): Design and Rationale for a Pilot Randomized Controlled Trial
*Kim Madden - McMaster University - Hamilton, Canada
Breanne Flood - St. Joseph's Healthcare Hamilton - Hamilton, Canada
Monica Malek - St. Joseph's Healthcare Hamilton - Hamilton, Canada
Janie Astephen Wilson
Jean-Eric Tarride - McMaster University - Hamilton, Canada
Vickas Khanna - McMaster University - Hamilton, Canada
Anthony Adili
*Email: maddenk@mcmaster.ca
Background: Total knee arthroplasty (TKA) is a common surgery for end-stage knee osteoarthritis. Partial knee arthroplasty (uni- or bi-compartmental) is also a treatment option for patients with arthritis present in only one or two knee compartments. Partial knee arthroplasty can preserve natural knee biomechanics, but these replacements may not last as long as total knee replacements. Partial knee replacements are underutilized because they are technically demanding and there is uncertainty about implant survival. Orthopaedic robots can help facilitate partial knee replacements by increasing accuracy and precision. We hypothesize that we can use orthopaedic robots to shift more patients away from TKA toward partial knee replacements. This trial will investigate the feasibility and assess clinical outcomes for a larger definitive trial.
Methods: This is a protocol for a parallel randomized pilot trial of 64 patients with uni- or bicompartmental knee arthritis. Patients are randomized to either receive robot-assisted partial knee arthroplasty or standard care TKA. The primary outcome for this pilot trial is investigating the feasibility for larger trial. Clinical and economic outcomes include joint awareness, return to activities, knee function, patient global impression of change, persistent post-surgical pain, re-operations, resource utilization and cost-effectiveness, health-related quality of life, radiographic alignment, knee kinematics during walking gait, and complications. We will follow participants for 24 months after surgery.
Results: We have randomized 31 participants to date (16 standard care TKA, 15 robot-assisted partial). 66% of participants are female, the mean age is 62 (SD 7.5), 60% were working at the time of their surgery, 63% used a walking aid before surgery. All participants randomized to robot-assisted partial knee replacement received bi-compartmental (medial and patellofemoral) replacements. Mean surgical time was 83 minutes (SD 18) in the robot-assisted group and 60 minutes (SD 23) in the standard group. 9 participants have completed their 12 month post-op visit. Additional follow-up visits are ongoing.
Discussion: The RoboKnees pilot study is the first step in determining whether we can shift more patients toward partial knee replacements by leveraging the improved accuracy and precision of orthopaedic robots. Conclusions from this study will be used to design the future large-scale trial. This study will inform surgeons about the potential benefits of partial knee replacements using an orthopaedic robot to improve precision and accuracy of implant placement.
#8716
Pre- and Postoperative Knee Phenotype Distribution Following Robot-Arm Assisted Lateral Unicompartmental Knee Arthroplasty: A Retrospective Study of 305 Patients
*Roderick Vossen - Hospital of Special Surgery - New York, United States of America
Lindsey Ruderman - Hospital for Special Surgery - New York, USA
Tarik Bayoumi - Hospital for Special Surgery - New York, USA
Hendrik Zuiderbaan - Hospital for Special Surgery - New York, USA
Andrew Pearle - Hospital for Special Surgery - New York, USA
G.M.M.J. Kerkhoffs - Amsterdam UMC - Amsterdam, Netherlands
*Email: vossenr@hss.edu
Introduction: Variation of coronal alignment and phenotype distribution in healthy knees and knees with generalized osteoarthritis (OA) have been described and appear to differ. Phenotype distribution for lateral compartment OA (lcOA) has not yet been described, and the relationship between phenotypes and patient reported outcome measurements (PROMs) has been minimally studied. Therefore, this study aimed to evaluate Coronal Plane Alignment of the Knee (CPAK) phenotype distribution for patients with lcOA (fig. 1). The secondary aim of this study was to analyze PROMs between preoperative CPAK phenotypes.
Methods: A single surgeons' registry was reviewed to retrospectively identify patients who received robot-arm assisted unicondylar knee arthroplasty for lcOA. A radiographic analysis of 305 knees was performed to determine CPAK phenotypes. Pre- and postoperative phenotype distribution was described. PROMs were collected by digital questionnaire (Knee Injury and Osteoarthritis Outcome Score for Joint Replacement (KOOS JR), Kujala, patient satisfaction (5-point Likert scale and redo of surgery)). PROMs were analyzed between CPAK phenotypes, by sex and age groups, between phenotypes realigned to its pre-arthritic- compared to phenotypes realigned to a different phenotype and between phenotypes achieving Patient Acceptable Symptom Score and maximum thresholds and those that did not.
Results: Three-hundred-and-five knees were included (mean age: 64.0 ±11.0 years, mean BMI: 26.6 ±4.6 kg/m2, female: 58.0%, Caucasian: 89.5%). Total of seven CPAK phenotypes were observed preoperatively, with CPAK3 (54.1%) and CPAK6 (21.0%) being the most common compared to a all nine CPAK phenotypes that were observed postoperatively with CPAK6 (32.8%), CPAK3 (22.0%) and CPAK5 (21.3%) as most common (fig. 2 and 3). No significant differences were found in PROMs (FU 3.2 ± 1.5 years).Apart from CPAK2 that was significantly greater for male patients compared to female (22.6% vs 11.9%, p = 0.008)., no significant differences in distribution were seen by sex or age groups.
Conclusion: LcOA CPAK distribution was variable and differed compared to healthy- and generalized OA CPAK distribution as valgus aligned phenotypes were predominant. Although some differences in CPAK distribution between male- and female patients were significant, no significant relation could be analyzed between PROMs and CPAK phenotypes.
References:
1. MacDessi SJ et al. Coronal plane alignment of the knee (CPAK) classification. Journal of bone and joint surgery. British volume. 2021;103-B(2):329-337. https://search.proquest.com/docview/2484183959. doi: 10.1302/0301-620X.103B2.BJJ-2020-1050.R1.
2. Hirschmann MT et al. Phenotyping of hip–knee–ankle angle in young non-osteoarthritic knees provides better understanding of native alignment variability. Knee Surg Sports Traumatol Arthrosc. 2019;27(5):1378-1384. https://link.springer.com/article/10.1007/s00167-019-05507-1. doi: 10.1007/s00167-019-05507-1.
Figures
Figure 1
Figure 2
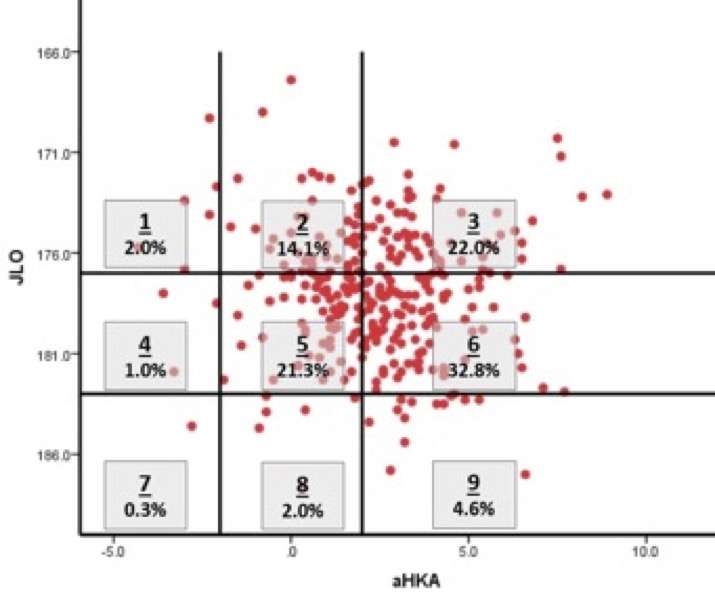
Figure 3#8705
Novel Technique for Accurate Placement of Components in Resurfacing UKA
*Robert Eberle - Maxx Orthopedics - Apex, USA
Daniel Hatz, MD - US Air Force - Omaha, USA
Martin Redish, MD - ParkRidge Bone & Joint - Chattanooga, USA
*Email: eberlewriting@gmail.com
INTRODUCTION
Resurfacing unicondylar knee arthroplasty (UKA) using an inlay, all-polyethylene tibial component lost popularity and has been all but forgotten due to the inability to train surgeons. The purpose is to describe an established novel technique that advances the reproducibility of training and outcomes utilizing new instrumentation and power tools specifically designed for this procedure supported with clinical and radiographic outcomes.
METHODS
Between December 2018 and May 2022, the lead author (MR) performed 942 resurfacing UKAs utilizing an inlay all-polyethylene tibia. Of these cases, 50 consecutive, non-selected patients with either the medial or lateral compartment degeneration and a minimum of 2-years follow-up were retrospectively reviewed. We introduce a novel UKA technique utilizing component specific cutting jigs, high-speed burrs and a custom reciprocating tibial rasp to prepare the tibial pocket and femoral surface. Short term clinical and satisfaction results are reported. Radiographic analysis of these cases included coronal alignment, posterior slope, centrality of the femoral component on the tibial component, and knee alignment.
RESULTS
Overall, 95% of patients were satisfied with the surgical results. There were no revisions or recommendation for revisions in this study cohort post-operatively through 2-years. Postoperative radiographic evaluation revealed coronal alignment, posterior slope, alignment of components (center-on-center alignment) were within ±1% or ±1mm of the component targets.
CONCLUSION
We have introduced and recommend a novel technique for minimally invasive resurfacing UKA using an inlaid all-polyethylene tibial component is a viable option for treatment of unicondylar OA of the knee. Short-term radiographic and clinical results have demonstrated early success. This technique is ideal for use in an ASC. It can be performed safely as an outpatient in patients in their 70s or 80s. Continuance of the study across multiple surgical centers / surgeons with long-term follow-up is warranted.
#8406
Change in Fixed Flexion Deformity With Medial Unicompartmental Knee Arthroplasty: Does This Prompt Review of Existing Clinical Criteria?
*Warran Wignadasan - University College London Hospital - London, United Kingdom
Babar Kayani - University College Hospital London - London, United Kingdom
Andreas Fontalis - University College London Hospital - London, United Kingdom
ahmed magan - University College London Hospital - London, GB
Alastair Chambers - UCLH - London, United Kingdom
Fares Haddad - University College London Hospital - London, United Kingdom
*Email: warran.wignadasan@gmail.com
Introduction
Unicompartmental knee arthroplasty (UKA) is an succussful form of treatment for patients with unicompartmental knee arthritis. A residual fixed flexion deformity (FFD) has been shown to be related to worse functional outcomes. Change in FFD with UKA has not been well exploed and reported on. Our study aims to assess change in FFD in patients undergoing medial UKA with robotic arm-assistance.
Methods
This prospective cohort study which includes 134 patients (73 males and 61 females with a mean age of 62 ± 5.7 years) undergoing robotic-arm assisted medial UKA between 2018 and 2022. Patients were divided into four study groups based on the degree of preoperative FFD: 0.5-3 degrees, 3–6 degrees, 6-9 degrees, and >9 degrees. Intraoperative optical motion capture technology was used to assess pre- and postoperative FFD. The Oxford Knee Score (OKS) was assessed in all patients pre-operatively and at one-year follow-up. Clinical FFD was also measured pre- and post-operatively in the coutpatient clinic setting.
Results
This study found statistically significant reduction in mean pre-operative to post-operative FFD for each of the four study groups: 0.5-3 degrees (pre op FFD = 1.38, post op FFD = 0.46 degrees, P = 0.023), 3 - 6 degrees (pre op FFD = 4.25 degrees, post op FFD = 1.90, P = <0.001), 6 – 9 degrees (pre op FFD = 7.11 degrees, post op FFD = 3.63, P < 0.001), >9 degrees (pre op FFD = 11.0 degrees, post op FFD = 6.37, P value < 0.001). There was a statistically significant association between the degree of the initial intraoperative FFD and the absolute change in the intraoperative FFD (0.5° to ≤ 3°: change in FFD 0.88° ± 1.49°; 3° to ≤ 6°: change in FFD 2.90° ± 1.53°; 6° to ≤ 9°: change in FFD 3.49° ± 1.44°; and > 9°: change in FFD 4.60° ± 1.95°, p = 0.002). There was a moderately strong correlation between the initial intraoperative FFD and change in intraoperative FFD (Pearson’s correlation coefficient = 0.71, p = 0.001) following UKA. The overall OKS for the study population improved from preoperative values to postoperative values at one-year follow-up (22.18 ± 7.75 vs 44.32 ± 3.42 respectively, p<0.001).
Conclusion
This is the first study to use optical motion capture technology to measure the change in FFD during robotic-arm assisted medial UKA. The FFD reduced by approximately 50% in all four cohorts. These findings may help to revise existing clinical criteria for medial UKA.
#8384
Outcomes of Image-Based Robotically Assisted Revision From Unicompartmental to Total Knee Arthroplasty
*Fabio Mancino - University College London Hospitals NHS Foundation Trust - London, United Kingdom
Andreas Fontalis - University College London Hospital - London, United Kingdom
ahmed magan - University College London Hospital - London, GB
Ricci Plastow - University College London Hospitals NHS Foundation Trust - London, United Kingdom
Fares Haddad - University College London Hospital - London, United Kingdom
*Email: fabio.mancino90@gmail.com
Background
Unicompartmental knee arthroplasty (UKA) is a cornerstone treatment for single compartment knee osteoarthritis (OA). Failure of a UKA often leads to a complex revision that may require a higher level of constraint, augments to manage potential bone loss, and stem extension, with reported inferior outcomes compared to primary TKA. The utility of robotic-arm assistance in the management of a failed UKA is a relatively underexplored technique. The aim of our study was to evaluate robotic-arm assistance in the setting of revision UKA to TKA surgery and compare surgical and patient reported outcome measures (PROMs) with primary robotic-arm assisted TKA.
Methods
Our study encompasses a consecutive series of 16 patients that underwent revision from a UKA to a TKA with robotic-arm assistance (Mako Surgical Corporation, Kalamazoo, Michigan) between 2019 and 2022. Patients were matched using baseline characteristics 1:2 with a control group (32 patients) of patients that underwent primary robotic-assisted TKA during the same time. Data were prospectively collected and included blood loss, length of stay (LOS), intraoperative and post-operative complications. Furthermore, pre-operative and post-operative PROMs including the Forgotten Joint Score (FJS) and Oxford Knee Score (OKS) were evaluated.
Results
16 patients with a mean age of 70 years (range, 59 to 85) and BMI of 28 (range, 21 to 39) were included in the revision group. The minimum follow-up (FU) was 6 months (mean, 24 months; range, 6 to 36 months). Baseline characteristics and PROMs were comparable between the two groups. The main indication was progression of OA (64%), followed by pain (19%), instability (13%), and valgus collapse (6%). A Posterior Stabilized (PS) polyethylene insert was used in 88% of the cases (14 of 16 cases) with a mean thickness of 11mm, and there was no necessity for using metal augments. The mean haemoglobin (Hb) drop in the revision group was 32 g/L vs 30 g/L in the control group (p=0.561). Mean Length of Stay was comparable between the groups at 76 vs 67 hours, respectively (p=0.195). There was one intraoperative complication in the UKA to TKA revision group (tibial fracture) and none in the primary TKA group. No participants underwent revision surgery or had an infection. No significant differences were noted between pre- and postoperative hip-knee-ankle angle (HKA, mean 180° vs 179°, p=0.267) and knee flexion in the revision group (118° vs 121°, p=0.207). FJS and OKS improved in the revision and control group by 44 vs 53 points (p=0.109), and by 17 vs 22 points, respectively (p=0.131).
Conclusion
The main finding of this study is that robotic-arm assistance in revision UKA to TKA surgery is safe and potentially advantageous, given it resulted in comparable blood loss, LOS, PROMs and clinical outcomes compared to primary robotic-assisted TKA. More importantly, no patients needed additional fixation (augment or stem extension). Further validation of these findings is needed by more data and by seeing revisions for different UKA failure modes addressed this way.
#8204
Length of Stay and Discharge Dispositions in Total Knee Arthroplasty and Unicompartmental Knee Arthroplasty: A Comparison of Robotic-Arm Assistance and Conventional Techniques.
*Andreas Fontalis - University College London Hospitals - London, United Kingdom
Isabella Catrina Haddad - University College London Hospital - London, United Kingdom
Christian Donovan - University College London Hospitals NHS Foundation Trust - London, United Kingdom
Rhody David Raj - niversity College London Hospitals NHS Foundation Trust - London, United Kingdom
Ricci Plastow - University College London Hospitals NHS Foundation Trust - London, United Kingdom
Sam Oussedik - University College London Hospitals NHS Foundation Trust - London, United Kingdom
Ayman Gabr
Fares Haddad - University College London Hospital - London, United Kingdom
*Email: andreasfontalis@gmail.com
Introduction
In-hospital length of stay (LOS) and discharge dispositions following arthroplasty could act as surrogate measures for improvement in patient pathways and have major cost saving implications for healthcare providers. With the ever-growing adoption of robotic technology in arthroplasty, it is imperative to evaluate its impact on LOS. The objectives of this study were to identify whether any differences were evident between conventional (CO TKA and UKA) and robotic arm-assisted Total Knee Arthroplasty (RO TKA) and Unicompartmental Knee Arthroplasty (RO TKA) in relation to inpatient LOS and discharge dispositions. In addition, we aimed to identify factors and patient characteristics related to delayed discharge in our study population. and Unicompartmental Arthroplasty (RO UKA) versus conventional technique
Methods
This large-scale, single institution study included patients of any age undergoing primary TKA ( N = 1,375) or UKA (N = 337) for any cause between May 2019 and January 2023. Data extracted included patient demographics, LOS, need for Post Anaesthesia Care Unit (PACU) admission, anaesthesia type, readmission within 30 days and discharge dispositions. Univariate and multivariate logistic regression models were also employed to identify factors and patient characteristics related to delayed discharge.
Results
The median LOS in the RO TKA group was 76 hours (54, 104) versus 82.5 (58, 127) in the CO TKA group, p<0.001 and 54 hours (34, 77) in the RO UKA versus 58 (35, 81) in the CO UKA, p = 0.031 (Figures 1 and 2). Discharge dispositions were comparable between the two groups. A higher percentage of patients undergoing CO TKA required PACU admission (8% versus 5.2%, p = 0.040). In our multivariate models, age, need for PACU admission, ASA score > 2, general anaesthetic and utilisation of the conventional technique were significantly associated with LOS > 3 days in TKA. In UKA patients, only female gender, ASA > 2 and need for PACU admission reached statistical significance in our model.
Conclusion
Our study showed that robotic-arm assistance was associated with a shorter LOS in patients undergoing primary UKA and TKA and no difference in the discharge destinations. Our results suggest that robotic-arm assistance could be advantageous in partly addressing the upsurge of knee arthroplasty procedures and the concomitant health care burden; however, this needs to be corroborated by long-term cost effectiveness analyses and data from randomised controlled studies.
Figures
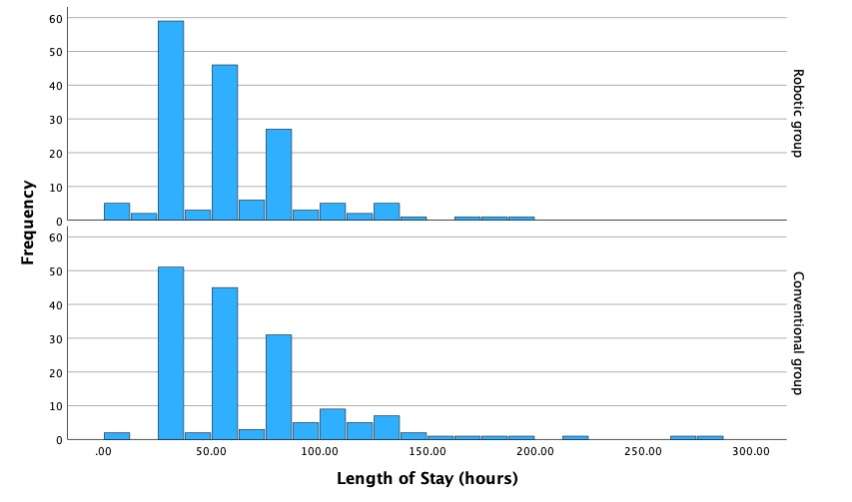
Figure 1
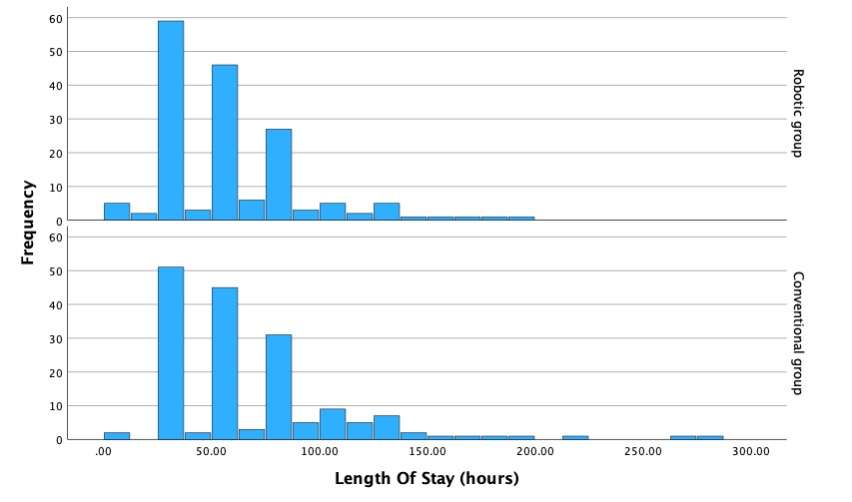
Figure 2#8585
Enhancing Precision in Total Knee Arthroplasty: A Novel Classification for Assessing Bone Resection Accuracy of Image-Based Robotic-Assisted Surgery Compared to Conventional Techniques
*Cecile Batailler - Lyon, France
Sebastien Lustig - Hôpital de la Croix Rousse - Lyon, France
*Email: cecile-batailler@hotmail.fr
Introduction
Robotic-arm assisted total knee arthroplasty (TKA) has improved accuracy in achieving preoperatively planned bone resection and final joint alignment, with precision within 1 mm. Nevertheless, the bone cuts accuracy has been assessed only on soft bone or cadavers. This study aimed 1) to describe and validate a new classification system of bony surfaces after bone resection during TKA, and 2) to compare the quality of bony surfaces between robotic-assisted TKA versus conventional TKA.
Methods
This prospective comparative cohort study included 60 consecutive primary TKA for end-stage primary osteoarthritis between November 2021 and June 2022. The exclusion criteria were previous femoral or tibial osteotomy or fracture, ligament insufficiency, and associated procedure (osteotomy or extensor mechanism procedure). Thirty TKA were performed by an image-based robotic-assisted system. The control group included 30 conventional jig-based TKA. Standardized intraoperative photographs with the same camera were collected following femoral and tibial bone resection to assess the quality of the bone cuts and the congruency between bone-implant. Six intraoperative photographs were taken for each case, three without the implant and three with the implant. The proposed classification calculated a score (on 100) based on the quality of the bone cuts and the congruency between the implant and cut. Six blinded observers individually reviewed the intraoperative photographs two times and allocated a score to each patient. Inter-and intra-observer reliabilities were assessed. The scores were also compared between both groups.
Results
The demographic data were similar between both groups. In the robotic group, the mean age was 73.1 years old ±7.1, the mean body mass index was 29.1 kg/m2 ±4.2, the mean preoperative knee alignment was 173.3° ±4.5°. In the conventional group, the mean age was 71.5 years old ±8.6 (p=0.49), the mean body mass index was 29.5 kg/m2 ±4.9 (p=0.28), the mean preoperative knee alignment was 173.7° ±4.2° (p=0.96).
The classification showed very good to excellent intra-observer and inter-observer agreements (from 0.74 to 0.96). The mean classification was 94.4 ±5.0 [81;100] in the robotic group and 77.6 ±10.9 [63;97] in the conventional group (p<0.0001). The cut quality part was 48.4 ±2.4 in the robotic group and 39.1 ±3.5 in the conventional group (p<0.001). The congruency part was 46.0 ±3.8 in the robotic group and 30.3 ±4.2 in the conventional group (p<0.0001).
Conclusion
The new classification of bone resection quality had very good to excellent intra-observer and inter-observer reliabilities. The robotic-assisted system for primary TKA allowed more accurate bone cuts and better contact bone-implant compared to the conventional technique.
#8135
Intraoperative Dynamic Planning With Robotics Improves Patellar Tilt Following Primary Total Knee Arthroplasty
Travis Weiner - Columbia University Medical Center - New York, USA
Mouhanad El-Othmani - Columbia University Medical Center - New York, USA
Alexander Neuwirth - Columbia University Medical Center - New York, USA
Jeffrey Geller - Columbia University Medical Center - New York, USA
Roshan Shah - Columbia University Medical Center - New York, USA
*John Cooper - Columbia University - New York, USA
*Email: jcooper02@gmail.com
Introduction
Anterior knee pain remains a commonly reported complaint following total knee arthroplasty (TKA) and has been shown to correlate to the patella as a source. While robotic knee systems have been shown to improve femoral and tibial component accuracy, the impact of robotics on patella tracking and tilt has not been adequately studied. Therefore, we sought to compare patellar tilt in robotic and conventional TKA.
Methods
We retrospectively reviewed 602 consecutive primary TKAs by a single surgeon over a 4-year period. Implant utilization varied over the study period and included implants from four different manufacturers. The population was divided into two cohorts: robotic vs. conventional. Conventional knees were performed with a measured resection technique with post-resection soft tissue balancing. Robotic knees were performed with one of three robotic platforms. Patellar tilt was measured using merchant view radiographs (Figure 1). Chi-square tests were used to examine for differences between categorical variables and t-tests for continuous variables. Binary logistic regression was used to control for differences between cohorts.
Results
A total of 260 robotic knees and 311 conventional knees were included. There were no differences in demographics between groups. A binary logistic regression showed no significant association between decreasing patellar tilt and a lack of patellar resurfacing (p = 0.217, 95% CI, 0.974-1.121). The absolute value of patellar tilt was used as a neutral of 0° was considered the goal for patellar tilt during surgery, and both increasing lateral and medial tilt away from neutral were considered as increasingly imperfect. The mean patellar tilt in the robotic cohort was 1.96° compared to 2.89° of tilt in the conventional cohort (p=0.0001) (Figure 2). The percentage of outliers where the patellar tilt exceeded 3 degrees from neutral was significantly higher among the conventional cohort (34% vs 20%, p=0.00018) (Table 1).
Conclusion
Robotic TKA leads to an improvement in patellar tilt, with a smaller percentage of outliers, compared to conventional TKA. The results of this study might further support the notion of improved flexion gap balancing and general alignment with robotic TKA and consistency of results with lower incidence of outliers. These findings could partially explain the improved pain with robotic TKA.
Figures
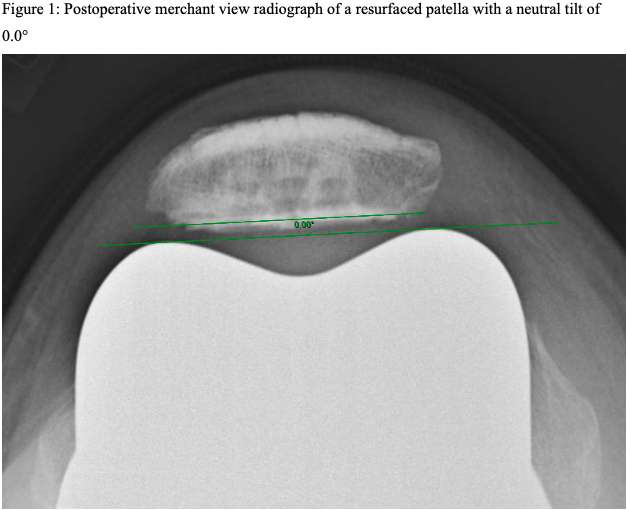
Figure 1
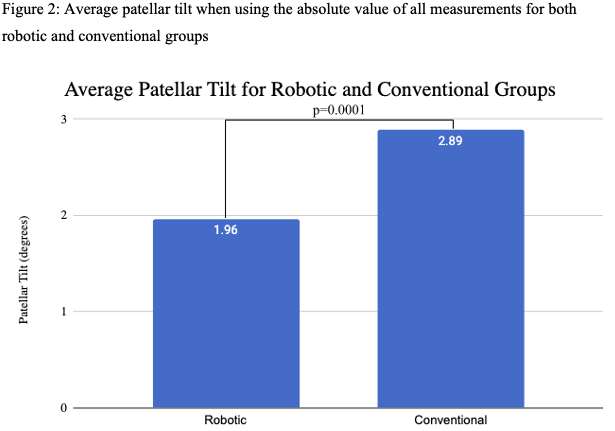
Figure 2
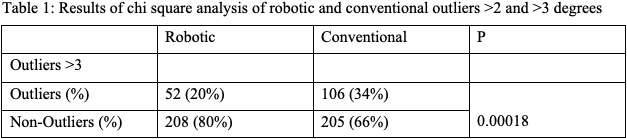
Figure 3#8149
Use of Thinner Polyethylene Liners Associated With Robotic-Assisted Total Knee Arthroplasty Compared to Navigation-Guided and Manual Techniques
*Tony Shen - Hospital for Special Surgery - New York, USA
Ryan Cheng - Hospital for Special Surgery - New York, USA
Mark P. Figgie - Hospital for Special Surgery - New York, USA
Geoffrey H Westrich - Hospital for Special Surgery - New York, USA
*Email: shent@hss.edu
Background: The thickness of a polyethylene liner used for a total knee arthroplasty (TKA) is reflective of the quantity of native bone resected, particularly on the tibia. The purpose of this study was to examine whether the use of a robotic-assisted technique during TKA results in the use of thinner polyethylene liners and improved bone conservation compared to navigation-guided and manual TKAs.
Methods: We retrospectively identified all polyethylene liners implanted during 16,401 primary unilateral TKAs performed on 14,253 patients between January 2018 and June 2022 at our institution. TKAs were separated by technology used: (1) image based robotic-assisted (n = 1,279), (2) imageless navigation (n = 8,348), and (3) manual (n = 6,774). The primary outcome of interest was polyethylene liner thickness.
Results: Polyethylene liners greater than 15mm in thickness were more frequently used during navigation guided (5.1%) and manual (4.0%) TKAs compared to robotic-assisted TKAs (1.9%) (both p < 0.001). Polyethylene liners 11mm and thinner were more frequently used during robotic-assisted TKAs (84%) compared to navigation-guided (70%) and manual (74%) procedures (both p < 0.001).
Conclusion: The thickness of polyethylene liners used during TKA vary depending on the type of technology used intra-operatively. This finding may be due to the ability of image based robotic-assisted techniques to consistently facilitate conservative bony resections and optimal ligament balancing across different types of anatomy and deformity during TKA.
#8136
In-Vivo Accuracy of a New Robotically-Assisted System for Total Knee Arthroplasty: A Prospective Cohort Study
*Faseeh Zaidi - University of Auckland - Auckland, New Zealand
Connor Fitz-Gerald - Auckland City Hospital - Auckland, New Zealand
Scott Bolam - University of Auckland - Auckland, New Zealand
Michael M Hanlon - Auckland City Hospital - Auckland, New Zealand
Jacob T Munro - University of Auckland - Auckland, New Zealand
Thor F Besier - University of Auckland - Auckland, New Zealand
Andrew Paul Monk - University of Auckland - Auckland, New Zealand
Craig Michael Goplen - University of Alberta - Edmonton, Canada
*Email: szai535@aucklanduni.ac.nz
Introduction: Recent technological advancements have led to the introduction of robotic-assisted total knee arthroplasty to improve the accuracy and precision of bony resections and implant position. However, the in vivo accuracy is not widely reported. The primary objective of this study is to determine the accuracy and precision of a cut block positioning robotic arm.
Methods: Seventy-seven patients underwent total knee arthroplasty with various workflows and alignment targets by three arthroplasty-trained surgeons with previous experience using the ROSA® Knee System. Accuracy and precision were determined by measuring the difference between various workflow time points, including the final pre-operative plan, validated resection angle, and post-operative radiographs. The mean difference between the measurements determined accuracy, and the standard deviation represented precision.
Results: The accuracy and precision for all angles comparing the final planned resection and validated resection angles was 0.90° ± 0.76°. The proportion within 3° ranged from 97.9% to 100%. The accuracy and precision for all angles comparing the final intra-operative plan and post-operative radiographs was 1.95 ± 1.48°. The proportion of patients within 3° was 93.2%, 95.3%, 96.6%, and 71.4% for the distal femur, proximal tibia, femoral flexion, and tibial slope angles when the final intra-operative plan was compared to post-operative radiographs. No patients had a postoperative complication requiring revision at the final follow-up.
Conclusions: This study demonstrates that the ROSA Knee System has accurate and precise coronal plane resections with few outliers. However, the tibial slope demonstrated decreased accuracy and precision on post-operative short-leg lateral radiographs with this platform.
#8616
Posterior Stabilizing Implant Kinematics Following Robotic Arm-Assisted Total Knee Arthroplasty
*Sean Higinbotham - University of Denver - Denver, United States of America
Azhar Ali - Stryker Orthopaedics - Mahwah, USA
Xiangyi (Cheryl) Liu - Stryker - Mahwah, USA
Cheryl Blackwood - Boulder Centre for Orthopedics & Spine - Boulder, USA
C. Brian Blackwood - Blouder Centre for Orthopedics & Spine - Boulder, USA
Kevin Shelburne - University of Denver - Denver, USA
*Email: sean.higinbotham@du.edu
Introduction: With the advent of robotic-assisted surgical systems, total knee arthroplasty (TKA) implants can be placed with improved precision and reduced variability1. Early results indicate that enhanced accuracy can lead to more consistent patient satisfaction with TKA2. Our study aimed to quantify in-vivo knee-joint kinematics using high-speed stereo radiography (HSSR) in TKA patients who underwent CT-based robotic-assisted surgery and assess how mechanical alignment affects pivot location across various activities.
Methods: Twelve patients (height: 1.76±0.1 m, mass: 85.5±22 kg, BMI: 27±5, age: 65±6 years) received a Triathlon Posterior Stabilizing total knee implant in a preoperatively planned mechanical alignment and functionally positioned using Mako total knee robotic assisted surgery system. One-year, postoperative, patients were measured performing five activities of daily living (gait, stair descent, lunge, supine leg press3, and seated knee extension) using HSSR. The 3D position of the femoral and tibial implants was tracked to the radiography images using DSX and described relative to the native bone position in which the implants were surgically placed. Medial and lateral low points of the femoral component were identified as the lowest point relative to the transverse plane of the tibial tray and were used to calculate anterior-posterior (AP) compartment translations. Tibial-axial-pivot location was quantified as the center-of-rotation of the femoral low-points relative to the tibial plateau across the flexion range.
Results: Average varus-valgus (VV) range of motion (ROM) was 1.38º±0.82º in gait, 1.46º±0.72º in stair descent, 2.88º±1.81º in lunge, 3.86º±1.60º in supine leg press, and 4.78º±1.64º in seated knee extension. AP low point translations in the medial compartment between femoral and tibial components during the five dynamic tasks showed the greatest mean ROM in stair descent (6.68±2.87 mm) and least in leg press (3.14±1.23 mm). The greatest mean AP ROM in the lateral compartment occurred during leg press (9.29±1.73 mm) and least in gait (3.07±1.74 mm) (Figure 1). Across all activities, 136 instances (39%) of central pivot, 105 medial pivot (30%), 67 no pivot (19%) and 44 lateral pivot (13%) (Figure 2) were observed. AP center-of-rotation was characterized by a weak, anterior-medial to posterior-lateral, slope relationship (r =-0.26, p<0.01) (Figure 3).
Conclusion: Our results highlight the motion of a Triathlon Posterior Stabilizing implant system that is functionally positioned utilizing a robotic assisted surgery system. A central rotation pattern emerged as the primary pivot location with the lateral low point consistently exhibiting greater posterior translation with increased flexion across all activities and medial exhibiting the same pattern except in lunge, seated knee extension, and extension portion of the leg press. The pivot locations observed in this patient cohort were similar to a healthy cohort described in literature.3 Compared to the healthy cohort, implanted patients had higher coefficient of variation in their VV motion in gait (59%|45%) and lunge (63%|41%), but lower variability in leg press (41%|42%) and seated knee extension (34%|38%).
References [1] Sires JD et al. J Knee Surg. 2021 [2] Marchand et al J Knee Surg 2019 [3] Hamilton et al. J Biomech 2022
Figures
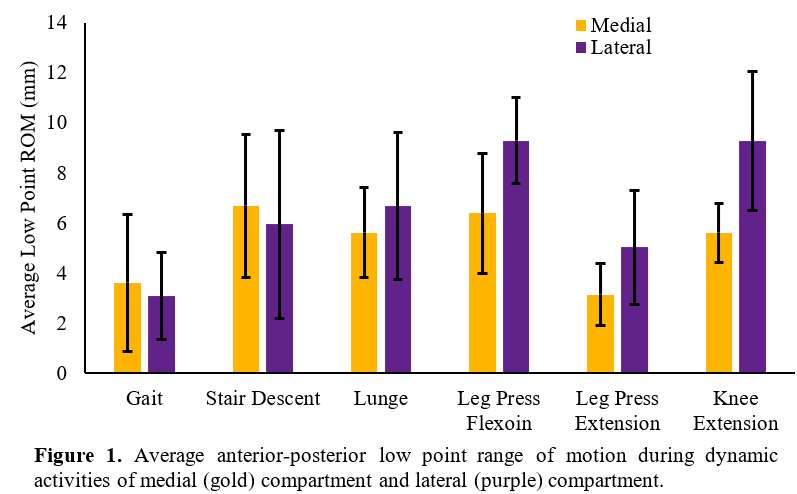
Figure 1
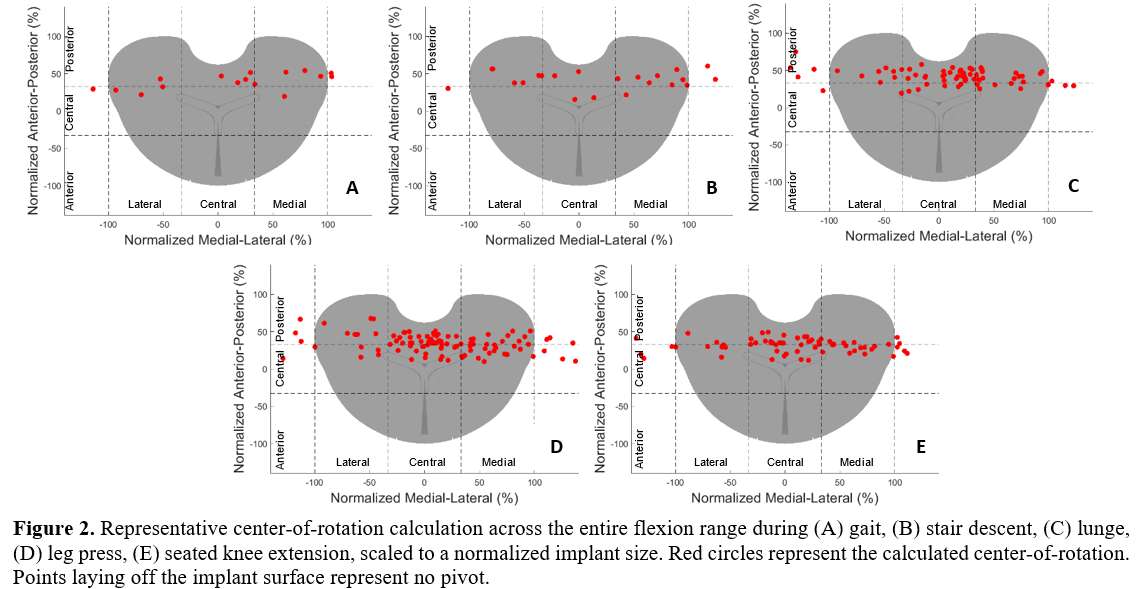
Figure 2
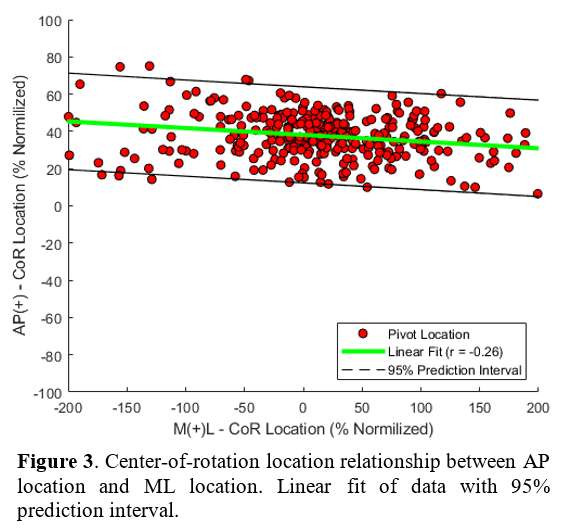
Figure 3#8303
Noise Related Injury During Robotic Total Knee Arthroplasty Compared to Manual Total Knee Arthroplasty
*Dianne Pagan - University of Miami Miller School of Medicine - Miami, United States of America
Michele D’Apuzzo - University of Miami Miller School of Medicine - Miami, USA
Victor Hernandez - University of Miami Hospital - Miami, USA
Jamie Carvajal - University of Miami Miller School of Medicine - Miami, USA
*Email: pagandianne@gmail.com
Introduction
Occupational noise exposure during the use of robotic equipment poses a risk to orthopaedic surgeons that has not yet been quantified in the literature. Noise exposure levels at or above 85 decibels as an 8-hour time-weighted-average (TWA) are considered hazardous. This study aimed to determine whether the noise of robotic instruments used in total knee arthroplasty (TKA) increases the risk of developing noise-induced hearing loss (NIHL) when compared to traditional instruments. We hypothesized that if noise measurements are compared during TKAs using the manual technique, the Mako Robotic-Arm Assisted Surgery, and the CORI Surgical System; then the Mako group will have the highest measurements.
Methods
Intraoperative audio was recorded during either manual, Mako, or CORI TKAs. The DecibelX application was used to record the duration of exposure, average decibel level, maximum decibel level, TWA, noise dose percentage of maximum allowable daily noise, and noise dose percentage projected forward over 8 hours. A one-way ANOVA was performed. Statistical significance was set at a 95% confidence interval.
Results
Fifty audio recordings were analyzed. The results indicated no significant difference in the means of neither duration of exposure nor maximum decibels. A significant difference in mean average decibel level was found, with post hoc testing showing a difference between Mako and manual TKAs (p < 0.001), and Mako and CORI TKAs (p = 0.002) (Figure 1). A significant difference in mean TWA was found, with post hoc testing showing a difference between Mako and manual TKAs (p = 0.018), and Mako and CORI TKAs (p = 0.043) (Figure 2). A significant difference in mean noise dose was found, with post hoc testing showing a difference between Mako and manual TKAs (p < 0.001), and Mako and CORI TKAs (p < 0.001) (Figure 3). A significant difference in mean projected noise dose was found, with post hoc testing showing a difference between Mako and manual TKAs (p < 0.001), and Mako and CORI TKAs (p < 0.001).
Discussion & Conclusion
Orthopaedic surgeons performing TKAs using both robotic and manual approaches are exposed to noise levels known to cause NIHL, with Mako TKAs having the highest levels. Further efforts to reduce noise levels made by orthopedic surgical instruments are warranted.
Figures
Figure 1
Figure 2
Figure 3#8651
Hip-Spine Classification Automation and Population Analysis Using Deep Learning
*Robert Ricotti - Hospital for Special Sugery - New York, USA
Seong Jun Jang - Hospital for Special Surgery - New York, United States of America
Theofilos Karasavvidis - Hospital for Special Surgery - New York, USA
Cale Pagan - Hospital for Special Surgery - New York, United States of America
Jonathan Vigdorchik - Hospital for Special Surgery - New York, USA
Peter K. Sculco - Hospital for Special Surgery - NYC, USA
David J. Mayman - Hospital for Special Surgery - New York, USA
Seth A. Jerabek - Hospital for Special Surgery - New York, USA
*Email: RicottiR@hss.edu
Concurrent spinal pathology often exists in the patient population undergoing total hip arthroplasty (THA). The hip-spine relationship influences lumbar lordosis (LL), acetabular anteversion, and pelvic rotation as one changes from a standing to a seated position. Spinal deformity, age, sex, and BMI can influence these parameters, and it is important to understand how they interact when planning for acetabular component placement in THA. The hip-spine classification was developed to better characterize spinopelvic mobility and guide operative management of THA patients. The objectives of this study were to 1) characterize the hip-spine classifications of 1,622 patients at a single institution, 2) utilize a novel deep learning (DL) method to provide validated measurements of spinopelvic parameters and mobility in the same patient cohort, and 3) analyze differences in hip-spine classification distributions based on age, sex, and BMI.
A multi-modal DL workflow was developed to automatically measure spinopelvic tilt (SPT), sacral slope (SS), pelvic incidence (PI), and LL on preoperative standing and seated lateral spinopelvic radiographs in 1,622 patients who underwent THA at our institution. The final DL workflow’s measurement accuracy was assessed using a hold-out set of 200 testing images (100 standing:100 sitting). These DL measurements were compared against two trained, blinded readers using inter-class correlation coefficients (two-way 147 mixed, single score, ICC3) and Bland-Altman plot analyses to assess bias. ICCs > 0.9 were defined as excellent, and the DL workflow was applied to the entire patient cohort to determine hip-spine classifications. The patients were then categorized into one of four hip-spine classifications: 1A) normal spinal alignment and mobility, 1B) normal spinal alignment and stiff spine, 2A) flatback deformity and normal mobility, and 2B) flatback deformity and stiff spine. Analyses were performed to investigate the relationship between age, sex, BMI, and preoperative hip-spine classification.
Mean age was 63.2 ± 11.5 years (range 13 to 83), mean BMI was 28.8 ± 5.7 (range 16.6 to 52.1), and 887 (54.9%) patients were female. The DL workflow demonstrated excellent accuracy in measuring SPT (ICC = 0.98) and PI-LL (ICC = 0.95) when compared to 2 different readers (Figure 1). The population breakdown of hip-spine classifications in our cohort of 1,622 patients as measured by the DL workflow can be found in Figure 2. Hip-spine classifications differed based on age (over 65 vs. under 65, p < 0.0001) and BMI (obese vs. non-obese, p = 0.01) (Figure 3). There were no differences in hip-spine classifications based on sex (p = 0.12). Additional univariate analyses did not indicate that age and BMI were confounders.
The DL workflow in this study provided accurate and valid measurements of SPT and PI-LL in our cohort of 1,622 patients, which is the largest series to date. Automation of the hip-spine classification streamlines preoperative planning for THA and helps the surgeon plan for changes in spinopelvic parameters following THA. Future studies are encouraged to use novel DL methodology to investigate automation of postoperative hip-spine classification measurements in relation to clinical outcomes.
#8504
Prediction of Pelvic Tilt on Anteroposterior Pelvic Radiographs: A Deep Learning-Enabled Analysis
*Cale Pagan - Hospital for Special Surgery - New York, United States of America
Seong Jun Jang - Hospital for Special Surgery - New York, United States of America
Theofilos Karasavvidis - Hospital for Special Surgery - New York, USA
Peter Sculco - Hospital for Special Surgery - New York, USA
David J. Mayman - Hospital for Special Surgery - New York, USA
Jonathan Vigdorchik - Hospital for Special Surgery - New York, USA
Seth A. Jerabek - Hospital for Special Surgery - New York, USA
*Email: paganc@hss.edu
Introduction: The assessment of sagittal spinopelvic alignment has gained significant interest, due to its impact on clinical outcomes and the risk of instability after total hip arthroplasty (THA). Developing a method to accurately measure spinopelvic tilt (SPT) using anteroposterior (AP) radiographs would minimize radiation exposure, reduce healthcare costs, and optimize surgical outcomes. Previous studies assessing coronal pelvic parameters such as the sacro-femoral -pubic (SFP), did not demonstrate strong correlations with SPT. The aim of this study was to identify a novel coronal plane parameter with a strong relationship to SPT, utilizing a large cohort of THA candidates with AP and lateral radiographs of the hip.
Methods: A retrospective review was conducted on 1,894 adult patients (1028 females and 864 males; mean age 62.9 SD 11.9) who underwent primary THA and received full-length standing AP and lateral radiographs. A deep learning (DL) model was developed to automate detection and analysis of 34 angles and 2,179 ratio combinations for all patients. All angles and ratios were derived from 19 key landmarks, consistently visible on AP radiographs, and were correlated with SPT as measured on lateral films (Fig. 1). Landmarks included the superior aspect of the iliac crests, center of femoral heads, ischial tuberosity, the superior and inferior aspects of the sacroiliac joints, pubic symphysis, and obturator foramen. The midpoint of all bilateral landmarks was utilized to derive all angles and ratios. The relationship between angles/ratios and SPT was assessed using Pearson correlation coefficient stratified by gender.
Results: Results: Statistical analysis between SFP and SPT demonstrated a Pearson’s correlation coefficient of 0.6 (p<0.01) (male = 0.63; female = 0.66). Analysis of angles demonstrated 9 parameters with a higher correlation coefficient when compared to SFP. The strongest correlation with SPT was demonstrated when assessing the angle formed by the midpoint of the inferior SI joints, center of femoral head, and superior pubic symphysis (r= 0.68 (p<0.01), male = 0.65; female = 0.72). Notably, analysis of ratios demonstrated 14 unique ratios with Pearson’s correlation coefficient greater than 0.7. The ratio derived from the pelvic height divided by the distance of the midpoint of the superior obturator foramina to the midpoint of the iliac crests demonstrated the strongest correlation coefficient of 0.74 (p<0.01) (male = 0.73; female = 0.78) (Fig. 2 and 3). Sex-specific correlations demonstrated stronger correlations for females across all parameters.
Conclusion: Strong correlation between coronal parameters and SPT was demonstrated. Novel angles and ratios on AP radiographs were found to be strongly correlated with SPT, specifically with a stronger correlation than SFP. Our next steps will include creating an interpretable and predictive model to determine SPT from these parameters.
Fig. 1 Automated detection of key anatomic landmarks from which angles and ratios were derived.
Fig. 2 The novel ratio derived from dividing the red line by the white line which demonstrated the highest correlation coefficient.
Fig. 3 Scatterplot demonstrating relationship of the novel ratio with SPT.
Figures
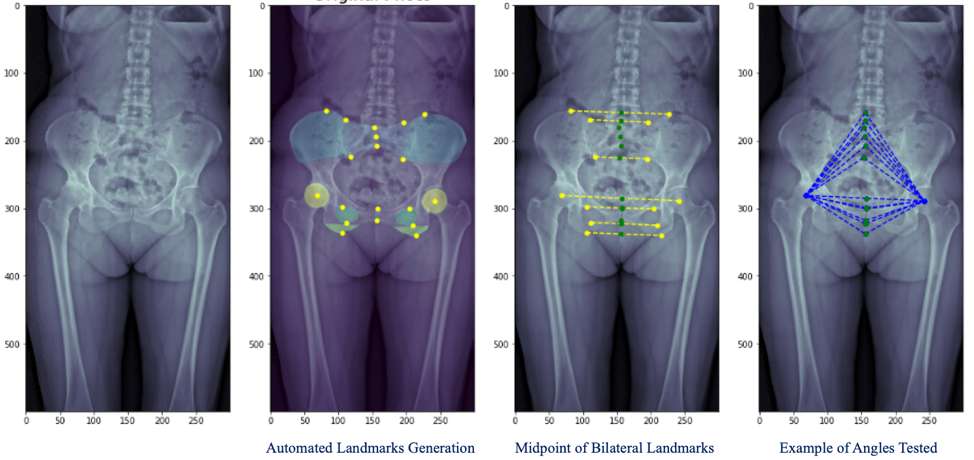
Figure 1
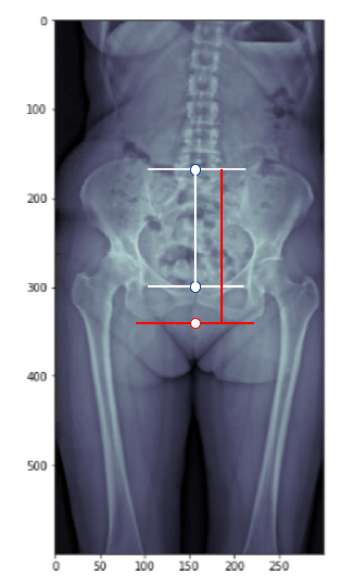
Figure 2
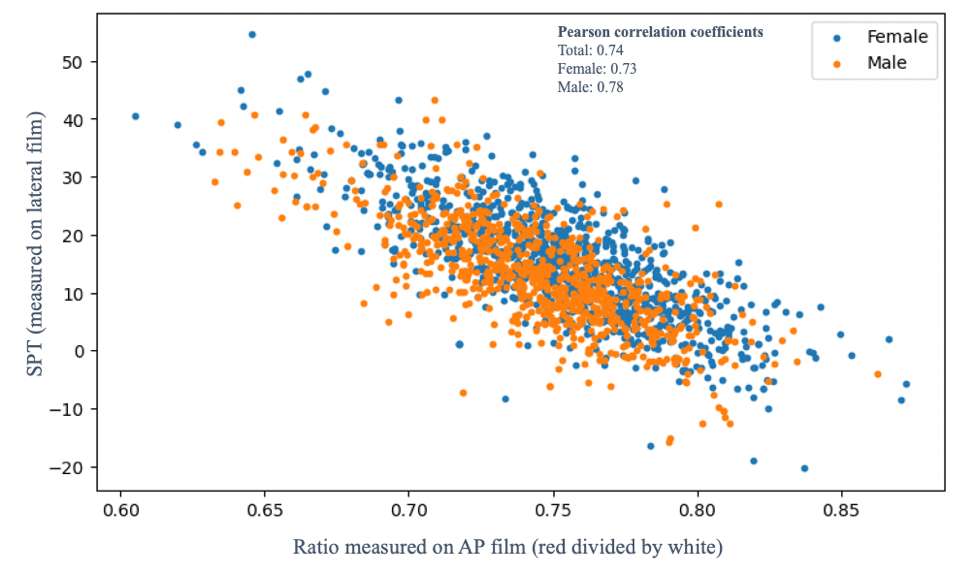
Figure 3#8445
Fully Automated AI-Based CT Analysis in Total Hip Arthroplasty
*Hassan Nemati - Ortoma AB - Gothenburg, Sweden
Andreas Pettersson - Ortoma AB - Gothenburg, Sweden
Linus Bystrom - Ortoma AB - Gothenburg, Sweden
*Email: hassan.nemati@ortoma.com
Proper placement of acetabular implant is essential for the success of total hip arthroplasty and long-term stability. Post-operative analysis of CT imaging data provides early detection of malpositioning of the acetabular implant and can help to foresee mechanical loosening and plan further treatment. However, currently available post-op CT analysis tools require several time-consuming manual or semi manual steps, including hip bone annotation, pre- to post-op image registration, and implant detection and localization.
In this study, the entire workflow of a novel orthopedic surgical platform that utilizes CT imaging with Artificial Intelligence (AI)-based algorithms and Machine Learning (ML) models to automate several critical aspects of total hip arthroplasty is presented. The complete work process encompasses three key modules: pre-operative planning, navigation during surgery, and follow-up analysis. The primary objective of the current study was to evaluate the performance of the follow-up analysis module. This module automatically extracts relevant information from pre-op and post-op CT imaging data to automatically determine the placement (angle and position) of the acetabular cup component relative to the hip bone. In addition, the follow-up analysis module provides visualization of the automatically localized landmarks, segmented bones and implants in slice and projection views, and 3D representation of the pelvis bone for both pre- and post-op scans (see Figures 1 and 2).
Clinical study was performed on 30 patients to evaluate the accuracy of the automatic detection of cup placement in the follow-up analysis module compared to the conventional manual procedure. The manual procedure consists of a) overlaying the cup component template onto the post-op CT image and adjusting its position manually until it matched the post-op cup component; b) annotating the hip bone in pre- and post-op CT images; and c) using a volume registration algorithm to align the hip bone in pre- and post-op images. Here, the cup placement (cup anteversion, inclination, and position) was presented in the patients coordinate system of the pre-operative CT image and used as the ground-truth.
Post-op CT imaging data from 30 surgeries were analyzed and results showed a high level of accuracy in automatic detection of cup placement, with a mean difference of -0.31 ± 0.87 (°) for anteversion, 0.17 ± 1.04 (°) for inclination, and 0.28 ± 0.78 (mm) for cup position. Furthermore, statistical test showed no significant (p<0.05) difference between the results of the fully automatic follow-up analysis module and the ground-truth.
The novel orthopedic follow-up analysis module, with the use of AI algorithms and ML models, provides a fast and efficient capability to analyze post-operative CT imaging data. The clinical results demonstrated its notable accuracy in determining the acetabular implant placement. Surgeons can visually inspect and verify the cup placement immediately after the post-op CT images are taken. The overall computation time for the automatic analysis is about 3 minutes which results in highly accurate implant detection and localization.
#8361
Automated Landmark Detection in Functional Lateral Radiographs Using Deep Learning
*Gerard Smith - Corin - Sydney, Australia
Edgar Wakelin - OMNI Life Sciences - Raynham, USA
Sanjeev Gupta - Royal Prince Alfred – Institute of Rheumatology and Orthopaedics, - Sydney, Australia
Christopher Plaskos - Corin - Raynham, USA
Jim Pierrepont - Corin - Pymble, Australia
*Email: gerard.smith@coringroup.com
Evaluation of patient-specific spinopelvic mobility in total hip arthroplasty (THA) requires detection of bony landmarks in lateral functional radiographs. Current manual landmarking methods are inefficient, time consuming, and subjective. This study proposes a series of deep learning models to automate image classification, object detection and key point detection for accurate derivation of spinopelvic mobility (SPM).
A series of deep learning models were developed to create an image processing pipeline capable of classifying functional radiographs and detecting anatomical landmarks. An international multicenter imaging database containing preoperative and postoperative anterior-posterior and lateral functional radiographs was utilized (HREC: Bellberry: 2020-08-764-A-2). A vision transformer image classifier model was trained to isolate three functional image positions: 1) standing, 2) contralateral step-up, and 3) flexed seated (Figure 1). A YoloV8 object detection model was employed to isolate the L1 vertebra before detecting the L1 endplate. A deep learning multi-stage convolutional neural network model was used to perform keypoint detection. All models subdivided available imaging into 70:20:10 train:validate:test groups respectively. Available imaging for each model is described in Table 1. Ground truth landmarks were manually captured and independently verified by qualified engineers during pre-operative planning with additional assistance of 3D computed tomography derived landmarks. Pelvic tilt (PT), sacral slope (SS), L1 endplate angle (relative to the horizontal) and the lumbar lordotic angle (LLA) were derived from the ground truth and predicted landmark coordinates. Interobserver variability was assessed with a subset of images landmarked by 9 qualified engineers.
The image classifier produced a classification accuracy of 99.6% and an area under the curve and F1-score of 0.999. The object detection algorithm used to isolate the L1 vertebra achieved a mean average precision at an intersection of union of 0.5 (mAP@50) of 99.3%, precision of 97.9%, and recall of 98.6%. The model produced a mean absolute error (MAE) ± standard deviation for PT, SS, and LL1 endplate error of 1.6°±2.1°, 3.2°±2.6°, 2.5°±2.4°, respectively. Furthermore, the LLA angle error was determined to be 4.2°±3.2°.The interobserver 95% confidence interval (CI) for engineer measured PT, SS, and LLA (1.9°, 1.9°, 3.1°, respectively) was comparable to the MAE values generated by the model. The mean prediction time was 1.9 seconds per image.
Image classification of routine pelvic radiographs can be performed with high accuracy. The landmark detection process produced landmarks with a MAE comparable performance to the gold standard manual annotation process. LLA prediction produced the lowest SPM accuracy, likely due to error propagation from the SS and L1 landmarks. Our model series, which incorporates image classification, object and keypoint detection deep learning algorithms, shows excellent performance when compared against the current gold standard manual annotation process.
Figures
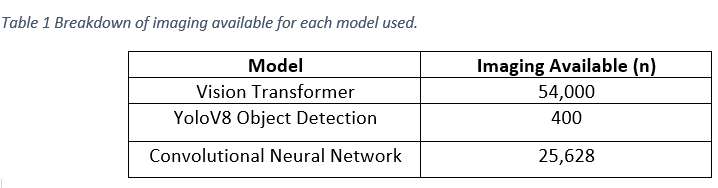
Figure 1
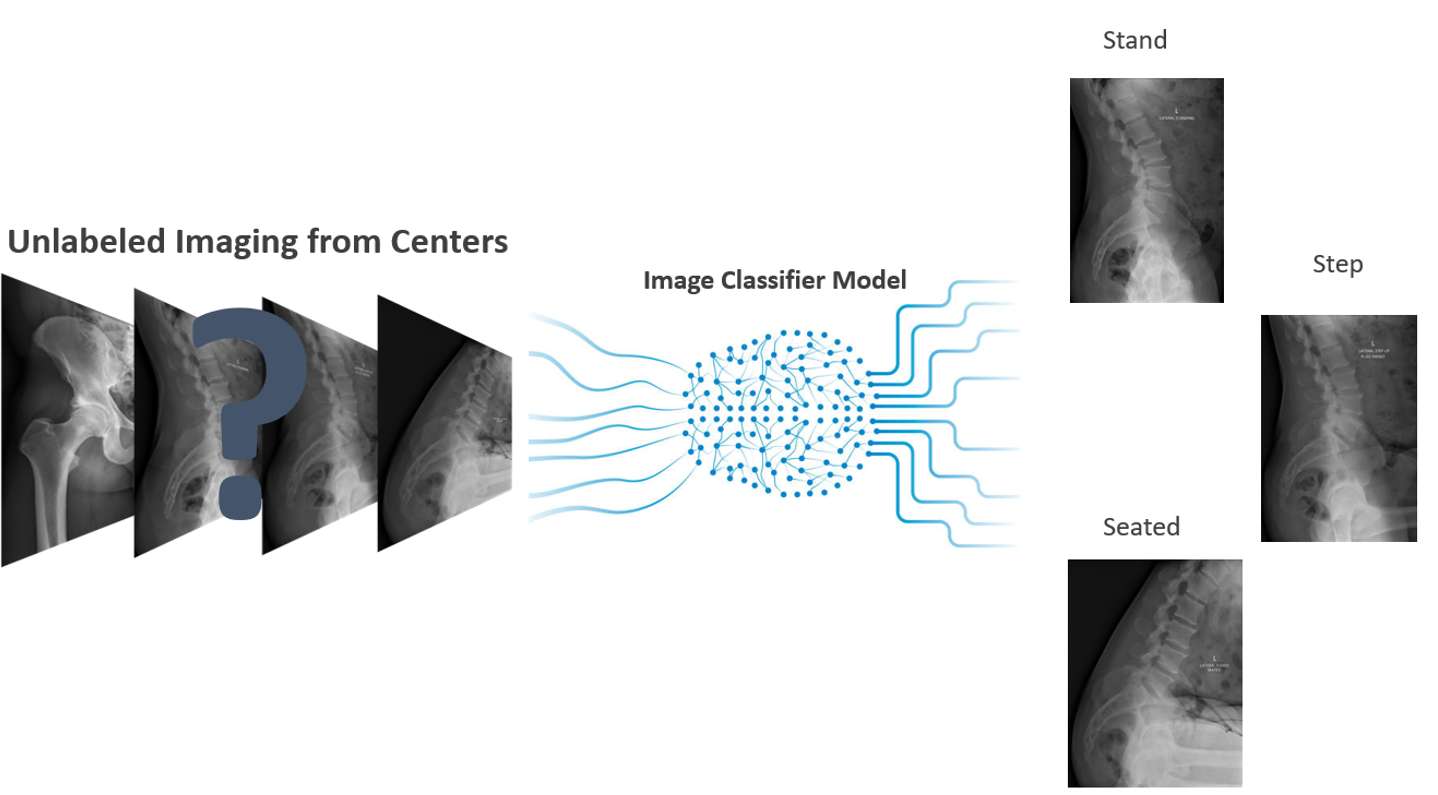
Figure 2
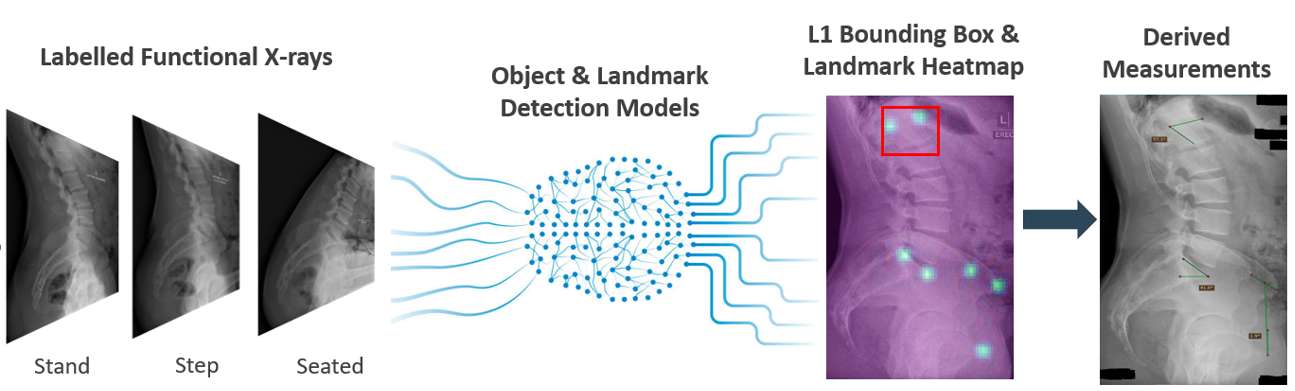
Figure 3#8506
Automation of Segmentation Using Computer Vision and Deep Learning Algorithms
Raquel Rodriguez - Stryker - Manchester, United Kingdom
*Laura Yanoso-Scholl - Stryker Orthopaedics - USA
Matthew Thompson - Stryker - Fort Lauderdale, USA
Kevin de Souza - Stryker - Manchester, United Kingdom
*Email: Laura.Scholl@stryker.com
Introduction: Published literature has described a post-operative computer tomography (CT) method to assess implant placement accuracy to plan where it has the advantage of using a direct methodology based on patients post-operative imaging. However, limitations include artifacts created from the metal implants that can cause ambiguity in measurements (CT).
Recently, computer vision algorithms have advanced including the use of active appearance models to optimize interpreting medical imaging by using differences between current estimate of appearance and target image. Deep reinforcement learning algorithms, that refine image processing through trial and error, have also been more widely used within the medical field. Application of these techniques offers value in automating the measurement of postoperative CT implant placement measurements and reduces impact of artifact and human error when assessing postoperative CTs.
The purpose of this study was to validate an analysis that incorporates computer vision and deep learning to auto-segment postoperative THA implants in comparison to an established manual process.
Methods: Postoperative CTs were collected and analyzed for 10 THA patients. They were segmented to isolate the acetabular cup and femoral stem implants using both a manual and automated process. The auto-segmentation analysis was performed by using active appearance modeling (AAM). An implant specific AAM was built from models of the implants which enabled improved accuracy of auto-segmentation in the presence of metal-induced image artifacts. Deep learning refinement was then applied using U-Net segmentation to improve performance in diseased areas. Then a corresponding projection was performed using the dense landmarks projected onto accurate refined surface. At the end of the process, the cup and stem models were saved as STL files. For manual-segmentation, a threshold value was selected to isolate the metal implants and disregard the less dense patient bone. The acetabular cup was then separated from the femoral components by manually editing the 3D masks.
The distance between the automated and manual implant surfaces were calculated to measure differences.
Results: Manual and auto-segmented implants were matched and differences between the segmentations were calculated (Figure 1). Those values are shared in Table 1. Regarding implant error, implant surface mean difference was 0.72 [mm] (SD = 1.85) for the femoral stem and 0.47 [mm] (SD = 0.60) for the acetabular cup.
Conclusion: The auto-segmentation process had a mean difference less than 1mm when compared to the manual-segmentation process. When visually inspecting all 10 patients the largest difference was observed at the proximal stem (trunnion) where the stem is inserted into the femoral head, resulting in large deviations. With metal artifact it was difficult to distinguish between the two with manual-segmentation. Auto-segmentation removed this difficulty and retained the trunnion structure. Based on this analysis, auto-segmentation proved to be a valid algorithm that eliminated error due to metal artifact and human error. Additionally, automation of this process reduced manual analysis time.
Figures
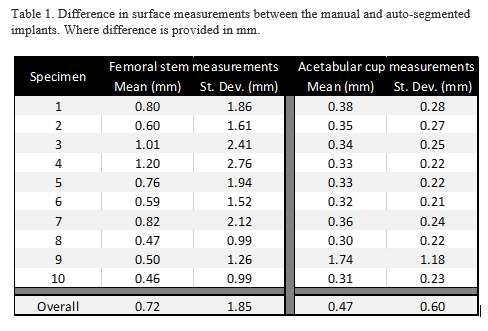
Figure 1
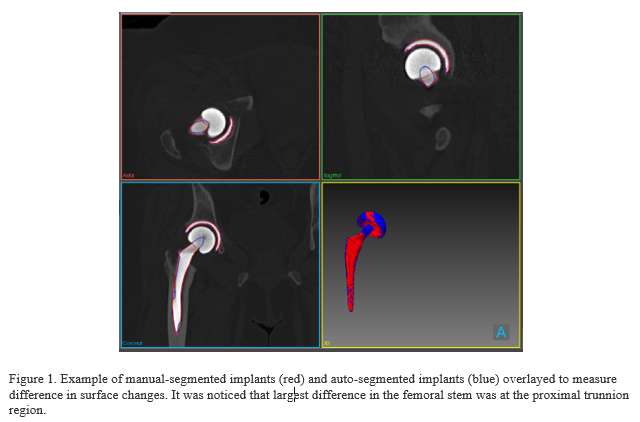
Figure 2#8345
Machine Learning Analysis of Radiographs Can Predict Failing Total Hip Replacements Before They Become Symptomatic
*Vipin Asopa - North Middlesex University Hospital - London, United Kingdom
Andrew Womersley - SWLEOC - London, United Kingdom
Jad Wehbe - SWLEOC - London, United Kingdom
Peter Harris - SWLEOC - London, United Kingdom
David Sochart - University of Salford - Manchester, United Kingdom
Keith Tucker - Norwich
Richard Field - South West London Elective Orthopaedic Centre - London, United Kingdom
*Email: vipin@asopa.net
Introduction
One-hundred thousand hip replacement are performed annually in the UK. However, in 2019, 8087 hip replacements were revised. Almost half of these revisions were for aseptic loosening.
Early identification or prediction of failure by using Machine Learning (ML) analysis of radiographs would allow earlier identification of poorly performing implants, improve implant design process and avoid unnecessary patient suffering, If ML fails to show an abnormality in a follow-up radiograph it would reassure the patient and surgeon. Unnecessary follow-ups could be deferred. If ML shows an abnormality, then there would be an indication for continued follow up. These advantages would represent savings in health expenditure.
The aim of this study is to investigate whether machine learning can identify and/or predict failing uncemented total hip replacements through the analysis of radiographs before patients become symptomatic and before the human eye identifies features of failure on a radiograph.
Methods
Consent was sought from 253 patients being followed up in a single design uncemented total hip replacement implant surveillance study between 2010-2017. Radiographs were downloaded from a PACS system and labelled according to side, projection and the presence of lucency, cortical hypertrophy and pedestal formation (factors thought to be associated with a loose stem) by 3 skilled observers. The Oxford Hip Score was obtained for all patients, before and at set time points after surgery.
Deep learning was performed using the RGB ResNet 18 model with radiographic images fed in chronological order. The model was trained according to revision status and radiographic features (as described). Data augmentation and cross validation were used to increase the available training data and improve verification of results. Adjustments to the model were carried out to improve the true and false positive rates.
Results
184 / 253 patients (mean age 62 (range 33-79), BMI 28 (range 18-40) who underwent an a single design uncemented total hip replacement consented to inclusion in this study.
Radiographs from 18 / 184 patients had all 3 features thought to be associated with increased risk of implant failure (lucency, cortical hypertrophy and pedestal formation). 6 / 184 patients were revised for aseptic loosening. A total of 2097 images were available for analysis. 166 patients were used for ML algorithm testing of 2 scenarios to detect patients who had undergone revision.
The ML algorithm identified failed hips before radiographic features and before patients became symptomatic. Figure 1 shows that the algorithm could detect a failing hip as far as 3 years before it become apparent clinically or radiologically apparent (EOC 059). The AUC was 82%, true positive rate 82 percent and false positive rate 20%.
Conclusions
ML algorithms can identify failing hips and predict failure years before features become visible on a radiograph or before patients become symptomatic and could help speed hip implant design, testing and clinical use.
Acknowledgements: Research department
Keywords:
Machine learning, implant failure, revision hip
Figures
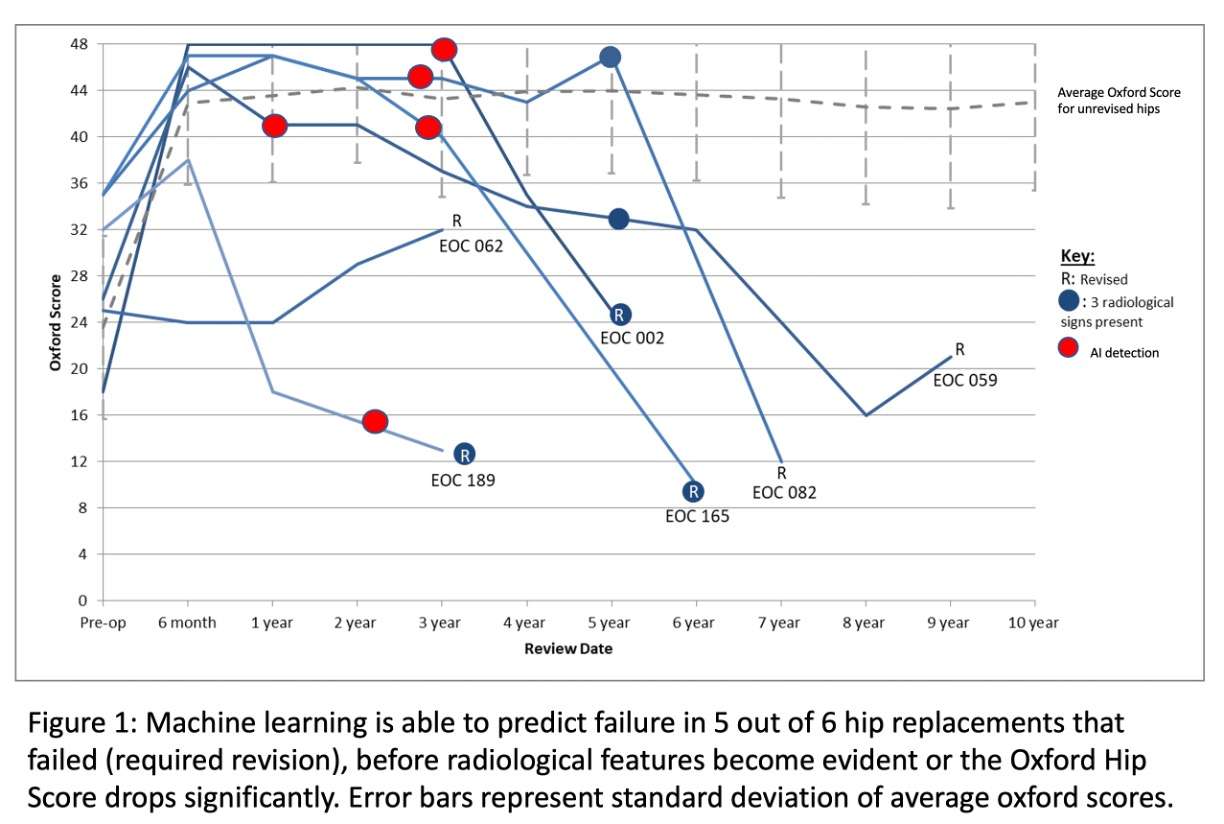
Figure 1#8792
A Phase 1b Open-Label, Dose-Escalating Study to Evaluate the Safety and Tolerability of PLG0206, in Patients Undergoing DAIR for Treatment of PJI Occurring After TKA: Interim Analysis
Atul Deshpande - Peptilogics - Pittsburg, United States of America
*David Huang - Peptilogics Inc - Pittsburg, USA
Nicolas Piuzzi - Cleveland Clinic - Cleveland, USA
Antonia Chen - Brigham and Women's Hospital - Boston, USA
Christopher Pelt - University of Utah - Salt Lake City, USA
Barbara Trautner - 5Michael E. DeBakey Veterans Affairs Medical Center - Houston, USA
Despina Dobbins - Peptilogics - Pittsburg, USA
Matthew Dietz - 7West Virginia University - Morgantown, USA
Nick Pachuda - New York, United States of America
*Email: David Huang
Aim: This study is prospectively assessing the safety and tolerability of PLG0206 administered via an irrigation solution for the treatment of periprosthetic joint infections (PJI) after total knee arthroplasty (TKA) during DAIR in combination with standard-of-care antibiotic therapy. Secondary objective is to assess the clinical efficacy compared to historical control on clinical outcomes at Days 21, 42, 90+ post-DAIR procedure.
Method: This study is a multi-center, Phase 1b open-label, dose-escalating study. PLG0206 was administered as a single dose irrigation for 15-minutes in the wound cavity of patients undergoing DAIR for treatment of PJI after TKA (NCT05137314). Two cohorts of patients received a single irrigation with a solution of PLG0206 at 3 mg/mL (n=7) or 10 mg/mL (n=7).
Results: All 14 patients presented with acute hematogenous PJI of the knee. In the low-dose cohort, 3/7 (42.9%) subjects reported adverse events (AEs) and 2/7 (28.6%) subjects reported a serious adverse event (SAE). In the high dose cohort, 1/7 (14.3%) subjects reported AE and 1/7 (14.3%) subjects reported SAE. All reported adverse events and serious adverse events (SAEs) were unrelated to PLG0206 except for one SAE (neurogenic knee pain) in the high dose cohort which is possibly related to PLG0206. No clinically relevant laboratory abnormalities, vital signs, or physical examination abnormalities were observed.
No recurrences were reported in six patients vs. historical control of 44% failures in DAIR patients at 90 days. One patient was lost to follow up in the low dose cohort.
Figure1: Patient demographic characteristics, microbiological cultures, and clinical outcomes by dose cohort and times since DAIR surgery
Conclusions: Following a single 15-minute irrigation of PLG0206 to the wound cavity of patients undergoing a DAIR procedure for treatment of PJI occurring after TKA, PLG0206 appears safe, well-tolerated and clinically efficacious at 90 days. Longer-term follow-up of the clinical outcomes for these patients are ongoing. These findings are encouraging and showcase the potential for PLG0206 as a curative solution for treating PJIs.
Figures
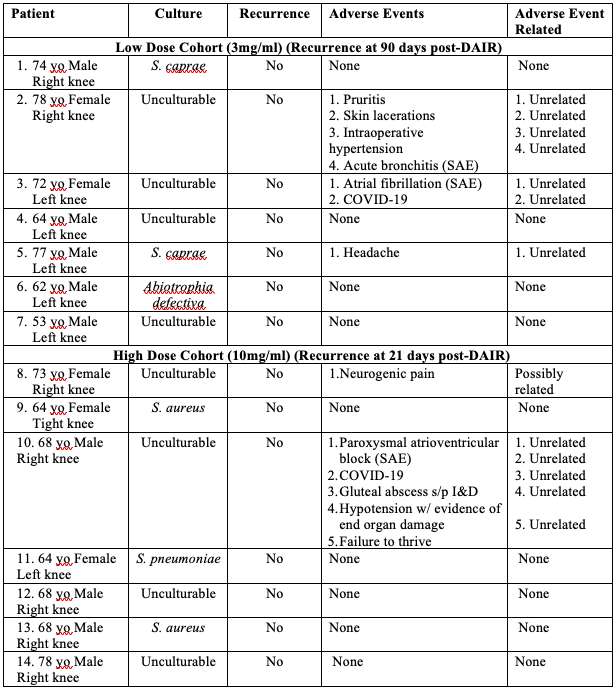
Figure 1#8162
The Results of 16S DNA-NexGen Sequencing (NGS) in Culture-Negative Periprosthetic Joint Infections
*Gerhard Maale - Medical Center Plano - Plano, USA
*Email: gerhardmaale53@gmail.com
INTRODUCTION: Traditional culture methods have long been used to identify the presence of organisms in periprosthetic joint infections (PJI). Culture retrieval is at best 50-60% recovery for one organism alone. With preoperative treatment of PJI with antibiotics, the rate is anticipated to be much lower. 16S Nexgen sequencing is thought to have a much higher recovery rate for patients pretreated with antibiotics. The question asked is whether Nexgen sequencing can recover more than one organism from draining sinus tracts and whether or not pretreatment with oral or systemic antibiotics has any effect on this recovery means.
METHODS: 97 patients were identified with open draining sinus tracts around PJI, including 69 knees, 20 hips, 5 shoulders, and 3 elbows. All patients had an average of 5-7 operations (historically) prior to referral. All wounds were open and culture-negative. Each open wound was swabbed and underwent 16S DNA-Nexgen sequencing by Microgen.
RESULTS: None of this patient population had a positive culture of their draining sinus wound. Of these 97 patients, 58 of the open wounds were monomicrobial, and the other 39 were found to be polymicrobial PJI, with an average of 1.67 bacteria/fungal species per patient. Of the patients with polymicrobial infections, 21 were identified to have both gram-positive and gram-negative bacteria on NexGen sequencing. 90 patients had preoperative treatment with antibiotics. 33 patients were treated with oral agents and 57 patients with IV antibiotics prior to referral.
DISCUSSION AND CONCLUSION: Our findings indicated better identification of organisms in PJI with 16S DNA-NexGen sequencing over classic culture. Additionally, preoperative antibiotic coverage did not alter the recovery of the organism/organisms with Nexgen sequencing. Furthermore, it identified resistant genomes in these organisms. It recovered multiple organisms in 40% of the cases. Twenty one % had both gram+ and gram – organisms recovered with NGS.
#8710
Intraosseous Vancomycin in Tourniquetless Primary Total Knee Arthroplasty
*Timothy Brown - Houston Methodist Hospital - Houston, USA
Thomas Sullivan - Houston Methodist Hospital - Houston, USA
Francesca Taraballi - Houston Methodist Research Institute - Houston, USA
Stefano Serpelloni - Houston Methodist Research Institute - Houston, USA
Pradyumna Gurusamy - Houston Methodist Hospital - Houston, USA
Kwan Park - Houston Methodist Hospital - Houston, USA
*Email: tsbrown2@houstonmethodist.org
Introduction
It is currently unknown whether intraosseous (IO) administration of vancomycin prior to tourniquetless total knee arthroplasty (TKA) is an effective method for antibiotic prophylaxis. The purpose of this study is to determine whether IO administration of vancomycin is as effective as intravenous (IV) administration prior to tourniquetless TKA, thereby eliminating the 1-2 hour infusion time.
Methods
This is a prospective, randomized, single-blinded controlled trial. Our current study size involves 4 patients in each arm: 4 given IV vancomycin, and 4 given IO vancomycin. The IV group received ancef and vancomycin in the pre-operative period approximately 1 hour prior to incision. IV Vancomycin was dosed using a weight-based scale. The IO group received ancef via IV, and vancomycin was delivered via IO technique (500mg) into the tibial tubercle, immediately prior to incision. Systemic samples for vancomycin levels were taken prior to the case, and at time of closure. Bone samples from the distal femur, proximal tibia, and synovium from the suprapatellar pouch were collected throughout the procedure. Tissue vancomycin concentrations were calculated after processing via high-performance liquid chromatography.
Results
No patient demographic or serum creatinine differences were present. Significant differences in systemic vancomycin levels (ug/mL) were found at the start of the case: IV= 32.7 ± 9.0, IO 0 ± 0, p<.01; and at the end of the case: IV = 18.7 ± 3.4, IO = 8.3 ± 1.0, p<.03. No significant differences were seen in the average vancomycin concentration in the distal femur: IV = 72.4 ± 23.1, IO = 79.2 ± 6.5 p= .79; or in the proximal tibia IV= 59.7 ± 29.9, IO = 73.9 ± 28.8, p= .74. Similarly, synovial levels were not significantly different, IV = 11.45 ± 6.8, IO = 10.4 ± 3.8, p= .9.
Conclusion
Our study shows that similar levels of vancomycin in femur, tibia, and synovium can be achieved using IO vancomycin in tourniquetless TKA setting compared to IV administration of vancomycin, without need for an extended infusion time. Additionally, total systemic levels in IO administration are significantly lower than in IV administration.
Figures
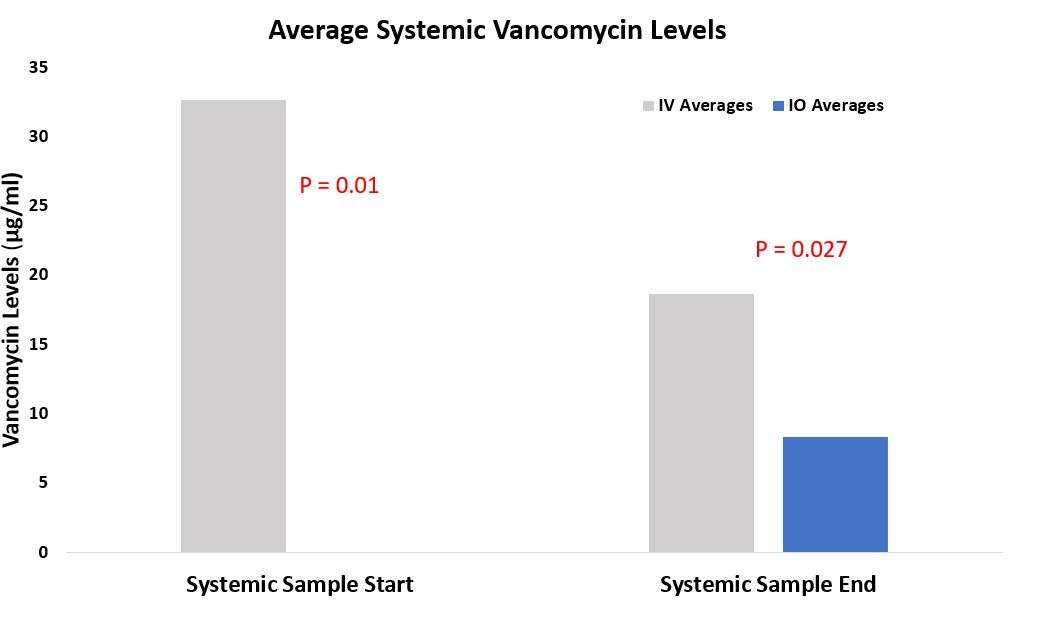
Figure 1
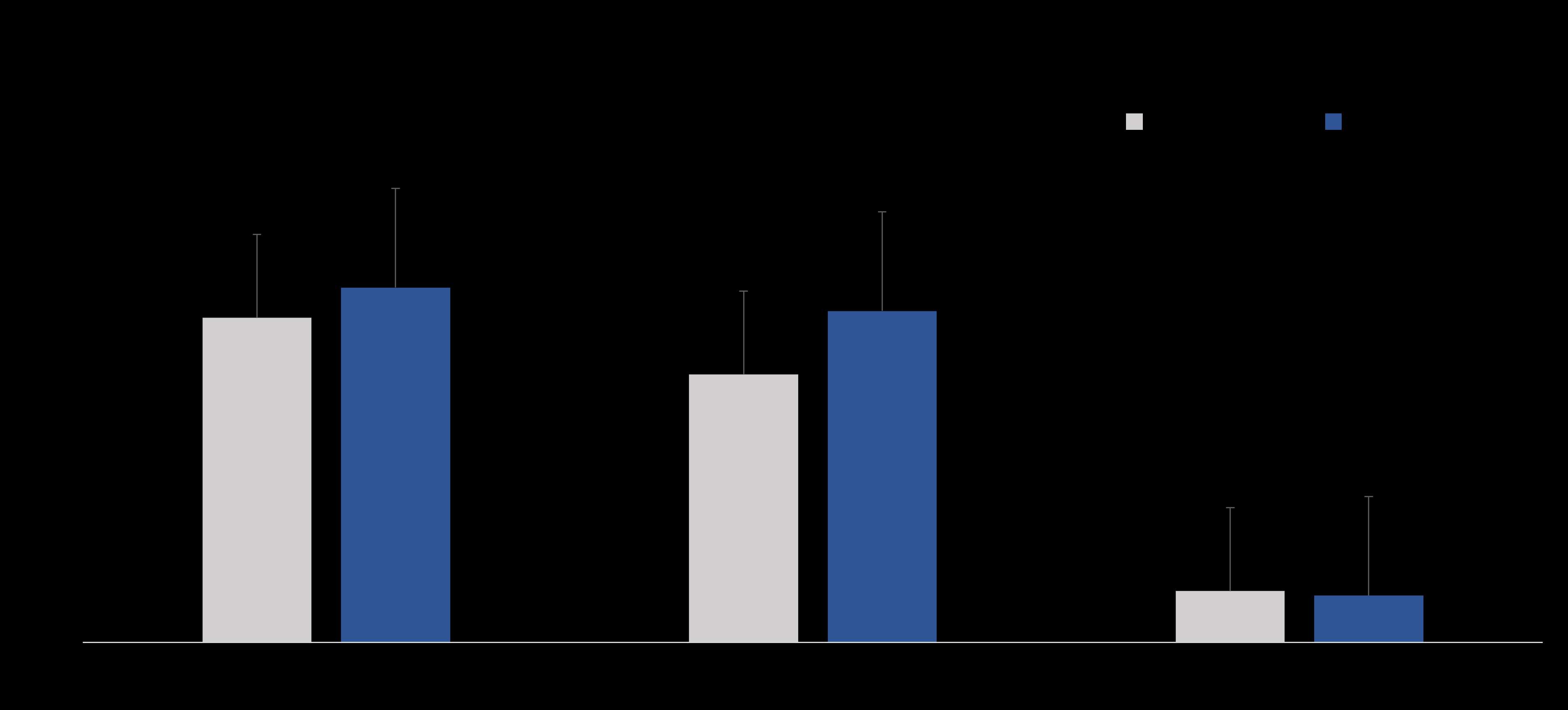
Figure 2#8708
High-Concentration Continuous Local Antibacterial Perfusion Therapy for Periprosthetic Knee Joint Infection
Yuki Suzuki - Hokkaido University - Sapporo, Japan
*Koji Iwasaki - Hokkaido University - Sapporo, Japan
Joutoku Zenta - Obihiro Kosei Hospital - Obihiro, Japan
Tomohiro Onodera - Hokkaido University - Sapporo, Japan
Masatake Matsuoka - Hokkaido University - Sapporo, Japan
Ryosuke Hishimura - Hokkaido University - Sapporo, Japan
Masanari Hamasaki - Hokkaido University - Sapporo, Japan
Eiji Kondo
Norimasa Iwasaki - Department of Orthopaedic Surgery Hokkaidao University School of Medicine
*Email: rockcape324@pop.med.hokudai.ac.jp
Introduction
Periprosthetic joint infections (PJIs) are among the most challenging pathologies following arthroplasty. Estimates show that PJI had a prevalence of 0.5%–1.9% after primary total knee arthroplasty (TKA) and 8%–10% after revision TKA [1-7]. PJI after TKA leads to higher morbidity, longer hospital stays, and higher health care costs [8, 9]. Therefore, improved outcomes for PJI after TKA is needed.
Managing PJI requires complex treatment strategies, including multiple surgical intervention and long-term antimicrobial treatment. Surgical treatments have consisted of debridement, intra venous antibiotics, and implant retention (DAIR) or the replacement of components through either a one- or two-stage procedure [10]. However, current findings show that up to 30% of cases develop recurrent infections [10]. Biofilm formation surrounding the implant [11] and insufficient antibiotic concentrations at the infected site via intravenous administration [12] have been identified as reasons for the low treatment success rate of PJI.
Recently, a novel method of continuous local antibiotic perfusion (CLAP) had been developed for difficult infectious pathologies, such as open fractures and osteomyelitis [13, 14]. This treatment aimed to maintain high local concentrations of antibacterial agents at the infection site. During CLAP therapy, high concentrations of antibiotics are continuously and directly injected into the local infected lesion, achieving sufficient localized antibacterial drug concentration levels. In addition, drainage could reduce the risk of excessive increase in serum concentrations of antibiotics and adverse effects. Although CLAP therapy is theoretically effective for PJI after TKA, no report has been available regarding its outcomes for PJI. Therefore, the current study aimed to evaluate the efficacy and safety of CLAP therapy for acute and chronic PJI after TKA.
Material and Methods
1. Patient recruitment
1-1. Informed consent
Institutional review board approval and written informed consent were obtained for this retrospective study. Patients were provided a detailed explanation that this treatment was an off-label use of antibacterial drugs. The treatment was approved by the Certified Clinical Research Review Board of our hospital as an off-label use of aminoglycoside antibiotics. The patients and/or their families were informed that the case data would be submitted for publication, to which they provided their consent.
1-2. Subjects
Patients diagnosed with PJI after TKA at our facility from May 2019 to June 2022 were retrospectively reviewed. The inclusion criterion was the ability to undergo 2 weeks of CLAP therapy. The exclusion criterion was a follow-up period of <6 months and those with severe chronic kidney disease (CKD) [estimated glomerular filtration rate (eGFR) <30 mL/min/1.73 m2] [15].
1-3. Diagnosis
PJI was diagnosed according to the guidelines established by the American Academy of Orthopaedic Surgeons and the International Consensus Group on PJI [16]. Patients who developed PJI after TKA were divided into two groups, (1) those with acute infection occurring within 1 month from the initial symptoms or diagnosis and (2) those with chronic infection occurring 1 month after the initial symptoms or diagnosis[10].
2. Treatment protocol
2-1. Surgical treatment
Patients with acute PJI underwent the combination therapy with DAIR and CLAP therapy. Meanwhile, patients with chronic infection underwent two-stage treatment. During the first stage, we removed all materials and performed aggressive debridement irrigation, and placement of cement spacers followed by CLAP therapy and systemic antibiotic administration. Systemic antibiotics, sensitive for the detected bacteria, were administered intravenously for 2 weeks, followed by at least 6 weeks of oral antibiotic administration after surgery. Antibiotics were discontinued after clinical signs suggested no infection. For those with chronic infection, the second-stage surgery (revision TKA) was undertaken after we confirmed no recurrence of infection after 4 weeks antibiotics withdrawal and at least 12 weeks after the first-stage surgery.
2-2. CLAP therapy
Equipment for CLAP therapy was introduced before wound closure during combined surgery. We have modified the previously reported CLAP method to make the treatment much easier and economical [13]. Two 5-Fr utility tubes (Atom Medical Corp, Tokyo, Japan) and a 15-Fr drain tube (Blake® drains, Ethicon, Raritan, NJ) were inserted into the main wound (Figure 1). Two utility tubes were connected to a syringe pump, after which a 15-Fr drainage tube was connected to the closed wound drainage system (J-VAC reservoirs™, Ethicon). A high concentration of gentamicin (GM; 60 mg/ saline 48 mL each) was continuously administered through the two inserted utility tube at 2.5 mg/h (2 mL/h) using a syringe pump. The total amount of GM was 120 mg/day at the start of CLAP. To perfuse the GM, the tips of the injection and drainage tubes were placed far apart from each other. The tip of the utility tube was placed where we suspected the main part of the infection was located, that is, mainly in the suprapatellar pouch and posterior part of tibiofemoral (TF) joint. The 15-Fr drain was placed far from the utility tube, mainly at the ventral side of TF joint space just posterior to the patella. The utility and drain tubes were fixed to the skin. The former was fixed using two horizontal mattress sutures surrounding the tube, whereas the latter was fixed by wrapping ends of the simple drain hole suture around the tube and tightly tied to prevent leakage.
CLAP was performed for 2 weeks similar to the original method [13, 14]. Serum GM concentrations were monitored three times a week after surgery and controlled under 2.0 µg/mL to prevent GM side effects. When GM concentrations exceed 2.0 µg/mL, the amount of GM was reduced by 80 mg/day. When side effects of GM were observed, such as ototoxicity and renal dysfunction, CLAP was withdrawn immediately. Ototoxicity was assessed by an otolaryngologist before and just after CLAP. During CLAP therapy, the knee was kept in extension and fixed with a knee brace. Weight bearing was prohibited for the involved extremity, and crutches or a wheelchair was used during CLAP.
3. Evaluation
3-1. Treatment and related outcomes
The follow-up details included clinical examination, C-reactive protein (CRP), and radiographic imaging every month for the first 3 months and then every 3 months thereafter. Successful treatment was defined the absence of clinical symptoms, such as swelling, redness and local heat, and negative CRP levels without recurrence at the final follow-up.
3-2. CLAP related outcomes
CLAP related outcomes such as any drain problem, serum and drain GM concentration, eGFR and side effects were observed. Data are presented as mean ± standard deviation, except for days [described as median (interquartile range)]
Results
Patient demographics
Among the included patients, 14 [5 males and 9 females with aged 69.5 ± 11.0 years old (range, 49–87 years old)] developed PJI after TKA during study period (Table 1). No patients were excluded and dropped out during follow-up. Among the 14 PJI patients, 5 and 9 were diagnosed with acute and chronic infection. The follow-up period was 491 days (304 to 632 days).
Treatment and related outcomes
CLAP therapy combined with DAIR was performed for all five cases with acute PJI, among whom one underwent revision TKA and the others underwent primary TKA. The period from final TKA was 62 days (29–204 days), whereas the period from diagnosis of PJI to surgery was 1 day (1 to 3 days). Four knees had a positive culture: methicillin-sensitive Staphylococcus aureus (MSSA) with methicillin-resistant Staphylococcus haemolyticus (one knee), Escherichia coli (one knee), Staphylococcus epidermidis (one knee), and Staphylococcus aureus (one knee). The follow-up period was 285 days (264–526 days). All cases with acute infection had successfully preserved implants.
Among the nine knees with chronic PJI, seven underwent two-stage revision surgery, whereas the remaining two were treated with DAIR as desired by the patients. However, these two cases failed to obtain infection control, followed by additional two-stage revision surgery. Seven of the nine patients with PJI who underwent two-stage revision surgery were developed PJI after primary TKA, whereas the remaining two knees who underwent DAIR and failed developed PJI after revision TKA. The period from TKA was 1,021 days (316–2,335 days), whereas the period from the first infection diagnosis to surgery (performing CLAP) was 63 days (17–91 days). All knees with chronic PJI after TKA had a positive culture with the following pathogens: Staphylococcus lugdunensis (three knees), MSSA (two knees), MSSA with Candida (one knee), Staphylococcus epidermidis (one knee) and Bacillus species (one knee). The follow-up period was 495 days (360 to 635 days). At the final follow-up period, all cases archived infection control. No loosening of the implant was observed, and all cases were able to walk and perform their daily routine at home.
CLAP outcomes and the side effect
All 14 patients were able to continue CLAP for 2 weeks. No drain-related problems, such as drain obstruction, leakage, or drain site infection, were observed. Serum GM concentrations reached maximum within a week in almost all cases. Four patients had serum GM concentrations over 2.0 µg/mL (Table 2). GM concentrations decreased in all cases after the GM dosage was reduced. The mean eGFR was 75.7 ± 31.2 mL/min/1.73 m2 preoperatively and 73.6 ± 24.0 mL/min/1.73 m2 at the final follow-up period. No side effects of GM, such as renal function failure or ototoxicity, were noted throughout the treatment and follow-up period.
Discussion
This is the first study to introduce CLAP therapy for patients with PJI after TKA. All cases with acute PJI were able to retain their implants with CLAP therapy combined with DAIR, whereas all cases with chronic PJI underwent revision surgery and achieved infection control. Although four cases had GM concentrations over 2.0 µg/mL, no side effects of GM, such as renal function failure or ototoxicity, were observed throughout the follow-up periods.
Radical DAIR has been the standard therapy for acute PJI [16, 17]. A previous review on DAIR for acute PJI after TKA reported success rates ranging from 16% to 60% [10]. DAIR was often combined with continuous irrigation or local antibiotics treatment, with success rates ranging from 73% to 88% [13, 18, 19]. In the present study, all acute PJI cases who underwent CLAP therapy combined with DAIR were able to retain their metal implants, indicating that CLAP therapy with DAIR may have superior effects on acute PJI over conventional DAIR.
Revision surgery remains the standard strategy for chronic PJI. Several reports on revision arthroplasties for chronic PJI have been available, with success rates ranging from 72%–93% [10, 20]. Among those who developed chronic infection after TKA, all seven cases who underwent two-stage revision surgery with CLAP therapy were able to successfully undergo revision TKA without recurrence of infection and any complications. These excellent results indicate that CLAP can be a novel and effective treatment approach for chronic PJI.
The increased success rate of CLAP combined therapy for PJI can be attributed to several reasons. First, CLAP can provide sufficient local concentration of GM while controlling the same through monitoring. Second, the effects of GM is concentration-dependent [21]. Third, high concentrations of GM are bactericidal for drug-resistant microbes, including methicillin-resistant Staphylococcus aureus (MRSA) [14]. Additionally, high concentrations of GM are also more effective against biofilms than other antibiotics [13, 14]. High positivity rates for MRSA cultures (24%–82%) and biofilm formation are characteristics of PJI [22-24]. MRSA has been associated with greater PJI treatment failure than other specimens have [25]. The antibiotic minimum inhibitory concentration of GM against MRSA was reported as 0.06-64 µg/mL, and minimum biofilm eradication concentration was 1-256µg/mL [26]. Our study showed the mean GM concentration of drainage fluid of 763.0 µg/mL, although the serum GM concentration was controlled under 2.0 µg/mL. These results indicated that CLAP therapy could provide the affordable GM concentration for MRSA and its biofilms.
Two cases failed to retain their implants with CLAP therapy combined with DAIR, both of which had chronic PJI. This indicates the limit of CLAP therapy combined with DAIR. Although CLAP therapy exhibits synergistic effects with DAIR for acute PJI and with staged revision surgery for chronic PJI, CLAP therapy combined with DAIR was not effective for chronic PJI. We believe that overconfidence in the effects of CLAP therapy might not be beneficial for the patients and that selecting adequate combined treatment with CLAP therapy depending on the status of the infection was key for achieving successful treatment of PJI.
Almost all cases reached maximum serum GM concentrations within 7 days, consistent with the findings reported in a previous study [21]. To prevent side effects, GM concentrations were monitored every 2 or 3 days. Consequently, there 5 cases (35.7%) had serum GM concentrations over 2.0 µg/mL temporarily. All cases with serum GM concentrations over 2.0 µg/mL had eGFR lower than 70 mL/min/1.73 m2 at the time of surgery (mean GFR was 50.1 ± 23.8 mL/min/1.73 m2). However, they did not suffer from ototoxicity and renal failure. Thus, frequent monitoring was important to prevent side effects of CLAP therapy. In addition, the amount of GM should be carefully controlled and may be reduced for cases with preoperative low eGFR.
The present study has some limitations worth noting. First, our sample size was quite small, and our follow-up period was relatively short. The incidence of PJI at our institution was very low due to several prophylactic treatments. Thus, obtaining samples with a long follow-up period was difficult [27]. Second, background characteristics varied among cases, making it difficult to compare therapeutic effects. However, this has been the first study to report on the outcomes and risks of CLAP therapy for both acute and chronic PJIs. The success rates and GM concentrations during CLAP therapy observed in our study can provide important information for the future treatment of PJI.
Conclusions
In conclusion, despite the need to monitor for ototoxicity and renal failure, CLAP therapy was safe and effective for the treatment of acute and most chronic PJIs.
References
1. Wilson MG, Kelley K, Thornhill TS. Infection as a complication of total knee-replacement arthroplasty. Risk factors and treatment in sixty-seven cases. J Bone Joint Surg Am 72(6): 878, 1990
2. Jamsen E, Huhtala H, Puolakka T, Moilanen T. Risk factors for infection after knee arthroplasty. A register-based analysis of 43,149 cases. J Bone Joint Surg Am 91(1): 38, 2009
3. McCleery MA, Leach WJ, Norwood T. Rates of infection and revision in patients with renal disease undergoing total knee replacement in Scotland. J Bone Joint Surg Br 92(11): 1535, 2010
4. Phillips JE, Crane TP, Noy M, Elliott TS, Grimer RJ. The incidence of deep prosthetic infections in a specialist orthopaedic hospital: a 15-year prospective survey. J Bone Joint Surg Br 88(7): 943, 2006
5. Bozic KJ, Kurtz SM, Lau E, Ong K, Chiu V, Vail TP, Rubash HE, Berry DJ. The epidemiology of revision total knee arthroplasty in the United States. Clin Orthop Relat Res 468(1): 45, 2010
6. Kurtz SM, Lau E, Schmier J, Ong KL, Zhao K, Parvizi J. Infection burden for hip and knee arthroplasty in the United States. J Arthroplasty 23(7): 984, 2008
7. Kurtz SM, Ong KL, Lau E, Bozic KJ, Berry D, Parvizi J. Prosthetic joint infection risk after TKA in the Medicare population. Clin Orthop Relat Res 468(1): 52, 2010
8. Klouche S, Sariali E, Mamoudy P. Total hip arthroplasty revision due to infection: a cost analysis approach. Orthop Traumatol Surg Res 96(2): 124, 2010
9. Osmon DR, Berbari EF, Berendt AR, Lew D, Zimmerli W, Steckelberg JM, Rao N, Hanssen A, Wilson WR, Infectious Diseases Society of A. Diagnosis and management of prosthetic joint infection: clinical practice guidelines by the Infectious Diseases Society of America. Clin Infect Dis 56(1): e1, 2013
10. Gehrke T, Alijanipour P, Parvizi J. The management of an infected total knee arthroplasty. Bone Joint J 97-B(10 Suppl A): 20, 2015
11. Macias-Valcayo A, Aguilera-Correa JJ, Broncano A, Parron R, Aunon A, Garcia-Canete J, Blanco A, Esteban J. Comparative In Vitro Study of Biofilm Formation and Antimicrobial Susceptibility in Gram-Negative Bacilli Isolated from Prosthetic Joint Infections. Microbiol Spectr 10(4): e0085122, 2022
12. Le Vavasseur B, Zeller V. Antibiotic Therapy for Prosthetic Joint Infections: An Overview. Antibiotics (Basel) 11(4), 2022
13. Himeno D, Matsuura Y, Maruo A, Ohtori S. A novel treatment strategy using continuous local antibiotic perfusion: A case series study of a refractory infection caused by hypervirulent Klebsiella pneumoniae. J Orthop Sci 27(1): 272, 2022
14. Maruo A, Oda T, Mineo R, Miya H, Muratsu H, Fukui T, Oe K, Kuroda R, Niikura T. Continuous local antibiotic perfusion: A treatment strategy that allows implant retention in fracture-related infections. J Orthop Surg (Hong Kong) 30(2): 10225536221111902, 2022
15. Chen TK, Knicely DH, Grams ME. Chronic Kidney Disease Diagnosis and Management: A Review. JAMA 322(13): 1294, 2019
16. Parvizi J, Tan TL, Goswami K, Higuera C, Della Valle C, Chen AF, Shohat N. The 2018 Definition of Periprosthetic Hip and Knee Infection: An Evidence-Based and Validated Criteria. J Arthroplasty 33(5): 1309, 2018
17. Della Valle C, Parvizi J, Bauer TW, Dicesare PE, Evans RP, Segreti J, Spangehl M, Watters WC, 3rd, Keith M, Turkelson CM, Wies JL, Sluka P, Hitchcock K, American Academy of Orthopaedic S. Diagnosis of periprosthetic joint infections of the hip and knee. J Am Acad Orthop Surg 18(12): 760, 2010
18. Liu CW, Kuo CL, Chuang SY, Chang JH, Wu CC, Tsai TY, Lin LC. Results of infected total knee arthroplasty treated with arthroscopic debridement and continuous antibiotic irrigation system. Indian J Orthop 47(1): 93, 2013
19. Royo A, Bertrand ML, Ramos L, Fernandez-Gordillo F, Guerado E. Is there still a place for continuous closed irrigation in the management of periprosthetic total knee infection? Open Orthop J 7: 205, 2013
20. Maltos AL, Portari GV, Saldanha JC, Bernardes Junior AG, Pardi GR, da Cunha DF. Scurvy in an alcoholic malnourished cirrhotic man with spontaneous bacterial peritonitis. Clinics (Sao Paulo) 67(4): 405, 2012
21. Selby NM, Shaw S, Woodier N, Fluck RJ, Kolhe NV. Gentamicin-associated acute kidney injury. QJM 102(12): 873, 2009
22. Mittal Y, Fehring TK, Hanssen A, Marculescu C, Odum SM, Osmon D. Two-stage reimplantation for periprosthetic knee infection involving resistant organisms. J Bone Joint Surg Am 89(6): 1227, 2007
23. Parvizi J, Azzam K, Ghanem E, Austin MS, Rothman RH. Periprosthetic infection due to resistant staphylococci: serious problems on the horizon. Clin Orthop Relat Res 467(7): 1732, 2009
24. Salgado CD, Dash S, Cantey JR, Marculescu CE. Higher risk of failure of methicillin-resistant Staphylococcus aureus prosthetic joint infections. Clin Orthop Relat Res 461: 48, 2007
25. Kurd MF, Ghanem E, Steinbrecher J, Parvizi J. Two-stage exchange knee arthroplasty: does resistance of the infecting organism influence the outcome? Clin Orthop Relat Res 468(8): 2060, 2010
26. Mottola C, Matias CS, Mendes JJ, Melo-Cristino J, Tavares L, Cavaco-Silva P, Oliveira M. Susceptibility patterns of Staphylococcus aureus biofilms in diabetic foot infections. BMC Microbiol 16(1): 119, 2016
27. Parvizi J, Saleh KJ, Ragland PS, Pour AE, Mont MA. Efficacy of antibiotic-impregnated cement in total hip replacement. Acta Orthop 79(3): 335, 2008
Figure captions
Figure 1. Scheme of the drain and tubes during continuous local antibiotic perfusion. The tip of the two were placed in the suprapatellar pouch and posterior part of tibiofemoral joint. The drainage tube was placed at the ventral side of the insert.
Table
Table 1. Demographic and surgical details of patients with periprosthetic joint infection (PJI) after total knee arthroplasty (TKA) treated by continuous local antibiotics perfusion (CLAP) combination.
|
Case
|
Age
(years)
|
Sex
|
Affected side
|
Comorbidity
|
Acute/chronic infection
|
Previous TKA surgery
(primary/revision)
|
Time from TKA (days)
|
Culture
|
Time from
infection diagnosis to surgery
|
Surgery
|
CRP negative achievement (days from surgery)
|
Time to 2nd surgery (days)
|
Follow-up period (days)
|
Success / failure
|
|
1
|
86
|
F
|
R
|
HT
|
Acute
|
Primary
|
29
|
-
|
0
|
DAIR, insert exchange
|
92
|
|
1189
|
Success
|
|
2
|
77
|
M
|
L
|
-
|
Acute
|
Primary
|
204
|
MSSA
|
11
|
DAIR, insert exchange
|
64
|
|
526
|
Success
|
|
3
|
77
|
F
|
L
|
AP, HT, DLP, DM, asthma, GERD
|
Acute
|
Primary
|
62
|
MSSA, Staphylococcus haemolyticus (MRS)
|
1
|
DAIR, insert exchange
|
71
|
|
264
|
Success
|
|
4
|
87
|
M
|
R
|
RA, HT, ARF, prosthesis cancer
|
Acute
|
Primary
|
623
|
Escherichia coli
|
1
|
DAIR, insert exchange
|
126
|
|
285
|
Success
|
|
5
|
70
|
F
|
R
|
DM, HT, DLP
|
Acute
|
Revision
|
21
|
Staphylococcus epidermidis
|
3
|
DAIR, insert exchange
|
46
|
|
193
|
Success
|
|
6
|
49
|
F
|
L
|
RA
|
Chronic
|
Primary
|
4113
|
Staphylococcus epedermidis
|
416
|
Implant removal, cement spacers, debridement
|
14
|
180
|
635
|
Success
|
|
7
|
61
|
F
|
R
|
-
|
Chronic
|
Primary
|
1021
|
MSSA
|
17
|
Implant removal, cement spacers, debridement
|
32
|
168
|
495
|
Success
|
|
8
|
73
|
F
|
L
|
DM, HT, DLP
|
Chronic
|
Primary
|
2616
|
MAC
|
27
|
Implant removal, cement spacers, debridement
|
27
|
|
448
|
Success
|
|
9
|
69
|
F
|
L
|
RA, hypothyroidism
|
Chronic
|
Primary
|
204
|
MSSA, Candida
|
91
|
Implant removal, cement spacers, debridement
|
159
|
112
|
1215
|
Success
|
|
10
|
71
|
F
|
R
|
-
|
Chronic
|
Primary
|
482
|
MSSA
|
72
|
Implant removal, cement spacers, debridement
|
168
|
148
|
1196
|
Success
|
|
11
|
74
|
M
|
L
|
HT, DLP
|
Chronic
|
Revision
|
1647
|
Staphylococcus lugdunensis
|
63
|
Implant removal, cement spacers, debridement
|
29
|
133
|
622
|
Success
|
|
12
|
58
|
F
|
R
|
Sjogren disease, DM, RA, asthma, HT, DLP
|
Chronic
|
Revision
|
316
|
Staphylococcus epidermidis
|
193
|
Implant removal, cement spacers, debridement
|
102
|
105
|
264
|
Success
|
|
13
|
70
|
M
|
L
|
HT, lung cancer, lymphedema, pulmonary fibrosis
|
Chronic
|
Primary
|
1844
|
Staphylococcus lugdunensis
|
5
|
DAIR, insert exchange
|
×
|
|
486
|
Failure
|
| |
71
|
M
|
L
|
Chronic
|
Primary
|
|
Staphylococcus lugdunensis
|
13
|
Implant removal, cement spacers, debridement
|
62
|
|
620
|
Success
|
|
14
|
51
|
M
|
L
|
-
|
Chronic
|
Primary
|
247
|
Staphylococcus hominis
|
5
|
DAIR, insert exchange
|
71
|
|
360
|
Failure
|
| |
52
|
M
|
L
|
Chronic
|
Primary
|
|
Bacillus species
|
28
|
Implant removal, cement spacers, debridement
|
56
|
|
420
|
Success
|
HT, hypertension; AP, angina pectoris; DLP, dyslipidemia; DM, diabetes mellitus; GERD, gastroesophageal reflux disease; ARF, acute renal failure; RA, rheumatoid arthritis; MSSA, methicillin-sensitive Staphylococcus aureus; MRS, methicillin-resistant Staphylococcus; MAC, Mycobacterium avium–intracellulare complex; DAIR, debridement, antibiotics and implant retention; CRP, c-reactive protein
Table 2. Blood test Results for renal function and gentamicin (GM) concentration.
|
Case
|
Preoperative eGFR(mL/min/1.73 m2)
|
Final eGFR
(mL/min/1.73 m2)
|
Maximum serum GM concentration (µg/mL)
|
The day serum GM concentration reached maximum (day)
|
Maximum drain GM concentration (µg/mL)
|
Success/failure
|
|
1
|
72.4
|
57.0
|
0.5
|
7
|
590.0
|
Success
|
|
2
|
51.7
|
71.9
|
0.6
|
6
|
853.5
|
Success
|
|
3
|
92.0
|
96.4
|
1.2
|
3
|
840.0
|
Success
|
|
4
|
7.0
|
30.2
|
2.1
|
3
|
80.0
|
Success
|
|
5
|
118.0
|
99.0
|
1.0
|
7
|
730.0
|
Success
|
|
6
|
131.4
|
127.1
|
1.0
|
3
|
1180.0
|
Success
|
|
7
|
68.3
|
81.5
|
0.6
|
1
|
303.0
|
Success
|
|
8
|
87.4
|
70.6
|
0.3
|
7
|
390.0
|
Success
|
|
9
|
99.5
|
70.2
|
1.0
|
14
|
1090.0
|
Success
|
|
10
|
75.1
|
69.9
|
2.2
|
7
|
567.0
|
Success
|
|
11
|
56.4
|
51.1
|
2.8
|
1
|
730.0
|
Success
|
|
12
|
118.6
|
109.2
|
0.9
|
3
|
1120.0
|
Success
|
|
13
|
45.3
|
46.8
|
3.4
|
3
|
2050.0
|
Failure
|
| |
42.8
|
51.9
|
1.9
|
1
|
315.3
|
Success
|
|
14
|
79.0
|
71.8
|
1.0
|
1
|
390.0
|
Failure
|
| |
66.8
|
72.7
|
0.7
|
5
|
979.2
|
Success
|
eGFR, estimated glomerular filtration rate
Figures
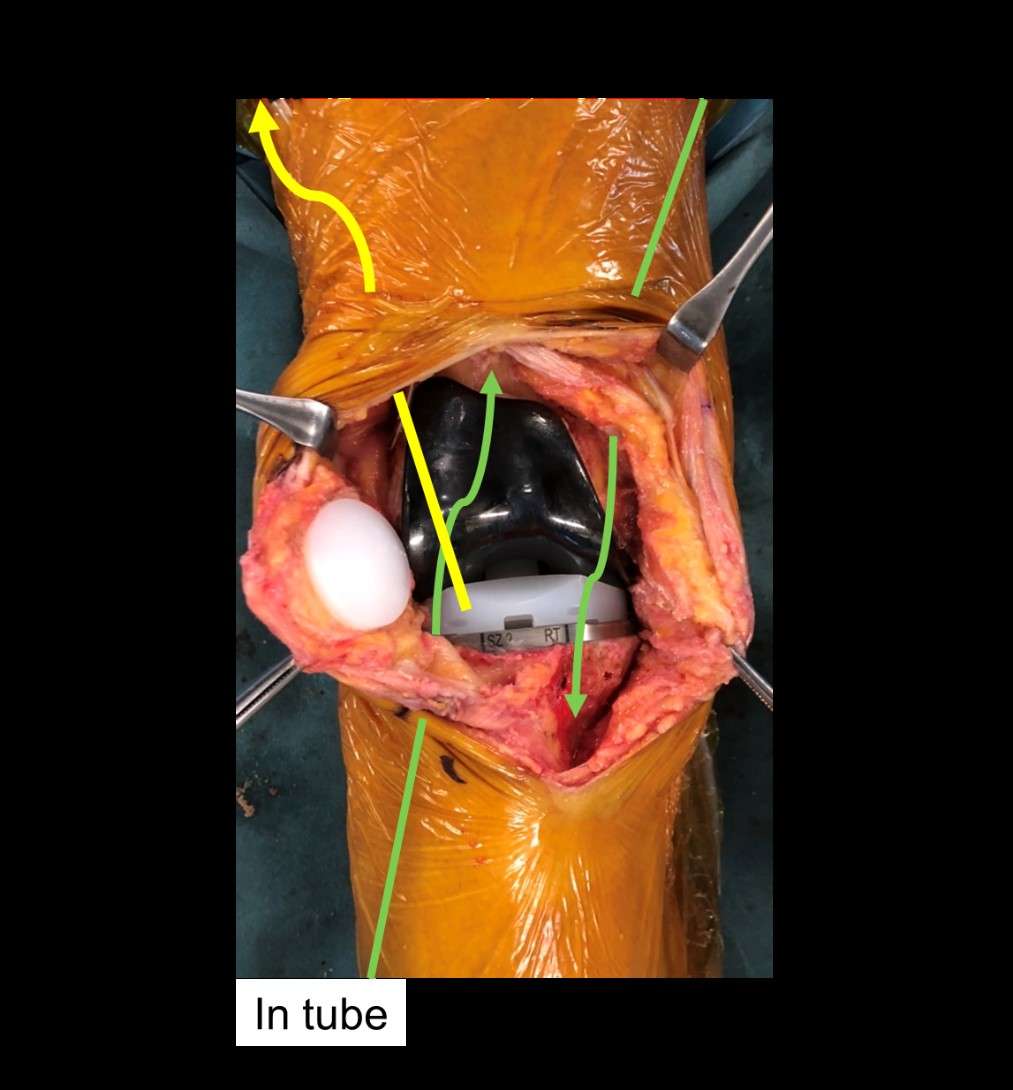
Figure 1#8764
Operating Room Airborne Microbial Load; Non-Scrubbed Staff Apparel Matters
Sabir K. Ismaily - UTHealth - Houston, USA
*David Rodriguez - University of Texas - Houston, USA
Hugh Jones - UT Health Science Center at Houston - Houston, USA
Andrey Zuskov - UT Health Houston - Houston, USA
Brian Crowley - UT Health Houston - Houston, USA
Kartik I. Reddy - UTHealth - Houston, USA
Humberto Aparicio - McGovern Medical School - Houston, USA
*Email: david.rodriguezquintana@uth.tmc.edu
Introduction
Periprosthetic joint infection is a leading cause of total joint replacement failure. In a previous study, we found the implanting stage of a primary total knee arthroplasty to be the most at risk for contamination. Statistical correlation was also found between the level of contamination, concentrations of airborne particles and the number of staff present. In this study, we focused on the apparel of non-scrubbed operating room (OR) staff to elucidate their contribution to the airborne microbial load in the OR.
Methods
We compared standard OR attire consisting of laundered hospital scrubs and jacket to disposable coveralls worn over scrubs. For one hour, four lab members conducted standardized maneuvers in an OR that simulate movements of the nurse, anesthesiologist, implant representative, and entering/exiting staff with an airborne particle counter and agar plates throughout the OR. After one hour, the staff changed apparel and repeated the test. Each session of both phases consisted of two tests by the same individual on the same day. The order of testing of each group was also alternated for each subsequent session.
Results
There was a 78% reduction in the settle rate of viable particles collected during the testing of coveralls (58.9 ±21 CFUs/m2/hr) compared to standard OR attire (269.1 ±24 CFUs/m2/hr) p=0.004. The concentration of airborne particles for the coveralls group was also 78% lower at 1053 ±75 particles/m3 compared to 4812 ±538 particles/m3 in the standard OR attire group, p=0.004.
Conclusion
Changing OR staff apparel from standard scrubs and jacket to coveralls in an OR with four staff members yielded a reduction in CFUs of 78% (p=0.004) as well as a similar reduction in particles measured of 78% (p=0.004). These results suggest that the shed rate of viable particles from non-scrubbed OR staff can be significantly reduced by changing their apparel.
#8668
X-Ray Visualized Biosensors for Implant Infection
*John DesJardins - Clemson University - Clemson, USA
Jeffery Anker - Clemson University - Clemson, USA
Caleb Behrend - OrthoArizona - Glendale, USA
Lindsey Calcutt - Aravis BioTech, LLC - Greenville, USA
Rong Wang - Clemson University - Clemson, USA
Josh Finkel - Aravis BioTech, LLC - Greenville, USA
*Email: jdesjar@clemson.edu
A common complication of implant surgeries is post-surgery infections. These may be caused by bacterial contamination during surgery or subsequent adhesion of microorganisms onto implant surface. Delayed diagnosis would lead to reduced function, increased morbidity and may require device removal. Therefore, early detection of infections is important for successful management of hip infections.
We have developed a pH responsive hydrogel sensor to measure pH levels in the joint fluid in order to detect, monitor, and study infection. This poly (acrylic acid)-based hydrogel swells at higher pH and shrinks at lower pH. The length changes of the hydrogel was determined by distance changes between an embedded radio-dense tantalum bead and a metal pinning wire in the two ends of the hydrogel. The pH sensor could be attached to prosthetic hips prior to implantation, enables routine radiographs to give functional chemical information.
The inter-observer reliability result shows measured pH values fit well with the actual pH values, the average inter-observer precision is 0.03 pH unit, and the accuracy is 0.08 pH unit.
Synovial fluid contains multiple infection biomarkers such as pH, lactate levels, C-reactive protein, α-defensin etc. The conventional procedure requires joint aspiration which is painful and is impractical for routine screening or serial monitoring during treatment. The developed X-ray based sensor provides painless, non-invasive and inexpensive detection.
In preliminary work, the hydrogel sensor has successfully been attached to the neck of a femoral hip stem. The sensor holder and the extra screws can fix the hydrogel position and prevent potential displacement over time. In this design, the hydrogel slides into the holder until the rod on the barb at the position of pH 7 and is pined by a metal wire from outside of the holder. Therefore, the sensor can be read without manually attaching radio dense markers and the hydrogel length is reproducible. Moreover, it can be miniaturized for subsequent animal study while maintaining the hydrogel’s reversibility.
Our preliminary results show the feasibility of measuring local pH in synovial fluid at hip implants. When used in combination with medical implants, this system will enable non-invasive early detection and monitoring of hip infection using plain radiography (X-ray imaging). Future work will improve the hydrogel sensor design to improve the sensor reproducibility and increase the imaging accuracy.
Figures
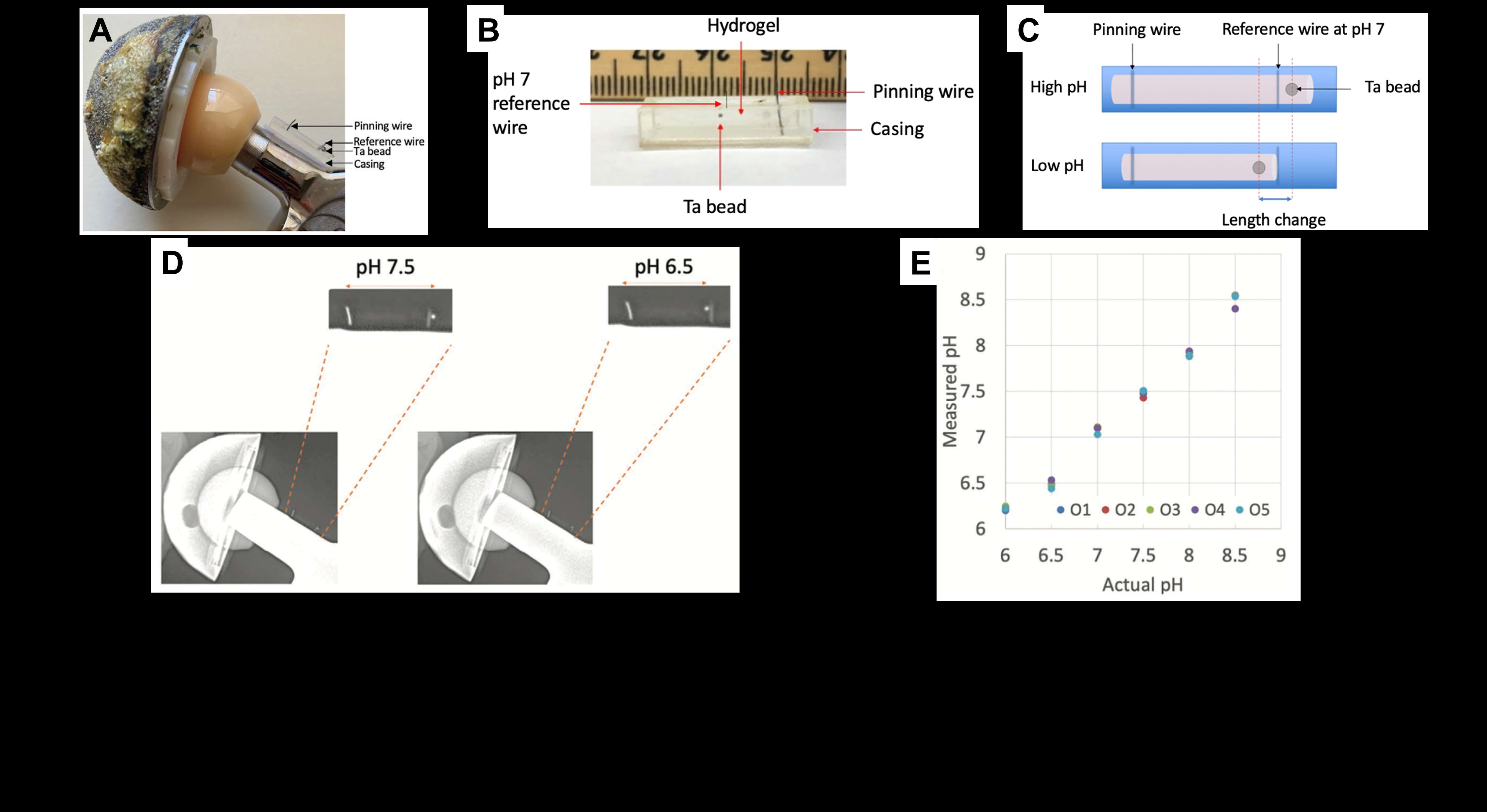
Figure 1#8749
Robotic Surgery With Functional Evaluation 3D Software for Total Hip Replacement Mitigate Spinopelvic Mobility Effect on Impingement
*Andrea Marcovigi - AOU Policlinico di Modena - Modena, Italy
Fabio Catani - University of Modena and Reggio Emilia - Modena, Italy
Francesco Zambianchi - University of Modena and Reggio-Emilia - Modena, Italy
Filippo Selleri - University of Modena and Reggio Emilia - modena, Italy
*Email: dr.marcovigi@gmail.com
Introduction:
The aim of the present study is to investigate whether the use of a 3D software for Functional Component Positioning Assessment (FCPA) in robotic arm assisted THR could influence the effect of spinopelvic mobility on prosthetic and bony impingement.
A secondary aim is to evaluate the reproducibility of prosthetic and bone impingement patterns with final component positioning.
Methods
This is a retrospective, observational study. 136 consecutive patients treated for Robotic Arm-Assisted THA with the same implant type were searched from surgical registry.
Sacral slope (SS) was measured from preoperative lateral pelvis X-rays in sitting and standing position, then the difference was calculated for every patient and population was divided in 3 groups: Stiff (?SS < 10°), Normal (10°<?SS<30°), Hypermobile (?SS>30°). All sacral slope data were loaded into the robotic software to set pelvis orientation.
FCPA virtual 3D tool (fig.1) allows the surgeon to simulate prosthetic hip motion in flexion/extension, adduction/abduction and internal/external rotation, evaluating also cup inclination and version variation during pelvic tilting from sitting to standing positions. This way it is possible to assess preoperatively any impingement occurring during hip motion.
Acetabular component positioning was initially set at 40° of inclination and 20° of anteversion with femoral stem position planned as close as possible to 15° of anteversion, compatible with meta-epiphyseal femoral version, to better achieve the optimal combined version.
Cup and stem anteversion were then adjusted according to impingement determinations of FCPA. The aim during implant planning via FCPA was to prevent any bony or prosthetic impingement in two set position of the hip. In the first one, with the pelvis in standing position, the hip was in 15° of extension and 15° of external rotation with neutral abduction; in the second one, with the pelvis in sitting position, the hip was in 90° of flexion and 35° of internal rotation with neutral abduction.
Postoperatively, FCPA was performed virtually both with preoperative and final components position, to evaluate bone or prosthetic impingment that might occur during 5 routine motor activities of daily living: maximum flexion, maximum extension, rising from a low sitting, shoe tying, rolling over. For all 5 motor tasks, type of impingement was classified as follows: Prosthesis on Prosthesis (PP) when contact occurred between stem neck and liner, Prosthesis on Bone (PB) when contact occurred between stem neck and pelvic bone and Bone on Bone (BB).
Results:
In rise from seated position impingement occurred in 9% of cases, in 7% of cases it was due to contact between bones.
In seated shoe tying impingement occurred in 6% of cases, in 5% of cases it was due to contact between bones.
In rolling over impingement occurred in 35% of cases, in 20% it was due contact between prosthetic components. (see complete dataset in figure 2)
Spinopelvic mobility did not correlate neither with bony nor with prosthetic impingement.
Conclusion:
Robotic arm assisted surgery in THA using a FCPA software appears to mitigate spinopelvic mobility effect on impingement
Figures
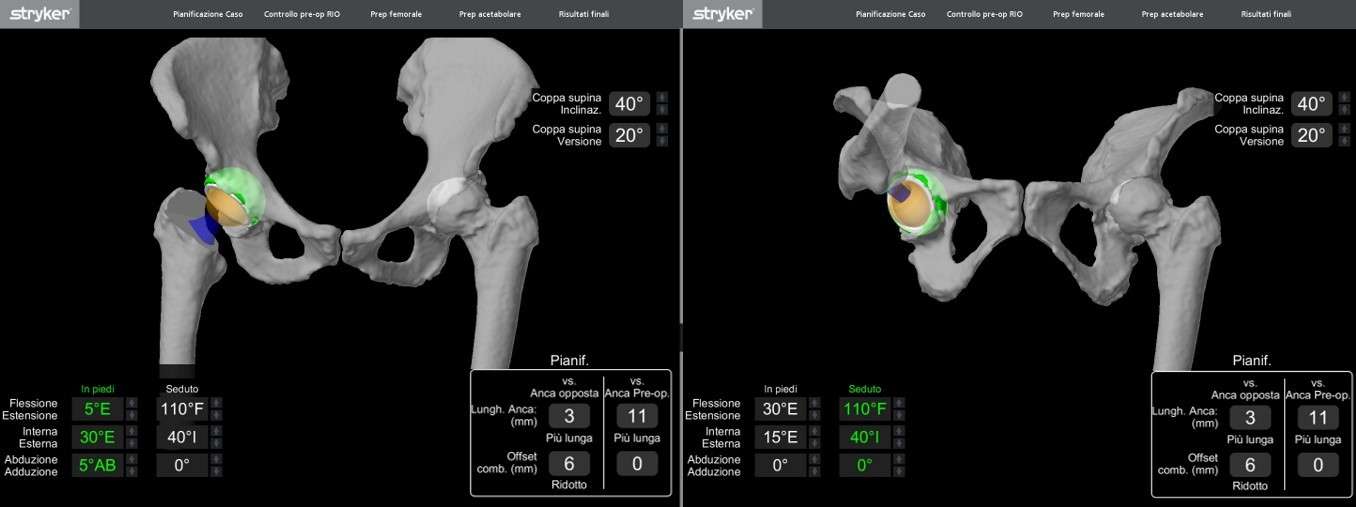
Figure 1
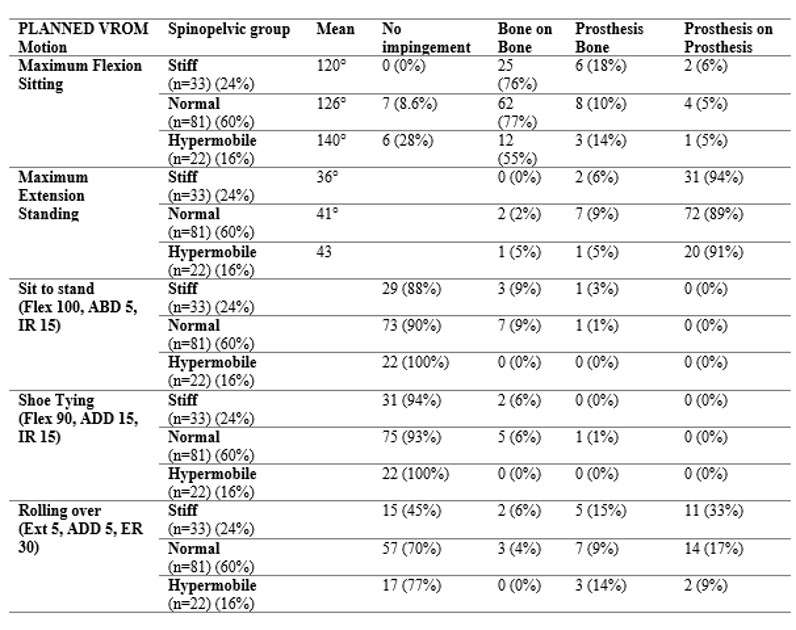
Figure 2#8340
Automated Digital Templating of Component Sizing Is Accurate in Robotic Total Hip Arthroplasty When Compared to Predicate Software
Graham Buchan - Cleveland Clinic Lerner College of Medicine - Cleveland, United States of America
Christian Hecht - Cleveland Clinic - Cleveland, USA
Sebastian Rodriguez-Elizalde - Humber Hospital - Toronto, Canada
Tamon Kabata - Kanazawa university - kanazawa, Japan
*Atul Kamath - Cleveland Clinic - Cleveland, USA
*Email: kamatha@ccf.org
Introduction: Accurate pre-operative templating of prosthesis components is an essential factor in successful total hip arthroplasty (THA), including robotically-assisted THA (RA-THA) techniques. While pre-operative planning for RA-THA can be performed with established predicate digital planners, a novel, robotic-optimized digital planner has been designed specifically to integrate with a fluoroscopy-based RA-THA system. Our present study sought to validate the accuracy of a novel, robotic-optimized THA planning software compared to a predicate THA planner for component sizing.
Methods: This study included a consecutive series of 199 patients who received manual THA (mTHA) and fluoroscopy-based RA-THA at a single institution. All cases were templated using a predicate pre-operative templating software. For RA-THA cases, the novel robotic-optimized pre-operative planner software was also used for templating. The differences between templated and implanted acetabular cup, femoral head, and stem component sizes were compared based on matching within 1, 2, and ≥3 sizes. Differences in templated and implanted femoral stem implant geometry were also compared.
Results: The robot-optimized pre-operative RA-THA plans demonstrated equivalent accuracy to that of predicate pre-operative plans for both RA-THA and mTHA cases (Table 1). Specifically, the robot-optimized pre-operative RA-THA plans and predicate pre-operative plans for both RA-THA and mTHA cases demonstrated equivalently high proportions of templated acetabular cups (90.4 vs. 86.8 vs. 82.8; p=0.421), femoral stems (76.0 vs. 65.1 vs. 67.7; p=0.096), and femoral heads (91.3 vs. 96.2 vs. 88.2; p=0.302) within +/-1 size of implanted components (Figure 1). No significant differences were detected in the proportion of matching templated and implanted stem geometry across the study cohorts (91.4 vs. 96.2 vs. 93.3; p=0.344) (Table 2).
Conclusion: The findings of this study demonstrate that this novel, robot-optimized pre-operative THA planner is highly accurate in predicting implanted component sizes. There was an 11% improvement in matching templated and implanted femoral stem components within +/-1 size, when compared to a predicate software. The direct linking of robotic planning software to intra-operative execution offers further distinct advantages over predicate software, including the direct linking of robotic planning software to intra-operative execution. This study can be used to justify the use of this robot-optimized THA templating, in lieu of predicate software, for the pre-operative planning of fluoroscopy-based RA-THA.
Keywords: Digital templating, Total hip arthroplasty (THA), Robotic-THA, Pre-operative planning
Figures
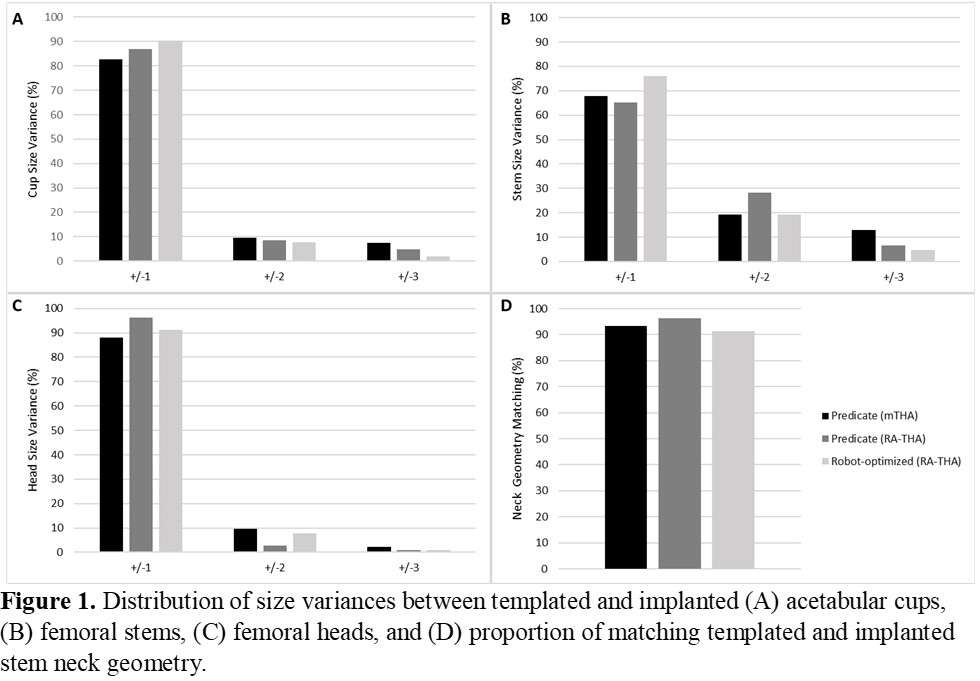
Figure 1
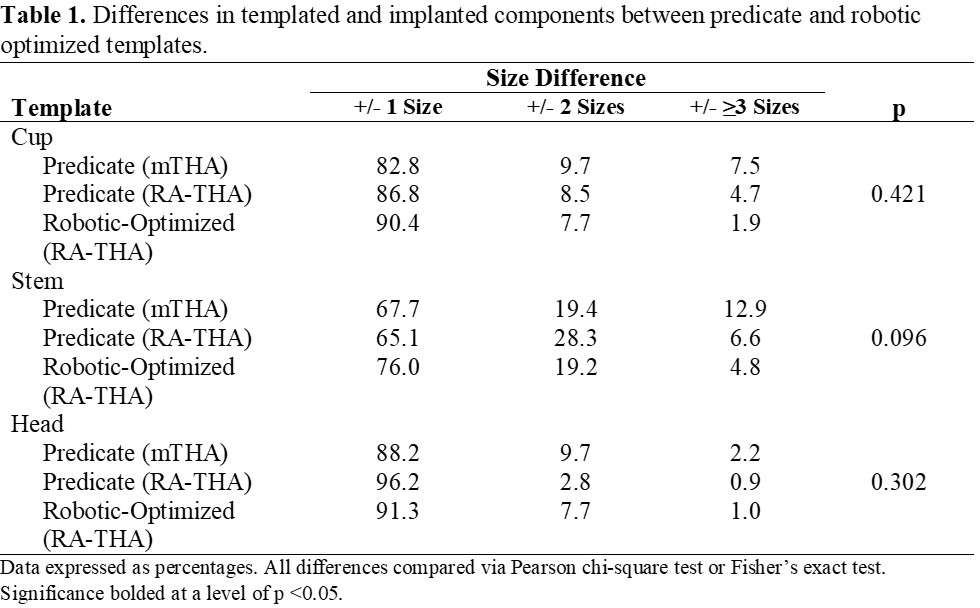
Figure 2
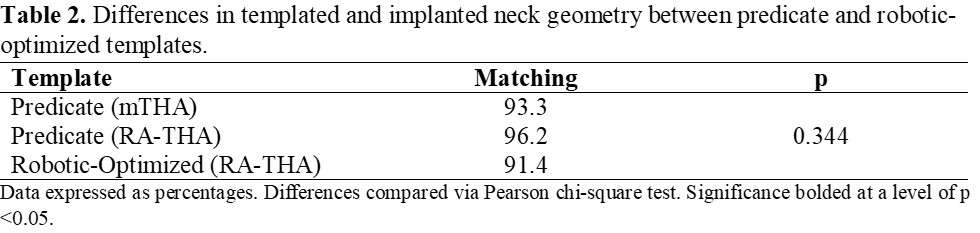
Figure 3#8790
Accuracy of Implant Placement for Robotic Assisted THA Based on a Multi-Center Cohort
Ajay Lall - American Hip Institute - Chicago, USA
Jonathan Vigdorchik - Hospital for Special Surgery - New York, USA
Robert Marchand - Ortho Rhode Island - South County, USA
Matthew Thompson - Stryker - Fort Lauderdale, USA
Seth A. Jerabek - Hospital for Special Surgery - New York, USA
Benjamin Domb - American Hip Institute - Des Plaines, USA
*Laura Yanoso-Scholl - Stryker Orthopaedics - USA
Geoffrey H Westrich - Hospital for Special Surgery - New York, USA
*Email: Laura.Scholl@stryker.com
Accuracy of implant placement for robotic assisted THA based on a multi-center cohort
Introduction: Image based robotic assisted total hip arthroplasty (THA) now allows a more accurate placement of the acetabular and femoral components based upon a preoperative plan that considers spinopelvic alignment and an intraoperative assessment of impingement modelling. The aim of this study was to prospectively evaluate implant placement to plan accuracy for robotic-assisted total hip replacement.
Methods: A prospective, multi-center study was performed on 54 consented patients who received an image based robotic-assisted THA along with intraoperative functional pelvic tilt and virtual ROM assessments with impingement modelling. A preoperative CT-scan was required for robotic surgery and final planned implant placement was recorded. CT-scans and patient reported outcomes (PROMS) were collected at 6-week follow-up. Accuracy measurements were performed using 3D analysis software to segment and evaluate preoperative and postoperative CT-scans to measure difference between final implant plan and postoperative placement. A Mann Whitney U test, with 95% confidence interval, was performed to assess statistical difference between planned and postoperative implant placement.
Results: In comparing the final implant plan to the 6 week postoperative CT scan for accuracy, there was a mean difference of only 1.7? (SD 1.2) for cup inclination and 2.0? (SD 1.3) for cup version. Comparing postoperative stem version to intraoperative broach version, stem version had a mean difference of only 1.9? (SD 1.6?) in anteversion. The difference in plan vs measured offset was 1.80mm (SD 1.40). For all variables, there was no statistical difference between planned and postoperative measurements. Center-of-head-rotation had a mean error of 1.48mm (SD 0.97) in medial-lateral axis, 1.32mm (SD 1.08) in anterior-posterior axis, and 1.37mm (SD 1.18) in superior-inferior axis. Leg length discrepancy was targeted to be corrected to be equal to the contralateral hip. It was measured to be restored to within 0.1mm (SD 2.7) compared to contralateral hip?. At 6-week follow-up, patients had mean 83.0 ± 10.6 HOOS, 81.3 ± 12.5 HHS, and no reported adverse events.
Discussion/Conclusion: Consideration of pelvic tilt and virtual ROM influenced implant placement plan, specifically for patients with stiff spinopelvic pathology. Robotic-assisted THA aided the surgeon in achieving accurate functional placement to plan for acetabular and femoral implants which resulted in excellent early patient outcomes. The accuracy of implant placement to plan based upon a CT scan analysis postoperatively may provide the surgeon confidence that they can achieve the values intraoperatively planned.
#8599
Predictability of Implant Templating and Positioning With a Robotic-Assisted Direct Anterior Approach
Joseph Nessler - St. Cloud Orthopedics - Sartell, USA
*Laura Yanoso-Scholl - Stryker Orthopaedics - USA
*Email: Laura.Scholl@stryker.com
INTRODUCTION: Direct anterior approach (DAA) continues to grow, with 56.2% of THA surgeons reporting they perform DAA. Some studies have suggested increased risk of femoral fracture. Fluoroscopy is often used to better visualize implant placement/sizing intra-operatively. 3D CT scans for pre-operative templating and intra-operatively guiding implant placement in robotic-assisted THA may eliminate the need for fluoroscopy. The purpose of this study was to evaluate predictability of templating and sizing for robotic-assisted THA DAA without use of fluoroscopy.
METHODS: 50 robotic-assisted DAA total hips were performed without use of fluoroscopy. Templating/sizing were performed using a pre-operative 3D CT. A virtual ROM tool was used to assess impingement and, if needed, implants were adapted to minimize impingement. A digital ruler feature was used to plan femoral neck resection (Figure 1). Landmarks on the bone were identified and using the digital ruler the surgeon localized planned level of neck osteotomy. Neck cut view on the robotic display helped the surgeon reproduce planned stem version. Data collected included, planned and final femoral implant size, version, and head-offset. Patients were followed to record any revision or peri-prosthetic fractures.
RESULTS: Stem, cup, and head offset sizing were performed using 3D CT. Size prediction was 100% for cups, 96% for stems and within ±1 size for 100% of stems. The surgeon targeted to restore leg-length to within ±2mm of the preoperative planned leg-length. Planned head offset averaged 2.07mm ± 2.9mm (range: -5 to 7.5mm) and the final offset used averaged 1.16mm ± 3.19mm (range: -5 to 7.5). The average change in head offset between planned/final was 1.88mm ± 2.16mm (range: 0 to 7.5).
The digital ruler guided osteotomy level of neck cut and was compared it to the seating height of the final broach. The variation of broach position to neck cut was average 0.23mm ± 1.74mm (range: 3mm short to 8mm long). After stem impaction, the actual version of the stem was captured and compared to the planned version. The average planned stem version was 14.0°±8.6° (range: -1° to 46°) and the average final stem version was 15.8°±10.1° (range: -11° to 51°). The average difference between planned and final version was 3.9°±3.2° (range: 0° to 11°). Final version values were evaluated in the virtual ROM tool, none caused a significant change in ROM to impingement. Trialing with the broach was only performed in cases where the longest or shortest head offset was pre-planned. Leg length was restored to within ±2mm of the preoperative planned leg-length in all cases.
At follow-up, there were no reported post-operative peri-prosthetic fractures or revisions.
DISCUSSION: In this study, 3D templating enabled the surgeon to pre-operatively predict implant sizing. 3D planning (digital ruler, neck cut view, sizing) provided an understanding of how the stem would seat prior to bone preparation. These features allowed the surgeon to eliminate the use of intra-operative imaging and, in most cases, component trialing in DAA. Ongoing studies are assessing the influence on surgical efficiency and reduction of OR time/streamlining instruments.
Figures
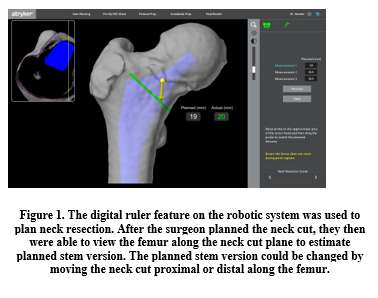
Figure 1
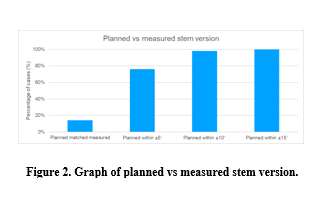
Figure 2#8325
Comparison of Computerised Tomography Based Planning of Conventional Total Hip Arthroplasty Versus Robotic-Arm Assisted Total Hip Arthroplasty: A Prospective Randomised Controlled Trial.
*Andreas Fontalis - University College London Hospitals - London, United Kingdom
Babar Kayani - University College Hospital London - London, United Kingdom
Dia Giebaly - University College London Hospital - London, United Kingdom
Jenni Tahmassebi - UCLH - London, UK
Alastair Chambers - UCLH - London, United Kingdom
Fares Haddad - University College London Hospital - London, United Kingdom
Ricci Plastow - University College London Hospitals NHS Foundation Trust - London, United Kingdom
*Email: andreasfontalis@gmail.com
Introduction
Achieving accurate implant positioning and restoring native hip biomechanics are key surgeon-controlled, technical objectives in Total Hip Arthroplasty. The primary objective of this study was to compare the reproducibility of the planned preoperative centre of hip rotation (COR) in patients undergoing robotic-arm assisted THA versus conventional THA. Secondary objectives were to ascertain the accuracy in achieving the planned combined offset, acetabular cup orientation and leg-length correction.
Methods
This prospective randomised controlled trial included 60 patients with symptomatic hip osteoarthritis, randomly allocated in a 1:1 ratio, to conventional THA or robotic-arm assisted THA(RO THA). Patients in both arms underwent pre- and post-operative CT scans and a patient-specific three-dimensional plan utilising the robotic software was created. The COR, combined offset, acetabular cup inclination and version and leg length discrepancy were measured on the pre- and post-operative CT scanogram at six weeks following surgery.
Results
Baseline characteristics were comparable between the groups. The mean absolute error for achieving the planned horizontal and vertical COR in the RO THA group was 1.9mm(1.3) and 1.4mm(1) versus 4.2mm(2.25),p<0.001 and 3mm(4),p=0.03 in the CO THA. The post-operative mean leg length discrepancy compared to the contralateral side was 0.75mm(0.91) in the RO THA versus 1.4mm(1.2), p=0.02. The root mean square for achieving the planned anteversion and inclination in the RO THA group were 2.9 and 2 versus 8.6 and 7. Patients in the RO THA group achieved a more accurate restoration of the combined offset, p=0.01.
Conclusion
This RCT showed that RO THA was associated with improved accuracy in restoring the native COR, better preservation of the combined offset and leg length correction. Differences between groups were particularly pronounced in relation to achieving the desired acetabular cup positioning. Looking at longer-term and registry data, will be key to evaluating whether the above findings translate to improved implant survival and superior outcomes.
#8502
Postoperative Complications and Readmission Rates in Robotic-Assisted and Manual Total Hip Arthroplasty
*Cole Howell - Albany Medical College - Albany, United States of America
Antonia Chen - Brigham and Women's Hospital - Boston, USA
Sietske Witvoet - Stryker - Amsterdam, Netherlands
Manoshi Bhowmik-Stoker - Stryker Orthopaedics - Mahway, USA
Andrea Coppolecchia - Stryker - Mahwah, USA
*Email: colehowell@gmail.com
Introduction: Total hip arthroplasty (THA) is one of the most common orthopaedic procedures performed today. However, postoperative complications continue to occur. The implementation of technology in surgery, including robotic-assisted platforms, aims to improve THA efficacy and patient outcomes. Nonetheless, the impact of robotic-assisted platforms on postoperative readmission and complication rates remains unclear. This study aims to compare 90-day postoperative complications, readmission, and emergency department (ED) rates between robotic-assisted THA (RA-THA) and manual THA (M-THA).
Methods: A retrospective review of a multi-hospital database was performed to identify patients who underwent THA between January 2016 and December 2021. Surgeons who performed both RA-THA and M-THA techniques at 73 geographically diverse hospitals within the United States (both rural and urban locations) were included. To account for covariates, RA-THA and M-THA cohorts were 1-to-1 matched based on patient gender, age groups (<50, 50-65, 65-80, >80y), and body mass index (BMI) groups (<20, 20-25, 25-30, 30-35, 35-40, >40kg/m2) resulting in 8,033 patients in each cohort (N=16,066). 90-day readmission and ED rates were compared between cohorts, as well as rates of readmission with >23 hours observation. Complications reported during readmission were classified according to the Clinical Classification Software (CCS) schema, based on ICD-10 codes, and compared between cohorts. Cohorts were statistically compared using Mann-Whitney U and Chi-squared tests.
Results: Of the patients who underwent M-THA, 54.0% were female and the mean age and BMI were 66.3±10.3 years and 29.8±5.6 kg/m2, respectively. In comparison, 53.2% of patients who underwent RA-THA were female and the mean age and BMI were 66.1±10.4 years and 29.9±5.7kg/m2, respectively. Baseline characteristics of both cohorts (age, gender, BMI and CCI scores) were similar between cohorts due to matching. All-cause 90-day readmission rates were 3.4% for RA-THA and 3.8% for M-THA (p=0.176). RA-THA patients had lower rates of readmission with >23 hours observation (2.6%) compared to M-THA (3.4%, p=0.006, Table 1). For readmissions with >23 hours observation, RA-THA patients had greater endocrine complications (RA-THA: 0.07%; M-THA: 0.0%, p=0.031), but less mechanical complications including dislocation (RA-THA: 0.09%; M-THA: 0.42%, p<0.001) and wound infection (RA-THA: 0.02%; M-THA: 0.11%, p=0.021, Table 2). However, RA-THA patients had higher rates of ED visits (8.4%) compared to M-THA patients (7.5%, p=0.041). With regards to ED visits, there was only a difference between cohorts for pulmonary complications, specifically shortness of breath without pulmonary embolism (RA-THA: 0.39%; M-THA: 0.20%, p=0.040, Table 3). Rates of readmission with >23 hours observation and ED visits between the two cohorts were not significantly different for all other postoperative complications.
Conclusion: The M-THA cohort had significantly higher rates of readmission with >23 hours observation due to dislocation and cellulitis compared to RA-THA. Conversely, the RA-THA cohort had higher rates of ED visits due to shortness of breath without pulmonary embolism. There was no significant difference in the overall 90-day readmission rate between the two cohorts.
Figures

Figure 1
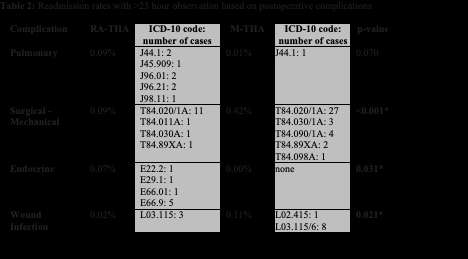
Figure 2
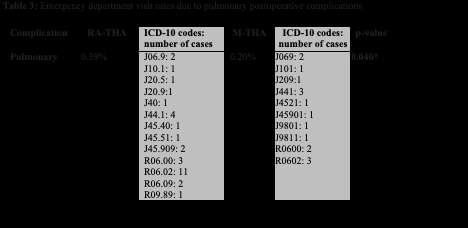
Figure 3#8571
In Vivo Three-Dimensional Weight Bearing Knee Kinematics Derived Using an Innovative and Automated Deep Learning Approach
*Viet-Dung Nguyen - The University of Tennessee at Knoxville - Knoxville, United States of America
Michael LaCour - University of Tennessee - Knoxville, USA
Richard Komistek - The University of Tennessee - Knoxville, USA
*Email: vnguye28@vols.utk.edu
Introduction: Fluoroscopic analyses of total knee arthroplasty (TKA) kinematics have been the gold standard for weight bearing kinematics analyses for 30 years, enabling an objective evaluation of postoperative knee joint kinematics and the development of better implant designs. Common parameters of interest during these evaluations include range of motion, condylar motion patterns, and femorotibial rotation patterns. Unfortunately, the conventional approach involves labor intensive manual work. This research aims to reduce the workload of knee kinematic analyses by utilizing a novel approach of automated 3D-to-2D image registration using deep learning to derive in vivo, weight bearing knee kinematics.
Methods: The overall process framework, illustrated in Figure 1, entails the following steps: image processing and segmentation, 3D-to-2D registration, and the resulting kinematics analyses. The Center for Musculoskeletal Research (CMR) research lab has amassed a substantial dataset of TKA fluoroscopy data spanning over three decades. Accordingly, this dataset has previously been subjected to systematic image processing, camera calibration, and manual registration, therefore serving as training data for a deep learning model utilized in the image segmentation process (DNN1) as well as another DNN model, referred to as DNN2, which takes a fluoroscopic image as input and outputs the corresponding transformation matrix of the 3D model. Following the automated segmentation and registration, the initial pose is refined via an optimization process to achieve optimal registration results, as demonstrated in the bottom image of Figure 2. In this study, clinically applicable parameters obtained from these algorithms, such as LAP, MAP, LSI, MAP, and AXR, were compared to ground truth manual registration techniques from previous fluoroscopic evaluations.
Results: In comparison with the ground truths by manual approach, the automated approach exhibits the average errors of 0.29±0.16 (mm) for LAP, 0.18±0.12 (mm) for MAP, and 0.24±0.16 (degrees) for AXR, as seen in Figure 3, demonstrating the high degree of accuracy attained.
Conclusion: The primary purpose of this research is to evaluate clinically applicable parameters, such as LAP, MAP, and AXR. These kinematic outputs help surgeons and engineers to evaluate the condylar rollback, extensor mechanism moment arms, impingement, etc. of TKA patients with high accuracy. Therefore, this automated approach provides clinically applicable design feedback automatically, almost in real-time. This approach can also be readily adapted for use in the analysis of kinematics in other joints, including the hip and ankle.
Figures
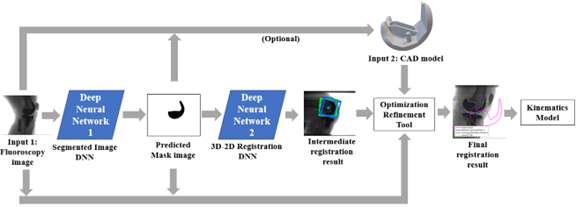
Figure 1
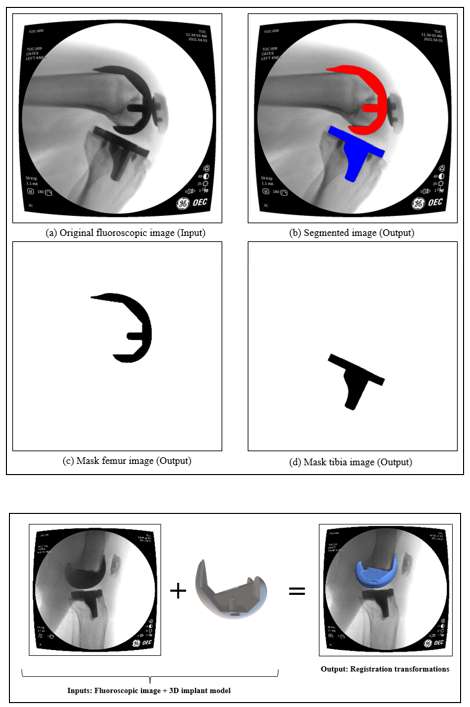
Figure 2
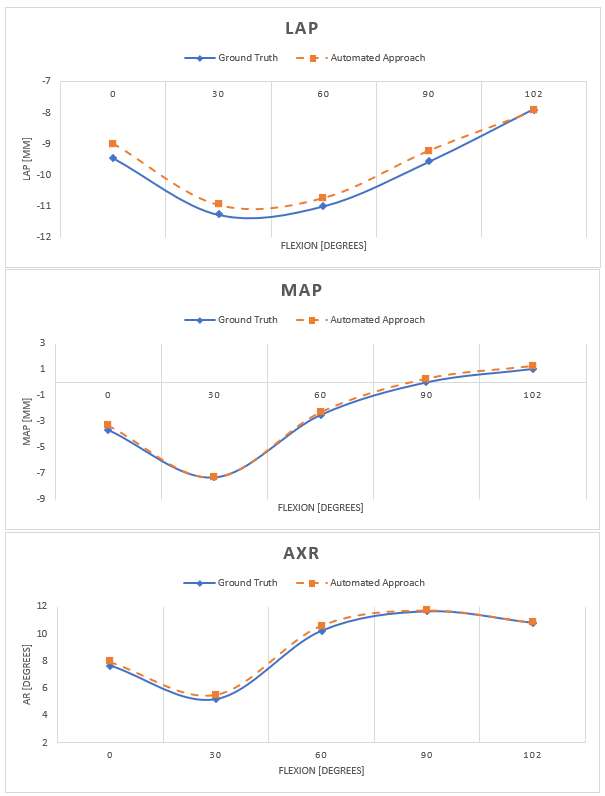
Figure 3#8436
Clinical Evaluation of an AI-Based 2D-to-3D Knee Bone Reconstruction for Patient-Specific Instrumentation in Total Knee Arthroplasty
*Hassan Nemati - Ortoma AB - Gothenburg, Sweden
Andreas Pettersson - Ortoma AB - Gothenburg, Sweden
Linus Bystrom - Ortoma AB - Gothenburg, Sweden
*Email: hassan.nemati@ortoma.com
While CT-based 3D planning offers numerous advantages over traditional 2D planning methods for total knee arthroplasty, it has not yet become a widely adopted practice. Many orthopedic surgeons prefer the traditional 2D planning approach owing to their familiarity with the workflow and the ready accessibility of X-ray scanner in most hospitals. On the other hand, with the advance in artificial intelligent (AI)-based algorithms it is possible to construct a 3D model of the patient’s knee joint using only X-ray images. This way, not only the common X-ray based workflow can be used, but also a patient specific 3D model is provided to offer additional capabilities of planning in 3D.
In this study, a novel 2D-to-3D platform is evaluated that utilizes X-ray images with AI-based algorithms and machine learning (ML) models to reconstruct the knee joint in 3D. The input to the platform is two X-ray images and the output is the automatic generation of 3D models for the knee joint, i.e., femur and tibia. The platform then determines an appropriate implant size for femoral and tibia components based on the 3D models (See Figure 1).
The primary objective of the present study was to investigate whether this AI-based platform can produce accurate 3D model of the knee joint that have clinical relevance for determining the appropriate size of the knee implants. A clinical study was performed on 60 patients and the knee implant size based on the 3D models are compared to the surgeon’s planning of implant size using the traditional 2D planning.
The analysis is performed on real X-ray images for patients who underwent total knee arthroplasty. The size of femoral and tibia components in the traditional 2D planning for 60 patients were compared with the suggested implant size based on the automatic generation of 3D knee joint. The mean and standard deviation difference for the femoral component was -0.15 ± 0.63. For the tibia component, the sizes were labeled with C, D, E, F, G, H, and J. Among all 60 patients, there were only 10 mismatches (± 1 size difference) between the surgeon’s planning in 2D and the suggestion on the 3D platform.
The novel 2D-to-3D platform, with the use of AI-based algorithms and ML models, provides a fast and efficient capability to plan knee implant components in 3D with high accuracy. In addition, since the 3D model of the knee joint is provided, additional features including the knee join kinematic simulation is included in the platform. Furthermore, 3D planning allows surgeons to view the knee joint from multiple angles and perspectives, enabling them to better assess the joint's structure and select a more accurate implant size, position, and orientation. The high accuracy, customization, and reduced surgery time associated with 3D knee arthroplasty planning can result in reduced pain and higher patient satisfaction.
Figures
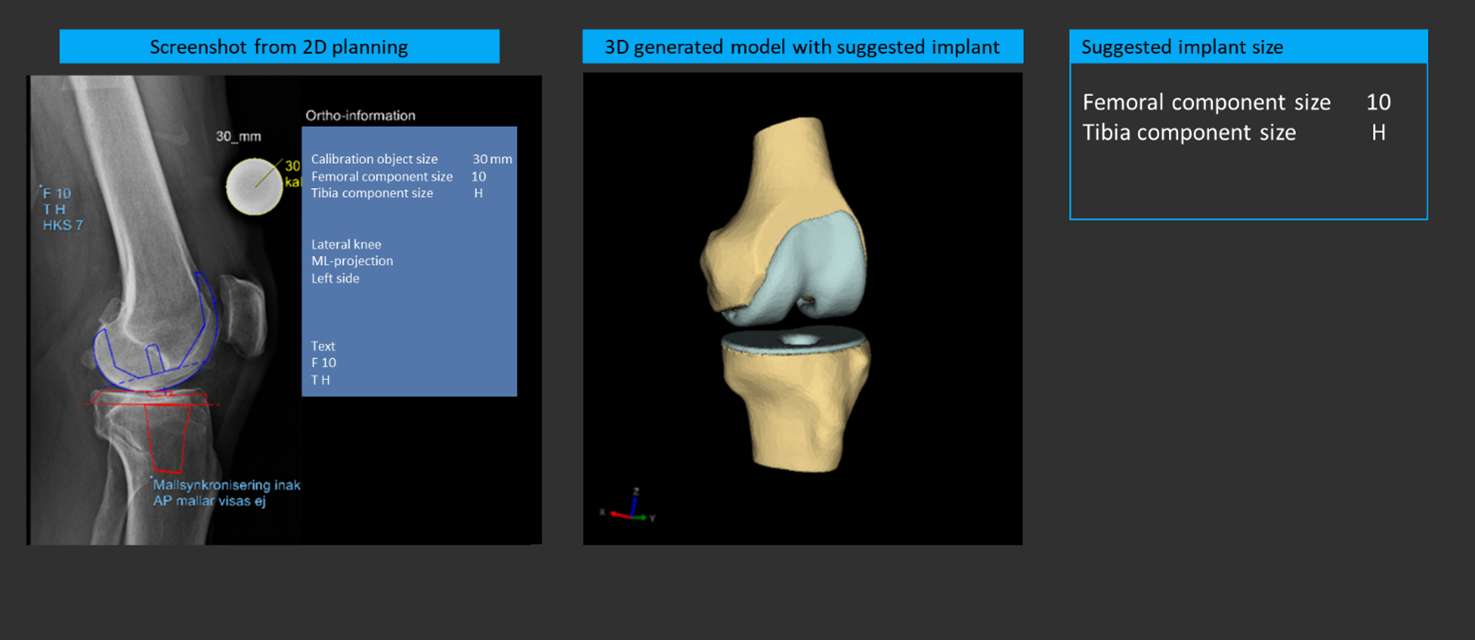
Figure 1#8172
Using Patient Anatomy, Demographics and Machine Learning Algorithm to Predict Bone Mineral Density for Cementless Application
*Amit Mane - DePuy Synthes - Warsaw, USA
Chase Maag - DePuy Synthes - Warsaw, USA
*Email: amane5@its.jnj.com
Introduction: Cementless TKA offers benefits of biologic fixation, preservation of native bone, improved surgical efficiency etc.1,2. It relies on good quality and viable host bone for successful osseointegration1. Pre-operative bone mineral density (BMD) assessment can provide early insight into quality of a host bone. However, expensive essential imaging techniques could limit access to this information. Recently, machine learning (M-L) algorithms have been utilized to predict post-TKA infection risks, blood transfusion rate, post-operative readmission3 etc. Implementing M-L algorithms to predict BMD pre-operatively could help select patients for Cementless procedures. Therefore, the objective of this study was to develop a M-L algorithm to predict proximal tibial BMD using the anatomic and demographic information.
Methods: CT scans of 2461 lower legs were used. Six scans included density measurement device-Phantom. The study was split in three steps (Fig.1). First, using six tibial scans and Phantom calibration matrices, proximal tibial BMDs (10mm inferior to plateau) were determined. Recently, in CT scans without Phantom, air-fat-muscle densities have been used to determine BMD4. Using this Phantomless techniques, tibial BMDs in same six scans were estimated. The RMSEs were calculated to ensure accuracy of Phantomless technique. In second step, the Phantomless technique was used to determine proximal tibial BMD in 2461 CT scans (Fig.1). The BMD values within the virtual cube at the proximal tibia center were estimated, and later averaged to represent a single density value. Additionally, various lower leg anatomic variables and patient demographic information were collected. Third step included development of Neutral Network (NN) that would predict BMD using anatomic and demographic information. The NN was a multilayer perceptron (MLP) consisting of 1942 hidden units with 12 layer depth. After preprocessing, 2398 samples were left and split into train/validation sets of 2396 and 2. A batch size of 2 and Adam optimizer was used in training with Huber loss function. Training was terminated using early stopping method on validation loss.
Results: RMSE between Phantom and Phantomless BMD for all six proximal tibiae ranged between 9.1-17.1mg/cc (Fig.2). The mean BMD of 2398 proximal tibiae determined using Phantomless technique was 75.8±45.9mg/cc (Fig.2). The patient demographics and anatomic features are summarized in Fig.2. The NN MLP achieved RMSE of 9.5mg/cc over the training data with RMSE of 1.8mg/cc on the validation set (Fig.3).
Conclusion: Good host bone quality is an essential requirement for successful osseointegration1,2. Clinicians have recommended avoiding patients with decreased bone density1. However, limited guidelines are available for patient selection in Cementless applications. The study developed simple and cost-effective M-L algorithm to determine BMD within 9.54 mg/cc, using a priori patient demographic and anatomy. These parameters can be obtained from whole leg radiographs, consequently making the methodology more suitable in clinical environment. Results can also be further strengthened by including diverse ethnicities to represent global population, while implementing algorithm to cortical bone, Femur etc. In summary, the research highlighted ability of Artificial Intelligence and M-L algorithms to predict proximal tibial BMD for the Cementless applications.
References: 1.Asokan et.al.-2021, 2.Meneghini et.al.-2018, 3.Kwon et.al.-ORS2023,Poster#779-779,780,784, 4.Stitzerl etl.al.-2015
Figures
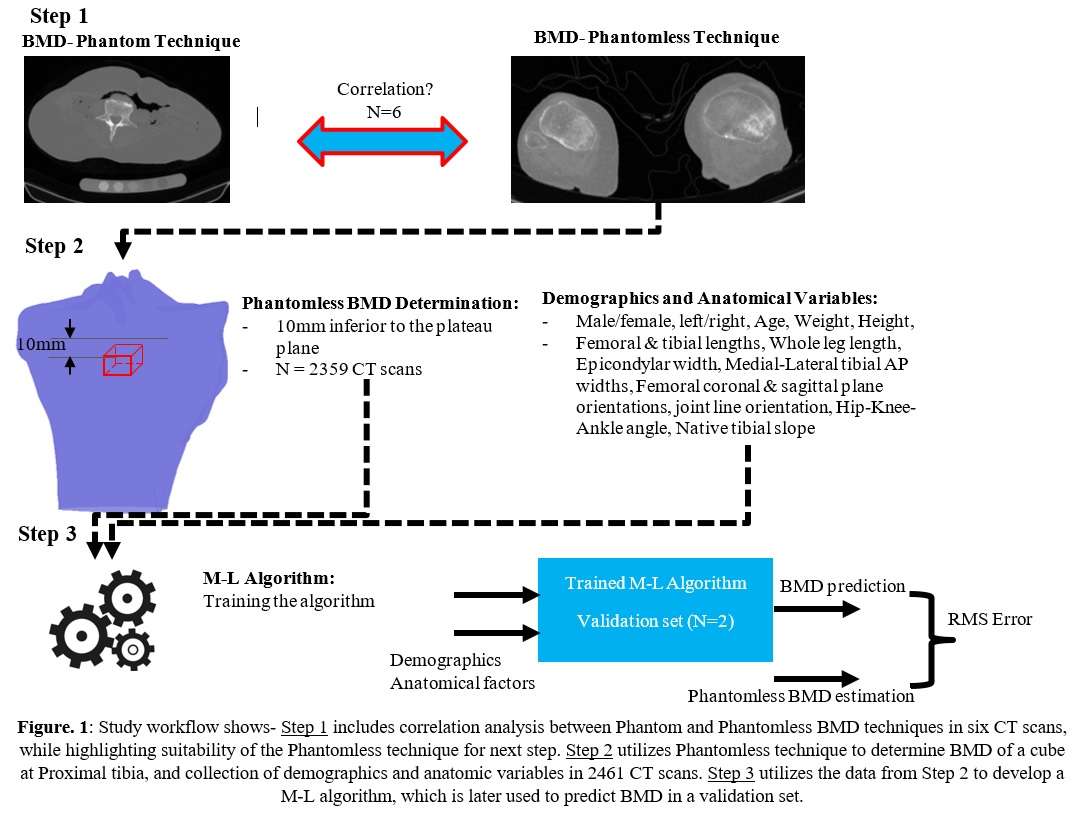
Figure 1
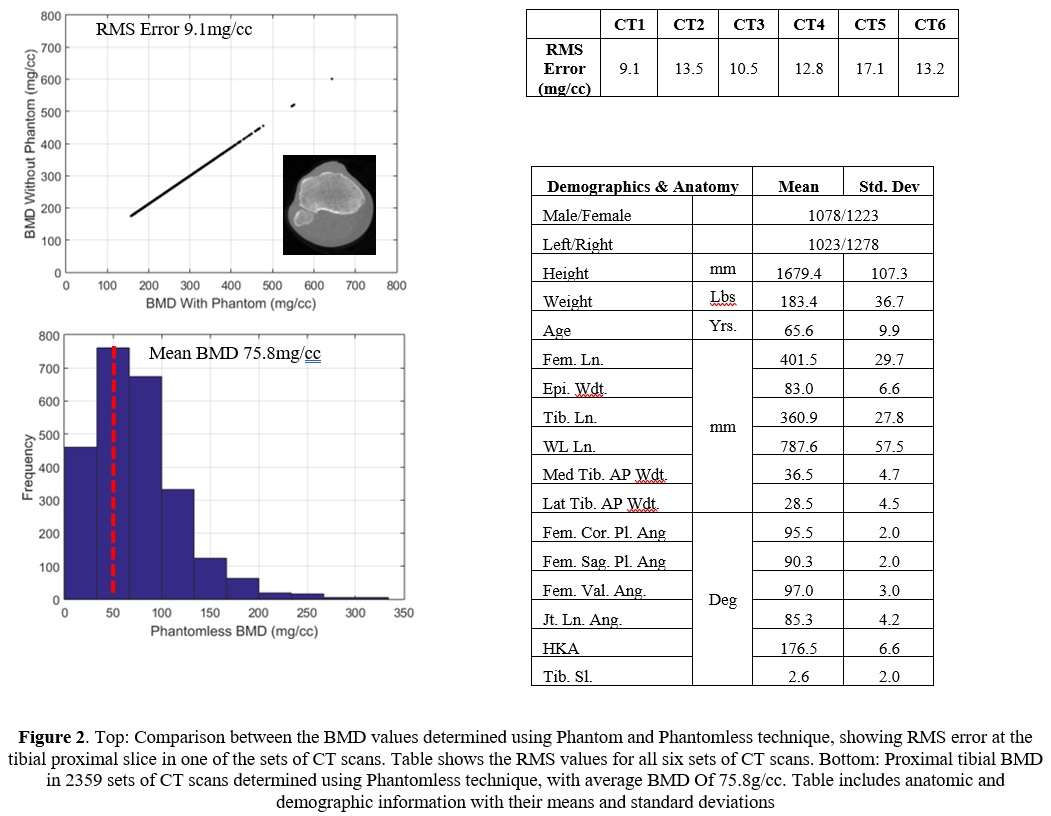
Figure 2
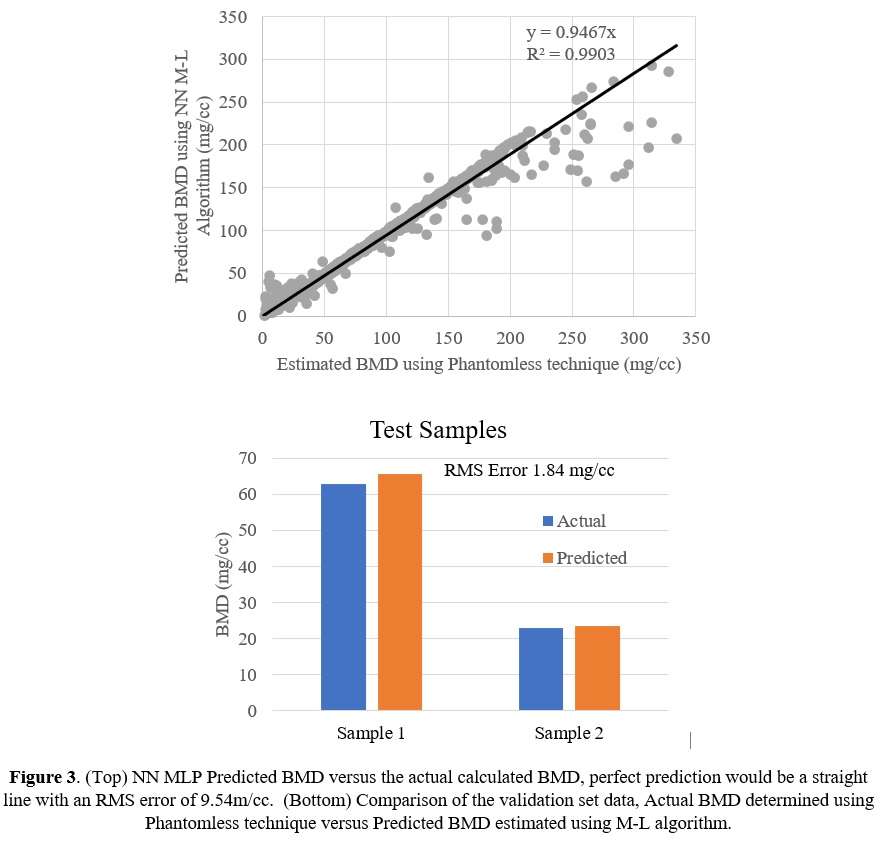
Figure 3#8569
Is Patient Data Needed When Creating Machine Learning Algorithms for Classifying Activities During TKA Recovery?
Andrew Meyer - Stryker - Mahwah, USA
*Matthias Verstraete - Stryker - Fort Lauderdale, USA
Christopher DiCesare - Exponent - Farmington Hills, USA
Scott McLean - Exponent - Farmington Hills, USA
*Email: matthias.verstraete@stryker.com
Introduction
Recently developed IMU technologies have enabled remote monitoring of patient recovering from total knee arthroplasty (TKA). However, due to the quantity of data and uncertainties inherent in unsupervised data collection, clinical interpretation of IMU-derived outcomes can be challenging. As a result, machine learning algorithms are being developed to process these datasets and provide clinically relevant information to practitioners. Creating these algorithms, however, requires large quantities of labeled data from patient populations, which is oftentimes costly to acquire. As a result, datasets from healthy subjects are often used in place of patient data, which may result in algorithms that function poorly when applied to clinical populations.
In this study, we investigated the generalizability of accelerometer-based activity classifiers trained using only healthy subjects to a TKA population (<18 weeks post-surgery).
Methods
Lateral shank-mounted triaxial accelerometer data were collected at 50 Hz from 10 healthy (5M5F, 44+/-10) and 13 post-TKA patients (4M9F, 66+/-11 years, 3-17 weeks post-surgery) performing 7 different activities. Synchronous video data was used to label the accelerometer data.
A machine learning model was trained to predict the activity using the accelerometer data. Features including the standard deviation, minimum and maximum accelerations, inter-quartile range, absolute range, mean absolute difference, energy, and impulse were computed for 2.4 second windows with a 50% overlap between time adjacent windows. Two extreme gradient boosted decision tree (XGBoost) classifiers were trained, one using data from the healthy population and one using pooled data from both populations. Model parameters were tuned using leave-one-subject-out cross validation. Two patients were withheld for testing the healthy patient classifier, and six patients (2 control, 4 TKA) were withheld for testing the pooled-data classifier.
Results
The healthy subject classifier performed well on the test subjects from the healthy population (Figure 1), although it had some difficulty differentiating stair ascent, stair descent, and level walking. The accuracy was 89.3% with a precision of 91.9% and a recall of 89.8%. Sitting and standing were combined since a shank mounted accelerometer yields the same reading for both activities.
When applied to the patient population, the healthy-subject classifier performed poorly (Figure 2), with an accuracy of 60.7%, precision of 58.1%, and a recall of 50.6%. The classifier was unable to reliably identify stair ascent and descent as well as walking in the patient population. After retraining the classifier using data from both healthy and TKA patients, these issues were partially resolved (Figure 3). Performance improved, with accuracy of 80.8%, precision of 85.1%, and recall of 80.0%. However, the classifier still had difficulty differentiating stair ascent/descent, level walking, and walking with an aid.
Conclusions
Machine learning algorithms trained using healthy subjects are not always generalizable to patient populations. In this case, the TKA patients’ activity performance was substantially different from the healthy subjects, especially their handling of stairs and walking. This result emphasizes the importance of dedicated algorithm development when developing medical devices, which should build on data obtained from patients with relevant demographics and pathological conditions when intended to support the treatment of these patients.
Figures
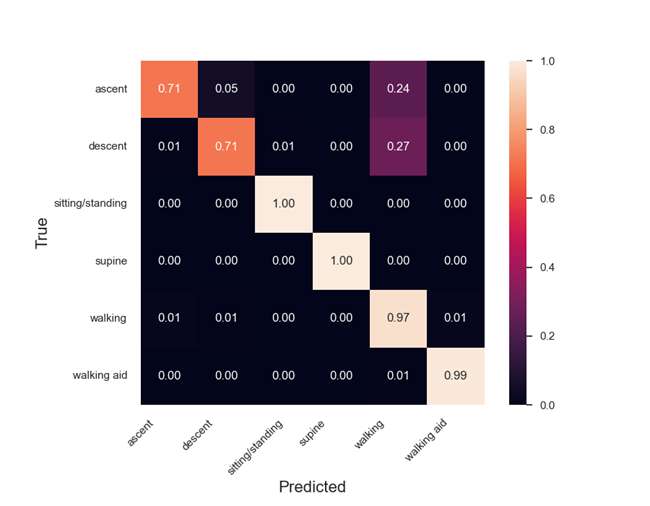
Figure 1
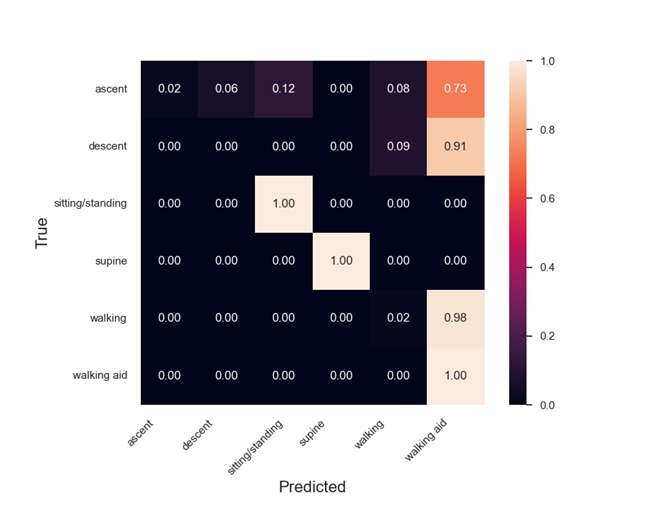
Figure 2
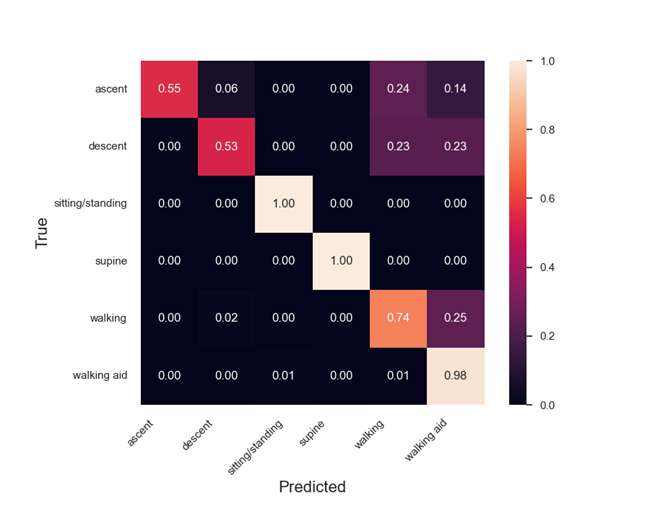
Figure 3#8360
Detection of Knee Range of Motion From Images Using Artificial Intelligence
*Ishaan Jagota - 360 Med Care - Sydney, Australia
Joshua Twiggs - University of Sydney - Sydney, Australia
Brad Miles - 360 Knee Systems - Pymble, Australia
Justin Roe - North Sydney Orthopaedic and Sports Medicine Centre - Sydney, Australia
Andrew Shimmin - Melbourne Orthopaedic Group - Melbourne, Australia
Jonathan Bare - Melbourne Orthopaedic Group - Melbourne, Australia
Chloe Franken - 360 Med Care - Pymble, Australia
Qipeng Shen - 360 Med Care - Epping, Australia
*Email: ishaan.jagota@360med.care
Introduction: Attainment of Range of Motion (ROM) of 0°-110° flexion is a key functional goal of Total Knee Arthroplasty (TKA) as well as an early post-operative indicator of functional outcome. Traditionally, knee ROM is measured by a trained examiner using a goniometer, which makes tracking progression over time in outpatient/remote management settings difficult. Artificial Intelligence (AI) algorithms have been developed which could be used to track incremental improvements to ROM after TKA with the use of regular photos. The aim of this pilot study was to assess the accuracy of the BlazePose AI landmark detection algorithm for measuring knee ROM relative to goniometer measurements taken by a physiotherapist.
Method: A single reference participant was recruited to this study and instructed to lie supine and extend their leg to the point of full extension, and then to flex their knee to the point of full flexion. A total of 438 images were taken using a smartphone camera. These images were taken simultaneously with goniometer measurements by a trained physiotherapist to create a set of known values to train a machine learning model on. Goniometer measurements and visual estimations were performed by three senior physiotherapists on a subset of 60 flexion and extension positions. Of the 438 images, 60 images were reserved for model validation and the remaining 378 images were divided into training and test sets with an 80-20 split. The images were then analysed using BlazePose to extract information such as three-dimensional coordinates of the hip, knee and ankle. A machine learning model was then trained and tested on the prediction of the angle as measured via goniometry. Following this, the validation set was processed via BlazePose, and the created machine learning model to predict the associated goniometer measurement. All statistical analysis was performed using Python.
Results: A very strong correlation was observed between the BlazePose and goniometer ROM measurements (R=0.95). The mean absolute difference between the two measurements was 4.3°±5.9°. This is comparable to the mean absolute difference between the physiotherapists’ goniometer measurements of 4.5°±5.7° and displayed greater reliability than the visual estimations of the physiotherapists (8.1°±9.7°). A Bland-Altman analysis (Figure 1) displayed that the differences between the two methods were distributed evenly around the mean, indicating no systematic bias between the two measurement techniques.
Conclusions: This study provides evidence that using the BlazePose AI landmark detection algorithm for measuring knee ROM from images is a reliable and accurate alternative to traditional goniometer measurements. The use of AI-based methods has the potential to enable daily remote follow-up of ROM progress through utilisation of smartphone applications and other digital tools, which could enable early detection of joint stiffness or lack of functional progress. Further research will investigate the efficacy of the model when implemented into a remote care program for detecting ROM complications during early follow-up and practical ease of use in patient self-administration.
#8224
Real-Time AI Surrogate Simulation Model for Enhanced TKA Outcomes: Validation and Applications in the Operating Theatre
*Ishaan Jagota - 360 Med Care - Sydney, Australia
Joshua Twiggs - University of Sydney - Sydney, Australia
Justin Roe - North Sydney Orthopaedic and Sports Medicine Centre - Sydney, Australia
Brad Miles - 360 Knee Systems - Pymble, Australia
David Liu - Gold Coast Centre for Bone and Joint Surgery - Tugun, Australia
Andrew Shimmin - Melbourne Orthopaedic Group - Melbourne, Australia
Jonathan Bare - Melbourne Orthopaedic Group - Melbourne, Australia
Qipeng Shen - 360 Med Care - Epping, Australia
*Email: ishaan.jagota@360med.care
Introduction
Joint dynamics (forces and motion within the joint) following Total Knee Arthroplasty (TKA) are a factor influencing patient outcome. The Dynamic Knee Score (DKS) is a validated Artificially Intelligent (AI) model derived from computational simulations of joint dynamics, enabling evaluation of potential impacts on patient outcome. The DKS takes 45 seconds to run each simulation, however, limiting potential use. This study evaluates the development of a surrogate model of the DKS evaluating many possible surgical plans instantaneously for real time AI surgical guidance.
Method
Initial validation of the DKS model as a predictive model of patient reported outcome was undertaken with a series of 1074 TKAs. Pre- and post-operative CT scans and 12-month Knee Injury and Osteoarthritis Outcomes Score (KOOS) were collected. Implants used were Corin (Cirencester, UK), APEX and MatOrtho (Surrey, UK) Saiph designs. Comparisons between the predicted outcome and postoperative KOOS were performed for validation (examples outlined in Figure 1 and Figure 2). Following this, a further 675 DKS simulations were performed and a series of anatomical and functional measurements of the implantation taken. These measurements covered native anatomy and functional offsets of the femoral, tibial and patellar components, and were used to derive an AI surrogate model that predicts the simulation and patient outcome from measurements alone. Validation of the accuracy of this surrogate model and determination of speed of evaluation was performed.
Results
Among the 1074 TKA patients from the DKS validation, patients for whom the surgery followed the best possible DKS plan demonstrated reduced postoperative impairment in knee catching, squatting, straightening, and descending stairs. The surrogate model was shown to recreate the simulation prediction with a correlation of 0.76. Significant functional measurement factors in predicting the behaviour of the simulation were the change in patella component, medialisation of the patellar apex, tibial rotation to Insall’s axis, the combined flexion of the components and alterations to the relative epicondylar offsets of the medial and lateral collateral ligament bundles. Evaluation of the model occurred c.10,000 times faster than the original simulation, allowing for c.200 surgical options to be evaluated per second in a navigated in-surgery decision making setting.
Conclusions
The AI DKS model reduces post-TKA difficulties in squatting, straightening, and patello-femoral function. The surrogate model evaluates in real-time and can be integrated into the operating theatre for improved patient-specific surgical planning and reduced post-operative functional difficulties. AI models may drive future advancements in personalized alignment strategies and surgical planning.
Figures
Figure 1
Figure 2#8268
A Clustering Algorithm to Support Intraoperative Planning During Robotic-Assisted Total Knee Arthroplasty
*Nathan Netravali - Smith + Nephew - Littleton, United States of America
Steve Yurick - Smith+Nephew - Pittsburgh, USA
Steven Nishiyama - Desert Orthopaedic Center - Las Vegas, USA
Thorsten M. Seyler - Duke University - Durham, USA
*Email: nnetravali@gmail.com
Introduction
Multiple alignment philosophies exist for total knee arthroplasty (TKA), with the common goal of improving patient satisfaction, implant longevity, and surgical reproducibility. However, there is no consensus regarding optimal placement technique for femoral and tibial components. More recently, robotic-assisted TKA (RA-TKA) has been shown to improve accuracy and alignment relative to conventional manual instrumentation. Handheld, image-free RA-TKA (HI-TKA) builds on this technology by providing intraoperative surface mapping, enabling real-time assessment of bone alignment to inform hip-knee-ankle angle planning and 3D component orientation.
The aim of this study was to assess trends in intraoperative HI-TKA planning, and resulting implant position, to inform a clustering algorithm based on patient deformity, implant type, and preference in surgical approach.
Methods
This global, multi-center, retrospective analysis was conducted on an intraoperative dataset of 2,976 HI-TKA procedures performed by 95 surgeons between July 2019 and November 2022. K-means clustering was applied to the dataset for 6 implant designs with the final surgical case plan as the input. The algorithm separated case plans into 2 clusters (referred herein as “Cluster A” and “Cluster B”) based on plan parameters (e.g., resection depth, component orientation). Due to inherent variation in planning values stemming from differences in implant design, clustering was applied separately for each implant type. Following clustering, the cases were separated into 8 deformity categories for each implant type based on level of preoperative varus/valgus long leg alignment (0-7° varus or valgus, >7° varus or valgus) and flexion contracture (0-10° or >10°).◊◊
Results
For a given implant type and deformity combination, implant placement values were averaged within cluster to generate reference values. Table 1 represents the cluster distribution across all HI-TKA procedures based on deformity category. Differences between Clusters A and B were noted for each implant type. Figure 1 illustrates a representative comparison between implant placement in Cluster A and B for one implant type (Journey◊ II BCS implant, Smith & Nephew) and deformity category (low varus [0-7°], high flexion [>10°]). For the Journey II BCS, on average, plans in Cluster A had lower femoral resection depths and higher tibial medial and lateral resection depths compared with plans from Cluster B. Surgeons that used a planning approach captured by Cluster B may have preferentially raised the joint line compared with those in Cluster A for this implant and patient deformity category.
Conclusion
Identification of clusters to inform surgical approach may enhance a surgeon’s ability to personalize and individualize implant placement and optimize intraoperative workflow, thus potentially leading to improved clinical efficiency.
◊Trademark of Smith+Nephew.
◊◊Patent Pending
Figures
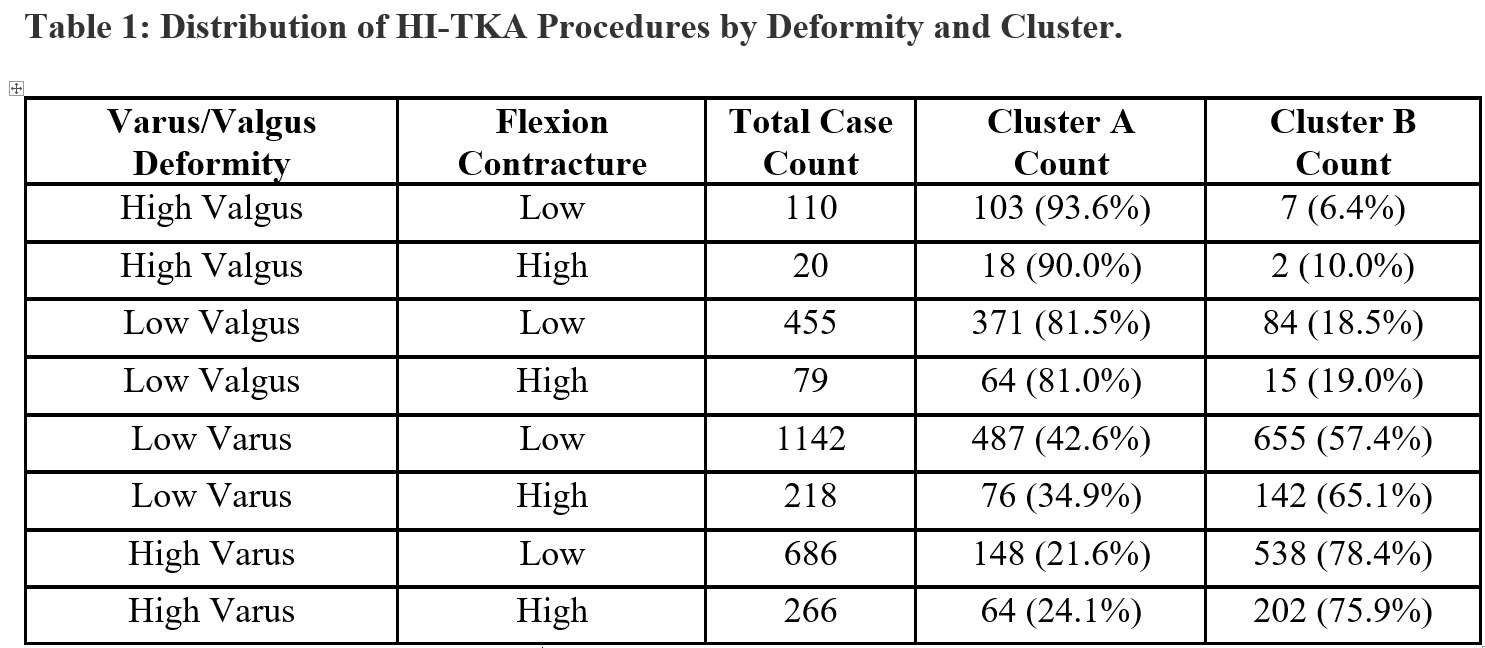
Figure 1
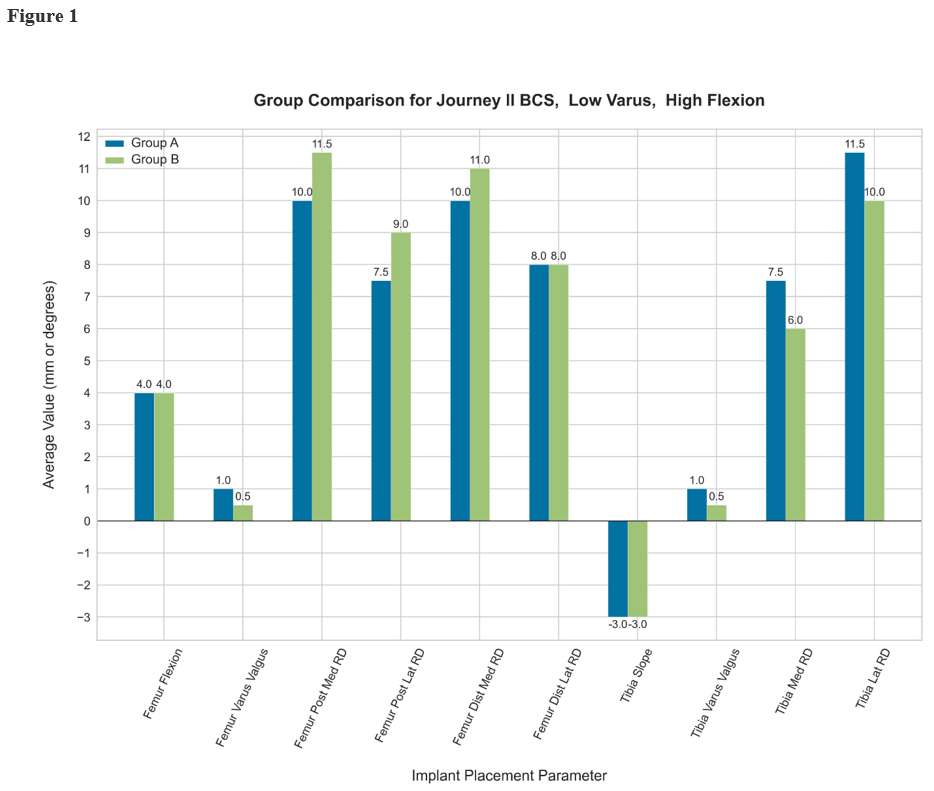
Figure 2#8100
Cement Loaded With High-Dose Gentamicin and Clindamycin Does Not Reduce the Risk of Subsequent Infection After Aseptic Total Hip or Knee Revision Arthroplasty: A Preliminary Study
*Jean-Yves Jenny - University Hospital Strasbourg - Illkirch, France
Ceyran Hamoudi - University Hospital Strasbourg - Strasbourg, France
*Email: Jean-yves.jenny@chru-strasbourg.fr
Introduction
Aseptic total hip (rTHA) and knee (rTKA) revision arthroplasty is associated with an increased risk of developing periprosthetic joint infections (PJI) compared to primary arthroplasty. Strategies to prevent this complication include prophylactic antibiotics, meticulous skin preparation and surgical site irrigation. When cemented implants are used, antibiotic-loaded bone cement (ALBC) may help to prevent infection. It has been previously demonstrated that dual ALBC may decrease the risk of subsequent infection after rTHA or rTKA for periprosthetic infection. The aim of this study was to quantify the prophylactic effect of high-dose gentamicin and clindamycin antibiotic-loaded bone cement (ALBC) during revision total hip (rTHA) or knee (rTKA) arthroplasty for aseptic reasons. The hypothesis was that the raw surgical site infection (SSI) rate is lower when this particular cement is used in comparison to cement loaded with standard-dose gentamicin during rTHA or rTKA for aseptic reasons.
Methods
This retrospective, single-center, observational study was conducted in compliance with the recommendations of the Helsinki declaration. It was approved by the institutional Ethics Committee, and all patients gave their written consent. 290 consecutive patients undergoing aseptic rTHA or rTKA were included. Two consecutive cohorts were defined based on the type of ALBC used. The surgical procedure was standardized, and prophylactic antibiotic were administered according to international recommendations. The first cohort (control group) involved 145 patients where ALBC with gentamicin only was used. The second cohort (study group) involved 145 patients where ALBC with high-dose gentamicin and clindamycin was used. All patients were followed for a minimum of 24 months. A surgical site infection (SSI) was defined according to the Musculoskeletal Infection Society definition. The occurrence of an SSI was recorded. The rate of repeat surgery for SSI was monitored. The susceptibility of the bacteria found in the SSI to gentamycin and clindamycin was noted. Any complication or side effect during the postoperative survey was documented, and its relationship to the bone cement was analyzed. The primary endpoint was the raw SSI rate after 24 months.
Results
The raw SSI rate was 8/145 (6%) in the control group and 13/145 (9%) in the study group (odds ratio 0.62, p=.26). There was a significant impact of the presence of any risk factor on the SSI rate (15/100 vs 6/169, odds ratio = 4.25, p=.002). There was no significant impact of any individual risk factor on the SSI rate. The rate of repeat surgery for SSI was not different in both groups. The bacteria found in the SSI were mainly resistant to gentamycin and clindamycin. No complication or side effect related to ALBC was observed in either group.
Conclusion
These results do not support the routine use of gentamicin and clindamycin ALBC for the fixation of revision implants after rTHA and rTKA for aseptic reasons.
#8383
Efficacy of a Vancomycin/Tobramycin-Doped Polyphosphate Dicalcium Phosphate Dehydrate (VAN/TOB-PDCPD) Composite in a Mouse Infection Model With 3D-Printed Porous Titanium Cylinders
David Markel - The CORE Institute - Novi, USA
*Therese Bou-Akl - Providence-Providence Park Hospital - Southfield, USA
Paula Dietz - Ascension Providence Hospital - Southfield, USA
Bin Wu - Providence-Providence Park Hospital - Southfield, USA
Weiping Ren - Wayne State University - Select Country
*Email: Therese.Bou-akl@ascension.org
INTRODUCTION: The efficacy of saline irrigation for treatment of periprosthetic infection (PJI) is limited in the presence of metallic implants. This study evaluated the therapeutic efficacy of antibiotic doped ceramic composite (PVA-VAN/TOB-PDCPD) after saline wash in a mouse infection model implanted with porous Ti cylinders with defined pore sizes.
METHODS: Study #1: Establishment of a pouch infection and biofilm formation. Mouse pouches were implanted with Ti cylinders (400, 700 and 100 um pore size) and inoculated with S. aureus at 1X103 CFU (low grade) or 1X106 CFU per pouch (high grade). Mice were sacrificed at 1 week for microbiological analysis.
Study #2: Treatment arm. Mouse pouches were implanted with 400 um Ti cylinders, inoculated as above and randomized into 4 groups: (1) no bacteria (negative control); (2) bacteria without saline wash (positive control); 3) saline wash alone and (4) saline wash + PVA-VAN/TOB-PDCPD. After seven days the pouches were opened and washed with saline alone or had an additional injection of PVA-VAN/TOB-PDCPD. Mice were sacrificed 14 days after pouch wash for testing.
RESULTS: Data from study #1 showed that neither low-grade (1X103 CFU) nor high-grade (1X106 CFU) pouch infections caused body deterioration of the mice/pouch. As shown in Fig. 1, the low-grade (1X103 CFU)infection was more significant in 400 um Ti cylinders than that in Ti cylinders with larger pore sizes (700 and 1000 um, p<0.05). A similar pattern of high- grade infections were observed among the three groups.
Data from study #2 showed that the saline wash alone was ineffective in eradicating both low and high-grade infections. The addition of PVA-VAN/TOB-PDCPD to the saline wash eradicated the Ti cylinder-associated infections demonstrated by negative bacterial cultures of the end pouch washouts; the end bacterial burden was significantly higher in the saline wash group vs. the saline plus PVA-VAN/TOB-PDCPD group (0.49±0.02 and 0.005±0.001 respectively, p<0.05) (Fig. 2). These results were supported by SEM and histology analysis (Fig. 3).
CONCLUSION: Porous Ti cylinders, especially those with smaller pore size, were vulnerable to bacterial infection that could not be treated by saline irrigation alone. Application of PVA-VAN/TOB-PDCPD directly into the surgical site alone or after saline wash represents a feasible approach of prevention and/or treatment of PJI.
Figures
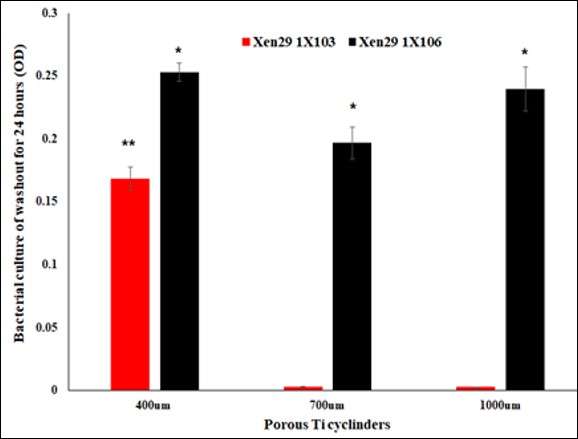
Figure 1
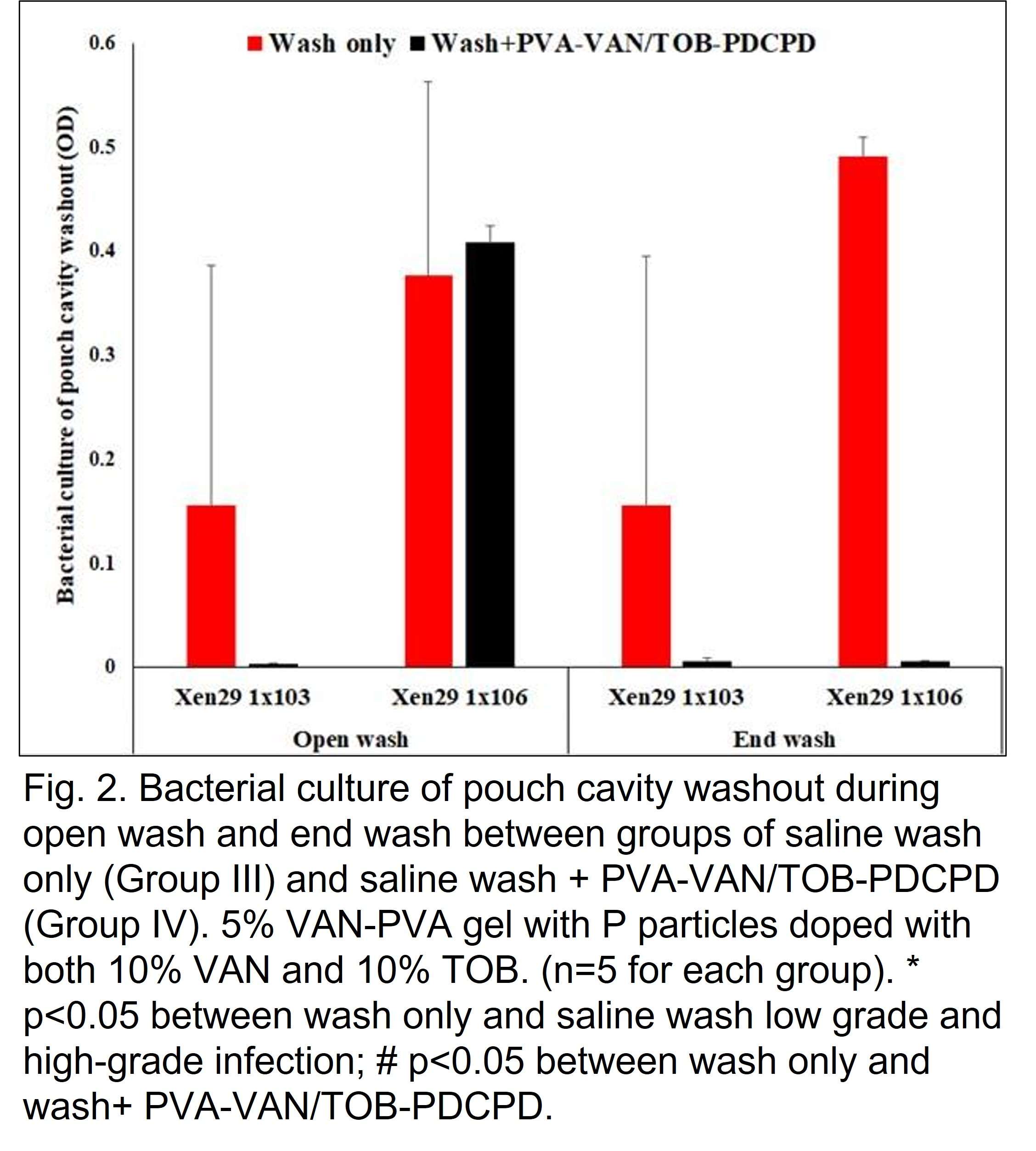
Figure 2
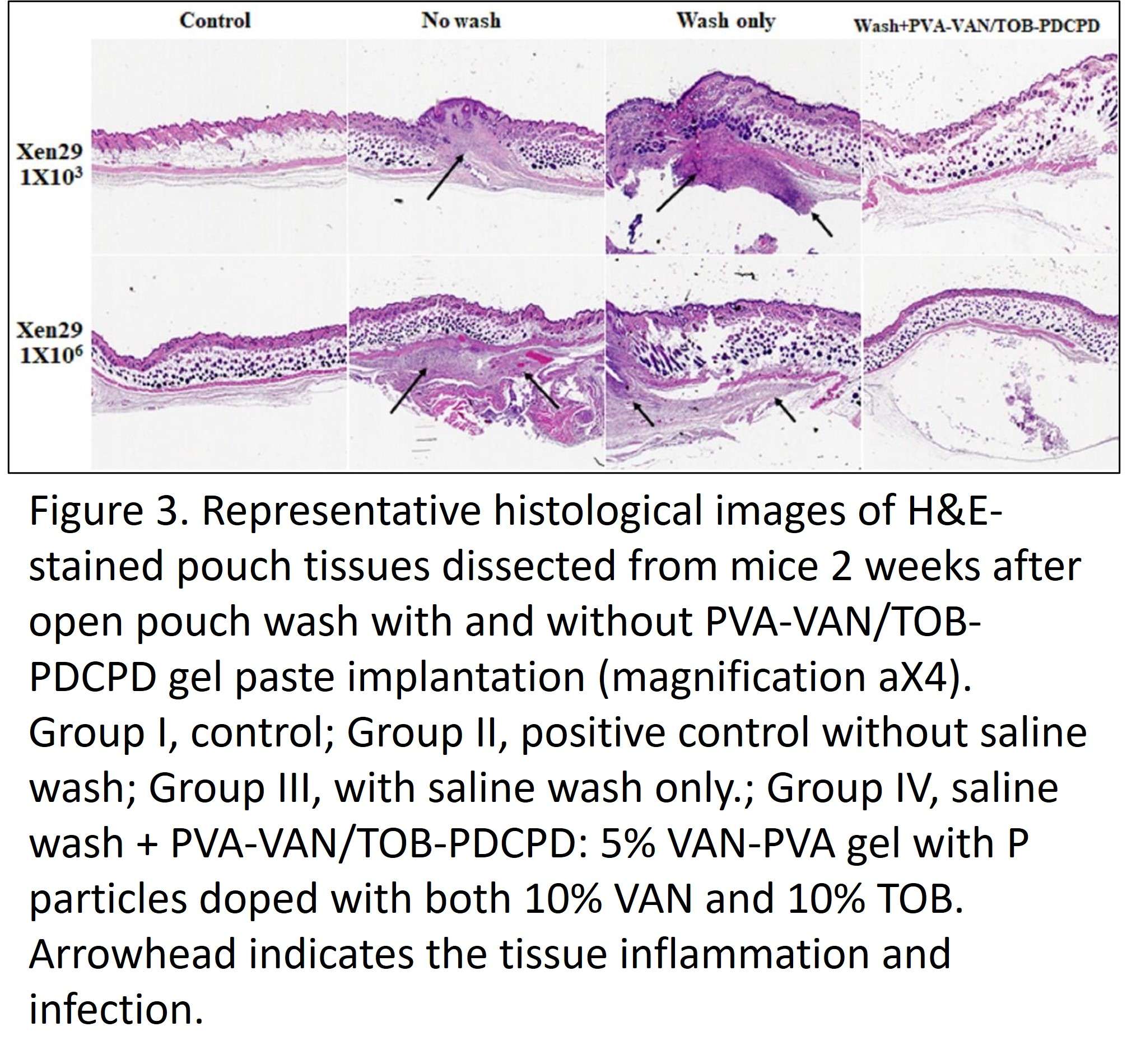
Figure 3#8778
Microfluidic Synthesis of Gentamicin Sulfate Loaded PLGA Microparticles
Mehmet Asik - USA
Aybike Reyhanli - Harvard Medical School and Mass General Hospital - Boston, United States of America
Ebru Oral - Massachusetts General Hospital - Boston, USA
*Orhun Muratoglu - Massachusetts General Hospital - Boston, USA
*Email: efrafferty@partners.org
Introduction
Periprosthetic joint infection (PJI) is a severe complication of total joint arthroplasty with a 1-2% incidence in primary surgical procedures (1). Long-term antibiotic therapy or surgical interventions are required to manage this complication (2). The average success of the treatment is as low as 54% (3) with a 5-year mortality rate of 21% (4). These poor outcomes are caused by reduced local drug availability in systemic administration and bolus local administration in the joint. We developed a local sustained drug delivery system with a biodegradable polymer, polylactic co-glycolic acid (PLGA), to achieve sustained gentamicin sulfate (GS) release to improve PJI treatment.
Method
GS PLGA microparticles were prepared with a double-emulsion technique in a custom-designed microfluidic device. W1 (water) phase contained 500 mg/ml GS in water. PLGA was dissolved in ethyl acetate and used as the oil (O) phase. W2 (water) phase was an aqueous solution with or without gentamicin sulfate. W1, O, and W2 phases were pumped into the microfluidics device to form microparticles. The nascent particles were collected into 85 ml of the W2 phase and left overnight on a shaker for solvent evaporation to remove ethyl acetate. The solidified microparticles were washed three times with water by centrifuging and removing the supernatant. The microparticles were imaged and frozen overnight. Following 48hr lyophilization, GS elution was determined in 3 ml phosphate buffered saline (PBS) at 0.25, 1,3,5 7,14, 21, and 28-day time points. The collected GS samples were derivatized to analyze with the fluorometric o-phthaldialdehyde (OPA) method (5).
Results
The microparticles were between 50-150 um in size (Figure 1). PLGA’s molecular weight and L/G ratio altered the release rate profile (Figure 2). The increase in the molecular weight of the PLGA extended the release duration of the GS. The lowest release rate on the first week (0.08 ug/mg/day) and shortest release duration (3 days) were observed with a 75/25 L/G ratio PLGA (753). On the other hand, higher molecular weight PLGAs (505&505g) had higher release rates than the lower molecular weight PLGA (503). For 503 and 505, the release rate dropped to 0.01 ug/mg/day on the 21st day and increased later due to increased degradation of particles on the 28th day.
Conclusion
The pharmacokinetic model calculation of a 160 mg application of microparticles indicated that a sustainable release of GS for 28 days over 10X MIC concentration is feasible. These results indicate the possible use of gentamicin microparticles either as prophylaxis or a treatment alternative to PJI.
Figures
Figure 1
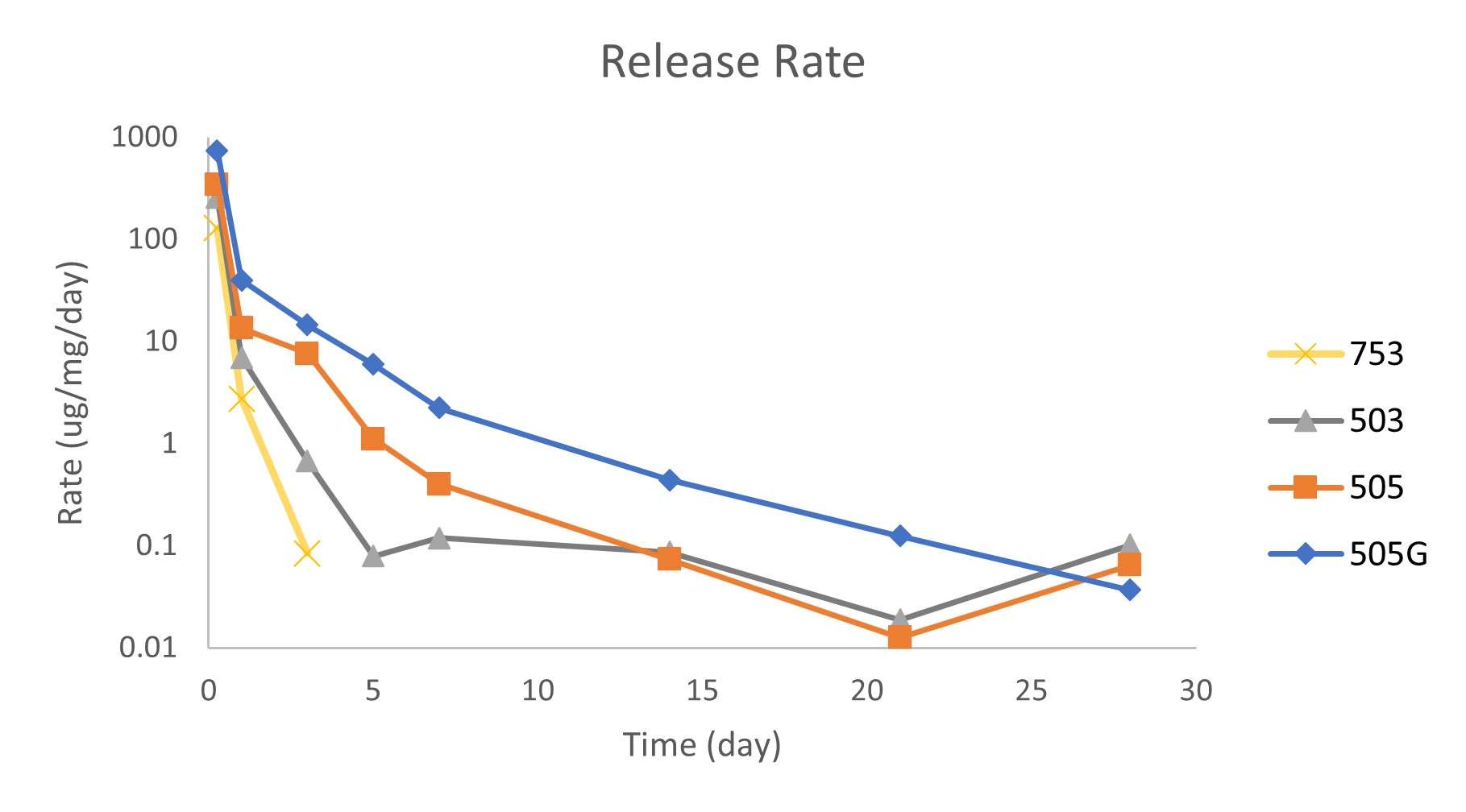
Figure 2#8709
Therapeutic Efficacy of an Erythromycin-Loaded Coaxial Nanofiber Coating in a Rat Model of Periprosthetic Joint Infection
David Markel - The CORE Institute - Novi, USA
Dexter Powell - Ascension providence Hospital - Southfield, USA
Bin Wu - Providence-Providence Park Hospital - Southfield, USA
Paula Dietz - Ascension Providence Hospital - Southfield, USA
*Therese Bou-Akl - Providence-Providence Park Hospital - Southfield, USA
Liang Chen - Wayne State University - Detroit, USA
Tong Shi - Wayne State University - Detroit, USA
Weiping Ren - Wayne State University - Select Country
*Email: Therese.Bou-akl@ascension.org
Abstract
Implant surface nanofiber (NF) coatings represent an alternative way to prevent/treat periprosthetic joint infection (PJI) via local drug release. We developed and characterized a coaxial erythromycin (EM)-doped PLGA/PCL-PVA NF coating. The purpose of this continuing study was to determine the efficacy of EM-NF coatings (EM0, no EM, EM100, 100 mg/ml and EM1000, 1000 mg/ml. wt/wt) in a rat PJI model. A strong bond of the EM-NF coating to the surface of titanium (Ti) pins was confirmed by in-vitro mechanical testing. In the rat PJI model, micro computed tomography (mCT) analysis showed that both EM100 and EM1000 NF coatings effectively reduced periprosthetic osteolysis compared to EM0 (vehicle control) at 8 and 16 weeks after implantation. Histological analysis of paraffin tissues demonstrated that both EM100 and EM1000 coatings effectively controlled infection and enhanced periprosthetic new bone formation. Hard tissue sections at 16 weeks had similar findings. The bone implant contact (BIC) of EM100 (35.08%) was higher than negative controls and EM0 (3.43% and 0%, respectively), and the bone area fraction occupancy (BAFO) of EM100 (0.63 mm2) was greater than controls and EM0 (0.390 mm2 and 0.0 mm2, respectively). The BAFO of EM100 was higher than that of EM1000 (0.3mm2). These findings may provide basis for a new implant surface fabrication strategy aimed at reducing the risks of defective osseointegration and PJI, and likely to improve the success rate of TJR.
KEYWORDS
Coaxial nanofibers, erythromycin (EM), periprosthetic joint infection (PJI), surface coating, drug release, osteointegration, rat model
1. Introduction
Septic and aseptic loosening are common failure mechanisms of total joint arthroplasty (TJA) 1; 2. Improving osseointegration and preventing periprosthetic joint infection (PJI) have great clinical benefits. An orthopedic implants that would promote rapid osseointegration and prevent PJI would be of great benefit particularly when placed into bone compromised by disease or physiology.
Erythromycin (EM), a commonly used antibiotic, is a 14-membered lactone ring macrolide. It is effective against most bacteria encountered in PJI.3; 4 EM has other biological effects well beyond its antimicrobial activity5. It has effectiveness in ameliorating chronic inflammation by down regulation of elevated NF-kB signaling6-8 without negatively effecting the NF-kB signaling7. EM also inhibited wear debris-induced inflammation and bone loss (in-vitro and in-vivo)7; 9; 10 by inhibiting osteoclast formation and enhancing osteoblast activity. The effects are immunomodulatory rather than immunosuppressive7; 8. This is important since PJI patients have some immunological dysfunction11. In addition, EM has a positive anabolic effect and has been shown to positively impact osteoblast growth and differentiation of murine MC3T3 pre-osteoblasts12. The challenge is delivering EM to periprosthetic tissue in a sustained and predictable way13; 14.
Implant surface fabrication techniques have been developed to improve osseointegration and prevent PJI via sustained periprosthetic drug delivery15-17. One of the most promising methods of modifying an implant surface is nanoscale coating via electrospinning18. By using coaxial electrospinning19, nanofibers (NFs) can be applied to an implant and used as controllable drug release devices. In a coaxial core-sheath system, drug release is affected by both the concentration gradient and the degradation rate of the sheath barrier20.
We developed a coaxial electrospun NF composed of polycaprolactone (PCL)/ poly(lactide-co-glycolide) (PLGA)sheath and polyvinyl alcohol (PVA)core polymers (PCL/PLGA-PVA) 21; 22. PCL and PLGA are FDA-approved hydrophobic polymers that can be used as local drug delivery devices23; 24. The degradation rate of the PCL/PLGA sheath fiber can be used to control the release pattern of embedded drugs24 and a PVA core fiber can be used as a drug reservoir22. PVA is a water soluble and biodegradable polymer25.. In addition, PLGA and PCL can be strongly bonded to a titanium (Ti) implant surface26. We recently created an EM-doped PCL/PLGA-PVA NFs (EM-NF) and directly deposited it onto a titanium (Ti) implant surface during electrospinning27. The core and sheath components of the coaxial PCL/PLGA-PVA NF coatings were loaded with EM at different concentrations for study: EM0 (no EM), EM 100 (100mg/ml), EM500 (500mg/ml) and EM1000 (1000mg/ml). A sustained EM release from EM-NFs for > 4 weeks was observed in vitro27. The eluents collected from EM-NFs showed strong zone of inhibition (ZOI) to S. aureus growth and the sizes of ZOI positively related to the amount of EM released. In addition, EM-NFs were nontoxic to rat bone marrow stem cells (rBMSCs) and stimulated cell growth and osteoblastic differentiation of rBMSCs27. The purpose of this continuing study was to evaluate the in vivo therapeutic efficacy of the EM-NFs coating in a rat infected tibia implantation PJI model.
2. Materials and Methods
Materials:
PVA(MW~205,000), PCL (MW~70,000-90,000), PLGA(MW~54,000-69,000) and EM were purchased from Sigma-Aldrich (St. Louis, MO). Titanium pins (0.8 mm diameter, 0.8 mm length with flat heads 1.94 mm diameter) were obtained from Shanghai Sixth Hospital (Shanghai, China). Sprague Dawley (SD) rats (female, BW 200g-300g) purchased from Charles River laboratories (Wilmington, MA). Staphylococcus aureus (SA) was obtained from ATCC (#49230).
Preparation of EM- doped coaxial PCL/PLGA (1:1)-PVA NFs
The PCL/PLGA solution was prepared by mixing 11% PCL and 15% PLGA (dissolved in chloroform/dimethylformamide (DMF) at the ratio 1:1 (v/v) with stirring)24; 27. PVA 15% solution was prepared by dissolving PVA powders in distilled water at 90 oC. Both PLGA/PCL(sheath) and PVA(core) solution were mixed with EM stock solution (5 mg/ml) before electrospinning. The final EM concentration in the sheath and core fibers were either 100mg/ml (EM100) or 1000mg/ml (EM1000). NFs without EM doping (EM0) were included as vehicle controls. The selection of the two EM concentrations (EM100 or EM1000) was based on the in vitro physiochemical and biological characterization of EM-NF formulations27. Coaxial electrospinning was achieved as previously described using a customized coaxial nozzle28 with 20 KV voltages applied. To achieve EM-NF coating, Ti pins were fixed on a specific designed collector, which has two horizontal and parallel embedded and electric-conductive sticks to hold the Ti pins. EM-NFs were directly deposited on the Ti surface. The coated pins were then soaked briefly in 70% ethanol, air-dried and sterilized by UV light irradiation overnight before animal experiment. The thickness of the EM-NF coating (~50 um) was determined by micro-caliper.
Bonding strength of Ti pins with EM-NF coating
An ex vivo porcine bone implantation model28 was used to determine whether the EM-NF coating remained intact during implantation. Fresh-frozen porcine knee specimens were purchased from a local slaughterhouse and stored at −20oC. The specimens were thawed overnight, and all soft tissues were removed before testing. A flat dissected bony surface was obtained after removing all superficial articular tissues. The tibial bone holes (diameter = 0.835 mm; depth = 10 mm) were made. Ti pins with EM-NF coating were slowly inserted into the bony holes using an Instron model 8841 Universal Materials Test Machine at the speed of 3 mm min−1. The porcine bones with implanted Ti pins were then soaked in sterilized PBS at 37oC for two days before the pullout of the implanted Ti pins. The maximum friction force required for both push in and pullout of Ti pins were recorded (n = 3).
Rat model of S. aureus-infected tibia implantation
The study wasapproved by the IACUC of Ascension-Providence Hospital and Wayne State University. Fifty-six (56) SD rats were divided into four groups (n=8 per group and each time point, Table 1). Rats were anesthetized by intraperitoneal injection (Xylazine at 8mg/kg and Ketamine at 75mg/kg). The right hind limb area was shaved followed by a rinse of 70% Alcohol. A 10 mm medial parapatellar incision was made and the patellar tendon laterally dislocated to expose the tibial plateau. The center of the tibial plateau was reamed to form a circular indent of 1.5mm diameter and 0.5mm depth. A pilot hole was created at the intercondylar eminence and a 1.0-mm Ti wire used to make a channel into the medullary canal. Pre-colonized NF coated Ti pins were inserted into the medullary canal to form a part of the knee joint. A mixed contamination model S. aureus was used: direct injection of broth (1 × 103 CFU) into the medullary cavity before pin insertion and one step pin dip-soak in 2 ml of culture (1 × 103 CFU) just before implantation. Wounds were closed in layers. Animals were housed individually and provided food and water ad libitum. Ketoprofen 5mg/kg was administered post-operatively and once daily for one week. Rats were sacrificed at 8 and 16 weeks after surgery, respectively.
Evaluation of periprosthetic osteolysis by micro computed tomography (μCT)
Baseline scanning was performed immediately postoperatively then at sacrifice (8 or 16 weeks) using a μCT (vivaCT 40, Scanco Medical AG, Brüttisellen, Switzerland) set at 70 kV, 114 μA, with 650 ms integration time and 40 μm resolution. Periprosthetic osteolysis area (mm2) was measured on defined sections 0.2 mm from the pin surface (Fig. 2A). Briefly, all 2D μCT slices were examined from the top of the pin to the distal end. The image slice with the largest area of periprosthetic osteolysis was selected and the contour around the bony margin of that area was measured with built in software. The calculated Ti pin area was 0.0064cm2.
Histological analysis of decalcified paraffin specimens
Tibia samples were fixed (10% formalin) for 24 hours then decalcified (10% ethylene diamine tetra-acetic acid (EDTA)). The Ti pins were gently removed with minimal loss tissues at the bone-implant interface. Paraffin embedded tissue sections (6 mm) were cut and stained with hematoxylin & eosin (H&E). Bone area (mm2) was the measured area of bone tissue in the region 200 µm from the pin surface. Presence of fibrous tissue, infection and inflammatory cellular infiltration were described. The histology slides were evaluated by an independent investigator blinded to treatment.
Histomorphometry of hard tissue sections
One tibia containing a pin from each group was fixed in 70% alcohol, dehydrated in graded ethanol solutions and embedded in methylmethacrylate without decalcification. Sections, 40-mm-thick, were created perpendicular to the implant’s long axis using a rotary diamond saw (SP1600, Leica, Germany) then polished (Leica SP2600). The sections were stained with 1% toluidine blue. Bone-to-implant contact (BIC) was calculated as the linear percentage of the interface with direct bone-to-implant contact to total interface of the implant in the cancellous bone. Bone area fraction occupancy (BAFO) was measured as the area percentage of bone tissue to the whole area, defined as a ring 200 mm from the implant surface.
Statistical Analysis
Values were expressed as mean ± standard deviation. All analyses were performed using Microsoft Excel 2016. ANOVA single factor was used for comparison between group means and a p-value < 0.05 was considered significant.
3. Results
Bonding strength of EM-NF coating on the surface of Ti pins
Using an ex vivo porcine bone implantation model, we found that the EM-NF coating remained intact after the test of push in and pullout (Fig. 1). A few samples of EM-NF coating were disrupted at the end of the pin that may have been caused by the uneven or rough surface of the created bone holes. The maximum shear force required for insertion of Ti pins coated with EM0, EM100 and EM1000 were 4.51N, 14.3N and 7.8 N, respectively. The maximum shear force required for pull out of Ti pins coated with EM0, EM100 and EM1000 were 2.2 N, 4.9 N and 4.0 N, respectively (Fig. 2C). EM doping increased the force required for both push in and pullout of Ti pins, especially for EM100 (p<0.05). Our data confirmed that the bonding strength of the EM-NFs to the smooth surface of the Ti pins was acceptable for the planned animal experiment.
Animal study
No rats were dropped from the study due to body deterioration (weight loss or fever), wound infection (swelling of the leg, loss of active motion in knee and ankle joints) or other complications
mCT evaluation of Osteolysis
The area of periprosthetic osteolysis was considered to reflect efficacy of infection control. EM-NF coating (EM100 and EM1000) effectively reduced S. aureus induced periprosthetic osteolysis vs EM0 control at both 8 and 16 weeks (Fig. 2). At 8 weeks, areas of osteolysis for EM100 and EM1000 were smaller than EM0 control and close to the negative control. The trend was even more significant at 16 weeks. Areas of osteolysis were smaller in EM100 than with EM0 (p=0.08).
Histological analysis of decalcified paraffin tissue sections
The inhibition of infection and osteolysis by EM-NFs was confirmed by histology (Fig. 3). Characteristic features of periprosthetic infection with osteolysis were seen in EM0: sequestrum, adjacent soft tissue abscesses, expanded bone loss around implants, significant bacterial growth, inflammation, and exudation. Treatment with both EM100 and EM1000 controlled local infection and enhanced periprosthetic new bone formation at 8 and 16 weeks. Some EM-NF matrix residue was observed at the interface of newly formed bone and the Ti pin surface at 8 weeks, but none was found at16 weeks indicating complete resorption of the NFs.
Hard tissue sections histology
The efficacy of EM100 and EM1000 coatings were further confirmed by 16 week hard tissue sections. The Bone implant contact (BIC) and bone area fraction occupancy (BAFO) within 200µm of the implanted Ti pin surfaces were used to evaluate the osseointegration around the implants (Fig. 4, Table 2). The BIC (%) of EM100 (35.08%) was higher than negative control (3.43%) and EM0 (0%). The BAFO of EM100 (0.63 mm2) was higher than negative control (0.390 mm2) and EM0 (0.0 mm2). The BAFO of EM 100 was also higher than EM1000 (0.3mm2).
4. Discussion
Strategies aimed at reducing PJI and the resultant lack of osseointegration should improve the success of TJA and increase implant longevity29. We developed EM-eluting coaxial PCL/PLGA-PVA NF coating (EM-NF) that had sustained and controllable drug delivery with prolonged bacterial growth inhibition. The degradation of the NF matches the period of an implant’s osseointegration 27. In this continuing study, it was demonstrated that the EM-NF coding could be strongly bound to a titanium surface and that the drug elution effectively inhibited periprosthetic infection and enhanced osseointegration up to 16 weeks after implantation in a rat PJI model. The data indicated that the structural and mechanical advantages of EM-NF coating shows potential to both prevent PJI and enhance osseointegration.
Implant coatings like hydroxyapatite (HA), have been widely used to promote increase implant osseointegration. But use drug delivery from HA is limited due to weak binding between loaded drug and HA surface and a resultant burst drug release30-34. PMMA cement has also been applied for antibiotic drug delivery and widely used for PJI prevention and treatment. But, the efficacy of antibiotics impregnated cement continues to be debated.35; 33; 36
A drug-eluting implant coating should deliver antibiotics at a level well above the minimum inhibitory concentration (MIC) for at least 6 weeks for PJI treatment22; 37. In our previous study, we noted a constant and sustained EM release from NFs up to 12 weeks when EM was embedded both in the core and sheath fiber solution before electrospinning27. The amount of EM released was proportional to the amount of EM doped27. The concentration of EM (EM100 and EM1000) released was higher than the concentration required for the MIC of S. aureus (1mg/ml)38 at all phases27. Furthermore, the EM released was stable and inhibited bacterial growth in-vitro27. Therefore, EM-NF coating is expected to provide a first line defense against infection particularly if an implant were placed in a contaminated or high risk surgical field.
We then evaluated the therapeutic efficacy of EM-NF coating in a rat PJI model39. This rat model has been widely used for the therapeutic efficacy evaluation of periprosthetic drug delivery40 and implant surface modification41. The rat model has an orthotropic weight bearing implant being subjected to sustained mechanical shear stresses in vivo. As shown in Fig. 2, EM-NF coating (EM100 and EM1000) effectively reduced S. aureus infection induced periprosthetic osteolysis compared to EM0 control at 8 and 16 weeks by mCT analysis. The rate, quantity and quality of periprosthetic new bone formation (osseointegration) are relied on for efficacy of infection control, as well as the physiochemical and biological properties of EM-NF coating42; 43. Consistent with the µCT findings, histological analysis of paraffin tissue sections (Fig.3) showed a typical features of periprosthetic infection with osteolysis in EM0 group. Treatment with both EM100 and EM1000 effectively controlled local infection and enhanced periprosthetic new bone formation at both 8 and 16 weeks. The EM-NF were biocompatible and completely resorbed over the study period. The therapeutic efficacies of EM-NF coating were further validated by the measurement of periprosthetic bone volume at 16 weeks (Fig. 4, Table 2).
Taken together, data from animal study demonstrated that both EM100 and EM1000 effectively inhibited S. aureus infection and enhanced osseointegration up to 16 weeks. However, EM1000 had lesser impacts on periprosthetic new bone formation compared to EM100, due to the local much higher EM concentration than that of EM100. Our finding is in agreement with out in vitro data that EM100 NF coating significantly enhanced the cell growth and differentiation of rat bone marrow cells that was significantly diminished by EM1000 treatment.27
There were some study limitations. First, additional microbiological analysis of implanted Ti pins and/or bone marrow cavity washout should be studied in the future to establish the status of infection control (bacterial growth and biofilm formation, etc.). Second, we need to develop noninvasive imaging techniques for the real-time evaluation of in-vivo EM release kinetics. Finally, the sample size was small (n=8) when considering the requirements of quantitative histological analysis for both hard tissue and paraffin section specimens.
5. Conclusions
Data from this animal study demonstrated that EM-NF coatings could be well bound to a titanium surface and were dually functional in enhancing osseointegration and eliminating bone infection up to 16 weeks. These findings may provide a new implant surface fabrication strategy aimed at reducing the risks of defective osseointegration and PJI, and likely to improve the success rate of TJR.
Acknowledgments
This work was in part supported by the Rehabilitation Research & Development Award by the Department of Veterans Affairs (RX001818-01).
Table 1. Rat groups
|
Group
|
Description
|
8 Weeks
|
16 Weeks
|
|
I
|
NF coating, no SA, negative control
|
4
|
4
|
|
II
|
EM0-NF coating, with SA, positive control
|
8
|
8
|
|
III
|
EM100-NF coating, with SA
|
8
|
8
|
|
VI
|
EM1000-NF coating, with SA
|
8
|
8
|
Table 2 BIC and BAFO measurement
|
Group
|
BIC (%)
|
BAFO
|
Bone to implant contact (BIC): The BIC was calculated as the percentage of implant surface in direct contact with the bone over the entire length of the implant within the section examined.
Bone area fraction occupancy (BAFO): The BAFO was the area occupied by the mineralized bone matrix within 200um of the implant within the section. Measurement was made by outlining the bone surface area from the total field within 200um radius of the implant and was expressed as percentage (implant area was removed).
|
|
Negative control
|
3.43
|
0.39
|
|
EM0
|
0
|
0
|
|
EM100
|
35.08
|
0.63
|
|
EM1000
|
0
|
0.3
|
References
1. Pulido L, Ghanem E, Joshi A, et al. 2008. Periprosthetic joint infection: the incidence, timing, and predisposing factors. Clin Orthop Relat Res 466:1710-1715.
2. Lee K, Goodman SB. 2008. Current state and future of joint replacements in the hip and knee. Expert Rev Med Devices 5:383-393.
3. Guardia A, Shi T, Bou-Akl T, et al. 2021. Properties of erythromycin-loaded polymeric dicalcium phosphate dehydrate bone graft substitute. Journal of Orthopaedic Research n/a:1-9.
4. Ruzaimi MY, Shahril Y, Masbah O, et al. 2006. Antimicrobial properties of erythromycin and colistin impregnated bone cement. An in vitro analysis. Med J Malaysia 61 Suppl A:21-26.
5. Giamarellos-Bourboulis EJ. 2008. Macrolides beyond the conventional antimicrobials: a class of potent immunomodulators. Int J Antimicrob Agents 31:12-20.
6. Ren W, Markel DC. 2011. Emerging Ideas: Can Erythromycin Reduce the Risk of Aseptic Loosening? Clin Orthop Relat Res 469:2399-2403.
7. WP R, XY L, BD C, et al. 2004. Erythromycin inhibits wear debris-induced osteoclastogenesis by modulation of murine macrophage NFkB activity. J Orthop Res 22:21-29.
8. Shinkai M, Henke MO, Rubin BK. 2008. Macrolide antibiotics as immunomodulatory medications: Proposed mechanisms of action. Pharmacol Ther 117:393-405.
9. WP R, W B, L M, et al. 2006. Erythromycin (EM) inhibits wear debris-induced inflammatory osteolysis in a murine model. J Orthop Res 24:280-290.
10. Ren W, Blasier R, Peng X, et al. 2009. Effect of oral erythromycin therapy in patients with aseptic loosening of joint prostheses. Bone 44:671-677.
11. Nich C, Takakubo Y, Pajarinen J, et al. 2013. Macrophages-Key cells in the response to wear debris from joint replacements. J Biomed Mater Res A 101:3033-3045.
12. Shen Y, Wang W, Li X, et al. 2014. Mitigative effect of erythromycin on PMMA challenged preosteoblastic MC3T3-E1 cells. ScientificWorldJournal 2014:107196.
13. Song W, Yu X, Wang S, et al. 2011. Cyclodextrin-erythromycin complexes as a drug delivery device for orthopedic application. Int J Nanomedicine 6:3173-3186.
14. Ren WP, Song W, Esquivel AO, et al. 2014. Effect of erythromycin-doped calcium polyphosphate scaffold composite in a mouse pouch infection model. J Biomed Mater Res B Appl Biomater 102:1140-1147.
15. Simchi A, Tamjid E, Pishbin F, et al. 2011. Recent progress in inorganic and composite coatings with bactericidal capability for orthopaedic applications. Nanomedicine: Nanotechnology, Biology and Medicine 7:22-39.
16. Anselme K, Ponche A, Bigerelle M. 2010. Relative influence of surface topography and surface chemistry on cell response to bone implant materials. Part 2: biological aspects. Proc Inst Mech Eng H 224:1487-1507.
17. Gao P, Nie X, Zou M, et al. 2011. Recent advances in materials for extended-release antibiotic delivery system. Journal of antibiotics 64:625-634.
18. Baker BM, Handorf AM, Ionescu LC, et al. 2009. New directions in nanofibrous scaffolds for soft tissue engineering and regeneration. Expert Rev Med Devices 6:515-532.
19. Gluck JM, Rahgozar P, Ingle NP, et al. 2011. Hybrid coaxial electrospun nanofibrous scaffolds with limited immunological response created for tissue engineering. Journal of Biomedical Materials Research Part B: Applied Biomaterials 99B:180-190.
20. Szentivanyi A, Chakradeo T, Zernetsch H, et al. 2011. Electrospun cellular microenvironments: Understanding controlled release and scaffold structure. Advanced Drug Delivery Reviews 63:209-220.
21. Song W, Yu X, Markel D, et al. 2013. Coaxial PCL/PVA electrospun nanofibers: osseointegration enhancer and controlled drug release device. Biofabrication 5:035006.
22. Song W, Seta J, Chen L, et al. 2017. Doxycycline-loaded coaxial nanofiber coating of titanium implants enhances osseointegration and inhibits Staphylococcus aureus infection. Biomedical materials (Bristol) 12:045008.
23. Makadia HK, Siegel SJ. 2011. Poly Lactic-co-Glycolic Acid (PLGA) as Biodegradable Controlled Drug Delivery Carrier. Polymers 3:1377-1397.
24. Chen L, Mazeh H, Guardia A, et al. 2019. Sustained release of strontium (Sr2+) from polycaprolactone/poly (D,L-lactide-co-glycolide)-polyvinyl alcohol coaxial nanofibers enhances osteoblastic differentiation. Journal of Biomaterials Applications 34:533-545.
25. Song W, Markel DC, Wang S, et al. 2012. Electrospun polyvinyl alcohol-collagen-hydroxyapatite nanofibers: a biomimetic extracellular matrix for osteoblastic cells. Nanotechnology 23:115101.
26. Xiao D, Liu Q, Wang D, et al. 2014. Room-temperature attachment of PLGA microspheres to titanium surfaces for implant-based drug release. Applied Surface Science 309:112-118.
27. Ren W, Yu X, Chen L, et al. 2022. Osteoblastic differentiation and bactericidal activity are enhanced by erythromycin released from PCL/PLGA-PVA coaxial nanofibers. Journal of Biomaterials Applications 37:712-723.
28. Song W, Yu X, Markel DC, et al. 2013. Coaxial PCL/PVA electrospun nanofibers: osseointegration enhancer and controlled drug release device. Biofabrication 5:035006.
29. Raphel J, Holodniy M, Goodman SB, et al. 2016. Multifunctional coatings to simultaneously promote osseointegration and prevent infection of orthopaedic implants. Biomaterials 84:301-314.
30. Pastorino D, Canal C, Ginebra MP. 2015. Drug delivery from injectable calcium phosphate foams by tailoring the macroporosityGÇôdrug interaction. Acta Biomaterialia 12:250-259.
31. Peter B, Pioletti DP, Laib S, et al. 2005. Calcium phosphate drug delivery system: influence of local zoledronate release on bone implant osteointegration. Bone 36:52-60.
32. Fosca M, Rau JV, Uskokovi? V. 2022. Factors influencing the drug release from calcium phosphate cements. Bioactive Materials 7:341-363.
33. Zhou Z, Seta J, Markel DC, et al. 2018. Release of vancomycin and tobramycin from polymethylmethacrylate cements impregnated with calcium polyphosphate hydrogel. Journal of Biomedical Materials Research Part B: Applied Biomaterials 106:2827-2840.
34. Anagnostakos K, Wilmes P, Schmitt E, et al. 2009. Elution of gentamicin and vancomycin from polymethylmethacrylate beads and hip spacers in vivo. Acta Orthop 80:193-197.
35. Hinarejos P, Guirro P, Leal J, et al. 2013. The use of erythromycin and colistin-loaded cement in total knee arthroplasty does not reduce the incidence of infection: a prospective randomized study in 3000 knees. J Bone Joint Surg Am 95:769-774.
36. Nien YH, Lin Sw, Hsu YN. 2013. Preparation and characterization of acrylic bone cement with high drug release. Materials Science and Engineering: C 33:974-978.
37. Lepretre S, Chai F, Hornez JC, et al. 2009. Prolonged local antibiotics delivery from hydroxyapatite functionalised with cyclodextrin polymers. Biomaterials 30:6086-6093.
38. Tang P, Low DE, Atkinson S, et al. 2003. Investigation of Staphylococcus aureus isolates identified as erythromycin intermediate by the Vitek-1 System: comparison with results obtained with the Vitek-2 and Phoenix systems. Journal of clinical microbiology 41:4823-4825.
39. Monzon M, Garcia-Alvarez F, Lacleriga A, et al. 2001. A simple infection model using pre-colonized implants to reproduce rat chronic Staphylococcus aureus osteomyelitis and study antibiotic treatment. J Orthop Res 19:820-826.
40. Back D, Pauly S, Rommel L, et al. 2012. Effect of local zoledronate on implant osseointegration in a rat model. BMC Musculoskeletal Disorders 13:42.
41. Cheng Z, Guo C, Dong W, et al. 2012. Effect of thin nano-hydroxyapatite coating on implant osseointegration in ovariectomized rats. Oral Surgery, Oral Medicine, Oral Pathology and Oral Radiology 113:e48-e53.
42. Kohgo T, Yamada Y, Ito K, et al. 2011. Bone regeneration with self-assembling peptide nanofiber scaffolds in tissue engineering for osseointegration of dental implants. Int J Periodontics Restorative Dent 31:e9-16.
43. Wang J, Shah A, Yu X. 2011. The influence of fiber thickness, wall thickness and gap distance on the spiral nanofibrous scaffolds for bone tissue engineering. Materials Science and Engineering: C 31:50-56.
Figures
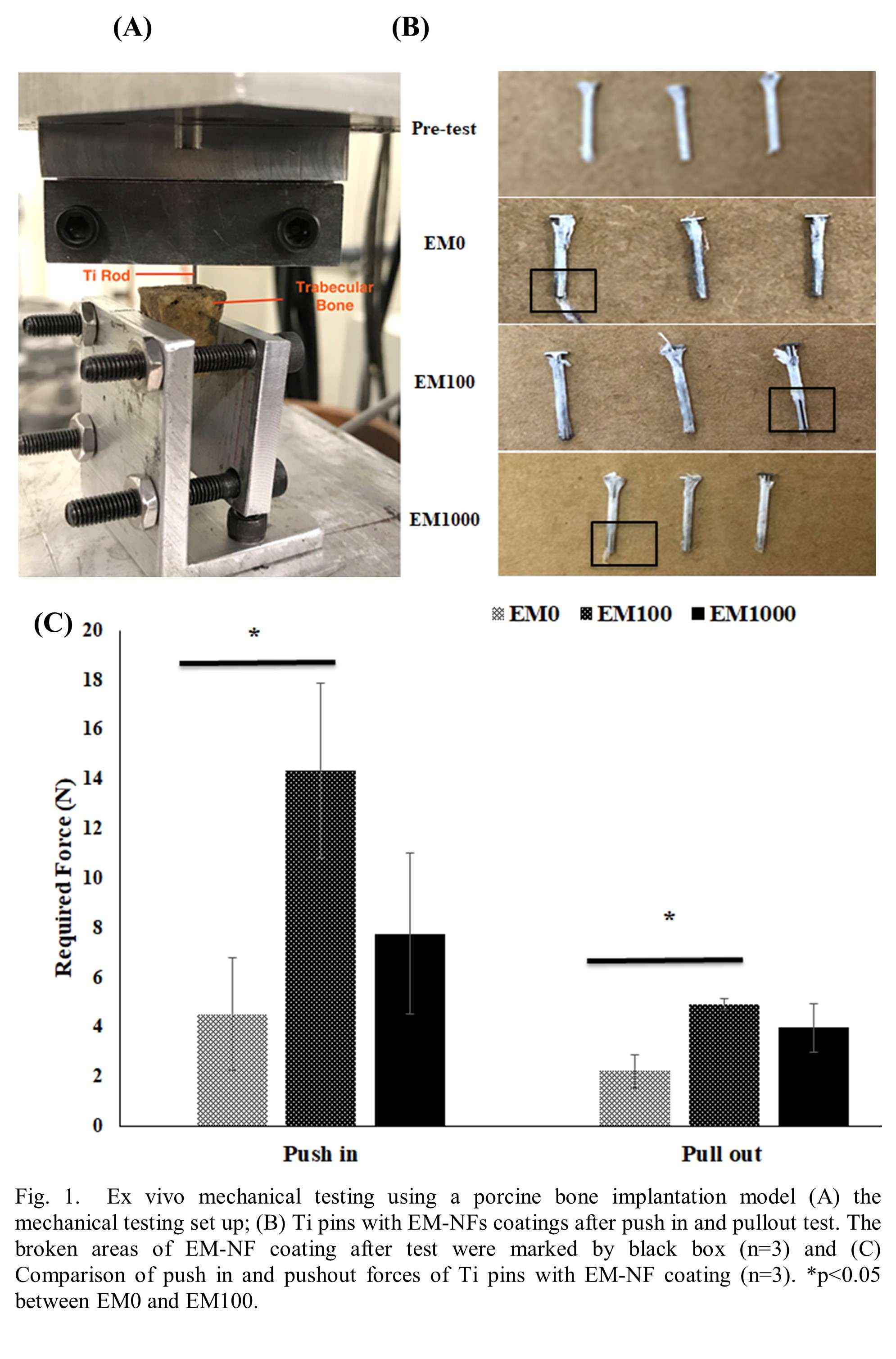
Figure 1
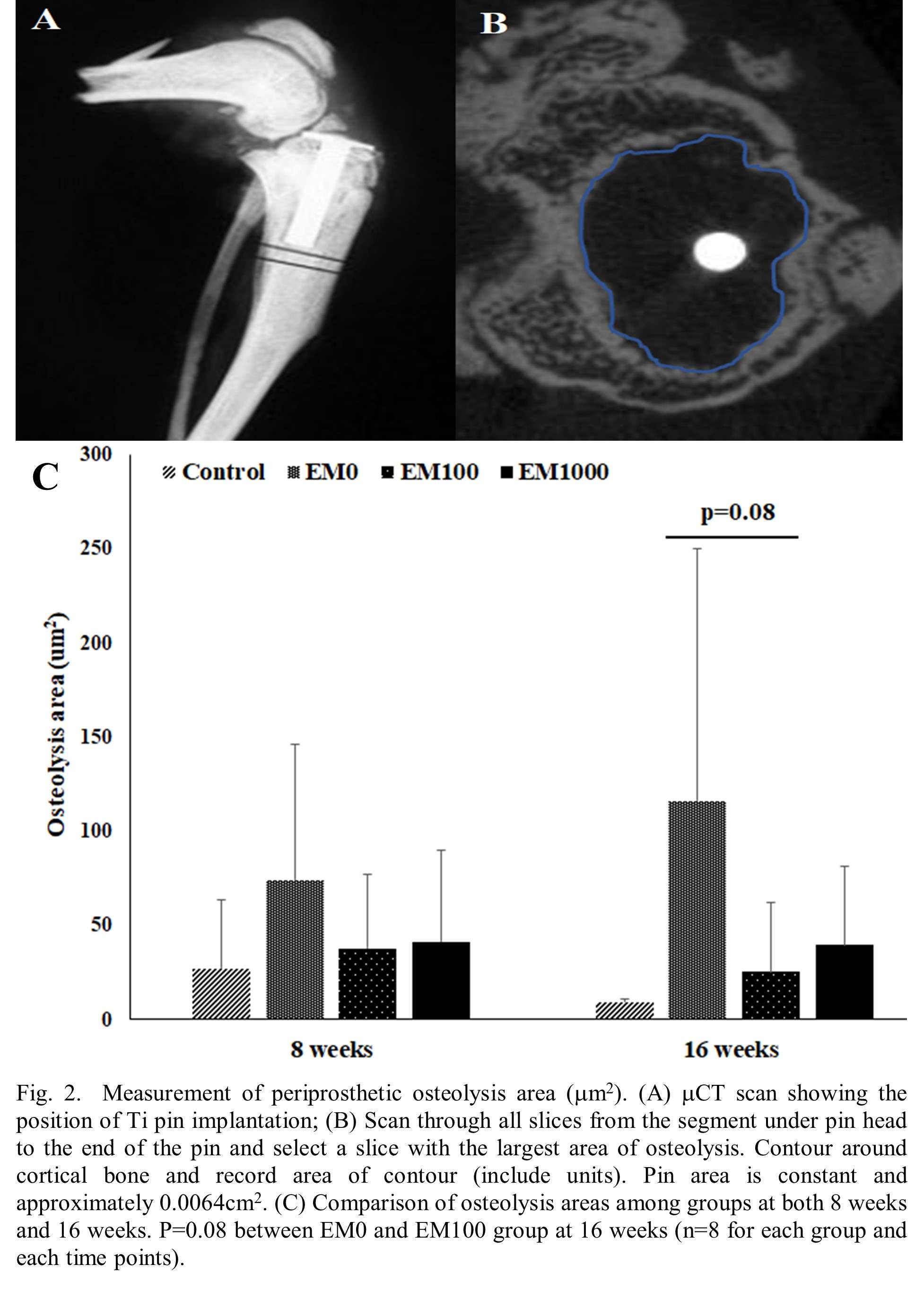
Figure 2
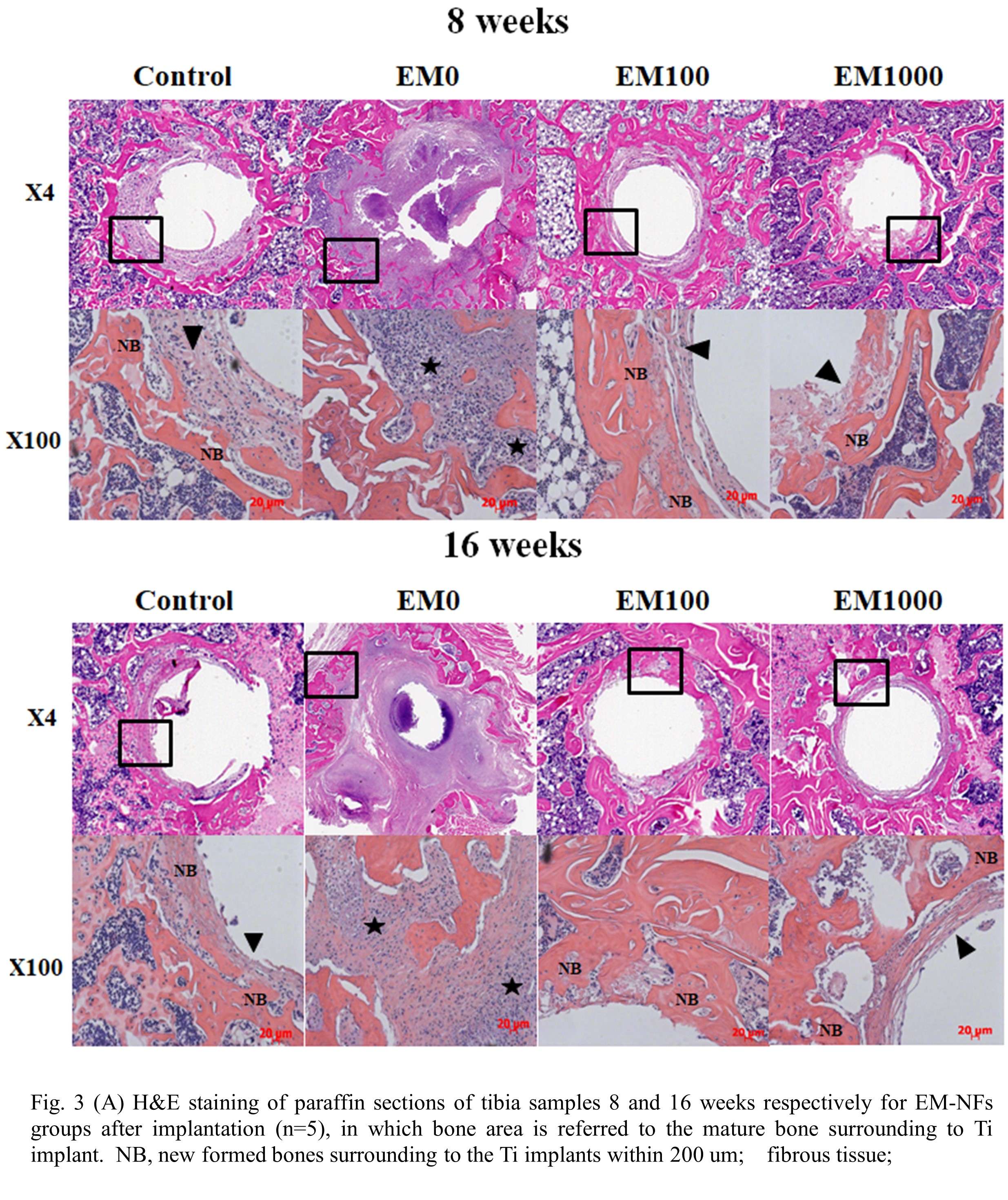
Figure 3
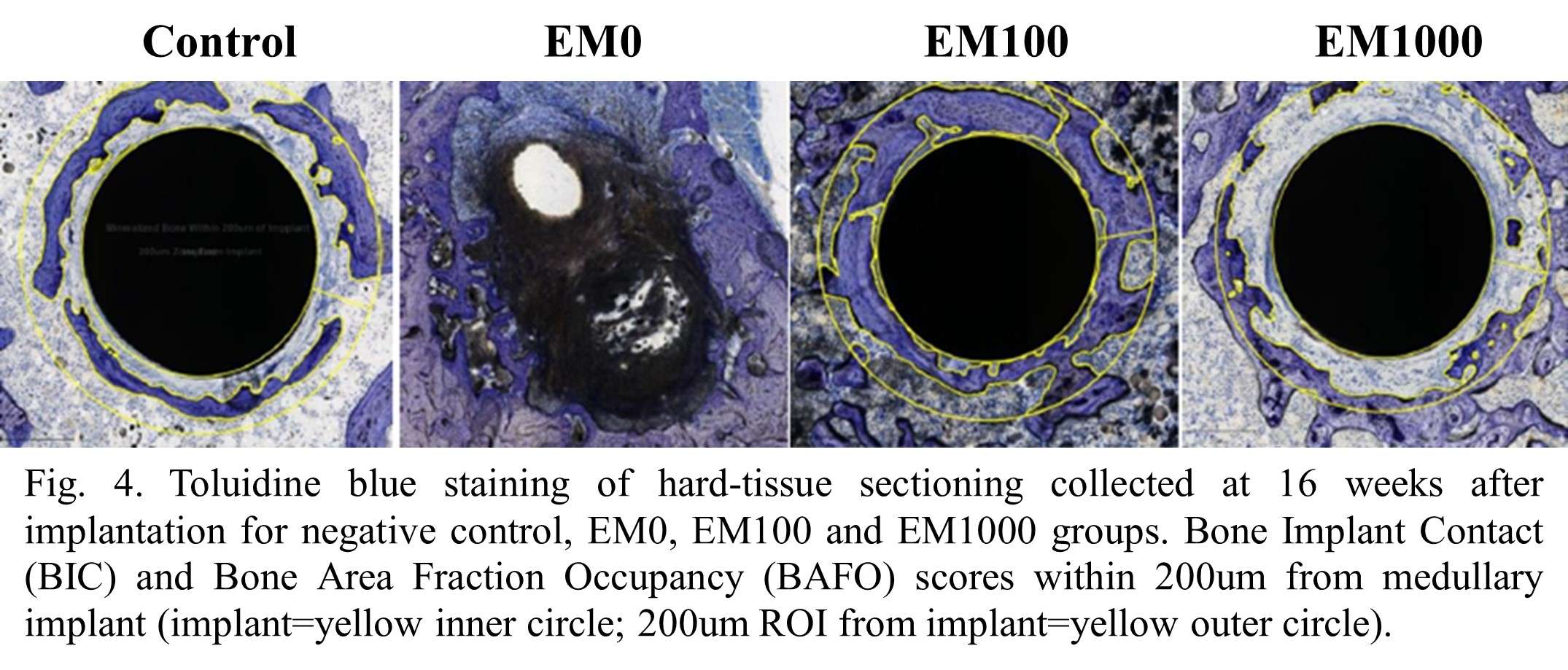
Figure 4#8740
Simultaneous Delivery of Antibiotic and Analgesic Drugs From UHMWPE for Joint Implants
Nicoletta Inverardi - Massachusetts General Hospital - Boston, United States of America
Keita Fujino - Massachusetts General Hospital - Boston, USA
Anthony Marzouca - Massachusetts General Hospital - Boston, USA
Amita Sekar - Massachusetts General Hospital - Boston, USA
Jean Yuh - Massachusetts General Hospital - Boston, USA
Eileen Walsh-Rock - Massachusetts General Hospital - Boston, USA
Mehmet Asik - USA
Orhun Muratoglu - Massachusetts General Hospital - Boston, USA
*Ebru Oral - Massachusetts General Hospital - Boston, USA
*Email: eoral@partners.org
Introduction
Local drug delivery in total joint arthroplasty may be advantageous to effectively address conditions such as pain and infection. We previously proved the feasibility of blending UHMWPE with antibiotics1 or analgesics2 and processing implants for local drug delivery. Our findings also suggested improved antibacterial activity of some drug combinations 3. In this study, we blended the antibiotic vancomycin and the analgesic bupivacaine in UHMWPE, and we studied the mechanical, elution and antibacterial properties of the compression molded blends. The purpose is to obtain a material that can release analgesic and antibiotic drugs and to investigate their simultaneous antibacterial action.
Methods
Vancomycin hydrochloride (VC) and bupivacaine hydrochloride (BP) were sieved with a 75 µm mesh and mixed with UHMWPE by mechanical turbulation (total drug loading: 1, 5 and 7 % w/w). The blends were compression molded (170 °C for 10 min 20 MPa, cooling under the applied pressure). Prior to molding, blends were dehydrated for 18-24h at 90 °C under vacuum. Samples for tensile testing were die-cut and tensile tests were run at a crosshead speed of 10 mm/min (ASTM D638-10, n=4). Machined samples (3×5×20 mm3, n=6) were eluted in 1.7 ml de-ionized water. The eluent was collected at given timepoints and the medium was replenished. Drug concentration was determined by LCMS/MS. The antibacterial properties of samples (3×5×10 mm3, n=3, control: virgin UHMWPE) were investigated by incubating them with 1.35ml of 105 CFU/ml Staphylococcus aureus (ATCC 12600) in Mueller Hinton broth at 37 °C under shaking. The bacteria viability was determined by a luminescence assay (BacTiter-Glo™, Promega)4; the remaining solution was centrifuged, and the bacteria were resuspended in fresh broth.
Results
The ultimate tensile strength (UTS) and elongation at break (EAB) decreased as drug loading increased. UTS higher than 30 MPa and EAB greater than 330% was obtained at a total drug loading equal or less than 5%. At these drug loading, the effective composition in eradicating bacteria was 1%VC+4%BP loaded UHMWPE. Compared to the 1%VC loaded UHMWPE, the composition with additional 4%BP showed an increased released in VC. This, together with the additive action between VC and BP, can explain the improved antibacterial effect when comparing 1%VC loaded UHMWPE (no eradication) to 1%VC+4%BP loaded UHMWPE (full eradication at day 2)[Fig. 1].
Conclusion
This study provides a first proof-of-concept of the beneficial effect of dual antibiotic and analgesic-eluting UHMWPE in addressing both post-operative pain and peri-prosthetic joint infection after joint arthroplasty.
References
- Suhardi et al., Nature Biomed Eng (2017)
- Grindy et al., Acta Biomaterialia (2019)
- D. Gil et al., Diagn Microbiol Infect Dis. (2020)
- A. Sekar et al., Journal of Visual Experimentation (2023)
Acknowledgements
National Institutes of Health Grant R01AR077023.
Keywords: UHMWPE, drug delivery, periprosthetic joint infection
Figures
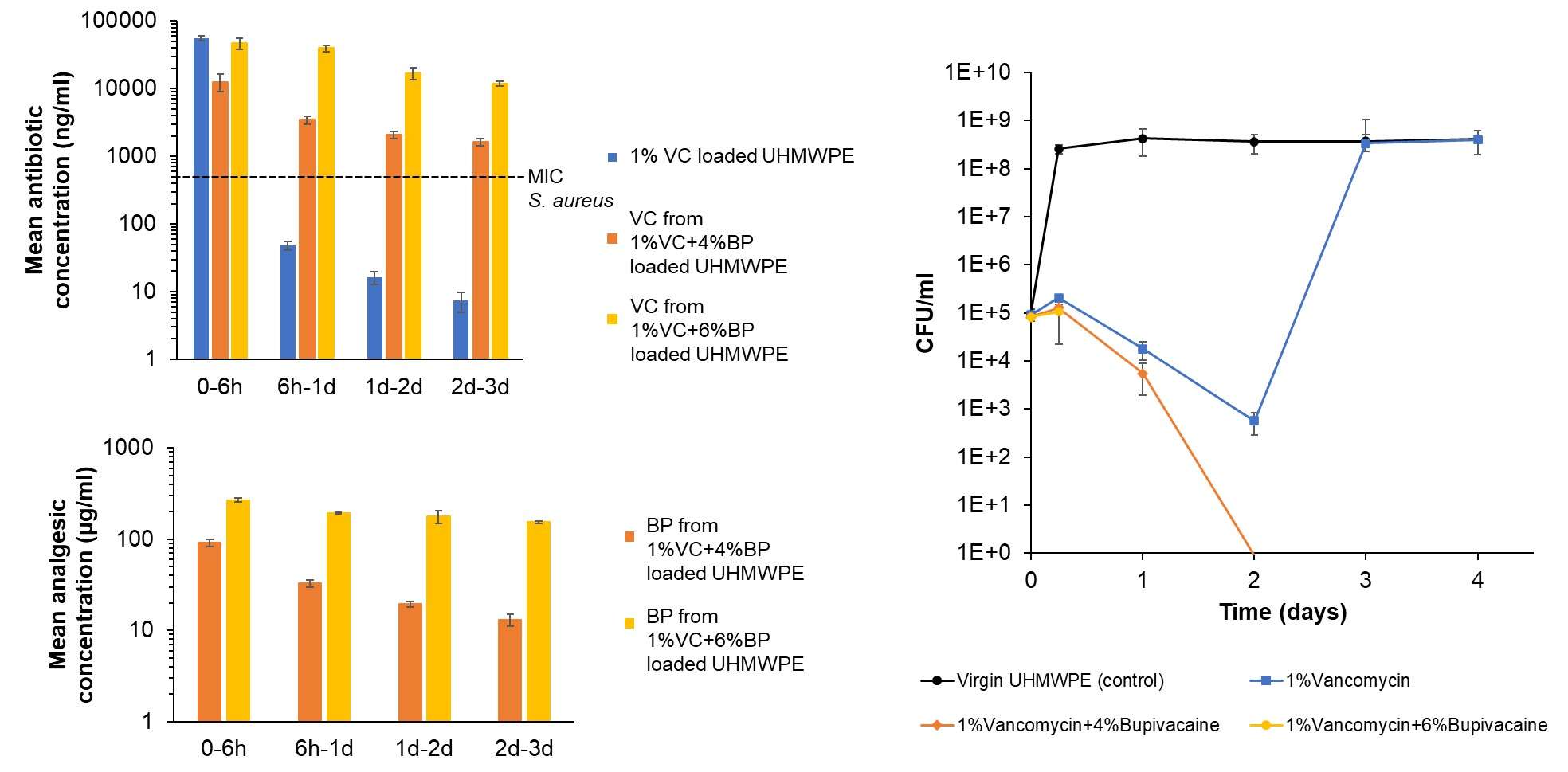
Figure 1#8779
A Novel Method to Determine the Longitudinal Antibacterial Activity of Drug-Eluting UHMWPE
Amita Sekar - Massachusetts General Hospital - Boston, USA
Sashank Lekkala - Massachusetts General Hospital - Boston, United States of America
*Ebru Oral - Massachusetts General Hospital - Boston, USA
Orhun Muratoglu - Massachusetts General Hospital - Boston, USA
*Email: eoral@partners.org
INTRODUCTION: Periprosthetic joint infections, the majority of which occur shortly after joint replacement, constitute almost 25% of total knee revision surgeries, and complete eradication of bacterial infection poses a major challenge[1]. A promising way to tackle this problem is to develop drug-delivery devices to ensure local sustained delivery of efficient concentrations of antibiotics to support systemic administration[2]. Conventional antibacterial testing methods are lacking in providing real-time and longitudinal antibacterial efficacy data which can be correlated to the elution profile of antibiotics from these devices[3]. Here we report a direct and versatile methodology to determine the antibacterial efficacy of antibiotic-eluting UHMWPE implants[4].
METHODS: Staphylococcus aureus strains ATCC 12600 (gentamicin and vancomycin susceptible), L1163 (gentamicin susceptible, vancomycin-intermediate), and L1101 (gentamicin resistant, vancomycin-intermediate) were used in this study. Gentamicin and vancomycin-loaded (7% w/w) UHMWPE blocks were prepared by blending with GUR1020 UHMWPE powder and consolidated using compression molding[2]. The cut strips (3×5×20 mm3) were eluted in 1.7ml deionized water over a series of time points. The drug concentration was measured using o- pthaldialdehyde (OPA) method and UV spectroscopy for gentamicin and vancomycin respectively. Drug-loaded and virgin UHMWPE strips (control) were placed in a 3 mL syringe filled with 1.35 mL of Mueller Hinton broth (MHB) containing 105 CFU/mL of bacteria. The syringe setup was placed on a shaking incubator at 37 °C until the indicated time points of 6 hours, Day 1, Day 2, Day 3, Day 4, Day 5, Day 6, and Day 7. Real-time ATP-based luminescence assay was performed on samples (100 µL) taken at each time point to determine the viability of bacteria. The remaining suspension was centrifuged at 10,000 × g and the pelleted bacteria was resuspended in fresh MHB to partially simulate continuous synovial turnover and ensure maintenance of the same bacterial population throughout the study. Adherent bacteria on the surface were also determined at the end of Day 7.
RESULTS: The drug release from a ~2 cm2 antibiotic-loaded UHMWPE strip demonstrated a burst release at 6 hours followed by a steady release rate with a release concentration greater than MIC until 7 days (Figure 1). Gentamicin eluted from the implant material strips was effective in eradicating the susceptible strains (ATCC 12600, L1163) within 3 days (>3log reduction) but was ineffective in eradicating resistant strain L1101. Concurrently, eluted vancomycin effectively eradicated L1163 in 1 day, ATCC 12600 in 2-3 days, and failed to completely eradicate persistent subpopulations of L1101 exhibiting vancomycin-intermediate resistance (Figure 2). The viable adherent bacteria count on surfaces of both gentamicin and vancomycin-eluting UHMWPE also validated the varying efficacy profile of drug-eluting implant against the S. aureus strains
CONCLUSION: Using this semi-static method, it was possible to differentiate both the extent and the rate at which the implant materials affected the different strains. The study also revealed the antibacterial efficacy of drug concentrations eluted from <2% of implant surface implying that antibiotics could be loaded onto non-load bearing regions of implants without interfering with their functionality.
Figures
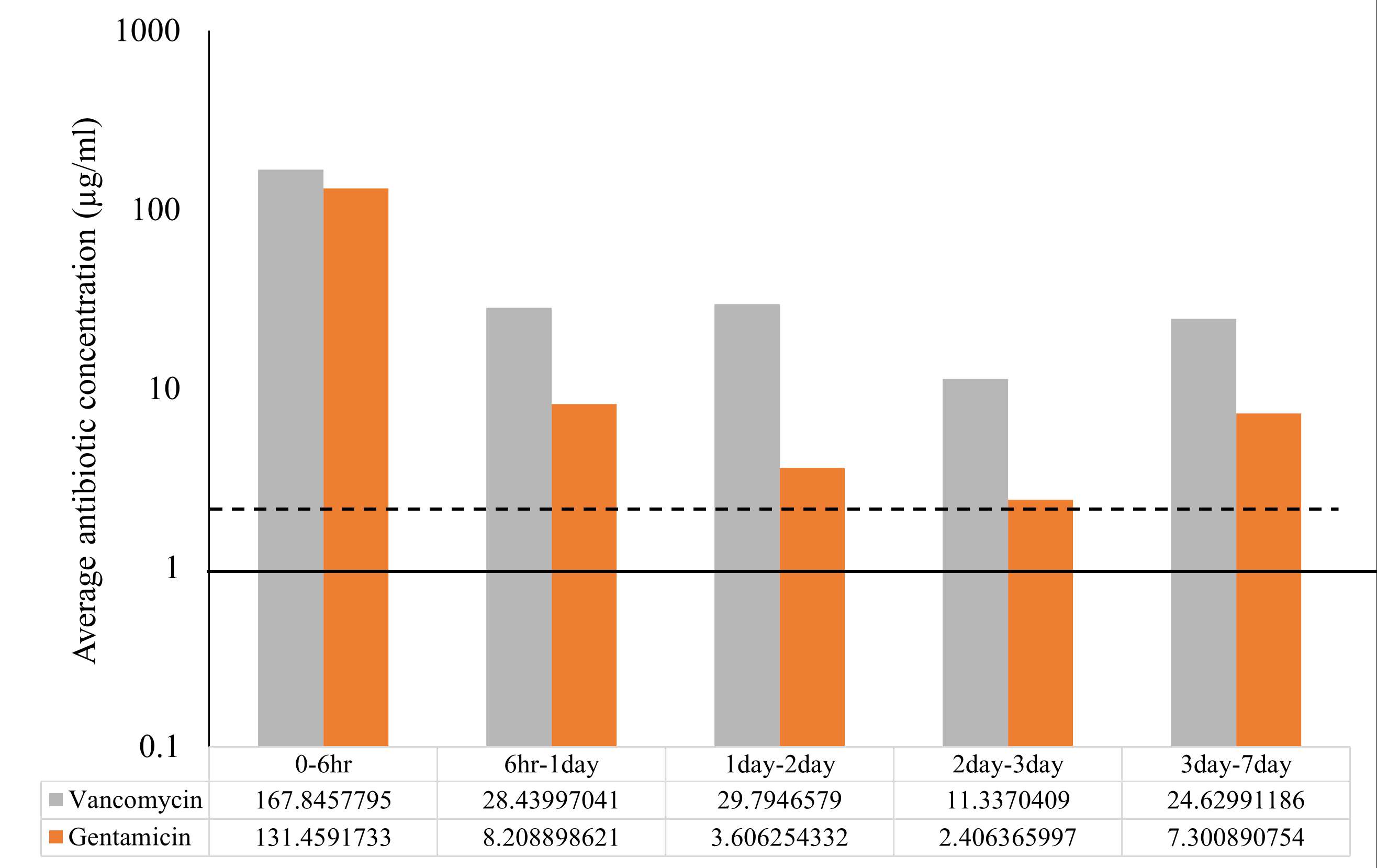
Figure 1
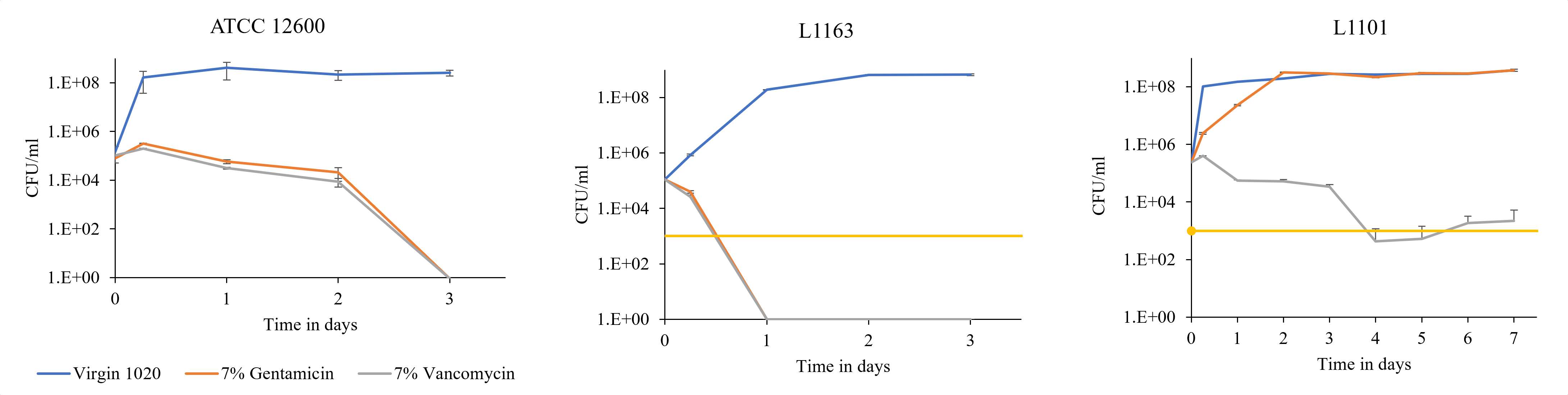
Figure 2#8477
3D-Printed Silicon Nitride-PEEK Composite Demonstrates Antimicrobial Effect in Vitro
*Paul DeSantis - Drexel University - Philadelphia, United States of America
Ryan Bock - SINTX Technologies - Salt Lake City, USA
Noreen Hickok
Steven Kurtz - Drexel University - Philadelphia, USA
*Email: pmd67@drexel.edu
INTRODUCTION: Aseptic loosening and infection are prevalent reasons for revision in total knee and total hip arthroplasty [1,2]. An in vivo experiment where rats were induced with Staphylococcus epidermidis found after 3 months that implants made from silicon nitride (Si3N4), a synthetic non-oxide ceramic, had strong antibacterial and osseointegrative properties when compared to titanium and polyetheretherketone (PEEK) [3]. Due to its ceramic nature, Si3N4 can be susceptible to brittle fracture on its own [4], however it is proposed that a composite material made from both Si3N4 and PEEK could have the potential to combine the favorable antimicrobial properties of Si3N4, with the biomechanical properties of PEEK. In addition to having similar mechanical properties to bone, PEEK is a semi-crystalline thermoplastic that can be 3D-printed into patient-specific geometries, making it useful in many arthroplasty procedures.
METHODS: Pure PEEK filament and a 15 vol% Si3N4/PEEK composite (SN/PEEK) were extruded into rods with 1.75 mm diameter and a length of 14 mm. As-fired-silicon-nitride (AFSN) rods were manufactured with the same dimensions and acted as Si3N4 controls. One colony of S. epidermidis (ATCC 14990) was added to 5 mL of Tryptic Soy Broth and placed in a shaking incubator overnight at 37°C and 180 rpm. A subculture was prepared and standardized to 105 CFU/mL in its growth phase using a 0.5 McFarland standard. Sterile rod samples (n=6) were placed in media containing 10% human serum, 7% dextrose, and 1X PBS in a 48-well plate. Samples were inoculated with 103, 104, or 105 CFU/mL in each well and incubated for 24 hours at 37°C and 95 rpm in a shaking incubator. Samples were then aseptically removed, rinsed 3X in PBS, and incubated in 10% Trypsin. Serial dilutions of the samples were placed on Petrifilms and statistical significance was determined using a Kruskal-Wallis test with multiple comparisons. Three independent experiments were performed for each level of inoculum.
RESULTS: After 24 hours of incubation, the average number of adherent bacteria for each level of inoculum and each material is shown in Figure 1. For each level of inoculum, the Kruskal-Wallis test had an overall p-value less than 0.001 and AFSN and SN/PEEK were both found to have significantly less bacteria than pure PEEK samples. AFSN samples were consistently found to have the fewest CFU/mL overall, supporting the antimicrobial effect reported in previous literature. SEM images comparing surface characteristics of AFSN and SN/PEEK composite are shown in Figure 2.
CONCLUSION:. These experiments confirm that a composite material made from both PEEK and Si3N4 will retain some of the antimicrobial properties observed in pure Si3N4 samples, while also allowing for the material to be used in the 3D-printing process. This allows for the creation of custom 3D-printed orthopedic implants that reduce the risk of infection when compared to traditional implant materials such as titanium alloy and pure PEEK.
REFERENCES: [1] YG Koh, Mater. Sci. Eng. C (2019) [2] R Pivec, The Lancet (2012) [3] WR Walsh, Clin. Orthop. Relat. Res. (2016).[4] TJ Webster, Acta biomater. (2012).
Figures
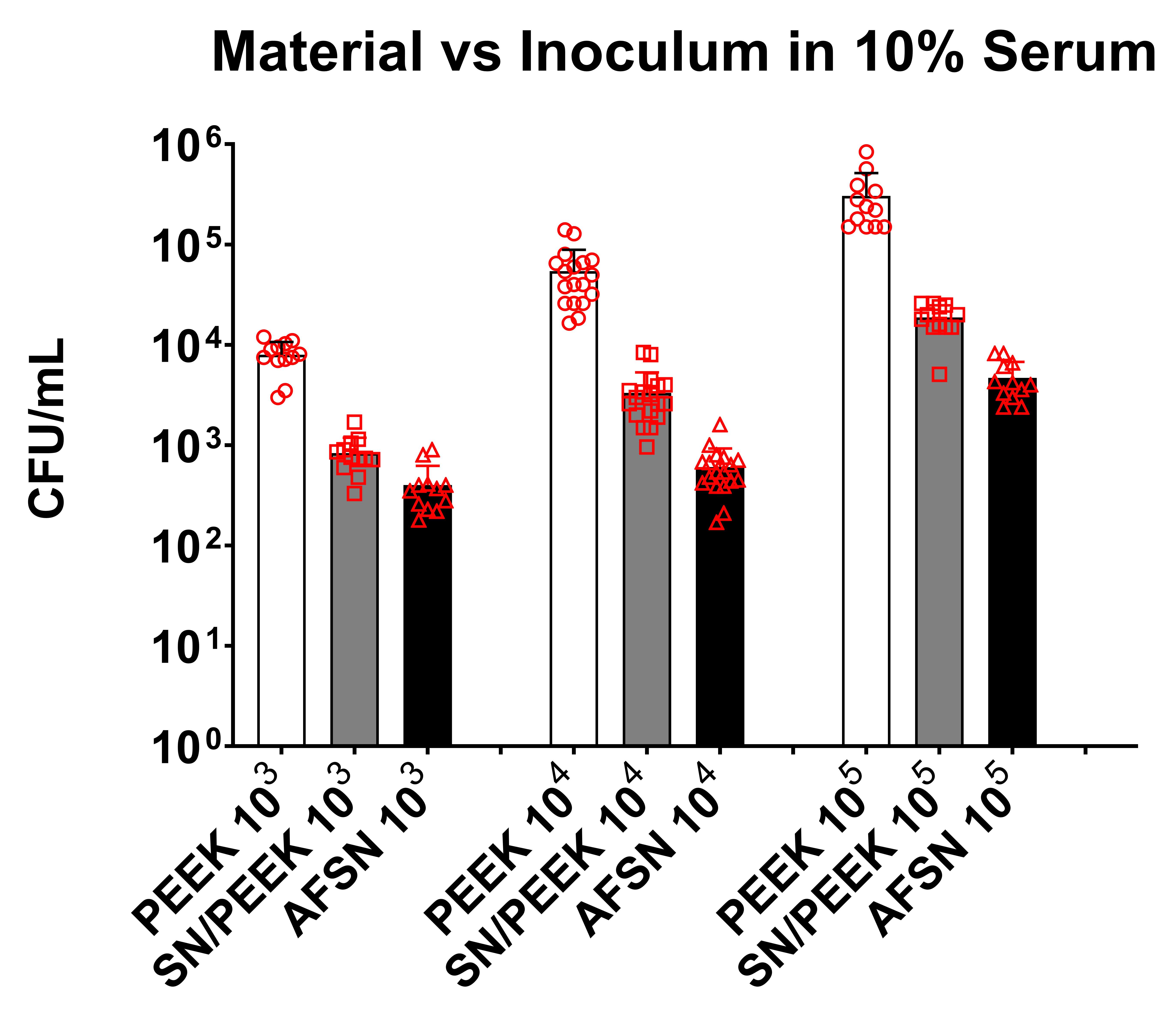
Figure 1
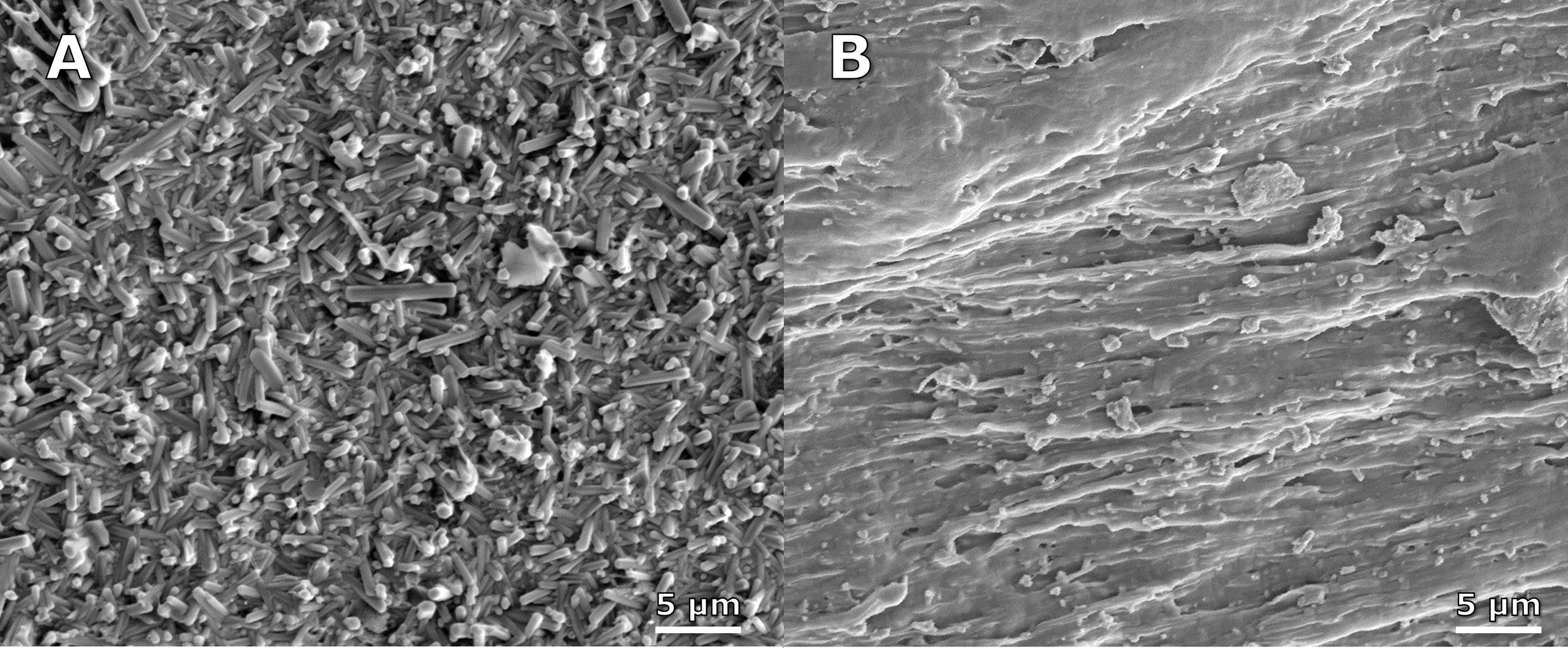
Figure 2#8719
H1, a Cementless Ceramic Anatomic Hip Resurfacing: The Results of a 250pt Study at 1-5 Yrs.
*Justin Cobb - Imperial College - London, United Kingdom
Susannah Clarke - Imperial College London - London, United Kingdom
Camilla Halewood - Imperial College London - London, United Kingdom
Robert Wozencroft - Imperial College London - London, United Kingdom
Jonathan Jeffers - Imperial College London - London, United Kingdom
Amy Maslivec - Imperial College London - London, United Kingdom
*Email: j.cobb@imperial.ac.uk
Abstract
This is an application for the HAP Paul award from the entire MSk Lab, who have worked on this latest project for the last decade. it encompasses novel device design, additive manufactured instruments, web based planning, and gait analysis as an objective metric of function.
A novel anatomic cementless ceramic hip resurfacing device was developed, to enable women and smaller men to have access to this more conservative form of arthroplasty. After pre-clinical testing, we designed a single arm multi-centre study which would satisfy the demands of regulators in the EU and USA.
Materials and Methods: 250 patients were recruited to a first in human study. Instruments were designed for single use, and additively manufactured. Outcome was measured using PROMs, imaging and gait analysis.
Results: 250 H1 devices were implanted between September 2017 and March 2022 by 8 surgeons in 6 hospitals. With between 5.5 and 1.2 yrs of follow up, there have been no deaths or lost to follow up. 6 have been revised. The median Harris Hip Score reached 100/100 at 3 months following surgery. Gait analysis confirmed that the asymmetric preoperative gait was restored to a highly symmetric pattern even at 6.5km/hr. Imaging studies showed good osseomechanical integration.
Discussion: the clinical and radiographic results appear equivalent to the current state of the art resurfacing hip arthroplasties. Gait analysis confirms that these patients function symmetrically even at higher speeds. The clinical results are as good in women and in men of smaller sizes.
Introduction
A new era of hip arthroplasty emerged in 1990 when Bargar and Paul reported their first CT planned, robot assisted canine hip arthroplasty (at the meeting of the ISSCP in Nice hosted by Aubaniac).Since that first enabling technology, research groups around the world have developed alternative assistive technologies, while device designers have looked at alternative materials, coatings and designs of device. This paper sets out our rationale for concentrating on device design and instruments in the quest to improve the outcome of arthroplasty while reducing the human cost of the procedure.
Hip Resurfacing Arthroplasty (HRA) with a metal-on-metal articulation is an alternative to total hip arthroplasty (THA), which enables patients to return to nearer normal levels of activity, as confirmed using gait analysis in a recent randomised controlled study1. Total hip replacement, while universally acknowledged to be a successful procedure, is not able to return patients to normal levels of activity2. Randomised trials previously had failed to capture this difference in activity3, 4, perhaps owing to the ceiling effects of the metrics commonly used, such as the Harris Hip score (HHS) and the Oxford Hip Score (OHS)4. Both of these scores have a modal score postoperatively of 100%, making any clinically important differences in outcome hard to demonstrate using PROMs5, 6.
The procedure of resurfacing has had a mixed history, with generations of devices being developed, introduced and then withdrawn owing to the failures of the bearing surfaces or the biological reaction to wear debris7, 8. Most recently, in the 1990s, Metal-on-Metal resurfacing was developed successfully using ‘as cast’ Cobalt Chrome alloy9. A recent multinational paper confirmed that good results long term survival rates could be obtained by ordinary hip surgeons10. However, some devices were associated with raised levels of metal ions in the blood, leading to the withdrawal of most devices11. In 2023, only a few devices are approved for use – the Birmingham Hip Resurfacing (BHR, Smith&Nephew) being the only one licenced for use in most countries. In longer term follow up, this demonstrate very low revision rates even in younger patients, while other devices such as the ASR, Durom and Cormet had features which predisposed to early failure10.
Ceramic on ceramic(CoC) bearings are well established in THA, with superior durability in comparison with ceramic on older polyethylene (CoP) bearing couples at 10 years in patients under 6012, and markedly superior durability over metal on polyethylene (MoP) bearings at 20 years13. In the longer term, CoC bearings have also been associated with lower rates of infection14, 15 . On the other hand, component fracture remains a recognised mode of failure for both femoral head and acetabular liners, dependent on the interaction between the metal taper and the ceramic16. in the absence of any metal tapers, making a monobloc ceramic device attractive.
Squeaking of the hip articulation is a type of adverse event particular to hard-on-hard bearings17. The incidence reported in ceramic bearings is highly dependent on the design of the acetabular and femoral metal components, varying from 2% to over 20%18.
In response to these documented shortcomings of THA in younger patients, and the problems associated with MoM HRA, we developed a ceramic HRA, H119.
We surmised that the clinical outcome of patients receiving this device would resemble that of patients with BHR devices, and the modern short stemmed cementless devices which are used for younger more active adults. A trial was powered to demonstrate functional non-inferiority to the BHR, in the absence of metal ions. Additional elements were included to ensure that the study met the standards set in Europe and the USA. In the UK, the only published standard comes from the Orthopaedic Device Evaluation Panel (ODEP)19. For the European market, the General Safety and Performance Requirements of the Medical Device Regulation (MDR) must be met for the purposes of CE marking20. This new regulation is better aligned with the federal drug administration (FDA) which uses a composite clinical outcome score(CCOS)21. The CCOS used to capture the clinical outcome of the most recently approved hip resurfacing device was therefore adopted.
Our primary hypothesis was therefore that H1 would perform safely and effectively. Our secondary hypothesis was that H1 would allow patients of any size and gender to function as well as the larger men who are still allowed access to the BHR.
Materials and Methods
The H1 device (figure 1) and the instrumentation used was first reported to ISTA after completion of the safety study22. A set of single use instruments were developed and used in all cases (figure 2).
Trial Design and Oversight: A multi-centre single arm non-inferiority study was designed. The details have been published 23.
This was divided into two parts, a safety study followed by an efficacy study. The safety study used Computer Tomography Stereo Analysis (CTSA) to detect migration of components 24, and metal ion levels to confirm absence of elevated cobalt and chromium levels in the blood25. All adverse events were recorded whether device or procedure related. The efficacy study used the benchmark published by the Orthopaedic Device Evaluation Panel (ODEP) as a threshold, combined with subjective and objective outcomes collected using the Oxford Hip Score (OHS)
26 and the Harris Hip Score (HHS)55 as both have been used to report outcome following HRA. A subgroup of patients local to the lead site were offered entry into a gait analysis study before and after surgery.
The trial was co-ordinated by the clinical trial manager (M A-L) in the MSk Lab of Imperial College. Independent oversight of the trial was carried out by Professor Olof Skoldenberg of the Karolinska Institute. The East of England Research Ethics Committee approved the initial study design.
Patients: Patients between the ages of 18 and 70 with a painful hip considering arthroplasty were offered entry into the study. Patients were not eligible if their bone mass was low, or they had a condition which predisposed them to infection. The full list of exclusions is published in the trial protocol8.
Trial Procedure:
After consenting, patients underwent hip resurfacing using a posterior approach as described by Derek McMinn27. The H1 devices were inserted using instruments derived from the BHR operative technique22. For Acetabular preparation, three single use reamers 1mm apart were used, with the final reamer being one mm smaller than the device. A trial was inserted to confirm orientation and rotation of the component, ensuring that the psoas tendon was not impinged upon by the anterior recess. The acetabular component was impacted in place, and any correction of version was obtained with a rim punch before final impaction. On the femoral side, the neck axis was obtained using a ring guide, which was 1mm smaller than the femoral neck, ensuring the neck would not be notched. A top head cut was made to ensure that no machined neck would be left uncovered, before a one-piece sleeve and chamfer cutter was applied to a fixed depth. After a final check that the head should not be seated further, the definitive head was impacted with a head impactor which controlled rotation, ensuring that the flexion and extension facets were facing correctly. Postoperative management was exactly as for BHR, with full weight bearing as tolerated from day 1. Impact sports were discouraged for 12 months following surgery in keeping with other resurfacing protocols28.
Outcomes
Outcome data was obtained at frequent early timepoints in the safety study, with both subjective and objective assessments at 6 weeks, 3 months, 6 months and 1 year, using clinical and CTSA review. Efficacy timepoints were at 6 weeks, 6 months, 1, 2, and 3yrs, with clinical and plain radiograph review to satisfy the benchmark of ODEP 3A.
Statistical Analysis
The ODEP benchmarks are published by the Orthopaedic Device Evaluation Panel, using the single metric of ‘all-cause revision’. Currently the 3A benchmark demands that 3 centres, with at least 3 surgeons outside the development centre have inserted at least 150 devices with at least 72 inserted for at least 3 yrs. The failure rate, as reported by the all-cause revision rate should be such that the lower bounds of 95% confidence should be less than 5%.
Radiographic failure was reported if radiolucent lines or bone resorption had increased in any zone on two consecutive time points.
For non-inferiority when compared with the BHR, the levels of cobalt and chromium metal ions in the blood should be in the normal range, with none at the level reportable to the MHRA of 7parts per billion.
Clinically an increase of 14 points in the OHS and 20 points in the HHS is needed for the procedure to be considered successful. All deaths, serious adverse events and adverse events were to be reported and collated.
Gait was measured preoperatively and at one year following surgery on an instrumented treadmill using a well published protocol.
Results
In May 2023, 125 H1 had been inserted for over 3 yrs, while all 250 had been inserted for over 12 months (table 1).
|
Cohort
|
All cases
|
Females
|
Small implants (≤46mm)
|
|
Number of cases
|
250
|
69/250
|
69/250
|
|
Number of patients
|
224
|
63/224
|
62/224
|
|
Number of unilateral cases
|
198
|
57/198
|
54/198
|
|
Number of bilateral cases
|
52
|
12/52
|
15/52
|
The entire cohort had an average age of 53, and 69 were women, and 58 were classified as small (46mm or smaller, table 2).
|
|
All cases
|
Females
|
Small implants (≤46mm)
|
|
Age at time of surgery, years
|
53 ±9
|
53 ±9
|
54 ±9
|
|
Number of female cases
|
69/250
|
69/69
|
58/69
|
|
Height, mm
|
175 ±8
|
168 ±6
|
167 ±6
|
|
Weight, kg
|
81 ±14
|
69 ±11
|
69 ±11
|
|
BMI, kg/m²
|
26 ±4
|
25 ±4
|
25 ±4
|
|
Current Smoker
|
5/250
|
3/69
|
4/69
|
|
High Alcohol Use (daily drinker)
|
20/250
|
5/69
|
5/69
|
While most patients experienced a straightforward recovery with clinical and radiographic evidence of return to health and activity (figure 2). Adverse and serious adverse incidents were collected and recorded fully. 6 serious adverse events occurred (Table 3).
|
SAE Description
|
Immediate PostOp
|
0-6wk
|
6wk-3m
|
3m-6m
|
6m-1 year
|
1-2 year
|
>2 year
|
|
Cases at time point
|
N=250
|
N=250
|
N=250
|
N=248
|
N=184
|
N=106
|
N=92
|
|
Device-related serious adverse events
|
|
Revision for symptomatic femoral crush
|
0/250
|
0/250
|
0 /250
|
0/248
|
1/184
|
0/106
|
0/92
|
|
Procedure-related serious adverse events
|
|
Revision for femoral neck fracture
|
0/250
|
0/250
|
0/250
|
1/248
|
1/184
|
0/106
|
0/92
|
|
Revision for repeat dislocation
|
0/250
|
0/250
|
0/250
|
1/248
|
0/184
|
0/106
|
0/92
|
|
Revision for psoas impingement
|
0/250
|
0/250
|
0/250
|
0/248
|
0/184
|
1/106
|
0/92
|
|
Wound complications
|
0/250
|
0/250
|
1/250
|
0/248
|
0/184
|
0/106
|
0/92
|
|
Sciatica
|
0/250
|
0/250
|
0/250
|
1/248
|
0/184
|
0/106
|
0/92
|
|
Unrelated adverse events
|
|
Traumatic brain injury
|
0/250
|
0/250
|
0/250
|
0/248
|
0/184
|
1/106
|
0/92
|
|
Traumatic neck injury
|
0/250
|
0/250
|
0/250
|
0/248
|
0/184
|
1/106
|
0/92
|
3 of these were for Vancouver type B type fractures (figure 2), one was for recurrent dislocation and one for groin pain caused by psoas impingement. All 5 of these resulted in revision to a DM primary hip. Another patient sustained a traumatic brain injury in collision with a van while training for a cycle race. This severe adverse event is not related to the procedure or the device. The H1 remains in situ, but the patient is now severely brain damaged. There have been no deaths and no infections. The 4 year survival is 97%, with the lower confidence bound of 95.2% (figure 3).
One hundred and three adverse events were reported (Table 4). 24 of these related to crushes of the femoral head bone, resulting in slight varus shift of the femoral component at a mean of 9 months following surgery. 21 of these were temporarily symptomatic, while 3 were only detected at routine follow up.
|
AE Description
|
Immediate PostOp
|
0-6wk
|
6wk-3m
|
3m-6m
|
6m-1 year
|
1-2 year
|
>2 year
|
|
Cases at time point
|
N=250
|
N=250
|
N=250
|
N=248
|
N=184
|
N=106
|
N=92
|
|
Device-related adverse events
|
|
Pain
|
0/250
|
1/250
|
0/250
|
2/248
|
2/184
|
2/106
|
1/92
|
|
Grinding sensation
|
0/250
|
0/250
|
0/250
|
0/248
|
0/184
|
1/106
|
0/92
|
|
Minor femoral head movement
|
0/250
|
0/250
|
1/250
|
1/248
|
4/184
|
3/106
|
2/92
|
|
Asymptomatic femoral crush
|
0/250
|
0/250
|
0/250
|
0/248
|
1/184
|
1/106
|
1/92
|
|
Symptomatic femoral crush
|
0/250
|
0/250
|
0/250
|
9/248
|
12/184
|
1/106
|
0/92
|
|
Procedure-related adverse events
|
|
Pain
|
2/250
|
2/250
|
3/250
|
8/248
|
2/184
|
7/106
|
0/92
|
|
Stiffness
|
0/250
|
0/250
|
0/250
|
1/248
|
0/184
|
0/106
|
0/92
|
|
Psoas Impingement
|
0/250
|
1/250
|
0/250
|
0/248
|
0/184
|
0/106
|
1/92
|
|
Atrial Fibrillation (post-operative)
|
1/250
|
0/250
|
0/250
|
0/248
|
0/184
|
0/106
|
0/92
|
|
Swelling (post-operative)
|
2/250
|
1/250
|
0/250
|
0/248
|
0/184
|
0/106
|
0/92
|
|
Post-dural headache
|
1/250
|
0/250
|
0/250
|
0/248
|
0/184
|
0/106
|
0/92
|
|
Wound complications
|
1/250
|
2/250
|
0/250
|
0/248
|
0/184
|
0/106
|
0/92
|
|
Rash
|
0/250
|
2/250
|
0/250
|
0/248
|
0/184
|
0/106
|
0/92
|
|
Blood loss (intra-operative)
|
1/250
|
0/250
|
0/250
|
0/248
|
0/184
|
0/106
|
0/92
|
|
Heterotopic Ossification
|
0/250
|
0/250
|
0/250
|
0/248
|
0/184
|
1/106
|
0/92
|
|
Unrelated adverse events
|
|
Extra scan taken
|
1/250
|
0/250
|
0/250
|
0/248
|
1/184
|
0/106
|
0/92
|
|
Shoulder injury/surgery
|
1/250
|
0/250
|
0/250
|
0/248
|
2/184
|
0/106
|
0/92
|
|
TKR
|
0/250
|
0/250
|
0/250
|
1/248
|
0/184
|
0/106
|
0/92
|
|
Issue with contralateral hip
|
0/250
|
0/250
|
0/250
|
0/248
|
0/184
|
2/106
|
2/92
|
|
Fall
|
0/250
|
0/250
|
0/250
|
0/248
|
0/184
|
1/106
|
1/92
|
|
Acute DVT
|
0/250
|
0/250
|
0/250
|
1/248
|
0/184
|
0/106
|
0/92
|
|
Neurological Issues
|
0/250
|
0/250
|
0/250
|
0/248
|
1/184
|
0/106
|
0/92
|
The stereoanalysis for implant migration shows the bone implant interface to be stable - it is reported in a separate submission to ISTA.
Whole blood levels of cobalt and chromium did not change following surgery, remaining in the normal range as expected (table 5).
|
Measure
|
Pre-Op
|
3 months
|
6 months
|
1 year
|
2 years
|
|
Number of data points
|
N=34
|
N=27
|
N=31
|
N=27
|
N=28
|
|
Mean Blood Cobalt Ion level
|
0.30 ±0.09
|
0.33 ±0.10
|
0.34 ±0.12
|
0.32 ±0.11
|
0.35 ±0.15
|
|
Mean Blood Chromium Ion level
|
0.43 ±0.17
|
0.42 ±0.07
|
0.46 ±0.19
|
0.42 ±0.08
|
0.55 ±0.25
|
In functional terms, most patients have had an unremarkable postoperative experience, recovering rapidly. The OHS reported a subjective rise of 20 points from a median of 26 preop to 48 at 6 months after surgery(Figure 5). The HHS reported an objective rise of 40 points from a median of 60 preop to a median of 100 at 3 months after surgery(figure 6). Subgroup analysis by size or gender or the presence of a femoral crush did not demonstrate any detectable differences.
Gait analysis was carried out before and one year after surgery in 81 patients. Their walking speed increased from an average of 5.7km/hr to 7.2km/hr (table 6).
|
|
Pre Op
|
Post Op
|
P Value
|
|
Top Walking speed (km/hr)
|
5.7 (1.01)
|
7.2 (0.72)
|
.00
|
|
Top Walking speed (m/s)
|
1.6 (0.28)
|
2.0 (0.20)
|
.00
|
|
Cadence (Steps/min)
|
47.4 (2.2)
|
45.1 (1.2)
|
.06
|
|
Step length (cm)
|
64.2 (2.3)
|
69.1 (2.1)
|
.02
|
|
Step Width (cm)
|
0.8 (0.3)
|
0.8 (0.4)
|
.65
|
Gait symmetry was also restored, from being asymmetric before surgery at slower speeds to being highly symmetric at higher speeds following surgery (figure 7)
Discussion
This single arm study set out to document the safety and efficacy of a novel hip resurfacing device for clinical use with results interpretable in different regulatory environments.
To satisfy regulatory demands, the safety of the procedure and the efficacy of the device need to equivalent to the predicate device – the BHR.
Safety has been demonstrated in three ways: by the normal levels of cobalt and chromium, an all-cause revision rate which met the benchmark set by ODEP, and by the stability of the device implant interfaces using CTSA and radiology.
The adverse events recorded were no more prevalent than expected. They included a number of femoral crushes, resulting in the femoral head tilting and subsiding by a few degrees and a few millimetres. The extent of this ‘crush’ is of a similar size and prevalence to that reported for short cementless stemmed implants29, 30. As has been found with short cementless stems, these events appear to be benign and self limiting, and a consequence of surgical technique. The prevalence of these events can be reduced from 25% to 2% by inserting the femoral component in valgus as recommended in 197831.
Efficacy was demonstrated using conventional metrics – the Oxford Hip Score and the Harris Hip Score. Using both of these metrics, the gain in function exceeded the benchmarks demanded, with both scores recording a median of 100% suggesting that the full extent of improvement in function was not captured by these metrics.
Finally the gait analysis recorded in 81 patients prospectively records a symmetric gait at higher speeds, something not yet recorded for any total hip32, 33.
The metrics demanded of any new medical device will continue to evolve as safety and efficacy standards rise. For a novel resurfacing device, the ceramic material avoided the entire issue of metal ions in the blood. However, the benchmark set by ODEP of all cause revision rate could have been a problem for two reasons. First the ease of revision: one patient had a revision for groin pain, as the procedure is a simple one, while three patients had revisions for Vancouver B1 type fractures. The incidence of periprosthetic fractures around or below a well fixed femoral stem is not reported by joint registries. Indeed the incidence is ignored as it cannot easily be captured for the treatment often takes place in trauma theatres, by trauma teams on a site which is remote from any registry related arthroplasty service. So the simple and safe treatment of an adverse event which is related to the procedure but is not device specific, is treated as a failure by the registry when a fracture occurs following HRA, while the more substantial but less effective treatment of plate and cable fixation of the same class of fracture following THA is not even noted. Fortunately only three patients with an H1 sustained such a fracture. Just one more would have meant that the H1 would not have met the ODEP 3A benchmark.
In conclusion, with 5 years of clinical experience the H1 device appears to function in a similar way to the BHR, but without any elevation of metal ions in the blood. The clinical outcomes when measured both subjectively and objectively appear to be independent of gender and size of component.
1. Gerhardt D, Mors TGT, Hannink G, Van Susante JLC. Resurfacing hip arthroplasty better preserves a normal gait pattern at increasing walking speeds compared to total hip arthroplasty. Acta Orthop. 2019 Jun;90(3):231-6. Epub 2019/04/02.
2. Bahl JS, Nelson MJ, Taylor M, Solomon LB, Arnold JB, Thewlis D. Biomechanical changes and recovery of gait function after total hip arthroplasty for osteoarthritis: a systematic review and meta-analysis. Osteoarthritis Cartilage. 2018 07;26(7):847-63. Epub 20180221.
3. Costa ML, Achten J, Parsons NR, Edlin RP, Foguet P, Prakash U, et al. Total hip arthroplasty versus resurfacing arthroplasty in the treatment of patients with arthritis of the hip joint: single centre, parallel group, assessor blinded, randomised controlled trial. BMJ. 2012 Apr 19;344:e2147. Epub 2012/04/21.
4. Lavigne M, Therrien M, Nantel J, Roy A, Prince F, Vendittoli PA. The John Charnley Award: The functional outcome of hip resurfacing and large-head THA is the same: a randomized, double-blind study. Clin Orthop Relat Res. Feb;468(2):326-36. Epub 2009/06/23.
5. Kalairajah Y, Azurza K, Hulme C, Molloy S, Drabu KJ. Health outcome measures in the evaluation of total hip arthroplasties--a comparison between the Harris hip score and the Oxford hip score. J Arthroplasty. 2005 Dec;20(8):1037-41.
6. Porter M. National Joint Registry for England and Wales, 9th Annual Report 2012. Available from: http://www.njrcentre.org.uk/njrcentre/Portals/0/Documents/NJR%208th%20Annual%20Report%202011.pdf.
7. Amstutz HC, Graff-Radford A, Gruen TA, Clarke IC. THARIES surface replacements: a review of the first 100 cases. Clin Orthop Relat Res. 1978 Jul-Aug(134):87-101.
8. Wagner H. Surface replacement arthroplasty of the hip. Clin Orthop Relat Res. 1978 Jul-Aug(134):102-30.
9. Daniel J, Pradhan C, Ziaee H, Pynsent PB, McMinn DJ. Results of Birmingham hip resurfacing at 12 to 15 years: a single-surgeon series. Bone Joint J. 2014 Oct;96-B(10):1298-306. Epub 2014/10/03.
10. Van Der Straeten C. Hip resurfacing arthroplasty in young patients: international high-volume centres' report on the outcome of 11,382 metal-on-metal hip resurfacing arthroplasties in patients ?50 years at surgery. Hip Int. 2020 Sep:1120700020957354. Epub 2020/09/09.
11. Langton DJ, Jameson SS, Joyce TJ, Gandhi JN, Sidaginamale R, Mereddy P, et al. Accelerating failure rate of the ASR total hip replacement. J Bone Joint Surg Br. 2011 Aug;93(8):1011-6.
12. El-Desouky II, Helal AH, Mansour AMR. Ten-year survival of ceramic-on-ceramic total hip arthroplasty in patients younger than 60 years: a systematic review and meta-analysis. J Orthop Surg Res. 2021 Nov 18;16(1):679. Epub 20211118.
13. Swarup I, Lee YY, Chiu YF, Sutherland R, Shields M, Figgie MP. Implant Survival and Patient-Reported Outcomes After Total Hip Arthroplasty in Young Patients. J Arthroplasty. 2018 09;33(9):2893-8. Epub 20180419.
14. P. H, A. D, Flouzat LC-H. DIFFERENT BEARINGS ON EACH SIDE HAVE DIFFERENT INCIDENCE OF INFECTION IN PATIENTS WITH BILATERAL TOTAL HIP ARTHROPLASTY. Orthopaedic Proceedings. 2018;100-B(SUPP_11):25-.
15. Madanat R, Laaksonen I, Graves SE, Lorimer M, Muratoglu O, Malchau H. Ceramic bearings for total hip arthroplasty are associated with a reduced risk of revision for infection. Hip Int. 2018 May;28(3):222-6.
16. Howard DP, Wall PDH, Fernandez MA, Parsons H, Howard PW. Ceramic-on-ceramic bearing fractures in total hip arthroplasty: an analysis of data from the National Joint Registry. Bone Joint J. 2017 Aug;99-B(8):1012-9.
17. Imbuldeniya A, Munir S, Chow J, Walter WL, Zicat BA, Walter WK. Factors affecting squeaking in metal on metal hip resurfacings. Hip Int. 2014;24(4):340-6. Epub 20140508.
18. Walter WL, O'toole GC, Walter WK, Ellis A, Zicat BA. Squeaking in ceramic-on-ceramic hips: the importance of acetabular component orientation. J Arthroplasty. 2007 Jun;22(4):496-503. Epub 20070328.
19. Tucker K, Gregg P, Kay P, Porter M, Howard P, Pickford M, et al. Monitoring the introduction and performance of a joint replacement: the United Kingdom metal-on-metal alert. J Bone Joint Surg Am. 2011 Dec;93 Suppl 3:37-42.
20. Weszl M, Rencz F, Brodszky V. Is the trend of increasing use of patient-reported outcome measures in medical device studies the sign of shift towards value-based purchasing in Europe? Eur J Health Econ. 2019 Jun;20(Suppl 1):133-40. Epub 2019/05/18.
21. Administration FD. Clinical Data Presentations for Orthopedic Device Evaluations. 2004; Available from: https://www.fda.gov/regulatory-information/search-fda-guidance-documents/clinical-data-presentations-orthopedic-device-applications-guidance-industry-and-fda-staff.
22. Cobb JP, Halewood C, Wozencroft R, Logishetty K, Jeffers JR, Clarke S, editors. H1 Anatomic Ceramic Hip Resurfacing: Results of a 20 Patient Safety Study. International Society of Technology in Arthroplasty Annual Meeting 2018; 2018; London, UK.
23. Al-Laith M, Clarke S, Halewood C, Wozencroft R, Guest B, Van Der Straeten C, et al. A Prospective, Non-Randomized, Consecutive Series, Multicentre, Observational Study to Evaluate The Clinical Outcome of Ceramic-On-Ceramic Hip Resurfacing Arthroplasty Using The Ceramic, Non-Porous, Non-Cemented H1 Hip Resurfacing Arthroplasty. 2022.
24. SG C, JP C. Low dose CT-based Spatial Analysis (CTSA) to
measure implant migration after ceramic hip resurfacing arthroplasty (HRA) – a
phantom study. Journal of Engineering in Medicine. 2023.
25. Beaulé PE, Kim PR, Hamdi A, Fazekas A. A prospective metal ion study of large-head metal-on-metal bearing: a matched-pair analysis of hip resurfacing versus total hip replacement. Orthop Clin North Am. 2011 Apr;42(2):251-7, ix.
26. Harris KK, Price AJ, Beard DJ, Fitzpatrick R, Jenkinson C, Dawson J. Can pain and function be distinguished in the Oxford Hip Score in a meaningful way? : an exploratory and confirmatory factor analysis. Bone Joint Res. 2014 Nov;3(11):305-9.
27. DJW M. Modern Hip Resurfacing: Springer; 2009.
28. Girard J, Miletic B, Deny A, Migaud H, Fouilleron N. Can patients return to high-impact physical activities after hip resurfacing? A prospective study. Int Orthop. 2013 Jun;37(6):1019-24. Epub 2013/03/05.
29. Schaer MO, Finsterwald M, Holweg I, Dimitriou D, Antoniadis A, Helmy N. Migration analysis of a metaphyseal-anchored short femoral stem in cementless THA and factors affecting the stem subsidence. BMC Musculoskelet Disord. 2019 Dec 12;20(1):604. Epub 20191212.
30. Panichkul P, Bavonratanavech S, Arirachakaran A, Kongtharvonskul J. Comparative outcomes between collared versus collarless and short versus long stem of direct anterior approach total hip arthroplasty: a systematic review and indirect meta-analysis. Eur J Orthop Surg Traumatol. 2019 Dec;29(8):1693-704. Epub 20190730.
31. Freeman M. Some anatomical and mechanical considerations relevant to the surface replacement of the femoral head. Clinical orthopaedics and related research. 1978 (134):19-24.
32. van Oldenrijk J, Scholtes VAB, van Beers LWAH, Geerdink CH, Niers BBAM, Runne W, et al. Better early functional outcome after short stem total hip arthroplasty? A prospective blinded randomised controlled multicentre trial comparing the Collum Femoris Preserving stem with a Zweymuller straight cementless stem total hip replacement for the treatment of primary osteoarthritis of the hip. BMJ Open. 2017 Oct;7(10):e014522. Epub 2017/10/16.
33. Beaulieu ML, Lamontagne M, Beaulé PE. Lower limb biomechanics during gait do not return to normal following total hip arthroplasty. Gait Posture. 2010 Jun;32(2):269-73. Epub 20100611.
Figures

Figure 1
Figure 2
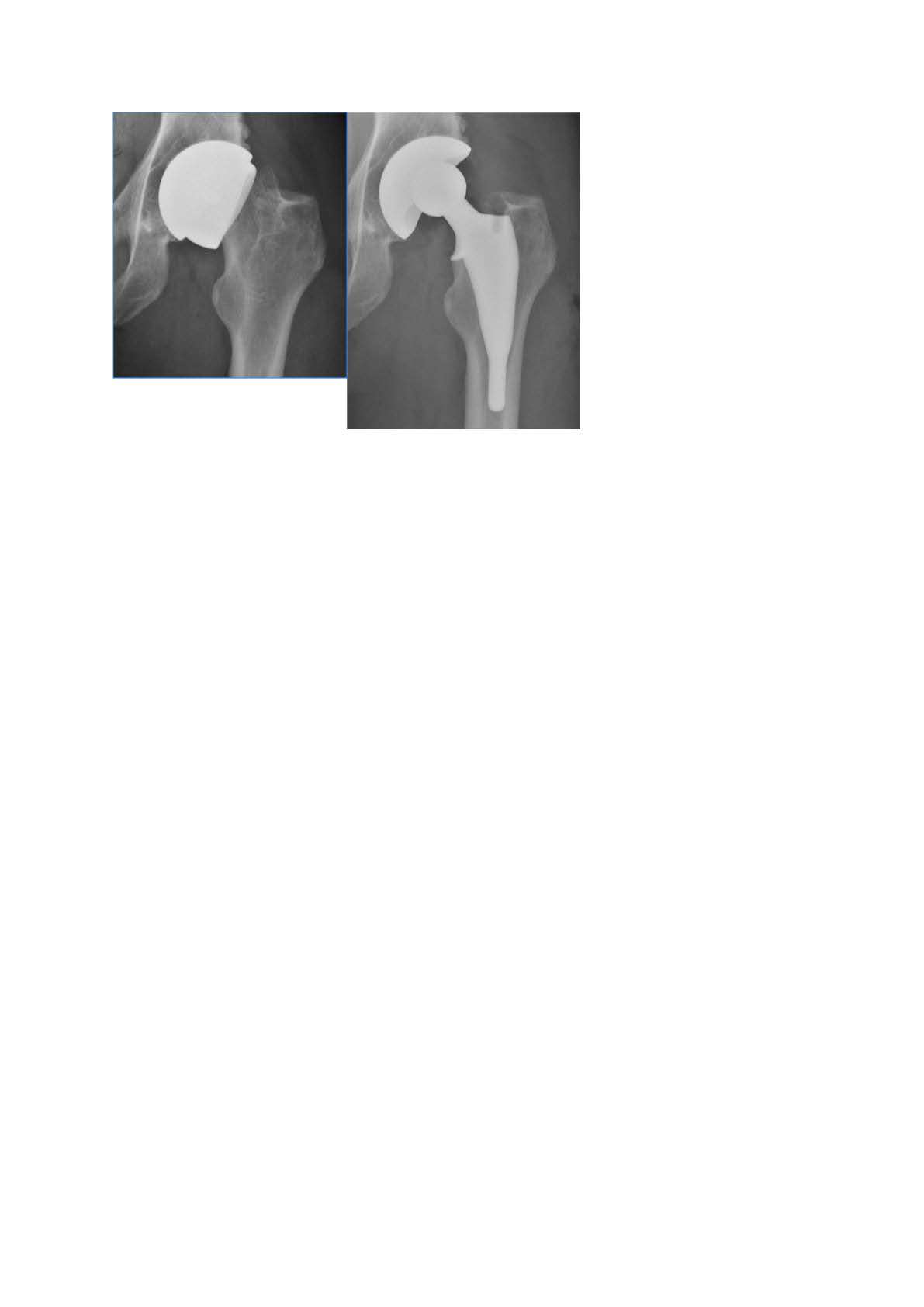
Figure 3
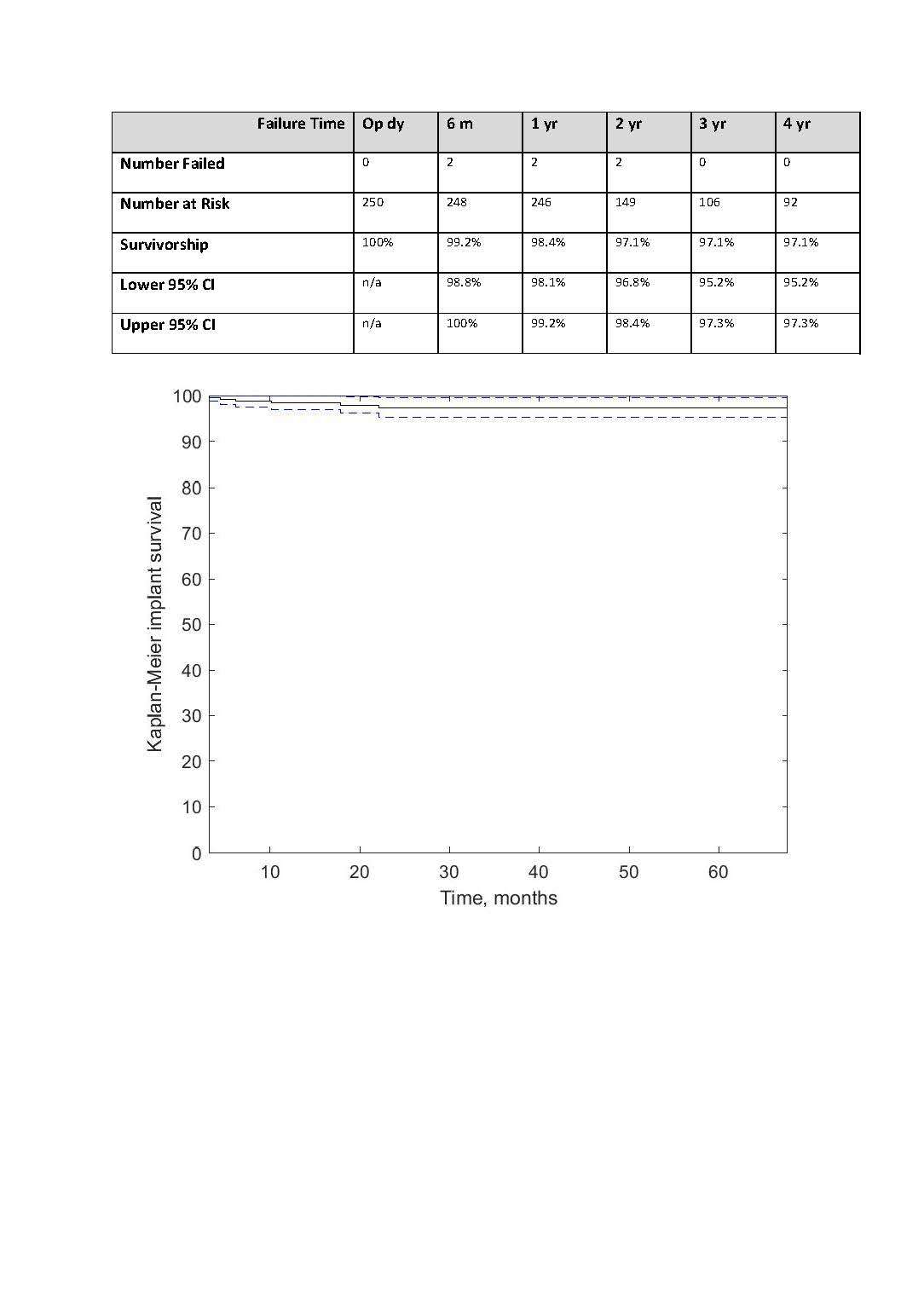
Figure 4
Figure 5
Figure 6
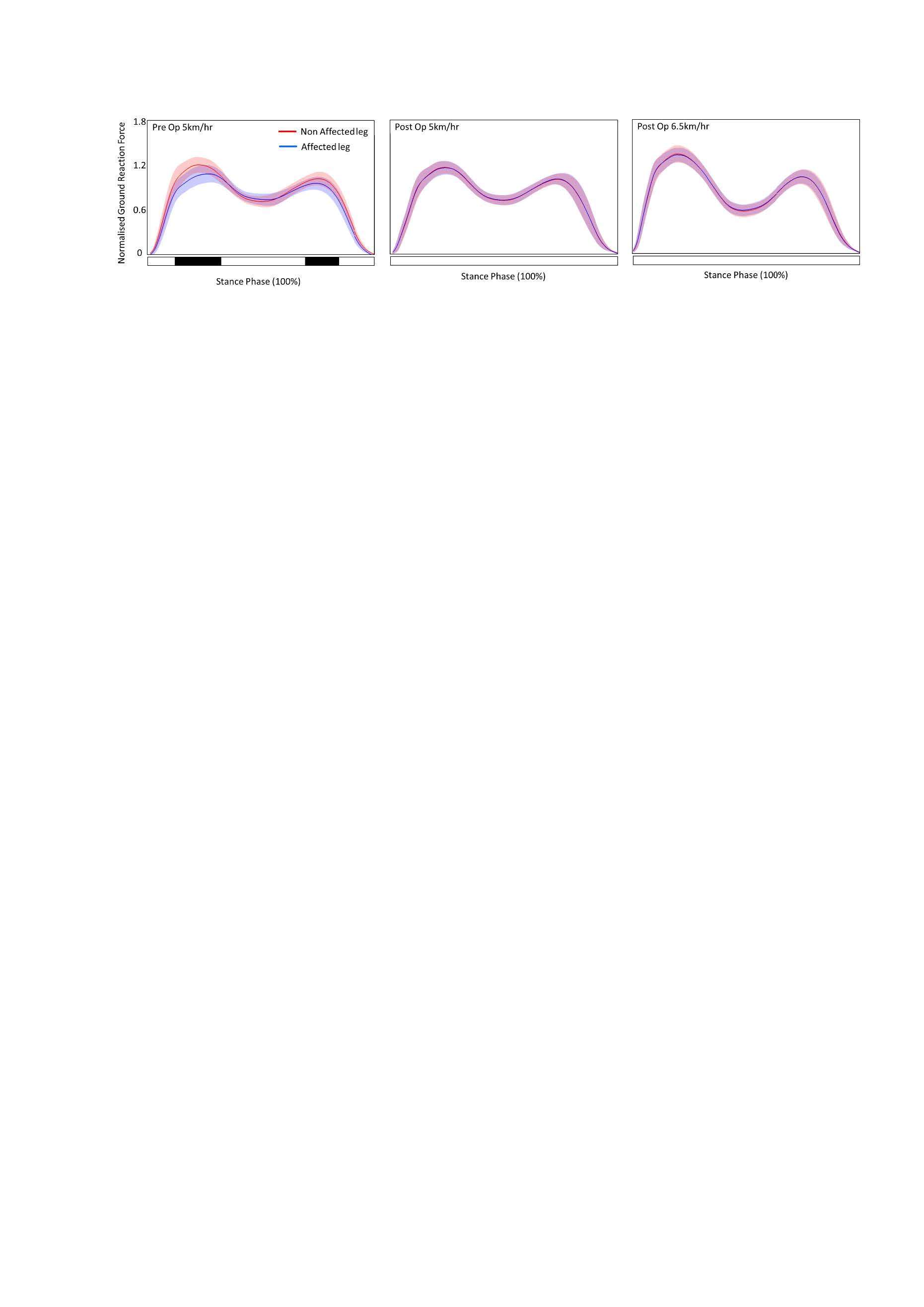
Figure 7#8621
Toward Functional Reconstruction of the Pre-Diseased State in Robotic-Assisted Total Knee Arthroplasty
*Periklis Tzanetis - University of Twente - Enschede, Netherlands
Rene Fluit - University of Twente - Enschede, Netherlands
Kevin de Souza - Stryker - Manchester, United Kingdom
Seonaid Robertson - Stryker - Manchester, United Kingdom
Bart Koopman - University of Twente - Enschede, Netherlands
Nico Verdonschot - Twente - Nijmegen, Netherlands
*Email: p.tzanetis@utwente.nl
Abstract
Background: The surgical target for optimal positioning in robotic-assisted total knee arthroplasty remains a subject of ongoing discussion. One of the proposed targets is to recreate the knee’s functional behavior to its pre-diseased state. The aim of this study was to optimize implant positioning, starting from mechanical alignment (MA), toward restoring the pre-diseased status, including ligament strain and kinematic patterns, in a patient population.
Methods: We utilized a statistical shape and appearance model-based approach to segment the pre-operative computed tomography of 20 osteoarthritic patients that identified the subchondral osteophyte-free surfaces and estimated cartilage from the segmented bones; these geometries were used to construct patient-specific musculoskeletal models of the pre-diseased knee. Subsequently, implantations were simulated using the MA method, and a previously developed optimization technique was employed to find the optimal implant position that minimized the root-mean-square deviation between pre-diseased and post-operative ligament strains and kinematics.
Results: There were evident biomechanical differences among the simulated patient models, but also trends that appeared reproducible at the population level. Optimizing the implant position significantly reduced the observed peak strain deviations from 22.8% to below 5.3% for all but the anterolateral ligament across the population; and concomitantly reduced the kinematic deviations from 3.6±1.5 mm and 4.5±1.7° with MA to 2.6±1.4 mm and 3.5±1.9° relative to the pre-diseased state. To achieve this, the femoral component consistently required translational adjustments in the anterior, lateral, and proximal directions, while the tibial component required a more posterior slope and varus rotation in most cases.
Conclusion: These findings confirm that MA-induced biomechanical alterations relative to the pre-diseased state can be reduced by optimizing the implant position and may have implications to further advance pre-planning in robotic-assisted surgery that strives to restore the pre-diseased knee function.
Keywords
Robotic-assisted total knee arthroplasty, pre-diseased knee, mechanical alignment, optimal implant position, musculoskeletal modeling
Introduction
Total knee arthroplasty (TKA) is a highly effective operation in treating end-stage knee osteoarthritis (OA), yet some patients report limited function of the implanted joint, impacting overall satisfaction after surgery [1]. Optimal positioning of the implant has been described as one of the determinants of post-operative functional outcomes [2] and remains a subject of discussion [3]. Knee implants are traditionally placed according to mechanical alignment (MA) principles, striving to restore a neutral mechanical axis of the lower limb. This systematic approach, however, disregards the patient-specific, pre-diseased knee anatomy and may induce non-physiological tension of the peri-articular soft tissues and, consequently, altered kinematics [4]. Previous research suggests that restoring constitutional varus knees to neutral may be sub-optimal, necessitating some degree of medial soft-tissue releases to achieve symmetric, balanced flexion and extension gaps [5]; the effects of over-correcting pre-operative varus deformity have been shown in several studies [6,7]. It therefore appears necessary to define a more individualized positioning target based on the patient’s knee phenotype [8]. Utilizing robotic-assisted TKA (RA-TKA) enables pursuing alternative alignment strategies, allowing for a personalized approach to implant positioning [3]. Restoration of the pre-diseased functional status in a personalized manner can be defined as a logical target for RA-TKA. Recently, the functional alignment technique with a pre-operative MA plan has been proposed to re-establish joint line obliquity of the knee, as prior to the onset of the disease, subject to the mediolateral soft-tissue laxity profiles, whilst restoring pre-diseased knee kinematics [9,10]. Furthermore, the surgical precision with robotic assistance contributes to making these positioning targets more reproducible, with minimal post-operative outliers [11], although the degree of improvement in functional outcomes appears inconsistent [12–14]. This implies that optimal implant position may vary based on the unique morphology of the patient’s knee, and is important to pre-operatively simulate the biomechanical effects of implant positioning in order to ensure optimal post-operative joint function. Previously, we have used machine-leaning-based morphing techniques to recreate the knee morphology that was present before the onset of osteophytes, referred to here as the pre-diseased state. Leveraging this information, we developed a musculoskeletal model-based optimization workflow, which mechanically aligned the knee implant and, subsequently, optimized its position in order to reproduce the pre-diseased ligament strains and tibiofemoral kinematics [15].
The aim of this study was first to quantify deviations in ligaments strains and consequent tibiofemoral kinematics comparing mechanically implanted with their corresponding pre-diseased knees over a population of patients and, second, to utilize the pre-defined optimization technique, fine-tuning the implant position, in order to recreate the pre-diseased functional profiles of each patient as closely as possible. This study ultimately sought to establish how much we could gain by employing a personalized approach relative to MA, how large the component positional changes were with respect to MA, and whether these changes exhibited clear patterns. We hypothesized that adjusting the position of the individual components would ensure a closer correspondence between the pre-diseased and post-operative biomechanical situation than using MA alone. However, we expected that the required positional adjustments would be variable due to the inherent anatomical variation and differences in strain and kinematic profiles between patients.
Material and Methods
The data used in this study were part of the knee functional flexion axis dataset [16]. Pre-operative CT scans of bilateral lower extremities were collected from 20 OA patients, featuring different stages of osteophyte severity, who underwent primary TKA, performed using the Stryker Knee Navigation system (Stryker, Kalamazoo, MI, USA). These images were automatically segmented using a statistical shape and appearance model-based methodology to reconstruct the hip, ankle, and arthritic knee bone surfaces [17]. An osteophyte volume detection algorithm, provided by Imorphics (Stryker, Manchester, UK), was employed to subtract the osteophytic features from the outer surface of the cortical bone and recreate the osteophyte-free femoral and tibial bone geometries [17–19]. Related techniques have been validated elsewhere [20]. The articular cartilage of the knee was estimated by applying a magnetic resonance image-derived training shape model that follows an established segmentation protocol [21]. Anatomical landmarks were extracted from the segmented hip, knee, and ankle bones to determine the origin and orientation of the femoral, tibial, and patellar reference frames based on a pre-defined convention [22]. As described in an earlier study [17], the generated knee structures of this cohort of patients were used to morph the geometry of a musculoskeletal reference model and estimate the ligament attachment sites, applying a non-linear morphing technique [23]; these patient-specific models constituted the pre-diseased knees (Figure 1). Strains of the anterior cruciate ligament (ACL), posterior cruciate ligament (PCL), deep medial collateral ligament (dMCL), superficial medial collateral ligament (sMCL), lateral collateral ligament (LCL), anterolateral ligament (ALL), oblique popliteal ligament (OPL), and posterior capsule (PC) were computed for each patient, simulating a knee extension motion from 60° to 0° with only the gravitational force acting along the longitudinal axis of the system. Tibial kinematics relative to the femur, including anterior-posterior (AP), lateral-medial (LM), proximal-distal (PD) translations, and external-internal (EI) and varus-valgus (VV) rotations were estimated over the same arc of motion based on Grood and Suntay’s definition [24].
The patient-specific models were implanted with the Triathlon single-radius, cruciate-retaining total knee system (Stryker Orthopaedics, Mahwah, NJ, USA) according to the principles of MA. Sizing of the femoral component ensured anatomical congruence with the condyles, preventing anterior cortex notching greater than 3 mm, as associated with periprosthetic fracture [25], and mediolateral overhang exceeding 3 mm, which may cause soft-tissue impingement and consequent post-operative pain [26]; accordingly, the size selection of the tibial component ensured tibial coverage with minimal tray overhang at the cortical bone edges. The implants had a tibial insert thickness of 9 mm which is a common choice in clinical practice [27] and has been shown to offer a good compromise between the depth of tibial resection and strains of the collateral ligaments [28]. However, the surgeons could opt for a thinner or thicker insert, depending on the intra-operative stability assessment. The patellae were not resurfaced. The MA-TKA models simulated the same activity as the pre-diseased models to study the effect of mechanical implantation on the ligament strains and kinematics. Differences in the predicted strain and kinematic curves between the pre-diseased and MA-TKA models were quantified using the root-mean-square deviation (RMSD). An optimization algorithm that we developed in previous work [15] was subsequently utilized to find the optimal position of the components that minimized the RMSD between the pre-diseased and post-operative biomechanical profiles. The objective function of this optimization problem consisted of three distinct components: the first two, equally weighted components, involved the summation of the RMSD for each individual ligament strain and kinematic variable, respectively, while the third pertained to a penalty factor added to the resulting function value, as provided in Equation (1). In Equation (1), x indicates the degrees of freedom of the components subject to the lower and upper bounds, denoted as lb and ub, respectively; the boundary conditions were defined relative to MA. More precisely, the translational variation of the femoral component was constrained to within ±6 mm in the AP, LM, and PD directions, whereas the translational placement of the tibial component was maintained unchanged to ensure convergence to a global optimum position. The rotational variation of the femoral and tibial components was confined within ±3° and ±6°, respectively, for flexion-extension (FE) and within ±6° for EI and VV rotations. Further, n indicates the dimension of the solution space equal to the number of x positional variables (n=9), m is the number of strain or kinematic variables involved in the objective function, and wi is the weighting factor assigned to the ith ligament or kinematic variable, which was calculated such that the strain and kinematic errors were given equal importance. Finally, t denotes the number of discrete time steps of the model simulation (t=60) from 0° to 60° (θend), and yi,θindicates the predicted strain or kinematic value of the ith ligament or kinematic variable, respectively, at flexion angle θ. A penalty factor, denoted as P, equal to 103 was applied to the objective function if the force residual at any given flexion angle, as computed by the model’s force-dependent-kinematics solver [29], exceeded the specified tolerance of 5 N, thus ensuring dynamic consistency of the simulations. In case multiple positioning solutions resulted in similar objective function values, indicating minimal deviations relative to the pre-diseased state, the solutions closest to MA were chosen to facilitate their clinical feasibility [30]. Figure 1 provides a schematic description of the study’s workflow spanning pre-operative image segmentation to musculoskeletal modeling and model-based implant positioning optimization. To assess the effect of optimization on minimizing the strain and kinematic deviations between the pre-diseased and MA-TKA models, we used the non-parametric Wilcoxon signed-rank test, and set the significance level to 0.05.
Results
Large variations in the pre-diseased ligament strain and tibiofemoral kinematic profiles were observed among the 20 patient cases. The standardized MA could not accommodate these individual profiles. Consequently, adopting a personalized, targeted approach became necessary to achieve optimal implant positioning. Strains of the ligaments in the MA-TKA models exhibited deviations of up to 22.8% in the medial collateral (dMCL, sMCL), 6.2% and 13.8% in the lateral collateral (LCL) and anterolateral (ALL) ligaments, respectively, and up to 5.3% in the posterior ligamentous structures (PCL, OPL, PC) relative to the pre-diseased state. The predicted knee kinematics showed an RMSD of 3.6±1.5 mm for translations and 4.5±1.7° for rotations compared to their corresponding pre-diseased knee over all patients. The optimization process resulted, on average, in a 47.3% reduction of the objective function value compared to the MA position among patients. Figure 2 depicts the progression of the objective function value for an individual, representative patient case, starting from the MA position and reaching the optimal position. For all patients, optimizing the implant position reduced the strain deviations to less than 5.3% in the dMCL and sMCL, 4.1% and 8.5% in the LCL and ALL, respectively, and 3.7% in the PCL, OPL, and PC structures in relation to the pre-diseased state; and resulted in kinematic deviations of 2.6±1.4 mm and 3.5±1.9°. Intra-patient comparison revealed significantly lower deviations in the AP and LM translations and VV rotation with the optimized TKA models compared to MA controls. Similarly, the reduction in ligament strains was significant for all ligaments except for the PCL (Figure 3). The required adjustments in the position of the femoral and tibial components to reproduce the biomechanical status of the pre-diseased knees are summarized in Table 1.
Discussion
This study investigated the effect of mechanically and optimally aligning a knee implant aiming to reproduce physiological ligament strains and tibiofemoral kinematics over a patient population. To achieve this, we compared MA-TKA models with their pre-diseased counterparts and, subsequently, utilized a formerly established optimization technique to optimize the implant position, thereby recreating the pre-diseased knee functional status as closely as possible. The findings of this study suggest that adjusting the position of the individual components relative to MA results in a more closely match between the pre-diseased and post-operative biomechanical conditions. We found variable optimal positions among patients, but we could identify distinct positional patterns within the patient population that enabled achieving the examined surgical target.
MA of the knee implant, as utilized in this study, had a clinically important effect on the strains of the ligaments with concomitant changes in the tibiofemoral kinematics. The dMCL and sMCL strains were consistently larger in the MA-TKA models, exhibiting average deviations of 10.6% and 6.8%, respectively, compared to the pre-diseased state among patients (Figure 3). This corroborates the work of Delport et al. [31], who showed that restoration to absolute neutral MA can increase the collateral strain deviations from the native knee during passive flexion-extension. Provenzano et al. [32] identified the onset of fiber plastic deformation at 5.1% strain in the medial collateral ligamentous structures; sub-failure strains above the specified threshold can induce nearly complete ligament disruption and, post-operatively, increased medial laxity, hindering the overall functional stability. In the current study, the observed higher strains in the medial collateral ligaments could be associated to the relatively large valgus angulation, as reflected in the VV results (Figure 3). Surgeons may consider using a thinner tibial insert as an option to slightly relax the collateral ligaments, with caution advised since downsizing the insert might increase knee laxity in multiple directions [33] with consequent, aberrant kinematics. This consideration, however, was not assessed in this study. Although the LCL showed a relatively low average strain deviation at 2.3% from the pre-diseased knee, individual deviations, such as those observed in one patient case (6.2%), cannot be disregarded and may be a plausible cause of pain when exceeding the ultimate ligament strain [34]. The ALL reported clinically relevant strain deviations averaged at 6.3%, which could be due to larger anterior tibial translation during flexion [35]. Such deviations may have consecutively contributed to the increased EI rotational deviations, as this ligament restricts internal tibial rotation [36], although the functional role of the ALL remains inconsistent [37].
Optimizing the implant position resulted in a statistically significant reduction in the average strain deviations of the medial (<2.9%), lateral (1.4%) collateral ligaments and posterior capsular structures (<1%) (Figure 3) within the clinically acceptable range [31,32,38]. These results are consistent with other research that emulated native collateral ligament elongation patterns using a patient-specific, model-driven response surface methodological technique [39]. Although, statistically, there was a significant reduction of the ALL strain deviation at 4.8% on average from the pre-diseased knee, some individual patient models yet exhibited deviations of up to 8.5%, well above the pre-defined damage threshold [32] that are likely to be related to the nearly unchanged EI angulation, and could logically lead to anterolateral knee pain. Modifying the objective function to give more weight to the strain of the particular ligament could potentially mitigate this issue. Following the ligament adjustments, there was a concomitant reduction of the observed average kinematic deviations by 27.8% for translations and 22.2% for rotations, resulting in an RMSD of 2.6 mm and 3.5°, respectively (Figure 3). This aligns with a recent study [40], which approximated native kinematics within a similar order of deviations based on cadaveric model-based artificial neural network optimization. We should note, however, that the AP translational deviations in the current study remained somewhat higher than in the pre-diseased situation for some patients, with one case reaching up to 9.3 mm, which may be in part due to the altered geometry and conformity of the articular surface. Patient-specific implants have been shown to reduce such deviations, exhibiting femoral rollback closer to the normal knee than standard designs since they better match the shape of the patient’s knee [41,42].
The optimal position of the implant was found to be within ±4 mm and ±4° from MA in most patients, which appears to be a clinically feasible deviation from the MA position of the components [43–45], although some patients required slightly larger changes. This implies that MA is a reasonable functional target that can be improved utilizing techniques similar to the one proposed in this study. The precise positional adjustments revealed certain directional pathways toward achieving optimal placement of the individual components. More specifically, the femoral component was placed in a more anterior, lateral, and proximal position, rotated externally relative to the tibia, with increasing varus alignment of the tibial component. Coronal alignment of the femoral component was more variable and less clear, but the majority of patients still required some degree of varus. Bellemans et al. [5] described that a relevant proportion of the normal knees has a limb alignment of 3° varus or more; therefore, slight under-correction to approximate pre-diseased alignment, as might have occurred in our study, could be more physiological for these patients [46], potentially producing functional results close to the pre-diseased situation. Rotating the femoral component externally within 5° (Table 1) using the central referencing technique seems acceptable, with minimal compromise of the mediolateral condylar symmetry [47,48]. The surgeons should, however, be aware of component overhanging that could arise at the posterior aspect of the lateral condyle, which can cause impingement with the capsular fibers. Further, it is important to consider that excessively rotating the femoral component either internally or externally has consequences on patellar tracking [49], potentially inducing anterior knee pain [50]. At the tibial level, Innocenti et al. [51] showed that re-positioning the tibial component to 2° and up to 6° varus intuitively increased the strain in the LCL beyond physiological capacity, as compared to neutral mechanical configuration, which could affect the clinical outcomes in a detrimental way. These findings, however, were not confirmed in the current study, possibly because the concurrent rotational adjustment of the femoral component compensated for any such effect. Furthermore, the tibial component consistently required more posterior slope in the optimal position relative to MA. This is in agreement with an earlier investigation, which suggested that increasing the tibial slope with reference to the center of the tibial plateau is relevant to restoring the biomechanics of the native knee [52]. Surgeons often strive for a slope between 0° and 7° based on the implant manufacturer’s recommendations; the average slope in the present study was 3.1±1.3°, falling inside this range. Nonetheless, it is important to consider individual variations in native tibial slopes, as insufficient slope correction may inhibit the overall stability of the joint [53]. Overall, it should be noted that the observed positioning trends may have been influenced by the standard implant design used; hence they should be interpreted within the context of this choice. Recent evidence suggests that alternative designs, such as the bi-cruciate retaining prostheses, have a direct influence on post-operative knee biomechanics [54], and it is, therefore, possible that utilizing other designs may generate different positional changes of the components relative to those defined by MA.
The strength of this simulation-based study is that it thoroughly examines the feasibility of post-operatively restoring the pre-diseased knee function in a population of OA patients, which would be unfeasible in clinical trials and/or cadaveric experiments. Furthermore, it attempts to quantify the knee ligament strains and kinematics as they were before the onset of the disease in a patient-specific biomechanical situation and, subsequently, use this information to optimize the position of the implant with respect to the existing MA technique. This could help guide the formulation of a personalized surgical plan, feeding a robotic system with the specified target for its precise execution. Our methodology can further aid in selecting different TKA designs or designing a more personalized implant that better matches the patient’s pre-diseased articular geometry, employing the osteophyte-free statistical shape model-based technique, and maintains alignment results close to normal kinematics.
The present study had some limitations. First, the accuracy of the model predictions has not been assessed against experimental measurements of soft-tissue strains and knee kinematics. Previous validation of comparable models [23,55] revealed kinematic errors in the order of observed deviations, which accentuates the relevance of performing dynamic, in vivo measurements of knee mechanics in response to implant position to determine the accuracy and reliability of the model outputs before the patient-specific clinical application. Second, the ligament mechanical properties were not patient-specific but derived from comparable intact knee models in the literature and remained unmodified in the implanted configuration, although they are likely to change over time due to OA. Nonetheless, using the same ligament properties for both pre-diseased and implanted models ensured equal levels of uncertainty in their predictions, which eventually allowed us to isolate the influence of implantation on the joint’s mechanics. Third, the models were limited to simulating only a gravity-assisted flexion-extension activity; in principle, it would be possible to analyze other motor tasks, as previously reported [28], yet the particular activity can be performed intra-operatively to assess whether the joint is adequately aligned and whether the surrounding soft tissues are appropriately tensioned to allow for a balanced motion during flexion and extension. We should also emphasize that the study results are specific to a single-radius, cruciate-retaining prosthesis. It would be interesting to test other implant designs to determine whether the observed deviations and positioning trends persist or are largely affected by design. Finally, given that the proposed method lacks clinical validation, a clinical trial is necessary to assess whether it aids in improving post-operative functional outcomes and, ultimately, patient satisfaction.
In conclusion, mechanically aligned TKA can induce clinically relevant changes in the strains of the ligaments and tibiofemoral kinematics compared to the knee’s pre-diseased condition, which could, however, be mitigated by optimizing the position of the components. Optimal placement was variable and patient-specific, yet certain trajectories in the spatial positioning of the femoral and/or tibial components were identified at the population level for the implant design studied. These findings provide relevant information to quantify the positional target in robotic-assisted TKA in order to reconstruct the pre-diseased functional state of the knee as closely as possible and thereby improve knee function and overall patent satisfaction.
Funding
This research was funded by Stryker European Operations Ltd., Ireland.
References
[1] Bourne RB, Chesworth BM, Davis AM, Mahomed NN, Charron KDJ. Patient satisfaction after total knee arthroplasty: who is satisfied and who is not? Clin Orthop Relat Res 2010;468:57–63. https://doi.org/10.1007/s11999-009-1119-9.
[2] Rivière C, Iranpour F, Auvinet E, Howell S, Vendittoli PA, Cobb J, et al. Alignment options for total knee arthroplasty: a systematic review. Orthop Traumatol Surg Res 2017;103:1047–56. https://doi.org/10.1016/j.otsr.2017.07.010.
[3] Oussedik S, Abdel MP, Victor J, Pagnano MW, Haddad FS. Alignment in total knee arthroplasty. Bone Jt J 2020;102 B:276–9. https://doi.org/10.1302/0301-620X.102B3.BJJ-2019-1729.
[4] Rivière C, Iranpour F, Auvinet E, Aframian A, Asare K, Harris S, et al. Mechanical alignment technique for TKA: are there intrinsic technical limitations? Orthop Traumatol Surg Res 2017;103:1057–67. https://doi.org/10.1016/j.otsr.2017.06.017.
[5] Bellemans J, Colyn W, Vandenneucker H, Victor J. The Chitranjan Ranawat Award: is neutral mechanical alignment normal for all patients? The concept of constitutional varus. Clin Orthop Relat Res 2012;470:45–53. https://doi.org/10.1007/s11999-011-1936-5.
[6] Anijs T, Wolfson D, Verdonschot N, Janssen D. Population-based effect of total knee arthroplasty alignment on simulated tibial bone remodeling. J Mech Behav Biomed Mater 2020;111:104014. https://doi.org/10.1016/j.jmbbm.2020.104014.
[7] Vanlommel L, Vanlommel J, Claes S, Bellemans J. Slight undercorrection following total knee arthroplasty results in superior clinical outcomes in varus knees. Knee Surgery, Sport Traumatol Arthrosc 2013;21:2325–30. https://doi.org/10.1007/s00167-013-2481-4.
[8] Slevin O, Hirschmann A, Schiapparelli FF, Amsler F, Huegli RW, Hirschmann MT. Neutral alignment leads to higher knee society scores after total knee arthroplasty in preoperatively non-varus patients: a prospective clinical study using 3D-CT. Knee Surgery, Sport Traumatol Arthrosc 2018;26:1602–9. https://doi.org/10.1007/s00167-017-4744-y.
[9] Kayani B, Konan S, Tahmassebi J, Oussedik S, Moriarty PD, Haddad FS. A prospective double-blinded randomised control trial comparing robotic arm-assisted functionally aligned total knee arthroplasty versus robotic arm-assisted mechanically aligned total knee arthroplasty. Trials 2020;21:1–10. https://doi.org/10.1186/s13063-020-4123-8.
[10] Clark G, Steer R, Wood D. Functional alignment achieves a more balanced total knee arthroplasty than either mechanical alignment or kinematic alignment prior to soft tissue releases. Knee Surgery, Sport Traumatol Arthrosc 2022;31:1420–6. https://doi.org/10.1007/s00167-022-07156-3.
[11] Hampp EL, Chughtai M, Scholl LY, Sodhi N, Bhowmik-Stoker M, Jacofsky DJ, et al. Robotic-arm assisted total knee arthroplasty demonstrated greater accuracy and precision to plan compared with manual techniques. J Knee Surg 2019;32:239–50. https://doi.org/10.1055/s-0038-1641729.
[12] Ollivier M, Parratte S, Lino L, Flecher X, Pesenti S, Argenson JN. No benefit of computer-assisted TKA: 10-year results of a prospective randomized study. Clin Orthop Relat Res 2018;476:126–34. https://doi.org/10.1007/s11999.0000000000000021.
[13] Winnock de Grave P, Kellens J, Tampere T, Vermue H, Luyckx T, Claeys K. Clinical outcomes in TKA are enhanced by both robotic assistance and patient specific alignment: a comparative trial in 120 patients. Arch Orthop Trauma Surg 2022:1–9. https://doi.org/10.1007/s00402-022-04636-6.
[14] Agarwal N, To K, McDonnell S, Khan W. Clinical and radiological outcomes in robotic-assisted total knee arthroplasty: a systematic review and meta-analysis. J Arthroplasty 2020;35:3393–409. https://doi.org/10.1016/j.arth.2020.03.005.
[15] Tzanetis P, Fluit R, Souza K de, Robertson S, Koopman B, Verdonschot N. Pre-planning the surgical target for optimal implant positioning in robotic-assisted total knee arthroplasty. Bioengineering 2023;10:543. https://doi.org/10.3390/bioengineering10050543.
[16] Oussedik S, Scholes C, Ferguson D, Roe J, Parker D. Is femoral component rotation in a TKA reliably guided by the functional flexion axis? Clin Orthop Relat Res 2012;470:3227–32. https://doi.org/10.1007/s11999-012-2515-0.
[17] Tzanetis P, de Souza K, Robertson S, Fluit R, Koopman B, Verdonschot N. Numerical study of osteophyte effects on pre-operative knee functionality in patients undergoing robotic-assisted total knee arthroplasty. J Orthop Res 2022 (submitted).
[18] Motesharei A, Batailler C, De Massari D, Vincent G, Chen AF, Lustig S, et al. Predicting robotic-assisted total knee arthroplasty operating time: benefits of machine-learning and 3D patient-specific data. Bone Jt Open 2022;3:383–9. https://doi.org/10.1302/2633-1462.35.BJO.
[19] Bowes MA, Kacena K, Alabas OA, Brett AD, Dube B, Bodick N, et al. Machine-learning, MRI bone shape and important clinical outcomes in osteoarthritis: Data from the Osteoarthritis Initiative. Ann Rheum Dis 2021;80:502–8. https://doi.org/10.1136/annrheumdis-2020-217160.
[20] Morton AM, Akhbari B, Moore DC, Crisco JJ. Osteophyte volume calculation using dissimilarity-excluding Procrustes registration of archived bone models from healthy volunteers. J Orthop Res 2020;38:1307–15. https://doi.org/10.1002/jor.24569.
[21] Hunter DJ, Bowes MA, Eaton CB, Holmes AP, Mann H, Kwoh CK, et al. Can cartilage loss be detected in knee osteoarthritis (OA) patients with 3-6 months’ observation using advanced image analysis of 3T MRI? Osteoarthr Cartil 2010;18:677–83. https://doi.org/10.1016/j.joca.2010.02.010.
[22] Carbone V, Fluit R, Pellikaan P, van der Krogt MM, Janssen D, Damsgaard M, et al. TLEM 2.0 - A comprehensive musculoskeletal geometry dataset for subject-specific modeling of lower extremity. J Biomech 2015;48:734–41. https://doi.org/10.1016/j.jbiomech.2014.12.034.
[23] Marra MA, Vanheule V, Fluit R, Koopman BHFJM, Rasmussen J, Verdonschot N, et al. A subject-specific musculoskeletal modeling framework to predict in vivo mechanics of total knee arthroplasty. J Biomech Eng 2015;137:020904. https://doi.org/10.1115/1.4029258.
[24] Grood ES, Suntay WJ. A joint coordinate system for the clinical description of three-dimensional motions: application to the knee. J Biomech Eng 1983;105:136–44. https://doi.org/10.1115/1.3138397.
[25] Zalzal P, Backstein D, Gross AE, Papini M. Notching of the anterior femoral cortex during total knee arthroplasty. characteristics that increase local stresses. J Arthroplasty 2006;21:737–43. https://doi.org/10.1016/j.arth.2005.08.020.
[26] Mahoney OM, Kinsey T. Overhang of the femoral component in total knee arthroplasty: Risk factors and clinical consequences. J Bone Jt Surg 2010;92:1115–21. https://doi.org/10.2106/JBJS.H.00434.
[27] Rajamäki A, Niemeläinen M, Junnila M, Lehtovirta L, Karsikas M, Ponkilainen V, et al. Thicker polyethylene inserts (≥ 13 mm) increase the risk for early failure after primary cruciate-retaining total knee arthroplasty (TKA): a single-centre study of 7643 TKAs. Knee Surgery, Sport Traumatol Arthrosc 2023;31:1018–25. https://doi.org/10.1007/s00167-022-07189-8.
[28] Tzanetis P, Marra MA, Fluit R, Koopman B, Verdonschot N. Biomechanical consequences of tibial insert thickness after total knee arthroplasty: a musculoskeletal simulation study. Appl Sci 2021;11:2423. https://doi.org/10.3390/app11052423.
[29] Andersen MS, De Zee M, Damsgaard M, Nolte D, Rasmussen J. Introduction to force-dependent kinematics: theory and application to mandible modeling. J Biomech Eng 2017;139:091001. https://doi.org/10.1115/1.4037100.
[30] Schelker BL, Nowakowski AM, Hirschmann MT. What is the “safe zone” for transition of coronal alignment from systematic to a more personalised one in total knee arthroplasty? A systematic review. Knee Surgery, Sport Traumatol Arthrosc 2022;30:419–27. https://doi.org/10.1007/s00167-021-06811-5.
[31] Delport H, Labey L, Innocenti B, De Corte R, Vander Sloten J, Bellemans J. Restoration of constitutional alignment in TKA leads to more physiological strains in the collateral ligaments. Knee Surgery, Sport Traumatol Arthrosc 2015;23:2159–69. https://doi.org/10.1007/s00167-014-2971-z.
[32] Provenzano PP, Heisey D, Hayashi K, Lakesand R, Vanderby R. Subfailure damage in ligament: a structural and cellular evaluation. J Appl Physiol 2002;92:362–71. https://doi.org/10.1152/jappl.2002.92.1.362.
[33] Mueller JKP, Wentorf FA, Moore RE. Femoral and tibial insert downsizing increases the laxity envelope in TKA. Knee Surgery, Sport Traumatol Arthrosc 2014;22:3003–11. https://doi.org/10.1007/s00167-014-3339-0.
[34] Smeets K, Slane J, Scheys L, Claes S, Bellemans J. Mechanical analysis of extra-articular knee ligaments. Part one: native knee ligaments. Knee 2017;24:949–56. https://doi.org/10.1016/j.knee.2017.07.013.
[35] Vincent JP, Magnussen RA, Gezmez F, Uguen A, Jacobi M, Weppe F, et al. The anterolateral ligament of the human knee: an anatomic and histologic study. Knee Surgery, Sport Traumatol Arthrosc 2012;20:147–52. https://doi.org/10.1007/s00167-011-1580-3.
[36] Parsons EM, Gee AO, Spiekerman C, Cavanagh PR. The biomechanical function of the anterolateral ligament of the knee. Am J Sports Med 2015;43:669–74. https://doi.org/10.1177/0363546514562751.
[37] Noyes FR, Huser LE, Levy MS. Rotational knee instability in ACL-deficient Knees: role of the anterolateral ligament and iliotibial band as defined by tibiofemoral compartment translations and rotations. J Bone Jt Surg - Am Vol 2017;99:305–14. https://doi.org/10.2106/JBJS.16.00199.
[38] Guo Z, Freeman JW, Barrett JG, De Vita R. Quantification of strain induced damage in medial collateral ligaments. J Biomech Eng 2015;137:071011. https://doi.org/10.1115/1.4030532.
[39] Quilez MP, Delport HP, Wirix-Speetjens R, Wesseling M, Perez Anson MA, Jonkers I, et al. Can standard implants reproduce the native kinematics of a TKA patient? Epic Ser Heal Sci 2019;3:311–306. https://doi.org/10.29007/7dsg.
[40] Dejtiar DL, Bartsoen L, Wesseling M, Wirix-Speetjens R, Sloten J Vander, Perez MA. Standard cruciate-retaining total knee arthroplasty implants can reproduce native kinematics. Epic Ser Heal Sci 2020;4:61–4. https://doi.org/10.29007/lj2j.
[41] Patil S, Bunn A, Bugbee WD, Colwell CW, D’Lima DD. Patient-specific implants with custom cutting blocks better approximate natural knee kinematics than standard TKA without custom cutting blocks. Knee 2015;22:624–9. https://doi.org/10.1016/j.knee.2015.08.002.
[42] Koh YG, Park KM, Kang KT. The biomechanical effect of tibiofemoral conformity design for patient-specific cruciate-retaining total knee arthroplasty using computational simulation. J Exp Orthop 2019;6. https://doi.org/10.1186/s40634-019-0192-6.
[43] Parratte S, Pagnano MW, Trousdale RT, Berry DJ. Effect of postoperative mechanical axis alignment on the fifteen-year survival of modern, cemented total knee replacements. J Bone Jt Surg 2010;92:2143–9. https://doi.org/10.2106/JBJS.I.01398.
[44] Bonner TJ, Eardley WGP, Patterson P, Gregg PJ. The effect of post-operative mechanical axis alignment on the survival of primary total knee replacements after a follow-up of 15 years. J Bone Jt Surg - Ser B 2011;93 B:1217–22. https://doi.org/10.1302/0301-620X.93B9.26573.
[45] Abdel MP, Ollivier M, Parratte S, Trousdale RT, Berry DJ, Pagnano MW. Effect of postoperative mechanical axis alignment on survival and functional outcomes of modern total knee arthroplasties with cement: A concise follow-up at 20 years. J Bone Jt Surg - Am Vol 2018;100:472–8. https://doi.org/10.2106/JBJS.16.01587.
[46] Victor J. Optimising position and stability in total knee arthroplasty. EFORT Open Rev 2017;2:215–20. https://doi.org/10.1302/2058-5241.2.170001.
[47] Slevin O, Moser LB, Hirschmann MT. Is there an optimal TKA component position? In: Basics in Primary Knee Arthroplasty. Springer International Publishing; 2022, pp. 299–309. https://doi.org/10.1007/978-3-030-58178-7_26.
[48] Bonnin MP, Saffarini M, Nover L, Van Der Maas J, Haeberle C, Hannink G, et al. External rotation of the femoral component increases asymmetry of the posterior condyles. Bone Jt J 2017;99B:894–903. https://doi.org/10.1302/0301-620X.99B7.BJJ-2016-0717.R1.
[49] Verlinden C, Uvin P, Labey L, Luyckx JP, Bellemans J, Vandenneucker H. The influence of malrotation of the femoral component in total knee replacement on the mechanics of patellofemoral contact during gait: An in vitro biomechanical study. J Bone Jt Surg - Ser B 2010;92:737–42. https://doi.org/10.1302/0301-620X.92B5.22603.
[50] Bell SW, Young P, Drury C, Smith J, Anthony I, Jones B, et al. Component rotational alignment in unexplained painful primary total knee arthroplasty. Knee 2014;21:272–7. https://doi.org/10.1016/j.knee.2012.09.011.
[51] Innocenti B, Bellemans J, Catani F. Deviations from optimal alignment in TKA: is there a biomechanical difference between femoral or tibial component alignment? J Arthroplasty 2016;31:295–301. https://doi.org/10.1016/j.arth.2015.07.038.
[52] Marra MA, Strzelczak M, Heesterbeek PJC, van de Groes SAW, Janssen DW, Koopman BFJM, et al. Anterior referencing of tibial slope in total knee arthroplasty considerably influences knee kinematics: a musculoskeletal simulation study. Knee Surgery, Sport Traumatol Arthrosc 2018;26:1540–8. https://doi.org/10.1007/s00167-017-4561-3.
[53] Okazaki K, Tashiro Y, Mizu-uchi H, Hamai S, Doi T, Iwamoto Y. Influence of the posterior tibial slope on the flexion gap in total knee arthroplasty. Knee 2014;21:806–9. https://doi.org/10.1016/j.knee.2014.02.019.
[54] Arnout N, Victor J, Vermue H, Pringels L, Bellemans J, Verstraete MA. Knee joint laxity is restored in a bi-cruciate retaining TKA-design. Knee Surgery, Sport Traumatol Arthrosc 2020;28:2863–71. https://doi.org/10.1007/s00167-019-05639-4.
[55] Vanheule V, Delport HP, Andersen MS, Scheys L, Wirix-Speetjens R, Jonkers I, et al. Evaluation of predicted knee function for component malrotation in total knee arthroplasty. Med Eng Phys 2017;40:56–64. https://doi.org/10.1016/j.medengphy.2016.12.001.
Figures
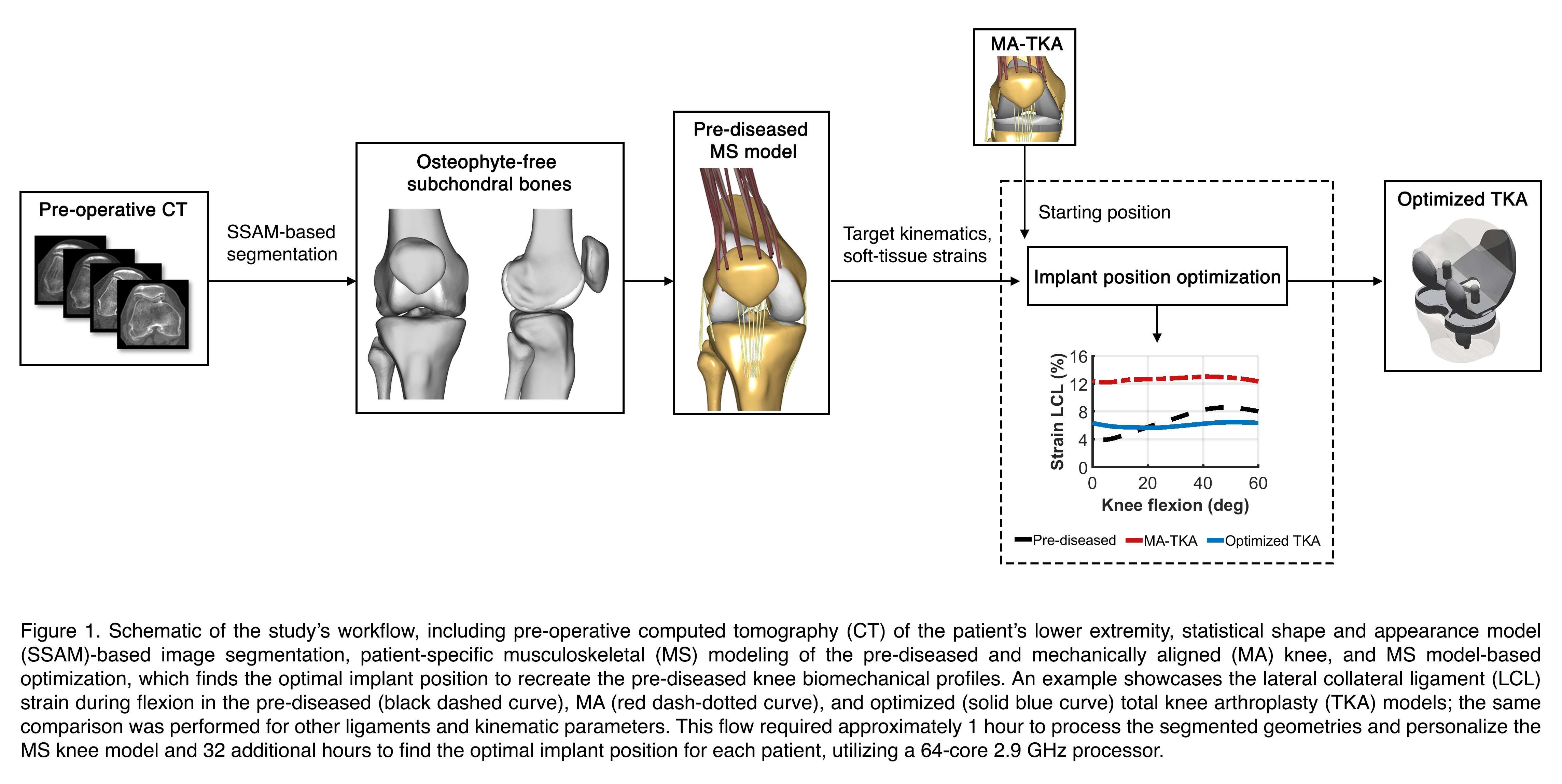
Figure 1
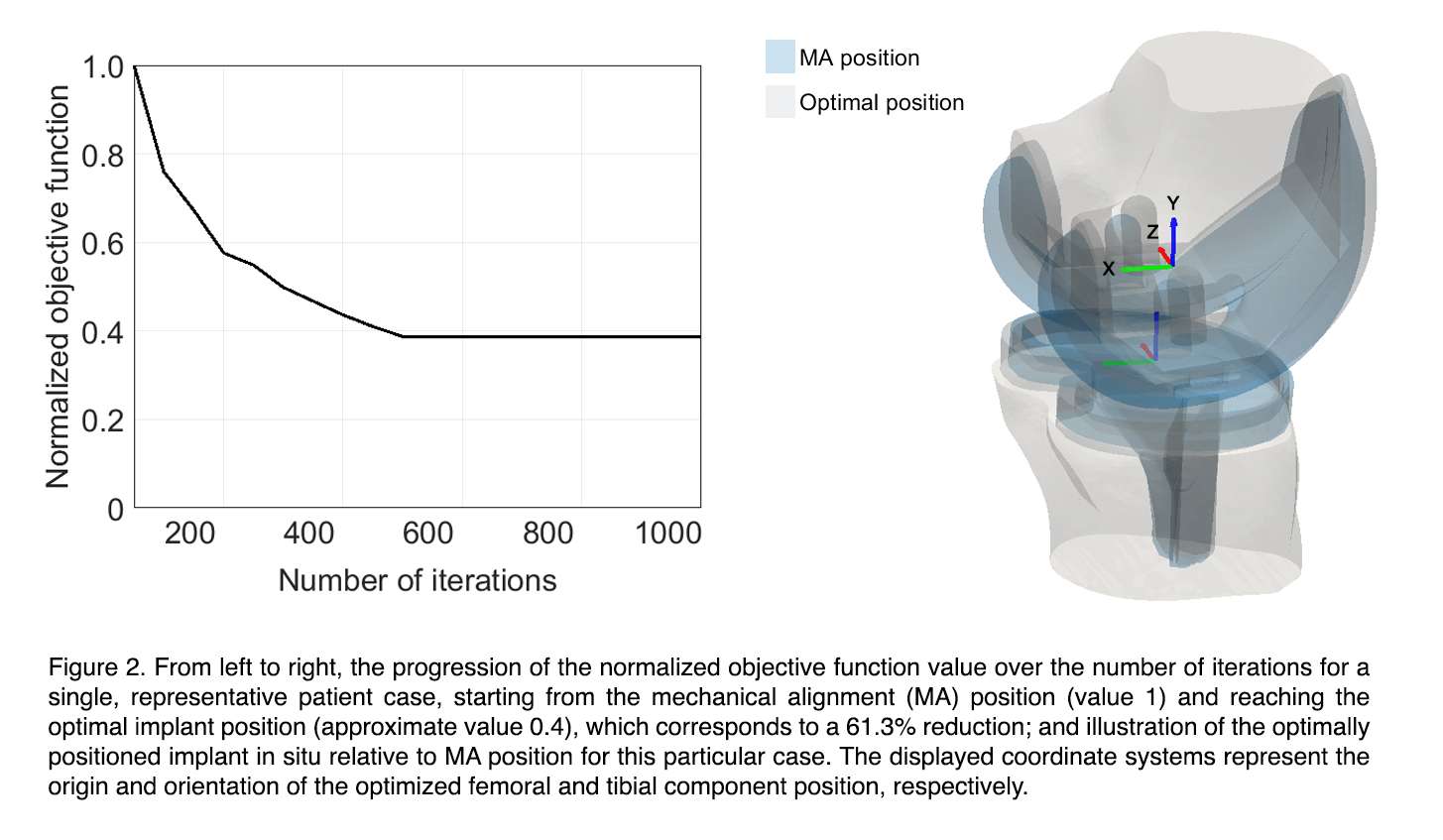
Figure 2
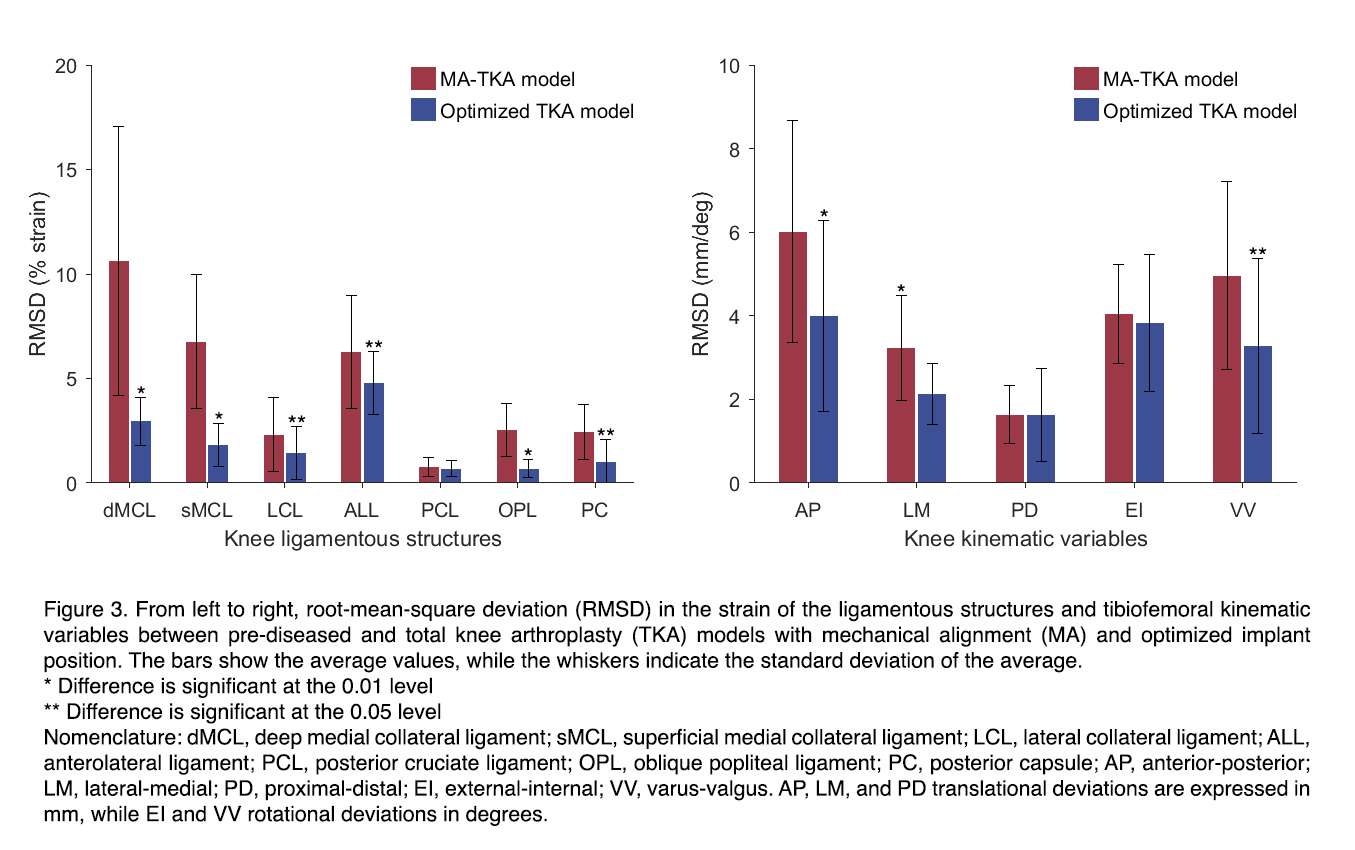
Figure 3
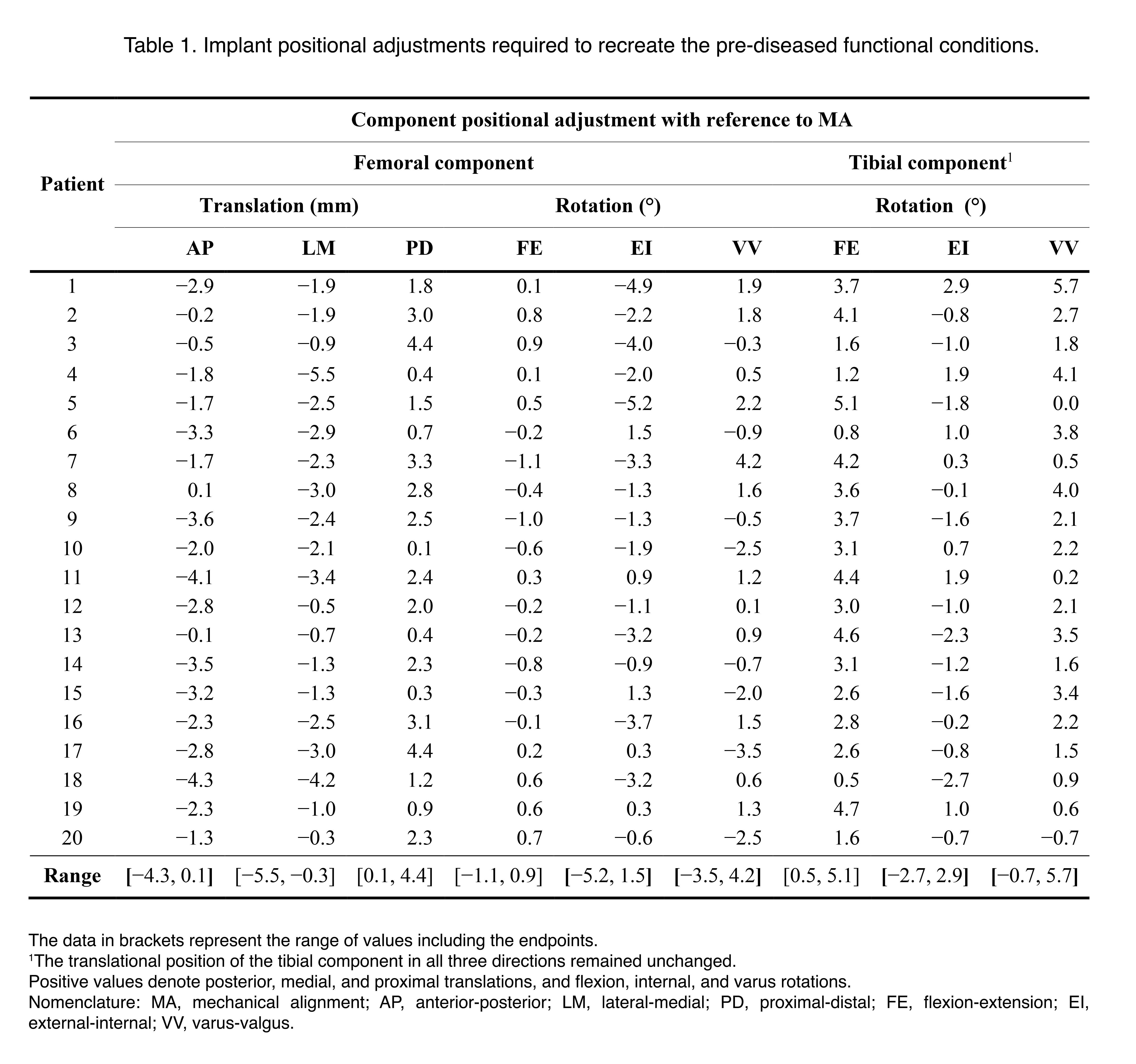
Figure 4
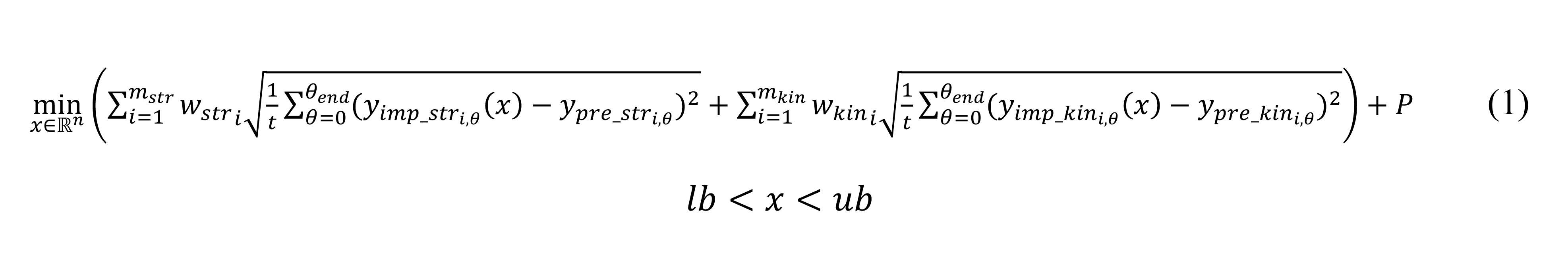
Figure 5#8844
Introduction: Aims for modern implant design
*Nico Verdonschot - Radboudumc - Nijmegen, Netherlands
*Email: nico.verdonschot@radboudumc.nl
#8845
Implant design matters
*Scott Banks - University of Florida - Gainesville, USA
*Email: banks@ufl.edu
#8847
Current concepts in implant testing
*Jeffrey Bischoff - Zimmer, Inc. - Warsaw, USA
*Email: jeff.bischoff@zimmerbiomet.com
#8846
New coatings and materials in modern implant design
*Steven Kurtz - Drexel University - Philadelphia, USA
*Email: smk38@drexel.edu
#8848
Additive manufacturing: Technical possiblities and challenges
*Timothy Wright - Hospital for Special Surgery - New York, USA
*Email: wrightt@hss.edu
#8849
Additive manufacturing: Clinical opportunities and challenges
*Joseph Lipman - Hospital for Special Surgery - New York, USA
*Email: lipmanj@hss.edu
#8552
Surface Damage and Oxidation of Highly Crosslinked Polyethylene Knee Inserts Implanted More Than 10 Years
*Tabitha Derr - Drexel University - Philadelphia, USA
Shabnam Aslani - Drexel University - Philadelphia, USA
Daniel MacDonald - Drexel University - Philadelphia, USA
Greg Klein - Hartzband Center for Hip and Knee Replacement - Hackensack, USA
Nicolas Piuzzi - Cleveland Clinic - Cleveland, USA
Steven M. Kurtz - Drexel - Philadelphia, USA
*Email: tabitha.derr@gmail.com
Introduction
Highly cross-linked polyethylene (HXLPE) was originally introduced to improve wear and oxidation resistance in total knee arthroplasty (TKA). Different manufactures have implemented different thermal treatments to help reduce oxidation. These include remelting and sequentially annealing. Short term studies have found good wear and oxidation results for HXLPE inserts; however, it is important to understand how time in vivo affects the wear of these materials. The purpose of this study was to investigate the wear and oxidative performance of HXLPE knee inserts implanted for greater than 10 years and compare the different thermal treatments used to reduce oxidation.
Methods
14 HXLPE TKA inserts implanted for greater than 10 years were retrieved during revision surgery as part of an IRB approved multi-institutional implant retrieval program. The articulating surface, backside and post of the inserts were evaluated for 7 surface damage mechanisms, which include burnishing, pitting, scratching, abrasion, delamination, surface deformation and embedded debris, according to the semiquantitative Hood scoring method. The inserts were sectioned along the midline and medial condyle. 200 µm thick slices were obtained with a microtome and boiled in heptane to remove lipids. Oxidation indices (OI) for the articulating surface, backside, AP surfaces and post were measured with Fourier-transform infrared spectroscopy according to ASTM 2102. Mann-Whitney U Test was used to compare remelted and sequentially annealed components.
Results
The TKA cohort had an average implantation time of 11.9 ± 1.4 years, an average patient BMI of 32.02 ± 7.84 kg/m2, an average age at insertion of 57 ± 8 years, and a median UCLA score of 7 (IQR=3). The components were revised for infection (n=5/14), instability (n=5/14), loosening (n=3/14) and stiffness (n=1/14). The most prevalent modes of surface damage on the articulating surface were burnishing, pitting, and scratching. No difference was found in total damage scores for the articulating, backside, or post (Mann-Whitney U Test; p=0.345, p=0.282, and p=0.750 respectively). Oxidation was higher in the AP surfaces for sequentially annealed components (1.400 ± 1.870) when compared to remelted (0.371 ± 0.622) (Mann Whitney U Test; p=0.029). Oxidation indices did not differ for the articulating surface, backside, or post (Mann-Whitney U Test; p=0.081, p=0.755, and p=1.000 respectively). Additionally, of these regions, only the articulating surface had mean oxidation indices above 1 (sequentially annealed = 2.817 ± 2.244 and remelted = 1.017 ± 1.278).
Conclusion
In this study we examined the in vivo surface damage and oxidation for HXLPE TKA inserts with long term implantation times. Surface damage did not differ between components and oxidation was only found to differ in the AP region where it was higher in annealed components. This indicates that both remelted and annealed components performed similarly in vivo when implanted for periods of time beyond 10 years. Additionally, oxidation was low in all areas except the articulating surface. This study was limited in component numbers; therefore, it will be important to analyze a greater number of implants in each category to better understand the affect time has on in vivo wear of HXLPE.
Figures
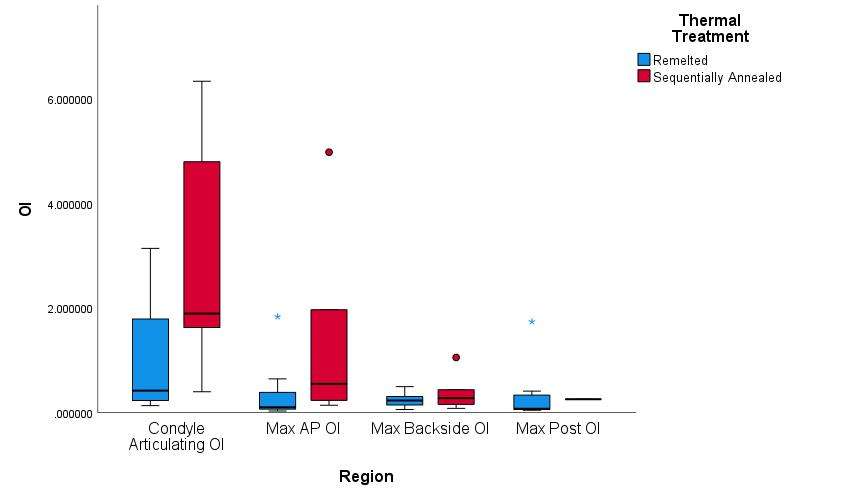
Figure 1
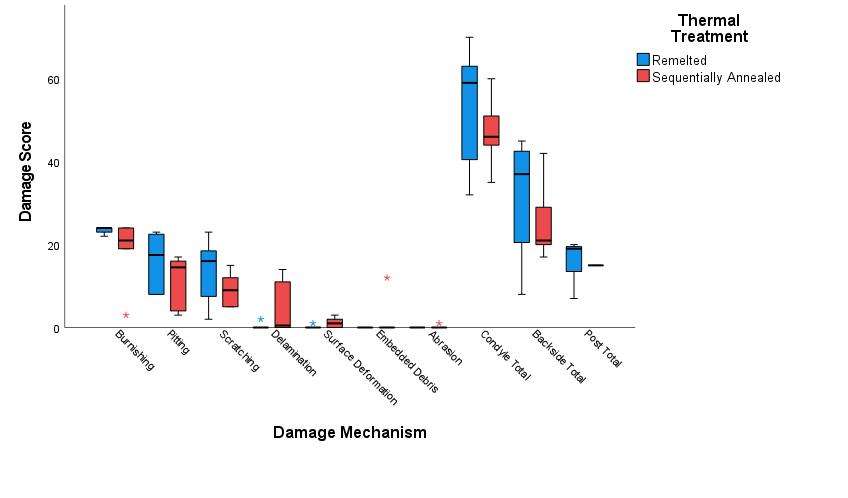
Figure 2#8544
Effect of Storage Method on Oxidation Rate of UHMWPE
*Zachary Currier - Dartmouth College - Norwich, United States of America
Barbara Currier - Dartmouth College - Hanover, USA
Taylor J Floyd - Dartmouth College - Hanover, USA
Kori Jevsevar - Dartmouth College - Hanover, USA
Douglas Van Citters - Dartmouth College - Hanover, USA
*Email: zachary.m.currier@dartmouth.edu
Introduction:
Over the last 30 years, the effect of exposure of UHMPWE total joint components to oxygen (shelf and in vivo) and to oxidizing species has been well documented [1, 2]. It is further known that retrieved devices will oxidize on the shelf even in the absence of free radicals due to the oxidation of absorbed species [3]. Oxidation is known to change density, mechanical properties, and chemical nature of the polyethylene, reducing the value of studies commissioned more than a few months after revision surgery. We hypothesize that storage in low temperatures will meaningfully slow post-explant oxidation rates through rendering the Arrhenius exponent more negative. The objective of this study is to test the hypothesis using retrieved devices (across the spectrum of radiation doses and thermal treatments) analyzed at revision and after storage on the shelf or in a freezer.
Methods:
Using an IRB-approved total joint arthroplasty retrieval collection, 32 UHMWPE tibial bearings (5 gamma barrier, 8 annealed, 19 remelted) from four different manufactures were analyzed for oxidation. Because this study includes, in part, oxidation due to absorbed species, oxidation was reported as maximum ketone oxidation index (KOI) due to this measure’s reduced sensitivity to absorbed esters retained in retrieved devices. Baseline KOI was measured within 3 months of retrieval on explants surgically removed between 2015-2021. Sections from these bearings were stored on the shelf in air and in the freezer in air (-70°C) for mean 5.8 years (range 1.1 – 7.3 years). KOI was remeasured using Thermo-Scientific iN10-FTIR microscope, and the oxidation delta was determined. Statistical analyses used SPSS, with p-value < 0.05 considered statistically significant.
Results:
Figure 1 shows oxidation measurements of components as-received and after storage. Consistent with earlier studies, UHMWPE implants oxidized over time in vivo and on the shelf after explant. UHMWPE stored at -70°C showed less change in oxidation as compared to UHMWPE stored on the shelf, as exemplified in individual scans shown in Figure 2. As-received oxidation was compared to after-storage oxidation (freezer and shelf) using paired-samples t-tests. Freezer-stored devices showed oxidation not significantly different than as-received oxidation regardless of polyethylene treatment type, (t(31) = 1.081, p = 0.288). Shelf stored devices showed oxidation significantly different than as-received oxidation measurements regardless of whether the devices contained free radicals at implantation, (t(31) = -4.616, p < 0.001). Devices with higher initial as-received oxidation exhibited a greater change in oxidation on the shelf. Oxidation change was not statistically distinguishable from zero for freezer-stored devices in each polymer treatment group (p=0.288).
Conclusion:
Storing implants in the freezer slowed the oxidation process significantly, which in turn helps preserve the mechanical and chemical properties of the material for future studies. Gamma-barrier and annealed HXL devices demonstrated greater oxidation changes on the shelf as compared to remelted HXL devices, but these same devices showed near zero oxidation changes when stored at -70°C.
Figures
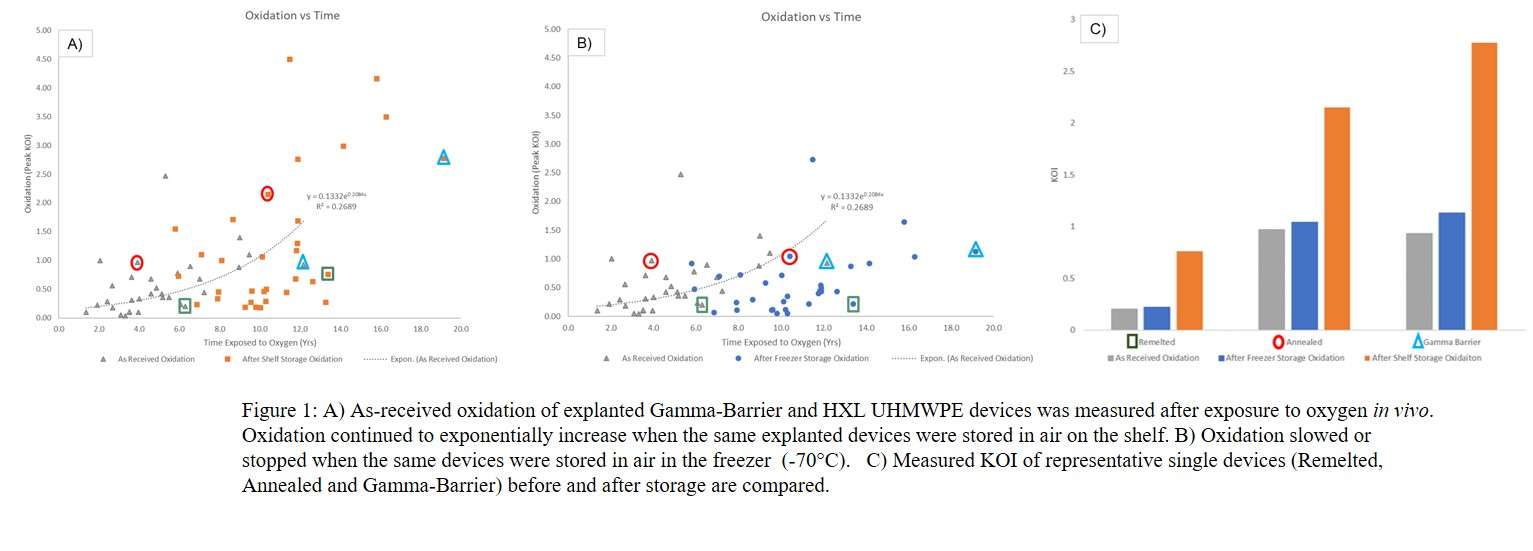
Figure 1
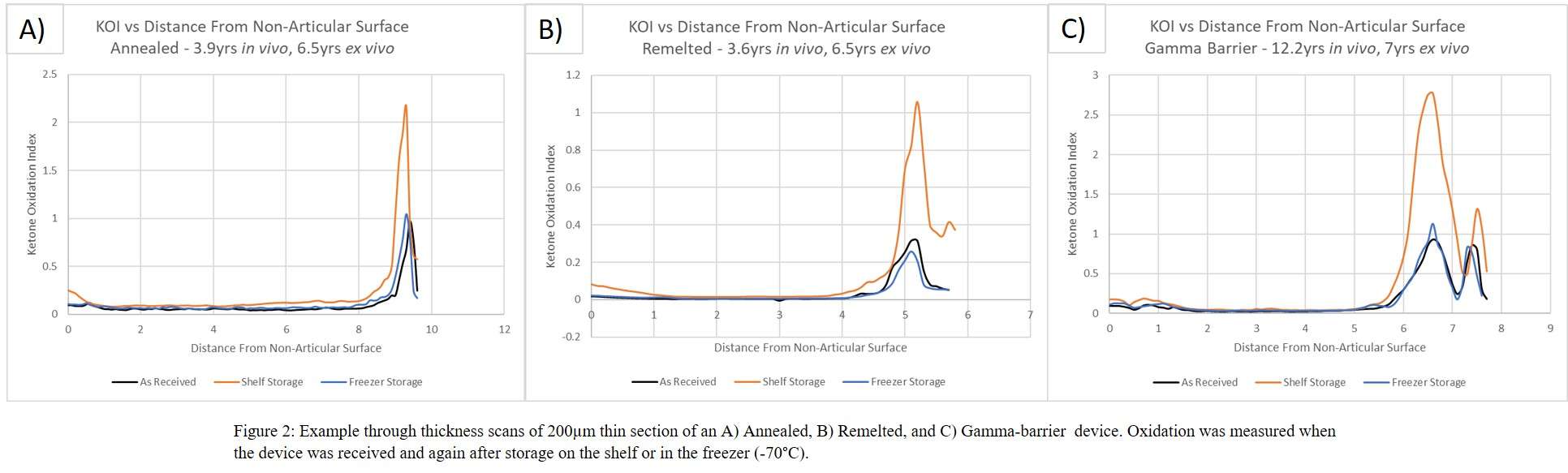
Figure 2
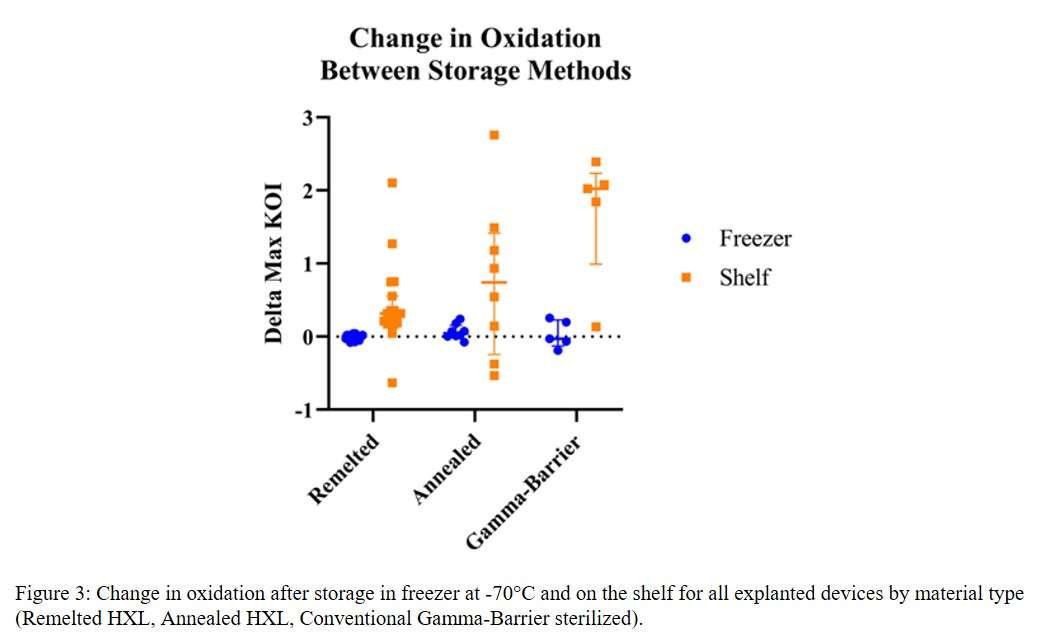
Figure 3#8575
Oxidative Degradation Profile of Retrieved UHMWPE Prosthetic Knee Liner: A Spectroscopic Analysis
*Songyun Liu - University of Illinois at Chicago - Chicago, USA
Douglas Van Citters - Dartmouth College - Hanover, USA
Joshua Jacobs - Rush University Medical Center - USA
Robin Pourzal - Rush University Med Ctr - Chicago, USA
*Email: Songyun_Liu@rush.edu
Introduction: The use of highly cross-linked UHMWPE (HXLPE) has successfully combatted early total knee arthroplasty (TKA) failure due to particle induced osteolysis. Nonetheless, studies have shown that in vivo oxidation of HXLPE components is related to the accelerated wear in TKA. Currently, there exist two generally accepted oxidation pathways: (1) a free radical mediated mechanism; and (2) an absorbed pro-oxidative species mechanism. The latter is believed to be affected by synovial fluid, which has a complex composition that varies among individuals. In this study, we are presenting a combined Fourier Transform Infrared spectroscopic imaging (FTIRI) and Raman spectroscopic mapping approach to generated spatial maps of full-depth oxidation profiles and absorbed organic constituents to better understand in vivo chemical alterations and its impact on wear and clinical outcomes.
Methods: 13 retrieved tibial liners, with a minimum postretrieval shelf time, from 5 designs and 4 manufactures were analysed. The average time in situ was 124.2 months (60.3, 197.5). Liners were cut in the sagittal plane of the medial condyle to expose a vertical cross-section. Thin sections (~200 μm thick) were removed parallel to the exposed cross-section using a microtome for FTIRI/Raman analysis. For FTIRI, a large field of view was achieved via mosaic mode for a full-depth scans reaching from the articulating surface to the backside. All imaging data were collected using an Agilent Cary 670/620 system with 2 cm-1 spectral resolution (wavenumber range: 3750-900 cm-1). Because absorbed species are of interest, devices were not extracted. Thus, the spatial distribution of the oxidation profile was calculated using the ester-insensitive ketone oxidation index (KOI), defined as the peak center of around 1715 cm-1 (ketone peak) referenced against the peak center at 1368cm-1. Data processing was done using CytoSpec software. Absorbed species were also assessed by Raman micro-spectroscopy (Horiba) using a 10x objective and 532nm laser.
Results: 12 of 13 liners exhibited a strong subsurface oxidation band (hundreds of microns below the articulating surface) and an increased oxidation zone in the subsurface of the backside. The average maximum KOI was 0.89 months ± 0.54, ranging from 0.05 to 2.3. For comparison with the ASTM oxidation index based on the carbonyl band (1736cm-1 vs. 1368cm-1), the corresponding chemical images of one liner are showcased in Figure 1. Our Raman data identified the occurrence of different concentration of β-carotene infiltrates within the surface of 11 tibial liners. β-carotene is an antioxidant characterized by Raman peaks at 1005cm-1, 1155cm-1, and 1514cm-1 (Fig.2).
Conclusion: This study highlights the heterogeneity of oxidation profiles of HXLPE liners across manufacturers, both first and second generation. Imaging revealed a complex oxidation progression scenario that likely results from a competitive interplay between the absorption of anti- and pro-oxidative species, which could affect the mechanical property of these liners. Interestingly, the liner without oxidation exhibited the strongest β-carotene signal at the surface. The cause of in vivo oxidation is multifactorial warranting further research. The chemical state of wear debris generated from chemically altered surfaces also needs to be considered with respect to its bio-reactivity.
Figures
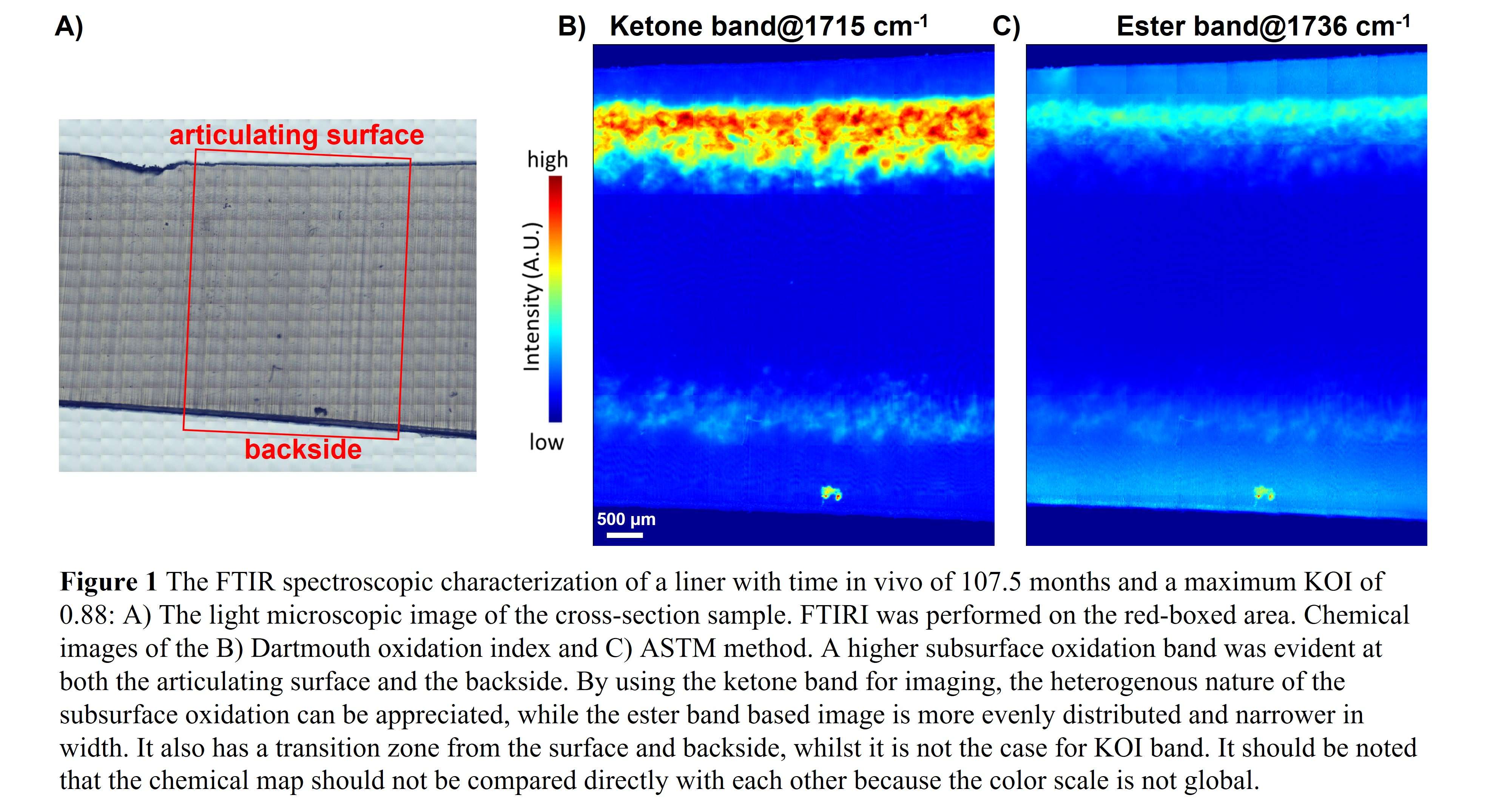
Figure 1
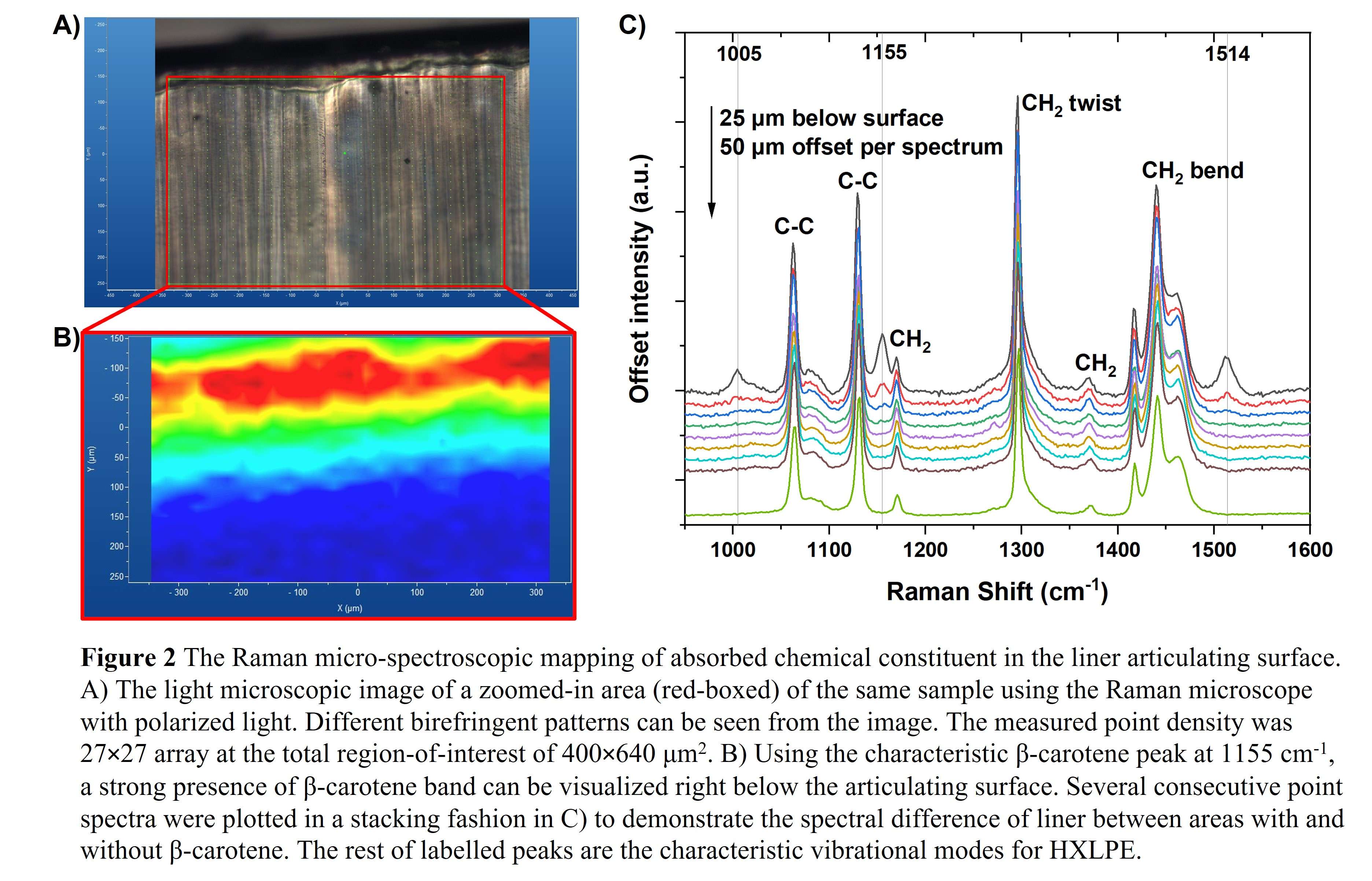
Figure 2#8547
Femoral Head Penetration Rates and Oxidation of Highly Crosslinked Polyethylene Hip Liners Implanted More Than 10 Years
*Tabitha Derr - Drexel University - Philadelphia, USA
Josh Thekkumthala - Drexel University - Philadelphia, USA
Daniel MacDonald - Drexel University - Philadelphia, USA
Greg Klein - Hartzband Center for Hip and Knee Replacement - Hackensack, USA
Arthur L Malkani - Louisville, USA
Steven M. Kurtz - Drexel - Philadelphia, USA
*Email: tabitha.derr@gmail.com
Introduction
Highly cross-linked polyethylene (HXLPE) was originally introduced to improve wear and oxidation resistance in total hip arthroplasty (THA). Several different thermal treatments are used to help limit the oxidation of these HXLPE liners. These include remelting, annealing and sequentially annealing. Studies of HXLPE have found promising results for shorter-term implantation studies; however, it is important to know the effect of longer-term implantation on the HXLPE materials. The purpose of this study was to investigate the wear and oxidative performance of HXLPE hip liners implanted for greater than 10 years and observe the effects of different thermal treatments of the HXLPE.
Methods
51 THA liners from various manufacturers were retrieved during revision surgery as part of an IRB approved multi-institutional implant retrieval program. Thickness measurements were taken in triplicate in loaded and unloaded regions of the liner using a digital micrometer (accuracy=0.001). The penetration rates for the liners were calculated as the difference between the mean thickness of the unloaded and loaded regions divided by the implantation time. Liners were sectioned along the superior and inferior axis and 200 µm thick slices of were obtained using a microtome. The polyethylene slices were boiled in heptane to remove lipids that were absorbed in vivo. Oxidation indices for rim, locking mechanism, articulating surface and backside regions of the superior and inferior axis were measured using Fourier-transform infrared spectroscopy according to ASTM 2102. Mann-Whitney U Test was used to determine statistical difference between annealed and remelted HXLPE components. Sequentially annealed polyethylene was excluded from the comparison because we did not possess an adequate number of components in our study.
Results
The cohort had an average implantation time of 12.5 ± 2.0 y, average patient of BMI 28.8 ± 5.6 kg/m2, and median UCLA score of 7 (IQR-3). The components were revised most often for instability (n = 15/51) and loosening (n = 14/51). The penetration rate for the whole cohort was 0.052 ± 0.190 mm/y. The penetration rate averaged 0.062 ± 0.185 mm/y for annealed components and 0.018 ± 0.030 mm/y for remelted components. Penetration rates did not differ between the remelted and annealed components (Mann-Whitney U Test, p=0.140). Oxidation indices were found to be significantly higher in the annealed components for all regions (Mann-Whitney U Test, p<0.001).
Conclusion
In this study we examined the in vivo penetration rates and oxidation for HXLPE THA liners with long term implantation times. The average penetration rate for the cohort was low and did not differ significantly between annealed and remelted. Oxidation was found to be higher in the annealed components for all regions. All remelted components had oxidation indices below the clinically relevant level of 1. It is important to note though that this study did not include the most current iteration of sequentially annealed components which have been found to have lower oxidation in the rim than the annealed components in a shorter-term study.
Figures
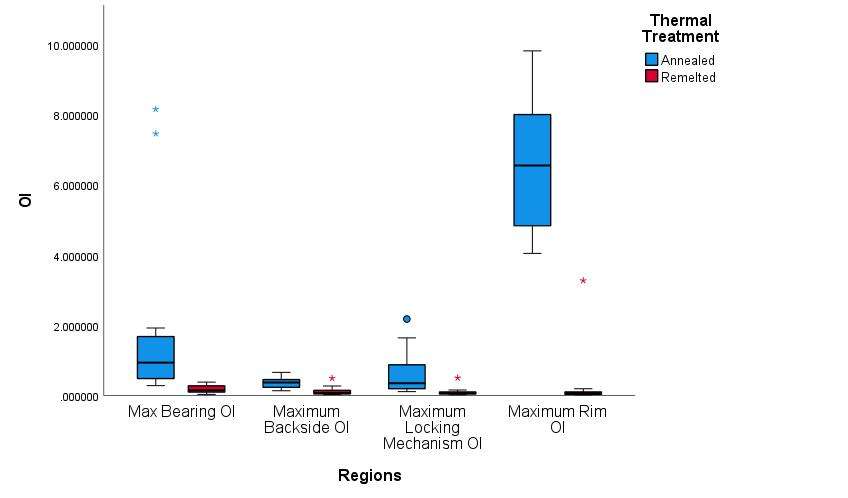
Figure 1#8620
Determination of Particle Induced Osteolysis in THA With Highly Crosslinked Polyethylene Liners
Songyun Liu - University of Illinois at Chicago - Chicago, USA
Deborah Hall - Rush University Medical Center - Chicago, USA
Corina Dommann-Scherrer - Cantonal Hospital Winterthur - Winterthur, Switzerland
Peter Wahl - Kantonsspital Winterthur - Winterthur, Switzerland
*Robin Pourzal - Rush University Med Ctr - Chicago, USA
*Email: robin_pourzal@rush.edu
Introduction: The use of highly crosslinked polyethylene (HXLPE) liners has resulted in much better wear behavior, lower incidence of particle induced osteolysis and increase implant longevity in total hip arthroplasty (THA). However, it is unclear if osteolysis is truly eliminated or only postponed. The problem is that late particle induced osteolysis is difficult to diagnose because pathologists can no longer see HXLPE debris due to its smaller size, and certain periprosthetic tissue hallmarks of osteolysis, such as foreign body giant cells, are no longer obvious. Objective: to locate polyethylene debris within periprosthetic tissue of two THA cases with HXLPE liners and obvious osteolysis.
Methods: Two THA cases (87- and 61-year-old, male) with implantation times of 7. 6 and 6.8 years and the same type of HXLPE liner were reviewed. Both were removed due to pain and implant loosening with obvious radiolucency visible along the femoral stem. The acetabular wear of both liners was assessed with an optical coordinate measuring machine and volumetric loss was calculated. Pseudo-capsule tissue was histopathologically analyzed. Two consecutive tissue sections were prepared for a) H&E staining and b) 5-μm unstained section was placed on a 25mm×2mm BaF2 disc (IR transparent) for Fourier Transform Infrared Spectroscopic Imaging (FTIRI) analysis.
Results: Both acetabular liners exhibited prominent wear scars (Fig. 1) with wear volumes of 115.5 mm3 and 208.28 mm3 (wear rate: 15.2 mm3/year and 30.6 mm3/year), respectively. The maximum linear penetrations were 207.9 μm and 315.6 μm (linear wear rate: 0.0274 mm/year and 0.0464 mm/year), respectively. The macrophage infiltration of both cases exhibited no obvious presence of polyethylene debris. For instance, in Case one (Fig. 2D), under polarized light at most one birefringent particle could be detected per field of view. However, using the characteristic peak representing the absorbance of methylene group (νδ(CH2)), centered at 2918 cm-1 and 2850 cm-1, respectively, chemical images generated by FTIR-I clearly showed the prominent presence of polyethylene within the tissue (Fig. 2E). Aside from identifying the aggregation of PE debris, another finding was glycogen deposition within the hypertrophic synovial membrane of the neo-capsule (Fig. 2G). Using Case two as an example, the presence of polyethylene was co-localized with the presence of cells as indicated by characteristic IR signals, centered at 1081 cm-1, associated with nucleic acid (Fig. 3B & D), whereas areas without either cells or polyethylene exhibited most dominantly the presence of Amide I, indicative of the collagenous tissue matrix (Fig. 3C).
Conclusions: This study has shown that particle induced osteolysis can still occur THA with HXLPE liners even under 10 years in situ. However, it has also demonstrated the clinical difficulty of determining the presence of PE debris within periprosthetic tissue. FTIRI is an ideal tool to determine the presence of fine PE particle accumulations intracellularly. More retrieval analysis is needed to determine new histopathological hallmarks of osteolysis related to HXLPE liners and potential increased bioreactivity of these much finer sub-micron PE debris. The overall precedence of late osteolysis will be important to understand the longevity and clinical outcomes of current generation of HXLPE.
Figures
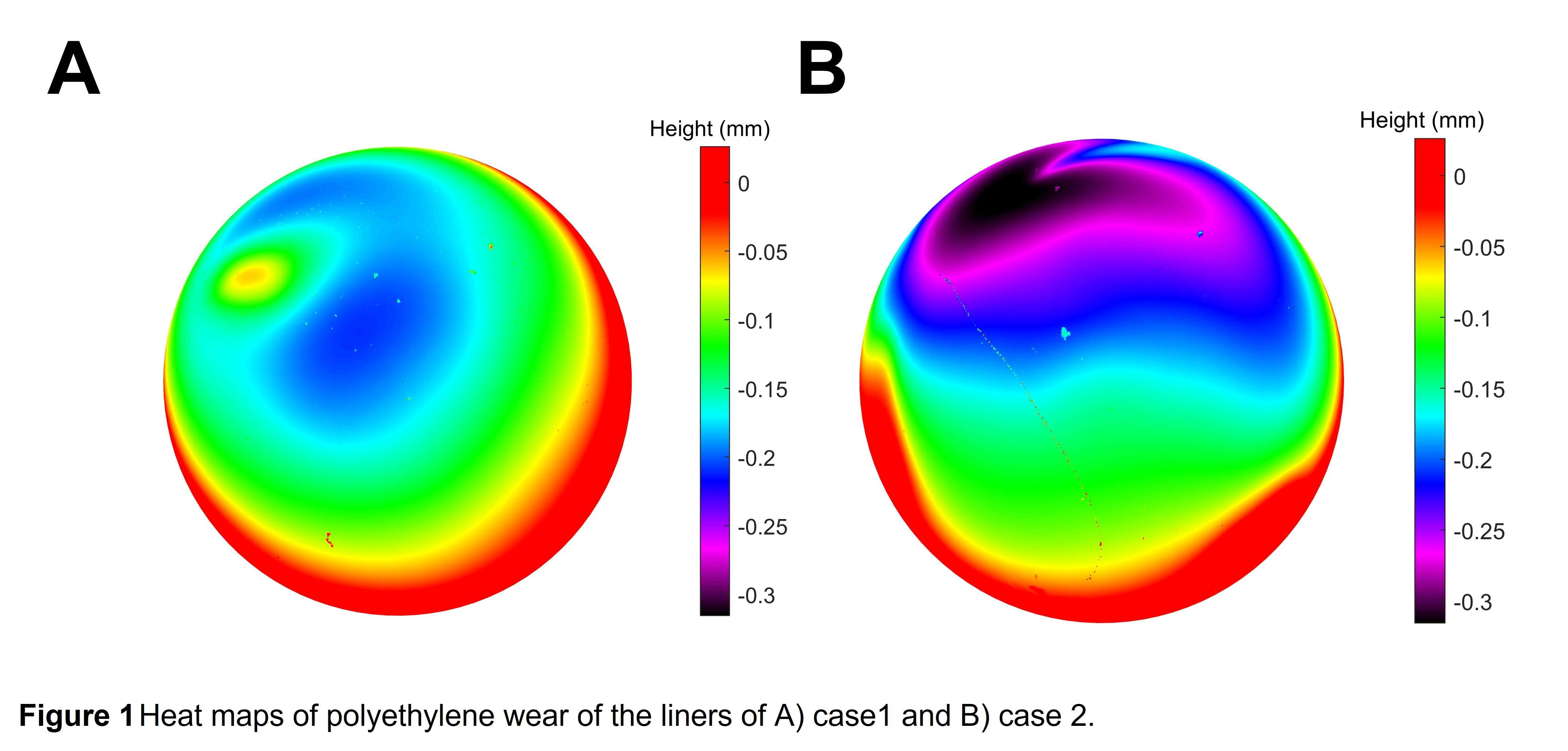
Figure 1
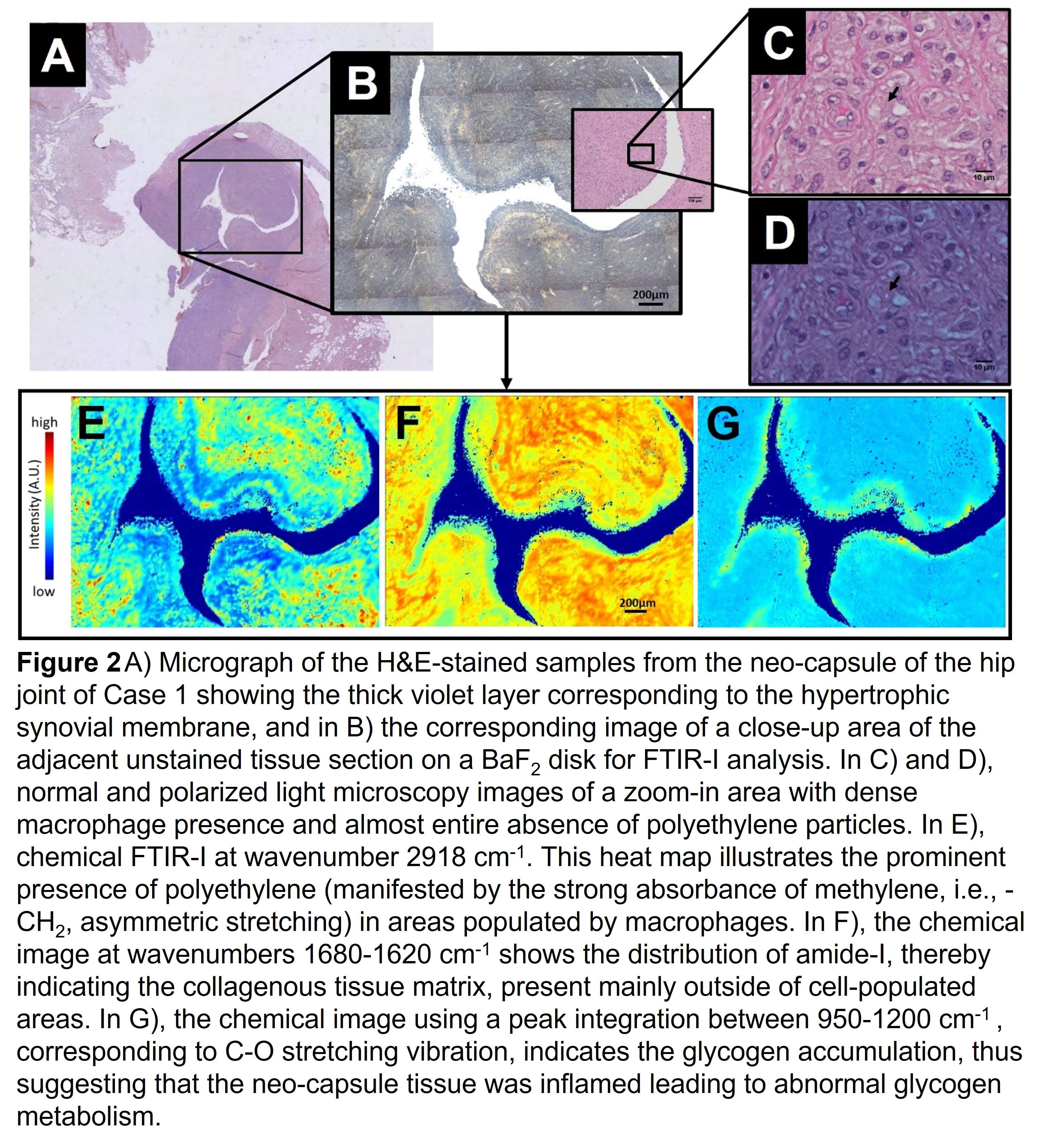
Figure 2
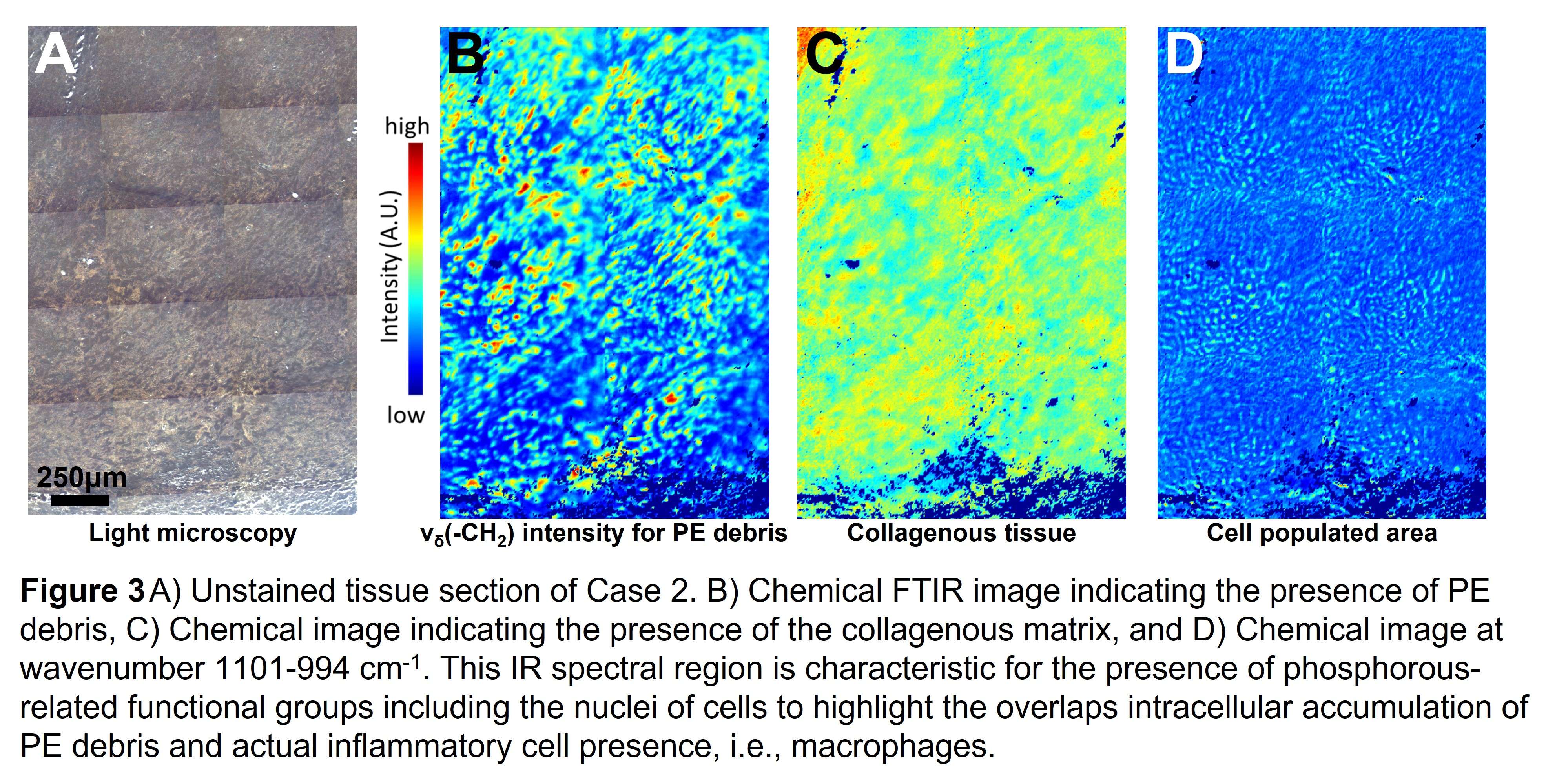
Figure 3#8446
Influence of Ageing in a Wet in-Vitro Environment on the Thermomechanical Properties of PMMA-Based Bone Cement
*Magnus Reulbach - Hannover Medical School - Hannover, Germany
Crystal Emonde - Hannover Medical School - Hannover, Germany
Patrick Evers - Leibniz University Hannover - Garbsen, Germany
Hannah Behnsen - Leibniz University Hannover - Hannover, Germany
Florian Nuernberger - Leibniz University Hannover - Hannover, Germany
Henning Windhagen - Hanover Medical School - Hannover, Germany
Eike Jakubowitz - Hanover medical Schhol - Hannover, Germany
*Email: reulbach.magnus@mh-hannover.de
Introduction:
Total hip replacement (THR) is the most successful and frequently performed elective surgery [1]. About 30–50% of all primary THR are fixated with bone cement (PMMA) [2–4]. Loosening of THR is still the main reason for revision surgery [5] and the removal of still fixed parts of cemented stems during revision surgery is a challenging task that can result in periprosthetic bone damage [6]. Heating of PMMA above its glass transition temperature (TG) provides a promising approach for a gentler removal of the fixed prosthesis [7]. However, the TG values of PMMA in literature vary between 65 and 126°C [7–9], and it seems to decrease in liquid environments. In addition, nothing is known about the correlation between increasing water uptake and decreasing TG to date. Reliable data on TG is essential for developing a gentler method of removing cemented THR by heating PMMA.
Hypothesis: TG correlates with PMMA water absorption up to its saturation and decreases significantly up to this point.
Methods:
TG was determined thermally using differential scanning calorimetry (DSC) according to ASTM-E2716-09 as well as mechanically using the Vicat softening temperature (VST, thermal-mechanical method) according to DIN-EN-ISO-306. With Palacos® (Heraeus Medical GmbH, Wehrheim, Germany) a widely used PMMA cement [4] was studied. The effect of water absorption was investigated storing PMMA samples 1, 3, 7, 14, 28, and 56 days in both Ringer’s solution (37 °C) and air (30% humidity and 37 °C). For VST three samples (10x10x2 mm) were tested for each storage interval and each condition. Absorption was determined measuring the relative weight change of the VST samples after each interval. For verification, VST and TG of a PMMA based bone cement spacer after 12 weeks in-situ were determined. For statistics an analysis of variance with post hoc t-tests and Pearson’s correlation coefficient were calculated.
Results and Discussion:
Samples stored in Ringer’s solution showed a relative water uptake of 0.67%±0.08% after one day and increased up to 1.57%±0.03% after 14 days being consistent with literature [9]. A further uptake could not be detected. VST decreases significantly (p<0.01) from 108.0±1.1 °C to 93.4±4.5 °C after 14 days and then stagnates without further significant changes (p≥0.21) (Figure 1). Water absorption correlates significantly with the VST (r=-0.93, p<0.01) (Figure 2). TG decreased from 95.6°C after one day in air to 85.4°C after 56 days in Ringer’s solution. TG (87.2 °C) and VST (90.8±1.3 °C) of the spacer are close to the values determined for samples stored in Ringer’s solution for 56 days (Figure 3).
Conclusion:
For subsequent validation tests to gentler removal of THRs by PMMA heating, the interval of a final softening temperature reduction and water absorption after 14 days in Ringer's solution provides important information.
Acknowledgements:
Funded by the Deutsche Forschungsgemeinschaft (DFG, German Research Foundation) – SFB/TRR-298-SIIRI – Project-ID 426335750
[1]Pivec et al. 2012
[2]German Arthroplasty Registry Report 2021
[3]Reed et al. 2022
[4]W-Dahl et al. 2021
[5]Feng et al. 2022
[6]Masri et al. 2005
[7]Ghanem et al. 2017
[8]Kuehn et al. 2005
[9]Ayre et al. 2014
Figures
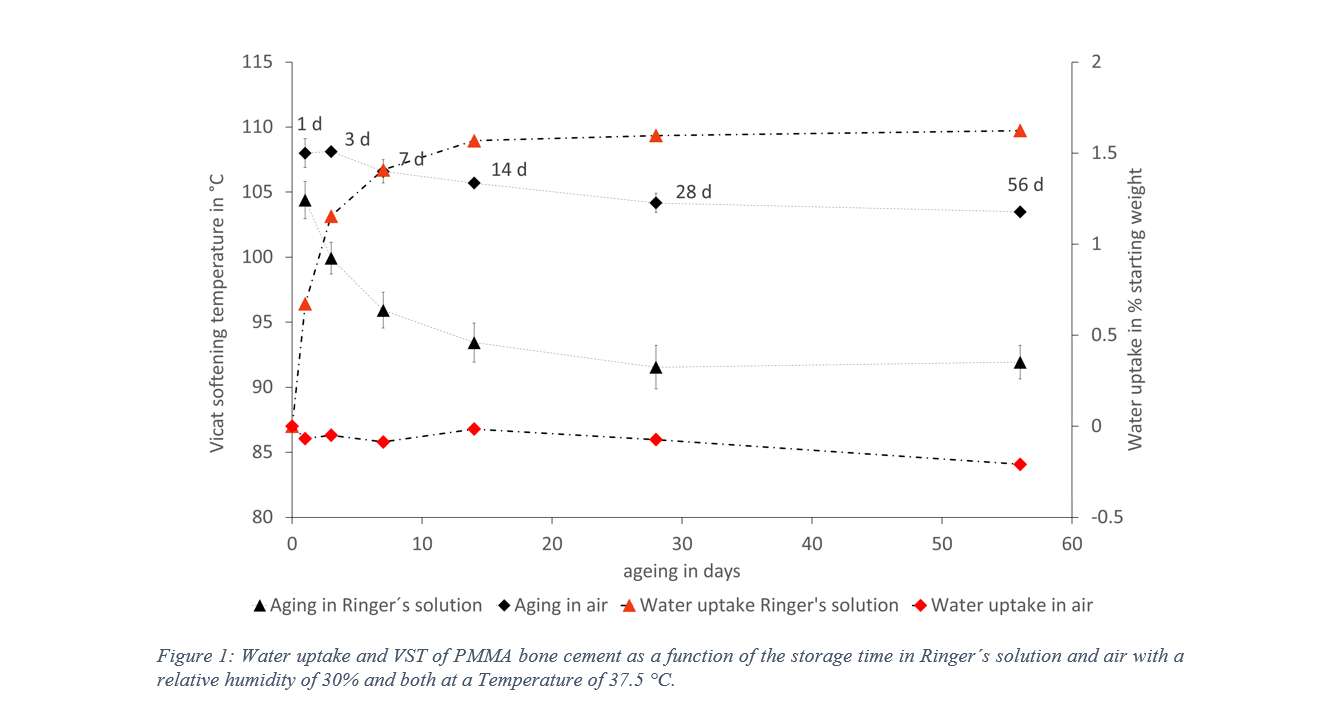
Figure 1
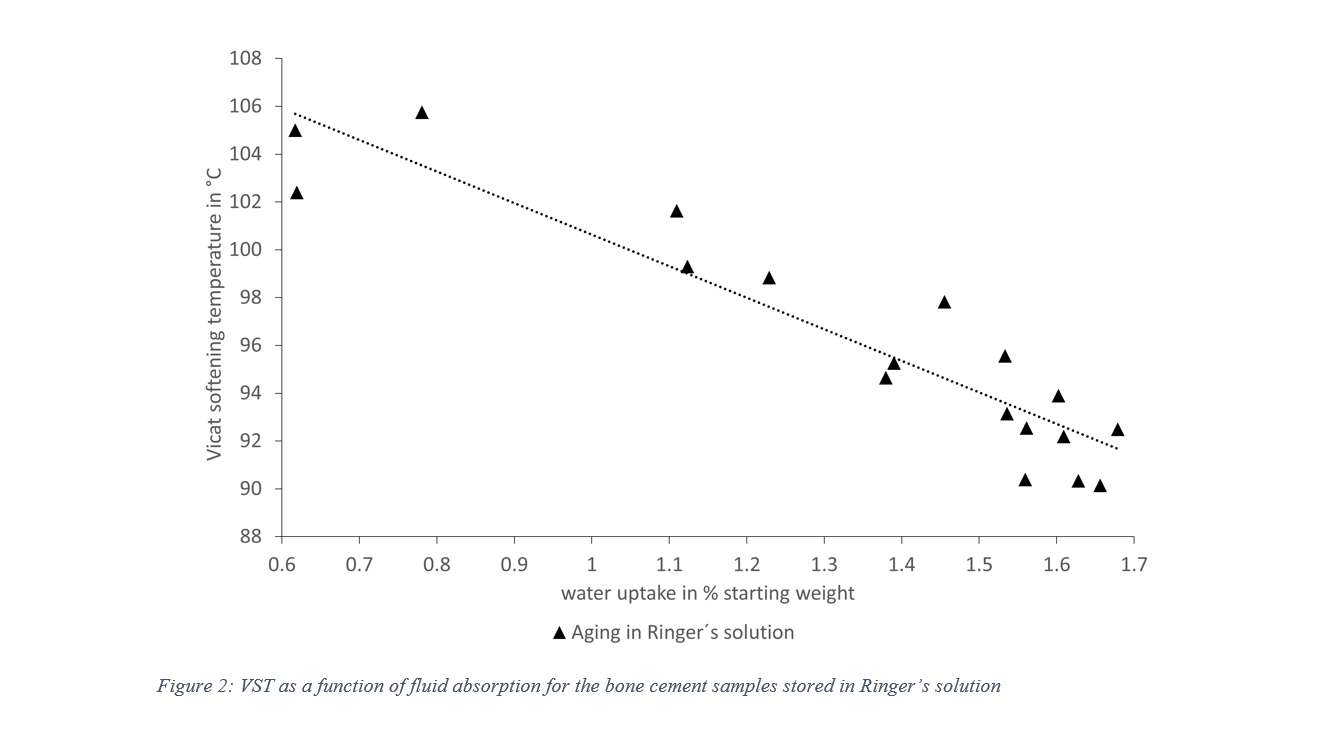
Figure 2
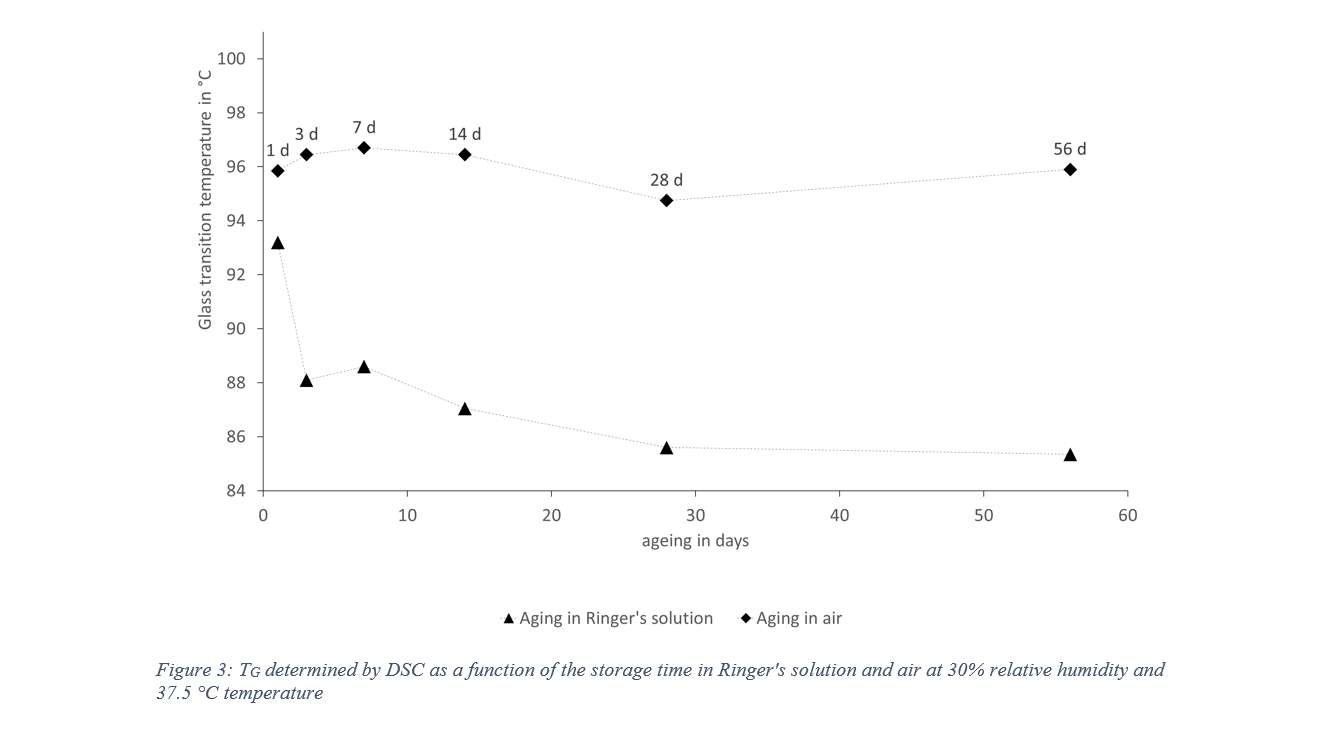
Figure 3#8623
Scapular Morphology Variation and Surgical Placement of Glenosphere Component Affects RTSA Biomechanics. a Controlled Predictive Simulation Study.
*Pavlos Silvestros - University of Victoria - Victoria, Canada
George Athwal - University of Western Ontario - London, Canada
Joshua Giles - University of Victoria - Victoria, Canada
*Email: pavlossilv@gmail.com
INTRODUCTION: Reverse Total Shoulder Arthroplasty (RTSA) has become a common method for restoring shoulder function in patients with arthropathy. Despite RTSA accounting for over 50% of shoulder replacements the biomechanical implications and functional outcomes of these operations are still to be fully understood, in part because the effects and interactions of patient bone morphology and construct placement remain unknown. Previous research has studied the effect of construct placement on the glenoid but have not controlled for morphological variation. Computational anatomy and musculoskeletal simulation methods can provide biomechanical information to help identify causal relationships across a patient population and surgical placement strategies. The aim of our study was to evaluate the effect of common glenosphere placements and scapular morphology variation on RTSA biomechanics.
METHODS: A previously developed clinical statistical shape model (SSM) generated scapular morphologies by independently modulating two modes of variation (principal components 2 and 5; part of larger dataset Nmodes=6) across four levels (±1, ±3 SD) for each mode. A baseline condition following expert orthopaedic surgeon guidelines defined a 39 mm diameter glenosphere orientated to correct for superior glenoid inclination and positioned with a 7 mm inferior overhang and 2 mm lateralisation from the inferior most point of the glenoid on each scapula. Two more sets of models with an additional +5 and +10 mm of glenosphere lateralisation for each instance were also completed. The new glenohumeral joint geometries from the digital surgeries were used to define musculoskeletal shoulder models. Muscle driven predictive simulations (OpenSim 4.3) were completed that required the models to achieve a forward reaching task in 3D space whilst minimising muscular effort and completion time. Differences between the level of lateralisation across the duration of the simulated task were inferred using Statistical Parametric Mapping through an ANOVA with a post-hoc Bonferroni correction.
RESULTS: Inter- and intra-mode morphological variation resulted in changes to the deltoid moment arms and force generating capacities which in turn influenced resultant joint loading. Resultant joint loads increased with glenosphere lateralisation from the baseline condition for each instance of morphological variation. The magnitude of the increase however was not of the same order for the two modes of variation. Mode 1 showed prolonged significant differences (p<0.05) between placements at the 55-65% and 80-90% phases of the task. Mode 2 showed smaller significant differences for shorter duration at the 84% and 80% points of the task for 5 mm against baseline and 10 mm conditions respectively.
DISCUSSION: These findings are the first to show that surgical placement decisions for RTSA glenospheres will affect resulting patient biomechanics differently depending on their morphology and how it varies compared to an average patient. Specifically, in the presented study, resultant joint loading was more sensitive to changes in inferior acromion translation (Mode 1) compared to superior acromion rotation (Mode 2). By controlling for patient morphology (using SSMs), constructs placement (using MSK modeling), and “what if?” effects on biomechanics during a task (using predictive simulations) datasets can be generated that can augment surgical planning technologies and improve patient outcomes.
Figures
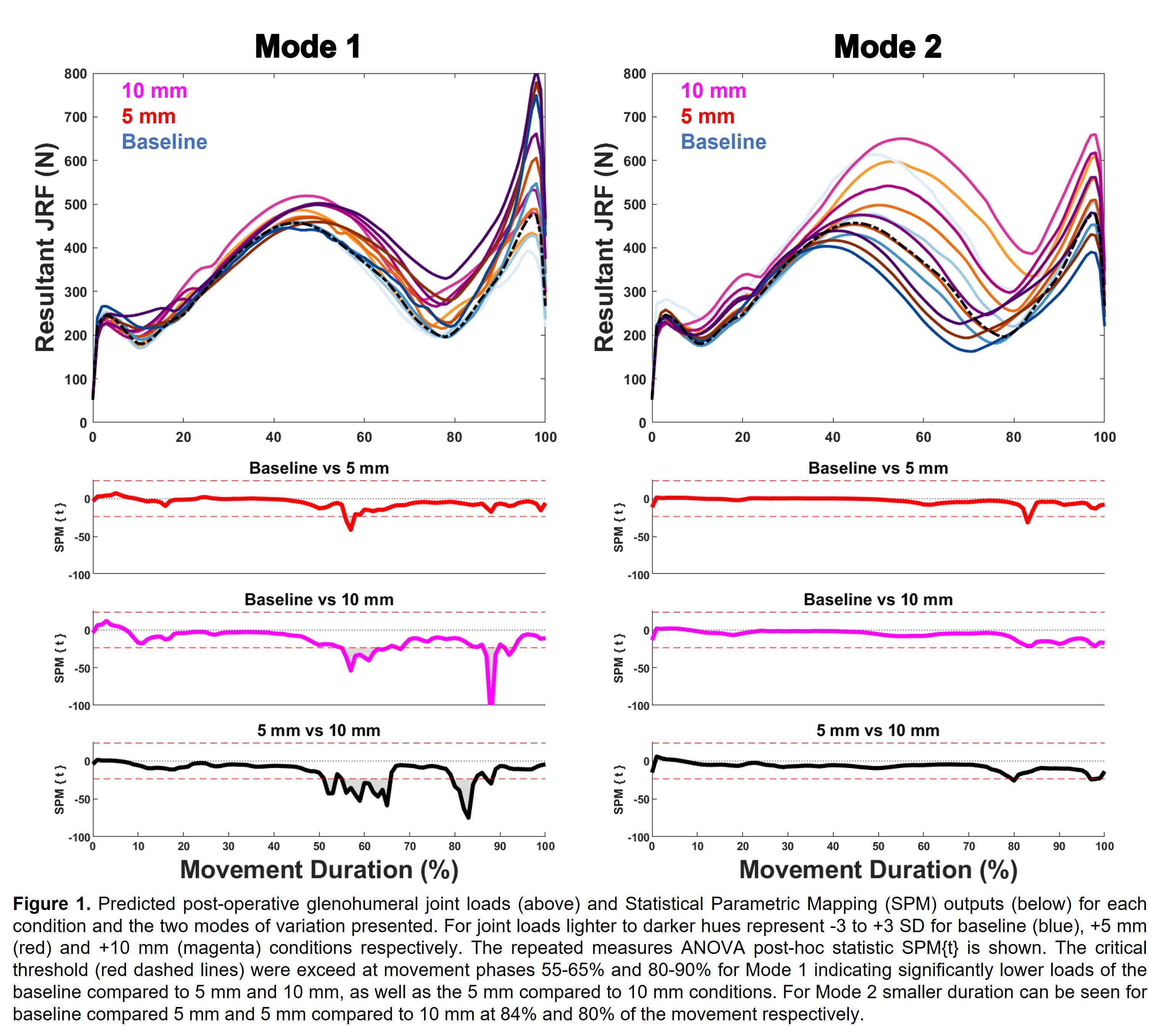
Figure 1#8226
Assessing Variability in Glenoid Version and Inclination Measurements: Implications for Total Shoulder Arthroplasty
*Estelle Liu - 360 Med Care - Sydney, Australia
Benjamin East - Newcastle Orthopaedic Research Institute - Newcastle, Australia
Joshua Twiggs - University of Sydney - Sydney, Australia
Brad Miles - 360 Knee Systems - Pymble, Australia
*Email: estelle@360med.care
Introduction:
Glenoid malalignment of the component in Total Shoulder Arthroplasty (TSA) can contribute to poor Range of Motion (ROM) and implant survivorship postoperatively. Native glenoid inclination and anteversion are commonly used references, particularly in the growing practice of 3D planning for TSA. There is a lack of consensus on how inclination and anteversion should be evaluated in 3D, however. This study sought to determine the degree of similarity between a ‘sphere fit’ and ‘plane fit’ calculation of native glenoid orientation across a case series of patients.
Methods:
A case series of 27 shoulders in 26 patients who received primary TSA for osteoarthritis was studied. Each patient received a preoperative CT scan of the unilateral shoulder. The CT scans were segmented and landmarked to produce three landmark sets: the anterior, posterior, superior and inferior glenoid rim points excluding osteophytes (the ‘rim’ measurement), five glenoid face points excluding the rim (‘face’ measurement), and a combination of the former sets to make up nine glenoid surface points (‘all points’ measurement) (Figure 1). Glenoid version and inclination were calculated from both a) a best-fit plane from each landmark set and b) from the glenoid centre to the best-fit sphere centre from each landmark set (Figure 2), for a total of 6 candidate measurements. Statistical analysis was conducted in R Studio.
Results:
Moderate to strong correlations were observed for all glenoid version measurements (r>0.89, p<0.05) and all inclination measurements (r>0.68, p<0.05). The mean absolute difference between glenoid version defined from a best-fit-plane calculated from rim points and face points was 2.3°±2.7° with 8 patients outside ±3° (29.6%). The same methods resulted in a mean absolute difference in glenoid inclination of 3.1°±2.4° with 14 patients outside ±3° (51.9%). Glenoid version measured from a best-fit-plane and best-fit-sphere defined from rim points were comparable, with a mean absolute difference of 0.9°±1.0° and 1 patient outside ±3°. The mean absolute difference between glenoid inclination measured using the same methods was 1.5°±1.3° with 4 patients outside ±3° (14.8%).
Conclusion:
All methods were able to discern between patients with higher or lower glenoid version and inclination. The largest discrepancies existed between glenoid orientations defined from the glenoid rim compared to the glenoid face, highlighting the importance of considering the entire glenoid. The best-fit-plane and best-fit-sphere models were comparable for the glenoid version measurement, but less clear for the glenoid inclination. Future research will involve correlating these measurements against intraoperative surgeon observation to determine which method best reflects intuitive surgeon evaluation in theatre.
Figures:
Figure 1: (A) Rim measurement landmarks (blue) made up of the anterior, posterior, superior and inferior glenoid rim points excluding osteophytes. (B) Glenoid measurement landmarks (blue) on the glenoid face.
Figure 2: (A) Best-fit plane (red) and (B) best-fit sphere (yellow) with the projection axes from the glenoid centre (black) used to calculate glenoid version and inclination.
Figures
Figure 1
Figure 2#8795
An in Vitro Mechanical Analysis of Glenoid Peg-Cement Interfacial Behaviour Through Neural Network-Based Segmentation and Digital Volume Correlation
Jakub Targosinski - University of Western Ontario - London, Canada
*Louis Ferreira - University of Western Ontario - London, Canada
Jonathan Kusins - The University of Western Ontario - London, Canada
Andrew Nelson - The University of Western Ontario - London, Canada
George Athwal - University of Western Ontario - London, Canada
*Email: louis.ferreira@sjhc.london.on.ca
Introduction
Aseptic loosening of the glenoid component is the largest single mode of failure in anatomic shoulder arthroplasty. The rocking horse mechanism, whereby cyclic eccentric loading of the glenoid implant rim causes progressive damage to the cement-bone interface of the implant, has been implicated as the mechanical progenitor of aseptic loosening. Digital volume correlation (DVC) used in concert with micro-computed tomography can provide more localized information about the role of failure of specific locations of interest in the glenoid implant such as the interface between glenoid implant peripheral pegs, the bone cement mantle, and the trabecular bone of the glenoid. Neural network-enhanced segmentation techniques can enable high-accuracy segmentation of distinct components of the peg-cement-implant interface, allowing for a DVC-based mechanical analysis of each component individually. This study applies a neural network-based segmentation approach in concert with DVC to delineate the behaviour of each individual component of the interface in an in vitro glenoid component peripheral peg model.
Methods
10 (n=5 for both tensile and compressive loading) bone cores were recovered from humeral osteotomies post-total shoulder arthroplasty. The bone cores were implanted with a modelled glenoid peripheral peg and the resulting constructed specimens were loaded using a radiolucent hexapod robot inside of a cone beam micro-CT scanner. Stepwise compressive and tensile displacements that emulate glenoid lift-off were applied to the peg constructs, and micro-CT images were captured of the peg-cement-bone interfaces in both an unloaded and loaded state (Fig. 1). A U-net based neural network was used to segment the components of the peg interface into distinct components. DVC-derived maximum and minimum principal strains were calculated at multiple locations of interest inside the peg-cement-bone interface for each separate component and were examined for any site-specific significant relationships (Fig. 1).
Results
There was a significant relationship between location along the axial length of the peg and magnitude of maximum and minimum principal strains within the bone in the tensile loading of the peg constructs (F(3)=97.3, p<.001). There was also a significant relationship between lateral distance from the pegs and the magnitude of maximum and minimum principal strains within the interface (F(1)=965.4, p<.001). In the compressive loading, both relationships were also present, but the magnitude of strain measured was much lower: maximum and minimum principal strains in the interface were highest closest to the root of the peg with a mean magnitude of 3.5*104µε and 4*104 µε at maximum displacement in the tensile loading, respectively (Fig. 2).
Conclusion
This in vitro study used DVC in combination with micro-CT imagery segmented using a neural network-enabled approach in order to elucidate the specific mechanical role of each element of the peg-cement-bone interface under the articulating surface of glenoid components. It was found that the highest area of bone strain was at the root of the peg; bone strains were also higher in the tensile loading mode, implicating a tensile mode of failure near the root of the peg as the likeliest area of failure for the trabecular structure supporting the glenoid component.
Figures
Figure 1
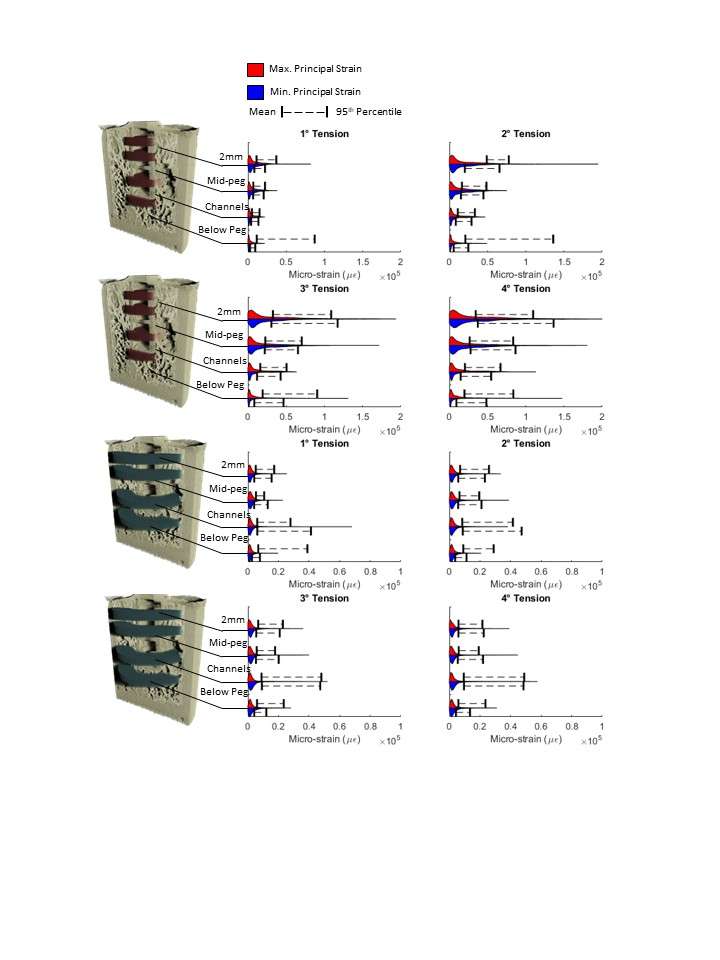
Figure 2#8221
Beyond the Glenoid: Importance of Humeral Component Orientation in Total Shoulder Arthroplasty
*Estelle Liu - 360 Med Care - Sydney, Australia
Joshua Twiggs - University of Sydney - Sydney, Australia
Brad Miles - 360 Knee Systems - Pymble, Australia
Benjamin East - Newcastle Orthopaedic Research Institute - Newcastle, Australia
*Email: estelle@360med.care
Introduction:
Glenoid component version is a critical consideration in Total Shoulder Arthroplasty (TSA) with widespread use of preoperative planning and patient-specific instrumentation to deliver the planned version. However, it is the combination of glenoid and humeral orientations that influence joint stability, muscle forces, and range of motion; not the glenoid alone. Despite this, the humeral component is commonly retroverted 30° from the forearm axis intraoperatively in all patients (previously shown to overestimate retroversion by 10°), thus orienting patients at approximately 20° retroversion to the transepicondylar axis (TEA), while the true humeral version to the TEA for each patient is unknown. This study investigates the relative variability of the humeral version to glenoid version and the resultant patient variation in combined glenohumeral version in a TSA population.
Methods:
A case series of 27 shoulders in 26 patients who received primary TSA for osteoarthritis was studied. Each patient received a preoperative CT scan extending beyond the distal humerus, which was segmented and landmarked to calculate the anatomic humeral version and anatomic glenoid version. Combined glenohumeral version was calculated as the sum of the anatomic humeral and glenoid versions. Statistical analysis was conducted in R Studio.
Results:
The mean humeral retroversion, glenoid retroversion and combined glenohumeral retroversion were 12.5°±11.2°, 11.4°±5.9° and 23.9°±12.3°, respectively (Figure 1). The variance in humeral version was significantly greater than glenoid version (p=0.004). There was a weak trend between humeral and glenoid version, where patients with higher glenoid retroversion were likely to have a less retroverted humerus, but this was not statistically significant (r=0.37, p = 0.06). In our series, 15 patients (56.5%) had a humeral retroversion outside of 20°±5°. Interpatient variation in humeral version between patients with similar glenoid version was significant: two reference patients with comparable glenoid retroversion (Patient A: 21.2°, Patient B: 20.5°) exhibited a difference in humeral retroversion and combined retroversion greater than 40° (A: 21.4°, B: -20.2° humeral; A: 42.6°, B: 0.3° combined).
Conclusion:
Our study shows that the standard intraoperative technique may cause over half of patients to experience a change greater than 5° from their preoperative humeral version due to the large population variation. Patient-specific humeral version needs to be considered during TSA to maximise internal and external rotation and achieve soft-tissue balance. The weak correlation between glenoid and humeral version suggests a compensatory mechanism to achieve balance as osteoarthritis progresses, but the absence of a strong correlation highlights the need to consider planning the orientation of the humeral component independently to the glenoid.
Figure:
Figure 1: Violin plot comparing the spread of glenoid, humerus and combined retroversion.
Figures
Figure 1#8199
Influence of Surgical Variability on Humeral Stress Shielding
*Christine Mueri - Zimmer Biomet - Winterthur, Switzerland
Adam Henderson - Zimmer Biomet - Winterthur, Switzerland
Jeffrey Bischoff - Zimmer, Inc. - Warsaw, USA
Ghislain Maquer - Zimmer Biomet - Winterthur, Switzerland
Philippe Favre - Zimmer Biomet - Winterthur, Switzerland
*Email: christine.mueri@zimmerbiomet.com
Introduction:
In silico clinical trials (ISCT) have been proposed to enrich clinical data for device performance assessment and have the potential to provide additional insights in the factors potentially influencing the surgical outcome. Stress shielding (SS) around the humeral stem is a potential complication after total shoulder arthroplasty that may lead to long term implant loosening. Clinical studies have highlighted that a large relative stem size (RSS) tended to increase bone resorption [1,2]. The aim of this study was to evaluate if surgical variability besides implant sizing may influence humeral stress shielding.
Methods:
A cohort of 34 virtual humeri was taken from an internal 3D bone database, and virtual surgery was performed with the same implant as in a prior clinical study [1] (Bio-Modular, Zimmer Biomet) (Fig. 1A-C). To study the influence of RSS and implant alignment on SS, a stem size matching the size of the humeral canal was implanted as well as one size larger, and implant orientation was additionally varied by ±1° medially/laterally. The implanted bones were then meshed, and contact definitions and boundary conditions including joint reaction and muscle loads representative of daily usage were applied (Figure 1D-F). Bone material properties were assigned based on the greyscale value of the CT image (Figure 1E). SS was assessed in the same regions of interest as in the clinical study [1] i.e. lateral (L1 (1/3 of the stem length), L2 (2/3)) and medial (M1 (1/3), M2 (2/3)) aspect of the humeral stem (Fig 2.A) by comparing the relative change in strain energy density (SED) in the intact bone and after implantation as a common metric for the potential of bone resorption or bone deposition.
Results:
For bone models that predicted severe SS, the highest reduction in SED was observed in the lateral aspect of the humeral stem (L1) followed by the proximal medial aspect (M1), which was consistent with the findings of the clinical study [1] (Fig 2). Implant sizing had a significant effect on stress shielding, with one size larger stems resulting in a higher reduction in SED in all four evaluated regions (Fig. 3A). Medial tilting of the stem also had a significant effect on SS with increased reduction in SED in all regions of interest but especially in the proximal lateral aspect L1 (Fig. 3B), whereas lateral tilting showed no significant effect compared to an aligned stem placement.
Conclusion:
This study confirmed the findings of previous studies [1,2] showing that the risk of SS increases with a larger RSS. In addition, surgical variation in terms of implant alignment was found to also influence the risk for SS, where a medial stem tilting can also result in higher SS especially in the proximal lateral aspect of the humerus. These findings highlight the benefits of ISCT to study the effects of specific surgical variabilities that would not always be possible with traditional clinical studies.
References:
[1] Nagels et al., J Shoulder Elbow Surg. 2003;12:35-9
[2] Raiss et al., J Shoulder Elb Surg 2019;28:715–23
Figures
Figure 1
Figure 2
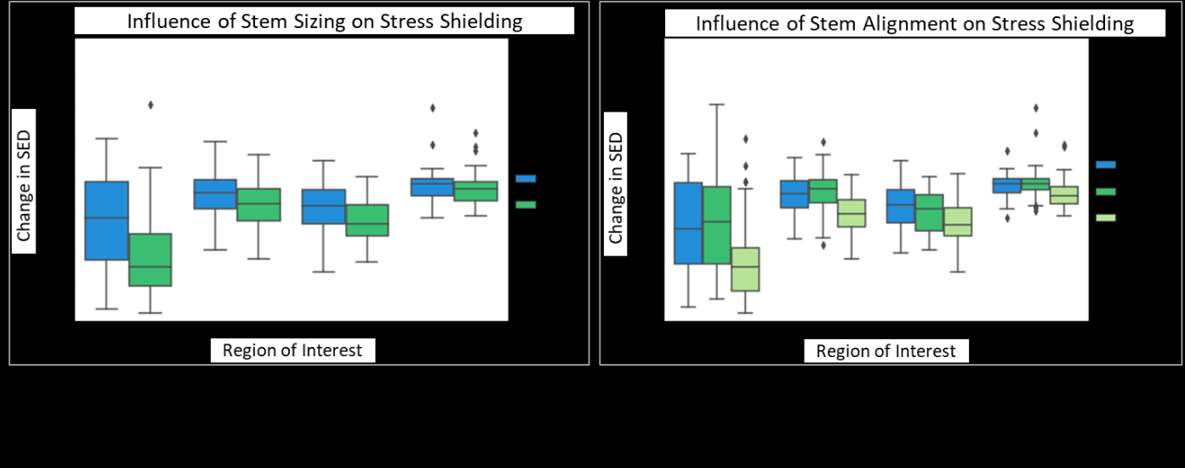
Figure 3#8631
The Effect of Neck Shaft Angle on Stemless Reverse Humeral Component Fixation
*David Cunningham - University of Western Ontario - London, Canada
Gregory Spangenberg - University of Western Ontario - London, Canada
G. Daniel G. Langohr - The University of Western Ontario - London, Canada
George Athwal - University of Western Ontario - London, Canada
James Johnson - SJHC - London, Canada
*Email: dcunni9@uwo.ca
Purpose:
Stemless humeral implants have gained popularity as an alternative to traditional stemmed devices; however, despite their increased use, there is a lack of literature available on the fixation of reversed stemless humeral components. One important variable is neck-shaft angle (NSA) which has previously been shown to have an effect on range of motion [1] and risk of scapular notching [2]. Accordingly, this finite element analysis investigated the effect of NSA in the time-zero fixation of stemless reversed humeral components.
Method:
Eight patient-specific (sex: male, age: 70 ± 21 years, mass: 70 ± 9 kg, height: 177 ± 5 cm) three-dimensional models of the proximal humerus were created from CT data using Mimics (Materialise, Leuven, Belgium). A generic implant model, synthesized from commercially available stemless reversed humeral implants was created (Figure 1). A matrix transformation was utilized to incrementally vary neck shaft angle about an axis at the lateral apex of the osteotomy on the 135° resection plane to permit investigation of neck shaft angles of 130°, 135°, 140°, 145°, and 150°. Four different loading scenarios, built from previously published in-vivo telemetrized implant data [3] and corrected for the individual body weight of each subject were assessed in Abaqus CAE (Dassault Systèmes Corp). The four loading states identified were chosen to represent an array of activities a patient may experience postoperatively while still adhering to standard postoperative instruction. Bone—implant distractive micromotion was employed as the outcome variable.
Results:
The greatest implant-bone distraction was observed with a neck shaft angle of 130°. In all implant loading scenarios, significantly lower magnitudes of micromotion were elicited when the neck shaft angle was increased (P = 0.0001). An average reduction of 17% in bone-implant distraction was observed for every 5° increase in neck shaft angle.
Conclusion:
Implantation neck shaft angle is parameter that appears to affect the stability of stemless reverse humeral components at the time of implantation. At lower, more varus, neck shaft angles, increased bone-implant distractions were observed during simulated activities of daily living. Therefore, humeral head osteotomies performed at higher neck shaft angles may improve the initial fixation stability of stemless humeral components.
Figure 1: A generic stemless reversed humeral implant model synthesized from commercially available stemless humeral implant designs.
Figure 2: Percent of Initial Contact Area for the 5 different neck shaft angles (NSA) and 4 loading scenarios.
[1] B. S. Werner, J. Chaoui, and G. Walch, “The influence of humeral neck shaft angle and glenoid lateralization on range of motion in reverse shoulder arthroplasty,” J. Shoulder Elb. Surg., vol. 26, no. 10, pp. 1726–1731, 2017.
[2] B. K. Jeon et al., “Combined effect of change in humeral neck-shaft angle and retroversion on shoulder range of motion in reverse total shoulder arthroplasty - A simulation study,” Clin. Biomech., vol. 31, pp. 12–19, 2016.
[3] P. Damm and J. Dymke, “Orthoload Database,” Julius Wolff Institute, 2021. [Online]. Available: https://orthoload.com/database/. [Accessed: 26-Jul-2021].
Figures
Figure 1
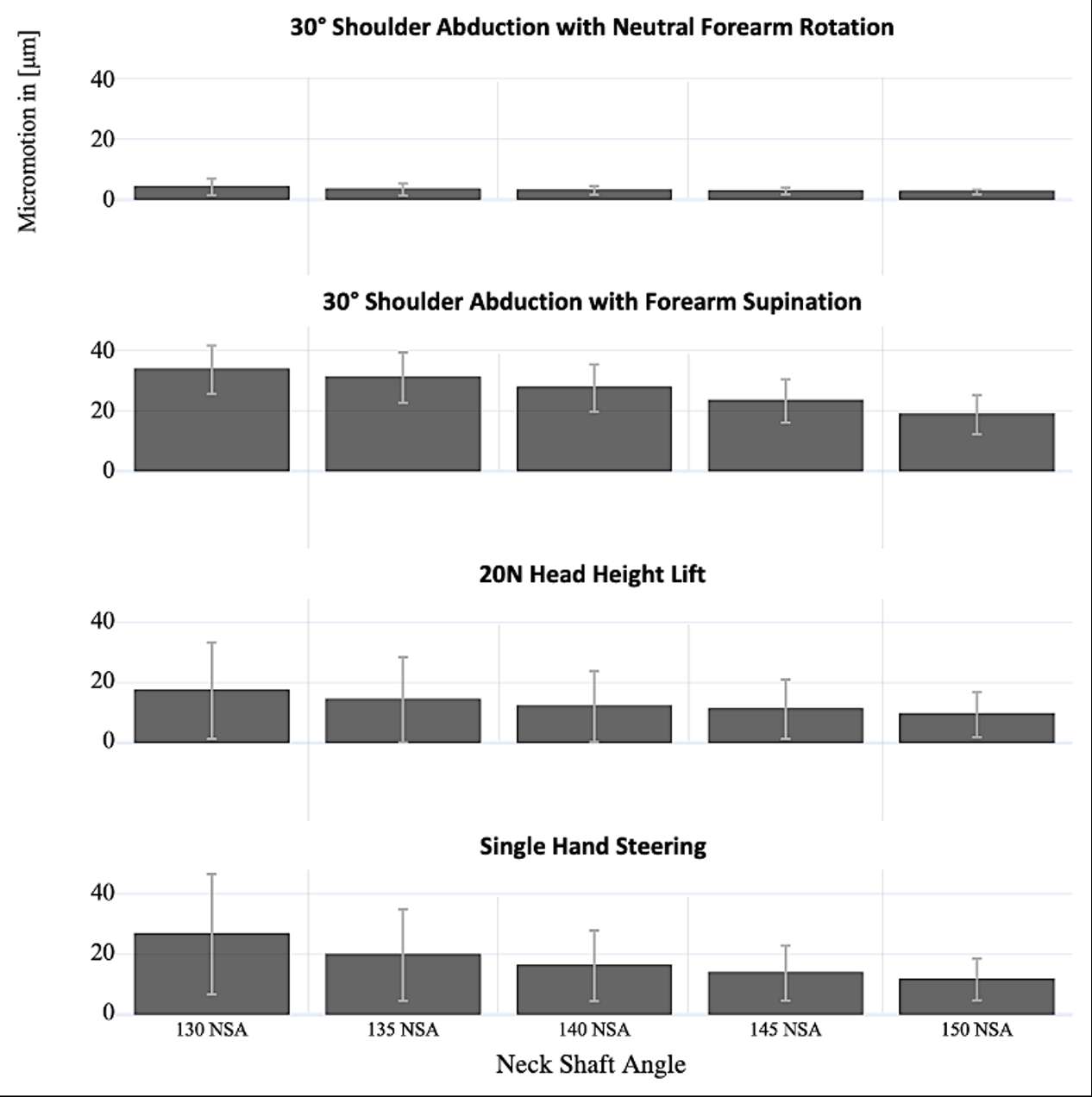
Figure 2#8850
Navigated restricted medial pivot KA
*Morteza Meftah - NYU Hospital for Joint Diseases - New York, USA
*Email: morteza.meftah@nyulangone.org
#8851
Robotic TKA: 3D planning prior to cuts
*Martin Roche - Holy Cross Hospital - Fort Lauderdale, USA
*Email: martin@mroche.com
#8852
Restricted kinematic: Start with tibia
*Philip Winnock De Grave - AZ Delta - Roeselare, Belgium
*Email: philip.winnockdegrave@azdelta.be
#8853
Mitigating the post-op TKA tsunami
*Andrew Wickline
*Email: awickline23@gmail.com
#8854
Optimization of TKA outcomes with AI
*Stefano Bini - University of California San Francisco - San Francisco, USA
*Email: Stefano.Bini@ucsf.edu
#8437
Retrieval Analysis of Femoral and Tibial Metal Augments in Total Knee Arthroplasty
*Hannah Spece - Drexel Implant Research Center - Philadelphia, USA
Caroline Ries - Drexel Universtiy - Philadelphia, USA
Paul DeSantis - Drexel University - Philadelphia, United States of America
Greg Klein - Hartzband Center for Hip and Knee Replacement - Hackensack, USA
Nicolas Piuzzi - Cleveland Clinic - Cleveland, USA
Matthew Kraay - Case Western Reserve - Cleveland, USA
Clare Rimnac - Case Western Reserve Hospital - Cleveland, USA
Steven Kurtz - Drexel University - Philadelphia, USA
*Email: hannahspece@gmail.com
Introduction: Metal augments are often used alongside revision total knee arthroplasty (TKA) components to help stabilize the joint and compensate for bone loss. Off-the-shelf components range in size, shape, and fixation method, and the choice of augment depends on the bone defect being addressed. Despite their widespread use, it is unclear which augment characteristics are most often used in TKA procedures with bone loss. The purpose of this retrieval study was to characterize the augments present in a large cohort of retrieved TKA implants. In addition, we assess the reasons for revision and in vivo damage of the augmented devices.
Methods: 166 augmented femoral components and 72 augmented tibial trays were retrieved during TKA revision surgery as part of a multi-institutional implant retrieval program. The cohort represented 202 patients and included designs from 12 manufacturers. Operative notes were reviewed to determine patient and procedure details, and augment characteristics were recorded. A categorization scheme was created, and the augments were classified according to shape as: full/half block and wedge for tibial trays and distal/posterior block and L-shaped for femoral components (Figure 1).
A subcohort of devices implanted >10 years was selected for further investigation of the augment-implant interface. In order to remove bone cement and allow for disassembly, the subcohort components were boiled in toluene for 6 hours and sonicated in water. Augment fixation screws were then removed if present. The exposed interface surfaces were assessed visually and using digital microscopy. One particularly damaged component also underwent X-ray fluorescence (XRF) for characterizing metal debris.
Results: The cohort had a mean implantation time of 3.9 ± 4.2 y (range: 0 to 22.4y), and mean patient BMI of 33.5 ± 8.4 kg/m2. Thecomponents were revised most often for infection (47%, n = 94/202), loosening (25%, n = 51/202), and instability (11%, n = 22/202). 112 were implanted during revision TKA and 43 were implanted during a primary procedure (47 not reported). For the femoral components, 130 included distal blocks, 106 had posterior blocks, 1 had an anterior wedge, 5 had half L-shaped, and 1 had full L-shaped. For the tibial components, 21 included full block augments, 45 had half blocks, and 4 had wedges. The augments ranged in size from 4 to 20 mm for femoral and 5 to 20 for tibial. Multiple augments were sometimes stacked to increase their height.
For the long-term subcohort, the augment-implant interface surfaces showed minimal signs of damage or corrosion. This may be attributable to the thin layer of bone cement often present at the interface, even for screw-fixated augments. In one case, metal staining was observed in the cement of a device that was revised at 14 years for loosening and extensive metallosis (Figure 2). The metal was identified as Ti6Al4V, matching the augment metal. The device itself was CoCr.
Conclusion: This cohort of retrieved augmented TKA components suggests these interfaces may generally contribute little metal debris or corrosion. However, the case of metallosis is concerning and warrants further study.
Figures

Figure 1
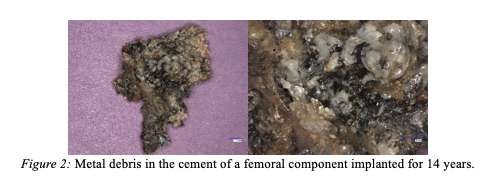
Figure 2#8619
Bio-Inert to Bioactive: Improving the Biological Response of Ti-6Al-4V via Silicon Nitride and Aqueous PEKK Solutions
*Jackson Hendry - SINTX Technologies - Salt Lake City, USA
Tony Decarmine - Oxford Performance Materials - South Windsor, USA
Douglas Hoxworth - SINTX Technologies - Salt Lake City, USA
James Porteus - Oxford Performance Materials - South Windsor, USA
Sonny Bal - Sintx - USA
Thomas Webster - 3School of Health Sciences and Biomedical Engineering - Tianjin, China
Ryan Bock - SINTX Technologies - Salt Lake City, USA
*Email: jhendry@sintx.com
INTRODUCTION: Many current implantable devices leverage titanium alloy's (Ti-alloy) mechanical properties to include intricate porous structures that facilitate bone ingrowth. However, the increased surface area associated with this also increases the risk of infection. Alternatively, silicon nitride (Si3N4) is a ceramic material that both inhibits biofilm formation and exhibits improved osteogenic properties relative to Ti-alloy [1]. While monolithic Si3N4 is an approved material for use in commercial spinal fusion devices, its ceramic properties present challenges for producing complex geometries and applications where the device will undergo significant tensile loading. Using a high-strength polyetherketoneketone (PEKK) matrix to bond Si3N4 particles to a Ti-alloy implant surface combines the mechanical properties of titanium with the antibacterial and osteogenic performance of Si3N4. Aqueous PEKK solution (OXPEKK-SC, OPM) functions as a suspension medium, allowing dispersed ß-Si3N4 particles to uniformly cover the surface of intricate titanium implants. Thin, composite Si3N4-PEKK films on titanium implants could improve titanium implants’ functionality by reducing the incidence of post-operative implant infections while simultaneously enhancing osseointegration.
METHODS: Si3N4-PEKK composite coatings of high- and low-concentration adhered to Ti6Al4V ELI (Grade 23 ASTM F136) tiles (?15mm x 1mm thick) by dip coating them into submicron ß-Si3N4 particles suspended in an aqueous PEKK solution (OXPEKK®-SC, Oxford Performance Materials). Dried composite dip-layers were heated to peri-melting temperatures to form contiguous composite films (Fig.1.). Cytotoxicity was assessed on eluants of disc samples in accordance with ISO 10993-5 using fibroblasts (ATCC 3T3). Surface energies were evaluated using the Owen-Wendt model with measured contact angles for deionized water, glycerol, and dichloromethane. Protein adsorption for mucin, casein, lubricin, fibronectin, and vitronectin was assessed using ELISA assays. In vitro osteoblast (human fetal osteoblasts, ATCC CRL-11372) adhesion (4 hours) and mineralization (7, 14, and 21 days) were assessed. Bacteria CFUs were evaluated at 24 and 48 hours in vitro using five strains: MRSA (ATCC 43300), S. aureus (ATCC 14222), E. faecalis (ATCC 19433), S. epidermidis (ATCC® 14990), and P. aeruginosa (ATCC 10145). Coupons treated with the composite coating were characterized by visible light microscopy, scanning electron microscopy, and confocal laser scanning microscopy.
RESULTS: Compounding ß-Si3N4 particles into Si3N4-PEKK coatings significantly increased the surface energy of PEKK. Increased surface energy promoted the adsorption of critical proteins that inhibit biofilm-forming bacteria strains while simultaneously stimulating bone cell functions. Si3N4-PEKK coatings exhibited superior osteoblast adhesion and calcium deposition over Ti6Al4V benchmark samples (p<0.01, three replicates of n=3) (Fig. 2.). Si3N4-PEKK showed a >1-log10 colony reduction of multiple clinically relevant biofilm-forming bacteria strains after 48 hours of exposure (Fig.3.). The composite Si3N4-PEKK samples and benchmark base materials (Si3N4 and PEKK) each exhibited a cytotoxicity score of zero. Thin composite Si3N4-PEEK coatings demonstrate the successful merger of biomaterial technologies and offer a potential benefit of coating existing implantable devices using a non-line-of-sight method to improve their performance in cases where infection risk is elevated.
Figures
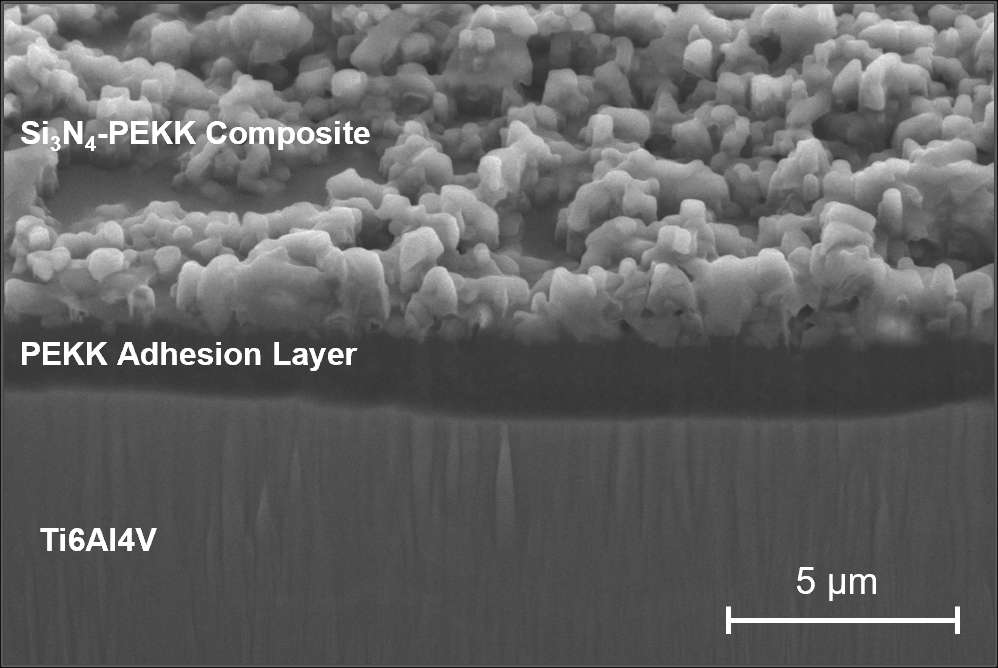
Figure 1
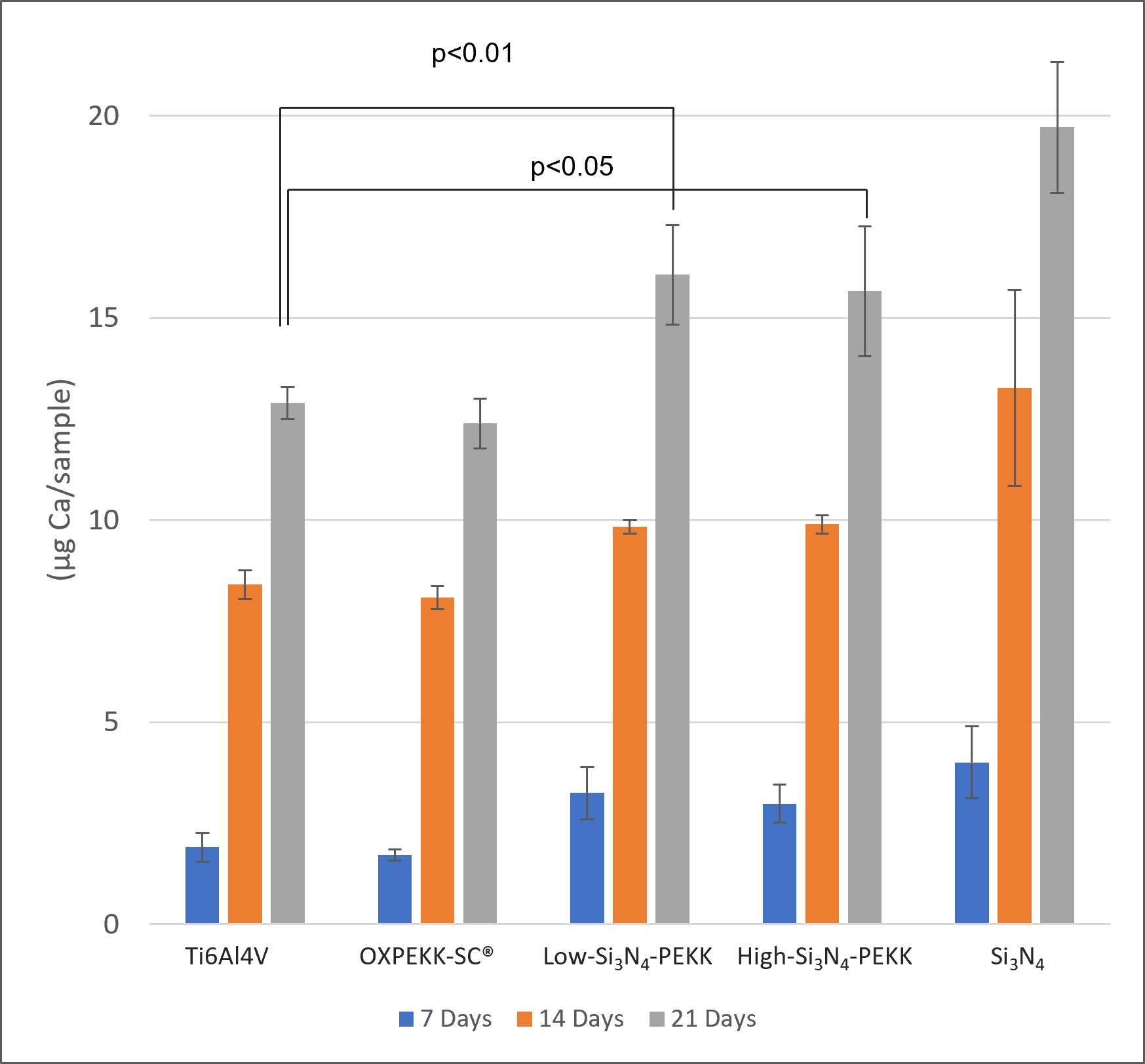
Figure 2
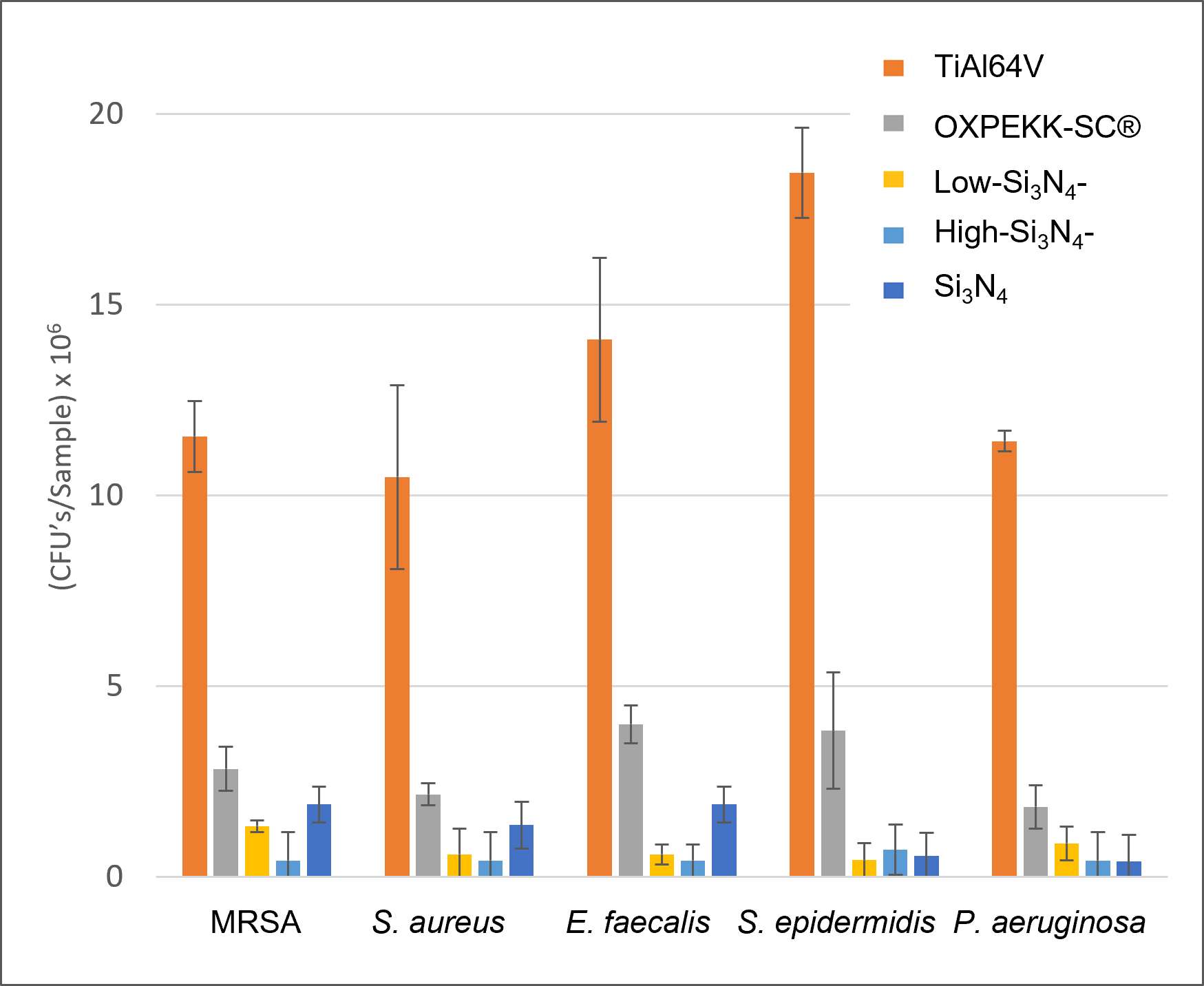
Figure 3#8346
Excellent Results of Large-Diameter Ceramic-on-Ceramic Bearing in Total Hip Arthroplasty at Minimum Ten Years Follow-Up
*Gautier Beckers - Hôpital Maisonneuve Rosemont - Montréal, Canada
Martin Lavigne - Maisonneuve-Rosemont Hospital - Montreal, Canada
Vincent Masse - Maisonneuve-Rosemont Hospital - Montreal, Canada
Mina Wahba Morcos - Hôpital Maisonneuve-Rosemont - Montréal, Canada
Pascal-Andre Vendittoli - Maisonneuve-Rosemont Hospital, Montreal University - Montreal, Canada
*Email: gautier.beckers@gmail.com
Introduction:
Large-diameter head (LDH) total hip arthroplasty (THA) increases hip range of movement and stability while avoiding component impingement. Combined with ceramic-on-ceramic (CoC) bearings wear resistance, it may be an ideal implant for young and active patients. This study reports the minimum 10-year results of the first 276 LDH CoC THAs implanted at our institution We report the reoperation/revision rate, different patient-reported outcome measures (PROMS), the whole blood metallic ions, and the radiographic evaluation.
Methods:
The 276 THAs were performed with a posterior approach using the Maxera cup and Delta ceramic LDH (Zimmer Biomet). The patients age at surgery was 53.8 (10.3 ; 16 – 73). The women/men ratio was 1.6. 264 (96%) hips were reviewed at a mean of 6 years (min 4 – max 7)and 230 (83%) hips at a mean of 10 years (min 10 – max 12) postoperatively. Nine deaths occurred during the follow-up. Adverse events, reoperation, and revision and their specific causes were recorded. UCLA activity score, WOMAC score, Forgotten Joint Score (FJS), patients’ satisfaction and joint perception question were evaluated at medium- and long-term follow-ups. Reoperations and implant revisions were recorded. Whole blood titanium (Ti), chromium (Cr) and cobalt (Co) levels were measured with high-resolution ICPMS at the last follow-up.Continuous variables are presented by the mean (standard deviation; minimum–maximum) and were compared with the Wilcoxon Signed Rank test. Categorial data were compared with the Chi-square test.
Results:
At the last follow-up, there were three revisions (1.3%): one early revision for acetabular cup insufficient primary fixation, one traumatic peri-prosthetic acetabular fracture and one for probable deep chronic infection (severe osteolysis without identified germ). There were five re-operations (2.1%): one was for a suspicion of acute prosthetic joint infection, one for a sciatic neuropathy which required a femoral-shortening osteotomy, and three for a traumatic periprosthetic femoral fracture. No hip dislocation was reported. Mean UCLA activity score, WOMAC score, and FJS significantly UCLA were 5.5 (1.8; 0 - 9), 90.9 (12.7; 42.7 – 100.0), and 80.4 (20.5; 18.8 – 100) respectively. There was a statistically significant decrease in these PROMS between the midterm and last follow-up evaluation (P<0.05), but the difference was inferior to the minimally important difference. Most patients (72.7%) perceived their THA as natural or artificial without limitation, and 100% were either strongly satisfied or satisfied. Mean Co, Cr and Ti ion levels were 0.27 (0.15; 0.1 – 1.1), 0.32 (0.2; 0.2 – 2.2), and 2.37 (1.0; 1.1 – 5.6), respectively. No progressive radiolucent line, osteolysis or implant loosening was observed at radiographic evaluation.
Conclusion
LDH CoC THAs have demonstrated a very low revision rate in a young and active group of patients, and no dislocation was observed. Patients' functional outcomes, satisfaction and joint perception were excellent. The systemic Ti levels were low and related to the uneventful and unavoidable passive corrosion of implant surfaces and does not reveal any indirect signs of trunnionosis, The tested LDH CoC THA provides long-term implant survivorship with unrestricted activity while avoiding implant impingement, liner fracture at insertion, and hip instability.
#8633
How Well Do Oxinium Femoral Heads Perform Compared to Ceramic and CoCrMo? - a Retrieval Perspective
*Carlo Maria Canossi - University of Illinois at Chicago - Bologna, Italy
Deborah Hall - Rush University Medical Center - Chicago, USA
Bianca Romay - Rush University Medical Center - Chicago, USA
Jennifer Wright - Rush University Medical Center - Chicago, USA
Stephanie McCarthy - Rush University Medical Center - Chicago, USA
Michael Godoy - Rush University Medical Center - Chicago, USA
Robin Pourzal
*Email: carlomaria.canossi@mail.polimi.it
Introduction: Bearing surface wear and fretting corrosion at modular taper junctions are major causes of failure in total hip replacements (THR) with CoCrMo femoral heads and polyethylene (PE) liners. The corrosion issue can be mitigated with the use of ceramic heads. A newer alternative are Oxinium heads that consist of Zirconium with 2.5% Niobium, and undergo a heat-treatment to obtain a 5μm-thick outer zirconia layer. Oxinium combines the abrasion resistance and low friction of a ceramic with the workability and toughness of a metal. Most available data are based on in vitro tests while data on the in vivo performance is limited. Objective: to evaluate surgically retrieved Oxinium THRs with respect to its in vivo wear and corrosion behavior.
Methods: We studied 27 Oxinium heads with an average time in situ of 22.4 months, and roughly time matched groups of 23 ceramic and 26 CoCrMo heads for reference. All heads were coupled with a PE liner. An optical coordinate-measuring-machine (CMM) was used to analyze the head bearing surface, head taper and the PE liner. Selected areas of the heads were analyzed in a scanning electron microscope (SEM). The PE liner damage was scored from 1-4 under a light-microscope based on the presence and severity of grooves/scratches, pitting/embedded debris, and polishing for a total possible score of 12.
Results: 3D-reconstruction of the heads based on CMM data showed for all groups that the original shape was ellipsoidal with the Oxinium heads exhibiting a higher shape deviation compared to ceramic and metal (Stdev = 0.0016, 0.00043 and 0.00077, respectively). The average angular mismatch, defined as the difference between head and the stem taper angles, for the Oxinium, ceramic and metal groups were 0.11º, 0.09 º and -0.04º. Thus, Oxinium heads had proximal engagement which also correlated with proximal wear bands in 3 cases. Due to the short implantations times, extensive volume loss was not observed in most heads, tapers and liners across all groups. Two Oxinium heads exhibited prominent areas where the outer zirconia layer was removed [Fig. 1], resulting in wear volumes of 8.9mm3 and 0.39mm3. Most Oxinium heads exhibited pits [Fig. 2], that appeared to originate from the substrate. Oxinium head taper damage was minimal compared to CoCrMo. Too few PE liners could be scanned due to surgically induced damage from revision to conduct a meaningful comparison of volumetric wear. The PE damage score was not different between the three femoral head materials. However, a trend indicated that less polishing wear occurred on liners coupled with Oxinium.
Conclusion: Oxinium heads seem to perform better than metal heads with respect to corrosion but appear less favorable with respect to the bearing surface wear performance compared to both metals and ceramics. Inconsistencies on the substrate surface resulted in weak links that may lead to crack initiation within the zirconia layer leading to removal of the protective layer and exposing the vulnerable substrate. The performance of Oxinium heads has to be further monitored as retrievals with longer implantation times become available.
Figures
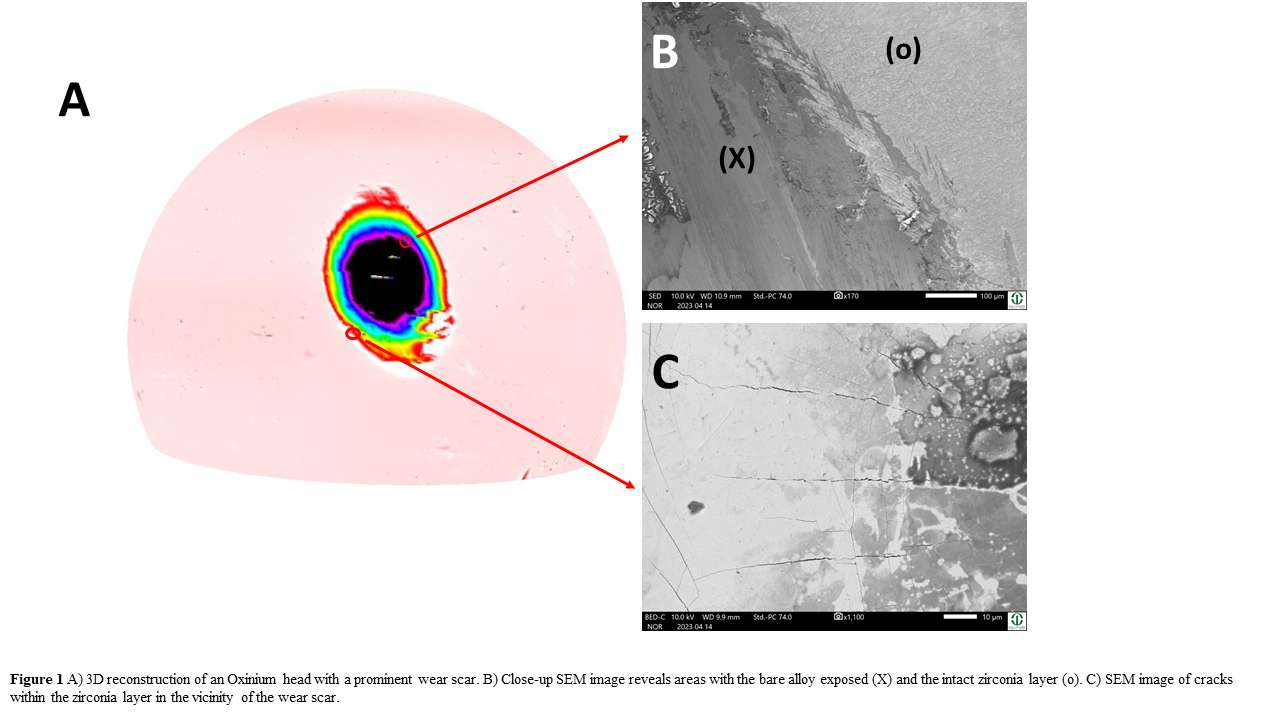
Figure 1
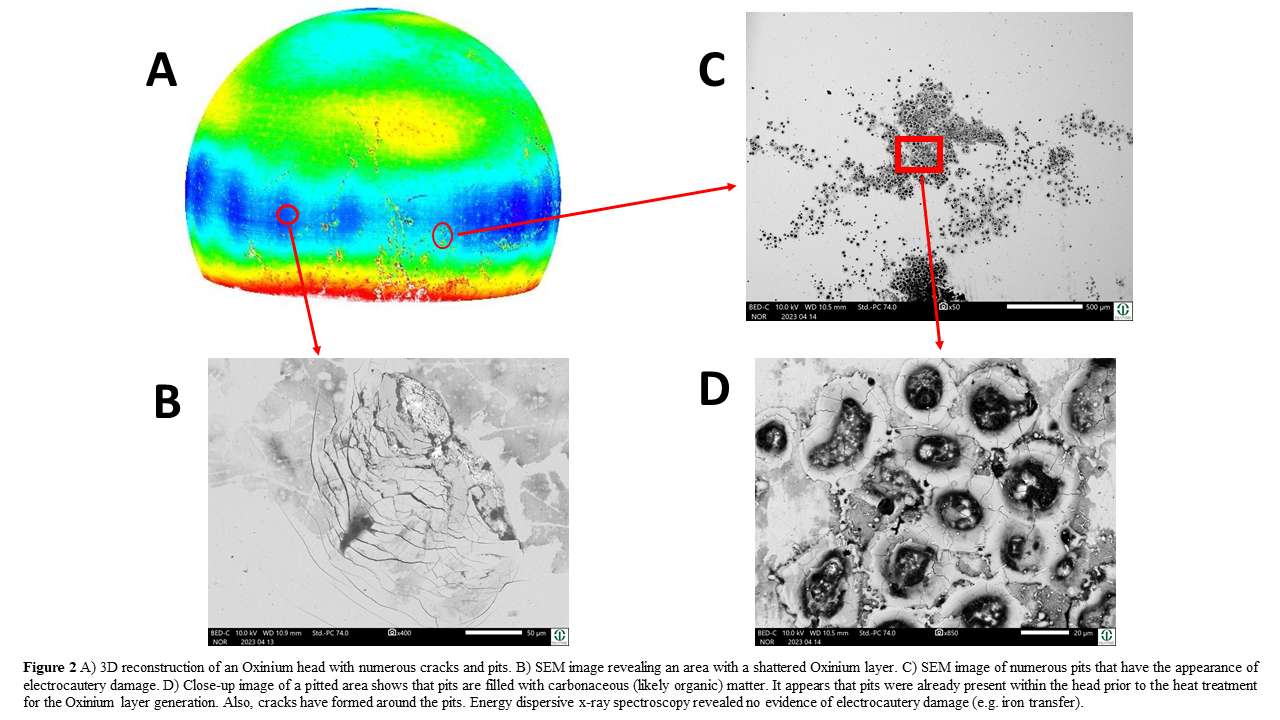
Figure 2#8628
Synovial Fluid May Affect Ti-6Al-4V and CoCrMo Ion Release
*Madison Brown - University of Tennessee Health Science Center - Memphis, USA
Michael Kurtz - Clemson University - Charleston, USA
Lisa Phan - University of Tennessee Health Science Center - Memphis, USA
Hwaran Lee - Clemson University
Charley Goodwin - Clemson University - Charleston, USA
Lilliana Taylor - Clemson University - Charleston, USA
Jeremy Gilbert - Clemson University - Charleston, USA
William Mihalko - University of Tennessee - Memphis, USA
*Email: mbrow275@uthsc.edu
Introduction: Total knee arthroplasty (TKA) is now one of the most performed elective surgeries with estimates predicting 1.26 million annual TKAs by 2030. However, the satisfaction rate may be as low as 80% and tens of thousands of patients are subjected to revision surgery. TKA systems consist of a Ti-6Al-4V baseplate and CoCrMo femoral component. Patient biology may initiate corrosion at the device interface in vivo, facilitating auto-catalytic behavior where initial corrosion promotes further corrosion. In this study, we evaluate the corrosion potential of synovial fluid (SF) post TKA from a necropsy retrieval program. We hypothesizethat the unique biology of each cadaver may be responsible for inducing a varying level of ion release during in vitro linear polarization testing.
Methods: Synovial fluid was extracted from 14 cadavers post TKA. In a three-electrode system, Ti-6Al-4V (ASTM F136) or CoCrMo (ASTM F1537) open circuit potential was recorded for 65 min using SF as the electrolyte solution. Platinum wire and Ag/AgCl were used as counter and reference electrodes respectively. Next, the working electrode was linearly polarized from -1 to 1 V. Inductively coupled plasma mass spectroscopy (ICP-MS) was used to quantify ion content following sample digestion. Data were normalized with respect to solution volume and electrode surface area.
Results: Ion analysis of SF shows a linear correlation between the release of the following ion pairs: Co and Cr; Ti and V. The highest recorded ion content for Co and Cr was 2054 and 772 μg/cm2 respectively. For Ti and V, 121 and 5.5 μg/cm2 were the most recorded in an individual patient’s necropsy sample. K-means clustering analysis of ion release reveals low (cluster 1), medium (cluster 2), and high (cluster 3) ion content for both CoCrMo (Figure 1) and Ti-6Al-4V (Figure 2) ion pairs. The high ion group was significantly higher than the medium ion group and the medium group significantly higher than the low group (both p < 0.001). EIS results (not shown) show a transition from Randles-like phase angle behavior to a coated model as CoCrMo ion release increases.
Conclusion: This study supports the hypothesis that the corrosion potential of SF may vary by patient. Clustering reveals three linearly increasing ion content groups with levels higher than physiological levels. Linearly polarizing CoCrMo to 1 V exceeds the alloy’s approximately 0.3 V breakdown potential and likely explains the 10-fold increase in ion content when compared with samples exposed to a Ti-6Al-4V working electrode. A coated response in the impedance of the high ion-content synovial fluid samples exposed to CoCrMo may be indicative of the increased working electrode surface area after corrosion. Future work will assess the corrosion potential of patient synovial fluid pre-arthroplasty to determine whether corrosion properties can predict revision or low patient satisfaction.
#8535
Electrosurgery Induced Damage to Ti-6Al-4V Implant Alloy Surfaces
Mohsen Karshenas - Clemson University - Charleston, United States of America
*Jeremy Gilbert - Clemson University - Charleston, USA
*Email: jlgilbe@clemson.edu
Introduction: Electrocautery techniques are utilized during orthopedic surgeries to cut and cauterize tissue. During implantation or revision surgeries, the electrocautery blade may induce an arc down on the metal implant surface causing multiple high temperature events including oxidation, residual stress development, cracking, melting, metal vaporization, molten particle formation and others [1]. Such arc damage can affect the wear behavior, corrosion resistance and fatigue crack initiation resistance of the alloy. When cautery damage is present in high stress regions it may result in early fatigue failure as has been reported. Electrocautery damage on the implants is not yet fully understood and the extent of surface microstructure alteration has not been studied. In this study, we explored how electrocautery can alter the Ti-6Al-4V alloy microstructure and composition and investigated the range of damage modes present. We hypothesize that arcing between stainless steel electrode blade and the Ti-6Al-4V implant alloy surface can change the normal surface microstructural characteristics of this alloy.
Methods: Polished Ti-6Al-4V samples were used to induce electrocautery damage in monopolar cutting mode and output power range from 40-150 Watts in different time intervals (1s to 3s) with a CONMED System 2450 electrosurgical generator (Utica, NY). Digital optical microscopy (DOM), backscattered electron (BSE), and scanning electron microscopy (SEM) of the alloy surface were performed. Then an SEM/EDS analysis was performed on damaged areas. Characterization and categorization of the types and extent of damage were made.
Results: The initial characteristic damage in monopolar cutting mode, 150 Watts output power in 2 seconds observed on the Ti-6Al-4V surface (Figure 1). In the center zone of damage, the cracks can be clearly observed (Figure 2). The SEM/EDS analysis showed transferred iron ions, the chemical segregated elements, the oxidized reacted layers, particle generation, plasma etching of the microstructure and intermixing of alloying elements from both the Ti-6Al-4V and the 316L SS blade caused by the electrocautery spark (Figure 3). The results indicated that as both the blade tip and the metal surface are susceptible to metal melting and evaporation, it can cause a mixture of iron and Ti-6Al-4V elements, leading to the formation of new alloys. The middle area or the heat-affected zone (HAZ) has been affected by the heat of the plasma of EC (Figure 1A). This causes surface discoloration, as seen in the rainbow pattern with rippled regions. The outer zone of damage is base metal (Figure 1C).
Conclusion: The induced electrocautery damage during orthopedic surgeries can alter the surface condition of the Ti-6Al-4V implant in multiple ways that may impact biocompatibility, corrosion resistance and wear and fatigue behavior. Plasma arcing can be seen as discolored patterns on the surface. There are oxide films, local melting, and cracks as well as distributed new particles. The chemical segregated batches of fundamental elements of the implant alloy on the surface supported our hypothesis showing that iatrogenic damage caused by electrocautery during surgeries has consequences that are not yet well understood.
References: 1. Gilbert et al, J Arthroplasty, 2017. 2. Zobel et al.
Acknowledgements: Wyss Endowment
Figures
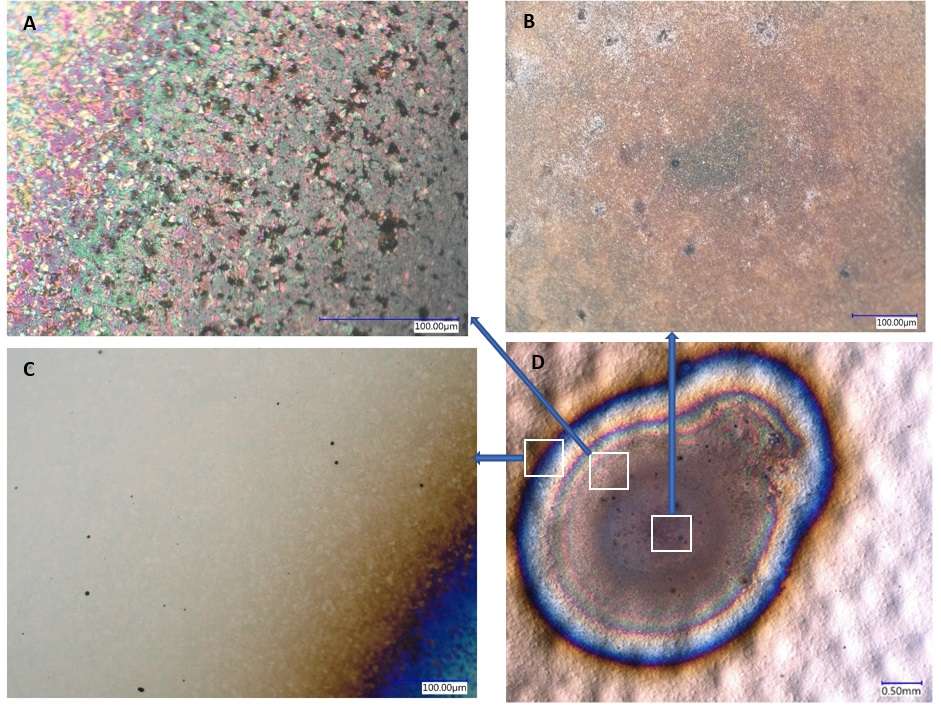
Figure 1
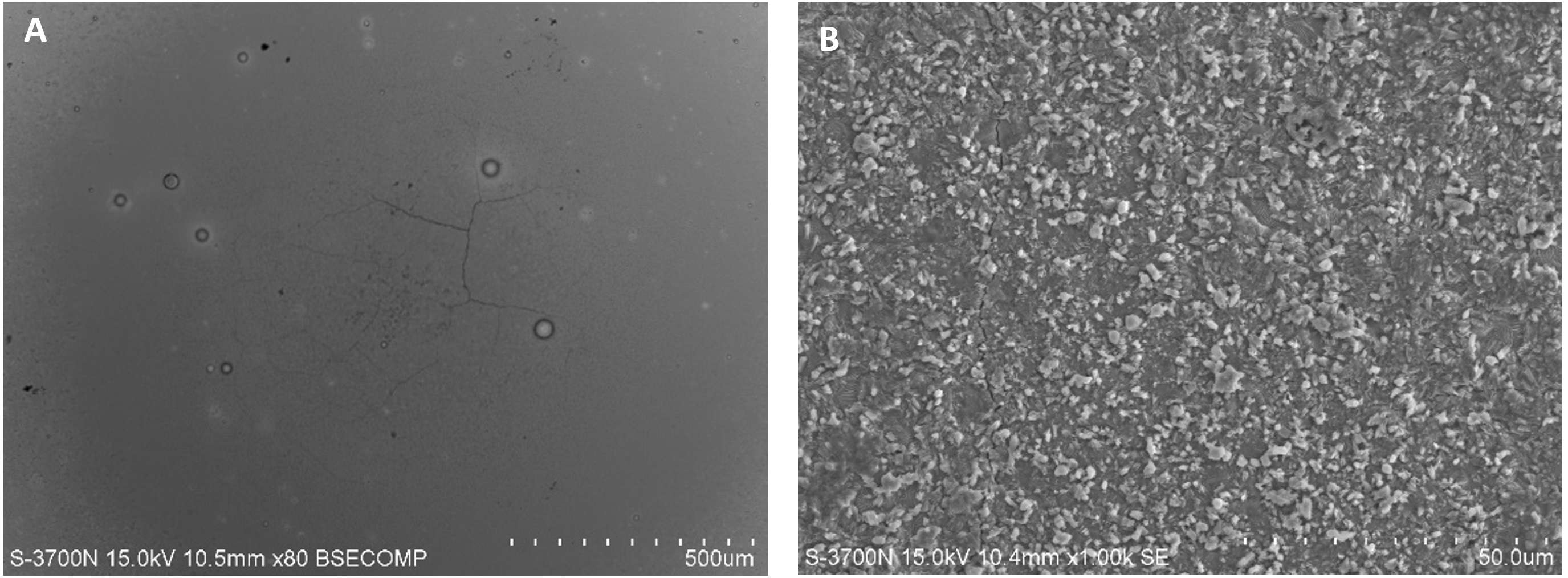
Figure 2
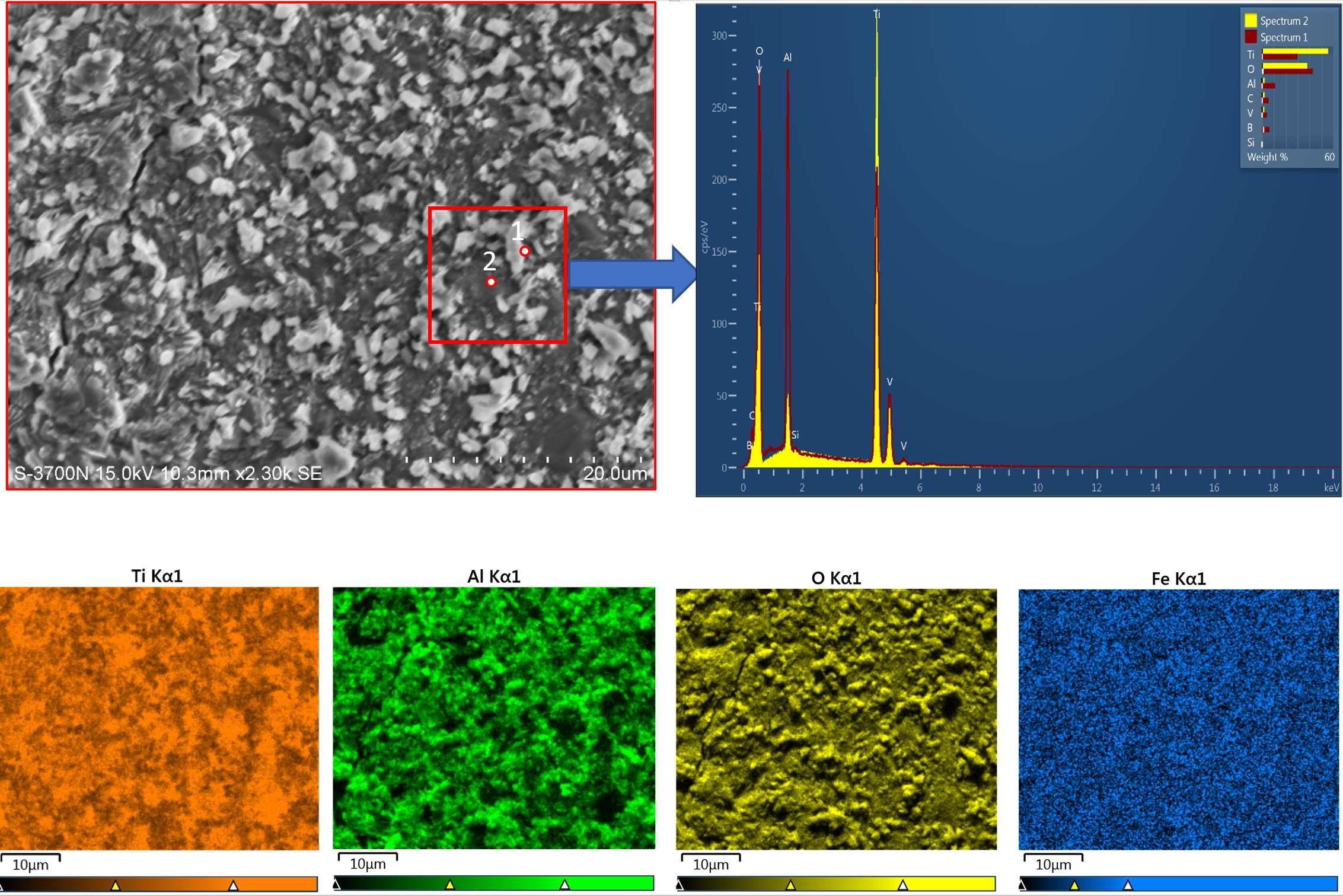
Figure 3#8776
A New Standard Test Method for Testing Reverse Total Shoulder Replacements.
*Hani Haider - UNMC - Omaha, USA
Joel Weisenburger - The University of Nebraska Medical Center - Omaha, USA
Ryan Siskey - Exponent - Philadelphia, USA
Ruth Heckler - DJO Surgical - Austin, USA
Nichole Plata - Enovis - Austin, United States of America
*Email: hhaider@unmc.edu
Reverse Total Shoulders (RTS) are essentially ball-in-socket systems, with highly conforming articulation of large contact area, with moderate stress. Telemetry measurements showed that shoulder loading could reach 2.38x bodyweight (BW) when moving a 2kg load. Osteolysis is a concern in RTS, as wear has been reported clinically in retrievals. Similar to hips, the long-term wear of RTS is therefore important. The purpose of this study was to develop a wear testing method for RTS, utilizing physiologically realistic forces/motions.
By synthesis of data from telemetrized implants we inferred that the magnitude and direction of the average force vector in the shoulder were similar across patients. We estimated and aligned this average vector to the compressive FZ axis of an AMTI Hip Simulator (Watertown, MA) by building custom fixtures with compound angles. These optimized the RTS range of motion (ROM) inside a physiologically relevant shoulder motion envelope within the hip simulator’s full ROM. The simulator’s “hip” motions were 3D transformed into shoulder motions, and the process iterated tens of times until the shoulder motion was maximized, kept realistic, optimized, and provided cross-shear. With this optimum alignment, the RTS could articulate between 38°-79° elevation, in the elevation planes between 15°-45°. We placed the maximum force of 2.38 BW (~1,700 N) at maximum elevation angle (79°) and a minimum of 10% (R=0.1, 170 N) at the lowest elevation angle (38°). The resulting motion (and load) resembled an orchestra conductor carrying a heavy baton, with modest ROM along a V-shaped path and then reversing back, at a cyclic frequency of 0.75 Hz (Fig 1).
Two groups of RTS were tested (N=3). The 32mm glenospheres were CoCr and were paired with GUR1020 ultra-high-molecular-weight-polyethylene (UHMWPE) humeral bearings that were either conventional or highly cross-linked (HXL) (150 kGy) and stabilized with vitamin-E (blended, 0.1% by weight). The bearings were secured into Ti humeral stems. Testing was conducted for 2 million cycles (Mc), lubricated with diluted bovine serum (30 g/L, 37°C). The wear of the UHMWPE bearings and glenospheres was measured at standard intervals and corrected with the weight gain of active load soak controls.
The average wear rates of conventional and HXL vitamin-E UHMWPE humeral bearings were 23.9±6.71 and 2.69±0.416 mg/Mc, respectively (Fig. 2, p=0.031). The use of HXL vitamin-E UHMWPE significantly reduced the wear when compared to conventional UHMWPE (89% wear reduction). The CoCr glenospheres paired with conventional and HXL vitamin-E UHMWPE humeral bearings wore similarly at 0.575±0.290 mg/Mc and 0.504±0.121 mg/Mc, respectively (p=0.724) and showed some minor scratching.
The RTS wear test method presented here produced physiologically realistic alignment, forces, and motions, including cross shear, and produced wear not dissimilar that seen on RTS retrievals. After much discussion at the International Organization for Standards (ISO) level among world leading labs and the US Food and Drug Agency (FDA), this method we developed is being considered for an ISO standard test method.
Figures
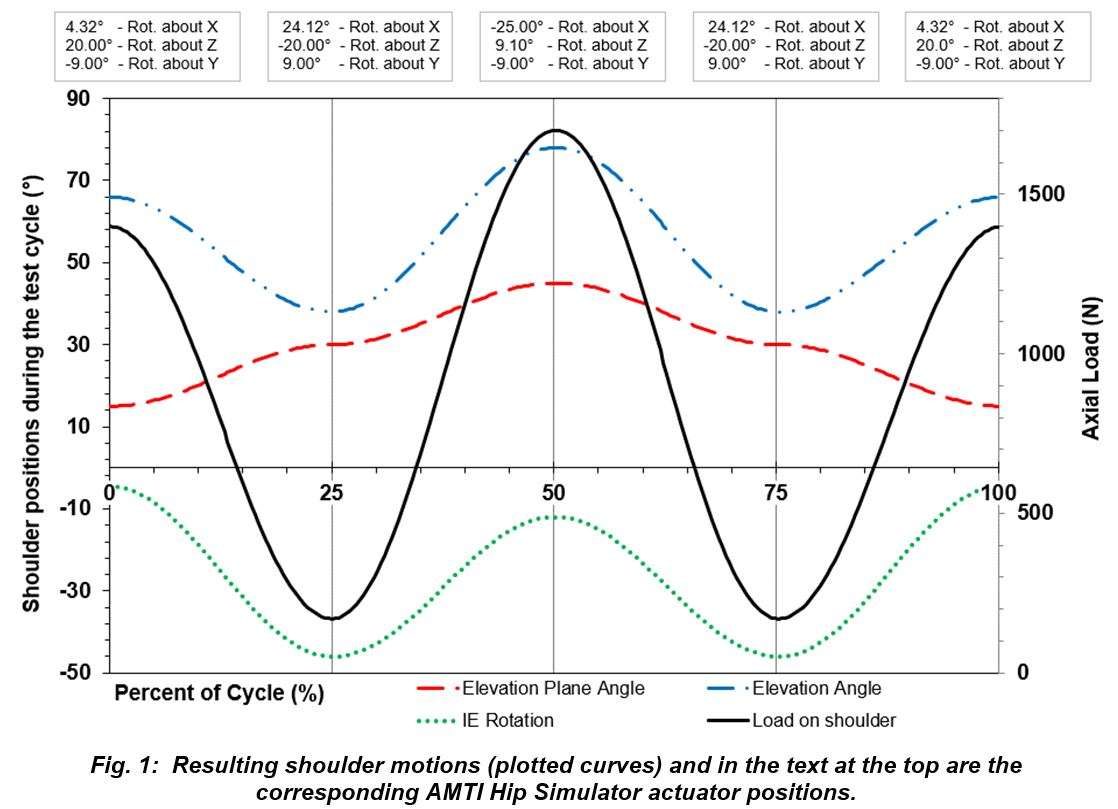
Figure 1
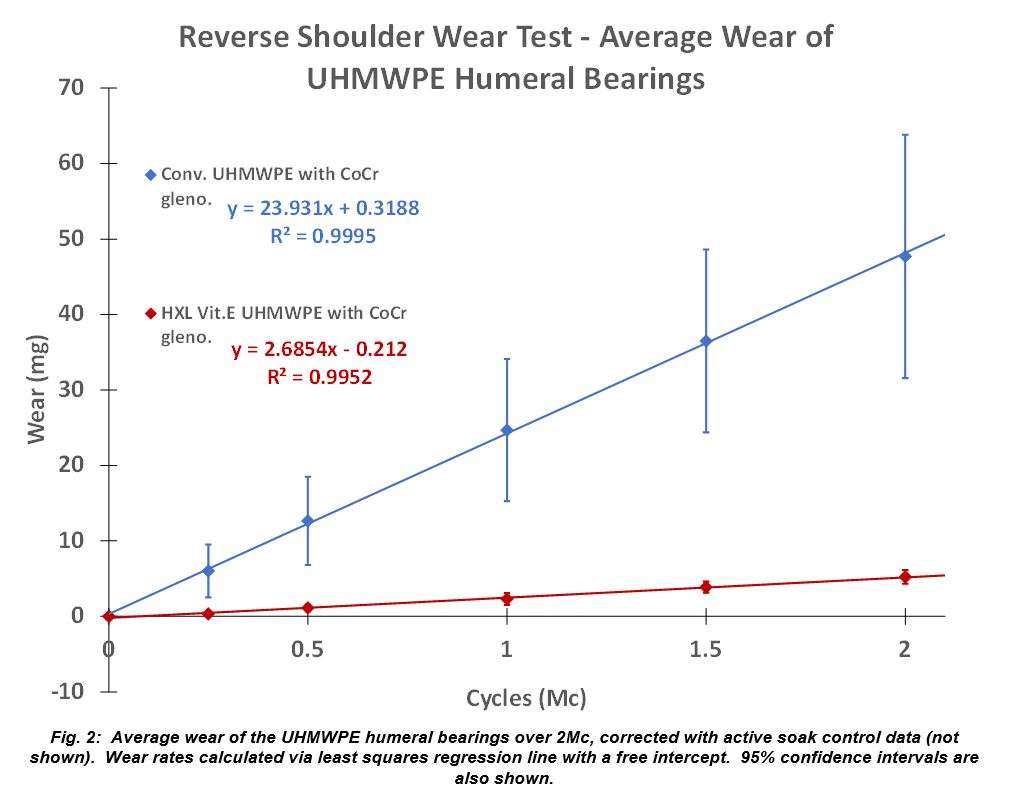
Figure 2#8440
Validation of Reverse Shoulder Scapular Notching Risk for an in Silico Clinical Trial
*Rodney Summers - Zimmer Biomet - Warsaw, USA
Jeffrey Bischoff - Zimmer, Inc. - Warsaw, USA
Adam Henderson - Zimmer Biomet - Winterthur, Switzerland
*Email: rodney.summers@zimmerbiomet.com
INTRODUCTION
Clinical trials cannot routinely cover all possible clinical scenarios of contemporary medical devices due to factors such as patient availability for enrollment and loss due to follow-up. Additionally, they cannot necessarily provide a physiological or mechanistic explanation for the origin of complications. In silico clinical trials (ISCT) can augment clinical trials with predictions of clinical performance from a virtual cohort, by providing results earlier and more comprehensively than a traditional clinical trial. ISCT can also provide a more detailed explanation for the root cause of any complications, enabling enhanced patient stratification and refined implant indications for use. The focus of this study is to determine if the clinical observation of scapular notching following reverse total shoulder arthroplasty can be predicted in an in silico model using adduction limit as a surrogate for notching incidence.
METHODS
Numerical models of 38 full shoulders were obtained from segmented and reconstructed CT. Each virtual model underwent simulated reverse shoulder arthroplasty (RSA) according to contemporary surgical techniques, using generalized implant geometries (Figure 1). Following reconstruction, each joint was subjected to a simulated range of motion assessment to identify the adduction limit (first contact between bearing and bone). Perturbations on inferior overhang (5mm vs 0mm) and inferior tilt (10° vs 15°) of the glenosphere were included to evaluate the impact of surgical approach on risk of notching. Key measures were prosthesis-scapular neck angle (PSNA) and peg-glenoid rim distance (PGRD) (Figure2). The in silico approach was evaluated by comparing in silico predictions to clinical rates of notching1. Only the prevalence of notching was reported in the clinical study; adduction limits were not.
RESULTS
Increasing PGRD by 5mm (i.e., no inferior overhang) meaningfully increased the adduction limit (reduced ROM). A 5° increase in PSNA significantly impacted adduction limit, with a smaller PSNA allowing for more ROM. The in silico clinical study showed statistically significant increased adduction limit (representing decreased shoulder ROM) with increased PSNA and PGRD (p<0.001) (Figure 3) which is consistent with clinical observation of increased incidence of scapular notching with increased PSNA and PGRD.
CONCLUSIONS
The present in silico assessment of scapular notching risk successfully replicated observations of a previous retrospective clinical study1 which stratified parameters associated with scapular notching. Additionally, the model enabled direct assessment of the impact of implant position and bone resection on ROM on a patient-specific basis, adding mechanistic insight beyond what can be obtained via a clinical study. This study provides confidence in the clinical utility of future ISCT applications of the modelling approach for supporting design of new RSA systems and refining surgical techniques via a comprehensive consideration of patient anatomies, implant characteristics, and surgical approaches. Although the model does not include soft tissue effects, which likely impact the quantitative predictions of in vivo range of motion, a qualitative assessment of shoulder ROM for RSA systems can lead to improved patient outcomes and reduce the risk of adverse events such as scapular notching2.
REFERENCES
- Simovitch et al. JBJS. 2007
- Roche et al. J. SES. 2009
Figures
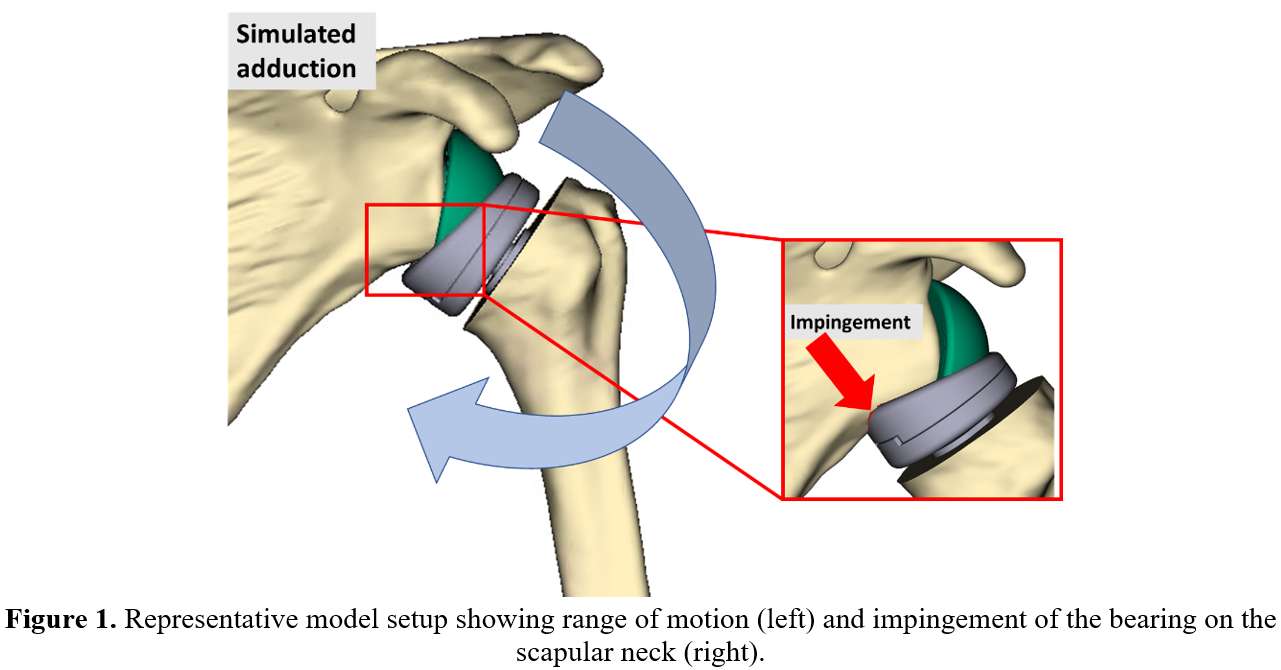
Figure 1
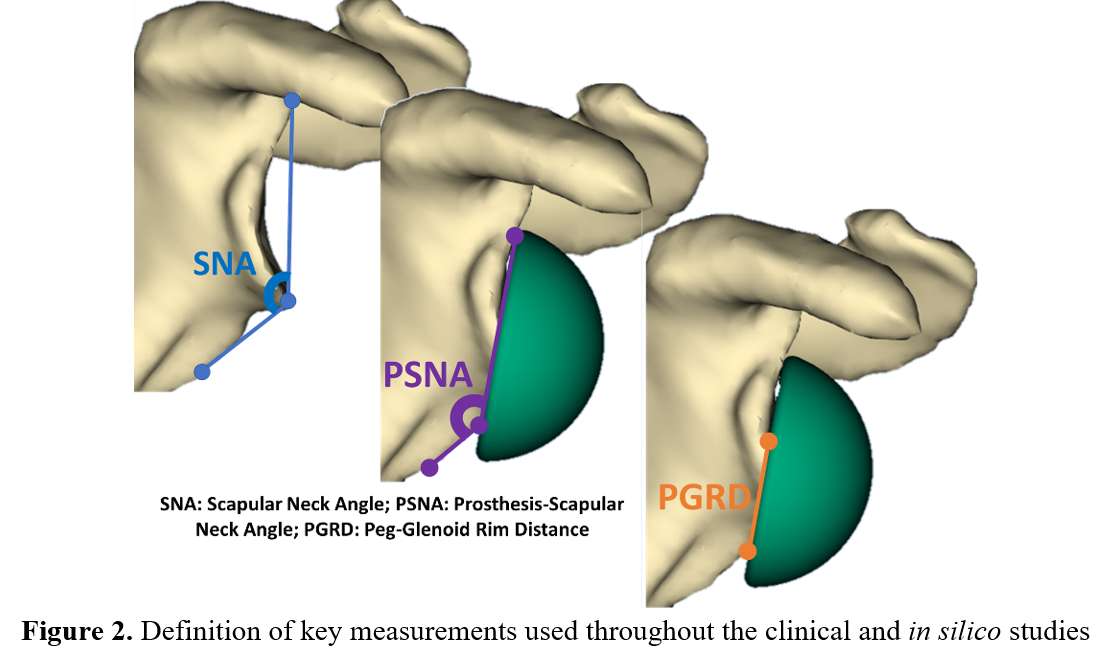
Figure 2
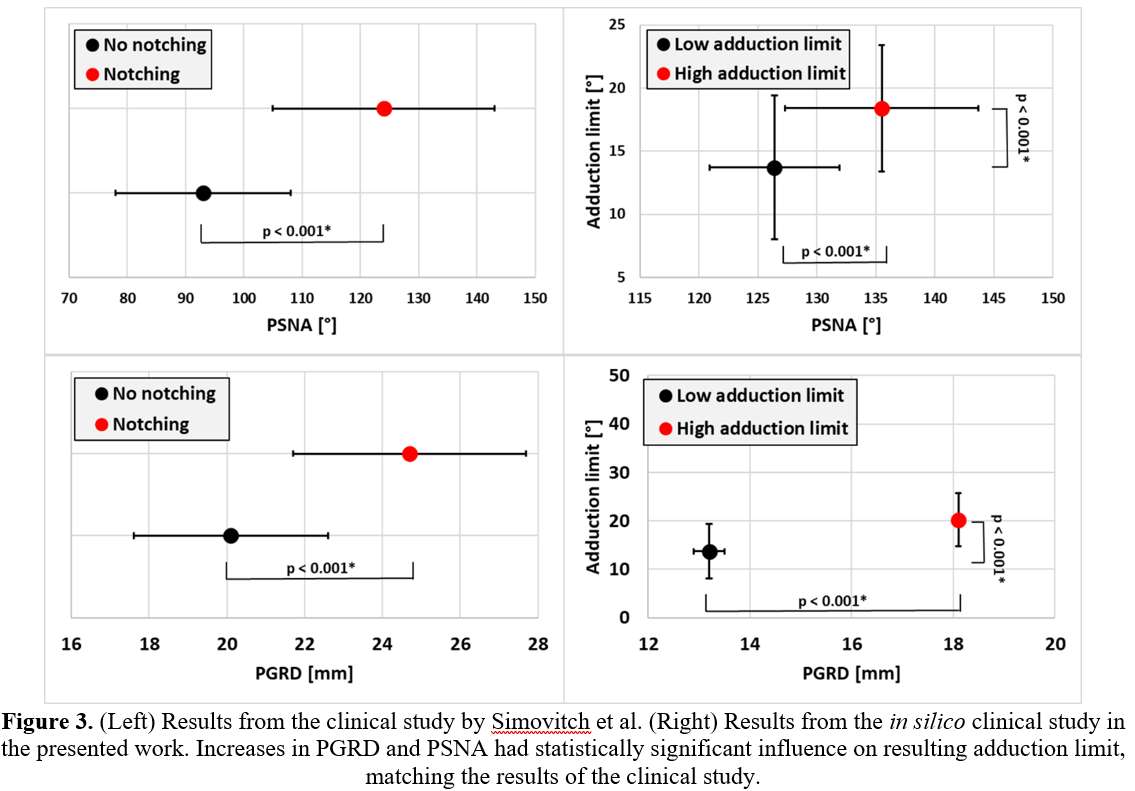
Figure 3#8207
Navigation in Reverse Shoulder Arthroplasty: How the Lateralization of Glenosphere Can Affect the Clinical Outcome
*Luigi Tarallo - University of Modena and Reggio Emilia - Modena, Italy
Fabio Catani - University of Modena and Reggio Emilia - Modena, Italy
Alessandro Dona - University of Modena and Reggio Emilia - Modena, Italy
Marta Montanari - University of Modena and Reggio Emilia - Modena, Italy
*Email: ltarallo@unimore.it
Introduction: One of the main causes of reverse shoulder arthroplasty (RSA) failure is attributable to the malpositioning of the glenoid component. Postoperative instability is the most common complication reported from 2.4% to 31% in the current literature Initial experiences with computer-assisted surgeryhave shown promising results in increasing the accuracy and repeatability of placement of the glenoid component and screws. The aim of this study was to evaluate the functional clinical results, in terms of joint mobility and pain, by correlating them with intraoperative data regarding the positioning of the glenoid component. The hypothesis was that the lateralization more than 25mm of the glenosphere can led to better stability of the prosthesis but should pay in term of a reduced range of movement and increased pain.
Methods: 50 patients were enrolled between October 2018 and May 2022; they underwent RSA implantation assisted by Guided Personalized Surgery (GPS) navigation system (Equinoxe reverse shoulder prosthesis). Active range of motion, ASES score and VAS pain scale were recorded before surgery. Preoperative data about glenoid inclination and version were collected by pre-op X-Rays and CT scans. Intraoperative data - inclination, version, medialization and lateralization of the glenoid component - were recorded using computer-assisted surgery. 46 patients had been further clinically and radiographically re-evaluated at 3-month, 6-month, 1-year, and 2-year follow-up.
Results: We found a statistically significant correlation between anteposition and glenosphere lateralization value (DM -6.057mm; p=0.043). Furthermore a statistically significant correlation has been shown between abduction movement and the lateralization value (DM -7.723mm; p= 0.015). No other statistically significant associations were found when comparing the values of glenoid inclination and version with the range of motion achieved by the patients after reverse shoulder arthroplasty.
Conclusion: Navigation can be used as a reference to improve our knowledge of the impact that intraoperative parameters can have on the patient's clinical outcome. We observed that the patients with the best anteposition and abduction results had a glenosphere lateralization between 18mm and 22mm. When increasing the lateralization above 22mm or reducing it below 18mm, on the other hand, both movements considered decreased their range.
Keywords: RSA navigation system, glenosphere lateralization, clinical and functional outcomes.
Figures
Figure 1#8660
Outcomes of Augmented vs Standard Baseplates in Reverse Shoulder Arthroplasty
Carlos Fernandez - JFK / U Miami Orthopedic Surgery Program - Lantana, USA
*Vani Sabesan - Cleveland Clinic Florida - Weston, USA
Sarah Girshfeld - Florida Atlantic University Charles E Schmidt College of Medicine - Boca Raton, USA
Brandon Macknofsky - Florida Atlantic University Charles E Schmidt College of Medicine - Boca Raton, USA
Clyde Fomunung - Palm Beach Shoulder Service - Lantana, USA
Howard Routman - Atlantis Orthopedics - Atlantis, USA
Ali Mohamed - FAU - Boca raton, USA
Garrett Jackson - Chicago, United States of America
*Email: sabes001@gmail.com
Background: Despite the increased utilization of shoulder arthroplasty (RSA) since the early 2000s, it is not indicated for all shoulder pathologies and is met with specific difficulty in cases of glenoid bone loss. Augmented glenoid baseplates have shown encouraging results as a treatment for managing glenoid bone loss, but with limited studies assessing the efficacy of the treatment for reverse shoulder arthroplasty. The purpose of this study was to compare the functional and patient reported outcomes (PROs), complications, and revisions of patients undergoing RSA with augmented baseplates versus standard baseplates.
Methods: A retrospective review of 447 patients treated with primary RSA by a single fellowship trained surgeon at a single institution was performed. There were 279 patients in the standard group (SG) and 168 patients in the augmented group (AG), which was further divided by the type of augment into three subgroups (superior, posterior, superior/posterior). Demographic variables, range of motion (ROM), PROs, and pain scores were collected from preoperative and latest follow-up visits, in addition to complication and revision rates. PROs included Simple Shoulder Test (SST), University of California Los Angeles (UCLA) Shoulder, American Shoulder and Elbow Surgeons (ASES), Constant Shoulder, and Shoulder Pain and Disability Index (SPADI) scores. For each variable, the augmented groups were compared to the SG and statistical analysis was performed.
Results: Compared to the SG, there were significantly more males in the AG (p<0.01) and fewer patients with the diagnosis of RCT (p=0.04). At an average follow-up of 26.0 months, the AG and each of the augmented subgroups performed as well, or better, than the SG on all functional and PROs. No significant difference was found in rates of complications, revisions, humeral radiolucent lines, glenoid loosening, or scapular notching.
Conclusions: Even when glenoid wear is present, augmented baseplates perform equally or better than standard baseplates in primary RSA, with no significant difference in early complication rates. Despite concerns about over-tightening with augmented baseplates, both treatments demonstrated comparable functional improvements and pain relief. This suggests that augmented baseplates are not only effective for glenoid wear, but also provide successful patient outcomes.
#8634
Evaluating the Effect of Simulating Extreme Physiological Loads on Interface Fixation of Stemless Reverse Humeral Component Fixation
*David Cunningham - University of Western Ontario - London, Canada
G. Daniel G. Langohr - The University of Western Ontario - London, Canada
George Athwal - University of Western Ontario - London, Canada
James Johnson - SJHC - London, Canada
*Email: dcunni9@uwo.ca
Purpose:
The use of stemless anatomic humeral implants has emerged as a viable alternative to traditional stemmed devices. Despite the growing popularity of these stemless devices, evaluation of the fixation remains limited. Previously, investigations have focused on implant response to varied abduction motions [1], [2], or to uniaxial loading protocols intended on replicating the magnitudes and eccentricities of abduction motions [3]. However, the shoulder articulation is exposed to a much more diverse array of loading directions and magnitudes that may not be encompassed by these protocols [4]. It is therefore important to fully assess all potential physiological loading conditions. This finite element study assessed a loading method to more thoroughly investigate initial fixation of a stemless humeral component.
Method:
Five patient-specific (sex: male, age: 67 ± 24 years, mass: 66 ± 6 kg, height: 178 ± 3 cm) three-dimensional models of the proximal humerus were created from CT data using Mimics (Materialise, Leuven, Belgium). A generic implant model (Figure 1) was positioned into the humeral models at a 135° neck shaft angle. Three physiologically extreme boundary loads and one 30° abduction motion load, created from published in-vivo telemetrized implant data [5] and corrected for the individual body weight of each subject were assessed in Abaqus CAE. The three boundary loads identified were created to describe 95% of activities with data available. Bone—implant distractive micromotion was employed as the outcome variable.
Results:
The Superior-Anterior load resulted in significantly more micromotion when compared to the simple 30° abduction load (30° Abduction: 2.98 ± 0.57 µm, Anterior: 3.82 ± 1.32 µm, Superior-Anterior: 7.53 ± 1.29 µm, Superior: 4.68 ± 2.03 µm) (P < 0.0001). However, all loads elicited significantly different implant positions of maximum distraction (30° Abduction: 19.5 ± 5.14°, Anterior: -40.5 ± 5.70°, Superior-Anterior: 20.72 ± 5.46, Superior: 69.94 ± 3.76°); each posing a different challenge to implant initial fixation (P < 0.0001).
Conclusion:
Humeral component loading is an important parameter during the evaluation of implant performance. Due to the diverse array of loading states experienced by the shoulder, it is likely important to utilize loading protocols that sufficiently encompass the myriad of loading magnitudes and directions that may be experienced by a patient postoperatively; as results of these analyses may vary dependent on the implant design, loading magnitude, and/or the direction of loading.
Figure 1: A generic stemless reversed humeral implant.
Figure 2: Maximum micromotion and angular position of the point of maximum micromotion for each of the (4) evaluated loads.
[1] N. Razfar et al., “Comparison of proximal humeral bone stresses between stemless, short stem, and standard stem length: A finite element analysis,” J. Shoulder Elb. Surg., vol. 25, no. 7, pp. 1076–1083, 2016.
[2] ASTM, “ASTM F2028-17 Standard Test Methods for Dynamic Evaluation of Glenoid Loosening or Disassociation,” pp. 1–15, 2018.
[3] P. Favre et al., “In vitro initial stability of a stemless humeral implant,” Clin. Biomech., vol. 32, pp. 113–117, 2016.
[4] P. Damm and J. Dymke, “Orthoload Database,” Julius Wolff Institute, 2021. [Online]. Available: https://orthoload.com/database/. [Accessed: 26-Jul-2021].
Figures
Figure 1
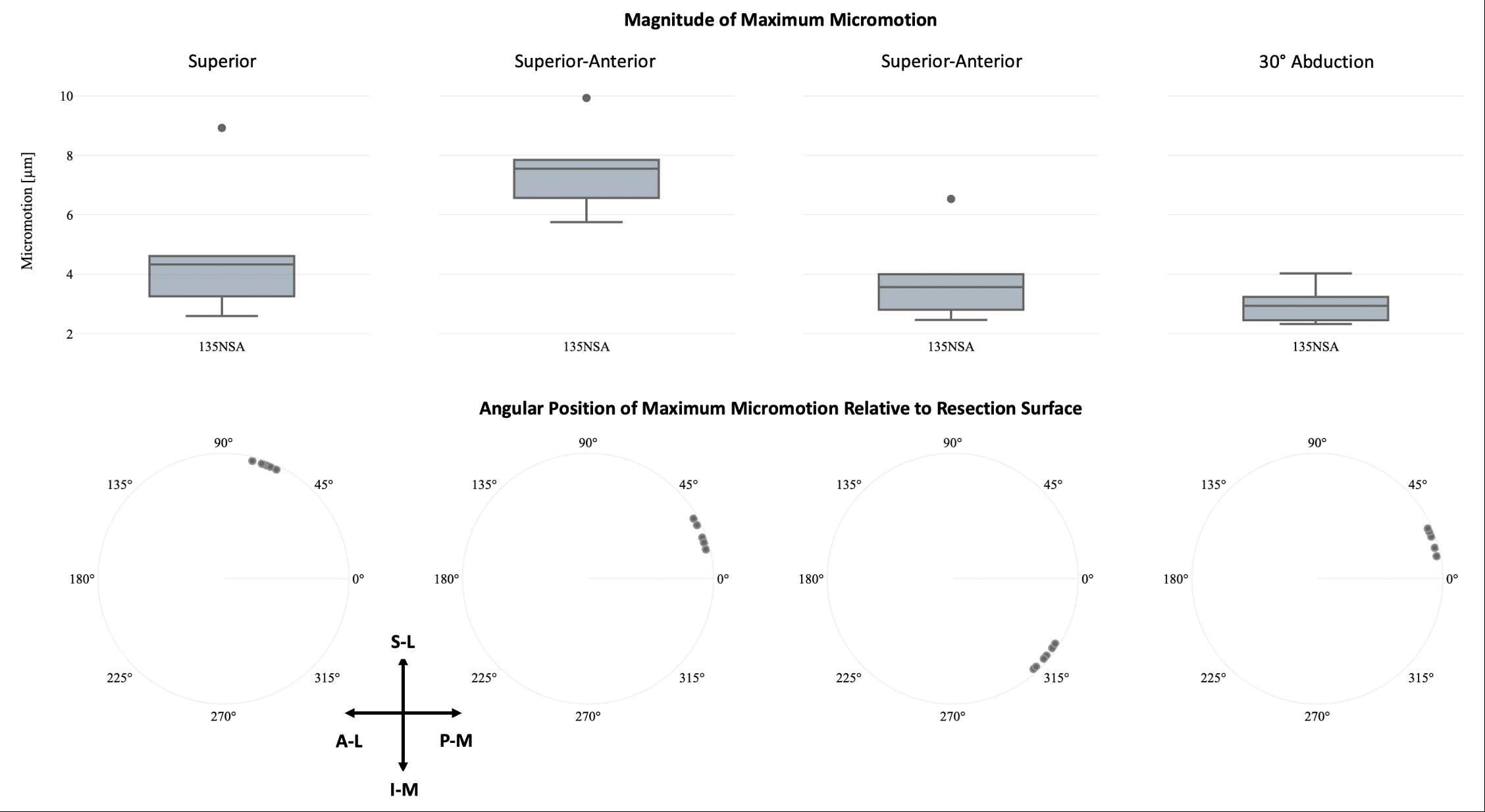
Figure 2#8680
Automated Bicipital Groove Detection in 3D Humerus Models Using Computer Vision
*Gregory Spangenberg - University of Western Ontario - London, Canada
Fares Uddin - King Hamad University Hospital - Busaiteen, Bahrain
Kenneth J. Faber - Western University / Hand and Upper Limb Clinic - London, Canada
G. Daniel G. Langohr - The University of Western Ontario - London, Canada
*Email: gspangen@uwo.ca
Purpose: Patient specific 3D printed guides for shoulder arthroplasty exist for the glenoid but currently no commercially available system exists for the humerus. A primary barrier to adoption is the process of designing a custom guide for each patient that references specific alignment landmarks. The humerus bicipital groove is often used as a reference during the implantation of a shoulder prosthesis to guide the recreation of native retroversion. [1] It has also been used as a fixation landmark for instrumentation that guides the humeral osteotomy.[2] Therefore, an automated algorithm that identifies the bicipital groove would prove useful in the implementation of humeral component 3D printed patient specific guides.
Methods: Segmented DICOM images from 27 cadaveric shoulder CT scans were used to generate 3D models of the humerus. The bicipital groove of each shoulder was virtually digitized by an upper limb orthopaedic surgeon to provide validation data. A patient-specific coordinate system is generated using “shoulder”, a python package [3]. 2D axial slices are taken along the canal-axis, and a Gaussian-kernel change-point algorithm detects the surgical neck [4]. The perimeter of each slice until the distal cutoff of the surgical neck is unwound into polar coordinates. Cysts are identified by the algorithm as regions where the polar perimeter doubles back onto itself and are then removed. The second derivative of the weighted average is calculated to permit identification of radial depressions seen in figure 1. The bicipital groove region was estimated using an optimized combination of the following relationships: (i) the maximum radial mean lies between the endpoints of the articular surface, (ii) 2 of the 3 largest peaks of the 2nd derivative of the radial mean are endpoints of the articular surface which contain the maximum radial mean between them, and (iii) the minimum radial mean is highly correlated to bicipital grove location. Following optimization, the estimated region of the bicipital groove is used to calculate the nearest local radial minima of each axial slice shown in figure 1. The local minima are finally transformed back to the original coordinate system of the CT scanned bone as shown in figure 2.
Results: The accuracy of the algorithm was evaluated against the digitized data and along equal quartiles bounded proximally by the top of the lesser tuberosity and distally by the surgical neck. The root mean squared error from proximal to distal quartiles was 0.87mm, 0.82mm, 0.91mm, and 1.01mm respectively. The absolute difference in distance between algorithmic and digitized bicipital groove loaction is also shown in Figure 3. No significant differences between the algorithmic estimates and digitized locations were detected.
Conclusion: The bicipital groove can be automatically identified with sufficient accuracy to enable its use as a reference feature for the generation of a 3D printed patient specific guide for the humerus.
References:
[1] Itamura et al. JSES (2002)11(4):322–6. [2] Cavanagh et al. JSES-Int. (2021)5(5):875–880. [3] Spangenberg. PYPI (2022) Shoulder:https://pypi.org/project/shoulder/ [4] Garreau et Al. Electron.J.Statist. (2018)12(2):4440–4486
Figures
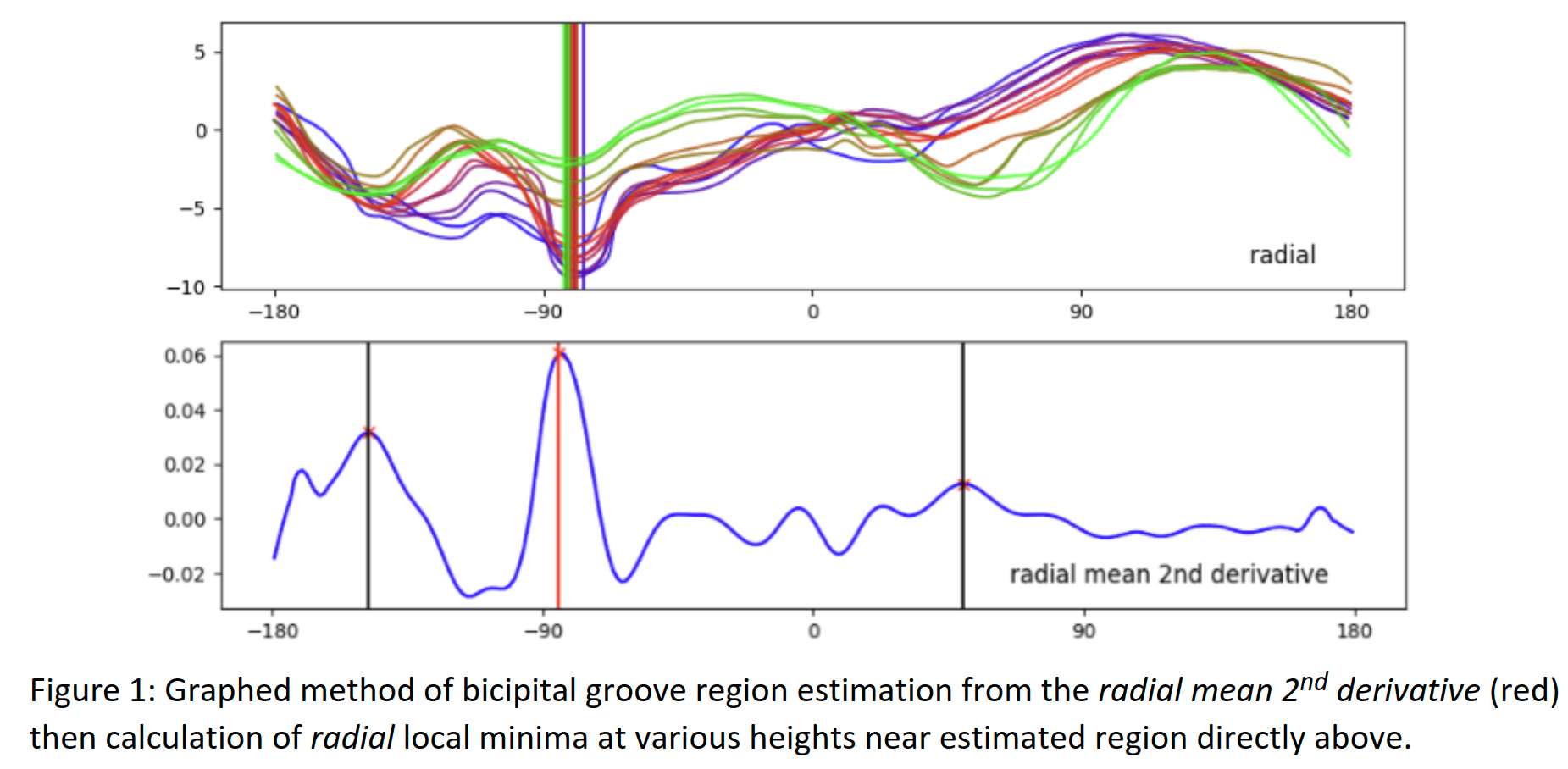
Figure 1
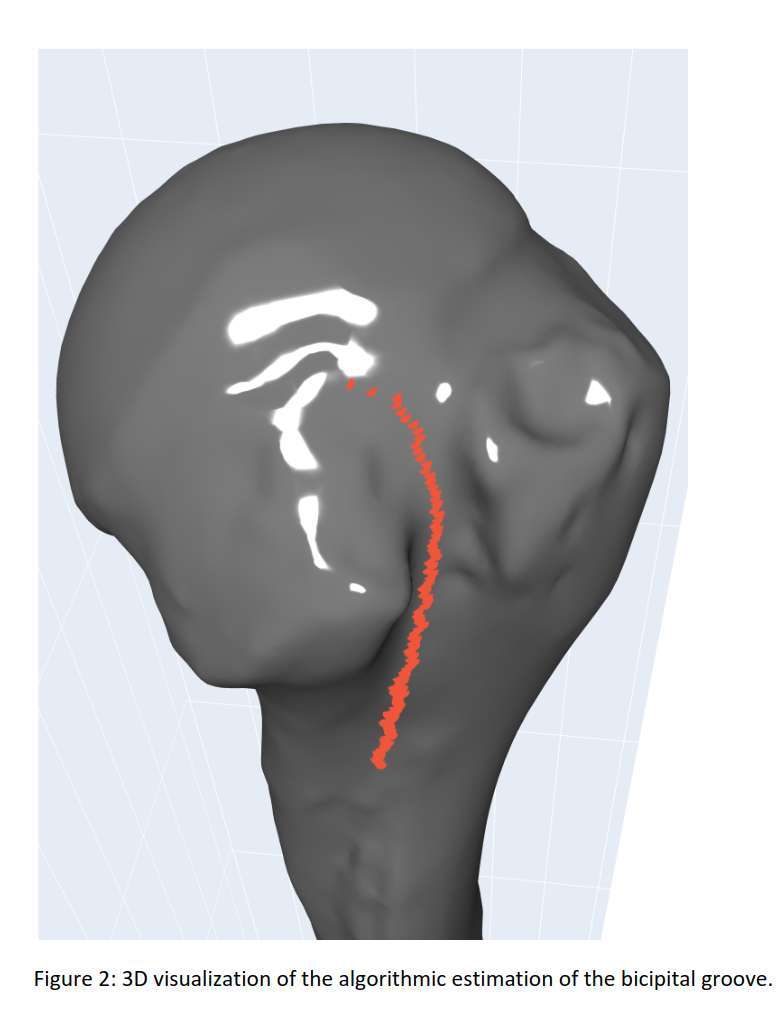
Figure 2
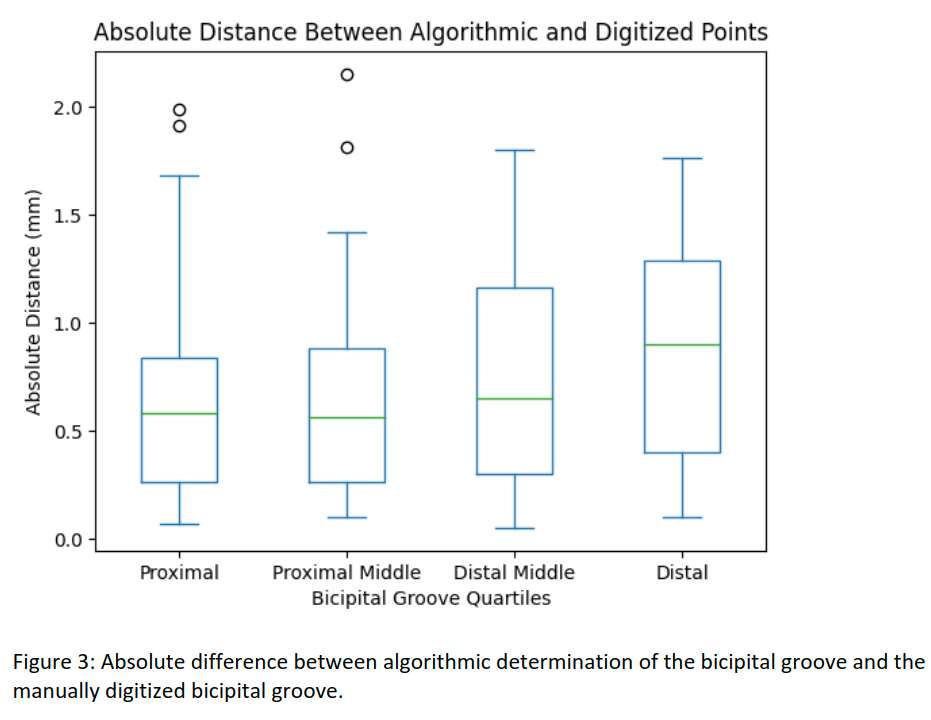
Figure 3#8529
Association of C. Acnes With Histopathologic and Tribological Characteristics of Retrieved Shoulder Arthroplasties
*Deborah Hall - Rush University Medical Center - Chicago, USA
Alexander Hornung - Rush University Medical Center - Chicago, USA
Niraj Lawande - Rush University Medical Center - Chicago, USA
Stanley Liu - Rush University Medical Center - Chicago, USA
Gregory Nicholson - Rush University Medical Center - Chicago, USA
Robin Pourzal - Rush University Med Ctr - Chicago, USA
Grant Garrigues - Rush University Medical Center - Chicago, USA
*Email: deborah_hall@rush.edu
Introduction: Clinical failure from underlying periprosthetic joint infection (PJI) remains a frequent cause for revision after total shoulder arthroplasty (TSA) with Cutibacterium acnes being commonly associated with PJI. While C. acnes infection has been correlated with prosthetic loosening, it is unknown if chemical changes within the periprosthetic environment alter the wear and corrosion behavior of the prosthesis. Thus, the current study aims to identify specific patient, implant and histopathological characteristics associated with C. acnes infection.
Methods: A retrospective review of 130 patients treated with revision shoulder arthroplasty at a single institution between 2017 and 2021 was performed. Patients with definite PJI due to highly virulent (e.g., S. aureus) were excluded from this analysis. The presence of C. acnes infection was categorized according to the 2018 International Consensus Meeting (ICM) definitions. Group 1 (unlikely) and Group 2A (definite or probable) were initially compared. Group 1 was also compared with a more inclusive Group 2B (definite, probable, or possible). Damage to the implant was microscopically assessed. A semi-quantitative system was used to score sections of periprosthetic tissue for the extent and type of cellular response. Demographics, operative, and radiographic information were also compared between the groups.
Results: In total, 117 patients met the inclusion criteria. There were 103 patients in the unlikely PJI group (Group 1), 14 patients with either definite or probable C. acnes PJI (Group 2A), and 19 patients (Group 2A plus five possible PJI patients) in Group 2B. Histopathological analysis revealed higher lymphocyte counts in Group 2A (p=0.004) and Group 2B (p=0.018) compared to Group 1. Group 2A also exhibited significantly higher plasma cell scores (p=0.022). Group 2B was more likely to have marked neutrophil scores than Group 1 (p=0.033). Both C. acnes groups (Group 2A and 2B) exhibited significantly higher preoperative white blood cell counts (p=0.027 and p=0.013, respectively). There was no significant difference in other preoperative inflammatory markers (e.g., ESR and CRP). Moreover, there were no differences in the incidence of radiographic humeral loosening or implant damage.
Discussion: A marked lymphocytic response was present in both C. acnes groups, whereas a distinct plasma cell response was associated with Group 2B compared to Group 1. Together, these responses may result from prolonged inflammation and could serve as a future diagnostic marker for C. acnes infection. Histologic elevation of polymorphonuclear leukocytes (PMNs) in C. acnes PJI has shown mixed results in the literature; here, there was no significant difference between Group 2A and Group 1; however, Group 2B exhibited significantly greater PMNs compared to Group 1. We did not appreciate a sex predominance in the C. acnes groups, which diverges from previous reports showing an increased incidence of C. acnes in male patients. Moreover, there was no difference in humeral loosening for the C. acnes groups on post-operative radiographs, which differs from prior studies of revision shoulder arthroplasty. Finally, there were no differences in PE or metal wear, suggesting that the inflammatory response to C. acnes is likely not to be directly degenerative to the implant components.
#8855
Sides matter: Preoperative dynamic spinopelvic assessment techniques
*russell bodner - Silver Spray Laboratory, Kishwaukee hospital - USA
*Email: drbodner926@gmail.com
#8856
Robotic THA: The power of 3D planning
*Fares Haddad - University College London Hospital - London, United Kingdom
*Email: fsh@fareshaddad.net
#8857
Fixation challenges in post traumatic and revision hip arthroplasty
*David Rodriguez - University of Texas - Houston, USA
*Email: david.rodriguezquintana@uth.tmc.edu
#8858
Augmented reality in THA
*Stephen Murphy - New England Baptist Hospital - Boston, USA
*Email: stephenbmurphymd@gmail.com
#8859
Resurfacing is back
*Justin Cobb - Imperial College - London, United Kingdom
*Email: j.cobb@imperial.ac.uk
#8426
Wear Simulation of Metal-on-Polyethylene Total Hip Arthroplasty Under Varying Third-Body Conditions
Ilya Borukhov - Stryker Orthopedics - Mahwah, USA
Rafael Baez - Stryker Orthopaedics - Mahwah, USA
*Sally LiArno - Stryker Orthopaedics - Bergenfield, USA
*Email: Sally.LiArno@stryker.com
INTRODUCTION
Roughening of the metallic femoral surface in-vivo after total hip arthroplasty (THA) has been reported previously and can occur due to recurrent dislocations or third-body abrasive damage[1,2]. Sources of third-body abrasive particles can vary greatly and can include bone, bone cement, metallic, or ceramic debris. Soon-to-be published revisions of ISO 21535 will require wear testing under aggressive third-body conditions although no standard for it exists currently. To facilitate development of a clinically relevant third-body wear test, we evaluated metal-on-polyethylene THA bearings under various third-body conditions and measured the gravimetric wear of the bearings as well as surface damage generated.
METHODS
A 32mm fixed bearing THA (Trident X3, LFIT CoCr, Stryker, Mahwah, NJ) was evaluated under four conditions: 1) clean (n=2), 2) 5g/L bone cement (n=2), 3) 5mg Ti6Al4V particles (n=3), and 4) 5mg Al2O3 particles (n=3). For the bone cement condition, SimplexP (Stryker, Mahwah, NJ) was cryomilled, sieved (100-250µm) and added to the serum prior to the start of every interval. For the embedded debris condition, Ti6Al4V (100-150µm) and Al2O3 (100-250µm) were embedded into the polyethylene insert prior to the start of the test only. A hip joint simulator (MTS, Eden Prairie, MN) was used for testing with cups positioned superior. Testing was conducted for 1.5 million cycles (mc), per ISO 14242-3:(R2019), with a modified load profile consisting of a physiological Paul curve with a peak axial force of 2450N [3]. To assess surface damage, surface texture parameters were measured using a white light interferometer (WLI) (NewView6300, Zygo, Middlefield, CT). A minimum of four images, 0.702-x-0.526mm, were captured in the damaged region of each femoral component. Surface roughness (Sa,nm) and average peak-to-valley height (Rz,µm) were reported.
RESULTS
Head weight loss was negligible in the clean control, increased slightly with addition of bone cement, further increased with embedment of Ti6Al4V, and greatest for the Al2O3 group (Table 1). After 0.5mc, the Al2O3 particles remained embedded while most Ti6Al4V particles had worn away/dislodged. With regards to the polyethylene insert, wear rate was highest for the Al2O3 group due to dramatic roughening of the femoral heads. Head Sa compared to the clean control was, on average, 1250X, 56X and 6X rougher for Al2O3, Ti6Al4V and bone cement groups respectively. Representative WLI images can be seen in Figure 1.
DISCUSSION
Third-body wear mechanisms in-vivo are complex and variable making it particularly difficult to simulate in-vitro. In this study, multiple third-body conditions were simulated with wide varying results. The surface roughness obtained using bone cement and Ti6Al4V debris more closely matched that of retrievals, with Ito et.al. reporting a roughness of 120±110nm (range:10-450 nm)[1]. This is also in agreement with the investigators own retrieval analysis conducted on CoCr femoral components. Ceramic particles of similar size appeared to be too aggressive in comparison. As test methods develop to evaluate next generation bearing materials under clinically relevant conditions, particular emphasis should be placed on particle source, size, as well as method, and frequency of introducing the particles to the bearings.
REFERENCES
[1]Ito.,J.Arthroplasty., 25(2):302-308,2010.
[2]Mai.,Am.J.Orthop.,39(10):495-500,2010.
[3]Paul,JP, Proc.Inst.Mech.Engrs.181(3J):8-15,1966
Figures
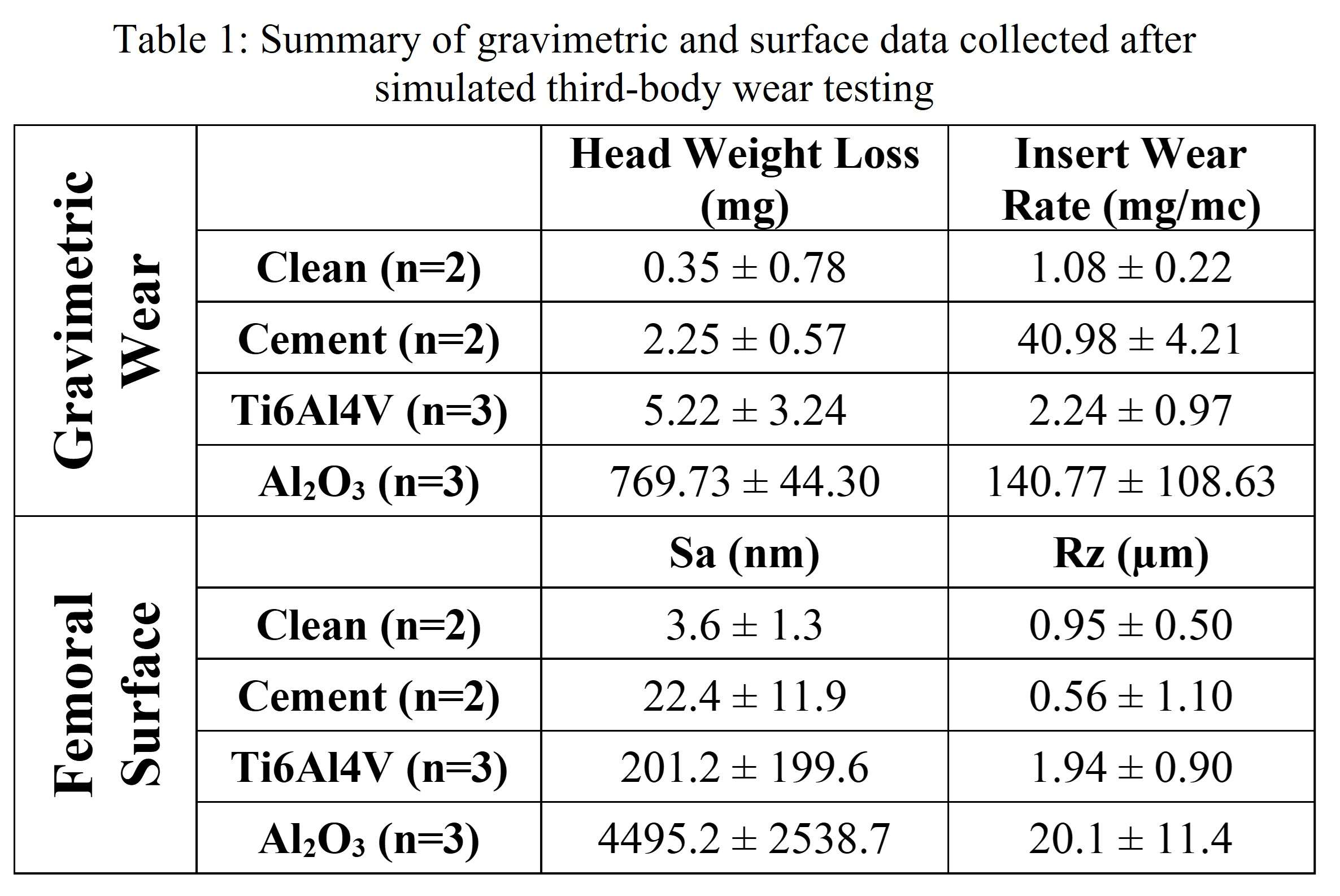
Figure 1
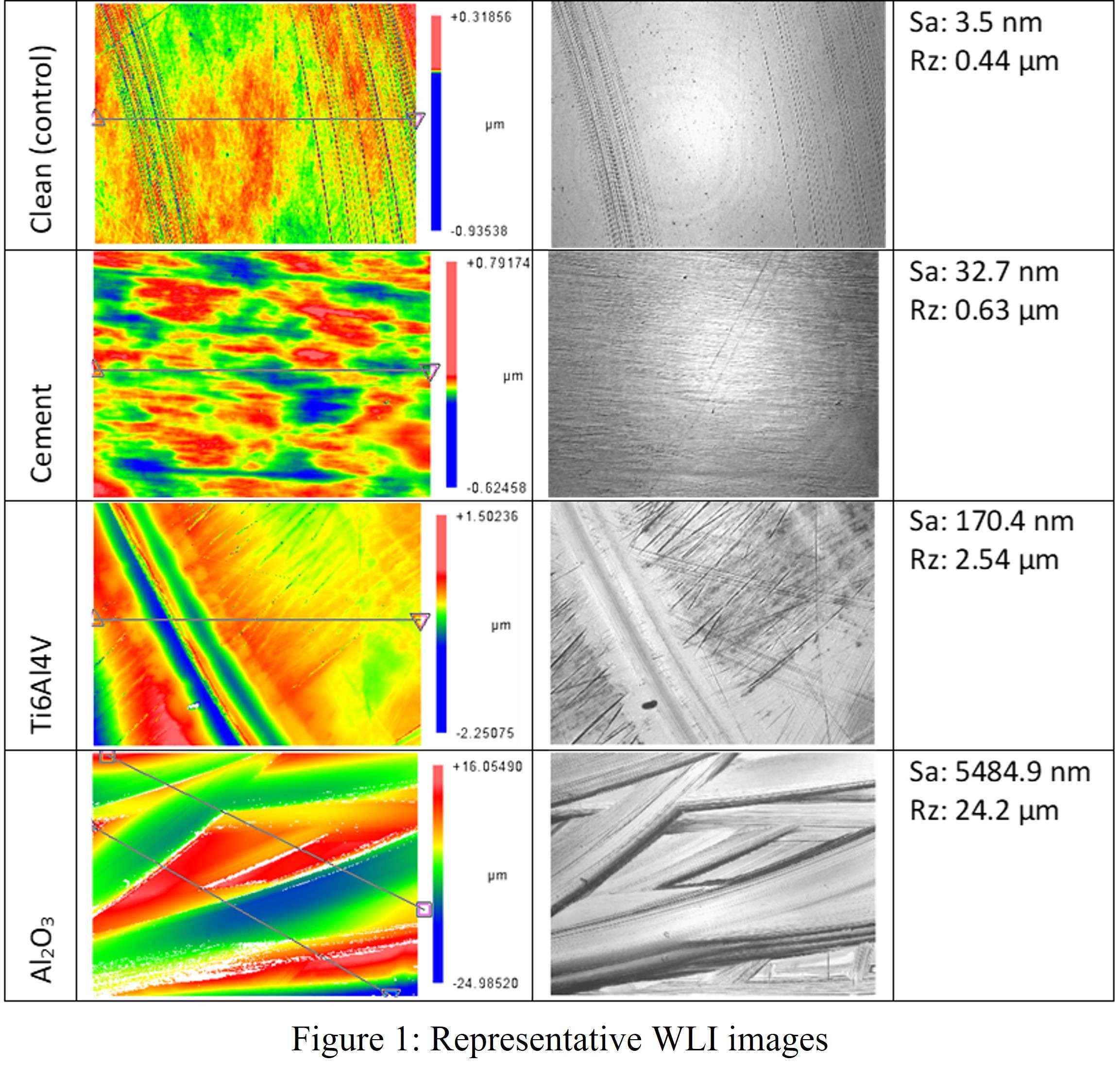
Figure 2#8566
Wear, Corrosion, and Adverse Tissue Response in 2nd Generation Small-Diameter Metal-on-Metal Total Hip Arthroplasties
*Stephanie McCarthy - Rush University Medical Center - Chicago, USA
Peter Wahl - Kantonsspital Winterthur - Winterthur, Switzerland
Deborah Hall - Rush University Medical Center - Chicago, USA
William Elzemeyer - Rush University Medical Center - Chicago, USA
Pat Campbell - Los Angeles, USA
Sophia Sangorgio - Orthopaedic Institute for Children/UCLA - Los Angeles, USA
Edward Ebramzadeh - Orthopaedic Institute for Children/UCLA - Los Angeles, USA
Douglas Van Citters - Dartmouth College - Hanover, USA
Joshua Jacobs - Rush University Medical Center - USA
Robin Pourzal - Rush University Med Ctr - Chicago, USA
*Email: stephanie_mccarthy@rush.edu
Introduction: Adverse local tissue responses (ALTR) to bearing wear and modular junction corrosion products led to revisions of a variety of >40mm large diameter (LD) third-generation metal-on-metal (MOM) hip arthroplasties. CoCrMo femoral head use in THA has been declining in favor of ceramic heads less susceptible to tribocorrosion processes. However, second-generation 28 and 32mm MOM THA have performed well, mostly in Europe, with excellent survival rates up to 20 years and few reports of ALTR or corrosion. The study objective was to characterize wear and corrosion behavior as well as associated tissue responses of a cohort of second-generation small diameter (SD) MOM THA.
Methods: 40 SD MOM modular heads and metal liners were collected (35 consecutively retrieved by a single surgeon, 5 short-term retrievals from three additional institutions). Median (range) time in-situ was 17.7 years (0.6, 26.7). Common reasons for revision included adverse reaction to metal debris, periprosthetic fracture, and pain. Head tapers were assessed using a modified Goldberg damage score (1 minimal, 2 mild, 3 moderate, 4 severe). Bearing surfaces and head tapers were measured with a Redlux optical coordinate-measuring-machine to assess implant wear, taper corrosion, and volumetric material loss. A subset of implants (n=25) was sectioned and etched for metallographic analysis, and alloys were characterized in a scanning electron microscope. Hip capsule tissue was available for 73% of cases. H&E-stained histological sections were analyzed for synovial lining integrity, inflammatory infiltrate, and tissue organization. Pearson’s Correlation and linear regression models were used to identify damage trends.
Results: Median (range) bearing surface material loss was 19.3 mm3 (5.8, 273.8). There was no linear correlation between wear and time (Fig.1). Taper damage score distribution was 17.5% minimal, 35.0% mild, 20.0% moderate and 27.5% severe. Median (range) head taper material loss was 0.3 mm3 (0.01, 9.4). 52.5% of head tapers had no measurable wear. Metallurgical analysis showed heads were made of high-carbon alloy with characteristic carbide presence (Fig.2A). 75% of cases had imprinting of stem taper topography in the head taper. Six heads exhibited a chemically-driven column damage-like pattern in the modular junction (Fig 2B&C). Local tissue responses were mild to moderate, with marked particle-laden macrophages but little to no lymphocytic responses.
Conclusion: This cohort of SD MOM bearings had an overall lower wear rate than known wear rates of various third-generation LD MOM devices. High wearing cases were associated with dislocation or fracture, leading to altered tribological behavior. Neither wear nor corrosion was associated with lymphocytic responses, likely due to relatively low rates of metal release compared to contemporary LD MOM THA. Microstructural appearance and subsequent damage progression differed between these implants and low carbon, contemporary heads. Imprinting was present but was milder than that seen in low-carbon alloy, likely due to the carbide presence. Column damage occurred but was different from the low-carbon condition, with visible bands of carbides creating areas with a similar appearance to column damage. These results indicate a different overall wear and corrosion behavior of 2nd generation SD MOM compared to later generations of MoM THA.
Figures
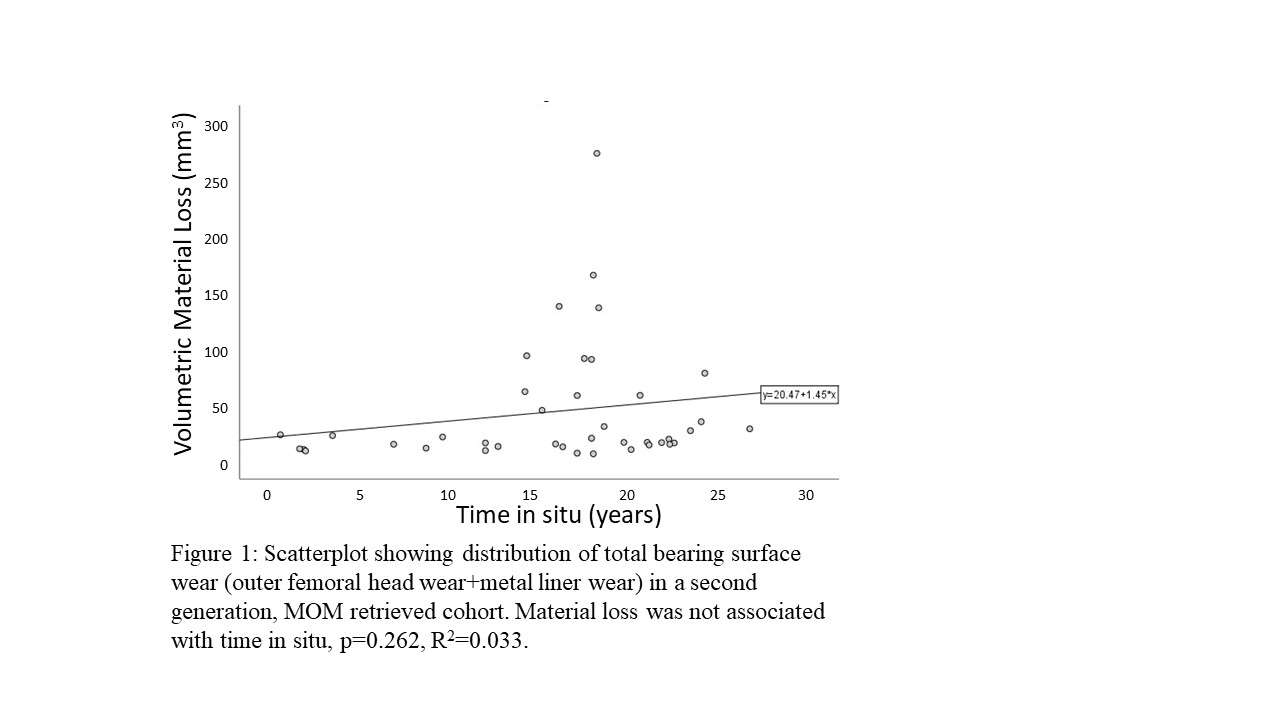
Figure 1
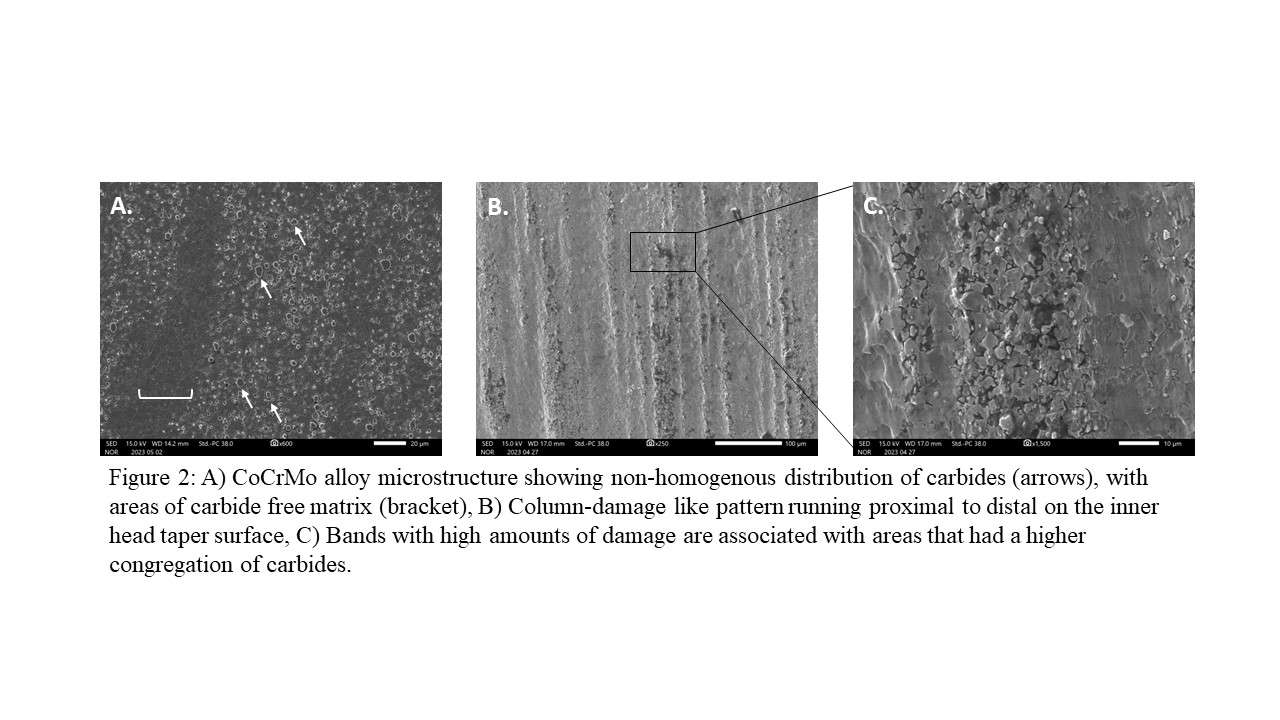
Figure 2#8607
Additively Manufactured Ti-6Al-4V Oxide Degradation Precipitates Pit Nucleation
Michael Kurtz - Clemson University - Charleston, USA
Kazzandra Alaniz - Clemson University - Charleston, USA
*Jeremy Gilbert - Clemson University - Charleston, USA
*Email: jlgilbe@clemson.edu
Introduction: Retrieval studies document selective dissolution on Ti-6Al-4V implants. We can reproduce this damage in the lab by combining cathodic activation and inflammatory species. While various conventionally manufactured titanium alloy damage modes have been characterized, little is known about the long-term in vivo behavior of additively manufactured (AM) Ti-6Al-4V. In this study, we expose AM Ti-6Al-4V to cathodic activation and inflammatory species, documenting changes to the alloy’s surface and impedance properties over time. We seek to answer the following research questions: (1) How might AM Ti-6Al-4V corrosion initiate and progress when exposed to adverse electrochemical events? (2) What changes occur at the oxide interface during corrosion? We hypothesize a mechanism where an initial degradation of the oxide film promotes corrosion at the metal-solution interface.
Methods: Mirror polished AM Ti-6Al-4V samples were cathodically activated at -0.4 V for 6 h, 12 h, 18 h, and 24 h. Electrolyte solution comprised of 0.1 M H2O2/phosphate buffered saline (PBS). Following electrochemical testing, sonicated samples were imaged using scanning electron microscopy (SEM). SEM micrographs were analyzed using ImageJ to quantify the extent of corrosion. Following a 60 s open circuit potential, and an initial baseline electrochemical impedance spectroscopy (EIS) recording, EIS was captured every hour for 24 h at -0.4 V. The near β alloy AM Ti-29Nb-21Zr was used as a comparison alloy. Captured EIS spectra were fit using a Randles constant phase element (CPE) circuit and the phase angle symmetry technique. Extracted circuit element values were plotted vs. time. All electrochemical tests were run in triplicate (n =3) except for the 24 h cathodic activation (n = 5). To determine the effect of time on dissolution, a one-way analysis of variance was used (α = 0.05).
Results: SEM micrographs document pit nucleation at 12 h (Figure 1A), a process that continues until 18 h (Figure 1B). At 24 h (Figure 1C) Existing pits grow in width, evidenced by the increase in contrast. ImageJ analysis of each micrograph shows a significant increase in the % dissolved after 24 h (Figure 1D, p = 0.001). Note the decrease in oxide polarization resistance (Rp) prior to pit initiation. AM Ti-6Al-4V Log adjusted Rp, CPE capacitance (Q, Q-2) and CPE exponent (α) decrease over time (Figure 2A-D). In contrast, AM Ti-29Nb-21Zr oxide function remains comparatively stable. Plots of Q vs. α reveal two clusters corresponding with dissolved and control surface states. As the oxide degrades and the AM Ti-6Al-4V surface dissolves, α decreases and Q increases.
Conclusion: Both the impedance and micrograph data suggest an in vitro dissolution mechanism that begins with the degradation of the oxide film, followed by pit nucleation, and then pit growth. This mix of qualitative and quantitative results support our original hypothesis. Additionally, the oxide’s CPE capacitance and exponent may be predictive of the surface state of the alloy. EIS spectra captured on rehydrated samples after dissolution fail to recover to pre-corroded levels. Future work will investigate this dissolution mechanism in more physiologically representative conditions.
Figures
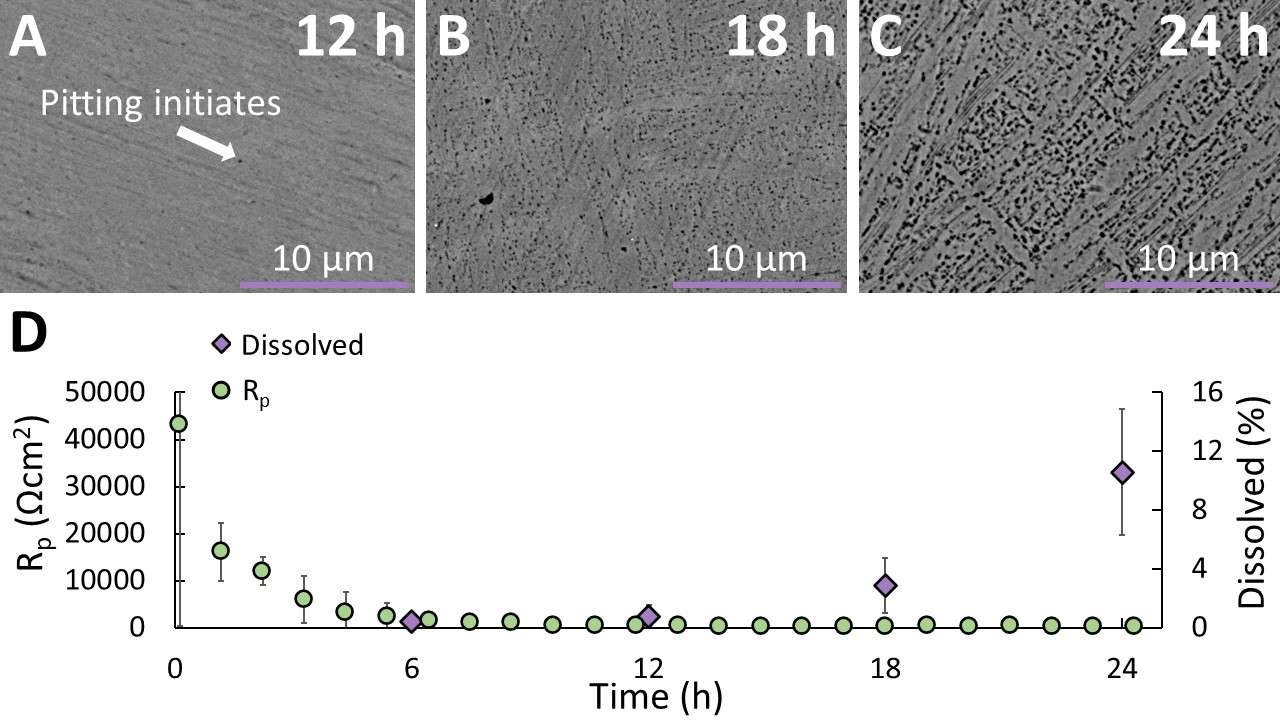
Figure 1
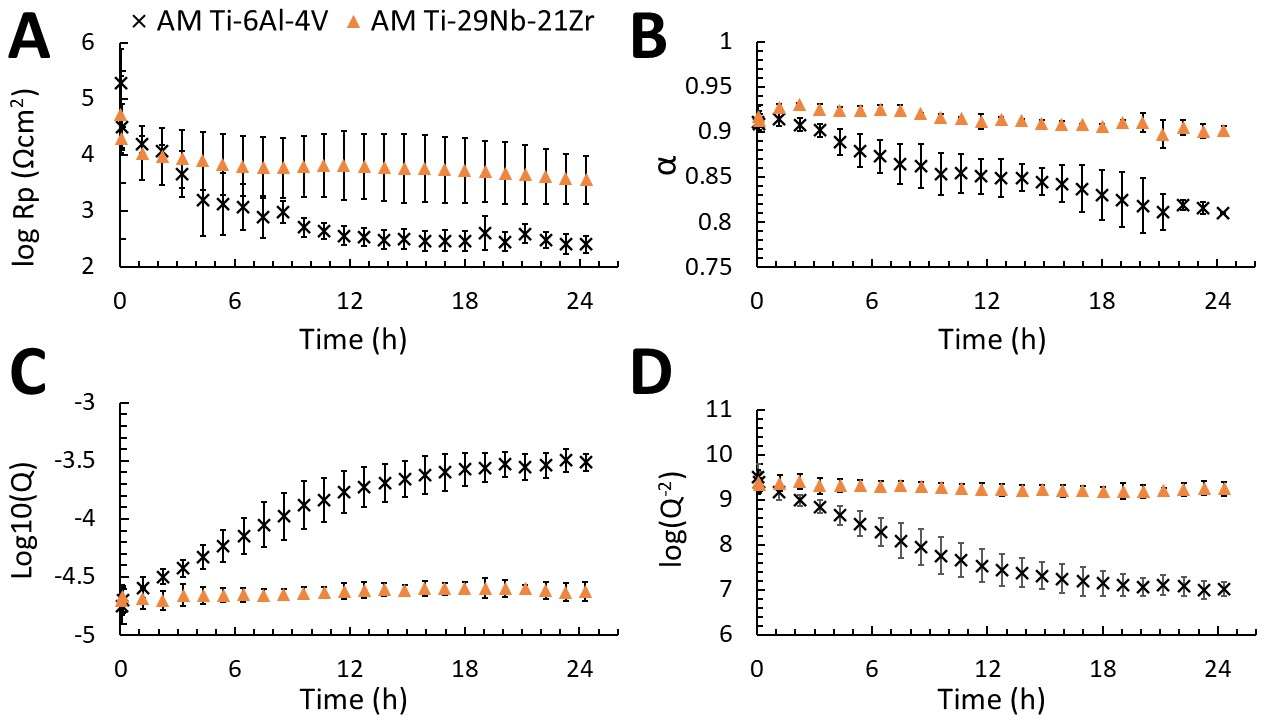
Figure 2
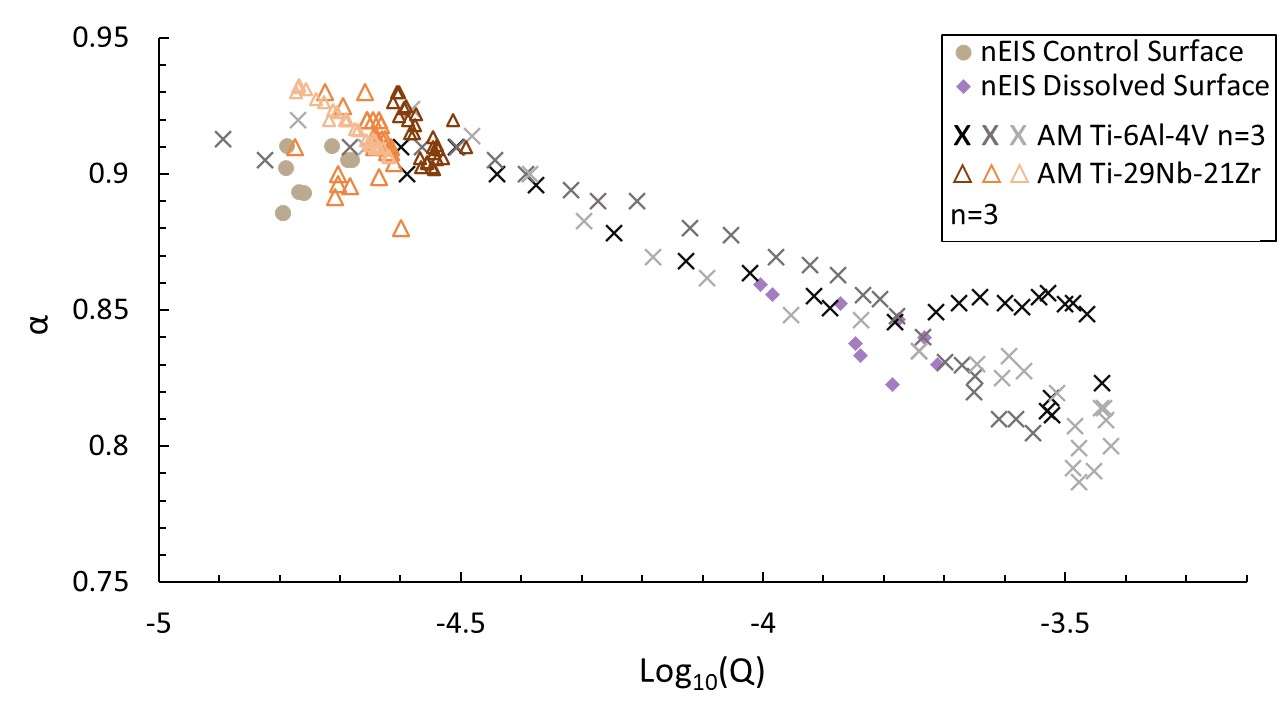
Figure 3#8581
The Effect on Fretting Corrosion of Four Taper Design Parameters Using Design of Experiments Principles and a Novel in-Vitro Fretting Current Test Method
*Adam Shallenberg - DJO Surgical - Austin, USA
Eric Ouellette - Exponent - Philadelphia, USA
*Email: adam.shallenberg@djoglobal.com
Introduction:
A Design of Experiments (“DOE”) approach was leveraged to determine the effects of stem taper length (10.2 vs. 16mm), diameter (11.7 vs. 12.7mm), surface finish (16 Ra vs. 3000 Rz (coarse microthread)), and neck angle (127° vs. 132°) on fretting corrosion in a modular femoral head taper junction.
Methods:
A four factor, full factorial DOE was designed to test the 16 unique trunnion combinations with a 32mm/-4mm offset CoCr modular head. The junction was assembled per F2009 except with a 4kN assembly load. Other factors that might influence fretting corrosion were held constant.
Specimens were assembled into a custom fixture and the junctions were submerged in 37° C neutral phosphate buffered saline (PBS). A three-electrode electrochemical cell was established using the stem trunnion sample as the working electrode with a potential of +50 mV vs. OCP.
Each assembly was exposed to a 1.0 MC fatigue regime (3000N, R=0.1, f=5hz) interrupted by a low frequency, incremental cyclic loading interval (180 cycles, 1000N-5000N increasing in 500N increments, R=0.1, f=1hz). The challenge load was applied after 0, 100,000, 500,000, and 1 MC fatigue cycles. Load and fretting current data were recorded during the low frequency intervals.
The current data was analyzed using Minitab. SEM/EDS analysis was performed on select specimens after disassembly to investigate the junction surfaces.
Results:
All test specimens experienced a reduction in fretting current after 1.0 MC. Surface finish was the only individual factor with a significant effect on fretting current in the new condition and after 1 MC. The coarse microthreaded surface finish resulted in lower fretting current. After 1.0 MC, long tapers with a large diameter and short tapers with a small diameter experienced lower fretting current. Configuration 5 (long/large/coarse/high angle) and Configuration 9 (short/small/smooth/low angle) had the lowest and highest currents, respectively.
SEM with elemental analysis on Configurations 5 and 9 identified oxides consistent with corrosion. The material in the Configuration 5 head (roughened stem trunnion) was focused in the regions of fretting while material in the Configuration 9 head (smooth stem trunnion) was evenly distributed. Flattening of the proximal microthread peaks, superimposed fretting damage, and some apparent CoCr transfer to the titanium trunnion were observed in Configuration 5. The machining marks of the Configuration 9 trunnion were substantially altered by fretting. Less corrosion product was apparent on the coarse microthread stem trunnions.
Conclusion:
We present a new hybrid fretting corrosion methodology that leverages aspects of previously published short-term incremental cyclic fretting corrosion tests, with a moderate length 1 MC fatigue style fretting corrosion test. This methodology provides additional information at multiple intervals in the test, which may not be available in 10 MC testing with an endpoint measurement using ICP-MS.
Within the range of values tested in this study, the coarse microthread surface finish was protective against fretting current especially when combined with a long/large or short/small stem trunnion. A unitization process of decreasing junction micromotion with increasing fatigue loading may explain the consistent reduction in fretting current with fatigue cycles regardless of taper design.
Figures

Figure 1
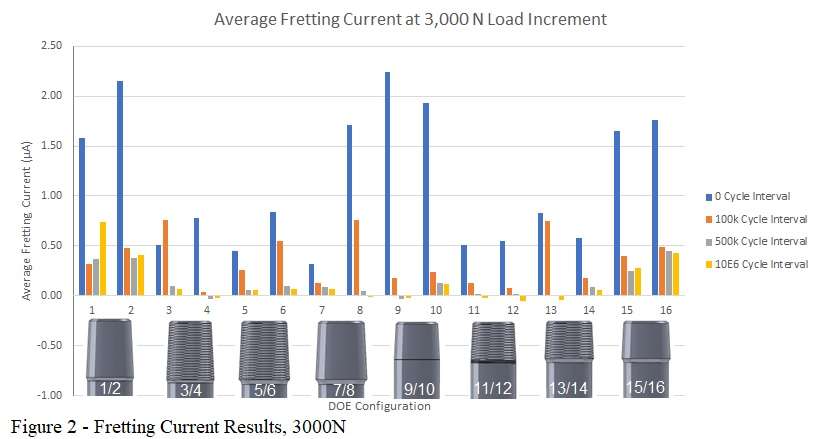
Figure 2
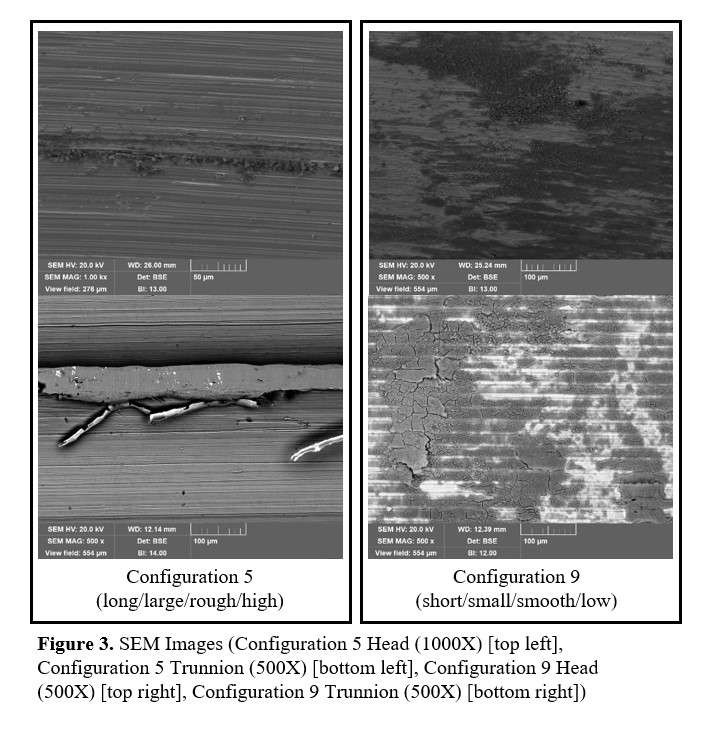
Figure 3#8615
Failure Following Revision Total Hip Arthroplasty After Cobalt-Chrome Femoral Heads Are Placed on a Retained Femoral Stem
*Gloria Coden - Bronx, United States of America
Nicholas Pagani - Tufts Medical Center - Boston, USA
David M. Ramsden - New England Baptist Hospital - Boston, USA
Thomas Zink - Tufts Medical Center - Boston, USA
Daniel Ward - New England Baptist Hospital - Boston, USA
James V Bono - Boston, USA
Carl Talmo - New England Baptist Hospital - Boston, USA
*Email: gscoden@gmail.com
Introduction: Failure due to trunnionosis has been reported with cobalt-chrome (CoCr) heads in total hip arthroplasty (THA). However, there is limited data on the use of these heads in the revision setting. The purpose of this study was to analyze the outcomes of patients who underwent revision THA with retained femoral component and received a CoCr femoral head on a used trunnion.
Methods: We retrospectively reviewed 107 revision THAs with a retained femoral component and received a CoCr femoral head with a V40 taper between February 2006 and March 2019. We compared these patients to 90 revision THAs with a retained femoral component who received a C-taper CoCr femoral head between May 2013 and June 2022. Demographics, implant details, and postoperative complications, including need for repeat revision were recorded. Univariable and multivariable analyses were performed. Odds ratios (OR) and 95% confidence intervals (CI) were calculated. Significance was set at p<0.05.
Results: Patients were more likely to undergo a repeat revision if they had a larger head size implanted (mean=35.7 versus 33.0 millimeters, OR=1.1, 95% CI=1.02-1.2, p=0.016), implantation with a titanium–molybdenum–zirconium–iron femoral stem compared to a titanium stem (26.9% versus 10.0%, OR=3.3, 95% CI=1.4-7.7, p=0.006), and implantation with a V40 taper head compared to a C-taper head (22.4% versus 6.7%, OR=4.0, 95% CI=1.6-10.4, p=0.004). A multivariable regression showed that a V40 taper CoCr head was a risk factor for undergoing a repeat revision compared to a C-taper head (OR=3.0, 95% CI=1.02-9.0, p=0.046), but not age (p=0.3), gender (p=0.3), body mass index (p=0.9), American Society of Anesthesiologists score (p=0.3), head size (p=0.1), head offset (p=0.5), cemented compared to a uncemented femoral stem (p=0.2), number of components revised (p=0.4, p=0.7), and metallosis during initial revision (p=0.9). Of the patients who underwent a repeat revision, patients implanted with a C-taper head underwent repeat revision sooner than with a V40 head (131.2 versus 1135.4 days, p=0.045).
Conclusion: The placement of a new V40 taper CoCr femoral head on a used trunnion during revision THA with retained femoral component carries a higher risk of reoperation than with a C-taper CoCr femoral head (22.4% versus 6.7%, p=0.004). Therefore, we urge caution with using a CoCr femoral head on a retained trunnion. A titanium–molybdenum–zirconium–iron femoral stem (p=0.006) and larger head size (mean=35.7 versus 33.0 millimeters, p=0.016) also increase the risk of repeat revision THA. We recommend that patients who were implanted with a V40 CoCr femoral head at revision THA undergo long term monitoring, since many of these patients only underwent repeat revision THA years after the initial revision.
#8601
Selective Dissolution of Additively Manufactured Ti-6Al-4V
Michael Kurtz - Clemson University - Charleston, USA
Peter Kurtz - Clemson University - Charleston, USA
Audrey Wessinger - Clemson University - Charleston, USA
Aldo Moreno-Reyes - Clemson University - Charleston, USA
*Jeremy Gilbert - Clemson University - Charleston, USA
*Email: jlgilbe@clemson.edu
Introduction: Additively manufactured (AM) titanium devices are implanted with increasing rates. At the device interface, AM generates complex geometries, promoting osseointegration. While early registry returns of AM titanium devices are favorable, the long-term in vivo response remains unknown.
Retrieval studies of conventionally manufactured titanium devices document severe corrosion including Ti-6Al-4V β phase dissolution. Previous studies reproduce this damage in vitro by combining negative potential excursions and inflammatory simulating solutions. In this study, we aim to answer the following questions: (1) Does the AM Ti-6Al-4V β phase selectively dissolve when exposed to cathodic activation and inflammatory species? (2) How does the oxide structure and function change following dissolution? We hypothesize that despite a different microstructure, AM Ti-6Al-4V will be susceptible to corrosion damage modes documented on conventionally manufactured devices.
Methods: Cylindrical Ti-6Al-4V samples were printed with an SLM 125HL machine, stress relieved at 600 oC for 3 h, and mirror-polished. Atomic force microscopy (AFM) quantified baseline oxide structure and nearfield impedance spectroscopy (nEIS) captured control spectra (n = 9). Backscattered electron (BSE) scanning electron microscopy (SEM) images of the alloy surface and precursor powder were acquired. Selective dissolution was induced by cathodically activating samples at -0.4 V for 48 h in 0.1 M H2O2/phosphate buffered saline solution (PBS, n = 3). Selective dissolution was confirmed using SEM. Post-test, AFM images of dissolved surfaces were captured at 50, 5, and 1 µm resolutions. Three nEIS spectra were acquired from three corroded samples (n = 9). Control and dissolved nEIS spectra were fit with a Randles constant phase element (CPE) circuit using phase angle symmetry. Statistical analysis of log-adjusted circuit element values was conducted using a two-sample t-test (α = 0.05).
Results: An SEM micrograph of the AM Ti-6Al-4V powder precursor (Figure 1A) reveals spherical particles of various widths. After printing and stress relieving, alloy microstructure (Figure 1B) appears martensitic with α lathes. AFM Height (Figure 1C) and deflection (Figure 1D) images show oxide domes. Note the relatively small scale of recorded heights. Following cathodic activation in 0.1 M H2O2/PBS solution, BSE and secondary micrographs (Figure 2A-B) document preferential dissolution of the β phase. Dark, curved crevices appear where prior β grains were dissolved from the surface. AFM height (Figure 2C-E) and deflection (Figure 2F-H) images confirm the β dissolution and depict an oxide with increased height variation. Bode plots (Figure 3A-B) show a phase angle shift for dissolved samples. Significant differences (p < 0.05) were recorded for circuit element values (Figure 3C-F).
Conclusion: The AM Ti-6Al-4V β phase selectively dissolved from the alloy surface when exposed to inflammatory species and cathodic activation, supporting our hypothesis. Structurally, the oxide films over dissolved and control surfaces appear similar, though differences in oxide height over dissolved surfaces was larger. Functionally, changes in the oxide film and alloy surface manifest as modifications to the oxide’s capacitance. Both the CPE parameter (Q) and exponent (α) may be predictive of the surface’s dissolution state, though further research is required.
Figures
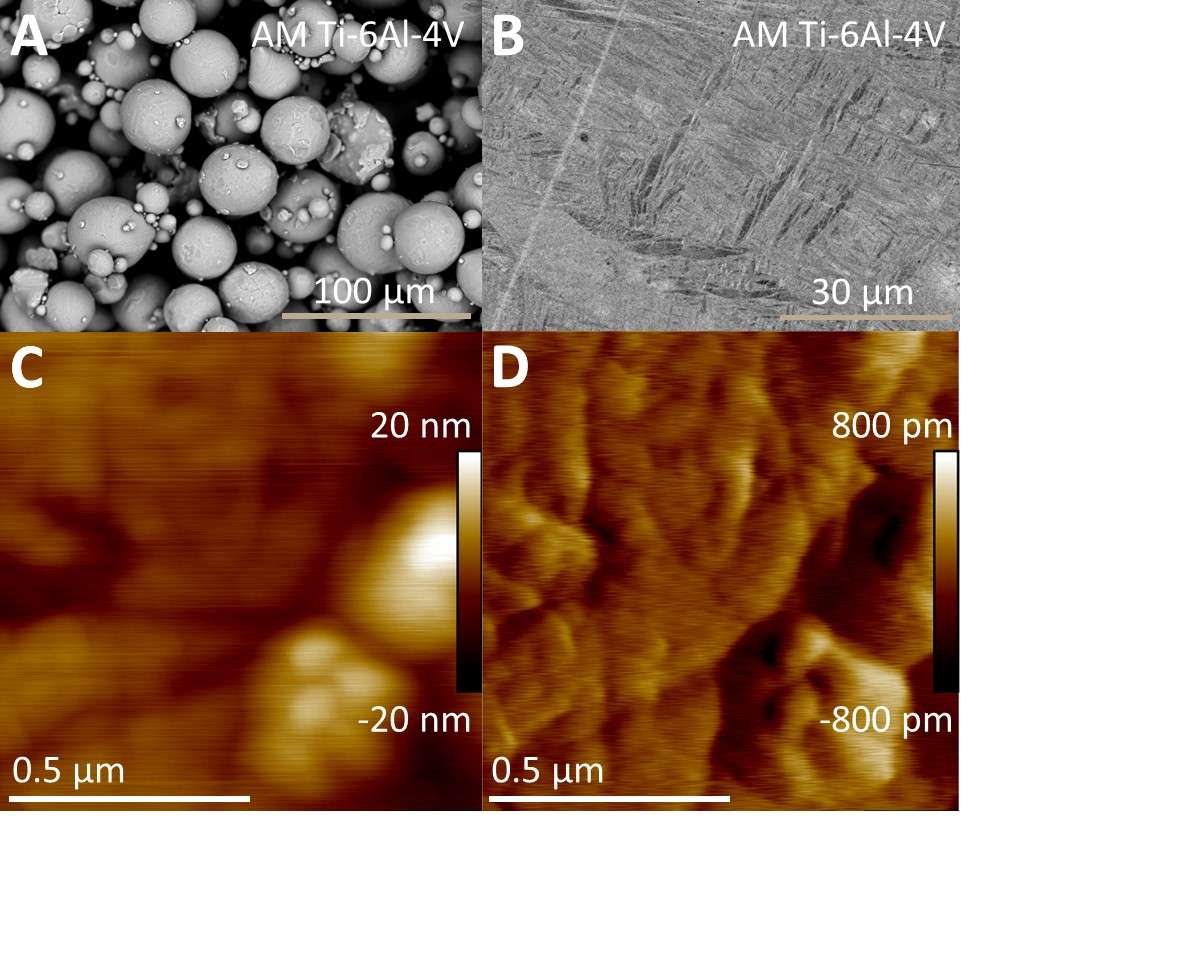
Figure 1
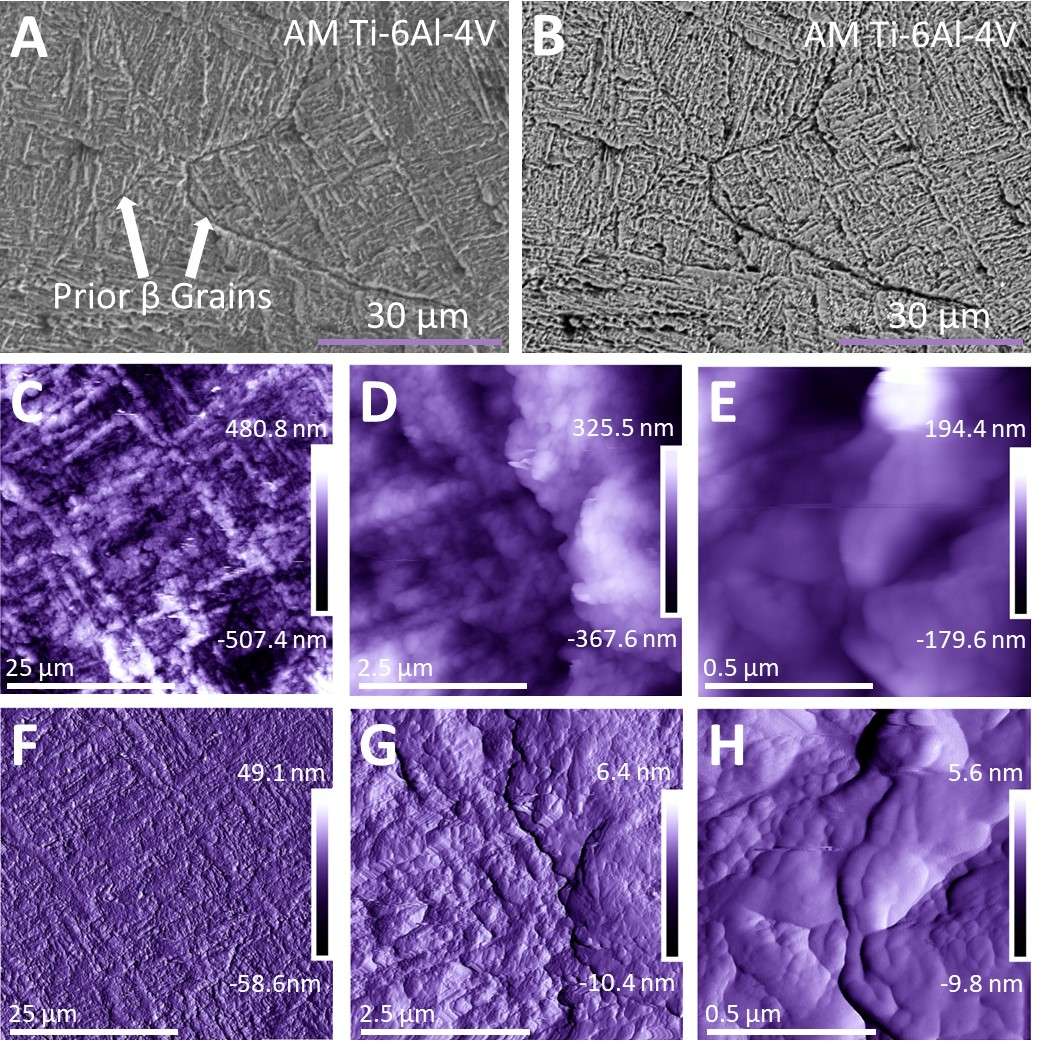
Figure 2
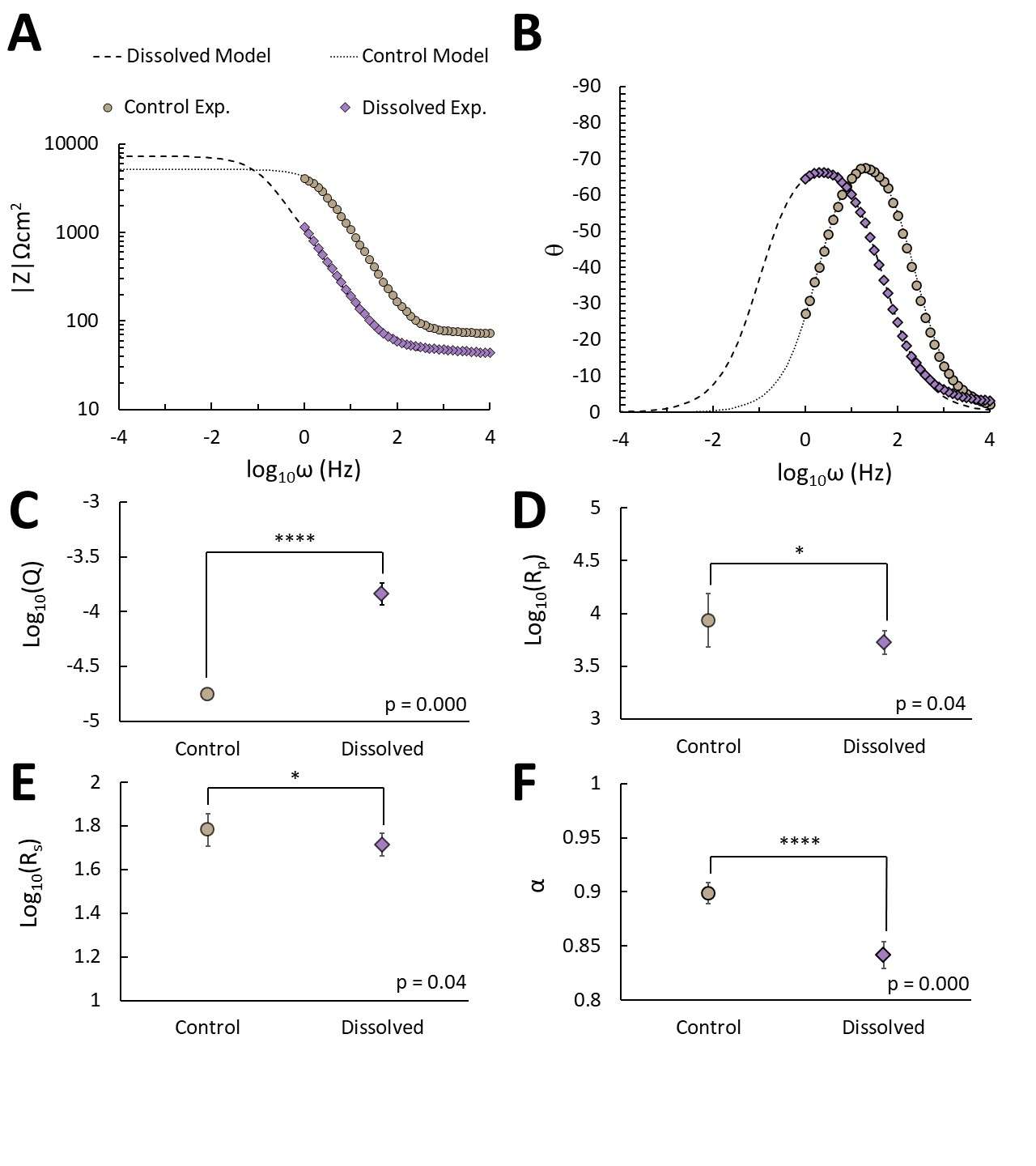
Figure 3#8355
Inducible Displacement of Cementless Femoral and Tibial Components at Multiple Squat Positions
*Rebecca Hext - Western University - London, Canada
Jordan Broberg - Western University - London, Canada
Brent Lanting - London Health Sciences Centre - London, Canada
Matthew Teeter - Western University - London, Canada
James Howard - London Health Sciences Centre - London, Canada
*Email: rhext@uwo.ca
Introduction: Cementless total knee replacement (TKR) designs have porous metal surfaces that allow for bone ingrowth to create a biological fixation mechanism that may provide an advantage for long-term fixation. While multiple studies utilizing radiostereometric analysis (RSA) have shown sufficient fixation of cementless components, few studies have investigated the inducible displacement. Of these studies, few if any investigate the displacement of both the femoral and tibial component. The goal of this study was to assess inducible displacement of cementless femoral and tibial TKR components during a knee bend.
Methods: Thirty-four patients were analyzed. Supine and weightbearing RSA exams were performed at 1-year post-operation. The weightbearing exams consisted of a standing exam (0°), and multiple squat positions with the operated knee at flexion angles of 20°, 40°, and 60°. Model-based RSA (MBRSA) software was used to measure inducible displacement, the micromotion measured between supine and weightbearing exams. Inducible displacements were reported as maximum total point motion (MTPM) and as 3D translations at different points of interest, called fictive points, placed around both the femoral and tibial component (Figure 1). The poses of the femoral and tibial components were also used to calculate the true flexion angle of the operated knee for each examination. Correlations between the flexion angle of the operated knee and inducible displacement were calculated.
Results: For inducible displacement of the femoral component, MTPM was 0.70 ± 0.36 mm at the 0° exam, 0.84 ± 0.46 mm at the 20° exam, 1.01 ± 0.45 mm at the 40° exam, and 1.20 ± 0.71 mm at the 60° exam. For inducible displacement of the tibial component, MTPM was 1.22 ± 0.67 mm at the 0° exam, 1.25 ± 0.46 mm at the 20° exam, 1.56 ± 0.41 mm at the 40° exam, and 1.63 ± 0.46 mm at the 60° exam. Inducible displacement of the femoral component increased with knee flexion angle, with the strongest correlations at the anterior flange tip (r2 = 0.18, p = 0.0001, Figure 2), medial peg (r2 = 0.15, p = 0.0005), and lateral posterior condyle fictive points (r2 = 0.13, p = 0.0013). Inducible displacement of the tibial component also increased with knee flexion angle, with the strongest correlations at the stem tip (r2 = 0.51, p < 0.0001, Figure 3), anterolateral (r2 = 0.22, p < 0.0001), and anteromedial fictive points (r2 = 0.19, p < 0.0001).
Conclusion: The MTPM for the standing displacements were, on average, within ranges expected for stable implants for both the femoral and tibial components. Tibial components had greater inducible displacements, on average, than femoral components. For both components, an increased knee flexion angle in a weightbearing squat position correlated with greater inducible displacements. The strongest correlation was observed at the anterior flange tip for the femoral component and at the stem tip for the tibial component. As many activities of daily living require ranges of knee flexion, it is important to consider the increased inducible displacement of cementless TKR components at higher knee flexion angles.
Figures
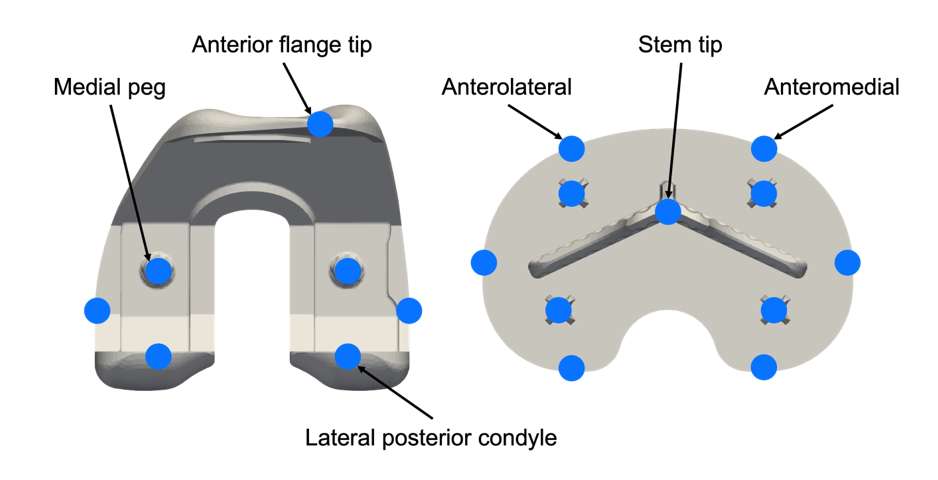
Figure 1
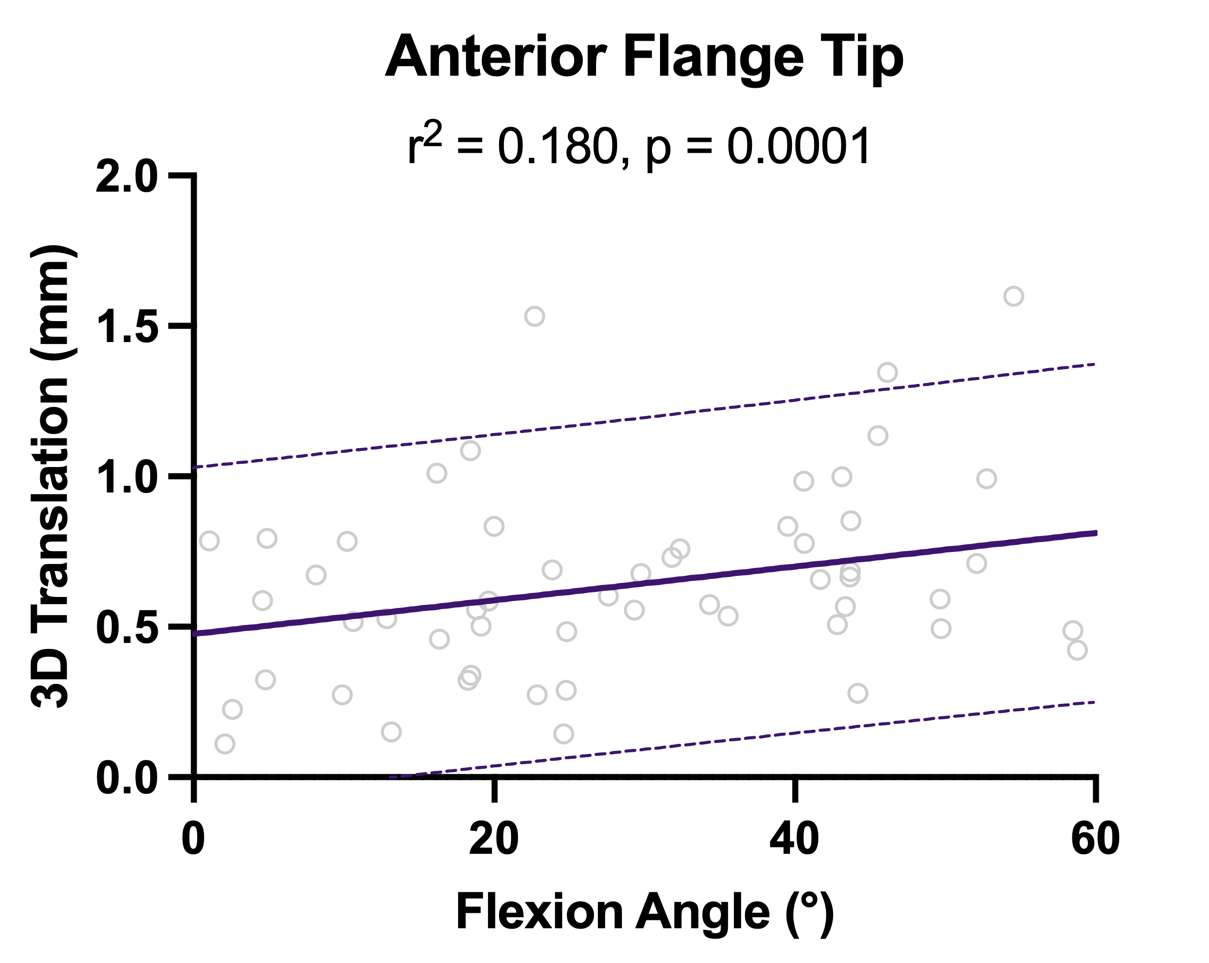
Figure 2
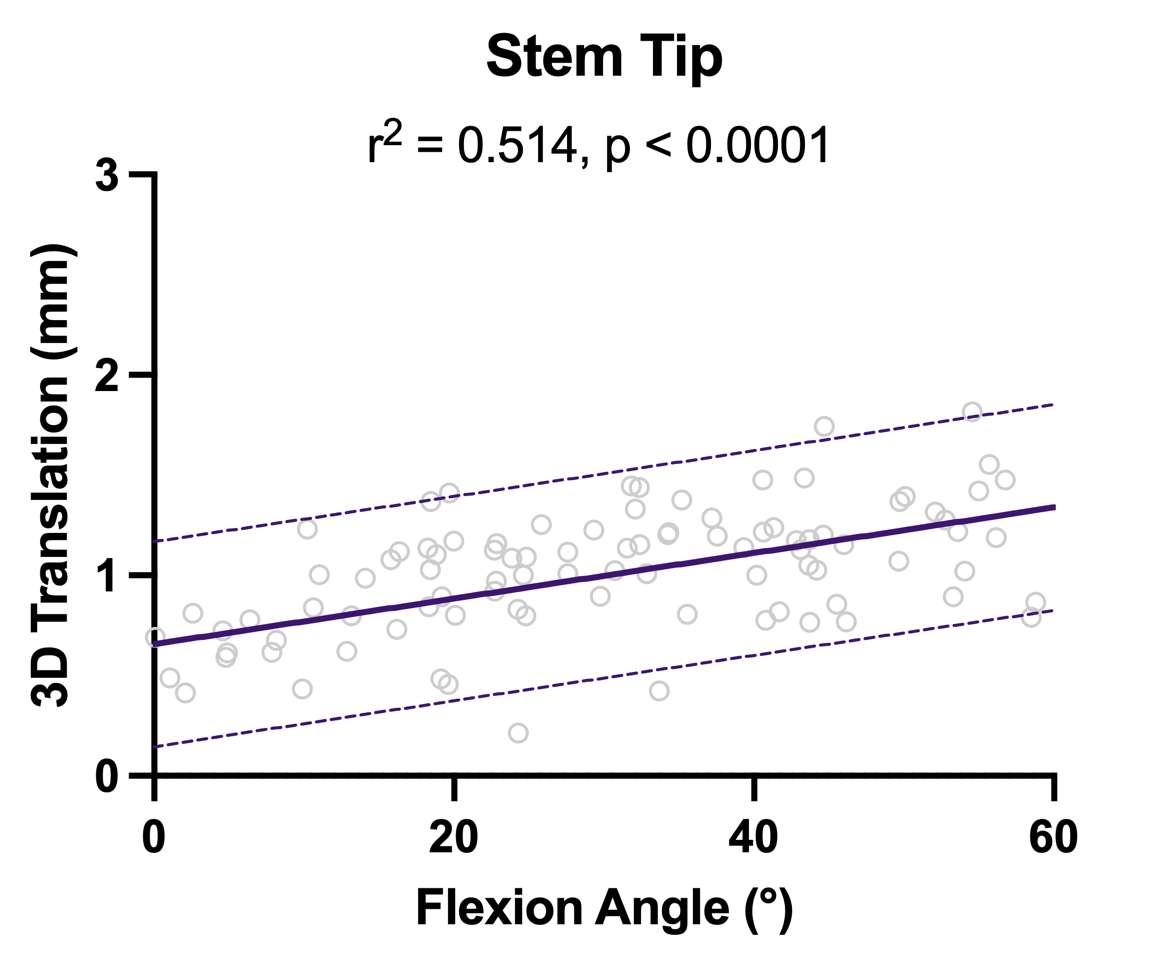
Figure 3#8124
Tibial Baseplate Migration Two Years After Unrestricted Kinematically Aligned Total Knee Arthroplasty Indicates Low Risk of Baseplate Loosening in Individual Patients
Abigail Niesen - University of California, Davis - Davis, USA
Pranav Tirumalai - University of California, Davis - Davis, USA
Stephen Howell
*Maury Hull - University of California Davis - Sacramento, USA
*Email: mlhull@ucdavis.edu
INTRODUCTION: In unrestricted kinematically aligned (KA) total knee arthroplasty (TKA), there are concerns about long-term tibial baseplate fixation because components are aligned to restore the patient’s pre-arthritic joint line regardless of the preoperative deformity, which can set the proximal medial tibial angle (PMTA) in varus according to mechanical alignment (MA) criteria. The possible increased stress at the baseplate-cement-bone interface from using a medially constrained (MC) implant design and adding constraint from retaining the posterior cruciate ligament retention (PCL) further heightens concern regarding long-term fixation. Using radiostererometric analysis (RSA) to quantify baseplate movement over time (termed migration), two stability limits that indicate long-term stability in individual patients are a change in maximum total point motion (ΔMTPM) (i.e., largest displacement of a point on the baseplate) between 1 and 2 years < 0.2 mm and anterior tilt < 0.8°. Accordingly, the purposes of this study were to determine for unrestricted KA+MC+PCL: (1) the number of patients who exhibited ΔMTPM > 0.2 mm, (2) the number of patients who exhibited anterior tilt > 0.8°, and (3) whether patient-specific alignment and loading variables were associated with migration in six degrees of freedom (6DOF) or in MTPM.
METHODS: Thirty-five patients underwent unrestricted, cemented, caliper-verified KA TKA with an MC implant and PCL retention (GMK Sphere, Medacta International, Castel San Pietro, Switzerland). Biplanar radiographs were acquired postoperatively on the day of surgery and at 1.5, 3, 6, 12, and 24 months. Baseplate migration in 6DOF and the MTPM were determined at each follow-up time point relative to the day of surgery using model-based RSA to identify those patients with migration above the two stability limits. The PMTA, posterior tibial slope angle (PTSA), sex, and body mass index (BMI) were analyzed for an association with migration in 6DOF and in MTPM using linear mixed models. Migrations in 6DOF were normally distributed. However, for MTPM, a log base 10 transformation was applied to achieve a normal distribution using a Shapiro-Wilk test. Significance was p < 0.05.
RESULTS: Preoperative age, body mass index, and Oxford Knee Score (OKS) were 68 ± 7 years, 31 ± 5 kg/m2, and 23 ± 8 (mean ± SD), respectively. Thirty-two of the 35 patients were available for analysis at 2 years. One of 32 patients exhibited positive ΔMTPM > 0.2 mm (Fig 1) and anterior tilt > 0.8° (Fig 2) though the postoperative OKS of 44 points indicated high function. PMTA, PTSA, sex, and BMI were not associated with baseplate migration in 6DOF or the MTPM (p ≥ 0.0905).
CONCLUSION: After unrestricted KA TKA using a MC insert design and PCL retention, results showed low risk of long-term baseplate loosening as 31 of 32 patients exhibited tibial baseplate migration below the two stability limits. The low risk of loosening regardless of the postoperative PMTA counters the MA TKA experience that varus alignment promotes early loosening.
Figures
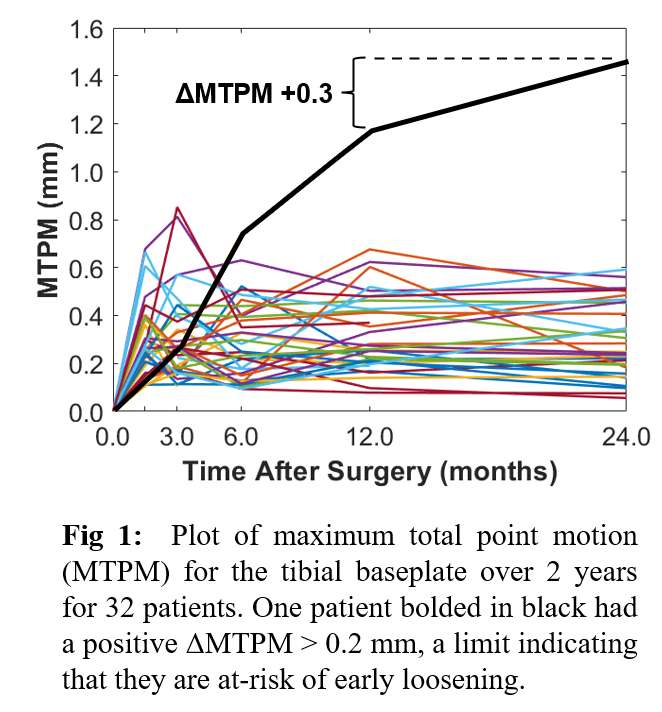
Figure 1
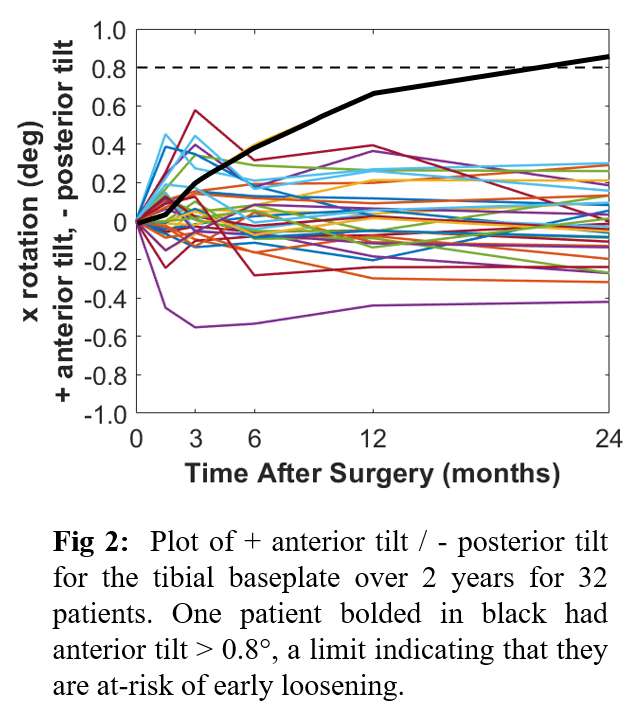
Figure 2#8486
Preclinical Testing of Stemless Shoulder Implant Stability
*Kyle Snethen - Zimmer Biomet - Warsaw, USA
Yang Son - Zimmer Biomet - Warsaw, USA
Christine Mueri - Zimmer Biomet - Winterthur, Switzerland
Marc Bandi - Zimmer GmbH - Winterthur, Switzerland
Jeffrey Bischoff - Zimmer, Inc. - Warsaw, USA
*Email: kyle.snethen@zimmerbiomet.com
Introduction: Stemless total shoulder arthroplasty (TSA) has increased in acceptance over the past decade. Stemless humeral implants facilitate bone preservation, reduce stress shielding and decrease surgical time compared to traditional stemmed implants but require adequate initial stability between implant surfaces and proximal humeral bone to support osseointegration for long-term fixation. Standardized testing provides guidance to device manufacturers to prove adequate risk mitigation and allows regulatory bodies to benchmark performance between devices. There are currently no consensus technical standards for assessing the initial stability of stemless shoulder implants. The objective of the present study was to develop a test method for assessing the initial stability of a stemless humeral implant for application in anatomic TSA.
Methods: Twelve samples consisted of a Comprehensive® Nano Stemless Shoulder (Zimmer Biomet, Warsaw, IN) humeral component, an assembled humeral head and implanted into 20PCF cellular rigid polyurethane foam (Sawbones®) prepared with surgical instruments. Samples were evenly divided into two groups to simulate different surgical conditions and evaluate if the test could distinguish between more and less adverse conditions regarding implant stability. The first group exhibited nominal conditions with the humeral head flush against the resection and the second group exhibited worst-case conditions with a 2mm gap between the humeral head and the resection and a 1cm cyst in the broach cavity. Initial stability was characterized as the relative motion of the humeral head to the adjacent foam surface, or micromotion, during cyclic loading. Three load steps of 100N, 500N and 870N were applied to settle the implant and simulate different levels of activity. Cyclic loading was applied (R=0.1 @ 1Hz) at 30o with respect to the resection to the humeral head via a compatible polyethylene bearing for 100 cycles each load step. Digital image correlation tracked the motion of the humeral head and foam surface during loading (Figure 1). Micromotion was evaluated at cycles 1, 25, 50, 75 and 100 for the 870N load step and normalized to the average nominal micromotion at cycle 100. A two-way ANOVA and Tukey’s post-hoc pairwise comparisons determined if cycle count was a significant factor (α=0.05) on the micromotion comparison between surgical conditions.
Results: The maximum micromotion occurred at the inferior region of the humeral head for all test samples. Averaged micromotion for both test groups converged within 5% by the 75th load cycle (Figure 2). The worst-case surgical condition exhibited significantly greater micromotion than the nominal condition across all cycle counts with average micromotion 2.2 times greater at the 100th cycle.
Conclusion: A test method was developed for evaluating the initial fixation of stemless TSA and demonstrated the ability to distinguish between surgical conditions expected to influence implant stability. The Nano stemless shoulder was chosen for evaluation due to extensive history of clinical success. It is recognized that many experimental factors can influence micromotion measurements; thus, the developed method is intended for comparative testing between devices and not against an absolute micromotion criterion potentially providing the foundation for a standardized test for assessing initial stability of stemless TSA.
Figures
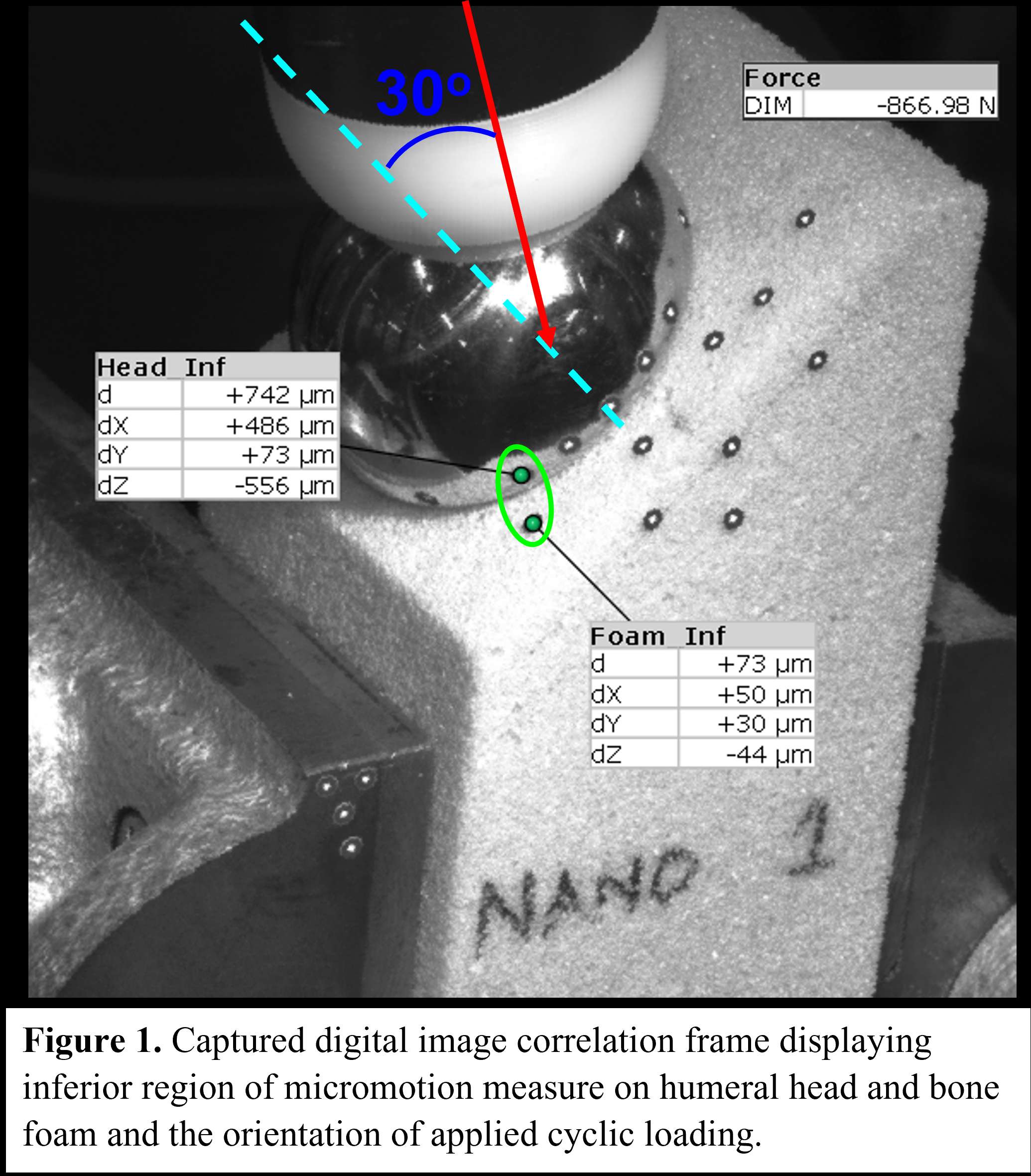
Figure 1
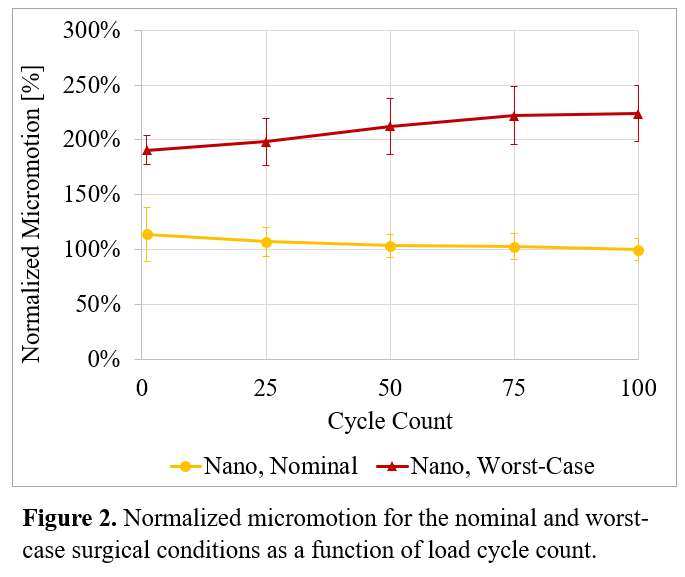
Figure 2#8744
Comparing the Kinematics of TKA During Quadriceps-Controlled Squat Simulations Versus Directly Controlled Flexion With Superimposed Quadriceps Forces
*Alexandre Galley - Western University - London, Canada
Ilya Borukhov - Stryker Orthopedics - Mahwah, USA
Brent Lanting - London Health Sciences Centre - London, Canada
Ryan Willing - Western University - London, Canada
*Email: agalley@uwo.ca
Introduction: Conventional joint motion simulators for pre-clinical testing of total knee arthroplasty (TKA) designs manipulate the knee using force, displacement, or simulated muscle control. For the first time, a rig capable of all three control modes but is currently under development [1]. The muscle actuator system (MAS) can generate gravity-dependent, quadriceps-controlled motions and is mounted onto a force/displacement-controlled robotic knee testing system (VIVO, AMTI, Watertown, MA, USA). The purpose of this work is to compare the kinematics of the knee during squats when flexion is muscle-driven and gravity-dependent, versus squats where muscle forces are still applied but flexion is prescribed.
Methods: A phantom TKA joint comprising cruciate sacrificing components (Triathlon PS, Size 4, Left, Stryker Orthopaedics, Mahwah, NJ, USA), and a Kevlar® strap extensor mechanism with a patellar button (Triathlon X3 Symmetric patella, 31mm x 9mm, Stryker Orthopaedics, Mahwah, NJ, USA) was mounted to the joint motion simulator (Figure 1). In the first test, a baseline continuous motion was produced by directly controlling joint flexion/extension from 20°-100°, with a 60N compressive force applied and all remaining degrees of freedom (DoF) maintained at 0N/0Nm. In the second test, flexion angle was controlled indirectly by the quadriceps. The quadriceps actuator applied an extension torque that acted in opposition to a flexion torque created by a 60N gravity force acting from the hip to the ankle. Quadriceps forces at 5° increments of flexion were extracted. For the third test, the joint flexion angle was controlled directly by the VIVO. The corresponding flexion angle-dependent quadriceps forces measured in the second test were applied. Tibiofemoral (TF) and patellofemoral (PF) kinematics were measured using an optical tracking system (Optotrak Certus, NDI, Waterloo, ON, Canada) and compared using mean signed differences (PF kinematics from the first test were neglected as no quads forces were used).
Results: Flexion tests that included a quadriceps (2nd and 3rd) resulted in mean anteriorly offset tibial positioning when compared to the baseline (7.1 ± 0.9mm and 6.4 ± 1.2mm, respectively), and the anterior/posterior (AP) kinematics of those two tests were indistinguishable (-0.7 ± 0.9mm) (Table 1). Mean differences for internal/external (IE) kinematics are less severe between tests, and varus/valgus (VV) kinematics are nearly uniform across the three tested motions. Patellofemoral kinematic differences between the 2nd and 3rd tests are small, recorded in terms of medial/lateral (ML) patellar translation (0.3 ± 0.2mm) and patellar flexion with respect to the femur (0.2 ± 0.9°).
Conclusions: Tibiofemoral kinematics between muscle-driven flexion (2nd test) and flexion being prescribed with identical forces present (3rd test) are in close agreement. Both differ from the baseline (no muscles) in terms of TF kinematics considered. The baseline cannot be used for a comparison of PF kinematics, as no quads forces were used. However, these results demonstrate that, if accurate quadriceps forces are known a priori, actually driving flexion motions in closed-loop quadriceps force control may not be necessary, as similar TF and PF kinematics can be generated.
References:
[1] A. Galley et al. (2023). ORS 2023 Annual Meeting. Paper #853.
Figures
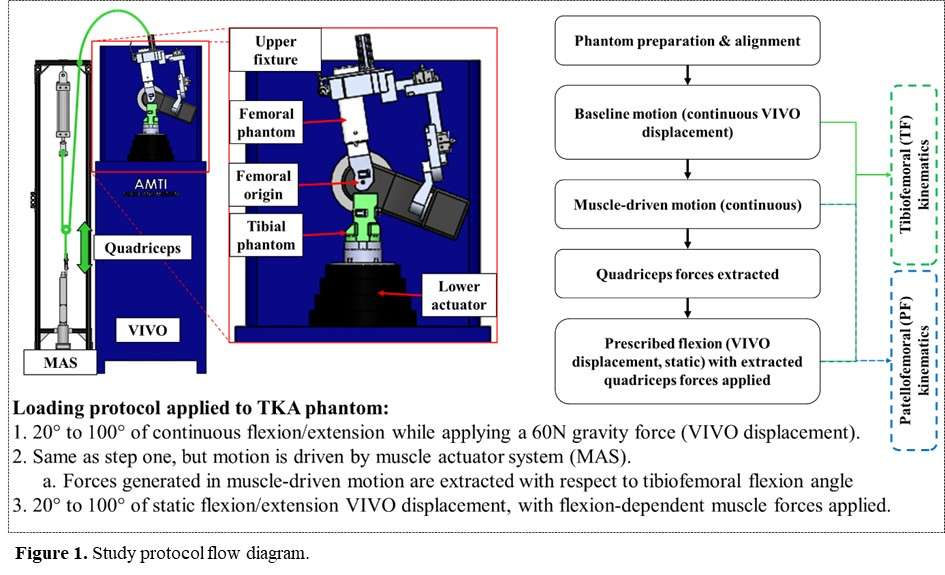
Figure 1
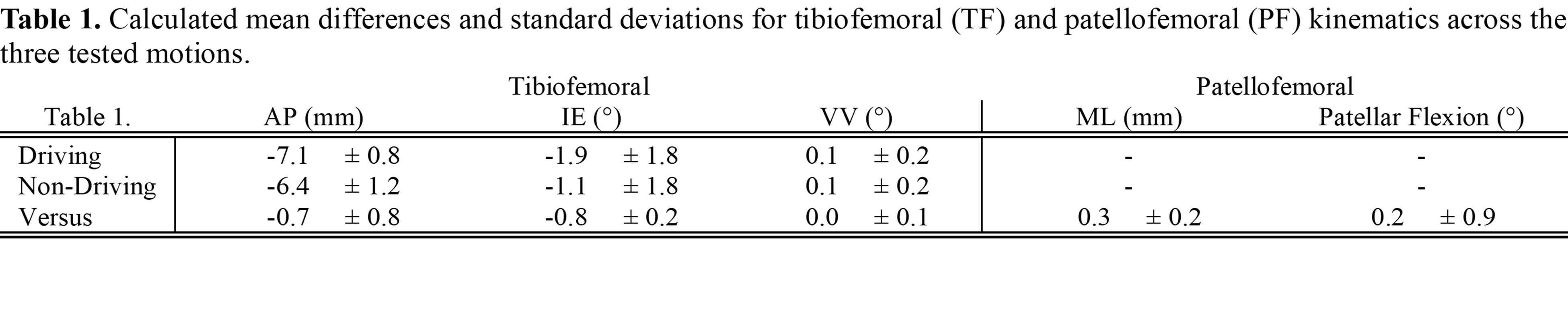
Figure 2
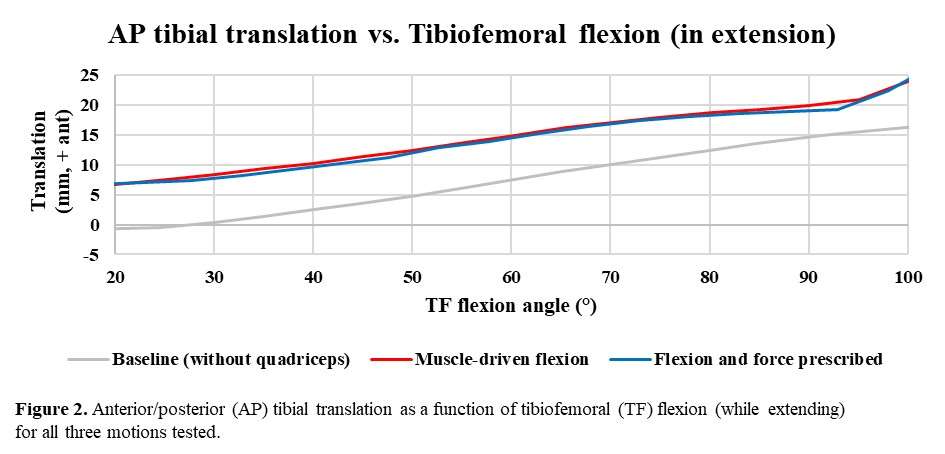
Figure 3#8354
In-Vitro Comparison of Glenoid Cartilage Wear on Metal and Ceramic in Shoulder Hemiarthroplasty
*Hazimah Mahmud - Imperial College London - London, United Kingdom
Dong Wang - University of Exeter - Exeter, GB
Anthony Bull - Imperial College - United Kingdom
Roger JH Emery - Imperial College - London, United Kingdom
Andrew Amis - Imperial College of London - London, UK
Peter Reilly - Imperial College London - London, United Kingdom
Ulrich Hansen - Imperial College London - London, UK
*Email: siti.mahmud18@imperial.ac.uk
While hemiarthroplasty improves shoulder functions and provides pain relief in patients, it has a high revision rate of up to 30% at 9 years after surgery [1], of which 83% of the revisions was for painful glenoid erosion. To improve hemiarthroplasty outcomes by minimising glenoid wear, ceramic has been gaining attention as an alternative counter-bearing surface to metal because it is hard, inert, abrasion-resistant and is not subject to gradual surface roughness changes as with metal [2]. Ceramic is also more wettable than metal, providing better joint lubrication and so, less adhesive wear on the glenoid [2]. There are limited studies evaluating implant materials on in-vitro glenoid wear during realistic joint articulation. Therefore, this study aimed to compare the in-vitro glenoid cartilage wear on metal and ceramic counterface using a shoulder wear simulator.
17 fresh-frozen human cadaveric shoulders (MedCure) were assigned to the Al2O3 ceramic group (4 males, 4 females, age 58.3 ± 3.7 years) and the CoCr metal group (4 males, 5 females, age 61.7 ± 3.4 years). The resected glenoid and the size-matched humeral head implant (Mathys) were set up in a six-station shoulder wear simulator to replicate ‘washing the opposite axilla’, a low-load daily activity, by applying the appropriate joint motion and loading profiles obtained from an earlier musculoskeletal modelling study. The joint was kept lubricated by filling the test cell with 500mL diluted calf serum and 15ppm Pro-Clin 300 as the anti-microbial agent. Each wear test was performed for a total of 500,000 cycles at 1.2Hz. At every interval of 125,000 cycles, the glenoid was imaged with a micro-computed tomography (Versa 510, Zeiss), then processed in Fiji (ImageJ) and Matlab R2019b software to characterise the glenoid wear by calculating the change in cartilage thickness (Figure 1). Statistical analyses were performed in GraphPad Prism, with a p ≤ 0.05 level of significance.
The mean thickness of the native cartilage prior to wear testing was 2.2 ± 0.4mm for the ceramic group and 2.1 ± 0.4mm for the metal group. At the end of the wear test, the cartilage thickness decreased significantly to 1.6 ± 0.4mm (p = 0.011) for ceramic and 1.5 ± 0.4mm (p = 0.0075) for metal. The mean cartilage wear between the ceramic and metal was not significantly different through the wear test (p > 0.05), but when looking at the individual wear data (Figure 2), the wear for ceramic tests had a very large variation, making it more unpredictable than metal. The region with the most wear was inconsistent between the two groups (Figure 3): the wear for ceramic was highest at the inferior-anterior region and the wear for metal was highest at the superior-anterior region, although they are not significantly different to other regions (p > 0.05).
Ceramic has better tribological properties than metal, but ceramic had a less predictable glenoid cartilage wear behaviour. This study did not find evidence that the use of ceramic in shoulder hemiarthroplasty with healthy cartilage is a much better alternative to conventional metal humeral head.
Figures
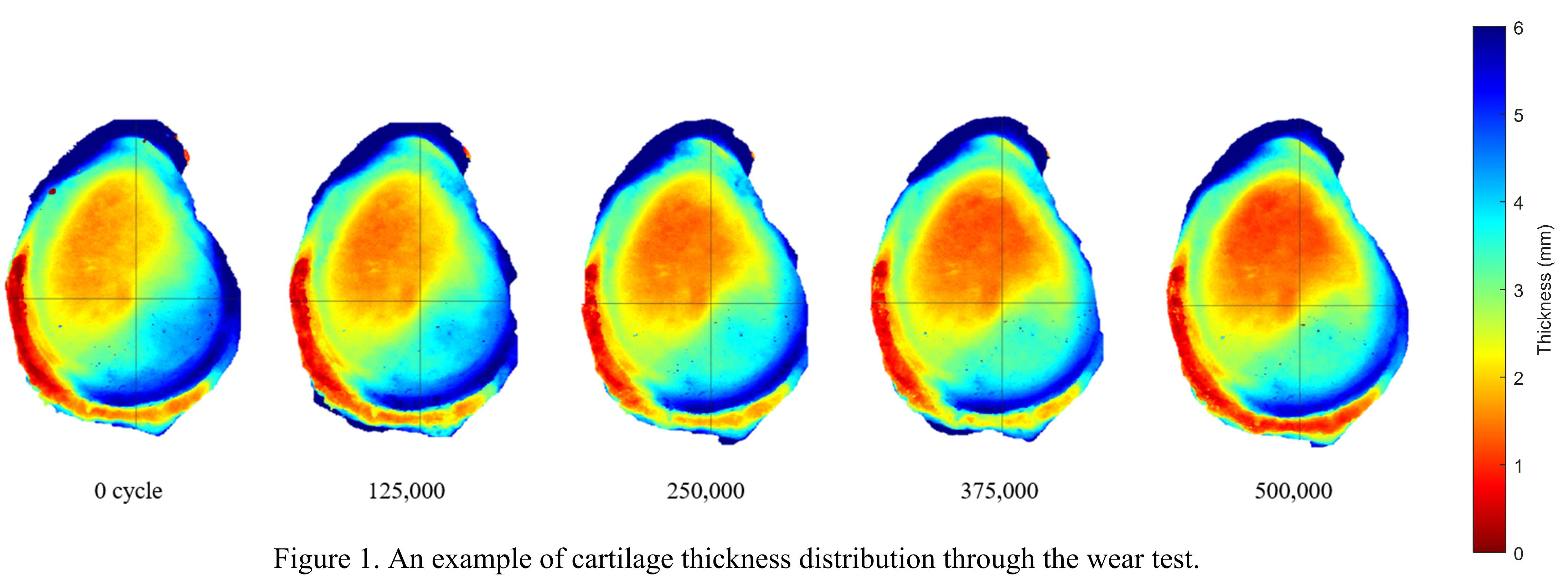
Figure 1
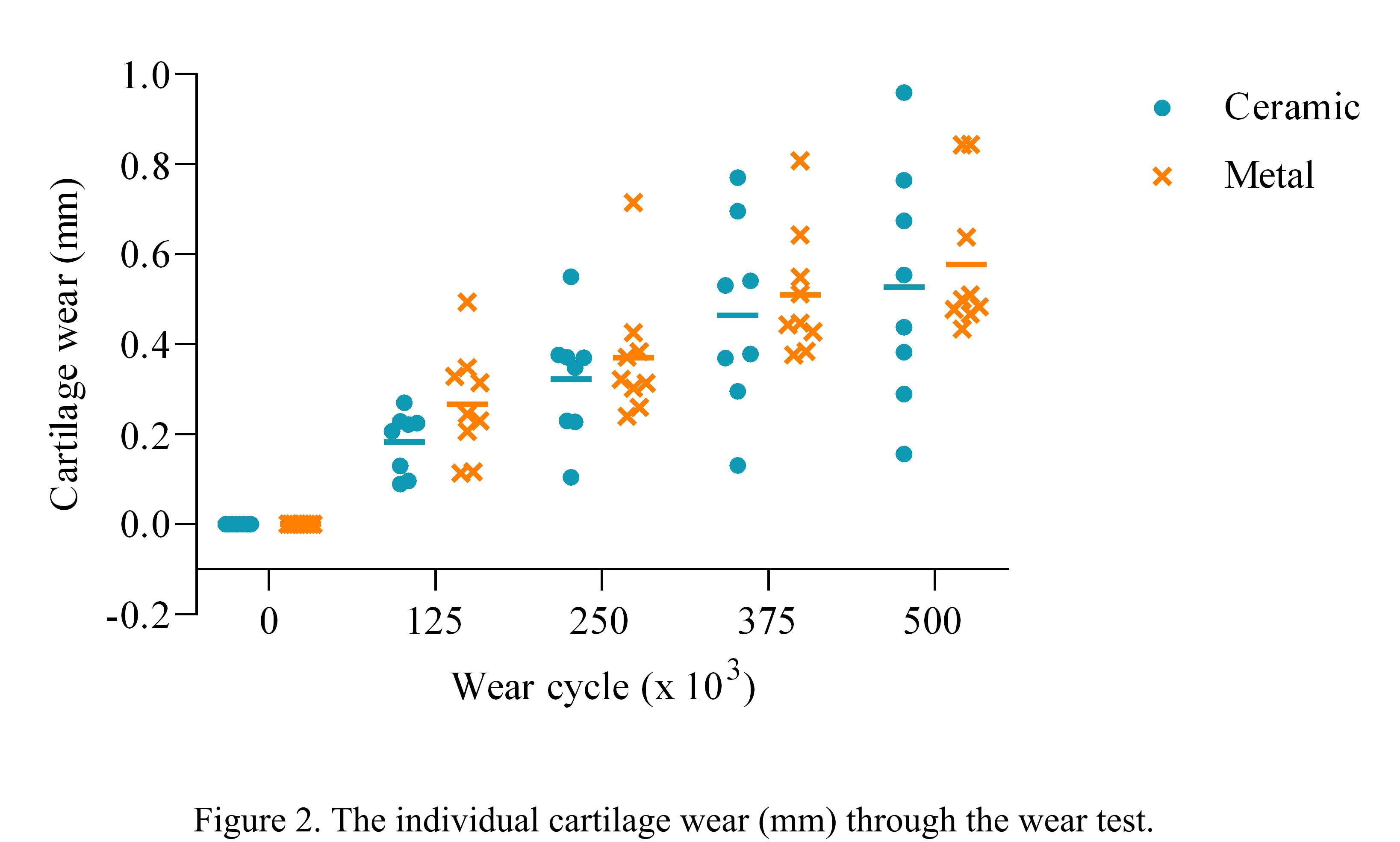
Figure 2
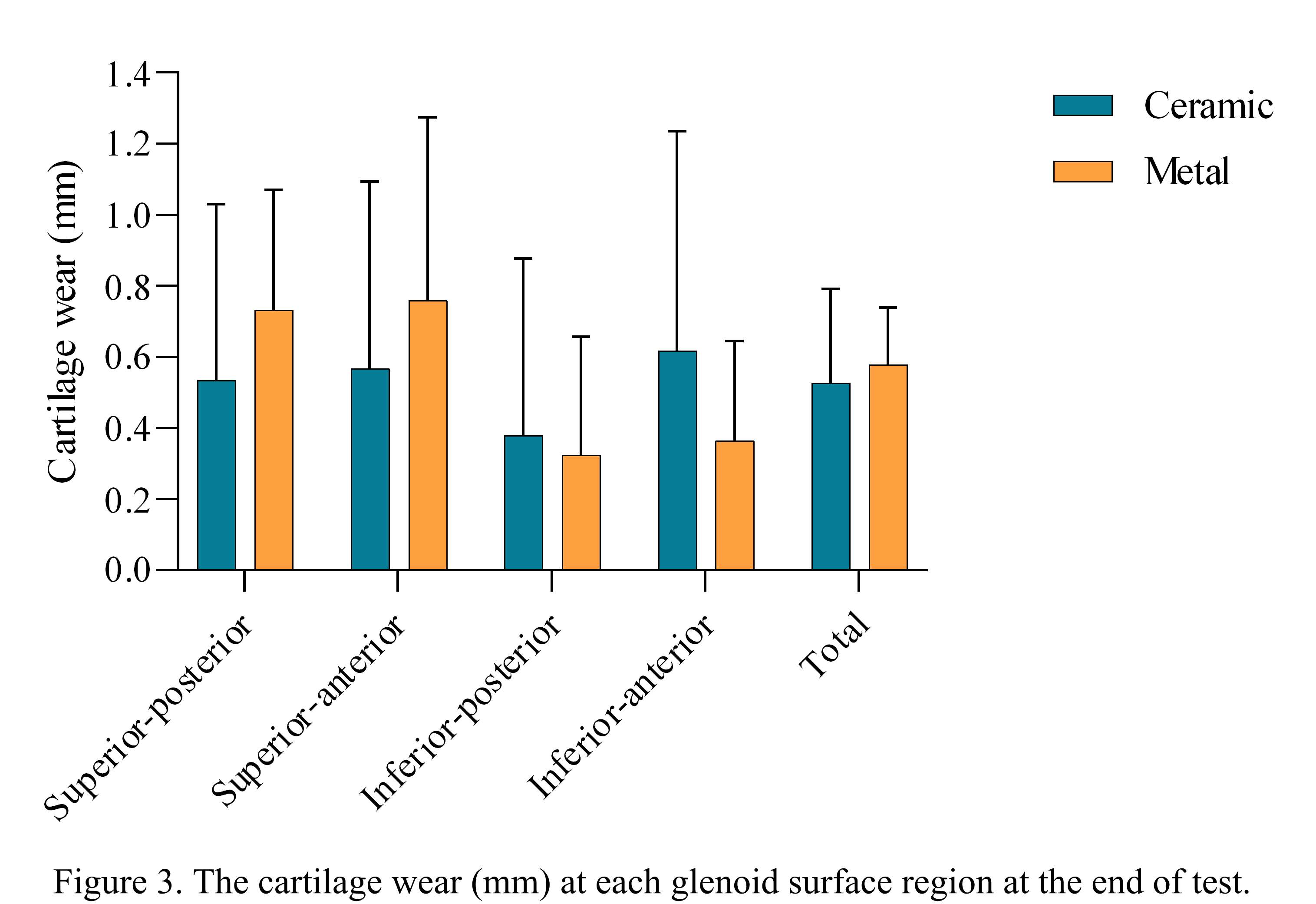
Figure 3#8386
Primary Stability of a New TendoClip Fixation Procedure for Tuberosity Reattachment on Total Shoulder Endoprothesis
*Maximilian Uhler - Laboratory of Biomechnics and Implant Research - Heidelberg, Germany
Thilo Patzer - Schoen Clinic Dusseldorf - Dusseldorf, Germany
Joerg Fleischer - Marienhaus Clinic St. Wendel - St. Wendel, Germany
Daniel Knopp - Heinrich Heine University Dusseldorf - Dusseldorf, Germany
Philippe Kretzer - Laboratory of Biomechanics and Implant Research, University of Heidelberg - Heidelberg, Germany
*Email: maximilian.uhler@med.uni-heidelberg.de
Introduction:
The refixation of the tuberosity in inverse shoulder endoprosthesis leads to a significant improvement in function with regard to stability and active rotation. However, tuberosity reattachment with the classic 3D suture cerclage is time-consuming and often leads only to insufficient stability and thus poor healing. The new Tendo Clip technique with screw-retained titanium clips enables simpler and time-saving refixation of the tuberosities with sufficient primary stability.
Methods:
The present study investigated two different refixation procedures for the treatment of 4-fragment fractures of the proximal humerus using a biomechanical test. For this purpose, 8 paired humerus specimens were treated with inverse shoulder endoprostheses. After randomization into two treatment groups, the manually created 4-fragment fractures according to Neer (1970) were refixed using conventional 3D suture cerclages on one side. The other treatment group was characterized by fixation of the tubercle fragments using a novel TendoClip procedure. The fixation of the fracture fragments in cerclage restorations was attached to the prosthesis body with conventional fixation threads according to the manufacturer's instructions. In the TendoClip restoration, fixation was based on a screw connection (see Figure 1).
For the experimental comparison of the different restoration methods, it was first necessary to develop a suitable test setup and define a loading pattern. The specimens of both treatment groups were subjected to a cyclically increased load. The torques generated by a single-axis hydraulic material testing machine were converted into tensile forces by the test setup, whereby these were physiologically introduced via the rotator cuff muscle tendons remaining on the bone fragments. The cyclically increased load was applied until failure of the respective fixation. During the investigations, the relative movements of the individual fracture fragments to the stem of the humerus were measured as an indicator of the primary stability of the fitting procedure.
Results:
It could be demonstrated that the tensile forces generated in the first 4 load levels resulted in a significant difference when considering the relative motions between the TendoClip and Cerclage groups. The experimental studies showed that 5 out of 8 specimens in the TendoClip group could be loaded for more than 1000 cycles (10 load levels). In the cerclage group, this was only achieved on one preparation. The recorded failure mechanisms also showed that the fixed bone fragments of the Cerclage procedure were significantly more frequently affected by loosening and visible relative movements compared to the TendoClip procedure (see Figure 1 and Figure 2). Nevertheless, limitations of the TendoClip restoration could also be observed. In 2 of 8 cases, a bone fragment was torn out of the fixation.
Conclusion:
The present study has concluded that the presented TendoClip procedure can be an alternative to conventional fixation procedures in multifragmentary proximal humerus fractures and can improve the fixation of the tuberosity fragments. This was demonstrated by the measurement results of the relative movements between the individual bone fragments.
Figures
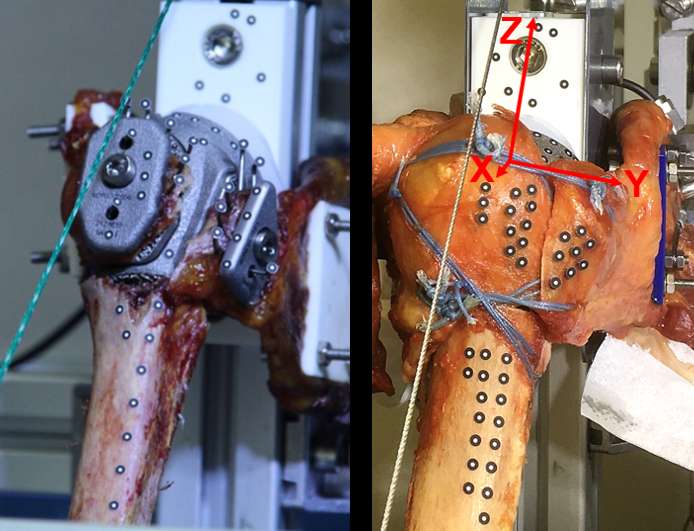
Figure 1
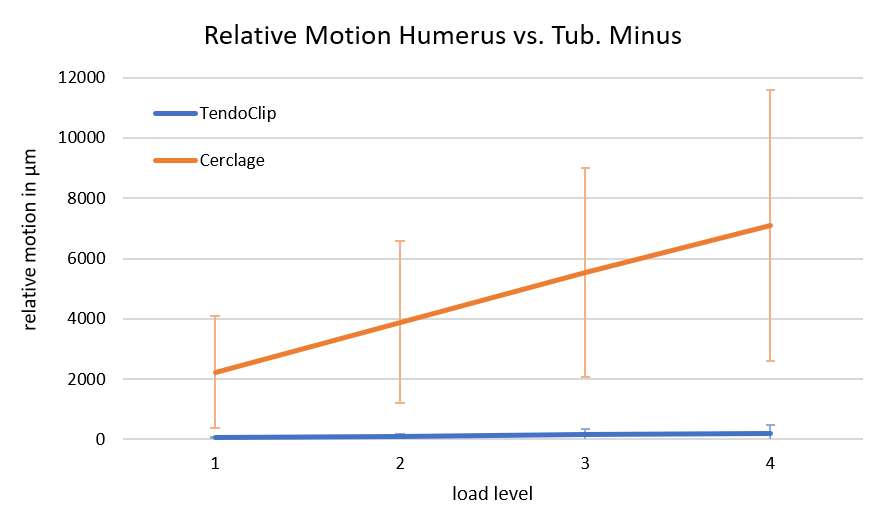
Figure 2
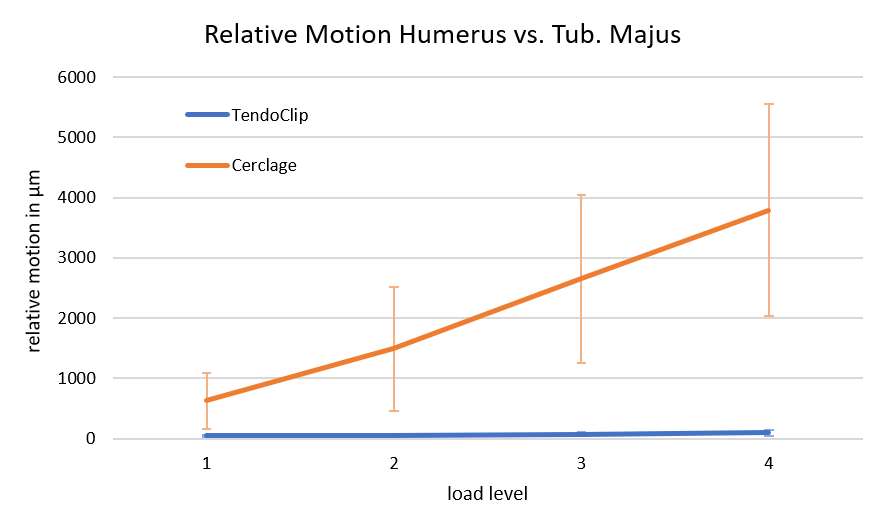
Figure 3#8470
Analyzing the Effects of Cruciate Ligaments in UKA Subjects Using a Forward Mathematical Model of the Knee
*Seth Coomer - University of Tennessee in Knoxville - Knoxville, USA
Richard Komistek - The University of Tennessee - Knoxville, USA
Michael LaCour - University of Tennessee - Knoxville, USA
Bradley Meccia - University of Tennessee Knoxville - Knoxville, USA
*Email: scoomer2@utk.edu
INTRODUCTION: Understanding the roles of the soft tissues affecting the knee is vitally important when designing and analyzing both total knee arthroplasty (TKA) and unicompartmental knee arthroplasty (UKA). Since the UKA retains more of its native knee features, including the cruciate ligaments, soft tissue stability may play an even more important role. The objective of this study is to analyze UKA subjects in a validated forward solution mathematic model (FSMM) to further understand the roles of important soft tissues structures.
METHODS: All the major ligaments at the knee joint are present in this model, validated using telemetry and fluoroscopy, including cruciate, collateral, patellofemoral, and patellar ligaments. The ligaments are modeled as nonlinear springs with multiple bundles of fibers and can be simulated as well-functioning or as deficient. The model has 10 virtual subjects with subject-specific bone models with subject-specific soft tissue attachments, gathered from CT scans. Each subject was virtually implanted with two medial UKAs (Sigma and Preservation, DePuy Synthes) and performed a deep knee bend to 120? flexion. To further analyze the role of the ACL, one subject had its ACL properties modified to replicate an ACL deficient subject. These results were then compared with the fluoroscopic results of a previously analyzed UKA subject with a ruptured ACL [1]. The anteroposterior (AP) motion of the medial contact point (MCP) of the femur with respect to the tibial plateau was monitored throughout the activity.
RESULTS: For a subject with an intact ACL, the MCP started at 4.80 mm at full extension and showed steady posterior rollback to -8.11 mm. The ACL deficient subject in the model started more posterior at -2.8 mm and translated to -21.1 mm at 80° flexion and then slid anteriorly to -11.2 mm at 120° flexion (Figure 1). The previous fluoroscopic study reported similar findings, where ACL intact subjects began 6.19 mm anteriorly and consistently rolled in the posterior direction to -4.36 mm at 90° flexion, while ACL deficient subject began at -6.78 mm and rolled back to -12.71 mm at 45° flexion, then experienced an anterior slide to -2.12 mm at 90° flexion [2] (Figure 2). The average ACL force was similar between the two different medial UKA implants, with Sigma ACL forces beginning at 0.70 xBW at full extension and decreasing to 0.42 xBW at 60° flexion, while Preservation revealed ACL forces of 0.68 xBW at full extension, decreasing to 0.41 xBW at 60° flexion (Figure 3a). The Sigma UKA PCL force was 0.18 xBW at full extension and 0.92 xBW at 120° flexion, while Preservation subjects had a force of 0.19 xBW at full extension and 0.96 x BW at 120° flexion (Figure 3b).
CONLCUSIONS: The data from this study demonstrated that a properly functioning ACL maintains a more anterior position of the femur throughout a DKB activity and prevents significant anterior sliding in late flexion. ACL health should be considered in potential UKA candidates, as ACL function plays an important role in UKA kinematics.
Figures
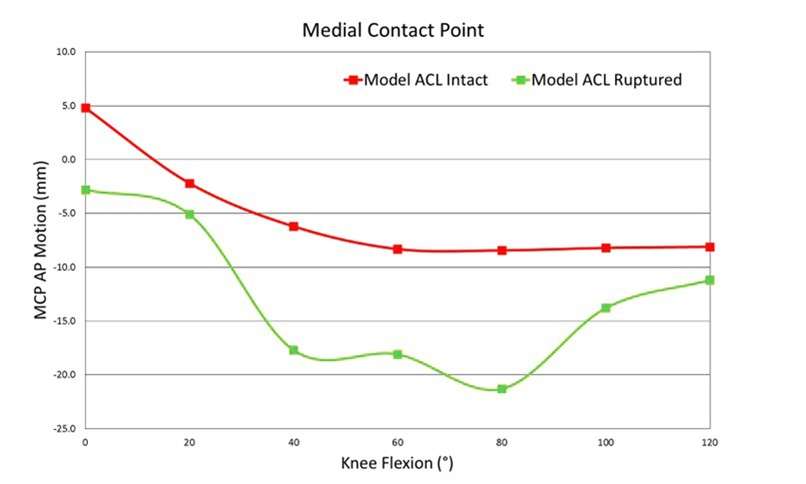
Figure 1
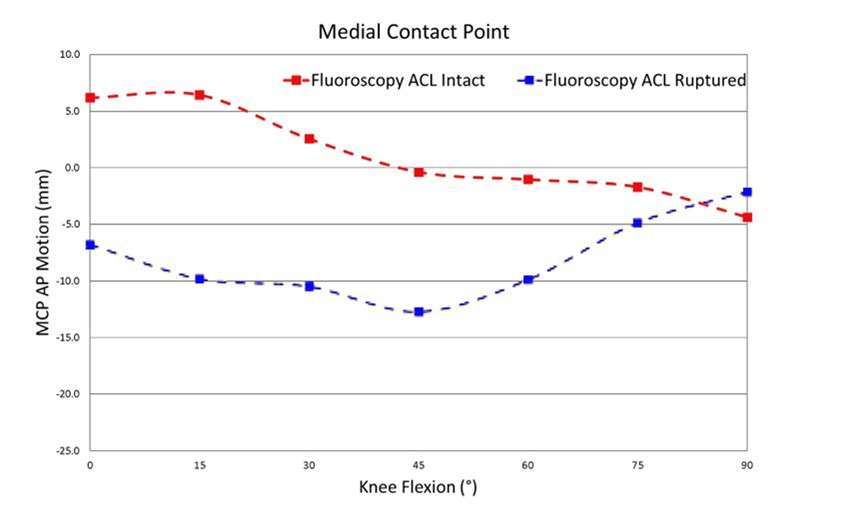
Figure 2
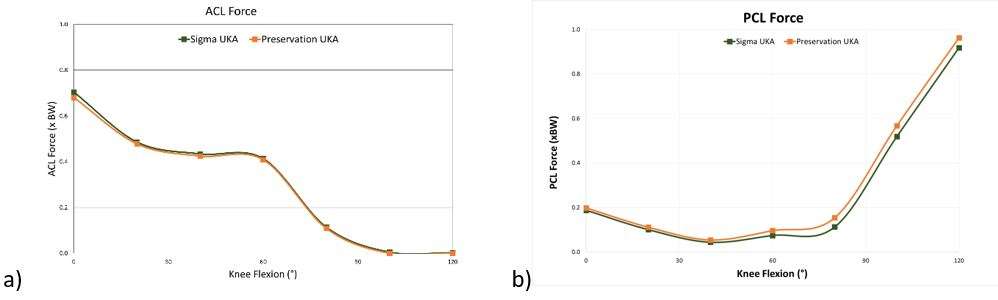
Figure 3#8532
Interference and Keel Size Influence Micromotion in Cementless Unicompartmental Knee Replacement (UKR): Mechanical Analysis Using a High-Accuracy Micromotion Measurement Setup
*AZMI RAHMAN - University of Oxford
David Heath - NDORMS, University of Oxford - Oxford, United Kingdom
Stephen Mellon - University of Oxford - Oxford, United Kingdom
David Murray - University of Oxford - Oxford, United Kingdom
*Email: azmissrahman@gmail.com
Introduction: In cementless UKR, early post-operative tibial fractures are 7x more common in very small tibias (Size AA/A compared to larger sizes). The likelihood of such fractures has been shown to be reduced when a smaller keel is used. The mechanical effect of interference and keel size on primary fixation have not been assessed mechanically, and it is unclear if a smaller keel may impair primary fixation (prior to bone remodelling and ingrowth into bone-implant interfaces). This study assesses the effect of interference and keel design variants on sagittal micromotion using existing and prototype keel designs of the a cementless UKR tibial component.
Method: A low-cost, high-resolution uniplanar Digital Image Correlation setup was developed, and validated to be accurate to 50 micrometres. 3D-printed titanium tibial components were designed and printed: standard, no-interference, no-keel, and small. Variants were implanted into bone-analogue foam which were machined to a CT-reconstructed small tibia, using identical surgical instrumentation and technique. Tibias were loaded to 200N axially in physiological loading positions: 8mm posterior to midpoint representing shallow flexion (e.g., a step-up motion), and 15mm posterior to midpoint representing deep flexion (e.g., a lunge). Sagittal micromotion was measured and calculated during loading and unloading.
Results: In all tests, anterior lift-off was the largest micromotion observed. In shallow flexion, a standard keel moved more than the no-interference and no-keel variants (340μm vs 63μm vs 30μm, p=0.002). In deep flexion, the no-interference and no-keel variants moved more than the standard (826μm vs 1003μm vs 521μm, p=0.039). The small keel experienced less micromotion than the standard keel in both shallow flexion (245μm vs 340μm p=0.233, overall p=0.009) and deep flexion (378μm vs 521μm p=0.265, overall p=0.006).
Values and statistical comparisons listed above are for largest micromotion in each load-unload sequence; if overall micromotion was considered, all differences stated above were significant (F-test comparison, greatest p=0.006).
Conclusion: Counterintuitively, interference increases micromotion in shallow flexion (e.g., a step-up motion), likely due to implant pivoting around the fixed bone-keel interface – such motion is known to be safe in patients in the early post-operative period. However, the keel is protective in deep flexion (e.g., a lunge), limiting drastic micromotion. To avoid excess implant micromotion prior to remodelling, patients should be advised against deep knee flexion early post-operatively. The micromotion setup produced for this study enabled accurate and precise measurement of implant motion and can be used for assessment of other mechanical factors influencing micromotion.
Figures

Figure 1
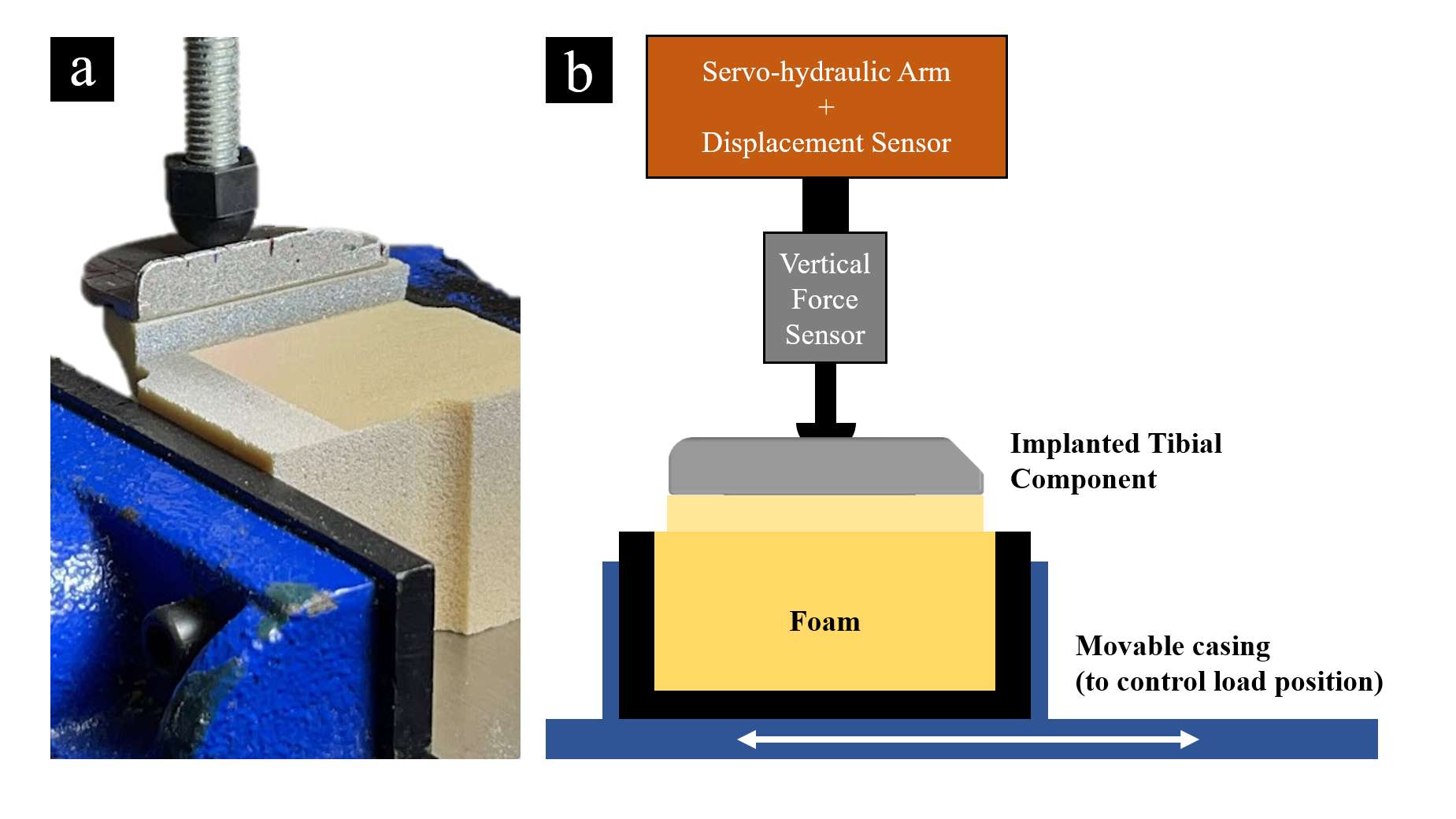
Figure 2
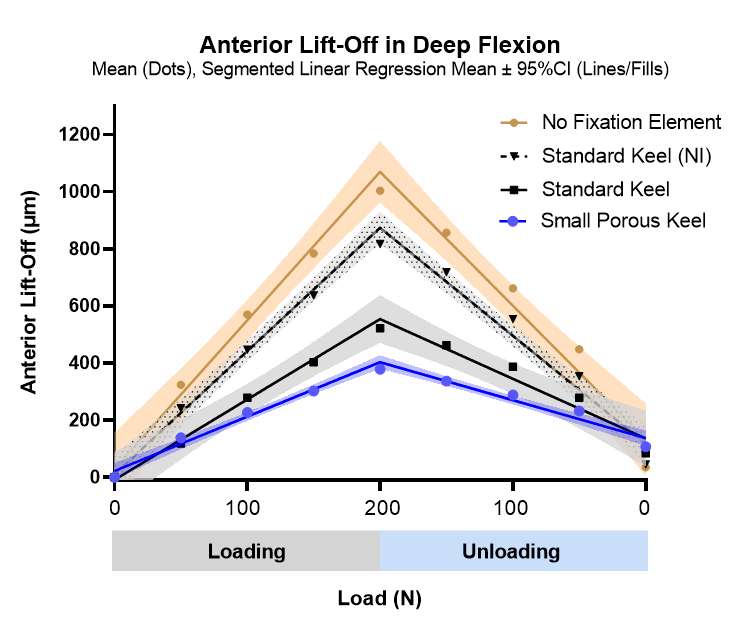
Figure 3#8372
Factors Affecting Correction of Coronal Alignment and Its Prediction After Oxford UKA
*Arun Mullaji - Breach Candy Hospital - Mumbai, India
*Email: arunmullaji@gmail.com
Introduction: The Oxford technique is based on soft-tissue balancing of gaps; meniscal bearing dislocation remains the main concern with this implant. There may be a tendency to use a thicker insert resulting in overcorrection into valgus. There is currently with no method to avert this complication.
Aims: to determine factors that influence correction of coronal alignment after OUKA and whether these can be used to predict alignment, thereby assisting the surgeon in obtaining optimal alignment.
Methods: This is a retrospective study of prospectively collected data of 337 consecutive knees (228 females, 109 males; 164 left and 173 right knees) undergoing Oxford UKA by a single surgeon using Phase 3 instrumentation in patients ranging from 47-88 y (mean 65.4). Preoperative long leg scanograms, and short radiographs were obtained and following measurements made: MLDFA, HKA, MPTA, VCA, JLCA. Intra-operative measurements were made of thickness of medial femoral osteophyte resected (OT), intercondylar distance (ICD), thickness of tibial resection, posterior condylar resection thickness, spigot size, bearing thickness used. Preoperative HKA deformity was grouped into 4 classes -mild, moderate, severe, extreme. Preoperative JLCA was grouped into JLCA 1-4, 5-8, >8 deg. Postop HKA was grouped into undercorrected, optimal, acceptable and overcorrected classes. Tibial and femoral implant proudness were derived from resection and implant data. Using trigonometric analysis, the angular correction (tan) achieved by the combined implant thickness used was computed taking the ICD into account. Statistical analysis was performed using Jamovi (Version 2.3.21.0.) We performed descriptive statistics, ANOVA, chi-square test, correlation, and ordinary least squares regression for regression analysis. A p value <.05 was considered statistically significant.
Results: Preop HKA mean 170o (152-179) was corrected to a mean of 176o (164-186). Mean correction achieved was 5.5o. Overall acceptable or optimal alignment was achieved in the majority of patients, as well as in the subgroups. Isolated cases of few degrees of overcorrection were seen in all groups. Undercorrection was seen in severe and extreme deformities. The osteophyte thickness, preop HKA, JLCA and ICD were significant predictors of correction achieved. A formula for computing femoral resection (by milling) and bearing thickness to achieve desired correction will be presented.
Conclusion:This novel study highlights the factors that influence correction of coronal alignment after OUKA and presents a formula for predicting alignment, thereby assisting the surgeon in obtaining optimal alignment.
Figures
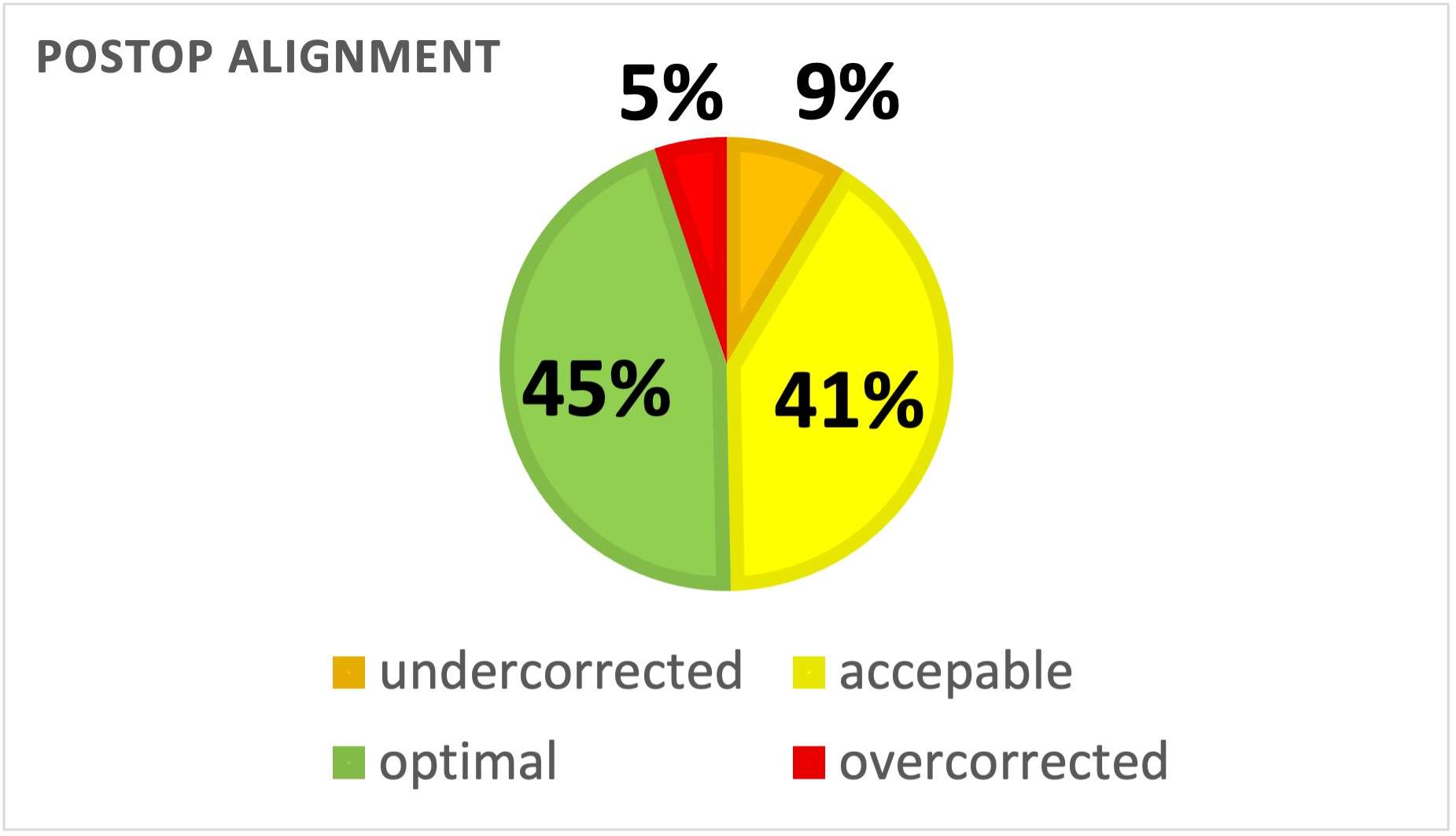
Figure 1
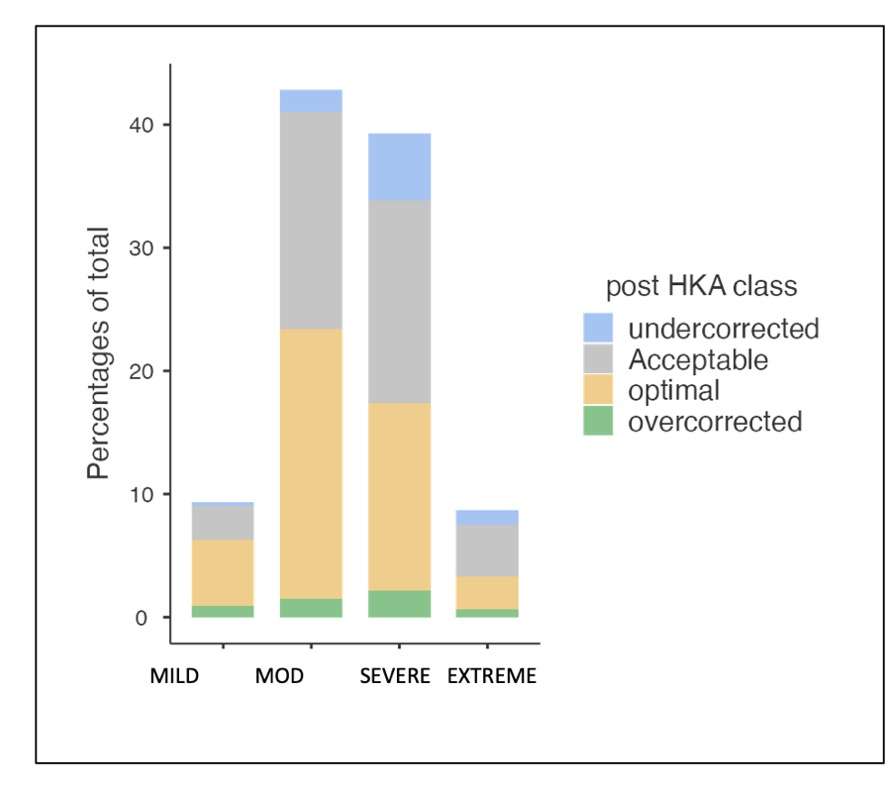
Figure 2#8745
Clinically Important Differences Are Often Not Achieved in Patient-Reported Outcomes for Robot-Assisted and Navigated Unicompartmental Total Knee Arthroplasty: A Systematic Review
*Vinaya Rajahraman - New York, United States of America
Kyle Lawrence - NYU Orthopedic Hospital - New York, USA
David Bloom - NYU Orthopedic Hospital - New York, USA
Alana Prinos - Langone Orthopedic Hospital - New York, USA
Muhammad Haider - NYU Orthopedic Hospital - New York, USA
Joshua Rozell - NYU Langone Orthopedic Hospital - New York, USA
Ran Schwarzkopf - NYU Langone Medical Center Hospital for Joint Diseases - New York, USA
Muhammad Haider - NYU Orthopedic Hospital - New York, USA
Armin Arshi - New York University Langone Health - New York, United States of America
*Email: v.rajahraman@gmail.com
Introduction:
Technology is increasingly incorporated into unicompartmental knee arthroplasty (UKA) by way of computer-assisted navigation (N-UKA) and robot-assisted surgery (R-UKA) in order to improve alignment, implant positioning, and gap balancing. Whether the addition of intraoperative technology aids in achievement of the minimal clinically important difference (MCID) in patient-reported outcomes (PROMs) compared to conventional UKA (C-UKA) remains unknown. The goal of this systematic review was to assess whether differences in PROMs between C-THA and technology-assisted UKA reached MCID values.
Methods:
PubMed/MEDLINE/Cochrane Library were systematically reviewed for studies that compared PROMs between primary C-UKA, the control group, and N-UKA or R-UKA. Delta improvements between groups were compared to established MCID values. Additional radiographic and clinical differences were assessed. Review of literature yielded four (N=328) N-UKA and seven (N=526) R-UKA studies with C-UKA cohorts as control groups for analysis.
Results:
Differences in preoperative and postoperative PROMs were reported as statistically significant in three of four studies (75%) comparing N-UKA and C-UKA, however none of the studies reported values that reached the MCID. Differences in preoperative and postoperative PROMs were reported as statistically significant in four of seven studies (57.1%) comparing R-UKA and C-UKA, however only three of the studies (42.9%) reported values that reached the MCID. Improved radiographic outcomes for N-UKA and R-UKA were reported in 75% and 57.1% of studies respectively. Only one study reported improved revision rates with R-UKA compared to C-UKA.
Conclusion:
Though studies may report better improvements in PROMs in N-UKA and R-UKA compared to C-UKA, these often may not achieve clinically significant values. Future studies comparing clinical outcomes between technology-assisted UKA and C-UKA should report PROMs within the context of validated MCID values.
#8239
Do Pain and Patient Reported Outcome Measures Vary by Patient Pre-Operative Physical Activity Levels in Partial Knee Arthroplasty Patients?
*Roberta Redfern - Zimmer Biomet - Pemberville, USA
Mike Anderson - Zimmer Biomet - Lehi, USA
Dave Van Andel - Zimmer Biomet - Grand Rapids, USA
Jason Cholewa - Zimmer Biomet - Warsaw, USA
*Email: roberta.redfern@zimmerbiomet.com
Physical activity (PA) is suggested to reduce pain associated with osteoarthritis, however evidence suggests that patients with knee pain may avoid PA as a result of pain. Research has demonstrated a heterogeneous effect of preoperative PA on functional outcomes following arthroplasty. The goal of this study was to investigate pain and patient-reported outcome measures (PROMs) as a function of objectively collected pre-operative PA levels in patients scheduled to undergo partial knee arthroplasty (PKA).
Secondary data analysis of a large multicenter prospective observational cohort study investigating the use of a smartphone-based care management platform for self-directed rehabilitation following lower limb arthroplasty. Patients were provided a smartwatch for objective, passive collection of steps pre-operatively through up to one year following the procedure. Patients were eligible for inclusion in this analysis if scheduled to undergo PKA with at least 90 days of follow-up data available for evaluation. Patients were categorized based on average pre-operative step counts according to quartiles, where patients performing steps in 0-25th percentile were classified as low PA, 25th-75th percentile as medium PA, and those with step counts in the 75th-100th percentile were classified as high PA. Step counts, numeric pain ratings, KOOS JR, and EQ5D5L scores were compared by groups pre-operatively and at 1- and 3-months post-operatively.
536 patients undergoing PKA were included. The median average daily step count was 5013.4 (IQR 3465.2 – 7422.3). Pre-operative pain scores did not vary significantly by PA level on ANOVA (p=0.08), ranging from 5.17±2.11 in high PA subjects to 5.70±1.89 in the low PA group. On ANOVA, pre-operative KOOS JR (56.48±11.75 vs 51.50±11.68, p<0.05) and EQ5D5L index scores (80.40±15.37 vs 72.26± 17.45, p<0.05) varied only between the high and low PA groups. At 3 months post-operative, average step counts continued to vary among activity groups, however patients in the low and medium PA groups exceeded pre-operative step counts, while high activity patients reached 96% of their pre-operative average steps. Numeric pain scores were similar across PA levels at 3 months post-operatively. Patients in the medium PA group demonstrated the greatest pain reduction at 3 months (3.23 points), compared to 2.61-point reduction high PA, and 3.06-point reduction in the low PA group but did not reach significance. Change in EQ5D5L index scores was similar across PA levels at 1 and 3 months. At 3 months post-operatively, KOOS JR scores were similar among PA levels, however subjects in the low PA group appreciated greater change than those with high pre-operative PA (21.16 vs 16.52, p<0.05).
Patients who exhibit low levels of activity as measured by step counts pre-operatively demonstrate higher levels of pain and lower levels of function prior to partial knee arthroplasty. However, greater improvements in objective activity and patient reported function as measured by PROMs are observed in patients with lower pre-operative activity.
#8714
Outcomes of Patellofemoral Arthroplasty: Robotic-Assistance Produces Superior Mid-Term Outcomes
*Jonathan Katzman - NYU Langone - New York, United States of America
Weston Buehring - SUNY Downstate College of Medicine - New York, United States of America
Patrick Connolly - NYU Langone Health - New York, USA
Ran Schwarzkopf - NYU Langone Medical Center Hospital for Joint Diseases - New York, USA
Ivan Fernandez-Madrid - NYU Hospital for Joint Diseases - New York, USA
*Email: jonathankatzman98@gmail.com
Introduction
The efficacy of robotic-assisted Patellofemoral Arthroplasty (PFA) remains an ongoing matter of debate. This study seeks to compare midterm clinical and patient-reported outcomes for patients who underwent robotic-assisted and conventional PFA surgeries.
Methods
A retrospective review found 237 knees in 211 patients which underwent patellofemoral arthroplasty between June 2011 and October 2021 at our institution. After excluding indications other than primary osteoarthritis and cases with less than one year of follow-up, the final study cohort consisted of 90 conventional operations and 94 robotic-assisted operations. Propensity score matching was utilized to address to significant differences in the sex distribution between groups.
Results
Overall, there was a revision-free survivorship rate of 89.7% with an average time to follow-up of 4.6 years (range, 1.2 to 11.1). 29 knees (15.8%) required various non-conversion reoperation procedures. The all-cause revision rate, accounting for revision PFAs and conversions to TKA, was greater for the conventional matched cohort compared to the robotic-assisted matched cohort (P = 0.014). The mean time to revision was also longer for the robotic-assisted cohort (P = 0.026) yielding a Kaplan-Meier survivorship curve that shows differences between the cohorts (P = 0.041). All revisions following robotic-assisted PFA were caused by progression of osteoarthritis, whereas conventional PFAs also required revision due to aseptic loosening and malposition. Rates of infection resulting in irrigation and debridement were higher for conventional cases (P = 0.041). Patients in the robotic-assisted matched cohort showed greater improvements in Knee injury and Osteoarthritis Outcome Score for Joint Replacement (KOOS, JR.), PROMIS Pain Intensity, PROMIS Pain Interference, PROMIS Mobility, and PROMIS Physical Health. There were no significant differences in length of stay, discharge disposition, range of motion, or PROMIS Mental Health.
Conclusion
PFA is an effective treatment for addressing advanced arthritis in the patellofemoral compartment of the knee. Robotic-assistance may improve clinical and patient-reported outcomes.
#8476
Analyzing UKA Mechanics Using a Forward Solution Mathematical Model of the Knee
*Seth Coomer - University of Tennessee in Knoxville - Knoxville, USA
Richard Komistek - The University of Tennessee - Knoxville, USA
Michael LaCour - University of Tennessee - Knoxville, USA
Bradley Meccia - University of Tennessee - Knoxville, United States
*Email: scoomer2@utk.edu
INTRODUCTION: Studies evaluating total knee arthroplasty (TKA) have documented abnormal kinematic patterns, often opposite of the normal knee [1]. Although there are ample studies documenting TKA results, in vivo studies evaluating the kinematics of unicompartmental knee arthroplasty (UKA) are less common. Accordingly, there is minimal data available to support UKA kinematics being more normal-like in pattern and magnitude. The objective of this study is to advance a forward solution mathematical model (FSMM) previously validated for TKA [2] to analyze UKA subjects and compare results against fluoroscopy.
METHODS: The FSMM, validated using telemetry and fluoroscopy, has 10 virtual subjects with subject-specific bone models and soft tissue attachment sites gathered from CT and MRI data. Previously published modeling techniques for TKA [2] were utilized in the UKA model, but the UKA model has additional features, including separate patellofemoral surfaces for the native bone and femoral component (Figure 1) as well as inclusion of all ligaments and native bone structures. Each subject, virtually implanted with a medial Preservation UKA and a medial Sigma UKA, performed a deep knee bend (DKB) to 120? knee flexion. Key results include condylar motion, soft tissue forces, contact forces, and flexion facet center (FFC) motion. These results were then compared with results from previous fluoroscopic studies.
RESULTS: In the model, the Sigma UKA demonstrated anterior motion of the FFC during early flexion and a slight posterior motion in later flexion, with the FFC moving on average from -7.49 mm at full extension to -5.65 mm at 120° flexion. The average FFC motion for subjects in the fluoroscopy study implanted with the Sigma UKA demonstrated a similar pattern with slightly more anterior motion, with the FFC moving from -7.92 mm at full extension to -5.03 mm at 100° flexion [3] (Figure 2).
Patellofemoral forces occurring between the patella and the native bone for the Sigma UKA subjects were 0.11 x BW at full extension and increased to a peak force of 2.44 x BW at 100° flexion, while the Preservation UKA subjects had a force of 0.12 x BW at full extension and increased to a peak force of 2.40 x BW at 100° flexion. Patellofemoral forces occurring between the UKA components was 0.07 x BW at 60° flexion and 0.99 x BW at 120° flexion, while the Preservation UKA subjects experienced a force of 0.11 x BW at 80° flexion and increased to 0.88 x BW at 120° flexion (Figure 3).
CONLCUSIONS: The results across multiple implant types demonstrated good correlation between the FSMM and previous fluoroscopic studies. This model also produced more detailed results on patellofemoral forces, determining the magnitudes of forces on the components. Mathematical modeling can be a valuable tool to supplement component design and evaluation for both TKA and UKA, as models such as these provide rapid feedback to component position and design changes. Accordingly, this FSMM has been advanced to properly assess UKA subjects and provides unique insights into multiple UKA designs.
Figures
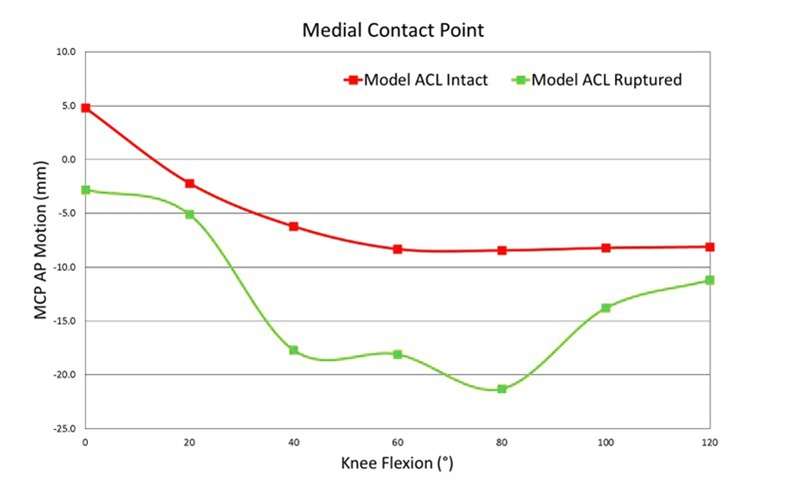
Figure 1
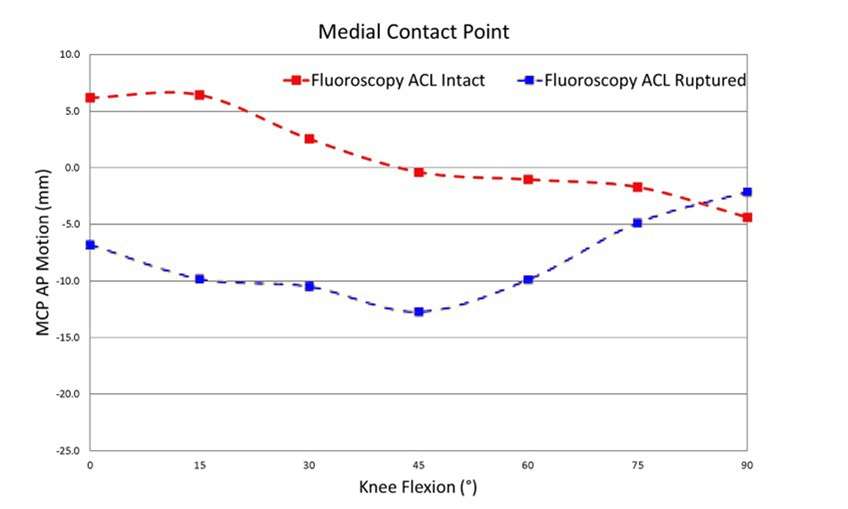
Figure 2
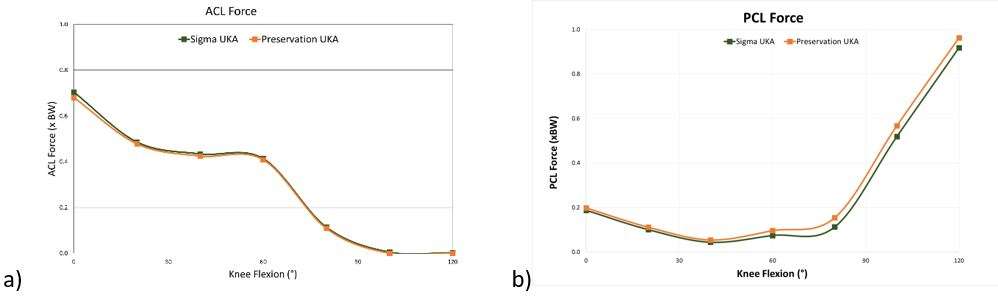
Figure 3#8411
Effect of Component Positioning on Bearing Overhang in Mobile Bearing UKA
*Eik Siggelkow - Zimmer GmbH - Winterthur, Switzerland
Marc Bandi - Zimmer GmbH - Winterthur, Switzerland
Iris Blatter - Zimmer Biomet - Winterthur, Switzerland
*Email: eik.siggelkow@zimmerbiomet.com
Introduction: Mobile bearingunicompartmental knee arthroplasty (UKA) shows excellent long-term results with a survival rate of 91% (15 years) [1]. This may be even improved by considering the effect of component placement to the resulting bearing overhang (BO) which may be a factor contributing to bearing wear, bearing dislocation or pain due to soft tissue impingement [2, 3]. This study analyzed component placement variations in relation to resulting BO for passive knee flexion as a first step in determining component placement combinations with minimized BO.
Methods: One specimen specific virtual biomechanics knee (VBK) model was virtually implanted with a mobile bearing UKA, using the appropriate component sizes and placements adhering to the current surgical technique (Oxford, Zimmer Biomet, tibia base plate: C, femur: medium, bearing: medium). The VBK model consisted of the tibia/femur bone and cartilage 3D geometry derived from CT and MRI data sets. Passive soft tissue contributions were incorporated using a phenomenological ligament model validated against robot experimental testing [4]. Component placements were then varied as follows: 1) medial-lateral (ML) femur placement (-2.5 mm = lateral to 2.5 mm = medial) combined with tibia internal-external (IE) rotation (-8° = internal to 8° = external), 2) combined with femur IE rotation (see 1), 3) pure IE rotation of femur and tibia component (see 1, 2). For each placement parameter combination, a passive knee flexion was simulated and the largest BO throughout the arc of flexion was calculated.
Results: Femur ML/tibia IE: Femur medial placement of 2.5mm combined with tibia internal rotation (figure 1) showed a peak BO of 3.8 mm (-4° tibia rotation). Femur ML/IE: Similarly, femur medial placement combined with femur internal rotations (figure 2) resulted in a peak BO of 3.4 mm (-2° femur rotation). Lateralized femur placement resulted in lower but not minimal BO (2 mm) for both component rotations. Tibia/femur component IE rotation variation resulted in BO below 2 mm (figure 3). Lowest BO was found to be close to the neutral component placement (area 1a: 2° tibia, 4° to 0° femur rotation, area 1b: 0 to 2° tibia, -6° femur rotation). Highest BO was found at 8° tibia external rotation combined with femur component rotational placements between -6° and 8° (area 3a) and for -8° tibia internal rotation with -6° to -8° femur internal rotation (area 3b).
Conclusions: Rotational placement (IE) of femur and tibia component showed low sensitivity to BO (< 1.8 mm). Component positioning around neutral position (+4° to -8° tibia IE rotation, +8° to -6° femur IE rotation) resulted in lowest BO. Tibia external rotations > 4° resulted in highest BO. Medialized femur component placement (> 1.5 mm) showed to have a higher impact on BO than component rotations. Since these data represent one specimen specific model more research is needed.
References: [1] Pandit et al. BJJ, 2015, [2] Martin et al., JOR, 2019; [3] Bradley et al. JBJS, 1987; [4] Siggelkow et al, Proceedings CMBBE 2012
Figures
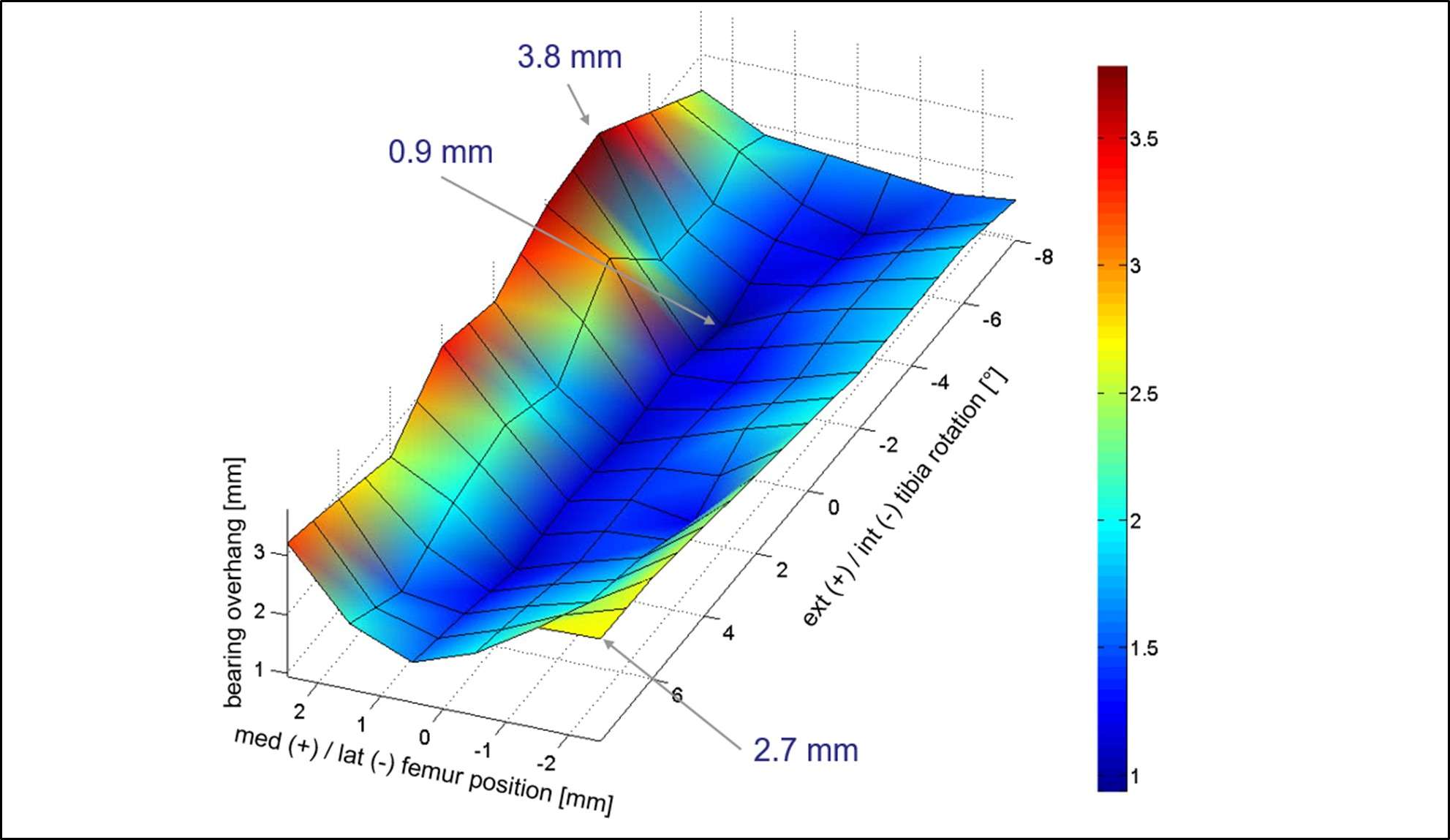
Figure 1
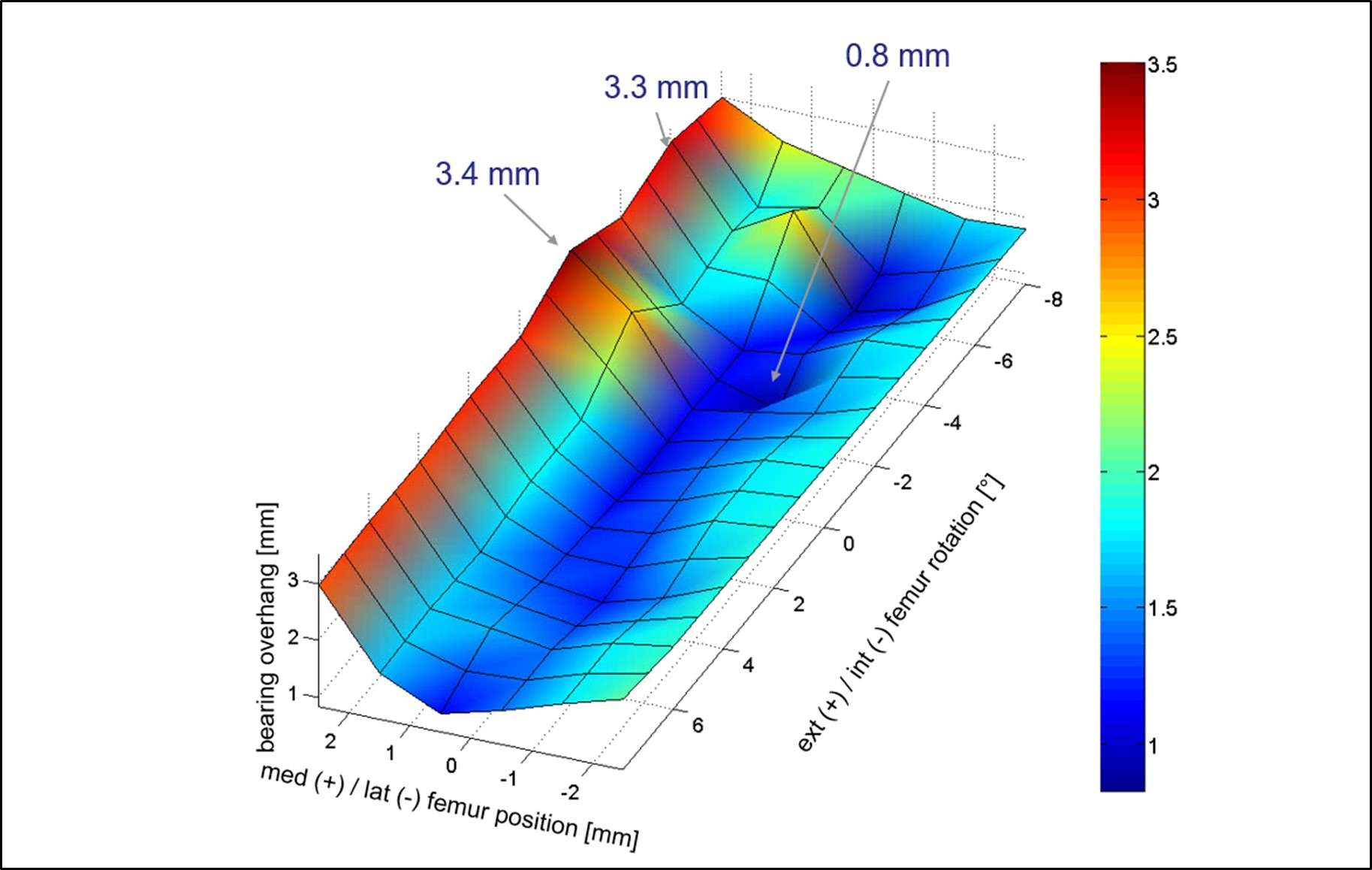
Figure 2
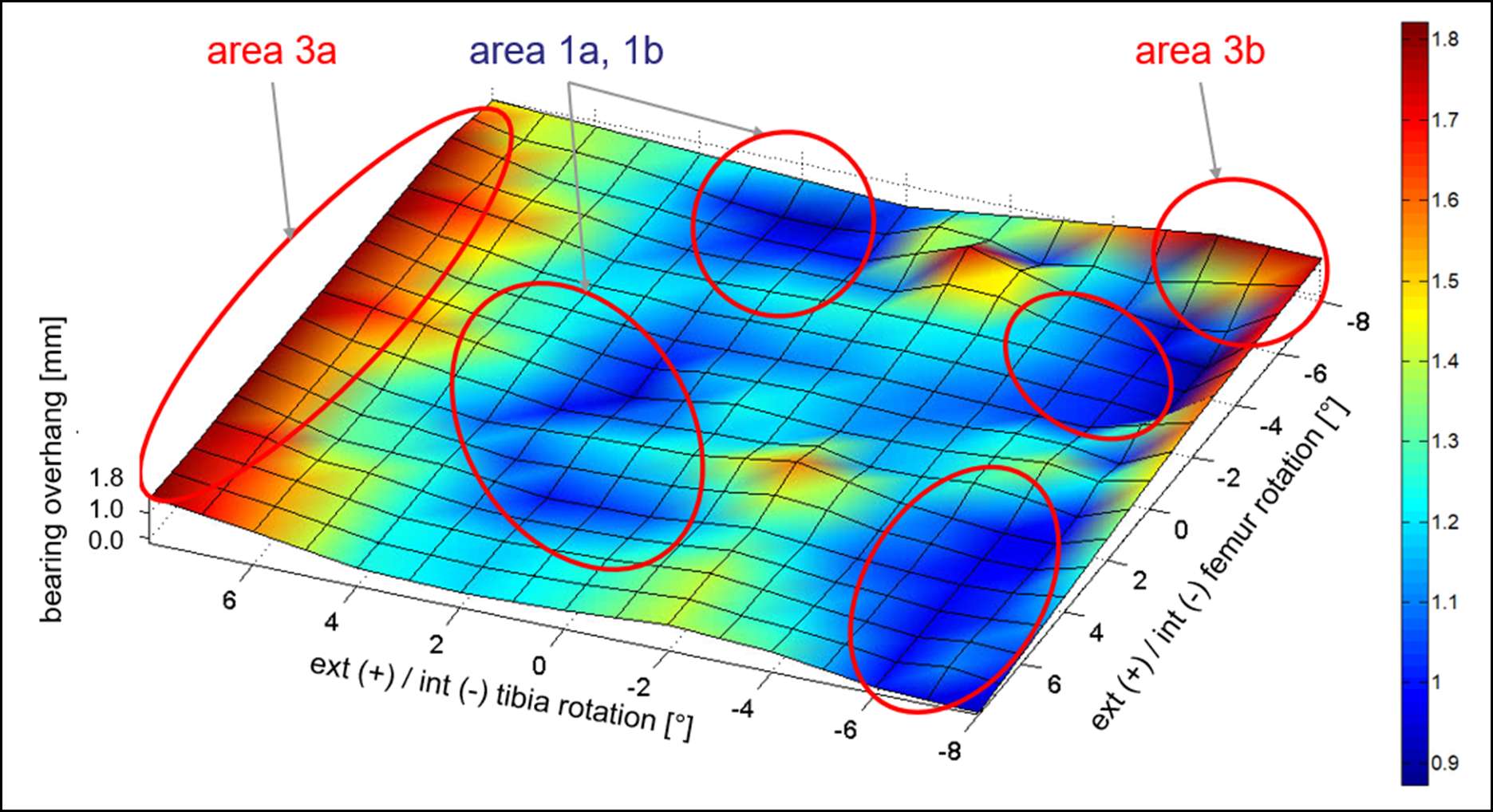
Figure 3#8300
Robotic-Arm Assisted Lateral Unicompartmental Knee Arthroplasty: Case Series With Minimum 5-Year Follow-Up
Kevin Mekkawy - Hospital for Special surgery - West Palm Beach, United States of America
Hugo Rodriguez - Hospital for Special Surgery - Miami, United States of America
Rushabh Vakharia - Holy Cross Hospital - Ft. Lauderdale, USA
Tsun Yee Law - Holy Cross Orthopedic Institute - Oakland Park, USA
*Martin Roche - Holy Cross Hospital - Fort Lauderdale, USA
*Email: martin@mroche.com
Introduction: Lateral unicompartmental knee arthroplasty (UKA) has been shown to be a successful treatment modality for isolated lateral osteoarthritis (OA) of the knee. The reproduction of proper knee kinematics, limb alignment, as well as proper soft tissue balancing, and component positing have been shown to be of the utmost importance for a successful UKA. Robotic assistance has shown to be a reliable tool in order to replicate these factors, as compared to manual instrumentation alone (Figures 1 and 2). Recent studies have shown the potential of robotic-assisted surgery in controlling these surgical factors for medial UKA, however, studies assessing outcomes of robotic-assisted lateral UKA (RAUKA) are lacking. Therefore, a retrospective single center study was performed to assess outcomes of lateral RAUKA.
Materials and Methods: Patients who underwent lateral RAUKA from a single surgeon at a central institution between January 2008 and June 2017 were identified. All patients received a lateral UKA with a fixed-bearing metal backed onlay tibial component. Patients over the age of 18, with at least a 5-year follow-up and a lateral UKA were contacted by phone and asked a series of questions to determine satisfaction and survivorship. Each patient was asked in a ‘yes’ or ‘no’ manner, if they have had their implant revised or reoperated for any reason, and a 5-point Likert scale was used to assess satisfaction.
Results: Of a total of 67 patients (70 knees), no patients declined participation within the study, 7 patients (7 knees) were deceased, 5 patients (5 knees) had disconnected numbers and 5 patients (5 knees) were lost to follow up (i.e. could not be contacted by phone). Data was collected from 50 patients (53 knees). Of the patients that responded: 32 (60%) were right knees; 32 (60%) were female, and average follow-up was 7.6 years (5 - 14). Of the 53 knees, 1 had a revision (98% survivorship) (Figure 3). Excluding the revision, 51 (98%) of the included cases were either “very satisfied” or “satisfied” with their surgery.
Conclusions: Robotic-arm assisted lateral UKA was found to have high survivorship and a satisfaction rate in patients that had at least a 5-year follow-up. In the future, larger prospective comparison studies with longer follow-ups are necessary to adequately compare survivorship and satisfaction rates of robotic-assisted lateral UKA to conventional UKA.
Figures
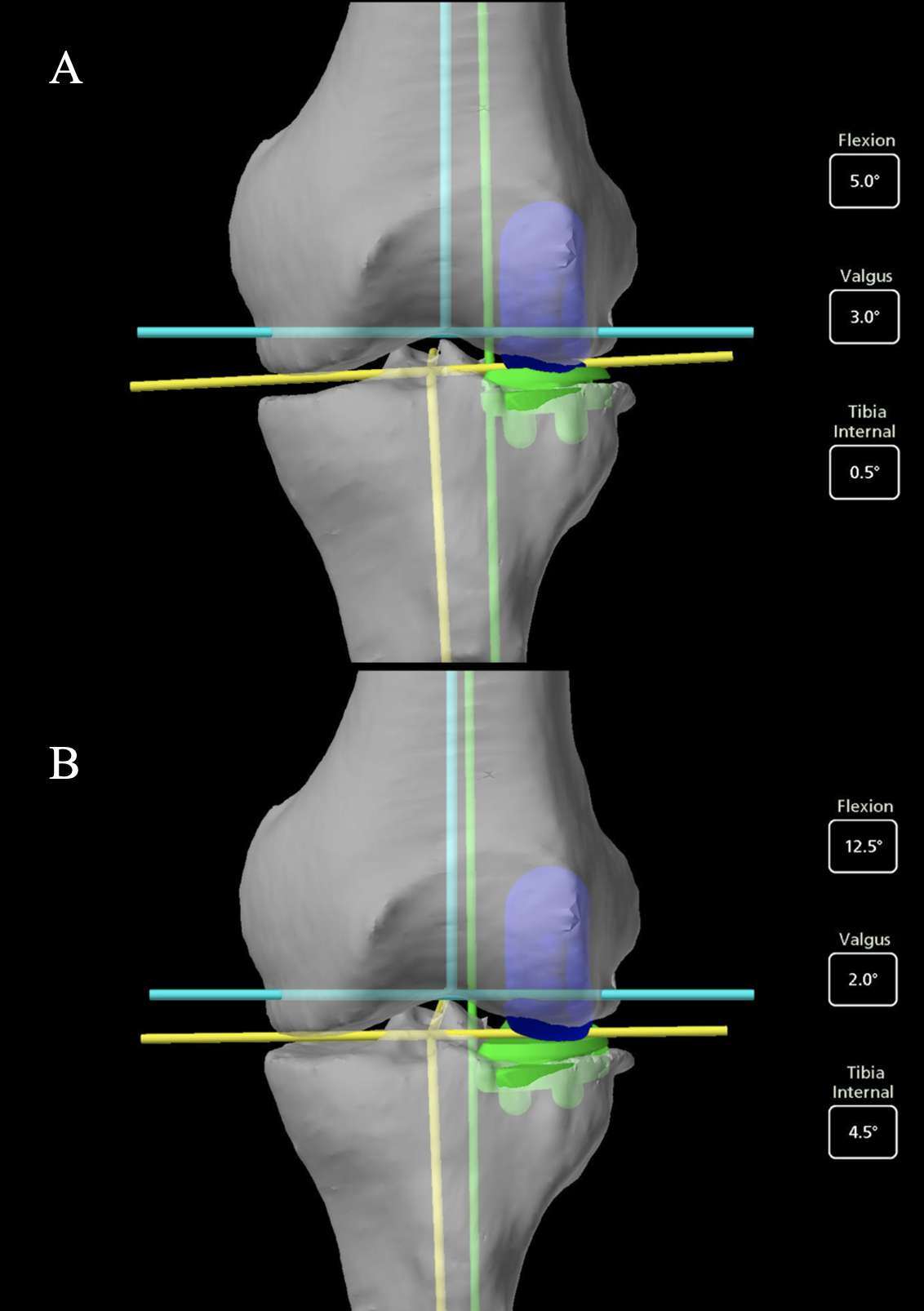
Figure 1
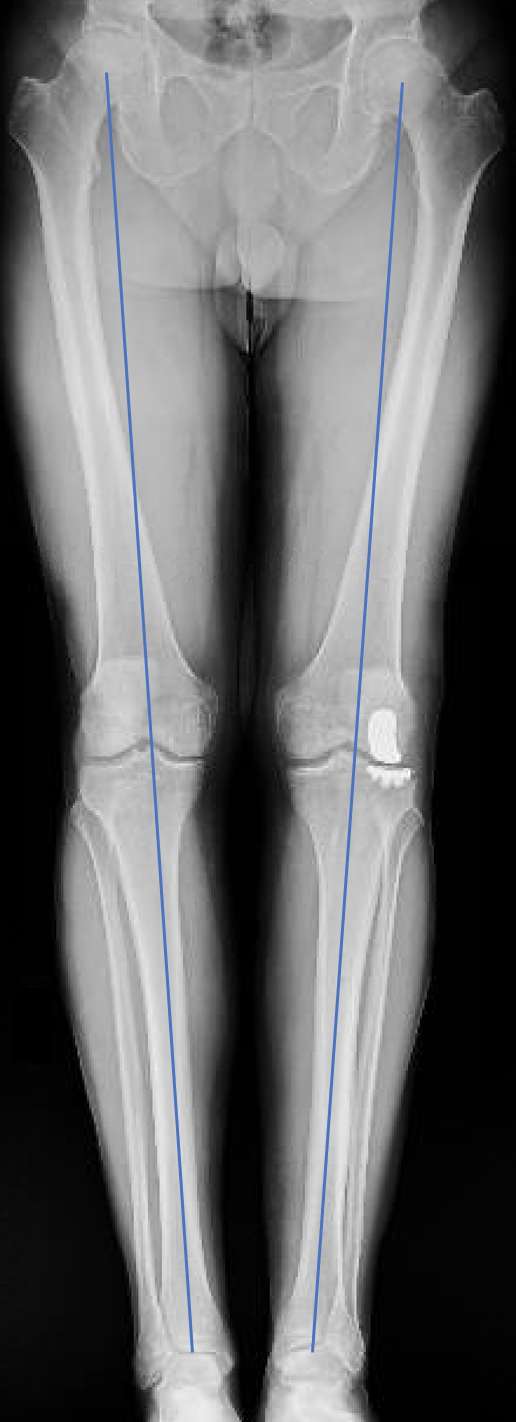
Figure 2
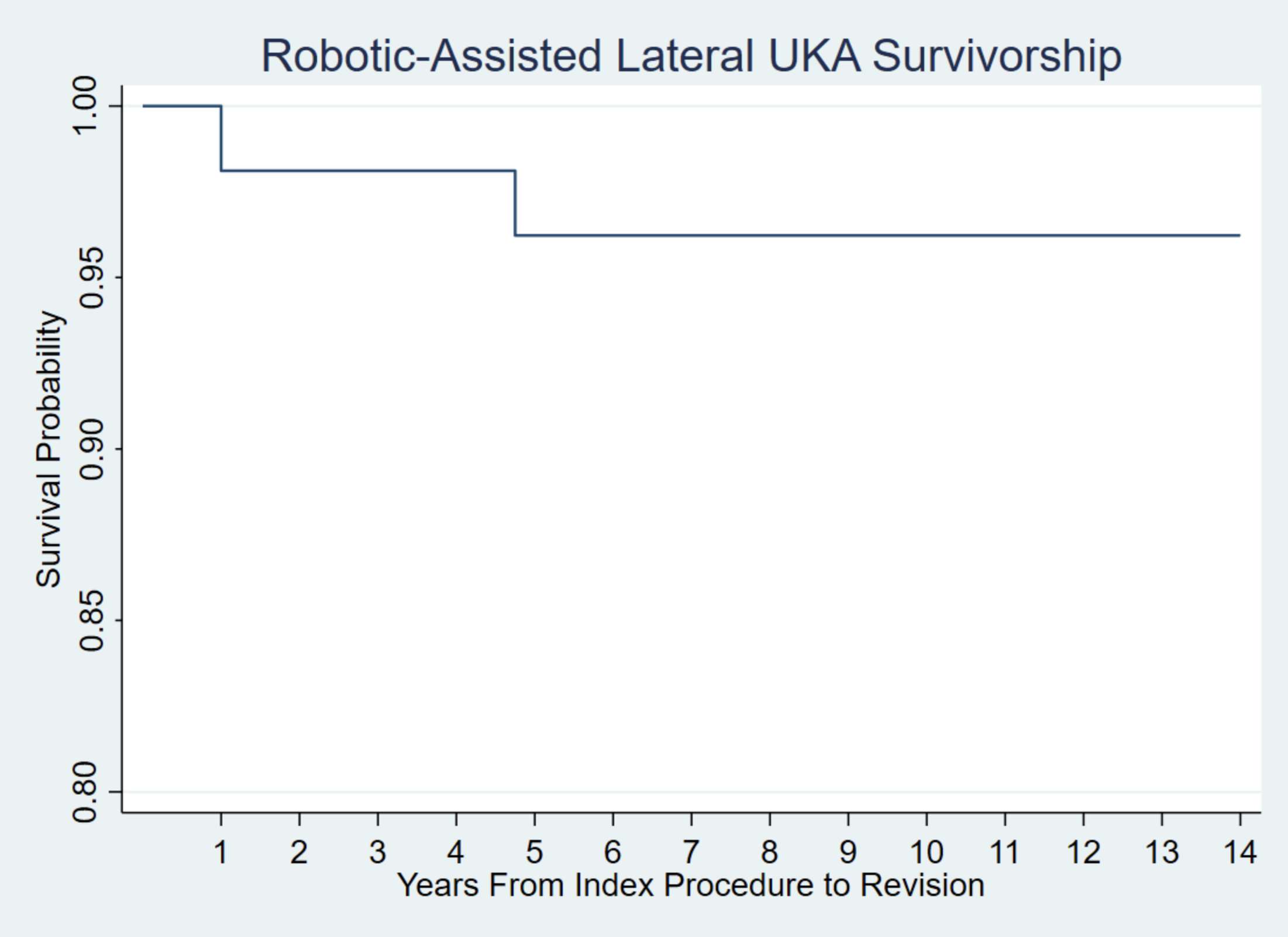
Figure 3#8410
Robotic Arm-Assisted Lateral Unicompartmental Knee Arthroplasty: Outcomes at Short Term Follow Up
*Warran Wignadasan - University College London Hospital - London, United Kingdom
Justin Chang - Division of Orthopaedic Surgery, Humber River Hospital, Toronto, Ontario, Canada - Toronto, Canada
Andreas Fontalis - University College London Hospital - London, United Kingdom
Ricci Plastow - University College London Hospitals NHS Foundation Trust - London, United Kingdom
Fares Haddad - University College London Hospital - London, United Kingdom
*Email: warran.wignadasan@gmail.com
Introduction
It has previously been reported that over 40% of patients who undergo total knee arthroplasty (TKA) would be suitable for a unicompartmental knee arthroplasty. UKA is less invasive than TKA and preserves the unaffected compartments of the knee. Advancements in technology has led the the development of robotic arm-assistance, which aims to improve precision and accuray of bone cuts in order to enhance component alignment, optimise soft tissue balance and minimise surgical trauma. Robotic-arm assisted medial compartment unicompartmental knee arthroplasty (RA UKA) is associated with improved accuracy of implant positioning and excellent early functional outcomes . However, there is paucity of evidence regarding outcomes following RA UKA for isolated lateral compartment osteoarthritis. The aim of this study was to assess the short-term clinical and patient reported outcomes of lateral compartment UKA, utilising robotic-arm assistance.
Methods
our study is a retrospective study of prospectively collected data of 21 consecutive patients who underwent lateral RA-UKA. The study included 9 (42.9%) males and 12 (57.1%) females with a mean age of 63.4 ± 9.2 years. The Oxford Knee Score (OKS) was measured pre-operatively and at one-year post-operatively, while range of motion (ROM) and complications were also recorded. All procedures were conducted using the MAKO Robotic Arm Interactive Orthopaedic System (Stryker Corp, Mako Surgical Corp, Ft. Lauderdale, FL). All patients received the Restoris MCK Partial Knee implant (Stryker Corp, Mako Surgical Corp, Ft. Lauderdale, FL).
Results
We found a statistically significant improvement in OKS at one year follow up compared to baseline pre-operartive scores (21.8 ± 5.6 vs 45.2 ± 2.8 respectively; p<0.001). There was also a significant improvement in ROM associated with lateral RA UKA when comparing pre-operative values to at one year follow up (123.5° ± 8° vs 131.5° ± 6.3° respectively; p<0.001). None of the patients in our study underwent revision surgery within the follow up period.
Conclusion
In our study, lateral RA UKA resulted in significant improvements in clinical and patient reported outcomes with low complications rates. Further long-term comparative studies are needed to assess the utility of lateral RA-UKA versus conventional UKA.
#8707
Durability Enhancement of a Triboelectric Energy Harvester for Load Monitoring in Total Knee Replacement
*mahmood chahari - binghamton university - Binghamton, United States of America
Emre Salman - Stony Brook University - Stony Brook, USA
Milutin Stanacevic - Stony Brook University - Stony Brook, USA
Ryan Willing - Western University - London, Canada
Sherry Towfighian - Binghamton University - Binghamton, USA
*Email: mchahar1@binghamton.edu
ABSTRACT: The objective of this study is to develop a robust energy harvester capable of converting a wide range of pressure into electricity to power a load sensor within the knee implant. By developing a robust and self-powered system, the study aims to enhance the monitoring capabilities of knee implants, providing valuable insights into detecting the aberrant loading that can reduce the lifetime of total knee replacement (TKR). To efficiently convert pressure into electricity, a cuboid-array-structured triboelectric nanogenerator (TENG) operating in vertical contact mode is designed and incorporated into the TKR. The proposed TENG consists of a cuboid-patterned silicone rubber layer that is sandwiched between two thin aluminum layers. By introducing dopamine-modified BaTiO3 nanoparticles, it becomes possible to improve electrical stability and mechanical durability of the silicone rubber.
INTRODUCTION: Knee osteoarthritis (KOA) is a rising joint disease in older individuals, and total knee replacement (TKR) is an effective intervention for chronic knee pain. However, TKR failures are often attributed to misalignment or overload. Implantable sensors can monitor joint forces, detect issues, and protect patients. Researchers have explored smart knee implants with load-monitoring capabilities, including strain gauges, electromagnetic generators, and piezoelectric energy harvesters. The most common type is piezoelectric material, which are toxic and brittle. Using TENGs, we address these drawbacks and have developed a highly durable energy harvester based on cuboid-array-structured TENG for effective joint health monitoring and early detection of implant misalignment in knee implants.
METHODS:
The electrical outputs of the self-powered embedded triboelectric pressure sensor were measured at various forces and frequencies under material testing system MTS simulating walking and running activities at 1 to 3 Hz.
RESULTS:
Under dynamic compressive loading of 2200N magnitude, each left and right TENG integrated into the knee implant package has the capacity to generate an estimated power output of approximately 15μW at operating frequency of 1 Hz that is capable of running a front-end electronic system which consumes 5.35 μW. Moreover, at a higher frequency of 3 Hz, the output power can be enhanced to approximately 43μW. The triboelectric nanogenerator exhibits remarkable mechanical durability, functioning reliably more than 30,000 cycles under a 2200N load.
CONCLUSION: The proposed robust and self-powered pressure sensor shows outstanding mechanical stability, with the output voltage stability after 30,000 dynamic loading cycles. The harvested power of 15 μW obtained at 1 Hz frequency allows powering analog to digital converter for continuous joint load monitoring. The durability of the cuboid-patterned TENG proposed in this work was significantly improved compared to previous research [1]. With better signal stability, a broad detection range, and longer lifetime, this harvester shows promise as a wireless and durable pressure sensor for detection of aberrant loading in knee implants.
REFERENCES:
[1] N. Hossain, … G. Y.-J. of, and undefined 2021, “Effect of dielectric material and package stiffness on the power generation in a packaged triboelectric energy harvesting system for total knee replacement,” asmedigitalcollection.asme.org, Accessed: May 16, 2023. [Online]. Available: https://asmedigitalcollection.asme.org/biomechanical/article-abstract/143/10/101009/1109470
Figures
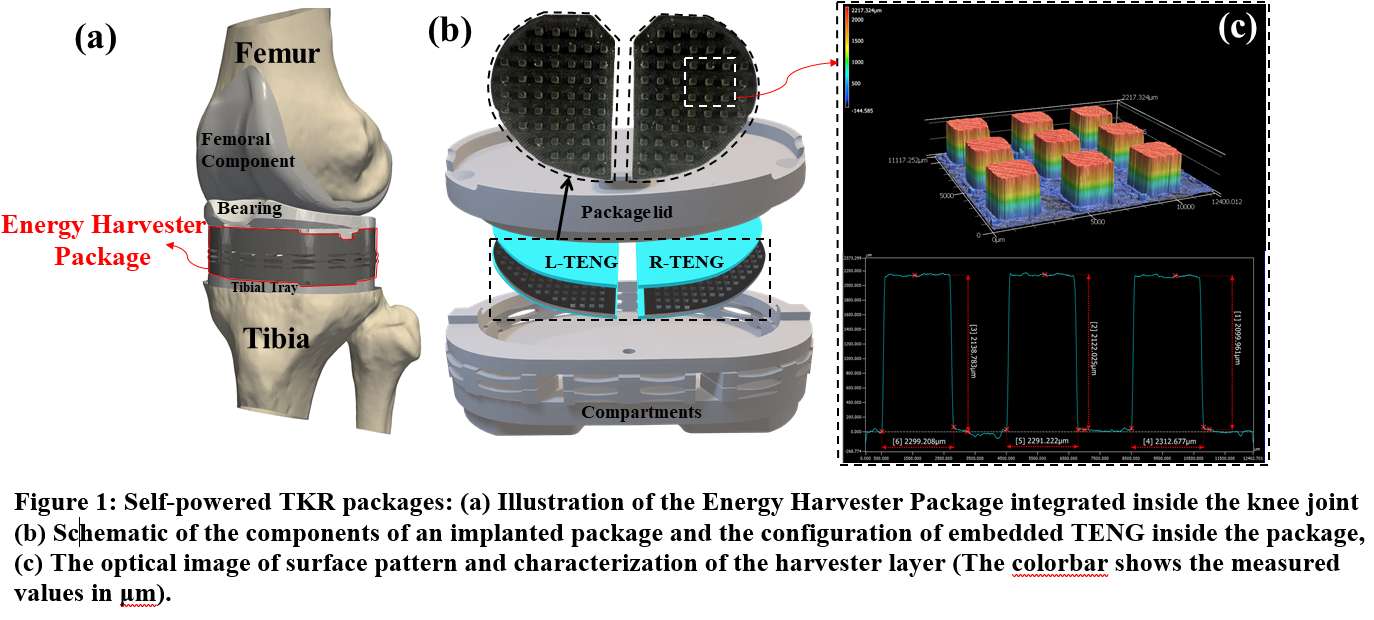
Figure 1
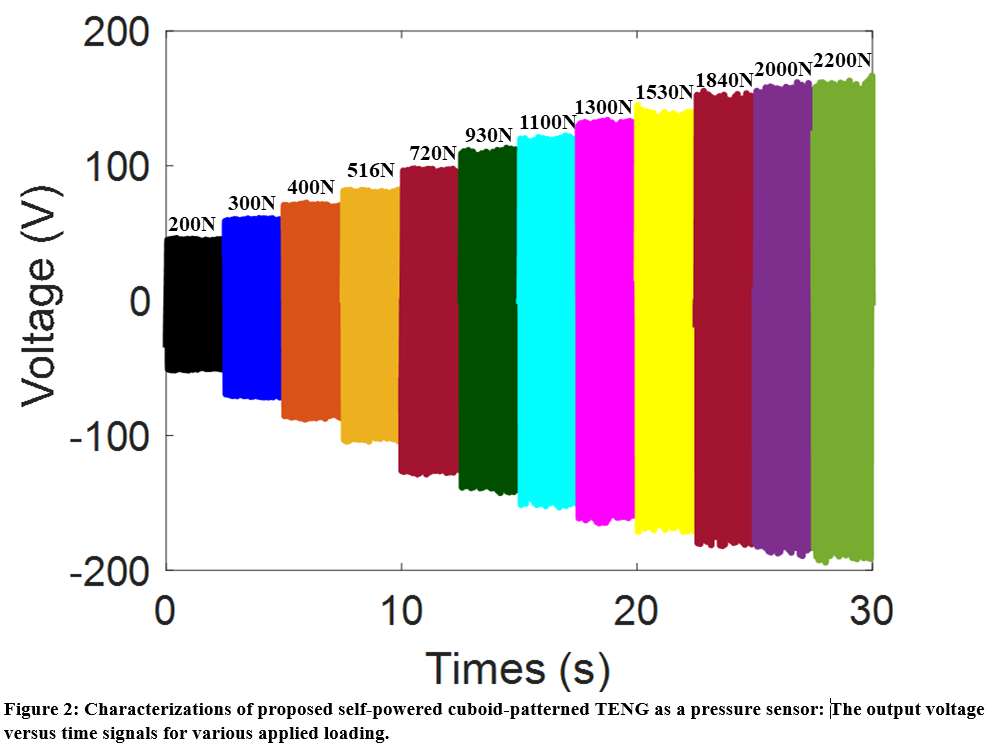
Figure 2
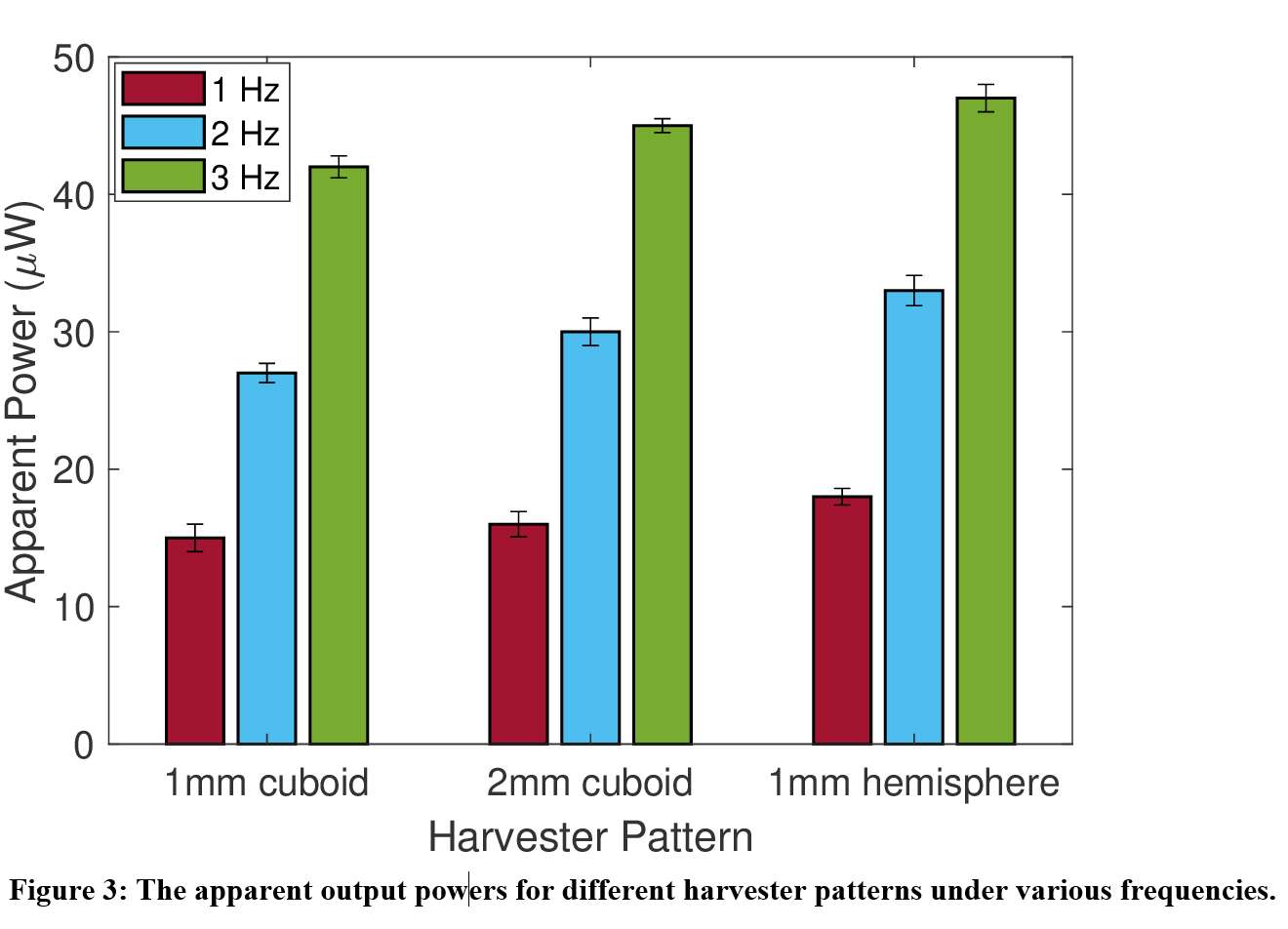
Figure 3#8700
Patient Compliance at One Year Following Sensor Enabled Total Knee Arthroplasty Procedures
Patrick Verta - Canary Medical - Carlsbad, USA
*Patrick Aubin - Canary Medical - Carlsbad, USA
Genne' DeHenau McDonald PT - Canary Medical - Gainesville, United States of America
Fred Cushner - Hospital for Special Surgery - New York, USA
*Email: paubin@canarymedical.com
Introduction
The addition of wearable technology to the clinical pathway offers the potential for remote post-operative patient assessment beyond the scope of current, questionnaire-based patient-reported outcome measures. However, one of the main limitations of wearables is patient compliance, which is known to be low negatively affected by inconsistent device placement, daily wear, periodic need for charging, and internet connectivity. New monitoring implantable total joint prostheses have the potential to reduce the burden on patients and improve patient compliance. This paper evaluates patient compliance at multiple intervals following the indicated TKA procedure.
Methods
A retrospective analysis of the first 398 patients who were implanted with the smart prosthesis. The percentage of patients with their device still connected to their home base station and reporting data was measured at Days 60, 86, 183, and 320. To compare to the implantable device compliance. This was compared to a literature review of healthy and orthopedic patient compliance with wearables was performed.
Result
During the immediate post operative period, patient compliance was high with 93.7 % (373/398 patients), transmitting at 60 days post op and 92 (366/398 patients), percent at 3 months. At 6 months compliance was 85.8 percent and 78.8 percent at 1 year (Table 1). Compared to historical controls for wearables [1-4], compliance was higher throughout the one year follow up period with compliances diminishing slightly at one year post operatively (Table 1). Reasons for noncompliance included patient discontinuing base station and patients requesting to opt out.
Conclusion
Patient compliance at all time points post operatively were significantly higher for the implanted devices as compared to historically reported externally worn activity trackers. These results indicate that implantable devices have reduced burden on the patient as compared to wearable activity trackers which have to be worn continuously and charged periodically. Innovative smart orthopedic devices that enable remote patient monitoring have the potential to transform healthcare by promoting patient engagement, which may ultimately lead to better patient outcomes.
References
- Yu SP, et al. Ann Phys Rehabilitation Medicine, 2022
- Cushner, F. “AAOS Annual Meeting, 2019.
- Lyman S, et al. HSS Journal. 2020
- Hermsen S, et al. Journal Medical Internet Research, 2017.
Figures
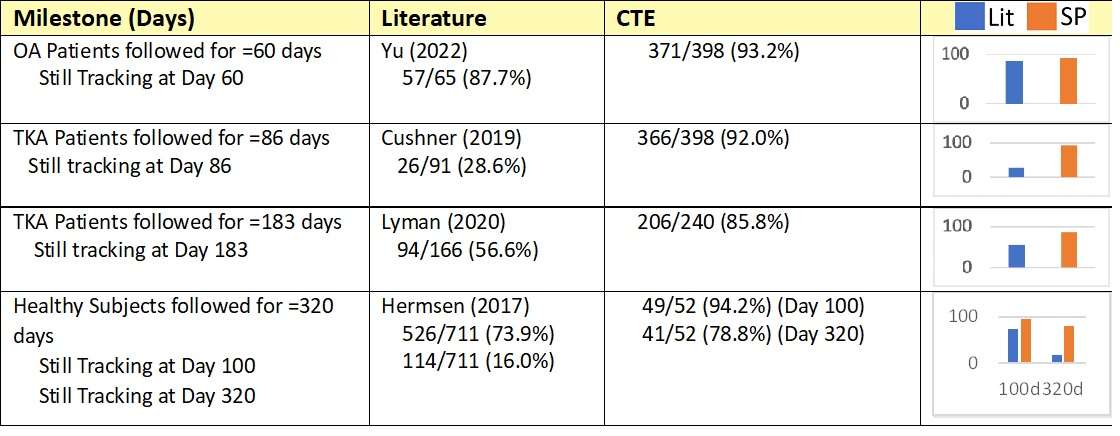
Figure 1#8546
Objective Tracking of Physical Activity in Knee Osteoarthritis Patients Awaiting Joint Arthroplasty
*Kaitlin Genge - Dalhousie University - Halifax, Canada
Annemarie Laudanski - Dalhousie University - Halifax, Canada
Glen Richardson - Dalhousie University & Capital Health - Halifax, Canada
Michael Dunbar - Dalhousie University - Halifax, Canada
Janie Wilson - Dalhousie University - Ancaster, Canada
*Email: Kt763307@dal.ca
Introduction: Physical activity (PA) is an important part of overall health, and activity levels in osteoarthritis (OA) patients awaiting knee arthroplasty are significantly lower than in healthy age-matched individuals1. PA levels are often captured using self-report surveys, however these may be biased or confounded by other factors and may not reflect true activity levels2. Previous studies have reported objectively-measured step-counts in arthroplasty patients3, however limited literature explores how pre-operative step-counts relate to patient characteristics and clinical outcomes. This study’s objective was to examine relationships between objective step-count measures and relevant patient-specific variables (BMI, pain, self-reported PA, and mental health) in individuals awaiting knee arthroplasty.
Methods: End-stage knee OA patients were recruited from the participating surgeons’ arthroplasty wait lists. Questionnaires were used to capture demographic and patient-reported outcome measures (PROMs), including pain (OKS-PCS), PA (UCLA Activity Score), and mental health (PHQ8). To objectively monitor patients’ PA, inertial measurement units (Axivity, AX6) were placed on the shin of the participants’ surgical leg during a scheduled clinic visit. Raw accelerometer data were collected over 7-day periods, and step-counts were calculated in a custom MATLAB (v.9.13) code. Means and standard deviations for each participant and the group were calculated for the central 6 full collection days. Correlation analyses were used to examine associations between objectively-measured step-counts and BMI, OKS-PCS, UCLA, and PHQ8 scores (α=0.05).
Results: Results from the first 9 recruited participants (6F/3M) showed an average daily step-count of 5686 ± 3631(Table 1), consistent with published step-count averages and variability in similar populations4. Higher BMI (Figure 1) and lower self-reported PA levels (Figure 2) were significantly associated with lower step-counts (p=0.023, r2=0.55 and p=0.016, r2=0.59, respectively). An inverse relationship between PA and BMI is consistent with comparisons between patient-reported PA and BMI found in literature5. Our comparison of objective PA with BMI further supports this relationship. Despite a significant correlation, the relationship between self-reported PA and objectively-measured step-count highlights a lack of specificity, with several individuals scoring their PA as 3/10, but having daily step-counts ranging from 2590 to 10008. Preliminary statistics showed no significant correlations between objectively-measured step-count with self-reported pain (p=0.25, r2=0.18) or mental health (p=0.49, r2=0.072).
Conclusion: The combination of objective PA measurements with PROMs suggests the existence of complex interactions between patient behaviors and subjective outcomes. Extensive wait times in Nova Scotia provide a unique opportunity to evaluate physical decline in relation to other relevant patient variables longitudinally throughout the wait period. Continuous and longitudinal recruitment, combined with further multivariate modeling of objectively-measured PA with PROMs and clinical outcomes will help identify patient-specific characteristics relating to pre-operative physical decline, and provide insight into the relationship between PA and surgical outcomes and, therefore, how PA should be considered in surgical timing and triage decisions.
References:
[1] de Groot et al., 2008 Osteoarthr. Cartil., 16(4); 436–442
[2] Prince et al., 2008 IJBNPA, 5(56)
[3] Sašek et al., 2021 J. Clin. Med, 10(24); 5885
[4] Lützner et al., 2014 CORR, 472(12); 3933-3940
[5] Raud et al., 2020 Sci. Rep, 10(1)
Figures
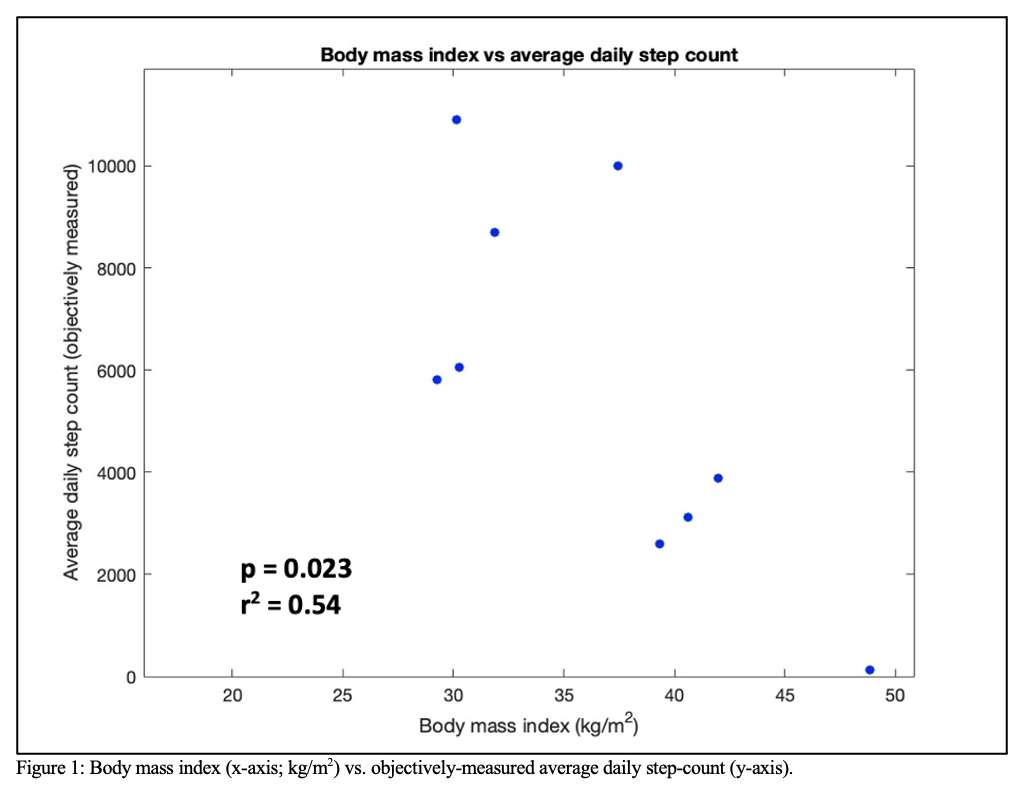
Figure 1
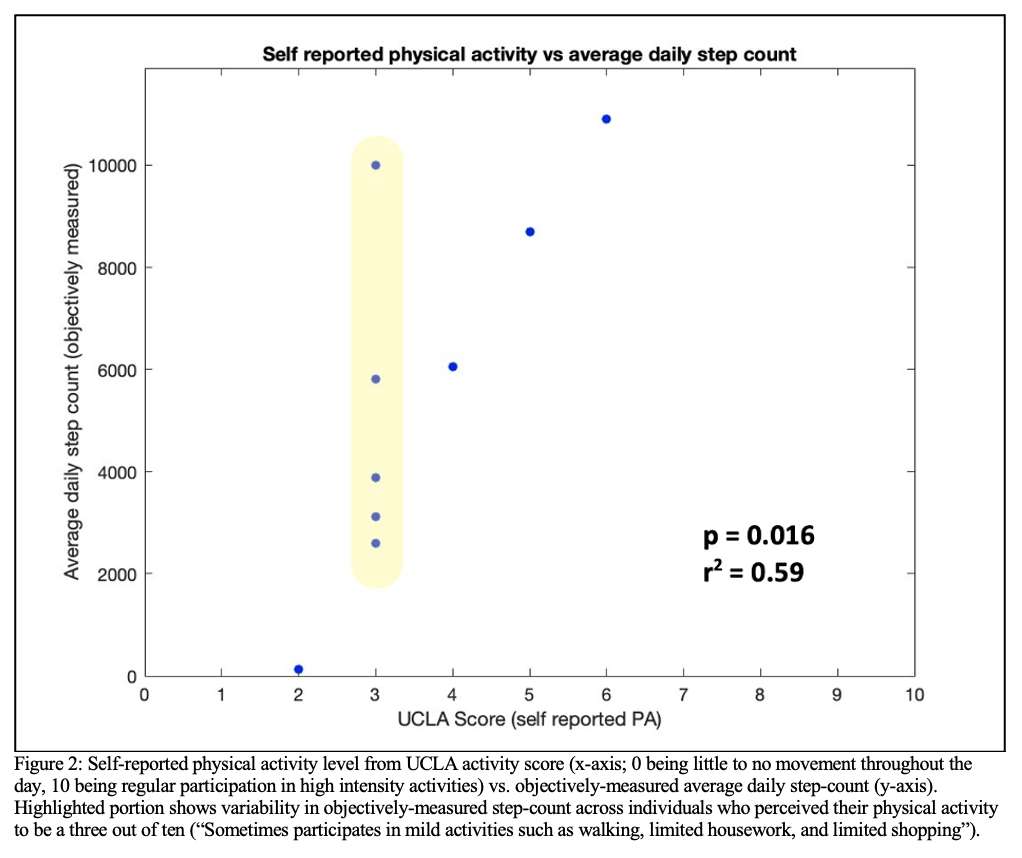
Figure 2
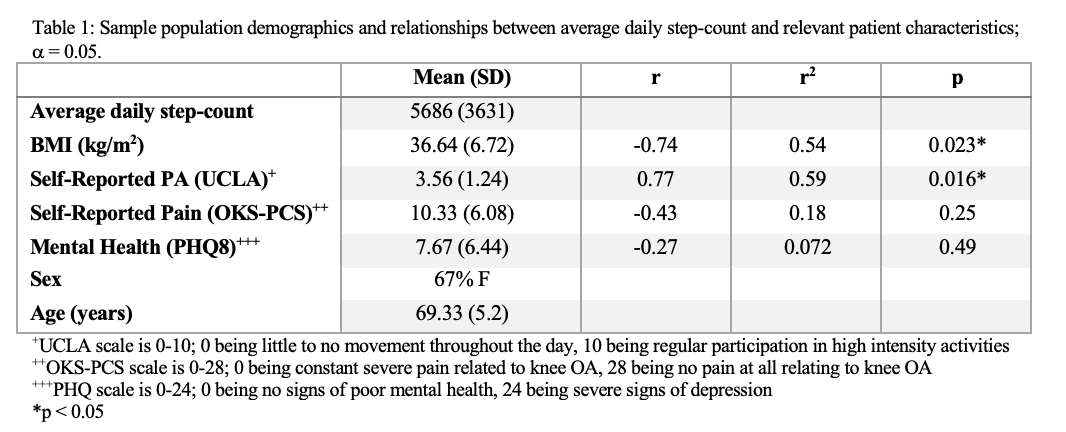
Figure 3#8247
Joint-Specific Patient-Reported Outcome Measures Demonstrate High Reliability When Completed by Electronic Methods
Jess Lonner - Rothman Institute - Philadelphia, USA
*Roberta Redfern - Zimmer Biomet - Pemberville, USA
Anna N Ren - Zimmer Biomet - Gibsonia, USA
Scott Abshagen - Zimmer Biomet - Warsaw, USA
Robert E Neher - Zimmer Biomet - Roanoke, USA
Mike Anderson - Zimmer Biomet - Lehi, USA
Dave Van Andel - Zimmer Biomet - Grand Rapids, USA
Matthew Miller
*Email: roberta.redfern@zimmerbiomet.com
Introduction
Collection of patient-reported outcome measures (PROMs) has been more frequently incorporated in clinical research over a variety of medical disciplines, and particularly within the field of orthopedics. PROMs collection has historically been time-consuming and expensive. Given the potential advantages of electronic PROMs collection, this method has been increasingly utilized in research trials and daily practice. While general PROMs research has suggested that electronic and paper collection elicit equivalent results, few studies have specifically investigated the reliability of electronic collection methods for joint--specific PROMs, such as those utilized in total joint arthroplasty populations.
Methods
Secondary analysis of data from a multicenter, prospective observational cohort study. Patients undergoing total hip arthroplasty (THA), total knee arthroplasty (TKA), or partial knee arthroplasty (PKA) were prescribed the use of a smartphone-based care management platform for self-directed rehabilitation post-operatively. Collection of joint-specific PROMs included Hip dysfunction and Osteoarthritis Outcome Score (HOOS JR) for THA patients and Knee injury and Osteoarthritis Outcome Score (KOOS JR) for TKA patients. PROMs were collected during 5 study-specific intervals; patients were invited to complete questionnaires electronically via the mobile application or on paper during in-person clinic visits. While not intended by the study protocol, a portion of patients completed the same interval survey via both electronic and paper methods. Electronic and paper interval scores were compared to investigate reliability of electronic collection using Pearson and Spearman correlation techniques and Intraclass Correlation Coefficients (ICC) over the entire cohort, as well as by procedure and survey type. Score variance as a function of days between completions by method was also investigated by multivariate longitudinal model.
Results
In total, 467 patients completed 571 PROMs surveys by both electronic and paper method. The average age of subjects was 63.9±9.0 years, mean BMI was 30.1±6.3 kg/m2. Linear correlation over all procedures and survey intervals suggested excellent reliability, with Pearson correlation 0.88 (p<0.0001), Spearman correlation 0.88 (p<0.0001) and ICC=0.87. Adjustment for BMI and age did not affect Pearson or Spearman results. Considering only HOOS JR, Pearson and Spearman correlation coefficients were 0.88 and 0.89, respectively, with ICC=0.88. Similarly, KOOS JR demonstrated high reliability with Pearson and Spearman correlation coefficients of 0.87 and 0.89, respectively, with ICC=0.87. Longitudinal model considering age, BMI, gender, survey, visit, and days between responses suggested only days between response was associated with difference in survey results (p=0.01), where larger differences between electronic and paper methods were associated with longer times lapsed between survey completions.
Conclusions
Electronic collection of the HOOS JR and KOOS JR joint-specific PROMs commonly utilized in arthroplasty populations is reliable, providing similar results as compared to paper methods. Only days between survey completion was associated with differences in scores after including survey and visit in longitudinal models. The advantages of electronic PROMs collection in this population do not appear to be accompanied by loss of data integrity.
#8498
Validation of the MotionSense Wearable Device for Measuring Knee Flexion During Treadmill Walking
*Alexandra Ligeti - University of Strathclyde - Glasgow, GB
Lauren Forsyth - University of Strathclyde - Glasgow, United Kingdom
Mark Blyth
Philip Riches - Univeristy of Strathclyde - Glasgow, United Kingdom
*Email: alexandra.ligeti.2016@uni.strath.ac.uk
Introduction: With 100,000 total knee arthroplasty (TKA) procedures taking place in the United Kingdom annually, and 94% of these procedures occurring in individuals 50 years and older, the demand for home-based rehabilitation is high, however, compliance is poor. Wearable technologies, such as MotionSenseTM (Stryker, US), can remotely support post-operative TKA rehabilitation by providing personalised rehabilitation and tracking of home exercises, enabling healthcare professionals to continuously monitor rehabilitation progress remotely. Validation of such devices across a range of potential ability levels against a known kinematic model in activities of daily living is important for confident interpretation of resulting clinical data. The aim of this study therefore was to validate the accuracy of MotionSenseTM against a clinical motion capture standard.
Methods: Upon receiving NHS ethics approval, twenty able-bodied young individuals (age 24 ± 4 years, mean ± SD) and 14 older participants (71 ± 5 years) volunteered and consented to the study. Retroreflective markers and MotionSenseTM sensors were attached to the lower limb (Figure 1). Volunteers walked for 5 minutes at a self-selected comfortable speed on a treadmill. Vicon PlugInGaitTM determined knee flexion (100 Hz) and the MotionSenseTM sensors exported data in real-time (~50Hz) to a mobile device on which a proprietary algorithm determined knee flexion. Following up-sampling to 1000Hz, cross-correlation was used to time synchronise the measurements in gait cycle windows identified from peak flexion to peak flexion. As the zero point for knee flexion depends on marker placement, the mean knee flexion was subtracted from each data set before calculating a root mean square error (RMSE) between the technologies, determined in each gait cycle window. T-tests compared the older and the younger populations and significance was taken at the 5% level.
Results and Discussion: Fewer gait cycles were collected and analysed on older compared to younger volunteers (93 ± 45 vs 170 ± 107, mean ± SD, p < 0.001). The older volunteers walked slower than the younger group (0.94 ± 0.12 ms-1 vs 1.17 ± 0.07 ms-1 , p < 0.001). RMSE values are similar to previous studies’ RMSE data and demonstrate an excellent agreement between the technologies with a pooled RMSE < 3.5° (Table 1). Walking speed may affect accuracy, as the MotionSenseTM was more accurate in the older group albeit non-significantly (p = 0.21).
Conclusion: MotionSenseTM performed accurately during treadmill walking in both older and young populations. The difference between the technologies may be considered clinically negligible given the inherent variation in such analyses. Further research should be conducted on TKA patients validating the technology for this population.
Figures
Figure 1
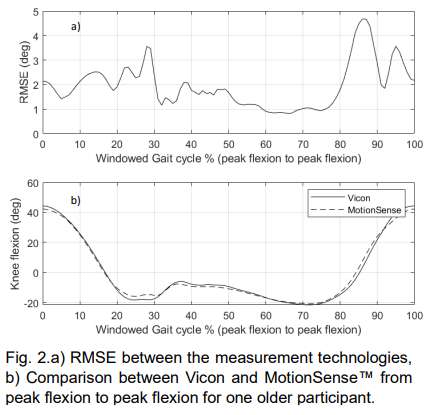
Figure 2
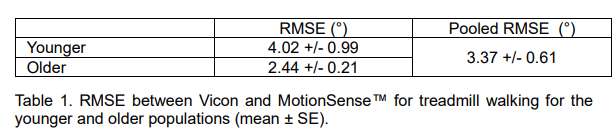
Figure 3#8487
A Proposed Method to Objectively Track Recovery Rates of Patients Following Total Knee Arthroplasty
*Richard Bolander - TracPatch - Orland Park, United States of America
S. David Stulberg - Northwestern Memorial Hospital - Chicago, USA
Danielle Marshall - University of Miami/Jackson Memorial Hospital - Miami, United States of America
Victor Hernandez - University of Miami Hospital - Miami, USA
*Email: rbolander@tracpatch.com
Introduction
Wearable devices provide a way to continuously monitor patients pre and post-surgery using objective metrics. The derived data allows for the ability to set expectations for daily recovery standards and identify cases where patients fall outside of the norms for their matched cohorts. To define these standards, an aggregate of patient recovery data needed to be captured and analyzed. In this abstract, we calculated the daily recovery rates across three metrics associated with the function of the knee following total knee arthroplasty.
Method
Data were collected from the TracPatch System, which consists of two wearables that are used to track the position of the leg (max and min) position of the leg each minute. 481 patients from 11 surgeons and 8 institutions were retrospectively analyzed. Devices were worn on average of 44±27 days and for 12± 4 hours per day. The population consisted of 62% female and had mean age of 67. 82% of females and 90 % of males had BMI greater than 30. 95% of patients experienced a midline incision.
To assess the functional limits of the knee each day, the system utilizes the daily progress tests, by means of a sitting lift or seated heel slide, that result in a min and max knee position. The total daily activity was quantified using step counts. To characterize the total daily behavior the position of the leg was classified into six categories (1: -5 to 15, 2: 15 to 45, 3: 45 to 75, 4: 75:115, 5, 115 to 130, 6: Active) for each minute in which the knee was in slight motion. Each category was then calculated as a percentage of a daily distribution.
Results
Boxplots were generated for Flexion and Extension by day and reported in Figure 1. Boxplots for the total daily step count are provided in Figure 2. A representation of total daily activity is provided in Figure 3.
Conclusion
The results of this study provide a method to characterize the daily recovery of a patient across multiple metrics. Each metric progresses at a different rate and measure of function than the current best practice of measuring flexion and extension and 4 or 6 week follow-up appointments.
Figures
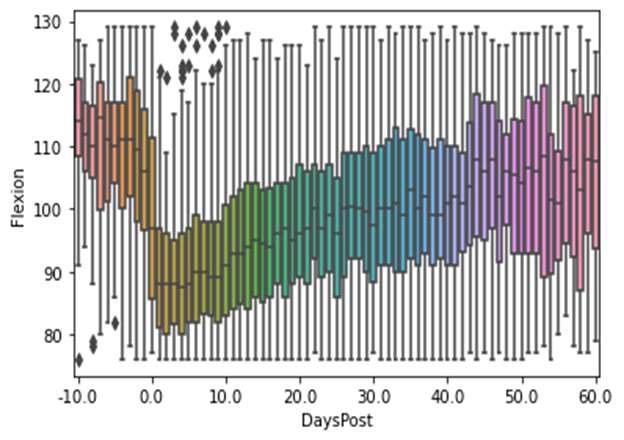
Figure 1
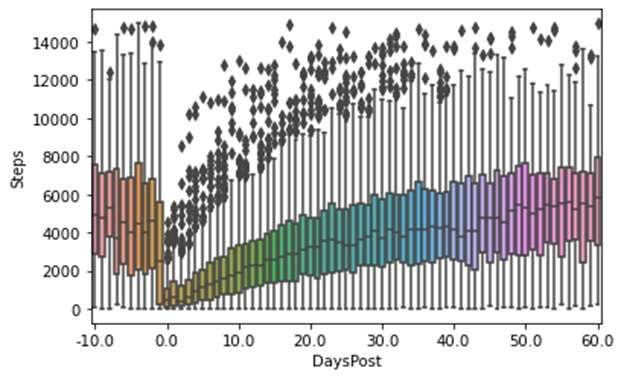
Figure 2
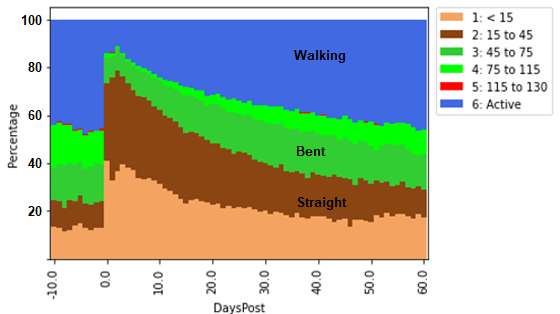
Figure 3#8490
Characterization of the Relative Change in Objective and Subjective Metrics by Baselining Patients With Wearable Technology Prior to Total Knee Arthroplasty
*Richard Bolander - TracPatch - Orland Park, United States of America
Sarah Bolander - Exhibitor: TracPatch - USA
S. David Stulberg - Northwestern Memorial Hospital - Chicago, USA
Danielle Marshall - University of Miami/Jackson Memorial Hospital - Miami, United States of America
Victor Hernandez - University of Miami Hospital - Miami, USA
*Email: rbolander@tracpatch.com
Wearable sensors and associated supporting technologies (i.e. patient applications) can provide both objective (joint position, step counts, etc.) and subjective data (i.e. pain scores and PROMS) to track a patient’s episode of care. Establishing a subjective and objective baseline of a patient’s experience may arguably be beneficial for multiple reasons including, setting recovery expectations for the patient and demonstrating effectiveness or success of the intervention.
In this abstract we characterized a subset of patients (n=80 from 7 surgeons) that wore the TracPatch system at least 6 days prior to total knee arthroplasty and provided data up to 50 days post intervention. The 5-day average prior to surgery for total step counts (activity), achieved flexion on a progress test (functional limit) and VAS daily pain score were calculated. The difference from baseline was then calculated for each patient for each day post-surgery and reported as averages. The compliance of conducting any exercise using the TracPatch app was also calculated and reported as the number of unique patients each day post-surgery.
On average, a patient will experience a deficit of 4000 steps immediately following surgery that will return to near baseline levels 50 days post intervention. A 30-degree deficit will be observed in flexion that will return at a similar rate as steps. VAS scores will worsen with an increase of approximately 3 points immediately following surgery. However, pain will decrease by two points relative to baseline between 40 and 50 days. For exercise compliance, we observed a period immediately following surgery where patients did not want to complete exercises until 7 days post intervention, in which post-surgical exercise compliance peaked. Then beyond that point exercise compliance then steadily decreased over time, presumably due to lack of interest or through the lack of perceived need due to increased functionality and decreased pain.
The results of this abstract demonstrate the concept that tracking a patient’s presurgical subjective and objective data provides a reference for postsurgical outcomes. On average, a patient will regain near similar functionality and decreased pain 40 to 50 days following intervention. Applications for this data include benchmarking for evaluating intervention success as well as setting patient expectations. The observation of increased pain and decreased exercise compliance following surgery highlights the need to train patients on wearable technologies prior to surgery so that adequate training and comfort with using the system can occur. Future evaluations will include increased sample sizes and responses reported for relevant cohorts and intervention strategies.
Figures
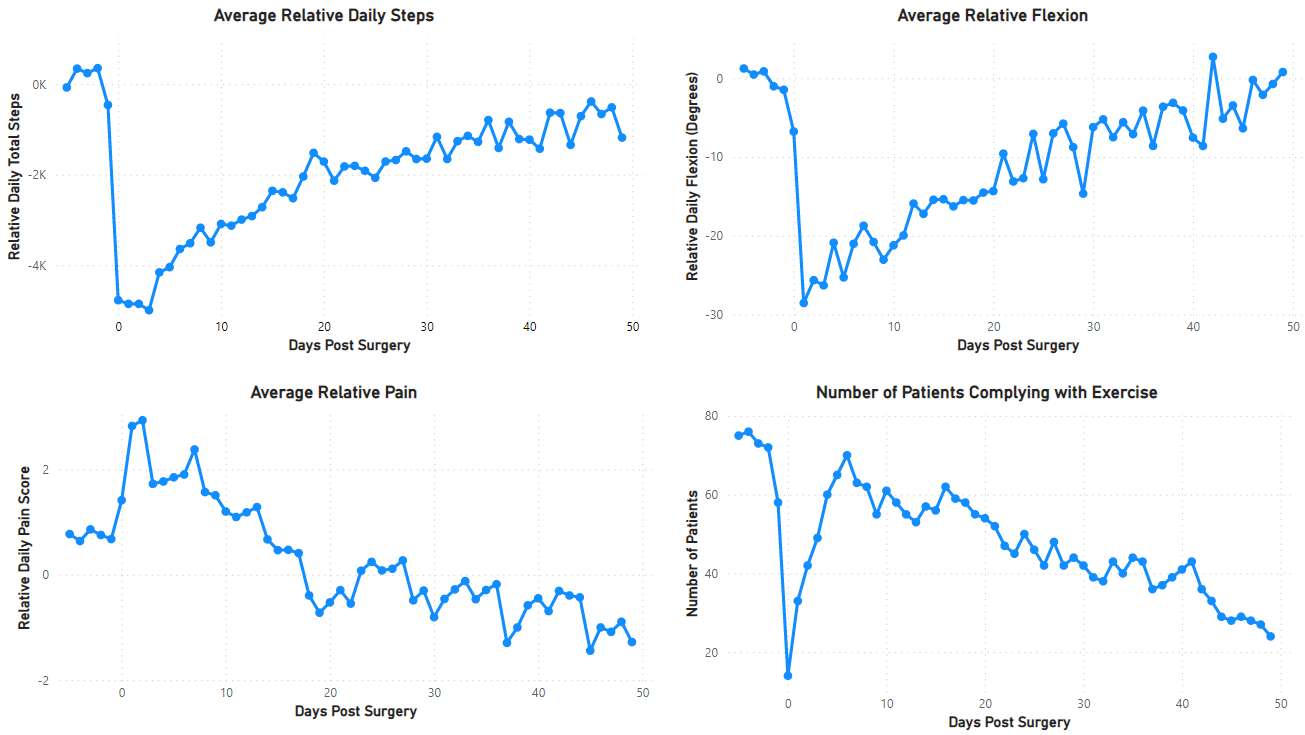
Figure 1#8272
Assessment of Color Sensor Technologies for Telehealth Applications
*Kevin Abbruzzese - Stryker - Mahwah, USA
Vincent Alipit - Stryker - Mahwah, USA
Andre Freligh - Stryker Orthopaedics - MAHWAH, United States of America
*Email: kevin.abbruzzese@stryker.com
Introduction:
Color sensing technologies can detect and measure the color of an object or surface based on the intensity of red, green, and blue wavelengths and represented as a digital signal. Sensors that convey color spectrum information can be a valuable tool to aid in telehealth applications where subjective assessments are often based on visual perception. Telehealth applications that assess physiological conditions or pathologies based on color, like skin conditions, urological disease, wounds, and even thermography assessments may benefit from objective and quantifiable methods [1]. The wound healing continuum classifies wounds appearance according to color as a practical way of guiding chronic wounds assessment and intervention [2]. Several color sensor technologies were evaluated to determine the accuracy of color measurements.
Methods:
RGB measurements were obtained from Nix Mini 2 Color Sensor, TCS34725 RGB Sensor, and a virtual reality interface using the Azure Kinect camera using a standard set of BEHR® paint swatches, Table 1. The default colors for red, green, blue, and yellow were evaluated for each paint sample (N=8). Colorimetric data was assessed to determine the difference between two colors based on Euclidean distance, chromaticity, and Euclidean chromaticity measurements from acquired RGB intensity values ranging from 0-255 [3]. Euclidean distances were calculated as the square root of the difference of the sum of the squares of the RGB values. Chromaticity estimates were considered to determine the differences between colors based on colorfulness (hue and saturation). Euclidean distances for chromaticity values were estimated to determine device accuracy.
Results:
An Anderson-Darling test was used to assess normality for Euclidean distance, chromaticity, and Euclidean chromaticity distance. Euclidean data was normally distributed and an ANOVA test was used to assess significance between Euclidean distance data. No significant differences (p=0.22) were detected between the Nix color sensor (42.72±26.55), the TCS34725 sensor (49.85±24.92), or the Azure Kinect (67.0±30.7), Figure 1. A Kruskal Wallis test was used to assess significance for chromaticity values between devices. No significant differences were detected between devices for chromaticity values of red, green, and blue, Table 2. An ANOVA test was used to assess significance for 2D chromaticity values. No significant differences (p=0.355) were detected between the Nix color sensor (0.096±0.074), the TCS34725 sensor (0.058±0.022), and the Azure Kinect (0.099±0.074).
Conclusion:
The purpose of this study was to assess the accuracy of using color sensors or technologies to measure the differences between RGB color values. The results of the study indicate that any of the tested devices are capable of measuring RGB colors with no significant differences detected between the control RGB values for Euclidean distance, chromaticity, and 2D Euclidean chromaticity. The TCS34725 color sensor performed more similarly to the Nix color sensor based on Euclidean distance estimates, suggesting comparable luminance. The respective colors are limited compared to the variety of colors that can be seen in the real world. Further research is needed to better understand the sensitivity of RGB estimates. This can help ensure accuracy and effectiveness in potential colorimetric telehealth assessments.
Figures
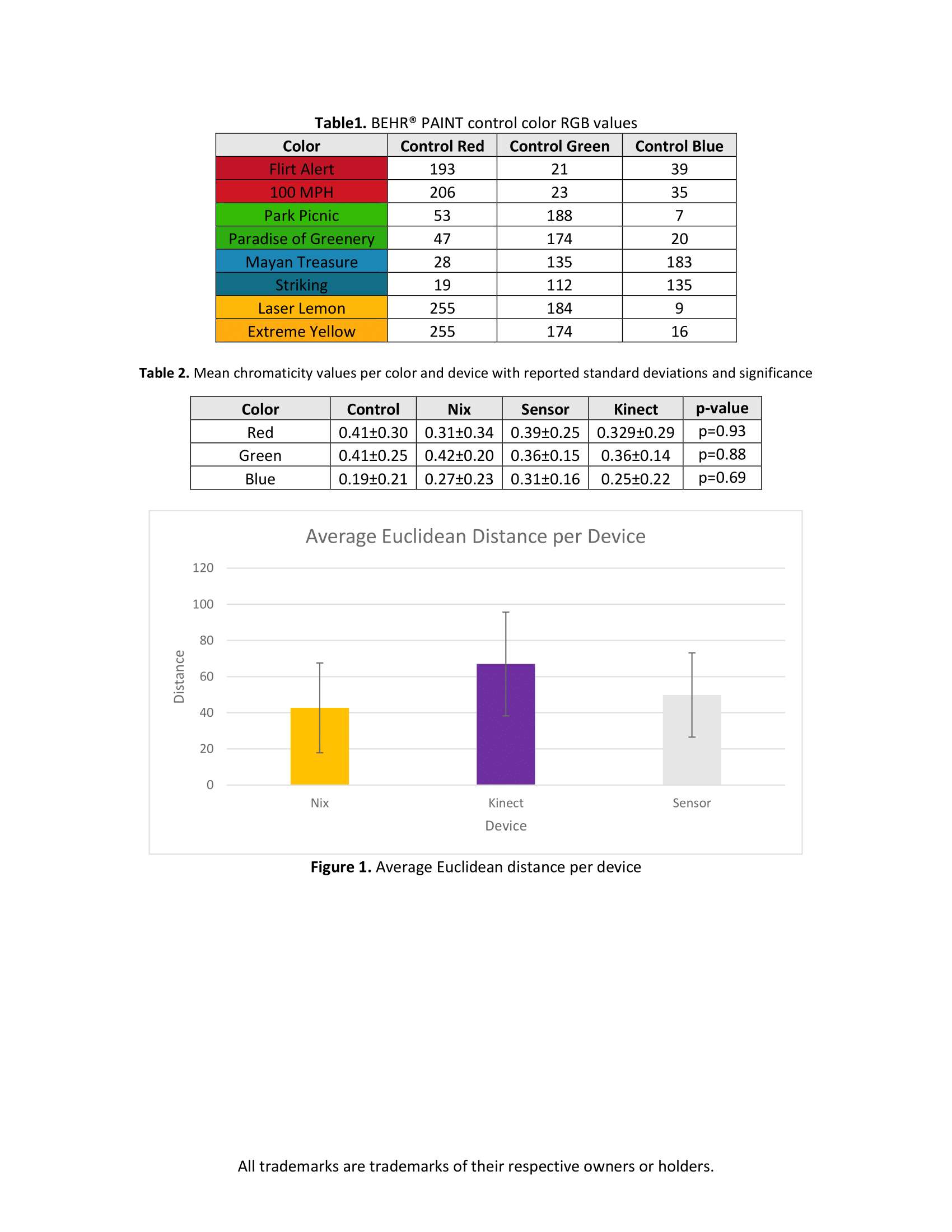
Figure 1#8508
An Optoelectronic Motion Capture Protocol for in Clinic Orthopaedic Gait Analysis
*Monica Malek - St. Joseph's Healthcare Hamilton - Hamilton, Canada
Kim Madden - McMaster University - Hamilton, Canada
Vickas Khanna - McMaster University - Hamilton, Canada
Anthony Adili
Habeeb Khan - St Josephs Hospital - Hamilton, Canada
Janie Wilson - Dalhousie University - Ancaster, Canada
*Email: malekm10@mcmaster.ca
Purpose: optoelectronic motion capture has been used extensively in orthopaedic research to capture functional deficits during walking in patients before and after surgery. Despite significant research uptake, clinical translation of these tools has been poor. Motion capture is typically done in specialized lab environments. However, this presents problems for patient access, data throughput, and participant recruitment and retention in research. Therefore, we aimed to implement a motion capture system in our orthopaedic clinic for ease of patient access. Our objectives were to 1) design and install a motion capture setup and protocol in the orthopaedic clinic hallway for instrumented gait data collection, and 2) collect pilot kinematic gait data from patients before and after joint replacement surgery to demonstrate feasibility and preliminary validity of the setup.
Methods: We designed the system layout and integration and installed a 14-camera optoelectronic motion capture system (Optitrack; optitrack.com) in the hallway of the orthopedic clinic within the constraints of a busy clinic environment and a hallway considerably narrower (put dimensions here) than typical motion capture labs. A novel retroreflective marker placement protocol was created to account for the suboptimal viewing volume and minimal sagittal plane viewing coverage. Six healthy adult volunteers were recruited to capture walking gait trials in the hallway setup at two time points, one week apart, to examine day-to-day-repeatability of kinematic outcomes with the system. Patients participating in our RoboKnees randomized trial on robotic assisted (Mako; stryker.com) knee arthroplasty walked the length of the hallway five times at their self-selected walking speed pre-operatively, three months, six months, and 12 months post-operatively to assess gait changes over time. Three-dimensional lower extremity joint angles and stride characteristics were modeled from the motion capture data using Visual 3D (C-Motion Inc; c-motion.com).
Results: To date, sixteen participants scheduled for knee arthroplasty participated in the gait analysis during their regularly scheduled preoperative visit and at three, six and 12 months postoperatively. Patients were on average 61 (±6) years old with a BMI of 35 (±8). Mean pre-operative walking speed of preoperative patients was 0.94 (±0.14) m/s. 3D knee angle metrics were found to be within variability limits of literature values from specialized gait labs on similar populations. This preliminary demonstrates feasibility of our in-clinic motion capture setup.
Conclusion: We have designed and installed an optimized setup for instrumented gait analysis in an orthopaedic hallway. Our preliminary results demonstrate feasibility and face validity. We will use the system for future studies assessing function before and after hip and knee arthroplasty and other orthopaedic procedures. The setup has fostered great collaboration potential among a multidisciplinary team of biomedical engineers, kinesiologists, trial methodologists, and orthopaedic surgeons.
#8743
Assessing Knee Range of Motion Using the MotionSense Wearable Device in Total Knee Arthroplasty Patients During Treadmill and Stair Walking: A Case Study
Alexandra Ligeti - University of Strathclyde - Glasgow, GB
Lauren Forsyth - University of Strathclyde - Glasgow, United Kingdom
Mark Blyth
*Philip Riches - Univeristy of Strathclyde - Glasgow, United Kingdom
*Email: philip.riches@strath.ac.uk
Introduction: Although 100,000 total knee arthroplasty (TKA) procedures take place in the U.K. annually, there is a lack of standardisation for TKA rehabilitation protocols [1]. Knee range of motion (ROM), given its importance in walking and activities of daily living demonstrates the largest change in the first 4 weeks post-TKA [2], with greater ROM and walking ability associated with greater patient satisfaction [3]. Wearable technologies present a solution to enhance home-based rehabilitation by remotely and continuously monitoring and assessing patient progress, particularly over the initial phase of rehabilitation. For implementation into home-based rehabilitation, it is important for wearable sensors to provide accurate and reliable information. The aim of this study was to assess knee range of motion using the MotionSenseTM sensors and App and to validate the results against a clinical motion capture standard in a TKA patient pre- and post-TKA surgery.
Methods: Following approvals, a 71-year-old female (Weight: 89.6kg; Height: 1.71; BMI: 30.75kg/m2) consented and visited the Human Performance Laboratory in the Clinical Research Facility at the Glasgow Royal Infirmary pre-TKA surgery and 1-week post-TKA surgery. Retroreflective markers were attached, as per the Vicon PlugInGaitTM model. In addition, MotionSenseTM sensors were attached unilaterally to the lower limb (Figure 1). The participant completed treadmill walking, consisting of 5 minutes walking at a self-selected comfortable speed on a level treadmill, and stair ascent/descent, completed on a flight of 4 stairs. The ascent/descent was completed 3 times per trial, with 3 trial repetitions, totalling 9 ascents/descents of the stairs. Knee flexion was determined by Vicon PlugInGaitTM and by an proprietary algorithm embedded on a mobile App. Following up-sampling to 1000Hz, cross-correlation time synchronised the measurements in gait cycle windows identified from peak flexion to peak flexion. A root mean square error (RMSE) between the technologies was determined and averaged across each gait cycle window.
Results: Treadmill walking was completed at the same speed pre- and post-TKA (0.7m/s), however more gait cycles were collected and analysed pre-TKA (18 vs 15). For the stair ascent/descent 13 more gait cycles were collected and analysed pre-TKA (32 vs 19). Knee ROM was similar during both walking and stair ascent/descent, but decreased following surgery (table 1). For both activities the participant remained in flexion, with limited ability to extend the knee post-TKA. The RMSE data demonstrated an excellent agreement between the technologies for both walking and stair ascent/descent pre- and post-TKA. For walking, the RMSE values were 1.59°±0.65 on average (±SE) (pre-TKA:2.23°; post-TKA:0.95°. For the stair ascent/descent the RMSE values were 1.27°±0.04 on average (±SE) (pre-TKA: 1.23°; post-TKA: 1.30°).
Conclusion: MotionSense performed accurately during treadmill walking and stair ascent/descent activity. At one-week post-TKA participant ROM reduced as expected. This is an ongoing study and will benefit from recruiting more patients and conducting further follow-up assessments.
References
[1] D. F. Hamilton et al., BMJ, p. m3576, 2020.
[2] Kornuijt, G. J. L. et al., Musculoskelet. Surg., 103, 3, 289-297, 2019.
[3] Van Onsem S, Knee Surg. Sports Traumatol. Arthrosc., 26, 11, 3272-3279, 2018.
Figures
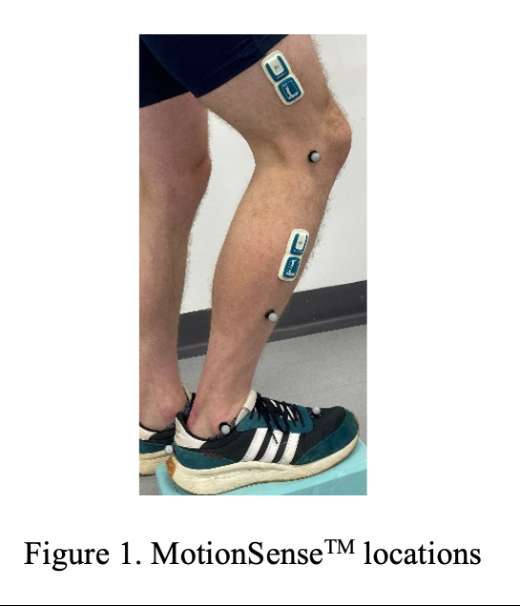
Figure 1
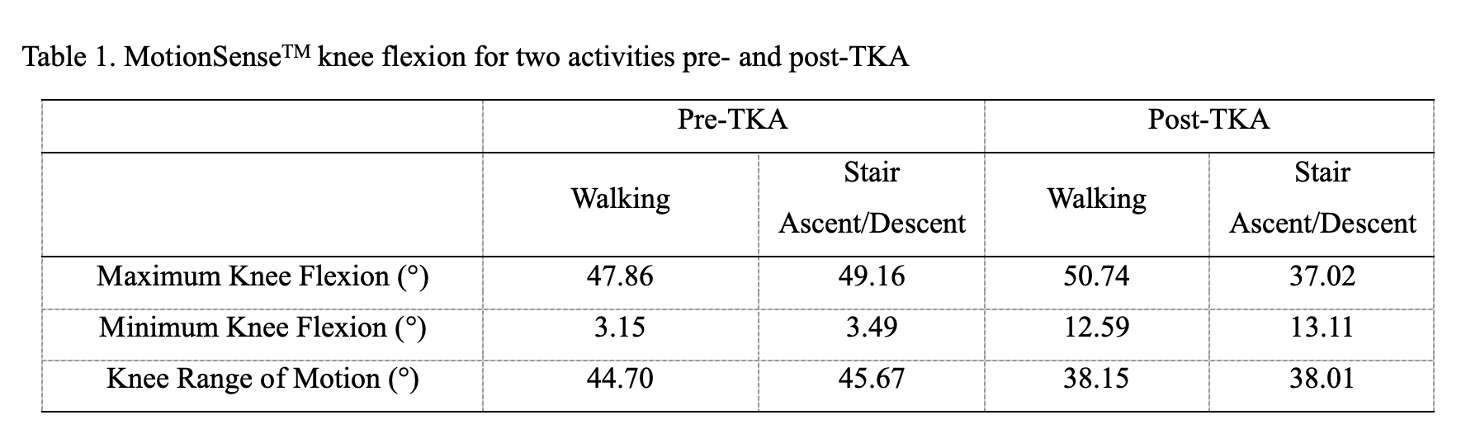
Figure 2#8134
Comparison of Osteosynthesis With and Without Additional Plate for Vancouver Type B1 and Type C Periprosthetic Fractures of Femur
JI WAN KIM - University Of Ulsan, Asan Medical Center - Seoul, Select Country
*Keun-Ho Kim - Asan Medical Center - Seoul, South Korea
*Email: d200626@amc.seoul.kr
Introduction: This study aimed to compare the clinical and radiologic outcomes of well-fixed periprosthetic femoral fractures (PFF) after hip arthroplasty according to augmentation with attachment plate.
Patients and methods: The medical records of patients who underwent reduction and internal fixation of Vancouver type B1 and C periprosthetic femoral fractures after hip arthroplasty between June 2006 and June 2021 were retrospectively obtained from a single center. Data on demographic data, injury mechanism, fracture pattern including open fractures and Vancouver classification, cause of hip arthroplasty, time interval between fracture and arthroplasty, and surgical method were recorded. Functional outcomes including Koval and HHS Score at postoperative 1 year were reviewed. The need for reoperation was also reviewed. Radiologic findings included union time and anatomical alignment of femur at the last radiologic follow-up.
In this study, nonunion and malunion was regarded as an unsatisfactory outcome. We compared surgical outcomes according to augmentation of attachment plate use.
Results: Thirty two patients were included. Their mean age was 79.5 years (SD: 8.8), and the mean follow-up period was 18.4 months. The fractures resulted from high-energy (6 cases) and low-energy (26 cases) injuries. No case involved open fractures. There were 21 cases of Vancouver type B1 fractures and 11 cases of type C fractures. There were 15 patients of use of attachment plate (group 1); 17 patients of non-use (group 2). In group 1, the number cortices purchased with the proximal screws were more than that of group 2 (14.2 ± 4.1 vs 3.4 ± 3.9, p < 0.001), while proximal wire was more frequently used in group 2 (0.2 ± 0.4 vs 1.3 ± 1.2, p = 0.004). Distal screws were also used more in group 1(13.5 ± 4.6 vs 5.6 ± 4.6, p < 0.001). One patient in group 1 (6.7%) and 7 (41.2%) in group 2 had a nonunion or delayed union (p = 0.07).One patient (6.7%) in group 1 had nonunion and she achieved bone union after osteosynthesis with bone graft. In group 2, 7(41.2%) had a nonunion 5(29.4%), or delayed union 2(11.8%), which showed no significant difference (p = 0.069). The union times were 24.0 ± 12.7 and 41.5 ± 53.2 weeks in group 1 and group 2, respectively. No significant difference between the two groups (p = 0.678) was noted. Beals-Towers criteria revealed excellent or good outcomes of 100% in group 1 and 88.2% in group 2 respectively. Group 2 has more common healing problemcomes (6.7% vs 41.2%, p = 0.031).
Conclusion: I
The augmentation with the attachment plate improved clinical outcome in patients with well-fixed PFF. We believe that the increased number of proximal cortices of screw purchase and the longer plate provided adequate fixation and might yield satisfactory outcomes.
#8773
Biomechanical Evaluation of a Cerclage Suture System for Fixation of Femoral Fracture Fragments in Revision THA
Shuyang Han - University of Texas Health Science Center at Houston - Houston, USA
Robert Frangie - University of Texas - Houston, United Kingdom
Nicholas D. Lanfermeijer - UTHealth - Houston, USA
Jonathan E. Gold - UTHealth - Houston, USA
Sabir K. Ismaily - UTHealth - Houston, USA
Andrew Yoo - UTHealth - Houston, USA
Philip Noble - Institute of Orthopedic Research and Education - Houston, USA
*David Rodriguez - University of Texas - Houston, USA
*Email: david.rodriguezquintana@uth.tmc.edu
Introduction: Periprosthetic fracture of the femur is a common complication after total hip arthroplasty. The choice of fixation method for the femur fracture plays a critical role in the overall success of intraoperative fixation obtained during the procedure. While traditional metal cerclage cables have been widely used, newer non-metal systems have emerged as potential alternatives, offering advantages such as improved soft tissue interaction. The objective of this study was to biomechanically evaluate and compare the efficacy between a commonly used CoCr cerclage cable and a cerclage suture system for fixation of femoral fracture fragments.
Methods: Six pairs of cadaveric specimens were utilized in this study. An ETO fracture was first created using a validated methodology. The fractures were then fixed with either a CoCr cerclage cable (Group A) or a TigerTape (Arthrex, FL) cerclage (Group B). Six pairs of retroreflective markers were rigidly fixed along the fracture to measure the movement of the ETO fragment relative to the femur in four zones (Figure 1). Subsequently, revision THA with a tapered fluted stem (Arcos, ZimmerBiomet) was carried out. Throughout the implantation process, the change in distance between each pair of markers was measured using a high-resolution laser scanner at five time points: after reduction, after reaming, after stem insertion, after reaming for proximal body, and after proximal body insertion. Following implantation, each specimen was subjected to multi-axial loading for 500 cycles under 70% of the peak forces and moments during walking. The motion of the fragments and the stem with respect to the distal femur was measured using an 8-camera motion capture system (Vicon, UK).
Results: No significant difference in the three-dimensional marker displacement was observed during each step of revision THA between Group A and Group B (p>0.05). After implantation, the average motion across all marker positions was 0.53±0.86mm vs 0.33±1.11mm in the anterior direction, 0.51±0.49 vs 0.42±0.56mm in the inferior direction, and -0.30±0.53 vs 0.23±0.80mm in the lateral direction. After 500 cycles of multiaxial loading, stem subsidence in Group A was 0.43±0.56mm vs 0.45±0.53mm in Group B (p>0.05, Figure 2). In terms of marker displacement after the testing, there was no significant difference between the two groups in all four zones (Figure 3).
Conclusion:
The present study compared the efficacy of CoCr cerclage and TigerTape cerclage for periprosthetic fracture fixation. The results did not reveal any significant differences between the two fixation methods in terms of the measured parameters. Larger clinical studies with long term follow-up are necessary to assess clinical outcomes and provide insights to guide the selection of the optimal fixation method. Ultimately, the choice of fixation method should consider multiple factors, including surgeon preference, patient characteristics, and bone quality tailored to each revision THA procedure.
Figures
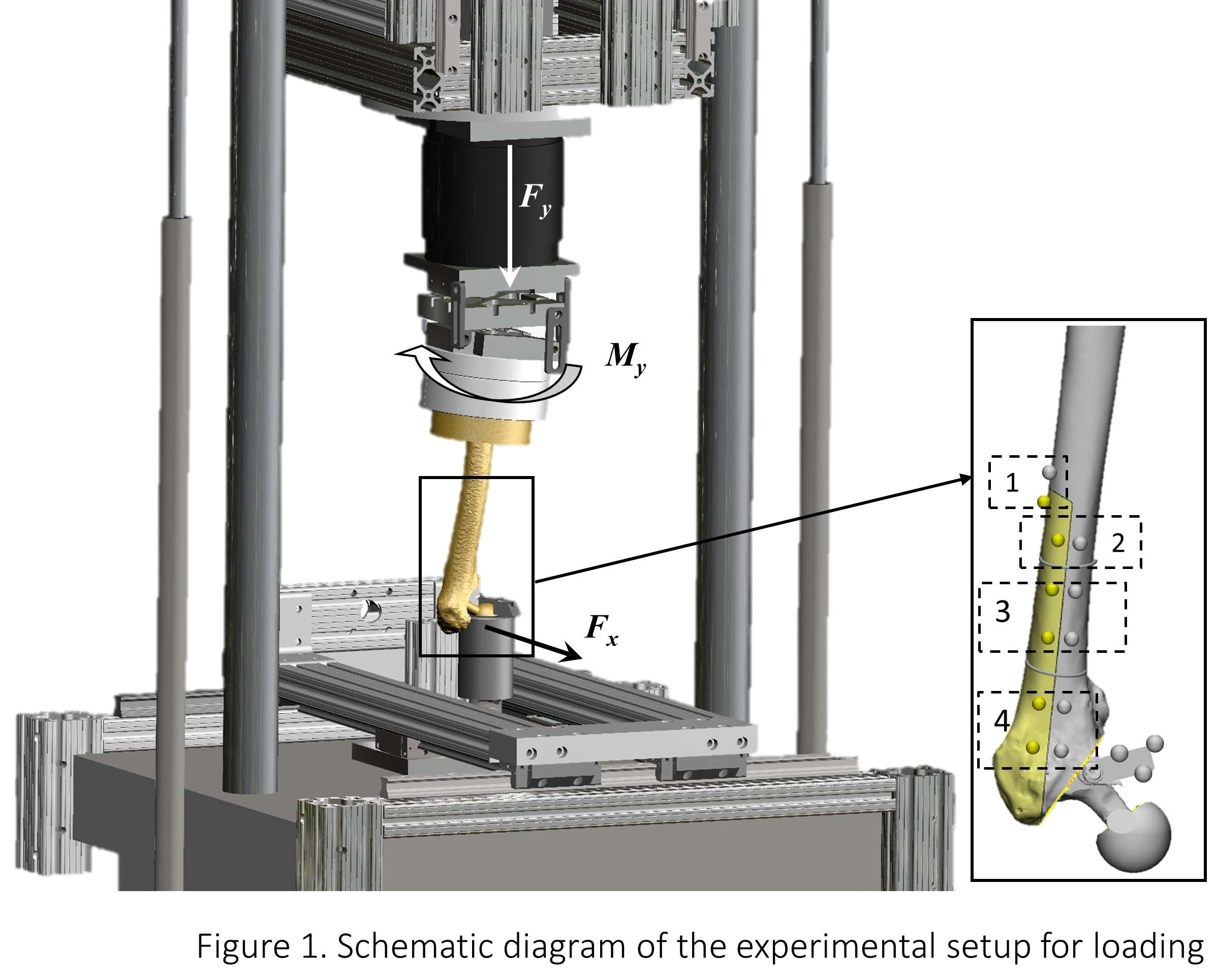
Figure 1
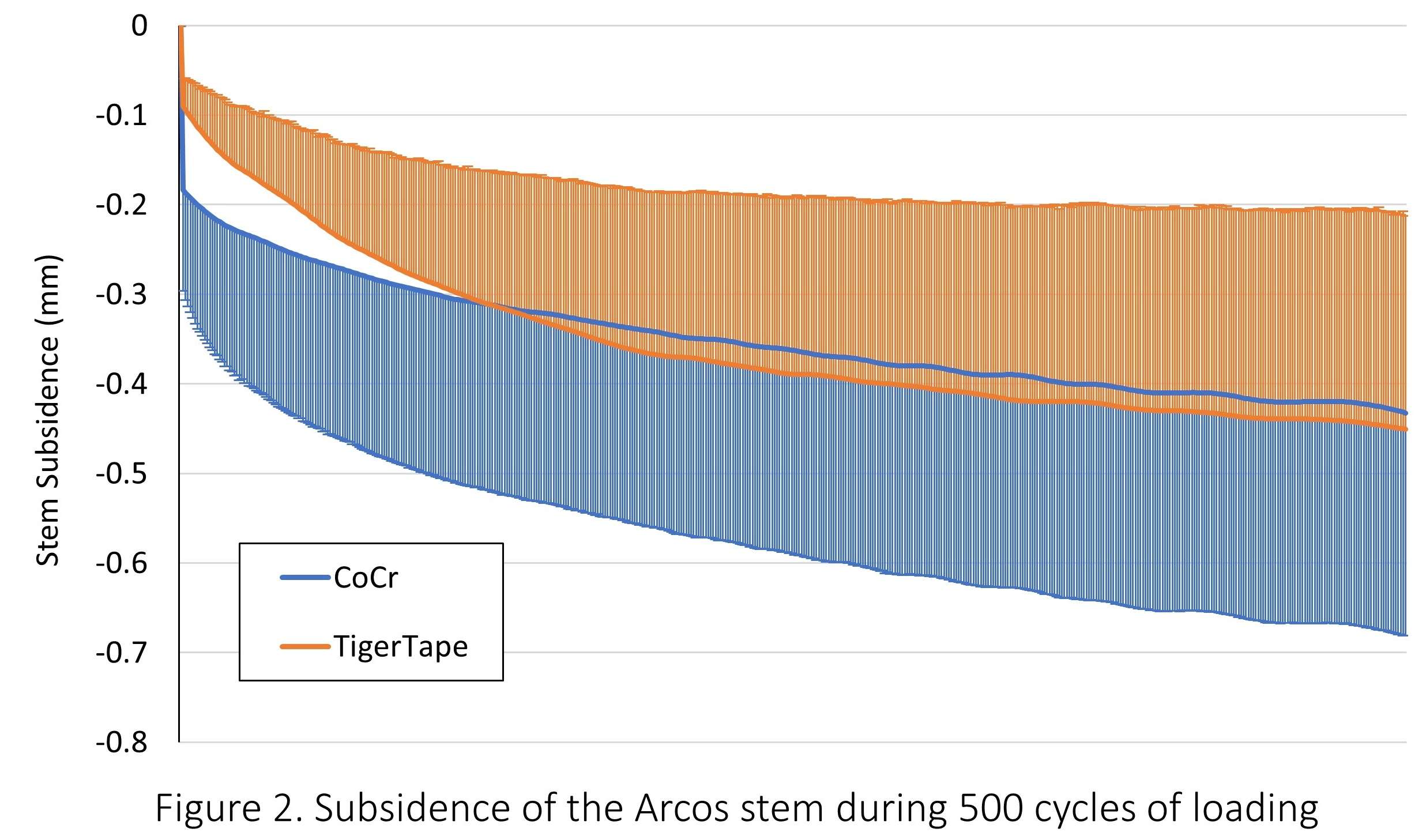
Figure 2
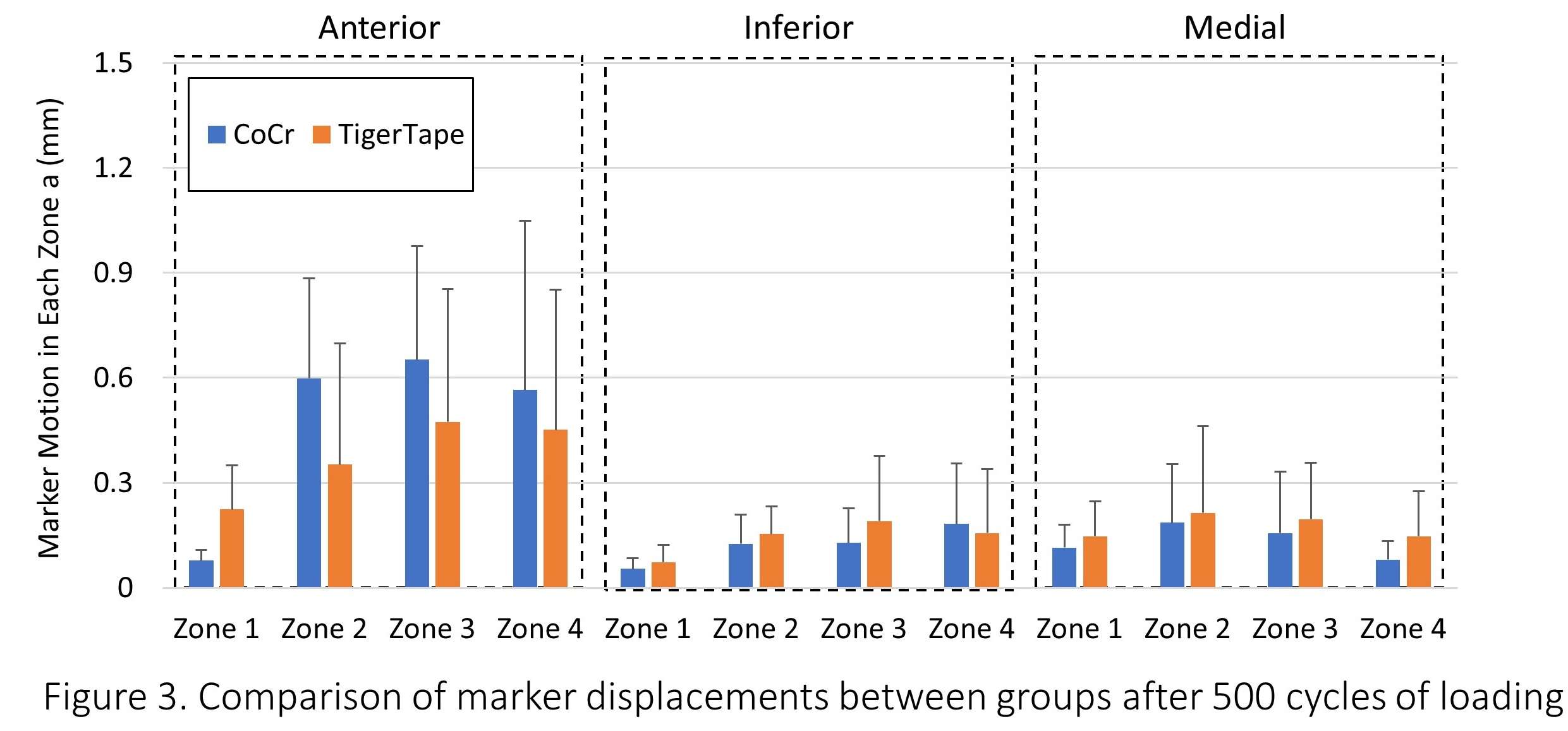
Figure 3#8456
Non-Metallic Cerclage Fixation Methods for Periprosthetic Hip Fractures Offer Promising Outcomes: A Systematic Review
*Benjamin Domb - American Hip Institute - Des Plaines, USA
Ali P. Parsa - American Hip Institute - Des Plaines, USA
Zayd Chishti - American Hip Institute - Des Plaines, USA
Sheema K. Saeed - American Hip Institute - Des Plaines, USA
Andrew D. Carbone - American Hip Institute - Des Plaines, USA
Julio Nerys-Figueroa - American Hip Institute - Des Plaines, USA
Mark F. Schinsky - American Hip Institute - Des Plaines, USA
Ady Kahana - American Hip Institute Research Foundation - Chicago, United States of America
*Email: DrDomb@americanhipinstitute.org
Purpose: To systematically review the literature and compare the outcomes and results of conventional metallic versus modern non-metallic cerclage systems in periprosthetic femur fractures (PFFs).
Methods: A literature search of the PubMed, MEDLINE, and Google Scholar databases was performed in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines for clinical studies reporting on periprosthetic femur fractures (PFFs) in total hip arthroplasty (THA) patients and cerclage fixation techniques.
Results: Eight studies with 1,362 patients (1,366 hips) were included. The mean age ranged from 48.2 to 81.7 years, and the study population was 79.4% female. Infection rate at the site of the PFF or ETO was 0.22% (3 of 1366 hips), all occurring in patients with non-metallic cerclage fixation. 88.9% of subsidence (16 out of 18) occurred following metallic cerclage , while the other two occurred following non-metallic fixation. Significant stem subsidence was substantially higher in hips with metallic device fixation 5.1% Vs. 0.19% . Non-metallic fixation had a 93.9% clinical or radiologic healing rate .Loss of fixation as a rate of 0.6% was higher in metallic fixation. The mean postoperative HHS scores were 72.9 and 72.5 in the non-metallic and metallic groups, respectively.
Conclusion: The findings of this systematic review suggest that available non-metallic cerclage fixation methods demonstrate similar clinical success as conventional methods, with even fewer complications, when utilized in hip arthroplasty. The current use of novel materials appears to be safe and reliable for these surgeries and can be considered a dependable option for surgeons.
#8514
Effectiveness of Prophylactic Cerclage Fixation for Prevention of Femoral Fracture Propagation
Kartik I. Reddy - UTHealth - Houston, USA
Nicholas D. Lanfermeijer - UTHealth - Houston, USA
Jonathan E. Gold - UTHealth - Houston, USA
Sabir K. Ismaily - UTHealth - Houston, USA
Philip Noble - Institute of Orthopedic Research and Education - Houston, USA
David Rodriguez - University of Texas - Houston, USA
*Shuyang Han - University of Texas Health Science Center at Houston - Houston, USA
*Email: hanshuyang123@gmail.com
INTRODUCTION
Prophylactic cerclage cables have been widely advocated for preventing the propagation of fractures during total hip arthroplasty and postoperative activities. However, the placement of these cables in clinical practice varies, with recommendations spanning 0-20mm below the fracture site. Despite the clinical relevance, there is a lack of definitive biomechanical data guiding the optimal location for prophylactic cable placement. Therefore, this study was carried out to address two questions:
(1) What is the effective placement range of cerclage cables to prevent fracture propagation?
(2) Does cable tension change during loading, and what implications does this have on the stability of the cerclage technique?
METHODS
Femoral shafts from six fresh-frozen cadavers were used. Each specimen underwent a series of five axial loading tests, with a cobalt-chromium prophylactic cable placed at different distances (i.e., 5mm, 10mm, 15mm, 20mm, and a cableless control) from an induced fracture. The cerclage cable was equipped with a compressive load cell to ensure consistent initial cable tension across all tests and to measure the changes in cable tension during subsequent loading.
To prepare the specimens for testing, the femoral canal was reamed, followed by the insertion of a 4° taper using an MTS machine at a rate of 10mm/min (Figure 1). The tests were terminated upon crack propagation. The fracture was stained with blue ink to facilitate visualization. The ultimate force and changes in cable tension were then compared among different groups using a repeated-measures ANOVA, followed by post hoc analysis using Fisher's protected least significant difference (PLSD) test.
RESULTS
There were significant variations in the effectiveness of cerclage cable placement at different distances from the initial fracture. In comparison to the cableless control group, the 5mm group requires a significantly higher ultimate force (normalized: 331.6±58.2N/mm vs. 154.6±28.7N/mm) to propagate the fracture (p=0.004), corresponding to 3.3 times the body weight of an average person (Figure 2). A significant difference was also seen between the 10mm group and the control (p=0.004). However, when the cable was placed at 15mm and 20mm, the ultimate force was not significantly different from the control group (p=0.385 and 0.426).
The tension in the cerclage cable exhibited distinct patterns in different groups. With the cable at 5-10mm, the cable tension first remained relatively stable and then increased rapidly in the later stage (Figure C). Conversely, when the cable was placed at 15-20mm below the fracture, the tension in the cable decreased an average of 3% throughout the test.
CONCLUSION
The results of this biomechanical study underscore the critical importance of precise cable placement. When the prophylactic cable was positioned within the 5-10mm range, the cable effectively resisted fracture propagation and experienced increased tension as the loading progressed. However, cables placed in the 15-20mm range exhibited limited effectiveness. These findings emphasize the significance of accurate cable placement for achieving optimal biomechanical outcomes and fracture prevention in clinical practice.
ACKNOWLEDGEMENTS
The cerclage cables were donated by ZimmerBiomet.
Figures
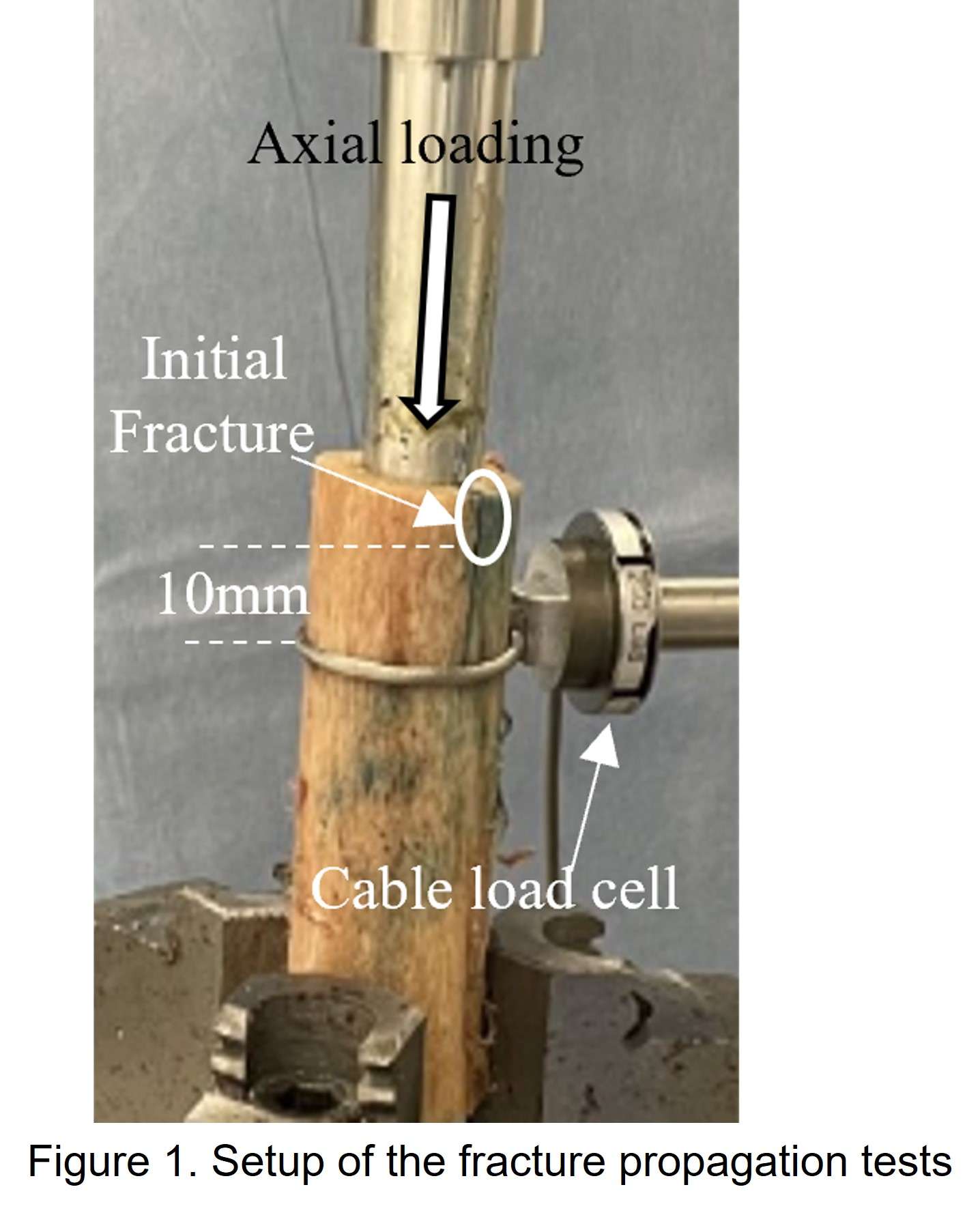
Figure 1
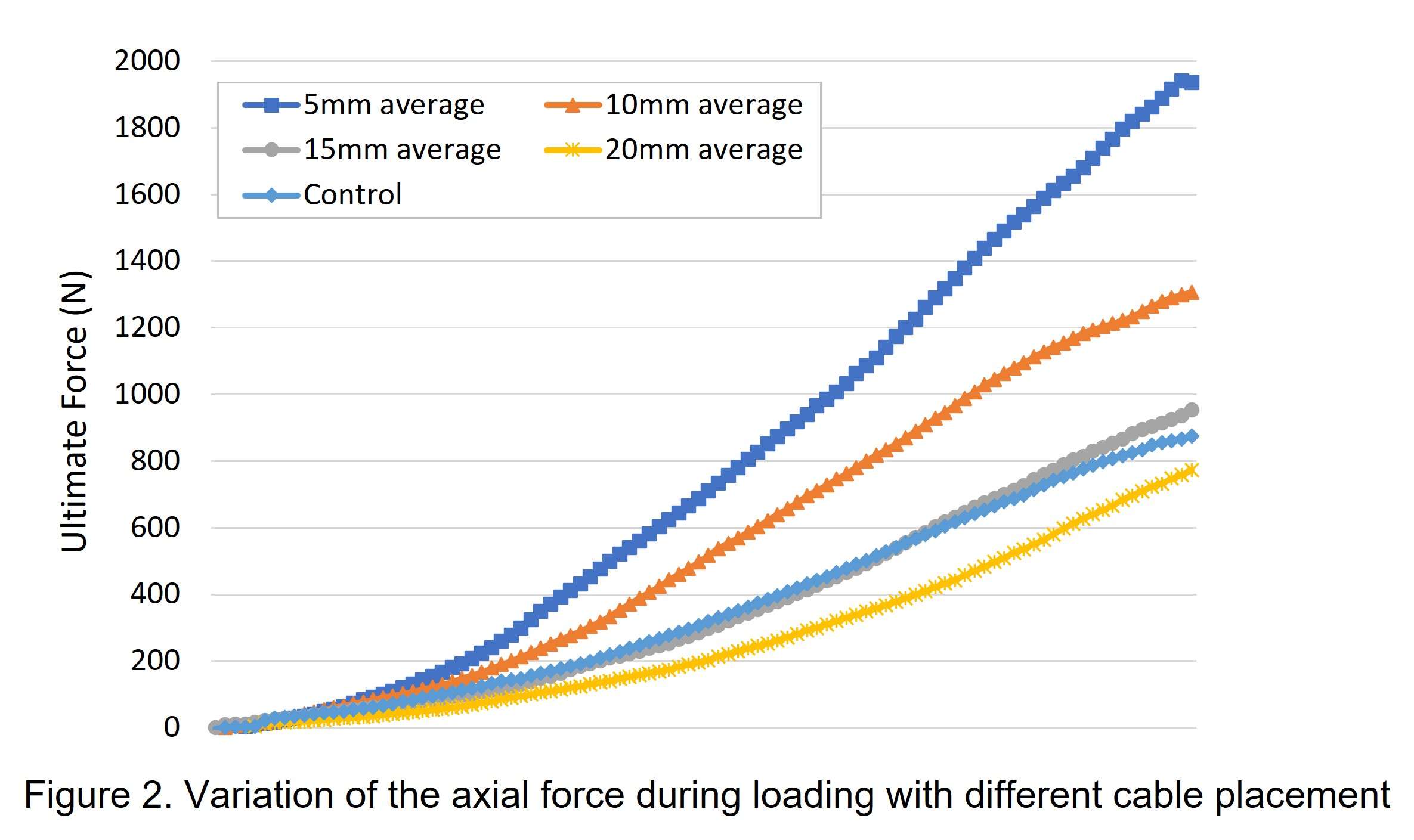
Figure 2
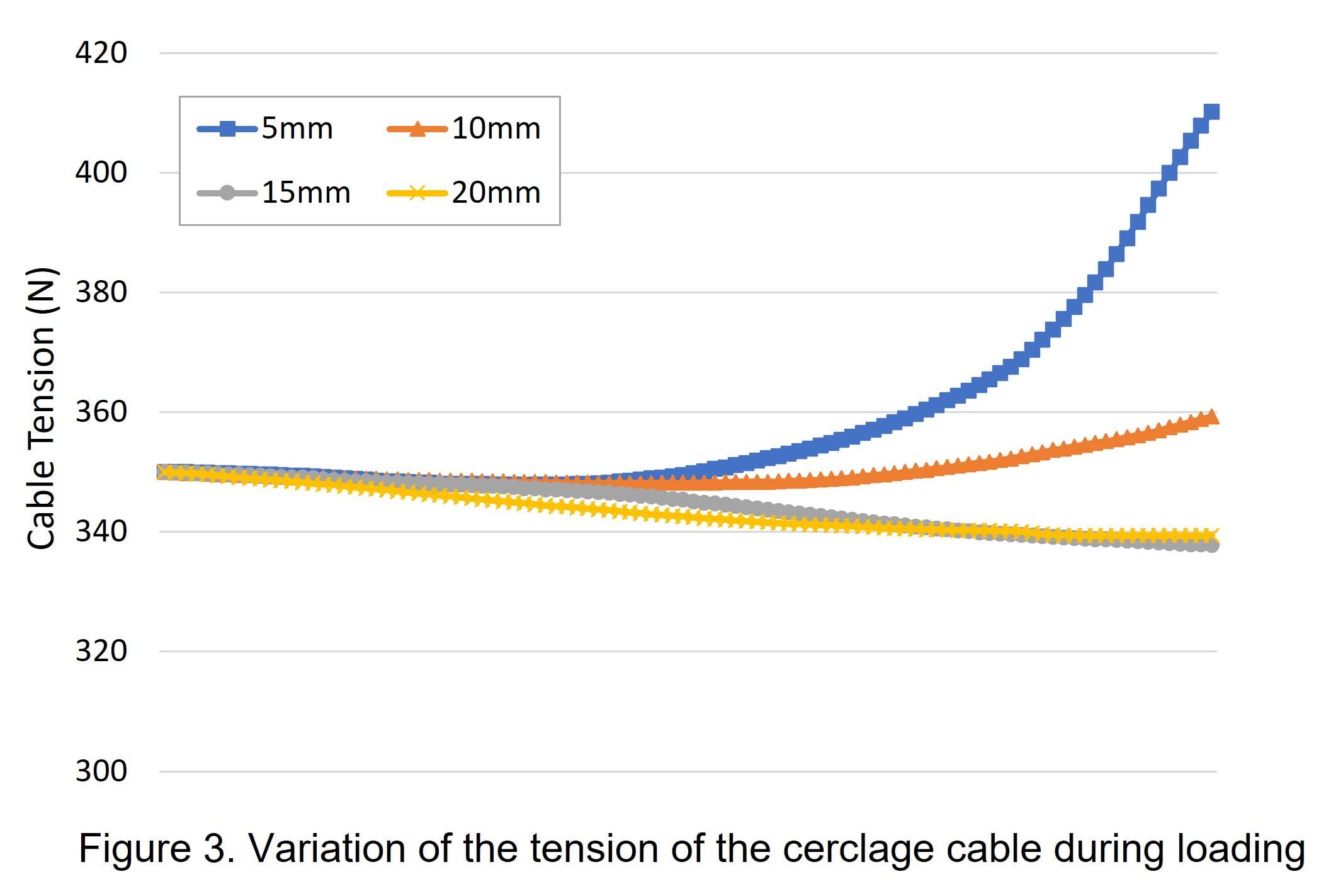
Figure 3#8326
Treatment of Osteoporosis After Femoral Neck Fracture Improves Survival After Periprosthetic Femoral Fracture
*Yasuhiko Takegami - Nagoya University - Nagoya, Japan
Yusuke Osawa - Nagoya University - Shizuoka, Japan
Hiroki Iida - Nagoya University - Nagoya, Japan
Yuto Ozawa - Nagoya University - Nagoya, Japan
Hiroto Funahashi - Nagoya university - Nagoya, Japan
Takamune Asamoto - Nagoya University - Nagoya, Japan
Hiroaki Ido - Nagoya University - Nagoya, Japan
Shinya Tanaka - Nagoya university - Nagoya, Japan
Keiji Otaka - Nagoya university - Nagoya, Japan
*Email: takegami@med.nagoya-u.ac.jp
Purpose: Periprosthetic femoral fracture (PPF) is a debilitating complication that can occur following total hip arthroplasty (THA) or hemiarthroplasty (HA) in femoral neck fractures. This study aimed to investigate the effect of osteoporosis treatment on the prognosis of PPF patients after femoral neck fracture.
Methods: A multicenter retrospective study named as TRON was conducted. The study population included 156 PPF patients who had undergone hemiarthroplasty for femoral neck fracture between January 2010 and December 2019. Patients were divided based on whether they had received osteoporosis treatment before PPF injury. A log-rank test was used to compare survival rates. We conducted a Cox proportional hazards analysis to identify factors associated with the survival rate after PFF injury.
Results: Twenty-seven of the 156 patients had received osteoporosis treatment prior to PPF injury. The 1-year and 2-year overall survival rates after PPF were 80.9% and 75.3%, respectively. The log-rank test revealed that the 1-year survival rate with and without osteoporosis treatment was 89.5% and 78.1%, respectively (P=0.012). In the Cox proportional hazards analysis, age, BMI, presence or absence of surgery, and presence or absence of osteoporosis treatment showed independent associations with the survival rate after PFF injury. The hazard ratio for the presence of osteoporosis treatment was 0.22 (95% confidence interval 0.07–0.75, P=0.015).
Conclusion: The survival prognosis after PPF was improved in patients who received osteoporosis treatment before surgery. After a proximal femoral fracture, osteoporosis treatment should be performed not only for the purpose of preventing secondary fractures, but also for improving the prognosis in the unlikely event of PPF.
Figures
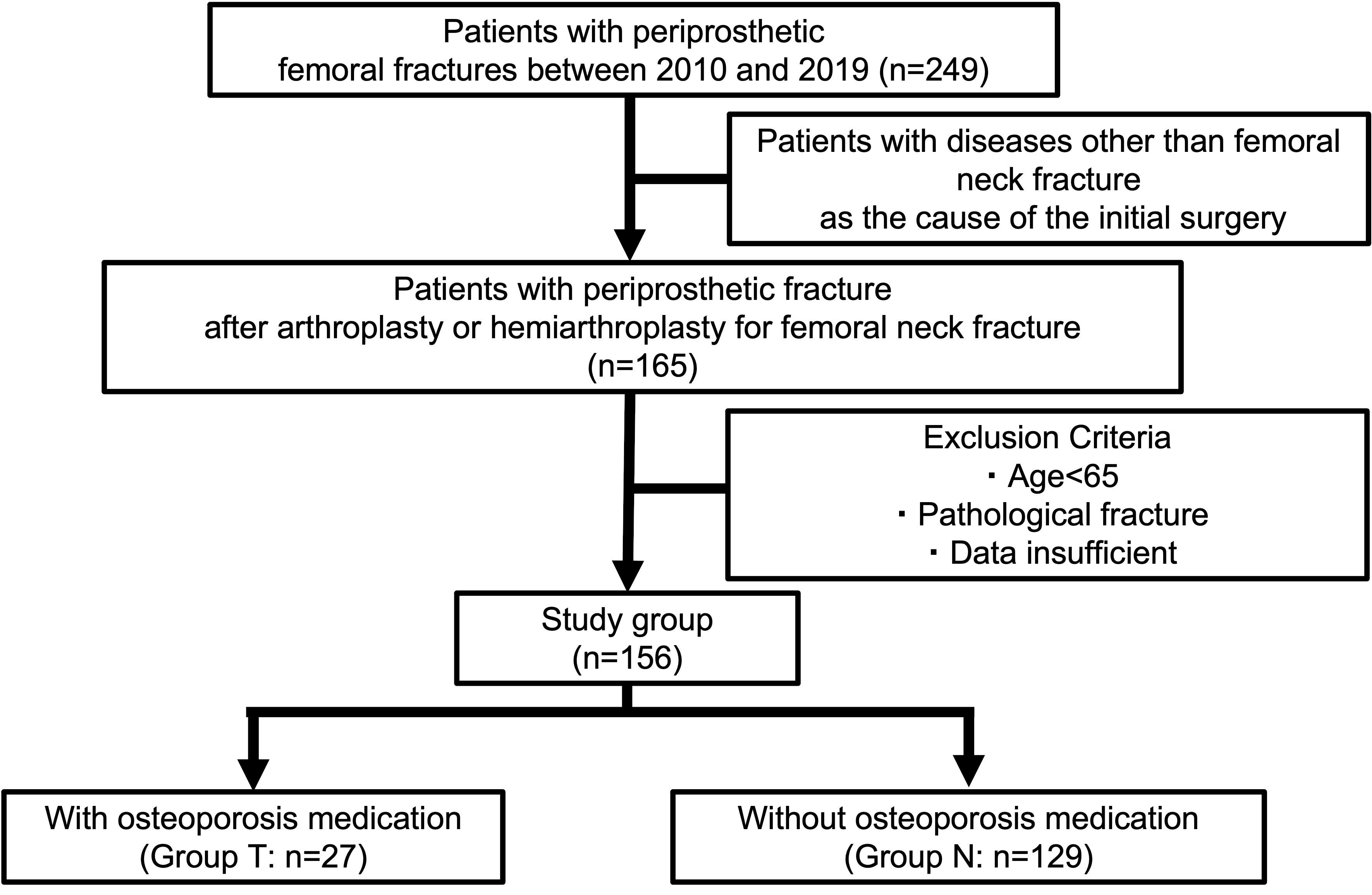
Figure 1
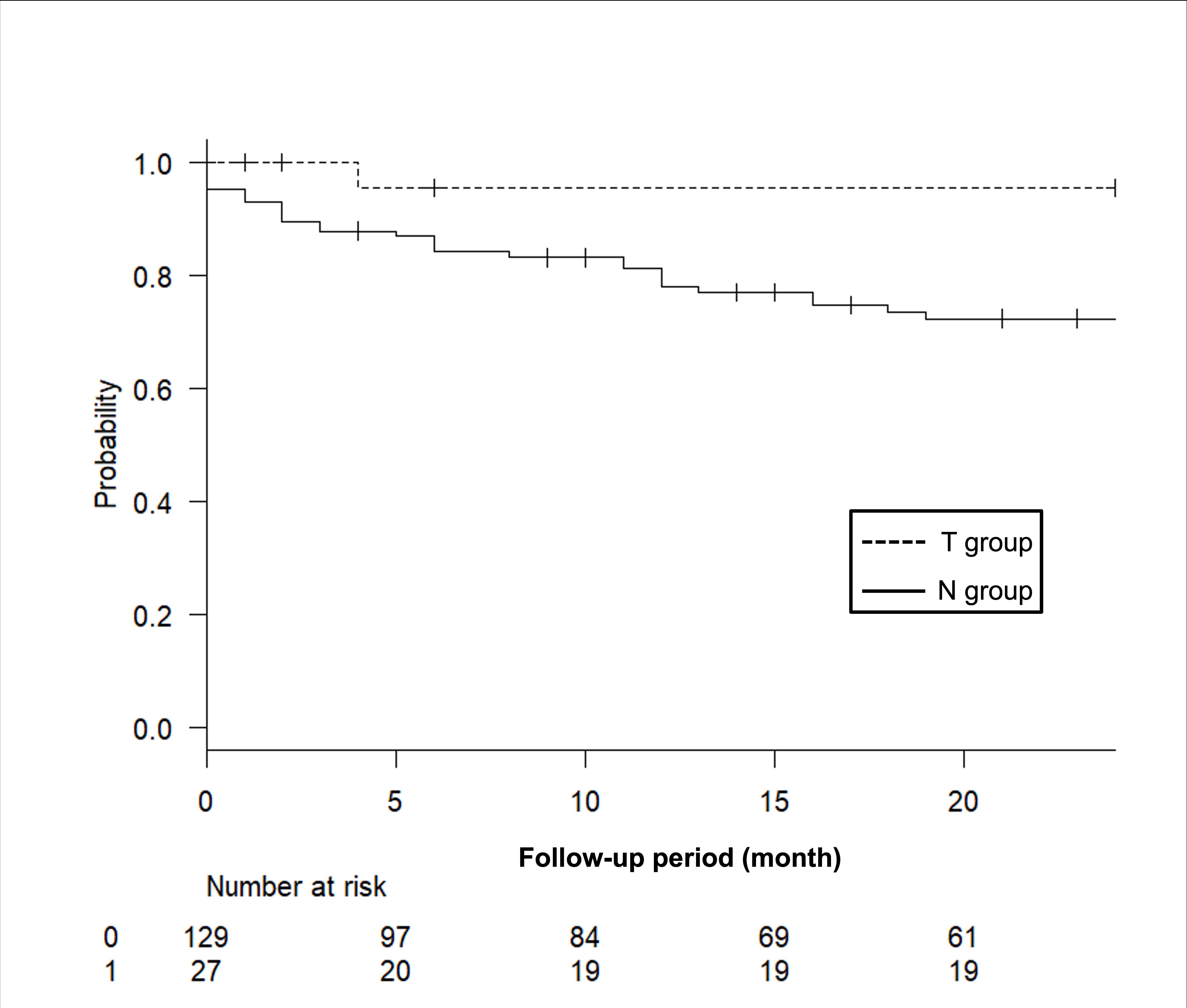
Figure 2#8505
Midterm Clinical Performance of a Tapered Wedge Femoral Stem
Yogesh Mittal - The Orthopaedic Center - Tulsa, USA
*Ahmad Faizan - Stryker - Mahwah, USA
Manoshi Bhowmik-Stoker - Stryker Orthopaedics - Mahway, USA
Jianhua Shen - Stryker - Mahwah, USA
*Email: ahmad.faizan@stryker.com
Introduction:
A second generation tapered wedge stem was designed using a large number of CT scans with the intent to improve proximal femur fit [1]. This stem design has been shown to significantly reduce the incidence of periprosthetic femoral fracture within 30 days postoperative [2]. This stem design has also been shown to reduce early adverse events in patients with Dorr type C bone, where traditional cementless stems have been controversial due to fear of fracture and subsidence [3]. The objective of this study was to longitudinally follow patients with a second generation cementless tapered wedge stem to characterize survivorship, reasons for revision, adverse events, and patient reported outcome measures in all bone types.
Methods:
Data was collected as part of a post-market, prospective, multicenter clinical trial. One hundred and thirty five patients received a primary total hip arthroplasty with the same implant design from March 2012 through February 2015. Mean age was 59.73±8.01 years, mean height 172.3±9.4 cms, mean BMI 27.5±3.6, 53.3% of female patients, and a primary diagnosis of end stage osteoarthritis. Patients completed a EQ5D, Oxford Hip Score (OHS) and modified Harris Hip Scores (HHS) pre operatively, and post operatively at 1, 3, 5, and 7 year followup. Minimal Clinically Important Difference (MCID) was determined for all patient reported measures while a Kaplan-Meier Survival curve was established for implant survivorship.
Results:
HHS (Figure 1), OHS, and EQ5D showed excellent improvements between preoperative and one year visits and maintained a positive trend through 7 year followup. The MCID showed improvement in all joint specific outcome measures (Figure 2) while general health VAS remained unchanged over time. Three patients were revised in this study resulting in 97.6% all cause survivorship at 7 years. One suffered a traumatic fall within a month postoperative (stem revised), and two had reoccurring dislocations within the first 2 years following surgery (stems retained during revision). (Figure 3)
Conclusion:
The CT based stem design which was previously shown to have better radiographic fit [4] and reduced periprosthetic fracture [2] demonstrated excellent survivorship and clinical outcomes at 7 years follow up. None of the revisions were due to stem failure.
References:
- Faizan A, Wuestemann T, Nevelos J, Bastian AC, Collopy D. Development and verification of a cementless novel tapered wedge stem for total hip arthroplasty. J Arthroplasty. 2015 Feb;30(2):235-40. doi: 10.1016/j.arth.2014.09.023. Epub 2014 Oct 2. PMID: 25449589
- AN Fleischman, MM Schubert , C Restrepo, AF Chen , RH Rothman. Reduced Incidence of Intraoperative Femur Fracture With a Second-Generation Tapered Wedge Stem. J Arthroplasty. 2017 Nov;32(11):3457-3461.
- J Lindner, J Napier, A Feher, et al. Cementless tapered wedge stems in patients undergoing primary total hip arthroplasty with Dorr C bone—are complication risks increased? Ann Transl Med 2019 | http://dx.doi.org/10.21037/atm.2019.08.124
- Issa K, Pivec R, Wuestemann T, Tatevossian T, Nevelos J, Mont MA. Radiographic fit and fill analysis of a new second-generation proximally coated cementless stem compared to its predicate design. J Arthroplasty. 2014 Jan;29(1):192-8. doi: 10.1016/j.arth.2013.04.029. Epub 2013 May 21. PMID: 23706811
Figures
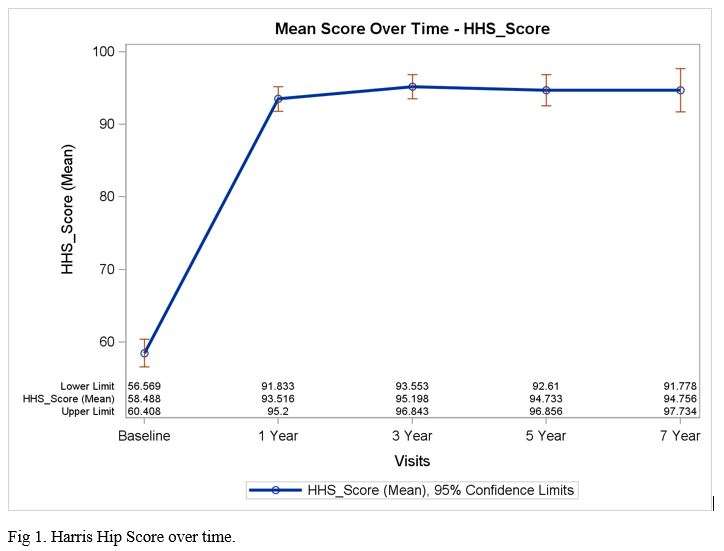
Figure 1
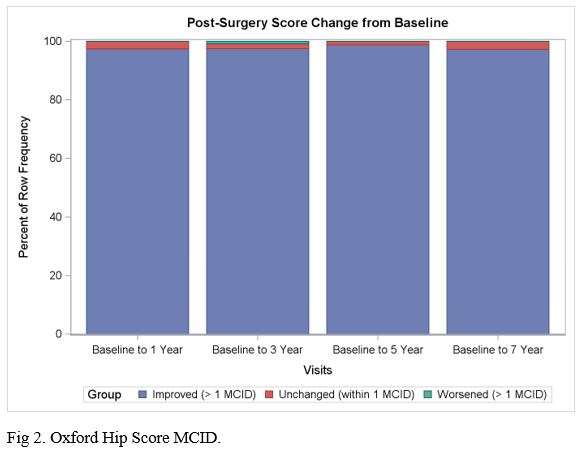
Figure 2
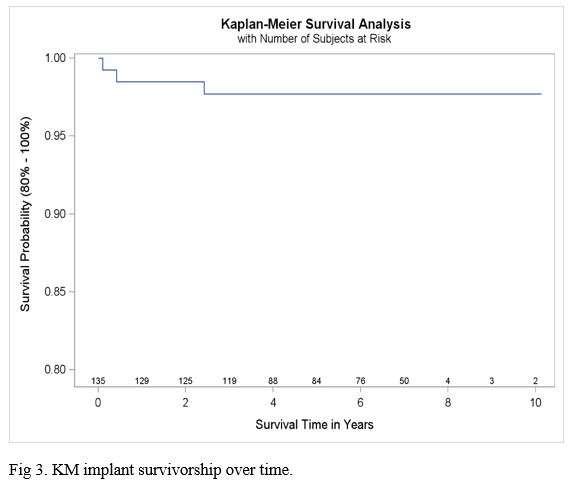
Figure 3#8095
Cementless Hemi-Arthroplasty in a Centenarian (103 Years Old): High Risk for Periprosthetic Fracture?
Georg-Antonio Bernecker - Kantonsspital Baselland - Bruderholz, Switzerland
*Andrej Nowakowski - Kantonsspital Baselland - Bruderholz, Switzerland
*Email: andrej.nowakowski@unibas.ch
Introduction
Elderly patients with displaced femoral neck fractures are thought to have poorer outcomes and are at higher risk of having systemic and local complications when compared to younger patients. A treatment option generally recommended for the elderly, low mobility and frail population is hemi-arthroplasty. Traditionally, many authors recommend the use of cemented femoral stems due to the decreased risk of having periprosthetic fractures. More recently, however, cementless implantation techniques have received increasing attention due to their decreased surgery time and the avoidance of cement associated cardiopulmonary complications (BCIS; bone cement implantation syndrome).
Methods
Presented here is a case of a 103-year-old female patient who suffered a displaced femoral neck fracture type Garden III/ Pauwels II, which was treated with a cementless hemi-arthroplasty.
Intraoperatively a thorough inspection of the femoral calcar following osteotomy of the femoral head was performed. No signs of calcar fractures, indentations or fissures that could potentially lead to a higher risk of postoperative complications such as a periprosthetic fracture, were identified.
During surgery the patient was cardio-pulmonal stable and her haemoglobin values were stable during aftercare. The patient was mobilized and allowed full weight bearing with crutches on postoperative day 1.
Results
Time from incision to suture was 25 minutes. Despite the patient’s direct oral anticoagulant (Apixaban) medication, intraoperative blood loss was only 120 ml. Standardized follow-ups with plain radiographs 5 days, 6 weeks and 3 month postoperative did not show any signs of subsidence. At 3 months follow-up, the patient was ambulating painless and autonomous with her walking stick just like she had done before the accident. No postoperative complications have been noted up to date (9 Mth).
Conclusion.
We recommend NOT to generally rule out the use of cementless stems when performing hemi-arthroplasties in the elderly population with suspected poor bone quality. Instead, we recommend a thorough evaluation of the calcar and femur when using cementless stems. Notably, the frail elderly patients benefit as well from shorter surgery time, less blood loss and last but not least the decreased risk of suffering from BCIS. Therefore, future studies including implant survival rates, counting number of postoperative complications such as periprosthethic fractures and BCIS, are needed.
#8155
Analysis of Incidence and Risk Factor for Periprosthetic Fracture After Total Knee Arthroplasty in Korea Between 2010 and 2020 Based on National Registry.
MinKi Kim - Seoul national university - Seoul, Korea (Republic of)
Jisu Park - SMG–SNU BORAMAE MEDICAL CENTER - Seoul, Korea (Republic of)
Tae Jung Kim - SMG-SNU Boramae Medical Center/Seoul National University College of Medicine - Seoul, Korea (Republic of)
Tae Woo Kim - Seoul National University College of Medicine, SMG-SNU Boramae Medical Center - Seoul, South Korea
Moon Jong Chang - SMG-SNU Boramae Medical Center - seoul, South Korea
Chong Bum Chang - Seoul National University College of Medicine - Seoul, South Korea
*Seung-Baik Kang - Boramae Medical Center/ Seoul National University College of Medicine - Seoul, South Korea
*Email: ossbkang@gmail.com
Abstract
Background : With the increased use of total knee arthroplasty (TKA), socioeconomic burden of periprosthetic fracture (PPF) which is one of the major complication after TKA is also rapidly increasing. However, the study that analyze the epidemiology of PPF after TKA based on national registry is very limited..
Purpose : This study aimed to (1) document the incidence of PPF after TKA in Korea between 2010 and 2020 based on the national registry data, and (2) analyze the risk factor PPF after TKA.
Materials and Methods : Using the Health Insurance Review and Assessment Korean database, the annual incidence, and medical cost of PPF after TKA in Korea between 2010 and 2020 were evaluated, and stratified by age and gender. The annual incidence of PPF was defined as the number of new PPF case per year, and presented as a population-adjusted rate per 100,000 Korean population, and PPF rate was defined as total PPF patients per total TKA patients. Medical comorbidities including coronary heart disease, cerebrovascular disease, pulmonary disease, dementia, peptic ulcer, liver disease, diabetes, hemiplegia, renal disease, cancer, Parkinson disease, and osteoporosis were evaluated as possible risk factors for PPF using the cox hazards ratio (HR).
Results : Overally, 2.4 % of TKA patients (14,429 PPF / 608,605 TKAs) experienced PPF between 2010 and 2020 in Korea[fig. 1][fig. 2]. The annual incidence of PPF after TKA in Korea gradually increased by 155% from 1.2 in 2010 to 3.1 in 2020. The PPF rate was similar between different age groups, and female patients showed higher PPF rate compare to male patients. Cox hazard model identified severe liver disease (HR 1.303; CI, 1.015-1.674), hemiplegia (HR 1.244; CI, 1.021-1.515), dementia (HR 1.206; CI, 1.118-1.301), Diabetes (HR 1.181; CI, 1.141-1.221), renal disease (HR 1.159; CI, 1.057-1.270), cerebrovascular diseaseas (HR 1.148; CI, 1.099-1.201), osteoporosis (HR 1.101; CI, 1.064-1.142) as risk factors for PPF after TKA.[fig. 3]
Conclusion
Between 2010 and 2020, overall PPF rate after TKA were 2.4% in korea, and annual PPF incidence gradually increased. Severe liver disease, hemiplegia, and dementia were identified as strong risk factors for PPF after TKA. The results of this study shows necessity of appropriate health care strategy to prevent and reduce PPF after TKA, especially in high risk group.
#8455
Collared Stems Can Reduce Early Subsidence in Primary Total Hip Arthroplasty: A Systematic Review on Randomized Clinical Trials
*Benjamin Domb - American Hip Institute - Des Plaines, USA
Julio Nerys-Figueroa - American Hip Institute - Des Plaines, USA
Ali P. Parsa - American Hip Institute - Des Plaines, USA
Andrew J. Curley - American Hip Institute - Des Plaines, USA
Sam Charif - American Hip Institute - Des Plaines, USA
Mark F. Schinsky - American Hip Institute - Des Plaines, USA
*Email: DrDomb@americanhipinstitute.org
Purpose: To provide an updated review of literature on the impact of using a femoral collared stem on subsidence, PROs, and revision rate.
Methods: A literature search of the Pubmed and Medline databases was performed during January 2023 in accordance with Preferred Reporting Items for Systematic Reviews and Meta-Analyses guidelines. Articles were screened for adult patients who underwent THA and for randomized controlled trials that evaluated collared and collarless subsidence and PROs. Exclusion criteria included trials that included non-THA patients and did not report PROs. Additional data collection included patient demographics, stem-calcar contact, subsidence, CFR, stem orientation, surgical approach, femoral neck height, Dorr Type, PROs, complications, and revisions.
Results: Five studies met inclusion criteria. Mean patient ages ranged from 58.5 to 72.4 years old, and the mean BMI ranged from 26.6 to 29.8 kg/m². The mean reported follow-up of the included clinical trials ranged from 1 to 9.6 years. Two studies reported mean early subsidence at two weeks postoperatively which was 0.36, 0.99 mm for collared stems and 0.52, 3.22mm for collarless stems and proved to be statistically significant. All studies demonstrated improved PROs at the most recent follow-up. Revision rates ranged from 4 to 11.3%, but these were not statistically significant.
Conclusions: This systematic review of literature demonstrated that implantation of collared stem compared to collarless may reduce early post-operative subsidence but was not superior to collarless stems for patient-reported outcomes and revision rate as both stems had similar results.
#8501
Evaluation of Femoral Morphological Parameters Used in Pre-Operative Planning
*Jarrod Nachtrab - University of Thennessee - Knoxville, USA
Thang Nguyen - University of Tennessee - Knoxville, USA
Michael LaCour - University of Tennessee - Knoxville, USA
Richard Komistek - The University of Tennessee - Knoxville, USA
*Email: jnachtra@vols.utk.edu
INTRODUCTION:
Total Hip Arthroplasty (THA) has been improving rapidly due to the success of new procedures brought about in the modern era. One advancement was the classification of femoral canals using Dorr Type classifications, separating the femoral canal into Type A, Type B, and Type C. This allows for more robust stem fits within the canal. However, this only tells us what is happening within the canal and does not consider external geometry. Maintaining a patient’s anatomical center (including version and neck angle) has been shown to improve overall kinematic outcomes. Unfortunately, most stems on the market today also only cover a small range of neck angles. The objective of this study is to determine what other femoral morphology should be considered besides just the femoral canal when planning a THA.
METHODS:
A total of 60 proximal femurs from segmented CT scans (20 Type A, 20 Type B, and 20 Type C) were evaluated in this study. Proximal femoral bone morphological data was gathered using a previously validated pre-operative planning tool. This included: Dorr Canal Type, anatomical head center location, neck angle, and femoral head diameter (Figure 1). The same pre-operative planning tool was used to simulate implantation of three stem systems shown in Figure 2: Tri-Lock, Corail, and Emphasys and a new system where the femur and cup were designed together. For each stem system, the distance from implanted head center (IHC) to the anatomical head center (AHC) and canal bone removal volume was calculated.
RESULTS:
Across all 60 bones, regardless of canal type, the average femoral neck angle was found to be 127.3±10.2° (106.3°-147.7°). The stems used in this study covered a much smaller range, 125°-135°, with the majority landing at 130° for the neck angle. The average femoral head diameter was found to be 46.6±3.8mm (39.6mm-52.6mm), and the chosen implanted heads were 36mm (11/60) and 40mm (49/60) based on the found femoral head diameter per subject. The IHC to AHC found for each of the tested implant systems are in Table 1.
CONCLUSION:
The Emphasys stem employed a variable femoral neck offset within the standard and high offset catalogs, unlike the Tri-Lock and Corail stems. This could have played a role in it having the smallest IHC to AHC distance on average. Regardless, there were still outlier subjects that the tested stem systems did not fit as well. These cases occurred for subjects that had a neck angle that was outside of the range of neck angles the tested implants covered. The stem shape of the Emphasys and Corail yielded a better overall fit within the canal, leading to much less extra bone removal after broaching. With all of this in mind, the data suggests that the orthopaedic community may be putting too much weight on canal shape and not enough into matching the external morphology of the femur. The canal classification is important but supplementing that information with the neck angle and/or version angle of the subject’s femoral anatomy could lead to further improved outcomes.
Figures
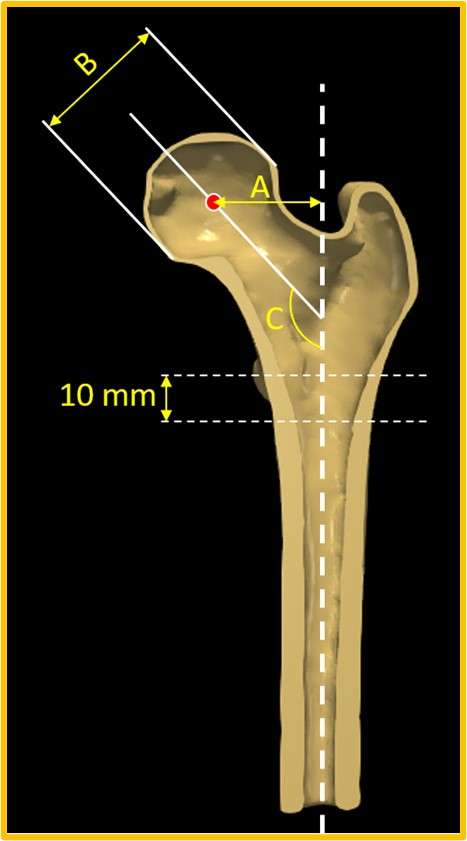
Figure 1
Figure 2
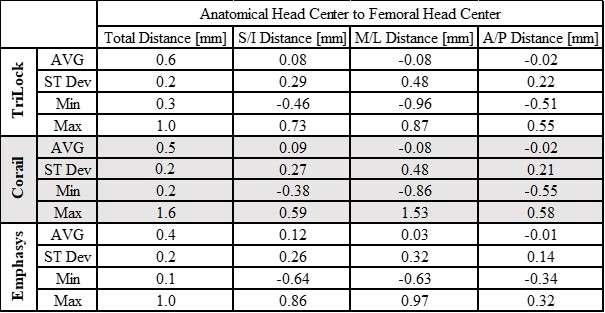
Figure 3#8737
Stricter Correction of Lower Extremity Length Is Required During Total Hip Arthroplasty in Patients With Ankylosing Spondylitis
Chan Jin Park - Chonnam National University Hwasun Hospital - Gwangju, Korea (Republic of)
*TAEK-RIM YOON - Chonnam national university hwasun hospital - Gwang-ju, Korea (Republic of)
*Email: tryoon@naver.com
Introduction: Patients with ankylosing spondylitis often have fusions in the spine and sacroiliac joints, such that it is difficult to compensate for the difference in length of the lower extremities.
Methods: We retrospectively measured the difference in lower extremity length after total hip arthroplasty(THA) in 89 patients with ankylosing spondylitis from June 2004 to February 2021 at Our institute. Patients were divided into two groups based on a difference in lower extremity length of 5 mm or 10 mm. Clinical outcomes were investigated using the Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC) and Harris Hip Score (HHS). In addition, these points are investigated : patient satisfaction with the operation; whether there was a current difference in leg length; and whether there was a limping gait.
Results: The group with a leg length difference of 5–10 mm rather than <5 mm had significantly worse WOMAC pain and stiffness. The survey revealed statistically significant differences in patient satisfaction with the operation, limping gait, and whether back pain had improved.
Conclusion: For patients with ankylosing spondylitis, reducing the difference in length of the lower extremities to <5 mm, which is more accurate than the current standard of <10 mm, may produce greater improvement in clinical outcomes after hip arthroplasty.
Keywords: Ankylosing spondylitis; coronal balance; leg length inequality; total hip arthroplasty
#8290
Statistical Shape Model Enabled Pre-Operative Templating Can Improve Femoral Offset and Neck-Shaft Angle Measurement From Standard Radiographs
Oscar Denton - Queens University Belfast - Belfast, GB
*Alex Lennon - Queen's University Belfast - Belfast, United Kingdom
David Beverland - Musgrave Park Hospital - Belfast, United Kingdom
Janet Hill - Musgrave Park Hospital - Belfast, United Kingdom
Nicholas Dunne - Dublin City University - Dublin, Ireland
*Email: a.lennon@qub.ac.uk
Introduction
Total hip arthroplasty targets restoration of patient-specific joint geometry. Medical images are used to define the patient-specific geometry and a modular stem is used to best match this. The most accurate is CT scanning, which allows for an accurate 3D joint reconstruction. However, cost, duration and radiation exposure prevent routine CT use. Instead, radiographs are used as standard. Projection from 3D to 2D during radiograph acquisition introduces magnification and projection error, which can significantly misrepresent the true shape. The aim of this work is to improve the prediction of femoral offset (FO) and neck-shaft angle (NSA), key variables when deciding on a modular stem, based on standard radiographs. This would allow more reliable templating of modular stem combinations without additional imaging steps.
Methods
Orientation and shape of a femur were optimised against the projected geometry to correct for misrepresented features.
Initially, the target shapes, the projected femur geometry in anteroposterior (AP) and lateral radiographs, were processed to 500 equally spaced points marking the projected contour of the shape. These were generated from a statistical shape model (SSM), with rotations randomly applied from a uniform distribution between ±25° flexion, ±5° abduction and ±30° rotation. Rotation of the lateral femur was constrained to within 75° and 105° of the AP case, where 90° would represent orthogonal radiographs.
Artificial contours can then be generated by varying 3D shapes using an SSM, rotating the shape about 3 axes independently for both AP and lateral cases, followed by ray casting to simulate radiograph acquisition (Fig. 1), and a variable to define a distal cut-off of the projected contours due to limited detector size (Fig. 2).
A similarity between the target contour and an artificial contour was calculated by summing the distance between paired points, i.e. the 500 evenly spaced points starting from the distal lateral corner of each contour. Evaluating the similarity between artificial and target contours allows the framing of an optimisation problem, which matches artificial contours to the target contours.
For each optimisation, the output is a 3D geometry, defined as an SSM sample, orientations in AP and lateral radiographs and distal cut-offs that are most likely to have produced the target contours. These are used to define predicted FO and NSA.
Results
RMSE of radiographic FO (i.e. based only on the projected contour) versus anatomic was 7.0 mm (trend to underpredict), whilst RMSE of radiographic NSA was 5.3° (trend to overpredict); top row of Fig. 3. When predicting FO and NSA via the optimisation, RMSE for FO was reduced to 3.6mm (~50% reduction), and RMSE for NSA was reduced to 3.5° (~35% reduction); bottom row of Fig. 3.
Conclusion
Computational testing indicates that SSM-enabled 2D-3D reconstruction is capable of pre-operative templating both FO and NSA, from asynchronous AP and lateral radiographs, more accurately than the standard technique of direct radiographic measurement. Future work will focus on automating contour extraction directly from radiographs and validating the technique based on real radiographs.
Figures
Figure 1
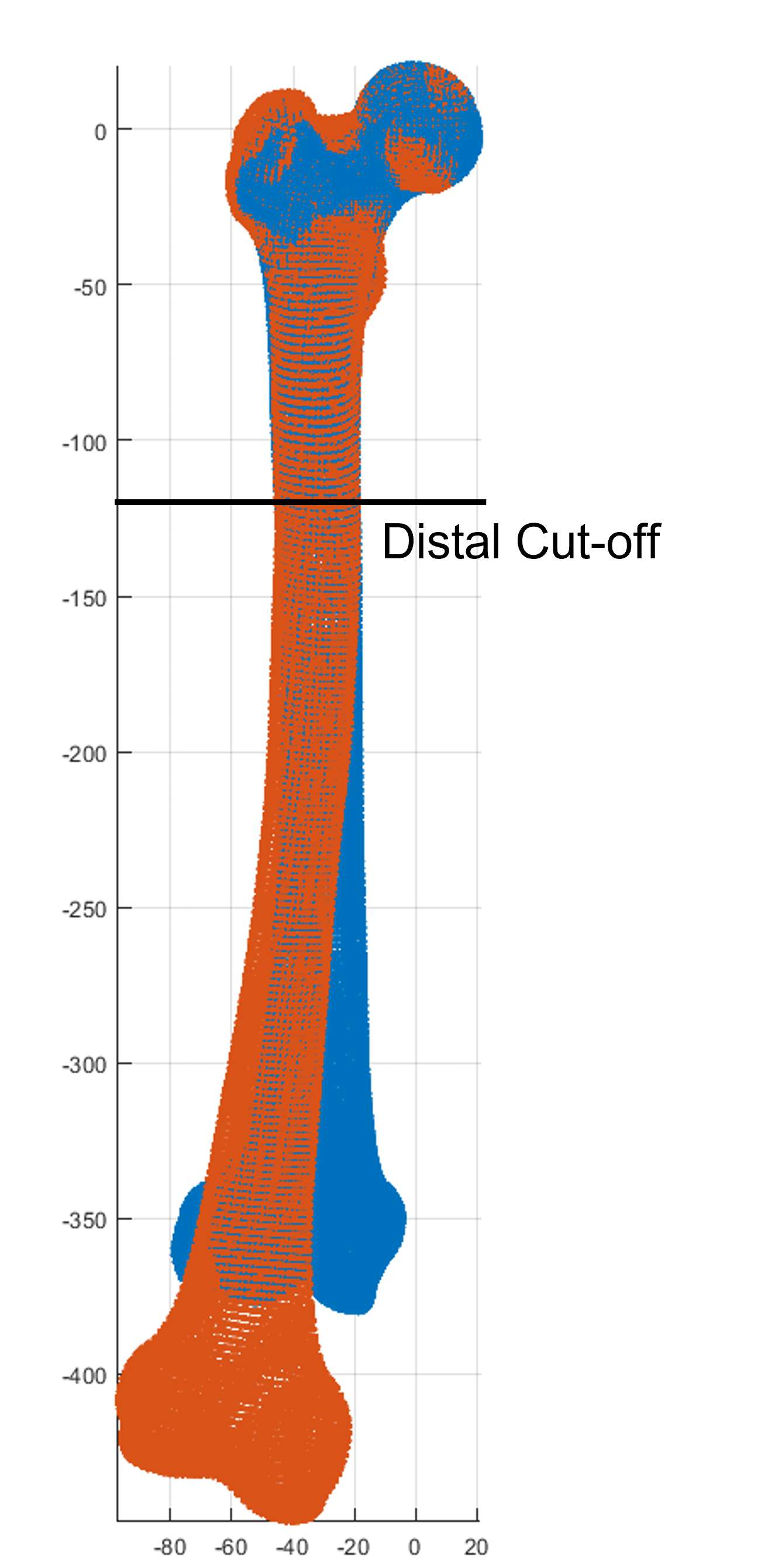
Figure 2
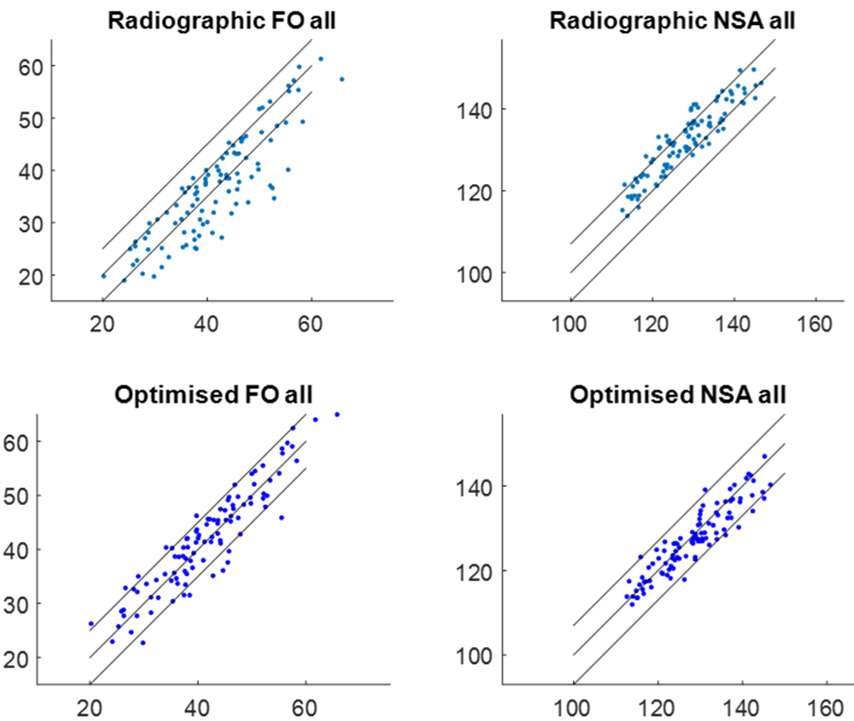
Figure 3#8166
Development of a 3D Planner for Personalized Periacetabular Osteotomy in Patients With Hip Dysplasia
*Kartik Logishetty - Imperial College - London, United Kingdom
Simon Harris - United Kingdom
Justin Cobb - Imperial College - London, United Kingdom
*Email: k.logishetty@imperial.ac.uk
Introduction: Symptomatic adult hip dysplasia is surgically managed by periacetabular osteotomy (PAO) to restore acetabular coverage. The success of PAO relies on accurate planning and execution, which can be challenging due to the patient-specific nature of the procedure, and the challenges in achieving lateral coverage without anterior impingement or excessive anteversion. This study aimed to develop a 3D planner software for personalized planning of PAO in patients with hip dysplasia, accounting for factors such as location and angulation of cuts, and patient-specific pelvic tilt.
Methods: A 3D planner was designed using computer-aided design (CAD) software, modeling the relevant bony structures. The planner incorporated customizable Bernese PAO cuts, simulated movements of the acetabular fragment, and real-time feedback on fragment position and femoral head coverage. The software was also capable of correcting acetabular tilt and predicting impingement to optimize surgical outcomes. To validate the 3D planner, a cohort of patients with hip dysplasia who had undergone PAO was included in the study. Preoperative CT scans were used to create 3D models, and the software-generated plans were compared to actual postoperative CT scans, focusing on femoral head coverage, and acetabular indices including a novel CT-derived Tonnis angle.
Results: The 3D planner was effective in simulating PAO and assisting in personalized surgical planning, providing real-time feedback on femoral head coverage as a percentage and sectoral coverage, optimizing fragment position, and considering the patient's pelvic functional position. The software also identified areas of potential impingement between the fragment and the pelvis, allowing for adjustments to achieve desired femoral head coverage with minimal fragment movement. The planner's predictions were found to be in good agreement with postoperative CT-based radiographic outcomes, demonstrating the software's potential for enhancing PAO planning and execution.
Discussion: This study introduces a 3D planner software for personalized planning of PAO in patients with hip dysplasia. The planner allows surgeons to customise the location and angulation of the Bernese PAO cuts and provides real-time feedback on femoral head coverage, enabling optimal fragment positioning. It also enables correction of acetabular tilt and detects potential impingement sites. Future studies may investigate the incorporation of this 3D planner into intra-operative navigation.
#8522
Effects of Different Surgeon Preferences With Stem Head Sizes in Total Hip Arthroplasty
*
Jarrod Nachtrab - University of Thennessee - Knoxville, USA
Garett Dessinger - The University of Tennessee - Knoxville, USA
Richard Komistek - The University of Tennessee - Knoxville, USA
Michael LaCour - University of Tennessee - Knoxville, USA
*Email: thangnguyen4796@gmail.com
Introduction: Hip dislocation is a significant complication after total hip arthroplasty (THA) that can cause pain, instability, implant wear, and revision surgery. It has been documented that increased the femoral head diameter can reduce the risk for dislocation by increasing the head-neck ratio and required jump distance of the femoral head. In this study, a previously validated forward solution hip model was used to investigate the additional effects that increasing head size may have on postoperative mechanics in different alignment scenarios. The primary objective of this study is to evaluate the effects of increasing femoral head size with THA, specifically on hip separation, contact bearing mechanics related to bearing surface force, contact area and contact stress, and hip muscle forces.
Methods: In this study, eight total subjects were analyzed using forward solution dynamic modeling of the lower extremity during stance phase of gait (Figure 1). The femoral components were positioned using two different techniques – an anatomical fit position and a canal fit position as defined in previous publications – resulting in variations in component offsets and stem version angles. We combined these two alignment methods with two femoral head size preferences, resulting in four potential combinations shown in Figure 1 for all 8 subjects. All analyses were conducted using an EMPHASYS stem and EMPHASYS cup with a neutral liner (DePuy Synthes).
Results: The results indicated that using larger head sizes results in a reduction of hip separation, an increase in femoral head contact area, and a decrease in contact stress within the acetabular liner. Specifically, using a larger femoral head reduced hip separation by 7% (Figure 2). The results also revealed that using the largest femoral head size led to an increase in the contact area between the femoral head and the acetabular liner, with the femoral head sizes of 36 and 40 providing almost twice the contact area of the sizes 28 and 32. However, one alignment scenario, using the largest femoral head sizes slightly increases hip force by 2.43% and muscle forces for the iliopsoas, gluteus medius, and tensor fasciae latae muscles by 2.72%, 4.49%, and 2.75%, respectively (Figure 3).
Conclusion: This study yielded results that are consistent with those of previously published, indicating improved overall stability using larger femoral heads. In addition, these findings also suggest that larger head size can also reduce contact stress on the polyethylene liner through an increase in contact area, although it may also increase hip and muscle forces and cause impingement of adjacent muscles under certain scenarios. Overall, the benefits of using a larger head size seem evident herein, and therefore it is advisable to use larger femoral head sizes when reasonable, at the discretion of the surgeon.
#8697
Biomechanical Analysis of Fixation Implant Options for Intertrochanteric Femur Fractures
*Wei Zeng - New York Institute of Technology - Old Westbury, United States of America
Kidar Arjune - New York Institute of Technology - Old Westbury, USA
Jericho Lee - New York Institute of Technology - Old Westbury, USA
*Email: zeng.work@gmail.com
Introduction
Intertrochanteric fractures are a common type of injury in the elderly population due to trauma or accidents and result in a high rate of patient mortality and morbidity. Treatment for intertrochanteric femur fractures usually involves surgical intervention. The specific surgical technique used depends on the fracture pattern and the patient's overall health. Common implant options include the use of screws, plates, or rods to stabilize the fracture and promote healing. The choice of implant depends on various factors, including the fracture pattern, patient characteristics, and surgeon preference. In this study, we aim to evaluate the biomechanical performance of three internal fixation implants for intertrochanteric femur fracture under physiological loading conditions using FEA.
Methods
Three commonly used internal fixation implants for intertrochanteric fractures, namely the dynamic hip screw (DHS), proximal femoral nail antirotation (PFNA, see Fig. 1), and proximal femur locking compression plates (PF-LCP), were evaluated. In order to analyze the biomechanical responses of these implants under physiological loading conditions, a series of finite element (FE) models of femur-implant systems were developed, which were simulated to assess the qualitative and quantitative aspects of the biomechanics of bone and devices. For each type of implant, four FE models were created to examine the strain in the bone and the equivalent stress in the devices during walking and stair-climbing scenarios. These simulations replicated the hip contact force using both static and dynamic loadings, providing a comprehensive evaluation of the implants' performance. To avoid or limit potential numerical artifacts on a single element or very few elements, the 95th percentile of strain and equivalent stress based on the 95th percentile peak element response was used to characterize the model outcomes.
Results
In comparison to the DHS and LCP, the components of the PFNA exhibited lower stress levels during force-controlled simulation. Additionally, the femur fragments with PFNA implants demonstrated reduced levels of bone strain. While the maximum equivalent stress on each implant was higher to a certain extent during stair climbing compared to walking scenarios, the PFNA generally exhibited a lower risk of femur bone fracture and implant failure in comparison to the DHS and LCP.
Conclusion
The PFNA approach offers minimal invasiveness and superior biomechanical characteristics compared to the DHS or LCP side plate. By inserting into the femur's medullary canal, the PFNA minimizes bending effects due to the reduced length of the cantilever beam. Consequently, it exhibits lower peak strain on the bone and the lowest maximum stress on device in all loading groups. Our study findings can serve as a valuable reference for implant selection. Furthermore, we anticipate that our research will inspire further exploration in the field of femoral fracture treatment and the development of patient-specific orthopedic implants.
Acknowledgments
Supported by the Institutional Support of Research and Creativity (ISRC) Grant provided by the New York Institute of Technology (NYIT)
References
- Zeng, et al., Computer Methods and Programs in Biomedicine. 2020
- Gabler, et al., Annals of Biomedical Engineering. 2019
- Zeng, et al., Frontiers in Bioengineering and Biotechnology. 2023
Figures
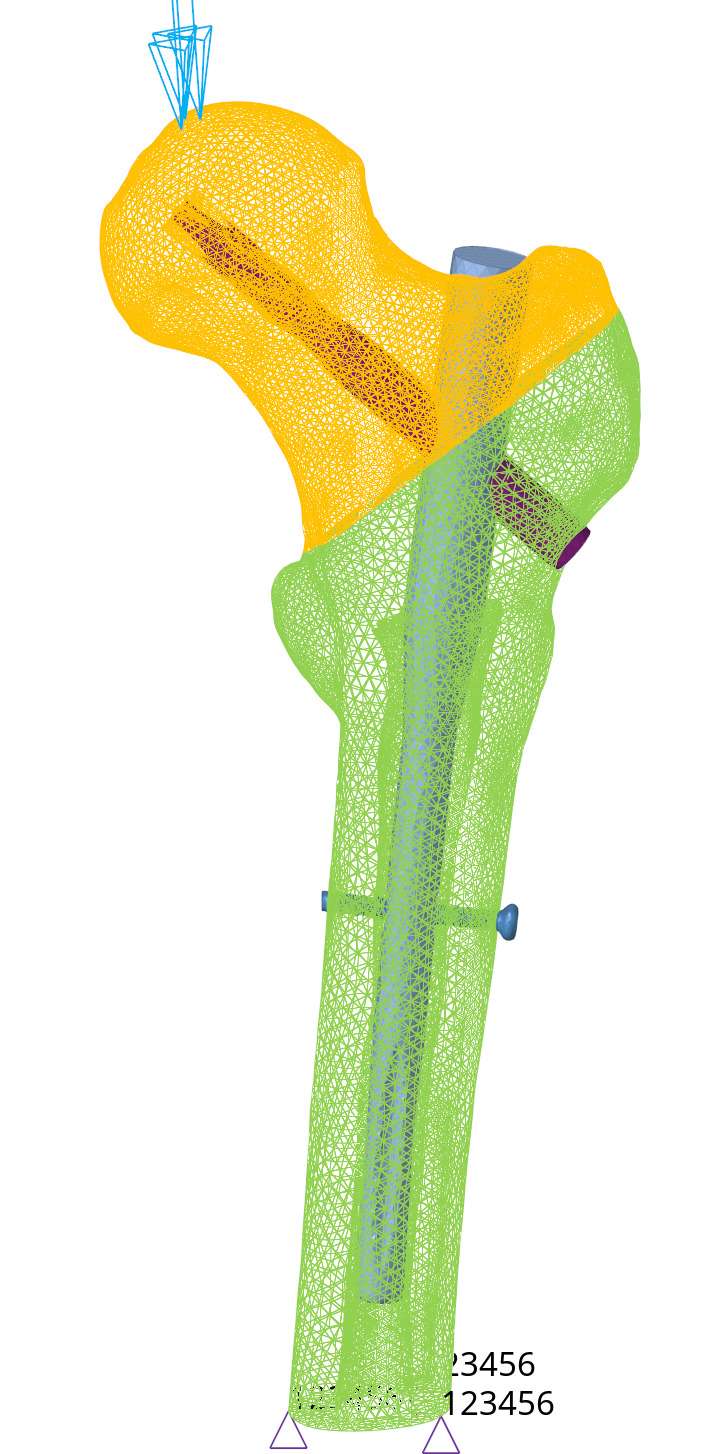
Figure 1#8509
Workers Compensation Patients Showed Improvement After Primary Total Hip Arthroplasty
*Benjamin Domb - American Hip Institute - Des Plaines, USA
Saiswarnesh Padmanabhan - American Hip Institute - Des Plaines, USA
Omkar Prabhavalkar - American Hip Institute - Des Plaines, USA
Paulo P. Padilla - American Hip Institute - Des Plaines, USA
Ali P. Parsa - American Hip Institute - Des Plaines, USA
Mark F. Schinsky - American Hip Institute - Des Plaines, USA
*Email: DrDomb@americanhipinstitute.org
BACKGROUND: Patients under workers’ compensation (WC) status are often excluded from clinical outcome studies due to their associated inferior outcomes. Therefore, the comparative studies based on WC are limited.
METHODS: Data was prospectively collected and retrospectively reviewed on all patients who underwent Primary Total Hip Arthroplasty (THA) during August 2009 and July 2020. Inclusion criteria were patients who underwent primary THA for the treatment of OA and had complete follow-up for the Harris Hip Score (HHS), Forgotten Joint Score (FJS), Visual Analog Scale (VAS), and satisfaction at minimum 2-year follow-up. Patients who had concomitant Gluteus Medius repairs were excluded from the study.
RESULTS: A total of 42 patients from 46 patients (91.5%) eligible WC patients met the inclusion criteria. WC patients demonstrated significant improvements from preoperative to a minimum 2-year follow-up for HHS and VAS for pain (p <.05). Though, the WC group had demonstrated lower preoperative and postoperative values when compared to the non-WC control group, improvement was not significant different (p >.05) for HHS and VAS. The non-WC control group had a larger mean FJS at the 2-year minimum follow-up (p <.05). The satisfaction levels were comparable between the two groups.
CONCLUSION: Patients with WC claims treated with hip replacement surgery showed significant improvement at a minimum 2-year follow-up. Patients with WC claims had comparable outcomes to patients without WC Claims.
#8391
High Risk of Dislocation After Total Hip Arthroplasty in Patients With Crowe Type IV Developmental Dysplasia of the Hip
Zi-Chuan Ding - West China Hospital, Sichuan University - Chengdu, China
*Zongke Zhou - Sichuan University - Chengdu, China
Weinan Zeng - Southwest Hospital, Third Military Medical University - Chongqing, China
*Email: zongke@126.com
Introduction
To investigate whether the risk of dislocation after total hip arthroplasty (THA) in patients with Crowe type IV developmental dysplasia of the hip (DDH) is high and to further identify the risk factors for postoperative dislocation in these patients.
Methods
This retrospective cohort study reviewed Crowe type IV DDH patients undergoing THA between January 2009 and December 2017 in our institution. Each Crowe type IV DDH patient was matched with three Crowe type I, II, or III DDH patients according to gender, side and date of operation. The primary outcome of this study was postoperative dislocation after THA. Occurrence, rate, classification, treatment and outcome of dislocation were documented in detail for all patients. The dislocation rates were compared between Crowe type IV DDH patients and Crowe type I, II, or III DDH patients. Demographic data, implant factors, and surgical factors were compared between the dislocation and no dislocation groups. Multiple logistic regression analysis was used to determine the independent risk factors for dislocation in Crowe type IV hips.
Results
A total of 131 Crowe type IV hips were followed up for a mean of 76.5 ± 28.1 months. Three hundred and ninety?three Crowe type I, II and III hips, including 261 type I hips, 94 type II hips, and 38 type III hips, were identified as controls and followed up for a mean of 76.4 ± 28.2 months. No significant difference was observed in follow?up time between two groups (P = 0.804). One or more dislocations occurred in 22 of the 524 dysplasia hips (4.20%). Of the 22 dislocated hips, 20 hips (90.9%) were successfully managed with non?operative treatment. Two patients (9.1%, one Crowe type I and one Crowe type IV) experienced recurrent dislocation and required revision surgery. Crowe type IV hips had a significantly higher postoperative dislocation rate than type I, II, and III hips (11.45% vs 1.78%, P < 0.001) (Fig 1-3). The use of a 22?mm femoral head (odds ratio [OR] = 23.55, 95% confidence interval [CI] = 1.901–291.788, P = 0.014), older age (OR = 1.128, 95% CI = 1.037–1.275, P = 0.031), and absence of false acetabulum (OR = 12.425, 95% CI = 1.982–77.879, P = 0.007) were identified as independent risk factors for dislocation in Crowe type IV hips.
Conclusions
Crowe type IV DDH patients were at a high risk of dislocation after THA, and using large femoral heads and improving abductor muscle strength may help decrease the rate of postoperative dislocation in such patients.
Figures
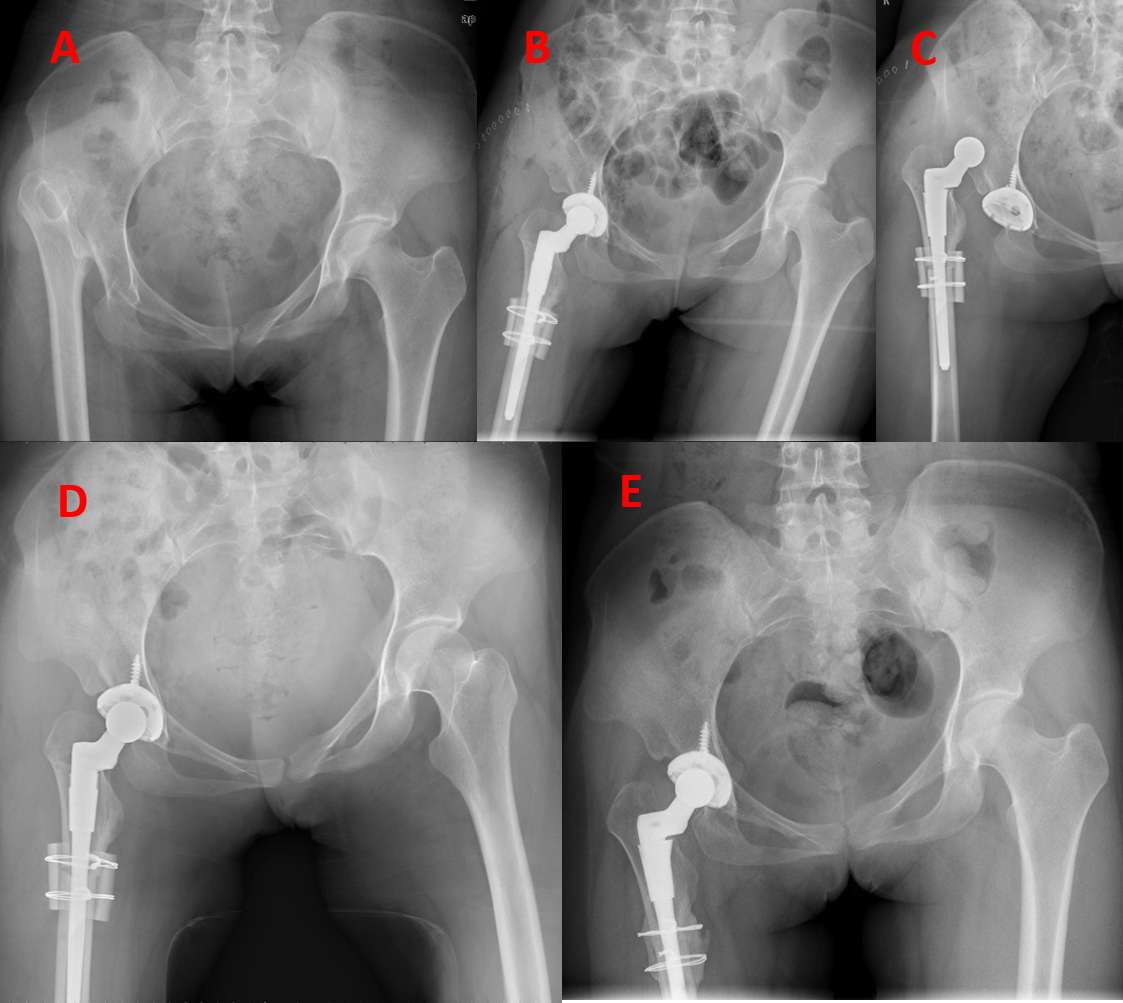
Figure 1
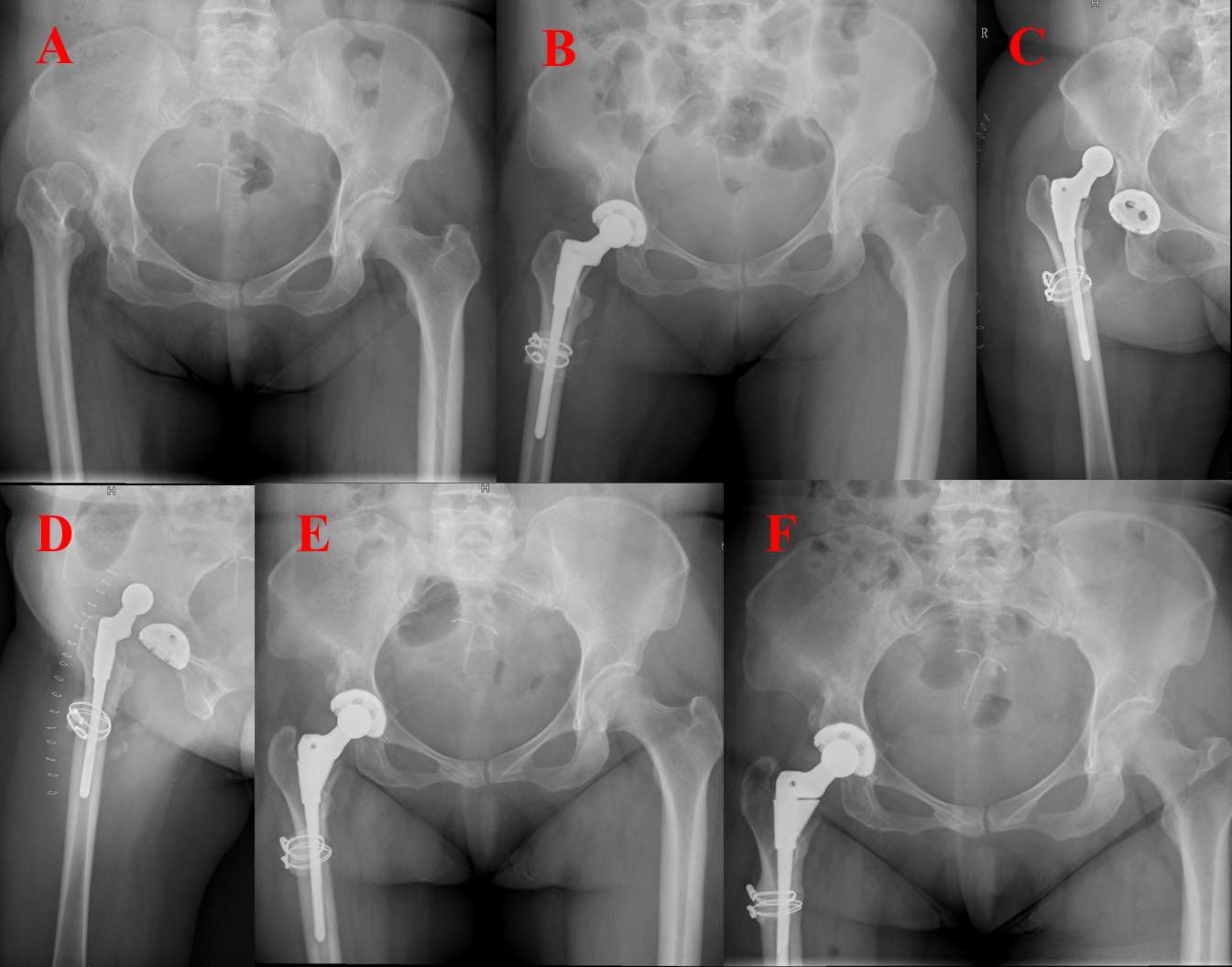
Figure 2
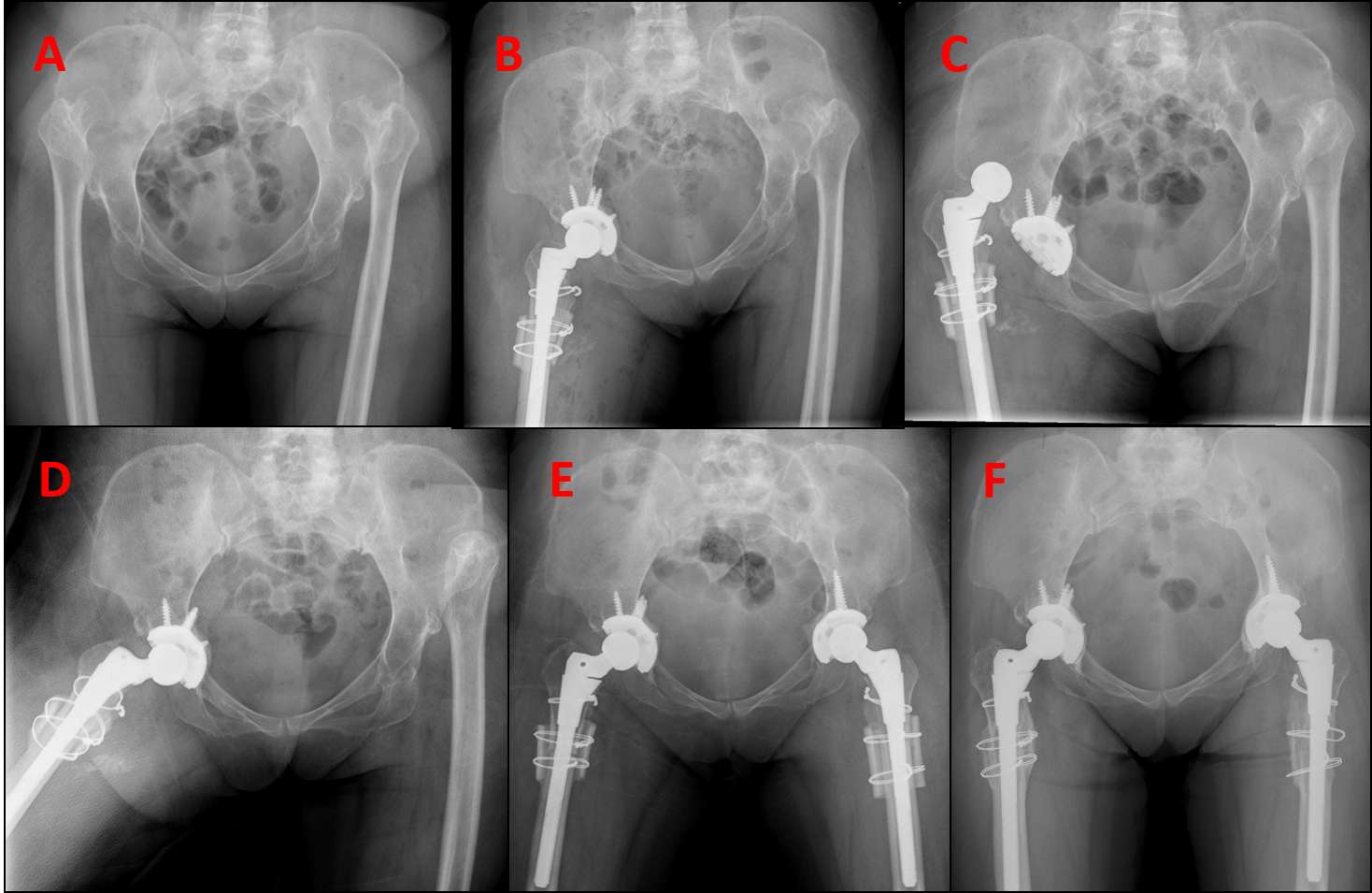
Figure 3#8646
Patient-Specific THA in Young Patients Require Extra-Small Hip Stems
*Ritvik Sarkar - Hospital for Special Surgery - New York, United States of America
Joseph Lipman - Hospital for Special Surgery - New York, USA
Timothy Wright - Hospital for Special Surgery - New York, USA
David J. Mayman - Hospital for Special Surgery - New York, USA
Mark P. Figgie - Hospital for Special Surgery - New York, USA
*Email: sarkarr@hss.edu
Introduction. Pediatric and young adult patients present unique challenges compared to the adult osteoarthritis population [1-2]. Nearly 20% of patients who undergo primary total hip arthroplasty (THA) at or before the age of 35 at our institution receive patient-specific implants [3]. While good outcomes have been reported, little design and clinical data are available on the patient-specific implants used to treat these patients.
Methods. We reviewed patient-specific implants designed at our institution from 2006 to 2019 for primary THAs in patients ≤35 years or those with a primary diagnosis of pediatric hip disease (juvenile inflammatory arthritis (JIA), developmental dysplasia of the hip (DDH), or skeletal dysplasia). Implant design parameters were collected from CAD models, engineering drawings, and preoperative CT scans. We collected patient demographics, primary diagnosis, and outcomes.
Results. We identified 57 primary patient-specific hip stems from 40 patients; 17 patients received patient-specific hip stems bilaterally. No patient-specific acetabular components were used.
The most common primary diagnosis (Table 1) was skeletal dysplasia (23 patients, 40%), followed by DDH (8 patients, 14%), then JIA (5 patients, 9%). Mean age at time of surgery was 36 years (Fig. 1).
8 hips (14%) had a cemented femoral component. The remaining were uncemented, press-fit, metaphyseal-engaging stems. All acetabular components were uncemented and used highly cross-linked polyethylene liners.
Stem diameter was 8.0±1.2mm with a range from 5.5mm to 11mm. 46 stems (81%) had cylindrical cross-sections. 3 stems (5%) had elliptical cross-sections. All cemented stems were tapered with rectangular cross-sections. Stem length from lateral shoulder to the distal tip, was 105±23.5mm (Fig. 2). Lateral offset from the stem axis to the +0 head center was 35±6.5mm. Head height from the medial corner of the implant to the +0 head center was 22±5.3mm. 35 stems (61%) had a neck-stem angle below 135°, with 125° (22 stems, 39%) being the most common. 18 stems (32%) had a neck-stem angle of 135°. 4 stems (7%) had a neck-stem angle above 135°, up to a maximum angle of 145°.
Native femoral anteversion ranged from 33° retroverted to 70° anteverted with no clear pattern of distribution. All non-cemented stems were designed with fixed anteversion of 18±7.5°, which reduced anteversion by 7±23° from native.
48 stems (84%) had at least one of the following characteristics: stem diameter <8mm, stem length <100mm, or anteversion correction >10° from native. Of the remaining hips, 5 required patient-specific designs to navigate the unique shape of the proximal femur.
3 hips (5%) were revised, 1 for infection at 2 years, and 2 for aseptic loosening of the patient-specific femoral component at 1 year and 3 years.
Conclusion. Hip stems designed for this population are small diameter and short length with varus necks and low head centers. Stems are frequently shorter than commercially available small stem options and additionally require the ability to correct for native anteversion.
References. [1] Sedrakyan A, et al. J Bone Joint Surg Am. 2014; [2] Dessyn E, et al. HIP International. 2019; [3] Swarup I, et al. J Arthroplasty. 2018
Figures
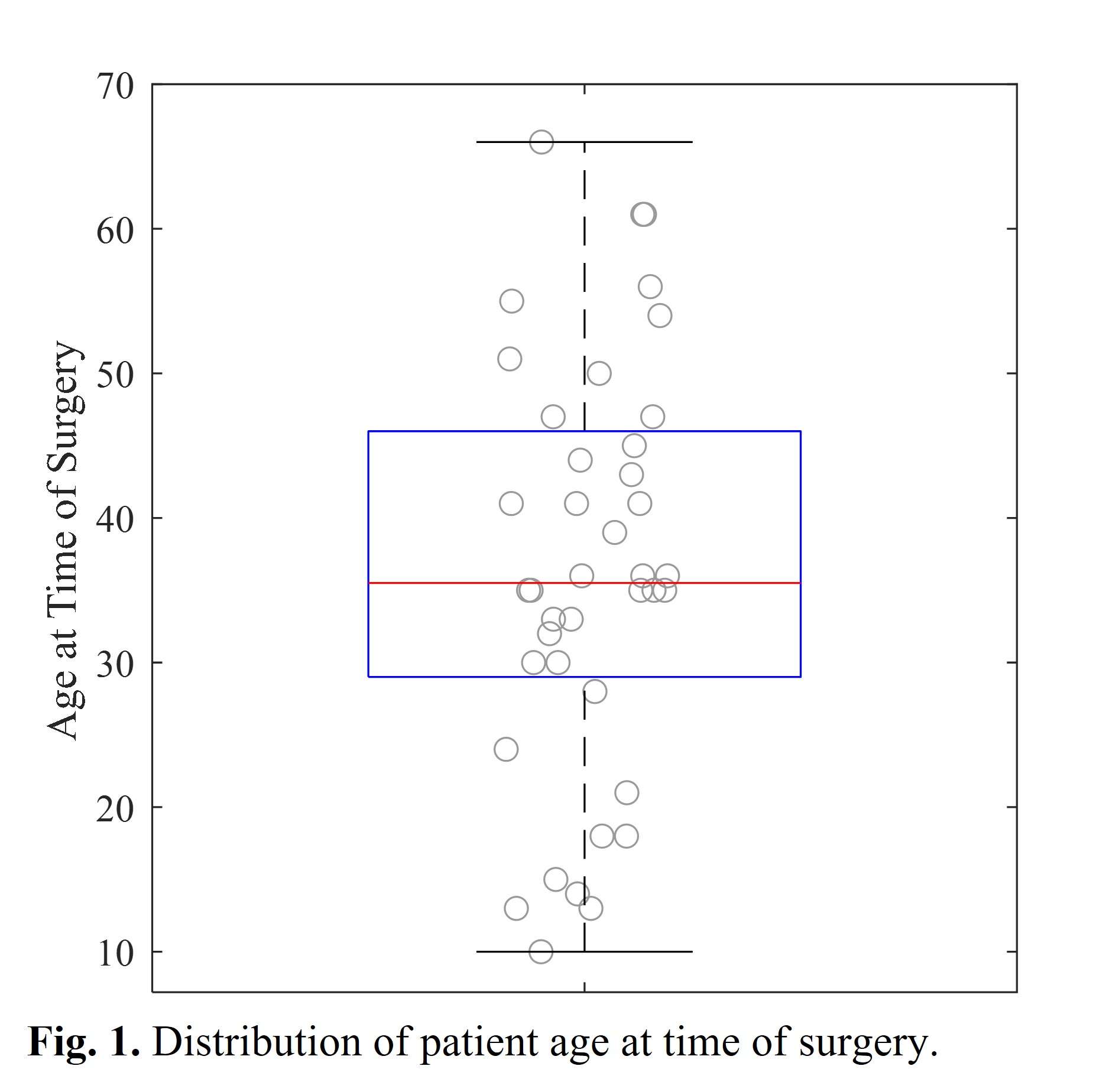
Figure 1
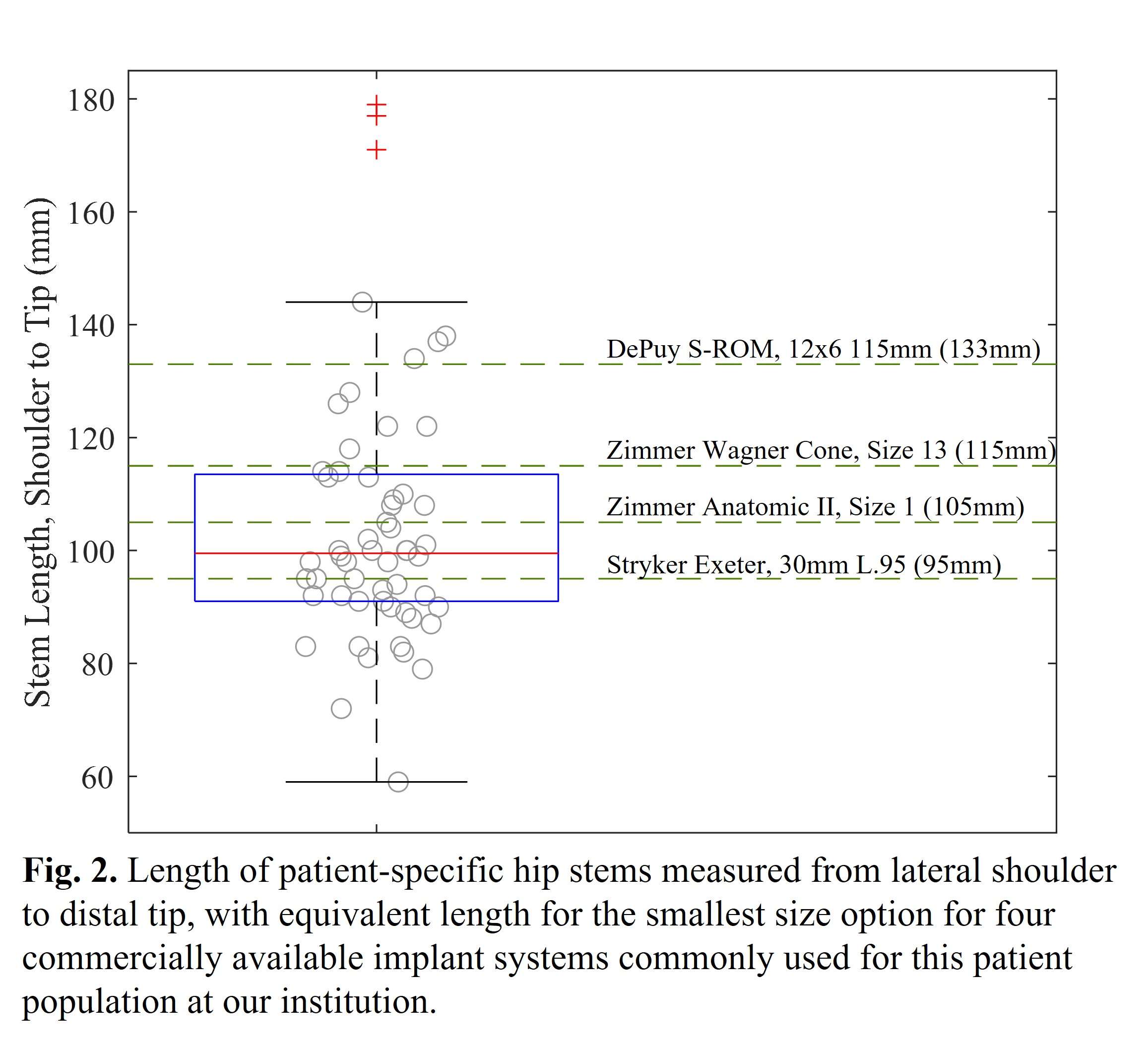
Figure 2
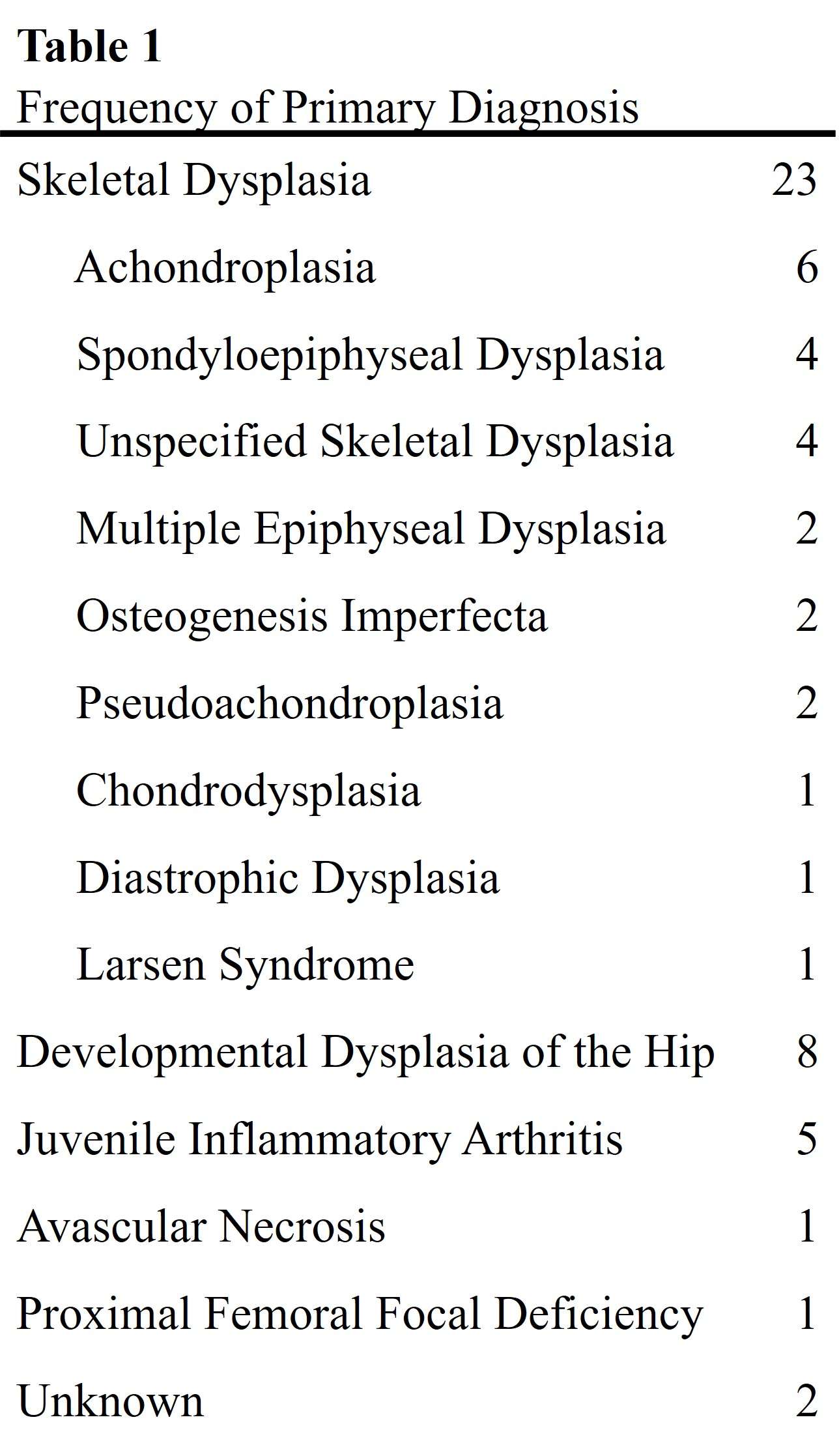
Figure 3#8363
Assessing the Feasibility of Mixed Reality Navigation for Intraoperative Guidance in Total Hip Arthroplasty
*Joshua Twiggs - University of Sydney - Sydney, Australia
Brad Miles - 360 Knee Systems - Pymble, Australia
Alana Sylvester - 360 Med Care - PYMBLE, Australia
William Walter - Specialist Orthopaedic Group - North Sydney, Australia
Tyson Doneley - Brisbane Orthopaedic Clinic - Spring Hill, Australia
Kaushik Hazratwala - Queensland Lower Limb Clinic - Pimlico, Australia
*Email: joshua_twigg@msn.com
Introduction: Correct orientation (inclination and anteversion) of the acetabular component in Total Hip Arthroplasty (THA) is essential to minimising the risks of implant failure and Range of Motion limiting impingement. Navigation allows surgeons to achieving planned inclination and anteversion values but requires markers at extra wound sites on the patient Mixed Reality (MR) is a potential alternate technology that combines the features of Virtual Reality (VR) and Augmented Reality (AR), anchoring three-dimensional holographic objects into the real world and enabling users to interact with virtual and physical objects simultaneously. The Microsoft HoloLens is one such head-mounted, MR device with potential applications in THA, but its accuracy is not well understood. The aim of this study is to investigate the accuracy of the HoloLens in navigating acetabular inclination and anteversion using a markerless prototype navigation application.
Method: A custom application was developed on the HoloLens for markerless navigation. The application allows a surgeon user to capture landmarks by pointing at the left & right Anterior Superior Iliac Spine and the Pubic Symphysis to reconstruct the Anterior Pelvic Plane (APP), in addition to the acetabular centre. From the acetabular centre, the HoloLens then projected a hologram of a cup axis at 40? inclination and 20? anteversion. Performing this procedure with a sawbones model, the user recreated the angle of the projected hologram with a cup impactor positioned in the acetabulum, and a 3D scan was taken of the scene. The 3D scan was reconstructed in ScanIP Simpleware to calculate the actual orientation and inclination achieved. This procedure was repeated 20 times and the mean accuracy determined.
Results: Across the 20 repeated measurements, mean accuracy was 2.7° (± 1.7°) in inclination and 3.3° (± 2.9°) in anteversion of the cup, and the difference in mean or variation in accuracy between these two values was not statistically significant. Of the 40 angular measurements, only 5 (12.5%) were out by more than 5° and none were out by 10°, comparable with current marker-based navigation systems.
Conclusion: MR has the potential to provide accurate intraoperative surgical guidance for cup inclination and anteversion in THA, without markers or infrastructure beyond a head mounted helmet. Further research and development is called for to adapt the prototype mixed reality application and test its performance in a cadaver lab setting.
#8656
Accuracy of Acetabular Cup Positioning Using Patient-Specific Augmented Reality Guidance
Dan Sun - Tufts University - Boston, USA
William Murphy - Harvard Medical School - Boston, USA
Patrick Lane - Surgical Planning Associates - USA
*Stephen Murphy - New England Baptist Hospital - Boston, USA
*Email: stephenbmurphymd@gmail.com
Introduction:
Malposition of the acetabular cup has been shown to contribute to postoperative complications following Total Hip Arthroplasty, including dislocations and revisions [1]. Surgeons have used many methods to achieve optimal placement of the acetabular cup, including preoperative planning, traditional navigation, robotics, and intraoperative radiography. More recently, a patient-specific Mixed Reality guidance solution has been developed that allows the surgeon to see 3D models of patient anatomy, surgical instruments and implants in relationship to the patient during surgery using a head-mounted device. The purpose of this study was to evaluate the accuracy of cup placement relative to the goal for surgeries performed using the Mixed Reality guidance platform.
Methods:
Fifty-seven patients underwent CT based preoperative planning for use of the Mixed Reality guidance system. This includes the generation of 3D models of the patient’s pelvis and femur and planned placement of 3D models of the components. 3D holograms were created for display during surgery. At the time of the procedure, a smart mechanical navigation tool was docked to the patient with a tracking image target located outside of the body in a predicted position. The holograms were then displayed into the patient during surgery on a headmounted device (Microsoft HoloLens 2) by anchoring and tracking the image target. The surgeon then aligned the real cup handle as closely as possible to the projection of the cup handle. All cases were performed by the senior author.
After surgery, all patients underwent standing EOS biplanar imaging at their postoperative appointment [2]. These two images were used to calculate the achieved cup orientation using a validated methodology [3]. This was compared to the planned cup orientation.
Results:
The mean error in achieved vs planned operative anteversion of the acetabular cup was -1.3° (SD: 2.0°, Min -7.0°, Max 2.0°), the mean error in operative inclination was -1.2° (SD: 2.5°, Min -6.0°, Max 5.0°). The mean absolute error was 1.8° (SD: 1.6°) for operative anteversion, and 2.2° (SD: 1.7°) for operative inclination. Demographic information for patients is summarized in table 1.
Conclusion:
The Mixed Reality guided acetabular components were well placed with low mean error, and all cases within +/- 7 degrees in both planes. Further studies may provide additional data on the accuracy of Mixed Reality guidance for accurate acetabular component placement in total hip arthroplasty.
References:
[1] Techniques for Optimizing Acetabular Component Positioning in Total Hip Arthroplasty: Defining a Patient-Specific Functional Safe Zone. JE Feng, AA Anoushiravani, N Eftekhary, D Wiznia, R Schwarzkopf, JM Vigdorchik; JBJS Reviews. 2019 Feb;7(2):e5. doi: 10.2106/JBJS.RVW.18.00049
[2] Geometry of the EOS® Radiographic Scanner, B. N. Groisser, Department of Mechanical Engineering Technion-Israel Institute of Technology Haifa, Israel, arXiv:1904.06711
[3] Sun, Daniel C., William S. Murphy, Andrew J. Amundson, Patrick M. Lane, Jens H. Kowal, and Stephen B. Murphy. "Validation of a Novel Method of Measuring Cup Orientation Using BiPlanar Simultaneous Radiographic Images." The journal of arthroplasty (2023)
Figures
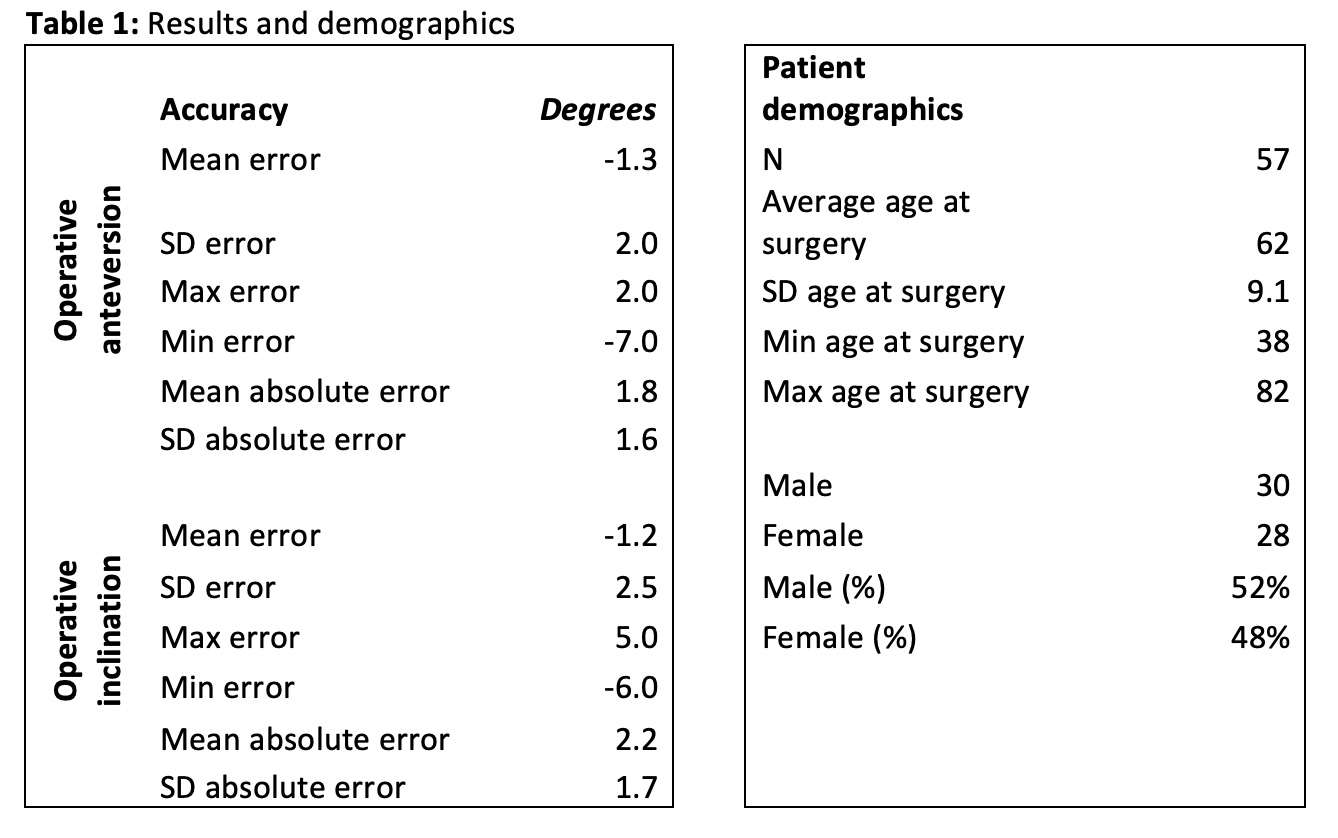
Figure 1
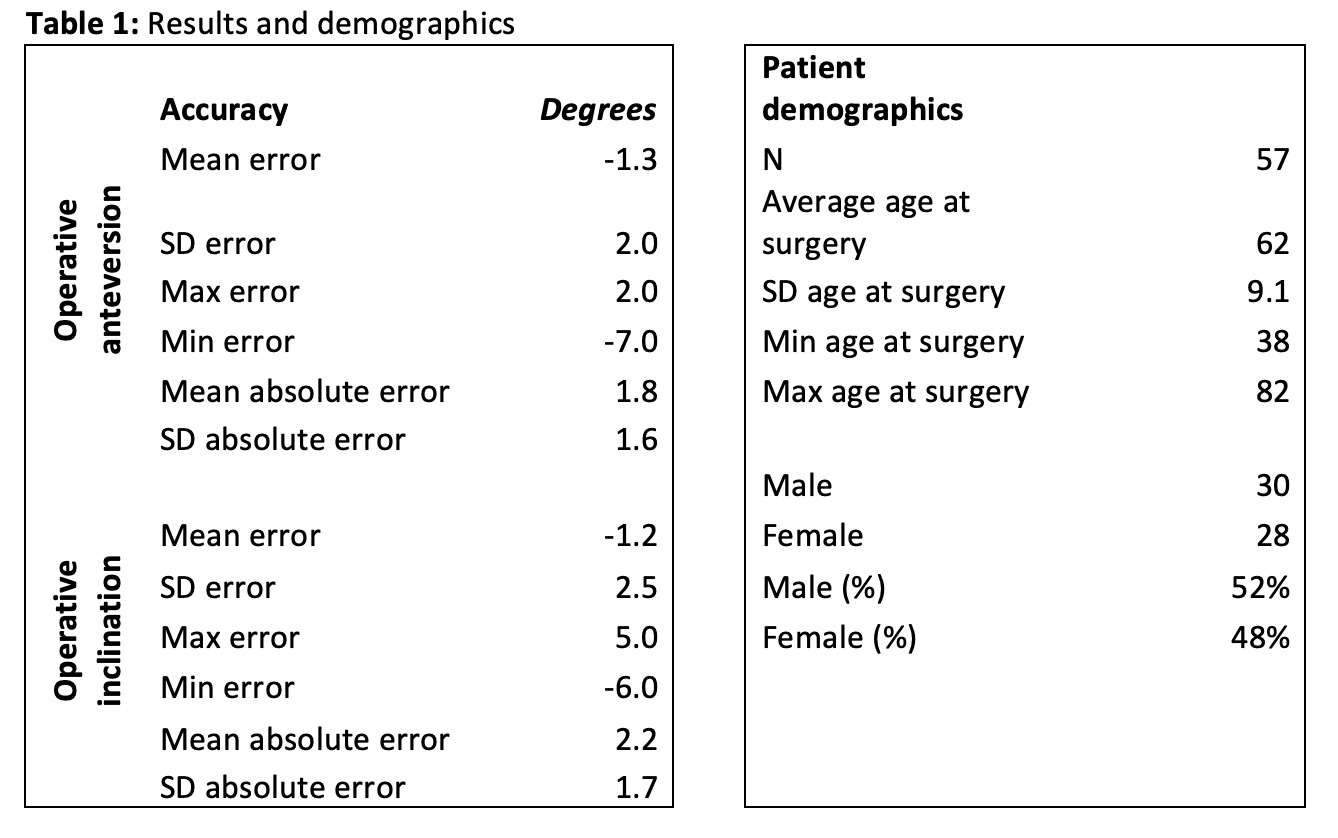
Figure 2#8229
Handheld Infrared Stereo Camera vs. Augmented Reality-Based Navigation System: A Comparison Study in Total Hip Arthroplasty
*Shinya Tanaka - Nagoya university - Nagoya, Japan
Yasuhiko Takegami - Nagoya University - Nagoya, Japan
Keiji Otaka - Nagoya university - Nagoya, Japan
*Email: shinn.0118@gmail.com
Introduction:
Currently, portable navigation systems (PNS) with different registration methods and angle measurement methods are available. Naviswiss is an accelerometer-based system that uses a handheld infrared stereo camera, while AR-Hip is an augmented reality-based system. When both are used in total hip arthroplasty(THA) in the lateral decubitus position, registration is performed in the supine position and the patient is repositioned to the lateral decubitus position. In this study, we compared the accuracy of these two different types of PNS.
Methods:
We retrospectively compared patients who underwent hip replacement with Naviswiss (Naviswiss AG, Brugg, Switzerland) or AR-Hip (Zimmer Biomet Japan, Tokyo, Japan) at Nagoya University Hospital from April 2019 to March 2022. All cases were performed in the lateral decubitus position with a posterior approach. The most appropriate stem was selected based on the shape of the femur and the cup was selected from the same manufacturer as the stem. The target angles of cup were 40° for radiographic inclination (RI) and 20° for radiographic anteversion (RA). Accuracy error(absolute values of the differences between the angles measured postoperatively and the target angles), and navigation error (absolute values of the differences between the angles measured postoperatively and the angles displayed on the navigation screen) were were compared.
Results:
There were 89 cases in the Naviswiss group and 69 cases in the AR Hip group. The mean cup placement angles for the Naviswiss and AR-Hip groups were RI 41.0° ± 4.5° and RA 19.5° ± 5.1°, and RI 40.0° ± 4.7° and RA 17.1° ± 5.3°, respectively. The accuracy error and navigation error for both inclination and anteversion showed no significant difference between the two groups (accuracy error: Naviswiss group, inclination 3.5° ± 3.0°, anteversion 4.1° ± 3.1°, AR-Hip group, inclination 3.5° ± 3.1°, anteversion 4.5° ± 4.0°; navigation error: Naviswiss group, inclination 3.5° ± 3.0°, anteversion 4.1° ± 3.1°, AR-Hip group, inclination 3.5° ± 3.1°, anteversion 4.5° ± 4.0°). There were no significant differences between the two groups in terms of operative time, intraoperative blood loss, or complications.
Conclusion:
The accuracy of an accelerometer-based portable navigation system using handheld infrared stereo cameras and an AR-based portable navigation system in THA in the lateral decubitus position was not significantly different. Both systems had good accuracy with errors within 5° for both inclination and anteversion. The method of performing registration in the supine position and surgery in the lateral position was found to be useful in the use of portable navigation systems.
#8611
Accuracy of Acetabular Cup Placement in DA THA: A Handheld Portable Image-Based Navigation System May Outperform a Robotic Platform.
*Eric M Slotkin - Reading Hospital, Orthopaedic Associates of Reading, - West Reading, Pennsylvania, USA
Stefan Kreuzer - INOV8 Orthopedics - Houston, USA
Alejandro Gonzalez Della Valle - Hospital for Special Surgery - New York, USA
*Email: eslotkindo@gmail.com
Introduction:
Malposition of the acetabular component during total hip arthroplasty (THA) is a leading cause of complications and need for revision. With the advent of lumbosacral orientation in THA, surgeon understanding of the at-risk patient has led to a more patient-specific and narrowed component safe zone. Robotic-assisted THA purports to improve accuracy of component positioning with many reports demonstrating over 92% of components within 10 degrees of inclination and anteversion compared to intraoperative system output. Many intraoperative enabling technologies have been brought to market to improve surgeon accuracy and to address this specific issue. This study aimed to evaluate the intraoperative accuracy output of acetabular cup position values using a handheld miniaturized portable navigation system (Naviswiss, AG) compared to post-operative CT scans.
Methods:
A total of 108 direct anterior approach total hip (DA THA) surgeries utilizing the intraoperative navigation device were performed over a six-month period. Intraoperative device output for measured acetabular component inclination and anteversion were recorded and compared with values derived from post operative CT scans.
Results:
Post operative CT analysis of acetabular component positioning compared to intraoperative values from the navigation unit demonstrated 90.74% and 89.81% were within 3 degrees of inclination and anteversion, respectively. Expanded, 97.22% and 94.44% were within 5 degrees of intraoperative inclination and anteversion, respectively. One post operative scan yielded an inclination value greater than 5 degrees but less than 10 degrees, and two scans showed an anteversion value great than 5 degrees but less than 10 degrees. No scan demonstrated an absolute inclination or anteversion measurement difference greater than 8 degrees from the intraoperative navigation unit value. Overall, 92.59% of components were within 5 degrees and 100% were within 8 degrees for both inclination and anteversion compared to post operative CT measurements.
Conclusion:
This handheld portable navigation system yielded highly accurate intraoperative component positioning values confirmed by post operative CT scans during DA THA. These values demonstrated results superior to the accepted accuracy within 10 degrees reported with robotic assisted THA. These smaller, portable, and more accessible intraoperative units may provide surgeons improved accuracy and availability in a number of surgical settings for use in total hip arthroplasty.
#8140
Obesity Is Not Associated With the Accuracy of Registration of Cup Placement in Muscle-Sparing Total Hip Arthroplasty Using CT-Based Navigation
*Hiroshi Watanabe - Nippon Medical School - Tokyo, Japan
Mari Yokouchi - Nippon Medical School Musashi Kosugi Hospital - Kawasaki, Japan
Yuta Mouri - Nippon Medical School Musashi Kosugi Hospital - Kawasaki, Japan
Takuya Uematsu - Nippon Medical School - Tokyo, Japan
Tokifumi Majima - Nippon Medical School - Tokyo, Japan
*Email: watanabehiroshi@nms.ac.jp
Background
Portable navigation systems have become popular in total hip arthroplasty (THA). However, computed tomography (CT)-based navigation systems still have many advantages regarding the accuracy of cup placement in severe conditions, such as in minimally invasive surgery (MIS), low-volume surgeons, and obese patients. The objectives of this study were to evaluate the accuracy of cup placement with its learning curve in consecutive THA cases using CT-based navigation in those conditions.
Methods
This study retrospectively investigated 75 consecutive patients who underwent THA using a direct superior approach by a low-volume surgeon. All patients were categorized into three groups (N = 25) using a time sequence as initial, intermediate, and recent groups [Table 1]. We investigated operative time, body mass index (BMI), and the registration error of the cup alignment by comparing intraoperative and postoperative values.
Results
Regarding inclination, the absolute error in the initial group was significantly greater than that of the intermediate and recent groups [Fig. 1]. Regarding anteversion, there were no significant differences in the absolute errors between the groups [Fig. 2]. Operative time was significantly correlated with consecutive case numbers (r = −0.770; P = 0.0001) [Fig. 3]. Meanwhile, there were no significant differences in the absolute errors between BMI > 25 vs < 25 kg/m2 groups for inclination and anteversion [Fig. 4, 5]. However, the operative time in the BMI > 25 kg/m2 group was significantly greater compared with the BMI < 25 kg/m2 group.
Conclusion
CT-based navigation offers excellent accuracy of cup orientation regardless of obesity, including MIS and low-volume surgeon situations. Proficiency in CT-based navigation requires as few as 25 cases.
Figures
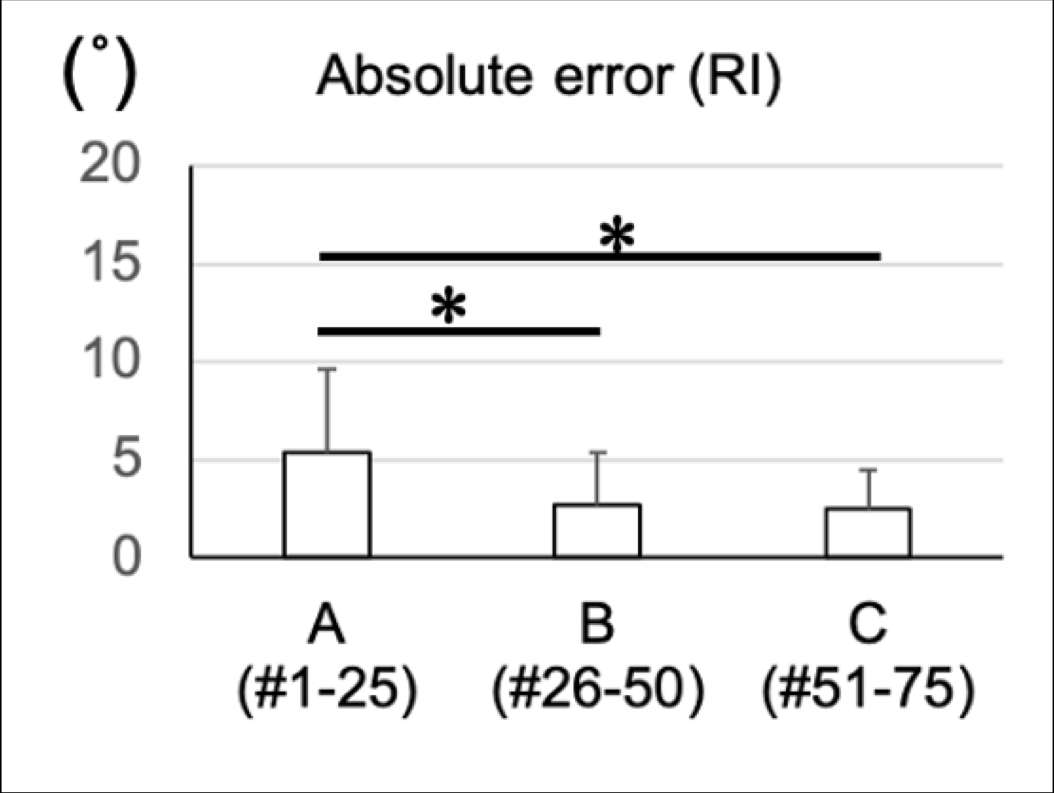
Figure 1
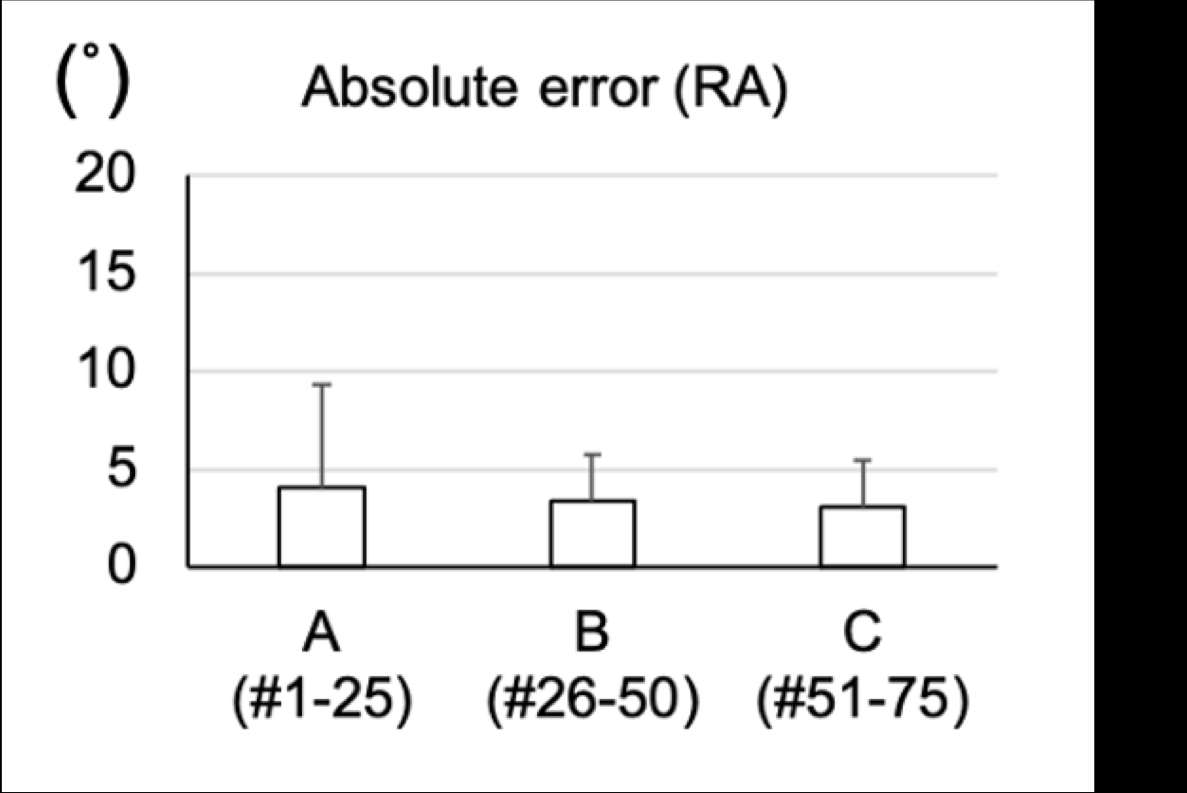
Figure 2
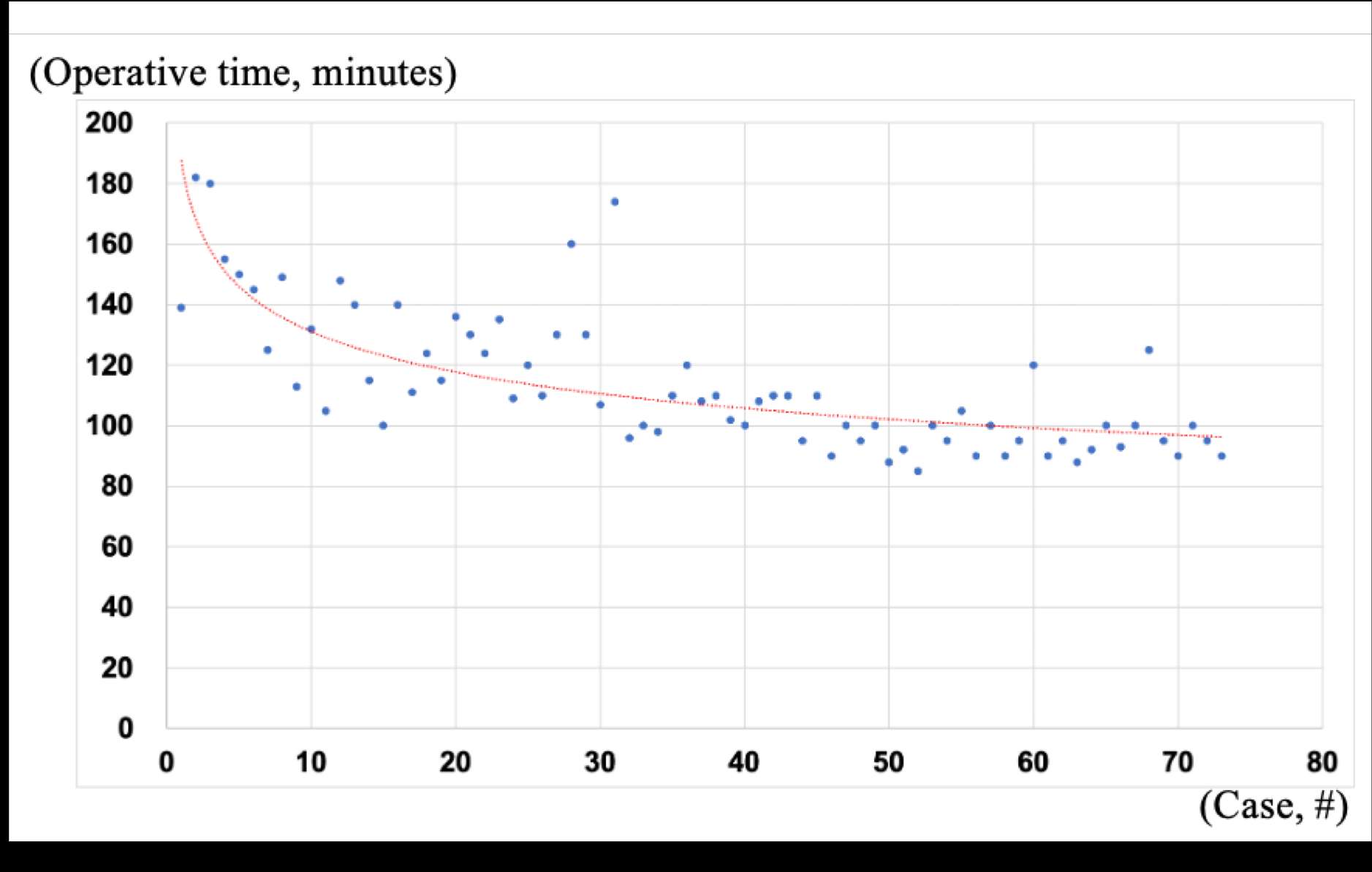
Figure 3
Figure 4
Figure 5
Figure 6#8119
Cup Positioning Accuracy of Direct Anterior Approach Using a Non-Invasive, Image-Based Software Navigation System in Primary Total Hip Arthroplasty
*David Fawley - DePuy Synthes - Warsaw, USA
Thierry Bernard - DePuy Synthes - West Chester, USA
J. Craig Morrison - Nashville, USA
John Redmond - Southeast Orthopaedic Specialists - Jacksonville, USA
*Email: dfawley1@its.jnj.com
Introduction
Computer-assisted total hip arthroplasty (THA) has been in use for over a decade. There has been increased interest in non-invasive navigation in recent years. A non-invasive software navigation system (SNS) was developed to provide accurate digital templating and intraoperative surgical guidance for offset, leg length, anteversion and inclination (abduction). These data are determined using a completely non-invasive imaging approach and do not rely on hardware typically associated with computer assisted navigation. The aim of this study was to review cup positioning accuracy, operative, and early clinical outcomes after THA via direct anterior approach (DAA) with a non-invasive SNS.
Methods
Data were collected within a multi-center, prospective, company-sponsored clinical study (NCT04191733). All cases were performed via DAA. All clinical assessments were summarized. Cup positioning success was defined as a composite endpoint. Both cup inclination and cup version needed to be within 10 degrees of surgeon target to be considered a success. The paired t-test was used for evaluation of changes from baseline for clinical outcomes scores. Cup positioning was measured by a third-party (Medical Metrics, Inc.) using AP hip and AP pelvis plain films.
Results
228 subjects were analyzed; 85 in the SNS group, 143 in the non-SNS group. Patient age, gender and primary diagnoses were similar between groups. Mean BMI was larger in the SNS group (p=0.0001) (Figure 1). In SNS, 42.4% of subjects were obese, compared to 22.4% in the non-SNS. In the non-SNS group, fluoroscopy was used intraoperatively to help place the cup in 69.2% of cases. Cup positioning outcomes were excellent for both groups (Figure 2). SNS showed more variation in inclination compared to target (p=0.0094), and less for version (p<0.0001). Mean inclination success was not significantly different between groups. Mean version success was 98.7% for SNS and 87.7% for non-SNS (0.0039). Mean overall success was 96.2% for SNS and 81.9% for non-SNS (p=0.0026). Mean proximal femur and pelvis vertical alignment (an estimate of leg-length discrepancy) was 3.1 (SD 2.7) mm for SNS and 4.1 (3.1) mm for non-SNS (p=0.02). Scatterplots showing the cup position for each group are presented in Figure 3.
Conclusion
A non-invasive, image-based software navigation system used in anterior approach can be a useful tool for helping surgeons execute their target cup position. In our study, compared to a group of DAA cases without use of the software, surgeons were highly successful hitting their cup positioning targets (96.2% compared to 81.9%). Our evaluation also suggests that use of the SNS may also help to decrease leg-length discrepancy, similar to what others have reported1. Limitations of our study include a non-randomized design and different surgeons within each group. Continued study of this SNS, ideally under a randomized study design, would be valuable.
References
- O’Leary R, Saxena A, Arguelles W, Hernandez Y, Osondu CU, Suarez JC. Digital Fluoroscopic Navigation for Limb Length Restoration During Anterior Total Hip Arthroplasty. Arthroplasty Today; 18 (2022).
Figures
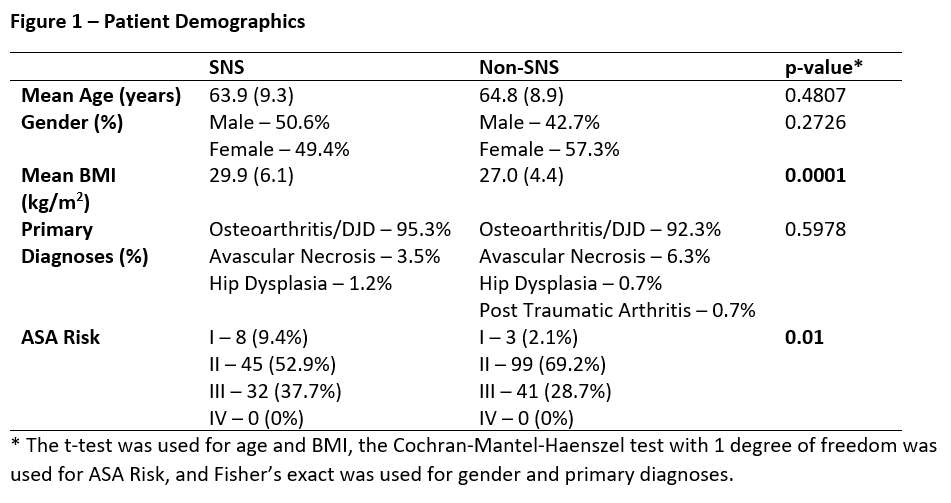
Figure 1
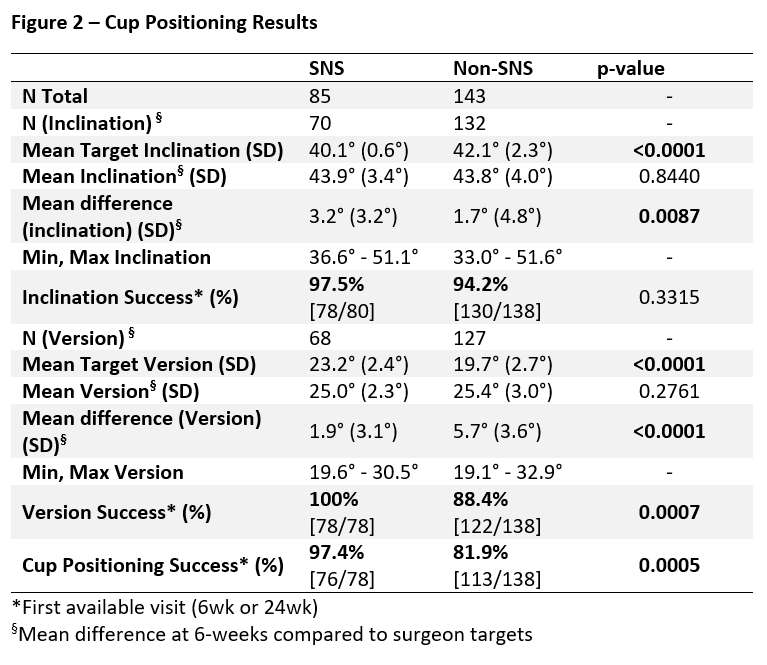
Figure 2
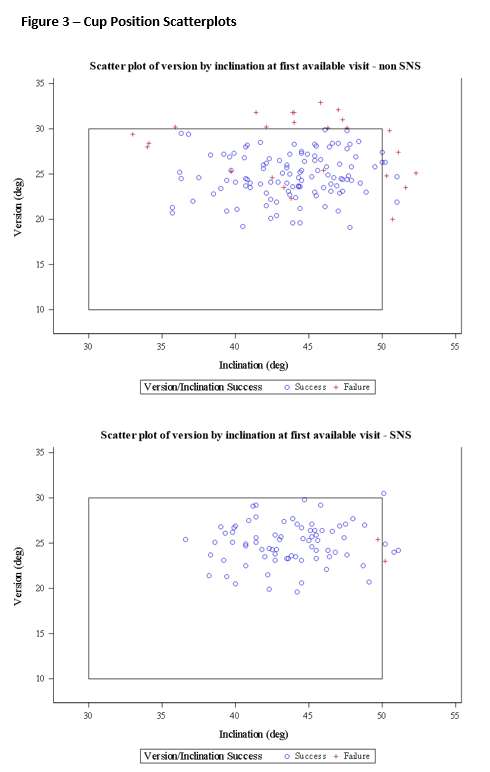
Figure 3#8511
Accuracy of Planned Femoral Neck Cut in Total Hip Arthroplasty Using Robotic-Arm Assisted With Navigation Guidance.
*Benjamin Domb - American Hip Institute - Des Plaines, USA
Paulo P. Padilla - American Hip Institute - Des Plaines, USA
Saiswarnesh Padmanabhan - American Hip Institute - Des Plaines, USA
Julio Nerys-Figueroa - American Hip Institute - Des Plaines, USA
Mark F. Schinsky - American Hip Institute - Des Plaines, USA
Mark Schinsky - American Hip Institute Research Foundation - Chicago, United States of America
Mark F. Schinsky - American Hip Institute - Des Plaines, USA
*Email: DrDomb@americanhipinstitute.org
Objective: This study aimed to evaluate the accuracy of the planned femoral neck cut in patients undergoing robotic-arm assisted total hip arthroplasty (rTHA) with navigation-guided femoral neck cut, and to compare it with postoperative radiographs.
Methods: A retrospective analysis was conducted on 70 patients who underwent rTHA with the use of femoral neck cut guidance. Postoperatively, radiographs were obtained to measure the actual femoral neck cut. Femoral neck cut value was defined as the distance from the superior aspect of the lesser trochanter to the most distal aspect of the femoral cut.
Results: The mean planned femoral neck cut was 25.5mm (± standard deviation), while the mean measured femoral neck cut angle was 25.9mm (± standard deviation). Statistical analysis revealed no statistically significant difference between the planned and measured femoral neck cut angles (p= 0.65). Mean difference was noted to be 2.03 mm. Furthermore, a strong correlation (r2=0.82) was observed between the planned and measured femoral neck cut angles, indicating a high level of accuracy in achieving the planned neck cut.
Conclusion: This study demonstrates that THA with femoral neck cut guidance enables precise execution of the planned femoral neck cut. The implementation of this surgical technique enhances the precision and reproducibility of femoral neck cuts in THA, potentially leading to improved clinical outcomes.
Keywords: Total hip arthroplasty, MAKO robot version 4.0, navigation guidance, femoral neck cut, postoperative radiographs.
#8356
The Learning Curve Associated With Conversion From Fluoroscopic Direct Anterior Hip Arthroplasty to Robotic-Assisted Direct Anterior Hip Arthroplasty
*
Kara Sawaya - Trinity Health Ann ARbor - Ypsilanti, USA
Amy J. Harshberger - Trinity Health Ann Arbor - Ypsilanti, USA
*Email: mamasini@comcast.net
Title:
The learning curve associated with conversion from fluoroscopic direct anterior hip arthroplasty to robotic-assisted direct anterior hip arthroplasty
Introduction:
Prolonged use of fluoroscopy during orthopaedic procedures may result in significant radiation exposure and lead to an increased risk of thyroid and breast cancer in orthopaedic surgeons [1,2]. In total hip arthroplasty (THA), direct anterior (DA) approach is generally performed with fluoroscopic guidance to assist with implant positioning. Robotic-assisted (RA) THA provides an alternative to fluoroscopic guidance, thus reducing radiation exposure. The aim of this study was to assess the learning curve associated with the adoption of RA-THA with DA approach with regards to surgical time, use of fluoroscopy, and implant placement.
Methods:
A retrospective, non-randomized evaluation of learning curve with the adoption of RA-THA by assessing surgical time was conducted on a consecutive series of 85 DA cases performed by a single surgeon. 47 cases had manual THA with fluoroscopy and 38 cases had RA-THA. Surgical time was defined as skin to skin. All cases were performed on a standard OR table and had an acetabular placement target of 40? inclination and 20? anteversion. An independent reviewer blinded to the surgical technique used the Widmer method to measure inclination and version [3].
Results:
Manual fluoroscopic group had a mean age of 66±10 and BMI of 28.7±6.3. RA-THA group had a mean age of 68±8.9 and BMI of 29.0±5.0. Mean surgical time for the manual group was 88±21 minutes and surgical time for RA-THA group was 101±14 minutes. Figure 1 shows the change in surgical time of the RA-THA group. After 15 RA-THA cases, surgical time reached time neutral compared to the manual group and the following 10 consecutive RA-THA cases had the same average time as the manual cases.
The first 17 RA-THA cases utilized fluoroscopy to verify implant position until the surgeon became comfortable with accuracy of the RA system. After case 17, fluoroscopy was abandoned in all subsequent RA-THA cases. Mean fluoroscopic use was 31±11.4 seconds for the manual group and 3.8±2.0 seconds for the first 17 RA-THA cases. Mean radiation dose delivered to the surgical field was 5.61±5.71mGy.
Manual THA resulted in mean acetabular inclination of 41.3±4.4? and anteversion of 22.4±3.0?, where 14 cases had more than ±5°variation in inclination or version from target. RA-THA resulted in mean acetabular inclination of 42.0±4.2? and anteversion of 22.3±3.9?, where 13 cases had more than ±5° variation in inclination or version from target. There was no change in RA-THA placement accuracy after case 17.
Conclusion:
DA THA can be performed with RA-THA and achieve equivalent acetabular placement without fluoroscopy. Surgical time was higher for the RA-THA group during the learning curve, but then decreased and was consistent with the manual group after 15 cases. Elimination of fluoroscopy provides a safer procedure for surgeons by eliminating radiation exposure. An additional benefit outside this study scope was the elimination of protective lead and future studies are proposed to understand the effects of eliminating wearing lead on the surgical teams’ experience.
#8158
The Use of Robotic-Arm-Assistance to Achieve Functional Cup Positioning in THA
*ahmed magan - University College London Hospital - London, GB
Andreas Fontalis - University College London Hospital - London, United Kingdom
Fares Haddad - University College London Hospital - London, United Kingdom
Fabrice Glod - Hôpitaux Robert Schuman-Fondation Hôpitaux Robert Schuman - Luxembourg-City, Luxembourg
Pierre Putzeys - Hôpitaux Robert Schuman-Fondation Hôpitaux Robert Schuman - Luxembourg-City, Luxembourg
*Email: ahmedmagan@gmail.com
Introduction: An essential factor for achieving stability in total hip arthroplasty (THA) is accurate implant positioning tailored to individual patient phenotype. It is widely accepted that no universal target exists, and variations in spinopelvic mobility mandate adjustments to the surgical plan, bringing the concept of functional component positioning to the fore. This study evaluates functional outcomes and PROMs utilising robotic-arm-assistance (RAA) to achieve functional implant positioning.
Methods: This prospective cohort study includes 150 patients undergoing primary uncemented THA. After attaining a CT scan and sitting and standing lateral lumbar radiographs, RAA was utilised to execute the patient-specific plan. Radiological parameters pertaining to implant positioning were recorded. The Oxford Hip Score (OHS), Hip Disability and Osteoarthritis Outcome Score (HOOS) and Forgotten Joint Score (FJS) were assessed pre-operatively and at one year.
Results: Mean inclination was 41.9 ± 3.7, mean anteversion 21.3 ± 2.5 and mean combined version 33.4 ± 7.5. Improvement from baseline was statistically significant for all PROMs and ROM. After one year, the median HOOS was 94.4 (Q1, Q3 80 to 98.8), median OHS 47 (Q1, Q3 42 to 48) and median FJS 93.7 (Q1, Q3 77.1 to 100). No dislocations occurred in this cohort during the follow-up period.
Conclusion: Functional implant positioning utilising RAA yielded excellent PROMs and functional outcomes one year post-operatively. Patient-specific component positioning was significantly affected by individual spinopelvic motion. Tailored implant positioning and RAA may offer a viable solution for reducing dislocation rates. However, comparative studies with larger sample sizes are key for validation.
#8336
Improved Accuracy of a Novel Fluoroscopy-Based Robotically Assisted THA System Compared to Manual THA
Graham Buchan - Cleveland Clinic Lerner College of Medicine - Cleveland, United States of America
Christian Hecht - Cleveland Clinic - Cleveland, USA
David Liu - Gold Coast Centre for Bone and Joint Surgery - Tugun, Australia
Lipalo Mokete - Charlotte Maxeke Johannesburg Academic Hospital, Johannesburg - Sandton, South Africa
Daniel Kendoff - HELIOS Kliniken Berlin-Buch, Schwanebecker Chaussee - Berlin, Germany
*Atul Kamath - Cleveland Clinic - Cleveland, USA
*Email: kamatha@ccf.org
Introduction: Accurate acetabular cup position remains a persistent challenge in total hip arthroplasty (THA). Studies investigating the early outcomes of robotic-assisted THA (RA-THA) systems have shown improved cup placement compared to unassisted manual THA (mTHA) approaches. However, contemporary robotic platforms are reliant on pre-operative CT imaging and the efficacy of these systems remain inconclusive in the current literature. The goal of this study was to analyze the accuracy of a novel, fluoroscopy-based RA-THA system compared to a fluoroscopy-guided mTHA approach and determine the effect of the robotic system on operative time.
Methods: We performed a retrospective cohort analysis on a consecutive series of 198 patients who received mTHA and RA-THA between March 2021 and July 2022. The primary outcome of interest was the accuracy of acetabular component placement, defined by average cup inclination and anteversion. Acetabular cup orientation was determined by analyzing post-operative standing anteroposterior (AP) pelvic obtained at routine 6-week post-operative follow-up visits. Secondary outcomes included the proportion of acetabular cups positioned within the Lewinnek safe zone, operative time, and overall room time.
Results: The RA-THA group demonstrated significantly higher accuracy of acetabular anteversion to target compared to the manual group (18.5 vs. 21.7?; p<0.001), and significantly lower variance in the RA-THA group for both acetabular anteversion (26.0 vs. 44.5; p=0.008) and inclination parameter (26.8 vs. 46.7; p=0.007) (Figure 1). The RA-THA treatment group also had a significantly greater proportion of acetabular cups placed within the Lewinnek safe zone compared to the mTHA group (81.6 vs. 59.0%; p<0.001) (Figure 2). The RA-THA cohort had longer operative times compared to mTHA group (39.0 vs. 35.3 mins; p=0.003), but no difference was seen in total operating room time between groups (101.2 vs. 101.2 mins; p=0.982) (Table 1).
Conclusion: This study demonstrates that the use of a novel, fluoroscopy-based, pin-less THA robotic platform increased the accuracy of acetabular cup placement, including a 22.6% improvement in safe zone placement, compared to a fluoroscopy-guided mTHA approach. Additionally, the additive technology did not increase overall case time. Given the improved accuracy with the fluoroscopy-based robotic technique used in this study, our findings can be used to justify the further investigation of fluoroscopy-based THA robotic assistance systems in clinical practice.
Keywords: total hip arthroplasty (THA); accuracy; robotic-assisted surgery; fluoroscopy; robotic THA; hip replacement
Figures
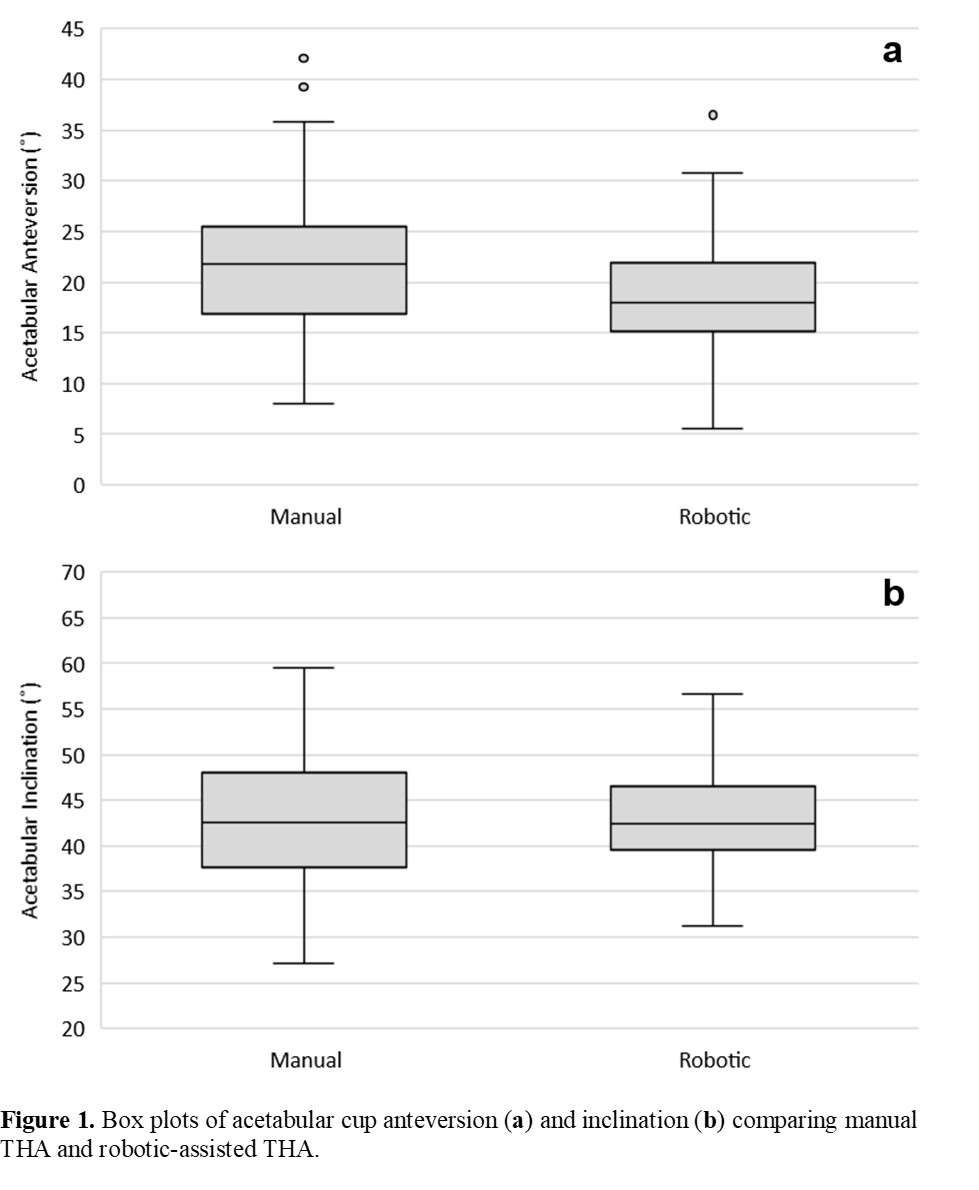
Figure 1
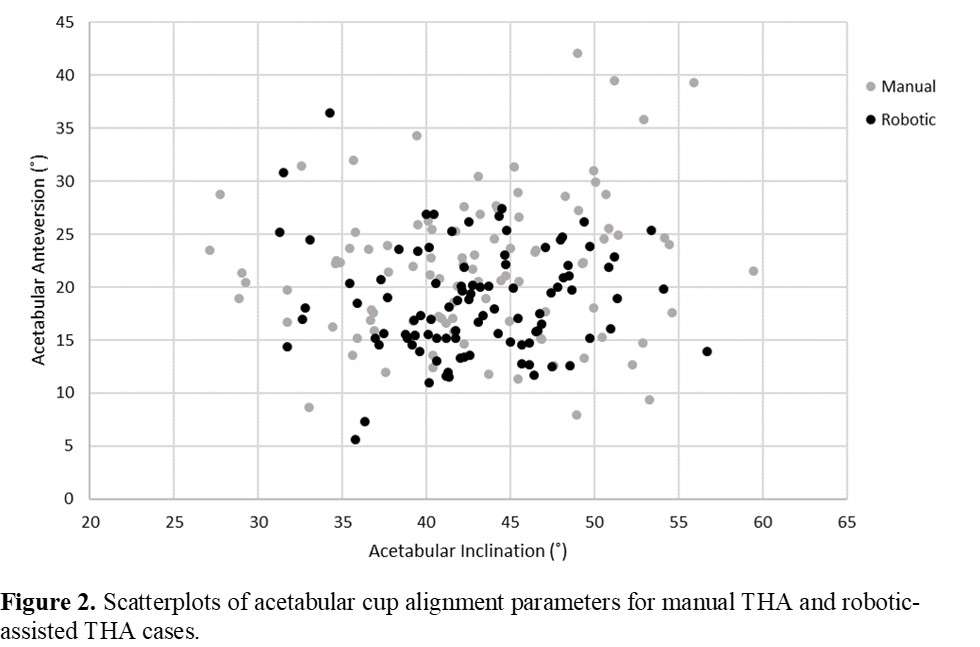
Figure 2
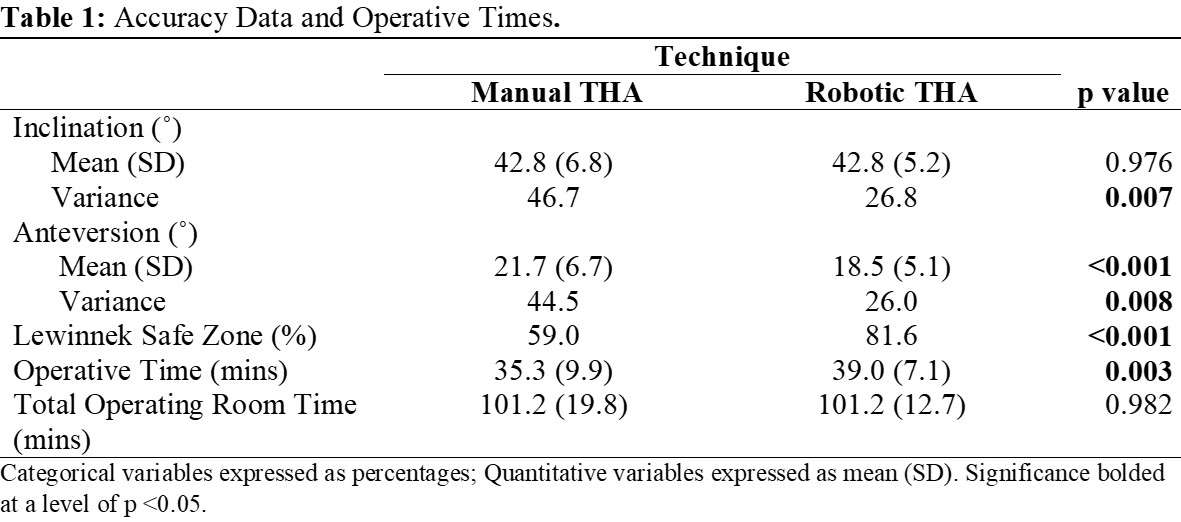
Figure 3#8762
Synovial White Blood Cell Count and Differential to Predict Successful Management of Acute Periprosthetic Joint Infection With Debridement Antibiotics, and Implant Retention
*Itay Ashkenazi - NYU Langone Health - New-York, United States of America
Jeremiah Thomas - NYU Langone Health - Scarsdale, United States of America
Akram Habibi - NYU Langone Orthopedic Hospital - New York, United States of America
Theodore Di Pauli Von Treuheim - NYU Langone Health - New York, USA
Jonathan Katzman - NYU Langone - New York, United States of America
Claudette Lajam - NYU Hospital For Joint Diseases - New York, USA
Vinay Aggarwal - NYU Hospital for Joint Diseases - New York, USA
Ran Schwarzkopf - NYU Langone Medical Center Hospital for Joint Diseases - New York, USA
Benjamin Schaffler - NYU Orthopedic Hospital - New York, USA
*Email: itay.ashkenazi@gmail.com
Introduction
Utilization of synovial white blood cell (WBC) count and percentage of synovial polymorphonuclear cells (PMN%) from joint aspiration for predicting outcomes of debridement, antibiotics, and implant retention (DAIR) for acute periprosthetic joint infection (PJI) (<3 months from index total joint arthroplasty) is not well documented. This study aimed to assess the value of synovial WBC and PMN% in predicting DAIR outcomes and as a prognostic tool for decision making.
Methods
This was a retrospective review of 131 consecutive patients who were diagnosed with an acute hip or knee PJI between September 2011 and May 2022. After excluding patients without a minimum of one-year follow-up or who underwent treatment other than DAIR, 83 patients remained for final analyses. Surgical outcome was defined by the Musculoskeletal Infection Society (MSIS) outcome reporting tool (Tiers 1 to 4). Tiers 1 and 2 were considered successful treatment while Tiers 3 and 4 were considered failed treatment. Receiver operating characteristic curve (ROC) analyses were performed to estimate optimal thresholds of WBC and PMN%. Kaplan-Meier survival analyses with log-rank test were also performed.
Results
Synovial WBC counts (area under the curve (AUC) 0.790) demonstrated superior accuracy in predicting DAIR outcomes when compared to PMN% (AUC 0.677). The calculated WBC count threshold (5,168 cells/mL) demonstrated sensitivity of 94% and specificity of 59%. The optimal PMN% count threshold (88.5%) showed 69% sensitivity and 65% specificity. There was a significant difference in failure free survival at latest follow-up between the cases with WBC count higher vs lower than 5,168/mL (p<0.001), as well as for cases with PMN% over 88.5% (p=0.009).
Conclusion
Synovial WBC count (<5,168/mL) demonstrated very high sensitivity to confirm successful DAIR. Both WBC count and PMN% (<88.5%) thresholds can significantly determine DAIR survival and should be considered when counseling patients on successful management for acute PJI.
Figures
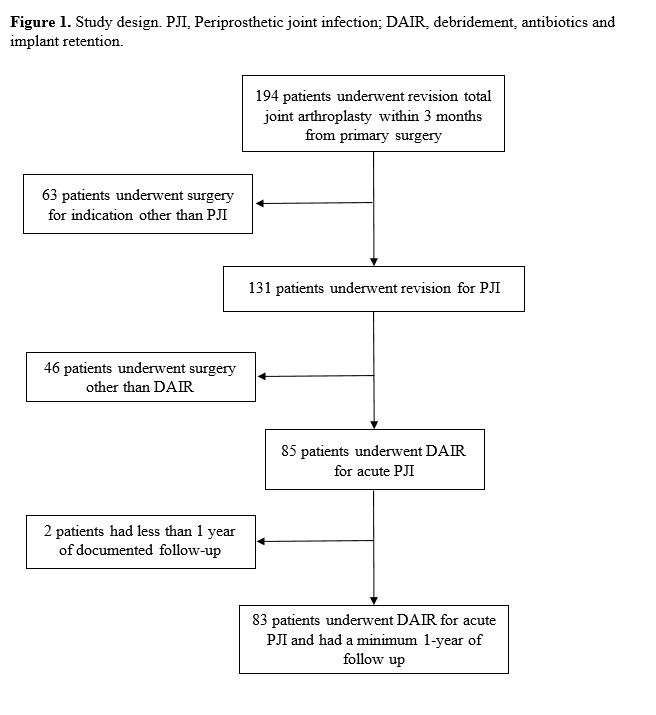
Figure 1
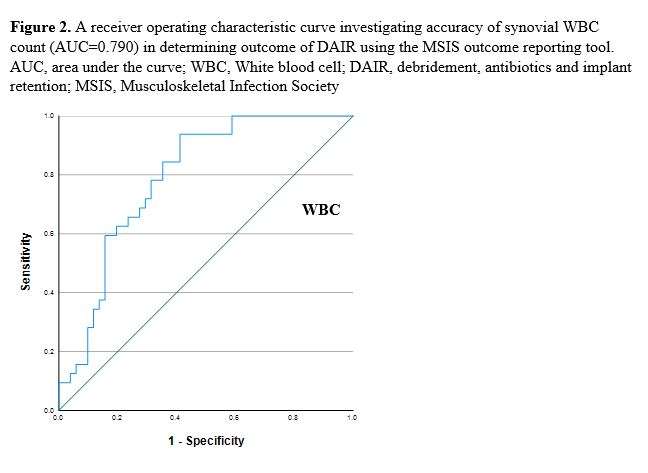
Figure 2
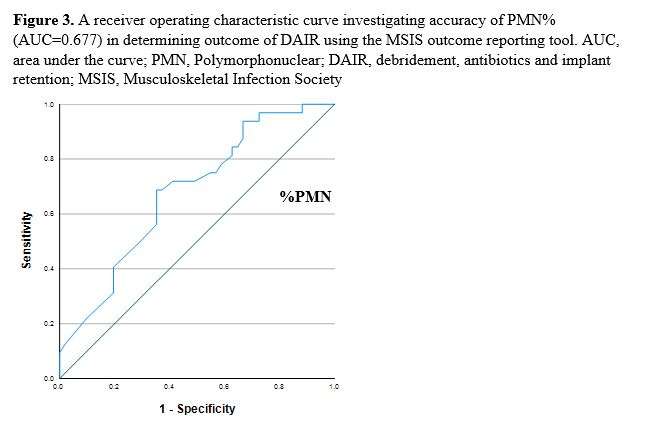
Figure 3
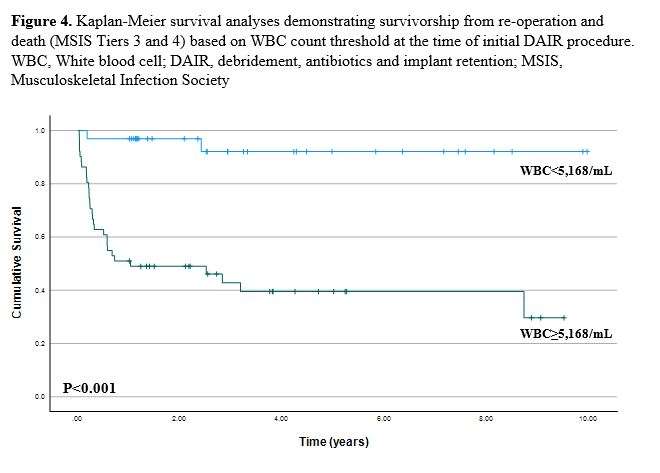
Figure 4
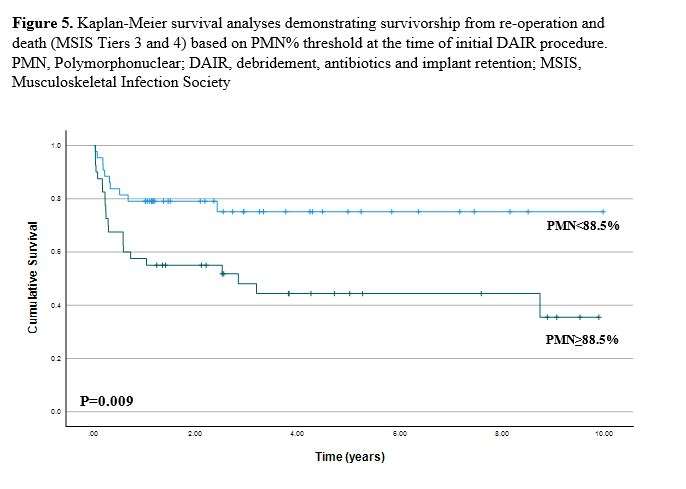
Figure 5
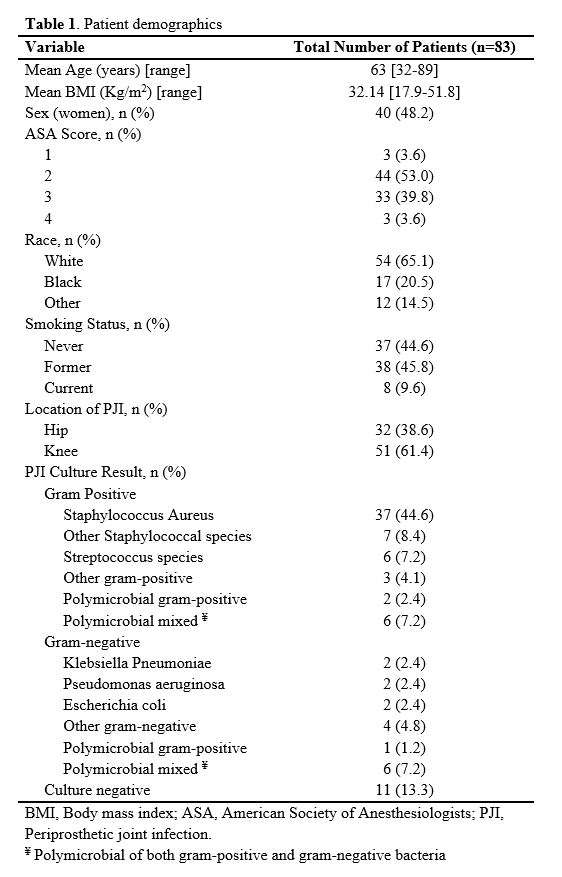
Figure 6
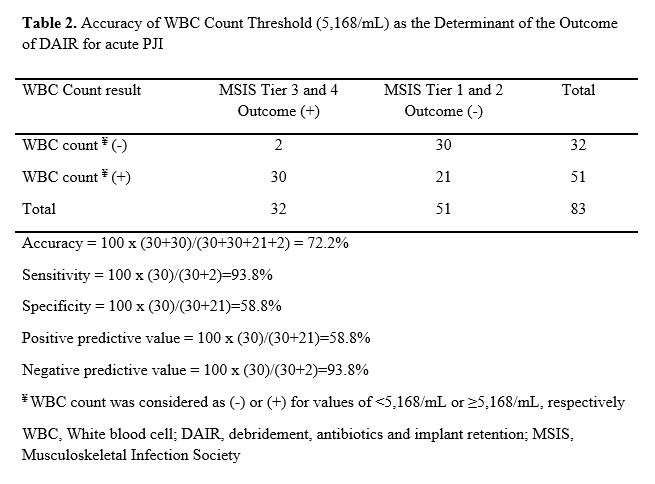
Figure 7
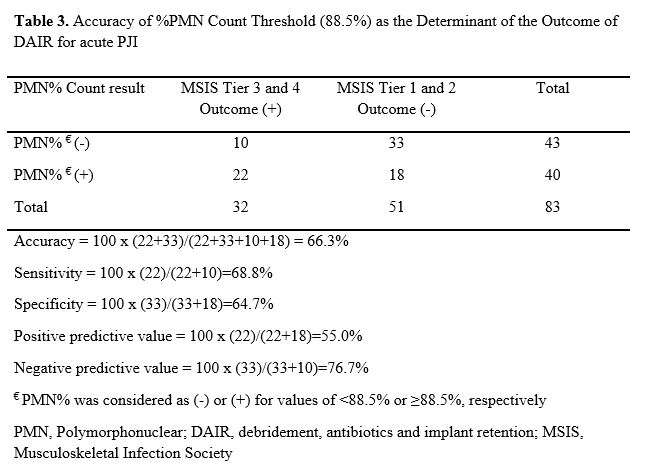
Figure 8#8102
Treatment Failure or New Infection ? Persistent Infection After One-Stage Exchange for Hip and Knee Periprosthetic Joint Infection.
*Jean-Yves Jenny - University Hospital Strasbourg - Illkirch, France
*Email: Jean-yves.jenny@chru-strasbourg.fr
Introduction
A failure rate range of 15 to 36% has been described after one-stage exchange for periprosthetic joint infection. Failure is traditionally attributed to persistence or recurrence of the initial infection. However, each surgical procedure carries its own risk of iatrogenic infection. The risk of postoperative infection following a hip or knee implant exchange for aseptic reasons has been estimated at 5% to 10%. Thus, it must be considered the possibility that a post-treatment infection is actually due to a new infection, acquired during the implant exchange, and independent of the initial infection.
The goal of this study was to perform an comprehensive analysis of the failed cases to define if it was related to persistence or recurrence of the index infection or development of a new infection.
Methods
A retrospective analysis was conducted. All cases of periprosthetic joint infection treated by one stage exchange at a referral tertiary care center between 2017 and 2018 were included. 171 cases were selected: 96 men and 75 women with a mean age of 69 years. Hip joint was involved in 97 cases and knee joint in 74 cases. Surgery was conducted in a standardized manner, and antibiotic treatment was administered following the more recent recommendations. All cases were followed for at least two years. The occurrence of a surgical site infection (SSI) according to the definition of the MSIS was recorded. A comprehensive analysis of all available pre-, per- and postoperative bacteriological samples was performed. Persistence of the index infection was considered if the pathogen(s) responsible for the index infection and for the SSI were identical. Occurrence of a new infection was considered if the pathogen(s) responsible for the index infection and for the SSI were different.
Results
33 failures were recorded (19%). Persistence or recurrence of the index infection was observed in 5 cases (15% of the failures). Occurrence of a new infection was observed in 25 cases (76% of the failures). Three cases could not be classified (9% of the failures).
Conclusion
Eradication of periprosthetic joint infection by one stage exchange was observed in 97% of the cases. The main reason for a SSI during the follow-up time was the occurrence of a new infection with a different pathogen (15% of the index cases). These new pathogens were probably introduced into the surgical field during revision surgery. Greater efforts should be given for prevention of bacterial contamination during revision surgery, while treatment of the index infection seems to be very successful. It would be interesting to obtain the same information after two-stage exchange involving two successive revision surgeries.
#8761
Surgical Vest Decreases Contamination With Sterile Surgical Helmet Systems
Sabir K. Ismaily - UTHealth - Houston, USA
Andrew Konopitski - UT Health Houston - Houston, USA
Hugh Jones - UT Health Science Center at Houston - Houston, USA
Kenneth Mathis - UT Health Houston - Houston, USA
*David Rodriguez - University of Texas - Houston, USA
*Email: david.rodriguezquintana@uth.tmc.edu
Introduction
Sterile surgical helmet systems (SSHS) are frequently utilized when performing total joint arthroplasty in order to protect the surgeon and maintain the sterile field. Many surgeons use a standard gown in combination with the SHSS. We believe the positive pressure created by the SHSS in combination with an uncovered back seam may result in contamination of the surgical field directly behind the surgeon and assistants. The goal of this study was to determine if pairing a surgical vest with the SSHS would result in reduced contamination.
Methods
In a custom sterile isolation chamber, an orthopaedic fellow donned the SHSS with one of three surgical gown configurations: 1.) Standard gown (SG) with no vest 2.) Standard gown with a surgical vest (SG+V) 3.) Toga style (TS) gown. Surgical movements were simulated for 1 hour by sawing and cutting on a sterilized polyethylene block. Contamination was measured with agar settle plates positioned directly behind the subject. Agar plates would be incubated and CFUs later counted. Power analysis required that each gown configuration be tested 12 times. CFU averages were compared with student t-test and analysis of variance (ANOVA) with p < 0.05 indicating significance.
Results
Both the addition of a sterile vest to a standard surgical gown, or use of a toga style gown, resulted in less contamination than a standard surgical gown alone. Standard gowning grew 331.7 ± 52 CFU/m2/hr, Toga style grew 170.5 ± 41.9 CFU/m2/hr, and Standard + Vest grew 182.2 ± 30.8 CFU/m2/hr (SG vs. TS p = 0.01; SG vs SG+V p = 0.02, TS vs SG+V p > 0.05).
Conclusion
In order to reduce contamination of the surgical field directly behind the surgeon and assistants, all surgical participants should cover the back seam of a standard gown with a vest or don a toga style gown.
#8551
Differences in Infections Between Total Hip Arthroplasty in Outpatient vs Inpatient Facilities
*Lucas Voyvodic - Brooklyn, United States of America
Ariel Rodriguez - Maimonides Medical Center - Brooklyn, United States of America
Judy Lee - SUNY Downstate Health Science University - Brooklyn, USA
Matthew Magruder - Maimonides Medical Center - Brooklyn, USA
Alejandra Moncayo - SUNY Downstate College of Medicine - Brooklyn, USA
Nicole Ackerman - SUNY Downstate College of Medicine - Brooklyn, USA
Jack Choeuka - Maimonides Medical Center - Brooklyn, USA
Orry Erez - Maimonides Medical Center - Brooklyn, USA
Gabriel Lama - Maimonides Medical Center - Staten Island, United States of America
*Email: lucasvc129@gmail.com
INTRODUCTION: The purpose of this study was to investigate whether outpatient operations have higher rates of: 1) surgical site infections (SSIs); 2) peri-prosthetic joint infections (PJIs) compared to in-patient facilities following primary THA; 3) to compare the same day and 90-day costs associated with primary THA in both groups; and 4) compare costs in patients that developed postoperative infections with patients that did not in both the inpatient and outpatient setting.
METHODS: A retrospective query from January 1st, 2010, to March 31st, 2021 was performed using an administrative claims database for patients who underwent primary THA. Study group patients were those who underwent THA as outpatients, and control patients were those who underwent surgery as an inpatient. Primary outcomes assessed included SSIs and PJIs at 90-days and 2-years following surgery, as well as same day and 90-day costs associated with primary THA.
RESULTS: Patients undergoing outpatient primary THA did have significantly decreased incidence of developing SSIs at 90 days (0.6% vs. 0.9%; OR: 0.64, p<0.001), SSIs at 2 years (0.8% vs. 1.4%; OR: 0.54, p<0.001), PJIs at 90 days (0.4% vs 0.9; OR: 0.43, p<0.001) and PJIs at 2 years (0.5% vs. 1.4% %; OR: 0.38, p<0.001. Outpatient THA had significantly lower same day ($3,961.60 vs $10,500.99; p<0.001) and 90-day costs ($6,128.78 vs $13,896.54; p<0.001) than the inpatient cohort. Additionally, patients that developed PJI or SSI within 90 days postoperatively had a significantly higher 90 day cost than patients that did not develop a postoperative infection.
CONCLUSION: The outpatient transition of THAs has potential to decrease healthcare costs and risk of infection as well as increase patient satisfaction. This result is useful for understanding risk of complications for physicians offering outpatient THAs, as well as the cost associated with this procedure.
#8741
Effectiveness of Combined Adipose-Derived Mesenchymal Stem Cell and Antibiotic Therapy in Rat Model of Periprosthetic Joint Infection
Yuki Yamamuro - Kanazawa University School of Medical Science - Kanazawa, Japan
*Tamon Kabata - Kanazawa university - kanazawa, Japan
Katsuhiro Hayashi - Kanazawa University - Kanazawa, Japan
Yoshitomo Kajino - Kanazawa University - Kanazawa, Japan
Daisuke Inoue - Kanazawa University - Kanazawa, Japan
Takaaki Ohmori - Kanazawa Univercity - Kanazawa, Japan
Junya Yoshitani - Kanazawa University - Kanazawa, Japan
Takuro Ueno - Kanazawa University - Kanazawa, Japan
Ken Ueoka - Kanazawa University - Kanazawa, Japan
Atsushi Taninaka - Kanazawa university - Kanazawa, Japan
Tomoyuki Kataoka - Kanazawa University - Kanazawa, Japan
Yoshitomo Saiki - Kanazawa University - Kanazawa, Japan
Yu Yanagi - Kanazawa University - Kanazawa, Japan
Hiroyuki Tsuchiya - Kanazawa University - Kanazawa, Japan
*Email: tamonkabata@yahoo.co.jp
Periprosthetic joint infection (PJI) is characterized by biofilm infection, which is difficult to alleviate while preserving implant integrity. Furthermore, long?term antibiotic therapy may increase the prevalence of drug?resistant bacterial strains, necessitating a non?antibacterial approach. Adipose?derived stem cells (ADSCs) exert antibacterial effects; however, their efficacy in PJI remains unclear. This study investigates the efficacy of combined intravenous ADSCs and antibiotic therapy in comparison to antibiotic monotherapy in a methicillin?sensitive Staphylococcus aureus (MSSA)?infected PJI rat model. The rats were randomly assigned and equally divided into 3 groups: no?treatment group, antibiotic group, ADSCs with antibiotic group. The ADSCs with antibiotic group exhibited the fastest recovery from weight loss, with lower bacterial counts (p = 0.013 vs. no?treatment group; p = 0.024 vs. antibiotic group) and less bone density loss around the implants (p = 0.015 vs. no?treatment group; p = 0.025 vs. antibiotic group). The modified Rissing score was used to evaluate localized infection on postoperative day 14 and was the lowest in the ADSCs with antibiotic group; however, no significant difference was observed between the antibiotic group and ADSCs with antibiotic group (p < 0.001 vs. no?treatment group; p = 0.359 vs. antibiotic group). Histological analysis revealed a clear, thin, and continuous bony envelope, a homogeneous bone marrow, and a defined, normal interface in the ADSCs with antibiotic group. Moreover, the expression of cathelicidin expression was significantly higher (p = 0.002 vs. no?treatment group; p = 0.049 vs. antibiotic group), whereas that of tumor necrosis factor (TNF)?α and interleukin(IL)?6 was lower in the ADSCs with antibiotic group than in the no?treatment group (TNF?α, p = 0.010 vs. no?treatment group; IL?6, p = 0.010 vs. no?treatment group). Thus, the combined intravenous ADSCs and antibiotic therapy induced a stronger antibacterial effect than antibiotic monotherapy in a MSSA?infected PJI rat model. This strong antibacterial effect may be related to the increased cathelicidin expression and decreased inflammatory cytokine expression at the site of infection(Fig.1).
#8452
Symptomatic Benign Prostatic Hyperplasia: An Optimizable Risk Factor for Periprosthetic Joint Infection After Elective Primary Total Knee Arthroplasty
Boyi Jiang - Sichuan University West China Hospital - Chengdu, China
*Zongke Zhou - Sichuan University - Chengdu, China
*Email: zongke@126.com
Abstract
Background:Symptomatic benign prostatic hyperplasia often leads to lower urinary tract symptoms and causes stasis of urine in the bladder and urinary tract, making it a risk factor for postoperative urinary retention (POUR) after total knee and hip arthroplasty (TKA/THA). Such retention and the potential need for repeated catheterizations increase the following risk of urinary tract infection (UTI). These considerations have led to the suggestion that sBPH increases the risk of periprosthetic joint infection (PJI) after TKA and THA because organisms gain access to the joint through transient bacteremia. However, a subsequent large retrospective study failed to find an association between sBPH and the risk of PJI within two years after primary TKA and THA. Whether symptomatic BPH, which is common among middle-aged and older men, affects the risk of periprosthetic joint infection (PJI) remains controversial.
Methods:We retrospectively analyzed medical data from 948 men who underwent primary TKA or THA at our institution between 2010 and 2021.We comparedtheincidence of postoperative complications such as PJI, UTI, and postoperative urinary retention (POUR) between 316 patients (193 hips and 123 knees) who did and 632 patients who did not have sBPH; the two groups of patients were matched to each other in a 1:2 ratio based on numerous clinical demographic variables. In the subgroup analyses, we stratified sBPH patients according to whether they began anti-sBPH medical therapy prior to arthroplasty.
Results:Periprosthetic joint infectionfollowing primary TKA was significantly more common among sBPH patients than among patients who did not have sBPH (4.1 vs 0.4%; P = 0.029), as were UTI (P = 0.029), and POUR (P < 0.001). Patients who had sBPH also had an increased incidence of UTI (P = 0.006) and POUR (P < 0.001) following THA. Among sBPH patients, those who started anti-sBPH medical therapy before TKA suffered significantly lower incidence of PJI than those who did not (0 vs 9.1%; P = 0.016); Premedication can also lower the incidence of POUR following TKA (P < 0.001) and THA (P = 0.01)
Conclusion:Symptomatic BPH is an independent risk factor for PJI in men after primary TKA, and it increases the risk of post-TKA and THA urinary retention and UTI. Starting anti-sBPH therapy before TKA and THA may reduce the risk of these complications and benefit sBPH patients. Therefore, we recommend sBPH patients receive perioperative medical therapy for the optimization of THA and TKA outcome.
Keywords
Periprosthetic joint infection, symptomatic benign prostatic hyperplasia, total knee and hip arthroplasty, urinary tract infection, medical therapy
#8377
Efficacy of Commercially Available Irrigation Solutions on Removal of Staphylococcus Aureus Infected Porous Titanium Implants- an in-Vitro Study
Joseph Seta - Ascension Providence Hospital - Southfield, USA
Paula Dietz - Ascension Providence Hospital - Southfield, USA
Fadi Aboona - Ascension Providence Hospital - Southfield, USA
Martin Weaver - Ascension Providence Hospital - Southfield, USA
*Therese Bou-Akl - Providence-Providence Park Hospital - Southfield, USA
Weiping Ren - Wayne State University - Select Country
David Markel - The CORE Institute - Novi, USA
*Email: Therese.Bou-akl@ascension.org
INTRODUCTION: Little research has established the efficacy/superiority of commercially available irrigation solutions in the treatment of periprosthetic joint infections (PJI) in the presence of porous titanium (Ti) implants. This study aimed to compare the in vitro bactericidal efficacy of five commercially available irrigation solutions in the presence of infected 3D printed porous Ti discs.
METHODS: Porous Ti discs (2x4 mm) with different pore sizes (400, 700 and 1000um) were placed into S. aureus suspension (Xn29, 1x106 CFU/ml) and incubated at 37oC for 3 hours (acute)(n=6) or 3 days (biofilm)(n=4). The Ti discs were then irrigated with 5 different solutions (saline, Bacitracin, Clorpactin, Irrisept and Bactisure) for 15 seconds, followed by 4 consecutive rounds of saline sonication (5 min each). The sonicate solutions were collected after each round for bacterial culture. Statistical analysis was performed using one-way ANOVA followed by Tukey Kramer Post Hoc test. The morphology and bacteria residence/biofilm of cultured Ti discs prior to sonication were analyzed by scanning electron microscopy (SEM).
RESULTS: Irrigation of infected disks with saline consistently left high CFU/ml bacterial counts in sonicate solution in both acute (Fig 1A-C) and biofilm groups (Fig 1D-F). In the acutely infected group of 400um pore size (ANOVA returned a p=0.001) for sonication #1 statistical differences were found with saline irrigation compared to Irrisept and Bactisure as well as bacitracin irrigation compared to both Irrisept and Bactisure. Bactisure irrigation in the acute infection group led to the lowest levels of CFU/ml in sonicate solutions across all pore sizes. Samples with smaller pore sizes (400um) consistently had much higher CFU/ml after irrigation both for acute and biofilm groups. In the biofilm group, irrigation with saline, Bacitracin, Clorpactin and Irrisept showed high concentrations of remaining bacteria across all pore sizes; and low concentrations observed in the Bactisure group without significant statistical difference (in sonicate #1 ANOVA returned a p=0.14). SEM demonstrated biofilm formation in unwashed as well as saline irrigated disks, and small areas of biofilm remaining on the Bactisure washed disks (Fig 2).
CONCLUSION: Irrigation of infected porous Ti disks using saline, Bacitracin and Clorpactin failed to adequately reduce the bacterial load regardless of pore size of the disks. Of note, the smallest pore size (400um) Ti disks consistently retained higher amounts of bacteria, even after irrigation which highlights the difficulty in mechanically removing bacteria from porous Ti disks. Irrigation with Irrisept reduced bacteria in the acute setting but did not sufficiently clear biofilm. Bactisure was the only irrigation solution which demonstrated consistent reduction in the bacterial burden in both the acute and biofilm groups, with more significant effect seen in smaller pore size due to the higher initial bacteria burden. It is important to note that Bactisure was designed specifically to target biofilms, whereas Irrisept is advertised as an antimicrobial wound lavage. The biofilm presence was noted in all groups using SEM, the size and number of biofilms following irrigation with Bactisure was significantly reduced. These results are of particular importance when considering irrigation of modern porous coated implants which have a high propensity to harbor bacteria and further emphasizes the difficulty in removing biofilms, particularly in the setting of PJI.
Figures
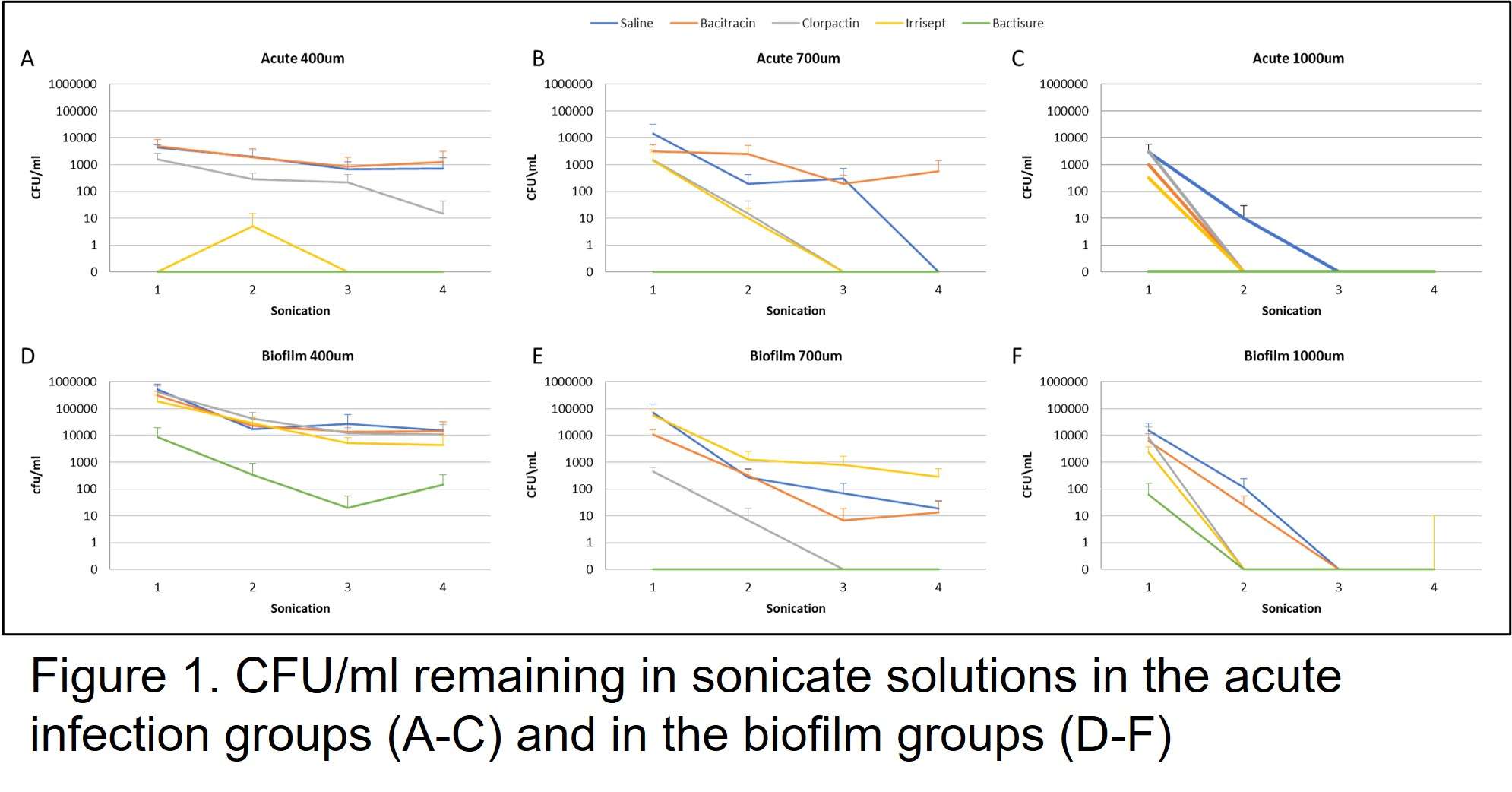
Figure 1
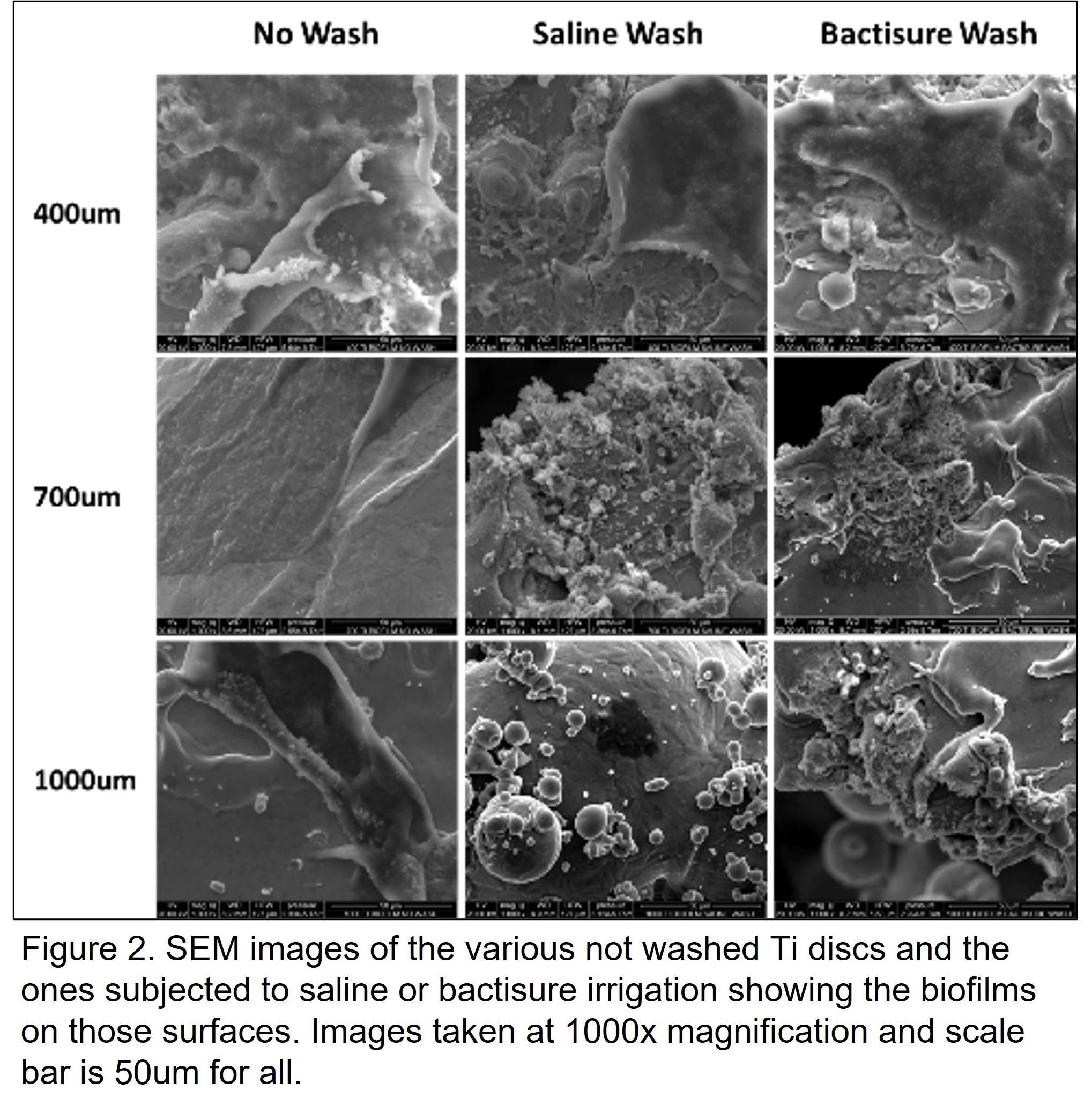
Figure 2#8161
Metal-Ion Allergies Associated With Higher Risk of Infection After Total Knee Arthroplasty
*Gerhard Maale - Medical Center Plano - Plano, USA
*Email: gerhardmaale53@gmail.com
INTRODUCTION: Multidirectional instability (MDI) associated with metal ion allergies presents with arthrofibrosis and mimics infection as a cause of failures following 1.5% of primary total knee arthroplasty (PTKA) patients. As part of the patient workup in the 276-patient MDI cohort, Lymphocyte Transformation Tests (LTT) were done, and 82.5% had metal ion allergies, with the most common being Nickel (72.5%). Vascular flow phase imagery with tri-phase bone scans demonstrate increased vascular flow to the surrounding capsular areas and thickened synovium on CT scans. In this cohort, is the infection rate higher at the time of replacement compared to historical values?
METHODS: All patients with known metal ion allergies by LTT were reviewed for infection. The organisms recovered were noted. All patients were diagnosed by the Consensus definition of Periprosthetic Joint Infections (PJI).
RESULTS: Out of the 200 patients experiencing nickel allergies, 21 (10.5%) experienced PJI following a PTKA, all of which required subsequent revisions. For the 21 PJI patients, 12 were female and 9 were male. The average age was 66 with a range 44 -79. Comparing this rate with historical rates of infection of PTKAs that ranged from 0.4 to 4.5%, Fisher’s exact test demonstrated significant increases in infection rates with people with metal-ion allergies, with the p-value of 0.005.
CONCLUSION: Infection is a major cause of concern in joint arthroplasties. Infection rates among patients with MDI associated with metal allergies are significantly greater after PTKA compared to the general population. The underlying cause of this increased rate of infection is unknown but thought to be associated with the increase in vascularity of the joint capsule as demonstrated by the vascular flow phase imagery in MDI patients due to the metal allergy.
#8415
Do Older Patients Benefit From Medially Tighter Flexion/Extension Gaps in TKA? an Analysis of 1-Year Functional Outcomes Stratified by Age
*Matthew Hickey - The University of British Columbia - Vancouver, Canada
Antony J Hodgson - The University of British Columbia - Vancouver, Canada
Carolyn Anglin - University of Calgary - Calgary, Canada
Bassam Masri - University of British Columbia / Department of Orthopaedics - Vancouver, Canada
Frederic Picard
*Email: matthew.hickey@hiphealth.ca
Introduction
In total knee arthroplasty, soft tissue balancing targets are partially driven by alignment philosophy. The development of technology-assisted TKA allows surgeons to quantitatively measure and achieve desired flexion and extension gaps intra-operatively. However, the impact of patient-specific factors such as age on optimal joint balance targets is currently unknown. This study retrospectively investigated the impact of patient demographics on optimal post-operative joint balance for improved functional and pain outcomes (1-year Oxford Knee Score, OKS).
Methods
We analysed 1171 navigated TKA cases which used a gap balancing procedure undertaken at our institution. We then assessed correlations between mediolateral gap differences in both flexion and extension with 1-year OKS using Spearmen correlation. Patients were then stratified by age at index (thresholds: 70 and 80 years old) and then further divided into subgroups depending on if post-operative flexion/extension gaps (measured during trialing) were either tighter medially or laterally (i.e. which compartment gap was the smallest). 1-year OKS was then compared between subgroups using a Wilcoxon Rank-sum non-parametric test. The minimally important clinical difference of ?OKS=5 between populations was applied.
Results
Gap balance favoring medially tighter in extension significantly correlated with 1-year OKS (p<0.002). Patient subgroups older than 70 years, 80 years, (Figures 1-2) who were medially tighter in extension (p<0.01, p<0.03) or flexion (p<0.03, p<0.03) had a statistically detectable increase in 1-year OKS. Additionally, laterally tighter patients in extension who were older than 80 years had a clinically significant decrease in 1-year OKS compared to those who were tighter medially (median OKS: 36 vs. 41).
Discussion
In this study, we found that patients with medially tighter gaps in extension significantly correlated with1-year OKS. In addition, we found that older patients may benefit from having slightly tighter medial compartments compared to laterally tight. In the native knee, the medial compartment compared tends to be tighter than the lateral compartment (Shalhoub 2019), therefore, it is possible that patients with tighter medial compartments post-TKA may better match their pre-arthritic state, improving patient reported outcomes measures. Currently, we are aiming to evaluate preoperative flexion/extension balance and its relationship to postoperative knee balance and patient-reported outcome scores. This effect was more pronounced in the subset of older patients, with patients older than 80 years old with laterally tight compartments exhibiting a median reduction in OKS of 5 points compared to those who were tighter medially, just reaching the MICD. This may be attributed to the natural decline in proprioception during aging (Ferlinc 2019), affecting individual patients’ ability to adapt to a substantially different flexion/extension balance (becoming laterally tighter as opposed to medially tighter) post-TKA. Overall, this study suggests that a tighter medial compartment in extension, better reflecting that of the native knee, may improve functional outcomes at 1-year and that this effect may be more pronounced in the older TKA patient population.
Figures
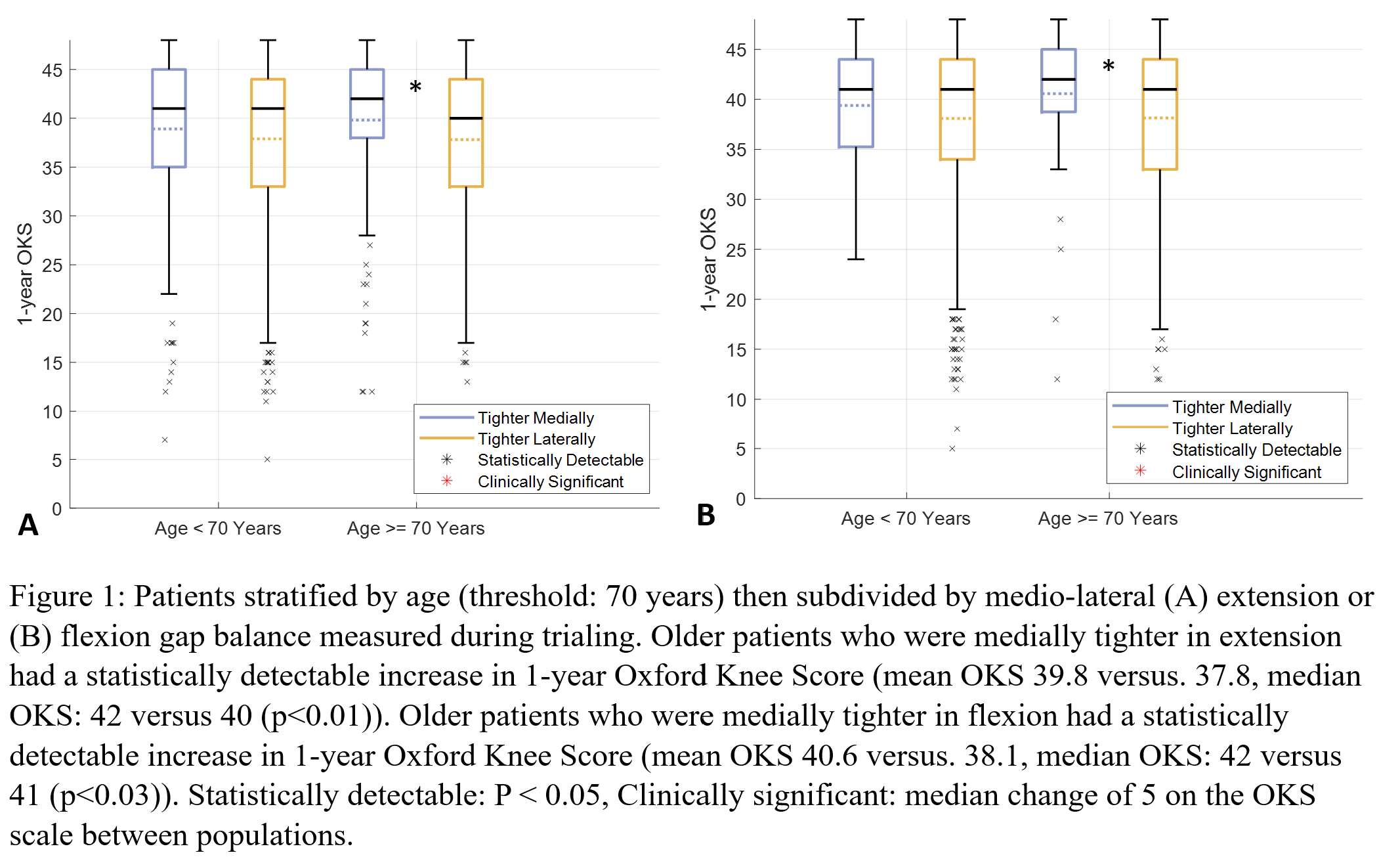
Figure 1
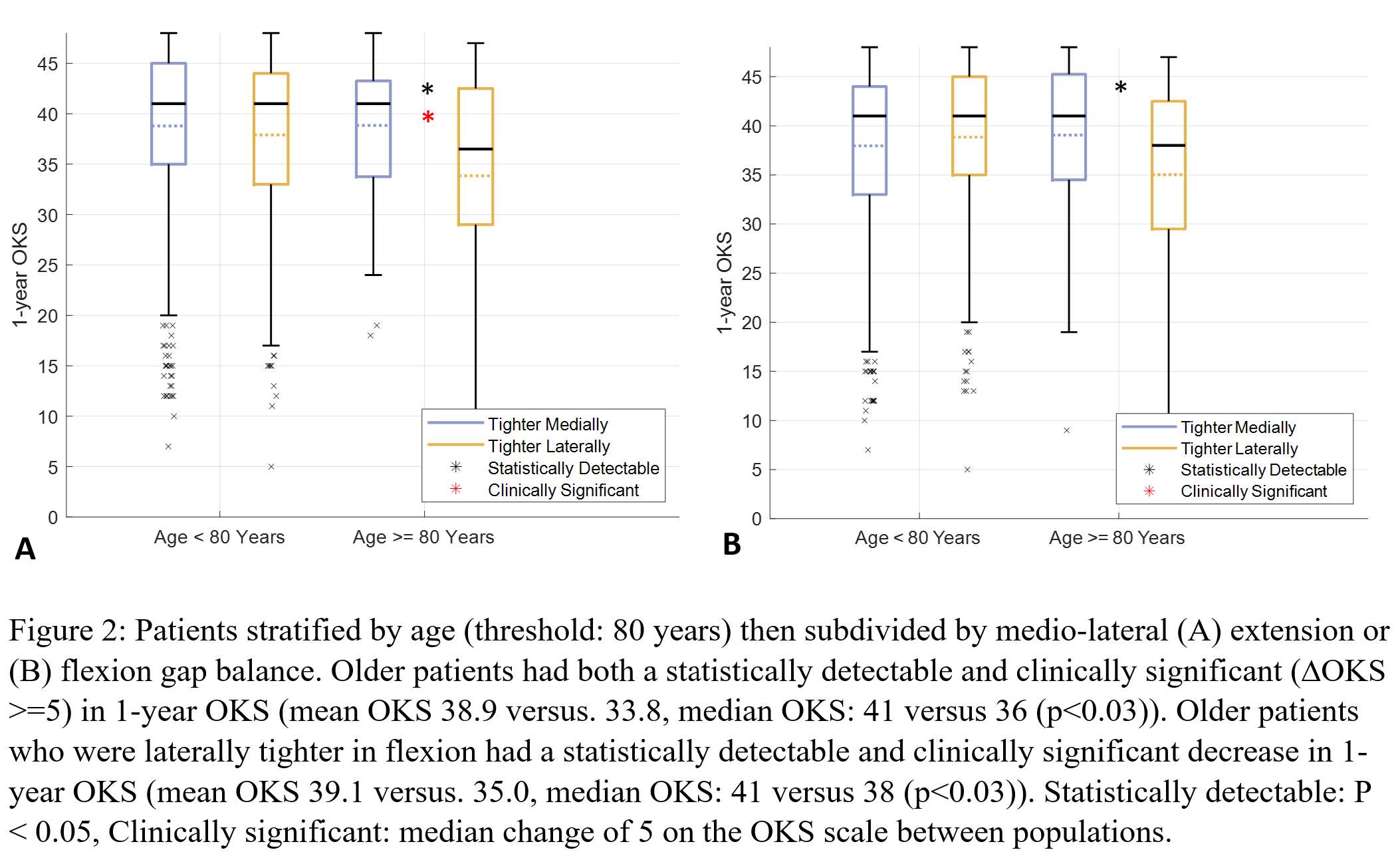
Figure 2#8489
Preoperative Medial Laxity Correlates With Poorer and Postoperative Lateral Laxity Correlates With Improved PROMs Scores
Eric M Slotkin - Reading Hospital, Orthopaedic Associates of Reading, - West Reading, Pennsylvania, USA
*Alex Orsi - Corin - Raynham, USA
Corey E. Ponder - Oklahoma Sports and Orthopedics Institute - Oklahoma, USA
John Keggi - Orthopaedics New England - Middlebury, USA
Jeffrey Lawrence - Gundersen Health System - viroqua, USA
Stephen McMahon - Monash University - Melbourne, Australia
Simon Coffey - Nepean Hospital - Sydney, Australia
Edgar Wakelin - OMNI Life Sciences - Raynham, USA
Christopher Plaskos - Corin - Raynham, USA
Paramjeet Gill - UCSF-Fresno - Fresno, USA
*Email: Alex.Orsi@coringroup.com
Preoperative and postoperative ligament laxity can be characterized intraoperatively using digital robotic tensioning. Understanding how knee joint laxity affects preoperative and early post-operative patient reported outcomes (PROMs) may aid surgeons in tailoring intra-operative balance and laxity to optimize outcomes for specific patients.
This study aims to determine if preoperative and postoperative ligament laxity is associated with PROMs, and if laxity thresholds impact PROMs during early post-operative recovery.
514 patients were retrospectively reviewed. BMI was 32±8kg/m2. Mean age was 66±9 years. 63% were female. Medial and lateral knee joint laxity was measured preoperatively using a digital robotic ligament tensioning device after a preliminary tibial resection, and postoperatively during femoral trialling.
Linear regressions were performed between laxity in extension (10°), midflexion (45°), and flexion (90°) and KOOS12-Summary scores (preoperative, 6-weeks, 3-months, and 6-months).
Welch’s t-tests determined significant differences between laxity groups for the various PROM’s scores at all time points.
Correlations were found between preoperative KOOS12-Summary scores and preoperative medial midflexion and flexion laxity (p<0.01).
Lower preoperative KOOS12-Summary scores were observed in patients with ≥7 mm of preoperative medial laxity compared to patients with <7 mm of preoperative medial laxity in extension (29.8±14.8 vs.39.4±14.3, p<0.001), midflexion (34.8±14.8 vs. 39.6±14.3, p<0.01), and flexion (34.7±14 vs. 39.4±14.5, p<0.05).
Patients with ≥7 mm of preoperative medial flexion laxity compared to patients with <7 mm of preoperative medial flexion laxity had greater KOOS12-Summary scores at 6-weeks (p<0.01), 3-months (p<0.05), and at 6-months (p<0.05). These patients also had a greater Δ KOOS12-Summary scores, relative to their preoperative score, at all time points (p<0.01).
Correlations were also found between 3-month KOOS12-Summary scores and postoperative lateral flexion laxity (p<0.01).
Higher KOOS12-Summary scores were observed in patients with ≥0.5 mm of postoperative lateral flexion laxity compared to patients with <0.5 at 3-months (74.5±16 vs. 69.1±16.3, p<0.01) and at 6-months (81±15.5 vs. 74.6±18.6, p<0.05).
Patients with excessive preoperative medial laxity have worse preoperative PROMs score, however they achieve higher postoperative PROMs scores compared to those without excessive preoperative medial laxity. Furthermore, postoperative lateral flexion laxity is associated with improved PROMs scores at 3 and 6-months.
#8185
Intraoperative Gap Assessment Including Mid-Flexion in Total Knee Arthroplasty Using Navigation With Joint Stability Graphs
*Sang Jun Song - Seoul, South Korea
Cheol Hee Park - Department of Orthopaedic Surgery, College of Medicine, Kyung Hee University Seoul - Seoul, South Korea
Hyun Woo Lee - Kyung Hee University - Seoul, South Korea
*Email: tesstore@empas.com
Purpose: Mid-flexion instability can result in unfavorable clinical symptoms including pain, swelling, and unstable feeling in daily activity after total knee arthroplasty (TKA). Most previous studies measured the gaps discontinuously at the specific angle of knee flexion when evaluating the soft tissue balancing in the mid-flexion range. A recent software program of navigation, which is called a joint stability graph or balance in motion, provides the continuous gap information throughout the full range of motion (ROM) on three-dimensional geometries (Fig. 1). The purpose of the present study was to assess continuous gaps in the replaced knee throughout the full ROM after TKA using a joint stability graph, and to analyze the gap laxity in the mid-flexion range.
Methods: One hundred TKAs were performed using imageless navigation with a joint stability graph. While positioning guides for each respective cut, the surgeon can safely preview the resection’s impact for the resulting joint gaps, and control the soft tissue balance at the knee flexion of 0° (extension) and 90° (flexion). The gap between the femoral component and insert were evaluated throughout the full ROM using the joint stability graph. The mechanical axis (MA) and change of joint line height were radiographically evaluated. Post-hoc power analyses using a significant alpha value of 0.05 were performed on the proportion of the mid-flexion instability as a primary outcome to determine whether the sample had sufficient power. The power was determined to be sufficient (100%).
Results: The flexion-extension gap differences in each medial and lateral compartment, and the mediolateral gap differences in flexion and extension were all ≤3 mm. There were 29 cases with convex joint stability graphs in the medial and laeral compartments and 24 cases with the convex graph in the lateral compartment only. The other 47 cases demonstrated rectangular patterns in the joint stability graph (Fig. 2). None of the knees had mid-flexion instability, which is defined by a peak mid-flexion gap that is 3 mm greater than the smaller value of flexion or extension gap. The average MA was well corrected from varus 11.1° to varus 0.8° postoperatively. The proportion of postoperative well-aligned knees (MA ≤3°) was 88%. The joint line height was well preserved (14.6 mm vs.14.4 mm, r=0.742, p=0.001).
Conclusion: The joint stability graph in TKA using the navigation can effectively evaluate the continuous gap throughout the ROM, including the mid-flexion range. Mid-flexion instability was uncommon in primary TKAs with appropriate alignment and proper preservation of the joint line.
Figures
Figure 1
Figure 2#8262
Knee Stiffness Evaluations Using an Advanced Total Knee Arthroplasty Simulator Model for Loaded Ligament Assessments
*Kevin Abbruzzese - Stryker - Mahwah, USA
Azhar Ali - Stryker Orthopaedics - Mahwah, USA
Andre Freligh - Stryker Orthopaedics - MAHWAH, United States of America
Scott Logan - Stryker Orthopaedics - Mahwah, USA
Stefano Bini - University of California San Francisco - San Francisco, USA
Jared Weir
Michael Mont - Sinai Hospital of Baltimore - Baltimore, USA
*Email: kevin.abbruzzese@stryker.com
Introduction
Achieving optimal outcomes in total knee arthroplasty (TKA) relies on maintaining an appropriate balance of soft tissues. Surgeons often rely on subjective manual techniques to assess soft-tissue balance, which may vary depending on their experience and introduce variability. To address this, a newly-developed pendulum knee drop (PKD) technique, offers a quantifiable approach to reliably estimate the soft tissues of the knee joint. This is a non-invasive test that passively assesses soft-tissue stiffness by measuring leg oscillations. This study utilized the PKD test with varying simulated ligament tensions to evaluate stiffness using an advanced knee simulator model (AKS) of a simulated robotic-assisted TKA.
Methods
The AKS model was designed using a three-dimensional (3D) Computed Tomography scan of a patient undergoing a left TKA who had a varus deformity and moderate osteophytes and was manufactured using 3D printing. The model underwent robotic-assisted TKA. Incremental loads were applied to the joint line after femoral and tibial resections in the range of 5 to 25 pounds (lbs) with a tensioning device. To assess knee stiffness, the pendulum knee drop test (PKD) was performed 3 times per loading condition. A single inertial measurement unit was placed on the AKS shank to measure knee range of motion throughout the passive leg drop. Stiffness estimates were calculated as the logarithmic decrement coefficient between the first two successive peaks of knee flexion/extension measurements. Kruskal Wallis and Tukey post hoc tests were used to assess significance and locations of differences between loading conditions.
Results
No significant differences were detected between the 5 and 8 lb conditions. A significant increase in stiffness was observed between incremental loading conditions (p=0.032) (Figure 1). A significant increase in stiffness was observed between the 10, 12, and 25 lb conditions. The 25 lb loaded condition resulted in the greatest stiffness as compared to all other loading conditions. Overall, stiffness estimates exhibited a 4th order polynomial trend (R²=1), suggesting a nonlinear relationship between load and stiffness.
Conclusion
This study aimed to investigate the effect of varying loading conditions at the joint line to determine the impact on knee soft-tissue stiffness following TKA using the PKD test in a simulated knee model. A significant increase in knee stiffness was observed with increased load. This suggests that incremental tension across the joint line can lead to change in stiffness of the soft tissue around the knee. These results may have important clinical implications, as they could aid in the selection of the appropriate implant size for patients undergoing TKA or enhance bony resection decisions. Overall, the study provides valuable insights into the relationship between joint loading conditions and knee stiffness and provides a method to quantify physiological properties of the knee.
References
[1]. Meloni MC, Hoedemaeker RW, Violante B, Mazzola C. Soft tissue balancing in total knee arthroplasty. Joints. 2014 May 8;2(1):37-40. PMID: 25606540; PMCID: PMC4295665.
Figures
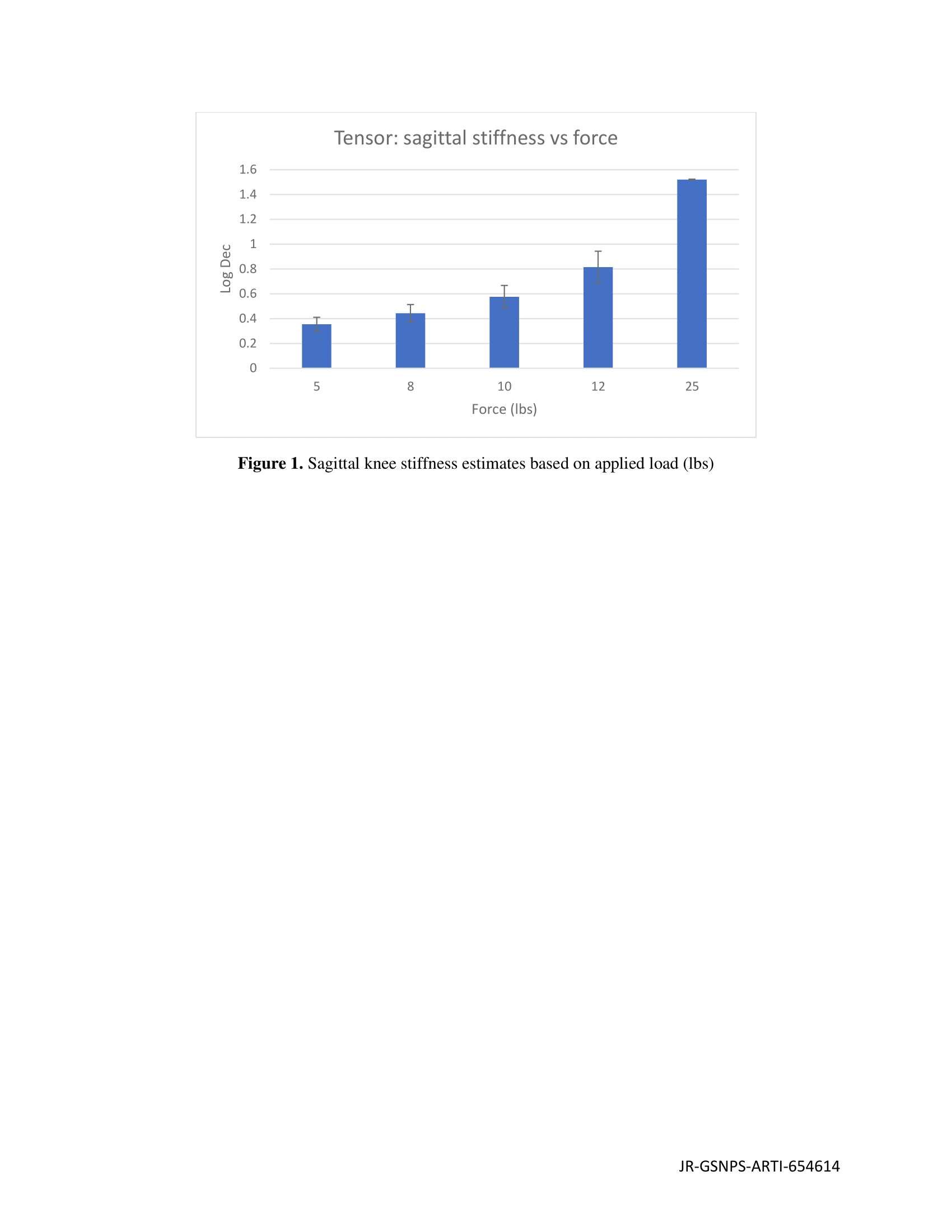
Figure 1#8127
Comparison of Two Methods Used to Assess Gaps in Total Knee Arthroplasty
*Saskia Anna Brendle - Aesculap AG / LMU Munich - Tuttlingen, Germany
Ingrid Dupraz - Aesculap AG - Tuttlingen, Germany
Sven Krueger - Aesculap AG Research & Development - Tuttlingen, Germany
Joachim Grifka - Regensburg University Medical Center - 93077 Bad Abbach, Germany
Peter E. Mueller - Hospital of the Ludwig-Maximilians-University of Munich - Munich, Germany
Thomas M. Grupp - Aesculap AG - Tuttlingen, Germany
*Email: saskia.brendle@aesculap.de
Introduction
A well-balanced knee is key to achieve good functional outcomes after total knee arthroplasty (TKA). Therefore, preserving the soft tissue envelope is of great importance [1]. Intra-articular spreaders have been used in the last decades to assess the gaps intra-operatively. Currently, several robotic systems propose the assessment of gaps by performing a varus-valgus stress on the joint. This method has the potential to assess gaps pre-operatively and at several stages during surgery. However, to our knowledge, the difference between the gaps assessed with varus-valgus stress, compared to the gaps assessed with intra-articular spreaders, has not been investigated so far. The objective of this study was to quantify the medial and lateral tibiofemoral gaps of native knees at different flexion angles prior to tibia and anterior cruciate ligament (ACL) resection by applying both, distraction force and varus-valgus stress on the joint.
Methods
In this in vitro study, twelve fresh-frozen human cadaveric knees with intact capsule were tested on a six-degrees-of-freedom joint motion simulator (Advanced Mechanical Technologies Inc., Watertown, USA) by applying a distraction force or a varus-valgus stress at different flexion angles (0°, 30°, 45°, 60°, 90°) with a resulting force on the medial and lateral collateral ligaments similar to the force applied by a surgeon when using an intra-articular spreader, maintaining all other forces/moments at 0 N/Nm. Prior to testing, the femur and tibia of each specimen were subjected to a complex 3D fitting process (ARAMIS 12M, GOM Metrology GmbH, Braunschweig, Germany) using segmented CT scans to measure the tibiofemoral gaps medially and laterally during distraction force and varus and valgus stress along the mechanical axis of the tibia using the same reference points (Figure 1). The measured gaps were standardized to the medial gaps at 0° flexion distraction force to allow comparison of the specimens. Mean standardized gaps and standard deviations were calculated from all twelve specimens.
Results
For both methods, the native medial and lateral gaps were tightest in extension, increased until 30° flexion, then remained mostly constant and decreased again slightly at 90°. In addition, the lateral gaps were larger than the medial gaps over the entire range of motion for both methods. The gaps measured during varus-valgus stress strongly correlated with but were always smaller than the gaps measured during distraction force over the entire arc of flexion (Figure 2).
Conclusion
It was shown that the gaps measured by applying a varus-valgus stress on the joint strongly correlated with but were always smaller than the gaps acquired by distracting the joint. One reason might be the impact of the reference points used to measure the gaps. Compared to distraction force, which generates parallel gaps, varus-valgus stress leads to a pie-shaped opening of the medial and lateral compartments. Therefore, different computation of the reference points may be necessary. A further reason may be that the joint is differently stressed with both methods. This needs to be investigated in further clinical studies and taken into account in navigated or robotic-assisted TKA.
References
1. Rivière et al. 2020
Figures
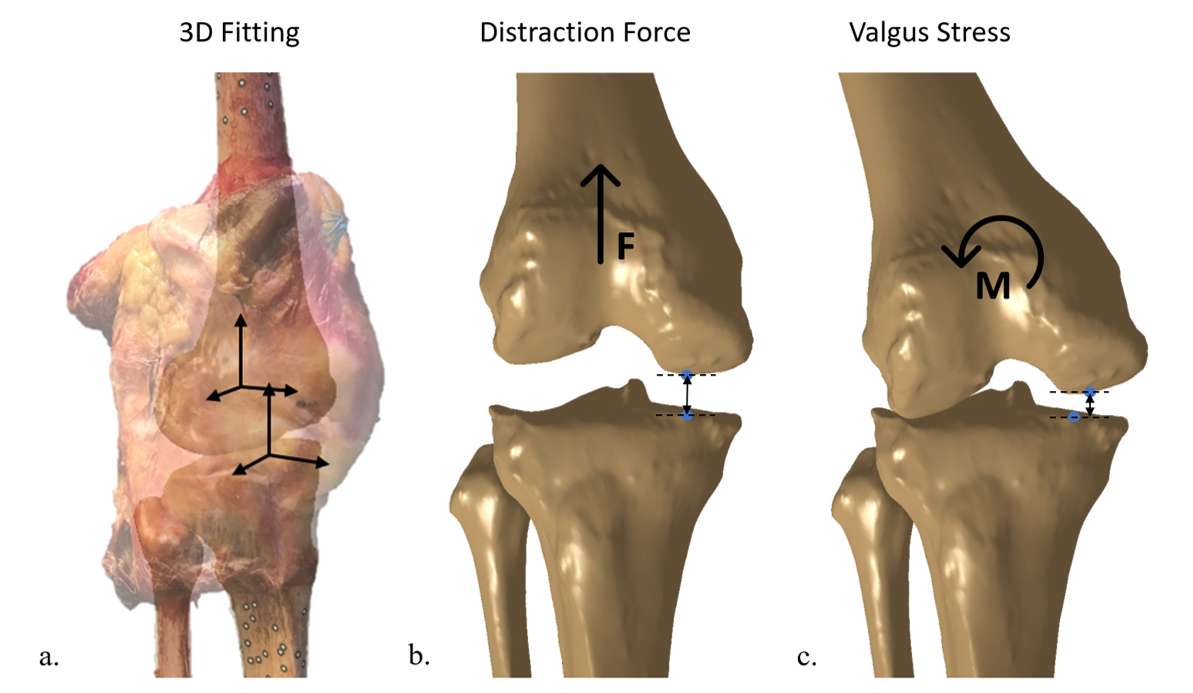
Figure 1
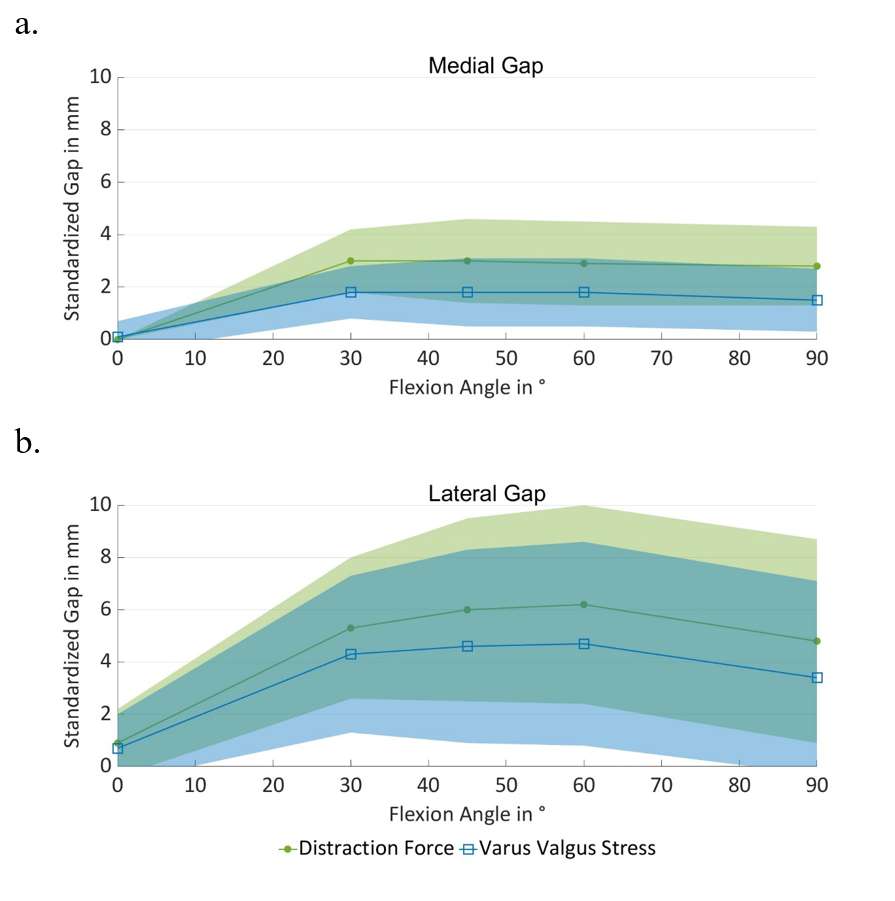
Figure 2#8145
Influence of PCL Retention and Sacrifice on Knee Balance and Bony Resection With Functional Alignment During Robot-Assisted Total Knee Arthroplasty.
*Suhas Boenerjous-Abel - Bronx-Lebanon Hospital - Bronx, USA
*Email: drsuhas09@gmail.com
Introduction: The decision to retain or sacrifice the posterior cruciate ligament (PCL) during total knee arthroplasty (TKA) is a subject of debate among arthroplasty surgeons. Prior studies have reported varying effects of PCL resection on gaps, with flexion gap being more affected.Most of these studies were in-vitro using mechanical tensioners on cadavers that do not replicate normal physiological elasticity. Additionally, previous in-vivo studies have solely investigated the impact of PCL removal on gaps, without considering its effects on bone cuts and overall ability to achieve preemptive balance. The primary aim of this study was to determine the number of knees that could achieve pre-balance with and without the PCL and to analyze the effects of PCL removal on gaps and bone cuts. Furthermore, we also attempt to utilize the initial gap assessment data to predict which knees can be balanced through PCL retention or sacrifice.
Methods: This was a prospective study of 100 consecutive patients undergoing primary Mako robot assisted TKA (RATKA). Patients with primary unilateral varus osteoarthritis of the knee were included. Initial gap assessment and pre balancing without soft tissue release was performed with the PCL retained, and knees were classified as balanced or unbalanced. Subsequently, the PCL was removed in all cases, and re-balancing was performed to evaluate changes in the proportion of knees balanced and the impact on gaps and bone resection thickness. Furthermore, the medio-lateral differences in initial gaps were recorded in all cases to predict which knees may require PCL sacrifice to achieve further balance.
Results: The study included 100 patients with a mean age of 59.2 (SD-8.56) with female preponderance (N=73, 73%). Mean pre-operative varus deformity was 10.5 degrees (SD=2.14). 69 knees (69%) were balanced using functional alignment with the PCL retained. PCL sacrifice in the balanced group resulted in preferential increase in flexion gap. Rebalancing these knees lead to decrease in post femur cut by ( 2.5 mm postmedial, 2.1 mm posterolateral) Removal of the PCL in remaining 31 knees helped achieve further pre-balance in 15 knees which had isolated flexion medial tightness. 16 knees which had medial tightness both in flexion and extension remained unbalanced even with PCL resection, requiring additional soft tissue releases to achieve balance.
Conclusion: With functional alignment and dynamic balancing during RATKA, majority of the knees balanced with PCL intact. PCL resection increased chances of attaining pre-balance and lead to preferential increase in flexion gaps. Rebalancing the knees in the balanced group after PCL resection was associated with decrease in thickness of posterior femoral bone resection making it more bone conserving. We could predict knees with isolated flexion medial tightness and a mediolateral (ML) extension gap difference of 5mm or < can be balanced with removal of the PCL. Knees with medial tightness both in flexion and extension and a ML extension difference of >7 mm could not be balanced even after removal of the PCL requiring further soft tissue releases.
Figures
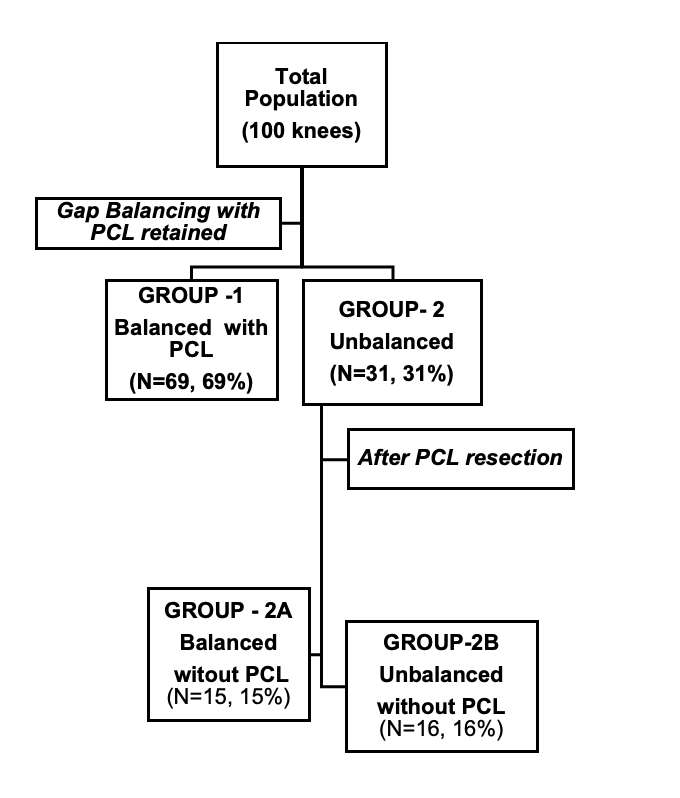
Figure 1
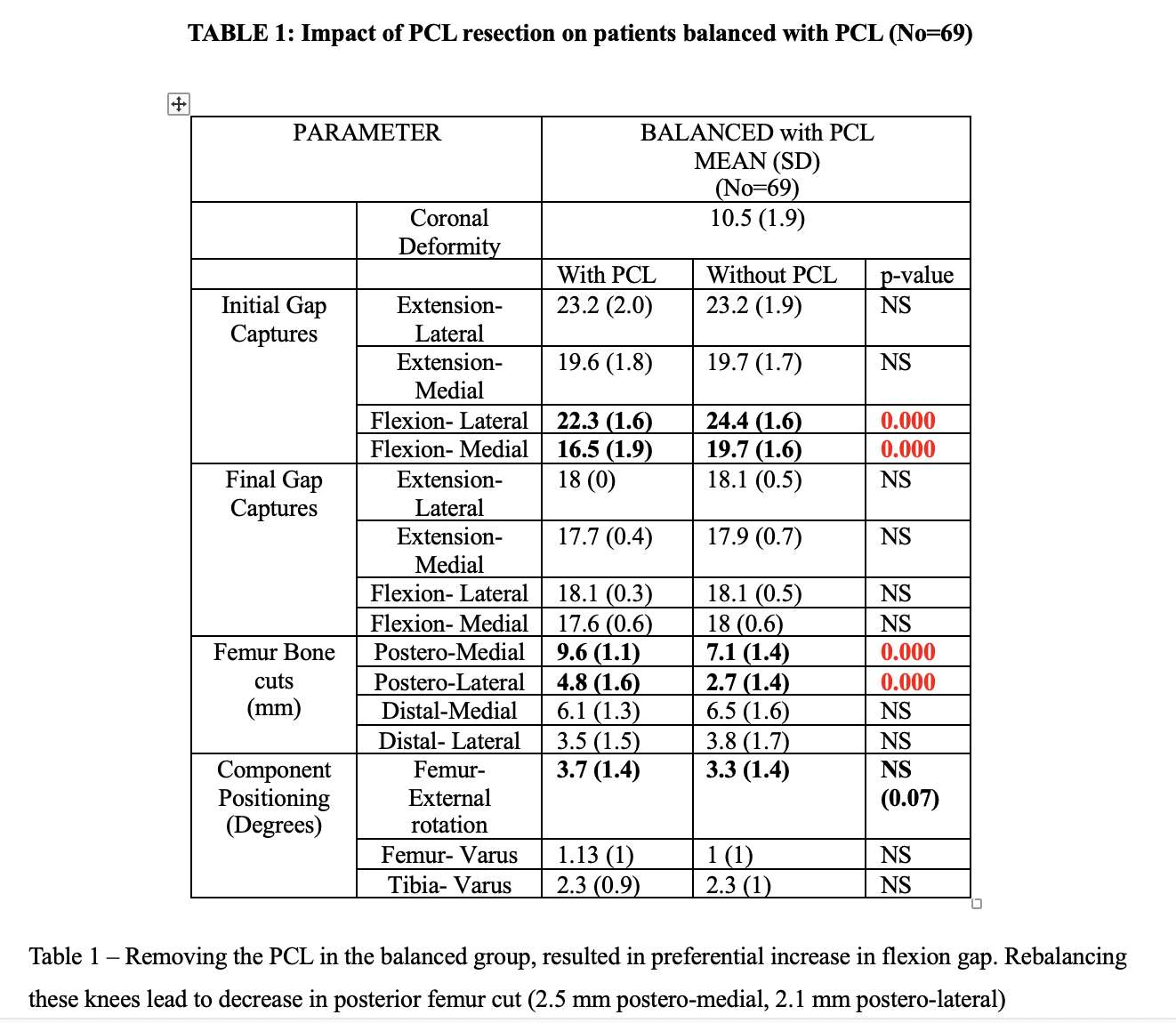
Figure 2
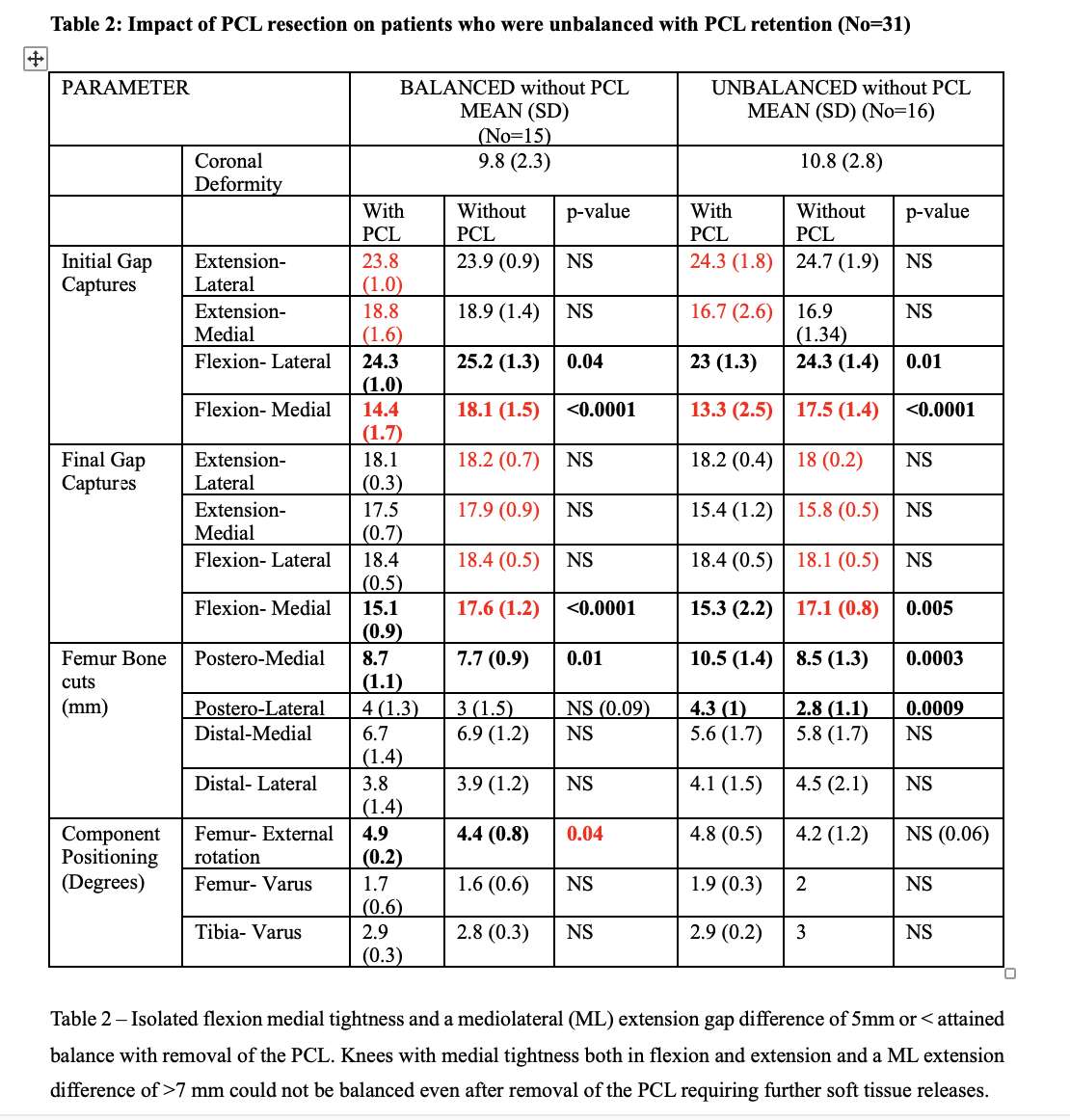
Figure 3#8750
Functional Laxity of Total Knee Arthroplasty Implants Measured During Simulated Squat Motions
*Alexandre Galley - Western University - London, Canada
Ilya Borukhov - Stryker Orthopedics - Mahwah, USA
Brent Lanting - London Health Sciences Centre - London, Canada
Ryan Willing - Western University - London, Canada
*Email: agalley@uwo.ca
Introduction: Stability of total knee arthroplasty (TKA) implants can be assessed pre-clinically using joint motion simulators that manipulate the knee in force, displacement, or simulated muscle-control. Our muscle actuator system (MAS) [1] can generate gravity-dependent, quadriceps-controlled motions on a force/displacement-control robotic knee testing system (VIVO, AMTI, Watertown, MA, USA). Stability can be assessed using laxity tests that apply forces across the joint and measure resulting displacements. This is usually done on robotic knee testing systems but is challenging with conventional muscle-driven knee simulators. The purpose of this study was to measure the laxity of a TKA design during gravity-dependent, muscle-driven motions with the combined VIVO-MAS. Furthermore, the tensions of virtual collateral ligaments were measured during tests to better understand their contributions to joint stability.
Methods: A phantom TKA knee was created using cruciate-sacrificing components (Triathlon PS, Size 4, Left, Stryker Orthopaedics, Mahwah, NJ, USA) and mounted within the VIVO-MAS. To replicate their contributions in passively stabilizing the knee, virtual medial and lateral collateral ligaments (MCL, LCL) were created using literature-derived parameters [2], [3]. A baseline motion was first produced with the VIVO-MAS of 20°-90° flexion/extension under quadriceps-control. Quadriceps extension directly opposed a flexion moment created by a 75N gravity force acting from hip-to-ankle. Laxity during motion, or functional laxity, was evaluated by repeating this test with superimposed anterior/posterior (AP) forces (±80N), internal/external (IE) torques (±4Nm) or varus/valgus (VV) torques (±8Nm). Tibiofemoral (TF) kinematics and simulated ligament forces were recorded. Mean and peak deviations from baseline motions caused by each superimposed load were calculated, and the angles associated with the peak deviations were recorded.
Results: The mean anterior functional laxity (2.8±0.8mm) was smaller than the mean posterior functional laxity (6.2±1.4mm) (Figure 1), and the peak (3.9mm) occurred at 20° whereas the peak posterior functional laxity (8.0mm) occurred at 40°. The IE and VV functional laxities displayed more symmetric behaviour (Table 1). During anterior laxity testing, mean MCL engagement increased by 18±9N whereas the increase for the LCL was only 2±17Nm with similar behaviour during posterior laxity tests (Figure 2). The MCL shows less mean engagement during internal laxity tests (8±7N) than the LCL (24±14N). The MCL’s engagement (20±3N) is less than the LCL’s (32±5N) during varus laxity tests. Engagement behaviour is reversed when the opposing loads are applied.
Conclusions: Functional laxities were largest while in extension. In flexion, higher quadriceps forces stabilized the joint by increasing joint contact forces. The AP functional laxity was asymmetric, which is expected due to the quadriceps’ involvement. The IE and VV laxities were symmetric, which is expected due to the symmetric articular surfaces of the implants used. Ligament forces behaved analogously when opposing loads were applied. These data provide insights in knee joint stability during functional activities, potentially supplementing data gained from simple laxity tests alone.
References:
[1] A. Galley et al. (2023). ORS 2023 Annual Meeting. Paper #853.
[2] K. Bloemker et al. (2012). The Open Biomedical Engineering Journal. 6(1): 33-41.
[3] K. Cho et al. (2018). BMC Musculoskeletal Disorders. 19(1): 266.
Figures
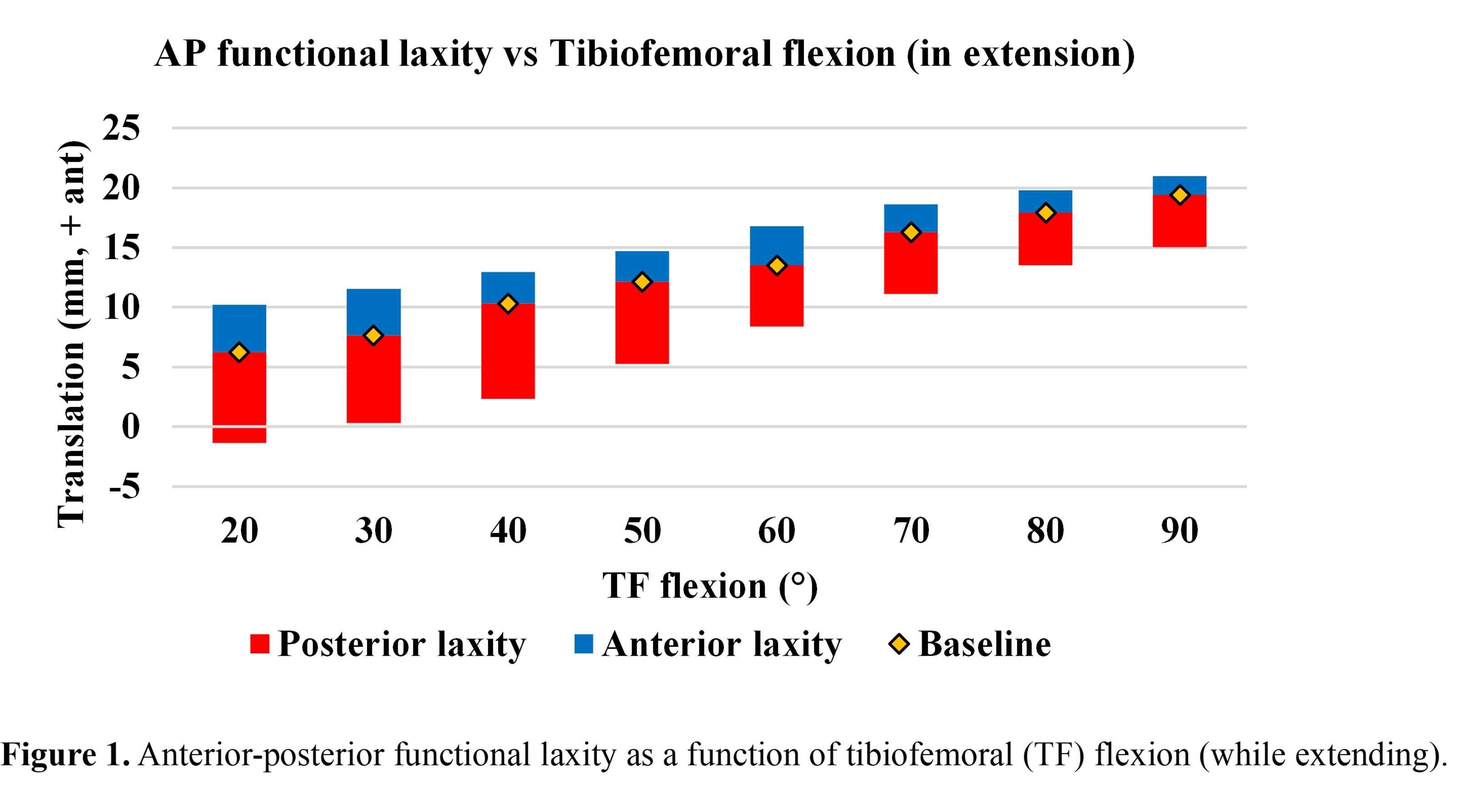
Figure 1
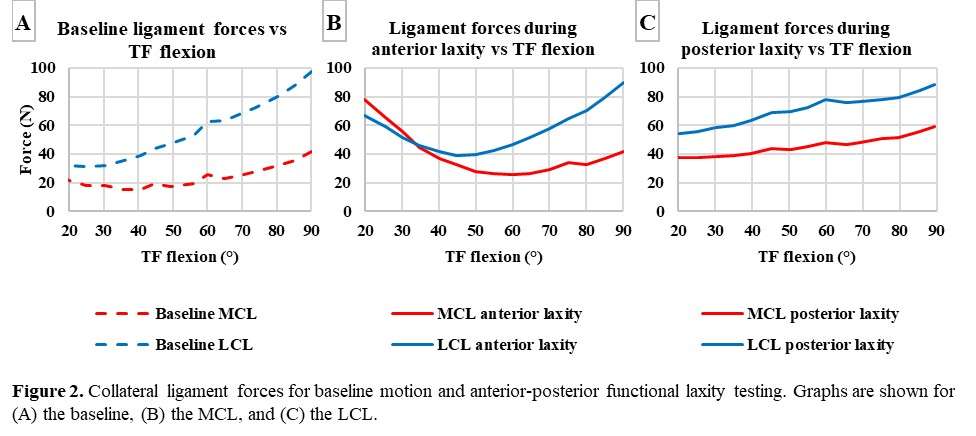
Figure 2
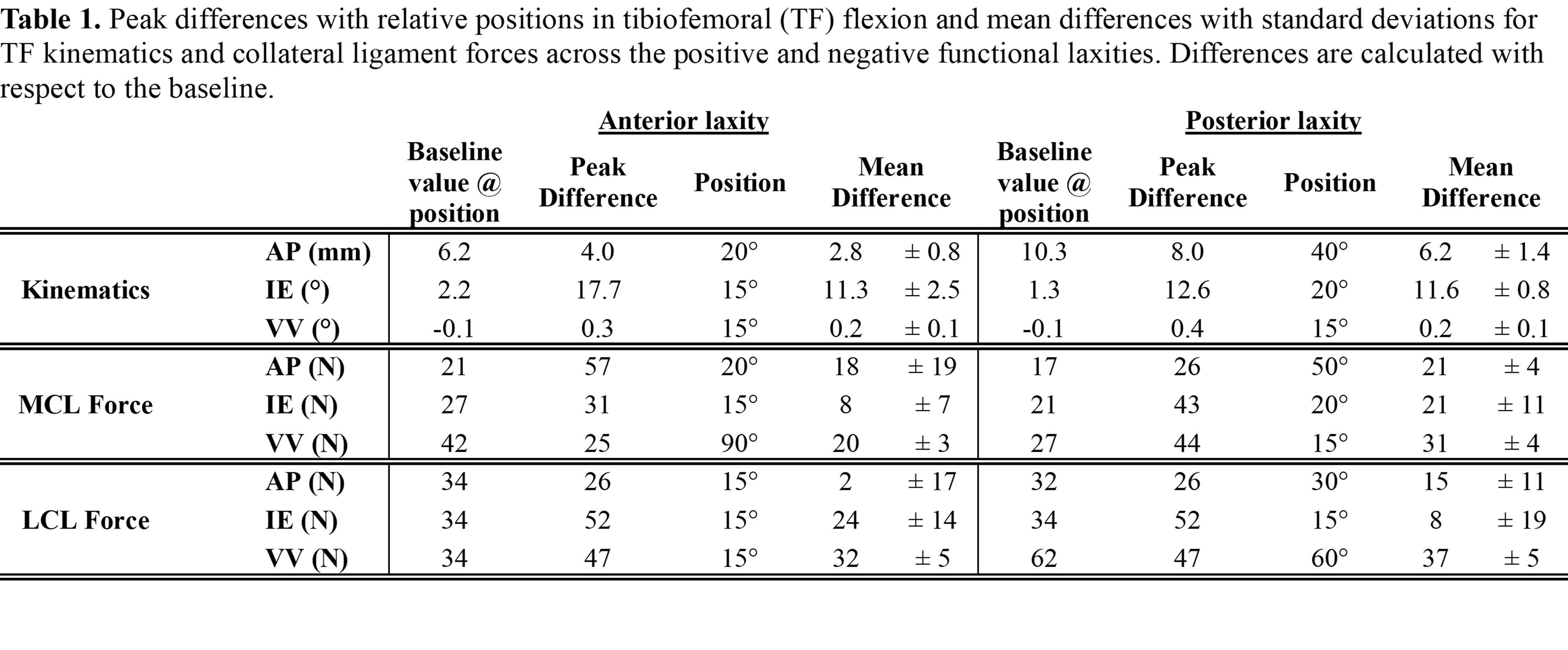
Figure 3#8142
Soft Tissue Laxity Is Highly Variable in Patients Undergoing Total Knee Arthroplasty
Travis Weiner - Columbia University Medical Center - New York, USA
Roshan Shah - Columbia University Medical Center - New York, USA
Alexander Neuwirth - Columbia University Medical Center - New York, USA
Jeffrey Geller - Columbia University Medical Center - New York, USA
*John Cooper - Columbia University - New York, USA
*Email: jcooper02@gmail.com
Introduction
One major goal of total knee arthroplasty (TKA) is achieving balanced medial and lateral gaps in flexion and extension. Poor balance may lead to instability, pain, increased polyethylene wear, aseptic loosening, and ultimately may result in revision TKA. While bone resections are planned by the surgeon, soft tissue laxity is largely intrinsic and patient-specific in the absence of additional soft tissue releases. We sought to determine variability in soft tissue laxity in patients undergoing TKA.
Methods
We retrospectively reviewed 113 patients undergoing robotic TKA using a single robotic knee system by two fellowship-trained arthroplasty surgeons. Preoperative knee deformity was collected. Data from a dynamic intraoperative stress exam were collected to quantify maximal medial and lateral opening in flexion (85-95 degrees) and extension (-5-20 degrees). The robot measured the resultant gaps (in millimeters) of the medial side with valgus stress and similarly of the lateral side with varus stress. T-tests were used to examine for differences between continuous variables.
Results
Of the 113 knees, 71 (62.8%) had a preoperative varus deformity, 36 (31.9%) had a preoperative valgus deformity, and 6 (5.3%) had a neutral preoperative alignment.
A valgus stress opened the medial compartment a mean of 4.3 +/- 2.3mm (0.0-12.4mm) in extension and 4.6 +/- 2.3mm (0.0-12.9mm) in flexion. A varus stress opened the lateral compartment a mean of 5.4 +/- 2.4mm (0.3-12.6mm) in extension and 6.2 +/- 2.5mm (0.0-13.4mm) in flexion (Table 1).
The medial compartment of varus knees opened significantly more to valgus stress than valgus knees in both extension (5.2mm vs 2.6mm; p<0.0001) and flexion (5.4mm vs 3.3mm; p<0.0001) (Table 2). The lateral compartment of valgus knees opened significantly more to varus stress than varus knees in both extension (6.7mm vs 4.8mm; p<0.0001) and flexion (7.4mm vs 5.8mm; p=0.0003) (Table 3).
Conclusion
Soft tissue laxity is highly variable in patients undergoing TKA, contributing anywhere from 0-13mm to the post-resection gap. Only a small part of this variability is predictable by preoperative deformity. These findings have implications for either measured-resection or gap-balancing techniques and would create a pathway for a “hybrid” or “patient-specific” balancing technique. Importantly, these workflow changes might make a substantial impact on patient function and satisfaction.
Figures
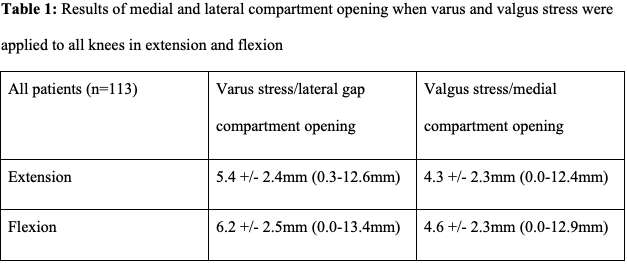
Figure 1
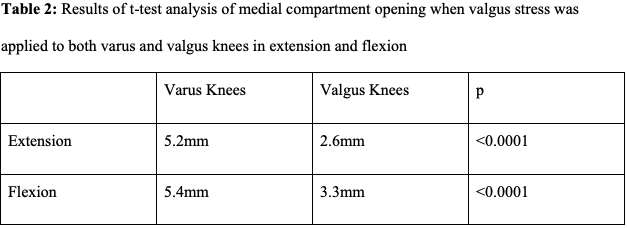
Figure 2
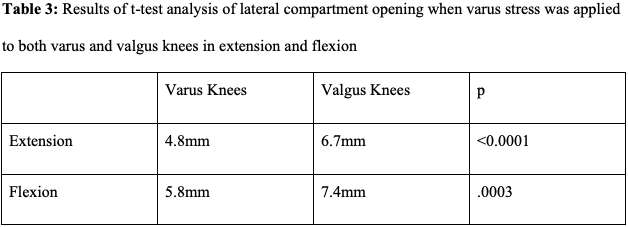
Figure 3#8215
Manual Surgeon-Applied Stress and a Ligament Tensor Instrument Provide a Similar Assessment of Pre-Resection Flexion Laxity During Robotic Total Knee Arthroplasty
Travis Weiner - Columbia University Medical Center - New York, USA
*John Cooper - Columbia University - New York, USA
*Email: jcooper02@gmail.com
Introduction
Modern robotic total knee arthroplasty (TKA) systems allow surgeons to perform an individual assessment of a patient’s soft tissue laxity prior to performing bone resections, which can be used to alter planned bone resections for the goal of achieving balanced gaps. While assessment of the soft tissue laxity in extension is relatively straightforward, there are several ways to assess soft laxity in flexion. The purpose of this study was to compare two common methods for assessment of flexion gap laxity when performing robotic TKA.
Methods
This was a retrospective review of data collected from patients undergoing primary robotic TKA by one of two fellowship-trained arthroplasty surgeons experienced in robotic TKA. Maximal flexion laxity in the medial and lateral compartments was first quantified to the nearest 0.5mm by the robotic system using a dynamic, manual, surgeon-applied stress after removal of all osteophytes. These data were used to plan for the desired balanced flexion gap by a combination of (a) choosing the appropriate femoral size, (b) translating the femoral component anteriorly or posteriorly, and (c) rotating the component internally or externally. Flexion laxity of the medial and lateral compartments was quantified for a second time after the distal femoral and proximal tibial resections were performed, using a ligament tensor instrument specifically designed for the flexion space. These new data were used to plan for the same desired flexion gap using the same three variables above. Operative time in seconds was recorded for each flexion gap assessment method. Paired t-tests were used to assess for differences between methods, with significance set at p=0.05.
Results
Data from both methods of flexion gap assessment were available in 19 patients. With the same flexion gap goal, both methods produced identical recommendations for the femoral component sizing (p=1.00) and near-identical recommendations for anterior-posterior translation of the femoral component (tensor method +0.03mm less anterior, range 0-0.5mm; p=0.67). There was not a significant difference between femoral component rotation, with the tensor method recommending a mean 0.05 degrees further external (range 0 to 3 degrees) than the manual method (p=0.84). Flexion gap assessment with the tensor method took a significantly longer amount of OR time than the manual method (40.5 seconds vs 19.2 seconds; p<0.0001).
Conclusion
Tensioning the flexion space with a manual surgeon-applied stress and with a ligament tensor device produced near-identical laxity data, suggesting that surgeons may comfortably choose either method as a reliable method of assessing maximal soft tissue laxity of the flexion space.
#8533
Efficacy of a Novel, Fully-Integrated Tensioning Device for the Evaluation of Joint Laxity During Total Knee Arthroplasty
*steven haas - Hospital for Special Surgery - New York, United States of America
Jimmy Chow - Orthopedic Institute of the West - Phoenix, USA
Steve Yurick - Smith+Nephew - Pittsburgh, USA
Kenneth Urish - University Of Pittsburgh - Sewickley, USA
Joseph Signorelli - Memorial Medical Center - Ashland, USA
Michael Ast - Hospital for Special Surgery - New York, USA
*Email: haass@hss.edu
Introduction
Achieving optimal soft tissue balance in total knee arthroplasty (TKA), in conjunction with good implant alignment and fixation, has been shown to improve postoperative outcomes. Soft tissue balance is attained by careful selection of implant size, modifications to implant alignment, and soft tissue release. However, assessments of joint laxity, which critically informs these adjustments, are typically done intraoperatively and manually. Thus, forces applied to distract the joint can vary considerably by surgeon, flexion angle, and patient characteristics. This has important consequences on decision making related to insert thickness and implant position.
The aims of this preliminary investigation were to quantify intraoperative repeatability of a novel fully-integrated digital tensioning device (CORI◊ Digital Tensioner, Smith & Nephew) during robotically assisted TKA using a handheld, image-free surgical system (HI-TKA), and to assess early patient-reported outcome measures (PROMs).
Methods
HI-TKA procedures were performed by one surgeon at one study site from March 2022 - January 2023 as the first in an ongoing, prospective, multi-center study. Patients requiring a cemented TKA as a primary indication were included in this study. Patients with a diagnosis of post-traumatic osteoarthritis and those needing revision surgery, a nonstandard implant, or bilateral TKA were excluded. PROMs were collected before surgery and 6 weeks and 6 months postoperatively. PROMs included the 2011 Knee Society Score (KSS) Satisfaction score and EuroQol-5D Visual Analog Scale (EQ-5D VAS) and Index Score. During TKA, the lateral and medial condylar gaps were measured throughout the stressed range of motion (ROM) (0 – 100°). Three trials each were performed using the CORI◊ Digital Tensioner device and a manual technique. All measurements were acquired prior to bone resection. A force setting of 100N ± 10N was used for tensioner measurements. Measurement variability was defined as the average of the standard deviation of gap measurements at each 10-degree increment of flexion. Implant position was based on a tensioner measurement made following the repeatability trials.
Results
Six patients (mean age, 72.2 years; 4M/2F) underwent HI-TKA. Mean KSS Satisfaction scores increased with time from surgery (preop, 19.0±10; 6 weeks, 27.0±8; 6 months, 32.4±5; respectively) (n=5 at 6 months). Mean EQ-5D VAS and Index scores increased from the preoperative (67.8±21 and 0.59±0.2, respectively) to 6-week (75.8±12 and 0.65±0.2, respectively) time points, and were maintained or decreased at 6 months (75.8±16 and 0.56±0.5, respectively) (n=5 at 6 months). There was a 64.6% reduction in lateral gap measurement variability (Figure 1A) and 67.5% reduction in medial gap measurement variability (Figure 1B) over the stressed ROM compared to the manual technique (n=4).
Conclusion
These represent the first results published to date on this novel tensioning system. Preliminary results from this ongoing study indicate variability in gap measurements was reduced when using the CORI◊ Digital Tensioner compared to a manual approach. Future work should compare PROMs between TKA procedures performed with and without use of the CORI◊ Digital Tensioner device to evaluate clinical benefit of this approach.
◊Trademark of Smith & Nephew, Inc.
Figures
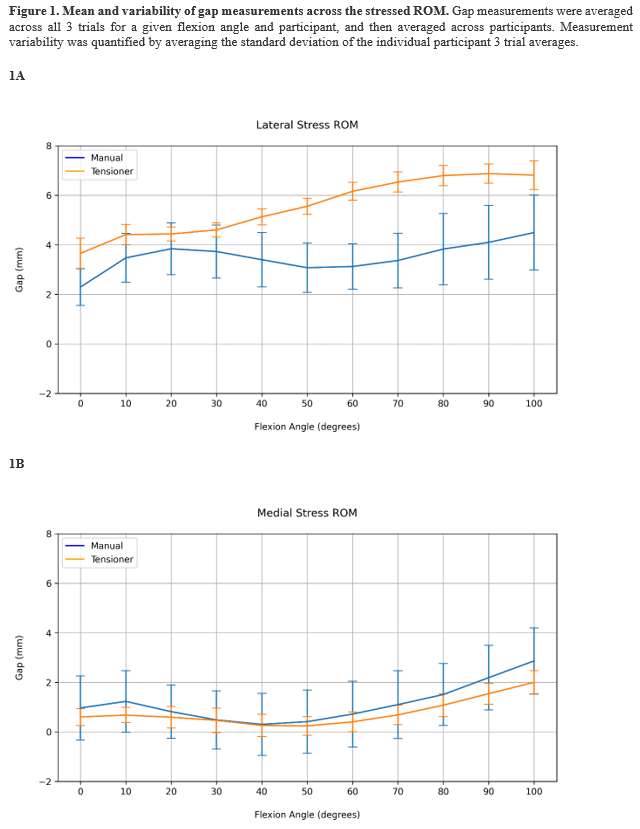
Figure 1#8232
Cup Safe Zone and Optimal Stem Anteversion in Total Hip Arthroplasty for Patients With Highly Required Range of Motion
Yukihiro Habe - Osaka University - Osaka, Japan
Hidetoshi Hamada - Osaka University - Suita, Japan
Keisuke Uemura - Osaka University - Suita, Japan
Kazuma Takashima - Osaka University Graduate School of Medicine - Suita, Japan
*Nobuhiko Sugano - Osaka University Graduate School of Medicine - Suita, Japan
*Email: n-sugano@umin.net
Introduction
In reducing the risk of dislocation after total hip arthroplasty (THA), it is ideal to control the cup alignment according to the stem anteversion to avoid implant impingement during various activities of daily living. There are many reports of impingement simulation of THA, but the selected activities are different, resulting in different safe zones. Internal rotation of 30° at 90° of hip flexion has been selected as an assumed position at risk for posterior dislocation in several ROM simulations 1). However, it has been reported that internal rotation of 40° at 90° of hip flexion is required in various activities on the floor 2). When this position is considered, the results of the impingement-free cup-safe zone are quite different.This study aimed to assess the effects of internal rotation angle at hip 90° flexion on the size of the safe zone according to the stem anteversion.
Methods
The prosthesis used a flat liner, and a 32-mm or 40-mm femoral head and stem. Implant impingement was evaluated using collision detection in the 3D CAD software Solid Edge (SIEMENS). The initial position of the stem was set at 5° of flexion and 5° of adduction. The stem anteversion angle was set from -15° to 55° with an increment of 5°.
14 different activity hip positions reported by Widmer were selected, including 30° internal rotation at 90° flexion (required 30° IR) 1). “Required 40° IR” was required 30° IR added with 40° internal rotation at 90° flexion, which is required for sitting on the floor in the Japanese lifestyle 2). “Required 50° IR” was required 40° IR added with 50° internal rotation at 90° flexion.
The radiographic inclination and anteversion angles of the liner impingement were measured. The angle at which impingement occurs in multiple stem positions was measured, and the range of angles at which impingement does not occur was defined as a safe zone. Safe zones formed by a different stem anteversion were compared.
Results
As internal rotation at 90° of flexion increased, the safe zone decreased (Figure 1). With a 32-mm head, the stem anteversion with the maximum safe zone areas was 15°/25°/35° in required 30° IR/40° IR/50° IR, respectively (Figure 2). With a 32-mm head, the optimal stem anteversion at 40° of cup inclination was 15°/25°/35° in required 30° IR/40° IR/50° IR, respectively (Figure 3). The safe zone area of the 32-mm head was smaller than that of the 40-mm head.
Conclusion
The safe zone area was reduced by the increase in required IR at 90° flexion. When the required IR at 90° flexion increases, the safe range of the cup anteversion shifts to a higher value and desirable stem anteversion range becomes narrow, so the stem and cup target alignment should be adjusted according to the patient's lifestyle and demand.
Reference
1. Widmer KH. Clin Orthop Relat Res. 2020 Aug;478(8):1904-1918.
2. Miki H et al. J Arthroplasty. 2007 Oct;22(7):946-52.
Keywords
Total hip arthroplasty, computer simulation, safe zone, range of motion, combined anteversion
Figures
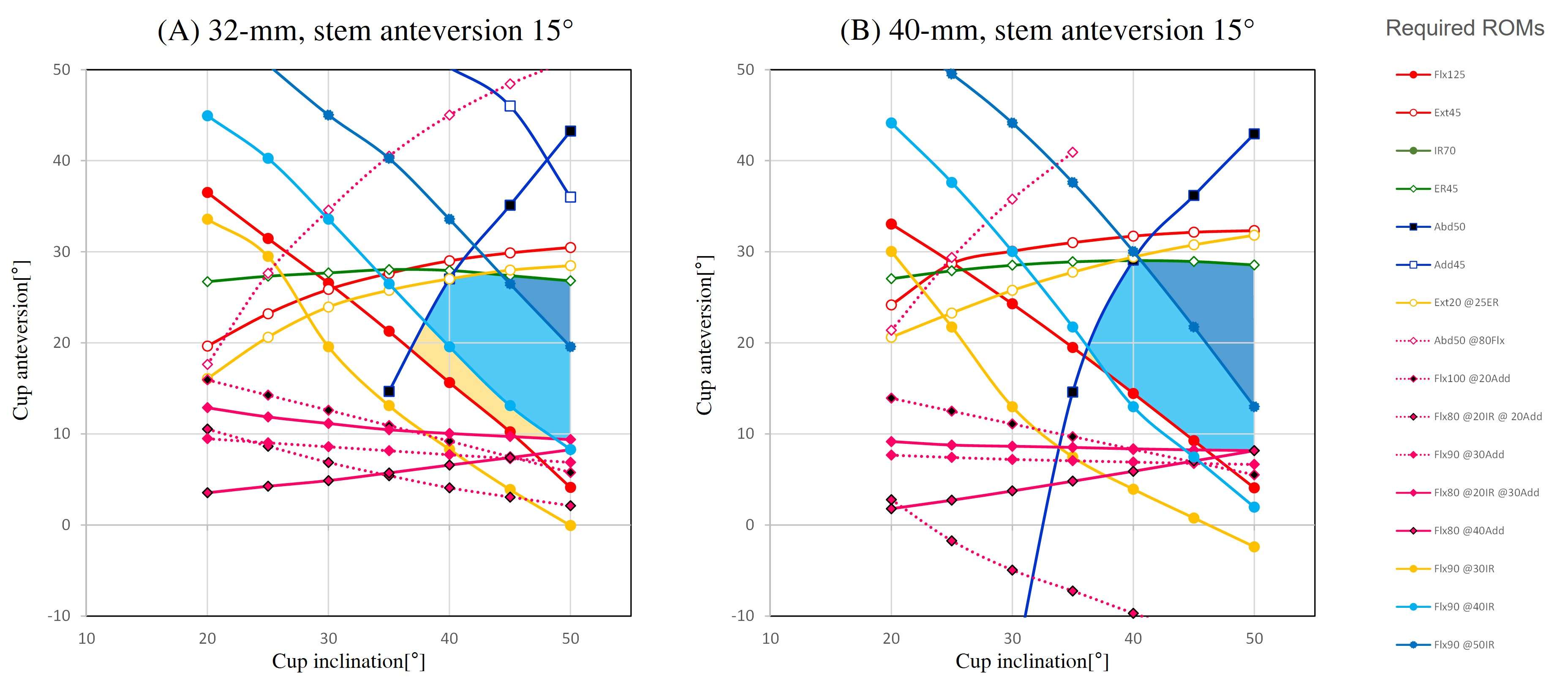
Figure 1
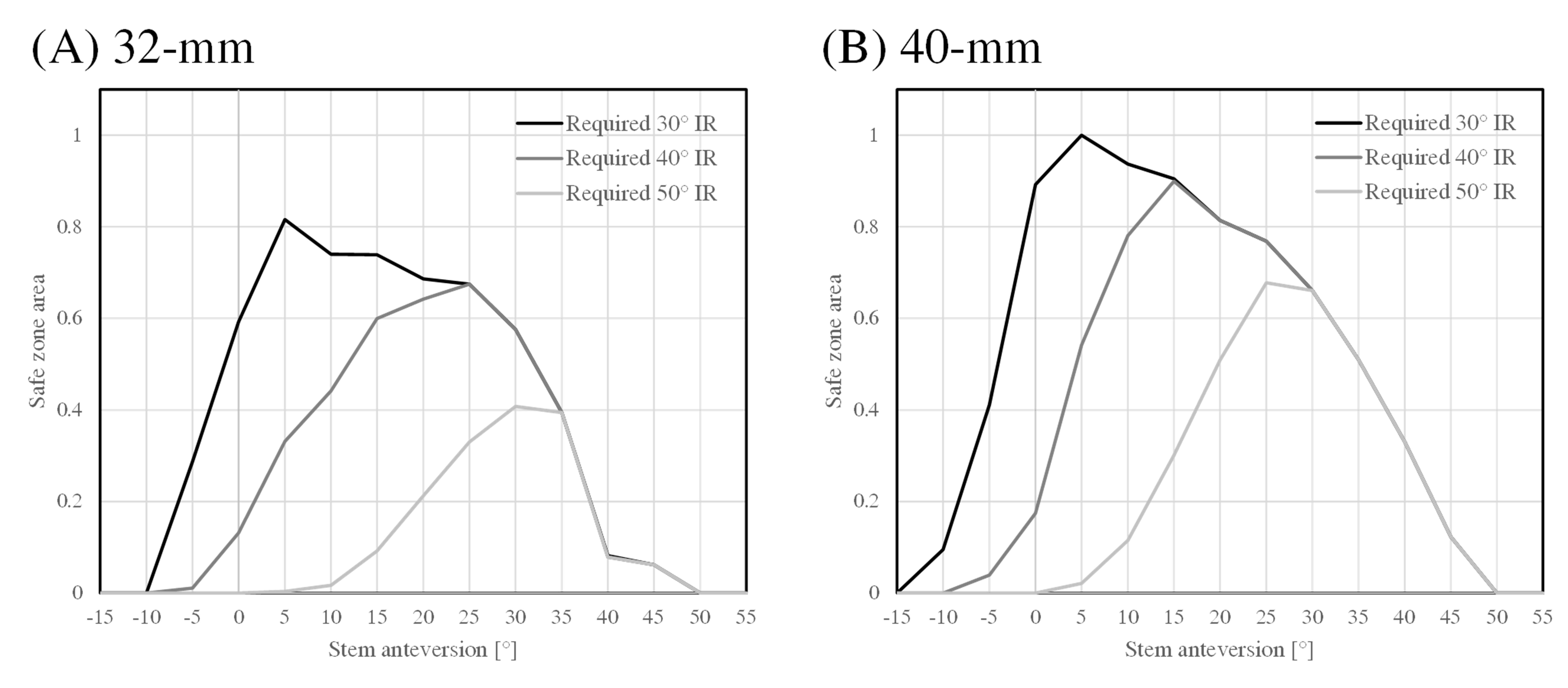
Figure 2
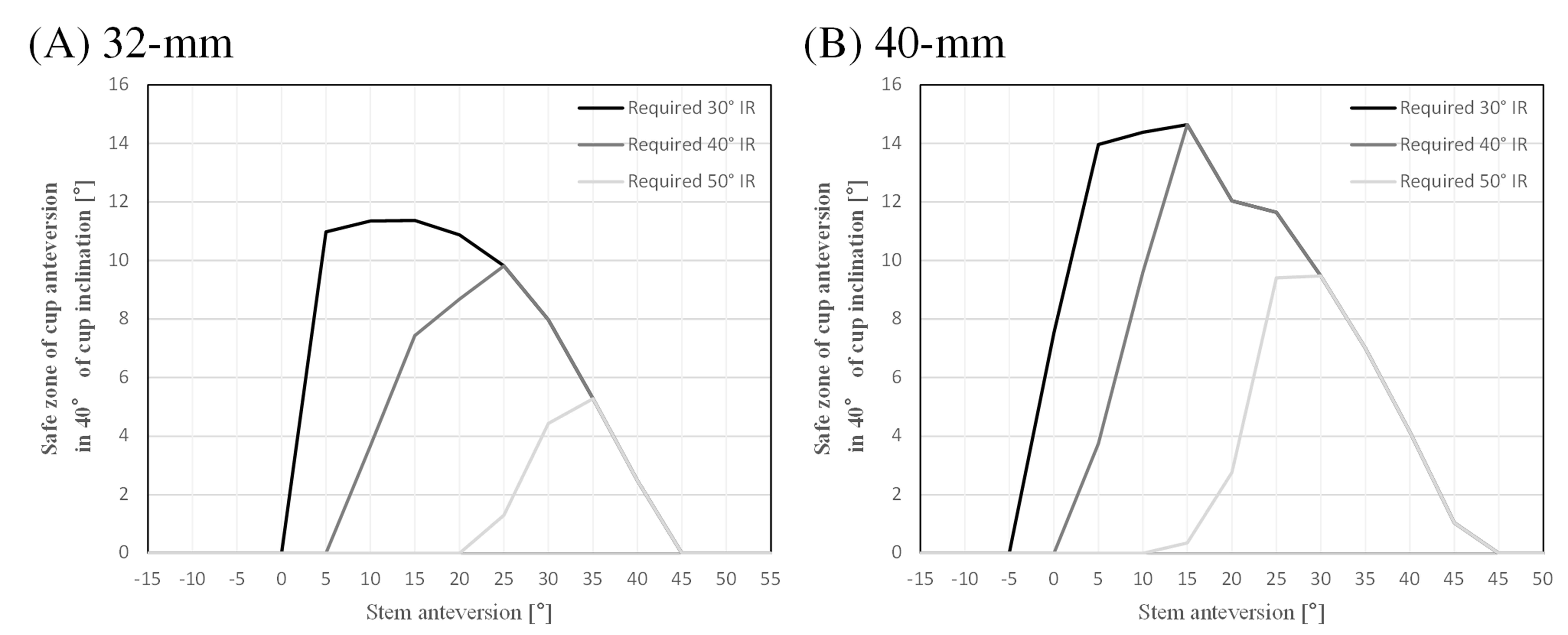
Figure 3#8395
Femoral Neck Resection Height Influences Hip Internal Rotation Range of Motion
Lilian Lim - FormusLabs - Auckland, New Zealand
Nicholas John Giori - Palo Alto, USA
David Liu - Gold Coast Centre for Bone and Joint Surgery - Tugun, Australia
Alex Carleton - FormusLabs - Auckland, New Zealand
Duncan Bakke - Formus Labs - Auckland, New Zealand
*Marco Schneider - Formus Labs - Auckland, New Zealand
Thor F Besier - University of Auckland - Auckland, New Zealand
*Email: marco@formuslabs.com
Introduction
Careful implant sizing and positioning in total hip arthroplasty (THA) can preserve hip biomechanics, maximise range of motion, and avoid impingement and subsequent risk of dislocation [1]. Patient-specific solutions are important to restore hip function to meet high patient expectations. Varying neck resection, among optimisation of component selection and positioning, is one method of minimising the risk of impingement. The aim of this study was to use a computational model to determine the influence of femoral neck cut height on internal rotation impingement in a cohort of patients.
Methods
Eighteen patients who had undergone routine primary THA were included in this study. The mean age of the participants was 66 yrs (range 50–85 yrs; 5 males and 13 females). Patient pelvis and femur anatomies were segmented into 3D models automatically by 3D preoperative THA planning software, FormusHip (Formus Labs, NZ). The femoral head and acetabular centres were co-registered and defined as the joint centre. The femoral head was removed from the femur model with varying levels of additional clearance in increments of 2.5 mm to simulate different ‘resection heights’. Femurs were placed at 90° flexion and rotated internally until impingement, which was defined as the femur being within 5 mm of the hemipelvis (Figure 1).
Results
Increased head clearance was correlated with an increase of internal rotation before impingement. Mean rotation before impingement was 15.5° at 2.5 mm of head clearance and 38.5° at 15 mm of head clearance. For every millimetre of femoral neck removed, internal rotation at impingement was increased by an average of 2°. 11 cases impinged at 0 degrees with 2.5mm of clearance. Seven cases impinged at 0 degrees with 5.0mm of clearance. 2 cases impinged at 0 degrees with 7.5mm of clearance. The relationship between internal rotation at impingement and head clearance was patient-specific.
Conclusion
A trade-off exists between optimal implant positioning and the maximisation of ROM to avoid impingement. Based on computational simulations, we found that the amount of femoral resection to avoid impingement was highly individualised across patients. During surgery, neck resection may influence the size, position, and orientation of the stem, which may influence the joint centre. Our study highlights the importance of patient-specific 3D planning of femoral neck resection in total hip arthroplasty to minimize the risk of impingement while preserving hip function. Next steps involve exploring the influence of implant sizing and positioning on femoral neck resection and impingement.
References
- Malik A, Maheshwari A, Dorr LD. Impingement with total hip replacement. J Bone Joint Surg Am. 2007 Aug;89(8):1832-42. doi: 10.2106/JBJS.F.01313. PMID: 17671025.
Figures
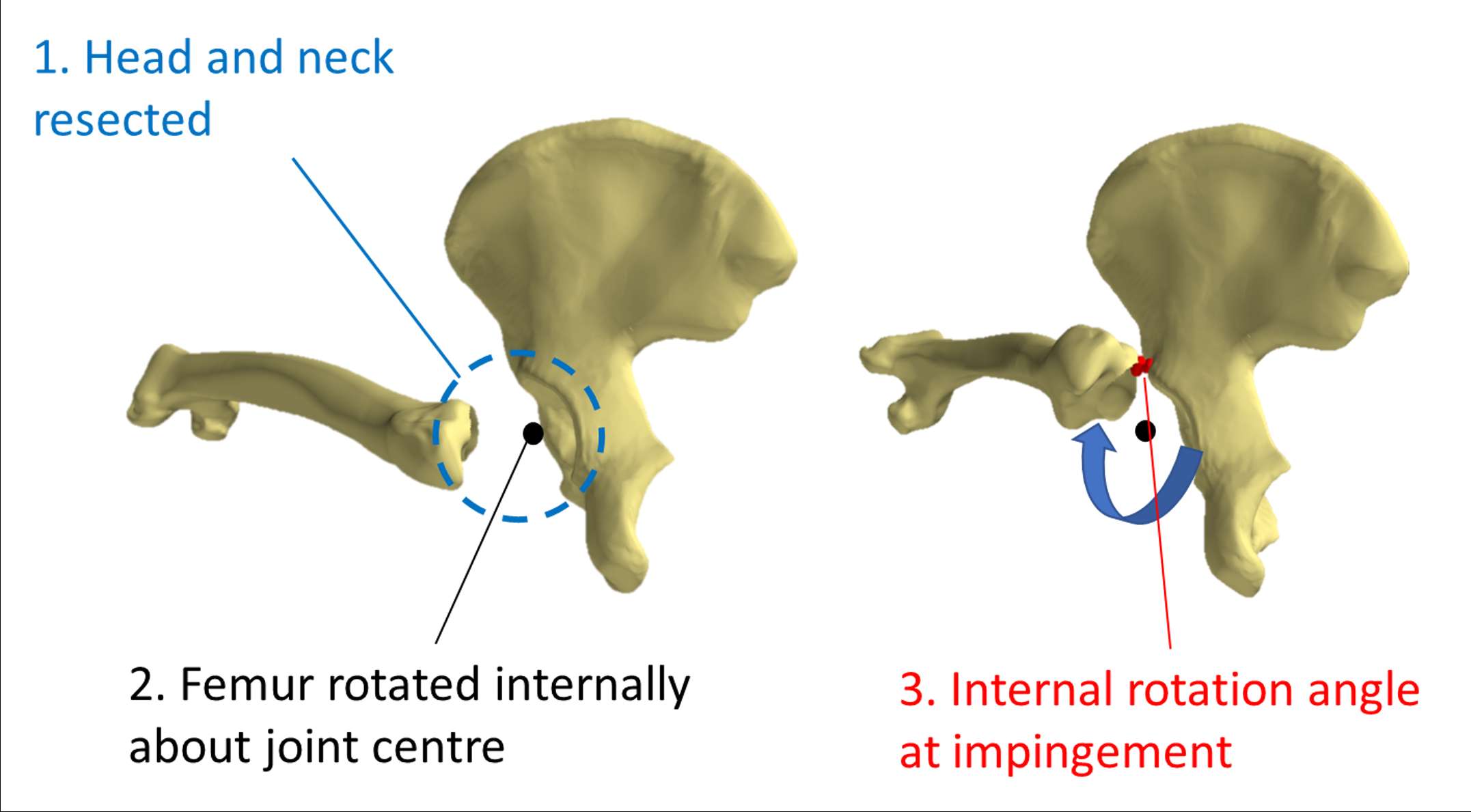
Figure 1
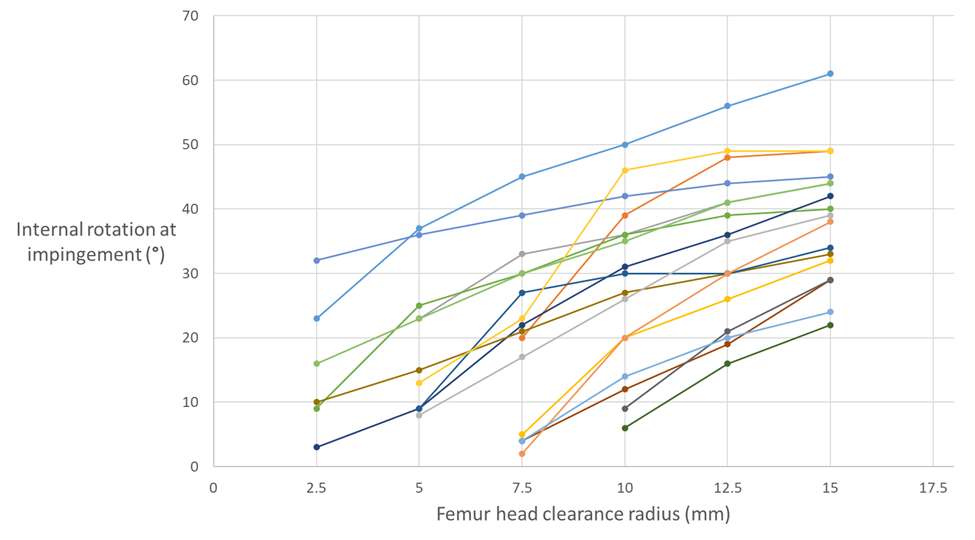
Figure 2#8112
Some Offset Restoration Options Can Paradoxically Lead to Decreased Range of Motion in Primary Total Hip Arthroplasty: A 3D Computer Simulation Study
*Aidin Eslam Pour - Yale University - New Haven, United States of America
Daniel Wiznia - Yale University - New Haven, USA
Steven Tommasini - Yale University - New Haven, USA
Claire Donnelley - Yale University - New Haven, USA
Wei Shao Tung - Yale University - New Haven, USA
*Email: apourmd@icloud.com
Introduction: Femoral offset restoration in total hip arthroplasty (THA) results in optimal biomechanics and range of motion (ROM) free of bone-bone impingement assessable. We hypothesize that difference in implant design features significantly affects bone-bone impingement risk in primary THA.
Methods: A cohort of 43 primary robotic-arm assisted THA were included in this retrospective computer simulation study. Accounting for sagittal pelvic tilt, maximum external rotation at 0° of hip flexion, and maximum internal rotation at 90° and 100° of hip flexion prior to bone-bone impingement were measured. To influence offset, we included neutral/extended polyethylene liners, neutral/plus prosthetic head, and standard/high offset Insignia stem, and Accolade-II stem with 132°/127° neck angle.
Results: Extended polyethylene liner use resulted in decreased bone-bone impingement for both Insignia/Accolade-II stems, but also decreased prosthetic ROM in hip extension (mean -4.5° to 5°, range -10° to 0°) and hip flexion (mean -3°to -3.7°, range -10° to 0°) due to decrease in the head diameter. Using a plus head or different stem offset/neck angle options resulted in either: 1) no improvement in ROM (up to 60% in Accolade-II stem; up to 28% in Insignia stem) or, 2) paradoxical increase in bone-bone impingement (up to 19% in Accolade-II stem; 7% in Insignia stem).
Discussion: Counterintuitively, a subset of patients experience a paradoxical increase in bone-bone impingement when transitioning from standard to high offset or varus necks due to pelvic and proximal femur bone shape. For this group of patients, preoperative personalized 3D modelling may help guide implant choice to optimize the outcomes.
Figures#8329
Which Short External Rotator Muscles Should Be Preserved to Prevent Dislocation After Total Hip Arthroplasty?
*Yoshiaki Ito - Shonan Kamakura Joint Reconstruction Center - Kamakura, Japan
Daisuke Suzuki - Hokkaido Chitose Collage of Rehabilitation - Chitose, Japan
Fumiya Kizawa - Sapporo Medical University - Sapporo, Japan
Arata Kanaizumi - Sapporo Medical University - Sapporo, Japan
Toshihito Hiraiwa - University of Toyama - Toyama, Japan
Satoshi Nagoya - Sapporo Medical University - Sapporo, Japan
*Email: ito-since1976@nifty.com
Introduction: The external rotation torque exerted by the short external rotator muscles acts as a dynamic stabilizer that resists internal rotation of the hip joint. Therefore, preservation of the short external rotator muscles when performing total hip arthroplasty is expected to increase dislocation resistance. However, it is not clear which short external rotator muscles exert the most external rotation torque at each hip flexion angle. Our purpose was to analyze which short external rotator muscles can be effectively preserved to prevent dislocation after total hip arthroplasty by measuring and comparing the hip external rotation torque exerted by individual muscles due to changes in their course with hip flexion.
Methods: Nine fresh-frozen cadavers and 15 hip joints were used in the experiment. The forces of the short external rotator muscles were estimated by measuring the physiological cross-sectional area (PCSA) of individual muscles separately for the piriformis, obturator internus, conjoined tendon, and obturator externus. Muscle-string models were created on the pelvis-femur with a matched course for each short external rotator muscle, and a load of 5 N per cm2 of PCSA for each muscle was applied as a load equivalent to muscle force. In this condition, the external rotation torque of each short external rotator muscle was measured for each 15° of hip flexion 0 - 105°. The sum of the external rotation torque was determined, and the contribution of each muscle to the external rotation torque was calculated for each angle of flexion.
Results: For the piriformis, obturator internus, and conjoined tendon, external rotation torque peaked at 15° hip flexion, and then decreased as the hip flexed. Conjoined tendon had the most external rotation torque at 0 - 45° hip flexion (flex 0, 15, 30° p < 0.01, 45° p < 0.05 Dunnett). The piriformis changed its action from external to internal rotation torque between 75 - 90° of flexion. The obturator externus had the lowest torque of the short external rotator muscles at 0° hip flexion, but the external rotation torque increased as the hip flexed. At 60° of flexion, the external rotation torque of the conjoined tendon and the obturator externus were almost equal, and at 75° of flexion or more in deep flexion, the obturator externus had the highest external rotation torque (flex 75° p < 0.05, 90, 105° p < 0.01 Dunnett?.[Fig.1]
Conclusion: The muscles most effective in resisting dislocation varied according to hip flexion angle. The obturator externus had the highest external rotation torque at hip flexion angles of 75° or greater, which is the dislocation position, and preservation of the obturator externus was considered most effective in resisting dislocation, which included dynamic factors based on muscle force.
Figures
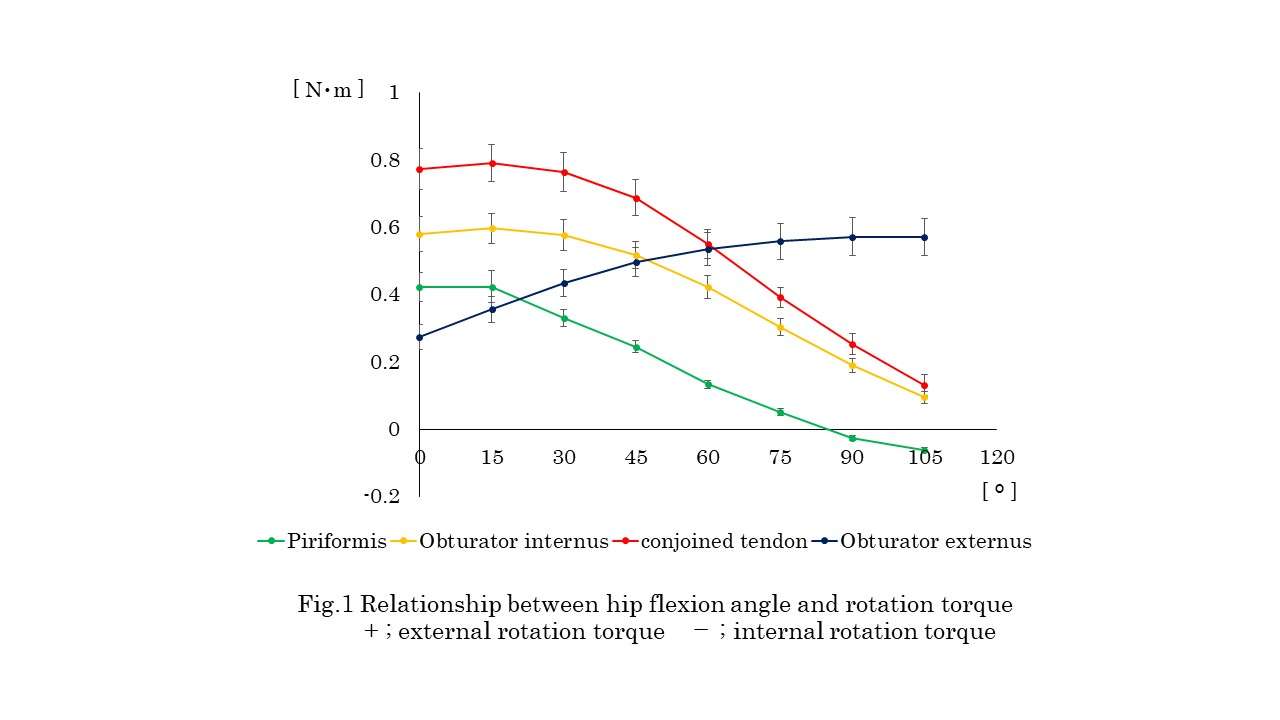
Figure 1#8369
Validation of a Novel RSA-Based Impingement Metric to Assess Component-on-Component Impingement Risk in Vivo.
*Shahnaz Taleb - Western University - London, Canada
Jordan Broberg - Western University - London, Canada
Matthew Teeter - Western University - London, Canada
*Email: staleb3@uwo.ca
Introduction. Component-on-component impingement in total hip arthroplasty may lead to limited range of motion and function, pain, dislodgement of the modular liner or loosening of the implant due to increased stress on the liner rim, accelerated metal wear, subluxation, and dislocation. Because of its consequences, many clinical studies are looking at methods to reduce the risk of impingement following hip arthroplasty. However, the assessment of component-on-component impingement in clinical studies is largely limited to radiographic qualitative assessment of the hip joint, finite element analyses, and cadaver studies. There is a need for more precise measurements of impingement in vivo in the research setting. Radiostereometric analysis (RSA) is a minimally invasive dual-plane radiographic technique used to monitor 3D movements of musculoskeletal joints and is the gold standard for measuring implant kinematics. We aimed to validate a novel RSA-based impingement risk metric to measure component-on-component impingement.
Methods. A phantom experiment of a standard metal-on-polyethylene total hip system with similar radiographic properties as bone was performed. The phantom femur was rigidly attached to a composite translation stage of 0.002 mm translational accuracy and 0.02 rotational accuracy. RSA examinations were performed as typical for a traditional weight-bearing RSA exam for large joints. An initial baseline radiograph was taken, followed by images at translation increments of 0.25 mm, 0.5 mm, 1.00 mm, 2.00 mm, and 5.00 mm in each orthogonal plane. Images at five impinged positions were also taken. All images were measured using commercial model-based RSA software. Double-exposure radiographs were taken to measure repeatability. RSA measurements were compared against true micrometer displacement. Implant component poses from RSA were then used to transform implant component STLs to the imaged position. A circumference around the femoral head skirt located where impingement with the polyethylene liner would occur was defined. The distance between the closest point about this circumference and the edge of the polyethylene liner was defined as the impingement distance. A lower impingement distance suggests a greater risk of impingement occurring.
Results. No significant differences were found between micrometer and RSA measurements for Tx (p = 0.77), Ty (p = 0.17), Tz (p = 0.31). No significant differences in RSA were found between first and second exposure measurements Tx (SD = 0.08 mm), Ty (SD = 0.07 mm), Tz (SD = 0.04 mm). Similar results were observed when comparing impingement distances measured from each exposure (SD = 0.09 mm). For impinged positions, distances between the closest point of the head skirt to the polyethylene plane ranged from 0.22 mm to 0.53 mm (Fig. 1). In a neutral position, the distance measured was 16.56 mm (Fig. 2).
Conclusion. We were able to create and validate an RSA-based risk metric to assess hip impingement in vivo. A 0.55 mm threshold may be proposed to define impingement where distances approaching 0.55 mm are at a greater risk of impingement and distances < 0.55 mm are considered impinged. The simplified metric will be a useful tool for future clinical studies in component-on-component impingement.
Figures
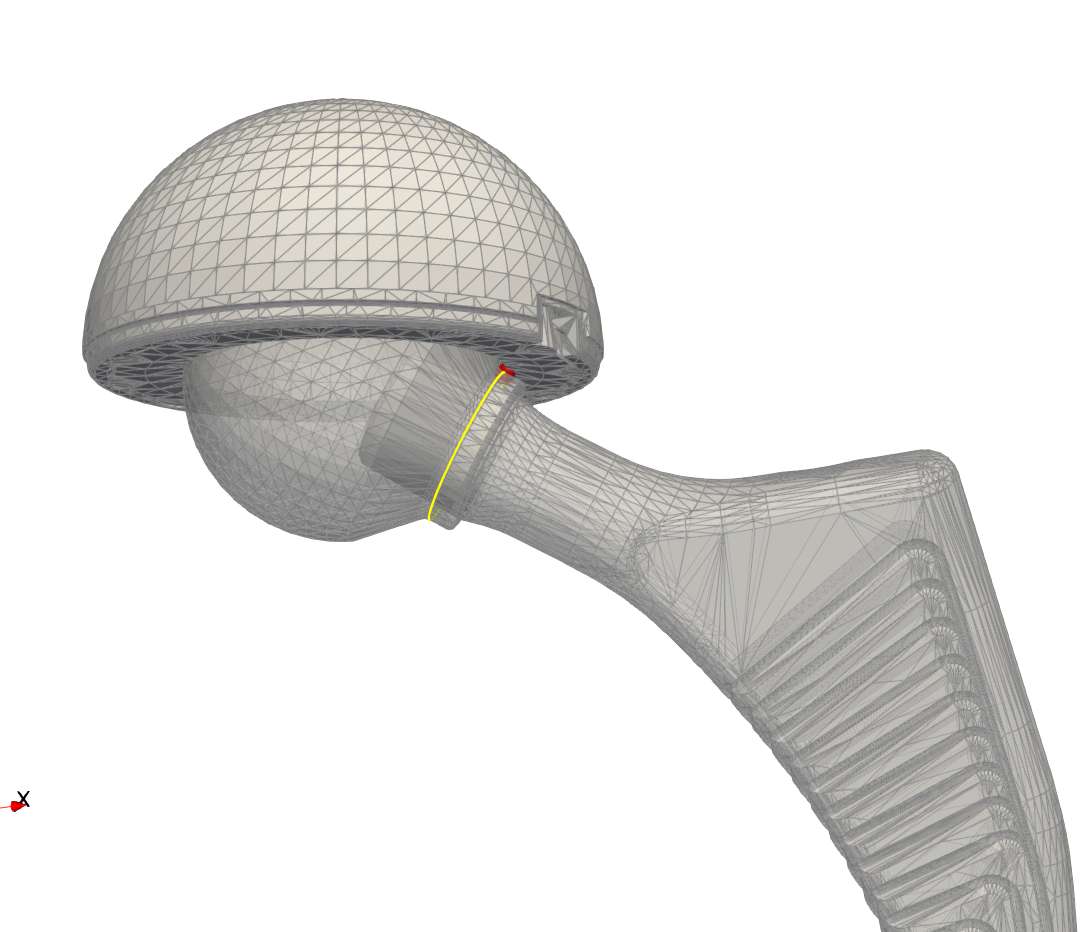
Figure 1
Figure 2#8233
Utilizing a Dual Modular Retroversion Neck Can Provide the Impingement-Free Safe Zone of the Cup Alignment for Patients With a High Femoral Anteversion
Yukihiro Habe - Osaka University - Osaka, Japan
Hidetoshi Hamada - Osaka University - Suita, Japan
Keisuke Uemura - Osaka University - Suita, Japan
Kazuma Takashima - Osaka University Graduate School of Medicine - Suita, Japan
*Nobuhiko Sugano - Osaka University Graduate School of Medicine - Suita, Japan
*Email: n-sugano@umin.net
Introduction
In reducing the risk of dislocation after total hip arthroplasty (THA), it is ideal to control the cup alignment according to the stem alignment to avoid implant impingement during various activities of daily living. There are many reports of impingement simulation of THA showing that no cup-safe zone exists when stem anteversion is more than 45°. Modular neck stem that is capable of correcting excessive femoral anteversion angles have shown efficacy in providing the impingement-free safe zone for a highly anteverted stem, however, detailed reports on the safe zone are scarce. We asked; (1) What is the effect on the impingement-free safe zone area when changing from a straight neck to a retroverted neck? (2) What is the optimal radiographic anteversion (RA) safe range at radiographic cup inclination (RI) 40° which is recommended as the target to avoid edge loading and to increase jumping distance?
Methods
The prosthesis used a flat liner, a 32-mm femoral head, and a modular neck stem, which allowed for interchangeable use of a straight neck (ST) and a 15° retroverted neck (V15). Implant impingement was evaluated using collision detection in the 3D CAD software Solid Edge (SIEMENS). The initial position of the stem was set at 5° of flexion and 5° of adduction. The stem anteversion angle was set from -10° to 65° with an increment of 5°. 15 different activity hip positions reported by Widmer and Miki were selected, including 40° internal rotation at 90° flexion 1, 2). The radiographic inclination and anteversion angles of the liner impingement were measured. The angle at which impingement occurs in multiple stem positions was measured, and the range of angles at which impingement does not occur was defined as a safe zone. Safe zones formed by a different stem anteversion were compared.
Results
(1) The ST safe zone area was the largest at 25° stem anteversion and disappeared at 50°. The V15 safe zone area was the largest at 40° stem anteversion and disappeared at 65° (Figure 1, 2). The V15 safe zone area at 40° stem anteversion was larger than that of the ST safe zone area at 25° stem anteversion (Figure 2).
(2) The RA safe range at RI of 40° disappeared at stem anteversion of 45° or greater for ST and at stem anteversion of 60° or greater for V15 (Figure 3).
Conclusions
This study demonstrated the usefulness of the 15° retroversion neck to provide RA safe zones in an excessive stem anteversion up to 60°. The use of V15 allows for a cup RA safe zone at 40° RI in stem anteversion 25° to 55°.
Reference
1. Widmer KH. Clin Orthop Relat Res. 2020 Aug;478(8):1904-1918.
2. Miki H et al. J Arthroplasty. 2007 Oct;22(7):946-52.
Keywords
Total hip arthroplasty, computer simulation, safe zone, range of motion, combined anteversion
Figures
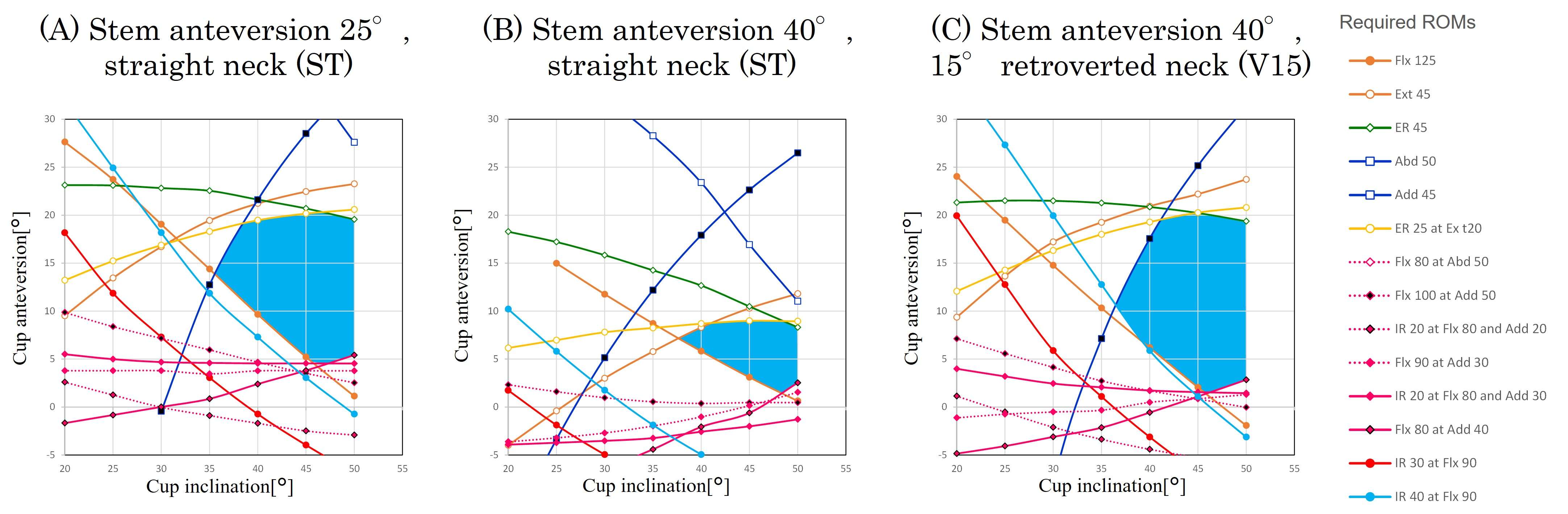
Figure 1
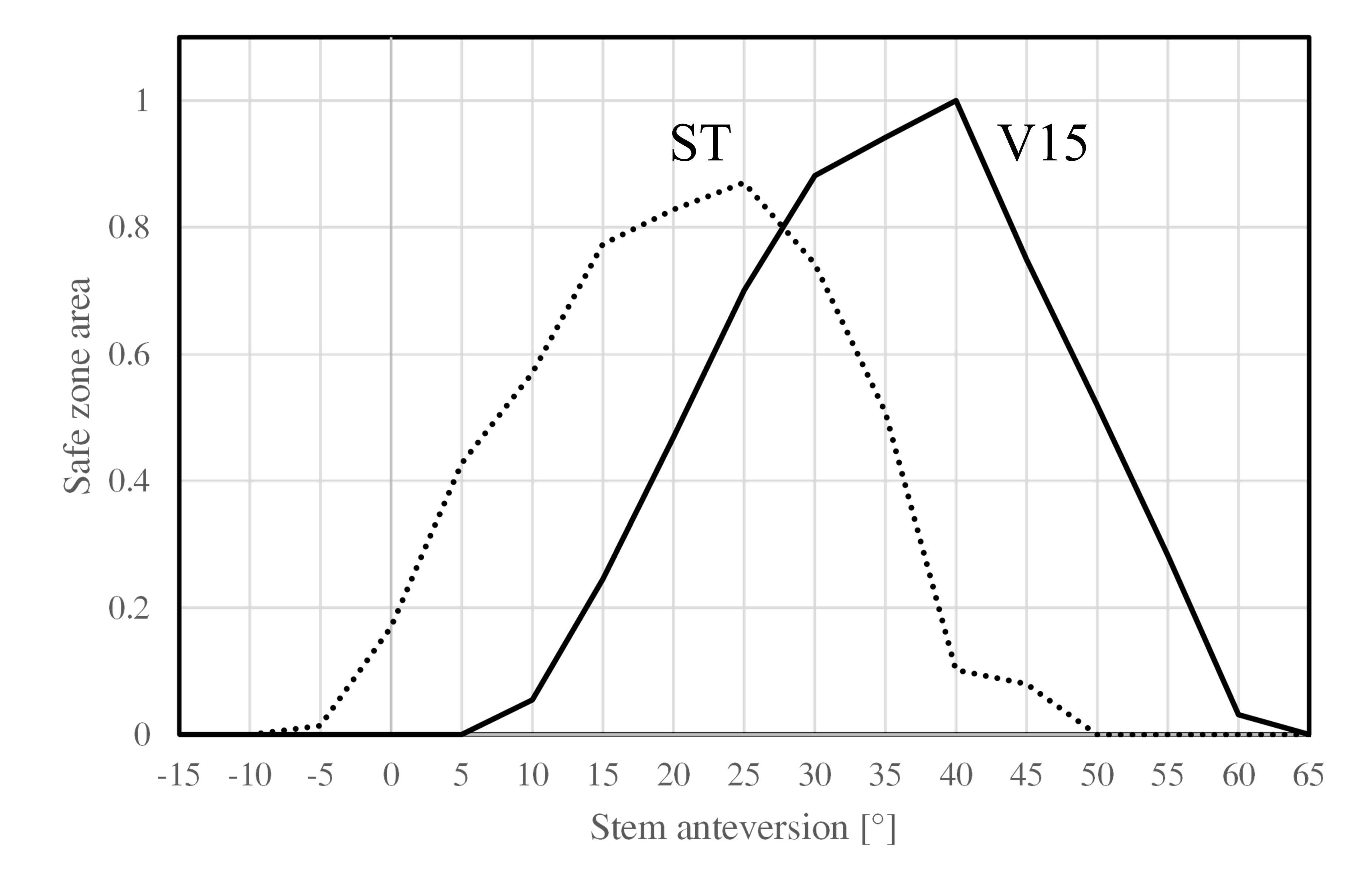
Figure 2
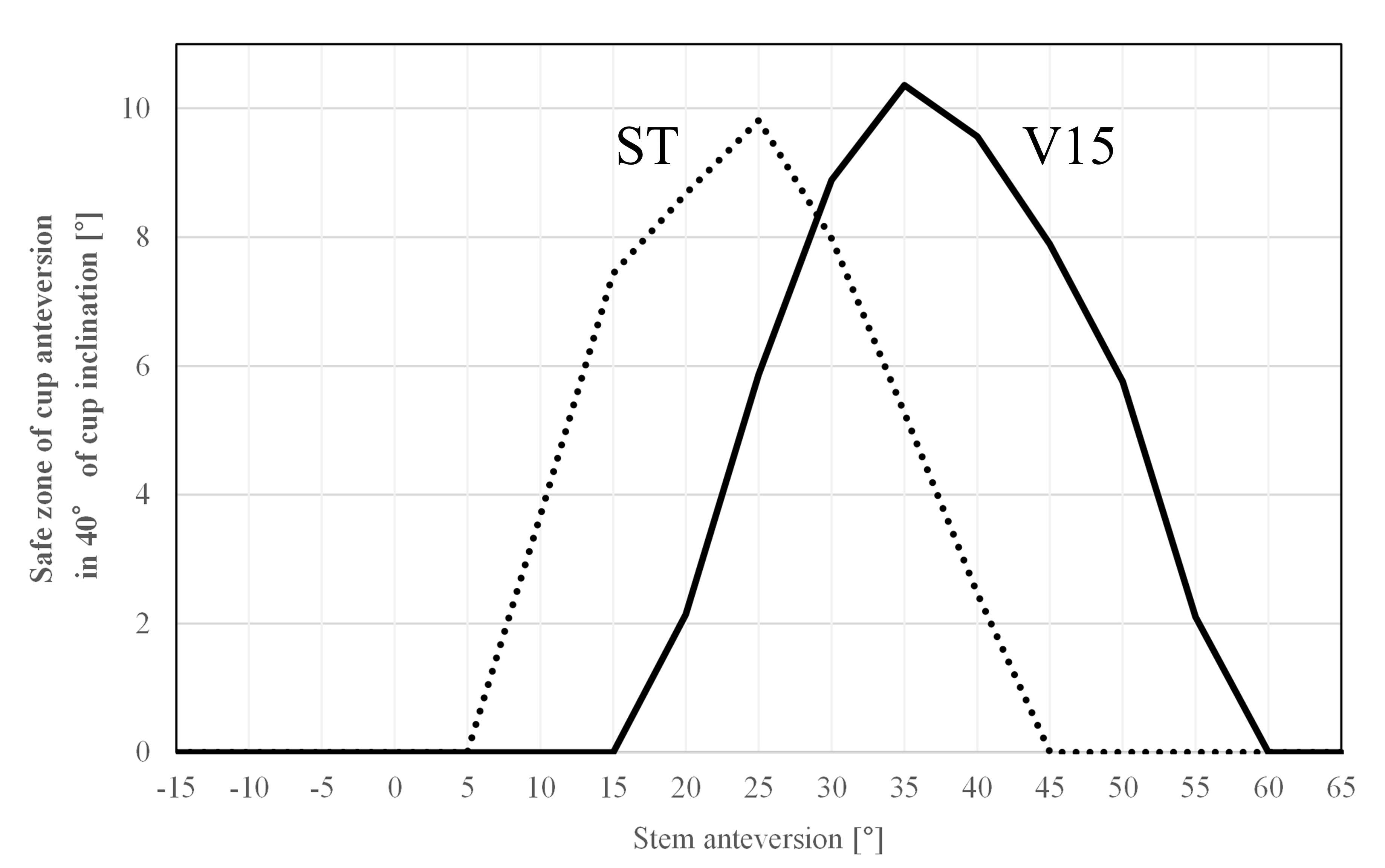
Figure 3#8485
Impingement Evaluation of Dual Mobility Total Hip Replacement Constructs
*Ioan Cracaoanu - DePuy Synthes - Leeds, United Kingdom
Elizabeth Hippensteel - DePuy Synthes - Warsaw, USA
Robert Wright - DePuy Synthes - Leeds, United Kingdom
Danielle Gehron - Depuy Synthes - Warsaw, USA
Anne Osantowski - Depuy Synthes - Warsaw, USA
*Email: icracaoa@its.jnj.com
Impingement between the femoral neck stem and acetabular liner and/or shell in total hip replacement may occur in vivo due to patient anatomy (including joint laxity), surgical positioning and/or implant design, or combination of these factors. In vitro testing of impingement is complex due to the multifactorial nature of the different implant components and systems as well as the need to address and standardize this type of analysis across testing laboratories. The purpose of this study is to evaluate the effect of impingement in two dual mobility (DM) hip replacement systems utilizing the guidance established in ASTM F2582-20.
Hip simulation neck impingement testing for two hip joint implant designs was conducted per ASTM F2582-20 in three separate groupings to address all possible failure mechanisms. A Pinnacle DM hip system (DePuy Synthes Joint Reconstruction) was evaluated alongside a novel DM system. The objectives of the three studies were as follows:
-
Group 1: Effect of impingement on the DM metal liner and shell on the locking mechanism.
-
Group 2: Effect of impingement between the femoral stem and shell on fatigue strength of the acetabular shell.
-
Group 3: Effect of impingement on the DM metal liner (lipped and non-lipped styles) and shell on femoral stem mechanical strength.
Polyethylene components were artificially aged per ASTM F2003-02(2015). An AMTI VIVO joint simulator (Fig. 1) was used to generate impingement between the femoral neck and DM liner/shell using profiles described in ASTM F2582-20. Samples were tested in bovine calf serum lubricant (37?C ± 2?C) for 1.0 million cycles (MC). Damage assessment was performed every 0.2 MC and the new impingement angle determined. Quantification following dynamic impingement included: liner push-out per ASTM F1820-13 (Groups 1-3); unsupported shell fatigue per ASTM F3090-20 (Group 2 and Group 3 non-lipped samples), and neck fatigue per ISO 7206-6:2013 (Group 3).
All liners, stems, and shells coupled with non-lipped liner designs sustained damage during dynamic impingement. Shells coupled with lipped liners were unaffected as the lipped design covered the contact region of the shell. Photographic evidence of the impingement contact areas (Fig. 2) and the impingement angle was recorded.
|
|
Pre-Impingement
|
Post- Impingement
|
|
Pinnacle DM
|
2562±440N
|
3499±361N
|
|
Novel DM
|
3970±160N
|
3879±302N
|
The ASTM F2582-20 method for in vitro impingement and evaluation is an adverse scenario which creates severe damage to the femoral neck and shell/liner construct in hip replacement components. This study evaluated this method on two dual mobility (DM) hip replacement systems. Additional work is required to determine the clinical relevance of these adverse testing conditions.
Figures

Figure 1

Figure 2#8449
Impingement in Dual Mobility Hip Joint Replacement Under Adverse Conditions According to ASTM F2582:2020
*Mazen Al-Hajjar - DePuy Synthes - Leeds, GB
Oscar O'Dwyer Lancaster-Jones - DePuy Synthes Joint Reconstruction - Leeds, United Kingdom
Ioan Cracaoanu - DePuy Synthes - Leeds, United Kingdom
Megan Hadley - DePuy Synthes - Leeds, United Kingdom
*Email: malhaija@its.jnj.com
INTRODUCTION:
In vitro testing of impingement is very complex due to the many factors that can influence its occurrence in vivo. The aim of this study was to understand the consequences of impingement on a dual mobility (DM) total hip replacement (THR) system in a scenario where the polyethylene mobile bearing (MB) becomes fixed due to adverse condition as described in ASTM F2582:2020.
METHODS:
Dual mobility bearings consisting of CoCr femoral head on a titanium femoral stem articulating against a polyethylene MB were used in this study. The femoral stems were replaced with fixtures representing the same geometry and material as the femoral stems. The MB was fixed using a restrictor plate that allowed the taper fixture to contact the mobile bearing during impingement. Two polyethylene materials, a novel antioxidant (AOX) polyethylene and a conventional moderately crosslinked polyethylene, were artificially aged (two weeks per ASTM F2003) prior to testing. Worst-case MB and femoral head sizes were chosen for this study.
A multi-station electromechanical ProSim simulator was used to apply the kinematics specified in the impingement standard, namely: flexion / extension of 0°/ 10°, adduction / abduction of 0°/ 5° (from initial contact) and internal / external rotation of 5°/ 5° applied in a sinusoidal waveform about the centre of rotation of the machine and the articular bearing (Figure 1). A constant axial compressive load of 600N was applied vertically through the acetabular cup/ fixed polyethylene bearing and all cycles performed at a frequency of 1Hz. New-born calf serum (protein concentration 30g/L) was used as a lubricant. Temperature was maintained at 37?C ± 2?C within the test chamber. Each test ran for a total of 1 million cycles with qualitative assessment of the damage performed every 0.2 million cycles.
RESULTS:
All aged conventional moderately cross-linked samples showed either crack initiation or fracture at the impingement site at 0.2 Mc, whereas there was no damage in the form of fracture or crack observed after 1.0 Mc for antioxidant polyethylene samples (Figure 2). All polyethylene components showed signs of wear and plastic deformation. The antioxidant polyethylene MB samples had an increased lever-out torque after impingement compared to non-impingement baseline. This was also the case for the moderately crossed linked polyethylene.
DISCUSSION:
The conventional moderately crosslinked polyethylene material affected by artificial aging were challenged under this impingement test method resulting in polyethylene fracture for one of the test groups. Lever-out testing did not distinguish between the different severity of damage that occurred on the different polyethylene components suggesting that it may not be an appropriate method to biomechanically assess the retention of the femoral head in the MB post-impingement as described in ASTM F2582:2020.
CONCLUSION:
Impingement in THR can result in dislocation, loss of fixation and excess release of wear debris and reduction in strength of the different parts of the hip joint system. This study presents an adverse impingement scenario for dual mobility systems resulting in fracture in older polyethylene materials and highlighting the benefits of antioxidant polyethylene materials under such conditions.
Figures
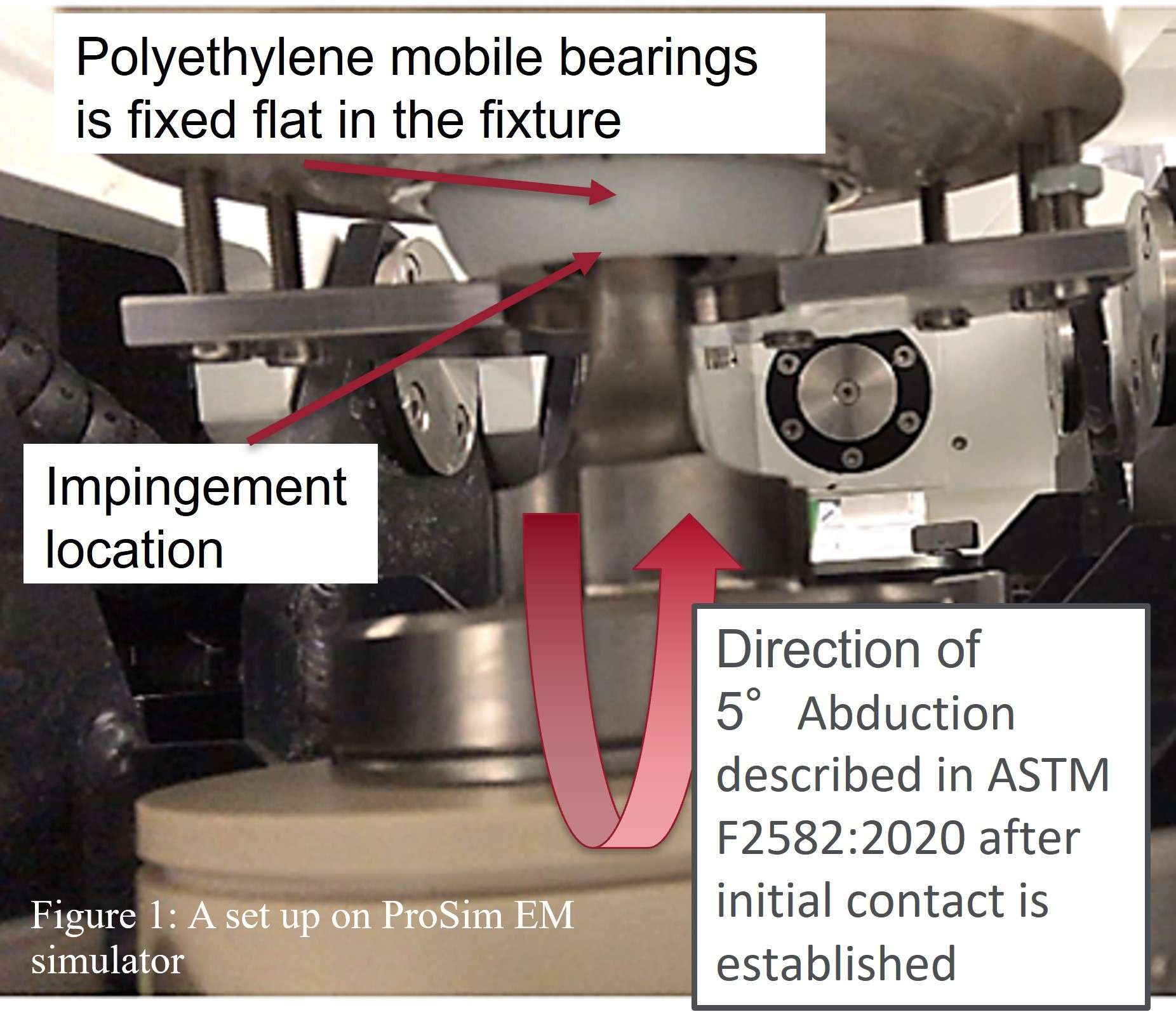
Figure 1

Figure 2#8527
Prevalence of Dual Mobility Usage in Patients at Risk of Adverse Spinopelvic Mobility
*Edgar Wakelin - OMNI Life Sciences - Raynham, USA
Gerard Smith - Corin - Sydney, Australia
Christopher Plaskos - Corin - Raynham, USA
Michael O'Sullivan - Mater Clinic - Wollstonecraft, Australia
Andrew Shimmin - Melbourne Orthopaedic Group - Melbourne, Australia
Jim Pierrepont - Corin - Pymble, Australia
*Email: edgar.wakelin@coringroup.com
Introduction
Dual mobility (DM) total hip arthroplasty (THA) aims to reduce the rate of dislocation in high-risk cases by increasing the dislocation jump height. A number of adverse spino-pelvic mobility (SPM) risk factors have been identified which contribute to increased dislocation risk, however, the utilization of DM implants in patients with adverse SPM is unknown. This study uses a large industry database to review the utility of DM in high-risk patients.
Methods
A retrospective analysis of the CorinRegistry™ was performed for THA cases planned and executed between August 2019–March 2023. All cases received a pre-operative CT scan and lateral standing and flex seated x-rays for OPS™ pre-operative planning. During planning, the following adverse SPM risk factors were identified: Pelvic Incidence - Lumbar Lordosis (PI-LL) ≥ 20°; Standing Pelvic tilt (PT) ≤ -10°; Lumbar Flexion (LF) ≤ 20°; Seated Pelvic Tilt - Standing Pelvic Tilt (Seat2StandPT) ≥ 20°; Standing Pelvic Tilt minus Supine Pelvic Tilt (Stand2SupPT) < -13°. The frequency of planned DM implants as validated by the surgeon are compared against the SPM risk factors and to the whole cohort.
Results
A total of 16,180 THAs were extracted from the CorinRegistry from 80 contributing surgeons. Demographics were: 64±11 years, 47% male with surgical approach: 62% posterior, 35% direct anterior approach and 2.8% lateral. Of the 80 surgeons, 50 (63%) reported planning a THA with DM implants. Of these surgeons, DM usage was planned on average 10.0% of the time, in which 33% used DM ≤ 2.5% of the time, 56% ≤ 10%, and 79% ≤ 20% of the time, Figure 1.
The presence of adverse SPM significantly impacted the decision to plan for DM implants. Increasing the proportion of planned DM implants to: PI-LL: 27.7%, PT: 19.8%, LF: 26.5%, Seat2StandPT: 30.3%, Stand2SupPT: 24.9% (p < 0.0001 in all cases). When a combination of adverse SPM factors were present the prevalence of DM implant plans increased to a maximum of 42.9% when all 6 were identified. THAs with DM implants planned reported increased age (70.6±10.7 vs 63.9±11.4, p < 0.0001), greater proportion of women (64% vs 53%, p < 0.0001) and higher posterior lateral approach (82% vs 69%, p < 0.0001).
Conclusion
Despite increased DM utility in high-risk patients, a large proportion of high-risk patients do not receive DM implants during planning. Increased DM usage in a high-risk population may reduce rate of dislocation in THA.
Figures
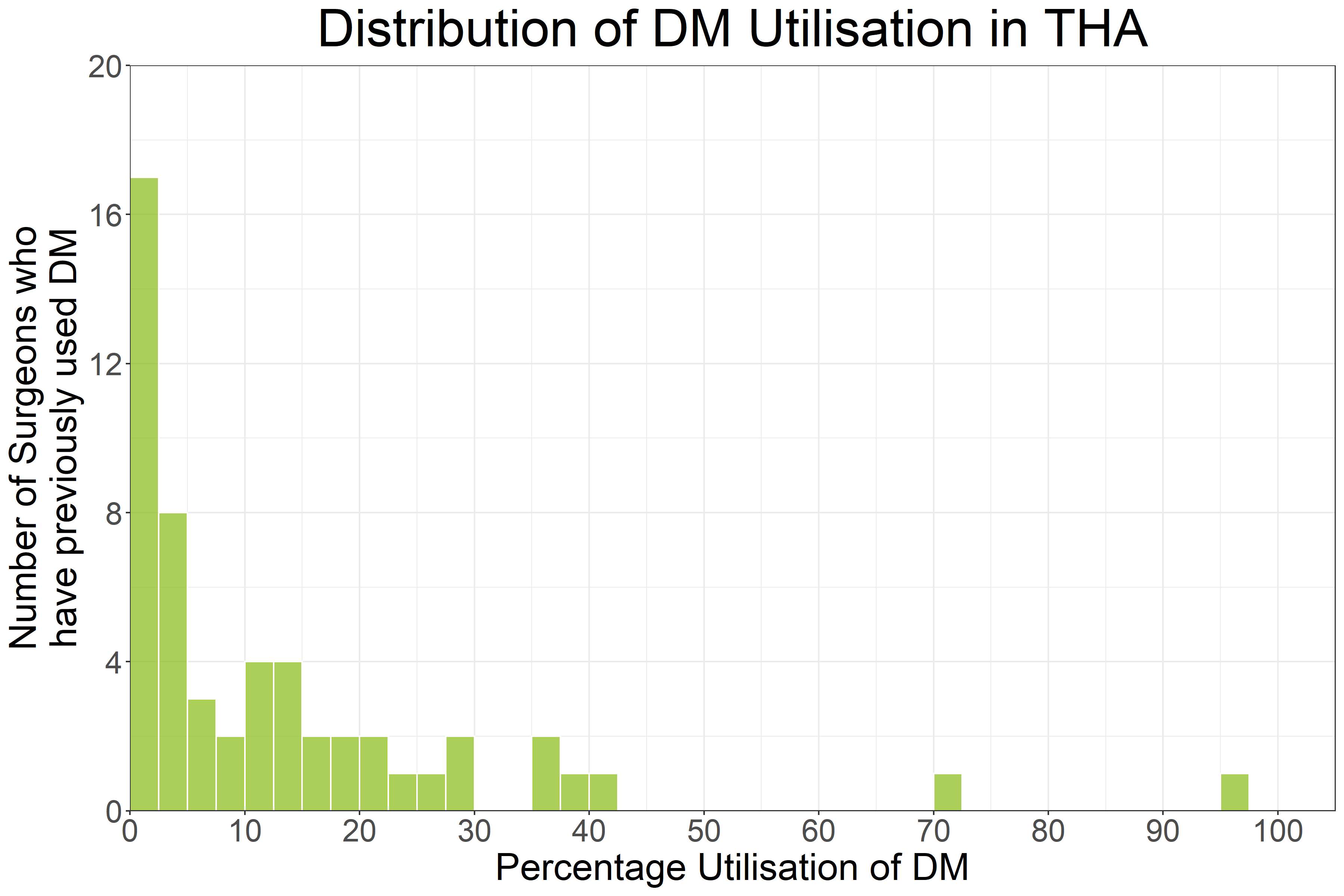
Figure 1#8540
In-Vitro Wear of Dual Mobility Total Hip Replacements With Artificially Aged Antioxidant Polyethylene Bearings.
*Megan Hadley - DePuy Synthes - Leeds, United Kingdom
Elizabeth Hippensteel - DePuy Synthes - Warsaw, USA
Corey Laukhuf - DePuy Synthes - Warsaw, USA
Clive Wilson - DePuy Synthes - Leeds, United Kingdom
*Email: mhadley1@its.jnj.com
Introduction
Dual mobility (DM) total hip replacements are increasing in popularity as a solution to address joint instability. An increased jump distance and possible wider range of motion reduce risk of impingement and dislocation, leading to better performance for patients.
Antioxidant polyethylene (AOX) bearings are also increasingly used in total hip arthroplasty to combat the challenges presented by long term oxidative degradation and associated reduction in mechanical properties and wear resistance.
This study compared several DM designs with wear of a conventional single bearing (THA), examining the effect of bearing diameter, use of antioxidant polyethylene compared with moderately cross-linked polyethylene (XLPE) and the effect of artificial aging of the polyethylene on implant wear during a standard walking simulation.
Method
Four specimen types were evaluated:
- DM (28mm head) XPLE
- THA (40mm head) AOX
- DM (28mm head) AOX
- DM (36mm head) AOX
The 28mm DM moderately cross-linked polyethylene was selected as a comparative size to the AOX DM specimens. The 40mm THA was selected as a worst-case comparator for the bearing type.
XLPE was manufactured from GUR 1050 UHMWPE, and AOX from GUR 1020 UHMWPE.
For each group, non-aged specimens were compared with artificially aged components pre-treated at 70°C under O2 at 5 atm. for two weeks per ASTM F2003-02(2015). All specimens were subjected to a minimum of 6MC of wear simulation of standard walking per ISO14242:1 (2014). New-born calf serum with a protein concentration of 30g/L was used as lubricant. Testing was performed using a ProSim 10 station Wear Simulator (Simulation Solutions, UK) or AMTI ADL hip simulator (AMTI, MA).
Wear was measured gravimetrically every 0.5MC, per ISO14242:2 (2016). Load soak controls were used to compensate for any fluid uptake. Differences in wear between specimen groups were evaluated using a one-tailed student T test.
Results
|
Specimen type
|
Wear rate – non aged (mg/MC)
|
Wear rate – aged (mg/MC)
|
|
DM XLPE 28mm
|
13.43±3.12
|
18.18±4.46
|
|
THA AOX 40mm
|
7.27±1.16
|
8.17±0.93
|
|
DM AOX 28mm
|
2.00±0.69
|
1.22±0.77
|
|
DM AOX 36mm
|
7.29±2.07
|
2.59±0.47
|
(±95% confidence intervals)
The wear rate of DM XPLE bearings increased significantly with aging (p=0.023). In contrast, no significant increase with aging was observed for any of the AOX bearings (THA AOX 40mm p=0.063, DM AOX 28mm p=0.997, DM AOX 36mm p=0.996).
Conclusion
This study indicates that while a significant increase in wear is observed for an XLPE DM bearing following aging, there was no corresponding increase all AOX bearings. We observed a consistently lower wear rate on all AOX bearings than XLPE, with a significant difference between the highest wearing AOX group and lowest wearing XLPE group (p=0.996). Wear rates of aged 28mm AOX DM bearings are significantly lower than 36mm (p=0.00), however both were significantly lower than the worst case clinically available AOX THA design (p=0.00).
This study indicates that DM bearings in combination with AOX polyethylenes offer benefits in long term wear reduction can be achieved, decreasing the need for invasive revision procedures.
Figures
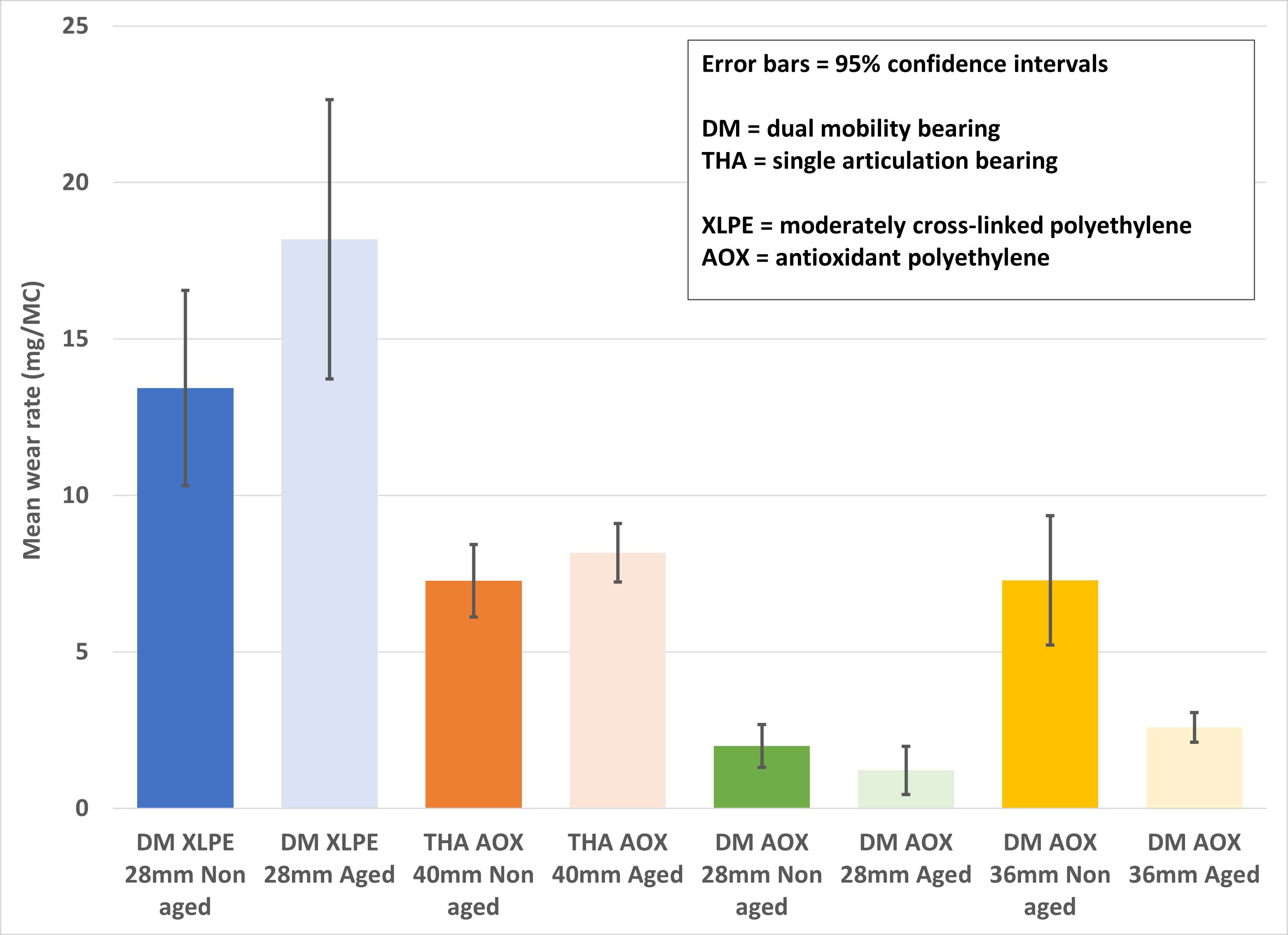
Figure 1#8699
Valgus Knee Osteoarthritis Treated With Unrestricted Kinematically Aligned TKA Is Efficient and Leads to Results Comparable to Varus Osteoarthritis
*Anand Dhaliwal - California Northstate University - Elk Grove, USA
Alexander Nedopil - University of Wuerzburg - Wuerzburg, Germany
Stephen Howell
Maury Hull - University of California Davis - Sacramento, USA
*Email: Anand.Dhaliwal7125@cnsu.edu
Background: Unrestricted kinematically aligned (KA) total knee arthroplasty (TKA) with manual instruments restores the patient’s pre-arthritic joint lines, thus relying on healthy ligaments to set the varus-valgus alignment of the tibial component and provide postoperative knee stability, and restore normal knee function. In many surgeons treating patients with valgus knee osteoarthritis (OA), the concern prevails that the medial collateral ligament (MCL) is elongated and insufficient with the consequence that unrestricted KA is avoided to treat valgus knee deformity. The purpose of this cohort study was to test 1) whether unrestricted KA TKA of patients with valgus deformity results in a higher early revision rate due to instability compared to patients with varus deformity, and 2) whether patients with a valgus deformity have inferior functional outcomes than patients with a varus deformity or patellofemoral OA after unrestricted KA TKA.
Methods: Patients treated from November 2019 to October 2021 with unrestricted KA TKA for all deformities by one surgeon were prospectively followed. Analysis of variance (ANOVA) determined differences in preoperative knee alignment (- valgus / + varus), distal femoral joint line orientation, and proximal tibial joint line orientation, the Kruskall-Wallis-test determined differences in patient-reported outcome measures (PROMs), and the Pearson’s Chi-Square test determined differences in patient satisfaction between varus, valgus, and patellofemoral (PF) osteoarthritic deformities.
Results: During the study period 112 consecutive patients were operated, of whom 101 patients (90%) provided information about any reoperation and completed PROMs at least 1 year after TKA. Of the 101 included patients, 74 had varus OA, 24 valgus OA, and 3 PF OA. One patient with varus OA had a one-stage revision surgery for infection. The mean preoperative knee angle, femoral and tibial joint line orientations were 0.5 ± 7.9°, 82.2 ± 3.4°, and 84.8 ± 3.7° in the varus group, -9.4 ± 3.2°, 81.4 ± 2.5°, and 89.6 ± 2.7° in the valgus group, and -8.0 ± 2.5°, 80.2 ± 4.3°, and 90.1 ± 5.1° in the PF group (p = 0.001811, p = 0.6375, p = 0.01467). The median Forgotten Joint Score (FJS) / Oxford Knee Score (OKS) / and KOOS Jr. Score and the percentage of satisfied patients with valgus OA was 71/42/76 and 87.5%, 80/42/80 and 93% in patients with varus OA, and 94/46/94 and 100% in patients with PF OA (p > 0.1636).
Conclusions: Within the ranges observed in this study, performing unrestricted KA TKA for valgus OA is efficient and associated with comparable patient outcomes to varus and PF OA. Surgeons, who are limiting the principle to restoring the patient’s pre-arthritic joint line orientations to varus and PF OA, can use the results from this study to expand the indication of unrestricted KA TKA to valgus OA.
Level of Evidence IV
Key Words: knee arthroplasty; kinematic alignment; valgus deformity; patient-reported outcomes
#8093
Title: Emerging Utilization of Cementless Total Knee Arthroplasty: A Nationwide Analysis From 2015 to 2021
*Anthony S Unger - George Washington University - Washington, USA
Amil R. Agarwala - Department of Orthopaedic Surgery, George Washington University School of Medicine and Health Scienc - washington, USA
Emile-Victor Kuyla - aDepartment of Orthopaedic Surgery, George Washington University School of Medicine and Health Scien - washington, USA
Alex Gua - aDepartment of Orthopaedic Surgery, George Washington University School of Medicine and Health Scien - washington, USA
Gregory Golladay - West Hospital - Richmond, USA
Savyasachi C. Thakkar - Department of Orthopaedic Surgery, Johns Hopkins Medicine - baltimore, USA
Gautam Siram - Summit Orthopedics - chevy chase, USA
Sandesh Rao - dWashington Orthopaedics and Sports Medicine - checvy chase, USA
*Email: ungeranthony@gmail.com
Introduction
Modern cementless total knee arthroplasty (TKA) fixation has shown comparable long-term outcomes to cemented fixation, questioning the current indications for this fixation. The purpose of this study was to observe 1) patient factors associated with undergoing cemented vs cementless TKA and 2) the change in utilization of cementless TKA from 2015 to 2021.
Methods
A retrospective analysis of patients undergoing cemented and cementless TKA fixation from 2015 to 2021 was conducted using the PearlDiver Database. Patient factors were compared based on TKA fixation type. Subsequently, the percentage utilization of cementless fixation as the TKA fixation type was observed for each year to observe the percentage change for all patients as well as for different sub-groups. Of the 618,157 patients who underwent TKA, 589,153 (95.3%) underwent cemented fixation and 29,004 (4.7%) underwent cementless fixation.
Results
Patients who underwent cementless fixation were significantly younger, more likely to be male, and significantly less likely to have osteoporosis, dementia, and chronic kidney disease when compared to those with cemented fixation (p<0.05 for all). From 2015 to 2021, the utilization of cementless TKA significantly increased by 242%. For all age groups, insurance types, regions, sex, and comorbidities, the utilization of cementless TKA significantly increased (p<0.05 for all).
Discussion
Although the utilization of cementless fixation significantly increased in all patient populations, there is still no consensus on when to cement and in whom. Clinical practice guidelines are needed to ensure safe and effective utilization of cementless fixation.
#8306
Modular Tibial Stems in Primary Total Knee Arthroplasty, a High-Volume Arthroplasty Institution’S Protocol
*Mohamed Elkabbani - Mansoura University - Mansoura, Egypt
Amr Osman - Burjeel Hospital for Advanced Surgery - Dubai, United Arab Emirates
Ehab Elbaz - Sheikh Khalifa medical city Ajman - ajman, United Arab Emirates
Mohamed ADI
Samih Tarabichi - Dubai, UAE
Diego Zamagni - ETH Zurich - Zurich, Switzerland
*Email: Mohamedalkappany@daad-alumni.de
Background
Stems are intramedullary extensions of either the femoral or tibial component of a total knee arthroplasty with several suggested biomechanical advantages such as decreased risk of aseptic loosening, reducing micromotion, and resisting component tilting and liftoff. However Such benefits come at the expense of some drawbacks, including stress shielding, possibility of periprosthetic fracture, complicated revision, and pain of the stem tip.
While TKA stems are largely reserved for revision TKA, their indications in primary TKA are still debatable. The aim of this study is to review and discuss the indications and outcomes of various tibial components stems in primary total knee arthroplasty.
Methods
In this study, we retrospectively reviewed radiographic and clinical outcomes of 30 primary TKA patients implanted with modular stemmed tibial components and followed up for a minimum of 24 months in a high volume arthroplasty institution. Demographic data (age, sex, BMI, primary diagnosis, and outcome measures including oxford knee score), and any revisions were identified.
Results
There was no incidence of tibial radiolucent lines, excellent functional outcomes, and no complications associated with stem modularity at time of last follow up.
Conclusions
While there is currently no consensus on using stems in primary total knee arthroplasty, short stem extensions are attractive options in selected primary TKR patients that may provide a survival benefit in complex primary TKA while allowing for less bone removal, no cortical bone contact, and easier future revisions if needed.
#8337
FE Study on the Influence of Patient-Related Factors on the Primary Fixation of a Cementless PEEK Tibial Component
*Corine Post - Radboud university medical centre - Nijmegen, Netherlands
Thom Bitter - Raboud University Nijmegen Medical Centre - Nijmegen, Netherlands
Adam Briscoe - Invibio Ltd - Lytham St. Annes, United Kingdom
Nico Verdonschot - Radboudumc - Nijmegen, Netherlands
Dennis Janssen - Radboud University Nijmegen Medical Centre - Nijmegen, Netherlands
*Email: Corine.Post@radboudumc.nl
Introduction
The use of polyetheretherketone (PEEK- OPTIMATM) for the cementless tibial component is of interest because of its possible solution to avert peri-prosthetic bone resorption due to stress-shielding. A new material for the cementless tibial component may have implications for the primary fixation, quantified by the micromotions on the bone-implant interface. These micromotions may be influenced by patient-related factors. Therefore, the aim of this finite element (FE) study was to investigate the influence of patient-related factors, including sex, age and BMI, on the resulting micromotions of a cementless PEEK tibial component.
Methods
In this study, CT-scans of 74 healthy knees were included along with the patient information on sex, weight, length and age. Consecutively, a workflow was created to develop FE models of these 74 tibiae including tibial tray and insert. The tibial tray was simulated with a Young’s modulus for either PEEK (3.7 GPa) or titanium (109 GPa). A musculoskeletal model was used to derive the implant-specific tibiofemoral contact forces including centers of pressure of a gait and squat activity. The contact forces were incrementally applied during four loading cycles. To quantify the primary fixation, the 95th percentile of the maximum resulting micromotions was defined.
Results
The largest resulting micromotions were found at the anterior side and posterior lateral side of the tibial tray (Figure 1). The PEEK components generated significantly larger micromotions than the titanium components (mean PEEK: 67.63 µm, mean titanium: 38.69 µm, p<0.001). No significant differences in micromotions were seen between the sex and age groups (Figure 2). Higher BMI resulted in larger resulting micromotions (Figure 2). The difference between all three BMI groups was statistically significant (p<0.001).
Conclusion
The current FE study indicates that a higher BMI results in larger micromotions, while variations in sex and age did not significantly influence the primary fixation. The current analysis provides some preliminary insights on primary fixation of cementless TKA components. We are currently performing a more in-depth multivariate analysis to identify the underlying mechanisms, such as interactions with bone quality. In addition, we plan on performing a more detailed analysis of outliers to investigate potential risk factors.
Acknowledgements
PEEK-OPTIMATM is a trademark of Invibio Ltd. Implant geometry was supplied by Maxx Orthopaedics Inc.
Figures
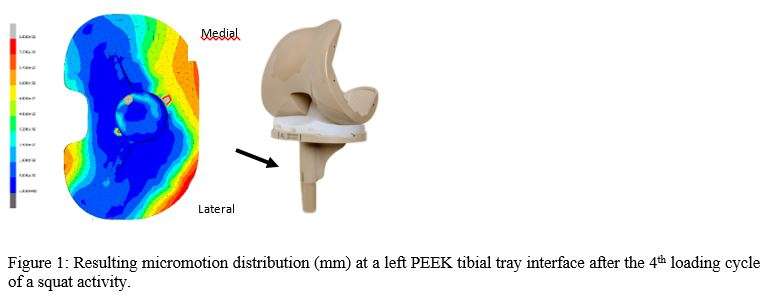
Figure 1
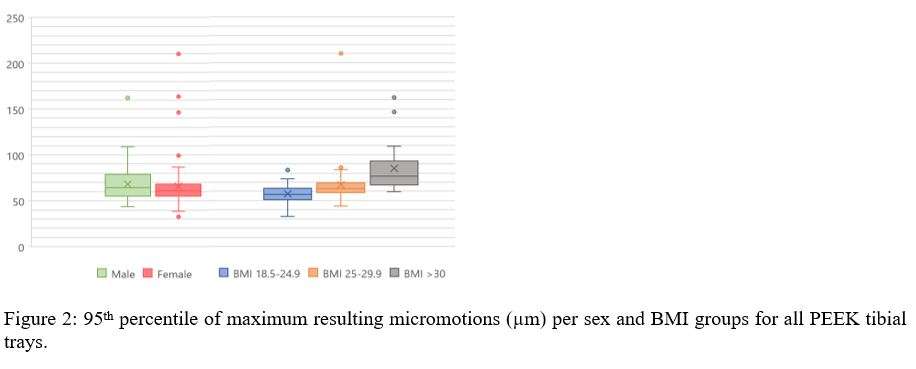
Figure 2#8548
Monocryl Sutures Lead to Less Wound Drainage Following Total Knee Arthroplasty: A Cadaveric Study
*Lucas Voyvodic - Brooklyn, United States of America
Ariel Rodriguez - Maimonides Medical Center - Brooklyn, United States of America
Ivan Golub - Maimonides Medical Center - Brooklyn, USA
Chaim Miller - Maimonides Medical Center - Brooklyn, USA
Che Hang Jason Wong - Maimonides Medical Center - Brooklyn, USA
*Email: lucasvc129@gmail.com
INTRODUCTION:
Wound drainage is a complication that has been associated with worse outcomes following total knee arthroplasty, including periprosthetic joint infection (PJI) and subsequent need for revisions. Wound drainage is a complication that has been associated with worse outcomes, including periprosthetic joint infection (PJI) and subsequent need for revisions. The purpose of this study was to determine which closure results in the least amount of wound drainage, and theoretically, lead to a decrease in wound complications.
METHODS:
A standard midline incision for TKA approach was performed on twenty cadaveric knee specimens. The wounds were closed with either staples and dermabond or monocryl only. The dressing consisted of five 4”×8” gauze sponges were stacked directly on top of one another regardless of study group. The knees were then injected with 150mL of methylene blue. Each layer of gauze was examined by illuminating the gauze through a glass panel, placing a graph paper over it, and recording the number of boxes of graph paper overlapping any drainage stain on the gauze to quantify the volume of drainage left in the dressing from each wound. This technique for measuring wound drainage was developed by El-Gazzar et. al when they studied the use of Dermabond following TKA.
RESULTS:
The group of specimens closed with staples and Dermabond were found to have significantly more wound drainage than the group closed using Monocryl sutures (102.11 graph units vs 18.44 graph units: p-value=0.0254).
CONCLUSION:
This study compared two closure techniques on cadaveric specimens with standard midline TKA incisions. The results showed that wounds closed with staples and Dermabond drained more than those closed with Monocryl sutures alone. This result should be used by surgeons when determining closure technique to minimize the risk of wound complications.
#8387
Development and Validation of a Mechanically Fidelic Knee Surgical Training Model
*Kieran Bennett - Flinders University - Adelaide, Australia
Parham Foroutan - Flinders University - Adelaide, Australia
Ella Fairweather - Flinders University - Adelaide, Australia
Sammuel Sobey - Fusetec - Adelaide, Australia
Nick Litchfield - Fusetec - Adelaide, Australia
Mark Roe - Fusetec - Adelaide, Australia
Karen Reynolds - Flinders University - Adelaide, Australia
John Costi - Flinders University - Adelaide, Australia
Mark Taylor - Flinders University - Adelaide, Australia
*Email: kieran.bennett@flinders.edu.au
Introduction
Current knee surgical training models provide anatomically correct bony geometry but often lack functional biomechanical accuracy1. This inaccuracy has resulted in limited opportunities for surgeons to practice techniques like computer navigated total knee arthroplasty, which relies on measurements of joint mechanics. In this work, we aimed to design, manufacture, and experimentally validate a knee surgical training model to reproduce the flexion dependent varus-valgus (VV) and anterior-posterior (AP) mechanics of cadaveric knees, while maintaining anatomic accuracy. The design and validation were undertaken using a combination of probabilistic finite element modelling (FEM) and mechanical testing in a six degree-of-freedom (6DOF) hexapod robot.
Methods
The knee FEM was based on an adult male, and included a reduced ligament set (lateral and medial collateral, anterior and posterior cruciate, and the anterolateral ligament). These ligaments were modelled as point-to-point linear springs, and all bones were modelled as rigid bodies. Latin hypercube sampling was used to adjust the reference strain (slack length) of each ligament to explore the range of manufacturing tolerances that may be produced.
Seven physical knee models were manufactured using a J750 Digital Anatomy 3D Printer (Stratasys, Rehovot, Israel). The proximal femur and distal tibia were embedded into aluminum potting cups to match the orientation and alignment of the FEM. The potted knee models were mounted in a custom-built 6DOF hexapod robot with knee kinematic axes aligned with the robot’s axes.
In both the FEM and hexapod experiments, knee kinematics were measured at extension, mid-flexion, and full flexion. At each flexion angle: 1) all off-axis shear forces and moments were minimized using an adaptive load control algorithm. 2) 20 N of superoinferior (SI) tensile force was applied to ensure the ligaments were taught. 3) (a) ±10 Nm VV moment was applied with all other rotation and translation axes (except SI translation) constrained; (b) ±100 N AP load was applied with all three rotations axes, and mediolateral displacement constrained.
Results
The FEM 5-95% boundary shows general agreement with cadaveric data for both VV and AP loading at all three flexion angles (VV mechanics in extension shown in Figure 1). The manufactured models reproduced the overall constraint predicted by the FE models and were within the bounds of the cadaveric data. Four of the seven manufactured models showed greater varus rotations due to applied load, and two showed greater valgus rotations, when compared to both the FEM results and cadaveric data.
Conclusion
This research has developed a knee surgical training model that can replicate and constrain the AP and VV motions in cadavers, reducing the dependence on cadaveric specimens.
References
1. Clifton W et al, Clinical Anatomy, 33:428-430, 2020
Figure Label
Figure 1. Comparisons between the 5-95% FEM, seven manufactured models (M1-M7), and the mean and standard deviation cadaveric knees for varus (-ve) – valgus (+ve) moment-rotation profile in the extended position.
#8153
A Novel Hybrid Bicruciate Retaining Total Knee Arthroplasty Tibial Component Design
*Diego Zamagni - ETH Zurich - Zurich, Switzerland
Mohamed Elfekky - Dubai Health Authority - Dubai, United Arab Emirates
Samih Tarabichi - BURJEEL HOSPITAL FOR ADVANCED SURGERY - Dubai, United Arab Emirates
*Email: dzamagni97@gmail.com
More than 20% of total knee replacement (TKR) patients reveal to be unsatisfied by their implant; an unacceptably high rate nowadays, compared to the 7% dissatisfaction rate of total hip arthroplasty (THA). The main underlying reasons of this failure can be attributed to abnormal kinematics, poor proprioceptive outcomes and discomforts associated to the current standard arthroplasties. While in the latter the anterior cruciate ligament (ACL) is sacrificed, a diametrically opposed approach called bicruciate retaining (BCR) TKR spares both cruciate ligaments. Although this anatomical approach is supported by many publications in terms of knee motion, patient preference and joint feeling, it failed to solidly establish on the market, mainly due to design flaws and a highly challenging surgical procedure. The contemporary BCR implants that should solve the problematics related to both ACL-sacrificing and old BCR designs are still far from this achievement, according to their early clinical results. Further design improvements are therefore required, especially in the tibial implant and polyethylene (PE) bearings, representing the most problematic components of BCR TKAs, with the first often associated to loosening and anterior fatigue breakage, whereas the second to dissociation and wear. In this direction, a novel BCR TKA tibial component was designed in 3D Studio Max software, by Autodesk. Each single feature was created upon a meticulous design rationale and conceived to overcome the past and modern BCR designs flaws and weaknesses. As a result, a revolutionary hybrid BCR TKA design was devised, featuring a mobile bearing (MB) lateral compartment and a fixed bearing (FB) medial, opposed to the current TKRs offering a single, unique, either MB or FB, approach for both knee condyles [Fig. 1, 2]. In this regard, an innovative “hybrid bearing interface” was designed with a strengthened partial peripheral lock on a flat medial side and a convex bearing-guiding wall on a domed lateral tibial plateau. Such a hybrid configuration reflects the compartmental disparity of the human knee and recreates the natural medial stability and lateral mobility of the joint. Moreover, when used in combination with fixed medial pivot and mobile lateral biconcave inserts, it reproduces a ball-in-socket femorotibial articulation, which, as in THA, leads to maximum implant stability and minimal PE wear. In addition to this breakthrough, several improvements were introduced to the contemporary BCR designs, including an anatomical, cruciate-friendly central cutout and spiked hexagonal pegs along with an anterior hemispherical component to provide robust immediate and long-term cementless tibial fixation [Fig. 2, 3]. In conclusion, although in silico, laboratory and clinical tests are needed to prove its theoretical advantages over the current implants, our novel hybrid BCR TKA design could represent a decisive turning point in the total knee replacement field, finally closing the gap between TKA and THA once and for all.
Figures

Figure 1

Figure 2

Figure 3#8538
Evaluation of an Initial Robotic-Assisted Direct Anterior Approach Cohort Receiving a Collared Stem Implant: Short-Term Results
*Laura Yanoso-Scholl - Stryker Orthopaedics - USA
Kevin Marchand - Ortho Rhode Island - South County, USA
Kelly Taylor - Ortho Rhode Island - South County, USA
Daniel Erwin - Ortho Rhode Island - Wakefield, USA
Robert Marchand - Ortho Rhode Island - South County, USA
Zachary Guerrieo - Ortho Rhode Island - Wakefield, USA
*Email: Laura.Scholl@stryker.com
INTRODUCTION: Metaphyseal filling, collared stems have become popular with direct anterior approach (DAA), based on their ease of lateralization and insertion through less-invasive surgical exposures. To aid with DAA, robotic-assisted technology provides 3D-CT preoperative planning and intraoperative guidance to accurately assess stem version. With other femoral stems, this has been shown to provide more accurate implant planning and improved patient outcomes. The purpose of this study was to understand predictability and patient outcomes for a collared stem system through a DAA in combination with a robotic-assisted system during a surgeon’s initial cases.
METHODS: A high-volume surgeon, experienced with robotic-assisted DAA, adopted use of a collared metaphyseal filling stem. Intra-operative data and patient outcomes out to 6-months post-operative were collected prospectively during the surgeon’s first 75 cases. A Student’s t-test (α=0.05) was used for statistical comparisons.
RESULTS: Patients’ profile: 67% female, 53% right hips, average age of 68.6yrs ± 9.3, average BMI of 28.6 ± 6.7 and average ASA score of 2.3 ± 0.5. 22.6% of femurs were Type A, 69.4% Type B, and 8.1% Type C.
Intra-operative assessment reported average stem version, estimated by visual assessment, was 13.25 ± 3.81° compared to 15.67 ± 7.94° measured by the robotic system where average difference between the measurements was 5.41 ± 4.38° (p<0.05). At final broach, the broach sat at the calcar level for 92% of cases and after stem impaction, the stem sat at the calcar level for all but three cases. For those cases, the surgeon confirmed the stem was stable before completing the case. For all cases, the surgeon reported broaching effort like a traditional tapered wedge stem broach system for a tapered wedge stem.
No periprosthetic fractures were recorded. Compared to pre-operative, patients had statistically significantly improved reduced-WOMAC, PROMIS10 Mental, and HOOS JR scores at 6-months post-operative. Although improved, PROMIS10 Physical was not statistically significant. 6-months post-operative, average FJS was 80.9 ± 25.2, 64% reported operative hip felt like a natural joint, 7% reported it felt like an artificial joint with no restriction, and 29% reported it felt like an artificial joint with minimal restrictions.
DISCUSSION: Literature has shown the use of robotic-assisted technology is more accurate in predicting femoral version compared to a surgeon’s visual assessment.4 Results from this study support that finding. It was found that visual estimation values were improved compared to those reported in literature and this is potentially due to the collar on the medial side of the stem that may serve as a visual guide to help the surgeon estimate femoral version.
Dorr Type A femurs have been associated with fracture if exposed to excessive impaction. It was encouraging to find, after fully impacting the stem in Type A femurs, the stem sat at the calcar level for all cases and there were no reports of femoral fractures. 6-months post-operative, patients had significant improvement in outcomes with all patients reporting minimal to no restrictions with their new joint. These patients will continue to be followed as they reach further timepoints.
#8338
Improved Short-Term Outcomes for a Novel, Fluoroscopy-Based Robotic-Assisted Total Hip Arthroplasty System Compared to Manual Technique With Fluoroscopic Assistance
Graham Buchan - Cleveland Clinic Lerner College of Medicine - Cleveland, United States of America
Christian Hecht - Cleveland Clinic - Cleveland, USA
Peter Sculco - Hospital for Special Surgery - New York, USA
James B Chen - Hospital for Special Surgery - New York, USA
*Atul Kamath - Cleveland Clinic - Cleveland, USA
*Email: kamatha@ccf.org
Introduction: While robotic-assisted total hip arthroplasty (RA-THA) has been associated with improved accuracy of component placement, the perioperative and early postoperative outcomes of fluoroscopy-based RA-THA systems have yet to be elucidated. Our present study sought to elucidate the early outcomes associated with fluoroscopy-based RA-THA focusing on hospital stay, time to discharge, and outcomes during the first 90 days after surgery.
Methods: This retrospective cohort analysis included a consecutive series of patients who received manual, fluoroscopy-assisted THA (mTHA) and fluoroscopy-based RA-THA at a single institution. Our primary outcome of interest were rates of complications within 90 days of surgery. Secondary outcomes includes length of hospital stay (LOS), discharge disposition, and postoperative visual analog scale (VAS) pain scores. Inclusion criteria for this study were patients greater than 18 years of age who underwent primary unilateral direct anterior approach (DAA) THA by the primary study surgeon at a single institution. All patients received THA for a diagnosis of osteoarthritis, avascular necrosis, or rheumatoid arthritis.
Results: No differences existed between groups with respect to demographic data or perioperative recovery protocols. The RA-THA cohort had a significantly greater proportion of outpatient surgeries compared to the mTHA cohort (37.4% vs. 3.8%; p<0.001) (Figure 1) and significantly lower LOS (26.0 vs. 39.5 hours; p<0.001). There were no differences in baseline pain scores (6.4 vs. 6.1; p=0.296), highest in-hospital pain scores (7.6 vs. 8.1; p=0.105), and average in-hospital pain scores (4.8 vs. 4.9; p=0.416) between the RA-THA and mTHA study cohorts. The RA-THA cohort had significantly lower patient-reported VAS pain scores at 2-week follow-up visits (2.5 vs. 3.3; p=0.048), but no difference was seen after 6-week follow visits (2.5 vs. 2.8; p=0.468) (Table 1). There were no significant differences in discharge disposition or lengths of stay in SNF/IRFs for patients discharged to rehabilitation facilities. No major complications, including fractures, dislocation, joint infections, or surgical revisions, were detected in the study sample within the 90-day postoperative period. There was no significant difference in rates of minor complications between the RA-THA and mTHA cohorts (1.9% vs. 6.7%; p=0.083) during the 90-day postoperative period (Table 2).
Conclusion: Fluoroscopy-based RA-THA demonstrates low rates of postoperative complications, improved post-operative pain profiles, and increased outpatient procedures when compared to manual, fluoroscopy-assisted THA. Given the low rates of complications and shortened LOS, these results demonstrate that the introduction of fluoroscopy-based RA-THA is safe within the 90-day postoperative period.
Keywords: total hip arthroplasty (THA); outcomes; robotic-assisted surgery; fluoroscopy; robotic THA; hip replacement
Figures
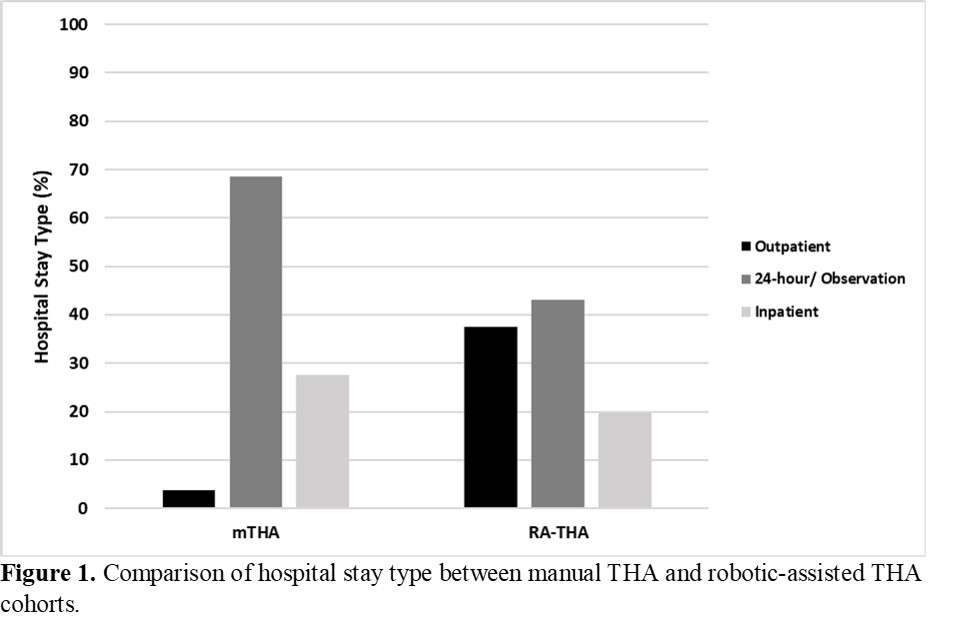
Figure 1
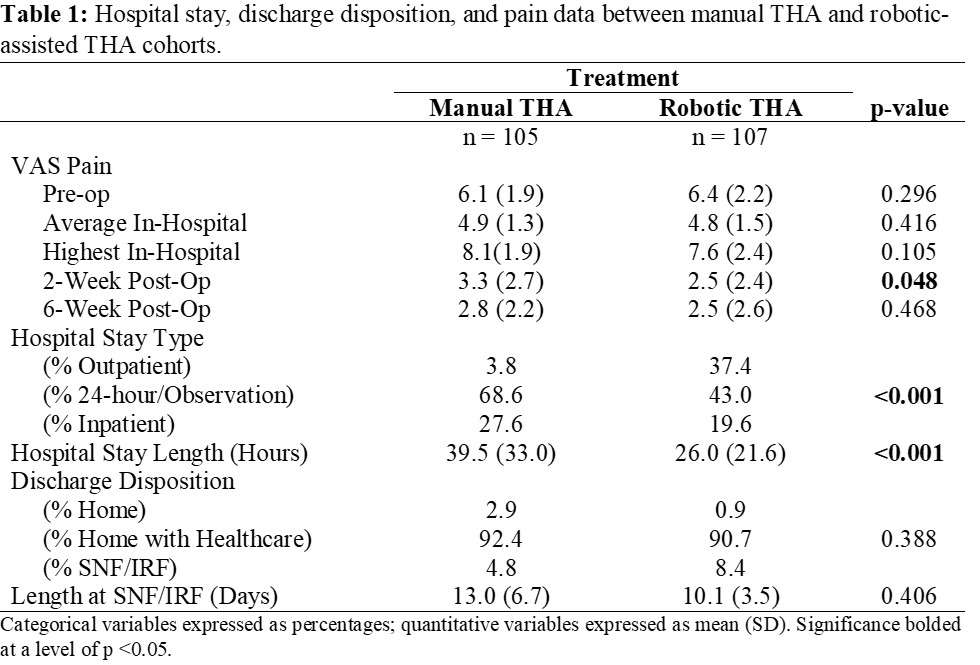
Figure 2
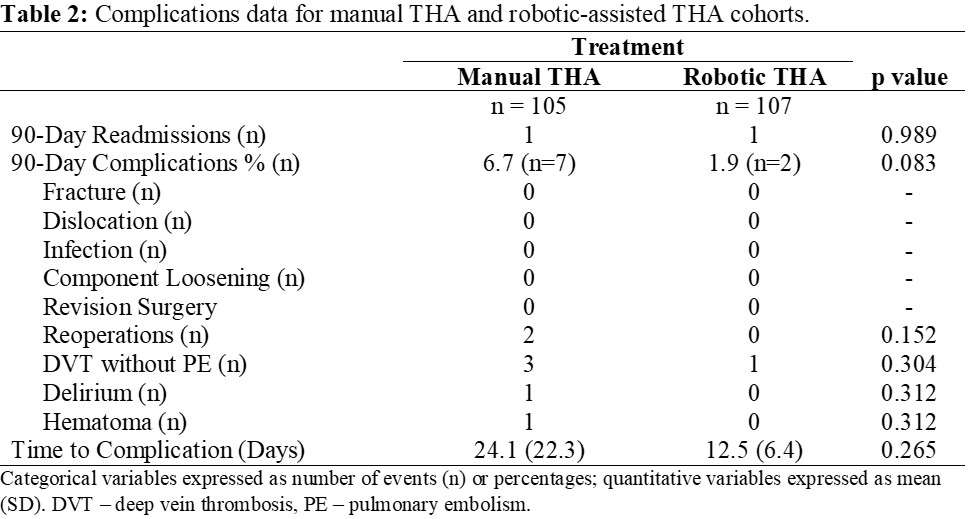
Figure 3#8208
Length of Stay and Discharge Dispositions Following Robotic-Arm Assisted Total Hip Arthroplasty and Predictors of Delayed Discharge.
*Andreas Fontalis - University College London Hospitals - London, United Kingdom
Warran Wignadasan - University College London Hospital - London, United Kingdom
Fabio Mancino - University College London Hospitals NHS Foundation Trust - London, United Kingdom
Crystallynn Skye The - University College London Hospitals NHS Foundation Trust - London, United Kingdom
Ricci Plastow - University College London Hospitals NHS Foundation Trust - London, United Kingdom
ahmed magan - University College London Hospital - London, GB
Fares Haddad - University College London Hospital - London, United Kingdom
*Email: andreasfontalis@gmail.com
Background
Post-operative length of stay (LOS) and discharge dispositions following arthroplasty could act as surrogate measures for improvement in patient pathways. With the increasing use of robotic technology globally in arthroplasty, it is vital to analyse its impact on LOS. The aims of our study were to compare LOS and discharge dispositions following robotic-arm assisted Total Hip Arthroplasty (RO-THA) with the conventional technique (CO-THA) and identify predictors of delayed discharge.
Methods
This large-scale, single institution study included patients of any age undergoing primary THA (N = 2,031) for any cause between May 2019 and January 2023 at a tertiary centre. Data collected included patient demographics, LOS, type of anaesthetic used, need for Post Anaesthesia Care Unit (PACU) admission, readmission within 30 days of hospital discharge and discharge dispositions. Univariate and multivariate logistic regression models were also utilised to identify factors and patient characteristics associated with delayed discharge.
Results
The median LOS in the RO-THA group was 54 hours (34, 78) versus 60 (51, 100) in the CO-THA group, p<0.001. Discharge dispositions were comparable between the two groups. A higher proportion of patients undergoing CO-THA required PACU admission post-operatively (7.2% versus 5.2%, p = 0.238), however this did not reach statistical significance. Our multivariate model demonstrated that age, female gender, need for admission into PACU, ASA>2 and utilisation of CO-THA were associated with a LOS >2 days.
Conclusion
Our study showed that robotic-arm assistance was associated with a shorter LOS in patients undergoing primary THA and no difference in the discharge destinations. Our results indicate that robotic-arm assistance could be beneficial in partly tackling the increased demand for hip arthroplasty procedures and the associated health care burden; however, this needs to be further validated by long-term cost effectiveness analyses and data from randomised controlled studies.
#8293
The Effect of Surgical Approach on the Outcomes of Outpatient Total Hip Arthroplasty at a Single Ambulatory Surgery Center in New England
*Jacob Laperche - Frank H. Netter School of Medicine Quinnipiac University - Middletown, United States of America
James Dove - Brown University Department of Orthopaedics - Providence, USA
Valentin Antoci - University Orthopedics - Providence, USA
Eric Cohen - University Orthopedics - Providence, USA
Michael Kutschke - Brown University Orthopaedics Department - Providence, USA
David Painter - Warren Alpert Medical School Brown University - Providence, USA
Alexander Homer - Warren Alpert Medical School Brown University - Providence, USA
*Email: jacoblaperche@gmail.com
Introduction: Primary total hip arthroplasty (THA) is increasingly being performed in the outpatient setting. Prior studies have suggested that the direct anterior approach (DAA) has earlier mobilization and decreased pain in the early post-operative period compared to anterolateral (AL) and posterolateral (PL) approaches suggesting it is the ideal approach for the outpatient setting. However, there is little known regarding the differences in same-day discharge rates and complications of total hip approach in same-day total hip arthroplasty in the ambulatory surgery center (ASC) setting.
Objective: To determine the effects of THA approach on the same-day discharge rates, readmissions, and complications in a single ASC.
Methodology: A retrospective chart review was performed between July 2019 and October 2021 for all patients who underwent primary THA in a single ASC. Successful same-day discharge, surgical approach, length of surgery, estimated blood loss (EBL), complications, and readmission events were recorded for each patient. Using SPSS version 28.0.1.1 complications were compared using Pearson Chi-Square while EBL and surgery length were compared with one-way ANOVA (α=0.5).
Results: A total of 326 total hip arthroplasties were included in this study. There were 17 total complications (5.2%), including direct admissions to the emergency department, wound complications, instability, infection, and revision surgery. Among all complications there were 5 direct admissions, making the successful same-day discharge rate 98.5%. Complications were not associated with approach with 8 complications (4.2%) from the DAA, 3 complications (4.5%) from the AL, and 6 (8.6%) from the PL approach. Direct admissions were not associated with approach with 1 (0.5%) from the DAA, 3 (4.5%) in the AL approach, and 1 (1.4%) in the PL approach. Readmission rates were associated with approach with 0 readmissions in the DAA and AL cohorts and 3 (4.3%) in the PL cohort.
Conclusion: In the ASC setting, patients undergoing THA regardless of approach showed no difference in successful same-day discharge or complications. Same-day THA can be safely performed in the DAA, AL, and PL approach to the hip.
Figures
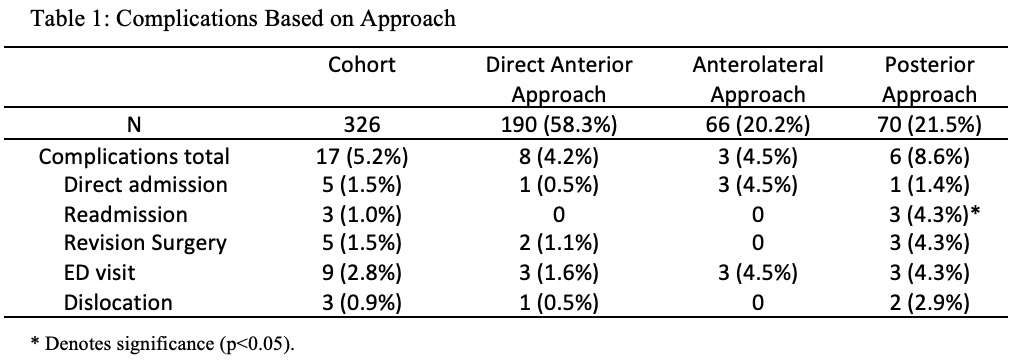
Figure 1#8597
Length of Stay and Discharge Dispositions After Total Hip Arthroplasty: A Propensity Matched Comparison of Robotic Assisted and Manual Techniques
*Laura Yanoso-Scholl - Stryker Orthopaedics - USA
Diviya Rajesh - Harvard - Boston, USA
Sietske Witvoet - Stryker - Amsterdam, Netherlands
Andrea Coppolecchia - Stryker - Mahwah, USA
Antonia Chen - Brigham and Women's Hospital - Boston, USA
*Email: Laura.Scholl@stryker.com
Introduction: Robotic technology using preoperative 3D CTs continues to gain popularity in the field of total hip arthroplasty (THA) for its potential to improve accuracy of component placement and patient outcomes. Nonetheless, there is minimal data around the impact of robotic-assisted THA (RA-THA) on hospital length of stay (LOS) and discharge location using a large patient cohort. The purpose of this study was to compare LOS, discharge locations, and readmission rates for propensity matched cohorts of RA-THA vs. manual THA (M-THA).
Methods: A retrospective review of a multihospital database was performed to identify patients who underwent THA between January 2016 and December 2021 from surgeons who performed both RA-THA and M-THA techniques at 77 geographically diverse hospitals within the US (both rural and urban locations). To account for covariates, RA-THA and M-THA cohorts were 1-to-1 matched based on patient gender, age groups (<50, 50-65, 65-80, >80y), and body mass index (BMI) groups (<20, 20-25, 25-30, 30-35, 35-40, >40kg/m2) resulting in 8,536 patients in each cohort (N=17,072). LOS, same day discharge (<23 hours), discharge disposition and 90-day all-cause readmission rates were compared between cohorts. Cohorts were statistically compared using Mann-Whitney U and Chi-squared tests.
Results: Of the patients who underwent M-THA, 54.1% were female and mean age and BMI were 66.3±10.4 years and 29.6±5.8kg/m2, respectively. In comparison, 53.3% of patients who underwent RA-THA were female and mean age and BMI were 66.2±10.4 years and 29.8±5.8kg/m2, respectively. Considering surgeon case volume, 65.9% of RA-THA cases were performed by a high-volume surgeon (>50 cases annually) versus 71.9% of M-THA cases. Mean LOS was significantly shorter for RA-THA patients (1.39±0.85 days) compared to M-THA patients (1.48±0.91 days, p<0.001, Figure 1). 5.3% of RA-THA patients and 5.6% of M-THA patients were discharged on the same day (LOS<23 hours, p=0.38). Of the RA-THA cases with same day discharge, 28.4% were done by a surgeon who did less than 50 cases annually versus 13.4% of M-THA cases. There were statistically significantly more RA-THA cases discharged home without home healthcare compared to M-THA (47.9% versus 45.5%, p=0.001). There were statistically significantly less RA-THA cases discharged to a skilled nursing facility (SNF) compared to M-THA (5.6% versus 6.9%, p=0.001). 3.0% of the RA-THA versus 3.4% of the M-THA cases had an all-cause 90-day readmission event (p=0.26).
Discussion: Compared to M-THAs, RA-THA procedures had a shorter average LOS, similar percentage of patients with a LOS<23h, higher percentage of patients discharged home without home healthcare, less patients with SNF discharge, and a similar all-cause 90-day readmission rate. These results may be of interest to surgeons who participate in bundled payment programs and engage in cost savings. Future analysis should compare other short-term outcome measures, such as complication rates, patient reported outcome scores, satisfaction, and total cost of care.
Figures
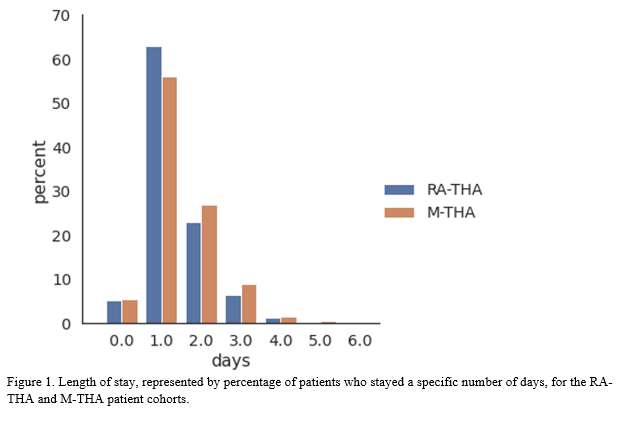
Figure 1#8202
Femoral Nerve Palsy Following Total Hip Arthroplasty via an Anterolateral Supine Approach
*Yuto Nishioka - Itami City Hospital - Itami, Japan
Atsunori Ohnishi - Itami city hospital - Itami, Japan
Takaaki Nakai - Itami city hospital - Itami, Japan
Tsuyoshi Nakai - Itami city hospital - Itami, Japan
*Email: nishiokay2020@gmail.com
Introduction: Femoral nerve palsy (FNP) is a relatively uncommon but devasting complication following total hip arthroplasty (THA). Although there is limited literature on FNP in THA, several studies have suggested that FNP was more common with anterior approach than posterior approach. This study aimed to investigate the incidence, risk factors, and prognosis of FNP after THA via anterolateral supine approach (ALS-THA).
Methods: Between January 2018 and August 2022, 913 patients with 1064 hip joints (165 men and 899 women, mean age 69.1 years old) who underwent primary ALS-THA at a single hospital were retrospectively investigated. Incidence of FNP was calculated, and association between BMI, operation time and intraoperative blood loss and FNP was evaluated. The course of the disease was also followed up.
Results: The 1064 cases included 966 with osteoarthritis of the hip, 49 with idiopathic osteonecrosis of the femoral head, 7 with rheumatoid arthritis, 12 with rapidly destructive hip arthritis, 6 with subchondral fragility fractures, and 24 with trauma. Five patients (0.49%) developed postoperative FNP. All patients were women, osteoarthritis, and left-side cases. The patients were a mean age of 70.8 years, mean BMI of 26.5, mean operative time of 61.6 minutes, and mean blood loss of 295 ml. The patients were found to have no knee extension between 1 and 6 days postoperatively, and two of them also had paresthesia of medial thigh. All patients were treated conservatively (taking Vit. B12 and low-frequency therapy), and all showed improvement to MMT levels of 3 to 5. The mean hospital stay was 54 days, and all patients were discharged home. In this study, all patients with FNP postoperatively were women but the difference was not statistically significant. Moreover, intraoperative blood loss was not significantly high, and anticoagulants were not regularly used in the all patients.
Conclusion: FNP after ALS-THA in our hospital was extremely rare and the incidence was 0.49%. But it causes weakness of the quadriceps muscles and sensory loss in the thigh, resulting in longer rehabilitation period and lower patient satisfaction. Although there were no obvious risk factors of FNP after ALS-THA in this study, the surgical procedure including a location of the intraoperative retractor could be one of them. Further investigation is needed.
#8746
Patient Reported Outcome Differences for Navigated and Robot-Assisted Total Hip Arthroplasty Frequently Do Not Achieve Clinically Important Differences: A Systematic Review
*Vinaya Rajahraman - New York, United States of America
Kyle Lawrence - NYU Orthopedic Hospital - New York, USA
David Bloom - NYU Orthopedic Hospital - New York, USA
Hayley Raymond - Langone Orthopedic Hospital - New York, USA
Casey Cardillo - NYU Orthopedic Hospital - New York, USA
Joshua Rozell - NYU Langone Orthopedic Hospital - New York, USA
Ran Schwarzkopf - NYU Langone Medical Center Hospital for Joint Diseases - New York, USA
Armin Arshi - New York University Langone Health - New York, United States of America
*Email: v.rajahraman@gmail.com
INTRODUCTION: Total hip arthroplasty (THA) using computer-assisted navigation (N-THA) and robot-assisted surgery (RA-THA) has been increasingly adopted to improve implant positioning and offset/leg length restoration. Whether clinically meaningful differences in patient reported outcomes (PROMs) compared to conventional THA (C-THA) are achieved with intraoperative technology has not been established. This systematic review aimed to assess whether published relative PROM improvements with technology use in THA achieved minimal clinically important differences (MCIDs).
METHODS: PubMed/MEDLINE/Cochrane Library were systematically reviewed for studies comparing PROMs for primary N-THA or RA-THA with C-THA as the control group. Relative improvement differences between groups were compared to established MCID values. Reported clinical and radiographic differences were assessed. Review of N-THA and RA-THA literature yielded six (N=2,580) and 10 (N=2,786) studies, respectively, for analysis.
RESULTS: Statistically significant differences in postoperative PROM scores were reported in two of six (33.3%) studies comparing N-THA with C-THA, though only one (16.7%) reported clinically significant relative improvements. Statistically significant differences in postoperative PROMs were reported in six of 10 (60.0%) studies comparing RA-THA and C-THA, though none reported clinically significant relative improvements. Improved radiographic outcomes for N-THA and RA-THA were reported in 83.3% and 70.0% of studies, respectively. Only one study reported differences in revision rates with technology use.
CONCLUSION: Reported PROM scores in studies comparing N-THA or RA-THA to C-THA often do not achieve clinically significant relative improvements. Future studies reporting PROMs should be interpreted in the context of validated MCID values to accurately establish the clinical impact of intraoperative technology.
Figures
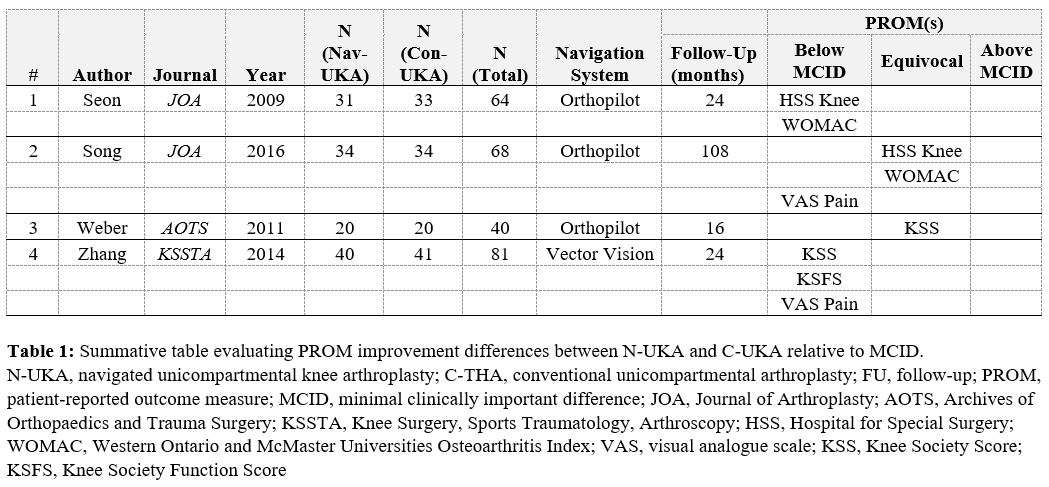
Figure 1
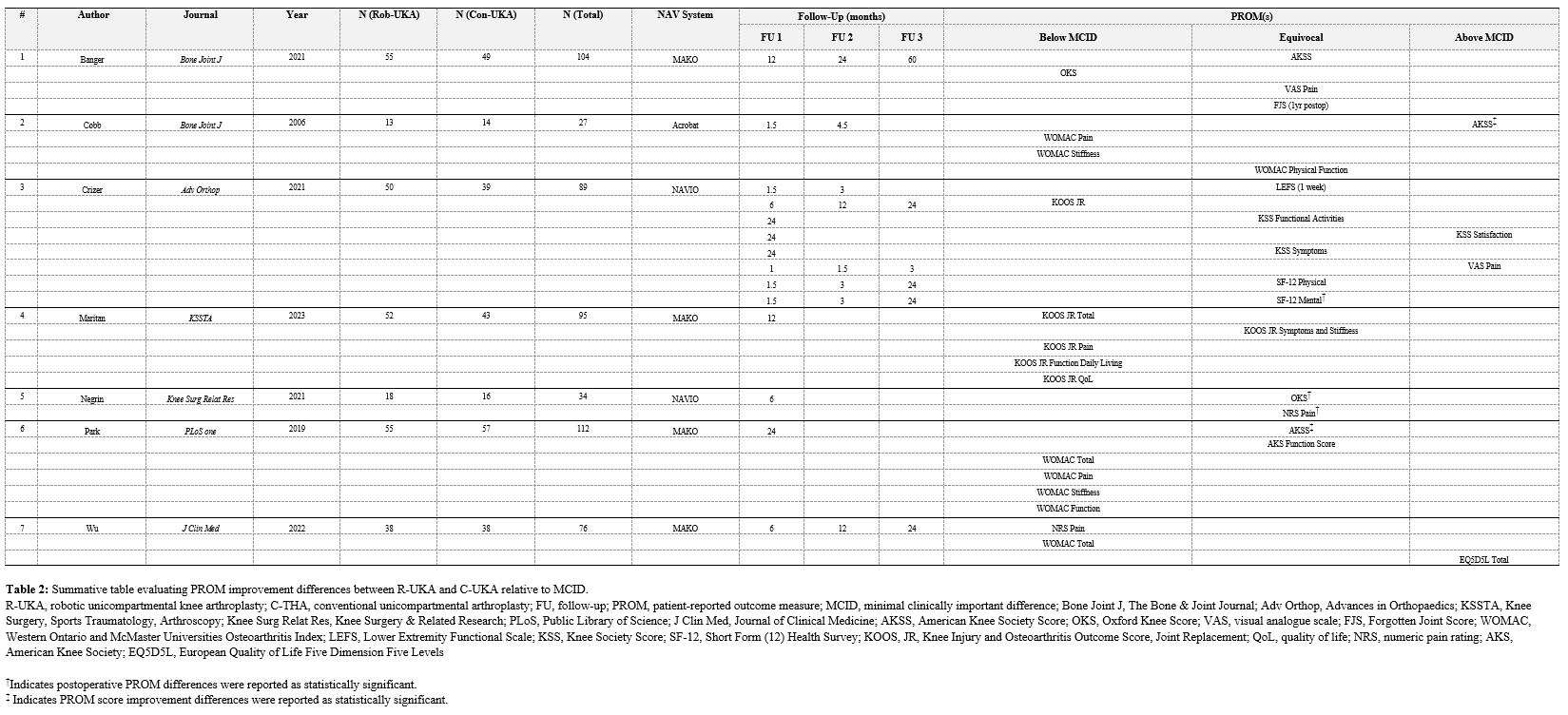
Figure 2
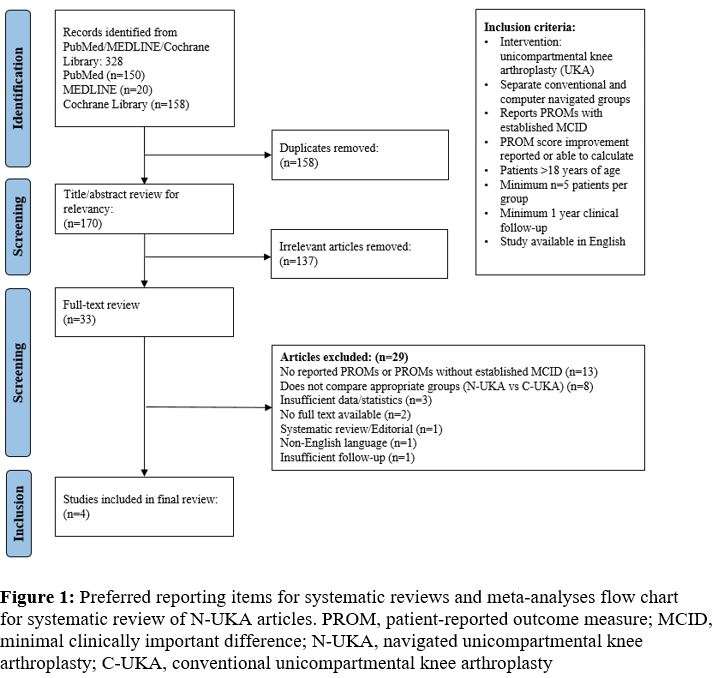
Figure 3
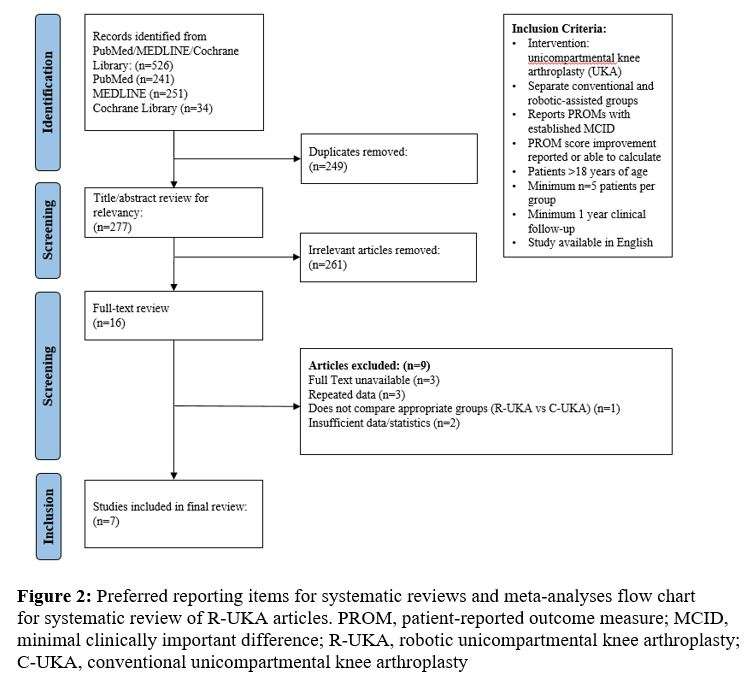
Figure 4#8753
Effect Technology Has on Short-Term Outcomes in Total Hip Arthroplasty
*Weston Buehring - New York, United States of America
Hayley Raymond - Langone Orthopedic Hospital - New York, USA
Alana Prinos - Langone Orthopedic Hospital - New York, USA
Omid Barzideh - NYU Orthopedic Hospital - New York, USA
Morteza Meftah - NYU Hospital for Joint Diseases - New York, USA
Matthew Hepinstall - NYU Langone Health - New York, USA
Patrick Connolly - NYU Langone Health - New York, USA
*Email: westonbuehring2013@gmail.com
Introduction
The utilization of technology in total hip arthroplasty (THA) has significantly increased over the years, particularly within our institution. However, conflicting data exists regarding the effect of robotic- and computer-assisted surgery (CAS) on short-term clinical outcomes. The aim of this study was to describe the effect of different surgical preferences on postoperative outcomes following THA.
Methods
13,268 patients who underwent primary, elective THA from January 2016 to December 2022 were retrospectively reviewed. Three cohorts were created based on the utilization of technology: robotics, manual (no technology), and CAS. Patient demographics and short-term outcomes data, including Activity Measure for Post-Acute Care (AM-PAC) scores, which is a validated tool used to quantify postoperative functional status, was collected and analyzed using ANOVA and multivariate logistic regressions.
Results
1402 robotic-assist (10.6%), 7213 manual (54.4%), and 4653 CAS (35.0%) cases were included in our analysis. Mean lengths of stay (LOS) favored robot-assisted over manual and CAS cases (42.04 vs. 67.71 vs. 42.31 (hours); p<0.001), as did patient discharge to home (95.5 vs. 87.9 vs. 93.1%; p<0.001). In addition, 30-day (1.4 vs. 2.3 vs. 2.0%; p=0.045) and 90-day readmission rates (3.1 vs. 4.5 vs. 4.1; p=0.021) were lowest in the robot-assisted cohort, as was rate of dislocation within 90-days (0.2 vs. 0.7 vs. 0.4; p<0.001). Multivariate analysis demonstrated use of robotics to be significantly associated with lower odds of 30-day (p=0.023) and 90-day readmission (p<0.001), independent from surgical approach, use of intraoperative fluoroscopy, dual-mobility implants, or baseline demographic characteristics. Multivariate analysis also showed patients had higher odds of achieving a perfect AM-PAC score on postoperative day 0 with robotic-assisted THA.
Conclusion
The present study demonstrates favorable short-term clinical outcomes, including shorter LOS, greater discharge to home, lower 30-day and 90-day readmission rates, and greater odds of achieving a perfect same day AM-PAC score, for patients receiving robot-assisted surgery compared to manual and CAS THA.
#8331
Collecting Long-Term (5 to 10-Years) Patient-Reported Outcome Measures May Be Unnecessary for Total Hip Arthroplasties
Pedro Rullan - Cleveland Clinic Foundation - Cleveland, USA
Ignacio Pasqualini - Cleveland Clinic Foundation - Cleveland, USA
Alison Klika - Cleveland Clinic - Cleveland, USA
Jianhua Shen - Stryker - Mahwah, USA
Manoshi Bhowmik-Stoker - Stryker Orthopaedics - Mahway, USA
*Emily Hampp - Stryker - Mahwah, USA
Nicolas Piuzzi - Cleveland Clinic - Cleveland, USA
*Email: emily.hampp@stryker.com
Introduction: The clinical relevance ratio (CRR) was developed to account for the loss of follow-up in clinical studies reporting patient-reported outcomes measures (PROMs). However, no study has tested its use with original outcome data for total hip arthroplasties (THA). Therefore, this study aimed to (1) determine the proportion of patients that had a clinically significant improvement in PROMs at each follow-up visit following THA; and (2) calculate the CRR over time for PROMs following THA.
Methods: Eightindependent studies reporting PROMs preoperatively and postoperatively out to 10 years for 2,540 patients who underwent primary THA in Europe or the United States were aggregated. 2,653 THAs performed from 1996 to 2021 were included. A distribution-based minimal clinically important difference (MCID) threshold was used to determine which patients had a clinically significant improvement in PROMs. The CRR was calculated by dividing the number of cases that met the MCID threshold by the number of cases at the beginning of the study. The maximum follow-up time was ten years.
Results: The proportion of THA patients that had a clinically significant improvement in PROMs at each follow-up visit is summarized separately for U.S. and E.U. studies. For U.S. studies, MCID attainment was higher for Harris Hip Score (HHS), Physical Composite Score (PCS), and European Quality of Life-5 Dimensions Questionnaire subscale for Time Trade-Off (EQ5DTTO) compared to other PROMs. For E.U. studies, MCID attainment was greatest for HHS and Oxford Hip Score (OHS). General health PROMs for EQ5D-Visual Analog Scale (VAS) and Mental Composite Score (MCS), as well as the Lower Extremity Activity Scale (LEAS), had the lowest percentages of score improvements. Overall, most improvements in PROM scores stabilized 1 year post-operatively (Figure 1). However, while the proportion of cases with clinically significant improvements in PROM scores for THA was stable after a short period of fluctuations at early follow-up visits, the CRR decreased remarkably over time (Figure 2). The tipping point where the CRR began decreasing for THA studies was mainly at the 1-year follow-up time point.
Conclusion: The clinical relevance ratio for PROMs decreases significantly after short-term follow-up periods for THA patients. Long-term PROM collection at 5 to 10 years and analysis may be unnecessary following THA. Arthroplasty surgeons should focus on 1-year PROMs to assess clinically significant improvements after THA.
Figures
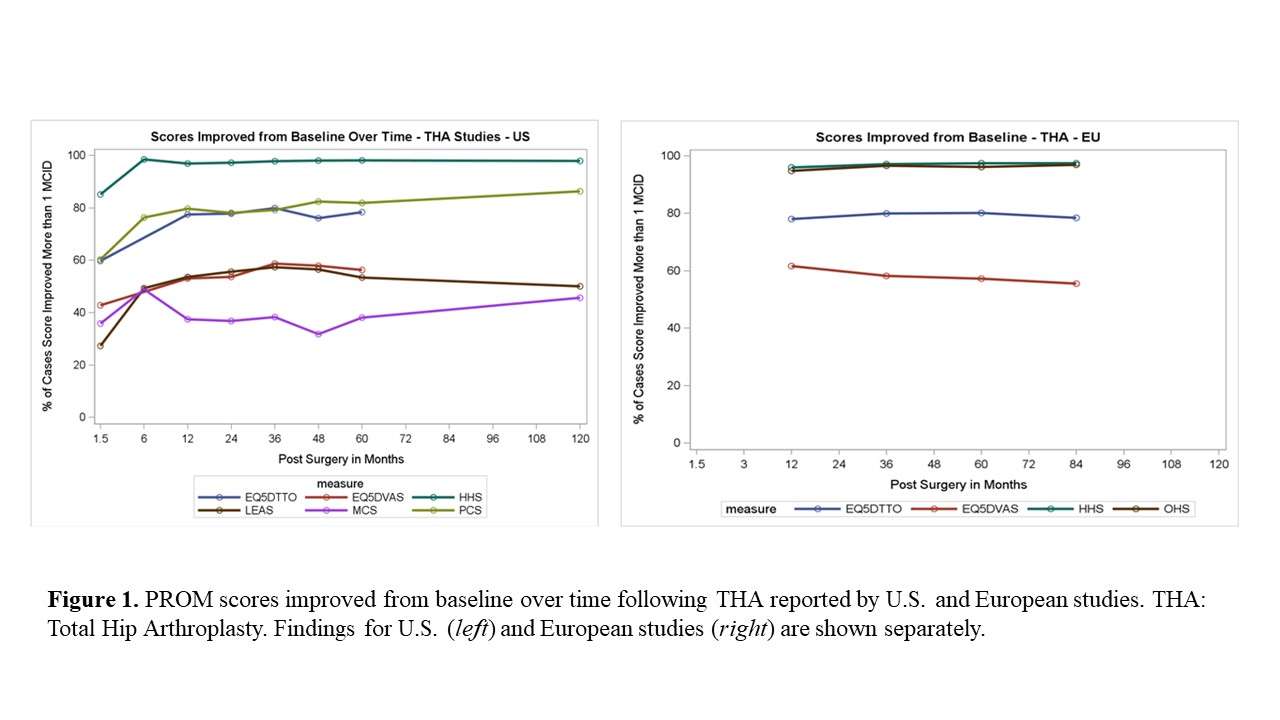
Figure 1
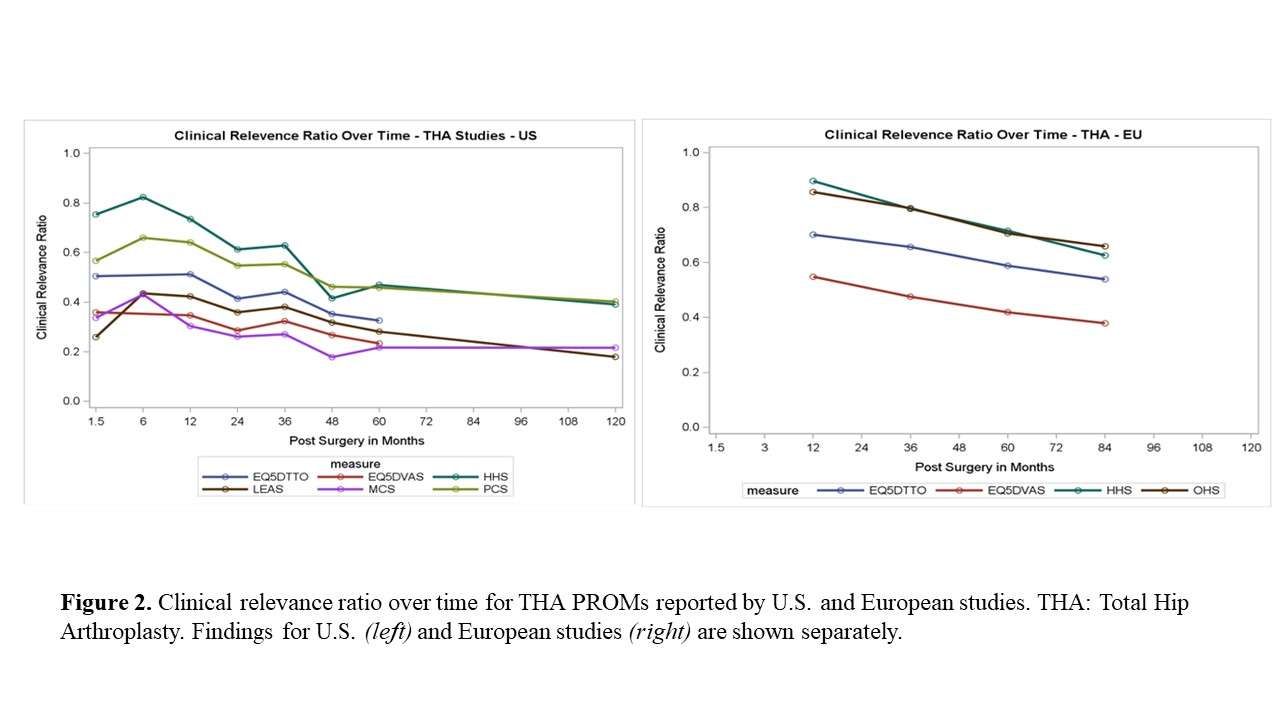
Figure 2#8648
A Novel Implant Planning Software Tool for Hip Stem Alignment
*Gokce Yildirim - Vent Creativity Corporation - Weehawken, USA
Rachel Alexander - Vent Creativity Corporation - Weehawken, USA
Dimitrios Georgopalis - Vent Creativity - Weehawken, USA
*Email: gyildirim@ventcreativity.com
Introduction: Hip replacement surgery often relies primarily on the coronal X-Ray of a patient for implant sizing and position. However, the final position of the stem may differ from the intended location both in the coronal plane, but also in axial and sagittal planes, creating a potential for leg length discrepancy, dislocation, or excessive wear of the articulating materials.
Methods: CT data is used to generate previously validated accuracy of both mesh and intensity point clouds models1. Eleven cadaver hips were CT scanned. Representative collared femoral hip stems were generated with enough sizing options to accommodate general surgical planning needs. Femoral scans were autolandmarked and autoplanned for neck cut planes by the previously validated system1. The head center, neck axis, anatomical femoral axis, and neck cut plane were used as initial conditions and the implant was placed automatically by the system into the bone canal. Once the initial placement is completed, an iterative optimization method was used to generate all possible implant sizes, and positions to preserve two major conditions: head center location ±5mm, and maximum stem surface contact with cortical bone with minimum interference. Finally, postop scans were autosegmented to generate the bone and implant models to compare to.
Results: Automated result replicated femoral head center, stem sizing and stem orientation in general agreement with surgical result according to the post plan manual analysis. 3D planning generated significant axial rotation from the initial conditions, reducing dense bone removal on posterolateral corner, while preserving head center and canal fit.
Conclusion: To date, 3D planning tools have required surgeon input; however, an automated surgical planning tool may provide value in efficient and repeatable fit evaluation for implant development or surgical planning. Limited data was available for this analysis. Further study is required. System requires longitudinal studies to determine the effectiveness of picking clinically successful outcomes along with the implant positions.
Figures
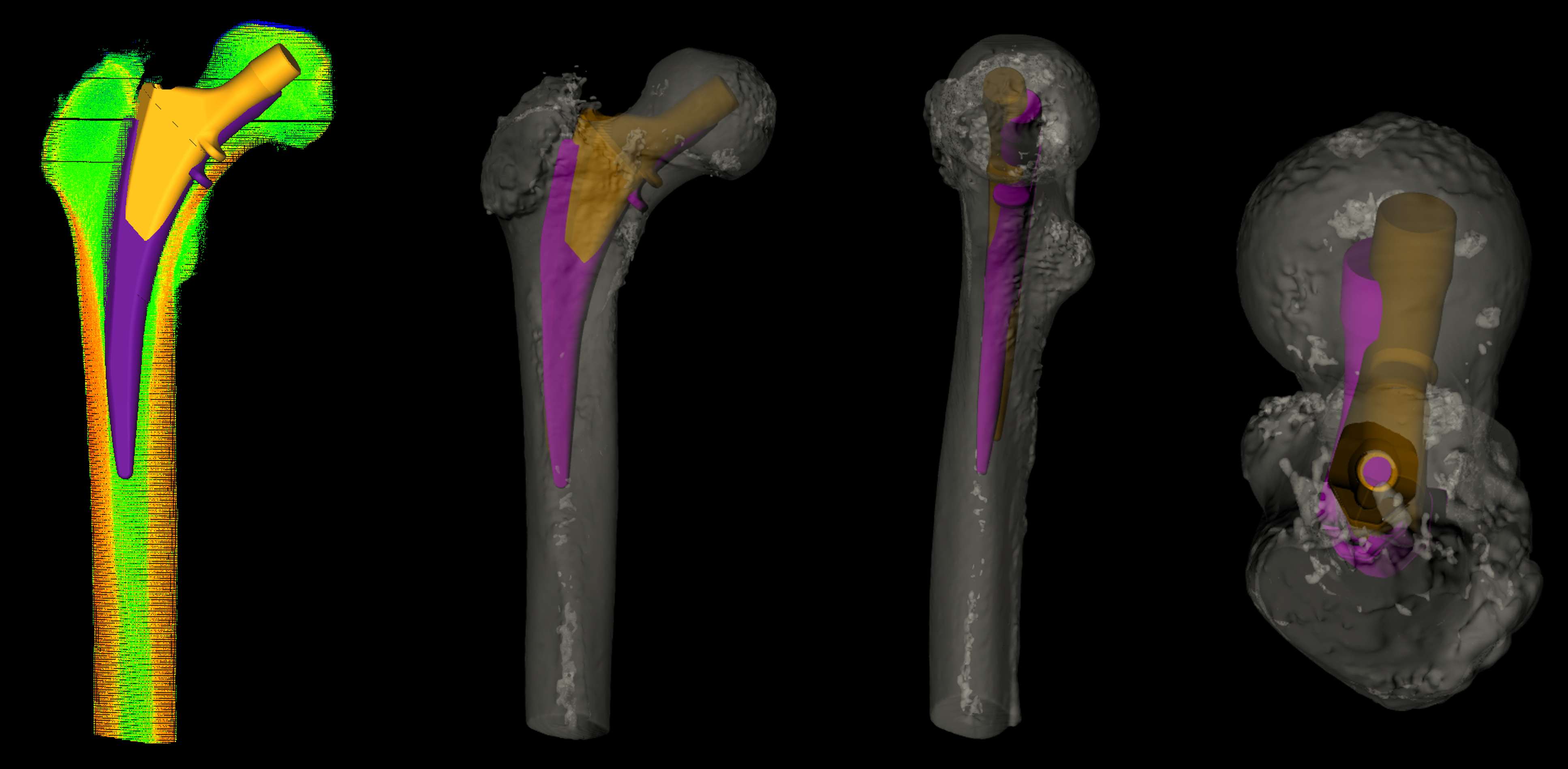
Figure 1
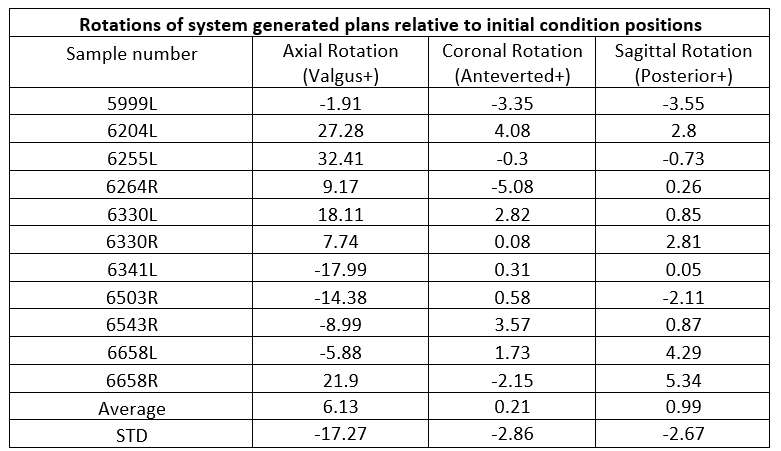
Figure 2#8831
Introduction: Techniques of invention discovery
*Morteza Meftah - NYU Hospital for Joint Diseases - New York, USA
*Email: morteza.meftah@nyulangone.org
#8832
Minimal viable product
*Ashvin Dewan - New York, United States of America
*Email: ashvin.dewan@casectrl.com
#8833
Commercializing your great orthopedic product idea
*Dan Justin - New York, United States of America
*Email: danfjustin@gmail.com
#8834
With whom should I spin out? The importance of the cap table for investors
*Jonathan Jeffers - Imperial College London - London, United Kingdom
*Email: j.jeffers@imperial.ac.uk
#8517
Robotically-Assisted Total Knee Arthroplasty Leads to Improved Early Recovery Over Manual Total Knee Arthroplasty: A Comparative Study Utilizing Daily Remote Monitoring
*Charles Hannon - St. Louis, United States of America
Maria Schwabe - Washington University School of Medicine - St. Louis, USA
Jacqueline King - Washington University in St. Louis - St. Louis, USA
Venessa Riegler - Washington University in St. Louis - St. Louis, USA
Ryan Nunley - Washington University-Saint Louis - Saint Louis, USA
Robert Barrack - Washington University School of Medicine - Saint Louis, USA
*Email: charles.p.hannon@gmail.com
Introduction: Robotically-assisted total knee arthroplasty (RA-TKA) leads to improved surgical accuracy and precision compared to manual instrumentation, but the clinical benefits remain unknown. The purpose of this study was to compare the early clinical outcomes including pain, opioid consumption, activity level, and patient reported outcomes between robotically-assisted and manual total knee arthroplasty (TKA) utilizing daily remote patient monitoring.
Methods: We prospectively enrolled 94 RA-TKAs between September 2021 – November 2022 and compared them to a consecutive series of 114 manual TKAs that were prospectively enrolled between November 2020 – October 2021 in a prior study with the same protocol. All patients utilized a wrist-based activity monitor and a smartphone app-based patient engagement platform for one week preoperatively and 90 days postoperatively to collect daily step counts, Visual Analog Scale (VAS) pain scores, opioid consumption, as well as weekly Oxford Knee Score (OKS) and monthly Forgotten Joint Score (FJS). There were no differences between groups in sex or BMI. The robotic group was younger (mean age 65 v. 53, p = 0.06). Independent sample t-tests were used for continuous data and Chi-squared and Fisher’s exact tests were used for discrete data. Linear mixed models were used for longitudinal analysis of patient outcomes over the study period.
Results: RA-TKA led to decreased pain (mean VAS 3.6 v. 4.2; p = 0.01) and opioid consumption in the first 7 days postoperatively (mean 1 less pill on average per day; p = 0.004) compared to manual TKA. RA-TKA patients stopped opioids on average 6 days sooner compared to manual TKA patients (mean 24 days v. 30 days; p = 0.04). There were no differences in days to stopping gait aids (mean 31 days v. 35 days; p = 0.09). There was no difference in length of surgery (p=0.4) between groups, but RA-TKA patients stayed on average 3 more hours in the hospital than manual TKA patients (p=0.0). RA-TKA led to improved OKS scores compared to manual TKA for the first 5 weeks postoperatively (p=0.01). There were no differences in FJS scores at all times points or OKS scores beyond 5 weeks. There were no differences in step count (p=0.64), complications (p=0.2), or readmissions (p=0.5) between groups.
Conclusion: Using a smartphone-based patient engagement platform and wrist based wearable activity sensor to collect daily patient data, we found that robotic-assistance in TKA led to incremental improvements in pain, opioid consumption, and OKS scores compared to manual TKA in the early recovery after primary TKA.
Level of evidence: III.
Keywords: robotically-assisted; manual; total knee arthroplasty; recovery
#8495
Cementless TKA With Robotic Technology Shows Excellent One-Year Outcomes in Young, Obese Patients
*Melanie Caba - Stryker Corporation - Mahwah, United States of America
Christina O'Neill - Stryker - Mahwah, United States of America
Kelly Taylor - Ortho Rhode Island - South County, USA
Zachary Guerrieo - Ortho Rhode Island - Wakefield, USA
Emily Hampp - Stryker - Mahwah, USA
Kevin Marchand - Ortho Rhode Island - South County, USA
Robert Marchand - Ortho Rhode Island - South County, USA
*Email: melanie.caba@stryker.com
Introduction:
The number of primary total knee arthroplasty (TKA) cases performed annually in the U.S. is increasing at an exponential rate, with an increasing demand from younger and obese patients [1,2]. Existing evidence has shown patients with these demographics are at an increased risk of postoperative complications such as revisions following TKA [1,2]. CT-based Robotic-assisted (RA) TKA can provide more accurate component placement to plan and may be a beneficial treatment to help improve clinical outcomes. The purpose of this study was to evaluate survivorship and outcomes of cementless RA-TKA cases in young, obese patients.
Methods:
A retrospective review was conducted on 559 cementless RA-TKA cases of patients under the age of 65 from a single, high-volume surgeon. From this cohort, BMI was used to classify cases of a young obese cohort (age<65, BMI≥35) and a young nonobese cohort (age<65, BMI<35). Data collected from each case included operative time, six-week postoperative flexion, preoperative and postoperative WOMAC pain and WOMAC function scores out to one year and postoperative adverse events. Minimally clinical important difference (MCID) was assessed for one year PRO outcomes. Two-sample t-tests and chi-squared tests with α=0.05 in SAS 9.4 were used to assess significant difference.
Results:
The young obese cohort (mean BMI 40.8±4.9) consisted of 204 cases (36%) and the young nonobese cohort (mean BMI 29.1±3.6) consisted of 355 cases (64%). There was no significant difference in mean operative time (p=.51) or flexion values at six-weeks postoperative for both cohorts (p=.44). There was no significant difference in the rate of revisions (0.5% vs 1.1%, p=.44) or MUAs (6.4% vs 4.0%, p=.20) between the young obese and young nonobese cohorts, respectively. Firgure 1 displays no significant difference in all mean PRO values at preoperative and postoperative timepoints. Figure 2 shows over 80% of patients in the two cohorts reached MCID for WOMAC pain and WOMAC function at one year postoperative and the percent of cases to reach MCID was not significantly different.
Discussion:
The results of this study showed that young obese patients who received cementless CT-based RA-TKA had similar outcomes to young nonobese patients. Following cementless RA-TKA for both cohorts, there was excellent survivorship and a large percent of patients reached MCID for 1-year pain and function PRO scores. Additionally, there was no significant difference in mean operative time or 6-week postoperative flexion values. The utilization of cementless RA-TKA has demonstrated to be a viable treatment option for this patient demographic.
References:
[1] Nam D, Kopinski JE, Meyer Z, Rames RD, Nunley RM, Barrack RL. Perioperative and Early Postoperative Comparison of a Modern Cemented and Cementless Total Knee Arthroplasty of the Same Design. J Arthroplasty. 2017 Jul;32(7):2151-2155. doi: 10.1016/j.arth.2017.01.051. Epub 2017 Feb 7. PMID: 28238584.
[2] Goh GS, Wells Z, Ong CB, Small I, Ciesielka KA, Fillingham YA. Does Body Mass Index Influence the Outcomes and Survivorship of Modern Cementless Total Knee Arthroplasty? J Arthroplasty. 2022 Nov;37(11):2171-2177. doi: 10.1016/j.arth.2022.05.041. Epub 2022 May 27. PMID: 35644461.
Figures
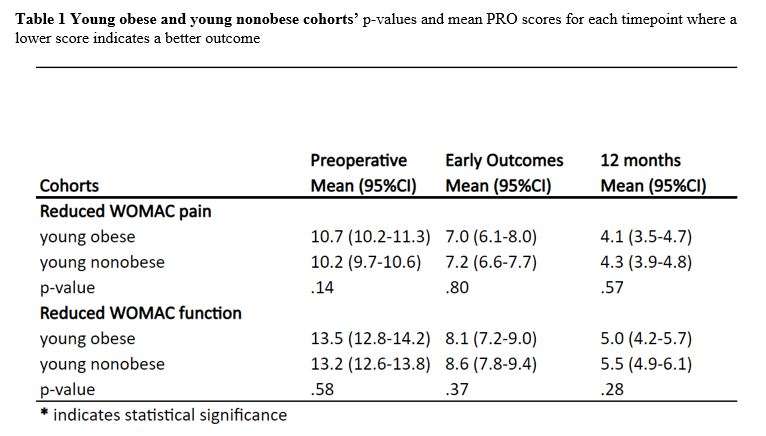
Figure 1
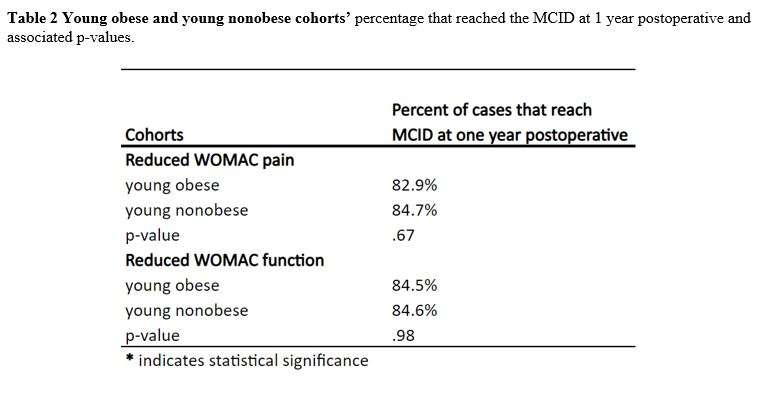
Figure 2#8807
Robotic Assisted Total Knee Arthroplasty Results in Smaller Femoral Components and Larger Tibial Baseplates Than Manual Replacement
Neelaab Nasraty - Yale University - Hamden, United States of America
Ran Schwarzkopf - NYU Langone Medical Center Hospital for Joint Diseases - New York, USA
Matthew Hepinstall - NYU Langone Health - New York, USA
Claire Donnelley - Yale University - New Haven, USA
Vinaya Rajahraman - New York, United States of America
Daniel Waren - NYU Langone Health - New York, USA
Stephanie Frezzo - NYU - NEW YORK, USA
Jenna Bernstein - YALE UNIVERSITY - NEW HAVEN, USA
*Daniel Wiznia - Yale University - New Haven, USA
*Email: daniel.wiznia@yale.edu
Introduction: A variety of robotic systems have been introduced for total knee arthroplasty (TKA). Several of these systems utilize three-dimensional (3D) bone modeling and intraoperative ligamentous balancing data to assist the surgeon in personalizing implant size and position. It has yet to be established how 3D modeling utilizing computed tomography (CT) influence surgeon selection of implant sizes. This study evaluated the effect of CT-based 3D modeling, ligamentous balancing data, and robotic arm assistance on implant selection.
Methods: We reviewed 645 TKAs performed with a single prosthetic design at two academic medical centers between 2016 and 2022. Robotic arm surgical assistance was utilized in 304, whereas 341 were conventionally-instrumented. Robotic surgeries were preformed using a single platform with pre-operative 3D planning based on CT scan. Femoral and tibial implant sizing was compared between robotic and conventional techniques. A secondary analysis examined tibial polyethylene thickness. Multivariate analyses assessed for confounding and effect modification on the basis of demographics including age, sex, height, weight, and patient-reported race.
Results: The two cohorts exhibited no significant differences in age (p=0.33), weight (p = 0.29), or race (p=0.24). The robotic cohort had fewer women (58.9% vs. 66.7% p=0.04) and was taller on average (66.3 in vs. 65.0 in p< 0.001). Robotic knees were 90.1% cruciate retaining (CR), whereas manual knees were 57.8% CR and 42.2% posterior stabilized (PS). On multivariate analysis, robotic TKAs had larger tibial components (p < 0.001) and smaller femoral components (p=0.017). Polyethylene liner thickness was not significantly associated with any tested metric.
Conclusions: Robotic arm-assisted TKA with CT-based 3D planning was associated with larger mean tibial component size and smaller mean femoral component size when compared to conventionally-instrumented TKAs despite accounting for patient demographics. Observed differences likely reflect differences in the data informing implant selection; effects on clinical outcomes warrant further study.
#8143
Robotic Total Knee Arthroplasty Is Associated With Thinner and Less Constrained Polyethylene Inserts
Travis Weiner - Columbia University Medical Center - New York, USA
William Crockatt - Columbia University Medical Center - New York, USA
Roshan Shah - Columbia University Medical Center - New York, USA
Jeffrey Geller - Columbia University Medical Center - New York, USA
Alexander Neuwirth - Columbia University Medical Center - New York, USA
*John Cooper - Columbia University - New York, USA
*Email: jcooper02@gmail.com
Introduction
Accurate pre-resection assessment of gap measurements during total knee arthroplasty (TKA) may reduce the need for thicker polyethylene inserts or those with higher constraint by allowing the surgeon to address potential imbalance through guiding bony resections and implant position. Thicker polyethylene inserts have been associated with higher rates of instability, loosening, and infection requiring revision surgery as compared to thinner inserts. Constrained inserts have also been shown to have higher revision rates due to polyethylene wear or premature aseptic loosening. This study aimed to determine whether robotic-assistance with pre-planning allowed for the use of thinner and less-constrained polyethylene inserts compared to conventional methods.
Methods
Records were retrospectively reviewed for 408 patients who underwent primary TKA ??by a single fellowship-trained adult hip and knee reconstruction surgeon between January 2018 and September 2022. Patients were divided into two cohorts based on the technique utilized – conventional, manual methods with a jig-based system (CM-TKA, 169 knees) or robotic-assisted TKA (RA-TKA, 237 knees). Operative notes were reviewed for implant brand, thickness of the polyethylene insert, degree of constraint of the polyethylene insert, and whether robotic assistance was used to complete the operation. Statistical analysis was performed using Chi-square tests for categorical variables and t-tests for continuous variables.
Results
There were no significant differences in demographic characteristics between the RA-TKA and CM-TKA groups. Statistically significant differences were observed between cohorts with the RA-TKA group having a smaller mean polyethylene insert thickness (11.0mm ± 1.3mm vs. 11.7mm ± 1.7mm, p<0.0001) (Table 1), a higher rate of use of the thinnest 10mm insert (43% vs 34%, p=0.048), a lower rate of use of “outlier” insert sizes ≥14mm (5% vs 18%, p<0.0001) (Table 2), and a lower rate of use of constrained inserts (4% vs 18% of knees, p<0.0001) (Table 3).
Conclusion
In a review of 408 consecutive total knee arthroplasty patients, use of robotic-assisted techniques allowed for the use of thinner polyethylene inserts, fewer “outlier” polyethylene sizes, and reduced need for constrained inserts compared to conventional, manual methods. These findings suggest RA-TKA may better allow surgeons to intra-operatively address gap imbalance prior to bony resections and may ultimately reduce the need for thicker and more highly constrained implants.
Figures

Figure 1
Figure 2
Figure 3#8701
Novel Robotic Technique to Investigate TKR Stability
*Sander Holthof - Imperial College London - London, GB
Andrew Amis - Imperial College of London - London, UK
Richard van Arkel - Imperial College London - London, United Kingdom
Michael Rock - DePuy Synthes Joint Reconstruction - Leeds, United Kingdom
*Email: s.holthof@imperial.ac.uk
Introduction:
Instability after TKA affects up to 22% of patients. Patient factors, surgical technique and implant designs might all be contributing factors, though computer modelling and fluoroscopy studies suggest that implant design may be the root cause of instability, specifically mid-flexion instability. These studies also hypothesised mid-flexion instability might be related to femoral radius changes in multi-radius (J-curve) designs. The aim of this study was to explore how implant design affects stability across a complete range of knee flexion-extension.
Methods:
A simVITRO (simVITRO, USA) controlled six-degree-of-freedom robotic arm was using for testing. Kinematics were calculated in a trans-epicondylar axis system. Three cruciate-retaining (CR) implant designs were tested: a CR Femur with a J Curve and a medially congruent tibial insert (JMC), CR femur with gradually reducing radius (GradiusTM) and medially stabilising tibial insert (GMS), and a CR femur with a single radius and symmetrical tibial insert (SRS). All implants were flexed from 0-140° of flexion with a compression force of 710N. The tests were first run with no anterior-posterior tibial force (neutral path), then with 90N of anterior tibial force, and then 90N of posterior tibial force and 6-DOF kinematics were recorded. Each implant was tested 6 times.
Results:
The novel test revealed differences between neutral path motion, AP stability and flexion/extension kinematics. The JMC implant showed large changes in AP stability over small flexion regions (e.g. the stability envelope increased 94% between 20-40° flexion) [Fig.2]. Further, no tibial anterior translation was detected in low flexion (<25°) [Fig.1]. The SRS did not have any sudden stability changes in flexion, but had the largest a large AP laxity envelope throughout the flexion cycle, compared to the other implants (235% larger, p<0.05) [Fig.4], reflecting lower bearing conformity. The GMS implant exhibited a smooth increase in AP stability envelope until around 90° of flexion[Fig.3], and also exhibited coupled internal tibial rotation in higher flexion and when an anterior tibial force was applied [Fig.5].
Conclusions:
The new testing technique revealed distinct functional differences between implant designs. Preclinical lab testing can provide important data related to functional and mid-flexion stability, independently of surgical technique and patient-specific factors. This more rigorous testing reveals important kinematic differences that are not detected with current standards at fixed angles of flexion (ASTM F1223). Having a better understanding of inherent kinematic and stability differences due to implant design would allow surgeons to make more informed choices surrounding implant designs based on patient needs.
Figures
Figure 1
Figure 2
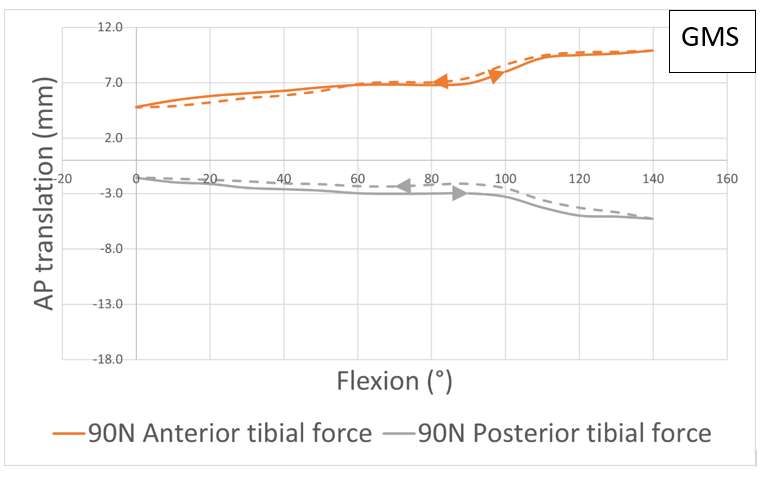
Figure 3
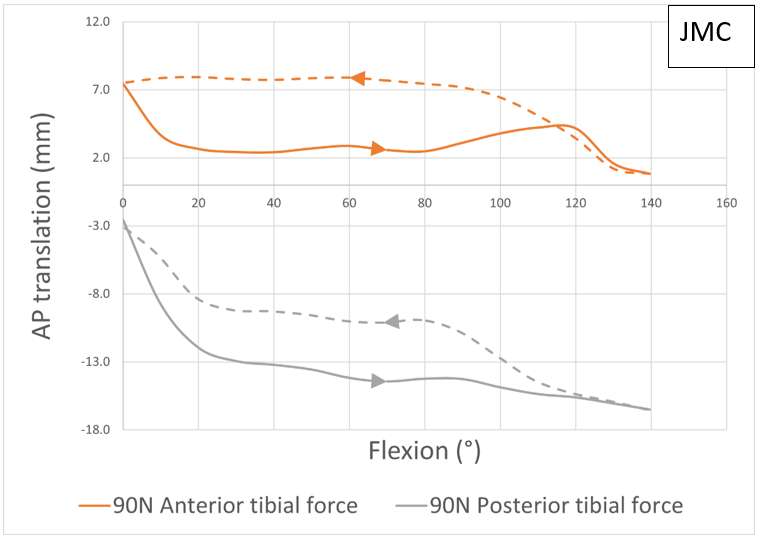
Figure 4
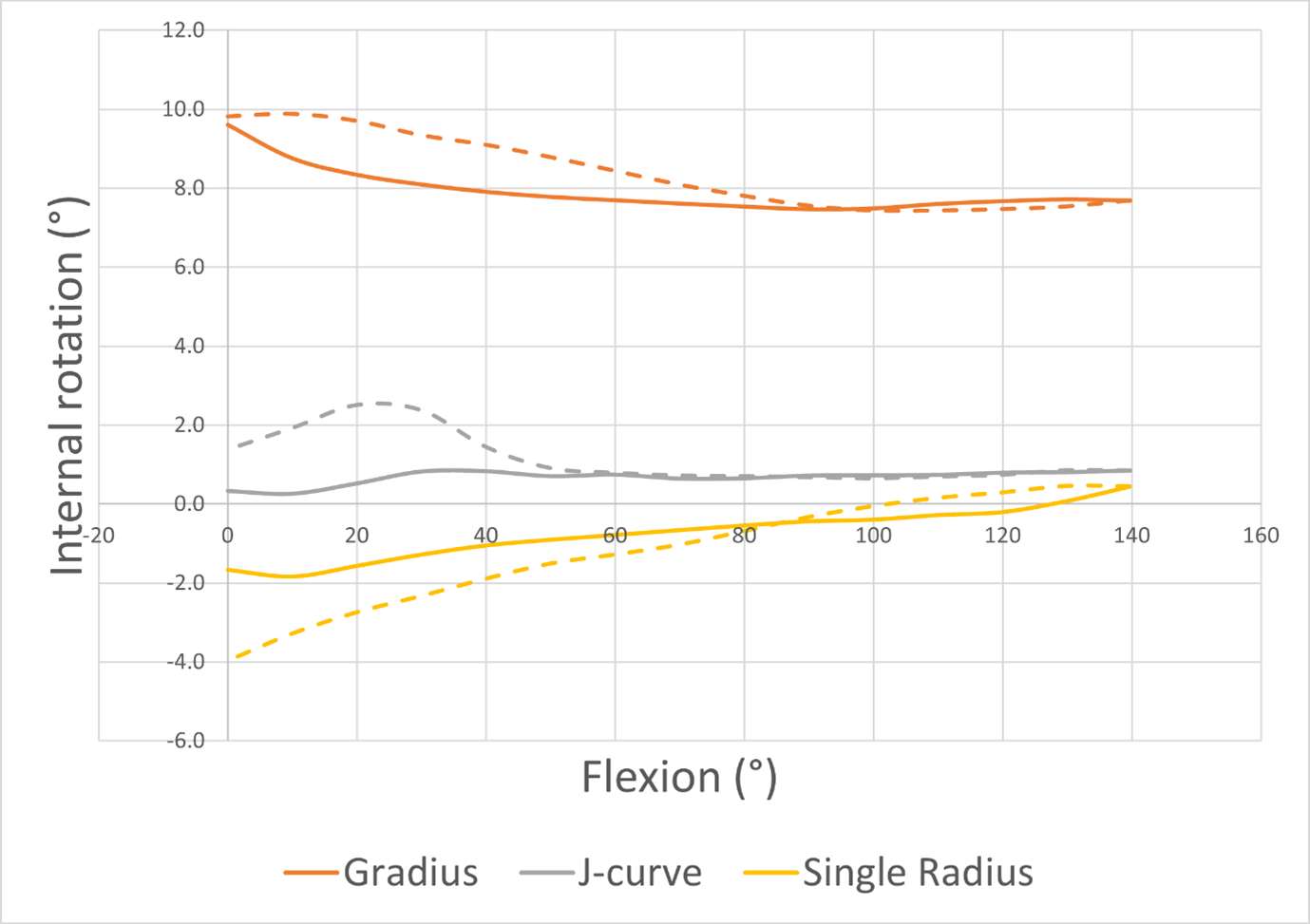
Figure 5#8401
Clinical Translation of Statistical Shape Modelling in Hip Revision Surgery
Sara De Angelis - University College London - London, GB
Anna Di Laura - Royal National Orthopaedic Hospital and Dept. MechEng at UCL - London, United Kingdom
Alister Hart - Royal National Orthopaedic Hospital - London, United Kingdom
*Johann Henckel - Royal National Orthopaedic Hospital - London, United Kingdom
Harry Hothi - London Implant Retrieval Centre - Stanmore, United Kingdom
Angelika Ramesh - University College London - London, United Kingdom
*Email: j.henckel@ucl.ac.uk
Introduction
Total hip arthroplasty (THA) is a widespread orthopaedic procedure used to restore hip joint function when severe acetabular defects are present. Current treatment options present a high revision rate due to implant failure. The assessment of the defect is important to guide the positioning of the acetabular components and reduce the need for revision surgeries. The mirroring of the contralateral hemipelvis has been previously used to measure hip joint parameters. However, this technique cannot be applied when the contralateral hemipelvis is pathological or metalwork is present.
We used a statistical shape model (SSM) as a tool to help derive landmarks that are often absent in hip joints of patients with large acetabular defects. Our aim was to improve surgical planning of hip revision surgery using SSM technology by comparing the model to real-life clinical cases.
Methods
This retrospective cohort study involved 38 patients with Paprosky type IIIB defects. An SSM was built on 50 healthy pelvises and used to virtually reconstruct the native pelvic morphology for all cases. Within the cohort, 18 of the 38 patients had healthy contralateral side. The SSM was then compared with the preoperative computerized tomography (CT)?based plan for all patients whose surgery was planned without the SSM technique. The outcome measures were the difference in CoR between the SSM and 1) the diseased hip, 2) the plan and 3) the contralateral healthy hip.
Results
The largest difference in CoR was found between the 38 diseased hips and their corresponding SSMs (1), with a median of 31.17 mm (interquartile range [IQR]: 43.80–19.87 mm) [Fig. 1]. The median difference in CoR between the plan and the SSM (2) was 8.53 mm (IQR: 12.76–5.74 mm) [Fig. 2]. In seven cases, the surgeon chose a high CoR to maximise bony fixation. The median difference in CoR between the SSM and the healthy contralateral side (3) was 7.84 mm (IQR: 10.13–5.13 mm).
Conclusions
This is the first study to investigate whether SSM can be used to plan the reconstruction of pelvises with large acetabular defects and measure hip joint parameters using a large sample of clinical cases. The SSM successfully reproduced a reconstruction of the native anatomy of pathological hemipelvises and derived their native hip joint centre [Fig. 3]. Our findings show that the model can be used as an important tool to aid preoperative planning and implant design.
Figures
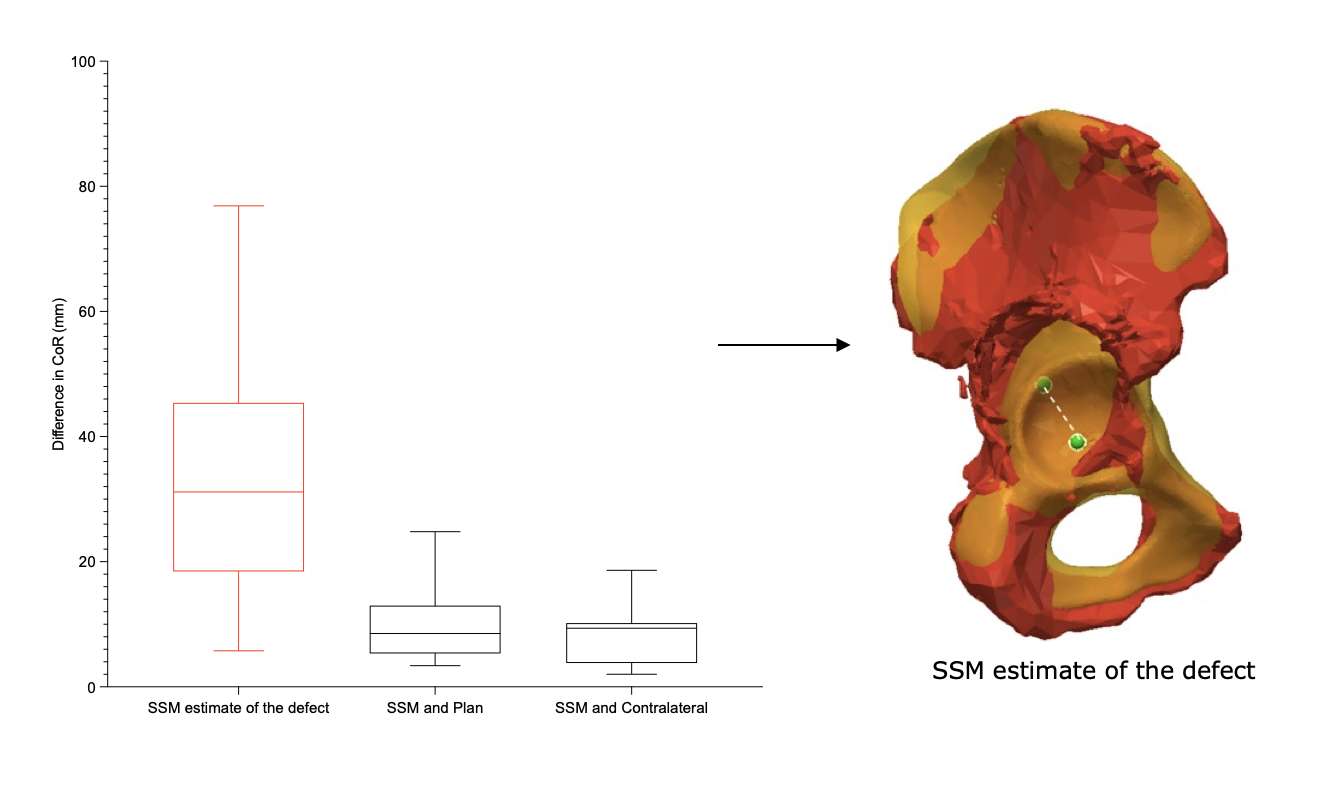
Figure 1
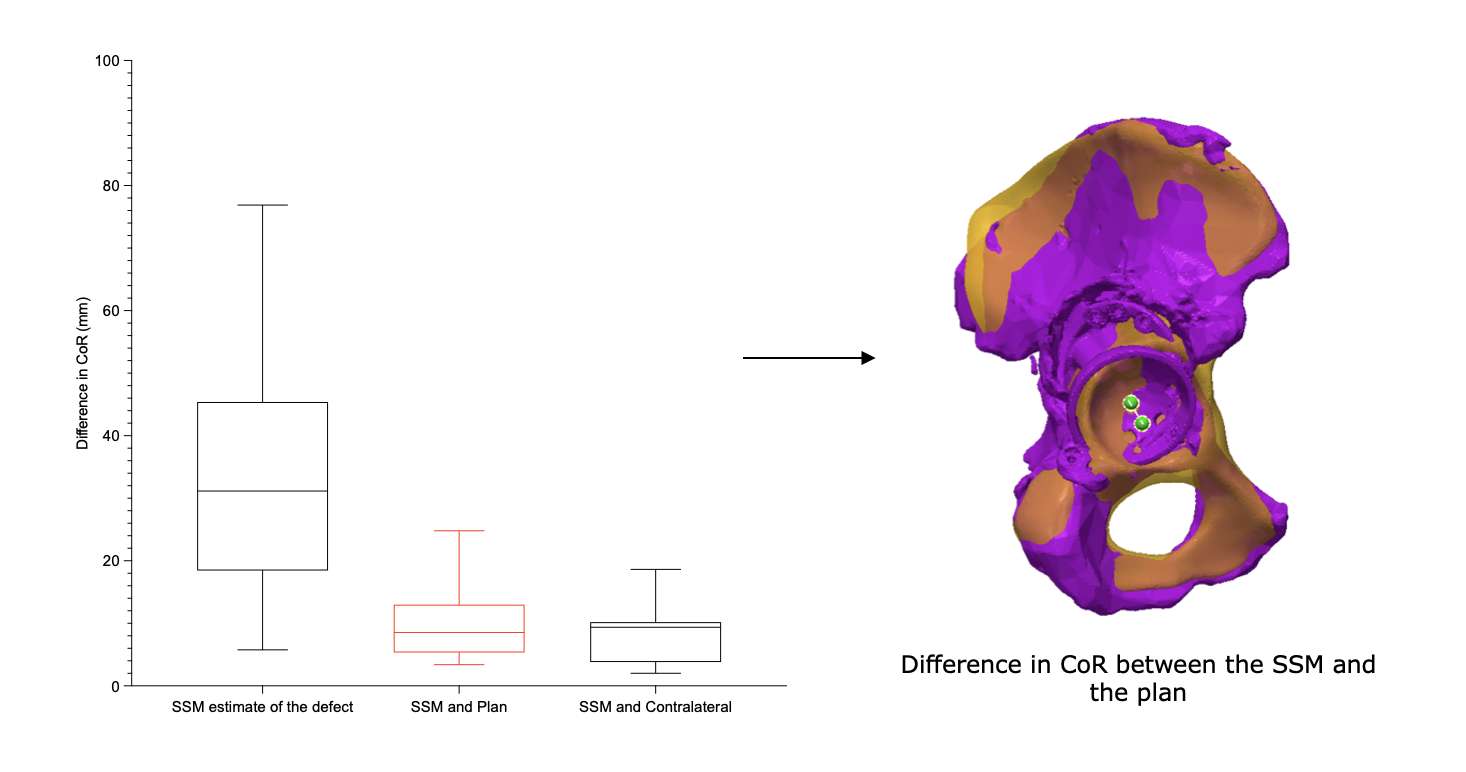
Figure 2
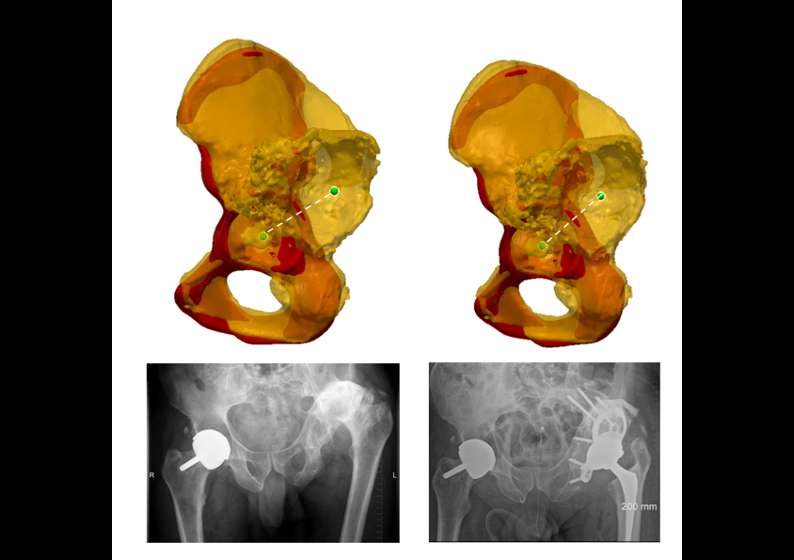
Figure 3#8389
Prediction and Validation of Cortical Bone Contact in Cementless Femoral Stems
Lilian Lim - FormusLabs - Auckland, New Zealand
Nynke Rooks - Formus Labs - Auckland, New Zealand
Bogdan Solomon - Centre for Orthopaedic and Trauma Research, Adelaide Medical School, The University of Adelaide - Adelaide, Australia
Stuart Callaray - Centre for Orthopaedic and Trauma Research, Adelaide Medical School, The University of Adelaide - Adelaide, Australia
Egon Perilli - Medical Devices Research Institute, College of Science and Engineering, Flinders University - Adelaide, Australia
*Marco Schneider - Formus Labs - Auckland, New Zealand
Thor F Besier - University of Auckland - Auckland, New Zealand
*Email: marco@formuslabs.com
Introduction
The contact area between a cementless femoral stem and the femoral canal is critical to achieving fixation in Total Hip Arthroplasty (THA). Pre-operatively predicting the location and area of contact between the stem and bone from CT imaging data would aid in optimising implant selection and placement required for long-term success. However, limited data are available for validating these predictions, with published values varying greatly [1]. In this study, we collect and compare the location and area of contact between cementless stems and the femoral canal predicted from pre-operative CTs and calculated from post-operative CT imaging.
Methods
Pre-operative and post-operative CT scans were taken of 7 cadaveric hip joint specimens (2 right, 5 left) where THA using Taperloc Complete Full Profile stems was performed [2]. The post-operative CT scans were manually segmented to obtain post-operative models of the implants, inner cortical bone, and external femoral bone surfaces. CAD models of the stems were then registered to the segmented stems in the pre-operative position. Pre-operative CT scans were processed by the Formus Hip planner (Formus Labs, Auckland, NZ), which automatically segmented the hemipelvis, femur external surface, and the inner cortical bone of the femur, and virtually implanted CAD models of stems size-matched to the post-operative implants. The post-operative coordinate system was then registered to the pre-operative coordinate system using the external femur surfaces. A point-to-mesh distance calculation between the stems below the base of the neck and the inner cortical bone was performed, where distance values of zero or more (i.e. overlap) were considered contact. Contact areas for the porous section of the stem were also calculated. Contact area was expressed as a percentage relative to the surface area of the entire stem below the base of the neck. The uncertainty in the segmentation of the inner cortical surface and its effect on contact area was accounted for by varying the distances by ± 1 voxel.
Results
Contact areas were variable across the specimens with a mean of just 12% (tolerance range 8-22%). Predicted contact areas compared favourably to measured, with a mean of 14% (tolerance range 6-20%) (Figure 1). When considering the porous section of the stem only, the averages were 12% and 13% (tolerance 6–23% and 8–20%) for the predicted and delivered values, respectively (Figure 2). Regarding location, stem contact was greatest at Gruen zones 1 and 7 and across the anterior surface, zone 8. A few stems showed contact at zones 3 and 5, but these did not extend across the anterior or posterior surfaces.
Conclusion
Cementless stems make relatively small contact with the femoral canal and the location and magnitude of contact is highly variable. Automated 3D pre-operative planning can predict stem positions and contact to aid surgeons in implant selection and positioning. MicroCT imaging and computational modeling of the same specimens will provide more insight into this complex interaction.
References
-
Reimeringer, M., & Nuño, N. (2016). J Biomech, 49, 1064-1070.
-
Grace, T., et al. (2022). J Orthop Res, 40, 396-408.
Figures
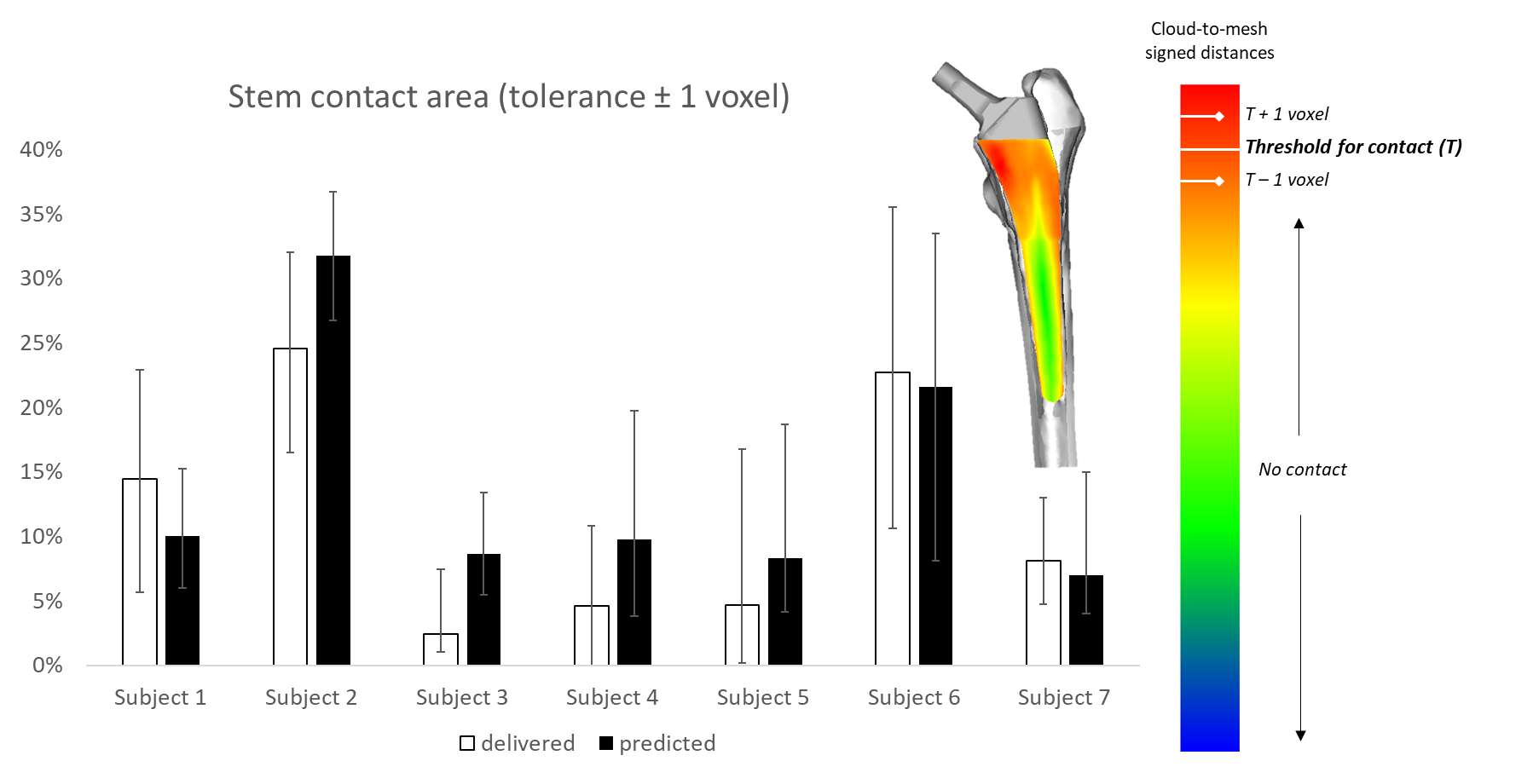
Figure 1
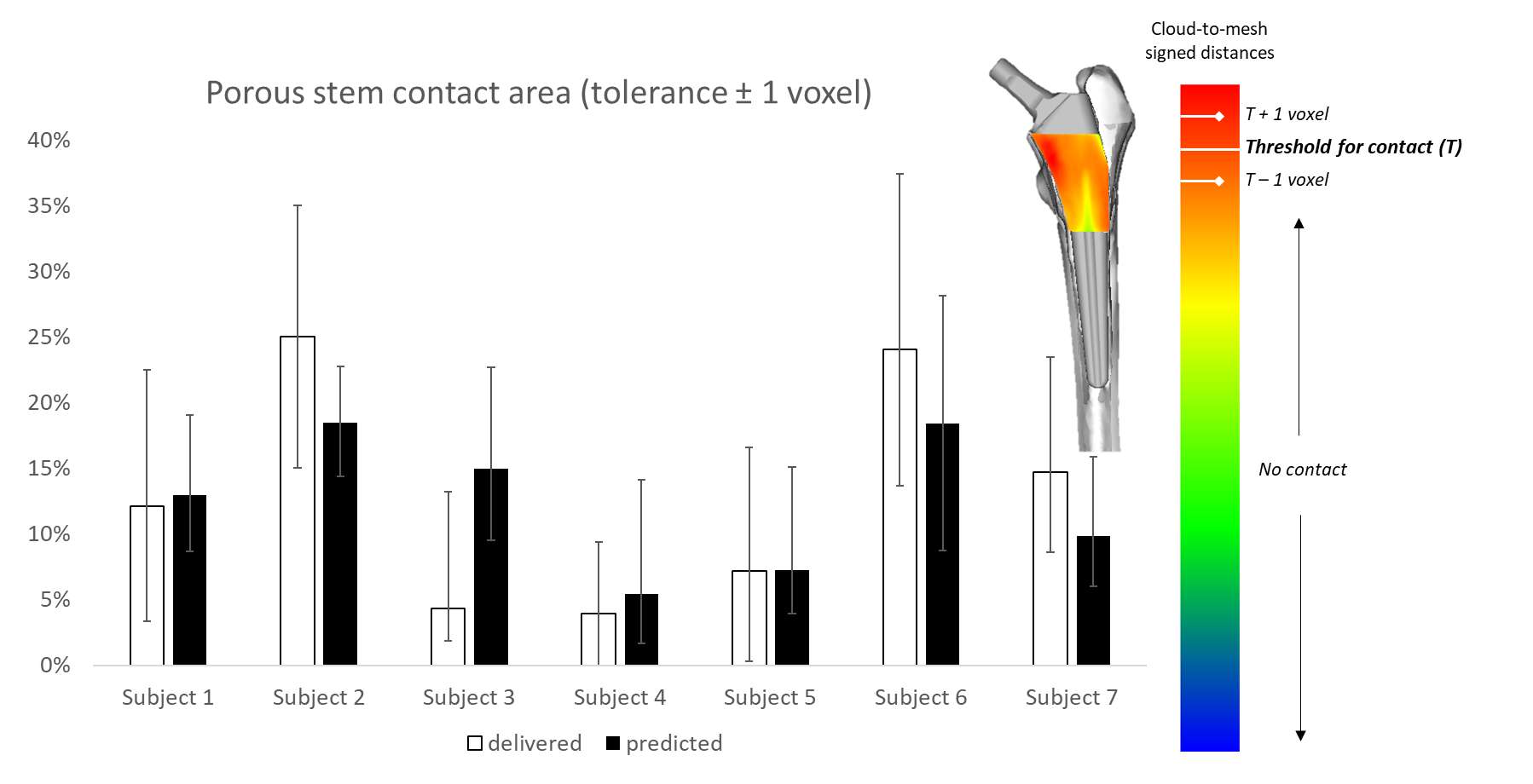
Figure 2#8298
Contribution of the Medial Iliofemoral Ligament to Hip Stability After Total Hip Arthroplasty Through Direct Anterior Approach
*Jennifer Bido - Hospital for Special Surgery - New York, United States of America
Jose Rodriguez - Hospital for Special Surgery - New York City, USA
Fernando Quevedo Gonzalez - Hospital for Special Surgery - New York, USA
Kate Meyers - Hospital for Special Surgery - New York, USA
*Email: bidoje@hss.edu
INTRODUCTION: Dislocation after total hip arthroplasty (THA) is one of the primary reasons for THA revision in the US [1]. During THA through the direct anterior approach (DAA), the iliofemoral ligament, which provides the main resistance to external rotation of the hip [2], must be partially transected. While transecting this ligament creates concerns for anterior dislocation of the hip, especially during external rotation with the leg in extension, the extent of the transection remains variable: some surgeons keep the medial aspect of the ligament intact, others transect and repair it, and others transect the ligament without repairing it. Our current ability to adequately manage the anterior hip capsule during DAA-THA is hampered by a lack of understanding of the biomechanical consequences of these surgical choices. Therefore, we asked two questions: (1) what is the contribution of the medial iliofemoral ligament to resisting anterior dislocation after DAA-THA? (2) How much resistance to anterior dislocation can be obtained by repairing the medial iliofemoral ligament?
METHODS: Seven cadaveric hemi-pelvis to knee specimens were procured. A fellowship trained orthopaedic surgeon performed THA through a standard DAA, using a cementless acetabular cup and a cemented femoral component. The specimens were CT scanned before and after implantation (Fig 1). Prior to testing, the external rotation ROM of each specimen to impingement (either intra- or extra-articular) in 10? of extension was computationally determined. 3D-printed specimen specific guides were used to define the anatomic coordinate systems of the femur and pelvis (Figs. 1 and 2). Each specimen was tested on a six-degree of freedom robotic manipulator (KR300 Ultra 2500, Kuka) utilizing hip-testing specific control software (SimVitro)(Fig. 2). During testing, the pelvis was placed in 10? of extension. The femur was externally rotated until reaching the specimen-specific impingement target. Throughout testing, 10N forces were applied to ensure the femoral head remained seated in the acetabular liner. Total external rotation torque was recorded throughout the motion of each hip with the medial iliofemoral ligament intact, after transecting the ligament, and after repair. Each test was repeated three times. Torque at impingement was calculated for each condition. To isolate the contribution of the native ligament, the impingement torque for the transected state was subtracted for both the intact and repaired conditions.
RESULTS: The contribution of the iliofemoral ligament varied between specimen with an average contribution of 54% (SD 31%) of the total torque at the instant of impingement (Fig. 3). When the ligament was repaired after being transected, it contributed to 12% (8%) of the torque to impingement across specimens, thus only restoring 22% of the native resistance against dislocation.
CONCLUSION: The medial iliofemoral ligament was a significant contributor to the hip torque at impingement during external rotation of the hip when intact. Repairing the ligament could only restore one fifth of its ability to generate torque to resist anterior hip dislocation.
REFERENCES: [1] AJRR 2021 report. [2] van Arkel; J Bone Joint Surg. 2015;97:484–491.
ACKNOWLEDGMENTS: The authors would like to thank Exactech for providing the implants for this research
Figures
Figure 1
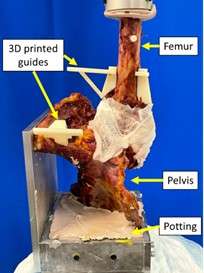
Figure 2
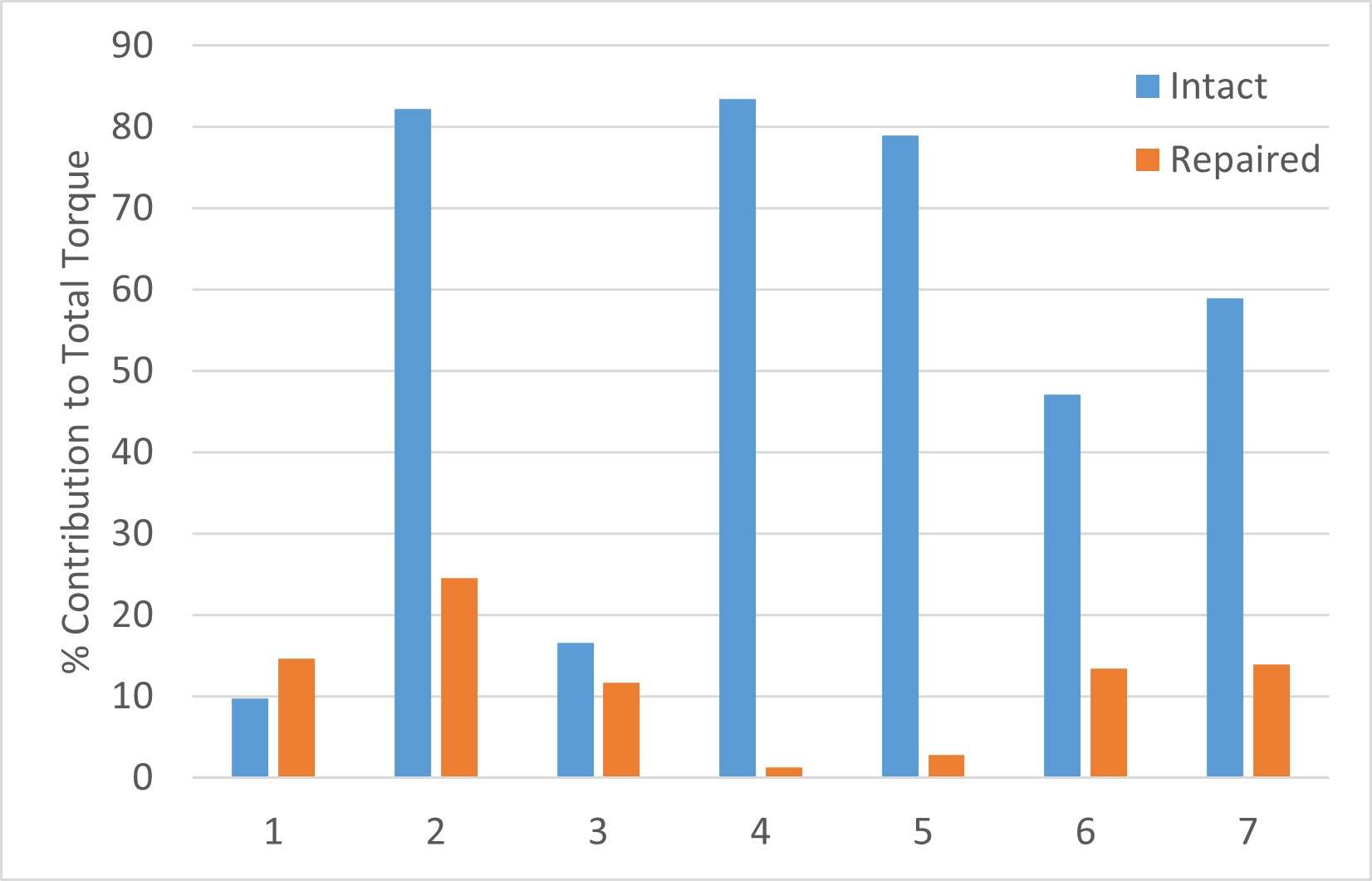
Figure 3#8366
Iliopsoas Tendonitis After Total Hip Arthroplasty: Development of an Instantaneous Risk Prediction Simulation
*Joshua Twiggs - University of Sydney - Sydney, Australia
Max Hardwick-Morris - 360 Med Care - Sydney, Australia
Brad Miles - 360 Knee Systems - Pymble, Australia
Kaushik Hazratwala - Queensland Lower Limb Clinic - Pimlico, Australia
Tyson Doneley - Brisbane Orthopaedic Clinic - Spring Hill, Australia
William Walter - Specialist Orthopaedic Group - North Sydney, Australia
*Email: joshua_twigg@msn.com
Introduction: Iliopsoas tendonitis occurs in 4-30% of patients after total hip arthroplasty (THA) and 18-30% of patients after hip resurfacing arthroplasty (HRA). Despite these relatively high incidences, there are few attempts at computationally modelling iliopsoas tendonitis. We have previously developed a simulation that can quantify iliopsoas impingement and validated this model in two studies; one of patients who underwent THA and one of patients who underwent HRA surgery. The purpose of this study was to translate these results into a predictive score that can be used to define a patient’s risk of groin pain related to iliopsoas tendonitis.
Methods: Validation of the iliopsoas impingement detection simulation occurred in previous case-controlled investigations of both THA and HRA patients (Figure 1). In these studies, cohorts of symptomatic patients and asymptomatic patients were simulated in the novel impingement detection model in supine and standing positions. Parameters measured included impingement, pelvic tilt, cup orientation, and cup prominence. Using this data, we generated logistic regression models to determine if the model’s output was a strong predictor of iliopsoas tendonitis. Receiver operating characteristic (ROC) curves were also generated to determine the model’s sensitivity, specificity, and area under the curve (AUC).
Results: For the THA patients, the logistic regression models determined that the impingement values significantly predicted the probability of groin pain, and the simulation had a sensitivity of 74%, specificity of 100%, and an AUC of 0.86. For the HRA patients, the logistic regression models determined that the impingement values also significantly predicted the probability of groin pain, and the simulation had a sensitivity of 83%, specificity of 100%, and an AUC of 0.95. Using these logistic regression models, a novel risk for groin pain scores was. These scores are calculated instantaneously.
Conclusion: Using case-controlled investigations, we developed predictive simulations that quantify a patient’s likelihood of postoperative iliopsoas tendonitis on a scale of 0-100% (Figure 2). Interestingly, the simulations both have a baseline risk of groin pain, irrespective of cup position and orientation. This reflects previous literature that has linked iliopsoas tendonitis to factors such as leg length, offset, and prosthetic femoral head diameter. This simulation has clinical applications. First, the risk of groin pain scores could be used in 3D preoperative templating to guide decisions about planned cup position and orientation. Second, the simulation could be coupled up with image-based navigation to report a risk of groin pain intraoperatively based on cup size, cup orientation, and ream-depth.
Figures
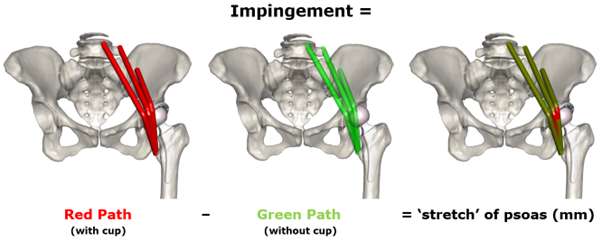
Figure 1
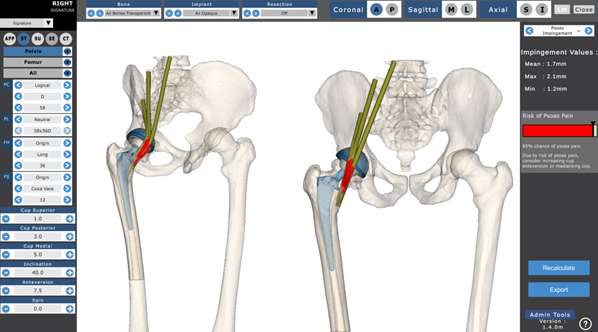
Figure 2#8210
Impingement During Dislocation Prone Activities of Daily Living in Dual Mobility and Standard Acetabular Cups: A Geometric Model
Mackenzie Smeeton - University of Leeds - Leeds, United Kingdom
Simon Williams - University of Leeds - Leeds, United Kingdom
Graham Isaac - DePuy International Ltd - Leeds, Select Country
James Anderson - DePuy Synthes Joint Reconstruction - Leeds, United Kingdom
Tim Board - Wrightington, Wigan & Leigh NHS Foundation Trust - Wigan, United Kingdom
*Ruth Wilcox - University of Leeds - Leeds, United Kingdom
Sophie Williams - University of Leeds - Leeds, United Kingdom
*Email: r.k.wilcox@leeds.ac.uk
Introduction
Dual mobility (DM) hip replacements (THRs), introduced to reduce dislocation, are widely used but function in-vivo is not fully understood.
The aim of this study was to compare the incidence of impingement of a modular dual mobility with that of a standard cup during various activities of daily living.
Methods
A geometrical model of one subject’s bony anatomy [1] was developed with a THR implanted (Corail® stem, Pinnacle® cup (DePuy Synthes)). Three acetabular cup combinations were modelled: 52mm cup and 32mm neutral polyethylene (PE) liner (32/52STD); 52mm cup with metal DM liner, 45mm PE mobile liner and 22mm head (22/45/52DM); 54mm cup with metal DM liner, 47mm PE mobile liner and 28mm head (28/47/54DM). Joint motions were taken from kinematic data of seven activities of daily living associated with dislocation [2] and walking. The acetabular component was positioned in a range of inclination and anteversion (30°-70°, 0°-50° respectively in 5° increments). The occurrence of impingement (implant-implant, bone-bone, or implant-bone) was assessed for each component combination, orientation and activity. Implant-implant impingement can occur through neck to metal or PE liner (DM and standard cups respectively) or neck to PE mobile liner (DM only) (Fig.1).
The results comprise a colour coded matrix which sums the number of impingement events for each cup position, activity, and implant variant. Simple range of movement (ROM) calculations were also made.
Results
Neck to PE mobile liner impingement, occurred for both DM sizes, for all activities, and most cup placement positions (Fig 2). Even under standard walking conditions there were only a small number of positions which avoid impingement for the DM cups indicating that the PE head is likely to move at the start of walking activity.
There were no cup placement positions that avoided neck to metal or PE liner impingement in all activities, for DM or STD components (Fig 3). All cups had a range of placements where impingement occurred in only three activities and the least number of impingement events occurred at the high end of likely component placement positions. Impingement predominantly comprised implant-implant and bone-bone contact.
DM metal liner geometry affected the simple ROM (32/52STD 124.7°, 28/47/54DM 123.5° and 22/45/52DM 118.8°). ROM of DM cups increases with cup diameter.
Conclusions
Consistent with DM philosophy, neck to PE mobile liner impingement (resulting in PE mobile liner motion) occurred for all activities of daily living, nearly all cup positions and is also likely to occur at start-up for standard walking.
Neck to liner impingement frequency is comparable between both DM sizes (metal liner) and a standard PE liner.
In this study, modular DM is not associated with increased ROM compared to a standard liner of similar size.
References: [1] Pryce et al, Proc I MechE, Part H, 2022, on-line ahead of print. [2] Nadzadi et al. J.Biomech, 2003, 36, 277
Acknowledgements: Greg Pryce for model development and Figure 1
Key Words: THR, Dual Mobility, Impingement
Figures
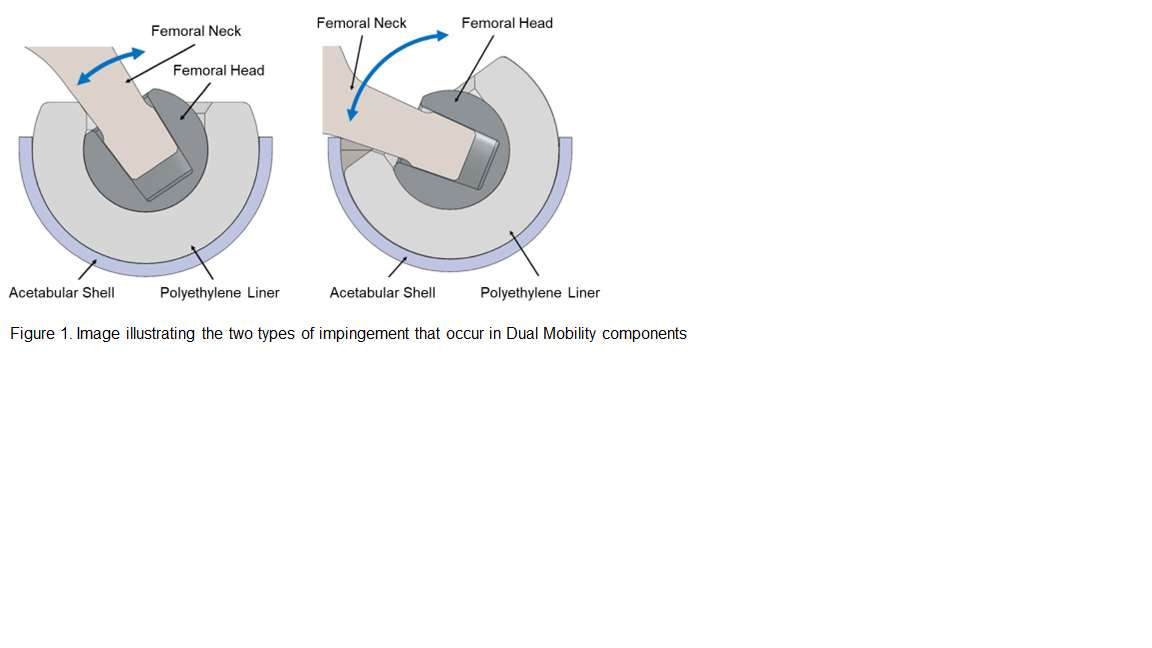
Figure 1
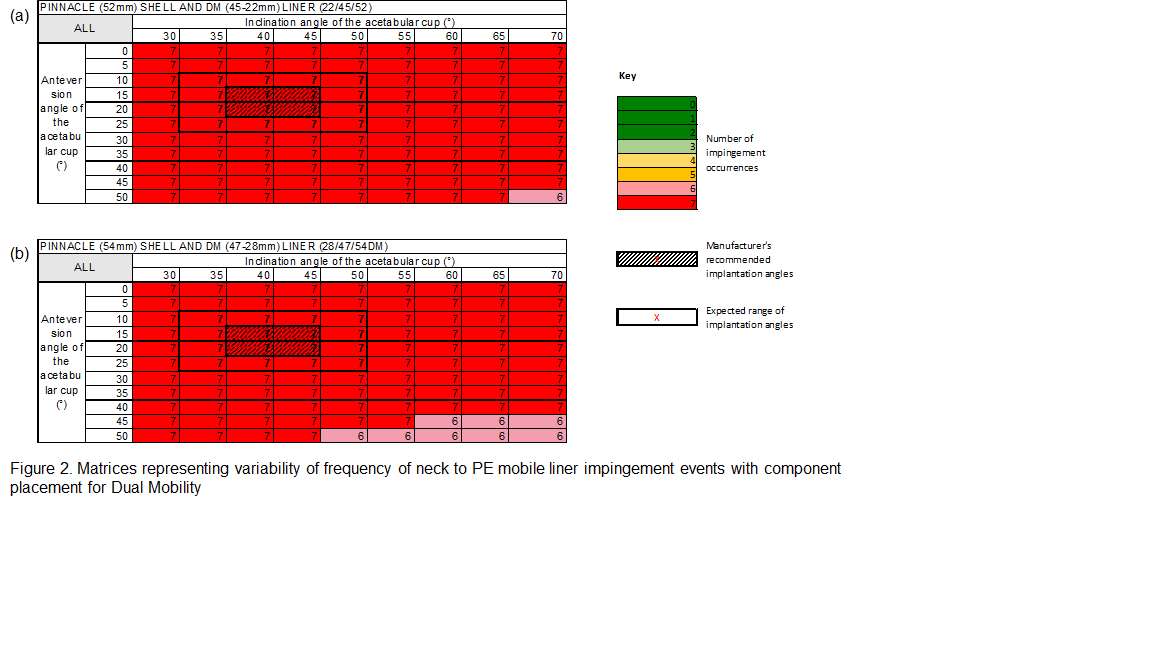
Figure 2
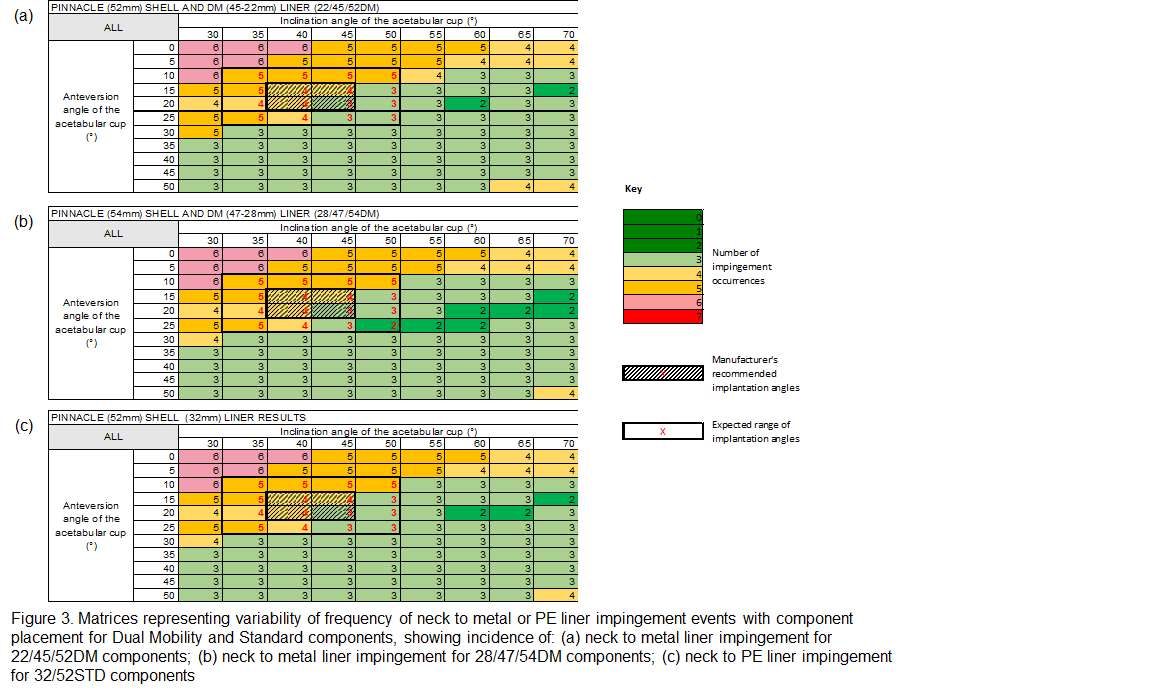
Figure 3#8365
Anteversion vs. Medialisation: Comparing Strategies to Mitigate Iliopsoas Impingement in Total Hip Arthroplasty
*Joshua Twiggs - University of Sydney - Sydney, Australia
Brad Miles - 360 Knee Systems - Pymble, Australia
Max Hardwick-Morris - University of Sydney - Sydney, Australia
William Walter - Specialist Orthopaedic Group - North Sydney, Australia
Tyson Doneley - Brisbane Orthopaedic Clinic - Spring Hill, Australia
Kaushik Hazratwala - Queensland Lower Limb Clinic - Pimlico, Australia
*Email: joshua_twigg@msn.com
Introduction: Iliopsoas tendonitis occurs in between 5-30% of patients after hip arthroplasty and is mainly attributed to impingement between the cup and iliopsoas. We have developed and validated a novel simulation for detecting and quantifying iliopsoas impingement. This simulation has been used to identify preoperative risk factors,but the current study was aimed at profiling the contribution of cup position and orientation to iliopsoas impingement.
Methods: 413 patients received lower-limb CT scans and lateral x-rays that were segmented, landmarked, and measured using a validated preoperative planning protocol. Implants (Depuy; Corail/Pinnacle) were positioned according to standardised parameters, including a 40°/20° standing cup orientation and recreation of the native centre of rotation (COR). The anatomy and implants were simulated in the standing reference frame with a novel computational model that detects iliopsoas impingement. This process is summarised in Figure 1. Definitions of patients at-risk and not at-risk of tendonitis were defined from a previous validation study (Figure 2). To determine the influence of cup position and orientation on iliopsoas impingement, at-risk patients underwent additional simulations with increased anteversion (+5°), medialisation of the cup by 3mm, and both increased anteversion and medialisation of the cup.
Results: The number of patients at-risk of iliopsoas tendonitis due to iliopsoas impingement were as follows: 104 patients at 40°/20°; 67 patients at 40°/25°; 87 patients at 40°/20° with a medialised cup; and 38 patients at 40°/25° with a medialised cup. An increase in anteversion from 20° to 25° led to a 36% reduction in patients at-risk of iliopsoas tendonitis, whereas a 3mm medialisation of the cup led to a 16% reduction. The iliopsoas impingement when the cup anteversion was increased from 20° to 25° was, on average, 70% less, whereas the impingement when the cup was medialised by 3mm was, on average, only 23% less.
Conclusion: The aim of this study was to profile the contribution of acetabular cup position and orientation to iliopsoas impingement using a previously validated simulation. Using an initial cup position that recreated the native COR and an orientation of 40°/20° in standing, our results demonstrate that there is a greater reduction in patients who are at-risk of iliopsoas tendonitis through an increase in anteversion of 5° when compared to a medialisation of the cup by 3mm. This may indicate it is advantageous to increase anteversion instead of medialising the cup when a patient is at-risk of iliopsoas impingement (with consideration of other consequences, such as posterior prosthetic impingement).
Figures
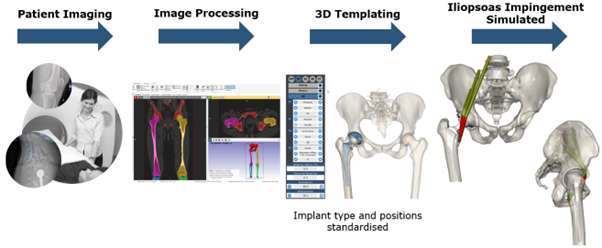
Figure 1
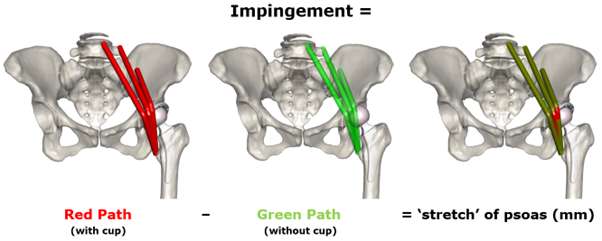
Figure 2#8824
Medtech Innovation Accelerator: Early development milestones that increase your chance of success
*Brittney Martinez - New York, United States of America
*Email: britt@pbcbiomed.com
#8826
Venture Investor: Venture funding in 2023; What are we looking for?
*Matt Harbaugh - New York, United States of America
*Email: matt@mountainstatecapital.com
#8825
Pitching Strategics
*Kristine Ilaria - New York, United States of America
*Email: kristine.ilaria@smith-nephew.com
#8576
An Automated Real-Time Approach for Image Processing and Segmentation of Fluoroscopic Images and Videos Using a Single Deep Learning Network
*Viet-Dung Nguyen - The University of Tennessee at Knoxville - Knoxville, United States of America
Michael LaCour - University of Tennessee - Knoxville, USA
Richard Komistek - The University of Tennessee - Knoxville, USA
*Email: vnguye28@vols.utk.edu
Introduction: Total knee arthroplasty outcomes can be evaluated using kinematics analyses and 3D-to-2D fluoroscopic image registration. Unfortunately, the 2D aspect of the registration process relies heavily on accurate image segmentation and processing to enable a 3D model to be accurately overlayed atop a 2D processed image. There have been many attempts to automate this image processing segmentation using both non-learning and learning approaches, but previous work faces difficulties such as challenging blurry images, components reaching out of the captured image field, overlapped images, or a combination of the above. This work aims to overcome these challenges using a single deep learning neural network to automate image segmentation and processing.
Methods: Over 30 years, a large database was established, containing over 30,000 fluoroscopic TKA images with various implant types. By using voxelization projection, those known registration data can produce segmented images of both femur and tibia, which can be cleaned, verified, and labeled carefully to serve as the testing and training datasets for the next step of deep learning. These images are separated randomly into a training set (90%) and testing set (10%), then the training data are fed into a deep neural network model that is an extension of the ResNet model. This single deep neural network model consists of one input layer representing the fluoroscopic image and three output layers for bounding box, boundary (mask), and label (femur or tibia) information of the processed fluoroscopic image.
Results: The predicted segmented images of the testing set are compared with their ground truth for the validation of the model’s performance. The original fluoroscopy images (top-left), along with their corresponding segmented images (top-right) with the corresponding masks for both femur (red) and tibia (blue) are shown in Figure 1. The masks of both the femur and tibia (bottom images) in Figure 1 are perfectly aligned atop the corresponding silhouettes, as evident from the visualization. In comparison with the ground truth voxelization projection, the average Mean Average Precision (mAP) score for femur and tibia segmentation are 85.61 and 88.28 respectively, which are higher than the existing segmentation learning model’s performance. The deep learning model robustly allows for segmentation of every frame within a fluoroscopic video, as seen in Figure 2, showing promising real-time use. Finally, the proposed model’s performance is tested in many challenging cases, again showing satisfactory results. The segmentation result of a blurry fluoroscopic image can be seen in Figure 3.
Conclusion: Image processing and segmentation is an essential tool in 3D-to-2D image registration for TKA analysis. While manual segmentation is time-consuming, prone to human error, and subject to inter-observer variability, automated image segmentation provides a more objective and reproducible analysis of medical images. The simulation results show a great performance in predicting segmented images of fluoroscopy images. These techniques can also be expanded to additional fields of medical imaging analysis, such as CT scan segmentation and Ultrasound analyses.
Figures
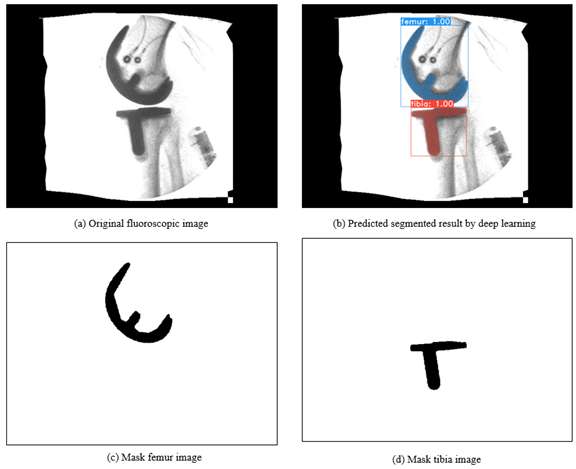
Figure 1
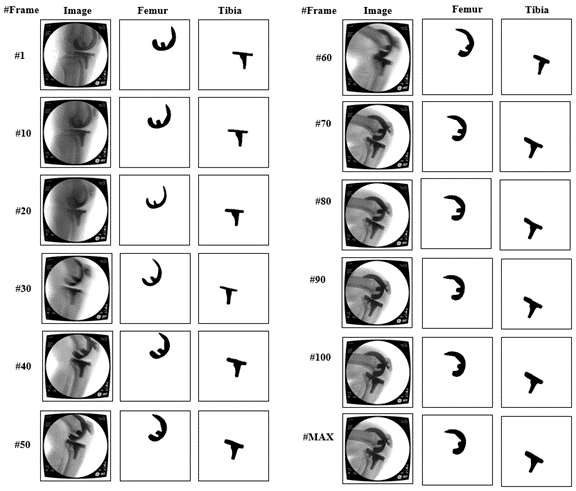
Figure 2
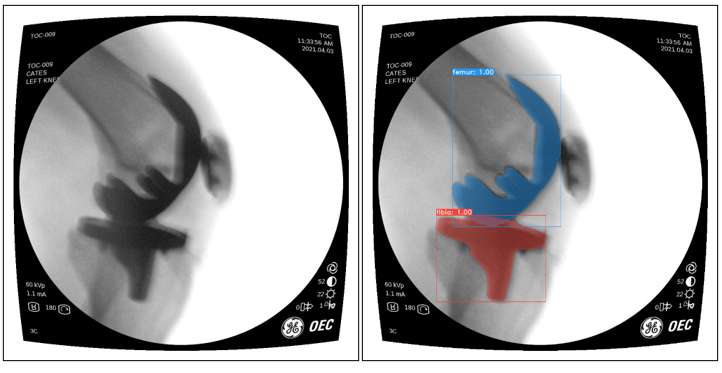
Figure 3#8560
Toward Overcoming Single-Plane Limitations in Total Knee Arthroplasty Kinematics Measurements
*Andrew Jensen - University of Florida - Gainesville, USA
Scott Banks - University of Florida - Gainesville, USA
*Email: ajensen123@ufl.edu
Introduction: Roughly 20% of patients receiving total knee arthroplasty are dissatisfied [1]. Unfortunately, clinicians do not have the tools to clinically assess joint dynamics. Recent advancements have shown that it is possible to autonomously measure TKA kinematics in a clinical setting using single-plane fluoroscopic imaging equipment [2]. However, there still exists limitations in using single-plane imaging because of symmetric projective ambiguities in the model-image registration step (dubbed “symmetry traps”) (Figure 1.) [2,3]. This abstract proposes three novel methods: one to calibrate single-plane kinematics using bi-plane data, and two to autonomously solve “symmetry traps”.
Purpose/Aim of Study: Our study aims to answer the following questions: (1) How well does a calibration constant derived from gold-standard bi-plane measurements correct single-plane kinematics [3]? (2) How effective are automated symmetry-trap correction algorithms?
Materials and Methods: Kinematics from a previously reported study by the authors were used for analysis. These kinematics were obtained using a previously reported method [2] that leverages convolutional neural network (CNN) segmentations and contour alignment for model-image registration.
First, we attempt to adjust single-plane kinematics using a calibration constant determined from a Bland-Altman plot between single-plane and gold standard bi-plane data. The root-mean-squared differences between human-supervised kinematics and adjusted kinematics will be measured. Then, we evaluate two methods of overcoming “symmetry traps”. The first method is an algorithm that selects between a pose and it’s symmetric counterpart by choosing the orientation that minimizes absolute ad/abduction angle relative to the femoral component (“blind method”). For the second method, we create a spline that connects all ad/abduction angles for frames outside the “Ambiguous Zone” [2], and select poses within the “Ambiguous Zone” by selecting orientations closer to the newly created spline. (“spline method”). The sensitivity and specificity of each method will be reported.
Results/Findings: The Bland-Altman calibration adjustment reduced RMS differences between autonomous and human-supervised varus/valgus values from 1.64° to 1.38° (0.26° improvement). The sensitivity and specificity of the “blind” method are 61.7% and 66.8%, respectively. The sensitivity and specificity of the spline method are 1.1% and 99.0%, respectively.
Discussion: Decreased RMS differences using Bland-Altman calibration constant highlights inherent limitations in single-plane measurements. More gold-standard data should be used to ensure this method is robust to changes in both single-plane and bi-plane measurements. The high sensitivity of the “blind” method for solving symmetry traps suggests that this method does correct frames that fall into symmetry traps. However, further work must be done on determining those frames accurately to increase specificity. The spline method shows a high ability to preserve correct poses (high specificity), but unfortunately doesn’t seem to accurately capture the metrics necessary to define symmetry ambiguities (extremely low sensitivity). Further work can be done to increase the importance of relationships between other kinematic measures, instead of just focusing on ad/abduction.
Conclusion: Novel methods were introduced for overcoming single-plane limitations of measuring TKA kinematics. Further work is needed to achieve clinically acceptable accuracy.
[1] Bourne et al. CORR, 2010
[2] Jensen et al, arXiv, 2022
[3] Broberg et al, JOR, 2023
Figures
Figure 1#8661
Does CT Imaging Improve Tibial Defect Prediction for Zonal Fixation?
Marco Brenneis - Hospital for Special Surgery - New York, USA
*Friedrich Boettner - Hospital for Special Surgery - New York, United States of America
Sebastian Braun - Hospital for Special Surgery - New York, USA
Fernando Quevedo Gonzalez - Hospital for Special Surgery - New York, USA
Peter K. Sculco - Hospital for Special Surgery - NYC, USA
Dimitrios Flevas - Hospital for Special Surgery - New York, USA
*Email: drboettner@email.de
Purpose
Improvements in metal artifact reduction protocols for Computer Tomography (CT) allow for improved assessment of bone loss around tibial components prior to revision total knee arthroplasty (rTKA). The purpose of this study was to evaluate the accuracy of preoperative CT-based AORI grading and to correlate CT-based volumetric defect measurements with intraoperative AORI findings.
Methods
99 patients undergoing rTKA with preoperative CT-images were identified in a institutional revision registry. CT-image segmentation with 3D-Slicer Software was used to create 3D tibial bone defects which were then graded according to the AORI classification (Figure 1). These 3D-CT gradings were compared to preoperative X-ray and intraoperative AORI grading. Volumetric 3D-bone defect measurements were used to investigate the relationship between AORI classification and volumetric defect size in the three anatomic zones of the tibia.
Results
Substantial agreements between preoperative 3D-CT AORI and intraoperative AORI (kappa=.663;P<0.01) and fair agreements between preoperative X-ray AORI and intraoperative AORI grading (kappa=.304;P<0.01) were found (Figure 2). Moderate correlations between volume of remaining bone and intraoperative AORI grading were found in epiphysis (rS=-0.529;P<.001), metaphysis (rS=-0.557;P<.001) and diaphysis (rS=-0.421;P<.001). Small volumetric differences between AORI I vs. AORI II defects and relatively large differences between AORI II and AORI III defects in each zone were detected.
Conclusion
3D-imaging of bone defects improved the prediction of intraoperative tibial bone loss. CT-imaging appears to be an accurate tool for revision planning based on the concept of zonal fixation. The relatively small difference in defect volume between AORI I, IIa and IIb suggests that updated CT-based classifications might hold benefits for the planning of rTKA.
Figures
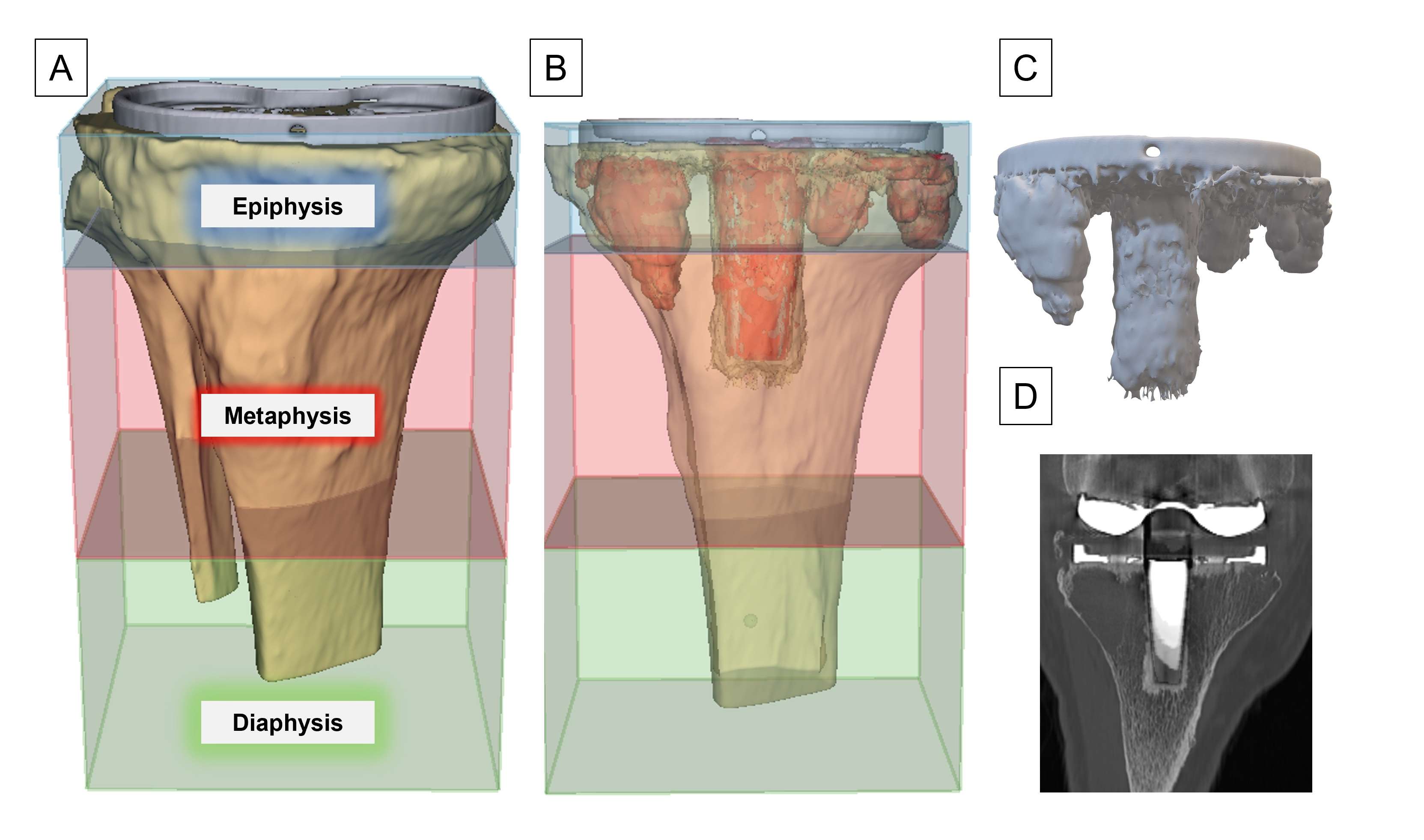
Figure 1
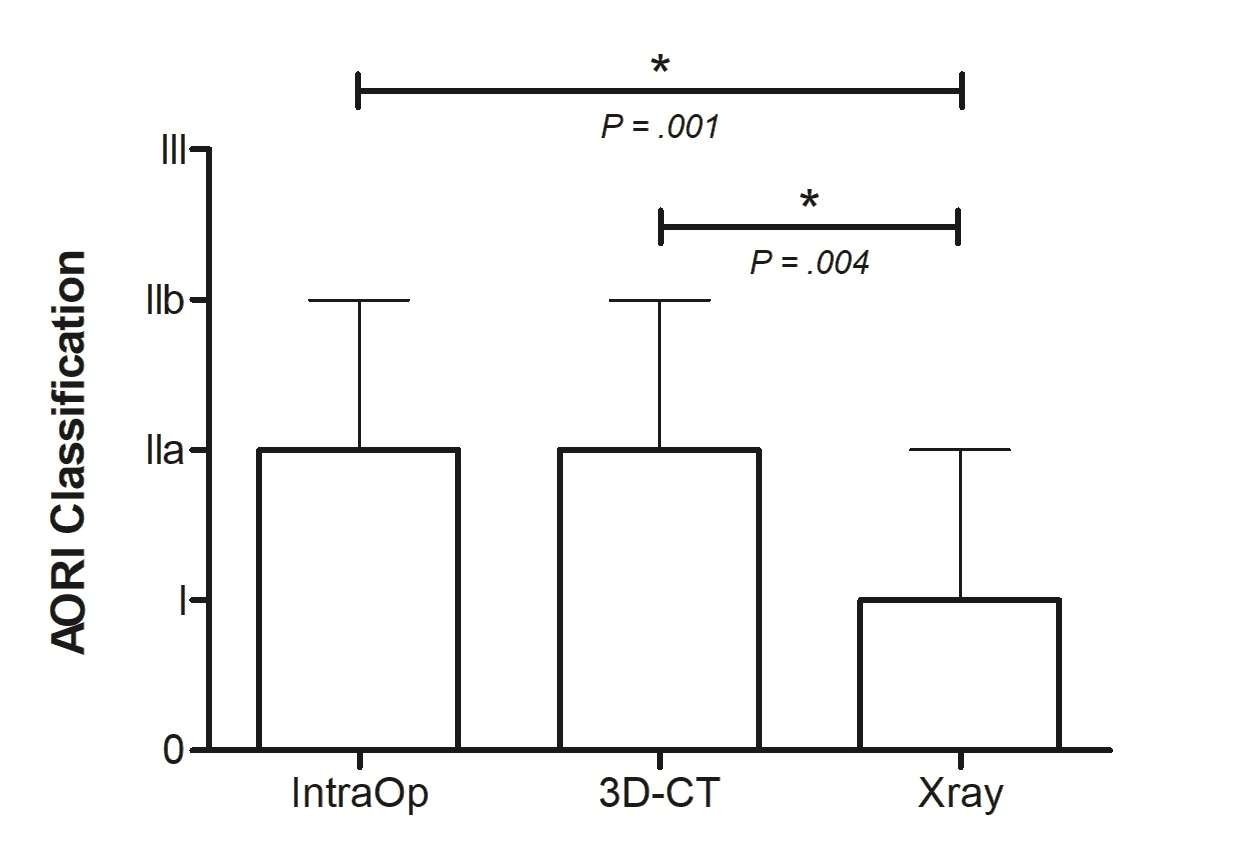
Figure 2#8182
Registration of Knee Articulating Surface Utilizing a Portable Handheld Laser Scanner: A Cadaveric Study
*Akram Habibi - NYU Langone Orthopedic Hospital - New York, United States of America
Morteza Meftah - NYU Hospital for Joint Diseases - New York, USA
Gordon Goodchild - Caira Surgical - New York, USA
*Email: akramhabibi01@gmail.com
INTRODUCTION: Image-based navigation systems for total knee arthroplasty require intra-operative registration of anatomical landmarks to the pre-operative images. This registration will orient the computer coordinate system to the surgical coordinate system and enable real-time tracking of the patient anatomy relative to the scan data and surgical plan. The accuracy of the registration of the anatomic is directly related to the overall accuracy of the navigation system. Unfortunately, this process is long, tedious, and involves multiple steps with an optical tracking probe to identify landmarks. We hypothesized that use of a handheld laser scanner can achieve an accurate and fast registration of cartilage surface with MRI images.
METHODS: MRI scans were obtained for six cadavers and images were segmented to produce 3D models of the articulating surface of tibia and femur using commercial software (MIMICS, Materialise). A portable, hand-held laser scanner (E4D Technologies) was used to generate surface models of the same tibia and femur. The 3D scans were registered to the segmented MRI scans. Multiple scans were performed by 4 operators to calculate inter- and intra-observer reliability. An iterative closest point (ICP) algorithm was then used to align the handheld scans to the MRI scans (Figure 1). The accuracy of the registration fit was determined by the Root Mean Square (RMS) difference between the two surfaces in the region of interest (avoiding cartilage).
RESULTS: There were a total of 72 scans. All 6 cadaveric knees were successfully registered to their MRI images with <1mm mean RMS error for both femoral and tibial scan (Figure 2). Each knee was well within the error threshold objective of 1 mm RMS error. The mean time for successful registration was 54 seconds (± 24 sec). The inter- and intra-observer reliability were 0.92 and 0.87, respectively.
CONCLUSION: All six cadaveric knee specimens were successfully scanned and multiple operators were able to successfully scan a knee within the targeted time with minimal training. Accurate registration of the tibia and femur using a handheld 3D scanner was achieved for all six cadaveric knees with RMS errors consistently less than 1 mm for all scans and operators, demonstrating proof-of-concept of a handheld scanning and registration processes. Use of a hand-held scanner has the potential to revolutionize the current registration process and reduce the intra-operative time to less than one minute. Further advancement of this scanning technique will utilize the intra-operative scans to identify bony landmarks and avoid the need for pre-operative 3D images.
Figures
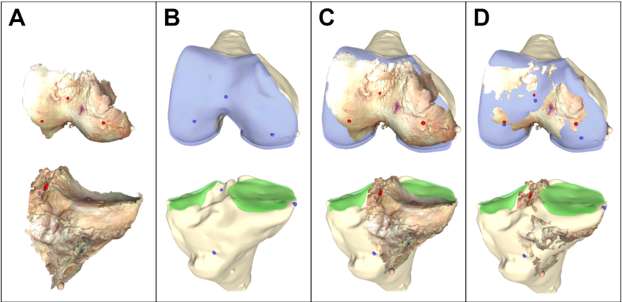
Figure 1
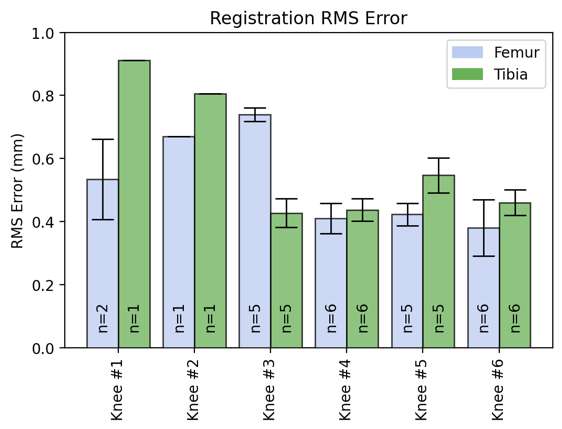
Figure 2#8217
Functional PET/MRI of Knee Synovial Macrophage Activity Before and After Total Knee Arthroplasty
*Zachary Koudys - Western University - London, Canada
Mathew G Teeter - Schulich School of Medicine and Dentistry, Western University and London Health Sciences Centre - London, Canada
Brent Lanting - London Health Sciences Centre - London, Canada
Tom Appleton - Western University - London, Canada
Jonathan Thiessen - Lawson Health Research Institute - London, Canada
*Email: zkoudys@uwo.ca
INTRODUCTION: Total knee arthroplasty (TKA) is a surgery with high success rates and good patient outcomes. However, 6.9% of knee replacement surgeries are revisions of old implants due to ongoing pain, stiffness, and loosening. Revision TKA is more expensive and has worse patient outcomes. Recent studies have begun to explore the role of the inflammatory response in poor TKA outcomes. Inflammation is an important predictor of pain in osteoarthritis (OA) and synovial inflammation may play a role in knee stiffness after TKA. Macrophages are the dominant immune cells of the synovium and regulate knee inflammation, and when activated, they upregulate mitochondrial translocator protein (TSPO) expression. The current standard of care features Magnetic Resonance Imaging (MRI) to assess structural changes in the joint. Positron Emission Tomography (PET) can be used to image important biological processes at the cellular level. [18F]FEPPA is a PET tracer that targets TSPO with high specificity. The goal of this work was to validate the use of [18F]FEPPA PET/MRI in the assessment of macrophage activation in knee synovial tissue. This method may allow non-invasive imaging of important inflammatory processes and understand the role of activated macrophages in ongoing pain, stiffness, and loosening after TKA.
METHODS: To validate the use of [18F]FEPPA in imaging activated macrophages in vivo, synovial tissue was gathered from 12 participants with end stage OA who underwent primary TKA. Knee synovial tissue was sectioned and embedded on slides. Tissue sections were incubated in [18F]FEPPA and imaged by autoradiography for 6 hours. Adjacent tissue sections were incubated with DAPI, CD68 antibody, and TSPO antibody for immunofluorescent analysis of the actual macrophage activation. 6 of the full cohort were imaged in a 3T hybrid PET/MRI after injection with [18F]FEPPA and a 45-minute uptake period. MR of both knee joints was performed with sequences including 3D Dual Echo Steady State (DESS), Bilateral T1 weighted and Fast Spin Echo (FSE) with metal artifact reduction if contralateral TKA was present. Standardized Uptake Values (SUV) were calculated from the measured PET signal, injected dose, and patient mass.
RESULTS: [18F]FEPPA tracer uptake calculated from autoradiography correlated to the true macrophage activation measured through TSPO+ immunofluorescence with r = 0.85 and p = 0.0029. SUV calculated from PET/MRI was correlated to true macrophage activation found through immunofluorescence with r = 0.90 and p = 0.083. Attenuation correction maps corrected for metal artefacts enabled visualization and measurement of tracer uptake surrounding the femoral and tibial components.
CONCLUSION: Understanding the role of knee joint inflammation may be important in managing pain and stiffness after TKA. The underlying causes of poor TKA outcomes are often unclear. [18F]FEPPA PET/MRI uptake in knee synovial tissue was validated to correlate to the true macrophage activity. This tool may be able to diagnose a cellular response as the cause of pain or dissatisfaction and assist in earlier clinical dissatisfaction management after TKA. Future work using [18F]FEPPA PET/MRI could assess different reaction types to metal or plastic implant debris, joint stiffness and fibrosis, and periprosthetic infection.
Figures
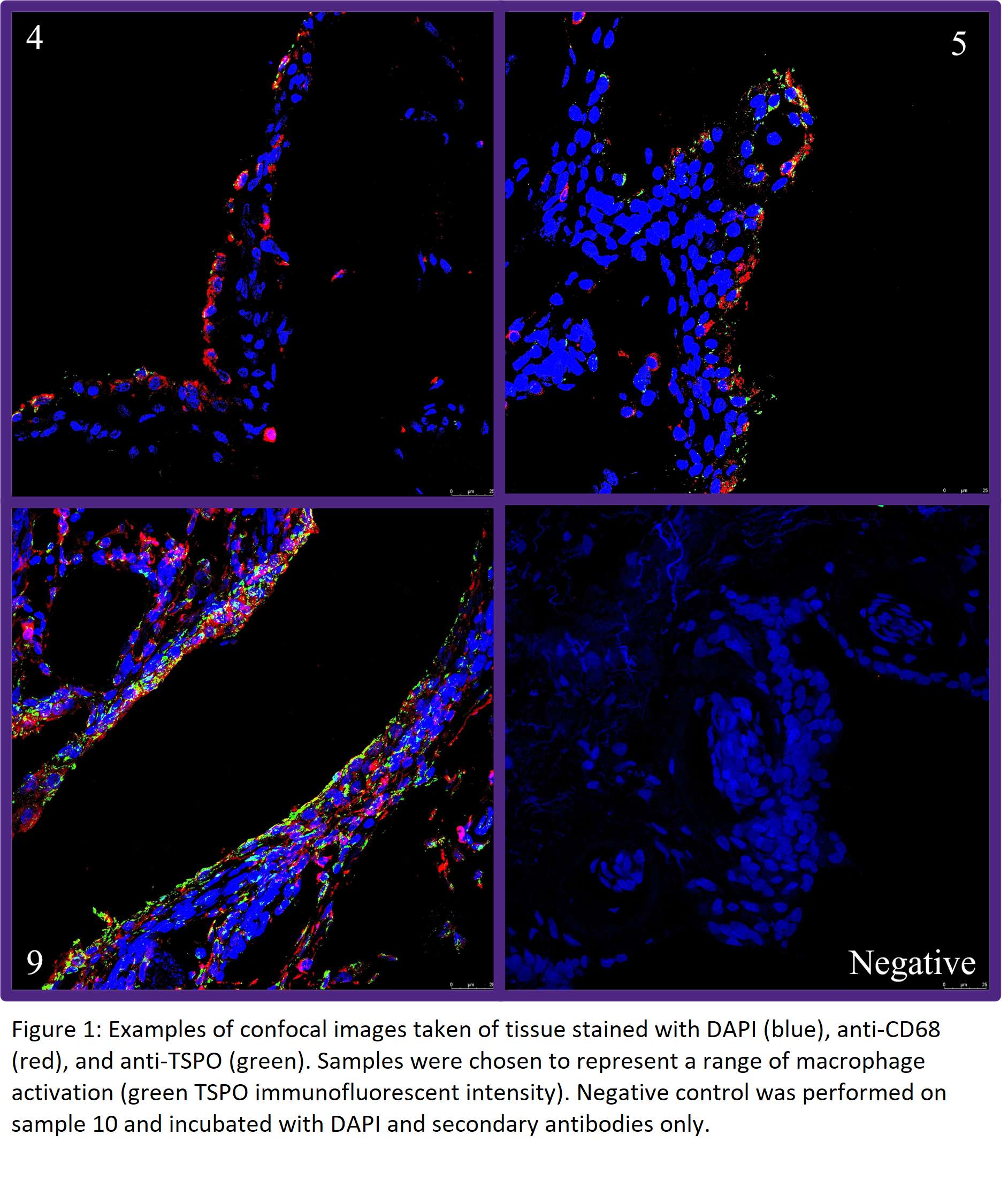
Figure 1
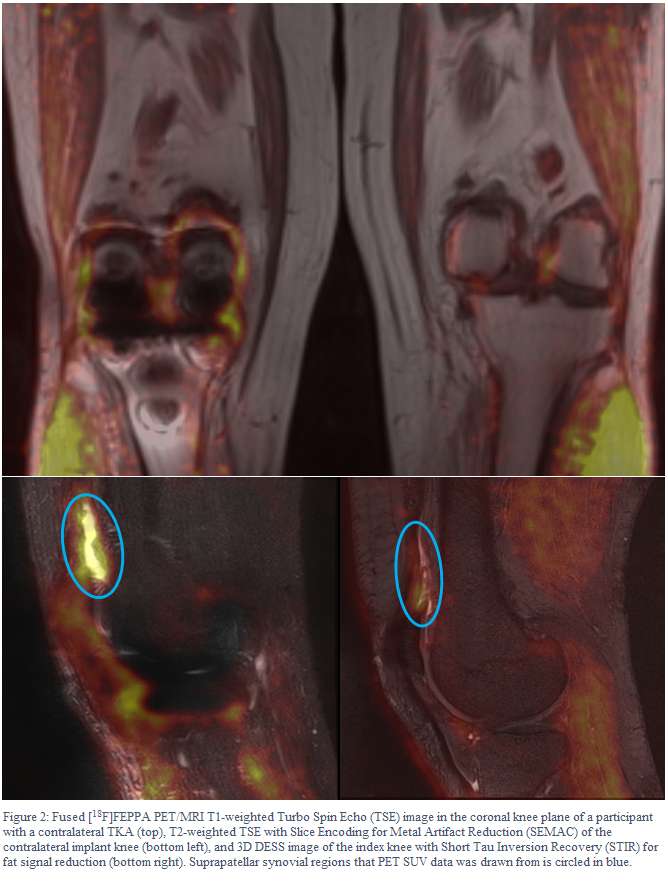
Figure 2
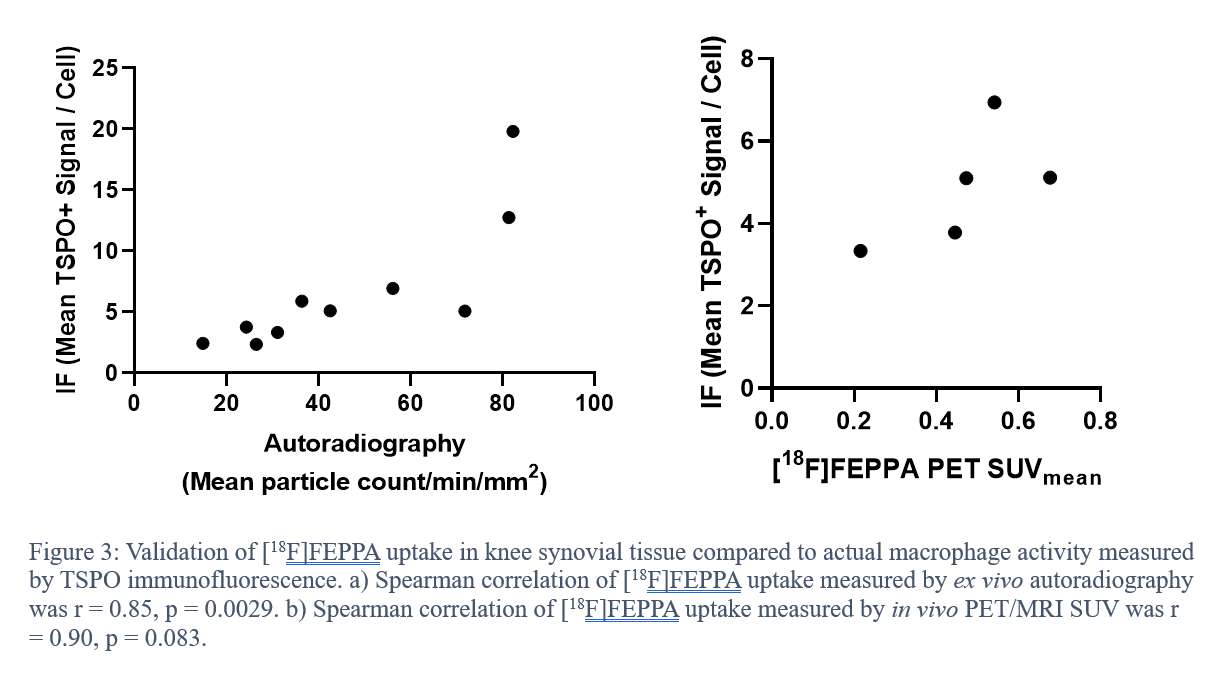
Figure 3#8350
Surgical Bootcamps With Virtual Reality Deliver Technical Skills and Trainee Confidence: A Randomised Controlled Trial
*Ria Varma - Imperial College London - London, GB
Monil Karia - Imperial College London - London, United Kingdom
Sachit Mehta - Imperial College London - London, United Kingdom
Thomas Edwards - Imperial College London - London, United Kingdom
Justin Cobb - Imperial College - London, United Kingdom
Gareth Jones - Imperial College - London, United Kingdom
Kartik Logishetty - Imperial College - London, United Kingdom
*Email: ria.varma19@imperial.ac.uk
Virtual Reality has been validated as a training tool, but has not previously been integrated into a surgical curriculum or into bootcamps for junior residents. We mapped the national orthopaedic training curriculum with VR training, to deliver a hybrid bootcamp of lectures, sawbone demonstration, and hands-on training for total hip arthroplasty (THA). We assessed its feasibility, and conducted a randomised controlled trial, comparing the effectiveness VR versus expert-delivered lectures for learning technical skills and improving confidence.
A total of 14 surgical trainees (one female, 13 males; mean age 28 years (26 to 30)) participated in this 1:1 randomised controlled trial. All had no prior experience of assisting in or performing anterior approach THA. The trainees were divided into two groups of 7; a VR group and control group. The initial completed one hour of fully immersive VR training in a simulation laboratory, while the latter received an interactive lecture alongside the op-tech as conventional preparation for learning THA. Subsequently, participants performed a sawbone THA, assessed independently by two hip surgeons blinded to candidates’ training exposure. Primary outcomes were technical and non-technical surgical performance, which were evaluated by a validated national Procedure Based Assessment (PBA) specific to Total Hip Arthroplasty. Secondary outcomes were acetabular component orientation, knowledge assessment, and perceptions of training received.
After a single session, VR-trained participants performed at a similar or higher level to controls. They achieved a median PBA level of 3a (procedure performed with minimal guidance or intervention (needed occasional help)) versus control surgeons who obtained a median of 2b (guidance or intervention required for key steps only). The VR arm also achieved superior acetabular component placement in the coronal pelvic plane (inclination; median degrees from target 4 (IQR 2 to 7) vs 10 (IQR 10 to 17)) and comparable with controls for axial plane (anteversion; mean degrees from target 9 (sd 7) vs 8 (sd 9)). Knowledge assessment yielded a median of 6 (4 to 6) and 5 out of 10 for VR and control groups respectively. Training was scored favourably by both arms, with VR training scoring consistently higher for all domains. Mean perceived anatomical knowledge gained was scored 4.7 versus 3.9 and surgical instrument knowledge was 4.7 versus 3.7 (both out of 5). Trainees scored VR training higher for its ability to help them achieve core surgical training competencies; 4.9 versus 4.1 out of 5.
This study showed that a VR-augmented bootcamp is a feasible and effective method for teaching hip arthroplasty. The VR group displayed similar or higher levels of technical and non-technical surgical performance as indicated by the PBA scores. Moreover, superior outcomes were demonstrated not only in the acetabular component orientation, but the VR group also displayed higher scores in their perceived training quality and subsequent confidence. We recommend the widespread delivery of VR-augmented bootcamps for training junior residents.
#8448
Periprosthetic Fracture Rates Using Collared Stems in Uncemented Primary Total Hip Arthroplasty With a Posterior Approach
Robert Ricotti - Hospital for Special Sugery - New York, USA
Dimitrios Flevas - Hospital for Special Surgery - New York, USA
Ruba Sokrab - Hospital for Special Surgery - New York, USA
Jonathan Vigdorchik - Hospital for Special Surgery - New York, USA
David J. Mayman - Hospital for Special Surgery - New York, USA
Seth A. Jerabek - Hospital for Special Surgery - New York, USA
Thomas Sculco - Hospital for Special Services - New York, USA
Peter K. Sculco - Hospital for Special Surgery - NYC, USA
*Nicholas Schiller - Hospital for Special Surgery - Miami Beach, United States of America
*Email: n.schiller1@umiami.edu
Periprosthetic femur fracture (PFF) is a major complication following total hip arthroplasty (THA) that carries significant morbidity, mortality, and economic burden. Uncemented femoral stems are highly preferred in primary THA, but are associated with higher PFF risk than cemented stems. Collared stems have shown promise in reducing PFF rates following uncemented primary THA when compared to collarless stems, while maintaining a similar prosthetic design (Figure 1). The purpose of this study is to assess PFF rate among two surgeons before and after switching from a collarless to collared stem design.
This retrospective study included 1,888 uncemented primary THAs using the posterior approach performed by two attending surgeons at one institution from January 2016 to December 2022. Both surgeons switched from a collarless stem design (Synergy, Smith & Nephew; Summit, DePuy Synthes) to a collared design (Actis, DePuy Synthes) in mid-2020, which was the only change in their surgical practice. Data was collected regarding stem design, frequency of PFF, and requirement for revision surgery. Periprosthetic fractures were identified and confirmed using medical records and radiographic imaging. Fracture rates and percentages between collared and collarless stems were then analyzed. Power analysis confirmed over 80% power for the sample, and a Fisher’s Exact Test was performed to determine if there was a significant association between collared and collarless stem use on PFF rates and revision.
A total of 1,888 uncemented primary THAs were eligible for analysis. Demographics of the collarless and collared stem groups can be found in Figure 2. 1,123 (59.5%) patients received a collarless stem, and 765 (40.5%) patients received a collared stem. In total, 17 (0.9%) PFFs occurred over the study period. There were 16 fractures (1.4%) out of 1,123 collarless stems, and 1 fracture (0.1%) out of 765 collared stems (p = 0.002). The majority of fractures (N = 14, 82.4%) occurred within 90 days following THA. Revision rate for PFF decreased from 0.9% in the collarless stem group to 0.1% in the collared stem group (p = 0.03).
Collared stems were associated with a significant decrease in PFF rate compared to collarless stems in uncemented primary THA, as well as a lower revision rate. Future studies are encouraged to continue to investigate PFF and other complications with a collared stem design.
#8573
How Does a Collar Impact the Risk of Periprosthetic Femoral Fracture for Two Distinct Patient Types?
Ryan Helbock - Hospital for Special Surgery - New York, USA
*Fernando Quevedo Gonzalez - Hospital for Special Surgery - New York, USA
Joseph Lipman - Hospital for Special Surgery - New York, USA
Simarjeet Puri - Hospital for Special Surgery - New York, USA
Timothy Wright - Hospital for Special Surgery - New York, USA
Jonathan Vigdorchik - Hospital for Special Surgery - New York, USA
Elizabeth B. Gausden - Hospital for Special Surgery - New York, USA
Peter Sculco - Hospital for Special Surgery - New York, USA
*Email: quevedogonzalezf@hss.edu
Introduction: Periprosthetic femoral fracture (PFF) is a prominent cause of revision for primary total hip arthroplasty (THA) [1,2]. Prior biomechanical research indicates that femoral components with a collar provide protection against PPF in normal cadaveric bone tested with simplified loads [3,4]. Our goal was to evaluate the biomechanical impact of a collar in representative THA patients, under loading conditions that occur during daily activities. We created a biomechanical computational model to answer the following question: in patients who sustained PFF, would a collar have provided biomechanical protection?
Methods: Under IRB approval, we identified seven patients (6 females, age 51-75, BMI 26.2-40.0) that received robotically assisted primary THA and suffered an early PFF. Each PFF case patient was matched to a control patient who also underwent robotically assisted primary THA but without PFF. Matching criteria included age, weight, height, sex, race, and implant design, size, and offset. At our institution, CT-scans are standard-of-care for robotically assisted THA, which allowed us to create finite element models including patient-specific geometry and distribution of bone material properties (Fig. 1). We reverse engineered the femoral implants by 3D scanning the components. We used JointTrack Auto (https://github.com/BRIO-lab/Joint-Track-Machine-Learning) to align the bone and implant geometries to anteroposterior X-rays obtained in the recovery unit to reproduce the patient-specific implantation. A generic collared version of each implant was created, assuming perfect collar-calcar contact. Models were meshed with 1-3mm linear tetrahedral elements. The bone was assigned elastic non-homogeneous material properties from the CT-scan [5], and the implants were assumed to be titanium alloy. We assumed line-to-line frictional contact between implant and bone. All models were loaded with the maximum hip contact force and muscle forces from stair ascent [6], adjusted for bodyweight. Bone at the interface with the implant with strain greater than 50% of the static failure strain of femoral bone tissue was assumed at risk of failure [7]. We compared the “as-implanted” and collared conditions with paired t-tests with a significance of 0.05.
Results: Adding a collar reduced (p=0.02) the interfacial bone volume at risk of PFF of the cases by 7.5%; however, the collar did not impact the PFF risk for controls (reduction of 1.0%, p=0.06). Cases and controls had no different (p=0.65) percentage of bone at risk of failure in the “as-implanted” condition (Fig. 2). The collar reduced the risk of PFF in the majority of the patients (13 of 14), having greater impact for patients at higher risk of PFF.
Conclusion: A collar can mitigate the strains that could lead to PFF for select higher risk patients, but the beneficial effect of the collar is not universal. The collar demonstrated greater benefit to patients that experienced PFF after THA compared to controls that did not.
References: [1] AJRR 2022 Annual Report; [2] Bendich, et al., J Arthroplasty. 2022; [3] Lamb, et al., Bone Joint J. 2019; [4] Konow, et al., Bone Joint J. 2021; [5] Morgan, et al., J Biomech. 2003; [6] Heller, et al., J Biomech. 2005; [7] Morgan and Keaveny, J Biomech. 2001.
Figures
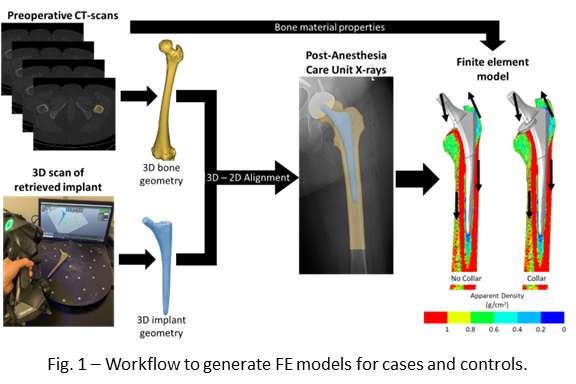
Figure 1
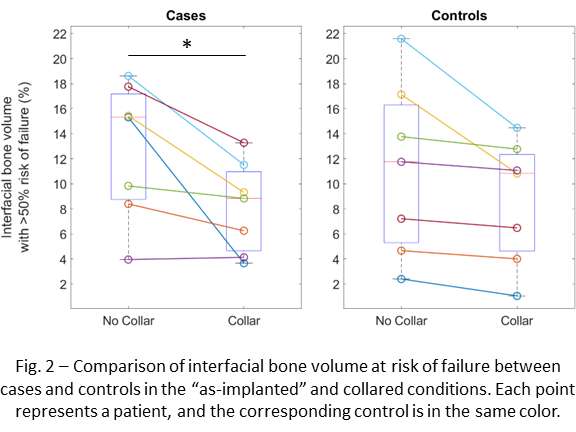
Figure 2#8335
Effect of Bearing Size and Concavity on Outer Articulation Wear of a Dual Mobility Construct as Compared to Fixed Bearings
*Ilya Borukhov - Stryker Orthopedics - Mahwah, USA
Sally LiArno - Stryker Orthopaedics - Bergenfield, USA
Rafael Baez - Stryker Orthopaedics - Mahwah, USA
Tomasz Bazel - Stryker Orthopaedics - Mahwah, USA
*Email: ilya.borukhov@stryker.com
INTRODUCTION
In-vitro wear studies have shown that when the dual mobility (DM) construct of a total hip arthroplasty (THA) is allowed to articulate freely, the wear performance of the bearing is similar, if not improved, compared to fixed bearings of the same size[1]. Soon-to-be published revisions to the ISO 21535 will require wear testing of DM constructs in both unconstrained, dual articulation conditions, as well as constrained conditions for which only the outer bearing articulates. There is, however, limited literature on the effect of bearing size and concavity on the outer bearing only wear performance of a DM construct. We evaluated the wear characteristics of the outer, convex, bearing of several sizes of a DM construct and compared the wear performance to historical data on similar sizes of fixed, concave, bearing constructs.
METHOD
A modular DM THA consisting of a CoCr liner and polyethylene insert was evaluated. The polyethylene insert that articulated against the CoCr liner was modified post-production to constrain all motion to the outer bearing surface (Figure 1). All polyethylene inserts used were sequentially crosslinked and annealed (X3, MDM, Stryker, Mahwah, NJ). The outer bearing sizes (n=2 each) evaluated were 36, 38, 40, and 58 mm. A hip joint simulator (MTS, Eden Prairie, MN, USA) was used for testing with cups positioned superior at 50? inclination (Figure 1). Testing was conducted per ISO 14242-3 (R2019) with a modified load profile consisting of a physiological Paul curve with a peak axial force of 2450N [2]. Testing was conducted for 2 million cycles (mc) and a linear regression was assessed between bearing size and wear rate. Historical evaluations of fixed bearing devices (LFIT CoCr, Trident X3, Stryker Orthopaedics, Mahwah, NJ) were reported for comparison.
RESULTS
For the outer bearing of the DM construct, wear rate ranged from 3.09-8.44 mm3/mc and there was a trend for larger size bearings to generate more wear (R2=0.69) (Figure 2). Historical data of fixed, concave, bearing devices ranging from 28–44mm showed a similar trend (R2=0.26); however, the regression line was shifted by -2.1 mm3/mc. The slopes of the two regression lines were similar (0.17 mm3/mc?mm).
DISCUSSION
Wear of THA devices is dependent on bearing size, with larger bearings exhibiting higher contact area and contact sliding distance which would theoretically generate more wear. The results of this study confirm this with larger DM constructs generating more wear when constrained to only articulate on the outer bearing. In addition, the convex outer articulation of a DM bearing generated more wear than the concave inner articulation of a fixed bearing THA. This is likely due to the orientation of the polyethylene insert and kinematics during the hip wear simulation. The polyethylene insert is oriented superior and stationary for a fixed bearing THA, while for a DM THA, when constrained, is oriented inferior and moving. This difference in orientation likely generates more contact sliding distance on the polyethylene insert in the DM THA, generating more wear.
REFERENCES
[1]Loving L,J.Orthop.Res,33(3):398-404,2015Mar. [2]Paul,JP,Proc.Inst.Mech.Engrs.181(3J):8-15,1966
Figures
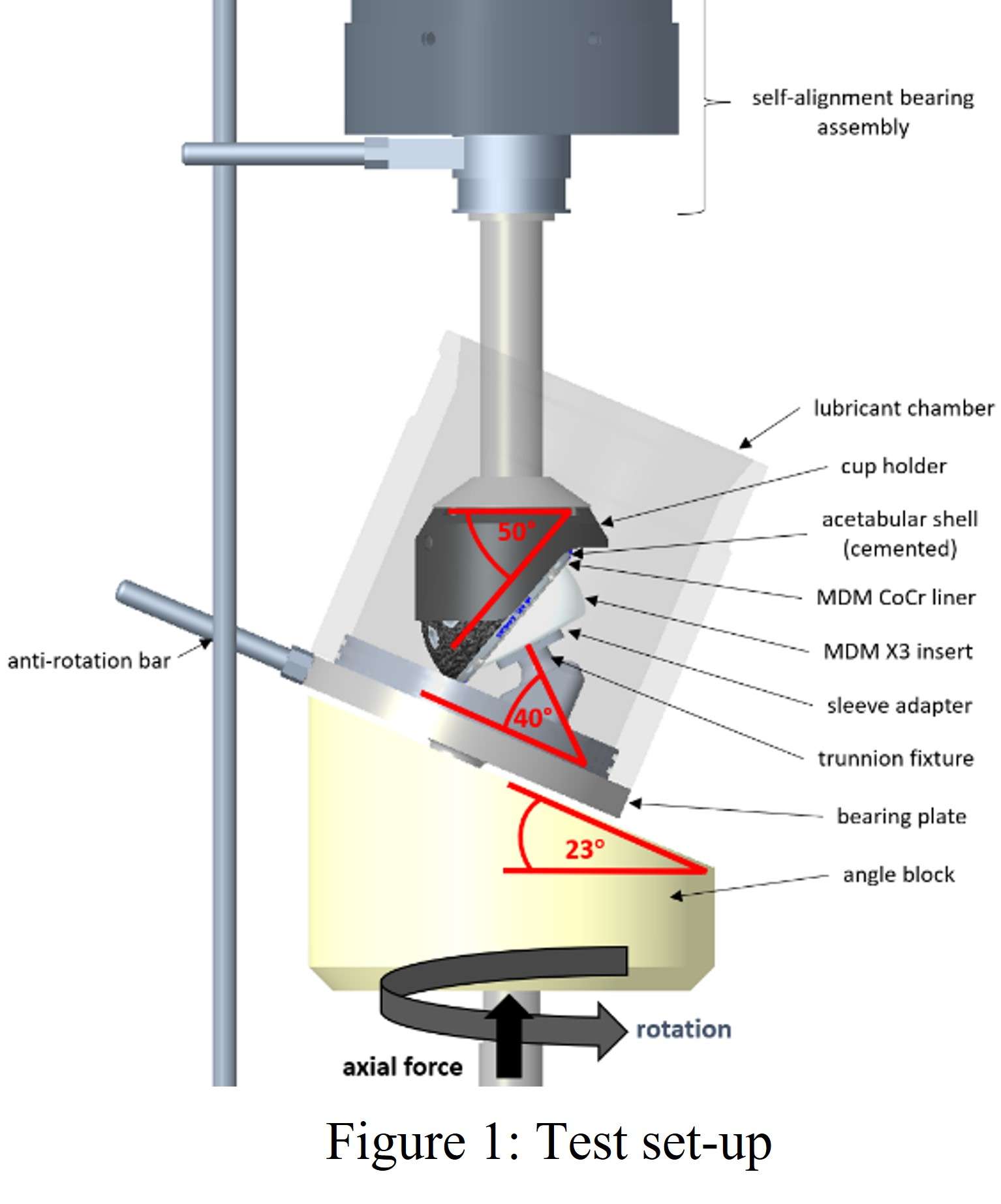
Figure 1
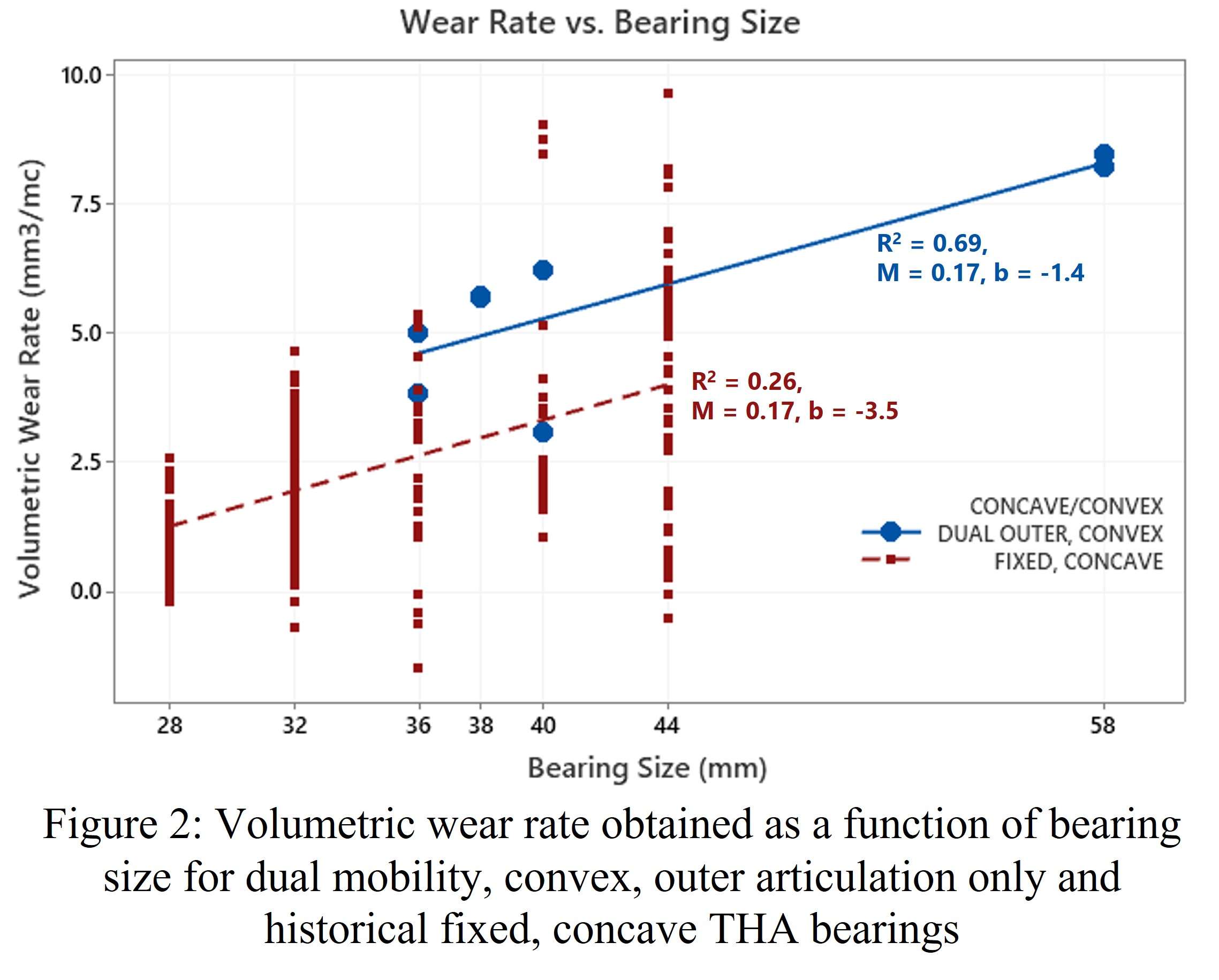
Figure 2#8404
Monitoring of in Vitro Motion of Unconstrained Dual Mobility Polyethylene Liners for Total Hip Replacement Under Different Kinematic Conditions
Mackenzie Smeeton - University of Leeds - Leeds, United Kingdom
Matthew Shuttleworth - University of Leeds - Leeds, Select Country
James Anderson - DePuy Synthes Joint Reconstruction - Leeds, United Kingdom
Graham Isaac - DePuy International Ltd - Leeds, Select Country
Tim Board - WWL NHS Trust - Wigan, United Kingdom
*Ruth Wilcox - University of Leeds - Leeds, United Kingdom
Robert Kay - University of Leeds - Leeds, GB
Sophie Williams - University of Leeds - Leeds, United Kingdom
*Email: r.k.wilcox@leeds.ac.uk
Introduction
Despite their increasing use and broadening of indications, the functional mechanisms of Dual Mobility (DM) Total Hip Replacements (THRs) are not well understood particularly with regards to the unconstrained polyethylene (PE) liner motion. Current experimental methodologies are not suitable to assess the motion of these components under clinical conditions. The aim of this pilot study was to use a non-line-of-sight tethered sensing system to monitor the orientation of DM polyethylene liners in-vitro under physiologically relevant loading, displacement, and lubrication conditions.
Methods
Three BI-MENTUM™ DM component sets (DePuy Synthes) were tested on a single-station, six-axis hip joint simulator, which applied twin-peak axial loading and rotational displacements to the components to recreate standard gait as specified by ISO 14242-1 for 3,600 cycles. Each component set underwent three repeats and the orientation of the unconstrained liner was monitored using a validated inertial tracking system (1). PE liner movement was described in terms of inclination, azimuth and precession as described in Figure 1. Single components were then tested three times under adverse conditions (high cup inclination angle, low swing phase load (100N compared with 300N) and standard gait at 2Hz).
Results
The surface between the femoral head and liner appeared to be the primary articulation site for the implants with minimal engagement of the outer bearing (i.e., PE mobile liner/shell interface) observed. Under standard gait conditions the most noticeable observation was that the liners also appeared to steadily rotate about the femoral axis throughout each test. However, generally intra- and inter-component variability of the liner motions was observed (Figure 2). This highlights the potential complexity and sensitivity of DM mechanics, suggesting that small variations to the in-vivo environment (e.g., component position) may have a significant impact on the mechanical behaviour of the implant.
Differing adverse conditions had different effects when compared to standard gait (Figure 3). High cup angle produced a similar profile but with more variability in one repeat. Standard gait at 2Hz produced movement profiles which had some similarities to standard gait at 1Hz. However reduced swing phase load produced a movement profile which was both more rapidly changing and erratic than that of standard gait. The tests in the last two conditions were truncated to avoid sensor tether damage.
Conclusion
Pilot data generated in this study suggests that motion primarily occurs at the inner bearing (i.e., head/liner interface) of these devices, although the intra- and inter-component behaviour of the unconstrained DM liner exhibits variability of its motions. Preliminary data suggests that adverse test conditions may influence behaviour of DM liners, but further research is required to confirm. Outputs from this investigation will inform a more extensive study.
References: Shuttleworth et al. Sensors. 2023, 23(2), 904.
Acknowledgements: This work was supported by the EPSRC (EP/R003971/1) and NIHR Leeds Biomedical Research Centre
Figures
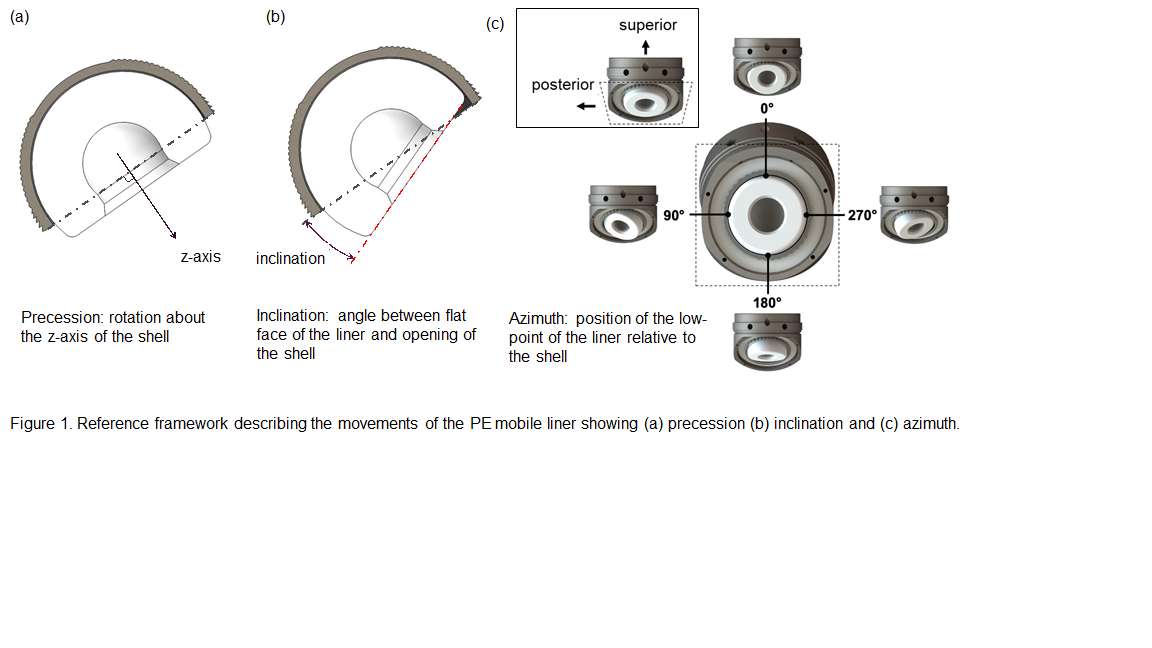
Figure 1
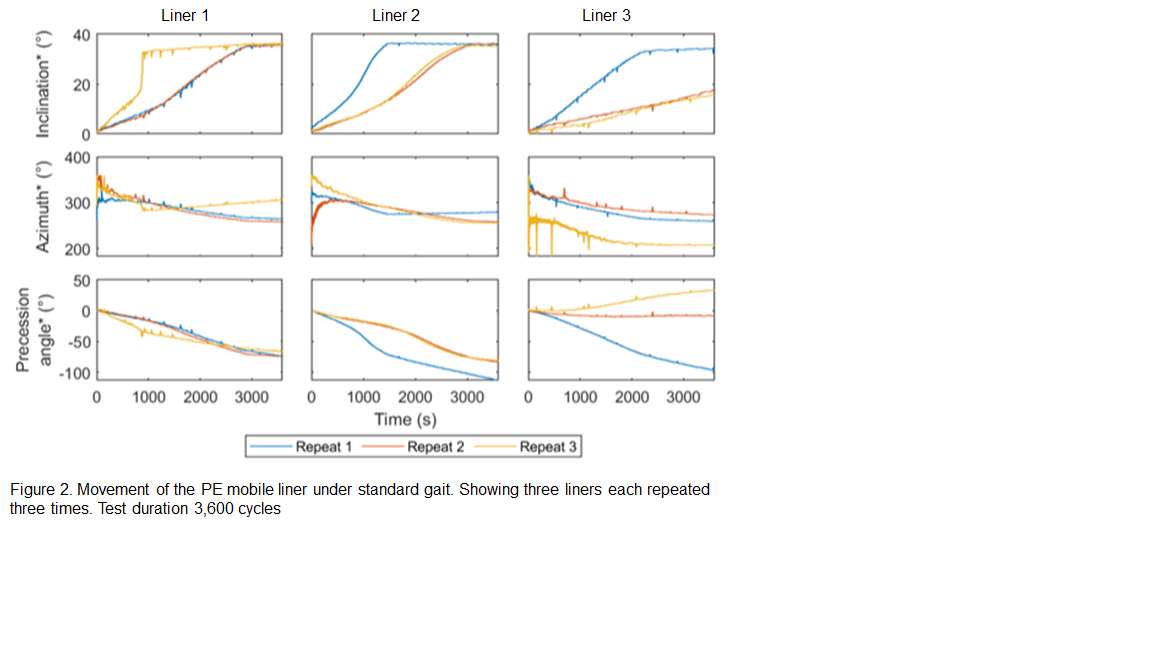
Figure 2
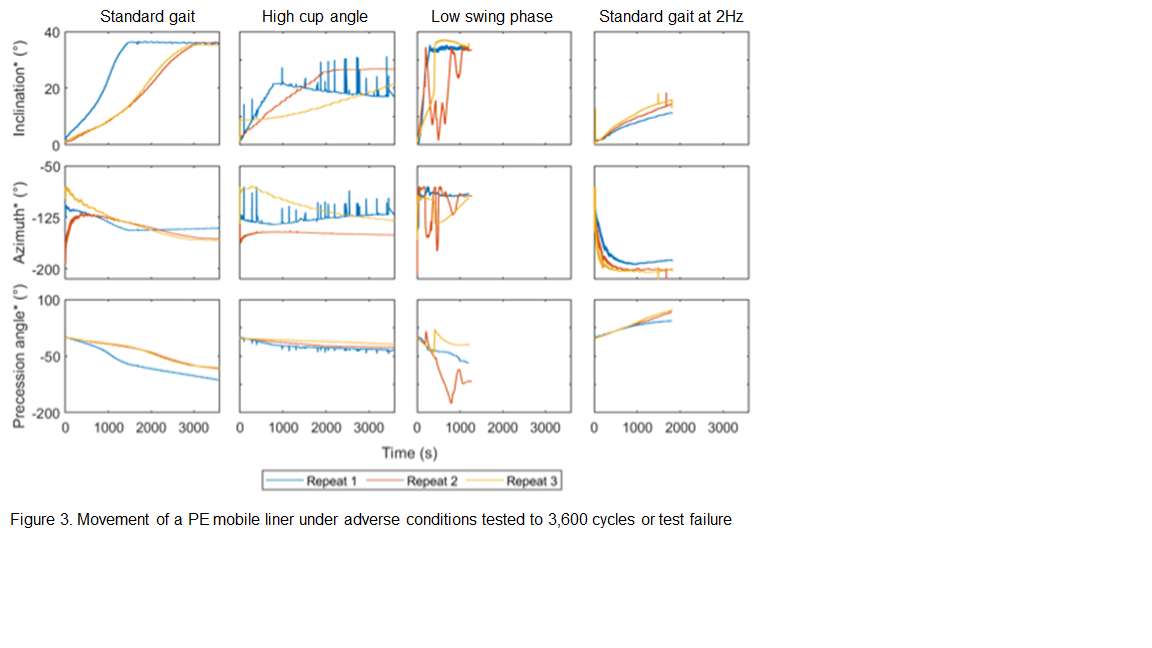
Figure 3#8499
Are Bone Sparing Stems Truly Bone Sparing?
*Jarrod Nachtrab - University of Thennessee - Knoxville, USA
Thang Nguyen - University of Tennessee - Knoxville, USA
Michael LaCour - University of Tennessee - Knoxville, USA
Richard Komistek - The University of Tennessee - Knoxville, USA
*Email: jnachtra@vols.utk.edu
INTRODUCTION:
Some stem systems are being marketed as bone sparing, but does that truly mean they remove less overall bone? Most companies only consider the location of the neck cut for this claim, without consideration for the canal reaming that must also be done. The objective of this study is to compare bone removal between three stem systems, looking at both the location of the neck cut with respect to the lesser trochanter as well as a volumetric reaming within the canal, to get a better idea of overall bone removal for a given stem system.
METHODS:
A total of 58 femurs from segmentation of CT scans were evaluated in this study. A previously validated pre-operative planning tool was used to evaluate the proximal portion of each subject-specific femoral bone model. The three stem systems used in this study, Tri-Lock, Corail, and Emphasys are shown in Figure 1. Of these three stem systems, only Tri-Lock has been marketed as bone sparing and Emphasys is an implant system designed with the stem and the cup together. After virtual implantation, the location of the Lowest Calcar Point (LCP) with respect to the Letter Trochanter (LT) was used to determine the location of the neck cut. Additionally, the volume of bone removal within the canal needed for each stem was calculated by finding the volume of the stem that overlapped with the cortical bone surrounding the canal.
RESULTS:
When comparing the overall distances from the LT to the LCP, Tri-Lock had an average total distance of 34.63mm±4.39mm (24.19mm–48.15mm), Corail had an average total distance of 31.40mm±4.22mm (22.72mm–42.01mm), and Emphasys had an average total distance of 33.26mm±4.06mm (24.29mm–45.28mm). Larger numbers indicate a more proximal neck cut. When looking in only the superior/inferior direction, LT to LCP distances in the SI direction were as follows: Tri-Lock had an average of 30.37mm±5.40mm (14.88mm–45.38mm), Corail had an average of 25.53mm±5.43mm (13.62mm–38.13mm), and Emphasys had an average of 28.14mm±5.06mm (17.75mm–41.10mm). The rest of the data for the LT to LCP distances can be found in Table 1. The volumetric data of the bone that needed to be removed through reaming the canal was found for each stem system: Tri-Lock required 0.462cm3±0.319cm3 (0.018cm3–1.274cm3) on average, Corail required 0.018cm3±0.045cm3 (0.000cm3–0.226cm3) on average, and Emphasys required 0.006cm3±0.023cm3 (0.000cm3–0.141cm3) on average.
CONCLUSION:
The results of this study do show that Tri-Lock is the most bone sparing of the three according to the LT to LCP distance, which is typically used to signify a bone sparing stem system. However, Tri-Lock also required the most cortical bone removal through reaming of the canal when compared to the other two stem systems, indicating a tighter fix proximally for this stem, while both Corail and Emphasys had minimal to no amounts of bone removal required within the canal. This shows that marketing a stem as bone sparing could be misleading.
Figures
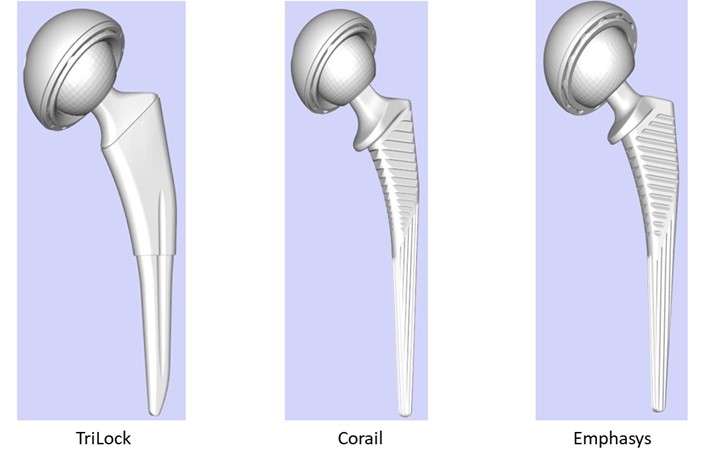
Figure 1
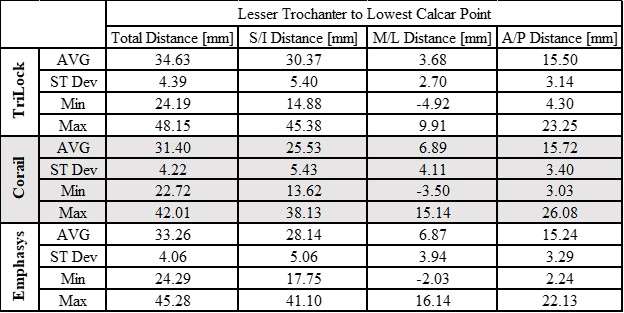
Figure 2#8178
Measured Impaction Forces During Implantation Are Affected by the Sensor Position and the Implant Mounting
*Peter Schlieker - TUHH Hamburg University of Technology - Hamburg, Germany
Michael Morlock - TUHH Hamburg University of Technology - Hamburg, Germany
Gerd Huber - TUHH Hamburg University of Technology - Hamburg, Germany
*Email: peter.schlieker@tuhh.de
INTRODUCTION
Impaction forces during implantation cannot be directly measured at the point of interest (PoI), such as taper connections or the implant-bone-interface. Consequently, there is a certain inertia and stiffness between the PoI and the force sensor, which is particularly relevant for dynamic processes. Comparison between different studies is difficult, due to different implantation instruments and different measuring positions (mallet, top or bottom of the impactor, stem). The impedance of the associated soft tissue is also commonly completely disregarded. The aim of this study was to investigate whether forces measured at different positions are comparable and whether soft tissue impedance has to be considered.
METHODS
Metal ball heads were repeatedly assembled to stem tapers (DePuy Synthes, MA; n = 8 each), which were attached to a platform with a high mass (167 kg) by different stiffnesses (0.6 N/mm, 1.9 N/mm, 4.0 N/mm, 5.0 N/mm, rigid) to simulate soft tissue impedance (total n = 64). The 1st assembly served as preconditioning and reference measurements (4.0 N/mm) were repeated at the 2nd, 5th, 10th and 15th measurement. After disassembly, parts were cleaned, randomly reassigned and reused. Forces were measured at (i) the tip of the surgical mallet (Fig. 1; 9041A, Kistler, CH), (ii) at the head impactor close to the tip and (iii) at the stem below the taper (both 9333A, Kistler, CH). Data acquisition was performed at 800 kHz (NI-9775, National Instruments, TX). Noise was removed with 4th order zero-phase Butterworth low-pass filters with cut-off frequencies adjusted for each sensor position (mallet sensor: 20 kHz, impactor sensor: 10 kHz, stem sensor: 15 kHz).
RESULTS
Peak forces at the mallet varied from 4.7 kN to 12.4 kN. Impactor and stem forces were normalized to the mallet peak force. The normalized forces at the impactor and the stem were similar for all reference measurements (Fig. 2, impactor: p = 0.135, stem: p = 0.409). The measured forces decreased from the mallet to the impactor by 65.2 % and to the stem by 78.8 % (p < 0.001). The stiffness below the stem did not affect the measured forces; except in the rigid situation, when the normalized forces were higher at both measurement positions (Fig. 3, both p < 0.001).
CONCLUSION
Mallet impaction with its hard metal-on-metal contact causes a short high dynamic impulse, which is severely reduced at the PoI by the masses and stiffnesses of the instruments. Consequently, only forces measured at the same position should be compared. Force ratios can only be applied if they are determined for the respective instruments [2]. The soft tissue influence should not be neglected since rigid clamping in-vitro might overestimate the acting forces in-vivo.
REFERENCES
[1] Doyle et al., J BIOMECH 2019
[2] Krull et al., BONE JOINT RES 2018
ACKNOWLEDGEMENT
DePuy Synthes provided the implants and surgical instruments, which is gratefully acknowledged.
Figures
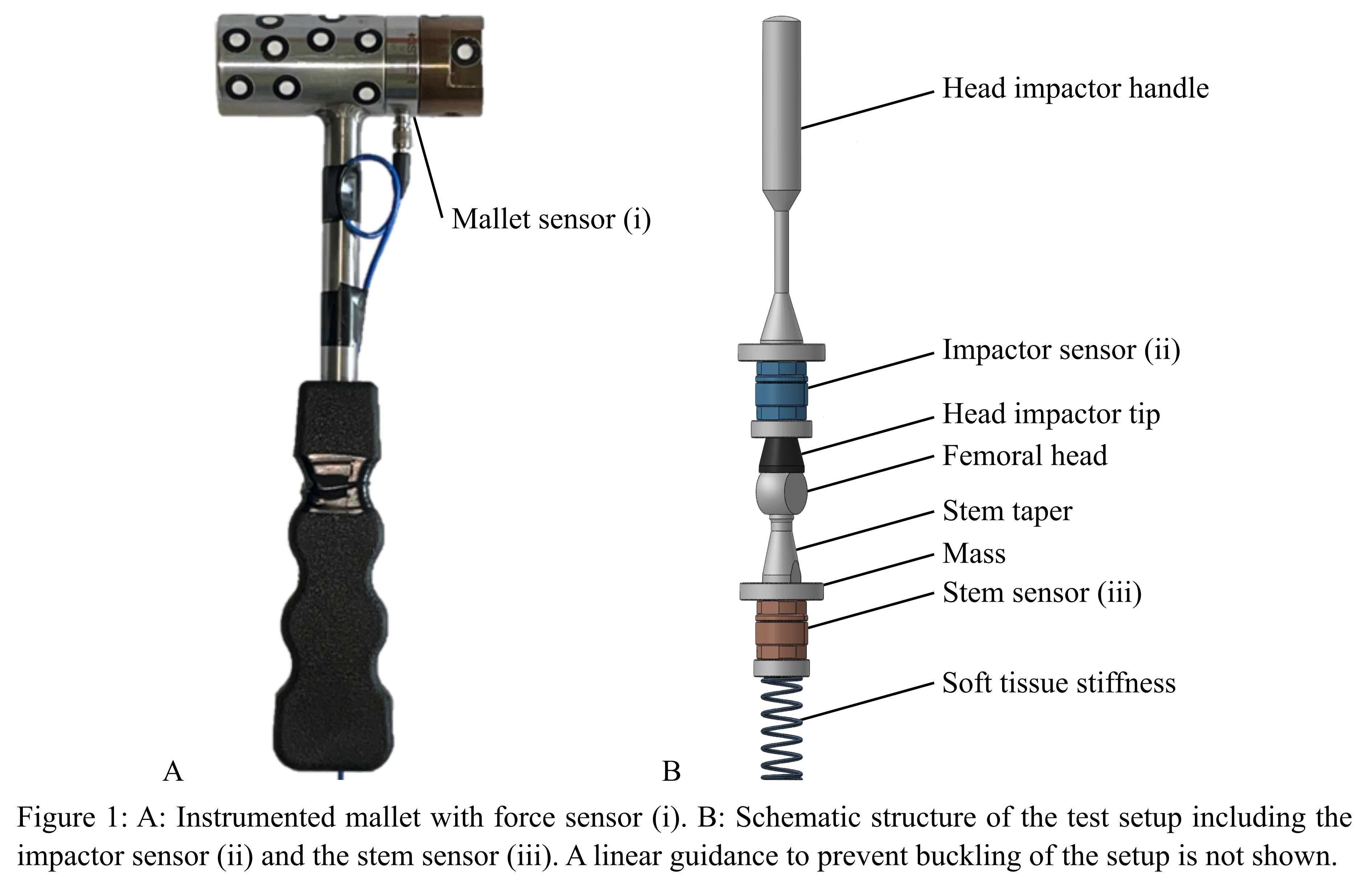
Figure 1
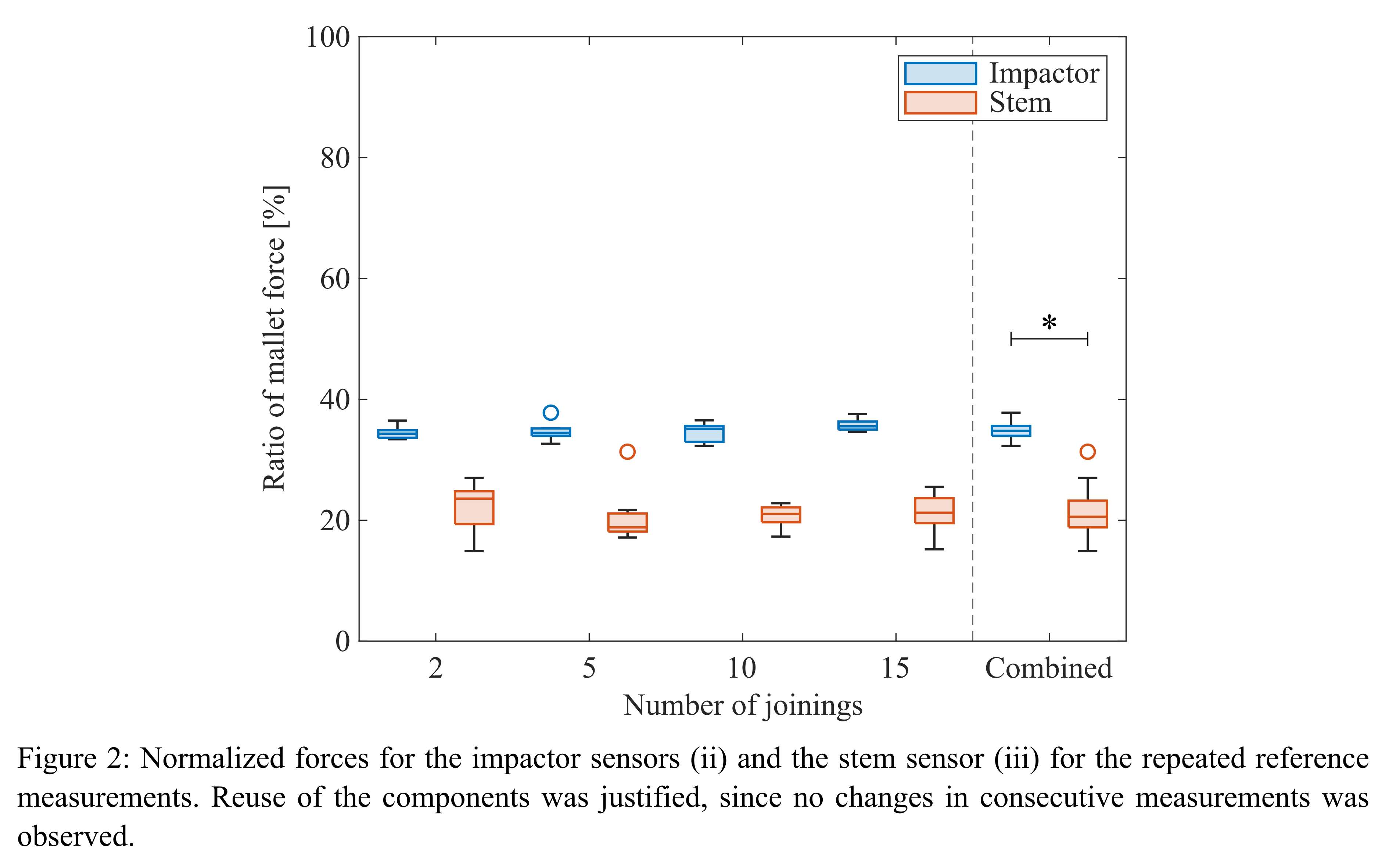
Figure 2
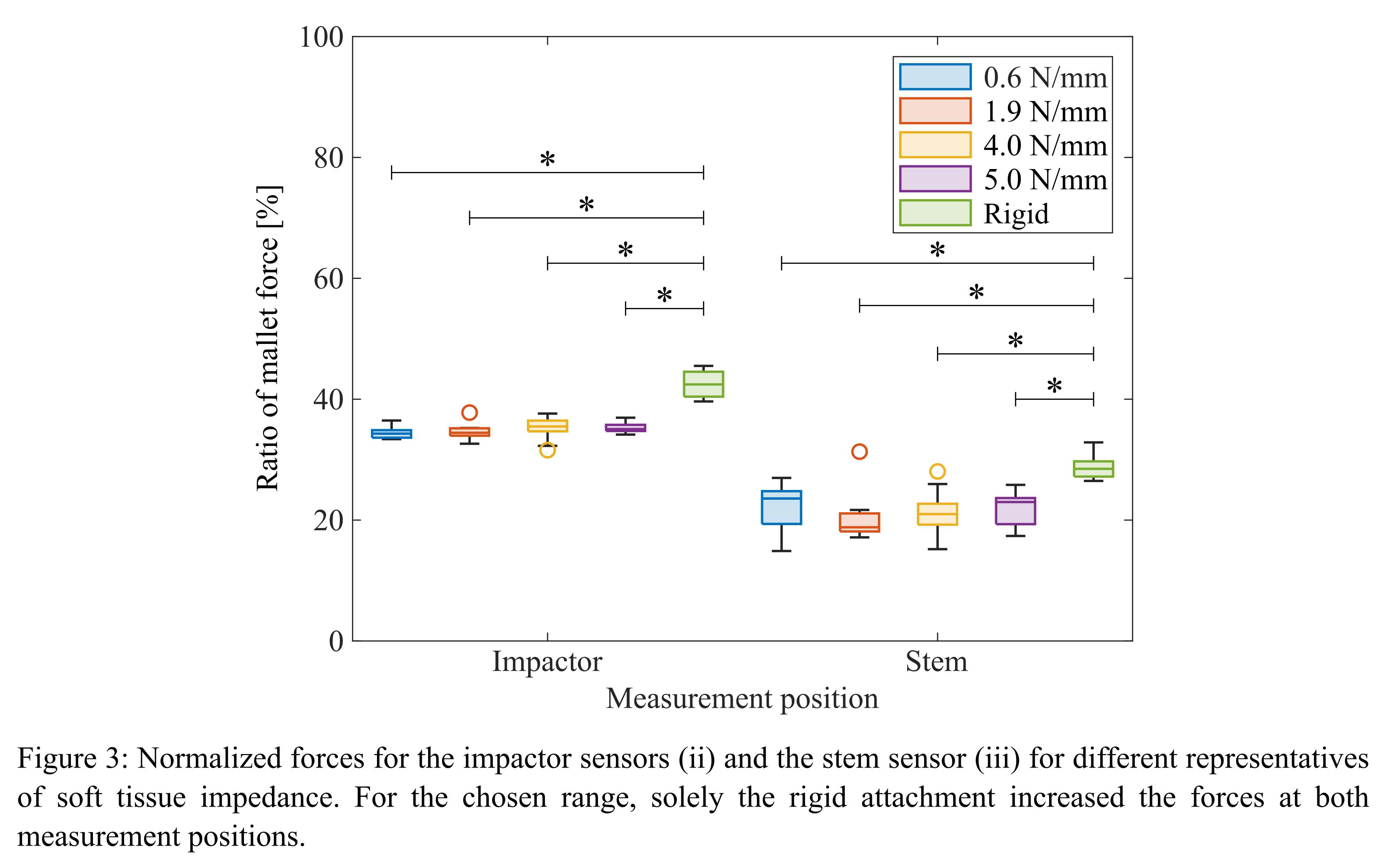
Figure 3#8835
Introduction to intellectual property
*William Brogan - Tysons Corner, United States of America
*Email: bbrogan@mh2law.com
#8836
Innovation in academia
*Orhun Muratoglu - Massachusetts General Hospital - Boston, USA
*Email: omuratoglu@partners.org
#8837
Negotiating with strategics: How much disclosure is okay and how to know when to stop
*Addie Harris - Haventure - Warsaw, United States of America
*Email: addie.harris@gmail.com
#8860
How to Successfully Interact with FDA
Holly Rhodes - MCRA - Washington, DC, United States of America
#8442
The Effect of Inter-Subject Variability in TKA Joint Mechanics During Gait on Implant Fixation: A Holistic Evaluation of TKA Biomechanics With a Subject-Specific Computational Workflow
*Jonathan Glenday - Hospital for Special Surgery - New York, USA
Peter Sculco - Hospital for Special Surgery - New York, USA
Jonathan Vigdorchik - Hospital for Special Surgery - New York, USA
Cynthia Kahlenberg - Hospital for Special Surgery - New York City, USA
David J. Mayman - Hospital for Special Surgery - New York, USA
Eytan Debbi - Hospital for Special Surgery - NY, USA
Joseph Lipman - Hospital for Special Surgery - New York, USA
Timothy Wright - Hospital for Special Surgery - New York, USA
Fernando Quevedo Gonzalez - Hospital for Special Surgery - New York, USA
*Email: glendayj@hss.edu
Introduction: Approximately two thirds of total knee arthroplasty (TKA) failures are related to either joint mechanics (e.g., instability) or fixation mechanics (e.g., aseptic loosening) [1]. While joint and fixation mechanics are interrelated [2], biomechanical studies often treat them separately, hampering our understanding of how joint kinematics and forces combine to influence component fixation. Therefore, our goal was to investigate the influence of joint kinematics and forces on TKA component fixation. To this end, we developed a subject-specific computational workflow to holistically assess TKA biomechanics.
Methods: Our computational workflow integrates a multibody musculoskeletal model to evaluate joint mechanics with a finite element (FE) model to evaluate fixation mechanics (Fig.1). We implemented our workflow using data from the third, fourth, fifth, and sixth Grand Challenge Competitions to Predict In Vivo Knee Loads (GC3-GC6) [3], which include preoperative and postoperative CT-scans and motion analysis data of gait trials with an instrumented TKA. We adapted a generic lower extremity musculoskeletal model [4] to the anatomy and TKA implantation of each subject. We utilized an enhanced static optimization algorithm (https://simtk.org/projects/opensim-jam) to determine the subject-specific tibiofemoral joint forces, contact locations, and kinematics throughout the stance phase of gait, which we directly transferred to corresponding subject-specific FE models to evaluate the implant fixation. For the FE models, the tibia was fixed 150 mm distal to the resection and modeled with non-homogenous elastic modulus (E) determined from the preoperative CT-scan, using proximal tibia density-modulus relationships [5,6] and a Poisson’s ratio (υ) of 0.3. The tibial component, insert, and cement were modeled as a titanium alloy (E=114 GPa, υ=0.33), ultra-high molecular weight polyethylene (E=463 MPa, υ=0.46), and polymethylmethacrylate (E=2.2 GPa, υ=0.33), respectively. We tied the bone-cement interface and considered cohesive contact between implant and cement [7]. We computed the risk of cement-implant debonding by computing the interfacial failure index (FI) [8].
Results: Across subjects, the contact forces predicted by the musculoskeletal model matched the corresponding experimentally measured forces from the instrumented TKA (Fig. 2a-c), capturing the inter-subject variability in force magnitude and contact locations during stance (Fig. 2d). The variability in joint mechanics translated to fixation mechanics; FI peaked at 11% (GC3), 47% (GC4), 50% (GC5), and 47% (GC6) of gait (Fig. 3a) but did not coincide with the greatest joint loading, which occurred at 47% (GC3), 44% (GC4), 49% (GC5), and 13% (GC6) of gait. Across subjects, the highest FI values were consistently located at the posterior-distal end of the stem (Fig. 3b).
Conclusion: Our workflow identified inter-subject variability in the risk of debonding during gait that was not directly attributed to peak joint loading, underscoring the combined influence of joint kinematics and forces on fixation mechanics.
References: [1] AJRR Annual Report 2022. [2] Teeter et al., J Arthroplasty 2017. [3] Fregly et al., J Orthop Res 2011. [4] Arnold et al., Ann Biomed Eng 2010. [5] Morgan et al., J Biomech 2003. [6] Snyder & Schneider. J Orthop Res 1991. [7] de Ruiter et al., J Exp Orthopaed 2017. [8] Zelle et al., J Biomech 2011.
Figures
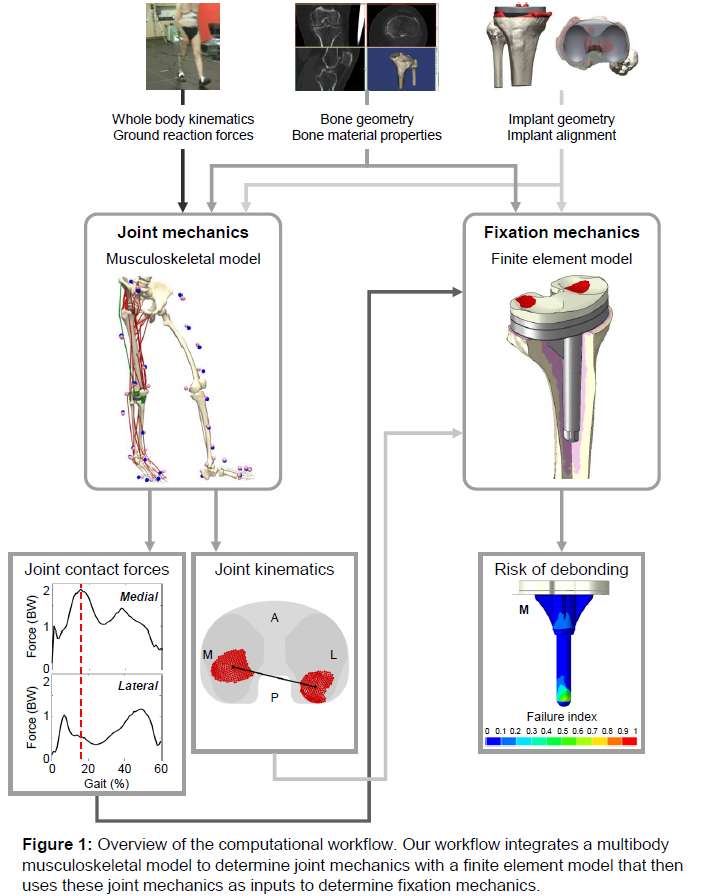
Figure 1
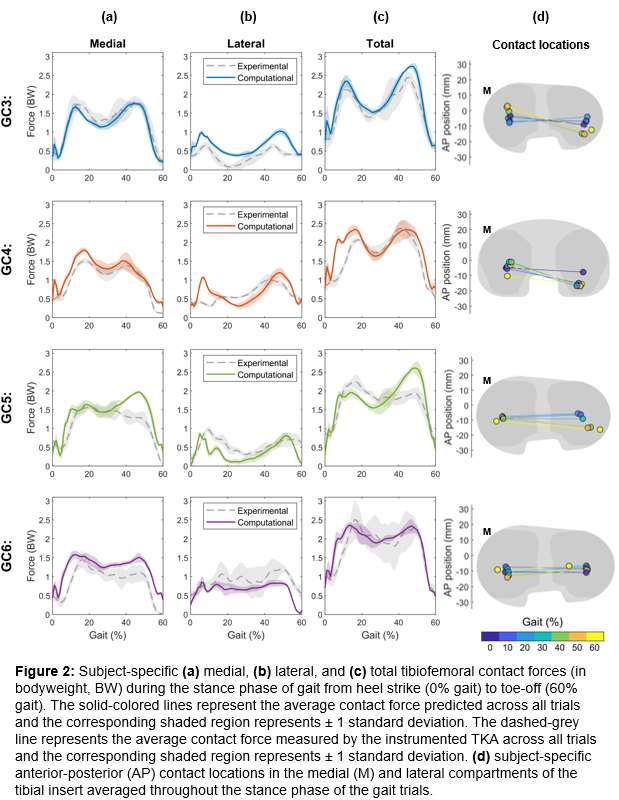
Figure 2
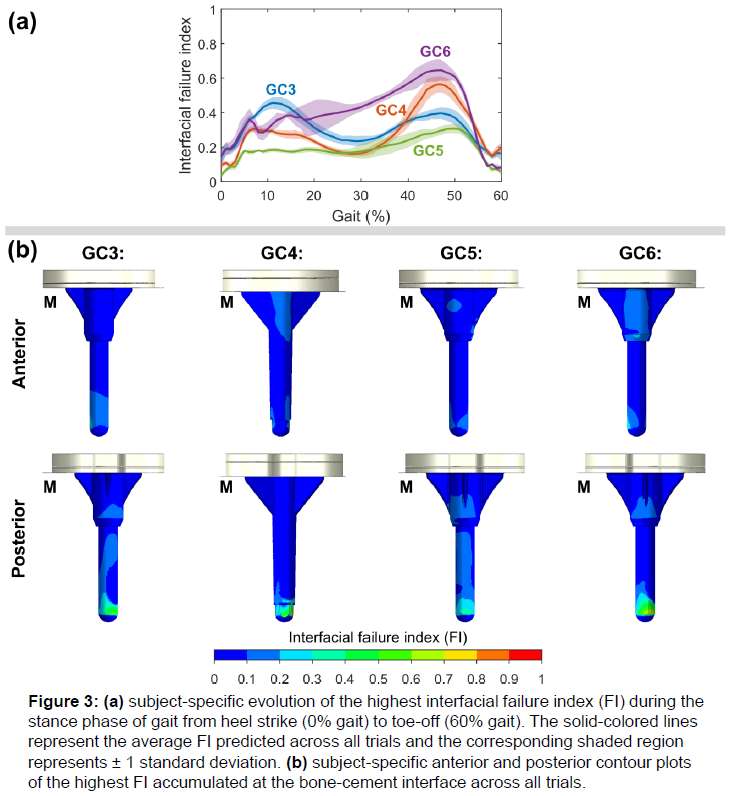
Figure 3#8539
Differences in Neuromuscular Activation Before and After Surgery Based on Gait Biomechanics Phenotypes Among Total Knee Arthroplasty Recipients
*Annemarie Laudanski - Dalhousie University - Halifax, Canada
Kathryn Young-Shand - Dalhousie University - Halifax, Canada
Cheryl Hubley-Kozey - Dalhousie University - Halifax, Canada
Syed S. R. Abidi - Dalhousie University - Halifax, Canada
Michael Dunbar - Dalhousie University - Halifax, Canada
Glen Richardson - Dalhousie University & Capital Health - Halifax, Canada
Janie Wilson - Dalhousie University - Ancaster, Canada
*Email: annemarie.laudanski@dal.ca
Introduction
Standardized surgical approaches currently utilized in total knee arthroplasty (TKA) do not guarantee patient satisfaction [1,2]. The heterogeneity of OA patients has led to our recent phenotyping efforts, revealing 4 distinct clusters predominantly separated by sex and knee biomechanics during gait, and their associated cluster-specific post-TKA kinematic and kinetic improvements [3]. However, neuromuscular differences during important dynamic tasks such as walking between the identified phenotype clusters have yet to be explored which could inform cluster-specific rehabilitation and surgical approaches. Therefore, the objective of this study was to examine cluster-associated differences in neuromuscular patterns during gait before and after TKA.
Methods
This investigation constitutes a secondary analysis of 114 patients with severe knee OA scheduled for primary TKA surgery recruited for gait analysis one-week pre and one-year post-TKA. All patients provided informed consent and subsequently performed self-paced overground walking trials while muscle activity was measured using surface electromyography (Bortec Inc.) from the medial and lateral gastrocnemii, quadriceps (vasti and rectus femoris), and hamstrings. Electromyographic (EMG) signals were full wave rectified, low pass filtered (6Hz), amplitude normalized to a percentage of maximum voluntary isometric contractions, and time normalized to 100% of the gait cycle. Principal component (PC) models described previously [4] were used to determine key amplitude and shape characteristics within each muscle group. Two-factor ANOVAs were used to examine cluster and session (pre- and post-TKA) main and interaction effects for the EMG PC scores (p < 0.025) by muscle group.
Results
Patients were assigned to one of four previously established clusters based on sex and pre-TKA biomechanics (Table 1) [3]. Significant cluster and session effects were found in PC3 of the gastrocnemii where cluster 3 exhibited more constant activation early to midstance pre-TKA (p<.001) which decreased post-TKA (p<.001) (Figure 1, Table 2). More prolonged hamstring activation from early to midstance was observed in clusters 2 and 3 (PC2, p<0.001) pre-TKA and resolved post-TKA (p<0.001), while a significant cluster effect in PC4 revealed greater activation during late-stance and early swing in cluster 3. Finally, a significant cluster effect in PC2 of the quadriceps highlighted greater midstance activation in clusters 2 and 3 pre-TKA (p<0.001), which decreased significantly post-TKA (p<0.001). Cluster 3 also demonstrated a higher ratio between mid stance and swing in quadriceps activations both pre- and post-TKA (PC3). Similar to trends reported in kinematic and kinetic measures [3], both higher functioning clusters (1 and 4) demonstrated less musculoskeletal improvements than those observed in the lower functioning clusters.
Conclusions
Muscle activation pattern differences exist between the previously identified OA clusters [3] suggesting heterogeneity between groups based on sex and functional ability. Further investigations into these biomechanical and neuromuscular differences may suggest the need for cluster or patient-specific surgical considerations in addition to cluster-targeted pre- and post-rehabilitation strategies for maximizing patient outcomes.
References
[1] Astephen Wilson et al., J Orthop Res37:1754-1759, 2019.
[2] Naili et al., Knee Surgery, Sport, Traumatol Arthrosc. 25:3378-3386, 2017
[3] Young-Shand et al., J Orthop Res. 41(2):335-344, 2023
[4] Hubley-Kozey et al., J Electromyogr Kinesiol. 16(4):365-78, 2006
Figures
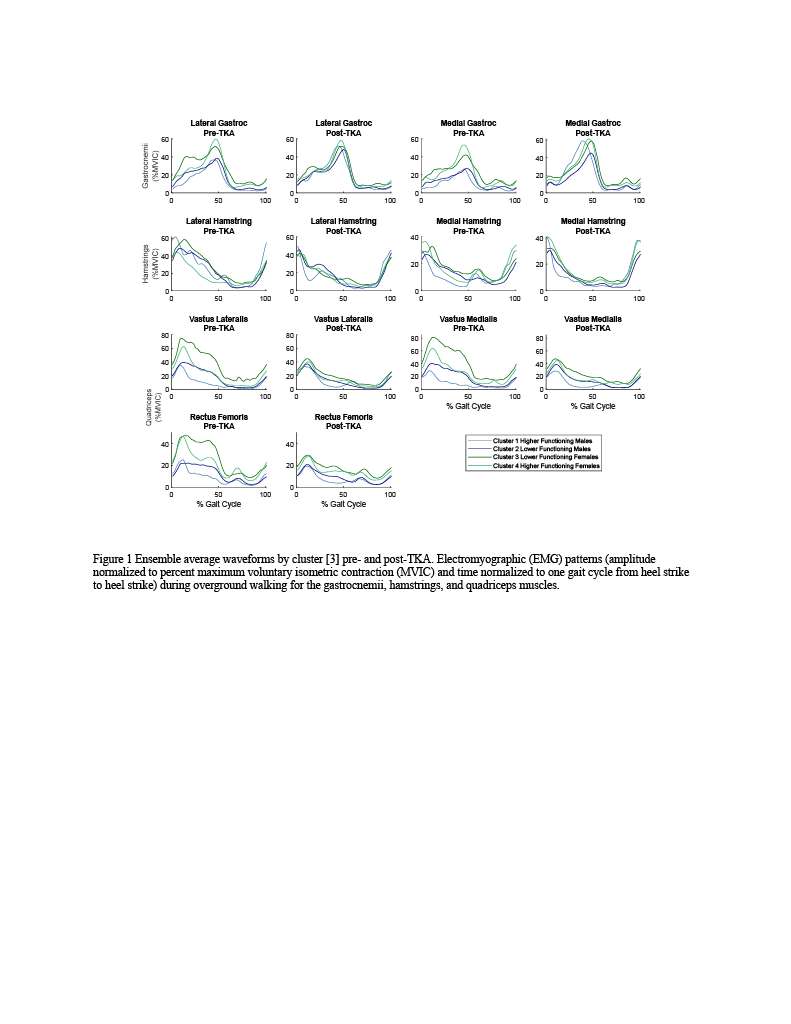
Figure 1
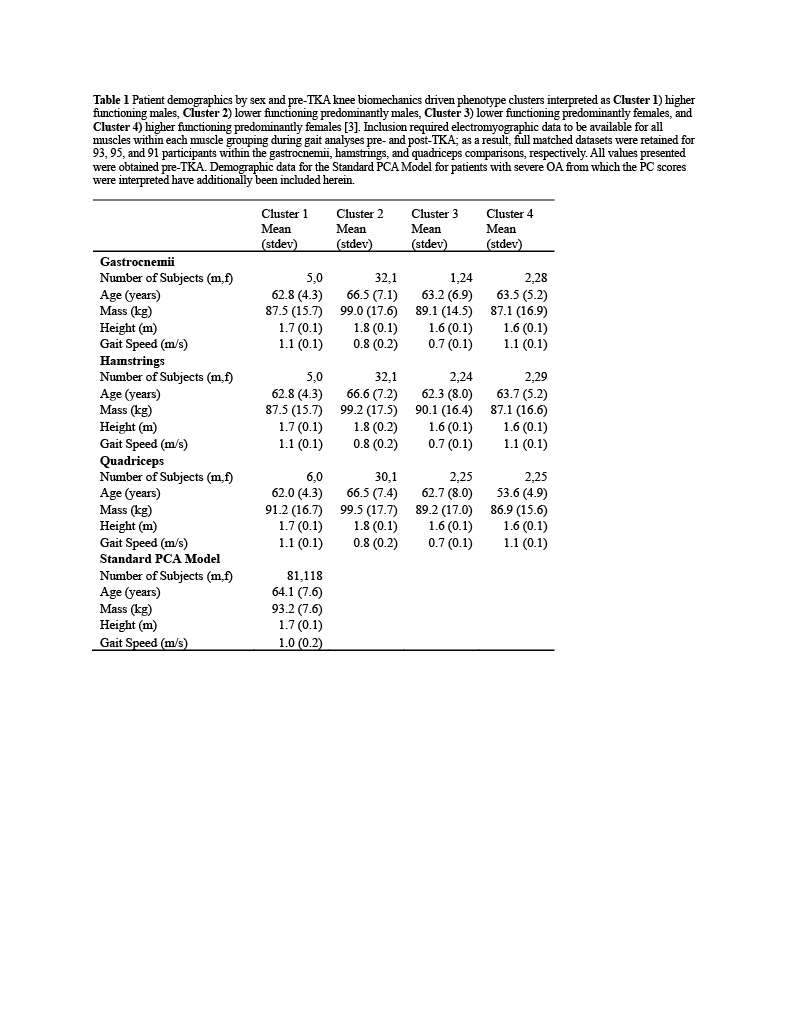
Figure 2
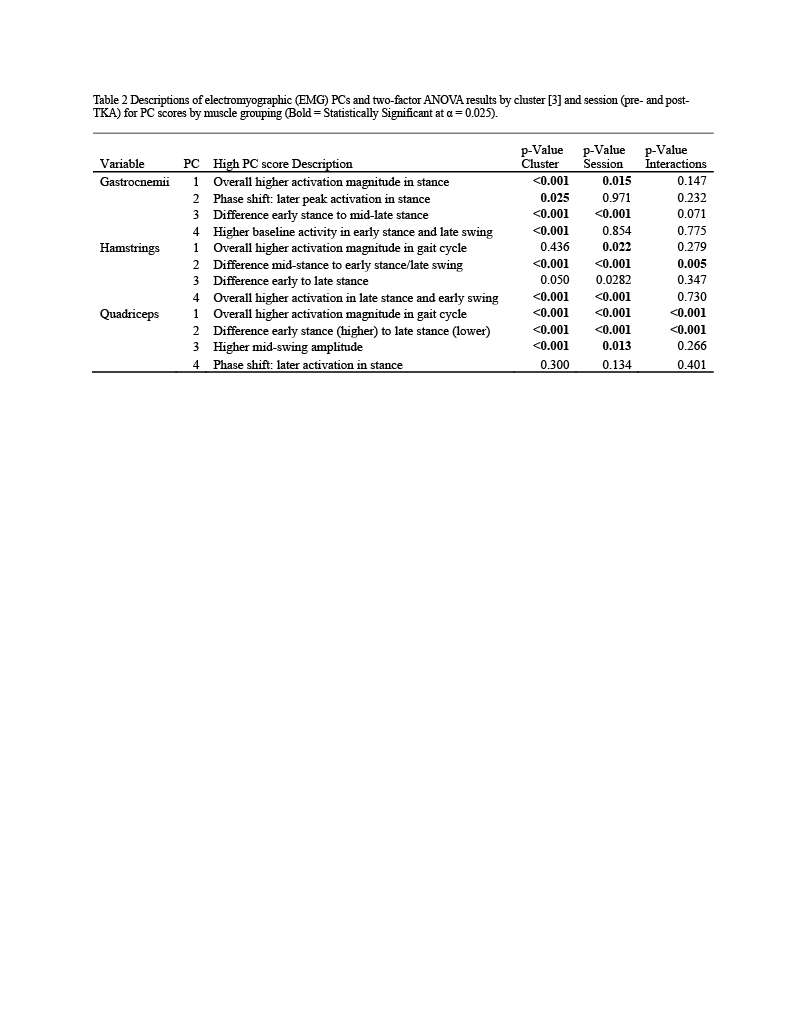
Figure 3#8491
Impact of PCL Resection on Coronal and Sagittal Joint Balance in TKA
*Edgar Wakelin - OMNI Life Sciences - Raynham, USA
Adam Edelstein - Northwestern University - Chicago, USA
Christopher Plaskos - Corin - Raynham, USA
Linda Suleiman - Northwestern Unversity - Chicago, USA
*Email: edgar.wakelin@coringroup.com
Aim
The posterior cruciate ligament (PCL) is a main antero-posterior and medial stabilizer of the knee, particularly in flexion. Some studies have investigated the impact of PCL resection on small samples of cadaveric or clinical knees, however large population studies of the impact of PCL resection on joint laxity and balance throughout flexion has not been performed. We aim to characterize the pre-operative joint balance in a PCL retaining and PCL sacrificing knee cohort and determine the impact of PCL resection on joint balance throughout flexion and impact on component alignment.
Methods
A retrospective analysis of the CorinRegistry was performed, inclusion criteria were: robotically assisted total knee arthroplasty in which a digital joint tensioner was used in a tibial first approach. A total of 3989 cases were returned in which 764 had a PCL retaining and 3225 a PCL sacrificing workflow. Intra-operative landmarks were obtained using the robotics system, and all resections recorded. After the tibial resection was performed, a digital joint tensioner was inserted into the knee and pre-femoral resection gaps were recorded. The tibial resection thickness was then subtracted from the joint gaps. Medial and lateral gaps were compared throughout flexion, and the change in femoral posterior resection thickness and femoral rotation to achieve balance was calculated. Sagittal changes are defined as the gap in flexion subtracted from the gap in extension. Student’s t-tests are used to compare gaps between groups, and chi-square tests used to compare percentages with a critical p-value of 0.05.
Results
The PCL sacrificing and retained cohort report similar pre-operative deformity (3.9±5.5° vs 4.4±5.3° varus), age (68.3±8.7 vs 67.4±8.9), sex (57%F vs 56%F) and flexion deformity (3.3±6.2° vs 2.8±6.2°). The medial compartment reported greater laxity throughout flexion in the PCL sacrificed cohort of between 0.5 – 1.8 mm, table 1, in which the greatest medial opening occurs in flexion. Conversely, the lateral side reported no change in flexion, but tightening in midflexion and extension with PCL resection (0.5 – 0.7 mm), table 1.
The medial compartment maintained sagittal stability in the PCL retaining group (Ext to Flex difference of 0.1 mm), however, the PCL sacrificing group reported a greater flexion gap of 1.2±2.4 mm compared to extension, table 1. No difference was found between groups in sagittal stability in the lateral compartment with an increased gap of 1.6 – 1.8 mm, table 1.
To achieve similar medial sagittal balance to the PCL retaining cohort, PCL sacrificed knees require 1.3±2.4 mm less medial posterior resection, in which >20% require ≥3mm reduction (table 2). To achieve similar flexion balance, PCL sacrificed knees require 2.0±3.1° more internal rotation and over a quarter of knees require more than 3° more internal rotation, table 2, figure 1.
Conclusion
PCL sacrifice results in a significant change to knee joint gaps and balance, particularly in flexion. To achieve similar stability, PCL sacrificing TKA will require on average reduced medial posterior femoral resection thickness and more internal femoral rotation compared to similar knees using a PCL retaining workflow.
Figures
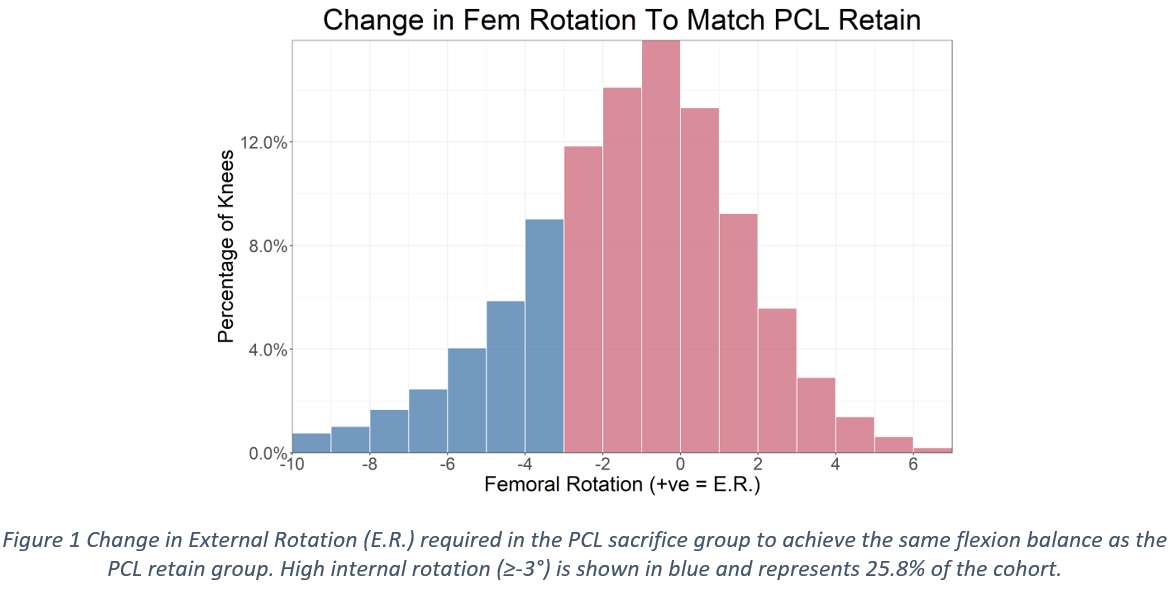
Figure 1
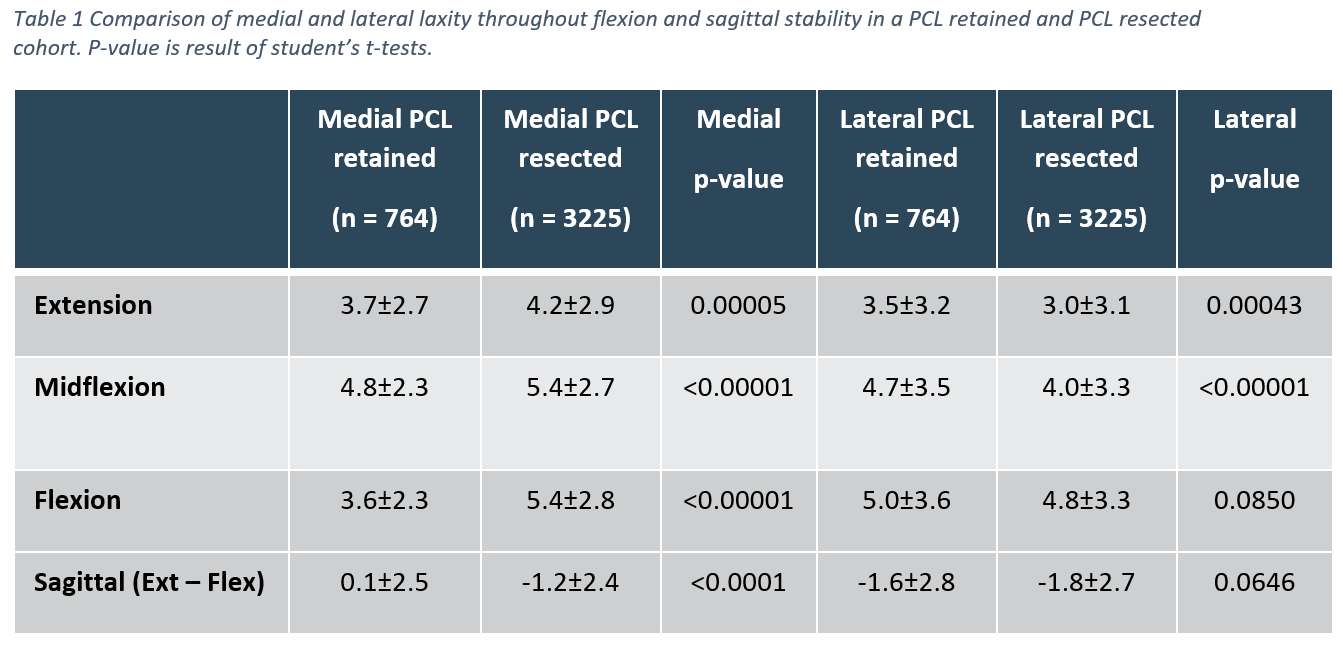
Figure 2
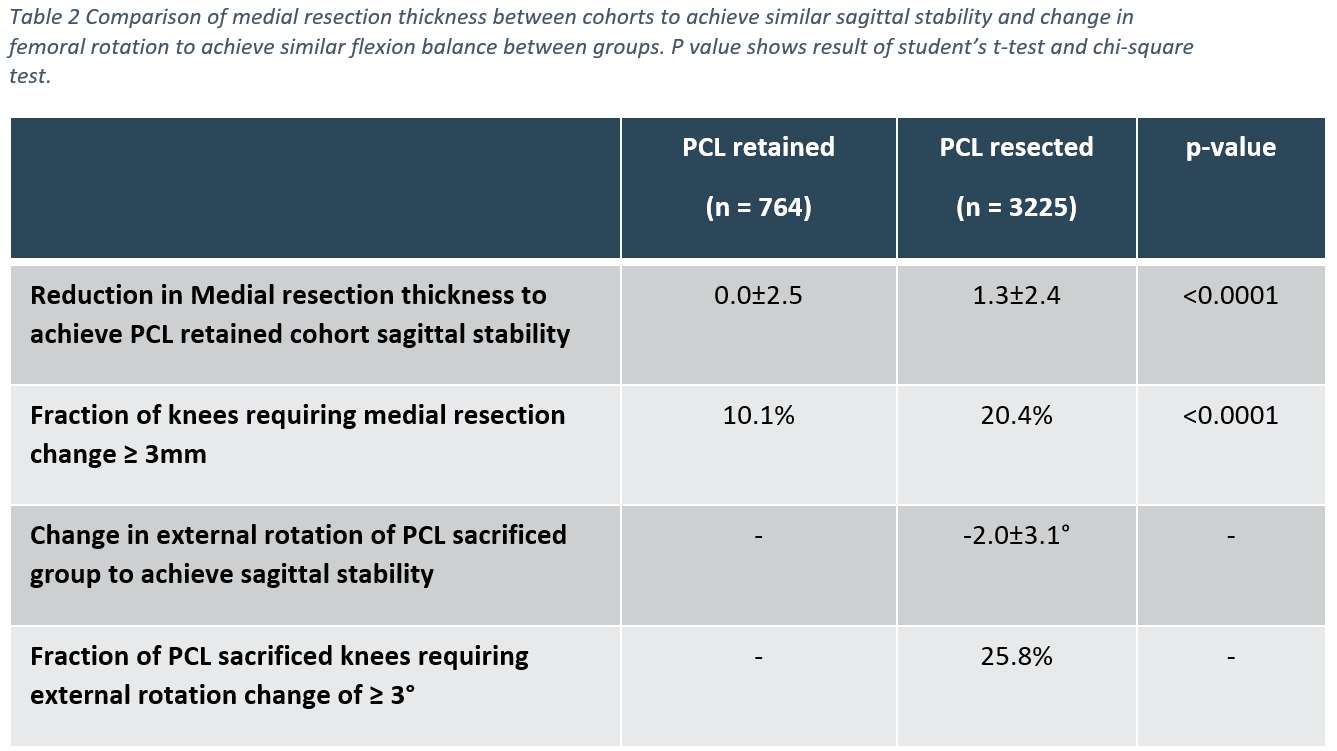
Figure 3#8271
Role of the Posterior Cruciate Ligament in Femoral Rollback in Medial Congruent Total Knee Arthroplasty: A Computational Study
*Reza Pourmodheji - Hospital for Special Surgery - New York, United States of America
Jacob M. Hirth - Hospital for Special Surgery - New York, USA
Brian Chalmers - Hospital for Special Surgery - New York CIty, USA
Cynthia Kahlenberg - Hospital for Special Surgery - New York City, USA
William Long - NYU Langone Health - New York, USA
Timothy Wright - Hospital for Special Surgery - New York, USA
Geoffrey H Westrich - Hospital for Special Surgery - New York, USA
David J. Mayman - Hospital for Special Surgery - New York, USA
Carl Imhauser - USA
Peter Sculco - Hospital for Special Surgery - New York, USA
*Email: pourmodhejir@hss.edu
Introduction: Medial congruent (MC) polyethylene designs in total knee arthroplasty (TKA) are intended to enhance antero-posterior stability and reproduce the “medial pivot” behavior of the native knee joint [1]. Medial congruent designs have a smaller sagittal radius of curvature on the medial compartment than on the lateral compartment. The design allows retention or resection of the posterior cruciate ligament (PCL), however, little is known on how the PCL impacts femoral rollback in this new semi-congruent articulation [2, 3]. We developed a computational framework to quantify how the PCL affects femoral rollback in a MC-TKA to investigate how PCL retention impacts posterior translation of the medial and lateral femoral condyles during knee flexion. We hypothesized that retaining the PCL will increase the posterior translation of the medial and lateral femoral condyles in flexion.
Methods: Computational models derived from 10 independent cadaveric left legs (five males, five females; age: 63.7±10.5 years) were virtually implanted with an MC tibial insert and cruciate retaining femoral component (Persona, Zimmer-Biomet, Warsaw, IN) using measured resection technique and the tibial component at 5° of posterior slope (Fig.1). The computational model used a multibody dynamics framework, which included 33 line-elements representing the PCL, collaterals, and capsular ligaments, and a rigid body contact formulation to describe the interaction of the two articular surfaces. We modeled two conditions: PCL-retained and PCL-resected. For both conditions, the knee was flexed from 0 to 90° under 500 N of compression, which represented a test of passive flexion. At 0° and 90° of flexion, contact points between the femoral component and the medial and the lateral tibial articular surfaces of the tibial insert were obtained for both PCL conditions. Femoral rollback was defined as the anterior-posterior (AP) distance between tibiofemoral contact points at 0° and 90° of flexion, with anterior defined as positive. To compare femoral rollback between the PCL-retained and PCL-resected conditions, we used a nonparametric Wilcoxon signed-rank test.
Results: PCL retention resulted in greater medial and lateral femoral rollback compared to PCL resection with a median increase in rollback of 2.5 mm (p=0.002) and 3.4 mm (p=0.0009), respectively (Fig. 2-3).
Conclusion: Retaining the PCL increased posterior translation of both femoral condyles throughout knee flexion, confirming our hypothesis. However, with PCL retention, lateral femoral condyle rollback was more variable, ranging from 3.1 to 9.2 mm. Since the PCL is highly nonisometric through a range of flexion, small variations in its anatomy may strongly influence the magnitude of force carried by the PCL and, consequently, its influence on femoral rollback. Our finding corroborates with clinical observations of difficulty obtaining consistent levels of femoral rollback with PCL retention, particularly for the lateral compartment.
Acknowledgment: The Clark and Kirby Foundations. Implants donated by Zimmer Biomet, Inc.
References: [1] Frye et al. Arhoplast. Today. 2021. [2] Scott et al., J Arthroplasty, 2008. [3] Most et al. Clin. Orthop. Relat. Res. 2003.
Figures
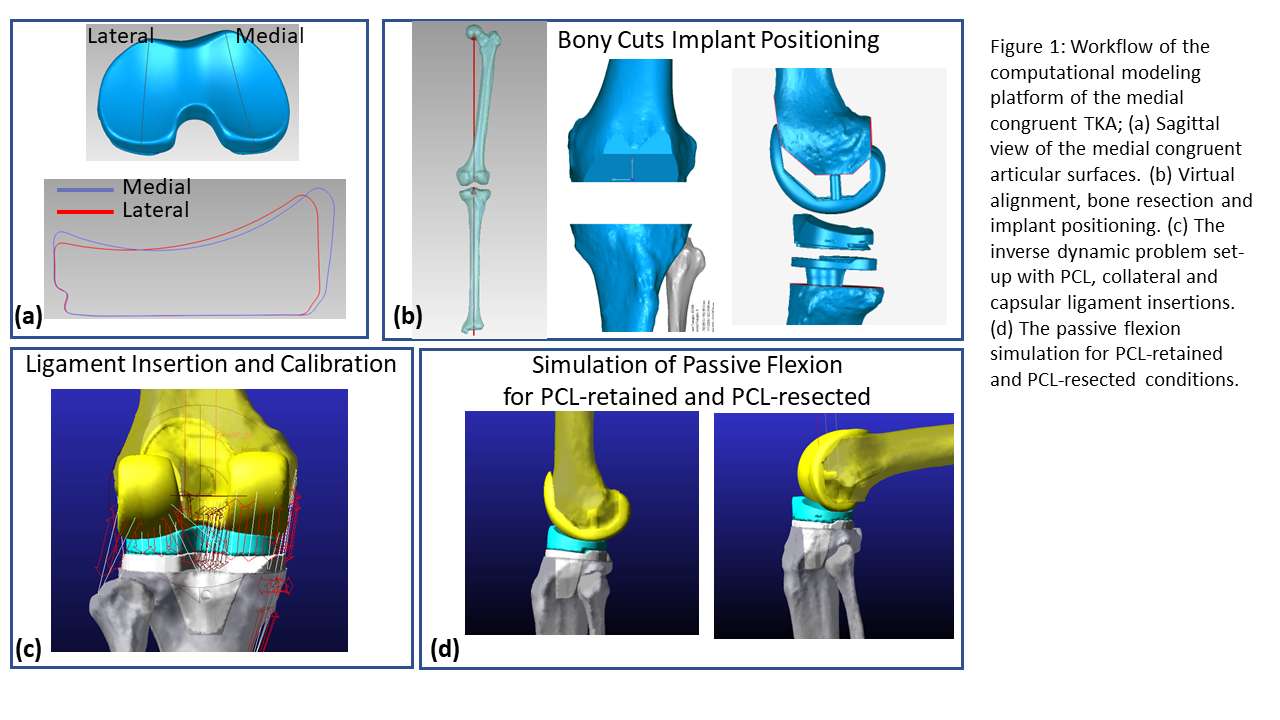
Figure 1
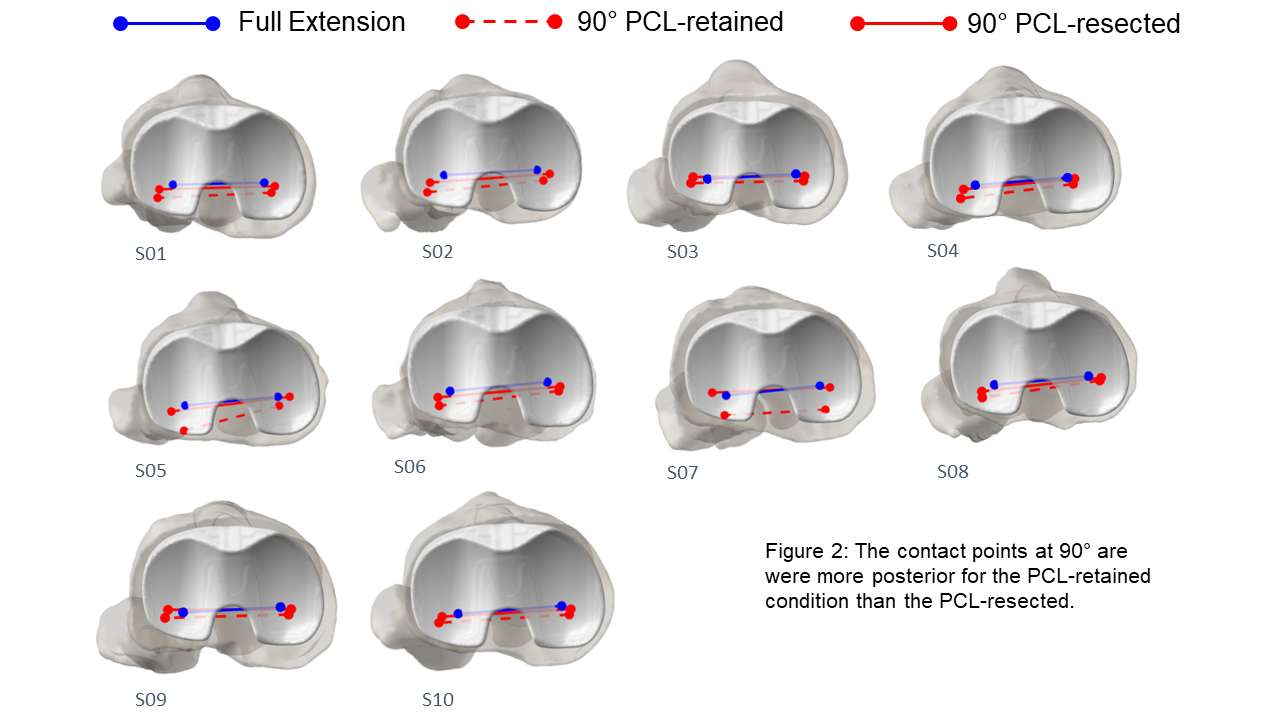
Figure 2
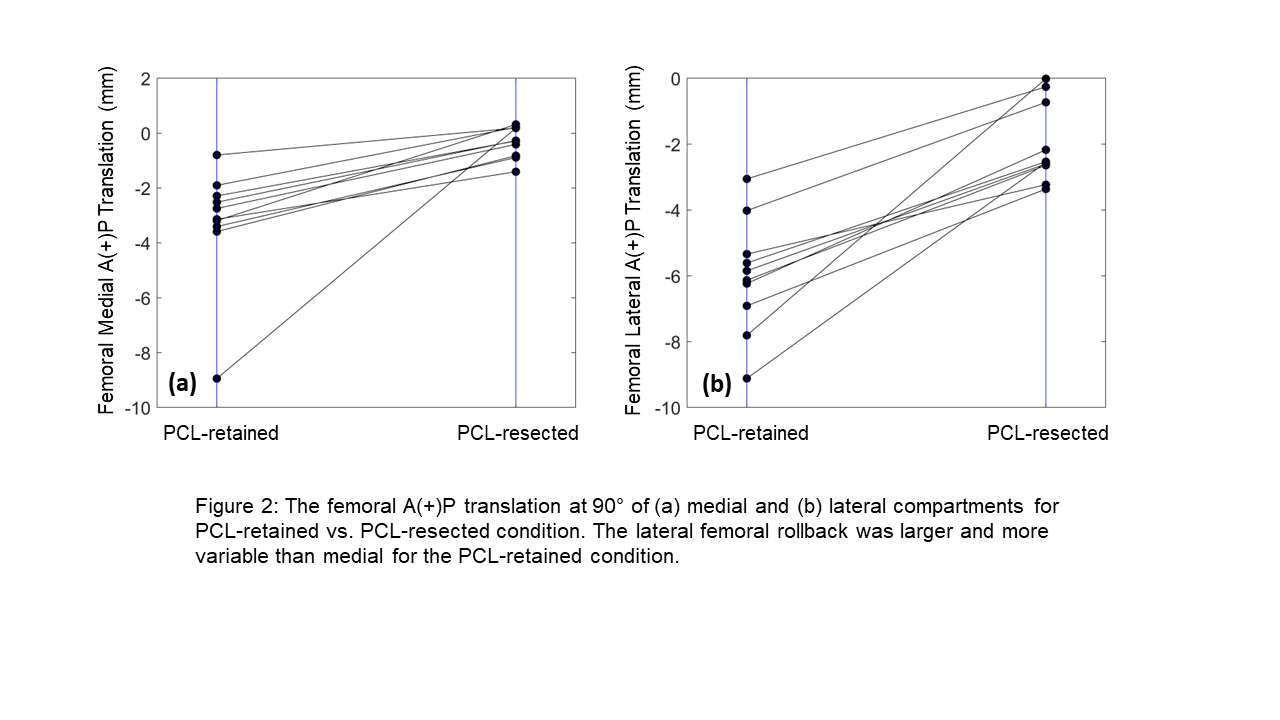
Figure 3#8736
Posterior Stabilized Implants Require Greater Hamstrings Activity Compared to Medial Ball-and-Socket Implants During Stair Descent
Erik Kowalski - University of Ottawa - Ottawa, Canada
Alexandre Pelegrinelli - State University of Londrina - Londrina, Brazil
Nicholas Ryan - University of Ottawa - Ottawa, Canada
Geoffrey Dervin - The Ottawa Hospital - Ottawa, Canada
*Mario Lamontagne - University of Ottawa - Ottawa, Canada
*Email: mlamon@uottawa.ca
Introduction
Different implant designs can impact knee biomechanics and muscle activity during gait. However, their effect during more demanding tasks, such as descending a staircase, is unclear. This study aimed to evaluate knee biomechanics and muscle activations of the quadriceps, hamstrings, and gastrocnemius muscles in patients one year after total knee arthroplasty (TKA) with either a posterior stabilized (PS) or medial ball-and-socket (MBS) implant and compare them to a group of healthy controls.
Methods
Twenty-eight TKA patients were randomized to either a PS (n=14, female= 6, age= 65.6±8.1, BMI=30.3±3.9kg/m2, Zimmer Biomet NexGen with PS inserts) or MBS (n=14, female=6, age=63.7±5.7, BMI=27.4±3.5kg/m2, MicroPort Orthopedics Evolution Medial Pivot with cruciate sacrificing inserts), and were compared with 14 healthy controls (female=6, age=64.4±5.6, BMI=24.9±2.1kg/m2). All TKA patients underwent TKA with the same surgeon using the same surgical approach; midline incision with a subvastus approach, and the surgeon utilized a mechanical neutral alignment. The protocol required patella resurfacing, and PCL was released in all patients. Patients visited the biomechanics lab approximately 12 months after TKA, where knee biomechanics and muscle activity were measured as they descended a three-step staircase.
Fourteen wireless electromyography (EMG) sensors were placed on the quadriceps (vastus medialis, rectus femoris, and vastus lateralis), hamstring (biceps femoris, semitendinosus), and gastrocnemius (medial and lateral heads) muscles of both limbs and reflective markers were placed on anatomical landmarks. A maximal voluntary isometric contraction (MVIC) was recorded for each muscle. EMG signals and knee biomechanics were recorded as participants completed five trials down a 3-step staircase. Variables of interest were the knee biomechanics and linear envelope normalized to MVIC. Knee biomechanics and muscle linear envelopes were compared across the entire cycle using statistical parametric mapping.
Results
Linear envelopes of EMG were averaged together for each muscle group (i.e., hamstrings: semitendinosus + biceps femoris) and compared between the groups. No differences in quadriceps or gastrocnemius linear envelopes existed between the groups. The PS group descended the stairs with greater hamstring muscle activation throughout single limb support compared to the MBS (8 to 51% stair descent cycle (%SDC)) and control (13 to 44%SDC) groups (Figure 1). MBS and PS groups had less knee flexion angle than the CTRL group throughout the weight acceptance and forward continuance phases. However, the MBS group did achieve more knee flexion than the PS group during this period (0 to 31%SDC). Knee joint moments and powers were similar between the MBS and PS groups, but neither reached the level of the control group (Figure 1).
Conclusion
Lower knee flexion angles and increased hamstring muscle activity indicated that the PS group descended the stairs with a stiffer knee gait pattern than the MBS group. The medial congruent tibial insert of the MBS implant may provide greater passive stability as patients required less muscle activity than the PS group. The PS implant does not have this passive stability. It requires greater hamstring muscle activation to prevent anterior sliding of the implant during stair descent, especially when in single-limb support.
Figures
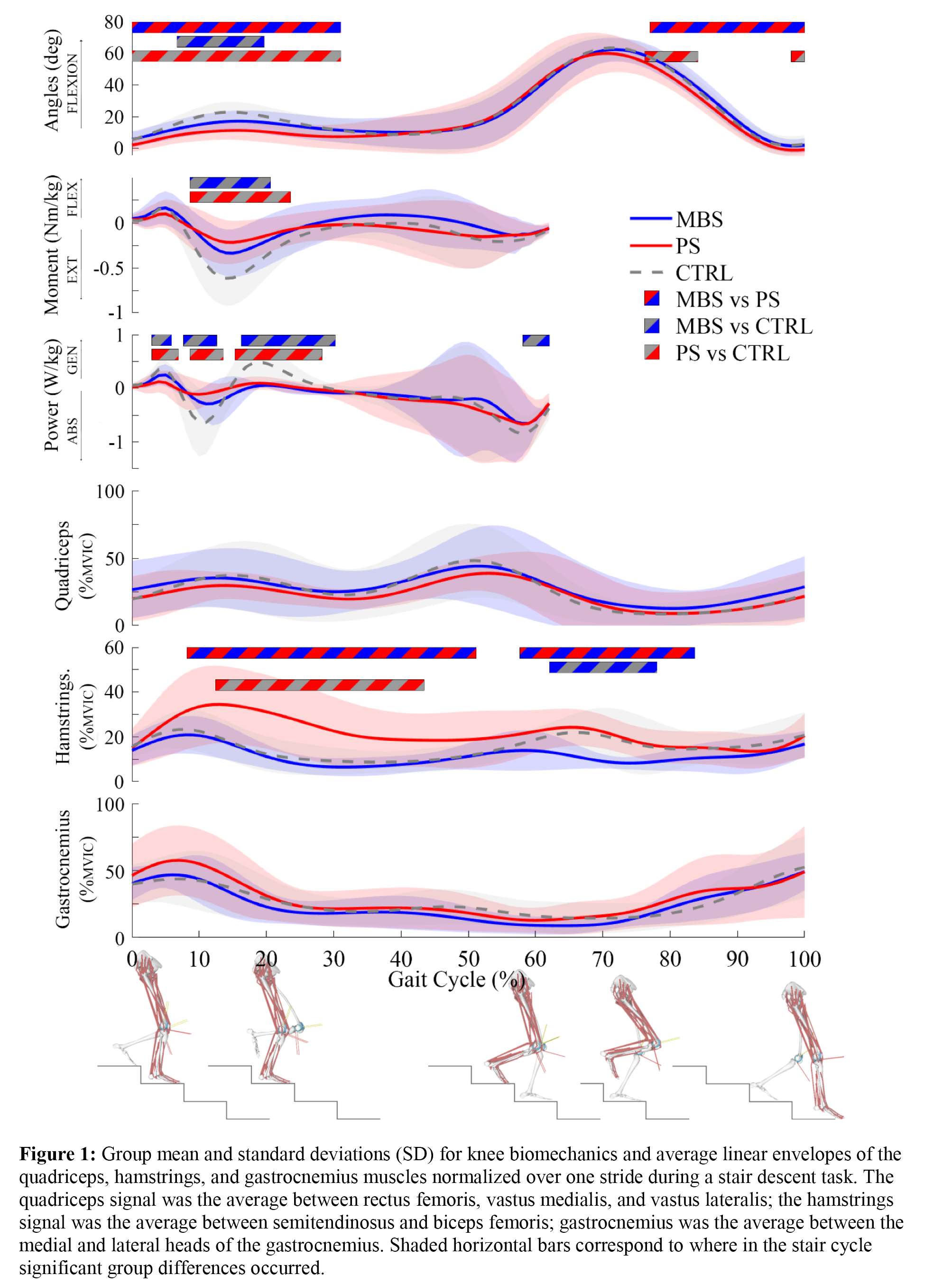
Figure 1#8249
Patellar Mechanics of a Novel Hinge Knee System: A Finite Element Analysis
*Azhar Ali - Stryker Orthopaedics - Mahwah, USA
Kenneth Pascale - Stryker - Mahwah, USA
Xiangyi (Cheryl) Liu - Stryker Orthopaedics - Mahwah, USA
Viktor Erik Krebs - Cleveland, USA
*Email: azhar.ali@stryker.com
Introduction: Hinge knee systems provide joint replacement solutions for revision, oncology and/or patients with severe bone and soft tissue pathologies. Historical hinge systems have reported patellar maltracking and extensor deficiency, limiting patient function [1]. The objective was to evaluate patellofemoral (PF) mechanics for a novel hinge design (NHD) and compare its extensor function to the natural knee condition and historical implant designs with demonstrated clinical success using a patient-specific finite element model simulating a seated knee extension activity.
Methods: A previously-validated, force-driven finite element model of a healthy subject was leveraged for comparison of PF mechanics between the natural condition, non-hinged knee systems (condyle-stabilized - CS, total stabilized TS), and hinged knee systems (PHD S and PHD M, NHD) [2,3]. The model simulated a seated knee extension captured using 50 Hz dynamic bi-plane fluoroscopy, motion capture, and electromyography. Implant components were virtually aligned using a mechanical alignment approach by an experienced surgeon to achieve the best fit for the patient model. Primary knee systems (CS, TS) and NHD were matched in size and alignment, but PHD components were translated anteriorly (~3mm) relative to the NHD hinge axis to achieve clinically-relevant fit between femoral boss and canal (Figure 1). The patella was resurfaced using a 36mm dome patella and aligned to maximize coverage and restore native patellar thickness. The simulation applied a PID-controlled quadriceps force calibrated to match the subject’s fluoroscopic flexion, enabling prediction of PF kinematics, extensor loads, and effective moment arm.
Results: The novel hinge design increased maximum patellar flexion by 4.1° compared to the historical system, and avoided lateral patellar subluxation of up to 4 mm in deep flexion, achieving closer to natural patellar tracking consistent with the non-hinged knee systems (CS, TS) (Figure 2). The novel design required 10.3% and 12.2% less quadriceps force to extend the knee across the flexion range when compared to PHD small and medium, respectively (Figure 3). Also, NHD achieved quadriceps force similar to TS with differences of 5.9% across the flexion range. Additionally, patellar tendon forces were 13.6% and 9.5% lower in NHD than PHD small and medium at 10°. Consistent with reduced quadriceps forces, NHD demonstrated increased effective moment arm by up to 3.2 mm in mid-flexion (30°-90°) compared to PHD, and effective moment arm within 1 mm of the TS system.
Conclusion: The novel hinge design restored PF kinematics and extensor efficiency closer to that of the non-hinged knee systems (CS, TS) as compared to predicate PHD. Despite anteriorizing PHD femoral components to achieve better canal fit, NHD demonstrated improved extensor function through mid-flexion with reduced quadriceps forces and increased effective moment arm. NHD may enable hinge patients to perform challenging activities of daily living, such as chair rise, with greater ease. Improved patellar tracking and decreased patellar tendon forces may potentially reduce risk of pain and tendon rupture. The current study demonstrated improved patellar mechanics and extensor function in a novel hinge design for revision, oncology and severely diseased patients.
Figures
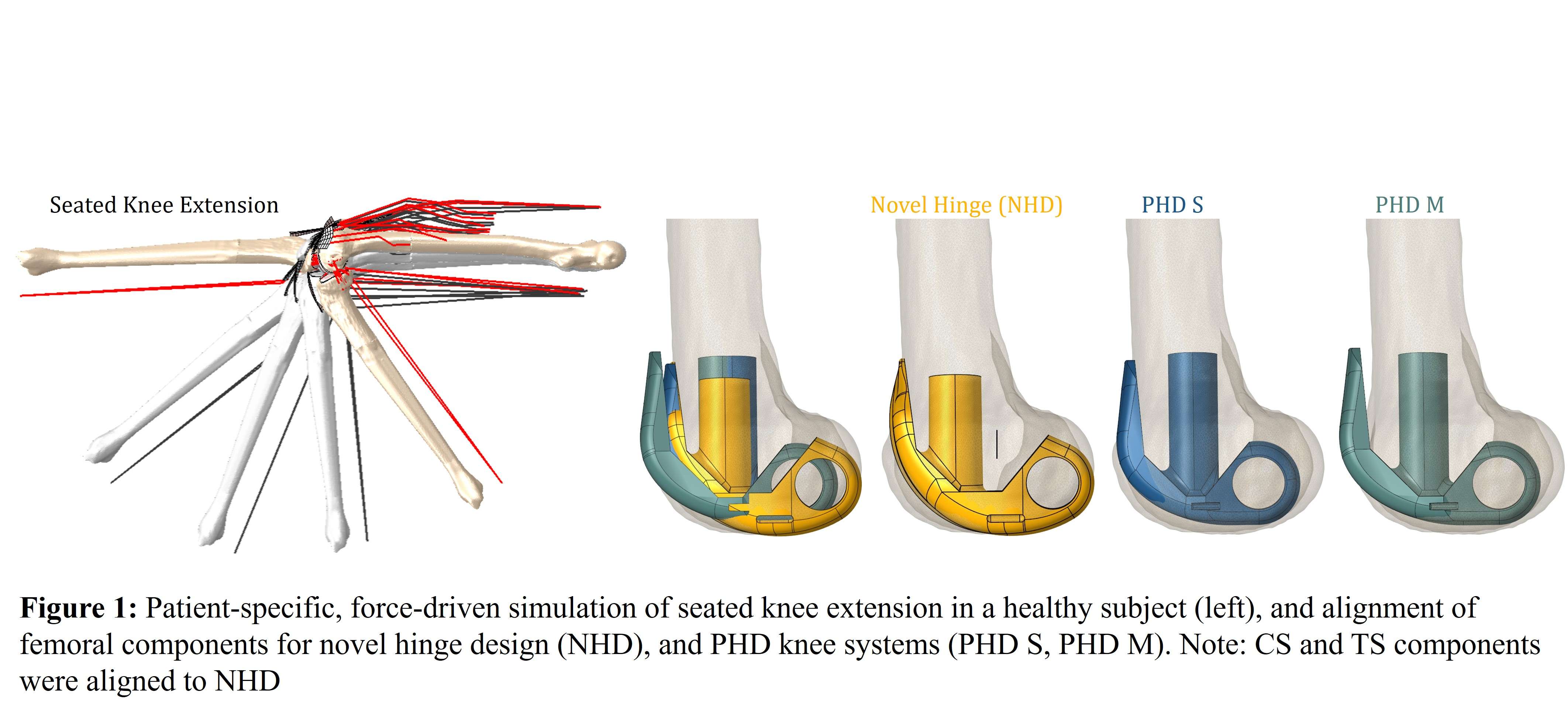
Figure 1
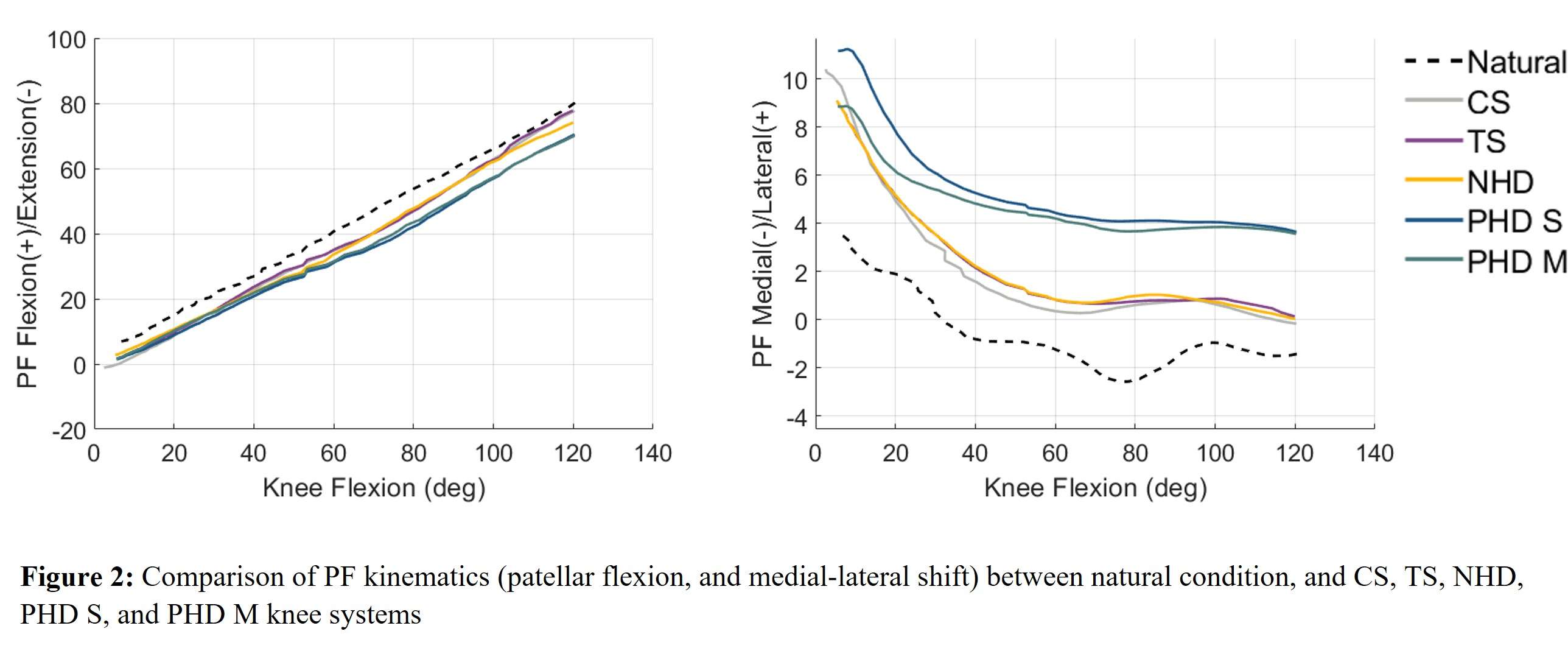
Figure 2
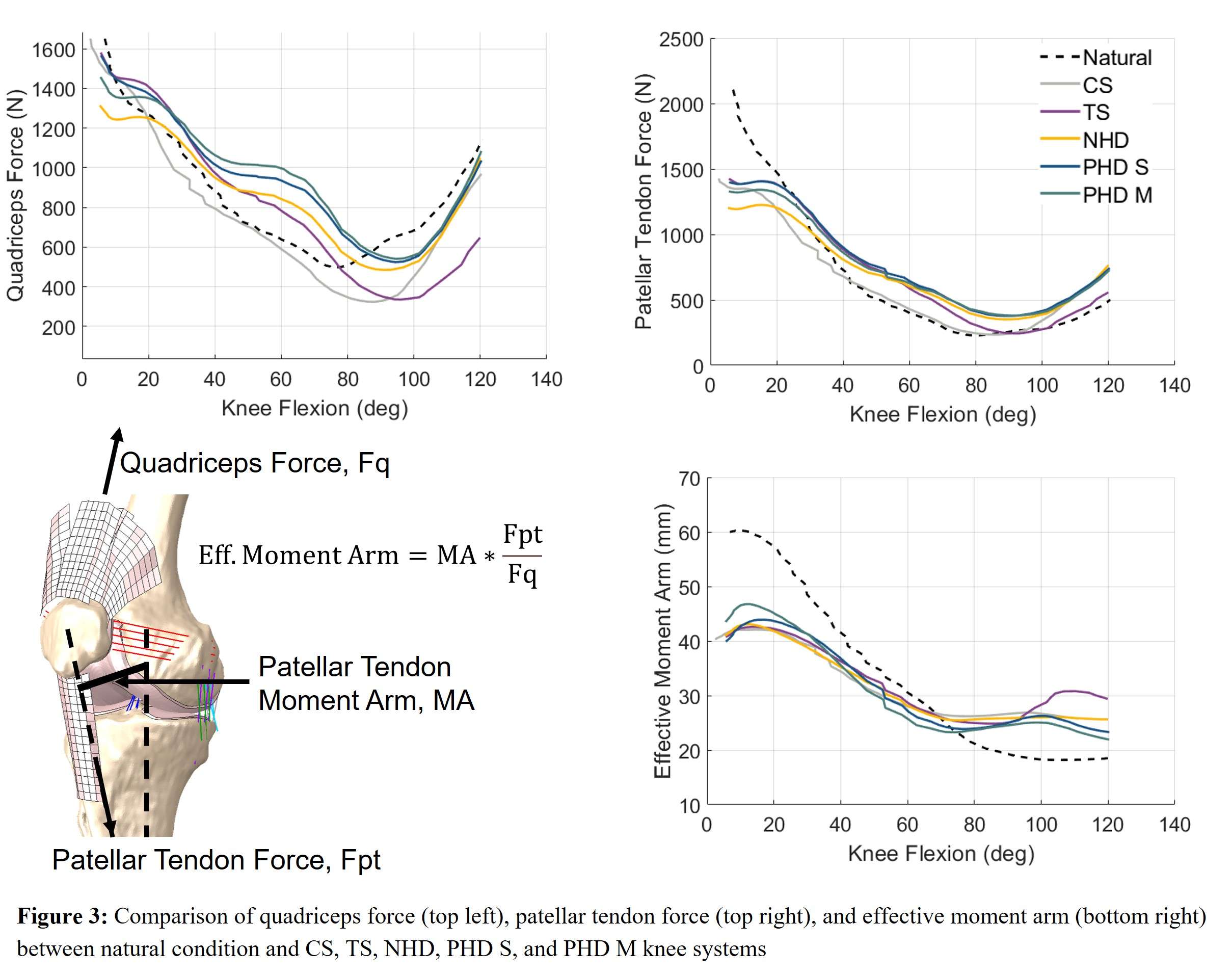
Figure 3#8600
KneeHub - Engaging the Arthroplasty Community to Develop Credible Simulations of the Knee
*Jason Halloran - Washington State University - Spokane, USA
Thor Andreassen - University of Denver - Denver, USA
Thor F Besier - University of Auckland - Auckland, New Zealand
Snehal Chokhandre - Cleveland Clinic - Cleveland, USA
Shady Elmasry - Exponent - Phoenix, USA
Carl Imhauser - USA
Peter J. Laz - University of Denver - Denver, USA
Nynke Rooks - Formus Labs - Auckland, New Zealand
Marco Schneider - Formus Labs - Auckland, New Zealand
Kevin Shelburne - University of Denver - Denver, USA
Ahmet Erdemir - Cleveland Clinic - Cleveland, United States of America
*Email: jason.halloran@wsu.edu
Introduction
Lack of reproducibility is a pervading issue in both clinical and preclinical research, with estimates of irreproducibility reaching 75% to 90%. Simulation-based research is not an exception and this issue has particular relevance in knee arthroplasty. In arthroplasty, virtual prototyping has influenced implant design and informed surgical and rehabilitation approaches1,2. The knee biomechanics community has taken significant strides to share modeling data3, deliver software to build individualized knees4, and understand the influence of modeling decisions5. Modeling and simulation in biomechanics inherit the challenges of computational approaches in any scientific discipline. Alleviating these challenges are prerequisites for establishing validity and credibility. Recent initiatives focus on verification and validation (ASME VVUQ40) or on broadly applicable credible practices (IMAG/MSM Ten Simple Rules). Yet, a foundational concern emerges due to deviations in modeling and simulation of the same knee for the same purpose, i.e., the art of the modeler, or even, deviations of a workflow when it is implemented again by the same modeler or by others, i.e., reproducibility, all contributing to uncertainty. The absence of reproducibility, as dictated by the “context of use”, is a significant barrier for adoption of simulation. An underappreciated concept is how independent modeling workflows contribute to irreproducibility. Our NIH-sponsored “KneeHub” project tackled this issue; showing that when target simulation scenarios and the source data to build models remained the same, variations in modeler’s choices introduced uncertainties that impacted reproducibility5 (Fig. 1). This lack of consistency motivates the next phase to develop reproducible, consensus-based modeling and simulation workflows for knee biomechanics. This paper summarizes our previous findings and presents the follow-up work to engage ISTA members to participate in the development of the consensus-based workflows, which will be evaluated to quantify their reproducibility (Fig. 2).
Methods
Previous activities included the delivery and documentation of modeling processes and products for five independent teams across four phases: Development, Calibration, Benchmarking, and Reuse. The deliverables were analyzed for differences in modeling approaches and simulation predictions (Fig. 1). Next we will use a structured Delphi process to define two relevant contexts of use and to establish corresponding consensus modeling workflows (Fig. 2), which will facilitate an evaluation of reproducibility.
Results
We showed that even when source data and target simulation scenarios remain the same, modelers exhibit variations in model form, coordinate system definition, and calibration strategy5. These choices resulted in a wide range of simulation predictions (e.g., post-calibration RMSEs from 1° to 20° in internal-external rotation) (Fig. 1).
Conclusion
The success of developing and evaluating consensus workflows relies on expert feedback. ISTA is an ideal venue to publicize the activities of our community-directed effort and provides the opportunity for arthroplasty-related needs to inform the project moving forward.
Acknowledgments
NIH-NIBIB R01EB024573
References
1. Clary, et al. J. Biomech. 46, 2013.
2. Elmasry, et al. J. Biomech. 120, 2021.
3. Chokhandre & Erdemir. J. Mech. Behav. Biomed. Mater. 112, 2020.
4. Baldwin, et al. Comput. Methods Programs Biomed. 97, 2010.
5. Rooks, et al. J. Biomech. Eng. 143, 2021.
Figures
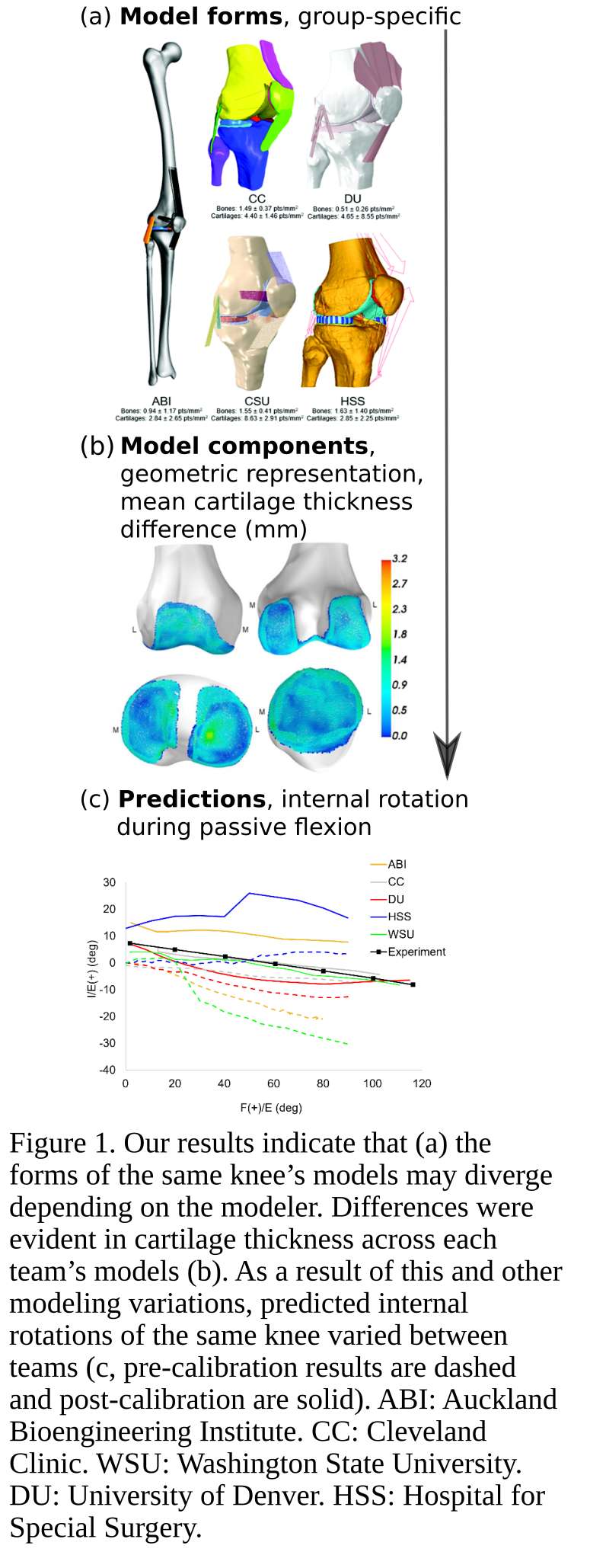
Figure 1
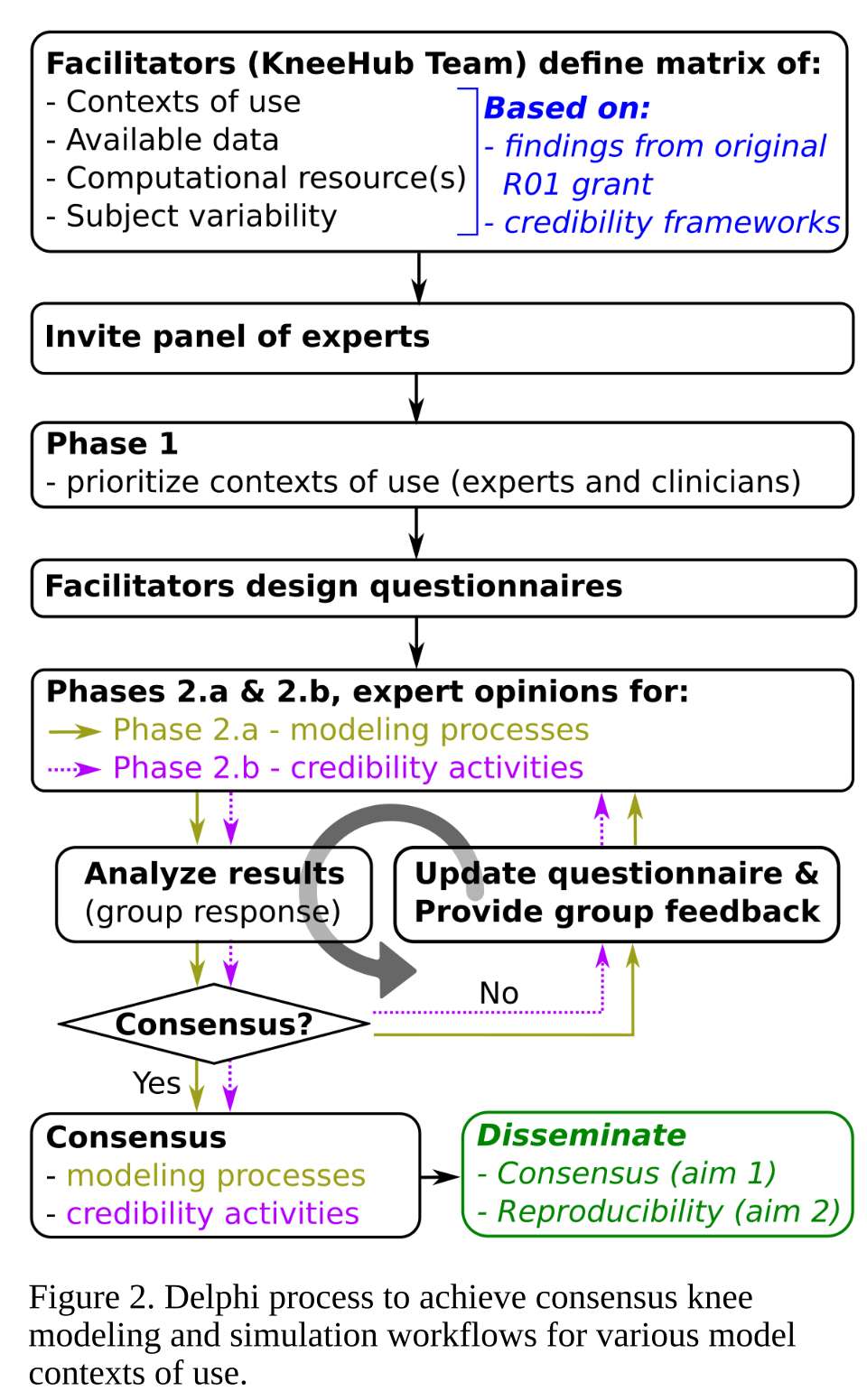
Figure 2#8327
Open Wedge High Tibial Osteotomy Alters Patellofemoral Joint Kinematics of the Knee: A Multibody Simulation Study
*Lennart Schroeder - Julius-Maximilians-University of Wuerzburg - Wuerzburg, Germany
Sonja Grothues - RWTH Aachen University - Aachen, Germany
Josef Brunner - Ludwig Maximilian University of Munich - Munich, Germany
Klaus Radermacher - RWTH Aachen University - Aachen, Germany
Boris M. Holzapfel - Ludwig Maximilian University of Munich - Munich, Germany
Maximilian Joergens - Ludwig Maximilian University of Munich - Munich, Germany
Julian Fuermetz - BG Unfallklinik Murnau - Murnau, Germany
*Email: lennartschroeder@gmx.de
Background: Predicting kinematics of the knee after open-wedge high tibial osteotomy (owHTO) may increase native joint survivorship and futher postpone the need for total knee arthroplasty. With the help of 3D imaging and planning, the morphological changes, such as an increase in tibial tuberosity trochlea groove distance (TT-TG), are increasingly better understood. However, the effects of the procedure on knee kinematics have not yet been sufficiently investigated. Therefore, the aim of this study was to investigate the morphological and kinematic changes of the knee joint, in particular the patellofemoral joint, using a modified multibody simulation model.
Methods: owHTO with an open tibial wedge of 6mm to 12mm (1mm intervals) was virtually performed on each of eight three-dimensional computer-aided-design-models derived from computer tomography scans of full-leg cadaver specimens. By using a multibody simulation model of the native knee, for each owHTO version an individual biomechanical simulation model was built and knee flexion from 10° to 90° was simulated. Morphologic and alignment parameters as well as tibiofemoral and patellofemoral kinematic parameters, each resulting in 5120 data points each over the course of knee flexion, were evaluated.
Results: An approximally linear increase in TT-TG (~0.4mm/1mm) as well as a lateral translation and tilting of the patella was observed. In addition, valgisation lead to a medial translation of the tibia, which changed the tibiafemoral contact area. The largest biomechanical effect was observed in tibiofemoral internal-external rotation. A decrease in the degree of tibial internal rotation during knee flexion of approximately 0.5° was observed for each 1-mm increase in tibial coronal correction.
Conclusion: The simulation of different owHTO showed manifold effects on morphological parameters and on the kinematics of the knee joint. The effects on the patellofemoral joint, especially with a biplanar cut towards proximal, should be considered when performing owHTO. The increase in TT-TG and tibial internal rotation leads to lateralisation and tilting of the patella, which may increase retropatellar pressure and is a potential risk factor for anterior knee pain.
#8617
Finite Element Modeling of Patient-Specific Total Shoulder Arthroplasty
*Ignacio Rivero Crespo - University of Denver - 9085 E. Mississippi Ave, United States of America
Kevin Shelburne - University of Denver - Denver, USA
Casey Myers - University of Denver - Denver, USA
Thor Andreassen - University of Denver - Denver, USA
David Weinstein - Colorado Center of Orthopaedic Excellence - Colorado Springs, USA
Peter Laz - University of Denver - Denver, USA
*Email: iaki.riverocrespo@du.edu
INTRODUCTION: Whether using standard or patient-specific instrumentation, there are differences between the planned and executed location of implants in total shoulder arthroplasty (TSA) [1]. Prior finite element (FE) simulations have relied on implanted versions of generic models of the shoulder or applied kinematics from healthy individuals. The objective of this study was to simulate the impact of surgical variation in TSA using patient-specific models guided by each patient’s kinematics measured with high-speed stereo-radiography (HSSR). The simulations were used to investigate (1) the change in center of pressure (COP) by varying implant placements, and (2) the impact of using each patient’s contralateral kinematics as a preoperative target.
METHODS: Six subjects, each with one TSA implanted shoulder: 3 anatomic (aTSA) and 3 reverse (rTSA) performed abduction, flexion, and internal-external rotation with the arm abducted to 90. Activities were captured using HSSR and joint kinematics obtained through fluoroscopic tracking techniques [2]. Explicit FE models were created using patient-specific rotations from fluoroscopy data and joint force data obtained from OrthoLoad (Fig. 1) [3]. Contact was modeled using a pressure-overclosure relationship following Fitzpatrick et al. [4]. Stability ratio was defined as the ratio of shear force to compression force. Simulations included a design of experiments (DOE) for variations in glenoid implant alignment following ranges reported in literature [1]. Variations in the humeral stem version alignment were based on the allowed variability in the implant design. Patient-specific implanted and native contralateral kinematic profiles were applied to each FE model. Lastly, a sensitivity analysis was performed to assess the importance of variations in kinematics and loads on COP.
RESULTS: Overall, variation in humeral stem version was found to have a higher influence on the center of pressure and implant stability compared to the perturbation of the glenoid implant. For the aTSA subjects, increasing retroversion ranging from 5°-30° caused the COP to shift towards the implant center (Fig. 2). Additionally, aTSA subjects showed similar stability ratios when using subject-specific and contralateral kinematics across all activities. However, rTSA subjects showed an increase in stability ratio when using contralateral kinematics. The sensitivity analysis showed kinematics have an increased influence on COP compared with changes to the loads (Fig. 3).
CONCLUSION: This study uses patient-specific kinematics and FE analysis to evaluate the effects of different surgical and patient-specific parameters on COP and implant stability. Previous studies suggest that humeral stem retroversion improved implant stability [2]. In agreement, this study shows improved stability and movement towards a central COP following implant retroversion. Additionally, this study suggests that while the contralateral kinematics could be used as a pre-operative target for aTSA patients, it might not be a reasonable target for rTSA patients as restoring kinematics can affect implant stability. The variability observed in this study further emphasizes the need for patient-specific approaches to determine proper placement of TSA components.
REFERENCES: 1. Iannotti et al. J. Bone Jt. Surg. (2019). 2. Walden, Master’s Thesis, University of Denver (2019). 3. Bergmann et al. Charite Universitaetsmedizin Berlin (2008). 4. Fitzpatrick et al. J. Biomech. Eng. (2010).
Figures
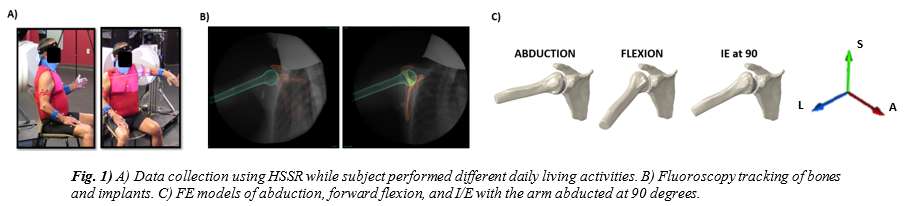
Figure 1
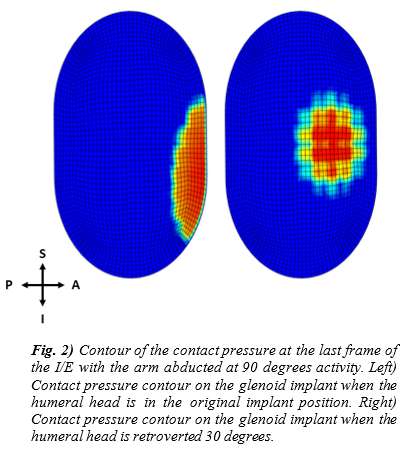
Figure 2
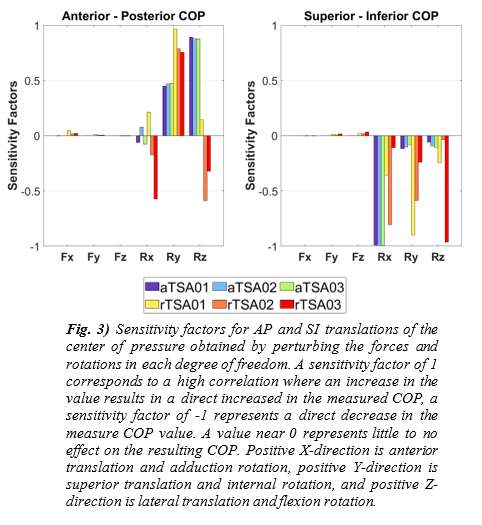
Figure 3#8258
Including Muscle and Ligament Forces in a Patient-Specific Finite Element Model of Reverse Shoulder Arthroplasty Affects Glenoid Micromotion
*Jonathan Glenday - Hospital for Special Surgery - New York, USA
Andreas Kontaxis - Hospital For Special Surgery - New York, USA
Benjamin Johnston - Cornell University - Ithaca, USA
Fernando Quevedo Gonzalez - Hospital for Special Surgery - New York, USA
*Email: glendayj@hss.edu
Introduction: Reverse shoulder arthroplasty (RSA) alleviates pain and restores shoulder function to patients with rotator cuff arthropathies [1]. Many studies have investigated the biomechanics of RSA; however, current finite element (FE) investigations of micromotion of the uncemented glenoid fixation utilize simplistic loading profiles and do not consider the effect of the complex, multiaxial loads typically observed during activities of daily living (ADL). Moreover, these models often omit loading applied by muscles and ligaments (‘soft tissues’). The objective of our study was to use a patient-specific musculoskeletal model [2] to predict joint contact and soft tissue loads and then transfer these loads to a custom, matching FE model to evaluate glenoid fixation (Fig. 1). We hypothesized that peak bone-implant micromotion would not occur during peak joint contact loading and that simulating soft tissue forces would increase micromotion compared to FE simulations with only joint contact loading.
Methods: Musculoskeletal and FE models were customized using preoperative CT scans of five patients with rotator cuff arthropathies. The scapulae, humeri, and soft tissue attachments were customized to the patient-specific anatomy and a commercial RSA system (Zimmer-Biomet, Warsaw, IN) was virtually implanted. The musculoskeletal model simulated a ‘reaching for an object at head height’ ADL and predicted the soft tissue forces and glenohumeral (GH) joint contact forces. These forces were transferred to the matching FE models. Here, GH contact forces were applied at the glenoid sphere center and scapular soft tissue forces were applied at their bony attachments. The scapula was modeled as non-homogenous, with an elastic modulus (E) determined from the CT data [3] and a Poisson’s ratio (υ) of 0.3. The glenoid sphere was modeled as rigid and the baseplate and locking screws were modeled as a titanium alloy (E=113.8 GPa, υ=0.3). All bone-implant interfaces were modeled as frictional (µ=0.6). The scapula was fixed at the medial border and acromioclavicular joint. We simulated two loading configurations: 1) GH contact force only, and 2) GH contact and soft tissue forces (Fig. 1a-b) and evaluated bone-implant micromotion.
Results: Throughout the ADL, compression represented 50%-92% of the net GH contact force (Fig. 2). At peak loading (31% of the cycle), compression was 88% of the net force. The greatest peak micromotion was observed when compression accounted for 72% of the net force (79% of the cycle). Peak micromotion was lower (p=0.046) when only GH contact forces were applied (5 ± 2 µm) than when GH contact and the soft tissue forces were applied (18 ± 11 µm).
Conclusions: We found that the peak micromotion during the simulated ADL did not occur during peak joint contact loading, but instead when the ratio of compression to shear forces was smaller. Furthermore, including soft tissue forces led to increased micromotion; however, all peak micromotions were within acceptable osseointegration limits. Future computational studies of glenoid fixation should consider entire activity cycles and include soft tissue loading to avoid underestimating fixation mechanics.
References: [1] Boileau. JSES (2006). [2] Kontaxis and Johnson. Clin Biomech (2009). [3] Morgan. J Biomech (2003).
Figures
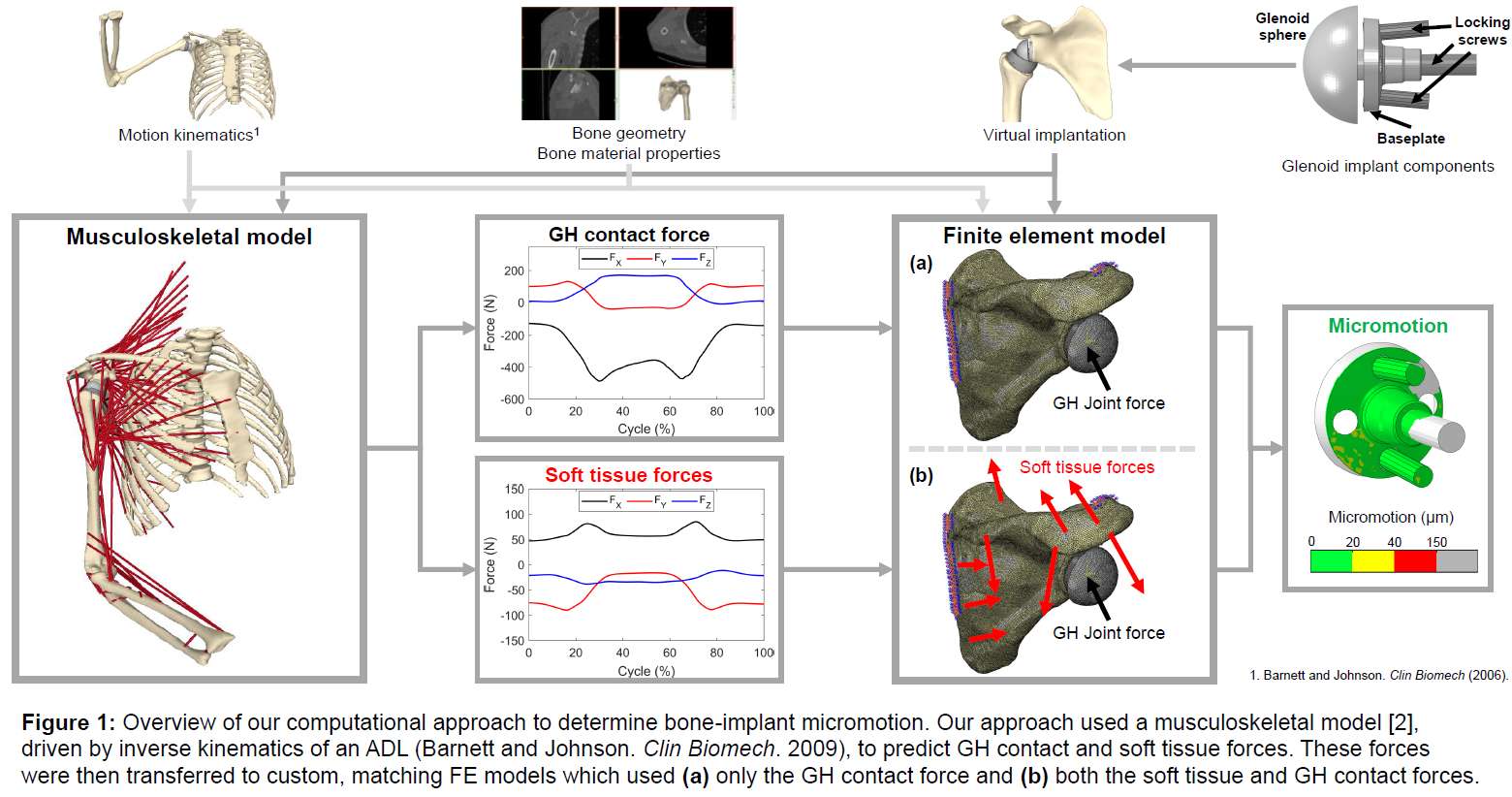
Figure 1
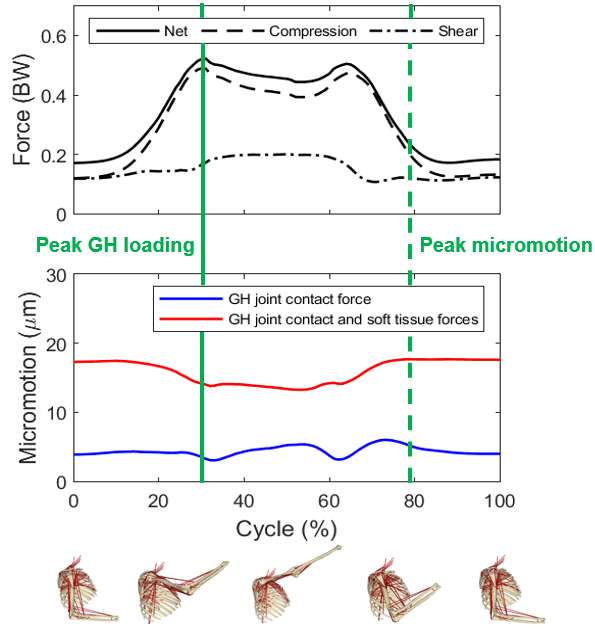
Figure 2#8248
Knee Functional Positioning Sensitivity Study Using Finite Element Analysis
*Azhar Ali - Stryker Orthopaedics - Mahwah, USA
Jerry D'Alessio - Stryker - Mahwah, USA
Xiangyi (Cheryl) Liu - Stryker Orthopaedics - Mahwah, USA
Seth A. Jerabek - Hospital for Special Surgery - New York, USA
David J. Mayman - Hospital for Special Surgery - New York, USA
*Email: azhar.ali@stryker.com
Introduction: There is growing interest in functional knee positioning philosophies for total knee arthroplasty (TKA), with surgeons seeking to align TKA implants to replicate a patient’s native anatomy and soft tissue balance. While there can be thousands of potential solutions to achieve a balanced TKA given an alignment philosophy, preferred boundaries for component position and gap targets for desired laxity, the prioritization of surgical parameters for optimal placement remains unknown [1]. The objective of this study was to develop a comprehensive implant positioning sensitivity study using patient-specific finite element models of three healthy subjects to identify and rank the most impactful surgical parameters for knee function.
Methods: Using a previously-validated experimental and computational approach, patient-specific, force-driven, finite element models were developed to reproduce the native knee mechanics for three healthy subjects performing single-leg lunge and stair descent activities [2,3]. Virtual surgeries were performed using Triathlon CR on each subject model to represent 100 implant alignment combinations spanning the allowable surgical boundaries. Latin Hypercube sampling was used to simultaneously perturb 9 surgical parameters corresponding to femoral and tibial rotations/translations. A total of 17 surgical parameters were computed in relation to the component positions (Figure 2). Medial congruency and trochlear ML fit were computed as the difference between the bone and implant surface along the condyle and trochlear dwell. Custom, automated scripts were used to optimize size and alignment of components for unprescribed implant positions. For each subject (n=3), activity (n=2), and implant alignment (n=100), patient-specific finite element models predicted knee mechanics (i.e., TF/PF kinematics, joint load, ligament forces), and the root mean square errors (RMSE) between the implanted simulations and natural condition were evaluated (n=600 simulations, Figure 1). Total sensitivity was defined as the contribution of each surgical parameter to the combined model error from all model outputs, subjects, and activities.
Results: Following virtual surgeries, femoral and tibial components were size-matched in 56% of simulations, size-down femur in 40% of simulations and size-up femur in 4% of simulations on average across 3 subjects. Femoral and tibial resection depths ranged from 1.4 mm to 10.8mm across all subjects and alignment combinations (Figure 2). Overall, medial congruency had the greatest total sensitivity to knee mechanics, and significantly affected medial joint load (Figure 3). Femoral and tibial resections that reproduced the native joint line improved kinematics and compartmental forces relative to the natural condition. Greater trochlear fit significantly improved PF kinematics and extensor efficiency. Tibial slope significantly affected anterior-posterior kinematics, but an opposing trend in lunge and stair descent led to minimal combined impact on knee function (Figure 3, top).
Conclusion: Advanced 3D surgical parameters (medial congruency and trochlear ML fit) consider overall implant fit to the native anatomy and have the greatest impact on knee performance. Using 3D CT-based surgical planning, medial congruency, trochlear fit and joint line restoration can be optimized to potentially improve patient outcomes. While functional positioning enables surgical planning based on anatomy and soft tissue, the current study quantifies the prioritization of surgical parameters to best restore native function.
Figures
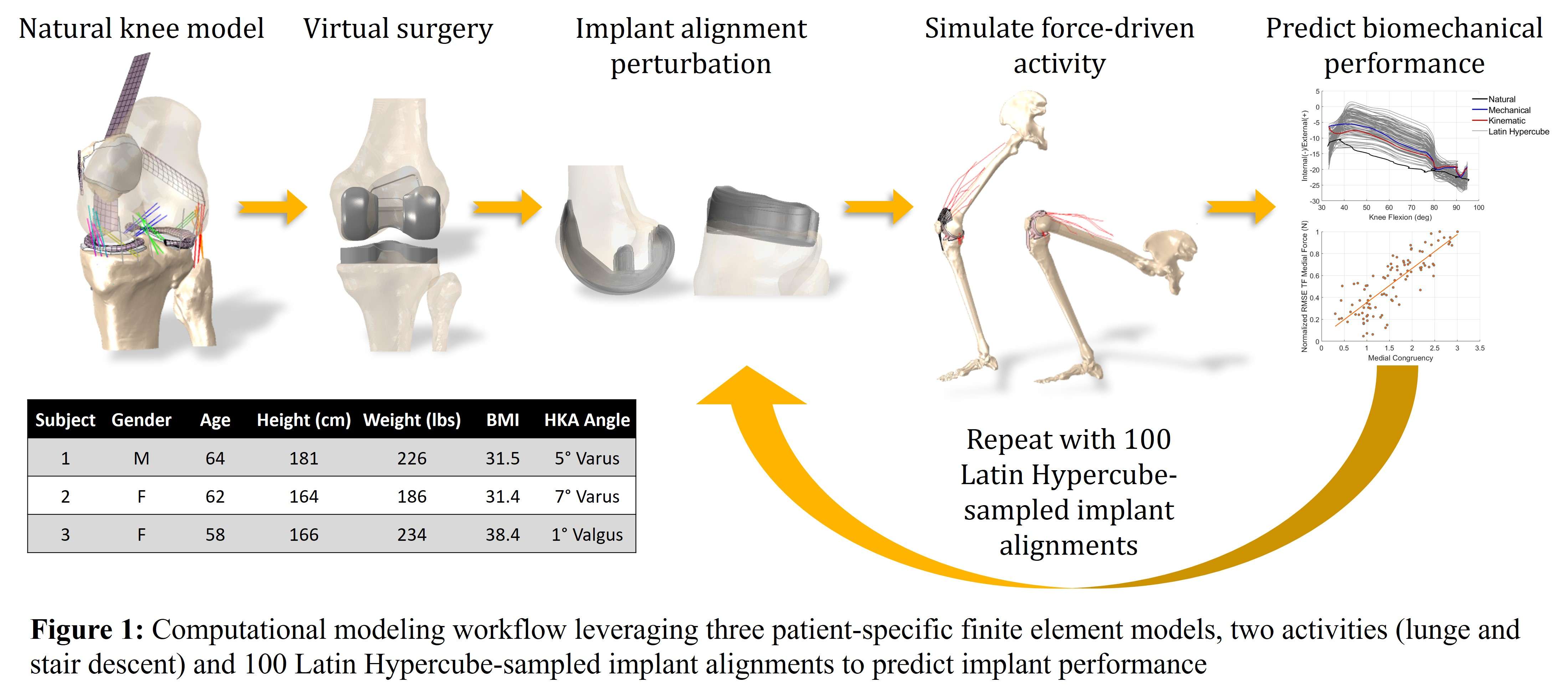
Figure 1
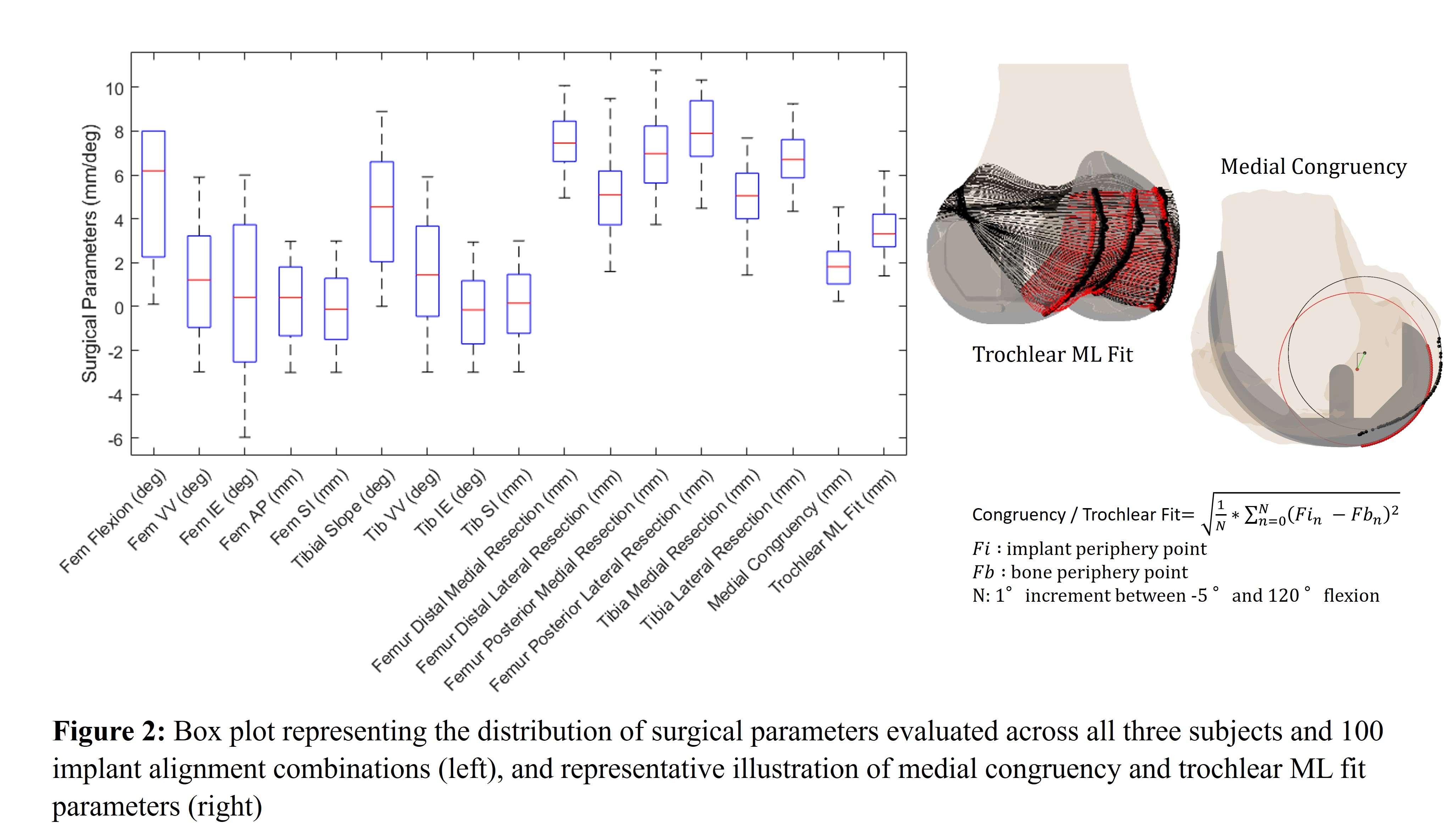
Figure 2
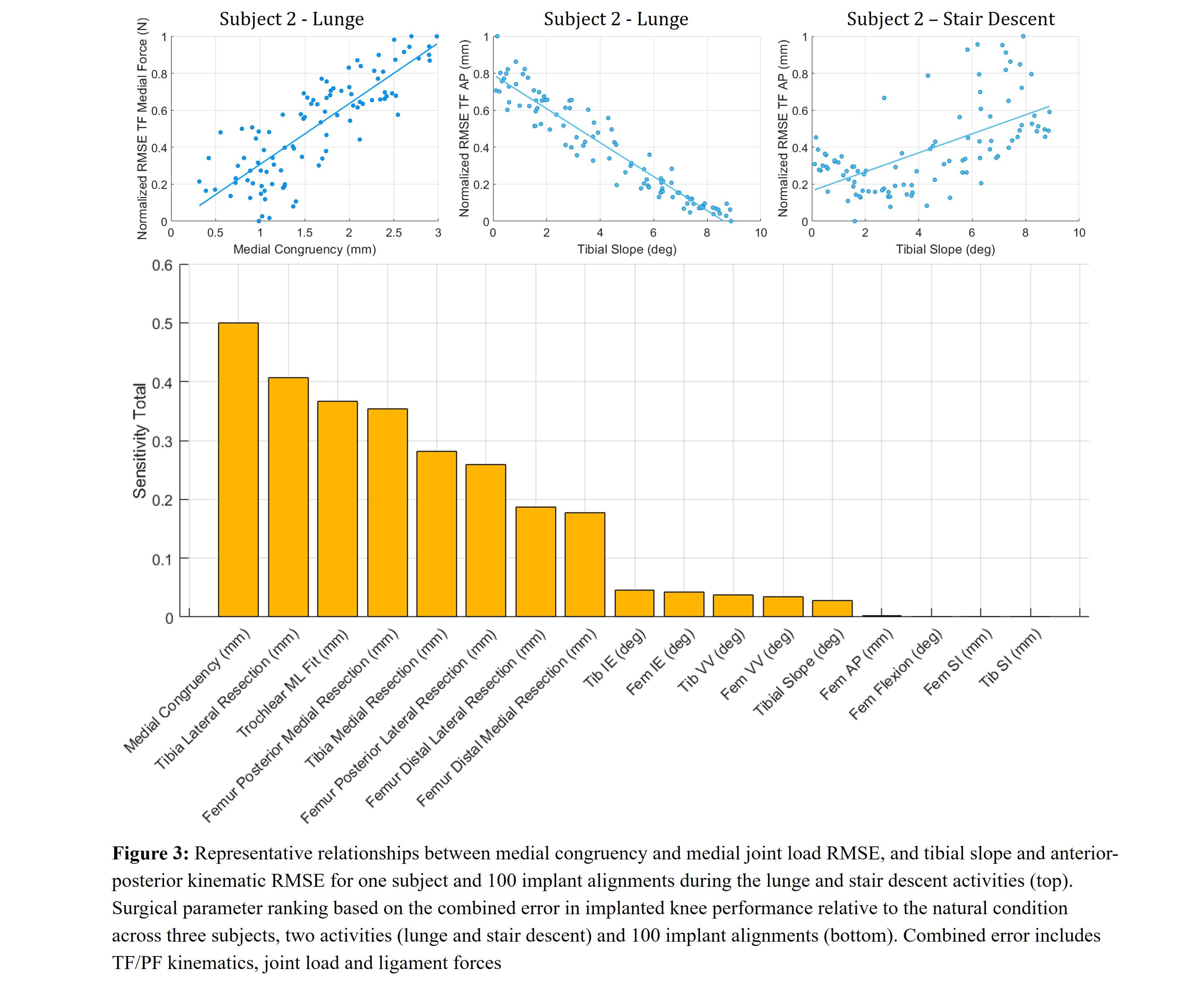
Figure 3#8129
Up to 69% Stress Relaxation in Bovine Trabecular Bone in 24 Hours: The Potential to Improve Models of Primary Fixation of Press-Fit Implants
*Thomas Gersie - RadboudUMC - Nijmegen, Netherlands
Thom Bitter - Raboud University Nijmegen Medical Centre - Nijmegen, Netherlands
David Wolfson - DePuy International Ltd - Leeds, United Kingdom
Robert Freeman - DePuy International Ltd. - Leeds, United Kingdom
Nico Verdonschot - Radboudumc - Nijmegen, Netherlands
Dennis Janssen - Radboud University Nijmegen Medical Centre - Nijmegen, Netherlands
*Email: thomas.gersie@radboudumc.nl
Introduction
Computational models of total joint reconstructions rely on the accuracy of material models used to capture the bone material properties. While trabecular bone is typically modelled as a linear elastic material, its mechanical response is time-dependent, displaying a distinct stress-relaxation response. The viscoelastic behavior may have a significant effect on the simulation of the primary fixation of press-fit implants. Therefore, in the current study we quantified the long-term nonlinear stress relaxation in experiments with bovine trabecular bone.
Methods
34 Trabecular femoral bovine bone cylinders were harvested. Before testing, the bone mineral density (BMD) was determined using CT images. A uniaxial compressive strain (0.2 to 0.8%, specimens immersed in physiological saline at 37°C) was applied for 24 hours on 16 samples to determine the test duration for the multiple stress relaxation experiments. Experimental data ranging from 10 to 120 minutes was extrapolated and fitted with a power law function, and compared to the full 24-hour power law fit. The remaining samples were tested on four consecutive days, allowing 24 hour to recover after each experiment, at increasing static strains from 0.2 to 0.8%, with 0.2% increments. A simplified Schapery model (SSM) and a Modified superposition model (MSM) were fit to the multiple stress relaxation experiments.
Results
After 24 hours, stress relaxation ranging from 41.0 to 68.7% was observed. Up to 52.9% occurred in the first 10 minutes (Figure 1). Following the optimal relaxation duration experiments, a constant displacement was applied for 30 minutes and the resulting data was extrapolated to 24 hours. Since, no relation was found between BMD and time-dependent behavior (Figure 2), only a sample-by-sample approach was used to fit the two nonlinear viscoelastic models to the repeated stress relaxation experiments. The SSM and MSM were adequate in fitting the nonlinear stress relaxation response (Figure 3).
Conclusion
This study demonstrated the significant amount of stress relaxation occurring in trabecular bone. Holding the constant strain for a minimum of 30 minutes led to an acceptable extrapolation of the data to 24 hours. Stress relaxation was not influenced by BMD, while it varied nonlinearly strain. Up to 52.9% stress relaxation occurred within 10 minutes, which in clinical practice is still during surgery. This highlights the importance of incorporating viscoelastic behavior in orthopedic simulations, such as those related to implant loosening, to ensure more accurate results. The next step is to incorporate the viscoelastic behavior in finite element simulations of primary fixation of femoral and tibial total knee arthroplasty components to demonstrate the influence of bone relaxation on primary fixation, which may further optimize the implant design process.
Acknowledgements
This collaboration project is co-funded by the PPP allowance made available by Health~Holland, Top Sector Life Sciences & Health, to stimulate public-private partnerships, and DePuy Synthes (Leeds, UK).
Figures
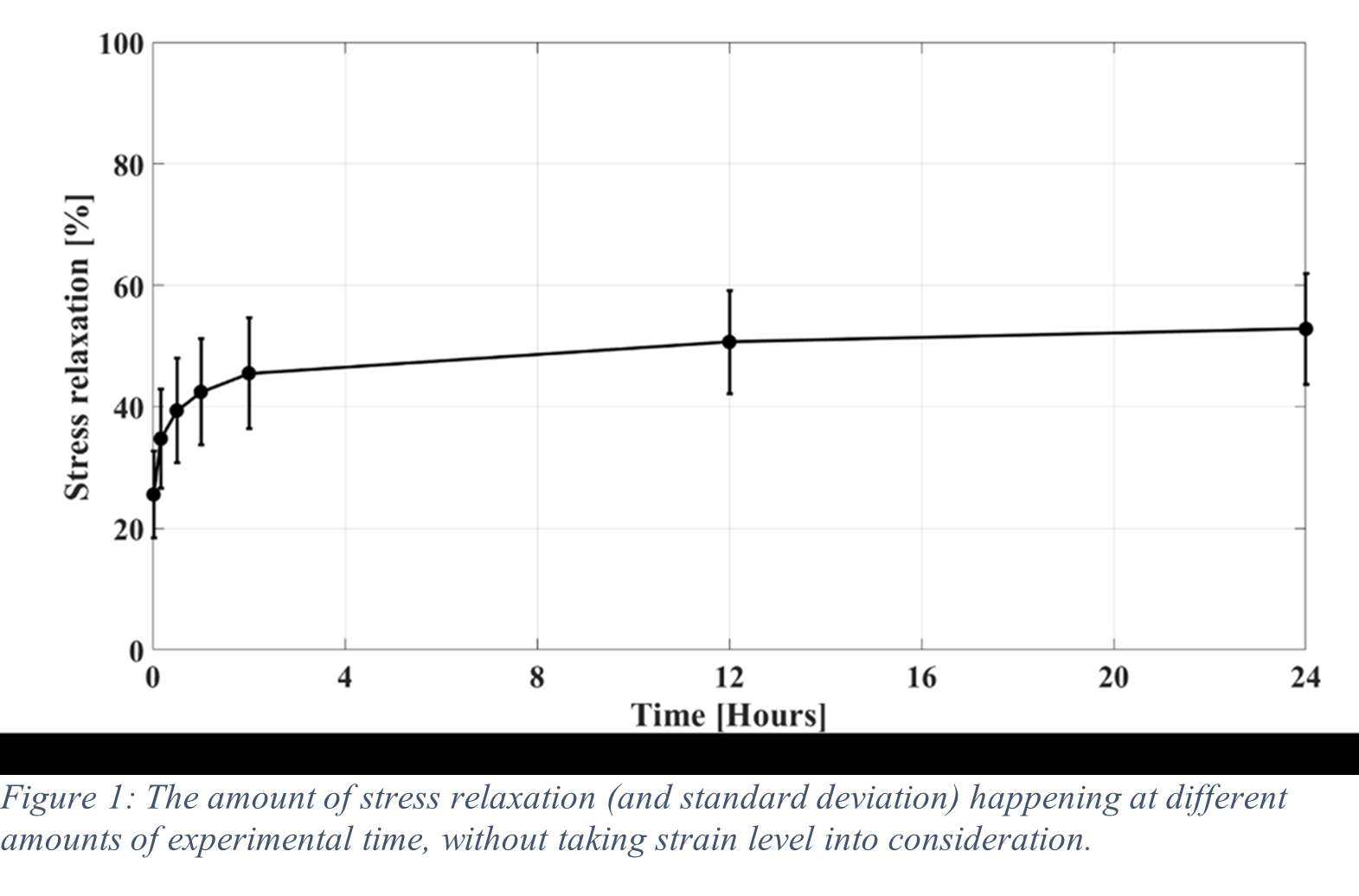
Figure 1
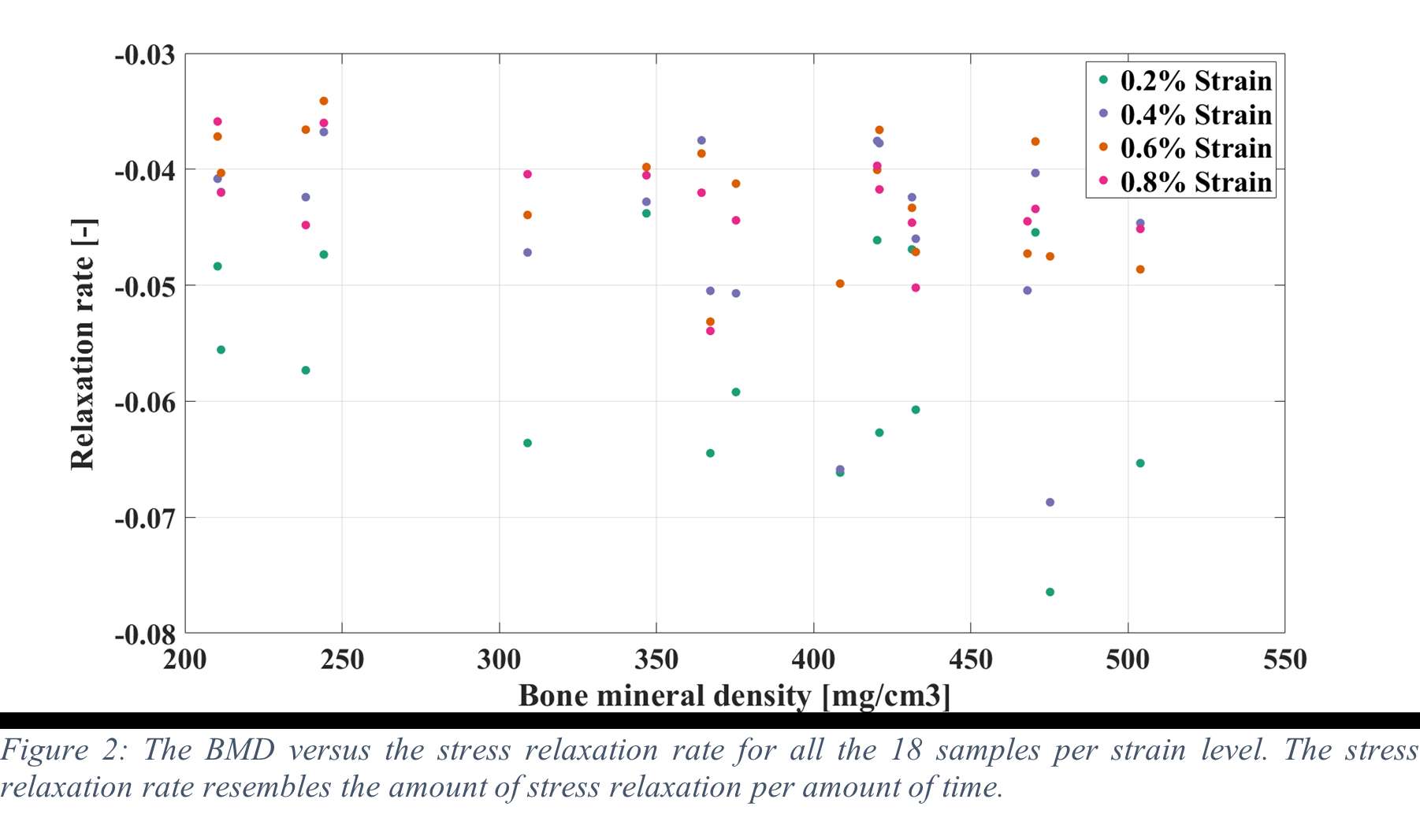
Figure 2
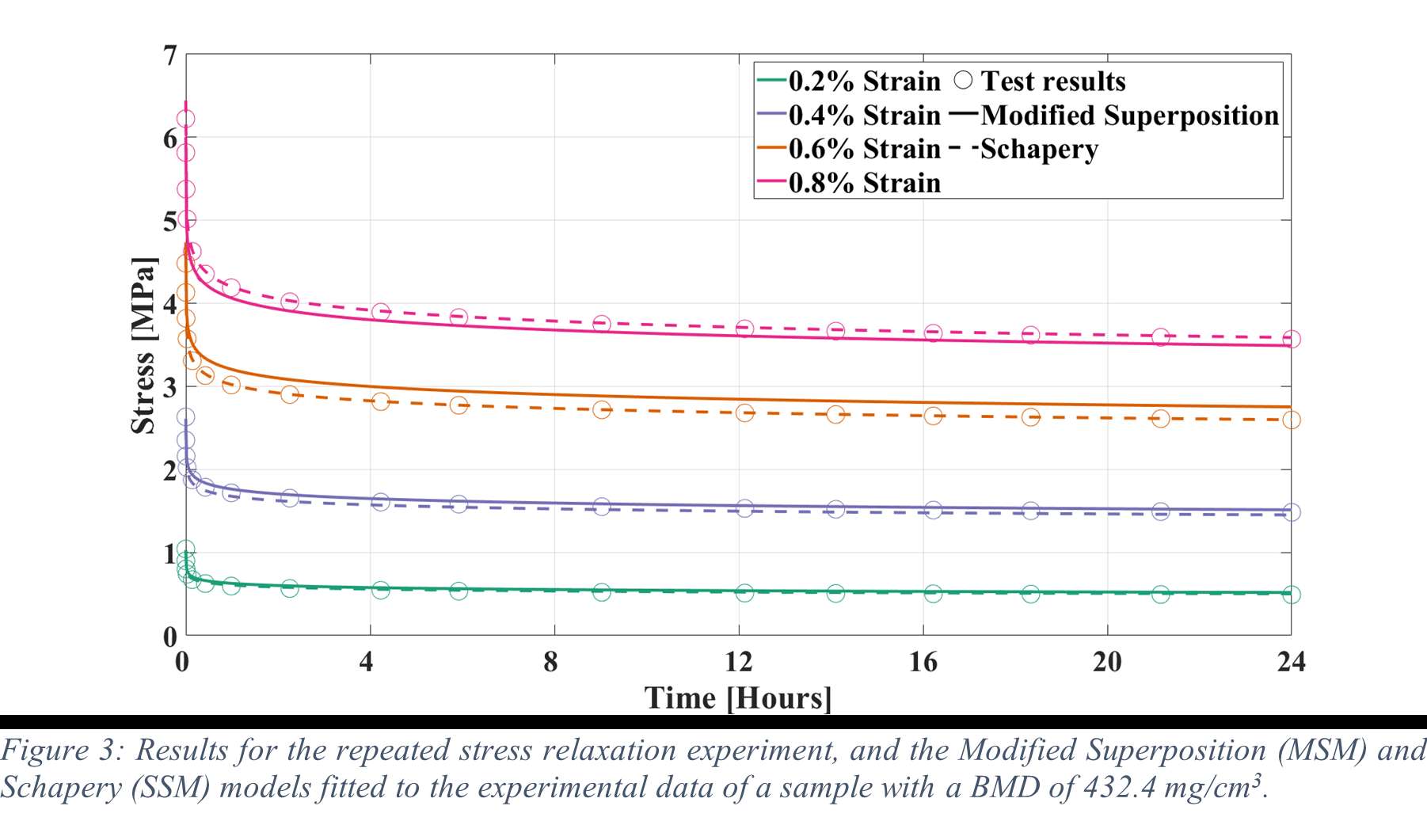
Figure 3#8838
"Inside the Box" A Systematic Approach to Breakthrough Thinking
*Drew Boyd - Cincinnati, United States of America
*Email: darla@drewboyd.com
#8827
What do strategics look for when assessing a start up
*Kristine Ilaria - New York, United States of America
*Email: kristine.ilaria@smith-nephew.com
#8828
Acquisition watch outs - Headwinds and tailwinds
*Robert Cohen - Stryker - Mahwah, USA
*Email: robert.cohen@stryker.com
#8829
Accelerating innovation through acquisitions, how R&D & M&A need to work hand in hand
*Stephen White - DePuy Synthes - Warsaw, USA
*Email: SWhite14@ITS.JNJ.com
#8830
Dealing in the post covid era - a change in market outlook
*Simran Sabharwal - Smith & Nephew - New York, United States of America
*Email: simran.sabharwal@smith-nephew.com
#8118
Completion of Patient Reported Outcomes Measures Improved With Use of an Arthroplasty-Specific Mobile Application Results From a Randomized Controlled Trial
Matthew Miller
*Roberta Redfern - Zimmer Biomet - Pemberville, USA
Mike Anderson - Zimmer Biomet - Lehi, USA
Scott Abshagen - Zimmer Biomet - Warsaw, USA
Dave Van Andel - Zimmer Biomet - Grand Rapids, USA
Jess Lonner - Rothman Institute - Philadelphia, USA
*Email: roberta.redfern@zimmerbiomet.com
Introduction
The collection of patient-reported outcome measures (PROMs) has historically been reported as costly and time-consuming, with low compliance rates that may impact reimbursement. Little research has reported the effects of mobile applications to support PROMs collection following arthroplasty.
Methods
Secondary analysis of data from a multicenter randomized controlled trial was performed. Patients were randomized to utilize a smartphone-based care management platform (app) for self-directed rehabilitation and completing joint-specific PROMs (HOOS JR/KOOS JR) via the application at prescribed intervals or on paper during clinic visits. Control patients received practice standard of care and completed PROMs via emailed hyperlink or during clinic visits. Compliance with protocol-defined timepoints were calculated through one year post-operatively. Comprehensive Joint Replacement (CJR) submission rules (pre-operative PROMs -90 – 0 days and post-operative PROMs 270-425 days) were also applied to compare compliance by group assignment. Patients were categorized by age (65 years or older) to determine the rate of compliance and electronic completion of surveys within Medicare-eligible subjects.
Results
384 and 451 patients in the app and control groups, respectively, were eligible for analysis. Age (62.6±9.6 vs 63.3±9.5, p=0.35), sex (59.4% vs 58.9% female, p=0.87), and procedure (p=0.91) were similar between arms. Compliance was higher in the app group preoperatively (97.1% vs 87.6%, p<0.0001) and at every timepoint postoperatively, including at one year (71.7% vs 51.6%, p<0.0001). This trend persisted across procedure types. On logistic regression, including age, sex, procedure, and pre-operative PROMs scores, intervention arm was the strongest predictor of completion of all PROMs, where app users were 4.4 times more likely to be compliant at all timepoints (OR 4.493, 95%CI 3.302 – 6.113, p<0.0001). Similarly, app users were twice as likely to be compliant with CJR guidelines (OR 2.08, 95%CI 1.515 – 2.856, p<0.0001). The majority of patients in the app group (77.5%), completed PROMs using the application as opposed to paper methods. In this cohort, patients older than 65 years exhibited higher compliance at all timepoints than those <65 years of age. Significantly higher CJR compliance was observed in patients over 65 in the app group compared to the control group (74.1% vs 52.2%, P,0.0001). Considering patients who were compliant with CJR timeframes for pre- and post-operative PROMs, only 9.5% of patients ≥65 years completed surveys electronically via hyperlink whereas 66.7% of patients in the intervention arm completed both surveys within the mobile application (p<0.0001).
Conclusion
A smartphone mobile application that engages patients during recovery after knee and hip joint arthroplasty improves compliance with completion of pre- and post-operative PROMs compared to other electronic and paper collection methods.
#8567
Characterizing the Trajectory of Patient Recovery Following Total Knee Arthroplasty Using Implanted Sensors
Fred Cushner - Hospital for Special Surgery - New York, USA
Jeffrey Yergler - South Bend Orthopaedics - South Bend, USA
Barbara Elashoff - Canary Medical - Carlsbad, USA
Giles Scuderi
Patrick Aubin - Canary Medical - Carlsbad, USA
*Genne' DeHenau McDonald PT - Canary Medical - Gainesville, United States of America
Patrick Verta - Canary Medical - Carlsbad, USA
*Email: gmcdonald@canarymedical.com
Background: Knowing how quickly and how well patients recover from total knee arthroplasty requires objective patient outcome data. The collection of such data has been limited to wearable technology that carries certain drawbacks. The purpose of the current study is to analyze TKA patient post-op activity levels using embedded sensor technology for the first time.
Methods: Two hundred fifty-eight patients underwent a primary TKA with the smart implanted canturioTM tibial extension (CTE) between October 4, 2021, and July 15, 2022, by thirty-three surgeons. All kinematic data (step count, walking speed, stride length, cadence, functional knee range of motion, and tibia range of motion) were collected and stored in a HIPAA-compliant cloud data management platform. Estimates of the 5th through 95th percentile values of step count, walking speed, stride length, cadence, functional knee range of motion, and tibia range of motion were calculated for each parameter. Data were divided into 4 mutually exclusive cohorts (men <65y or ≥65y; women <65y or ≥65y). Simple linear regression on the Week 12 percentiles using Week 6 percentile as explanatory variable was performed for each gait parameter.
Results: Ninety percent of patients had more than 80 days of data transmitted during the 12-week period. The average age in the study population was 63.6 years with 138 (54%) women. Summaries of the six gait parameters in the form of recovery curves show faster recovery in the initial 6 weeks than in the second (Figure 1). Older patients (≥65 years) walked slower, with shorter stride lengths. The 6-week percentiles demonstrated a strong, linear correlation to the 12-week percentiles for each gait parameter, with correlation coefficients ranging from 0.87 to 0.92 (Figure 2). All gait parameters have C-statistics >0.8 for the concordance between weeks 6 and 12.
Conclusions: Our results show a high compliance rate with data collection and transmission using a smart implant. Assessment at 6 weeks may have predictive power for identifying poor recovery at week 12, suggesting a potential screening tool. A future study is needed to validate these findings in an independent set of patients.
Keywords: total knee arthroplasty, recovery curve, clinical outcome
Figures
Figure 1
Figure 2#8081
Real-Time Patient Recovery Tracking Using Wearable Technology Following Total Knee Arthroplasty
*Rohan Mangal - University of Miami Miller School of Medicine - Miami, United States of America
Andrew Pierce - Herbert Wertheim College of Medicine - Miami, USA
Danielle Marshall - University of Miami Miller School of Medicine - Miami, USA
Richard Bolander - TracPatch - Orland Park, United States of America
*Email: rohan.mangal@med.miami.edu
Introduction
Functional recovery after total knee arthroplasty (TKA) is typically measured using patient-reported questionnaires or physician measurements at discrete time points. Using wearable devices, surgeons can now monitor patients’ rehabilitation progress continuously through objective data. Knowing the normal recovery curves of knee range of motion (ROM) after TKA in the early postoperative weeks may allow for prompt identification of patients who fail to meet basic recovery milestones. The aim of this study was to analyze the daily recovery patterns in the first six weeks after TKA using a wearable device.
Methods
This study retrospectively analyzes patients who underwent TKA between 2020 and 2022. Patients were treated by 11 surgeons from 8 institutions. Eligibility criteria included all patients ages 18 or older who underwent a primary unilateral TKA and owned a smartphone. The wearable device was applied in the OR during their TKA procedure and patients were instructed on using the device after the operation. Measurements including knee ROM, daily steps, leg position, and time wearing the device were collected continuously over six weeks. Data was analyzed using the chi-square test for categorical variables and Mann-Whitney U test for nonparametric continuous variables in Python. Boxplots were created and the Granger causality test was calculated to demonstrate the effect of gender and BMI on recovery.
Results
481 patients were retrospectively reviewed. The mean age was 65 and 69 for males and females, respectively (range 50-80). Females comprised 61% (n=295) of study participants. 82% of female and 90% of male patients had a BMI >30. Average daily wear time of the device was 12 hours (± 4) for a total of 45 days (± 27). At baseline, knee ROM was -2° to 110°. On the first postoperative day, mean knee ROM was 10° to 88° and -2° to 100° at six weeks postoperatively. In the first postoperative week, patients spent on average 30% of their time with their knee in near or full extension (-2° to 15°). Recovery was nonlinear, with greatest improvements in knee ROM and step count occurring in the first 30 days. Patients reached their preoperative step count levels between 30-40 days on average. Obese female and morbidly obese male and female patients demonstrated slower and more varied step count recoveries.
Conclusion
This is the first study to provide normative data on the day-to-day functional recovery of patients following TKA using wearable sensor technology. This data has not previously been captured during standard clinic visits. Knee ROM, step count, and time spent in knee extension recovered to baseline in a nonlinear manner after TKA. Understanding the recovery timeline following TKA can allow surgeons to accurately counsel patients preoperatively and intervene more promptly when recovery does not fall within the standard kinetics.
#8481
Smartphone-Based Step-Count Measures Correlate With H/KOOS12-Function and UCLA Activity PROMs During Early THA and TKA Recovery
Eric M Slotkin - Reading Hospital, Orthopaedic Associates of Reading, - West Reading, Pennsylvania, USA
*Alex Orsi - Corin - Raynham, USA
Christopher Plaskos - Corin - Raynham, USA
Stephen McMahon - Monash University - Melbourne, Australia
Edgar Wakelin - OMNI Life Sciences - Raynham, USA
Corey E. Ponder - Oklahoma Sports and Orthopedics Institute - Oklahoma, USA
Jeffrey Lawrence - Gundersen Health System - viroqua, USA
John Keggi - Orthopaedics New England - Middlebury, USA
Simon Coffey - Nepean Hospital - Sydney, Australia
Paramjeet Gill - UCSF-Fresno - Fresno, USA
*Email: Alex.Orsi@coringroup.com
Smartphone-based apps that measure step-count and patient reported outcomes (PROMs) are being increasingly used to quantify recovery in total hip (THA), total knee (TKA), and collectively total joint arthroplasty (TJA). However, optimum patient-specific activity level before and during TJA early-recovery is not well characterized. This study investigated 1) correlations between step-count and PROMs and 2) how patient demographics impact step-count preoperatively and during early postoperative recovery.
Smartphone step-count and PROM data collected using a commercially available patient engagement mobile app from 1,395 THA (mean±SD age: 64±10 years, mean±SD BMI: 29±7 kg/m2, 53% female) and 1,762 TKA (mean±SD age: 66±9 years, mean±SD BMI: 31±9 kg/m2, 59% female) patients was retrospectively reviewed. Mean daily step-count was calculated over three time-windows: 60 days prior to surgery (preop), 6-7 weeks postop (6wk), and 13-14 weeks postop (3mo).
Linear correlations between step-count and H/KOOS12 Function and UCLA activity scores were performed. Age >65years, BMI >35, and sex were used for demographic comparisons. An ROC analysis helped determine optimum thresholds for dividing patients by step count level: low (<2500 steps/day), medium (2500-5500 steps/day), and high (>5500 steps/day).
Welch’s t-tests determined significant differences in mean step-counts between demographic groups and in mean PROMs between step-count groups.
UCLA and HOOS-12 Function correlated with step-count at all time-windows (p<0.01). High vs low step count individuals had higher UCLA scores preoperatively (Δ1.4, p<0.001), at 6wk (Δ0.9, p<0.001), and 3mo (Δ1.5, p<0.001), and higher HOOS12 Function scores preoperatively (Δ9.3, p<0.001), at 6wk (Δ5.6, p<0.001), and 3mo (Δ6.3, p<0.001) [Fig. 1].
Males had greater mean daily step-count preoperatively (Δ1.1k p<0.001), at 6wk (Δ1.4k, p<0.001), and at 3mo (Δ1.5k, p<0.001). Younger patients had greater step-count preoperatively (Δ1.2k, p<0.001), at 6wk (Δ1.4k, p<0.001), and 3mo (Δ1.8k, p<0.001). Low BMI patients had greater mean daily step-count preoperatively (Δ1.5k, p<0.001), at 6wk (Δ1.4k, p<0.01) and 3mo (Δ1.6k, p<0.01) [Fig. 2].
Correlations were found between PROMs and step count preoperatively (UCLA, KOOS12 Function, p<0.01), at 6 weeks (UCLA, KOOS12 Function, p<0.05) and at 3 months (UCLA, p<0.001). High vs. low step count individuals had higher UCLA scores preoperatively (Δ1.2, p<0.001), at 6wk (Δ0.6, p<0.01), and at 3mo (Δ1.0, p<0.001), and higher KOOS12 Function scores preoperatively (Δ5.3, p<0.05), and at 6wk (Δ6.3, p<0.01) [Fig. 1].
Males had greater step-count preoperatively (Δ1.3k, p<0.001), at 6wk (Δ1.4k, p<0.001), and at 3mo (Δ1.0k, p<0.01). Younger patients had greater step-count preoperatively (Δ1.0k, p<0.001), and at 3mo (Δ0.8k, p<0.01). Low BMI patients had greater step-count preoperatively (Δ1.0k, p<0.001), and at 6wk (Δ0.8k, p<0.01) [Fig. 2].
Daily step-count is significantly impacted by patient demographics and correlates with PROMs, as patients with high step count exhibit improved PROMs. Generic recovery profiles may therefore not be appropriate for benchmarking across diverse populations.
Figures
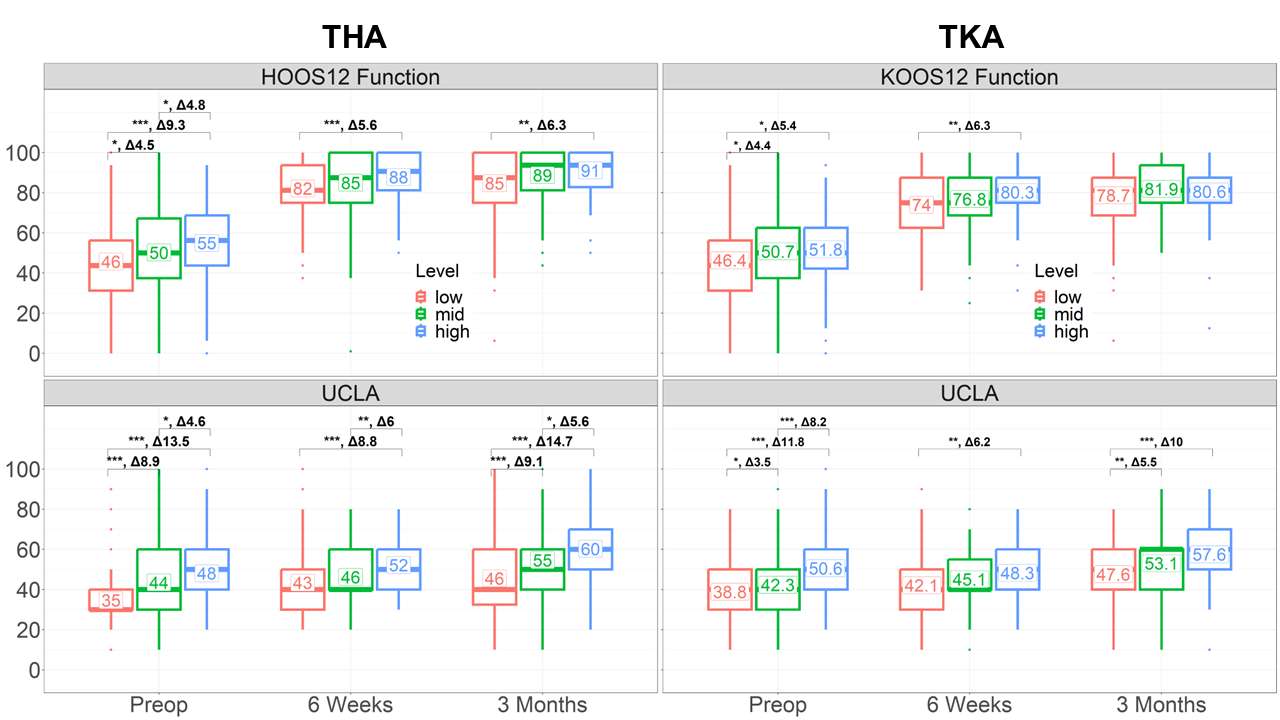
Figure 1
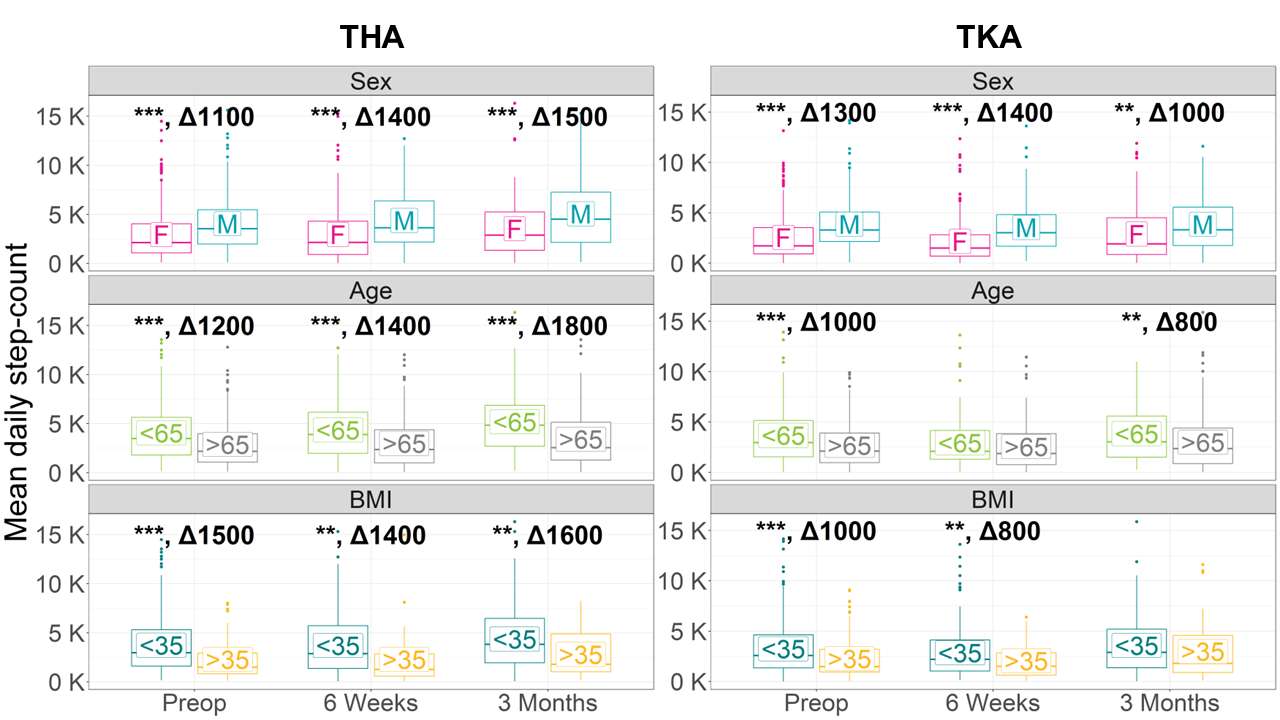
Figure 2#8242
Do Pain and Patient Reported Outcome Measures Vary by Patient Pre-Operative Physical Activity Levels in Total Knee Arthroplasty Patients?
*Roberta Redfern - Zimmer Biomet - Pemberville, USA
Mike Anderson - Zimmer Biomet - Lehi, USA
Jason Cholewa - Zimmer Biomet - Warsaw, USA
Dave Van Andel - Zimmer Biomet - Grand Rapids, USA
*Email: roberta.redfern@zimmerbiomet.com
Patients with osteoarthritis of the knee are often advised to engage in physical activity to reduce pain, however patients may avoid PA due to the same associated joint pain. Effects of pre-operative PA on functional outcomes have been heterogenous, with few reports demonstrating positive impacts of pre-habilitation programs. Our goal was to investigate patient-reported outcomes and satisfaction as a function of pre-operative PA levels in patients undergoing total knee arthroplasty (TKA).
Secondary data analysis of a large multicenter prospective observational cohort study investigating a smartphone-based care management platform for self-directed rehabilitation following arthroplasty. Patients were provided a smartwatch for passive, continuous step count collection pre-operatively through one year. Patients were eligible for inclusion in this analysis if at least 90 days of follow-up data were available following TKA. Activity was categorized based on the cohort’s step count quartiles into low, medium, and high pre-operative PA (low: 0-25th percentile, medium: 25th-75th and high: 75th-100th percentile). Steps counts, numeric pain ratings, KOOS JR, EQ5D5L, and satisfaction were compared by ANOVA with post-hoc Tukey pairwise comparisons according to activity group.
In total, 1941 TKA patients were included. The median average pre-operative step count over the cohort was 5210.5 (IQR 3085.8 – 6764.9). Age and BMI varied by PA groups, increasing with decreasing activity groups (all, p<0.05). Pain scores also increased with decreasing activity level (all pairwise comparisons, p<0.05). Similarly, the high PA group presented with the highest pre-operative KOOS JR and EQ5D5L Index scores, which decreased with decreasing activity level group (all pairwise comparisons, p<0.05). Patient reported pain 3 months post-operative was similar between activity levels, however change in pain from pre-procedural levels was lowest in the high PA group (2.54 points), differing from both the medium PA (2.95-point reduction) and low PA (3.06-point reduction). Low and medium PA patients increased physical activity following TKA at 3-months, reaching 176% and 104% of pre-operative step counts whereas high PA patients returned to 88% of their pre-operative activity. Greater improvements in the EQ5D5L were also observed in the low and medium PA groups compared to high PA (both, p<0.05). Similarly, patients in the low and medium PA groups demonstrated greater improvements in function by KOOS JR scores at 3 months than those with high pre-operative PA (20.2 and 18.7 versus 9.1-point increase, respectively, p<0.05). Patient satisfaction at 3 months was similar between all groups.
Patients undergoing TKA who present with higher levels of physical activity as measured by step counts report lower levels of pain and higher function pre-operatively. However, these patients demonstrate smaller improvements in pain, function, and health status compared to those with lower pre-operative activity following the procedure.
#8681
Assessing Interface Strength of Conductive Polymeric Composite Electrodes Toward Orthopedic Bearing-Embeddable Load Sensors
*Peder Solberg - Dartmouth College - Hanover, United States of America
Zhe Xu - Dartmouth College - Hanover, USA
John XJ Zhang - Dartmouth College - Hanover, USA
Douglas Van Citters - Dartmouth College - Hanover, USA
*Email: peder.solberg.th@dartmouth.edu
Introduction:
Sensors can provide continuous, in vivo feedback regarding the nature of loads placed on a patient’s implant. Such data enable real-time personalization of patient rehabilitation plans1,2. Current smart implants allow for detection of kinematic gait parameters but can’t directly detect the loads placed through the implants3. Sensors embedded within polymeric bearings might allow for other valuable data to be derived from smart implants such as load magnitude and direction, impact absorption, and force concentration. Several technical challenges must be addressed first to ensure the electrical and mechanical reliability of these sensor-bearing systems. We hypothesize that soft, porous piezoceramic materials can be successfully paired to ultra-high molecular weight polyethylene (UHMWPE) based conductive polymeric composite (CPC) electrode materials, allowing sensors to be integrated directly into the UHWMPE materials already used for orthopedic bearings (Fig. 1). The purpose of this study was to assess the ability of this system to maintain electrical contact and resist mechanical interface delamination in loaded scenarios.
Methods:
Piezoelectric porous ceramic was engulfed in an electrically insulating matrix to create a flexible sensor with rough surfaces. These sensors were then surrounded by a CPC powder made with a conductive carbon black similar to that used as a colorant in black UHMWPE sutures. Sensor-powder assemblies were cold pressed then compression molded in neat UHMWPE at 175°C until well consolidated into sensor-electrode assemblies. Once cool, 500µm-by-10mm samples were cut normal to the sensor plane and tested in tension to determine maximum stress before failure. With adhesive failure expected at the material interface, this provided a direct measure of the mechanical integration of the sensors to the CPC electrode material and an indirect measure of electrical contact. CPCs with carbon concentrations of 10wt%, 5wt%, 1wt%, and 0wt% were assessed to determine the tradeoff of conductivity for interface strength.
Additionally, the ability of the electrode material to pick up the voltage generated on the piezo surface was assessed for 5wt% carbon black electrodes by placing a compressive load on the material and assessing voltage output.
Results:
Results indicate that good mechanical integration can be achieved between UHMWPE-based CPC electrodes and porous piezoceramics. Mechanical integration of the sensor-electrode boundaries (stress >0 MPa) was achieved for all four materials but was better at lower carbon concentrations (Fig. 2). Preliminary electrical results also demonstrated a signal on the order of tens of millivolts under hand-applied pressure to the system (Fig. 3), while a non-piezoelectric control demonstrated no meaningful signal.
Conclusion:
This work demonstrates that piezoceramics can be successfully integrated into UHMWPE-based electrode materials. Both mechanical and electrical properties were evaluated at the sensor-electrode interface. The interface strength decreases with increasing concentration of carbon powder, which is expected due to the increased presence of stress concentrators in the material with the addition of solid-state additives.
References
1.De Maria Marchiano et al. J Pers Med,2021;11(3):216.
2.Burny et al. Med Eng Phys,2000;22(7):469-79
3.Cushner et al. Orthop Proc,2021;103-B(SUPP_9):18-18
Acknowledgments:
The NH EPSCoR BioMade Project is supported by NSF’s Research Infrastructure Improvement Award #1757371.
Keywords:
Smart implants, piezoceramic sensor, UHMWPE
Figures
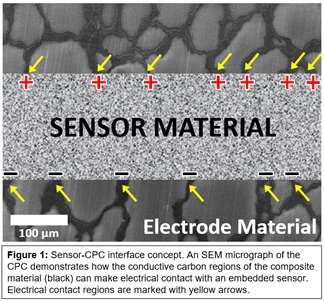
Figure 1
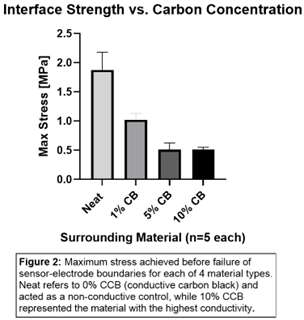
Figure 2
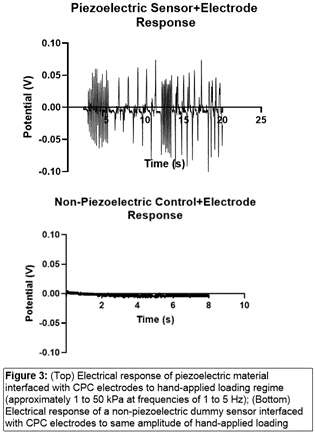
Figure 3#8111
Does Semaglutide Use Decrease Complications and Costs Following Total Knee Arthroplasty?
*Matthew Magruder - Maimonides Medical Center - Brooklyn, USA
Vincent Yao - Sophie Davis Biomedical Education Program at the CUNY School of Medicine - New York, USA
Ariel Rodriguez - Maimonides Medical Center - Brooklyn, United States of America
Mitchell Ng - Maimonides Medical Center - Brooklyn, United States of America
Orry Erez - Maimonides Medical Center - Brooklyn, USA
*Email: mmagruder@maimonidesmed.org
Introduction: Diabetes and obesity are two of the most common risk factors associated with complications following total knee replacement (TKA). Semaglutide (e.g. OzempicÒ) is an effective medication for the management of diabetes; importantly, it has recently been demonstrated to induce significant, long lasting weight loss. However, the medication’s effect on TKA outcomes has yet to be elucidated. Therefore, the aim of this study is to evaluate whether patients who are taking Semaglutide at the time of total knee arthroplasty demonstrate: 1) fewer medical complications; 2) fewer implant related complications; 3) fewer readmissions; 4) less costs.
Methods: A retrospective query was performed from January 1st, 2010, to March 31st, 2021, using the administrative claim database. Patients who underwent primary TKA for osteoarthritis who had a diagnosis of DM and an active prescription for Semaglutide at the time of the procedure were included in this study. Patients who were taking Semaglutide were successfully propensity score matched to controls based on age, gender, BMI, insulin status, metformin use, and Elixhouser Comorbidity index (ECI), yielding a total of 50,853 TKA patients (Semaglutide = 8,495; control = 42,358). The outcomes of interest included 90-day post-operative medical complications, 2-year implant related complications, 90-day readmissions, in-hospital length of stay, and day-of-surgery and 90-day episode of care costs. Multivariate logistical regression was used to calculate odds ratios (ORs), 95% confidence intervals, and p-values. A p-value of <0.003 was used as the significance threshold after using a Bonferroni correction.
Results: Patients taking Semaglutide had higher incidence and odds of developing a deep vein thrombosis (0.9% vs 0.6%; OR 1.5; p=0.002), pneumonia (2.9% vs 2.0%; OR 1.48; p<0.001) and having a hypoglycemic event (2.1% vs 1.4%; OR 1.53; p<0.001), but significantly lower odds of sepsis (0.0% vs 0.5%; OR 0.23; p<0.001). All other medical complications did not differ between cohorts (Table 1). Further, the Semaglutide cohorts had lower odds of both PJI (2.4% vs 3.4%; OR 0.69; p<0.001) and revision 4.1% vs 4.8%; OR 0.83; p=0.003); no other implant related complications were different between cohorts. Finally, Semaglutide cohort had similar same-day surgical costs but lower 90-day costs ($15,447.83 vs $17,349.53; p<0.001).
Conclusion: Semaglutide use at the time of primary TKA was associated with decreased risk of sepsis, PJI, readmission, revision surgery and costs. However, patients were also at an increased risk of DVT, PNA and hypoglycemic events. The large decrease risk of sepsis likely stems from Semaglutide’s immunomodulatory effects, suppressing immune system overreaction characteristic of sepsis. Providers should use this investigation in their surgical decision-making, as well as counsel patients who are taking Semaglutide about the post-operative benefits and risks.
Figures
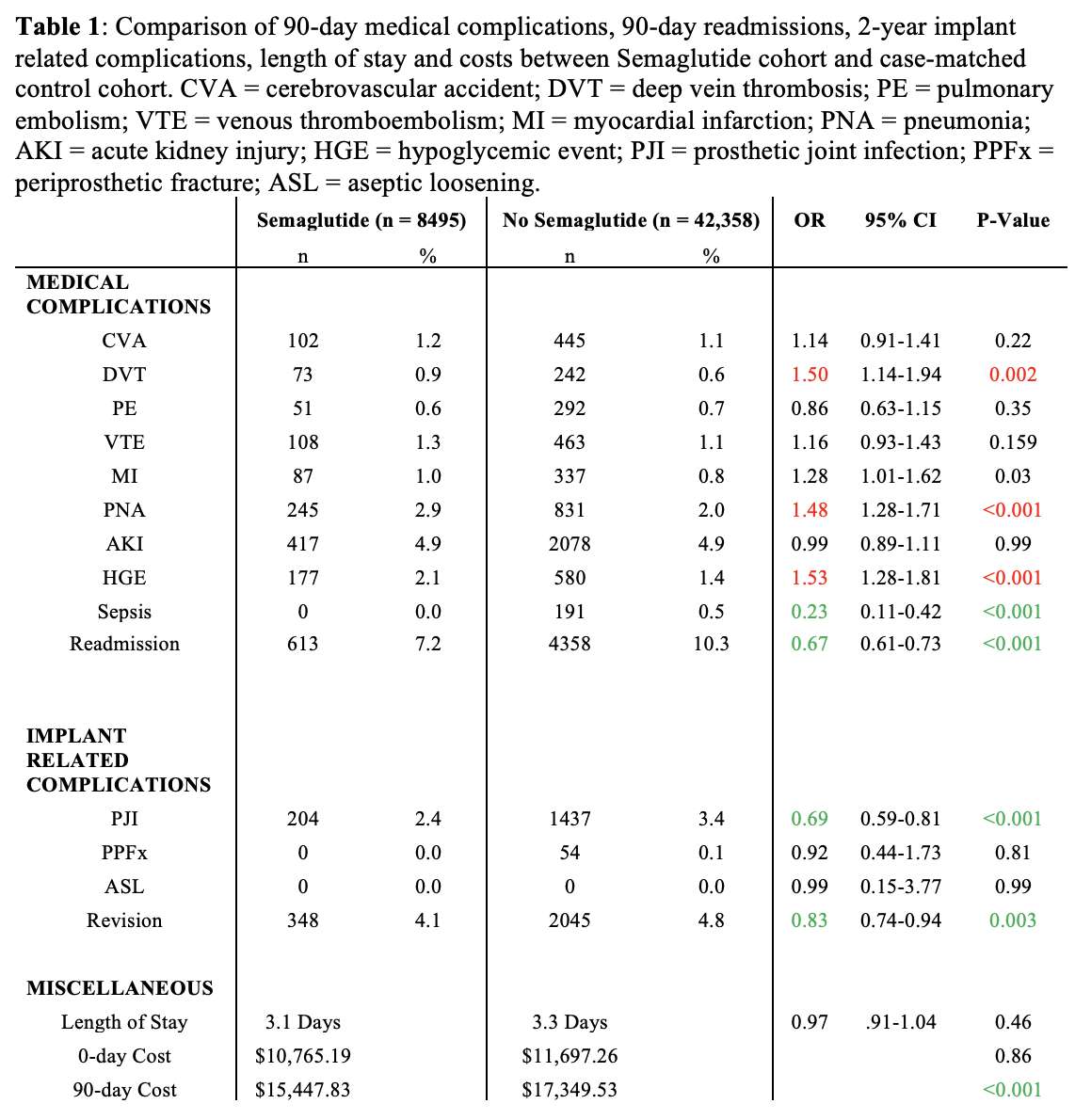
Figure 1#8109
Post-Operative Complications, Readmissions, Lengths of Stay and Cost Analysis of Patients With Atrial Septal Defects Undergoing Total Joint Arthroplasty
*Matthew Magruder - Maimonides Medical Center - Brooklyn, USA
Adam Gordon - maimonides Medical Center - Brooklyn, USA
Mitchell Ng - Maimonides Medical Center - Brooklyn, United States of America
Salvatore Capotsoto - Renaissance School of Medicine at Stony Brook University - Stony Brook, USA
Che Hang Jason Wong - Maimonides Medical Center - Brooklyn, USA
Peter Sculco - Hospital for Special Surgery - New York, USA
Gabriel Lama - Maimonides Medical Center - Staten Island, United States of America
*Email: mmagruder@maimonidesmed.org
Introduction: Atrial septal defects (ASDs) are one of the most common congenital heart defects. Patients with ASDs have 35-40-year mortality rates statistically similar to that of the general public, and therefore many will develop osteoarthritis and require a total joint arthroplasty (TJA). Thus, the study aims to determine whether patients who have ASDs undergoing TJA have higher rates of 1) medical complications, 2) readmissions, 3) in-hospital length of stay, and 4) costs.
Methods: Using an administrative claim database (PearlDiver), a retrospective query from January 1, 2010 to October 31, 2020 was performed. All patients who underwent primary total knee arthroplasty (TKA) and total hip arthroplasty (THA) for osteoarthritis with a diagnosis of ASD at the time of the procedure were included. ASD patients were successfully 1:5 propensity score matched with controls based on age, gender, rates of depression, hypertension, hypothyroidism, obesity, diabetes mellitus, congestive heart failure, history of stroke, atrial fibrillation and tobacco use, yielding a total of 45,695 TKA patients (ASD = 7,635, control = 38,060) 18,407 THA patients (ASD= 3,084, control= 15,323). Outcomes evaluated included 90-day medical complications, 90-day readmissions, in-hospital lengths of stay, and day-of-surgery and 90-day episode-of-care costs. Medical complications evaluated included cerebrovascular accidents (CVA), deep vein thrombosis (DVT), venous thromboembolism (VTE), pulmonary embolism (PE), pneumonia, myocardial infarction (MI), urinary tract infection (UTI), superficial skin infection (SSI), and transfusions. Multivariate logistical regression was used to calculate odds ratios (ORs), 95% confidence intervals (95% CIs), and p-values. Welch’s t-tests were used to test for significant differences in lengths of stay and costs of care between cohorts. A p value <0.001 was the significance threshold.
Results: ASD patients had higher incidence and odds of total medical complications after TKA (43.12% vs 22.10%; OR 2.09; p<0.001) and THA (49.16% vs 25.15%; OR 2.08; p<0.001). The medical complications that demonstrated the highest increases in risk were DVTs, CVAs, MIs, VTEs and PEs. However, ASD patients were not significantly more likely to be readmitted after TKA (5.30% vs 4.73%; OR 1.13; p=0.033) or THA (6.00% vs 5.71%; OR 1.05; p=0.531). Length of stay was not significantly longer in ASD patients undergoing TKA (3.24 days vs 3.24 days; p=0.805) but was significantly longer after THA (5.30 days vs 3.76 days; p<0.001). Same-day surgery costs were not significantly larger in ASD patients after TKA ($23,892.53 vs $23,453.40; p=0.066) but was after THA ($23,981.93 vs. $23,579.18; p<0.001). 90-day episode of care costs were not statistically different between cohorts.
Conclusion: Patients with ASDs are at increased risk of total 90-day medical complications when undergoing primary TJA than propensity matched controls. This patient population demonstrates the highest increases in thromboembolic complications, including DVTs, CVAs, MIs, VTEs and PEs. Providers may consider pre-operative cardiac clearance, using less tourniquet time, or more significant post-operative anticoagulation in this patient population to mitigate these risks.
Figures
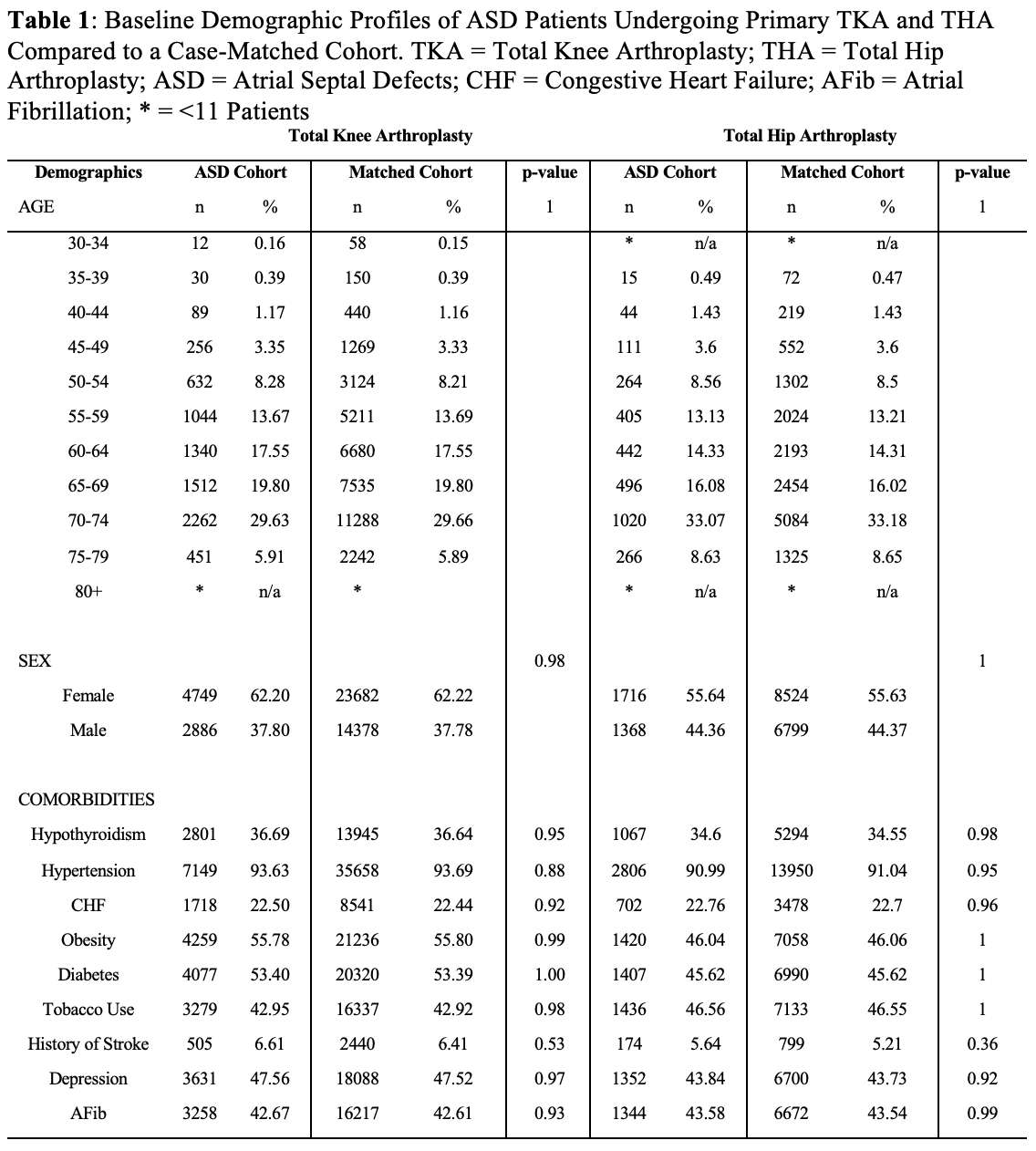
Figure 1
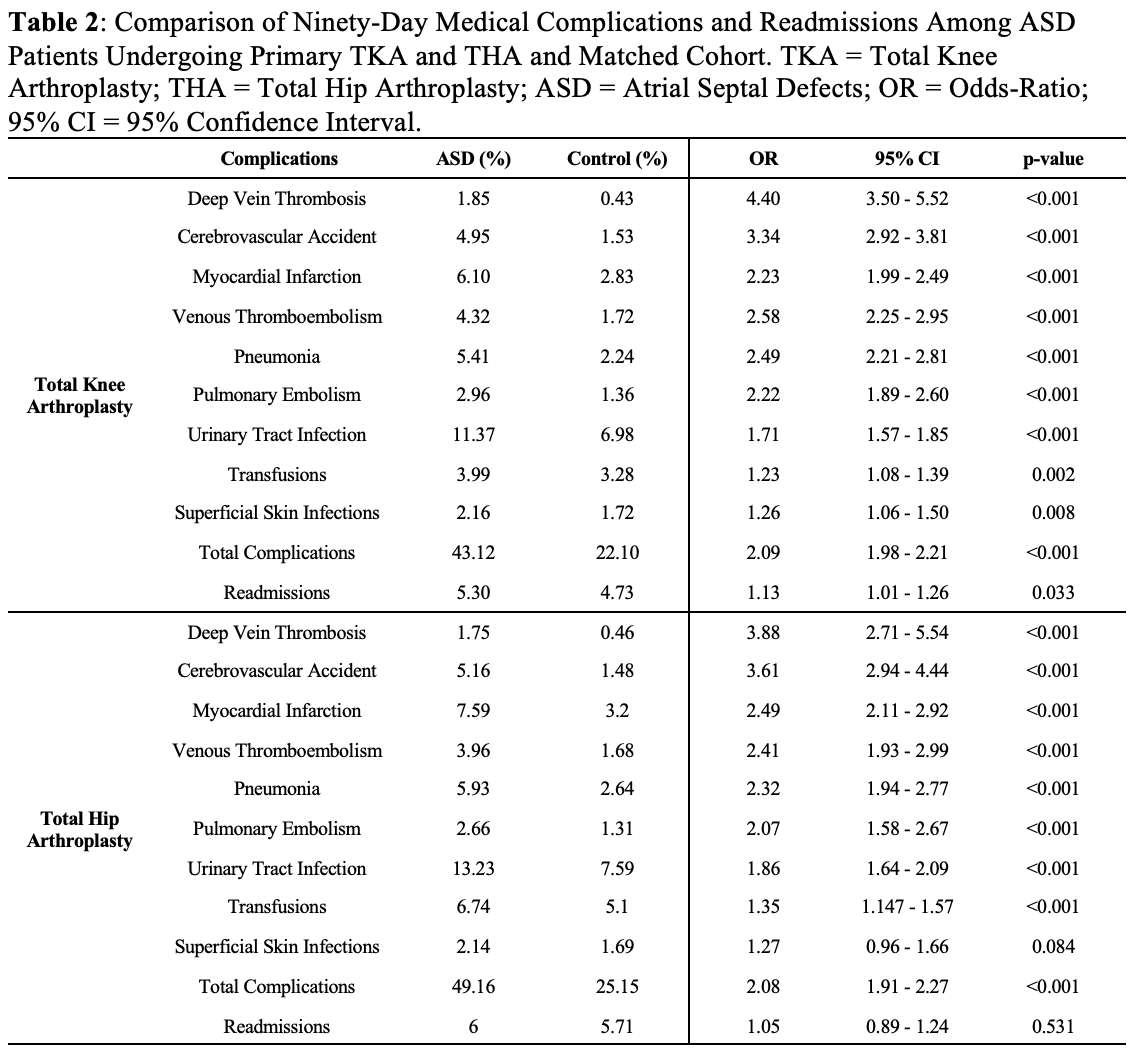
Figure 2
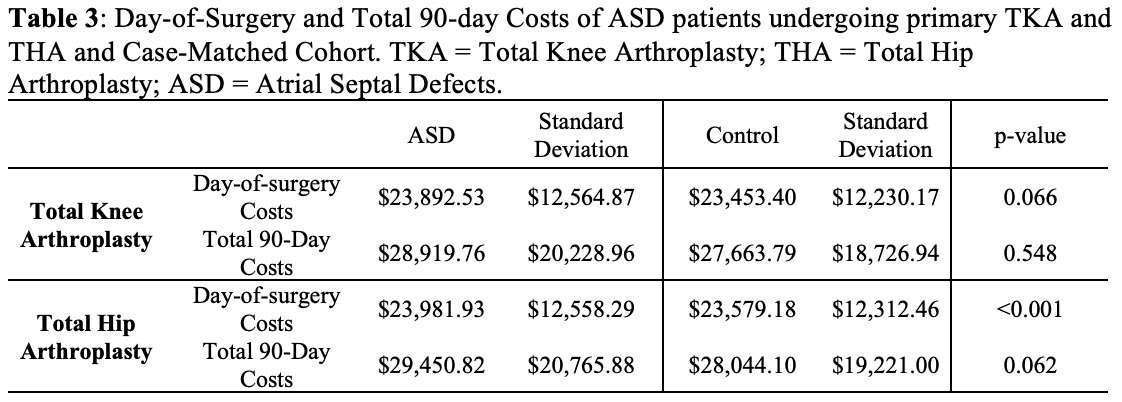
Figure 3#8177
Influence of Patient Age on Revision and Mortality Rates in Elective THA Procedures Differentiated by Stem Type and Stem Fixation
*Michael Morlock - TUHH Hamburg University of Technology - Hamburg, Germany
Oliver Melsheimer - EPRD Deutsche Endoprothesenregister gGmbH - Berlin, Germany
*Email: morlock@tuhh.de
Introduction: The preferred use of cemented stems has been suggested for patients aged 75 years and over (1). Recently a more detailed look at design variants for uncemented stems has shown that selective uncemented stem fixation might also be appropriate for the elderly population (2) with possibly a reduced mortality (3). The German Arthroplasty Register EPRD was used for a detailed analysis of revision risk and mortality dependent on stem type and fixation up to 7 years in different patient age cohorts.
Methods: Elective primary THA cases for Coxarthrosis using uncemented cups (excluding support cups) were selected from the EPRD data base (n0= 294,579; ; n3= 151,035; n7= 11,429). Stem designs were classified in 4 types (uncemented, uncemented with collar, uncemented short, cemented). Single stem designs with at least 300 cases in follow up or with type variants were analysed individually using a non-inferiority approach. Revision and mortality rates for stem types were compared in 4 age cohorts: below 60 years (“YOUNG”), between 60-70 (“MID-I”), between 70-80 (“MID-II”) and above 80 (“OLD”).
Results: The mortality rate distinctly increased with time in situ and age cohort (Figure 1) The increase was affected by stem design and fixation: cemented fixation was associated with the highest mortality rate after three and seven years in all age cohorts (all p < 0.04). Revision risk also increased with time in situ and was affected by stem design and fixation (Figure 2): the uncemented type was associated with the highest revision rate in all age cohorts (all p<0.001) but the YOUNG cohort (p= 0.08). In the MID-I cohort, collar showed a lower revision rate than the three other types (all p<0.02, with SHORT only a trend p=0.18). In the OLD cohort, collar and cemented showed a lower revision rate than the two other types (all p<0.014, with SHORT only a trend p=0.06). A non-inferiority analysis of single stem designs implanted in the OLD cohort highlights the higher revision rate for uncemented stems in this cohort while also demonstrating the consistency of single designs inside the type groups (Figure 3).
Discussion: Uncemented stems should not be used in patients above 80 years due to their higher revision risk. Uncemented stems with a collar are shown to exhibit a lower revision risk as uncemented stems in all age cohorts, questioning the use of collarless variants if collared ones are available. If cementing should be avoided, collared stems seem to be a good alternative.
The one stem design available in 3 types (Corail uncemented, collar, cemented) demonstrates the benefit of a collar on this kind of uncemented stem design with a clearly lower revision risk as the collarless variant and even a lower risk as the cemented variant in old patients (Figure 3).
References: (1) Tanzer M et al. CORR 2018; (2) Wilson J et al. JoA 2023; (3) Della Valle A et al. JoA 2022
Figures
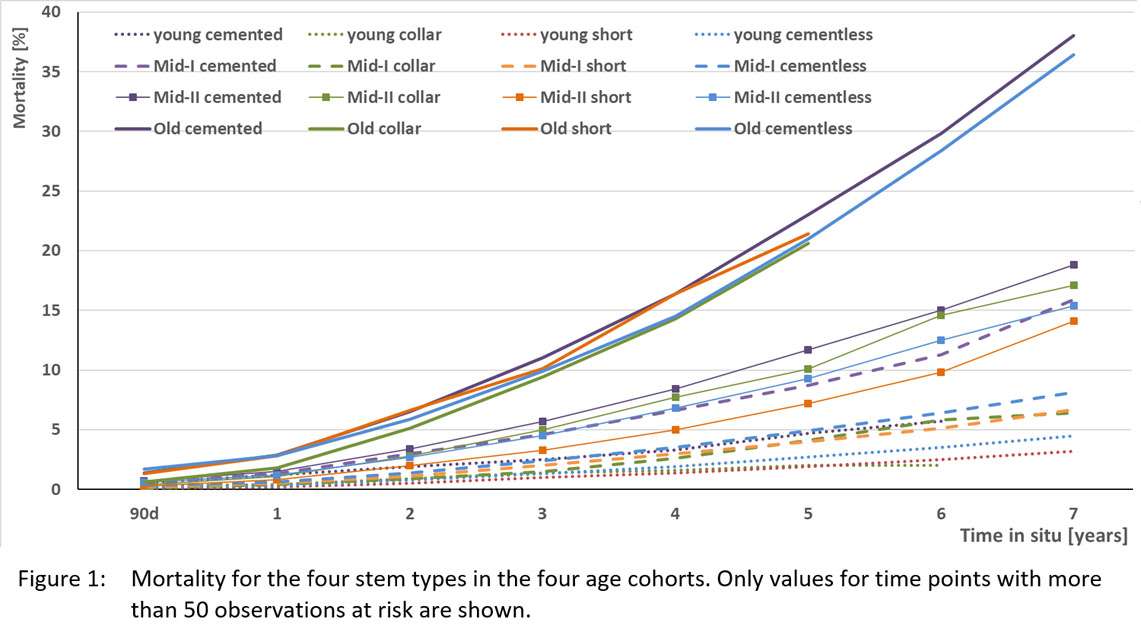
Figure 1
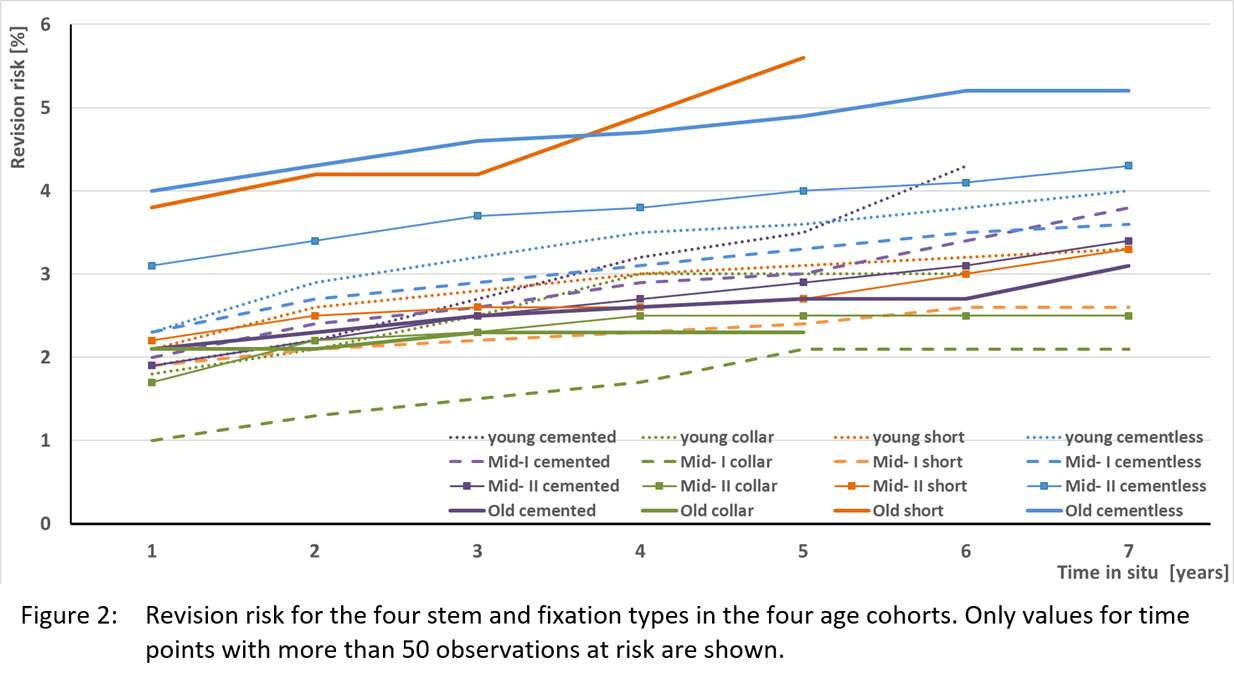
Figure 2
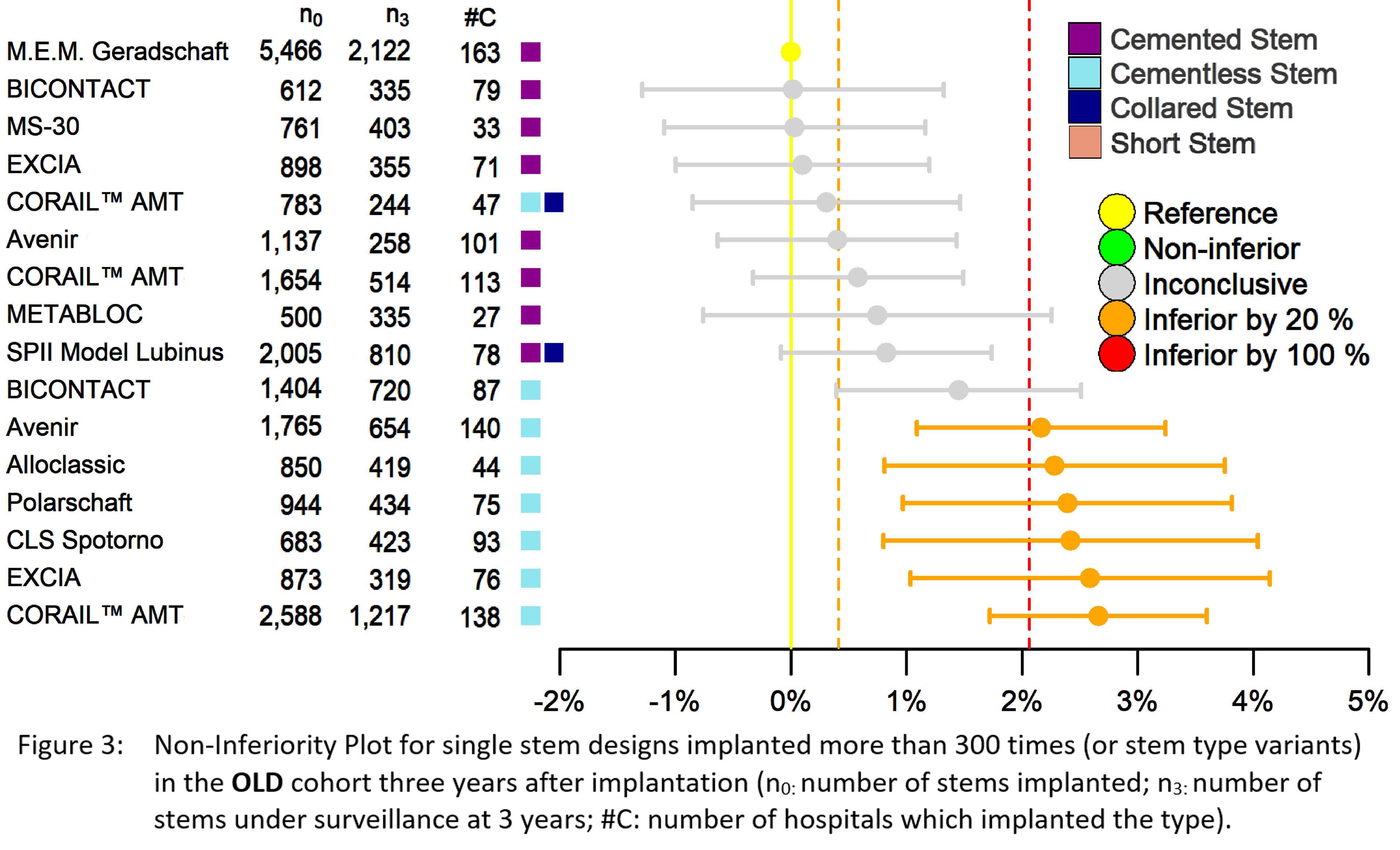
Figure 3#8139
Effects of Perioperative Intravenous Dexamethasone on the Severity of Persistent Postsurgical Pain After Total Knee Arthroplasty: A Prospective, Randomized, Double-Blind, Placebo-Controlled Trial
Nitchanant Kitcharanant - Faculty of medicine, Chiangmai University - Chiangmai, Thailand
*Warakorn Jingjit - Chiang Mai University - Chiang Mai, Thailand
Prangmalee Leurcharusmee - Chiang Mai University - Chiang Mai, Thailand
*Email: boyortho@hotmail.com
Effects of Perioperative Intravenous Dexamethasone on the Severity of Persistent Postsurgical Pain After Total Knee Arthroplasty: A Prospective, Randomized, Double-Blind, Placebo-Controlled Trial
ABSTRACT
Background: Despite the significant recent successes of total knee arthroplasty (TKA), some patients still experience persistent postsurgical pain (PPSP). Severe acute postoperative pain has been shown to be the major risk factor for PPSP. Recently published studies have provided additional documentation of the benefits of systemic corticosteroids in the perioperative setting. It is not clear whether perioperative intravenous dexamethasone has an effect on severity of PPSP after TKA.
Methods: Forty-eight patients undergoing unilateral TKA were included in a randomized, double-blind, placebo-controlled trial receiving intravenous Dexamethasone 10 mg or saline immediately prior to induction of spinal anesthesia and a second and third dose of Dexamethasone 10 mg or saline postoperatively at 24 and 48 hrs. All patients underwent spinal anesthesia and received a standardized, multimodal analgesic regime. The primary outcome was modified WOMAC scores for pain at 12 weeks postoperative as assessed by using the modified Thai version of the Western Ontario and McMaster Universities (WOMAC) osteoarthritis index for knee osteoarthritis questionnaire, and secondary outcomes were pain during a five-meter walk, pain during 45 degrees active knee flexion, maximal pain at rest over the last 24 hrs, visual analogue scale values for nausea, and rescue opioid and anti-emetic medicine consumption.
Results: Modified WOMAC scores for pain at 12 weeks postoperative were lower in the Dexamethasone group (p=0.021). Pain during a five-meter walk was significantly lower in the Dexamethasone group at 24, 30, 48, 54 and 72 hrs after operation (p<0.01). Pain during 45 degrees active knee flexion was lower in the Dexamethasone group at 24, 30, 48, 54 and 72 hrs postoperative (p<0.01). Maximal pain at rest was lower in the Dexamethasone group at postoperative 0-24, 24-48, and 48-72 hrs (p<0.01). Visual analogue scale values for nausea postoperatively at 6, 24, 30, 48 and 54 hrs were also lower in the Dexamethasone group (p<0.02). Opioid and anti-emetic medicine consumption were lower in the Dexamethasone group during the first 0-24 and 24-48 hrs (p<0.01). No wound complications were observed in all patients.
Conclusions: Perioperative intravenous Dexamethasone significantly decreased persistent postsurgical pain when comparing to placebo at 12 weeks following total knee arthroplasty. In addition, perioperative intravenous Dexamethasone improved pain in early postoperative setting, reduces postoperative nausea and vomiting, and reduced opioid and anti-emetic medicine consumption.
Keywords: Intravenous Dexamethasone, persistent postsurgical pain, total knee arthroplasty
Introduction
Despite the significant recent successes of total knee arthroplasty (TKA) (1), some patients still experience persistent postsurgical pain (PPSP) (2-4). The International Association for the Study of Pain (IASP) defines PPSP as pain that has occurred after surgery and persisted for at least three months, which is not common for normal healing process (8). While most patients recovering from the surgery felt that the pain subsided within three months after TKA (2, 6), about 10-34% of patients are dissatisfied with long-term pain outcome (7).
The consequences of under-recognized PPSP in surgical patients importantly include the impacts of long-term analgesic use (11). Elderly patients who received opioid after surgery are at risk of becoming long-term opioid users and chronic uses of nonsteroidal anti-inflammatory drugs (NSAIDs), thus increasing the risk of gastro-intestinal ulcers and bleeding, renal insufficiency, stroke, and myocardial infarction (12). Uncontrolled acute postoperative pain can result in prolonged hospitalization and delayed mobilization due to inability to participate in physical therapy and rehabilitation and resulting in patient dissatisfaction and poor quality of life after the surgery. Severe acute postoperative pain has been shown to be the major risk factor for PPSP (2, 5).
Recent meta-analyses have shown that intermediate dose Dexamethasone (0.11 to 0.2 mg/kg) can be safely used to reduce postoperative pain at movement and to minimize opioid consumption as well as to decrease postoperative nausea and vomiting (18). However, it is not clear whether perioperative intravenous Dexamethasone has an effect on severity of persistent postsurgical pain (PPSP) after total knee arthroplasty (TKA). The research hypothesis is that TKA patients experience less severe PPSP after administration of perioperative intravenous Dexamethasone. This study aims to investigate the effect of perioperative intravenous Dexamethasone on the severity of PPSP after TKA and compare that with a placebo.
Methods
Patients and study design
The study protocol and consent form were both approved by the Research Ethics Committee of the Faculty of Medicine, Chiang Mai University, Chiang Mai, Thailand (COA no. ORT-2558-03245). The clinical trial was registered at ClinicalTrials.gov and the registration number is NCT02760459.This study was conducted in accordance with the principles described in the Declaration of Helsinki and all of its later amendments.
From September 2016 to March 2019, seventy-one patients were assessed for eligibilty. Only primary knee osteoarthritis patients undergoing unilateral TKA were eligible. We excluded patients with any history of active rheumatic diseases, previous musculoskeletal injury of the same knee, previous surgery on the same knee, psychiatric disorders or cognitive impairment, contraindicated to spinal anesthesia, poorly controlled diabetes mellitus (HbA1C > 7.5), ischemic heart disease or peripheral arterial disease or cerebrovascular disease, hepatic insufficiency (Child-Pugh score > 5), and renal insufficiency (Creatinine clearance < 30 mL/min) (n=22).
In this prospective, double-blind, placebo-controlled study, forty-nine patients were randomly assigned, using a block of four randomization technique, to one of two groups: the steroid group (Dexamethasone) (n= 24) or the control group (normal saline solution) (n=25). The steroid group received intravenous Dexamethasone 10 mg immediately prior to induction of spinal anesthesia and received a second and third dose of intravenous Dexamethasone 10 mg postoperatively at 24 and 48 hr. The control group received sterile intravenous normal saline solution serving as placebo immediately prior to induction of spinal anesthesia and postoperatively received a second and third dose of the placebo at 24 and 48 hr. Every patients received a standardized, multimodal analgesic regime. One patient in the placebo group had postoperative myocardial infarction and therefore needed to be excluded from the study, leaving 48 patients for final analysis.
Surgery, anesthesia and postoperative analgesia
Every surgeries were performed by a single surgeon (WJ). The anesthesiologist was responsible for the administration of the study drug during the preoperative period. Neither the surgeon nor the anesthesiologists were aware of all patient group allocation. Nurses who prepared the study drugs were not allowed to participate in any outcome assessment.
All patients had the same preoperative and postoperative pain protocol consisting of preoperative pain management for 1 week with Paracetamol (Beramol 500 mg.) given 1 tab orally every 6 hrs, Etoricoxib (Arcoxia 60 mg.) given 1 tab orally after breakfast, and Omeprazole (Miracid 20 mg.) given 1 tab orally before breakfast. Patients were asked to complete documents to confirm the number of pills taken. Lorazepam (Anta 0.5 mg.) was prescribed the night before surgery. Cefazolin (Cefamezin 1 gm.) was given intravenously 30 minutes before the operation; Clindamycin (Rosil 600 mg.) was used instead if the patient was allergic to Cefazolin. Three ampoules of Tranexamic acid (Transamin 750 mg.) were given 30 minutes preoperatively.
The steroid group received intravenous Dexamethasone 10 mg immediately prior to induction of spinal anesthesia and received a second and third dose of Dexamethasone 10 mg postoperatively at 24 and 48 hrs. The control group received sterile intravenous normal saline solution, serving as placebo, immediately prior to induction of spinal anesthesia and received a second and third dose of placebo postoperatively 24 and 48 hrs. Both Dexamethasone and normal saline solution were administered as an intravenous bolus. Spinal anesthesia administered was performed using 0.5% Bupivacaine hydrochloride (Astrazeneca).
A tourniquet was inflated to a pressure of 300 mmHg before skin incision and was deflated after wound closure. A standard medial parapatellar arthrotomy and posterior-stabilized total knee prosthesis (Zimmer, LPS-Flexed) were used in all patients. The implant was fixed with cement and the patella was not resurfaced.
Postoperatively, Cefazolin (Cefamezin 1 gm.) was given intravenously every 6 hrs for 3 days, then switched to Cephalexin (Keflex 500 mg.) 1 tab orally after meals and at night for 2 weeks. During the postoperative period, a research assistant nurse was assigned to administer the intravenous dexamethasone or placebo depending on the patient’s allocated group, but that person was not involved in any outcome assessment. The research assistant who was blinded to the treatment allocation, handed the pain assessment questionnaire to the patients.
Postoperative pain management consisted of Parecoxib (Dynastat 40 mg.) given intravenously every 12 hrs only on Post-op day 0 and Post-op day1 plus Etoricoxib (Arcoxia 90 mg.) given 1 tab orally after breakfast and Paracetamol (Beramol 500 mg.) given 1 tab orally every 6 hrs. Morphine 3 mg. was given intravenously upon request once every 4 hrs and was titrated for breakthrough pain. For postoperative nausea and vomiting management, Ondansetron (Onsia 4 mg.) was given intravenously upon request every 6 hrs and was titrated for breakthrough nausea and vomiting. Tolperisone (Mydocalm 50 mg.) was also be given 1 tab orally every after meals. Omeprazole (Miracid 20 mg.) was prescribed 1 tab orally before breakfast to prevent gastro-intestinal side effects from corticosteroids and Cox-2 inhibitor drugs. During the first 24 hrs after surgery, all patients were advised to rest in bed but were allowed to perform ankle pump exercises. All patients were encouraged to walk with a walker the morning after surgery. Every patients had their blood sugar assessed regularly. All patients were allowed to discharge from the hospital when they were able to ambulate independently with a walker for 20 meters and flex the operated knee at least 90 degrees. All patients had the same home medication protocol consisting of oral antibiotics for 2 weeks and pain management with Etoricoxib (Arcoxia 60 mg.) 1 tab orally after breakfast and Omeprazole 1 tab orally before breakfast. Patients were asked to fill out documents to confirm the number of pills taken.
All patients had appointments postoperatively at weeks 2, 6 and 12. Wound assessment was performed by the orthopedic surgeon (WJ). The research assistant nurse assessed the Modified WOMAC scores for pain at each follow-up.
The primary outcome measure was the Modified WOMAC score for pain (VAS 0-500) postoperatively at week 12 comparing the Dexamethasone group and the placebo group to test the hypothesis that Dexamethasone was superior to placebo (Superiority test) using a minimally clinically important difference (MCID) of 12 points (47).
Secondary outcome measures were (1) visual analogue scales for pain during a five-meter
walk (VAS 0-100) postoperatively at 24, 30, 48, 54 and 72 hrs; (2) visual analogue scales for pain during 45 degrees active knee flexion (VAS 0-100) postoperatively at 24, 30, 48, 54 and 72 hrs; (3) Visual analogue scales for maximum pain at rest over the last 24 hours (VAS 0-100) postoperatively at 0-24, 24-48 and 48-72 hrs. Each pain outcome was assessed using a visual analogue scale of 0-100 mm, in which 0 indicates no pain and 100 indicates the worst pain; (4) Visual analogue scales for nausea (VAS 0-100) postoperatively at 6, 24, 30, 48, 54 and 72 hrs. This outcome was assessed using a visual analogue scale of 0-100 mm, in which 0 indicates no nausea or vomiting and 100 indicates the worst nausea or vomiting; (5) Opioid consumption (mg.) postoperatively at 0-24 hrs, 24-48 hrs, 48-72 hrs; (6) Anti-emetic medicine consumption (mg.) postoperatively at 0-24 hrs, 24-48 hrs, 48-72 hrs; (7) Modified WOMAC scores for pain using the modified Thai version of the Western Ontario and McMaster (WOMAC) osteoarthritis index for knee osteoarthritis questionnaire postoperatively at week 2 and 6; (8) Wound complications (including periprosthetic joint infection and inadequate wound healing). Periprosthetic joint infection was diagnosed using the criteria outlined by the Musculoskeletal Infection Society [49]. Inadequate wound healing was defined as delayed wound healing or wound dehiscence and was evaluated postoperatively at weeks 2, 6 and 12 by the orthopedic surgeon (WJ).
Statistical analysis
Sample size analysis was calculated. Twenty-four knees per group were required to detect a minimally clinically important difference (MCID) of 12 points at 12 weeks using the modified WOMAC score for pain (47), with a SD of approximately 16.72 points in each group. A one-sided hypothesis test at an alpha level of 0.05 and power of 80% were used to evaluate statistical significance. There was no interim analysis. Therefore, 48 patients were recruited into the study. Patients were included in the primary analysis on the basis of intention to treat. Student’s t-test and chi-square test with an alpha of 0.05 and a power level of 0.80 were used to compare the continuous and categorical data, respectively. A p-value < 0.05 was considered statistically significant. All data was analyzed using the Statistical Package for Social Sciences (SPSS Version 17.0, SPSS Inc., Chicago, IL, USA).
Results
Forty-eight patients were included for final analysis (Table 1). Modified WOMAC scores for pain at 12 weeks postoperative were significantly lower in the Dexamethasone group (p=0.021) (Table 2). Pain during a five-meter walk was significantly lower in the Dexamethasone group at 24, 30, 48, 54 and 72 hrs after operation (p<0.01) (Table 3). Pain during 45 degrees active knee flexion was lower in the Dexamethasone group at 24, 30, 48, 54 and 72 hrs postoperative (p<0.01) (Table 4). Maximal pain at rest was lower in the Dexamethasone group at postoperative 0-24, 24-48, and 48-72 hrs (p<0.01) (Table 5). Visual analogue scale values for nausea postoperatively at 6, 24, 30, 48 and 54 hrs were also lower in the Dexamethasone group (p<0.02) (Table 6). Opioid and anti-emetic medicine consumption were lower in the Dexamethasone group during the first 0-24 and 24-48 hrs (p<0.01). No wound complications were observed in all patients.
Table 1. Patient demographic and clinical characteristics
|
Patient characteristics
|
Dexamethasone group
(n = 24)
|
Placebo group
(n = 24)
|
p-value
|
|
Age (years), mean ± SD
|
66.967.82
|
64.505.73
|
0.221
|
|
Female sex, n (%)
|
24 (100.0%)
|
22 (91.7%)
|
0.489
|
|
Body mass index (kg/m2), mean ± SD
|
26.354.65
|
26.444.05
|
0.939
|
|
Pain problems elsewhere, n (%)
|
19 (79.2%)
|
19 (79.2%)
|
0.999
|
|
Preoperative modified WOMAC scores for pain (0-500), mean ± SD
|
227.6358.31
|
234.1356.96
|
0.698
|
Data are presented as mean ± SD or number (%)
Abbreviations: p-value = Student’s t-Test and Chi-square test
p-value < 0.05 indicates statistical significance
SD standard deviation, n number
WOMAC Western Ontario and McMaster
Table 2. Modified WOMAC scores for pain
|
Outcome measure
|
Dexamethasone
group
(n = 24)
|
Placebo group
(n = 24)
|
p-value
|
|
At 2 weeks postoperative
|
54.7943.25
|
84.8846.78
|
0.025
|
|
At 6 weeks postoperative
|
31.6722.78
|
48.5431.12
|
0.037
|
|
At 12 weeks postoperative
|
9.3817.77
|
23.7523.56
|
0.021
|
Data are presented as mean ± SD
Abbreviations: p-value = Student’s t-Test
p-value < 0.05 indicates statistical significance
SD standard deviation, n number
WOMAC Western Ontario and McMaster
Table 3. Visual analogue scales for pain during a five-meter walk
|
Outcome measure
|
Dexamethasone
group
(n = 24)
|
Placebo group
(n = 24)
|
p-value
|
|
At 24 hrs postoperative
|
47.9215.60
|
68.9613.75
|
<0.001
|
|
At 30 hrs postoperative
|
34.5815.32
|
54.3817.90
|
<0.001
|
|
At 48 hrs postoperative
|
23.7515.27
|
43.5418.56
|
0.001
|
|
At 54 hrs postoperative
|
15.4212.85
|
39.7917.10
|
<0.001
|
|
At 72 hrs postoperative
|
11.6712.04
|
32.7914.81
|
<0.001
|
Data are presented as mean ± SD
Abbreviations: p-value = Mann-Whitney test
p-value < 0.05 indicates statistical significance
SD standard deviation, n number
Table 4. Visual analogue scales for pain during 45 degrees active knee flexion
|
Outcome measure
|
Dexamethasone
group
(n = 24)
|
Placebo group
(n = 24)
|
p-value
|
|
At 24 hrs postoperative
|
43.7519.30
|
63.9625.32
|
<0.001
|
|
At 30 hrs postoperative
|
37.5019.39
|
55.4219.39
|
0.001
|
|
At 48 hrs postoperative
|
26.6716.85
|
44.7923.20
|
0.002
|
|
At 54 hrs postoperative
|
20.2118.68
|
41.8824.26
|
0.001
|
|
At 72 hrs postoperative
|
14.5815.60
|
38.5420.61
|
<0.001
|
Data are presented as mean ± SD
Abbreviations: p-value = Mann-Whitney test
p-value < 0.05 indicates statistical significance
SD standard deviation, n number
Table 5. Visual analogue scales for maximal pain at rest
|
Outcome measure
|
Dexamethasone
group
(n = 24)
|
Placebo group
(n = 24)
|
p-value
|
|
At 0-24 hrs postoperative
|
68.7519.18
|
86.4610.27
|
0.001
|
|
At 24-48 hrs postoperative
|
44.5813.51
|
57.9210.62
|
0.001
|
|
At 48-72 hrs postoperative
|
26.8811.21
|
46.468.40
|
<0.001
|
Data are presented as mean ± SD
Abbreviations: p-value = Mann-Whitney test
p-value < 0.05 indicates statistical significance
SD standard deviation, n number
Table 6. Visual analogue scales for nausea
|
Outcome measure
|
Dexamethasone
group
(n = 24)
|
Placebo group
(n = 24)
|
p-value
|
|
At 6 hrs postoperative
|
21.2512.96
|
58.3319.71
|
<0.001
|
|
At 24 hrs postoperative
|
6.259.70
|
30.0027.82
|
0.001
|
|
At 30 hrs postoperative
|
1.254.48
|
14.3817.53
|
<0.001
|
|
At 48 hrs postoperative
|
0.000.00
|
13.1320.42
|
<0.001
|
|
At 54 hrs postoperative
|
0.000.00
|
5.2110.16
|
0.010
|
|
At 72 hrs postoperative
|
0.000.00
|
1.885.68
|
0.077
|
Data are presented as mean ± SD
Abbreviations: p-value = Mann-Whitney test
p-value < 0.05 indicates statistical significance
SD standard deviation, n number
Discussion
This is the first double-blind, randomized, placebo-controlled study investigating the effects of steroid on severity of persistent pain after knee arthroplasty. The most important finding of the current investigation was that the severity of chronic pain after total knee arthroplasty as assessed by the modified WOMAC score was different in patients who received and those who did not receive perioperative dexamethasone. This supports the hypothesis that the use of perioperative intravenous Dexamethasone not only improved pain in early postoperative setting and reduced opioid consumption but could also decrease the severity of chronic pain after total knee arthroplasty.
The etiology of PPSP is as yet unclear. Kehlet suggested that it is a consequence either of ongoing inflammatory process or of a presentation of neuropathic pain resulting from surgical injury to the peripheral nerves (5). Among the risk factors for developing PPSP, the most impact is the intensity of acute postoperative pain (5, 9). Severe acute postoperative pain puts the patients at risk of developing PPSP as it can induce central nervous system sensitization (5). Age, gender, psychological disorders, pain problems elsewhere in the body and previous experience with pain are also potential risk factors for developing PPSP (5, 10).
In that regard, recent published studies have provided additional documentation of the benefits of systemic corticosteroids in perioperative setting (13). Corticosteroids are already well known for their anti-inflammatory effects (13, 14) and their effectiveness in preventing postoperative nausea and vomiting (PONV) (15, 16). Current evidence shows that systemic corticosteroids can reduce acute postoperative pain effectively (17, 18). Recent randomized trials have shown corticosteroids administered preoperatively to be effective for reducing postoperative pain after total knee arthroplasty (TKA) and total hip arthroplasty (THA) (19-23, 30-34). Only a few studies have investigated the effectiveness in reducing PONV and pain when Dexamethasone was administered postoperatively. Ramundstad et al. demonstrated that Methylprednisolone 125 mg, which is equivalent to 25 mg Dexamethasone, given intravenously 24 hours after lower limb orthopedic surgery could reduce the severity of postoperative pain and provided less opioid consumption (35). Our study also shows the same results and supports the previous literature findings.
There is a growing body of evidence on how the immune system involves in the development of chronic pain. The importance of interactions between the immune and nervous systems in pain were recently fully appreciated (51). An inflammation that activates pain pathways was a response from by the body’s innate immune cells after injury. Immune and glial cells modulate the pain pathway by releasing several mediators to interact on peripheral nociceptors. This interaction intensifies the synaptic strength and induces peripheral sensitization, and if left untreated, can contribute to chronic pathological pain via central sensitization. Systemic dexamethasone has been demonstrated to have an inhibitory effect on central and peripheral inflammation and subsequently prevents the development of chronic postsurgical pain (52, 53). Considering these evidence, it seems that dexamethasone has a desirable property to prevent the transition from acute to chronic pain states.
Our study had its limitations. We solely followed the patients for 12 weeks and only the longer-term follow-up study could demonstrate the effectiveness of dexamethasone in reducing PPSP at longer time points. In addition, the use of corticosteroids to treat postoperative pain after TKA is not widely used due to the concerns of complications (17) such as delayed wound healing (14) and increase the risk of infection (39). These are the results from suppression of the immune system which is more commonly seen in chronic or long-term corticosteroid users (38). Chronic corticosteroid use also independently increases the risk for adverse gastrointestinal events (40). Because our study was not powered to detect these complications, there is a need for future studies to determine the safety of corticosteroid use in longer-term follow-up. Nevertheless, these adverse events are most commonly found in long-term glucocorticosteroid therapy, and occur only very rarely when steroids are administered for less than 5 days (17, 27, 41-44), indicating that the dosage and duration of administration of Dexamethasone utilized in this study are safe. We also did not observe patients with wound complications or periprosthetic joint infection after using the current protocol.
Conclusions
Perioperative intravenous Dexamethasone could significantly decreased persistent postsurgical pain when comparing to placebo at 12 weeks following total knee arthroplasty. In addition, perioperative intravenous Dexamethasone improves pain in early postoperative setting, reduces postoperative nausea and vomiting, and reduces opioid and anti-emetic medicine consumption.
References
1. Callahan CM, Drake BG, Heck DA, Dittus RS. Patient outcomes following tricompartmental total knee replacement. A meta-analysis. JAMA. 1994;271(17):1349-57.
2. Grosu I, Lavand'homme P, Thienpont E. Pain after knee arthroplasty: an unresolved issue. Knee Surg Sports Traumatol Arthrosc. 2014;22(8):1744-58.
3. Filos KS, Lehmann KA. Current concepts and practice in postoperative pain management: need for a change? Eur Surg Res. 1999;31(2):97-107.
4. Shang AB, Gan TJ. Optimising postoperative pain management in the ambulatory patient. Drugs. 2003;63(9):855-67.
5. Kehlet H, Jensen TS, Woolf CJ. Persistent postsurgical pain: risk factors and prevention. Lancet. 2006;367(9522):1618-25.
6. Vilardo L, Shah M. Chronic pain after hip and knee replacement. Tech Reg Anesth Pain Manag. 2011;15:110–115.
7. Beswick AD, Wylde V, Gooberman-Hill R, Blom A, Dieppe P. What proportion of patients report long-term pain after total hip or knee replacement for osteoarthritis? A systematic review of prospective studies in unselected patients. BMJ open. 2012;2(1):e000435.
8. Classification of chronic pain. Descriptions of chronic pain syndromes and definitions of pain terms. Prepared by the International Association for the Study of Pain, Subcommittee on Taxonomy. Pain Suppl. 1986;3:S1-226.
9. Puolakka PA, Rorarius MG, Roviola M, Puolakka TJ, Nordhausen K, Lindgren L. Persistent pain following knee arthroplasty. Eur J Anaesthesiol. 2010;27(5):455-60.
10. Wylde V, Hewlett S, Learmonth ID, Dieppe P. Persistent pain after joint replacement: prevalence, sensory qualities, and postoperative determinants. Pain. 2011;152(3):566-72.
11. Steyaert A, Lavand'homme P. Postoperative opioids: let us take responsibility for the possible consequences. Eur J Anaesthesiol. 2013;30(2):50-2.
12. Alam A, Gomes T, Zheng H, Mamdani MM, Juurlink DN, Bell CM. Long-term analgesic use after low-risk surgery: a retrospective cohort study. Arch Intern Med. 2012;172(5):425-30.
13. Kehlet H. Glucocorticoids for peri-operative analgesia: how far are we from general recommendations? Acta Anaesthesiol Scand. 2007;51(9):1133-5.
14. Rhen T, Cidlowski JA. Antiinflammatory action of glucocorticoids--new mechanisms for old drugs. N Engl J Med. 2005;353(16):1711-23.
15. De Oliveira GS, Jr., Castro-Alves LJ, Ahmad S, Kendall MC, McCarthy RJ. Dexamethasone to prevent postoperative nausea and vomiting: an updated meta-analysis of randomized controlled trials. Anesth Analg. 2013;116(1):58-74.
16. Henzi I, Walder B, Tramer MR. Dexamethasone for the prevention of postoperative nausea and vomiting: a quantitative systematic review. Anesth Analg. 2000;90(1):186-94.
17. Salerno A, Hermann R. Efficacy and safety of steroid use for postoperative pain relief. Update and review of the medical literature. J Bone Joint Surg Am. 2006;88(6):1361-72.
18. De Oliveira GS, Jr., Almeida MD, Benzon HT, McCarthy RJ. Perioperative single dose systemic dexamethasone for postoperative pain: a meta-analysis of randomized controlled trials. Anesthesiology. 2011;115(3):575-88.
19. Backes JR, Bentley JC, Politi JR, Chambers BT. Dexamethasone reduces length of hospitalization and improves postoperative pain and nausea after total joint arthroplasty: a prospective, randomized controlled trial. J Arthroplasty. 2013;28(8 Suppl):11-7.
20. Lunn TH, Kristensen BB, Andersen LO, Husted H, Otte KS, Gaarn-Larsen L, et al. Effect of high-dose preoperative methylprednisolone on pain and recovery after total knee arthroplasty: a randomized, placebo-controlled trial. Br J Anaesth. 2011;106(2):230-8.
21. Jules-Elysee KM, Lipnitsky JY, Patel N, Anastasian G, Wilfred SE, Urban MK, et al. Use of low-dose steroids in decreasing cytokine release during bilateral total knee replacement. Reg Anesth Pain Med. 2011;36(1):36-40.
22. Jules- Elysee KM, Wilfred SE, Memtsoudis SG, Kim DH, YaDeau JT, Urban MK, et al. Steroid modulation of cytokine release and desmosine levels in bilateral total knee replacement: a prospective, double-blind, randomized controlled trial. J Bone Joint Surg Am. 2012;94(23):2120-7.
23. Koh IJ, Chang CB, Lee JH, Jeon YT, Kim TK. Preemptive low-dose dexamethasone reduces postoperative emesis and pain after TKA: a randomized controlled study. Clin Orthop Relat Res. 2013;471(9):3010-20.
24. Haynes RC. Adrenocortical steroids, In: Goodman A, Rall TW, Nies AS, et al, eds. Goodman and Gilman's The Pharmacological Basis of Therapeutics. 8th ed. New York, NY: Pergamon Press, 1990:1446
25. Holte K, Kehlet H. Perioperative single-dose glucocorticoid administration: pathophysiologic effects and clinical implications. J Am Coll Surg. 2002;195(5):694-712.
26. Gilron I. Corticosteroids in postoperative pain management: future research directions for a multifaceted therapy. Acta Anaesthesiol Scand. 2004;48(10):1221-2.
27. Lunn TH, Kehlet H. Perioperative glucocorticoids in hip and knee surgery - benefit vs. harm? A review of randomized clinical trials. Acta Anaesthesiol Scand. 2013;57(7):823-34.
28. Gan TJ, Meyer T, Apfel CC, Chung F, Davis PJ, Eubanks S, et al. Consensus guidelines for managing postoperative nausea and vomiting. Anesth Analg. 2003;97(1):62-71, table of contents.
29. Wang JJ, Ho ST, Tzeng JI, Tang CS. The effect of timing of dexamethasone administration on its efficacy as a prophylactic antiemetic for postoperative nausea and vomiting. Anesth Analg. 2000;91(1):136-9.
30. Kardash KJ, Sarrazin F, Tessler MJ, Velly AM. Single-dose dexamethasone reduces dynamic pain after total hip arthroplasty. Anesth Analg. 2008;106(4):1253-7, table of contents.
31. Mathiesen O, Jacobsen LS, Holm HE, Randall S, Adamiec-Malmstroem L, Graungaard BK, et al. Pregabalin and dexamethasone for postoperative pain control: a randomized controlled study in hip arthroplasty. Br J Anaesth. 2008;101(4):535-41.
32. Rasmussen ML, Mathiesen O, Dierking G, Christensen BV, Hilsted KL, Larsen TK, et al. Multimodal analgesia with gabapentin, ketamine and dexamethasone in combination with paracetamol and ketorolac after hip arthroplasty: a preliminary study. Eur J Anaesthesiol. 2010;27(4):324-30.
33. Lunn TH, Andersen LO, Kristensen BB, Husted H, Gaarn-Larsen L, Bandholm T, et al. Effect of high-dose preoperative methylprednisolone on recovery after total hip arthroplasty: a randomized, double-blind, placebo-controlled trial. Br J Anaesth. 2013;110(1):66-73.
34. Cui Z, Liu X, Teng Y, Jiang J, Wang J, Xia Y. The efficacy of steroid injection in total knee or hip arthroplasty. Knee Surg Sports Traumatol Arthrosc. 2014.
35. Romundstad L, Breivik H, Niemi G, Helle A, Stubhaug A. Methylprednisolone intravenously 1 day after surgery has sustained analgesic and opioid-sparing effects. Acta Anaesthesiol Scand. 2004;48(10):1223-31.
36. Franklin J, Lunt M, Bunn D, Symmons D, Silman A. Risk and predictors of infection leading to hospitalisation in a large primary-care-derived cohort of patients with inflammatory polyarthritis. Ann Rheum Dis. 2007;66(3):308-12.
37. McDonough AK, Curtis JR, Saag KG. The epidemiology of glucocorticoid-associated adverse events. Curr Opin Rheumatol. 2008;20(2):131-7.
38. Gedalia A, Shetty AK. Chronic steroid and immunosuppressant therapy in children. Pediatr Rev. 2004;25(12):425-34.
39. Stuck AE, Minder CE, Frey FJ. Risk of infectious complications in patients taking glucocorticosteroids. Rev Infect Dis. 1989;11(6):954-63.
40. Messer J, Reitman D, Sacks HS, Smith H, Jr., Chalmers TC. Association of adrenocorticosteroid therapy and peptic-ulcer disease. N Engl J Med. 1983;309(1):21-4.
41. Sauerland S, Nagelschmidt M, Mallmann P, Neugebauer EA. Risks and benefits of preoperative high dose methylprednisolone in surgical patients: a systematic review. Drug Saf. 2000;23(5):449-61.
42. Clune JE, Greene AK, Guo CY, Gao LL, Kim S, Meara JG, et al. Perioperative corticosteroid reduces hospital stay after fronto-orbital advancement. J Craniofac Surg. 2010;21(2):344-8.
43. Kim K, Brar P, Jakubowski J, Kaltman S, Lopez E. The use of corticosteroids and nonsteroidal antiinflammatory medication for the management of pain and inflammation after third molar surgery: a review of the literature. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2009;107(5):630-40.
44. Waldron NH, Jones CA, Gan TJ, Allen TK, Habib AS. Impact of perioperative dexamethasone on postoperative analgesia and side-effects: systematic review and meta-analysis. Br J Anaesth. 2013;110(2):191-200.
45. Koog YH, Wi H, Jung WY. Eligibility criteria in knee osteoarthritis clinical trials: systematic review. Clin Rheumatol. 2013;32(11):1569-74.
46. Altman R, Asch E, Bloch D, Bole G, Borenstein D, Brandt K, et al. Development of criteria for the classification and reporting of osteoarthritis. Classification of osteoarthritis of the knee. Diagnostic and Therapeutic Criteria Committee of the American Rheumatism Association. Arthritis Rheum. 1986;29(8):1039-49.
47. Ehrich EW, Davies GM, Watson DJ, Bolognese JA, Seidenberg BC, Bellamy N. Minimal perceptible clinical improvement with the Western Ontario and McMaster Universities osteoarthritis index questionnaire and global assessments in patients with osteoarthritis. J Rheumatol. 2000;27(11):2635-41.
48. Kuptniratsaikul V, Rattanachaiyanont M. Validation of a modified Thai version of the Western Ontario and McMaster (WOMAC) osteoarthritis index for knee osteoarthritis. Clinical rheumatology. 2007;26(10):1641-5.
49. Parvizi J, Zmistowski B, Berbari EF, Bauer TW, Springer BD, Della Valle CJ, et al. New definition for periprosthetic joint infection: from the Workgroup of the Musculoskeletal Infection Society. Clinical orthopaedics and related research. 2011;469(11):2992-4.
50. Kelly AM. The minimum clinically significant difference in visual analogue scale pain score does not differ with severity of pain. Emerg Med J. 2001;18(3):205-7.
51. Ren K, Dubner R. Interactions between the immune and nervous systems in pain. Nat Med. 2010;16:1267–1276.
52. Pinto-Ribeiro F , Moreira V , Pêgo JM, Leão P , Almeida A, Sousa N. Antinociception induced by chronic glucocorticoid treatment is correlated to local modulation of spinal neurotransmitter content. Mol Pain. 2009;5:41.
53. Javan M, Kazemi B, Ahmadiani A, Motamedi F . Dexamethasone mimics the inhibitory effect of chronic pain on the development of tolerance to morphine analgesia and compensates for morphine induced changes in G proteins gene expression. Brain Res. 2006; 1104:73–79.
#8110
History of Diabetic Foot Ulcer Is Associated With Increased Risk Prosthetic Joint Infection and Sepsis After Total Joint Arthroplasty
*Matthew Magruder - Maimonides Medical Center - Brooklyn, USA
Vincent Yao - Sophie Davis Biomedical Education Program at the CUNY School of Medicine - New York, USA
Ariel Rodriguez - Maimonides Medical Center - Brooklyn, United States of America
Mitchell Ng - Maimonides Medical Center - Brooklyn, United States of America
Che Hang Jason Wong - Maimonides Medical Center - Brooklyn, USA
Michael Mont - Sinai Hospital of Baltimore - Baltimore, USA
*Email: mmagruder@maimonidesmed.org
Introduction: Foot ulcers are a common sequelae of diabetes mellitus (DM), which confers increased risk of cellulitis, osteomyelitis, and other lower extremity infections. The effect of a history of diabetic foot ulcer (DFU) on total joint arthroplasty (TJA) outcomes has not been investigated. Therefore, the aim of this study is to evaluate whether patients with a history of DFU have increased risk of 1) post-operative superficial skin infections (SSI), 2) prosthetic joint infections (PJI), 3) sepsis and 4) revisions following total joint arthroplasty.
Methods: Using an administrative claim database (PearlDiver), a retrospective query from January 1, 2010 to October 31, 2020 was performed. All patients who underwent primary total knee arthroplasty (TKA) and total hip arthroplasty (THA) for osteoarthritis with a diagnosis of DFU at the time of the procedure were included. DFU patients were successfully 1:5 propensity score matched with controls based on age, gender, body mass index, DM, rheumatoid arthritis, inflammatory bowel disease, end-stage renal disease, chronic liver disease, smoking status and peripheral vascular disease, yielding a total of 33,155 TKA patients (DFU = 5,529; control = 27,626) and 17,146 THA patients (DFU= 2,862; control= 14,284). Outcomes evaluated included 90-day and 2-year rates of prosthetic joint infections and superficial skin infections, as well as 90-day development of sepsis and 2-year revisions. Multivariate logistical regression was used to calculate odds ratios (ORs), 95% confidence intervals (95% CIs), and p-values. Welch’s t-tests were used to test for significant differences in lengths of stay and costs of care between cohorts. A p value <0.001 was the significance threshold.
Results: Patients with a history of DFU had significant increased risk of developing sepsis within 90 days of primary TKA (OR 4.59; p<0.001) and THA (OR 4.87; p<0.001). DFU did not confer an increased risk of PJI at 90-days for TKA (OR 0.8; p=0.1) or THA (OR 0.85; p=0.34), but by 2 years following index procedure for both TKA (OR 1.51; p<0.001) and THA (OR 1.55; p<0.001). Risk of SSI were also increased following primary TKA and THA at 90-days and 2-years post-op. Finally, risk of 2-year revisions were also increased in the DFU cohort.
Conclusion: Patients with a history of DFU who underwent total joint arthroplasty demonstrated a significantly increased risk of the development of post-operative sepsis and PJI. Further, patients demonstrated increased risk of SSI and revision surgery. Providers should use this investigation in their decision-making prior to surgery, and to counsel patients with DFU about the post-operative risks.
Figures
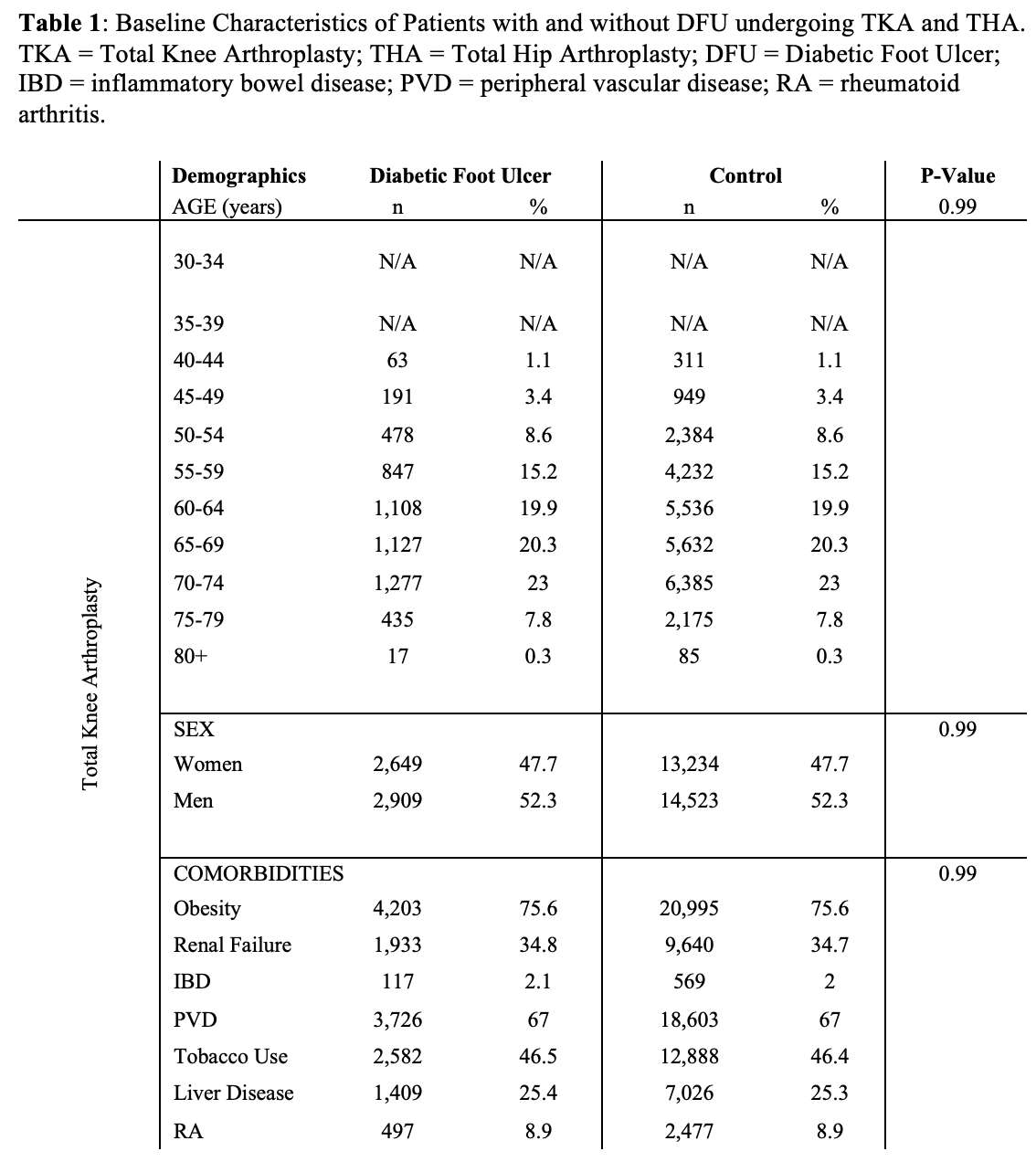
Figure 1
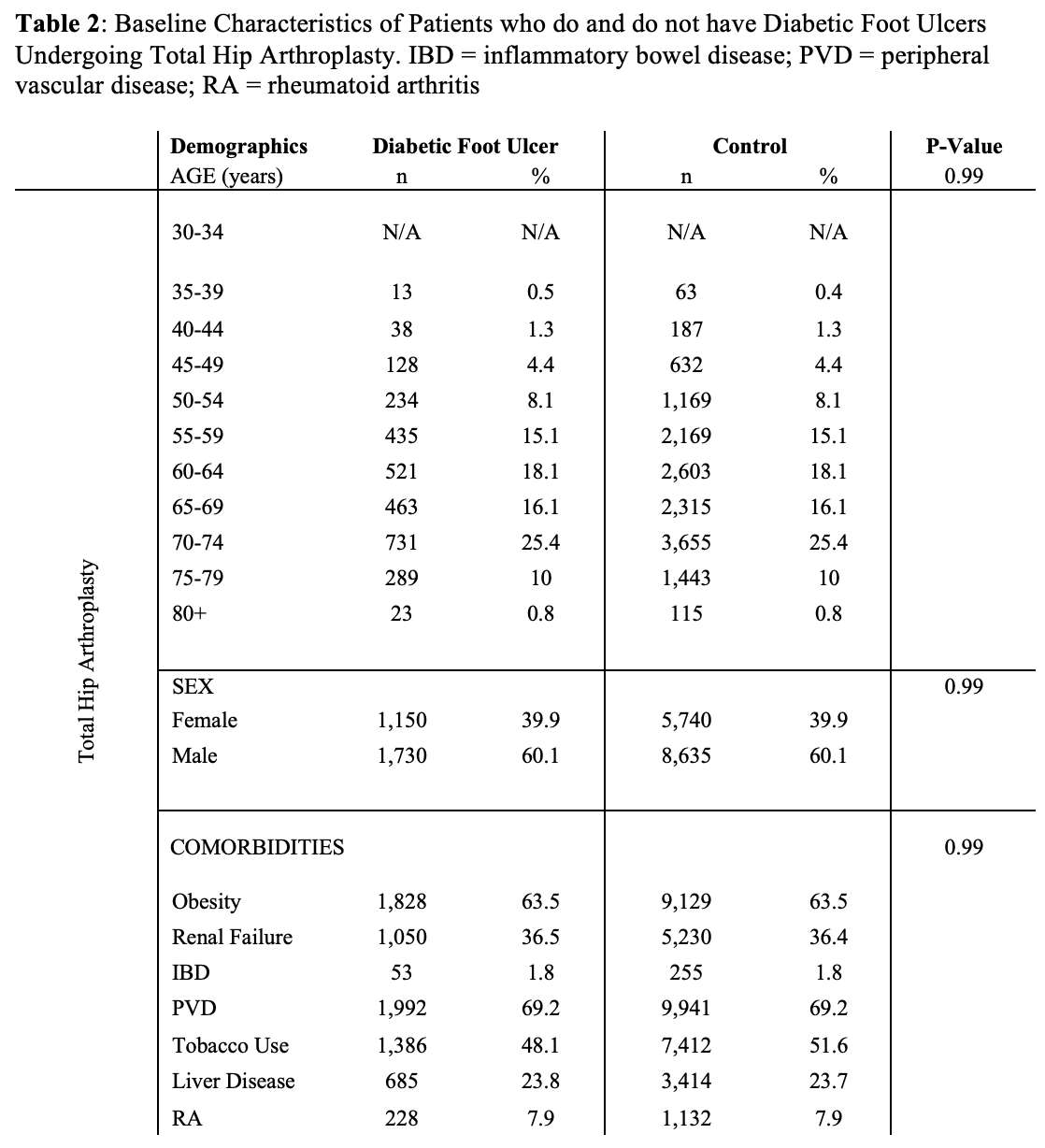
Figure 2
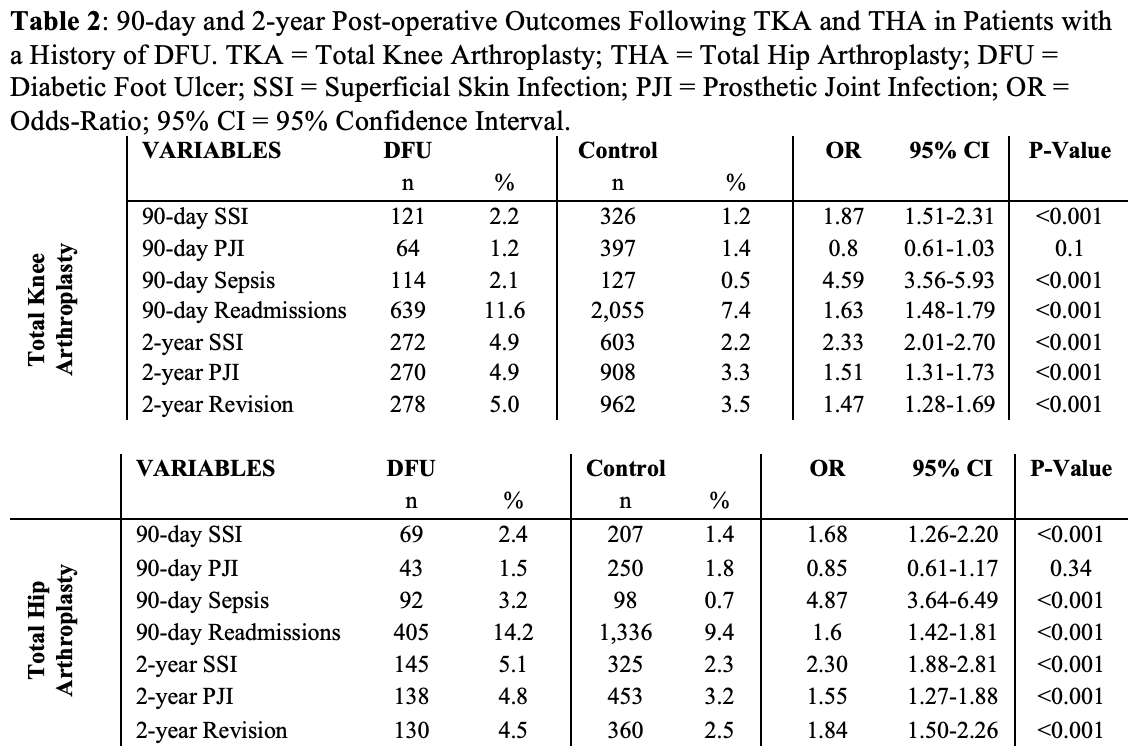
Figure 3#8108
Inflammatory Bowel Disease Patients Undergoing Total Hip Arthroplasty Have Higher Risk of Implant-Related Complications
*Matthew Magruder - Maimonides Medical Center - Brooklyn, USA
Shabnam Parsa - Renaissance School of Medicine at Stony Brook University - Stony Brook, USA
Adam Gordon - maimonides Medical Center - Brooklyn, USA
Mitchell Ng - Maimonides Medical Center - Brooklyn, United States of America
Che Hang Jason Wong - Maimonides Medical Center - Brooklyn, USA
Gabriel Lama - Maimonides Medical Center - Staten Island, United States of America
*Email: mmagruder@maimonidesmed.org
Introduction: Inflammatory bowel disease (IBD) is a systemic inflammatory disease that is thought to result from uncontrolled immune-mediated gut inflammatory response in genetically predisposed patients to an environmental trigger that interacts with the host gut microbiome. Recent studies have found that IBD patients are at increased risk of medical complications after total hip arthroplasty (THA). The purpose of this study was to evaluate whether IBD patients are at increased risk of implant-related complications after THA.
Methods: A retrospective study from January 1st, 2010 to October 31st, 2020 using an administrative claims database was performed. Patients and complications were queried through the use of International Classification of Disease, Ninth and Tenth revision (ICD-9/10) codes. The database was first queried for all patients who underwent primary THA. This cohort was subsequently filtered for all patients with a diagnosis of IBD (which included diagnosis codes for both Crohn’s Disease and ulcerative colitis). Inclusion criteria comprised patients who underwent primary THA with a diagnosis of IBD at the time of surgery. Exclusion criteria included any patients who were taking a corticosteroid medication at the time of surgery, as this could potentially confound the data analyses. To reduce the effects of confounding, study group patients were randomly ratio matched 1:5 based on age, sex and the Charlson Comorbidity Index (CCI); producing a total of 66,146 patients (IBD patients = 11,025, controls = 55,121 Table 1). Matching was successful as there was no statistical differences with the aforementioned matching parameters between the two cohorts (Table 1). Outcomes evaluated included periprosthetic fracture, aseptic loosening, prosthetic joint infection, and THA revision within 2-years of index procedure. Chi-square analyses were used to compare the matched cohorts. The association of IBD and implant complications was evaluated using logistical regression to calculate odds ratios (ORs), 95% confidence intervals (95% CIs), and p-values. A p-value <0.0001 was used as the significance threshold.
Results: Patients with IBD had a greater incidence and odds of total implant complications (7.03% vs. 3.98%; OR 1.76; p<0.0001) compared with matched controls (Table 2). IBD patients had significantly higher incidence and odds of developing periprosthetic fracture (0.50% vs. 0.20%; OR 2.46; p<0.0001), aseptic loosening (1.45% vs. 0.84%; OR 1.75; p<0.0001), prosthetic joint infection (2.87% vs. 1.77%; OR 1.64; p<0.0001) and THA revisions (2.21% vs. 1.17%; OR 1.91; p<0.0001) (Figure 1).
Discussion: Patient’s undergoing primary THA with a history of an IBD had significantly higher odds of developing implant related complications than matched controls. Periprosthetic hip fractures demonstrated the largest increased risk, which is likely due to IBD patients increased risk of poor bone quality and osteoporosis. Providers should use this study to appropriately educate their patients on the risks of surgery. Future research should stratify severity of IBD and evaluate whether severity correlates with a step-wise increase in implant-related complications.
Figures
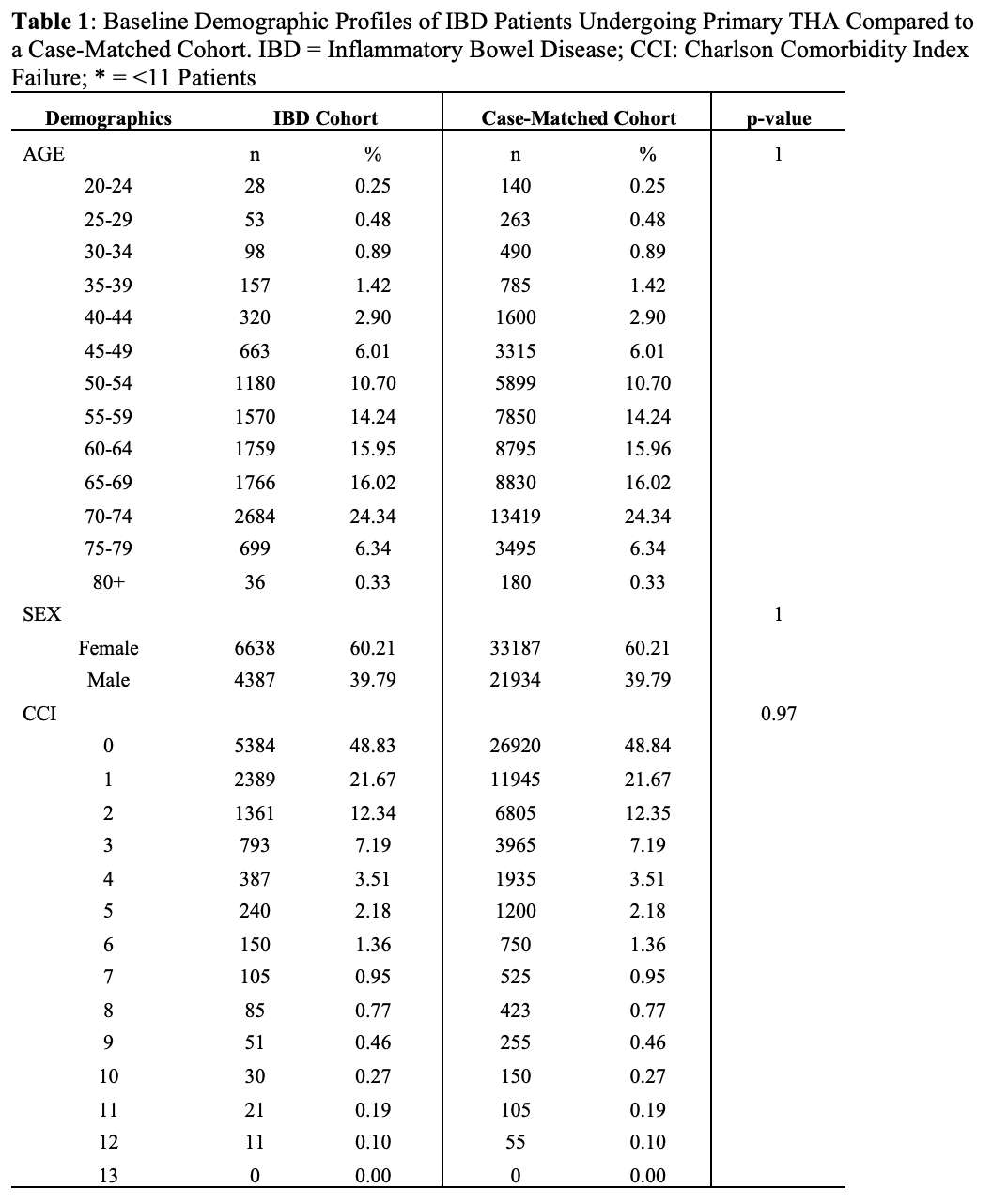
Figure 1
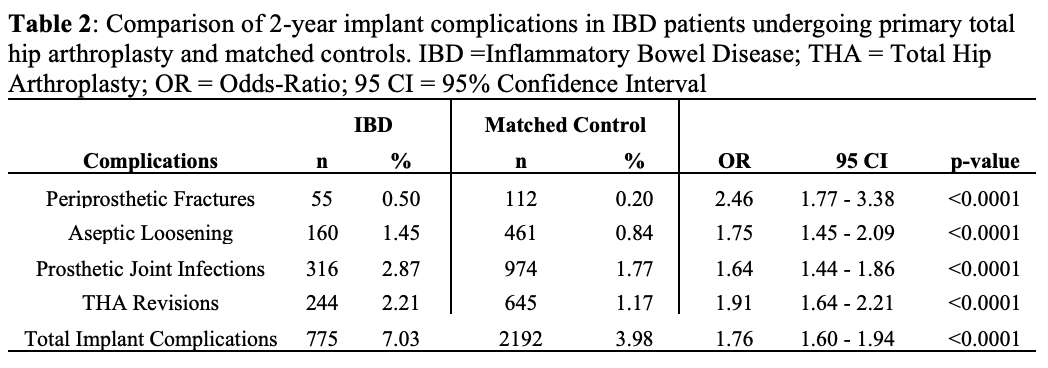
Figure 2
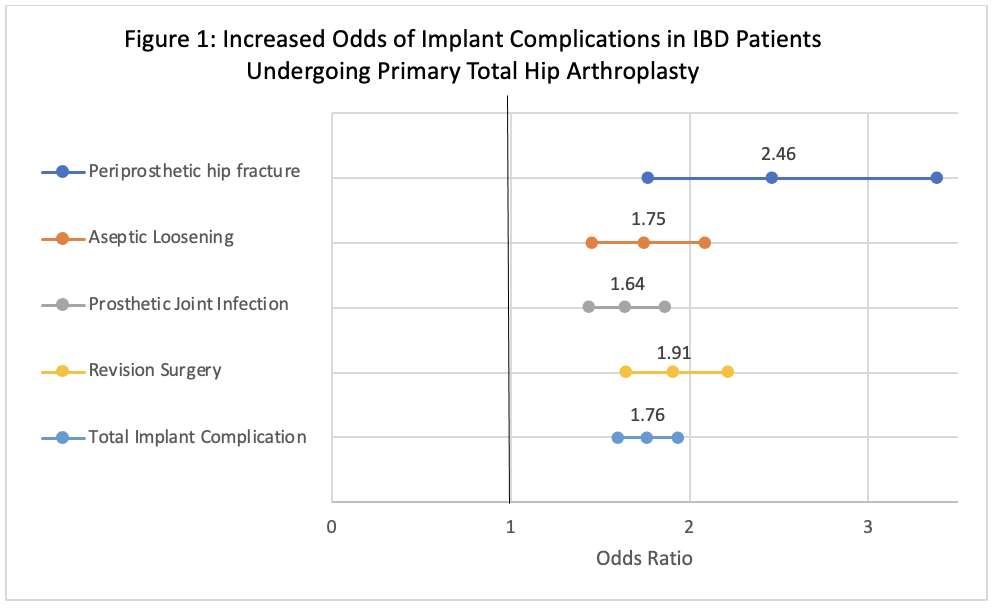
Figure 3#8839
The innovation journey of an orthopedic surgeon: Dimer, infection, and NASA
*Arthur Kreitenberg - New York, United States of America
*Email: akriet@msn.com
#8840
Successful Haventure startups in surgical robotics: A few tips & tricks
*Stephane Lavallee - SURGIVISIO - Grenoble, France
*Email: sl@stephanelavallee.com
#8841
Innovation in publication: Time for a change!
*Ira Kirschenbaum - Bronx-Lebanon Hospital - Bronx, USA
*Email: IKIRSCH@bronxleb.org
#8842
H1: Years in the making
*Susannah Clarke - Imperial College London - London, United Kingdom
*Email: sgc05@imperial.ac.uk
#8843
The integration of robotics, sensors, and data into the digital ecosystem
*Martin Roche - Holy Cross Hospital - Fort Lauderdale, USA
*Email: martin@mroche.com
#8695
Tracking Functional Recovery After Total Knee Arthroplasty: A Case for Wearable Activity Monitors
*Danielle Marshall - University of Miami/Jackson Memorial Hospital - Miami, United States of America
Andrew Pierce - Herbert Wertheim College of Medicine - Miami, USA
Dianne Pagan - University of Miami Miller School of Medicine - Miami, United States of America
Victor Hernandez - University of Miami Hospital - Miami, USA
Richard Bolander - TracPatch - Orland Park, United States of America
Rohan Mangal - University of Miami Miller School of Medicine - Miami, United States of America
*Email: marshalld447@gmail.com
Introduction
Functional recovery after total knee arthroplasty (TKA) is typically tracked using patient-reported outcome measures (PROMs) or physician measurements at discrete time points. Using wearable devices, surgeons can now monitor patients’ rehabilitation progress continuously through objective data. Currently, there is a lack of quality studies evaluating the efficacy of using wearable devices to track patient functional recovery after TKA. The primary objective of this study aimed to compare functional recovery in terms of postoperative knee range of motion (ROM) in patients using the wearable activity device versus standard postoperative clinical monitoring.
Methods
This was a prospective, randomized clinical trial for patients ≥18 years of age undergoing primary unilateral TKA who owned a smartphone between 2018 and 2023. Patients who consented to participate were randomized in a 1:1 ratio to either the wearable or conventional cohort. Patients who were assigned to the wearable group received the device postoperatively on the day of the operation and were instructed on device usage. Knee ROM was collected continuously over six weeks for the wearable cohort and via physical exam at two and six weeks postoperatively for the conventional cohort. Data was analyzed using the chi-square test for categorical variables and one-way ANOVA for continuous variables. Boxplots were created to demonstrate the effect of age, sex, and BMI on recovery, with statistical significance set at p < 0.05.
Results
A total of 148 patients were prospectively randomized to the study (74 in each cohort). Differences in preoperative characteristics between cohorts are summarized in Table 1. The results showed that at postoperative weeks two, four, and six there was no significant difference in knee ROM (both flexion and extension) between the conventional and wearable groups (Figure 1). The results showed no significant difference in flexion at postoperative week six when comparing patient BMI or sex (Figure 2). There was a significant difference in postoperative flexion at six weeks based on patient age, with patients ≥80 years and those <80 years old attaining a mean flexion of 91.7º±3.7º and 106.8º±13.6º, respectively (p = 0.05).
Conclusion
This study directly compared the use of a wearable device versus the standard postoperative evaluation of functional recovery following TKA. Our results show that wearable device ROM measurements were consistent with those obtained on standard postoperative physical exam. However, clinical measurements were less sensitive to changes in ROM as evidenced by the subtle but important differences in return to full extension captured by the wearable device but not on clinical exam. Age ≥80 years predicted decreased recovery of flexion postoperatively, while BMI and sex demonstrated no measurable impact. The continuous recording of postoperative objective ROM data is valuable to surgeons as this reduces the time-consuming burden of data collection in the clinic and does not rely on patient recall and a rigid postoperative follow up schedule. In the future, clinicians may choose to remotely monitor patients' activity and schedule in-person clinic visits at personalized times (eg, inflection points in postoperative recovery such as unexpected decreases in mobility).
Figures
Figure 1
Figure 2
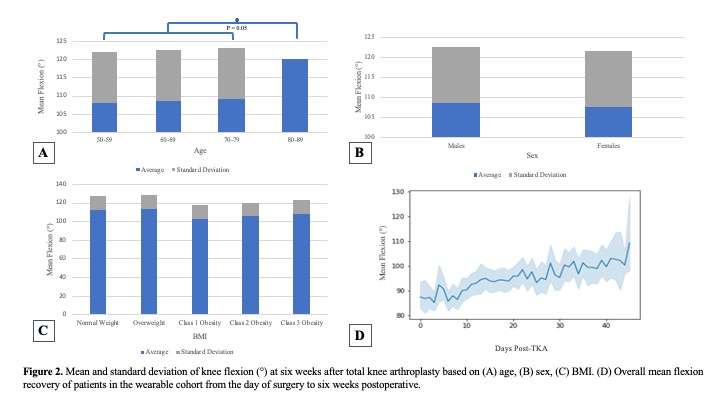
Figure 3#8434
Comparing Commercially Available Wearable Motion Sensors to Optical Motion Capture Measuring Knee Range of Motion Before and After TKA
Skye Richards - University of Rhode Island - Warwick, United States of America
Robert Marchand - Ortho Rhode Island - South County, USA
*Ryan Chapman - University of Rhode Island - Kingston, United States of America
*Email: rmchapman@uri.edu
Introduction: In the US, ~600,000 total knee arthroplasties (TKA) occur annually. Post-TKA, range of motion (ROM) recovery is important for successful outcomes. However, ROM measurements typically occur via simplified, imprecise techniques (e.g. goniometry) in controlled settings that do not consider real-world experiences. Therefore, methods for capturing knee ROM in real-world settings are necessary. One recently developed modality is wearable inertial measurement unit (IMU) motion sensors. However, the accuracy of these wearables is inadequately described. Accordingly, the objectives of this study were to evaluate 1) wearable sensor accuracy pre/post-TKA and 2) patient sensor self-application accuracy.
Methods: This IRB approved prospective cohort study compared two sagittal knee angle measurement techniques (wearable IMU sensors vs. optical motion capture (MOCAP)) in 20 patients undergoing unilateral TKA. Patients were assessed at our biomechanics laboratory ~3 weeks pre-TKA having self-applied wearables (unaligned sensors) per their surgeon’s instructions (lateral shank/thigh, Figure 1A). Optical MOCAP markers were placed on their pelvis/bilateral lower extremities (Figure 1B). Wearable sensor sagittal knee angles (fs=50Hz) and optical MOCAP position data (fs=100Hz) were captured simultaneously during all movements (heel/wall slides, standing knee bends, seated march, long arc quads, sit-to-stand, timed-up-and-go, treadmill gait). Unaligned sensor data were mathematically rotated in-silico to correct for sensor-to-leg alignment errors and stored as a new knee angle measurement (aligned sensors). All patients then underwent TKA via the medial parapatellar approach by one surgeon using the same implant make/model. Three weeks post-TKA, patients repeated the data capture process. All MOCAP 3D position data were processed in Visual3D computing sagittal knee angles. Unaligned sensor, aligned sensor, and optical MOCAP sagittal knee angles were compared using correlation analyses and generated cross-plots (optical MOCAP vs. IMU-based sagittal knee angle).
Results: 20 TKA patients (10M, 67.7±7.4yrs, BMI=32.0±7.1) were enrolled. Wearable IMU accuracy was equal pre-/post-TKA, and data were combined accordingly. Knee angle measurements were compared (MOCAP vs. unaligned sensors vs. aligned sensors). An example subject walking (Figure 2) highlights typical knee angle DC offset errors in unaligned sensors versus aligned sensors and optical MOCAP. Across all subjects/activities (Figure 3A), unaligned sensors yielded strong, significant correlations with MOCAP with a high coefficient of determination (R2=0.98). However, 13.7° of average erroneous extra knee flexion was present with unaligned sensors. Mathematically correcting unaligned sensors to aligned sensors yielded the same R2 but reduced the offset error to 1.3° erroneous knee extension. Throughout all activities, mean/mean absolute value error (Figure 3B) for unaligned sensors vs. MOCAP were -17.2±5.4° and 17.3±5.3°, respectively. Comparing aligned sensors vs. MOCAP resulted in mean/mean absolute value error -0.5±5.1° and 3.3±3.9°, respectively.
Conclusion: Wearable sensors capturing data outside of well-controlled clinical/laboratory settings provide richer information regarding patient recovery, facilitating improved clinical decision making. However, sensor and patient variability need to be assessed for these novel wearables. This wearable sensor was accurate across a variety of activities when aligned with the leg mechanical axes (<3.5° error). However, patient wearable sensor self-application induced a significant DC offset error (>15°). Thus, patient sensor application training and sensor-to-leg alignment procedures are critical for accurate knee angle measurements.
Figures
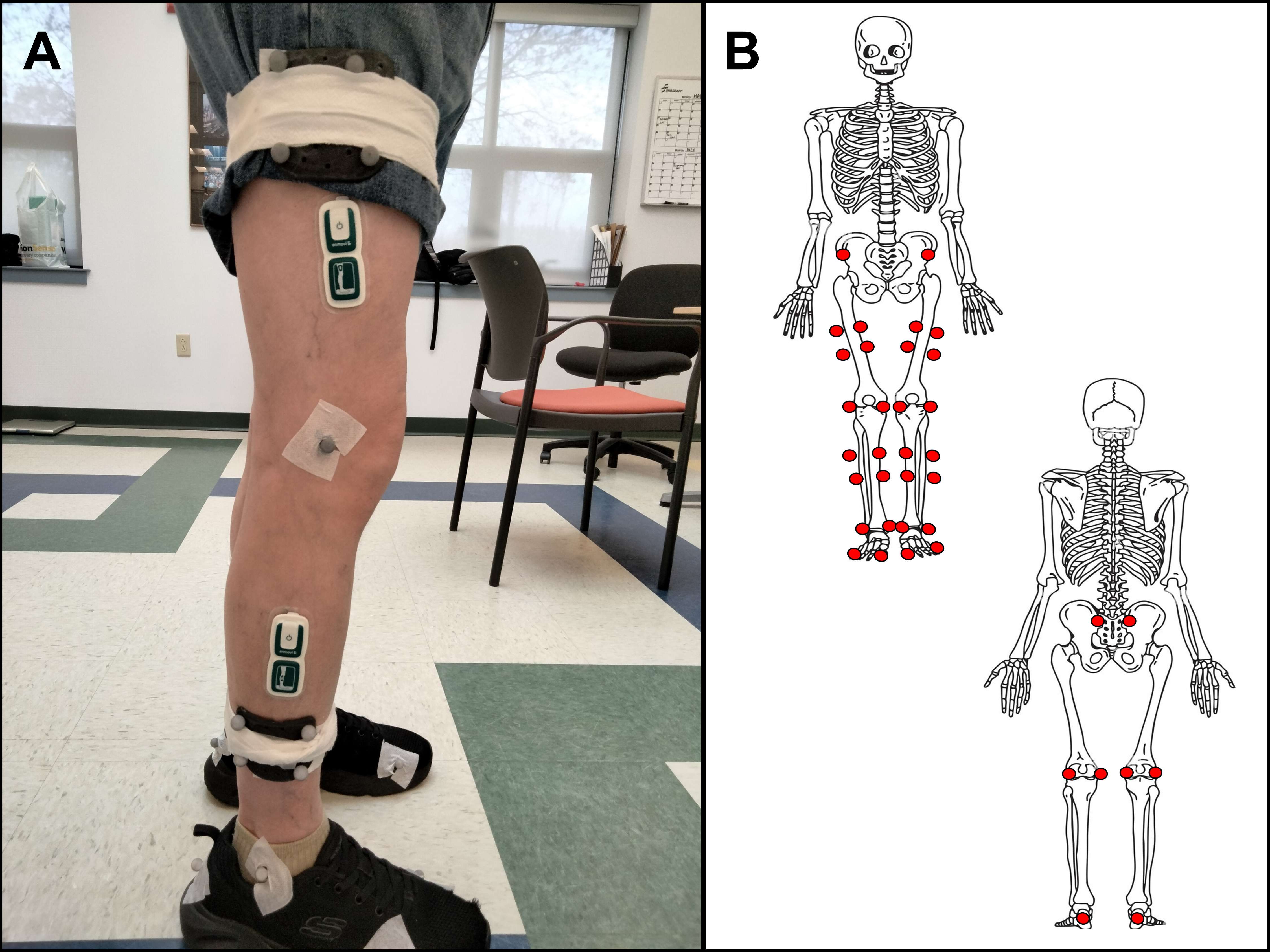
Figure 1
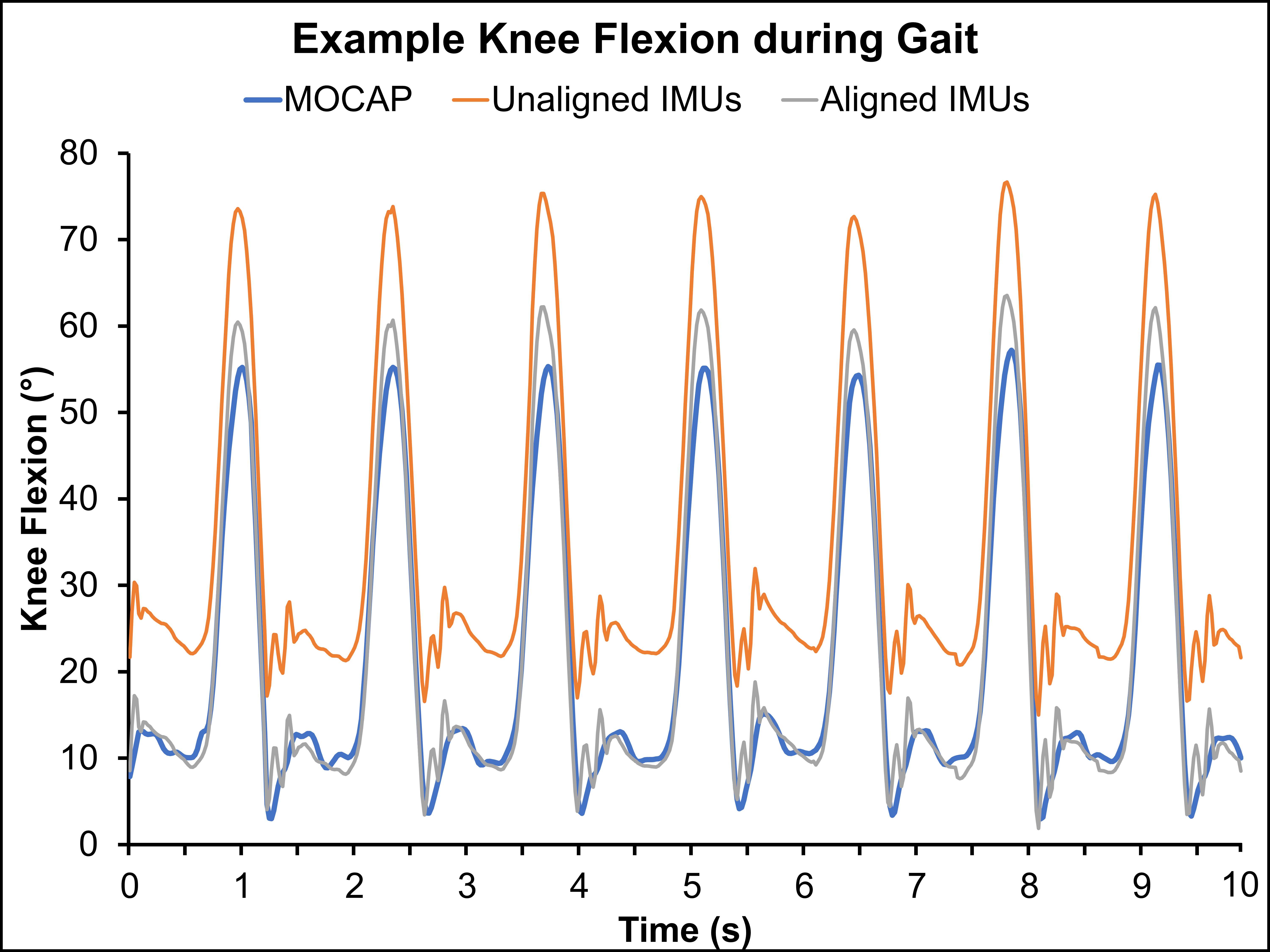
Figure 2
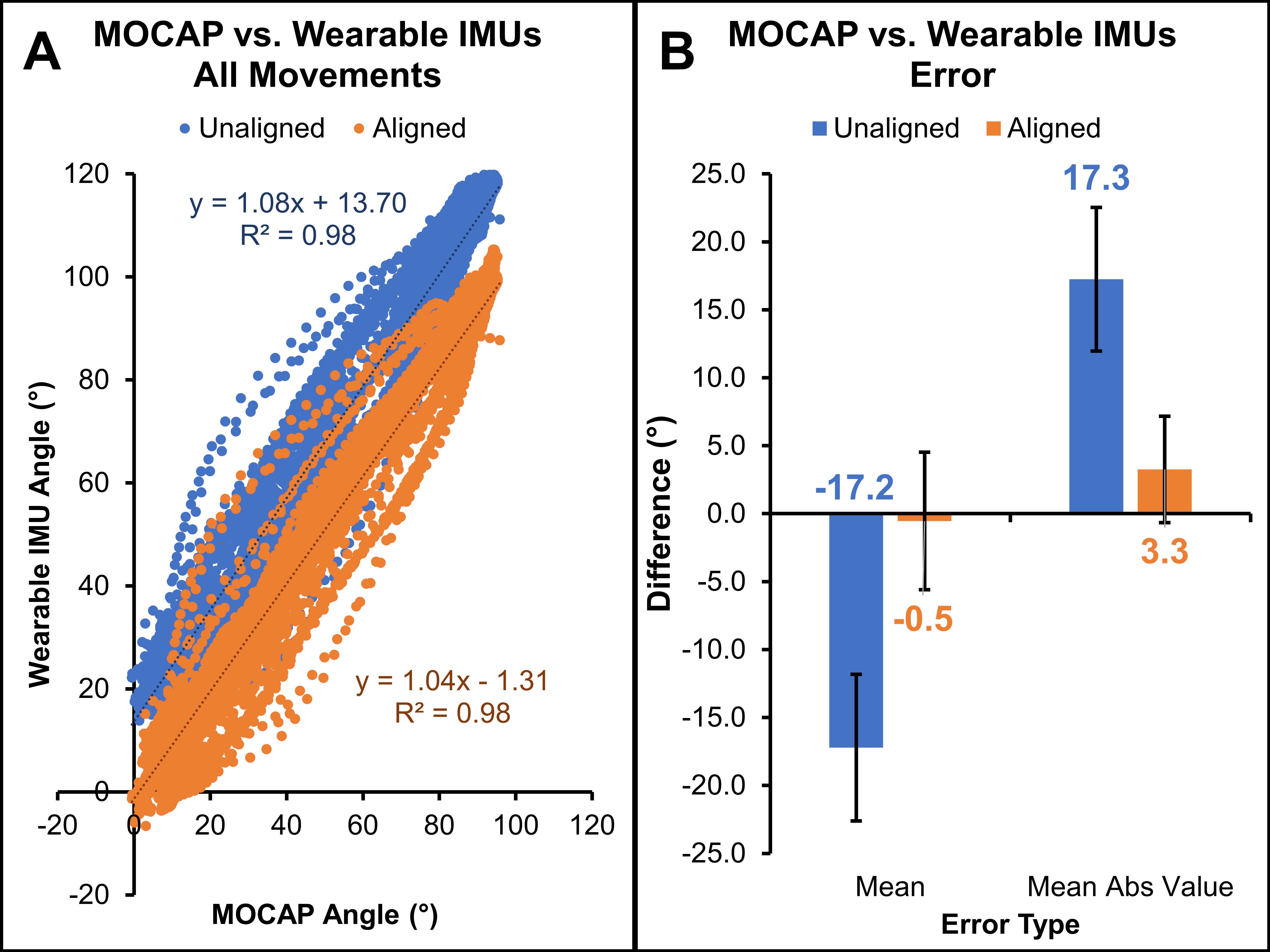
Figure 3#8493
The Relationship Between Cadence and Total Daily Step Counts in Patients Following Total Knee Arthroplasty
*Richard Bolander - TracPatch - Orland Park, United States of America
S. David Stulberg - Northwestern Memorial Hospital - Chicago, USA
Ralph Mobbs - Prince of Wales Private Hospital - Sydney, Australia
*Email: rbolander@tracpatch.com
Surface sensor wearable technologies provide the ability to continually monitor patients pre and post intervention. It has been reported that total daily step counts will decrease post operatively and then return to or exceed preoperative baselines. While those reports have summarized at the weekly level, this abstract reported on the total daily step counts and their relationship to cadence (steps/min). Cadence is known as a general indicator of health where 100 steps per minute is defined as moderate intensity physical activity and is an indicator of walking speed and overall movement capability. This abstract reviewed metrics of cadence and compared them to total daily steps counts to determine whether different recovery patterns from total daily step count could be observed.
In this analysis, 481 patients from 11 surgeons and 8 institutions wore the TracPatch system in a range anywhere between 10 days pre surgery to 60 days post-surgery. While not directly reported to the patient or provider, the system measures the number of steps taken per minute. The distribution of total steps in the day, mean step counts per minute and max steps in a minute for each day for each patient was reported.
The inter-quartile range for total daily steps ranged between 50 and 1000 immediately following surgery and between 3000 and 7000 50 days post-surgery. It was observed that the interquartile range increased as the number of days post-surgery increased. This was likely associated with a patient’s preference. The max achieved cadence inter quartile range remained constant throughout recovery by approximately 30 steps per minute. However, the median immediately following surgery was approximately 50 steps per minute and 50 days post was approximately 90. For average steps in a minute, the median following surgery and 50 days post was approximately 20 and 30 with an interquartile range of 10. This indicates that patients were generally not walking consistently for at least minute for numerous walking bouts that will occur throughout the day. However, the measure may indicate a behavior associated with either the ability to walk further or faster overtime.
The presented analysis suggests that metrics associated with cadence have more consistent interquartile ranges than total daily step count, where there is a greater dispersion in outcomes as the days post-surgery increases. The differences in total daily steps may be more heavily influenced by factors such as gender, BMI, pain levels or individual motivation. Further research will investigate whether markers of cadence may be less susceptible to co-variates for comparing recovery rates across patients.
Figures
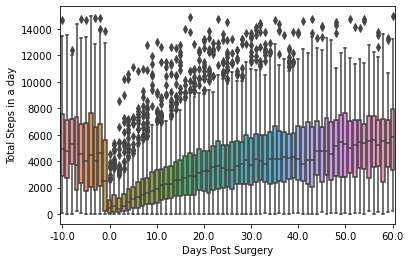
Figure 1
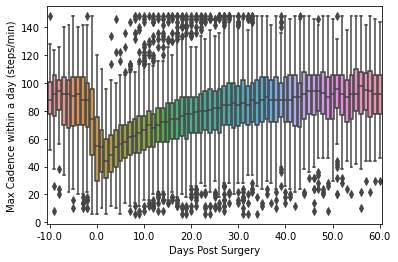
Figure 2
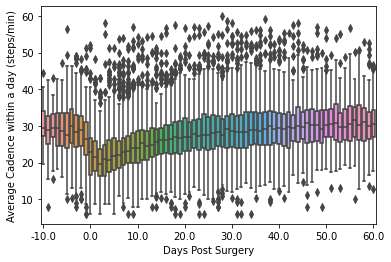
Figure 3#8342
The Sensor Enabled TKA - a Novel Approach to Pre- and Post-Op Gait Analysis
*Kevin Gemmell - Canary Medical - Carlsbad, USA
Heidi Sharipov - Canary Medical - Carlsbad, USA
Patrick Aubin - Canary Medical - Carlsbad, USA
Peter Schiller - Canary Medical - Carlsbad, USA
Jeffrey Gross - Canary Medical - Carlsbad, USA
William Hunter - Canary Medical - Carlsbad, USA
Fred Cushner - Hospital for Special Surgery - New York, USA
*Email: kgemmell@canarymedical.com
Introduction: Gait analysis and performance outcome measures are powerful tools to evaluate mobility and knee function following total knee arthroplasty. The ability to capture preoperative data with an external wearable and/or postoperative data with an implanted Self-Monitoring Analysis and Reporting Technology (SMART) knee in the clinic environment enables objective assessment with the patient acting as their own control. The observed in-clinic gait and functional performance data, complemented with postoperative, at-home, remotely-collected gait data from the SMART implant, enhance the clinical utility of these tools.
The objective of this study was to evaluate the use of the novel canturio™te implant and CanarE (external wearable) to capture kinematic parameters within a clinic environment in comparison to gold standard measurement methods.
Methods: Fifteen subjects (7 Male, 8 Female, Height 171.1 cm ± 11.0 cm) wore an externally mounted canturio™te implant or a CanarE wearable while performing multiple trials of a Level Walking test, a Stair Climb test, and an Active/Passive Prone Range of Motion (ROM) test. The canturio™te and CanarE wearable were affixed medially on the proximal aspect of the tibia to minimize relative motion of the device with respect to the test subject’s tibia during activity. A contact tachometer was used to measure walking speed during the Level Walking test; a stopwatch was used to time the step-over-step and step-to-step Stair Climb Test, and a motion capture system was used to track and calculate ROM during the Prone ROM tests. The canturio™te or CanarE for each test was used with independent measurement data for comparative analysis. Statistical analysis was based on a procedure for two one-sided tests (TOST) using the t-distribution method. The following calculated gait parameters were assessed:
- Level Walking Test: Average Walking Speed, Cadence, Stride Length, Functional Knee ROM, and Tibial ROM
- Stair Climb Test: Stairs per Minute
- Active/Passive Prone ROM Test: ROM
Results: Based on a clinical equivalence margin of 0.32 m/sec [Foucher et al. 2016] for average walking speed, 18 stairs/min [Benaim et al. 2019] for average stairs per minute, and 14.6 deg [Austin et al. 2008] for ROM, the independent measures of walking speed, stairs per minute, and ROM all demonstrated P-values less than 0.001 for all level walking speeds, ascending and descending stairs with step-over-step and step-to-step gait patterns, and Active/Passive ROM for both the canturio™te implant and CanarE wearable (Table 1).
Conclusion: Statistical equivalence agreement was demonstrated between the canturio™te and CanarE as compared to the gold standard measurement systems used in standard of care clinical gait assessment. Based on these results, the canturio™te and CanarE wearable can provide accurate and easily accessible gait analytics when utilized in different mobility tests.
Assessing patient functional performance and gait parameters following total knee replacement allows clinicians to monitor recovery progress, identify potential complications, and tailor rehabilitation strategies, ultimately enhancing patient mobility and quality of life. By evaluating gait, therapists can detect abnormalities, measure biomechanical changes, and track improvements, thus contributing to more personalized care, a successful post-operative outcome, and reducing the risk of future injuries.
Figures
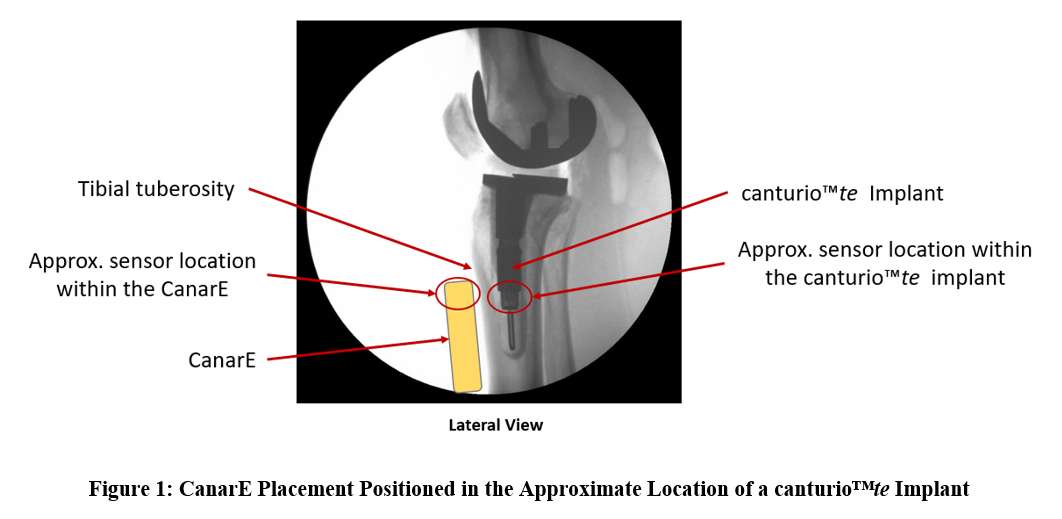
Figure 1
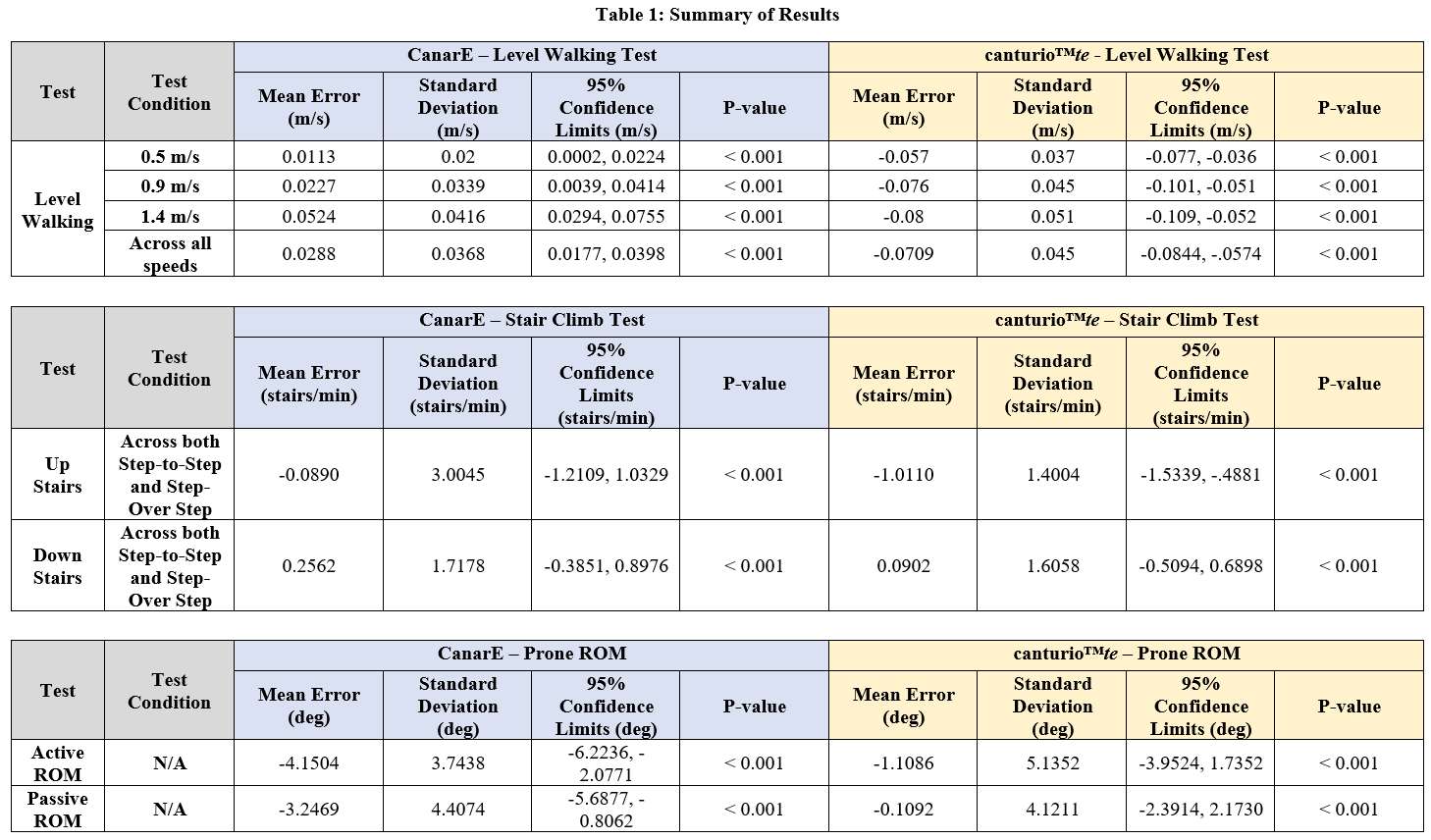
Figure 2#8559
Wearable Sensor Data as a Digital Biomarker for Patients Requiring a Manipulation After TKA: a Novel Concept Illustrated Using a Prospective Pilot Study
*Matthias Verstraete - Stryker - Fort Lauderdale, USA
Ricardo Antunes - Stryker - Glasgow, GB
Emily Hampp - Stryker - Mahwah, USA
Paul Jacob - Oklahoma Joint Reconstruction Institute - Oklahoma City, USA
Robert Marchand - Ortho Rhode Island - South County, USA
*Email: matthias.verstraete@stryker.com
Abstract
Arthrofibrosis and associated stiffness is a common complication after knee arthroplasty surgery. This complication is commonly treated using a manipulation under anesthesia, though conservative physiotherapy options are also indicated in the early post-operative phase. This paper explores the opportunity of using digital biomarkers extracted from knee-worn wearable sensors to identify patients developing stiffness.
A total of 101 primary knee patients were prospectively enrolled in a clinical study aiming to quantitatively assess the post-operative recovery. From this cohort, two patients were identified by the leading surgeons to be in need for a manipulation. After completion of the clinical data collection, the ability of various digital biomarkers in identifying the patients in need for a manipulation was assessed.
Our study indicates that patients’ daily range of motion was a significant predictor for patients requiring a manipulation. This statistically significant effect was observed starting two weeks after surgery. The other digital biomarkers evaluated in the study, such as cadence and number of hourly steps, were not correlated with the patients need for a manipulation.
This study thus suggests that patients in need for a manipulation can be identified early on in their post?operative journey when using wearable sensor technology to monitor patients’ daily activities. This study furthermore indicates the benefit of clearly defined clinically relevant metrics when developing these remote monitoring solutions, though patient compliance is recognized as a challenge when implementing these technologies in collecting real world data.
Keywords: knee arthroplasty, remote monitoring, complications, stiffness, wearables, digital health
Introduction
Arthrofibrosis is a common complication after total knee arthroplasty (TKA), reflected by stiffness that can be debilitating to patients causing pain, fatigue, abnormal gait, difficulty rising from chairs and difficulty with daily activities. The prevalence of stiffness has been reported between 4 and 16% after primary TKA [1-5]. Todays’ preferred treatment for stiffness following total knee arthroplasty is to perform a manipulation under anesthesia (MUA). Stiffness can also be treated conservatively, while revision arthroplasty is be indicated for some patients [5-7]. Literature indicates that an early treatment and identification is beneficial and preferred by surgeons, as improved outcomes have been reported when treated in the first twelve weeks [5,8-10]. The current identification of patients suffering from stiffness is however mainly based on physician assessment during the post-operative follow?up visits that take place 6 to 12 weeks post-operatively.
With the advance of remote monitoring tools, wearable sensors are now available that facilitate the remote tracking of patients’ functional recovery after surgery. Today, various types of sensors are available commercially for clinicians and patients to that extent. Some focus on generic monitoring of patients’ recovery (e.g. activity tracking watches or wrist bands) while others focus on the specific pathology (e.g. knee-worn sensors that track the patients’ range of motion) [11-16].
This paper evaluates the potential of using knee-worn wearable sensor data to identify patients at risks for complications following total knee surgery. More specifically, it was hypothesized that the functional data collected using a knee-worn sensor could serve as a digital biomarker to help identify patients at risk of needing a manipulation under anesthesia.
Methods
During a prospective study, a series of 101 primary total knee patients were recruited at two centers under IRB. Following consent, the patients engaged in the use of a knee-worn wearable sensor accompanied with a mobile app installed on their smartphone (MotionSense, Stryker, United States) [13]. Patients with known skin conditions or no smartphone were excluded from the study. The wearable sensors are attached by the patients on the lateral aspect of their operated knee in the morning; the first sensor is located a hand-width above the knee and the second sensor about the same distance below the knee. In the evening, the patients take off the sensors such that they can be recharged overnight. The wearable sensors connect through Bluetooth to the patients’ phone, where a dedicated mobile application provides the patient with the quantitative insights in their recovery as well as a daily task board that can consist of physiotherapy exercises, the collection of pain scores and patient reported questionnaires and a remote gait analysis. While the sensors are attached to the patients’ legs throughout the day, patients’ activities of daily living are monitored (ADLs). This is reflected by the patients’ knee active time, functional range of motion and step count. The former is defined as the amount of time the patients are moving their knee more than 0.1 degree per second relative to the number of hours the patients are wearing the sensors for the day. Analogous, the reported number of steps is normalized by the number of hours the patient is using the sensors. Both the data from the daily tasks and ADLs are uploaded to a HIPAA-compliant cloud database at regular time intervals, allowing clinicians to remotely monitor the patients’ functional recovery through an online dashboard.
Data analysis was completed using custom Python (v3.9.0) scripts to characterize the various digital biomarkers during a) Remote Gait Analysis in terms of Range of Motion (ROM) (delta between maximum and minimum flexion) and cadence (steps/minute) and b) Activities of Daily Living (ADL) in terms of ROM (delta between maximum and minimum flexion), steps (steps/hour), and knee active time (% worn time). Aggregate, bi-weekly values were determined for each metric up to 12 weeks postoperatively and further detailed analysis were performed at the 2, 6 and 12 week interval reflecting time points where patients would traditionally return to the clinic for a follow-up visit. These detailed analyses included a statistical evaluation of the MUA patients’ digital biomarkers compared to the remainder of the population using a linear mixed effects model (LME) run using the Python statsmodels package (v0.13.5). The LME fitted a random intercept allowing for patient-to-patient variability while accounting for the time relative to surgery and clinically identified need for an MUA as co-variables. Patients that underwent a known MUA as reported by the lead surgeons were separated from the other, well-recovering patients. Statistical significance was defined as a p-value below 0.05.
Results
Compliance The patients had a mean age of 66.6 years (49 to 79 year) and average BMI of 31.0 (23.6 to 41.8). When patients used MotionSense on a given day, they were wearing the sensors for over ten hours per day (median [Q1:Q3]: 10.3 hours [9.9:10.7]). Looking across their episode of care patients started using MotionSense two days after surgery and wore the MotionSense sensors for 67.5 days (see Table 1).
Complications From the 101 patients enrolled in the study, two patients (referred to as MUA1 and MUA2) underwent a manipulation under anesthesia (prevalence of 1.98%). In addition, one patient reported an infection and was excluded from the analyses presented in the remainder of this paper.
Remote Gait Analysis Patients’ compliance with the remote gait analysis was lower than patients’ compliance with wearing the sensors during daily activities, resulting in some weeks with missing data. Looking at the available data obtained from remote gait analysis, the patients’ functional range of motion during walking indicated both patients in the bottom 15% of the population up to six weeks after surgery (range 0.02 to 0.15, Table 2). Statistically significant differences were only observed for the later time intervals, no statistical difference was observed two weeks after surgery (Table 3). For patients requiring an MUA, cadence was generally lower (range 0.04 to 0.52, Figure 1) as compared to non-MUA patients, albeit differences between groups were non-significant at all time intervals (Table 3).
Activities of Daily Living: During daily activities, the patients’ range of motion, knee active time and steps per hour was measured using the wearable sensors. The daily range of motion consistently showed both patients were at the lower end of the population (range 0.01 to 0.09, Table 2). The average daily steps in each hour did not show a clear differentiation between the MUA and non-MUA patients (range 0.05 to 0.35, Table 2). The same applies to the knee active time (range 0.01 to 0.62, Table 2). In line with these observations, using the LME model, statistically significant differences between the MUA and non-MUA cohort were identified at all time intervals for the daily range of motion as measured using the wearable sensors. The hourly step count and active time were not significantly different between the MUA and non?MUA group at all time intervals (Table 3).
Discussion
During a prospective study, a series of 101 primary total knee patients were instructed to use a knee-worn wearable sensor that allows assessing a patients’ range of motion, daily activity, and gait characteristics remotely. These key metrics served as digital biomarkers and were compared between patients requiring an MUA and those not requiring an MUA as indicated by the expert-opinion of the senior surgeons and after data collection was completed.
The use of wearable sensors allows establishing a quantitative baseline for patient recovery after knee replacement surgery, thereby providing an important reference frame to (remotely) assess patients’ post?operative recovery. In our pilot study, two patients underwent a manipulation after identification by the surgeon during in-clinic follow-up visits. The observed MUA prevalence in our study is therefore in line with published literature [17,18]. In the early post-operative phase, both patients were trending at the bottom of the cohort when looking at their range of motion (both during ADLs and remote gait analysis). This statistically significant effect was already observed two weeks after surgery when looking at the functional range of motion passively collected while the patients were wearing the sensor. As such, the functional range of motion measured with a knee-worn wearable sensor could be seen as a digital biomarker with the potential to characterize the patients’ risk for a manipulation under anesthesia. No statistically significant differences were observed using other functional metrics (such as patients’ walking cadence, step count or knee active time). Since it is well-understood that achieving a functional range of motion in the early post?operative phase is indicative for good recovery and improved patient satisfaction [19], this research suggests that clinically relevant measures such as range of motion present a more sensitive digital biomarker for identifying patients in need for an MUA compared to metrics which are less directly linked to the clinical decision-making such as the patients’ cadence when walking or hourly number of steps. This supports the use of a knee-worn, joint-specific wearable sensor over more generic activity trackers that are not able to measure the patients’ knee flexion on a regular basis.
Since the current study flagged the patients with developing stiffness as early as two weeks after surgery, this also holds the potential to engage these patients in a dedicated physiotherapy program early on. Such programs have shown to be effective in reducing the need for a manipulation [20-22]. In turn, significant cost savings could be anticipated from the insights gained from wearable sensor data. Indeed, over 25% of the 90-day readmissions after TKA are linked to stiffness [23] and patients requiring a manipulation are more likely to need revision surgery [24]. Each revision surgery is thereby associated with a total cost of 49 360 USD, including consumption of significant hospital resources and a median length of stay of five days [25]. For those patients where such modified physiotherapy programs would however not be effective in preventing an MUA, the early identification using the presented digital biomarkers still holds the potential to create a more effective intervention with superior clinical outcomes. Indeed, numerous studies have reported on the beneficial effects of an early execution of an MUA (e.g. first 12 weeks [26]). We can therefore conclude that the presented sensor technology, in combination with the remote monitoring of the patients, holds the potential to give surgeons more insights in their clinical decision-making during the regular post-operative follow-up visits. Rather than relying on a single, in-clinic datapoint, gathered during a post-operative follow-up visit, the use of these knee-worn sensors can now allow the surgeons to review a patients’ individual progression over the last weeks during these follow-up visits, in turn accelerating their clinical decision-making. As a result, these knee-worn wearable sensors provide a more patient-specific treatment and allow to manage patients by exception rather than providing the same prescriptive and standardized care-pathway to all patients undergoing total knee arthroplasty.
This study however indicated potential challenges with patient compliance when deploying wearable sensor technology. During the first two months after surgery, patients complied well with the prescribed use of their sensors allowing for monitoring their daily activities. While our metrics have now shown that this window should be sufficient for assessing the complication of interest, it is recognized that the patient compliance in the third month after surgery was more challenging. It is hypothesized that this is driven by a good number of patients achieving their functional recovery targets (i.e. at or above their pre-operative levels). However, to transform the post?operative care delivery and achieve a patient-specific approach where patients are managed by exception, clinicians will need to rely on solutions that provide a high level of patient compliance. To achieve this, the problem of patient compliance shall be addressed from various angles. First, patient engagement shall be addressed by engaging design and user experience experts in the development process to drive patient engagement in the adoption of digital technologies. Second, this also opens the door for the use of smart implants pending their ability to capture clinically relevant and interpretable metrics with sufficient accuracy.
This pilot study has some important limitations. First, the study is underpowered to allow for the widespread use and development of predictive algorithms towards the identification of patients at risk for a manipulation. Indeed, this paper intended to only describe a limited case series of patients requiring a manipulation and the associated potential of leveraging wearable data as a digital biomarker during remote monitoring, larger series with appropriate identification of patients with complications will be needed to move towards predictive algorithms. This study is further limited in that it does not look at the efficacy of the manipulation, instead the study was primarily designed to monitor patients in the early post-operative phase focusing on the first 90 days after primary total knee surgery and such data analyses was thus not possible. A third limitation of this study is that it only looked at one commonly reported complication after total knee surgery. Other complications were not evaluated and will require a more comprehensive dataset to understand the potential of this sensor technology.
Conclusion
In conclusion, this study identified the patients’ remotely tracked range of motion as a potential digital biomarker, allowing to assess a patients’ risk for a manipulation under anesthesia in the early post-operative phase. The pilot data presented in this study suggests that patients at risk can already be identified two weeks after surgery. Patient compliance with these technologies facilitating remote monitoring can however be a challenge, requiring dedicated attention during the development process.
References
[1] Kim J, Nelson CL, Lotke PA. Stiffness after total knee arthroplasty. Prevalence of the complication and outcomes of revision. J Bone Joint Surg Am. 2004 Jul;86(7):1479-84. PMID: 15252096.
[2] Yercan HS, Sugun TS, Bussiere C, Ait Si Selmi T, Davies A, Neyret P. Stiffness after total knee arthroplasty: prevalence, management and outcomes. Knee. 2006 Mar;13(2):111-7. doi: 10.1016/j.knee.2005.10.001. Epub 2006 Feb 20. PMID: 16490357.
[3] Erkan S, Yercan HS, Okcu G, Ozalp RT. Total diz artroplastisi sonras? diz sertli?ine neden olan faktörler [Factors causing stiff knee after total knee arthroplasty]. Eklem Hastalik Cerrahisi. 2011;22(1):16-21. Turkish. PMID: 21417981.
[4] Sharkey PF, Lichstein PM, Shen C, Tokarski AT, Parvizi J. Why are total knee arthroplasties failing today--has anything changed after 10 years? J Arthroplasty. 2014 Sep;29(9):1774-8. doi: 10.1016/j.arth.2013.07.024. Epub 2014 Jul 5. PMID: 25007726.
[5] Sunil Kumar KH, Mamarelis G, Pettit M, Khanduja V. Management of Stiffness following Total Knee Arthroplasty: International Survey on Surgeon Preferences. SICOT J. 2021;7:30. doi: 10.1051/sicotj/2021008. Epub 2021 Apr 30. PMID: 33929314; PMCID: PMC8086424.\
[6] Fitzsimmons SE, Vazquez EA, Bronson MJ. How to treat the stiff total knee arthroplasty?: a systematic review. Clin Orthop Relat Res. 2010 Apr;468(4):1096-106. doi: 10.1007/s11999-010-1230-y. Epub 2010 Jan 20. PMID: 20087698; PMCID: PMC2835585.
[7] Rockov ZA, Byrne CT, Rezzadeh KT, Durst CR, Spitzer AI, Paiement GD, Penenberg BL, Rajaee SS. Revision total knee arthroplasty for arthrofibrosis improves range of motion. Knee Surg Sports Traumatol Arthrosc. 2023 May;31(5):1859-1864. doi: 10.1007/s00167-023-07353-8. Epub 2023 Feb 21. PMID: 36809514; PMCID: PMC10090018.
[8] Rahardja, R., Mehmood, A., Coleman, B. et al. Early manipulation under anaesthesia for stiffness following total knee arthroplasty is associated with a greater gain in knee flexion. Knee Surg Sports Traumatol Arthrosc 31, 979–985 (2023). https://doi.org/10.1007/s00167-022-07128-7
[9] Namba RS, Inacio M. Early and late manipulation improve flexion after total knee arthroplasty. J Arthroplasty. 2007 Sep;22(6 Suppl 2):58-61. doi: 10.1016/j.arth.2007.02.010. Epub 2007 Jul 26. PMID: 17823017.
[10] Issa K, Banerjee S, Kester MA, Khanuja HS, Delanois RE, Mont MA. The effect of timing of manipulation under anesthesia to improve range of motion and functional outcomes following total knee arthroplasty. J Bone Joint Surg Am. 2014 Aug 20;96(16):1349-57. doi: 10.2106/JBJS.M.00899. PMID: 25143495.
[11] Bolam SM, Batinica B, Yeung TC, Weaver S, Cantamessa A, Vanderboor TC, Yeung S, Munro JT, Fernandez JW, Besier TF, Monk AP. Remote Patient Monitoring with Wearable Sensors Following Knee Arthroplasty. Sensors (Basel). 2021 Jul 29;21(15):5143. doi: 10.3390/s21155143. PMID: 34372377; PMCID: PMC8347411.
[12] Patterson JT, Wu HH, Chung CC, Bendich I, Barry JJ, Bini SA. Wearable activity sensors and early pain after total joint arthroplasty. Arthroplast Today. 2020 Mar 6;6(1):68-70. doi: 10.1016/j.artd.2019.12.006. PMID: 32211478; PMCID: PMC7083735.
[13] Antunes R, Jacob P, Meyer A, Conditt MA, Roche MW, Verstraete MA. Accuracy of Measuring Knee Flexion after TKA through Wearable IMU Sensors. J Funct Morphol Kinesiol. 2021 Jul 5;6(3):60. doi: 10.3390/jfmk6030060. PMID: 34287303; PMCID: PMC8293382.
[14] Constantinescu D, Pavlis W, Rizzo M, Vanden Berge D, Barnhill S, Hernandez VH. The role of commercially available smartphone apps and wearable devices in monitoring patients after total knee arthroplasty: a systematic review. EFORT Open Rev. 2022 Jul 5;7(7):481-490. doi: 10.1530/EOR-21-0115. PMID: 35900191; PMCID: PMC9297050.
[15] Crawford DA, Duwelius PJ, Sneller MA, Morris MJ, Hurst JM, Berend KR, Lombardi AV. 2021 Mark Coventry Award: Use of a smartphone-based care platform after primary partial and total knee arthroplasty: a prospective randomized controlled trial. Bone Joint J. 2021 Jun;103-B(6 Supple A):3-12. doi: 10.1302/0301-620X.103B6.BJJ-2020-2352.R1. PMID: 34053272.
[16] Bahadori S, Immins T, Wainwright TW. A review of wearable motion tracking systems used in rehabilitation following hip and knee replacement. J Rehabil Assist Technol Eng. 2018 Jun 18;5:2055668318771816. doi: 10.1177/2055668318771816. PMID: 31191937; PMCID: PMC6453074.
[17] Rubinstein RA Jr, DeHaan A. The incidence and results of manipulation after primary total knee arthroplasty. Knee. 2010 Jan;17(1):29-32. doi: 10.1016/j.knee.2009.07.001. Epub 2009 Aug 6. PMID: 19664928.
[18] Thorsteinsson H, Hedström M, Robertsson O, Lundin N, W-Dahl A. Manipulation under anesthesia after primary knee arthroplasty in Sweden: incidence, patient characteristics and risk of revision. Acta Orthop. 2019 Oct;90(5):484-488. doi: 10.1080/17453674.2019.1637177. Epub 2019 Jul 4. PMID: 31269851; PMCID: PMC6746267.
[19] Van Onsem S, Verstraete M, Dhont S, Zwaenepoel B, Van Der Straeten C, Victor J. Improved walking distance and range of motion predict patient satisfaction after TKA. Knee Surg Sports Traumatol Arthrosc. 2018 Nov;26(11):3272-3279. doi: 10.1007/s00167-018-4856-z. Epub 2018 Feb 8. PMID: 29423545
[20] Jette DU, Hunter SJ, Burkett L, Langham B, Logerstedt DS, Piuzzi NS, Poirier NM, Radach LJL, Ritter JE, Scalzitti DA, Stevens-Lapsley JE, Tompkins J, Zeni J Jr; American Physical Therapy Association. Physical Therapist Management of Total Knee Arthroplasty. Phys Ther. 2020 Aug 31;100(9):1603-1631. doi: 10.1093/ptj/pzaa099. PMID: 32542403; PMCID: PMC7462050.
[21] Manrique J, Gomez MM, Parvizi J. Stiffness after total knee arthroplasty. J Knee Surg. 2015 Apr;28(2):119-26. doi: 10.1055/s-0034-1396079. Epub 2014 Dec 16. PMID: 25513992.
[22] Rodríguez-Merchán EC. The stiff total knee arthroplasty: causes, treatment modalities and results. EFORT Open Rev. 2019 Oct 7;4(10):602-610. doi: 10.1302/2058-5241.4.180105. PMID: 31754466; PMCID: PMC6836076.
[23] Schairer WW, Vail TP, Bozic KJ. What are the rates and causes of hospital readmission after total knee arthroplasty? Clin Orthop Relat Res. 2014 Jan;472(1):181-7. doi: 10.1007/s11999-013-3030-7. PMID: 23645339; PMCID: PMC3889434.
[24] Parkulo TD, Likine EF, Ong KL, Watson HN, Smith LS, Malkani AL. Manipulation Following Primary Total Knee Arthroplasty is Associated With Increased Rates of Infection and Revision. J Arthroplasty. 2023 Mar;38(3):567-572.e1. doi: 10.1016/j.arth.2022.09.027. Epub 2022 Sep 30. PMID: 36191695.
[25] Bhandari M, Smith J, Miller LE, Block JE. Clinical and economic burden of revision knee arthroplasty. Clin Med Insights Arthritis Musculoskelet Disord. 2012;5:89-94. doi: 10.4137/CMAMD.S10859. Epub 2012 Dec 5. PMID: 23239930; PMCID: PMC3520180.
[26] Sala J, Jaroma A, Sund R, Huopio J, Kröger H, Sirola J. Manipulation under anesthesia after total knee arthroplasty: a retrospective study of 145 patients. Acta Orthop. 2022 Jun 21;93:583-587. doi: 10.2340/17453674.2022.3167. PMID: 35727106; PMCID: PMC9214639.
Figures
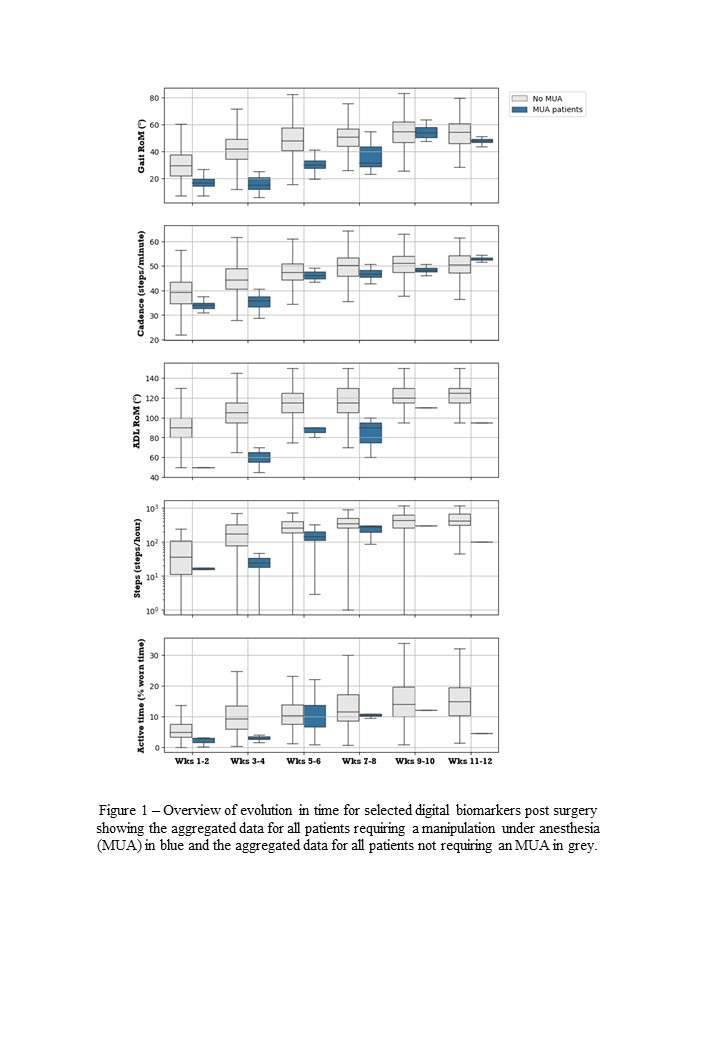
Figure 1

Figure 2
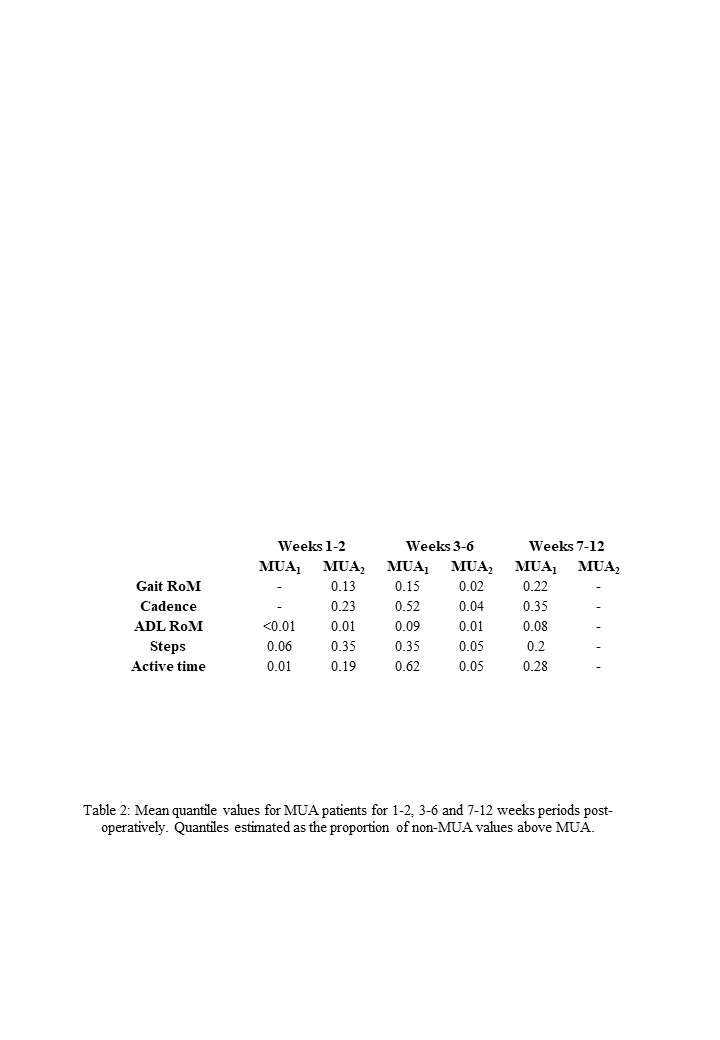
Figure 3
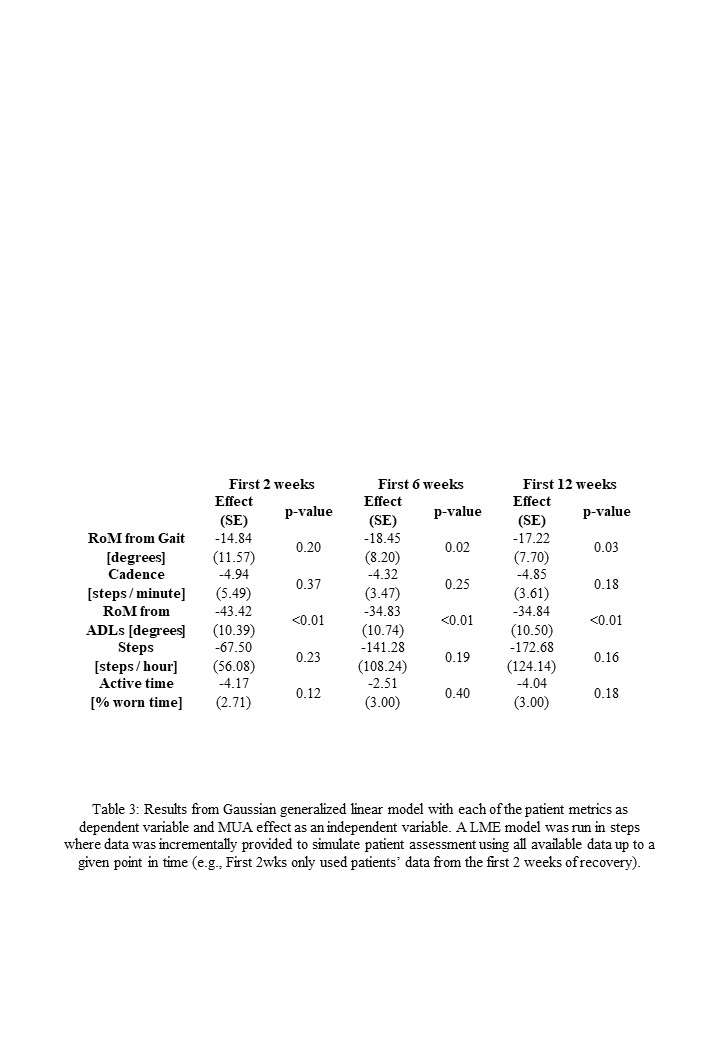
Figure 4#8353
Effects of Real-Time Biofeedback Using Instrumented Insoles on Recovery After Total Knee Arthroplasty
Victor Cheuy - University of Colorado San Francisco
*Jennifer Stevens-Lapsley - University of Colorado - Denver, United States of America
Cory Christiansen - University of Colorado - AURORA, USA
Meghan Connors - University of Colorado - AURORA, USA
Amy Peters - University of Colorado - AURORA, USA
Ryan Koonce - University of Colorado - AURORA, USA
Craig Hogan - University of Colorado - AURORA, USA
Jennifer Stevens-Lapsley - University of Colorado - Aurora, USA
*Email: jennifer.stevens-lapsley@cuanschutz.edu
Introduction: This work is part of a parent clinical trial to determine if physical rehabilitation that focuses on movement training restores healthy movement patterns after TKA and reduces the risk of osteoarthritis (OA) progression in the contralateral knee. The movement pattern training program (MOVE) involves task training that is augmented with real-time biofeedback delivered via instrumented insoles.
Methods: 138 participants were enrolled in the parent randomized controlled trial (NCT03325062). Participants were allocated to one of two dose-equivalent treatment groups: standard rehabilitation plus movement training (MOVE) or standard rehabilitation without movement training (CONTROL). Movement training promoted between-limb symmetry and surgical knee loading during activity-based exercises. Movement training strategies included real-time biofeedback using in-shoe pressure sensors (Loadsol, Novel Electronics Inc) in both the clinic and home settings through an iOS device, and verbal, visual, and tactile cues from the physical therapist. While detailed study results will be presented on the instrumented insole data, results will not be available until June 2023. Instead, data on a pilot precursor group of patients (n=8; 63.3 ± 6.5 years) scheduled to undergo TKA for end-stage osteoarthritis (OA) are presented until clinical trial data are available. Patient enjoyment of the insoles was measured using the intrinsic motivation index (IMI). Weight-bearing asymmetry (WBA) and knee extension moment asymmetry (KEMA) ratios during standing, over-ground walking, and rising from a chair were quantified using an eight-camera motion analysis system with embedded force plates (ratio=surgical/non-surgical). Quadriceps strength asymmetry (QSA) ratios were assessed using an electromechanical dynamometer. Functional performance was assessed using the five times sit-to-stand test (FTSST) and six-minute walk (6MW) test. Descriptive statistics and paired t-tests were utilized to analyze changes over time.
Results: All pilot participants were able to use biofeedback both in clinic and at home and rated the insoles at a high level of enjoyment on the IMI (median=6.8 out of 7). WBA during bilateral stance, over ground walking, and rising from a chair returned to baseline by the end of intervention (all p>0.05) and subjects exhibited symmetrical weight-bearing by six months on all tasks (WBA ratio = 1.0, 0.99, 0.98 respectively). KEMA and QSA ratios recovered to baseline by the end of intervention (all p>0.05) and by six months QSA mean performance improved. 6MW and FTSST performance recovered to baseline levels by the end of intervention (both p>0.05) and by six months mean performance had improved beyond baseline. Results will be expanded upon to include clinical trial results for all participants.
Conclusion: Previous studies have found that patients consistently exhibit lower-limb movement asymmetry during dynamic tasks after TKA. However, subjects in the MOVE program demonstrated symmetrical weight-bearing on all tasks at 6 months. KEMA and QSA also improved to higher levels than typically reported. Recovery of functional performance was also enhanced with the MOVE intervention, as patients typically do not recover to baseline levels until 2-3 months after TKA. In summary, real time weight-bearing biofeedback training is feasible to implement into clinical practice and may lead to improved movement symmetry and functional recovery after TKA.
#8763
The Financial Impact of Patient Comorbidities on Total Hip Arthroplasty Procedures - a Matched Cohort Analysis of Patients With and Without High Comorbidity Burden
*Itay Ashkenazi - NYU Langone Health - New-York, United States of America
Jeremiah Thomas - NYU Langone Health - Scarsdale, United States of America
Jonathan Katzman - NYU Langone - New York, United States of America
Muhammad Haider - NYU Orthopedic Hospital - New York, USA
Morteza Meftah - NYU Hospital for Joint Diseases - New York, USA
Roy Davidovitch - NYU Langone Health - New York, USA
Ran Schwarzkopf - NYU Langone Medical Center Hospital for Joint Diseases - New York, USA
*Email: itay.ashkenazi@gmail.com
Introduction
The impact of increased patient comorbidities on the cost-effectiveness of total hip arthroplasty (THA) procedures is lacking. This study aimed to compare revenue, costs and short-term surgical outcomes between patients with and without a high comorbidity burden (HCB).
Methods
We retrospectively reviewed 14,949 patients who underwent an elective, unilateral THA between 2012 and 2021. Patients were stratified to HCB (Charlson comorbidity index [CCI]≥5 and American Society of Anesthesiology [ASA] scores of 3 or 4) and non-HCB groups, and were further 1:1 propensity matched based on baseline characteristics. Perioperative data, revenue, costs and contribution margins (CM) of the inpatient episode were compared between groups. 90-day readmissions and revisions were also compared between groups. Of the 11,717 patients who had available financial data (n=1,017 HCB, n=10,700 non-HCB), 1,914 patients were included in the final matched analyses (957 per group).
Results
Total (P<0.001) and direct (P<0.001) costs were significantly higher for HCB patients. Comparable revenue between cohorts (P=0.083) resulted in significantly decreased CM in the HCB patient group (P<0.001). The HCB patients were less likely to be discharged home (P<0.001) and had significantly higher 90-day readmission rates (P=0.049), specifically for non-orthopedic indications (P=0.002).
Conclusion
In our propensity-matched cohort, increased THA costs for HCB patients were not matched by increased revenue, resulting in decreased CM. Higher rates of non-home discharge and readmissions in the HCB population add to additional financial burden. This may affect the ability of hospitals to cover indirect costs, threatening access to care for HCB patients who require THA. Adjustments to the current reimbursement models should better account for the increased financial burden of HCB patients undergoing THA, to ensure access to care for all patient populations.
Figures
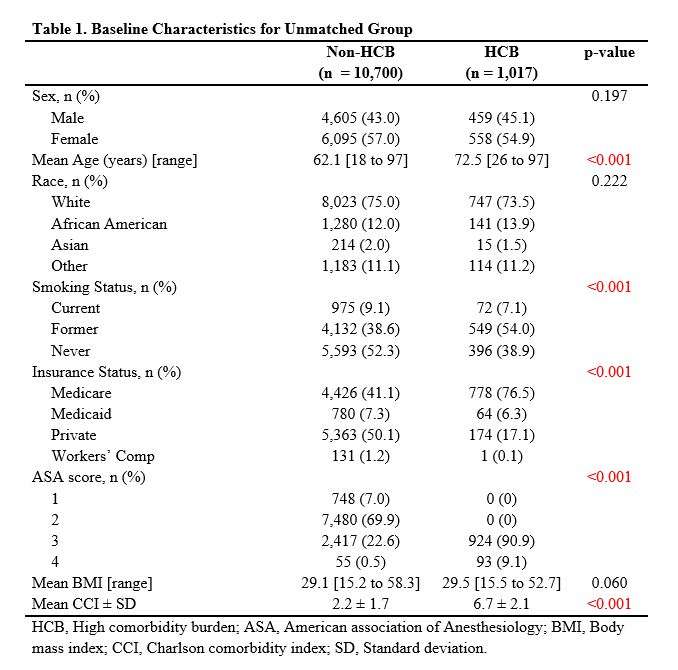
Figure 1
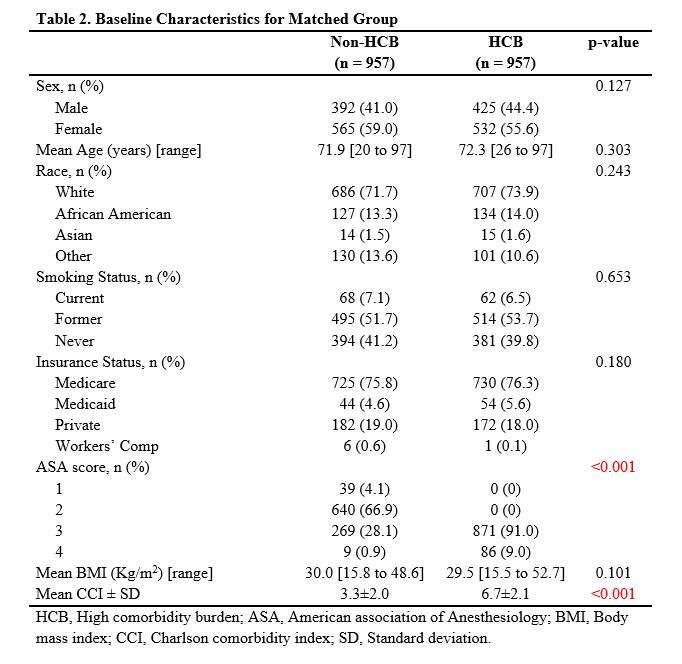
Figure 2
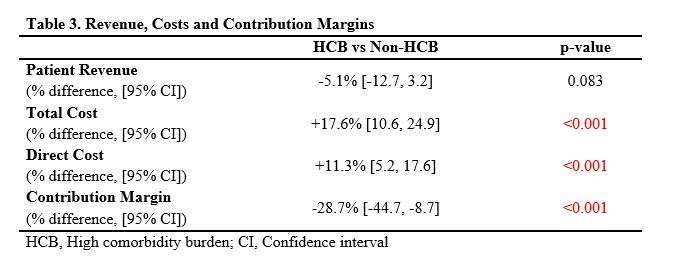
Figure 3
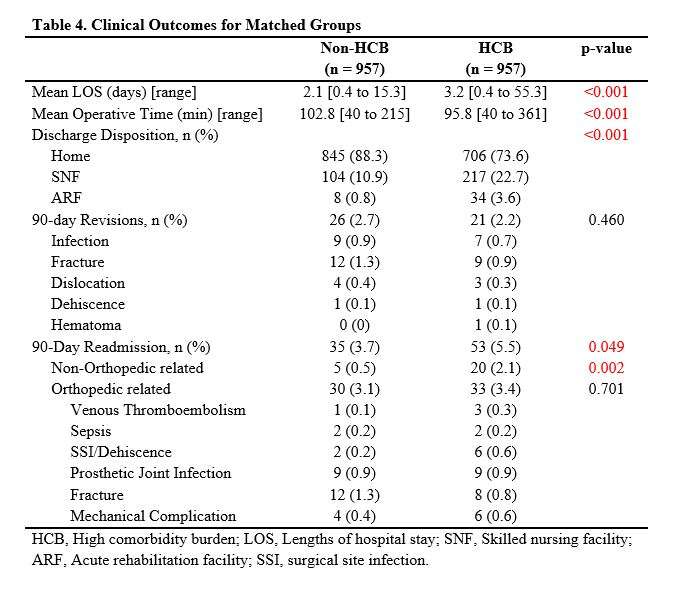
Figure 4#8787
A Decade of Risk Reduction Following Total Knee Arthroplasty? Consecutively Evaluating the Relative Risk of Patient Factors and Postoperative Complications From 2012 to 2022
*Kyle Lawrence - NYU Orthopedic Hospital - New York, USA
Itay Ashkenazi - NYU Orthopedic Hospital - New York, USA
Jonathan Katzman - NYU Langone - New York, United States of America
Sam Barzideh - NYU Langone - New York, USA
Claudette Lajam - NYU Hospital For Joint Diseases - New York, USA
Morteza Meftah - NYU Hospital for Joint Diseases - New York, USA
Ran Schwarzkopf - NYU Langone Medical Center Hospital for Joint Diseases - New York, USA
*Email: kyle.lawrence@nyulangone.org
BACKGROUND: Effect of perioperative risk optimization of complications among high-risk patients following total knee arthroplasty (TKA) have not been evaluated over time. We aimed to assess absolute and relative risk reductions (ARR, RRR) over the last decade of postoperative complications in patients with established risk factors.
METHODS: Overall, 20,130 primary TKAs from an academic center between 2012-2022 were analyzed. Changes in length of stay (LOS) and home discharge were assessed over time. Absolute risk (AR) of 90-day readmissions, emergency department (ED) visits, and two-year revisions (for patients with two-year follow-up) were assessed within each two-year interval for all patients and for patients with the following risk factors: age>70, body mass index (BMI)>40, smoking, and diabetes. We calculated ARR and RRR for each consecutive time interval relative to the initial interval.
RESULTS: Average LOS (4.3 to 1.6 days, P<0.001) and non-home discharge rates (53.0% to 5.7%, P<0.001) decreased throughout the study period, and this was consistent across risk factors. During the past decade, 90-day readmission risk reductions were observed in BMI>40 (RRR: 0.87, ARR: -0.16), diabetes (RRR: 0.31, ARR: -0.02) and age>70 (RRR: 0.39, ARR: -0.02). Risk of 90-day ED visits increased for all patients (AR: 0.024 to 0.049), and diabetes represented the risk factor with the highest increase in 90-day ED risk (AR: 0.010 to 0.060). Considering all patients, two-year revision risk remained consistent (RRR: 0.01, ARR: 0.00), however for age>70, BMI>40, smoking, and diabetes (ARR: 0.149, 0.138, 0.317, and 0.353, respectively), two-year revision risk decreased throughout the decade.
CONCLUSION: Decreased 90-day readmission and two-year revision risk in patients with established risk factors suggests improvement in the perioperative risk optimization of these patients over the last decade. Two-fold increase in ED utilization without subsequent increase in readmission, alongside decreased LOS and non-home discharge, suggests the need for improved patient education regarding expected postoperative recovery.
Figures
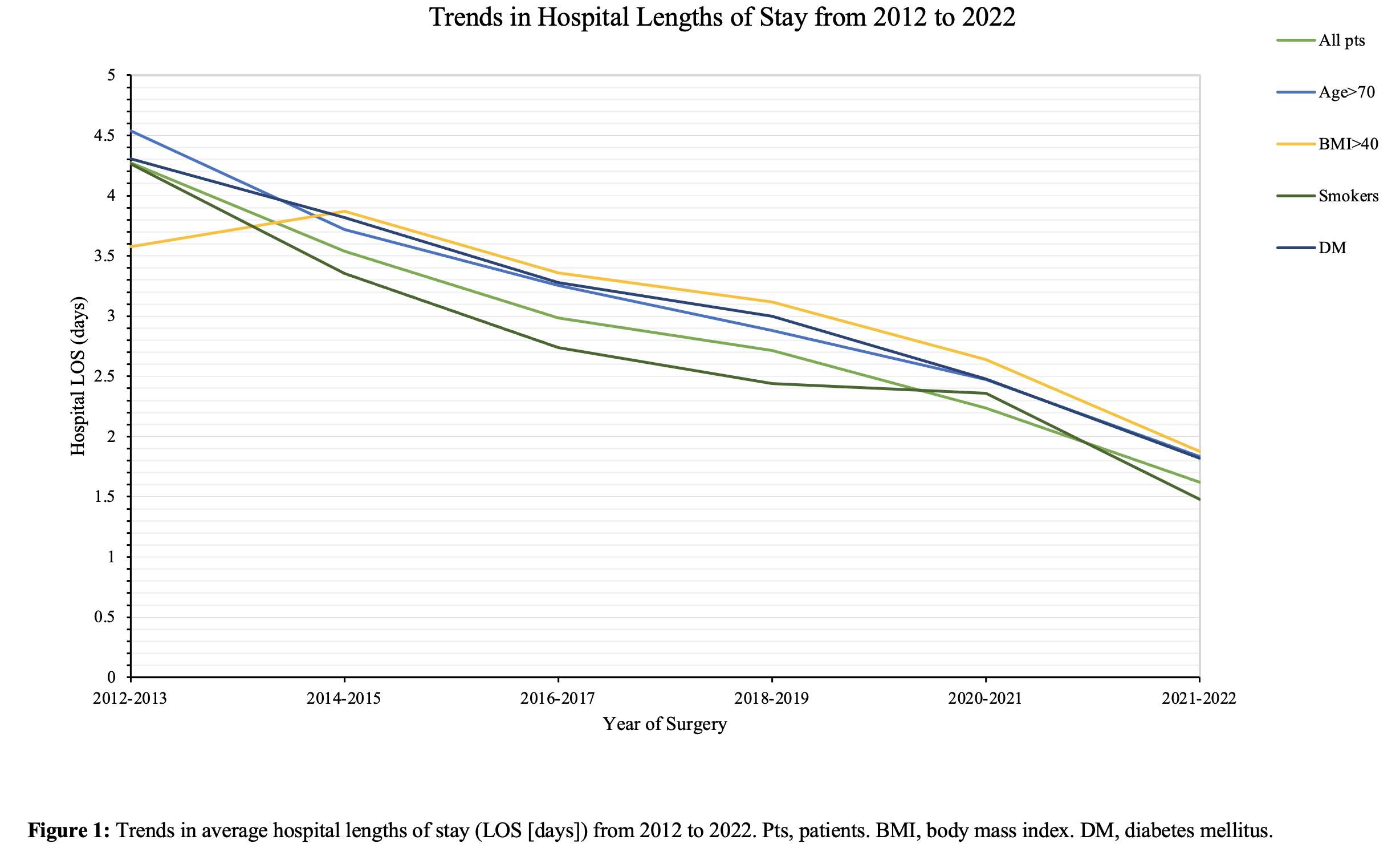
Figure 1
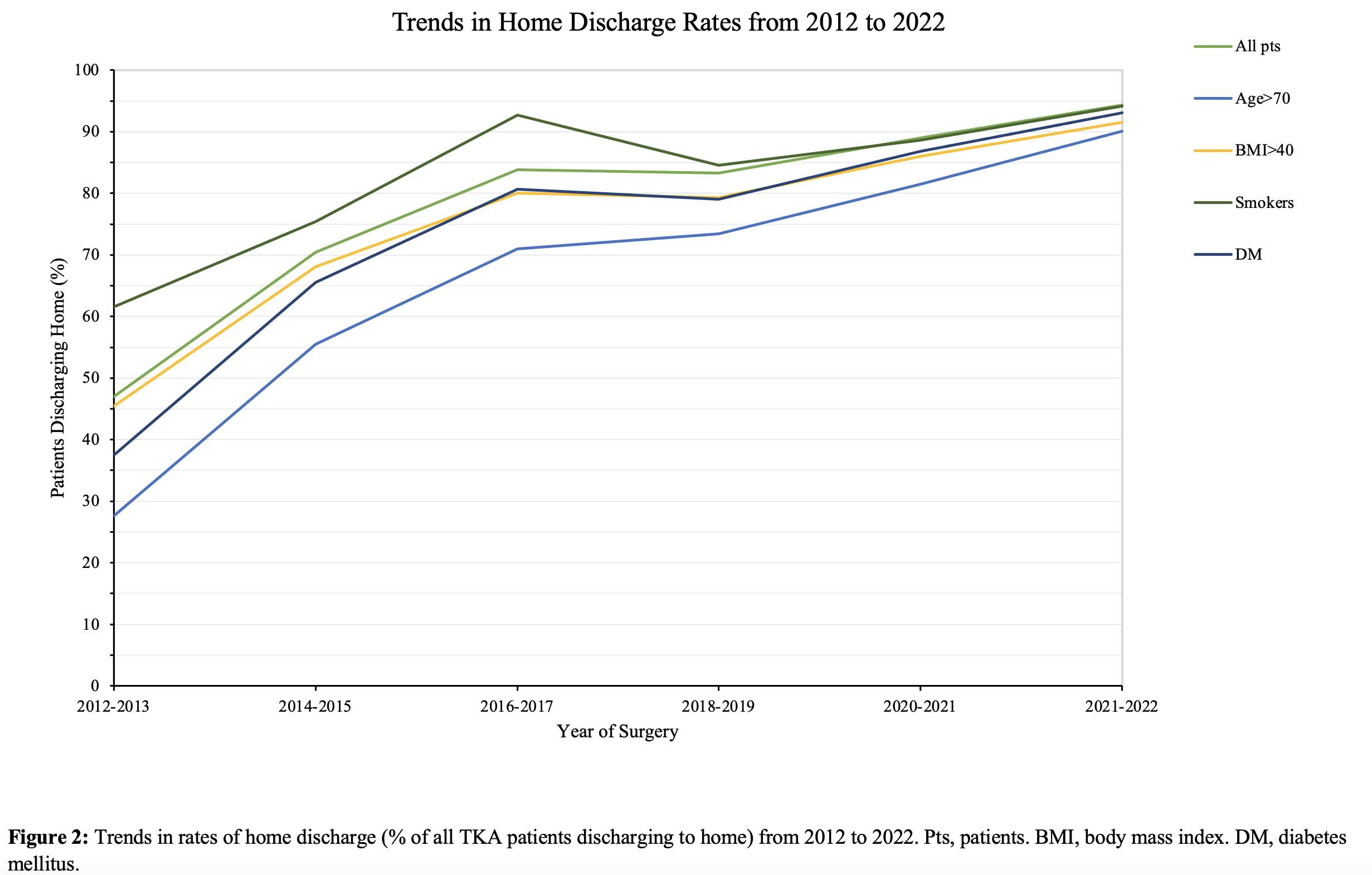
Figure 2
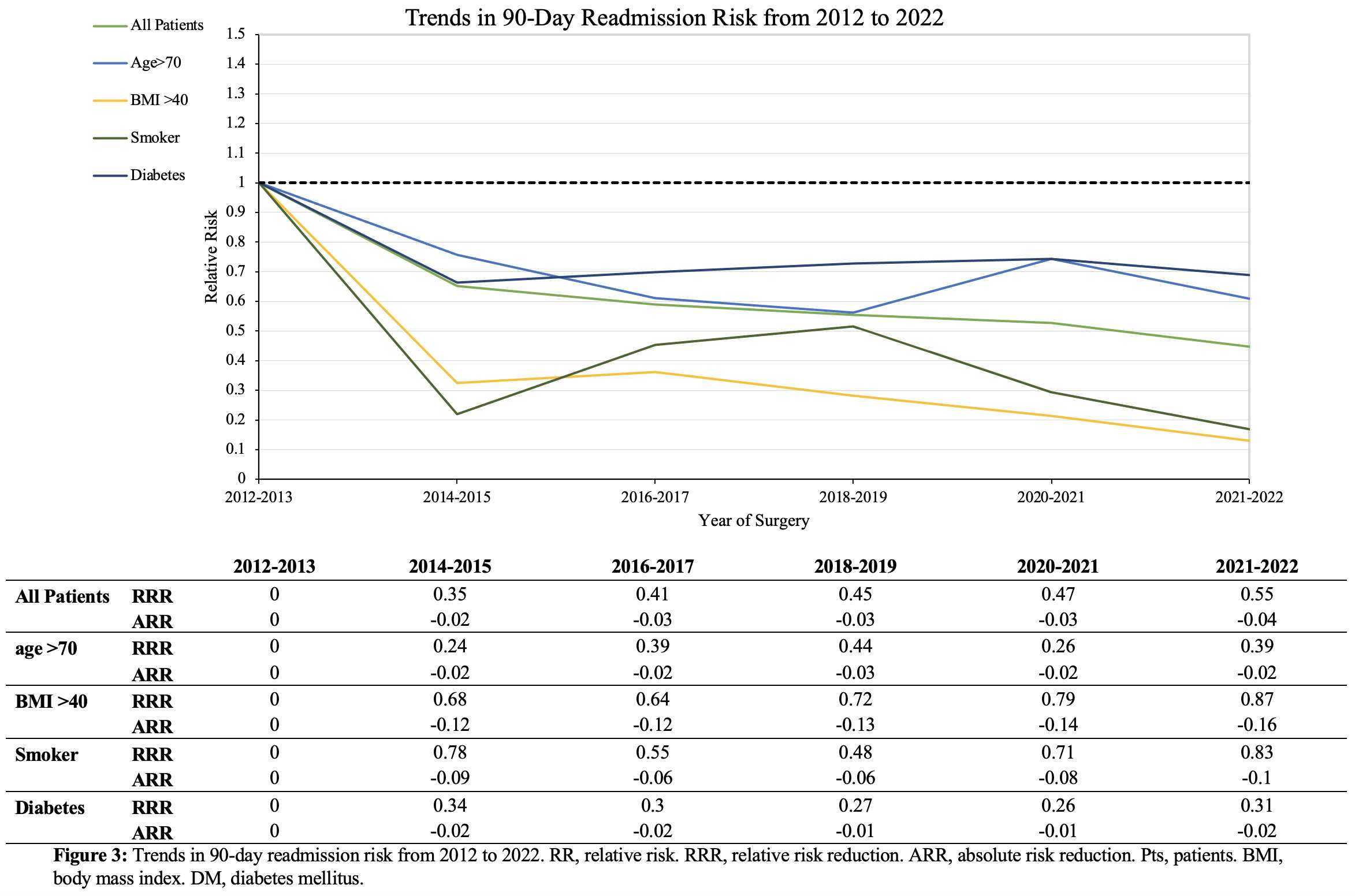
Figure 3
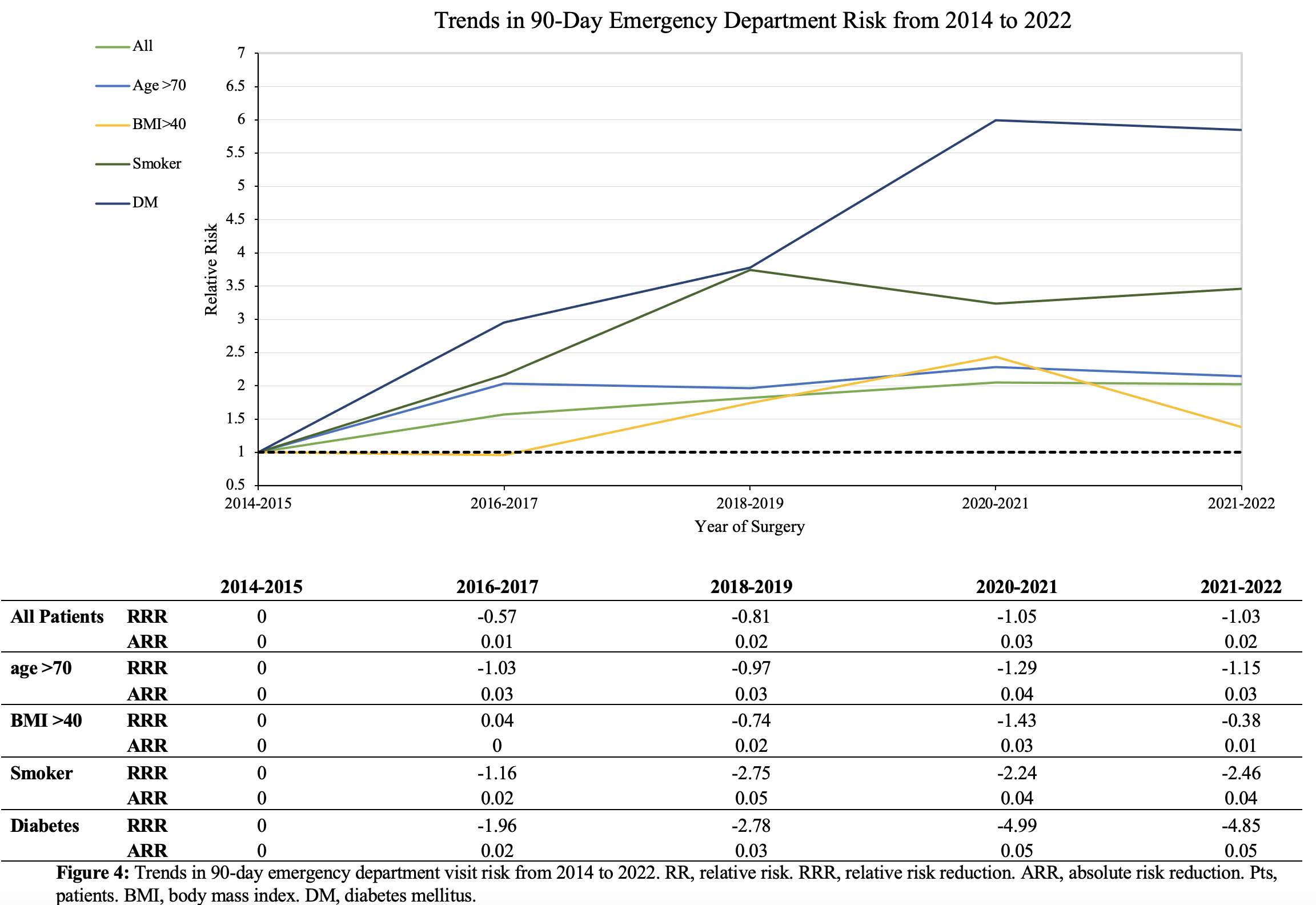
Figure 4
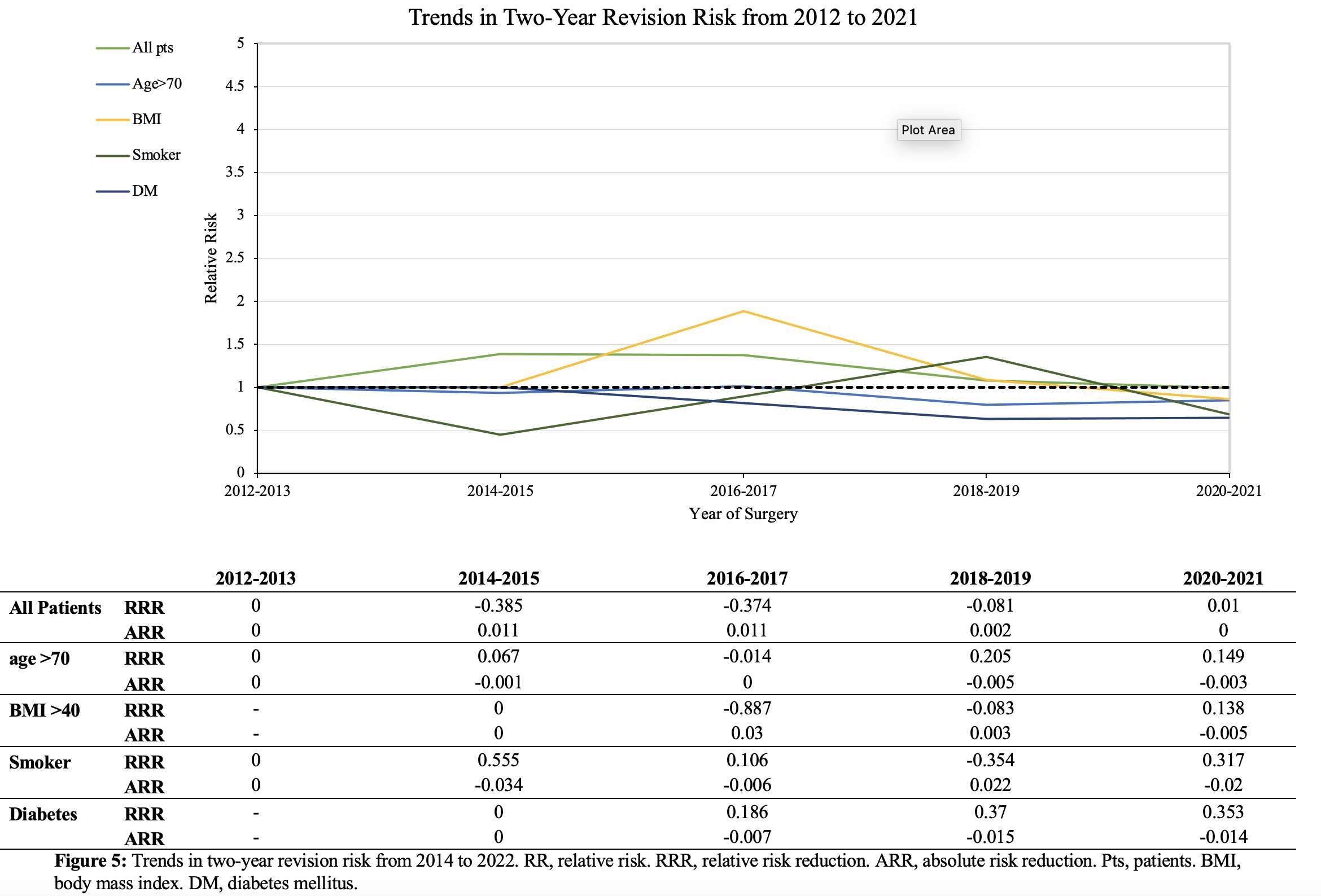
Figure 5#8104
Complication Rate Is Not Increased After Ambulatory Total Hip Arthroplasty in Comparison to Fast-Track Total Hip Arthroplasty. a Propensity-Matched Prospective Comparative Study.
*Jean-Yves Jenny - University Hospital Strasbourg - Illkirch, France
Aymard De Ladoucette - Clinique De l'Union - Saint Jean Cedex, France
*Email: Jean-yves.jenny@chru-strasbourg.fr
Introduction
Prolonged hospital stay is associated with higher patient morbidity and mortality, as well as high[adl1] costs for the healthcare system. Therefore, the last decade saw[adl2] a concerted effort to reduce length of stay, minimise cost and improve efficacy, which resulted in various fast-track (FT), also called enhanced recovery after surgery, protocols for total hip arthroplasty (THA). Current evidence suggest that FT is efficient compared to conventional pathways. However, concerns remain with regards to safety of fast-track and especially ambulatory procedures. The purpose of this study was to compare outcomes of propensity-matched patients that received FT THA in[adl3] ambulatory versus non-ambulatory settings, focussing on the occurrence of postoperative complications. The hypothesis was that 90-day postoperative complication rates of ambulatory FT THA would not be higher than after non-ambulatory FT THA.
Methods
This is a prospective study of consecutive patients that received FT THA at various rates of ambulatory and non-ambulatory surgery by 10 senior surgeons (10 centres). The decision between ambulatory and non-ambulatory surgery was made on a case-by-case basis depending on the surgeon and patient. All patients provided written informed consent for the [adl4] use and publication of their data, and the study was approved by the institutional review board. All patients were prospectively followed until 90 days after surgery. Postoperative complications, readmissions and reoperations were collected. The severity of the postoperative complications was assessed according to Clavien-Dindo. Patients completed Oxford Hip Score (OHS) at the latest follow-up. The primary outcome was the postoperative complication rate within the first 90 days following surgery.
To compare ambulatory versus non-ambulatory FT THA, a propensity score based on age, sex, body mass index (BMI), and American Society of Anaesthesiologists (ASA) score was developed using the “matchit” algorithm. The authors aimed for 1:2 (ambulatory:non-ambulatory) optimal propensity score matching (ratio, 1/2) without replacement, using logistic regression of the treatment on the covariates.
Results
Compared to non-ambulatory FT THA, patients scheduled for ambulatory FT THA had no significant differences in 90-day total postoperative complication rates (10.7% vs. 12.9%, p=n.s.). There was no difference in 90-day readmission rates and reoperation rates, in the severity of the postoperative complications and in the time of occurrence of the postoperative complications between the two groups. There was no significant difference in the mean 90-days OHS score between patients that has ambulatory (42.6±6.0 points) or non-ambulatory (42.3±6.0 points) FT THA (p=n.s.).
Conclusion
These findings support the hypothesis that 90-days postoperative complication rates are similar for ambulatory versus non-ambulatory FT THA, and refute fears of some surgeons who might be reluctant to perform ambulatory THA due to greater risk of early postoperative complications that could be more difficult to manage after discharge.
#8698
Surgical Risk Stratification by Using Machine Learning in Ambulatory Surgical Centers
*Amir Pourmoghaddam - Memorial Bone and Joint Research Foundation - Houston, USA
David Balderree - INOV8 Orthopedics - Houston, USA
Stefan Kreuzer - INOV8 Orthopedics - Houston, USA
*Email: amirpm@gmail.com
The number of outpatient surgical procedures have been growing over the past decade in Ambulatory Surgical Centers (ASCs). Previous studies indicated that patients receiving joint replacement surgeries in ASCs achieve comparable or superior infection rates, patient satisfaction rate, and functional recovery outcomes compared to the patients in hospitals (1, 2). In addition, ASCs offer cost-effectiveness by lowering overhead costs, reduced facility fees, and no overnight stay reducing hospital stays costs while maintaining quality of care (3). However, not all patients might be a good candidate to receive treatment in ASCs particularly patients with complex physiological background or those requiring specialized care. These patients might be better suited to be treated at hospitals. The selection of surgical facility should consider various patient factors, complexity of cases, and healthcare team recommendations. Therefore, surgical risk stratification has become a crucial step to minimize postoperative risks and can be an important process to assign patients to proper surgical site based on their risk factors profiles. This approach enables healthcare professionals to manage and mitigate risks effectively, ensuring patient safety and optimized outcomes.
The objective of this study is to analyze surgical risk stratification in cases performed at our surgical center. We utilized a machine learning approach to develop a process that aids in selecting the optimal surgical location, ASC or Hospital. The machine learning approach utilizes patient risk profiles and historical patients’ post-operative recovery profiles to identify the best surgical site for each patient.
The ongoing study involved analyzing 951 hip and knee arthroplasty cases, ranging in age from 20 to 80 years and BMI between 18 and 45. The dataset was divided into a 90% training phase and a 10% testing phase. Out of 14 evaluated models, 10 exhibited over 49% accuracy (Figure 1), with the logistic regression model yielding 70.59% accuracy and the Support Vector Machine demonstrating the highest accuracy at 75.29%. Noteworthy factors in the logistic regression model included alcohol use history, sleep apnea, COPD, previous adverse events, anesthesia issues, blood clot history, cancer history, pulmonary and cardiovascular issues, anemia, hypertension, and infection history (Figure 2).
Our ongoing study highlights the value of machine learning in identifying surgical risks in Ambulatory Surgical Centers (ASCs). Further investigation is needed to enhance the model's accuracy by analyzing a larger and more diverse set of cases from multiple centers. This will provide valuable insights for optimizing risk prediction and improving patient outcomes, revolutionizing surgical risk stratification and personalized care in ASCs.
- Fleischman AN, Austin MS, Purtill JJ, Parvizi J, Hozack WJ. Patient factors that influence outcome after total knee arthroplasty. J Arthroplasty. 2015;30(9 Suppl):12-15. doi:10.1016/j.arth.2015.02.042
- Sepúlveda V, Marcano AI, Gutiérrez Delgado E, et al. Total hip and knee arthroplasty: Better outcomes and cost-effectiveness in a certified ambulatory surgical center compared with hospital setting. J Arthroplasty. 2021;36(6):1945-1950. doi:10.1016/j.arth.2020.12.021
- Bovonratwet P, Bozic KJ, Ondeck NT, et al. Ambulatory Surgery Centers and Outpatient Hospitalization for Primary Total Knee Arthroplasty: Evidence from California Statewide Data. J Arthroplasty. 2021;36(5):1519-1525.e3. doi:10.1016/j.arth.2020.11.027
Figures
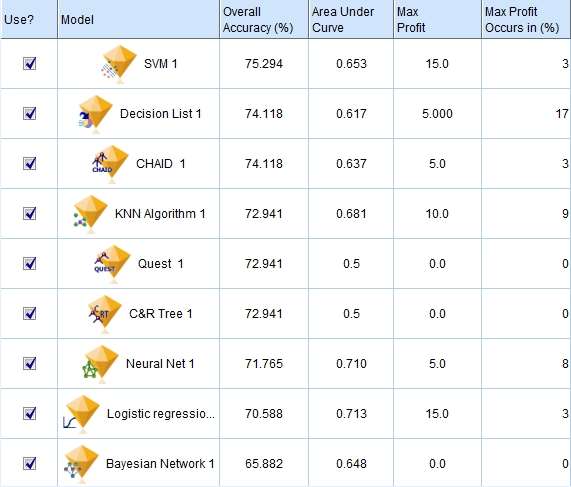
Figure 1

Figure 2#8147
Development and Validation of a Novel Risk Assessment Instrument to Assess Candidacy for Ambulatory Hip or Knee Arthroplasty Based on Machine Learning Techniques
Matthew Wickersham - Weill Cornell Medical College - New York, USA
Nicholas Bartelo - Weill Cornell Medical College - New York, USA
Scott LaValva - Hospital for Special Surgery - New York, USA
Edwin Su - Hospital for Special Surgery - New York, USA
Mathais Bostrum - Hospital for Special Surgery - New York, USA
Michael Ast - Hospital for Special Surgery - New York, USA
Olivier Elemento - Weill Cornell Medical College - New York, USA
*Tony Shen - Hospital for Special Surgery - New York, USA
*Email: shent@hss.edu
BACKGROUND: Patient selection is critical to the safety of ambulatory hip or knee arthroplasty; those selected for outpatient arthroplasty should be at minimal risk of requiring a hospital-based intervention postoperatively. Using a machine learning approach, we aimed to develop a risk assessment instrument that identifies patients who are at higher risk of requiring inpatient postoperative care based on their preoperative characteristics.
METHODS: We reviewed 17,792 patients who underwent a primary total hip or total knee arthroplasty between 2016-2019 at an orthopaedic specialty hospital using the electronic medical record. Features associated with these patients were catalogued in a machine learning software package. Patients were divided into a group which required a hospital-based intervention (HBI) during their postoperative admission (n=12,820) and those who did not (n=4,972). HBIs included abnormal vital signs, abnormal laboratory testing requiring monitoring or intervention, blood transfusion, urinary retention requiring catheterization, uncontrolled nausea, and uncontrolled pain requiring intravenous breakthrough medication. Supervised machine learning models were applied to predict the risk for HBI. The most predictive features were used to develop a risk assessment instrument. Using these same methods, the performance of this model was assessed in an external cohort of patients through the National Institutes of Health (NIH) All of Us database.
RESULTS: Using features that are routinely available at a preoperative visit, the best performing model carries an AUROC of 0.72. The predictive capability of this model far exceeds current clinical selection criteria, which carries an AUROC of 0.56. When applied to external cohort of patients, the model carries a comparable AUROC of 0.68.
CONCLUSION: A machine learning approach can provide an objective, data-driven approach in predicting the need for a hospital-based intervention following THA or TKA. This approach may be directly applied to optimize patient selection for ambulatory joint replacement surgery.
#8537
The Value of a Total Joint Hotline for Triaging Post-Operative Patients in an Effort to Decrease Unnecessary Emergency Department Visits
*Jimmy Daher - Ochsner Medical Center - New Orleans, USA
Bhumit Desai - Ochsner Medical Center - New Orleans, United States of America
Willard Moore III - Ochsner Medical Center - New Orleans, USA
Cruz Velasco-Gonzalez - Ochsner Medical Center - New Orleans, USA
George Chimento - Ochsner Health System - New Orleans, USA
*Email: jimmy.daher@lau.edu
Introduction:
Total joint arthroplasty (TJA) has been shown to be a safe and successful procedure. However, post-operative issues may arise prompting Emergency Department (ED) visits or hospital readmissions which lead to significant health care costs and compromise patient outcomes. Government and commercial payors monitor ED utilization as well as readmission rates in post-operative patients. While some patients have a problem that is best handled in the ED, many do not. A Total Joint Hotline (TJH) was established at our institution as a means of triaging post-operative patient concerns in an effort to decrease ED utilization in patients that could safely be treated elsewhere. The purpose of this study was to determine if the TJH decreased unneeded ED visits.
Methods:
The TJH was implemented in March 2021. Patients were assigned a wristband at their pre-operative clinic visit with a phone number answered directly by a member of the adult reconstruction care team. Patients were instructed to call with any issues encountered during their post-operative course and their concerns were triaged by either providing reassurance, arranging clinic follow-up, or directing them to the ED. A retrospective review of patients undergoing TJA from March 2020-February 2022, was performed. Patients were divided between 2 groups (pre TJH implementation vs post TJH implementation). ED visits and readmission data within 30 days of surgery were collected, utilizing the institution’s electronic medical records, which also gave access to outside facilities. Patients with calls regarding emergency medical issues such as suicidal ideation, acute severe cardiopulmonary symptoms, anaphylaxis, or a fall with inability to stand were excluded, as these patients’ self-directed to the ED. Statistical analysis included chi-square, t-test, and logistic regression with Firth correction for data spareness, with alpha set at 0.05.
Results:
A total of 1410 TJAs were performed during the study period (Fig. 1). 3.8% of TJH patients presented to the ED (29/768) vs 5.8% of non-TJH patients (37/642). Although there was a very strong trend, with the numbers available, there was no statistical significance in the number of patients that presented to the ED between both groups (p=0.0785, Figure 1). However, there is a 36% decreased odds ratio for ED visits in TJH patients [unadjusted OR, 0.64 (0.39,1.06)]. Importantly, 34.5% of TJH patients were readmitted compared to 10.8% of non TJH patients (p=0.0302 OR,4.0 (1.14,14.1)), demonstrating that the TJH decreased the number of unnecessary ED visits.
Conclusion:
The proportion of patient ED visits was higher in the non TJH group whereas the readmission rate was lower indicating that most of the postoperative issues in TJA patients can be addressed without an ED visit. The TJH is an effective tool in optimizing postoperative care while decreasing unnecessary visits to the ED.
Figures
Figure 1#8730
Utilization of Preoperative EOS Imaging to Prevent Adverse Events Following Total Hip Arthroplasty
*Weston Buehring - New York, United States of America
Itay Ashkenazi - NYU Orthopedic Hospital - New York, USA
Benjamin Schaffler - NYU Orthopedic Hospital - New York, USA
Morteza Meftah - NYU Hospital for Joint Diseases - New York, USA
Matthew Hepinstall - NYU Langone Health - New York, USA
Ran Schwarzkopf - NYU Langone Medical Center Hospital for Joint Diseases - New York, USA
Alana Prinos - Langone Orthopedic Hospital - New York, USA
*Email: westonbuehring2013@gmail.com
Introduction
Previous studies have shown the use of intraoperative technology in total hip arthroplasty (THA) to provide favorable outcomes and lessen the burden of adverse events. With the increased utilization of biplanar radiographs for both procedural planning and characterization of postoperative alignment, this study sought to describe the effect preoperative dynamic EOS imaging has on the prevention of adverse outcomes following THA.
Methods
We retrospectively reviewed 11,814 cases of patients who underwent primary, elective THA from January 2016 to December 2021. Patient demographics and clinical data were collected and compared between patients who did or did not have preoperative standing and sitting EOS imaging (EOS Imaging System; Paris, France) (n=3,484, 29.5%; n=8,330, 70.5%, respectively) using chi-squared test and multivariate logistic regressions.
Results
The rate of patients with preexisting spinal fusion was higher in the EOS cohort (2.2 vs. 0.6%, respectively, p<0.001). Rate of dislocation was found to be significantly lowered in the EOS group (0.9 vs. 1.4%; p=0.034). Other outcomes data also favored cases in which preoperative EOS images were obtained, including length of stay (40.15 vs. 67.16 hours; p<0.001), rate of discharge to home (94.1 vs. 80.0%; p<0.001), and 90-day readmission rate (3.5 vs. 6.0%; p<0.001). Multivariate analysis demonstrated preoperative EOS to be significantly associated with lower odds of dislocation, independent from surgical approach, coexisting spinal fusion, utilization of dual-mobility implants, and the intraoperative use of fluoroscopy.
Conclusion
This study demonstrates the validity and efficacy of obtaining EOS imaging for preoperative planning of THA. Preoperative EOS was independently associated with decreased risk for dislocations, and even in a cohort of significantly more spinal fusion cases, the rate of dislocation in the EOS cohort was significantly lowered. Physicians should consider the incorporation of the EOS imaging system into their practice to decrease adverse events.
#8203
Plain Long-Leg Radiographs Versus Computerised Tomography in Planning for Robotic-Arm Assisted Total Knee Arthroplasty: A Multicentre Cohort Study
*Andreas Fontalis - University College London Hospitals - London, United Kingdom
Thomas Luyckx - UZ Leuven - Leuven, Belgium
Thomas Vanspauwen - Hôpitaux Robert Schuman - Luxembourg-City, Luxembourg
Robin Moreels - AZ Delta Roeselare - Roeselare, Belgium
Rhody David Raj - niversity College London Hospitals NHS Foundation Trust - London, United Kingdom
Fabio Mancino - University College London Hospitals NHS Foundation Trust - London, United Kingdom
Philip Winnock De Grave - AZ Delta - Roeselare, Belgium
Ricci Plastow - University College London Hospitals NHS Foundation Trust - London, United Kingdom
Fares Haddad - University College London Hospital - London, United Kingdom
Pierre Putzeys - Hôpitaux Robert Schuman-Fondation Hôpitaux Robert Schuman - Luxembourg-City, Luxembourg
*Email: andreasfontalis@gmail.com
Introduction
Traditionally, arthroplasty surgeons have utilised long leg radiographs to measure native alignment; however, in cases where robotic-arm assistance is employed, it is also possible to obtain them from the computed tomography (CT) used for pre-operative planning. The objective of this study was to compare the correlation of measuring the lower limb constitutional alignment with traditional long leg radiographs versus CT used for pre-operative planning in robotic-arm assisted knee arthroplasty.
Methods
This international, multicentre cohort study across 3 tertiary centres, encompassed 290 patients undergoing primary, robotic-arm assisted TKA or UKA, for whom long leg alignment views and CT scanogram were available pre-operatively. The constitutional alignment was established by measuring the medial proximal tibial angle (MPTA), lateral distal femoral angle (LDFA), hip knee alignment (HKA), arithmetic hip knee alignment (aHKA), joint line obliquity (JLO) and Coronal Plane Alignment of the Knee (CPAK).
Results
Mean age was 69 years (range 40 to 87). The mean difference in measurements between the two imaging modalities was 0.34° ± 2.5° for HKA; 0.9 ± 3, JLO; 1.2 ± 1.8, aHKA; 1 ± 2.2, MPTA; LDFA -0.15 ± 1.8. No statistically significant difference was evident for any of the measured variables between the groups(independent samples t-test). Bland-Altman plots for all variables showed that approximately 95% observations were within the limit of agreement. The Pearson's corelation coefficient was 0.929 for HKA; 0.78 for MPTA; 0.859 for aHKA; 0.832 for LDFA and 0.714 for JLO, p<0.001 for all analyses.
Conclusion
In our study we found no significant difference and very high correlation between long-leg radiographs and the CT scanogram obtained during pre-operative planning in robotic-arm assisted knee arthroplasty, with respect to calculating lower limb alignment. These findings suggest that the need for long-leg radiographs in robotic-arm assisted arthroplasty may be obsolete, as the constitutional alignment can be accurately measured from the CT used for pre-operative planning.
#8184
Fluoroscopy Versus Imageless Optical Navigation in Direct Anterior Approach Total Hip Arthroplasty
*Marc Anthony Manzo - Temerty Faculty of Medicine, University of Toronto - Toronto, Canada
Johnathan Lex - University of Toronto - Toronto, Canada
Sebastian Rodriguez-Elizalde - Humber River Hospital - Toronto, Canada
Ryan Perlus - Division of Orthopaedic Surgery, Humber River Hospital, Toronto, Ontario, Canada - Toronto, Canada
Barry Cayen - Humber River Hospital - Toronto, Canada
Justin Chang - Division of Orthopaedic Surgery, Humber River Hospital, Toronto, Ontario, Canada - Toronto, Canada
*Email: marc.manzo@mail.utoronto.ca
Background: The direct anterior approach (DAA) for total hip arthroplasty (THA) has grown in popularity due to reduced pain, earlier mobilization, and decreased length of stay. Intraoperative fluoroscopy is commonly used in DAA THA to improve component positioning and minimize leg length discrepancy (LLD). However, fluoroscopy alone has been shown to be an unreliable tool to accurately measure these parameters and exposes the patient and surgical team to additional radiation. Imageless optical navigation systems have been shown to reduce the frequency of positional outliers in acetabular cup position in direct lateral and posterior approach THA. The aim of this study was to compare the accuracy of fluoroscopy to that of an imageless optical navigation system in DAA THA.
Methods: A retrospective cohort study was performed of 640 primary DAA THA procedures performed with intra-operative fluoroscopy (n=300 patients) or imageless optical navigation (n=304 patients). Accuracy was compared between fluoroscopy and imageless navigation by measuring acetabular cup inclination, anteversion, and LLD from postoperative anteroposterior pelvic radiographs. Interobserver reliability of each measurement was determined using Cronbach’s alpha. The proportion of components placed within the Lewinnek safe zone was evaluated, as well as those placed within a more precise target range of ± 5 degrees from the surgical target (40° ± 5° inclination and 20° ± 5° anteversion).
Results: There was a difference between fluoroscopy and navigation THA in mean acetabular inclination (40.4° SD: 4.7° vs. 42.0° SD: 3.74° respectively, p<0.001) and anteversion (17.9° SD: 4.7° vs. 20.3° SD: 3.6° respectively, p<0.001). According to the Lewinnek criteria, there was a difference in the proportion of components placed within the anteversion range (fluoroscopy: 71.3% vs. navigation: 83.8%, p<0.001) but not inclination (71.7% vs 76.9% respectively, p=0.147). However, there was no significant difference between fluoroscopy and navigation in the percentage of components positioned within the Lewinnek safe zone targets for both inclination and anteversion (90.3% vs 88.8% respectively, p=0.519). When using the more precise targets, navigation increased the proportion of acetabular components positioned correctly (fluoroscopy: 50.3% vs navigation: 65.6%, p<0.01). Mean LLD was higher with use of fluoroscopy compared to navigation (5.5 mm SD: 4.1 mm vs. 4.6 mm SD: 3.4 mm respectively, p<0.001).
Conclusion: Both fluoroscopy and imageless optical navigation result in accurate component positioning for DAA THA. Navigation was more precise and associated with improved acetabular anteversion placement and restoration of LLD. Navigation is an accurate alternative to fluoroscopy while also reducing radiation exposure to the patient and operating team.
#8341
Efficacy of a Novel, Fluoroscopy-Based Robotic-Assisted Total Hip Arthroplasty System in Restoring Limb Length and Offset
Graham Buchan - Cleveland Clinic Lerner College of Medicine - Cleveland, United States of America
Christian Hecht - Cleveland Clinic - Cleveland, USA
*Atul Kamath - Cleveland Clinic - Cleveland, USA
*Email: kamatha@ccf.org
Introduction: Optimizing leg length discrepancy (LLD) and restoring global and femoral offset (GO, FO) are integral to improving the stability and longevity of total hip arthroplasty (THA). A novel robotic-assisted THA (RA-THA) platform has been developed to utilize pre-operative templating and intraoperative fluoroscopic imaging to guide the restoration of native biomechanics. We sought to evaluate the effectiveness of this novel, pinless, fluoroscopy-based RA-THA system to restore templated LLD and offset parameters.
Methods: We performed a retrospective analysis on a consecutive series of patients who underwent fluoroscopy-based RA-THA at our institution. The primary outcome was the difference between achieved and templated LLD, GO, and FO parameters. Secondary outcomes Secondary outcomes include the difference in achieved versus templated acetabular cup anteversion and inclination values. All patients were targeted for 40°/15° of inclination/ anteversion for cup positioning. Preoperative templated LLD and offset target values were collected from preoperative planning templates generated by a robot-optimized THA planner. Achieved values were collected from postoperative surgeon reports automatically generated from the robot at the end of the procedure.
Results: The mean difference between achieved and preoperatively templated values of LLD (3.0 ±4.7mm), GO (-3.5 ±7.0mm), and FO (-0.75 ±5.4mm) were all within 3.5mm of establishing equalized leg length and offset (Table 1). The proportion of patients with a difference in achieved and templated values <10mm were 89% for LLD, 79% for GO, and 90% for FO. Additionally, the average achieved values of acetabular cup anteversion (0.8 ± 2.6?) and inclination (-1.4 ± 3.9?) were within 1.5? of targeted values. For 43 of the 98 (44%) patients in this study, the surgeon referenced intraoperative robotic data to adjust femoral components from the preoperative plan in order to optimize LLD and offset parameters.
Conclusions: The results of our present study demonstrated that fluoroscopy-based RA-THA is associated with high levels of accuracy in restoring key biomechanics of the hip. In a large number of patients, the surgeon used intraoperative robotic data to more closely achieve LLD and offset goals. This demonstrates the ability of this system to merge preoperative data with intraoperative, actionable data provided by the robotic software to restore leg length and global/femoral offset parameters.
Keywords: Digital templating, Total hip arthroplasty (THA), Robotic-THA, Pre-operative planning, limb length discrepancy (LLD), offset
Figures
Figure 1#8183
In Vitro Wear of a Novel Vitamin E Crosslinked Polyethylene Lumbar Total Joint Replacement
*Steven Kurtz - Drexel University - Philadelphia, USA
Ryan Siskey - Exponent - Philadelphia, USA
Ron Yarbrough - 3Spine - Chattanooga, USA
Scott Hodges - 3Spine - Duxbury, USA
S. Craig Humphreys - 3Spine - Duxbury, USA
*Email: smk38@drexel.edu
Introduction: Anterior total disc replacement (ADR), conceived for degenerative disc disease in the anterior lumbar spine using conventional polyethylene bearings. A novel, lumbar total joint replacement (TJR) design has been developed that treats degeneration across all three columns of the lumbar spine (anterior, middle, and posterior columns) (Fig. 1). The objective of the study was to test in vitro whether Vitamin E stabilized highly crosslinked polyethylene (VE-HXLPE) mitigates the biomechanical performance risks of wear, abrasion, and impingement, comparable to its predecessor lumbar ADR designs.
Methods: A lumbar TJR with bilateral VE-HXLPE superior bearings and CoCr inferior bearings was evaluated under clean, impingement, and abrasive conditions. ISO 18192-1 guided clean and abrasive testing. For abrasive testing, CoCr components were scratched to simulate in vivo abrasion. Impingement was assessed per ASTM F3295. Devices were tested for 10 million cycles (MC) under clean conditions, 5 MC under abrasion, and 1 MC under impingement.
Results: Wear rates under clean and abrasive conditions were 1.2 ± 0.5 and 1.1 ± 0.6 mg/MC. VE-HXLPE components demonstrated evidence of burnishing and multidirectional microscratching consistent with microabrasive conditions with the cobalt chromium spherical counterfaces. Under impingement, wear rates ranged between 1.7 ± 1.1 (smallest size) to 3.9 ± 1.1 mg/MC (largest size). No functional or mechanical failure was observed across any of the wear modes.
Conclusion: Wear and osteolysis continue to be important clinical concerns in spinal arthroplasty. In this study we performed an in vitro wear study of a novel lumbar total joint replacement design that replicates the biomechanics and kinematics of the intervertebral disc and facet joint complex.Overall, we found that that a VE-HXLPE-on-CoCr total joint replacement met or exceeded the benchmarks established by traditional ADRs, with wear rates previously reported in the literature ranging between 1-15 mg/MC. The adverse testing results support that the VE-HXLPE is resistant to abrasive damage, and is reasonably forgiving under high stress impingement conditions. The potential clinical benefits of this novel TJR design, which avoids long-term facet complications through facet removal with a posterior approach, were found to be balanced by the in vitro tribological performance of the VE-HXLPE bearings.
Figures
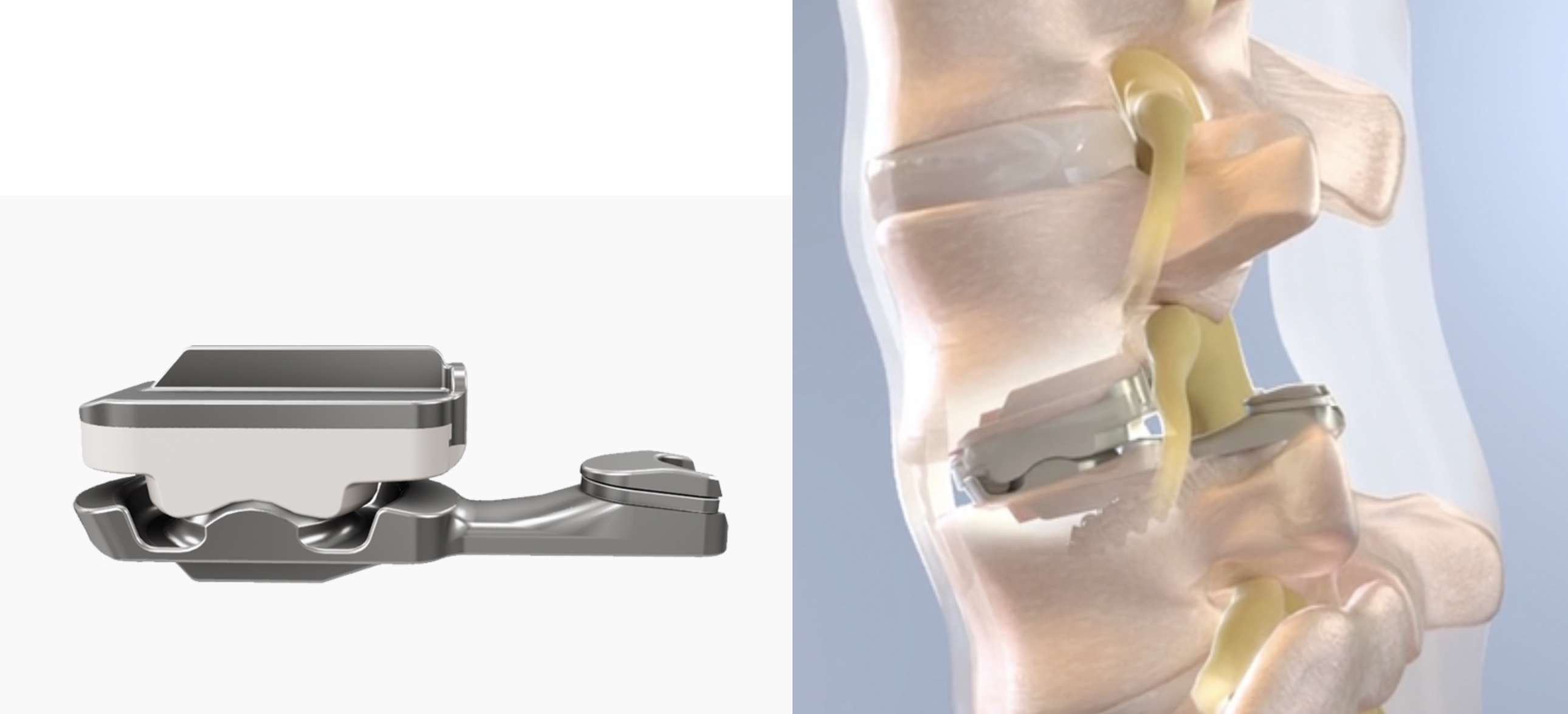
Figure 1#8367
Early Acetabular Cup Migration May Be a Source of Error in Assessment of Intra-Operative Placement Accuracy.
*Shahnaz Taleb - Western University - London, Canada
Jennifer Polus - University of Western Ontario - London, Canada
Brent Lanting - London Health Sciences Centre - London, Canada
Matthew Teeter - Western University - London, Canada
*Email: staleb3@uwo.ca
Background: Proper positioning of the acetabular cup is essential for long-term total hip arthroplasty (THA) success. Conventional freehand and mechanically guided techniques can result in suboptimal cup placement, even in the hands of experienced surgeons. Computer navigation and robotic techniques have been of interest to improve cup placement, with their accuracy often reported by comparing the intraoperative to six-weeks postoperative position; however, early component migration is known to occur within the first six-weeks. This study aimed to assess the potential error in cup position measurements caused by early acetabular cup migration within the first six-weeks following THA.
Methods: Patients were enrolled pre-operatively as part of a prospective RCT. A single fellowship-trained arthroplasty surgeon performed all surgeries using the direct anterior approach with adjuvant fluoroscopy with no (n = 7), one (n =16), or two (n = 10) screws. No significant differences were found in placement change between groups; therefore, all patients were combined for the analysis. Acetabular cup inclination and anteversion angles were measured by two raters from radiographs taken intra-operatively and at six-week post-operation. Inclination was measured as the angle between the bi-ischial line and a line tangent to the opening of the acetabular cup. Anteversion was measured using the technique described by Lewinnek et al. as version = arcsin(D1/D2) where D1 denotes the short axis of the acetabular cup while D2 is the long axis of the acetabular ellipse (Figure 1) [1]. A radiostereometric analysis examination was performed on the day of surgery as well as 6-weeks post-operation to assess for total rotation.
Results: Thirty-three patients were included in our analysis. Mean inclination angles were 31.2° intra-operatively and 32.8° at six-weeks post-operation (maximum difference = 11.1°). Mean anteversion angles were 23.5° and 29.3° intra-operatively and at six-weeks post-operation (maximum difference = 15.3°). Patients with change greater than 10° in inclination (n=1) and anteversion (n=5) had one screw and no screws, respectively. Mean anterior tilt, internal rotation, and valgus rotation between day of surgery and six-weeks post-operation were 1.33°, 0.98°, and 0.80° respectively. Mean total rotation at 6-week follow-up was 1.95° with a maximum rotation of 4.98°.
Conclusion: This study demonstrated that early migration of acetabular cups may introduce an error in studies reporting computer navigation accuracy in acetabular cup placement based on post-operative imaging follow-ups. Part of the difference reported between intended and achieved cup positioning is due to in vivo acetabular cup migration, rather than navigation errors. As such, the accuracy and precision of computer navigation systems may be higher than previously reported.
References: [1]Lewinnek GE, Lewis JL, Tarr R, Compere CL, Zimmerman JR. Dislocations after total hip-replacement arthroplasties. vol. 60. 1978.
Figures
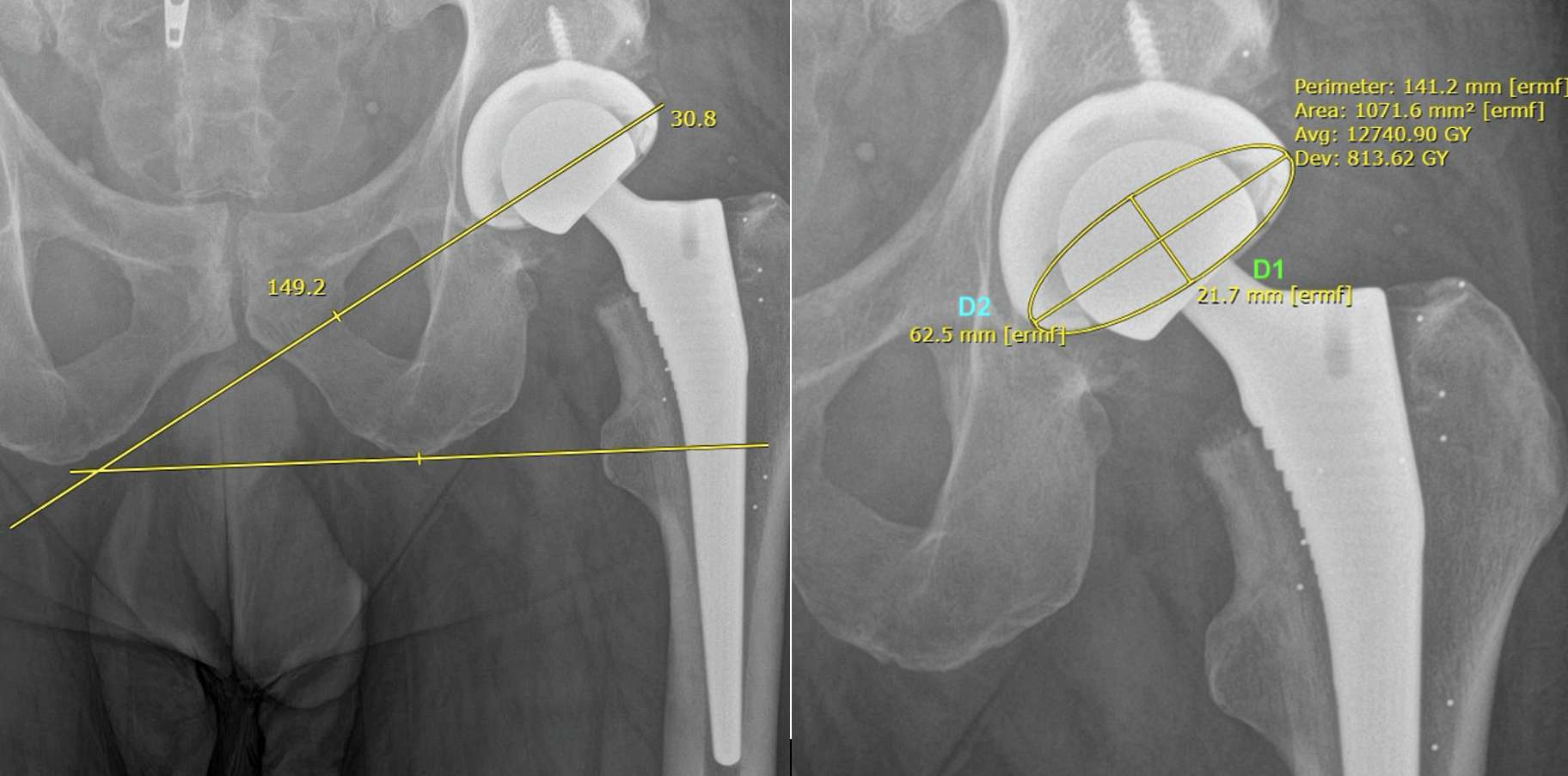
Figure 1#8238
Does Quality of Life Improve in Patients With Significant Comorbidities Following Total Hip Arthroplasty?
*Roberta Redfern - Zimmer Biomet - Pemberville, USA
Dave Van Andel - Zimmer Biomet - Grand Rapids, USA
Mike Anderson - Zimmer Biomet - Lehi, USA
Jason Cholewa - Zimmer Biomet - Warsaw, USA
*Email: roberta.redfern@zimmerbiomet.com
Presence of comorbidities may affect outcomes following total hip arthroplasty (THA). Psychological comorbidities such as depression or anxiety, as well as physical conditions including back pain or other joint pain have been suggested as factors affecting post-operative outcomes. This study aimed to compare the impact of THA on health-related quality of life and functional outcomes between patients with high and low levels of comorbidity.
Secondary data analysis from a multicenter, prospective observational cohort study. A smartphone-based care management platform with smartwatch was prescribed to all subjects for self-directed rehabilitation following THA. Participants completed the EQ5D5L and HOOS JR pre-operatively and 12 months post-operatively. A comorbidity score was calculated based on presence of items in previously validated scores, and included depression, anxiety, and previous lumbar or cervical surgery. Low-comorbidity and high-comorbidity groups were created by dividing the population by the 95th percentile score. Pre- and post-operative HOOS JR, EQ5D5L index, and EQ-VAS were compared between groups, as were changes from baseline.
Comorbidity and pre-operative HOOS JR scores were available for evaluation in 1616 patients. The 95th percentile score was 3.0; patients were grouped by scores less than or greater than 2 points. HOOS JR scores did not differ significantly at baseline in high vs low comorbidity groups (53.12±12.92 vs 51.11±12.58, p=0.07). High comorbidity patients rated their baseline health-related quality of life lower by EQ5D5L index (0.486±0.265 vs 0.408±0.305, p=0.004) and EQ-VAS (72.59±16.53 vs 66.91±19.58, p=0.001). At one year post-operatively, change in HOOS JR was significantly greater in the high comorbidity group (37.01±15.61 vs 33.09±15.92, p=0.01) though not clinically significant. Differences in EQ5D5L index and EQ-VAS scores persisted between groups at one year, however improvements over baseline were equivalent in high and low comorbidity groups by EQ5D5L index (0.403±2.72 vs 0.391±0.301, p=0.69) and EQ-VAS (12.78±17.44 vs 14.39±18.24, p=0.38).
Patients undergoing THA in the highest 5th percentile of comorbidity scores demonstrated clinically similar joint function pre- and post-operatively. These patients reported lower health-related quality of life throughout the entire study period, however, appreciation of similar improvements on EQ5D5L and EQ-VAS suggest equivalent benefit for those with significant comorbid conditions following THA.
#8348
Patient Characteristics Associated With Discordance in Patient Reported Outcome Measures Following Primary Total Hip Arthroplasty
*Natalie Pahapill - Hospital For Special Surgery - New York, United States of America
Daniel Driscoll - Hospital for Special Surgery - New York, USA
Vanessa Ruiz - Hospital for Special Surgery - New York, USA
Mihir Dekhne - Hospital For Special Surgery - New York, USA
Catherine MacLean - Hospital For Special Surgery - New York, USA
Alexander Mclawhorn - HSS - New York, USA
*Email: pahapilln@hss.edu
Introduction:
Patient Reported Outcome Measures (PROMs) are important standards in measuring surgical outcomes and informing care decisions in orthopedics, especially as insurance companies shift towards collecting PROMs to assess value and improvement. Metrics like Minimal Clinically Important Difference (MCID) and Substantial Clinical benefit (SCB) are used to access PROM significance and change in response to the HOOS Jr. following total hip arthroplasty. Discordance in this study refers to a mismatch between these PROM metrics and the Patient Acceptable Symptom State (PASS). Past work has shown that there are patients who report satisfaction following surgery yet did not meet MCID. Likewise, there are patients who report low satisfaction, yet surpass MCID. This study aimed to identify patient characteristics that are associated with an increased likelihood of developing post-operative PROM discordance following total hip arthroplasty. We hypothesize that there are certain pre-operative and post-operative patient characteristics that may be associated with the increase in the likelihood a patient reflecting discordant PROMs.
Methods:
This retrospective study drew data from a joint replacement registry at a single institution and included all patients who underwent a primary unilateral THA between June 2019-December 2021 that completed preoperative and one-year post-operative PASS and HOOS Jr. questionnaires. Patients that underwent bilateral or staged THA were excluded. Patients were divided into discordant (D), or non-discordant (ND) groups based on their one-year post-operative PROMs [Fig. 1]. Records were reviewed for 14 patient characteristics [Fig. 2, Fig. 3]. Univariate and multivariate analyses were performed with a significance level of P ≤ .05.
Results:
In the PASS vs MCID group (n=1188), 16% of patients were discordant. In the univariate analysis, increased age (p=0.002), smoking history (p=0.016), and post-operative concurrent lumbar procedure were significant (p = 0.028). In the multivariate analysis, rate of post-operative lumbar procedures (OR=2.75, 95% CI: 1.153-6.198, p = 0.024) and other preoperative orthopedic procedure (not lumbar) (OR = 2.12, 95% CI: 1.003-4.188, p=0.049) may be associated with an increased likelihood of a patient being discordant. In the PASS vs SCB (n=1188) group, 19.9% of patients were discordant. In the univariate analysis, age (p<0.001), pre-operative PROMIS-10 mental health score (p=0.016), insurance status (p=0.010), and pre-operative concurrent orthopedic procedures (not lumbar) (p=0.033) were significant. In the multivariate analysis, male sex (OR=1.57, 95% CI: 1.162-2.129, p=0.003) and any pre-operative concurrent orthopedic procedure (not lumbar) (OR = 2.25, 95% CI: 1.128-4.313, p = 0.022) may be associated with an increased likelihood of a patient being discordant.
Conclusion
This study explored various characteristics that may lead to an increased probability of PROM discordance in PASS vs MCID and PASS vs SCB in patients who underwent primary unilateral total hip arthroplasty. Identifying factors that may lead to PROM discordance can help identify areas in which PROM measurement, collection, and metrics may need modification. Furthermore, knowledge about factors associated with discordance may lend providers insight into how a patient will perform on their post-operative PROMs, allowing for adjustment of treatment plans. Further studies warrant exploration of additional patient characteristics and consideration of other PROM measures and metrics.
Figures
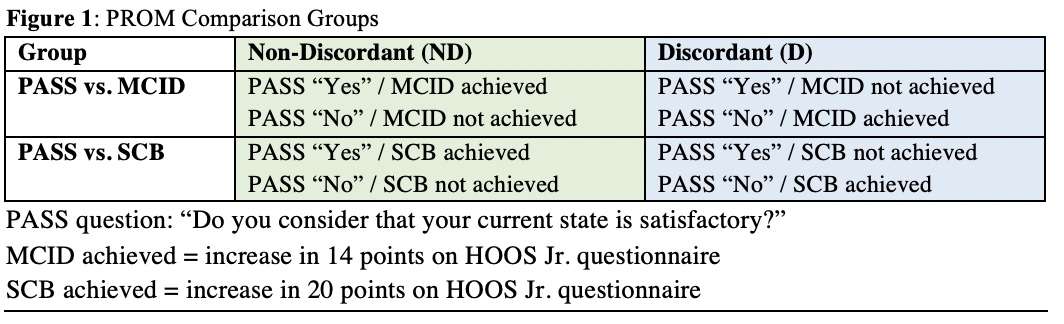
Figure 1
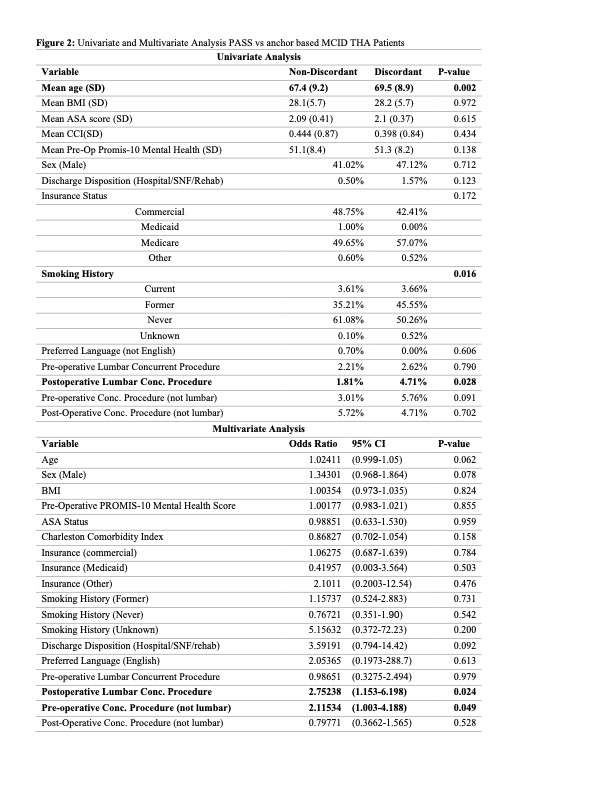
Figure 2
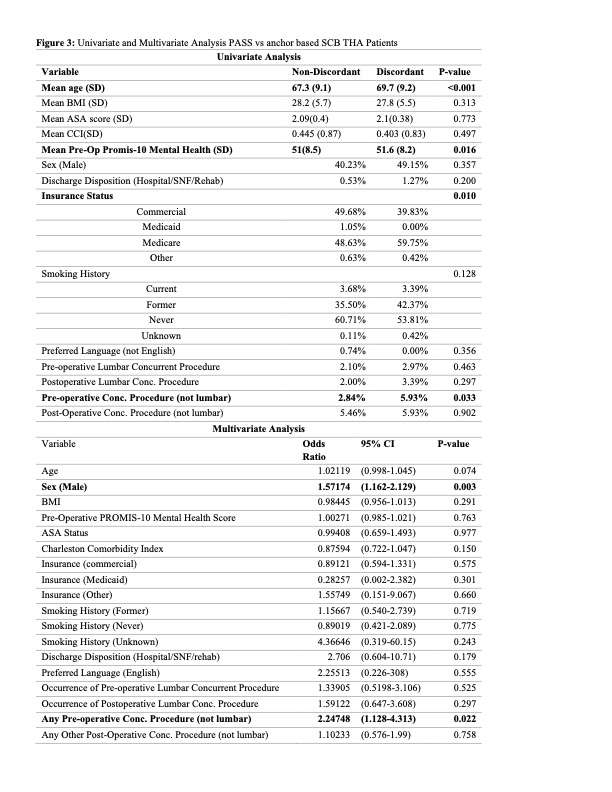
Figure 3#8269
Surgical Approach and Functional Recovery Outcomes After Primary Total Hip Arthroplasty in the Early Postoperative Period
*David Fawley - DePuy Synthes - Warsaw, USA
Thierry Bernard - DePuy Synthes - West Chester, USA
Rodrigo Diaz - DePuy Synthes - Palm Beach Gardens, USA
clay thomason - Carolina Orthopaedic and Sports Medicine Center - gastonia, United States of America
Brian Gladnick - Carrell Clinic - Dallas, United States of America
John Redmond - Southeast Orthopaedic Specialists - Jacksonville, USA
Jefferson Craig Morrison - Southern Joint Replacement Institute - Nashville, USA
René ten Broeke - Maastricht University Medical Centre - Maastricht, Netherlands
Kory Johnson - Orthopaedic Associates of Michigan - Byron Center, United States of America
Luigi Zagra - IRCCS Galeazzi Orthopedic Institute - Milan, Italy
Mohammad Burney - North Texas Medical Research Institute - Rockwall, United States of America
Eric Matthew Heinrich - Austin, USA
Michael Alexiades - New York, USA
*Email: dfawley1@its.jnj.com
Introduction
Extensive research has been conducted to review recovery after total hip arthroplasty (THA) in relation to surgical approach. However, it is more difficult to quantify the course of recovery throughout the early recovery period. Standard patient reported outcomes measures (PROMs) typically only allow collection of data at a single timepoint, and while this is valuable, it does not allow review of changes over time. A novel questionnaire was used within two prospective, multi-center studies to collect patient reported early functional recovery metrics between 1- and 12-weeks postoperatively. The objective of the study was to compare early recovery between a group of direct anterior approach (DAA) and lateral decubitus approach (LDA) cases.
Methods
Data were collected within two prospective, multi-center studies (NCT04191733 and NCT03189303). Subjects were asked to report when they could first accomplish functional recovery tasks after their primary THA. In the LDA study, only lateral decubitus approaches were used (posterior, anterior lateral). In the DAA study, only direct anterior approach was used. A time to event analysis with Kaplan-Meier methodology was conducted for each question, where the event was the time at which the subject was first able to achieve the task in question. Time to event curves exhibit the cumulative percent of subjects who were able to achieve the task. Curves were compared with a log-rank test to compare LDA vs. DAA. Additionally, normal approximation p-values were provided for the LDA vs. DAA estimates at each timepoint.
Results
170 subjects were analyzed in LDA, 218 in DAA. Patient age and gender were similar between groups. BMI was higher in the LDA group (p=0.0049), and ASA risk was higher in the DAA group (p=0.0123) (Figure 1). Of the eleven functional recovery questions asked, only two (ability to stand from a chair without assistance, and ability to accomplish primary goal) were not statistically different. Distributions for all other questions were significantly different. Graphs showing recovery over time can be viewed for each question in Figure 2, and p-values for each timepoint are presented in Figure 3. Recovery between the two different approaches in LDA were reviewed and the there were no statistical differences detected between groups for the functional recovery outcomes.
Conclusion
Early functional recovery was excellent for both groups. DAA showed significant improvements in functional recovery outcomes in most categories, especially in the early postoperative period. By 10-weeks postoperatively, recovery outcomes were similar between groups for all questions. These results are compelling in suggesting the benefit of continued study of time to recovery in the early postoperative period after THA, and it would be beneficial to review these recovery data in randomized, controlled studies to account for variability in patient recovery pathways, to compare to minimally invasive LDA techniques, and to explore the association with objective assessments.
Figures
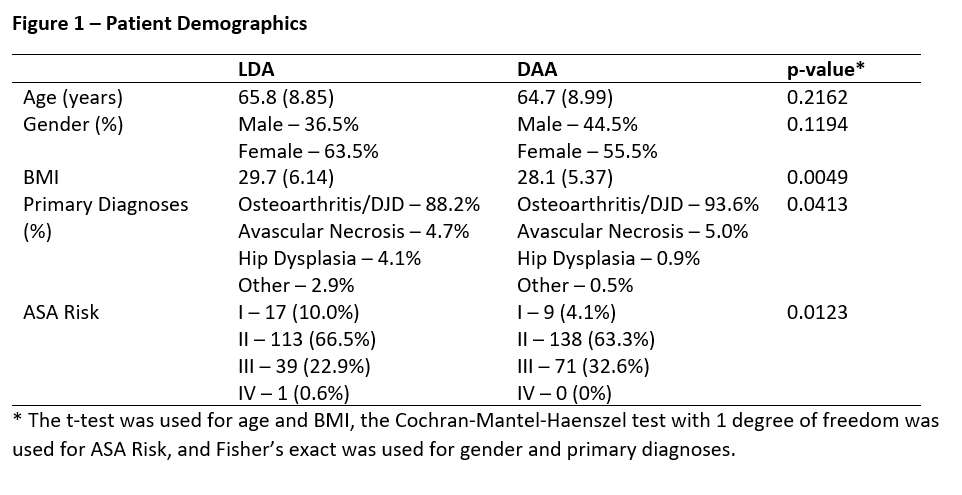
Figure 1
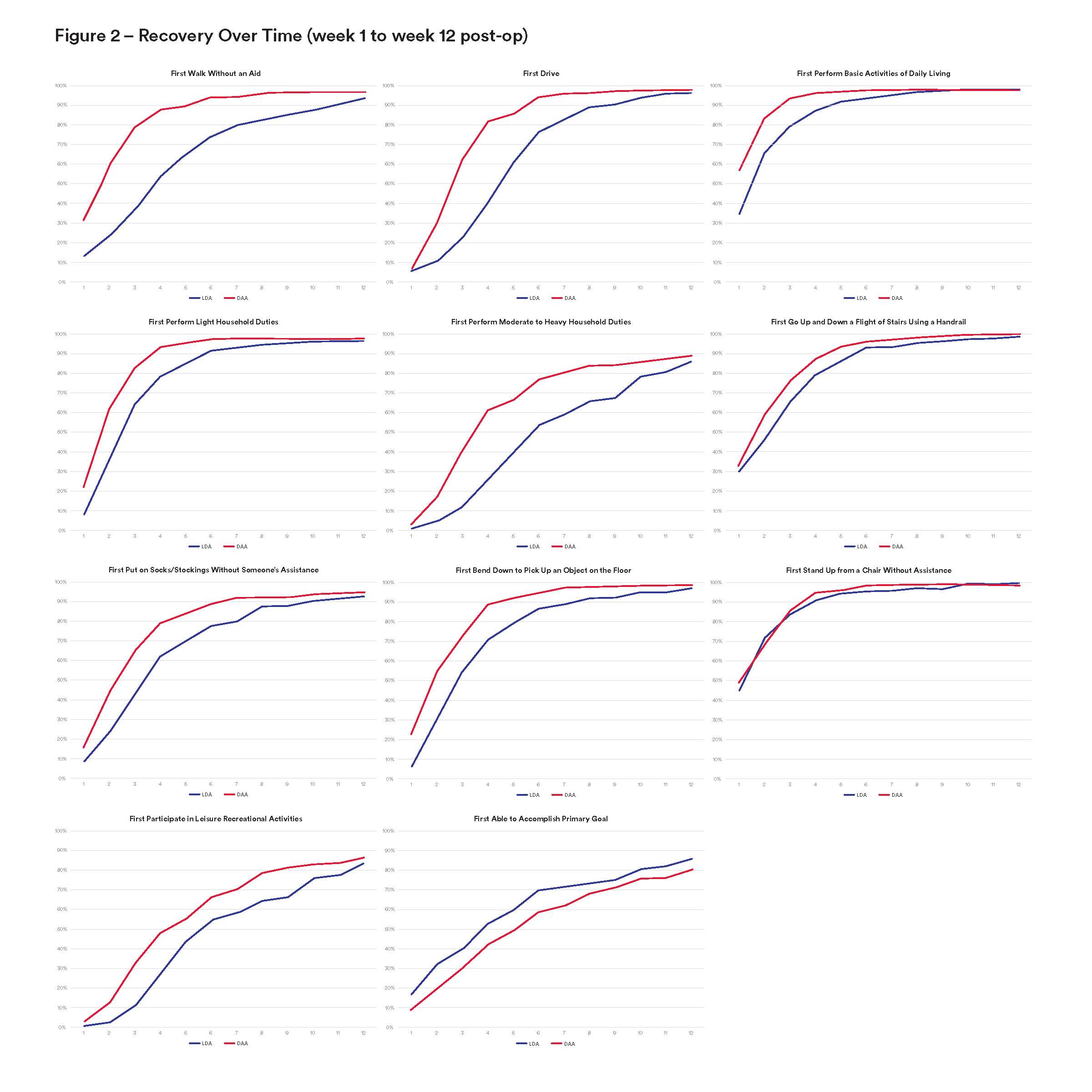
Figure 2
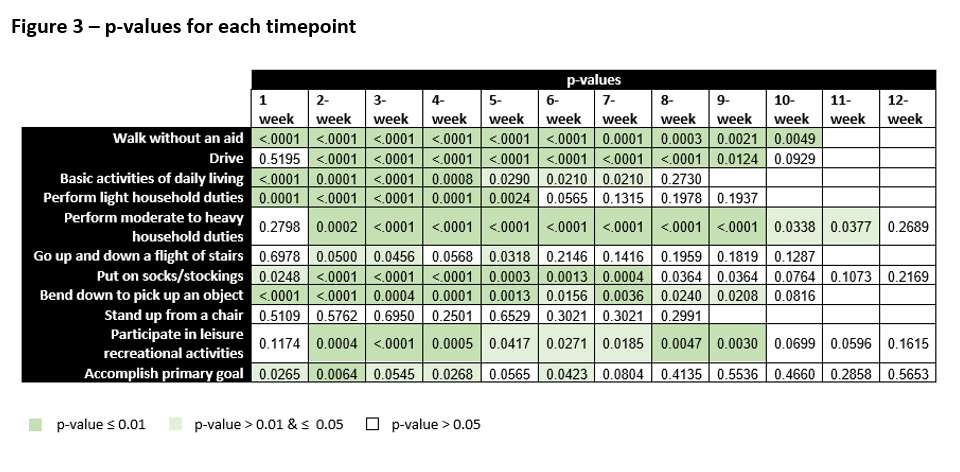
Figure 3#8285
Sex Specific Post-Operative Outcomes of Primary Total Hip Arthroplasty: The Performance of Total Hip Arthroplasty Procedures Leads to Worse Outcomes in Men
*Mitchell Ng - Maimonides Medical Center - Brooklyn, United States of America
Zhongming Chen - Lenox Hill Hospital - New York, USA
Matthew Magruder - Maimonides Medical Center - Brooklyn, USA
Andriy Kobryn - SUNY Downstate College of Medicine - Brooklyn, USA
Nikhil Vasireddi - Case Western Reserve University School of Medicine - Cleveland, USA
Jeremy Dubin - Hospital for Special Surgery - New York, USA
Afshin Razi - Maimonides Medical Center - Brooklyn, USA
Che Hang Jason Wong - Maimonides Medical Center - Brooklyn, USA
Michael Mont - Sinai Hospital of Baltimore - Baltimore, USA
*Email: mitchng77@gmail.com
INTRODUCTION: The demand for primary total hip arthroplasty (THA) is expected to increase significantly in the coming years, and women are expected to account for the greatest proportion of this increased demand. The purpose of this study was to determine using a national database, the effect of sex on 90-day outcomes in primary THA patients while matching for confounding variables. Specifically, we evaluated: (1) in-hospital lengths of stay; (2) 90-day readmission rates; (3) 90-day medical complications; (4) and total global 90-day episode of care (EOC) costs in men and women.
METHODS: Using the 100% Medicare Standard Analytical Files (SAF), a query from January 1st, 2005 to March 31st, 2014 from a nationwide database was performed to analyze patients who received a primary THA. The series was divided into two cohorts: men (n = 436,737) and women (n = 436,737). Men and women patients were matched according to age and Elixhauser-Comorbidity Index (ECI). Uni- and multi-variable regression analyses were performed to analyze the effects of sex on in-hospital lengths of stay, 90-day readmission rates, 90-day medical complications, and total global 90-day episode of care (EOC) costs.
RESULTS: Men had greater overall 90-day medical complications compared to women following primary THA (1.28 vs. 1.19%, p<0.001). Men were found to have higher rates of acute kidney failure (0.12 vs 0.05%, p<0.0001), acute pancreatitis (0.02 vs. 0.01%, p<0.0001), cerebrovascular accidents (0.03 vs. 0.01%, p<0.0001), deep vein thromboses (0.06 vs. 0.04%, p<0.0001), and myocardial infarctions (0.02 vs. 0.01%, p<0.0001). Women were found to have higher rates of acute post-hemorrhagic anemiae (0.31 vs. 0.30%, p<0.001) and urinary tract infections (0.40 vs. 0.28%, p<0.0001) compared to men. Men had shorter in-hospital LOS (3.42 vs. 3.54 days, p<0.001), but greater 90-day readmission rates (7.67 vs. 6.39% p<0.0001). Both cohorts had similar total global 90-day episode of care costs ($14,869.85 ± $12,333.50 vs. $14,957.34 ± $10,915.61, p = 0.36).
CONCLUSION: Men undergoing THA have a greater number of overall 90-day medical complications and readmission rates while women have higher incidence of UTI and post-hemorrhagic anemia, and longer LOS. Understanding sex-based differences in complication rates and outcomes can help surgeons with pre-operative counseling and targeted pre-operative optimization.
#8751
The Impact of Obesity on Total Hip Arthroplasty Outcomes When Performed by High Volume Surgeons – a Propensity Matched Analysis From a High-Volume Urban Center
*Jeremiah Thomas - NYU Langone Health - Scarsdale, United States of America
Itay Ashkenazi - NYU Orthopedic Hospital - New York, USA
Kyle Lawrence - NYU Orthopedic Hospital - New York, USA
Joshua Rozell - NYU Langone Orthopedic Hospital - New York, USA
Morteza Meftah - NYU Hospital for Joint Diseases - New York, USA
Ran Schwarzkopf - NYU Langone Medical Center Hospital for Joint Diseases - New York, USA
Muhammad Haider - NYU Orthopedic Hospital - New York, USA
Alana Prinos - Langone Orthopedic Hospital - New York, USA
Hayley Raymond - NYU - New York, United States of America
*Email: jeremiahjosephthomas@gmail.com
Introduction:
Previous data suggest that obesity does not impact surgical outcomes following total knee arthroplasty performed by high-volume (HV) surgeons. However, this effect has yet to be studied in total hip arthroplasty (THA) patients. This study aimed to evaluate impact of patient obesity on THA outcomes when surgery is performed by HV surgeons.
Methods:
A retrospective analysis of patients who underwent primary, elective THA between January 2012 and December 2022 with a HV surgeon (top 25% surgeons by number of annual primary THA) was performed. Patients were stratified by their body mass index (BMI) into three cohorts: BMI≥40 (morbidly-obese, MO), 30≤ BMI<40 (non-morbidly obese, NMO) and BMI<30 (non-obese, NO) and 1:1:1 propensity matched based on baseline characteristics. A total of 13,223 patients were evaluated, of which, 669 patients were included in final matched analysis (223 patients per group). The average number of annual THA performed for HV surgeons was 171 cases.
Results:
MO patients had significantly longer surgical time (P<0.001) and hospital lengths of stay (P<0.001). Rates of 90-day readmissions (P=0.211) and all-cause, septic and aseptic revisions at latest follow-up (2.9 ± 2.6 years) (P=0.268, P=0.903 and P=0.168, respectively) were comparable between groups. In a sub-analysis for Non-HV surgeons, MO patients had a significantly greater risk of revision (P=0.021) and trended towards significantly greater readmissions (P=0.056).
Conclusion:
Clinical outcomes and complication rates after THA performed by a HV surgeon are similar regardless of patient obesity status. Patients with MO may experience improved outcomes and reduced procedural risks if they are referred to HV surgeons.
Figures
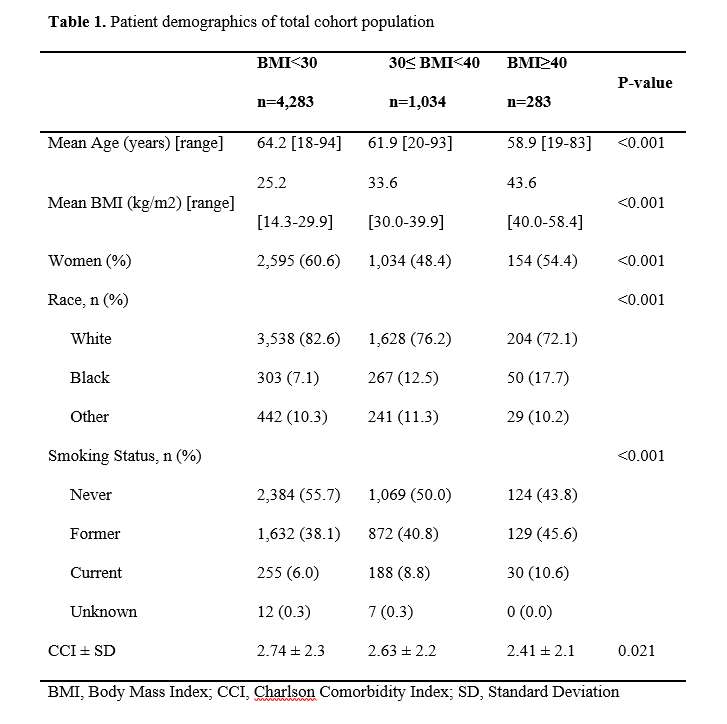
Figure 1
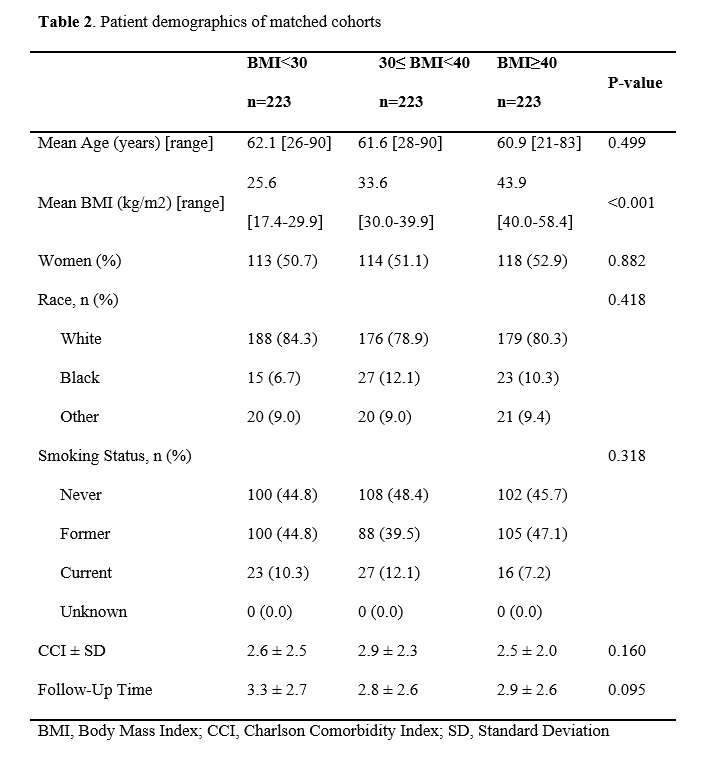
Figure 2
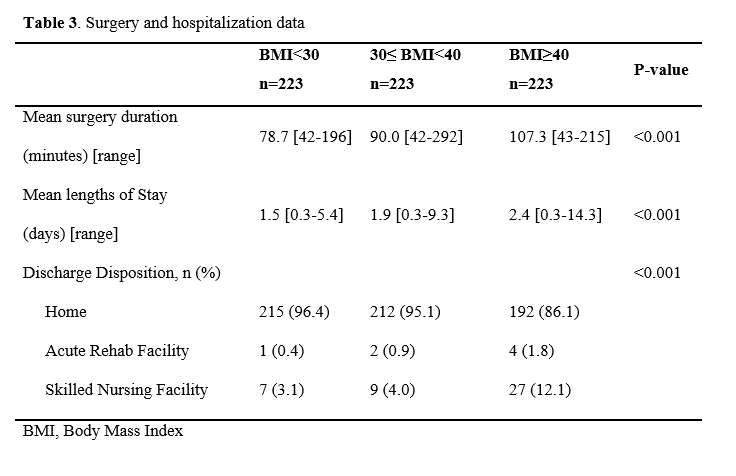
Figure 3
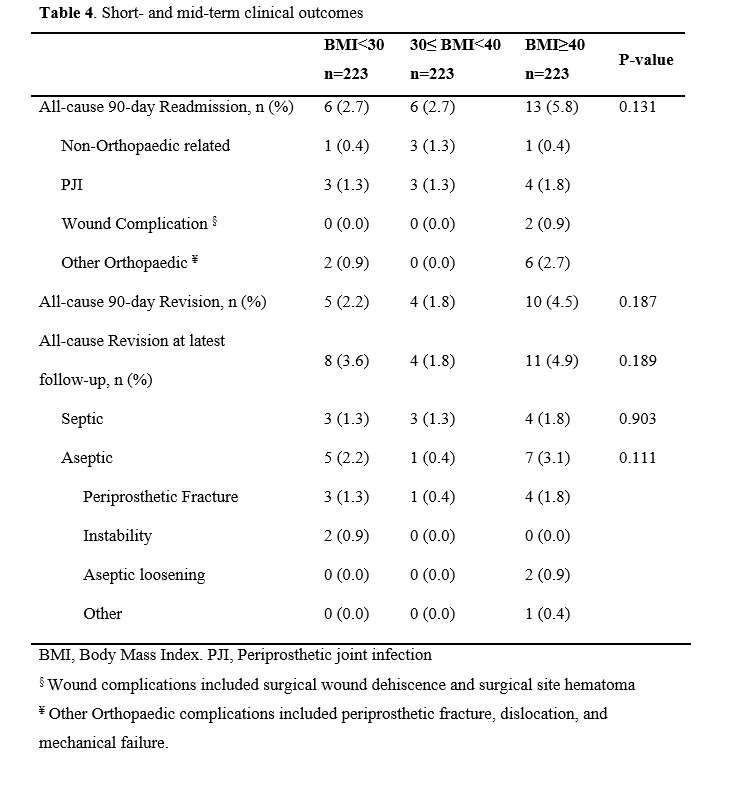
Figure 4
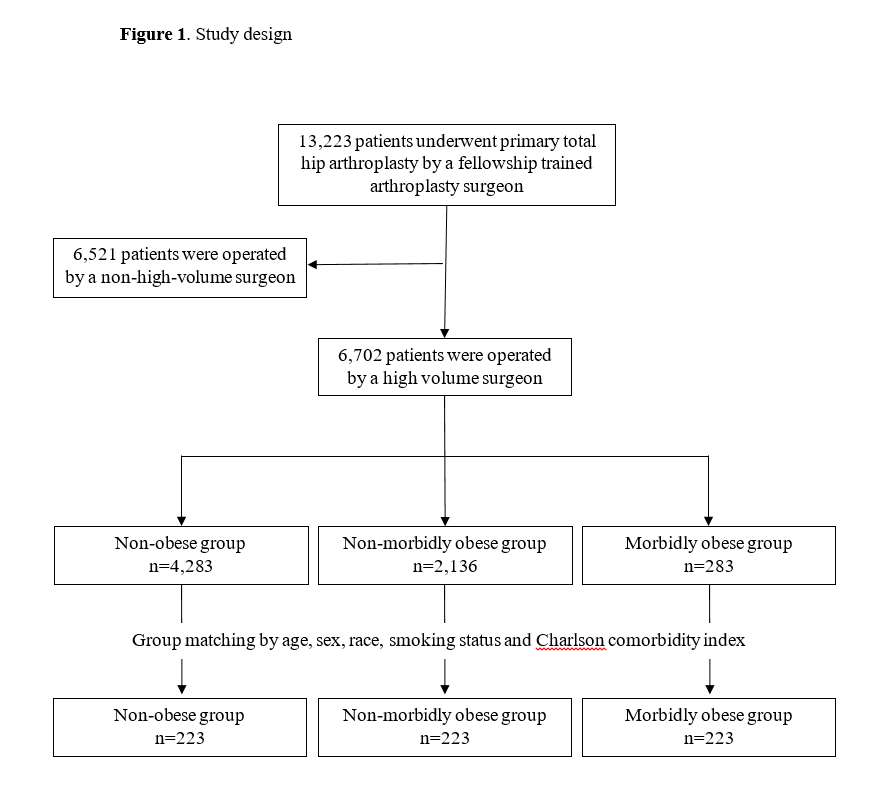
Figure 5
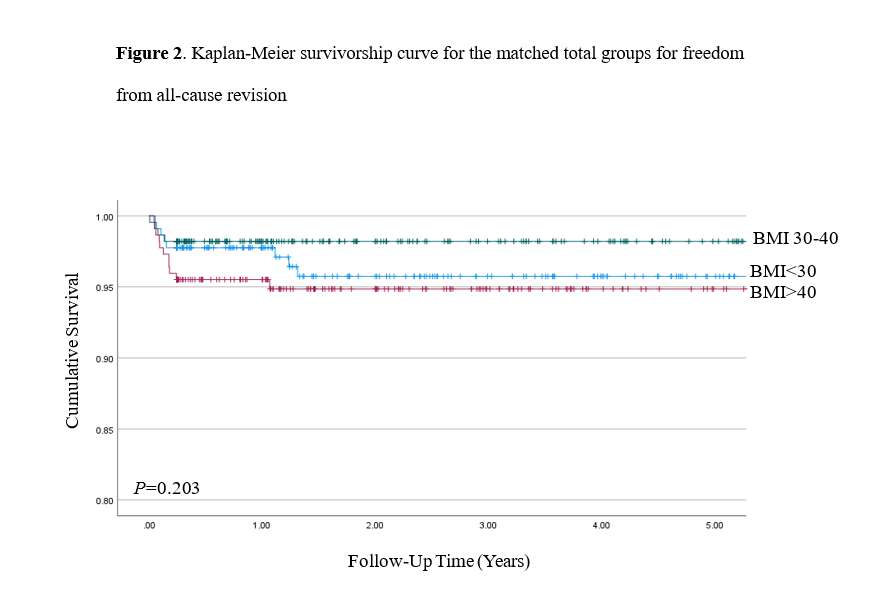
Figure 6
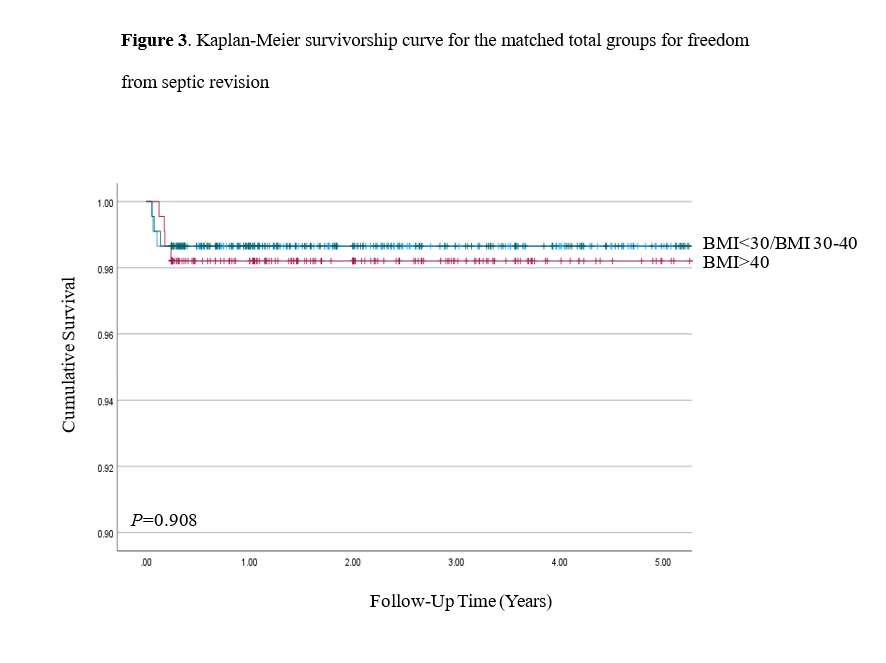
Figure 7#8758
Robot-Assisted Surgery Provides Greater Improvement in Patient-Reported Outcome Measures Following Total Hip Arthroplasty
*Weston Buehring - New York, United States of America
Emily Ronan - Langone Orthopedic Hospital - New York, United States of America
Omid Barzideh - NYU Orthopedic Hospital - New York, USA
Patrick Meere - New York University Hospital for Joint Diseases - New York, USA
Matthew Hepinstall - NYU Langone Health - New York, USA
Morteza Meftah - NYU Hospital for Joint Diseases - New York, USA
*Email: westonbuehring2013@gmail.com
Introduction
Use of technology in total hip arthroplasty (THA) continues to rise, but data are sparse regarding any effects technology may have on clinical outcomes. Therefore, the aim of this study was to explore any influence robot-assistance and computer navigation may have on patient-reported outcome measures (PROMs) after THA.
Methods
We retrospectively reviewed 2,905 primary, elective THA cases from January 2016 to April 2022 with complete preoperative, 3-month postoperative, and 1-year postoperative PROM data available. Three cohorts were created based on technology utilization: robotic (n=195, 6.7%), manual (n=1140, 39.2%), or navigation (n=1570, 54.1%). Patient demographics along with Hip disability and Osteoarthritis Outcome Score, Joint Replacement (HOOS, JR) and pain scores (PROMIS Pain Interference) were collected and compared using ANOVA tests.
Results
While preoperative and 3-month HOOS, JR scores remained similar across the 3 groups, 1-year HOOS, JR (80.5 vs. 74.0 vs. 74.0; p=0.03), delta HOOS, JR at 3-months postoperative (20.3 vs. 17.9 vs. 16.3; p=0.01), and delta HOOS, JR at 1-year postoperative (33.4 vs. 27.2 vs. 25.8; p=0.04) scores all significantly favored the robotic cohort compared to the manual and navigation groups, respectively. In addition, pain score improvements were significantly greater in robot-assist cases compared to manual and CAS cases, with greater improvements in PROMIS Pain Interference scores at 3 months (12.5 vs. 10.1 vs. 9.7; p=0.02) and 1-year (11.06 vs. 10.03 vs. 8.42; p=0.02) postoperative.
Conclusion
We observed improved HOOS, JR and pain scores at 3-months and 1-year postoperatively in robot-assisted THA compared to manual and navigated cases. This has not been a consistent finding in the limited research on this topic; additional well-powered, prospective, randomized trials are warranted.
#8429
Patient Satisfaction and Patient-Reported Outcomes Do Not Vary by BMI Class in Total Hip Arthroplasty
Nicolas Piuzzi - Cleveland Clinic - Cleveland, USA
Trevor Murray - Cleveland Clinic - Cleveland, USA
Matthew Deren - Cleveland Clinic - Cleveland, USA
*Roberta Redfern - Zimmer Biomet - Pemberville, USA
Dave Van Andel - Zimmer Biomet - Grand Rapids, USA
Craig L Israelite - Philadelphia, USA
Charles Nelson - University of Pennsylvania - Philadelphia, USA
*Email: roberta.redfern@zimmerbiomet.com
Introduction
Obesity has been identified as a risk factor for post-operative complications in patients undergoing total hip arthroplasty (THA). However, studies investigating the impact of patients’ body mass index (BMI) class on functional outcomes have provided conflicting results. This study aimed to investigate patient-reported outcomes, pain, and satisfaction as a function of BMI class in a large cohort of patients undergoing THA.
Methods
1736 patients within a large prospective observational study were included in this study and categorized into BMI classes by pre-operative height and weight. BMI distribution over the cohort demonstrated 9 (0.5%) underweight, 394 (22.7%) healthy weight, 654 (37.7%) overweight, 378 (21.8%) Class I, 196 (11.3%) Class II, and 105 (6.0%) Class III. Pre- and post-operative HOOS JR, satisfaction, and pain scores were compared by BMI class using one-way ANOVA with pairwise comparisons. 89.3% of patients provided PROMs data at 90 days and 65% provided this information at 1 year post-operatively.
Results
Pre-operative HOOS JR decreased with increasing obesity class over the cohort (Table1, p<0.0001). Pairwise comparisons revealed healthy weight patients reported significantly higher pre-operative HOOS JR than overweight patients, as well as patients in all classes of obesity (Table 1, p<0.05). Pre-operative patient-reported pain scores increased with BMI class (ANOVA, p<0.0001). Class I – III patients reported higher pain than those with healthy weights pre-operatively; Class III individuals also reported significantly higher pain than the Class I obesity group. Overall, no outcomes varied by BMI class post-operatively. Changes in HOOS JR scores from baseline suggest larger improvements with increasing BMI class, where Class III patients reported an increase of 33.7±15.6 points at 90 days compared to 26.1±17.1 in healthy weight individuals (Table 1 p=0.002). On pairwise comparisons, Class II and Class III patients reported larger HOOS JR improvements than healthy and overweight patients at 90 days post-operative. The Class II group demonstrated the largest proportion reaching MCID at this point (96.5%) compared to 87.4% in healthy weight individuals (p=0.003). Change in satisfaction (21.3±13.0, p=0.02), and pain scores (-5.0±3.0, p<0.0001) were largest in the Class III obesity group at 90 days post-operatively compared to 18.98±12.19 points improvement in satisfaction and -3.98±2.62 pain reduction in healthy weight patients. All obesity classes versus healthy weight exhibited significant change in pain at 90 days post-operatively on pairwise comparisons (p<0.0001). Class III patients continued to demonstrate the largest improvement in HOOS JR at one-year post-operative (41.1±17.07), where healthy weight individuals improved by 34.01±15.23 (p=0.002). Distribution of patients reaching MCID at one year did not differ by class (94.6% - 100%, p=0.35).
Conclusion
Overall patients of higher BMI class reported greater improvements in hip function, pain, and joint satisfaction following THA compared to healthy weight individuals. Individuals with the highest BMI class appeared to appreciate the greatest benefits as measured by HOOS JR, satisfaction, and pain scores. While risk/benefit shared decision making remains a personalized and case by case requirement of THA, this study highlights that the utilization of BMI cutoff points BMI may not be warranted based on pain and functional improvement.
#8294
Prior Shoulder Arthroscopy Is Associated With Worse Outcomes Following Primary Reverse Shoulder Arthroplasty
Garrett Jackson - Chicago, United States of America
Christopher Brusalis - Rush University Medical Center - Chicago, USA
Clyde Fomunung - Palm Beach Shoulder Service - Lantana, USA
Carlos Fernandez - JFK / U Miami Orthopedic Surgery Program - Lantana, USA
*Vani Sabesan - Cleveland Clinic Florida - Weston, USA
Howard Routman - Atlantis Orthopedics - Atlantis, USA
*Email: sabes001@gmail.com
Introduction: Expanded indications for reverse shoulder arthroplasty (RSA) have led to its frequent use as an end-stage treatment when patients have persistent rotator cuff pathology or recurrent symptoms following shoulder arthroscopy. However, the extent to which prior shoulder arthroscopy impacts clinical outcomes following RSA remains poorly characterized. The aim of the present study was to evaluate the impact of previous ipsilateral shoulder arthroscopy on patient-reported outcomes and range of motion following RSA.
Methods: All patients who underwent RSA for either rotator cuff arthropathy or glenohumeral osteoarthritis from June 2014 to September 2019 by a single surgeon were retrospectively identified through a prospectively collected database. Patients who underwent RSA following prior ipsilateral shoulder arthroscopy were propensity-matched based on sex and age to a control group of patients who underwent RSA without previous shoulder surgery. Prior shoulder arthroscopy procedures included rotator cuff repair (70%), anterior instability repair (5%), and diagnostic arthroscopy and debridement (25%). Patient-reported outcomes (PROs), including the Simple Shoulder Test (SST), American Shoulder and Elbow Surgeons score (ASES), University of California-Los Angeles (UCLA) score, and active range of motion were measured preoperatively and at a minimum 2 years postoperative.
Results: Forty patients (n=20 RSA with prior arthroscopy (RSAPA), n=20 control RSA) were analyzed in the cohort. Mean duration of follow-up for patients with prior ipsilateral shoulder arthroscopy and control patients was similar (p = 0.449) (RSAPA= 40.9 ± 14.4 months and RSA=44.5 ± 16 months. All PROs improved postoperatively (p < 0.0001). Improvements in PROs for RSAPA were diminished relative to RSA control group patients, including SST (RSAPA=4.6 vs RSA=8, p = 0.002), ASES (RSAPA=40.5 vs RSA=56.4, p = 0.011), and UCLA (RSAPA=14.5 vs RSA=21.9, p = < 0.0001) scores. Sixty-five percent of patients who had prior shoulder arthroscopy and 95% of control patients without prior shoulder arthroscopy rated their postoperative forward elevation as “normal” (p = 121). One patient (5%) with prior shoulder arthroscopy required revision due to recurrent instability, whereas no patients in the control group required revision surgery.
Conclusion: Patients who underwent RSA following prior ipsilateral shoulder arthroscopy had inferior two-year postoperative clinical outcomes scores and decreased shoulder range of motion compared to a propensity-matched control group of patients who underwent RSA without prior shoulder arthroscopy. This is concerning given the rates of shoulder arthroscopy and the utilization of RSA as a salvage procedure. Further research is needed to understand the relationship of prior arthroscopy and risk factors that negatively impact outcomes after RSA.
#8308
Comparison of Clinical Outcomes Using Inlay Versus Onlay Humeral Trays in Reverse Shoulder Arthroplasty for Patients With Cuff Tear Arthropathy
*Stephen Weber - Sacramento Knee & Sports Medicine - Sacramento, USA
Edward McFarland - Johns Hopkins School of Medicine - Baltimore, USA
Prashant Meshram - Johns Hopkins - Baltimore, USA
Uma Srikumaran - Johns Hopkins - Baltimore, USA
Punyawat Apiwatanakul - Johns Hopkins - Baltimore, USA
*Email: webersc@earthlink.net
The inlay design of humeral tray in reverse shoulder arthroplasty (RSA) has been suggested to have the advantage of better humeral side fixation, but there are concerns of greater tuberosity fracture. In comparison, onlay humeral tray in RSA are suggested to have the advantages of impingement free range of motion and reduced scapular notching, but there are concerns of increased scapula stress fractures. The aim of this study was to compare the clinical results among patients with CTA undergoing RSA with two prostheses having lateralized glenosphere and 135° NSA, but which differed in the position of the humeral tray as either inlay or onlay design.
Methods:
This was a retrospective study of prospectively obtained data from a single institutional database of shoulder division of a tertiary care center and was approved by our institutional review board. The database was searched for all patients who underwent primary RSA between 2009 to 2017 (N=511). To be included, patients with a diagnosis of and cuff tear arthropathy had to be treated with a RSA prostheses having a lateralized glenosphere and 135? NSA either with an inlay or onlay humeral tray design. 102 patients met the inclusion criteria and had a minimum of 2 years follow up (mean, 44, range 24-125 months). Of the included 102, 63 (62%) had implanted a RSA design with an inlay humeral tray (inlay group) and 39 (38%) had onlay tray (onlay group). All patients underwent a preoperative and postoperative evaluation including a physical examination for range of motion (ROM), radiographs, and multiple PROs (ASES, SST, and WOOS score). The clinical significance was evaluated using published minimal clinically important difference (MCID) values.
Results
Preoperatively there were no significant differences in the two groups demographically except for more proportion of females in the inlay group (75% vs 56%, P=0.04). The preoperative PROs and ROM were not statistically different between the inlay and onlay groups. The comparison of final follow up PROs and ROM including external rotation were not statistically or clinically significantly different between inlay or onlay groups.
There was no statistically significant difference between the inlay and onlay design for baseplate loosening (3% vs 5%, P=0.63) and revision (0% vs 5%, P=0.07). Of 3 patients in the onlay group who required a revision, the reason was baseplate failure in one patient, instability in another patient, and periprosthetic shoulder infection in the third patient. The rate of acromial stress fracture (3 vs 5%, P=0.63) and prosthesis dislocation (0 vs 2.5%, P=0.20) were also similar between inlay and onlay groups (Table 3). There was no difference between the onlay and inlay groups postoperatively for the rate scapula notching neither by incidence (21% vs 8%, P=0.08) nor by distribution.
Conclusion
This study found that at a two-year minimum follow up, the position of humeral tray as either inlay or onlay did not influence the clinical outcomes of function, range of motion, complications including baseplate loosening and acromial stress fracture, and scapula notching.
Figures
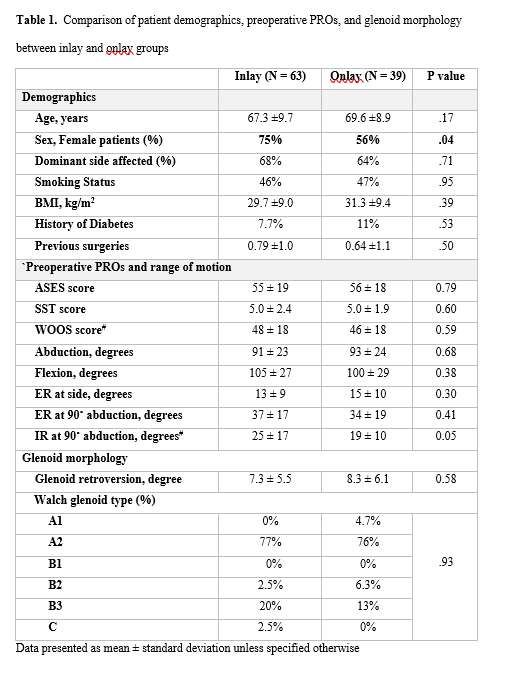
Figure 1
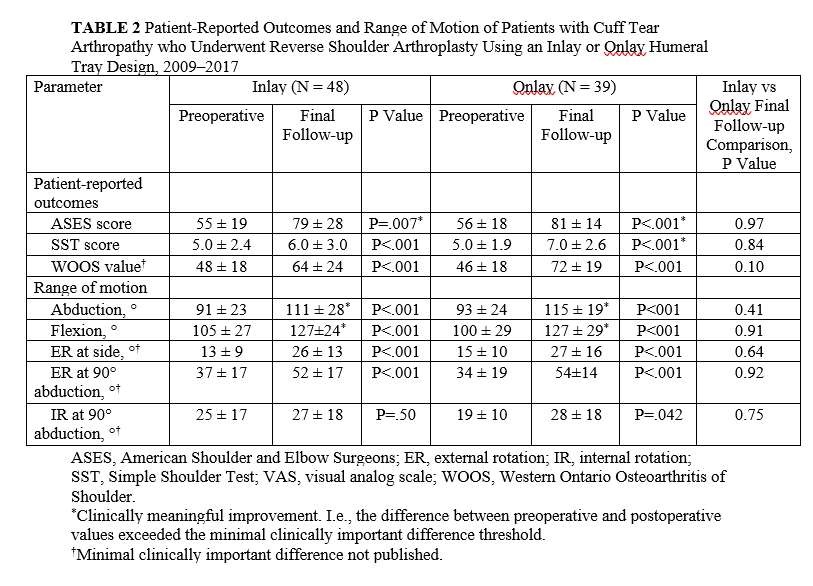
Figure 2#8637
Effect of Steroid Injections on Outcomes Following Shoulder Arthroplasty
Carlos Fernandez - JFK / U Miami Orthopedic Surgery Program - Lantana, USA
*Vani Sabesan - Cleveland Clinic Florida - Weston, USA
Clyde Fomunung - Palm Beach Shoulder Service - Lantana, USA
Sarah Girshfeld - Florida Atlantic University Charles E Schmidt College of Medicine - Boca Raton, USA
Brandon Macknofsky - Florida Atlantic University Charles E Schmidt College of Medicine - Boca Raton, USA
Howard Routman - Atlantis Orthopedics - Atlantis, USA
Benjamin Lack - New York, United States of America
*Email: sabes001@gmail.com
Introduction:?Steroid injections are well-known short-term treatments for glenohumeral osteoarthritis; however, many patients eventually require more definitive management with surgical treatment. Recent literature has called into question the utility and safety of steroid injections prior to shoulder surgery due to increased infection and revision rates. Conclusive data regarding the relationship of preoperative injection and postoperative outcomes is lacking. The purpose of this study was to determine the impact of ipsilateral preoperative injections on clinical outcomes following shoulder arthroplasty (SA).
Methods:?A retrospective study was performed on 563 patients who underwent SA by a single fellowship-trained orthopedic surgeon from 2017-2020. Patients were divided into two groups: 240 received a preoperative injection (IG) and 323 were in the control group (CG). Patient reported pain and satisfaction, simple shoulder test (SST), shoulder pain and disability index (SPADI), complications, reoperations, and range of motion (ROM) were compared between groups. Change (delta) in clinical and functional outcomes were calculated from the pre- to postoperative period and compared.
Results:?The cohort was comprised of 55% females with an average age of 71.1, BMI of 29.6, and mean follow-up of 21.5 months. The IG group had a significantly greater proportion of females (64%; p<0.01) and older age of 72.9 (p<0.01). The number of comorbidities between groups was comparable. There was a significantly greater postoperative improvement in range of motion with forward elevation (70 vs. 80, p=0.025) and abduction (60 vs. 70, p=0.030) in patients who did not have a corticosteroid injection within the past year. Patients in the IG group had significantly greater delta in reported outcomes on the SST (+6.25 vs. +5.37, p = 0.005). Patient satisfaction, complication rates (p=0.98) and reoperation rates (p=0.98) were comparable between groups.
Conclusions:?Ipsilateral shoulder injections prior to SA leads to less improvements in function but better patient satisfaction without an increase in complications. Surgeons can consider continuing to use injections as a viable first line management option before SA without concerns for increased complications, but further research is needed on the impact of these injections on postoperative function and pain.
#8310
Comparison of Outcomes of Reverse Shoulder Arthroplasty in Patients With Dislocation Arthropathy With Matched Cohort of Patients With Glenohumeral Osteoarthritis With Severe Glenoid Bone Loss
*Stephen Weber - Sacramento Knee & Sports Medicine - Sacramento, USA
Punyawat Apiwatanakul - Johns Hopkins - Baltimore, USA
Prashant Meshram - Johns Hopkins - Baltimore, USA
Ridge Maxson - Johns Hopkins - Baltimore, USA
Uma Srikumaran - Johns Hopkins - Baltimore, USA
Edward McFarland - Johns Hopkins School of Medicine - Baltimore, USA
*Email: webersc@earthlink.net
Dislocation arthropathy (DA) of the shoulder is due to glenohumeral dislocations with or without previous stabilization surgery. Reverse shoulder arthroplasty (RSA) has emerged as a favorable treatment option in patients with DA but the literature of clinical results is limited to few studies with small sample size. The goal of this study was to compare the clinical results of RSA in patients with DA with patients having glenohumeral osteoarthritis with intact cuff and severe bone loss.
Methods This is a retrospective matched cohort study of 13 patients with DA who were treated with RSA by one surgeon between 2011 and 2019 and a 3 to 1 matched control group of 39 patients with osteoarthritis and similar amounts of glenoid bone loss. All patients in both groups had a minimum of two years of follow-up and were treated with the same RSA system: a lateralized glenosphere, a 135° neck shaft angle, and an uncemented humeral stem (Figure 1). Glenoids were reamed eccentrically until there was at least 90% coverage of the baseplate. No bone grafting or augmented glenoid components were utilized.
Results The implant survival at follow-up was 100% in the DA group and 98% (38/39) in the OA group. In the DA group there were no signs of baseplate loosening, osteolysis nor notching in any patient. The complication rate was similar in the two groups (15% vs 13%, P=0.41). Both groups showed statistically significant improvements in PROs (SST, ASES, WOOS, SANE, satisfaction) and ROM following RSA procedure. At final follow-up there was there was no statistically significant difference between the groups in PROs and ROM (Figure 2).
Conclusion With the available numbers, this study found that the clinical results of RSA for DA treated with eccentric reaming are comparable to the results of a matched cohort of OA patients with similar treatment. At the short term follow up, RSA with eccentric glenoid reaming is a valid treatment strategy in patients with DA but studies with larger sample size and longer follow up are warranted.
Figures
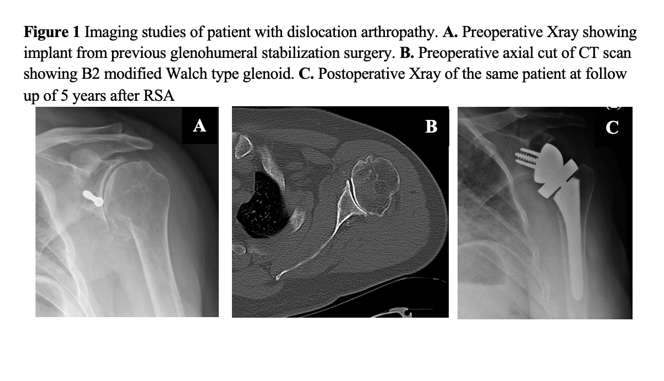
Figure 1#8594
Acromiohumeral Distance: Can Radiographic Factors Impact Outcomes After Reverse Shoulder Arthroplasty?
*Carlos Fernandez - JFK / U Miami Orthopedic Surgery Program - Lantana, USA
Vani Sabesan - Cleveland Clinic Florida - Weston, USA
Clyde Fomunung - Palm Beach Shoulder Service - Lantana, USA
Feyikemi Ogunfuwa - Florida Atlantic University Charles E Schmidt College of Medicine - Boca Raton, USA
Ajay Desai - Florida Atlantic University Charles E Schmidt College of Medicine - Boca Raton, USA
Howard Routman - Atlantis Orthopedics - Atlantis, USA
Jake Goguen - New York, United States of America
*Email: carlosfernandezpeaguda@gmail.com
Introduction: Reverse shoulder arthroplasty (RSA) is highly effective in restoring clinical function and reducing pain, however optimizing outcomes and minimizing complications is a priority. Various risk factors including radiographic measurements and biomechanical factors have emerged as possible tools useful to predict clinical outcomes and potential complications after RSA. The purpose of this study was to investigate the association between radiographic measurements for arm lengthening and clinical outcomes in patients following RSA.
Methods:?A retrospective review of 63 patients who underwent RSA?performed by a single surgeon from August 2017 to February 2020 was performed. The preoperative and postoperative Acromiohumeral Distance (AHD) were obtained from radiographs and used to determine arm lengthening (AHDdelta). The β angle was also calculated from radiographs. Functional outcomes and patient reported outcomes including the Simple Shoulder Test (SST), Constant Score, American Shoulder and Elbow Surgeons Score (ASES), University of California at Los Angeles (UCLA) Shoulder Score, Shoulder Pain and Disability Index (SPADI), and Shoulder Arthroplasty Smart (SAS) Score were collected with a minimum of 1-year follow up.? Radiographic measurements were correlated to clinical, functional, and patient satisfaction outcome scores using Pearson’s correlation coefficient tests.
angle was also calculated from radiographs. Functional outcomes and patient reported outcomes including the Simple Shoulder Test (SST), Constant Score, American Shoulder and Elbow Surgeons Score (ASES), University of California at Los Angeles (UCLA) Shoulder Score, Shoulder Pain and Disability Index (SPADI), and Shoulder Arthroplasty Smart (SAS) Score were collected with a minimum of 1-year follow up.? Radiographic measurements were correlated to clinical, functional, and patient satisfaction outcome scores using Pearson’s correlation coefficient tests.
Results: The mean age of the cohort was 73.3 ± 8.7 years with an average BMI of 28.5 ± 5.8 at the time of surgery. The mean follow-up was 19 ± 7.3 months.?The mean arm lengthening and postoperative β angle were 2.36 ± 0.9cm and 89.6 ± 10.6°, respectively. Arm lengthening was significantly correlated to improvement of daily pain (r = 0.277, p = 0.030), with the most improvement observed in arm lengthening ranging from less than 0 cm to 1.5 cm. Postoperative β angle had significant correlations with improvement of SPADI and SST scores. All other correlations were not statistically significant.
Conclusion: As expected, the results of our study showed AHD increased postoperatively after RSA. Less arm lengthening had the greatest decrease in pain and optimal glenoid inclination correlated with improved outcome scores. There remains a debate regarding the optimal arm lengthening and inclination for RSA to restore active range of motion while minimizing complications. Establishing optimal arm lengthening parameters radiographically is needed to understand the relationship with clinical outcomes and guide surgeons on best practices for surgical techniques.
#8171
Muscle Activation Patterns and Torque Output During External Rotation (ER) After a Reverse Total Shoulder Arthroplasty Comparing Coper and Non-Coper ER Patient Populations
*Alexis Nelson - UTHSC - Memphis, Select Country
Blake Hajek - UTHSC - Memphis, USA
Jordan Justice - University of Tennessee Health Science Center - Memphis, USA
Willie Polio - Campbell Clinic - Memphis, USA
Tyler Brolin - Campbell Clinic - Germantown, United States of America
Shannon Hughes - UTHSC - Memphis, USA
David Bernholt - Campbell Clinic - Memphis, USA
Frederick Azar - Campbell Clinic - Memphis, USA
Thomas Throckmorton - Campbell Clinic - Germantown, USA
William Mihalko - University of Tennessee - Memphis, USA
*Email: anelso70@uthsc.edu
Introduction: Reverse total shoulder arthroplasty (RTSA) is a reliable treatment for restoring shoulder function. RTSA patient outcomes to return to functional external rotation (ER) is less predictable and needed for activities of daily living. ER muscle activation patterns with RTSA are not well understood in teres minor deficient patients. Therefore, the purpose of this study was to examine the timing of upper extremity muscle activation patterns and torque output during shoulder ER and internal rotation (IR) in teres minor deficient patients who have a well-coping and non-well-coping RTSA during external rotation.
Methods: Preliminary data of fourteen teres minor deficient patients who had a well-coping (n=9, Coper) and non-well-coping (n=5, Non-Coper) RTSA for external rotation were recruited to participate. Biomechanical analysis of their post-operative shoulder during ER and IR were performed in the moderate neutral position (MN) and abduction (AB) position. Seven surface EMGs and 2 needle EMGs were placed prior to participation. The dynamometer recorded torque and position at (100 Hz) and electromyography (2000 Hz; EMG), simultaneously. Patients performed 5 movement trials in both AB and MN conditions on the dynamometer. Visual 3D was used to identify external rotation time series. Custom software (MATLAB, 2021a) was utilized to perform Teager-Kaiser energy operator on EMG signals to determine EMG onset and offset from which integrated RMS EMG was calculated.
Results: In the patient groups, the sequence of iEMG (microvolts*msec) muscle activation timing from maximal IR to maximal ER began with the pectoralis major and scapular stabilizers (upper trapezius (15.7), middle trapezius (21.6), and latissimus dorsi (19.9)), followed to external rotation the anterior deltoid (18.6) and serratus anterior (28.2) activated past neutral into ER. The non-Coper muscle activation patterns were similar compared to the Coper group, however the teres major assisted from neutral through ER and altered similarly in the AB position (39.7) (Figure 2A and 2B). No differences were found for peak ER torque output in the Coper compared to the non-Coper group for either MN or AB position (Figure 1A and 1B).
Conclusion: After RTSA, individually mapped muscle activation patterns and peak ER torque output were similar in the Coper compared to the non-Coper group for external rotation. Muscle activation patterns are altered in the moderate neutral compared to abduction position. Our findings show the important role of the anterior deltoid (AD) and serratus anterior (SA) to help perform ER. This preliminary study elaborates the complexity of the upper extremity for rehabilitation post RTSA operations where rehabilitation should include the altered anatomy muscle activations of the RTSA shoulder to recover ER fully. A limitation to the study was power was not sufficient to reach significance, further enrollment and data collections are required to analyze differences between patient groups, however this study provides potential trends to follow once data collections are completed. Future studies should analyze EMG intensity in addition to activation timings.
Figures
Figure 1
Figure 2#8255
Evaluation of Deltoid Elongation in Preoperative Planning of Reverse Shoulder Arthroplasty Is a Potential Decision Support Tool to Prevent the Risk of Scapula Fracture
*Sanne Vancleef - Leuven, Belgium
Filip Jonkergouw
Katrien Plessers - Materialise - Leuven, Belgium
Roel Wirix-Speetjens - Materialise NV - Leuven, Belgium
Sebastian DeBoodt - Materialise - Leuven, Belgium
*Email: sanne.vancleef@materialise.be
Introduction
Inadequate muscle elongation in reverse shoulder arthroplasty (RSA) could lead to instability, limited range of motion and acromial fractures. However, only a limited number of studies assessed the optimal elongation, suggesting that deltoid elongation between 10 and 20% could improve shoulder joint function[1,2]. Additionally, Acott et al suggest that over-tensioning the deltoid could lead to scapular fractures [3]. The goal of this study was to perform a retrospective analysis to assess whether the elongation defined by a personalized 3D model integrated in pre-operative planning software aligns with those reported in literature, and to evaluate the muscle elongation of cases where a scapular fracture occurred.
Methods
The 3D mathematical model introduced by Pitocchi et al [4] was integrated in TRUMATCH™ Personalized Solutions Shoulder System (Materialise, Leuven). A retrospective analysis of 356 surgeon approved, anonymized RSA plans was conducted. Muscle elongations of the deltoid, infraspinatus, teres minor and subscapularis were exported from the planning software for each case. The mean and standard deviation of each muscle were calculated, and a boxplot was generated in Python. Additionally, pre- and post-operative CT scans of two patients with scapular fractures were processed in the planning software to derive muscle elongations.
Results
The mean (±standard deviation) elongation was 13.2% (±4.3%), -11.7% (± 5.3%), -19.7% (± 7.6%), -9.9% (± 8.0%) for the deltoid, infraspinatus, teres minor and subscapularis muscle respectively. Fig. 1 shows the Whisker boxplot of the measured muscle elongations and highlights the 2 cases in which a scapular fracture occurred.
Conclusion
Overall, RSA was found to lengthen the deltoid and shorten the rotator cuff, which aligns with the findings of Roche et al. Moreover, the measured deltoid elongations are consistent with previously reported values. Notably, the deltoid elongation in the two scapular fracture cases exceeded the 75th percentile of the retrospective cases, supporting the hypothesis that over-tensioning the deltoid may increase scapula fracture risk. However, other risk factures such as bone density, may need to be considered to help surgeons refine their surgical plan and to reduce scapular fractures risk. This study did not differentiate between the muscle elongation associated with different implant systems, which will be explored in future research.
References
[1] L. De Wilde, et al., ‘Consequences of deltoid muscle elongation on deltoid muscle performance: a computerised study’, Clinical Biomechanics, vol. 17, pp. 499–505, 2002
[2] C. P. Roche et al., ‘Impact of Inferior Glenoid Tilt, Humeral Retroversion, Bone Grafting, and Design Parameters on Muscle Length and Deltoid Wrapping in Reverse Shoulder Arthroplasty’, Bull Hosp Joint Dis, vol. 71, no. 4, pp. 284–293, 2013
[3] T. R. Acott, et al., ‘A quantitative analysis of deltoid lengthening and deltoid-related complications after reverse total shoulder arthroplasty: A retrospective case-control study’, Curr Orthop Pract, vol. 31, no. 2, pp. 126–132, 202AD
[4] J. Pitocchi et al., ‘Automated muscle elongation measurement during reverse shoulder arthroplasty planning’, J Shoulder Elbow Surg, vol. 30, no. 3, pp. 561–571, Mar. 2021
Keywords: muscle elongation, reverse shoulder arthroplasty, scapular fractures, pre-op planning
Figures
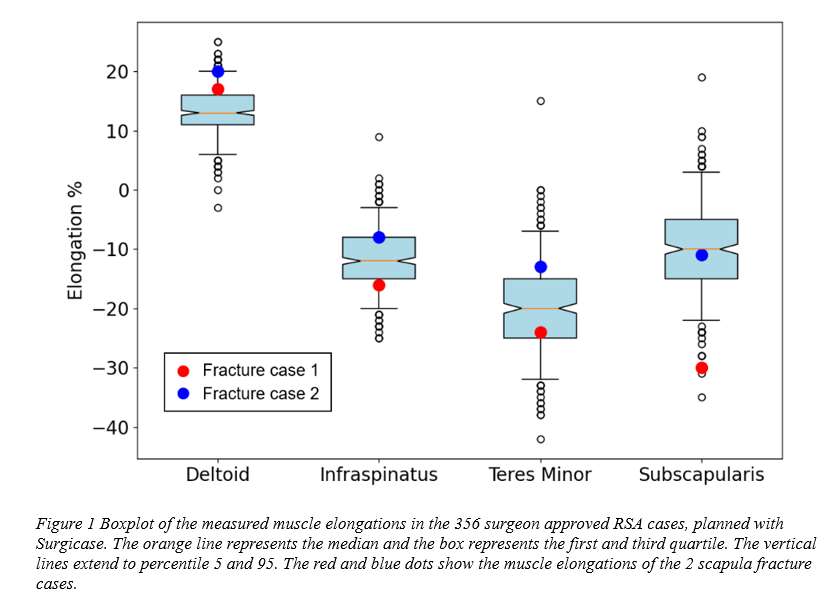
Figure 1#8639
Reverse Shoulder Arthroplasty for Proximal Humeral Fractures: Better Treated Acute Versus Chronic Malunions?
Carlos Fernandez - JFK / U Miami Orthopedic Surgery Program - Lantana, USA
*Vani Sabesan - Cleveland Clinic Florida - Weston, USA
Feyikemi Ogunfuwa - Florida Atlantic University Charles E Schmidt College of Medicine - Boca Raton, USA
Clyde Fomunung - Palm Beach Shoulder Service - Lantana, USA
Howard Routman - Atlantis Orthopedics - Atlantis, USA
Jake Goguen - New York, United States of America
*Email: sabes001@gmail.com
Proximal humeral fractures are common in patients over the age of 60 years, but the optimal management remains debated. Reverse shoulder arthroplasty (RSA) has recently emerged as the preferred operative management option for complex proximal humeral fractures in the elderly. Although RSA seemingly provides satisfactory clinical and functional outcomes in patients with complex proximal humeral fractures (PHF), little is known of the clinical and functional outcomes of patients with delayed RSA treatment of proximal humerus fractures. The purpose of this study was to compare postoperative clinical outcomes in patients with acute PHFs versus malunions of PHF treated with RSA.
A retrospective clinical evaluation of 42 patients who underwent RSA performed by two fellowship-trained shoulder surgeons in a single institution from 2012 to 2022 was performed. The acute group consisted of 29 patients while 13 patients were in the malunion group. Preoperative and postoperative clinical outcomes including range of motion, Simple Shoulder Test (SST), Constant Score, ASES Shoulder Score, UCLA Score, Shoulder Pain and Disability Index (SPADI), and Shoulder Arthroplasty Smart (SAS) score were recorded and assessed using paired t-test.
The mean age of patients included in this study was 72 years old with an average BMI of 25.6 at the time of surgery and average follow up of 7 months. The majority of the cohort was Caucasian (97.6%) and female (85.7%). Demographics were comparable between the acute and malunion groups. Overall, the postoperative range of motion was 105° of active forward elevation, 96.3° of active abduction, 22.5° of active external rotation, and 2.4° for internal rotation for the cohort. When comparing acute versus malunions, those in the malunion group had significantly higher SPADI scores when compared to the acute group (63.3 vs 40.24, p = .012). At final follow up, 62.1% of patients rated their satisfaction as better or much better. Regarding complications, there was 1 case of scapular notching and no patients required revision surgery. The range of motion, complications, satisfaction, and other outcomes were not significantly different between the groups.
RSA for proximal humerus fractures resulted in a 60% patients satisfaction rate with minimal complications. Regardless, if patients treated in the acute setting or for malunions with an RSA there were comparable outcomes and patient reported satisfaction. Ultimately, patients and surgeons can work together for optimal timing of RSA for proximal humerus fractures in the elderly without sacrificing improvements in function or patient reported outcomes.
#8252
Can Standard Test Specimens Inform the Mechanical Behaviour of Real Implant Designs?
*Stylianos Kechagias - Imperial College London - London, United Kingdom
Jonathan Jeffers - Imperial College London - London, United Kingdom
Kabelan Karunaseelan - Imperial College London - DUNSTABLE, United Kingdom
Reece Oosterbeek - University of Oxford - Oxford, United Kingdom
*Email: s.kechagias20@imperial.ac.uk
Introduction
Modern cementless implants consist entirely or partially of lattice structures to provide a stiffness close to the one of bone that maintains bone homeostasis and promotes bone in-growth. For this purpose, lattice porosity and topology are designed to maximise its biological and mechanical function. Mechanical characterisation is done by testing a small cylindrical or rectangular specimen which is assumed representative of the utilised lattice. Testing standards (e.g., ISO 13314 and ASTM D1621) suggest specimens to be adequately large with an aspect ratio (length to diameter/width ratio ζ) between 1 and 2. Although it is well-known that aspect ratio can affect the mechanical properties of other engineering materials, no previous work has investigated what happens in lattices. Yet, lattices in orthopaedic implants are designed outside the aspect ratio bounds of standardised specimens, for example ζ<1 in tibial trays while ζ>1 in femoral stems. This study investigates the effect of aspect ratio in the mechanical behaviour and properties of lattice structures.
Methods
Lattice structures of three topologies were designed using different aspect ratios (ranging from 0.5 to 3) either as cylindrical or rectangular specimens. The selected topologies were a body-centred cubic (BCC) structure (80% porosity), an octet-truss structure (70% porosity) and a stochastic structure with high connectivity (90% porosity). This enabled to take into consideration different micro-architectures (stochastic vs. periodic), different specimen shapes (cylindrical vs. rectangular) and different range of porosities.
Specimens were fabricated with uniform strut thickness of 230 μm using Selective Laser Melting and pure titanium powder. Quasi-static compression tests were performed to determine the elastic modulus and ultimate strength from the acquired stress-strain curves (n=5 per specimen type), while digital image correction was used to capture the developed strains inside selected specimens.
Results
Regardless of aspect ratio, all BCC specimens exhibited a ductile behaviour with failure through layer-by-layer collapse (struts in this case bend), while the stochastic and octet-truss lattices exhibited a quasi-brittle behaviour and failed through shear band formation (struts experience axial loading). This indicated that the loading mode of struts is intrinsic of lattice’s topology and independent of aspect ratio. DIC images however demonstrated that the strain distribution inside the specimens heavily depended on aspect ratio and topology. This altering patterns of strain distribution led the mechanical properties of each topology to converge differently for altering aspect ratio. Specimens with aspect ratio between 1 and 2 had statistically similar properties, however stiffness increased up to 40% for decreasing aspect ratio (ζ<1) in the BCC lattices [Fig. 1] and increased up to 30% for increasing aspect ratio (ζ>2) in both quasi-brittle structures [Fig. 2,3]. On the contrary, ultimate strength increased for decreasing aspect ratio in all cases with the maximum difference being 24%.
Conclusion
Aspect ratio does not alter the micro-deformation of a lattice; however, the local strain distribution and mechanical properties of lattices drastically change for extreme aspect ratios. This is important to consider when designing implants that should match the stiffness of bone since standardised specimens are not indicative of structure’s behaviour in real applications.
Figures
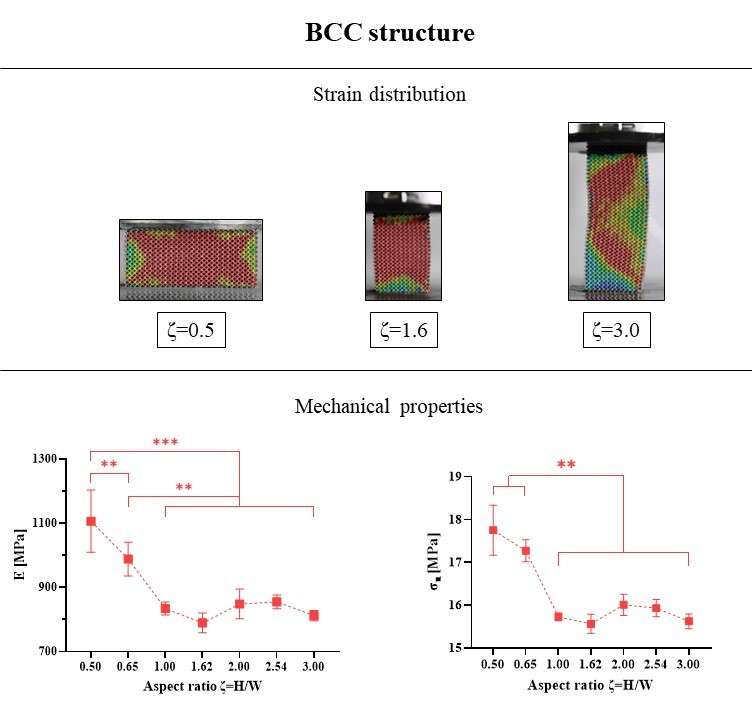
Figure 1
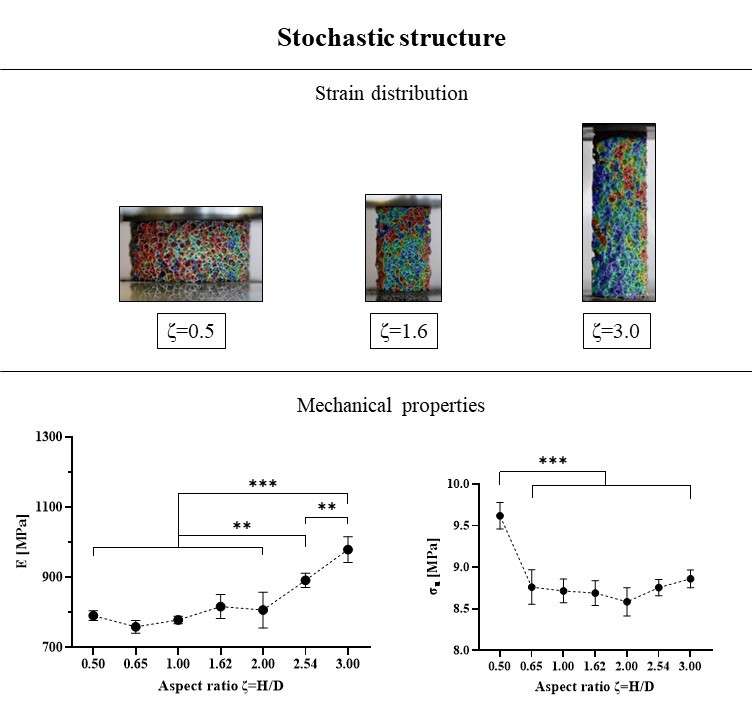
Figure 2
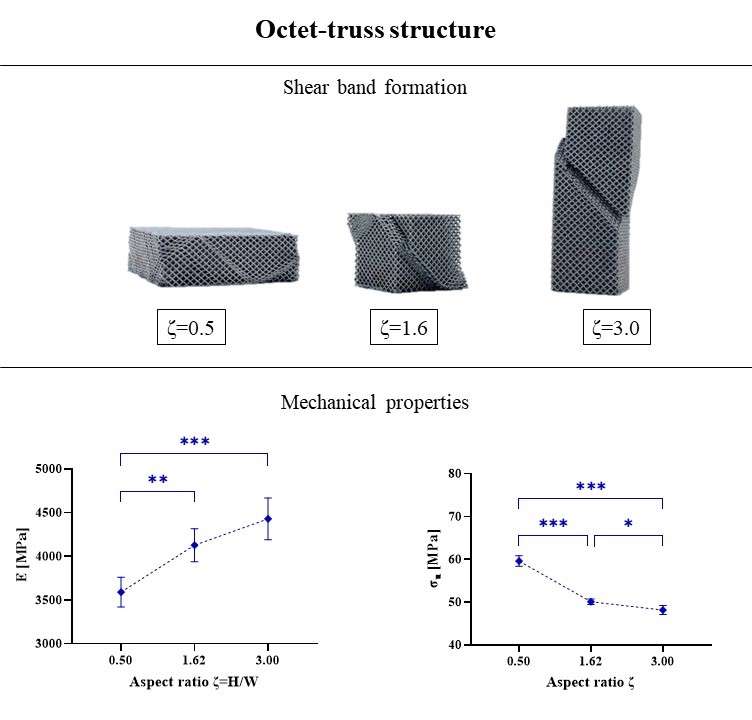
Figure 3#8230
Structural Defects and Variable Porosity in 3D Printed Cups From 6 Manufacturers
*Harry Hothi - London Implant Retrieval Centre - Stanmore, United Kingdom
Johann Henckel - Royal National Orthopaedic Hospital - London, United Kingdom
Anna Di Laura - Royal National Orthopaedic Hospital and Dept. MechEng at UCL - London, United Kingdom
Alister Hart - Royal National Orthopaedic Hospital - London, United Kingdom
*Email: h.hothi@ucl.ac.uk
Introduction:
3D printing is rapidly being adopted to manufacture orthopaedic implants due to the advantages of being able to print complex shaped designs, with greater control of porous structures to enhance bone growth. This clinical advantage is particularly seen in the printing of custom-made titanium cups to treat patients with large acetabular defects.
There is a known risk however of structural defects occurring in 3D printed components in the form of voids, which may impact their structural integrity. There are also no established standards to guide the design of bone-facing porous structures, meaning that manufacturers may employ different approaches to this.
The aim of this study was to investigate the differences in the porous structures and potential structural defects between a series of 3D printed titanium implants.
Methods:
We analysed 12 unused, final-production custom-made 3D printed acetabular cups that had been produced by 6 orthopaedic manufacturers, Figure 1. We performed high resolution micro-CT imaging of each cup using previously optimised and published scanning parameters. The scan images were segmented using an automated thresholding process and 3D models of each cup were reconstructed for analysis.
These were first used to characterise the morphometric features of the porous layers: (1) the level of porosity, (2) pore size, (3) thickness of porous struts and (4) the depth of the porous layers.
We then examined the internal cup structures to identify the presence of any voids and to characterise: (1) the total number of voids, (2) their volume and volume fraction, (3) sphericity, (4) size and (5) location.
Results:
There was a variability between designs in the level of porosity (34% to 85%, Figure 2), pore size (0.74 to 1.87mm), strut thickness (0.28 to 0.65mm), and porous layer depth (0.57 to 11.51mm). One manufacturer printed different porous structures between the cup body and flanges; another manufacturer printed two differing porous regions within the cup body.
We found 5 cups contained a median (range) of 90 (58-101) structural voids, Figure 3. The median volume of the voids and the resulting volume fractions of these cups were 5.17 (1.05-17.33) mm3 and 99.983 (99.972 – 99.998) % respectively. The median void sphericity and size were 0.47 (0.19-0.65) and 0.64 (0.27-8.82) mm respectively. The voids were predominantly located adjacent to screw holes, within flanges and at the transition between the flange and main cup body; these were between 0.17 and 4.66mm from the cup surfaces.
Conclusion:
We found a wide variability between manufacturers in the porous titanium structures they 3D print. Longer term clinical data will help explain how this variability impacts the integrity of bony fixation.
Whilst no fractures of 3D printed implants have been reported clinically, the size, shape and location of the structural voids identified are such that there may be an increased risk of crack initiation from them, potentially leading to a fracture. Regulators, surgeons and manufacturers should be aware of this variability in final print quality.
Figures

Figure 1
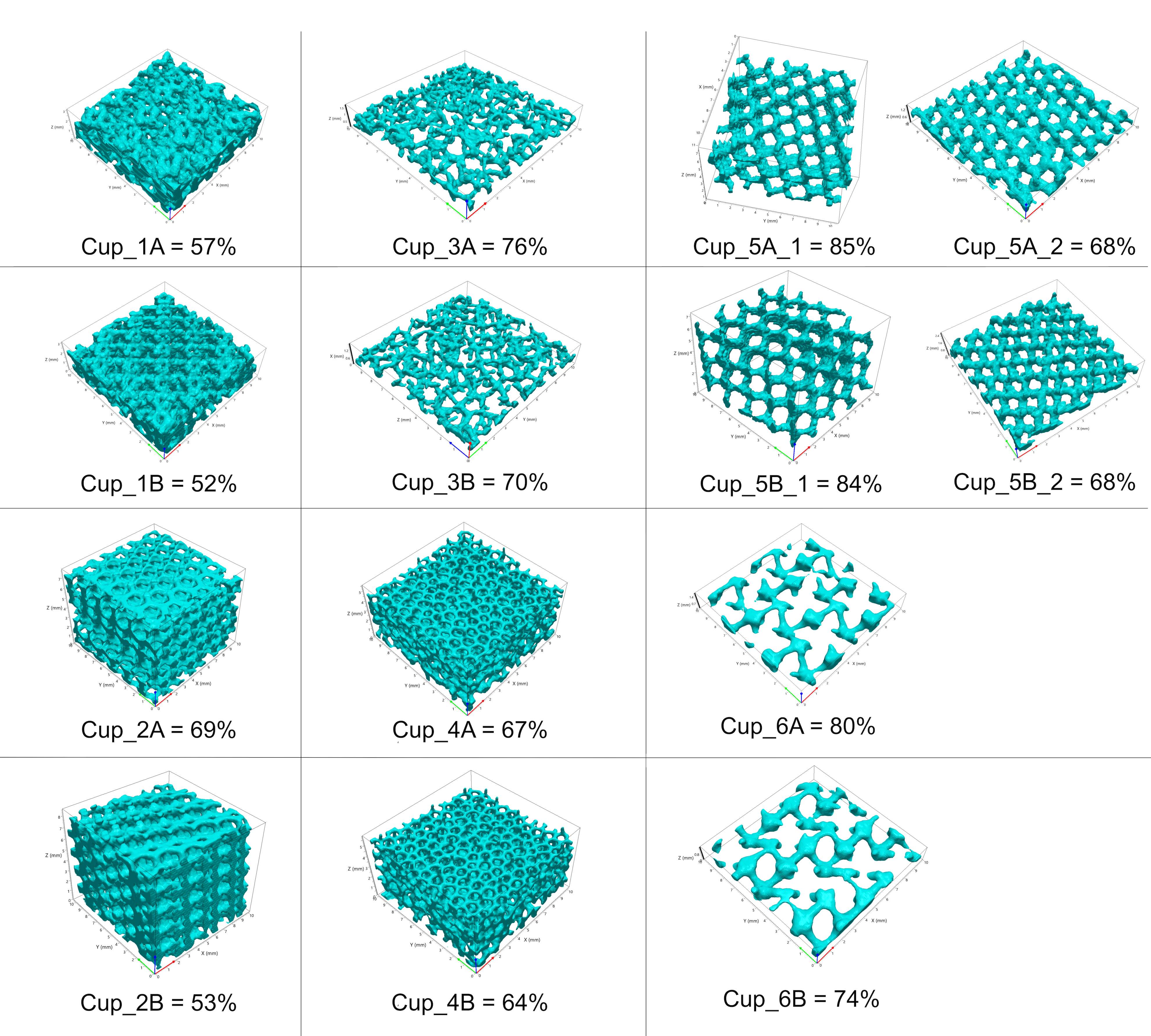
Figure 2
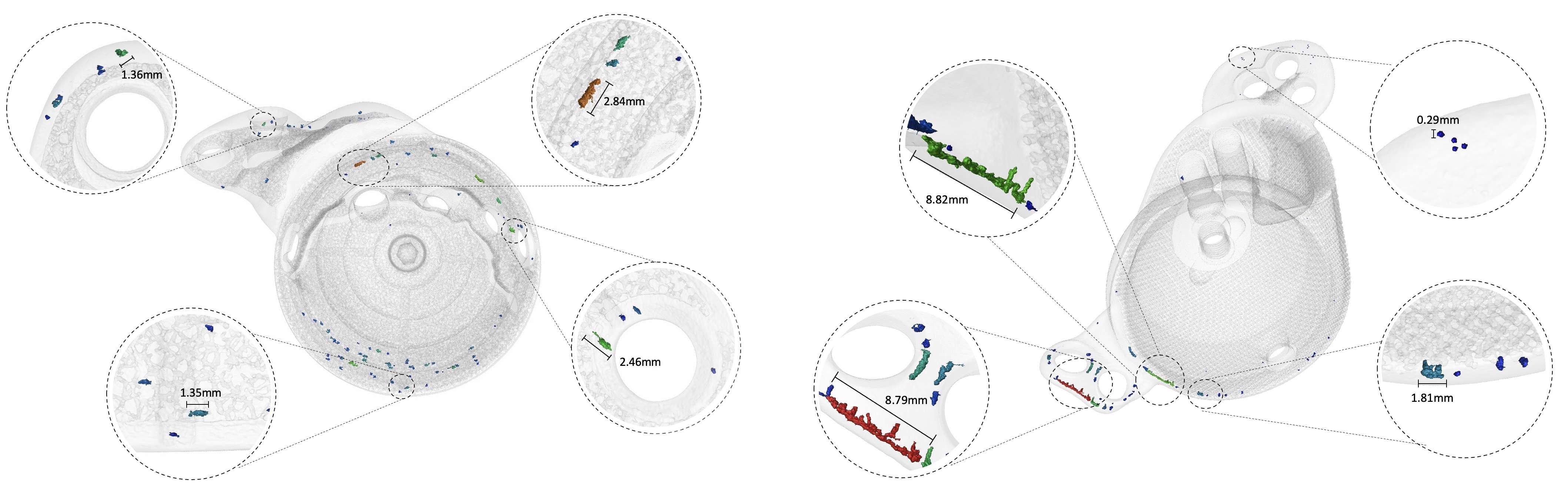
Figure 3#8349
Custom 3D Printed Implants for Acetabular Reconstruction: Mid-Term Functional and Radiological Results
*Anna Di Laura - Royal National Orthopaedic Hospital and Dept. MechEng at UCL - London, United Kingdom
Johann Henckel - Royal National Orthopaedic Hospital - London, United Kingdom
Harry Hothi - London Implant Retrieval Centre - Stanmore, United Kingdom
Alister Hart - Royal National Orthopaedic Hospital - London, United Kingdom
*Email: anna.laura.14@ucl.ac.uk
Introduction
Paprosky type-3B acetabular defects present the greatest technical challenge in revision hip surgery because of severe osteolysis involving the medial wall, anterior and posterior columns, and medialization of the hip centre. Implant fixation and mechanical stability can be compromised due to the heterogeneity in remaining pelvic bone stock and quality. In the present study, we report clinical and radiological outcomes of patients with massive acetabular defects treated with custom 3D printed implants and dual mobility bearings.
We aimed to report our experience in the management of patients with type-3B defects using custom 3D printed titanium implants.
Our primary and secondary objectives were the assessment of clinical and radiological outcomes at the minimum follow-up time of 36 months.
Methods
We reviewed the database of patients who consecutively underwent acetabular reconstruction with a custom 3D printed implant and dual mobility bearings for the treatment of Paprosky type-3B defects between 2016 and 2019. Functional and radiological outcomes were assessed. Radiological evaluation was performed immediately postoperatively, at 6 months and annually thereafter and imaging reviewed for radiolucency signs, implant stability and congruency. Radiographs and tri-planar CT reformats were evaluated by an orthopaedic surgeon and an engineer expert in implant imaging for component integrity, evidence of new bone formation at the bone-implant interface and migration Fig1. The outcome measures were: 1) Implant survivorship, 2) Oxford Hip Score (OHS) pre- and post-surgery, 3) Complications (dislocation, neurovascular injury, infection, iatrogenic fracture), 4) Radiographic evidence of loosening, 5) Radiographic evidence of migration.
Results
A total of 26 patients with minimum follow-up of 36 months were identified, including 17 women. The median age was 69 years (range, 49 to 90), 4 patients had pelvic discontinuity. The median follow-up was 53 months (range, 36 to 77). The cumulative implant survivorship was 100%. The Oxford Hip Score improved significantly, from a median of 8 (range, 2 to 21) preoperatively to 32 (range, 14 to 47) postoperatively (p=0.0001). One patient suffered transient sciatic nerve palsy. There was 1 early dislocation resolved with a non-surgical treatment, 1 infection re-occurrence in a patient with a long history of infection, 0 fractures. Radiographically, bone in-growth was observed at the bone/implant interface of 24 patients (92%), there were no cases of implant loosening, no radiological migration was observed at the last follow up, year 3 and beyond Fig.2.
Conclusion
We presented the largest study with 3 to 6 years clinical and radiological follow-up of patients with Paprosky type-3B defects. Our study shows good short-term results with the use of the custom acetabular implants and a dual mobility bearing type. We found that the implant had an excellent survival rate, good clinical outcomes and a low complication rate. The treatment of severe acetabular defects with the use of custom 3D printed titanium implants featuring a dual mobility bearing is a viable option and offers a substantial improvement of symptoms and in the quality of life.
Figures
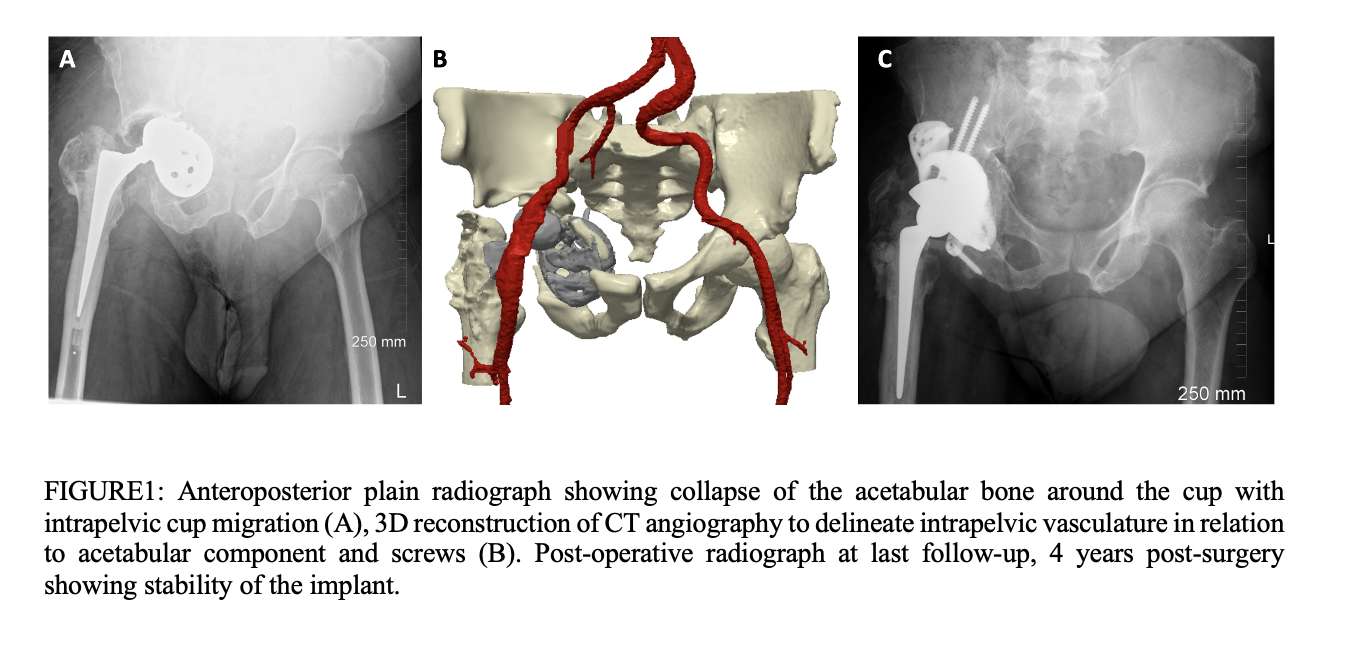
Figure 1
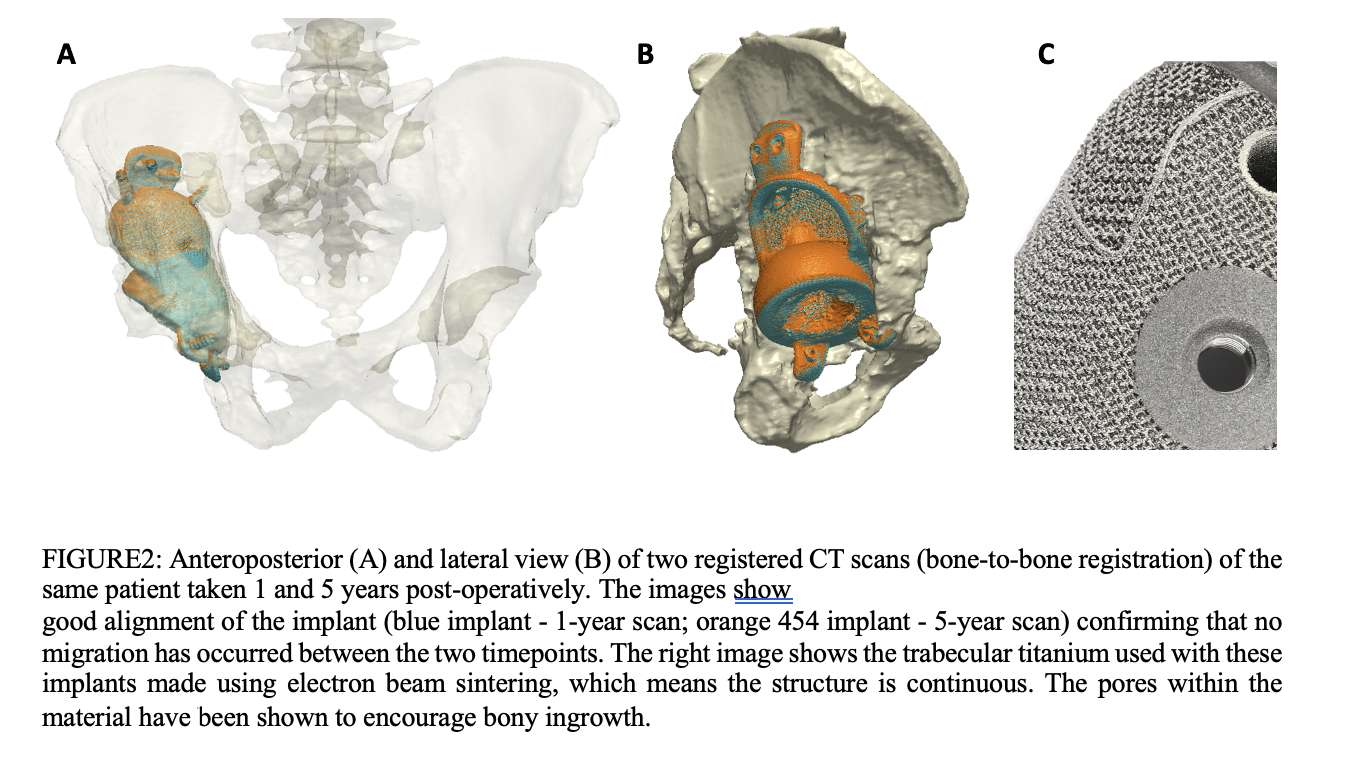
Figure 2#8782
Effect of Microporosities and Inclusions on Tibial Components of Knee Prosthesis
Renir Reis Damasceno Neto - LEBm / UFSC - Florianopolis, Brazil
Eduardo Alberto Fancello - Mechanical Design and Analysis Group (GRANTE) - Florianopolis, Brazil
Carlos Rodrigo De Mello Roesler - FAPEU - Florianopolis, Brazil
*Edison da Rosa - Federal University of Santa Catarina - Florianópolis, Brazil
Patricia Ortega Cubillos - UFSC - Florianopolis, Brazil
*Email: edison.rosa@ufsc.br
INTRODUCTION
Knee prostheses are generally manufactured from Co-Cr-Mo alloys, in accordance with ASTM F75, through a lost wax casting process. This casting process, like other processes, leaves small defects inside the material in the form of impurities, notably non-metallic inclusions. These inclusions, if located in regions of stress concentration, often develop a material fatigue failure process.
OBJECTIVES
The objective of the research is to develop a methodology that allows evaluating the quality of prostheses, specifically in terms of their fatigue life. The process considers the existing inclusions, the stress state and the mechanical properties of the material.
METHODOLOGY
The methodology adopted in this work starts from the characterization of impurities present in the material, especially non-metallic inclusions. A stress analysis, using the finite element method, identifies the critical points, in which the presence of porosity and inclusions is more worrying, and allows an assessment of the probable fatigue life. It was possible to develop an integrated procedure, evaluating, for the geometries of the tibial components of knee prostheses, the critical points and the state of stress in typical conditions of use, assessing fatigue life for use of approximately 2 million cycles per year.
RESULTS
The article presents a first version of an integrated procedure evaluating the three stages of fatigue failure, explicitly considering the effect of microdiscontinuities, notably non-metallic inclusions, including the analysis of the predominant mechanism of the deleterious effect of the presence of inclusions, on the nucleation life, according to the models available in the literature, in particular those of Murakami. If the cracks lead to a cyclic stress, in which DK, calculated by the parameter √(area), is less than DKth, the nucleated crack remains dormant, otherwise it propagates. The double perturbation that starts to act inside the material, due to the coexistence of a crack, together with a population of inclusions, affects the failure criterion associated with KIC, for example, according to Priest's models.
CONCLUSION
The proposed approach using a distribution of inclusions in a tibial component, obtained by radiographic evaluation, indicates an insufficient fatigue life which should demand a revision in the casting process in order to reduce the size and number of inclusions in the critical region, as well as a redesign of the prosthesis details.
#8234
Partially Molten Titanium Particles on 3D Printed Acetabular Cups
*Harry Hothi - London Implant Retrieval Centre - Stanmore, United Kingdom
Johann Henckel - Royal National Orthopaedic Hospital - London, United Kingdom
Anna Di Laura - Royal National Orthopaedic Hospital and Dept. MechEng at UCL - London, United Kingdom
Alister Hart - Royal National Orthopaedic Hospital - London, United Kingdom
*Email: h.hothi@ucl.ac.uk
Introduction:
3D printing is rapidly being adopted by the orthopaedic industry. This technology enables manufacturers to create implants with complex shapes, produce thinner walled components and more closely control bone facing porous structures.
These components however require several post-processing methods after being printed to address imperfections introduced as a result of current limitations in 3D printing technology. A key printing challenge is the generation of partially molten titanium particles which have been shown to be present across the surface of porous structures. Their formation is caused by large thermal gradients resulting in free neighbouring powder particles being heated below their melting point and attaching to the printed part.
Previous experimental studies have reported that these particles may illicit an adverse biological response, although this has not been reported clinically. Sandblasting or chemical etching is commonly used to remove these during the post-processing stage however it has been suggested that they may still be present on the porous structures of final-production implants. The aim of this study was to use a semi-quantitative analysis method to characterise the presence of partially molten particles on a series of unused 3D printed cups.
Methods:
The porous structures of 6 unused 3D printed acetabular cups, from 6 manufacturers were analysed using scanning electron microscopy (SEM), Figure 1. 10 images of struts were captured across the surface of each cup and 5 images of struts at the sub-surface level. Image analysis software was then used to identify each particle present on each SEM scan, with an outcome of (1) the number of particles per mm2 and (2) the diameter of each particle.
We compared these surface parameters across the different designs and also looked at any differences in the presence of particles between the surface and sub-surface level of the porous structure of each cup.
Results:
Partially molten particles were present on the struts of the porous structures in all cups examined; there was a large variability between designs in the size and number of particles (p<0.01). There were a median (range) of 34 (1-227) particles/mm2 on the outer strut surfaces. The inner surfaces had a median of 70 (1-423) particles/mm2, Figure 2. We also observed a variability in the number of surface particles present at different regions within the same cup, Figure 3.
The median (range) diameter of all particles identified across all cups was 36 (10-122) mm. Multiple comparison tests revealed that similarities in diameter between four cups, with a median of 31 (10-76) mm, and a similarity between the other two cups which had a median particle diameter of 68 (20-122) mm.
Conclusion:
This is the first study to quantify and compare the presence of partially molten titanium particles on the porous structures of final-production 3D printed acetabular cups. The evidence of these particles across all designs suggests that current post-processing methods have not fully addressed how to remove these. We recommend that the release of these particles in situ is monitored, potentially using blood titanium level testing.
Figures

Figure 1
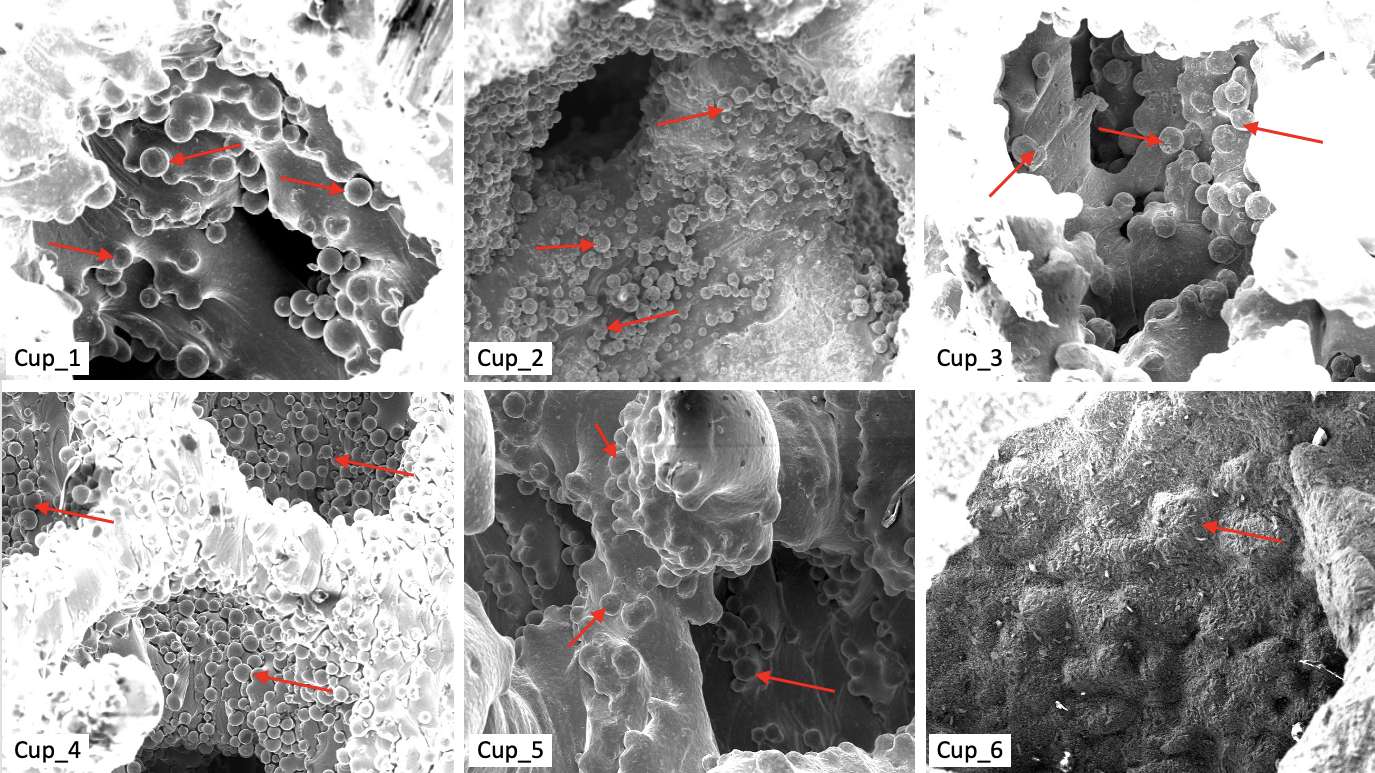
Figure 2
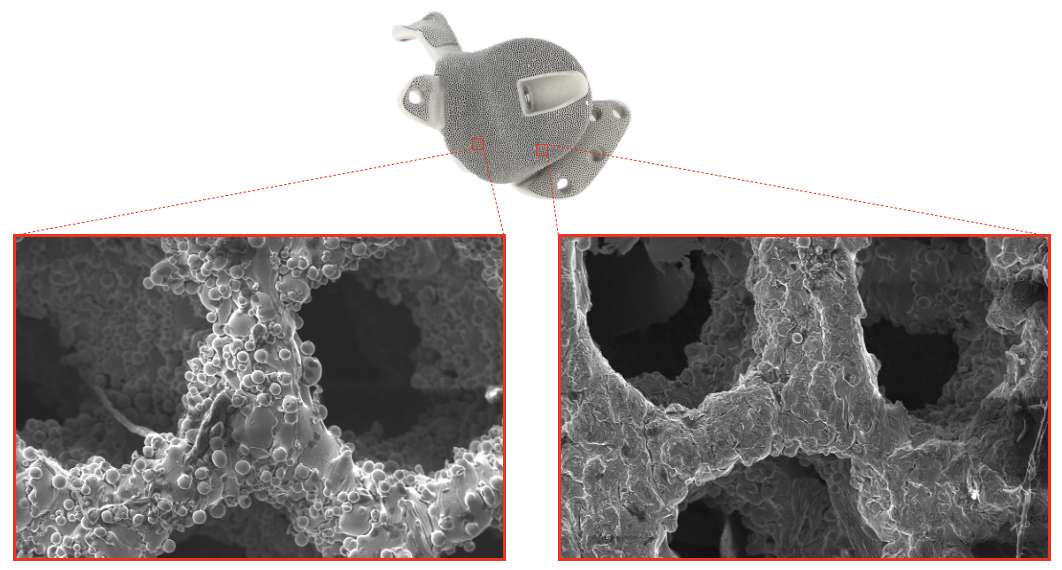
Figure 3#8461
An Investigation on the Mechanism of Action of Bactiguard Coating Used on Orthopaedic Devices
*Saurabh Lal - Zimmer Biomet - Swindon, United Kingdom
Erika Sodergren - Bactiguard AB - Tullinge, Sweden
Henry Ng - Bactiguard AB - Tullinge, Sweden
Anna Ericsson - Bactiguard AB - Tullinge, Sweden
Devendra Gorhe - Zimmer Biomet - Warsaw, USA
Lucia Pontiroli - Zimmer Biomet - Swindon, United Kingdom
Billy Sodervall - Bactiguard AB - Tullinge, Sweden
Imran Khan - Zimmer Biomet Inc - Swindon, United Kingdom
*Email: saurabh.lal@zimmerbiomet.com
Introduction: Bactiguard coating is a thin layer of silver, gold, and palladium bound to the substrate through covalent bonds. It is currently applied to orthopaedic implants and catheters for reducing microbial adhesion and subsequent risk of biofilm formation. The purpose of this study was to investigate the applicability of various mechanisms of action (MoAs) to Bactiguard coating that are typically associated with anti-adhesive and antimicrobial coatings.
Methods: The galvanic interaction between the noble metals of the coating was evaluated by PeakForce TUNA Atomic Force Microscopy (AFM) and Electrostatic Force Microscopy (EFM). Microbial adhesion was evaluated by using a modified version of the Ahearn test1,2. Microbial inhibition was evaluated by Zone of Inhibition (ZoI) test3. Escherichia coli (J53 with silver-resistance plasmid) was used to test adhesion and ZoI for silver-resistant bacteria. The silver release was evaluated by immersion test4. The generation of Reactive Oxygen Species (ROS) was tested by dichlorodihydrofluorescein diacetate (DCFDA) assay5. Changes in the pH of the surrounding media were evaluated before/after soaking. Bacterial cell morphology and membrane integrity were evaluated by scanning electron microscopy.
Results: PeakForce TUNA AFM and EFM tests confirmed the generation of small galvanic currents (approximately up to 250 pA) by the Bactiguard coating. PeakForce TUNA AFM test results are shown in Figure 1. The microbial adhesion test demonstrated about 83.5% to 100% reduction in the adhesion of various microorganisms associated with implant-related infections on Bactiguard-coated Ti6Al4V samples [Figure 2]. Microbial inhibition tests showed the absence of a ZoI in each test. The absence of antimicrobial silver-based MoAs was observed by microbial adhesion test indicating 99.97% reduction in the adhesion of silver-resistant bacteria [Figure 2], microbial inhibition test showing no ZoI with silver-resistant bacteria, and immersion test showing large margins (29- to 50-fold lower concentration) of silver release from the MIC values for microorganisms associated with implant-related infections. The absence of a pH-based MoA was confirmed by no significant differences in pH between coated and uncoated samples. The absence of ROS generation as a MoA was confirmed by no significant differences in ROS levels between coated and uncoated samples [Figure 3]. The absence of MoAs relying on damage to cell morphology or membrane integrity was confirmed by scanning electron microscopy.
Conclusion: This study concluded that the observed reduction in microbial adhesion from the surface of medical devices with Bactiguard coating is due to a non-eluting, galvanic MoA.
References:
1. Gabriel et al., Effects of silver on adherence of Bacteria to Urinary Catheters: In vitro studies. Current Microbiology 30: 17-22, 1995.
2. Ahearn et al., Effects of Hydrogel/Silver Coatings on In Vitro Adhesion to Catheters of Bacteria Associated with Urinary Tract Infections. Current Microbiology 41: 120-125, 2000.
3. A modified version of the Zone of Inhibition testing according to ASTM2149-10.
4. A modified version of the immersion test according to ISO10993-15.
5. AB113851 - Abcam Cellular ROS Assay Kit.
Figures
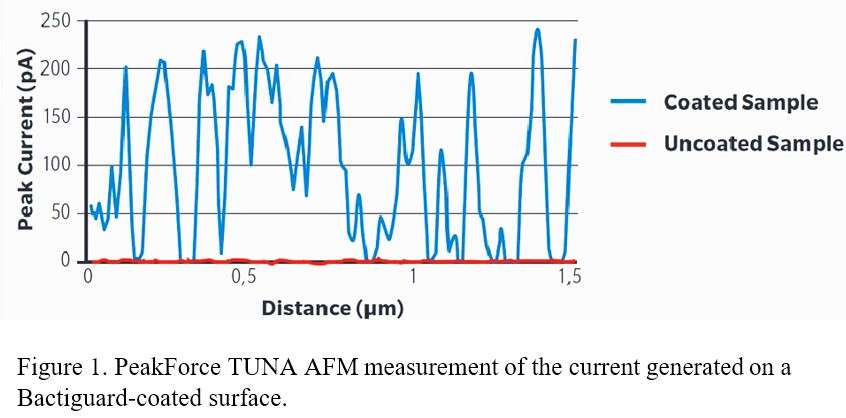
Figure 1
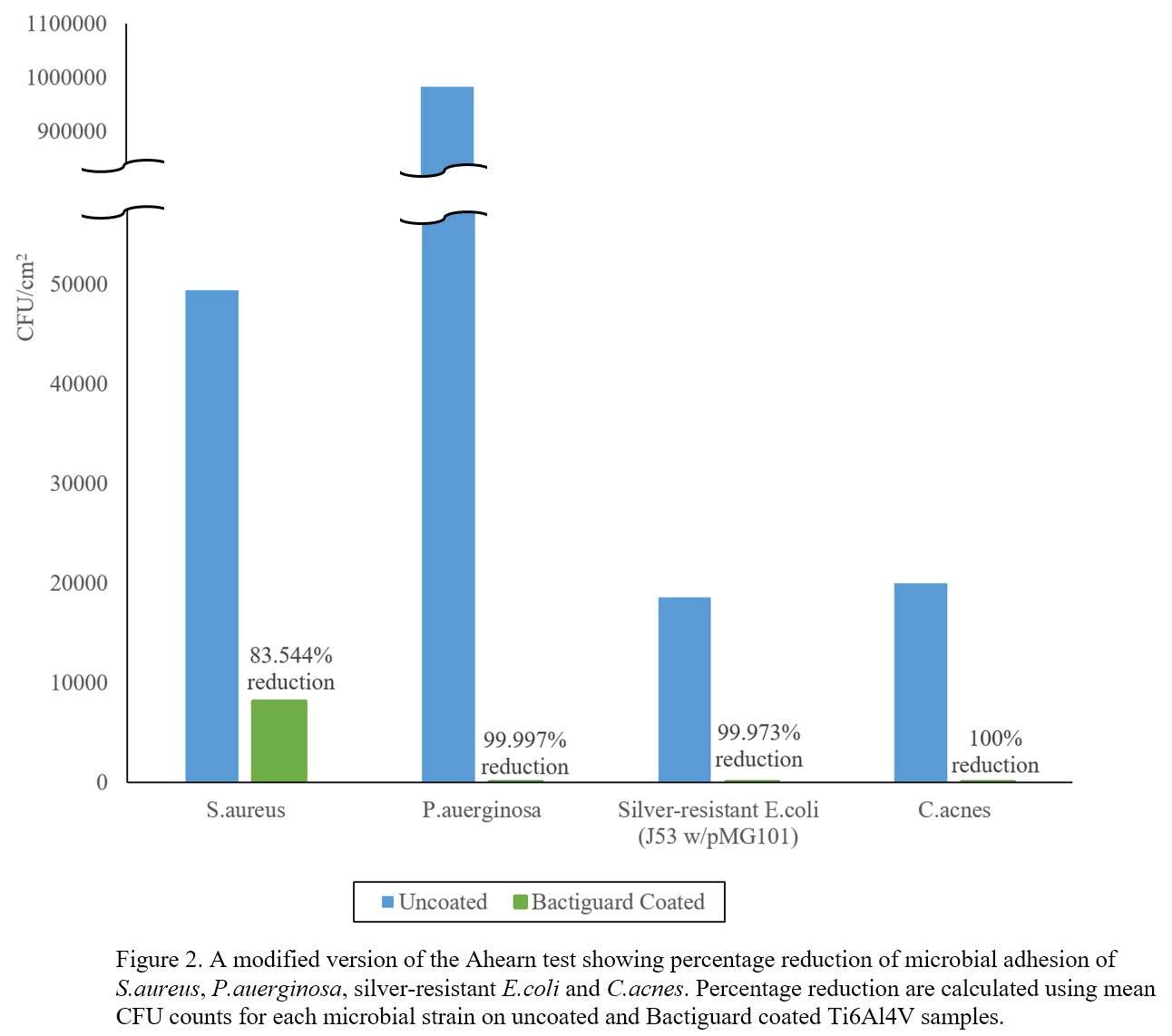
Figure 2
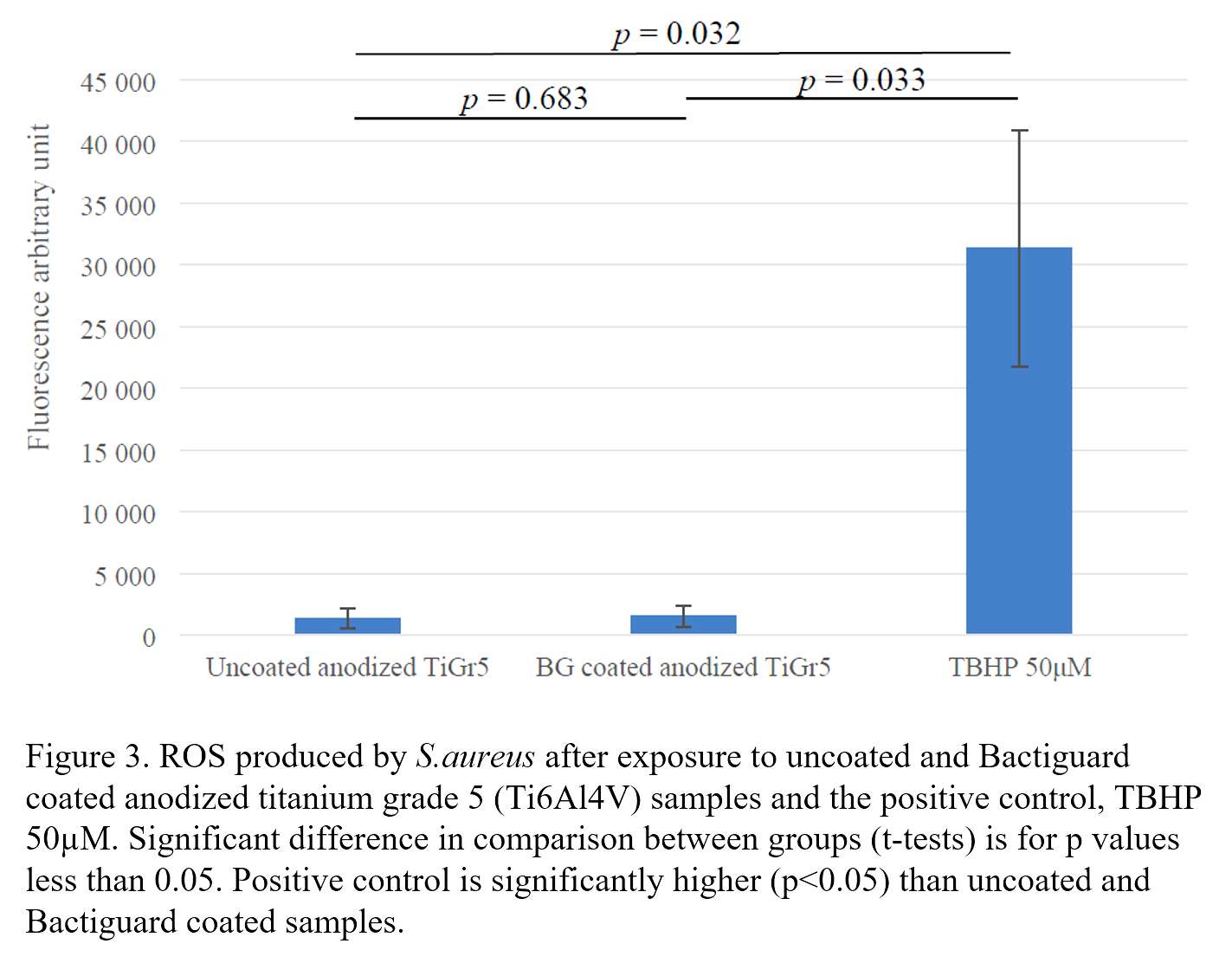
Figure 3#8371
Surface Roughness of Titanium Implants and Its Impact on Bacterial Adherence: Implications for Periprosthetic Joint Infection Prevention
Drew Clippert - Brown University/ University Orthopedics - Providence, United States of America
*Valentin Antoci - University Orthopedics - Providence, USA
Jacob Laperche - Frank H. Netter School of Medicine Quinnipiac University - Middletown, United States of America
Caitlin Barrett - University Orthopedics - Providence, USA
Dioscaris Garcia - Brown University - Providence, USA
Jillian Glasser - University Orthopedics Inc. - Providence, USA
*Email: valentin.antoci@gmail.com
INTRODUCTION: Periprosthetic joint infection (PJI) is currently one of the most pressing issues in the field of orthopedics, and Staphylococcus aureus is commonly implicated in these infections. Complications of PJI can result in revision surgery, longer hospital stays, increased antibiotic use, and increased cost. Most research on orthopedic infection is focused upon treatment and prevention, but research on how characteristics of implant materials affect bacterial adherence could provide insight into the optimal properties for orthopedic materials. Total hip arthroplasty (THA) implants commonly incorporate partially textured titanium components to promote osseointegration; although this texture has been shown to facilitate bacterial adherence and growth. This project focused on the difference of Staphylococcus aureus bacterial adherence for titanium implants based upon surface roughness.
METHODS: Smooth and rough AI6V4Ti clinical titanium (Ti) samples were inoculated with 1x10^4 colony-forming units (CFU)/mL Staphylococcus aureus for 6, 12, or 24 hours respectively and washed with phosphate-buffered saline (PBS) to remove non-adherent bacteria. Samples were then imaged with confocal laser scanning microscopy after being incubated with SYTO-9 and propidium iodide from a LIVE/DEAD® BacLight™ Bacterial Viability Kit. Areas of fluorescence were used to quantify percent bacterial surface coverage. Scanning electron microscopy (SEM) was used to visualize surface colonization and biofilm formation along with implant topography.
RESULTS: Confocal imaging showed bacterial growth was significantly greater (p<0.05) on rough Ti implants than smooth Ti implants at 6, 12, and 24 hours, and this disparity increased with increasing incubation time. SEM imaging confirmed these findings, which showed greater bacterial colonization on rough implants. Additionally, rougher Ti samples had significantly different and more textured microtopography compared to smooth samples.
CONCLUSION: This study illustrates that surface characteristics of titanium implants can have a significant effect on bacterial adherence. The increased colonization of rough Ti implants could be due to a larger surface area provided by the valleys and ridges in which bacteria are able to adhere. Clinically, this increased colonization of rough implants could be indicative of an increased chance of infection. These results could allow for the design of THA implants with the optimal surface characteristics to limit bacterial adherence and viability while maintaining osseointegration properties.
#8362
Design, Material, and Manufacturing Factors That Affect the Fixation of Metallic Implants to Bone: Evidence From Total Knee Replacements
*Elexis Baral - Hospital for Special Surgery - New York, USA
Aarti Shenoy - Hospital for Special Surgery - New York, USA
Geoffrey H Westrich - Hospital for Special Surgery - New York, USA
Thomas Sculco - Hospital for Special Services - New York, USA
Timothy Wright - Hospital for Special Surgery - New York, USA
*Email: barale@hss.edu
Introduction: Posterior stabilized total knee arthroplasty (PS TKA) is a successful operation to restore knee function. However, recently higher failure rates have been associated with femoral component loosening at the cement-implant interface, potentially related to the surface roughness of the component backside. This phenomenon has been prominent in TKAs retrieved at our institution, all by a single manufacturer (A). To explore this issue, we compared surface roughness values and finishes of components across different manufacturers to determine if a correlation existed between surface roughness and component failure. We had two aims: 1. Identify backside regions prone to burnishing on retrieved components from Manufacturer A, and 2. Compare roughness values and surface finish of pristine components of manufacturer A against those from pristine components from 2 competing manufacturers (B and C).
Methods: 32 retrieved PS TKAs from manufacturer A, revised for loosening, were graded for burnishing using an established protocol. The backside of the component was divided into 14 zones (Fig. 1) that were each graded on a scale of 0 (no burnishing) to 2 (moderate burnishing). Grading was performed under light microscopy at 20X (Fig. 2). Any region with >50% adhered cement was excluded. A laser-scanning profilometer was used to evaluate the surface roughness of 5 pristine components, including 3 components from Manufacturer A, and one each from Manufacturers B and C.
Results: Burnishing results from Manufacturer A showed the most undamaged regions to be in the anterior and posterior flanges (zones 1 and 2 and 9 and 10, respectively. Profilometry data revealed a large variation of roughness among the 5 samples. Manufacturer C had the highest mean roughness value (Ra = 12.9 ±0.72 µm), and Manufacturer B had the lowest (3.7 ±0.25 µm). The three components from Manufacturer A had roughness values of 4.1 (±0.46) µm, 7.6 (±1.1) µm and 5.4 (±0.38) µm. Line scans of the sampled surfaces illustrated differences in texture and topography (Fig. 3).
Conclusions: Surface roughness was similar across the pristine components implying that surface roughness might not be a determining factor for implant-cement debonding. Different locations along the femoral component had varying burnishing scores, indicating differences in loading while the components had been implanted. Further investigation is needed to better understand if surface finish or treatments correlate with femoral loosening.
Figures
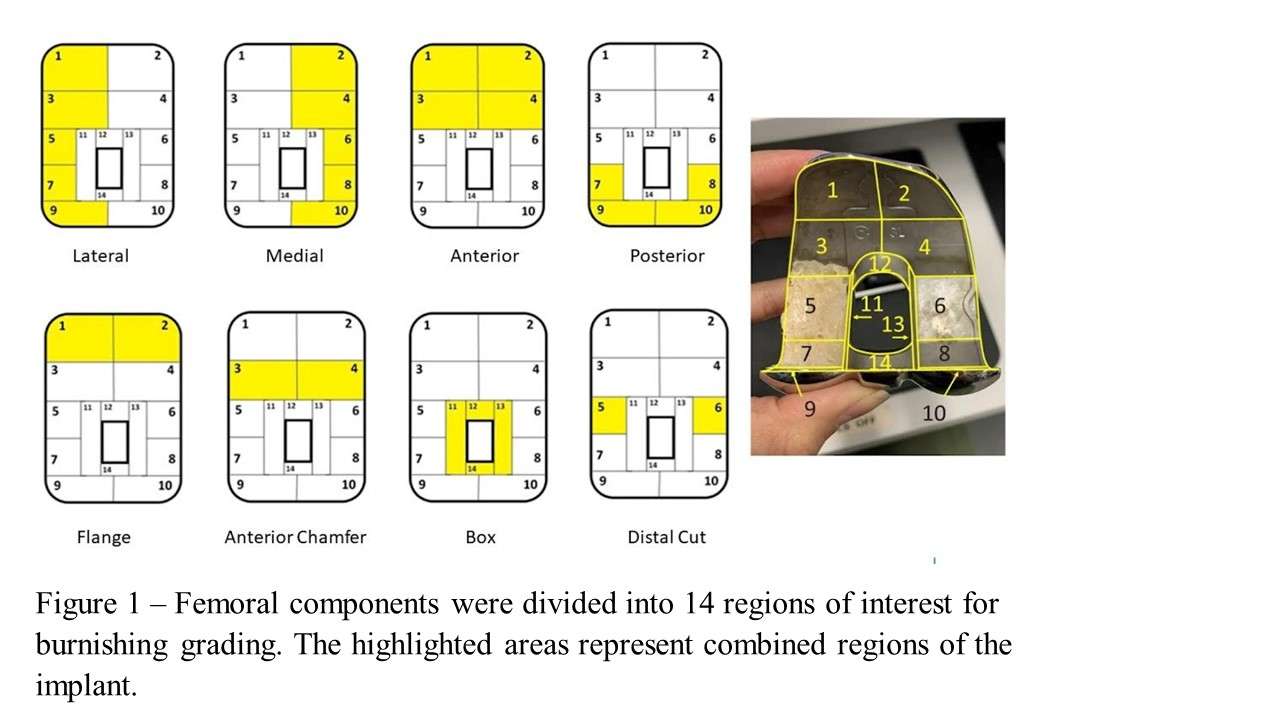
Figure 1

Figure 2
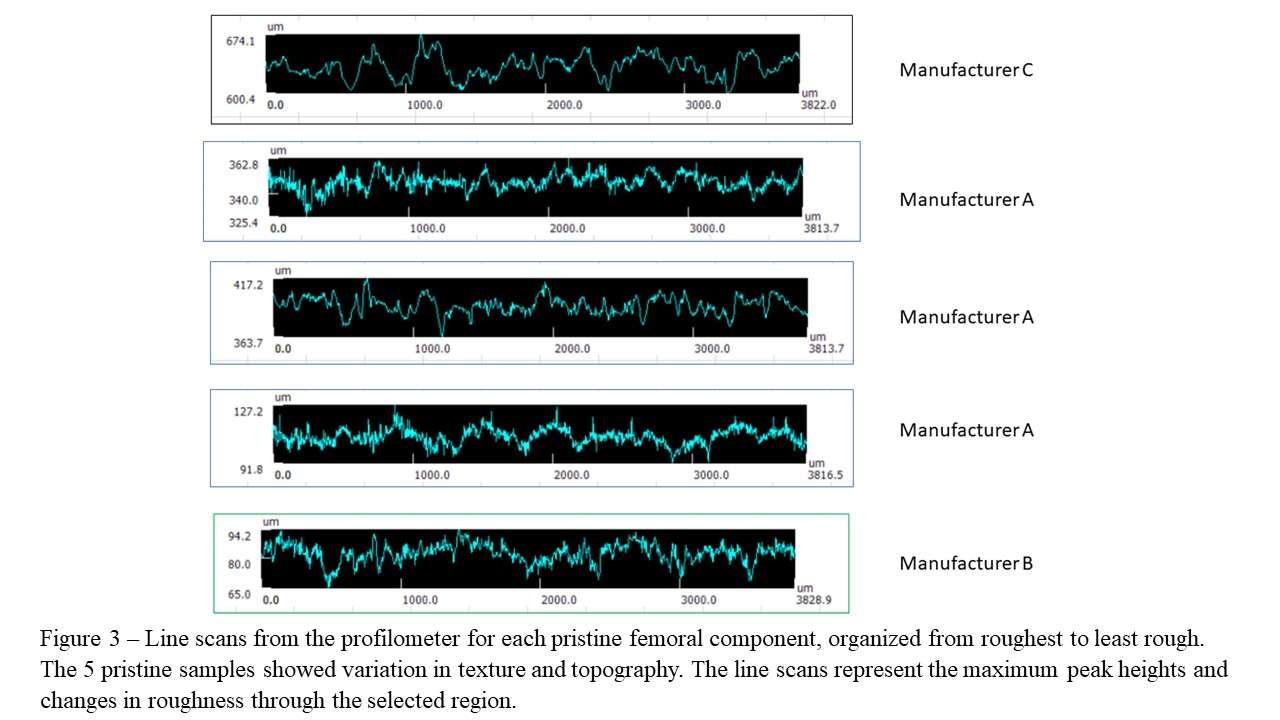
Figure 3#8727
Electronic Surgeon-Patient Communication Following Primary Total Hip Arthroplasty: Effects on Postoperative Outcome Measures and Patient Reported Outcome Scores
Akram Habibi - NYU Langone Orthopedic Hospital - New York, United States of America
Spencer Ward - NYU Langone Orthopedic Hospital - New York, USA
Jonathan Katzman - NYU Langone - New York, United States of America
Ran Schwarzkopf - NYU Langone Medical Center Hospital for Joint Diseases - New York, USA
*Joshua Rozell - NYU Langone Orthopedic Hospital - New York, USA
Casey Cardillo - NYU Orthopedic Hospital - New York, USA
*Email: Joshua.rozell@nyulangone.org
Introduction: With the integration of messaging capabilities in today’s electronic health records, patients and providers are able to exchange real-time information postoperatively. The purpose of our study was to assess the effect of messaging rates and timing on postoperative outcomes and patient reported outcomes (PRO) after primary total hip arthroplasty (THA).
Methods: A database of primary THA patients at a single academic institution from January 2018 to February 2023 was queried. A total of 7,515 patients were included with 1,071 patients sending messages within two weeks (early) and 625 patients sending messages at two to eight weeks (late) postoperatively. Patient demographics, length of stay (LOS), 90-day emergency department (ED) visits, 90-day readmissions, revisions, Patient-Reported Outcomes Measurement Information System (PROMIS) and hip disability and osteoarthritis outcome score (HOOS) were collected. Patients who sent messages early were younger (63.0 vs 65.4 vs 64.3 years; P<0.001) and had lower American Society of Anesthesiology (ASA) Scores (ASA III: 26.1 vs 35.5 vs 30.7%; P<0.001) compared to non-messengers and late messengers, respectively.
Results: Non-messengers had longer LOS (50.3 vs 30.6 vs 43.1 hours; P<0.001) and higher rates of non-home discharges (10.49 vs 3.2 vs 4.8; P<0.001) compared to patients who messaged early and late, respectively. Most messages were sent within two weeks (1.65 vs 1.41; P<0.001). There was no difference in 90-day ED visits (P=0.642), 90-day readmission (P=0.753), and revisions (P=0.859) between all three groups. Changes in PRO scores did not significantly differ between groups when assessed at three and six months, and at one-year. Patients who sent more messages by eight weeks had a higher chance of all-cause revisions (odds ratio 1.13; P=0.012). Linear regression models of PROMs did not demonstrate significant changes in scores based on number of messages sent.
Conclusion: Younger patients with less comorbidities are more likely to send messages after THA while non-messengers had longer LOS and higher rates of non-home discharges. Patients with higher rates of messages at eight weeks had higher chances of all-cause revisions. The long-term impact of patient-surgeon connection on subjective outcomes still remains largely unknown. Future studies should assess messaging content and its relationship to patient outcomes.
#8283
Trends in Surgical Management of Osteonecrosis of the Femoral Head: A 2010 to 2020 Nationwide Study
*Mitchell Ng - Maimonides Medical Center - Brooklyn, United States of America
Adam Gordon - maimonides Medical Center - Brooklyn, USA
Nicolas Piuzzi - Cleveland Clinic - Cleveland, USA
Afshin Razi - Maimonides Medical Center - Brooklyn, USA
Che Hang Jason Wong - Maimonides Medical Center - Brooklyn, USA
Lynne Jones - USA
Michael Mont - Sinai Hospital of Baltimore - Baltimore, USA
Matthew Magruder - Maimonides Medical Center - Brooklyn, USA
Ariel Rodriguez - Maimonides Medical Center - Brooklyn, United States of America
*Email: mitchng77@gmail.com
INTRODUCTION: The incidence of osteonecrosis of the femoral head (ONFH) is estimated at more than 20,000 patients annually in the U.S. Our study aimed to provide a 10-year analysis: 1) evaluating total operative procedures with rates normalized to the population; 2) determining trends of arthroplasty versus joint-preserving procedures; and 3) quantifying specific operative techniques in patients <50 versus >50 years of age.
METHODS: A total of64,739 patients who were diagnosed with ONFH and underwent hip surgery were identified from a nationwide database between 2010 and 2020. The percentage of patients managed by each operative procedure was calculated and normalized to the overall population annually. Patients were grouped into joint-preserving versus non-joint-preserving (arthroplasty) procedures, and divided by age under/over 50 years. Linear regression modelling was performed to evaluate trends/differences in procedural volume by year.
RESULTS: The number of operative procedures to treat ONFH has relatively declined from 2010 to 2020. The relative proportion of joint-preserving procedures increased (8.6 to 11.2%) during this time period. There were significantly more joint-preserving procedures in patients aged <50 years relative to >50 years (15.3 vs. 2.7%, p<0.001). Overall, THA was the most common procedure (57,033;88.1%) relative to hemiarthroplasty (3,875;6.0%), core decompression (2,730;4.2%), bone graft (467;0.7%), and osteotomy (257;0.4%).
CONCLUSION: Surgical management of patients who have ONFH remains predominantly arthroplasty procedures (94% overall). Our findings suggest an increase in joint-preserving procedures, particularly core decompression, in patients <50 years (15.3%). Our findings provide insight into surgical management trends for ONFH, and suggest opportunities for joint-preserving procedures.
#8301
YouTube Is a Poor Source of Patient Information for Patellofemoral Joint Arthroplasty
*Harkirat Jawanda - Rush University Medical Center - Chicago, USA
Garrett Jackson - Chicago, United States of America
Zeeshan Khan - Rush Univeristy Medical Center - Chicago, USA
Mitchell Pfennig - Loyola - Chicago, USA
Christopher Brusalis - Rush University Medical Center - Chicago, USA
Anjay Batra - Rush - Chicago, USA
Nikhil Verma - Rush University Medical Center - Chicago, USA
Jorge Chahla - Rush University Medical Center - Chicago, USA
*Email: Harkirat.jawanda@rushortho.com
Background: YouTube™ is the most popular video-streaming platform on the internet, and a significant source of information for patient education. There is no current study that evaluates the educational content on YouTube™ for the topic of patellofemoral joint arthroplasty.
Purpose: This study aimed to investigate the currently published online YouTube™ content on patellofemoral joint arthroplasty and assess its reliability and quality of educational content.
Methods: A YouTube™ search was conducted for patellofemoral arthroplasty using “patellofemoral joint replacement” and “patellofemoral joint arthroplasty” as search terms. The top 60 unique videos were rated for accuracy, reliability, and educational content using the Journal of the American Medical Association (JAMA) criteria, DISCERN score and the Global Quality Score (GQS).
Results: Among 60 unique videos, the majority were published by a healthcare organization (68%) and presented by a physician (65%). Thirty-seven videos (62%) specifically addressed patellofemoral arthroplasty and 23 videos (38%) focused on other topics without addressing patellofemoral arthroplasty. The mean scores and standard deviations for all videos were the following: JAMA 2.0 ± 0.7; DISCERN 45.2 ± 12.4; and GQS 2.2 ± 1.1. Videos published within the last year had a significantly greater average GQS (p = 0.04) and trended towards a significantly greater average DISCERN score (p = 0.08).
Conclusion: Current patellofemoral arthroplasty content on YouTube™ is of relatively low educational quality. Although more recently published videos trend towards higher quality, the number of videos focused on other topics when searching for “patellofemoral arthroplasty” or “patellofemoral replacement” remains a potential source of confusion. These findings reinforce the value of physician guidance and vetting of online healthcare information for patients.
#8728
Surgeon-Patient Communication Using the Electronic Portal: Impact on Postoperative Outcomes and Patient Reported Outcome Measures Following Total Knee Arthroplasty
Akram Habibi - NYU Langone Orthopedic Hospital - New York, United States of America
Spencer Ward - NYU Langone Orthopedic Hospital - New York, USA
Jonathan Katzman - NYU Langone - New York, United States of America
Ran Schwarzkopf - NYU Langone Medical Center Hospital for Joint Diseases - New York, USA
*Joshua Rozell - NYU Langone Orthopedic Hospital - New York, USA
Hayley Raymond - NYU - New York, United States of America
*Email: Joshua.rozell@nyulangone.org
Introduction: Patient using the electronic medical record portal can have real time communication with their surgeon following total knee replacement (TKA). The purpose of this study was to evaluate the impact of timing and number of messages sent to the surgeon after TKA on patient reported outcomes (PRO).
Methods: A retrospective review of primary TKAs performed at a single academic institution from January 2018 to February 2023 was conducted. Of 9,353 patients included, 1,219 patients sent messages within two weeks (early) of surgery, and 507 patients sent messages between two to eight weeks (late) after surgery. Patient demographics, length of stay (LOS), 90-day emergency department (ED) visits, 90-day readmissions, revisions, Patient-Reported Outcomes Measurement Information System (PROMIS) and hip disability and osteoarthritis outcome score (HOOS) were collected. Regression models were utilized to control for patient comorbidities to assess outcomes and PRO. Patients who did not send messages were older (67.3 vs 64.5 vs 65.8 years; p<0.001), and patients who messaged early had a lower American Society of Anesthesiology (ASA) Scores (ASA III: 37.1 vs 43.6 vs 41.4%, p<0.001) compared to patients who did not message or messaged between two to eight weeks, respectively.
Results: Patients who messaged within two weeks following surgery had shorter hospital length of stay (41.2 vs 53.40 vs 47.45 hours, p<0.001) and were more likely to be discharged home (99.5 vs 96.8 vs 97.6%, p<0.001) compared to both non-messengers and late messengers. Patients who messaged late were more likely to experience a 90-day readmission following surgery (3.3 vs 3.2 vs 5.3%, p=0.05). There was no significant difference in 90-day ED visits (p=0.091) and revision rates (p=0.752). Most messages were sent within two weeks (1.76 vs 1.48; p<0.001). There was no significant difference in PROMs regardless of message timing, and there was no association between messaging timing and perioperative outcomes or PROMs.
Conclusion: Older patients may be less familiar with emerging technologies and are less likely to send messages following TKA. Those who send messages early may be more likely to understand the postoperative recovery leading to less readmissions. These findings highlight the need to educate older patients on post-operative recovery pre-operatively to avoid unnecessary readmissions.
#8754
The Effect of Technology on Short-Term Clinical Outcomes in Total Knee Arthroplasty
*Weston Buehring - New York, United States of America
Jonathan Katzman - NYU Langone - New York, United States of America
Muhammad Haider - NYU Orthopedic Hospital - New York, USA
Benjamin Schaffler - NYU Orthopedic Hospital - New York, USA
Matthew Hepinstall - NYU Langone Health - New York, USA
Morteza Meftah - NYU Hospital for Joint Diseases - New York, USA
Muhammad Haider - NYU Orthopedic Hospital - New York, USA
*Email: westonbuehring2013@gmail.com
Introduction
Technology has been increasingly employed in total knee arthroplasty (TKA), with robot-assisted surgery (RAS) and computer-assisted navigation (CAN) being notable examples. However, the impact of these advancements on short-term outcomes and resource utilization in TKA remains uncertain. This study aims to investigate the effects of different surgical techniques on postoperative outcomes following TKA.
Methods
A retrospective analysis of our center was performed; 15530 primary, elective TKA cases between January 2016 and December 2022 were analyzed. Cases were categorized into three groups based on type of technology: 1936 cases (12.5%) in the RAS group, 3048 (19.6%) in the CAN group, and 10546 (67.9%) in the manual group. Demographic data and short-term outcome measures, including the Activity Measure for Post-Acute Care (AM-PAC) scores, which is a validated tool used to quantify postoperative functional status, were collected and compared using ANOVA and multivariate logistic regression.
Results
Over the 6-year study period, there was an increase in the proportion of RAS (7.3% to 17.9%) and CAN (7.0% to 44.6%) cases, while the percentage of manual cases decreased from 85.7% to 37.4% (p<0.001). The RAS group exhibited the shortest mean length of hospital stay (38.79 hours), followed by the CAN group (47.30 hours) and the manual group (56.69 hours) (p<0.001). Furthermore, the RAS and CAN cohorts had significantly higher rates of discharge to home compared to the manual group (95.1% in RAS, 94.4% in CAN, and 79.2% in manual; p<0.001). Although there were no significant differences in 30-day readmissions, the 90-day readmission rate was lowest in the RAS group (2.4% in RAS, 3.0% in CAN, and 3.5% in manual; p=0.04). In addition, perfect AM-PAC scores on postoperative day 0 (POD0) were more often observed in the RAS cohort relative to the CAN and manual groups (31.1% vs. 21.8% vs. 8.3%, respectively; p<0.001). Multivariate analysis demonstrated that use of RAS was independently associated with reduced odds of 90-day readmission (p=0.031), after adjusting for implant type and baseline demographics. Multivariate analysis also showed RAS and CAN cohorts as having significantly greater odds of achieving a perfect AM-PAC score on POD0 (p<0.001).
Conclusion
We observed favorable short-term outcomes associated with the integration of robot-assisted surgery in TKA, including shorter hospital stays, increased likelihood of discharge to home, and lower 90-day readmission rates. RAS holds promise as a valuable technology for enhancing postoperative outcomes after TKA.
#8488
Parametric Analysis of Factors Affecting Osteochondral Grafts in the Knee
*Gavin Day - iMBE, University of Leeds - Leeds, GB
Alison Jones - University of Leeds - Leeds, United Kingdom
Marlene Mengoni - University of Leeds - Leeds, United Kingdom
Ruth Wilcox - University of Leeds - Leeds, United Kingdom
*Email: g.day1@leeds.ac.uk
Introduction
Osteochondral grafting is a treatment for articular cartilage damage which involves replacing the damaged region with an autograft or allograft consisting of a cylinder of bone with a layer of cartilage, or with a synthetic biomaterial. There is uncertainty regarding the factors that affect the short-term stability of these grafts and evidence of graft subsidence and poor integration. The purpose of this study was to investigate the parameters that affect the immediate stability of osteochondral grafts within a tibiofemoral joint using finite element (FE) models.
Methods
This study utilised previously derived subject-specific FE models of a human tibiofemoral joint that were built and validated against experimental contact pressure data for six cases with different osteochondral graft conditions [1] (Fig.1:A). The models contained element-wise material properties of the bone derived from CT scan data, and calibrated friction properties between the graft and host bone from an independent experimental study of 12 osteochondral graft push-in tests [2]. Parametric tests were performed to investigate the effect of changing the density-modulus relationship for the bone component of the graft (±50%), graft length and graft site mismatch (10mm ± 2mm), and the effect of graft site dilation on graft stability. Graft site dilation tests used experimental push-in testing (n=8) for FE method validation [3], (Fig.1:B). Push-in force comparisons were made using the force at 1 mm of displacement below flush with surrounding cartilage.
Results
The study found a strong correlation between the computational models and experimental data in regards to the effect of graft site dilation on push-in force, even though there was no significant difference observed between dilated and undilated sites in the experimental results (Fig.1:B). Changes to the graft bone density, graft length and site length mismatch and graft site dilation had little effect on the resultant tibial contact pressure in the knee joint model. However, large changes were found internally when measuring peak stress, strain and internal contact pressure on the graft for all of the parameters (Fig.1:C,D).
Conclusion
The use of FE models in this study provided useful insights into the immediate stability of osteochondral grafts within a tibiofemoral joint, and enabled patient and clinical variables to be evaluated systematically in ways that are not possible using experimental methods alone. The agreement measured between experimental and computational models continues to add confidence to the modelling approach. The study highlights the importance of selecting appropriate grafting parameters and site preparation, as large changes were found in the stress and strain within the bone elements when varying properties and site preparation. Therefore, caution should be exercised while selecting the graft properties to prevent damage to the bone and graft prior to bony integration. Further research is required to investigate the tradeoffs between dilated and undilated graft sites.
References
1. Day et al. ESB 2022 2. Day et al. 2022 JMBBM 3. McCall et al, ORS 2023
Acknowledgements
This work was supported by the EPSRC, EP/P001076. The authors would like to thank the tissue donors and their families.
Figures
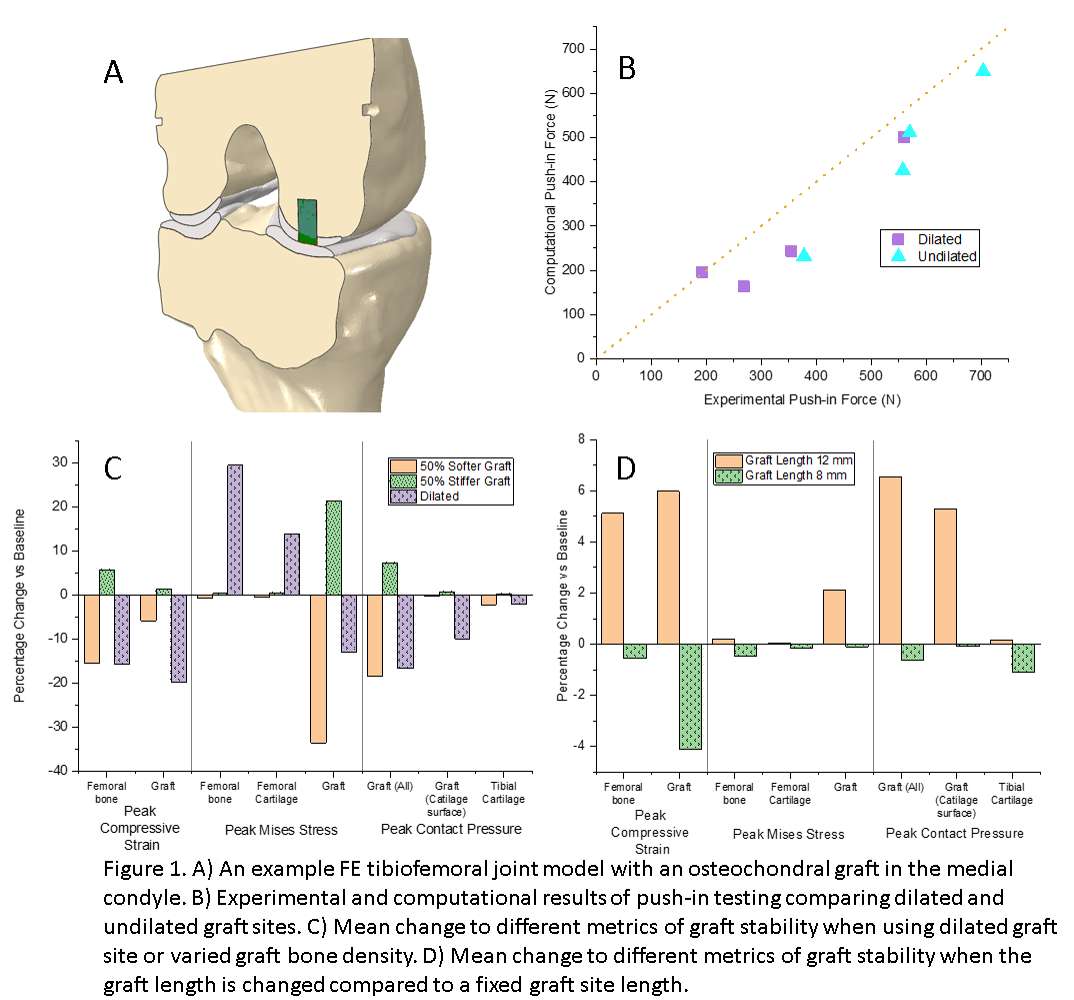
Figure 1#8641
Does Medicare Insurance Affect Access to Complex Revision Shoulder Arthroplasty Subspecialty Shoulder Care?
Carlos Fernandez - JFK / U Miami Orthopedic Surgery Program - Lantana, USA
*Vani Sabesan - Cleveland Clinic Florida - Weston, USA
Anna Redden - FAU - boca raton, USA
Clyde Fomunung - Palm Beach Shoulder Service - Lantana, USA
*Email: sabes001@gmail.com
Introduction: Revision shoulder arthroplasty (RSA) has increased due to the exponential growth of shoulder arthroplasty but requires more complex and lengthy procedures with increased risk for postoperative complications. Because revision shoulder arthroplasty is such a highly specialized procedure, it is usually performed by subspecialty trained surgeons. Access to these subspecialized surgeons is sometimes limited based on patient insurance status. The purpose of this study was to determine whether access to care for revision shoulder arthroplasty differed from access to care for a primary shoulder arthroplasty in a Medicare population.
Methods: Three highly populated and diverse counties in South Florida were selected for data collection with 69 orthopedic offices identified by Google search. Investigators contacted each office to schedule an appointment for primary or revision shoulder arthroplasty for a family member using a blocked phone number. Calls were repeated with a waiting period of at least five days. A standardized script of this simulated orthopedic patient case with Medicare insurance was used to limit interpersonal variation. The ability to schedule a new patient appointment, waiting period for the scheduled appointment (time between date of call and date of appointment in business days), and whether the appointment was with a shoulder specialist, or a non-specialist, were recorded. Clinics accepting Medicare that performed primary or revision shoulder arthroplasty were included.
Results: Of the 69 offices called, 34 offices accepted Medicare and the remaining 35 did not accept Medicare or did not have a specialist trained to perform RSA. Ten offices did not perform revision shoulder arthroplasty surgeries. Patients seeking appointments for a primary RSA did not have to wait longer when wanting to see a shoulder specialist compared to a general orthopedic surgeon (12.3 days vs 8.9 days, p = 0.197). This finding was similar for patients seeking an appointment for rRSA as the wait time was similar for a shoulder specialist (13.6 days vs 12.8 days, p = 0.864). No correlations existed between median age and wait times, or median income and wait times. There was no significant difference in wait times for primary versus revision shoulder arthroplasty, for either general orthopedic surgeons or shoulder subspecialists. There was also no difference in phone call duration for primary and revisions (p=0.503).
Conclusions: The results of our study indicate no significant difference in access to care between patients seeking a primary versus revision shoulder arthroplasty with a general orthopedic surgeon versus a shoulder specialist. Revision shoulder arthroplasty appears to be evaluated and performed at equivalent wait times by both general orthopaedic surgeons and shoulder specialists. More research needs to be done on the importance of subspecialty training and outcomes as well as access to care to optimize patient success for these complex revision SA surgeries.
#8305
Pandemic Effect on Patient Anxieties, at-Risk Populations, and the Impact on Orthopedic Volume Trends
Drew Clippert - Brown University/ University Orthopedics - Providence, United States of America
Caitlin Barrett - University Orthopedics - Providence, USA
*Valentin Antoci - University Orthopedics - Providence, USA
Jacob Laperche - Frank H. Netter School of Medicine Quinnipiac University - Middletown, United States of America
Dioscaris Garcia - Brown University - Providence, USA
*Email: valentin.antoci@gmail.com
Introduction: Limited research has been done on the impact of Covid-19 on orthopedic patient perspectives or practice trends, despite the significant impact on orthopedic surgery over the last few years. Our study aims to investigate surgical impact and patient perceptions in orthopedic surgery through analysis of surgical volume, direct patient survey, and the direct correlation between specific patient factors and Covid-19 anxiety levels.
Methods: Using an IRB-approved anonymous survey, we collected information regarding demographics, basic health history, and Covid-related anxiety levels from 206 patients, with descriptive statistics calculated for all sections. Factor analysis with varimax rotation was conducted to group Covid-related questions. Descriptive statistics were used to measure how demographic factors influenced perspectives on Covid-19 risk and healthcare utilization during the pandemic. Additionally, clinical practice trends were collected to better understand the impact on surgical volume and associated rebound after a catastrophic global event like the pandemic.
Results: Patients were moderately worried about contracting and surviving Covid-19, but still prioritized their orthopedic care despite the associated risks. Nevertheless, a typical orthopedic practice dropped 30-40% of visits between week 5 and 7 of Covid-19, with complete recovery by week 32. Telehealth visits comprised 20% of week 7 orthopedic visits. Additionally, we found that patients with public insurance (p=0.02), previous hypertension diagnosis (p=0.006), currently unemployed (p=0.001), and lower self-assigned health levels (p=0.002) were more worried about their Covid-19 risk and impact on their care compared to the general population. For some patients, this anxiety translated into greater concern for and/or less utilization of healthcare services during the pandemic.
Conclusion: The COVID-19 pandemic is largely resolved, but the impact and understanding of population dynamics are critical for future preparation and likely re-organization of healthcare systems. The current study delineates patient perceptions related to Covid-19 and larger orthopedic practice trends, further underlying the greater risks for compromised populations. Due to high levels of patient anxiety around Covid-19 and difficulties finding safe medical care, telehealth communication services proved a promising technology in orthopedics and medicine in general. Future technologies like artificial intelligence may then optimize care even further.
#8328
Decreasing Trends in Arthroscopic Treatment of Osteoarthritis of the Knee Following Publication of the 2013 AAOS Clinical Practice Guidelines: A Large Database Analysis
*Kent Kern - Corewell Health/Michigan State University - Grand Rapids, United States of America
Tyler Madden - Corewell Health - Grand Rapids, USA
Karl Roberts - Corewell Health/Michigan State University - Grand Rapids, USA
*Email: kkern4343@gmail.com
Introduction: The primary purpose of this study was to analyze the utilization trends of arthroscopic lavage/debridement for patients with an isolated diagnosis of knee osteoarthritis (OA) following the publication of the 2013 AAOS clinical practice guideline (CPG) regarding non-arthroplasty management of knee OA. The 2013 CPG recommended strongly against the use of arthroscopy for treatment of knee OA. A secondary analysis was performed to evaluate trends in conservative treatment options over this same time frame including physical therapy (PT), corticosteroid injections (CSI), and viscosupplementation injection (VSI). The 2013 CPG was inconclusive in its recommendation for CSI, recommended strongly against use of VSI, and had a strong recommendation in favor of exercise/physical activity. The purpose of examining trends for both arthroscopic and conservative treatment options is to evaluate how they have changed in relation to one another following the publication of the 2013 CPG.
Methods: This analysis was performed using the IBM MarketScan Commercial and Medicare Supplemental Databases from 2012-2019, which contains healthcare data for more than 43.6 million individuals. This study included patients who were ≥ 21 years of age who underwent one or more knee arthroscopies for the primary diagnosis of knee osteoarthritis without concomitant pathology including loose bodies or meniscal tears. Inclusion required continuous enrollment for 12 months before surgery to allow for analysis of interventions such as PT, CSI and VSI in the year prior to arthroscopy. Both obesity and demographic data including age, sex and geographic distribution were included for analysis. Data included was produced as actual rates and subgroup analysis was performed based on age cohorts.
Results: The overall rate of arthroscopic intervention in 2012 was 59.15 per 1,000 patients and decreased 14.91 points to 44.24 per 1,000 patients in 2019 [Fig 1]. This decreasing trend in arthroscopic treatment equates to an average decrease of 4.0% year over year from 2012-2019 with an overall decrease of 32.0%. The three age cohorts with highest utilization of arthroscopy were the 40-49, 50-59 and 60-69 age cohorts [Fig 2]. The 40-49 group demonstrated a 41.5% decrease in arthroscopy rates between 2012 and 2019, the 50-59 group decreased by 37.2% and the 60-69 group decreased by 35.9% [Fig 3]. In patients who underwent arthroscopy, utilization of VSI in the year prior to surgery decreased from 8.4% in 2013 to 5.8% 2019, while CSI increased from 49.6% to 55.9% and PT increased from 28.6% to 34.5%.
Conclusion: This analysis demonstrates physician adherence to the 2013 AAOS CPG which strongly recommended against arthroscopic treatment for an isolated diagnosis of knee OA. Both overall and age cohort specific arthroscopic utilization rates consistently decreased year over year for all cohorts in accordance with the publication of the 2013 CPG. The secondary analysis demonstrated an overall decrease in VSI with a corresponding increase in CSI and PT utilization.
Figures
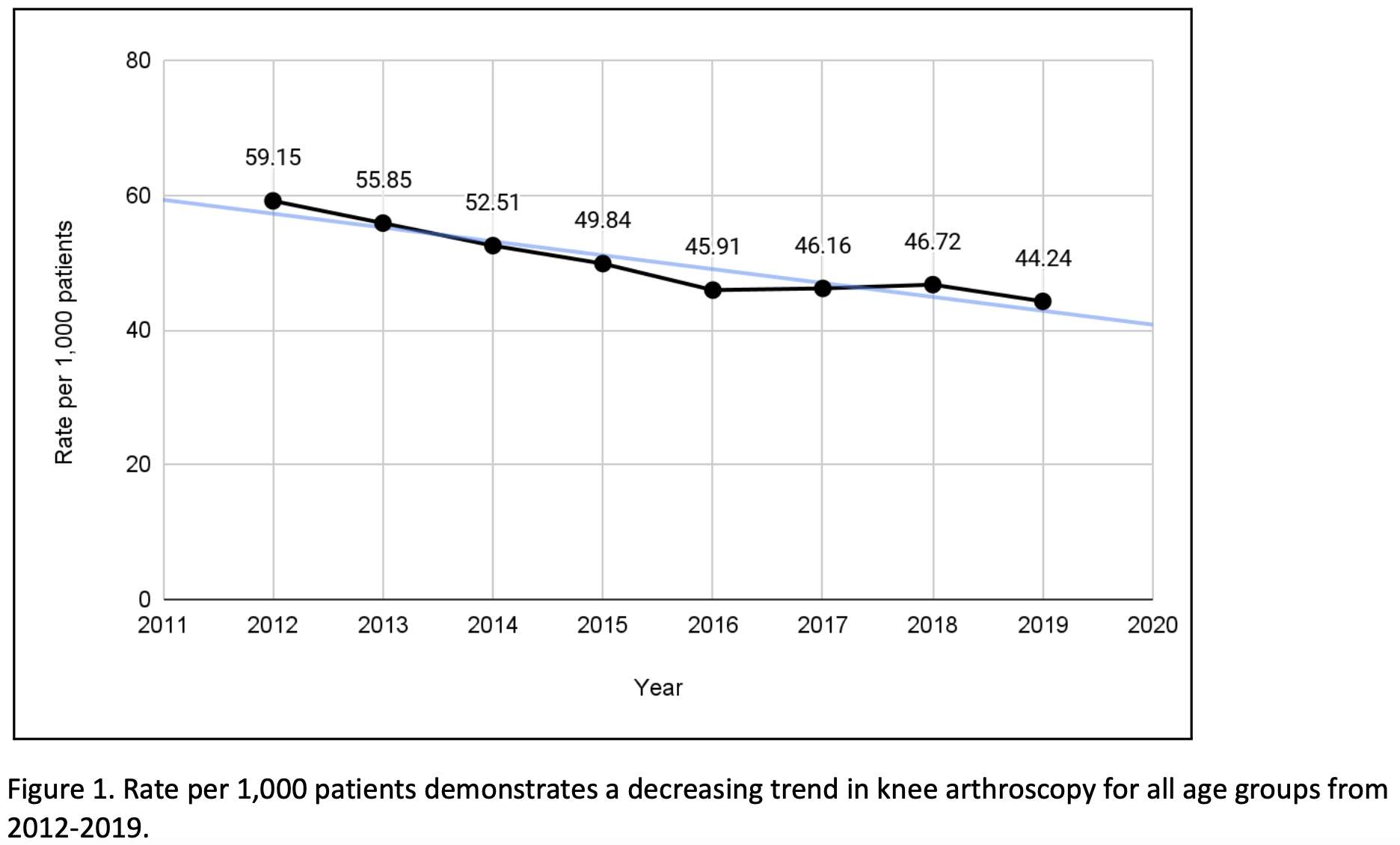
Figure 1
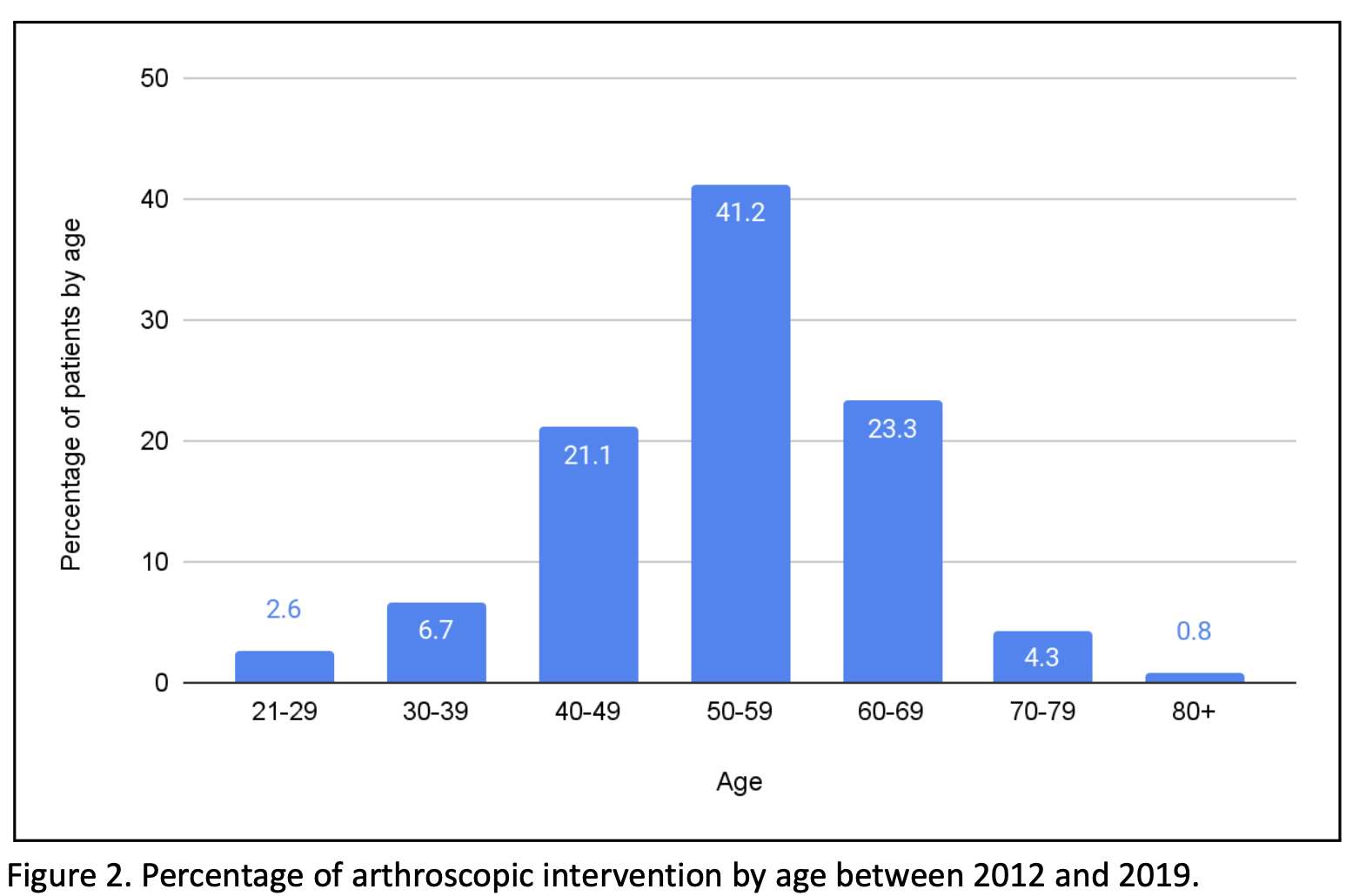
Figure 2
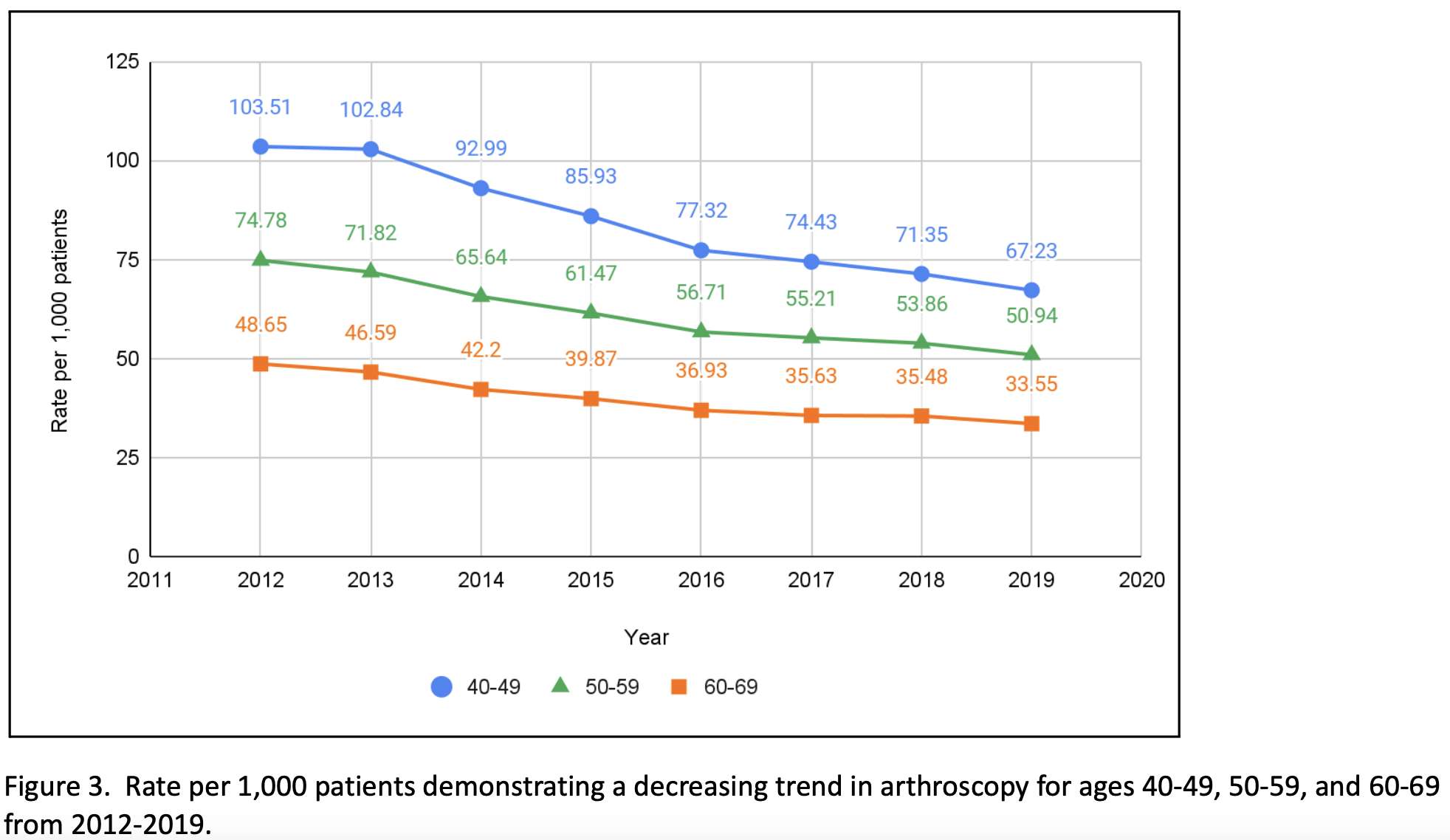
Figure 3#8323
Data Collection by Digital Patient Engagement App
Maarten-Paul Van de Kerkhove - ZorgSaam Ziekenhuis - Terneuzen, Netherlands
*Anne Karelse - Ghent University Hospital - Gent, Belgium
*Email: anne.karelse@telenet.be
Data collection by Digital Patient Engagement App
Introduction
Fast-track surgery is highly desirable because of economic health care, reduction in complications and patient satisfaction perspectives. If we want to improve this procedure a change in thinking strategy is mandatory asking two important questions: Why is my patient still in hospital? and, How is my patient doing outside the hospital? Because of the shortened hospital stay there is an information gap. Telemedicine and digital aids are proven to be effective. We used and optimised the My mobility app to the needs and wishes of our practice to guide patients through the procedure with the aim of improving patient information, preparation, expectations, and follow-up, all ultimately to improve healthcare, costs, and patient satisfaction. We describe a 2-year process of trial and adjustments to develop the app that meets our requirements.
Methods
All patients requiring a TKR or THR are included for surgery in Rapid Recovery programs; all patients are equally informed, walking at day of surgery and aim at early discharge. A nurse is committed to the pre and postoperative support, and data management. Information is provided with the My Mobility app that runs on smart phones or watch, starting preoperatively the moment the operation is planned, until 1 year postoperatively. It provides patients with preoperative information, enables two-way messaging, video consultation, provides and encourages exercises. It monitors pain experience, and it collects subjective and objective data as PROMs and clinical improvement (Fig.1). The connection of wearables enables monitoring and collecting mobility, functional and gate analysis data.
Results
The creation of a team around the patient and the app is essential to make this work. This must include all care givers: surgeon, nurses, physiotherapists, outpatient care givers, assistants, and the ICT-department and each is given a clear responsibility. Information is key in outcome of the program and all care givers should give the same information and repeat it. There should be one program leader from the inside team that is responsible for the adherence of patients to the app and wearable, monitors the dashboard of patients progress and answers the questions in the chat.
Conclusion
Digital patient engagement apps are used to engage, inform, and improve the patient’s adherence to treatment plans. To add this to an orthopaedic practice a complete set-up with a dedicated team from the start is essential. Providing the same information to patients and repeat it is the key to success. The common goal is data collection as interpretation and study can improve the outcome of your program and finally improve health care and patient satisfaction.
Figures
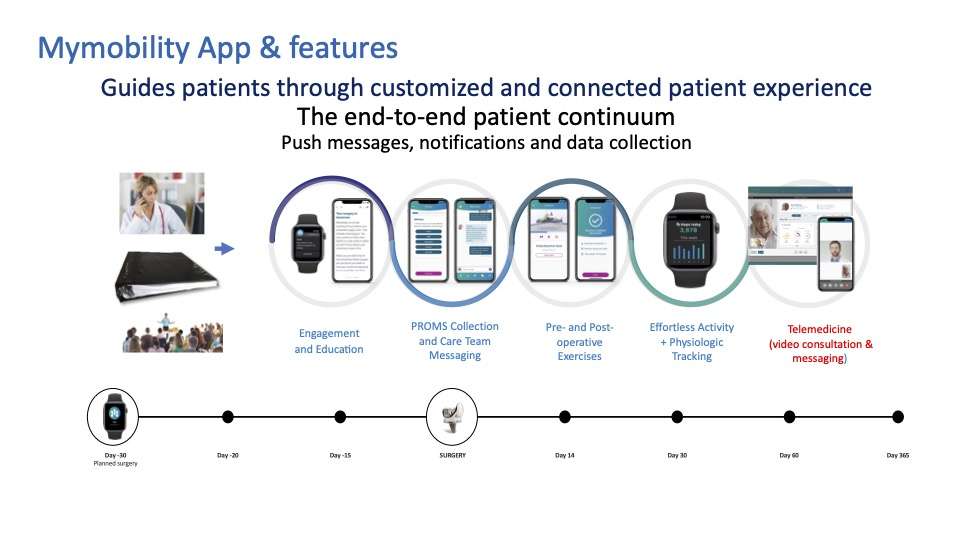
Figure 1#8718
Short-Term Outcomes of a Novel, Personalized Revision Knee System
*Emily Ronan - Langone Orthopedic Hospital - New York, United States of America
Jonathan Katzman - NYU Langone - New York, United States of America
Alana Prinos - Langone Orthopedic Hospital - New York, USA
Hayley Raymond - Langone Orthopedic Hospital - New York, USA
Daniel Waren - NYU Langone Health - New York, USA
Ran Schwarzkopf - NYU Langone Medical Center Hospital for Joint Diseases - New York, USA
Vinay Aggarwal - NYU Hospital for Joint Diseases - New York, USA
*Email: emarie7100@gmail.com
Introduction: One of the most common indications for revision total knee arthroplasty (rTKA) in orthopedic patients is aseptic loosening, which results from failed implant fixation and osseointegration. The use of multi-zonal fixation, highly porous metal material, and durable polyethylene bearing surfaces have been successful in increasing implant stability and longevity. This study sought to determine the initial clinical outcomes of a novel revision knee system that utilizes the above factors to promote success following rTKA.
Methods: All data were collected retrospectively from a single tertiary academic medical center. Patients were included if they received an rTKA using tibial and/or femoral components from the Persona® Revision Knee System of Zimmer Biomet from September 2020 to December 2022. Demographic variables, intraoperative data, and surgical outcomes data were collected for each patient. In addition, implant details (such as fixation type and cone and/or augment use) were collected.
Results: Of all 35 revisions, 31 were total revisions, 3 were tibial revisions, and 1 was a femoral revision. The most common indication for revision was aseptic loosening (51.4%), followed by instability (22.9%), periprosthetic joint infection (PJI) (11.4%), polyethylene wear (8.6%), and arthrofibrosis (5.7%). Among the implanted femoral stems, 16 (45.7%) underwent cemented fixation, and 19 (54.3%) underwent hybrid fixation. Among the implanted tibial stems, 21 (60.0%) underwent cemented fixation, and 14 (40.0%) underwent hybrid fixation. Of all 35 cases, 29 (85.9%) included at least one tibial and/or femoral cone, and 24 (68.6%) cases included augments. Three procedures incurred 90-day complications, including cellulitis (5.7%) and acute on chronic anemia with PE (2.9%). There was only one reoperation (irrigation and debridement; 2.7%) within the cohort. There were no component-replacing re-revisions in the cohort.
Conclusion: The results of our study demonstrate that a novel revision knee system incorporating multi-zonal fixation, porous metal material, and durable polyethylene bearing surfaces is successful in promoting implant stability while minimizing postoperative complications and reoperations. This study is limited by its relatively small sample size and minimal postoperative data, as many of our cases had <1 year of follow-up data. Nonetheless, our study shows promising initial results for a novel revision knee system. Future studies should continue to investigate these implants in the long-term.
Figures
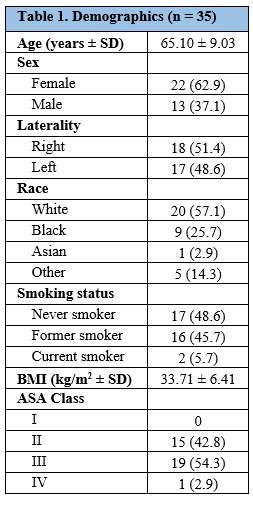
Figure 1
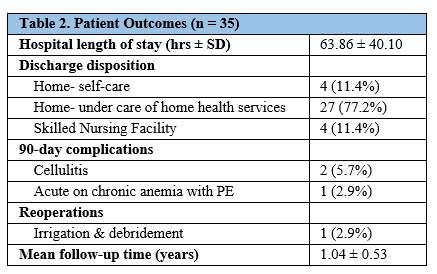
Figure 2
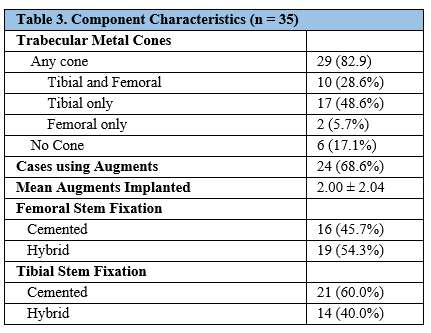
Figure 3#8419
Efficacy of Custom Made Porous Cones in TKA Re-Revisions: A Biomechanical Comparative Study
*Edoardo Bori - BEAMS Department ULB - Bruxelles, Belgium
Marika Padalino - Université Libre de Bruxelles - Bruxelles, Belgium
Gianluca Piovan - IRCCS Sacro Cuore Don Calabria Hospital - Negrar, Italy
Bernardo Innocenti - Universite' Libre De Bruxelles - Bruxelles, Belgium
*Email: edoardo.bori@gmail.com
Introduction
In the case of knee prosthesis revision, prosthetic stability is crucial. In patients who have undergone multiple revisions, the presence of extremely weak and fragile bones is common, making it extremely challenging to achieve good fixation. To address this issue, patient-specific porous titanium cones have been developed to ensure good bone-prosthesis interface, with limited stress peaks and an even distribution of forces along the entire bone. To verify the distribution of stresses on both the tibia and femur induced by the use of these patient-specific cones compared to the use of traditional fixation techniques, a biomechanical analysis based on previously validated finite element models was conducted. A further parameter analyzed was then the use of porous titanium (Ti-Por) compared to solid (conventional) titanium.
Methods
Finite element models based on previous literature works were developed [1].
Five configurations were analyzed:
- TKA revision with the use of porous titanium cone (Ti-Por).
- TKA revision with the use of solid titanium cone (conventional).
- TKA revision with the use of cemented stem.
- TKA revision with the use of press-fit stem.
- TKA revision with the use of large resection prostheses
All configurations were analyzed testing two standard load conditions (see Figure 1)
- Full Extension (0° of flexion): The tibia is fixed distally, and an axial load of 2200 N is applied to the proximal surface of the femur.
- Sitting-to-Standing (90° of flexion): The tibia is fixed distally, and a vertical load of 1000 N is applied to the proximal surface of the femur.
Results
The average von Mises stress distributions were extracted and studied in the distal region of the femur and the proximal region of the tibia for both the full extension configuration at 0° (See Figure 2) and for the sitting-to-standing one at 90° (See Figure 3).
Discussion
Full Extension 0°:
The porous and solid cones returned a uniform stress distribution in the distal part of the femur, while the presence of the stem induced stress shielding (especially the press-fit stem). The large resection instead showed higher stresses in this area, but with potential risk of fracture.
The proximal tibia showed overall similar distributions, with the Ti-Por presenting the most homogeneous one.
Sitting-to-Standing 90°:
The cones distributed more uniformly the stresses in the distal part of the femur, with higher values found in the case of Ti-Por cone. The press-fit stem induced instead high stress, with a risk of fracture, while the cemented stem and the large resection prosthesis induced stress shielding in the distal femur.
The proximal tibia returned stress peaks for both stems and large resection prosthesis, while both cones presented instead more homogeneous distributions.
Conclusions
The use of cones returned overall more homogeneous stress distribution in the bone and avoided the occurrence of stress-shielding, instead found for the other prosthetic options; for these reasons, the use of cones should be kept into account by the surgeon when defining the approach for the specific patient.
References
- https://doi.org/10.1007/s00402-020-03670-6
Figures
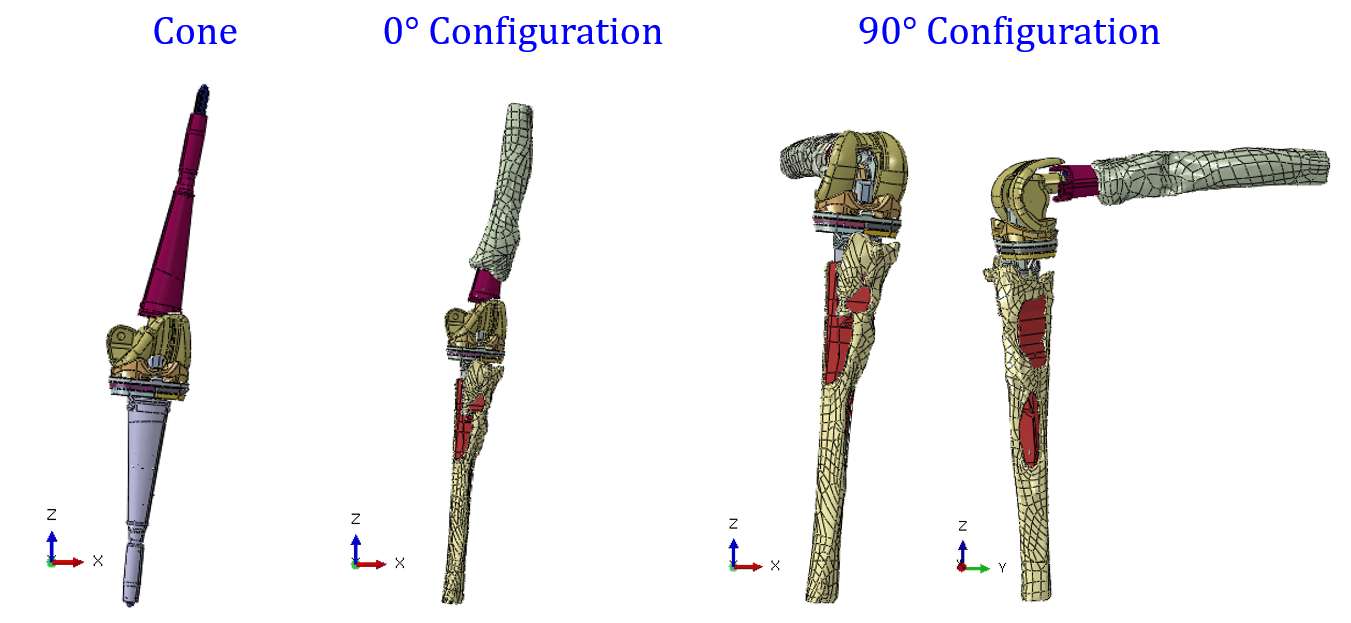
Figure 1
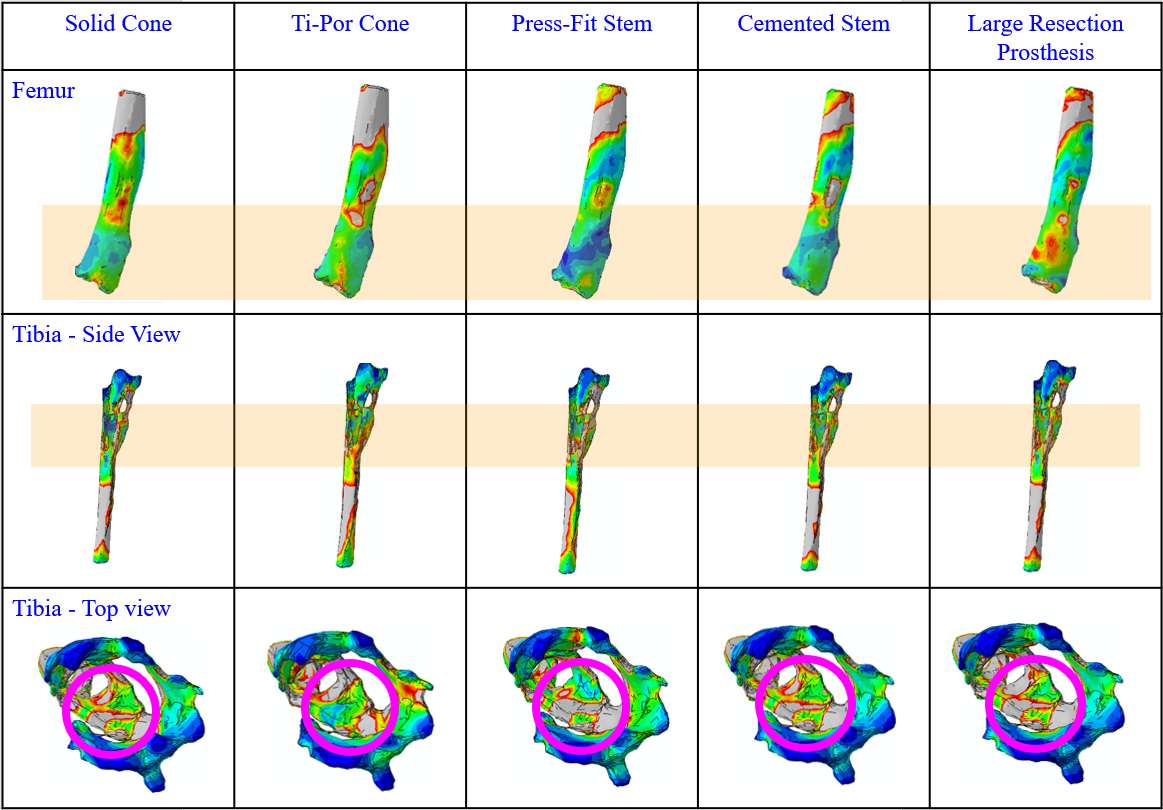
Figure 2
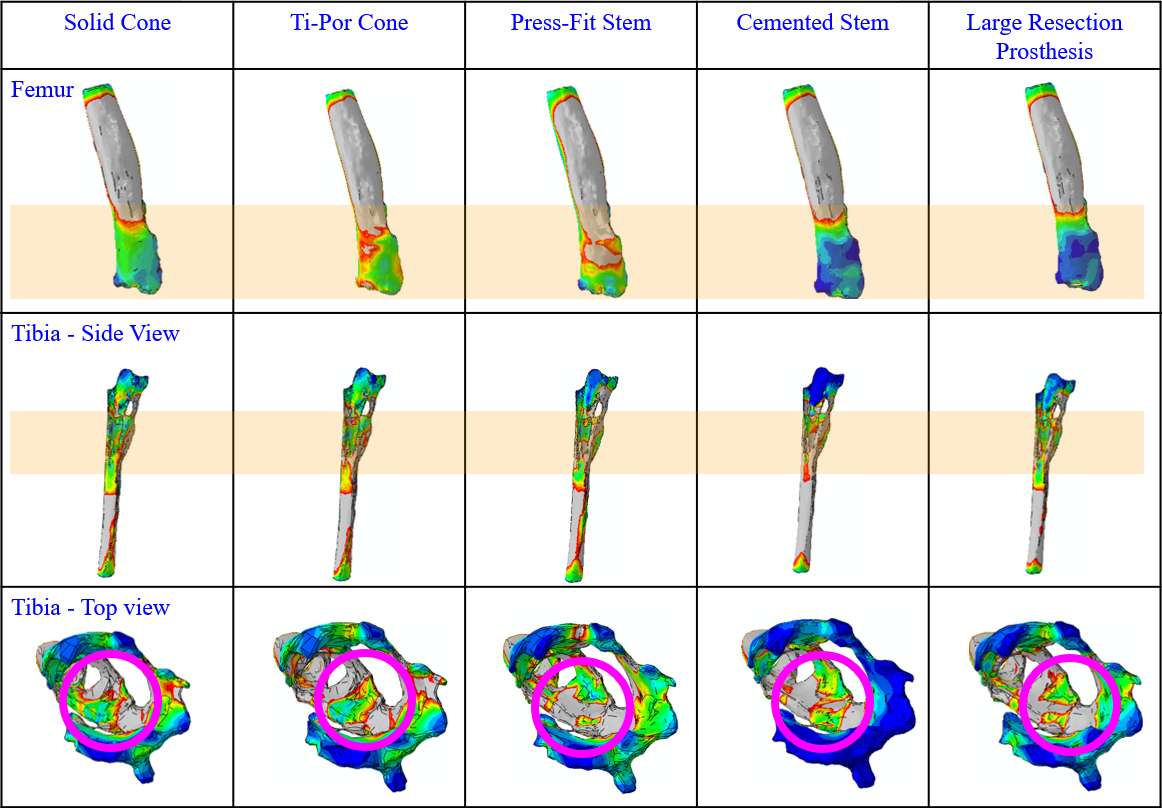
Figure 3#8126
In-Silico Method for Pre-Clinical Assessment of Custom-Made Knee Implants
*Georg Hettich - Aesculap AG - Tuttlingen, Germany
Josef-Benedikt Weiß - Aesculap AG - Tuttlingen, Germany
Thomas M. Grupp - Aesculap AG - Tuttlingen, Germany
*Email: georg.hettich@aesculap.de
Introduction:
In severe cases of revision total knee arthroplasty (TKA), a custom-made knee implant may represent one of the few remaining treatment options to restore joint function. Compared to a patient-matched implant, where the implant design is validated within a design envelop, the design of a custom-made implant is rare and absolutely unique. Pre-clinical assessment of the mechanical properties of such a custom-made implant is therefore very challenging since time constraints often prevents a biomechanical test and pure simulation results are limited in their transferability to the clinical situation.
This study suggests and applies an in-silico assessment method to support surgeons and engineers in their decision whether a design of a custom-made implant is mechanically reliable.
Methods:
The assessment of the custom-made implant relies on a comparable reference implant and its already performed biomechanical tests as well as a finite element analysis (FEA) of the reference and the custom-made implant. The assessment comprises six steps (Figure 1): (1) identification of the main potential failure mechanism of the reference implant and its acceptance criterion, (2) reproduction of the already performed biomechanical test of the reference implant via FEA, (3) identification of the endurance limit and the value of the corresponding FEA quantity, (4) definition of this value as the acceptance criterion for the custom-made implant FEA, (5) reproduction of the biomechanical test via custom-made implant FEA, (6) conclusion, whether the acceptance criterion for the custom-made implant is fulfilled or not.
This method was applied to two exemplary cases of custom-made implants to assess their mechanical properties. The results of the FEA in terms of tensile stress and micromotion were compared between the reference implant and the custom-made implant and confirmed by biomechanical tests of the custom-made implants.
Results:
The FEA results of the reference implants compared to the custom-made implants show a decrease in maximum tensile stress (Figure 2) and micromotion, respectively. Since the reference implants successfully performed the biomechanical test, the method predicted that the custom-made implants also would perform the biomechanical tests successfully. This prediction was confirmed by biomechanical tests of the custom-made implants.
Conclusion:
The suggested method allows to assess the mechanical properties of a custom-made implant solely by a FEA without performing a biomechanical test with it. The transferability of the FEA results is achieved by the comparison to the biomechanical test of a reference implant and its FEA results, where a acceptance criterion for the FEA was derived. This method represents an important contribution in the pre-clinical evaluation of custom-made implants to achieve a sustainable treatment in severe revision TKA cases.
Keywords:
Custom-made implants, Finite Element Analysis, Total Knee Arthroplasty
Figures
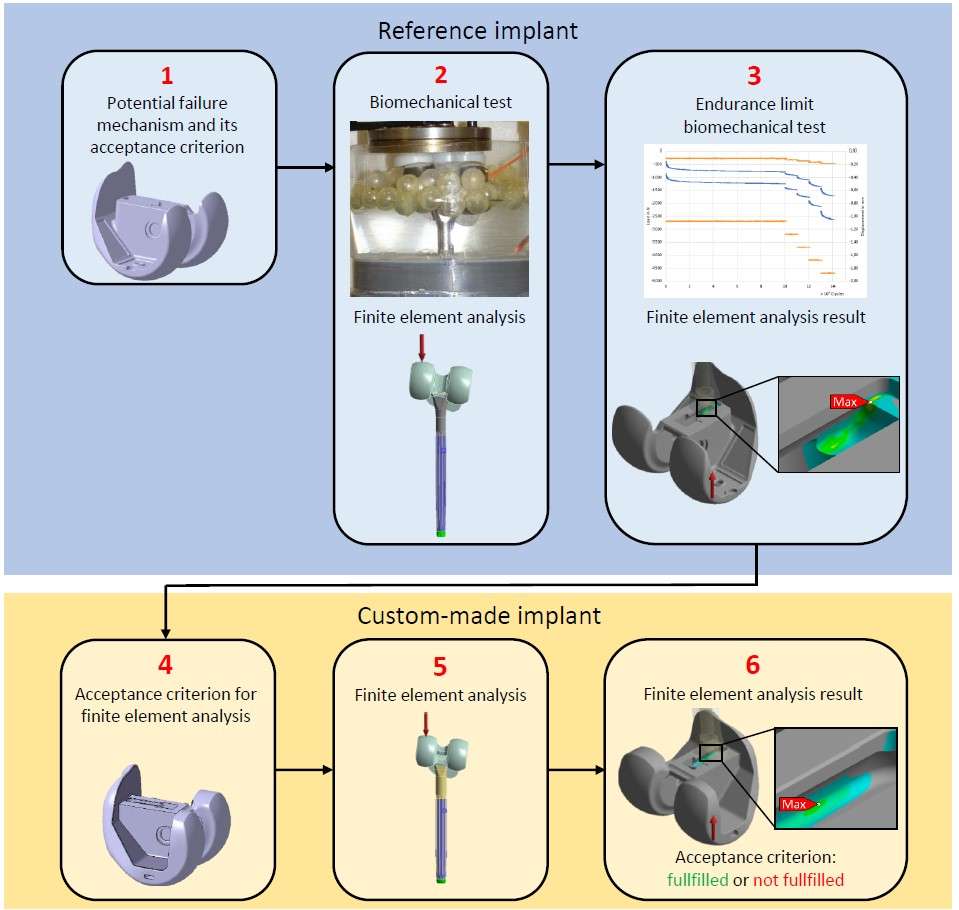
Figure 1
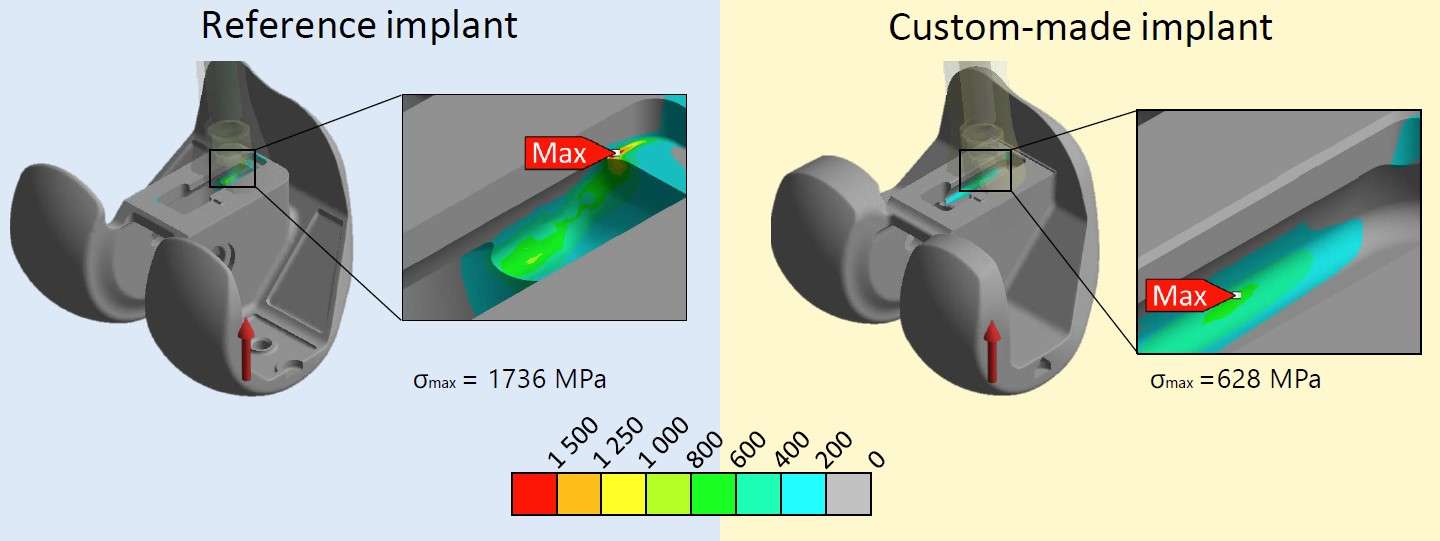
Figure 2#8603
Improved Clinical Outcomes for Personalised Alignment Strategies Using Assistive Technology.
*Philippe Van Overschelde - Medisch Centrum Latem - Sint-Martens-Latem, Belgium
*Email: vanoverschelde@gmail.com
Introduction
Total knee arthroplasty remains the standard of care for treating end-stage osteoarthritis of the knee joint. Approximately 15 - 20 % of the patients remain dissatisfied following surgery. To improve outcomes, some authors suggest a personalized alignment strategy. We investigated the use of the CPAK classification in the decision making process regarding the alignment strategy
Methods
In this study all TKA candidates got a preop and postop a full leg X-rays. The full leg X-rays allow to assign a patient to a CPAK phenotype. Based on this classification the decision was made to use a kinematic alignment protocol for CPAK type I, II and IV and a mechanical alignment protocol for CPAK type III, VI. In both groups the Knee + augmented reality solution (Pixee Medical, Besancon, France) was used together with the implantation of medial pivot knee design. The primary research goal is to evaluate the clinical outcome score (FJS and KSS with a minimum 1y FU) for the two different phenotype groups. The secondary research goal was to compare the results with a previously operated cohort of 250 patients divided into varus and valgus knees treated with a pure mechanical alignment strategy with conventional jigs for all cases.
Results
This retrospective study evaluated 180 patients operated with the augmented reality solution. All patients were followed up for at least one year. The median values for FJS and KSS were 80 and 39 respectively for the kinematic alignment group (CPAK phenotype I, II and IV). The median values for FJS and KSS were 79 and 39 respectively for the mechanical alignment group (CPAK phenotype III, VI.). The median value for the FJS for the 250 patients cohort was 77 for the varus cases and 58 for the valgus cases.
Conclusion
The use of a classification tool, hence the CPAK phenotyping, in combination with assistive technology allowed us to introduce a personalised alignment strategy with improved outcomes for our patients and thus reducing the number of patients that remain dissatisfied after surgery. Further research to finetune the alignment strategy is ongoing and includes the combination of static and dynamic evaluation in a 3D setting.
#8257
Does Femoral Flange Design Influence Patellofemoral Mechanics in Patient Specific Alignment?
*Amit Mane - DePuy Synthes - Warsaw, USA
Rebecca Boldt - DePuy Synthes - Warsaw, USA
Chase Maag - DePuy Synthes - Warsaw, USA
Ian Leslie - DePuy Synthes Joint Reconstruction - Leeds, United Kingdom
Michael Hirschmann - Kantonsspital Baselland - Basel, Switzerland
Richard Komistek - The University of Tennessee - Knoxville, USA
*Email: amane5@its.jnj.com
Introduction: Patient specific alignment (PSA) in TKA has been introduced to restore natural knee kinematics by restoring native alignment1,2. Orthopedic manufacturers have determined that PSA is allowable with their respective TKA designs. However, the majority of these designs were developed in the era of Mechanical alignment. This raises question of their suitability in PSA, in which the trochlear groove is often internally rotated3. One way to address this is to investigate the sensitivity of the parameters, such as patellofemoral (PF) biomechanics to PSA positioning perturbations4. Therefore, objective of this work was to assess the PF biomechanics in PSA, with various patellar and femoral perturbations, using two femoral components with distinctive flange designs.
Methods: Previously validated Forward dynamics model simulated deep knee bend activity (Fig.1)5. Implants included were ATTUNE™ Medial Stabilized construct (DePuy Synthes), Construct-AMS, and a ball-and-socket type construct, Construct-eMP. Constructs were simulated in PSA with a 3? joint line orientation in extension and 0? femoral rotation in flexion; this baseline PSA is referred as neutral configuration. For perturbation analysis, patellar component was moved 5mm mediolaterally (ML) at neutral patellar height and at 5mm Alta position (Fig. 1). Later, femur was rotated 3? internally and externally. The simulation was repeated for both constructs. The changes in medial (MPFL) and lateral (LPFL) PF ligament strains, patella-tibial ML shift and tilt from their corresponding magnitudes at neutral configurations were estimated. Change in Quadriceps lengths at all configurations were reported.
Results: In Construct-AMS, the MPFL and LPFL strains increased by average of 2.7±0.3% and 1.3±0.3% from its neutral configuration respectively, whereas, in Construct-eMP, these strains increased by 6.8±0.3% and 3.6±0.3% respectively(Fig. 2). In early flexion (<40?), patellar ML shift increased by 2.5mm in Construct -AMS and 4.6mm in Construct -eMP (Fig. 3). In later flexion, both patellae showed smaller variations in ML shift; however, in Construct-AMS patella remained closer to its neutral configuration path than that of Construct-eMP. During early flexion, the peak elongation of Rectus Femoris, Vastus Medialis and Vastus Lateralis muscles were smaller for Construct -AMS than Construct-eMP (Fig. 3).
Conclusion: The trochlear groove of the Construct-eMP is significantly more conforming than that of the Construct-AMS (Fig.2). The effects of these differences could have influenced observed trends in PF biomechanics. The Construct-eMP experienced larger changes in PF ligament strains, motions and quadricep elongation from its neutral position than Construct-AMS. These trends may indicate that the constrained PF compartment may be less forgiving to variation in implant placement. The femur used in Construct-AMS is characterized with the GLIDERIGHT™ PF articulation with funnel shaped articular geometry. These features could have allowed patella more freedom to find its position in-harmony with the surrounding soft tissues. Similar results were reported previously6-8. Further, a study utilizing Construct-AMS demonstrated that variation in the femoral rotation did not impact patient outcomes9. In summary, the femoral flange design and PF congruencies could alter PF biomechanics in PSA.
References: 1.Nisar et.al.-2020, 2.Xiao et.al.-2020, 3.Riviere et. al.-2018, 4.Niki et.al.-2020, 5.Khasian et.al.-2020, 6.Ferris et.al.-ASME-BioE-2012, 7.Shalhoub et.al.-ESB-2012, 8.Fitzpatrick et.al.-ORS-2012, 8.Murgier et. al.-KSSTA-2020
Figures
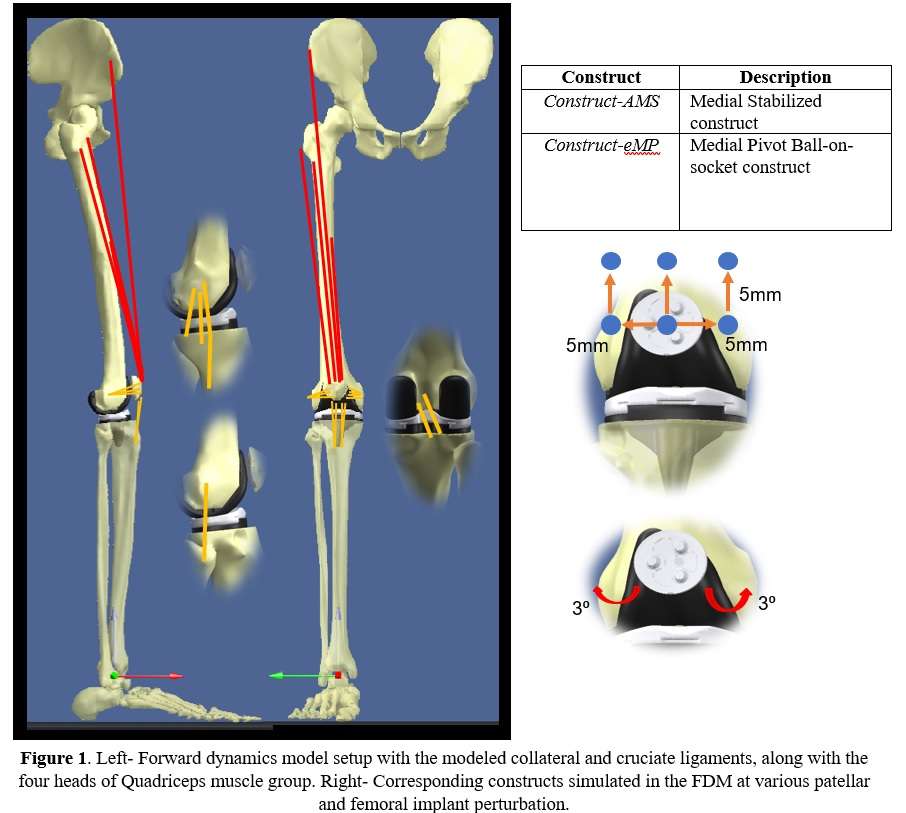
Figure 1
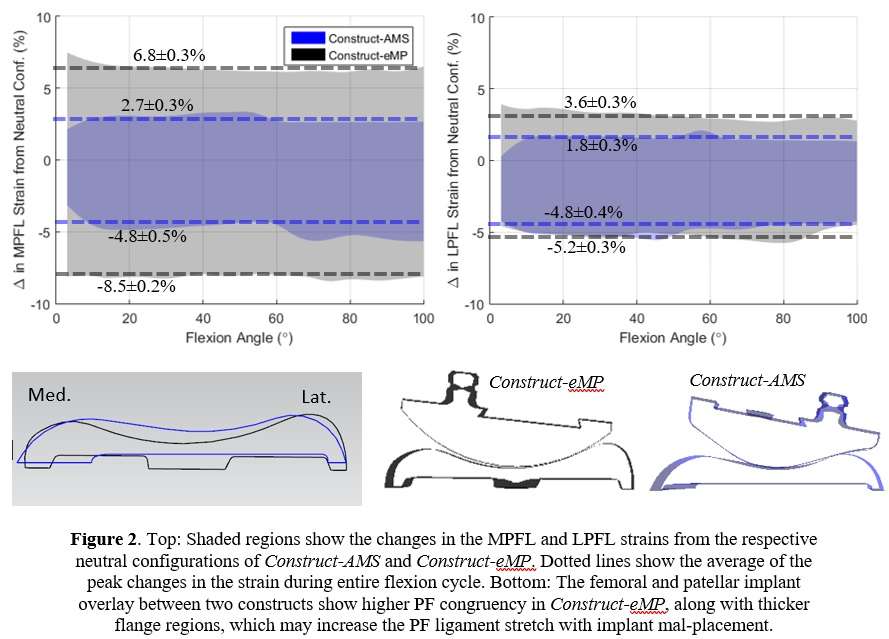
Figure 2
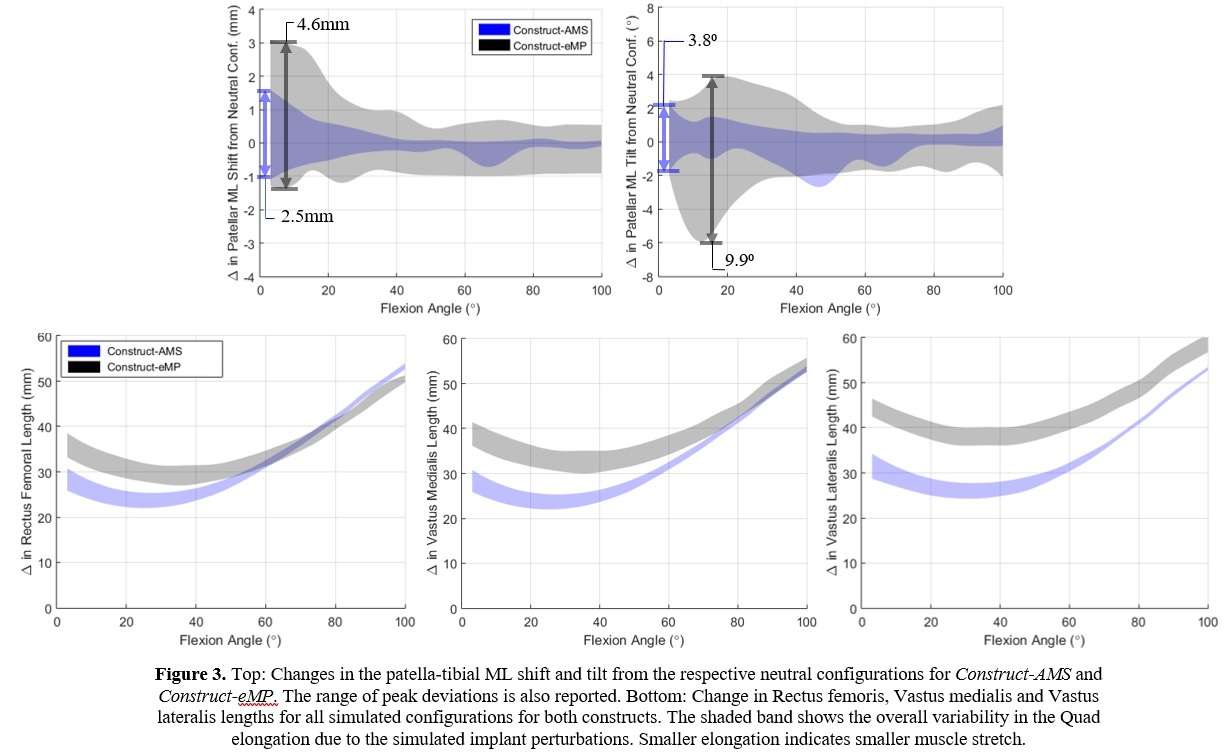
Figure 3#8347
3D-Printed Patient-Specific Instrumentation Helps Achieve the Intended Prosthetic Femoral Version
*Anna Di Laura - Royal National Orthopaedic Hospital and Dept. MechEng at UCL - London, United Kingdom
Johann Henckel - Royal National Orthopaedic Hospital - London, United Kingdom
Harry Hothi - London Implant Retrieval Centre - Stanmore, United Kingdom
Alister Hart - Royal National Orthopaedic Hospital - London, United Kingdom
*Email: anna.laura.14@ucl.ac.uk
Introduction:
Implantation of the femoral component with suboptimal version is associated with instability of the reconstructed hip joint. High variability of Prosthetic Femoral Version (PFV) has been reported in primary Total Hip Arthroplasty (THA). Three-dimensional (3D) Patient-Specific Instrumentation (PSI) has been recently developed and may assist in delivering a PFV within the intended range.
We performed a pilot study to better understand whether the intra-operative use of a novel PSI guide (Figure1), designed to deliver a PFV of 20°, results in the target range of PFV in primary cemented THA.
Methods:
We analysed post-operative Computed-Tomography (CT) data of two groups of patients who underwent primary cemented THA through posterior approach; 1. A group of 11 patients (11 hips) for which the surgeon used an intra-operative 3D-printed stem positioning guide (experimental) 2. A group of 24 patients (25 hips) for which the surgeon did not use the guide (control). The surgeon aimed for a PFV of 20°, and therefore the guide was designed to indicate the angle at which the stem was positioned intra-operatively. PFV angles were measured using the post-operative 3D-CT models of the proximal femurs and prosthetic components in both groups. Our primary objective was to compare the PFV in both groups (Figure 2). Our secondary objective was to evaluate the clinical outcome.
Results:
Mean (± SD) values for the PFV was 21.3° (± 4.6°) and 24.6° (± 8.2°) for the experimental and control groups respectively. In the control group, 20% of the patients reported a PFV outside the intended range of 10° to 30° anteversion. In the experimental group, this percentage dropped to 0%. Satisfactory clinical outcome was recorded in both groups (Figure 3).
The experimental group had a median follow up time of 11 months (10 to 13 months). The median follow-up time for the control group was 23 months (16 to 32 months). No intra-operative complications such as fracture, indicating wrong implant planning, size or implantation, have been recorded, resulting in an overall functional clinical outcome. At the most recent follow-up, none of the hips had been revised for any reason.
Conclusion:
The intra-operative use of a PSI PFV guide helped the surgeon avoid suboptimal PFV in primary cemented THA. Further studies are needed to evaluate if the PSI guide directly contributes to a better clinical outcome.
Figures
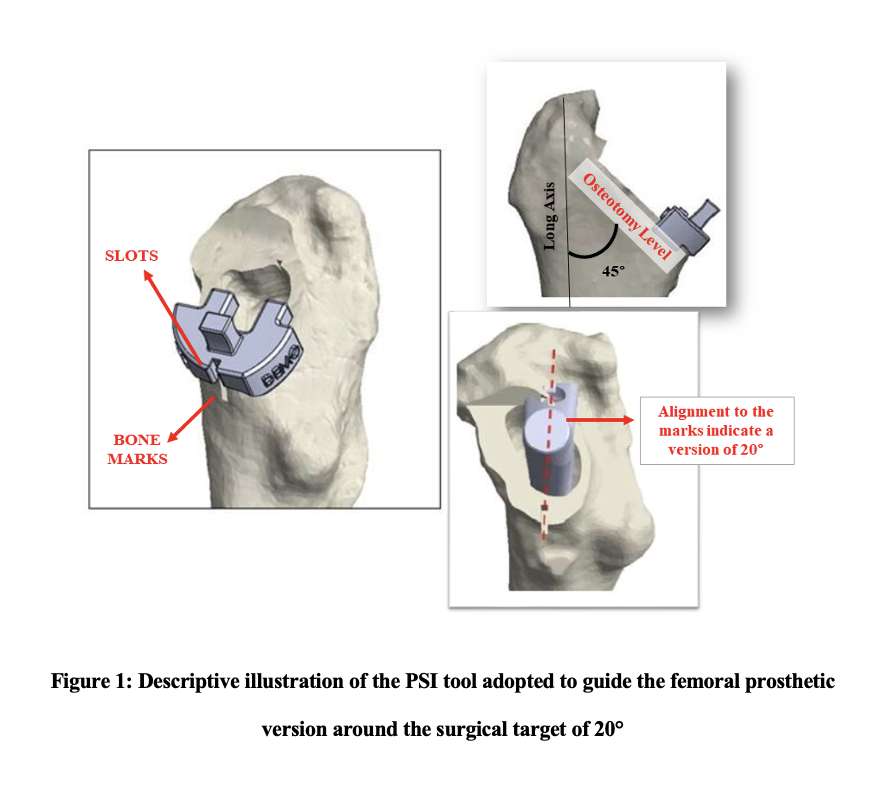
Figure 1
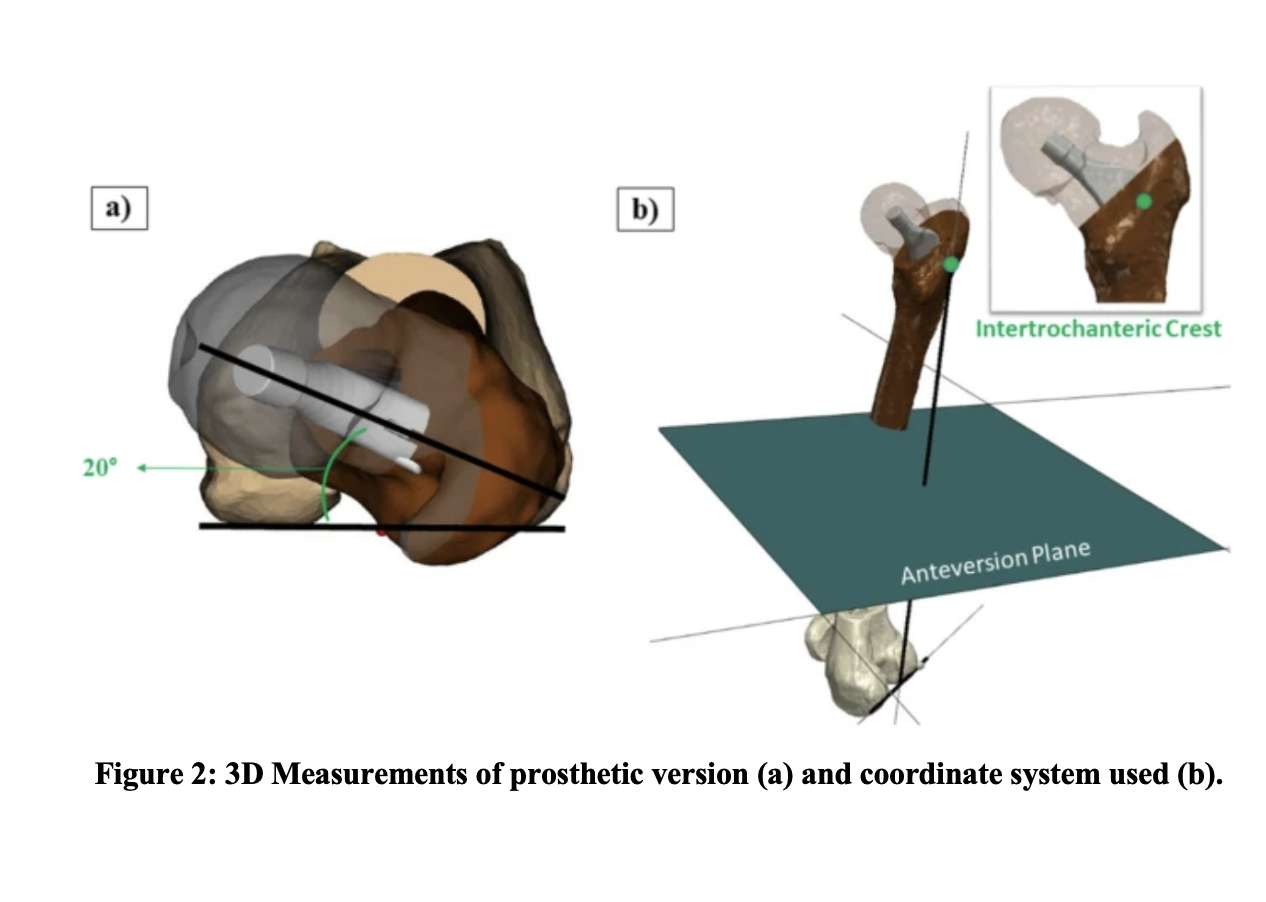
Figure 2
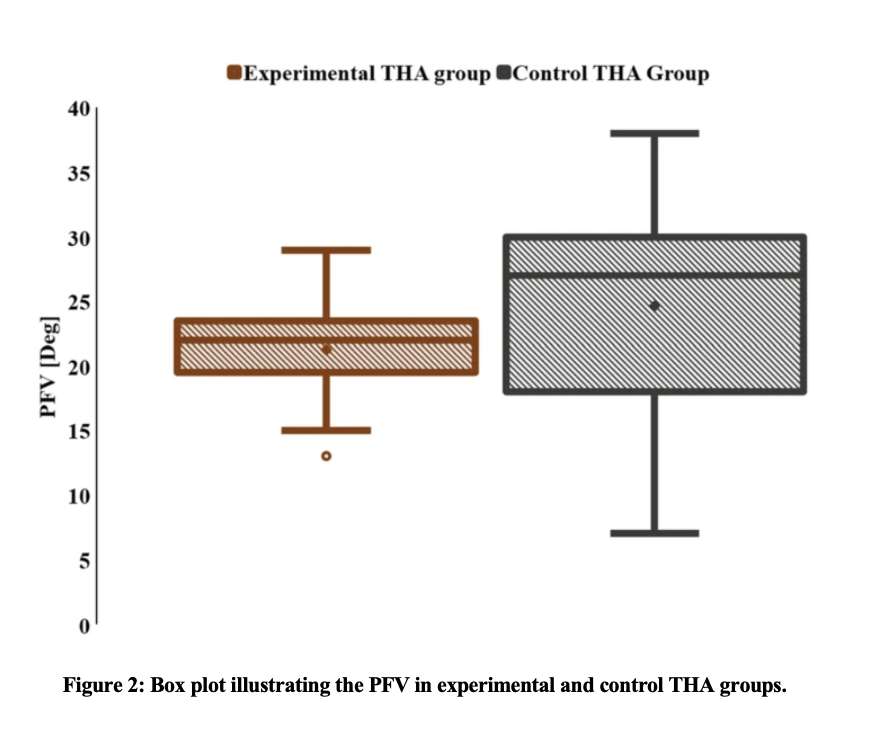
Figure 3#8800
Improved Postoperative Radiographic Alignment Associated With Patient-Specific Instrumentation Total Ankle Arthroplasty
*Gloria Coden - Bronx, United States of America
Kurt Hofmann - New England Baptist Hospital - Boston, USA
Nicholas Veale - Boston Sports & Shoulder Center - Waltham, USA
*Email: gscoden@gmail.com
Introduction: The rate of total ankle arthroplasty (TAA) is projected to continue to increase. While the most significant predictor of complications after total ankle arthroplasty is implant malalignment, this can be technically challenging to achieve. Patient-specific instrumentation (PSI) was designed to improve the accuracy of implant positioning in TAA. Therefore, we sought to compare radiographic alignment and postoperative function in patients who underwent a TAA with versus without PSI guides.
Methods: We retrospectively reviewed 25 ankles who underwent TAA using PSI with minimum 1 year follow-up. These were compared to 50 ankles who underwent TAA without PSI, which were chosen as historical controls. Demographics were similar between each group, including age (p=0.6), gender (p=0.4), body mass index (p=0.4), American Society of Anesthesiologists score (p=0.1), laterality (p=0.9), preoperative diagnosis (p=0.5), smoking status (p=0.1), history of diabetes mellitus (p=0.7). Patients in the PSI cohort had better preoperative plantarflexion (32.9 versus 26.2 degrees, p=0.001) and total range of motion (41.9 versus 34.5 degrees, p=0.017), but similar preoperative dorsiflexion (9.0 versus 8.3 degrees, p=0.6). Patients in the PSI cohort had more preoperative talar tilt (8.0 versus 4.0 degrees, p=0.002), but similar preoperative coronal angle (0.4 versus 0.5 degrees, p=1.0) and sagittal translation (2.0 versus 1.3 degrees, p=0.6).
Results: Postoperative coronal angle (-0.9 versus 1.8 degrees, p<0.001) and sagittal angle (-2.6 versus 5.9 degrees, p<0.001) were more accurate with the PSI guides compared to the manual guides. Postoperative range of motion was similar between the PSI and manual groups, including dorsiflexion (9.8 versus 10.7 degrees, p=0.6), plantarflexion (32.1 versus 29.2 degrees, p=0.2), and total range of motion (41.8 versus 39.9 degrees, p=0.6) at 1 year postoperatively. Patients with PSI had decreased American Orthopaedic Foot and Ankle Society (AOFAS) pain scores (27.2 versus 32.4, p=0.041), but there were similar AOFAS function (40.1 versus 39.6, p=0.9), AOFAS alignment (9.3 versus 9.1, p=0.8), and AOFAS total scores (75.8 versus 80.5, p=0.3) between cohorts. At minimum 1 year follow-up, there was no difference in the number of TAAs that required reoperations (3 versus 16 TAAs, p=0.1).
Conclusion: The use of PSI is associated with more accurate postoperative coronal and sagittal alignment compared to manual TAA. While patients with PSI had decreased AOFAS pain scores, both cohorts had similar postoperative range of motion and overall AOFAS scores. While there were a similar number of reoperations in each cohort, this retrospective review was limited to patients with minimum 1 year follow-up. Further research is needed to determine if the improved alignment with the use of PSI implants is associated with better survivability long term.
#8676
Patient-Specific Instrumentation for Total Ankle Arthroplasty
*Gloria Coden - Bronx, United States of America
Nicholas Veale - Boston Sports & Shoulder Center - Waltham, USA
Kurt Hofmann - New England Baptist Hospital - Boston, USA
*Email: gscoden@gmail.com
Introduction: The rate of total ankle arthroplasty (TAA) is projected to continue to increase. While the most significant predictor of complications after total ankle arthroplasty is implant malalignment, it can be technically challenging to achieve this, especially in patients who underwent previous foot and/or ankle surgery. The alternative to TAA is an ankle arthrodesis, which limits a patients range of motion and changes gait biomechanics. However, patient-specific instrumentation (PSI) was designed to improve the accuracy of implant positioning in TAA, especially in patients with unique anatomy. Therefore, we sought to present the case of a patient who underwent a TAA after triple arthrodesis with retained hardware using PSI.
Methods: We present the case of a 66-year-old male who underwent a left triple arthrodesis 1 year preoperatively. Post-operatively, ankle range of motion was 5 degrees of dorsiflexion and 15 degrees of plantarflexion. The patient had an American Orthopaedic Foot and Ankle Society (AOFAS) score of 32, Ankle Osteoarthritis Score (AOS) of 6.67, Patient Reported Outcomes Measurement Information System (PROMIS) physical health score of 32.4, and a PROMIS mental health score of 53.3. Radiographs demonstrated a valgus deformity with a coronal alignment of -2.0 degrees, talar tilt of 14.0 degrees, and tibiotalar sagittal translation of 10.1 degrees anteriorly. The patient continued to have 7 out of 10 left ankle pain on the Visual Analog Scale (VAS) despite conservate treatment and was therefore indicated for a left TAA. Due to the patient’s triple arthrodesis with retained hardware, we chose to use PSI due to the unique anatomy and to identify interfering hardware.
Results: The patient underwent a TAA with PSI for posttraumatic arthritis. There were no intraoperative complications. Operative time was 161 minutes. The preoperative plan was accurate and predicted the patient’s implant sizes. At 3 months postoperatively, the patient has 1 out of 10 pain on VAS and walks unassisted with a reciprocating gait. The patient has similar dorsiflexion of 5 degrees, but improved plantarflexion of 30 degrees. AOFAS score improved to 67, AOS decreased to 1.61, PROMIS physical health improved to 44.9, and PROMIS mental health score improved to 48.3. Radiographs at 3 months postoperatively demonstrate a coronal alignment of -5.0 degrees and a sagittal alignment of the tibial angle of -1.0 degrees. The patient reports that he is satisfied with his outcome and that the function of his ankle is much better.
Conclusions: TAA is an alternative to ankle arthrodesis and can be successfully performed in patients with previous foot and ankle surgery, including a triple arthrodesis. Patient specific instrumentation is useful for preoperative planning to correct the ankle deformity and identifying interfering hardware. It is important for surgeons to be aware of this technology, especially when preforming TAA in patients with challenging anatomy.
#8098
Solving the Anchorage Problem Distal to the Isthmus as an Essential Feature of a Customized Spacer
Alexander Vacariu-Seelig - Kantonsspital Baselland / University of Basel - Bruderholz, Switzerland
*Andrej Nowakowski - Kantonsspital Baselland - Bruderholz, Switzerland
*Email: andrej.nowakowski@unibas.ch
Purpose:
After surgery, immediate full weight bearing is especially important in the elderly population. Besides reducing postoperative morbidity, it also increases patient well-being and satisfaction. Especially in two-staged surgeries, it is a surgeon’s goal to achieve a situation in which the patient is fully mobile inbetween the two surgeries. However, in some unique cases this may be a challenge for surgeons.
Material/Methods:
An 88-year-old patient suffered a chronic implant-associated infection with a difficult to treat germ, which implemented a two-stage revision approach. The patient had undergone several previous surgeries, including implantation of a cemented long-stem prosthesis, which lead to cement leakage due to a femoral defect in the lateral shaft area at the isthmus. Hence, it was necessary to achieve stabile anchoring distal of the isthmus to allow weight bearing in the interval between the two interventions. To allow distal anchorage, a gamma nail was sawed off proximally to build up the custom-made spacer. The following custom-made spacer was implanted. Additionally modified with transversal drill-holes, this distal part of the nail got cemented into a cement sleeve using two 10ml syringes as a guide form. After curing of the cement, the modified nail could be locked distally to be further built up with a conventional long spacer.
Results:
The patient could be mobilized very well with the custom-made implant until the reimplantation of the new prosthesis could be performed. Even though the patient was of age, there was no thrombosis, no embolism, and no excessive muscle atrophy. She was quickly mobile again after reimplantation. One year postoperatively, the 89-year-old patient, still free of infection, presented with a safe and limp-free gait. She still lives at home and is independently mobile. There are no contractures, a negative Trendelenburg and unrestricted and pain-free mobility.
Conclusion:
For individual cases, the industry does not always have a suitable (interim) implant. So with our custom made spacer distal anchoring was possible as well as postoperative mobilization. We think that the mobility and the preserved preload of the abductors during the spacer phase reduced immobility-associated complications such as pneumonia, deconditioning, thromboembolic events, sarcopenia, etc.
#8309
Use of Custom Glenoid Components for Reverse Total Shoulder Arthroplasty
*Stephen Weber - Sacramento Knee & Sports Medicine - Sacramento, USA
Punyawat Apiwatanakul - Johns Hopkins - Baltimore, USA
Prashant Meshram - Johns Hopkins - Baltimore, USA
Andrew Harris - Johns Hopkins - Baltimore, USA
Joel Bervell - Johns Hopkins - BAltimore, USA
Piotr Lukasiewicz - Johns Hopkins - Baltimore, USA
Ridge Maxson - Johns Hopkins - Baltimore, USA
Matthew Best - Johns Hopkins - Baltimore, USA
Uma Srikumaran - Johns Hopkins - Baltimore, USA
Edward McFarland - Johns Hopkins School of Medicine - Baltimore, USA
*Email: webersc@earthlink.net
Introduction: Severe glenoid bone loss in reverse total shoulder arthroplasty (RTSA) requiring bone grafting may result in high failure rates. Custom glenoid components have been reported to be a viable solution for patients with large bone defects. However, the strengths and limitations of using these implants have not been described. The purpose of the present study was to evaluate short-term clinical and radiographic outcomes after RTSA using a custom glenoid baseplate and to report the benefits and short-term complications associated with its use.
Methods: This was a single-institution, retrospective case series of 29 patients for whom a custom glenoid component was created between 2017 and 2022 for extensive glenoid bone loss. Ultimately, 25 patients (10 primary, 15 revision) were studied for between 1 and 51 months, with 9 having a minimum of 2-year follow-up. Patients were evaluated preoperatively and at intervals for up to 5 years. All received preoperative physical examination, plain radiographs, and computed tomography (CT) as well as planning and creation of the custom implant with the manufacturer’s engineers. Patient-reported outcome measures were recorded preoperatively for all patients and repeated 1 and 2 years postoperatively. Intra- and postoperative complications were reported.
Results: Of 25 patients, a custom implant was unable to be matched in 4, who were treated with either eccentric reaming and placement of standard RTSA components or an incompletely seated custom component. For these 4, time from CT scan to implantation averaged 7.6 months (range 6.1–10.7 months), compared with 5.5 months (range 2–8.6 months) for those implanted without difficulty. Intraoperative complications were 5 greater tuberosity fractures, 1 proximal acromial fracture, and 1 medial calcar fracture of the proximal humeral shaft. In 8 patients (32%) the central screw did not provide compression, but none of these had failed at most recent follow-up. There was no failure of the glenoid component in any patient, including the 9 with at least 2-year follow-up, in whom there were significant and minimal clinically important difference changes from preoperatively to postoperatively in visual analogue scale score for pain, all patient-reported outcome measures, and range of motion in abduction and internal rotation up the back.
Conclusion: In the present study, prolonged time of >6 months from CT scan to device implantation resulted in bone loss rendering the implants unusable. Satisfactory short-term radiographic and clinical follow-up at a minimum of 2 years can be achieved with a well-fitting device.
Figures
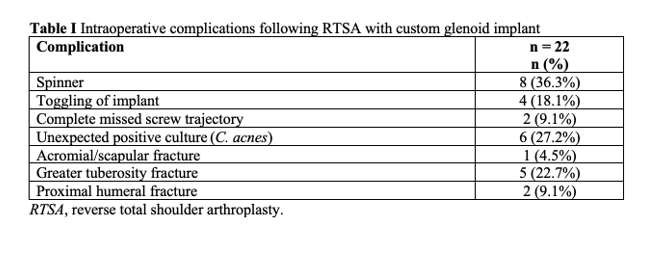
Figure 1#8433
Patient Satisfaction and Patient-Reported Outcomes Do Not Vary by BMI Class in Knee Arthroplasty
Nicolas Piuzzi - Cleveland Clinic - Cleveland, USA
Trevor Murray - Cleveland Clinic - Cleveland, USA
Matthew Deren - Cleveland Clinic - Cleveland, USA
*Roberta Redfern - Zimmer Biomet - Pemberville, USA
Craig L Israelite - Philadelphia, USA
Charles Nelson - University of Pennsylvania - Philadelphia, USA
Dave Van Andel - Zimmer Biomet - Grand Rapids, USA
*Email: roberta.redfern@zimmerbiomet.com
Introduction
Although obese patients have been reported to be at increased risk of peri- and post-operative complications associated with total knee arthroplasty (TKA), and body mass (BMI) cutoffs for patient selection have been proposed, this remains a controversial topic. Therefore, this study aimed to investigate patient-reported outcomes, satisfaction, and pain of as a function of BMI class at 90-day and 1-year postoperatively.
Methods
Subjects within a large prospective observational study were categorized into Centers for Disease Control (CDC) BMI classes according to pre-operative values. 2,365 patients with 3 months potential post-operative follow-up were included. The average age of subjects was 64.7±9.0; 61.6% were female. BMI distribution over the cohort demonstrated 7 (0.3%) underweight, 340 (14.4%) healthy weight, 731 (30.9%) overweight, 666 (28.2%) Class I, 391 (16.5%) Class II, and 230 (9.7%) Class III obesity. Age decreased with increasing BMI class (model p<0.0001), where patients in the Class III group were 58.6±14.9 years compared to 65.0±19.9 in the healthy weight group. Pre- and post-operative KOOS JR, satisfaction, and pain scores were compared by BMI class by ANOVA with pairwise comparisons. 84.1% of patients provided KOOS JR scores at 3 months; 1-year completion rate was 51.7%.
Results
Pre-operative KOOS JR and joint satisfaction scores decreased with increasing obesity class over the cohort, where healthy weight patients’ pre-operative KOOS JR scores and satisfaction scores were 55.6±12.3 and 15.0±8.2, respectively and decreased to 46.7±11.1 and 11.8±7.4, respectively in Class III patients (ANOVA models, p<0.0001). Pre-operative pain increased with BMI class (p<0.0001). However, neither KOOS JR, satisfaction, nor pain varied by BMI class post-operatively. 85.8% of Class III patients reached MCID at 90 days compared to 76.8% of the healthy weight group (p=0.03). Change in scores from baseline suggest larger improvements with increasing BMI class, where Class III patients reported the greatest improvements in KOOS JR (23.2±15.7, p=0.01) and pain scores at 90 days (-3.56±2.65, p<0.0001). Pairwise comparisons demonstrate greater pain reduction for Class II and III patients compared to overweight (-2.46±2.70) and healthy weight patients (-2.53±2.64) at 90 days. Class III patients continued to demonstrate greater improvements in KOOS JR at one-year post-operative; pairwise comparisons demonstrated significantly larger changes in Class III patients (31.1±17.8pts) compared to healthy (26.0±18.8) and overweight groups (29.5±17.1) as well as in Class II patients (30.8±18.0) compared to healthy weight individuals (all, p <0.05). The proportion reaching MCID by one year trended towards significance, with the highest success in Class I and lowest in healthy weight individuals (94.5% vs 86.7%, p=0.054).
Conclusion
Patients of higher BMI class reported greater and earlier improvements in knee function, pain, and knee satisfaction following TKA compared to healthy weight and overweight individuals. Risk/benefit discussions and shared decision-making remain paramount to the decision for surgical intervention. However, given that patients in the class II and III groups may appreciate the most benefit from TKA, rationing of this surgical intervention based on BMI alone may not be warranted.
#8374
Does Discharge Disposition or Length of Stay for Patients Undergoing Staged Bilateral Total Joint Arthroplasty Change Between First and Second Procedures?
Marcel M Dupont - Columbia University Medical Center - New York, USA
Alirio J deMeireles - Columbia University Medical Center - New York, USA
Timothy D Gossett - Columbia University Medical Center - New York, USA
Roshan Shah - Columbia University Medical Center - New York, USA
*John Cooper - Columbia University - New York, USA
*Email: jcooper02@gmail.com
Introduction: Staged bilateral total joint arthroplasty (TJA) is common, yet any differences in hospital length of stay (LOS) and discharge disposition between the first and second surgery has not been well studied. The purpose of this study is to determine whether discharge disposition and LOS differ between the first and second surgeries for staged bilateral TJA. Secondarily, we want to determine whether there are predictors of LOS or patient-reported outcome measures (PROMs) associated with LOS changes.
Methods: Data were retrospectively collected from all patients who underwent staged bilateral total knee (TKA) or total hip arthroplasty (THA), defined as both stages performed within a 12-month period, between March 2015 and August 2022 by four surgeons at a single tertiary academic institution. LOS after the first surgery (LOS_1) and the second surgery (LOS_2) were compared for TKA, THA, and combined TJA cohorts using paired t-tests. Discharge rates to home vs subacute rehab (SAR) and rates of ambulatory (same day discharge) surgery were compared between first and second surgeries using McNemar’s test for paired proportions. Multivariable regression analysis was performed to identify predictors of LOS and whether PROMs are associated with LOS changes.
Results: 433 staged bilateral TKA (N=272) and THA (N=161) were included. LOS_2 was significantly shorter than LOS_1 for the TJA cohort (1.39 vs 1.51 days, p=0.039) with 95% confidence interval (CI) for mean difference of [0.006, 0.221]. However, further analysis showed LOS_2
Conclusions: Our results show shorter LOS and higher rates of ambulatory surgery after second surgery vs. first for patients undergoing staged bilateral TKA but not THA. There were no differences in discharge rates to home vs. SAR between first and second surgery for either procedure. No factors were associated with average LOS, but factors associated with LOS_2 < LOS_1 include undergoing TKA, former smoking, and younger age. These findings may assist providers, patients, and other stakeholders with discharge planning for staged bilateral TJA.
Figures
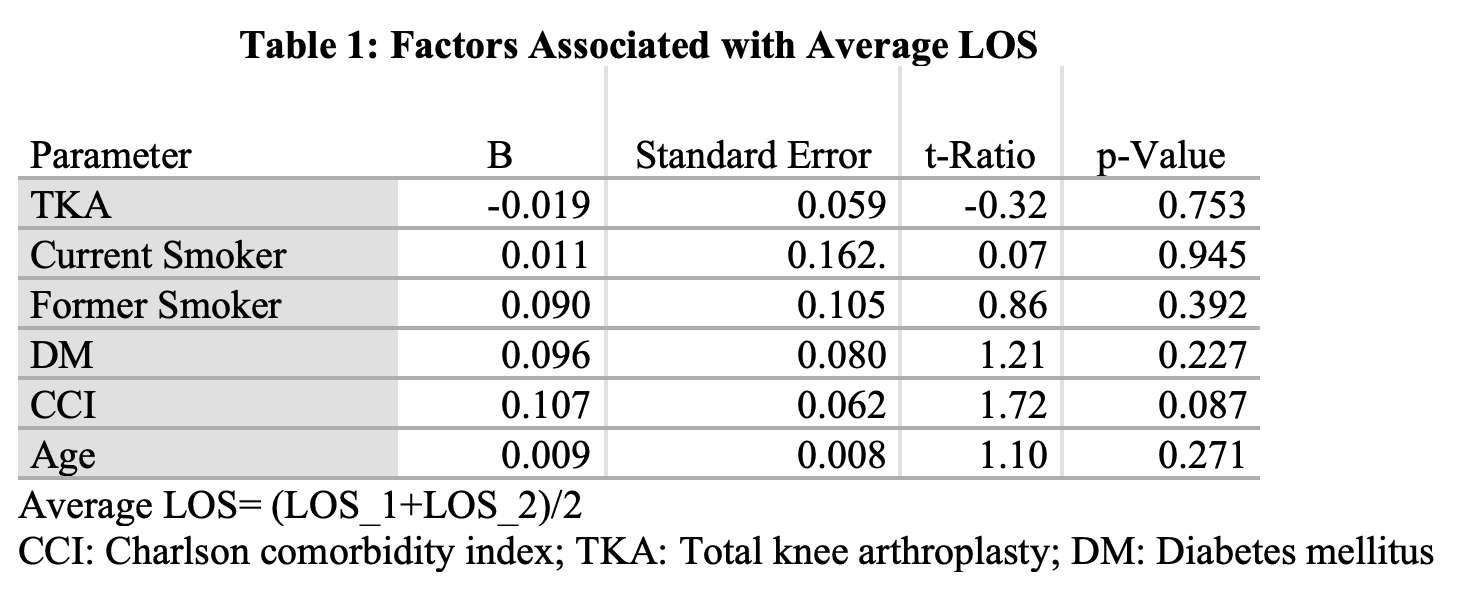
Figure 1
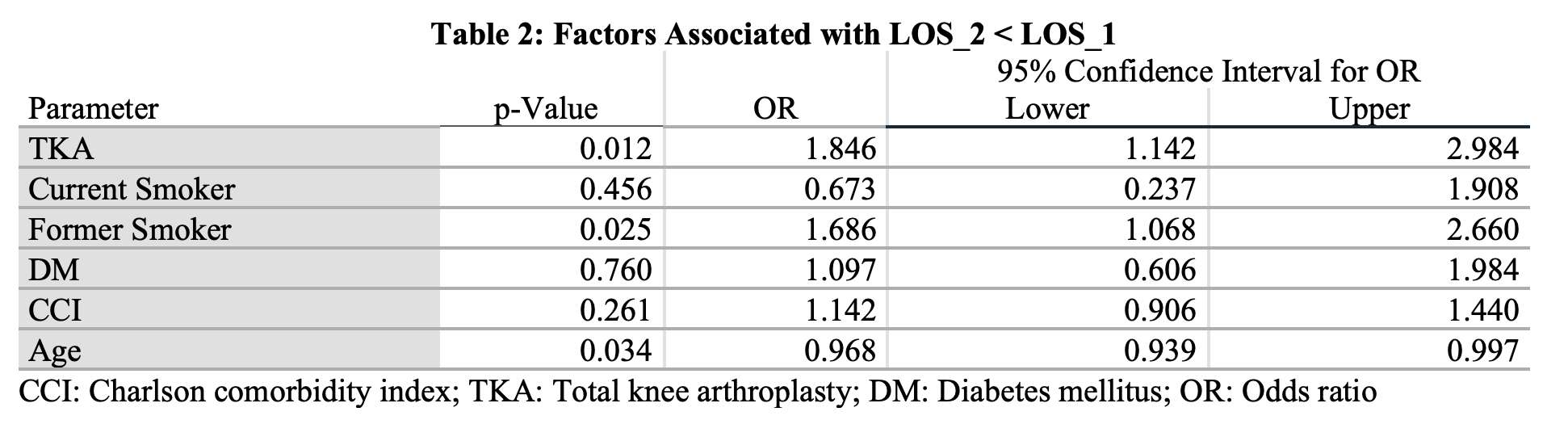
Figure 2
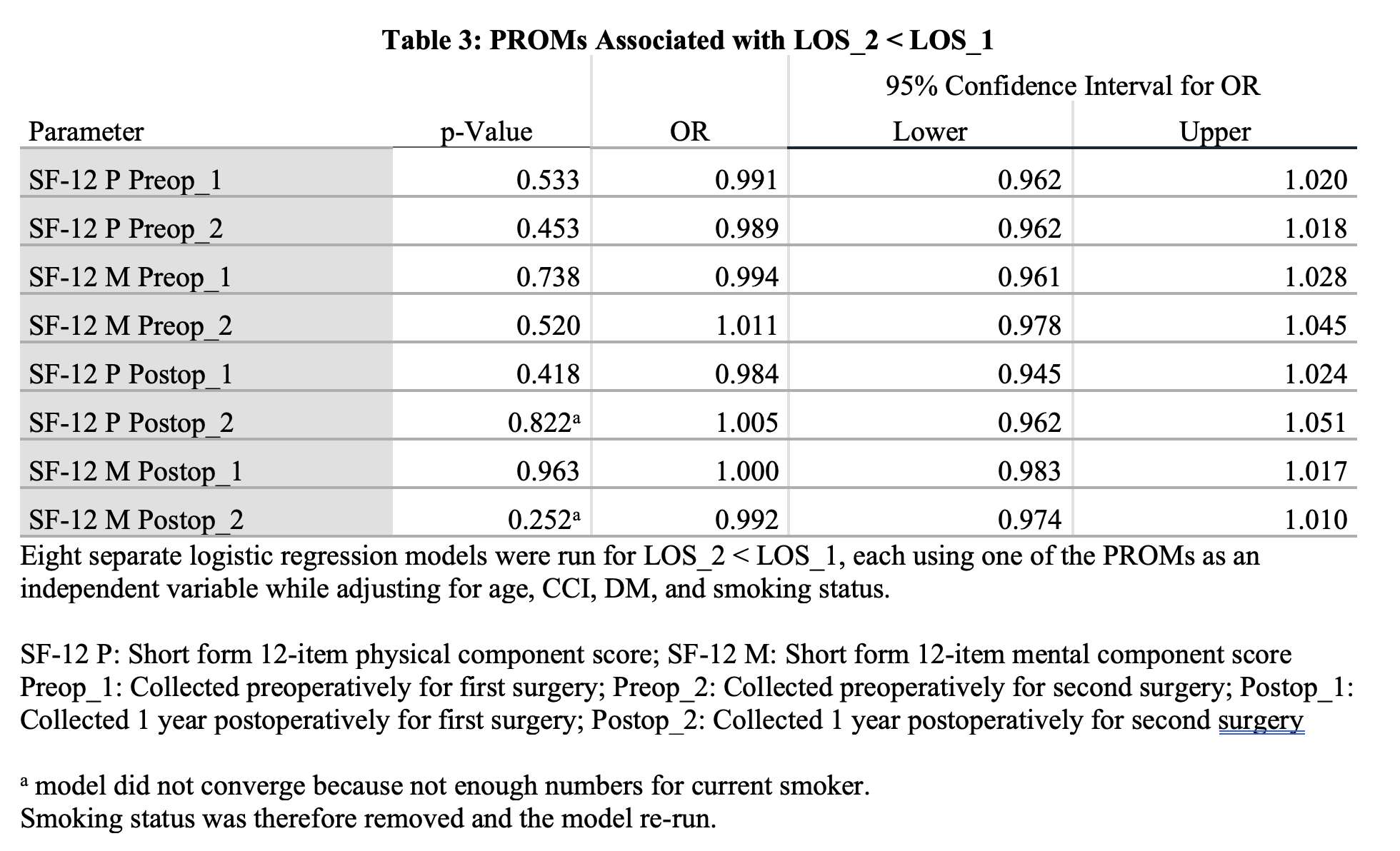
Figure 3#8467
Determining the Patient Acceptable Symptomatic State for the Forgotten Joint Score, the Modified Harris Hip Score, and the Visual Analog Scale at Minimum 5-Year Follow-Up Following Primary Total Hip A
*Benjamin Domb - American Hip Institute - Des Plaines, USA
Saiswarnesh Padmanabhan - American Hip Institute - Des Plaines, USA
david maldonado - UT Houston - Houston, United States of America
Andrew J. Curley - American Hip Institute - Des Plaines, USA
Mark F. Schinsky - American Hip Institute - Des Plaines, USA
Ady Kahana - American Hip Institute Research Foundation - Chicago, United States of America
*Email: DrDomb@americanhipinstitute.org
BACKGROUND: The patient acceptable symptomatic state (PASS) has not been determined for 5 years minimum follow up in Primary Total Hip arthroplasty. The purpose of this study was to determine minimum 5-year follow-up patient acceptable symptomatic state (PASS) thresholds for the Forgotten Joint Score, the Modified Harris Hip Score, and the Visual Analog Scale in Primary Total Hip Arthroplasty.
Methods: Data were prospectively collected and retrospectively analyzed for all primary THA patients between February 2014 and July 2017. The inclusion of patients was based on if they had answered the 5-year anchor question. Patients were excluded if they were worker’s compensation or if they had a concomitant Gluteus Medius repair. The anchor question being a binary “successful” treatment based on satisfaction, pain levels, and functional capacity. A receiver operator characteristic (ROC) analysis was performed to determine a threshold for the PASS. The ceiling effect of these scores were assessed to see which one is least affected. A multivariate logistic regression was used to identify predictors for achieving the PASS.
Results: The ROC analysis of the 5-year outcomes of 300 patients produced excellent areas under the curve (AUC) for the PASS. The AUC values for the PASS for the FJS, mHHS, and VAS are as follows 0.865, 0.868, and 0.888. The threshold values for the PASS for the FJS was 54.2 (sensitivity, 0.905; specificity, 0.741), for the mHHS was 68 (sensitivity, 0.963; specificity, 0.704), and for the VAS was 3.1 (sensitivity, 0.949; specificity, 0.741). The ceiling effect for the FJS was at 39.6%, for the mHHS was at 47.7%, and for the VAS it was at 60.6%.
Conclusion: After Primary Hip Arthroplasty, the minimum 5-year timepoint PASS thresholds for the FJS, the mHHS, and the VAS were 54.2, 68, and 3.1. From the ceiling effect analysis, it can be seen that there is a significant ceiling effect, but the Forgotten Joint Score is still the least affected.
#8593
I Cant Get No Satisfaction: One-Month Post-Operative Pain Predicts Three-Month Post-Operative Dissatisfaction in Total Knee Arthroplasty
*Mike Anderson - Zimmer Biomet - Lehi, USA
Jason Cholewa - Zimmer Biomet - Warsaw, USA
Roberta Redfern - Zimmer Biomet - Pemberville, USA
Dave Van Andel - Zimmer Biomet - Grand Rapids, USA
Karl Surmacz - Zimmer Biomet - Swindon, United Kingdom
*Email: Mike.Anderson@zimmerbiomet.com
Introduction
Dissatisfaction following total knee arthroplasty (TKA) continues to be reported as approximately 20% with conflicting data in the literature. Numerous studies report strong associations between pain and satisfaction; however, most studies have assessed these variables later (six to 12 months) in the post-operative period. The purpose of this study was to evaluate the relationship between one-month pain scores and three-month post-operative satisfaction and investigate if an immediate post-operative pain score cutoff exists between satisfied and dissatisfied patients.
Methods
This was a secondary analysis from data collected in a multicenter longitudinal cohort study comprised of TKA patients using a digital care management platform. Patients (n=1520) underwent primary TKA between February 2019 and February 2023. Pain was assessed at one-month post-operative via an 11-point numeric rating scale (NRS). Satisfaction was assessed at three months post-operative via the Knee Society composite satisfaction score (KSS). Patients were stratified into satisfied (KSS >30) and dissatisfied (KSS < 30) subgroups. Quantile regression was used to create a best fit line to identify a cutoff between the one-month NRS and the three-month KSS. Further, a logistic regression model was used to classify patients into good/poor satisfaction that included a co-morbidity index, one-month active flexion range of motion, anxiety/depression score, gender, age and body mass index (BMI). The importance of the features in the model was assessed using permutation importance method to create a best-fit line between satisfaction and NRS. The model was compared to simpler model using only the NRS as input.
Results
The majority of patients were female (58.4%). The mean age of the population was 64.4±8.8 years, and the mean BMI was 31.4±6.2 kg/m2. The median modified Charlson Comorbidity Index score was 1.0 (interquartile range, 0 – 2). The one month mean NRS and satisfaction scores were 3.7±2.0 and 29.4±8.6, respectively. A total of 624 (41.1%) patients were dissatisfied at three months post-operative. There was a moderate correlation between one-month NRS pain and three-month satisfaction (r =-0.39). Based on the best-fit line, the cut-off for dissatisfaction occurs with an NRS for pain of > 4.0 (Figure 1). The model achieves an AUC of 0.75 (std=0.03), with a maximum f1-score 0.66, corresponding to sensitivity = 0.80, specificity = 0.56. The model can be tuned to reduce false positives and increase precision.
Conclusion
A high proportion of patients were dissatisfied with their TKA procedure during the early post-operative period. As expected, there was a correlation between pain and satisfaction. More importantly, one-month post-operative pain scores greater than 4.0 were associated with patient dissatisfaction at three months post-operative. Assessing pain in the immediate post-operative period may provide clinicians with diagnostic data that may help detect patients at risk for a poor prognosis three months following TKA. Further research is needed to determine if these immediate post-operative scores are associated with early and long-term follow-up.
Figures
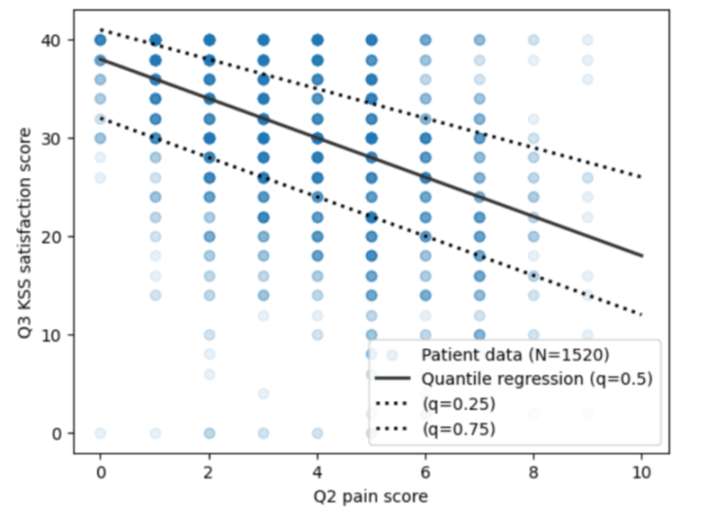
Figure 1#8364
Can We Link PROMIS to KOOS, JR and HOOS, JR Scores? Results From a Preliminary Analysis.
*Mary Hennekes - Henry Ford Health - Detroit, USA
Matthew Gasparro - Henry Ford Health - Detroit, USA
Christopher Rittle - Henry Ford Health - Detroit, USA
Joshua Castle - Henry Ford Health - Detroit, USA
Eric Makhni - Henry Ford - Detroit, USA
*Email: mhennek1@hfhs.org
Introduction:
Patient-reported outcome measures (PROMs) are some of the most useful measures to assess both the physical and behavioral impacts conditions can have on a patient’s life. Requiring completion of multiple PROM questionnaires at the time of a patient visit can increase survey burden and decrease patient satisfaction. Establishing relationships between PROMs can minimize survey burden and increase the amount of quality data collected.
The Patient-Reported Outcome Measurement Information Systems (PROMIS) physical function (PF) and pain interference (PI) questionnaires and the Knee Injury and Osteoarthritis Outcome Score (KOOS, JR) or the Hip Disability and Osteoarthritis Outcome Score (HOOS, JR) forms are several PROMs that can be useful in evaluating patient-perceived disability of knee and hip osteoarthritis. This study sought to determine whether any agreement exists between PROMIS-PF and PROMIS-PI and either KOOS, JR or HOOS, JR.
Methods:
After receiving institutional review board approval, a retrospective chart review was completed of all patients who presented to a total joint arthroplasty clinic or for surgery in 2022. At this institution, it is now routine practice to collect PROMIS-PF, PROMIS-PI and either KOOS, JR or HOOS, JR at patients’ preoperative visits, on the day of the OR, and postoperatively. Patients were excluded if they did not have one of the above completed scores on the day in question or if they presented for care other than for knee or hip osteoarthritis. A regression analysis was performed to calculate R2 values relating PROMIS-PF to both KOOS, JR and HOOS, JR and PROMIS-PI to both KOOS, JR and HOOS, JR. P-values were also calculated for each comparison.
Results:
At preliminary data collection, 256 patients presented for knee osteoarthritis or follow-up after total knee arthroplasty with 256 matched comparisons between PROMIS-PF and KOOS, JR and PROMIS-PI and KOOS, JR. 158 patients presented for hip osteoarthritis or follow-up after total hip arthroplasty with 158 matched comparisons between PROMIS-PF and HOOS, JR and 157 matched comparisons between PROMIS-PI and HOOS, JR. PROMIS-PF (p<0.001, 95% CI ) and PROMIS-PI (p<0.001) were both significantly associated with KOOS, JR scores. However, on regression analysis (Figure 1A, 1B), relationships were less agreeable with R2 values of 47% (adjusted R2 46.79%) and 50.91% (adjusted R2 50.72%), respectively. Only PROMIS-PI (p<0.001) was significantly associated with HOOS, JR scores. This relationship exhibited a moderate fit (R2 52.46%, adjusted R2 52.15%, Figure 2A) on regression analysis. PROMIS-PF and HOOS, JR were neither significantly associated (p=0.113) nor a good fit on regression analysis (R2 43.64%, adjusted R2 43.28%, Figure 2B).
Conclusions:
Preliminary data suggests that, while there is a significant association between PROMIS-PF and PROMIS-PI and KOOS, JR or HOOS, JR scores, the concordance between these questionnaires is moderate at best. While minimizing survey burden is one of the tenets of successful PROM completion, in patients presenting to total joint arthroplasty clinics, it may be necessary to incorporate both KOOS, JR/HOOS, JR and PROMIS-PF and PI forms for a more holistic understanding of the impact knee or hip osteoarthritis has on a patient’s daily life.
Figures
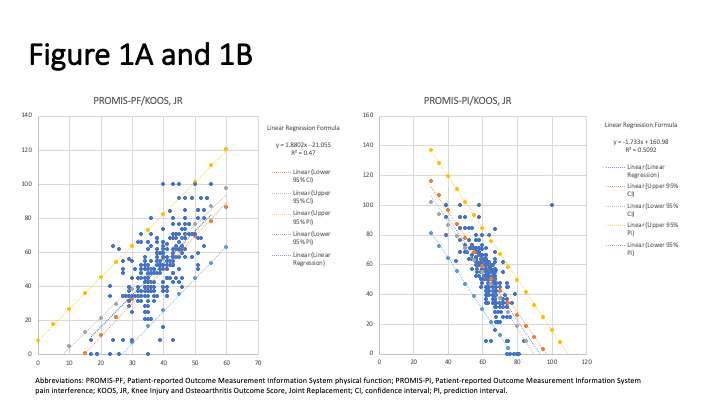
Figure 1
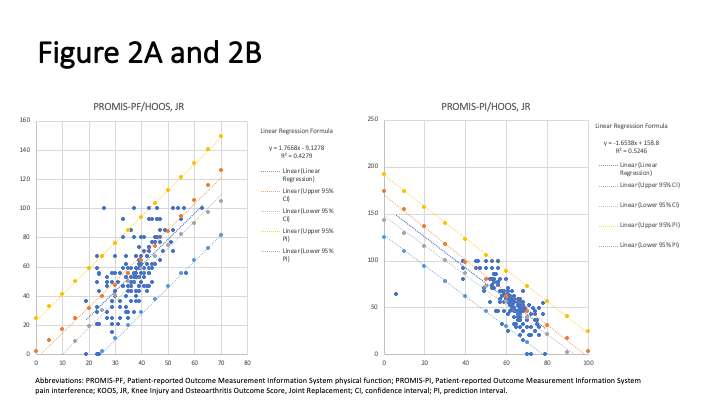
Figure 2#8378
Preoperative Synovitis and Satisfaction After Total Knee Arthroplasty for Osteoarthritis
*Jennifer Polus - University of Western Ontario - London, Canada
Holly Philpott - Western University - London, Canada
Trevor Birmingham - Western University - London, Canada
Edward Vasarhelyi - Western University - London, Canada
Steven MacDonald - London Health Sciences Centre - London, Canada
Brent Lanting - London Health Sciences Centre - London, Canada
Tom Appleton - Western University - London, Canada
Matthew Teeter - Western University - London, Canada
*Email: jpolus@uwo.ca
Introduction: Total knee arthroplasty (TKA) is a routinely successful procedure, yet one in five patients report that they are not satisfied with the outcome of their surgery. The synovium is frequently inflamed by osteoarthritis, and clinical symptoms of pain and stiffness are strongly correlated to the degree of synovitis. Since the synovium is not removed in TKA, it is possible that ongoing synovial inflammation may be an unrecognized source of knee pain and stiffness after surgery, resulting in dissatisfaction. The purpose of this study is to investigate the association between synovitis and satisfaction after TKA.
Methods: At one-year post-TKA, patients (n=95) were given a questionnaire that addressed their level of satisfaction with the outcome of their procedure on a five-point Likert scale. Patients who answered that they were not satisfied, minimally satisfied, or moderately satisfied were classified as dissatisfied and patients who answered that they were highly satisfied or very highly satisfied were classified as satisfied. Preoperatively and at one-year postoperatively, all patients underwent ultrasound (US) imaging and synovitis severity were scored according to the OMERACT knee US protocol. Patients also completed the Knee Injury and Osteoarthritis Outcome Score (KOOS) questionnaire before and one-year after surgery. We compared synovitis and patient outcomes between satisfied and dissatisfied patients preoperatively and postoperatively.
Results: In this cohort, 66 (69%) patients were satisfied, and 29 (31%) patients were dissatisfied. Preoperatively, dissatisfied patients had higher peak synovitis grade (mean difference = 0.434, 95% CI [0.085, 0.783], p=0.015), summed general synovitis score (mean difference = 0.759, 95% CI [0.152, 1.366], p=0.015), and maximal effusion depth (mean difference=1.548mm, 95% CI [0.141, 2.956], p=0.031), but there were no differences at one-year postoperation. Preoperatively, there were no differences in any of the KOOS subsections, however at one-year, dissatisfied patients had significantly poorer outcomes in all sections (p<0.001) including pain (mean difference=16.12, 95% CI [7.716, 24.53]) and quality of life (mean difference=27.47, 95% CI [18.03, 36.92]).
Conclusion: Despite similar baseline measures of patient-reported pain, dissatisfied patients had greater US measures of synovitis going into surgery and poorer outcomes after surgery. Synovitis is a known indicator of disease progression in osteoarthritis and the level of synovitis preoperatively may be a key predictor of postoperative satisfaction. The use of point of care US will allow surgeons to be aware of the degree of synovitis at the time of surgery and may help in perioperative decision making.
#8316
The Impact of Patient Grit, Resilience and Ability to Cope With Stress on Outcomes Following Total Joint Arthroplasty
*Jacob Laperche - Frank H. Netter School of Medicine Quinnipiac University - Middletown, United States of America
Drew Clippert - Brown University/ University Orthopedics - Providence, United States of America
Valentin Antoci - University Orthopedics - Providence, USA
Caitlin Barrett - University Orthopedics - Providence, USA
*Email: jacoblaperche@gmail.com
Introduction: As mental health becomes a growing topic within the field of medical research, there has been increased study into how mental and emotional factors affect a patient’s outcome following surgery. However, many studies only focus on pain management skills, depression, and anxiety levels, while coping ability is often ignored. This study’s purpose is to determine if a patient’s coping skills influence length of stay, readmissions, pain, and functioning after total joint replacement.
Methods:127 patients in our orthopedic practice received an IRB-approved questionnaire, which included surveys that measured grit, resilience, and responses to stress at their preoperative clinic visit. Responses to the WHO Well-being Index and SF-8 Health Survey were also collected. At one year after surgery, hospital data and FORCE-TJR database information were collected to determine if coping skills affected outcomes after surgery. Linear regression and student’s t-tests were used to quantify this relationship, with a value of p<0.05 as the cutoff for significance.
Results: All 127 patients who received this questionnaire were matched in hospital records; however, only 50 had completed 12-month FORCE-TJR profiles. In statistical analysis, grit survey responses and total questionnaire scores were not significantly associated with increased length of stay, readmissions, pain, functioning, and quality of life after total joint replacement surgery. However, the effect of grit scores on pain at 12 months approached significance (p=0.0966).
Conclusion: While no significant correlation was seen between coping skills and functional outcomes, pain, and hospital data, classical thinking would suggest that patients undergoing total joint arthroplasty need a certain degree of grit to get through the required recovery and physical therapy. Since previous data has found correlation between the mental component of SF-36 and functional outcomes, further study is required on this topic.
#8333
Is Survivorship a Concern With Cementless Total Knee Arthroplasty?
Nicolas Piuzzi - Cleveland Clinic - Cleveland, USA
Jianhua Shen - Stryker - Mahwah, USA
Ignacio Pasqualini - Cleveland Clinic Foundation - Cleveland, USA
*Emily Hampp - Stryker - Mahwah, USA
Michael A Masini - Ypsilanti, USA
John W Noble - Center for Orthopedics - Lake Charles, USA
Manoshi Bhowmik-Stoker - Stryker - Mahwah, USA
*Email: emily.hampp@stryker.com
Introduction: The use of cementless constructs in total knee arthroplasty (TKA) has increased across the United States, with the American Joint Replacement Registry (AJRR) reporting 9.7% in 2019 and 18.8% in 2022 [AJRR 2022]. While there is growing evidence that biological fixation is a potentially better long-term option for morbidly obese and young (≤ 65 years) patients [Sinicrope 2019, Wang 2020], some cementless knee constructs have been recalled from the U.S. market over the last 10 years. The purpose of this study was to evaluate survivorship of a modern cementless TKA in comparison to a gold standard cemented knee design.
Methods: A prospective, multicenter, consecutive case-control series study was performed where 453 patients received a primary total knee. Cohort 1, 373 knees in 319 patients, received a fully cementless construct, while cohort 2, 147 knees in 134 patients, received a fully cemented construct of the same implant design. Patients were followed annually for five years for cemented and seven years for cementless to record survivorship and adverse events. Patient demographics were compared between groups using a Wilcoxon test for numeric variables and the chi-square test for categorical variables. A log-rank test was used to compare the Kaplan-Meier (KM) survival function between the two cohorts. All statistical tests were two-tailed, and p-values less than 0.05 were considered statistically significant.
Results: There were no significant differences in mean age (62.12 ± 4.03 vs 63.18 ± 4.14), BMI (30.99 ± 4.38 vs 30.99 ± 4.13) or gender (49% female vs 57% female) between cohort 1 and cohort 2, respectively. All patients had a primary diagnosis of end-stage osteoarthritis. The KM all-cause survivorship at five years was 98.2% (95% CI: 95.7% - 99.3%) for cementless and 97.5% (95% CI: 92.6% - 99.2%) for cemented (p>0.05), as shown in Figure 1. There were no statistical differences in the occurrences of adverse events (Figure 2).
Conclusion: In this study, cemented and cementless implants had similar rates of adverse events and survivorship out to seven-year follow-up. Results are promising for modern cementless knees which have enhanced geometry and fixation over predicate devices in the U.S. market.
Figures
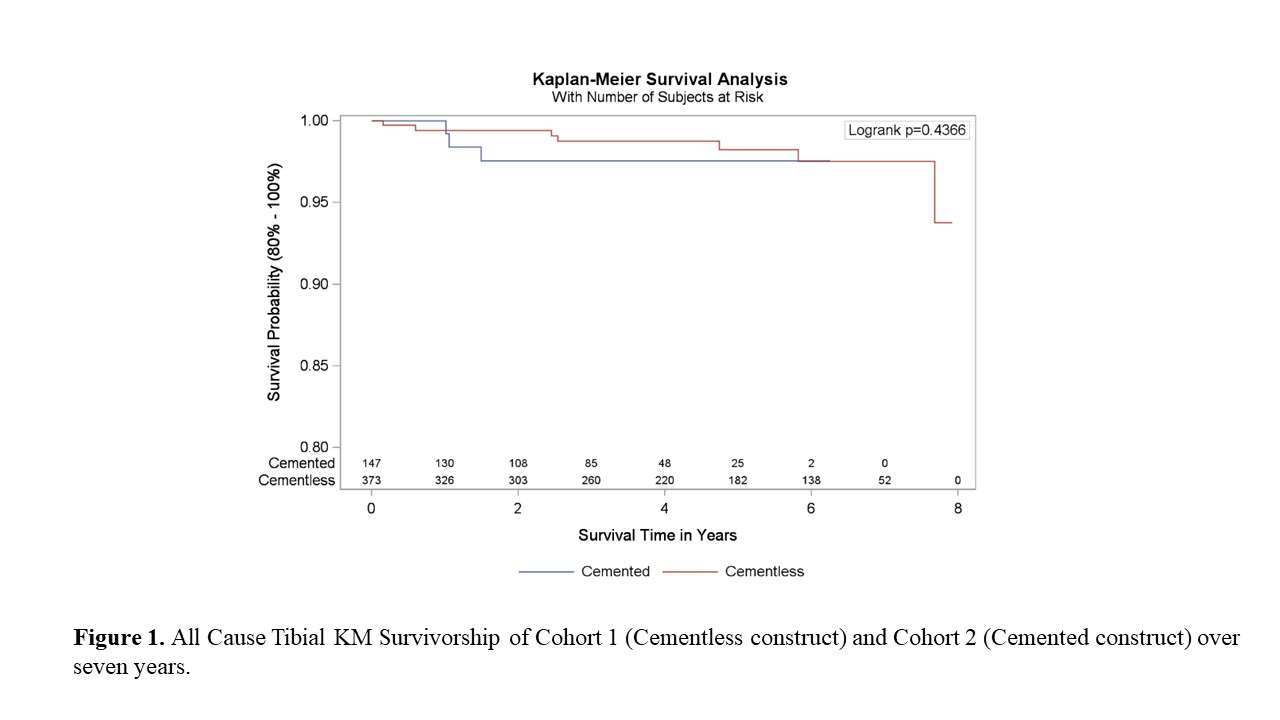
Figure 1
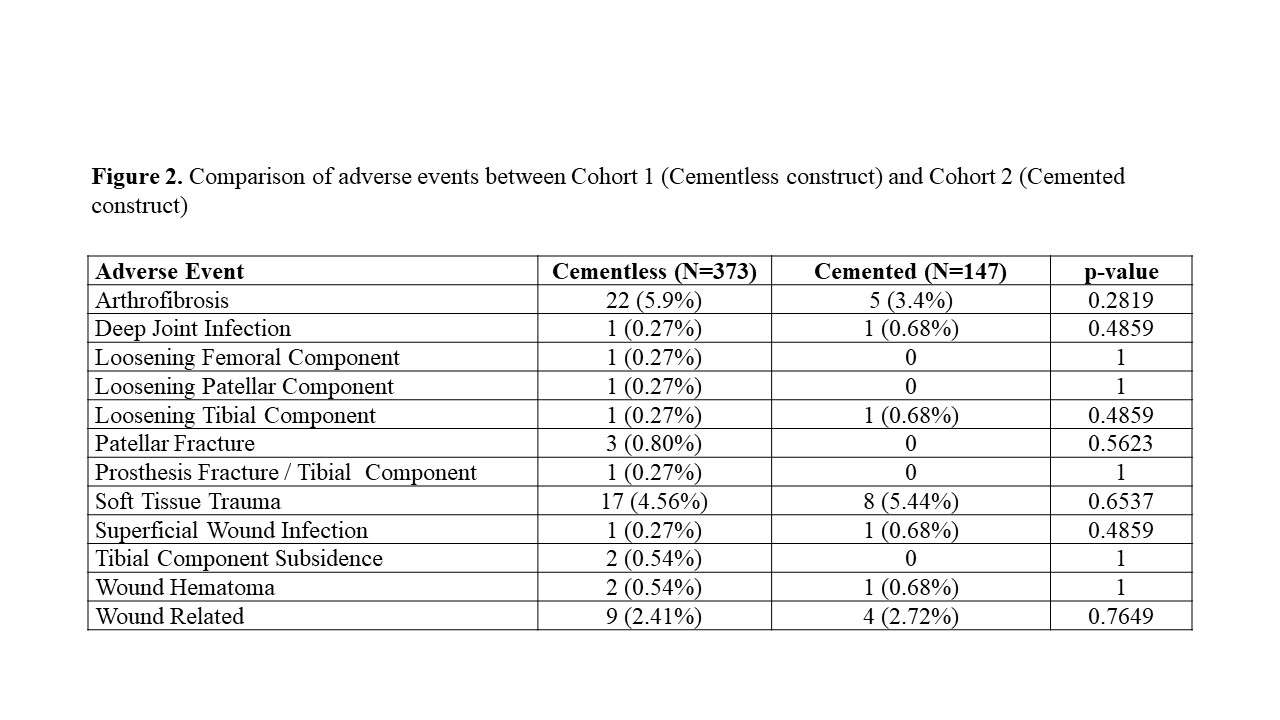
Figure 2#8674
Early Identification of Poorly Performing Implants in Michigan With the Example of the Vanguard XP
*Nicholas Frisch - Ascension - Bloomfield Hills, United States of America
David Markel - Providence Hospital and Medical Center - Southfield, USA
Richard Hughes - University of Michigan - Ann Arbor, USA
Brian Hallstrom - University of Michigan - Ann Arbor, USA
*Email: nick@frischortho.com
Introduction: Arthroplasty registries play a critical role in improving the quality of care for patients and performing post-market surveillance of medical devices. Without a global infrastructure for identifying outlier implants, it falls to each individual registry to report on implant performance in its annual report and/or publish in peer-reviewed literature. When an implant appears to have worse performance than other similar implants, the registry can potentially protect patients from further use of that implant by reporting their results. This paper reports the Michigan Arthroplasty Registry Collaborative Quality Initiative (MARCQI) findings specific to the Biomet Vanguard XP bicruciate-retaining total knee implant and explores opportunities to improve the timeliness and reliability of reporting.
Methods: Data collected by MARCQI’s 2019 report covered MARCQI activities from 2/15/2012 through 12/31/2018 and included the use of the Biomet Vanguard XP implant. Demographic data were analyzed using Chi-squared and independent two-group t-tests to determine if there were differences in cases between Vanguard XP and all other implants. The cumulative percent revision (CPR) was computed from the survival function, S(t), using CPR(t)=100*(1-S(t)). The S(t) was estimated using the Kaplan-Meier method. A log-rank test was used to assess differences in the CPR curve for the Vanguard XP and all other implants. A Cox proportional hazards model was also used to assess the impact of age and sex on the hazard function for revision. The comparative group used was all other TKA implants. Cumulative sum (CUSUM) charts adapted for arthroplasty were constructed for each individual surgeon in MARCQI.
Results: There were 148,832 knee arthroplasty cases in the MARCQI registry. When cases containing unknown/missing data and deaths were excluded, there were 507 that used a Vanguard XP implant combination and 134,605 cases that used other implants (Figure 1). The unadjusted cumulative precent revision (CPR) curve up to five years post-operatively (Figure 2) for the Vanguard XP differed from the CPR curve for all other implants in MARCQI (P<0.0001). The hazard ratios for the three factors included in the Cox proportional hazards model were all significantly different from unity: implant (2.76, 1.98 – 3.86, 95% CI), sex (0.80, 0.74 – 0.85, 95% CI), and age (0.96, 0.96 – 0.97, 95% CI). The top three reasons for revision were pain, arthrofibrosis, and aseptic loosening (Table 1). All of the surgeons who used the Vanguard XP experienced higher failure rates than before they used the implant.
Conclusion: Arthroplasty implant registries have a a critical role in identifying and reporting implant outcomes. The increased coverage of registry reporting is important going forward as practices and implant usage vary. The timing of reporting important and the development of thresholds and benchmarks for reporting in collaboration with industry could potentially save patients from the morbidity caused by implants that do not perform as well as anticipated. The Vanguard XP experienced higher early failure rates than other TKA implants within the MARCQI registry.
#8292
Patellar Resurfacing and Survivorship After Primary Total Knee Arthroplasty From the Michigan Arthroplasty Registry Collaborative Quality Initiative
*Kent Kern - Corewell Health/Michigan State University - Grand Rapids, United States of America
Brian Hallstrom - University of Michigan - Ann Arbor, USA
David Markel - The CORE Institute - Novi, USA
Thomas Zheng - University of Michigan - Ann Arbor, USA
Richard Hughes - University of Michigan - Ann Arbor, USA
Karl Roberts - Corewell Health/Michigan State University - Grand Rapids, USA
Tyler Madden - Corewell Health - Grand Rapids, USA
*Email: kkern4343@gmail.com
Introduction: Patellar resurfacing remains a controversial topic globally. Practices vary widely with resurfacing rates of 3.4% (Sweden), 11.8% (Germany), 38.4% (UK), 75.4% (Australia) and 89.7% (U.S.). This study analyzes survivorship of total knee arthroplasty (TKA) based on patellar resurfacing from the Michigan Arthroplasty Registry Collaborative Quality Initiative (MARCQI). MARCQI captures data on more than 97% of all elective total hip and knee arthroplasty cases performed in the state of Michigan.
Methods: This was a retrospective cohort study of cases enrolled in MARCQI from 2012 to 2019. Data was analyzed to determine the incidence, distribution and survivorship of TKA performed with and without patellar resurfacing. Descriptive statistics and cumulative percent revision (CPR) out to 5 years were calculated using Kaplan Meier estimates. Exclusion criteria included total hip arthroplasty cases, unicondylar knee arthroplasty cases, patellofemoral joint arthroplasty cases and all revision knee cases.
Results: This analysis included 162,292 primary TKAs. In MARCQI, 92.6% (150,347) of primary TKAs resurfaced the patella and 7.4% (11,945) were left unresurfaced. For cruciate retaining TKA, 88.9% were resurfaced and 11.1% were not. For posterior stabilized TKA, 95.9% were resurfaced and 4.1% were not. The overall CPR at 5 years for resurfaced TKAs was 3.09% (95% CI: 2.97, 3.20) and those without resurfacing was 4.40% (95% CI: 3.93, 4.94) with a hazard ratio of 1.41 (1.07, 1.87) after adjusting for sex, age and fixation (cemented/uncemented). Knees with an unresurfaced patella were associated with a statistically higher CPR at 2, 3, 4 and 5 years postoperatively with no overlap in confidence intervals.
Conclusion: Resurfacing the patella varies widely around the world and is still debated. Our statewide registry data supports that registries and studies should report survivorship separately based on the status of the patella as not resurfacing the patella has a significant effect on likelihood of revision and should be considered a confounding variable. This data also supports that surgeons should more strongly consider resurfacing the patella at the time of surgery to avoid increased rates of revision and potentially increased morbidity and cost to the patient and healthcare system.
Figures
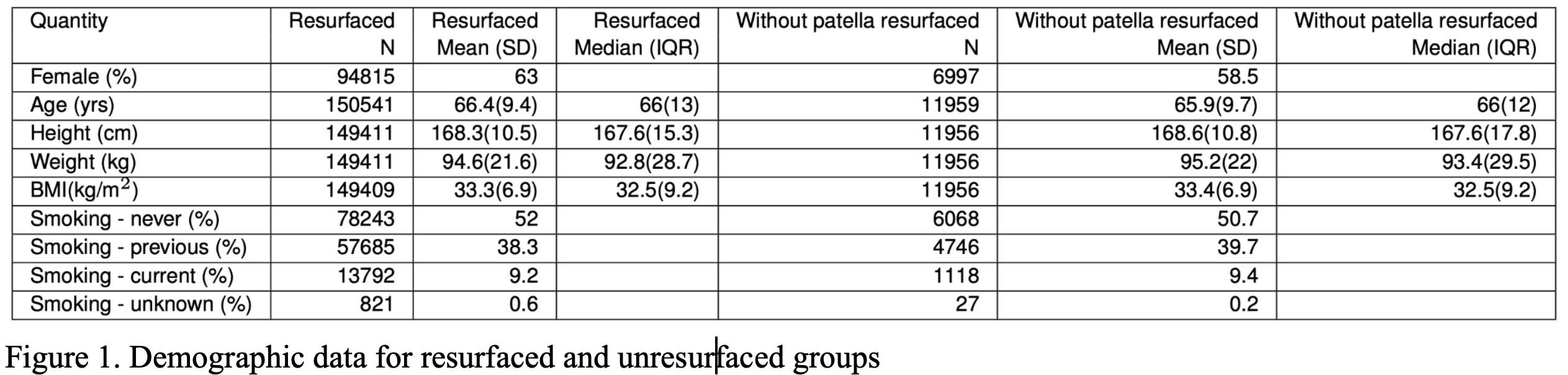
Figure 1
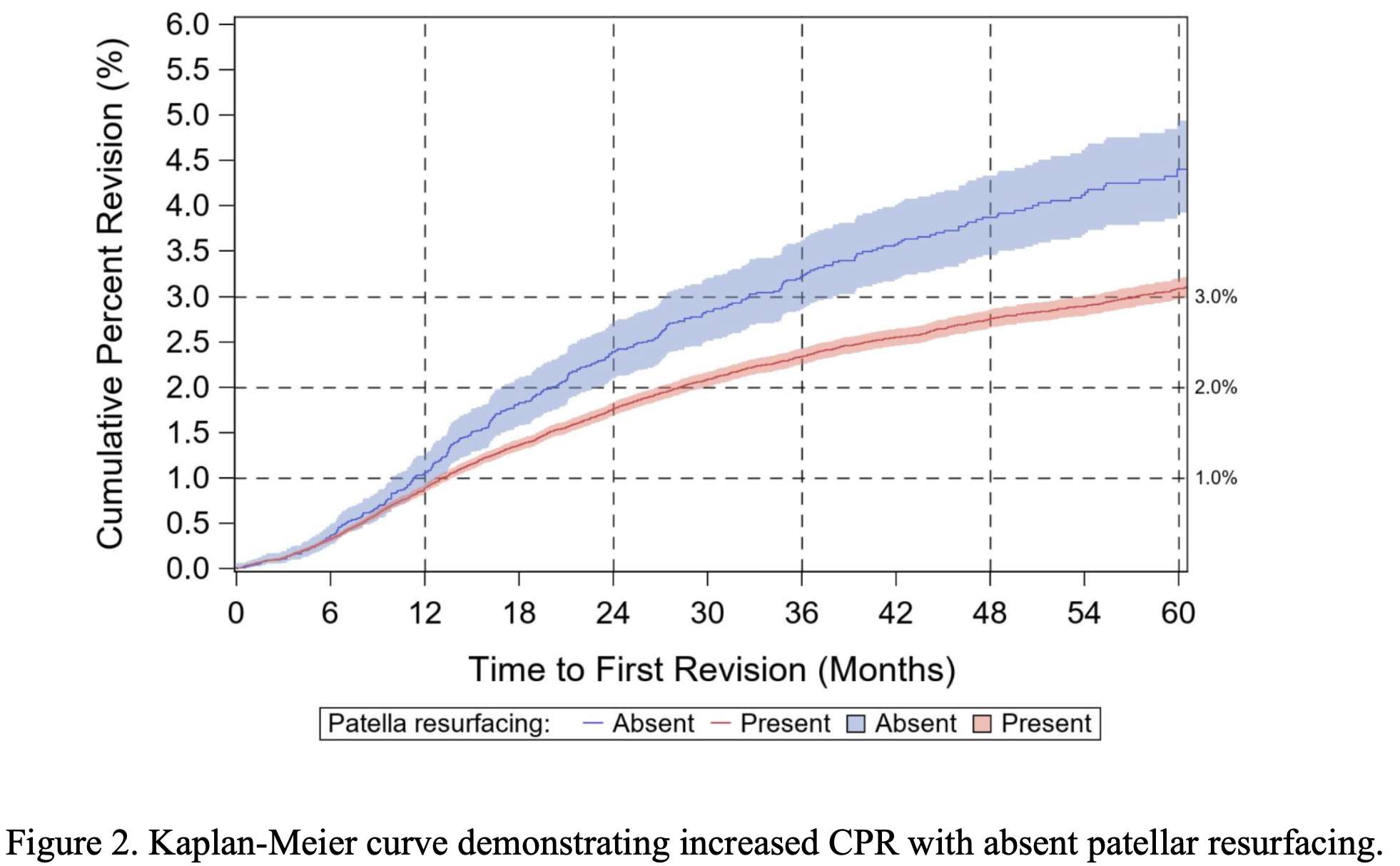
Figure 2
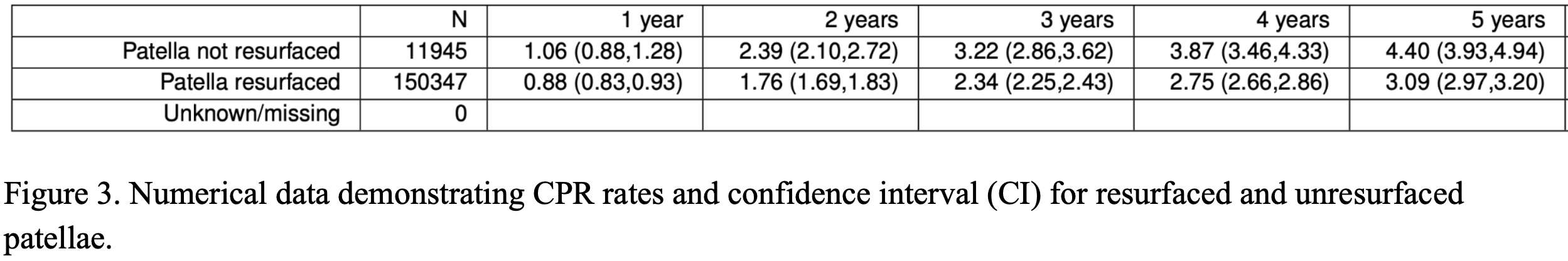
Figure 3#8267
Survivorship and Clinical Outcomes of a Single Design Revision Tibial Component in Primary and Revision Total Knee Arthroplasty: A Multi-Center Registry Review
*nader nassif - Hoag Orthopedic Institute - Newport Beach, United States of America
Kirk Kindsfater - Orthopaedic Center of the Rockies - Fort Collins, USA
David F Dalury - Baltimore, USA
Andrew Spitzer - Cedars-Sinai Medical Center - Los Angeles, United States of America
Donald Pomeroy - Arthroplasty foundation - Louisville, United States of America
John F Irving - New Haven, USA
William P Barrett - Renton, USA
*David Fawley - DePuy Synthes - Warsaw, USA
Sean Croker - Depuy Synthes - West Chester, USA
Robert Gorab - Hoag Orthopedic Institute - Irvine, United States of America
*Email: dfawley1@its.jnj.com
Introduction
Fixation of the tibial component can be challenging when undertaking complex primary and revision total knee arthroplasty procedures. A modified revision fixed bearing tibial component was released in 2017, with a rotating platform option available in 2018. This evaluation retrospectively reviewed the survivorship and clinical outcomes for this tibial component, used in both complex primary and revision total knee arthroplasty (pTKA and rTKA respectively). Data were collected as part of an ongoing, multi-center, company sponsored standard of care registry.
Methods
Clinical assessments were summarized. It is recognized that sites within the registry have different standard of care follow-up visits, therefore, standardized registry visit windows were established, which were back-to-back to include all follow-up data. Kaplan-Meier (KM) survivorship was performed with revision of any component as the endpoints. Two survivorship analyses were performed with differing censoring assumptions. unrevised subjects were censored at the last clinical follow-up [clinical assumption (CA)], and at the date of database extract [registry assumption (RA)]. In all cases survival estimates and graphs were truncated at 40 knees remaining at risk.
Results
A total of 905 augmented revision tibial components were utilized in this study group, 552 were implanted in pTKA and 353 in rTKA. Patient population included 339 (62.1%) females for pTKA and 180 (51.3%) for rTKA. Mean age (SD) was 64.1 (7.79) years for pTKA and 68.0 (8.50) years for rTKA. Mean BMI (SD) was 30.5 (5.83) for pTKA and 30.5 (5.91) for rTKA. Osteoarthritis was the primary diagnosis for 489 (88.6%) subjects with pTKA . For rTKA , diagnoses included aseptic loosening for 151 (42.9%), infection for 54 (15.3%), instability for 51 (14.5%), and pain/stiffness for 31 (8.8%) of subjects. Tibial sleeves were utilized in 1.45% (8/552) of pTKA procedures and 79.87% (282/353) of rTKA procedures. Tibial stems were utilized in 40.58% (224/552) of pTKA and 91.50% (323/353) of rTKA procedures. Three revisions were reported for pTKA: 2 for infection (0.37%), 1 for instability (0.18%). Five re-revisions were reported for rTKA : 3 for infection (0.85%), 2 for aseptic loosening (0.57%). For 2 of the re-revisions, the tibial component was not revised. In one case the insert was exchanged after infection, and in another, the femoral component loosened but the tibial component remained well-fixed. All-cause survivorship was 99.37% at 5 years for pTKA and 98.43% at 4 years for rTKA using RA. KM estimates are presented in Figure 1 and Figure 2. Mean American Knee Society Scores (pre-2011) are presented in Figure 3.
Conclusion
Short to mid-term survivorship identified satisfactory outcomes using an enhanced revision tibial component for additional fixation needs in more complex surgical scenarios. Analyses of outcomes in multi-site registry databases are challenging, due to site variability of protocols and diminished rates of clinical follow up. In an observational registry data setting, RA tends to overestimate survivorship , whereas CA has the potential to underestimate survivorship. This report included both analyses to improve data transparency and provide a more meaningful understanding of the incidence of revision.
Figures
Figure 1
Figure 2
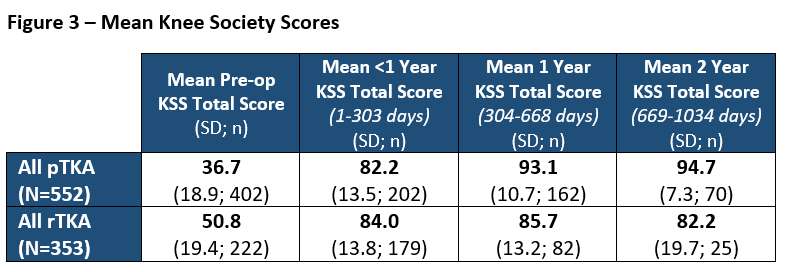
Figure 3#8770
Highly Porous Metaphyseal Cones in Revision Total Knee Arthroplasty
Filesha Haniff - Mahwah, United States of America
*Kevin Denehy - Bluegrass Orthopaedics - Lexington, USA
Emily Hampp - Stryker - Mahwah, USA
*Email: denehy@gmail.com
Introduction: Highly porous metaphyseal cones have been introduced to restore metaphyseal integrity for improved cement interdigitation to achieve durable fixation in revision total knee arthroplasty (TKA). The purpose of this study is to review the survivorship, surgical efficiency, clinical results, and complications of revision TKA using highly porous 3-dimensionally printed titanium metaphyseal cones.
Methods: This is a review of 197 revision TKAs among 193 patients using metaphyseal tibial (n=194) and femoral (n=69) cones. There were 66 cases utilizing both tibial and femoral cones. The mean age of the patients was 67 years (range 46 - 87), who had a mean follow-up of 21.1 months (range 0 – 67.7 months). There were 122 women and 71 men, who had a mean body mass index of 32 (range 20-45).
Results: Kaplan-Meier survival of the cones was 98.24% at 2 years (number at risk n=76). If infection was excluded, survivorship was 100%. There were no cases of aseptic loosening. The mean Knee Society Score improved from 56.7 points preoperatively to 88.1 points at the 2-year follow-up timepoint. The mean Knee Society Functional Score improved from 29.2 points preoperatively to 58.7 points at the 2-year follow-up timepoint. A total of 14 of the 193 patients required additional surgery with the following 17 events noted:11 events of manipulation under anesthesia; 3 events of irrigation and debridement with poly exchange and 1 event of irrigation and debridement; 1 event of patellar subluxation and 1 event of superficial wound infection. There were 3 intraoperative complications of femoral fracture, none of which were related to cone preparation or insertion. The median tourniquet time for revisions using just tibial cones was 107 minutes, while those utilizing both femoral and tibial cones was 98 minutes (p =0.0805).
Conclusion: Metaphyseal fixation is important for survivorship in revision TKA, which can be challenging due to cancellous and structural bone loss encountered at the time of revision. Prosthetic joint infection continues to be the leading cause of failure in revision TKA. The use of the highly porous titanium metaphyseal cones produced from 3-dimensionally printed technology used in this study demonstrated excellent short-term results with no cases of aseptic loosening and few complications from implantation. The use of a central femoral cone, in addition to a tibial cone, did not result in a significant increase in operative time. Further follow-up is required to determine if these results can be durable over a longer period.
#8114
Clinical and Biomechanical Evaluation of Midlevel Constrained and Posterior-Stabilized Polyethylene Inserts in Primary Total Knee Arthroplasty: An Analysis of 12,674 Cases
*Cynthia Kahlenberg - Hospital for Special Surgery - New York City, USA
Michael M. Kheir - The Rothman Institute at Thomas Jefferson University - Philadelphia, USA
Fernando Quevedo Gonzalez - Hospital for Special Surgery - New York, USA
Yu-Fen Chiu - Hospital for Special Surgery - New York, USA
Timothy Wright - Hospital for Special Surgery - New York, USA
Brian Chalmers - Hospital for Special Surgery - New York CIty, USA
Peter K. Sculco - Hospital for Special Surgery - NYC, USA
*Email: kahlenbergc@hss.edu
Introduction
Use of midlevel constraint polyethylene inserts is increasing in total knee arthroplasty (TKA). The purpose of this study was to compare, in a large cohort, the survivorship and reason for revision of both posterior-stabilized (PS) and midlevel inserts used in primary TKA.
Methods
We reviewed all cases of primary TKA performed at a single center from 2016-2019 using either a PS or midlevel constraint insert from one of six major manufacturers. Data elements collected included patient demographics, implants, reasons for revision, and whether a manipulation under anesthesia (MUA) was performed. We performed finite element (FE) analyses to quantify the varus/valgus and axial-rotation constraint of each midlevel constrained insert.
Results
Finite element analysis modeling of each midlevel insert demonstrated a range in varus/valgus constraint from ±1.1 to >5 degrees and a range in axial rotation constraint from ±1.5 to ±11.5 degrees (Fig 1).
Use of midlevel constraint increased over the study period (Fig 2). Among 9,163 PS knees and 3,511 midlevel knees, the 5-year survivorship free from all-cause revision was significantly lower (p=0.004) for midlevel inserts than PS inserts: 92.7% (95% CI=90.2-95.1%) vs 94.1% (95% CI=92.5-100%) (Fig 3). Five-year survivorship free from revision for mechanical problems was significantly lower (p=0.04) for midlevel inserts: 95.5% (95% CI=93.4-97.6%) vs 95.8% (95% CI=94.2-100%). When individually comparing each company’s midlevel insert to the same manufacturer’s PS insert, no differences were found in all-cause revision rates (p≥0.91) or revisions for mechanical problems (p≥0.97). No significant differences were found between the different companies’ midlevel inserts for all-cause revision (p=0.44), mechanical revision (p=0.76), or revision for aseptic loosening rate (p=0.35). Using propensity score matching between midlevel and PS groups, no significant differences were found in rates of MUA (p=0.717), all-cause revision (p=0.123), revision for aseptic loosening (p=0.066), and revision for instability (p=0.451).
Conclusion
We found minimal differences in survivorship rates between PS and midlevel constraint knees. When matched for multiple variables, no difference occurred in revision rates or rates of MUA between primary TKA performed with PS versus midlevel inserts.
Figures
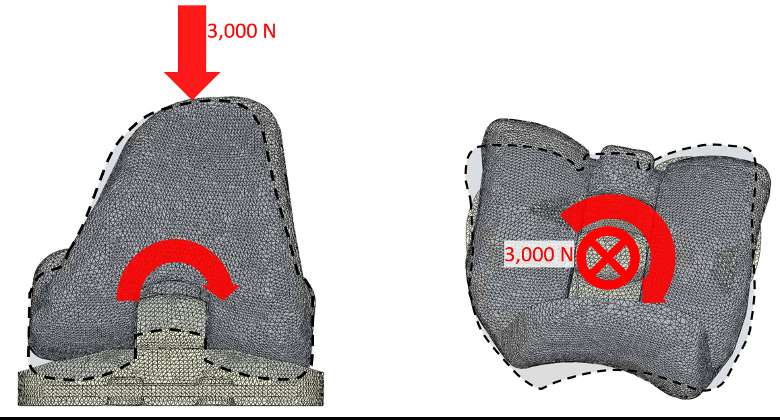
Figure 1
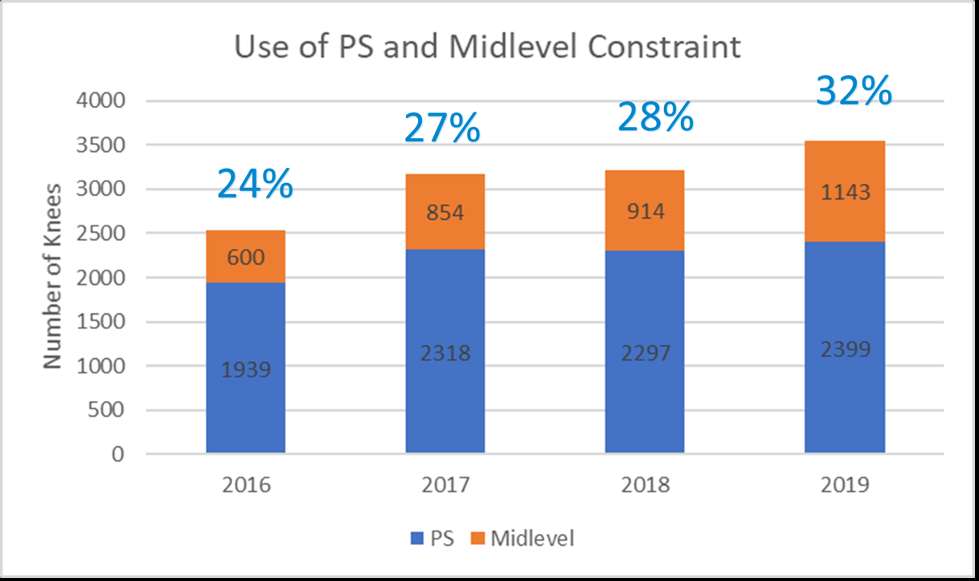
Figure 2
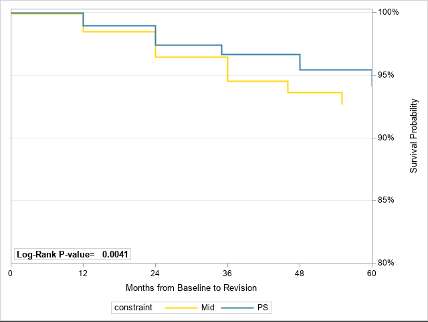
Figure 3#8399
Wear of a PEEK-OPTIMA Polymer-on-UHMWPE Total Knee Replacement
*Raelene Cowie - University of Leeds - Leeds, United Kingdom
Adam Briscoe - Invibio Ltd - Lytham St. Annes, United Kingdom
Louise M. Jennings - University of Leeds - Leeds, United Kingdom
*Email: r.cowie@leeds.ac.uk
Introduction
PEEK-OPTIMA™ polymer (unfilled PEEK) is being considered as the femoral component material of total knee replacements due to being more bioinert and having a modulus more similar to bone than cobalt chrome. The aim of this study was to quantify the wear of an all-polymer PEEK-OPTIMA™ polymer-on-UHMWPE total knee replacement and compare to conventional knee replacement materials (cobalt chrome-on-UHMWPE). This study builds on preliminary work1 of a similar device by increasing sample size, using sterilised knee replacements the same as those used in a clinical trial and a state-of-the-art electromechanical simulation system.
Methods
Six PEEK-OPTIMA™ polymer and six cobalt chrome femoral components of similar initial surface topography (Table 1) and geometry coupled with 12 all-polyethylene (ethylene oxide sterilised, non-crosslinked) tibial components. Four additional unloaded tibial components were used to compensate for moisture uptake by the polyethylene. The study was carried out in a six-station ProSim Electromechanical knee simulator with Leeds high displacement controlled kinematic conditions (Figure 1). The study was carried out in 25% bovine serum (~16g/l) diluted with 0.03% sodium azide solution under room temperature conditions as previously optimised for this all-polymer bearing couple2. The wear of the UHMWPE tibial components was determined gravimetrically (minimum every 1 million cycles) over the duration of the 5 million cycle study. The surface roughness of the articulating surfaces was assessed using a contacting Form Talysurf pre-test and at 5 million cycles. Statistical analysis was carried out to compare different material combinations using ANOVA with significance taken at p<0.05.
Results
The wear of the UHMWPE tibial components was 5.7±0.6 and 6.6±1.7mm3/MC against cobalt chrome and PEEK-OPTIMA™ femoral components respectively (Figure 2), there was no significant difference in UHMWPE wear rate against the different materials (p=0.27). Linear scratching was visible on the PEEK surface and at the conclusion of the study, the mean surface roughness of the PEEK polymer was significantly higher than cobalt chrome (p<0.05). After 5 million cycles wear simulation, polishing of the tibial components decreased their surface roughness but light scratching on tibial components articulating against PEEK polymer resulted in a significant difference in the surface roughness (Table 1).
Conclusion
There was no significant difference in UHMWPE wear against PEEK-OPTIMA™ polymer and cobalt chrome femoral components. A higher UHMWPE wear rate and lower variability in the data was measured in this study compared to previous investigations of this device for both materials1. The increase in sample size, changes in the simulator with improvements in profile following3, differences in polyethylene sterilisation and other aspects of this complex system all likely had a role in the increased wear and reduced variability. The investigations of the two implants were carried out side-by-side using the same simulator to give a direct comparison of the materials which is best practice. Scratching of the PEEK is consistent with previous investigations1,2. This study gives further confidence in the use of PEEK-OPTIMA™ polymer in the femoral component of total knee replacements in terms of UHMWPE wear.
1.Cowie R.M., et al.2016.JOEIM.230(11):1008-1015.
2.Cowie R.M., et al.2019.JMBBM.89:65-71.
3.Abdelgaied A, et al.2017.JOEIM.231(1):643-651.
Figures
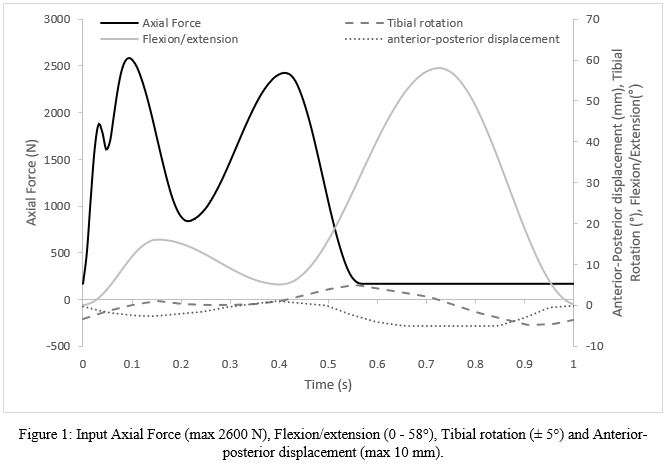
Figure 1
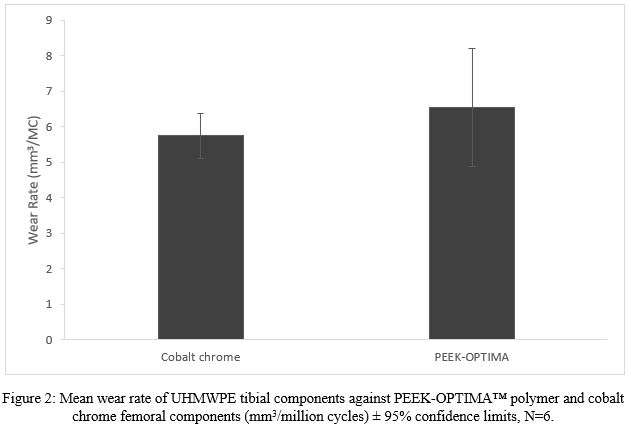
Figure 2
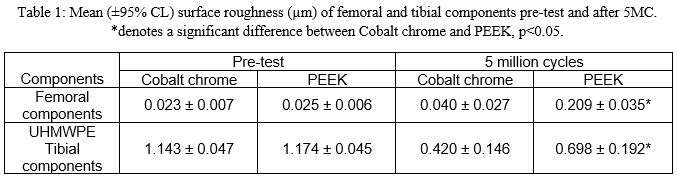
Figure 3#8423
The Effect of Multiple Variables on the Wear Performance of a Highly Crosslinked UHMWPE: A Retrospective Review
*Ilya Borukhov - Stryker Orthopedics - Mahwah, USA
Sally LiArno - Stryker Orthopaedics - Bergenfield, USA
*Email: ilya.borukhov@stryker.com
INTRODUCTION
In-vitro hip simulation has had a long history of reproducing clinical failures, wear mechanisms, and predicting clinical successes in total hip arthroplasty (THA)[1]. This has led to the development and widespread clinical use of highly-crosslinked polyethylene as well as common metallic and ceramic countersurfaces. As part of pre-clinical screening, researchers are required to evaluate worst-case design and material conditions to amplify differences in wear. This can be challenging when modern designs have multiple bearing sizes, polyethylene thicknesses, and head material combinations. Furthermore, the low wearing nature of modern highly-crosslinked polyethylene can exaggerate the impact of test variability. To assess the impact of multiple variables on the wear performance of a single highly-crosslinked polyethylene, we conducted a retrospective review of in-vitro wear studies and performed a multiple linear regression analysis.
METHOD
The material for all acetabular inserts for this analysis was sequentially irradiated and annealed polyethylene (X3, Stryker, Mahwah, NJ). Each acetabular insert was coupled with a CoCr, LFIT CoCr, Orthinox stainless steel, Alumina ceramic, or Biolox Delta ceramic femoral head. In all tests, an orbital hip joint simulator (MTS, Eden Prairie, MN, USA) was used for testing with cups positioned superior. Testing was conducted per ISO 14242-3:(R2019) with a modified load profile consisting of a physiological Paul curve with a peak axial force of 2450N[2]. Tests in which the volumetric wear rate was acquired with at least 2.5 million cycles (mc) using a linear regression was included. Outliers were identified and removed using a Grubbs outlier test (α=0.05). A multiple linear regression analysis was performed to assess the influence of bearing size, insert thickness, femoral head material, and total cycle count on measured volumetric wear rate. Significant was set at p ≤ 0.05.
RESULTS
A total of 245 samples, evaluated between the years 2002-2017, were included in this study. Bearing sizes were 22 (n=8), 28 (n=32), 32 (n=90), 36 (n=32), 40 (n=14), and 44 mm (n=62). Polyethylene thickness ranged from 3.8-21.6mm, with the most common thicknesses being 3.8 (n=70), 5.9 (n=81), 7.9 (n=50), and 9.9 mm (n=9). Head material included LFIT CoCr (n=179), Alumina (n=5), CoCr (n=39), Delta (n=11), and Orthinox stainless steel (n=4). Multiple linear regression analysis revealed bearing size to be the only statistically significant factor (p<0.001) accounting for 23% of the variance in the wear rate data. Polyethylene thickness (p=0.990), cycle count (p=0.763), and head material (p=0.382) were not statistically significant factors. Wear rate generally increased with increasing bearing size (Figure 1).
DISCUSSION
This study retrospectively evaluated the impact of bearing size, polyethylene thickness, head material, and cycle count on the wear performance of a clinically successful, highly-crosslinked polyethylene. The analysis revealed that bearing size was the only statistically significant factor, but it only accounted for 23% of the variability in the data. Head material was not a significant factor despite the theoretical advantages of ceramics over metals. To exaggerate differences between varying designs and materials, emphasis should be placed in developing test methods to evaluate THA bearings under aggressive conditions.
REFERENCES
[1]Wang A,Semin.Arthroplasty,17:49-55,2006. [2]Paul,JP.Proc.Inst.Mech.Engrs.,181(3J):8-15,1966
Figures
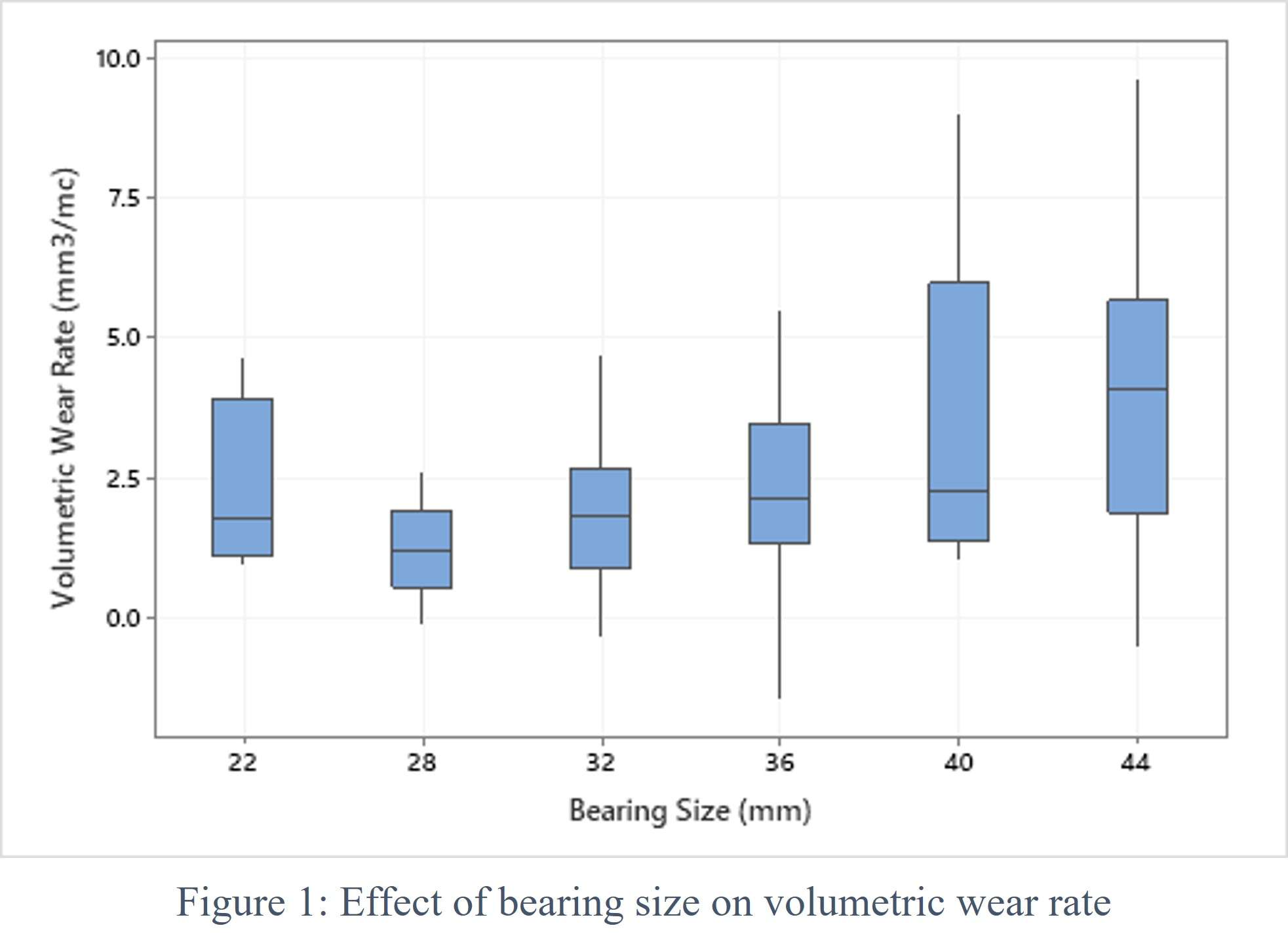
Figure 1#8515
Wear Performance of UHMWPE GUR 1050 After Mechanical Compression
*Lucas Gimenis de Moura - Federal University of Santa Catarina - Florianópolis, Brazil
Eduardo Alberto Fancello - Mechanical Design and Analysis Group (GRANTE) - Florianopolis, Brazil
Edison da Rosa - Federal University of Santa Catarina - Florianópolis, Brazil
Carlos Rodrigo De Mello Roesler - Universidade Federal de Santa Catarina - Florianópolis, Brazil
Clara Muniz Da Silva De Almeida - INMETRO - Duque de Caxias, Brazil
Marcia Maru - Inmetro - Duque De Caxias, Brazil
ARTHUR SANTOS - LEBm - UFSC/HU - Florianópolis, Brazil
*Email: lucas_lebm@outlook.com
Introduction
One of the preprocessing routes for improving the strength of semicrystalline polymers is by submitting the material to some grade of mechanical work to increase its crystallinity. In this work, the triboperformance of a commercial UHMWPE GUR 1050 after being subjected to permanent bulk microstructural modification by mechanical compression was investigated. The evaluations were performed in both compression direction and material flow direction.
Methods
Plate specimens of UHMWPE GUR 1050 of (36×36×6) mm3 size were subjected to plane-strain deformation in a home-made channel-die device, up to a compression ratio of 2.5. This resulted in a permanent deformed specimen at a compression ratio of 1.6. Tribological evaluation was performed through linear reciprocating dry sliding tests in non-deformed (ND) and deformed conditions, in the direction of compression (LD) and material flow (FD). The tests were run in triplicate in a multi-station hip simulator machine (AMTI-Boston) adapted to convert the original abduction-aduction angular movement into linear. Figure 1 presents details of the performed tests. The selected normal load of 280 N was equivalent to an average Hertz elastic contact pressure of about 30 MPa. The analyses included microstructural phase quantification by Raman spectroscopy (WITEc, Alpha300 system, 532 nm wavelength diode laser of 22 mW), wear volume by 3D profilometry (PGI830 Taylor Hobson) and dissipated energy by friction along the test run up to 1 million cycles. The dissipated energy was calculated as the area of the hysteresis loop of friction force values acquired per cycle test (see example in Fig.2d). The specimens were inspected through optical microscope (Zeiss AxioVision) with 5x objective lens.
Results
The plane strain compression created a complex plastic flow field in the polymer, whose direction was mainly orthogonal to the loading direction (see the deformed square grid, drawn before compression, in Fig.1b). This evidenced material became anisotropic. Increase in the crystalline phase was observed in the strained material (Fig. 2a) from 49% to 63%, mainly at the expenses of the amorphous phase, as compared to the result of non-deformed material. The wear volume of the pre-strained material was superior for about 36% (Fig.2b), indistinctly if tested in LD or FD, as compared to the non-deformed material (ANOVA, p < α, α = 0.05). The friction dissipated energy was higher for the specimen tested in LD (Fig.2e,), indicating more resistance to sliding, or worst self-lubrication performance in LD. The images of the worn area of the polymers tested up to 200,000 cycles (Fig.3) revealed that the wear regime was extremely severe, featuring strong manifestations of the two main mechanisms normally encountered in polymers, of abrasion and wave-like morphology formation. These continued up to the end of the test. The wear depth of dozens of micrometers (Fig. 2c), as well as the well visible generated wear particles (Fig. 3) also confirmed the high wear severity of the performed tests.
Conclusion
The results obtained in the present work demonstrated inferior triboperformance for the pre-strained material, in spite of the identified increase in the crystalline phase as compared to the non-deformed state.
Figures
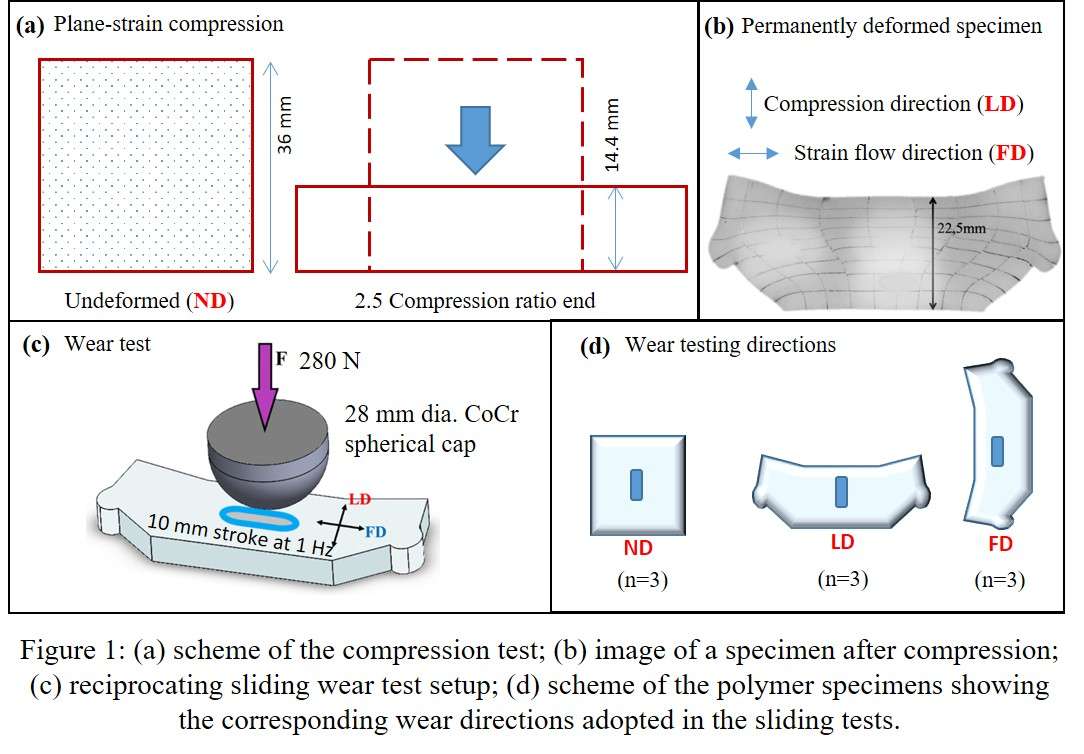
Figure 1
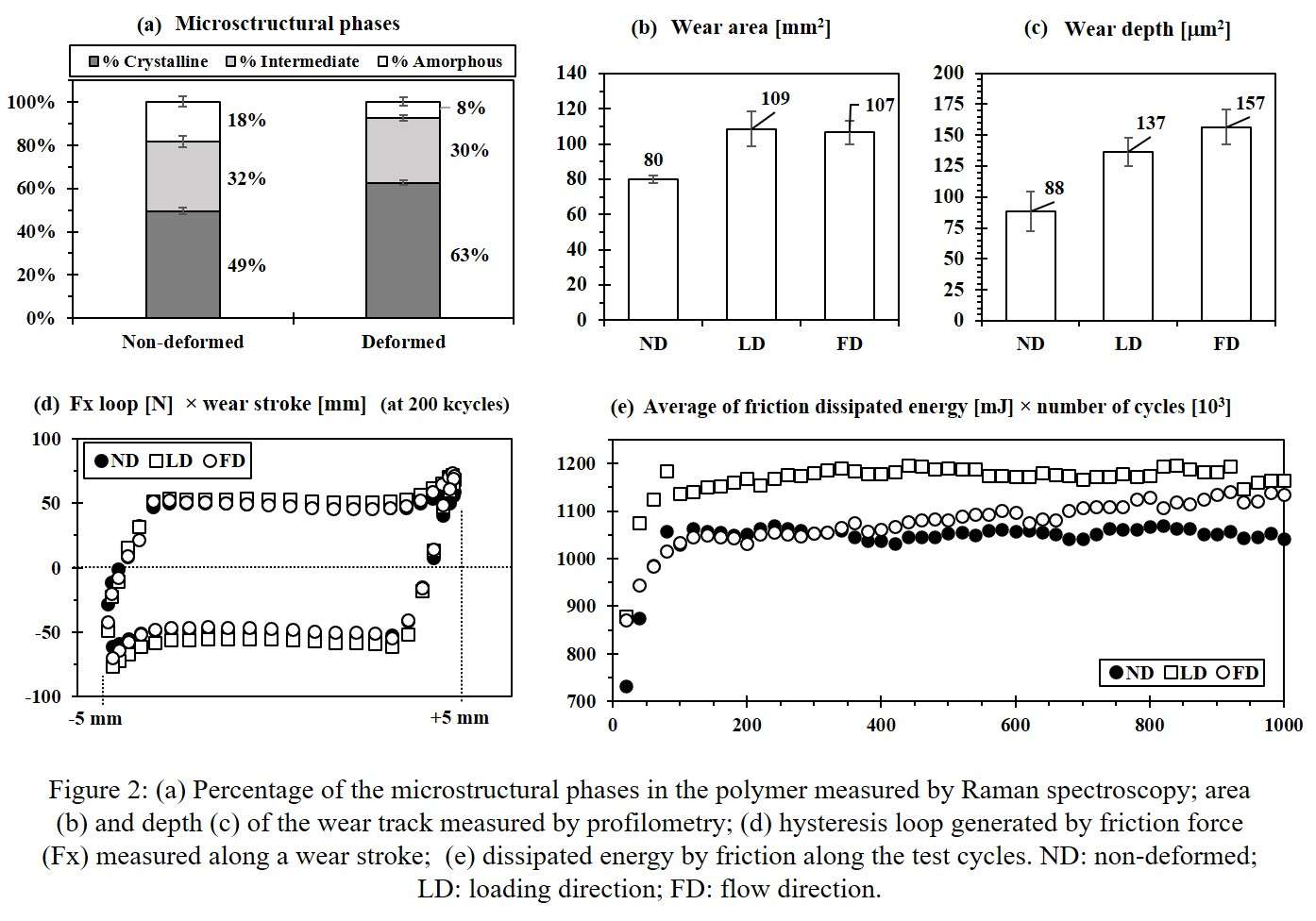
Figure 2
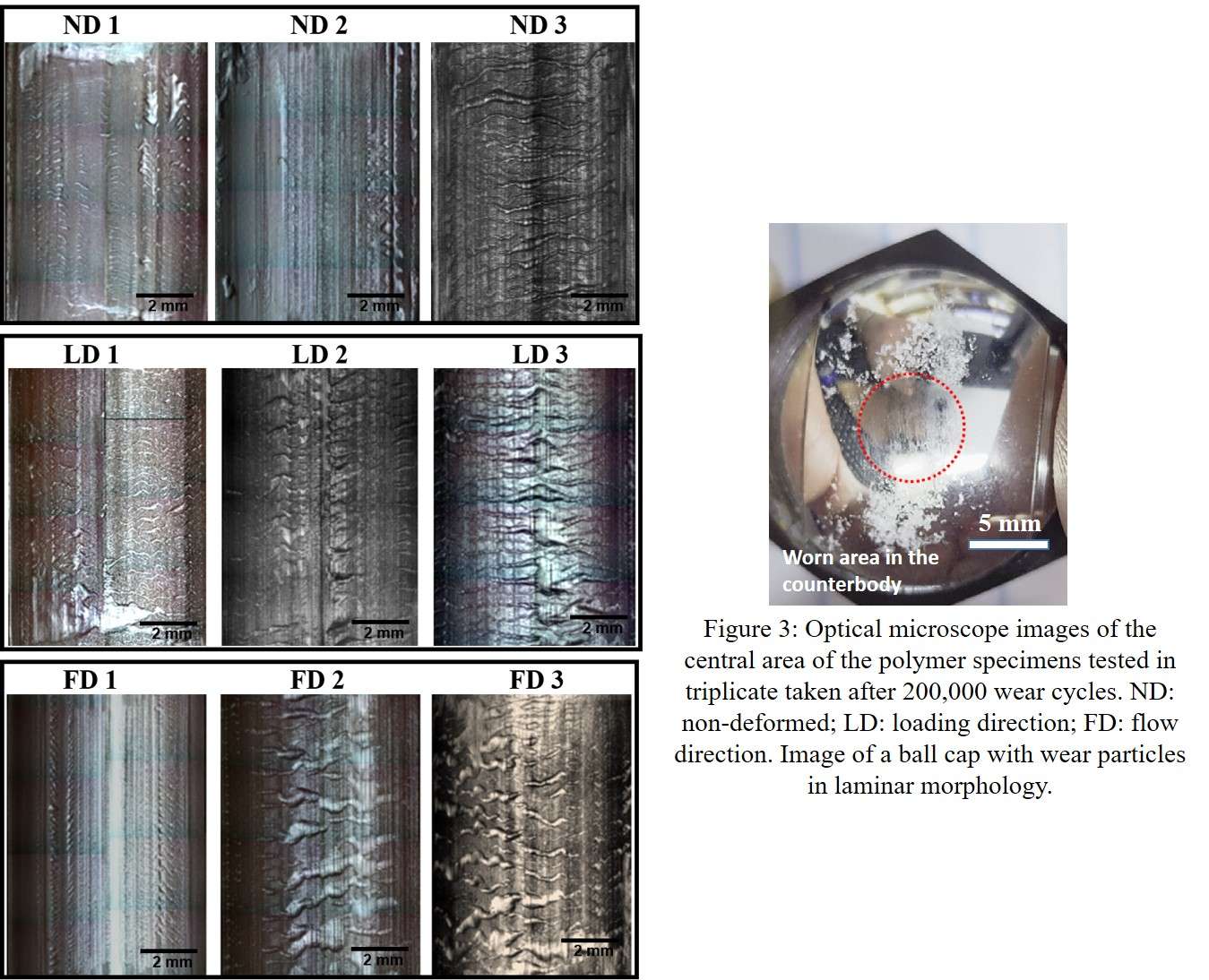
Figure 3#8678
Significant Wear Resistance of Vitamin E Grafted UHMWPE Reverse Shoulder Humeral Bearings Articulating With CoCrMo Glenospheres
*Diego Orozco Villasenor - Zimmer Biomet - Warsaw, USA
Ravikumar Varadarajan - Zimmer Biomet - Warsaw, USA
Kimberly Mimnaugh - Zimmer Biomet, Inc - Warsaw, USA
*Email: Diego.Orozco-Villasenor@zimmerbiomet.com
INTRODUCTION
Reverse total shoulder arthroplasty (RTSA) procedures have increased significantly year over year [1,2]. Instability, infection and loosening are three of the main reasons for revision of a primary RTSA [3]. Loosening of artificial joint replacements is linked to osteolysis caused by ultra-high molecular weight polyethylene (UHMWPE) wear debris [4]. With broadening use, including implantation in younger patients, an increased potential for debris-induced osteolysis, and subsequent implant loosening, is a significant concern. Conventional, highly cross-linked and vitamin E UHMWPE materials are currently used for humeral bearing devices in RTSA. However, with no standardized wear testing methods available and limited pre-clinical wear assessments using physiologically-relevant testing conditions for these polyethylene materials, their relative wear performance has been difficult to establish.
The objective of this study was to determine the wear performance of RTSA humeral bearings manufactured from a grafted vitamin E highly crosslinked polyethylene material.
METHODS
Humeral bearings, size 40 mm, manufactured from highly cross-linked UHMWPE (HXPE) stabilized with the antioxidant vitamin E (VE-HXPE), and humeral bearings, size 41 mm, manufactured from HXPE material, were articulated against compatible, size matching glenospheres manufactured from cobalt-chromium-molybdenum (CoCrMo) alloy. All humeral bearings were accelerated aged per section ASTM-F2003.
A previously evaluated in-vitro wear testing method for RTSA prostheses was implemented [5,6]. This wear test method reproduced the physiological kinematics and kinetics of an activity that intends to replicate key shoulder motions that occur in activity of daily life (Figures 1 and 2).
In vitro wear testing was performed for 4.0 million cycles (Mc) using a 12-station AMTI hip wear simulator (AMTI MA). All samples were submersed in lubricant throughout the duration of the test. The lubricant was a mixture of bovine calf serum (SH30073, Hyclone, Utah), sodium azide and disodium EDTA, diluted with deionized water to a protein concentration of 20 g/L. All samples were kept in sealed chambers with lubricant recirculated at 37±2°C.
Gravimetrically mass measurements were performed every 0.5 Mc. Load-soak controls were used to correct for the fluid absorption. Wear rates were calculated using a least-squares regression per ISO 14243-2. A 2-sample t-test statistic (α=0.05) was used to identify significant differences on the wear rates between test groups.
RESULTS
The VE-HXPE and HXPE humeral bearings exhibited wear rates of 3.4±0.5 mg/Mc and 22.6±3.9 mg/Mc, respectively (Figure 3). The VE-HXPE humeral bearings generated a mean average wear rate that was 85% lower than the wear rate generated by the HXPE humeral bearings (p < 0.01). All test samples (HXPE and VE-HXPE) completed the prescribed cycles without signs of delamination, cracks or gross deformation.
CONCLUSION
Humeral bearings manufactured from highly cross-linked UHMWPE stabilized with the antioxidant vitamin E (VE-HXPE) exhibited a significant wear rate reduction when compared with a similar device manufactured from HXPE without antioxidants. The lower wear rates, and the oxidative stability of the VE-HXPE material have been reported to provide long term stability [7]. A lower net amount of wear debris should help minimize the potential for osteolysis-induced loosening in the clinical setting.
Figures
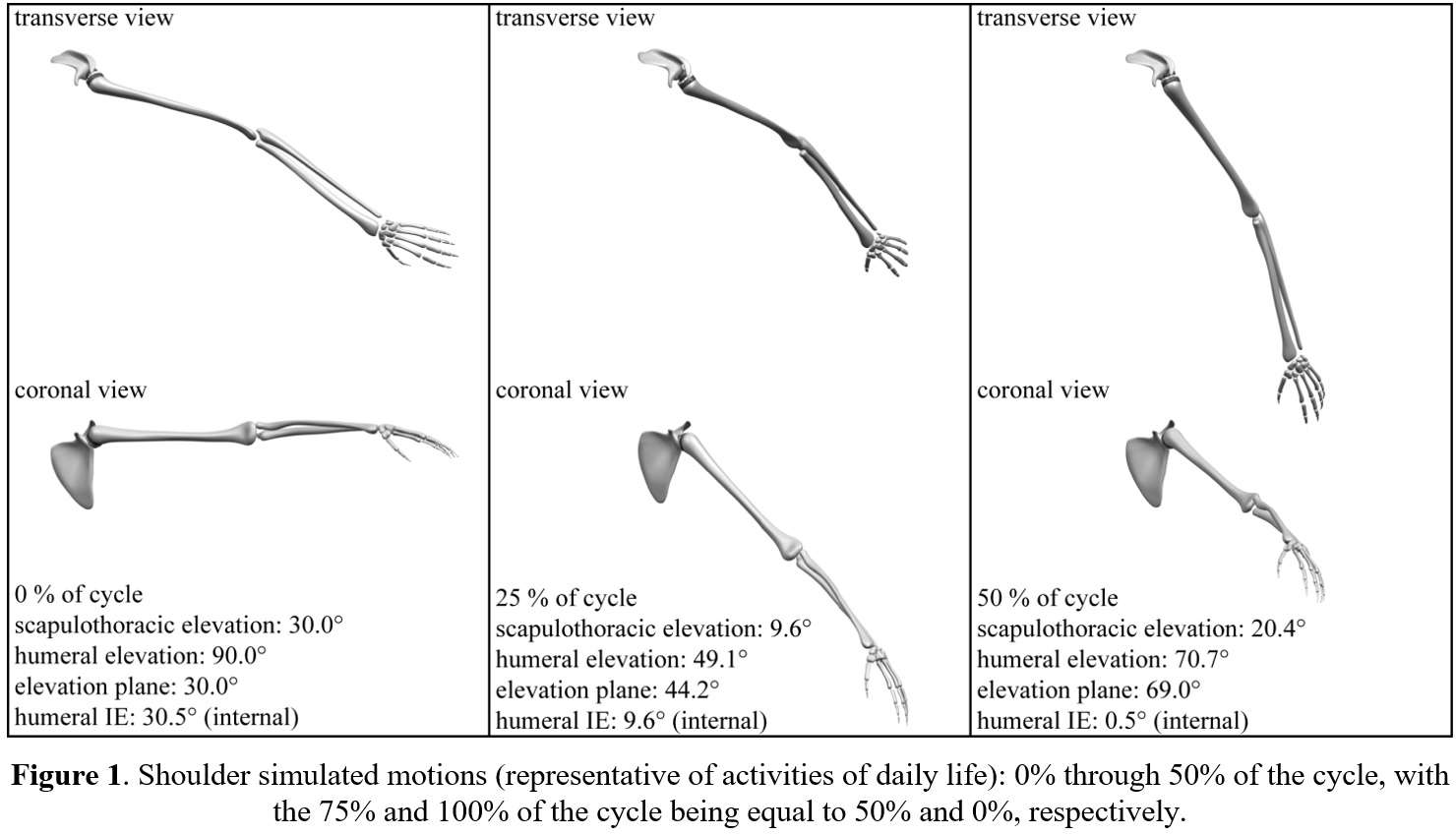
Figure 1
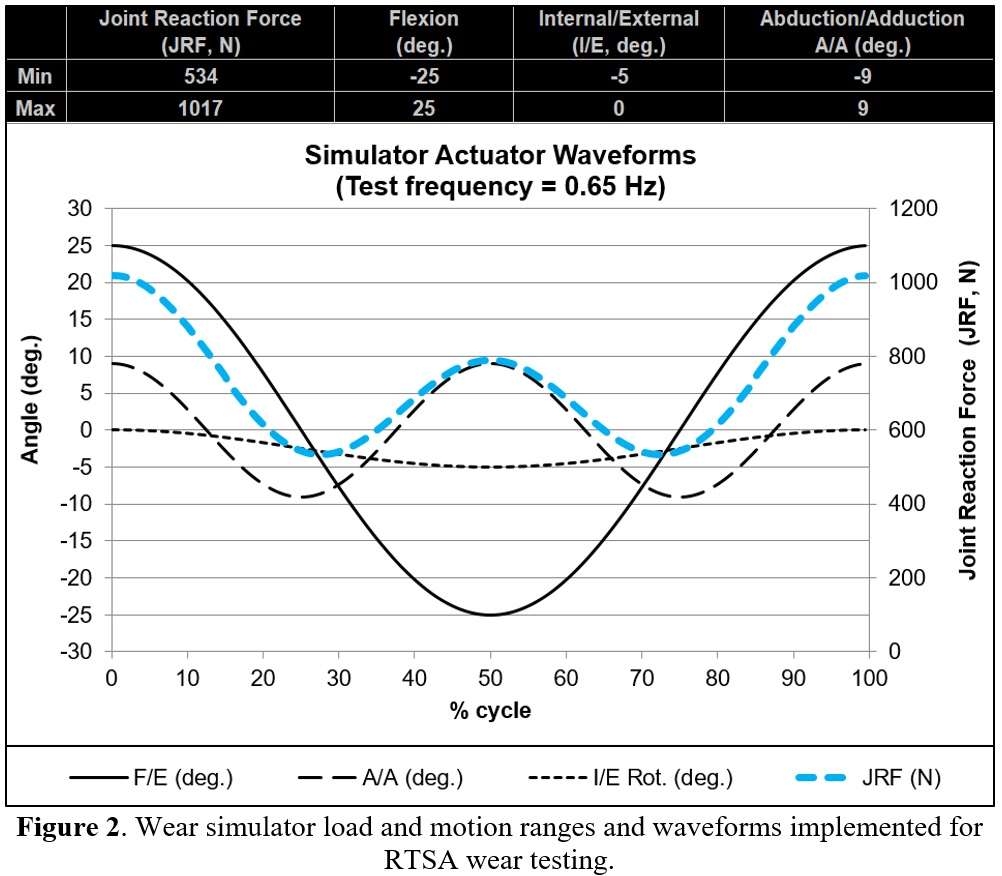
Figure 2
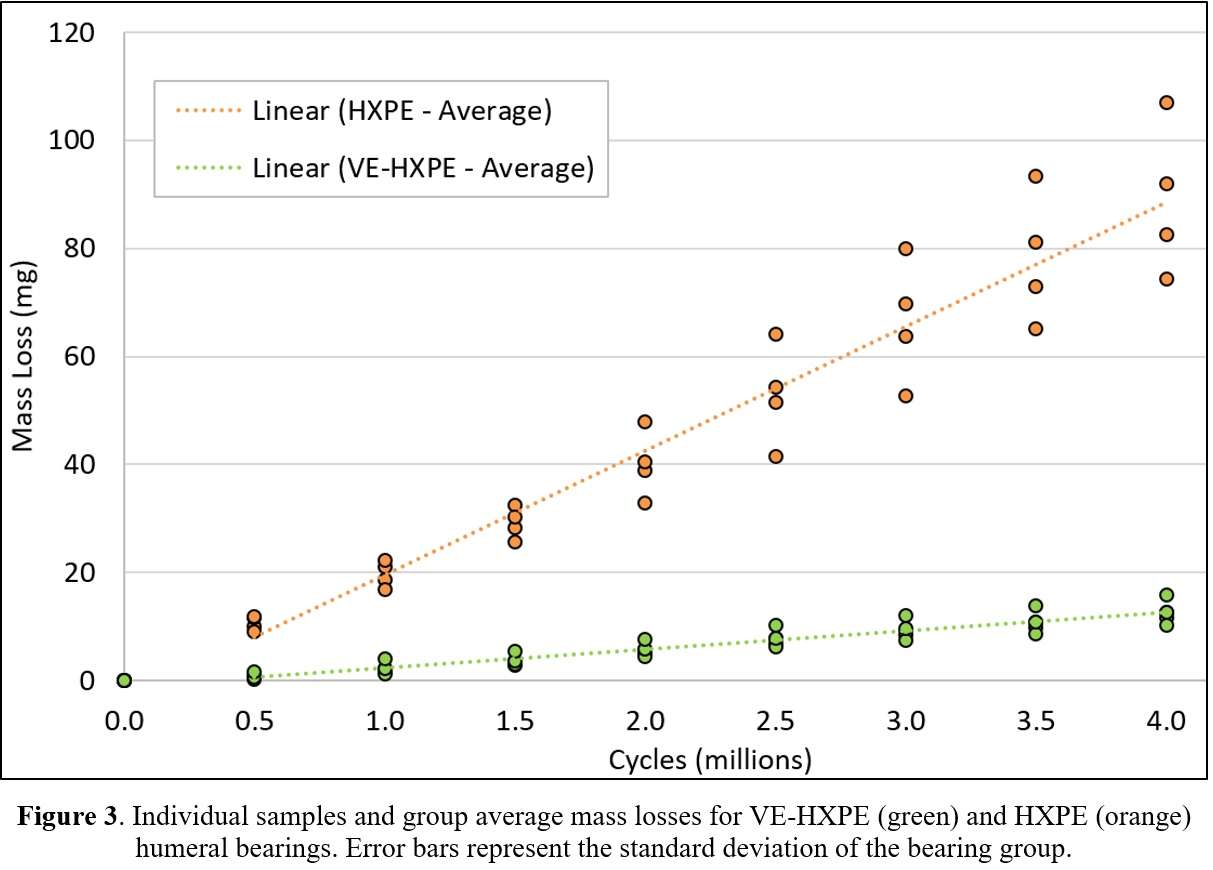
Figure 3#8484
Frictional Torque of the Outer Diameter of a Novel Dual Mobility Design
*Mazen Al-Hajjar - DePuy Synthes - Leeds, GB
Silvia Carbone - DePuy Synthes - Leeds, United Kingdom
Ioan Cracaoanu - DePuy Synthes - Leeds, United Kingdom
Megan Hadley - DePuy Synthes - Leeds, United Kingdom
*Email: malhaija@its.jnj.com
INTRODUCTION:
Dual-mobility (DM) articulations have recently gained popularity in addressing and managing hip instability in total hip arthroplasty (THA). Patients at risk of dislocation can rely on DM offering a larger jump distance and potentially greater range of motion which reduces impingement. Improvement in design options and material properties has contributed to diversification of THA offerings. The medium- to short-term results with DM THA have shown a low rate of instability and good overall survivorship in primary and revision THA.[1]
Whilst a larger head size reduces occurrence of dislocation, there are concerns that increased frictional torque can lead to higher risk of loosening. This study compares the frictional torque of the outer diameter articulation of a novel and two commercially available DM designs following ASTM F3143-20 Determination of Frictional Torque and Friction Factor for Hip Replacement Bearings under Standard Conditions Using a Reciprocal Friction Simulator.
METHODS:
This study (n=6) evaluated the frictional torque of the outer diameter (OD) of three DM designs, namely: a novel DM AOX polyethylene (PE) mobile bearing construct (shell size 64mm), BI-MENTUM DM (standard PE, shell size 65mm), and PINNACLE DM system (standard PE, shell size 66mm) (DePuy Synthes Joint Reconstruction) following ASTM F3142-20. A locking ring constrained the metal head within the MB allowing articulation to only between the polyethylene component and the metal cup. Friction testing: the test was conducted on AMTI VIVO joint simulator (Figure 1) for 126 cycles in “forward” profile and 126 in “reverse” in bovine serum (30mg/mL total protein) supplemented with sodium azide and EDTA. A Ceramic on Ceramic control sample was tested before and after test in the same lubricant. Data analysis: Eleven torque values around the peak load for each profile and each cycle were logged, extracted and the average of the forward and reverse torques was calculated. A one-way ANOVA analyzed the statistical difference of the three frictional torque means.
RESULTS:
The average frictional torque (±standard deviation) of the acetabular component articulating against the acetabular cup (Figure 2) for each DM design was:
- Novel DM AOX: 3.20 ±0.20 Nm
- BI-MENTUM DM: 3.27±0.22 Nm
- PINNACLE DM: 3.00±0.21 Nm
A one-way ANOVA comparison showed no statistical difference between the three means (p-value= 0.133).
DISCUSSION:
The purpose of this study was to compare the average frictional torque of the outer diameter of a novel DM design and two commercially available DM designs tested on an AMTI VIVO joint simulator under a simplified profile as recommended in ASTM F3143-20. The study showed no statistical difference in the outer diameter frictional torque of the three groups (p=0.133), confirming the novel design resistance to loosening performance is comparable to the marketed DM offering.
SIGNIFICANCE:
The frictional torque can be a reliable predictor of acetabular loosening. Larger bearing surfaces can lead to a higher frictional torque. Frictional torque measurement offers significant value in assessing the risk of implant loosening as the THA design and material performances are developed to address new patient needs.
REFERENCES:
[1] Darrith, B et al. O. 2018 BJJ
Figures

Figure 1
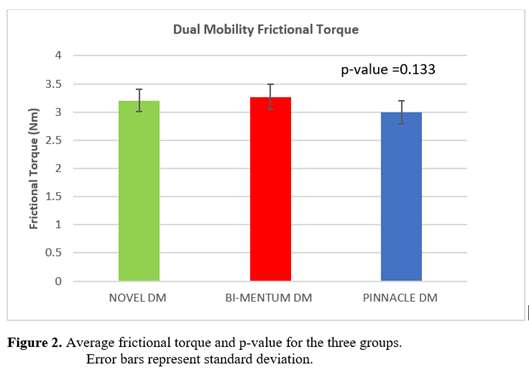
Figure 2#8190
Subluxation-Induced Adhesion of the Liner and Influence of Impaction to Prevent Liner Dissociation in Ceramic-Ceramic Total Hip Arthroplasty
*J. Philippe Kretzer - University of Heidelberg - Heidelberg, Germany
Sebastian Jaeger - Heidelberg University Hospital - Heidelberg, Germany
Mareike Schonhoff - Laboratory of Biomechanics and Implant Research - Heidelberg, Germany
Maximilian Uhler - Laboratory of Biomechnics and Implant Research - Heidelberg, Germany
*Email: philippe.kretzer@med.uni-heidelberg.de
In total hip arthroplasty ceramic liner fractures have often be associated to a surgical malseating off the liner in the cup.
Another potential explanation could be that the liner is disassociated due to increased adhesion of the liner to the head during small head subluxation movements.
The aim of this study was to determine adhesion forces between the liner and the head during subluxation movements of different velocities. Furthermore, the connection strength between the liner and the cup (taper connection) was studied to figure out if the adhesion forces may exceed the connection strengths leading to a disassociation of the liner from the cup.
Two different ceramic-ceramic implant systems (Mathys SeleXys and DePuy Synthes Pinnacle) with each two different diameters of joint articulation (28 and 36 mm) were studied.
The sliding pairs were immersed in serum as a joint fluid and pulled apart abruptly at different speeds (10-120mm/s). Hereby the adhesion forces between the sliding partners were measured.
In a further approach, it was analysed to what extent the joint strength of the taper connection between the cup and the liner depends on the impaction force of this connection.
For the small head diameter (28mm) with small clearance, adhesive forces were found to depend on the subluxation speed (Fig.1), while this effect was not so pronounced for larger head diameters and greater clearance. In individual cases, adhesive forces of up to 265N have been recorded.
With regard to the connection strength, there was a relevant influence of the implant system with lower connection strengths with the Mathys implants (Fig. 2). This can be well explained by the significantly larger taper angle of the Mathys implants.
If the results of the subluxation-related adhesion force and the taper connection strength are compared, it can be concluded that the Mathys implants should be impacted with forces of at least 2 kN so that the liner cannot loosen due to subluxation. For the DePuy Synthes implants, a joining force of 1 kN seems to be sufficient to hold the liner in place.
Figures
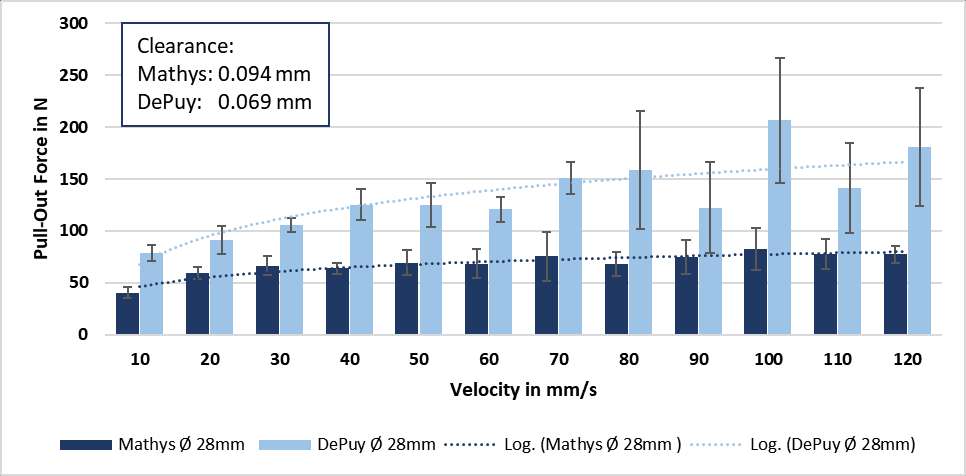
Figure 1
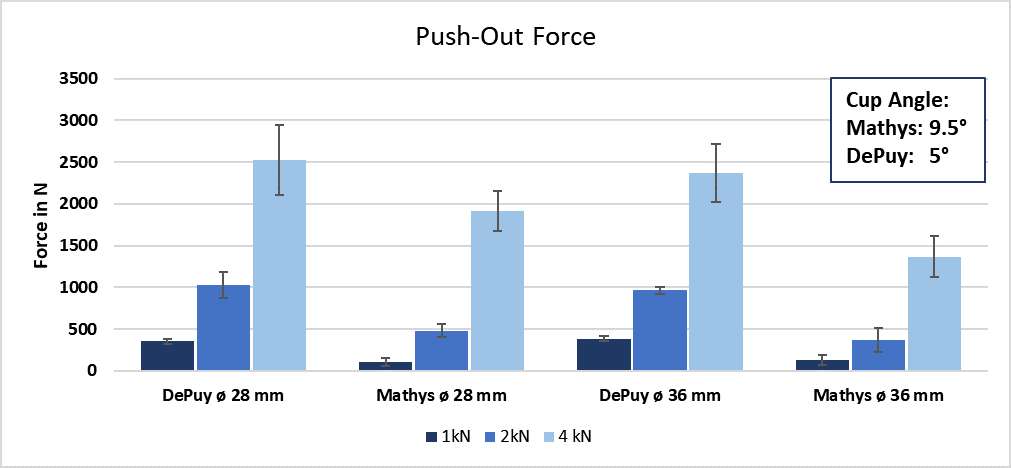
Figure 2#8132
Navigated Cup Placement in Total Hip Arthroplasty Is More Precise and Accurate Than the Free-Hand Technique: A Randomized, Controlled Trial
*Rene MihaliÄ - Valdoltra Orthopaedic Hospital - Ankaran, Slovenia
Jurij Zdovc - University of Ljubljana, Faculty of Pharmacy - Ljubljana, Slovenia
Janez Mohar - Orthopaedic Hospital Valdoltra - Ankaran, Slovenia
Rihard Trebse - ORTHOPAEDIC HOSPITAL VALDOLTRA - Ankaran, Slovenia
*Email: rene.mihalic@ob-valdoltra.si
Introduction
In total hip arthroplasty (THA), the gold standard for component placement is still the freehand technique, with up to 75% of cups placed out of the safe zone. To avoid cup position inaccuracies in the future, we designed a randomized, controlled trial to investigate if the use of the electromagnetic navigation (EMN) system in THA is more precise and accurate than the freehand technique, also in the hands of high-volume surgeons.
Methods
A pilot study validating the EMN system was the basis for power analysis. The patient demographic data included: diagnosis, age, sex, BMI, and Harris Hip Score (HHS). Patients were randomly assigned into two groups scheduled for THA. In the study group (EHIP), patients underwent navigated cup placement during THA, and in the control group (freehand), they underwent the conventional freehand technique. A modified Hardinge approach in the supine position was used, and cementless cups from the same manufacturer were implanted in all cases. In the EHIP group, a Steinman pin with a reference sensor was mounted above the acetabular edge before cup placement without additional tissue dissection. A specially designed device (Navi-frame) with the measuring sensor was pressed on both anterior superior iliac spines and the pubic tubercle to enable the anterior pelvic plane registration [Fig. 1]. Cup was impacted with the help of a conventional cup holder with a measuring sensor on it [Fig. 2]. In the freehand group, cups were placed freehand, aiming to place the cup around predefined target angles (42.5° for inclination and 15° for anteversion). Postoperatively, CT scans of the patient’s pelvises were performed, and the true cup position was determined by an independent person [Fig. 3]. From the differences between the target and true angles for every patient, we calculated the mean bias error (ME) and root mean squared error (RMSE), representing the method's accuracy and precision, respectively. The duration of surgery was also compared. The study was registered at ClinicalTrials.gov (NCT04101864).
Results
We included 42 patients in each group. We observed no significant demographic differences between groups. ME of inclination were 1.7° and 1.9° in the EHIP group and freehand group, respectively (p=0.9), and ME of anteversion were -1.7° and -4.5° in the EHIP group and freehand group, respectively (p<0.01). RMSE for inclination were 4.6 and 6.5° in the EHIP group and freehand group (p=0.02), respectively, and RMSE for anteversion was 2.8° and 8.0° in the EHIP group and freehand group, respectively (p<0.001). The median duration of surgery in the EHIP group and the freehand group was 70.4 minutes and 69.6 minutes, respectively (p=0.78).
Conclusion
The EMN is more precise and accurate than the freehand technique, especially for cup anteversion. Furthermore, there was no apparent significant difference in the duration of surgery comparing both groups. According to our results, navigated THA should be used primarily in anatomically complex cases or minimally invasive procedures where surgical landmarks are difficult to define.
Figures
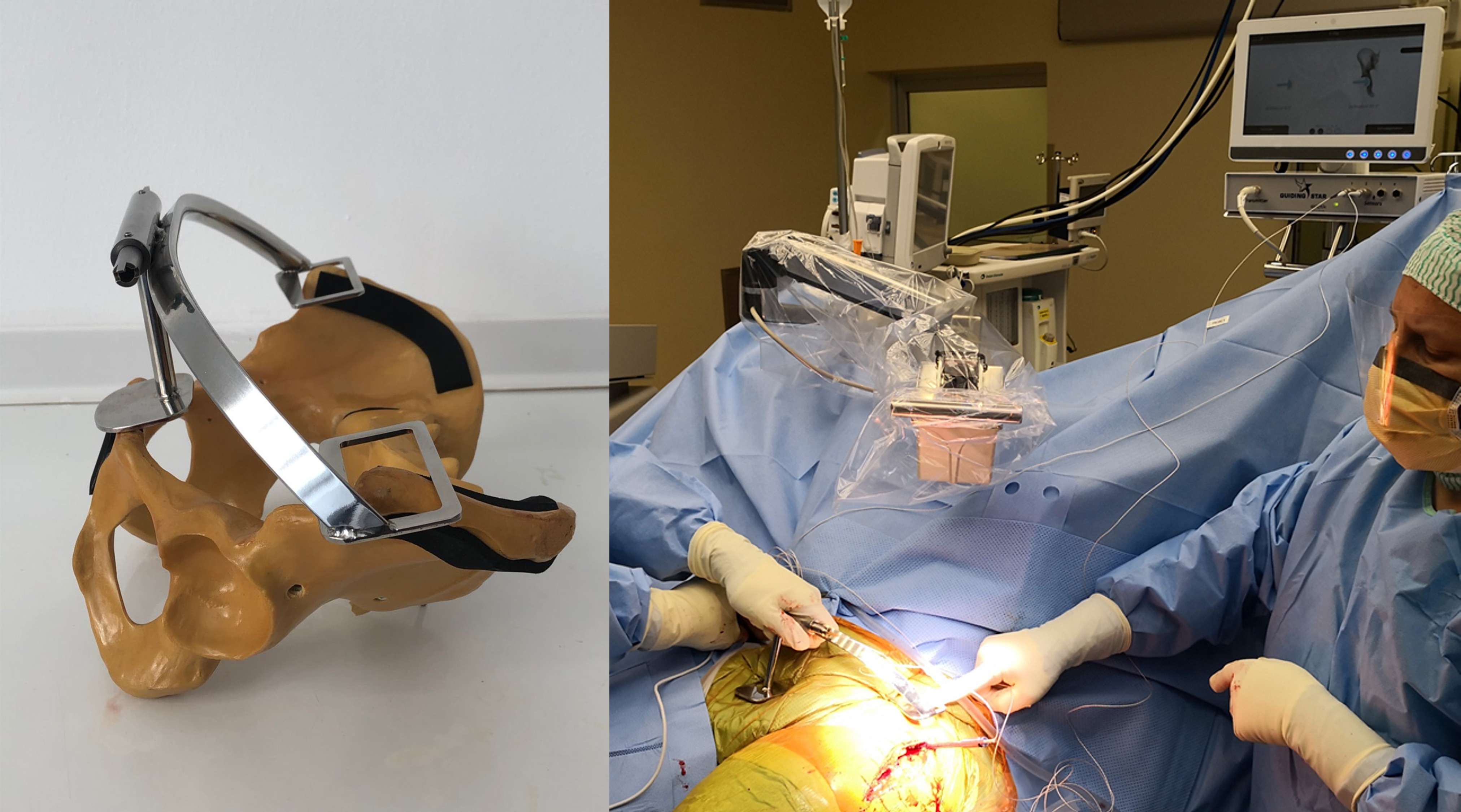
Figure 1
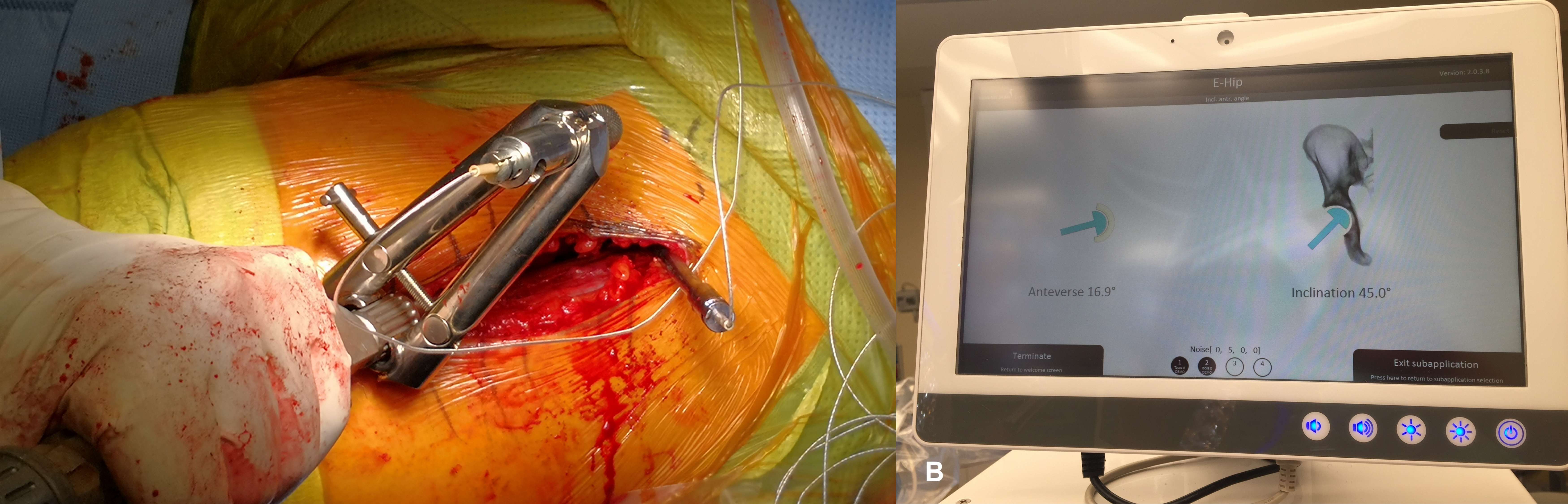
Figure 2
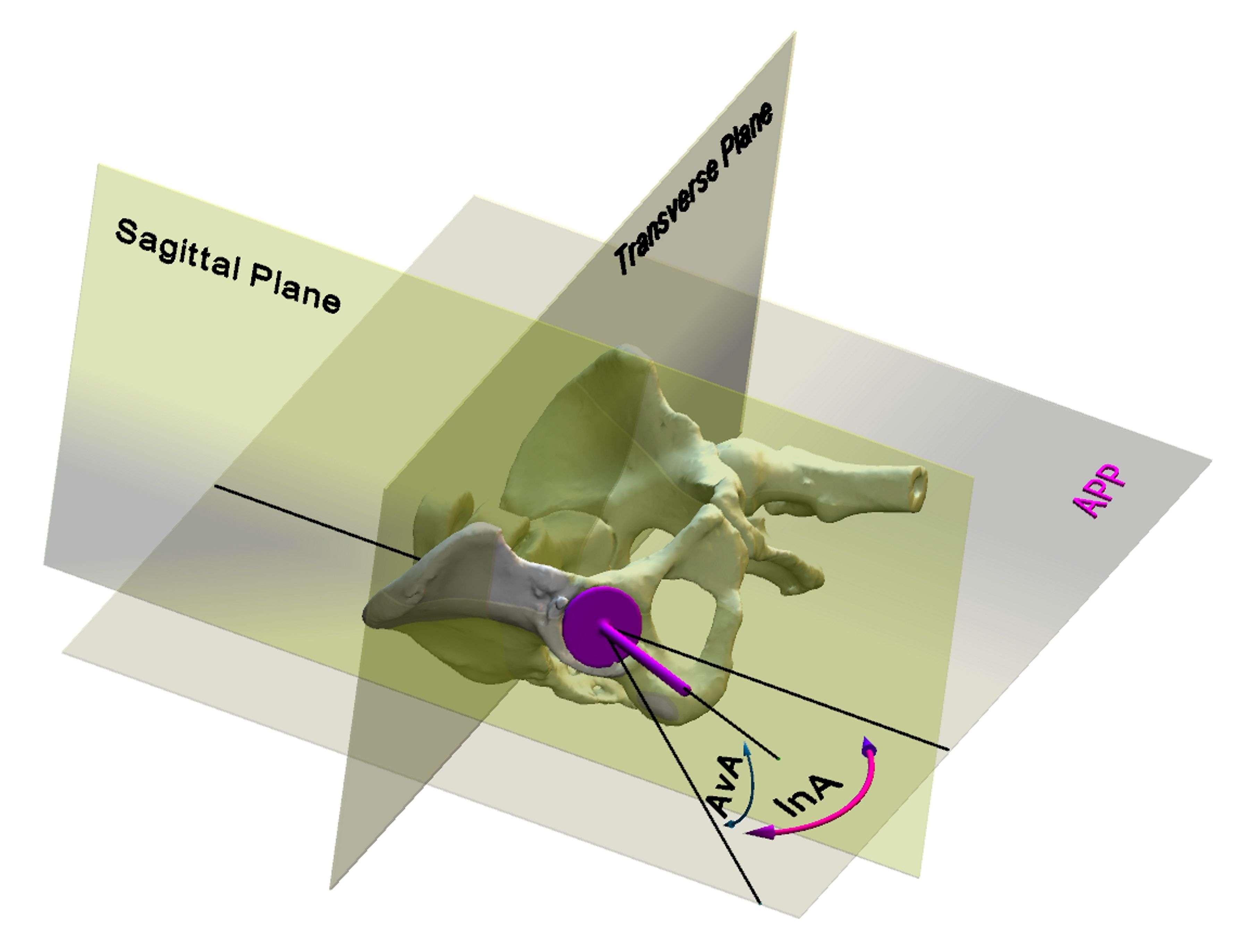
Figure 3#8534
Retrieval Analysis of Cages, Custom Acetabular Components and Cup Cages From Complex Revision Total Hip Arthroplasties
*Abdulganeey Olawin - Drexel University - Philadelphia, United States of America
Cemile Basgul - Drexel University - Philadelphia, USA
Steven Kurtz - Drexel University - Philadelphia, USA
Greg Klein - Hartzband Center for Hip and Knee Replacement - Hackensack, USA
P. Maxwell Courtney - University of Pennsylvania - Philadelphia, USA
Jonathan Garino - University of Pennsylvania - Philadelphia, USA
*Email: aoo27@drexel.edu
Pelvic discontinuity (PD), defined as a separation of the superior ilium and the inferior ischiopubic columns of the acetabulum, is classified as a Type IV pelvic deficiency by AAOS1,2. Treatment is based on the extent of discontinuity, available bone stock, and the potential for stable healing3. Fixations face high mechanical forces leading to persistent bone non-union. The use of cages, custom triflange prosthesis, and cup-cage constructs emerged as implant solutions to PD. Custom acetabular components are tailored to the patient's anatomy, while cup-cage constructs are available with existing implants. The objective of this retrieval study was to assess the performance of cages, custom acetabular components, and cup-cages used in the treatment of pelvic discontinuity. Specifically, the study focuses on analysing patient demographics, reasons for revision surgeries, and the characteristics of the implants employed.
A total of 11 implants, from 4 different manufacturers, including cup-cage constructs, custom acetabular components, and cages, were retrieved for analysis. Patient clinical data was reviewed, including revision reason, gender, number of previous revisions, implantation time, surgical sites, age, UCLA score, and BMI. Parameters such as implant size, number of sacroiliac joint screw-holes, ilium screw-holes, ischium screw-holes, and the presence of flanges were examined. Alloy determination was conducted using X-ray fluorescence. Damage mechanisms such as scratching, burnishing, corrosion, and fretting were further analysed using a digital microscope (Keyence VHX-700).
The cohort had an average implantation time of 4.69 ± 5.46 years, with a median of 3.05 years. The average BMI was 30.65 ± 5.29 kg/m2, with a median of 31.99 kg/m2. The gender distribution was balanced, with 56% female and 44% male patients. The mean age was 60.14 ± 9.85, with a median of 60.65. Revision surgeries were performed for several reasons, including loosening (6/11), infection (2/11), acetabular osteolysis (2/11), and acetabular fracture (1/11). Regarding implant characteristics, the median values for implant size, number of sacroiliac joint screw holes, ilium screw holes, ischium screw holes, and flanges were 64 mm, 14, 7, 2, and 3, respectively. The mode values were also similar, with 64 mm, 18, 8, 2, and 3, respectively. The majority of implants (10 out of 11) were made of titanium, while one implant was composed of chromium. One of the cages was broken. Scratching was observed in some implants, mainly occurring during surgery. Fretting was observed in only 2 implants and ¼ Higgs score was assigned4, and evidence of burnishing was found in one implant. No corrosion was observed in any of the implants.
This study investigates the in vivo performance of pelvic implants utilized in the treatment of pelvic discontinuity for total hip arthroplasty (THA). Loosening was the predominant reason for revision surgeries. The examined implants were predominantly intact, exhibiting infrequent occurrences of fretting and burnishing, along with surgery-related scratches. The study reveals diverse implant designs featuring varying screw-hole configurations in different regions, emphasizing the importance of patient-specific implants. Additionally, the presence of different flange numbers and locations further underscores the need for customized implant solutions.
Figures

Figure 1#8483
Assessment of Friction of Ceramic-on-Ceramic Hip Resurfacing Arthroplasty
*Gregory Pryce - Instite of Biogical and Medical Engineering - University of Leeds - Leeds, United Kingdom
Andrew Robert Beadling - University of Leeds - Leeds, United Kingdom
Danielle De Villiers - MatOrtho Ltd - Leatherhead, United Kingdom
Simon Collins - MatOrtho Limited - Leatherhead, United Kingdom
Michael Bryant - University of Leeds - Leeds, United Kingdom
*Email: G.M.Pryce@leeds.ac.uk
INTRODUCTION: Hip resurfacing arthroplasties (HRAs) typically have better clinical function in younger and more active male patients, this includes lower rates of dislocation and more regular gait compared to conventional total hip arthroplasties (THAs) [1]. However, the bearings of HRA’s are commonly manufactured from metals, which have been reported to increase the risk of metal ion release [2]. Clinical use of ceramic in THA has increased over the last decade [3], due to its good wear performance. Considering this, using a ceramic-on-ceramic (CoC) HRA may improve implant survivorship, as it eliminates the risk of ion release. The aim of this study was to assess friction of a CoC resurfacing during gait and jogging.
MATERIALS & METHOD: A ProSim Full-ISO electromechanical three-station hip simulator (SimSol, UK) was used with 64mm diameter (n=3) CoC HRAs (MatOrtho, UK). The acetabular cups were orientated at 30° inclination, and femoral heads were mounted vertically. Test lubricant used was foetal-bovine serum diluted with deionised water to a protein concentration of 30g/L, and sodium-azide (0.03%w/v) and ethylenediaminetetraacetic acid (EDTA, 3.1g/L) was added to prevent bacterial growth. The lubricant was heated to 37°C to replicate the conditions in-vivo. Kinematic and kinetic inputs of gait (ISO-14242) were applied for 2 million-cycles, followed by physiological jogging for 1 million-cycles. The jogging profile was derived from motion capture and multi-body musculoskeletal modelling [4]. Six-axis load cells, secured above the cup, were used to assess forces and torques at the bearing interface. Data was analysed using a custom MATLAB script (MathsWorks, USA) to determine frictional torques (FTs) during the testing in part reference to the methodology presented by Haider et al [5].
RESULTS: For both gait and jogging profiles, FT was observed to show a similar trend to the applied axial force. Peak FT occurred at the maximum applied axial force, whereas the lowest FT was determined at the lowest applied axial force (Fig.1A-B). Mean peak FT increased with increasing number of test cycles for both regimes, with jogging FT increasing at a higher rate.
CONCLUSIONS: The same trend between FT and applied axial force was observed by Haider et al. [5] with metal-on-polyethylene THAs, however the magnitude of FT during gait was greater in this study. This may be due to the different bearing combination and larger head diameter. Varying the inputs from gait to physiological jogging influenced the FT at the bearing interface. High FTs have been linked to higher wear; however, the FTs observed were of similar magnitude to other metal-on-metal HRAs [5]. The higher peak FT observed with jogging compared to gait, could result in higher wear clinically. Considering this, further investigation should be conducted to include activities of daily living, as this could impact friction at the bearing, especially in a HRA population who are likely to be more active than a THA cohort.
REFERENCES: [1]Marshall et al., Clin OrthopRelat Res 2013; [2] Shimmin et al., JBJS 2008; [3] National Joint Registry., 2023; [4] Lunn et al., J.Arthroplasty 2020; [5] Haider et al., J.Engineering in Medicine 2016.
#8608
Similar Physical Demands of Performing Arthroplasty Surgery and Clinic
*Gloria Coden - Bronx, United States of America
James Penn Miller - Tufts University School Of Medicine - Boston, USA
Lauren Schoeller - New England Baptist Hospital - Boston, USA
Carl Talmo - New England Baptist Hospital - Boston, USA
*Email: gscoden@gmail.com
Introduction: Assessing the long-term physical demands of a high-volume arthroplasty surgeon is beneficial to preserve longevity of a surgeon’s career, allowing surgeons to care for the maximum number of patients. While previous studies have described the physical toll of performing surgery, few studies have evaluated the physical demands of seeing patients in clinic, which often accounts for up to 60% of a surgeon’s clinical time. We sought to compare the physical demands of arthroplasty surgery compared to evaluating patients in the outpatient clinic.
Methods: We prospectively recorded the heart rate, respiratory rate, minute ventilation, cadence, and energy expenditure of a single fellowship-trained arthroplasty surgeon while seeing patients in the outpatient clinic (n=5 days) and while performing arthroplasty surgery at an ambulatory surgery center (n=28 days). All surgeries performed were either primary total hip or total knee arthroplasties. Chi-square and independent-samples t-tests were used to compare cohorts. Significance was set at p<0.05.
Results: The surgeon spent similar time each day at the outpatient clinic and at the ambulatory surgery center (399.0 versus 368.3 minutes, p=0.4). Cadence was 11.7 times higher when the surgeon was in the outpatient clinic compared to when the surgeon was performing surgery at the ambulatory surgery center (3.5 versus 0.3 steps per minute, p<0.001). Respiratory Rate was 1.5 times higher when operating at the ambulatory surgery center compared to seeing patients in the outpatient clinic (16.3 versus 10.9 respirations per minute, p<0.001). Minute ventilation was 2.2 times higher in the operating room at the ambulatory surgery center compared to in the outpatient clinic (20.7 versus 9.3 liters per minute, p<0.001). Heart rate (82.4 versus 79.1 beats per minute, p=0.1) and energy expenditure per minute (3.7 versus 3.5 Calories per minute, p=0.6) were similar while performing surgery at the ambulatory surgery center compared to seeing patients in outpatient clinic.
Conclusion: Overall, the physical demands of performing surgery at an ambulatory surgery center and treating patients in the outpatient clinic were similar. Surgeons should be aware of the high physical demands of both performing surgery and treating patients in the outpatient clinic, and further research is needed to identify techniques to increase the longevity of a surgeon’s career.
#8609
Higher Surgeon Energy Expenditure Associated With Performing Total Hip Arthroplasty Compared to Total Knee Arthroplasty
*Gloria Coden - Bronx, United States of America
James Penn Miller - Tufts University School Of Medicine - Boston, USA
Lauren Schoeller - New England Baptist Hospital - Boston, USA
Carl Talmo - New England Baptist Hospital - Boston, USA
*Email: gscoden@gmail.com
Introduction: Previously published studies have hypothesized that total hip arthroplasty (THA) requires the surgeon to expend more energy that total knee arthroplasty (TKA). However, techniques for performing these procedures have evolved, new technology is being used, and procedures are being performed more efficiently. Therefore, we sought to compare if primary THA had increased energy expenditure compared to primary TKA.
Methods: We prospectively recorded the heart rate, respiratory rate, minute ventilation, cadence, and energy expenditure of a single fellowship-trained arthroplasty surgeon over 26 days. Each day, the surgeon performed a combination of 5 primary THAs and TKAs. Patient demographics and location of surgery were retrospectively reviewed to evaluate differences in the physical demands of each surgical case. Chi-square and independent-samples t-tests were used to compare cohorts. Significance was set at p<0.05.
Results: Similar numbers of THA and TKA were performed at the inpatient hospital and the ambulatory surgery center (p=0.2). Age (p=0.5) and gender (p=0.6) were similar between patients who had a THA and TKA. Patients who had a TKA had on average a 1.1 times higher body mass index compared to THA (31.0 versus 28.1 kilograms/meter2, p=0.003). THA tended to have 1.2 times longer operative time compared to TKA (98.9 versus 84.0 minutes, p<0.001). THA had a statistically higher heart rate compared to TKA, although this is unlikely to be clinically significant (83.8 versus 80.7 beats per minute, p<0.001). Respiratory Rate was 1.1 times higher when performing THA compared to TKA (16.2 versus 15.1 respirations per minute, p<0.001). Minute ventilation was 1.2 times higher with THA than TKA (20.9 versus 18.0 liters per minute, p<0.001). Cadence was 1.6 times higher when performing TKA compared to THA (3.3 versus 2.1 steps per minute, p<0.001). THA had a 1.3 times higher energy expenditure per patient (388.0 versus 289.7 Calories per patient, p<0.001) and a 1.1 times higher energy expenditure per minute (3.9 versus 3.4 Calories per minute, p<0.001) compared to TKA.
Conclusion: THA is associated with longer operative time (98.9 versus 84.0 minutes, p<0.001) and increased energy expenditure per minute (3.9 versus 3.4 Calories per minute, p<0.001) compared to TKA. Despite THA and TKA procedures becoming more efficient, the physical burden on the surgeon has not significantly changed. Further research is needed to understand ways to decrease surgeon energy expenditure and promote career longevity.
#8765
Increased Patient Body Mass Index Is Associated With Increased Surgeon Physiologic Stress During Total Hip Arthroplasty
*Itay Ashkenazi - NYU Langone Health - New-York, United States of America
Ittai Shichman - NYU Langone Health - New York, USA
Alana Prinos - Langone Orthopedic Hospital - New York, USA
Jonathan Katzman - NYU Langone - New York, United States of America
Claudette Lajam - NYU Hospital For Joint Diseases - New York, USA
Ran Schwarzkopf - NYU Langone Medical Center Hospital for Joint Diseases - New York, USA
Joshua Rozell - NYU Langone Orthopedic Hospital - New York, USA
Kyle Lawrence - NYU Orthopedic Hospital - New York, USA
*Email: itay.ashkenazi@gmail.com
Introduction
While increased body mass index (BMI) in patients undergoing total hip arthroplasty (THA) increases surgical complexity, there is a paucity of objective studies assessing the impact of patient BMI on the cardiovascular stress experienced by surgeons during THA. Furthermore, the majority of the 22-modifiers appended to the operative Current Procedural Terminology code, which indicate increased operative work, are due to patient obesity. The aim of this study was to assess the impact of patient BMI on surgeon cardiovascular strain during THA.
Methods
We prospectively evaluated three fellowship-trained arthroplasty surgeons performing a total of 115 THAs. A smart-vest worn by the surgeons recorded mean heart rate, stress index (correlate of sympathetic activation), respiratory rate, minute ventilation, and energy expenditure throughout the procedures. Patient demographics as well as perioperative data including surgical approach, surgery duration, number of assistants, and the timing of the surgery during the day were collected. Linear regression was utilized to assess the impact of patient characteristics and perioperative data on cardiorespiratory metrics.
Results
Average surgeon heart rate, energy expenditure, and stress index during surgery were 98.50 beats/minute, 309.49 calories/hour, and 14.10, respectively. Higher patient BMI was significantly associated with increased hourly energy expenditure (slope: 4.71; standard error: 2.10, P=0.027), mean heart rate (slope: 0.24; standard error: 0.11, P=0.037), and stress index (slope: 0.08; standard error: 0.04 P=0.027) independent of surgical approach. Respiratory rate and minute ventilation were not associated with patient BMI. The number of assistants and time of surgery during the day did not impact cardiorespiratory strain on the surgeon.
Conclusion
The physiologic burden on surgeons during primary THA significantly increases as patient BMI increases. Current and future reimbursement models should account for higher physical strain on surgeons as an indicator for case complexity and difficulty.
Figures
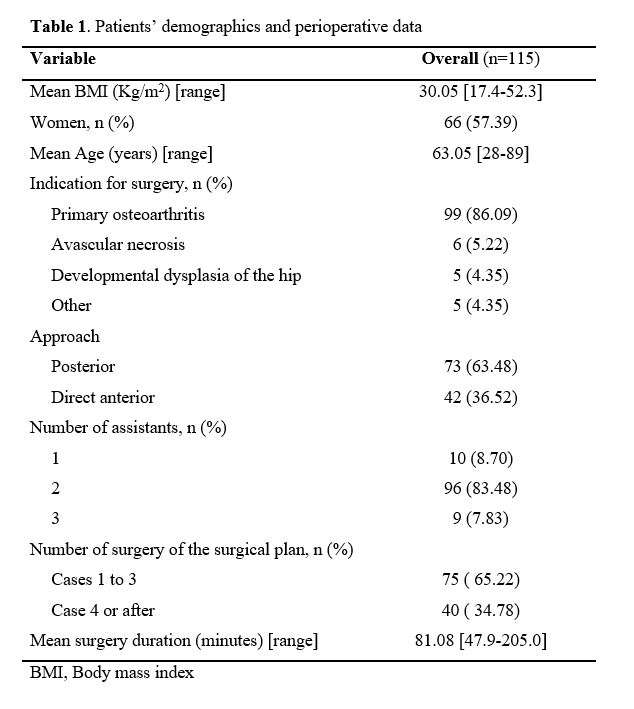
Figure 1
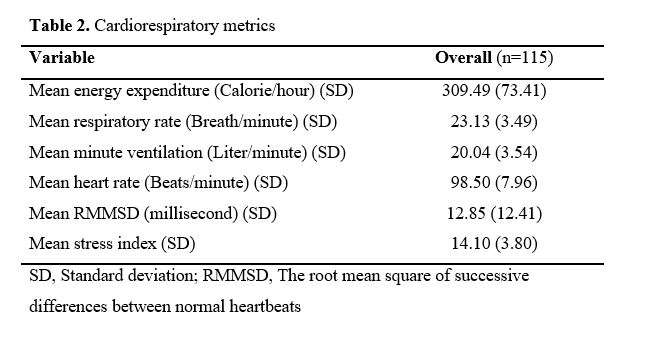
Figure 2
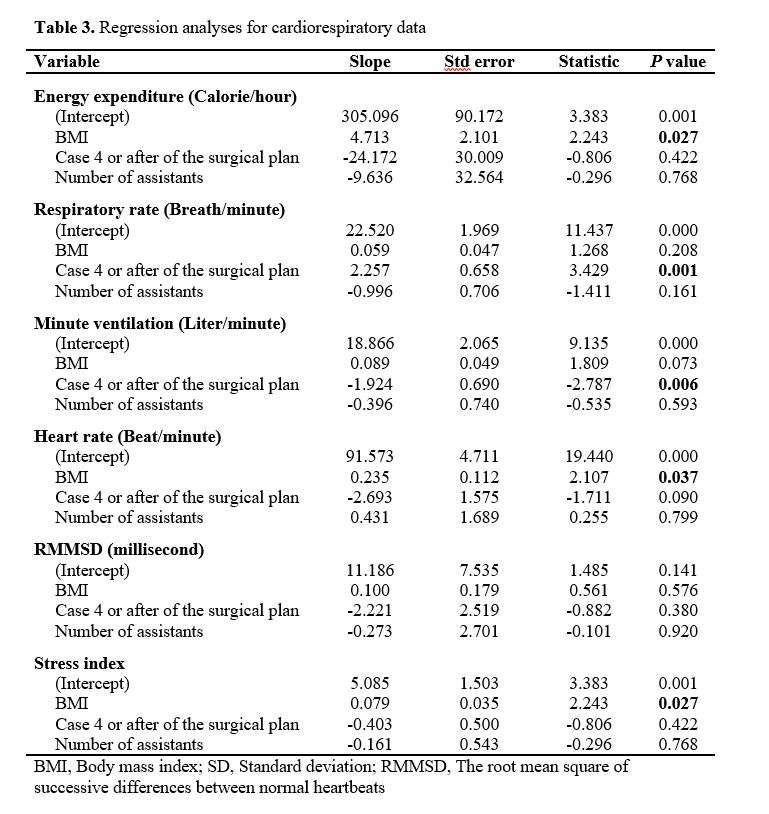
Figure 3#8604
Similar Surgeon Energy Expenditure in the Inpatient Hospital and Ambulatory Surgery Center Setting
*Gloria Coden - Bronx, United States of America
James Penn Miller - Tufts University School Of Medicine - Boston, USA
Lauren Schoeller - New England Baptist Hospital - Boston, USA
Carl Talmo - New England Baptist Hospital - Boston, USA
*Email: gscoden@gmail.com
Introduction: Recently, arthroplasty surgery has begun shifting from being performed in the inpatient hospital setting to being performed at ambulatory surgery centers. With ever increasing pressure to improve surgical efficiency, few studies have evaluated how the increased efficiency associated with ambulatory surgery centers effects the arthroplasty surgeon. We sought to compare the physical demands of performing arthroplasty surgery between an ambulatory surgery center and in the inpatient hospital setting.
Methods: We prospectively recorded the heart rate, respiratory rate, minute ventilation, cadence, and energy expenditure of a single fellowship-trained arthroplasty surgeon over 13 days each in the inpatient hospital setting and at an ambulatory surgery center. On all 26 days, a combination of 5 total hip arthroplasties (THA) and total knee arthroplasties (TKA) were performed. Patient demographics were retrospectively reviewed to evaluate differences in the physical demands of each surgical case. Chi-square and independent-samples t-tests were used to compare cohorts. Significance was set at p<0.05.
Results: Similar numbers of THA and TKA were performed at the inpatient hospital and the ambulatory surgery center (p=0.1). While older patients were 1.6 times more likely to have surgery at the inpatient hospital than at the ambulatory surgery center (67.4 versus 61.1 years, p<0.001), the distribution of gender (p=0.1) and body mass index (p=0.9) was similar between locations. The operative time was 1.4 times longer at the inpatient hospital than at the ambulatory surgery center (105.4 versus 77.7 minutes, p<0.001). However, the overall time spent at the hospital or ambulatory surgery center was similar (550.4 versus 562.7 minutes, p=0.6). Energy consumption per minute (3.6 versus 3.7 Calories per minute, p=0.7) and per day (1986.2 versus 2116.7 Calories per day, p=0.5) was similar at each location.
Conclusion: Despite similar patient populations, the surgical operative time at the inpatient hospital was significantly longer than at the ambulatory surgery center. This is likely due to improved efficiencies in the ambulatory surgery center setting. Over the entire day, the surgeon spends similar amounts of time in the hospital and surgery center and expends similar amounts of energy each day. Further research is needed to understand ways to decrease surgeon energy expenditure and promote career longevity.
#8253
Assessment of Color Sensor Technologies for Potential Wound Care Estimation
*Kevin Abbruzzese - Stryker - Mahwah, USA
Andre Freligh - Stryker Orthopaedics - MAHWAH, United States of America
Vincent Alipit - Stryker - Mahwah, USA
*Email: kevin.abbruzzese@stryker.com
Introduction:
Color sensing technologies can detect and measure the color of an object or surface based on the intensity of red, green, and blue wavelengths. This information can be converted to a digital representation of color and quantify spectrum of colors. This may aid in telehealth applications where qualitative assessments often rely on visual perception. Wound infection assessment by the classical clinical signs, such as redness, heat, swelling, and pain revealed to be of limited reliability when contrasted with a time-consuming wound sampling [1]. For remote assessment of wounds, the ability to accurately and reliably detect and monitor wound color can be an important tool for infection observation. A camera based virtual reality application with a color sensor was evaluated to determine the accuracy of color measurements observed in wound care.
Methods:
RGB measurements were obtained from Nix Mini 2 Color Sensor, TCS34725 RGB Sensor, and a virtual reality interface using the Azure Kinect camera using a standard set of BEHR® paint swatches, Table 1. Colorimetric data was assessed to determine the difference between two colors based on Euclidean distance, chromaticity, and Euclidean chromaticity measurements from acquired RGB intensity values ranging from 0-255 [3]. Euclidean distances were calculated as the square root of the difference of the sum of the squares of the RGB values. Chromaticity estimates were considered to determine the differences between colors based on colorfulness (hue and saturation). Euclidean distances for chromaticity values were estimated to determine device accuracy.
Results:
An Anderson-Darling test was used to assess normality for respective measurements. Euclidean data was normally distributed and an ANOVA test was used to assess significance between Euclidean distance data. Significant differences (p<0.005) were detected between the Nix color sensor (20.76±7.04), the TCS34725 sensor (100.4±53.4), and the Azure Kinect (55.74±22.87), Figure 1. The Nix color sensor resulted in significantly smaller Euclidean distances compared to the Kinect and TCS34725 sensor. The Azure Kinect resulted in significantly smaller error compared to the TCS34275 sensor. A Kruskal Wallis test was used to assess control and device chromaticity values. No significant differences were detected in chromaticity values between devices for red and green values, Table 2. Azure Kinect camera reported significantly higher values compared to all other groups.
Conclusion:
Three devices were used to assess RGB colors associated with wound care and evaluated based on Euclidean distances and chromaticity values. The Azure Kinect Camera and TCS sensor resulted in greater errors in color detection compared to the Nix color sensor. Camera based systems may be more susceptible to lighting conditions and result in less accurate color measurements with high blue light interference. Further research is needed to better understand conditions that can affect camera-based techniques and means to minimize artifact. Filters or post processing correction techniques may provide improvements in color measurements and would be independent of the camera. Applications like wound care assessment that require a higher degree of accuracy and precision may benefit from a color sensor to avoid artifact interference and provide greater color estimates among color spaces.
Figures
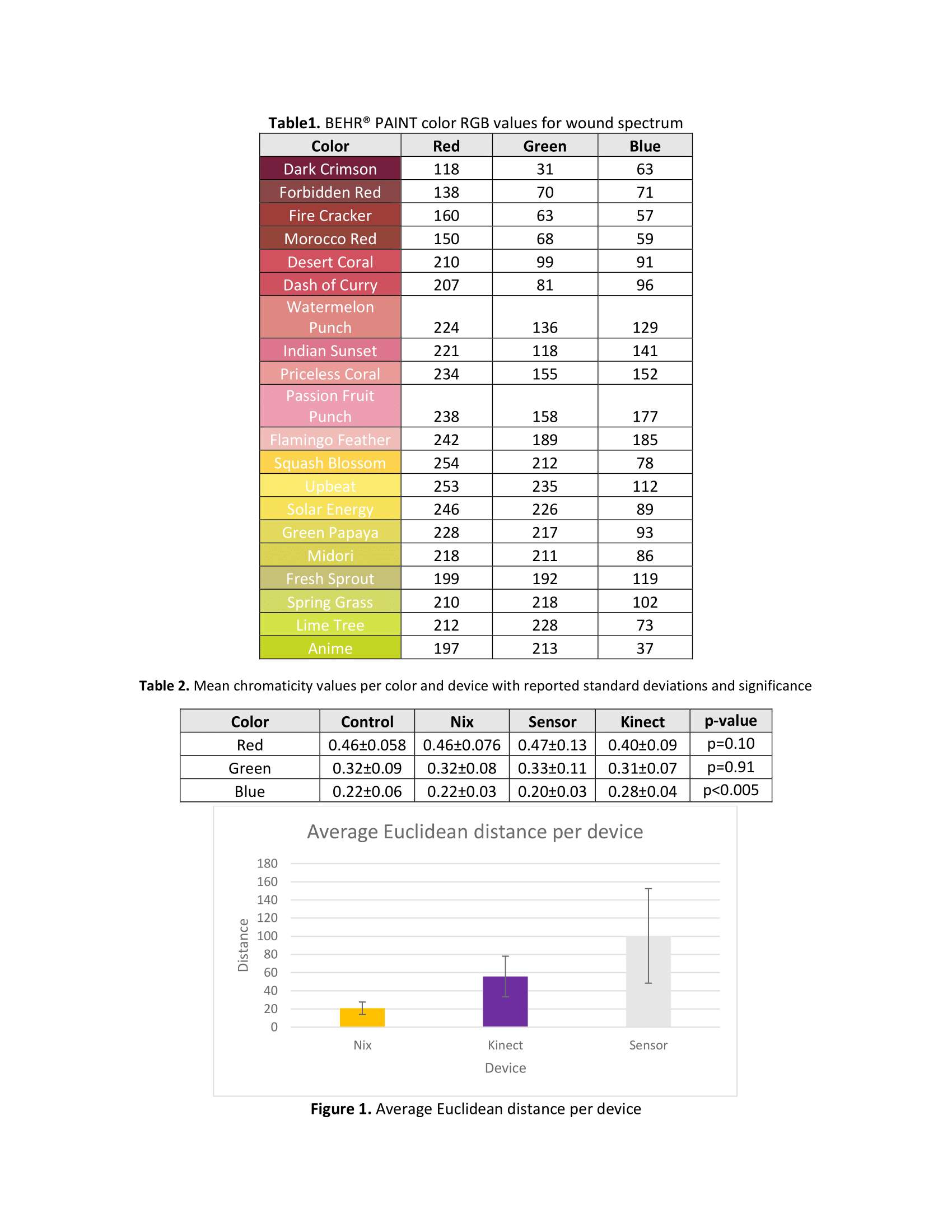
Figure 1#8647
Non-Invasive Active Acoustics as Biomarkers of Early Periprosthetic Joint Effusions, a Cadaveric Study
*Rahul Goel - Emory University - Atlanta, USA
Quentin Goossens - KULeuven - Leuven, Belgium
Trask Crane - Georgia Tech - Atlanta, USA
Göktu? Cihan Özmen - Georgia Tech - Atlanta, USA
Omer Inan - Georgia Tech - Atlanta, USA
Ajay Premkumar - Hospital for Special Surgery - New York, USA
*Email: rgoel09@gmail.com
Non-invasive Active Acoustics as Biomarkers of Early Periprosthetic Joint Effusions, a Cadaveric Study
1. INTRODUCTION
Knee joint effusions in the setting of total knee arthroplasty (TKA) may be associated with several different clinical entities, including prosthetic joint infection (PJI) and aseptic loosening, the two most common indications for TKA revision. There is a lack of available simple and operator independent non-invasive tools to detect small (<60 cc) effusions within the knee joint and characterize these effusions. This work presents a novel application of non-invasive active acoustics (AA) to identify, characterize, and potentially monitor periprosthetic joint effusions.
2. METHODS
Seven fresh frozen hip-to-toe cadavers (80.6 ± 10.2 years, BMI 26.0 ± 4.9 kg/m2) with knee replacements were included in this study. These included three cruciate retaining, three posterior stabilized TKA and one unicompartmental knee arthroplasty. Effusions were simulated by injecting 20mL increments (80mL max) of saline and bacteria (methicillin sensitive staphylococcus aureus) solutions into the joint space. AA were recorded after each injection by broad band exciting the tibia with a miniature shaker. Transducers attached to the skin measured the input force and output acceleration 2.2cm proximal to the input. The normalized spectral band power (BP) of the input / output frequency response functions was calculated.
3. RESULTS
The BP at 1255 ± 228 Hz was highly correlated (Pearson’s r = 0.83, Figure 1) with the injected volumes over all specimens (n = 33). Within specimen correlations varied between 0.80 and 1.00. The 20 cc volume BP was higher than the baseline (0 cc) (p = 0.03). The BP was on average lower for bacteria solution compared to saline solution, however not significantly (p > 0.05).
4. DISCUSSION and CONCLUSION
This cadaveric study demonstrates the potential utility of AA to quantify extremely small (20 cc) effusions in patients with total knee arthroplasties. The use of AA to discriminate between bacteria and saline effusions demonstrates promise but requires mores data to be confirmed.
#8612
Functional Metric to Support Identification of Anomalous Recovery in Remotely Monitored TKA Patients
*Ricardo Antunes - Stryker - Glasgow, GB
Paul Jacob - Oklahoma Joint Reconstruction Institute - Oklahoma City, USA
Robert Marchand - Ortho Rhode Island - South County, USA
Elaine Justice - Oklahoma Joint Reconstruction Institute - Oklahoma City, USA
Kelly Taylor - Ortho Rhode Island - South County, USA
Matthias Verstraete - Stryker - Fort Lauderdale, USA
*Email: ricardo.antunes@stryker.com
Patients are increasingly monitored remotely through a variety of technologies collecting physiological, activities and PROM data. This is also true in orthopedics where a variety of connected technologies and wearable sensors have come to market in the last years, including several targeting post-operative recovery. These technologies collect data on several parameters at higher granularity and reduced staff costs than can be achieved traditionally. Despite these advantages, the potentially large data volumes generated by these systems may increase the demand for clinical staff resources to process and derive insights and/or clinical actions from it. The potential increased demand for data interpretation by clinical staff may be ameliorated by using machine learning techniques that support clinical decisions driven by patient data. In the context of remote patient monitoring, these techniques can be used to appropriately summarize the state of a patient from multiple parameters and assess against normative models of recovery. We developed a score that summarizes the state of multiple patient metrics along the first 90 days of recovery following TKA surgery, that can be used to track the patient’s functional recovery using a single score.
Following IRB approval and completing the consent process, clinical data was collected from 101 patients undergoing primary total knee arthroplasty using the MotionSense wearable sensors along with the MotionSense® mobile application (Stryker, Mahwah, NJ). This system allows remote monitoring by clinicians of patients’ range of motion, step count, knee active time, and prescribed exercise completion, as well as pain scores and surveys. From the patient collected parameters, the mean and daily maximum number of steps, knee active time, cadence and range of motion were selected to be summarized into a functional knee score. The parameter values were used as input to a neural network that was trained to maximize the signal to noise ratio across the first 12 weeks of recovery.
The network training resulted in score values that on average increase through the recovery period (Figure 1). Despite not being fitted with information about the timing of the measurements, the variation of the score values captured recovery progression in the data, with larger week-to-week changes in the first 5 weeks of recovery. Generally, the deviation of a patient’s score from the mean trend can be used to assess the state of a patient’s recovery against the population level expectation.
The proposed metric allows aggregating multiple patient parameters into a single functional metric that can be easily monitored while reducing data monitoring load from practice staff.
Figures
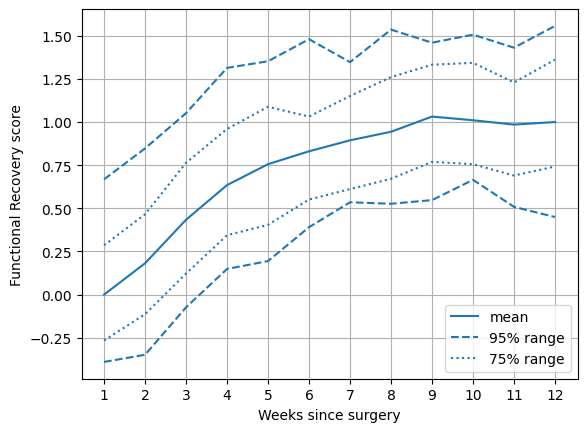
Figure 1#8519
Efficient and Non-Intrusive Knee Joint Loading Assessment Using Markerless Motion Capture During Walking Gait to Distinguish Knee Osteoarthritis Severity
*Jereme Outerleys - Queen's University - Kingston, Canada
Anastasija Mihic - Queen's University - Kingston, Canada
Jacob Calderone - Queen's University - Kingston, Canada
Vajra Keller - Queen's University - Kingston, Canada
Elise Laende
Kevin Deluzio - Queen's University - Kingston, Canada
*Email: jereme.outerleys@queensu.ca
INTRODUCTION
Joint loading patterns of the knee in the frontal and sagittal planes can discriminate knee osteoarthritis (OA) severity1,2, have been linked to disease progression3, and are targets for treatment. Joint moments are typically calculated using inverse dynamics with kinematic inputs from marker-based motion capture. Recent advances in markerless motion capture technology can measure kinematics that show high agreement with marker-based data with improved inter-session repeatabilty4. Reduced clothing constraints and shorter collection times associated with markerless methods are clear advantages for large-scale and longitudinal clinical investigations. The objective of this study was to investigate whether knee joint moments calculated using kinematic inputs from markerless motion capture can distinguish knee OA severity.
METHODS
Subjects were recruited from an orthopaedic clinic. Knee OA severity was determined clinically by advanced practice physiotherapists. Subjects with severe and moderate medial knee OA were included, yand a group of asymptomatic controls. Subjects walked back and forth across a 10m-walkway embedded with 4 force plates (AMTI, 1200 Hz) for 60s at self-selected walking speed in their own clothing and footwear. Video data were captured by 8 cameras (OptiTrack, 1080p, 60 Hz) digitally synchronized with force data. A calibration chessboard placed in a known location relative to each force plate aligned video and force coordinate systems. Video data were processed using Theia3D (Theia Markerless, v2021.3.02047) to obtain 3D kinematics from an inverse kinematics model with 3 degrees of freedom at the ankle, knee, and hip. The data were lowpass filtered at 8 Hz for kinematics and 50 Hz for force. Inverse dynamics was used (Visual3D, v2023.02.1) to obtain internal knee flexion and adduction moments resolved in the joint coordinate system and normalized by body mass. Moments were time normalized to stance phase and ensemble averaged over at least 3 strides. Principal component analysis extracted the first two modes of waveform variability1 (PCs), and ANOVAs with post-hoc comparisons (Tukey HSD) were used to test for differences across severity in PCs and gait speed (α < 0.05).
RESULTS AND DISCUSSION
Forty-two subjects were included in the analysis (24 severe, 10 moderate, 8 control). Groups were well matched in age (Table 1). PC1 captured overall waveform magnitudes while PC2 captured the difference between early and late stance. A severity effect was detected in PC2 for both the knee flexion and adduction moments (Table 1). The severe and moderate groups had stiffer flexion moment patterns compared to controls (Figure 1). The severe group also displayed a more sustained adduction moment pattern compared to moderates and controls. Gait speed decreased with severity and contributes to differences in kinetic outcomes in these populations. These data agree with previous work1,2 that report decreased sagittal moments and more sustained loading patterns in the frontal plane, with increasing levels of knee OA severity.
CONCLUSIONS
Kinematics from markerless motion capture produce joint loading outcomes that can distinguish between knee OA severities. This demonstrates the clinical utility of markerless motion capture by replicating clinical findings reported in the literature and more globally provides additional validity of the technology.
#8554
Feasibility and Repeatability of a Novel in-Clinic Markerless Motion Capture System for Arthroplasty Patient Gait Analysis
*Stephanie Civiero - Dalhousie University - Halifax, Canada
Michael Dunbar - Dalhousie University - Halifax, Canada
Glen Richardson - Dalhousie University & Capital Health - Halifax, Canada
Annemarie Laudanski - Dalhousie University - Halifax, Canada
Janie Wilson - Dalhousie University - Ancaster, Canada
*Email: s.civiero@dal.ca
INTRODUCTION: Total knee arthroplasty (TKA) patients have significant pre-operative deficits in functional gait mechanics1, and post-operatively, many patients do not achieve healthy gait function2. Therefore, objectively-measured variability in gait function must be explored to inform surgical decision-making and optimize functional outcomes. The industry standard for human gait analysis is laboratory-based optoelectronic motion capture, which has limitations regarding clinical uptake and participant recruitment, retention, and throughput. The purpose of this study was to design and evaluate a motion capture system and clinical gait analysis protocol within a clinical orthopaedic environment to efficiently measure key kinematic outcomes in gait for TKA surgical planning.
METHODS: The orthopaedic clinic space available for motion capture was a busy clinical hallway (20’long by 8’ wide). The camera number and placement, choice of system, and mounting considerations were all optimized to capture overground human gait kinematics. To enhance recruitment and minimize clinical disruptions, data collections could be no longer than 5 minutes, and the chosen system was required to be low-profile and easily set up/removed. To evaluate the system repeatability, a sub-study was completed, wherein gait data from 20 healthy participants (12F/8M) were collected on three separate visits. Knee joint angles relevant to TKA patient gait kinematics were calculated for each subject2-4 (Visual3D, C-motion). Standard deviations of the inter-session and -trial errors for each measure were calculated. Clinical protocol feasibility assessment is ongoing, with the recruitment of participants awaiting TKA occurring from participating surgeons’ wait lists. Mean and standard deviations of arthroplasty-relevant kinematic gait measures for this group were calculated and compared to outcomes from lab-based gait protocols in similar clinical populations for feasibility.
RESULTS: An innovative, laboratory-validated markerless gait analysis system5, which pairs optical motion capture with an AI-based human pose estimation algorithm (Theia3D, Theia Markerless), was chosen and ten magnetically mounted cameras (Sony, RX0ll) were installed in the identified hallway in the Halifax Infirmary orthopaedic clinic. This system met our criteria for time efficiency, minimal patient burden, and location flexibility. Preliminary inter-trial and inter-session repeatability errors for 9 healthy participants (6F/3M) reveal a maximum of 2.88? and 3.22? in the sagittal- and frontal-plane kinematic metrics respectively (Table 1). Initial clinical feasibility results from 9 pre-TKA patients (Figure 1) visually showed decreased range of motion (RoM) in knee flexion during both stance- and swing-phases and increased knee adduction RoM in stance when compared to the healthy adults (Figure 1). Reported kinematic values were also compared to literature values for arthroplasty patients1,6 (Table 2).
CONCLUSIONS: The installed markerless motion capture system and developed clinical gait protocol produced repeatable kinematic data comparable to those from laboratory-based protocols, and clinical arthroplasty gait outcomes comparable to literature1,6. We are therefore confident using the system for clinic-based analyses of knee kinematics in patients pre-TKA. to develop data-driven models for informing patient-specific surgical planning.
References
1Astephen et al., 2008 J.Orthop. Res. 26(3);332-341
2Hatfield et al., 2011 J.Arthroplasty 26(2);309-318
3Bonnefoy-Mazure et al., 2017 J.Arthroplasty 32(3);793-800
4Outerleys et al., 2021 JAB 37(2);130-138
5Kanko et al., 2021 J.Biomech. 127;110665
6Nagano et al., 2012 Knee 19(5);628-632
Figures
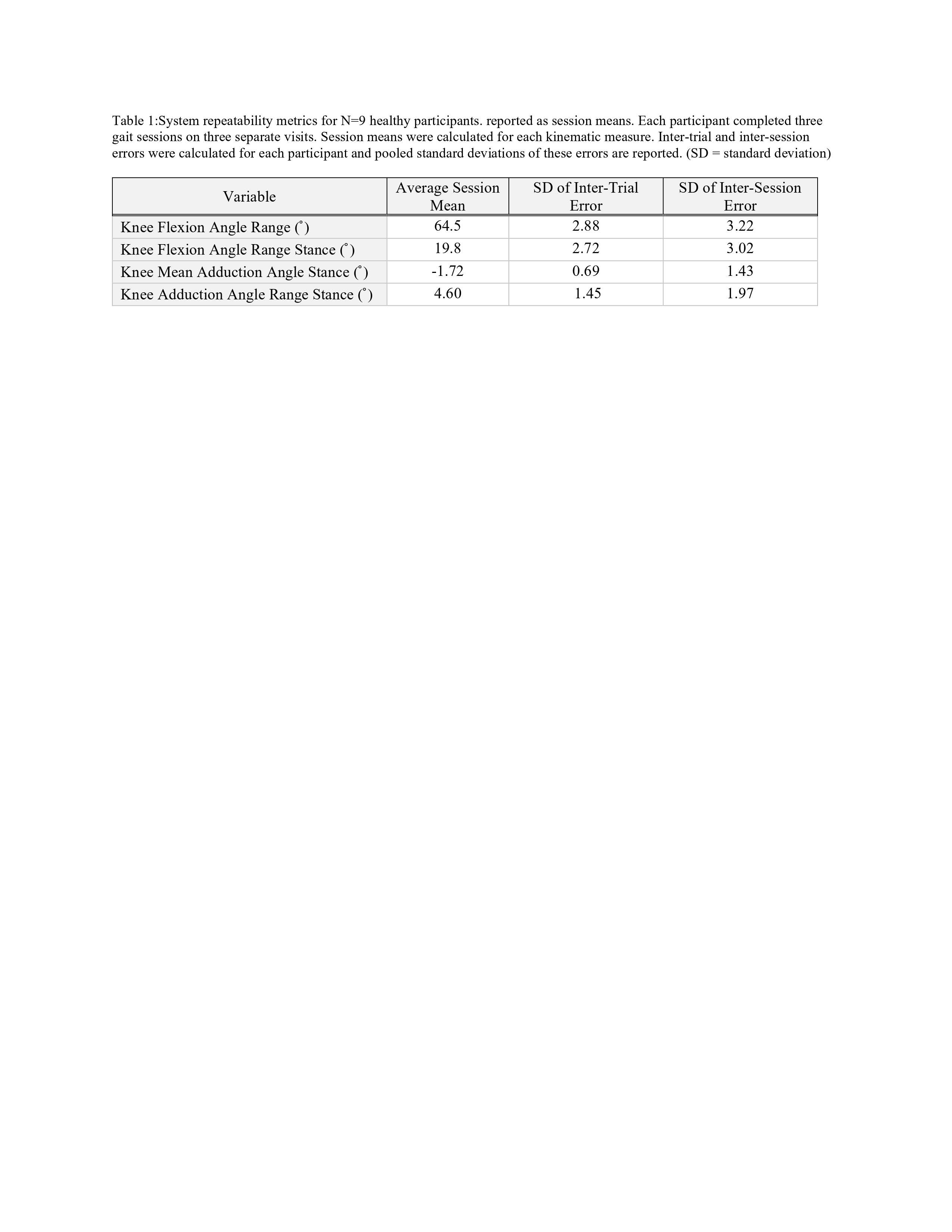
Figure 1
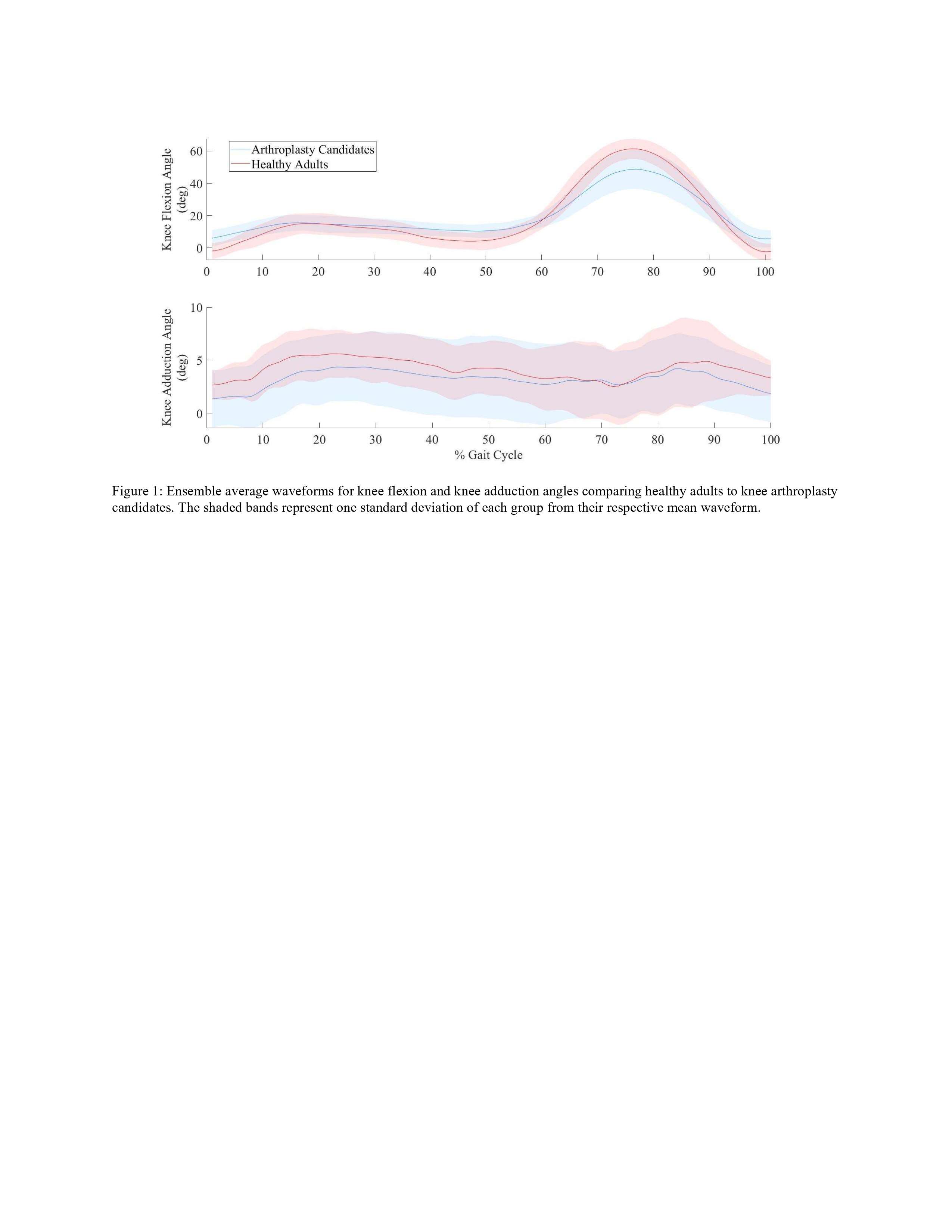
Figure 2
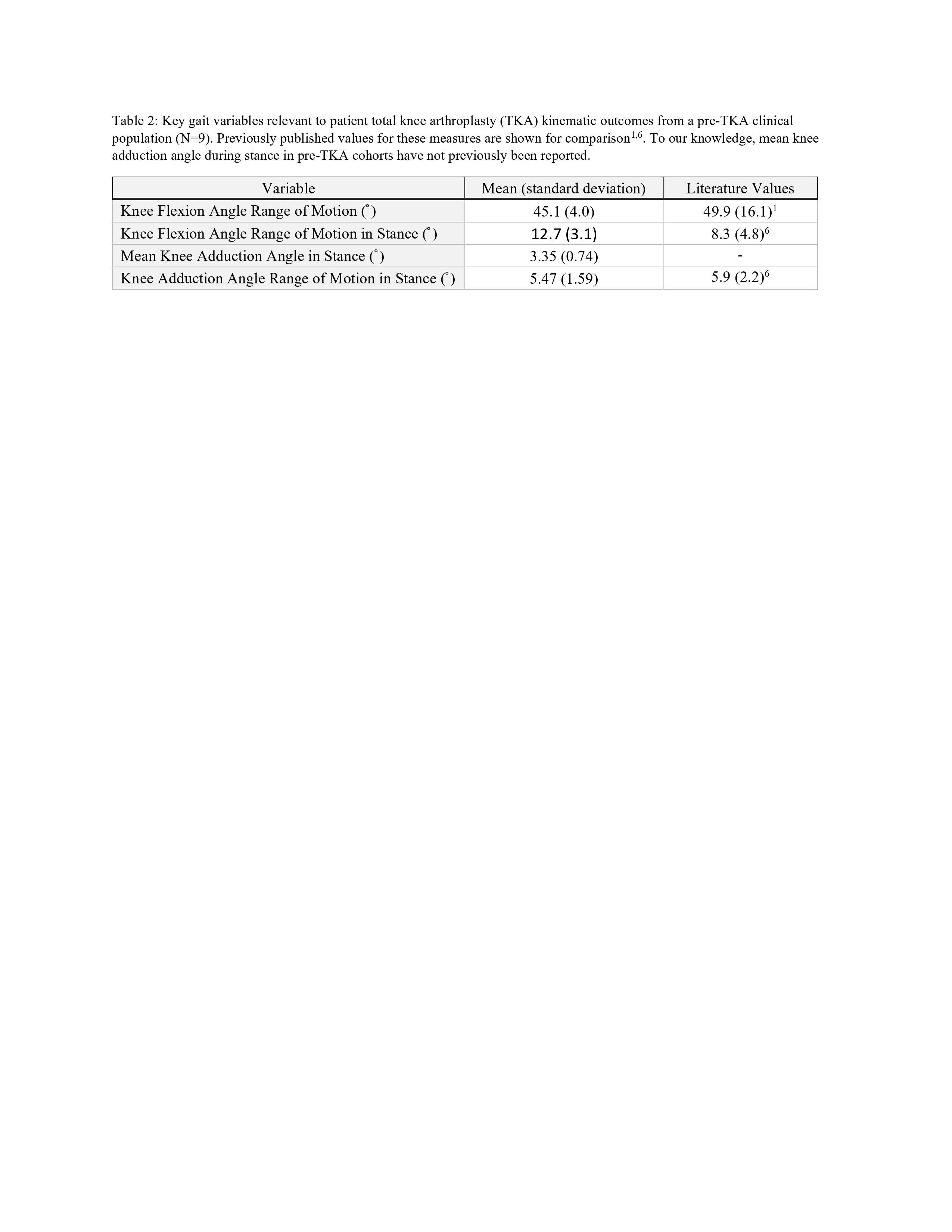
Figure 3#8558
Biomechanical Changes in Gait Kinematics Pre- and Post-TKA Detected With Markerless Motion Capture
*Elise Laende - Queen's University - Kingston, Canada
Jereme Outerleys - Queen's University - Kingston, Canada
Kevin Deluzio - Queen's University - Kingston, Canada
*Email: elise.laende@queensu.ca
Introduction
The use of biomechanical data to inform clinical decision making in orthopaedics has been rare, largely due to practical limitations in obtaining motion capture data. Traditional marker-based motion capture systems are resource- and time-intensive with significant data collection burden for patients. Markerless motion capture technology using computer vision and machine learning approaches to obtain biomechanical data from standard video images offers significant advantages for ease of data collection, but must demonstrate the ability to produce relevant outcome metrics in a clinical population. The objectives of our pilot study were to (1) determine the feasibility of using markerless motion capture technology to record kinematics during a variety of tasks in an orthopaedic population by examining the task completion by participants and ability of the markerless system to track body segments and (2) to compare gait waveform data collected on a severe knee osteoarthritis (OA) population to a control group, to replaced knee joints, and to historical marker-based data for similar groups.
Methods
Orthopaedic patients with knee OA were recruited directly from an orthopaedic assessment clinic after referral to an orthopaedic surgeon for total knee arthroplasty. Participants wore the clothes and shoes they had worn that day. Severe OA patients (n=77) performed functional tasks during markerless motion capture: timed up-and-go (TUG), stair ascent and descent, quiet standing (balance), walking at self-selected speed, and fast walking. Previous knee replacements in the contralateral leg were analyzed separately (n=17). The control group consisted of members of the community over 50 years of age (n=29). Markerless motion capture was performed using 8 commercially available video cameras (Sony RX0-II) recorded at 60 Hz and processed using Theia3D (Theia Markerless Inc.). Historical data using a marker-based motion capture system for severe OA post-knee replacement, and control subjects were obtained from a previous publication.
Results
The severe OA group was 56% female with mean age 69 years (SD 8). Kinematic data from the markerless system was calculated for all participants for all completed tasks with no discernable tracking issues. The control group (mean age 58 years, 59% female) had a self-selected walking speed of 1.3 m/s compared to 0.9 m/s for the severe OA group. Joint angle waveform data captured with the markerless system show similar patterns to historical data collected with marker-based motion capture for severe OA, post-knee replacement, and control groups (Figure 1).
Conclusion
This study demonstrated feasibility of using markerless motion capture on an orthopaedic population directly from a clinic visit with no restrictions on clothing. Kinematic data from markerless motion capture exhibited expected kinematic deviations based on historical marker-based gait data on similar populations. The ease of data collection and the standardized calculation of biomechanical metrics have important implications for clinical implementation as well as longitudinal and multi-centre studies.
Figures
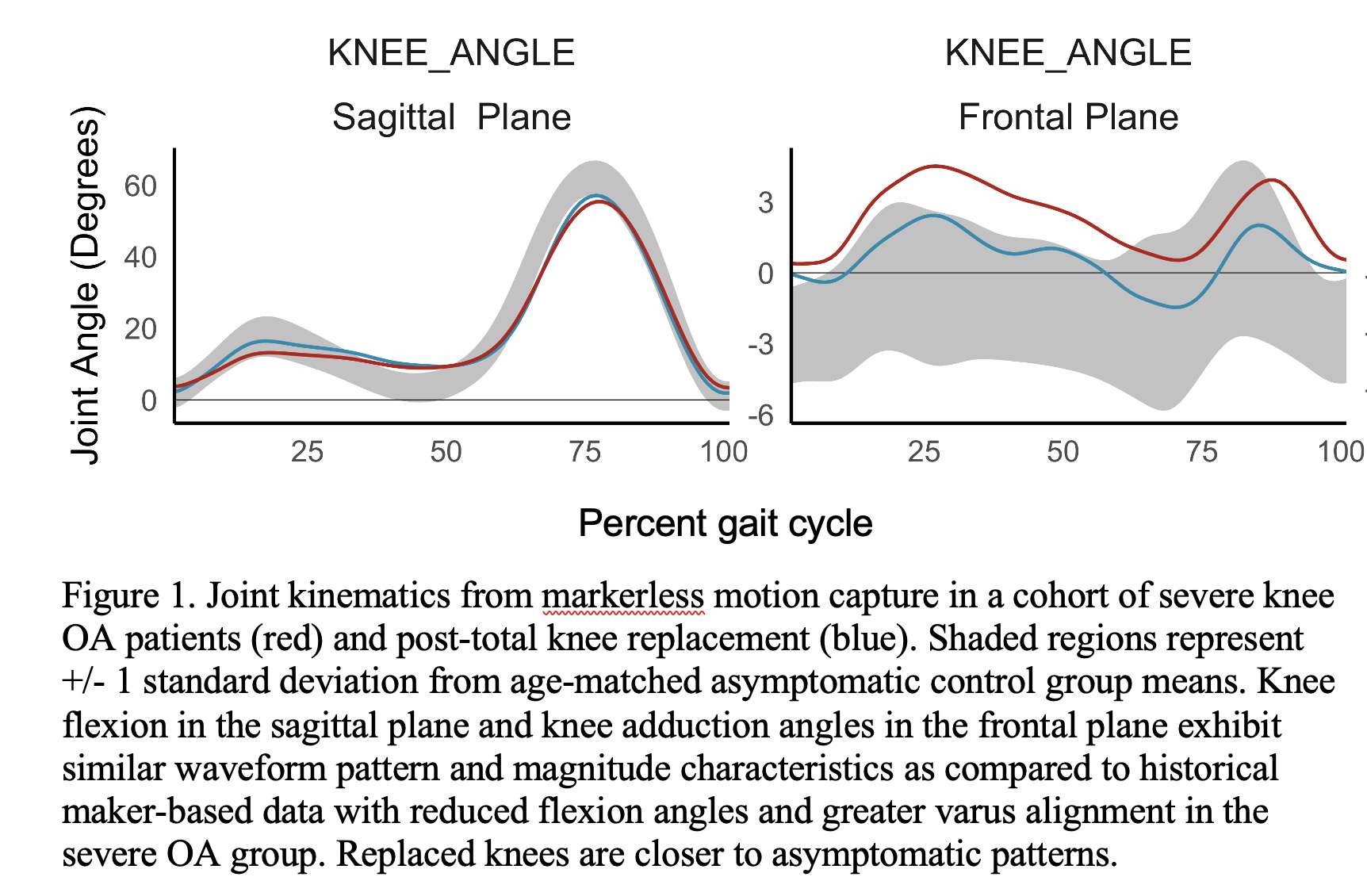
Figure 1#8299
Operative Time Associated With Increased Length of Stay After Single-Level Cervical Disc Arthroplasty: An Analysis of 3,681 Surgeries
*Mitchell Ng - Maimonides Medical Center - Brooklyn, United States of America
Nikhil Vasireddi - Case Western Reserve University School of Medicine - Cleveland, USA
Ahmed Emara - Cleveland Clinic - Cleveland, USA
Aaron Lam - Maimonides Medical Center - Brooklyn, USA
Nicholas Ahn - UH Cleveland Medical Center - Cleveland, USA
Amrit Khalsa - Perelman School of Medicine, University of Pennsylvania - Philadelphia, USA
John Houten - Maimonides Medical Center - Brooklyn, USA
Ahmed Saleh - Maimonides Medical Center - Brooklyn, USA
Afshin Razi - Maimonides Medical Center - Brooklyn, USA
*Email: mitchng77@gmail.com
Introduction: Cervical disc arthroplasty (CDA) most commonly serves as an alternative to anterior cervical discectomy and fusion (ACDF) to treat cervical spine disease, intended to decrease adjacent segment disease and secondary procedures. CDA acts as an attractive alternative to anterior cervical discectomy and fusion (ACDF), with the potential to preserve cervical mobility and minimizing the risk of developing adjacent segment disease (ASD). Nevertheless, with only 1,600 CDAs performed annually relative to the 132,000 ACDFs, CDA is considered a relatively novel procedure. The purpose of this study was to: 1) determine whether longer CDA operative time increases risk of 30-day post-operative complications; 2) analyze the association between operative time and subsequent healthcare utilization (length of stay (LOS); and 3) discharge disposition.
Methods: This was a retrospective study, identifying patients who underwent single-level CDA between January 2012 and December 2018 using the American College of Surgeons National Surgical Quality Improvement (ACS-NSQIP) database. Operative time was defined as duration of surgeon from initial skin incision to skin closure. The primary outcome of the study was healthcare utilization, determined through length of post-operative hospital stay (≤2 days vs. >2 days), discharge disposition (home vs non-home), 30-day re-operation or unplanned readmission. Secondary outcomes were measured through medical complications including any wound complications, infections, deep venous thrombosis, pulmonary embolism, and urinary tract infection. Differences in baseline patient demographics were identified through univariate analysis. Multivariate logistic regression was performed to identify associations between operative time (reference: 81-100 minutes) and healthcare utilization and medical/surgical outcomes, controlling for patient demographics.
Results: A total of 3,681 cases were performed during the time period of interest. Mean patient age was 45.52 years, with a mean operative time of 107.72±49.6 minutes. Higher odds of length of stay was demonstrated starting with operative time category 101-120 minutes (Odds ratio (OR): 2.164, 95% confidence interval (CI): (1.247 - 3.754), p=0.006), however not among discharge destination, 30-day unplanned readmission, or re-operation. There was no significant higher odds of any wound complication, infection, pulmonary embolism, deep venous thrombosis, or urinary tract infections associated with increased operative time (p>0.05).
Conclusion: Prolonged CDA operative time above the reference 81-100 minutes is independently associated with increased length of stay, however no other significant healthcare utilization parameters such as discharge disposition, re-admission or re-operation. Of note, there was no association between prolonged operative time and 30-day medical/surgical complications including wound complications, infections, pulmonary embolism, or urinary tract infection. As shorter surgeries are attractive for both logistic and financial reasons, it is important for surgeons to be aware they may be associated with more efficient subsequent healthcare utilization by patients as well.
#8577
Topology Optimization of Spinal Implants Under Physiologic Loads
*Richard Barina - University of Waterloo - Waterloo, Canada
Mihaela Vlasea - University of Waterloo - Waterloo, Canada
Stewart McLachlin - University of Waterloo - Waterloo, Canada
*Email: rbarina@uwaterloo.ca
Introduction: Instrumented spinal fusion using metallic implants is a common surgical procedure to stabilize the spinal column following injury and disease; however, high implant stiffness of the interbody cage can cause improper load sharing resulting in adjacent segment degeneration [1]. Metal additive manufacturing (AM) offers the potential to address this challenge using topology optimization (TO) to minimize the structural material required for a given loading scenario. This light-weighting design strategy can also reduce implant stiffness. However, there is currently no consensus on TO design strategies for spinal interbody cage implants due to a lack of direct comparison of the available TO techniques and limited experimental data to inform the design space optimization. The goal of this study was to compare single versus multi-objective TO design strategies for spinal implants under axial and multi-axial (physiologic) loading.
Methods: Physiologic loads to establish the boundary conditions for implant TO under axial and multi-axial loading were obtained from instrumented vertebral body replacements for flexion activities from the OrthoLoad online database [2]. Interbody cage implants with dimensions of 11Hx12Wx16L mm were then created for the available design space (Figure 1). Altair Inspire™ (Troy, MI) and nTopology (New York, NY) were used to perform single-objective (mass minimization with a safety-factor) and multi-objective (stress and displacement minimization) TO of the design space established between the two endplate surfaces. Deformation and stress analysis compared axial and multi-axial loading scenarios.
Results: Single (Figure 2) and multi-objective (Figure 3) TOs both produced tree-like implant designs with a small amount of compliance (maximum of 0.34mm displacement). This design keeps internal stresses below 50% yield strength while adhering to AM feature size constraints, which ensures the implants can be printed using metal AM. Both strategies show improved uniformity in the deformation. Further, multi-objective optimization (Figure 3) produced geometrically complex conically oriented struts, exhibiting more branching than the single-objective optimization. The multi-axial load case (Figure 2, b) generated diagonal struts along the circumference of the implant instead of mostly vertical struts as in the axial only case, likely to account for the added bending and shear loads on the implant.
Conclusion: This was a first attempt to look at improving spinal implant design using different TO strategies. New biomechanically informed design guidelines can be drawn from the results to improve future implant designs. The TO introduced organic branching structures to distribute axial loads, minimizing the stress of the superior endplate and evenly distributing the displacement. Because of these emerging structures, multi-objective TO can be more effective than single-objective TO at tailoring the desired mechanical response of a spinal implant. Furthermore, small off-axis loads (on the scale of 10 N) can drastically change the design of the implant and thus must be considered for robust implant design. Future design work will examine TO results from multiple patients and loading conditions associated with other daily activities.
References:
[1] Y. A. Othman et al. Ann. Transl. Med., vol. 7, no. Suppl 5, p. S170, Sep. 2019
[2] “Database 2017 «?OrthoLoad.” https://orthoload.com/database-2017/ (accessed Dec. 17, 2022).
Figures
Figure 1
Figure 2
Figure 3#8420
In-Situ Wear and Corrosion Performance Assessment of Mixed-Material Spinal Instrumentation Implant Systems Under Simulated Inflammatory Conditions
*Philipp Keller - University of Leeds - Leeds, GB
Richard Hall - University of Leeds - Leeds, United Kingdom
Andrew Robert Beadling - University of Leeds - Leeds, United Kingdom
Michael Bryant - University of Leeds - Leeds, United Kingdom
*Email: mnpke@leeds.ac.uk
Posterior spinal instrumentation, typically consisting of mixed-material cobalt-chromium and titanium alloy pedicle screw-rod assemblies, are modular components widely used to treat a range of spinal pathologies. However, evidence of wear and corrosion, also described as fretting-corrosion, has been reported at rod-screw junctions [1,2]. Micro-motion can cause the release of wear debris and corrosion by-products into the patient's body. Wear debris remains a clinical concern due to associated inflammation of periprosthetic spinal tissue leading to early surgical intervention and implant revision. This study aimed to develop a controlled fretting-corrosion experimental setup, to assess the influence of simulated inflammatory (SI) conditions on the wear and corrosion performance of commercially available spinal hardware.
A bespoke fretting tribometer with in-situ corrosion measurement has been deployed to investigate fretting between CoCrMo rods and unfastened Ti6Al4V screws. The test setup incorporated a 3-electrode corrosion cell, accommodated the rod-screw assembly, and ensured full immersion throughout testing. A total of 250000 cycles with a displacement of ±120µm were completed at 1Hz. Open circuit potential (OCP) and intermittent linear polarisation resistance measurements were recorded to quantify corrosion. Testing was conducted in two electrolytes at 37±2°C: Phosphate Buffered Saline (PBS) solution pH 7.4 as control and PBS+30mM Hydrogen Peroxide (H2O2) solution. The latter to replicate corrosive periprosthetic SI conditions. The material loss was determined gravimetrically. The wear scars were examined employing digital microscopy and scanning electron microscopy with energy-dispersive X-Ray spectroscopy.
Upon immersion, an increase in OCP was recorded for both electrolytes (Fig.1). An ennoblement in OCP from -0.04V to 0.32V was observed in H2O2 presence, confirming its oxidising effect [3]. Once fretting was initiated, a decrease to -0.4V in PBS and a protracted gradual decline to about -0.57V in PBS+H2O2 indicated mechanically induced surface damage. After fretting ceased, a recovery in OCP was observed; quicker in the PBS solution. In both electrolytes, the rod-screw contact operated within gross-slip regime, characterised by quasi-rectangular fretting loops and affirmed by energy area ratio values between 0.6 to 0.8 [4]. The gravimetric rod material loss was substantially increased from 221±35µg to 732±12µg with H2O2 addition (Fig.2). Likewise, the mass loss of the screw increased from 26±16µg and 37±28µg, respectively. The material loss patterns and corrosive rod discolouration appeared similar to those in clinical retrievals [2,5].
Differences in the implants' fretting corrosion performance were observed for two tested electrolytes. The presence of H2O2 significantly increased the corrosive degradation of the rod-screw interface. Based on the OCP data, hindered re-passivation of the rod-screw interface during fretting could be assumed. In accordance, the results revealed greater material loss in PBS+30mM H2O2 compared to PBS. Future work will analyse variations in screw tightening torque and the released wear debris to further the implications of fretting corrosion on spinal instrumentation.
ACKNOWLEDGEMENTS:
Funding from European Union's Horizon-2020 programme, Marie Sk?odowska-Curie grant No812765.
Implants provided by ChM sp.z o.o.
REFERENCES:
[1] Ayers et al., JMaterSciMaterMed, 2017., [2] Panagiotopoulou et al., JBiomedMaterResB, 2018., [3] Liu et al., JBiomedMaterResB, 2017., [4] Fouvry et al., ZAMM, 2000., [5] Singh et al., Spine, 2013.
Figures
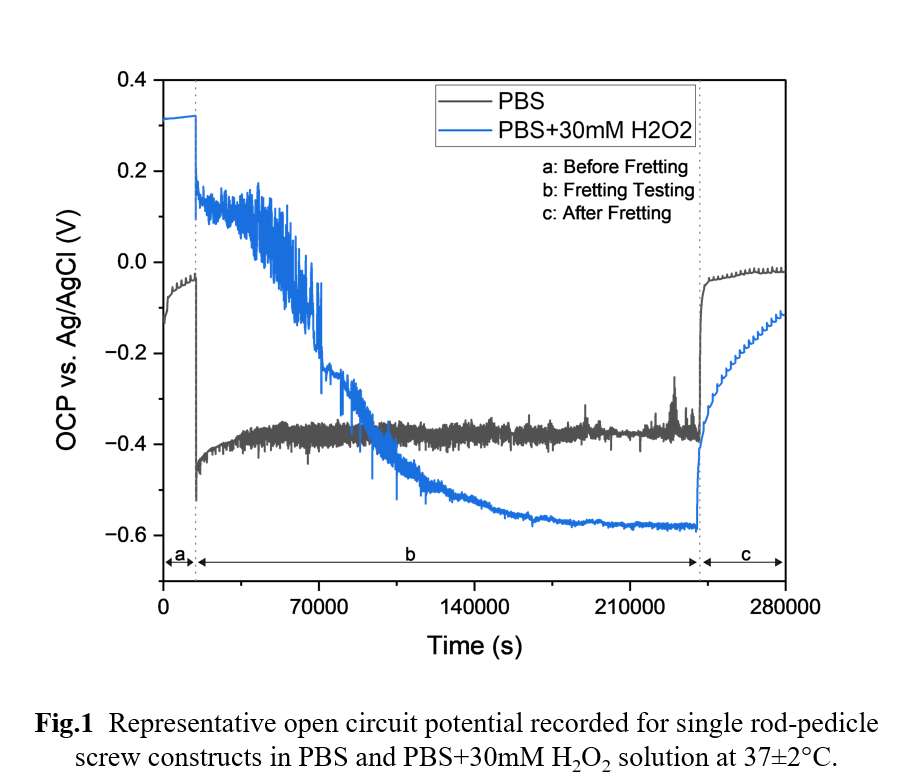
Figure 1
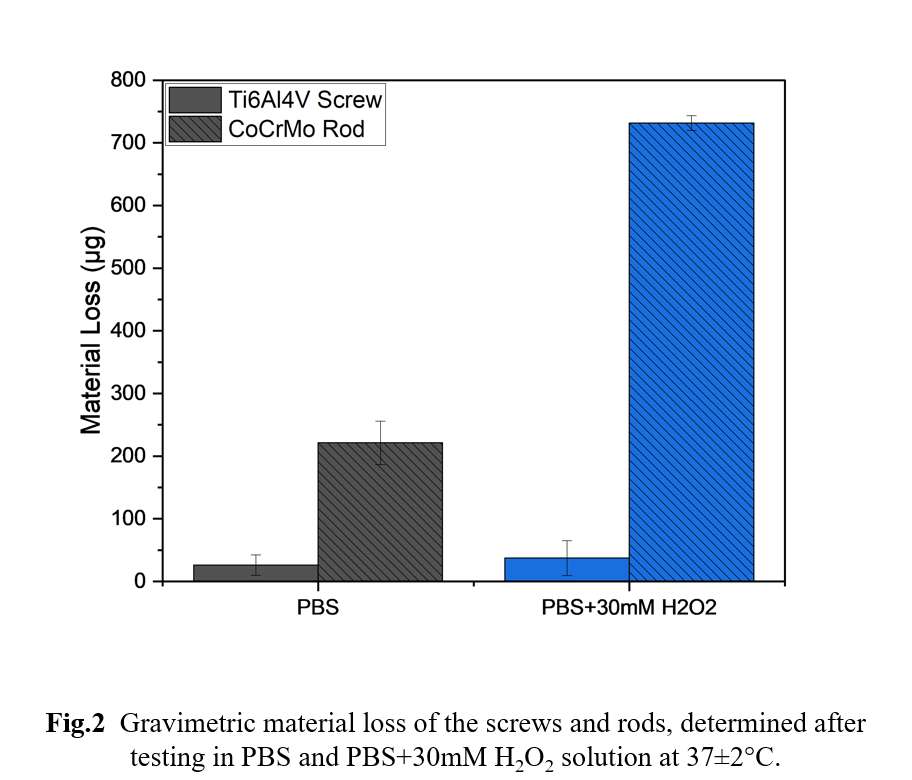
Figure 2
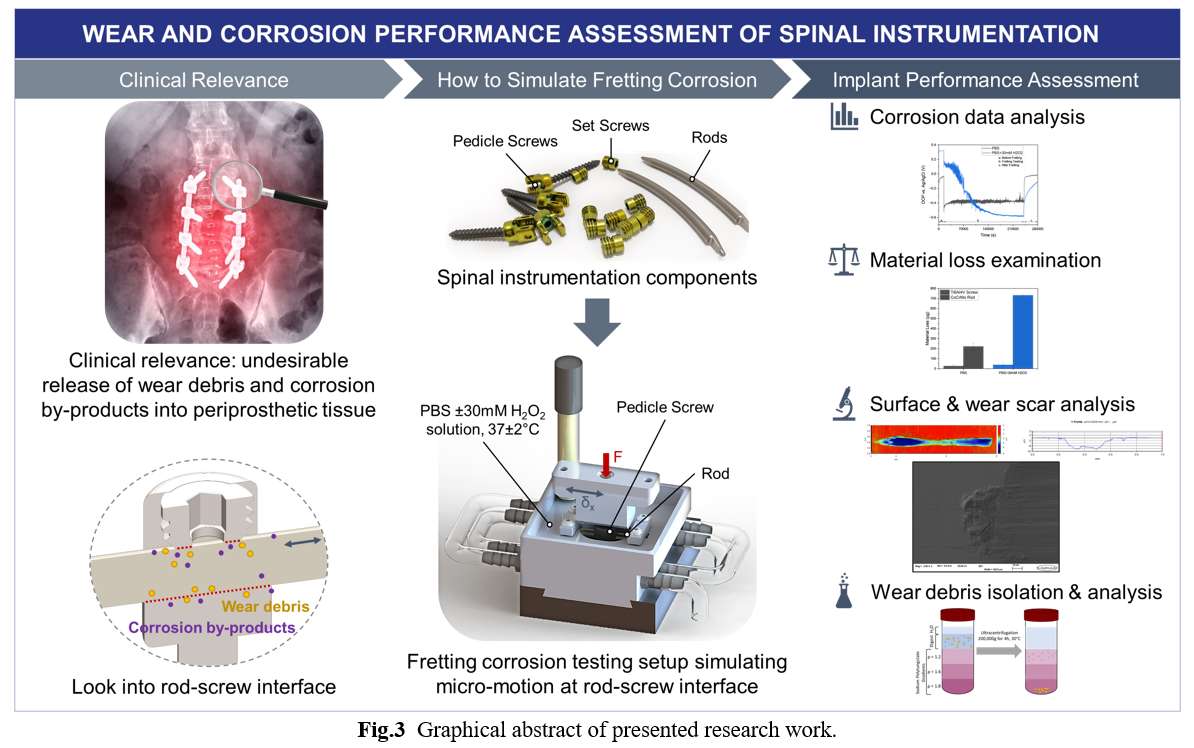
Figure 3#8497
Sneak Peak at a Comprehensive Retrieval Analysis of 200 Cervical Disc Replacements
Jenna Wahbeh - University of California, Los Angeles - Los Angeles, United States of America
Sang-Hyun Park - Orthopaedic Institute for Children /UCLA - Los Angeles, USA
Pat Campbell - Los Angeles, USA
Edward Ebramzadeh - Orthopaedic Institute for Children/UCLA - Los Angeles, USA
*Sophia Sangorgio - Orthopaedic Institute for Children/UCLA - Los Angeles, USA
*Email: ssangiorgio@mednet.ucla.edu
Introduction: The M6-C cervical disc replacement (OrthoFix, Plano, TX) has been implanted in over 70,000 patients worldwide since 2006; however, limited data has been presented on the long-term clinical outcome. All explanted devices received worldwide through post-market surveillance were inspected by an independent academic research center to identify patient-related, surgery-related, and device-related modes of failure. The goal of the present study was to identify the most common reasons for removal and to determine the role, if any, that multi-level surgery contributed to outcome.
Methods: A total of 216 M6-C devices from 187 patients were received by the retrieval center between 2016 and 2023. Devices with no clinical, radiographic, or surgical information, or devices that were first-generation device design were excluded. De-identified demographic information was collected and each device was analyzed through visual examination, photographic documentation, and analytical measurements. The surgeon-stated reason for revision was recorded. The cervical level and the number of levels treated with a disc replacement (single, 2-level, 3-level) and/or a hybrid procedure (TDR + 1 or more fusion(s)) was recorded. Visual and photographic documentation for all components focused on the mechanical condition, specifically distinguishing extraction damage from in vivo damage, and assessment of fixation status at the time of removal. Radiographic observations were included where available. Reasons for revision and levels treated were correlated with time to removal.
Results: Of the 216 devices received, 23 failed to meet the inclusion criteria. The final cohort included 193 devices from 171 patients implanted between 2009 and 2021 and removed between 2011 and 2023. Most devices were from single-level total disc replacement (TDR) procedures, followed by bi-level TDR only (Table 1), followed by hybrid procedures. The presence of an adjacent level device or fusion was not correlated with time to revision (P>0.10, Figure 1). Bony ongrowth and other indications of fixation were observed in 94.7% of devices. Mechanical device failure, osteolysis, and infection were the most common surgeon-reported reasons for removal (Figure 2). Devices that were removed due to infection were removed at a median time of 37 months in vivo, while devices removed for osteolysis were removed after a median time of 80 months.
Conclusions: The present study is the first large-scale retrieval analysis of cervical disc replacements of the same design. The results indicated that the number of levels treated with disc replacement and/or fusion, did not influence the time to removal. Further, the majority of the devices in the present study were assessed to be fixed at the time of removal. This is in contrast to the currently available data published in the MAUDE Database, which indicates that migration and/or loosening is the most common reason for TDR removal. It is important to note that the 216 devices received by the explant laboratory represent <1% of the total number of devices that have been implanted over the last 17 years. Nevertheless, this study provides valuable insights into the long-term performance of the M6-C device and highlights the need for ongoing retrieval analysis.
Figures
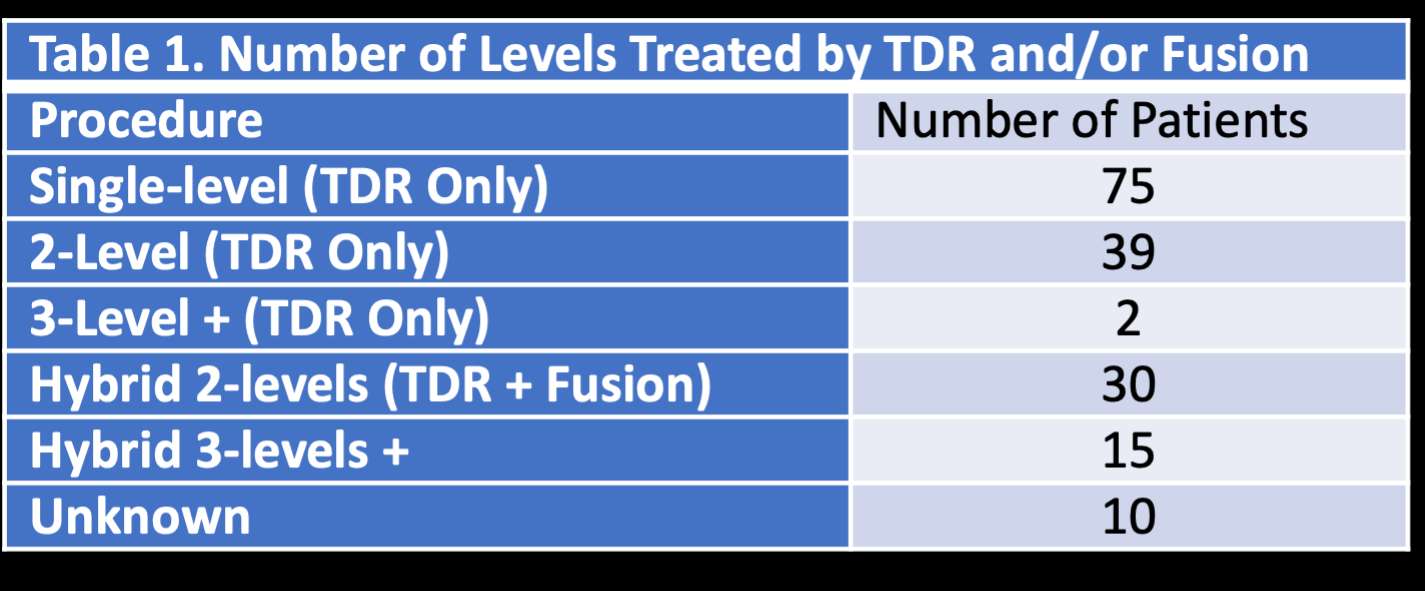
Figure 1
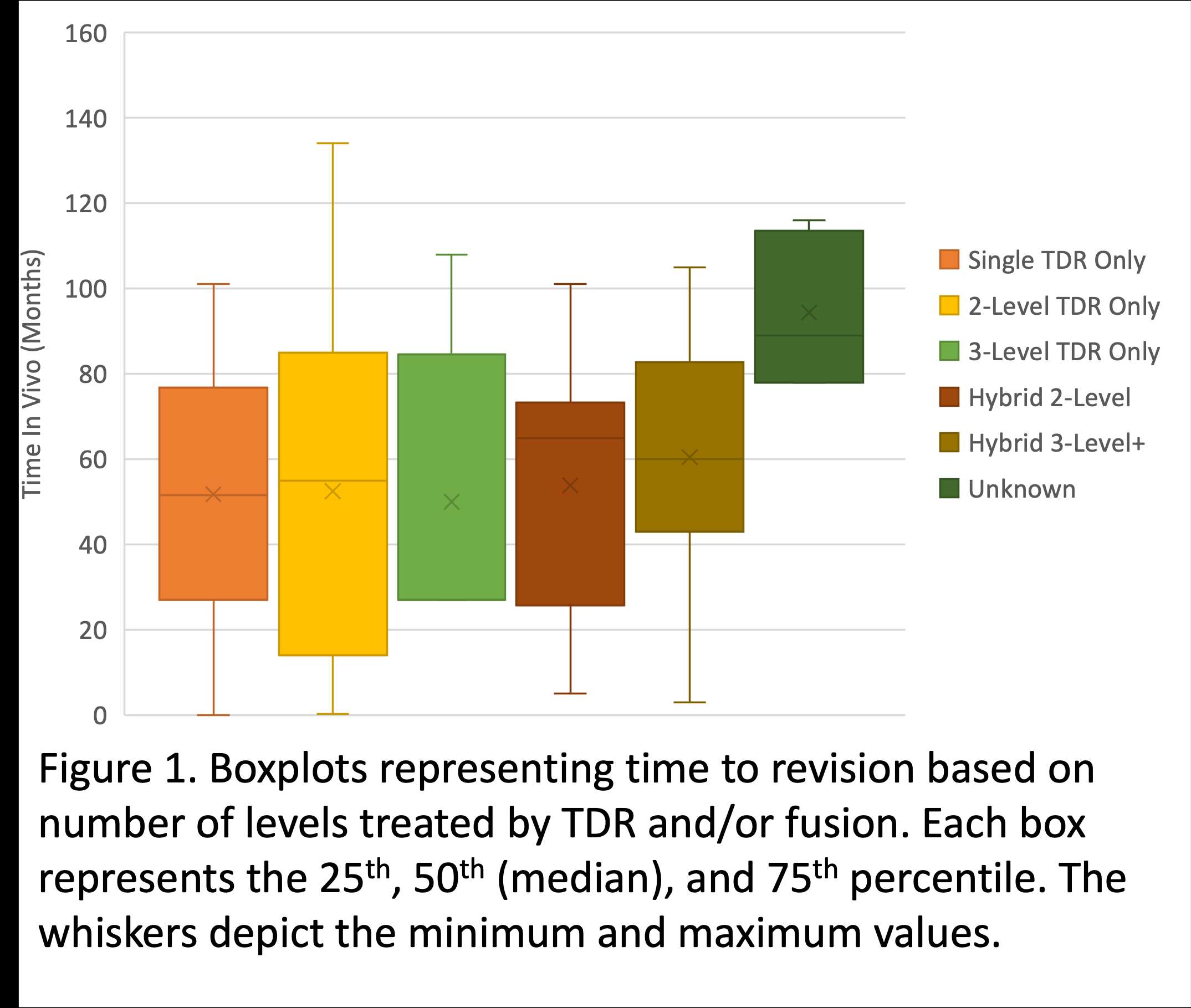
Figure 2
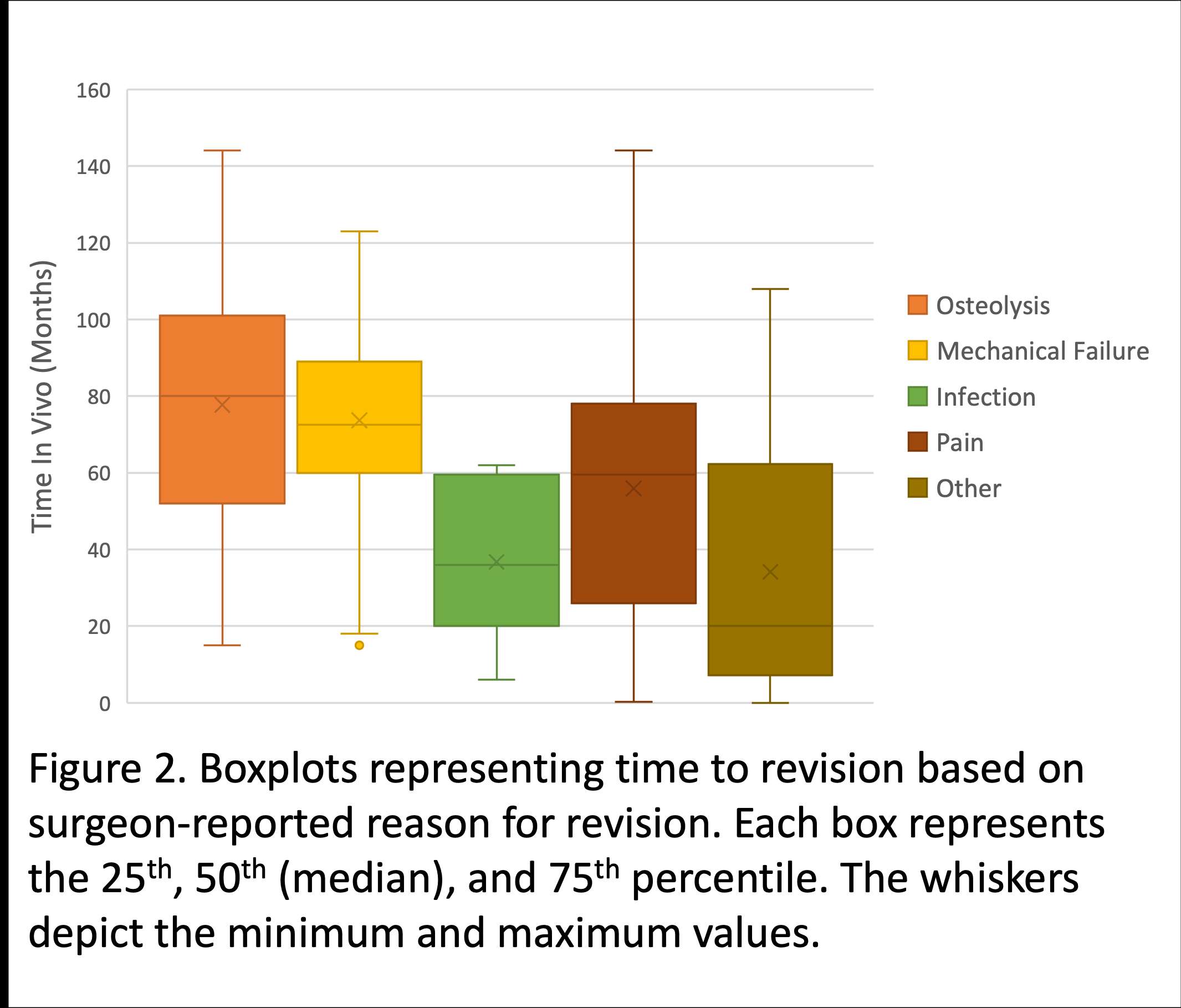
Figure 3#8659
Spinal Flexibility in Patients Undergoing Total Hip Arthroplasty
William Murphy - Harvard Medical School - Boston, USA
Andrew Amundson - USA
*Stephen Murphy - New England Baptist Hospital - Boston, USA
Patrick Lane - Surgical Planning Associates - USA
*Email: stephenbmurphymd@gmail.com
Introduction:
Surgeons increasingly use patient specific characteristics to set goals for implant positioning in Total Hip Arthroplasty (THA). Spinal stiffness, a measure of spinal pelvic motion, has recently come under increased study as a meaningful preoperative metric in THA. Spines that are more stiff increase the functional burden of bending on the hip joint, as the hip must make up for the lack of flexibility of the spine during motion [1]. This relationship has clinical consequences for impingement and stability after THA. The purpose of this study was to evaluate spine flexibility as measured on preoperative lateral radiographs.
Methods:
Ninety one consecutive patients undergoing THA underwent preoperative evaluation for surgical planning. As part of that evaluation, lateral radiographs were taken both in a standing and seating, forward bending position. Spine motion was defined as the angle between the inferior L1 endplate to the superior S1 endplate between standing and seated, forward bending position. A simple univariate regression with age was performed to determine significance at a p value of 0.05.
Results:
Demographics: Average age at surgery was 65.49 years (standard deviation 8.95, range 42-91). There were 49 (54%) women and 42 (46%) men. Average spinal motion was 31.45 degrees (standard deviation 13.76, range 0.50 – 59.90).
Age was significantly correlated with spinal motion, with each additional year of age associated with -0.58 degrees of spinal motion. P value was <0.001.
Regression coefficients are summarized in table 1.
Conclusion:
In our study, age was significantly correlated with decreased motion at the spine. This has consequences for both acetabular component goals and clinical impingement after total hip arthroplasty
References:
Ike, Hiroyuki MD; Dorr, Lawrence D. MD; Trasolini, Nicholas MD; Stefl, Michael MD; McKnight, Braden MD; Heckmann, Nathanael MD. Spine-Pelvis-Hip Relationship in the Functioning of a Total Hip Replacement. The Journal of Bone and Joint Surgery 100(18):p 1606-1615, September 19, 2018. | DOI: 10.2106/JBJS.17.00403
Figures
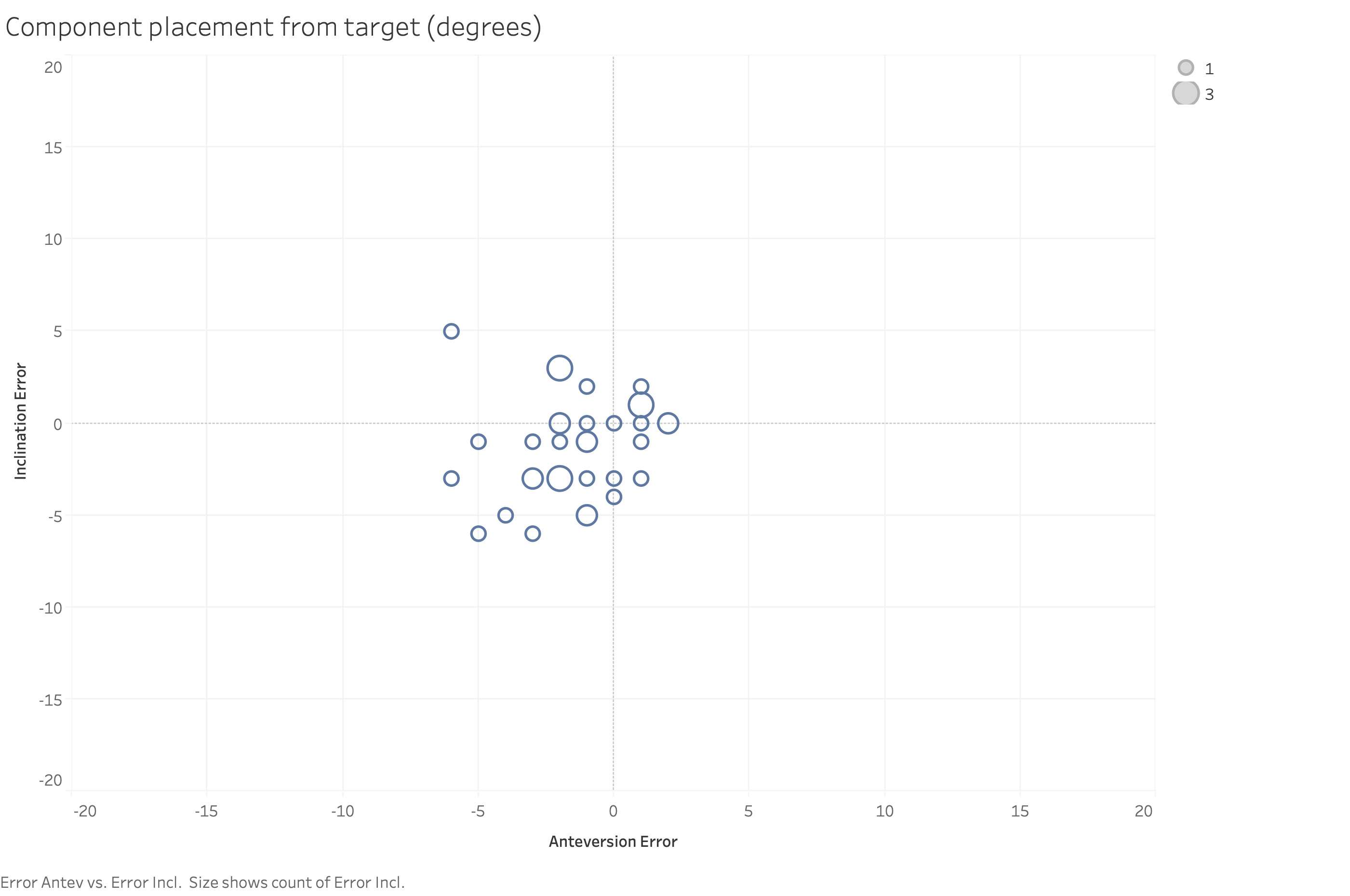
Figure 1
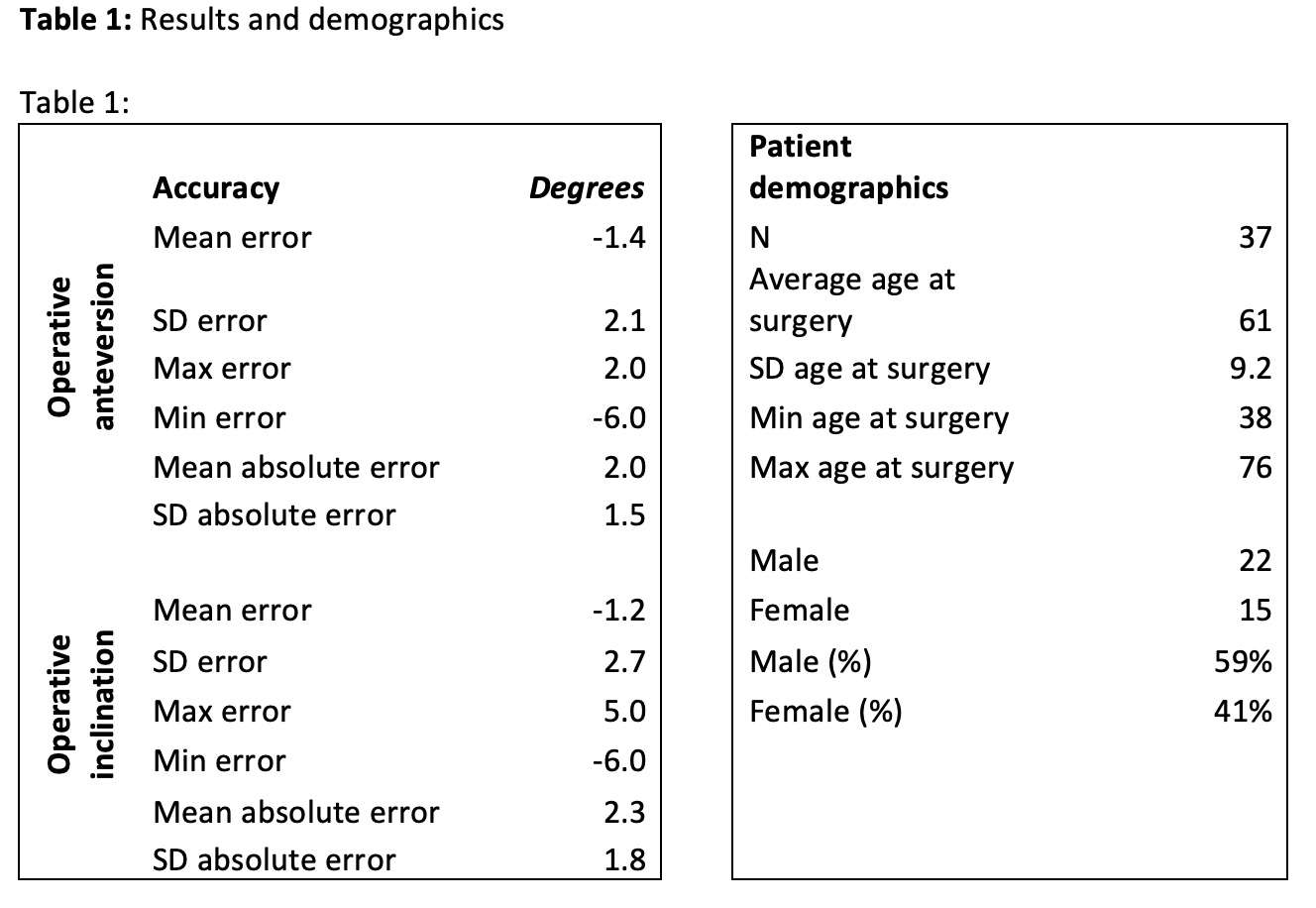
Figure 2#8198
Surgical Outcomes of Decompression Without Fusion for Lumbar Spinal Stenosis With Degenerative Scoliosis of More Than 20 Degrees
*Atsunori Ohnishi - Itami city hospital - Itami, Japan
Yuto Nishioka - Itami City Hospital - Itami, Japan
Takaaki Nakai - Itami city hospital - Itami, Japan
Junzou Hayashi - Itami city hospital - Itami, Japan
Tsuyoshi Nakai - Itami city hospital - Itami, Japan
*Email: atsunori0915@yahoo.co.jp
Introduction: Several studies have demonstrated good clinical outcomes of decompression alone for lumbar spinal stenosis with mild scoliosis. However, there have been few reports on clinical outcomes of decompression for moderate and severe scoliosis. The aim of this study was thus to retrospectively investigate surgical outcomes after decompression without fusion for lumbar spinal stenosis with degenerative scoliosis of more than 20 degrees.
Methods: 32 consecutive patients who had undergone decompression without fusion for lumbar spinal stenosis with coronal curvature of more than 20 degrees measured by Cobb method since September 2013 and were followed for at least 2 years after surgery were included in this study. The clinical outcome was assessed using Japanese Orthopaedic Association (JOA) score before surgery and at final follow-up with a mean of 36.2 months. Cobb angle and lumbar lordosis (LL) were measured using plain radiographs in the standing position. We also investigated additional fusion surgeries.
Results: The mean JOA score improved significantly from 13.0 points before surgery to 20.9 points at final follow-up (the mean recovery rate, 50.0%). The mean Cobb angle increased from 27.1 degrees before surgery to 28.6 degrees at final follow-up, although the difference was not significant. LL also showed no significant changes after surgery. In the good clinical outcome group (the recovery rate ≥ 50%; n = 17), the mean Cobb angle increased from 24.3 degrees preoperatively to 25.0 degrees postoperatively (p=0.57). On the other hand, in the poor clinical outcome group (the recovery rate < 50%; n = 15), the mean Cobb angle significantly increased from 30.4 degrees to 32.7 degrees (p=0.026). The preoperative Cobb angle tended to be larger in the poor outcome group than in the good outcome group (p=0.050). The postoperative Cobb angle was significantly larger in the poor outcome group. Neither pre- nor post-operative LL showed significant differences between the 2 groups. Additional fusion surgery was performed in two cases with the preoperative Cobb angle of 51 degrees and 29 degrees.
Conclusion: This study showed good clinical outcomes of decompression in patients with the mean Cobb angle about 25 degrees. Decompression without fusion is considered as one of the surgical options for lumbar spinal stenosis with moderate scoliosis. However, the clinical outcomes in patients with severe scoliosis were poorer than those with moderate scoliosis.
#8494
Presentation and Management of Infection in Total Disc Replacement: A Review of the Literature
*Hannah Spece - Drexel Implant Research Center - Philadelphia, USA
Armen Khachatryan - The Disc Replacement Center - Salt Lake City, USA
Frank M Phillips - Rush University Medical Center - Chicago, USA
Todd H Lanman - Lanman Spinal Neurosurgery - Beverly Hills, USA
Grant Garrigues - Rush University Medical Center - Chicago, USA
Hyun Bae - Cedars-Sinai Spine Center - Los Angeles, USA
Joshua Jacobs - Rush University Medical Center - USA
Steven Kurtz - Drexel University - Philadelphia, USA
*Email: hannahspece@gmail.com
Introduction: Total disc replacement (TDR) is widely used in the treatment of cervical and lumbar spine degeneration. TDR infection, although uncommon, can have devastating results.
Currently, given the low incidence of TDR infection, data and recommendations regarding treatment management are limited. The purpose of this review was to summarize literature reports of TDR infection and related treatment strategies. We queried: 1) What are the reported incidence rates of TDR infection; 2) What are the clinical characteristics of TDR infection; and 2) How is TDR infection managed?
Methods: We conducted a systematic search in PubMed and EMBASE using the search terms: (spine OR cervical OR thoracic OR lumbar) AND (disc OR disk) AND (replacement OR arthroplasty OR CDA OR TDR OR CDR) AND (infection OR bacteria OR Staphylococcus OR Propionibacterium OR acnes OR MRSA). English language studies that detailed the management of TDR infection cases and those that reported incidence rates of infection were included. Studies that reported infection rates only for special patient populations or rates not specific to TDR alone were excluded.
Results: A total of 128 unique studies were identified during our search. Twenty were included for review (Figure 1). There were 12 database studies and 8 case reports comprising 13 individual cases. Although clinical cohort studies also reported cases of infection, the information provided in generalized TDR outcome research was insufficient for inclusion in the present study.
The 12 database studies focused primarily on cervical spine; one focused on lumbar. The number of TDR cases included in each study ranged from 293 to 3976. Follow-up ranged from time until discharge to 5 years. Most studies reported the incidence of superficial, deep, and organ/space infection separately while others reported infection generally. Incidence rates were low, ranging from 0.03%-1.9% for cervical and 0.04%-0.24% for lumbar.
All but two of the case reports were for delayed infection. Patients primarily presented with dysphagia (cervical) or severe pain (lumbar). Preoperative imaging with CT or MRI was reported in each case and often elucidated periprosthetic tissue mass and/or fluid collection. All treatment involved vigorous debridement and irrigation, and the device was removed in 9 cases but left in situ in others (both cervical and lumbar) due to access difficultly and perceived removal risks. One case was successfully treated nonoperatively. Empirical and targeted antibiotic treatment varied, and Cutibacterium (formerly Propionibacterium) acnes was the most common organism found. Hypothesized causes of the delayed infection cases included latent, dormant implant contamination, esophageal/tracheal microperforation, and bacteremia.
Conclusion: Our review summarizes the state of clinical management for TDR infection, an infrequent but serious complication. Treatment strategy and success relies on several factors including patient symptoms and time to onset, comorbidities, microorganism type, and implant positioning/stability. While treatment strategies varied throughout the literature, they all followed the same prioritization: eliminate infection, and reconstruct the spine. The results will inform future work on the creation of an algorithm for TDR infection management.
#8587
Surgical Trends Demonstrate Increased Utilization of Cervical Disc Arthroplasty Over Anterior Cervical Discectomy and Fusion From 2016 to 2021
*Mitchell Ng - Maimonides Medical Center - Brooklyn, United States of America
Ariel Rodriguez - Maimonides Medical Center - Brooklyn, United States of America
Aaron Lam - Maimonides Medical Center - Brooklyn, USA
Ahmed Emara - Cleveland Clinic - Cleveland, USA
Nicholas Ahn - UH Cleveland Medical Center - Cleveland, USA
Amrit Khalsa - Perelman School of Medicine, University of Pennsylvania - Philadelphia, USA
John Houten - Maimonides Medical Center - Brooklyn, USA
Ahmed Saleh - Maimonides Medical Center - Brooklyn, USA
Afshin Razi - Maimonides Medical Center - Brooklyn, USA
*Email: mitchng77@gmail.com
Introduction: Anterior cervical discectomy and fusion (ACDF) has been the most common procedure to treat degenerative cervical conditions, but recently cervical disc arthroplasty (CDA) has arisen as a motion-preserving operation to decrease risk of adjacent segment disease. The objective of this study was to determine surgical trends between CDA versus ACDF over the past 5 years, quantifying surgical volume over time, comparing baseline patient demographics and resultant post-operative complications.
Methods: A total of 69,287 patients were identified from a nationwide database who underwent either ACDF (n=44,652) or CDA (n=24,635) from 2016 to 2021. The percentage of patients managed by each operative procedure was calculated overall and sub-divided by year. Baseline patient demographics were compared between operative groups, comparing resultant post-operative re-admission rates and 2 year revision rates. Linear regression modelling was performed to evaluate trends/differences in procedural volume by year.
Results: From 2016-2021, CDA constitute 35.6% of procedures, although the number/proportion of CDA procedures has significantly risen relative to ACDF (23.3% in 2016 to 43.2% in 2021, p<0.001). Patients undergoing CDA were younger and less likely to have diabetes, rheumatoid arthritis, obesity or tobacco use disorder relative to ACDF (p<0.0001 for all). Patients undergoing CDA had lower rates of re-admission (1.7 vs. 8.2%, p<0.0001) but higher 2-year revision rates (1.23 vs. 0.84%, p<0.0001).
Conclusion: Our findings quantify the increased surgical volume of CDA, both absolutely and relative to ACDF over the past 5 years. Of note, patients undergoing CDA have fewer baseline demographics, highlighting patient selection measures may be in place. This in turn has led to decreased re-admission rates for patients undergoing CDA, although both surgeons and patients should be aware of the increased 2-year revision risk of CDA relative to ACDF. Additional long-term studies evaluating patient reported outcomes and potential long-term complications of CDA are still required.
#8403
Can a Lumbar Vertebra Training Model Reproduce the Tactile Feel of Screw Insertion Into Cadavers?
*Parham Foroutan - Flinders University - Adelaide, Australia
Kieran Bennett - Flinders University - Adelaide, Australia
Ella Fairweather - Flinders University - Adelaide, Australia
Sammuel Sobey - Fusetec - Adelaide, Australia
Nick Litchfield - Fusetec - Adelaide, Australia
Mark Roe - Fusetec - Adelaide, Australia
Karen Reynolds - Flinders University - Adelaide, Australia
John Costi - Flinders University - Adelaide, Australia
Mark Taylor - Flinders University - Adelaide, Australia
*Email: parham.foroutan@flinders.edu.au
Introduction
Synthetic spine models have the potential to reduce the need for cadaveric specimens in surgical training and examination, and medical device demonstration. Current models have reproduced bony anatomy but are associated with a number of limitations including the inability to reproduce the tactile feel of screw insertion. Tactile feel is directly correlated to the screw insertion torque1; therefore, achieving an equivalent tactile feel of screw insertion in synthetic and cadaveric specimens requires matching the screw insertion torque into synthetic models with that of cadaveric specimens. In this work, we aimed to develop a lumbar vertebra surgical training model that reproduces the tactile feel of screw insertion into the pedicles of cadaveric tissue through a comparison of insertion torque.
Methods
Lumbar vertebrae (N = 7) were manufactured using a J750 Digital Anatomy 3D printer (Stratasys, Rehovot, Israel) with geometry based on an adult male. Pedicle screw insertion was undertaken using the DePuy Synthes Expedium VERSE dual threaded spinal system (screw size 6×40 mm) following the surgical guide. Models were prepared by drilling 4 mm diameter pilot holes into the bilateral pedicles, which were then tapped using the size 6 tapping tool. Models were mounted to a custom-built screw insertion testing apparatus consisting of a drill positioned perpendicular to the pedicles, and an 11 Nm torque transducer (model TRT-100, Transducer Techniques, USA) recording continuously at 20 Hz. During testing, the drill was driven at a constant 5 revolutions per minute, while a 9.8 N (1 kg) load was applied to the driver head to maintain bone?screw connection during insertion. Screw insertion torque was compared to the maximum torque reported for a similar screw insertion in cadaveric specimens2.
Results
The lumbar vertebra model required 1.77 ± 0.22 Nm (mean ± standard deviation) to complete five screw revolutions and showed minimal variation (coefficient of variation: CV = 0.12) in measured torque (Figure 1). This was comparable to the 1.95 ± 0.98 Nm (CV = 0.50) required for cadavers using a similar dual screw system2.
Conclusion
The lumbar vertebra training models presented in this work reproduced the insertion torque and hence tactile feel of cadaveric specimens during pedicle screw insertion. This equivalence suggests that these lumbar vertebrae models are well suited for use in surgical training and examination, as well as medical device demonstration.
References
1. Matsukawa, K. (2020), Journal of Orthopaedic Science.
2. Brasiliense, L.B.C, et al. (2013), The Spine Journal.
Figures
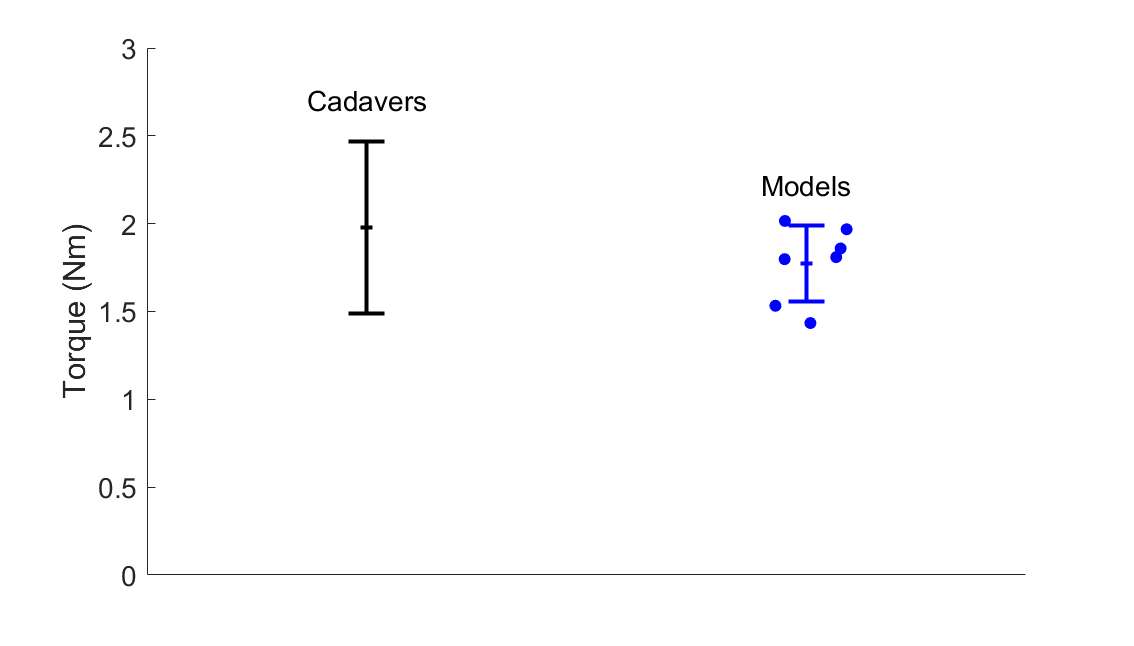
Figure 1#8755
Use of Technology Improves Mid-Term Clinical and Patient-Reported Outcomes in Cruciate-Retaining Total Knee Arthroplasty
*Weston Buehring - New York, United States of America
Patrick Meere - NYU Hospital for Joint Diseases - New York, USA
Omid Barzideh - NYU Orthopedic Hospital - New York, USA
Matthew Hepinstall - NYU Langone Health - New York, USA
Morteza Meftah - NYU Hospital for Joint Diseases - New York, USA
Benjamin Schaffler - NYU Orthopedic Hospital - New York, USA
Catherine Digangi - NYU Orthopedic Hospital - New York, USA
*Email: westonbuehring2013@gmail.com
Introduction
Cruciate-retaining design allows patients to retain their inherent level of lower limb proprioception and kinematics after total knee arthroplasty (CR-TKA). However, utility additional of robotic, or computer-assisted technology on outcomes of this design is not well established. Our study aimed to describe the effect of intraoperative technology on postoperative outcomes following CR-TKA.
Methods
We retrospectively reviewed 15,530 primary, elective TKA cases performed between January 2016 and May 2022, of which, 4,959 (31.9%) cases were identified as being cruciate-retaining based on implant type. The use of robotics (Stryker Mako) or navigation (Intellijoint, OrthAlign) were combined into a technology-cohort to comprise 2,174 cases (43.8%). 2,785 cases (56.2%) were the control cohort in which no technology was used. Patient demographics, clinical outcomes data, including Activity Measure for Post-Acute Care (AM-PAC) scores, which is a validated tool used to quantify postoperative functional status, and patient-reported outcomes measures (PROMs) in the form of Knee injury and Osteoarthritis Outcome Score, Joint Replacement (KOOS, JR) scores were collected and analyzed.
Results
Short-term outcomes favored the technology cohort when compared to the non-technology group, with shorter length of stay (42.96 vs. 60.57 (hours); p<0.001) and greater discharge to home (93.4 vs. 86.9%; p<0.001), respectively. Patients in the technology cohort also had a higher rate of achieving a perfect Activity Measure for Post-Acute Care (AM-PAC) score on postoperative day 0 (POD0) (24.0 vs. 5.9%; p<0.001). While rate of readmission was similar between both groups, rate of revision was lower in the technology group compared to the non-technology group (1.1 vs. 3.0%; p<0.001), with the most common cause for revision being implant instability. Lastly, the technology cohort showed significantly higher 6-week (55.23 vs. 50.49; p<0.001), 3-month (61.16 vs. 58.88; p=0.007), and 1-year (66.66 vs. 64.07; p=0.042) KOOS, JR scores relative to the non-technology group.
Conclusion
This study demonstrates favorable clinical and patient-reported outcomes associated with the incorporation of technology, in the form robotics or navigation, in CR-TKA. We found shorter LOS, greater discharge to home, higher AM-PAC scores on POD0, lower rate of revision, and higher postoperative PROM scores.
#8756
The Effect of Technology on Clinical and Patient-Reported Outcomes in Posterior-Stabilized Total Knee Arthroplasty
*Weston Buehring - New York, United States of America
Emily Ronan - Langone Orthopedic Hospital - New York, United States of America
Patrick Meere - NYU Hospital for Joint Diseases - New York, USA
Morteza Meftah - NYU Hospital for Joint Diseases - New York, USA
Matthew Hepinstall - NYU Langone Health - New York, USA
Catherine Digangi - NYU Orthopedic Hospital - New York, USA
*Email: westonbuehring2013@gmail.com
Introduction
The utility of technology, in the form of computer-assisted surgery (CAS) and robotics, in total knee arthroplasty (TKA) is not well defined. Theoretically, incorporation of such technology into the operating room makes execution of a defined plan more accurate and should reduce outliers. In order to eliminate the confounding factor of different implant designs, this study sought to examine the effect technology has on posterior-stabilized TKA (PS-TKA) cases. To date, no studies have described the effect the use or absence of technology, in the form of robotics or navigation, have on postoperative outcomes following PS-TKA.
Methods
We retrospectively reviewed 15,530 primary, elective TKA cases performed between January 2016 and May 2022, of which, 8,481 (54.6%) cases were identified as posterior-stabilized based on implant type. The use of robotics (Stryker Mako) or navigation (Intellijoint, OrthAlign) were combined into a technology-cohort to comprise 1,861 cases (21.9%), while a separate groups was created for cases in which no technology was used (6,620 cases, 78.1%). Patient demographics and short-term outcomes data, including Activity Measure for Post-Acute Care (AM-PAC) scores, which is a validated tool used to quantify postoperative functional status, and patient-reported outcomes measures (PROMs) in the form of Knee injury and Osteoarthritis Outcome Score, Joint Replacement (KOOS, JR) scores were collected and compared using chi-squared tests.
Results
Both cohorts shared similar baseline demographic characteristics. Short-term outcomes favored the technology cohort when compared to the non-technology group, with shorter length of stay (50.20 vs. 60.21 (hours); p<0.001) and greater discharge to home (91.2 vs. 83.2%; p<0.001), respectively. Patients in the technology cohort also had a higher rate of achieving a perfect AM-PAC score on postoperative day 0 (POD0) (18.0 vs. 8.8%; p<0.001). While rate of readmission was similar between both groups, rate of revision was lower in the technology group compared to the non-technology group (1.7 vs. 3.1%; p<0.001), with the most common indication for revision being implant instability. Lastly, the technology cohort showed significantly higher mean 1-year (68.05 vs. 63.01; p<0.001) KOOS, JR scores as well as improvement in 1-year KOOS, JR scores from preoperative levels (26.17 vs. 19.94; p<0.001) relative to the non-technology group.
Conclusion
This study highlights the favorable clinical and patient-reported outcomes associated with the incorporation of technology, in the form robotics or navigation, in PS-TKA, including shorter LOS, greater discharge to home, higher AM-PAC scores on POD0, lower rate of revision, and higher postoperative PROM scores.
#8598
Influence of Robotic Technology Updates on Hospital and Patient Reported Outcomes
*Laura Yanoso-Scholl - Stryker Orthopaedics - USA
Kevin Marchand - Ortho Rhode Island - South County, USA
Kelly Taylor - Ortho Rhode Island - South County, USA
Daniel Erwin - Ortho Rhode Island - Wakefield, USA
Zachary Guerrieo - Ortho Rhode Island - Wakefield, USA
Bob Marchand - ortho rhode island south county - Wakefield, United States of America
*Email: Laura.Scholl@stryker.com
Introduction: Literature has reported robotic-assisted total knee arthroplasty resulting in improved accuracy and clinical outcomes when compared to manual TKA.However, even with these findings, new learnings within the medical and technology fields challenge current robotic systems and create space for change and growth. As robotic systems are upgraded, it is important to continue to study the intraoperative and clinical effects of these upgraded systems. The purpose of this study was to evaluate hospital and patients reported outcomes as an experienced robotic-assisted TKA surgeon adopted a new software update.
Methods: A retrospective review was performed on cases completed by a single, high-volume, robotic surgeon following their adoption of a software update. A consecutive series of 146 patients were evaluated where all patients were diagnosed with end-stage osteoarthritis and received robotic-assisted TKA with cementless implants between the dates August 2022 and January 2023. Average age and BMI of this cohort was 68.4 years ± 8.0 and 29.2 kg/m ± 4.4, respectively. Where, 55% were female and 54% had TKA on their right knee.
Hospital reported outcomes including operative time, length-of-stay (LOS), and sizing of implants used. At 6-weeks, range-of-motion (ROM) was evaluated for the operated knee. Reduced-WOMAC scores were collected preoperatively, 2-weeks, 6-weeks, and 3-months postoperative. To assess patients’ clinical performance, minimally clinically important difference (MCID) was calculated for reduced-WOMAC using the distribution-based method by finding the difference between preoperative and 3-month outcome scores. Then calculating 0.5 times the standard deviation.
Results: Considering hospital reported outcomes, average surgical time was 61.3 minutes ± 9.6 where 50% of cases were performed in less than 60 minutes (Figure 1). Average LOS was 1.2 days ± 0.7 where 76% of patients returned home in first day. For 48% of cases the femoral implant was equal in size to the tibial implant and for 51% of cases, the femoral implant was a size smaller than the tibial implant.
At 6-weeks postoperative, average maximum flexion was 114.5° ± 12.0 where 86% of patients reached a maximum of 110°. At 3-months, the average reduced-WOMAC scores were statistically significantly less than at preoperative (Figure 1). MCID was calculated as 4.44 where mean values met MCID at 2-weeks, 6-weeks, and 3-month follow-up. There were no reported revisions at 3-month follow-up.
Discussion: When considering operative time for the same surgeon, their time stabilized at 62 minutes ± 2 after 1-year of experience with the robotic system1 where 38% of cases were performed under 60 minutes. Results from the current study were similar, indicating adoption of the updated software did not interfere with the surgeon’s average operative time. Femoral implants were either planned as equivalent size or one size smaller than the tibial implant for 99% of cases. Use of 3D CT planning allowed the surgeon to size the TKA implants to the patient’s anatomy and reducing the size of the femoral implant.
At 3-months postoperative, reduced-WOMAC was statistically significantly improved compared to preoperative. This cohort will continue to be followed to collect outcomes as patients reach longer-term follow-up.
Figures
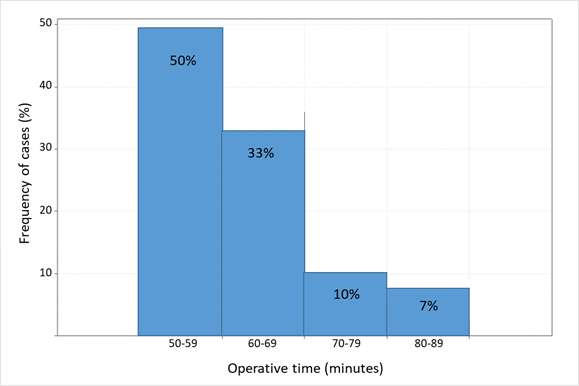
Figure 1
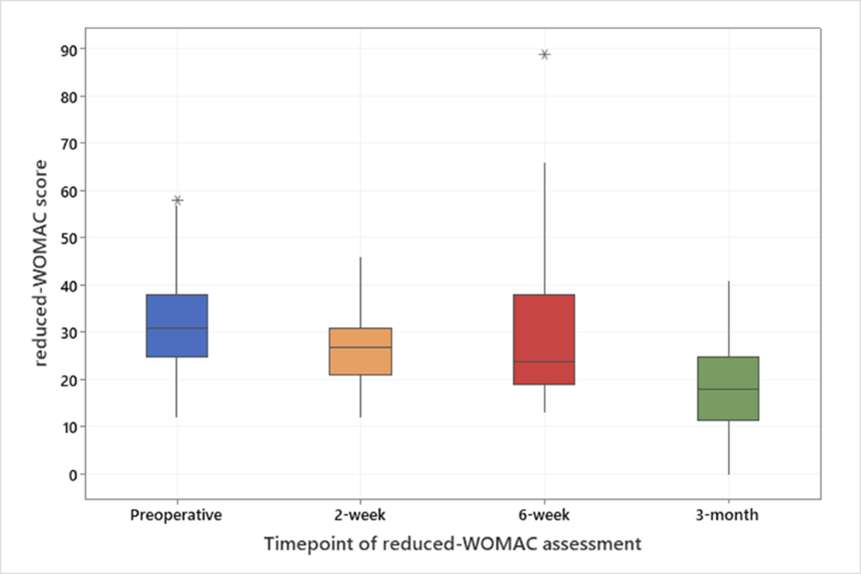
Figure 2
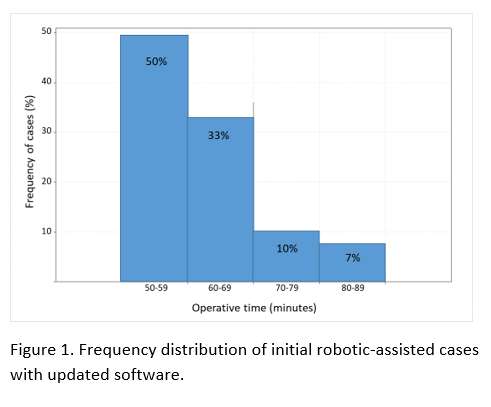
Figure 3
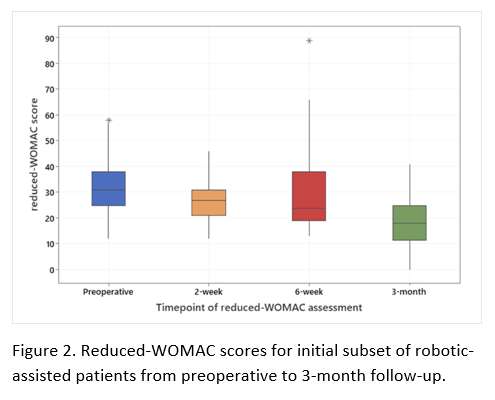
Figure 4#8496
Excellent Outcomes in Obese Patients - an Analysis of 1,564 Cementless Robotic-Assisted TKA Cases
*Christina O'Neill - Stryker - Mahwah, United States of America
Melanie Caba - Stryker Corporation - Mahwah, United States of America
Kelly Taylor - Ortho Rhode Island - South County, USA
Zachary Guerrieo - Ortho Rhode Island - Wakefield, USA
Laura Yanoso-Scholl - Stryker Orthopaedics - Mahwah, USA
Robert Marchand - Ortho Rhode Island - South County, USA
Kevin Marchand - Lewis Katz School of Medicine - Philadelphia, United States of America
*Email: christina.oneill@stryker.com
The growing prevalence of obesity in the U.S. population has been linked to the increasing demand for joint arthroplasty procedures, especially total knee arthroplasty (TKA). Previous evidence has shown that obese patients undergoing manual cementless TKA demonstrated lower failure and improved functional outcomes compared to manual cemented TKA. Robotic-assisted (RA) TKA has been introduced to help improve clinical outcomes and patient satisfaction. However, the outcomes of cementless TKA for obese patients using RA-TKA technology has not been previously characterized. The purpose of this study was to compare survivorship and outcomes of cementless TKA cases in obese and nonobese patient cohorts using RA-TKA technology.
A retrospective review was conducted on 1,564 cementless RA-TKA cases performed by a single, high-volume surgeon. From this cohort, BMI was used to classify cases for an obese cohort (BMI≥ 35) and nonobese cohort (BMI<35). Data was collected from each case including operative time, length of stay (LOS), blood loss, 6-week postoperative flexion, adverse events and PRO scores out to one-year postoperatively. PROs were collected through WOMAC pain, function, and total scores and KOOS-JR scores. A two-sample t-test with α=0.05 in SAS 9.4 was used to assess significant difference in PRO scores between the cohorts.
The obese cohort (mean BMI 40.5±4.7) consisted of 472 patients (30%) and the nonobese cohort (mean BMI 28.6±3.6) consisted of 1,092 patients (70%). Mean operative time for the obese cohort was greater by only four minutes compared to the nonobese cohort (75 minutes vs 71 minutes, p<.001). Mean LOS was 2.2 days in the obese cohort and 1.9 days in the nonobese cohort (p<.001). Mean blood loss for the obese cohort was 75.7 mL and 61.2 mL in the nonobese cohort (p=.014). Mean flexion values at 6-weeks postoperative for the obese cohort was 111.3° compared to 113.9° in the nonobese cohort (p<.001). Both cohorts had similar rates of revision (obese 0.42%, nonobese 0.46%, p=1.0), but the rate of manipulation under anesthesia (MUA) was higher for the obese cohort (4.2% vs 2.4%, p=.046). All mean preoperative PRO values reported significantly worse scores for the obese cohort compared to the nonobese cohort. For all postoperative PROs, the obese and nonobese cohorts had no significant difference in early outcome scores (4±2 weeks) and 12 month scores, indicating a similar recovery following TKA. Figure 1 shows mean scores and p-values for all PROs collected at each time point.
Given the increasing number of obese patients seeking TKA, it is important to identify optimal treatment for this population that may potentially help improve outcomes. This study demonstrated excellent PRO scores and survivorship in patients who received cementless RA-TKA, regardless of BMI. Additional studies to follow this patient demographic out to longer terms are needed.
Figures
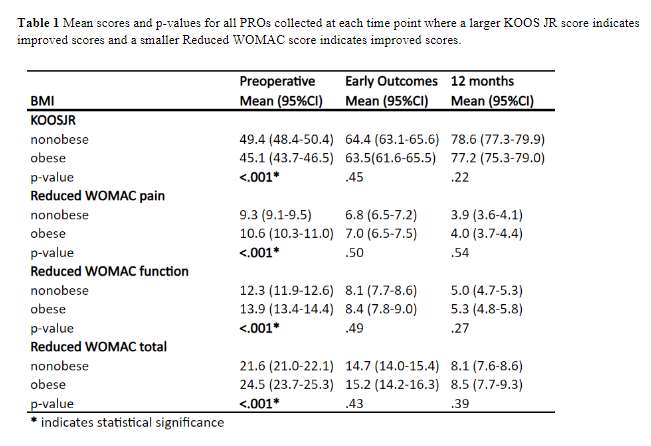
Figure 1#8176
Assessment of Accuracy and Early Outcomes During the Adoption Phase of a Novel Image-Free Robotic-Assisted System for Total Knee Arthroplasty
*Ian Leslie - DePuy Synthes Joint Reconstruction - Leeds, United Kingdom
Timothy Alton - Proliance Orthopedic Associates - Renton, USA
Erik Severson - Cuyuna Reginal Medical Center - Crosby, USA
Marcus Ford - Campbell Clinic Orthopaedics - Germantown, USA
James Lesko - DePuy Synthes Joint Reconstruction - warsaw, USA
Rodrigo Diaz - DePuy Synthes - Palm Beach Gardens, USA
Ronald Delanois - Sinai Hospital of Baltimore - Baltimore, USA
*Email: ileslie1@its.jnj.com
Introduction
Robotic-Assisted knee replacement is increasing in popularity due to increased accuracy of component positioning and the intraoperative data collection which can support patient specific techniques.1 This study focused on assessing the accuracy and early clinical outcomes of TKA using the recently introduced VELYS™ Robotic-Assisted solution during the learning phase, in comparison to manual TKA.
Methods
A multicenter, prospective non-randomized 1:1 cohort study of TKA patients was conducted at five sites. Subjects underwent TKA with either manual instrumentation or Robotic-Assistance (RA). RA procedures were the first conducted at each site, therefore, representing the adoption phase. The primary objective was a non-inferiority analysis of the accuracy (difference in actual vs. plan) of the Hip Knee Ankle Angle (HKA) for RA vs. manual, measured via post operative long leg radiographs. Further, the accuracy of the distal femoral v/v, tibial v/v and tibial posterior slope angles were measured via radiographs. All intraoperative and perioperative (90 days) adverse events (AEs) were collected along with the passive range of motion (ROM) and the following patient reported outcomes FJS, KOOS, EQ-5D, Pain, Satisfaction. A non-inferiority margin of 1.5 degrees was utilized for the HKA analysis. Continuous variables were assessed with a T-test and discrete outcomes with a Fisher’s extract test.
Results
One hundred participants were recruited for both the manual and RA groups. Age and gender were similar across groups; there was a possible difference in mean BMI although the range was similar (Figure 1). Mean preoperative KOOS, EQ-5D, Pain and ROM were similar (Figure 1). Intraoperatively the planned HKA and tibial cuts deviated more from neutral for the RA group than for manual. The RA cases were completed without any instances of soft tissue compromise. Surgeon satisfaction with the alignment, balance and implant fit was higher for the RA group than the manual group, however, RA was associated with increased surgical time (Figure 2). HKA accuracy was noninferior in the RA group compared to manual control under the 1.5 degree non-inferiority margin (P<0.0001). Further, improvements in the accuracy of the angle of the individual cuts for the RA group compared to manual were observed (Figure 3). The PROMs at 12 weeks were either equivalent or improved (FJS, Pain) for the RA group compared to manual. There was no significant difference in the incidence of AEs, however, there was a reduction in the number of AEs requiring surgical or medical intervention within 90 days for RA compared to manual (Figure 3).
Conclusion
The results of this study demonstrate the VELYS Robotic-Assisted solution can be safely adopted without adversely impacting the long leg alignment or rate of adverse events. The accuracy of the individual bone cuts was improved compared to manual which is consistent with the findings of a previous cadaveric study2. Further it was observed that FJS, pain and the incidence of AEs requiring intervention were improved at 12 weeks compared to manual instrumentation.
References
1. Zhang et al. KSSTA 2021. 2. Doan et al. JOA 2022
Figures
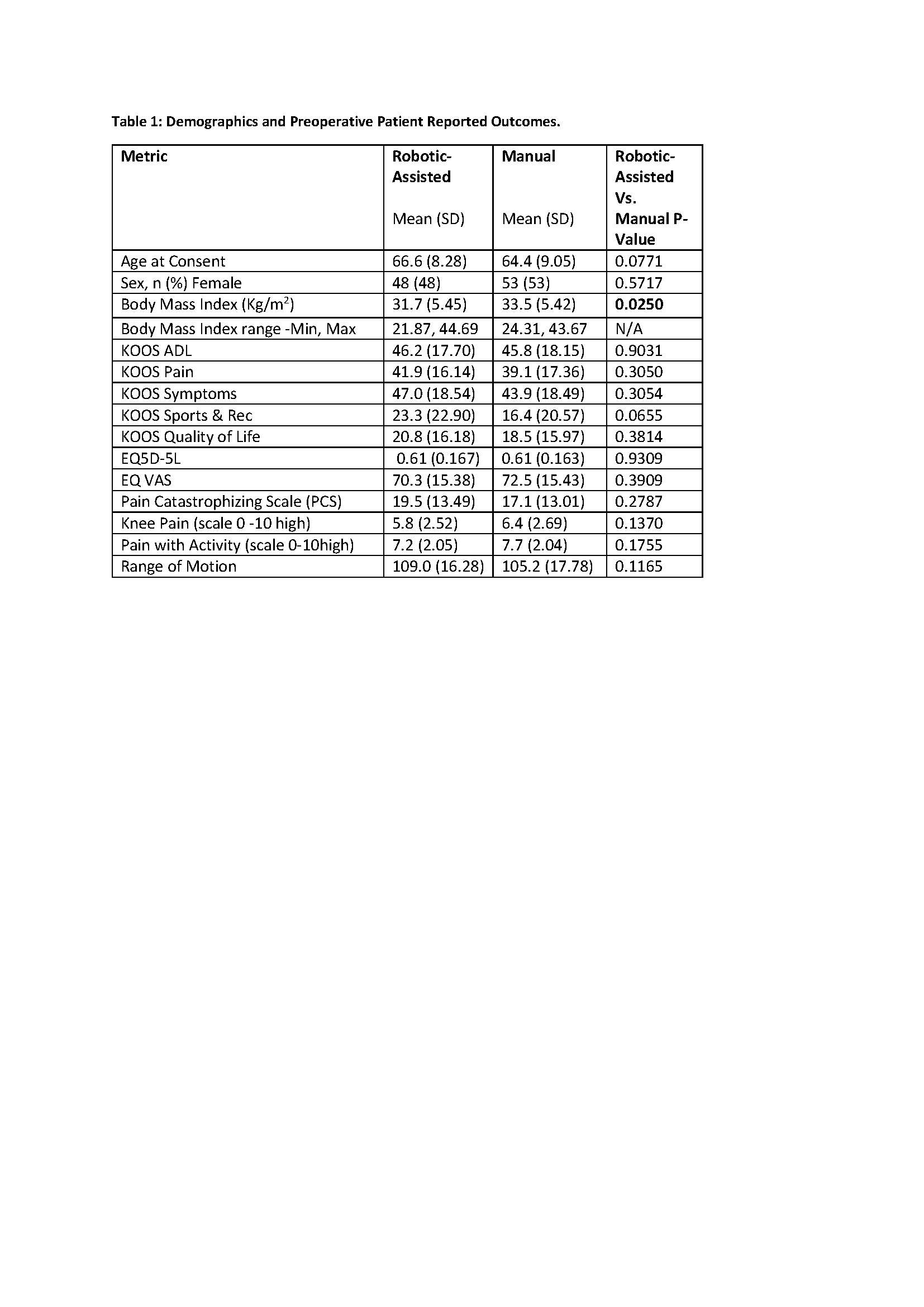
Figure 1
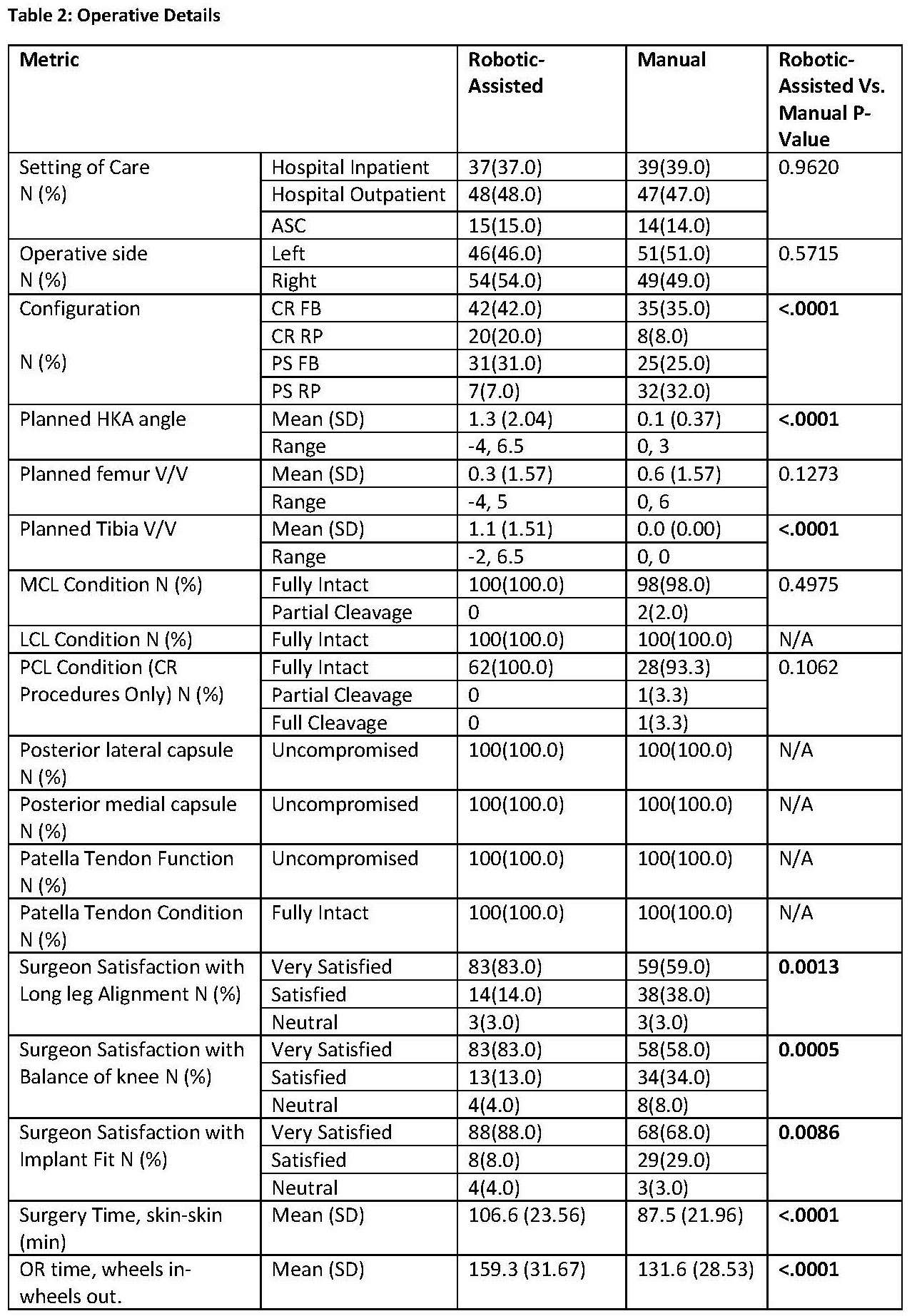
Figure 2
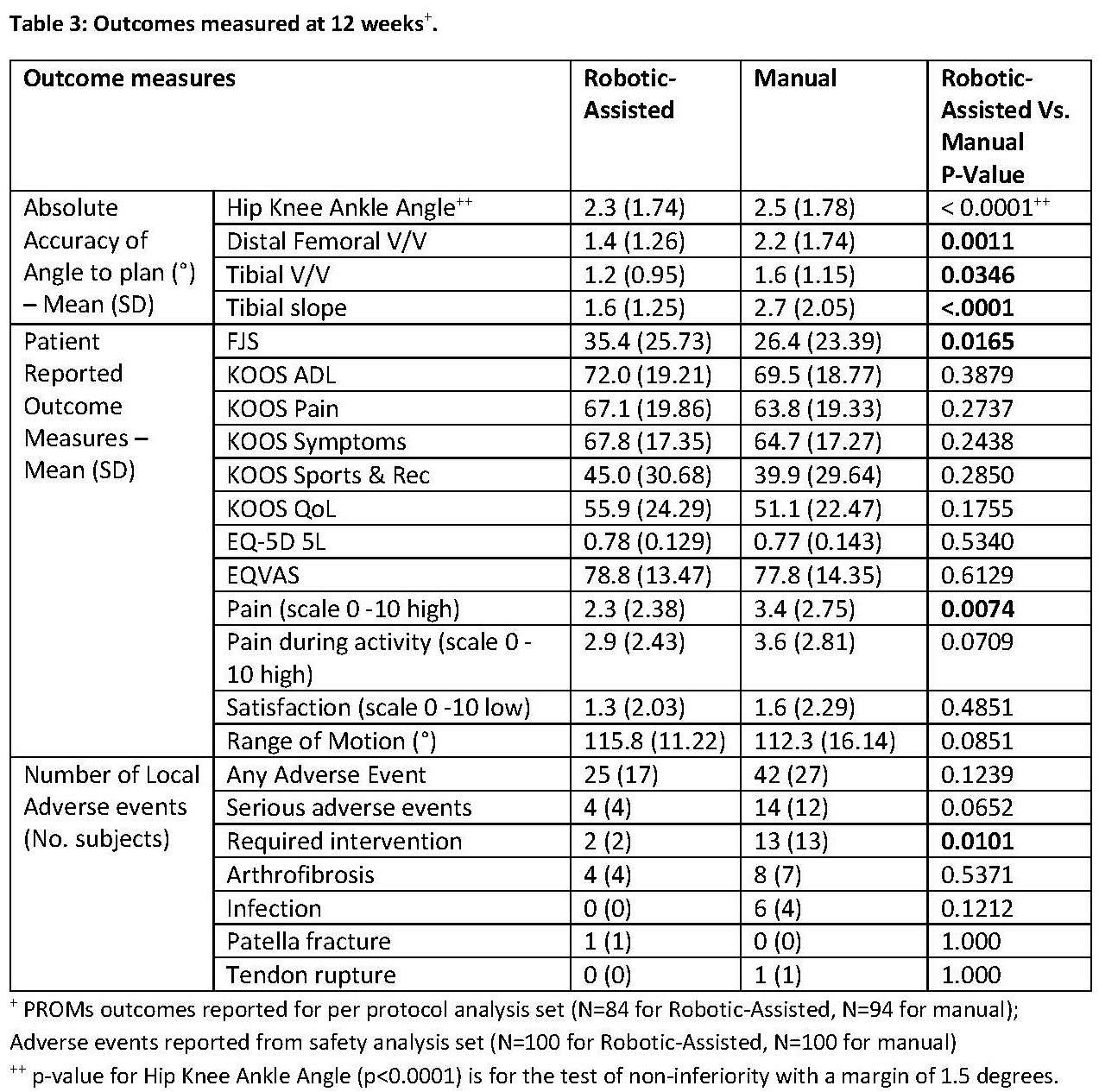
Figure 3#8582
The Impact of Image-Based Robotic-Assisted Total Knee Arthroplasty With Functional Positioning Principles: Anterior Compartment Restoration and One-Year Follow-Up Results.
*Cecile Batailler - Lyon, France
Moussa Kafelov - Croix Rousse hospital - Lyon, France
Sebastien Lustig - Sydney Orthopaedic Research Institute - Chatswood, Australia
*Email: cecile-batailler@hotmail.fr
Introduction
The functional positioning performed with an image-based robotic-assisted system restores the positioning of the trochlea groove in varus knees. Nevertheless, the anterior compartment is not always preserved with anterior under- or overstuffing. This study aimed to assess the functional outcomes according to the restoration of the anterior compartment at 12 months of total knee arthroplasty (TKA) performed with an image-based robotic-assisted system.
Methods
This retrospective study included 96 primary TKAs for end-stage varus osteoarthritis, performed with an image-based robotic-assisted system applying functional positioning principles between 2018 and 2022. The mean age was 69.5 years old ±7.6; the mean body mass index was 29.8 kg/m2 ±4.7. Kujala score, Forgotten joint score (FJS), Knee society score (KSS) knee, and KSS function were collected 12 months postoperatively. To assess the restoration of the anterior compartment, we measured the depth difference between the native and the prosthetic trochlea. Four sequential positions were assessed at which the patella is engaged in the femoral groove with knee flexion: at full extension, at 30° flexion, at 70° flexion, and 90° flexion. For each of these positions, we compared the highest point of the lateral native condyle and the lateral prosthetic condyle, the highest point of the medial native condyle and the medial prosthetic condyle, the deepest point of the native trochlear groove and the prosthetic trochlea.
Results
61.5% of TKA (n=59/96) had an understuffing of the anterior compartment. 42.7% (n=41/96) had an understuffing between 5 and 10 mm. 18.8% (n=18/96) had an understuffing superior to 10mm. The understuffing was mainly localized at 30° and 70° of flexion and on the lateral and medial condyles. Only 5.2% of TKA (n=5/96) had an anterior overstuffing between 5 and 10mm. At 12 months postoperatively, the mean Kujala score was 75.1 ±24.9 in the group without under- or overstuffing, 81.7 ±17.2 in the group with understuffing between 5 and 10 mm, 76.9 ±14.3 in the group with understuffing superior to 10 mm, and 74.2 ±26.2 in the group with overstuffing (p=0.59). The mean FJS score was 72.3 ±26.7 ; 77.2 ±23.5 ; 69.3±26.8; and 79.9 ±15.2, respectively (p=0.78). The mean KSS Knee score was 91.4 ±10.3 ; 91.6 ±11.3 ; 87.6 ±13.8; and 89.0 ±13.4, respectively (p=0.61). The mean KSS Function score was 90.0 ±12.6 ; 90.6 ±13.9 ; 93.3 ±9.7; and 94.0 ±8.9, respectively (p=0.76). There was no significant correlation between functional outcomes at 12 months and the restoration of the anterior compartment.
Conclusion
A moderate anterior understuffing was common after TKA was performed with functional positioning and an image-based robotic-assisted system. This anterior understuffing didn’t impact the functional outcomes at 12 months postoperatively, particularly the Kujala score. The functional positioning allowed the complete restoration of the anterior compartment in 33.3% of TKA. The shape of the implant could explain the difficulties in restoring the anterior compartment all along the arc of knee flexion.
#8462
Cementless Total Knee Arthroplasty Comparing Robotic-Assisted Technology and Manual Jig-Based Instrumentation
*Arthur Malkani - Jewish Hospital - Louisville, USA
Michael Stoltz - University of Louisville - Louisville, United States of America
Nolan Smith - University of Louisville - Louisville, United States of America
Sarag Abhari - University of Louisville School of Medicine - - Louisville, KY - Louisville, United States of America
Langan Smith - UofL Health - Louisville, United States of America
Madhusudhan Yakkanti - Louisville Orthopaedic Clinic - Louisville, USA
*Email: arthur.malkani@louisville.edu
INTRODUCTION:
Robotic-Assisted Total Knee Arthroplasty (RA-TKA) provides real time intraoperative information to help achieve accurate bone cuts, symmetric gap balancing, and the target implant placement and limb alignment. The purpose of this study was to evaluate clinical outcomes and Patient-Reported Outcome Measures (PROMs) following primary cementless TKA using robotic-assisted technology compared to traditional manual instrumentation.
METHODS:
This was a retrospective cohort study comparing the outcomes of 500 consecutive cementless primary RA-TKAs and 500 consecutive cementless primary TKAs using traditional manual instrumentation. Restricted kinematic alignment was utilized in the RA-TKA group versus neutral mechanical alignment in the manual cohort. Both cohorts had a minimum 2-year follow-up with both procedures performed during the same time period using the same implant design. In the RA-TKA group, 3 patients were deceased, and 29 were lost to follow-up (5.81%), with 468 cases available for review. In the manual TKA group, 10 patients were deceased, and 63 were lost to follow-up (12.6%), with 427 cases available for review. There was no significant difference in age or gender between the groups. The manual group had a significantly higher average BMI than the RA-TKA group (34.3 compared to 32.4, p-value=<0.00100). Outcome measures included range of motion, Knee Society Scores (KSS), Forgotten Joint Score (FJS-12), KOOS JR Score, overall satisfaction (5-point Likert scale), complications, and survivorship. Statistical analysis was performed using Student t-tests and Fisher’s exact tests.
RESULTS:
The RA-TKA had significantly higher KSS Function, KSS Knee, and KOOS JR scores compared to the manual group (86.1 vs 78.8, p<0.00100, 92.3 vs 83.8, p<0.00100, and 86.1 vs 80.4, p<0.00100 respectively). The RA-TKA group had higher post-operative knee flexion compared to the manual group (120 degrees compared to 117 degrees, p<0.00100). There was no significant difference in FJS-12 scores between the two groups, p=0.580. The RA-TKA group had a higher overall satisfaction rating (Likert scale 1-5) and a higher percentage of patients very satisfied or satisfied compared to the manual group (4.67 vs 4.55, p=0.0321, 94.0% vs 87.4%, p=0.00130 respectively). There were 15 revisions in the RA-TKA group compared to 25 in the manual group, p=0.0740. There was 1 case of prosthetic joint infection in the RA-TKA group compared to 7 in the manual group, p= 0.0164. Survivorship with all-cause failure as the endpoint was 96.8% at 3 years in the RA-TKA group compared to 94.15% in the manual group (p=0.0737). Survivorship with aseptic loosening as the endpoint was 99.8% at 3 years in the RA-TKA group compared to 99.1% in the manual group (p=0.201).
CONCLUSION:
RA-TKA demonstrates promising results with equal or superior post-operative PROMs and patient satisfaction compared to traditional manual instrumentation. RA-TKA using a cementless implant demonstrated a 96.8% survivorship and 94% patient satisfaction in this study. RA-TKA using a cementless implant appears to be a promising combination given the benefits of both biologic fixation and accuracy in achieving the target alignment and soft tissue balance.
#8512
Operative Time Learning Curve for an Image-Free Robotic-Assisted Total Knee Arthroplasty
*Cale Pagan - Hospital for Special Surgery - New York, United States of America
Theofilos Karasavvidis - Hospital for Special Surgery - New York, USA
Breana Siljander - Hospital for Special Surgery - New York, USA
Charles DeCook - Total Joint Specialists - Cumming, USA
Eytan Debbi - Cedars-Sinai Medical Center - Los Angeles, USA
Jonathan Vigdorchik - Hospital for Special Surgery - New York, USA
*Email: paganc@hss.edu
Introduction: Robotic-assisted total knee arthroplasty (RA-TKA) allows for more precise and accurate bone resection, implant position and joint alignment compared to manual TKA (M-TKA). The learning curve associated with the adoption of RA-TKA should be carefully considered, as it may lead to disruptions in operating room efficiency, increased complications, and higher costs. The current study aims to assess the operative time learning curve of RA-TKA analyzing a single-surgeon cohort.
Methods: The first 80 consecutive RA-TKA cases and last consecutive 80 M-TKA cases were assessed. The robotic cases included in this study represent the first imageless robotic cases of a single surgeon after transitioning from exclusively M-TKA. The learning curve cumulative summation (CUSUM) analysis was conducted to evaluate the RA-TKA operative times. This statistical tool quantifies the running total of differences between individual data points and the mean of all data points to assess stabilization of the surgical times. Three distinct phases constitute the learning curve: (1) the initial learning curve, (2) the plateau of the learning curve or period of increased competence, and (3) the post-learning period. The case number by which the CUSUM value entered the plateau was defined as the number of cases to proficiency. RA-TKA cases were further subdivided into sequential groups of 20 cases and compared to all M-TKA cases. Continuous variables were described with mean ± standard deviation and an independent t-test was conducted to compare surgical times between both techniques.
Results: Two distinct breakpoints (9 and 53) and three distinct phases were identified on the learning curve CUSUM plot (Fig. 1). Phase 1 (initial learning): the number of cases to proficiency was 9. Phase 2 (increased competence): a plateau between cases 10-52 indicates a stabilization of operative times. Phase 3 (post-learning): a downtrend of operative times between cases 53-80 depicts a period of optimized performance. The mean surgical time for RA-TKA was 42.4 ± 8.7 minutes, whereas the M-TKA group had a mean value of 35.3 ± 7.0 minutes (p<.001). Mean operative times for the first 20 RA-TKA cases and the last 20 RA-TKA cases were 48.0 versus 38.5 minutes (p< 0.05). Surgical times of the last RA-TKA group (cases 61-80) were similar when compared to the M-TKA group (p= 0.06).
Conclusion: Implementation of new robotic technology is expected to disrupt operative workflow. The RA-TKA is an enabling surgical tool that can be integrated efficiently into a surgical workflow with a rapid learning curve of 9 cases. Proficiency with the system can be expected to improve over time with eventual return to surgical times equal to or better than M-TKA.
Fig. 1 The CUSUM plot shows the cumulative sum of differences in surgical time compared to mean surgical time for RA-TKA. Red lines represent breakpoints at 9.44 and 52.71 (representing cases 9 and 53, respectively). Phase 1 represents the number of cases to proficiency. Phase 2 represents the competence phase. Phase 3 represents the period of optimized performance.
Fig. 2 RA-TKA Surgical times plotted chronologically by case number.
Figures
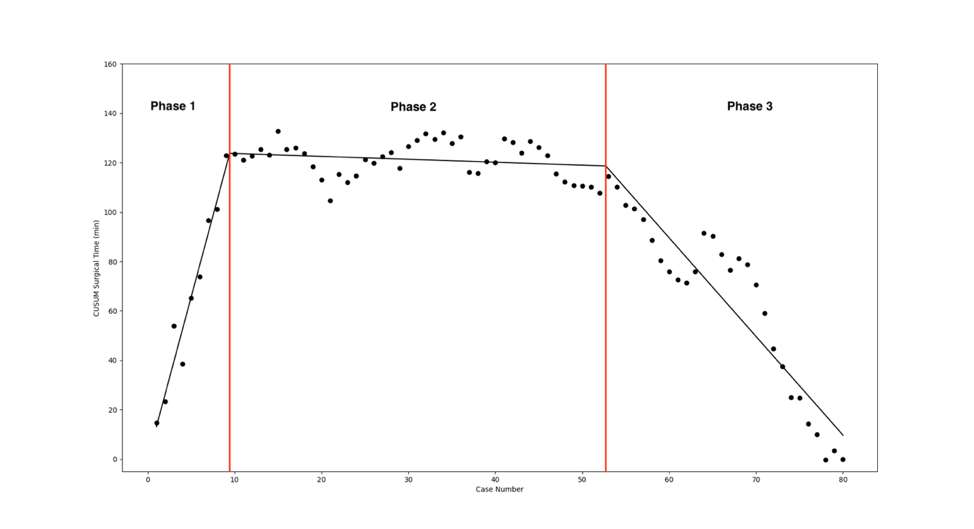
Figure 1
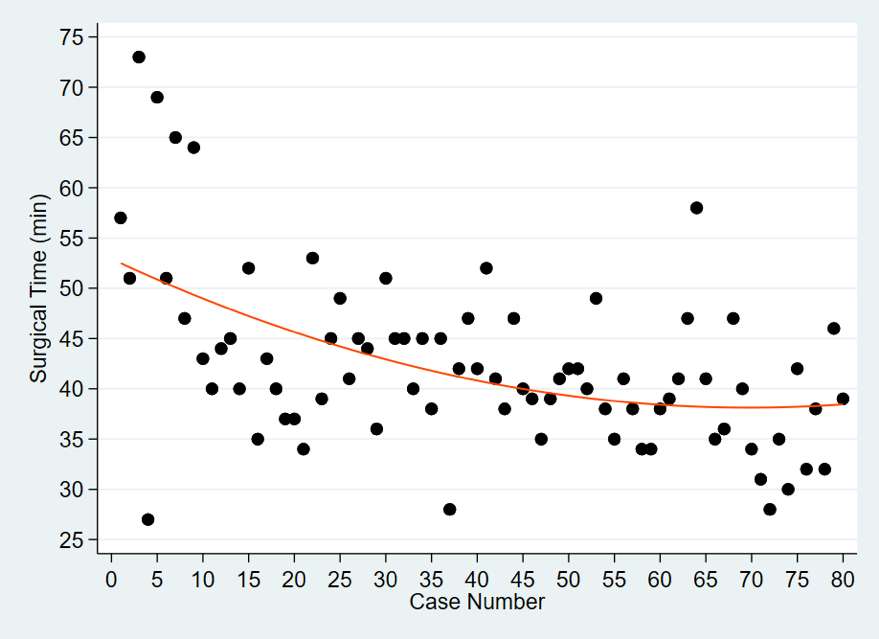
Figure 2#8211
Is TKA Surgical Performance Enhanced Using Augmented Reality? a Single Center Study on 76 Consecutive Patients
*Edoardo Bori - BEAMS Department ULB - Bruxelles, Belgium
Bernardo Innocenti - Universite' Libre De Bruxelles - Bruxelles, Belgium
Gianluca Castellarin
Elodie Barbieux - Université Libre de Bruxelles - Bruxelles, Belgium
Victor-Paul Grandjean - Université Libre de Bruxelles - Bruxelles, Belgium
Grace Jost - Université Libre de Bruxelles - Bruxelles, Belgium
*Email: edoardo.bori@gmail.com
Introduction
Augmented Reality (AR) is a recently introduced powerful tool which has already shown its potential in a wide spectrum of surgical procedures, including orthopaedics for the majorly addressed joints. This technology indeed allows, through the use of a dedicated visor, to visualize a series of information and/or images super-imposed to the user’s field of vision; thanks to this feature, therefore, it was introduced as a surgical assistant tool.
This single-center study, focused on total knee arthroplasty, aims to evaluate the outcomes of surgeries performed with AR assistance in terms of time of usage required by the system, blood loss and difference between the pre-planned positioning and the achieved one, defined in terms of tibial implant varus and slope angles.
Methods
Seventy-six consecutive patients were selected for this study. Pre-planning was performed according to the AR protocol and the aimed varus and slope angles were defined and used to instruct the AR system, which subsequently guided the tibial cuts intra-operatively (see Figure).
Total Knee Arthroplasty was performed, starting from the tibial cut with the assistance of AR tools; the time required to perform the calibration, registration, and fixation of the resection block was recorded. The varus and slope angles achieved were then recorded with an external measuring tool, in order to perform the comparison between these angles with the pre-planned ones; the average and standard deviation of the obtained differences were evaluated. The femoral cuts were then performed using the tibial ones as reference. The blood loss was measured by quantifying the volume of blood present in the drainage bag after its removal on the first postoperative day.
Results
The average usage time of the AR tool was 5±1 min, and the average blood loss was 450 ml (with no remarkable differences with patient from a control group operated without AR). Comparing planned and achieved varus angles, results showed an average difference of 0,578° ± 0,583°; a difference of 0,600° ± 0,751° was instead measured for the slope. In detail, for varus angles the differences were below 1° for 96% of the cases (and between 1° and 2° for the rest of the cases). Concerning the slope, 89% of the cases were under 1°, 9% between 1° and 2°, and 2% with more than 2°.
Conclusion
The results showed excellent accuracy of the surgical cuts according to the pre-planned ones, and no remarkable complications in surgical duration or bleeding when compared to traditional approaches. These outcomes highlight therefore the potential of this new technology as a valid and competitive option to be considered by the surgeons.
Figures
Figure 1#8530
The Effect of Tibial Component Fixation on Pain in Unicompartmental Knee Replacement (UKR): A 5-Year Radiographic and Patient-Reported Pain Study
*AZMI RAHMAN - University of Oxford
Katerina Dangas - University of Oxford - Oxford, United Kingdom
Stephen Mellon - University of Oxford - Oxford, United Kingdom
David Murray - University of Oxford - Oxford, United Kingdom
*Email: azmissrahman@gmail.com
Introduction: Cementless UKR is associated with less pain than structurally identical cemented UKR. Cementless UKR have been shown to experience fewer and smaller radiolucencies than cemented UKR, but there isn’t strong evidence associating this reduced radiolucency to reduced pain. This study compares radiographic and patient-reported outcomes (PROMs) of tibial component fixation between two large cohorts of cemented and cementless UKR.
Methods: 237 cemented and 191 cementless UKR were recruited and assessed using knee radiographs and the Intermittent and Constant OsteoArthritis Pain (ICOAP) scores five years after surgery. Aligned anterior-posterior knee radiographs were taken. Adequate alignment of the radiographs was assessed using vertical and horizontal rotation assessments calculated from implant projections, and rotation threshold values were used to select radiographs with <1mm radiographic malalignment. Quality of fixation was assessed in seven pre-defined bone-implant interfaces using a 3-pont scale (no radiolucency, partial radiolucency, complete radiolucency). Analysis was radiograph rotation and outcomes were performed using ImageJ software by 2 independent assessors. The ICOAP questionnaire were used to identify patients who experienced pain and patients who experienced no pain at time of assessment. Analysis of the relationship between radiolucency and pain was performed.
Results: All measurements had excellent inter-assessor agreement (ICC>0.9). Radiographs from 58 cemented and 46 cementless (24% of both cohorts) UKR were determined to be adequately aligned. Radiolucencies were significantly greater in the cemented cohort across all regions assessed (p<0.02 in all regions). However, both cemented and cementless cohorts had greatest radiolucency at the tibial wall (cemented:74%, cementless:40%), followed by the medial-most aspect of the tibial floor (cemented:44%, cementless:25%), followed by the lateral-most aspect of the tibial floor (cemented:24%, cementless:8%). Radiolucency was associated with incidence of pain in all regions of the tibial component in both cohorts: this was statistically significant in the floor in cemented (p=0.046) and cementless (p=0.033) cohorts, and keel in the cemented cohort (p=0.022).
Conclusion: Radiolucencies around the UKR tibial component are related to the incidence of pain. Cementless tibial components have significantly reduced radiolucencies than cemented, but radiolucencies persist present in them. These radiolucencies occur almost exclusively at the non-porous regions of the bone-facing implant surfaces, while porous interfaces had virtually no radiolucency. Applying a porous coat onto these non-porous regions of the bone-facing implant surfaces may further reduce radiolucency, and pain, in cementless UKR.
Figures

Figure 1
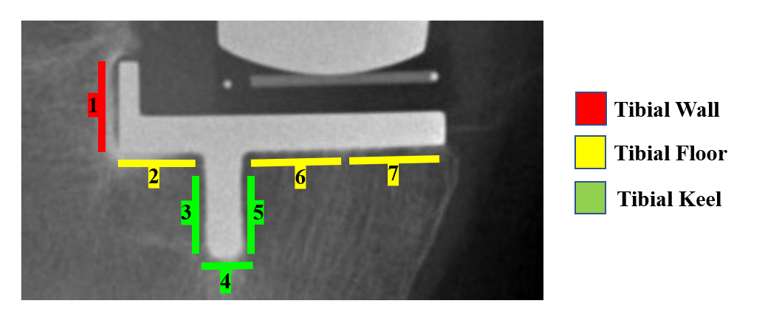
Figure 2
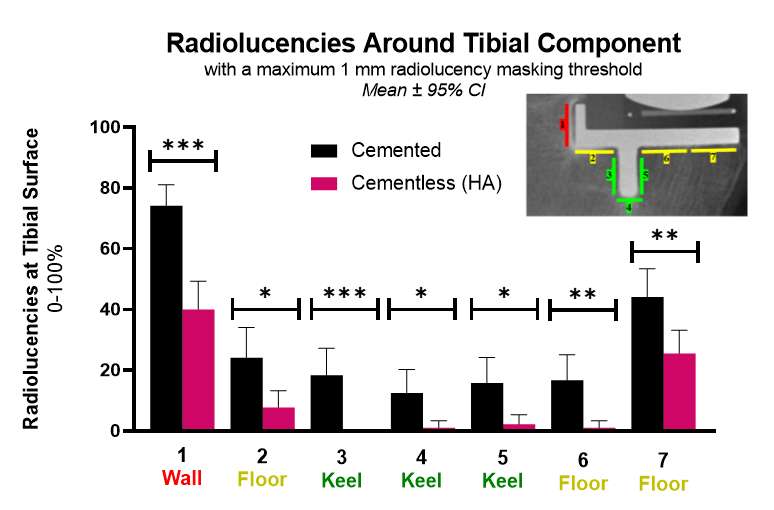
Figure 3
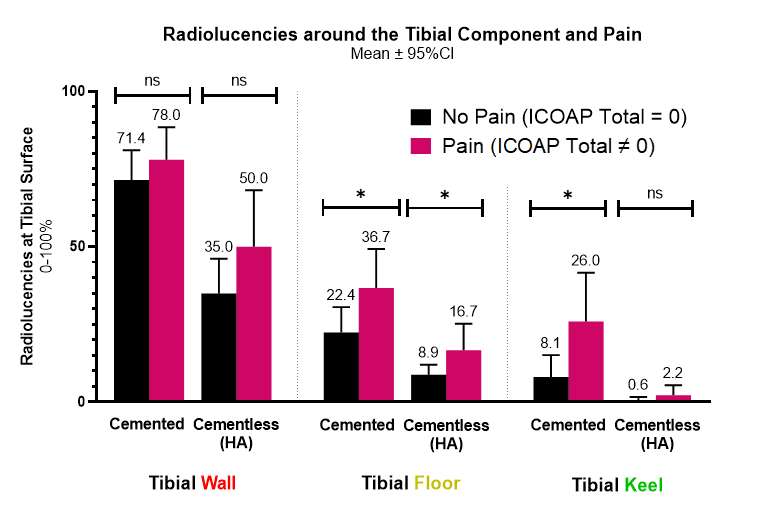
Figure 4#8332
Greater Patient Satisfaction With Cementless Total Knee Arthroplasty in a Case-Controlled Series
Nicolas Piuzzi - Cleveland Clinic - Cleveland, USA
Jianhua Shen - Stryker - Mahwah, USA
Ignacio Pasqualini - Cleveland Clinic Foundation - Cleveland, USA
*Emily Hampp - Stryker - Mahwah, USA
Michael A Masini - Ypsilanti, USA
John W Noble - Center for Orthopedics - Lake Charles, USA
Manoshi Bhowmik-Stoker - Stryker - Mahwah, USA
*Email: emily.hampp@stryker.com
Introduction: The use of cementless constructs in total knee arthroplasty (TKA) has seen a major increase in utilization, with the American Joint Replacement Registry (AJRR) reporting 9.7% in 2019 and 18.8% in 2022 [AJRR 2022]. This is associated with the increase of younger patients with greater BMI requiring surgery who have traditionally shown higher failure rates with cemented TKA [Abdel 2015]. There is growing evidence that biological fixation is a potentially better long-term option for these challenging patient types [Sinicrope 2019, Wang 2020], however patient’s perceptions and satisfaction against cemented TKA has not been well characterized. The purpose of this study was to evaluate patient reported outcomes, functional recovery and satisfaction in patients who received either a cementless or cemented TKA of the same design.
Methods: A prospective, multicenter, consecutive case-control series study was performed where 453 patients received a primary total knee. Cohort 1, 373 knee in 319 patients, received a fully cementless construct, while cohort 2, 147 knees in 134 patients, received a fully cemented construct of the same implant design. Patients completed a 2011 Knee Society Score (KSS), Oxford Knee Score (OKS), and Short Form 12 (SF12) preoperatively and at six weeks, six months, one and two years postoperative. Patient demographics were compared between groups using a Wilcoxon test for numeric variables and the chi-square test for categorical variables. The outcome scores were analyzed using a repeated measures mixed model, with multiple categorical factors and baseline scores as covariates. The model accounted for the correlation between repeated measures within each participant. All statistical tests were two-tailed, and p-values less than 0.05 were considered statistically significant.
Results: There were no significant differences in mean age (62.12 ± 4.03 vs 63.18 ± 4.14), BMI (30.99 ± 4.38 vs 30.99 ± 4.13) or gender (49% female vs 57% female) between cohort 1 and cohort 2, respectively. All patients had a primary diagnosis of end-stage osteoarthritis. OKS showed significantly higher scores in cohort 1 out to 2 years (p=0.0018) (Figure 1). SF12 Physical Composite Score was higher (p=0.0064) in cohort 1. KSS was significantly greater in the objective score (p<0.0001) (Figure 2) and functional score (p=0.0016). Patient satisfaction was greater at all timepoints from baseline expectation in cohort 1 (p< 0.0001) (Figure 3).
Discussion: In this case-controlled consecutive cohort study, the cementless construct group outperformed cemented knees in patient outcomes, satisfaction and function out to two years postoperative. This work suggests an excellent alternative to cemented knees within a short-term follow-up.
Figures
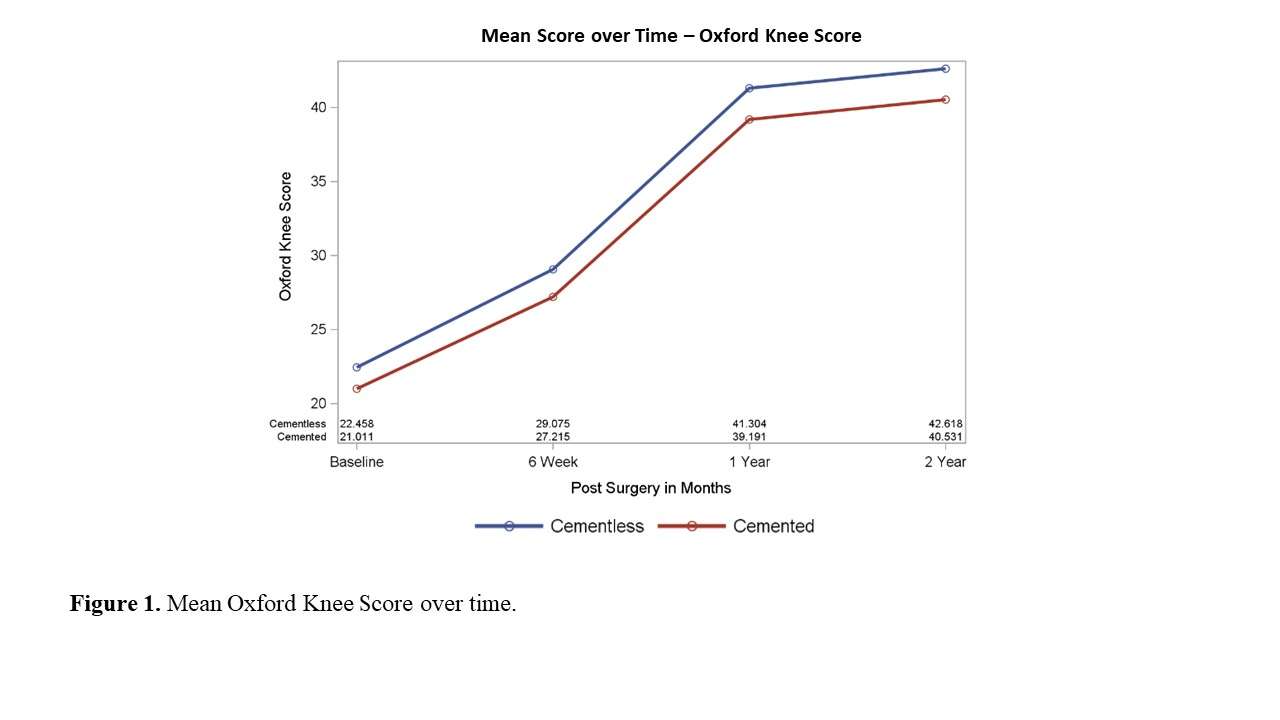
Figure 1
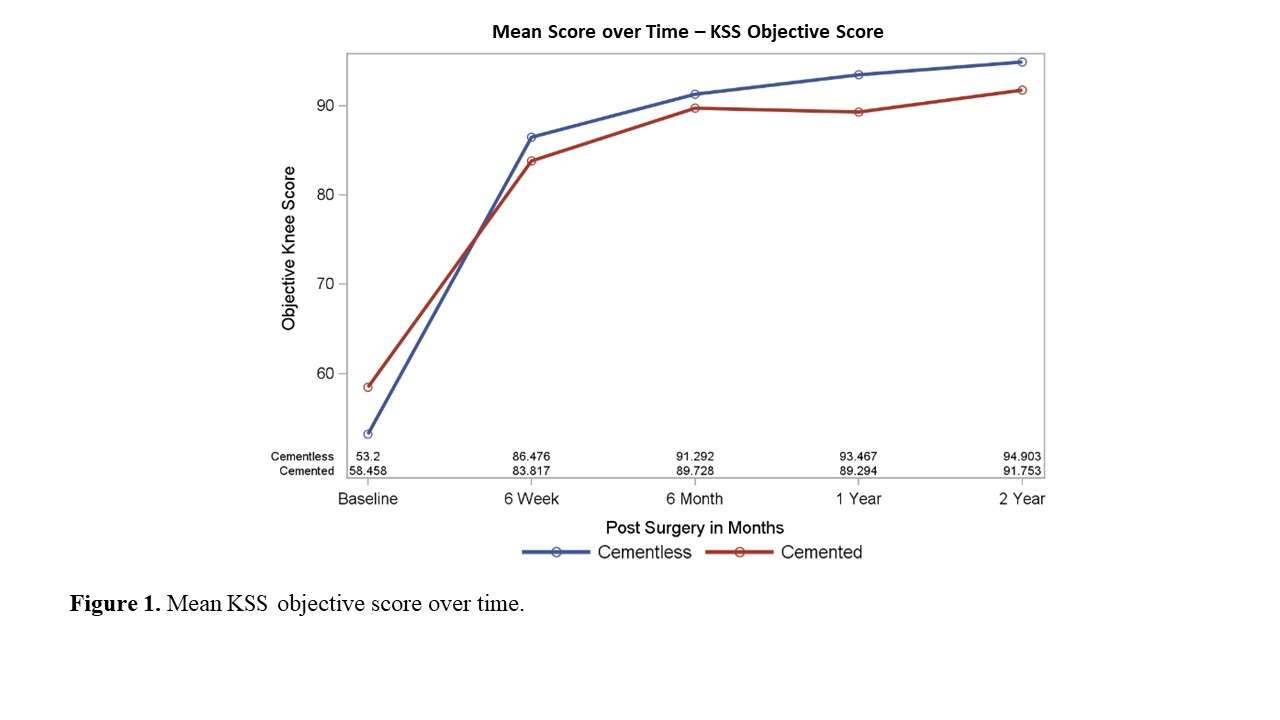
Figure 2
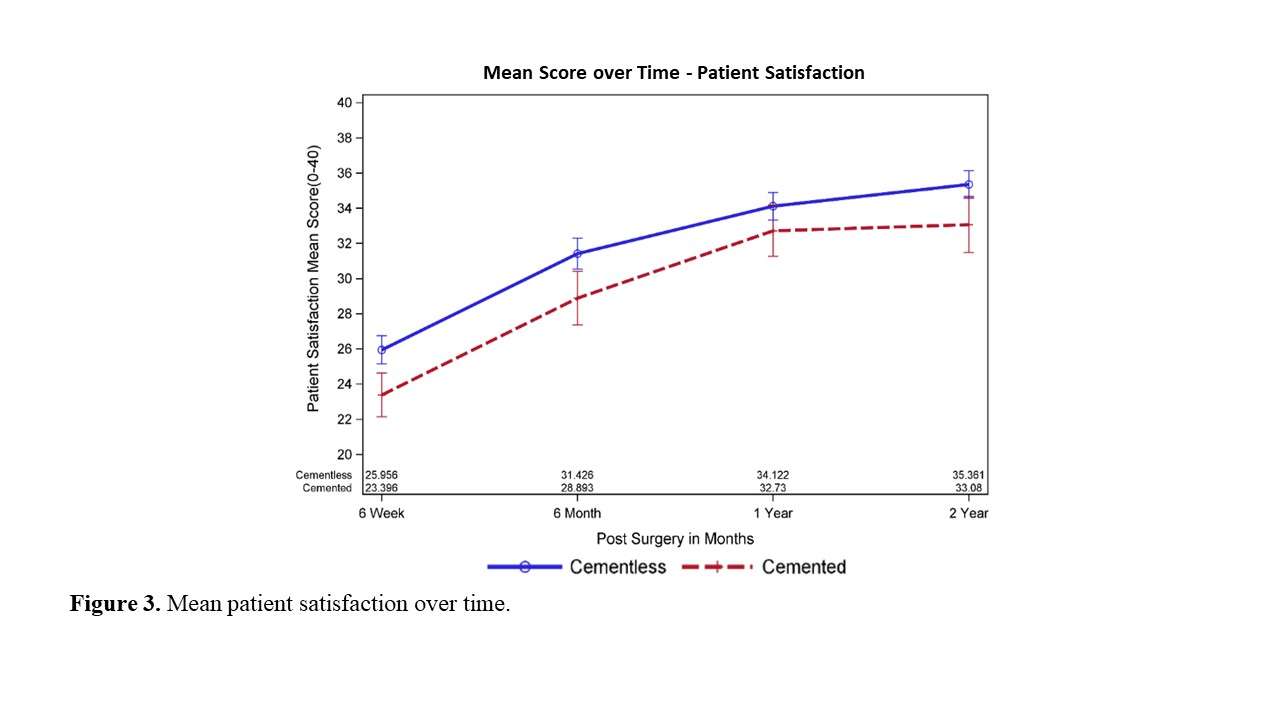
Figure 3#8451
Optimizing Intra-Operative Laxity and Balance for Improved 2 Year Pain Outcomes in TKA: A Prospective Cohort Study
*Edgar Wakelin - OMNI Life Sciences - Raynham, USA
Corey E. Ponder - Oklahoma Sports and Orthopedics Institute - Oklahoma, USA
Amber Randall - Flagstaff Bone and Joint - Flagstaff, USA
Jan Albert Koenig - Winthrop University Hospital - Rockville Centre, USA
Christopher Plaskos - Corin - Raynham, USA
Jeffrey DeClaire - The DeClaire LaMacchia Orthopaedic Institute - Rochester, USA
Jeffrey Lawrence - Gundersen Health System - viroqua, USA
John Keggi - Orthopaedics New England - Middlebury, USA
*Email: edgar.wakelin@coringroup.com
Aim
Integrated sensor and robotic-assisted technologies for total knee arthroplasty (TKA) allow surgeons to predictively plan and execute a desired joint alignment, laxity and tibiofemoral balance. Such technologies, however, are new to the market and although some have shown promise in improving short term patient reported outcome measures (PROMS), studies showing an impact on mid-term outcomes (i.e. ≥2-years) and beyond are lacking. The objective of this study was to determine if intra-operatively measured joint gaps are associated with 2-year pain outcomes in total knee arthroplasty (TKA) and whether balance and laxity windows could be defined in extension (10?), mid-flexion (40?) and flexion (90?) to optimize 2-year pain outcomes.
Methods
A prospective investigation of 310 robotically-assisted TKAs was performed. Final joint gap data was recorded using a digital tensioner, and component alignment data was recorded by the robotics system. In all cases a PCL sacrificing, tibia first approach was performed with an ultra-congruent tibial insert. The patella was resurfaced. Patient demographics and KOOS scores were recorded pre-operatively and KOOS scores and HSS satisfaction were recorded at 2-years post-op. A random search optimization algorithm was used to determine the global optimum laxity and balance window throughout flexion which maximized the improvement in 2-year KOOS pain scores. The windows were then combined to determine the impact of achieving optimal laxity and balance throughout flexion.
Results
Laxity and balance windows were defined in extension (Med lax: -2.0 to 2.5mm, Lat lax: -0.5 to 2.5mm, Balance: -3.0 to 0.0mm), mid-flexion (Med lax: -1.0 to 2.5mm, Lat lax: -0.5 to 3.0mm, Balance: -2.0 to 2.0mm) and flexion (Med lax: -2.0 to 3.5 mm, Lat lax: -2.0 to 1.5mm, Balance: -3.0 to 3.0mm), Figure 1. When all windows were satisfied, the greatest improvement in KOOS pain score was observed (100.0 vs 94.4, p < 0.0001), Figure 2A. The highest percentage of knees satisfying the Patient Acceptable Symptom State was also observed (93% vs 71%, p=0.0009) in knees which satisfied all windows, Figure 2B. This difference increased in knees which only satisfied 1-3 (29%) or 4-6 (69%) windows (p ≤ 0.0018). No optimal windows were found between component alignment and KOOS pain outcome (p ≥ 0.1180). High satisfaction was found across all groups (≥95%).
Conclusion
Intra-operatively measured joint gaps are associated with patient outcomes at 2-years after TKA. Optimal windows for improving patient outcomes were defined for laxity and balance but not for alignment indicating balance may have a greater impact on outcome than alignment.
Figures
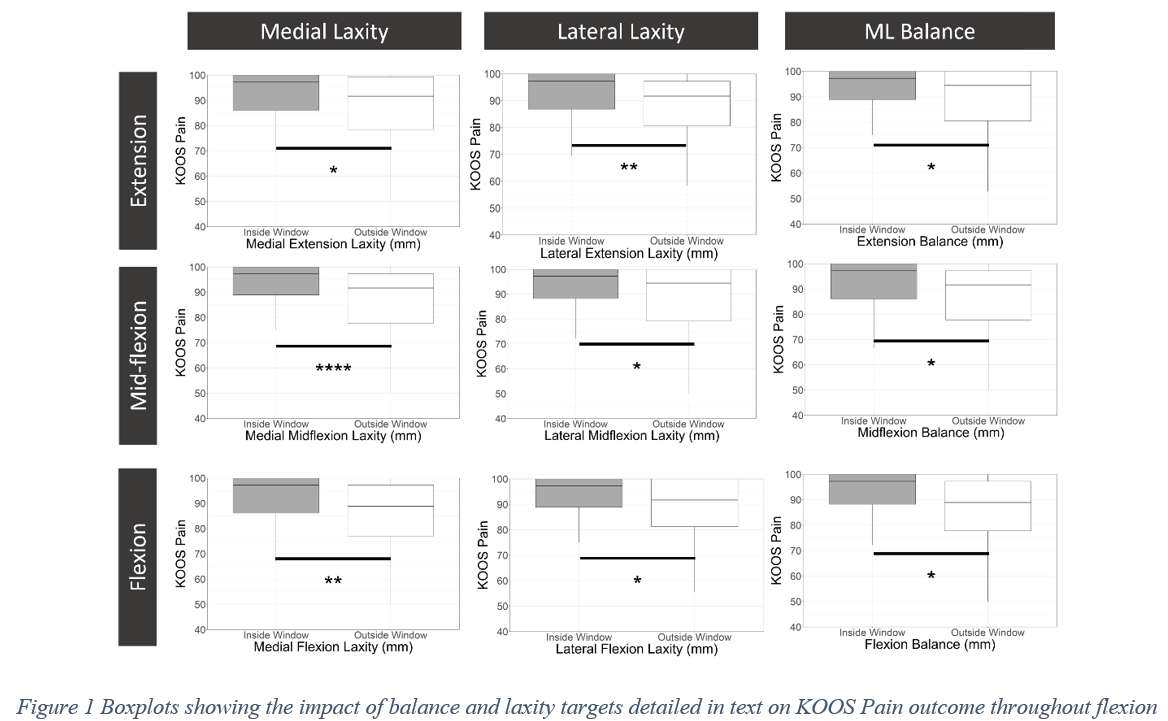
Figure 1
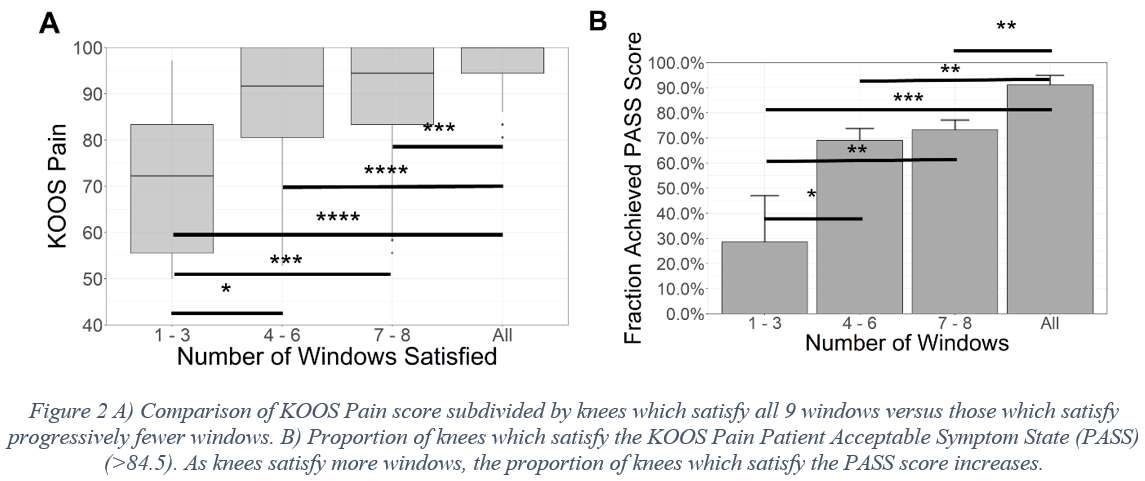
Figure 2#8322
With Preserved Medial Stability, Lateral Looseness Could Be Intraoperatively Allowed in PS-TKA for Varus Type Osteoarthritic Knees
Takao Inokuchi - Kobe Kaisei Hospital - Kobe City, Japan
*Hirotsugu Muratsu - Steel Memorial Hirohata Hospital - Himeji, Japan
Daiya Kitazawa - Dept. of Orthop Surg., Hyogo Prefectural Harima-Himeji General Medical Center - Himeji, Japan
Tomoyuki Matsumoto - Kobe University Graduate School of Medicine - Kobe, Japan
Akihiro Maruo - Steel Memorial Hirohata Hospital - Himeji, Japan
Ryosuke Kuroda - Department of Orthopaedic Surgery, Kobe University Graduate School of Medicine - Kobe, Japan
*Email: hiromuratsu@gmail.com
Introduction
For the varus type knees, medial release was performed to enlarge medial gap equalizing to the pathologically elongated lateral gap, which raised the risk of medial instability and worsen clinical outcome. The authors reported a new soft tissue balancing technique (medial preserving gap technique; MPGT). We prioritized the medial stability not aiming at perfect ligament balance and allowing lateral looseness. This study aimed to evaluate medial gap consistency and the influence of lateral looseness on postoperative clinical outcomes in PS-TKA with MPGT.
Methods
Nighty-one knees with varus type osteoarthritis underwent primary PS-TKA using the MPGT. Soft tissue balance with femoral trial component in place and patello-femoral joint reduced was quantitatively evaluated using offset type tensor with 40lbs. of joint distraction force. Both component gap and varus angle were measured at full extension and 90° of flexion. The medial compartment gap was calculated using trigonometric function (Fig 1). The patients were divided into two groups according to intraoperative varus angle at full extension; group N ≤ 3° (70 knees) and group V > 3° (21 knees). Clinical outcomes with Knee Society Score 2011 (KSS; symptoms, patient satisfaction, and functional activities) were compared pre and one-year postoperatively between 2 groups using 2 factors ANOVA (p < 0.05).
Results
Preoperative HKA angle (varus angle) was significantly higher in group V (18.9±7.0°) than N (12.4±5.6°). Mean intraoperative varus angle at extension was 1.3±1.2° in group N and 5.2±1.7° in group V (p<0.0001). The mean medial compartment gaps (mm) in group N and V were 11.0±1.6, 11.0±1.4 at extension and 14.2±2.4, 14.4±2.3 at flexion each respectively. No significant differences were found in medial compartment gaps between 2 groups at both extension and flexion. KSS scores with symptoms, satisfaction and functional activities in group N vs V were 8.8±4.4 vs 8.9±4.2, 18.1±8.3 vs 20.8±9.1 and 50.5±16.6 vs 54.6±14.3 preoperatively, and 18.9±5.9 vs 16.6±6.1, 30.5±5.7 vs 31.2±6.7 and 73.6±14.7 vs 71.7±18.6 postoperatively. Although both groups showed significant improvements with all scores 1 year postoperatively, there were no significant differences between 2 groups pre and postoperatively.
Discussions
Preoperative HKA angle in group V showed significantly more varus than group N, but medial compartment gaps showed no significant difference between 2 groups at both extension and flexion. Once we created adequate medial gap at extension for the implant thickness, aggressive medial release was not attempted to enlarge medial gap as much as the pathologically elongated lateral gap with permitting varus ligament imbalance; lateral looseness. these was feature of MPGT. The influence of lateral looseness with the condition of equal medial stability were not significant on 1-year postoperative clinical outcomes; symptoms, patient satisfaction, functional activities. This study showed that MPGT would be a safer fungible to preserve medial stability without excessive medial release for severe varus osteoarthritic knee. A residual asymmetrical joint gap; trapezoidal joint gap, could be allowed in PS-TKA using MPGT.
Conclusion
As far as medial knee stability was preserved, intraoperative lateral looseness did not affect clinical outcome in PS-TKA for severe varus type OA knees.
Figures
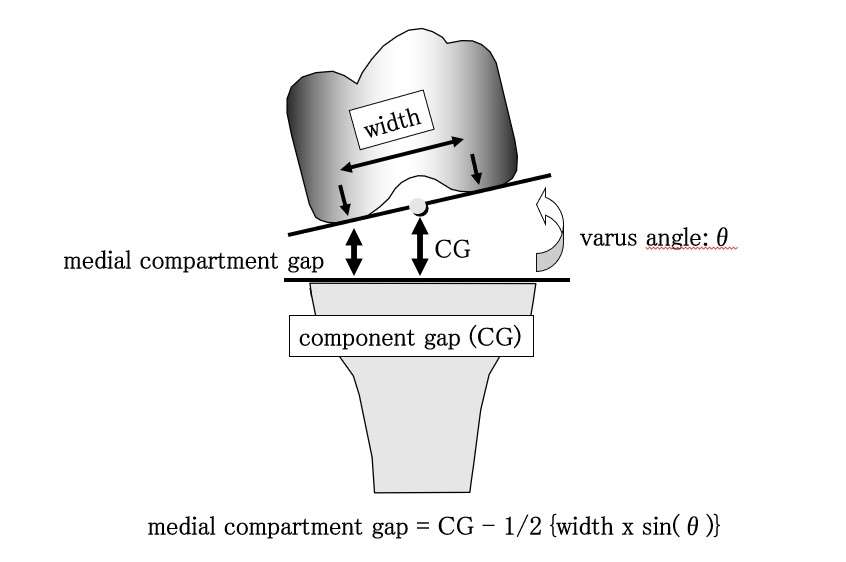
Figure 1
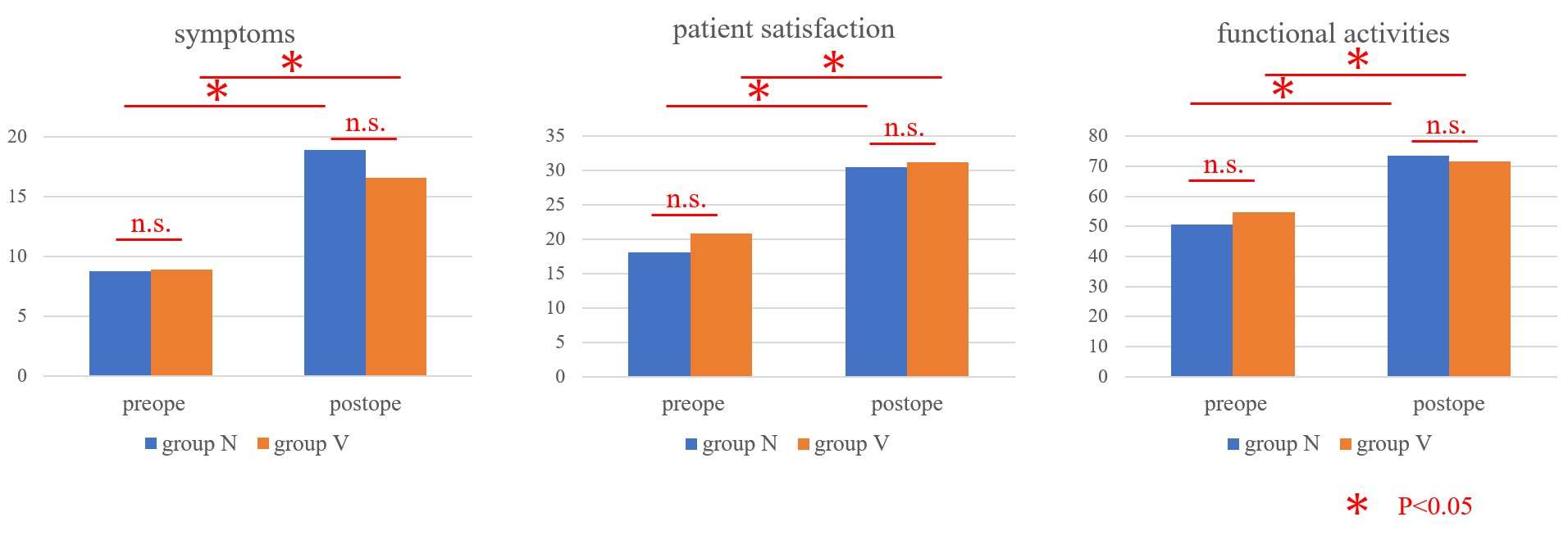
Figure 2#8170
Are Kinematics an Indicator of Outcome After Total Knee Arthroplasty?
*Joseph Lynch - Sydney Orthopaedic Research Institute - Chatswood, Australia
Pip Hodge - Australian National University - Canberra, Australia
Owen Rabak - Australian National University - Canberra, Australia
Diana M Perriman - Australian National University - Canberra, Australia
Jennifer Scarvell
Paul N Smith - Trauma and Orthopaedic Research Unit - Canberra, Australia
*Email: joe.lynch81@gmail.com
Introduction: The goal of total knee replacement (TKA) surgery is to relieve pain as well as to enhance function, range of motion, and joint stability. While in most cases TKA is successful, up to 20% of patients do not have a completely successful outcome [1]. Successful outcomes are typically evaluated through validated patient reported outcome measures (PROMs). Widely used PROMs include the Oxford Knee Score, Knee Society Score, and satisfaction visual analogue scales. Reasons for lack of self-reported success include ongoing pain, stiffness, and lack of expected function, especially in higher demand activities necessary for daily independence such as deep-kneeling and ascending and descending stairs/ladders [2,3]. Up to 80% of patients report ongoing difficulties performing deep-kneeling and ascending and descending tasks compared to people with healthy knees [4,5]. These difficulties negatively affect quality of life and satisfaction in patients following TKA, regardless of improvement compared to preoperative status [8]. Given both tasks are essential for daily independence and mobility, it is important to understand what factors are associated with poor outcomes in these activities.
Methods: This was a secondary analysis of a prospective RCT examining implant kinematics. 64 patients were included at minimum 1-year follow-up. Participants performed a step-up and deep-kneeling task which was imaged via continuous single-plane fluoroscopy. The 3D prosthesis CAD models were then registered to the fluoroscopy, yielding six-degree of freedom kinematic data. Patient reported outcome measures, including Oxford Knee Score, American Knee Society Score, surgical satisfaction and pain visual analogue scales were also collected. The associations between kinematics for both movements and PROMS were assessed using step-wise linear regressions controlled for sex, weight, height, and implant design.
Results: A higher total OKS was associated with more external rotation and more adduction at maximal flexion during kneeling, and more external rotation and minimum flexion during step-up. Improved AKSS was associated with increased internal-external rotation during step-up. Improved surgical satisfaction was associated with greater maximum flexion and more external rotation at maximal flexion during deep-kneeling; and more femoral internal rotation at terminal extension during step-up. An improved pain VAS score was associated with greater maximum flexion and more femoral external rotation during deep-kneeling; and greater internal femoral rotation during step-up.
Conclusion: The results of this study indicate that knee kinematics are associated with surgical satisfaction and other common PROMS, particularly at the limits of flexion and extension. These results have implications for the importance of rehabilitation and implant design. There needs to be a continued focus on optimising terminal flexion, extension and rotational range in total knee arthroplasty which may lead to an enhanced level function and patient outcomes.
References
1. Bourne RB, et al. Clin Orthop Relat Res 468(1): 57, 2010
2. Dunbar MJ and Haddad FS. Bone Joint J 96-B(10): 1285, 2014
3. Robertsson O et al. Acta Orthop Scand 71(3): 262, 2000
4. Wylde V, et al. EFORT Open Rev 4(7): 460, 2019
5. Byrne JM, et al. Clin Biomech (Bristol, Avon) 17(8): 580, 2002
#8225
In-Vivo Weight-Bearing Kinematics for Constrained Versus Traditional BCS TKA Cohorts Compared to the Normal Knee
*Michael LaCour - University of Tennessee - Knoxville, USA
Garett Dessinger - The University of Tennessee - Knoxville, USA
steven haas - Hospital for Special Surgery - New York, United States of America
Richard Komistek - The University of Tennessee - Knoxville, USA
*Email: mlacour@utk.edu
INTRODUCTION
Given the changes to a patient’s knee biomechanics following total knee arthroplasty (TKA), patients are often susceptible to instability and loss of functional outcomes postoperatively, especially in deep flexion activities. These factors, combined with patients who may have preoperative conditions or complex deformities, have led to the development of a variety of constrained TKA options. While constrained inserts may offer additional stability, whether patients will feel unrestricted or over-constrained is a topic of discussion. The objective of this study is to evaluate postoperative weight-bearing kinematics of both traditional and constrained Journey II Bi-Cruciate Substituting (BCS) TKA inserts and compare them to previously published non-implanted knee data.
METHODS
In vivo knee kinematics were assessed for 40 total subjects implanted by the same surgeon with a Journey II BCS TKA: 20 with a traditional polyethylene insert and 20 with a constrained polyethylene insert. Additionally, data for 10 non-implanted subjects from a previously published study were included as a comparison cohort. Each subject was asked to perform a weight-bearing deep knee bend activity while under fluoroscopic surveillance, and popular three-dimensional model fitting approaches were used to extract weight-bearing flexion and femorotibial condylar motion patterns. Specific parameters of interest included maximum weight-bearing range-of-motion (ROM), the anterior/posterior movement of the lateral condyle (LAP), the anterior/posterior movement of the medial condyle (MAP), and femorotibial axial rotation (AxRot).
RESULTS
The average ROM was 131.1° ± 9.3° for the Traditional Cohort, 123.6° ± 7.4° for the Constrained Cohort, and 139.0° ± 13.8° for the Non-Implanted Cohort. Both the Traditional Cohort and the Non-Implanted Cohort were statistically higher than the Constrained Cohort (p < 0.046).
All three cohorts had their lateral condyle initially positioned anterior to the midline (p < 0.032), similar to the screw-home mechanism. From full extension to maximum flexion, the average LAP was 12.4 mm ± 4.8 mm posterior for the Traditional Cohort, 15.6 mm ± 4.4 mm posterior for the Constrained Cohort, and 25.5 mm ± 11.1 mm posterior for the Non-Implanted Cohort (Figure 1). No participants in any cohort experienced lateral paradoxical anterior sliding. Both implanted cohorts were statistically lower in magnitudes than the Non-Implanted Cohort (p < 0.0007). All 3 cohorts experienced similar MAP magnitudes (p > 0.051) and patterns (Figure 2).
From full extension to maximum flexion, the AxRot was 5.6° ± 4.5° external for the Traditional Cohort, 5.8° ± 5.8° external for the Constrained Cohort, and 21.6° ± 10.3° external for the Non-Implanted Cohort (Figure 3). Both implanted cohorts experienced less AxRot than the Non-Implanted Cohort (p < 0.0001).
DISCUSSION
Although numerous studies have investigated the postoperative outcomes of the Journey II BCS TKA, no previous studies have investigated the constrained insert option in comparison to a traditional insert design and the normal knee. Subjects with a constrained insert still showed excellent postoperative kinematics and weight-bearing flexion, statistically comparable to the traditional insert, which indicates that the constrained insert allows the patients to feel more stable and less restricted by this TKA system.
Figures
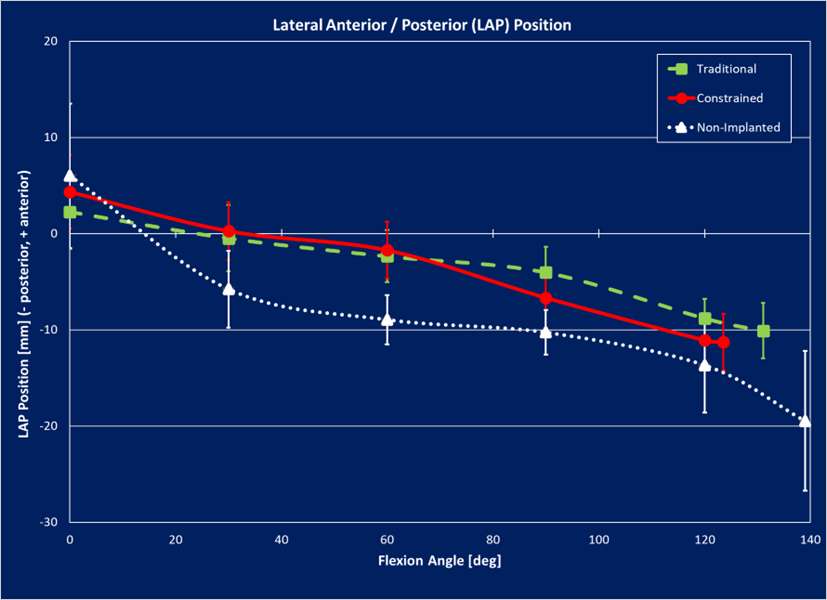
Figure 1
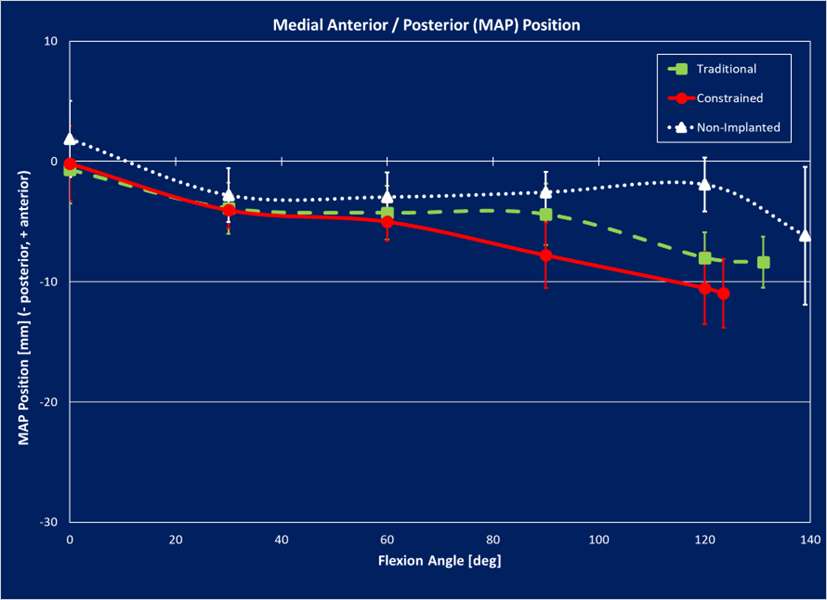
Figure 2
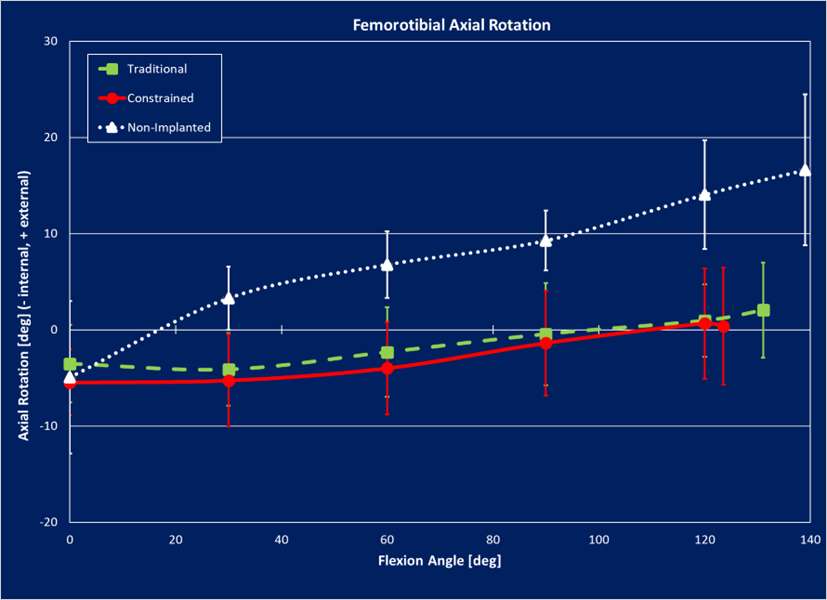
Figure 3#8624
Increased Posterior Pelvic Tilt From Pre-Operative Supine to Post-Operative Standing: Implications for Surgeons Utilising the Direct Anterior Approach
*Christopher Plaskos - Corin - Raynham, USA
Nathanael Heckmann - Keck School of Medicine of USC - Los Angeles, USA
Jonathan Bare - Melbourne Orthopaedic Group - Melbourne, Australia
Andrew Shimmin - Melbourne Orthopaedic Group - Melbourne, Australia
Jim Pierrepont - Corin - Pymble, Australia
*Email: christopher.plaskos@coringroup.com
Introduction: The aim of this study was to determine the change in pelvic tilt (PT) occurring from the preoperative supine to postoperative standing position following THA, and to identify factors associated with significant increases in posterior PT and resulting increases in functional acetabular cup anteversion.
Methods: In total, 933 primary THA with preoperative supine CT and standing lateral radiographs, and 1-year postoperative standing lateral radiographs were reviewed following IRB approval. Patients were operated on by one of four surgeons from two centers and followed a standardized imaging protocol. Preoperative supine PT was measured from CT as the angle between the anterior pelvic plane (APP) and the horizontal plane of the CT device. Standing PT was measured on standing lateral x-rays as the angle between the APP and the vertical line. Lumbar lordosis (LL), sacral slope (SS), and pelvic incidence (PI) were also measured, Figure 1. Positive and negative values indicate the pelvis is anteriorly and posteriorly tilted, respectively. Patients with ≥13° of posterior PT change from supine pre-op to standing post-op (corresponding to approximately a 10° increase in cup anteversion [1]) were grouped (Group A) and compared to those with less change (Group B) using unpaired student’s t-tests. Paired t-tests were used to compare changes in PT across the three positions.
Results: On average, the preoperative supine pelvic position changed from 3.8±6.0° to the postoperative standing position of -3.5±6.9° for a mean change of -7.4±4.5° (p<0.001). In total, 10.2% (95/933) had >13° posterior pelvic tilt from preoperative supine to postoperative standing, resulting in a corresponding increase in functional acetabular version ≥10°, Figure 2. Patients who had posterior pelvic tilt >13° were on average older (67.0 ± 8.0 vs 64.7 ± 8.7, p=0.011) and more likely to be female (66.3% vs 53.9%, p = 0.022) compared to patients with less posterior pelvic tilt. Preoperative factors associated with standing posterior pelvic tilt >13° were greater anterior supine PT (3.7° vs 5.3°, p=0.017), PI-LL mismatch (-2.4° vs 2.3°, p<0.013), greater posterior standing PT (-1.1° vs -4.7°, p<0.001) and a larger posterior change in PT from supine-to-standing (-4.8° vs -10.1°, p<0.001) and from supine-to-seated (-1.7° vs -6.0°, p=0.002), Table 1.
Conclusions: Approximately, one in ten patients experienced >13° posterior pelvic tilt postoperatively compared to their supine pelvic position, resulting in an increase in functional anteversion of more than 10°. Surgeons implanting acetabular components in the supine position should be aware of the potentially large increase in functional anteversion occurring in this subset of patients. Among the most important preoperative parameters indicative of posterior PT changes >13° from preoperative supine to postoperative standing were standing PT [2], supine-to-stand PT, supine-to-seated PT, and PI-LL mismatch.
References
[1] Lembeck B, Mueller O, Reize P, Wuelker N. Pelvic tilt makes acetabular cup navigation inaccurate. Acta Orthop 2005;76:517e23.
[2] Fujii J, Aoyama S, Tezuka T, Kobayashi N, Kawakami E, Inaba Y. Prediction of Change in Pelvic Tilt After Total Hip Arthroplasty Using Machine Learning. J Arthroplasty. 2022 Jul 2:S0883-5403(22)00682-9. doi: 10.1016/j.arth.2022.06.020.
Figures
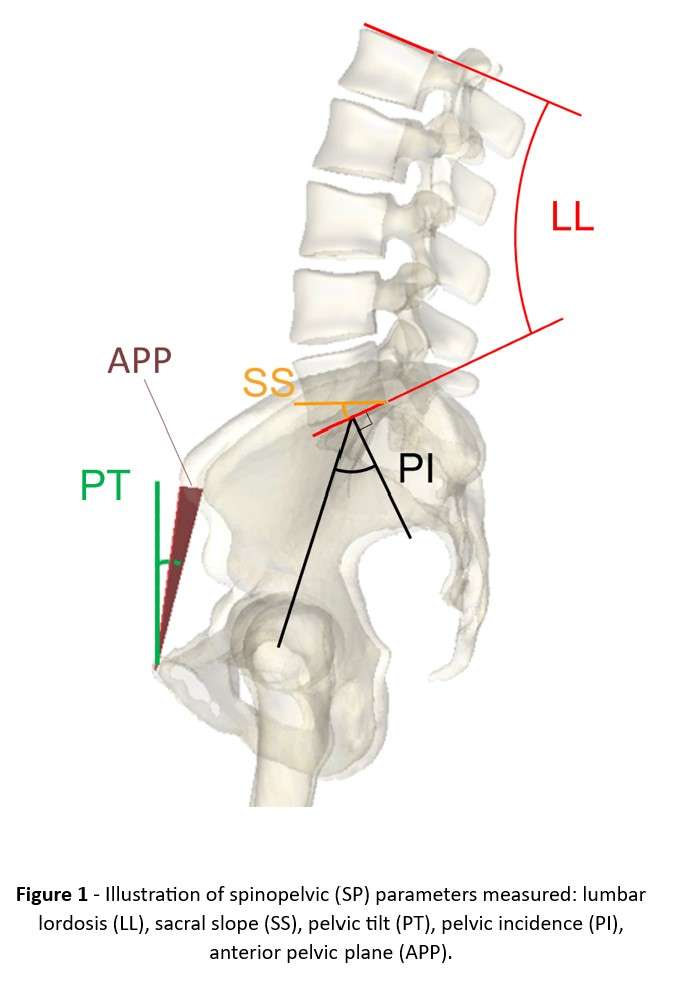
Figure 1
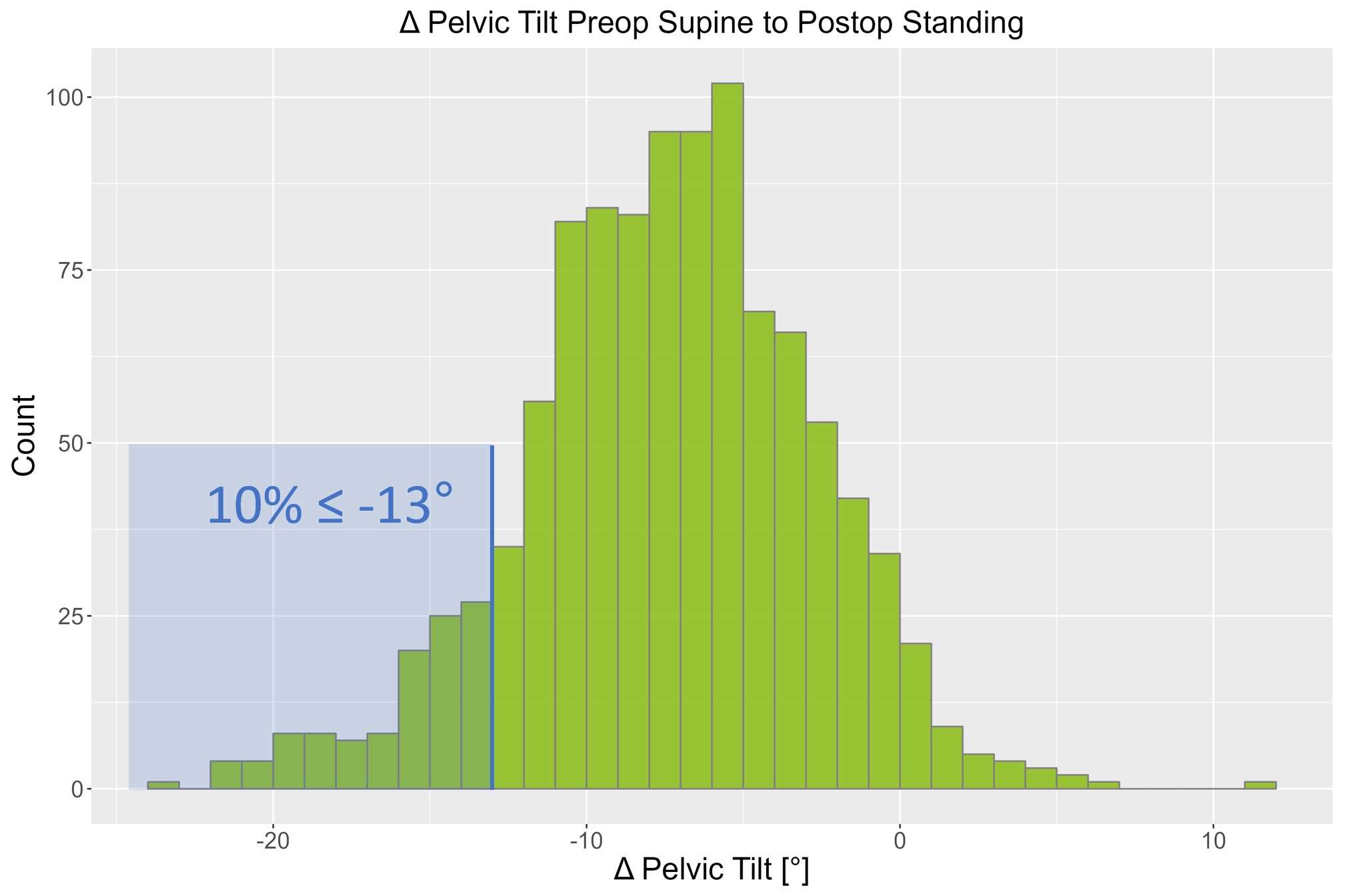
Figure 2
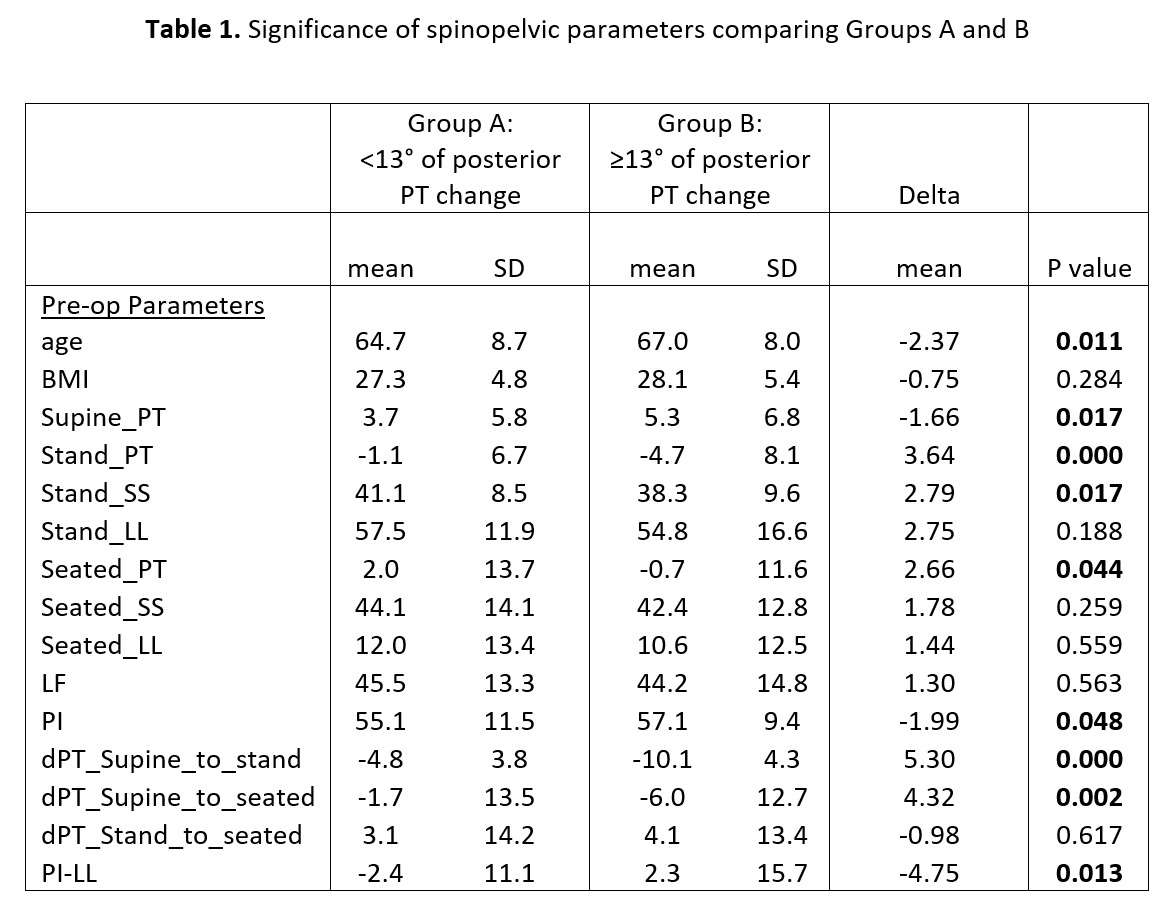
Figure 3#8181
Changes in Spinopelvic Tilt and Mobility After Total Hip Arthroplasty Using the Hip-Spine Classification: A Deep-Learning Enabled Analysis
*Seong Jun Jang - Hospital for Special Surgery - New York, United States of America
Theofilos Karasavvidis - Hospital for Special Surgery - New York, USA
Cale Pagan - Hospital for Special Surgery - New York, United States of America
Robert Ricotti - Hospital for Special Sugery - New York, USA
David J. Mayman - Hospital for Special Surgery - New York, USA
Seth A. Jerabek - Hospital for Special Surgery - New York, USA
Peter K. Sculco - Hospital for Special Surgery - NYC, USA
Jonathan Vigdorchik - Hospital for Special Surgery - New York, USA
*Email: seongjang22@gmail.com
Changes in Spinopelvic Tilt and Mobility after Total Hip Arthroplasty using the Hip-Spine Classification: A Deep-Learning Enabled Analysis
Abstract
Background: Understanding the relationship between spinopelvic mobility and spinopelvic tilt (SPT) is crucial to assess and reduce the risk of dislocation following total hip arthroplasty (THA). The purpose of this study was to apply a novel SPT measurement deep learning (DL) workflow in a large cohort of patients to determine the changes in SPT and mobility after THA.
Methods: A multi-modal DL workflow was developed to automatically measure SPT on spinopelvic radiographs in 504 patients who underwent THA using the hip-spine classification. Spinopelvic mobility was defined as hypermobile (ΔSPTstand-sit>30°), normal (10°ΔSPTstand-sit30°), and stiff (ΔSPTstand-sit10°). Analyses investigating the relationship between SPT, spinopelvic mobility, and hip-spine classifications were performed.
Results: The DL workflow had excellent accuracy in measuring SPT and spinopelvic mobility (ICC=0.96-0.98). Anterior SPT resolved (60%) or decreased (84%) in most patients. Postoperative changes in sitting and standing SPT were -4.110.9 (p<0.01, r=0.57) and 2.25.2 (p<0.01, r=0.83), respectively. 66% of patients with preoperative stiffness remained stiff whereas only 22% of patients with hypermobility remained hypermobile postoperatively. Patients identified using the hip-spine classification as either 1A or 2A (p<0.01), but not 1B or 2B (p>0.05), decreased in spinopelvic mobility following THA.
Conclusion: We leveraged DL to measure SPT and determined the relationship between pre- and postoperative SPT and mobility in patients who underwent THA using the hip-spine classification. Sitting and standing SPT changed postoperatively, and spinopelvic mobility change was dependent on preoperative mobility. The use of the hip-spine classification in THA planning demonstrated the resolution of anterior SPT and hypermobility.
Keywords: hip-spine classification; spinopelvic tilt, spinopelvic mobility, artificial intelligence; deep learning, total hip arthroplasty
Introduction
Total hip arthroplasty (THA) is the preferred surgical treatment for severe hip osteoarthritis and is one of the most successful surgical interventions, with a 10-year survival rate greater than 95%.[1,2] Despite the excellent outcomes of THA, revision surgery due to implant failure poses a challenge, occurring at a rate of approximately 4-5%.[3,4] Dislocation after primary THA has been identified as the most common etiology for revision surgery.[5,6] Although the causes of dislocation are multifactorial, component malposition has been implicated in most cases. Therefore, it is critical for the orthopedic surgeon to emphasize improved surgical technique, implant design, and assessment of patient-specific risk factors such as the hip-spine relationship to improve component positioning and ameliorate instability after THA.
The hip-spine relationship is essential in planning patient-specific acetabular component placement.[7,8] As one goes from a standing to seated position the pelvis rotates posteriorly, lumbar lordosis decreases, and the acetabular anteversion increases to accommodate the femur and avoid impingement and dislocation.[9-11] Spinal deformity and mobility are two issues that impact how the spine and pelvis interact. Spinopelvic mobility has been defined as the change in sacral slope (SS) or spinopelvic tilt (SPT) from the standing to seated position whereas spinal deformity is evaluated by measuring the pelvic incidence-lumbar lordosis mismatch.[12] By considering these parameters, classification systems and algorithms have been developed with patient-specific recommendations of optimal acetabular cup placement for spinopelvic pathology.[7,12,13] So far, studies have evaluated spinopelvic mobility utilizing change in sacral slope (ΔSSstand-sit) using these systems.[14] Despite increased awareness of the hip-spine relationship and its use for cup positioning, the changes in SPT and spinopelvic mobility following THA using these techniques have yet to be thoroughly understood.
Delineating SPT changes after THA utilizing the hip-spine classification would provide value in understanding how acetabular component placement driven by a patient’s specific hip-spine relationship will affect the patient’s postoperative sagittal alignment. These analyses require large cohort studies with radiographic measurements performed on multiple lateral spinopelvic films. The use of technology, specifically deep learning (DL), is well poised to provide objective and accurate assessments of spinopelvic parameters at a rapid pace. Indeed, it has already been used to measure spinopelvic parameters on lateral films with excellent accuracy[15] and to establish image registries for large-scale arthroplasty research.[16] Therefore, this study’s purpose was to develop and apply a novel multi-modal DL workflow to measure SPT and mobility in a large patient cohort and to then analyze predictable changes in SPT and mobility in patients who underwent THA using the hip-spine classification algorithm.
Methods
Patient Cohort and Images
A total of 5,096 THA cases performed by four surgeons using the posterior approach from 2012-2021 were retrospectively reviewed from one institution. Inclusion criteria included patients who 1) underwent unilateral THA; 2) completed sitting and standing spinopelvic imaging (EOS Imaging System™; EOS Imaging Inc, Paris, France) taken within 6-months preoperatively and at least 9-months postoperatively; 3) had preoperatively assessed hip-spine classification grades for acetabular component positioning.[8,12] Exclusion criteria included patients whose imaging (DICOM) was unavailable on the institutional cloud environment for the DL workflow.(Figure 1)
Spinopelvic Parameters: Spinopelvic Tilt and Mobility
Spinopelvic tilt (SPT) was defined as the angle subtended by a line connecting the sacrum endplate center and the center of the two femoral heads and/or implant cup. (Figure 2) Negative SPT values reflected anterior SPT, while positive values reflected posterior SPT. Spinopelvic mobility was defined as the SPT change from standing to sitting (ΔSPTstand-sit). Spinopelvic hypermobility was defined as ΔSPTstand-sit>30°, normal as 10°ΔSPTstand-sit30°, and stiff as ΔSPTstand-sit10°.[14,17] Although spinopelvic mobility can be defined as ΔSS from standing and sitting[14], the ΔSS and ΔSPT are geometrically equivalent[8] and ΔSPT was used in this study given its higher measurement reliability.[18]
Deep Learning Workflow: SPT Measurement Automation
A DL workflow consisting of three convolutional neural networks (CNN) was developed (Figure 3). Various CNNs have been used for computer vision tasks in orthopaedic research, including classification[19], segmentation[20], and object identification.[21] The three models included the following 1) a classification model identifying an image as a sitting or standing spinopelvic imaging; 2) a classification model identifying hip implant number in an image; and 3) a segmentation model identifying spinopelvic landmarks necessary for measurements.[15] These outputs were combined in an additional computer algorithm to automate SPT measurements on both sitting and standing images. Similar DL workflow pipelines for orthopaedic imaging analysis have previously been reported.[16] Initial training data for all three models included 1000 images from 250 randomly selected patients. The sample size was chosen based on other studies utilizing similar DL techniques to automate spinopelvic measurements on imaging[15] (See Supplemental Methods for Model Specifications and Metrics).
Spinopelvic Tilt, Spinopelvic Mobility, and Hip-Spine Classification
We utilized the DL workflow to automatically measure SPT on all sitting, standing, preoperative, and postoperative lateral films for the entire cohort.
Three distinct analyses after THA were conducted with the measured cohort.
1) Sitting SPT, standing SPT, and spinopelvic mobility (ΔSPT) measurements were compared pre and postoperatively to investigate spinopelvic parameter changes.
2) Patients were split into the three spinopelvic mobility groups (hypermobile, normal, and stiff) based on preoperative ΔSPT. The change in spinopelvic mobility (ΔSPTPost–ΔSPTPre) was compared across these three groups and by sex.
3) Patients were also split into the four hip-spine classifications (1A, 1B, 2A, and 2B) based on existing preoperative classification data, and the change in spinopelvic mobility (ΔSPTPost–ΔSPTPre) was compared across these four groups.
Statistical Analysis
The final DL workflow’s SPT measurement accuracy was assessed on an independent hold-out testing cohort of 200 images (100 sitting:100 standing). The DL-automated measurements were compared against two blinded trained readers using inter-class correlation coefficients (two-way mixed, single score, ICC3) and Bland-Altman plot analyses to assess bias. ICCs>0.90 were classified as excellent.[22]
Changes in sitting, standing, and ΔSPT measurements after THA were compared using a paired t-test or Wilcoxon signed-rank test after testing for normality. The Pearson’s R correlation was also determined. A chi-square test was used to determine differences in spinopelvic mobility groups before and after THA. To determine differences in spinopelvic mobility change after THA (ΔSPTPost–ΔSPTPre), an analysis of variance test and post-hoc Tukey comparisons or Kruskal-Wallis test and post-hoc Dunn’s test with Bonferroni adjustments were used to compare the three preoperative spinopelvic mobility groups and the four hip-spine classifications. All statistics were conducted in Python(V3.10) using scipy.stats(V1.9.1), scikit-posthocs(V0.7.0), statsmodel(V0.13.2), and pingouin(V0.5.2) packages.
Results
Image Cohorts
After inclusion and exclusion, 504 total (56.7% female, 61.511.3 years old) unique THA cases were analyzed. Mean follow-up imaging time was 427 days (170 days, minimum 272 days). Of these cases, 355 (54.0% female, 61.110.4 years old) had preoperatively graded hip-spine classifications. BMI were 28.45.4 and 28.45.5 for the two cohorts, respectively.
Deep Learning Workflow SPT Automation
The combined DL workflow calculated SPT at an average rate of 10.3 seconds/image (2,016 total images, 5.8 hours). The sitting vs. standing classification model had an area under the receiver operating characteristic (AUROC) of 1.00, the implant number classification model had an AUROC of 0.99, and the spinopelvic landmark segmentation model had a DSC of 0.88-0.90. Agreement of SPT measurements on an independent cohort of images was excellent (ICC 0.96-0.98) between readers and the final DL workflow.(Table 1) Bland-Altman plot analyses for combined sitting and standing measurements are depicted in Figure 4.
Changes in SPT Parameters and Anterior Tilt Resolution
SPT in the sitting and standing position and spinopelvic mobility were compared before and after THA.(Figure 5) Preoperative and postoperative standing SPT were strongly correlated (R=0.83, p<0.01) and postoperative SPT was higher (i.e. more posterior tilt) with paired differences of 2.25.2 (p<0.01). Preoperative and postoperative sitting SPT was moderately correlated (r=0.57, p<0.01), and postoperative sitting SPT was lower (i.e. more anterior tilt) after THA with paired differences of -4.110.9(p<0.01). Spinopelvic mobility (ΔSPTStand-Sit) was also moderately correlated postoperatively (r=0.46, p<0.01) and decreased with paired differences of -6.612.4 (p<0.01).
In this cohort, 25 patients had anterior tilt (negative SPTstand) preoperatively. 60% (15/25) no longer had anterior tilt after THA and 84% (21/25) had either a decrease in anterior tilt or resolution of anterior tilt with a paired difference 4.2 5.0 (p<0.01) (range -7.3-13.5).
Changes in Spinopelvic Mobility based on Preoperative Mobility
Significant differences in spinopelvic mobility groupings were found after THA (p<0.01).(Figure 6) Notably, 73/110 (66.4%) patients who had preoperative spinopelvic stiffness stayed stiff. Of patients who had spinopelvic hypermobility, 87/112 (77.7%) either had normal or stiff spinopelvic mobility after THA. There was a significant interaction between preoperative spinopelvic mobility and spinopelvic mobility change (p<0.01) and between sex and spinopelvic mobility change (p<0.01).(Figure 7) Patients with preoperative stiffness increased spinopelvic mobility by and patients with normal and hypermobile spinopelvic mobility decreased spinopelvic mobility by and , respectively. All differences were significant (p<0.05).
Changes in Spinopelvic Mobility based on Preoperative Hip-Spine Classification
Preoperative and postoperative SPT and mobility based on the preoperative hip-spine classification are depicted in Table 2. Patients with 2A and 2B classifications did not have any significant differences in spinopelvic mobility after THA (p=0.42-0.72). However, patients with 1A and 2A classifications exhibited decreases in spinopelvic mobility after THA (p<0.01) with patients in group 1A showing the greatest differences (p<0.05).
Discussion
The main findings are as follows: 1) a DL algorithm was developed to automatically measure SPT and spinopelvic mobility on lateral spinopelvic radiographs in ~10 seconds/image with excellent accuracy, 2) assessment of changes in SPT parameters revealed that 84% of patients with anterior tilt (negative SPTstand) preoperatively had either a significant decrease or resolution of anterior tilt after THA, 3) assessment of changes in spinopelvic mobility based on preoperative status demonstrated that 66% of patients who had preoperative spinopelvic stiffness stayed stiff, while 78% of patients who had spinopelvic hypermobility, either had normal or stiff spinopelvic mobility after THA. The latter was further verified by evaluation of changes in spinopelvic mobility based on preoperative hip-spine classification, showing that patients with 1B and 2B classifications did not have any significant differences in spinopelvic mobility after surgery, whereas significant decreases in spinopelvic mobility after THA were observed in patients with 1A and 2A classifications.
Recent research efforts leveraging DL have demonstrated great strides in automatically identifying clinically important features and extracting automated clinical measurements in different orthopaedic imaging modalities.[16,23,24] The present study utilized this approach to automate SPT measurements in lateral sitting and standing EOS imaging. After learning necessary features such as image modality and hip implant number, the DL workflow was able to incorporate this data with relevant bony landmarks segmentations to measure SPT in 2,016 lateral radiographs in approximately 10 seconds/image. All metrics, including AUROC for image modality and implant number classification, dice segmentation coefficient for bony landmark segmentation, and ICC for measurement agreement between human readers were between 0.88-1.00 for the entire workflow during validation and testing, indicating excellent results.[15,23,25] With the growing use of DL techniques in orthopedic research for data extraction, this study provides a powerful example of the immense potential to leverage artificial intelligence technology to investigate clinical outcomes after THA. Indeed, SPT measurements on over 2,000 images were collected in just hours, demonstrating the DL capability to reduce time and resource demands inherent in manual radiographic measurements for orthopaedic research, with potential to also streamline into clinical workflows.
Multiple studies have demonstrated increased dislocation risk after THA in patients with stiff spines compared with patients without spinal disease.[18,26,27] A preoperative analysis of patient’s spinopelvic movement is necessary to seek out the functional safe zone, which will determine a patient-specific acetabular position target.[8,12] Sagittal pelvic rotation (i.e., anterior or posterior tilt) has an important effect on acetabular orientation.[9,11,28] In a normal flexible spine, posterior pelvic tilt causes acetabulum opening and increases inclination and anteversion to allow the pelvis to roll back and the femur to come in parallel to the ground.[29] For every 1 change in PT, the surgeon should expect a 0.7 increase in anteversion.[29-31] Therefore, sagittal spinal balance is key in defining cup position targets. Since a hip flexion contracture can be the reason for anterior PT deformity in patients with a flexible spine, the acetabular component target should be an anatomical position between 20°-25° of anteversion and 40° of inclination, presuming that the flexion contracture may resolve postoperatively.[8,14,28,32] The current study supports this recommendation since most patients with preoperative anterior tilt had either a significant decrease or resolution of anterior tilt after THA, with a significant correction of 4.25.0 degrees after surgery.
The relationship between spinopelvic mobility and functional cup position has been assessed in patients undergoing THA, with hypermobile patients associated with greater change in anteversion and inclination due to changes in pelvic motion.[7,11] Some authors suggest lowering the cup anteversion target between 12-20 in patients with preoperative hypermobility to prevent excessive cup opening from standing to seated position.[33,34] However, there are studies showing spinopelvic hypermobility resolution following THA. In a cohort of 136 patients with preoperative hypermobility, 95% were no longer categorized as hypermobile one year after THA[14], while Stefl et al. showed that 11/19 hypermobile patients decreased their ΔSSstand-sit to below 30° into the normal category at the last postoperative timepoint (ranged from six weeks to one year).[7] Our results support these studies, as 78% of patients with preoperative spinopelvic hypermobility had normal or even stiff spinopelvic mobility after THA. These findings support that spinopelvic hypermobility is primarily hip-driven since correction of the underlying hip osteoarthritis leads to resolution. Adjusting targets for cup position based on spinopelvic hypermobility should be done with caution, as the adjusted target recommendations may exacerbate the decrease in anteversion and potentially increase the posterior dislocation risk.[14]
The hip-spine classification, introduced by Luthringer and Vigdorchik, allows surgeons to evaluate spinopelvic pathology and plan intraoperative management of cup placement.[8] In this study, 66% of patients who had preoperative spinopelvic stiffness stayed stiff, while 78% of patients who had spinopelvic hypermobility either had normal or stiff spinopelvic mobility after THA. The preoperative hip-spine classification was also associated with the magnitude of spinopelvic mobility change postoperatively. Specifically, stiff patients (1B and 2B classifications) did not have any significant differences in spinopelvic mobility after surgery, whereas significant decreases in spinopelvic mobility after THA were observed in patients with 1A and 2A classifications. These results imply that the hip-spine classification in THA is reliable despite the postoperative changes in pelvic mobility. As such, surgeons do not need to compensate for postoperative SPT change when treating patients with a 1B or 2B hip-spine classification. Presuming that most of stiff patients stay stiff after surgery, 30° anteversion to protect from posterior dislocation. In contrast, patients with a 1A or 2A hip-spine classification may have hip-driven changes in SPT with anterior SPT resolving and hypermobile SPT reducing to normal or even stiff categories. For this reason, adjusting component position based on preoperative pelvic position should be done with caution in these two groups (i.e., increasing anteversion (25°-30°) for anterior SPT or reducing acetabular anteversion (12°-15°) in the hypermobile pelvis). Instead, more traditional anatomic targets (20°-25°anteversion) are recommended based on these findings.
Limitations
This study had the following limitations. First, using data from a single institution, the outcomes represent the development and internal validation of a DL model, and comprehensive external validation is necessary. Second, pelvic rotation was unavoidable in some radiographs, which may affect measurements. However, all images were individually reviewed to ensure the correct measurement production before final analysis. This study also does not report direct clinical outcomes and efforts should be made to apply this model in larger cohorts to identify potential clinical value of those measurements in patient outcomes. Third, patients with contralateral hip osteoarthritis after THA were not excluded, which may affect spinopelvic mobility after THA.[14] Lastly, we did not measure anterior pelvic plane tilt, which arthroplasty surgeons commonly use to assess pelvic tilt. However, it is demonstrated in literature that SPT has greater interobserver and interobserver reliability, making it a more precise measurement.[18,35,36] This high agreement was also reflected by the ICCs in the present study.
Conclusion
We leveraged DL to measure SPT and determined the relationship between pre- and postoperative SPT and mobility in patients who underwent THA using the hip-spine classification. Sitting and standing SPT changed postoperatively, and spinopelvic mobility change was dependent on preoperative mobility. The use of the hip-spine classification in this large patient cohort demonstrated the resolution of anterior SPT and hypermobility. With the developed DL workflow, future work will investigate the relationship between all spinopelvic parameters related to the hip-spine classification including lumbar lordosis, pelvic incidence, and sacral slope, in larger cohorts of patients.
References
1. Ferguson, R.J., et al., Hip replacement. Lancet, 2018. 392(10158): p. 1662-1671.
2. Bayliss, L.E., et al., The effect of patient age at intervention on risk of implant revision after total replacement of the hip or knee: a population-based cohort study. Lancet, 2017. 389(10077): p. 1424-1430.
3. Kandala, N.B., et al., Setting benchmark revision rates for total hip replacement: analysis of registry evidence. BMJ, 2015. 350: p. h756.
4. Swarup, I., et al., Implant Survival and Patient-Reported Outcomes After Total Hip Arthroplasty in Young Patients. J Arthroplasty, 2018. 33(9): p. 2893-2898.
5. Bozic, K.J., et al., The epidemiology of revision total hip arthroplasty in the United States. J Bone Joint Surg Am, 2009. 91(1): p. 128-33.
6. Gwam, C.U., et al., Current Epidemiology of Revision Total Hip Arthroplasty in the United States: National Inpatient Sample 2009 to 2013. J Arthroplasty, 2017. 32(7): p. 2088-2092.
7. Stefl, M., et al., Spinopelvic mobility and acetabular component position for total hip arthroplasty. Bone Joint J, 2017. 99-B(1 Supple A): p. 37-45.
8. Luthringer, T.A. and J.M. Vigdorchik, A Preoperative Workup of a "Hip-Spine" Total Hip Arthroplasty Patient: A Simplified Approach to a Complex Problem. J Arthroplasty, 2019. 34(7S): p. S57-S70.
9. Lazennec, J.Y., et al., Hip-spine relationship: a radio-anatomical study for optimization in acetabular cup positioning. Surg Radiol Anat, 2004. 26(2): p. 136-44.
10. Philippot, R., et al., Pelvic balance in sagittal and Lewinnek reference planes in the standing, supine and sitting positions. Orthop Traumatol Surg Res, 2009. 95(1): p. 70-6.
11. Kanawade, V., L.D. Dorr, and Z. Wan, Predictability of Acetabular Component Angular Change with Postural Shift from Standing to Sitting Position. J Bone Joint Surg Am, 2014. 96(12): p. 978-986.
12. Vigdorchik, J.M., et al., 2021 Otto Aufranc Award: A simple Hip-Spine Classification for total hip arthroplasty : validation and a large multicentre series. Bone Joint J, 2021. 103-B(7 Supple B): p. 17-24.
13. Riviere, C., et al., The influence of spine-hip relations on total hip replacement: A systematic review. Orthop Traumatol Surg Res, 2017. 103(4): p. 559-568.
14. Sculco, P.K., et al., Preoperative spinopelvic hypermobility resolves following total hip arthroplasty. Bone Joint J, 2021. 103-B(12): p. 1766-1773.
15. Schwartz, J.T., et al., Deep Learning Automates Measurement of Spinopelvic Parameters on Lateral Lumbar Radiographs. Spine (Phila Pa 1976), 2021. 46(12): p. E671-E678.
16. Rouzrokh, P., et al., Applying Deep Learning to Establish a Total Hip Arthroplasty Radiography Registry: A Stepwise Approach. J Bone Joint Surg Am, 2022. 104(18): p. 1649-1658.
17. Grammatopoulos, G., et al., 2018 Frank Stinchfield Award: Spinopelvic Hypermobility Is Associated With an Inferior Outcome After THA: Examining the Effect of Spinal Arthrodesis. Clin Orthop Relat Res, 2019. 477(2): p. 310-321.
18. Buckland, A., et al., Sagittal Pelvic Orientation A Comparison of Two Methods of Measurement. Bull Hosp Jt Dis (2013), 2017. 75(4): p. 234-240.
19. Karnuta, J.M., et al., Artificial Intelligence to Identify Arthroplasty Implants From Radiographs of the Knee. J Arthroplasty, 2021. 36(3): p. 935-940.
20. Jang, S.J., et al., John Charnley Award: Deep Learning Prediction of Hip Joint Center on Standard Pelvis Radiographs. J Arthroplasty, 2022.
21. Thian, Y.L., et al., Convolutional Neural Networks for Automated Fracture Detection and Localization on Wrist Radiographs. Radiol Artif Intell, 2019. 1(1): p. e180001.
22. Koo, T.K. and M.Y. Li, A Guideline of Selecting and Reporting Intraclass Correlation Coefficients for Reliability Research. J Chiropr Med, 2016. 15(2): p. 155-63.
23. Karnuta, J.M., et al., Artificial Intelligence to Identify Arthroplasty Implants From Radiographs of the Hip. J Arthroplasty, 2021. 36(7S): p. S290-S294 e1.
24. Rouzrokh, P., et al., A Deep Learning Tool for Automated Radiographic Measurement of Acetabular Component Inclination and Version After Total Hip Arthroplasty. Journal of Arthroplasty, 2021. 36(7): p. 2510-+.
25. Rouzrokh, P., et al., A Deep Learning Tool for Automated Radiographic Measurement of Acetabular Component Inclination and Version After Total Hip Arthroplasty. J Arthroplasty, 2021. 36(7): p. 2510-2517 e6.
26. Perfetti, D.C., et al., Prosthetic Dislocation and Revision After Primary Total Hip Arthroplasty in Lumbar Fusion Patients: A Propensity Score Matched-Pair Analysis. J Arthroplasty, 2017. 32(5): p. 1635-1640 e1.
27. An, V.V.G., et al., Prior Lumbar Spinal Fusion is Associated With an Increased Risk of Dislocation and Revision in Total Hip Arthroplasty: A Meta-Analysis. J Arthroplasty, 2018. 33(1): p. 297-300.
28. Eftekhary, N., et al., A systematic approach to the hip-spine relationship and its applications to total hip arthroplasty. Bone Joint J, 2019. 101-B(7): p. 808-816.
29. Maratt, J.D., et al., Pelvic tilt in patients undergoing total hip arthroplasty: when does it matter? J Arthroplasty, 2015. 30(3): p. 387-91.
30. Wan, Z., et al., Imaging and navigation measurement of acetabular component position in THA. Clin Orthop Relat Res, 2009. 467(1): p. 32-42.
31. Lembeck, B., et al., Pelvic tilt makes acetabular cup navigation inaccurate. Acta Orthop, 2005. 76(4): p. 517-23.
32. Vigdorchik, J.M., et al., Does Prosthetic or Bony Impingement Occur More Often in Total Hip Arthroplasty: A Dynamic Preoperative Analysis. J Arthroplasty, 2020. 35(9): p. 2501-2506.
33. Ike, H., et al., The Effects of Pelvic Incidence in the Functional Anatomy of the Hip Joint. J Bone Joint Surg Am, 2020. 102(11): p. 991-999.
34. Lum, Z.C., et al., The Current Knowledge on Spinopelvic Mobility. J Arthroplasty, 2018. 33(1): p. 291-296.
35. Haffer, H., et al., Total Hip Replacement Influences Spinopelvic Mobility: A Prospective Observational Study. J Arthroplasty, 2022. 37(2): p. 316-324 e2.
36. Buckland, A.J., et al., Effects of Sagittal Spinal Alignment on Postural Pelvic Mobility in Total Hip Arthroplasty Candidates. J Arthroplasty, 2019. 34(11): p. 2663-2668.
37. Howard, J. and S. Gugger, fastai: A Layered API for Deep Learning. ArXiv, 2020. abs/2002.04688.
38. Zou, K.H., et al., Statistical validation of image segmentation quality based on a spatial overlap index. Acad Radiol, 2004. 11(2): p. 178-89.
Figure Legend
Figure 1: Patient and image selection workflow
Figure 2: Spinopelvic tilt measurement on a) sitting lateral spinopelvic imaging and b) standing lateral spinopelvic imaging
Figure 3: Deep learning workflow using three convolutional neural network models and image analysis algorithm to automate spinopelvic tilt on lateral standing and sitting imaging
Figure 4: Bland-Altman analysis comparing machine learning automated SPT measurements against the mean of two readers on an independent set of 200 radiographs
Figure 5: Scatter plot with linear regression comparing preoperative and postoperative a) sitting SPT b) standing SPT and c) spinopelvic mobility (ΔSPTStand-Sit). Black line represents perfect correlation with a slope of 1. Dark blue lines represent regression analysis for females and light blue lines represent regression analysis for males
Figure 6: Classification matrix (n = 504) of a patient’s preoperative and postoperative spinopelvic mobility classification based on spinopelvic hypermobility (ΔSPTstand-sit≥30°), normal mobility (10°ΔSPTstand-sit30°), and stiff mobility (ΔSPTstand-sit10°).
Figure 7: Grouped bar plot showing changes in spinopelvic mobility after THA based on a patient’s preoperative spinopelvic mobility.
Table 1: Interclass correlation coefficient of SPT measurement between readers and between automated deep learning measurements on independent testing cohort
Table 2: Spinopelvic tilt and mobility changes after THA based on preoperative hip-spine classifications
Supplemental Materials Legend
Supplemental Figure 1: Summary of sitting vs. standing image classification model training and validation metrics
Supplementary Figure 2: Summary of implant number classification model training and validation metrics
Supplementary Figure 3: Summary of spinopelvic segmentation model training and validation metrics
Supplemental Materials
Methods
Images were converted to PNG formats, resized (600x300 pixels), and normalized before model training. Images were annotated, reviewed, and appropriately excluded to provide accurate ground truths. Transfer learning using ResNet-34, data augmentation, and parameter optimization were conducted with the fast.ai (V2.3.0) library.[1] Transfer learning, which involves utilizing a preexisting network and training with new data for a similar task (i.e. image analysis), was used to create the models.[2] Data augmentation transformations included wrap, flip, zoom (max 2.5), and rotate (max = 40 degrees) for all models. Images were split in an 8:2 ratio for training and validation. All models were trained in PyTorch on a graphic processing unit (GPU) with 16 GB of memory. Classification matrices, accuracy metrics, and saliency maps were reported for the two classification models on the validation set (Supplemental Figures 1 and 2). The segmentation model was assessed using the multi-class dice segmentation coefficient [3] (Supplemental Figure 3).
1. Howard, J. and S. Gugger, fastai: A Layered API for Deep Learning. ArXiv, 2020. abs/2002.04688.
2. Weiss, K., Khoshgoftaar, T.M. & Wang, D. A survey of transfer learning. J Big Data 3, 9 2016. https://doi.org/10.1186/s40537-016-0043-6
3. Zou, K.H., et al., Statistical validation of image segmentation quality based on a spatial overlap index. Acad Radiol, 2004. 11(2): p. 178-89.
Acknowledgements
We would like to acknowledge and thank Marc Sturm and Mark Fontana for the creation of cloud infrastructure at the Hospital for Special Surgery to conduct these projects.
Figures
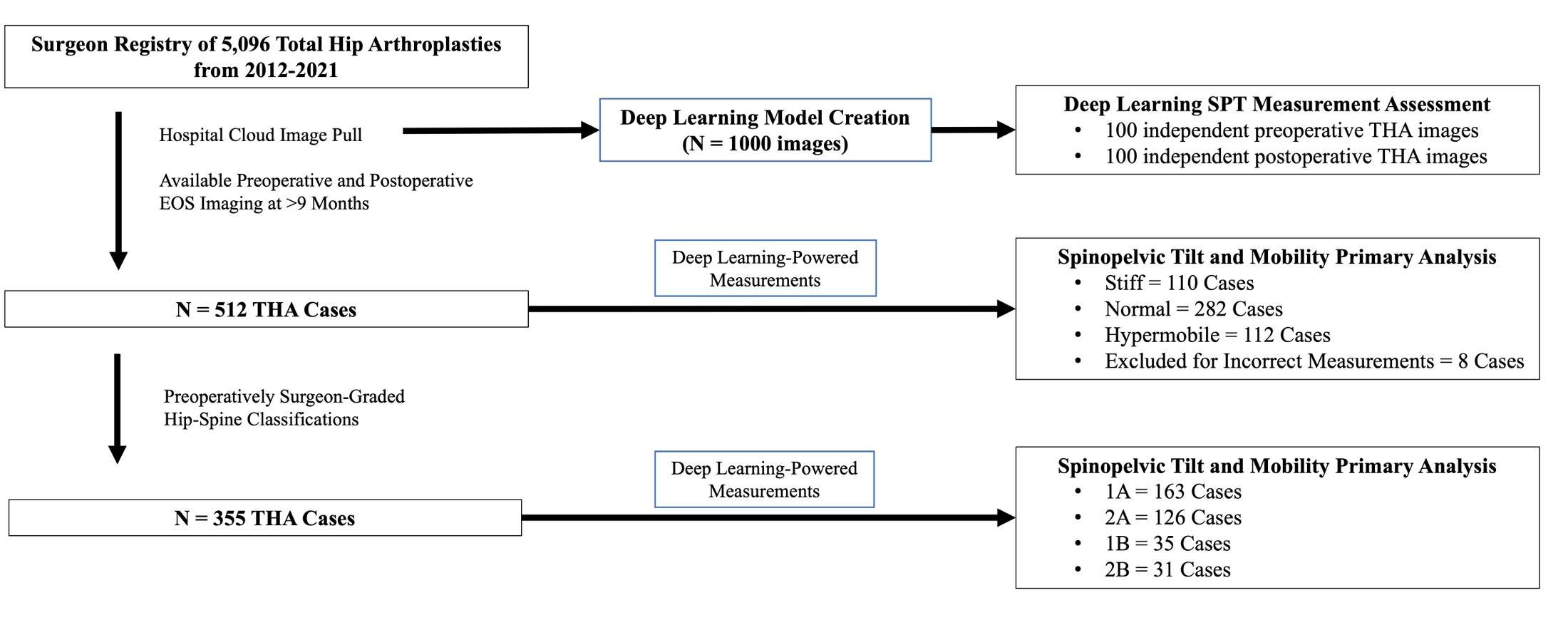
Figure 1
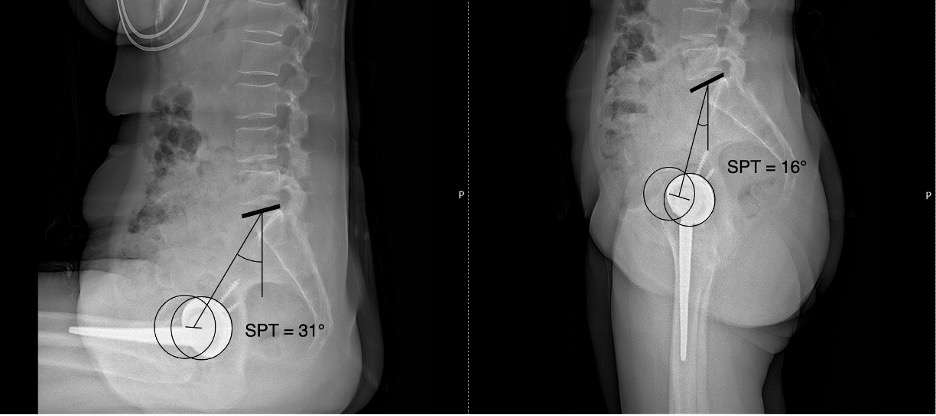
Figure 2
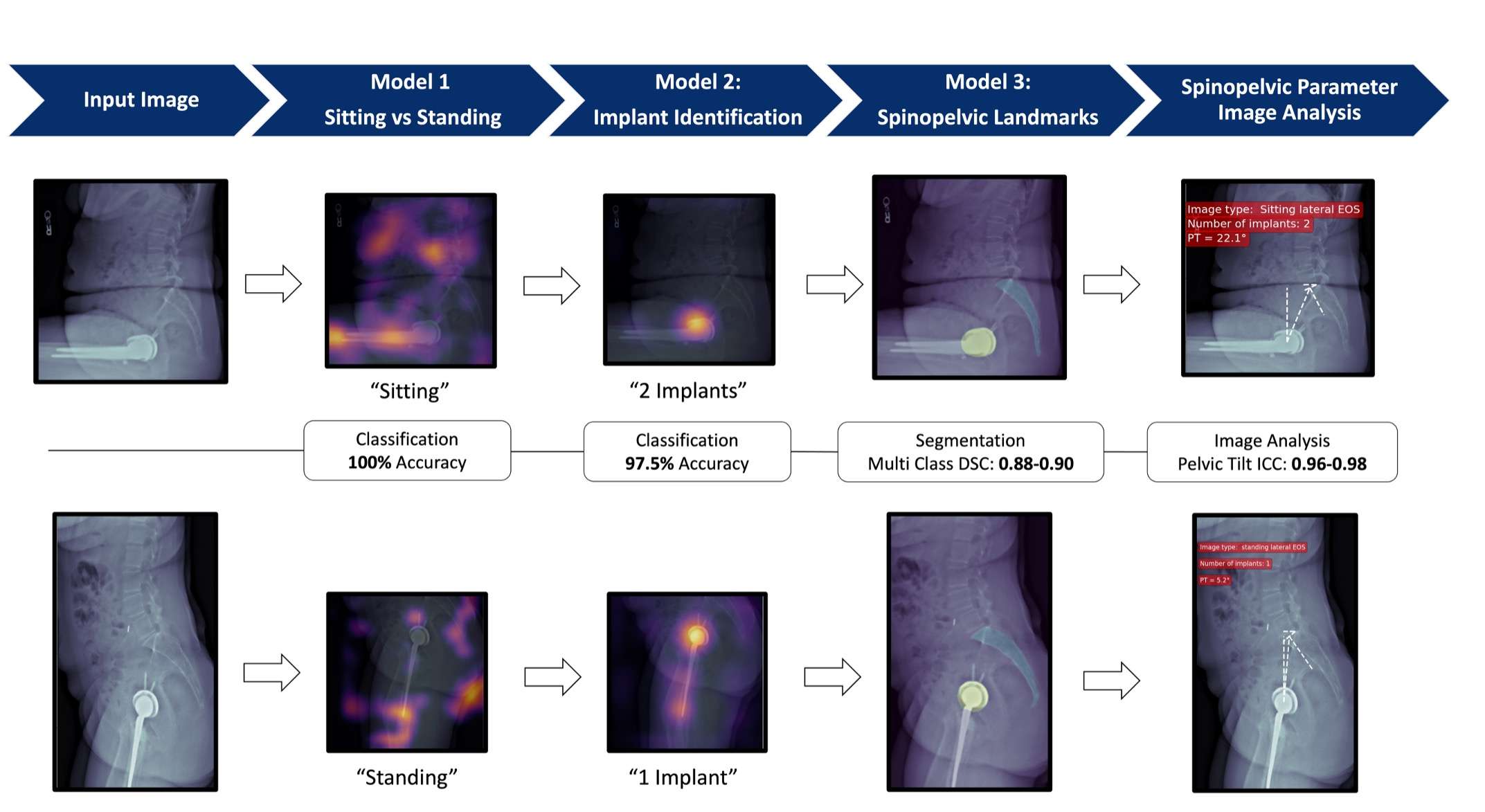
Figure 3
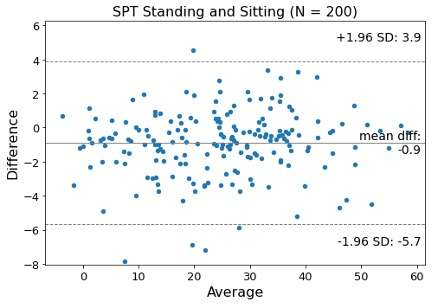
Figure 4
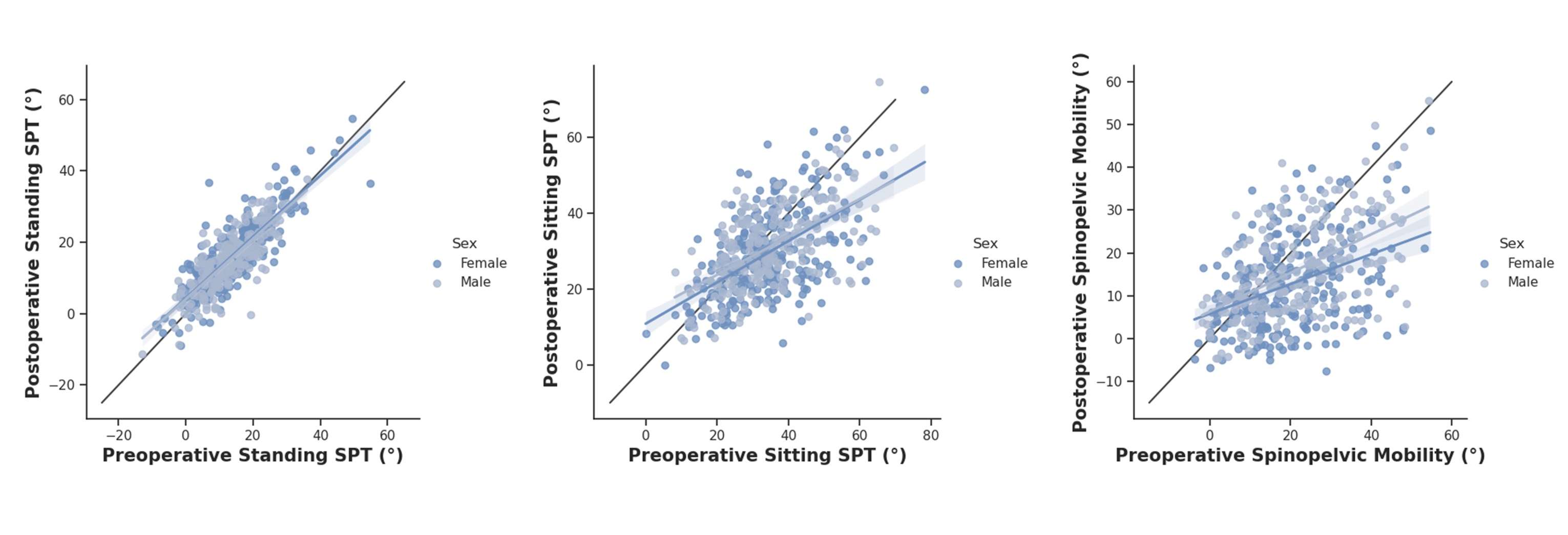
Figure 5
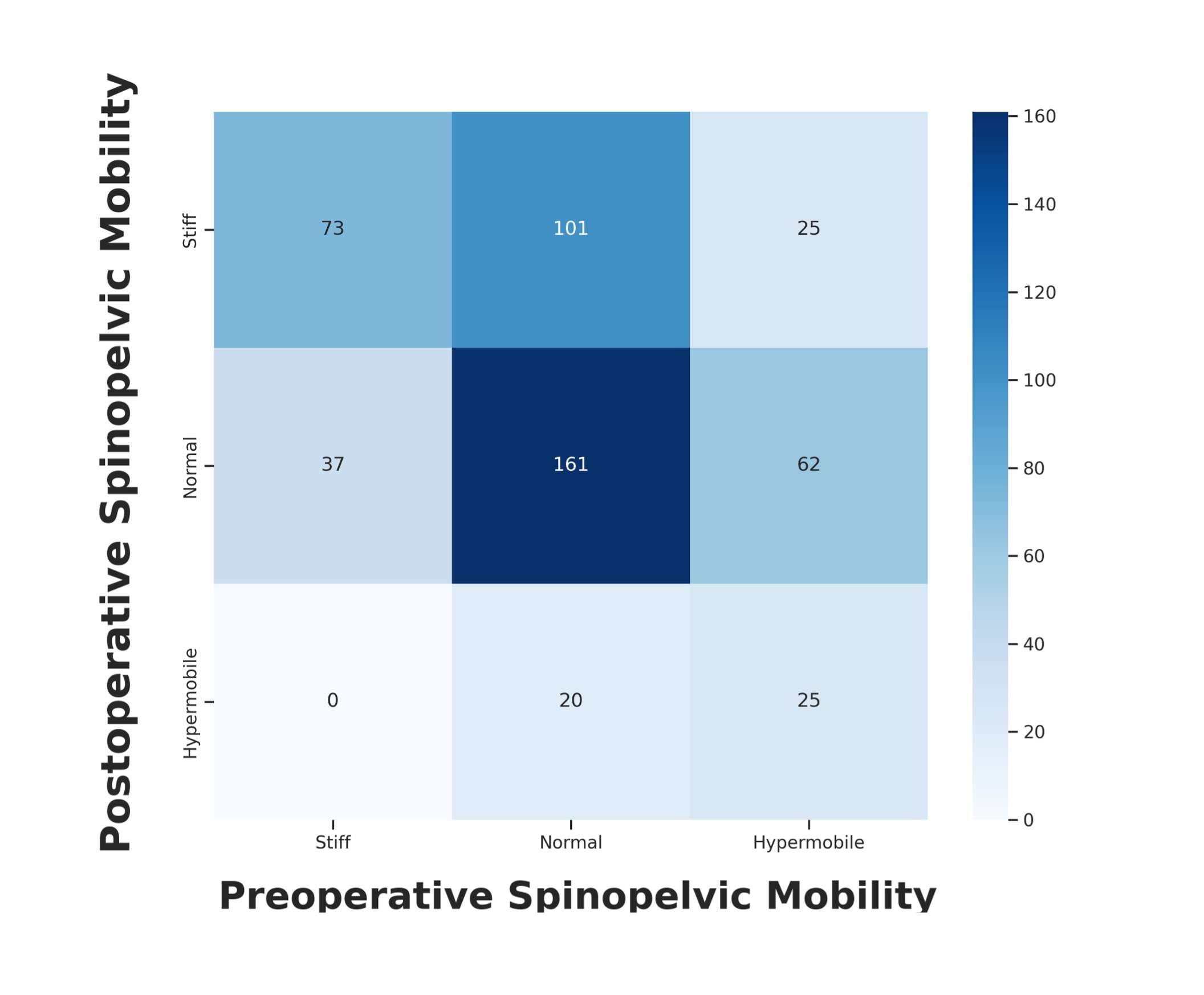
Figure 6
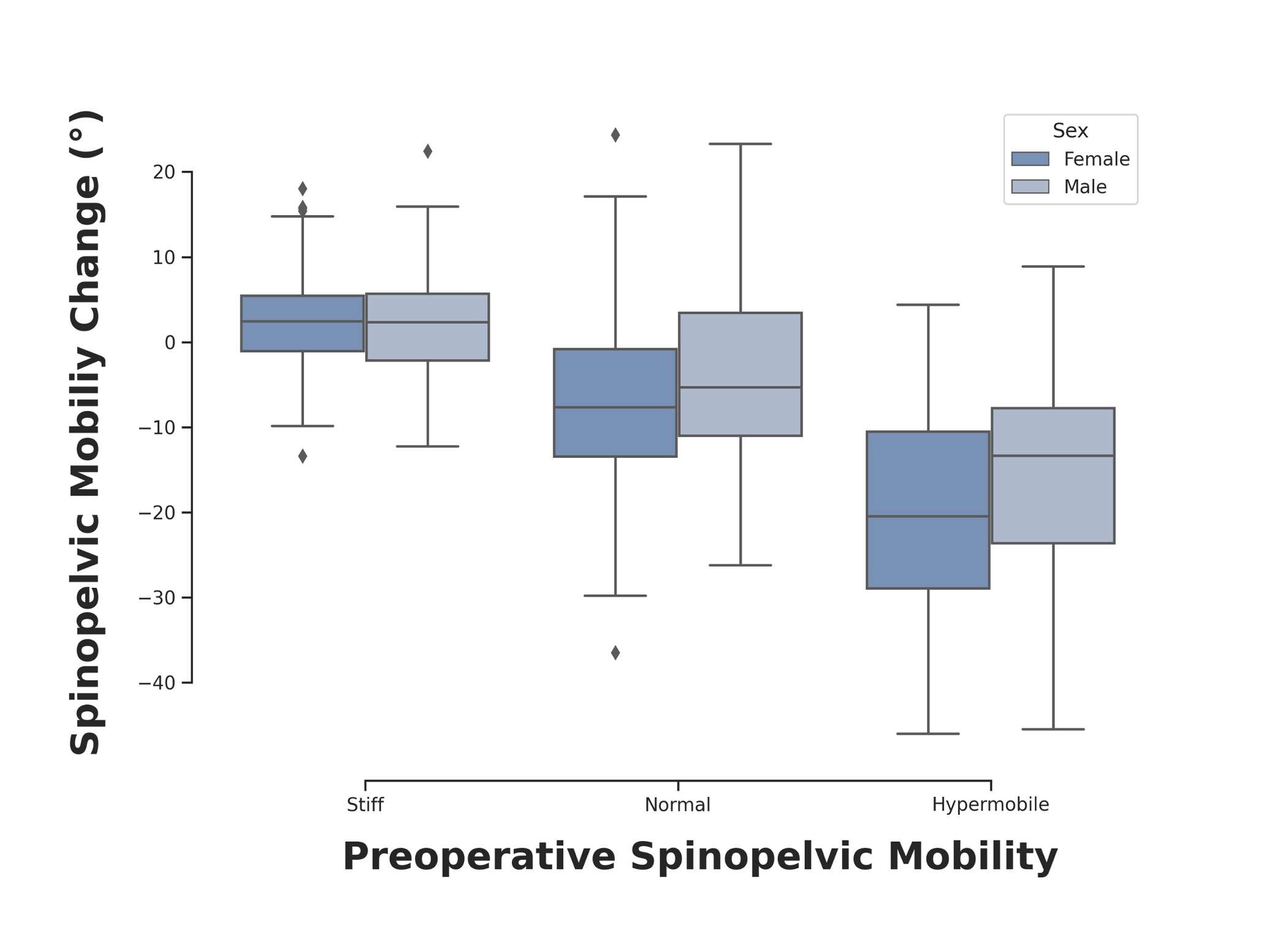
Figure 7
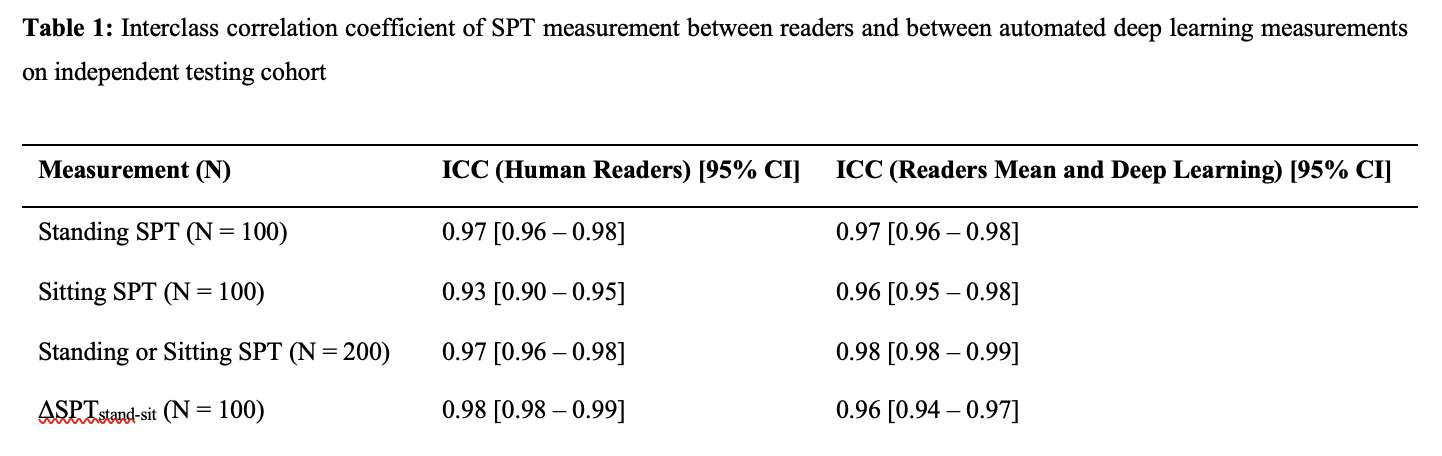
Figure 8
Figure 9
Figure 10
Figure 11
Figure 12#8266
Increased Cup Anteversion May Not Prevent Posterior Dislocation in Patients With Abnormal Spinopelvic Characteristics in Total Hip Arthroplasty
*Christopher Plaskos - Corin - Raynham, USA
Matthew Grosso - Hospital for Special Surgery - New York, United States
Jim Pierrepont - Corin - Cirencester, United Kingdom
Arjun Saxena - Rothman Orthopaedic Institute - Philadelphia, USA
*Email: christopher.plaskos@coringroup.com
Introduction
Previous studies have demonstrated that patients with abnormal spinopelvic (SP) parameters are at increased risk for dislocation following total hip arthroplasty (THA). Common recommendations to address this issue include increased acetabular anteversion to reduce the risk of dislocation. The aims of this study were to (1) assess the degree of variation of acetabular component placement and combined anteversion in a large cohort of dislocating THAs, (2) assess the SP characteristics of the cohort, and (3) examine the association between cup anteversion and reported direction of instability.
Methods
A commercial database of symptomatic hip arthroplasties referred for post-operative CT imaging and functional radiographic analysis was reviewed following Institutional Review Board approval. From this database, 322 cases referred for dislocation were reviewed. Exclusion criteria included: patients having >10mm of under-restoration of leg length discrepancy or hip offset discrepancy and those having >5mm of under-restoration of both leg length and hip offset, age >90, and resurfacing implants, leaving 245 cases in the final analysis. Cup and stem positioning and femoral head size were measured by registering 3D computer models of the implants within the CT image volume (Figure 1). SP parameters were measured in the standing and flex-seated positions on the functional lateral radiographs (Figure 2). Safe zones were defined for the acetabular component as: inclination: 30-50° (all positions); anteversion: 5-25° (APP); 15-35° (standing); 10-30° (supine), and for combined anteversion as 20-45° (APP); 30-55° (standing); 25-50° (supine). Spinopelvic characteristics were stratified by high, neutral, and low cup anteversion using thresholds of >35° and <15° anteversion in standing, respectively.
Results
In the dislocation cohort, 62%, 45%, and 42% of cups were within the safe zone in supine, standing, and the APP, respectively (p<0.001), figure 3. Similarly, 64%, 62% and 57% of hips were within the combined safe zone in supine, standing and the APP, respectively, with significantly fewer patients in the APP than supine safe zone (p=0.026). In the standing position, 25% (62/245) and 10% (25/199) of cups had high and low anteversion, respectively.
Patients with high vs neutral or low cup anteversion had significantly stiffer spines, more posterior pelvic tilt in standing, greater changes in pelvic tilt from stand to seated and supine to stand, higher sagittal imbalance, and higher hip user index and combined sagittal index parameters. Of the 45 patients with high cup anteversion and reported instability direction, 60% and 40% were reported to have posterior and anterior instability, respectively, with no differences in spinopelvic characteristics.
Conclusions
In this dislocating cohort, there is a decreased percentage of cups within the safe zone in the APP and standing position compared to the supine reference. In addition, we found that patients having poor SP characteristics and high cup anteversion can still dislocate, suggesting that adjusting cup anteversion alone may not be sufficient for preventing instability. Increased constraint, such as dual mobility or constrained liner constructs, may be required in these high-risk patients.
#8793
Variations in Acetabular Cup Inclination and Anteversion Measurements Obtained With Intraoperative Navigation Software Versus Postoperative Supine and Standing Radiographs
Louis Andrew Jordan - Hospital for Special Surgery - New York, United States of America
*Renee Ren - USA
Jonathan Spaan - Hospital for Special Surgery - New York, United States of America
Edwin Su - Hospital for Special Surgery - New York, USA
*Email: renyi@hss.edu
Introduction
Intraoperative C-arm imaging is widely used for direct anterior approach total hip arthroplasties (DAA THAs), but parallax and the limited field of view can compromise image quality. As implant malpositioning has been linked to accelerated wear and worse patient outcomes, there has been an increasing interest in the use of computer-assisted navigation software to improve intraoperative visualization and surgical outcomes. This is particularly important during DAA THAs, as this approach has been associated with a steeper technical learning curve. Little is known to what degree navigation may improve the precision and/or accuracy of intraoperative measurements, compared to C-arm fluoroscopy and postoperative radiographs (PRs) during DAA THAs.
The primary aim was to identify the degree of difference in acetabular cup inclination and anteversion measurements obtained on intraoperative images, and standing and supine anteroposterior PRs. The secondary aim was to identify patient factors that may influence measurement discrepancies between the imaging modalities.
Methods
From 2020-2021, 148 primary DAA THAs using the HANA operation table were analyzed. Computer-assisted navigation (RadlinkTM, El Segundo, California) obtained inclination and anteversion intraoperatively. Inclination was measured manually on calibrated C-arm images. Inclination and anteversion were measured on PRs using Einzel-Bild-Röntgen-Analyse (EBRA) software. Intra- and inter-rater reliability were good or excellent. Univariate and multivariate analyses considering age, sex, body mass index (BMI), acetabular cup and head sizes were conducted.
Results
Median (IQR) inclination was significantly lower on C-arm (38.50°, 36.50°-40.50°) than on navigation (39.00°, 38.00°-41.00°), postoperative supine PR (40.90°, 39.50°-43.00°) or standing PR (42.29°, 40.05°-45.94°) (p<.001 for all pairwise comparisons) [Figure 1]. Inclination measurements obtained with navigation were 1.90° lower than those obtained on supine PRs, and 3.29° lower than those obtained on standing PRs.
Median (IQR) anteversion differed between navigation (19.00°, 17.00°-21.00°) and PR supine (23.09°, 21.00°-26.00°) and standing (26.90°, 23.00°-30.06°) images (p<.001 for all pairwise comparisons) [Figure 2]. Navigation measurements were 4.09° lower than supine PRs and 7.90° lower than standing PRs.
Spearman correlation analysis revealed that with navigation, but not PR or C-arm, increasing BMI was associated with lower cup anteversion measurements (rs = –0.173, p=.037). However, regression analysis showed that BMI was not associated with differences in inclination or anteversion between imaging modalities (p>.05 for all comparisons). At 1-year follow-up, 82.35% of patients achieved the Minimal Clinically Important Difference for the Harris Hip Score.
Conclusions
Measurements obtained with navigation had a tighter range and were closer to measurements made on PRs than those made on C-arm images. Lower inclination measurements obtained on the C-arm suggest that parallax may horizontally stretch the outer edges of the C-arm image. The larger difference between C-arm and supine PRs versus navigation and supine PRs demonstrate that navigation can improve visualization of acetabular cup positioning to be more comparable to supine PR measurements. Inclination and anteversion was consistently higher on standing than on supine PRs, demonstrating that evaluation of implant positioning should consider patient positioning and changes in pelvic tilt as a confounding factor that may complicate evaluation of implant position.
Keywords: Computer-Assisted Navigation, Implant Positioning, Radiographic Imaging
Figures
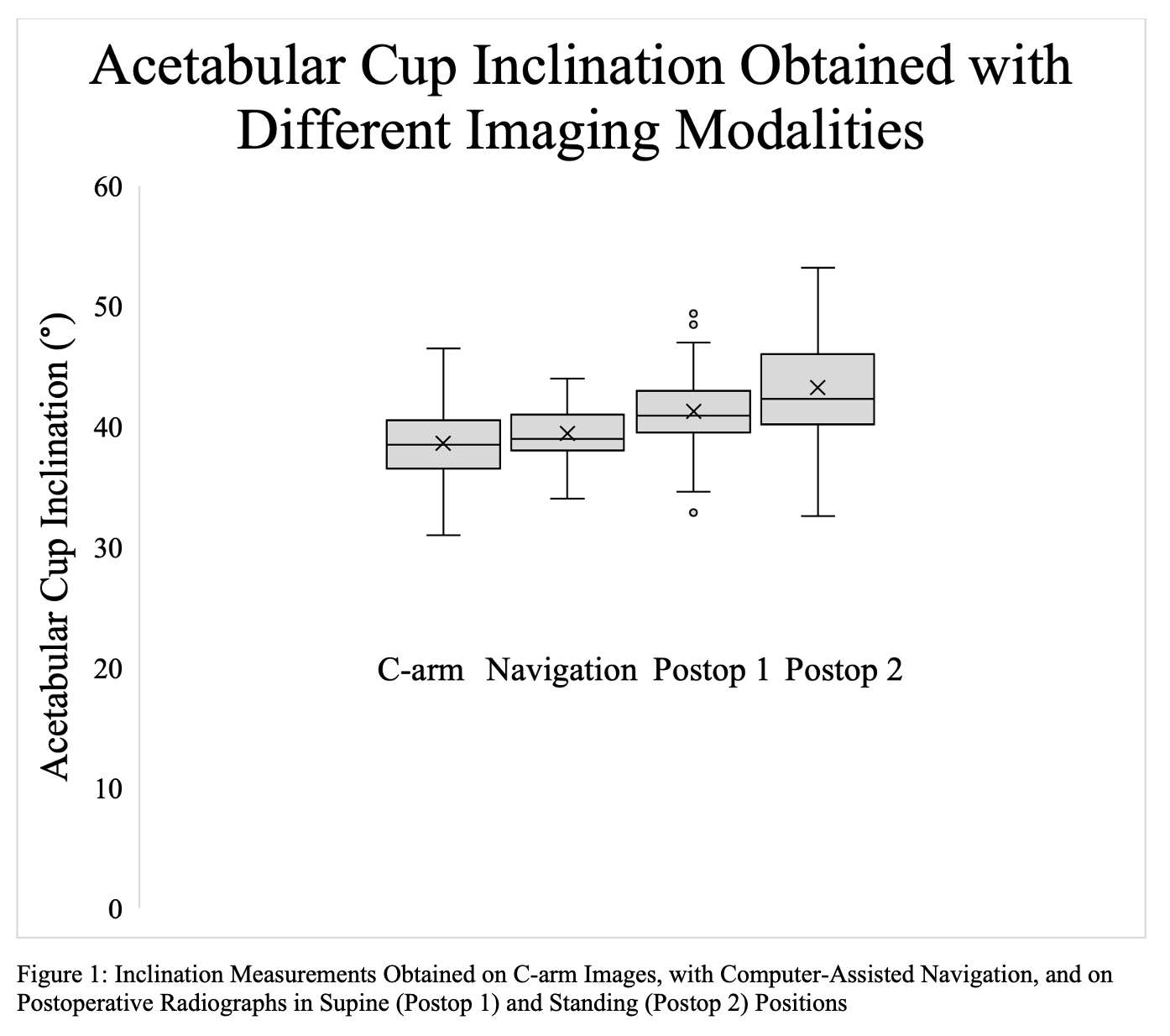
Figure 1
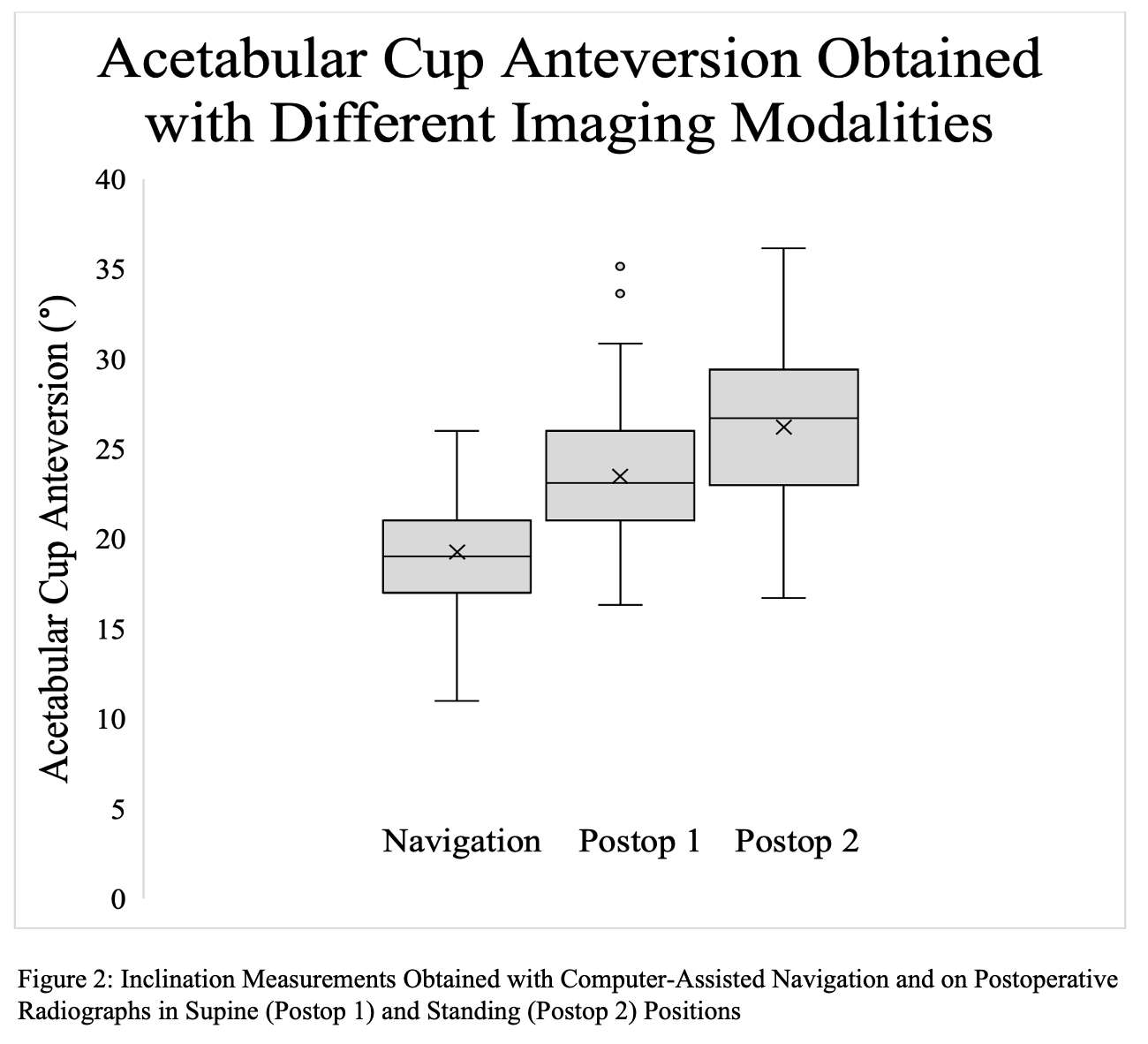
Figure 2#8344
Native Femoral Version Should Not Be Used as Reference in Uncemented Total Hip Arthroplasty
*Anna Di Laura - Royal National Orthopaedic Hospital and Dept. MechEng at UCL - London, United Kingdom
Johann Henckel - Royal National Orthopaedic Hospital - London, United Kingdom
Maria Moralidou - UCL - London, United Kingdom
Harry Hothi - London Implant Retrieval Centre - Stanmore, United Kingdom
Alister Hart - Royal National Orthopaedic Hospital - London, United Kingdom
*Email: anna.laura.14@ucl.ac.uk
Introduction
3-Dimensional surgical planning can precisely predict the size of the stem in uncemented primary Total Hip Arthroplasty (THA). Correct sizing usually results in optimal varus/valgus femoral alignment, however its effect on Prosthetic Femoral Version (PFV) is poorly understood. During implantation, conventional femoral stem designs tend to follow the metaphyseal twist of the internal femoral morphology, to eventually acquire the so-called “best-fit” position. Current 3D-CT planning systems use the Native Femoral Version (NFV) as a reference value to plan THA.
We aimed to assess the relationship between PFV and NFV in primary uncemented THA using 3D-CT analysis. Our primary objective was to quantify the difference between NFV and PFV. Our secondary objective was to evaluate the clinical outcome.
Methods
Pre- and post-operative CT imaging data was collected from 73 consecutive patients (81 hips) undergoing primary uncemented THA with a straight-tapered stem through posterior approach, reason for surgery was Osteoarthritis (OA) in all cases. The CT scans were used to generate the 3D virtual models of the native bony anatomy to measure NFV, post-operative CT scans were used to measure the PFV. An absolute comparison between the NFV and PFV was carried out, Fig 1. The clinical outcome was evaluated.
Results
The discrepancy between the NFV and PFV was low (<5°) in 43%, moderate (5) in 40%, high (>10°) in 11% and very high (>15°) in 6% of cases.
A NFV value between 5°and 10° and between 10° and 15° was reported in 15% and 16% of the patients respectively. Twenty-three per cent (23%) of the patients had a NFV value between 15° and 20° and 15% had a NFV between 20° and 25°. Finally, 10% of the patients had a NFV between 25° and 35° and 21% had a NFV of less than 5°. One female patient (1%) had retroversion of their native femur.
A PFV value between 5° and 10° and between 10° and 15° was reported in 16% and 25% of the femoral stems respectively. Twenty-one per cent (21%) of the femoral stems had a version between 15° and 20° and 7% had a version between 20° and 25°. Finally, 11% of the femoral stems were anteverted between 25° and 35° and 20% were anteverted less than 5°.
The discrepancy between NFV and PFV was low (<5°) in only 43% of cases and the lower and upper 95% limits of agreement were both high at 17 and 15 degrees Fig2,3. None of the hips have been revised for any cause at latest follow up with a median of 45 months post-op.
Conclusion
Planning and delivering PFV is challenging. The patient's NFV cannot be reliably used to predict the version of a straight, single-wedged, tapered, uncemented femoral stem. Surgeons should be aware of this and consider intra-operative anteversion guides and cemented stems to deliver the target version.
Figures
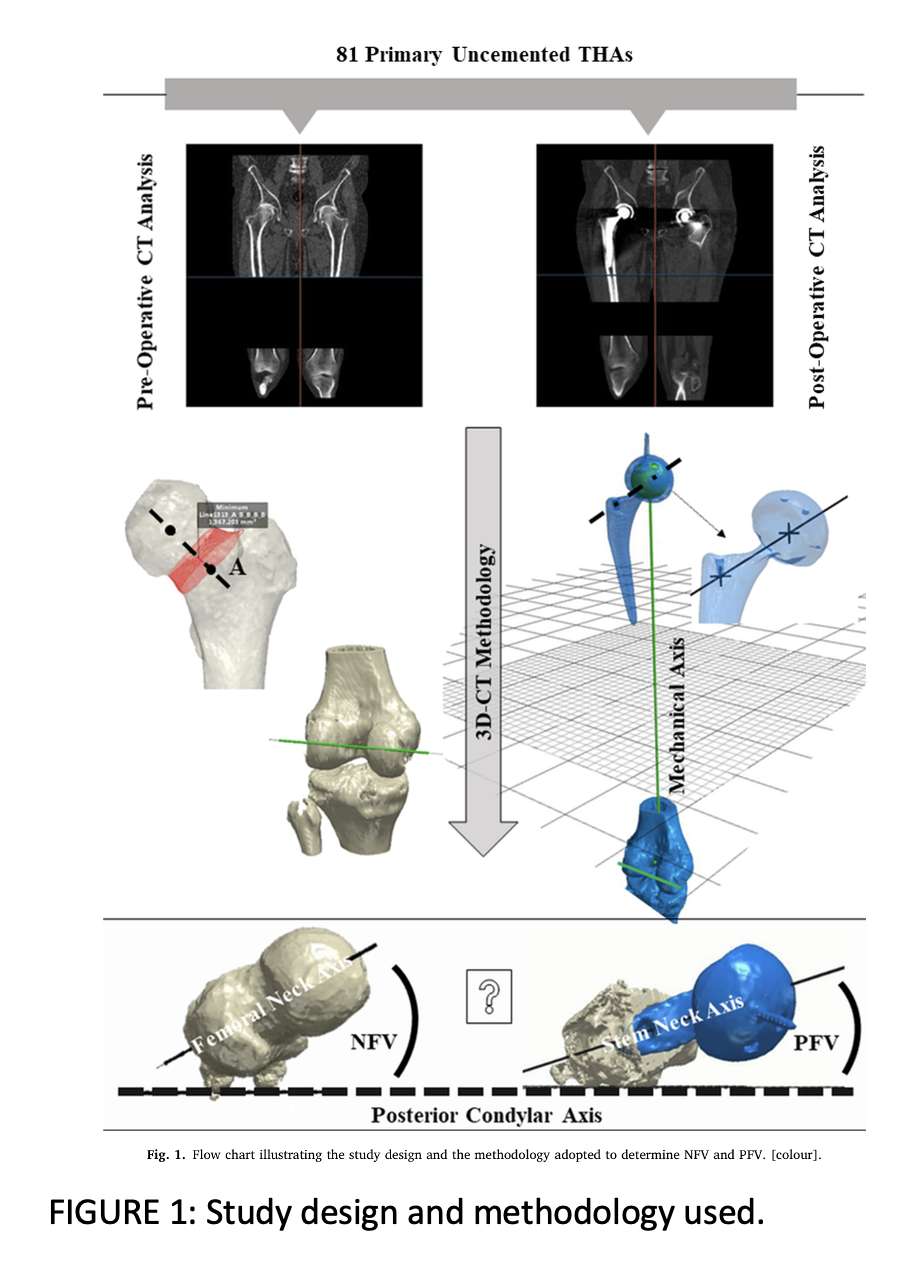
Figure 1
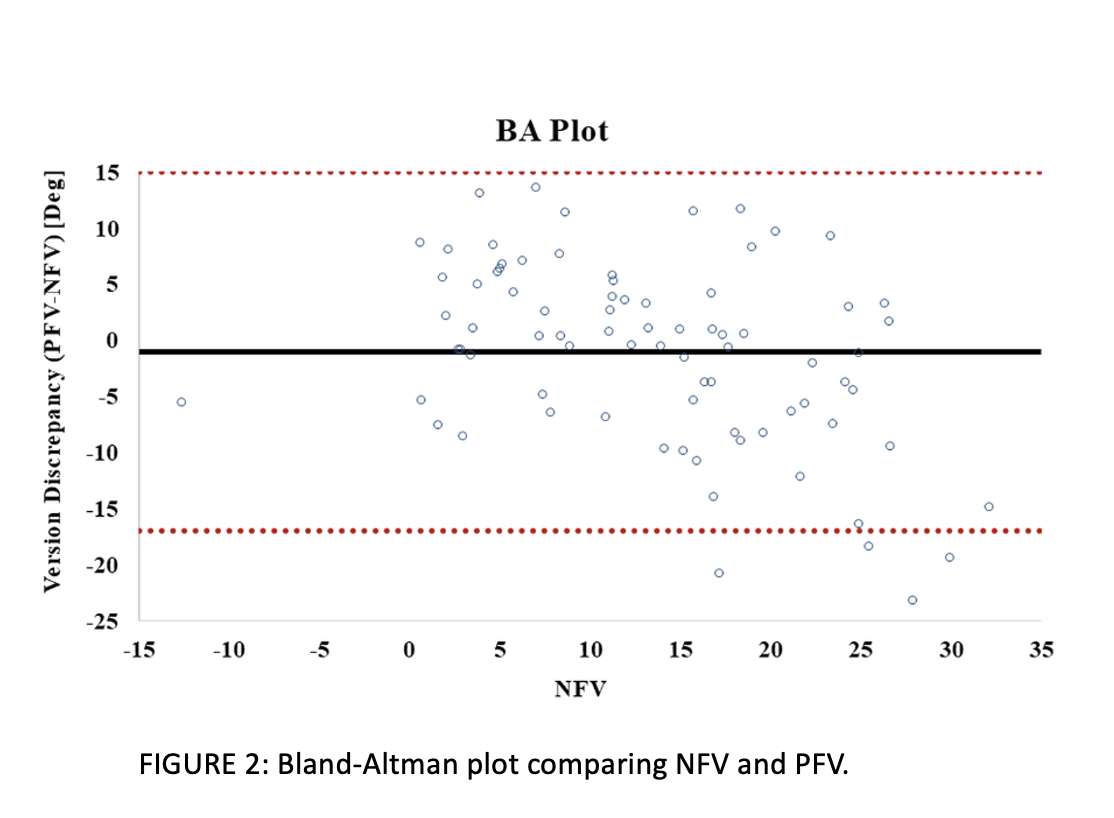
Figure 2
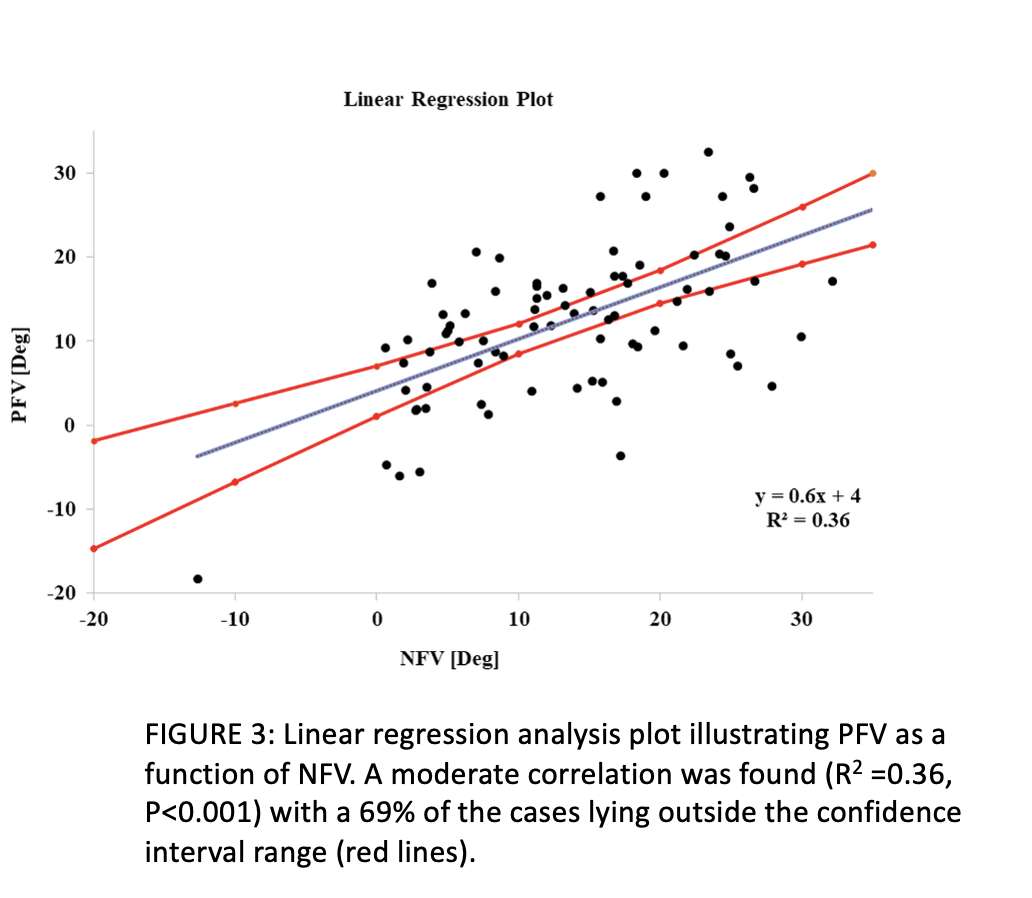
Figure 3#8658
A Novel Calibration Method to Enable IMU-Based Assessment of Spinopelvic Kinematics
*Thor F Besier - University of Auckland - Auckland, New Zealand
George Grammatopoulos - The Ottawa Hospital - Ottawa, Canada
Kyron Marsh - Pioneer Med - Auckland, New Zealand
*Email: t.besier@auckland.ac.nz
Introduction
Obtaining accurate dynamic kinematic reconstruction of spinopelvic motion in a clinical environment remains challenging. Inertial Measurement Units (IMUs) offer a promising solution to provide clinicians with dynamic kinematic assessment of patients before and after total hip arthroplasty. However, a key challenge limiting IMU use in clinical environments is the error associated with misalignment between the sensor and underlying bony segment, or sensor-to-segment (S2S) calibration. This study presents a novel S2S calibration method, using biplane X-ray imaging to obtain accurate orientation between multiple IMUs and underlying bony segments.
Methods
An assembly clip was designed and 3D printed to house an IMU sensor with an orientation fiducial of four stainless-steel beads (Figure 1). A multi-segment mechanical rig with an array of IMU assembly clips enabled static clinical spinopelvic poses to be reproduced. Simultaneous AP and lateral biplanar radiographs of the spinopelvic model in various static poses were generated using an EOS X-ray imaging device (Figure 2) (EOS, Paris, France). Computer vision algorithms were used to automatically detect each IMU orientation within the X-ray images from the unique projection of the IMU assembly clip stainless-steel beads. IMU sensor orientation output data was compared to the ‘ground truth’ orientation obtained from the X-ray images. Dynamic validation was performed using optical motion capture by replacing the stainless steel beads with reflective markers.
Results
A maximum calibration error of 5.4° was observed (across all poses, axes and IMU units). The mean average calibration errors observed were 0.67°, 0.37° and 2.77° in the global X, Y and Z axes respectively. The implementation of an additional Z-calibration method reduced maximum S2S calibration error to 0.64°. This method was replicated with optical motion capture to assess the accuracy of IMU output for spinopelvic motion investigation. Assuming the same static spinopelvic poses, a maximum IMU error of 7.60° was seen, with mean average errors of 0.93°, 0.67° and 3.24° in the global X, Y and Z axes respectively. For dynamic motion trials, maximum errors of 17.19° were recorded.
Conclusion
This study presents a novel calibration method to reduce sensor-to-segment calibration error of IMUs to underlying body segments. Static pose calibration errors were below acceptable margins for clinical application. However, the results from dynamic motion trials indicate that further refinements are needed to ensure accurate IMU orientations over a longer time frame. Across all trials, the global Z-axis produced the highest calibration errors originating from magnetometer sensor fusion. This study contributes to ongoing efforts towards developing accurate and reliable methods for clinical spinopelvic motion assessment.
Acknowledgements
We would like to thank Beyond Radiology for their support in providing EOS imaging for this study.
Figures

Figure 1
Figure 2
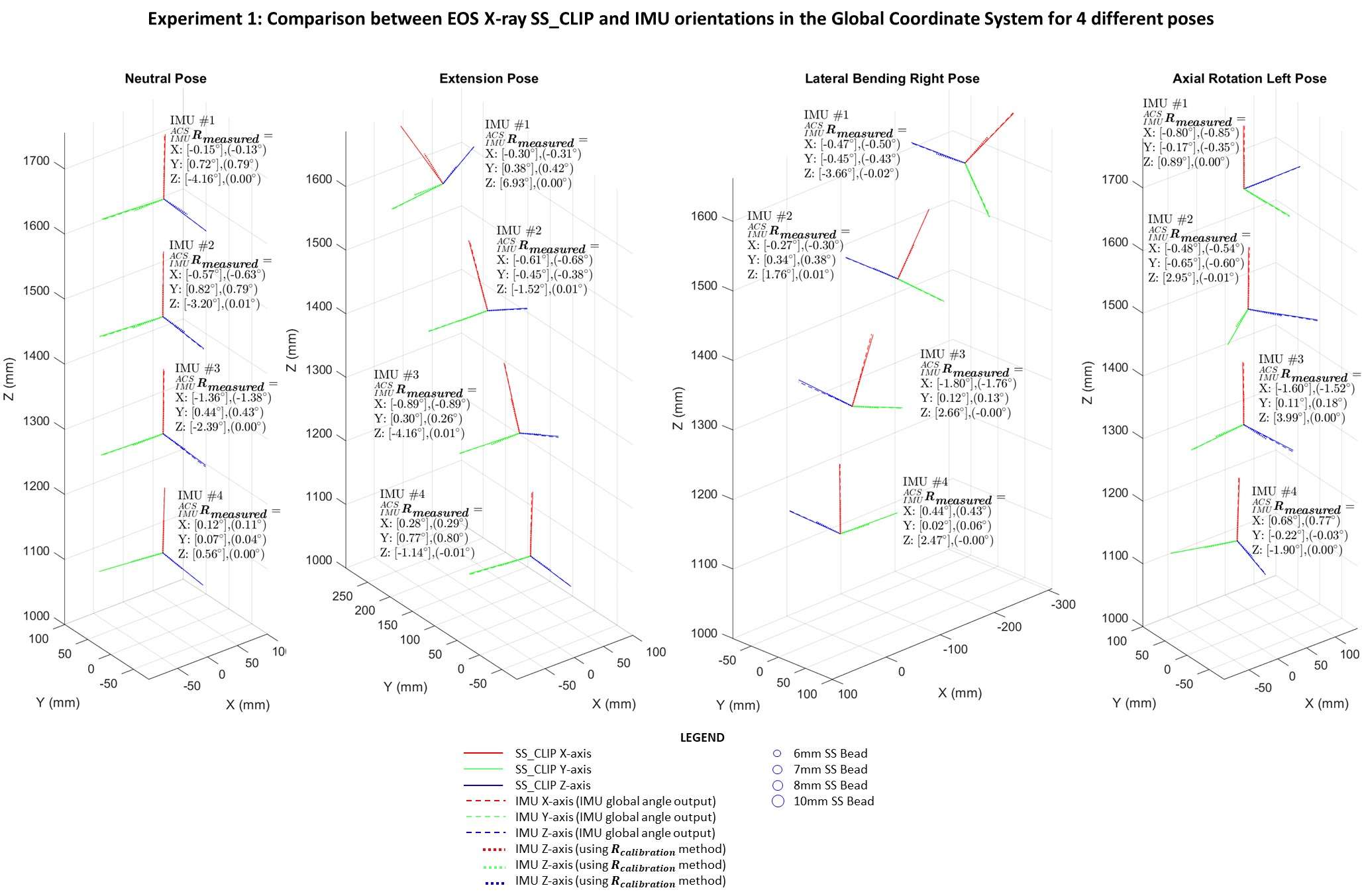
Figure 3#8280
Patient Specific Instrumentation in Laser Guide Navigated THA: Experience in the First 150 Cases, a CT Study
*Attilio Speranza - Sant'Andrea Hospital II University of Rome "La Sapienza" - rome, Italy
Raffaele Iorio
Dario Perugia - UNIVERSITA' DI ROMA "SAPIENZA" - ROME, Italy
veronica giuliani - sant'andrea la sapienza roma - roma, Italy
Natale Criseo - University of Rome La Sapienza - Rome, Italy
*Email: atsperanza@gmail.com
Background
Functional positioning of components in total hip arthroplasty (THA) and its relationship with individual spino-pelvic kinematics and patient’s anatomy are being extensively studied with the purpose to optimise the functional orientation of the implant during patient’s daily life activities, thus avoiding potential impingement and instability. Patient specific dinamic planning could be a game-changer, however, it should be accurately delivered intraoperatively. The main purpose of this study was to verify the reliability and accuracy of a patient-specific instrumentation (PSI) and laser-guided technique to replicate the preoperative dynamic planning in the first consecutive 150 cases.
Materials and methods
150 consecutive patients were prospectively enrolled and received a dynamic hip preoperative planning based on three functional lateral spinopelvic X-rays and a low dose CT scan. 3D printed PSI (including femoral and acetabular guides) and laser guided instrumentation were used intraoperatively. The accuracy of the system was measured using post-operative CT, as approved by the ethics committee, performed in 60 cases. Components size and orientation, osteotomy level, and change in hip-length and offset were measured and compared with the planned pre-operative values.
Results
The mean absolute deviation from the planned inclination and anteversion was 4.1° and 5.1° respectively. In 91% of cases, both inclination and anteversion were within +/- 10° of the planned values . Regarding osteotomy level, offset change, and limb length change, the mean deviation was respectively 1,3 mm, 2,2 mm, and 1,8 mm. No statistically significant difference was detected comparing the planned and the achieved values. One case of anterior dislocation was reported in the 150 patients treated, due to inaccurate positioning of the acetabular guide intraoperatively (anteverted 38°).
Conclusions
Patient-specific and laser-guided instrumentation is safe and accurately reproduce the dynamic planning in terms of components orientation, osteotomy level, leg length and offset.
#8133
Periacetabular Osteotomy Performed With Navigation and Patient-Specific Templates Is a Reproducible and Safe Procedure
*Rene MihaliÄ - Valdoltra Orthopaedic Hospital - Ankaran, Slovenia
Peter Brumat - Valdoltra Orthopaedic Hospital - Ankaran, Slovenia
Rihard Trebse - ORTHOPAEDIC HOSPITAL VALDOLTRA - Ankaran, Slovenia
*Email: rene.mihalic@ob-valdoltra.si
Introduction
Periacetabular osteotomy (PAO) is an effective surgical procedure for the treatment of developmental dysplasia of the hip with no or mild osteoarthritis. It is a very demanding, mostly free-hand technique with a long learning curve. We aim to present a modern surgical technique for the Bernese PAO using electromagnetic navigation (EMN) and patient-specific templates (PST) and compare the acetabular fragment reorientation accuracy under the EMN and traditional fluoroscopic control.
Methods
We retrospectively analyzed a prospective cohort of dysplastic hips scheduled for PAO. All patients selected for the surgery underwent preoperative computed tomography (CT) of the hip and pelvis for preoperative planning, design, and manufacturing of PST and for EMN purposes during surgery [Fig. 1]. All PAOs were performed using PST and EMN [Fig. 2]. Patient data included: age, sex, side, body mass index (BMI), number of complications, surgery duration, Harris Hip Score (HHS), lateral center-edge angle (LCEA), acetabular index (AI), and the length of follow-up. For the purpose of acetabular fragment reorientation analysis, patients were divided into two groups. In the study group (EMN), the acetabular fragment was reoriented with the help of EMN [Fig. 3]. In the control group (XR), the acetabular fragment was reoriented using fluoroscopy. We compared the difference between the planned and achieved position of the acetabular fragment and the outcomes between both groups.
Results
40 dysplastic hips were included, 30 in the EMN and 10 in the XR group. Both groups had no significant differences in patients’ characteristics and pre-surgical parameters. The average follow-up was 2.87 ± 1.13 years for the EMN group and 6.18 ± 0.92 years for the XR group. Two major complications occurred in the first four PAOs in the XR group (one transient peripheral peroneal nerve dysfunction and one popliteal deep venous thrombosis with no sequelae). No major complications were observed in the EMN group. One hip in each group was later converted to THA (2 and 5 years after PAO). There were 3 minor complications in the XR, and seven minor complications in the EMN group. The average absolute difference in planned and achieved LCEA and AI was 1.2° ± 1.5° and 1.1° ± 2° for the EMN and 7° ± 6.1° and 6.3° ± 6.3° for the XR group (p = 0.02; p = 0.03). The average surgery duration was 183 ± 32 minutes for the EMN and 203 ± 42 minutes for the XR group (p = 0.19).
Conclusion
Our study indicates that PAO performed with EMN and PST is a safe and reproducible procedure with a short learning curve. Additionally, navigated reorientation of the acetabular fragment is significantly more accurate than under fluoroscopic control.
Figures
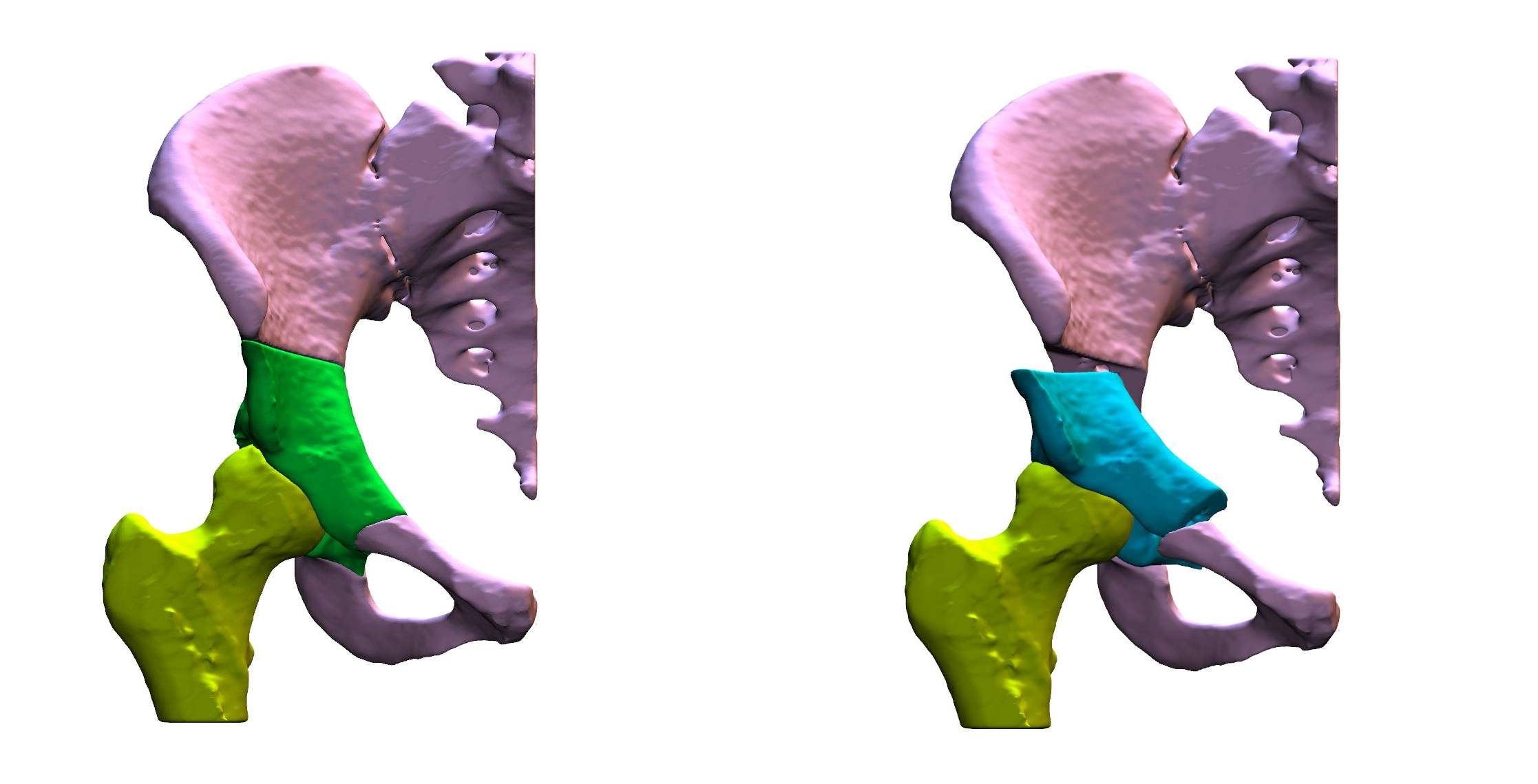
Figure 1
Figure 2
Figure 3#8636
Automated Generation of Patient-Specific Musculoskeletal Models to Predict Muscle Function Following Total Hip Arthroplasty
Nynke Rooks - Formus Labs - Auckland, New Zealand
Duncan Bakke - Formus Labs - Auckland, New Zealand
*Marco Schneider - Formus Labs - Auckland, New Zealand
Thor F Besier - University of Auckland - Auckland, New Zealand
*Email: marco@formuslabs.com
Introduction
Patient-specific musculoskeletal (MSK) models can assist surgeons to plan and execute their procedures by accounting for muscle-tendon function and analyzing the effects of implant design, position, size and alignment, on range of motion, muscle lengths, moment arms, and moment generation capacity. However, clinical adoption of MSK models is limited due to the high complexity and time cost in generating patient-specific parameters such as bone and joint anatomy. Articulated shape models can be used to generate patient-specific MSK models, including bone morphology and muscle attachments [1]. In this study, we evaluated the accuracy and speed of the Formus MSK model generation pipeline to create patient-specific MSK models for muscle function analysis.
Methods
The test dataset (n=10) consisted of post-mortem, de-identified CT images of adults (aged 53.3 ± 18.0 years, 5 males and 5 females) collected from the Victorian Institute of Forensic Medicine (Melbourne, Australia) and corresponding manually segmented ground truth anatomies including the pelvis, femurs, tibias, and fibulas. The CT images were processed using the Formus MSK model generation pipeline which uses a neural network to automatically segment the hemipelvises and femurs (Figure 1 A). A shape and pose optimization then fits an articulated shape model of the lower-limb which includes the pelvis, femurs, tibias, and fibulas (Figure 1 B), to generate a patient-specific MSK model including model articulations and embedded muscle attachments (Figure 1 C). Surface-to-surface mean absolute distance (MAD) was calculated post-registration on the test dataset to evaluate the accuracy of the sacrum, hemipelvis, femur, tibia, and fibula geometries of the MSK model against the segmented ground truths.
Results
After segmentation, patient-specific MSK models were generated in an average time of ~27 ± 11 minutes. Compared to automated segmentations, the Formus MSK model generation pipeline fitted the femur and hemipelvis models with an average MAD of 0.76 ± 0.004 mm. Compared to the manual segmentations, the femurs and hemipelvises were fitted with a mean MAD of 1.15 ± 0.08 mm. The tibias, fibulas, and sacrum geometries were predicted with a mean MAD of 1.93 ± 0.25 mm.
Conclusion
The entire process from CT scan to MSK model takes ~35 minutes, reducing the barrier to clinical adoption of MSK models. In this present study, errors in patient-specific geometries were lower than those produced through isotropic scaling (5.22 ± 1.37 mm) or the MAP client lower limb shape model (4.28 ± 0.80 mm) [1]. Similar to the MAP client, the MSK pipeline can generate patient-specific MSK models from skin-mounted markers. Embedded muscle attachments allow for patient-specific analysis of muscle function such as changes to muscle lengths and moment arms with surgery.
References
- Zhang, J., Fernandez, J., Hislop-Jambrich, J., & Besier, T. F. (2016). Journal of Biomechanics, 49, 3875-3881.
Figures
Figure 1#8216
Building Surgeon-Specific Predictive Models of Tibial Insert Thickness Using Knee Joint Laxity Signature
Prudhvi Tej Chinimilli - Exactech - Gainesville, USA
Wen Fan - Exactech - Gainesville, USA
*Laurent Angibaud - Exactech, Inc. - Gainesville, USA
Amaury Jung - Blue Ortho - La Tronche, France
James Huddleston - Stanford University - Woodside, United States of America
*Email: laurent.angibaud@exac.com
INTRODUCTION
Soft tissue balancing plays an important role in total knee arthroplasty (TKA) which affects post-operative clinical outcomes. One of the factors that contribute to successful soft tissue management relates to tibial insert thickness, an interoperative surgical decision that varies based on surgeon experience and preference. This study aims to 1) test the correlation between laxity curves and tibial insert thickness; 2) build surgeon-specific predictive models recommending tibial insert thickness based on the laxity curves.
METHODS
Within an anonymized instrumented CAOS system (ExactechGPS, Blue-Ortho) database, all cases with a tibia first technique performed by surgeons with more than 30 cases were retrospectively included, without any exclusions. During trial reduction, a trial femoral component was impacted onto the prepared distal femur and a mechanical intra-articular tibial distractor was introduced into the joint space which applied a quasi-constant distraction force once released, regardless of the joint gap. Then, the limb was manually taken through a full arc of motion and corresponding joint laxities were recorded by the CAOS system. Medial and lateral (ML) gaps were measured at 0° to 120° of flexion with small increments. For each surgeon, the correlation coefficients were calculated between each available flexion angle and tibial insert thickness. Then, the flexion that was associated with the highest correlation between either medial or lateral gap and tibial insert thickness was selected to build predictive models. For each surgeon, the data is split into a train-test ratio of 2:1. Random Forest and ordinal logistic regression models were trained, and the accuracy was evaluated by calculating proportions of exact predictions, predictions within 1mm, and predictions within 2mm using testing datasets.
RESULTS
Among 919 cases, 8 surgeons associated with 465 cases were selected for the correlation analysis. All surgeons showed correlation (r > 0.3) between ML gaps and tibial insert thickness except for surgeon 2, displayed in Figure 1. It can also be observed that there exists a relatively higher correlation between medial gaps and tibial insert thickness when compared to lateral gaps. The predictive accuracy with random forest and logistic regression models for each surgeon corresponding to three cases were displayed in Figure 2. It can be summarized that logistic regression performed slightly better than random forest for exact (52.5% vs 50.6%), 1mm difference (67.9% vs 66.5%), and 2 mm difference (94.1% vs 92.9%) predictions.
CONCLUSION
This study first investigated the correlation between knee joint laxity and tibial insert thickness in TKA with the tibial-first technique, and the findings demonstrated that the relationship tended to be surgeon specific. The predictive models performed reasonably accurately with a high correlation observed between joint gaps and tibial insert thickness. These models can guide surgeons to select the optimal tibial insert thickness during the surgery which improves surgical decision making and ensures joint stability postoperatively. The sample size is one of the limitations of this study which can impact the model training and testing. Further investigations of other potential predictors of tibial insert thickness will be performed to improve the performance of predictive models.
Figures
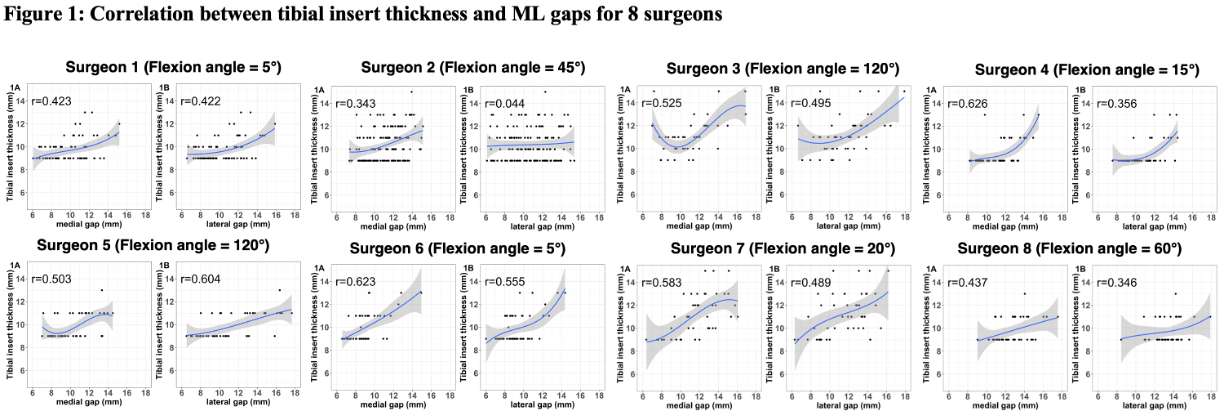
Figure 1
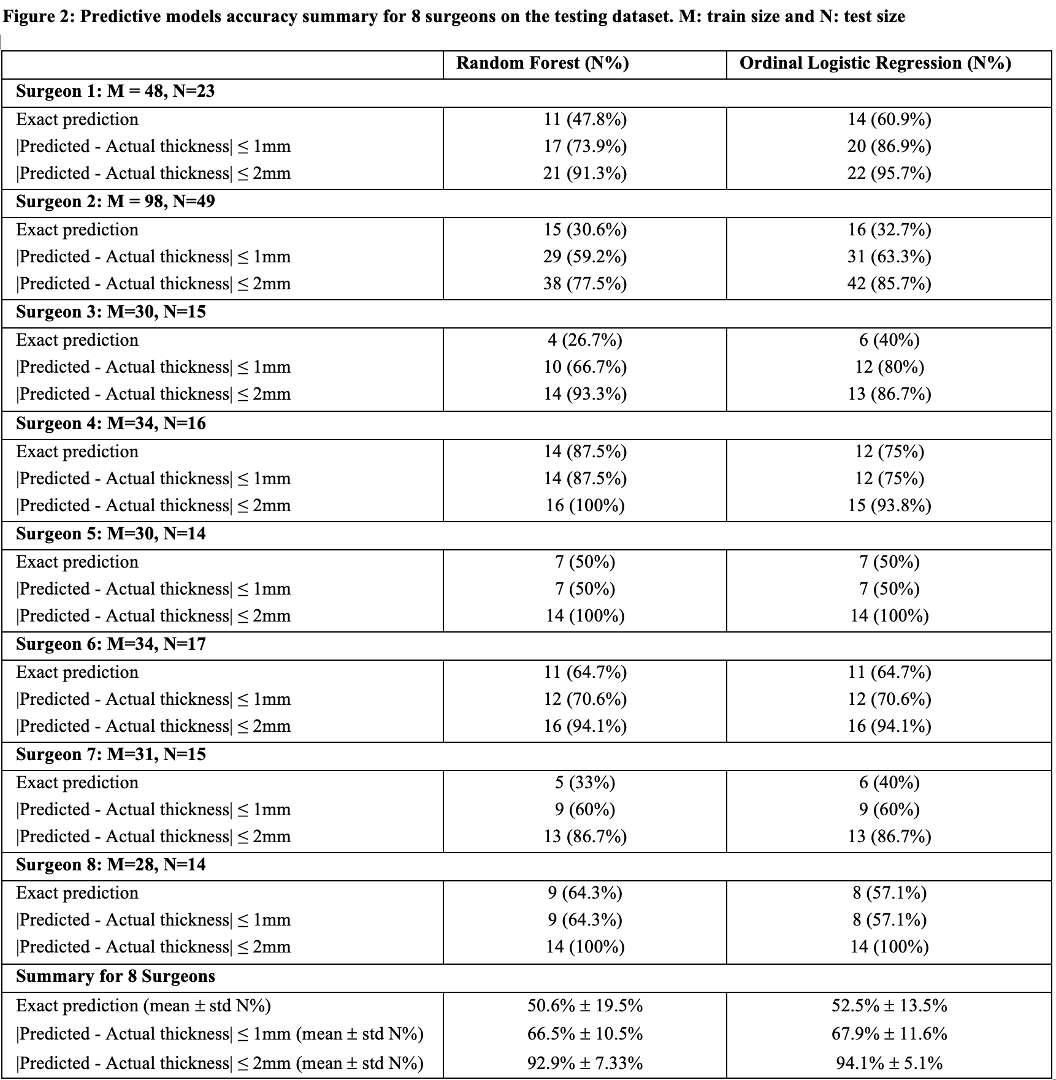
Figure 2#8652
A Comparison of Mixed Reality Navigation to Patient Specific Guides and Traditional Freehand Technique for Guide Pin Insertion in Glenoids With Severe Erosions
Cole Fleet - Western University - London, Canada
Ryan Gao - Roth | McFarlane Hand and Upper Limb Centre, St Joseph's Health Care - London, Canada
James Johnson - SJHC - London, Canada
*George Athwal - University of Western Ontario - London, Canada
*Email: gsathwal@hotmail.com
Background: Obtaining accurate guide pin position in severely eroded glenoids can be difficult using traditional three-dimensional (3D) preoperative planning with freehand guide pin insertion. Patient specific instrumentation (PSI) guides help to improve the accuracy of guide pin insertion but are associated with long production times and higher costs. The use of mixed reality for surgical visualization and holographic navigation (MR-NAV) is a newer technology with limited literature on its effectiveness and accuracy. Therefore, the purpose of this study was to evaluate and compare the accuracy in guide pin insertion with MR-NAV to that of traditional software planning (TSP) and PSI for glenoids with severe erosions.
Methods:15 computer tomography scans were obtained from patients (mean age 67±11 years) exhibiting type B2, B3, C, D, E2, and E3 glenoid erosion. The scans were automatically segmented and three models of each glenoid (with coracoid) were 3D printed. Guide pin position and orientation for each case was preoperatively planned by the senior author using validated preoperative planning software. Three methods of guide pin insertion were randomly evaluated with a surgeon who had been blinded to the preoperative planning. The first method employed TSP with freehand guide pin insertion, while the second method employed PSI guides which were preoperatively planned and 3D printed from titanium for each model. The third method utilized an MR-NAV system, which was used to complete the 3D glenoid model registration and guide pin insertion processes, while providing real-time holographic guidance (Figure 1). Once all guide pins had been inserted, an independent optical tracking system and custom digitization device was used to quantify the position and orientation of the guide pin relative to the glenoid model. The primary outcomes for this study were the absolute error in guide pin inclination, version, and entry point relative to the preoperative plan. The Total Global Error was also assessed, which was defined as the sum of the absolute error in both guide pin orientation and position. Statistical analysis was performed using a one-way repeated measures analysis of variance.
Results: MR-NAV and PSI produced similar errors in both version (1.0±0.9° versus 1.3±0.9°, p=1.000) and inclination (1.9±1.1° versus 2.4±1.0°, p=0.331). TSP exhibited significantly greater error in version compared to both MR-NAV and PSI (3.8±2.9°, p<0.027) and significantly greater error in inclination when compared only to MR-NAV (4.2±2.6°, p=0.025). Similar Total Global Error outcomes were observed between MR-NAV and PSI (4.7±1.0 [mm+deg] versus 4.4±0.8 [mm+deg], p=1.000), while TSP exhibited significantly greater Total Global Error (7.5±3.0 [mm+deg], p<0.009) compared to both MR-NAV and PSI techniques (Figure 2).
Discussion:MR-NAV and PSI are accurate techniques that can be used for guide pin positioning in the presence of severe glenoid erosion. Both methods significantly reduced the guide pin insertion error associated with TSP. MR-NAV systems allow the user real-time visualization of the surgical plan, and holographic intra-operative guidance, while likely reducing the costs and lead-time associated with single use patient specific guides.
Figures
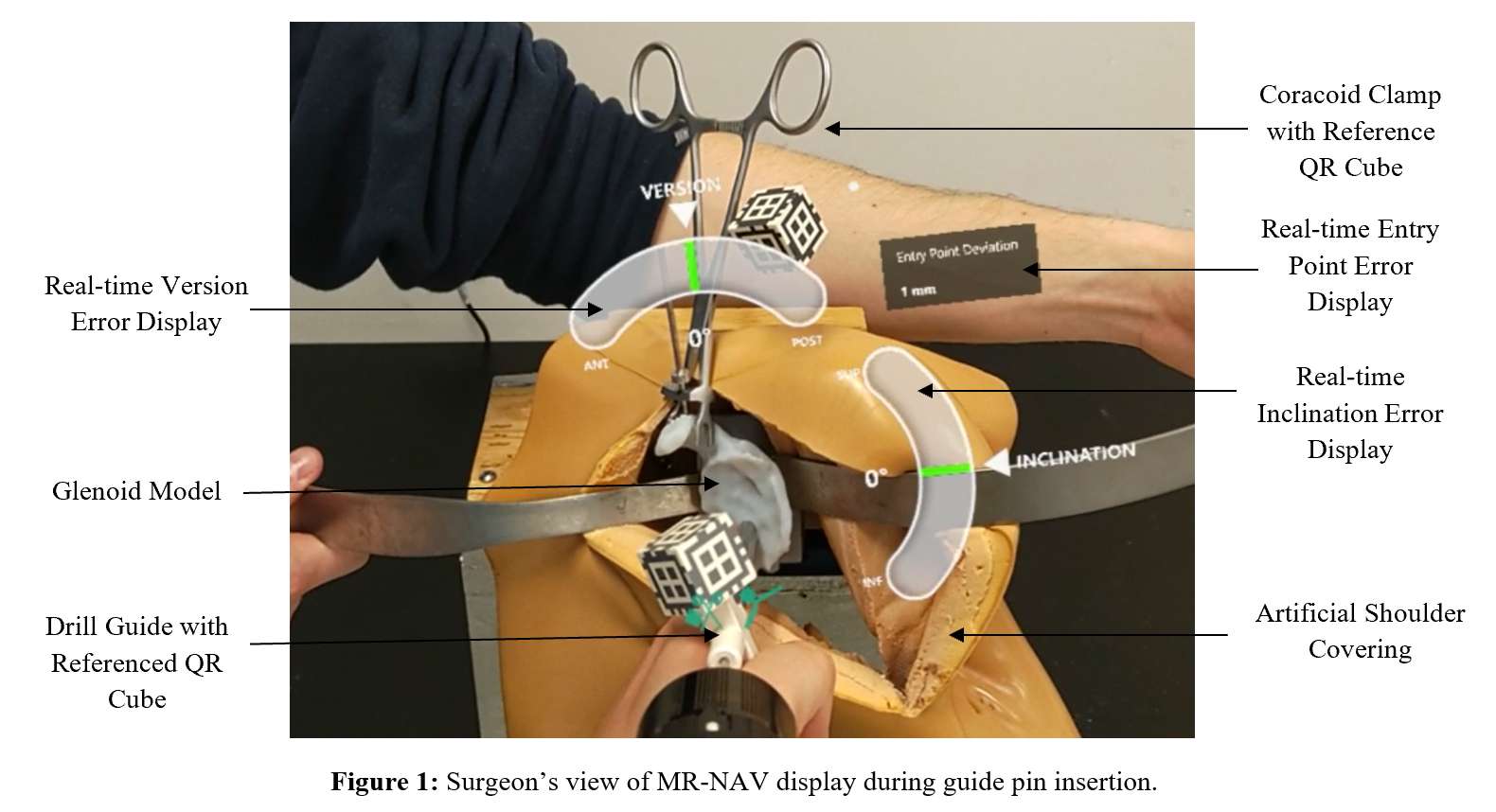
Figure 1
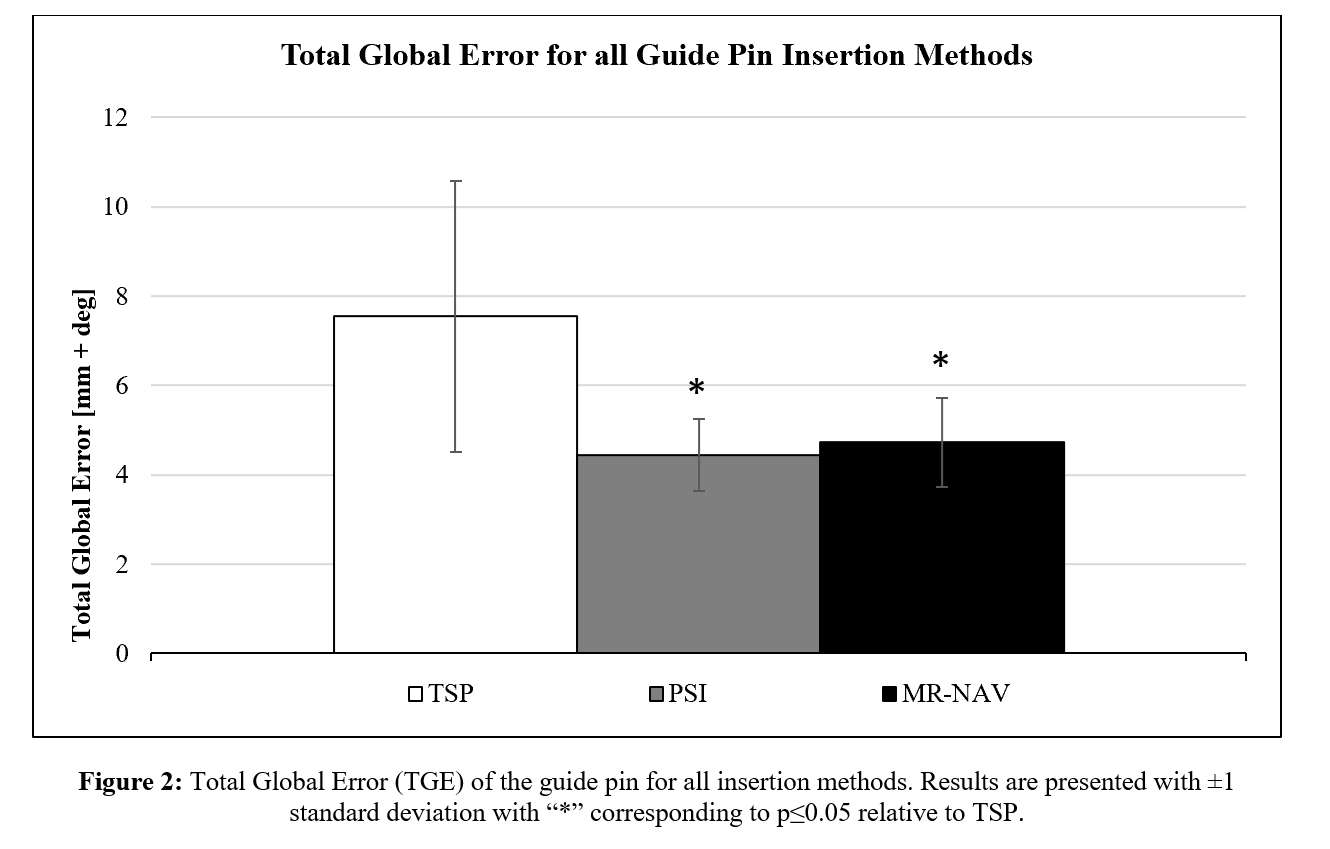
Figure 2#8742
The Placement Accuracy of Custom-Made Implants Utilizing Three-Dimensional Additive Manufacturing Technology for Cases With Massive Acetabular Bone Defects.
*Atsushi Taninaka - Kanazawa university - Kanazawa, Japan
Tamon Kabata - Kanazawa university - kanazawa, Japan
Yoshitomo Kajino - Kanazawa University - Kanazawa, Japan
Daisuke Inoue - Kanazawa University - Kanazawa, Japan
Takaaki Ohmori - Kanazawa Univercity - Kanazawa, Japan
Yuki Yamamuro - Kanazawa University School of Medical Science - Kanazawa, Japan
Tomoyuki Kataoka - Kanazawa University - Kanazawa, Japan
Yoshitomo Saiki - Kanazawa University - Kanazawa, Japan
Yu Yanagi - Kanazawa University - Kanazawa, Japan
Musashi Ima - Kanazawa university hosapital - Kanazawa, Japan
Takahiro Iyobe - Kanazawa university hospital - Kanazawa, Japan
Fujimaru Naoya - Kanazawa university hospital - Kanazawa, Japan
Hiroyuki Tsuchiya - Kanazawa University - Kanazawa, Japan
*Email: atsushi880628@yahoo.co.jp
Introduction
In total hip arthroplasty (THA), whether primary or revision surgery, significant bone defects in the acetabulum make the placement of the acetabular component challenging. The advent of 3D printing has made it possible to create custom-made implants that can be flexibly adapted to the bone defects of individual patients. The development of these custom-made implants has taken place all over the world, including in Japan. In this study, the accuracy of implant placement was examined in patients with severe acetabular bone defects who underwent THA using the T-REX® (Teijin Nakashima Medical Ltd. Japan), a custom-made implant developed in Japan.
Materials and Methods
We performed a retrospective study involving 10 patients (4 males and 6 females; mean age at surgery, 73.8 [57-87] years) who underwent primary and revision THA with T-REX® at our institution between October 2020 and July 2022. The diagnoses of the patients included three hips with high dislocation, three hips with periprosthetic infection, and four hips with aseptic loosening. Acetabular defects were Paprosky type IIB in three hips, IIIA in five hips, and IIIB in two hips. Primary THA was performed on three patients and revision THA on seven patients. A custom-made implant was designed and fabricated using 3D additive manufacturing technology. A pelvic model and an implant copy made to the same shape as the custom-made implant were prepared for use as a patient-specific guide and used intraoperatively to confirm the implant alignment and position (Figure 1). A 3D model of each pelvis and implant was constructed using CT data acquired postoperatively. The bilateral superior anterior iliac spine, pubic tuberosity, and superior posterior iliac spine were superimposed on the preoperative 3D model to verify the accuracy of the implant alignment and position errors.
Results
Evaluation of each installation alignment compared to its preoperative plan showed an average of 42.1 (34.9-48.4) degrees for inclination, with a mean absolute error of 3.92 ± 2.40 degrees, and an average of 20.1 degrees for anteversion, with a mean absolute error of 1.81 ± 1.34 degrees. Inclination and anteversion were within 3 degrees in three cases and within 5 degrees in six cases of the intended alignment. Rotation error was larger than 18 degrees in one case; in the remaining nine cases the error was within 7 degrees. The overall absolute error in rotation was relatively large at 5.48 ± 4.89 degrees from the preoperative plan.
In the evaluation of placement position error, it was possible to place the implants within 5 mm of the planned position in all cases and within 3 mm in six cases. Absolute errors of 1.87 ± 0.94 mm in the internal/external direction, 1.55 ± 0.98 mm in the anteroposterior direction, and 1.10 ± 0.77 mm in the vertical direction were observed(Figure 2).
Conclusion
T-REX®, a custom-made implant manufactured in Japan, is a viable treatment option for individuals with significant acetabular bone defects given that it can be accurately placed during THA and can provide firm initial fixation by taking advantage of its flexibility in design
Figures
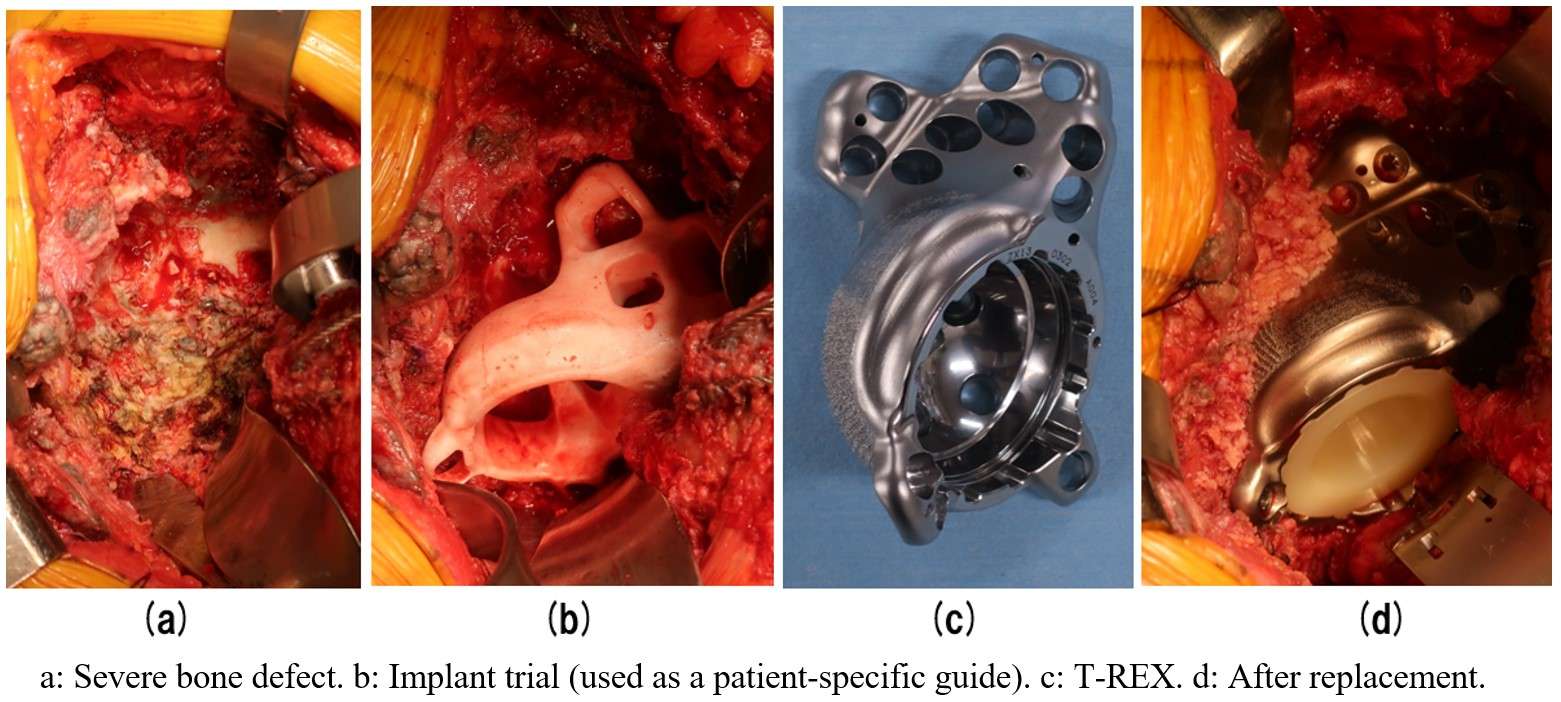
Figure 1
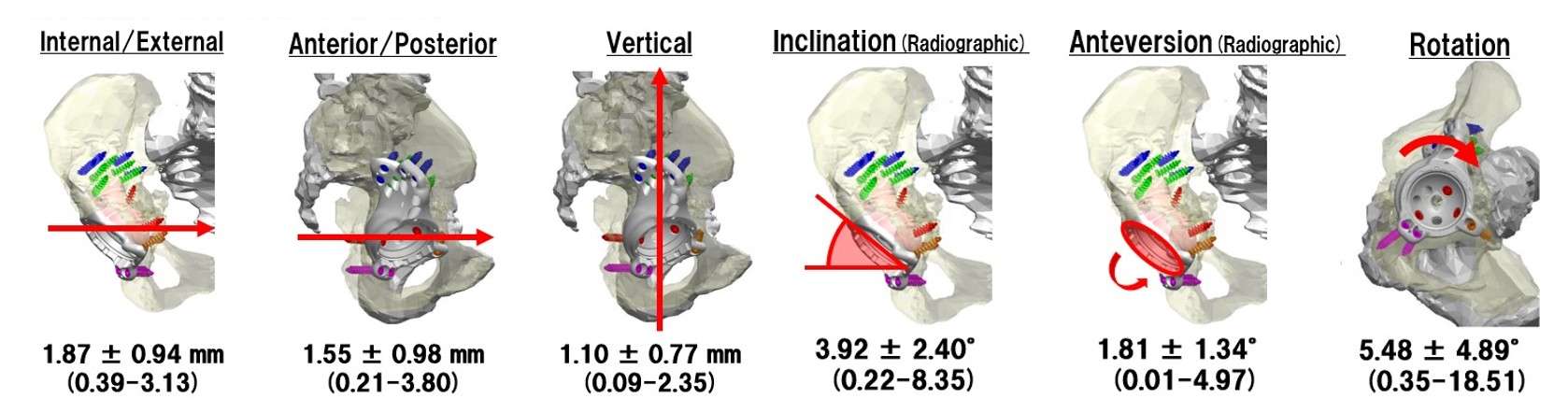
Figure 2#8629
Alignment in Total Knee Arthroplasty Is Only Part of the Equation: High Variability of Ligament Laxities for Each Knee Alignment Category
*Edgar Wakelin - OMNI Life Sciences - Raynham, USA
Christopher Plaskos - Corin - Raynham, USA
Gwo-Chin Lee - University of Pennsylvania - Philadelphia, USA
*Email: edgar.wakelin@coringroup.com
INTRODUCTION:
An increasing focus has been placed on targeting alternative alignments in total knee arthroplasty (TKA) in order to produce a more anatomic reconstruction. Kinematic TKA technique proposes that a balanced TKA can be produced by simply reproducing the native anatomy without the need for ligament releases. However, the anatomy of the knee is complex and highly variable. Therefore, the purpose of this study is to evaluate the in vivo medial and lateral ligament tensions of the knee across various limb alignment categories.
METHODS:
Using the ligament tensions acquired during 655 consecutive robotic assisted TKA with a dynamic ligament tensor, the relationship between medial and lateral collateral ligament laxity and overall limb alignment was established. Only knees with neutral or mechanical varus alignment were included and divided into 5 groups of increasing deformity: neutral 0-3° (n = 79), varus 3-5° (n = 146), 6-9° (n = 240), 10-13° (n = 138), and ≥14° (n = 53) varus, Figure 1. The distraction of the medial and lateral sides was compared across the various limb alignments using analysis of variance (ANOVA).
RESULTS:
For the varus knee, the ability to distract the medial collateral ligament in extension and flexion was proportional to the degree of varus deformity (p < 0.05), ranging from 4.1±2.4 mm (neutral) to 8.8±3.2 mm (≥14° varus) in extension (Figure 2, Left and Table 1) and 4.9±2.1 mm (neutral) to 8.7±3.2 mm (≥14° varus) in flexion. On the lateral side, the distraction of the lateral collateral ligament remained constant in both flexion and extension (p > 0.05), ranging from 1.3±2.7 mm (≥14° varus) to 2.2±2.5 mm (neutral) in extension (Figure 2, Right and Table 2) and 1.7±3.0 mm (≥14° varus) to 2.8±2.9 mm (neutral) in flexion. There was high variability of the stretch of the ligaments within each alignment category in which the standard deviation of the distractibility ranged from 2.0 to 3.2 mm.
CONCLUSION:
The anatomy and soft tissue identity of the knee is complex and highly variable. TKAs seeking to be more anatomic will not only need to restore alignment but also native soft tissue tensions. Targeting alignment alone may result in a TKA that is unbalanced and prone to early failure.
Figures
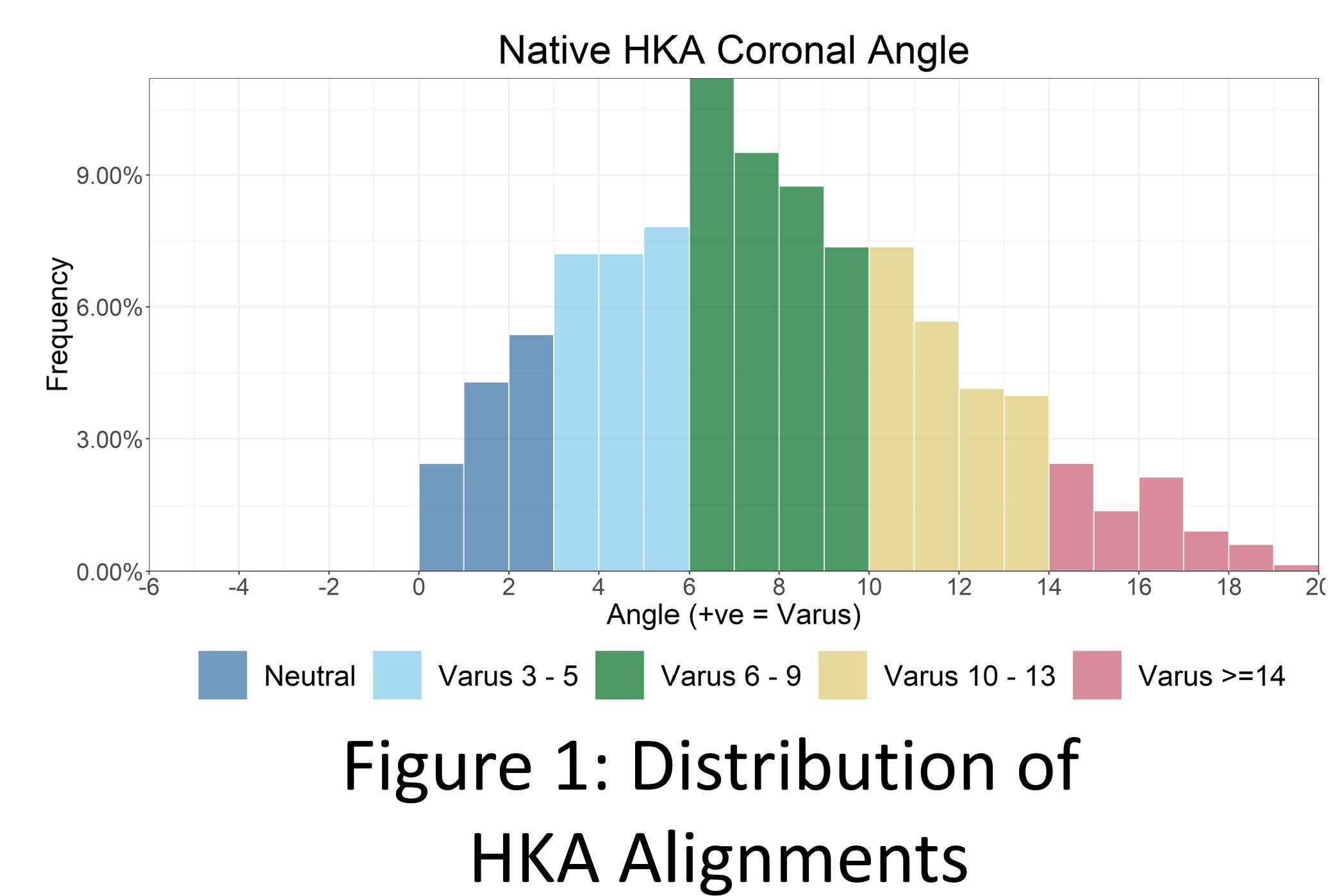
Figure 1
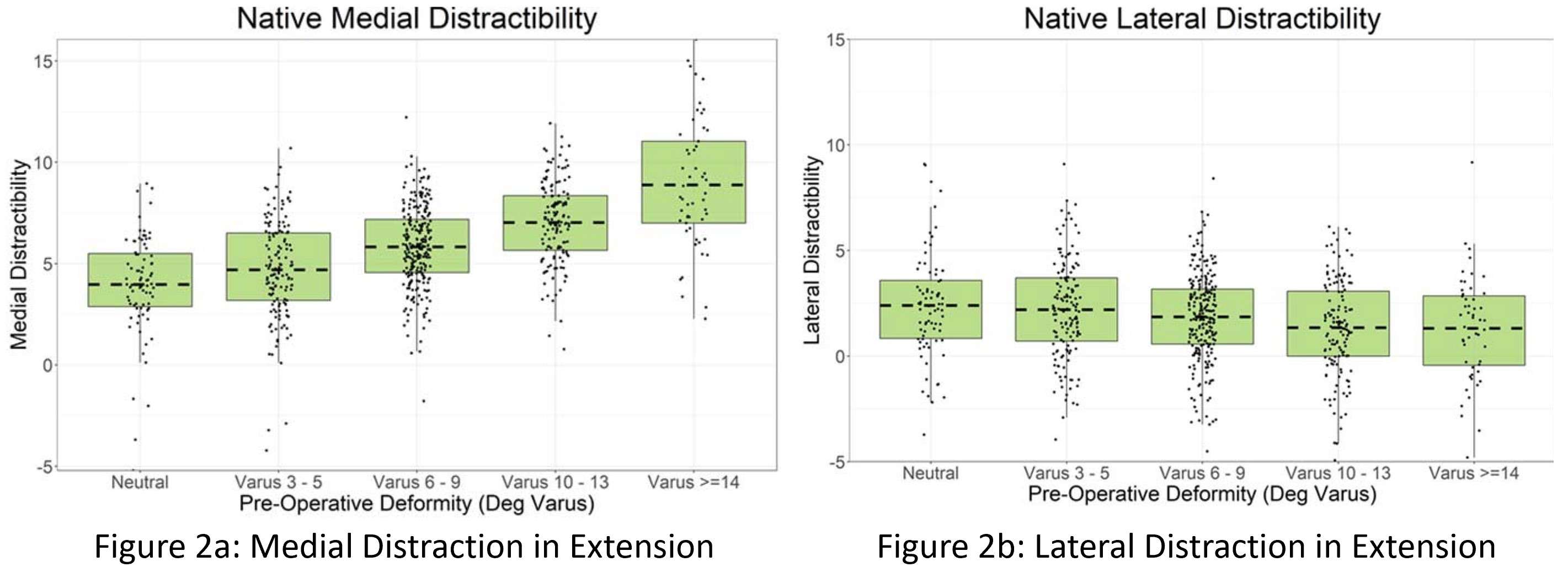
Figure 2
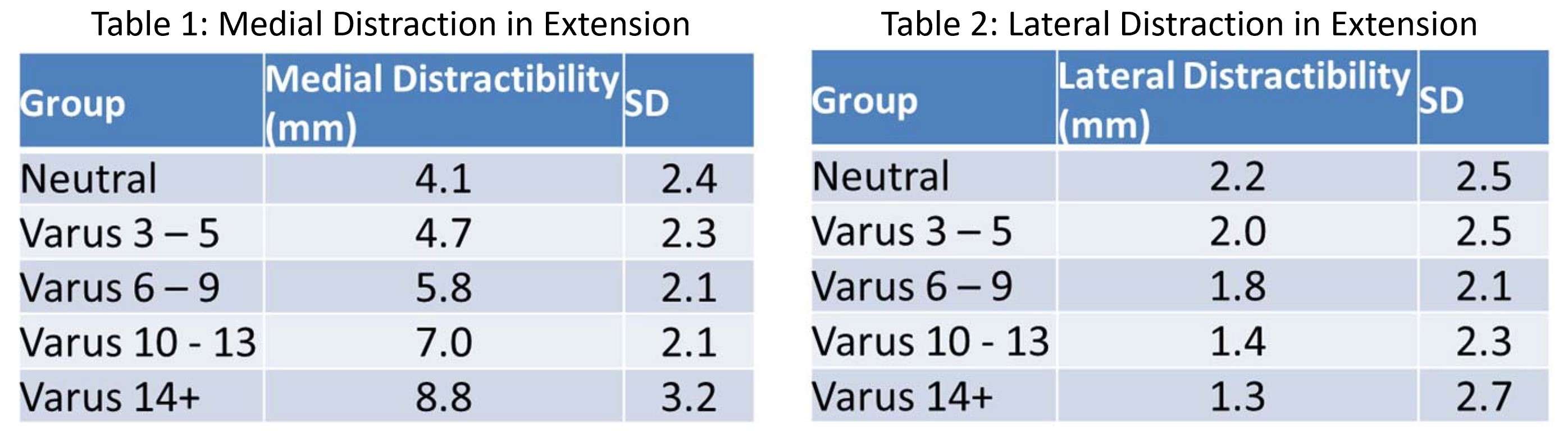
Figure 3#8796
Definition of the Laxity Goals During TKA Tends to Be Surgeon Specific
Prudhvi Tej Chinimilli - Exactech - Gainesville, USA
*Laurent Angibaud - Exactech, Inc. - Gainesville, USA
Wen Fan - Exactech - Gainesville, USA
François Boux de Casson - Blue-Ortho - Meylan, France
Amaury Jung - Blue Ortho - La Tronche, France
*Email: laurent.angibaud@exac.com
Introduction:
Alignment techniques in total knee arthroplasty (TKA) continue to evolve as technologies of implantation progress. The recent possibility of reliably characterizing the soft-tissue envelope enabled the development of TKA alignment techniques such as functional alignment, allowing the possibility of restoring the constitutional alignment while achieving proper soft-tissue balance. While these techniques offer guidelines for the bone cut parameters in terms of boundaries, the definition of the laxities is still unclear. In this regard, the objective of this study was to evaluate the laxity signatures set-up by surgeons at the time of the planning of the bone cut parameters.
Methods:
A retrospective review was performed on a proprietary cloud-based web database that archives technical cases logs performed using an instrumented CAS system. A total of 515 cases performed by 8 individual surgeons, with at least 30 cases each, were considered without any exclusions. The surgical technique encompassed the possibility of setting-up the femoral planning based on alignment, size, but also soft-tissue consideration acquired by placing an intra-articular tensioner between the proximal tibial cut and the native femur. For each case, the planned laxities were referenced relative to the planned medial laxity at 10° of flexion. Based on the potential impact of the conservation of the posterior cruciate ligament (PCL) or not, the cases were separated between CR (5 surgeons) and PS (4 surgeons). Surgeon 5 data is included in both PS and CR analysis due to more than 30 cases. Relative planned laxities were calculated for both medial and lateral compartments from 10° to 120° of flexion. Two Way ANOVA (Analysis of Variance) was used to compare the surgeon effect on the laxity definition. If the effect was significant, Tukey multiple comparisons of means were used to compare pair-wise laxity difference between surgeons. Significance level was set to 0.05.
Results:
Regardless of the conservation of the PCL and the side of the compartment, the relative laxities were significantly different between the 8 surgeons. The box and whisker charts are plotted displaying both medial and lateral laxity for each surgeon separately for PS and CR in Figures 1 and 2, respectively. It can be inferred that both medial and lateral median values at each flexion angle and overall laxity signatures look surgeon-specific. A further statistical analysis performed with Tukey multiple comparisons (Figure 3) inferred that for most of the pairwise comparisons, the laxity difference was found to be statistically significant which is marked in bold.
Conclusion:
The exact laxity required in TKA is yet to be determined. Some surgeons aim for equal rectangular gaps in both flexion and extension, some target trapezoidal gaps with added laxity on the lateral compartment compared to medial, while others plan for larger flexion gap than extension. Even though our study only considered cases using the same knee system and the same technique, the laxity goals were surgeon-specific. As recent studies suggest that laxity as small as 2mm may impact the outcomes, there exists an opportunity to develop solutions to further define the optimal laxity.
Figures
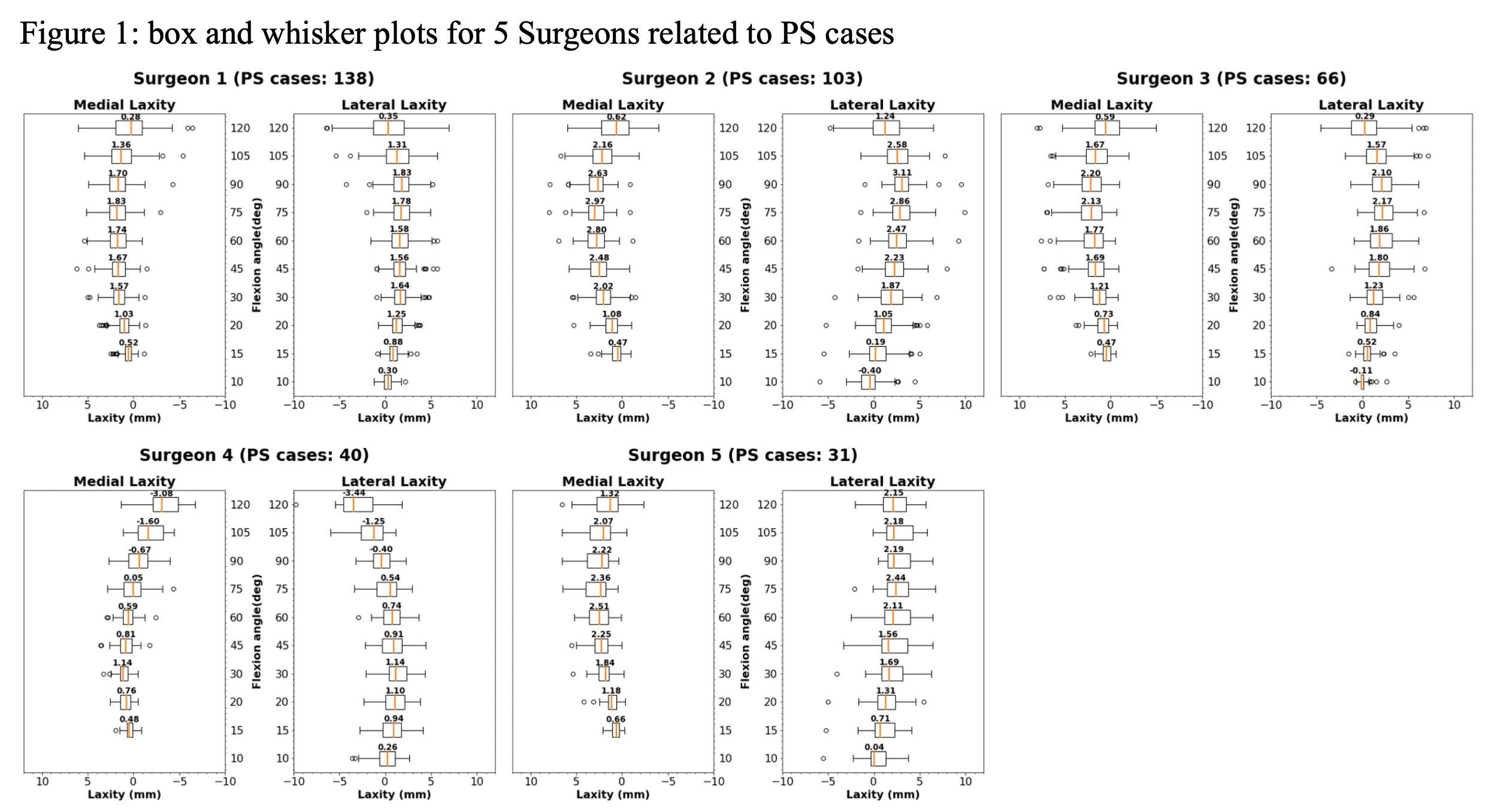
Figure 1
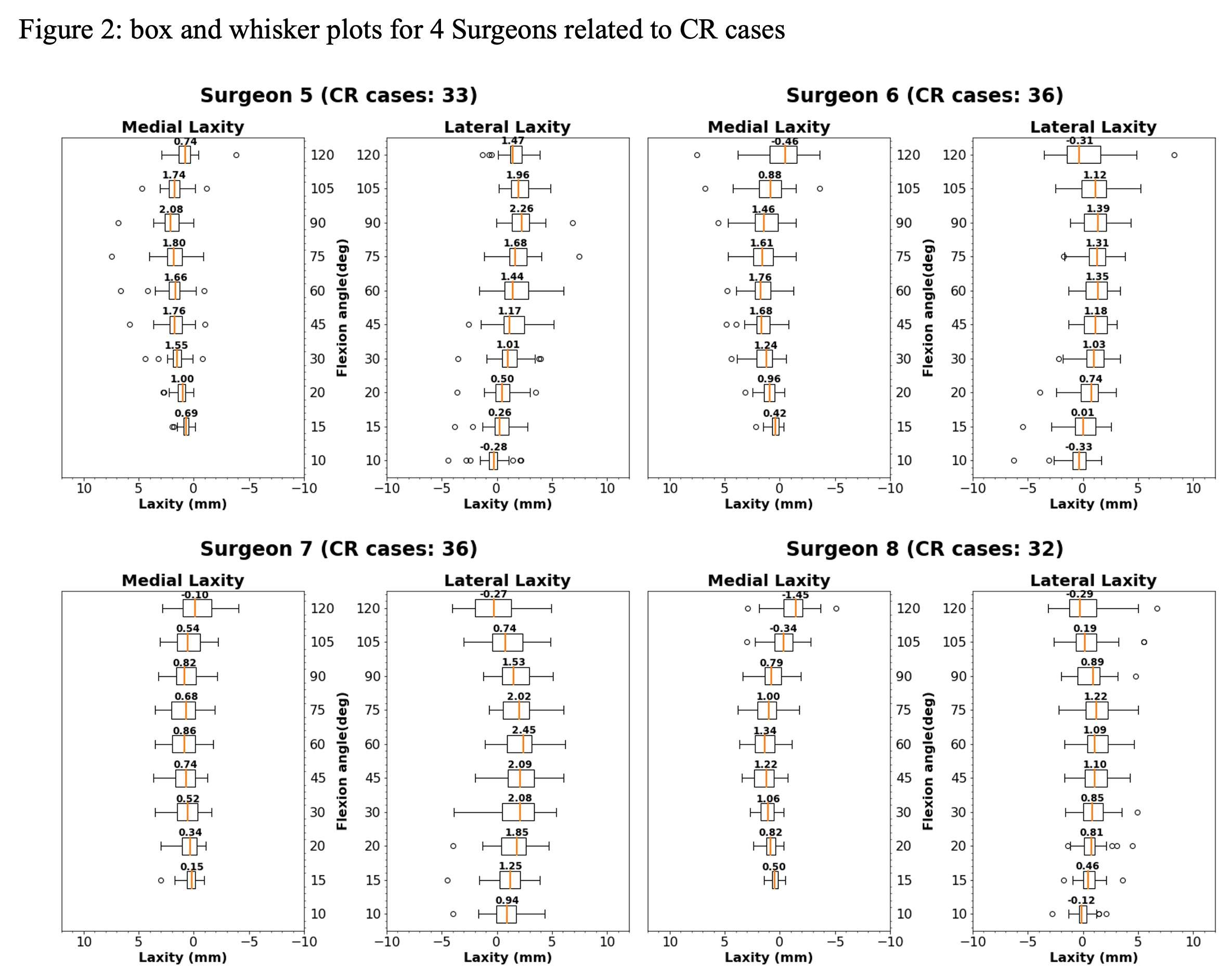
Figure 2
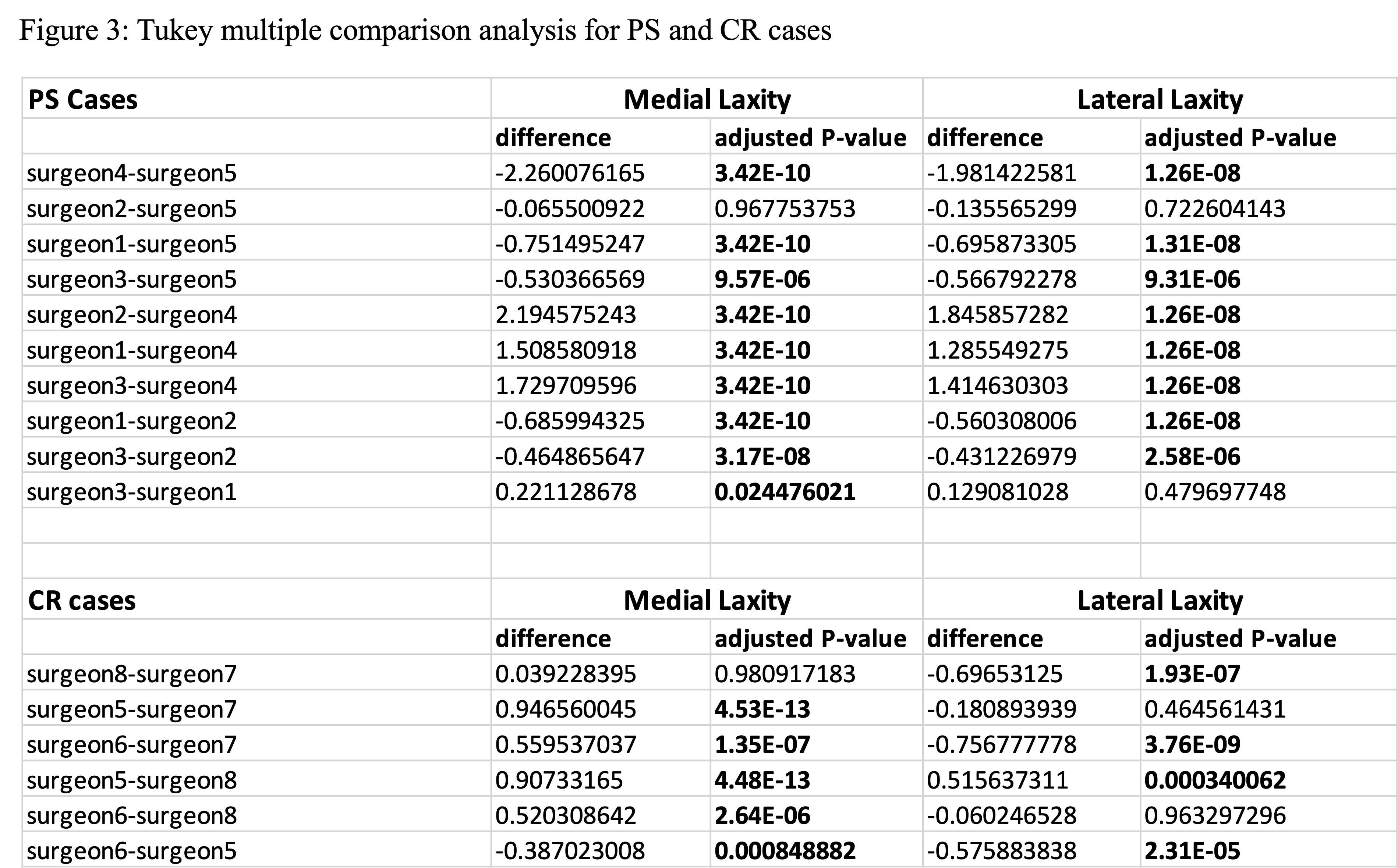
Figure 3#8591
Multiplanar in-Vivo Tibiofemoral Laxity: Comparison of Healthy Individuals to Patients Following TKA
*David Shamritsky - Hospital for Special Surgery - New York, United States of America
Erin Berube - Hospital for Special Surgery - New York, United States of America
Akinola Oladimeji - Hospital for Special Surgery - New York, USA
Brian Chalmers - Hospital for Special Surgery - New York CIty, USA
Stephen Lyman - Hospital for Special Surgery - New York, USA
Cynthia Kahlenberg - Hospital for Special Surgery - New York City, USA
William Long - NYU Langone Health - New York, USA
Geoffrey H Westrich - Hospital for Special Surgery - New York, USA
Eytan Debbi - Cedars-Sinai Medical Center - Los Angeles, USA
Peter Sculco - Hospital for Special Surgery - New York, USA
David J. Mayman - Hospital for Special Surgery - New York, USA
Timothy Wright - Hospital for Special Surgery - New York, USA
Carl Imhauser - USA
*Email: shamritskyd@hss.edu
Introduction:
Patients’ perceptions of knee instability following total knee arthroplasty (TKA) are a leading cause of dissatisfaction and revision surgery. The knee’s ligamentous restraints and articular surfaces are important contributors to joint stability which is commonly assessed via clinical exams of knee laxity. Unfortunately, laxity targets following TKA remain ill-defined with some surgeons suggesting the need to replicate native knee laxity. However, the inability to objectively and quantitively assess in vivo multiplanar knee laxity has posed a significant barrier to identifying these laxity targets. Using a custom arthrometer we have developed, we asked the following question: Does in vivo laxity in the anterior-posterior (AP), varus-valgus (VV), and internal-external rotation (IER) directions following TKA differ from that of the native knee?
Methods:
We used a custom arthrometer comprised of an instrumented linkage to measure knee laxity in the AP, VV, and IER directions [1] (Fig. 1). Tests were administered with the knee oriented at 20° of flexion. Two cohorts were tested: the operated leg of 11 patients at 1-year post-TKA (6 male/5 female; age: 65 ± 7 years, BMI: 29 ± 3; KOOS JR: mean 84 range 59-100); and both legs of 15 volunteers with no history of knee injury or surgery (8 male/7 female; age: 28 ± 6 years, BMI: 23 ± 3). Each leg was measured twice by two examiners manually applying 4 cycles of AP forces (10 N posterior to 30 N anterior), VV moments (±3 Nm), and IER moments (±2.5 Nm) to the lower leg. The net respective translations and rotations were calculated for each loading cycle and averaged for each leg [1]. In the healthy cohort, laxity data were averaged across both legs. We performed two analyses to answer our research question. First, we compared the laxities for both cohorts using a Mann-Whitney U test for unpaired, non-parametric data. Second, we evaluated relationships between VV and AP laxities and IER and AP laxities using linear regression and reported the coefficient of determination (R2) and the correlation coefficient (β) and corresponding p-values (α = 0.05).
Results:
Median and interquartile range of AP laxity in the TKA cohort were 2.2 mm and 2 mm less, respectively, than the healthy subjects (p<0.001) (Table 1). Similarly, median and range of IER laxity in the TKA cohort were 6.2° and 4.5° less, respectively, than the healthy subjects (p<0.01) (Table 1). AP laxity was positively correlated (p<0.05) with VV and with IER laxities in both the TKA cohort (β = 0.40 and 0.19, respectively) and the healthy cohort (β = 1.12 and 0.22, respectively) (Fig. 2).
Conclusion:
Lower median laxity and variability of laxity in the TKA population may be intuitive since the variable native articular geometries and ligamentous constraints are replaced with uniform, well-defined implant systems. Previous cadaveric work in native knee joints also found correlations between IER and AP laxities and VV and AP laxities [2]. Our finding of higher laxity and variability in the native cohort should be considered when recommending laxity targets in TKA and requires further study.
Figures
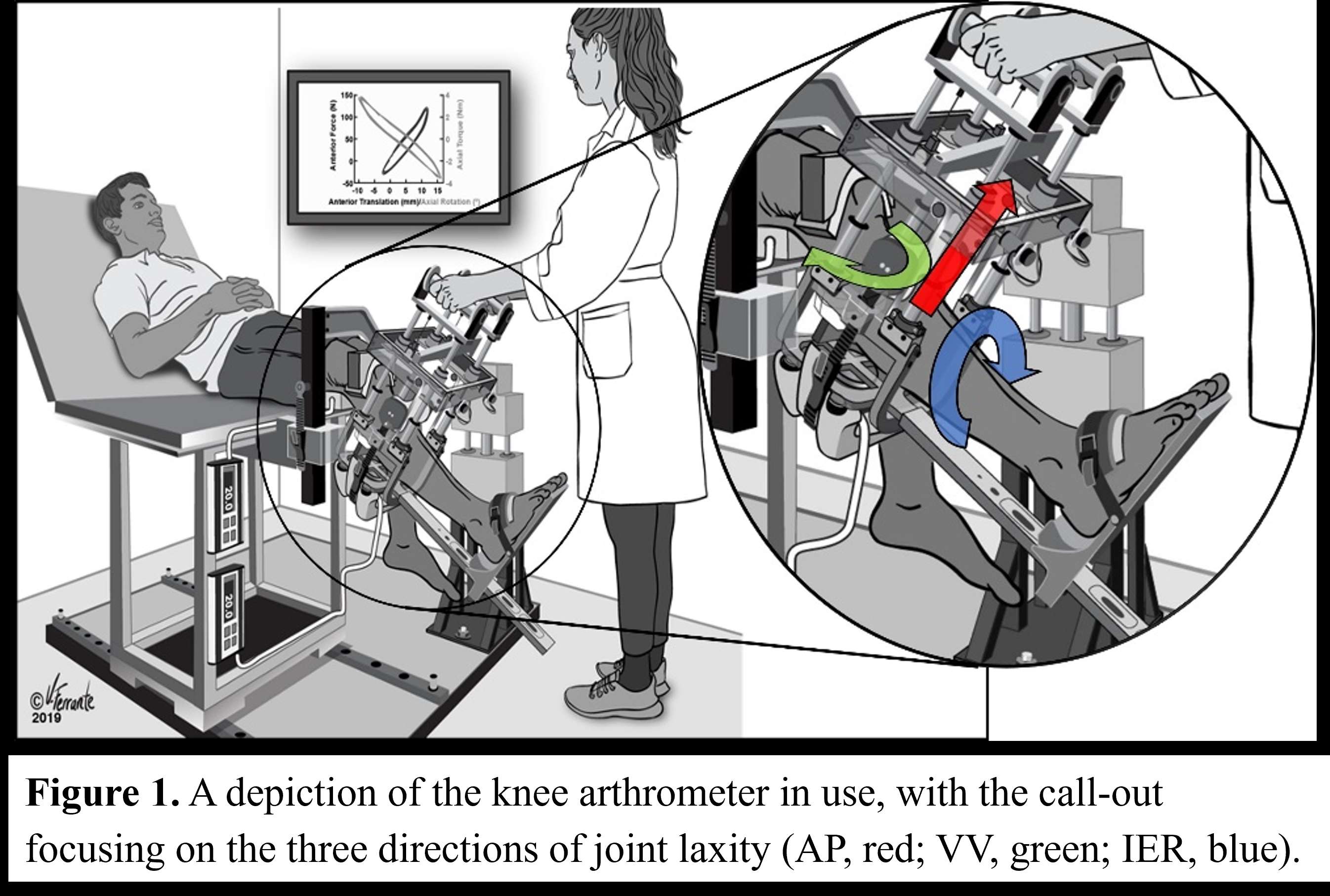
Figure 1
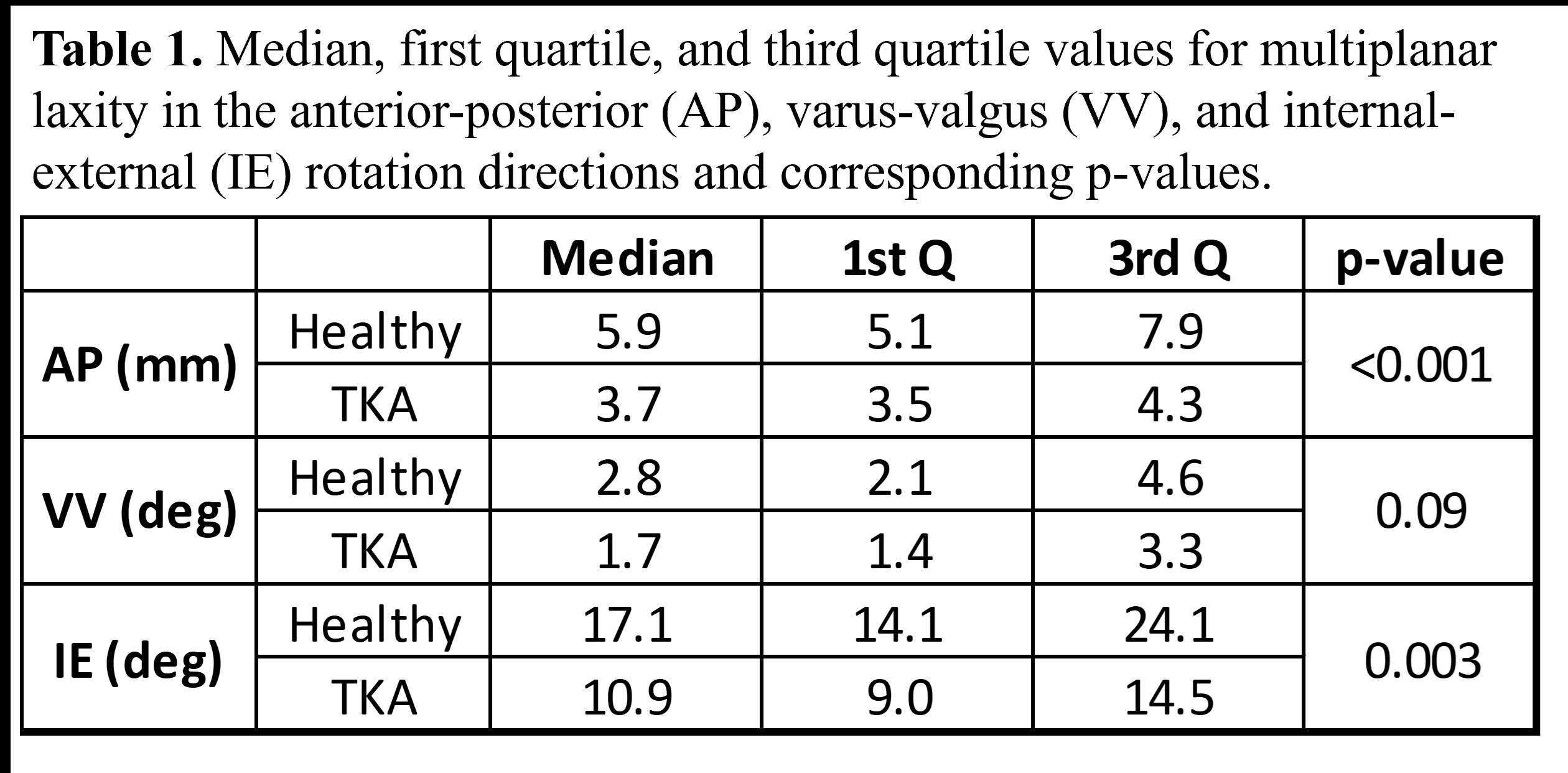
Figure 2
Figure 3#8260
Impact of Incremental Loads on Soft-Tissue Stiffness in a Simulated Total Knee Arthroplasty Model
*Kevin Abbruzzese - Stryker - Mahwah, USA
Azhar Ali - Stryker Orthopaedics - Mahwah, USA
Andre Freligh - Stryker Orthopaedics - MAHWAH, United States of America
Scott Logan - Stryker Orthopaedics - Mahwah, USA
Jared Weir
Stefano Bini - University of California San Francisco - San Francisco, USA
Michael Mont - Sinai Hospital of Baltimore - Baltimore, USA
*Email: kevin.abbruzzese@stryker.com
Introduction:
Achieving optimal outcomes in total knee arthroplasty (TKA) relies on maintaining an appropriate balance of soft tissues. Surgeons often rely on subjective manual techniques to assess soft tissue balance which can be difficult to quantify and replicate. To address this, a newly-developed pendulum knee drop (PKD) technique, offers a quantifiable approach to reliably estimate the soft tissues of the knee joint. The aim of this study was to assess the differential impact of incremental joint distraction loads using the PKD in a knee simulator model that underwent simulated robotic TKA as a measure of soft-tissue changes in the knee based on stiffness and damping.
Methods
The AKS model was designed using a three-dimensional (3D) Computed Tomographic scan of a patient undergoing a left TKA who had a varus deformity, moderate osteophytes, simulated soft-tissue, and was manufactured using 3-dimensional printing [1]. The AKS model underwent a simulated RATKA. Incremental loads were applied to the joint line after femoral and tibial resections in the range of 5 to 25 pounds (lbs) with a tensioning device at 90 degrees of knee flexion. The PKD test was performed 3 times per loading conditioning after tension was applied. Knee range of motion (ROM) oscillations were measured with an inertial measurement unit (IMU). Joint distances were measured by a robotic-arm system as the distance between the femoral bone and tibial plateau. Kinematics are described using lowest points of femoral condyles with respect to tibial coordinate system. Soft-tissue estimates for knee stiffness and damping were computed. Stiffness was computed as the change in joint load divided by the change in joint distance. Damping was calculated as the damping ratio. Analyses of variance assessed significant differences between loading conditions for stiffness and damping with a Tukey post hoc test used to locate respective differences.
Results
Average knee stiffness was significantly greater on the medial side at 90° compared to the lateral side for all loads (Figure 2). Significant differences were found between damping estimates for all loading conditions except 5 and 8 lbs, with 25 lbs resulting in the greatest damping (Figure 3). There was a high correlation between stiffness and damping (R²=0.98). Low point kinematics demonstrated an average posterior translation of 1.52mm as observed between the 5lb and 25lb load condition (Figure 1).
Conclusion
Incremental loading led to a significant increase in medial ligament tension where greater femoral posteriorization resulted in added tension on the PCL as inferred by a reciprocal increase in damping. Combining a tension device with the PKD test is a promising technique to estimate soft-tissue stiffness in a simulated TKA model as observed by the high correlation between knee stiffness and damping. These characterizations may reinforce objective decisions for soft-tissue balance in TKA.
References
- Faizan et al. “An advanced knee simulator can reproducibly be used for ligament balancing training during TKA”; ORS 2023, Poster #1656
Figures
Figure 1#8444
Midflexion Laxity in TKA: Alignment Matters, Implant Choice Does Not
*Alex Orsi - Corin - Raynham, USA
Christopher Plaskos - Corin - Raynham, USA
Eric M Slotkin - Reading Hospital, Orthopaedic Associates of Reading, - West Reading, Pennsylvania, USA
Enrique Forlenza - Rush University Medical Center - Chicago, USA
Stefan Kreuzer - INOV8 Orthopedics - Houston, USA
Vasili Karas - Rush University Medical Center - Chicago, USA
*Email: Alex.Orsi@coringroup.com
Implant selection and alignment technique may affect balance in TKA. The relative contribution of each of these factors is unknown. This study investigates the relative impact of single vs multi-radius implant design and alignment technique on balance variability using intra-operative displacement and tensioning measurements through range of motion.
Ligament balance data was collected using a robotic ligament tensioner at a static tensioning force of 70-90N in 154 consecutive TKAs. A tibia-first resection technique and a single-radius cruciate-retaining implant was used for all cases. Simulation software was used to virtually replace the single-radius with a multi-radius design of equivalent size, by aligning the implants to have equivalent gaps at 10° and 90° flexion.
Two alignment techniques were assessed by aligning the implants in gap balanced (GB) and measured resection (MR) positions.
Differences in mediolateral (ML) balance and mean laxity between the two implants and two techniques were compared throughout the flexion range at 0°, 20°, 30°, 45°, and 60°.
To estimate the pre-resection state of the knee, gaps prior to making any bone resections were calculated using the ligament tension and bone morphology data acquired.
Implant design had no effect on ML balance variability, while the effect due to technique ranged from 2±1.3 mm to 2.5±1.8 mm throughout the flexion range. Due to implant design having no effect on ML balance variability, the effects due to technique were significantly greater compared to implant design across all comparison angles (p<0.001) [Fig. 1].
Technique also had a greater contribution to mean laxity variability compared to the implant design at 0° (1.3±1 vs. 0.7±0.2 mm, p<0.001), 20° (1.5±1.2 vs. 0.4±0.2 mm, p<0.001), 30° (1.5±1.2 vs. 0.4±0.2 mm, p<0.001), 45° (1.5±1.1 vs. 0.2±0.2 mm, p<0.001), and 60° (1.5±1.1 vs. 0.2±0.2 mm, p<0.001) [Fig. 2].
Single-radius implant laxity was 0.7 mm looser at full extension and 0.4 mm tighter in midflexion compared to the multi-radius design.
MR had greater lateral ML imbalance compared to GB in extension (-0.7±3.1 vs 0.1±1 mm, p<0.01), midflexion (-1.4±2.7 vs -0.4±1 mm, p<0.001), and flexion (-2±2.9 vs -0.4±0.9 mm, p<0.001).
Pre-resection laxity had a standard deviation of 2.5 mm in extension and 2.5 mm in flexion.
Alignment technique contributes more towards laxity and balance variability than implant design. Implant geometry has no effect on ML balance variability. The laxity variability from alignment technique was similar to the variability measured from the arthritic knee population. Greater consideration should be given to alignment technique than implant geometry when achieving balance in TKA.
Figures
Figure 1
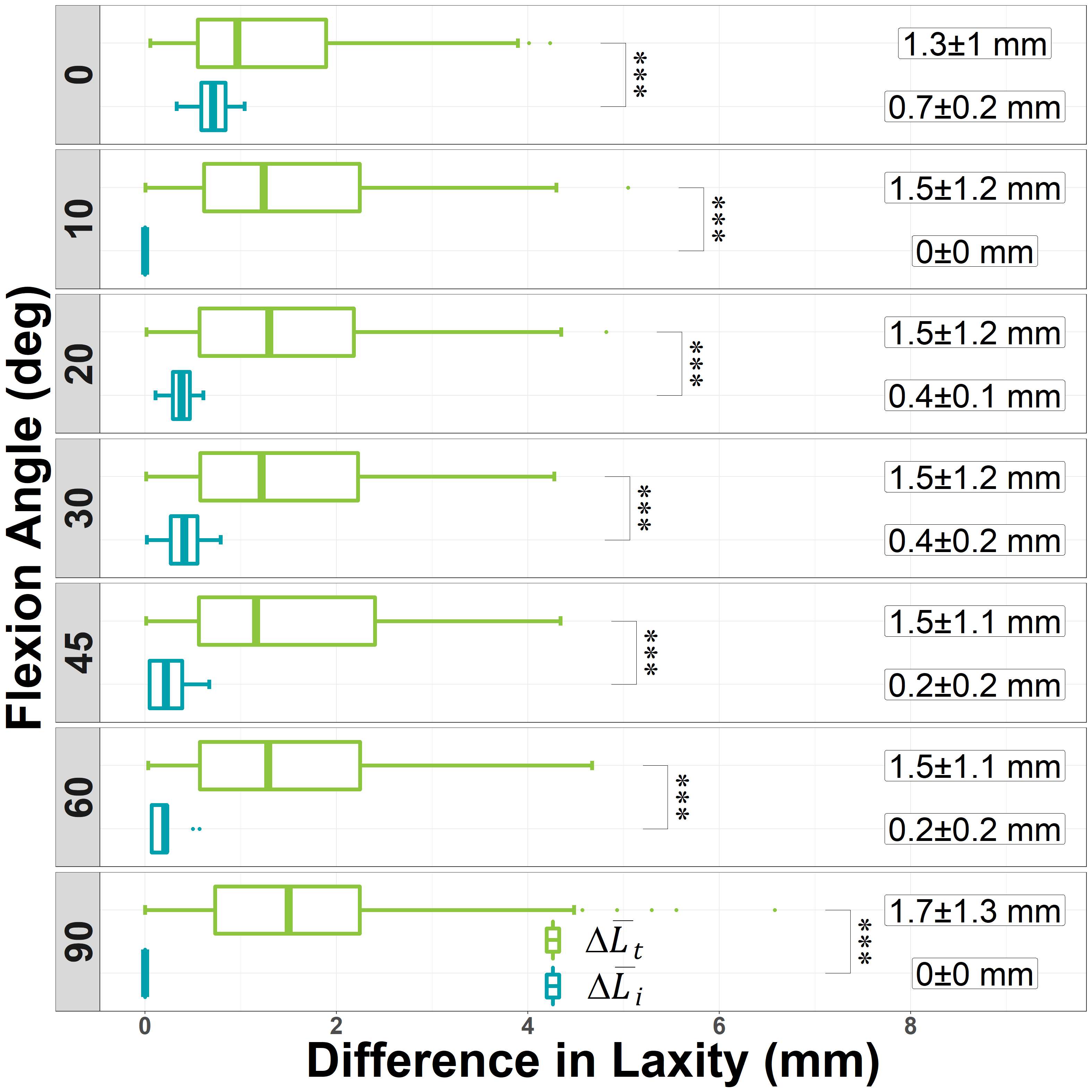
Figure 2#8769
Detecting Patterns of MCL Injury From Quantitative Knee Laxity Assessments
*Nicholas Dunbar - Houston, United States of America
Shuyang Han - University of Texas Health Science Center at Houston - Houston, USA
Jonathan E. Gold - UTHealth - Houston, USA
Kyle Borque - Houston Methodist Orthopedics and Sports Medicine - Houston, USA
Nicholas D. Lanfermeijer - UTHealth - Houston, USA
Lauren Pupa - Baylor College of Medicine - Houston, USA
*Email: nicholas.j.dunbar@uth.tmc.edu
Introduction
Advancements in detailed assessment of multi-ligament knee injuries can aid in patient selection and planning of surgical treatment. However, current medical imaging techniques to diagnose injury are costly to perform and rely on subjective evaluation. An alternative solution is computational evaluation of knee motions collected by high-resolution motion capture technology during routine laxity exams. Availability of knee kinematics data, which includes both intact and injured knee motions, is essential for developing computational methods that identify patterns of knee laxity that result from a specific knee ligament injury. In this study, given that the medial collateral ligament (MCL) is an important restraint to knee laxity and is commonly involved in multi-ligament knee injuries, we trained a computational method to detect patterns of experimentally measured knee laxity after resection of the MCL. The detected patterns may have the potential to differentiate MCL-deficient knees during routine clinical assessment.
Methods
Tibiofemoral kinematics from six fresh-frozen cadaveric knee specimens with intact soft-tissues were measured before and after MCL-resection in a simulated knee laxity assessment. Two repeated laxity assessments were performed on each knee for both the intact and injured conditions. The tibia was procedurally manipulated throughout its range-of-motion via an instrumented handle while marker trajectories and applied loads were continuously measured using high-resolution motion capture cameras and a six degree-of-freedom load cell. Knee laxity was defined by the upper and lower bounds of the varus/valgus (VV) and internal/external (IE) angles at every five degrees of knee flexion. To standardize the applied loads across assessment trials, laxity data was filtered by excluding data for which the forces and moments exceeded 30 N and 6 Nm, respectively. Statistical shape models (SSMs) were then created from multiple training datasets composed of intact and MCL-deficient knee laxity measurements from the first assessment trial. Three different SSMs were trained using VV only, IE only, and combined VV+IE laxity datasets. SSM fitting was performed on the second assessment trial by varying the coefficients of the shape modes which explained up to 95% of the variance in the laxity data. Finally, statistically significant differences in coefficients between intact and MCL-deficient knees were determined by a paired, two-tailed t-test (p<0.05).
Results
MCL-deficient knees had statistically different shape mode coefficients for the VV only (p≤0.02) and VV+IE SSMs (p≤0.047), but not for the IE only SSM (p≥0.07). Two, three, and four shape modes were identified for the VV only, IE only, and combined VV+IE SSMs, respectively (Fig. 1). The VV only shape modes corresponded to a shift in varus to valgus laxity and change in overall magnitude. Three of the four VV+IE shape modes could also differentiate MCL-deficient knees, including a pattern of combined IE+VV laxity and uniformity of IE laxity with flexion.
Conclusion
Objectively quantifying MCL injury is challenging due to variability in native knee laxity. Principle modes of variation identified using statistical shape models of knee laxity were able to differentiate the combined effects of MCL resection on internal/external and varus/valgus laxity compared to intact knees in an experimental setting.
Figures
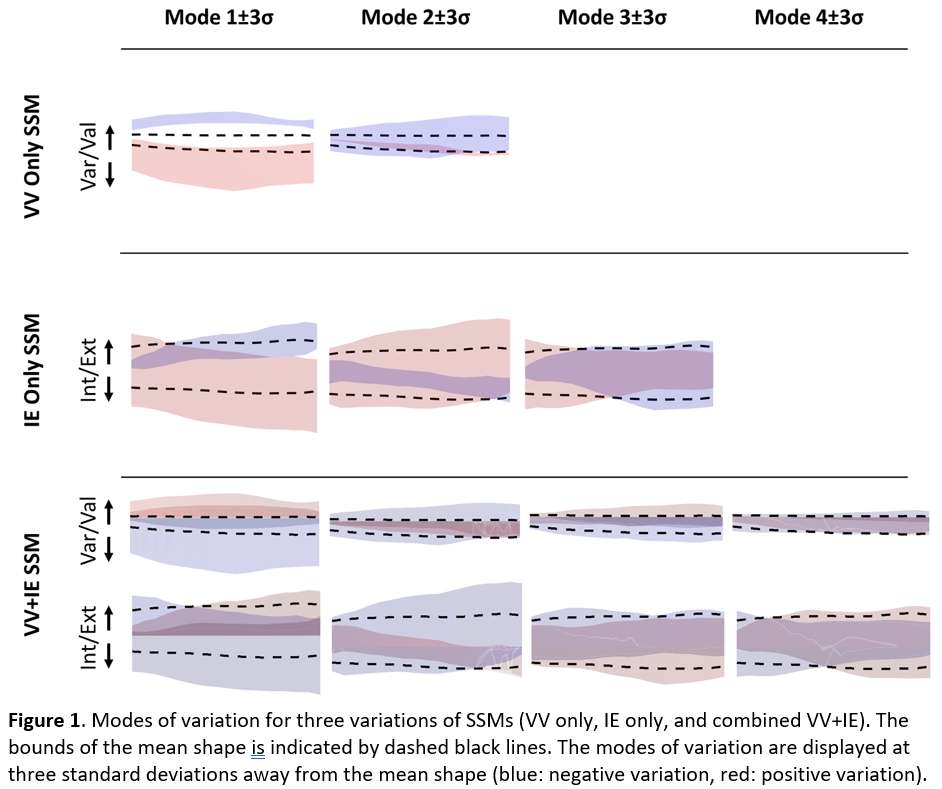
Figure 1#8799
Adverse Preclinical Testing of Ceramic-on-Ceramic Hip Resurfacing
*Andrew Robert Beadling - University of Leeds - Leeds, United Kingdom
Gregory Pryce - Instite of Biogical and Medical Engineering - University of Leeds - Leeds, United Kingdom
Danielle De Villiers - Queen Mary, University of London - London, United Kingdom
Simon Collins - MatOrtho Limited - Leatherhead, United Kingdom
Michael Bryant - University of Leeds - Leeds, United Kingdom
*Email: A.R.Beadling@leeds.ac.uk
INTRODUCTION: Metal-on-Metal resurfacing arthroplasty offered a good early solution for younger and more active patients by restoring function and preserving bone stock, making for an easier transition to a eventual total joint replacement [1]. They performed poorly, however, with higher than acceptable failure rates at five- and ten-year follow up [2]. This had not been captured in pre-clinical hip simulator studies, which follow a simplified loading and kinematic testing profile which is unrepresentative of in vivo conditions. When assessing new devices and materials care should be taken in preclinical testing to ensure they are tested under realistic biomechanics and representative conditions to gain a better understanding of how they are likely to perform. The present study examines a new Ceramic-on-Ceramic resurfacing device under patient derived profiles and adverse scenarios.
MATERIALS & METHOD: Ceramic-on-Ceramic resurfacing components (n=3) were articulated on a ProSim Full-ISO electromechanical hip simulator (SimSol, UK). The bearing diameter was 64 mm and the acetabular cups were orientated at 30° inclination from the horizontal. The test lubricant was foetalbovine serum diluted to a total protein concentration of 30 g/L with deionised water. EDTA (3.1 g/L) and Sodium Azide (0.03%w/v) was added to prevent bacterial growth. The lubricant wascirculated through the cell and heated to 37°C. The devices were articulated through an adverse testing regime beginning with 2 Mcycles of standard ISO-14242 load and kinematics [3]. Following this 1 Mcycles of a patient-derived adverse jogging profile were run in a stop-dwell-start configuration. The jogging profile was generated via motion capture of THR patients and resolved via a multi-body musculoskeletal model (AnyBody Technology) to obtain joint contact forces and rotations [4].Finally, third body debris (hydroxyapatite powder, 5 g/L) was added for a further 1 Mcycles of walking gait.
RESULTS: Figure 1 demonstrates the gravimetric mass loss of the acetabular cups over the course of the experiment. During the ISO walking and Jogging profiles, a minimal mass loss was detected. The variance between weigh points was often within the accuracy limit of the mass balance. Upon introduction of third body debris an uptick in the wear rate was noted; and the cups lost approximately 0.4 mg over the final million cycles. Figure 2 demonstrates volumetric analysis and white light interferometry of the femoral heads. No clear wear scar or significant surface damage / scratching was found on the components over the course of the experiment. Each head remained within manufacturing tolerances after the 4 Mcycles.
CONCLUSIONS: An adverse testing regime including realistic biomechanics and third body debris was used to assess the performance of a new ceramic-on-ceramic resurfacing device. Under normal operation and SDS jogging there was no consistent detectable mass loss from the acetabular components. The addition of third body debris did cause a detectable mass loss although significantly less than that from traditional Metal-on-Polymer bearings.
REFERENCES: [1] Goldsmith et al. Proc IMechE H, 2000,214(1). [2] Smith et al. Lancet, 2012, 380(9855). [3] BS ISO, 14242-1:2014. [4] Lunn et al. J Arthroplasty, 2020, 35(3).
Figures
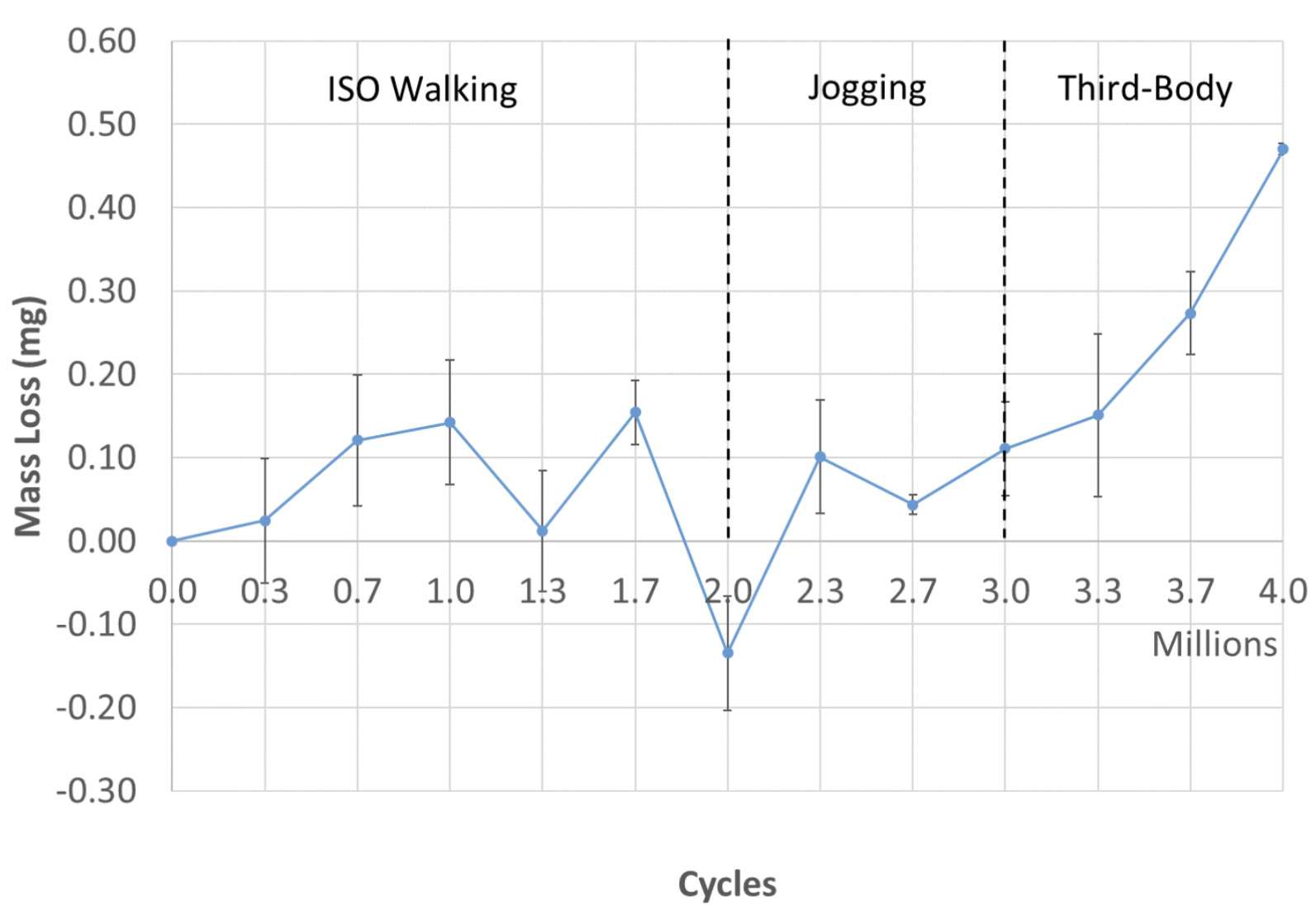
Figure 1
Figure 2#8388
Wear of a Ceramic-on-Ceramic Hip Resurfacing Under Activities of Daily Function
*Raelene Cowie - University of Leeds - Leeds, United Kingdom
Danielle De Villiers - MatOrtho Ltd - Leatherhead, United Kingdom
Simon Collins - MatOrtho Limited - Leatherhead, United Kingdom
Louise M. Jennings - University of Leeds - Leeds, United Kingdom
*Email: r.cowie@leeds.ac.uk
Introduction
Hip resurfacing is a bone conserving alternative procedure to Total Hip Replacement; particularly suited to younger or more active higher demand patients with good quality bone stock. Patients receiving a hip resurfacing often have better postoperative function and reduced dislocation than those with a Total Hip Replacement however, the use of metal-on-metal bearings in hip resurfacings has brought about issues relating to metal ion release that are well documented [1]. Hence there is a clinical need for alternative materials. Ceramics offer high wear resistance even under adverse conditions [2]. In this study, the wear of a ceramic-on-ceramic hip resurfacing was investigated under conditions representative of those the device would undergo during several activities of daily function.
Methods
Six 40/46mm ceramic-on-ceramic (Biolox Delta) hip resurfacing devices were studied (Figure 1) in two 3-station ProSim hip simulators. The study design is shown in Figure 2. In phase 1, 2 million cycles wear simulation was carried out with standard gait input kinematics as per ISO 14242-1 with 25% (16g/l) bovine serum as a lubricant, N=6. Phase 2 (N=3) investigated further activities of daily function using either a stop-dwell-start protocol [3], higher frequency and/or higher loading conditions representative of jogging. For the increased loading conditions, the shape of the input profile was consistent with that used for the gait cycle but the profile scaled to a peak load of 4.5kN. Frequencies investigated were either 1 or 1.25 Hz and the stop-dwell-start protocol consisted of 10 cycles, 5 second dwell (loaded at 1250N), 1 cycle, 5 second loaded dwell, 50 cycles [3]. The wear of the head and cup were measured gravimetrically.
Results
At the conclusion of each phase of the study, no wear scar or damage was visible on either the heads or cups. Under all conditions where the loading was 3kN, mean wear rates were 0.01mm3/million cycles or below, and even under the limits of measurement sensitivity. These values are a similar magnitude to the steady state wear rate of the same device tested under walking gait conditions [4]. And for conditions where loading was 4.5kN, mean wear rates were in the range 0.04-0.07mm3/million cycles (Figure 3). Increasing the applied load had a greater influence on the wear rate than either increasing frequency alone or using a stop-dwell-start protocol. Intermittent squeaking was apparent from some, but not all, implants during the stop-dwell-start protocol when the simulator stopped, despite the tolerances on all the implants being the same. Squeaking was not heard during any of the protocols where the implants were run continuously, indicating that repeated stopping of the in vitro simulation may disrupt the lubrication mechanism and potentially lead to squeaking.
Conclusion
Under all the activities of daily function investigated, the wear of the ceramic-on-ceramic hip resurfacing bearing couple was very low (mean <0.1mm3/million cycles) with no visual wear scar on the surface of the components.
[1] Pandit, H. et al (2008).JBJS(Br),90(7):847-851
[2] Al-Hajjar M. et al (2013),JOEIM,227(5):535-542
[3] Hadley M. et al (2018).JOEIM,232(12):1261-1270
[4] de Villiers, D., & Collins, S. (2020).,Biotribology,21:100117
Figures

Figure 1
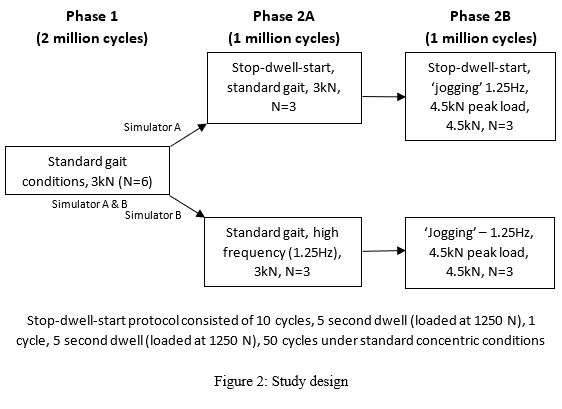
Figure 2
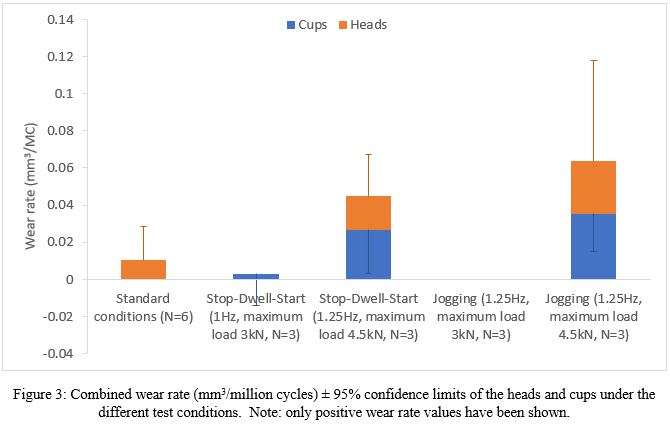
Figure 3#8475
Accuracy of the Birmingham Hip Resurfacing Components Placement Using the Anterior Approach With Dual-Plane Fluoroscopy and Computer Navigation: A Radiographic Evaluation
*Benjamin Domb - American Hip Institute - Des Plaines, USA
Paulo P. Padilla - American Hip Institute - Des Plaines, USA
Tracy George - American Hip Institute - Des Plaines, USA
david maldonado - UT Houston - Houston, United States of America
Payam W. Sabetian - American Hip Institute - Des Plaines, USA
Mark F. Schinsky - American Hip Institute - Des Plaines, USA
*Email: DrDomb@americanhipinstitute.org
Background: Precise and accurate component positioning is crucial in Birmingham Hip Resurfacing (BHR). A novel technique using the direct anterior (DA) approach with dual-plane fluoroscopy and computer navigation has been developed to minimize muscle damage and maximize accuracy.
Purpose: To evaluate the accuracy of component placement with dual-plane fluoroscopy and computer navigation, as compared to historical posterior approach-BHRs (PA-BHR).
Study Design: Cohort study; Level of evidence 3.?
Methods: Prospectively collected data from patients who underwent DA-BHR and PA-BHR from September 2019 to January 2022 were retrospectively reviewed. The DA-BHR radiographic parameters were compared to a control group of patients who underwent conventional PA-BHR. The position of the stem within the neck on lateral view was categorized according to zones 1 through 3 which corresponded to anterior, middle, and posterior thirds of the neck.
Results: Fifty-nine patients (59 hips) from the PA-BHR group and fifty-eight patients (58 hips) from the DA-BHR group were included with similar mean age, body mass index, total surgical time, and no intraoperative complications. The odds of patients in the direct anterior group being in the Callanan safe zone were 3.5 times higher than patients in the posterior group (OR, 3.5 [95% CI, 1.6, 7.7]). The odds of patients in the direct anterior group being in the Lewinnek safe zone were 5.3 times higher than patients in the posterior approach group (OR, 5.3 [95% CI, 2.1, 8.2]). The degrees in valgus of the stem when compared to native neck shaft angle were higher for DA-BHR groups in relation to the PA-BHR groups (5.6 ± 3.9 vs. 3.6 ± 4.9; P<0.05)). There was a greater percentage of patients in valgus for DA-BHR than in the PA-BHR group (98.3% vs. 89.8%). 98.3% of patients for the DA-BHR group had their femoral component centered in Zone 2 as compared to 66.1% in the PA-BHR (P<0.05). Global offset difference in the PA-BHR was greater than the offset difference in the DA-BHR cohort (4.4 ± 4.1 vs. 1.8 ± 2.3; P<0.05). Rate of femoral notching did not differ between groups.
Conclusion: BHR using the anterior approach with dual-plane fluoroscopy and computer navigation yielded greater consistency in the radiographic parameters when compared to manual PA-BHR. This approach may also benefit from other advantages of the direct anterior approach.
#8789
Hip Resurfacing in the Setting of Retained Proximal Femoral Instrumentation or Complex Deformity
Louis Andrew Jordan - Hospital for Special Surgery - New York, United States of America
Ajay Premkumar - Hospital for Special Surgery - New York, USA
*Renee Ren - USA
Jonathan Spaan - Hospital for Special Surgery - New York, United States of America
Edwin Su - Hospital for Special Surgery - New York, USA
*Email: renyi@hss.edu
Introduction
Total hip arthroplasty (THA) in the setting of significant retained femoral instrumentation or complex proximal femoral deformity may be challenging, and published reports of THA in this setting reveal sobering results. Hip resurfacing arthroplasty (HRA) is an alternative to THA and may avoid complex hardware removal or deformity correction at the time of hip arthroplasty.
This study investigates the largest cohort in the literature for metal-on-metal HRA in patients with retained femoral instrumentation or complex proximal femoral deformities. Intraoperative and postoperative complications, metal ion levels, and patient-reported outcomes are analyzed to understand the merits of HRA in this complex patient population.
Methods
Twenty-three patients who underwent elective HRA in the setting of significant proximal femoral deformity and/or retained femoral instrumentation were identified from a prospectively maintained registry. Pre and postoperative Lower Extremity Assessment Scores (LEAS), modified Harris Hip Scores (mHHS), HOOS JR scores, VAS pain levels, and metal ion levels were obtained.
Results
Of the 23 patients, a total of 17 patients had femoral instrumentation before their HRA, and 2 patients underwent staged preoperative hardware removal at an outside center prior to presentation. Of the 20 patients with prior surgery, 8 were previous femoral head pinning procedures (34.78%), 6 were prior proximal femoral osteotomies (26.09%), and 6 underwent prior open reduction internal fixation (26.09%) [Figure 1]. Causes of femoral deformities varied from prior trauma (30.43%), slipped capital femoral epiphysis (SCFE; 26.09%), polyostotic fibrous dysplasia (13.04%), Legg-Calvé-Perthes disease (13.04%), cerebral palsy (4.35%), developmental dysplasia (4.35%), and two cases had unknown congenital etiologies for their femoral deformities (8.70%) [Figure 2].
Median (IQR) follow-up was 5.03 (2.07−7.91) years, and no patients underwent intraoperative complications or revision surgery at their latest follow-up. The mean (SD) surgical duration was 94.40 (12.00) minutes, and postoperative length of stay was 1.74 (1.80) days. There were no intraoperative complications and all patients were discharged home. Median (IQR) postoperative LEAS, mHHS, VAS pain scale, and HOOS, JR. scores were 13.00 (9.25−13.00), 92.60 (92.40−100.00), 2.50 (0.75−10.00), and 92.34 (85.26−100.00), respectively. Fourteen patients completed postoperative serum metal ion level testing at a mean (SD) of 4.24 (2.85) years, where cobalt and chromium levels were 1.22 (0.36) ppb and 2.01 (0.80) ppb, respectively.
Conclusions
Compared to revision THA procedures, HRA is a viable option for patients with significant proximal femoral deformity and retained instrumentation. All subjects who completed patient-reported outcome surveys at follow-up demonstrated improvements from baseline scores, and all metal ion test results fell within acceptable limits. Thus, excellent results at mid-term follow-up can be achieved utilizing this strategy in this complex patient population.
Keywords: hip resurfacing arthroplasty, complex hip reconstruction, revision hip arthroplasty, proximal femoral anatomy, retained hardware
Figures
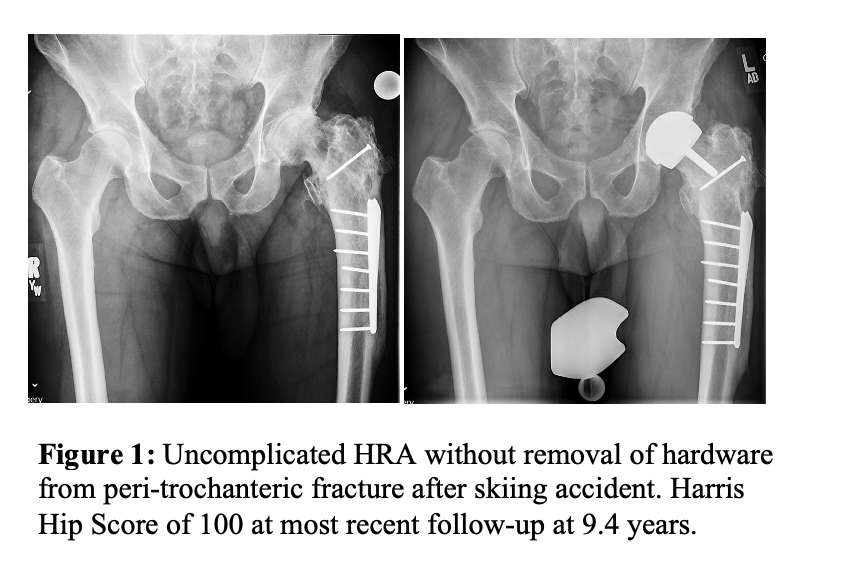
Figure 1
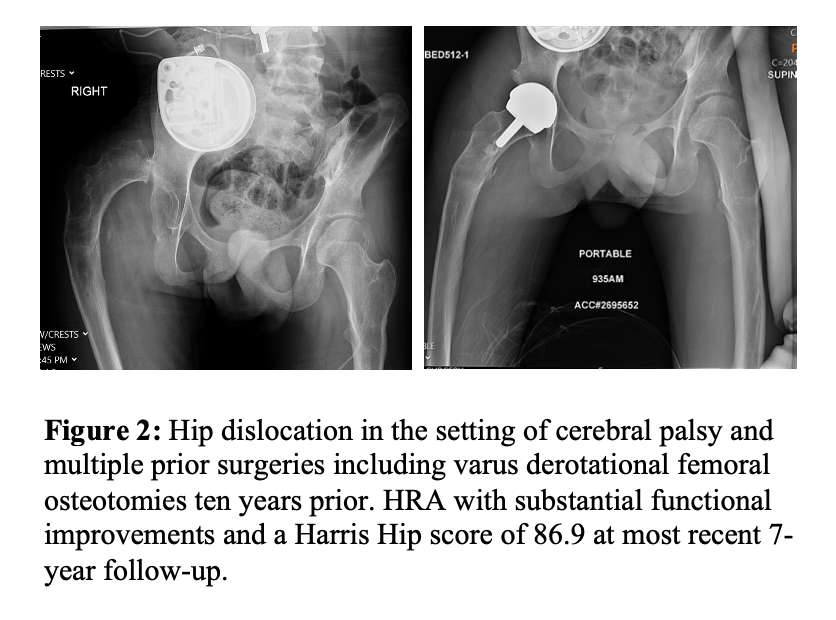
Figure 2#8368
Clinical Implications of Range of Motion Differences Between Total Hip Arthroplasty and Hip Resurfacing Arthroplasty: A Preoperative Simulation Study
*Joshua Twiggs - University of Sydney - Sydney, Australia
Max Hardwick-Morris - 360 Med Care - Sydney, Australia
Brad Miles - 360 Knee Systems - Pymble, Australia
William Walter - Specialist Orthopaedic Group - North Sydney, Australia
Kaushik Hazratwala - Queensland Lower Limb Clinic - Pimlico, Australia
Tyson Doneley - Brisbane Orthopaedic Clinic - Spring Hill, Australia
*Email: joshua_twigg@msn.com
Introduction: Dislocation is one of the most common complications in total hip arthroplasty (THA) and is primarily driven by bony or prosthetic impingement. Despite being indicated for younger, active patients, hip resurfacing arthroplasty (HRA) has been reported to have reduced range of motion (ROM), but lower rates of dislocation. However, femoral neck fracture is a common complication after HRA and may be caused by early impingement. The aims of this study were two-fold. First, to compare the ROM results of a patient cohort simulated with both THA and HRA implants. Second, to compare the simulated ROM with previously established references for pure hip joint flexion and external rotation (ER).
Methods: 55 patients from a single surgeon underwent 3D preoperative templating with both THA and HRA implants. All preoperative CT scans were segmented and landmarked, and x-rays were measured for functional parameters. All patients underwent 3D templating with the same implants (THA; Depuy Corail/Pinnacle and HRA; MatOrtho Adept). Implants were positioned according to conventional and standardised criteria and an example of both implants templated to a single patient’s anatomy is displayed in Figure 1. A novel ROM simulation was used to instantaneously calculate bony and prosthetic impingement limits in flexion and ER for all patients with both implant combinations (Figure 2). Previously established references fosr hip ROM were 110° for pure flexion and 45° for external rotation. ROM less than these values was considered a simulation fail.
Results: The mean ROM for the ER simulation with THA implants was 57.8°±16.7° (20.8°-105.5°), and 26.6°±13.3° (12.1°-67.3°) for HRA implants (p << 0.05). 50 patients (91%) had greater ROM with THA implants, and the mean difference in ROM was 31.2°. 10 patients (18%) failed the ER simulation with THA implants and 48 patients (87%) failed the ER simulation with HRA implants. The mean ROM for the flexion simulation with THA implants was 123.5°±10.0° (98.5°-142.3°), and 96.0°±20.4° (62.3°-149.8°) for HRA implants (p << 0.05). Again, 50 patients (91%) had greater ROM with THA implants, and the mean difference in ROM was 27.5°. 4 patients (7%) failed the flexion simulation with THA implants and 45 patients (81%) failed the flexion simulation with HRA implants.
Discussion: Our findings reinforce previous literature that has found significantly less ROM with HRA implants when compared to THA implants due to the preservation of the native femoral neck in HRA surgery. These results highlight the need for strict component positioning in HRA surgery to reduce the likelihood of early neck-on-cup impingement and subsequent femoral neck fracture.
Figures
Figure 1
Figure 2#8625
Could Alzheimer's Disease Pathology Be Associated With Metals Released From Total Hip Replacements?
*Robin Pourzal - Rush University Med Ctr - Chicago, USA
Puja Agarwal - Rush University - Chicago, USA
Sue E. Leurgans - Rush University Medical Center - Chicago, USA
Sonal Agrawal - Rush University Medical Center - Chicago, USA
Scott Ayton - University of Melbourne - Parkville, Australia
Ashley I Bush - University of Melbourne - Parkville, Australia
Deborah Hall - Rush University Medical Center - Chicago, USA
Julie A Schneider - Rush University Medical Center - Chicago, USA
Joshua J. Jacobs - Rush University Medical Center - Chicago, USA
*Email: robin_pourzal@rush.edu
INTRODUCTION: Both total joint arthroplasty (TJA) and Alzheimer’s Disease (AD) are prevalent in elderly populations. It is the goal of this study to determine if the presence of implant metals originating from TJA is associated with either higher implant metal content in the brain or AD pathology.
METHODS: Autopsy tissue from four brain regions (inferior-temporal-cortex (ITC), mid-frontal cortex, cingulate and cerebellum) of 699 (229 with TJA) participants from an ongoing longitudinal cohort study (Memory and Aging Project (MAP)) were analyzed. The ITC especially is known to be associated with early onset of AD. Diffuse and neuritic amyloid plaques and phosphorylated tau were assessed and summarized as standard measures of AD pathology. Implant metal (Co, Cr, Mo, Ti, Al) content in all four brain regions was determined by ICP-MS. The presence of total hip, knee and shoulder replacements (THA, TKA, TSA) was retrospectively conducted via a Medicare database search (ICD and CPT codes). The no-TJA-group was compared to the TJA group. Because of the higher likelihood of Co release in THA due to potential metal-on-metal bearings and modular junction corrosion, the TJA group was [further] divided into THA (N=146) and TKA/TSA group (N=83). Participants with more than one type of TJA were assigned to the THA group if THA was present. We used separate linear regression models adjusted for age, sex, education, and APOEε4-status for the associations of all metals (log-transformed) with global AD pathology, amyloid plaques, and phosphorylated tau.
RESULTS: The THA group had higher cobalt content across all brain regions combined (p=0.003) and within the ITC alone (p=0.051) compared to the no-TJA group, whereas the TKA/TSA group did not differ from the no-TJA group. We also observed more Ti in the ITC of patients with TJA compared to the no-TJA group (p=0.018). Across all tissue samples, Co was associated with higher amyloid load (β=0.35, standard error (SE)=0.16, p=0.027), phosphorylated tau (β=0.47, SE=0.18, p=0.011), and global AD pathology (β=0.19, SE=0.05, 0.0004) in the ITC. Across all tissue regions combined, Co was only associated with global AD pathology (β=0.2, SE=0.09, p=0.018). The presence of a TJA itself was not associated with AD pathology in either model. Interestingly, the presence of titanium, independent of the presence of a TJA, was negatively correlated with amyloid load (β=-0.67, SE=0.2, p=0.002) and global AD pathology (β=-0.15, SE=0.07, p=0.037) within the ITC.
CONCLUSION:This study has shown that the Co content of brain tissue was higher within the ITC in MAP participants with THA. Among all tested metals, Co was consistently associated with greater AD pathology, especially amyloid plaques within the ITC region. Interestingly, titanium appeared to have a beneficial association, however, the source of titanium is unclear. Although we found an association of cobalt with AD pathology, the cross-sectional nature of this study does not allow the determination of cause and effect. We also did not find a direct association between TJA of any type and greater AD pathology.
#8717
In-Vivo Wrist Range of Motion in Total Wrist Arthroplasty Measured With Biplane Videoradiography and Its Correlation With PROMs
*J.J. Trey Crisco - Brown University - Providence, USA
Kalpit Shah - Brown University - Providence, USA
Bardiya Akhbari - Harvard Univ - Boston, USA
Amy Morton - Brown University - Providence, USA
Douglas Moore - Brown University - Providence, USA
Scott Wolfe - HSS - New York, USA
Arnold-Peter Weiss - Brown University - Providence, USA
*Email: joseph_crisco@brown.edu
Introduction: The goal of total wrist arthroplasty (TWA) is to provide pain relief and preserve or improve wrist motion in patients with severe wrist pathology. However, TWA does not enjoy the high survival rates seen by other joint replacement implants. Though design iterations of TWA have improved over several decades and these implants are becoming a reliable option for patients with wrist arthritis, high complication rates predominantly involving the carpal component are still a major concern. The mechanism behind these failures remains unknown. At other joints, a mismatch between native kinematics and arthroplasty kinematics is a well-established mechanism for arthroplasty failure. However, there are limited in vivo biomechanical studies of TWA to determine if TWA kinematics match the native wrist. The goal of this study was to compare the kinematics of prosthetic total wrist arthroplasty and healthy wrists using biplanar videoradiography (BVR) during active range-of-motion (ROM). The advantages of BVR for kinematic tracking of implant motion is that measurements are made directly from videoradiography of the implants, without the error associated with skin motion artifact.
Methods: 6 patients who had a single total wrist implant design (Freedom wrist, Smith and Nephew, Memphis TN) (n=6, >6 months post-op) and control subjects (n=10) were recruited and examined under an approved IRB protocol. Biplanar videoradiography (BVR) was used to measure active flexion-extension, radial-ulnar deviation, and circumduction of control and prosthetic wrists. Surface models of the implants and bones were generated, coordinate systems of the implants and bones constructed, and wrist kinematics were calculated. The third metacarpal and radius for the control group, and the carpal and radial components for the TWA group were tracked with BVR using open-source 2D-to-3D registration software (Autoscoper, Brown University). Wrist kinematics was reported as the posture of MC3 with respect to the radius, relative to its posture in neutral position. Patient recorded outcome measurements (PROMs), including PRWHE, PROMIS Upper Extremity, and QuickDASH were also performed. An ordinary two-way ANOVA with post-hoc Sidak’s multiple comparison test was performed to compare ROM between cohorts (adjusted p-value <0.05 as significant).
Results: TWA subjects demonstrated significantly reduced wrist motion in all directions when compared to controls (extension: 22% reduction, flexion: 60%, ulnar deviation: 53%), except in radial deviation (28%) (Figure1). The envelope of circumduction was significantly reduced by 65% (Figure 2). PRWHE and QuickDASH scores were statistically better in the control than the TWA patients (P<0.04); PROMIS showed no difference (p=0.91). Using a regression analysis, improved PRWHE and QuickDASH scores, but not PROMIS, were associated with greater flexion, extension, and ulnar deviation ROM, but not with radial deviation ROM.
Conclusion: TWA patients exhibited reduced motion during ROM tasks when compared to control subjects. The reduced ROM correlated with reduction in most PROM metrics. While this study was limited to a small sample size and a single implant design, significant decreases in ROM and its correlation with PROMs were still found.
Figures
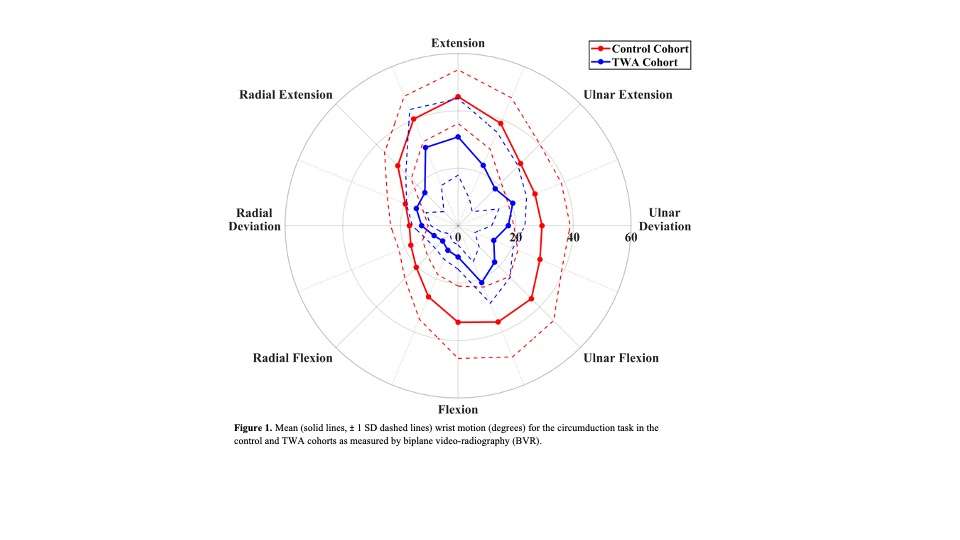
Figure 1
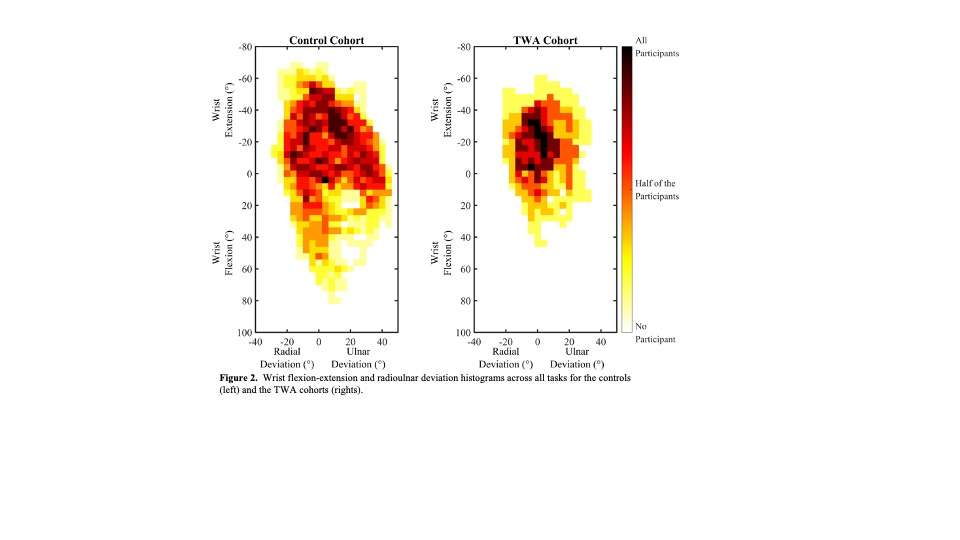
Figure 2#8650
Comparison of Micromotion in Three Trapezial Component Fixation Designs of a Thumb CMC Arthroplasty
*Ritvik Sarkar - Hospital for Special Surgery - New York, United States of America
Joseph Lipman - Hospital for Special Surgery - New York, USA
Timothy Wright - Hospital for Special Surgery - New York, USA
Robert Hotchkiss - Hospital for Special Surgery - New York, USA
Aaron Daluiski - Hospital for Special Surgery - NYC, USA
Daniel Osei - Hospital for Special Surgery - New York, USA
Fernando Quevedo Gonzalez - Hospital for Special Surgery - New York, USA
*Email: sarkarr@hss.edu
Introduction. Total joint arthroplasties of the carpometacarpal joint of the thumb suffer high rates of early aseptic loosening [1], particularly for the trapezial component [2]. The biomechanics of trapezial implant fixation remains poorly understood, and trapezial fixation features have not converged into an optimal design. As part of the development of a new carpometacarpal implant, our objective was to compare the primary stability (i.e., bone-implant micromotion) of three fixation designs for the trapezial component.
Methods. We evaluated three designs for cementless fixation of a trapezial component with a saddle-shaped articulation (Fig. 1): (A) a single large central hexagonal peg, (B) three smaller hexagonal pegs placed peripherally, and (C) a single central peg with a cruciform cross-section and two peripheral spikes. To evaluate their bone-implant micromotion, we created finite element models by segmenting out (Mimics, Materialise, Leuven, Belgium) the trapezium from the CT scans of 6 patients (2 male, 4 female, ages 67-83) with trapeziometacarpal arthritis. We virtually implanted each design into the trapezium by aligning the trapezial implant to a standardized joint coordinate system [3] and resecting 3mm below the center of the native trapezial saddle (Fig. 2). We meshed the bone-implant models with 1mm linear tetrahedral elements in Abaqus (Dassault Systemes, Providence, RI). The trapezial implant was assumed to be solid Ti-6Al-4V alloy (E=110 GPa, v=0.33). Bone was non-homogeneous, with material properties derived from preoperative CT scans [4-5]. We assumed a line-to-line frictional rough bone-implant interface with a friction coefficient of 0.6. We simulated a peak compressive load from pinch grip of 1080N [6-7]. The trapezium was rigidly fixed at the scaphotrapezial joint. We calculated micromotions along the bone-implant contact surfaces and compared them to <20µm threshold representing the most restrictive threshold micromotions for bone ingrowth [8].
Results. Design A had the largest average peak micromotion (51±17µm), followed by design B (41±12µm), then design C (28±7µm). In all cases, peak micromotion occurred near the proximal tip of the fixation features. While all cases had >60% of the surface area under the 20µm threshold (Fig. 3), design A had lower stable area (79±11%) than design B (85±8%) and design C (86±8%).
Conclusion. While differences were small and the amount of area required for a stable interface remains unknown, a design with a single central cruciform peg with peripheral spikes provided the greatest primary stability. Furthermore, such a design would also benefit from an easier surgical technique compared to designs requiring multiple parallel drill holes like design B.
References. [1] Huang K, et al. J Hand Surg Eur. 2015; [2] Hernandez-Cortes et al. J Hand Surg. 2011; [3] Cooney WP 3rd, et al. J Bone Joint Surg Am. 1981; [4] Morgan EF, et al. J Biomech. 2003; [5] Snyder SM and Schneider E. J Orthop Res. 1991; [6] Goislard de Monsabert B, et al. Medical Engin Phys. 2014; [7] Halilaj E, et al. J Biomech. 2015; [8] Jasty M, et al. J Bone Joint Surg Am. 1997
Figures
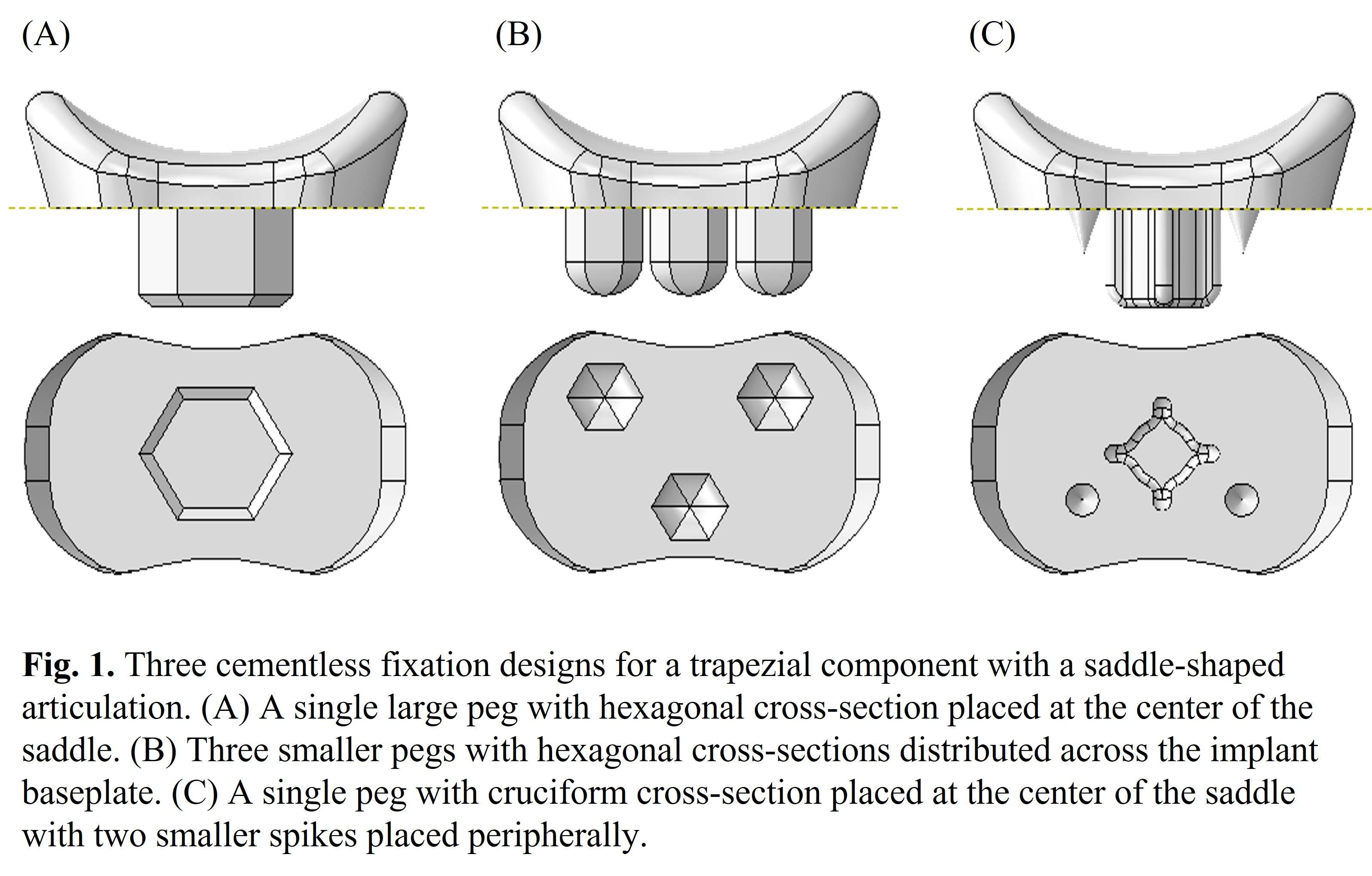
Figure 1
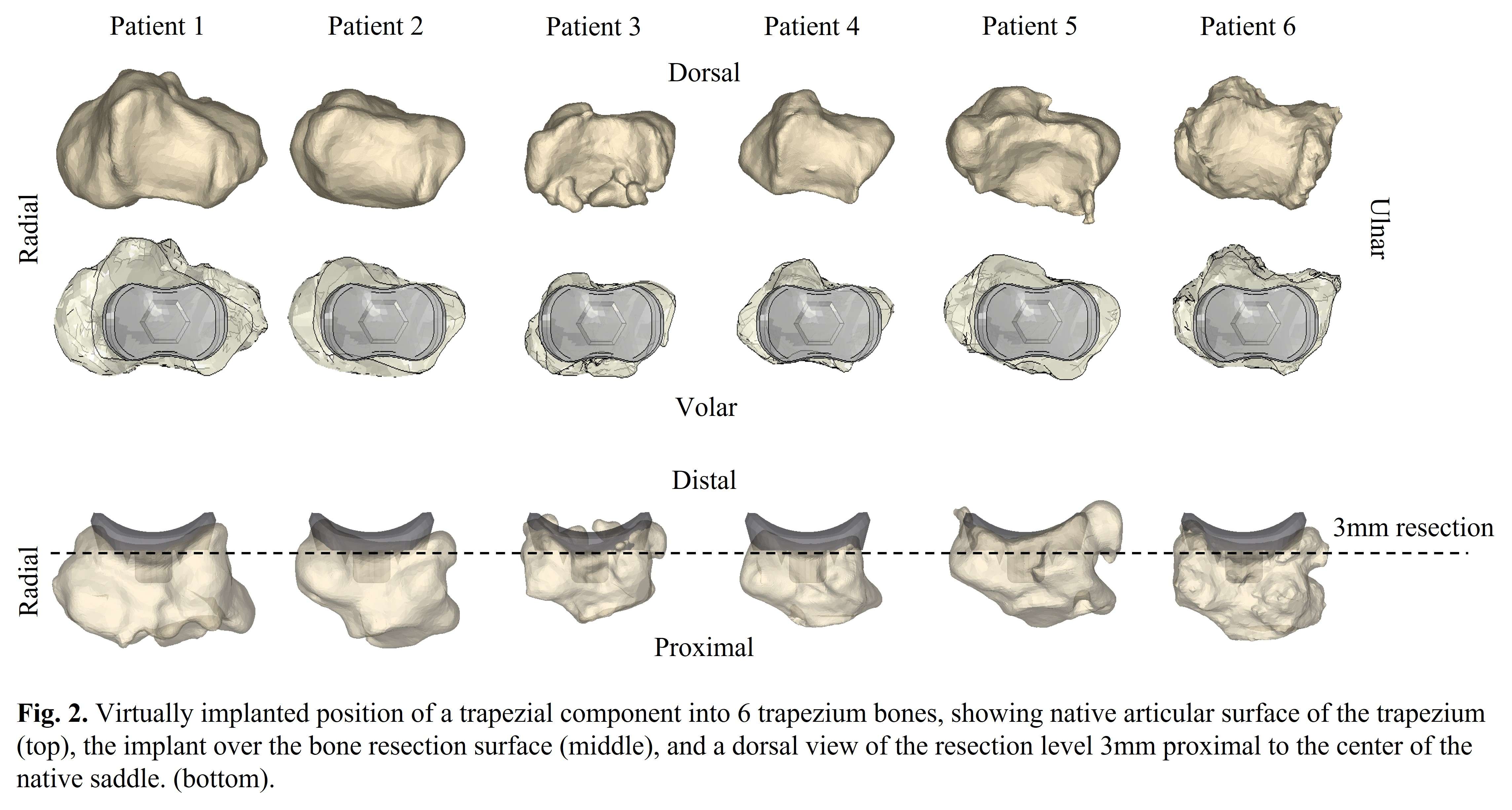
Figure 2
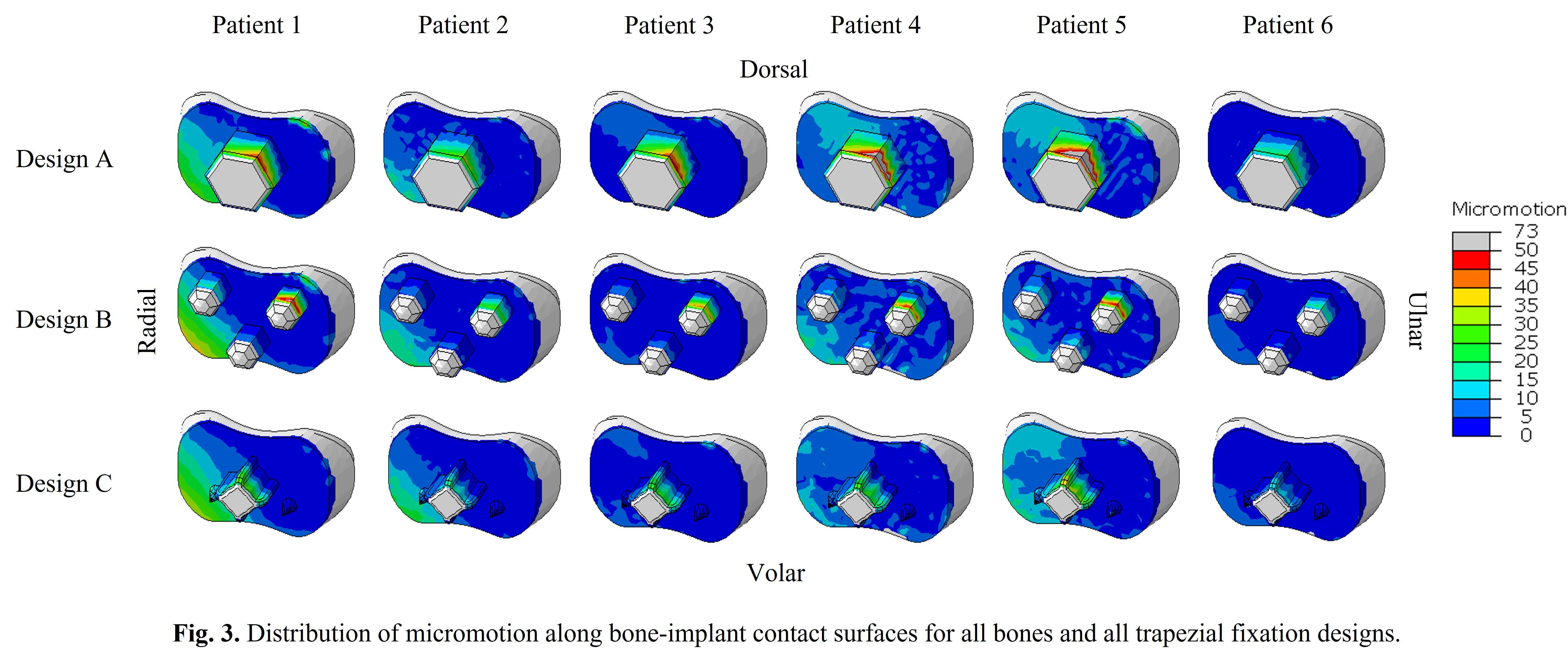
Figure 3#8564
Comparison of Muscle Forces and Characterization of Articular Loading During Active Flexion-Extension Motion of the Wrist Before and After Total Wrist Arthroplasty: An in-Vitro Cadaveric Study
*Elizabeth Norman - The University of Western Ontario - London, Canada
Emily Lalone - The University of Western Ontario - London, Canada
Assaf Kadar - St. Josephs Health Care - london, Canada
Nina Suh - Emory University - Atlanta, USA
G Daniel Langohr - Canada
*Email: enorman4@uwo.ca
Background: Total wrist arthroplasty (TWA) implants are a surgical option for those suffering from end-stage wrist arthritis that aims to reduce pain and restore near normal range of motion. Multiple generations of wrist arthroplasties have been designed and implemented over multiple years to reduce failure and increase longevity, however, relatively high complication rates persist [1]. Reported complications include extensor tendon rupture and dislocation of the carpal component. Loading within the distal carpal component and the effect of TWA reconstruction on the muscle forces required to actively flex and extend the wrist is currently not well understood. Therefore, the first objective of this study is to characterize the loading patterns within the carpal component to determine if high loading could be the cause of carpal component dislocation. The second objective is to compare the muscle forces required to actively flex and extend both a series of intact and TWA reconstructed cadaveric wrists to gain a better understanding of how these implants alter the native biomechanical state.
Methods: A load sensing generic TWA implant based on current commercially available devices was designed and manufactured for in-vitro use. Eight cadaveric upper limb specimens were acquired (average age: 76 +/- 10 yrs), and the wrist extensors (extensor carpi radialis brevis (ECRB), extensor carpi radialis longus (ECRL), extensor carpi ulnaris (ECU)) and flexors (flexor carpi ulnaris (FCU), flexor carpi radialis (FCR)) were tagged. Pronation/supination motion was then restricted using a Steinmann pin and the arm was then affixed to a previously validated active wrist motion simulator [2] that produces active motion using muscle forces while monitoring feedback wrist joint angles. Active flexion-extension motion was then simulated from 15 degrees of flexion to 15 degrees of extension while maintaining neutral radial-ulnar deviation, and the required muscle forces to achieve the motion were recorded. This was then repeated for the reverse direction (extension to flexion). Following this, the specimen was reconstructed with our custom load sensing generic TWA by an experienced Orthopaedic Surgeon, and testing was repeated for the reconstructed state. Articular loading was directly recorded using a six-degree-of-freedom load cell.
Results: The extensor muscle forces showed statistically higher forces in the reconstructed state compared to the intact state when the wrist moved from flexion to extension (p < 0.003), whereas the muscle force in the FCR decreased after the TWA was implanted (Table 1). In addition, the distal carpal component forces reached an average value of 115N when articulating from flexion to extension and slightly decreased to an average of 89N during the reverse movement (Figure 1).
Conclusion: The results of this work show that TWA reconstruction significantly increases the extensor muscle forces required to actively move the wrist from flexion to extension compared to the intact state. This may have an impact on wrist biomechanics as well as the long-term fixation of the TWA implant components, which is known to be a complication of these procedures.
References: [1] Bone Joint J 2022;104-B(10):1132–1141 [2] Padmore C. J Wrist Surg. 2019 Apr; 8(2): 124–131.
Figures
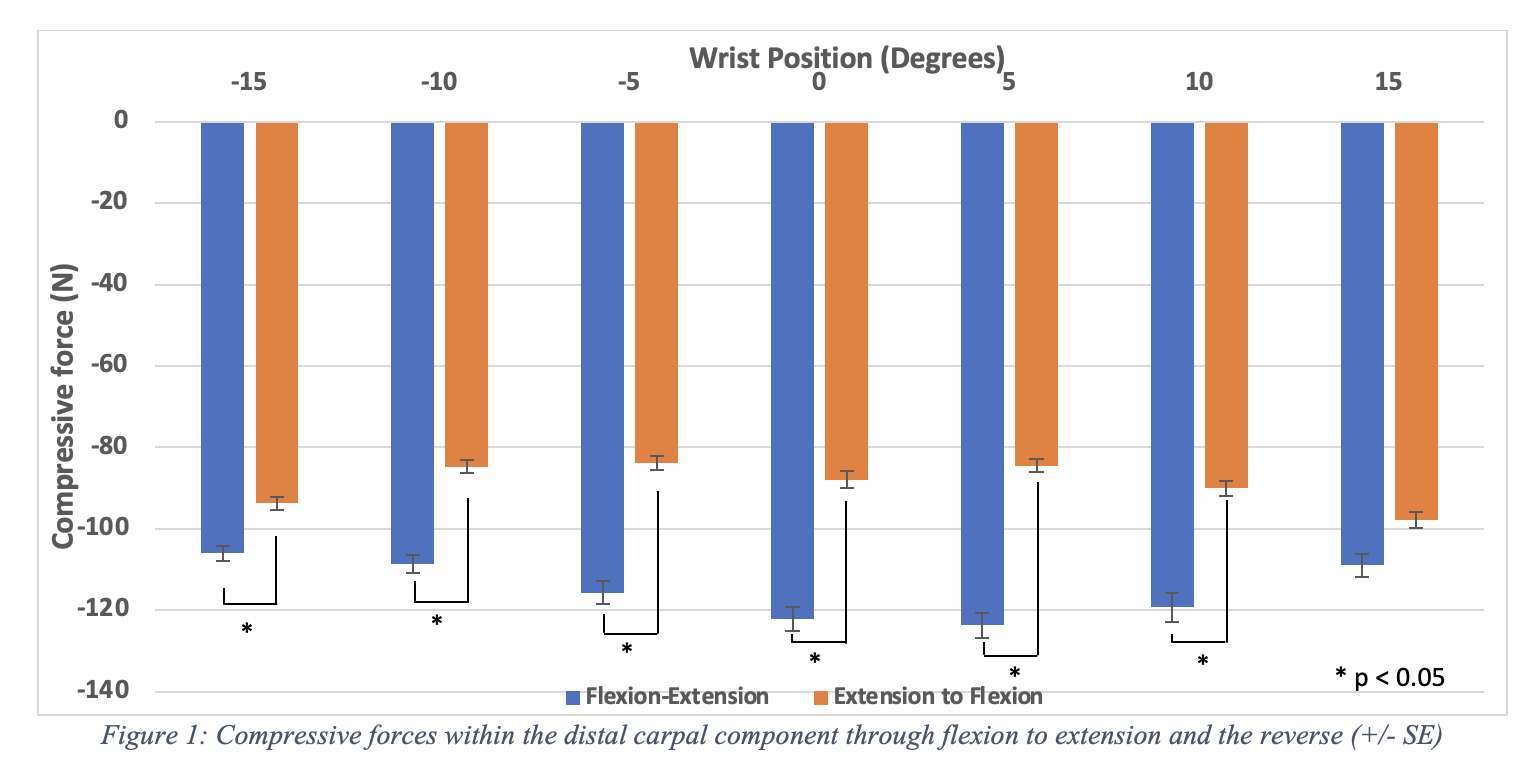
Figure 1
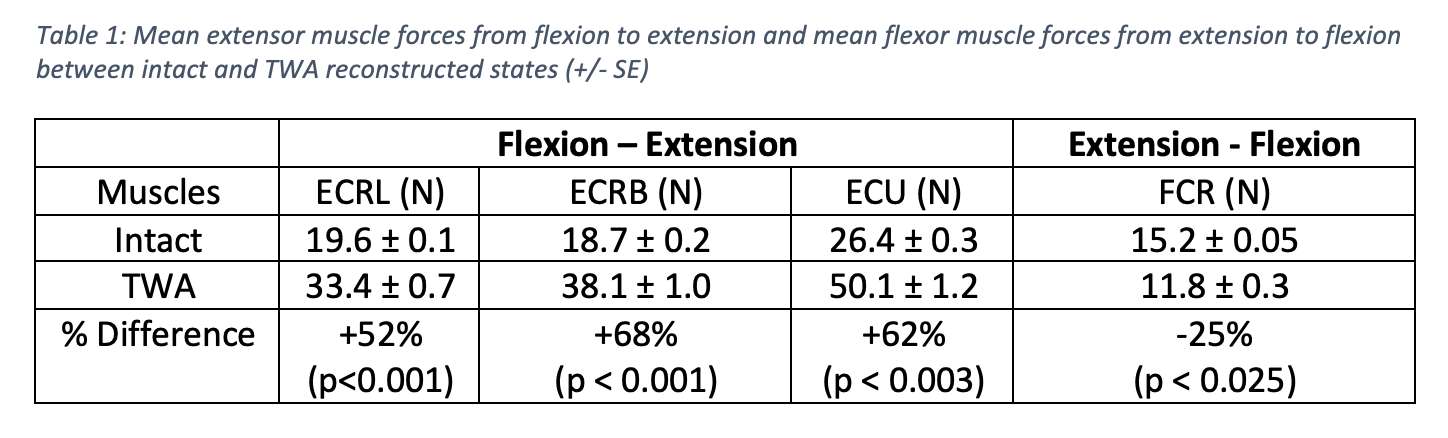
Figure 2#8721
1yr Follow-Up Demonstrates the KinematX Midcarpal Total Wrist Improves Wrist Motion, Strength, and Patient Reported Outcome Measures
*J.J. Trey Crisco - Brown University - Providence, USA
Scott Wolfe - HSS - New York, USA
William Runge - Hospital for Special Surgery - New Yook, USA
Alfred Hess - Florida Orthopaedic Institute - Tampa, USA
Barth Riedel - Loma Linda University Medical Center - Loma Linda, USA
*Email: joseph_crisco@brown.edu
Introduction: The history of total wrist arthroplasty (TWA) has been less successful than arthroplasty at larger joints. TWA design pathways have been fraught with obstacles and continue to evolve with our understanding of carpal mechanics. Despite early failures, total wrist prosthetic design has undergone a series of iterative modifications over the past 30 years that have enhanced durability and decreased complications. The KinematX™ total wrist arthroplasty (Extremity Medical, LLC) is a novel total wrist arthroplasty system whose design targets midcarpal articulations, thus preserving the anatomic location of the wrist’s center of rotation. The KinematX™ is indicated for osteoarthritis, post-traumatic arthritis, SLAC/SNAC wrist, Kienböck disease, and inflammatory arthritis. By restoring the midcarpal articulation, we hypothesize that the KinematX TWA will improve motion and patient outcomes with minimal risk of loosening.
Methods: From 2021-2022, 21 KinematX TWAs were performed by 5 surgeons at 5 institutions for a wide variety of degenerative wrist conditions and followed prospectively for a minimum duration of 12 months (range 12-26m). Range of motion, grip and pinch strength, radiographic evidence of implant loosening, and patient-reported outcome measures were recorded in a IRB-approved five-center HIPPA-compliant registry and statistically compared using a non-parametric signed rank test with a p value of 0.05.
Results: Of the 21 eligible patients, 18 had complete follow-up data and were included in the analysis. The average age was 71 years (range – 57-79). From preoperative to latest follow-up evaluation, extension improved from 38 to 50 degrees (p=0.007), flexion from 16 to 47 degrees (p=0.02), ulnar deviation from 20 to 27 degrees (p=0.002), and radial deviation from 5 to 17 degrees (p=0.004). Grip strength improved from 24 to 36 lb. (p=0.008), while pinch strength improved from 11 to 15 lb. (p=0.02). Patient-Reported Wrist Evaluation (PRWE) scores improved from 66 to 32 (p<0.001). One patient was revised for implant loosening, and a second patient has early radiolucencies in two zones.
Conclusions: Unlike previous total wrist designs,1–3 the midcarpal total wrist arthroplasty demonstrated statistical improvement of preoperative range of motion in all planes at minimum one year followup. Grip strength, and patient reported outcome measures all improved significantly through the latest follow-up, with a comparable complication profile. The KinematX midcarpal total wrist arthroplasty is a promising option to relieve pain and improve function for patients with a wide variety of debilitating arthritic disorders of the wrist.
References
1. Rossello MI, Zotta I, Rossello C, Formica M, Zoccolan A. Total Wrist Arthroplasty with Integra Freedom® Implants: A Pilot Study with a New Evaluation System. Indian J Orthop. 2022;56(6):1040-1047. doi:10.1007/s43465-022-00618-3
2. Boeckstyns ME, Herzberg G, Merser S. Favorable results after total wrist arthroplasty: 65 wrists in 60 patients followed for 5-9 years. Acta Orthop. 2013;84(1745-3682 (Electronic)):415-419. doi:10.3109/17453674.2013.823588
3. Pfanner S, Munz G, Guidi G, Ceruso M. Universal 2 Wrist Arthroplasty in Rheumatoid Arthritis. J Wrist Surg. 2017;6(3):206-215. doi:10.1055/s-0037-1598637
#8675
Total Ankle Arthroplasty Is Associated With Decreased Risk of Postoperative Total Knee Arthroplasty Compared to Ankle Arthrodesis
*Gloria Coden - Bronx, United States of America
Andrzej Brzezinski - New England Baptist Hospital - Boston, USA
Kurt Hofmann - New England Baptist Hospital - Boston, USA
*Email: gscoden@gmail.com
Introduction: There is a known association between ankle arthritis and ipsilateral knee arthritis. However, it is unknown if the increased range of motion and improved gait mechanics provided by total ankle arthroplasty (TAA) may delay the progression of knee arthritis compared to ankle arthrodesis (AA). We hypothesized that patients treated with TAA would have a lower incidence and longer time to total knee arthroplasty (TKA) compared to AA.
Methods: We retrospectively reviewed a matched cohort of 5726 AA and 5726 TAA performed between 1/1/2007 and 12/31/2021 using a commercial claims database. Mahalanobis nearest neighbor matching was performed based on age, gender, year of surgery, geographical region, and diagnosis. Mean follow-up was 2.2 years. Univariate and multivariate analyses were performed to assess for risk factors. Significance was set at p<0.05.
Results: Patients who underwent TAA were older (p<0.001), female (p<0.001), had earlier ankle surgery (p<0.001), and increased rates of ankle deformity (p<0.001) than AA, but rates of rheumatoid arthritis (p=0.54) and posttraumatic arthritis (p=0.53) compared to osteoarthritis were similar. Patients who underwent AA were 2.77 times more likely to undergo a postoperative TKA compared to patients who had a TAA (p<0.001). For those who underwent a TKA, patients with an AA (mean=3.48 years) tended to wait longer before proceeding with TKA than patients who underwent TAA (mean=2.62 years, p<0.001). Risk factors for undergoing postoperative TKA included having AA (p<0.001), earlier year of ankle surgery (p<0.001), older age (p<0.001), lack of ankle deformity (p<0.05), lack of obesity (p=0.047), and posttraumatic arthritis (p<0.001), while gender (p=0.55) and rheumatoid arthritis (p=0.75) were not.
Conclusion: There is a significantly higher incidence of patients undergoing TKA after AA compared to TAA (p<0.001). We recommend that surgeons consider TAA over AA, especially in young, active patients with minimal knee arthritis as a strategy to help delay the progression of knee arthritis. Patients who underwent AA tended to have a longer time interval to TKA (p<0.001), which may be related to the longer expected recovery from an AA compared to TAA. With improved rehabilitation protocols for TAA, patients may recover faster so that they can proceed with their ipsilateral TKA earlier.
#8664
Retrieval Analysis of the Evolution of an Ankle Design
Afton Limberg - Dartmouth College - Hanover, United States of America
*Peder Solberg - Dartmouth College - Hanover, United States of America
Zachary Currier - Dartmouth College - Norwich, United States of America
Rebecca Thomson - Dartmouth College - Hanover, United States of America
Frances Faro - Dartmouth Health - Lebanon, USA
Douglas Van Citters - Dartmouth College - Hanover, USA
*Email: peder.solberg.th@dartmouth.edu
Introduction:
Total ankle arthroplasty (TAA) is a viable treatment for end-stage arthritis, but revision rates for ankle replacements are higher than in hip or knee replacements. As a relatively low-volume procedure, less is known about the impact of ankle design features on failure modes compared to other joints. To this end, we assessed a single manufacturer’s fixed-bearing designs for reason for retrieval (RFR) and other metrics. The first design (hereafter D1) was characterized by an intramedullary stem and a saddle-shaped bearing surface. The second design (D2) is an updated version of D1 but with the ability to accommodate a longer tibial stem, two pegs added to the talar components, and a sulcus-shaped bearing surface for greater stability. The third design (D3) features a low-profile tibial component with complete visualization of the implant-bone interface, and a sulcus-shaped talar component1.
Methods:
An IRB-approved retrieval laboratory received retrieved components and surgeon-supplied RFR for 26 total ankles of 3 designs from a single manufacturer from 2010 to 2022. The polyethylene bearings of these retrievals were rated for clinical damage by three independent reviewers on a scale of 0 to 3. All designs were porous-coated and were rated for signs of bony ingrowth on a scale of 0 to 3. Polyethylene inserts received by the laboratory 6 months or less after retrieval (n = 15) were analyzed for oxidation using Fourier transform infrared spectroscopy.
Results:
Results in Figure 2 demonstrate the prominence of aseptic loosening as a failure mode among retrieved TAA components (35% overall). This is in line with failure modes expected for fixed-bearing ankle devices2. This was especially evident for D2, for which 5 of 7 devices (71%) were retrieved for aseptic loosening. This may be a result of the design changes successfully eliminating other failures experienced in D1 (subsidence, etc.). Oxidation rates of these components is on par with values for other ankle polymeric components as reported by Currier et al.3, though the newer designs (D2, D3) have half the oxidation rates of the older design (D1) as indicated in Figure 3. Poly bearing damage and bone ingrowth scores were similar across all three designs.
Conclusion:
Despite the small size of the retrieval record for TAA, these results indicate that evolved ankle designs offered by this single manufacturer appear to be related to the RFR, but they do not appear to greatly affect bone ingrowth or polymer damage. In addition, despite all bearings in this study being never irradiated, oxidation was found to occur in vivo. Lower oxidation in the newer designs suggests decreased stress and lower delivery of pro-oxidative species, likely related to the sulcus design. This work suggests several trends, and motivates the need for a systematic, multi-center collection of retrieved ankle components to increase failure mode assessment of TAA.
References
- Gross et al. J AAOS. 2018,May;26(10):353-359.
- Currier et al. Foot Ankle Int. 2019,Feb;40(2):131-138.
- Currier et al. JBJS 2023,Feb;105(4):293-301
Acknowledgments:
We thank Barbara Currier for her work collecting oxidation data for multiple retrievals in this study.
Keywords:
Ankle
Figures
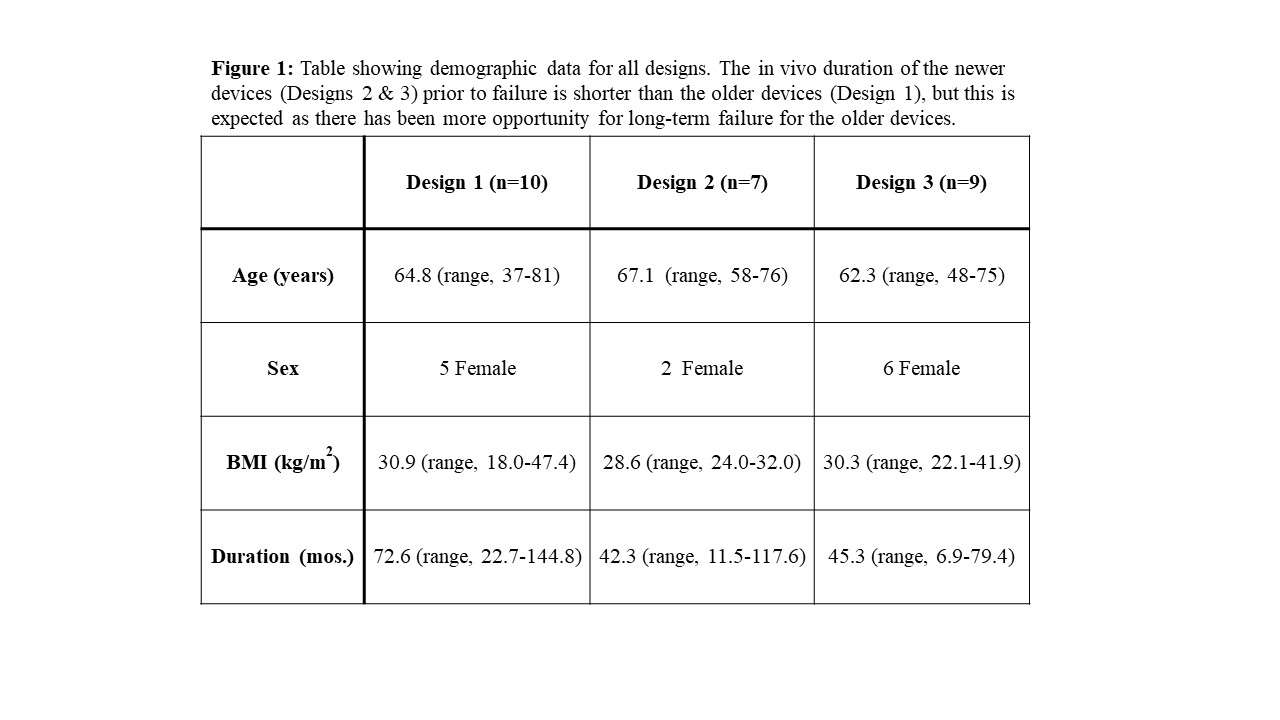
Figure 1
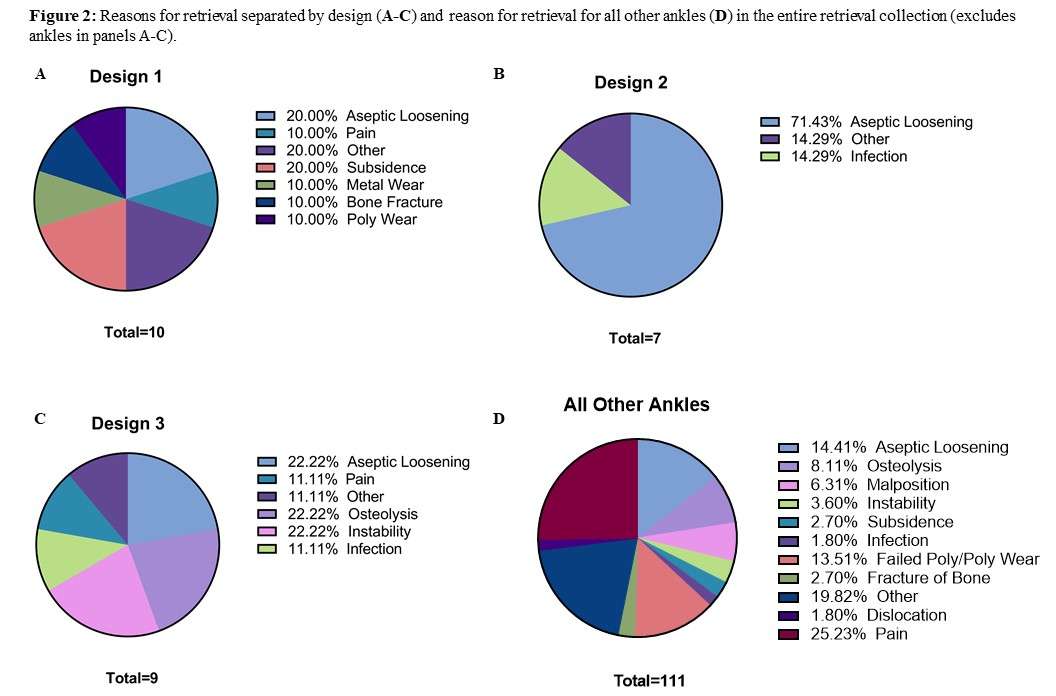
Figure 2
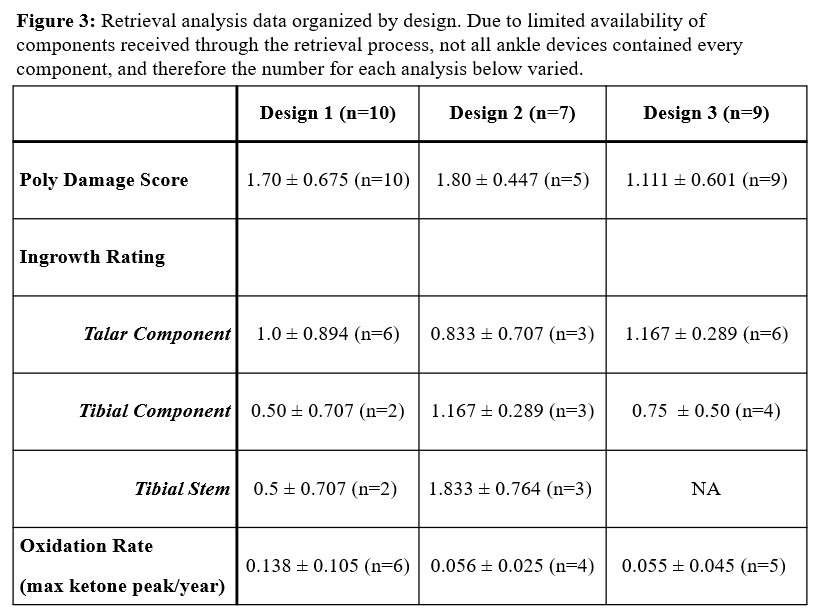
Figure 3#8653
Don't End Up Valgus - Consequences of Limb Alignment Over or Under-Correction
*Bradley Lambert - Houston Methodist Hospital - Houston, USA
Pradyumna Gurusamy - Houston Methodist Hospital - Houston, USA
Thomas Sullivan - Houston Methodist Hospital - Houston, USA
Jennifer Liu - Houston Methodist Hospital - Houston, USA
Stephen Incavo - The Methodist Hospital - Houston, USA
*Email: bslambert@houstonmethodist.org
Background: During total knee arthroplasty (TKA), it is common practice to correct malalignment to neutral (02) intraoperatively in order to modify the load distribution on the joint in a manner that is thought to be more biomechanically preferrable. However, little data exist on the clinical impact of achieving neutral limb alignment in the target zone (0) compared to either slightly undercorrecting (UC) or crossing over (CO) beyond the target zone. The purpose of this investigation was to compare patient reported outcomes (PROs) and knee range-of-motion (ROM) following TKA for varus and valgus patients corrected to either neutral (NEUT), UC, or CO.
Methods: In this retrospective analysis, 409 patients undergoing primary TKA at a single institution were studied. Limb alignment was assessed using digital radiograph software from high-resolution X-ray images taken pre- and post-operatively. Patients were divided into those with varus (>0) and valgus (<0) malalignment and then further divided based on post-operative alignment (NEUT, UC, or CO). UC or CO were defined as >2 of under (UC) or over (CO) correction from neutral. Within the varus and valgus patient groups, KOOS Jr. survey scores (PROs) were observed at Pre-op as well as at 6-weeks, 3-months, 6-months, and 1-year post-op. ROM measures were also collected at 2-weeks, 6-12 weeks, and >6-months post-op. An ANOVA repeated on time was used to compare PROs and ROM measures followed by a Bonferroni post-hoc test for pairwise comparisons. As a secondary aim, Chi-square analysis was used to examine the proportion of males and females within each group. Significance was set at p<0.05 for all analyses.
Results: Patient demographics are presented in Table 1. Significant findings for Varus and Valgus patients as well as a sex-based comparison of correction frequency is shown in the Figure. For varus patients, those in the CO group (overcorrected to -4.03°± 1.95 valgus) were observed to have lower KOOS Jr. scores at 3-months, 6-months, and 1-year post-op compared to those in the NEUT group (p<0.05). This finding was paired with reduced ROM at 6-12 weeks post-op in the CO group compared to NEUT and UC (p<0.05). Females were more frequently crossed over to valgus and males were more frequently undercorrected (p<0.05). For valgus patients, no differences in PROs were observed. However, those in the UC group (left in -4.39° ± 1.39 valgus) were observed to have reduced knee flexion at 6-12 weeks post-op. For Pre-op valgus patients, females were more frequently undercorrected and left in valgus.
Conclusions: For TKA patients with varus malalignment, limb alignment corrections crossing over beyond neutral into valgus (even if to a small degree) results in less favorable PROs and ROM compared to those corrected to neutral or who were left slightly varus. While no differences were observed for PROs within valgus patients, those left in valgus via under correction may result in less favorable ROM in the early stages of rehabilitation. In both instances (pre-op varus or valgus), females were more likely than men to be left in valgus alignment.
Figures
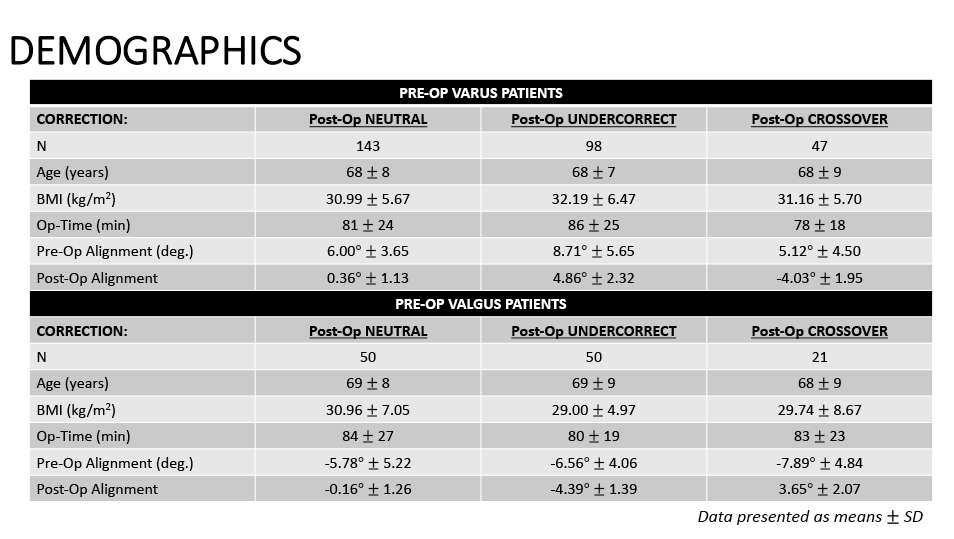
Figure 1
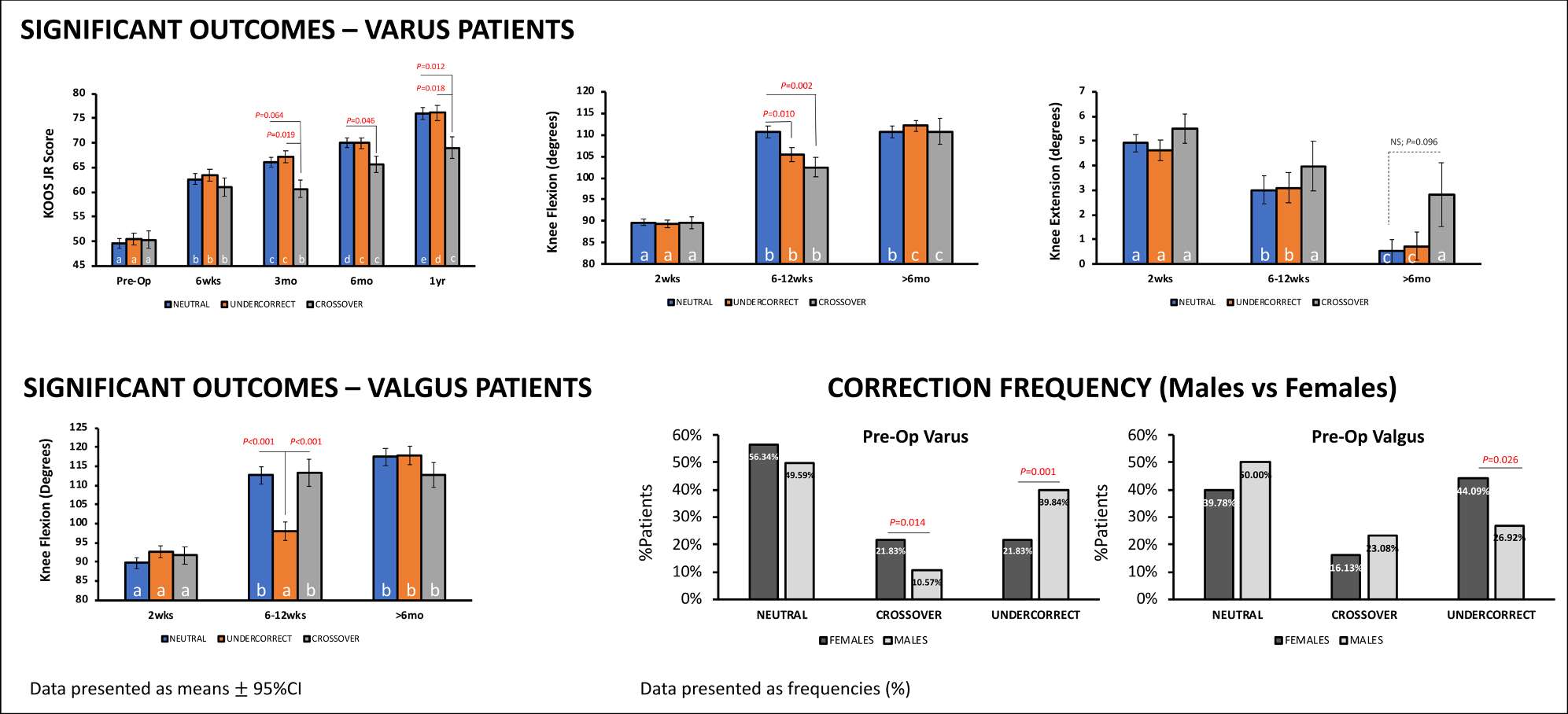
Figure 2#8583
Kinematic Alignment Best Restores the Patellofemoral Joint and Coronal Plane Alignment of the Knee: A Simulation Study Comparing Several Alignment Techniques
*Alex Orsi - Corin - Raynham, USA
Stefan Kreuzer - INOV8 Orthopedics - Houston, USA
Christopher Plaskos - Corin - Raynham, USA
*Email: Alex.Orsi@coringroup.com
Introduction:
Recent developments in total knee arthroplasty (TKA) component alignment techniques have focused on coronal plane alignment and tibiofemoral kinematics. Limited data exists on how TKA alignment affects the patellofemoral joint (PFJ). This study investigates how component alignment affects trochlear groove restoration and coronal plane alignment of the knee (CPAK).
Methods:
Native trochlear groove and implanted femoral component geometry data was collected from thirty-nine TKA performed using imageless navigation and a robotic ligament tensioner. Simulation software was utilized post-hoc to virtually implant the same implanted-size femoral component using five alignment techniques: gap balancing (GB) [1], mechanical alignment (MA) [2], unbounded kinematic alignment (KA) [3], restricted kinematic alignment (rKA) [4], and restricted inverse kinematic alignment (riKA) [4]. Femoral component geometry data from these simulations was collected.
Trochlear groove restoration was evaluated by comparing the geometric profiles of the native bone with the femoral component from the implanted and simulated techniques. A mean delta in trochlea position () was calculated for each technique in each case as the mean distance between the native bone and the implant geometry across nine points along the trochlear groove. These points were located at the midline, 10 mm medial, and 10 mm lateral to the midline at 20°, 45°, and 70° degrees of flexion along the trochlear groove, relative to the PFJ flexion axis [Fig. 1].
Native CPAK was calculated using intraoperative wear measurements from the distal femoral and proximal tibia. CPAK analyses compared each alignment technique against the native CPAK distribution. Mean absolute error (MAE) and signed error were calculated to determine the accuracy of each technique with respect to restoring native medial proximal tibial angle (MPTA), lateral distal femoral angle (LDFA), joint line orientation (JLO) and arithmetic hip-knee-ankle angle (aHKA). CPAK shift relative to native, defined as combined shift in JLO and aHKA using the square root of the sum of the squares, was evaluated for each technique.
Results:
All techniques under-stuffed the native trochlear groove. Both KA and rKA under-stuffed the trochlear groove by 3±1 mm and were closer to native compared to all other techniques (p<0.01) [Fig. 2]. GB was furthest from the native trochlea at 5±1 mm (p<0.05). The implanted technique trochlear position was more consistent than GB, MA, and riKA (p<0.05).
KA had the lowest MAE for LDFA, JLO, and aHKA, and the lowest CPAK shift [Fig. 3]. riKA had the lowest MAE and signed error for MPTA, while rKA had the lowest signed error for JLO. The implanted technique had the lowest signed error for aHKA.
Conclusions:
KA and rKA best restored the native trochlear groove, while the implanted technique showed superior repeatability. KA and rKA also restored CPAK to a more native distribution. However, the implanted technique and riKA also produced a relatively native CPAK distribution compared to GB and MA.
1. Orsi AD Arthroplast Today 2023, (10.1016/j.artd.2022.101090)
2. Orsi AD Arthroplast Today 2022, (10.1016/j.artd.2022.03.025)
3. Howell SM Surgery of the Knee 2012
4. Winnock de Grave P KSSTA 2023, (10.1007/s00167-023-07326-x)
Figures
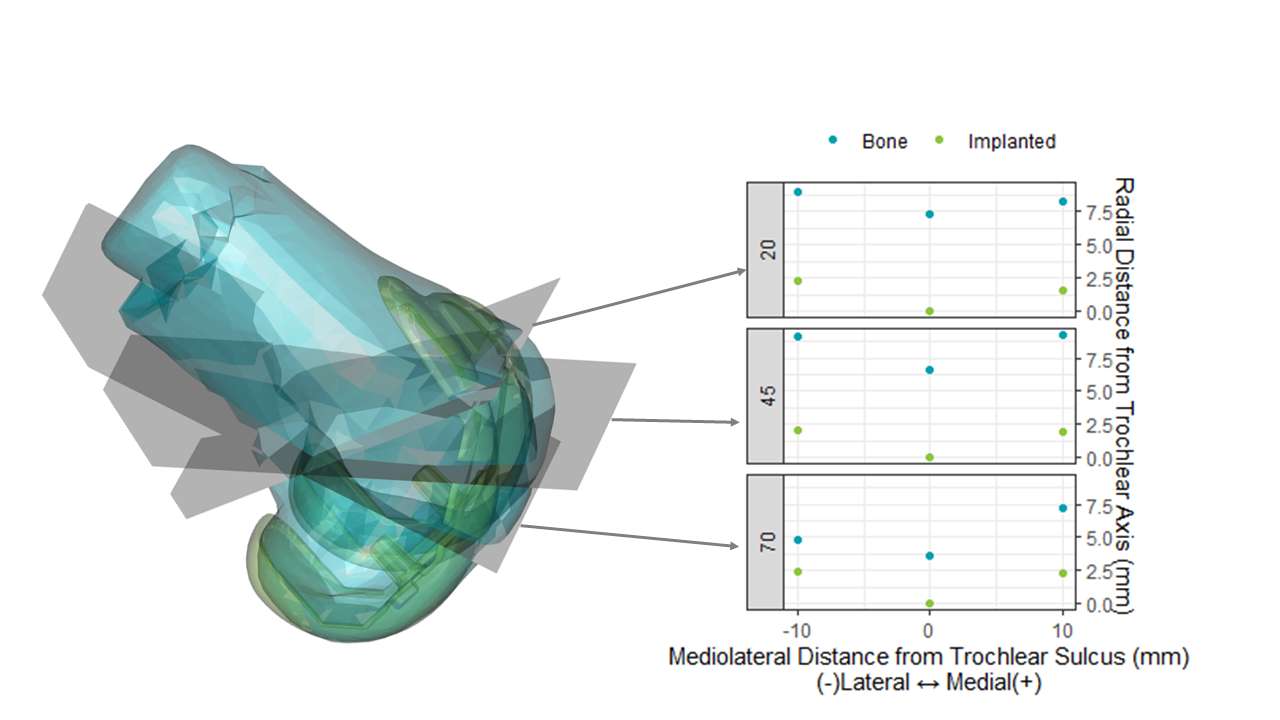
Figure 1
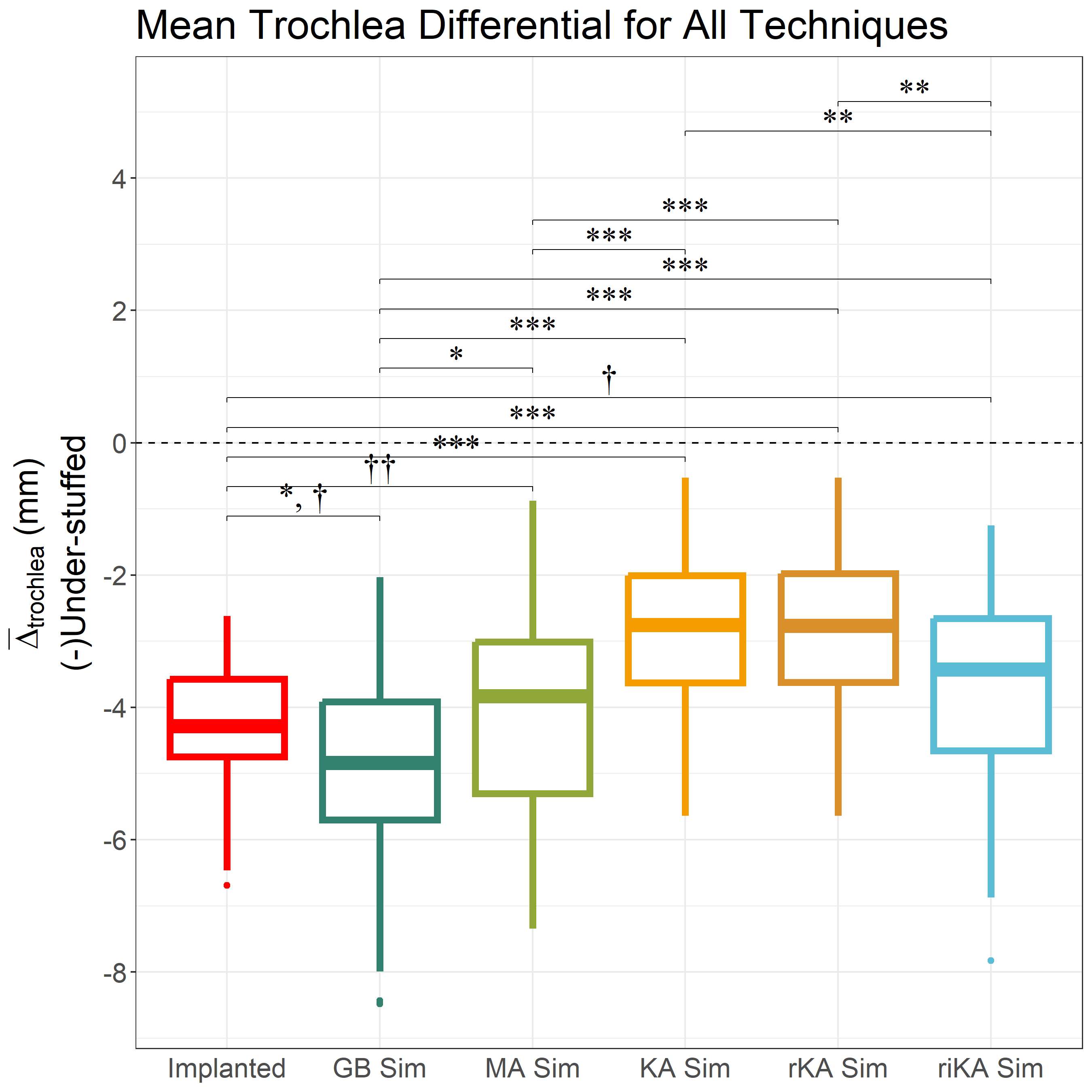
Figure 2
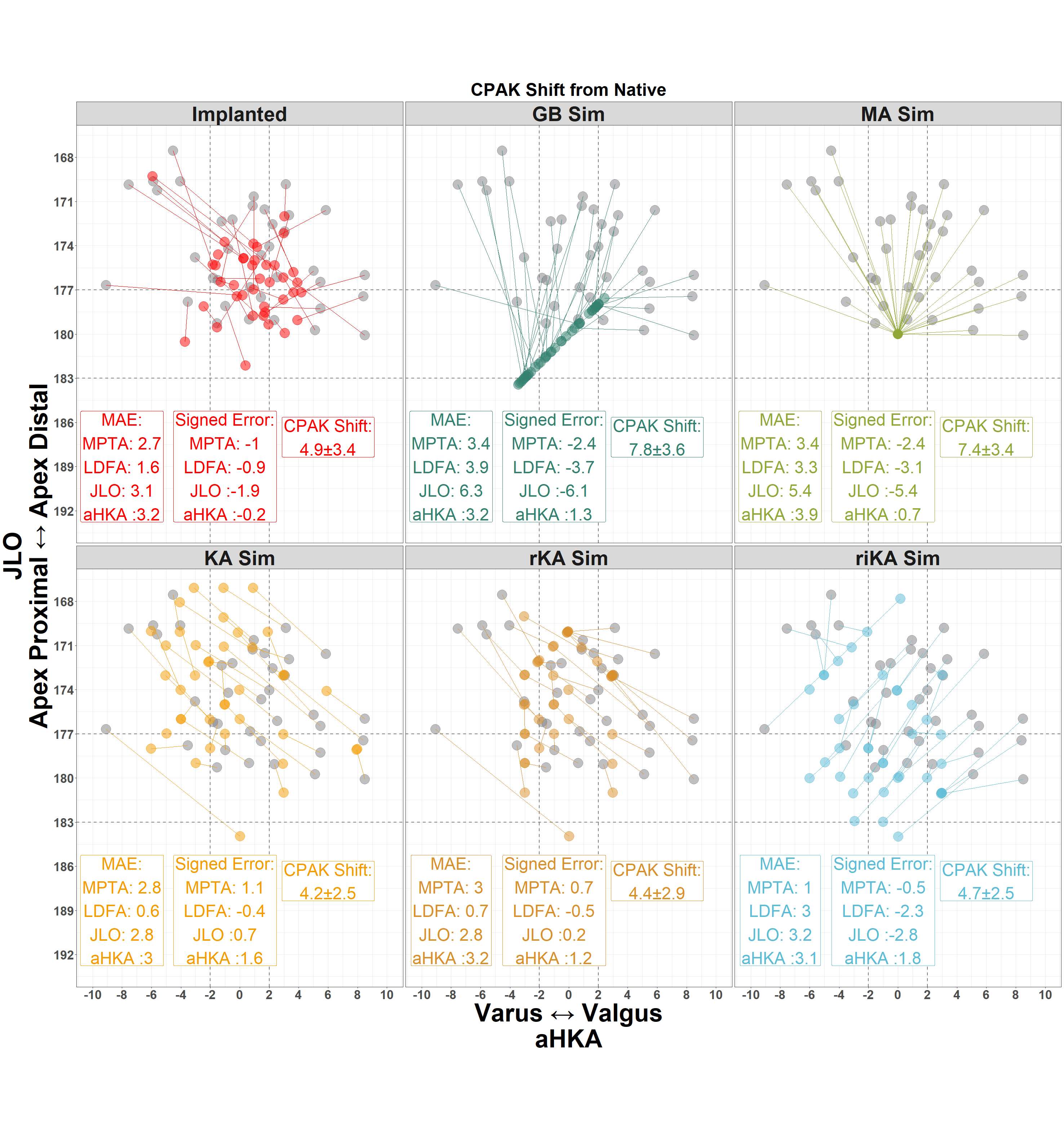
Figure 3#8416
Knee Adduction Moments in Kinematically and Mechanically Aligned Total Knee Arthroplasty in Level Walking and Stair Climbing
Ann-Kathrin Einfeldt - Hanover medical School - Hannover, Germany
*Eike Jakubowitz - Hanover medical Schhol - Hannover, Germany
Lars Tuecking - Hanover Medical School - Hannover, Germany
Max Ettinger - Hannover Medical School - Hannover, Germany
Henning Windhagen - Hanover Medical School - Hannover, Germany
*Email: jakubowitz.eike@mh-hannover.de
INTRODUCTION: The goal of kinematically aligned (KA) total knee arthroplasty (TKA) is to precisely restore the individual knee anatomy by maintaining the patient-specific joint line. As a result, these patients remain in a varus knee axis after surgery, as most patients with knee osteoarthritis show a varus deformity. In contrast, mechanically aligned (MA) patients have a more straightened leg axis. Therefore, it seems obvious that the knee adduction moment (KAM) will be greater in KA patients than in MA patients. However, it is still controversial whether the KAM is increased or decreased in KA patients [1,2]. Therefore, we hypothesized that the KAM reduction after bone consolidation is increased in MA compared to KA patients. The aim of the present randomized, observer-blinded, prospective study was to analyze the KAM changes in MA and KA patients.
METHTODS: 71 patients (33 KA, 38 MA) treated with the GMK Sphere TKA (Medacta) were included in the study. All patients were required to complete a 3D motion analysis for level walking and stair climbing (up and down) on the day before surgery and one year later. The difference between pre- and post-operative KAMs was calculated using inverse dynamics with the Plug-in Gait model and Nexus software (Vicon) and statistically evaluated using a t-test (p = 0.05)
RESULTS: Both groups show a reduction of KAM from pre- to post-OP in all three conditions (Fig. 1). The KAM reduction in KA compared to MA patients is greater in all three conditions. Looking at the maximum difference on average, KA patients show a significantly greater KAM reduction in the downstairs condition (p = 0.03). However, during level and upstairs walking this KAM reduction was not significantly higher (Tab. 1).
CONCLUSION: The hypothesis of a significantly greater KAM reduction in MA patients could not be confirmed. In contrast, KA patients showed a significantly greater reduction during the downstairs condition. We attributed this as a result of an increased knee stability for KA patients when confronted with more dynamic tasks. This study shows that despite a varus leg axis in KA patients the KAM reduced more than in MA patients, who have a straight leg axis.
REFERENCES: [1] Niki et al. (2018). Knee Surg Sports Traumatol Arthrosc, 26(6),1629-1635; [2] Du Ro et al. (2019). Knee, 26(3), 737-744
Figures
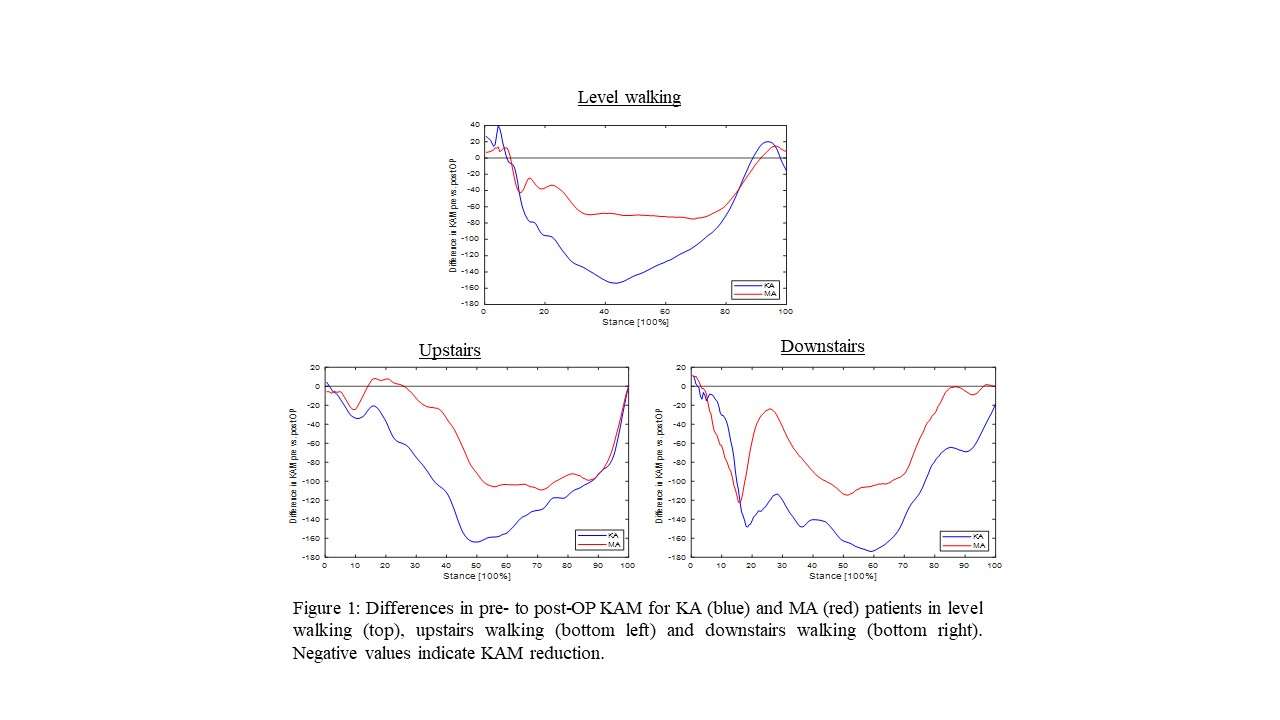
Figure 1
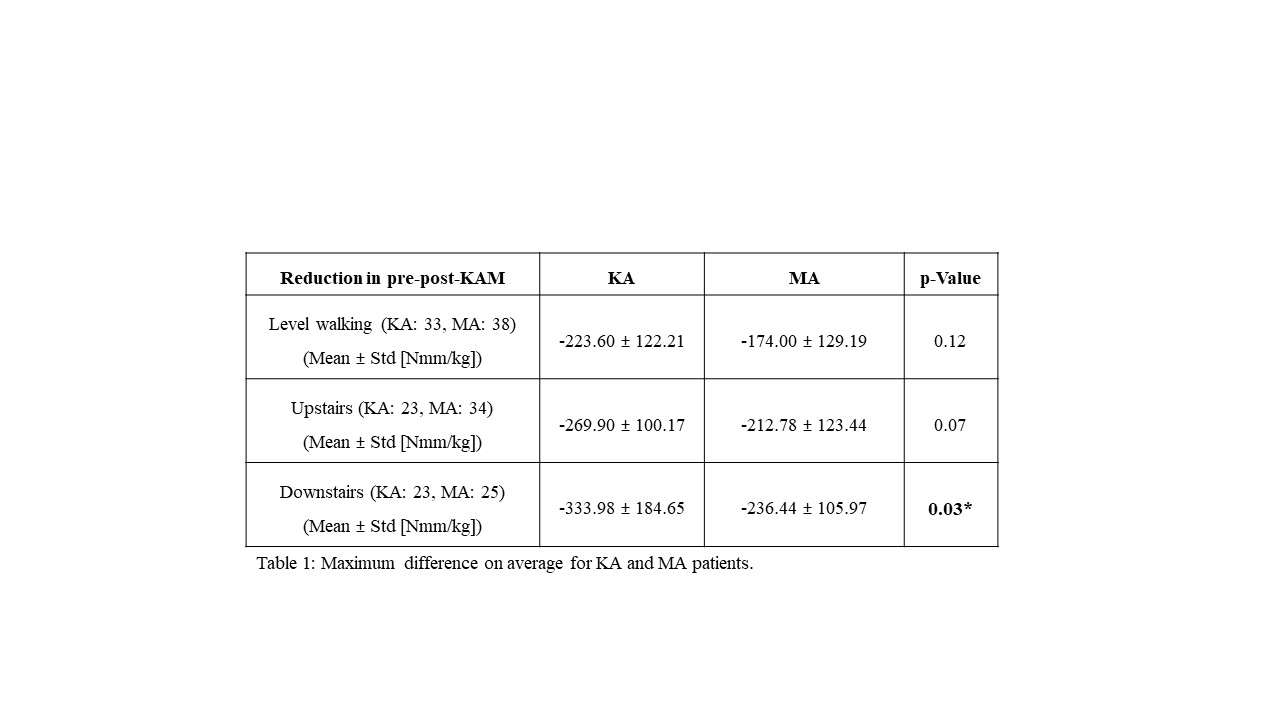
Figure 2#8413
Knee Kinematics While Stair Climbing After Mechanical and Kinematic Aligned TKA
Ann-Kathrin Einfeldt - Hanover medical School - Hannover, Germany
*Eike Jakubowitz - Hanover medical Schhol - Hannover, Germany
Max Ettinger - Hannover Medical School - Hannover, Germany
Lars Tuecking - Hanover Medical School - Hannover, Germany
Henning Windhagen - Hanover Medical School - Hannover, Germany
*Email: jakubowitz.eike@mh-hannover.de
INTRODUCTION: The goal of kinematic aligned (KA) total knee arthroplasty (TKA) is to precisely restore the individual knee anatomy by maintaining the patient-specific joint line. Compared to conventional mechanical alignment (MA), this hypothesis has already been indirectly confirmed using functional knee scores since a faster recovery and improved outcomes were observed. However, whether this improvement is directly associated with joint kinematics remains unanswered to date. Thus, conventional gait analysis in a level walking condition also come to controversial results. Therefore, the aim of the present randomized, observer-blinded, and prospective study was to analyse full knee joint kinematics (6-DOF) during up and downstairs walking after KA and MA TKA. A non-arthritic cohort served as a reference.
METHTODS: 74 patients (34 KA, 40 MA) treated with the GMK Sphere TKA (Medacta), and nine healthy controls were included. All patients had to complete a 3D motion analyses on an instrumented staircase the day before and one year after surgery. Kinematic data was acquired with a motion capture system with 12 infrared cameras (200 Hz). For detailed 6-DOF knee kinematics a Helen Hayes marker set was modified by using additional markers resulting in an over-determination of leg segments, and a quasi-static (frame to frame calculation) optimization algorithm was used to calculate joint movements using an inverse kinematics approach. The Forgotten Joint Score (FJS) and the Knee Society Score (KSS) were collected. For statistics, t-tests and Statistical Parametric Mapping (SPM) were used.
RESULTS: Post-op there are no significant differences between KA and MA patients in both conditions. In comparison with controls, MAs show a reduced knee extension (p = 0.02) and an increased knee adduction (p = 0.02, p < 0.01) in upstairs walking, whereas KAs show no differences towards controls (Fig.1). In the downstairs condition, there are no significant differences between all three groups in sagittal plane kinematics. In frontal plane rotations, both patient groups show greater adductions (p < 0.01) (Fig. 2). Post-op, KAs show a significantly increased FSJ score (KA = 63.7 vs. MA = 49.6, p = 0.01) whereas due to the KSS the groups do not differ functionally (KA = 80.5 vs. MA = 74.5, p = 0.13).
DISCUSSION: Contrary to expectations, MAs show a greater knee adduction post-OP in walking upstairs, indicating that the static leg axis does not reflect the joint angles in motion. Patients with KA appear to have better control of their knee in the frontal plane during that condition. In a more challenging task like downstairs walking, they also show a significantly increased knee adduction. Considering all results, KAs show fewer significant differences compared to controls than MAs, suggesting a more physiological gait pattern one year after TKA. This might be the reason why patients with KA are more likely to forget about their knee in everyday life.
ACKNOWLEDGEMENTS: The present work is supported by Medacta International. We thank Benjamin Fleischer-Lück for optimization coding.
Figures
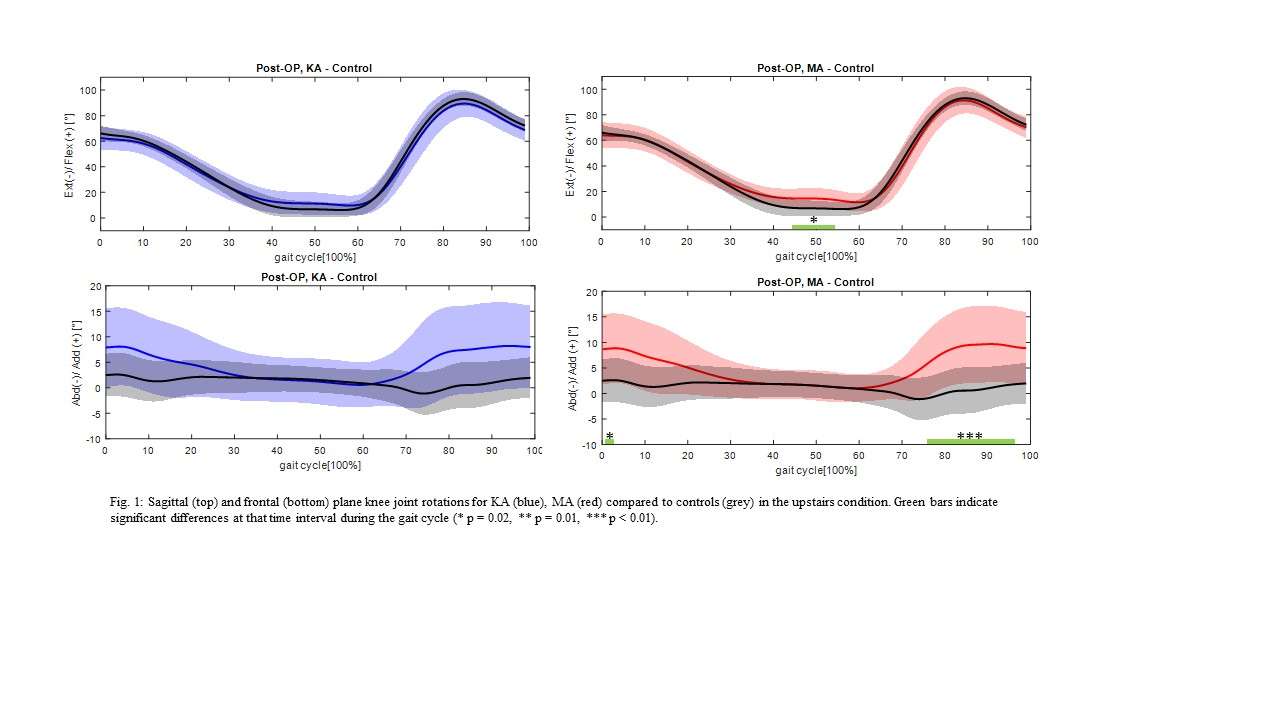
Figure 1
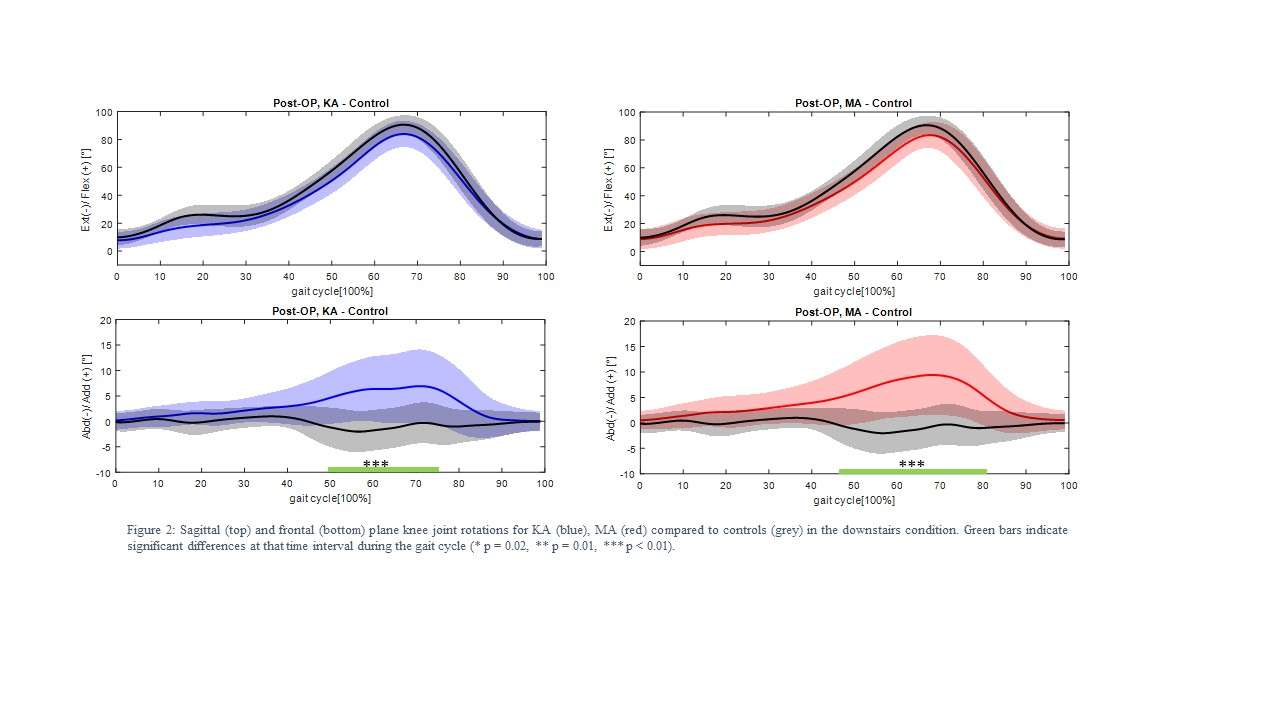
Figure 2#8457
No Difference in Alignment Between Kinematic and Mechanical Alignment Robotic-Assisted Total Knee Arthroplasty
*Theofilos Karasavvidis - Hospital for Special Surgery - New York, USA
Cale Pagan - Hospital for Special Surgery - New York, United States of America
David J. Mayman - Hospital for Special Surgery - New York, USA
Seth A. Jerabek - Hospital for Special Surgery - New York, USA
Jonathan Vigdorchik - Hospital for Special Surgery - New York, USA
*Email: Karasavvidist@hss.edu
INTRODUCTION: Traditionally, mechanical alignment (MA) has been considered the gold standard for total knee arthroplasty (TKA) and has prioritized neutral limb alignment to achieve an even load distribution on the implant. However, recent studies have suggested that kinematic alignment (KA), which aims to restore the natural alignment and joint line obliquity, may be a promising alternative. This study aims to compare KA and MA in terms of postoperative alignment and assess whether KA significantly deviates from the principle of aligning the limb as close to neutral alignment as possible.
METHODS: This retrospective study included 232 patients who underwent robotic-assisted TKA between 2016 and 2022 using an unrestricted kinematic alignment technique and a strict mechanical alignment technique (KA: 145, MA: 87). We measured the alignment using the lateral distal femoral angle (LFDA) and medial proximal tibia angle (MPTA), and the resultant arithmetic hip-knee-ankle angle (aHKA) in all cases. aHKA<0 indicated varus alignment, while aHKA>0 indicated valgus knee alignment. The primary outcome was the frequency of cases that resulted in an aHKA of ±4° of neutral (0°), as assessed on full-leg standing radiographs obtained at 6 weeks postoperatively. The secondary outcome was the change in coronal plane alignment of the knee classification (CPAK) type from preoperative to postoperative between the MA and KA groups. Power analysis indicated that the included number of patients would provide 95% power to detect a 2.5 degree difference in alignment. Statistical analysis consisted of paired T-tests to compare pre- and postoperative aHKA and chi-squared test to compare the frequency of cases that resulted in an aHKA of ±4° of neutral, as well as the rate of CPAK type change between KA and MA. P-value <0.05 was considered significant.
RESULTS: Mean preoperative aHKA was similar between the two groups (P=0.19). The KA group had a mean postoperative aHKA of -1.4±2.4° [Fig 1], while the MA group had a mean postoperative aHKA of -0.5±2.1° [Fig 2]. No significant difference in alignment was identified between KA and MA cases that resulted in HKA of ±4° of neutral (91.7% vs 96.6%, P=0.14). The MA group was associated with a significantly higher rate of CPAK type change from pre- to postoperatively (P<0.001).
CONCLUSION: KA achieved similar postoperative aHKA compared to MA and thus did not significantly deviate from the principle of aligning the limb as close to neutral alignment as possible. Surgeons should feel comfortable starting to introduce individualized alignment techniques because even without paying attention to boundaries, postoperative alignment will be within the MA safe zone.
Figures
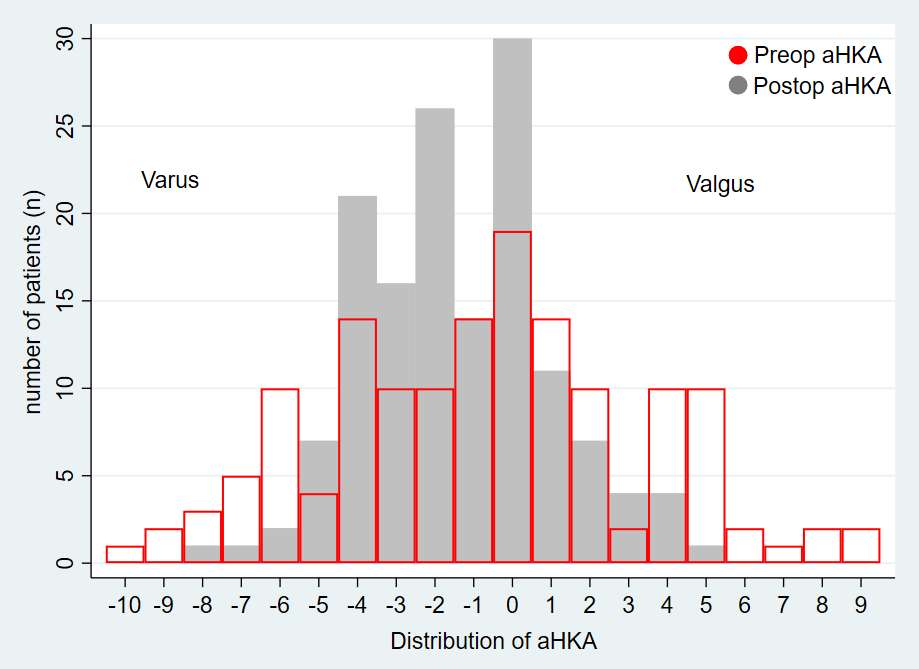
Figure 1
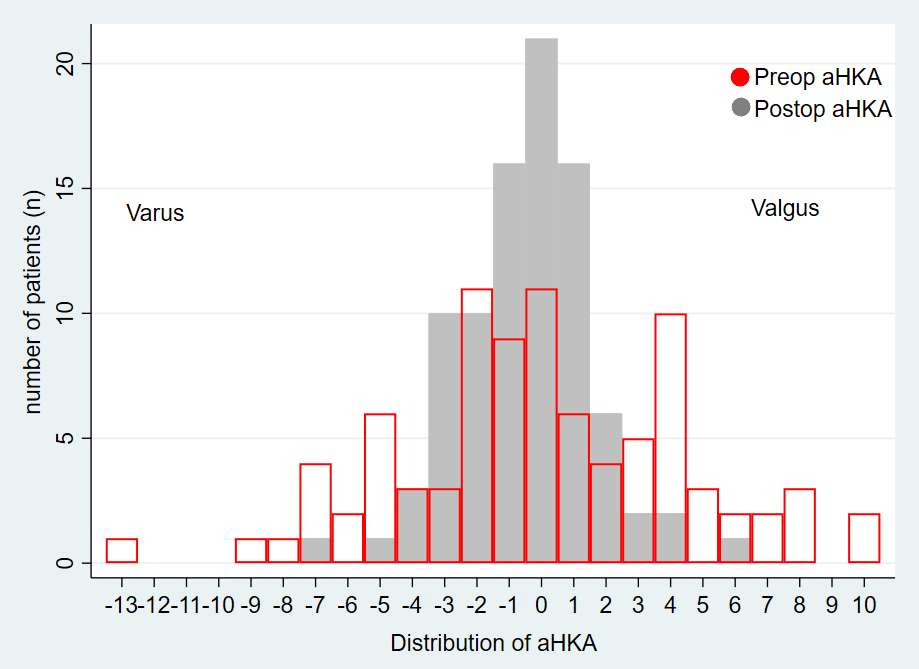
Figure 2#8802
Kinematic Alignment Produces Superior Clinical Results to Mechanical Alignment With Primary Medial-Pivot Knee Arthroplasty
*Trisha Vuong - Spokane, United States of America
David Scott - Spokane Joint Replacement Center - Spokane, USA
*Email: trisha.vuong@wsu.edu
Abstract
Introduction: The superiority of clinical outcomes in primary total knee arthroplasty (TKA) obtained with kinematic alignment (KA) versus mechanical alignment (MA) surgical approaches remains heavily contested. We performed a clinical trial comparing these two alignment approaches utilizing a true spherical "ball-in-socket" medial-pivot primary implant (MP). The hypothesis was that the KA group would have better clinical outcomes than the MA group.
Methods: In this prospective multicenter study, patients who underwent primary TKA implanted with a spherical MP device were enrolled and clinical outcomes collected. Institutional Review Board and informed consent was obtained. One site utilized an unrestricted KA protocol, and the others an MA approach. Surgical technique was standardized to include use of a tourniquet, medial parapatellar arthrotomy, eversion of the patella, sacrifice of the posterior cruciate ligament, manual instrumentation, and cement fixation. Forgotten Joint Scores (FJS) and flexion range of motion (ROM) were collected preoperatively and at 6-week, 6-month, 1-year, and 2-year follow up.
Results: 343 patients were enrolled at six sites, 101 KA knees and 242 MA knees. The FJS scores and ROM were significantly better in the KA group at 6-month through 2-year follow up. Maximum flexion at two years averaged 132° for the KA group and 121° for the MA group (p<0.0001). The KA group also had significantly better FJS scores at 6 months and one year: FJS was 64 (p≤0.0001) for the KA group and 55 for the MA group (p<0.014). The overall FJS score at the 2-year follow-up did not reach significance: 68 (KA) vs. 63 (MA) (p =0.17), but FJS question # 3 (walking 15 minutes) was significantly better in KA group (higher value indicates more noticeable symptoms) at the 6-month, 1-year, and 2-year follow up. At the 2-year follow up, FJS question #3 for the KA group and the MA group were 1.08 and 1.51 (p=0.025), respectively.
Conclusion: The KA group had significantly better ROM and FJS outcomes versus the MA group at 6 months through 2 years postoperative in this prospective multicenter trial of medial-pivot knees. The results demonstrated the superiority of KA to MA across multiple clinical outcomes.
Figures
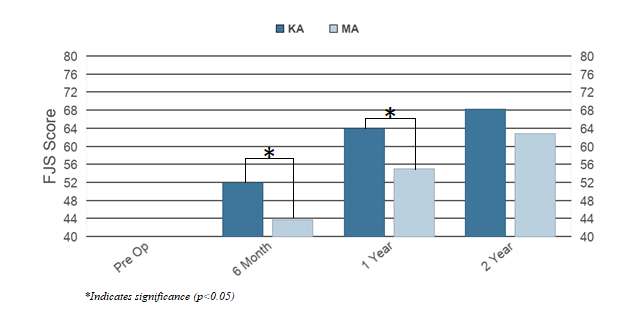
Figure 1
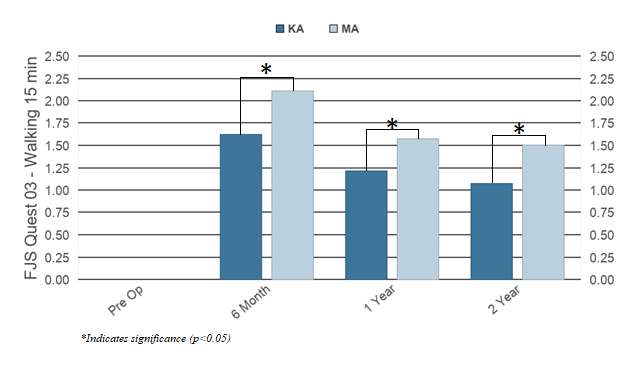
Figure 2
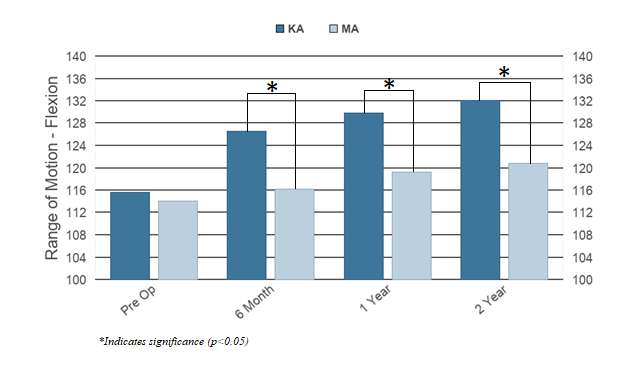
Figure 3#8352
Kinematics and Kinetics During Level and Downhill Walking in Total Knee Arthroplasty Using a Robotic Ligament Tensioner
*Markus Wimmer - Rush University - Chicago, USA
Takayuki Koya - Showa University Koto Toyosu Hospital - Tokyo, Japan
Ariane Lalles - Rush University Medical Center - Chicago, USA
Christopher Knowlton - Rush University Medical Center - Chicago, United States of America
Vasili Karas - Rush University Medical Center - Chicago, USA
*Email: markus_a_wimmer@rush.edu
Introduction: Robotic TKA holds promise in reliable ligamentous balancing through bony resection that may lead to improved outcomes, including more normal gait.The goal of this observational study was to capture functional parameters after robotic-assisted TKA and compare them with already recorded data of patients who received a manually implanted cruciate retaining prosthesis and those of an elderly healthy subject group.
Methods: Subjects who underwent robotic TKA receiving a Corin Unity CR implant were recruited from a single surgeon’s clinic for this ongoing IRB approved study. Subjects were included if they received a primary, unilateral TKA, were 8-14 months post-op and had BMI <35. Eligible subjects underwent a clinical exam before being gait tested. Passive reflective markers were placed on the skin and tracked with a multi-camera system. Simultaneously, ground reaction forces were collected during level and downhill walking (Figure). Subjects were instructed to walk at their usual pace, and five trials were recorded. Using inverse dynamics, knee kinematics and external moments were calculated and normalized to %bodyweight x height. For comparison, a data repository was assessed to obtain a manual TKA comparison group (single radius, cruciate retaining design), as well as an elderly healthy comparator group. One-way ANOVA with post-hoc Games-Howell analyses were performed on selected gait parameters. Statistical parametric mapping of the waveforms was also performed.
Results: Each group contained 10 subjects. The TKA groups were of similar age, but 8 years older than the Healthy group. Sex distribution was the same in all three groups. There were no differences in preferred walking speeds. The flexion-extension range during walking was similar in the Robotic and the Healthy groups, while the Manual group displayed a smaller range (p= 0.022 re. 0.008 for level and downhill). This was due to a lower peak flexion angle during swing. Conversely, during midstance, the knee of the Robotic group stayed significantly more flexed compared to Healthy (p<0.001). The tibial anterior-posterior displacement during walking was similar between Robotic and Healthy, but significantly lower than Manual (p=0.014 re. 0.007 for level and downhill).
The peak flexion moment, which occurs during the first half of stance in the sagittal plane, was higher in the Robotic group compared to the Manual group (p=0.01) and similar to Healthy indicating a normal quadriceps use. In contrast, the peak extension moment, occurring in the second half of stance, was lower in the Robotic group compared to Manual and Healthy (p=0.048 re. p<0.001). During downhill walking, the same differences came to light, but were amplified. In the frontal plane, the peak knee adduction moment of the Robotic group was similar to that of the Healthy group, while the Manual group showed a lower value (p=0.013). Again, these differences were also seen during downhill.
Conclusions: Some but not all of the gait parameters appear normalized after robotic surgery; subjects demonstrated healthy quadriceps use, which is interesting because the extensor mechanism is often compromised after TKA. On the contrary, the low extension moment suggests underutilizing the hamstrings. The exact reasons have to be identified.
Figures

Figure 1#8254
Different Patterns of Final Balance for Total Knee Arthroplasty Identified Using Intra-Operatively Collected Robotic Laxity Metrics, a Descriptive Analysis.
Mike Anderson - Zimmer Biomet - Lehi, USA
Peter McEwen - The Orthopaedic Research Institute of Queensland - Pimlico, Australia
Joseph Brook - Zimmer Biomet - London, United Kingdom
*Adam Henderson - Zimmer Biomet - Winterthur, Switzerland
Dave Van Andel - Zimmer Biomet - Grand Rapids, USA
Trevor Pickering - Mississippi Sports Medicine and Orthopaedic Center - Jackson, USA
*Email: adam.henderson@zimmerbiomet.com
Introduction
As digital health continues to advance, it is necessary to evaluate the ability of these technologies to capture clinically relevant data. Using robotic technology to assess intra-operative decisions associated with balancing the medial and lateral compartments in total knee arthroplasty (TKA) may allow us to evaluate the effects of different surgeon preferences regarding balance. The purpose of this feasibility study was to determine if surgeon patterns on balance in TKA could be identified via intra-operative data collection using a robotic system.
Methods
We performed a descriptive analysis of anonymized data from a commercial database for patients who underwent robotic assisted primary unilateral TKA between May 2019 and April 2023, and had their data uploaded into the commercial data warehouse (n=1,745) by one of ten surgeons in the USA or Australia. Institutional review boards deemed this study exempt from full review and provided a waiver of consent and authorization for secondary analysis.
Results
The average age of the population was 66.0 years (range, 30 – 88) and the majority were female (n=54%). The mean BMI was 32.2 kg/m2 (range, 19.3 – 55). There appears to be no difference in the initial intra-operative evaluation of balance between surgeons (Figure 1). At the final intra-operative evaluation of balance there are graphically distinct differences between surgeons, especially regarding the magnitude of within surgeon variation (Figure 2). It is apparent, that the majority of these surgeons preferred a tightly balanced knee with only a few surgeons extending beyond 1-2 mm difference between medial and lateral compartments.
Conclusion
To better understand the impacts of intra-operative decisions on post-operative outcomes the surgeon must be able to quantitatively assess intra-operative parameters. Our data demonstrate the ability of a robotic surgical assistant to passively collect these data points. Additionally, the data provided a visual representation of the different surgical philosophies on soft-tissue balance during TKA. Future studies are needed to determine how, or if, these different patterns affect immediate-, short-, and long-term outcomes.
Figures
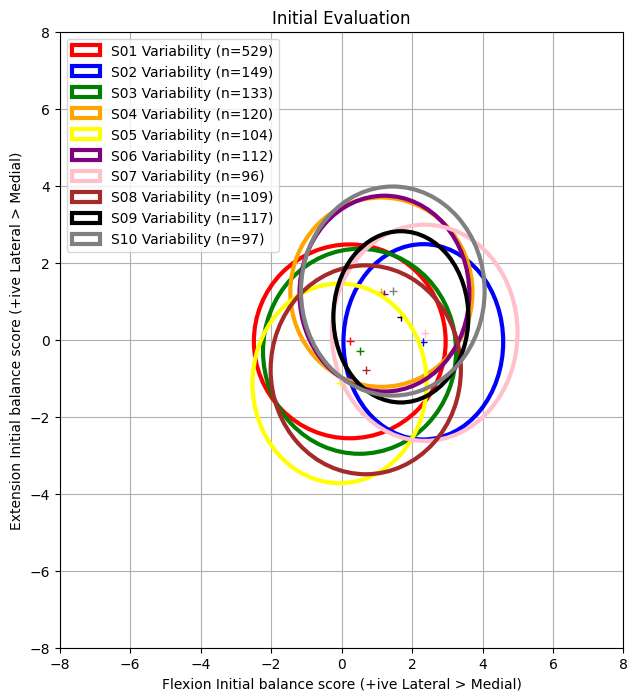
Figure 1
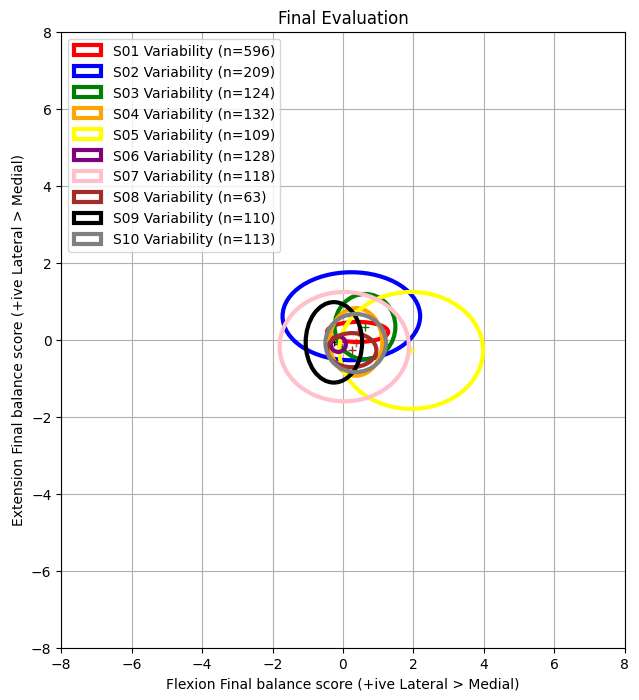
Figure 2#8282
Development of Smart Surgical Trial Components for Intra-Operative Assessment and Recording of Component ROM and Stability in Total Hip Replacement Surgery.
*Oliver Vickers - University of Leeds - LEEDS, GB
Tim Board - Wrightington, Wigan & Leigh NHS Foundation Trust - Wigan, United Kingdom
Peter Culmer - University of Leeds - Leeds, United Kingdom
Graham Isaac - University of Leeds - Leeds, United Kingdom
Robert Kay - University of Leeds - Leeds, GB
Sophie Williams - University of Leeds - Leeds, United Kingdom
*Email: o.g.vickers@gmail.com
Introduction: One of the most significant variables affecting outcomes in total hip replacement (THR) surgery is placement of components during surgery. Intra-operatively “trial” components are used by surgeons to assess component size and positioning to ensure optimal joint stability and range of motion (ROM). However, these current methods of assessment are subjective.
If trial THR components were instrumented with a sensing system capable of predicting femoral neck orientation (tilt and azimuth angle Figure 1), and femoral head subluxation, it could provide an objective method of predicting achievable component ROM, risk of impingement and joint stability.
This advance would enable quantification of the intra-operative assessment process and remove subjectivity, aiding clinician’s decision-making process and provide a method of recording/quantifying surgical performance.
Methods: The proprietary sensing system was integrated into 3D printed representations of a trial THR liner and femoral head.
The trial femoral head was positioned on a spigot stem fastened to a UR3 robotic arm. The robot could manipulate the stem and provided ground truth measurement of position and orientation of the trial femoral head and stem with respect to the trial liner. Copper foil was positioned on the components at the location of impingement contact and a continuity trigger was used to indicate if component impingement contact had occurred.
In the first test the stem was moved to 35 orientations (within one half of the liner’s hemisphere) at seven azimuth angles (0°,30°,60°,90°,270°,300°,330°) and five tilt angles (15°,30°.45°.60°,max tilt) the final tilt angle created an impingement contact. In the second test the robot started vertically with the trial femoral head located in the trial liner. The robot then moved the trial head 2mm and then 3mm out of the liner (along the central axis of the liner Figure 1) returning in between. The 2mm and 3mm separations were then repeated at 13 tilt angles (0°,5°,10°,15°,20°,25°,30°,35°,40°,45°,50°,55°,60°). Each test was repeated three times.
Results: Comparing angle measured by the robot versus the sensing system (Figure 2), when within the normal ROM of the components the RMSE±SD (across all three repeats) in the tilt and azimuth angle prediction was 1.6°±1.3° and 2.7°±2.6° respectively. Across all three repeats tilt angle at impingement was predicted at less than 1.2° from the actual stem tilt angle at impingement and the RMSE±SD of the predicted azimuth location of impingement was 1.3°±1.3°.
Comparing separation measured by the robot versus the sensing system (Figure 3), across all three repeats the tracking system correctly identified all separation occurrences at 3mm and 22/36 separation occurrences at 2mm with the remaining 14/36 overestimated as 3mm instead of 2mm.
Conclusion: The proprietary sensing method is real-time, non-contact and tracked stem orientation allowing the identification of an impingement point and recognised the occurrence of bearing surface separation >3mm.
The proposed device could enable the intra-operative assessment and recording of component ROM and stability therefore could be used in improving surgeon training and if used in surgery will provide intra-operative validation of component positioning, with the potential to improve surgical outcomes.
Figures
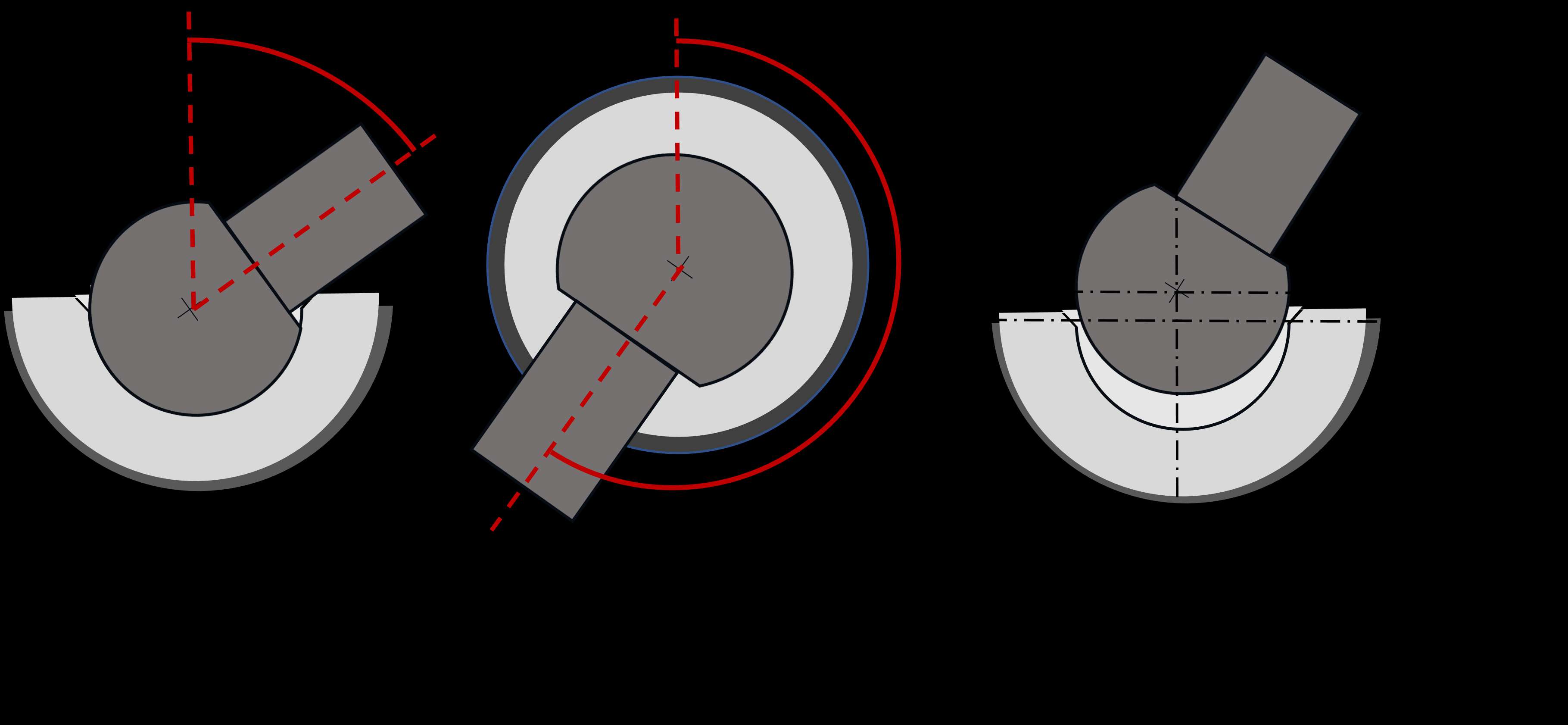
Figure 1
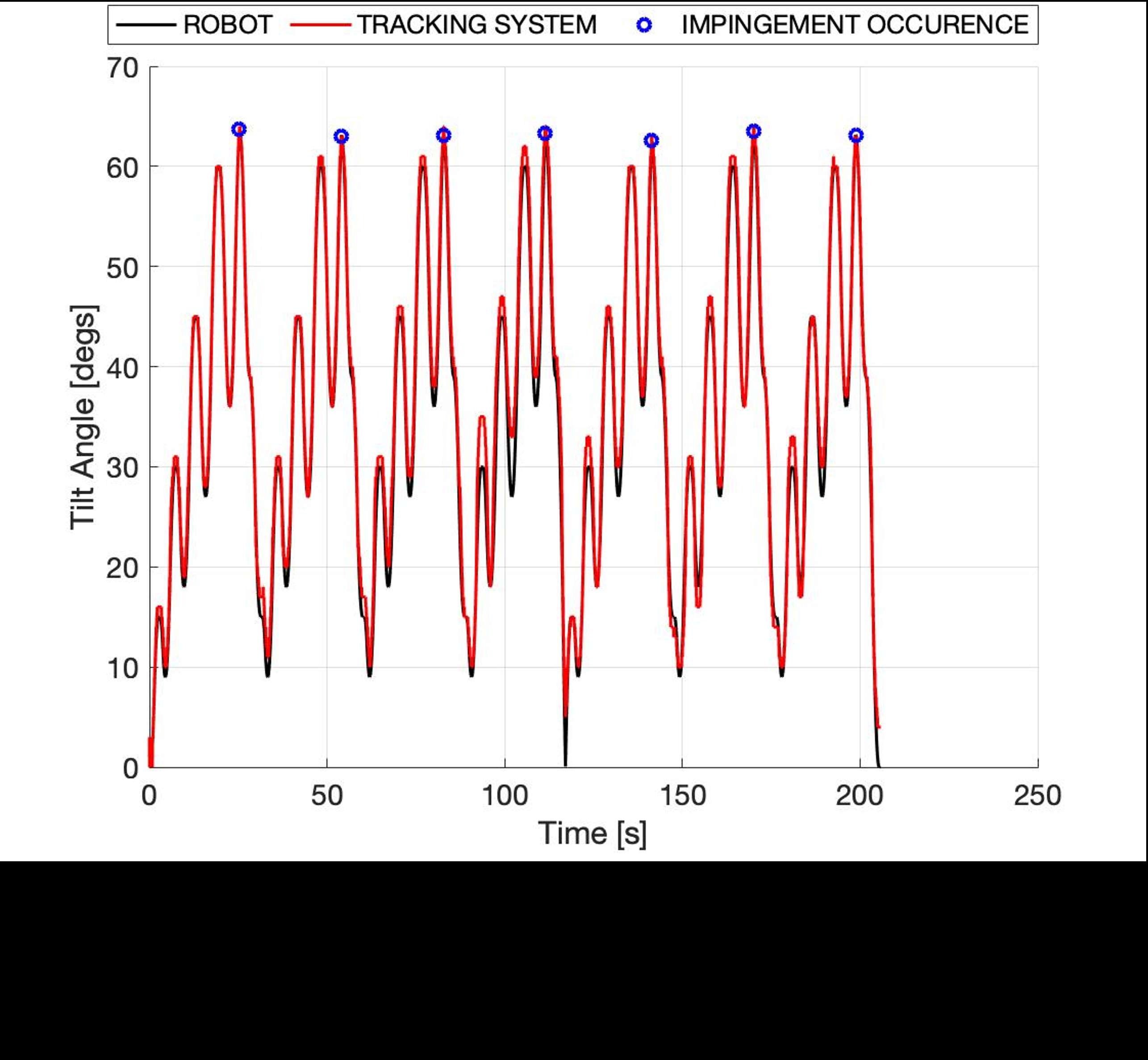
Figure 2
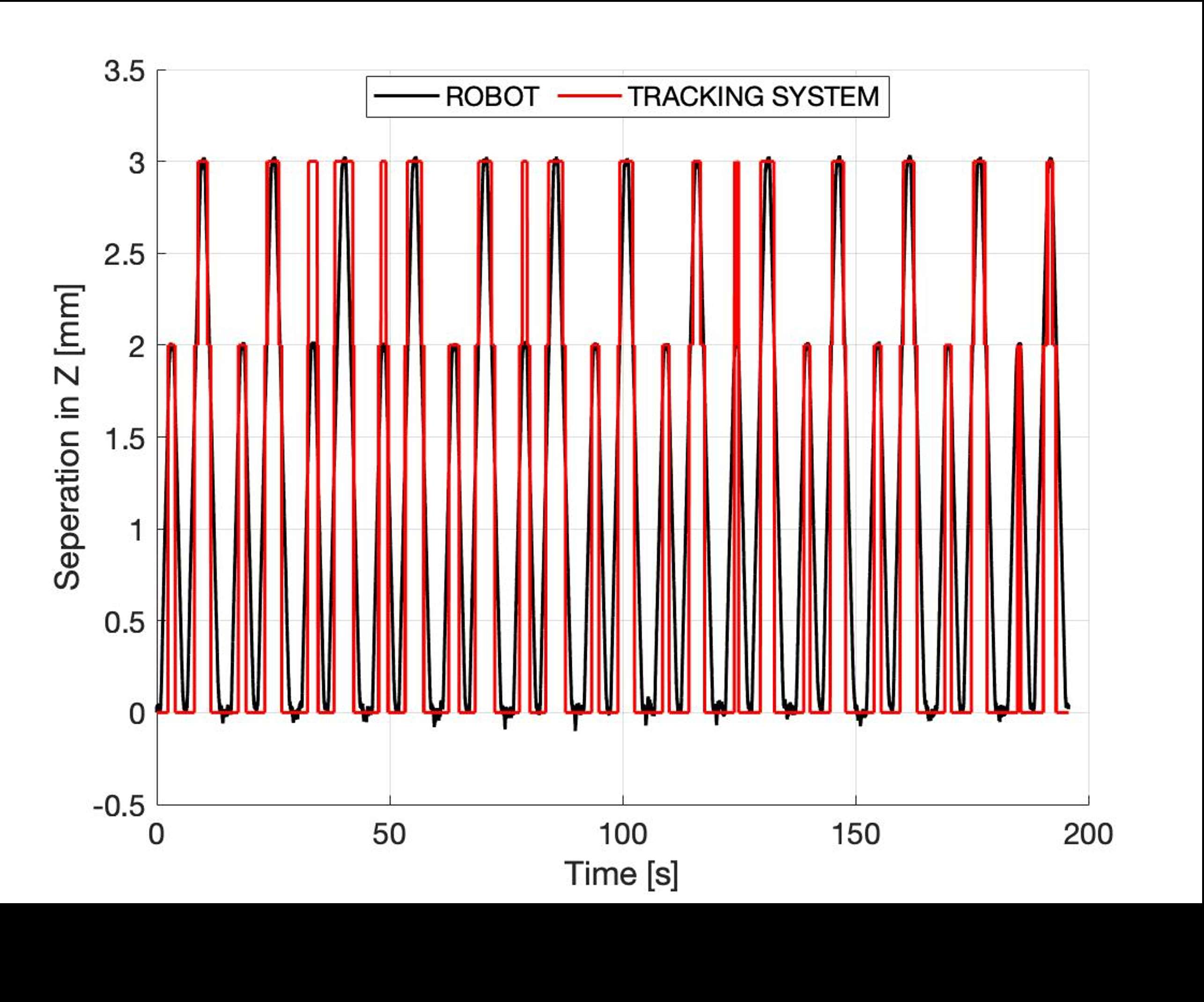
Figure 3#8418
Accuracy of a New Augmented Reality System for Total Knee Arthroplasty: A Cadaveric Study by Using the Nextar Tka
*Daniele Ascani - Medacta International SA - Chiasso, Switzerland
Massimiliano Bernardoni - Medacta International SA - Castel San Pietro, Switzerland
Geert Peersman
*Email: ascani@medacta.ch
Introduction
Knee arthroplasty, also known as knee replacement surgery, is a common procedure performed to alleviate pain and improve mobility in patients with advanced knee osteoarthritis or other knee conditions [1-2]. Precise positioning of the implants is crucial for the success of the surgery and to ensure the longevity of the prosthetic joint. However, traditional techniques for implant positioning can be limited in their accuracy and can result in complications such as implant malalignment or instability [3]. In recent years, there has been increasing interest in the use of augmented reality systems to improve the accuracy of implant positioning in knee arthroplasty [4]. This study aims to evaluate the accuracy of an augmented reality system for knee arthroplasty and to compare it to traditional techniques on cadavers. The results of this study could potentially provide valuable insights into the feasibility and effectiveness of using augmented reality technology to improve the accuracy and safety of knee arthroplasty surgeries.
Methods
The dataset used in this study consists of 7 knee specimens that have been scanned and loaded to the MySolution (Medacta International SA) platform to create the NextAR cases. Each knee has been preoperatively planned and executed using the NextAR TKA system. The NextAR TKA system is composed by a single-use tracking system (small camera infrared and active tracker), augmented reality glasses, and a medical grade PC monitor (Figure 1).
A total knee replacement procedure was performed on each knee following the NextAR TKA surgical technique, during the surgery the following surgical parameters were measured:
- Femoral varus/valgus angle
- Femoral flexion/extension
- Femoral internal/external rotation
- Tibial varus/valgus angle
- Tibial slope
The augmented reality allowed to accurately perform the registration of the CT scan on the specimen and the accurate execution of the bone cuts. Postoperative CT scans were performed to confront the intraoperative surgical parameters with the final position of the implants.
Results
The results showed a mean deviation below 1° (0,9°±0,1) between the values measured by the NextAR TKA and real position of the implants on postoperative images.
Conclusion
Overall, these cadaveric results suggest that the use of augmented reality systems for knee arthroplasty can lead to more accurate and personalized implant positioning, resulting in improved alignment and stability of the prosthetic joint without increasing the risk of complications.
The results showed an accuracy of 0,9° as deviation between the values measured by the NextAR and real position of the implants on the postoperative images.
The augmented reality was correctly used during the surgery with the following benefits (Figure 2):
- The data over imposed onto to the operative allowed to perform the surgical step with the sight always direct on the surgical area
- Light and wearable hardware worn under the surgical helmet
- Improvements to eyeballing traditional technique
- Improvement to the line-of-sight problem of the traditional navigation system
It can be concluded that the accuracy of the system is superior to the conventional surgical techniques [5] and PSI [6], and it is comparable to robotically-assisted systems [7].
Figures

Figure 1
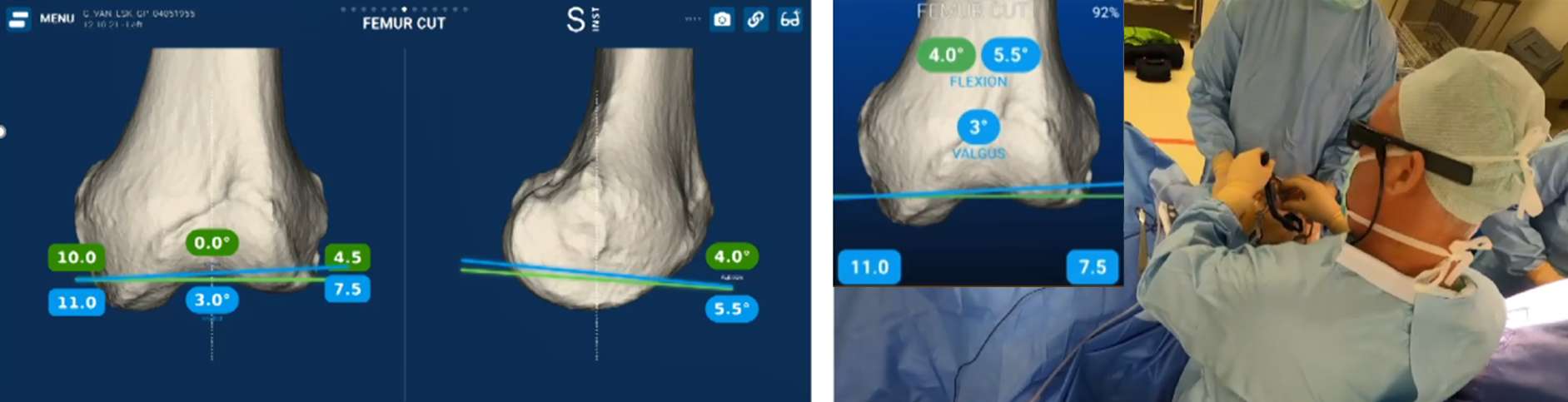
Figure 2#8801
Effect of Long-Term Gait Loading on a Piezoelectric Joint Load Sensor for Total Knee Arthroplasty
Brandon Hines - Tennessee Technological University - Cookeville, United States of America
Brent Lanting - London Health Sciences Centre - London, Canada
Ryan Willing - Western University - London, Canada
*Steven Anton - Tennessee Technological University - Cookeville, USA
*Email: santon@tntech.edu
Introduction: Smart devices have become ubiquitous to our daily lives, including smart implants which have built in sensing and communication circuitry for measuring implant performance data in vivo. The requirement for a battery may be a limitation for such devices, as they require additional space and put a limit on the useful life of the device. Energy-harvesting smart implants have been proposed as a solution to this concern, with examples employing triboelectric and piezoelectric energy harvesting and sensing as a means for self-powering. The long-term behavior of these systems when subjected to physiologically-relevant loading, however, has not been thoroughly investigated. The objective of this study is to evaluate the long-term performance of a piezoelectric, self-powered, load-sensing total knee arthroplasty implant, measured during 10,000 cycles of simulated gait using a joint motion simulator.
Methods: The smart implant evaluated in this study is a Stryker Triathlon size 3 with a modified polyethylene insert to include 6 piezoelectric transducers capable of measuring compartmental forces and contact points on the medial and lateral condyle. The locations of the transducers were selected by performing a parametric study to identify the best configuration for the purposes of sensing. The implant femoral and tibial components were cemented to femoral and tibial actuators of a six degrees of freedom joint motion simulator (AMTI VIVO, Watertown, MA) in a neutral alignment. The joint motion simulator prescribed femoral flexion, applied joint contact forces (anterior/posterior, medial/lateral, compression) and joint torques (abduction/adduction, internal/external) representative of those acting across the knee of an average 75 kg TKA patient, based on the Orthoload dataset (https://orthoload.com/). The simulator repeated these loads for 10,000 cycles at a rate of 1.2 seconds/cycle, sampling 10 cycles of joint loads and kinematics every 100 cycles, at 1000 samples/second. The voltage across each piezoelectric sensor was sampled using 2 16-bit NI 9215 cards at 1000 samples/second, the final 9,000 cycles. The experimental setup is shown in Figure 1.
Results: The study demonstrated the ability of the system to survive over a long-term gait loading scenario. The signal measured from the piezoelectric transducers exhibited some drift over the duration of the gait cycle as evident in Figure 2. The force profile measured by the piezoelectric transducers maintains the appearance of a realistic load profile over the duration of the study. The drift in the sensing signal could be evidence of deformation in the polyethylene insert as a result of the modifications made for the transducers. This could also be the result of some minor drift in the VIVO over the duration of the study.
Conclusion: This study indicates that piezo electric transducers embedded in the polyethylene component of a total knee replacement are capable of surviving long-term cyclic loading of a realistic gait loading profile. The signal maintained the appearance of a reasonable gait loading profile, but it did exhibit some drift over the duration of the study. This warrants further investigation to discover the possible causes of the drift in the signal.
Figures
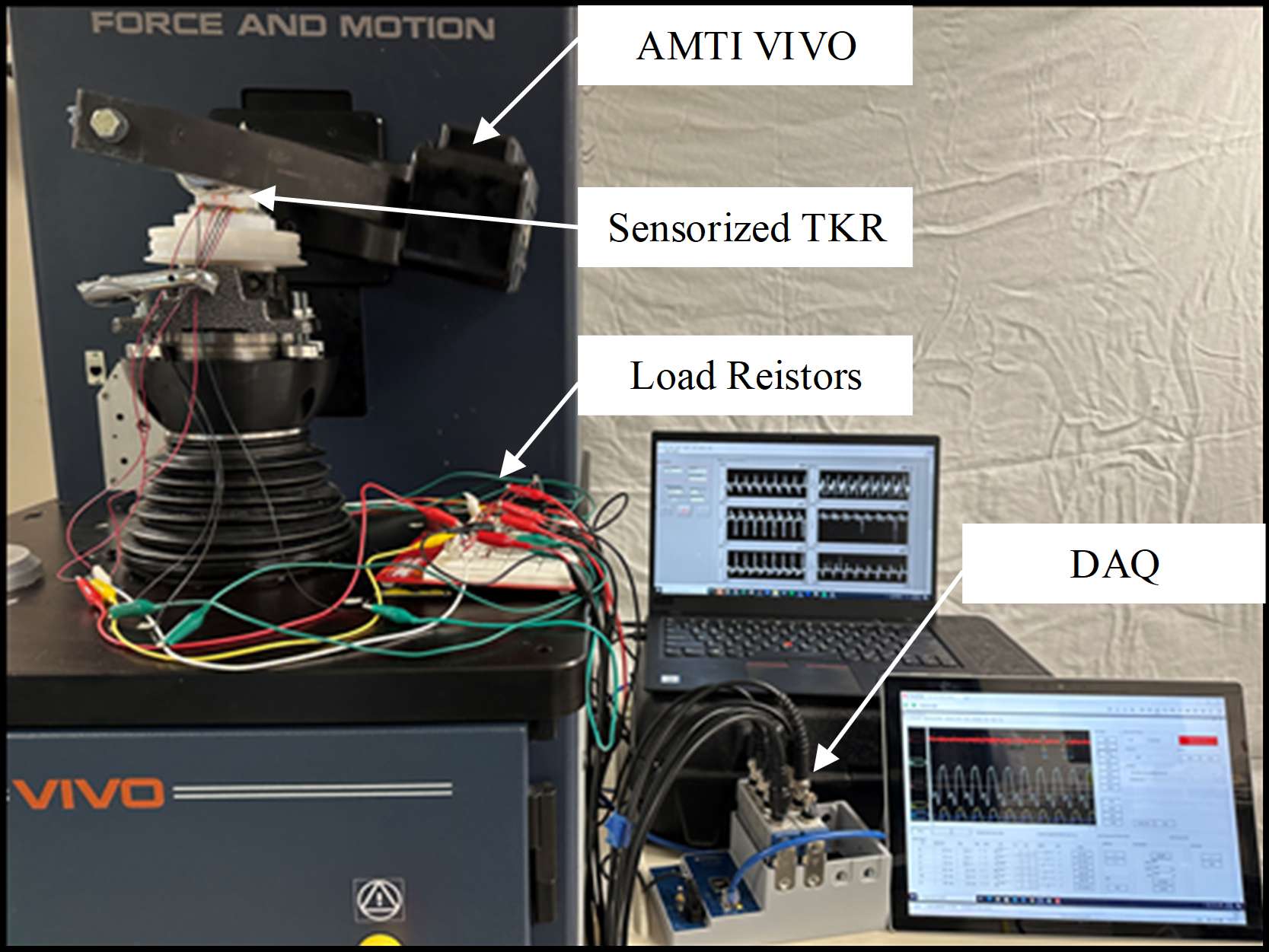
Figure 1
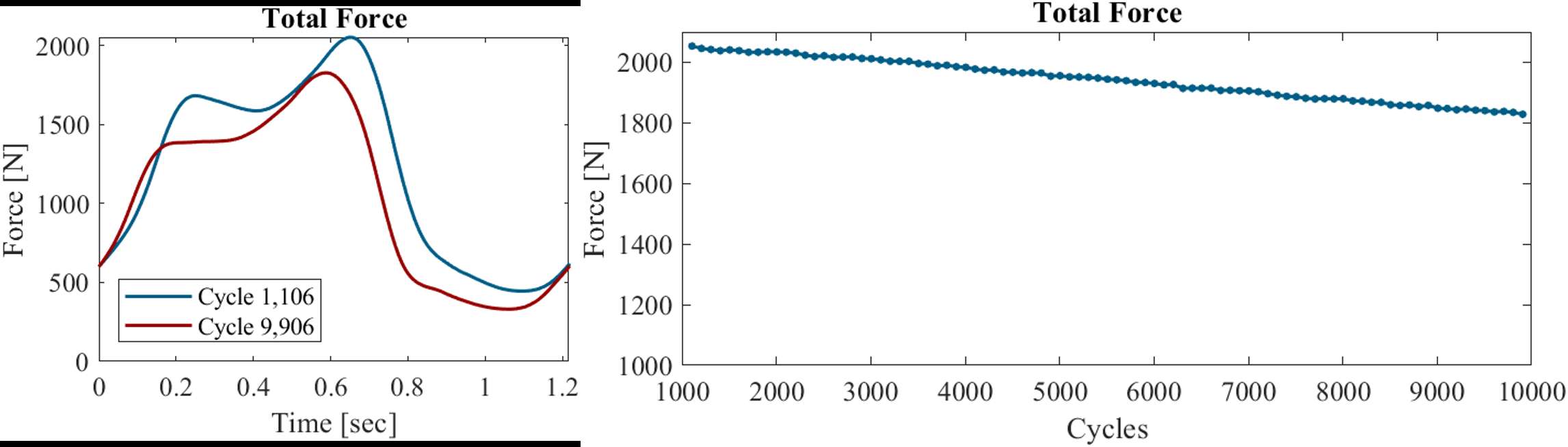
Figure 2#8550
Design and Virtual Evaluation of a Novel, Modular, Multi-Axis Contact Force Sensor for Arthroplasty
*Joshua Roth - University of Wisconsin-Madison - Madison, USA
Jason DePhillips - University of Wisconsin-Madison - Madison, USA
*Email: roth@ortho.wisc.edu
Understanding contact kinetics and kinematics of a joint following arthroplasty is critical to both intraoperative soft tissue balancing and preclinical development and evaluation of innovative implants and surgical techniques. For example, in the knee abnormal contact is associated with accelerated implant wear and decreased patient satisfaction (e.g., Kretzer 2010; Golladay 2019). Existing instrumented implants are unable to measure forces in all three directions and the center of pressure in each compartment and are designed for a specific implant design (e.g., Roth 2017; VERASENSE; Kirking 2006). Accordingly, our objective was to perform a thorough evaluation of the design and calibration approaches of our novel, modular, multi-axis contact force sensor for arthroplasty using a probabilistic finite element model (FEM). We used the tibiofemoral joint as a model system for this study, but our sensor is also applicable for the ankle and spine without modification, and the patellofemoral joint and shoulder with minor modifications to the sensor footprint.
To evaluate the design and calibration approaches, we developed an FEM of our modular, multi-axis contact force sensor. To mimic the strain gages in our design, we added 24 strain probes to the FEM where strain gages will be mounted (Figure 1). To emulate the complex loading of the knee during daily activities, we applied loads randomly sampled from the expected ranges of in vivo compression forces (0 to 4000 N) and shear forces (0 to 360 N) [5] at 80 locations to generate a dataset of 40,000 simulations (Figure 2). We extracted the 24 strain values from each simulation, and computed the output voltage assuming the gages were wired in quarter, half, and full Wheatstone bridges and added representative electrical noise (Figure 2). Finally, we virtually calibrated the multi-axis load/center of pressure-voltage relationships for all three bridge configurations using three methods: matrix algebra (MA), multiple linear regression with interactions (LR), and a neural network (NN). We randomly partitioned the virtual voltage datasets into a 70%/30% split for training/testing (28,000 train/12,000 test points) for all three methods. For each bridge configuration-calibration method combination, we computed the root-mean square (RMSE) errors of the forces in all three directions (compression-distraction, C-D; medial-lateral, M-L; anterior-posterior, A-P), and the M-L and A-P centers of pressure (CoP).
The NN method produced the lowest RMSE across bridge configurations (Figure 3). The quarter bridge-NN combination had slightly lower errors than the half bridge-NN combination (e.g., 11.9 N and 18.7 N, respectively for the C-D force). The greatest reductions of errors using the NN method were found in the C-D force measurements (41-55% depending on bridge configuration).
Our key finding was that, using the NN method with strain gages wired in either half or quarter bridges, our sensor is capable of achieving force errors between 0.3% and 4.0%, and CoP errors below 1.4 mm, which outperforms existing sensors. Our sensor will enhance the understanding of tibiofemoral contact kinetics and kinematics, enhance soft tissue balancing, and provide crucial data for the development of innovative implants and surgical treatments for arthroplasty applications.
Figures
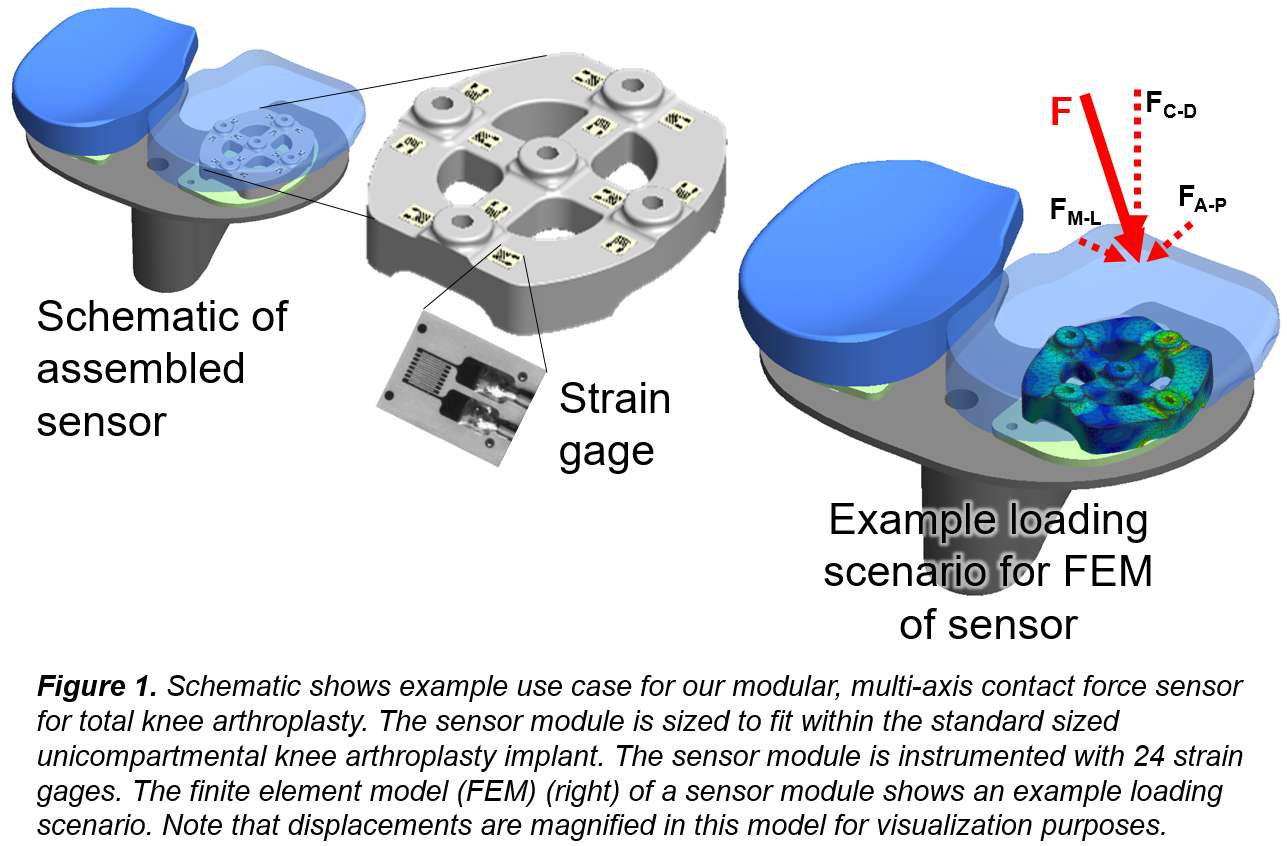
Figure 1
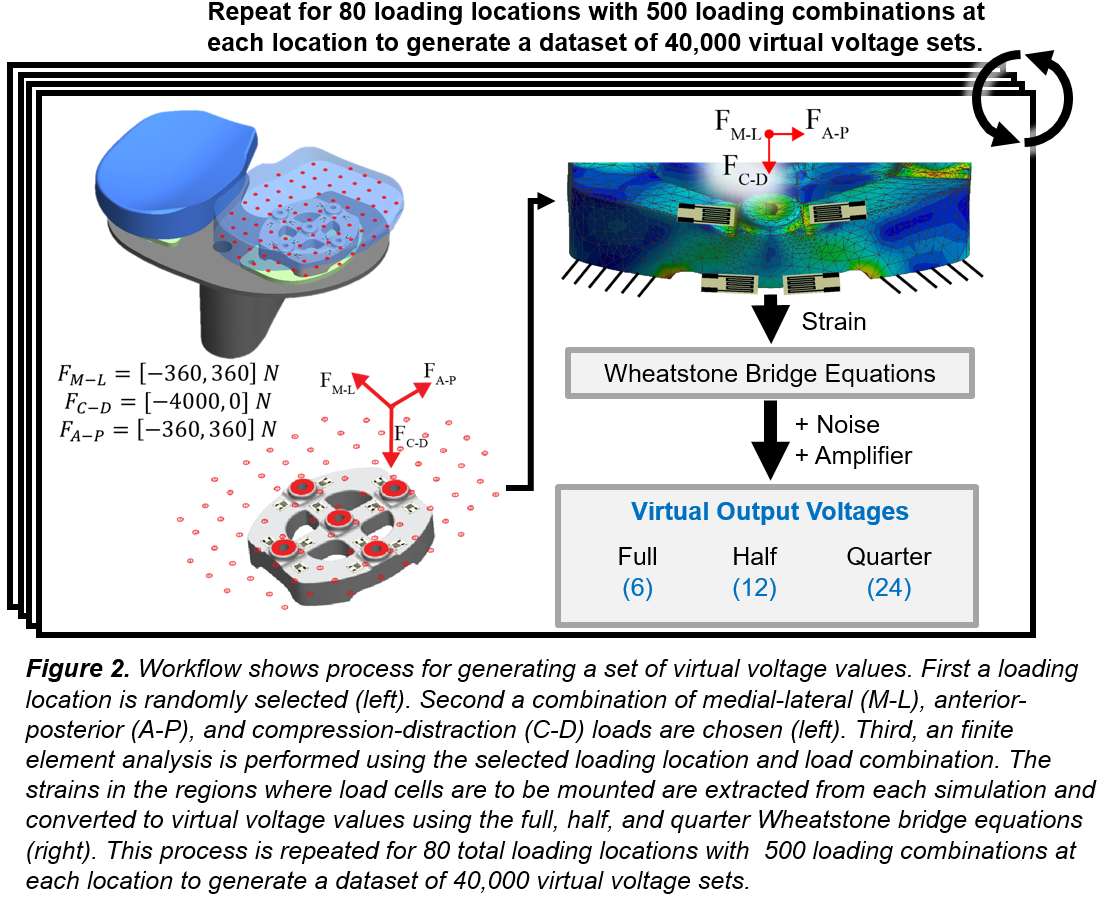
Figure 2
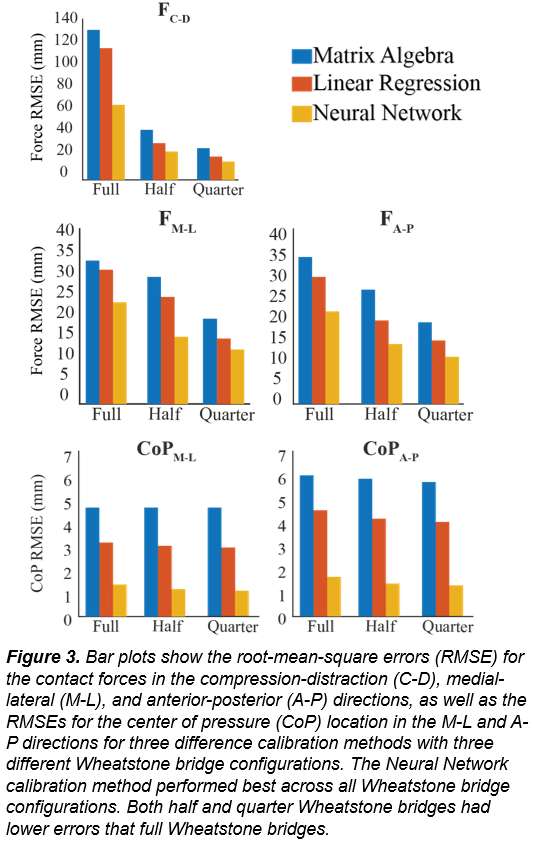
Figure 3#8206
Computational Design Optimization of the Bearing Geometries of a Cervical Total Disc Replacement
*Lucia Kölle - ETH Zürich - Zürich, Switzerland
Markus Flohr - CeramTec GmbH - Plochingen, Germany
S J Ferguson - Zurich, Switzerland
Benedikt Helgason - ETH Zurich - Zurich, Switzerland
*Email: lkoelle@ethz.ch
Introduction
Over 300 million people suffered from neck pain for more than three months in 2015 (1). When conservative treatment of neck pain fails for more than 6 weeks, surgery may be considered. One option is arthroplasty with a Total Disc Replacement (TDR). While TDRs generally perform well clinically, considerable reoperation rates have been reported. A meta-analysis found that within 7 years follow-up, 5.2% of patients had reoperation at the index level and 4.3% at adjacent levels (2).
To reduce complications connected to unphysiological postoperative biomechanics, TDRs should replicate the biomechanical functions of the biological structures they replace and be optimized for a specific spinal level. Therefore, this study aims to design TDR bearing geometries that replicate the moment-rotation curve of human C6/C7 anterior columns during coupled flexion/extension-anterior/posterior translation motions.
Methods
The design concept of the bearing geometries combines toric, cylindrical and spherical shapes. The optimization modified the geometry by altering the defined variables (Figure 1). A design optimization based on finite element (FE) simulations was set up (Figure 2) using LS-OPT V7.0.0, LS-PrePost V4.6.4, and LS-Dyna R13.0 (all: LSTC, USA) on a computer cluster with 60 cores and 320 GB RAM.
The design objective was to minimize the mean square error (MSE) between the TDR’s moment-rotation curve derived from the FE analysis, and an ex vivo measured moment-rotation response of C6/C7 anterior columns replaying their own pre-dissection kinematics (3). In the optimization, movement based on in vivo instantaneous centre of rotation data (4) and a load profile based on in silico data (5) were applied to the vertebra superior of the TDR. Manufacturing and anatomical constraints were imposed as bounds and a stress constraint prevented material failure. We used a metamodel based optimization that was sequential with domain reduction.
The implant material BIOLOX®delta (CeramTec GmbH, Germany), a zirconia toughened alumina ceramic, with decades of clinical use in hip replacements, was chosen.
Results
The design optimization took 5.5 hours and achieved a 99% reduction of the design objective (MSE) compared to the baseline design. In a coupled motion from neutral to flexion to extension, the moment-rotation curve exhibits nonlinearity and the behaviour of the optimized design matches that of the objective considerably better than the baseline design does (Figure 3).
Conclusion
This study shows that it is possible to use computational design optimization to generate bearing geometries for a TDR that replicate the moment-rotation curve of an anterior column during coupled motion. Using this approach, we expect that a level-specific design replicating the biomechanics of the biological structures it replaces, would reduce reoperation rates. The presented methodology could be adjusted for different design objectives and different applications.
References:
1. Hurwitz et al., Eur. Spine J., 27, 2018.
2. Badhiwala et al., J. Spine Surg., 6, 2020.
3. Hartman et al., Eur. Spine, J. 25, 2016.
4. Anderst et al., Spine., 38, 2013.
5. Bayoglu et al., Med. Eng. Phys., 68, 2019.
Acknowledgements:
Funding: European Union’s Horizon 2020 programme: Marie Sk?odowska-Curie grant agreement No 812765.
Figures
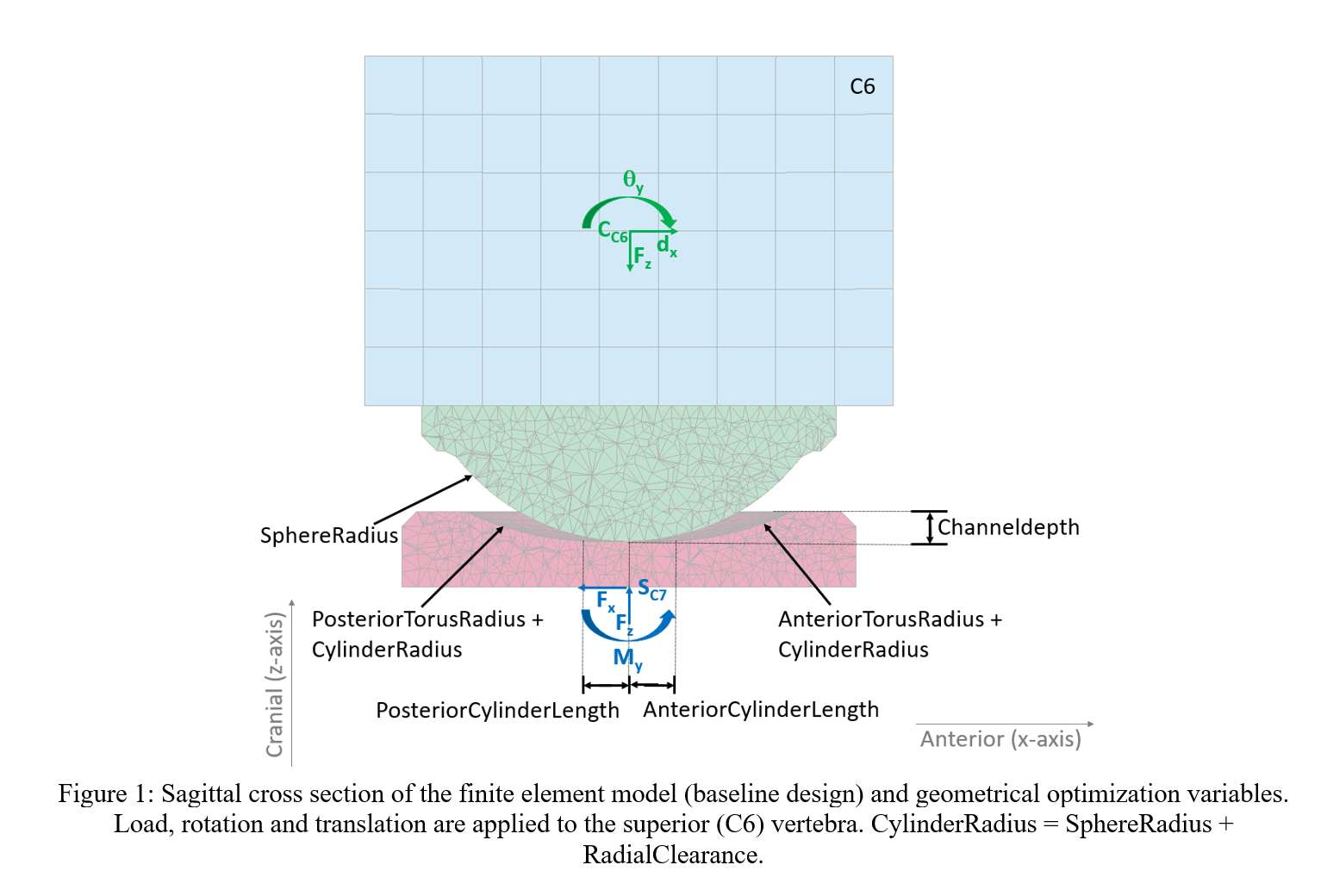
Figure 1
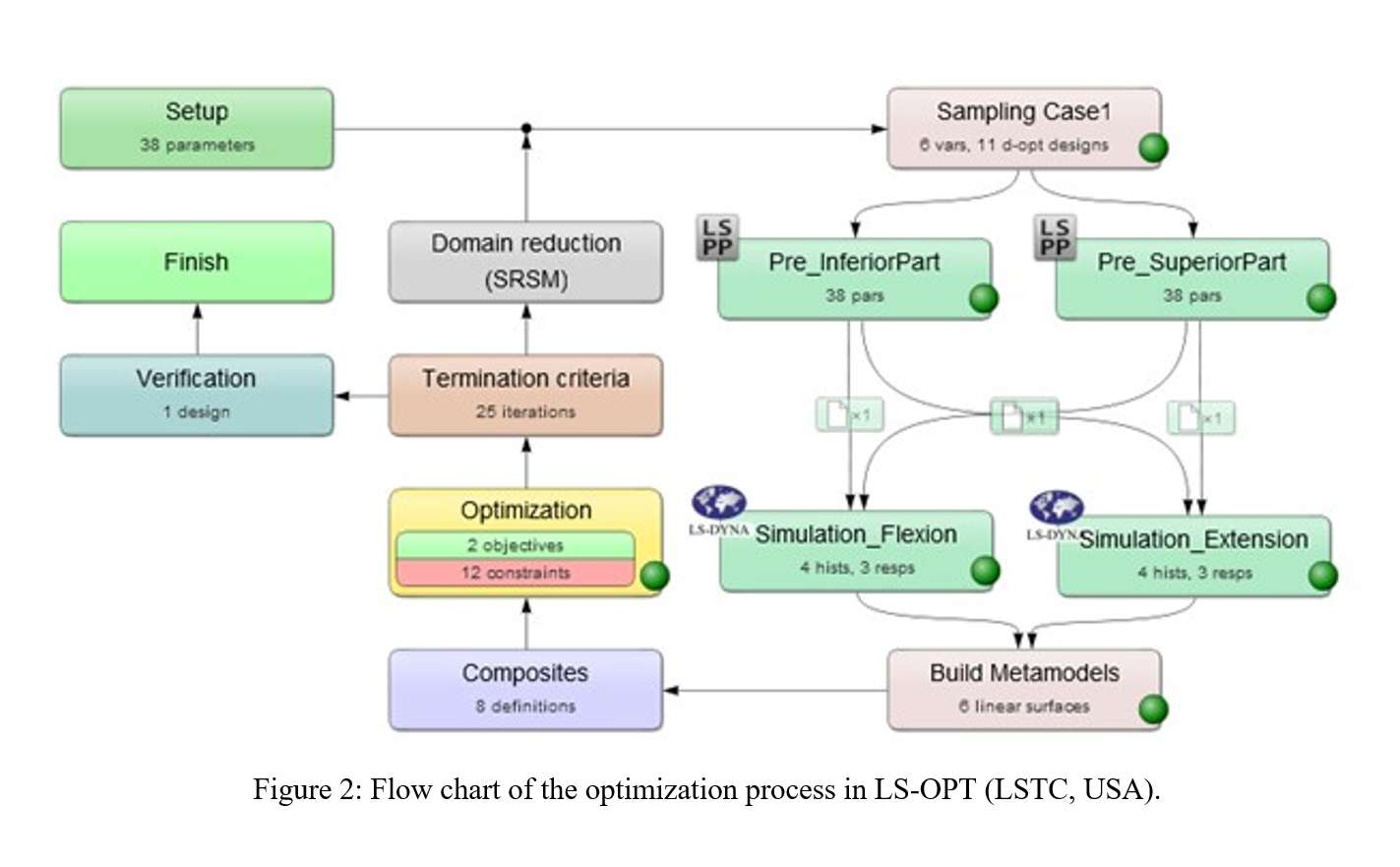
Figure 2
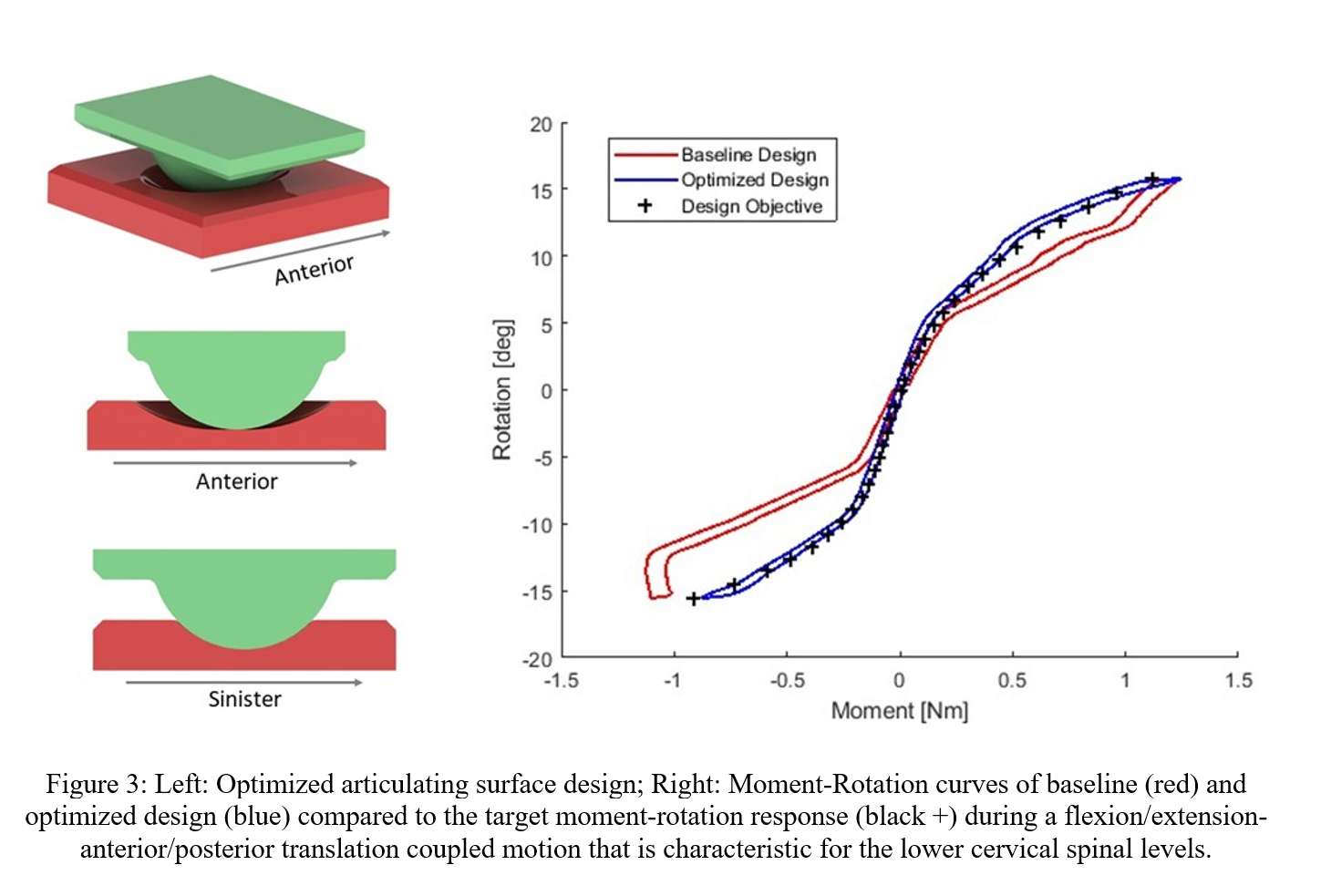
Figure 3#8274
Impact of Design Factors on Implant Stability in Cementless Hip Revision Arthroplasty: Experimental-Computational Studies Evaluating Different Implant Designs
Huizhou Yang - University of Denver - Denver, USA
William Fugit - Smith + Nephew - Memphis, USA
Paul Rullkoetter - University of Denver - Denver, USA
*Chadd Clary - University of Denver - Denver, USA
*Email: chadd.clary@du.edu
Introduction
Initial stability of cementless femoral stems after hip revision arthroplasty is crucial to restore hip biomechanics and ensure durable fixation. Various implant designs utilize different features to improve this stability in patients with proximal femoral defects. This study aimed to develop an experimental-computational framework for evaluating the fixation stability of novel cementless hip revision implants during activities of daily living.
Methods
Two cementless revision femoral stems were evaluated: RECLAIM™ Modular is a modular femoral system with a press-fit, splined, and tapered distal stem, RECLAIM Monobloc is a monobloc version of the same implant with additional splines between the primary splines, referred to as RECLAIM Advanced Splines (RAS), for enhancing cortical contact (Fig.1a). Each leg of a cadaveric lower limb specimen was broached to size for a primary implant to simulate a femoral defect, reamed, then implanted with RECLAIM Modular on one side and RECLAIM Monobloc on the contralateral side. The denuded femurs were potted into fixtures and mounted into an AMTI VIVOTM to simulate gait and stair descent (Fig.1b). Experimental boundary conditions were derived from OrthoLoad database (force-control for anterior-posterior, medial-lateral, and superior-inferior degrees-of-freedom). Relative displacements between the proximal exposed implant-bone interface were recorded using a digital image correlation system.
Finite element models of the specimen were developed in Abaqus/Standard from computed tomography scans (Fig.1b). Implant alignments were reconstructed using surface models of the implant and bone obtained from optical scans. The models were loaded with the boundary conditions from the experiment. The proximal implant-bone relative displacements were predicted and compared with measurements. After validation, virtual defects were added to the bone models to simulate different levels of bone loss (Paprosky Classifications Type-I, -II, and -IIIA) (Fig.1c). The two designs were then implanted into the same femur models and evaluated using the same activity loading. Implant-bone interface micromotions were investigated and compared. The models were additionally tested by applying a 10Nm torque and 1500N axial force to the implants to evaluate the axial and rotational stability, respectively. The corresponding implant rotation/subsidence and maximum bone strain energy density (SED) across the femurs were compared. Uncertainty bounds for the predictions were generated by perturbing the interference fit on the primary splines from 0-100µm.
Results
The average root-mean-square differences and corresponding correlations between the measured and predicted proximal implant-bone displacements were 7.9µm(7.0%)/0.02°(7.6%) and 0.93, respectively (Fig.2). For all evaluated bone defect types, the peak interface micromotions predicted for RECLAIM Modular was on average of 17.4µm(28.4%) larger than those predictions for RECLAIM Monobloc (Fig.3a). For rotational and axial stability tests, the rotation/subsidence of RECLAIM Monobloc were 15.4%/28.2% smaller than RECLAIM Modular (Fig.3b). The corresponding maximum SED across the femur was 47.4%/89.9% smaller for RECLAIM Monobloc (Fig.3c).
Conclusion
Overall, RECLAIM Monobloc showed better stability than RECLAIM Modular, especially in the case of Type-III defects. This was because RECLAIM Monobloc had relatively longer splines due to the monobloc design and RAS between the primary splines, which increased contact area with the bone, distributed the contact forces, and thereby reduced the implant rotation and subsidence motions.
Figures
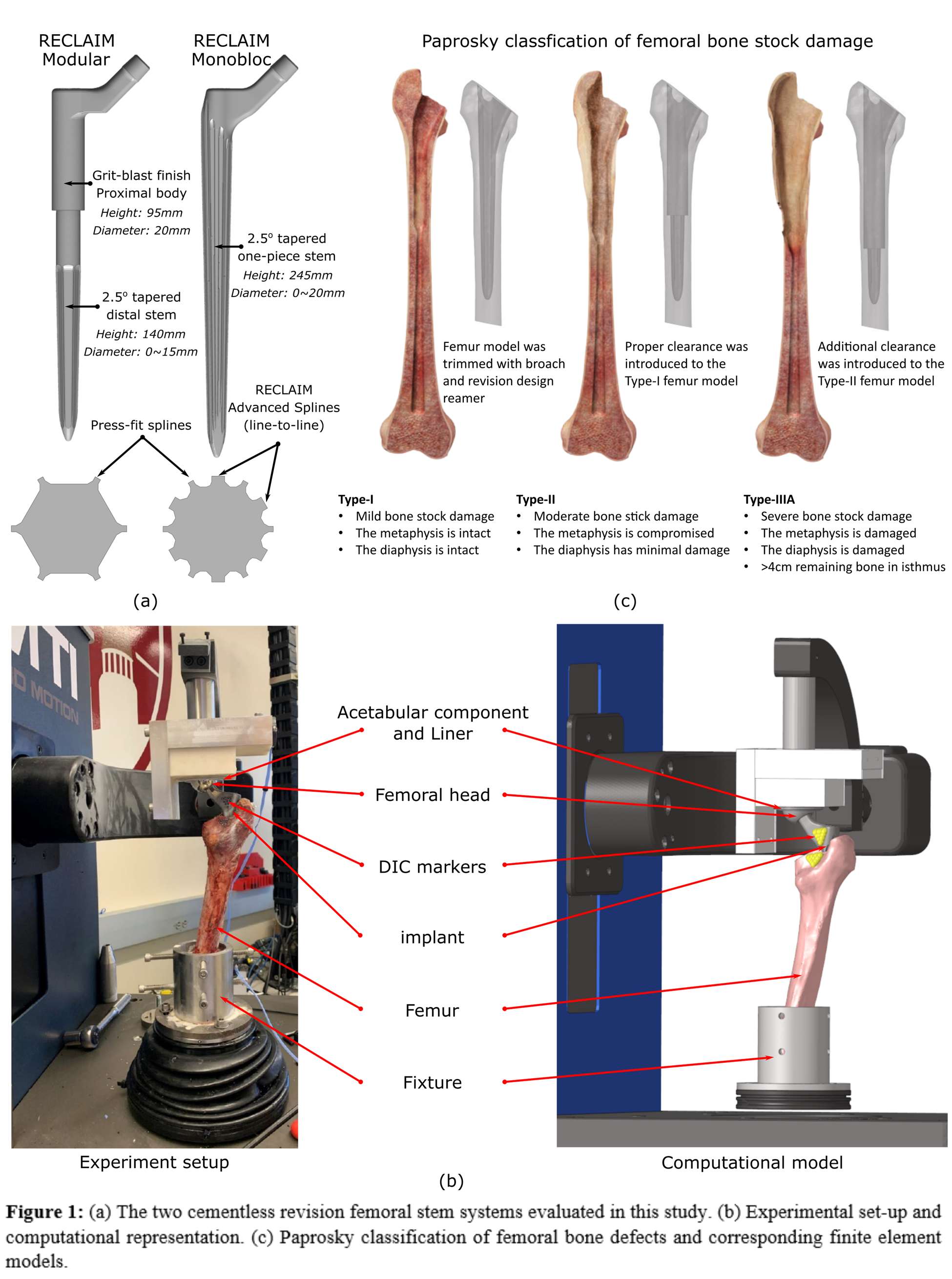
Figure 1
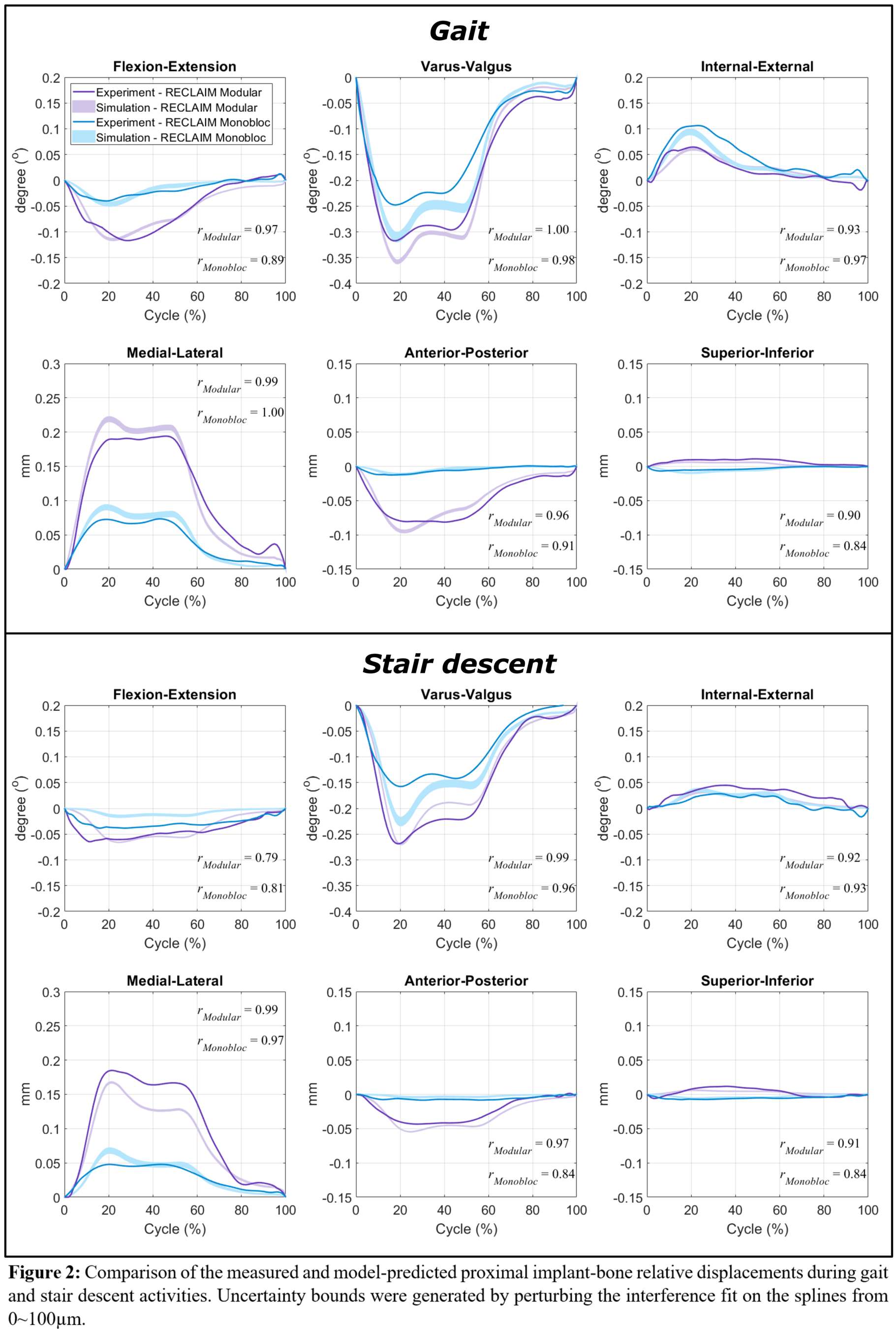
Figure 2
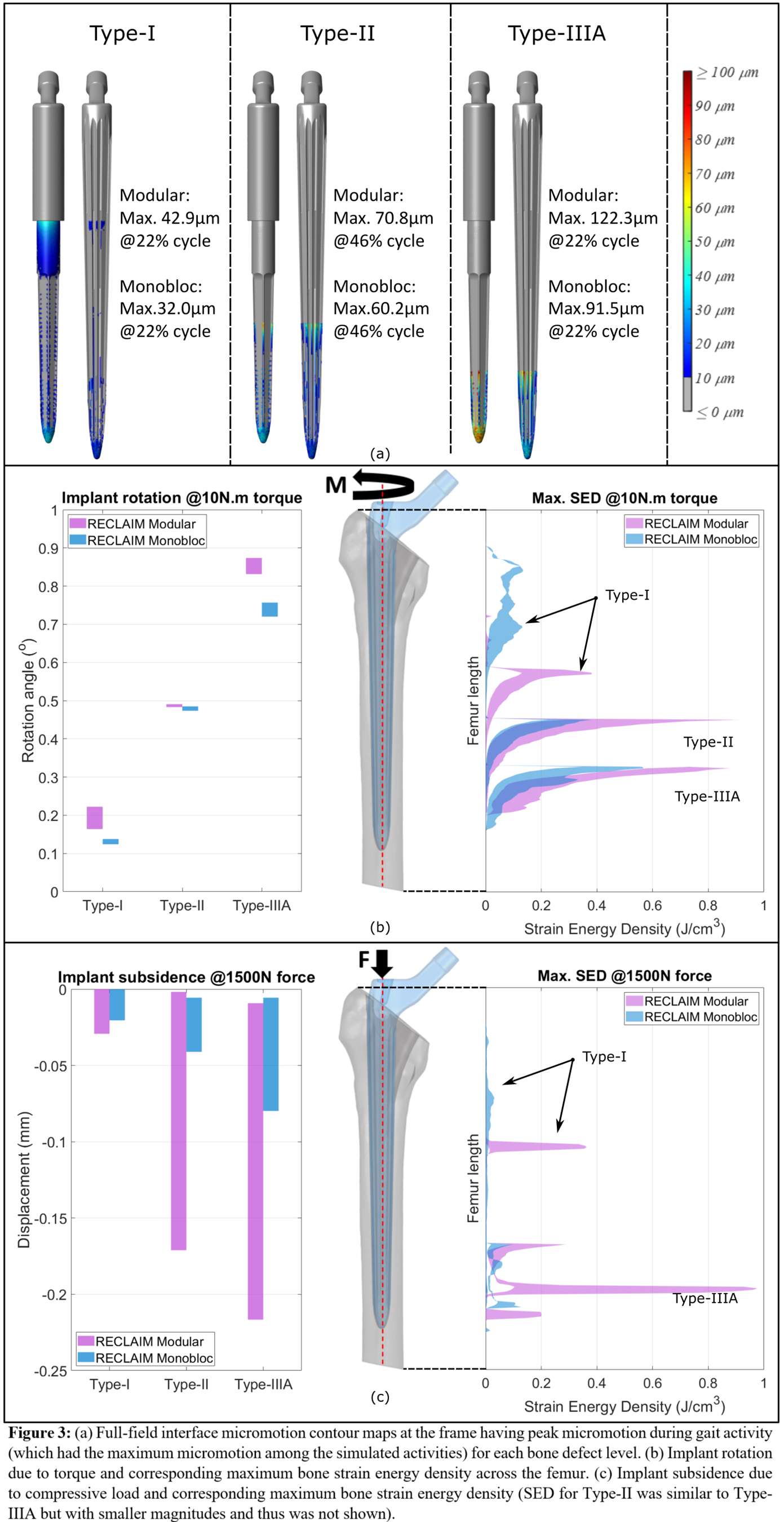
Figure 3#8141
How Do Small Design Changes Affect the Primary Stability of a Cementless Stem Design?
*Katja Glismann - TUHH Hamburg University of Technology - Hamburg, Germany
Tobias Konow - TUHH Hamburg University of Technology - Hamburg, Germany
Frank Lampe - Hamburg University of Applied Science
Gerd Huber - TUHH Hamburg University of Technology - Hamburg, Germany
Michael Morlock - TUHH Hamburg University of Technology - Hamburg, Germany
*Email: katja.glismann@tuhh.de
INTRODUCTION
Adequate primary stability, defined by minimal movement and rotation between the bone and a cementless stem, is needed to facilitate successful osseointegration and prevent the need for revision surgery due to implant loosening [1]. Loosening of the femoral component is observed more frequently in Dorr type A femurs, since small stem sizes appear stable to the surgeon due to distal capture of the stem tip while no press-fit in the proximal part is yet achieved [2]. Preoperative planning can prevent incorrect sizing by demonstrating that intra-operative canal reaming to widen the cavity for a larger stem size is needed. Canal reaming prolongs the operating time, requires more surgical equipment, and thus increases the risk of infection. The Emphasys™ stem (DePuy Synthes, US) was developed with changes to the broach design (different teeth, Fig.1) and minor design modifications to the femoral stem design Corail™ (DePuy Synthes, US, Fig.1) to reduce the necessity for canal reaming. This study compares relative motion and implant subsidence between these two stem designs as a measure of primary stability.
MATERIALS AND METHODS
Six pairs of fresh-frozen femurs (male, 54.7±11.2 years, two pairs of each Dorr Type), were implanted following templating with Emphasys™ and Corail™ stems on either side of a pair by an experienced surgeon using the respective instrumentation for each stem (Fig.1). CT- and laser-scans were taken pre- and post-implantation for subsequent contact analysis (Polyworks|Inspektor 19, InnovMetric Software, CA, Fig.2a). The implanted stems were subjected to cyclic loading to mimic walking (1Hz, 600 cycles 80-800N, 600 cycles 80-1600N, MTS, US). Digital image correlation was used to determine relative micromotion and rotations (25Hz, GOM, Germany). Implant subsidence and total rotation were calculated. Statistical analysis was performed using parametric paired T-Tests and non-parametric Kruskal-Wallis tests (α-level=0.05; SPSS 26, US).
RESULTS
A trend towards a larger bone-implant contact area was seen for Emphasys™ (p=0.07, power:0.3202, Fig.2b), pronounced in the posterior region of the stem (p=0.051, power:0.2777). While relative motions were quite similar for both designs at both force levels (p=0.133 and p=0.173), relative rotations were significantly higher for the Corail™ (p=0.028, Fig.3a). Stem subsidence was smaller for the Emphasys™ (p=0.028, Fig.3b) and a trend was observed towards lower rotation as well (p=0.075, power:0.1414). Rotation increased significantly with subsidence for both designs (Emphasys™: R²=0.964, p<0.001, Corail™: R²=0.889, p=0.005, Fig.3c).
DISCUSSION & CONCLUSION
The small design changes of broach and stem resulted in lower relative rotations and subsidence, suggesting a positive effect on the primary stability. This might be due to the sharper teeth distally on the Emphasys™ broaches and the trend towards a larger bone contact area of the Emphasys™ stem possibly due to the stem design changes. Clinical relevance of these differences has to be demonstrated clinically.
Present pre-clinical testing methods are able to identify the effect of small design changes, which affect the primary stability. This study could be used to validate Finite-Element-Model calculations investigating the effect of these changes.
Financial support by DePuy Synthes is kindly acknowledged.
REFERENCES
[1]Kienapfel et al., JoA:14,1999
[2]Magill et al., AT:6-1,2020
Figures
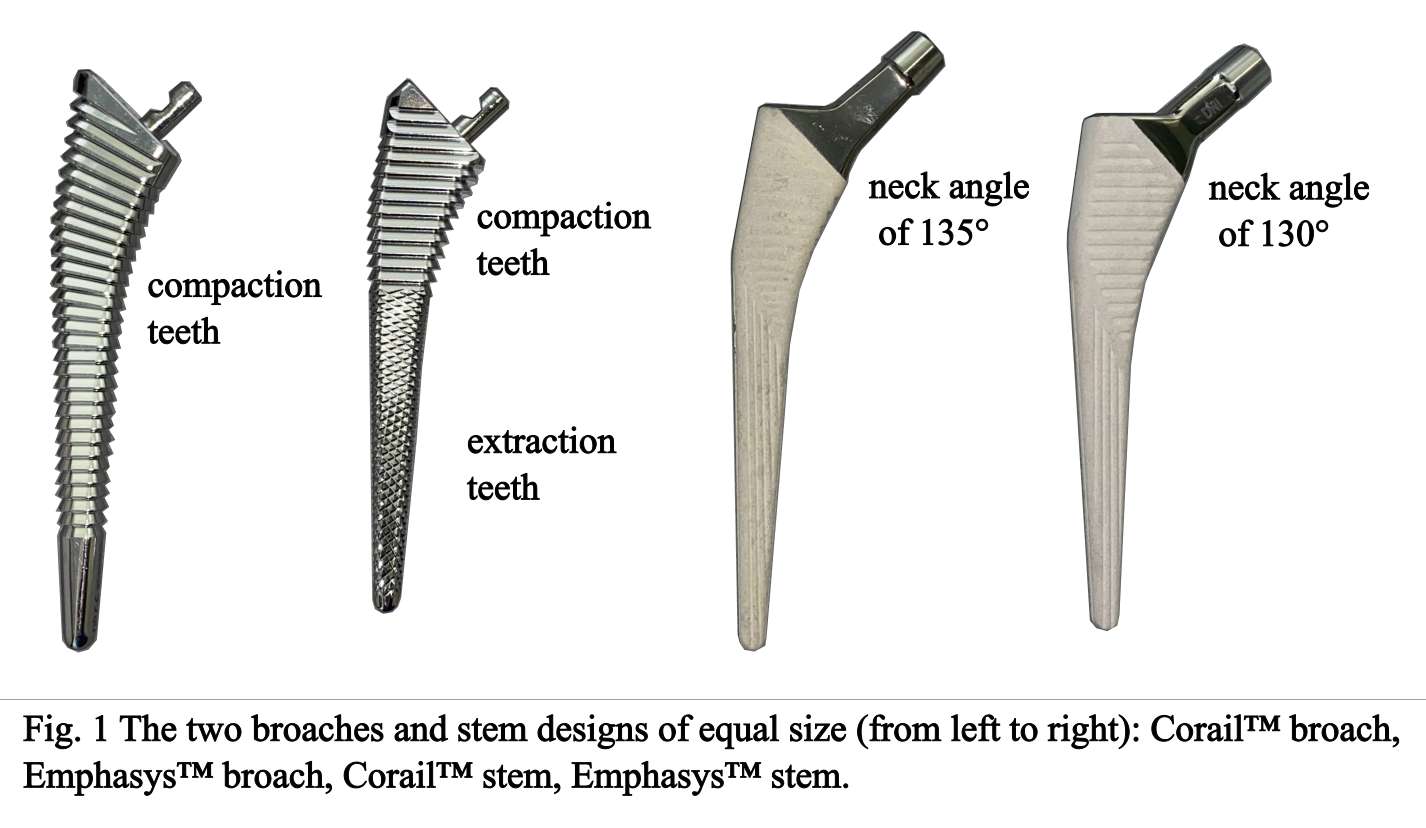
Figure 1
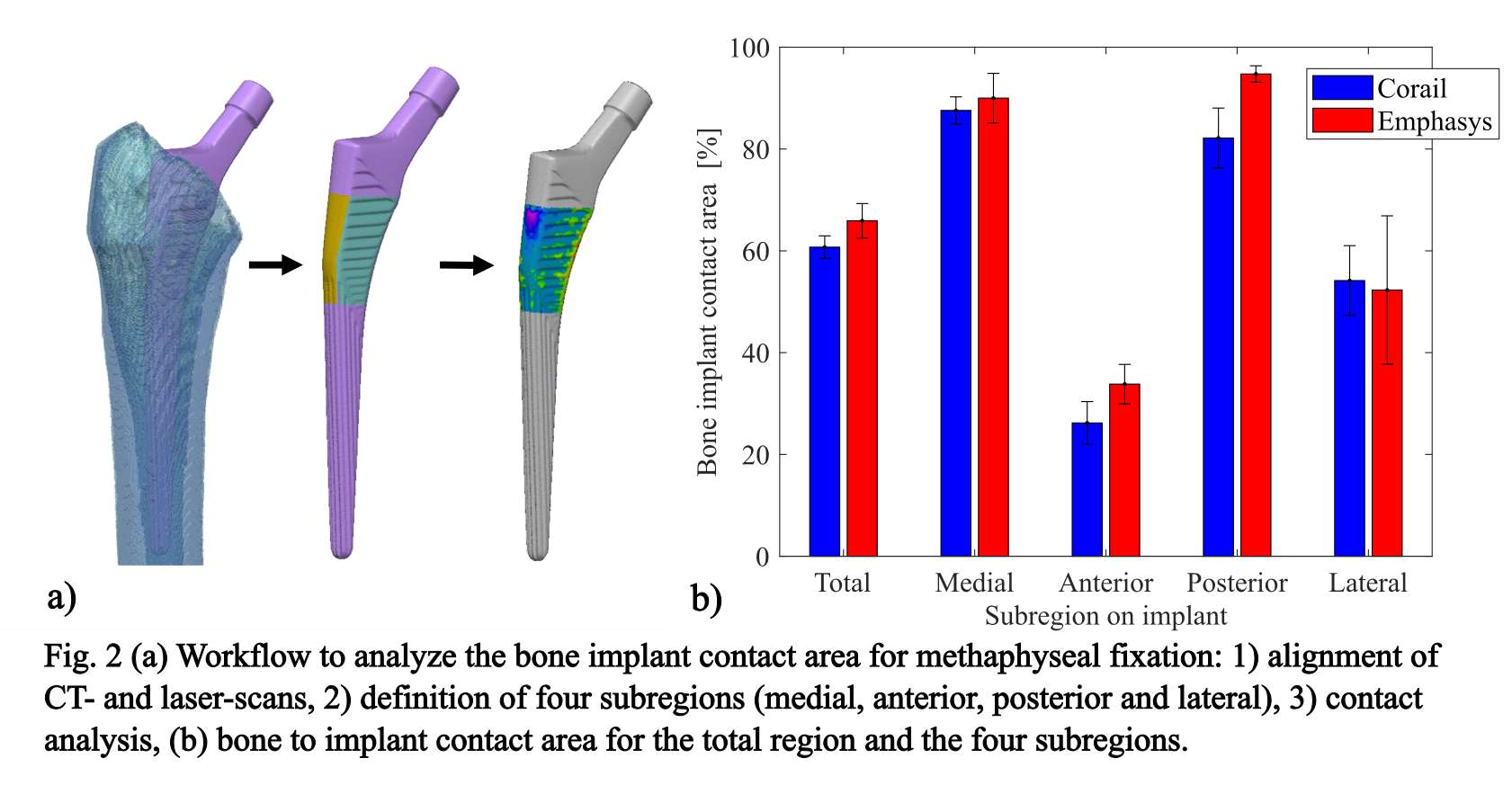
Figure 2
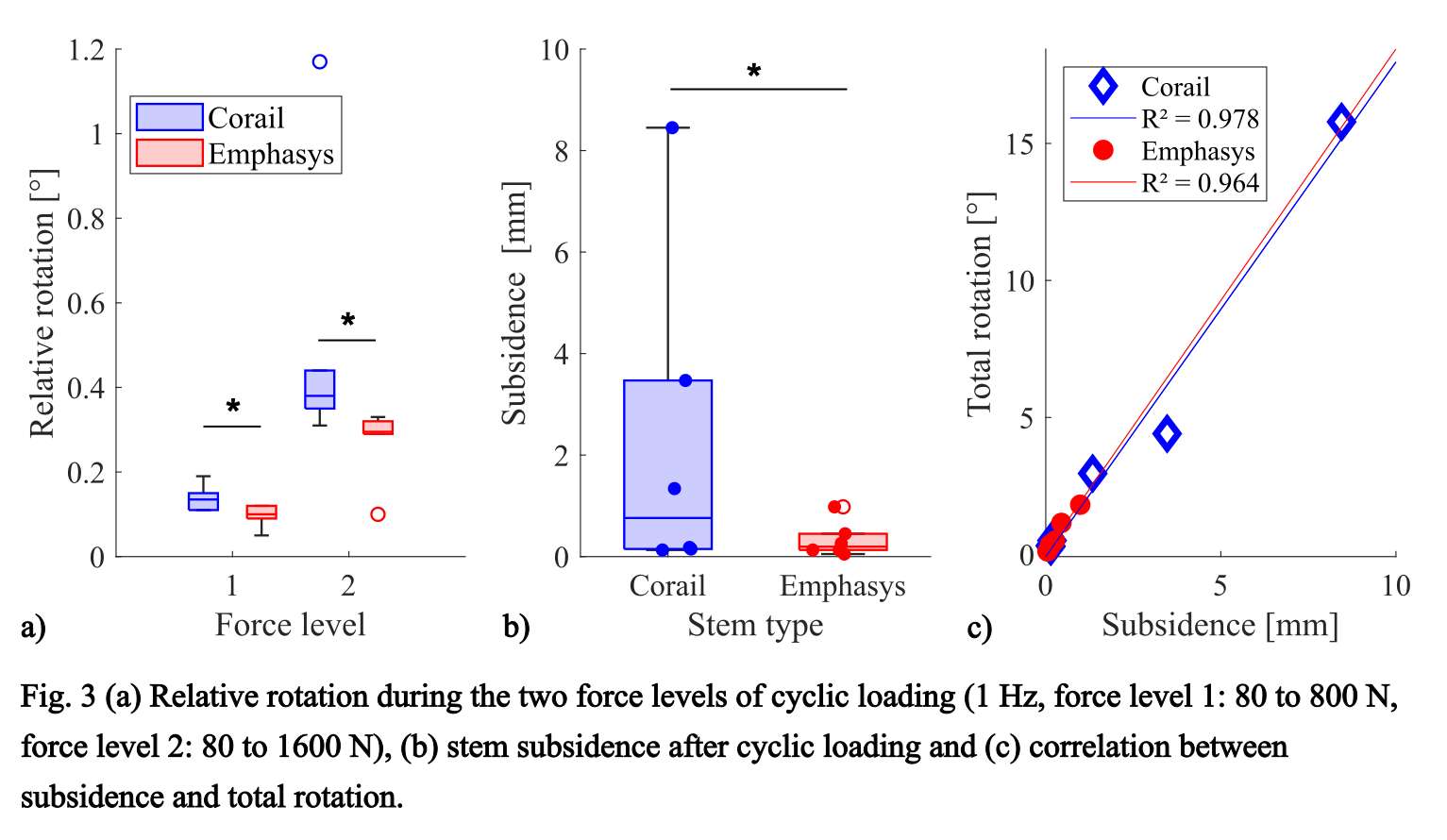
Figure 3#8715
Comparing Outcomes of Inlay and Onlay Components in Patellofemoral Arthroplasty
*Jonathan Katzman - NYU Langone - New York, United States of America
Weston Buehring - SUNY Downstate College of Medicine - New York, United States of America
Patrick Connolly - NYU Langone Health - New York, USA
Ran Schwarzkopf - NYU Langone Medical Center Hospital for Joint Diseases - New York, USA
Ivan Fernandez-Madrid - NYU Hospital for Joint Diseases - New York, USA
Casey Cardillo - NYU Orthopedic Hospital - New York, USA
*Email: jonathankatzman98@gmail.com
Introduction
Patellofemoral arthroplasty (PFA) has been shown to provide symptomatic improvement for isolated patellofemoral osteoarthritis. However, conflicting data exist regarding the optimal prosthetic design. Therefore, this study sought to compare clinical and patient-reported outcomes between inlay and onlay prostheses.
Methods
A single-center retrospective review found 237 knees in 211 patients which underwent PFA between 2011 and 2021. 53 cases were excluded from the final study cohort for having indications other than primary osteoarthritis or having less than one year of follow-up. Patients were grouped by prosthetic design and propensity score matching was utilized to address demographic differences between groups.
Results
In a matched analysis that yielded 63 inlay and 95 onlay prosthetic implants, no statistically significant differences in clinical outcomes were found between cohorts. Inlay implants trended slightly higher in rates of conversion to total knee arthroplasty (TKA) (11.1 vs. 8.4%; p=0.572) and manipulation under anesthesia (4.8 vs. 1.1%; p=0.146); however, the overall rate of reoperation (15.9 vs. 15.8%; p=0.989) and revision (11.1 vs. 10.5%; p=0.738) were found to be similar to the onlay prosthetic cohort. Kaplan-Meier survivorship analysis did not show differences between cohorts (p=0.549).
Patient-reported outcome measures (PROMs) favored inlay to onlay prosthesis with significantly higher mean Knee Injury and Osteoarthritis Outcome Score, Joint Replacement (KOOS, JR) scores at 12-weeks postoperative (63.38 vs. 45.39; p=0.031). Improvement in pain scores also favored the inlay prosthetic cohort with improvements in 12-week PROMIS Pain Intensity scores (-7.19 vs. -2.29; p=0.003) and 12-week PROMIS Pain Interference scores (-6.23 vs. -0.72; p<0.001).
Conclusion
This study found no significant differences in clinical outcomes between inlay and onlay prosthetic components. Higher KOOS, JR scores and improvement in pain scores does appear to favor the inlay prosthesis cohort.
#8523
The Keel Plays a Role in Periprosthetic Fractures of the Tibial Component in Cementless Unicompartmental Knee Replacement (UKR)
*AZMI RAHMAN - University of Oxford
David Heath - NDORMS, University of Oxford - Oxford, United Kingdom
Stephen Mellon - University of Oxford - Oxford, United Kingdom
David Murray - University of Oxford - Oxford, United Kingdom
*Email: azmissrahman@gmail.com
Introduction: In cementless UKR, primary fixation of the tibial component is achieved by press-fitting a keel (i.e. with interference) into a vertical slot cut into the proximal tibia. While the fixation of the keel into the tibial slot is essential to prevent implant loosening, it may also reduce the structural integrity of the proximal tibia, increasing the risk of peri-prosthetic fractures. Such fractures are rare in the western population (<1%), but relatively more common Asian populations (4-8%). The risk of fracture has been found to be 7 times more likely in very small tibias which receive the very small tibial components (Size AA and A), components which are not usually used in western populations. Despite these correlations, the mechanical and physiological processes underlying peri-prosthetic stress fractures are not known. This study explores the effect of keel-related features in fracture risk of these very small tibias.
Method: This in-vitro study compares the effect of keel and slot depth (standard vs 33% shallower vs no slot) and loading position (anterior/posterior gait range limits: mid-tibia vs 8mm posterior to midline) on fracture load and path. 3D-printed titanium tibial components were implanted in bone-analogue foam machined to a CT-reconstructed very small tibia which subsequently experienced a peri-prosthetic fracture. Implantation was performed using surgical instrumentation and technique. Implants were loaded to failure using a materials testing machine. Loads to fracture, and consequent bone fracture paths, were assessed.
Results: Introducing a standard slot reduces load to fracture by 50% (1421N vs 710N, p<0.0001). Press-fitting a standard keel into this slot further reduces load to fracture by 40% (710N vs 423N, p=0.0001). A shallower keel inserted into a shallower slot increases load to fracture substantially (shallow vs standard slot: 27% increase, 904N vs 710N p=0.0003, standard vs shallow slot+keel: 60% increase, 683N vs 423N p=0.0004). Standard-sized keel resulted in significantly more vertical fractures (standard 8.2° vs shallow 15.5° vs no keel 21°, degrees-to-vertical, p<0.0001). These vertical fractures are consistent with fracture patterns seen clinically. There was a small but significant difference in fracture load between loading positions with no keel (central 1422N vs posterior 1231N, 13% lower, p=0.0038), but this significance was lost when standard (11%, 423N vs 376N, p=0.330) and shallow (9%, 684N vs 624N, p=0.635) keels were inserted.
Conclusion: This study finds that the cementless tibial component keels contribute to peri-prosthetic tibial fractures: making a keel slot, and inserting a keel, both reduce the load needed to induce a fracture. These fractures also follow the path of the keel. As fractures are more common with very small tibial components (size A or AA), surgeons may wish to consider cementing these as this decreases the fracture risk. Smaller keel variants of these components may also decrease the fracture risk. In general, surgeons implanting cementless UKR tibias should avoid oversizing tibial components. They should also ensure keel slot is neither too long, by preventing the template from moving, nor too narrow, by ensuring a trial component can be inserted with finger pressure.
Figures

Figure 1
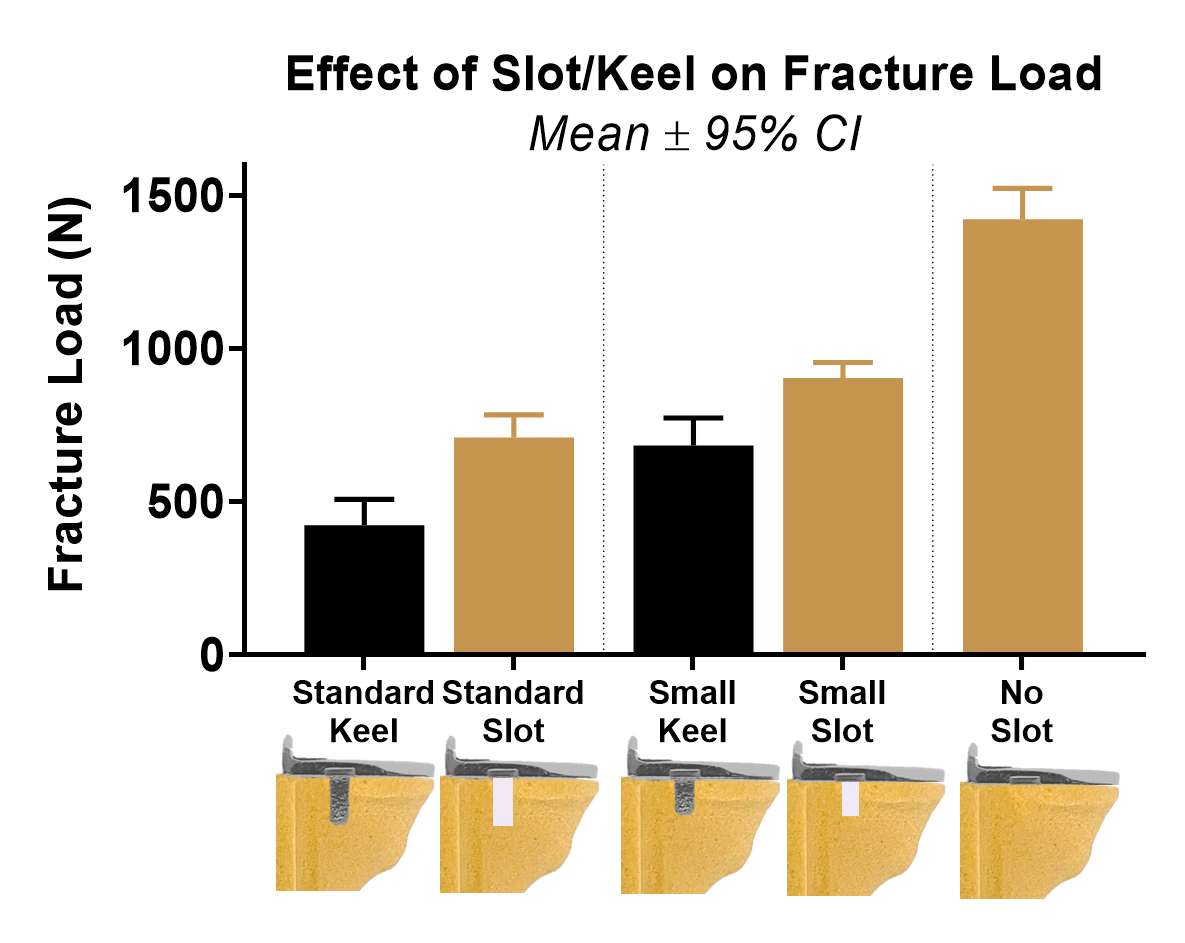
Figure 2
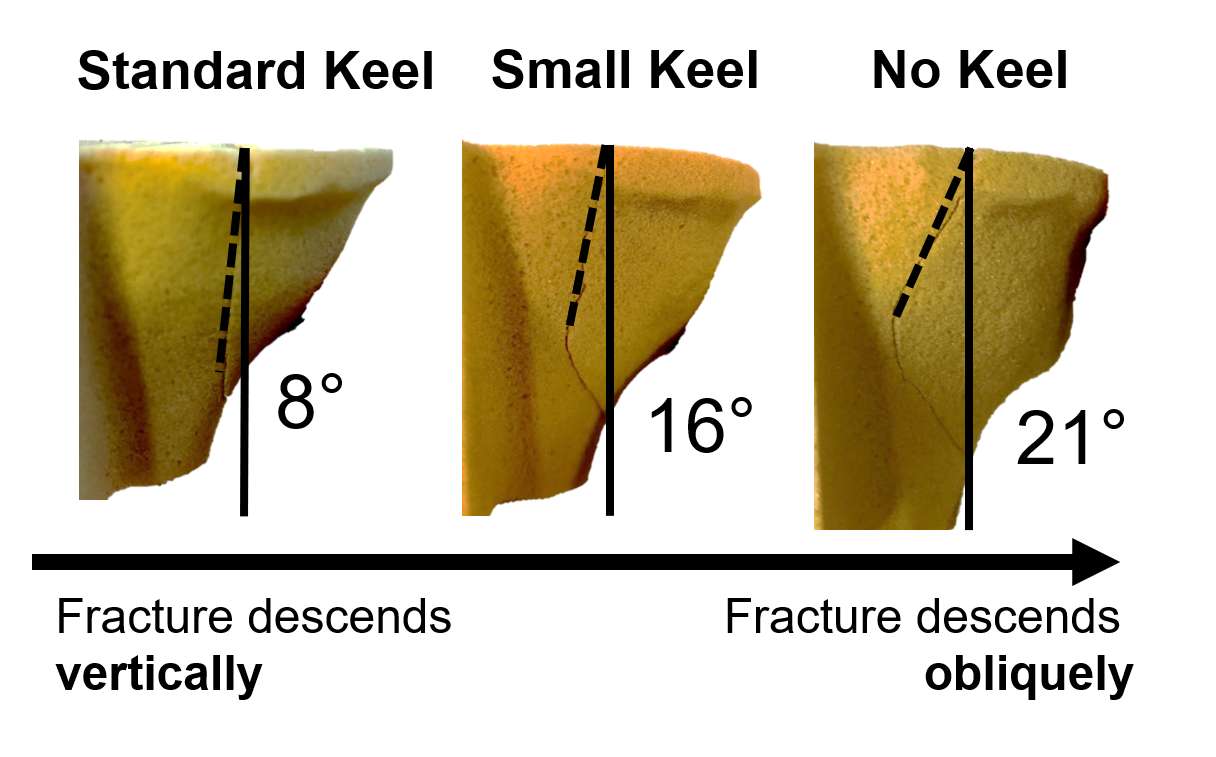
Figure 3#8251
Effect of Higher Patellofemoral Conformity on the Robustness of Patellar Biomechanics
*Thiago Amaral - Depuy Synthes - Fort Wayne, United States of America
Amit Mane - DePuy Synthes - Warsaw, USA
John Ryan Martin - Vanderbilt Health - Hendersonville, USA
Richard Komistek - The University of Tennessee - Knoxville, USA
*Email: tamaral2@its.jnj.com
Introduction: Implant malposition in TKA can lead to suboptimal functional performance1. Despite improvements in technique and technology, component placement variability in TKA continues to occur1. Patellofemoral (PF) congruency and implant position can impact the PF biomechanics. The degree of PF conformity could alter PF mechanics under such surgical variations, with higher congruency potentially forcing suboptimal patellar motion. Therefore, the objective of this study was to evaluate the sensitivity of PF compartment biomechanics to the surgical variation in two fixed bearing (FB) implant with different PF articular conformity.
Methods: A previously validated forward dynamics model (Fig. 1)2 was used to simulate deep knee bend (DKB) activity (5°-120° flexion). We studied ATTUNE™ Cruciate Retaining (CR) fixed bearing (FB) system (DePuy Synthes) with less conforming PF articulation, referred as ACR, and multi-radius FB medial congruent construct with higher PF conformity, referred as PMC (Fig. 1). Both constructs were simulated in mechanical alignment using respective surgical techniques. Surgical variation was simulated by rotating femur and tibial tray components 5? internally-externally (I/E) from their original orientations at neutral patellar height, and at 5mm Alta position (Fig. 1). At all configurations, medial and lateral PF ligament (PFL) strain, patellar mediolateral (M/L) shift and tilt with respect to tibia, and patellar I/E torques were normalized to the baseline motions. The worst-case deviations from baseline magnitudes were reported.
Results: For all measured outputs, PMC construct experienced larger changes from its respective baseline orientation (Fig. 2, 3). The largest difference for PFL strain, patellar shift and tilt for the PMC system was observed in the first 40° of flexion (Fig. 2, 3). The peaks in medial and lateral PFL strains (increase/decrease from neutral alignment) were 9.8%/-10.7% and 8.7%/-11.4% for PMC and 3.6%/-5.3% and 4.1%/-6.7% for ACR respectively (Fig. 2). For the M/L shift and tilt, the peaks were 2.5mm/-2.2mm and 8.7°/-10.3° for PMC and 1.0mm/-0.7mm and 4.1°/-3.8° for ACR respectively. Patellar I/E torques were higher for PMC across the entire flexion range (Fig. 3).
Conclusion: The PF articular conformity is critical and can influence the PF compartment biomechanics3. With ACR system, patella experienced the least amount of M/L shift and tilt from its corresponding baseline motions, concurrent with the least variations in the PFL strains (Fig. 2, 3). The ACR system utilized GLIDERIGHT™ PF articulation with funnel shaped femoral trochlear groove. The resultant PF interaction may have allowed PF compartment soft tissues to drive the patellar motions closer to its baseline alignment during tibial and femoral misplacements. The PMC system included a channeled shape trochlear groove, producing higher PF conformity. In this case, femoral component could carry patella and dictate its motions, consequently increasing PFL strains. Larger envelopes, i.e. high sensitivity to misplacement, of patellar M/L shift, tilt, and ligament strains observed in the PMC system could be attributed to the higher PF conformity. In summary, careful consideration of PF conformity and design can lead to improved patellar motions that are more forgiving and robust to component misplacement in TKA.
References: 1.Thompson et.al-2011, 2.Khasian et.al.-2020, 3.Howard et.al.-2018
Figures
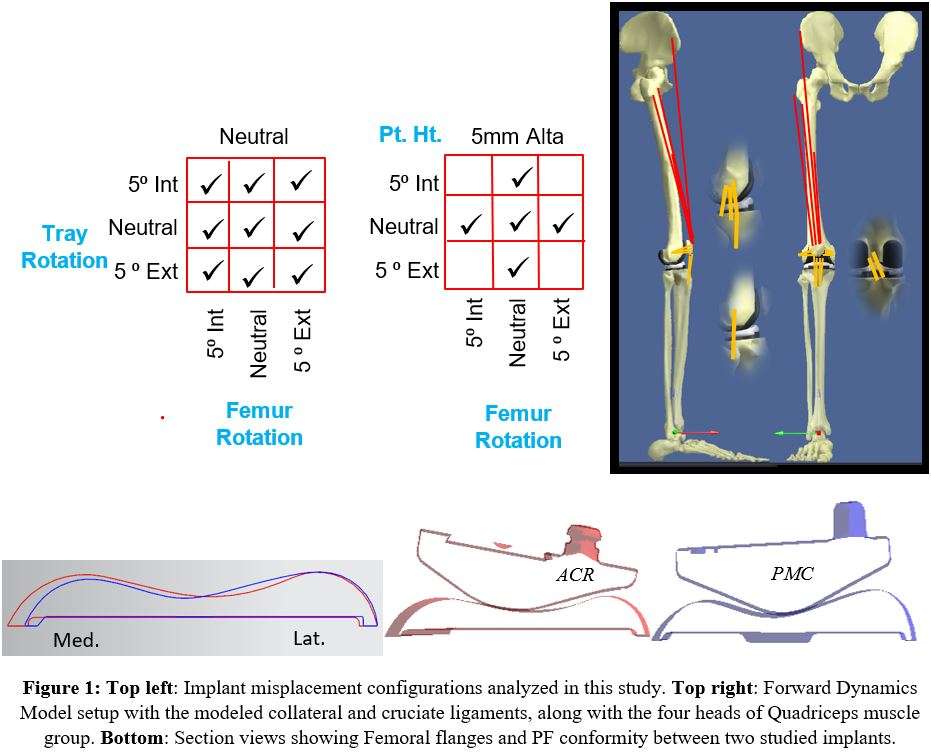
Figure 1
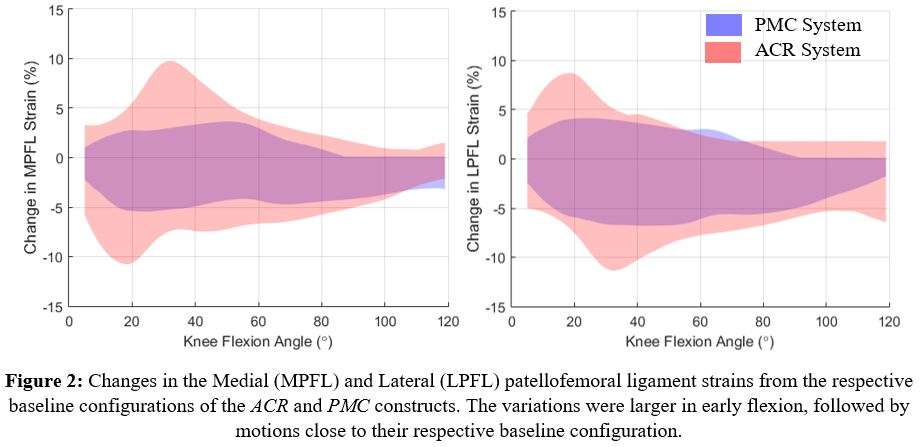
Figure 2
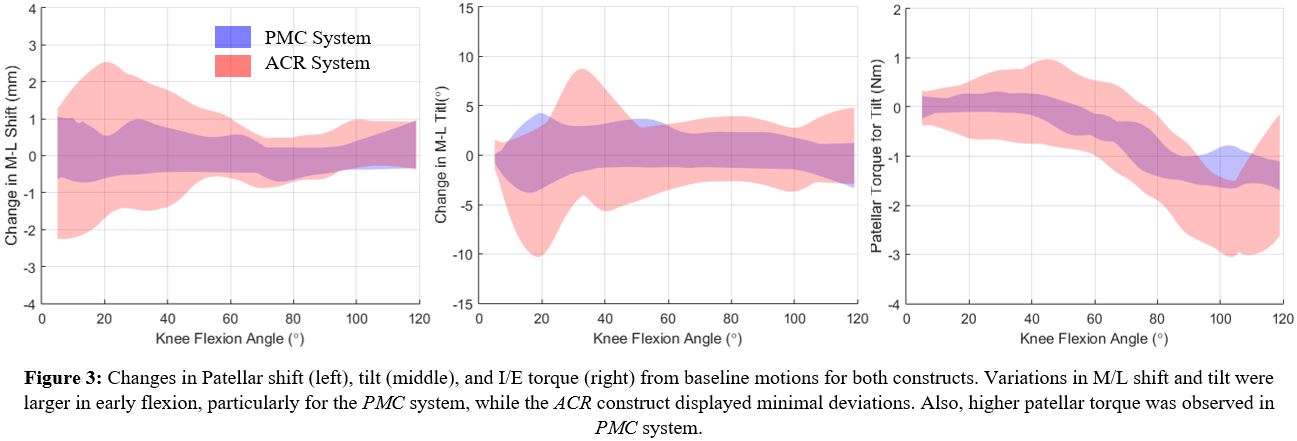
Figure 3#8458
Metal-Backed Cementless Patellar Components in Primary Total Knee Arthroplasty Using a Modern Design Implant: A Review of 707 Cases
*Arthur Malkani - Jewish Hospital - Louisville, USA
Alex Jouflas - University of Louisville - Louisville, United States of America
Arun Nadar - University of Louisville School of Medicine - Louisville, United States of America
Langan Smith - UofL Health - Louisville, United States of America
James Baker - UofL Health - Louisville, USA
Shikha Sachdeva - University of Louisville - Louisville, USA
Madhusudhan Yakkanti - Louisville Orthopaedic Clinic - Louisville, USA
*Email: arthur.malkani@louisville.edu
INTRODUCTION:
The use of cementless Total Knee Arthroplasty (TKA) implants has continued to increase over the past decade. First generation cementless metal-backed patellar components demonstrated high failure rates due to multiple factors including use of conventional polyethylene leading to osteolysis and bone loss. 2nd generation metal-backed patellar implants were developed with improvements in component design and polyethylene wear characteristics. Given the resurgence of cementless TKA usage especially in younger and active patients, the purpose of this study was to evaluate the clinical outcome of a 2nd generation cementless, metal-backed patellar component with a current design TKA implant.
METHODS:
This was a retrospective review from a single institution of 707 primary TKAs using a cementless, metal-backed patellar implant at a mean 7-year follow-up (range 2-12 years). 818 consecutive cementless TKAs were identified with 111 lost to follow-up (13.6%), leaving 707 with minimum 2-year follow-up available for review. There were 409 females and 298 males with mean age of 63 years (34-87) and mean BMI of 34.3 (18.8-64.5). Patient selection for cementless TKA was based on age and bone quality. All procedures were performed using the same cementless implant design, anesthesia, and postoperative protocols. Clinical outcomes evaluated included patient reported outcome measures (PROMs), complications, and revisions. Statistical analysis was performed to compare cohorts for pre- and post-operative patient metrics using t-tests: paired two sample for means.
RESULTS:
24 (3.4%) patients required revision surgery. 5 (0.71%) of these were due to patellar component complications: 1 (0.14%) for aseptic patellar loosening and 4 (0.57%) for polyethylene dissociation from the metal-backing. There were 19 (2.7%) non-patellar related revisions: 4 (0.57%) for tibial component aseptic loosening, 1 (0.14%) for femoral component aseptic loosening, 9 (1.3%) for prosthetic joint infection, 1 (0.14%) for popliteus impingement, and 4 (0.57%) for instability. Pre-operative Knee Score was 45.6 versus 91.1 post-operatively (p<0.001). Pre-operative Knee Functional Score was 45.7 versus 84.4 post-operatively (p<0.001). Survivorship of the metal-backed patellar component for all-cause failure was 97.5% at 12-years.
CONCLUSION:
Cementless TKA utilizing 2nd generation metal-backed patellar components with a modern design implant demonstrated a 97.5% survivorship at 12 years [Fig. 1]. Polyethylene dissociation from the metal-backing was the most common cause of patellar component failure. Advances in implant design and polyethylene wear properties have led to improved clinical results with cementless metal-backed patellar components. Given the increasing use of cementless TKA implants with metal-backed patellar components over the past decade, this study shows encouraging results with improved survivorship at mean 7-year follow-up compared to initial design metal-backed implants.
Keywords: primary TKA; cementless patella; metal-backed, TKA survivorship
Figures
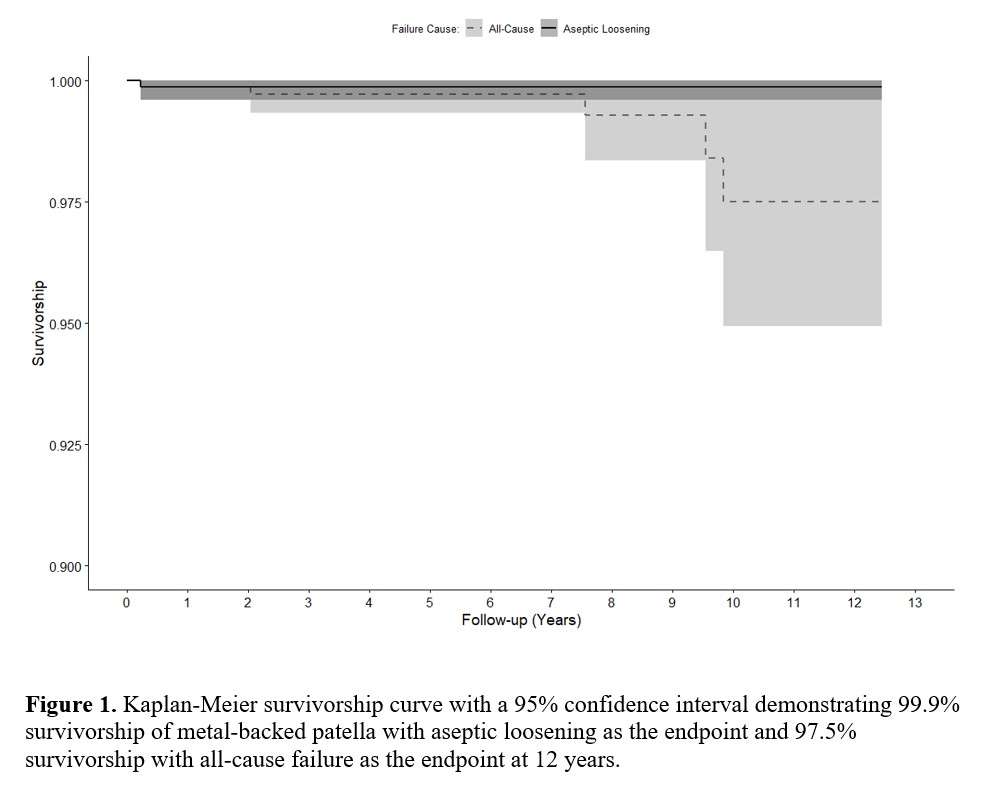
Figure 1#8706
Achieving Optimum Outcomes in TKA: First 100 Patients at 10-Years
*Robert Eberle - Maxx Orthopedics - Apex, USA
Sridhar Durbhakula - Washington Joint Institute - Bethesda, USA
Laura Rego, MA - OrthoBethesda - Adelphi, USA
*Email: eberlewriting@gmail.com
INTRODUCTION
Reporting the continuous, prospective outcomes of a post-surgical orthopaedic population without loss to follow-up at standard landmarks over time is rarely achieved in total knee arthroplasty (TKA). The purpose of this study was to advance the knowledge base of the 10-year minimum performance of a current primary TKA system through the continuous monitoring of a previously reported TKA population at 2 and 5 years.
METHODS
A prospective, continuous series of 100 primary posterior stabilized (PS) TKAs were performed in 96 patients by a single surgeon. Total Hospital for Special Surgery (HSS) knee scores and range of motion (ROM) was assessed for the entire cohort.
RESULTS
There were 10 patients lost to follow-up, and 3 additional patients were deceased, and all other patients were accounted for. The average patient age at surgery was 68.7 ±7.4 years. Two patients required incision and drainage for superficial wound infection of the indicated knees (<2 years). One patient at 4.5 years required tibial component and polyethylene insert revision following a motor vehicle accident resulting in a proximal tibial fracture and component loosening. There was no radiographic evidence of femoral component failure. There were no pre- or post-operative clinical or functional differences by gender and at the recent follow-up. In addition, there was an average significant increase in change of HSS knee score (p<0.001) and ROM (P<0.001) when compared to pre-operative baseline but no significant difference in HSS or ROM between the averages at 2-, 5- and 10-year outcome results.
CONCLUSIONS
The design charcteristic for component sizing and functional expectations were re-confirmed in the reported Western population cohort series, and observed optimum safety, performance, and efficacy through an average of 10-years. Further continued study efforts of this primary TKA system is warranted across multiple surgeons and all ethnic cultures.
#8227
Exploring Reward Modeling for Evaluating Total Knee Arthroplasty Simulations: A Pilot Study
Brad Miles - 360 Knee Systems - Pymble, Australia
*Ishaan Jagota - 360 Med Care - Sydney, Australia
Joshua Twiggs - University of Sydney - Sydney, Australia
Andrew Shimmin - Melbourne Orthopaedic Group - Melbourne, Australia
Jonathan Bare - Melbourne Orthopaedic Group - Melbourne, Australia
David Liu - Gold Coast Centre for Bone and Joint Surgery - Tugun, Australia
Justin Roe - North Sydney Orthopaedic and Sports Medicine Centre - Sydney, Australia
*Email: ishaan.jagota@360med.care
Introduction:
Joint dynamics, including forces and motion within the joint, following Total Knee Arthroplasty (TKA) are influenced by factors such as anatomy, alignment, and implant design. The Dynamic Knee Score (DKS) is an AI-based simulation model that predicts joint dynamics and evaluates the potential impact of surgical planning adjustments (Figure 1). However, the DKS model relies on post-operative Patient-Reported Outcome Measures (PROMs) as the ground truth, which limits data availability. To address sparse data challenges in AI, researchers have developed reward modeling, which involves comparing two datasets to inform an AI reward model that a second AI maximizer model attempts to fulfill. This pilot study aims to explore the applicability of reward modeling for TKA computer simulations.
Method:
We selected 240 videos of post-TKA deep knee bend simulations from previously evaluated patients in the 360MedCare registry. The videos were randomly paired and displayed side-by-side for assessment by simulation engineers based on clinical judgment. A reward model was then developed using Multivariate Adaptive Regression Splines (MARS) to fit the kinematic measurements of these simulations against the preferred and non-preferred simulations. We evaluated the model's accuracy in fitting the assessed preferences and the kinematic profile of the recommended TKA joint motion.
Result:
The reward model identified several factors contributing to a 'good' simulated knee in order of preference: the maximum extension reached when coming into full extension, avoidance of medial roll forward during flexion, medio-lateral force balance between condyles in mid-flexion, neutral tibio-femoral rotation at extension, minimal lateral patellar tracking in deep flexion, no patellar medial tilt in extension, and no patellar shear force in extension. The model's overall R2 was 0.4, indicating that it captures 40% of user preference variation. This performance is satisfactory, as the goal is not to achieve 100% accuracy due to noise from random pairings of evaluated simulations.
Discussion:
Sparse data presents a challenge for developing effective AI models in orthopedics. Techniques such as synthetic data generation and reward modeling have been employed in other fields to overcome this issue, and their application to TKA simulations appears promising. Further research is needed to characterize the useability of the reward model alongside a second AI maximiser model that determines optimal surgical plan.
#8131
Prioritization of Consequences After Total Knee Arthroplasty Contributing to a Poor Response: A Best-Worst Scaling Exercise Among Osteoarthritis Patients and Knee Specialists
*Petra Heesterbeek - Sint Maartenskliniek - Nijmegen, Netherlands
Malou Te Molder - Sint Maartenskliniek - Nijmegen, Netherlands
Lise Verhoef - Sint Maartenskliniek - Nijmegen, Netherlands
José Smolders - St Maartenskliniek - Nijmegen, Netherlands
Els Van Den Ende - Sint Maartenskliniek - Nijmegen, Netherlands
*Email: p.heesterbeek@maartenskliniek.nl
Introduction
Approximately 1 out of 5 patients has a poor response after total knee arthroplasty (TKA). To date no recommendations are available on the outcome domains that should be included in a definition for poor response to TKA because of osteoarthritis (OA). This hampers comparison of outcomes and improvement initiatives. Previous qualitative studies provide useful information on postoperative consequences for patients with OA contributing to poor outcome, but did not investigate their relative importance. The aim of this study was to prioritize consequences of TKA for patients with OA that contribute to a poor response, from the perspective of patients and knee specialists.
Methods
95 postoperative patients and 63 knee specialists (21 orthopedic surgeons, 10 physician assistants, 9 nurse practitioners and, 23 specialized physiotherapists) prioritized a set of 29 consequences, based on a previous qualitative study, using a Maximum Difference Scaling method. Hierarchical Bayesian analysis was used to calculate relative importance (RI) scores. Differences between patients versus knee specialists and satisfied patients versus dissatisfied patients were analyzed using Mann-Withney-U tests.
Results
79 patients were satisfied, 13 were dissatisfied and 3 patients did not know whether they were satisfied or dissatisfied with their TKAs. The highest ranked consequence for patients was “Inability to do normal activities such as walking, cycling, calm swimming and heavy household chores” while “No improvement in pain during the day” was ranked highest by knee specialists. There was one consequence that was in the patients’ top 5 but was not ranked in the top 5 of knee specialists: “No improvement in walking” (Figure 1). The top 5 of satisfied and dissatisfied patients included the same consequences, however “No improvement in pain with weight bearing/during activities” was significantly more important for dissatisfied patients (Figure 2).
Conclusion
The perspective of patients versus knee specialists and that of satisfied patients versus dissatisfied patients on the importance of consequences after TKA that contribute to a poor response are comparable. However, patients seem to be more focused on being able to perform valued activities, whereas knee specialists ranked pain relief higher. Pain, daily knee functioning and gait therefore are important outcome domains that should be included in a definition for defining poor response after TKA.
Figures
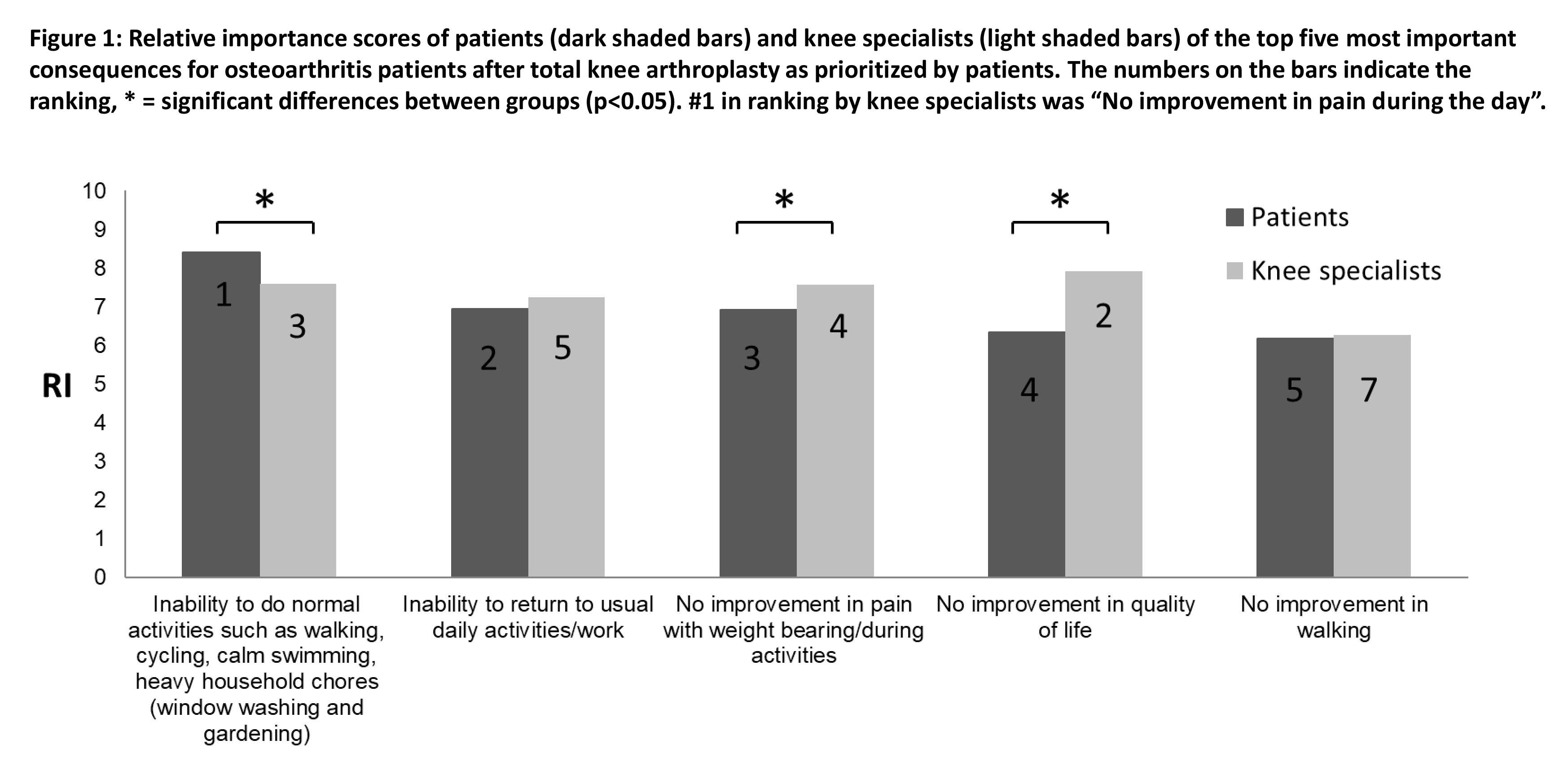
Figure 1
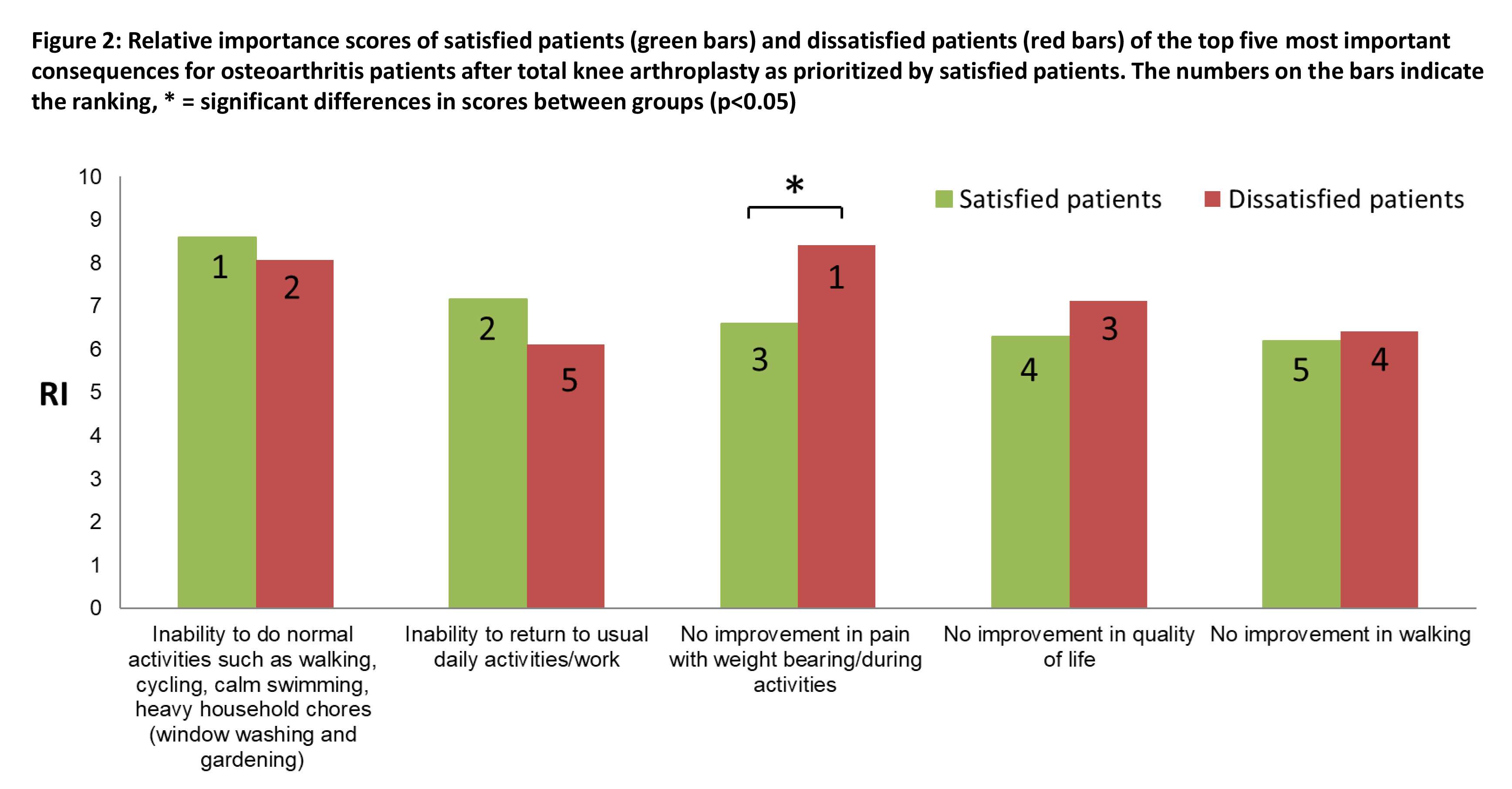
Figure 2#8503
Predicting Achievement of Clinically Meaningful Improvement in Patient-Reported Outcome Measures After Robotic-Assisted Total Knee Arthroplasty Using Machine Learning
Lisa Spahn Lundgren - Stryker - Amsterdam, Netherlands
Nathalie Willems - Stryker - Amsterdam, Netherlands
Sietske Witvoet - Stryker - Amsterdam, Netherlands
Maroussia Leidner - Freiburg, Germany
*Laura Yanoso-Scholl - Stryker Orthopaedics - USA
Daniele De Massari - Stryker - Eindhoven, Netherlands
Bob Marchand - ortho rhode island south county - Wakefield, United States of America
*Email: Laura.Scholl@stryker.com
Introduction: A central question in medical technology is estimating how a particular clinical intervention will ultimately affect the patient. For patients undergoing Total Knee Arthroplasty (TKA), surgery outcome is commonly assessed by means of patient-reported outcome measures (PROMs). The aim of this study was to develop and evaluate predictive modeling approaches to predict the achievement of minimal clinically important difference (MCID) in PROMs 1-year post-operatively. Such decision aid tools can be leveraged by hospital teams to more pro-actively manage patients and their post-operative expectations.
Methods: 430 robotic-assisted TKA patients were analyzed in this study. The following PROMs were collected preoperatively and 1-year post-operatively: KOOS JR, WOMAC Function, WOMAC Pain. Table 1 shows demographic information, case distribution and MCID status. Factors describing patient (demographics), diseased joint (derived from preoperative CT scan), implant size and implant position (derived from robotic system), and joint replacement procedure (e.g. inpatient/outpatient, surgery duration) were collected and selected as predictor variables. Four machine learning algorithms were trained to predict the probability of not achieving MCID status at 1-year post-TKA for each PROM survey, including logistic ridge regression, support vector machine, random forest classifier and the gradient boosting model CatBoost. “No MCID” was chosen as the target variable as it was the least represented class (see table 1) and likely of more interest to the surgical team. Models were evaluated by class discrimination (F1-score) and area under the receiver operating characteristic curve (AUC).
Results: For each PROM, the best model and its performance are shown in table 2. The overall best performing model, according to both F1 score and AUC, was ridge regression for WOMAC Function (AUC = 0.89, F1 = 0.69, sensitivity = 0.86, specificity = 0.84). Figure 1 shows the model's feature coefficients. Variables most strongly contributing to not achieving MCID status were preoperative PROMs, surgery duration and femoral resection depth (posterior and distal). Conversely, variables contributing to a positive outcome (achieving MCID), were medial/lateral alignment of the tibial component, whether the procedure was an outpatient surgery and whether the patient received Managed Medicare insurance. Less predictive variables were features describing the patient’s preoperative knee alignment, gender and surgery side.
Conclusion: This study developed machine learning models to predict MCID achievement at 1 year after TKA for three different PROMs. The developed models outperform state-of-the-art solutions from literature (see table 2), suggesting that they may be used for setting patient expectations about their post-operative outcomes after TKA. Interestingly, the most predictive features for likelihood of MCID include resection depths and implant positioning, which are directly related to the surgical plan, thus highlighting the potential of this tool for driving clinical decision making in a patient-specific manner.To further improve the predictive performance and interpretability of the models, possible next steps are repeating the study on a larger population and including additional variables, for instance socioeconomic variables or 3D metrics, such as joint congruency and patella tracking. Another advancement could be to consider alternative ways to better define surgery outcome, for instance based on joint mechanics.
Figures
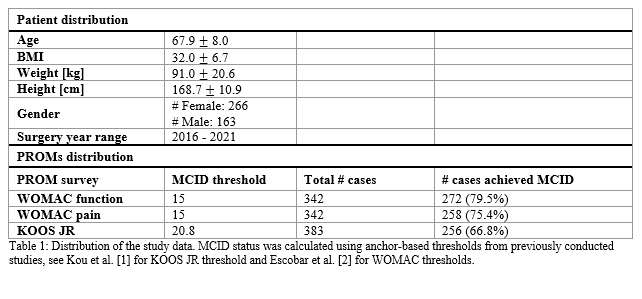
Figure 1
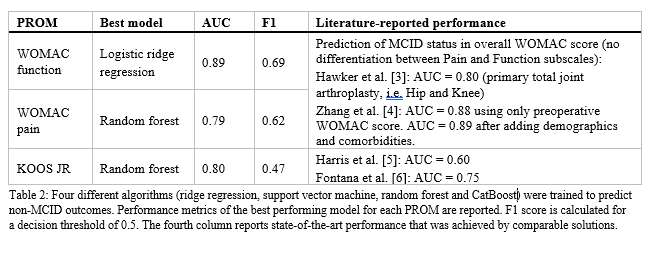
Figure 2
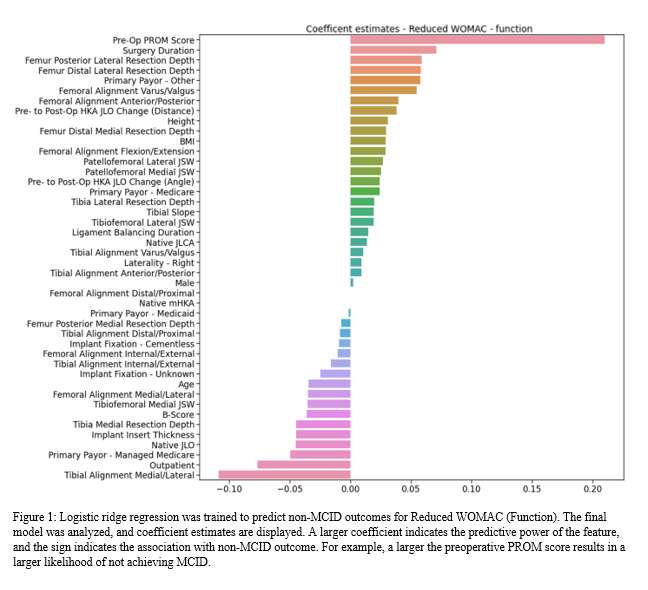
Figure 3#8380
Setting Expectations to Improve Satisfaction Following Total Knee Arthroplasty With Machine Learning
*Jennifer Polus - University of Western Ontario - London, Canada
Tom Appleton - Western University - London, Canada
Edward Vasarhelyi - Western University - London, Canada
Steven MacDonald - London Health Sciences Centre - London, Canada
Brent Lanting - London Health Sciences Centre - London, Canada
Matthew Teeter - Western University - London, Canada
*Email: jpolus@uwo.ca
Introduction: Total knee arthroplasty (TKA) is the only viable treatment for end-stage osteoarthritis, however, 20% of patients consistently report being dissatisfied with the outcome of their procedure. Patient dissatisfaction has been linked to unmet expectations of functional recovery. One way to potentially improve patient satisfaction is to counsel patients on what they can expect of their personal recovery. Our group has developed a tool to predict whether a patient is more likely to meaningfully improve on (responder) or keep (maintainer) their level of preoperative function after undergoing surgery. The purpose of this study is to assess the impact providing patients with a personal functional prediction has on their satisfaction and investigate relationships between patient satisfaction and functional recovery.
Methods: Patients (n=50) undergoing primary TKA are recruited and randomized to either receive the prediction of their functional recovery or to have surgery as normal. Preoperatively, patients perform the timed-up-and-go (TUG) walking test while instrumented with wearable sensors. Using the data collected from the wearable sensor system, the machine learning classifier predicts whether each patient is a functional responder or maintainer. Patients repeat the TUG test again at three-months and one-year postoperatively to track functional recovery. Prediction accuracy is evaluated by assessing whether each patient improved in total TUG time by the minimum clinically important difference (MCID = 2.27 seconds) from pre-operation to 3-months post-operation. Patients also complete the Knee Society Score (KSS) questionnaires to address their expectations and satisfaction.
Results: Patient recruitment and follow-up are ongoing. Thus far, 31 patients have consented to participate and 29 have undergone TKA. Patients were randomized to either receive their functional prediction (n=14) or have surgery with no knowledge of their prediction (n=15). Two patients were predicted to be “responders” and the remaining 27 were predicted to be “maintainers”. So far, 17 patients have completed their three-month follow-up. One predicted responder improved on their pre-operative function by 2.25 seconds and three predicted maintainers improved on their preoperative TUG times by more than 2.27 seconds, a clinically important difference that would make them responders. The current performance accuracy is 76.4% (13/17 correct predictions), in line with the trained and validated algorithm used that had an accuracy of 76%. Early analysis shows no difference in mean KSS expectation and satisfaction scores preoperatively or at three-months between patient groups.
Conclusion: Patient dissatisfaction following TKA is widespread and costly. This ongoing study is the first to evaluate an objective tool that utilizes wearable sensors and machine learning to help patients manage preoperative expectations of functional recovery. The algorithm is performing as expected, with a bias towards setting a lower expectation to ensure satisfaction with functional outcomes. As recruitment continues, the relationship between satisfaction, expectations, and functional ability will be further investigated and related to other patient outcomes.
#8541
Volume and pH of Synovial Fluid Prior to Total Knee Arthroplasty and the Relationship to Patient Reported Outcomes
*Madison Brown - University of Tennessee Health Science Center - Memphis, USA
Lisa Phan - University of Tennessee Health Science Center - Memphis, USA
Chandler Sears - University of Tennessee Health Science Center - Memphis, USA
Jordan Justice - University of Tennessee Health Science Center - Memphis, USA
Marcus Ford - Campbell Clinic Orthopaedics - Germantown, USA
John Crockarell - Campbell Clinic Orthopaedics - Germantown, USA
James Harkess - Campbell Clinic - Collierville, USA
James Guyton - Campbell Clinic Orthopaedics - Germantown, USA
Marc Mihalko - Campbell Clinic Orthopaedics - Germantown, USA
William Mihalko - University of Tennessee - Memphis, USA
*Email: mbrow275@uthsc.edu
Introduction: Total knee arthroplasty (TKA) is now one of the most performed elective surgeries with estimates predicting 1.26 million annual TKAs by 2030. However, the satisfaction rate may be as low as 80% and tens of thousands of patients are subjected to revision surgery. The effects of several patient-related factors such as age, gender, and weight have been investigated. However, little investigation has been done relating the qualities of synovial fluid (SF) to patient outcomes. Due to SF’s role of lubricating the joint and providing nutrients to surrounding structures, it is likely that patient’s SF may have an impact on the success of their TKA. The objective of this study is to investigate the relationships between SF volume, pH prior to surgery, and patient satisfaction.
Methods: After gaining consent from 113 patients, maximal SF was extracted just prior to TKA and volume and pH were recorded. Volumes over 10 mL were recorded as 10 mL. The patient’s stiffness, pain, and daily function were self-reported using a Knee injury and Osteoarthritis Outcome Score, Joint Replacement (KOOSJR) survey after three or more months post-op. Spearman’s rank correlation test was performed to examine each relationship between volume, pH, and KOOSJR score. Bimodal distribution of SF volumes prompted a Mann-Whitney test of KOOSJR scores with groups < 2 mL and ≥ 2 mL.
Results: SF volume ranged from 0-10 mL with an average of 4.8 ± 3.6. SF pH averaged 7.55 ± 0.31. KOOSJR scores ranged from 50-100 and averaged 77 ± 16 with 100 being a perfect score. No correlation was found between KOOSJR/pH (n=68, p=0.098). A strong negative correlation was found between volume/pH (n=61, p=0.003). A positive correlation was found between volume/KOOSJR (n=72, p=0.016). Additionally, patients with < 2 mL SF were found to have lower KOOSJR scores than those with ≥ 2 mL (n=72, p=0.004). Patients with < 2 mL SF had an average score of 69 ± 11 while patients with ≥ 2 mL had an average score of 81 ± 17 (shown in Figure).
Conclusions: There is a group of patients that are unsatisfied with the results of their total knee arthroplasty. The cause of this dissatisfaction may be multifactorial. It is important to consider environment in which the implant is being placed. Preliminary data shows a correlation between the volume of a patient’s SF and its pH which may correlate to the electrochemical properties of the SF. Additionally, patients those with higher KOOSJR scores (less pain) had higher levels of SF, which was not expected, as the body responds to OA by increasing SF levels to compensate for the disease. We will continue to enroll patients to evaluate these correlations while obtaining patient satisfaction scores after more time has passed after surgery.Future research will aim to better understand the causes of these correlations.
Figures
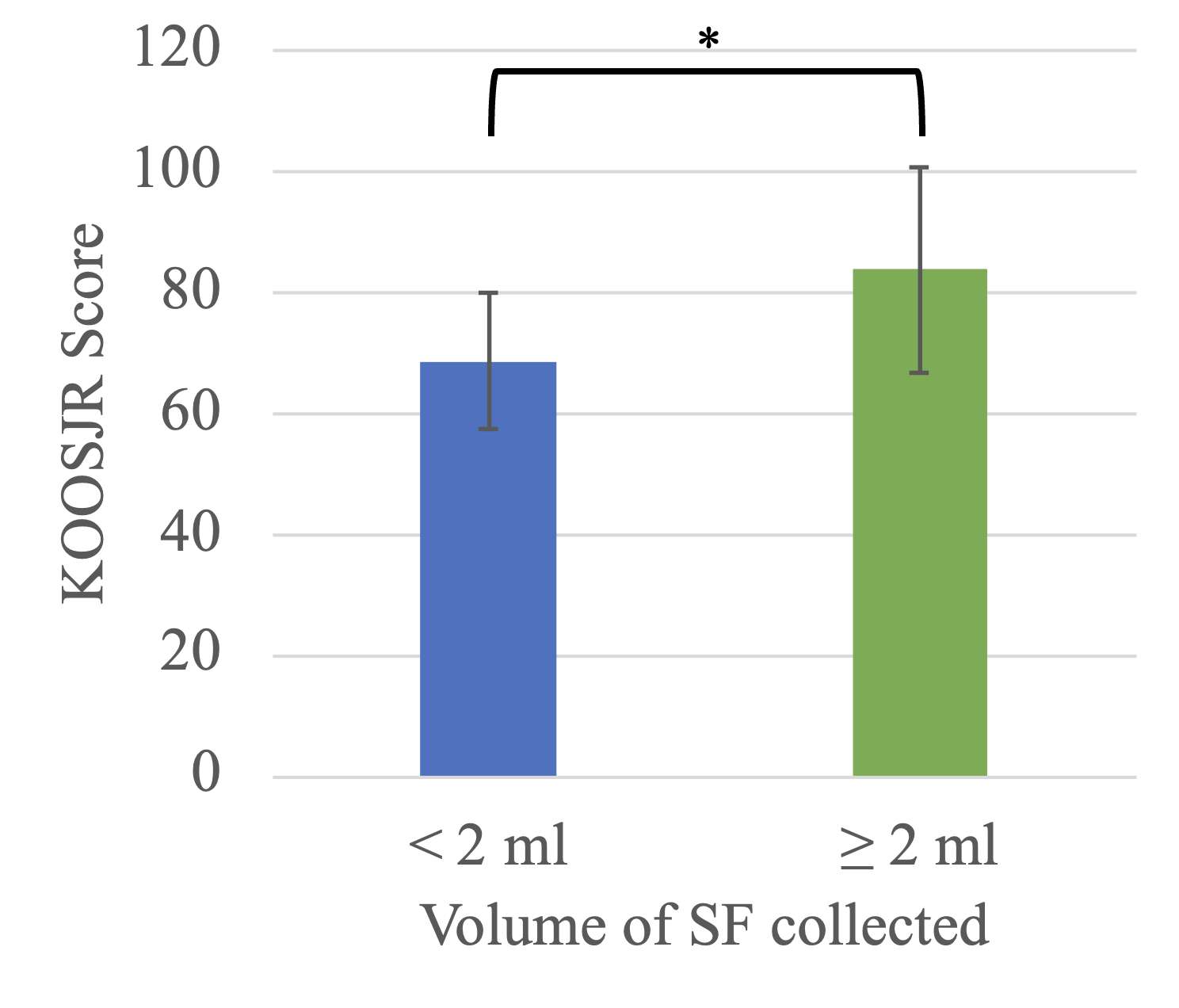
Figure 1#8528
The Patellar Tilt Differences After Total Knee Arthroplasty Between Supine and Standing Radiographic Views Are Not Related to the Rotation of Well-Aligned Femoral Components
*Fernando Quevedo Gonzalez - Hospital for Special Surgery - New York, USA
David J. Mayman - Hospital for Special Surgery - New York, USA
Theofilos Karasavvidis - Hospital for Special Surgery - New York, USA
Cale Pagan - Hospital for Special Surgery - New York, United States of America
Daniel Driscoll - Hospital for Special Surgery - New York, USA
Ryan Helbock - Hospital for Special Surgery - New York, USA
Peter Sculco - Hospital for Special Surgery - New York, USA
Eytan Debbi - Cedars-Sinai Medical Center - Los Angeles, USA
Timothy Wright - Hospital for Special Surgery - New York, USA
Jonathan Vigdorchik - Hospital for Special Surgery - New York, USA
Cynthia Kahlenberg - Hospital for Special Surgery - New York City, USA
*Email: quevedogonzalezf@hss.edu
Introduction: Radiographic evaluation of the patellofemoral joint in total knee arthroplasty (TKA) is generally performed with merchant views obtained in a supine position, which differs from the functional position of the patellofemoral joint [1]. Evaluation of the patella in functional position is important to understand knee function and anterior knee pain [2-3]. Our goals were to compare supine and weight-bearing standing merchant radiographs of the patella and determine whether the changes in patellar tilt are related to the position of the femoral component.
Methods: We retrospectively reviewed 11 patients (1 male, 10 females, ages: 52–83 years, BMIs: 22–36 kg/m2) who underwent robotic-assisted primary TKA with posterior-stabilized or cruciate retaining implants (Stryker, Mahwah, NJ) by a single surgeon (DJM) from July 2022 to March 2023 with resurfacing of the patella. Six weeks after TKA, patients underwent standard-of-care merchant radiographs in the supine position, with the knee in 45? flexion and the X-ray beam angled 30? relative to the femur [2], and a standing weight-bearing merchant view with a positioning device that ensured equivalence between both radiographic views (Fig. 1). We obtained the femoral component varus/valgus and internal/external rotations from the robotic TKA plan. Three independent observers measured the patellar tilt as the angle between the implant-bone interface and a line through the anterior aspects of the femoral condyles (Fig. 2) [4]. Lateral tilt (i.e., gap opening medially) was considered positive. Statistical analysis consisted of intra-class correlation coefficients (ICC) to determine agreement among observers, paired T-tests to compare tilt on supine and weight bearing merchant views, and Pearson correlation analysis to determine whether the supine tilt, weight-bearing tilt, or their difference correlated with the femoral component rotation. A p-value of 0.05 was considered significant.
Results: The observers had excellent agreement for supine (ICC=0.99) and standing (ICC=0.99) tilt; therefore, we averaged the measurements for analysis. Lateral tilt was smaller (p=0.04) and showed less spread on standing (mean 3.0?, range: 0.2?–8.0?) than supine merchant views (mean 6.7?, range: -0.1?–16.4?) (Fig. 3). Seven patients had decreased tilt on the standing view compared to the supine view (range: 1.8?–14.7?), three had increased tilt (range: 0.4?–2.6?), and one showed no change. The femoral component was in 1.1? valgus (range 1? varus – 2? valgus) and 1.5? internal rotation relative to the transepicondylar axis (range: 3.5? internal – 4? external). None of the of patellar tilt measurements were correlated with the varus/valgus or internal/external rotation of the femoral component.
Conclusions: post-TKA radiographs demonstrated significantly less patellar lateral tilt and more consistent patellar position on weight-bearing compared to during supine merchant views. The differences in tilt were not related to the coronal or axial rotation of the femoral component. Future studies will consider more extreme component alignment and explore the relationship between patellar position and anterior knee pain.
References: [1] Draper, et al., J Orthop Res. 2011; [2] Gharaibeh, et al. Knee. 2018; [3] Baldini, et al., J Bone Joint Surg. 2007; [4] Gomez, et al., Clin Orthop Relat Res. 1988
Figures
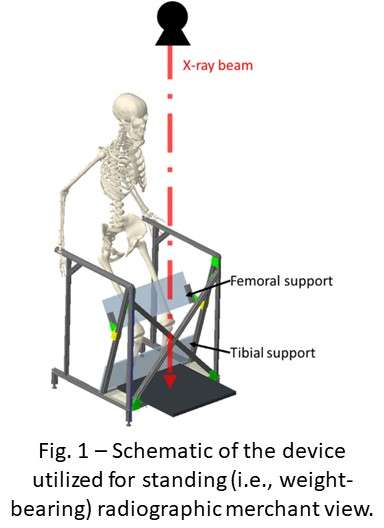
Figure 1
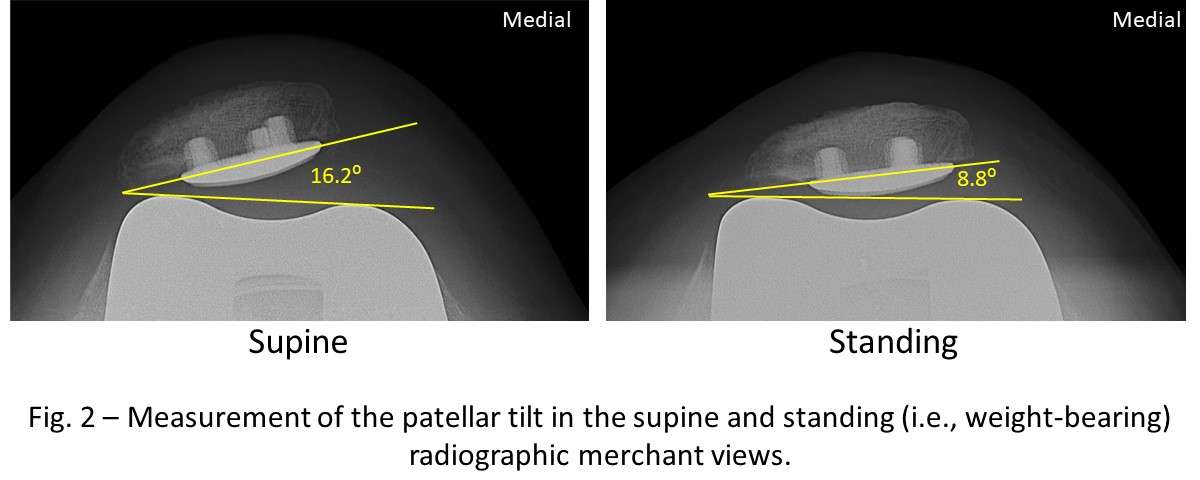
Figure 2
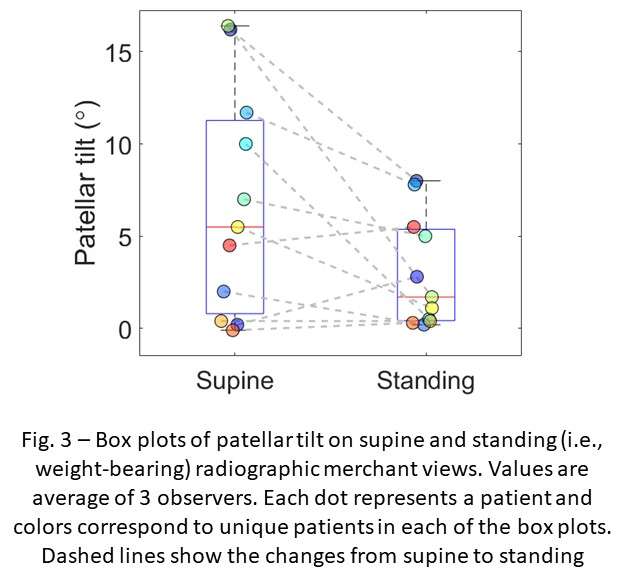
Figure 3#8712
Migration Analysis of a Cementless Monoblock Ceramic Acetabular Cup With a Titanium and Hydroxyapatite VPS Coating
*Susannah Clarke - Imperial College London - London, United Kingdom
Camilla Halewood - Imperial College London - London, United Kingdom
Kartik Logishetty - Imperial College - London, United Kingdom
Justin Cobb - Imperial College - London, United Kingdom
*Email: sgc05@imperial.ac.uk
Introduction:
The H1 Implant (Embody Orthopaedic Limited) is an all-ceramic hip resurfacing with a cementless monoblock cup and head. A titanium and hydroxyapatite vacuum plasma sprayed coating (Medicoat AG) is applied to the backside of the BIOLOX®delta cup (CeramTec GmbH) to encourage bone ongrowth. There is no commercially available product with a direct to ceramic bone ongrowth coating. To assess the stability of the cementless cup, a clinical migration study was undertaken.
Methods:
66 patients were implanted with 66 H1 Implants as part of a clinical investigation (ethics reference number 17/EE/0330, MHRA study number CI/2017/0040). A validated low-dose CT?based spatial analysis (CTSA) technique for measuring implant migration [1] was used to measure implant movement between 2 days and: 6 weeks; 3 months; 6 months; 12 months; and 24 months post-op. Tantalum beads were implanted into the surrounding pelvic bones, and the movement of the implant was measured using model-based techniques.
Results:
Figure 1 shows absolute cup translation over time for individual cups. The red dashed line shows the maximum error of the measuring technique and the green dashed line shows median translation. At 3 months, the mean (SD) absolute cup translation was 0.41mm (0.34mm) and at 24 months it was 0.42mm (0.32mm). The mean (SD) difference in cup translation between 3 months and 24 months was 0.00mm (0.11mm), which was not found to be significant (p>0.05).
Conclusion:
Early migration can be an indicator of future implant loosening and subsequent failure [2]. Migration analysis was undertaken on 66 all-ceramic cup implants with a direct to ceramic bone ongrowth coating, using a validated CT-based technique. Initial settlement was observed, as expected with a cementless implant. After the settlement, no further movement was observed. This acetabular cup with direct to ceramic bone ongrowth coating has been shown to be radiographically stable at 2 years.
References
1 Clarke et al. Low dose CT-based spatial analysis (CTSA) to measure implant migration after ceramic hip resurfacing arthroplasty (HRA): A phantom study. IMechE Part H. 237(3) (2023): 359-367
2 Pijls BG et al. Early proximal migration of cups is associated with late revision in THA: a systematic review and meta-analysis of 26 RSA studies and 49 survival studies. Acta Orthop 83(6) (2012): 583-591
Figures
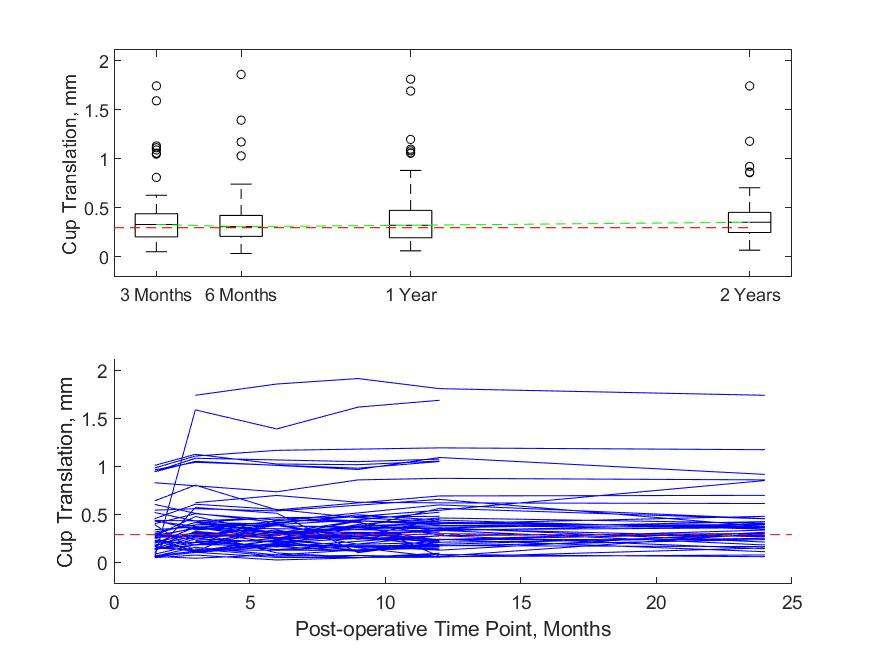
Figure 1#8180
Deep Learning Automation of Preoperative Radiographic Parameters Associated With Early Periprosthetic Femur Fracture After Total Hip Arthroplasty
*Seong Jun Jang - Hospital for Special Surgery - New York, United States of America
Kyle Alpaugh - Massachusetts General Hospital - Boston, USA
Kyle Kunze - Hospital for Special Surgery - New York, USA
David J. Mayman - Hospital for Special Surgery - New York, USA
Jonathan Vigdorchik - Hospital for Special Surgery - New York, USA
Seth A. Jerabek - Hospital for Special Surgery - New York, USA
Elizabeth B. Gausden - Hospital for Special Surgery - New York, USA
Peter K. Sculco - Hospital for Special Surgery - NYC, USA
Tim Li - Weill Cornell Medical College - New York, United States of America
*Email: seongjang22@gmail.com
Abstract
Background: The radiographic assessment of bone morphology impacts implant selection and fixation type in total hip arthroplasty (THA) and is particularly important in the context of minimizing periprosthetic femur fracture (PFF) risk. We utilized a deep learning (DL) algorithm to automate several femoral radiographic parameters and determined which automated parameters were associated with early PFF.
Methods: Radiographs from a publicly available database and from patients undergoing primary cementless THA at a high-volume institution (2016-2020) were obtained. A U-Net algorithm was trained with data augmentation and transfer learning and optimized to segment femoral landmarks for bone morphology parameter automation. Automated parameters were compared against that of a fellowship-trained surgeon and compared in an independent cohort of 100 patients who underwent THA (50 with early PFF and 50 controls matched by femoral component, age, gender, BMI, and surgical approach).
Results: On the independent cohort, the DL algorithm generated 1,710 unique measurements for 95 images (5% lesser trochanter identification failure) in 22 minutes. Medullary canal width, femoral cortex width, canal flare index, morphological cortical index, canal bone ratio, and canal calcar ratio all had good-to-excellent correlation with surgeon measurements (Pearson’s correlation coefficient:0.76-0.96). The metaphyseal morphologic indexes had a moderate correlation (0.50–0.63). Canal calcar ratio (0.43±0.08 vs. 0.40±0.07) and canal bone ratios (0.39±0.06 vs. 0.36±0.06) were higher (p<0.05) in the PFF cohort when comparing the automated parameters.
Conclusion: Deep learning-automated parameters demonstrated differences in patients with and without early PFF after cementless primary THA. This algorithm has the potential to complement and improve patient-specific PFF risk prediction tools.
Keywords: Bone Morphology; Artificial Intelligence; Deep Learning; Periprosthetic Femur Fracture, Total Hip Arthroplasty
Introduction
Periprosthetic femur fracture (PFF) after primary total hip arthroplasty (THA) is one of the major causes of early stem failure requiring revision surgeries (within 90 days of THA) in the United States.[1] It is a feared complication given its associated poor outcomes, and mortality ranges from 13-18% in the year following revision THA for this indication.[2-5] Several risk factors are associated with PFF following THA. These include demographic factors such as advanced age and female gender and medical factors such as osteoporosis and cementless fixation.[1, 6-10] In addition, there are preoperative radiographic risk factors for early PFF after cementless THA such as thinner distal cortices and a reduced amount of taper transitioning from the metaphyseal to diaphyseal regions.[11] In a single-institution, matched case-control study, Alpaugh et al. also determined that femoral component undersizing and misalignment were modifiable factors that increase risk of early PFF, especially in patients with at-risk native bone morphologies.[12]
To reduce the occurrence of early PFF, accurate and comprehensive assessments of patient bone morphology are an imperative component of medical decision-making. They not only guide surgeons in selecting the optimal implant type and size, but in also choosing the fixation technique. Nonetheless, bone morphology assessments are time-intensive and dependent on radiographic parameters that are prone to measurement biases. Deep learning (DL), a subset of artificial intelligence, has gained considerable attention as a set of computational methods capable of interpreting radiographs in a rapid and objective manner, with comparable accuracy to surgeons and radiologists.[13, 14] It offers a solution to the limitations of manual imaging evaluation, which requires expert guidance and time in assessing radiographic parameters. Namely, DL techniques have already successfully demonstrated efficacy in quantifying or predicting clinically significant parameters in orthopaedic surgery, such as identifying THA implant designs, predicting osteoporosis classification, estimating the hip joint center on pelvis radiographs, and, more recently, comparing leg-length discrepancy measurements.[15-19] Therefore, the same set of DL techniques could conceivably be applied appropriately to provide quantitative characterizations of femoral bone morphology, which traditionally requires the expert analysis of multiple radiographic parameters.
This study’s purpose was to 1) develop a DL algorithm capable of automating femoral bone morphology parameters and 2) to apply this tool in cohorts of patients with and without early PFF after THA to determine if automated measurements revealed significant differences in a clinical cohort. We hypothesized that 1) there would be no significant difference between the algorithm and fellowship-trained surgeon and 2) patients with early PFF will have significant differences in their bone quality radiographic parameters prior to THA.
Methods
Patient and Image Selection
Data was acquired from two different sources. Patient images were included from the Osteoarthritis Initiative (OAI) database, which consists of data collected from 4,796 patients (45-79 years old).[20] Inclusion criteria were patients who underwent a THA with an anteroposterior (AP) pelvis radiograph taken at baseline and at the last available study visit (96-months). Additionally, all patients undergoing primary cementless THA between 2016-2020 were also reviewed from a single high-volume institution (n=16,065) following institutional review board approval. Inclusion criteria included any patient who underwent THA with early (within 90 days) PFF who had available pre-operative radiographs. Exclusion criteria included patients who underwent THA for a diagnosis other than primary hip osteoarthritis.
Training and internal validation cohorts were derived from the total study population using a split with an 8:2 ratio (120 training, 30 validation). A hold-out testing set (n = 100) was used to ensure testing of the created DL models to new data. Critically, this hold-out testing set included 50 patients who had an early PFF after primary cementless THA and 50 matched patient controls who did not have an early PFF after THA. Patients were matched based on femoral component, patient age, gender, BMI, and surgical approach.(Figure 1). The femoral components included Taperloc Microplasty (Biomet; Warsaw, IN)(1), Summit (DePuy; Raynham, MA)(2), Trilock (DePuy)(1), Novation (Exactech; Gainesville, FL)(7), Master Lock (Medacta; Switzerland)(1), Anthology (Smith & Nephew; Nashville, TN)(3), Synergy (Smith & Nephew)(10), Accolade II (Stryker; Mahwah, NJ)(2), Secur-Fit Advanced (Stryker)(11), Secur-Fit Max (Stryker)(1), Anatomic (Zimmer; Warsaw, IN)(7), and Trabecular Metal (Zimmer)(5).
Measurement Descriptors
Preoperative femoral morphology measurements investigated in this study are described in Table 1 and Figure 2. These measurements were chosen based on preoperative radiographic parameters previously investigated in relation to early PFF following primary THA[12] and those scalable with a calibration marker. The cortical widths (CW), endosteal canal widths (EW), canal calcar ratio (CCR), canal flare index (CFI), morphologic cortical index (MCI), metaphyseal morphology (MMI), canal bone ratio (CBR) were included. The metaphyseal morphology index was defined as the ratio between the endosteal and cortical widths at 2cm above, 2cm below, and at the midpoint of the lesser trochanter.[12] These measurements were generated on the testing set by both the DL algorithm and a fellowship-trained surgeon for statistical comparison. All measurements were calibrated using a 25.4 mm marker ball.
Deep Learning Model
Convolutional neural networks (CNNs) are deep learning networks utilized for computer vision tasks. A U-Net is a type of CNN designed to identify image pixels belonging to specific objects (i.e. bony landmarks). The following landmarks were manually segmented (femoral cortex, medullary canal, implant prosthesis, lesser trochanter) to establish ground truths for the model. In this study, we developed a U-Net algorithm to first generate segmentations of landmarks and then applied an additional algorithm to convert these segmentations to radiographic parameters. (Figure 3 and Supplemental Methods)
Statistical Analysis
The U-Net model’s performance for landmark prediction was assessed using the multi-class dice coefficient.[21] To assess the DL-produced measurement accuracy, these values were compared against that of a fellowship-trained arthroplasty surgeon. The Pearson’s correlation, interclass correlation coefficient (ICC), paired t-tests/Wilcoxon tests, and Bland-Altman analyses were conducted for included measurements. To compare automated parameters between the early PFF and control cohort, independent t-tests or Mann-Whitney tests were conducted after determining variable normality. All statistics were conducted on a Jupyter Notebook(Python, Python Software Foundation, Wilmington, Delaware) using the Scipy.stats[22] package(V1.10.1) and Statsmodel package(V0.14.0).[23]
Results
Deep Learning Model Performance
The DL model was able to process each image and obtain all measurements in 14 seconds per image. The preoperative image model had a multi-class dice coefficient of 0.91, indicating excellent bony landmark segmentation.(Figure 3) In the testing set, the model failed to identify the lesser trochanter in 5 images (5% error rate) and preoperative measurements were obtained on 95 total images (1,710 total measurements) in under 22 minutes.(Figure 4)
Deep Learning vs. Surgeon Measurements
All cortical and endosteal canal width measurements had a Pearson’s correlation between 0.80-0.96 (p<0.01), indicating a strong correlation. For CCR, CFI, CBR, and MCI, the Pearson’s correlation was 0.78-0.81 (p<0.01). However, metaphyseal morphologic indices had moderate correlation (0.50–0.63).(Figure 5)
No significant differences were observed between the surgeon and DL algorithm for all CW measurements.(Table 2) However, the EW measurements at 2-cm, 7-cm and 10-cm below the lesser trochanter (p<0.05) were significantly different. When assessing the Bland-Altman plots, the algorithm measured the endosteal canal width 1.5-2.2-mm narrower on average than the surgeon.(Supplemental Figures 1 and 2) As a result, all ratios utilizing these direct measurements (CBR, MCI, and MM at 2cm below the lesser trochanter) were statistically different (p<0.05) between the algorithm and surgeon.
Surgeon-Measured Parameters: Periprosthetic Fracture vs. Matched Control Cohort
All bone morphology measurements conducted by the surgeon were compared between patients who underwent THA and had early PFF compared to matched patients who underwent THA and did not have early PFF (Table 3). Univariate analyses revealed that patients who had an early PFF had significantly lower endosteal widths at 2, 7, and 10 cm below the lesser trochanter. They also had significantly higher CCR (p=0.01), CBR (p<0.01) and significantly lower MCI (p<0.01) and CFI (0.01). Furthermore, the average MMI and MMI at 2 cm below the LT was significantly higher for the fracture cohort (p=0.01).
DL-Automated Parameters: Periprosthetic Fracture vs. Matched Control Cohort
All bone morphology measurements were compared between patients who underwent THA and had early PFF compared to matched control patients (Table 4). Patients who had an early PFF had significantly higher CCR (p=0.04) and CBR (p=0.02). They also had lower CFI (p=0.08) and MCIs (p=0.06), although these did not reach statistical significance. The direct endosteal canal width measurements were wider in the PFF cohort at 7-cm (12.4 2.3cm vs 11.6 2.0cm, p=0.07) and 10-cm (11.62.3cm vs 10.81.9cm, p=0.07), though these differences were likewise not statistically significant.
Discussion
The current study’s principle findings are as follows: (1) a DL model developed on a multi-center dataset constituting of preoperative radiographs demonstrated rapid and excellent performance for landmark segmentation concerning proximal femoral morphologic measurements and features; (2) DL measurements demonstrated strong correlations for cortical width, endosteal width, and morphological ratios with the exception of metaphyseal morphologic indices when compared to measurements performed by a fellowship-trained adult reconstruction and joint replacement surgeon; (3) automated morphologic measurements involving the proximal femur demonstrated significant differences in the CCR and CBR between patients sustained a PFF after THA compared with those who did not; however, surgeon measurements also demonstrated differences in the CFI and MCI; (4) the clinical implications of this DL approach include several opportunities for its incorporation into emerging patient-specific risk assessments tools for early PFF.
The current study demonstrated both feasibility and high performance for the development and application of a DL model to identify proximal femoral morphologic parameters accurately and consistently in the setting of THA. Importantly, these landmarks were converted into 18 distant femoral measurements in just 14 seconds, and this has value in decreasing the time and expertise burden required to manually measure and transfer this radiographic assessment for each patient. Furthermore, the metrics for landmark identification were excellent (multi-class dice coefficient of 0.91). This model’s performance is comparable to the performance of previously established DL models used for clinically relevant landmark prediction.[19, 24-27] Jang et al.[28] developed a DL algorithm to perform automated prediction of the hip joint center on standard AP pelvis radiographs with 3,172 patient images. Their landmark prediction model had a multiclass dice coefficient of 0.91, indicating excellent bony landmark segmentation for hip joint center estimation, which is comparable to the performance in the current study.
The majority of automated DL measurements were strongly correlated with those of a fellowship-trained orthopaedic surgeon, suggesting appropriate model robustness. Ensuring model accuracy is an essential process for determining whether the model interpretations of the images that it analyzes approximate how humans interpret and analyzes images in clinical practice.[29-31] Specifically, in the current study, model-human prediction agreement for CWs, EWs, CCR, CFI, CBR, and MCIs, indices were all above 0.74, with no significant differences observed between the surgeon and DL algorithm for all cortical width measurements (p>0.05, ICC:0.88-0.96). Interestingly, model-human measurements of metaphyseal morphologic indices had moderate correlations, while the model systematically measured the endosteal canal width 1.5-mm to 2.2-mm narrower on average than the surgeon, which was significantly different. Possibilities to explain this discrepancy include human subjectivity and/or systematic bias by the DL algorithm in determining the transition between the endosteal intramedullary space and cortex, which may occur secondary to anatomic variability or inconsistency in image resolution or X-ray penetrance. Given that measures such as CFI, CBR, CCR, MCI, and MM are all derived ratios from cortical and endosteal canal width measurements, there were also significant differences in these parameters as well between the surgeon and model. These findings are consistent with those of Yoon et al. in which a DL automated method to measure the CCR and cortical thickness index also had small, but significant, differences from a manual reader.[32]
Critically, this study investigated whether automated parameters still revealed clinical differences in patient cohorts that underwent THA. Both DL-automated calculations of CCR and CBR were significantly higher in patients who sustained PFFs, suggesting that of all the morphologic features studied that these two may specifically hold prognostic value. Prior studies have investigated the capabilities of DL to detect PFF on radiographs, but no study to date has used this approach to automatically identify prognostic measurements associated with PFF. For example, Alzaid et al.[33] applied DL on a dataset of 1,272 radiographs of periprosthetic fracture (comprising fractures around both total knee and total hip arthroplasties) and annotated them with bounding boxes to perform an object detection task. Their best-performing model detected the presence or absence of a periprosthetic fracture with 95% accuracy. Beyond being able to detect the presence or absence of a fracture, this study expands on capabilities and potential value of DL by detecting specific morphologic features associated with fractures. Both the surgeon measurements (statistically significant) and DL-automated measurements (trending toward significance) revealed greater endosteal widths at 2, 7, and 10 cm below the lesser trochanter in patients who sustained an early PFF. Although the measurements at these distal points were systematically lower by 1.5 to 2mm in the DL measurements compared to surgeon measurements, the greater endosteal widths in the fracture cohort for both surgeon and DL measurements align with the significant differences found in CCR and CBR. These findings indicate poorer radiographic bone quality in patients who suffered a fracture compared to those that did not. Importantly, these results concur with that of Bigart et al.[11] who demonstrated that the mean endosteal width at 10 cm distal to the lesser trochanter was greater in patients who sustained a PFF after THA. The endosteal widths were not significantly greater in their study, but they still resulted in significant differences in bone morphology indices of CCR, CBR, and CFI, which is what the current study found with the DL measurements.
The clinical implications of this DL approach include several opportunities for modifying treatment to mitigate early PFF risk. In patients for whom the algorithm identifies threshold values of CCR or CBR classifying them as at-risk for experiencing early PFF, the surgeon may consider use of a different fixation technique, a different stem type, or surgical approach to lower an individual patient’s PFF risk. Another strategy may consist of implicating a protected weight bearing protocol postoperatively prior to advancing to full weight-bearing in a patient that is at-risk.[34] Future studies are needed to externally validate and confirm that CCR and CBR remain differential markers of fracture risk prior to introducing such changes in treatment and postoperative protocols. Furthermore, this algorithm can be applied in larger powered cohort studies to investigate how stem factors such as collars and taper designs may mitigate some of these radiographic risk factors. Finally, Wyles et al. recently created a risk calculator for PFF after THA but radiographic assessments of the femur and hip were not included in the risk calculator.[35] However, studies investigating statistical modeling to predict the risk of clinically relevant outcomes such as the need for THA demonstrated improved performance with the addition of patient-specific DL-automated radiographic parameters.[36] Thus, given the existing literature highlighting the importance of radiographic assessments for PFF risk,[12] future studies could incorporate automated DL measurements to enhance tools for predicting and identifying higher risk patients.
There are several limitations that should be discussed. First, surgeon measurements were only available in whole numbers in the PACs system, whereas our DL model performed measurements to any specified decimal point, which somewhat limits the surgeon comparison with the DL model. This may account for some of the statistical differences observed in endosteal width, which differed slightly between model and human measurements. Second, external validation of this DL algorithm is necessary for confirming generalizability; however, data was included from both a single high-volume institution and a public database to increase data variability. Third, measurements were only performed on anteroposterior radiographs, and the utility of measurements performed on lateral orthogonal views remain unknown. Fourth, Alpaugh et al. demonstrated that femoral component undersizing and incongruity were correlated with increased early PFF risk[12]; however, DL automation of these patient-specific parameters remains uninvestigated and is of interest for future studies. Finally, it is possible that the study was underpowered to detect differences in certain morphologic features between the PFF and control cohorts; however, the algorithm was still able to discern statistical differences between CBR and CCR. It is possible that the remaining measurements could show statistical differences in larger studies, which is of interest for the future application of this algorithm.
Conclusions
This study demonstrated the use of an DL tool to extract radiographic morphologic features relevant to PFF after THA. The automated parameters showed significant differences, demonstrating significantly worse distal femoral bone quality in patients who sustained a PFF after THA compared to matched controls who did not.
References
1. Springer, B.D., et al., Perioperative Periprosthetic Femur Fractures are Strongly Correlated With Fixation Method: an Analysis From the American Joint Replacement Registry. J Arthroplasty, 2019. 34(7S): p. S352-S354.
2. Gausden, E.B., et al., Outcomes of Vancouver C Periprosthetic Femur Fractures. J Arthroplasty, 2021. 36(10): p. 3601-3607.
3. Shields, E., et al., Mortality and Financial Burden of Periprosthetic Fractures of the Femur. Geriatr Orthop Surg Rehabil, 2014. 5(4): p. 147-53.
4. Boylan, M.R., et al., Mortality Following Periprosthetic Proximal Femoral Fractures Versus Native Hip Fractures. J Bone Joint Surg Am, 2018. 100(7): p. 578-585.
5. Drew, J.M., et al., Survivorship After Periprosthetic Femur Fracture: Factors Affecting Outcome. J Arthroplasty, 2016. 31(6): p. 1283-1288.
6. Cooper, H.J., A.P. Jacob, and J.A. Rodriguez, Distal fixation of proximally coated tapered stems may predispose to a failure of osteointegration. J Arthroplasty, 2011. 26(6 Suppl): p. 78-83.
7. Cooper, H.J. and J.A. Rodriguez, Early Post-operative Periprosthetic Femur Fracture in the Presence of a Non-cemented Tapered Wedge Femoral Stem. HSS J, 2010. 6(2): p. 150-4.
8. Lamb, J.N., et al., A calcar collar is protective against early periprosthetic femoral fracture around cementless femoral components in primary total hip arthroplasty: a registry study with biomechanical validation. Bone Joint J, 2019. 101-B(7): p. 779-786.
9. Abdel, M.P., et al., Epidemiology of periprosthetic fracture of the femur in 32 644 primary total hip arthroplasties: a 40-year experience. Bone Joint J, 2016. 98-B(4): p. 461-7.
10. Tanzer, M., et al., Is Cemented or Cementless Femoral Stem Fixation More Durable in Patients Older Than 75 Years of Age? A Comparison of the Best-performing Stems. Clin Orthop Relat Res, 2018. 476(7): p. 1428-1437.
11. Bigart, K.C., et al., Does Femoral Morphology Predict the Risk of Periprosthetic Fracture After Cementless Total Hip Arthroplasty? J Arthroplasty, 2020. 35(6S): p. S359-S363.
12. Alpaugh, K., et al., Femoral Component Undersizing and Alignment are Risk Factors for Early Periprosthetic Femur Fracture. J Arthroplasty, 2022. 37(7S): p. S604-S610.
13. Burns, J.E., J. Yao, and R.M. Summers, Artificial Intelligence in Musculoskeletal Imaging: A Paradigm Shift. J Bone Miner Res, 2020. 35(1): p. 28-35.
14. Rouzrokh, P., et al., A Deep Learning Tool for Automated Radiographic Measurement of Acetabular Component Inclination and Version After Total Hip Arthroplasty. Journal of Arthroplasty, 2021. 36(7): p. 2510-+.
15. Karnuta, J.M., et al., Artificial Intelligence to Identify Arthroplasty Implants From Radiographs of the Hip. J Arthroplasty, 2021. 36(7S): p. S290-S294 e1.
16. Borjali, A., et al., Comparing the performance of a deep convolutional neural network with orthopedic surgeons on the identification of total hip prosthesis design from plain radiographs. Med Phys, 2021. 48(5): p. 2327-2336.
17. Yamamoto, N., et al., Deep Learning for Osteoporosis Classification Using Hip Radiographs and Patient Clinical Covariates. Biomolecules, 2020. 10(11).
18. Jang, S.J., et al., John Charnley Award: Deep Learning Prediction of Hip Joint Center on Standard Pelvis Radiographs. J Arthroplasty, 2022.
19. Jang, S.J., et al., Leg-Length Discrepancy Variability on Standard Antero-Posterior Pelvis Radiographs: An Analysis using Deep Learning Measurements. J Arthroplasty, 2023.
20. Lester, G., The Osteoarthritis Initiative: A NIH Public-Private Partnership. HSS J, 2012. 8(1): p. 62-3.
21. Zou, K.H., et al., Statistical validation of image segmentation quality based on a spatial overlap index. Acad Radiol, 2004. 11(2): p. 178-89.
22. Virtanen, P., et al., SciPy 1.0: fundamental algorithms for scientific computing in Python. Nat Methods, 2020. 17(3): p. 261-272.
23. Seabold, S. and J. Perktold. Statsmodels: Econometric and Statistical Modeling with Python. 2010.
24. Jang, S.J., et al., Comparison of tibial alignment parameters based on clinically relevant anatomical landmarks : a deep learning radiological analysis. Bone Jt Open, 2022. 3(10): p. 767-776.
25. Steele, J.R., et al., Deep Learning Phenotype Automation and Cohort Analyses of 1,946 Knees using the Coronal Plane Alignment of the Knee Classification. J Arthroplasty, 2023.
26. Jang, S.J., et al., Standardized Fixation Zones and Cone Assessments for Revision Total Knee Arthroplasty Using Deep Learning. J Arthroplasty, 2023.
27. Kunze, K.N., et al., Radiographic findings involved in knee osteoarthritis progression are associated with pain symptom frequency and baseline disease severity: a population-level analysis using deep learning. Knee Surg Sports Traumatol Arthrosc, 2023. 31(2): p. 586-595.
28. Jang, S.J., et al., John Charnley Award: Deep Learning Prediction of Hip Joint Center on Standard Pelvis Radiographs. J Arthroplasty, 2022. 37(7S): p. S400-S407 e1.
29. Oeding, J.F., et al., A practical guide to the development and deployment of deep learning models for the orthopedic surgeon: part II. Knee Surg Sports Traumatol Arthrosc, 2023.
30. Oeding, J.F., et al., A practical guide to the development and deployment of deep learning models for the Orthopedic surgeon: part I. Knee Surg Sports Traumatol Arthrosc, 2023. 31(2): p. 382-389.
31. Hill, B.G., et al., Deep Learning and Imaging for the Orthopaedic Surgeon: How Machines "Read" Radiographs. J Bone Joint Surg Am, 2022. 104(18): p. 1675-1686.
32. Yoon, S.-J., et al., Estimation and Comparison of Cortical Thickness Index and Canal-to-Calcar Ratio Using Manual Method and Deep Learning Method. Journal of Electrical Engineering & Technology, 2020. 15(3): p. 1399-1404.
33. Alzaid, A., et al., Automatic detection and classification of peri-prosthetic femur fracture. Int J Comput Assist Radiol Surg, 2022. 17(4): p. 649-660.
34. Kubiak, E.N., et al., Early weight bearing after lower extremity fractures in adults. J Am Acad Orthop Surg, 2013. 21(12): p. 727-38.
35. Wyles, C.C., et al., Creation of a Patient-Specific Total Hip Arthroplasty Periprosthetic Fracture Risk Calculator. J Arthroplasty, 2023.
36. Jang, S.J., et al., An Interpretable Machine Learning Model for Predicting 10-Year Total Hip Arthroplasty Risk. J Arthroplasty, 2023.
37. Schock, J., et al., Automated Analysis of Alignment in Long-Leg Radiographs by Using a Fully Automated Support System Based on Artificial Intelligence. Radiol Artif Intell, 2021. 3(2): p. e200198.
38. Zheng, Q., et al., Deep Learning Measurement of Leg Length Discrepancy in Children Based on Radiographs. Radiology, 2020. 296(1): p. 152-158.
39. Pizer, S.M., et al., Adaptive histogram equalization and its variations. Computer Vision, Graphics, and Image Processing, 1987. 39(3): p. 355-368.
40. Howard, J. and S. Gugger, fastai: A Layered API for Deep Learning. ArXiv, 2020. abs/2002.04688.
Table and Figure Legend
Table 1: Measurement Descriptors
Table 2: Measurement Comparison between Surgeon and Model
Table 3: Comparison Between Early Periprosthetic Fracture and Control Cohort: Surgeon Measured Parameters
Table 4: Comparison Between Early Periprosthetic Fracture and Control Cohort: Deep Learning Automated Parameters
Figure 1: Patient Selection Workflow for Model Training, Validation, and Testing
Figure 2: Anteroposterior Radiographs Parameters for Preoperative Assessment of Proximal Femoral morphology
Figure 3: Deep Learning Model and Image Processing Workflow. Patient Underwent Total Hip Arthroplasty on the Left Hip
Figure 4: Example of Manual Surgeon Measurement Compared to Automated Deep Learning Measurements
Figure 5: Surgeon vs. Deep Learning Measurements for Fracture (Blue) and Control (Orange) Cohorts for all 18 Measurements
Supplemental Materials Legend
Supplemental Figure 1: Bland-Altman Plot Analysis of Endosteal and Cortical Width Measurements in Millimeters
Supplemental Figure 2: Bland-Altman Plot Analysis of All Morphologic Ratio Measurements
Supplemental Materials
Two independent datasets (i.e. institutional and OAI database) were used to enhance the versatility and fidelity of the training data for DL algorithm creation for increased generalization. The sample sizes were chosen based on previous studies investigating the use of DL on radiographs for measurement automation and using a threshold of 0.85 for the dice coefficient during model training.[21, 37, 38] To increase variability in images that the model learned from, distinct image augmentations (i.e. crop, rotate, flip, contrast change) were introduced during each cycle of model training.
All images were resized to 512x512 pixels and normalized with adaptive histogram equalization to allow for contrast enhancement.[39] For both convolutional neural networks (CNN), a pre-trained deep learning model for image analysis, ResNet-34, was used as the base architecture. The fastai (V2.3.0)[40] deep learning library was used for model training, parameter optimization, and data augmentation. Batch size was set to 4. The multi-class dice coefficient was used as the optimization metric for the U-Net model. Both CNNs were trained for 150 epochs and the best-performing model was chosen on the validation set before testing. All models were trained in PyTorch on a 12 gigabyte NVIDIA Tesla K80 Graphics Processing Unit (GPU).
21. Zou, K.H., et al., Statistical validation of image segmentation quality based on a spatial overlap index. Acad Radiol, 2004. 11(2): p. 178-89.
37. Schock, J., et al., Automated Analysis of Alignment in Long-Leg Radiographs by Using a Fully Automated Support System Based on Artificial Intelligence. Radiol Artif Intell, 2021. 3(2): p. e200198.
38. Zheng, Q., et al., Deep Learning Measurement of Leg Length Discrepancy in Children Based on Radiographs. Radiology, 2020. 296(1): p. 152-158.
39. Pizer, S.M., et al., Adaptive histogram equalization and its variations. Computer Vision, Graphics, and Image Processing, 1987. 39(3): p. 355-368.
40. Howard, J. and S. Gugger, fastai: A Layered API for Deep Learning. ArXiv, 2020. abs/2002.04688.
Figures
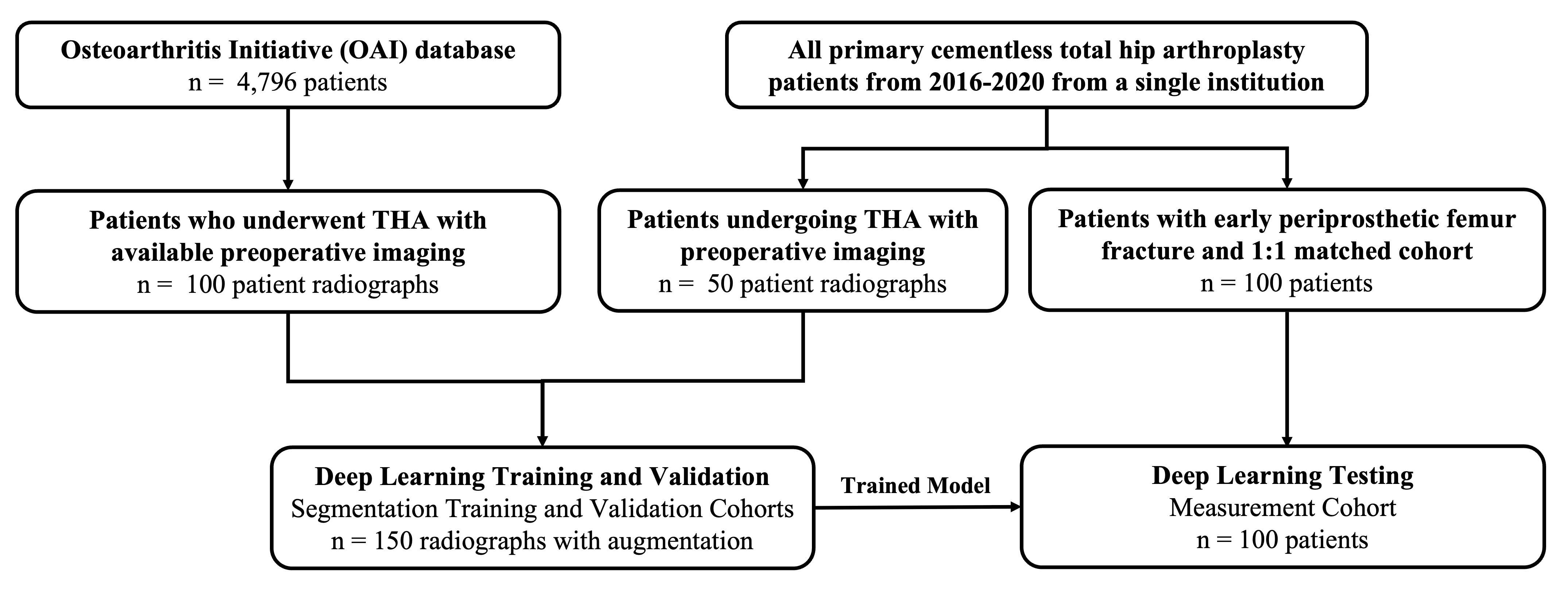
Figure 1
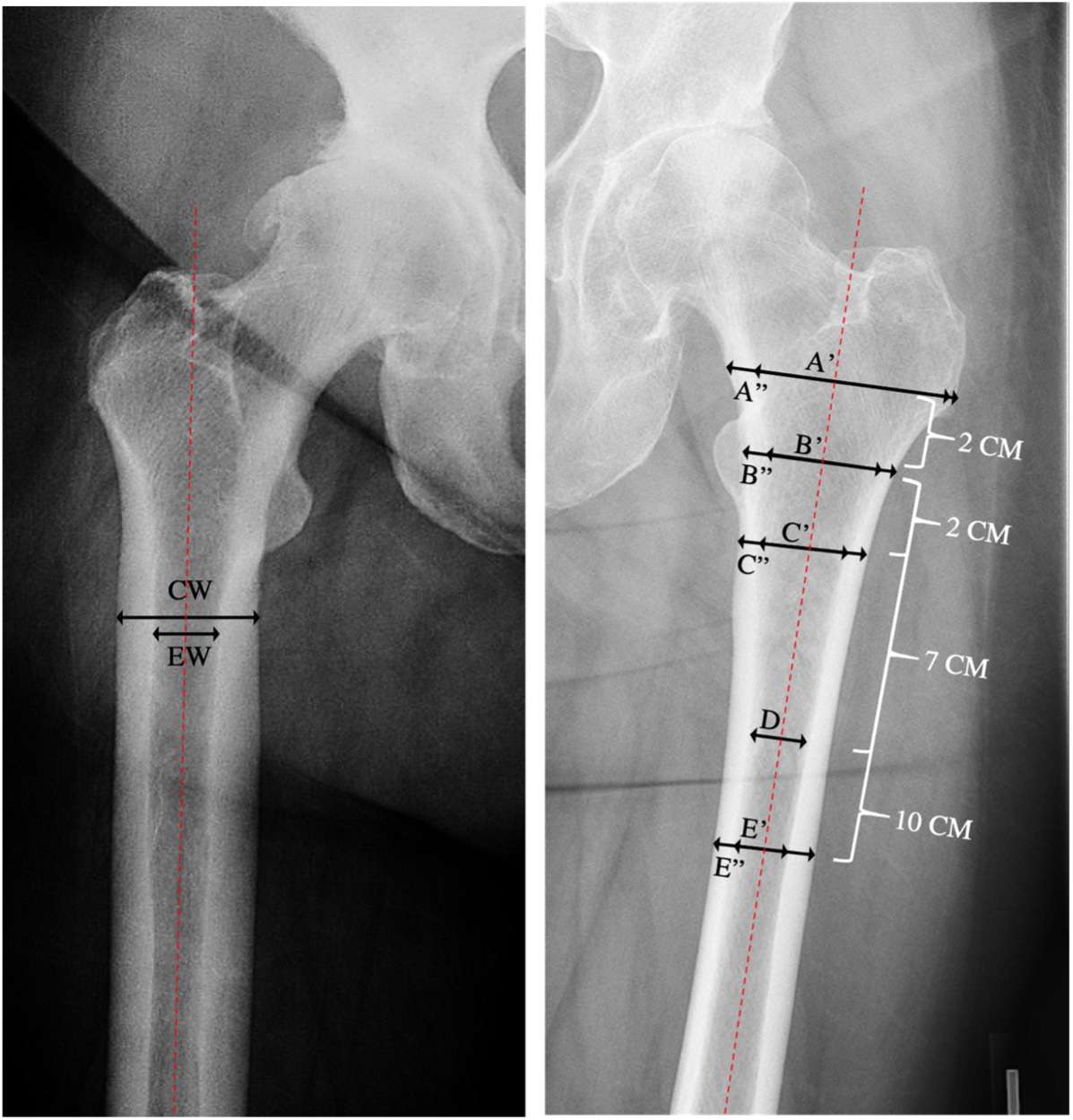
Figure 2
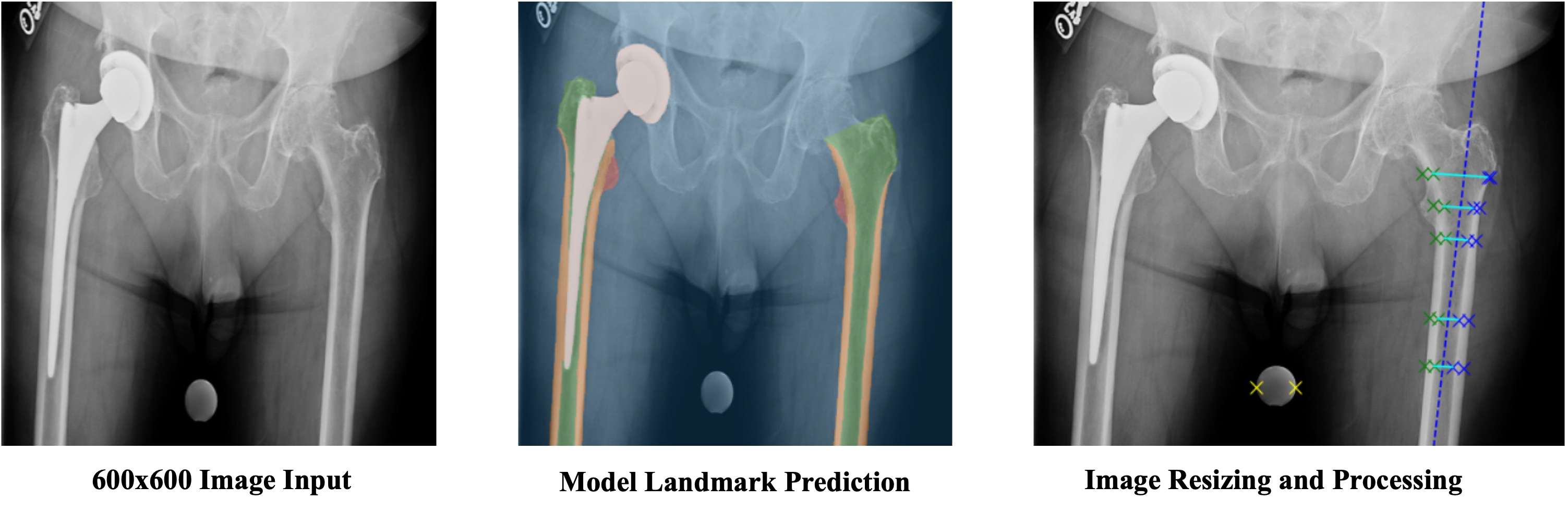
Figure 3
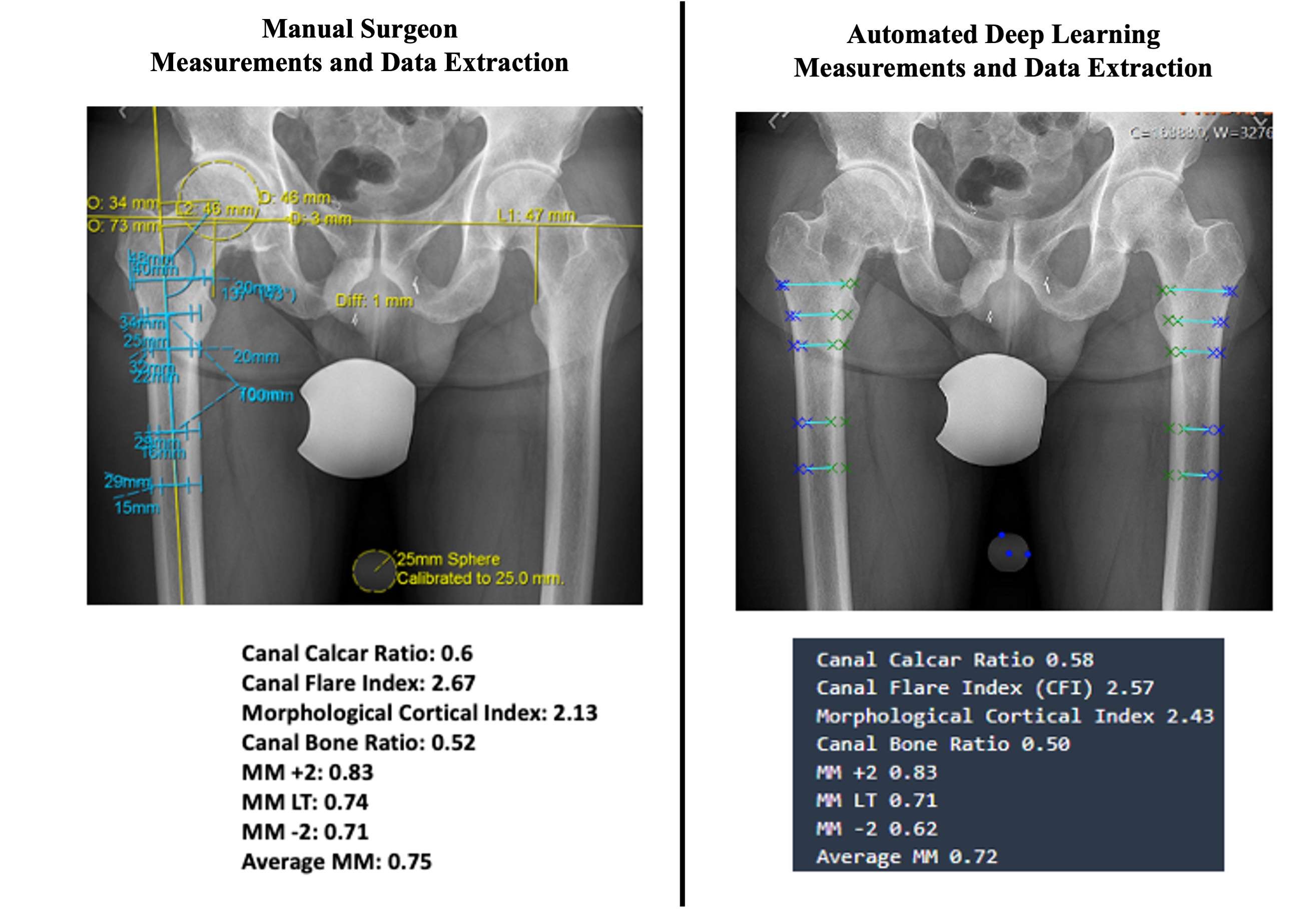
Figure 4
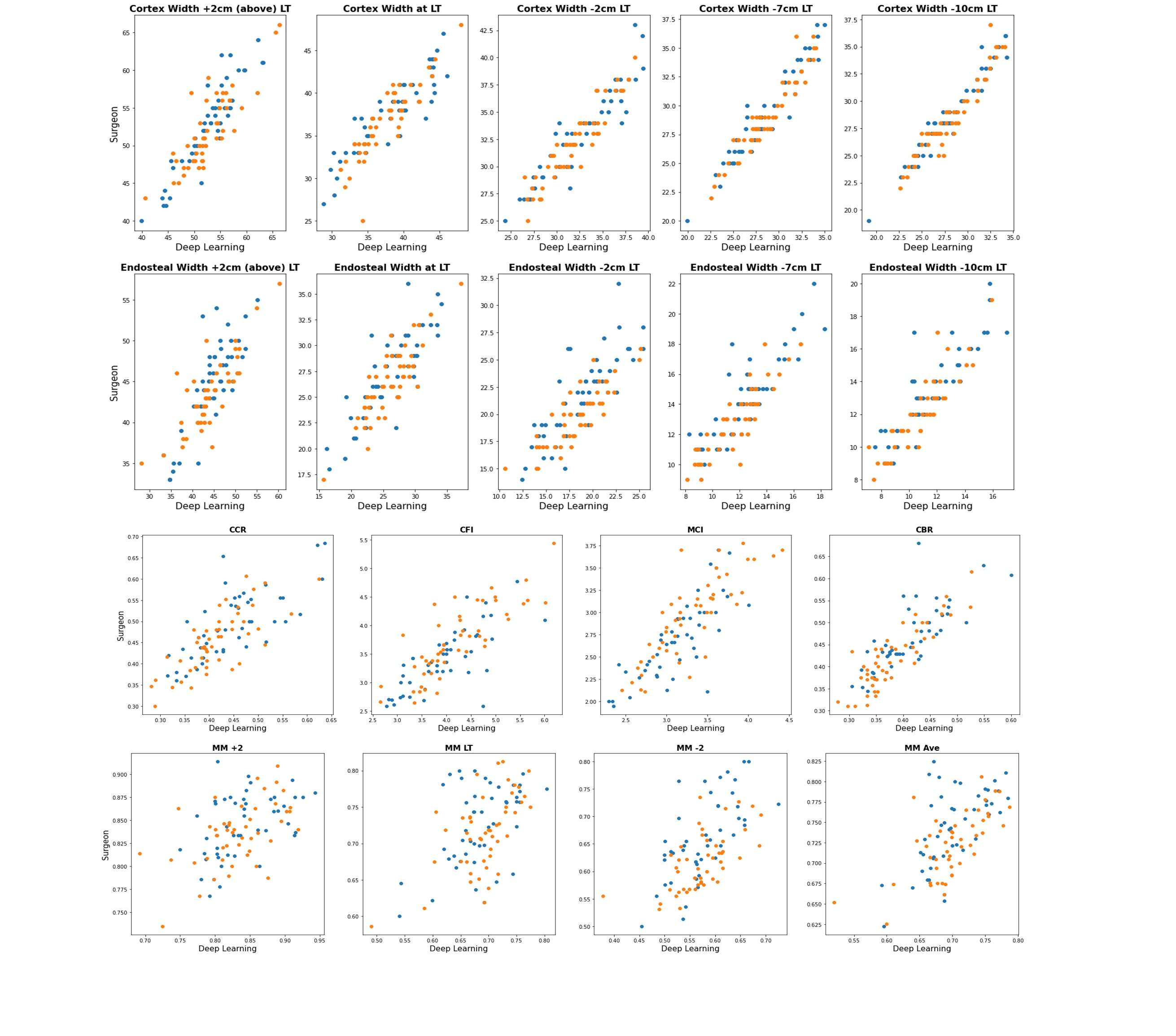
Figure 5
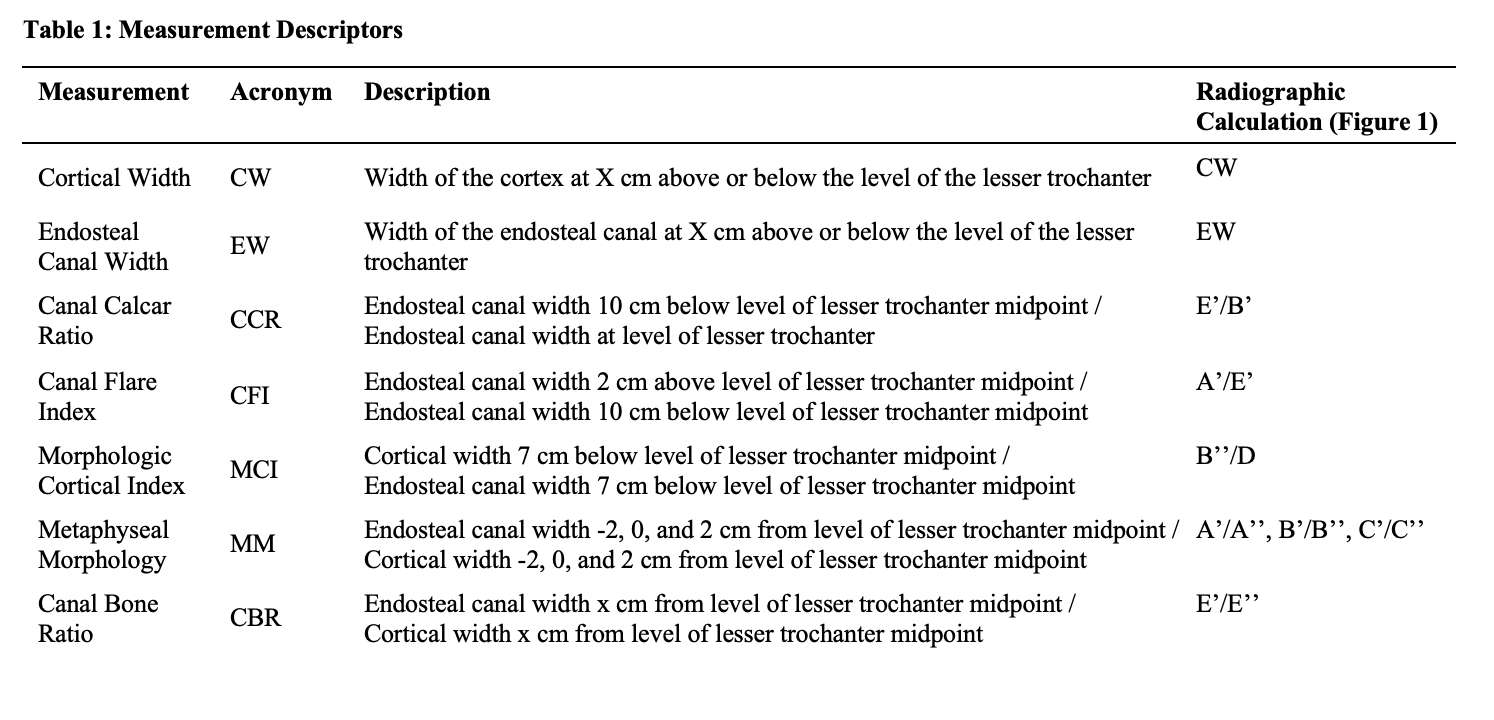
Figure 6
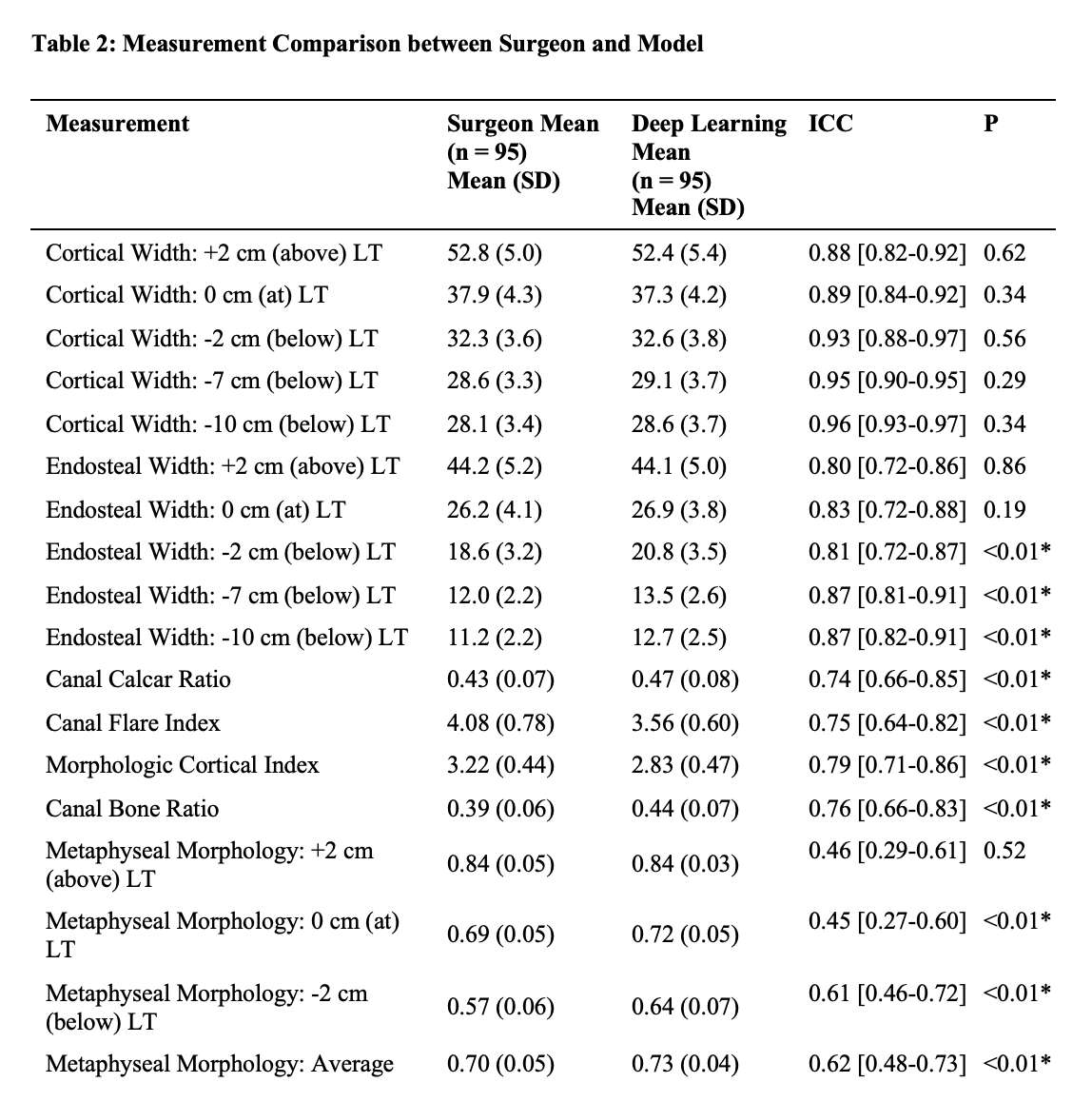
Figure 7
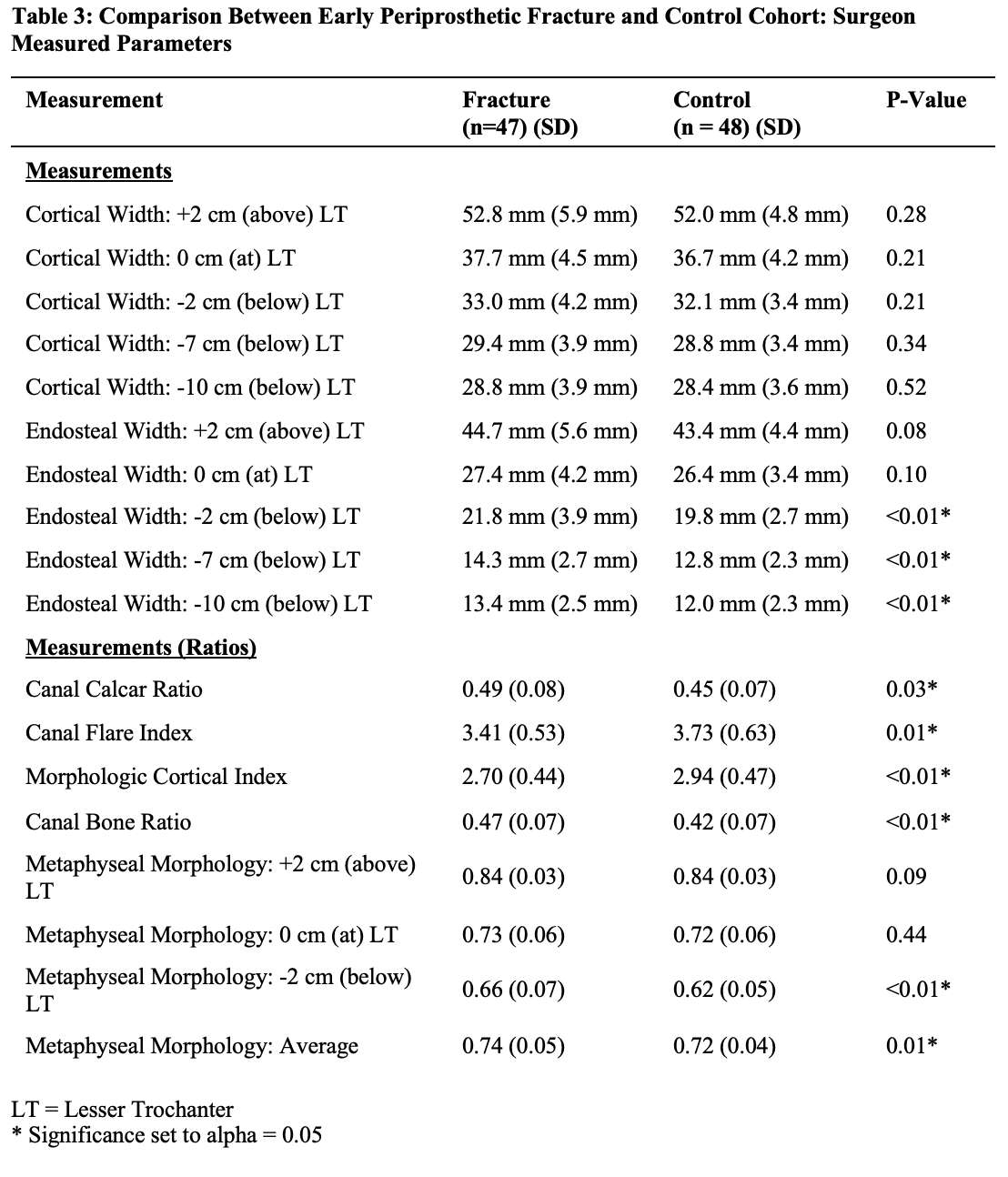
Figure 8
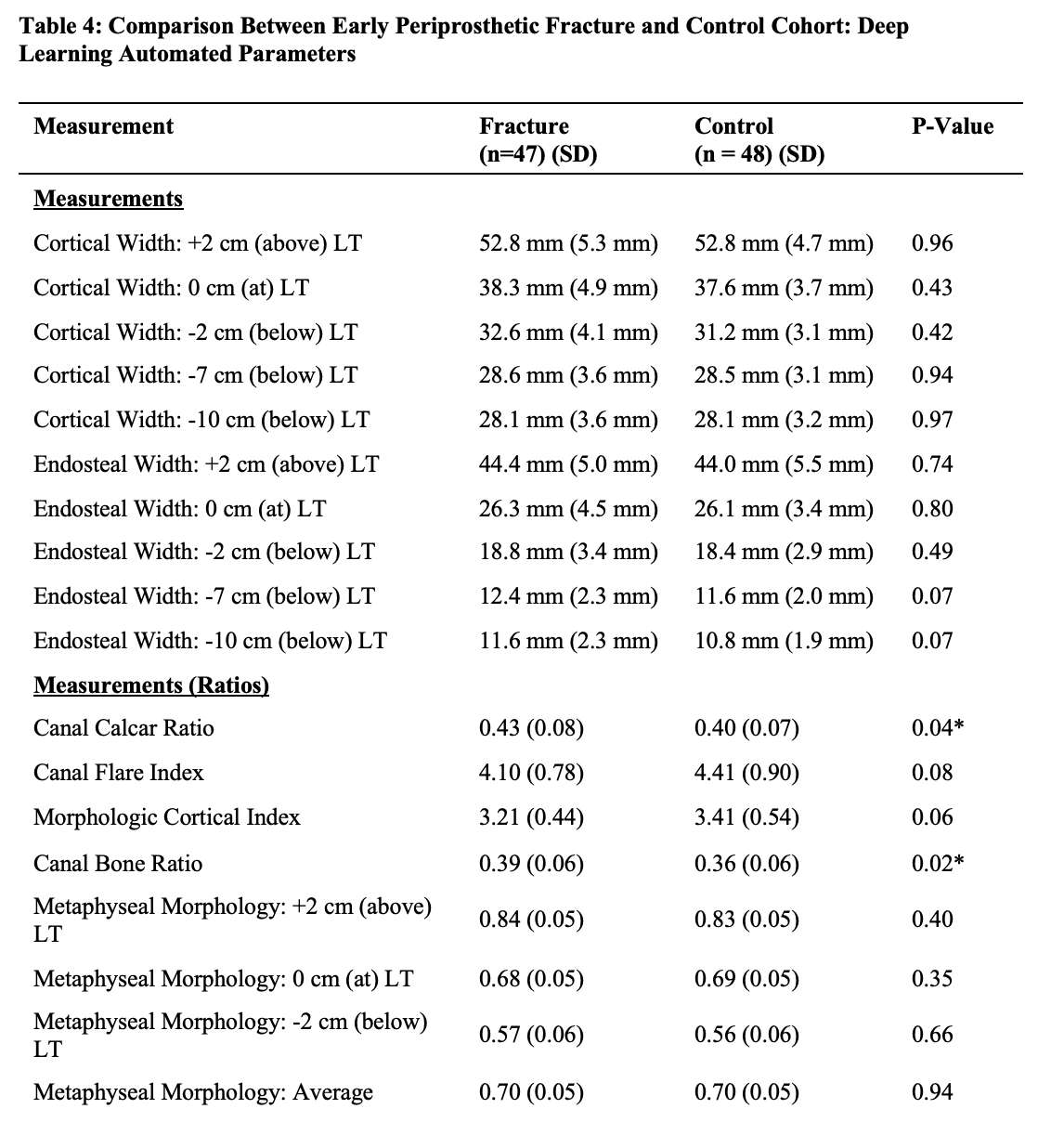
Figure 9
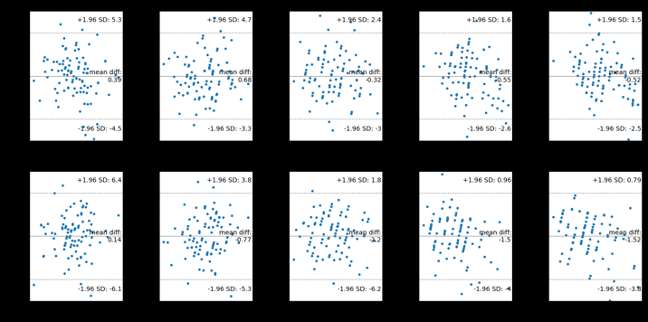
Figure 10
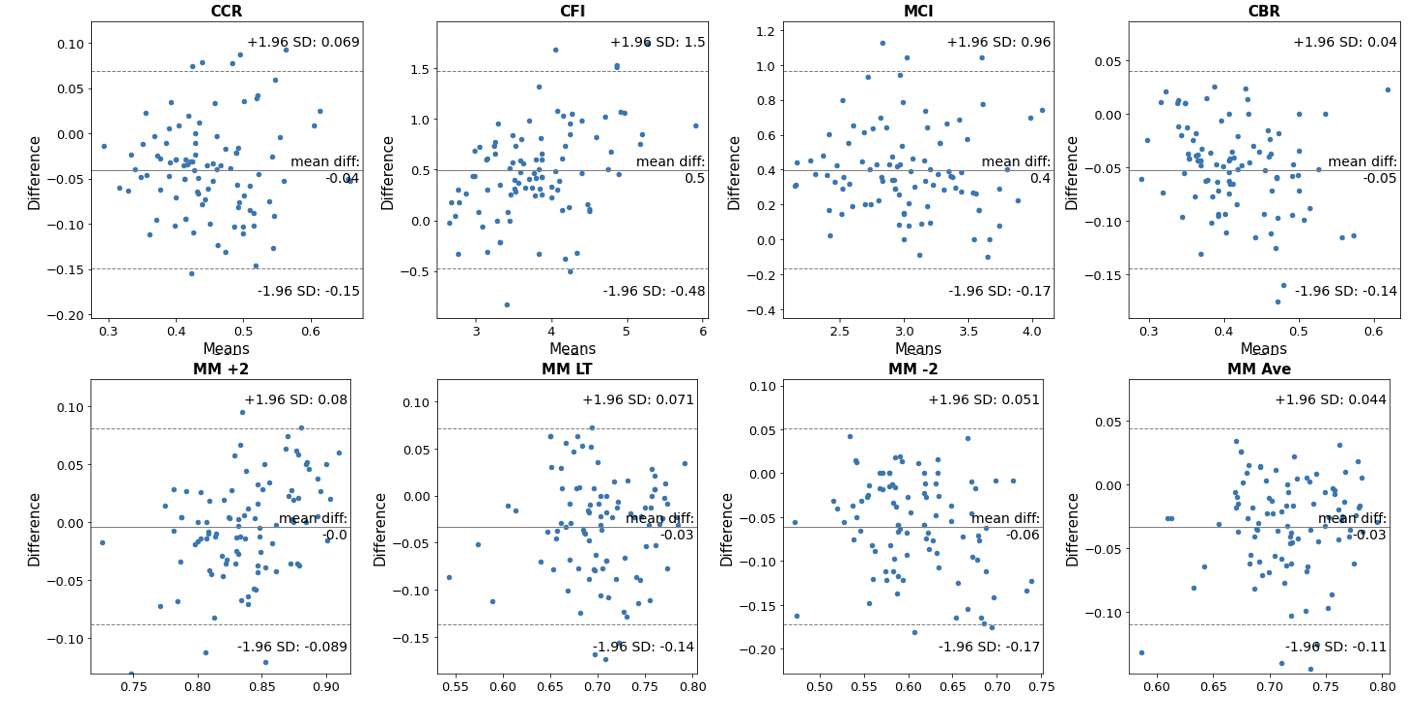
Figure 11#8414
Magnitude and Direction of Tibial Component Migration in Patients With Aseptic Loosening in Total Knee Arthroplasty
*Matthew Hickey - The University of British Columbia - Vancouver, Canada
Bart Kaptein - LUMC - Leiden, Select Country
Carolyn Anglin - University of Calgary - Calgary, Canada
Bassam Masri - University of British Columbia / Department of Orthopaedics - Vancouver, Canada
Antony J Hodgson - The University of British Columbia - Vancouver, Canada
*Email: matthew.hickey@hiphealth.ca
Introduction
The issue of which alignment strategy to use in total knee arthroplasties to improve implant survival and other clinical outcomes has become one of the most controversial topics in this field. Radiostereometric analysis (RSA) has been used to study implant migration patterns following the index procedure. Higher migration magnitudes (measured as the Maximum Total Point Motion (MTPM)) are considered indicative of loosening and associated with increased risk of revision. Unfortunately, many RSA-based studies principally report the scalar-valued MTPM rather than the full 6 degree-of-freedom relative motion, so such studies fail to distinguish between different forms of migration. We believe that important insights into loosening mechanisms could result from studying the 6 degree-of-freedom patterns of subsidence across patients who go on to revision. The purpose of this study is to report on a first analysis of 6 degree-of-freedom tibial migration patterns in a group of patients with implants classified as loose or needing revision who were monitored using RSA following their index procedure.
Methods
In a recent RSA cohort study (van Hamersveld 2019) that involved 85 patients with an 11-year median follow-up, 7 patients developed aseptic loosening, 3 of whom were eventually revised (Fig.1). The authors found that postoperative knee alignment or tibial component alignment that was mechanically in varus/valgus beyond ±3? yielded higher MTPM. Extending this study, we calculated the three translational and rotational parameters associated with each measurement (relative to the index surgery) and plotted the trends over time for each of the 7 patients. We then completed a linear regression to identify trends, calculated the statistical significance based on the null hypothesis that there were no changes over time, and applied a Bonferroni correction to account for the six multiple comparisons, adjusting the threshold for statistical significance to α=0.0083.
Results
When analysing tibial implant migration in aseptically loose components we observed two trends that achieved statistical significance: anterior translation of the tibial implant (6/7 migrated at least 1 mm, Fig.2C), and varus/valgus rotation (varus migration—see Fig.2F; range [1.0?-5.7?]). At least one of these trends was present in all 7 patients (Fig.3). Tibial component subsidence (superior/inferior translation) did not achieve statistical significance (Fig.2B), though its p-value (0.0087) was very close to the threshold; however, five patients exhibited virtually no subsidence, while two exhibited significant subsidence.
Discussion
While many studies use RSA to track migration of components, we found none that specifically focused on patients with loose components or that aimed to identify migration trends in this subset. Since RSA is not commonly used clinically and implant loosening is a rare occurrence, it is exceptionally rare to have RSA-based implant migration data for implants that are loose or recommended for revision. Yet the analysis of such patterns could provide significant insights into the causal mechanisms underlying aseptic loosening and need for revision surgery. Therefore, to facilitate future investigations, we recommend that authors doing RSA analyses of total knee implants present more complete 6 DOF implant migration data and include the raw implant migration data as supplemental material.
Figures
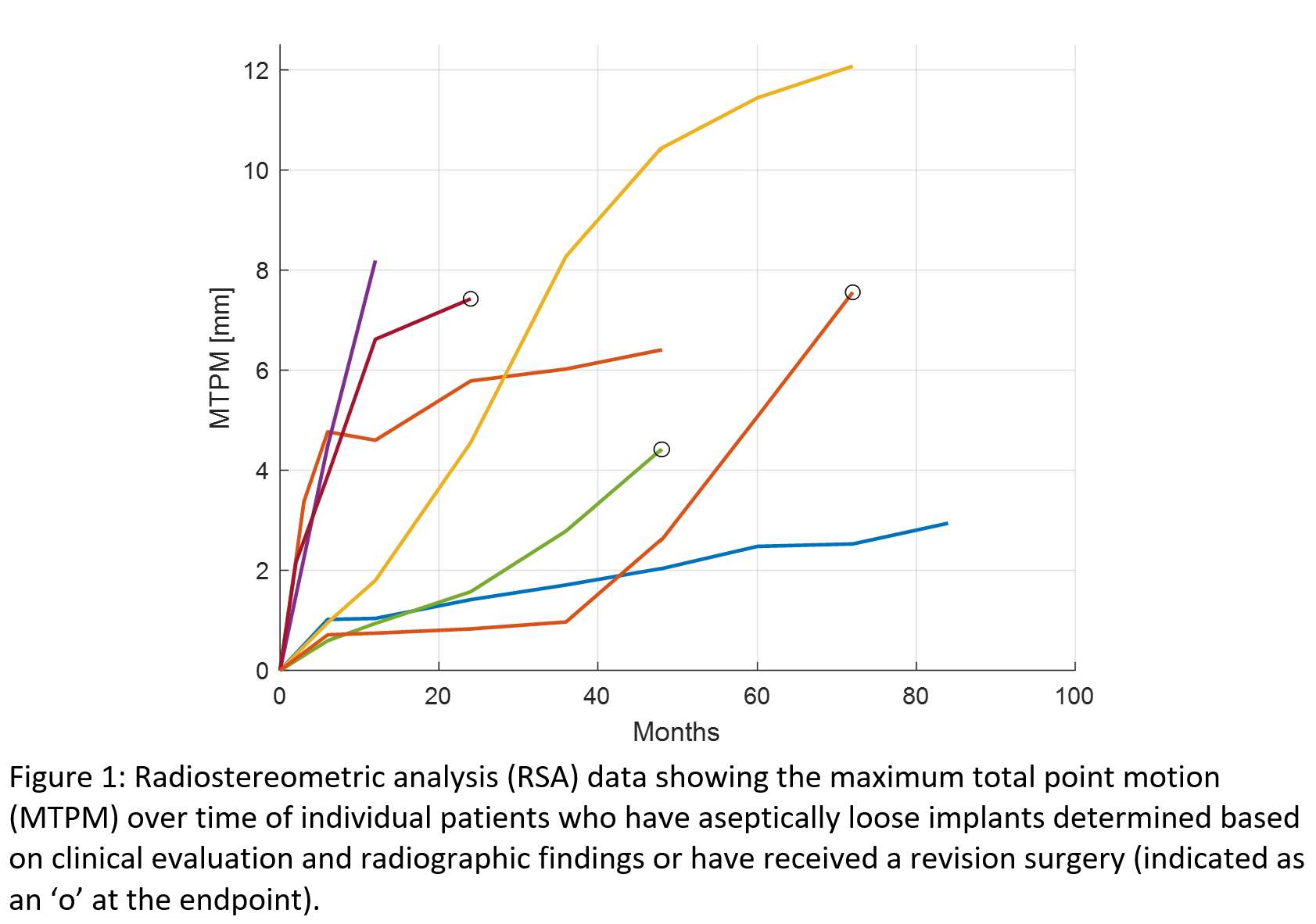
Figure 1
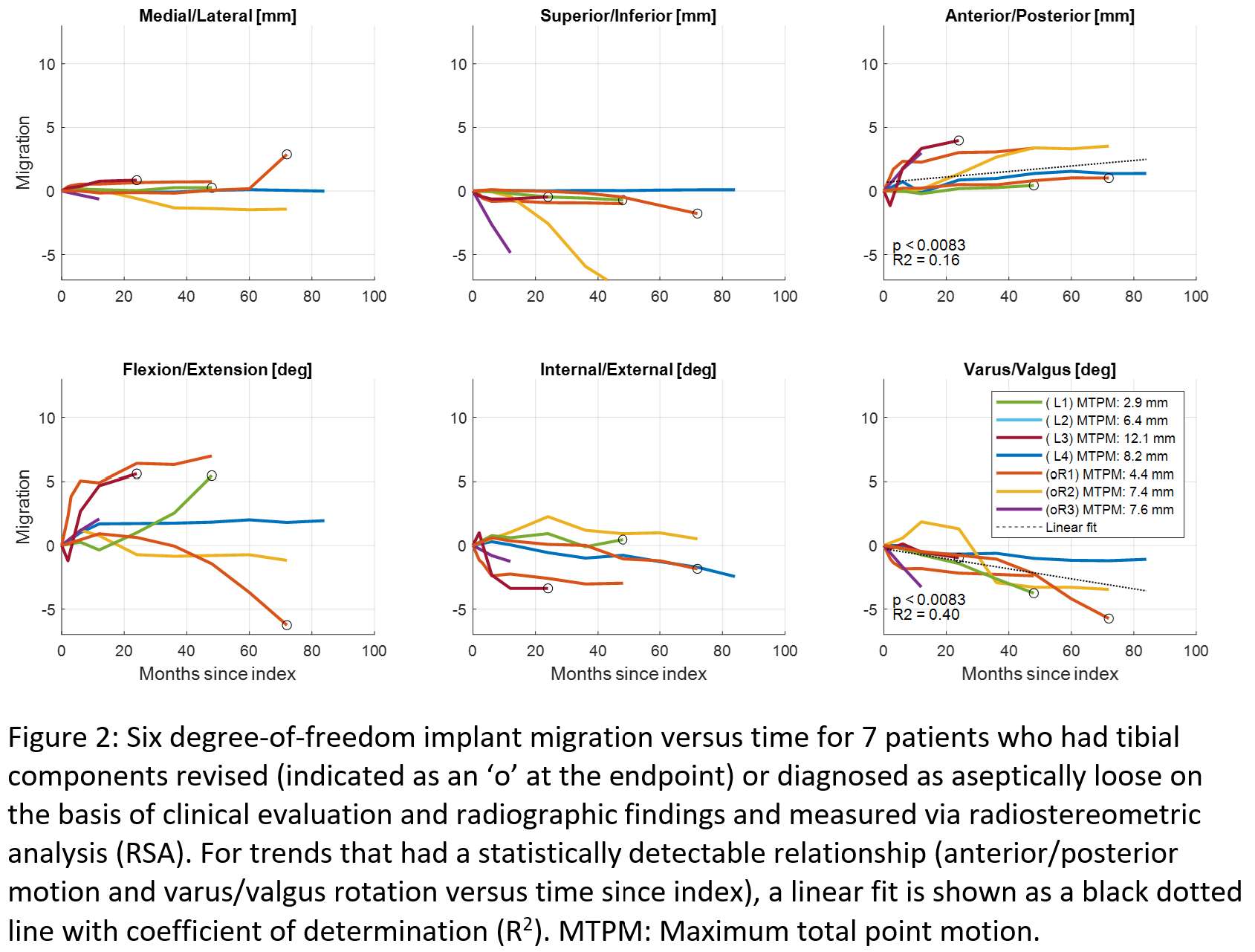
Figure 2
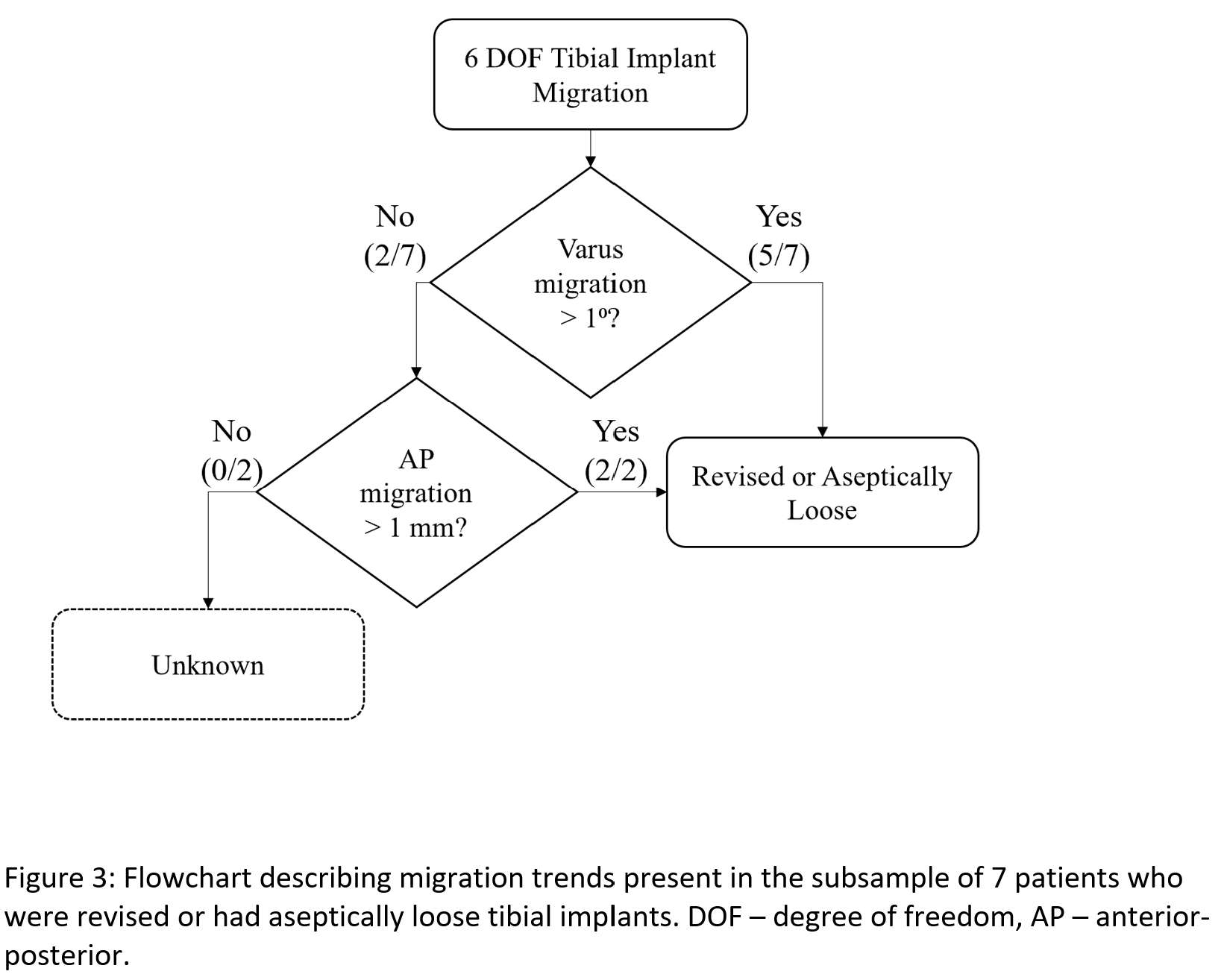
Figure 3#8261
Optimally Sized Tibial Implants Match Patient Coverage and Reduce Implant Stock
*Frederik Masure - Imperial College London - London, GB
Alex Liddle - Imperial College London - London, United Kingdom
Thomas Burge - Imperial College London - London, United Kingdom
Maxwell Munford - Imperial College London - London, United Kingdom
Jonathan Jeffers - Imperial College London - London, United Kingdom
*Email: fcm3718@ic.ac.uk
Introduction:
While performing a Unicompartmental Knee Arthroplasty (UKA), a surgeon determines a suitable implant size based on fit and minimising over/underhang (OUH). Poorly matching implant size to the patient can lead to instability, subsidence and loosening, resulting in long term clinical consequences. Existing off-the-shelf (OTS) implants are typically linearly sized with uniform spacing, neglecting any coupling between patient anatomy size, and shape.
This study proposed a method of optimising implant sizes to maximise coverage across the population. Secondly, it assessed the difference in implant sizing requirements between demographics in the US and the suitability of an existing implant’s geometry and sizes for that population.
Methods:
The study focused on UKA medial tibial components, a common surgery with frequent complications caused by inadequate sizing. A sample of 78 CT scans of patient tibiae were collected reflecting the ethnicity and sex demographic of the US population. Tibial resection was done computationally, using anatomical landmarks to replicate typical surgical technique.
A fully computational design of experiments (DOE) approach was used. The template for a commercially available fixed-bearing implant was scaled using full factorial dimensions of 17-35mm medio-laterally (ML) and 33-63mm anterio-posteriorly (AP) with 0.5mm intervals, creating 2183 modified implant templates. Each scaled template was located on the surface of the 78 tibia resections using an iterative closest point (ICP) method to minimise error. The maximum OUH was extracted for each implant size.
Optimally distributed implant sizes in ML and AP were found by maximising the total number of patient tibiae that could be treated within an OUH threshold between 1 and 3 mm for a given number of implants. This was achieved through a particle swarm optimisation (PSO) method. The data was compared to the actual sizes of the same commercially available implant which uses a linearly scaled size range.
Results:
Optimally sizing implants covered up to 23% more patients than linear scaling, depending on the OUH criteria, whilst using the same number of implants (Figure 1). For an OUH criteria of <2mm, optimal sizing covered a higher percentage of the population with 4 implants (77%) than 8 linearly scaled implants (73%) by having implants with non-linear scaling and spacing (Figure 2).
The study showed that, Caucasian and Black/African American men required the largest implants, with an average of 31mm in ML by 54mm in AP. Women in these demographics required smaller implants, averaging 28mm in ML by 48 mm in AP. The Asian demographic required smaller implants, with men requiring implants of average 26 mm ML by 49mm AP and women requiring 25 mm ML by 46 mm AP (Figure 3).
Conclusion:
Optimising implant sizing can achieve a substantially improved tibial coverage compared to conventional, evenly spaced and linearly scaled implants. This can aid in providing better overall outcomes for patients by reducing the OUH, as well as lessening the burden of implant and instrument stock required in surgery by reducing the OTS components needed.
Figures
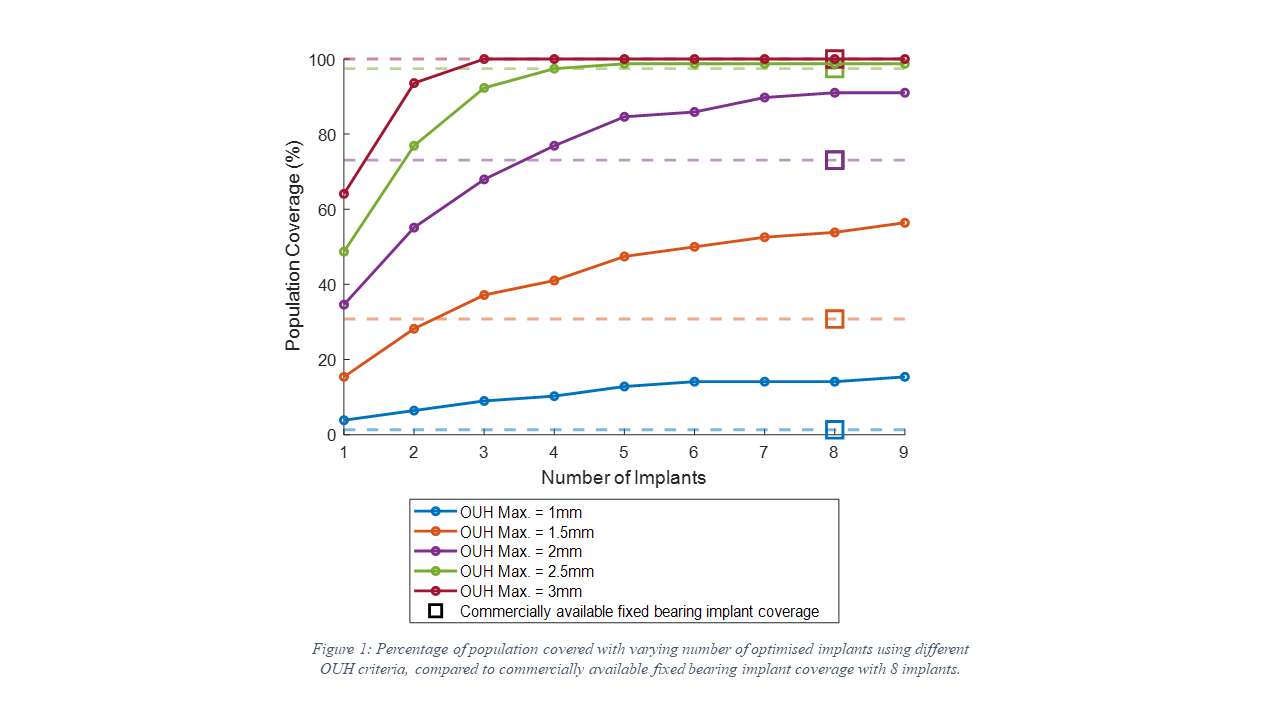
Figure 1
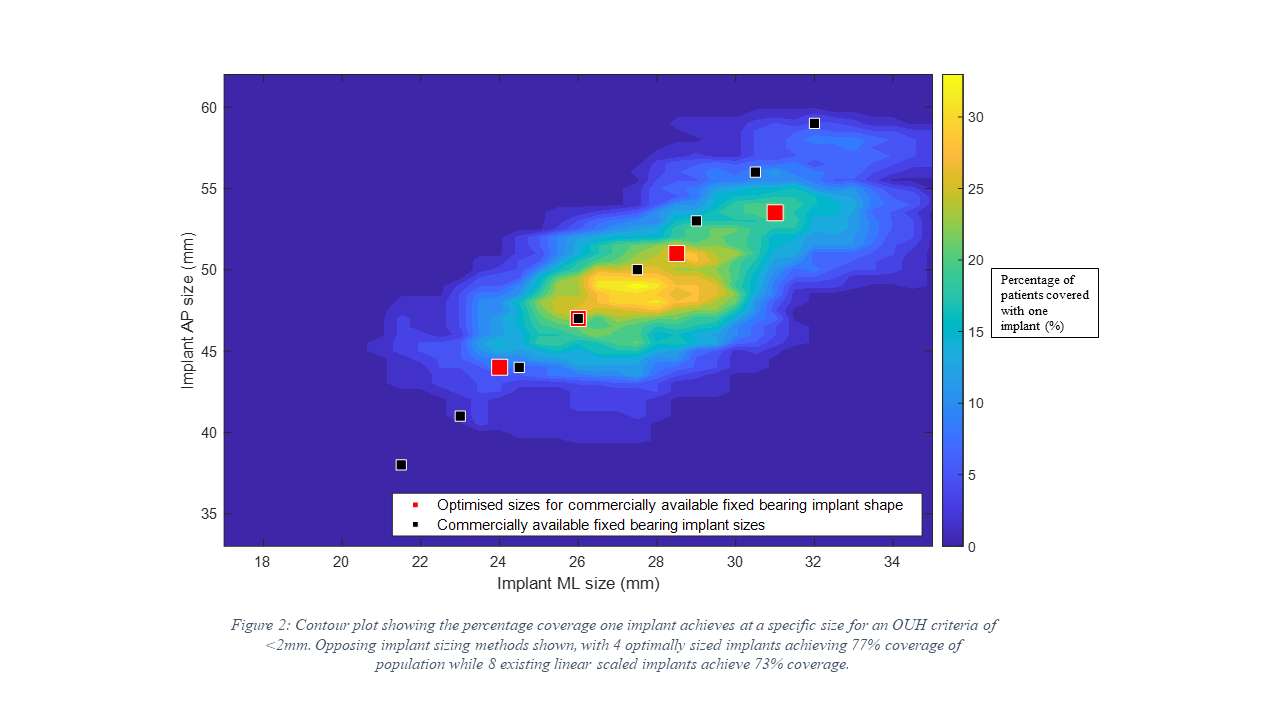
Figure 2
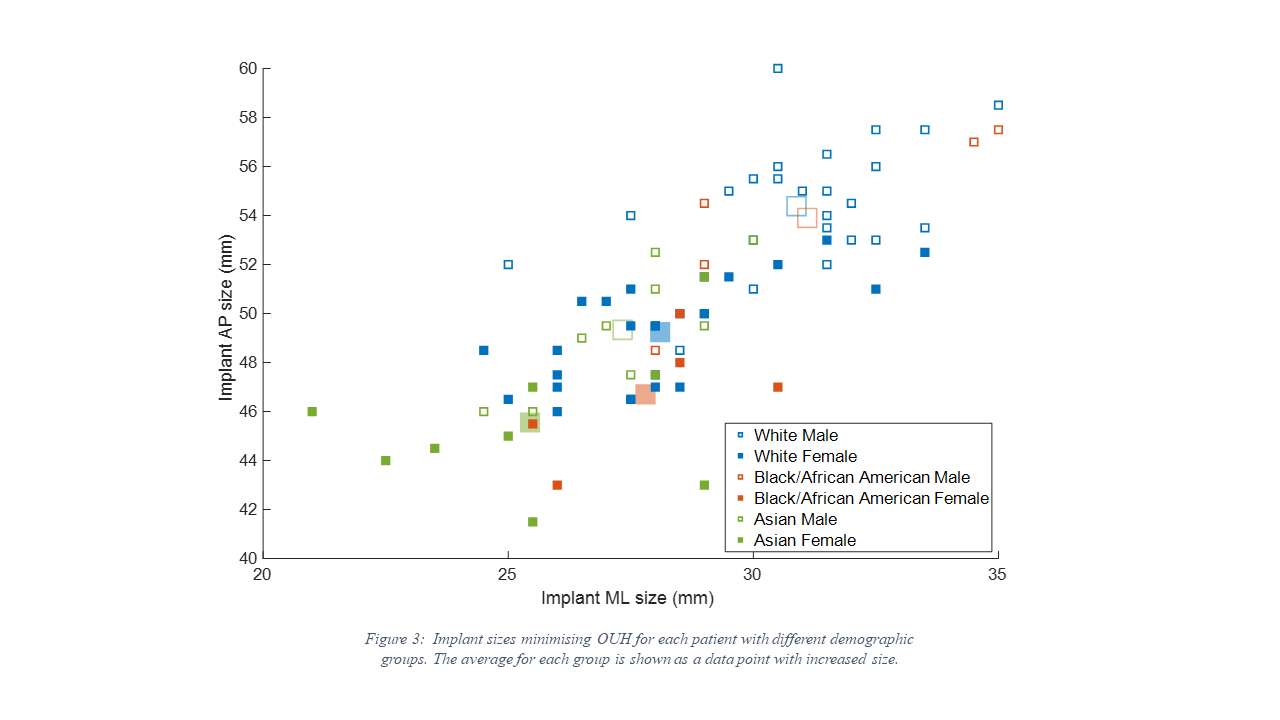
Figure 3#8724
TITLE: Total Knee Bearing Design Without Cruciate Ligaments
*Peter Walker - New York University Langone Orthopedic Hospital - New York City, USA
John Perez - New York University - New York, USA
Daniel Hennessy - NYU Langone Orthopedic Hospital - New York, USA
*Email: ptrswlkr@aol.com
TITLE: Total Knee Bearing Design without Cruciate Ligaments
INTRODUCTION: Non-cruciate designs, including the ultracongruent and medial pivot, are becoming more widely and lateral constraint, or a medial pivot. This study proposes the criterion of reproducing the laxity behaviour of the anatomic knee, laxity defined as the displacements of the femur on the tibia in the loaded knee, when shear and torque are applied. The purpose of this experimental study was to determine the radii of the lateral and medial femoral and tibial bearing surfaces which would most closely reproduce anatomic laxity patterns.
METHODS: An average femoral component was designed from 100 early arthritic knees. A fully-conformity tibial surface was made as an imprint of the femoral component in extension. The tibia was divided into 4 quadrants. Reduced conformity in each quadrant was achieved by flattening the original surface by a defined proportion. Nine combinations of lat/med and ant/post tibial surfaces were designed (Fig 1). Test components were 3D printed. A test machine applied combinations of compressive force, shear force, and torque (Fig 2). The Method was based on the ASTM standard for Constraint. Displacements and rotations of the femoral on the tibial surfaces were measured.
RESULTS: The laxities of the femur on the tibial quadrant surfaces were directly related to the conformity (Fig 3). When the tibial surfaces were asymmetric medial/lateral, the displacements were higher on the less conforming side, with combined axial rotation. The laxities were higher in flexion than in extension due to the reduced femoral radius in the sagittal plane. Also, the large anterior radius of the femoral and tibial surfaces anteriorly, caused higher anterior displacements. The largest lateral and medial displacements were 7 and 5mm, rotations reached 10 deg. Lowest values were close to zero. With the geometries tested, close to anatomic laxity could not be obtained. However reducing the radius of the femoral medial distal-anterior, was predicted to result in close to normal laxity.
CONCLUSIONS: The laxity patterns were determined by the tibial radii in the 4 quadrants. Lateral and medial asymmetry was necessary, as was different anterior to posterior radii. Anatomic laxity patterns could only be achieved for a smaller radius medial dist-ant femoral condyle, and different tibial-femoral conformity in the 4-quadrants. Such surfaces did not however induce femoral rollback in flexion, including on the lateral side. This could possibly be achieved by PCL retention, or bearing surface modifications.
REFERENCES
ASTM F-1223-14 Measurement of Constraint ….
Bandi et al. Can the knee’s envelope of motion…..ISTA 2016
Bergmann et al Standardized Loads…. Plos One 2015.
Boguszewski Markolf et al. Male-Female Differences in Knee Laxity. AJSM 2018.
Mueller et al. Results of a robotic cadaver study…. ORS 2021.
Reynolds Walker Buza. Mechanisms of anterior-posterior stability. J Biomech 2017.
Thomeer Pandy et al Articular contact motion at the knee…. JOR 2022.
Walker Borukhov LiArno. Obtaining anatomic motion and laxity…. The Knee. 2022.
Figures
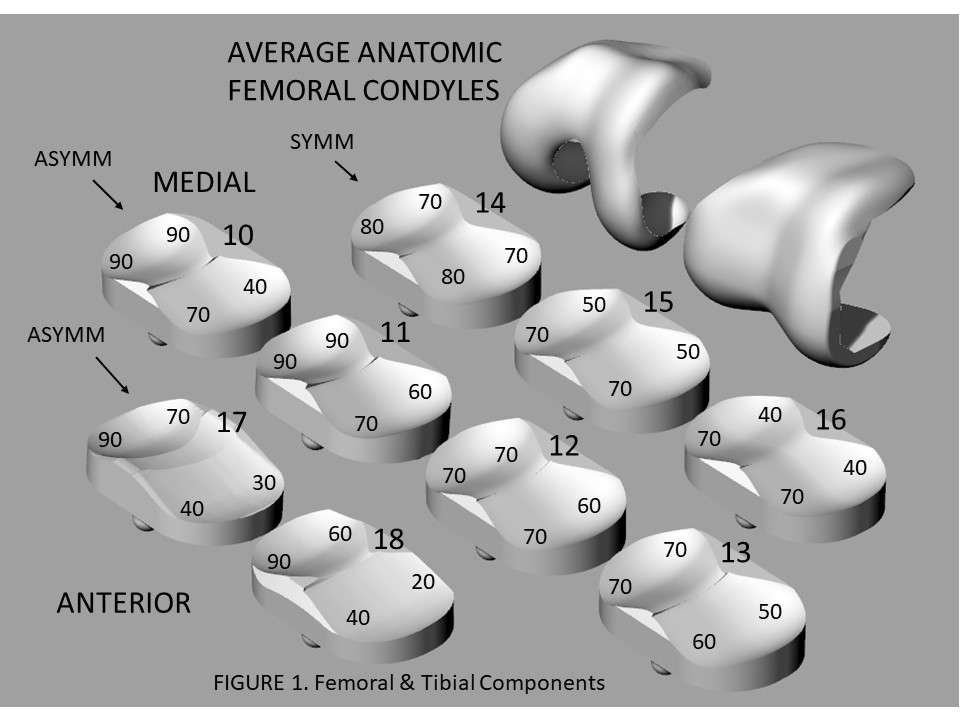
Figure 1
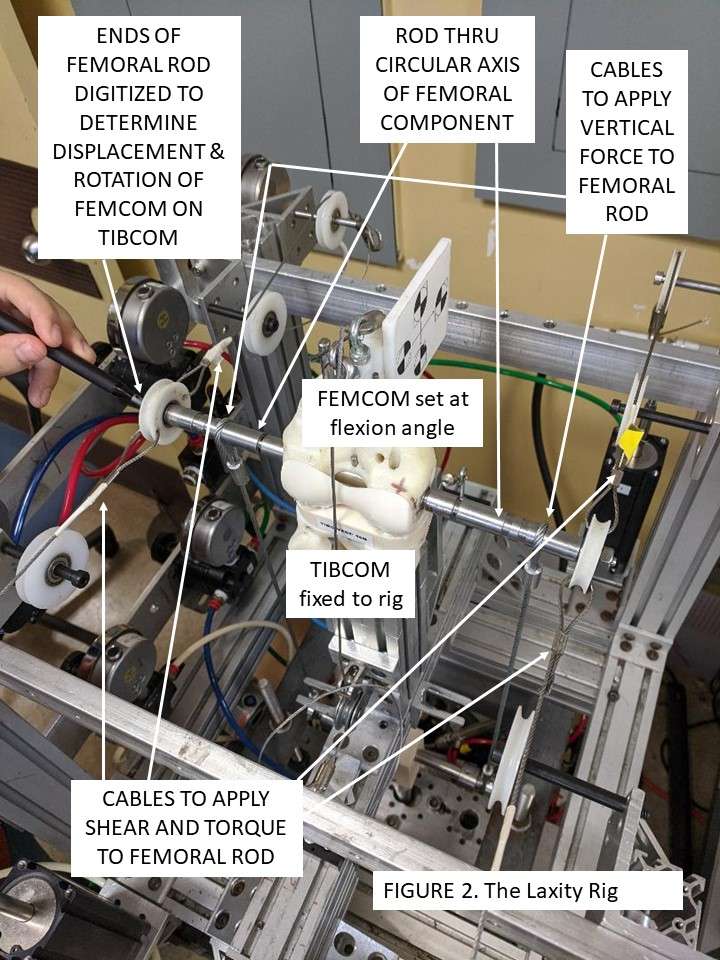
Figure 2
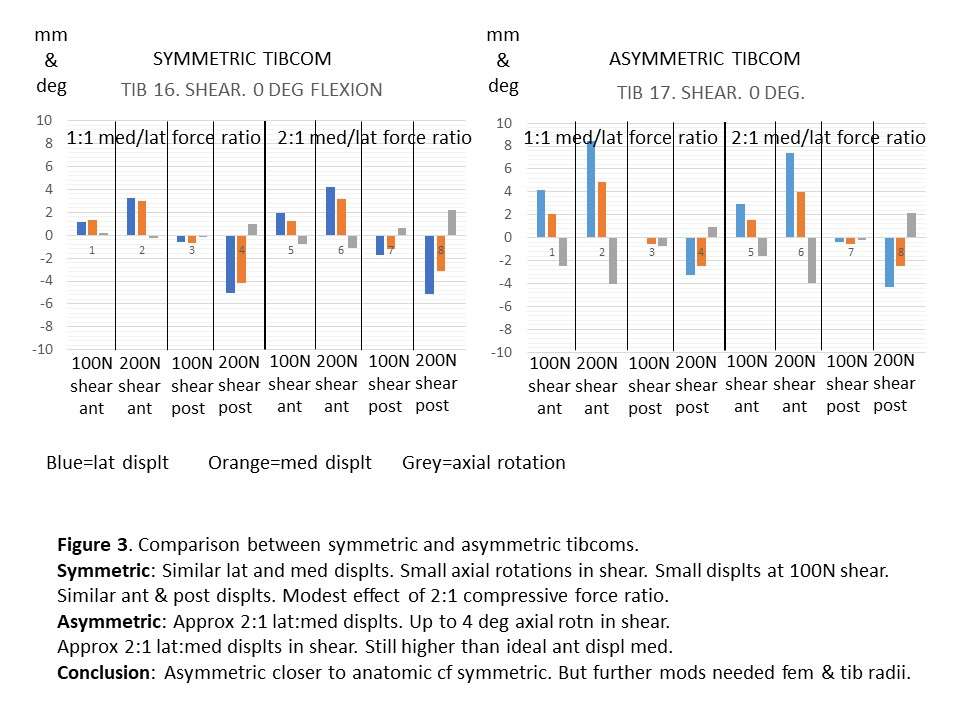
Figure 3#8222
Do Medial Congruent TKAs Offer Great Stability and More Normal-Like Kinematic Patterns? an in Vivo Fluoroscopic Study.
*Michael LaCour - University of Tennessee - Knoxville, USA
Garett Dessinger - The University of Tennessee - Knoxville, USA
Chad Krueger - Rothman Orthopaedic Institute - Philadelphia, USA
Richard Komistek - The University of Tennessee - Knoxville, USA
*Email: mlacour@utk.edu
INTRODUCTION
With increasing knee flexion, the normal knee experiences up to 27 mm of lateral condyle posterior motion and 10 mm of medial posterior motion, leading to external femorotibial rotation and a fan-like motion pattern (Figure 1). It is also documented that the medial condyle can move anteriorly, particularly in mid-to-late-flexion. Recently, the industry has been introducing polyethylene inserts with greater medial conformity, hoping to achieve less medial motion, more lateral rollback, and the associated fan-like motion. It has also been hypothesized that medial congruent total knee arthroplasties (TKAs) could be a replacement for posterior stabilized TKAs, leading to less femoral bone removal. The objective of this study is to determine the weight bearing kinematics for two TKAs having a medial congruent insert to assess if these TKAs experience more medial condyle stability, greater lateral condyle rollback, and more normal-like motion patterns.
METHODS
In vivo knee kinematics were assessed for 30 subjects implanted by the same surgeon: 15 with the Journey II Medial Dish TKA (J2MD, average postop = 6.7 months) and 15 with the Persona Medial Congruent TKA (PMC, average postop = 11.9 months). All surgeries were done with the same techniques and with resection of the PCL. Each subject was asked to perform a weight-bearing deep knee bend activity while under fluoroscopic surveillance, and the kinematics were derived using 3D model-fitting techniques. Medial (MAP) and lateral (LAP) condylar motion and femorotibial axial rotation (AxRot) were derived and compared to previous studies of the normal knee as well as PS and PCR TKA.
RESULTS
On average, subjects in this study experienced minimal condylar motion for both TKAs (Figure 2). The J2MD subjects experienced 2.0 ± 3.9 mm of posterior LAP from full extension to maximum flexion, and PMC subjects experienced 1.5 ± 3.3 mm. Three of 15 (20%) and 2/15 (13%) of the J2MD and PMC subjects experienced greater than 5.0 mm of posterior LAP, respectively. Five of the J2MD subjects and six of the PMC subjects experienced lateral condyle anterior motion. Both TKAs generally experienced anterior motion of their medial condyle, averaging 4.2 ± 2.2 mm and 2.5 ± 1.9 mm, for the J2MD and PMC groups, respectively. Finally, 14/15 (93%) of the J2MD Cohort experienced external AxRot, averaging 7.5 ± 4.4º, while 12/15 (80%) of the PMC Cohort experienced external AxRot, averaging 5.2 ± 5.2º (Figure 3).
CONCLUSION
Both groups did experience greater stability of their medial condyle compared to previous TKA studies, and a high incidence of subjects experienced medial anterior motion, similar to the normal knee. Unfortunately, the lateral motion was minimal for both TKAs, less than the normal knee as well as traditional PS and PCR TKAs. Nonetheless, both systems demonstrated good stability, and the added medial constraint was evident. With both cruciate ligaments resected and increased conformity on the medial insert, subjects did not experience much condylar motion, and it could be hypothesized that retaining the PCL may offer a benefit regarding posterior femoral rollback with increasing knee flexion.
Figures
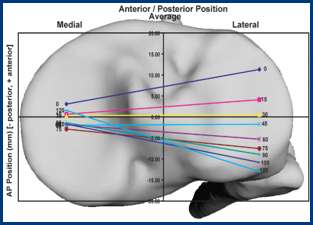
Figure 1
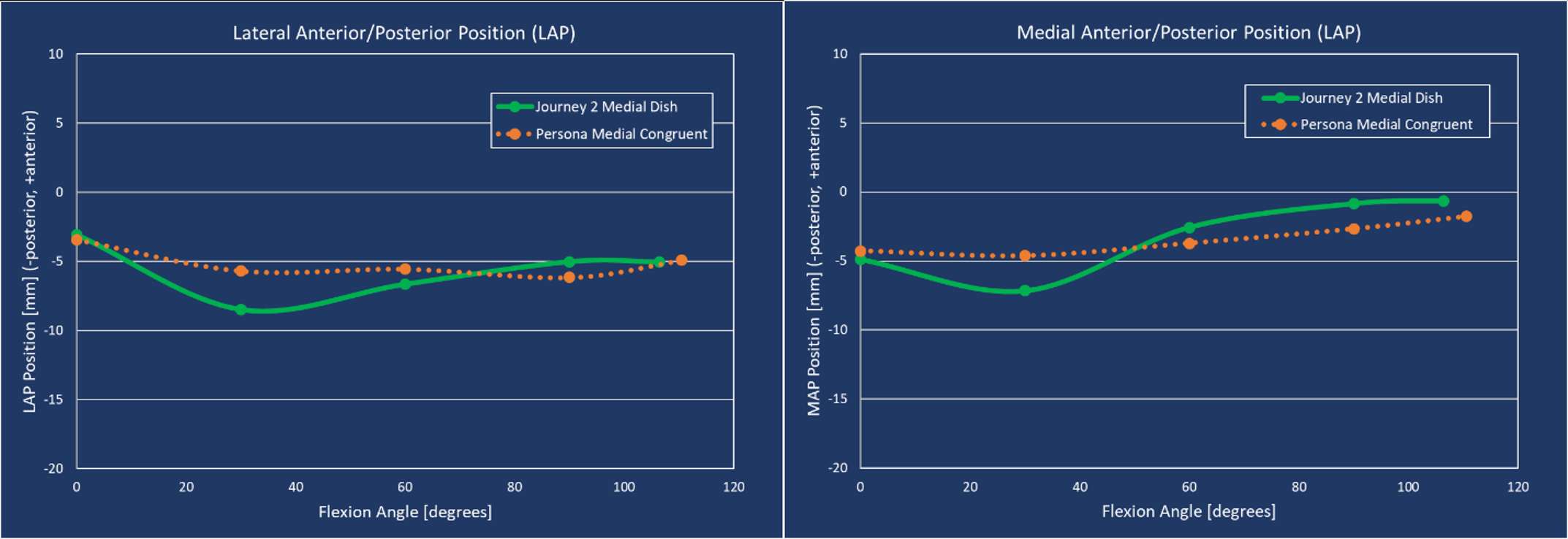
Figure 2
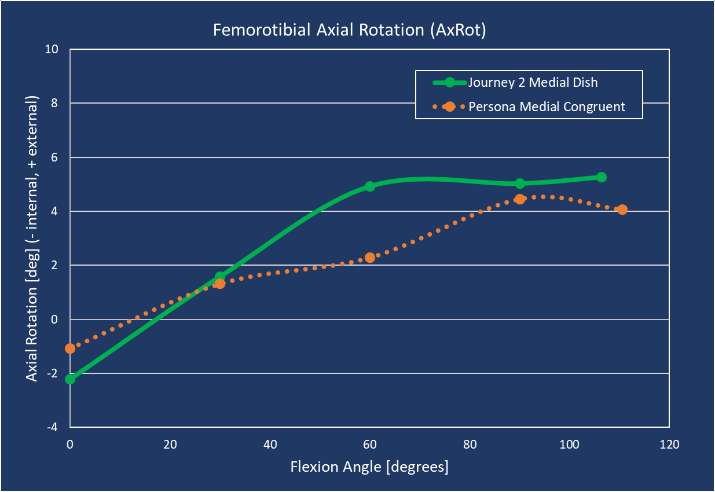
Figure 3#8324
Does Femoral Component Design Change Affect Loosening Rate of Femoral Component? a Retrospective Comparison Study With Conventional Design Versus High Flexion Design.
Youngchae Seo - Republic of Korea Air Force Education Command - Jinju, Korea (Republic of)
*Seung-Suk Seo - Department of Orthopedic Surgery, Haeundae Bumin Hospital - Busan, South Korea
*Email: wellknee@gmail.com
Introduction: High-flexion designs for total knee arthroplasty (TKA) have been introduced and promoted greater and safer knee flexion. However, loosening of the femoral component related to the deep flexion has been reported with high incidence. The purpose of this study is to evaluate clinical and radiological results of newly designed high-flexion TKA compared to conventional TKA at a long-term follow-up, and to analyze the implant survivorship based on the results.
Materials and Methods: This study included 165 patients (231 knees) who underwent TKA with Vega® Knee System (B Braun-Aesculap, Tuttlingen, Germany), which set as a study group, and 180 patients (259 knees) who underwent TKA with PFC Sigma PS® Knee System (DePuy, Warsaw, IN, USA), set as a control group, between March 2011 and February 2015. We evaluated the range of motion (ROM), Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC), Knee injury and Osteoarthritis Outcome Score (KOOS), radiographic results, prevalence of radiolucent line and complication rates in both patient groups respectively with an average 9.8 years of follow-up (range: 8.7-11.1 years).
Results: The mean ROM in the study group (131.1°) was greater than that in the control group (127.3°) at the final follow-up. In both groups, the WOMAC score and all 5 subscales of KOOS score (Pain, Symptoms, ADL, Sport/Rec and QOL) improved at the final follow-up. In the degree of improvement, the study group was significantly higher than the control group (P=0.03). There was no significant change in the alignment of implant measured as coronal and sagittal alignment of femoral and tibial components at the last follow-up compared to the immediate postoperative values in both groups (p>0.05). Radiolucent lines around femoral component was significantly higher in the study group (41 knees, 17.7%) than the control group (1 knees, 0.4%) on the last follow-up X-rays. There were 10 revision TKAs in the study group due to 9 cases of femoral component loosening (3.8%) and 1 case of infection. In the control group, there were 1 revision TKA caused by infection and 1 arthroscopic synovectomy for patella clunk syndrome. The survival rate of the study group (95.7%) was significantly lower than the control group (99.2%) at the postoperative 9.8 years.
Conclusion: The newly designed high-flexion TKA offers higher ROM compared to the conventional TKA. However, the prevalence of the radiolucent line and the aseptic loosening of the femoral component were significantly higher in high-flexion TKA than conventional TKA.
#8188
Kinematic Evaluation of Posterior-Stabilized Total Knee Arthroplasty With Fixed Bearing vs Mobile Bearing Insert Implanted With Mechanical Alignment: An in-Vivo Dynamic RSA Study
Stefano Zaffagnini - Researcher Istituti Ortopedici Rizzoli - Bologna University Sports - Bologna, Italia
Raffaele Zinno - University of Bologna - Bologna, Italy
Stefano Di Paolo - University of Bologna - Bologna, Italy
Nicola Pizza - IRCCS Istituto Ortopedico Rizzoli - Bologna, Italy
Giulio Maria Marcheggiani Muccioli - University of Bologna - Bologna, Italy
Laura Bragonzoni - University of Bologna - Bologna, Italy
*Domenico Alesi - IRCCS Istituto Ortopedico Rizzoli - Bologna, Italy
*Email: domenicoalesi@ymail.com
Introduction
To investigate in vivo kinematics of the same femoral design mechanically aligned posterior-stabilized (PS) total knee arthroplasty (TKA) with either fixed bearing (FB) or mobile bearing (MB) inlay, implanted by the same surgeon, using model-based dynamic radiostereometric analysis (RSA). The hypothesis was the finding of different kinematics behaviour between the two groups.
Methods
A cohort of 21 non-randomized patients (21 DePuy Attune PS-FB) was evaluated by dynamic RSA analysis at a minimum 9 month follow-up, while performing differently demanding daily living activities such as Sit-to-Stand (STS) Deep Knee Lunge (DKL). Kinematic data were compared with those of a cohort of 22 patients implanted with the same prosthetic design but with MB inlay. Anterior-posterior (AP) translations, varus-valgus (VV) and internal-external (IE) rotations of the femoral component respect to the tibial baseplate were investigated. Translation of medial and lateral compartment were analyzed using the Low Point method according to Freeman et al.
Questionnaires to calculate objective and subjective clinical scores were administered pre-operative and during follow up visit by the same investigator.
Results
FB TKA design showed lower AP translation during STS (6.8 ± 3.3 mm in FB vs 9.9 ± 3.7 mm in RP, p=0.0061*), lower VV rotation (1.9 ± 0.8° in FB vs 5.3 ± 3.3° in RP, p=0.0053*) and IE rotation (2.8 ± 1.1° in FB vs 9.5 ± 4.3° in RP, p=0.0013*) during DKL respect to MB TKA design.
PS FB group showed significantly lower translation of the Low Point of the medial compartment respect to the MB group(p < 0.01) [Fig. 1].
A significantly higher rate of patients with medial pivot in the FB group than in the MB group was detected in all examined motor tasks (STS - 81% in FB vs 57% in RP, Flexion phase of DKL - 89% in FB vs 57% in RP, Extension phase of DKL - 63% in FB vs 48% in RP) [Fig. 2].
Post-operative range of motion (ROM) clinically evaluated by the same operator using a goniometer was 117°±16° for FB group and 124°±13° for MB group.
No significant differences in post-operative range of motion (117°±16° for FB group and 124°±13° for MB group) and in clinical outcomes emerged between the two cohort.
Conclusions
Statistically significant differences were found between the two investigated designs in post-operative AP translations, VV and IE rotations of femoral respect to tibial component respectively in STS and DKL. Furthermore, FB cohort reported a significant higher percentage of medial pivot respect to MB cohort. Despite this, no differences in clinical outcomes were detected between groups. Both designs showed stable kinematics and represent a viable alternative in primary TKA.
Figures
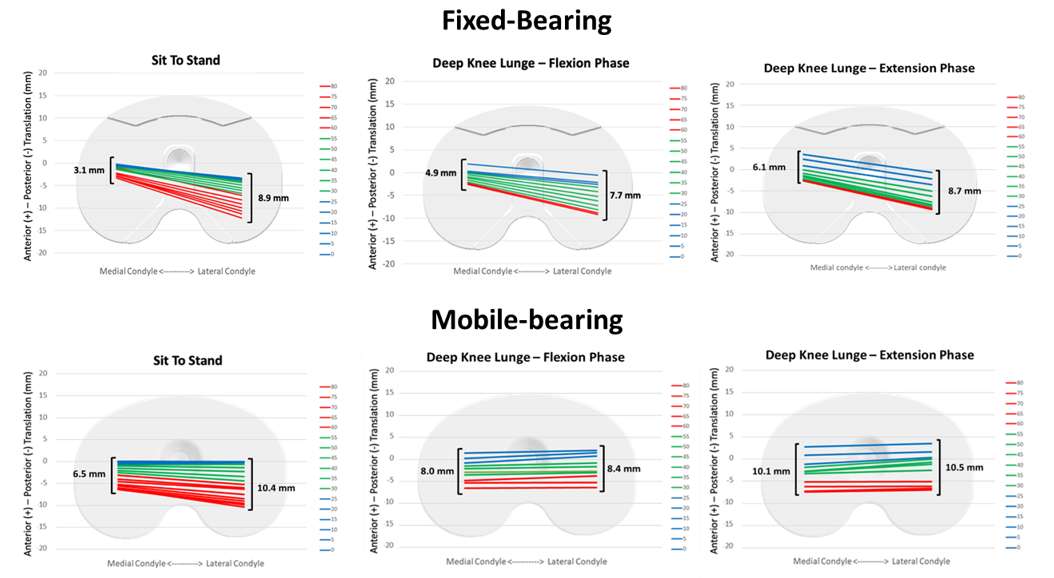
Figure 1
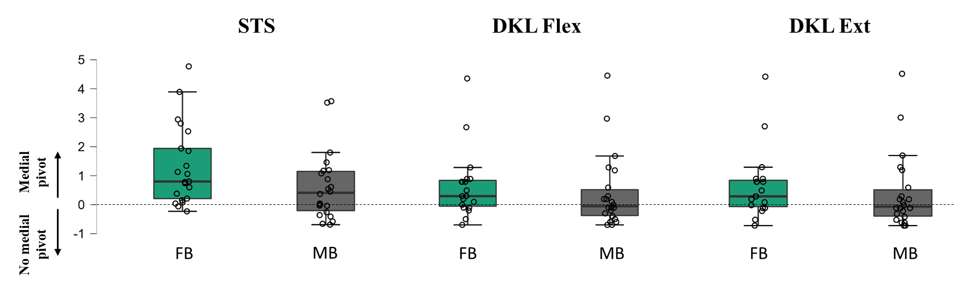
Figure 2#8507
Initial and Long-Term Bone Strains Associated With Solid and Lattice UKA
*Jennifer Stoddart - Imperial College London - London, United Kingdom
Maxwell Munford - Imperial College London - London, United Kingdom
Jonathan Jeffers - Imperial College London - London, United Kingdom
*Email: jcs113@ic.ac.uk
Introduction: Unicompartmental knee arthroplasty (UKA) is a successful treatment for osteoarthritis, but conventional solid metal implants disrupt the natural load transfer in the bone, leading to chronically strain-shielded and overstrained regions of bone. This can cause problems with long-term fixation and loss of bone density. Low-modulus titanium lattice UKA implants have previously been shown to maintain natural loading in the proximal tibia. Initial fixation in cementless implants is provided by an interference fit between implant fixation features and bone. It is unknown how introducing a low-modulus titanium lattice implant affects these initial bone strains in comparison to conventional solid implants.
Methods: A previously experimentally validated finite element model of a male proximal tibia with a medial UKA was prepared, both with a solid (E = 114GPa) and lattice (E = 980MPa) implant in a surgeon-planned position. Simulations representative of stages of osseointegration were investigated. First, an osseointegrated model where the implants were seated with the bone cuts flush to the geometry of the fixation pegs and keel, with a glued model describing the contact between the implant and bone. Medial condylar loads representing peak loads during sit-to-stand were applied. Second a primary fixation model where the keel and and peg cavities were modelled to prepresent a 0.25mm press fit between the implant and bone with sliding contact was investigated. Fixed displacement boundary conditions were applied to the proximal transverse surface of the implant in the primary fixation model. The distribution of strains in the bone was compared for the two implant materials.
Results: In the osseointegrated bone model, the 95th percentile minimum principal elastic strain in the medial condyle was 30% of the compressive yield strain of proximal tibial bone in the lattice implant, while in the solid implant it was 19%. The 95th percentile maximum principal strains were 23% of the tensile yield strain for the lattice implant and 15% for the solid implant.
In the initial fixation bone model, small differences were found between the results predicted in the lattice and solid implant models [Fig 1]; the mean equivalent elastic strains predicted in the medial tibial condyle were within +/-5% of each other [Fig 2]. As is expected with implant-bone interference interfaces, both elastic and plastic deformation was predicted in each implant model.
Conclusions: Implants made from a low-modulus lattice do not result in any substantial increase in the damage to the bone during initial fixation compared to conventional-material implants. Once the bone has adapted to the implant and osseointegration is achieved however, a low-modulus lattice UKA allows the bone around the implant to strain more than with a conventional solid implant, though the strain does not approach the yield strain of bone during activities of daily living.
Figures
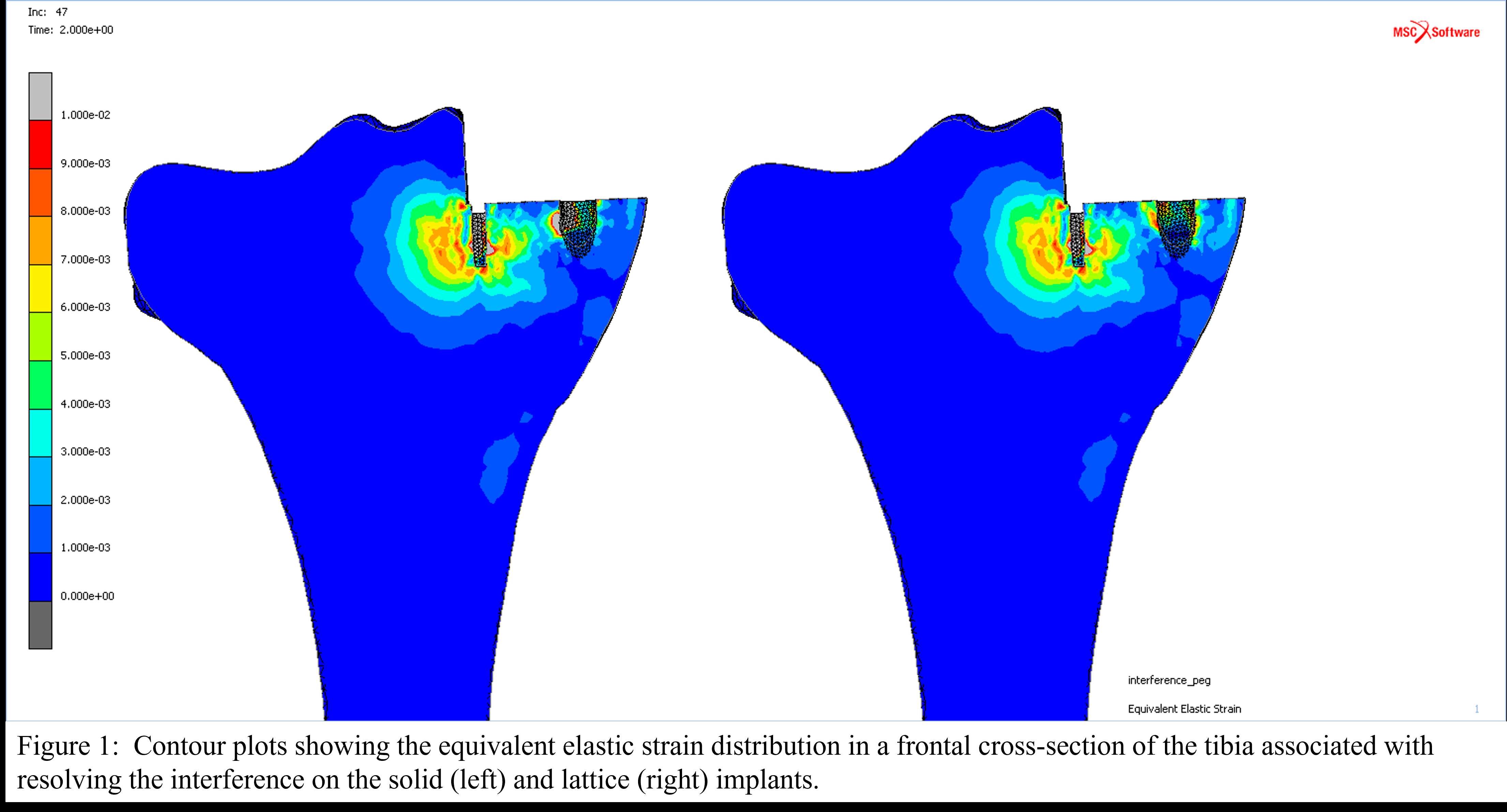
Figure 1
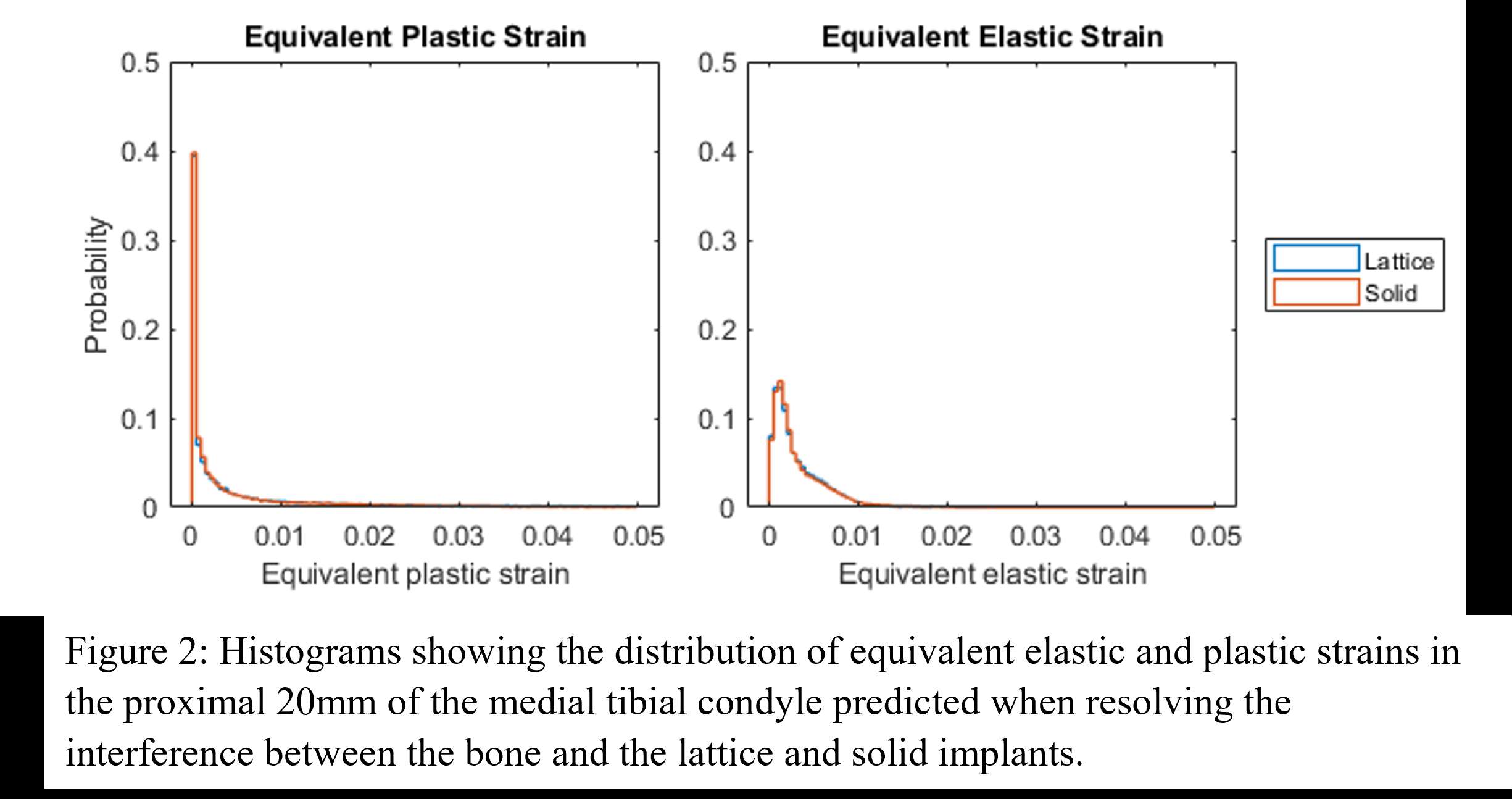
Figure 2#8259
3D Printed Porous Pegs Match Solid Peg Micromotion Performance
*Arron Hughes - OSSTEC - London, GB
Maxwell Munford - Imperial College London - London, United Kingdom
Jonathan Jeffers - Imperial College London - London, United Kingdom
*Email: arron@osstec.uk
Introduction
Initial stability of cementless tibial trays is crucial for effective osseointegration and long-term success of Unicompartmental and Total Knee Arthroplasty procedures (UKA & TKA). Traditional implants use solid metal press fit fixation features to obtain initial fixation and stability, but there is considerable interest in using additively manufactured (AM) lattice surfaces for bone ingrowth. These surfaces give greater porosity for bone ingrowth and provide a more compliant modulus to minimise the stiffness mismatch between implant and bone.
This study aims to compare the micromotion of AM lattice tibial pegs to a conventional peg used in tibial tray designs.
Methods
Two sets of tests were performed, micromotion and pull out. For micromotion testing – AM lattice tibial pegs and conventional solid pegs were manufactured using powder bed fusion, inserted into pre drilled 6mm holes in 15 PCF Saw Bone and subsequently cycled axially at 2Hz for 2000 cycles using a servohydraulic Instron 8874 with a load of 520N to 52N. Two LVDTs were used to measure the displacement of the saw bone and the peg sample during the test.
For pull out tests - AM lattice tibial pegs and conventional solid samples were axially inserted and removed into prepared 6mm holes in 15 PCF Saw Bone samples using a screw driven single axis Instron 5565 at a rate of 1mm/s
All pegs were manufactured in Ti alloy. The solid peg had a 0.25 mm interference fit. There were four designs of AM lattice pegs, with varying Elastic moduli (E=2.6GPa & E=3.6GPa) and radial interference fits (0.25mm, 0.5mm and 0.75mm).
Comparisons between all samples were made for micromotion, push in force and peak pull out strength using ANOVA, and Pearson correlation. For all AM lattice pegs, micromotion results were analysed relative to their solid metal counterparts by comparing normalised values for micromotion rather than raw data.
Results
Lattice hybrid pegs (Figure 1) showed comparable micromotion to conventional solid pegs (p=0.64) but with an average 15% reduction in push in force relative to the solid peg.
Increasing the radial interference from 0.25mm to 0.5mm reduced micromotion by 14% (p=0.039) while increasing required push in force by 51%. An increase to 0.75mm radial interference reduced micromotion by 1% (p=0.963) with a push in force increase of 27%. The correlation between pull out force and micromotion was found to be moderately strong at -0.733 and -0.688 for push in force and micromotion (Figure 3).
Conclusions
Titanium lattice cementless fixation features can achieve comparable micromotion performance to conventional solid fixation features while controlling and maximising the porosity for long term bone in-growth and reducing the risk of bone fracture by necessitating lower push in forces with the same radial interference.
Additively manufactured lattice tibial pegs for UKAs or TKAs can achieve both the short term requirements of initial fixation stability and reduction of micromotion, with sufficient porosity and strength to encourage long term bone growth and implant stability for a new generation of younger active patients.
Acknowledgments
Special thanks to Stephen Johnson for Rig Manufacture
Figures
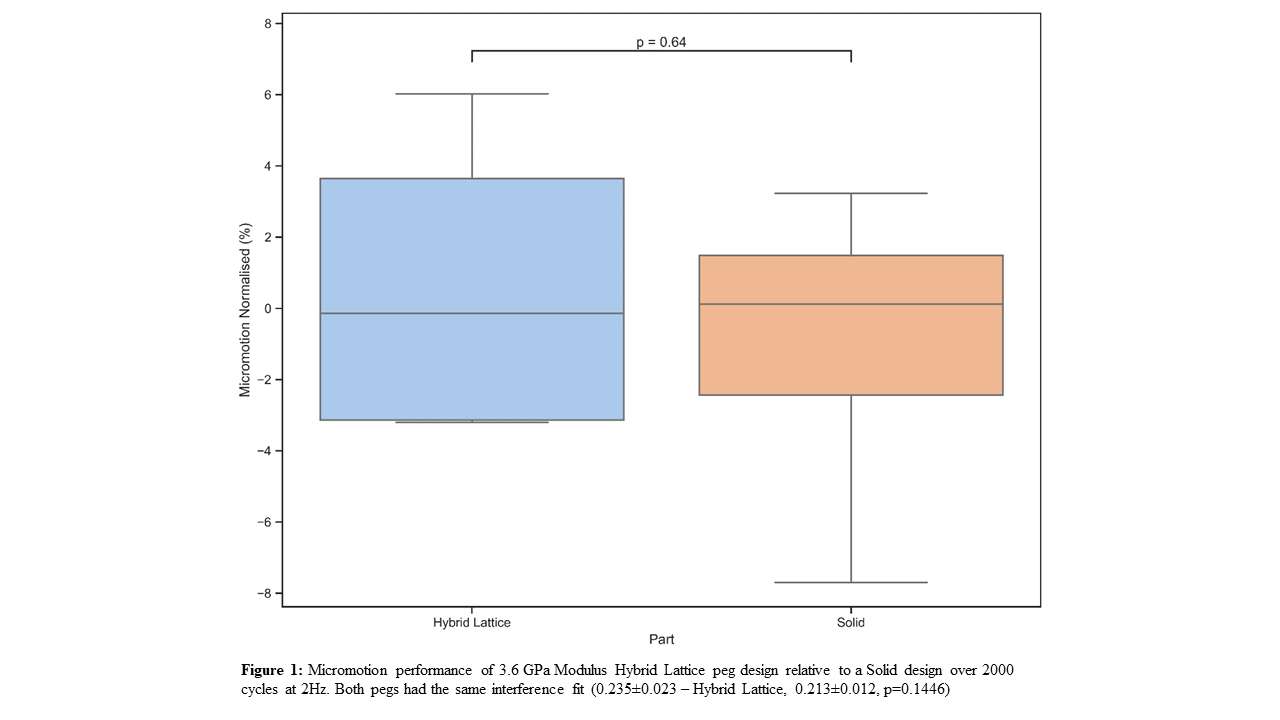
Figure 1
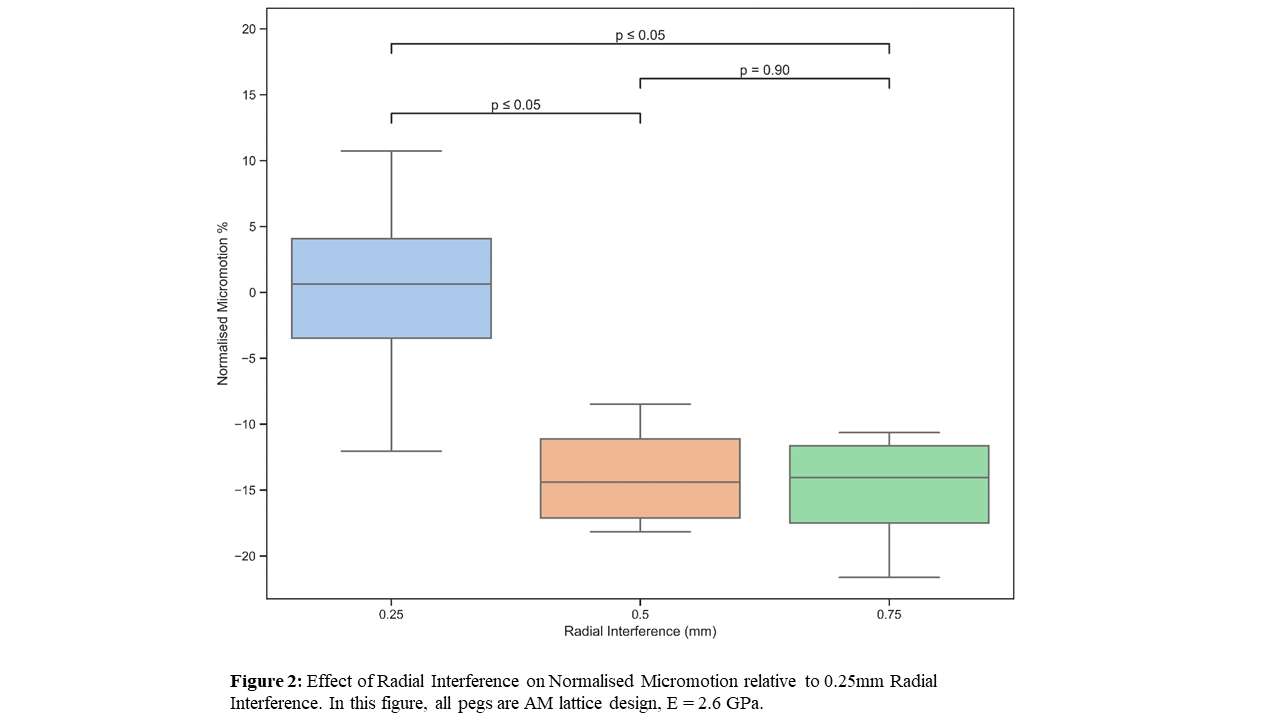
Figure 2
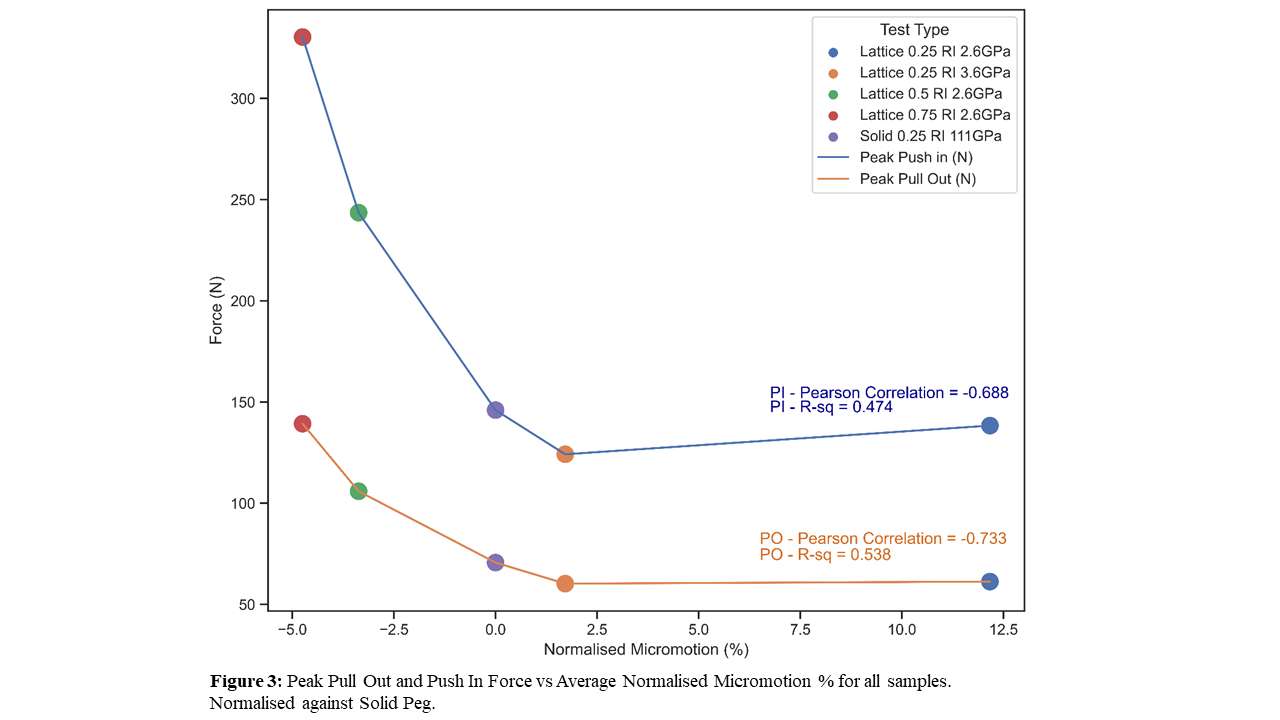
Figure 3#8194
Assessment of Hinged Knee Replacement Designs Using a Biomechanical Knee Simulator
Kenneth Pascale - Stryker - Mahwah, USA
Kevin Abbruzzese - Stryker - Mahwah, USA
Damon Servidio - Stryker - Mahwah, USA
*Emily Hampp - Stryker - Mahwah, USA
Rebecca Gonzalez - The Pennsylvania State University - University Park, USA
Lauren Hickox - The Pennsylvania State University - University Park, USA
Stephen Piazza - The Pennsylvania State University - University Park, USA
*Email: emily.hampp@stryker.com
Introduction: The ability to extend the knee and stabilize the patellofemoral joint under load is critical to functional recovery after revision knee arthroplasty. The capacity for knee extension and patellofemoral stabilization depends in part on the efficiency of the muscular extensor mechanism, which may be influenced by implant design. The purpose of this study was to compare extensor efficiency in terms of quadriceps force, quadriceps effective moment arm, patellofemoral force and patellar flexion between a clinically successful, legacy hinged knee design [Wignadasan 2021] and a novel design with an anatomical patellar track. A non-cadaveric knee simulator was used to assess performance between devices during a quadriceps-driven knee extension with range of motion consistent with a chair rise activity.
Methods: A non-cadaveric knee simulator based on the Oxford Rig design was used to model loaded knee extension, which was driven by a single linear actuator mounted on the femur representing the action of the quadriceps (Figure 1). A knee extension from ~105° to ~25° flexion was performed, and two hinged total knee replacements (legacy design and novel design) were compared. Implants were affixed to the femur and tibia segments. Quadriceps tension was monitored using a tension load cell in line with the actuator. Quadricep and patellar tendons were modeled with Kevlar straps. A 4-camera motion capture analysis system was used to track six degree of freedom segment motions at 100Hz using marker clusters affixed to the femur, tibia and patella (Figure 2). Quadriceps force, effective moment arm, patellofemoral force and patellar flexion were compared between devices. Both parametric and non-parametric tests were used to assess significance at the α = 0.05 level.
Results: The novel hinged implant demonstrated a significantly greater effective moment arm than the legacy implant at 90° of knee flexion (median: 18 mm vs. 14 mm, p < 0.001). The longer quadriceps effective moment arm of the novel design required 38% less quadriceps force at 90°, when compared to the legacy design (mean: 429 N vs. 699 N, p < 0.05). The novel hinged implant also demonstrated reduced patellofemoral force (mean: 490 N vs. 765 N, p < 0.001) and a lower patellar flexion angle (mean: 42° vs. 48°, p < 0.001) at 90° of knee flexion.
Conclusion: The novel hinged total knee replacement was able to extend the knee using less quadriceps force, which was in part due to a longer quadriceps effective moment arm. Additionally, the novel design demonstrated reduced patellofemoral force and patellar flexion at 90° of knee flexion, compared to the legacy design. These findings may be attributed to an anatomical patellofemoral geometry in the novel hinged implant, which may have implications for improved postoperative functional performance. Greater patellofemoral interaction may contribute to enhanced stability of the knee with biomechanically efficient movements. A limitation of the model was that mechanical analogs were used to mimic the effect of soft tissues. Future clinical studies are warranted on the potential benefits of the novel design in terms of functional recovery after revision knee arthroplasty.
Figures
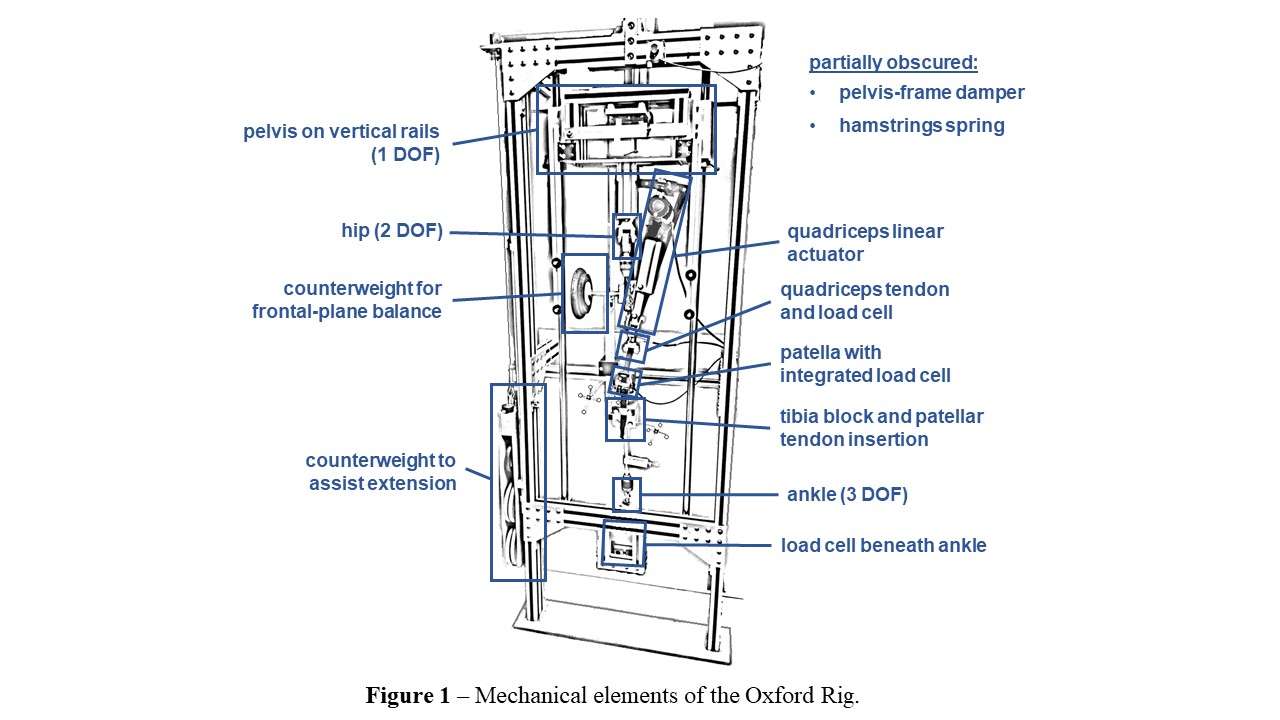
Figure 1
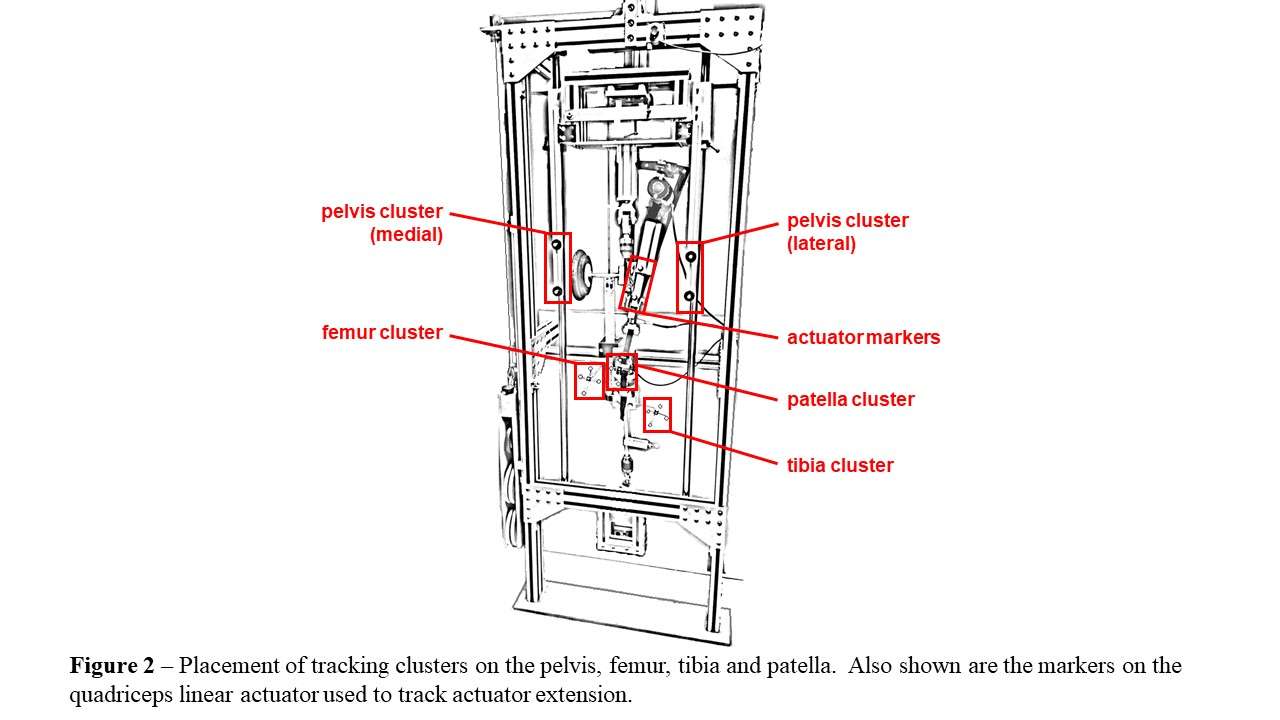
Figure 2POSTERS